Abstract
Introduction:
Typical chest pain symptoms are the cause that requires individuals to seek out medical care in Acute coronary syndrome(ACS). Evidence suggests, symptoms labelled as 'atypical 'is more common in women with ACS. The present study focuses on the need for the implementation of a gender specific approach in the current scenario by identifying gender based differences that exist in clinical presentations of the patients with ACS. Early identification of women's prodromal and acute symptoms of Myocardial Infarction is an important step in provision of appropriate treatment modality. Present study focus on need for implementation of gender-specific approach in current scenario by identifying gender based differences that exist in risk factors, clinical manifestations in patients presenting with MI.
Methodology:
Cross- sectional analytical study was conducted among 240 Participants (120 males and 120 females). Both men and women diagnosed with MI, who survived, stabilized after admission was included in the study. Consecutive sampling technique was used to select the participants. Data was collected on risk factors profile, clinical manifestations by administering structured questionnaire.
Results:
Risk factors such as history of diabetes, history of dyslipidemia was found to be homogenous among both men and women. 60% of men were ever smokers. Hypertension and known IHD was noted to be significant in women (p<0.002, p <0.001) but men presented with higher BMI (p<0.030). Females increasingly presented with atypical presentations when compared to males (p<0.005). Women commonly had squeezing and tightness type of pain and men reported tightness, burning, pricking type of pain (p<0.003). The majority of the women reported the onset of pain occurrence between 6am to 12 pm(p<0.004), whereas men significantly reported the onset of pain between 12 am -6 am(p<0.001).
Conclusion:
Gender based differences in risk factors and clinical presentation in men and women with myocardial infarction had been a focus in researches that emphasized need for focused assessment for women as they increasingly presents with atypical symptoms. The current study also supports the need of a gender specific approach to avoid delay in diagnosis and care of them.
Keywords: Acute coronary syndrome, clinical manifestations, gender differences in MI, myocardial infarction, risk factor
INTRODUCTION
Cardiovascular diseases (CVDs) are a major threat to the living society as its growth is devastating since decades. Primarily, these diseases occur due to interaction of many risk factors that are associated with an individual and modification of lifestyle plays an important role in improving heart disease.
Coronary artery disease (CAD) develops as a result of plaque deposition within coronary arteries. Formation of blood clot can result due to rupture of plaque causing ischemic changes in myocardium.[1] Myocardial infarction (MI) occurs as result of prolonged myocardial cell ischemia with involvement of myocardial necrosis. The statistics revealed by the WHO showed an estimation of 17.5 million people died with the cause of CVD, which constitutes about 31% of all global deaths, and cardiac diseases will be leading causes of disability.[2,3,4]
Several studies have been conducted in analyzing the epidemiology and case fatality of MI to identify the declining trends of mortality in CVD.[5] Evidence shows sex-specific patterns and a diverged trend in the incidence of MI with an increased incidence in women and elderly. The cardiovascular disease has perceived to be primarily concerned with men. However, mortality and morbidity of this disease are taking a leading role in women who constitute about 48% of Indian population. Women mostly present with atypical presentations, and higher index of suspicion is required while evaluating women with MI.[6,7,8]
METHODOLOGY
Cross-sectional analytical study was done among 120 males and 120 females who were admitted with MI in a tertiary care center in south India for the period of 1 year to identify difference in clinical presentation of patients with MI between men and women.
Typical chest pain is defined as sensation of pain in chest, mostly in the retrosternal region with nature of either squeezing, pressing, tightness, burning, heaviness which is radiating to neck, shoulder, and left arm. Other than these symptoms such as dizziness, sweating, shortness of breath, vomiting, palpitation, fainting, back pain, and fatigue was considered as atypical chest pain in this study.
Sample size was calculated in “n master 2.0” by using proportion of atypical clinical features in male and female as 29% and 42%, and considering finite population of MI patients in our settings during study period to be 250, and relative precision of 20%, with power as 80%, it was calculated to be sample size of 120 in each arm including 5% attrition.
Inclusion criteria were both men and women diagnosed with MI, who survived and stabilized. Consecutive sampling technique was used to select the participants. Structured questionnaire was developed as data collection tool. The tool consisted section A which included sociodemographic data, section B included risk factor survey including body mass index (BMI), blood pressure (BP), nature of work, family history of CAD, level of physical exercise, dietary pattern. history of smoking and alcoholism, comorbid illness. Section C included clinical presentation survey deals with description of pain, intensity of pain, location of pain, nature of pain, history of atypical presentation, time and circumstance of onset of pain. Permission was obtained from the Institute ethical committee, human studies, Reg. No: JIP/IEC/2016/1110. Ethical issues involved in the study were less than minimal risk. Informed consent was obtained from every participant after a brief explanation regarding the study by the investigator.
Statistical analysis
To compare the means of BP and weight, independent sample t-test was used. Chi-square/Fisher's exact test was used to compare the clinical characteristics, risk factors profile, location/nature of pain, and comparison of atypical manifestations. Mann–Whitney U-test was used for comparison of description of pain among men and women with MI.
RESULTS
The mean age of the participants was 54 versus 56 years among men and women. Groups were comparable in both systolic and diastolic pressure mean values. The male gender preponderance was noted in regard to higher BMI when compared to women (P < 0.030). Sedentary lifestyle pattern was prominent in women when compared to men which was significant at P < 0.000. The groups were found to be homogenous in other factors including family history of CAD, exercise pattern, dietary history, and previous history of MI [Table 1].
Table 1.
Clinical characteristics
| Name of variables | Male (n=120), n (%) | Female (n=120), n (%) | P # |
|---|---|---|---|
| Age (years), mean±SD | 54.8±10.04 | 56.8±9.84 | 0.107# |
| Blood pressure | |||
| Systolic BP | 117.54±18.75 | 127.88±18.98 | 0.672# |
| Diastolic BP | 74.46±10.64 | 79.97±11.39 | >0.995 |
| Underweight | 3 (2.5) | 5 (4.2) | 0.030* |
| Normal weight | 31 (25.8) | 51 (42.5) | |
| Overweight | 30 (25) | 25 (20.8) | |
| Obese | 56 (46.7) | 39 (32.5) | |
| Sedentary lifestyle | 53 (44.2) | 103 (85.8) | <0.001$ |
| Family history of CAD | 21 (17.5) | 32 (26.7) | 0.087$ |
| Previous MI | 19 (15.8) | 19 (15.8) | >0.995$ |
#Independent sample t-test, *Fisher’s exact test, $Chi square. SD: Standard deviation, BP: Blood pressure, MI: Myocardial infarction, CAD: Coronary artery disease
History of percutaneous coronary intervention, history of diabetes, and history of dyslipidemia were found to be homogenous among both men and women. Sixty percent of men were ever smokers. With regard to hypertension as comorbidity, there was increased frequency noted in women (P < 0.002). Furthermore, known ischemic heart disease was observed as comorbidity among women than men (P < 0.001). Further, 78.3% of female participants have attained menopause. Seventeen had hypertensive disorder during pregnancy, among them 70.6% had taken treatment regimen for hypertensive disorders [Table 2].
Table 2.
Risk factor profile in both genders
| Name of variable | Frequency (%) | P # | |
|---|---|---|---|
|
| |||
| Male (n=120), n (%) | Female (n=120), n (%) | ||
| Smoking | 72 (60) | 0 | <0.001** |
| Hypertension | 51 (42.5) | 75 (62.5) | <0.001** |
| Diabetes | 53 (44.2) | 65 (54.2) | 0.121 |
| Dyslipidemia | 21 (17.5) | 24 (20.0) | 0.620 |
| Prior PCI | 8 (6.7) | 2 (1.7) | 0.053 |
| Comorbid illness | |||
| Known IHD | 17 (14.2) | 39 (32.5) | 0.001** |
| Co-morbidities* | 103 (85.8) | 81 (67.5) | |
| History of menopause | 94 (78.3) | ||
| History of hypertension during pregnancy | 17 (14.2) | ||
#Chisquare test, **P<0.001, *Bronchial asthma, hypothyroidism, COPD. COPD: Chronic obstructive pulmonary disease, PCI: Percutaneous coronary intervention, IHD: Ischemic heart disease
Location of pain during MI did not vary among men and women including typical symptom of retrosternal pain radiating down left arm (23.7% vs. 24.3%). Although no statistical significance was noted in relation to atypical symptom between the groups, the increased frequency of pain was noted in the upper chest and intrascapular region among women (12.6% vs. 4.4%) [Table 3].
Table 3.
Comparison of location of pain among both genders with myocardial infarction
| Name of variable | Frequency (%) | P # | |
|---|---|---|---|
|
| |||
| Male (n=115), n (%) | Female (n=112), n (%) | ||
| Retrosternal radiating down left arm | 27 (23.9) | 27 (24.3) | 0.284 |
| Left upper chest | 52 (46.0) | 47 (42.3) | |
| Epigastric | 11 (9.7) | 11 (9.9) | |
| Upper chest, intrascapular | 5 (4.4) | 15 (12.6) | |
| Left shoulder down both arms | 8 (7.1) | 7 (6.3) | |
| Substernal radiating to neck and jaw | 12 (8.8) | 5 (4.5) | |
#Chi-square test
The onset of pain was very abrupt for both men and women. Constant mild chest pain was predominantly reported by men when compared to women, 28.3% versus 15% (P < 0.011). About 85% of females were presented with atypical manifestations such as dizziness, sweating, shortness of breath, vomiting, palpitation, fainting, back pain, and fatigue (P < 0.005) compared to 70% in men (P < 0.005) [Table 4].
Table 4.
Comparison of description of pain among men and women with myocardial infarction (n=240)
| Name of variable | Frequency (%) | P # | |
|---|---|---|---|
|
| |||
| Male (n=120), n (%) | Female (n=120), n (%) | ||
| Very abruptly | 43 (35.8) | 54 (45) | 0.092 |
| Began gradually and reached peak within several minutes | 4 (3.3) | 3 (2.5) | 0.368 |
| Began gradually and reached its peak after 30 min | 28 (23.3) | 19 (15.8) | 0.092 |
| Constant mild chest pain | 34 (28.3) | 18 (15) | 0.011* |
| On and off chest pain | 6 (5.0) | 18 (15) | 0.111 |
| No chest pain | 5 (4.2) | 8 (6.7) | 0.21 |
#Mann–Whitney U-test, *P<0.05
The groups were comparable in the perception of severe pain. However, women had more perception of moderate pain, whereas men had more perception of mild pain and overall, it was statistically significant at P < 0.032 [Figure 1].
Figure 1.
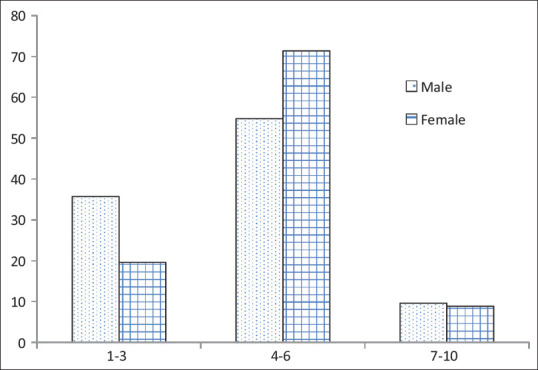
Comparison of intensity of pain among men and women with myocardial infarction # Mann–Whitney U-test, *P < 0.05. Numerical pain scale (scoring: 1–3 mild pain, 4–6 moderate pain, 7–10 severe pain)
Majority of women reported the onset of pain occurrence between 6 am and 12 pm (P < 0.004), whereas men significantly reported the onset of pain during 12 am to 6 am (P < 0.001). Women commonly had squeezing (23.2%) and tightness (40.4%) type of pain whereas men reported tightness (42.6%), burning (34.8%), pricking (4.5%) type of pain which was statistically significant at P < 0.003 [Figures 2 and 3].
Figure 2.
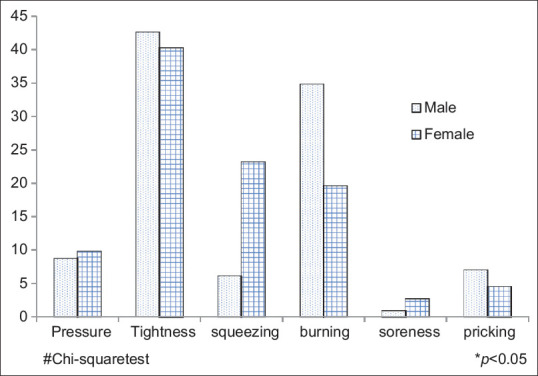
Comparison of nature of pain among men and women with
Figure 3.
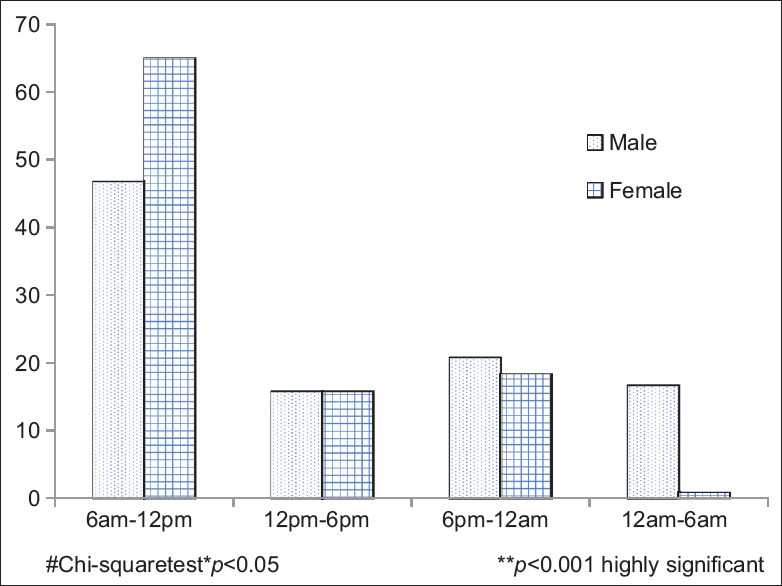
Comparison of time of onset of pain among men and women with myocardial infarction
DISCUSSION
Age distribution in the current study shows that the mean age of women is higher than men, however, there was no statistical significance in age distribution in both genders (P < 0.10). This distribution was comparable to findings of a European study where women had presentation of MI in later stage of life.[8] Duraes et al. had similar findings which showed that the mean age of women was higher compared to men, 60.5 years versus 56.3 years, respectively. The later presentation of MI among women is possibly due to the effect of protective hormone, estrogen in premenopausal stage which slows the progression of atherosclerosis.[9,10,11]
The current study shows that mean systolic and diastolic BP is higher among female participants when compared to men but was found statistically nonsignificant. Similar findings reported by Butala et al. showed that mean systolic BP among women was 134.1 and among men was 130.6 (P < 0.091).[12] Several contradicting studies reported gender-specific association of higher mean BP among women compared to men. The rising pattern of mean BP among female is assumed to be due to declining estrogen in postmenopausal stage.[13,14]
The present study findings reported higher BMI among males. In contrast to this, numerous studies showed similarity in BMI distribution among men and women.[12,14] The contradicting study findings in regard to BMI distribution may be possibly due to heterogeneity among population in terms of lifestyle, socioeconomic status, and dietary patterns.[15] Hypertension was significantly noted in women (P < 0.002). Many previous studies had consistent results showing hypertension as a major risk factor among women.[16,17,18]
The study findings show that predominant symptom exhibited by men and women is chest pain. When compared to men, females increasingly presented with atypical presentations such as dyspnea, nausea, vomiting, dizziness, sweating, and back pain (P < 0.005). In contrast to these findings, Berg et al. reported no significance in atypical presentations such as dyspnea, fatigue, neck pain, and vomiting but showed significant prevalence of nausea, back pain, dizziness, and palpitation among women.[19]
The present study did not find any gender-specific association in location of pain but shows higher presentation of females with intrascapular pain, this is contrast to earlier results which suggested increased pain in the right upper chest, sternum, and left side of chest in men whereas in women frequently reported pain in jaw, neck, throat, shoulder, left scapula had more frequency pain among men.[20,21]
Women commonly had squeezing and tightness type of pain whereas men reported tightness, burning, pricking type of pain which was significant at P < 0.003. In consistent to the current findings, male presentation of burning type of pain was reported by Bösner et al.[22]
The present study shows that majority of men had presented with STEMI (P < 0.004). Several studies reported similar findings of the current study, showing higher prevalence of STEMI among men when compared to women.[22,23,24,25,26]
CONCLUSION
The current study supports the need of a gender-specific approach in treatment and nursing care of the patients with MI, as the study revealed differences in presentation of MI that leads to the use of over-the-counter drugs and hospitalization delays. An insight to the public has to be made as disparities still exist in the treatment delay even though a complex health-care sector has evolved. This study can be a future reference since primary care physicians should be empowered about these differences so that they can refer the patients in time and the awareness that has to be made into the outskirts of the public regarding the disease presentations so that the treatment delays can be solved to an extent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.USA: 2016. Jun, [Last accessed on 2020 Jan 16]. What is Coronary Heart Disease – NIH Health CAD. Available from: https://www.nhlbi.nih.gov/health/ healthtopics/topics/cad . [Google Scholar]
- 2.Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, Ngu Blackett K, et al. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40:139–46. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva: World Health Organization; 2014. [Last accessed on 2020 Jan 16]. Global Status Report on Non Communicable Disease. Available from: http://www.who.int/nmh/publications/ncd_ statusreport_2014/en/ [Google Scholar]
- 4.Maskey A, Sayami A, Pandey MR. Coronary artery disease: An emerging epidemic in Nepal. J Nepal Med Assoc. 2003;42:122–24. [Google Scholar]
- 5.Roger VL. Epidemiology of myocardial infarction. Med Clin North Am. 2007;91:537–52. doi: 10.1016/j.mcna.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–8. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg RJ, Gore JM, Alpert JS, Dalen JE. Recent changes in attack and survival rates of acute myocardial infarction (1975 through 1981).The Worcester heart attack study. JAMA. 1986;255:2774–9. [PubMed] [Google Scholar]
- 8.Gobwald A, Schienkiewitz A, Nowossadeck E, Busch MA. Prevalence of myocardial infarction and coronary heart disease in adults aged 40-79 years in Germany: Results of the German Health Interview and Examination Survey for Adults (DEGS1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:650–5. doi: 10.1007/s00103-013-1666-9. [DOI] [PubMed] [Google Scholar]
- 9.Duraes AR, Bitar YS, Freitas AC, Filho IM, Freitas BC, Fernandez AM. Gender differences in ST-elevation myocardial infarction (STEMI) time delays: Experience of a public health service in Salvador-Brazil. Am J Cardiovasc Dis. 2017;7:102–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Malik FT, Klimuddin M, Ahmed N, Badiuzzaman M, Ahmed MN, Dutta A, et al. AMI in very young (aged ≤ 35yrs) Bangladeshi patients: Risk factors and coronary angiographic profile. Clin Trials Regul Sci Cardiol. 2016;13:1–5. [Google Scholar]
- 11.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18:598–602. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butala NM, Desai MM, Linnander EL, Wong YR, Mikhail DG, Ott LS, et al. Gender differences in presentation, management, and in-hospital outcomes for patients with AMI in a lower-middle income country: Evidence from Egypt. PLoS One. 2011;6:e25904. doi: 10.1371/journal.pone.0025904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Njølstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction.A 12-year follow-up of the Finnmark Study. Circulation. 1996;93:450–6. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- 14.Albrektsen G, Heuch I, Løchen ML, Thelle DS, Wilsgaard T, Njølstad I, et al. Risk of incident myocardial infarction by gender: Interactions with serum lipids, blood pressure and smoking.The Tromsø Study 1979-2012. Atherosclerosis. 2017;261:52–9. doi: 10.1016/j.atherosclerosis.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Mungreiphy NK, Dhall M, Tyagi R, Saluja K, Kumar A, Tungdim MG, et al. Ethnicity, obesity and health pattern among Indian population. J Nat Sci Biol Med. 2012;3:52–9. doi: 10.4103/0976-9668.95955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucholz EM, Strait KM, Dreyer RP, Lindau ST, D'Onofrio G, Geda M, et al. Editor's choice-sex differences in young patients with acute myocardial infarction: A VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6:610–22. doi: 10.1177/2048872616661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho KI, Shin ES, Ann SH, Garg S, Her AY, Kim JS, et al. Gender differences in risk factors and clinical outcomes in young patients with acute myocardial infarction. J Epidemiol Community Health. 2016;70:1057–64. doi: 10.1136/jech-2015-207023. [DOI] [PubMed] [Google Scholar]
- 18.Lichtman JH, Leifheit EC, Safdar B, Bao H, Krumholz HM, Lorenze NP, et al. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: Evidence from the VIRGO study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) Circulation. 2018;137:781–90. doi: 10.1161/CIRCULATIONAHA.117.031650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg J, Björck L, Dudas K, Lappas G, Rosengren A. Symptoms of a first acute myocardial infarction in women and men. Gend Med. 2009;6:454–62. doi: 10.1016/j.genm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Khan JJ, Albarran JW, Lopez V, Chair SY. Gender differences on chest pain perception associated with acute myocardial infarction in Chinese patients: A questionnaire survey. J Clin Nurs. 2010;19:2720–9. doi: 10.1111/j.1365-2702.2010.03276.x. [DOI] [PubMed] [Google Scholar]
- 21.DeVon HA, Ryan CJ, Ochs AL, Shapiro M. Symptoms across the continuum of acute coronary syndromes: Differences between women and men. Am J Crit Care. 2008;17:14–24. [PMC free article] [PubMed] [Google Scholar]
- 22.Bösner S, Haasenritter J, Hani MA, Keller H, Sönnichsen AC, Karatolios K, et al. Gender differences in presentation and diagnosis of chest pain in primary care. BMC Fam Pract. 2009;10:79. doi: 10.1186/1471-2296-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: Longitudinal population study. BMJ. 1998;316:1043–7. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moshki M, Zareie M, Hashemizadeh H. Sex differences in acute myocardial infarction. Nurs Midwifery Stud. 2015;4:e22395. doi: 10.17795/nmsjournal22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George L, Ramamoorthy L, Satheesh S, Saya RP, Subrahmanyam DK. Prehospital delay and time to reperfusion therapy in ST elevation myocardial infarction. J Emerg Trauma Shock. 2017;10:64–9. doi: 10.4103/0974-2700.201580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attard R, Dingli P, Doggen CJM, Cassar K, Farrugia R, Wettinger SB. The impact of passive and active smoking on inflammation, lipid profile and the risk of myocardial infarction? Open Heart. 2017;4:e000620. doi: 10.1136/openhrt-2017-000620. doi: 10.1136/ openhrt-2017-000620. [DOI] [PMC free article] [PubMed] [Google Scholar]


