Abstract
AIM
To explore whether human umbilical cord mesenchymal stem cell (hUCMSC)-derived exosomes (hUCMSC-Exos) protect rat retinal neurons in high-glucose (HG) conditions by activating the brain-derived neurotrophic factor (BDNF)-TrkB pathway.
METHODS
hUCMSC-Exos were collected with differential ultracentrifugation methods and observed by transmission electron microscopy. Enzyme-linked immunosorbent assays (ELISAs) was used to quantify BDNF in hUCMSC-Exos, and Western blot was used to identify surface markers of hUCMSC-Exos. Rat retinal neurons were divided into 4 groups. Furthermore, cell viability, cell apoptosis, and TrkB protein expression were measured in retinal neurons.
RESULTS
hUCMSCs and isolated hUCMSC-Exos were successfully cultured. All hUCMSC-Exos showed a diameter of 30 to 150 nm and had a phospholipid bimolecular membrane structure, as observed by transmission electron microscopy. ELISA showed the BDNF concentration of hUCMSCs-Exos was 2483.16±281.75. hUCMSCs-Exos effectively reduced the apoptosis of retinal neuron rate and improved neuron survival rate, meanwhile, the results of immunofluorescence verified the fluorescence intensity of TrKB in neurons increased. And all above effects were reduced by treated hUCMSCs-Exos with BDNF inhibitors. hUCMSC-Exos effectively reduced the apoptosis rate of retinal neurons by activating the BDNF-TrkB pathway in a HG environment.
CONCLUSION
In the HG environment, hUCMSC-Exos could carry BDNF into rat retinal neurons, inhibiting neuronal apoptosis by activating the BDNF-TrkB pathway.
Keywords: human umbilical cord mesenchymal stem cells, exosomes, diabetic retinopathy, retinal neurons, BDNF-TrkB pathway
INTRODUCTION
Diabetic retinopathy (DR) is a disease that severely and chronically damages vision disease. In China, the overall prevalence of diabetes is estimated to be 11.6% per year (95%CI, 11.3%-11.8%)[1]. To date, various anti-vascular endothelial growth factor (VEGF) methods have been applied in the clinic to prevent the development of diabetic retinal neuro-vasculopathy, including features such as retinal neovascularization and vitreous hemorrhage (VH). These therapies have proven to be effective in proliferative diabetic retinopathy (PDR), but recent results showed that anti-VEGF may impair the survival and function of neurons[2]. In vivo experiments showed that impaired acquisition or processing of the visual signal, including, abnormal electroretinogram (ERG) recordings, a decreased capacity for dark adaptation, and reduced contrast sensitivity, preceded the emergence of vascular endothelial lesions[3]. The mechanism of injury to retinal neurons is becoming a topic of great interest in DR research. The key to the successful repair of retinal neurons is the management of stem cells to maximize their therapeutic efficacy. Human umbilical cord mesenchymal stem cells (hUCMSCs) are widely used due to their therapeutic potential, including low immunogenicity, and their availability through noninvasive collection methods that raise no ethical issues[4]. This indicates that hUCMSCs are a promising candidate for allogeneic therapy in retinal regenerative treatment[5].
A clinical trial in DR and non-DR patients found that before the emergence of clinical signs of DR, brain-derived neurotrophic factor (BDNF) levels decreased in patients' serum and aqueous humor[6]. BDNF is well known for its neuroprotective effect in the central nervous system and may have various effects on the pathogenesis of some neurodegenerative and psychiatric disorders[7]. In a previous set of experiments, we injected hUCMSCs into the eyeballs of diabetic rats and found that these cells provided neuroprotection by effectively preventing a decline in BDNF levels, thus increasing retinal ganglion cell (RGC) survival in vivo[8]. However, mesenchymal stem cells (MSCs) have some defects that limit their future application, such as the low cell integration rate and unexpected abnormal growth after transplantation[9]. Recently, powerful evidence has indicated that the effects of MSCs are based on paracrine processes involving MSC-derived exosomes[10].
Exosomes are newly recognized as major paracrine factors released by various cell types, including MSCs. These membrane-bound vesicles measure 30-150 nm in diameter and carry proteins, mRNA, and miRNA, which serve as important media for intercellular communication[11]. Previous results confirmed the regulatory and protective mechanisms of exosome-carried cytokines in retinal neurons under ischemia and hypoxia[12]. However, research on the protective effects of neurotrophic factors in DR is scarce. Therefore, the purpose of this experiment was to investigate whether MSCs could reduce neuronal apoptosis in DR patients through BDNF carried by exosomes, which might increase the therapeutic potential of these stem cells. We hope to introduce a new concept for future DR treatment.
MATERIALS AND METHODS
Cell Culture
The hUCMSCs (Saier Biological Company, Tianjin, China) were cultured in six-well plates for 48h with Dulbecco's modified Eagle's medium/F12 (DMEM)/F12 containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 U/mL streptomycin. Retinal neurons were obtained from unicellular suspensions from 1- to 3-day-old Wistar rats.
Isolation and Characterization of hUCMSC-Derived Exosomes
Exosomes were collected by the differential ultracentrifugation method. Briefly, hUCMSCs were cultured in serum-free medium, and the supernatant culture medium (ScienCell, San Diego, California, USA) was collected and centrifuged (CP100WX/CR22N, Hitachi, Japan) at 4°C. After initial centrifugation at 300 g for 10min, 2000 g for 20min, and 10 000 g for 30min, the particles in the bottom were removed and discarded. Then, the exosomes were precipitated by ultracentrifugation of the supernatant at 100 000 g for 70min. The pellets were washed twice and resuspended in PBS. After being passed through a 0.22 µm filter, all exosome preparations were stored at -20°C until use.
The exosomes were diluted at a ratio of 1:10. A small amount of copper mesh was dipped into a 3% phosphotungstic acid solution and stained for 5min to deepen the background. The excess staining agent was removed with filter paper, and transmission electron microscopy was used to observe the exosomes.
All protein from the exosomes was extracted with lysis buffer, and the protein concentrations were measured with a BCA protein assay kit. The samples were boiled at 95°C for 5min, loaded onto a sodium dodecyl sulfate polyacrylamide gel for electrophoresis, and then transferred to a PVDF membrane. The primary antibodies included antibodies against CD63 (Invitrogen, Carlsbad, California, USA), CD9 (Invitrogen, Carlsbad, California, USA), and Calnexin (Invitrogen, Carlsbad, California, USA). The membranes were blocked with 5% nonfat dried milk and incubated with primary antibodies overnight at 4°C. The membranes were then incubated with secondary antibodies for 2h. The size distribution of exosomes was measured using a nanoparticle tracking and NanoSight analysis system (ZetaVIEW S/N 17-310; Particle Metrix, München, Germany).
Detection of BDNF in hUCMSC-Derived Exosomes by ELISA
To detect BDNF carried by hUCMSC-derived exosomes (hUCMSC-Exos), we performed an enzyme-linked immunosorbent assay (ELISA) in strict accordance with the manufacturer's instructions (R&D Systems, Minneapolis, Minnesota, USA).
Modeling a High-Glucose Environment
Rat retinal neurons were cultured [culture medium: high-glucose (HG) DMEM medium containing 10% FBS and 25 mmol/L D-glucose] at 37°C under a 5% CO2 atmosphere at saturated humidity and passaged until stable. The cells were divided into 4 groups: 1) control group: glucose 5.5 mmol/mL; 2) HG group: glucose 35 mmol/mL; 3) hUCMSCs-Exos group: glucose 35 mmol/mL, hUCMSCs-Exos 100 ng/mL; and 4) anti-BDNF aroup: glucose 35 mmol/mL, hUCMSCs-Exos 100 ng/mL, anti-BDNF, antibody 10 µg/mL (Santa Cruz, Dallas, Texas, USA).
Rat Retinal Neuron Activity Test
The retinal neurons were seeded on a 96-well plate at a concentration of 104/mL. Then, 100 µL cell medium and 20 µL MTT solution were added to each well and incubated with the retinal neurons for 4h. After 100 µL dimethyl sulfoxide (DMSO) was added, the plates were shaken at room temperature for 10min, and the optical density (OD value) was measured at 570 nm on a microplate reader. The above test was repeated 3 times, and the average value was taken.
Rat Retinal Neuron Apoptosis Test
For the cell apoptosis test, an Annexin V-FITC apoptosis detection kit (Bender MedSystems GmbH, Vienna, Austria) was used according to the manufacturer's instructions to perform fluorescence-assisted cell sorting (FACS) analysis to detect the level of apoptosis among retinal neurons. Briefly, 0.25% trypsin was used to isolate the retinal neurons of each group, and then the reaction was terminated with 10% FBS. The cells were collected, resuspended in 250 µL of binding buffer and stained with Annexin V-FITC and propidium iodide solution for 15min in the dark at room temperature. Samples were immediately analyzed by FACSCalibur™ flow cytometry.
Immunocytochemical Staining
The neurons were fixed with 4% neutral paraformaldehyde for 30min and blocked with 3% hydrogen peroxide-methanol at 37°C. Then, the samples were incubated with 1% bovine serum albumin (BSA) and 0.5% Triton X-100 for 20min. After being incubated with 10% normal goat serum, the samples were washed with PBS 3 times. TrkB expression was detected by immunostaining with rabbit anti-mouse TrkB polyclonal antibody (1:50; Cell Signaling Technology, Illinois, USA). After sequential incubation with biotinylated secondary antibody and avidin-biotin-peroxidase reagents, the cells were stained with 0.5 mg/mL horseradish peroxidase (HRP) substrate solution. The 4′,6-diamidino-2-phenylindole (DAPI) was used for nuclear staining. The stained sections were observed under a laser scanning confocal fluorescence microscope (Leica, Germany).
Statistical Analysis
Using SPSS 24.0 statistical software, the mean±standard deviation of each variable was calculated, and intragroup comparisons were performed using one-sample t-tests. All intergroup comparisons were performed by one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
RESULTS
Characterization of hUCMSC-Exos
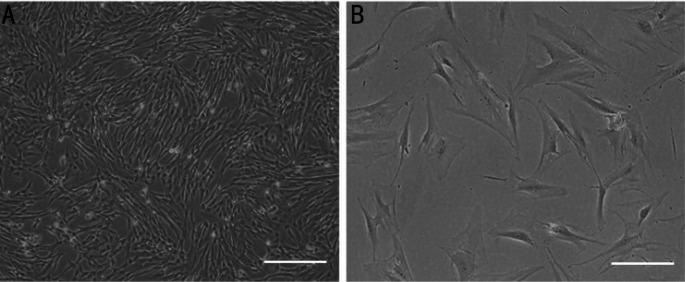
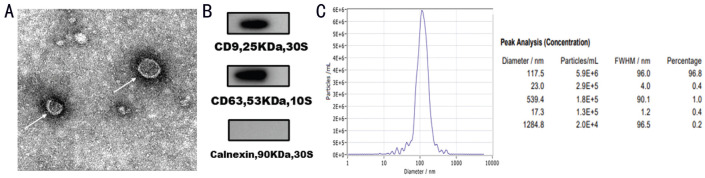
Images taken with an inverted microscope at 40× magnification showed that hUCMSCs grew in clusters and swirls (Figure 1A). The cells had fusiform or polygonal morphology, and some were multinuclear or binuclear, as observed at 100× magnification (Figure 1B). Exosomes were extracted from the supernatant of hUCMSCs by differential ultracentrifugation and observed under a transmission electron microscope. Under the transmission electron microscope, all vesicles were cup-shaped and had a bimolecular phospholipid membrane structure (Figure 2A) with a peak size near 117.5 nm, as shown by the NanoSight analysis system (Figure 2C). Furthermore, Western blot analysis showed that hUCMSC-Exos were positive for the surface marker proteins CD9 and CD63 but negative for Calnexin protein (Figure 2B). The protein concentration in hUCMSC-Exos was 0.48 µg/µL.
Figure 1. Observation of hUCMSC.
A: Observation under an inverted microscope showed vortex-like growth (magnification 40×). Scale bars: 200 µm. B: Under high magnification, most hUCMSCs have long fusiform shapes, and a few are polygonal (magnification 100×). Scale bars: 50 µm.
Figure 2. Identification of hUCMSC-Exos.
A: Transmission electron microscopy showed the teacup-like vesicular structure of hUCMSC-Exos (marked with arrows); these exosomes measured approximately 30-150 nm in diameter; B: hUCMSC-Exos were positive for the surface marker proteins CD9 and CD63 and negative for Calnexin; C: Analysis of the size distribution of hUCMSC-Exos with a NanoSight analysis system; the peak size is 117.5 nm. Scale bars: 100 nm.
ELISA Results for BDNF in hUCMSC-Exos
The concentration of the collected exosomes was adjusted to 1000 µg/mL, and ELISA showed that the BDNF concentration was 2483.16±281.75.
Protective Effect of hUCMSC-Exos on Retinal Neurons Under HG Conditions
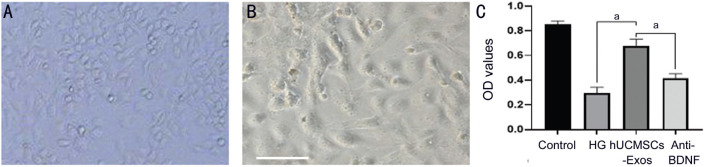
After 5d, rat retinal neurons were photographed under an inverted microscope (Figure 3A and 3B). According to the MTT results, the cell survival rates of the control group, HG group, hUCMSCs-Exos group, and anti-BDNF group were 0.851±0.028, 0.295±0.048, 0.674±0.057, and 0.413±0.039. The OD value of HG group was significantly lower than the control group (P<0.05), showing the impairment of neurons in HG environment. The hUCMSCs-Exos group was significantly higher than both HG group and anti-BDNF group (P<0.05), showing the participation of BDNF in hUCMSCs-Exos improves the survival rates of rat retinal neurons under HG. At the same time, we also found the HG group was significantly lower than anti-BDNF group (P<0.05; Figure 3C), and this may be related to other neurotrophic factors in hUCMSCs-Exos.
Figure 3. The morphology and vitality of rat retinal neurons.
A: Observation of rat retinal neurons on an inverted microscope 5d after treatment; B: Rat retinal neurons were observed under a high-power microscope 5d later. Scale bars: 50 µm. C: The mean OD values of rat retinal neurons in all groups; aP<0.05. Magnification 100×.
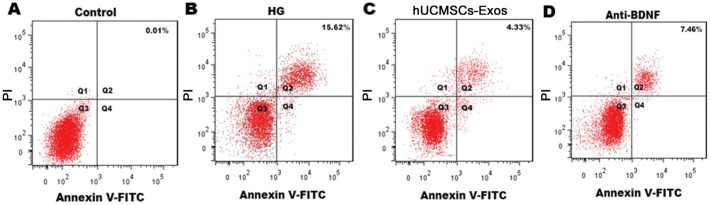
Consistent with the MTT results, the proportion of apoptotic cells in the control group, HG group, and hUCMSCs-Exos group and anti-BDNF group was 0.01%, 15.62%, 4.33%, and 7.46% respectively, with statistically significant differences among all groups (P<0.05; Figure 4), by specifically inhibiting BDNF in hUCMSCs-Exos, and the effect of hUCMSCs-Exo in reducing the rates of neurons apoptosis in HG environments is impaired.
Figure 4. Annexin V-FITC/PI double-staining flow cytometry showed the proportions of apoptotic neurons.
The apoptosis rate has been marked in each figure.
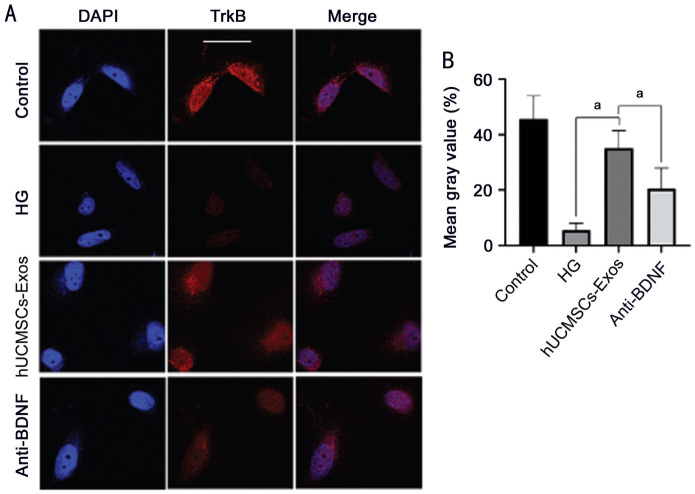
Effect of hUCMSCs-Exos on TrkB Expression in Retinal Neurons Under HG Conditions
The results of immunocytochemical staining were compared with mean gray value of TrkB protein between each group. The mean gray value in the HG group was significantly lower than that in the control group and the hUCMSCs-Exos group (P<0.05), therefore, the HG environment reduces the fluorescence intensity of TrkB protein; and the mean gray value in the hUCMSCs-Exos group was significantly higher than that in the anti-BDNF group (P<0.05). Also, inhibition of BDNF in hUCMSCs-Exos may reduce the expression intensity of TrkB under HG (Figure 5).
Figure 5. Immunocytochemical staining of TrkB in neurons.
A: The cell nuclei were stained with DAPI, and TrkB was stained as red; B: The mean TrkB expression of rat retinal neurons as determined by immunostaining; aP<0.05. Scale bars: 50 µm.
DISCUSSION
In this experiment, we examined the BDNF-TrkB pathway to explore the protective effect of hUCMSC-Exos in an HG environment, whereas the previous work concerning the protective effect of neurotrophic factors on retinal neurons, especially ganglion cells, focused on glaucoma-related disease[13]. BDNF is the most abundant of the various neurotrophic factors in the retina; by binding to the receptor TrkB and activating the extracellular signal-regulated kinase and phosphatidylinositol-3 kinase pathways, this protein could exert powerful neuroprotective effects in the retina[14]. BDNF is expressed in multiple cell types, including RGCs and Müller glial cells[15]. In a Western population, Shpak et al[16] found that BDNF levels in the plasma and tears of DR patients were significantly reduced compared to those of controls, while Liu et al[17] reported a reduction in plasma BDNF levels in Chinese patients with type II diabetes, which means that diabetes is a potential risk factor for DR and an independent marker of DR. It was confirmed both in vitro and in vivo that direct supplementation of BDNF in cell and rat DR models could protect neuronal function in HG environments by promoting TrkB expression and activating the TrkB/Erk/MAPK pathway[18].
Choosing appropriate donor cells to provide the exosomes was a major issue in our experimental design stage. Stem cell-based protection and regeneration of retinal neurons is an emerging and encouraging method in the field of ophthalmology[19]. hUCMSCs, widely known for characteristics such as a high reproduction rate, low immunogenicity, and the capacity to be subcultured stably, are stromal cells derived from the Wharton's jelly of the umbilical cord[20]. hUCMSCs have broad therapeutic prospects in the treatment of many diseases, and as we described above, they also have a notable protective effect on retinal neurons in diabetic rats[8].
Exosomes are 30-150 nm extracellular membrane vesicles of endocytic origin that were first discovered in the early 1980s[21]–[23]. Exosomes are released into the extracellular environment upon fusion of multivesicular bodies with the plasma membrane[24]. Existing evidence has suggested that exosomes can be secreted from almost all cell types. In addition, exosomes have been found in a variety of body fluids[21],[25]–[30]. We collected hUCMSCs-Exos by the classic differential ultracentrifugation method; although it is considered a time-consuming approach, it yields higher exosome quantity and purity than other methods. The use of Western blot analysis, transmission electron microscopy, and the NanoSight analysis system allows us to confirm the characteristics of the exosomes we isolated in this trial, such as their marker protein expression, their shape, and their mean diameter.
We used ELISA to confirm the presence of BDNF in hUCMSC-Exos and measured its content. The results supported the hypothesis that hUCMSCs could transfer BDNF to retinal neuronal cells through exosomes. Subsequently, an MTT assay found that the survival rate of neurons was significantly reduced after HG treatment, whereas both hUCMSC-Exos and anti-BDNF could improve cell viability; however, cell viability was inhibited by BDNF inhibitor compared with hUCMSC-Exos. Similar to the MTT test results, the difference in Annexin V-FITC/PI flow cytometry between the hUCMSC-Exo group and the anti-BDNF group indicated the inhibitory effect of hUCMSC-Exos on neuronal apoptosis in an HG environment. To further verify the mechanism involved in the protective effect of hUCMSC-Exos, we used immunofluorescence staining. The results showed that this inhibitory effect of apoptosis might be induced by exosomes through activation of the BDNF-TrkB pathway. During the experiment, we applied only the BDNF inhibitor, considering that hUCMSC-Exos carried varied cytokines and nucleic acids that might affect the apoptosis of retinal neurons. No other factors were altered, which may explain why the apoptosis of the anti-BDNF group was lower than that of the HG group.
In summary, hUCMSC-Exos protected rat retinal neurons in an HG environment, providing insights into stem cell therapy and the pathogenic mechanism of DR. Treatment with anti-BDNF significantly reduced this protective effect of hUCMSC-Exos, suggesting a BDNF-mediated mechanism. However, due to the limitations of the experimental conditions, we did not test other cytokines in exosomes and analyze their independent or joint effects; this direction remains to be explored in the future.
In conclusion, in previous experiments, we confirmed that hUCMSCs could protect retinal neurons in a rat model of DM by activating the BDNF-TrkB pathway. The present experiment further confirmed that exosomes could carry BDNF to retinal neurons, supplement their BDNF levels, and activate the BDNF-TrkB pathway to provide protection.
Acknowledgments
Foundations: Supported by Tianjin Science and Technology Project of China (No.14JCYBJC27400); Science and Technology Fund of Tianjin Eye Hospital (No.YKZD1901; No.YKYB1905).
Conflicts of Interest: Gao X, None; He GH, None; Zhang XT, None; Chen S, None.
REFERENCES
- 1.Xu Y, Wang L, He J, et al. 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 2.Latzer P, Shchyglo O, Hartl T, Matschke V, Schlegel U, Manahan-Vaughan D, Theiss C. Blocking VEGF by bevacizumab compromises electrophysiological and morphological properties of hippocampal neurons. Front Cell Neurosci. 2019;13:113. doi: 10.3389/fncel.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mrugacz M, Bryl A, Zorena K. Retinal vascular endothelial cell dysfunction and neuroretinal degeneration in diabetic patients. J Clin Med. 2021;10(3):458. doi: 10.3390/jcm10030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour AA, Yousefi M, Talebi M, Shamsasenjan K. Regenerative potential of Wharton's jelly-derived mesenchymal stem cells: a new horizon of stem cell therapy. J Cell Physiol. 2020;235(12):9230–9240. doi: 10.1002/jcp.29810. [DOI] [PubMed] [Google Scholar]
- 5.Drela K, Lech W, Figiel-Dabrowska A, Zychowicz M, Mikula M, Sarnowska A, Domanska-Janik K. Enhanced neuro-therapeutic potential of Wharton's Jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy. 2016;18(4):497–509. doi: 10.1016/j.jcyt.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Taşlipinar Uzel AG, UĞurlu N, Toklu Y, Çİçek M, Boral B, Şener B, ÇaĞil N. Relationship between stages of diabetic retinopathy and levels of brain-derived neurotrophic factor in aqueous humor and serum. Retina. 2020;40(1):121–125. doi: 10.1097/IAE.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 7.Numakawa T, Odaka H, Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int J Mol Sci. 2018;19(11):E3650. doi: 10.3390/ijms19113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Wang Y, Kong J, Dong M, Duan H, Chen S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Sci Rep. 2017;7(1):408. doi: 10.1038/s41598-017-00298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen YT, Ho YC, Lee YC, Ding DC, Liu PK, Tsai RK. The benefits and hazards of intravitreal mesenchymal stem cell (MSC) based-therapies in the experimental ischemic optic neuropathy. Int J Mol Sci. 2021;22(4):2117. doi: 10.3390/ijms22042117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan XL, Zhang YL, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, Feng L, Zelka R, Lopez J, Sharma M, Roth S. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146–160. doi: 10.1016/j.biomaterials.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu R, Shi Q, Yang H, Sha XY, Yu GC, Liu L, Zhong JX. Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension. Int J Ophthalmol. 2021;14(2):194–199. doi: 10.18240/ijo.2021.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12(10):1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 15.Pardue MT, Allen RS. Neuroprotective strategies for retinal disease. Prog Retin Eye Res. 2018;65:50–76. doi: 10.1016/j.preteyeres.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shpak AA, Gavrilova NA, Poliakova MA. Brain-derived neurotrophic factor in diabetic retinopathy and asymptomatic edema of the optic nerve head. Vestn Oftalmol. 2010;126(3):7–10. [PubMed] [Google Scholar]
- 17.Liu SY, Du XF, Ma X, Guo JL, Lu JM, Ma LS. Low plasma levels of brain derived neurotrophic factor are potential risk factors for diabetic retinopathy in Chinese type 2 diabetic patients. Mol Cell Endocrinol. 2016;420:152–158. doi: 10.1016/j.mce.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Tao LJ, Fu X, Zhao YC, Xu XL. BDNF protects retinal neurons from hyperglycemia through the TrkB/ERK/MAPK pathway. Mol Med Rep. 2013;7(6):1773–1778. doi: 10.3892/mmr.2013.1433. [DOI] [PubMed] [Google Scholar]
- 19.Mathew B, Poston JN, Dreixler JC, Torres L, Lopez J, Zelkha R, Balyasnikova I, Lesniak MS, Roth S. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1581–1592. doi: 10.1007/s00417-017-3690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13(9):1738–1755. doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- 22.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu WY, Bai XD, Zhang A, Huang JJ, Xu SX, Zhang JP. Role of exosomes in central nervous system diseases. Front Mol Neurosci. 2019;12:240. doi: 10.3389/fnmol.2019.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 26.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 27.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5(1):e8577. doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 30.Admyre C, Grunewald J, Thyberg J, Gripenbäck S, Tornling G, Eklund A, Scheynius A, Gabrielsson S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22(4):578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]