Abstract
AIM
To evaluate the efficacy of recombinant human nerve growth factor-loaded amniotic membrane (rhNGF-AM) on corneal epithelial and nerve regeneration in rabbit model.
METHODS
Freshly prepared human amniotic membrane (AM) were immersed into PBS buffer containing 100 or 500 µg/mL rhNGF for 15, 30, and 60min at 4°C. The in vitro release kinetics of rhNGF was measured with ELISA. For in vivo evaluation, the AM were immersed with 500 µg/mL rhNGF for 30min. Fifty-seven rabbits were selected to establish corneal epithelial defect model. In addition to the 19 rabbits in control group, 38 rabbits received AM transplantation with or without rhNGF after the removal of central epithelium. Corneal epithelial defect area, sub-epithelial nerve fiber density, corneal sensitivity, rhNGF contents in resident AM and corneas were measured after the surgery.
RESULTS
rhNGF was sustained release from the AM within 14d in vitro, with the positive correlation with initial immersion concentration. The immersion of AM in 500 µg/mL rhNGF for 30min achieved the most stable release within 14d. After transplantation in rabbit cornea, a high concentration of rhNGF in resident rhNGF-AM and cornea was maintained within 8d. Corneal epithelial healing, nerve fiber regeneration and the recovery of corneal sensitivity were significantly accelerated after the rhNGF-AM transplantation when compared to simple AM transplantation (all P<0.05).
CONCLUSION
Simple immersion of AM achieves the sustained release of rhNGF, and promotes corneal epithelial wound healing and nerve regeneration, as well as the recovery of corneal sensitivity in rabbit.
Keywords: recombinant human nerve growth factor, amniotic membrane, drug-loaded, corneal epithelium, nerve regeneration
INTRODUCTION
The human amniotic membrane (AM) contains many active ingredients such as nerve growth factor (NGF), epidermal growth factor, protease inhibitors and proteins that promote wound healing[1]–[2]. AM has anti-inflammatory, antifibrotic, and antiangiogenic properties, which makes it widely used in many ocular surface reconstruction surgeries[2]–[5], including the treatment of corneal epithelial defects caused by ocular trauma, infection, neurotrophic keratopathy (NK), surgical history or diabetes[6]–[9]. For a few refractory cases, such as persistent corneal epithelial defect (PCED), multiple AM transplants are required[10]–[11]. NGF has proven to be critical for promoting corneal epithelial regeneration and maintaining nerve integrity[12]–[13]. Recombinant human nerve growth factor (rhNGF) eye drops were approved for clinical treatment. The clinical studies revealed that rhNGF eye drops could safely and effectively relieve the symptoms of patients with dry eye and promote corneal healing in patients with NK[14]–[18]. The low-temperature storage conditions, long treatment period, and high cost limit its large-scale use[14]–[15]. Therefore, how to reduce the frequency of rhNGF eye drops and maintain their effective therapeutic effects is an urgent problem[19].
Accumulative evidence indicated that AM can be applied as a drug delivery vehicle, which can infuse drug molecules and slowly release them[20]–[22]. However, there is no information on the treatment using rhNGF-loaded AM transplantation. Therefore, we investigate the drug reservoir function of AM infused with rhNGF in vitro and further evaluate its effect on corneal epithelial defects in vivo. We found that rhNGF-loaded AM could release rhNGF on the ocular surface and maintain a higher concentration in the cornea, which revealed new possibilities for the treatment of corneal epithelial defects.
MATERIALS AND METHODS
Ethical Approval
This study was approved by the Ethics Committee of Shandong Eye Institute (Approval No.2019-23) and complied with the tenets of the Declaration of Helsinki. The animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research.
Animals
Adult male New Zealand rabbits, each weighing about 2 kg, were selected. They were purchased from the Jinan Xi Ling Jiao Experimental Animal Breeding Center and raised by the Animal Center of the Shandong Eye Institute. rhNGF was donated by Chen Wei of the Bioengineering Institute, China Academy of Military Medical Science. It was produced by expression of Chinese hamster ovary (CHO) cells. CHO is one of the best expression systems for exogenous eukaryotic genes. It is also the commonly used mammalian cell in biopharmaceuticals. The AM, collected one day before the experiment, was provided by the Eye Bank Center of Shandong Eye Institute. The mean thickness of AMs was calculated to be 175.60±11.28 µm.
rhNGF Release Kinetics of Amniotic Membrane In Vitro
AMs were placed on sterile gauze after washing with 0.9% NaCl solution. AMs (15×15 mm2) were cut and immersed in freshly prepared rhNGF solution (100 and 500 µg/mL) at 4°C. After 15, 30, and 60min, they were taken out, rinsed gently with phosphate buffered saline (PBS), and then transferred to a small petri dish containing PBS at room temperature. The AMs were taken out every day, rinse gently with PBS and placed in a new small petri dish containing PBS. The PBS solution, collected within 14d, was operated in the same mode for rhNGF detection by enzyme-linked immunosorbent assay (ELISA).
Establishment of Rabbit Corneal Epithelial Defect Model
We used a random number table to divide the 57 New Zealand rabbits into the following groups: normal control group (Control), AM transplantation group (AM group), and rhNGF-loaded AM transplantation group (rhNGF-AM group), 19 rabbits per group. According to the results of in vitro release of rhNGF from the rhNGF-AM, AM with an immersion of 500 µg/mL rhNGF for 30min was applied for the experiments. The central corneal epithelium (7.5 mm diameter) in anesthetized rabbits was removed using an Algerbrush II corneal rust ring remover (Alger, Lago Vista, TX, USA). Except for 19 rabbits in the Control group, the other two groups were given AM transplantation and rhNGF-loaded AM transplantation respectively. The AM was placed on the cornea and flattened, keeping the epithelium upward. At 2 and 4 mm behind the limbus, the AM was sutured intermittently with 10-0 nylon thread and fixed on the rabbit's superficial sclera. After the operation, 0.3% of ofloxacin ointment was applied to prevent infection.
Preparation of Amniotic Membrane and Cornea Extract
On postoperative day 2, 4, 6, 8, the rhNGF-AM and simple AM were removed from the eyeball after removing the sutures under topical anesthesia with proparacaine hydrochloride eye drops (Alcon, USA), and washed with a 0.9% NaCl injection. The AM tissues (10×10 mm2) were shredded on ice and ground for 3min in a tissue grinder, and then placed on ice for 15min and sonicated until the lysate was clear. After centrifugation at 12 000 rpm for 15min at 4°C, the supernatant of the upper AM was collected for rhNGF detection. The three corneas of rabbit in each group were harvested at 2, 4, 6, and 8d after epithelial abrasion, and total proteins were extracted with PBS. After centrifugated at 4°C, the supernatants were collected for rhNGF detection with ELISA.
rhNGF Detection by ELISA
The rhNGF ELISA test kit (ab99986, Abcom, Cambridge, Boston, UK) was used to detected NGF in samples, including PBS solution, AM and cornea extract. We added 100 µL of each standard and test ample into appropriate wells. They were gently shaken at 4°C overnight and washed with wash buffer. After washing, 100 µL of 1× biotin-labeled antibody was then added into each well, and shaken for 60min at room temperature. After addition of 100 µL of 1× horseradish peroxidase-labeled avidin, the mixture was then shaken and incubated at room temperature for 45min. After washing, 100 µL of substrate reaction solution was added into each well followed by adding 50 µL of a stop solution after incubation for 30min in darkness. The optical density (OD value) of each well was measured using a multifunctional microplate reader (SpectraMax i3x, Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 450 nm within 15min. Sample concentration was then calculated.
Corneal Epithelial Regeneration
After 0, 24, and 48h, corneal epithelial defects were visualized by staining with fluorescein sodium and photographed under a slit lamp microscope (BQ900; Haag-Streit, Bern, Switzerland). Image J (National Institutes of Health, Bethesda, MD, USA) was used to analyze the staining area and calculate the percentage of residual epithelial defect.
Corneal Sensitivity Measurement
A Cochet-Bonnet esthesiometer (Luneau Ophtalmologie, Chartres Cedex, France) was used to examine the change of corneal sensitivity at 7, 14, and 21d after the corneal epithelial removal. The rabbit was in a non-anaesthetized state during the measurement. We pushed the nylon thread of the esthesiometer to the maximal length (6 cm) and gently touched the central cornea of the rabbit to observe whether the rabbit had a blink reflex. If not, we shortened the length of the nylon filament thread by 5 mm and then gently touched the center cornea again until it caused an obvious blink reflex in the rabbit. We then determined the length of the nylon filament thread and recorded the result. This test was repeated at least three times.
Corneal Whole-mount Immunofluorescence Staining
Rabbits were sacrificed at 21d after the operation. The seven eyeballs of rabbit in each group were fixed with Zamboni stationary liquid for 60min and the corneas were then dissected around the scleral-limbal region and blocked by PBS containing 0.1% Triton X-100, 2% goat serum, and 2% bovine serum albumin for 2h. We then added an Alexa Fluor 488-conjugated neuronal class III b-tubulin antibody (Merck Millipore, Darmstadt, Germany), shaking gently overnight at 4°C. After washing five times, the cornea was spread on a glass slide and imaged and archived on an LSM880 Zeiss inverted microscope (Carl Zeiss Meditec, Jena, Germany). According to our previous studies[23]–[24], Image J software was used to calculate the density of the corneal sub-basal nerve fibers.
Statistical Analysis
Data are from at least three replicated experiments and are presented as means±standard deviation (SD). Statistical analysis was performed using SPSS 22.0 software (SPSS, Chicago, IL, USA), one-way analysis of variance and the Kruskal-Wallis test. Differences were considered statistically significance at P<0.05.
RESULTS
rhNGF Release In Vitro
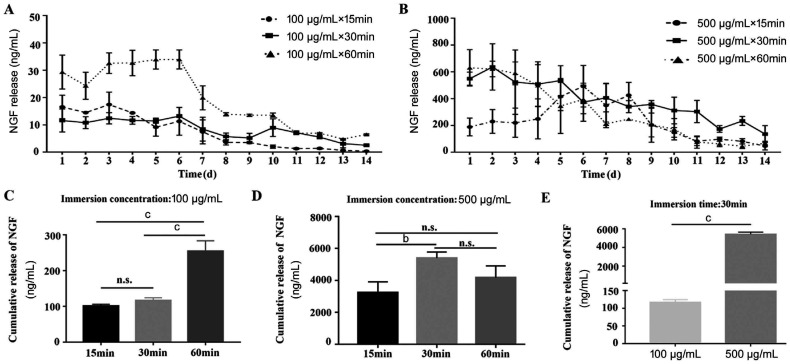
To examine the drug reservoir function of AM infused with rhNGF in vitro, the AM were immersed into PBS buffer containing 100 or 500 µg/mL rhNGF for 15, 30, and 60min at 4°C respectively. The release kinetics of rhNGF was measured with ELISA. The sustained release of rhNGF from AM was achieved for up to 14d (Figure 1A, 1B). The cumulative release of NGF after soaked in 100 µg/mL for 60min was more dramatically elevated than that for 15 or 30min, but with no significant difference between 15 and 30min (Figure 1C). The cumulative release of NGF after soaked in 500 µg/mL for 30min was significantly higher than that for 15 or 60min, while there was no significant difference between 15 and 60min (Figure 1D). Moreover, under the same immersion time, the cumulative release of NGF with an immersion concentration of 500 µg/mL was statistically greater than that with an immersion concentration of 100 µg/mL (Figure 1E).
Figure 1. The release kinetics of rhNGF from AMs.
A-B: The content of NGF released from AMs immersed with 100 and 500 µg/mL rhNGF for a period of 14d; C: Under the immersion concentration of 100 µg/mL, the cumulative release of NGF with immersion time of 60min was significantly higher than that with 15 or 30min; D: Under the immersion concentration of 500 µg/mL, the cumulative release of NGF with immersion time of 30min was significantly higher than that with 15 or 60min; E: Under the same immersion time, the comparation of cumulative release of NGF between 500 and 100 µg/mL. bP<0.01, cP<0.001, n.s.: Not significant.
Evaluation of rhNGF Release In Vivo
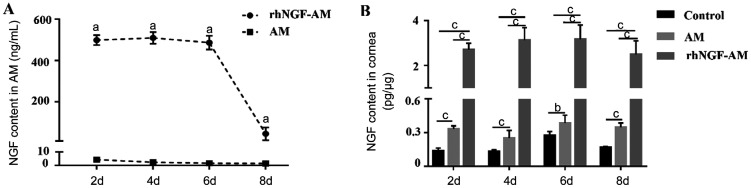
To evaluate the release of rhNGF in vivo, the content of NGF in AM and rhNGF-AM, the bioavailability of rhNGF in the cornea were measured at different time points after AM transplantation. As shown in Figure 2A, NGF was detectable both in rhNGF-AM and AM within 8d after transplantation and maintained a high level in rhNGF-AM within 6d after transplantation. The content of NGF at each time point in rhNGF-AM was much higher than that in AM. Moreover, the concentrations of rhNGF in corneas were significantly increased at 2, 4, 6, and 8d both in AM and rhNGF-AM group. However, the content of rhNGF-AM group was much higher than that of AM group (Figure 2B, n=3 per group). These results indicated that the sustained release of rhNGF in rhNGF-AM was successfully achieved.
Figure 2. The content of NGF from AMs and corneas.
A: A high concentration of rhNGF-AM was maintained within 6d and the content of NGF at each time point was significant different between the two groups; B: There were significant differences between the three groups at 2, 4, 6, and 8d postoperatively, and the content of NGF in the cornea of rhNGF-AM group were higher than that of the other two groups. aP<0.05, bP<0.01, cP<0.001.
rhNGF-AM Transplantation Promoted the Regeneration of Corneal Epithelium
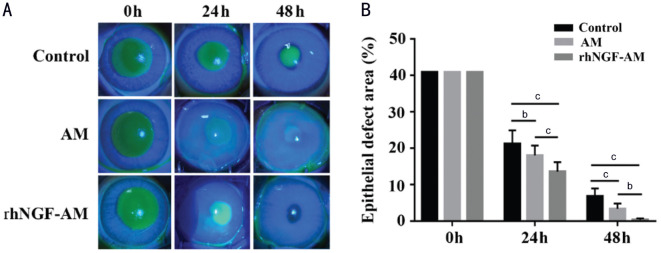
To assess the effects of rhNGF-AM on the regeneration of corneal epithelium, the AM transplantation with or without rhNGF were performed in rabbit cornea after the removal of central epithelium. rhNGF-AM transplantation efficiently promoted the regeneration rate of corneal epithelium, comparing with AM transplantation group and the control group (Figure 3A). The analysis results of residual epithelial defects showed a remarkable promotive effect of rhNGF-AM on corneal epithelial regeneration at 24 and 48h after epithelial removal (24h: 21.19%±3.72% in control group, 18.03%±2.71% in AM group, 13.54%±2.60% in rhNGF-AM group; 48h: 6.83%±2.1% in control group, 3.34%±1.45% in AM group, 0.34%±0.39% in rhNGF-AM group; Figure 3B, n=7 per group).
Figure 3. rhNGF-AM transplantation promoted the regeneration of corneal epithelium.
A: The residual epithelial defect was examined, with fluorescein staining, at 0, 24, and 48h after the removal of the corneal epithelium; B: The histogram of the residual epithelial defect presented as a percentage of the original wound area (n=7 per group). bP<0.01, cP<0.001.
rhNGF-AM Transplantation Promoted the Regeneration of Corneal Nerve Fibers
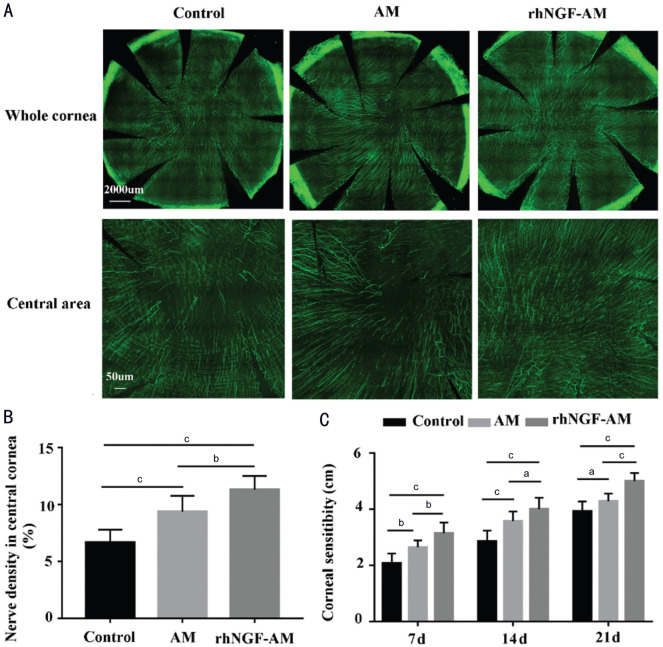
To explore the effects of rhNGF-AM on the regeneration of corneal nerve fibers, the corneal nerves of each group were detected at 21d after AM transplantation with or without rhNGF. When compared with AM and control group, the rabbit corneal nerve regeneration rate was faster in rhNGF-AM transplantation group (Figure 4A). The density of the central corneal sub-epithelial nerve fibers in rhNGF-AM group was much higher than in other groups (control: 5.73%±0.47%, AM: 9.27%±1.25%, rhNGF-AM: 11.91%±0.58%; Figure 4B, n=7 per group). Moreover, the recovery of corneal mechanical sensitivity in rhNGF-AM transplantation group was better than those in other two groups, with significant differences among the groups at 7, 14, and 21d after injury (7d, control: 2.07±0.35 cm, AM: 2.64±0.24 cm, rhNGF-AM: 3.14±0.38 cm; 14d, control: 2.86±0.38 cm, AM: 3.57±0.35 cm, rhNGF-AM: 4.00±0.41 cm; 21d, control: 3.93±0.35 cm, AM: 4.29±0.27 cm, rhNGF-AM: 5.00±0.29 cm; Figure 4C, n=7 per group). These results demonstrated that rhNGF-AM contributed to the regeneration of corneal nerve.
Figure 4. rhNGF-AM transplantation promotes the regeneration of rabbit corneal sub-epithelial nerves fibers and the recovery of corneal sensitivity.
A: Regenerated corneal nerve fibers examined with corneal whole mount staining at 21d after injury; B: Immunofluorescence intensity of central nerve fibers was calculated with Image J software; C: A Cochet-Bonnet esthesiometer was used to measure the mechanical sensitivity of the 3 groups at 7, 14, and 21d after the corneal epithelial removal (n=7 per group). aP<0.05, bP<0.01, cP<0.001.
DISCUSSION
AM transplantation is an effective approach for treating corneal wound healing and nerve regeneration due to the enrichment of various bioactive molecules. Some cases of delayed corneal epithelial wound healing need to be treated with drugs after AM transplantation[9]. However, the local application of eye drops after AM transplantation suffers from the problem of low bioavailability. Several pieces of evidence demonstrated that AM could load drugs and achieve a controlled and slow release[20]–[21]. The antibiotics-loaded AM acquired through immersion can increase the effective drug concentration of the aqueous humor and reduce the frequency of eyedrop applications, presenting an excellent therapeutic effect on bacterial keratitis and superior to eye drops[25]. Therefore, the drug-loaded AM can provide a dual role of AM and targeted drugs and also improve the ocular bioavailability of the attached drugs.
As a best-characterized member of the neurotrophic family, NGF not only stimulates the growth and survival of corneal epithelial cells and maintains limbal epithelial stem cell potential, but also supports corneal re-innervation[13] and promotes the repair of injured nerves[26]. Moreover, the rhNGF ophthalmic solution was approved for the treatment of NK with persistent epithelial defects. The biological function of rhNGF is closest to natural protein molecules[27]. The rhNGF expressed by CHO cells has high activity, low immune risk, and broad market prospects[28]. Therefore, rhNGF was selected as the target drug, and further investigate the possibility of rhNGF-AM transplantation on treating corneal epithelial defects. In this study, we successfully constructed rhNGF-AM by immersion. The simple immersion of AM not only achieved the long-term slow release of rhNGF, but also effectively promoted corneal epithelial wound healing and nerve regeneration.
The loading and release of drugs in AM were influenced by multiple factors, including the immersion time, temperature, drug concentration, AM thickness and others. Resch et al[29] found that the slow release of ofloxacin could be detected within 7h after AM was soaked in ofloxacin. Yelchuri et al[21] reported that sustained release of moxifloxacin AM was achieved for up to seven weeks. The soaking time length did not significantly affect the release of moxifloxacin. The cumulative amount of moxifloxacin released from thick AM was significantly more tremendous than with thin AM. In addition, the effect of AM immersion at 4°C was better than at room temperature[30]. In this study, we mainly investigated the effect of immersion time and drug concentration on rhGNF loading and release. When AM with similar thickness and size was immersed in different concentrations of rhNGF at 4°C, the release of rhNGF in AM could be sustained for at least 14d after soaking, with a positive correlation with initial immersion concentration. This was consistent with literature reports[20]–[21]. In this study, we shortened the preparation time before AM transplantation, by reducing the immersion time to 60min or less. The cumulative amount of rhNGF released from AM after immersion for 60min was much higher than after immersion for 15 or 30min, with no significant difference between 15 and 30min. However, the drug release curve showed that the release of rhNGF-AM between 7-14d after immersion for 30min was more stable than the curve for 15min, and with a higher release amount. When in 500 µg/mL rhNGF, the cumulative content of rhNGF with 30min immersion was significantly higher than that of 15 or 60min. The drug release curve showed that the rhNGF-AM after immersion for 30min could maintain significant sustained release. Therefore, the stable release of the rhNGF was essential for the treatment of ocular surface diseases with the rhNGF-AM transplantation.
Accumulative experimental and clinical studies have shown that the drug-loaded AM transplantation improved the therapeutic effect of the drug on the ocular surface[25]. As previous clinical data revealed the therapeutic effect of rhNGF on corneal healing of PCDE and corneal ulcers[18], we examined the rhNGF-AM on rabbit corneal epithelial wound healing and nerve regeneration. According to the dynamic release of rhNGF from the rhNGF-AM in vitro, the AMs immersed with 500 µg/mL rhNGF for 30min were selected for the subsequent experiments. Compared with the AM transplantation and the control group, rhNGF-AM transplantation significantly accelerated the corneal epithelial wound healing, the regeneration of the corneal subepithelial nerve and recovery of corneal sensitivity after injury. This was consistent with the effect of rhNGF eye drops obtained from other clinical and experimental research. We further analyzed the dynamic change of rhNGF in corneas after rhNGF-AM sutured on the ocular surface. We found that the content of NGF in corneas after rhNGF-AM transplantation was much higher than that with AM transplantation. Moreover, the high level of NGF in the rhNGF-AM transplantation group sustained for 6d, and dramatically decreased with time, but still detectable on the 8th day after transplantation. These results indicated that the continuous release of rhNGF after rhNGF-AM transplantation contributed to the accelerated corneal epithelial wound healing and nerve regeneration, suggesting the potential of rhNGF-AM transplantation.
Although we evaluated the sustained-release function of AM on rhNGF, there are still some limitations in this study. First, the concentration of immersed drugs affects the drug loading of AM. Therefore, the maximum threshold, stability and storage of rhNGF-AM are needed for determination, as well as other influencing factors. Second, whether the effect of rhNGF-AM transplantation is superior to rhNGF eye drops is required for further investigation using suitable pathological models, including wound-healing effect, the frequency, dosage and cost of medication.
In conclusion, the rhNGF-AM transplantation not only achieves the sustained release of rhNGF, but also accelerates corneal epithelial wound healing and nerve regeneration. These findings provide the rationale for the clinical application of rhNGF loaded AM to treat ocular disorders.
Acknowledgments
The authors thank Prof. Chen Wei of the Bioengineering Institute, China Academy of Military Medical Science for donating the rhNGF.
Authors' contributions: Wan LQ, conception and design, animal experiment conduction, data collection and analysis, writing the manuscript; Wang HF, Chen C, Li H, Zhang Y, and Xue JF, animal experiment conduction, the sample collection, and data analysis; Zhang YY and Zhou QJ, critical revision of the manuscript; Xie LX, conception and design, surgeon of this study.
Foundation: Supported by the Academic Promotion Program of Shandong First Medical University (No.2019ZL001).
Conflicts of Interest: Wan LQ, None; Zhang YY, None; Wang HF, None; Chen C, None; Li H, None; Zhang Y, None; Xue JF, None; Zhou QJ, None; Xie LX, None.
REFERENCES
- 1.Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111–121. doi: 10.5500/wjt.v4.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazari Hashemi P, Chaventre F, Bisson A, Gueudry J, Boyer O, Muraine M. Mapping of proteomic profile and effect of the spongy layer in the human amniotic membrane. Cell Tissue Bank. 2020;21(2):329–338. doi: 10.1007/s10561-020-09821-8. [DOI] [PubMed] [Google Scholar]
- 3.Mirzayev I, Gündüz AK, Özalp Ateş FS, Özcan G, Işık MU. Factors affecting recurrence after surgical treatment in cases with ocular surface squamous neoplasia. Int J Ophthalmol. 2019;12(9):1426–1431. doi: 10.18240/ijo.2019.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyakoshi A, Nishida Y, Tanaka A, Hayashi A. Histological equivalence of a hyper-dry amniotic membrane and the Ambio2TM after implantation in the rabbit conjunctiva. Ophthalmic Res. 2020;63(4):423–426. doi: 10.1159/000504579. [DOI] [PubMed] [Google Scholar]
- 5.Malla T, Jiang J, Hu K. Clinical outcome of combined conjunctival autograft transplantation and amniotic membrane transplantation in pterygium surgery. Int J Ophthalmol. 2018;11(3):395–400. doi: 10.18240/ijo.2018.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirostko B, Rafii M, Sullivan DA, Morelli J, Ding J. Novel therapy to treat corneal epithelial defects: a hypothesis with growth hormone. Ocul Surf. 2015;13(3):204–212.e1. doi: 10.1016/j.jtos.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan S, Cheng J, Dong Y, Xie L. Epithelial defects after penetrating keratoplasty in infectious keratitis: an analysis of characteristics and risk factors. PLoS One. 2018;13(11):e0208163. doi: 10.1371/journal.pone.0208163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193–204. doi: 10.1007/s10561-017-9618-5. [DOI] [PubMed] [Google Scholar]
- 9.Schuerch K, Baeriswyl A, Frueh BE, Tappeiner C. Efficacy of amniotic membrane transplantation for the treatment of corneal ulcers. Cornea. 2020;39(4):479–483. doi: 10.1097/ICO.0000000000002179. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SE, Medeiros CS, Santhiago MR. Pathophysiology of corneal scarring in persistent epithelial defects after PRK and other corneal injuries. J Refract Surg. 2018;34(1):59–64. doi: 10.3928/1081597X-20171128-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Röck T, Bartz-Schmidt KU, Landenberger J, Bramkamp M, Röck D. Amniotic membrane transplantation in reconstructive and regenerative ophthalmology. Ann Transplant. 2018;23:160–165. doi: 10.12659/AOT.906856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacchetti M, Bruscolini A, Lambiase A. Cenegermin for the treatment of neurotrophic keratitis. Drugs Today (Barc) 2017;53(11):585–595. doi: 10.1358/dot.2017.53.11.2722395. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Kang SS, Kim JY, Tchah H. Nerve growth factor attenuates apoptosis and inflammation in the diabetic cornea. Invest Ophthalmol Vis Sci. 2016;57(15):6767–6775. doi: 10.1167/iovs.16-19747. [DOI] [PubMed] [Google Scholar]
- 14.Sacchetti M, Lambiase A, Schmidl D, Schmetterer L, Ferrari M, Mantelli F, Allegretti M, Garhoefer G. Effect of recombinant human nerve growth factor eye drops in patients with dry eye: a phase IIa, open label, multiple-dose study. Br J Ophthalmol. 2020;104(1):127–135. doi: 10.1136/bjophthalmol-2018-312470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, Mantelli F, REPARO Study Group Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332–1343. doi: 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari MP, Mantelli F, Sacchetti M, Antonangeli MI, Cattani F, D'Anniballe G, Sinigaglia F, Ruffini PA, Lambiase A. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs. 2014;28(3):275–283. doi: 10.1007/s40259-013-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonini S, Lambiase A, Rama P, Filatori I, Allegretti M, Chao W, Mantelli F, REPARO Study Group Phase I trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1468–1471. doi: 10.1016/j.ophtha.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Mastropasqua L, Lanzini M, Dua HS, D'Uffizi A, Di Nicola M, Calienno R, Bondì J, Said DG, Nubile M. In Vivo evaluation of corneal nerves and epithelial healing after treatment with recombinant nerve growth factor for neurotrophic keratopathy. Am J Ophthalmol. 2020;217:278–286. doi: 10.1016/j.ajo.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Sheha H, Tighe S, Hashem O, Hayashida Y. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973–1980. doi: 10.2147/OPTH.S185184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer WJ, Grüterich M, Kook D, Sigg W, Kernt M, Messmer EM, Haritoglou C, Kampik A, Wolf A. Modification of amniotic membrane as a depot carrier for bevacizumab - an in-vitro model for a slow release mechanism. Curr Eye Res. 2013;38(4):445–450. doi: 10.3109/02713683.2012.757326. [DOI] [PubMed] [Google Scholar]
- 21.Yelchuri ML, Madhavi B, Gohil N, Sajeev HS, Venkatesh Prajna N, Srinivasan S. In vitro evaluation of the drug reservoir function of human amniotic membrane using moxifloxacin as a model drug. Cornea. 2017;36(5):594–599. doi: 10.1097/ICO.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 22.Hazarika M, Prajna NV, Senthilkumari S. Drug reservoir function of voriconazole impregnated human amniotic membrane: an in vitro study. Indian J Ophthalmol. 2021;69(5):1068–1072. doi: 10.4103/ijo.IJO_2649_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Gao N, Wu L, Lee PSY, Me R, Dai C, Xie L, Yu FX. Role of VIP and sonic hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic corneas. Diabetes. 2020;69(7):1549–1561. doi: 10.2337/db19-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Li W, Zhou Q, Li J, Wang X, Zhang J, Li D, Qi X, Liu T, Zhao X, Li S, Yang L, Xie L. MANF promotes diabetic corneal epithelial wound healing and nerve regeneration by attenuating hyperglycemia-induced endoplasmic reticulum stress. Diabetes. 2020;69(6):1264–1278. doi: 10.2337/db19-0835. [DOI] [PubMed] [Google Scholar]
- 25.Han YP, Li B, Yang JZ. Observation on the efficacy of the drug-soaking amniotic membrane transplantation for bacterial corneal ulceration. Chinese Journal of Ocular Trauma and Occupational Eye Disease. 2019;41(2):85–88. [Google Scholar]
- 26.Li BH, Kim SM, Yoo SB, Kim MJ, Jahng JW, Lee JH. Recombinant human nerve growth factor (rhNGF-β) gene transfer promotes regeneration of crush-injured mental nerve in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(3):e26–e34. doi: 10.1016/j.tripleo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Zheng HH, Jiang H. Progress of CHO expression system. Current Biotechnology. 2016;6(4):239–243. [Google Scholar]
- 28.Le W, Peng LJ, Shi QW, Ge YH, Liu HZ. Study on the high lever expression and bioactivity evaluation of recombinant human nerve growth factor. Chinese Pharmaceutical Affairs. 2014;28(6):601–606. [Google Scholar]
- 29.Resch MD, Resch BE, Csizmazia E, Imre L, Németh J, Szabó-Révész P, Csányi E. Drug reservoir function of human amniotic membrane. J Ocul Pharmacol Ther. 2011;27(4):323–326. doi: 10.1089/jop.2011.0007. [DOI] [PubMed] [Google Scholar]
- 30.Sara SH, Prajna NV, Senthilkumari S. Human amniotic membrane as a drug carrier - An in-vitro study using fortified cefazolin ophthalmic solution. Indian J Ophthalmol. 2019;67(4):472–475. doi: 10.4103/ijo.IJO_1336_18. [DOI] [PMC free article] [PubMed] [Google Scholar]