Abstract
The BACTEC MGIT 960 instrument is a fully automated system that exploits the fluorescence of an oxygen sensor to detect growth of mycobacteria in culture. Its performance was compared to those of the radiometric BACTEC 460 instrument and egg-based Lowenstein-Jensen medium. An identical volume of sample was inoculated in different media, and incubation was carried out for 6 weeks with the automatic systems and for 8 weeks on solid media. A total of 2,567 specimens obtained from 1,631 patients were cultured in parallel. Mycobacteria belonging to nine different taxa were isolated by at least one of the culture systems, with 75% of them being represented by Mycobacterium tuberculosis complex. The best yield was obtained with the BACTEC 460 system, with 201 isolates, in comparison with 190 isolates with the BACTEC MGIT 960 system and 168 isolates with Lowenstein-Jensen medium. A similar but not significant difference was obtained when the most-represented organisms, the M. tuberculosis complex, Mycobacterium xenopi, and the Mycobacterium avium complex, were analyzed separately and when combinations of a solid medium with the BACTEC MGIT 960 system and with the BACTEC 460 system were considered. The shortest times to detection were obtained with the BACTEC MGIT 960 system (13.3 days); 1.5 days earlier than that with the BACTEC 460 system (14.8 days) and 12 days earlier than that with Lowenstein-Jensen medium (25.6 days). The BACTEC MGIT 960 system had a contamination rate of 10.0%, intermediate between those of the radiometric system (3.7%) and the egg-based medium (17.0%). We conclude, therefore, that the BACTEC MGIT 960 system is a fully automated, nonradiometric instrument that is suitable for the detection of growth of tuberculous and other mycobacterial species and that is characterized by detection times that are even shorter than that of the “gold standard,” the BACTEC 460 system. The contamination rate was higher than that for the radiometric BACTEC 460 system and needs to be improved.
Although a variety of molecular biological methods have been shown to have the potential to provide direct detection of Mycobacterium tuberculosis complex from clinical specimens within a few hours (3, 5), culture still represents the cornerstone on which a definitive diagnosis of tuberculosis and other mycobacterioses relies. In recent years, the development of rapid, reliable methods for culture detection of acid-fast bacilli has been regarded as worthy of absolute priority (12, 13). Reasons for this renewed concern include the serious public health risk due to the reemergence of tuberculosis, the appearance of multidrug-resistant strains of M. tuberculosis, and the high incidence of Mycobacterium avium complex disease in patients with AIDS. Currently, mycobacterial culture can be performed with conventional solid media and by one of the available broth-based methods. Of these, the radiometric semiautomated BACTEC 460TB system (Becton Dickinson, Sparks, Md.), which was the first system to permit the significantly earlier detection of mycobacteria, is now widely accepted as the “gold standard” (4). It has several drawbacks, however: it involves the use of radioactive material, and reading of cultures is labor-intensive and is associated with a potential risk of cross-contamination. Furthermore the use of needles for inoculation of the vial involves the risk of stick injury. In recent years, several new nonradiometric technologies for growth and detection of acid-fast bacilli have been introduced; among these, the fluorimetric Mycobacteria Growth Indicator Tube (MGIT; Becton Dickinson) (7) and the MB-Redox system (Biotest, Dreieich, Germany) (10) are manual, while the ESP Culture System II (AccuMed, Chicago, Ill.) (15, 17), the MB/BacT system (Organon Teknica, Turnhout, Belgium) (8), the BACTEC 9000 MB system (Becton Dickinson) (6, 18), as well as the BACTEC MGIT 960 system (Becton Dickinson) are fully automated, continuously monitoring, walk-away systems.
The BACTEC MGIT 960 system is a noninvasive, nonradiometric system that uses the same technology used by manual MGIT and the BACTEC 9000 MB system. A ruthenium pentahydrate oxygen sensor embedded in silicon at the bottom of a tube containing 8 ml of modified Middlebrook 7H9 broth fluoresces following the oxygen reduction induced by aerobically metabolizing bacteria within the medium. A compact instrument incubates and tests, according to onboard algorithms, up to 960 culture tubes.
This paper summarizes the results of a multicenter clinical trial that compared the newly developed BACTEC MGIT 960 system, the radiometric BACTEC 460 system, and conventional solid medium for the recovery rates and time to detection of acid-fast bacilli from respiratory and extrapulmonary specimens.
MATERIALS AND METHODS
The investigation was carried on in three different Italian laboratories with 2,567 consecutive samples received with a request to determine the possible presence of mycobacteria. The specimens, most of which (94%) were smear negative, were obtained from 1,631 patients. Among the samples, 1,770 were respiratory (65% sputum samples, 22% bronchial aspirates, and 13% bronchial washings); among the nonpulmonary specimens, 380 were urine specimens and 137 were pleural fluid specimens, while 280 originated from various other body sites including stools, cerebrospinal fluid, ascitic fluid, pus, gastric juices, and biopsy specimens. While normally sterile body fluids (pleural fluid, pericardial fluid, cerebrospinal fluid, synovial fluid, and ascitic fluid) were concentrated by centrifugation only before being inoculated, respiratory specimens (sputum specimens, bronchial washings, and bronchoscopy specimens) and gastric fluid, urine, stool, pus, and tissue specimens were digested and decontaminated by the standard N-acetyl-l-cysteine–2% NaOH procedure (BBL MycoPrep; Becton Dickinson) (4). The supernatant was discarded, and the pellet was resuspended with sterile phosphate buffer to a final volume of 2 ml. The mixture was used both for preparation of a smear that was subsequently stained with auramine O and for inoculation of one BACTEC MGIT 960 tube (0.5 ml), one BACTEC 12B vial (0.5 ml), and two Lowenstein-Jensen slants (0.25 ml each).
Prior to inoculation BACTEC 12B and BACTEC MGIT 960 media were supplemented with the antibiotic mixture polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA) and growth supplement (Becton Dickinson); vials to be inoculated with cerebrospinal fluid were not supplemented with PANTA, as suggested for the BACTEC 460 system. BACTEC MGIT 960 tubes were incubated at 37°C in the BACTEC MGIT 960 instrument, in which they were automatically monitored each hour for fluorescence development for 42 days or until a positive signal developed. The BACTEC 12B vials were incubated at 37°C and were monitored with the BACTEC 460 instrument (9) twice per week for the first 2 weeks and weekly thereafter for an additional 4 weeks or until the growth index (GI) was >10; bottles with GIs of >10 were monitored daily until the achievement of a GI of >100. Solid media were incubated at 37°C for 8 weeks and were inspected weekly or until mycobacterial colonies were seen.
BACTEC MGIT 960 tubes that had a positive signal with the instrument, BACTEC 12B vials with GIs of ≥100, and the growth of colonies on Lowenstein-Jensen medium were considered positive results only after confirmation of the presence of mycobacteria by means of an acid-fast smear. Positive cultures that failed to reveal acid-fast bacilli in the smear were screened, without concentration, for contaminants by Gram staining, and if positive, they were considered contaminated and eliminated, while, if negative, they were incubated again; acid-fast smears and Gram-stained smears were retested in BACTEC MGIT 960 tubes and signaled again as positive for a maximum two additional times. Thereafter, the cultures were considered false positive. Mycobacteria grown in culture were identified by using nucleic acid probes (AccuProbe; Gen-Probe, San Diego, Calif.) (11) and, when negative, were tested both by high-performance liquid chromatography (14) and by biochemical tests (4).
The rates of recovery were compared and analyzed by McNemar's chi-square test. The paired t test was used to compare times to detection.
RESULTS
A mycobacterium was isolated with at least one of the three culture systems from 236 samples corresponding to 109 patients; the majority of isolates were obtained from respiratory specimens (n = 184), while 53 mycobacteria were isolated from extrapulmonary sites, with the majority of them being obtained from gastric juice (11 isolates) and urine (9 isolates) specimens. Microscopy was positive for 129 specimens (54%).
Members of the M. tuberculosis complex were the most frequently isolated mycobacteria, followed by Mycobacterium xenopi and the M. avium complex; other nontuberculous mycobacteria were represented by nine Mycobacterium gordonae, six Mycobacterium chelonae, and three Mycobacterium malmoense isolates and one isolate each of Mycobacterium fortuitum, Mycobacterium kansasii, and Mycobacterium terrae. The identification to the species level of organisms of the M. tuberculosis and M. avium complexes allowed detection of six isolates of Mycobacterium bovis among the M. tuberculosis complex and, among the M. avium complex, isolates of M. avium, Mycobacterium intracellulare, and the MAI-X group (16) as well.
The comparison of rates of recovery by individual system is shown in Table 1. The best yield was obtained with the BACTEC 460 system, but when compared with the BACTEC MGIT 960 system, it was not statistically significant; on the contrary, the two liquid media were significantly more sensitive than Lowenstein-Jensen medium both on the whole and among single species when the M. tuberculosis complex was considered separately.
TABLE 1.
Recovery of mycobacteria by individual systems and combinations of systemsa
| Mycobacterium or specimen | Total no. (%) recovered
|
|||||
|---|---|---|---|---|---|---|
| All media | BACTEC MGIT 960 system | BACTEC 460 system | Lowenstein-Jensen medium | BACTEC MGIT 960 system + Lowenstein-Jensen medium | BACTEC 460 system + Lowenstein-Jensen medium | |
| All | 236 | 190 (80) | 201 (85) | 167 (71) | 212 (90) | 225 (95) |
| M. tuberculosis complex | 169 | 149 (88) | 153 (92) | 124 (74) | 158 (94) | 160 (95) |
| M. xenopi | 24 | 13 (54) | 11 (46) | 17 (71) | 21 (87) | 22 (92) |
| M. avium complex | 22 | 21 (95) | 22 (100) | 16 (73) | 21 (95) | 22 (100) |
| Other MOTTb | 21 | 7 (33) | 15 (71) | 10 (48) | 12 (57) | 21 (100) |
| Smear positive | 228 | 122 (53) | 121 (53) | 103 (45) | 125 (55) | 127 (56) |
| Smear negative | 108 | 68 (63) | 80 (74) | 64 (59) | 87 (80) | 98 (91) |
More frequently occurring taxa are considered separately, as were smear-positive and smear-negative specimens.
MOTT, mycobacteria other than M. tuberculosis.
When the combinations of a liquid plus a solid medium were considered, the best performance was obtained with the BACTEC 460 system plus Lowenstein-Jensen medium (Table 1), which was more sensitive for the detection of both M. tuberculosis and other mycobacteria than the combination of the BACTEC MGIT 960 system and Lowenstein-Jensen medium (P = 0.04).
Fifty-two samples were found to be positive by only one method: 11 with the BACTEC 960 system, 24 with the BACTEC 460 system, and 17 with Lowenstein-Jensen medium (Table 2).
TABLE 2.
Mycobacteria detected by one system only
| Mycobacterium | No. of isolates detected
|
||
|---|---|---|---|
| BACTEC MGIT 960 system | BACTEC 460 system | Lowenstein-Jensen medium | |
| M. tuberculosis complex | 9a | 11a | 6 |
| M. gordonae | 6 | 3 | |
| M. xenopi | 2 | 3 | 6 |
| M. avium | 1 | ||
| M. chelonae | 1 | 1 | |
| M. fortuitum | 1 | ||
| M. kansasii | 1 | ||
| M. terrae | 1 | ||
One of the isolates was M. bovis.
Twenty-three microscopically positive samples from which mycobacteria failed to grow on all three media were all obtained from previously culture-positive patients who had been treated.
The mean times to detection for paired samples grown by the three methods were 13.34 days (standard deviation [SD], 7.73 days; median, 12 days) with the BACTEC MGIT 960 system, 14.80 days (SD, 7.77 days; median, 14 days) with the BACTEC 460 system, and 25.67 days (SD, 11.55 days, median, 24 days) with Lowenstein-Jensen medium, with all differences being statistically significant. The separate times to detection of the most frequently isolated mycobacteria reported in Table 3 emphasize the earlier detection with the BACTEC MGIT 960 system, which was moderate for M. tuberculosis complex (P = 0.03) but evident and highly significant for all nontuberculous mycobacteria. As expected, the times to recovery were shorter among smear-positive specimens (averages, 11.23 days with the BACTEC MGIT 960 system, 13.6 days with the BACTEC 460 system, and 22.91 days with Lowenstein-Jensen medium) than among smear-negative specimens (18.48, 19.00, and 32.38 days, respectively). For 20 samples, BACTEC MGIT 960 cultures gave a positive signal and the samples were incubated again, as no organism was detected in the broth by smear microscopy. The samples were found to be true positive about 5 or 6 days later and generally before the BACTEC 460 system gave a positive result.
TABLE 3.
Paired times to detection by individual methods
| Mycobacterium and smear result | No. of strains | Average time to detection (days)
|
||
|---|---|---|---|---|
| BACTEC MGIT 960 system | BACTEC 460 system | Lowenstein-Jensen medium | ||
| Total | 137 | 13.34 | 14.80 | 25.67 |
| M. tuberculosis complex | 113 | 14.25 | 14.88 | 25.08 |
| Positive | 80 | 12.50 | 13.14 | 22.56 |
| Negative | 33 | 19.58 | 19.12 | 31.18 |
| M. xenopi | 4 | 21.75 | 29.75 | 55.25 |
| Positive | 1 | 26 | 31 | 53 |
| Negative | 3 | 20.33 | 29.33 | 56 |
| M. avium complex | 16 | 5.94 | 8.56 | 23.31 |
| Positive | 12 | 5.25 | 8 | 22.92 |
| Negative | 4 | 8 | 4 | 24.50 |
| Other MOTTa, all smear positive | 4 | 9 | 22.25 | 22.25 |
MOTT, mycobacteria other than M. tuberculosis.
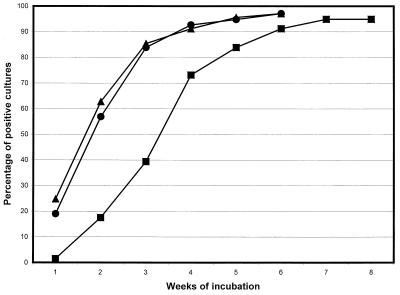
Cumulative detection times (Fig. 1) revealed both with the BACTEC MGIT 960 system and with the BACTEC 460 system a rate of positivity of over 80% within the 3rd week versus a rate of positivity of only 39% with Lowenstein-Jensen medium at the same time.
FIG. 1.
Cumulative percentages of mycobacteria detected weekly by individual methods. ▴, BACTEC MGIT 960 system; ●, BACTEC 460 system; ■, Lowenstein-Jensen medium.
The contamination rates were 3.70% with the BACTEC 460 system, 9.97% with the BACTEC MGIT 960 system, and 17.07% with Lowenstein-Jensen medium. Gram-positive cocci were the prevalent organisms responsible for contamination of BACTEC MGIT 960 tubes. For seven samples contaminants were also found in BACTEC MGIT 960 cultures positive for acid-fast bacilli. Fourteen samples had overgrowth in BACTEC MGIT 960 tubes but grew mycobacteria with at least one other culture medium. Contamination rates were practically the same at the three centers both for the BACTEC 460 system and for the BACTEC MGIT 960 system, while that for Lowenstein-Jensen medium was higher at one of the centers (22.4%). Four samples had false-positive results with the BACTEC MGIT 960 system; i.e., the tubes could not be tested to the end because of their repeated positive signals, even though they were negative for mycobacteria and contaminants by microscopy.
DISCUSSION
Rapid diagnosis of mycobacterial infections is critical; therefore, attempts to shorten the time needed for detection of such organisms deserve attention. The BACTEC MGIT 960 system is a fully automated, nonradiometric culture system which, due to continuous monitoring of O2 consumption, allows detection, without delay, of the mycobacteria growing within a liquid medium.
In our evaluation, the overall rates of recovery obtained with the BACTEC MGIT 960 and BACTEC 460 systems were clearly higher than those achieved with solid media, while in the comparison of the two liquid media, the rate of recovery of mycobacteria in the BACTEC MGIT 960 system was only slightly lower than that in the BACTEC 460 system. The use of one liquid medium and one solid medium is recommended by the Centers for Disease Control and Prevention (1), and nowadays the use of such a combination is acknowledged worldwide. When the combination with the solid medium was considered, the difference between the BACTEC MGIT 960 system and the BACTEC 460 system was further reduced. Most of the differences among various methods were for mycobacteria other than M. tuberculosis.
In regard to turnaround times, the mean detection times were significantly shorter for methods that used a liquid medium than for Lowenstein-Jensen medium. The BACTEC MGIT 960 system detected positive samples an average of 1.5 days earlier than the BACTEC 460 system did. This is a statistically significant difference that was also confirmed when the most-represented mycobacterial species were considered singularly. Such times to detection are certainly affected by the different reading frequencies of various methods; a more frequent inspection of radiometric cultures and solid media is, however, incompatible with the laboratory routine and with the limited reading speed of the BACTEC 460 instrument, particularly in laboratories with high workloads. The continuous growth monitoring, which allows the real-time detection of positive cultures, far from being a bias factor, therefore represents an important feature of automatic systems like the BACTEC MGIT 960 system.
All potentially pathogenic mycobacteria encountered in this trial grew well in the BACTEC MGIT 960 system, including notoriously fastidious organisms like M. bovis and M. malmoense; the only species represented by more than one isolate which failed to grow in the BACTEC MGIT 960 system was M. gordonae, a well-known environmental contaminant.
The high rate of contamination of the BACTEC MGIT 960 system is probably due to the fact that this system uses a highly rich medium; the BACTEC 460 system, which relies on the high degree of sensitivity of radiometric detection, uses a less rich medium that is consequently less liable to overgrowth. Interestingly, we noticed that the contamination rate seemed to decrease during the study at all three centers that participated in the evaluation; such improvement suggests the need to set a period during which technicians can become accustomed to handling the screw-cap vials.
Of great interest is the fact that in 12 instances, what was counted here as contamination was due to the growth in the BACTEC MGIT 960 system of nocardiae and, in one further case, of a Rhodococcus sp.; these organisms, whose detection is clinically important because of their pathogenicity, particularly in immunocompromised patients, represented 5% of the contaminants.
Only one evaluation of the BACTEC MGIT 960 system has been published so far (2). It reported recovery rates comparable to ours for the BACTEC MGIT 960 system and solid media and rates lower than ours for the BACTEC 460 system. The different ratio of the M. tuberculosis complex to the M. avium complex may well explain such a discrepancy as the M. tuberculosis complex growing better than the M. avium complex in the BACTEC 460 system. The M. tuberculosis complex represented more than 70% of our isolates but only 36% of the isolates in the other study. In both studies, but more evidently in ours, the BACTEC MGIT 960 system was characterized by the shortest times to detection. Although in both evaluations the contamination rate for the BACTEC MGIT 960 system was intermediate between those for the other methods, it appeared to be less favorable in our study than in another study.
Other automatic systems for the culture of mycobacteria have been introduced in recent times. Like with the ESP II system (15), mycobacteria belonging to the M. avium complex appear to benefit more than M. tuberculosis with the BACTEC MGIT 960 system. On the contrary, M. xenopi, characterized by poor or absent growth both with the ESP II system (15) and the MB/BacT system (8), grew easily and early in the BACTEC MGIT 960 system. On the other hand, the contamination rate for the BACTEC MGIT 960 system was the highest among those for automatic systems, thus emphasizing the higher risk of environmental contamination from the use of screw caps in comparison with that from the use of the rubber septum adopted by other systems. However, there are safety issues if needles are used for inoculation through a rubber septum.
The results reported here substantiate the fact that the BACTEC MGIT 960 system is a culture equivalent to the radiometric BACTEC 460 system. Recovery rates are very close to those of the radiometric method, while times to detection are even earlier. On the other hand, many good points characterize the system from the operative point of view: the radioactivity and the problems related to its use and disposal are not present, the full automation eliminates loading and unloading of tubes and minimizes the risk of bottle breakage, CO2 tanks are not required, the noninvasive monitoring of cultures eliminates the possibility of cross contamination, the use of screw caps on the tubes eliminate the need for use of needles and eliminates the risk of inadvertent needle pricks, the identification of samples by means of a bar code eliminates the risk of transcription errors, and maintenance is minimal. Furthermore, the space occupied by the BACTEC MGIT 960 instrument is very limited when one considers that it supports a heavy load (960 cultures corresponding to a daily capacity of 23 samples).
ACKNOWLEDGMENTS
We are grateful to Becton Dickinson for providing the instrumentation and reagents for evaluation.
We thank Uli Kunert and Salman Siddiqi for support.
REFERENCES
- 1.Centers for Disease Control and Prevention. Essential components of a tuberculosis prevention and control program. Morbid Mortal Weekly Rep. 1995;44-RR:1–16. [Google Scholar]
- 2.Hanna B A, Ebrahimzadeh A, Elliott L B, Morgan M A, Novak S M, Rüsch-Gerdes S, Acio M, Dunbar D F, Holmes T M, Rexer C H, Savthyakumar C, Vannier A M. Multicenter evaluation of the BACTEC MGIT 960 system for recovery of mycobacteria. J Clin Microbiol. 1999;37:748–752. doi: 10.1128/jcm.37.3.748-752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heifets L. Mycobacteriology laboratory. Clin Chest Med. 1997;18:35–53. doi: 10.1016/s0272-5231(05)70354-3. [DOI] [PubMed] [Google Scholar]
- 4.Nolte F S, Metchock B. Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 400–437. [Google Scholar]
- 5.Pfaller M A. Application of new technology to the detection, identification, and antimicrobial susceptibility testing of mycobacteria. Am J Clin Pathol. 1994;101:329–337. doi: 10.1093/ajcp/101.3.329. [DOI] [PubMed] [Google Scholar]
- 6.Pfyffer G E, Cieslak C, Welscher H M, Kissling P, Rüsch-Gerdes S. Rapid detection of mycobacteria in clinical specimens by using the automated BACTEC 9000 MB system and comparison with radiometric and solid-culture systems. J Clin Microbiol. 1997;35:2229–2234. doi: 10.1128/jcm.35.9.2229-2234.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfyffer G E, Welscher H M, Kissling P, Cieslak C, Casal M J, Gutierrez J, Rüsch-Gerdes S. Comparison of the Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli. J Clin Microbiol. 1997;35:364–368. doi: 10.1128/jcm.35.2.364-368.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohner P, Ninet B, Metral C, Emler S, Auckenthaler R. Evaluation of the MB/BacT system and comparison to the BACTEC 460 system and solid media for isolation of mycobacteria from clinical specimens. J Clin Microbiol. 1997;35:3127–3131. doi: 10.1128/jcm.35.12.3127-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqi S H. BACTEC TB system. Product and procedure manual, revision B. Towson, Md: Becton Dickinson Diagnostic Instruments System; 1989. [Google Scholar]
- 10.Somoskövi Á, Magyar P. Comparison of the Mycobacteria Growth Indicator Tube with MB Redox, Löwenstein-Jensen, and Middlebrook 7H11 media for recovery of mycobacteria in clinical specimens. J Clin Microbiol. 1999;37:1366–1369. doi: 10.1128/jcm.37.5.1366-1369.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockman L. DNA probes for the identification of mycobacteria. In: Isenberg H D, editor. Clinical microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. pp. 3.15.1–3.15.4. [Google Scholar]
- 12.Styrt B A, Shinnick T M, Ridderhof J C, Crawford J T, Tenover F C. Turnaround times for mycobacterial cultures. J Clin Microbiol. 1997;35:1041–1042. doi: 10.1128/jcm.35.4.1041-1042.1997. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenover F C, Crawford J T, Huebner R E, Geiter L J, Horsburgh C R, Good R C. The resurgence of tuberculosis. Is your laboratory ready? J Clin Microbiol. 1993;31:767–770. doi: 10.1128/jcm.31.4.767-770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tortoli E, Bartoloni A. High-performance liquid chromatography and identification of mycobacteria. Rev Med Microbiol. 1996;7:207–219. [Google Scholar]
- 15.Tortoli E, Cichero P, Chirillo M G, Gismondo M R, Bono L, Gesu G, Simonetti M T, Volpe G, Nardi G, Marone P. Multicenter comparison of ESP Culture System II with BACTEC 460TB and with Lowenstein-Jensen medium for recovery of mycobacteria from different clinical specimens, including blood. J Clin Microbiol. 1998;36:1378–1381. doi: 10.1128/jcm.36.5.1378-1381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viljanen M K, Olkkonen L, Katila M L. Conventional identification characteristics, mycolate and fatty acid composition, and clinical significance of MAIX AccuProbe-positive isolates of Mycobacterium avium complex. J Clin Microbiol. 1993;31:1376–1378. doi: 10.1128/jcm.31.5.1376-1378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods G L, Fish G, Plaunt M, Murphy M. Clinical evaluation of Difco ESP Culture System II for growth and detection of mycobacteria. J Clin Microbiol. 1997;35:121–124. doi: 10.1128/jcm.35.1.121-124.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanetti S, Ardito F, Sechi L, Sanguinetti M, Molicotti P, Delogu G, Pinna M P, Nacci A, Fadda G. Evaluation of a nonradiometric system (BACTEC 9000 MB) for detection of mycobacteria in human clinical samples. J Clin Microbiol. 1997;35:2072–2075. doi: 10.1128/jcm.35.8.2072-2075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]