Abstract
Purpose
Contrast-enhanced ultrasound (CEUS) is a useful tool to assess treatment response after percutaneous ablation or transarterial chemoembolization (TACE) of hepatocellular carcinoma (HCC). Here, we performed a systematic review and meta-analysis to evaluate the usefulness of CEUS in identifying residual tumor after locoregional therapy.
Methods
PubMed, Scopus, and Cochrane library databases were searched from their inception until March 8, 2021, for diagnostic test accuracy studies comparing CEUS to a reference standard for identifying residual tumors after locoregional therapy of HCC. The pooled sensitivity, specificity, accuracy, and diagnostic odds ratio (DOR) were obtained using a bivariate random effects model. Subgroup analyses were performed by stratifying the studies based on study design, type of locoregional therapy, CEUS criteria for residual tumor, timing of CEUS follow up, and type of standard reference.
Results
Two reviewers independently evaluated 1479 publications. After full-text review, 142 studies were found to be relevant, and 43 publications (50 cohorts) were finally included. The overall sensitivity of CEUS in detection of residual disease estimated from the bivariate random effects model was 0.85 (95% CI 0.80–0.89). Similarly, the overall specificity was 0.94 (95% CI 0.91–0.96). The diagnostic accuracy was 93.5%. The DOR was 70.1 (95% CI 62.2–148), and the AUROC was 0.95. Importantly, subgroup analysis showed no apparent differences in the diagnostic performance between locoregional therapy (TACE vs. ablation) and criteria used to define residual enhancement, timing of performing CEUS, study design, or type of reference standard.
Conclusion
CEUS is a highly accurate method to identify HCC residual tumor after TACE or percutaneous ablation.
Keywords: Contrast-enhanced ultrasound, Hepatocellular carcinoma, Locoregional therapy, Microwave ablation, Radiofrequency ablation, Transcatheter arterial chemoembolization, Treatment response
Introduction
Hepatocellular carcinoma (HCC) constitutes 75–85% of primary liver cancer cases and is the third leading cause of cancer mortality worldwide [1]. The incidence rate of HCC in the USA has increased in the last several decades and is projected to continue increasing [2]. Treatment indication for HCC patients depends on disease stage, liver function, and tumor burden [3]. The Barcelona Clinic Liver Cancer (BCLC) classification determines disease stage, which considers tumor extension, physical status, liver function, and cancer-related symptoms [4] and assists with interventional treatment determination.
The treatment of choice for HCC includes surgical resection and transplantation, which can be curative therapies [5]. Unfortunately, many patients are ineligible for surgical resection or transplantation due to advanced disease stages and comorbidities. HCC patients with BCLC stages 0-B can benefit from locoregional therapies, such as percutaneous ablation, transarterial chemoembolization (TACE), and transarterial radioembolization (TARE) to downstage or palliate disease [6]. Moreover, by downstaging disease and potentially preventing progression, locoregional therapies can serve as a bridge to transplantation [7].
TACE is a catheter-based therapy that embolizes a tumor-feeding artery and is suitable for patients with large or multinodular tumors [8]. Percutaneous ablation procedures can be categorized into thermal and chemical, and it is most suitable for patients with up to three nodules with each nodule less than 3 cm [9]. Thermal includes radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, and high-intensity focused ultrasound ablation (HIFUA), while chemical includes percutaneous ethanol injection (PEI). In the USA, MWA is generally the preferred method for percutaneous ablation treatment. TARE is a transcatheter intra-arterial procedure delivering radioactive yttrium 90 (Y90) to the target HCC lesions, and it might be considered in patients with HCC lesions beyond the “up-to-seven” criteria (new Milan criteria) with larger tumors and limited number of tumors, especially with portal vein thrombosis [10–13].
Currently, contrast-enhanced magnetic resonance imaging (CE-MRI) or contrast-enhanced computed tomography (CE-CT) is the reference standard for evaluating residual tumor post-treatment. CE-CT is reported to have a specificity of 100% and a sensitivity of 36% compared to histology, in a study of patients with liver transplantation shortly after ablation [14].The recommended guidelines by the Society of Interventional Radiology for follow-up imaging is 4–6 weeks post-treatment. This waiting period allows for optimal differentiation between post-treatment inflammation and viable tumor [15, 16]. Retreatment following locoregional therapies is often needed in this patient population, necessitating earlier detection of tumor viability for retreatment options.
Contrast-enhanced ultrasound (CEUS) has been studied as an alternative to CE-MRI and CE-CT, detecting HCC viability as soon as immediately post-treatment [17–19]. CEUS utilizes an ultrasound contrast agent (UCA) which consists of inert gas-filled microbubbles encapsulated in a lipid, protein, or polymer stable shell [20]. UCAs are 1–10 μm in diameter [21], allowing them to act as a blood pool agent retained in the blood vessels after peripheral injection [22]. Benefits of CEUS include its high temporal resolution, lack of ionizing radiation, lack of nephrotoxicity, cost effectiveness, and portability [23, 24]. Additionally, many of the artifacts generated on CT and MRI shortly after locoregional therapies do not cause artifacts on ultrasound, enabling earlier imaging to follow up.
This systematic review and meta-analysis aims to evaluate the role of CEUS to detect residual tumor after percutaneous ablation and TACE locoregional therapies to determine its diagnostic value in HCC patients. Furthermore, we evaluated the specific criteria of enhancement for residual disease on CEUS. This approach is expected to enable identification of optimal follow-up time and image criteria for the diagnosis of residual HCC following therapy.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy Studies (PRISMA-DTA) statement. The protocol has been registered with PROSPERO with registration number CRD42021235615. Since the current review was based on previously published studies, Institutional Review Board approval and patient consent were not required.
Literature search
We searched PubMed, Scopus, and Cochrane Library databases using the following keywords: contrast-enhanced ultrasound, Hepatocellular carcinoma, and ablation, TACE, and TARE. ((((therapeutic radioemboli*) OR (transarterial radioemboli*) OR (transhepatic arterial radioemboli*) OR (TARE)) OR ((therapeutic chemoemboli*) OR (transarterial chemoemboli*) OR (transhepatic arterial chemoemboli*) OR (TACE))) OR (Ablation)) AND (((Carcinomas, Hepatocellular) OR (Hepatocellular Carcinomas) OR (Liver Cell Carcinoma) OR (Liver cancer)) AND ((Contrast-enhanced ultrasound) OR (contrast-enhanced ultrasound) OR (contrast-enhanced ultrasonography) OR (contrast-enhanced ultrasonography) OR (contrast-enhanced sonography) OR (contrast-enhanced sonography) OR (CEUS))).
Eligibility criteria
The inclusion criteria consisted of: (1) a cohort study or a case–control study; (2) CEUS was used after locoregional therapies (TACE, TARE, and/or ablation) for HCC patients for evaluating treatment response; (3) a standard reference must be included, e.g., CT, MRI, or histology, etc.; (4) diagnostic criteria of CEUS in diagnosing residual HCC were mentioned; (5) either the absolute number of true-positive (TP), false-positive (FP), false-negative (FN), and true-negative (TN) results or the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) could be gathered or calculated from the full text. Exclusion criteria consisted of: (1) a review, conference abstract, case report, or letter; (2) language other than English; (3) animal studies; (4) studies using the same patient population with overlapping study period.
Study selection
Initial search records were imported to Endnote X9 (Clarivate Analytics, Philadelphia, USA). Then, two reviewers (YH and ES) independently screened the title and abstract of articles identified using the inclusion and exclusion criteria described above, and any disagreements were resolved when necessary by a third reader (WC) after reading the full text. The flow chart of the literature searching strategy is shown in Fig. 1.
Fig. 1.
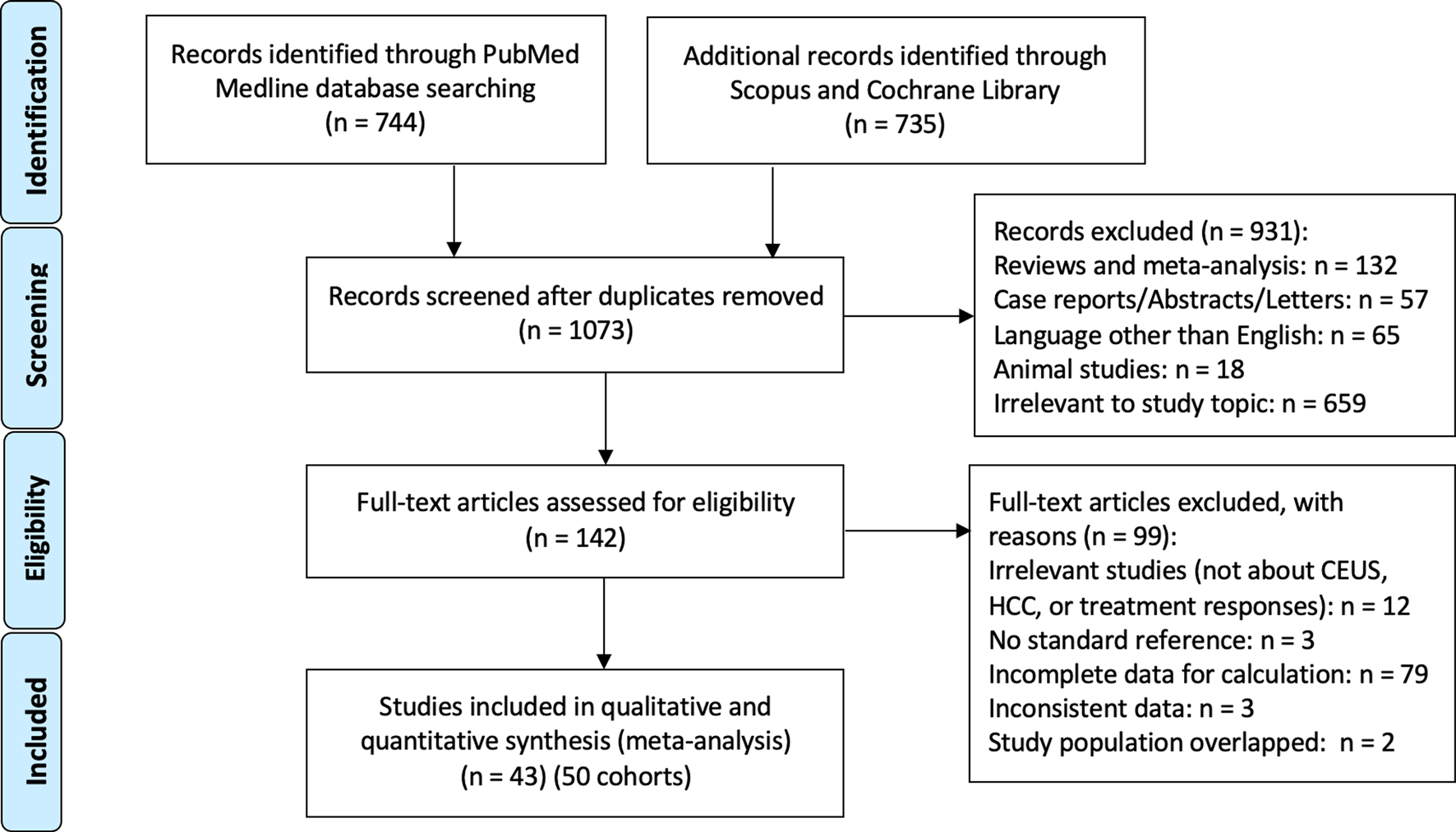
The PRISMA diagram of the literature retrieval, screening, and inclusion process
Data extraction
Two reviewers (YH and ES) independently extracted the data from the final included studies. Data were collected from each study: the last name of first author, publication year, study design, study time period, number of patients/nodules, age, gender, tumor diameter, type of locoregional therapy, ultrasound devices and contrast agent dosage of CEUS, reference standard, time of CEUS and the reference standard performed after locoregional, CEUS criteria for diagnosing residual disease, and data regarding TP, FP, TN, and FN rates were extracted.
Quality assessment for included studies
Two reviewers (YH and ES) independently assessed the study quality using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool. This is a revised quality assessment tool for a meta-analysis of diagnostic accuracy studies. QUADAS-2 consists of four key domains for risk of bias analyses—including patient selection, index test, reference standard, and flow and timing—and the three domains regarding applicability.
Dealing with missing data
If studies mentioned the correlation of CEUS with a standard reference but did not mention the exact case numbers detected by CEUS, we deemed the data to be incomplete. If the diagnostic criteria used for treatment response assessment on CEUS were not mentioned, or post-treatment imaging intervals were not mentioned, the study was also deemed to have incomplete data. Studies with incomplete data were excluded from analysis.
Statistical analysis
Statistical analysis was performed in R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria) and mada package (version 0.5.10). A bivariate random effects model was used for summary estimates of sensitivity and specificity with 95% confidence intervals. We also derived the diagnostic odds ratio (DOR), positive LR, negative LR, and summary receiver operating characteristic curve (SROC) from the pooled estimates.
SROC curves and the area under the curve (AUC) obtained from the fitted bivariate random effects model were used to summarize the overall test performance. SROC curves were plotted with the confidence region and prediction region. Heterogeneity between study results was assessed using visual inspection of the forest plot and SROC curves, as recommended for diagnostic test accuracy meta-analyses.
Before starting the analyses, we identified the following variables to be possible sources of heterogeneity: treatment type (ablation vs. TACE), study design (prospective vs. retrospective), timing of performing post-treatment CEUS, criteria for CEUS in diagnosing residual tumor (APHE vs. any other enhancement criteria), and reference standard (CT/MR vs histology/angiography). Following data collection, sub-analysis on these criteria was performed to determine their effects on CEUS performance.
Results
Study selection process
A total of 1479 studies were identified and retrieved for initial assessment. After duplicates were removed, two authors independently evaluated studies by titles and abstracts, and 142 publications remained for full-text review. Among them, 99 studies were further excluded after full-text review for the following reasons: not related to CEUS, HCC, or treatment response evaluations (n = 12), no standard reference (n = 3), incomplete data (n = 79), inconsistent data (n = 3), and study population overlapped (n = 2). Finally, 43 studies (50 cohorts) including 2993 cases of CEUS were ultimately enrolled in data synthesis and meta-analysis [25–67]. The study selection process is shown in Fig. 1.
Characteristics of studies
Descriptive characteristics of the included 43 studies are summarized in Table 1. The studies were published between 2000 and 2020. Thirty studies enrolled patients prospectively and 13 were retrospective. The number of HCC nodules in each study ranged from 12 to 266. Twenty-three studies used ablation as locoregional therapy, 14 studies used TACE as locoregional therapy, and the remaining 6 studies utilized both ablation and TACE for their patients. Our search yielded no study which utilized TARE. Contrast agents varied across studies, with 17 using Sonovue, 17 Levovist, 6 Sonazoid, 2 Definity, and 1 study used Optison and Imagent interchangeably. Out of the 50 cohorts, post-treatment CEUS were performed immediately or within 24 h in 8 cohorts, 1–7 days in 15 cohorts, 1–2 weeks in 3 cohorts, 2–4 weeks in 17 cohorts, longer than 1 month in 5 cohorts, unknown for 1 study (Kim 2006), and 1–30 days in 1 study (Kono 2007). In terms of the criteria used for CEUS in diagnosing residual tumor after locoregional therapy, 22 studies used arterial phase hyperenhancement (APHE), and 21 studies used “intratumorally enhancement”, “residual blood flow”, or “nodular enhancement”, etc., and we grouped them as “any enhancement”. Reference standards varied among studies, including CT, MRI, DSA, histology, and angiography. Other information such as age, gender, tumor diameter, ultrasound devices used, and dose of contrast agent are summarized in Table 1.
Table 1.
Study characteristics
| Study | Country | Study design | Study time period | # Patient (M/F) | # Nodules | Age (mean) | Tumor diameter (cm) | Locoregional treatment |
|
| ||||||||
| Ainora 2020 | Italy | Prospective | 12/2014–4/2016 | 94 (79/15) | 104 | 68 ± 9 | 1.0–7.0 (2.7) | PEI/RFA, TACE, combined |
| Cao 2018 | China | Prospective | 7/2016–10/2017 | 42 (31/11) | 42 | 24–71 (52.1 ± 13.1) | 1.4–4.8 (2.8 ± 1.0) | RFA |
| Cho 2015 | South Korea | Prospective | 11/2011–11/2012 | 12 (10/2) | 12 | 21–81 (63.9 ± 15.3) | 1.5–5 (3.2 ± 1.1) | TACE |
| Choi 2000 | South Korea | Prospective | 4/1999–10/1999 | 40 (31/9) | 45 | 35–82 (57) | 1.0–3.8 (2.7) | RFA |
| Choi 2003 | South Korea | Retrospective | 5/2000–12/2001 | 75 (63/12) | 81 | 31–72 (57) | 1.3–4.8 (2.5) | RFA |
| Cioni 2000 | Italy | Prospective | 10/1997–3/1999 | 47 (35/12) | 65 | 53–74 (65.7 ± 5.6) | 1.8–9.5 (4.3 ± 1.7) | TACE |
| Cioni 2001 | Italy | Prospective | – | 50 (39/11) | 61 | 45–78 (61.5) | 1.0–5.0 (2.5) | RFA |
| Dill-Macky 2006 | Canada | Prospective | 12/2002–2/2004 | 19 (15/4) | 21 | 43–83 (64) | 1.5–3.7 | RFA |
| Ding 2001 | China | Prospective | – | 26 (24/2) | 30 | 48–55 (66) | 1.0–6.0 (2.5 ± 1.4) | TACE, RFA, combined |
| Du 2015 | China | Prospective | 9/2011–1/2013 | 63 (55/8) | 78 | 41–67 (55 ± 7) | 0.8–2.9 (1.5 ± 0.4) | RFA |
| Gallotti 2009 | Italy | Prospective | 12/2005–12/2007 | 69 (44/14) | 90 | 28–89 (69) | 0.5–4.9 (2.6) | PEI/RFTA |
| Hirai 2002 | Japan | Prospective | 1/2000–3/2001 | 28 | 37 | 52–84 (68.8) | 1.0–8.0 (2.7) | TACE/RFA |
| Kim 2005 | Korea | Retrospective | 7/2002–7/2003 | 90 (66/24) | 94 | 35–90 (51) | 1.0–5.0 (2.1–1.3) | RFA |
| Kim 2006 | Canada | Prospective | – | 29 (19/10) | 31 | 36–74 (58) | 0.6–12.0 (2.5) | TACE |
| Kisaka 2006 | Japan | Retrospective | 3/2004–9/2004 | 29 (22/7) | 26 | 54–80 (68.6 ± 8.08) | 1.0–2.9 (1.6 ± 0.6) | RFA |
| Kono 2007 | USA | Prospective | – | 33 (21/12) | 33 | – | 1–10 (4.7) | TACE |
| Liu 2015 | China | Retrospective | 6/2007–12/2013 | 130 (122/8) | 130 | 17–80 (53 ± 12) | 1.0–21.4 (4.4 ± 4.1) | TACE |
| Lu 2007 | China | Prospective | 5/2004–3/2005 | 118 | 118 | 25–80 (56 ± 12) | 2.7 ± 12.0 | RFA/MWA |
| Luo 2010 | China | Prospective | 2/2007–11/2007 | 63 (38/25) | 63 | 53–80 (70) | 1.0–3.0 (2.2) | RFA |
| Meloni 2001 | Italy | Retrospective | 10/1999–2/2000 | 35 (24/11) | 43 | 47–75 (64) | 1.2–5.8 (3.6 ± 1.1) | RFA |
| Minami 2003 | Japan | Prospective | 3/1999–6/2001 | 17 | 19 | 52–87 (67.7) | 1.5–11 (3.9 ± 2.0) | TACE |
| Morimoto 2003 | Japan | Retrospective | 12/1999–3/2001 | 29 (17/12) | 29 | 58–79 (69.9) | – | TACE |
| Morimoto 2005 | Japan | Prospective | 1/2000–3/2002 | 48 | 72 | 52–79 (64.2) | 0.7–3.0 (2.1 ± 0.7) | RFA |
| Moschorius 2014 | Greece | Retrospective | 3/2008–4/2012 | 47 (37/10) | 80 | 51–84 (67.5 ± 8.5) | 2.3–16.3 (7.3) | TACE |
| Numata 2010 | Japan | Retrospective | 7/2007–10/2008 | 21 (12/9) | 21 | 65–80 (73) | 1.0–2.6 (16.3 ± 3.6) | HIFUA |
| Paul 2017 | India | Prospective | 2/2010–6/2015 | 42 | 57 | 24–72 (53.3 ± 12.5) | 1.0–15 (4.9 ± 2.9) | TACE |
| Pompili 2005 | Italy | Prospective | 3/2002–12/2003 | 47 (36/11) | 56 | 48–83 (70.8 ± 7.8) | 1.0–7.5 (3.3 ± 1.5) | PEI, RFA, TACE, combined |
| Pregler 2016 | Germany | Retrospective | 1/2014–1/2016 | 30 (25/5) | 30 | 54–73 (64) | (0.3 ± 0.7) x (3.2 ± 0.7) | MWA |
| Ricci 2009 | Italy | Prospective | 1/2001–5/2004 | 100 (65/35) | 100 | 62–76 | 2.6–4.8 (3.7 ± 1.1) | RFA |
| Shalvaggio 2010 | Italy | Prospective | 2/2005–12/2007 | 139 | 148 | 43–75 (59 ± 8.4) | – | RFA + TACE |
| Shaw 2015 | USA | Prospective | 2/2013–10/2013 | 14 (12/3) | 16 | 50–75 (62 ± 6) | 1.2–4.5 (2.5 ± 0.8) | TACE |
| Shima 2005 | Japan | Prospective | 4/2001–3/2004 | 51 (34/17) | 63 | 32–87 (70) | 1.0–9.0 (2.8) | TACE |
| Shimizu 2004 | Japan | Prospective | 10/2000–6/2001 | 40 (28/12) | 64 | 50–85 (66.3) | 1.0–6.0 | RFA |
| Shiozawa 2010 | Japan | Retrospective | 5/2002–10/2008 | 71 (51/20) | 87 | 46–86 (70 ± 8) | 0.8–6.5 (2.0 ± 1.0) | RFA, TACE, combined |
| Takizawa 2013 | Japan | Prospective | 9/2011–4/2012 | 32 (24/8) | 59 | 51–83 (70 ± 7) | 1.0–7.3 (2.9 ± 1.2) | TACE |
| Vilana 2003 | Spain | Prospective | 2/1999–4/2000 | 31 (22/9) | 31 | 67.87.7 | 1.5–5.0 (2.7 ± 0.8) | RFA + PEI |
| Vilana 2006 | Spain | Prospective | 7/2002–5/2003 | 41 (25/16) | 41 | 64.6 ± 11 | 1.3–4.1 (2.4 ± 0.7) | PEI, RFA |
| Wang 2016 | China | Retrospective | 7/2011–2/2013 | 75 (48/27) | 89 | 29–84 (57.5 ± 13.5) | 1.2–5.0 (3.7 ± 1.3) | RFA |
| Watanabe 2020 | Japan | Retrospective | 4/2014–6/2016 | 70 (43/27) | 89 | 44–87 (70 + 9.5) | 1.8 ± 1.1 | TACE |
| Wen 2003 | China | Prospective | 11/1999–3/2001 | 67 (47/20) | 91 | 43–85 (66) | 1.0–5.0 (2.4 ± 1.0) | RFA |
| Xia 2008 | China | Retrospective | 1/2007–8/2008 | 28 (24/4) | 43 | 60–85 (73.3) | 0.9–10.0 (2.9 ± 1.8) | TACE |
| Xu 2014 | China | Prospective | 5/2010–12/2011 | 72 | 83 | – | 2.4 ± 0.5 | RFA |
| Zheng 2013 | China | Prospective | 5/2007–5/2011 | 141 (132/9) | 266 | 21–87 (53.4 ± 12.1) | 0.6–5.7 (2.4 ± 1.0) | RFA/MWA |
|
| ||||||||
| Study | CEUS mode | Contrast agent and dosage | Time of post-treatment CEUS | CEUS criteria for residual disease | CEUS Binary TR Criteria | Reference standard | Time for reference standard | |
|
| ||||||||
| Ainora 2020 | CEUS | 2.4 ml SonoVue | 48 h | APHE | APHE | Helical CECT | 1 month | |
| Cao 2018 | 2D and 3D CEUS | 2.4 ml SonoVue | 1 month | Nodular arterially hyperenhancement area within of along the edge | APHE | CEMR | No more than 3 days after CEUS | |
| Cho 2015 | CEUS with low MI | 2.4 ml SonoVue | 4 weeks and 8 weeks | Partial or entire enhancement | Any enhancesment | Dynamic CEMRI | 12 weeks | |
| Choi 2000 | CEPD | 12.5 ml Levovist | 12–23 h | Presence of signal within the tumor | Any enhancement | CECT | 30 min | |
| Choi 2003 | CEUS with CHA | 4 g Levovist | Within 24 h | Nodular crescentic enhancing foci at the margins | Any enhancement | CECT | 1 month | |
| Cioni 2000 | CEPD | 2.5 g Levovist, 300 mg/ml | 3–4 weeks | Intratumoral blood flow signals | Any enhancement | CECT/CEMR | 3–4 weeks | |
| Cioni 2001 | CEPD | 2.5 g Levovist, 300 mg/ml | 3–7 days | Persistent intratumoral enhancement | Any enhancement | Dual-phase helical CT | 4–24 months | |
| Dill-Macky 2006 | PIH CEUS | 1.3 ml Definity | Within 1 h | APHE at the ablation margin or adjacent to the ablation zone | APHE | CT/MRI | 2 weeks to 1 month | |
| Ding 2001 | Pulse subtraction CHI | 2.5 g Levovist, 300 mg/ml | 6–10 days | Tumor perfusion flow same or higher than surroundings | Any enhancement | CECT | 6–10 days | |
| Du 2015 | CEUS with CPI | 1.5 ml SonoVue | 20–30 min | Persistent APHE | APHE | CEMR | 1 month | |
| Gallotti 2009 | CEUS nonlinear | 2.4 ml SonoVue | Immediately | Presence of residual enhancement | Any enhancement | CECT | 1 month | |
| Hirai 2002 | CEUS with CHA | 2.1 g Levovist, 300 mg/ml | 5–10 days | Residual intratumoral blood flow or residual tumor stain | Any enhancement | Dynamic CT | 3 months | |
| Kim 2005 | CEUS with ADI | 4 g Levovist, 300 mg/ml | Within 24 h | Focal areas with irregular peripheral enhancement | Any enhancement | Four-phase helical CT | 1 month | |
| Kim 2006 | CEUS with ADI | Levovist, 300 mg/ml | – | Intratumoral enhancement | Any enhancement | Angiography | – | |
| Kisaka 2006 | CEUS with VUS | Levovist, 300 mg/ml | 3 days | Distance measured on CEUS was not longer than measurement on VUS | Any enhancement | CECT | 3–5 days | |
| Kono 2007 | CEUS | 0.5–2 ml Optison and Imagent | 1–30 days | Flow present within the tumor including the rim | Any enhancement | Angiography/ CECT/CEMR | 1 month (CT), 3 months (MR) | |
| Liu 2015 | CEUS | 2.4 ml SonoVue | 0.5–3 months | mRECIST/ APHE | APHE | Histology/angiography | 0.5–3 months | |
| Lu 2007 | CEUS with low MI | 2.4 ml SonoVue | 1 month | APHE | APHE | CECT/CEMRI | 1 month | |
| Luo 2010 | 3D CEUS | 2 ml Sonazoid | 1 day | APHE | APHE | 3D CECT | 1 month | |
| Meloni 2001 | CEPD and PIH CEUS | Levovist, 300 mg/ml | 4 months | APHE | APHE | Dual-phase CECT | 4 months | |
| Minami 2003 | Coded phase harmonic sonography | 2.5 g Levovist, 400 g/l | 4–8 days | APHE | APHE | CECT | 2 months | |
| Morimoto 2003 | CEUS | Levovist, 300 mg/ml | 7 days | APHE and portal phase hypoenhancement | APHE | Histology | 7 days | |
| Morimoto 2005 | CEUS | Levovist, 300 mg/ml | 1 month | APHE | APHE | Histology | 4 weeks | |
| Moschorius 2014 | CEUS | 2.4 or 4.8 ml SonoVue | 7 days | mRECIST and diameters of viable lesions compared to baseline | Any enhancement | CT/MRI | 1 month | |
| Numata 2010 | 3D CEUS with CHA | 2 ml Sonazoid | Immediately, 1 week, and 1 month | APHE, homogenous enhancement (middle phase), and hypoechoic (late phase) | APHE | CECT/CEMR | 1 month | |
| Paul 2017 | CEUS | 2.4 ml SonoVue | 1 month | APHE and hypoenhancement during portal/ delayed phase | APHE | MRI | 1 month | |
| Pompili 2005 | Gray-scale harmonic CEUS | 2.4 ml SonoVue | Within 1 month | APHE | APHE | Multi-phasic spiral CT | 1 month | |
| Pregler 2016 | Dynamic CEUS | 2.4 ml SonoVue | 1 day | Irregular APHE and washout in the portal venous phase | APHE | CEMR | 6 weeks | |
| Ricci 2009 | CEUS with low MI | 2.4 ml SonoVue | 1 month | APHE | APHE | Spiral CT | 1 month | |
| Shalvaggio 2010 | PIH CEUS with MI | 2.4 ml SonoVue low | 1 month | Positive enhancement | Any enhancement | MDCT/angiography | 1 month | |
| Shaw 2015 | CEUS | 0.6–0.7 ml Definity | 1–2 weeks and 1 month | Tumor enhancement | Any enhancement | CT/MRI | 1 month | |
| Shima 2005 | CEPD | 7 ml Levovist, 300 mg/ml | 1 week | Enhancement in the arterial/portal phase or no ovalshaped perfusion defect the portal phase | Any enhancement | Histology/angiography | – | |
| Shimizu 2004 | CEUS with ADI | Levovist, 300 mg/ml | 1 day and 7 days | Residual vascularity in arterial phase and a defect in the post vascular phase in the delayed phase | Any enhancement | Dynamic CT | – | |
| Shiozawa 2010 | Pulse subtraction CHI | Sonazoid, 0.015 ml/kg | 34–1882 days (mean 267 days) | Nodular or crescent shaped APHE at the margins with a defect in the post vascular phase | APHE | Dynamic CT | 34–1882 days (median 267 days) | |
| Takizawa 2013 | CEUS with CHA | 2 ml Sonazoid | 1 day | Arterial and portal phase hypervascular enhancement | APHE | DSA/CECT | 2–6 months | |
| Vilana 2003 | CEPD | 2.5 g Levovist, 300 mg/ml | 1 month | APHE | APHE | Helical CT | 1 month | |
| Vilana 2006 | CEUS with CCI | 2.4 ml SonoVue | Within 24 h and 1 month | Persistence of contrast enhancement | Any enhancement | CT | 1 month | |
| Wang 2016 | Dynamic 3D CEUS | 1–1.5 ml SonoVue | 1 month | Nodular/crescent shaped APHE with portal venous phase subsiding | APHE | CECT | 1 month | |
| Watanabe 2020 | CEUS | 0.5 ml of Sonazoid | 1–2 days | Nodular enhancement | Any enhancement | CECT/angiography/CTHA | 4–16 weeks | |
| Wen 2003 | CEUS with CHA | Levovist, 400 mg/ml | 5–7 days | Safety margin detectability and hypervascularity | APHE | Dynamic CT | 5–7 days | |
| Xia 2008 | CEUS | Sonazoid, 0.01–0.02 ml/kg | 4–9 days | Intratumoral enhancement | Any enhancement | Dynamic CT | 5–9 days (median 7 days) | |
| Xu 2014 | Dynamic CEUS | 2.4 ml SonoVue | 20–40 days | Interior or rim APHE with portal phase subsiding | APHE | CECT/CEMRI | 1 month | |
| Zheng 2013 | CEUS | 2.4 ml SonoVue | 1 month | Lack of nonenhancement | Any enhancement | CECT | 1 month | |
TACE transarterial chemoembolization, RFA radiofrequency ablation, HIFUA high-intensity focused ultrasound ablation, PEI percutaneous ethanol injection, RFTA radiofrequency thermal ablation, MWA microwave ablation, CEUS contrast-enhanced ultrasound, MI mechanical index, PIH pulse inversion harmonics, CHA coded harmonic angio, VUS virtual ultrasonography, CHI contrast harmonic imaging, ADI agent detection imaging, CEPD contrast-enhanced power Doppler, CCI charge contrast imaging, APHE arterial phase hyperenhancement, CT computed tomography, MRI magnetic resonance imaging, CEMRI contrast-enhanced magnetic resonance imaging, CECT contrast-enhanced computed tomography, DSA digital subtraction angiography
Quality assessment
Detailed information for evaluating the quality of the studies is presented in Fig. 2. Overall, the risk of bias was found to be low, with most publications describing the study design with sufficient detail for quality assessment.
Fig. 2.
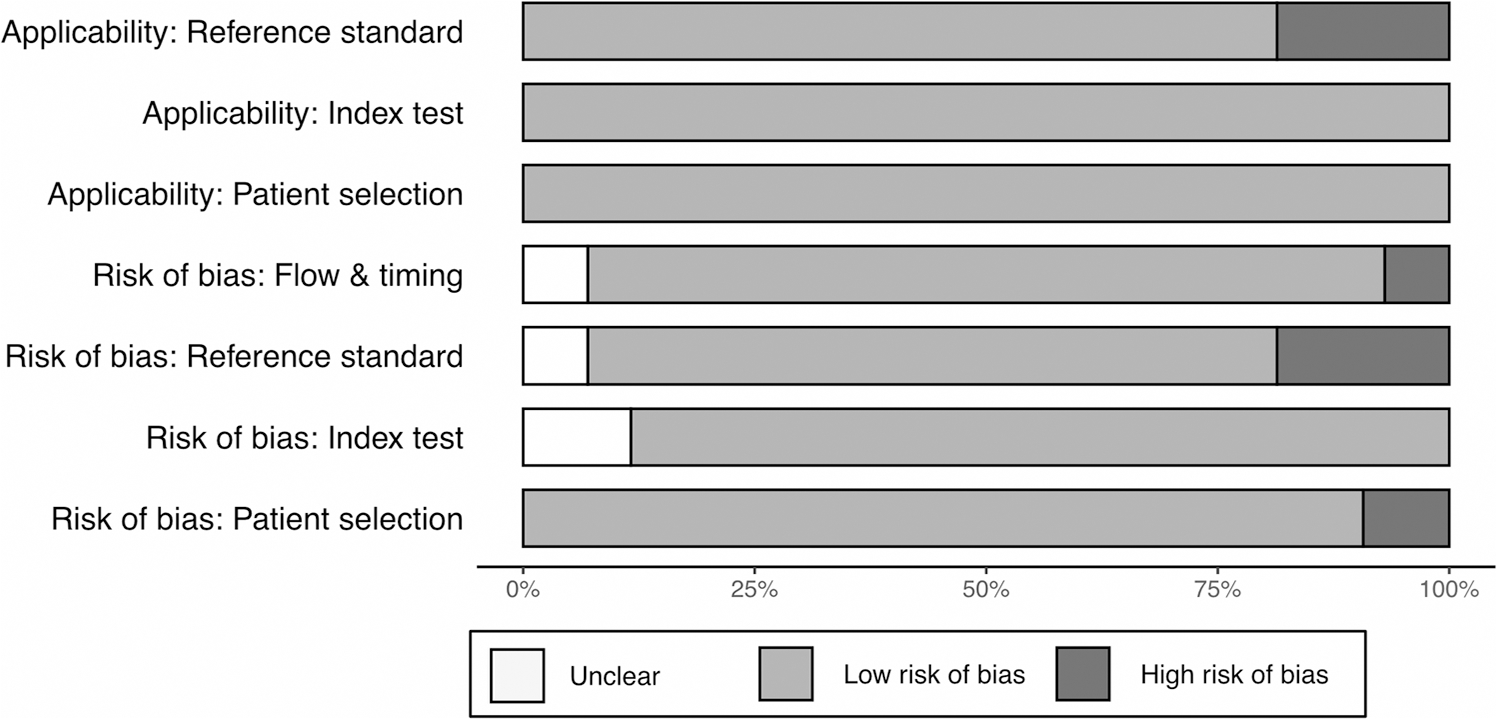
Risk of bias summary using the QUADAS-2 framework
Overall diagnostic accuracy
The overall sensitivity of all included studies was estimated to be 0.85 (95% CI 0.80–0.89), and the overall specificity was estimated to be 0.94 (95% CI 0.91–0.96). The Pooled DOR was 84.1 (95% CI 54.0–131.0), and log (DOR) was 4.56 (95% CI 4.13–5.00) (Supplementary Fig. 1). The AUC for the SROC was 0.95, and the diagnostic accuracy (fraction of correct tests) was 93.5%.
Subgroup analysis
Subgroup analyses were performed based on type of locoregional therapy and CEUS criteria for residual tumor, timing of CEUS post-treatment, study design, and the standard reference used to evaluate the influence of these factors on the overall effect. The summary of the subgroup analyses including sensitivity, specificity, positive likelihood ratio (+LR), negative likelihood ratio (−LR), positive predictive value (PPV), negative predictive value (NPV), prevalence (proportion of nodules that are residual after the first round of treatment, as identified by the reference standard), DOR, and AUC, are shown in Table 2.
Table 2.
Subgroup analyses results
| # Study cohorts | # Nodules | Fraction correct | Sensitivity | Lower CI | Upper CI | Specificity | Lower CI | Upper CI | |
|
| |||||||||
| Overall | 50 | 2993 | 0.935 | 0.850 | 0.803 | 0.887 | 0.937 | 0.913 | 0.955 |
| Therapy + Binary Criteria | |||||||||
| Ablation–APHE | 19 | 1050 | 0.948 | 0.747 | 0.643 | 0.828 | 0.956 | 0.935 | 0.970 |
| Ablation–Any enhancement | 8 | 747 | 0.960 | 0.829 | 0.705 | 0.908 | 0.971 | 0.928 | 0.989 |
| TACE–APHE | 6 | 318 | 0.918 | 0.931 | 0.860 | 0.967 | 0.803 | 0.602 | 0.917 |
| TACE–Any enhancement | 12 | 458 | 0.910 | 0.911 | 0.838 | 0.953 | 0.839 | 0.734 | 0.908 |
| Timing | |||||||||
| < 24 h | 8 | 471 | 0.921 | 0.684 | 0.449 | 0.851 | 0.956 | 0.891 | 0.984 |
| 1–14 days | 18 | 889 | 0.9294 | 0.684 | 0.449 | 0.851 | 0.957 | 0.891 | 0.984 |
| 3–4 weeks | 17 | 1258 | 0.949 | 0.837 | 0.771 | 0.887 | 0.958 | 0.933 | 0.973 |
| 1+ month | 5 | 311 | 0.913 | 0.815 | 0.545 | 0.942 | 0.942 | 0.843 | 0.980 |
| Study design | |||||||||
| Prospective | 34 | 2100 | 0.938 | 0.841 | 0.784 | 0.885 | 0.943 | 0.916 | 0.962 |
| Retrospective | 16 | 893 | 0.927 | 0.864 | 0.766 | 0.926 | 0.923 | 0.866 | 0.957 |
| Reference standard | |||||||||
| CT/MRI | 45 | 2668 | 0.934 | 0.840 | 0.787 | 0.882 | 0.940 | 0.918 | 0.957 |
| Histology/angiography | 5 | 325 | 0.942 | 0.922 | 0.844 | 0.963 | 0.916 | 0.654 | 0.984 |
|
| |||||||||
| + LR | − LR | NPV | PPV | Prev | DOR | Lower CI | Upper CI | AUC | |
|
| |||||||||
| Overall | 13.4 | 0.160 | 0.920 | 0.942 | 0.328 | 84.1 | 54.0 | 131 | 0.954 |
| Therapy + Binary Criteria | |||||||||
| Ablation–APHE | 16.8 | 0.265 | 0.891 | 0.958 | 0.175 | 63.6 | 31.7 | 128 | 0.962 |
| Ablation–Any enhancement | 4.71 | 0.087 | 0.934 | 0.865 | 0.748 | 163 | 51.1 | 517 | 0.947 |
| TACE–APHE | 28.4 | 0.177 | 0.885 | 0.972 | 0.147 | 54.9 | 12.5 | 240 | 0.944 |
| TACE–Any enhancement | 5.7 | 0.107 | 0.917 | 0.902 | 0.550 | 53.7 | 24.0 | 120 | 0.937 |
| Timing | |||||||||
| < 24 h | 15.5 | 0.332 | 0.750 | 0.948 | 0.146 | 47.9 | 10.5 | 220 | 0.929 |
| 1–14 days | 10.5 | 0.114 | 0.945 | 0.915 | 0.386 | 92.2 | 47.6 | 179 | 0.929 |
| 3–4 weeks | 19.6 | 0.170 | 0.929 | 0.955 | 0.253 | 116 | 60.5 | 222 | 0.963 |
| 1+ month | 13.5 | 0.198 | 0.989 | 0.795 | 0.682 | 71.1 | 20.0 | 253 | 0.958 |
| Study design | |||||||||
| Prospective | 14.8 | 0.168 | 0.916 | 0.947 | 0.293 | 88.3 | 51.0 | 153 | 0.954 |
| Retrospective | 11.1 | 0.147 | 0.926 | 0.928 | 0.410 | 76.3 | 36.9 | 158 | 0.953 |
| Reference standard | |||||||||
| CT/MRI | 14.0 | 0.170 | 0.949 | 0.932 | 0.551 | 82.3 | 51.8 | 131 | 0.956 |
| Histology/angiography | 11.0 | 0.088 | 0.913 | 0.942 | 0.301 | 129 | 27.5 | 604 | 0.948 |
APHE arterial phase hyperenhancement, TACE transarterial chemoembolization, CT computed tomography, MRI magnetic resonance imaging, CI confidence interval, +LR positive likelihood ratio, −LR negative ratio, NPV negative predictive value, PPV positive predictive ratio, Prev prevalence, DOR diagnostic odds ratio, AUC area under the curve
Note: For Timing, sum of study cohorts is not 50, as 2 studies [38, 40] were excluded because they did not report timing or had an interval too wide for analysis
Note: For Therapy + Binary Criteria, sum of study cohorts is not 50, as TACE and ablation were combined for treatment in five studies [35, 36, 51, 58, 60] and there were not detailed case numbers for each treatment
Note: Prevalence is defined by (true-positive cases + false-negative cases)/total cases
Locoregional therapy and criteria for residual tumor
We analyzed the diagnostic accuracy of CEUS in 4 subgroups: (1) TACE + APHE: The overall sensitivity of the 6 included cohorts was 0.93 (95% CI 0.86–0.98), and the overall specificity was 0.80 (95% CI 0.60–0.92), and the DOR was 54.9 (95% CI 12.5–24); (2) TACE + Any enhancement: the overall sensitivity and specificity of the 12 cohorts were 0.91 (95% CI 0.84–0.95) and 0.84 (95% CI 0.73–0.91) respectively, and the DOR was 53.7 (95% CI 24–120); (3) Ablation + APHE: the overall sensitivity and specificity of the 19 cohorts were 0.75 (95% CI 0.64–0.83) and 0.96 (95% CI 0.94–0.97), and the DOR was 63.6 (95% CI 31.7–128); (4) Ablation + Any enhancement: the overall sensitivity and specificity of the 8 cohorts in this group were 0.83 (95% CI 0.71–0.91) and 0.97 (95% CI 0.93–0.99), and the DOR was 163 (95% CI 51.1–517). There were no statistically significant differences between APHE vs. any enhancement within either the TACE or ablation groups (Table 2; Figs. 3, 4; p > 0.05).
Fig. 3.
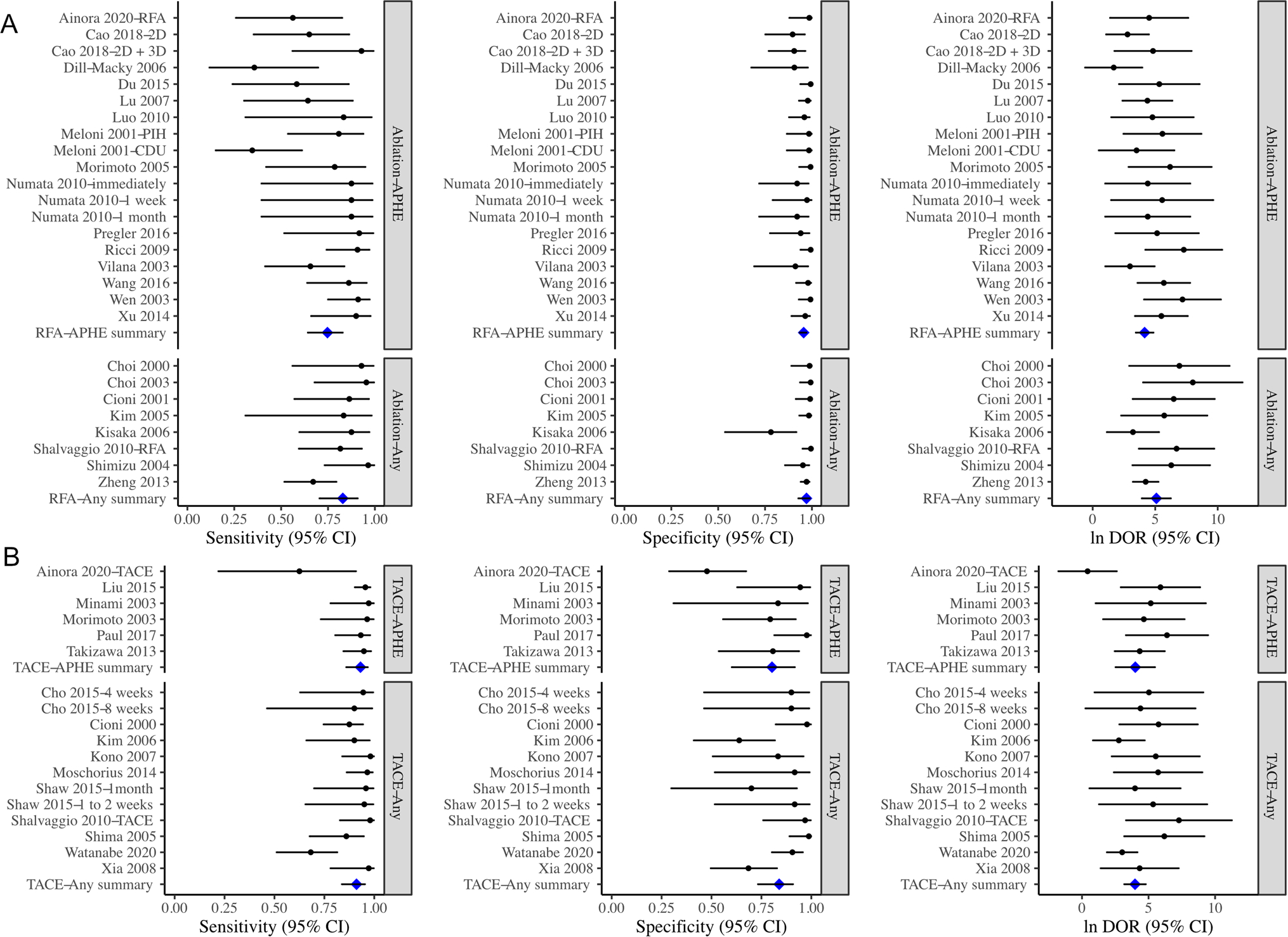
Forest plot showing the sensitivity, specificity, and log (DOR) of CEUS for identifying residual HCC tumors after ablation (A) or TACE (B). The enhancement criteria for identifying residual tumors were APHE only or any other enhancement criteria (including hypo and/or iso-enhancement). Pooled sensitivities, specificities, and log (DOR) were not significantly different between studies using APHE or studies using any other enhancement criteria. APHE arterial phase hyperenhancement, CEUS contrast-enhanced ultrasound, DOR diagnostic odds ratio, TACE transarterial chemoembolization
Fig. 4.
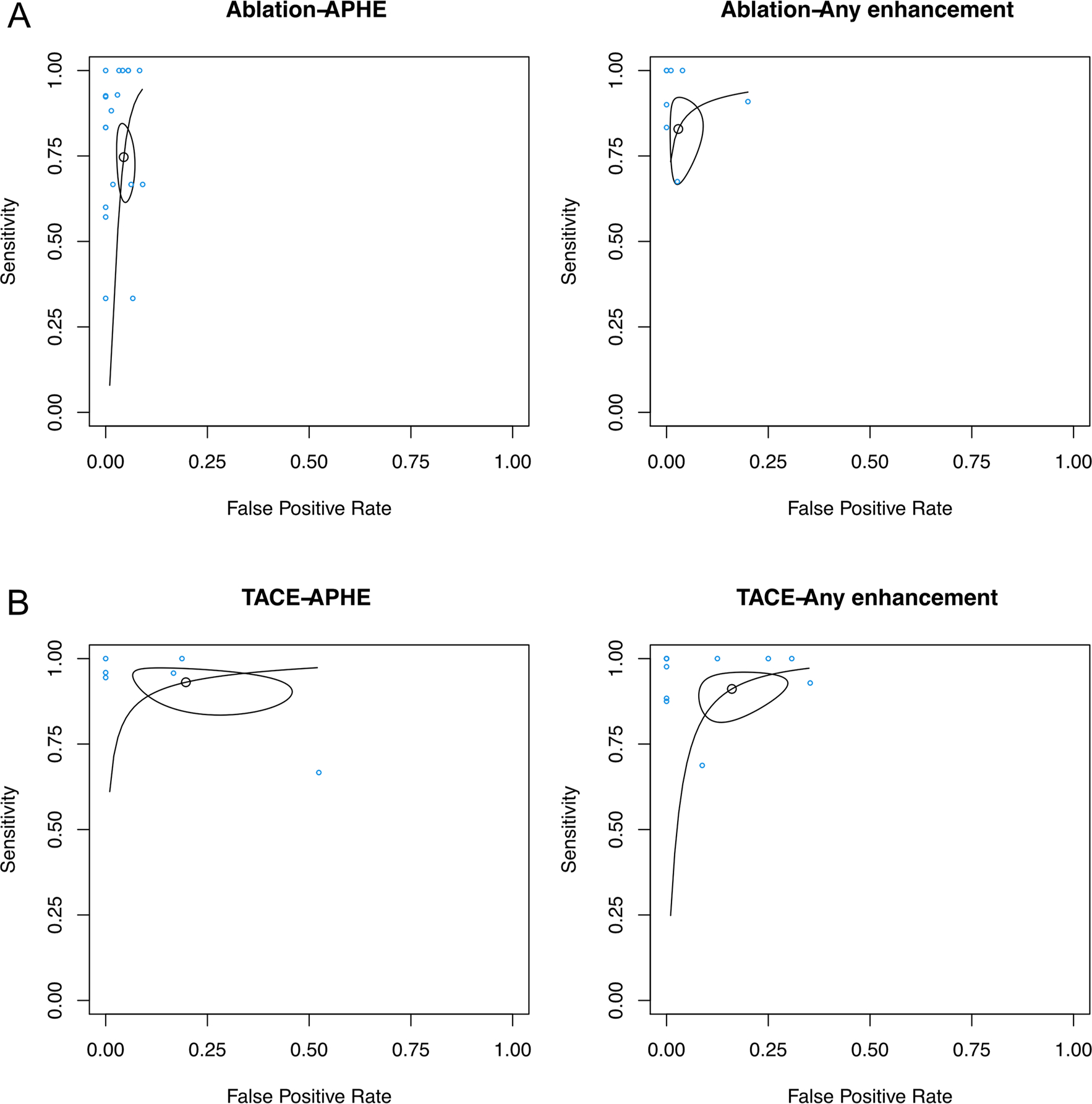
SROC curves of studies analyzed in Fig. 3, as stratified into four groups: A APHE or any other enhancement criteria following ablation, and B APHE or any other enhancement criteria following TACE. The points represent sensitivity and specificity of each individual study. The SROC curve in each subfigure summarizes the performance of each group of study. The size of the regions is correlated with imprecision of the summary estimates sensitivity and specificity. The shape of the regions is correlated with heterogeneity in the sensitivity/specificity parameters. It can be observed that in studies that utilized ablation, there is no significant difference in performance between APHE or any other enhancement criteria. The same can be stated for studies that utilized TACE. TACE studies have lower specificity (i.e., higher false-positive rate) than ablation studies, and marginally higher sensitivity than ablation studies
Timing of post-treatment CEUS
For post-treatment CEUS performed (1) immediately or within 24 h: the sensitivity of the 8 cohorts was 0.68 (95% CI 0.45–0.85), the specificity was 0.96 (95% CI 0.89–0.98), and the DOR was 47.9 (95% CI 10.5–120); (2) 1–14 days: the overall sensitivity and specificity of the 18 cohorts were 0.68 (95% CI 0.45–0.85) and 0.96 (95% CI 0.89–0.98), respectively, and the DOR was 92.2 (95% CI 47.6–179); (3) 3–4 weeks: there were 17 cohorts, and the sensitivity and specificity were 0.84 (95% CI 0.77–0.89) and 0.96 (95% CI 0.93–0.97), and the DOR was 116 (95% CI 60.5–222); and (4) longer than 1 month, the sensitivity and specificity of the 5 cohorts were 0.82 (95% CI 0.55–0.94) and 0.94 (95% CI 0.84–0.98), respectively, and the DOR was 71.1 (95% CI 20.0–253). There were no statistically significant differences between all the groups (Table 2; Figs. 5, 6; p > 0.05).
Fig. 5.
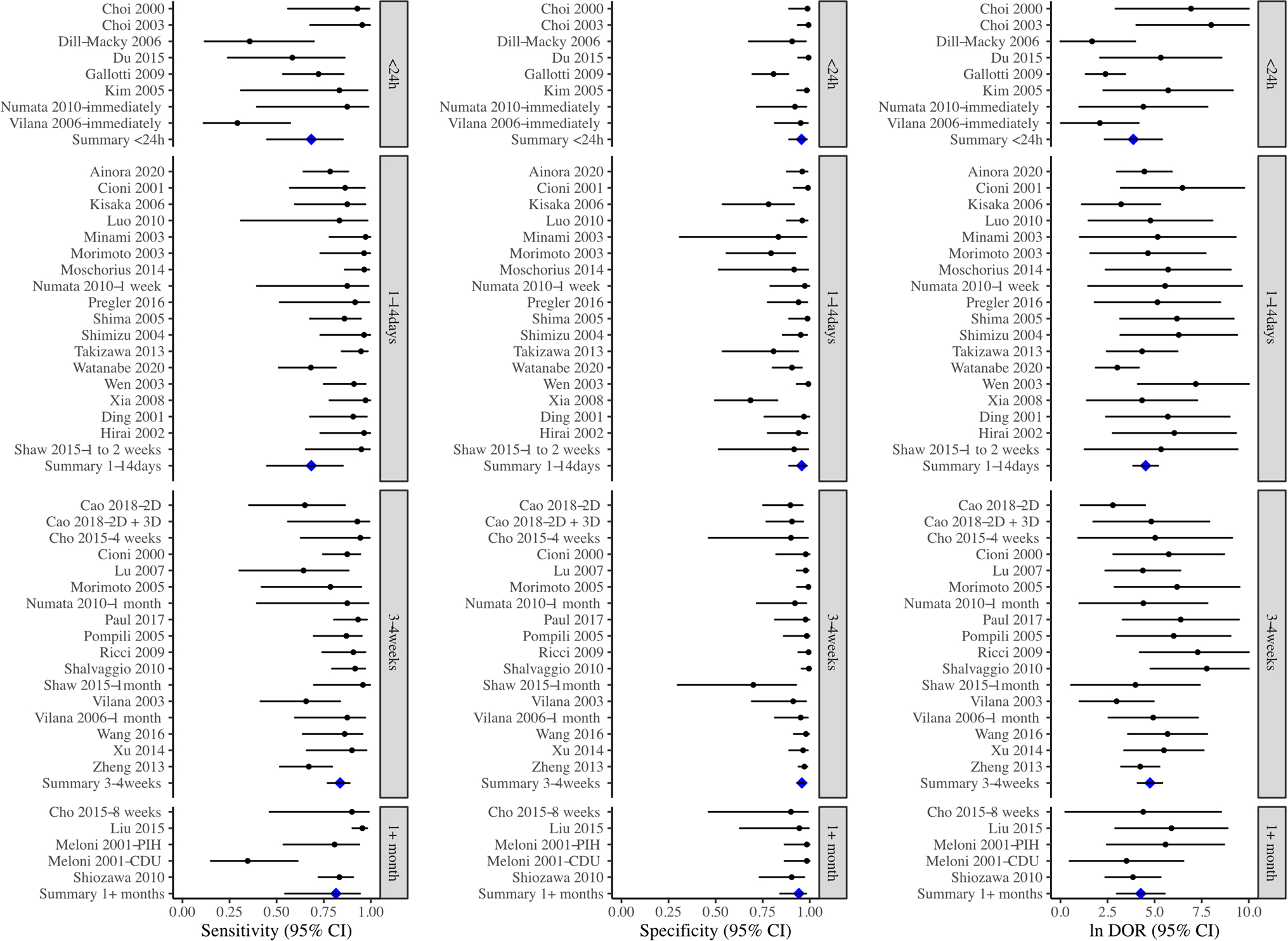
Forest plot showing the sensitivity, specificity, and log (DOR) of CEUS for identifying residual HCC tumors at different timepoints after treatments, stratified into five groups: less than 24 h, 1–14 days, 3–4 weeks, and greater than 1 month
Fig. 6.
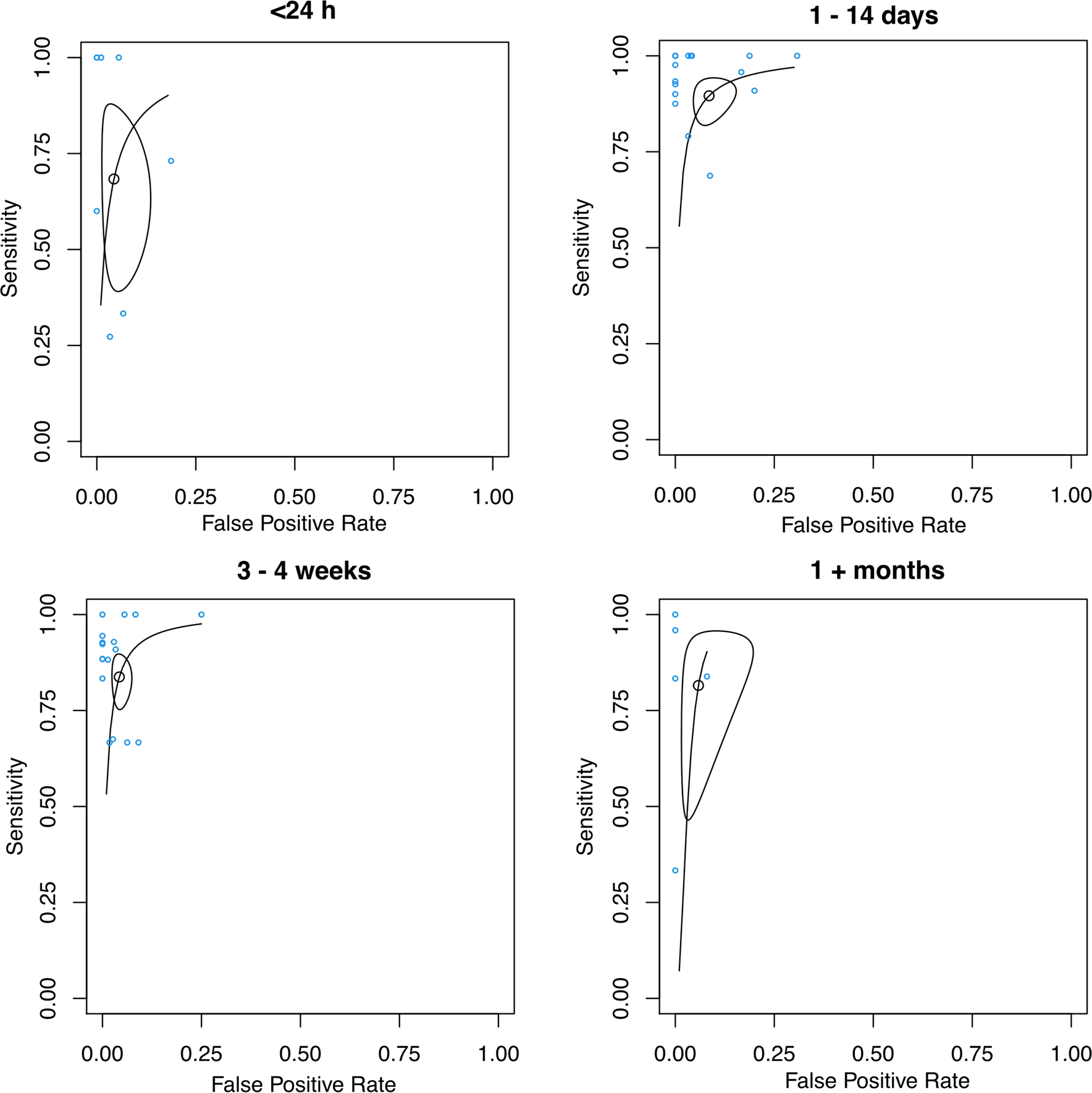
SROC curves of studies analyzed in Fig. 5. The points represent sensitivity and specificity of each individual cohort. The SROC curve in each subfigure summarizes the performance for each group of study. The size of the regions is correlated with imprecision of the summary estimates sensitivity and specificity. The shape of the regions is correlated with heterogeneity in the sensitivity/specificity parameters
Reference standard
Of the 45 cohorts that used CT/MRI as a reference standard, the overall sensitivity and specificity of CEUS were 0.84 (95% CI 0.79–0.88) and 0.94 (95% CI 0.92–0.96) respectively, and the DOR was 82.3 (95% CI 51.8–131); and for the remaining 5 cohorts that used histology/angiography as a reference standard, the overall sensitivity and specificity were 0.92 (95% CI 0.84–0.96) and 0.92 (95% 0.65–0.98), and the DOR was 129 (95% CI 27.5–604). There were no statistically significant differences between the two groups (Table 2; Supplementary Fig. 2; p > 0.05). There were only 2 cohorts that used histology, while 2 cohorts used a mix of histology/angiography, and so in this study histology and angiography were grouped together. Post-ablation MR or CT imaging varied from immediately after [60] to 4 months [44], although nearly all studies also performed the CT or MR imaging at 1 month (Table 1).
Study design
For the 30 prospective studies, the overall sensitivity and specificity of CEUS were 0.841 (95% CI 0.784–0.885) and 0.943 (95% CI 0.916–0.962) respectively, and the DOR was 88.3 (95% CI 51.0–153). And for the 13 retrospective studies, the overall sensitivity and specificity of CEUS were 0.864 (95% CI 0.766–0.926) and 0.923 (95% CI 0.866–0.957), and the DOR was 76.3 (95% CI 36.9–158). There were no statistically significant differences between the two groups (Table 2; Supplementary Fig. 3; p > 0.05).
APHE + washout vs. APHE-alone
Of the 19 ablation cohorts that used APHE as a criterion, these were divided into “APHE + washout” (n = 6) and “APHE-alone” (n = 13). The overall specificity were 95.5% (95% CI 91.5–97.6%) and 96.2% (95% CI 93.1–97.9), respectively, with no statistically significant differences between the two groups (p > 0.05). The overall sensitivity were 88.1% (95% CI 75.9–94.5%) and 69.8% (95% CI 56.7–80.3), respectively. Compared to the APHE + washout group, there was a lower sensitivity in the APHE-alone group (p = 0.035), which is due to outliers with very low sensitivities (Supplementary Fig. 4). After the two outliers from the APHE-alone group were removed, there were no statistically significant differences between the two groups in either sensitivity or specificity (p > 0.05).
Discussion
CEUS has been used for HCC diagnosis and post-treatment follow-up [17], and is actively developed in conjunction with standardization by the ACR CEUS LI-RADS working group [68]. Although many previous studies have been conducted to evaluate the diagnostic performance of CEUS in identifying HCC residual tumor in patients undergoing locoregional therapy, most of them were small single site studies, and a summary of the studies in this field is needed. Smaller prior meta-analyses have been performed in this area, but have not examined specific exam criteria which is needed to optimize implementation. To assess the overall performance of CEUS in post-treatment evaluation, Shi et al. explored the use of CEUS for post-treatment responses after RFA for HCC by a meta-analysis of 12 studies, and they found the overall success rate of CEUS is 91%, with higher success observed in studies using Sonazoid and CEUS performed within 24 h after RFA. However, RFA is only one of the many types of ablation, and is now used infrequently within the USA for the treatment of HCC [69]. Zhong et al. conducted a meta-analysis comparing the diagnostic accuracy between CEUS and CE-CT in assessing HCC residual tumor after TACE, and their results showed CEUS has 0.97 (95% CI 0.95–0.99) and 0.86 (95% CI 0.74–0.94) in sensitivity and specificity versus 0.72 (95% CI 0.67–0.76) and 0.99 (95% CI 0.95–1.00) of CE-CT, respectively. However, since only five studies using angiography/histology as a reference standard were included, the sample size was relatively low. Thus, we conducted this systematic review and meta-analysis to include all types of locoregional therapies and reference standards to quantitatively evaluate the diagnostic performance of CEUS in identifying residual tumor after treatment.
Importantly, our results showed there was no significant difference between using ‘any enhancement’ versus ‘APHE’ as a criterion for diagnosing residual tumor after TACE or ablation, which suggests that recurrent lesions after therapy may have different biological and imaging properties compared to the initial HCC tumor. In support of this, preclinical research found that incomplete RFA promotes angiogenesis, invasiveness, and metastasis of residual HCC via molecular pathways such as HIF-1alpha/VEGFA upregulation and beta-catenin signaling activation [70, 71]. Recently, another study found that recurrent HCC after RFA tends to have an irregular border, a more homogeneous enhancement, fewer inner necrotic areas, and less feeding vessels [72].
Although there is no widely accepted guideline for surveillance after locoregional therapy, post-treatment imaging should be performed at regularly scheduled intervals (usually 1 month after, and then 3–6-month intervals thereafter) to evaluate treatment response and to detect residual or new lesions [73, 74]. In most of studies included, post-treatment CEUS have been performed in 1–7 days (15 cohorts) and 3–4 weeks (17 cohorts) after locoregional therapy. This focus on follow-up time is important as residual disease may be retreated earlier than the current 1-month guidelines needed for cross sectional imaging. Some groups have also used CEUS multiple times during the ablative procedure to assess the immediate response and if residual tumor is present, re-ablation can be conducted earlier [75]. We performed subgroup analyses based on different timing of post-treatment CEUS, demonstrating that although there was no statistically significant difference among all the groups, CEUS performed within the first 24 h after locoregional therapy had a relatively lower sensitivity (68.4% compared to > 80% for all other subgroups). Evidence showed that when CEUS is performed immediately or within the first 24 h after RFA, hyperemia develops around the ablated area could hinder the diagnostic accuracy [25]. The reactive hyperemic rim caused by post-ablation effect can lead to false-positive cases by overdiagnosing the irregular borders as well as false-negative cases by failure to distinguish this area from a true residual tumor [76]. Besides, the intralesional air pockets formation and iatrogenic arterio-portal shunting after thermal ablation could also affect the imaging evaluation for residual tumors [77].
While noting that TACE and ablation are very different in nature, our results demonstrated that the performance of CEUS in detecting residual HCC post-locoregional therapy differs between studies which performed TACE compared to ablation. Sensitivity was higher in TACE (p < 0.0006), and specificity was higher in ablation (p < 0.0001). There are many reasons that may underlie this observation, such as rim hyperenhancement after ablation that may obscure residual tumors, or that image-guided ablation is usually performed on lesions easily seen on ultrasound compared to TACE-treated which tend to be higher in the hepatic dome.
We conducted another subgroup analysis in ablation studies, comparing APHE-only (13 cohorts) to APHE + washout (6 cohorts). Our expectation was that the additional of washout may result in a more stringent criteria, resulting in a CEUS test with higher specificity and potentially lower sensitivity. However, we did not detect meaningful differences in our analysis. The preliminary analysis highlights the need for head-to-head comparisons of APHE with or without washout in CEUS.
There are several limitations of this study. Firstly, there were high heterogeneity among studies, and subgroup analyses were not able to fully explore the sources of heterogeneities due to the limited number of studies in each subgroup. Secondly, the criteria of CEUS in diagnosing residual tumor after locoregional therapy varies across studies, especially for criteria other than APHE. As none of the studies used iso-enhancement as a diagnostic criterion for recurrent HCC, the question of whether it is more likely to see iso-enhancement of recurrent HCC lesions via CEUS than the de novo HCC, remains unresolved. And, out of the 25 cohorts that stated APHE as a criterion, only 7 (1 TACE and 6 ablation cohorts) of these simultaneously applied both APHE and delayed/portal phase washout in their criteria, and thus the effect of including washout also remains unresolved. Finally, the use of transarterial radioembolization continues to grow as a locoregional therapy for HCC [78]. Reports on the use of CEUS to monitor and predict response to transarterial radioembolization are promising but relatively sparse at this point [10, 79]. It is expected that the role of CEUS to monitor radioembolization will continue to grow and its diagnostic performance should be further examined in the future.
Conclusions
CEUS is a highly effective method to identify HCC residual tumor after locoregional therapy with an overall sensitivity of 85%, specificity of 94%, and AUROC of 0.95. This performance does not appear to be limited by follow up times > 24 h (up to 1 month), study design (prospective or retrospective) or enhancement criteria used to define residual disease (APHE-alone, APHE with washout, or any enhancement).
Supplementary Material
Acknowledgements
The authors want to sincerely thank members of the ACR CEUS LI-RADS working group Drs. Stephanie R. Wilson, Yuko Kono, and David Fetzer for their help with this manuscript.
Funding
This work was supported in part by NIH R01s CA215520, CA238241, and CA194307.
Conflict of interest
Yang Hai, Esika Savsani, and Weelic Chong have no relevant financial or non-financial interests to disclose. John Eisenbrey: GE Healthcare: grants and equipment support. Lantheus Medical Imaging: drug support and speaker fees. Siemens: equipment support. Book Royalties: Elsevier. Andrej Lyshchik: Research support: GE Healthcare, Bracco Diagnostics, Siemens Healthineers, Canon Medical Systems. Advisory Board: Bracco Diagnostics. Consulting: GE Healthcare, Bioclinica, WorldCare Clinical. Speaker Panel: GE Healthcare. Book Royalties: Elsevier.
Footnotes
Ethical approval and informed consent This is a meta-analysis of already published data and no ethical approval or incorment consent is required for this study.
Code availability Code is available upon reasonable request.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00261-021-03248-9.
Data availability
Code is available upon reasonable request.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021. [DOI] [PubMed] [Google Scholar]
- 2.Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A: Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin 2017, 67(4):273–289. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J: Hepatocellular carcinoma. Lancet 2012, 379(9822):1245–1255. [DOI] [PubMed] [Google Scholar]
- 4.Kinoshita A, Onoda H, Fushiya N, Koike K, Nishino H, Tajiri H: Staging systems for hepatocellular carcinoma: Current status and future perspectives. World J Hepatol 2015, 7(3):406–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Burroughs A, Bruix J: Hepatocellular carcinoma. Lancet 2003, 362(9399):1907–1917. [DOI] [PubMed] [Google Scholar]
- 6.Inchingolo R, Posa A, Mariappan M, Spiliopoulos S: Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J Gastroenterol 2019, 25(32):4614–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, Nalesnik M, Geller DA, Steel JL, Gamblin TC: Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol 2008, 15(11):3169–3177. [DOI] [PubMed] [Google Scholar]
- 8.Yim HJ, Suh SJ, Um SH: Current management of hepatocellular carcinoma: an Eastern perspective. World J Gastroenterol 2015, 21(13):3826–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong ZV, Tanabe KK: The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer 2014, 120(18):2824–2838. [DOI] [PubMed] [Google Scholar]
- 10.Eisenbrey JR, Forsberg F, Wessner CE, Delaney LJ, Bradigan K, Gummadi S, Tantawi M, Lyshchik A, O’Kane P, Liu JB et al. : US-triggered Microbubble Destruction for Augmenting Hepatocellular Carcinoma Response to Transarterial Radioembolization: A Randomized Pilot Clinical Trial. Radiology 2021, 298(2):450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piscaglia F, Ogasawara S: Patient Selection for Transarterial Chemoembolization in Hepatocellular Carcinoma: Importance of Benefit/Risk Assessment. Liver Cancer 2018, 7(1):104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei JY, Wang WT, Yan LN: Up-to-seven criteria for hepatocellular carcinoma liver transplantation: a single center analysis. World J Gastroenterol 2013, 19(36):6077–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR: Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J Gastroenterol 2019, 25(31):4360–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW: Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology 2005, 234(3):954–960. [DOI] [PubMed] [Google Scholar]
- 15.Yan FH, Zhou KR, Cheng JM, Wang JH, Yan ZP, Da RR, Fan J, Ji Y: Role and limitation of FMPSPGR dynamic contrast scanning in the follow-up of patients with hepatocellular carcinoma treated by TACE. World J Gastroenterol 2002, 8(4):658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DB, Nikolic B, Covey AM, Nutting CW, Saad WE, Salem R, Sofocleous CT, Sze DY: Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2012, 23(3):287––294.. [DOI] [PubMed] [Google Scholar]
- 17.Eisenbrey JR, Gabriel H, Savsani E, Lyshchik A: Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom Radiol (NY) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gummadi S, Eisenbrey JR, Lyshchik A: Contrast-enhanced ultrasonography in interventional oncology. Abdom Radiol (NY) 2018, 43(11):3166–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal S, Gui J, Merrill C, Wong JK, Burak KW, Wilson SR: Contrast-enhanced US in Local Ablative Therapy and Secondary Surveillance for Hepatocellular Carcinoma. Radiographics 2019, 39(5):1302–1322. [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Kim H, Han H, Lee M, Lee S, Yoo H, Chang JH, Kim H: Microbubbles used for contrast enhanced ultrasound and theragnosis: a review of principles to applications. Biomed Eng Lett 2017, 7(2):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appis AW, Tracy MJ, Feinstein SB: Update on the safety and efficacy of commercial ultrasound contrast agents in cardiac applications. Echo Res Pract 2015, 2(2):R55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung YE, Kim KW: Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography 2015, 34(1):3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wink MH, Wijkstra H, De La Rosette JJ, Grimbergen CA: Ultrasound imaging and contrast agents: a safe alternative to MRI? Minim Invasive Ther Allied Technol 2006, 15(2):93–100. [DOI] [PubMed] [Google Scholar]
- 24.Ranganath PG, Robbin ML, Back SJ, Grant EG, Fetzer DT: Practical advantages of contrast-enhanced ultrasound in abdominopelvic radiology. Abdom Radiol (NY) 2018, 43(4):998–1012. [DOI] [PubMed] [Google Scholar]
- 25.Ainora ME, Iezzi R, Ponziani FR, Garcovich M, Di Stasio E, Riccardi L, Annicchiarico BE, Abbate V, De Gaetano AM, Siciliano M et al. : Contrast-Enhanced Ultrasound in the Short-Term Evaluation of Hepatocellular Carcinoma after Locoregional Treatment. Dig Dis 2020, 38(6):522–533. [DOI] [PubMed] [Google Scholar]
- 26.Cao J, Dong Y, Mao F, Wang W: Dynamic Three-Dimensional Contrast-Enhanced Ultrasound to Predict Therapeutic Response of Radiofrequency Ablation in Hepatocellular Carcinoma: Preliminary Findings. Biomed Res Int 2018, 2018:6469703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YZ, Park SY, Choi EH, Baik SK, Kwon SO, Kim YJ, Cha SH, Kim MY: The usefulness of contrast-enhanced ultrasonography in the early detection of hepatocellular carcinoma viability after transarterial chemoembolization: pilot study. Clin Mol Hepatol 2015, 21(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi D, Lim HK, Kim SH, Lee WJ, Jang HJ, Lee JY, Paik SW, Koh KC, Lee JH: Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: usefulness of power Doppler US with a microbubble contrast agent in evaluating therapeutic response-preliminary results. Radiology 2000, 217(2):558–563. [DOI] [PubMed] [Google Scholar]
- 29.Choi D, Lim HK, Lee WJ, Kim SH, Kim YH, Kim SH, Lim JH: Early assessment of the therapeutic response to radio frequency ablation for hepatocellular carcinoma: utility of gray scale harmonic ultrasonography with a microbubble contrast agent. J Ultrasound Med 2003, 22(11):1163–1172. [DOI] [PubMed] [Google Scholar]
- 30.Cioni D, Lencioni R, Bartolozzi C: Therapeutic effect of transcatheter arterial chemoembolization on hepatocellular carcinoma: evaluation with contrast-enhanced harmonic power Doppler ultrasound. Eur Radiol 2000, 10(10):1570–1575. [DOI] [PubMed] [Google Scholar]
- 31.Cioni D, Lencioni R, Rossi S, Garbagnati F, Donati F, Crocetti L, Bartolozzi C: Radiofrequency thermal ablation of hepatocellular carcinoma: using contrast-enhanced harmonic power Doppler sonography to assess treatment outcome. AJR Am J Roentgenol 2001, 177(4):783–788. [DOI] [PubMed] [Google Scholar]
- 32.Dill-Macky MJ, Asch M, Burns P, Wilson S: Radiofrequency ablation of hepatocellular carcinoma: predicting success using contrast-enhanced sonography. AJR Am J Roentgenol 2006, 186(5 Suppl):S287–295. [DOI] [PubMed] [Google Scholar]
- 33.Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, Kawasaki T, Maekawa K: Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology 2001, 221(3):721–730. [DOI] [PubMed] [Google Scholar]
- 34.Du J, Li HL, Zhai B, Chang S, Li FH: Radiofrequency ablation for hepatocellular carcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in guiding and assessing early therapeutic response and short-term follow-up results. Ultrasound Med Biol 2015, 41(9):2400–2411. [DOI] [PubMed] [Google Scholar]
- 35.Gallotti A, D’Onofrio M, Ruzzenente A, Martone E, De Robertis R, Guglielmi A, Pozzi Mucelli R: Contrast-enhanced ultrasonography (CEUS) immediately after percutaneous ablation of hepatocellular carcinoma. Radiol Med 2009, 114(7):1094–1105. [DOI] [PubMed] [Google Scholar]
- 36.Hirai T, Ohishi H, Tokuno E, Takahashi M, Sakaguchi H, Anai H, Nishimoto Y, Hirohashi S, Kichikawa K: Qualitative diagnosis of hepatocellular carcinoma by contrast enhanced ultrasonography using Coded Harmonic Angio with Levovist. J Med Ultrason (2001) 2002, 29(1):3–9. [DOI] [PubMed] [Google Scholar]
- 37.Kim CK, Choi D, Lim HK, Kim SH, Lee WJ, Kim MJ, Lee JY, Jeon YH, Lee J, Lee SJ et al. : Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol 2005, 56(1):66–73. [DOI] [PubMed] [Google Scholar]
- 38.Kim HJ, Kim TK, Kim PN, Kim AY, Ko EY, Kim KW, Sung KB, Ha HK, Kim HC, Lee MG: Assessment of the therapeutic response of hepatocellular carcinoma treated with transcatheter arterial chemoembolization: comparison of contrast-enhanced sonography and 3-phase computed tomography. J Ultrasound Med 2006, 25(4):477–486. [DOI] [PubMed] [Google Scholar]
- 39.Kisaka Y, Hirooka M, Kumagi T, Uehara T, Hiasa Y, Kumano S, Tanaka H, Michitaka K, Horiike N, Mochizuki T et al. : Usefulness of contrast-enhanced ultrasonography with abdominal virtual ultrasonography in assessing therapeutic response in hepatocellular carcinoma treated with radiofrequency ablation. Liver Int 2006, 26(10):1241–1247. [DOI] [PubMed] [Google Scholar]
- 40.Kono Y, Lucidarme O, Choi SH, Rose SC, Hassanein TI, Alpert E, Mattrey RF: Contrast-enhanced ultrasound as a predictor of treatment efficacy within 2 weeks after transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol 2007, 18(1 Pt 1):57–65. [DOI] [PubMed] [Google Scholar]
- 41.Liu M, Lin MX, Lu MD, Xu ZF, Zheng KG, Wang W, Kuang M, Zhuang WQ, Xie XY: Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluating the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur Radiol 2015, 25(8):2502–2511. [DOI] [PubMed] [Google Scholar]
- 42.Lu MD, Yu XL, Li AH, Jiang TA, Chen MH, Zhao BZ, Zhou XD, Wang JR: Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007, 33(11):1736–1749. [DOI] [PubMed] [Google Scholar]
- 43.Luo W, Numata K, Morimoto M, Oshima T, Ueda M, Okada M, Takebayashi S, Zhou X, Tanaka K: Role of Sonazoid-enhanced three-dimensional ultrasonography in the evaluation of percutaneous radiofrequency ablation of hepatocellular carcinoma. Eur J Radiol 2010, 75(1):91–97. [DOI] [PubMed] [Google Scholar]
- 44.Meloni MF, Goldberg SN, Livraghi T, Calliada F, Ricci P, Rossi M, Pallavicini D, Campani R: Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. AJR Am J Roentgenol 2001, 177(2):375–380. [DOI] [PubMed] [Google Scholar]
- 45.Minami Y, Kudo M, Kawasaki T, Kitano M, Chung H, Maekawa K, Shiozaki H: Transcatheter arterial chemoembolization of hepatocellular carcinoma: usefulness of coded phase-inversion harmonic sonography. AJR Am J Roentgenol 2003, 180(3):703–708. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto M, Nozawa A, Numata K, Shirato K, Sugimori K, Kokawa A, Tomita N, Saitou T, Nakatani Y, Imada T et al. : Evaluation using contrast-enhanced harmonic gray scale sonography after radio frequency ablation of small hepatocellular carcinoma: sonographic–histopathologic correlation. J Ultrasound Med 2005, 24(3):273–283. [DOI] [PubMed] [Google Scholar]
- 47.Morimoto M, Shirato K, Sugimori K, Kokawa A, Tomita N, Saito T, Imada T, Tanaka N, Nozawa A, Numata K et al. : Contrast-enhanced harmonic gray-scale sonographic-histologic correlation of the therapeutic effects of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol 2003, 181(1):65–69. [DOI] [PubMed] [Google Scholar]
- 48.Moschouris H, Malagari K, Papadaki MG, Kornezos I, Stamatiou K, Anagnostopoulos A, Chatzimichael K, Kelekis N: mRECIST criteria and contrast-enhanced US for the assessment of the response of hepatocellular carcinoma to transarterial chemoembolization. Diagn Interv Radiol 2014, 20(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Numata K, Fukuda H, Ohto M, Itou R, Nozaki A, Kondou M, Morimoto M, Karasawa E, Tanaka K: Evaluation of the therapeutic efficacy of high-intensity focused ultrasound ablation of hepatocellular carcinoma by three-dimensional sonography with a perflubutane-based contrast agent. Eur J Radiol 2010, 75(2):e67–75. [DOI] [PubMed] [Google Scholar]
- 50.Paul SB, Dhamija E, Gamanagatti SR, Sreenivas V, Yadav DP, Jain S, Shalimar, Acharya SK: Evaluation of tumor response to intra-arterial chemoembolization of hepatocellular carcinoma: Comparison of contrast-enhanced ultrasound with multiphase computed tomography. Diagn Interv Imaging 2017, 98(3):253–260. [DOI] [PubMed] [Google Scholar]
- 51.Pompili M, Riccardi L, Covino M, Barbaro B, Di Stasi C, Orefice R, Gasbarrini G, Rapaccini GL: Contrast-enhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int 2005, 25(5):954–961. [DOI] [PubMed] [Google Scholar]
- 52.Pregler B, Beyer LP, Wiesinger I, Nießen C, Jung EM, Stroszczynski C, Wiggermann P: Microwave ablation of large HCC lesions: Added value of CEUS examinations for ablation success control. Clin Hemorheol Microcirc 2016, 64(3):483–490. [DOI] [PubMed] [Google Scholar]
- 53.Ricci P, Cantisani V, Drudi F, Pagliara E, Bezzi M, Meloni F, Calliada F, Erturk SM, D’Andrea V, D’Ambrosio U et al. : Is contrast-enhanced US alternative to spiral CT in the assessment of treatment outcome of radiofrequency ablation in hepatocellular carcinoma? Ultraschall Med 2009, 30(3):252–258. [DOI] [PubMed] [Google Scholar]
- 54.Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G: Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging 2010, 35(4):447–453. [DOI] [PubMed] [Google Scholar]
- 55.Shaw CM, Eisenbrey JR, Lyshchik A, O’Kane PL, Merton DA, Machado P, Pino L, Brown DB, Forsberg F: Contrast-enhanced ultrasound evaluation of residual blood flow to hepatocellular carcinoma after treatment with transarterial chemoembolization using drug-eluting beads: a prospective study. J Ultrasound Med 2015, 34(5):859–867. [DOI] [PubMed] [Google Scholar]
- 56.Shima T, Mizuno M, Otsuji H, Mizuno C, Obata H, Park H, Nakajo S, Okanoue T: Evaluation of transcatheter arterial embolization therapy on hepatocellular carcinomas using contrast-enhanced harmonic power Doppler sonography: Comparison with CT, power Doppler sonography, and dynamic MRI. Journal of Medical Ultrasonics 2005, 32(3):107–113. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu M, Iijima H, Horibe T, Yamada M, Suzuki S, Yanagisawa K, Seki T, Moriyasu F: Usefulness of contrast-enhanced ultrasonography with a new contrast mode, Agent Detection Imaging, in evaluating therapeutic response in hepatocellular carcinoma treated with radio-frequency ablation therapy. Hepatol Res 2004, 29(4):235–242. [DOI] [PubMed] [Google Scholar]
- 58.Shiozawa K, Watanabe M, Takayama R, Takahashi M, Wakui N, Iida K, Sumino Y: Evaluation of local recurrence after treatment for hepatocellular carcinoma by contrast-enhanced ultrasonography using Sonazoid: comparison with dynamic computed tomography. J Clin Ultrasound 2010, 38(4):182–189. [DOI] [PubMed] [Google Scholar]
- 59.Takizawa K, Numata K, Morimoto M, Kondo M, Nozaki A, Moriya S, Ishii T, Oshima T, Fukuda H, Okada M et al. : Use of contrast-enhanced ultrasonography with a perflubutane-based contrast agent performed one day after transarterial chemoembolization for the early assessment of residual viable hepatocellular carcinoma. Eur J Radiol 2013, 82(9):1471–1480. [DOI] [PubMed] [Google Scholar]
- 60.Vilana R, Bianchi L, Varela M, Nicolau C, Sánchez M, Ayuso C, García M, Sala M, Llovet JM, Bruix J et al. : Is microbubble-enhanced ultrasonography sufficient for assessment of response to percutaneous treatment in patients with early hepatocellular carcinoma? Eur Radiol 2006, 16(11):2454–2462. [DOI] [PubMed] [Google Scholar]
- 61.Vilana R, Llovet JM, Bianchi L, Sanchez M, Pagés M, Sala M, Gilabert R, Nicolau C, Garcia A, Ayuso C et al. : Contrast-enhanced power Doppler sonography and helical computed tomography for assessment of vascularity of small hepatocellular carcinomas before and after percutaneous ablation. J Clin Ultrasound 2003, 31(3):119–128. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Jing X, Ding J: Clinical value of dynamic 3-dimensional contrast-enhanced ultrasound imaging for the assessment of hepatocellular carcinoma ablation. Clin Imaging 2016, 40(3):402–406. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe Y, Ogawa M, Kumagawa M, Hirayama M, Miura T, Matsumoto N, Nakagawara H, Yamamoto T, Moriyama M: Utility of Contrast-Enhanced Ultrasound for Early Therapeutic Evaluation of Hepatocellular Carcinoma After Transcatheter Arterial Chemoembolization. J Ultrasound Med 2020, 39(3):431–440. [DOI] [PubMed] [Google Scholar]
- 64.Wen YL, Kudo M, Zheng RQ, Minami Y, Chung H, Suetomi Y, Onda H, Kitano M, Kawasaki T, Maekawa K: Radiofrequency ablation of hepatocellular carcinoma: therapeutic response using contrast-enhanced coded phase-inversion harmonic sonography. AJR Am J Roentgenol 2003, 181(1):57–63. [DOI] [PubMed] [Google Scholar]
- 65.Xia Y, Kudo M, Minami Y, Hatanaka K, Ueshima K, Chung H, Hagiwara S, Inoue T, Ishikawa E, Kitai S et al. : Response evaluation of transcatheter arterial chemoembolization in hepatocellular carcinomas: the usefulness of sonazoid-enhanced harmonic sonography. Oncology 2008, 75 Suppl 1:99–105. [DOI] [PubMed] [Google Scholar]
- 66.Xu X, Luo L, Chen J, Wang J, Zhou H, Li M, Jin Z, Chen N, Miao H, Lin M et al. : Acoustic radiation force impulse elastography for efficacy evaluation after hepatocellular carcinoma radiofrequency ablation: a comparative study with contrast-enhanced ultrasound. Biomed Res Int 2014, 2014:901642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng SG, Xu HX, Lu MD, Xie XY, Xu ZF, Liu GJ, Liu LN: Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol 2013, 19(6):855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson SR, Lyshchik A, Piscaglia F, Cosgrove D, Jang HJ, Sirlin C, Dietrich CF, Kim TK, Willmann JK, Kono Y: CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018, 43(1):127–142. [DOI] [PubMed] [Google Scholar]
- 69.Tombesi P, Vece FD, Sartori S: Radiofrequency, microwave, and laser ablation of liver tumors: time to move toward a tailored ablation technique? Hepatoma Research 2015, 1:52–57. [Google Scholar]
- 70.Kong J, Kong J, Pan B, Ke S, Dong S, Li X, Zhou A, Zheng L, Sun W-b: Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS ONE 2012, 7(5):e37266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang N, Wang L, Chai Z-T, Zhu Z-M, Zhu X-D, Ma D-N, Zhang Q-B, Zhao Y-M, Wang M, Ao J-Y: Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating β-catenin signaling. PLoS ONE 2014, 9(12):e115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu JY, Bai XM, Wang H, Xu Q, Wang S, Wu W, Yan K, Yang W: The Perfusion Features of Recurrent Hepatocellular Carcinoma After Radiofrequency Ablation Using Contrast-Enhanced Ultrasound and Pathological Stemness Evaluation: Compared to Initial Tumors. Front Oncol 2020, 10:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sainani NI, Gervais DA, Mueller PR, Arellano RS: Imaging After Percutaneous Radiofrequency Ablation of Hepatic Tumors: Part 1, Normal Findings. American Journal of Roentgenology 2013, 200(1):184–193. [DOI] [PubMed] [Google Scholar]
- 74.Ayuso C, Rimola J, García-Criado A: Imaging of HCC. Abdom Imaging 2012, 37(2):215–230. [DOI] [PubMed] [Google Scholar]
- 75.Lackey L 2nd, Peterson C, Barr RG: Contrast-enhanced ultrasound-guided radiofrequency ablation of renal tumors. Ultrasound Q 2012, 28(4):269–274. [DOI] [PubMed] [Google Scholar]
- 76.Meloni MF, Andreano A, Franza E, Passamonti M, Lazzaroni S: Contrast enhanced ultrasound: should it play a role in immediate evaluation of liver tumors following thermal ablation? European Journal of Radiology 2012, 81(8):e897–e902. [DOI] [PubMed] [Google Scholar]
- 77.Kim SK, Lim HK, Kim YH, Lee WJ, Lee SJ, Kim SH, Lim JH, Kim SA: Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics 2003, 23(1):107–121. [DOI] [PubMed] [Google Scholar]
- 78.Lemieux S, Buies A, A FT, Hallet J, Daigle G, Côté F, Provencher S: Effect of Yttrium-90 transarterial radioembolization in patients with non-surgical hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2021, 16(3):e0247958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delaney LJ, Tantawi M, Wessner CE, Machado P, Forsberg F, Lyshchik A, O’Kane P, Liu JB, Civan J, Tan A, Anton K, Shaw CM, Eisenbrey JR. Predicting Long-Term Hepatocellular Carcinoma Response to Transarterial Radioembolization Using Contrast-Enhanced Ultrasound: Initial Experiences. Ultrasound Med Biol 2021, 47(9):2523–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code is available upon reasonable request.


