Abstract
Background
Clinical scores for sepsis have been primarily developed for, and applied in High-Income Countries. This systematic review and meta-analysis examined the performance of the quick Sequential Organ Failure Assessment (qSOFA), Systemic Inflammatory Response Syndrome (SIRS), Modified Early Warning Score (MEWS), and Universal Vital Assessment (UVA) scores for diagnosis and prediction of mortality in patients with suspected infection in Low-and-Middle-Income Countries.
Methods
PubMed, Science Direct, Web of Science, and the Cochrane Central Register of Controlled Trials databases were searched until May 18, 2021. Studies reporting the performance of at least one of the above-mentioned scores for predicting mortality in patients of 15 years of age and older with suspected infection or sepsis were eligible. The Quality Assessment of Diagnostic Accuracy Studies tool was used for risk-of-bias assessment. PRISMA guidelines were followed (PROSPERO registration: CRD42020153906). The bivariate random-effects regression model was used to pool the individual sensitivities, specificities and areas-under-the-curve (AUC).
Findings
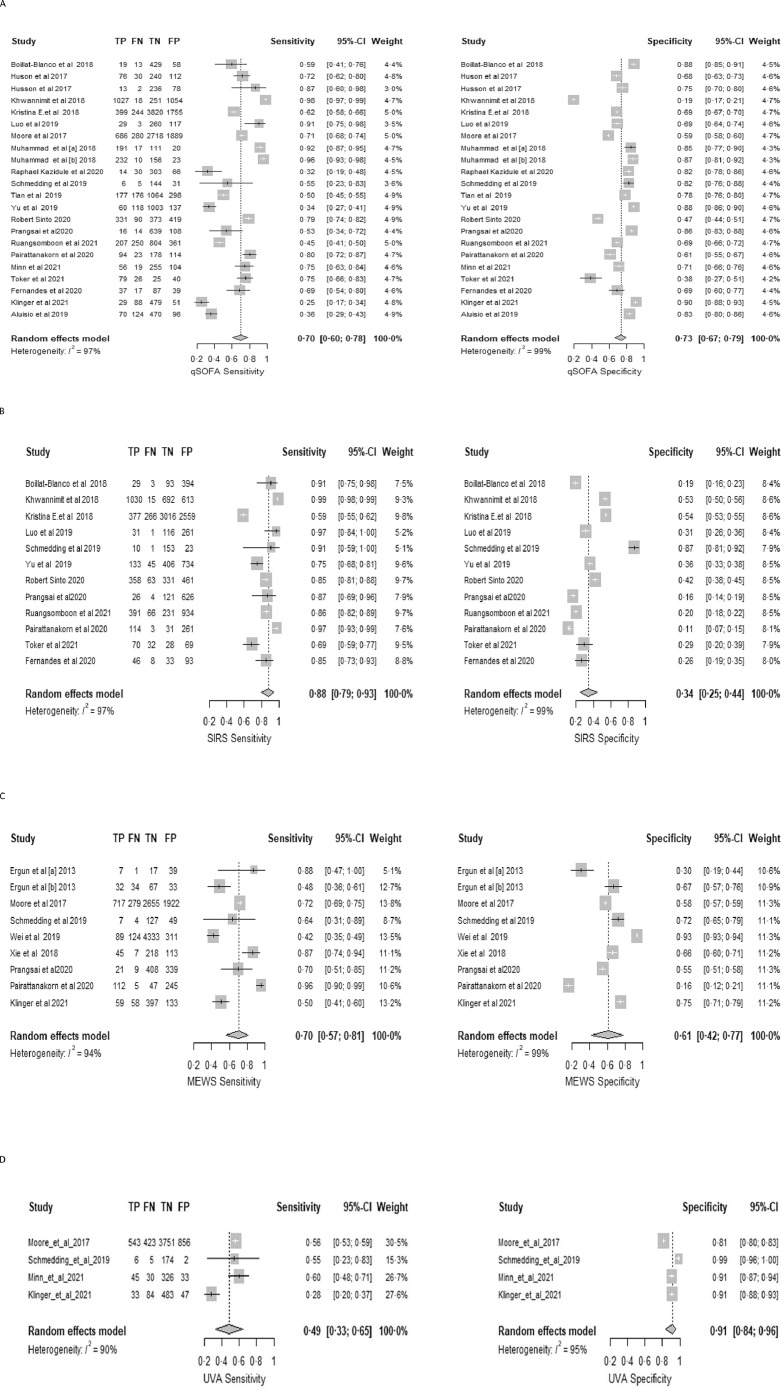
Twenty-four articles (of 5669 identified) with 27,237 patients were eligible for inclusion. qSOFA pooled sensitivity was 0·70 (95% confidence interval [CI] 0·60–0·78), specificity 0·73 (95% CI 0·67–0·79), and AUC 0·77 (95% CI 0·72–0·82). SIRS pooled sensitivity, specificity and AUC were 0·88 (95% CI 0·79 -0·93), 0·34 (95% CI 0·25–0·44), and 0·69 (95% CI 0·50–0·83), respectively. MEWS pooled sensitivity, specificity and AUC were 0·70 (95% CI 0·57 -0·81), 0·61 (95% CI 0·42–0·77), and 0·72 (95% CI 0·64–0·77), respectively. UVA pooled sensitivity, specificity and AUC were 0·49 (95% CI 0·33 -0·65), 0·91(95% CI 0·84–0·96), and 0·76 (95% CI 0·44–0·93), respectively. Significant heterogeneity was observed in the pooled analysis.
Interpretation
Individual score performances ranged from poor to acceptable. Future studies should combine selected or modified elements of different scores.
Funding
Partially funded by the UK National Institute for Health Research (NIHR) (17/63/42).
Key words: low-and-middle-income countries (LMICs), sepsis, severity scores, qSOFA, SIRS, MEWS, UVA
Research in context.
Evidence before this study
There was no earlier systematic review reporting the performance comparison of the four scores, and previous systematic reviews and meta-analyses included predominantly studies from high income countries (HICs). Variation in clinical presentation, aetiology of sepsis and limited access to intensive care unit between HICs and low-and-middle-income countries (LMICs) settings may contribute to differences between the performance of the scores.
Added value of this study
The performance of four available scores, Sequential Organ Failure Assessment (qSOFA), Systemic Inflammatory Response Syndrome (SIRS), Modified Early Warning score (MEWS), and Universal Vital Assessment (UVA) in the diagnosis of sepsis and in-patient mortality prediction in patients with suspected infection in LMICs was systematically reviewed for the first time. Currently used scores yield variable performance, ranging from poor to acceptable, in predicting mortality or sepsis diagnosis when applied to adult patients hospitalised with infectious diseases in LMIC settings. The sensitivity of qSOFA might be higher in LMICs than that reported from HICs. However, further validation studies are needed.
Implications of all the available evidence
Our analysis suggests a two-step approach to better predict worst outcome in patients with suspected infection in LMICs. The SIRS score has best sensitivity; it could assist clinicians in making the first triage, followed by the qSOFA, MEWS, or UVA scores to inform decisions about the appropriate level of care.
Alt-text: Unlabelled box
1. Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection; it is responsible for twenty percent of all-cause global mortality, the majority of which occurs in low-and-middle-income countries (LMICs) [1,2].
Sepsis is a syndrome that can manifest in affected patients as a broad constellation of symptoms and signs caused by the interplay of pathogens and host factors. To address the challenge of sepsis diagnosis, multiple sepsis diagnostic and mortality prediction models or scores have been developed.
The availability of sophisticated laboratory investigation tools and early warning scores are important instruments to improve diagnosis and management of sepsis in high-income countries (HICs), but applying those to LMIC settings is complex. The first early warning score published in 1997 was designed to enable detection of changes in illness severity using aberrations in vital signs. In 2001, the Modified Early Warning Score (MEWS) was published. It was created by assigning weighted scores to five physiological parameters (systolic blood pressure, pulse rate, respiratory rate, temperature and level of consciousness) based on severity of the abnormality [3]. Though not specific for sepsis, MEWS is intended to support medical staff in anticipating patients’ clinical deterioration.
Sepsis definitions have been modified significantly between 1991 and 2016 [4]. The current definition describes sepsis as organ dysfunction caused by dysregulated host response to infection. For clinical operationalisation, organ dysfunction is represented by an increase in the Sequential (sepsis-related) Organ Failure Assessment (SOFA) score of two points or more [5]. The preceding sepsis screening tool utilised the systemic inflammatory response syndrome (SIRS) criteria but was suggested to be abandoned by the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) task force due to concerns about its lack of specificity in identifying patients with severe disease resulting in potential over-treatment of patients with milder disease [5]. SOFA, however, requires laboratory values which may not be readily available outside of a highly resourced intensive care unit (ICU). Accordingly, the quick SOFA (qSOFA) score was proposed as a screening tool to identify patients at high risk of poor outcome [5].
MEWS, SIRS and qSOFA were mainly developed in HICs. LMICs share a high burden of many infectious diseases, for which sepsis is the common final pathway. Studies validating these scores in LMICs are limited, with performances differing from one study to another [[6], [7], [8], [9]]. Recently, the Universal Vital Assessment (UVA) score was developed, using multiple cohorts of patients from sub-Saharan African countries with suspected infection, demonstrating good performance in predicting in-hospital mortality [10]. The UVA score included points for systolic blood pressure, Glasgow Coma Scale score, temperature, oxygen saturation, respiratory and heart rates, and humon immunodeficiency virus (HIV) serostatus [10]. Its performance has been assessed by now in several studies in Africa [7,11]. In LMICs, there are limited healthcare resources and ICU facilities. As a consequence, severe and critically-ill patients cannot always be admitted to an ICU. Therefore, an applicable triage score that is easily applied by frontline clinicians is paramount in order to prioritise care. Previous systematic reviews assessed the performance of the qSOFA, SIRS, and MEWS scores, but did not focus on LMICs [12]. Compared with SIRS, qSOFA showed better specificity for predicting mortality but lower sensitivity for identifying patients with sepsis in patients with suspected infection [6,13]. It is well-known that setting and study population might influence the accuracy of screening and diagnostic testing [14]. This systematic review and meta-analysis assessed the performance of four available scores (qSOFA, SIRS, MEWS, UVA) in the diagnosis of sepsis and in-patient mortality prediction in adult non-pregnant and non-surgical patients with suspected infections in LMICs.
2. Methods
2.1. Search strategy and selection criteria
This systematic review and meta-analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement extension for Diagnostic Test Accuracy (DTA) [15]. An electronic search of the published literature was conducted on November 04, 2019 and updated on May 18, 2021 in PubMed, Science Direct, Web of Science, and the Cochrane Central Register of Controlled Trials. We used the following search terms: ‘qsofa’, ’sofa’, ‘sirs,’ ‘UVA’, ’MEWS’, ‘sequential organ failure assessment’, ‘systemic inflammatory response syndrome’, ‘Universal Vital Assessment’, ’Early Warning Score’, ‘sepsis’, ‘infections’. In addition, we used a filter suggested by the Cochrane Collaboration based on the World Bank list of low-income, lower-middle-income or upper-middle-income countries [16]. Articles resulting from these searches and relevant references cited in those articles were reviewed. The full search strategy used is reported in Supplementary File S1.
Studies which recruited patients 15 years of age and older with suspected infection or sepsis were eligible for inclusion using the following criteria: (1) full-length reports published in peer-reviewed journals; (2) observational studies or clinical trials of adult (>15 years old) patients; (3) studies that describe data about sepsis assessment using at least one of the four scores; and (4) studies that report the relationship between the sepsis screening criteria and at least one of the following outcomes: sensitivity or specificity for diagnosis of sepsis (organ dysfunction, SOFA ≥2), deaths that occurred in hospital, or any post-hospital discharge outcomes. According to the published definitions, qSOFA was considered positive when at least two variables met fulfilment criteria’; SIRS when at least two criteria were met; MEWS when at least five score criteria were met; and UVA when at least five score criteria were met. The details of each score are provided in Supplementary File S2. We excluded studies which were not performed in LMICs, studies which did not report sensitivity, specificity, or data to calculate the score performance characteristics, and studies limited to specific patient populations (such as COVID-19 or pneumonia patients). Two investigators (WNN & LBD) independently screened studies for eligibility; disagreement was resolved by consensus. If WNN and LBD did not agree after discussion, a third investigator (BRA) was consulted. The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020153906; available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020153906) and amended once.
2.2. Data extraction and quality assessment
The following data were extracted from the original studies: first author; year of publication; country of origin; study design; sample size; mortality rate; patient selection criteria; score evaluated, objectives and outcomes. In case of missing information, we contacted the respective corresponding authors. BRA and JRE independently extracted potentially relevant studies and reviewed each study according to the pre-defined eligibility criteria. The primary outcome was overall mortality (in-hospital or 28/30 days mortality). The secondary outcome was diagnosis of sepsis (acute organ dysfunction).
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool was used to assess the risk of bias in diagnostic test accuracy [17], as recommended by the Cochrane Collaboration. Consensus on the risk of bias was sought by two reviewers (BRA and WNN). A detailed quality assessment is provided in Supplementary File S3; articles rejected are listed in Supplementary File 4.
2.3. Data analysis
Statistical analysis was conducted using RevMan5.4 (Nordic Cochrane Center, Copenhagen, Denmark) [18] and RStudio 4.0.2 (250 Northern Ave, Boston, USA) [19]. We generated true positives, false negatives, false positives, and true negatives based on sensitivity, specificity, and 95% confidence intervals (CIs) of each study using RevMan5.4. We used the packages ‘meta’ [20] and ‘mada’ (version 0.5.10) [21] in CRAN-R to produce the meta-analysis forest and funnel plots. Between studies, statistical heterogeneity was assessed using the I2 statistic and Cochran's Q test; I2 values of more than 50% indicated a significant level of heterogeneity. Funnel plots were used to assess publication bias (Supplementary File S5). Pooled sensitivity and specificity were calculated using a bivariate random-effects regression model. The summary receiver operator curves were constructed, and the area under the curve (AUC) was used to appreciate the discriminatory performance of each score.
2.4. Ethics information
No ethical clearance was required for this systematic review and meta-analysis.
2.5. Role of the funding source
The supporting funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author, Martin P. Grobusch accessed the dataset, and the decision to submit for publication was jointly taken by all authors.
3. Results
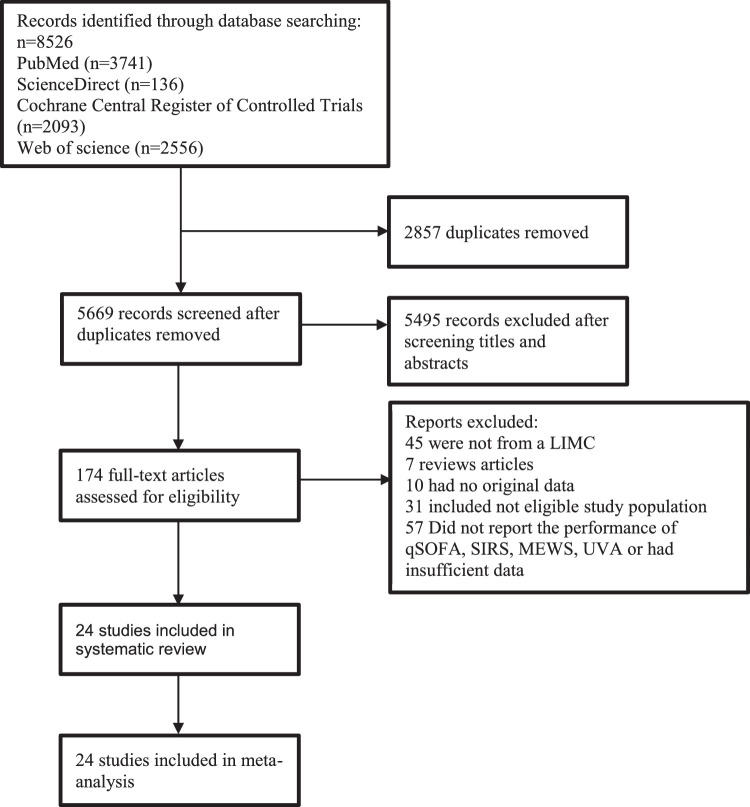
In total, 8526 published articles were initially identified (3741 articles from PUBMED, 136 articles from Science Direct, 2093 articles from the Cochrane Library, and 2556 on Web of Science). After removing duplicate articles, 5669 potentially eligible articles were screened. Of these articles, 5495 were excluded on the basis of title and abstract. A total of 174 articles underwent full-text review. One hundred fifty-five articles were excluded for the reasons presented in Figure 1. Finally, a total of 24 articles met our inclusion criteria for the systematic review and meta-analysis.
Figure 1.
Diagram of the study selection process.
All studies were published between 2013 and 2021. Characteristics of the studies included are presented in Table 1. The number of patients per study ranged from 64 to 6218, and the overall mortality rate in each study ranged from 3·8 % to 61·0%. Across studies, the two most-frequently reported conditions were respiratory tract infections and malaria.
Table 1.
Characteristics of studies included
| Author and year of publication [Reference] | Countries | Type of study | Department | Mean (SD)or Median age (Interquartile range) years | Mortality proportion (Number of death /Total number of patients, %) | Objectives of studies | Most-frequent infection n (%) | Score evaluated |
|---|---|---|---|---|---|---|---|---|
| Schmedding et al (2019)[30] | Gabon | Prospective | Emergency department | 38 (28–53) | 11/187(6) | To evaluated the ability of the qSOFA score to predict mortality in patients presenting to the emergency department, and compared the performance of qSOFA with the SIRS criteria, MEWS, and UVA scores. |
Malaria 97 (51) | qSOFA, SIRS, UVA, MEWS |
| Boillat-Blanco et al (2018)[31] | Tanzania | Prospective | Emergency department | 30 (23–40) | 32/519(6) | To evaluate the prognostic accuracy of qSOFA for 28-day all-cause mortality in febrile adult patients treated at emergency departments and to compare it with SOFA and SIRS. | Respiratory tract infection 223 (43) | qSOFA, SIRS |
| Raphael_Kazidule et al (2020)[32] | Malawi | Prospective | General wards | 40 (18–98) | 44/413(10) | To evaluate the predictive value of a qSOFA score of 2 for mortality among hospitalised adults and among those with suspected infection. | Not reported | qSOFA |
| Luo et al (2019)[33] | China | Prospective | General wards | 55(40-67) | 32/409(7.8) | To evaluate the ability to diagnostic sepsis and predict 28-day mortality | Respiratory tract infection 234 (57) | qSOFA, SIRS |
| Yu et al (2019)[27] | China | Retrospective | Emergency department | 62 (47–74) | 178/1318(13.5) | To determine the ability of qSOFA to predict in hospital mortality in a multicenter cohort of patients who presented with clinical symptoms of systemic infection. | Respiratory tract infection 712 (54) | qSOFA, SIRS |
| Tian et al (2019)[34] | China | Retrospective | General wards | 79(61–85) | 353/1716(21) | 1-To evaluate the accuracy of qSOFA for the diagnosis of sepsis-3 2-To evaluate the performance of qSOFA as one predictor of outcome in patients with suspicion of infection | Respiratory tract infection 1248 (73) | qSOFA |
| Wei et al (2019)[35] | China | Retrospective | Emergency department | 44.5(18.3) | 213/4857(4.4) | To evaluate the performance of MEWS in predicting the outcomes of adult patients presenting to the emergency department (ED) | Respiratory tract infection 1059 (22) | MEWS |
| Xie Xiaohua et al (2018)[36] | China | Prospective | Emergency department | 59.6(18.3) | 52/383(13.6) | To validate the performance of MEWS in a Chinese emergency department and to determine the best cut-off value for in-hospital mortality prediction | Respiratory tract infection 54 (14) | MEWS |
| Rudd et al (2018)[37] | Bangladesh, Haiti,India, Indonesia, Myanmar, Rwanda, Sierra Leone, Sri Lanka, Thailand, and Vietnam | Retrospective | General wards | 38(36-55) | 643/6218(10) | To assess the association of qSOFA with excess hospital death among patients with suspected infection in LMICs and to compare qSOFA with the systemic inflammatory response syndrome (SIRS) criteria | Malaria 1461 (24) | qSOFA, SIRS |
| Huson et al (2017)[38] | Malawi | Prospective | General wards | 35(26-47) | 106/458(23) | To determine the predictive value of qSOFA in Malawian patients with suspected infection | Not reported | qSOFA |
| Moore et al (2017)[29] | Gabon, Malawi, Sierra Leone, Tanzania, Uganda and Zambia | Retrospective | General wards | 36(27-49) | 966/5573(18) | To determine predictors of mortality UVA score and compare the performance of the UVA score in predicting mortality with that of MEWS and qSOFA. | Not reported | UVA, qSOFA,MEWS |
| Muhammad et al [a] (2018) [39] | Pakistan | Prospective | Intense care unit | 60.2(17.9) | 208/339(61) | To determine a comparison between the qSOFA score and SOFA when applied to septic shock patients in the Emergency Department for prediction of in-hospital mortality in the setting of a tertiary care hospital ED in a low-middle income country. | Respiratory tract infection 211 (62) | qSOFA |
| Muhammad et al [b] (2018) [39] | Pakistan | Prospective | Intense care unit | 59.6(17.2) | 242/421(57.5) | To determine a comparison between the qSOFA score and SOFA when applied to severe sepsis patients in the Emergency Department for prediction of in-hospital mortality in the setting of a tertiary care hospital ED in a low-middle income country. | Respiratory tract infection 187 (44) | qSOFA |
| Ergun et al [a](2013)[40] | Turkey | Prospective | Emergency department | Not reported | 8/64(12.5) | To determine the ability of the mMEDS score, the MEWS score and the CCI to predict prognosis in patients presenting to the ED of our hospital who are diagnosed with sepsis |
Not reported | MEWS |
| Ergun et al [b](2013)[40] | Turkey | Prospective | Emergency department | Not reported | 66/166(39·8) | To determine the ability of the mMEDS score, the MEWS score and the CCI to predict prognosis in patients presenting to the ED of our hospital who are diagnosed with sepsis |
Not reported | MEWS |
| Khwannimit et al (2018)[41] | Thailand | Retrospective | Intense care unit | 62(44-75) | 1045/2350(44·5) | To compare the SOFA score and qSOFA to SIRS criteria ability in predictive of in hospital mortality and organ failure | Respiratory tract infection 1174 (50) | qSOFA, SIRS |
| Huson et al (2016)[38] | Gabon | Retrospective | All wards | 34 (24-46) | 15/329(4·56) | To determine the predictive value of qSOFA in patients with suspected infection in a hospital with limited supportive care facilities, in Gabon. | Malaria 122 (37) | qSOFA |
| Sinto R, et al(2020)[42] | Indonesia | Prospective | Emergency department | 51 (38-60) | 454/1213(37·4) | To investigate the prognostic accuracy of the qSOFA and lactate criteria (defined as two or more qSOFA criteria, and venous lactate concentration higher than the defined cut-off) in an emergency department of a hospital with limited resources, in comparison with established prognosis criteria and screening criteria | Respiratory tract infection 808 (66·6) | qSOFA, SIRS |
| Prangsai et al(2020)[43] | Thailand | Retrospective | Emergency department | 67 (53–79) | 30/777(3.8) | To evaluate the accuracy of early warning scores (NEWS, MEWS, MEDS and SOS) and compare them with qSOFA and SIRS in detecting sepsis and predicting hospital admission and mortality in patients with suspected infection presenting at EDs | Primary bacteraemia 235 (30) | qSOFA, SIRS MEWS |
| Ruangsomboon et al (2021)[9] | Thailand | retrospectively | Emergency department | 72.6 (15.4) | 457/1622(28.18) | To validate and compare the clinical utility of REMS, SIRS, qSOFA, and NEWS in predicting in-hospital mortality and mortality within 7 days of admission in ED patients with suspected sepsis | Respiratory tract infection 982 (61) | qSOFA, SIRS |
| Pairattanakorn et al (2020)[44] | Thailand | prospective | all wards | 65.74 (17.84) | 117/409 (28.6) | To determine the diagnostic performance of SIRS score, qSOFA score, SOFA score, MEWS, and NEWS for sepsis detection and mortality prediction in adult patients suspected of having sepsis at Siriraj Hospital, Mahidol University, Bangkok, Tailand | Respiratory tract infection 138 (33·7) | Qsofa, SIRS MEWS |
| Minn et al (2021)[45] | Myanmar | prospective | General wards | 48 (29-64) | 75/434(17.28) | To determine the ability of several commonly used disease severity scores to predict the clinical course of patients with evidence of community-acquired sepsis in resource-limited tropical settings like Myanmar | Not reported | qSOFA UVA |
| Toker et al (2021)[46] | Turkey | prospective | Emergency department | 72.5(13.7) | 191/365(52.32) | To investigate the predictive capacity of the SOFA score, SIRS, qSOFA, and qSOFA + lactate criteria (qSOFA+L) criteria in the diagnosis and prognosis of sepsis |
Not reported | qSOFA, SIRS |
| Fernandes et al (2020)[47] | India | prospective | Emergency department | 47.5 (18.1) | 54/180(30) | To assess the prognostic accuracy of qSOFA score in predicting adverse outcomes in patients with suspected infections and to compare it with the SIRS (Systemic Inflammatory Response Syndrome) and the SOFA (Sequential Organ failure Assessment Score) | Respiratory tract infection 56 (31) | qSOFA, SIRS |
Twenty-three studies reported qSOFA (26,460 participants) score performance; twelve (15,401 participants) reported SIRS performance, nine reported MEWS (13,063 participants), and four reported UVA (6841 participants). Six studies compared the accuracy of qSOFA and SIRS, five studies compared qSOFA and MEWS, and three studies compared qSOFA and UVA criteria. One study compared all four scores. Two studies reported the performance of qSOFA in the diagnosis of sepsis. All studies included were observational. More than half were prospective. The studies were well designed, the quality assessment demonstrated a low risk of bias. The detailed QUADAS-2 assessment is presented in Supplementary File S3.
For mortatlity, the pooled sensitivity of qSOFA across all included studies was 0·70 (95% CI 0·60–0·78); the pooled specificity was 0·73 (95% CI 0·67–0·79) (Figure 2A); and the pooled AUC was 0·77 (95% CI 0·72–0·82). SIRS pooled sensitivity and specificity for predicting mortality were 0·88 (95% CI 0·79 -0·93) and 0·34 (95% CI 0·25–0·44) (Figure 2B), respectively; the pooled AUC was 0·69 (95% CI 0·50–0·83). MEWS pooled sensitivity and specificity were 0·70 (95% CI 0·57 -0·81) and 0·61 (95% CI 0·42–0·77) (Fig. 2C), respectively; the pooled AUC was 0·72 (95% CI 0·64–0·77). UVA sensitivity and specificity were 0·49 (95% CI 0·33 -0·65) and 0·91 (95% CI 0·84–0·96) (Figure 2 D), respectively; the pooled AUC 0·76 (95% CI 0·44–0·93). In the subgroup analysis assessing the performance of qSOFA in ICU vs outside of ICU, the sensitivity and the AUC of qSOFA in predicting mortality was better in studies assessing its performance in ICU compared with non-ICU areas (sensitivity 0·96 [95% 0·90; 0·98] vs 0·61 [95% CI 0·52; 0·68]); AUC: 0·95 [95% CI 0·90- 0·97] vs 0·72 [95 % 0·68- 0·75] respectively). The specificity did not differ considerably (0·67 [95% CI 0·13; 0·87] vs 0·74 [95% CI 0·69; 0·79]). Due to a limited number of studies assessing the other scores, a subgroup analysis was not performed.
Figure 2.
Forest plots for mortality by A qSOFA; B SIRS; C MEWS and D UVA scores.
In those studies simultaneously reporting the accuracy of a positive qSOFA score and positive SIRS criteria for predicting mortality, the qSOFA score was more specific but less sensitive than SIRS; qSOFA, MEWS, and UVA performed similarly (Table 2).
Table 2.
Pooled performance characteristics comparison of qSOFA and SIRS criteria for predicting mortality in patients with suspected infection
| Scores | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
|---|---|---|---|
| qSOFA vs SIRS | |||
| qSOFA | 0·72 (0·58-0·82) | 0·67(0·55-0·79) | 0·74(0·68–0·78) |
| SIRS | 0·88(0·79- 0·93) | 0·34(0·25- 0·44) | 0·56(0·40-0·76) |
| qSOFA vs MEWS | |||
| qSOFA | 0·58(0·35-0·78) | 0·78(0·62-0·88) | 0·73(0·63-0·79) |
| MEWS | 0·74(0·58-0·86) | 0·55(0·35-0·74) | 0·69(0·65-0·74) |
| qSOFA vs UVA | |||
| qSOFA | 0·50 (0·17; 0·82) | 0·79(0·51; 0·94) | 0·69(0·53 -0·78) |
| UVA | 0·45(0·24; 0·68) | 0·92(0·82; 0·96) | 0·77(0·47 -0·87) |
Three studies that reported the prognostic performance of positive qSOFA scores in predicting acute organ dysfunction; the pooled sensitivity, specificity, and AUC were 0·43 (95% CI 0·30 -0·57), 0·85 (95% CI 0·78 -0·90), and 0·76 (95% CI 0·5 -0·86), respectively. The pooled performance of SIRS in the diagnosis of acute organ dysfunction was as follows: sensitivity 0·87 (95% CI 0·58 -0·97); specificity 0·30 (95% CI 0·11 -0·59); and AUC 0·62 (95% CI 0·35 -0·85).
4. Discussion
In this systematic review and meta-analysis, the prognostic capability of qSOFA, SIRS, MEWS, and UVA for predicting mortality and organ dysfunction in adult patients with suspected infection or sepsis in LMICs was evaluated. The performance of qSOFA outside of ICU in our systematic review is lower to what was reported in the original study assessing the performance of qSOFA (0·72 (95% CI 0·68-0·75) vs 81(95% CI 0·80–0·82)) [22]. It is also lower than the performance (0·78 (95% CI, 0·72–0·84)) reported in 2018 in a systematic review including both HICs and LMICs [23]. The sensitivity of qSOFA in this systematic review, however, is higher than that determined by the systematic review which included studies from HICs only (0·76; 95% CI 0·59–0·88 vs 0·58; 95% CI 0·47–0·67) [24]. The difference in the performance of qSOFA in HICs as compared to LMICs could be due to patient characteristics and differences in the respective infectious disease burden, as well as variation in healthcare resources, and the degree to which definitive diagnostics are available (such as CT or MRI scans, bronchoscopy etc.). Furthermore, the mechanisms that lead to life-threatening acute organ dysfunction from infections such as malaria, tuberculosis, and HIV which are more prevalent in LMICs can differ from those of classic bacterial sepsis [25,26]. Some studies suggest the combination of qSOFA with biomarkers, such as C-reative protein and procalcitonin, to increase its sensitivity [27]. The Sepsis 3.0 task force designed qSOFA criteria to replace SIRS to identify patients with suspected infection who would require early diagnosis and treatment. However, our meta-analysis demonstrates that qSOFA had a poor sensitivity for predicting mortality as compared with SIRS. To that end, SIRS should not be abandoned as it could provide utility in a staged approach with qSOFA, whereby SIRS is used as a primary screening tool to identify patients requiring a high level of care, and qSOFA is applied subsequently for predicting mortality; an approach which to the best of our knowledge has not yet been investigated systematically.
The comparison of MEWS and qSOFA performance (AUC) in predicting mortality showed that they performed similarly (0·73 (95% CI 0·63-0·79) vs 0·69 (95% CI 0·65-0·74)). However, the sensitivity of MEWS is higher than that of qSOFA. When a high sensitivity trigger is used, it is more likely that the patient will be identified sooner. However, if a ‘sepsis bundle’ is administered to patients who ultimately do not have sepsis, there is a risk of over-treatment, and there are substantial concerns about excessive fluid administration and antibiotic use [28] In LMIC settings, patients with HIV have an increased risk for developing sepsis [25]. In our meta-analysis, the UVA score had the highest specificity (0·92, 95% CI 0·82; 0·96). The UVA was reported as an appropriate score to assess in-hospital mortality risk in adults, and derived exclusively from data from six sub-Saharan African countries [29]; further prospective validation would be helpful.
Pathogen spectrum and clinical presentation of sepsis may be different between LMICs and high-income settings. Due to the lack of human resources and ICU facilities in LMICs, there is a need to develop reliable triage scores to determine who requires the highest available level of care. Our meta-analysis demonstrates that there is no single top-performing score. Future studies should investigate the performance of amalgamated (i.e. combining the best of different scores) and combined scores (staged using sensitivity score, followed by the more specific) in various countries.
Our review has several limitations. First, there was considerable heterogeneity between the studies included. Second, the definition of suspected infection varied among studies; and due to the retrospective design of many studies, these differences would have introduced selection bias. Third, we were unable to directly compare the four scores because there was one study simultaneously reporting the performance of these scores.
In conclusion, there is not a single score which ultimately identifies, with accuracy, patients with suspected infections or sepsis at high risk of death or clinical condition deterioration. Amongst the scores readily at hand, SIRS could be applied to first screening to identify those patients requiring high-level care, followed by qSOFA, MEWS, or UVA scores, based both on published performance indicators and subject to local availability of data collection tools, for mortality prediction. There is a need to perform further studies to validate the UVA score. In general, future studies should investigate the performance of combined or sequential use of scores, or their amalgamation, i.e. optimalisation by combining selected or modified elements of different scores. This altogether could help to further improve patient triage in resource-limited environments and serve as a standard for mortality risk in future studies.
Contributors
MPG and BRA conceptualised the study. BRA, MPG and AAA determined the methodological approach. BRA,JRE, WNN and LBN primarily investigated the data and conducted the formal data analysis. MPG and AAA provided and organised study resources. BRA, MPG, GMN curated the data. BRA wrote the original draft, with input from MPG. BRA, TH, JR, STJ, and MPG, together with all co-authors, did the editing and the writing of the final manuscript version. MPG supervised the project. MPG and AAA administered the project. All authors contributed to the writing of the final manuscript version, and have read and agreed to the published version of the manuscript.
Data sharing statement
Data will be made available upon request made to the corresponding author. The analysis code used in this study is available online.
Declaration of Competing Interest
None of the author has any competing interests to declare.
Funding
This research was partially funded by the National Institute for Health Research (NIHR) (17/63/42) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK government.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101184.
Appendix. Supplementary materials
References
- 1.World Health Organization . WHO; Geneva: 2020. Sepsis.https://www.who.int/news-room/fact-sheets/detail/sepsis (accessed Aug 6. [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludikhuize Goossens A, Borgert J, Binnekade M, Dongelmans D, Subbe C. Standardized measurement of the Modified Early Warning Score results in enhanced implementation of a Rapid Response System: a quasi-experimental study. Resuscitation. 2014;85:676–682. doi: 10.1016/j.resuscitation.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J, Yang J, Mei J, Jin Y, Lu Y. Head-to-head comparison of qSOFA and SIRS criteria in predicting the mortality of infected patients in the emergency department: a meta-analysis. Scand J Trauma Resusc Emerg Med. 2018;26:56. doi: 10.1186/s13049-018-0527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmedding M, Adegbite BR, Gould S, et al. A Prospective Comparison of Quick Sequential Organ Failure Assessment, Systemic Inflammatory Response Syndrome Criteria, Universal Vital Assessment, and Modified Early Warning Score to Predict Mortality in Patients with Suspected Infection in Gabon. Am J Trop Med Hyg. 2019;100:202–208. doi: 10.4269/ajtmh.18-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aluisio AR, Garbern S, Wiskel T, et al. Mortality outcomes based on ED qSOFA score and HIV status in a developing low income country. Am J Emerg Med. 2018;36:2010–2019. doi: 10.1016/j.ajem.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruangsomboon O, Boonmee P, Limsuwat C, Chakorn T, Monsomboon A. The utility of the rapid emergency medicine score (REMS) compared with SIRS, qSOFA and NEWS for Predicting in-hospital Mortality among Patients with suspicion of Sepsis in an emergency department. BMC Emerg Med. 2021;21:2. doi: 10.1186/s12873-020-00396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore CC, Hazard R, Saulters KJ, et al. Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub-Saharan Africa. BMJ Glob Health. 2017;2:0344. doi: 10.1136/bmjgh-2017-000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinger A, Mueller A, Sutherland T, et al. Predicting mortality in adults with suspected infection in a Rwandan hospital: an evaluation of the adapted MEWS, qSOFA and UVA scores. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephen AH, Montoya RL, Aluisio AR. Sepsis and Septic Shock in Low- and Middle-Income Countries. Surg Infect. 2020;21:571–578. doi: 10.1089/sur.2020.047. [DOI] [PubMed] [Google Scholar]
- 13.Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome Criteria for the Diagnosis of Sepsis and Prediction of Mortality. Chest. 2018;153:646–655. doi: 10.1016/j.chest.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Leeflang MMG, Rutjes AWS, Reitsma JB, Hooft L, Bossuyt PMM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ Can Med Assoc J. 2013;185:E537–E544. doi: 10.1503/cmaj.121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 16.The Cochrane Collaboration. Filters for PubMed (NLM), MEDLINE (Ovid), Embase (Ovid), and CENTRAL (Cochrane Library) to help identify studies relevant to LMIC. /lmic-filters (accessed March 28, 2021).
- 17.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 18.The Cochrane Collaboration Review Manager (RevMan) 2020 [Google Scholar]
- 19.Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R:A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 20.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health. 2019 doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philipp Doebler (2020). mada: Meta-Analysis of Diagnostic Accuracy. R package version 0.5.10. https://CRAN.R-project.org/package=mada. .
- 22.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J-U, Sin CK, Park HK, Shim SR, Lee J. Performance of the quick Sequential (sepsis-related) Organ Failure Assessment score as a prognostic tool in infected patients outside the intensive care unit: a systematic review and meta-analysis. Crit Care. 2018;22:28. doi: 10.1186/s13054-018-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y-C, Luo Y-Y, Zhang X, et al. Quick Sequential Organ Failure Assessment as a prognostic factor for infected patients outside the intensive care unit: a systematic review and meta-analysis. Intern Emerg Med. 2019;14:603–615. doi: 10.1007/s11739-019-02036-0. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JM, Feasey NA, Rylance J. Aetiology and outcomes of sepsis in adults in sub-Saharan Africa: a systematic review and meta-analysis. Crit Care. 2019;23:212. doi: 10.1186/s13054-019-2501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudd KE, Seymour CW, Aluisio AR, et al. Association of the Quick Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA) Score With Excess Hospital Mortality in Adults With Suspected Infection in Low- and Middle-Income Countries. JAMA. 2018;319:2202–2211. doi: 10.1001/jama.2018.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Nie L, Liu A, et al. Combining procalcitonin with the qSOFA and sepsis mortality prediction. Medicine (Baltimore) 2019;98:e15981. doi: 10.1097/MD.0000000000015981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLymont N, Glover GW. Scoring systems for the characterization of sepsis and associated outcomes. Ann Transl Med. 2016;4:527. doi: 10.21037/atm.2016.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore CC, Hazard R, Saulters KJ, et al. Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub- Saharan Africa. BMJ Glob Health. 2017;2 doi: 10.1136/bmjgh-2017-000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmedding M, Adegbite BR, Gould S, et al. A Prospective Comparison of Quick Sequential Organ Failure Assessment, Systemic Inflammatory Response Syndrome Criteria, Universal Vital Assessment, and Modified Early Warning Score to Predict Mortality in Patients with Suspected Infection in Gabon. Am J Trop Med Hyg. 2019;100:202–208. doi: 10.4269/ajtmh.18-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boillat-Blanco N, Mbarack Z, Samaka J, et al. Prognostic value of quickSOFA as a predictor of 28-day mortality among febrile adult patients presenting to emergency departments in Dar es Salaam, Tanzania. PLOS ONE. 2018;13 doi: 10.1371/journal.pone.0197982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayambankadzanja RK, Schell CO, Namboya F, et al. The Prevalence and Outcomes of Sepsis in Adult Patients in Two Hospitals in Malawi. Am J Trop Med Hyg. 2020;102:896–901. doi: 10.4269/ajtmh.19-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo J, Jiang W, Weng L, et al. Usefulness of qSOFA and SIRS scores for detection of incipient sepsis in general ward patients: A prospective cohort study. J Crit Care. 2019;51:13–18. doi: 10.1016/j.jcrc.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Tian H, Zhou J, Weng L, et al. Accuracy of qSOFA for the diagnosis of sepsis-3: a secondary analysis of a population-based cohort study. J Thorac Dis. 2019;11:11. doi: 10.21037/jtd.2019.04.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei X, Ma H, Liu R, Zhao Y. Comparing the effectiveness of three scoring systems in predicting adult patient outcomes in the emergency department. Medicine (Baltimore) 2019;98:e14289. doi: 10.1097/MD.0000000000014289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie X, Huang W, Liu Q, et al. Prognostic value of Modified Early Warning Score generated in a Chinese emergency department: a prospective cohort study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-024120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudd KE, Seymour CW, Aluisio AR, et al. Association of the Quick Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA) Score With Excess Hospital Mortality in Adults With Suspected Infection in Low- and Middle-Income Countries. JAMA. 2018;319:2202–2211. doi: 10.1001/jama.2018.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huson MAM, Katete C, Chunda L, et al. Application of the qSOFA score to predict mortality in patients with suspected infection in a resource-limited setting in Malawi. Infection. 2017;45:893–896. doi: 10.1007/s15010-017-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baig MA, Sheikh S, Hussain E, et al. Comparison of qSOFA and SOFA score for predicting mortality in severe sepsis and septic shock patients in the emergency department of a low middle income country. Turk J Emerg Med. 2018;18:148–151. doi: 10.1016/j.tjem.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Çıldır E, Bulut M, Akalın H, Kocabaş E, Ocakoğlu G, Aydın ŞA. Evaluation of the modified MEDS, MEWS score and Charlson comorbidity index in patients with community acquired sepsis in the emergency department. Intern Emerg Med. 2013;8:255–260. doi: 10.1007/s11739-012-0890-x. [DOI] [PubMed] [Google Scholar]
- 41.Khwannimit B, Bhurayanontachai R, Vattanavanit V. Comparison of the performance of SOFA, qSOFA and SIRS for predicting mortality and organ failure among sepsis patients admitted to the intensive care unit in a middle-income country. J Crit Care. 2018;44:156–160. doi: 10.1016/j.jcrc.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 42.Sinto R, Suwarto S, Lie KC, Harimurti K, Widodo D, Pohan HT. Prognostic accuracy of the quick Sequential Organ Failure Assessment (qSOFA)-lactate criteria for mortality in adults with suspected bacterial infection in the emergency department of a hospital with limited resources. Emerg Med J. 2020;37:363–369. doi: 10.1136/emermed-2018-208361. [DOI] [PubMed] [Google Scholar]
- 43.Wattanasit P, Khwannimit B. Comparison the accuracy of early warning scores with qSOFA and SIRS for predicting sepsis in the emergency department. Am J Emerg Med. 2021;46:284–288. doi: 10.1016/j.ajem.2020.07.077. [DOI] [PubMed] [Google Scholar]
- 44.Pairattanakorn P, Angkasekwinai N, Sirijatuphat R, Wangchinda W, Tancharoen L, Thamlikitkul V. Diagnostic and Prognostic Utility Compared Among Different Sepsis Scoring Systems in Adult Patients With Sepsis in Thailand: A Prospective Cohort Study. Open Forum Infect Dis. 2021;8:ofaa573. doi: 10.1093/ofid/ofaa573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mar Minn M, Aung NM, Kyaw DZ, et al. The comparative ability of commonly used disease severity scores to predict death or a requirement for ICU care in patients hospitalised with possible sepsis in Yangon, Myanmar. Int J Infect Dis. 2021;104:543–550. doi: 10.1016/j.ijid.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 46.Kilinc Toker A, Kose S, Turken M. Comparison of SOFA Score, SIRS, qSOFA, and qSOFA + L Criteria in the Diagnosis and Prognosis of Sepsis. Eurasian J Med. 2021;53:40–47. doi: 10.5152/eurasianjmed.2021.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes S, Wyawahare M. Utility of quick sepsis-related organ failure assessment (qSOFA) score to predict outcomes in out-of-ICU patients with suspected infections. J Fam Med Prim Care. 2020;9:3251–3255. doi: 10.4103/jfmpc.jfmpc_150_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.