Abstract
Purpose:
The objective of the current study was to evaluate the correlation between obesity and the use of depo-medroxyprogesterone (DMPA) in regards to weight gain and changes in bleeding patterns.
Materials and Methods:
A retrospective chart review of women receiving 150mg DMPA via intramuscular injection at inpatient and outpatient clinics at the University of Mississippi Medical Center (UMMC) between June 1, 2012 to December 31, 2016 was conducted. Body mass indices (BMI) were assessed at baseline and at the time of final injection. Race, medical history, age at first DMPA injection, number and timing of injections, reported side effects, indication for DMPA use, and reason for discontinuation, if applicable were collected.
Results:
Of the 240 patients included in the study, 3.4% were underweight, 30.8% were normal weight, 23.3% were overweight, 15% were Class I obese, 9.6% were Class II obese, and 17.9% were Class III obese. 87.9% of the population were African American. Contraception was the most common indication for use. Women gained (2.40kg; 95% CI 1.34 – 3.45) while they were on DMPA (p<0.01), which after adjusting for confounding variables was associated with age at initial injection (beta coefficient = −0.13; p=0.01). Amenorrhea was the most commonly reported change in bleeding pattern among these women.
Conclusion:
Women who started DMPA at an earlier age gained the most weight over time, independent of initial BMI. Similar rates of amenorrhea were found among all BMI categories.
Keywords: birth control, Depo-Provera, Obesity, weight gain
Introduction
Depo-medroxyprogesterone acetate (DMPA) is one of the most common forms of reversible contraception in the United States and worldwide. DMPA is highly effective with 0.2–0.3% unintended pregnancy rate in the first year for perfect use and 6% for typical use [1]. DMPA is an injectable progestin-only contraceptive that works by suppressing ovulation [2]. Despite the low failure rate of DMPA, many users discontinue using it within their first year due to side effects such as weight gain [1,3,4].
Obesity has expanded by 7.2% (from 30.5% to 37.7%) between 1999 to 2014 among adults ages 20 and older, while it has grown by 3.3% among youth ranging 2–19 years of age (13.9% to 17.2%) [5]. More alarming, the incidence of obesity (BMI > 30 kg/m2) was reported to be 36.5% in women between the ages of 20–39 years and 20.9% in girls between the ages of 12–19 years in the NHANES database for 2015–2016 [6]. Despite the increase in obesity, the number of studies that examine the effectiveness, weight gain, and side effects of DMPA in overweight and obese women remains limited. The main objective of this study was to evaluate weight gain, and changes in menstrual flow or bleeding patterns between non-obese (BMI≤30kg/m2) and obese women (BMI≥30 kg/m2).
Materials and Methods
Upon authorization from the UMMC Institutional Review Board, the UMMC Enterprise Data Warehouse was queried for CPT or J codes used for therapeutic, prophylactic and/or diagnostic injection or infusion of 150mg medroxyprogesterone acetate which resulted in four codes (96372, 99211, J1050, J1055) used between June 1, 2012 and December 31, 2016. Women between the ages of 16 and 56 who started DMPA injections (150mg intramuscular) at UMMC during this time frame were included in the study. Women were excluded if they received another form of contraception within three months prior to receiving DMPA, had incomplete medical records while on DMPA, or received only one dose of DMPA. For study purposes medical records were considered incomplete if they did not include information on weight or height and/or did not have a consistent recorded history of DMPA administration throughout the study period.
A retrospective chart review of electronic medical records was conducted to obtain demographic information and each patient’s history of DMPA use. Baseline BMI was calculated from weight and height data collected at the initial visit for DMPA injection, as well as final BMI at final DMPA injection. The following BMI categories were used: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), Class I Obese (30.0–34.9 kg/m2), Class II Obese (35.0–39.9 kg/m2), and Class III Obese (≥40 kg/m2). These categories are currently used by the World Health Organization to classify obesity and are commonly accepted measure to define obesity [7,8]. Variables retrieved from clinical notes included race, medical history, age at first DMPA injection, number and timing of DMPA injections, reported side effects attributable to DMPA (i.e. self-reports or perception of weight gain/loss, changes in menstrual cycle, hot flashes, pain, headaches) indication for DMPA use, and reason for discontinuation, if applicable. All data was collected and managed using REDCap electronic data capture tools hosted at UMMC [9].
Statistical Analysis
We used descriptive and inferential statistical methods to analyze the data collected. Summary statistics such as mean, proportion, or percentage were computed and graphical representations were established. Analysis of variance (ANOVA) was used to compare the means of continuous variables such as age, weight gain and number of DMPA injections among different BMI categories. Further analysis was done using multivariate linear regression to adjust for covariates (number of DMPA injections, age at initiation, BMI level and race). In addition, either Pearson’s chi-squared test or Fisher’s exact test was applied to examine any associations among categorical variables (these were predominately the BMI categories, variables listed in Table 1 with the exception of age and the comorbidities listed in the Data Supplement). Associations of p<0.05 were considered statistically significant. All computations were done using R statistical software (version 3.3.1) and Graph Pad Prism 7.02.
Table 1.
Baseline demographics and clinical characteristics of women in the study.
| Under-weight (n=8) |
Normal Weight (n=74) |
Over-weight (n=56) |
Class I Obese (n=36) |
Class II Obese (n=23) |
Class III Obese (n=43) |
P value | |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 16.7±1.9 | 22.1±1.8 | 27.5±1.6 | 32.6±1.3 | 37.1±1.5 | 47.5±7.7 | <0.0001 |
| Age (yr) | 24.1±6.1b,c | 26.2±8.5a,b,c | 29.1±10 | 30.8±9.3 | 32.4±9.5 | 31.2±10.3 | 0.009 |
| Race (%) | |||||||
| African American | 75 | 86.4 | 89.3 | 86.1 | 91.4 | 90.7 | 0.80 |
| Caucasian | 25 | 10.8 | 7.1 | 11.1 | 4.3 | 9.3 | |
| Asian | 0 | 1.4 | 1.8 | 0 | 0 | 0 | |
| Other | 0 | 1.4 | 1.8 | 2.8 | 4.3 | 0 | |
| Insurance Status (%) | |||||||
| Medicaid | 62.5 | 33.7 | 28.5 | 55.6 | 30.4 | 48.8 | 0.13 |
| Medicare | 12.5 | 14.9 | 14.3 | 5.6 | 26 | 16.4 | |
| Private | 25 | 39.2 | 42.9 | 30.6 | 43.6 | 20.9 | |
| Uninsured | 0 | 12.2 | 14.3 | 8.2 | 0 | 13.9 | |
| Clinical Characteristics | 0.79 | ||||||
| Anemia (%) | 0 | 26.9 | 34.6 | 19.2 | 7.7 | 11.5 | |
| Asthma (%) | 0 | 41.9 | 22.6 | 16.1 | 9.7 | 9.7 | |
| BTL* (%) | 3.8 | 25 | 32.7 | 15.4 | 9.6 | 13.5 | |
| Sleep Problems (%) | 0 | 28.6 | 14.3 | 0 | 14.3 | 42.9 | |
| Smoker (%) | 6.3 | 31.3 | 25 | 31.3 | 0 | 6.3 | |
| Uterine Fibroids (%) | 0 | 20 | 26.7 | 20 | 13.3 | 20 | |
| Indication for DMPA use | 0.12 | ||||||
| Abnormal bleeding (%) | 12.5 | 8.5 | 21.1 | 22.7 | 18.8 | 25 | |
| Contraception | 62.5 | 75.6 | 53.5 | 52.3 | 50 | 57.7 | |
| Dysmenorrhea | 0 | 3.6 | 12.7 | 9.1 | 12.5 | 5.8 | |
| Menorrhagia | 0 | 4.9 | 5.6 | 9.1 | 15.6 | 3.8 | |
| Other | 25 | 6 | 7.1 | 6.8 | 3.1 | 7.7 | |
A statistically significant relationship of p<0.05 is denoted between the indicated noted groups and
Class I Obese,
Class II Obese, and
Class III Obese. Results are mean±SD.
Note-
BTL- bilateral tubal ligation
Results
There were 357 potential participants identified on initial screening, however, 117 were excluded due to being lost-to follow-up, received another hormonal contraception, or having incomplete medical records of DMPA use, leaving 240 women meeting the inclusion criteria for the study (Figure 1). The major demographic data for the groups are reported in Table 1. Women with Class I obesity were older at the start of DMPA administration compared to women with normal BMI (30.7±9 vs. 26.2±8.5yrs; p=0.02). Women with Class II obesity were older compared to women with an underweight BMI (p=0.03) or with normal BMI (p=0.006). Similar results were seen among Class III Obese women who were significantly older than underweight women (p=0.05) and women with normal BMI (p=0.006; Table 1). There was not a significant difference in race (p=0.80) or insurance status among participants (p=0.13). Common medical conditions (i.e. uterine fibroids, sleep apnea/insomnia, anemia) were not found to be significantly different among women when assessed across BMI (p=0.79; Table 1). There were no pregnancies among women who received DMPA within the schedule periods. We examined the common comorbidities associated with metabolic syndrome and found a correlation between the incidence of diabetes/pre-diabetes, dyslipidemia, and hypertension with BMI class (Data Supplement).
Figure 1. Flow chart of cases reviewed for the current study.
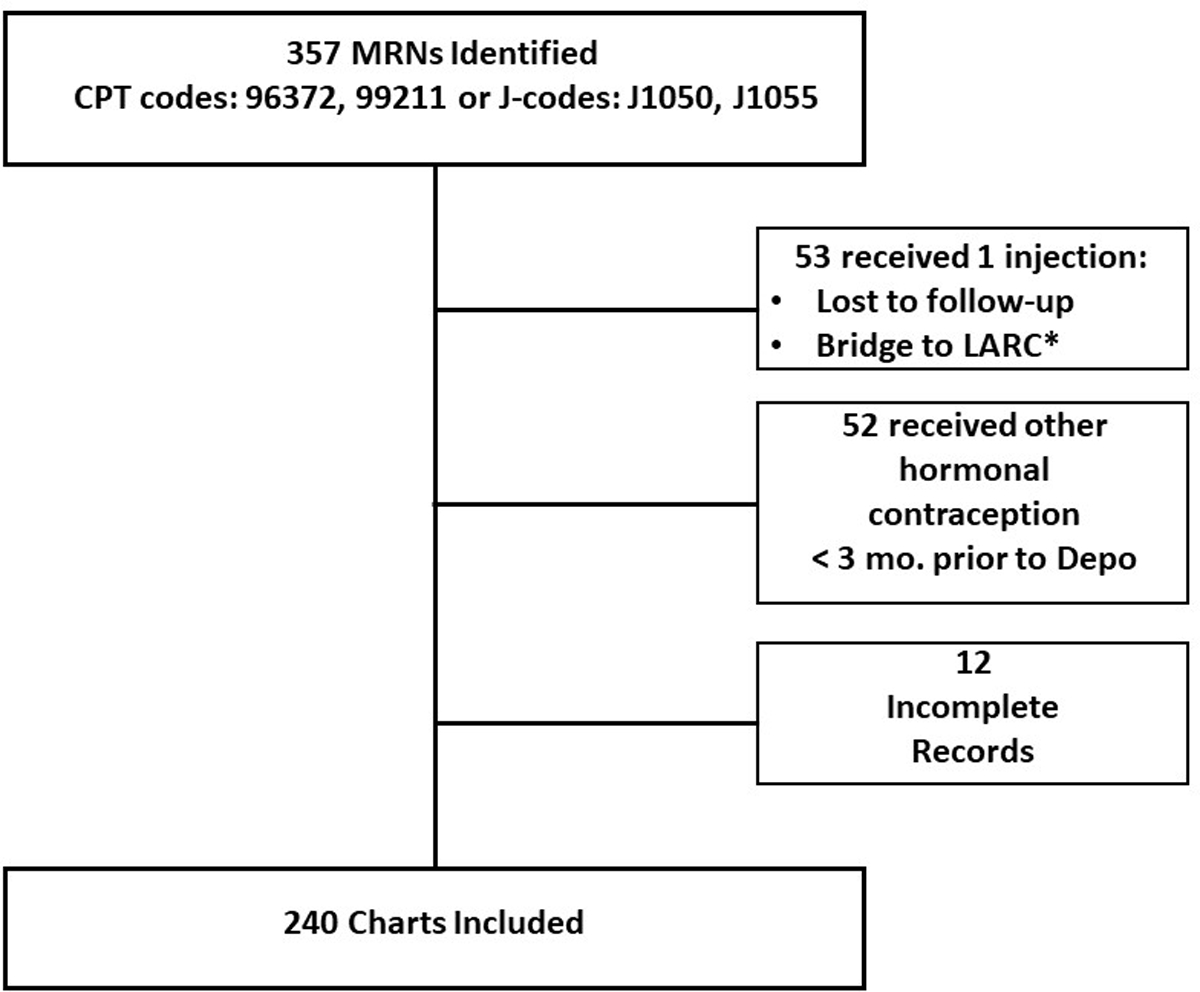
*LARC—long-acting reversible contraception.
Indication vs BMI
The most common indication for DMPA was contraception (73%), followed by abnormal uterine bleeding, dysmenorrhea, and menorrhagia. There was not a statistically significant difference in the indication for instituting DMPA treatment among women across BMI groups (p=0.12; Table 1). Other reasons for use included menstrual hygiene and cycle suppression. There was one patient with no listed indication for initiated DMPA therapy.
Weight gain while on DMPA
Overall women did gain weight while on DMPA (2.40kg; 95% CI 1.34 – 3.45). Further analysis was conducted adjusting for age, BMI, number of injections and race. While weight gain was found to decrease with increasing age (beta coefficient =−0.13; p=0.02; Figure 2), number of injections was not statistically significantly association with weight gain on DMPA (beta coefficient =0.14; p=0.14; Figure 3).
Figure 2. Weight gain is associated with age at DMPA initiation.
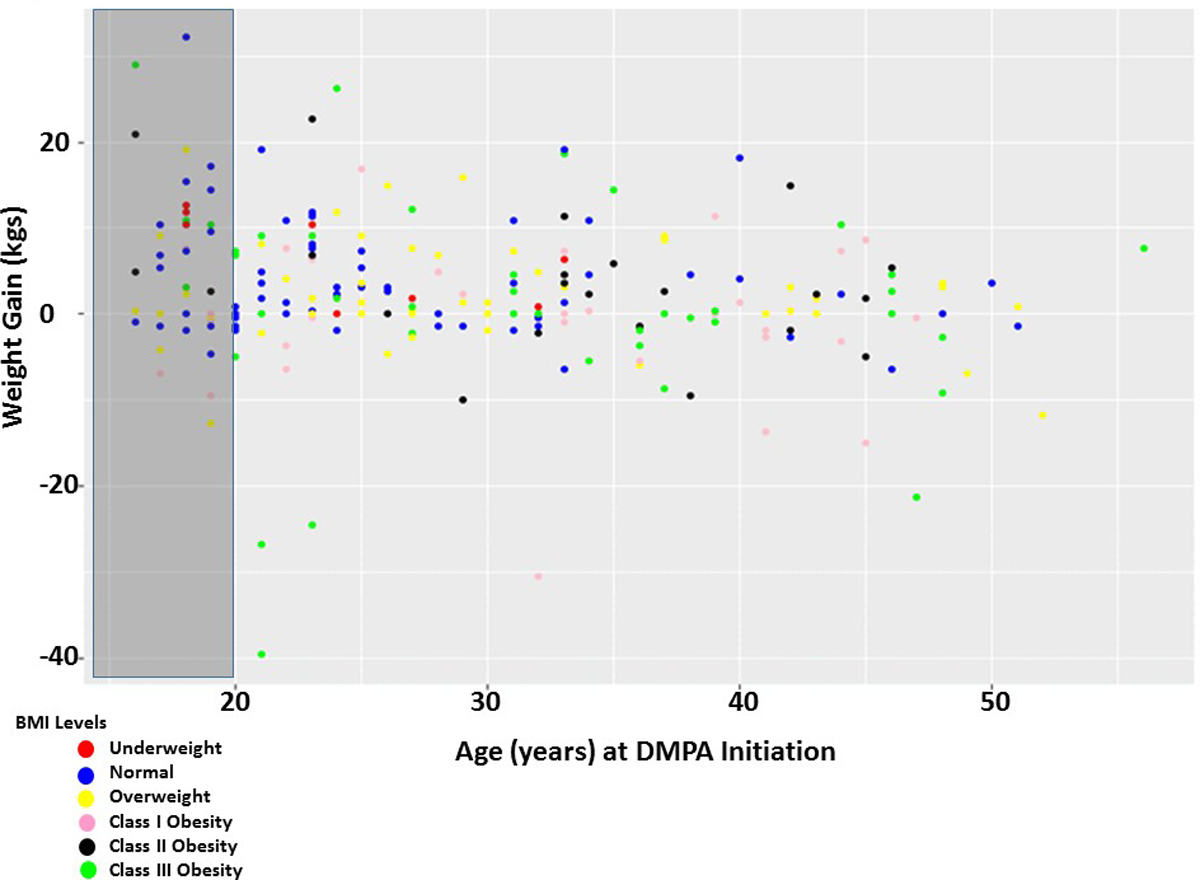
Each point represents a patient within their respective BMI classification at DMPA initiation, their age at DMPA initiation and their weight loss or gained while on DMPA. Weight loss/gained was calculated (weight at study end – weight at study beginning). The gray box indicates women who were adolescents (age 16–19) at the beginning of the study. The color of the dot corresponds to the BMI category.
Figure 3. Weight gain on DMPA is not dependent on BMI class.
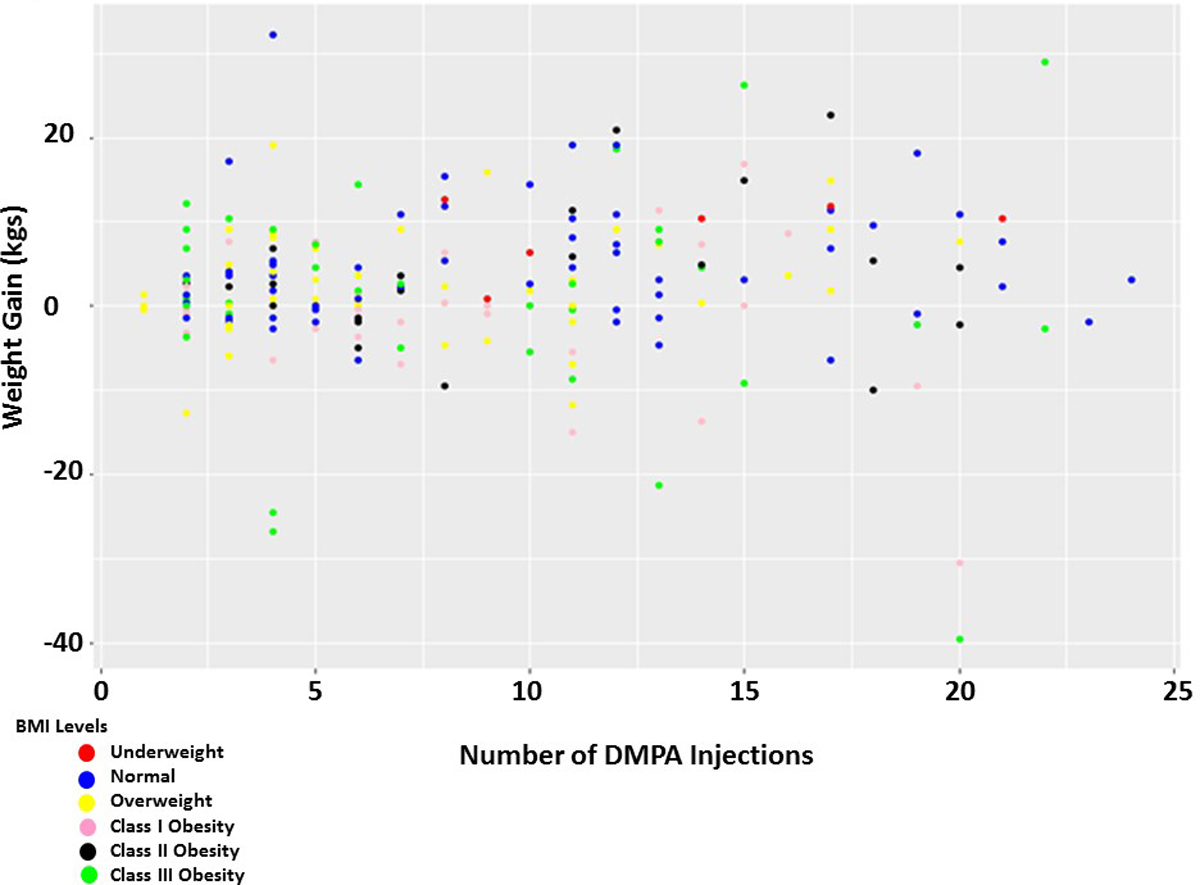
Weight gain, or loss, is not dependent on the number of DMPA injections or BMI class. Each point represents a patient within their respective BMI classification at DMPA initiation, the number of DMPA injections they received and the total amount of weight gained while on DMPA. Weight loss/gained was calculated (weight at study end – weight at study beginning). The color of the dot corresponds to the BMI category.
To determine if BMI at DMPA initiation was correlated with weight gain while on DMPA, weight gain was examined across the BMI categories and no significant differences were present (p=0.07). Women who started the study as underweight tended to gain weight (6.8±5.24 kg), whereas, women in the Class I obese category tended to lose weight (−0.5±8.19 kg). There was no statistically significant difference in weight change among adolescents (16–19 years of age) when compared across the BMI categories (p=0.08); however, collectively adolescents gained the most weight (5.35±1.4 kgs) compared to adults (1.75±0.56kgs; p=0.01) with DMPA use.
Bleeding Patterns versus BMI
Overall, there was no statistically significance difference in bleeding patterns across the various BMI classes (p=0.09). Changes in bleeding patterns from baseline (i.e. before DMPA initiation) among women were self-reported and included: reduced bleeding, intermenstrual spotting, and heavy bleeding. The most common pattern was amenorrhea, accounting for 52% of the study population (p<0.0001). Nonetheless, when the incidence of amenorrhea was compared across BMI classes, there was no significance (p=0.12).
There were only six documented cases of patient-reported weight gain, one of which was due to placement of a feeding tube in an underweight patient and 5 were patient complaints. There were no complaints of acne, fatigue, nor decreased libido as a side effect of DMPA administration. However, there were complaints of headaches (n=1), vaginal dryness (n=1), vasomotor symptoms (n=1), hot flashes (n=2), and changes in mood (n=1) among some of the study participants. At the end of the study, complete DMPA continuation and/or discontinuation data was available for 161 patients. Of these women, 70.4–87.5% chose to remain on DMPA. Side effects, desire for conception, and changes in birth control therapy were the most common reasons for discontinuation. Of the women who met the original inclusion criteria, 79 were either lost to follow-up or had no reason listed for choosing to end DMPA therapy. There was no significant variance across BMI classes for DMPA continuity, cessation, nor lost to follow-up (p=0.88).
Morbid obesity and DMPA
We conducted a sub-analysis on women in the Class III obese group to determine if these women were more likely to gain weight on DMPA or have differences in their side effect profile. There was no statistically significant variance in the age of DMPA initiation (p=0.42) or for weight gain between subcategories (p=0.49; Table 2). Similar to the general study population of women, amenorrhea was the most common side effect, affecting 76% of morbidly obese women (p<0.001).
Table 2.
Subcategories of morbidly obese women on DMPA. Weight change (BMI at the end of study period – BMI beginning of study period). Data is expressed in mean± standard error mean.
| Class III Obesea (40–49.9kg/m2) n=30 |
Class III Obeseb (50–59.9kg/m2) n=9 |
Class III Obesec (60+kg/m2) n=4 |
P value | |
|---|---|---|---|---|
| BMI (kg/m2) | 43.47±3.21 | 53.04±0.77 | 65.41±3.05 | <0.0001 |
| Age (yr.) | 30.5±1.93 | 30.8±3.57 | 37.8±2.53 | 0.42 |
| Weight Change (kgs) | 3.07±1.78 | −0.91±5.13 | 1.47±3.08 | 0.47 |
Discussion
Findings and Interpretation
Complaints of weight gain is a common theme among women receiving hormonal contraception, especially DMPA, and often serves as a reason for discontinuation [10–12]. In the current study, we demonstrated that the use of DMPA is linked to weight gain despite BMI class, but was dependent on age of commencement. After we adjusted for the number of DMPA injections (i.e. length of time on DMPA) and BMI, women who began therapy at an earlier age were susceptible to more weight gain throughout their time on DMPA. BMI was not statistically associated with bleeding patterns among women taking DMPA, which included the attainment of amenorrhea. None of the women who adhered to the DMPA schedule became pregnant regardless of their BMI or length of time on DMPA, which is similar to previous reports [1].
73% of the women in this study reported to use DMPA for contraception, whereas the remaining women used DMPA for reasons ranging for menstrual cycle suppression to medical therapy for uterine fibroids. The use of DMPA for off-label non-contraceptive reasons such as uterine fibroids, menstrual cycle suppression and for other reproductive health reasons has been reported and is not unique to our population [13–15]. However, regardless of the indication for using DMPA there was not a statistical difference among women in different BMI categories for their primary reason for using DMPA.
We also looked at additional factors associated with obesity and DMPA use such as sleep disturbances and did not find any significant differences among the different BMI categories. This is in contrast to studies by Brown et al who reported that women taking DMPA had sleep disturbances [16]. However, as sleep disturbances are prevalent among obese patients, it is possible that any disturbances experienced by obese women in the current study may have been due to their weight and not DMPA [17].
Strengths and weaknesses of the study
The main strength of our study is the large sample size of obese patients, as studies of contraception among obese and morbidly obese women are limited. It has been suggested that decreased physical activity plus increased caloric intake and/or body mass composition may be among the reasons why adolescents are more susceptible to weight gain while on hormonal contraception [18]. However, as the data in the current study was collected from routine gynecological DMPA visits physical activity and nutritional status data was not collected and serves as a study limitation. A systemic review by Silva et al examining dietary intake and eating behavior among DMPA users was not able to determine if DMPA contributes to changes in eating behavior which could possibly serve as an explanation for weight gain by its users [19]. Another study, though small with only 43 patients, reported that despite a decrease in appetite black adolescents gained more weight and body fat on DMPA compared to white adolescents who were on DMPA for the same amount of time, suggesting racial differences in weight gain and body composition while on DMPA [12]. Future prospective studies are needed to determine if lifestyle modifications of diet and exercise have an effect on weight gain among DMPA users, especially among younger females.
One limitation to the study is the lack of racial diversity among the population, which was 87.7% black. Because of the limited diversity in the patient population, results may not be applicable to the general population. This is especially true as some studies have reported differences in weight gain among different racial and ethnic groups [12,20]. Additionally, although there were a large number of obese women, the number of women who were underweight was considerably low. As the study design included a retrospective chart review, data verification via direct patient or provider contact was not an option, thus providing another study limitation. In spite of these limitations, this study provides important information regarding weight gain and bleeding patterns associated with DMPA use among the different BMI classes, including morbidly obese women.
Differences in results and conclusions
Our findings align with other studies that have reported positive associations between DMPA use and weight gain in younger women [21–24]. The effect of weight gain while on DMPA being independent of BMI displays a disparity from what has been reported by Le et al [25], who found that body mass indices <30 kg/m2 increased the risk for weight gain within the first six months of DMPA therapy. However, Pantoja reports results similar to our findings, in which women in the normal and overweight categories gained more weight while on DMPA compared with women in the obese categories [26]. Pantoja went on to suggest that the change in weight could be associated with metabolic alterations occurring in women of the normal and overweight classes [27,28].
Earlier studies have shown an increase in visceral fat among DMPA users, which is also linked to metabolic syndrome [29,30]. It has been conveyed that up to 40% of women discontinue DMPA due to side effects and weight gain; however, there were only five complaints of weight gain in the current study [3,31]. Amenorrhea was the most commonly acknowledged change in bleeding patterns across all groups of these women, as has been previously informed in other studies [32,33]. Although it has been suggested that the efficacy of progestin-only contraceptives such as DMPA may be reduced in obese women [34], we did not have any pregnancies in the current study.
Unanswered questions and future research
As certain contraceptives can worsen health conditions in morbidly obese women, we evaluated the incidence of common comorbidities associated with metabolic syndrome among study participants, regardless of their onset. Obese women were more likely to be hypertensive, dyslipidemia and have diabetes, all of which obesity is a major risk factor for [35]. While both new onset of dyslipidemia and diabetes have been reported in women using DMPA [36,37], hypertension has not been associated with DMPA usage [38]. As we did not conclude if these conditions were diagnosed before or after DMPA initiation, future studies will need to determine if DMPA increases hypertension and/or diabetes in obese patients. It is also possible that the high incidence of these comorbidities is due to the predominantly black population of our study, as this racial group is more prone to development of such conditions [39]. Future studies also need to include women who are on other forms of contraception (both hormonal and non-hormonal) to determine if the weight-gain seen in the current study is truly due to DMPA or other factors.
Conclusion
Weight gain during DMPA therapy was most notable among younger women. Moreover, obese women were not more susceptible to weight gain and did not have more side-effects from DMPA usage relative to non-obese women.
Supplementary Material
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- 1.Trussell J Contraceptive Failure in the United States. Contraception 2011;83(5):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobstein R, Polis C. Progestin-only contraception: injectables and implants. Best Pract Res Clin Obstet Gynaecol 2014;28:795–806. [DOI] [PubMed] [Google Scholar]
- 3.Peipert J, Zhao Q, Allsworth J, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol 2011;117(5):1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez L, Edelman A, Chen M, et al. Progestin-only contraceptives: Effects on Weight. Cochrane Database Syst Rev 2013;CD008815. [DOI] [PMC free article] [PubMed]
- 5.Ogden C, Carroll M, Fryzek J, et al. Prevalence of Obesity Among Adults and Youth: United States 2011–2014. NCHS Data Brief 2015;219:1–8. [PubMed] [Google Scholar]
- 6.Hales C, Carroll M, Fryar C, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS data brief Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 7.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894(i-xii):1–253. [PubMed] [Google Scholar]
- 8.Nejat E, Polotsky A, Pal L. Predictors of chronic disease at midlife and beyond - the health risks of obesity. Maturitas 2010;65:106–111. [DOI] [PubMed] [Google Scholar]
- 9.Harris P, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallo M, Lopez L, Grimes D, et al. Combination contraceptives: Effects on weight. Cochrane Database Syst Rev 2011. [DOI] [PubMed]
- 11.Haider S, Darney P. Injectable contraception. Clin Obstet Gynecol 2007;50(4):898–906. [DOI] [PubMed] [Google Scholar]
- 12.Bonny A, Britto M, Huang B, et al. Weight gain, adiposity, and eating behaviors among adolescent females on depot medroxyprogesterone acetate (DMPA). J Pediatr Adolesc Gynecol 2004;17(2):109–115. [DOI] [PubMed] [Google Scholar]
- 13.Sangkomkamhang U, Lumbiganon PL, M, Mol B. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Syst Rev 2013;28(2):CD008994. [DOI] [PubMed] [Google Scholar]
- 14.Venkatachalam S, Bagratee J, Moodley J. Medical management of uterine fibroids with medroxyprogresterone acetate (Depo Provera): a pilot study. J Obstet Gynaecol 2004;24(7):798–800. [DOI] [PubMed] [Google Scholar]
- 15.Fraser I, Dennerstein G. Depo-Provera use in an Australian metropolitan practice. Med J Aust 1994;160(9):553–556. [PubMed] [Google Scholar]
- 16.Brown S, Morrison L, Larkspur L, et al. Well-being sleep, exercise patterns and the menstrual cycle: a comparision of natural hormones, oral contraceptives and depo-provera. Women Health 2008;47(1):105–121. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie R, Patel S. The epidemiology of sleep and obesity. Sleep Health 2017;3(5):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangan S, Larsen P, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol 2002;15(2):79–82. [DOI] [PubMed] [Google Scholar]
- 19.Silva P, Qadir S, Fernandes A, et al. Dietary intake and eating behavior in depot medroxyprogesterone acetate users: a systematic review. Braz J Med Biol Res 2018;51(6):e7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickery Z, Madden T, Zhao Q, et al. Weight Change at 12 Months in Users of Three Progestin-Only Contraceptive Methods. Contraception 2013;88(4):503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risser W, Gefter L, Barratt M, et al. Weight change in adolescents who used hormonal contraception. J Adolesc Health 1999;24(6):433–436. [DOI] [PubMed] [Google Scholar]
- 22.Lange H, Belury M, Secic M, et al. Dietary Intake and Weight Gain Among Adolescents on Depot Medroxyprogesterone Acetate. J Pediatr Adolesc Gynecol 2015;28(3):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonny A, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolscent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006;160(1):40–45. [DOI] [PubMed] [Google Scholar]
- 24.Bonny A, Secic M, Cromer B. A longitudinal comparison of body composition changes in adolescent girls receiving hormonal contraception. J Adolesc Health 2009;45:423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Y, Rahman M, Berenson A. Early Weight Gain Predicting Later Weight Gain Among Depot Medroxyprogesterone Acetate Users. Obstet Gynecol 2009;114:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantoja M, Medeiros T, Baccarin M, et al. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception 2010;81(2):107–111. [DOI] [PubMed] [Google Scholar]
- 27.Pantoja M, Medeiros T, Baccarin M, et al. Variation of weight among users of the contraceptive with depot-medroxyprogesterone acetate according to body mass index in a six-year follow-up. rev Bras Ginecol Obstet 2009;31(8):380–384. [DOI] [PubMed] [Google Scholar]
- 28.Steward R, Bateman L, Slentz C, et al. The impact of short-term depot-medroxyprogesterone acetate treatment on resting metabolic rate. Contraception 2016;93(4):317–322. [DOI] [PubMed] [Google Scholar]
- 29.Berenson A, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol 2009;200(3):329.e1–329.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark M, Dillon J, Sowers M, et al. Weight, fat mass, and central distribution of fat incrase when women use depot-medroxyprogesterone acetate for contraception. Int J Obes 2005;29(10):1252–1258. [DOI] [PubMed] [Google Scholar]
- 31.Paul C, Skegg D, Williams S. Depot medroxyprogesterone acetate. Patterns of use and reasons for discontinuation. Contraception 1997;56:209–214. [DOI] [PubMed] [Google Scholar]
- 32.Hubacher D, Lopez L, Steiner M, et al. Menstrual pattern changes from levonorgestrel subdermal implants and DMPA: systematic review and evidence-based comparisons. Contraception 2009;80(2):113–118. [DOI] [PubMed] [Google Scholar]
- 33.Arias R, Jain J, Brucker C, et al. Changes in bleeding patterns with depot medroxyprogesterone acetate subcutaneous injection 104mg. Contraception 2006;74(3):234–238. [DOI] [PubMed] [Google Scholar]
- 34.Stanczyk F, Burke A, Hong K, et al. Morbid obesity: potential effects of hormonal contraception. Contraception 2018;98(3):174–180. [DOI] [PubMed] [Google Scholar]
- 35.Lavie C, McAuley P, Church T, et al. Obesity and cardiovascular diseases: implications regarding fitness, fatness and severity in the obesity paradox. J Am Coll Cardiol 2014;63:1345–1354. [DOI] [PubMed] [Google Scholar]
- 36.Batista G, Souza A, Marin D, et al. Body composition, resting energy expenditure and inflammatory markers: impact in users of depot medroxyprogresterone acetate after 12 months follow-up. Arch Endocrinol Metab 2017;61(1):70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berenson A, Rahman M, Wilkinson G. Effect of injectable and oral contraceptives on serum lipids. Obstet Gynecol 2009;114(4):786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakry S, Merhi Z, Scalise T, et al. Depot-medroxyprogesterone acetate: an update. Arch Gyneccol Obstet 2008;278:1–12. [DOI] [PubMed] [Google Scholar]
- 39.Gaillard T The metabolic syndrome and its components in African-American women: Emeriging trends and implications. Front Endocrinol 2018;8:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


