Abstract
Background
There is limited information on the long-term outcomes of ICDs in patients with inherited arrhythmia syndromes.
Methods
Prospective registry study of inherited arrhythmia patients with an ICD. Incidence of therapies and complications were measured as 5-year cumulative incidence proportions and analyzed with the Kaplan-Meier method. Incidence was compared by device indication, diagnosis type and device type. Cox-regression analysis was used to identify predictors of appropriate shock and device complication.
Results
123 patients with a mean follow up of 6.4 ± 4.8 years were included. The incidence of first appropriate shock was 56.52% vs 24.44%, p < 0.05 for cardiomyopathy and channelopathy patients, despite similar ejection fraction (61% vs 60%, p = 0.6). The incidence of first inappropriate shock was 13.46% vs 56.25%, p < 0.01 for single vs. multi-lead devices. The incidence of first lead complication was higher for multi-lead vs. single lead devices, 43.75% vs. 17.31%, p = 0.04. Patients with an ICD for secondary prevention were more likely to receive an appropriate shock than those with primary prevention indication (HR 2.21, CI 1.07–4.56, p = 0.03). Multi-lead devices were associated with higher risk of inappropriate shock (HR 3.99, CI 1.27–12.52, p = 0.02), with similar appropriate shock risk compared to single lead devices. In 26.5% of patients with dual chamber devices, atrial sensing or pacing was not utilized.
Conclusion
The rate of appropriate therapies and ICD complications in patients with inherited arrhythmia is high, particularly in cardiomyopathies with multi-lead devices. Risk-benefit ratio should be carefully considered when assessing the indication and type of device in this population.
Keywords: Inherited Arrhythmia, Cardiac Defibrillator, Cardiomyopathy, Channelopathy
1. Introduction
Patients with heritable arrhythmogenic conditions may require an implantable cardiac defibrillator (ICD) for primary or secondary prevention of sudden cardiac death [1], [2]. In this population, ICD implantation often occurs at a relatively young age, conferring a prolonged exposure to the risks associated with an implantable device [3], [4].
This study aimed to assess the incidence of device related outcomes including appropriate shock, inappropriate shock, and lead complications in patients with genetic cardiomyopathies and channelopathies implanted with ICDs.
2. Methods
2.1. Study design
This work is an analysis of prospective registry including all patients with an ICD followed at the Cardiovascular Genetics Program at New York University Langone Health between 2008 and 2020. The study was approved by the NYU School of Medicine IRB 09–0297.
Patients meeting these criteria were entered into an IRB approved registry. Clinical information was obtained from the patient’s electronic medical records. The patient’s diagnosis was categorized as either a cardiomyopathy or channelopathy according to the primary clinical feature of the condition. Channelopathies included Brugada Syndrome, Idiopathic VF, Long QT Syndrome, Short QT Syndrome, Catecholaminergic Polymorphic VT (CPVT), and Progressive Cardiac Conduction Disease (PCCD). Cardiomyopathies included Arrhythmogenic Cardiomyopathy, Hypertrophic Cardiomyopathy, Dilated Cardiomyopathy, PRKAG2 cardiomyopathy, Danon’s Disease and Primary Carnitine Deficiency. Patients were treated with antiarrhythmic therapy as indicated, at maximally tolerated doses. Each device shock was reviewed and adjudicated as appropriate or not by expert clinicians. Patients with either permanent atrial fibrillation or those who were atrial-paced<2% of the time were accounted as having an uncertain indication for a dual chamber device.
Outcomes included appropriate and inappropriate shock and lead related complications including lead fracture/noise, lead dislodgement, or unspecified lead failure as assessed by abnormal impedance, sensing, or thresholds.
2.2. Statistical analysis
Continuous variables are reported as either means ± the standard deviation or as medians ± the interquartile range. Means were compared using an unpaired T Test. Medians were utilized when data was not normally distributed and comparisons between medians were made using the Mann-Whitney Test. Categorical variables are reported as their raw frequency and their relative frequency with respect to the total and compared using the Fischer Exact Method. Outcomes were compared according to device indication (primary vs secondary prevention), diagnosis category (cardiomyopathy vs channelopathy) and device type (single vs multi-lead ICD). Cumulative 5-year incidence proportions were calculated and compared using the Fischer Exact Test. Outcomes were also assessed using the Kaplan-Meier Method. Comparison between survival curves were made using the log rank test and hazard ratios were calculated and compared using the Mantel-Haenszel method. A cox proportional hazard ratio was generated. For predictors of outcomes, we included age at implantation, gender, BMI, proband status, diagnosis type, presence of symptoms, cardiovascular comorbidities, family history of SCD or syncope, ejection fraction, left atrial (LA) volume index, indication for ICD placement, device type (single vs. dual chamber), and percent of atrial and ventricular pacing. Each variable was assessed using single variate analysis with a plan to subsequently generate a multivariable model using only the significant values. All incidence proportions and hazard ratios are displayed with their 95% confidence interval. Analysis was performed using Microsoft excel, SPSS and graphpad prism 9.
3. Results
3.1. Demographics.
A total of 123 patients (41.5% females) were included in the registry (Table 1). The mean age at analysis was 45.6 ± 14.7 and the mean age at device implant was 38.3 ± 15.7. The mean follow-up time was 6.4 years ± 4.8 years. Channelopathy was diagnosed in 77 (62.6%) patients while 46 (37.4%) patients had a primary diagnosis of cardiomyopathy. There was no difference in median ejection fraction between patients with a cardiomyopathy and a channelopathy (61% vs 60%, p = 0.6). The most frequent cardiomyopathy was arrhythmogenic cardiomyopathy (17 patients, 37%) followed by hypertrophic cardiomyopathy (15 patients, 32.6%). Other cardiomyopathies included Dilated Cardiomyopathy (11 patients, 23.9%), PRKAG2 Cardiomyopathy (1 patient, 2.2%), Danon Disease (1 patient, 2.2%), and Primary Carnitine Deficiency (1 patient, 2.2%). The most frequent channelopathies were J-wave syndromes including Brugada Syndrome and Early Repolarization (28 patients, 36.4%), and idiopathic VF (17 patients, 22.1%). Other channelopathies included Long QT syndrome (15 patients, 19.5%), Short QT Syndrome (8 patients, 10.4%), Catecholaminergic Polymorphic Ventricular Tachycardia (6 patients, 7.8%), and Progressive Cardiac Conduction Deficit (1 patient 1.3%). In addition, 2 patients with an unknown primary diagnosis but suspected channelopathy were counted as having an unknown channelopathy. A pathogenic/likely pathogenic variant mutation was identified in 85 (69.1%) of the patients. The most commonly involved genes were SCN5A, PKP2, KCNH2, and MYBPC3. Seventy-four (60.2%) patients had an ICD implanted for primary prevention while 49 (39.8%) patients had an ICD for secondary prevention. 37 patients (48.1%) with channelopathies had an ICD placed for primary prevention, while 37 (80.4%) patients with cardiomyopathies had an ICD placed for primary prevention. 40 patients (52%) with channelopathies had an ICD placed for secondary prevention, while only 9 (19.6%) patients with cardiomyopathies had an ICD placed for secondary prevention (p = 0.0005). Single chamber ICDs were implanted in 87 (70.7%) patients while 36 (29.3%) patients had a multi lead device. Thirty-four (27.6%) patients had dual chamber devices while 2 (1.6%) patients had biventricular devices. Of patients with a dual chamber device, 9 (26.5%) had either permanent AF (8.8%) or had < 2% atrial pacing (17.7%).
Table 1.
Baseline Characteristics.
| Total | Cardiomyopathy | Channelopathy | Primary | Secondary | Single-Lead | Multi-Lead | |
|---|---|---|---|---|---|---|---|
| Number | 123 | 46 (37.4%) | 77 (62.6%) | 74 (60.2%) | 49 (39.8%) | 87 (70.7%) | 36 (29.3%) |
| Age | 45.6 ± 14.7 | 47.0 ± 15.2 | 44.8 ± 14.5 | 46.6 ± 15.3 | 44.2 ± 13.9 | 45.4 ± 14.0 | 46.2 ± 16.5 |
| Gender (Male) | 72 (58.5%) | 27 (58.7%) | 45 (58.4%) | 42 (56.8%) | 30 (61.2%) | 54 (62.1%) | 18 (50%) |
| BMI | 26.4 ± 5.6 | 27.9 ± 6.5* | 25.5 ± 4.9* | 26.5 ± 5.4 | 26.2 ± 6.1 | 26.3 ± 5.5 | 26.4 ± 6.1 |
| BSA | 1.9 ± 0.2 | 2.0 ± 0.2* | 1.9 ± 0.2* | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.3 | 1.9 ± 0.2 |
| Proband | 98 (79.7%) | 37 (80.4%) | 61 (79.2%) | 52 (70.3%)* | 46 (93.9%)* | 69 (79.3%) | 29 (80.6%) |
| Age at Implant | 38.3 ± 15.7 | 40.8 ± 15.7 | 36.7 ± 15.6 | 39.7 ± 16.1 | 36.1 ± 14.9 | 38.0 ± 14.3 | 38.7 ± 18.8 |
| Follow up time | 6.4 ± 4.8 | 5.5 ± 4.6 | 6.9 ± 4.9 | 5.7 ± 4.4 | 7.4 ± 5.3 | 6.4 ± 4.5 | 6.4 ± 5.6 |
| Symptomatic | 115 (93.5%) | 45 (97.8%) | 70 (90.9%) | 66 (89.2%)* | 49 (100%)* | 80 (92%) | 35 (97.2%) |
| Comorbidities | 60 (48.8%) | 28 (60.9%)* | 32 (41.6%)* | 43 (58.1%)* | 17 (34.7%)* | 39 (44.8%) | 21 (58.3%) |
| Family History | 51 (41.5%) | 19 (41.3%) | 32 (41.6%) | 37 (50.0%)* | 14 (28.6%)* | 37 (42.5%) | 14 (38.9%) |
| + Genetics | 85 (69.1%) | 39 (84.8%)* | 45 (58.4%)* | 51 (68.9%) | 34 (69.4%) | 54 (62.1%)* | 31 (86.1%)* |
| EF | 59.1% ± 10.8% | 58.5% ± 13.9% | 59.7% ± 6.5% | 59.9% ± 11.5% | 57.9% ± 9.5% | 60.5% ± 10.4% | 56.5% ± 11.2% |
| LAVI | 26 ± 12.3 | 29.2 ± 14.9* | 22 ± 5.8* | 27.5 ± 14.4 | 23.5 ± 7.0 | 24.2 ± 12.8 | 30.3 ± 9.8 |
| % V Paced | 9.5% ± 27.3% | 21.5% ± 39.5%* | 2.2% ± 11.2%* | 12.5% ± 31.3% | 5.1% ± 19.5% | 5.0 ± 20.4%* | 20.8% ± 37.7%* |
| % A Paced | 42% ± 40.4% | 53% ± 40.6% | 31.7% ± 38.6% | 47.7% ± 38.6% | 30.1% ± 43.5% | N/A | 38.5% ± 39.3% |
Data are represented as frequency (relative proportion) or as mean ± SD.
Abbreviations: % A Paced: Percent of time paced form the atria, % V Paced: Percent of time paced from the ventricle, BMI: Body Mass Index, BSA: Body Surface Area, EF: Ejection Fraction, LAVI: Left Atrial Volume Index
Indicates p < 0.05 for the comparisons of Cardiomyopathy vs. Chennlopathy, Primary vs. Secondary and Single lead vs. Multi lead devices.
3.2. Outcomes
3.2.1. Appropriate Shock.
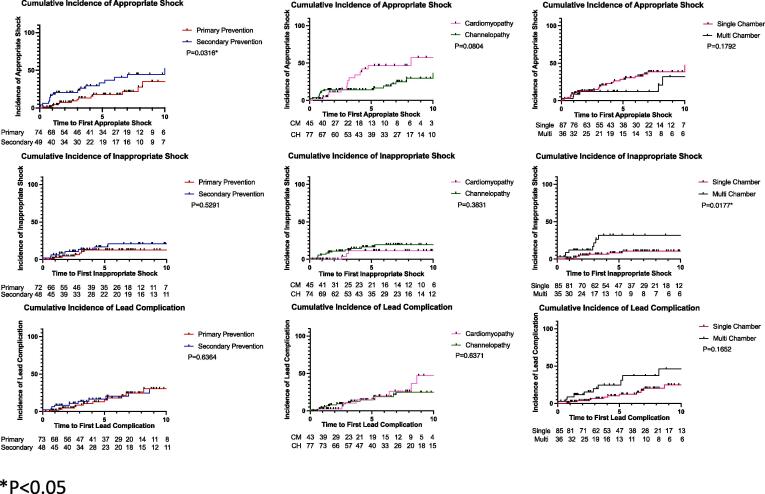
Outcomes are summarized in Table 2, Table 3 and depicted in Fig. 1. The 5-year cumulative incidence of appropriate shocks was 35.29% (25%-47.16%) in the overall cohort. The incidence of appropriate shocks was higher in patients with a cardiomyopathy compared to those with a channelopathy (56.52%, 95% CI 36.81%-74.37% vs. 24.44%, CI 14.24%-38.67%, p = 0.015) despite no differences in ejection fraction and a higher proportion of primary prevention devices in the cardiomyopathy group. The incidence of appropriate shocks in cardiomyopathy patients with primary prevention devices only was significantly greater than the incidence of appropriate shock in patients with channelopathy and primary prevention devices (52.94%, 95% CI 30.96%-73.84% vs 4.35%, 95% CI 0.22%-20.99%, p = 0.0007). The incidence of appropriate shocks was higher in patients with a secondary prevention indication as compared to those with a primary prevention indication (50%, 95% CI 32.63%-67.38% vs. 25%, 95% CI 14.19%-40.19%, p = 0.04). The hazard ratio for appropriate shock of an ICD placed for secondary compared to primary prevention was 2.21 (1.07–4.56), p = 0.03. The hazard ratio for the incidence of appropriate shock in patients with cardiomyopathy and primary prevention devices compared to patients with channelopathy and primary prevention devices was 5.04 (1.64–15.48), p = 0.005. There was no difference in appropriate shock rate between patients with a single lead ICD compared to multi lead device. Device indication (primary vs secondary) was the only significant predictor of appropriate shock on cox regression analysis (Table 3, HR 2.1, CI 1.04–4.35, p = 0.039).
Table 2.
5-year cumulative incidence of appropriate therapy and ICD complications.
| Overall |
Diagnosis Type |
Indication |
Device Type |
||||
|---|---|---|---|---|---|---|---|
| Cardiomyopathy | Channelopathy | Primary | Secondary | Single-Lead | Multi-Lead | ||
| Appropriate Shock | 35.29% | 56.52%* | 24.44%* | 25.00%* | 50.00%* | 38.46% | 25.00% |
| Inappropriate Shock | 23.53% | 13.04% | 28.89% | 22.50% | 25.00% | 13.46%* | 56.25%* |
| Lead Complication | 23.53% | 26.09% | 22.22% | 20.00% | 28.57% | 17.31%* | 43.75%* |
Indicates p < 0.05.
Table 3.
Cox regression predictors of appropriate shock, inappropriate shock and lead complication.
| Appropriate Shock | Inappropriate Shock | Lead Complication | |
|---|---|---|---|
| Age at Implant | 0.99 (0.97–1.01) | 1.00 (0.97–1.03) | 1.01 (0.98–1.03) |
| Gender | 0.59 (0.28–1.26) | 1.72 (0.64–4.62) | 2.13 (1.02–4.46) * |
| BMI | 1.06 (1.00–1.12) | 1.00 (0.91–1.10) | 1.00 (0.93–1.08) |
| Proband | 0.34 (0.10–1.11) | 0.84 (0.24–2.97) | 0.63 (0.22–1.81) |
| Symptomatic | 0.34 (0.05–2.47) | 0.72 (0.09–5.47) | 0.49 (0.07–3.66) |
| Comorbidities | 0.62 (0.30–1.27) | 0.39 (0.13–1.11) | 0.85 (0.41–1.78) |
| Family History | 1.76 (0.81–3.83) | 1.09 (0.40–3.02) | 1.03 (0.47–2.23) |
| EF | 1.96 (0.02–157.63) | 0.02 (0.00–5.45) | 0.04 (0.00–5.44) |
| LAVI | 1.03 (1.00–1.07) | 1.04 (0.98–1.09) | 1.02 (0.98–1.07) |
| % V Paced | 1.10 (0.24–5.10) | 2.33 (0.49–11.13) | 3.56 (0.96–13.14) |
| % A Paced | 14.26 (0.78–261.04) | 1.10 (0.18–6.87) | 2.68 (0.49–14.64) |
| Indication | 2.13 (1.04–4.35) * | 1.37 (0.51–3.68) | 1.15 (0.55–2.40) |
| Diagnosis type | 0.54 (0.26–1.10) | 1.70 (0.55–5.28) | 0.82 (0.38–1.78) |
| Device Type | 1.80 (0.74–4.40) | 0.32 (0.12–0.86) * | 0.59 (0.28–1.27) |
Abbreviations: % A Paced: Percent of time paced form the atria, % V Paced: Percent of time paced from the ventricle, BMI: Body Mass Index, EF: Ejection Fraction, LAVI: Left Atrial Volume Index
Indicates p < 0.05.
Fig. 1.
Cumulative incidence curves of first appropriate therapy and first device complication.
3.2.2. Inappropriate Shock.
A total of 19 patients received a total of 53 inappropriate shocks (mean 2.8 ± 2.6 shocks per patient). The 5-year cumulative incidence of inappropriate shocks was 23.53% (15.03%-34.86%) in the overall cohort. The most common etiologies of first inappropriate shock were lead failure (8 events, 42.1%) and SVT (7 events, 36.8%). Other causes of first inappropriate shock included oversensing (2 events, 10.53%), and EM interference (1 event, 5.3%). Of the 7 patients who experienced an inappropriate shock due to SVT, 5 had a single lead ICD and 2 had a dual chamber device. In addition, one patient had an inappropriate shock with no identifiable etiology. Patients with multi-lead devices had a significantly higher rate of inappropriate shock as compared to those with a single lead device (56.25%, CI 33.18%-76.9% vs. 13.46%, CI 6.68%-25.27%, p = 0.001). The hazard ratio of a multi-lead device for an inappropriate shock was 3.99 (1.27–12.52, p = 0.02). On Cox regression analysis, device type was the only significant predictor of inappropriate shock (HR 0.32, CI 0.12–0.86, p = 0.024).
3.2.3. Lead Complication.
A total of 39 lead complications occurred in 31 patients for a 5-year cumulative incidence of rate of 23.53% (15.03%-34.86%) in the overall cohort. 7 patients were implanted with a Sprint Fidelis Lead. The most frequent type of lead complication was lead fracture/noise, which occurred in 23 patients (59% of lead complications). There was no difference in lead complication rate between patients with a cardiomyopathy and channelopathy, nor between primary prevention and secondary prevention devices. The lead complication rate was significantly higher in patients with a multi lead device when compared to the rate in those with a single lead device, 43.75% CI 23.10%-66.82% vs. 17.31%, CI 9.38%-29.73%, p = 0.04. On Cox regression analysis, female relative to male gender was the only significant predictor of lead complication (HR 2.13, CI 1.02–4.46, p = 0.045).
4. Discussion
Implantable cardiac defibrillators have been utilized in patients with inherited arrhythmia syndromes to prevent sudden cardiac death but have been associated with relatively high complication rates. In this study of patients with inherited arrhythmic syndromes who had an ICD placed for prevention of SCD the main findings are:
-
1.
Appropriate shocks and device complications are frequent in inherited arrhythmia patients with an ICD.
-
2.
Appropriate shock is more frequent in patients with a primary cardiomyopathy diagnosis as compared to primary channelopathy diagnosis, regardless of left ventricular ejection fraction at implantation.
-
3.
Inappropriate shock driven by lead failure is significantly more frequent and partially preventable in patients with multi lead devices.
Our observed 5-year incidence of appropriate shock of 38.2% is higher than data reported previously in a similar population [5], [6]. A large meta-analysis of patients with inherited arrhythmia identified a 12.5%-30.5% extrapolated 5-year risk of appropriate shock in patients with primary and secondary prevention indication, respectively [4]. The 5-year incidence of lead complications seen in this study of 23.53% was similar to that seen in prior studies [5], [6].
The higher appropriate shock event rate observed in our study may be partially driven by our patient population which includes a relatively large representation of Arrhythmogenic Cardiomyopathy. This translates to a higher rate of appropriate shock concentrated among our patients with cardiomyopathy, and particularly ARVC, which is a condition known to carry a high rate of appropriate shock [7]. It is worth noting that the excess risk for appropriate shock in the cardiomyopathy group was independent of left ventricular ejection fraction, which is an important predictor of therapy in general populations with ICD. It is therefore important to consider that in patients with heritable cardiomyopathies such as Arrhythmogenic Cardiomyopathy and HCM, LV function does not predict arrhythmic risk, and even when LV dysfunction occur, a concealed arrhythmic phase often precedes overt structural and functional changes [8]. As expected, patients with an ICD placed for secondary prevention were at increased risk for appropriate shock as compared to primary prevention.
Complication rates were significantly higher in patients with multi-lead devices compared to single lead ICDs confirming previous observations from typical mixed populations of patients with ICDs [9], [10]. Patients with multi-lead devices also had a significantly higher inappropriate shock rate. This finding can be explained by the fact that lead failure was the primary cause of inappropriate shock, conferring a greater risk in those with multi-lead devices. Clinical guidelines including ACC/ESC/HRS consensus statements have defined the indications for dual chamber device placement, discouraging empiric implantation of an atrial lead in patients receiving an ICD. In turn, the addition of an atrial lead to ICD system should be generally reserved for patients with significant sinus node dysfunction. [11], [12], [13] Indeed, we found that 26.5% of our patients with a dual lead ICD had either persistent atrial fibrillation or very low atrial pacing requirements. This suggests that in a substantial proportion of our followed patients, implanted in multiple outside institutions, a single lead rather than dual lead ICD could have been utilized, potentially reducing complication rate. Alternatively, subcutaneous ICDs could be considered in this population as a method of reducing the rate of lead complications though additional information on how the safety and efficacy of these devices compares to transvenous models is needed [14]. These considerations are of particular importance in patients implanted at a younger age where the amortized risk of lead related complication is high.
Taken together, our data confirms a substantial risk of both appropriate shock and device related complications in patients with inherited arrhythmia. In recent years, there has been progress in the ability to risk stratify patients with conditions such as Long QT Syndrome,[15] CPVT [16] and Brugada syndrome,[17], [18] resulting in better characterization of those who are truly at a very high risk. In addition, therapy with Quinidine for Brugada syndrome[19], Nadolol for LQTS [20] and Flecainide for CPVT[21], and interventions for left sympathetic denervation have been demonstrated to substantially reduce arrhythmic risk, even in symptomatic patients with ventricular arrhythmia [22], [23]. Consequently, many patients may be safely treated without the need for an ICD. Our data stresses the importance of avoiding unnecessary device implantation. As recently recommended by society guidelines,[24], [25] management of patients with complex heritable cardiovascular disease by specialized inherited arrhythmia centers with specific expertise may help ensure that the right patients receive the right treatments.
4.1. Limitations
This study includes a limited sample size. This is partially mitigated by data collection which was prospective as part of a dedicated registry of all patients followed in our program. The number of patients with individual conditions was too small to allow subgroup analysis by diagnosis, which could be investigated in future studies. In addition, our study did not examine the role of device manufacturer or device programming on outcomes which should be evaluated in future studies. However, all devices were programmed based on HRS recommendations [26].
5. Conclusion
The observed rate of appropriate ICD shock and ICD-related complications in young patients with inherited arrhythmia syndromes is high particularly in patients with cardiomyopathies, those with a secondary prevention indication, and those with a multi-lead device. These findings further emphasize the importance of ICD therapy in this population and the importance of careful risk/benefit considerations when assessing the indication and type of device in this population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None declared.
References
- 1.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Hocini M., Pison L., Proclemer A., Larsen T.B., Madrid A., Blomstrom-Lundqvist C. Diagnosis and management of patients with inherited arrhythmia syndromes in Europe: results of the European Heart Rhythm Association Survey. Europace. 2014;16:600–603. doi: 10.1093/europace/euu074. [DOI] [PubMed] [Google Scholar]
- 3.Sherrid M.V., Daubert J.P. Risks and Challenges of Implantable Cardioverter-Defibrillators in Young Adults. Prog Cardiovasc Dis Nov-Dec. 2008;51:237–263. doi: 10.1016/j.pcad.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Nordkamp L.R.A.O., Postema P.G., Knops R.E. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–454. doi: 10.1016/j.hrthm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Quast A.B.E., Brouwer T.F., Kooiman K.M. Comparison of complications and shocks in paediatric and young transvenous and subcutaneous implantable cardioverter-defibrillator patients. Netherlands Heart Journal. 2018;26:612–619. doi: 10.1007/s12471-018-1186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen L.D.D., Christiansen M.K., Pedersen L.N. Implantable cardioverter-defibrillator therapy and device-related complications in young patients with interited cardiomyopathies or channelopathies: a 17-year cohort study. Europace. 2018;20:1849–1855. doi: 10.1093/europace/euy081. [DOI] [PubMed] [Google Scholar]
- 7.Orgeron G.M., James C.A., Te Riele A. Implantable Cardioverter-Defibrillator Therapy in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy: Predictors of Appropriate Therapy, Outcomes, and Complications. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.117.006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Voorn S.M., te Riele A.S.J.M., Basso C., Calkins H., Remme C.A., van Veen T.A.B. Arrhythmogenic cardiomyopathy: pathogenesis, pro-arrhythmic remodelling, and novel approaches for risk stratification and therapy. Cardiovascular Research. 2020;116:1571–1584. doi: 10.1093/cvr/cvaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins N.M., Grubisic M., Andrade J.G. Long-term complications, reoperations and survival following cardioverter-defibrillator implant. Heart. 2018;104:237–243. doi: 10.1136/heartjnl-2017-311638. [DOI] [PubMed] [Google Scholar]
- 10.Baskar S., Bao H.K., Minges K.E., Spar D.S., Czosek R.J. Characteristics and Outcomes of Pediatric Patients Who Undergo Placement of Implantable Cardioverter Defibrillators Insights From the National Cardiovascular Data Registry. Circ-Arrhythmia Elec. 2018;11 doi: 10.1161/CIRCEP.118.006542. [DOI] [PubMed] [Google Scholar]
- 11.Gillis A.M., Russo A.M., Ellenbogen K.A. HRS/ACCF Expert Consensus Statement on Pacemaker Device and Mode Selection Developed in partnership between the Heart Rhythm Society (HRS) and the American College of Cardiology Foundation (ACCF) and in collaboration with the Society of Thoracic Surgeons. Heart Rhythm. 2012;9:1344–1365. doi: 10.1016/j.hrthm.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Kusumoto F.M., Schoenfeld M.H., Barrett C.N. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society (vol 74, pg e51, 2019) Journal of the American College of Cardiology. 2019;74:1016–1018. doi: 10.1016/j.jacc.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Kusumoto F.M., Schoenfeld M.H., Barrett C. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: Executive Summary. Journal of the American College of Cardiology. 2019;74:932–987. doi: 10.1016/j.jacc.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Basu-Ray I., Liu J., Jia X. Subcutaneous versus tranvenous implantable defibrillator therapy: a meta analysis of case-control studies. JACC: Clinical Electrophysiology. 2017:1475–1483. doi: 10.1016/j.jacep.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 15.I. Goldenberg, Genotype-Phenotype Correlation in Congenital LQTS: Implications for Diagnosis and Risk Stratification. Cardiac Repolarization: Basic Science and Clinical Management 2020141-164.
- 16.Pflaumer A., Wilde A.A.M., Charafeddine F., Davis A.M. 50 Years of Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) - Time to Explore the Dark Side of the Moon. Heart Lung and Circulation. 2020;29:520–528. doi: 10.1016/j.hlc.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Honarbakhsh S., Providencia R., Garcia-Hernandez J. A Primary Prevention Clinical Risk Score Model for Patients With Brugada Syndrome (BRUGADA-RISK) JACC Clin Electrophysiol. 2021;7:210–222. doi: 10.1016/j.jacep.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Ojeda J., Arbelo E., Jorda P. The role of clinical assessment and electrophysiology study in Brugada syndrome patients with syncope. American heart journal. 2020;220:213–223. doi: 10.1016/j.ahj.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Mazzanti A., Tenuta E., Marino M. Efficacy and Limitations of Quinidine in Patients With Brugada Syndrome. Circ-Arrhythmia Elec. 2019;12 [Google Scholar]
- 20.Mazzanti A., Maragna R., Vacanti G. Interplay Between Genetic Substrate, QTc Duration, and Arrhythmia Risk in Patients With Long QT Syndrome. J Am Coll Cardiol. 2018;71:1663–1671. doi: 10.1016/j.jacc.2018.01.078. [DOI] [PubMed] [Google Scholar]
- 21.Kannankeril P.J., Moore J.P., Cerrone M. Efficacy of Flecainide in the Treatment of Catecholaminergic Polymorphic Ventricular Tachycardia A Randomized Clinical Trial. Jama Cardiology. 2017;2:759–766. doi: 10.1001/jamacardio.2017.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe H., Chopra N., Laver D. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nature Medicine. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niaz T., Bos J.M., Sorensen K.B., Moir C., Ackerman M.J. Left Cardiac Sympathetic Denervation Monotherapy in Patients With Congenital Long QT Syndrome. Circ-Arrhythmia Elec. 2020;13 doi: 10.1161/CIRCEP.120.008830. [DOI] [PubMed] [Google Scholar]
- 24.Stiles M.K., Wilde A.A.M., Abrams D.J. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm. 2021;18:E1–E50. doi: 10.1016/j.hrthm.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.G. Conte, A. Wilde, E.R. Behr, et al. Importance of Dedicated Units for the Management of Patients With Inherited Arrhythmia Syndromes. Circ Genom Precis Med Apr 2 2021CIRCGEN120003313. [DOI] [PMC free article] [PubMed]
- 26.Stiles M.K., Fauchier L., Morillo C.A., Wilkoff B.L. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter- defibrillator programming and testing. Heart Rhythm. 2019;17:e220–e228. doi: 10.1016/j.hrthm.2019.02.034. [DOI] [PubMed] [Google Scholar]