Graphical abstract
Abbreviations: 3-CQA, 3-O-caffeoylquinic acid; 4-CQA, 4-O-caffeoylquinic acid; 5-CQA, 5-O-caffeoylquinic acid; AA, ascorbic acid; EGCG, epigallocatechin gallate
Keywords: Caffeoylquinic acid, Isomerization, Ultrasound, Degradation kinetics, Weibull equation
Abstract
Caffeoylquinic acids are existed in many plant species with various biological and pharmacological activities. 3-O-caffeoylquinic acid and 4-O-caffeoylquinic acid are two isomers of caffeoylquinic acids, which may be degraded and transformed to their isomers in processing. The present paper found that the stability of 3- and 4-O-caffeoylquinic acid had decreased with the increasing solution alkalinity. 3-O-caffeoylquinic acid was more stable than 4-O-caffeoylquinic acid at the same condition. During degradation, 3- and 4-O-caffeoylquinic acid were partially converted to their isomers. Additionally, ultrasonic effects on the degradation and isomerization of 3- and 4-O-caffeoylquinic acid at different pH were studied. Ultrasound facilitated the degradation and isomerization of these compounds. The degradation kinetics were described by the Weibull equation. The protective effect of ascorbic acid and epigallocatechin gallate were also explored. Ascorbic acid and epigallocatechin gallate could alleviate the degradation of 3- and 4-O-caffeoylquinic acid under certain conditions.
1. Introduction
Caffeoylquinic acid (CQA) is one of the phenylpropanoids existing in coffee beans, Lonicerae Japonicae Flos, chrysanthemum, sweet potato and many other traditional Chinese medicines and foods. Based on the number or the position of caffeoyl groups, caffeoylquinic acids are classified into various derivatives, among which mono-CQAs are the most abundant [1]. Various biological and pharmacological effects of CQAs have been reported, such as antioxidant, antimutagenic, antihypertensive and anti-carcinogenic activities [2]. These potential health benefits have led the CQAs to many promising applications in pharmaceuticals, foodstuffs, and cosmetics [3].
However, CQAs are not stable and can be degraded or isomerized under some conditions. During blanching, baking and storage, the contents of 5-CQA and 3,5-diCQA decreased rapidly with the increase of temperature and time [4], [5], [6]. It has been reported that 3-CQA and 4-CQA are prone to degradation and isomerization due to the influence of temperature, pH, light and other factors [7]. Zhu et al. found that the transformation between 5-CQA, 3-CQA and 4-CQA were related to pH value. During the degradation of 5-CQA and 3-CQA, 4-CQA was produced preferentially, while during the degradation of 4-CQA, 3-CQA was produced preferentially [8]. Kamiyama et al. [9] studied the degradation of 5-CQA in aqueous solutions with different pH values. The degradation rate of 5-CQA within 2 h was 8.46% at pH 3.4, 49.92% at pH 4, 63.59% at pH 6, and 99.99% at pH 12, illustrating a pH-depended stability. Apparently, processing conditions have a great influence on the stability of some CQAs. Since ascorbic acid (AA) and epigallocatechin gallate (EGCG) have been acclaimed as the potent antioxidants and free-radical scavengers, they may inhibit the degradation of 3- and 4-CQA [10], [11].
Nowadays, ultrasound has been developed for the extraction of many bioactive substances from plant resources in light of its thermal and mechanical benefits to increase the yield of extracts. For example, it was reported to help extract phenolic substances from artichoke by-products, including 5-CQA and 1,5-diCQA [12]. Saleh et al. found that ultrasound-assisted extraction was faster than traditional methods for the extraction of chlorogenic acid. However, due to the high cavitation activity, the chlorogenic acid level decreased after 15 min of the treatment [13].
Therefore, studying the stability of mono-CQAs under ultrasonic condition is of great practical significance to expand their applications. In the present work, (1) the ultrasonic effect on the stability of 3- and 4-CQA at different pH was studied; (2) protective effects of AA and EGCG on 3- and 4-CQA were studied under several pH conditions; (3) the degradation kinetics and isomerization pattern of 3- and 4-CQA were explored at different pH with and without ultrasound, which are expected to lay a theoretical foundation for further clarification of the degradation pathway and mechanism of CQAs.
2. Materials and methods
2.1. Materials
Puerarin (≥99.71%), 3-CQA (≥99.37%), 4-CQA (≥99.39%), 5-CQA (≥99.07%) and EGCG were purchased from Chengdu Must Biotechnology Co., Ltd (Chengdu, China). Methanol and acetonitrile for HPLC were obtained from Scharlau Co., Ltd (Barcelona, Spain). AA was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). All other chemicals were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
2.2. Ultrasonic experimental set-up
The solutions of 3-CQA/4-CQA at different pH were prepared by mixing 2 mL of 3-CQA/4-CQA (1 mg/mL, in 50% methanol) with 18 mL of different buffer solutions (i.e., 0.2 M acetic acid buffer, pH 4.69; 0.2 M phosphoric acid buffer, pH 7.05; 0.15 M boric acid buffer, pH 7.96; 0.1 M carbonate buffer, pH 9.22). After shaking and mixing, the solutions were treated by a probe ultrasound machine (JY92-IIDN ultrasonic cell pulverizer, Ningbo Scientz Biotechnology Co., Ltd., China) with a 6 mm ultrasonic probe creating 22 kHz frequency. The samples were sonicated under the ultrasonic power of 270 W using a duty cycle of 50% (2 s on, 2 s off). During the ultrasonic process, the desired temperatures of samples were controlled at 40℃ by a circulating water bath (QYDC − 1006, Shanghai Joyn Electronic CO., Ltd., China). The liquid height (distance from liquid surface to bottle bottom) was kept at 3 cm. At each time intervals (10, 30, 60, 90, 120, 150, 180, 210 min), 100 μL sample was taken out and immediately mixed with 10 μL puerarin (2 mg/mL, in 50% methanol) and 890 μL mobile phase (0.1% phosphoric acid-acetonitrile, 4:1). Puerarin was added as an internal standard. Additionally, the solutions of 3-CQA and 4-CQA were treated at 40℃ water bath as the blank control. 100 μL sample was taken out at the each time point and mixed with puerarin as mentioned above, the same as the ultrasound-treated one. Each sample was measured in triplicate.
2.3. Caffeoylquinic acid determination
The content of caffeoylquinic acid was measured using an Agilent 1260 HPLC system (Agilent, US), equipped with a UV detector. Filtered samples (10 μL) were injected into a Phenomenon Luna-C18 column (250 mm × 4.6 mm, 5 μm) at 35℃. Samples were eluted by mobile phases A (acetonitrile) and mobile phase B (0.1% phosphoric acid) at a flow rate of 0.8 mL/min with the gradient program as follows: 0–11 min, A 10%–18%; 11–16 min, A 18%; 16–20 min, A 18%–10%. The detection wavelength was 330 nm. The concentration was calculated by internal standard method.
2.4. Effects of EGCG and AA
The solution of 3- and 4-CQA (2 mL, 2.8 mmol/L) in 50% (v/v) methanol was mixed with 16 mL buffer solutions of different pH (as mentioned in 2.3) respectively. Before the ultrasonic treatment, 2 mL of the antioxidant (EGCG or AA) was added. The final molar concentration ratio of the antioxidant and CQAs was 2:1, 1:1 or 1:2. Then the same ultrasonic treatment procedures as mentioned above were repeated.
2.5. Degradation kinetics at different pH
The degradation kinetics of 3- and 4-CQA at different pH were fitted by the Weibull model, a probability distribution function [14], [15]:
where k is the reaction rate constant; n is a shape constant (n > 1, the function is an increasing function; n < 1, the function is a decreasing function; n = 1, the function satisfies the exponential distribution); Ci is the concentration of CQAs at a certain time, and C0 is the initial concentration.
According to the above equation, the values of degradation parameters k and n can be calculated by nonlinear least square fitting with Origin 2019 software. The half-life of degradation (t1/2) can be calculated as follow:
2.6. Statistical analysis
All experiments were conducted in triplicate, leading to achieve the evaluation by the mean ± standard deviation (SD). The figures were plotted by Origin Software Version 8.5 (OriginLab Corp., MA, USA).
3. Results and discussion
3.1. Degradation kinetics of 3-CQA
A typical chromatogram is shown in Fig. S1, in which the peaks of 5-CQA, 3-CQA, 4-CQA and the internal standard have shown good separation resolutions.
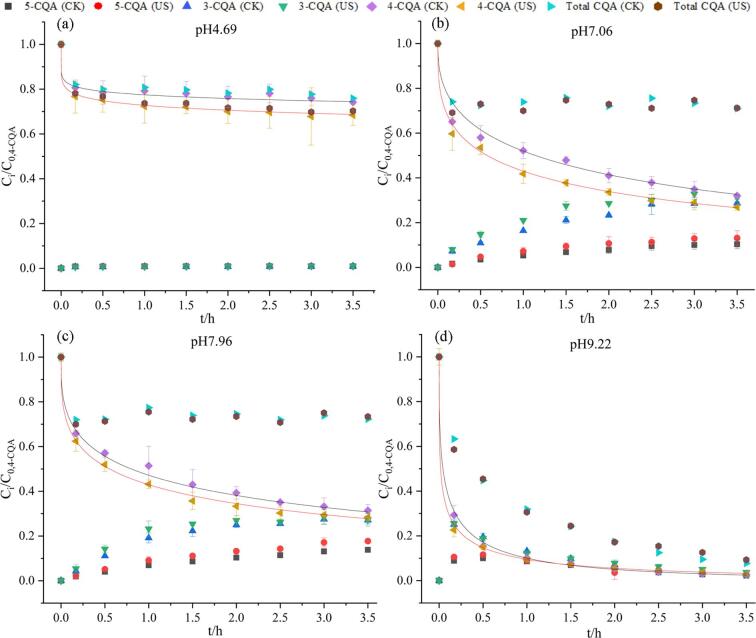
The degradation kinetics of 3-CQA under an ultrasonic treatment at different pH(pH 4.69、7.06、7.96 and 9.22)are shown in Fig. 1. The kinetics parameters were calculated and are listed in Table 1. The degradation curves of 3-CQA (solid curve in Fig. 1) in different pH buffer solutions were obtained by using the origin 2019 software and Weibull model. The Weibull distribution is a probability distribution function, used to fit the degradation kinetics of compounds.
Fig. 1.
Ultrasonic effects on the degradation and isomerization of 3-CQA at different pH: (a) pH 4.69, (b) pH 7.06, (c) pH 7.96, (d) pH 9.22, CK: Control, US: Ultrasound.
Table 1.
Kinetic parameters of ultrasonic degradation of 3-CQA and 4-CQA at different pH.
| pH | treatment | 3-CQA | 4-CQA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k | n | t1/2/h | R2 | k | n | t1/2/h | R2 | ||
| 4.69 | CK | 4.09E-07 | 0.109 | 85,457 | 0.995 | 7.77E-06 | 0.116 | 5447 | 0.999 |
| US | 6.56E-05 | 0.159 | 1512 | 0.999 | 1.84E-04 | 0.133 | 347 | 0.999 | |
| 7.06 | CK | 0.051 | 0.374 | 7.303 | 0.990 | 0.373 | 0.430 | 1.142 | 0.996 |
| US | 0.176 | 0.443 | 2.490 | 0.999 | 0.623 | 0.358 | 0.577 | 0.999 | |
| 7.96 | CK | 0.263 | 0.531 | 1.903 | 0.998 | 0.451 | 0.360 | 0.800 | 0.998 |
| US | 0.377 | 0.440 | 1.154 | 0.992 | 0.603 | 0.333 | 0.552 | 0.999 | |
| 9.22 | CK | 3.854 | 0.514 | 0.127 | 0.999 | 8.860 | 0.381 | 0.043 | 0.998 |
| US | 6.712 | 0.420 | 0.062 | 0.999 | 17.35 | 0.300 | 0.017 | 0.998 | |
CK: Control without treatment, US: Samples treated with ultrasound.
The stability of 3-CQA under the ultrasonic condition was closely related to the pH value of the system. 3-CQA was stable at pH 4.69 (as shown in Fig. 1a), but its degradation was accelerated by ultrasound. In addition, there was almost no isomerization of 3-CQA to 4-CQA or 5-CQA at pH 4.69. In comparison, under the neutral and alkaline conditions, the stability of 3-CQA was weakened. Moreover, the isomerization of 3-CQA to the other two isomers (4-CQA and 5-CQA) was enhanced (shown in Fig. 1 and Table S1). With the increase of alkalinity, the degradation degree of 3-CQA gradually increased. On the other hand, ultrasound significantly promoted the degradation and isomerization of the samples, which is ascribed to the ultrasonic stress. This result is in agreement with that of another study made by Cuéllar-Villarrea et al. who also observed the degradation of 3-CQA and other nutraceuticals in carrots by ultrasound [16]. Besides, the reactive species (e.g., OH radicals and hydrogen peroxide) formed by ultrasonic cavitation can react with CQAs, which contributed to the reduction of concentration of CQAs [17].
Among the above mentioned different pH conditions, pH 7.96 resulted in the most remarkable isomerization of 3-CQA. A similar phenomenon was observed by Dawidowicz and Typek [18]. From the experimental results, we can see that the isomerization of 3-CQA produced more 4-CQA than 5-CQA. The reason may be that 3-CQA was transformed to 4-CQA first, and then further transformed to 5-CQA. It can be observed from the content change of 5-CQA along with the treatment time (Fig. 1b&c). At pH 7.96 and pH 7.06, no 5-CQA was detected within 10 min.
At pH 9.22 (Fig. 1d), 3-CQA was degraded significantly no matter it was treated with or without the ultrasonic treatment. However, ultrasound obviously accelerated the decrease of 3-CQA content. The contents of 4-CQA and 5-CQA increased when 3-CQA decreased at the same time within 90 min. The decreased content of 3-CQA was much higher than the increased contents of 4-CQA and 5-CQA. Moreover, the total caffeoylquinic acid content decreased within the processing time, indicating that the degradation was stronger than the isomerization at pH 9.22. The contents of 4-CQA and 5-CQA increased first and then decreased with treatment time. The content increase of 4- and 5-CQA is due to the transformation of 3-CQA, while the content decrease is due to the severe degradation of 4- and 5-CQA. With the ultrasound treatment, 5-CQA content was higher than that of the control within 60 min, while 4-CQA content was similar to the control (Fig. 1d). It indicated that ultrasound facilitated the isomerization of 3-CQA to 5-CQA instead of 4-CQA. For the sample without the ultrasound, the concentration of 5-CQA increased slowly, reached the maximum value at 30 min, maintained to 60 min, and then decreased slowly. Compared with the control, ultrasound advanced the time when the content of 5-CQA began to decrease from 60 min to 30 min (Fig. 1d). It may be because that ultrasound not only accelerated the isomerization of 3-CQA to 5-CQA, but also accelerated the degradation and isomerization of 5-CQA.
At pH 7.96 (Fig. 1c), the total concentration of caffeoylquinic acid did not change much, but was decreased after the ultrasonic treatment. With the increasing treatment time, the concentration of 3-CQA decreased, while the concentration of 4-CQA and 5-CQA increased as the result of isomerization (Fig. 1c). Ultrasound promoted the concentration decrease of 3-CQA. Meanwhile, the concentration of 4-CQA in the ultrasonic samples increased before 120 min, which was always higher than that in the control, illustrating that ultrasound accelerated the isomerization of 3-CQA to 4-CQA. However, after 120 min, the concentration of 4-CQA in ultrasonic samples decreased with time, even lower than that in the control. This indicated that ultrasound promoted the degradation of 4-CQA after 120 min. The concentration of 5-CQA in the ultrasonic sample increased with time and was always higher than that in the control, confirming the ultrasonic-accelerated transformation of 3-CQA into 5-CQA. There are two possible reasons for the different changes of 4-CQA and 5-CQA caused by the ultrasound: (1). Because the stability of 4-CQA was lower than that of 5-CQA, the prolongation of ultrasonic time can lead to 4-CQA degradation more easily; (2). When the concentration of 4-CQA reached a certain level, ultrasound could promote the conversion of 4-CQA to 5-CQA.
At pH 7.06 (Fig. 1b), the concentration of total caffeoylquinic acid didn’t change significantly along with time. The concentration of 3-CQA decreased continuously, similar to pH 7.96. After ultrasonic treatment, the concentration of total caffeoylquinic acid and 3-CQA both declined compared to the control. Because of the ultrasound-accelerated isomerization of 3-CQA to 4-CQA, the concentration of 4-CQA in ultrasonic samples increased with time before 150 min, and was always higher than that of the control. After 150 min, the concentration of 4-CQA decreased slightly, but still higher than that of the control. The concentration of 5-CQA in the ultrasonic sample increased with treatment time, and was always higher than that in the control. It may be the same reason as pH 7.96 that ultrasound accelerated the degradation of 4-CQA and the isomerization of 4-CQA to 5-CQA.
The degradation kinetic parameters k, n and t1/2 of 3-CQA under different pH conditions are listed in Table 1. It can be seen in Table 1 that with the increase of pH, k increased while t1/2 decreased. The t1/2 value of ultrasonic samples at different pH was substantially smaller than that of the untreated control. The degradation rate instant k of ultrasonic samples was larger than that of the untreated control. It can be inferred that the degradation of 3-CQA was accelerated by ultrasound. With the increase of pH, the increased t1/2 value and the decreased k value showed that the stability of 3-CQA reduced.
3.2. Degradation kinetics of 4-CQA
Weibull model was used to describe the degradation of 4-CQA in different pH buffers (solid curve in Fig. 2). The change of concentration of 4-CQA and 5-CQA was also analyzed. The degradation kinetic parameters k, n, R2 and t1/2 of 4-CQA at different pH are shown in Table 1. The stability of 4-CQA under ultrasonic condition has a great relationship with pH. It can be seen from Fig. 2 that 4-CQA is most stable at pH 4.69. With the increase of alkalinity, the degradation degree gradually increased. Ultrasound facilitated the degradation of samples. Furthermore, under neutral and alkaline conditions, 4-CQA is easy to be degraded and isomerized to the other two isomers (i.e., 3-CQA and 5-CQA). The isomerization is most obvious under neutral conditions (shown in Fig. 2 and Table S2). Since higher pH favors the degradation of the CQA isomers [18].
Fig. 2.
Ultrasonic effects on the degradation and isomerization of 4-CQA at different pH: (a) pH 4.69, (b) pH 7.06, (c) pH 7.96, (d) pH 9.22, CK: Control, US: Ultrasound.
The isomerization of 4-CQA to both 3- and 5-CQA was observed. The content of converted 3-CQA was higher than that of 5-CQA at all pH conditions. In most cases, ultrasound could facilitate the isomerization of 4-CQA. For example, Alves Filho et al. [17] studied ultrasound induced hydrolysis of CQAs, and found that ultrasound could increase the acyl migration between 5-CQA, 4-CQA and 3-CQA isomers.
At pH 9.22 (Fig. 2d), the concentration of 4-CQA and total caffeoylquinic acid decreased with the increase of treatment time. Within 90 min of treatment time, the concentration of 4-CQA was further decreased by ultrasound. The concentration of 3-CQA increased in the first 10 min, and then decreased with time. The concentration of 5-CQA increased first until 30 min, and then decreased. These phenomena indicated that the degradation of 3-CQA and 5-CQA was quite remarkable at pH 9.22. Ultrasound promoted the isomerization of 4-CQA to 5-CQA, thus the concentration of 5-CQA in the ultrasonic sample was higher than that in the control. In contrast, the concentration of 3-CQA in the ultrasonic sample was lower than that in the control. This phenomenon was ascribed to the better acceleration of ultrasound to the degradation of 3-CQA than the conversion of 4-CQA to 3-CQA. The concentration of total caffeoylquinic acids in the ultrasonic samples was lower than that in the control before 120 min, but higher than that in control after 120 min. Since the stability of 3-CQA and 5-CQA is higher than that of 4-CQA, ultrasound-promoted isomerization is conducive to slowing the reduction of total caffeoylquinic acids.
At pH 7.96 (Fig. 2c), with the prolongation of treatment time, the concentration of 4-CQA decreased, while the concentration of 3-CQA and 5-CQA increased. Ultrasound accelerated the degradation of 4-CQA, as well as the isomerization of 4-CQA to 3-CQA and 5-CQA. However, the concentration of total caffeoylquinic acids did not change much, which was lowered by ultrasound before 150 min.
At pH 7.06 (Fig. 2b), the concentration of 4-CQA decreased continuously, whereas the concentration of total caffeoylquinic acids changed little. Ultrasound accelerated the concentration decrease of 4-CQA. With ultrasound treatment, the concentration of 3-CQA increased with time until it reached the maximum value at 180 min, which was always higher than that in the control. Meanwhile, the concentration of 5-CQA in the ultrasonic sample was always higher than that in the control, and raised with time. It indicated that ultrasound accelerated the isomerization of 4-CQA to both 3-CQA and 5-CQA.
As shown in Table 1, the rate constant k increased and t1/2 decreased significantly with the increase of pH, revealing a decreased stability of 4-CQA with the increase of pH. The t1/2 value of ultrasonic samples at different pH was lower than that of the control, while the k value of ultrasonic samples was higher than that of the control. It was due to the ultrasound-accelerated degradation and isomerization of 4-CQA. The difference of k value between ultrasonic samples and the control increased with the increase of pH. This is because with the increase of pH, the stability of 4-CQA decreased, which made it easier to be attacked by ultrasound. Comparing the results of both 3- and 4-CQA, it can be found that 3-CQA was more stable than 4-CQA at all pH conditions (Table 1).
3.3. Effect of AA
Because of the instability of 3- and 4-CQA at pH 7.06, 7.96 and 9.22, AA was added to protect CQAs from degradation. The degradation curves are shown in Fig. S2 & S3, and the parameters of degradation kinetics are listed in Table 2. The degradation rate constant k and the half-life time t1/2 of degradation are key criteria to evaluate the degradation rate.
Table 2.
Kinetic parameters of degradation of 3-CQA and 4-CQA at different pH with and without AA.
| pH | AA content | treatment | 3-CQA | 4-CQA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | n | t1/2/h | R2 | k | n | t1/2/h | R2 | |||
| 7.06 | 0 | CK | 0.051 | 0.374 | 7.303 | 0.990 | 0.373 | 0.430 | 1.142 | 0.996 |
| US | 0.176 | 0.443 | 2.490 | 0.999 | 0.623 | 0.358 | 0.577 | 0.999 | ||
| 1:2 | CK | 0.081 | 0.408 | 5.000 | 0.999 | 0.286 | 0.400 | 1.398 | 0.988 | |
| US | 0.153 | 0.429 | 2.789 | 0.997 | 0.504 | 0.380 | 0.756 | 0.999 | ||
| 1:1 | CK | 0.086 | 0.417 | 4.822 | 0.998 | 0.308 | 0.411 | 1.329 | 0.995 | |
| US | 0.148 | 0.429 | 2.867 | 0.998 | 0.545 | 0.350 | 0.642 | 0.997 | ||
| 2:1 | CK | 0.054 | 0.385 | 7.098 | 0.997 | 0.294 | 0.391 | 1.334 | 0.996 | |
| US | 0.129 | 0.434 | 3.324 | 0.998 | 0.552 | 0.342 | 0.619 | 0.993 | ||
| 7.96 | 0 | CK | 0.263 | 0.531 | 1.903 | 0.998 | 0.451 | 0.360 | 0.800 | 0.998 |
| US | 0.377 | 0.440 | 1.154 | 0.992 | 0.603 | 0.333 | 0.552 | 0.999 | ||
| 1:2 | CK | 0.176 | 0.474 | 2.625 | 0.999 | 0.340 | 0.291 | 0.834 | 0.995 | |
| US | 0.306 | 0.465 | 1.484 | 0.999 | 0.552 | 0.336 | 0.608 | 0.998 | ||
| 1:1 | CK | 0.207 | 0.512 | 2.362 | 0.999 | 0.516 | 0.354 | 0.689 | 0.999 | |
| US | 0.291 | 0.491 | 1.630 | 0.999 | 0.597 | 0.290 | 0.474 | 0.996 | ||
| 2:1 | CK | 0.188 | 0.511 | 2.592 | 0.999 | 0.270 | 0.384 | 1.423 | 0.998 | |
| US | 0.256 | 0.441 | 1.700 | 0.999 | 0.378 | 0.360 | 0.956 | 0.991 | ||
| 9.22 | 0 | CK | 3.854 | 0.514 | 0.127 | 0.999 | 8.860 | 0.381 | 0.043 | 0.998 |
| US | 6.712 | 0.420 | 0.062 | 0.999 | 17.35 | 0.300 | 0.017 | 0.998 | ||
| 1:2 | CK | 3.147 | 0.638 | 0.179 | 0.963 | 6.447 | 0.446 | 0.068 | 0.999 | |
| US | 5.949 | 0.466 | 0.077 | 0.992 | 9.443 | 0.390 | 0.041 | 0.999 | ||
| 1:1 | CK | 2.397 | 0.422 | 0.175 | 0.986 | 6.418 | 0.399 | 0.062 | 0.997 | |
| US | 3.436 | 0.464 | 0.132 | 0.991 | 5.693 | 0.455 | 0.078 | 0.993 | ||
| 2:1 | CK | 2.229 | 0.388 | 0.174 | 0.982 | 3.408 | 0.594 | 0.158 | 0.998 | |
| US | 3.255 | 0.386 | 0.119 | 0.965 | 5.137 | 0.543 | 0.099 | 0.999 | ||
CK: Control without treatment, US: Samples treated with ultrasound.
At pH 7.06, the addition of AA showed no obvious rules on the degradation of 3-CQA without ultrasound, but decreased the degradation rate of 3-CQA with ultrasound slightly. It inferred that AA didn’t show significant effect on 3-CQA degradation at pH 7.06, but it can lighten the ultrasonic attack to 3-CQA. At pH 7.96 and 9.22, the introduction of AA prevented 3-CQA from degradation whether with or without ultrasound. When the ratio of the added amount of AA to the amount of 3-CQA were 1:1 and 2:1, the increase of pH led to an enhanced protective effect of AA on 3-CQA degradation. With ultrasound, the protection of AA on 3-CQA was accumulated with the increasing dosage of AA. The protection of AA on 3-CQA was more notable under ultrasonic treatment. Therefore, AA protects 3-CQA from degradation mainly by attenuating the adverse effect of ultrasound, followed by inhibiting the degradation and isomerization of CQAs. The degradation of 4-CQA showed a similar result. The protective effect of AA on 4-CQA was greater under ultrasonic treatment. As mentioned above, it is confirmed by the results that 4-CQA was more unstable than 3-CQA whether with or without ultrasound. AA showed the most obvious protective effect on 4-CQA at pH 9.22 with the highest k value and the lowest t1/2 value.
It has been reported that significant losses of AA were observed after the ultrasound processing [19]. The formation of free radicals should be responsible for the degradation of AA, similar to the degradation of 3-CQA. AA, as a potent antioxidant and free-radical scavenger, donates single reducing equivalents cycling between the fully reduced AA and the radical anion, monodehydroascorbate. Furthermore, monodehydroascorbate reacts preferentially with other radicals [20]. Thus AA played a competitive role with CQAs, the degradation of CQAs reduced due to the consumption of free radicals by AA. Meanwhile, the added AA decreased the pH of the solution, which also profited the stability of CQAs. At pH 7.06 & 7.96, the isomerization was slightly inhibited by AA, since it stabilized CQAs in its original conformation. However the isomerization was promoted at pH 9.22 by adding AA. This presumably due to the stronger protective effect of AA on CQAs, which increased viability of isomers.
3.4. Effect of EGCG
EGCG was added with different molar concentration ratio of 3-CQA/4-CQA at pH 7.06, 7.96 and 9.22. The degradation curves were shown in Fig. S4 & S5. Based on the Weibull model, the parameters of degradation kinetics were calculated and are listed in Table 3.
Table 3.
Kinetic parameters of degradation of 3-CQA and 4-CQA at different pH with and without EGCG.
| pH | EGCG content | treatment | 3-CQA | 4-CQA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | n | t1/2/h | R2 | k | n | t1/2/h | R2 | |||
| 7.06 | 0 | CK | 0.051 | 0.374 | 7.303 | 0.990 | 0.373 | 0.430 | 1.142 | 0.996 |
| US | 0.176 | 0.443 | 2.490 | 0.999 | 0.623 | 0.358 | 0.577 | 0.999 | ||
| 1:2 | CK | 0.091 | 0.433 | 4.706 | 0.999 | 0.313 | 0.392 | 1.255 | 0.990 | |
| US | 0.185 | 0.426 | 2.282 | 0.999 | 0.621 | 0.416 | 0.667 | 0.995 | ||
| 1:1 | CK | 0.063 | 0.398 | 6.284 | 0.991 | 0.276 | 0.313 | 1.125 | 0.978 | |
| US | 0.197 | 0.477 | 2.352 | 0.998 | 0.614 | 0.401 | 0.653 | 0.995 | ||
| 2:1 | CK | 0.121 | 0.409 | 3.369 | 0.999 | 0.612 | 0.336 | 0.549 | 0.993 | |
| US | 0.243 | 0.436 | 1.774 | 0.996 | 0.950 | 0.392 | 0.413 | 0.999 | ||
| 7.96 | 0 | CK | 0.263 | 0.531 | 1.903 | 0.998 | 0.451 | 0.360 | 0.800 | 0.998 |
| US | 0.377 | 0.440 | 1.154 | 0.992 | 0.603 | 0.333 | 0.552 | 0.999 | ||
| 1:2 | CK | 0.188 | 0.485 | 2.494 | 0.998 | 0.467 | 0.316 | 0.670 | 0.999 | |
| US | 0.330 | 0.481 | 1.414 | 0.999 | 0.616 | 0.318 | 0.513 | 0.999 | ||
| 1:1 | CK | 0.165 | 0.458 | 2.721 | 0.998 | 0.197 | 0.302 | 1.507 | 0.997 | |
| US | 0.299 | 0.456 | 1.496 | 0.999 | 0.492 | 0.388 | 0.791 | 0.999 | ||
| 2:1 | CK | 0.154 | 0.467 | 2.961 | 0.990 | 0.879 | 0.281 | 0.308 | 0.999 | |
| US | 0.278 | 0.452 | 1.598 | 0.991 | 1.647 | 0.245 | 0.136 | 0.950 | ||
| 9.22 | 0 | CK | 3.854 | 0.514 | 0.127 | 0.999 | 8.860 | 0.381 | 0.043 | 0.998 |
| US | 6.712 | 0.420 | 0.062 | 0.999 | 17.35 | 0.300 | 0.017 | 0.998 | ||
| 1:2 | CK | 3.028 | 0.455 | 0.148 | 0.997 | 4.631 | 0.446 | 0.095 | 0.947 | |
| US | 5.751 | 0.398 | 0.069 | 0.994 | 8.817 | 0.370 | 0.042 | 0.922 | ||
| 1:1 | CK | 2.266 | 0.412 | 0.181 | 0.993 | 6.247 | 0.324 | 0.052 | 0.986 | |
| US | 3.139 | 0.447 | 0.140 | 0.996 | 9.817 | 0.331 | 0.034 | 0.974 | ||
| 2:1 | CK | 1.609 | 0.367 | 0.229 | 0.980 | 15.60 | 0.138 | 0.004 | 0.956 | |
| US | 2.214 | 0.412 | 0.186 | 0.981 | 11.73 | 0.252 | 0.020 | 0.921 | ||
CK: Control without treatment, US: Samples treated with ultrasound.
The addition of EGCG slightly increased the k value and decreased t1/2 value of 3-CQA degradation at pH 7.06, indicating small facilitation to the degradation of 3-CQA. At pH 7.06, the degradation of 4-CQA first decreased and then increased with the continuous increase of EGCG amount. The best protective effect was appeared when the molar concentration ratio of EGCG to 4-CQA was 1:1. At pH 7.96, the degradation of 3- and 4-CQA were retarded by EGCG both in the presence and absence of ultrasound. Furthermore, the protective effect was increased with the increasing concentration of EGCG. It is difficult to determine whether the protective effect of EGCG results from its protection of CQAs or the inhibition of ultrasonic effect. At pH 9.22, EGCG could protect 3- and 4-CQA from degradation to a certain extent. The degradation of 3-CQA slowed down with the increasing amount of EGCG, while the degradation rate of 4-CQA first decreased and then increased. Generally, EGCG effectively slowed down the degradation of 3- and 4-CQA at alkaline conditions. Similar to AA, EGCG promoted the isomerization of 3- and 4-CQA at pH 9.22 but inhibited it at pH 7.06 & 7.96.
EGCG is effective in attenuating free radicals and reactive oxygen species, resulting from the three adjacent hydroxyl (OH) groups at positions C-3′, −4′, and −5′ on its the B ring and the gallate moiety at carbon 3 of the C-ring [21]. The radical effect caused by ultrasonic cavitation is an important reason responsible for the ultrasonic degradation of CQAs. EGCG could protect H2O2-induced damage [22]. Therefore, the degradation of CQAs could be hindered in the presence of EGCG. The stability of EGCG is pH depended, decreasing in neutral and alkaline conditions with the increasing pH. The epimerization and degradation rates of EGCG increase with the increasing pH [23]. Epigallocatechin (EGC) and gallic acid (GC) are the degradation products of EGCG, while gallocatechin gallate (GCG) is the epimerization product. Both epimerization and degradation reaction of EGCG could be enhanced by ultrasound [24]. It has been reported that the products of the degradation showed higher scavenging activity and antioxidant capacity than the EGCG [25]. Therefore, with the increase of pH, the enhancement of the degradation and epimerization of EGCG may lead to better scavenging activity, which induce better protective effect on CQAs.
4. Conclusions
In conclusion, the degradation of 3- and 4-CQA was greatly affected by pH. Generally, 3- and 4-CQA were extremely unstable with severe degradation and a little isomerization at alkaline conditions. At acidic conditions, 3- and 4-CQA were relatively stable with little degradation and isomerization. At neutral and weakly alkaline conditions, 3- and 4-CQA showed similar degradation and isomerization degree. Moreover, ultrasound obviously promoted the degradation of 3- and 4-CQA, as well as their isomerization. Furthermore, the addition of AA and EGCG could impede the degradation of 3- and 4-CQA induced by ultrasound or alkalinity. Our study provided the fundamental research on the degradation pattern of 3- and 4-CQA with ultrasound which is beneficial to the application of ultrasound in the extraction of CQAs and the processing of food containing CQAs.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31871763), the Open Project Program of the Beijing Laboratory of Food Quality and Safety, Beijing Technology and Business University (FQS-202110), Open Foundation of Beijing Advanced Innovation Center for Food Nutrition and Human Health (20182008), and Key Research and Development Project of Zhejiang Province (2020C02052).
CRediT authorship contribution statement
Danli Wang: Data curation, Writing – original draft. Jiayuan Liu: Investigation, Methodology, Validation. Shaoping Qiu: Methodology, Visualization. Jingjing Wang: Investigation, Data curation. Gongshuai Song: Writing – review & editing. Bingquan Chu: Visualization, Visualization. Ling Li: Formal analysis. Gongnian Xiao: Resources, Conceptualization. Jinyan Gong: Supervision, Funding acquisition. Fuping Zheng: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105812.
Contributor Information
Jinyan Gong, Email: gongjinyan1982@163.com.
Fuping Zheng, Email: zhengfp@btbu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Skała E., Makowczyńska J., Wieczfinska J., Kowalczyk T., Sitarek P. Caffeoylquinic acids with potential biological activity from plant in vitro cultures as alternative sources of valuable natural products. Curr. Pharm. Des. 2020;26(24):2817–2842. doi: 10.2174/1381612826666200212115826. [DOI] [PubMed] [Google Scholar]
- 2.Narita Y., Inouye K. Degradation kinetics of chlorogenic acid at various pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions. J. Agric. Food. Chem. 2013;61(4):966–972. doi: 10.1021/jf304105w. [DOI] [PubMed] [Google Scholar]
- 3.Dawidowicz A.L., Typek R. Transformation of 5-O-caffeoylquinic acid in blueberries during high-temperature processing. J. Agric. Food. Chem. 2014;62(45):10889–10895. doi: 10.1021/jf503993q. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser A., Kammerer D.R., Carle R. Impact of blanching on polyphenol stability and antioxidant capacity of innovative coriander (Coriandrum sativum L.) pastes. Food Chem. 2013;140(1-2):332–339. doi: 10.1016/j.foodchem.2013.02.077. [DOI] [PubMed] [Google Scholar]
- 5.Li Y.-J., Zhang C.-F., Ding G., Huang W.-Z., Wang Z.-Z., Bi Y.-A., Xiao W. Investigating the thermal stability of six caffeoylquinic acids employing rapid-resolution liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. Eur. Food Res. Technol. 2015;240(6):1225–1234. [Google Scholar]
- 6.Xue M., Shi H., Zhang J., Liu Q., Guan J., Zhang J., Ma Q. Stability and degradation of caffeoylquinic acids under different storage conditions studied by high-performance liquid chromatography with photo diode array detection and high-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules. 2016;21(7):948. doi: 10.3390/molecules21070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y., Qiao L., Ye X., Liu D., Zhang X., Huang H. The sonodegradation of caffeic acid under ultrasound treatment: Relation to stability. Molecules. 2013;18(1):561–573. doi: 10.3390/molecules18010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu P., Miao X., Chen Y. Degradation kinetics of chlorogenic acid, cryptochlorogenic acid, and neochlorogenic acid at neutral and alkaline pH values. Acta Pharmaceutica Sinica. 2016;51(1):122–126. [PubMed] [Google Scholar]
- 9.Kamiyama M., Moon J.-K., Jang H.W., Shibamoto T. Role of degradation products of chlorogenic acid in the antioxidant activity of roasted coffee. J. Agric. Food. Chem. 2015;63(7):1996–2005. doi: 10.1021/jf5060563. [DOI] [PubMed] [Google Scholar]
- 10.Tu Y., Njus D., Schlegel H.B. A theoretical study of ascorbic acid oxidation and HOȮ/O2̇− radical scavenging. Org. Biomol. Chem. 2017;15(20):4417–4431. doi: 10.1039/c7ob00791d. [DOI] [PubMed] [Google Scholar]
- 11.Krupkova O., Ferguson S.J., Wuertz-Kozak K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. The Journal of Nutritional Biochemistry. 2016;37:1–12. doi: 10.1016/j.jnutbio.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 12.R. Punzi A. Paradiso C. Fasciano A. Trani M. Faccia M.C. de Pinto G. Gambacorta Phenols and antioxidant activity in vitro and in vivo of aqueous extracts obtained by ultrasound-assisted extraction from artichoke by-products Natural Product Communications 9 9 2014 1934578X1400900 10.1177/1934578X1400900924. [PubMed]
- 13.Saleh I.A., Vinatoru M., Mason T.J., Abdel-Azim N.S., Aboutabl E.A., Hammouda F.M. A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara scolymus L. (artichoke) leaves. Ultrason. Sonochem. 2016;31:330–336. doi: 10.1016/j.ultsonch.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Cunha L.M., Oliveira F.A.R., Oliveira J.C. Optimal experimental design for estimating the kinetic parameters of processes described by the weibull probability distribution function. J. Food Eng. 1998;37(2):175–191. [Google Scholar]
- 15.Manso M.C., Oliveira F.A.R., Oliveira J.C., Frias J.M. Modelling ascorbic acid thermal degradation and browning in orange juice under aerobic conditions. Int. J. Food Sci. Technol. 2001;36(3):303–312. [Google Scholar]
- 16.Cuéllar-Villarreal M.D.R., Ortega-Hernández E., Becerra-Moreno A., Welti-Chanes J., Cisneros-Zevallos L., Jacobo-Velázquez D.A. Effects of ultrasound treatment and storage time on the extractability and biosynthesis of nutraceuticals in carrot (Daucus carota) Postharvest Biol. Technol. 2016;119:18–26. [Google Scholar]
- 17.Alves Filho E.G., Sousa V.M., Rodrigues S., de Brito E.S., Fernandes F.A.N. Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104911. [DOI] [PubMed] [Google Scholar]
- 18.Dawidowicz A.L., Typek R. The influence of pH on the thermal stability of 5-O-caffeoylquinic acids in aqueous solutions. Eur. Food Res. Technol. 2011;233(2):223–232. [Google Scholar]
- 19.Ordóñez-Santos L.E., Martínez-Girón J., Arias-Jaramillo M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017;233:96–100. doi: 10.1016/j.foodchem.2017.04.114. [DOI] [PubMed] [Google Scholar]
- 20.Njus D., Kelley P.M., Tu Y.-J., Schlegel H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radical Biol. Med. 2020;159:37–43. doi: 10.1016/j.freeradbiomed.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad R.S., Butt M.S., Huma N., Sultan M.T., Arshad M.U., Mushtaq Z., Saeed F. Quantitative and qualitative portrait of green tea catechins (Gtc) through hplc. Int. J. Food Prop. 2014;17(7):1626–1636. [Google Scholar]
- 22.He J., Xu L., Yang L.e., Wang X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med. Sci. Monit. 2018;24:8198–8206. doi: 10.12659/MSM.911175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y.-Q., Yu P., Zhou W. Combined effect of pH and temperature on the stability and antioxidant capacity of epigallocatechin gallate (EGCG) in aqueous system. J. Food Eng. 2019;250:46–54. [Google Scholar]
- 24.Zhu Y., Zhang J., Chen F., Hu X., Xu D., Cao Y. Epimerisation and hydrolysis of catechins under ultrasonic treatment. Int. J. Food Sci. Technol. 2021;56(1):312–320. [Google Scholar]
- 25.Battestin V., Macedo G.A., De Freitas V.A.P. Hydrolysis of epigallocatechin gallate using a tannase from Paecilomyces variotii. Food Chem. 2008;108(1):228–233. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.