Abstract
Status epilepticus (SE) is a neurological emergency, and delayed management can lead to higher morbidity and mortality. It is thought that prolonged seizures stimulate stem cells in the hippocampus and that epileptogenesis may arise from aberrant connections formed by newly born cells, while others have suggested that the acute neuroinflammation and gliosis often seen in epileptic hippocampi contribute to hyperexcitability and epilepsy development. Previous studies have identified the expression of homeodomain-only protein (HOP) in the hippocampal dentate gyrus (HDG) and the heart. HOP was found to be a regulator of cell proliferation and differentiation during heart development, while it maintains the ‘heart conduction system’ in adulthood. However, little is known about HOP function in the adult HDG, particularly in the SE setting. Here, a HOP immunohistochemical profile in an SE mouse model was established. A total of 24 adult mice were analyzed 3–10 days following the SE episode, the ‘acute phase’. Our findings demonstrate a significant downregulation of HOP and BLBP protein expression in the SE group following SE episodes, while HOP/Ki67 coexpression did not remarkably differ. Furthermore, coexpression of HOP/S100β and HOP/Prox1 was not observed, although we noticed insignificant HOP/DCX coexpression level. The findings of this study show no compelling evidence of proliferation, and newly added neurons were not identified during the acute phase following SE, although HOP protein expression was significantly decreased in the HDG. Similar to its counterpart in the adult heart, this suggests that HOP seems to play a key role in regulating signal conduction in adult hippocampus. Moreover, acute changes in HOP expression following SE could be part of an inflammatory response that could subsequently influence epileptogenicity.
Abbreviations: HOP, Homeodomain Only Protein; HDG, Hippocampal Dentate Gyrus; HF, Hippocampus Formation; SE, Status Epilepticus; Ctrl, control tissue; SGZ, subgranular zone; SVZ, subventricular zone; GCL, granule cell layer; NSC, Neural stem cells; RGL cell, Radial glia-like cell; GFAP, Glial fibrillary acidic protein; GFP, green fluorescent protein; EGFP, enhanced green fluorescent protein; IHC, Immunohistochemistry; BrdU, 5-Bromo-2′-deoxyuridine; BLBP, Brain lipid-binding protein; DCX, Doublecortin; Prox1, Prospero Homeobox 1; S100β, S100 calcium-binding protein B
Keywords: HOP, Homeodomain-Only Protein, Epileptogenicity, Status Epileptics, Seizure-induced neuroinflammation, Hippocampal Formation, Neurocardiology
Highlights
-
•
Homeodomain-only protein (HOP) is exclusively expressed in adult hippocampal dentate gyrus.
-
•
In adult HDG, HOP-expressing cells could represent a unique terminally-differentiated, highly specialized glial subtype.
-
•
. A single episode of status epilepticus (SE) does not affect cell proliferation nor differentiation in adult HDG.
-
•
HOP protein is downregulated following SE and could influence epileptogenicity.
-
•
Similar to its function in adult heart, HOP seems to play critical role in regulating adult ‘hippocampus conduction system’.
-
•
HOP protein could represent a molecular target for acute management of SE to minimize its cumulative effects.
1. Introduction
Status epilepticus (SE) is a common neurological emergency associated with significant morbidity, such as later development of epilepsy (Chin et al., 2006, DeLorenzo et al., 1996). SE is defined as a ‘continuous seizure lasting more than 30 min, or two or more seizures without full recovery of consciousness between any of them’ (Betjemann and Lowenstein, 2015, Cherian and Thomas, 2009). Mesial temporal lobe epilepsy is a common form of human epilepsy (Engel J., 2001), and the temporal lobes are the most common brain regions to develop epileptogenicity (Blair, 2012), where hippocampal onset accounts for at least 80% of all temporal lobe seizures (Tatum, 2012). Although a previous study showed that prolonged seizure activity stimulates dentate granule cell neurogenesis and contributes to hyperexcitability (Parent et al., 1997), a recent report showed that the frequency of recurrent seizures was not permanently reduced when seizure-induced new neurons were experimentally ablated (Varma et al., 2019). Moreover, glial activation and neuronal loss have been remarkably observed in the hippocampi of epileptic patients (De Lanerolle et al., 2010, Scharfman, 2000).
The hippocampus is a part of the central nervous system that is essential for memory and learning (O’Keefe and Dostrovsky, 1971). Previous reports have shown that a single acute seizure episode can impair learning and memory (Holley and Lugo, 2016, Martinos et al., 2012) and seizure-induced hippocampal injury has been observed using different techniques, such as magnetic resonance imaging (Choy et al., 2010, Scott, 2003). This indicates that even a single episode of status epilepticus can have a significant impact on the hippocampus, particularly if not managed in a timely fashion. While neurogenesis is considered by some researchers to be the underlying epileptogenic mechanism, neuroinflammation has also been reported, not only as a neurogenesis inducer (Mo et al., 2019) but also as a detrimental cause of neurogenesis (Ekdahl et al., 2003), which seems more compatible with the ILAE histopathological classification. Neuronal loss seen in surgical hippocampal specimens is a hallmark (Blümcke et al., 2013, Dam, 1980). Seizure-induced neuroinflammation has been extensively reported using standard immunohistochemistry and recent genetic techniques (Dixit et al., 2016, Choy et al., 2014, Shapiro et al., 2008, Dhote et al., 2007). Some cytokines produced by immune cells, despite their classical role as effectors in the immune system, have a significant impact on synaptic transmission and neuronal excitability (Vezzani et al., 2016).
During embryogenesis, organ formation requires a ‘tightly regulated balance between cell proliferation and differentiation to generate and maintain the size and shape of the mature organ’ (Shin et al., 2002). Members of the homeodomain family play key roles in organogenesis (Gehring et al., 1994). The homeodomain family is encoded by a specific DNA sequence, the homeobox, that consists of regulatory genes coding for specific transcription factors (Shin et al., 2002; McGinnis and Krumlauf, 1992). HOP was first reported in developing murine hearts, where it is involved in cardiomyocyte proliferation and differentiation (Chen et al., 2002; Shin et al., 2002). However, HOP was found to regulate the cardiac conduction system in adult murine hearts, and HOP knockout experiments in adult mice have shown defects in cardiac conduction (Fishman, 2020, Hatcher and Basson, 2009, Ismat et al., 2005). The adult heart continues to express HOP, which seems to have a different function than during embryogenesis. A recent fate-mapping report has shown that HOP-expressing cells seen in the mouse neuroepithelium are the origin of HOP-expressing cells found in the adult dentate gyrus (Berg et al., 2019). Given that little is known about HOP function in the adult HDG, particularly in the setting of SE, our hypothesis was that considering its function in the adult heart, HOP could also play a key role in regulating signal conduction in adult hippocampus. Moreover, knowing that epilepsy is considered a conduction-related disorder and that it has an obvious impact on the hippocampus, we investigated HOP protein expression in the hippocampus using a status epilepticus model in adult mice.
2. Experimental procedures
2.1. Animals
All procedures involving animals and their care were performed in accordance with the policies of the Institutional Animal Care and Use of Tokyo Medical University. A total of 24 GFAP-EGFP transgenic mice (n = 12 Ctrl; n = 12 SE; age range: P9–11 weeks in all figures) were used to identify astrocytes in the hippocampus, where enhanced GFP is characteristically ‘found throughout the cell, even in astrocytic fine processes’ (Suzuki et al., 2003). Only male mice were used to avoid any hormonal effects during the sampling procedures. No wild type mice were used in the present work. The mice were housed under a 12-hour light/dark cycle, with ad libitum access to food and water throughout the experimental procedures.
2.2. Status epilepticus ‘acute-phase’ model
The status epilepticus model was established as described previously (Turski et al., 1983), with a slight modification of the drug dosing. Briefly, adult male GFAP-EGFP transgenic mice were pretreated with atropine methyl bromide (Sigma–Aldrich A6883, dissolved in saline, 5 mg/kg, intraperitoneally ‘i.p.’) to block the peripheral effects of pilocarpine, and ~15 min later, pilocarpine hydrochloride (Sigma–Aldrich P6503, dissolved in saline, 280 mg/kg i.p.) was given to induce convulsive seizures. Seizure activities were seen within an average of 15 min following pilocarpine injection. In accordance with the standard definition of SE, (Betjemann and Lowenstein, 2015), the seizures were terminated ~ 1 hr after pilocarpine injection using diazepam (Wako Pure Chemical Industries 045–18901, dissolved in 30% ethanol in saline, 10 mg/kg, i.p.).
Seizure behaviors were scored using Racine's scale (Racine, 1972), which is divided into 5 stages: (1) Mouth and facial movements. (2) Head nodding. (3) Forelimb clonus. (4) Rearing. (5) Rearing and falling. Some mice did not show noticeable seizure behavior; thus, only those mice that showed continuous, convulsive seizures (stage 4–5) were included in our analysis. The control mice received atropine, saline instead of pilocarpine, and then diazepam in the same strategy used in the ‘pilocarpine’ group. As expected, all control mice showed no abnormal behavior. Inflammation in the brain occurs acutely within 1–5 days following brain injury, including seizure activities (Thelin et al., 2017, Shapiro et al., 2008). Thus, the acute phase in our study was defined by the sacrifice timing. The mice were sacrificed within three to a maximum of ten days after the seizure induction session. This time-locked approach was meant to investigate the changes in the hippocampus that occurred specifically during the acute phase.
2.3. Tissue preparation
Three to a maximum of ten days after the seizure induction session, the mice were anesthetized and perfused transcardially with phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in 0.2 M phosphate buffer (PB). Their brains were removed, postfixed in 4% PFA overnight at 4 °C, cryoprotected in 10% sucrose for 6 hr, and then in 20% sucrose overnight at 4 °C. Then, the brains were embedded in O.C.T. compound (Sakura Finetek), snap-frozen using liquid nitrogen and stored at − 80 °C until the cryosection procedure. The hippocampi were cut into 40 µm slices perpendicular to the septotemporal axis (coronal plane) using a cryostat (CM1850, Leica) and then stored in cryoprotectant solution (30% ethylene glycol and 25% glycerin in 0.1 M PB) at − 20 °C until the immunohistochemistry procedure.
2.4. Immunohistochemistry
Standard immunohistochemistry was performed using the free-floating staining method. Briefly, after washing with phosphate-based saline (PBS), the tissue sections were first blocked with 1% bovine serum albumin in PBS-T (0.5% Triton X-100) for 30 min at room temperature (RT). Next, the sections were incubated with the primary antibodies overnight at 4 °C. Following washing, the sections were incubated with secondary antibodies for 2 hr at RT. Then, the sections were washed and incubated with Hoechst 33342 (1:1000 PBS, Life Technologies) for 15 min at RT and mounted on glass slides. The primary and secondary antibodies were diluted with PBS-T. For the detection of all antibodies, no antigen retrieval method was used. The antibodies used are listed in Table 1.
Table 1.
Antibodies list.
| Antibody/Species | Dilution | Vendor | Cat. number |
|---|---|---|---|
| Mouse anti-HOP | 1:200 | Santa Cruz | Sc398703 |
| Goat anti-DCX | 1:500 | Santa Cruz | sc-8066 |
| Chicken anti-GFP | 1:1000 | abcam | ab13970 |
| Rabbit anti-S100β | 1:500 | abcam | ab41548 |
| Rabbit anti-Prox1 | 1:1000 | Millipore | Ab5475 |
| Guinea pig anti-BLBP | 1:500 | Frontier Institute | Af291-1 |
| Rabbit anti-Ki67 | 1:500 | Cell signal | D3B5 |
| Donkey anti-mouse + Alexa 488 | 1:500 | Jackson | 715-545-151 |
| Donkey anti-mouse + Alexa 647 | 1:500 | Jackson | 715-606-151 |
| Donkey anti-goat + Alexa 546 | 1:1000 | Life Technologies | A11056 |
| Donkey anti-rabbit + Alexa 647 | 1:500–1000 | Invitrogen | A32795 |
| Donkey anti-rabbit + Alexa 546 | 1:500 | Life Technologies | A10040 |
| Donkey anti-chicken + Alexa 488 | 1:500 | Jackson | 703-546-155 |
| Donkey anti-guinea pig + Cy3 | 1:100 | Jackson | 706-165-148 |
2.5. Image acquisition and analysis
All images were acquired using a Zeiss confocal microscope (LSM 700, Carl Zeiss using ZEN black software). In a status epilepticus rat model, immunostaining of the proliferating markers did not show a qualitative difference among the tissue sections taken from different hippocampal regions (Parent et al., 1997). Thus, six randomly selected tissue sections from a minimum of three mice per group (SE vs. Ctrl) were analyzed and averaged from each animal. Only cells located in the innermost edge of the granule cell layer were included in our analysis. Thus, this work does not represent a ‘stereological determination of the exact cell number’. The images were analyzed and adjusted using the Fiji-ImageJ package. To facilitate quantification and data presentation of the colocalization profile, some colors seen in the figures have been converted digitally (via software) during the image postprocessing step (e.g., magenta to green). In all analyses, ‘n’ indicates the number of biological repeats (number of animals), and all data are presented as the mean ± SEM. Differences between groups (SE vs. Ctrl) were determined using unpaired Student's t-tests, and a p value of less than.05 was considered statistically significant. Of note, the present study is a brief experimental report that employed standard immunohistochemistry only and used a limited number of animals. Moreover, our data analysis was performed based on a single optical section approach. Thus, further studies overcoming the aforementioned limitations would enhance the generalizability of our findings and conclusions.
3. Results
3.1. Expression of HOP protein continues in adult HDG
In the adult mouse hippocampus, HOP-labeled cells are located at the innermost edge of the GCL (Berg et al., 2019, Toni et al., 2008). Conversely, HOP was not detected in the subventricular zone (SVZ) of the lateral ventricle of the adult forebrain (Li et al., 2015, Toni et al., 2008), although many researchers consider each SGZ and SVZ to be ‘neurogenic niches throughout life under normal conditions’ (Ming and Song, 2011). To first localize HOP expression in the hippocampus of adult mice, we performed standard immunohistochemical localization of HOP protein.
In agreement with previous reports (Berg et al., 2019, Li et al., 2015, Toni et al., 2008), we found that HOP was strongly expressed in the innermost edge of the GCL in adult mice, where approximately every 2–3 HOP-labeled cells seemed to be juxtaposed and arranged in seemingly irregularly spaced intervals along the entire GCL edge (Fig. 1A). Furthermore, we noticed randomly distributed, rather weak expression of HOP in the molecular layer of the hippocampus formation (HF). Overall, the HOP-labeled cells assumed a seemingly pyramidal-shaped cell body that was almost always located in the innermost edge and was mostly associated with radially oriented processes of different lengths spanning almost the entire width of the GCL. However, no discernible processes arising from cell bodies were observed directed toward the hilus region in each experimental group (Ctrl and SE). Overall, the location and morphology of the HOP-expressing cells in the SE tissue sections did not show a noticeable change compared to that in the control tissue, suggesting that SE episodes did not cause acute structural changes in the HOP-expressing cells.
Fig. 1.
HOP proliferation rate was not increased following SE (A) Hippocampi of Ctrl and SE adult mice immunostained with antibody against HOP (green), Ki67 (red), and the nuclei were stained blue with Hoechst 33342 (DAPI) show that HOP is almost exclusively expressed in the innermost edge of granule cell layer in adult mice; the so-called subgranular zone (SGZ). Ki67 expression was not increased following SE, and the colocalization with HOP in both SE tissue (upper panel, where c-d images are higher magnification of the inset seen in image a) and Ctrl tissue (lower panel, where f-h images are higher magnification of the inset seen in image e) seem statistically and biologically insignificant. Note: HOP antibody was originally immuostained with Magenta color and later was converted digitally into Green to enhance image visualization. Scale bar (of all images)= 30 µm. (B) Quantification of proliferation (Ki67) and HOP-expressing cells in SGZ per single coronal optical section; n = 3 animals/group, two-tailed t-test; data shown represent mean ± SEM. In both cases; total Ki67 and Ki67/HOP colocalization, the p-value were insignificant. Abbreviations: HOP: Homeodomain only protein; SE: status Epilepticus tissue; Ctrl: Control tissue.
3.2. The HOP proliferation rate was not increased following SE
Neurogenesis entails cell proliferation to maintain stem cell pools, while terminal differentiation usually coincides with proliferation arrest and permanent exit from the division cycle. Therefore, ‘imbalance between cell proliferation and proper differentiation are often the hallmark of cancer cells’ (Ruijtenberg and van den Heuvel, 2016). Currently, it is thought that so-called neural stem cells (NSCs) ‘remain quiescent under normal conditions’ (Lugert et al., 2010), whereas seizures stimulate and deplete them (Fu et al., 2019). HOP has been described as a quiescent NSC marker in the adult dentate gyrus (Berg et al., 2019), while other studies have shown that HOP in the adult dentate gyrus did not regulate cell proliferation (Mühlfriedel et al., 2005, Toni et al., 2008, Li et al., 2015). Thus, we used a status epilepticus model in adult mice as described previously (Turski et al., 1983) and analyzed the hippocampal tissue using HOP and Ki67 markers to assess the proliferation rate of the HOP-labeled cells, particularly with regard to SE.
Here, we found that HOP-labeled cells in the control tissue were not proliferating (Fig. 1A), which is consistent with a previous report, where almost all HOP-labeled cells in adult HDG were not proliferating (Li et al., 2015, Berg et al., 2019). In our data, the number of Ki67-positive cells showed no significant difference in the SE group compared to the Ctrl group (Fig. 1B; SE: 16.6 ± 2.36 cells versus Ctrl: 15.2 ± 1.47 cells, p = 0.7). Furthermore, SE did not increase the HOP/Ki67 proliferation rate (Fig. 1B; SE: 3.44 ± 0.2 cells versus Ctrl: 2.17 ± 0.07 cells, p = 0.06), although rare HOP-labeled cells overlapped with Ki67 in both the Ctrl and SE tissue (3 out of 16 cells in SE and 2 out of 15 cells in the Ctrl group). Furthermore, Ki67 seemed to be slightly more highly expressed in SE tissue than in Ctrl tissue if we considered the entire hippocampus in the analysis, namely, the molecular layer and hilus region. However, this issue was out of our present scope. Moreover, upregulating Ki67 in SE tissue in regions other than the GCL and the cornu ammonis regions could indicate that cells other than neurons seem to be introduced, most likely immune cells, since acute microglial and astrocyte activation following pilocarpine-induced seizures in rats has been reported previously (Shapiro et al., 2008). Together, our data suggest that HOP-labeled cells in the SGZ are not proliferating and that SE did not increase the HOP/Ki67 proliferation rate. This suggests that HOP-expressing cells in adult HDG are possibly mature cells that have already exited the cell cycle.
3.3. HOP in HDG is significantly downregulated following SE
It has been reported that seizures activate so-called ‘neural stem cell’ proliferation and thus deplete their pool (Fu et al., 2019, Jessberger and Parent, 2015). In our previous experiments, we found that HOP-labeled cells were not proliferating and that no significant proliferation difference in Ctrl versus SE tissue was observed. Thus, as a follow-up experiment, we further investigated the notion of seizure-induced NSC depletion using the GFAP-EGFP mouse line (Suzuki et al., 2003) and BLBP (brain lipid-binding protein) for coimmunostaining experiments with HOP.
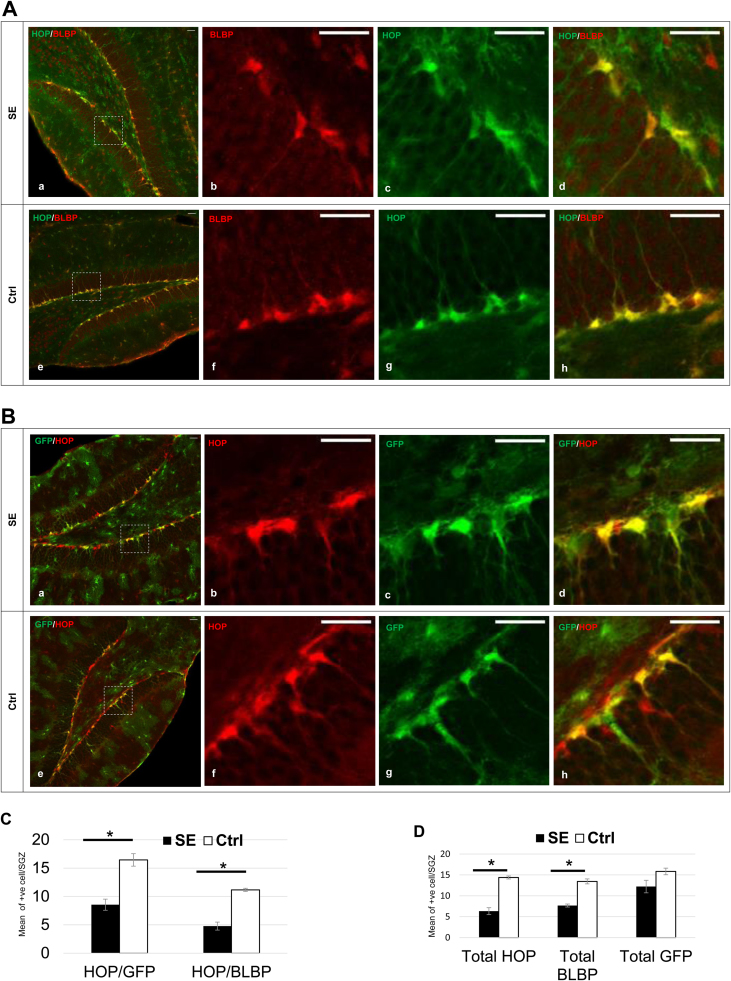
Here, we found almost complete HOP/BLBP overlapping in HDG, although not all BLBP were colocalized HOP (Fig. 2A) and a significant decrease of HOP/BLBP colocalized cells in SE tissue compared to that in Ctrl tissue has been observed (Fig. 2C; HOP/BLBP: SE: 4.78 ± 0.7 cells versus Ctrl: 11.2 ± 0.28 cells, p = 0.006). Next, we performed HOP coimmunostaining with GFP. In the HDG, HOP colocalized with the majority of the GFP (Fig. 2B and C; HOP/GFP: SE: 8.56 ± 0.98 cells versus Ctrl: 16.4 ± 1.1 cells, p = 0.01). Interestingly, the total HOP count and total BLBP count seemed simultaneously downregulated following SE (Fig. 2D; total HOP: SE: 6.33 ± 0.83 cells versus Ctrl: 14.4 ± 0.35 cells, p = 0.005; total BLBP: SE: 7.67 ± 0.36 cells versus Ctrl: 13.4 ± 0.6 cells, p = 0.006). However, in contrast to HOP and BLBP, total GFP seemed insignificantly affected by SE (Fig. 2D; total GFP: SE: 12.2 ± 1.51 cells versus Ctrl: 15.8 ± 0.79 cells, p = 0.18). Together, we found that HOP colocalized with BLBP and the majority of GFP, suggesting that these cells possibly represent the same cell population, which was found to be significantly and acutely decreased following the SE episodes.
Fig. 2.
HOP expression in HDG is significantly downregulated following SE. (A) Hippocampi of adult mice immunostained with antibody against HOP (green) and BLBP (red) show that total HOP expression in hippocampal dentate gyrus is significantly downregulated following SE. HOP almost always colocalized BLBP in SGZ. Similar to HOP, total BLBP has also been significantly decreased following SE episode. SE tissue seen in the upper panel, where c-d images are higher magnification of the inset seen in image a, and Ctrl tissue seen in the lower panel, where f-h images are higher magnification of the inset seen in image e). Note: HOP antibody was originally immuostained with Magenta color and later was converted digitally into Green to enhance image visualization. Scale bar (of all images)= 30 µm. (B) Hippocampi of adult mice immunostained with antibody against GFAP-GFP (green) and HOP (red) show that almost always colocalized BLBP in SGZ. Similar to HOP and BLBP, total GFP has also decreased to a great extent, but not significantly after SE episode. SE tissue seen in the upper panel, where c-d images are higher magnification of the inset seen in image a, and Ctrl tissue seen in the lower panel, where f-h images are higher magnification of the inset seen in image e. Scale bar (of all images)= 30 µm. (C) Quantification of colocalized HOP/BLBP and HOP/GFP as seen in SGZ per each coronal section; n = 3 animals/group; * P < 0.05, two-tailed t-test; data shown represent mean ± SEM. (D) Quantification of total HOP, total BLBP, and total GFP as seen in SGZ per each coronal section; n = 3 animals/group; * P < 0.05, two-tailed t-test; data shown represent mean ± SEM. In contrast to HOP and BLBP, total GFP seem not significantly affected by SE.
3.4. In SE, HOP rarely colocalized with DCX but never colocalized with Prox1
It has been reported that seizures increase cell neurogenesis in the dentate granule cell layer, which contributes to hyperexcitability (Morgan and Soltesz, 2008, Parent et al., 1997). However, other evidence has shown that ‘seizure-induced newborn neurons possess anticonvulsive effects resulting in overall decreased excitability’ (Danzer, 2019, Jakubs et al., 2006). In the present study, we did not observe increased proliferation following SE, although HOP protein was significantly downregulated. At this point, we were not sure whether the HOP-labeled cells had already died and were eliminated or whether they had been differentiated into other cell types as a result of the SE effects. Thus, we wanted to assess the notion of seizure-induced neuronal differentiation.
To do this, we costained HOP with the commonly used neuronal marker Prox1, which is a specific marker for mature dentate granule cells (Oliver et al., 1993), and DCX as a marker of migrating neuroblasts, although a previous report showed that ‘survival and maturation of adult-generated hippocampal neurons’ did not require DCX (Dhaliwal et al., 2016, Merz and Lie, 2013). Moreover, DCX is also expressed in astrocytes (Verwer et al., 2007).
Here, we found that HOP rarely colocalized with DCX in Ctrl tissue and it was even rarer in SE tissue (Fig. 3A). Moreover, no significant difference was observed in the SE versus Ctrl tissue (Fig. 3C; SE: 1.89 ± 0.25 cells versus Ctrl: 2.67 ± 0.15 cells, p = 0.1), which is consistent with a previous report, where no DCX- or T-box brain protein 2 (Tbr2)-expressing cells colocalized with HOP in the SGZ (Li et al., 2015). Furthermore, HOP/Prox1 colocalized cells were never observed in the present study, in either SE or Ctrl tissues (Fig. 3B), and to our knowledge, no data on the Prox1/HOP colocalization pattern in the adult hippocampus has been reported previously. The HOP did not proliferate or colocalize with neuronal markers. Instead, HOP was colocalized with glial markers, namely, BLBP and GFAP-EGFP. This suggests that HOP-expressing cells represent mature cells that are obviously distinctive from the neuronal cell population, particularly granule cells. Moreover, an acute single episode of SE did not cause neuronal differentiation and HOP-expressing cells are not stem cells or neurons but are most likely a unique terminally differentiated glial subtype.
Fig. 3.
Following SE, HOP rarely colocalized DCX, and never colocalized Prox1. (A) Hippocampi of Ctrl and SE adult mice immunostained with antibody against DCX (red) and HOP (green) show that HOP was almost did not colocalized DCX. These finding were seen in both Ctrl and SE tissue with no difference. SE images seen in the upper panel, where c-d images are higher magnification of the inset seen in image a, while Ctrl tissue images shown in the lower panel, where f-h images are higher magnification of the inset seen in image e. Scale bar (of all images)= 30 µm. (B) Hippocampi of Ctrl and SE adult mice immunostained with antibody against Prox1 (red) and HOP (green) show that HOP has never colocalized Prox1, and this staining pattern was seen in both Ctrl and SE tissue with no difference. Scale bar (of all images)= 30 µm. Note: HOP antibody in Figs. A and B was originally immuostained with Magenta color and later was converted digitally into Green to enhance image visualization. (C) Quantification of co-localized HOP/DCX as seen in SGZ per each coronal section; n = 3 animals/group; two-tailed t-test; data shown represent mean ± SEM. No count for HOP/Prox1 was performed since colocalization in both SE and Ctrl tissues was obviously not observed.
3.5. S100β expression was not significantly affected by SE
A previous study showed that S100β expression defines a ‘late developmental stage after which GFAP-expressing cells lose their NSC potential’ (Raponi et al., 2007). Moreover, S100β is more suitably used to visualize the overall distribution and changes in the number of astrocytes throughout the CNS (Zhang et al., 2019). Thus, to further explore the identity and fate of S100β-expressing cells, we coimmunostained S100β with BLBP and GFAP-EGFP.
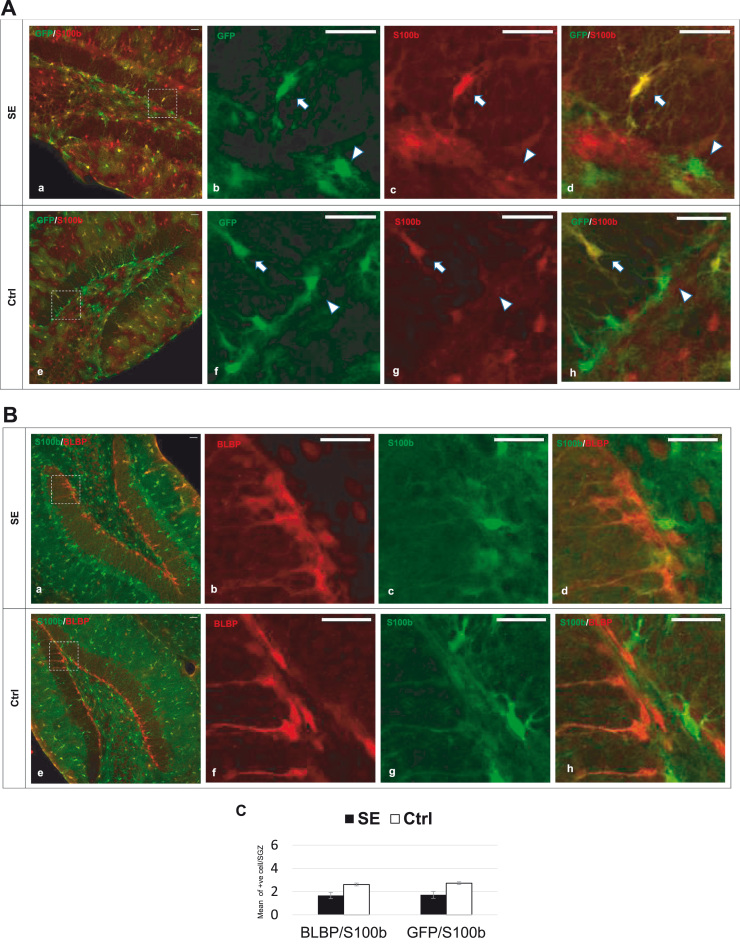
Here, we found that S100β in the HDG rarely colocalized with GFP (Fig. 4A) and that the SE episode did not cause significant changes in this colocalization pattern (Fig. 4C; S100β/GFP; SE: 1.72 ± 0.28 cells versus Ctrl: 2.72 ± 0.12 cells, p = 0.08). BLBP was extensively expressed in the HDG but rarely colocalized with S100β (Fig. 4B and C; S100β/BLBP; SE: 1.67 ± 0.27 cells versus Ctrl: 2.61 ± 0.12 cells, p = 0.08), suggesting that S100β seems to represent an almost distinct cell population separate from the BLBP and GFP cell populations. Moreover, a single episode of SE seems insufficient to differentiate GFP and BLBP into any other cell type, including S100β-labeled cells. Although rare S100β/GFP and S100β/BLBP might be considered differentiated cells, this is not necessarily due to the side effects of SE given that no significant difference in SE tissue was observed compared to that in control tissue. Collectively, SE did not cause acute differentiation of GFAP-EGFP and BLBP into S100β, although such differentiation might occur chronically since ‘S100β-immunoreactive astrocytes have been found to be threefold higher in patients with intractable epilepsy than that found in control patients’ (Griffin et al., 1995).
Fig. 4.
S100b expression are not significantly affected by SE, (A) Hippocampi of adult mice immunostained with antibody against GFAP-GFP (green) and S100b (Red) show that S100b has almost never co-localized GFP, particularly in SGZ (arrowheads), although rarely colocalized pattern seen in other regions, such as molecular layer and upper part of granular layer (arrows). SE episode did not cause a significant change on this colocalization pattern. Scale bar (of all images)= 30 µm., (B) Hippocampi of adult mice immunostained with antibody against BLBP (Red) and S100b (Green) show that S100b were rarely co-localized BLBP and that SE, episode did not cause a significant change on this colocalization pattern. Note: S100b antibody in Fig. B was originally immuostained with Magenta color and later was converted digitally into Green to enhance image visualization. Scale bar (of all images)= 30 µm. (C) Quantification of co-localized S100b/BLBP and S100b/GFP as seen in SGZ per each coronal section; n = 3 animals/group; two-tailed t-test; data shown represent mean ± SEM. In both cases, no statistical difference was observed.
4. Discussion
A previous study described HOP as a marker of quiescent neural stem cells (NSCs) in the adult HDG (Berg et al., 2019) and found that seizures activate the NSC pool and increase adult-born neurons in the HDG (Parent et al., 1997). Here, we found that HOP-expressing cells in the HDG did not proliferate or coexpress neuronal antibodies following SE episodes, while HOP expression was acutely downregulated compared to that in control mice. Moreover, HOP-expressing cells in the HDG seem to be a unique terminally differentiated, highly specialized glial subtype. Given that acute seizure activity can trigger inflammatory responses within the brain (Rana and Musto, 2018, Dey et al., 2016, Shapiro et al., 2008), in addition to the role of HOP in regulating the cardiac conduction system, we speculate that HOP seems to be closely involved in such inflammation processes and that HOP protein could play a critical role in regulating signal conduction in adult hippocampus given the effect of inflammation on the electrical behavior of cardiac tissue (Zhou and Dudley, 2020).
HOP is a critical transcription factor widely expressed in a variety of tissues, where it plays an indispensable role throughout life (Hng et al., 2020, Friedman et al., 2018, Jones et al., 2015). HOP was initially reported in the heart and brain, among other organs, during the developmental stage (C. H. Shin et al., 2002; Chen et al., 2002) and it continues to be expressed in the heart and brain in adulthood (Li et al., 2015, Risebro et al., 2012, Ismat et al., 2005). Previous studies have shown that HOP is expressed in cardiac tissue and ‘subserves at two stages: expansion of the ventricular myocardium during the embryonic period and later restriction of cardiomyocyte proliferation’ (Friedman et al., 2018). Recently, HOP has gained further attention in the neuroscience field following a report showing that ‘HOP retains common molecular signatures across development’ (Berg et al., 2019). The functionality of HOP has been investigated in different settings. However, to our knowledge, the present study is the first to investigate HOP expression changes in a seizure setting. Consistent with previous studies, we found that HOP was specifically expressed in the innermost GCL in adult mice. Moreover, the expression pattern of HOP protein in SE tissue did not discernibly differ from that in Ctrl tissue.
It has been reported that HOP-expressing cells in the HDG represent quiescent stem cells since they coexpress Sox2, GFAP, BLBP, and Nestin (Berg et al., 2019, Braun et al., 2003). However, other reports showed that not all Sox2 + cells in the SGZ expressed HOP (Li et al., 2015, Zweifel et al., 2018). Similarly, our data showed that not all HOPs in the SGZ expressed BLBP, suggesting that HOP-expressing cells that colocalized with BLBP seem to be a different cell subpopulation than those that did not. The concept of cell quiescence means that the cells can be activated and regain their proliferation capacity. Using Ki67, we did not identify proliferating HOP/Ki67 cells in either the Ctrl or SE tissues. In contrast, another study showed that BrdU colocalized with neuronal markers in the GCL, and this was increased in an animal model of status epilepticus (Parent et al., 1997). However, we see no obvious conflict with this finding since we studied the acute phase of changes following SE, while Parent et al. (1997) investigated the chronic phase, although proliferation seems unlikely to occur in a delayed fashion. However, Parent et al. (1997) reported that the ‘number of BrdU-labeled cells in the SGZ returned to baseline approximately 1 month following SE’. Collectively, this is questioning their role in epileptogenesis and synaptic reorganization. However, investigating HOP protein in the chronic phase following SE could provide further insight using endogenous antigens such as Ki67, which has most of the benefits of BrdU and none of its costs (Kee et al., 2002).
In our data, only a few overlapping HOP/Ki67 cells were noticed, with a similar level in the SE and Ctrl tissue, suggesting that SE has less of an effect on the overall proliferation level, particularly HOP-expressing cells. Not only does the average of 2–3 cells that showed HOP/Ki67 overlap seem biologically insignificant, but there is also a strong possibility that this rare overlap could in fact be due to high background autofluorescence on confocal microscopy. Collectively, HOP expression in adult HDGs does not seem to be involved in maintaining cell stemness. Instead, HOP has been described as a ‘negative regulator of hippocampal stem cell survival’ (Toni et al., 2008). Astrocyte and microglial proliferation and subsequent glial scarring along with neuronal degeneration have been previously reported following seizures in a temporal lobe epilepsy animal model (Guo et al., 2017, Loewen et al., 2016, Shapiro et al., 2008). Thus, we speculate that dysregulation of HOP expression, if recurrent and chronic, could lead to such a result, and future works might prove this.
Although widely considered to be NSC markers, GFAP is also used as a ‘routine identifier of astrocytes in the healthy CNS’ (Lewis et al., 1984). Variable upregulation of GFAP expression was also observed in reactive astrogliosis (Sofroniew, 2009). In fact, a previous study found that reactive astrogliosis seems to be the cause of the development of spontaneous astrogliosis-associated seizures (Robel et al., 2015). GFAP was found to ‘label astrocytes in the hippocampus’ (Zhang et al., 2019) and has long been recognized as an astrocyte maturation marker (Gomes et al., 1999). In the hippocampus, HOP is expressed in mature astrocytes (Li et al., 2015).
Likewise, BLBP has also been used as a marker for mature astrocytes. Although some authors have reported that some cells resemble radial glial cells in morphology and express BLBP, these cells gradually differentiated into mature astrocytes, ‘while few if any neurons appear to be derived from radial glial cells’ (McDermott et al., 2005). Furthermore, BLBP (aka, FABPB or B-FABP) was observed to be localized primarily to astrocytes in the adult brain; thus, it has been used as a marker of mature astrocytes (Owada et al., 2006). Pathologically, BLBP is expressed in glioma cells (Tian et al., 2018, Han et al., 2017), and intrinsic astrocyte heterogeneity significantly contributes to glioma pathogenesis (Irvin et al., 2017). Moreover, BLBP-expressing astrocytes have also been detected in experimental autoimmune encephalomyelitis (Kipp et al., 2011).
In our data, we found that HOP in the HDG almost always colocalized with BLBP, and similarly, the majority of GFP colocalized with HOP. These findings suggest that these three markers, namely, HOP, BLBP, and GFAP-EGFP, do not seem to be specific markers for the so-called NSCs, and their expression variation appears to reflect astrocytic heterogeneity across HDGs. The simultaneous downregulation of HOP, BLBP and, to a large extent, GFP, suggests that these markers may be part of the overlapping signaling pathway that is somehow involved in regulating the acute inflammatory response, particularly when it is triggered by seizure activities. HOP-expressing cells in the HDG could represent a unique glial subtype.
HOP protein downregulation seen in our findings following SE episodes might indicate that some of these downregulated cells might already be differentiated into glial cells or into neuronal committed cells. However, DCX rarely colocalized with HOP, with no significant differences observed in the SE group compared to the Ctrl group. This suggests that DCX seems not to be affected by a single episode of SE. Similarly, HOP never colocalized with Prox1, suggesting that HOP did differentiate into neurons or migrating neuroblasts and the rarely colocalized HOP/DCX seen in our data could represent cell types other than migrating neuroblasts. This possibility is supported by a previous report, where ‘DCX identified a range of morphological cell types in temporal lobe epilepsy, including immature populations, glial and microglial cell types’ (Liu et al., 2018). Similarly, we found rare instances of colocalized HOP/S100β in the HDG following SE episodes. It is highly possible that these rare HOP/S100β cells seen following SE episodes could represent acute pathologic expression of the S100β protein in certain vulnerable glial cells, resulting in gliosis. Interestingly, gliotic scarring or so-called hippocampal sclerosis is commonly described in the hippocampi of epileptic patients (Thom et al., 2009, Gates and Cruz‐Rodriguez, 1990).
In conclusion, the present study shows that HOP was acutely downregulated following seizure activity and that subsequent epilepsy development could be explained, at least in part, as a consequence of chronic HOP downregulation. Given that previous reports have presented a compelling set of evidence that HOP is involved in regulating the cardiac conduction system, we speculate that HOP protein also seems to be a regulator of the hippocampal signal conduction, which would support the recently emerging neurocardiology field. Moreover, HOP-expressing cells seem to be a unique terminally differentiated highly specialized glial subtype located exclusively at the innermost edge of the adult HDG. Such a unique location of the HOP protein could also explain its functionality. Impaired HOP regulation seems to be a critical factor in the development of convulsions, and further investigation of HOP regulation mechanisms in the adult hippocampus will contribute to the development of new strategies for investigating and treating epileptic disorders. Moreover, HOP protein could also be helpful for classifying epileptic surgical specimens as a novel tool in the epilepsy neuropathology field.
CRediT authorship contribution statement
The authors confirm contribution to the paper as follows: study conception and design: Alshebib YA. data collection: Alshebib YA. analysis and interpretation of results: Alshebib YA. Tomokatsu Hori. Taichi Kashiwagi; draft manuscript preparation: Alshebib YA; Taichi Kashiwagi. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
The authors wish to express special thanks to our neuroanatomy laboratory members at Tokyo Medical University, particularly Dr. Natsu Sasaki-Takahashi, for assistance in sample preparation and Dr. Yuko Gonda for continuous scientific advice and support.
Disclosures
The authors have no competing interests to declare.
References
- Berg D.A., Su Y., Jimenez-Cyrus D., Patel A., Huang N., Morizet D., Bond A.M. A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell. 2019;177(3):654–668. doi: 10.1016/j.cell.2019.02.010. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betjemann J.P., Lowenstein D.H. The Lancet Neurology. Lancet Publishing Group; 2015. Status epilepticus in adults. June 1. [DOI] [PubMed] [Google Scholar]
- Blair R.D.G. Temporal lobe epilepsy semiology. Epilepsy Res. Treat. 2012;2012:1–10. doi: 10.1155/2012/751510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I., Thom M., Aronica E., Armstrong D.D., Bartolomei F., Bernasconi A., Spreafico R. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54(7):1315–1329. doi: 10.1111/epi.12220. [DOI] [PubMed] [Google Scholar]
- Braun N., Sévigny J., Mishra S.K., Robson S.C., Barth S.W., Gerstberger R., Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur. J. Neurosci. 2003;17(7):1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Chen F., Kook H., Milewski R., Gitler A.D., Lu M.M., Li J., Epstein J.A. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110(6):713–723. doi: 10.1016/S0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Cherian A., Thomas S.V. Annals of Indian Academy of Neurology. Medknow Publications; Wolters Kluwer: 2009. Status epilepticus. July 1. [DOI] [Google Scholar]
- Chin R.F., Neville B.G., Peckham C., Bedford H., Wade A., Scott R.C. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368(9531):222–229. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- Choy M.K., Cheung K.K., Thomas D.L., Gadian D.G., Lythgoe M.F., Scott R.C. Quantitative MRI predicts status epilepticus-induced hippocampal injury in the lithium-pilocarpine rat model. Epilepsy Res. 2010;88(2–3):221–230. doi: 10.1016/j.eplepsyres.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Choy M.K., Dubé C.M., Ehrengruber M., Baram T.Z. Inflammatory processes, febrile seizures, and subsequent epileptogenesis. Epilepsy Curr. 2014;14(SUPPL.1):15–22. doi: 10.5698/1535-7511-14.s2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam A.M. Epilepsy and Neuron Loss in the Hippocampus. Epilepsia. 1980;21(6):617–629. doi: 10.1111/j.1528-1157.1980.tb04315.x. [DOI] [PubMed] [Google Scholar]
- Danzer S.C. Adult neurogenesis in the development of epilepsy. Epilepsy Curr. 2019;19(5):316–320. doi: 10.1177/1535759719868186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo R.J., Hauser W.A., Towne A.R., Boggs J.G., Pellock J.M., Penberthy L., Ko D. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029–1035. doi: 10.1212/WNL.46.4.1029. [DOI] [PubMed] [Google Scholar]
- Dey A., Kang X., Qiu J., Du Y., Jiang J. Trends in Pharmacological Sciences. Elsevier Ltd; 2016. Anti-Inflammatory Small Molecules to Treat Seizures and Epilepsy: From Bench to Bedside. June 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal J., Xi Y., Bruel-Jungerman E., Germain J., Francis F., Lagace D.C. Doublecortin (DCX) is not essential for survival and differentiation of newborn neurons in the adult mouse dentate gyrus. Front. Neurosci. 2016;9(JAN):494. doi: 10.3389/fnins.2015.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhote F., Peinnequin A., Carpentier P., Baille V., Delacour C., Foquin A., Dorandeu F. Prolonged inflammatory gene response following soman-induced seizures in mice. Toxicology. 2007;238(2–3):166–176. doi: 10.1016/j.tox.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Dixit A.B., Banerjee J., Srivastava A., Tripathi M., Sarkar C., Kakkar A., Chandra P.S. RNA-seq analysis of hippocampal tissues reveals novel candidate genes for drug refractory epilepsy in patients with MTLE-HS. Genomics. 2016;107(5):178–188. doi: 10.1016/j.ygeno.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Ekdahl C.T., Claasen J.H., Bonde S., Kokaia Z., Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J J. Mesial temporal lobe epilepsy: What have we learned. Neuroscientist. 2001;7(4):340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- Fishman G.I. Transcriptional regulation of the cardiac conduction system. Transac. Am. Clin. Climatol. Assoc. 2020;131:48–54. 〈http://www.ncbi.nlm.nih.gov/pubmed/32675842〉 [PMC free article] [PubMed] [Google Scholar]
- Friedman C.E., Nguyen Q., Lukowski S.W., Tam P.P.L., Powell J.E., Palpant N.J. Single-cell transcriptomic analysis of cardiac differentiation from human PSCs reveals HOPX-dependent cardiomyocyte maturation. Cell Stem Cell. 2018;23:586–598. doi: 10.1016/j.stem.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.H., Iascone D.M., Petrof I., Hazra A., Zhang X., Pyfer M.S., Chin J. Early Seizure activity accelerates depletion of hippocampal neural stem cells and impairs spatial discrimination in an Alzheimer’s disease model. Cell Rep. 2019;27(13):3741–3751. doi: 10.1016/j.celrep.2019.05.101. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J.R., Cruz‐Rodriguez R. Mesial temporal sclerosis: pathogenesis, diagnosis, and management. Epilepsia. 1990;31:S55–S66. doi: 10.1111/j.1528-1157.1990.tb05860.x. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Affolter M., Bürglin T. Annual Review of Biochemistry. Annual Reviews Inc; 1994. Homeodomain proteins. [DOI] [PubMed] [Google Scholar]
- Gomes F.C.A., Garcia-Abreu J., Galou M., Paulin D., Moura Neto V. Neurons induce GFAP gene promoter of cultured astrocytes from transgenic mice. GLIA. 1999;26(2):97–108. doi: 10.1002/(SICI)1098-1136(199904)26:2<97::AID-GLIA1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Griffin W.S.T., Yeralan O., Sheng J.G., Boop F.A., ak R.E., Rovnaghi C.R., Van Eldik L.J. Overexpression of the neurotrophic cytokine S100β in human temporal lobe epilepsy. J. Neurochem. 1995;65(1):228–233. doi: 10.1046/j.1471-4159.1995.65010228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D., Zou J., Wong M. Rapamycin attenuates acute seizure-induced astrocyte injury in mice in vivo. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-03032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Li H., Zhang Y., Qin J., Yang Q., Wang L., Xia C. Brain lipid-binding protein promotes proliferation and modulates cell cycle in C6 rat glioma cells. Int. J. Oncol. 2017;51(5):1439–1448. doi: 10.3892/ijo.2017.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher C.J., Basson C.T. Circulation Research. Lippincott Williams & Wilkins; 2009. Specification of the cardiac conduction system by transcription factors. September 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hng C.H., Camp E., Anderson P., Breen J., Zannettino A., Gronthos S. HOPX regulates bone marrow-derived mesenchymal stromal cell fate determination via suppression of adipogenic gene pathways. Sci. Rep. 2020;10(1):11345. doi: 10.1038/s41598-020-68261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley A.J., Lugo J.N. Effects of an acute seizure on associative learning and memory. Epilepsy Behav. 2016;54:51–57. doi: 10.1016/j.yebeh.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin D.M., McNeill R.S., Bash R.E., Miller C.R. Intrinsic astrocyte heterogeneity influences tumor growth in Glioma Mouse Models. Brain Pathol. 2017;27(1):36–50. doi: 10.1111/bpa.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismat F.A., Zhang M., Kook H., Huang B., Zhou R., Ferrari V.A., Patel V.V. Homeobox protein Hop functions in the adult cardiac conduction system. Circ. Res. 2005;96(8):898–903. doi: 10.1161/01.RES.0000163108.47258.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K., Nanobashvili A., Bonde S., Ekdahl C.T., Kokaia Z., Kokaia M., Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52(6):1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jessberger S., Parent J.M. Epilepsy and adult neurogenesis. Cold Spring Harbor Perspect. Biol. 2015;7(12) doi: 10.1101/cshperspect.a020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A., Opejin A., Henderson J.G., Gross C., Jain R., Epstein J.A., Hawiger D. Peripherally induced tolerance depends on peripheral regulatory T cells that require Hopx to inhibit intrinsic IL-2 expression. J. Immunol. 2015;195(4):1489–1497. doi: 10.4049/jimmunol.1500174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N., Sivalingam S., Boonstra R., Wojtowicz J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115(1):97–105. doi: 10.1016/S0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kipp M., Gingele S., Pott F., Clarner T., van der Valk P., Denecke B., Beyer C. BLBP-expression in astrocytes during experimental demyelination and in human multiple sclerosis lesions. Brain Behav. Immun. 2011;25(8):1554–1568. doi: 10.1016/j.bbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- De Lanerolle N.C., Lee T.S., Spencer D.D. Histopathology of human epilepsy. Epilepsia. 2010;51(SUPPL. 5):37. doi: 10.1111/j.1528-1167.2010.02823.x. [DOI] [Google Scholar]
- Lewis S.A., Balcarek J.M., Krekt V., Shelanskit M., Cowan N.J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments (cDNA expression library/cytoskeleton/astrocytes) Proc. Nati. Acad. Sci. USA. 1984;81:2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Matarin M., Reeves C., McEvoy A.W., Miserocchi A., Thompson P., Sisodiya S.M., Thom M. Doublecortin-expressing cell types in temporal lobe epilepsy. Acta Neuropathol. Commun. 2018;6(1):60. doi: 10.1186/s40478-018-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Takeda N., Jain R., Manderfield L.J., Liu F., Li L., Epstein J.A. Hopx distinguishes hippocampal from lateral ventricle neural stem cells. Stem Cell Res. 2015;15(3):522–529. doi: 10.1016/j.scr.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen J.L., Barker-Haliski M.L., Jill Dahle E., Steve White H., Wilcox K.S. Neuronal injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy. J. Neuropathol. Exp. Neurol. 2016;75(4):366–378. doi: 10.1093/jnen/nlw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M., Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Martinos M.M., Yoong M., Patil S., Chin R.F.M., Neville B.G., Scott R.C., de Haan M. Recognition memory is impaired in children after prolonged febrile seizures. Brain. 2012;135(10):3153–3164. doi: 10.1093/brain/aws213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott K.W., Barry D.S., McMahon S.S. Journal of Anatomy. Wiley-Blackwell; 2005. Role of radial glia in cytogenesis, patterning and boundary formation in the developing spinal cord. (September) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-N. January 24. [DOI] [PubMed] [Google Scholar]
- Merz K., Lie D.C. Evidence that doublecortin is dispensable for the development of adult born neurons in mice. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G. li, Song H. Adult neurogenesis in the Mammalian Brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.J., Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc. Natl. Acad. Sci. USA. 2008;105(16):6179–6184. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M., Eyo U.B., Xie M., Peng J., Bosco D.B., Umpierre A.D., Wu L.J. Microglial P2Y12 receptor regulates seizure-induced neurogenesis and immature neuronal projections. J. Neurosci. Official J. Soc. Neurosci. 2019;39(47):9453–9464. doi: 10.1523/JNEUROSCI.0487-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlfriedel S., Kirsch F., Gruss P., Stoykova A., Chowdhury K. A roof plate-dependent enhancer controls the expression of Homeodomain only protein in the developing cerebral cortex. Develop. Biol. 2005;283(2):522–534. doi: 10.1016/j.ydbio.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Oliver G., Sosa-Pineda B., Geisendorf S., Spana E.P., Doe C.Q., Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech. Develop. 1993;44(1):3–16. doi: 10.1016/0925-4773(93)90012-M. [DOI] [PubMed] [Google Scholar]
- Owada Y., Abdelwahab S.A., Kitanaka N., Sakagami H., Takano H., Sugitani Y., Kondo H. Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur. J. Neurosci. 2006;24(1):175–187. doi: 10.1111/j.1460-9568.2006.04855.x. [DOI] [PubMed] [Google Scholar]
- O’Keefe J., Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Parent J.M., Yu T.W., Leibowitz R.T., Geschwind D.H., Sloviter R.S., Lowenstein D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rana A., Musto A.E. Journal of Neuroinflammation. BioMed Central Ltd; 2018. The role of inflammation in the development of epilepsy. May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raponi E., Agenes F., Delphin C., Assard N., Baudier J., Legraverend C., Deloulme J.C. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. GLIA. 2007;55(2):165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risebro C.A., Petchey L.K., Smart N., Gomes J., Clark J., Vieira J.M., Yanni J., Dobrzynski H., Davidson S., Zuberi Z., Tinker A., Shui B., Tallini Y.I., Kotlikoff M.I., Miquerol L., Schwartz R.J., Riley P.R. Epistatic rescue of Nkx2.5 adult cardiac conduction disease phenotypes by prospero-related homeobox protein 1 and HDAC3. Circ. Res. 2012;111(2):19–31. doi: 10.1161/CIRCRESAHA.111.260695. [DOI] [PubMed] [Google Scholar]
- Robel S., Buckingham S.C., Boni J.L., Campbell S.L., Danbolt N.C., Riedemann T., Sontheimer H. Reactive astrogliosis causes the development of spontaneous seizures. J. Neurosci. 2015;35(8):3330–3345. doi: 10.1523/JNEUROSCI.1574-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijtenberg S., van den Heuvel S. Cell Cycle. Taylor and Francis Inc; 2016. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. January 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H.E. Vol. 911. New York Academy of Sciences; 2000. Epileptogenesis in the parahippocampal region. Parallels with the dentate gyrus; pp. 305–327. (Annals of the New York Academy of Sciences). [DOI] [PubMed] [Google Scholar]
- Scott R.C. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126(11):2551–2557. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- Shapiro L.A., Wang L., Ribak C.E. Vol. 49. Blackwell Publishing Inc; 2008. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats; pp. 33–41. (Epilepsia). [DOI] [PubMed] [Google Scholar]
- Shin C.H., Liu Z.P., Passier R., Zhang C.L., Wang D.Z., Harris T.M., Olson E.N. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110(6):725–735. doi: 10.1016/S0092-8674(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R., Watanabe J., Arata S., Funahashi H., Kikuyama S., Shioda S. A transgenic mouse model for the detailed morphological study of astrocytes. Neurosci. Res. 2003;47(4):451–454. doi: 10.1016/j.neures.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Tatum W.O. Mesial temporal lobe epilepsy. J. Clin. Neurophysiol. 2012;29:356–365. doi: 10.1097/WNP.0b013e31826b3ab7. [DOI] [PubMed] [Google Scholar]
- Thelin E.P., Tajsic T., Zeiler F.A., Menon D.K., Hutchinson P.J.A., Carpenter K.L.H., Helmy A. Frontiers in Neurology. Frontiers Media S.A; 2017. Monitoring the neuroinflammatory response following acute brain injury. July 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M., Eriksson S., Martinian L., Caboclo L.O., McEvoy A.W., Duncan J.S., Sisodiya S.M. Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: neuropathological features. J. Neuropathol. Exp. Neurol. 2009;68(8):928–938. doi: 10.1097/NEN.0b013e3181b05d67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., Shi J., Qin J., Jin G., Han X., Li H. Brain lipid binding protein mediates the proliferation of human glioblastoma cells by regulating ERK1/2 signaling pathway in vitro. In Vitro Cell. Develop. Biol. Anim. 2018;54(2):156–162. doi: 10.1007/s11626-017-0220-8. [DOI] [PubMed] [Google Scholar]
- Toni, A. De, Toni, A. De, Zbinden, M., Epstein, J.A., Ruiz, A., Prochiantz, A., & Caillé, I. (2008). Regulation of survival in adult hippocampal and glioblastoma stem cell lineages by the homeodomain-only protein HOP cell lineages by the homeodomain-only protein HOP, (May). 10.1186/1749-8104-3-13. [DOI] [PMC free article] [PubMed]
- Turski W.A., Cavalheiro E.A., Schwarz M., Czuczwar S.J., Kleinrok Z., Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983;9(3):315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Varma P., Brulet R., Zhang L., Hsieh J. Targeting Seizure-induced neurogenesis in a clinically relevant time period leads to transient but not persistent seizure reduction. J. Neurosci. Official J. Soc. Neurosci. 2019;39(35):7019–7028. doi: 10.1523/JNEUROSCI.0920-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R.W.H., Sluiter A.A., Balesar R.A., Baayen J.C., Noske D.P., Dirven C.M.F., Swaab D.F. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain. 2007;130(12):3321–3335. doi: 10.1093/brain/awm264. [DOI] [PubMed] [Google Scholar]
- Vezzani A., Fujinami R.S., White H.S., Preux P.M., Blümcke I., Sander J.W., Löscher W. Acta Neuropathologica. Springer Verlag; 2016. Infections, inflammation and epilepsy. February 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Ma Z., Zou W., Guo H., Liu M., Ma Y., Zhang L. The appropriate marker for astrocytes: comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. BioMed. Res. Int. 2019;2019 doi: 10.1155/2019/9605265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Dudley S.C. Evidence for inflammation as a driver of atrial fibrillation. Front. Cardiovascular Med. 2020;7:62. doi: 10.3389/fcvm.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel S., Marcy G., Lo Guidice Q., Li D., Heinrich C., Azim K., Raineteau O. HOPX defines heterogeneity of postnatal subventricular zone neural stem cells. Stem Cell Rep. 2018;11(3):770–783. doi: 10.1016/j.stemcr.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]