Highlights
-
•
Self-efficacy is negatively correlated with perceived stress in young adult drinkers.
-
•
Binge vs. non-binge drinking men show diminished PCC thickness and dmPFC GMV.
-
•
The metrics are positively/negatively each correlated with self-efficacy/stress.
-
•
Path analyses show daily drinks → neural metrics → low self-efficacy → high stress.
Keywords: Alcohol use disorder, Cerebral morphometrics, Impulsivity, Loss-of-control, Anxiety and depression
Abstract
Studies have identified cerebral morphometric markers of binge drinking and implicated cortical regions in support of self-efficacy and stress regulation. However, it remains unclear how cortical structures of self-control play a role in ameliorating stress and alcohol consumption or how chronic alcohol exposure alters self-control and leads to emotional distress. We examined the data of 180 binge (131 men) and 256 non-binge (83 men) drinkers from the Human Connectome Project. We obtained data on regional cortical thickness from the HCP and derived gray matter volumes (GMVs) with voxel-based morphometry. At a corrected threshold, binge relative to non-binge drinking men showed diminished posterior cingulate cortex (PCC) thickness and dorsomedial prefrontal cortex (dmPFC) GMV. PCC thickness and dmPFC GMVs were positively and negatively correlated with self-efficacy and perceived stress, respectively, as assessed with the NIH Emotion Toolbox. Mediation and path analyses to query the inter-relationships between the neural markers and clinical variables showed a best fit of the model with daily drinks → lower PCC thickness and dmPFC GMV → lower self-efficacy → higher perceived stress in men. In contrast, binge and non-binge drinking women did not show significant differences in regional cortical thickness or GMVs. These findings suggest a pathway whereby chronic alcohol consumption alters cortical structures and self-efficacy mediates the effects of cortical structural deficits on perceived stress in men. The findings also suggest the need to investigate multimodal neural markers underlying the interplay between stress, self-control and alcohol use behavior in women.
1. Introduction
1.1. Structural brain characteristics of binge drinking and heavy alcohol use
Frequently observed in young adults, binge drinking is associated with severe health consequences (Garcia et al., 2020, Powers et al., 2016, Stolle et al., 2009, Woo et al., 2017, Zullig et al., 2001). Numerous studies have examined the effects of alcohol misuse, including binge drinking, on the brain. For instance, morphometric studies have associated binge, heavy and/or dependent drinking with smaller volumes of the frontal, temporal, and parietal cortex, hippocampal complex, insula, cerebellum, and dorsal striatum (Gröpper et al., 2016, Rando et al., 2011, Syaifullah et al., 2021). Some studies reported larger volumes of nucleus accumbens in binge drinkers (Howell et al., 2013, Sousa et al., 2020) but another work reported otherwise (Mackey et al., 2019). Studies examining cortical thickness likewise associated thinning of frontal, temporal, parietal and occipital cortex, posterior cingulate, precuneus, and insula with heavy drinking (Bae et al., 2016, Cservenka and Brumback, 2017, Mackey et al., 2019). In a longitudinal study, the number of binge drinking episodes in the prior year predicted decrement in frontal and parietal cortical thickness in youth (Pfefferbaum et al., 2016).
Some of the studies reported sex differences, with adolescent male and female binge drinkers showing thinner and thicker left frontal cortices, respectively, as compared to their non-drinking counterparts (Squeglia et al., 2012). Another study showed that, compared to non– or light-drinking individuals, male and female binge drinkers showed lower and higher volumes, respectively, in prefrontal, temporal, motor, somatosensory cortical and striatal regions (Kvamme et al., 2015). Frequency of heavy drinking was associated with reduced frontal cortical thickness with larger effects in men (Morris et al., 2019). In contrast, a more recent work reported more negative effects of alcohol use on left orbitofrontal cortical thickness in women than in men (Thayer et al., 2016). Further, a descriptive review did not reveal sex differences in subcortical volumes or cortical thickness in relation to alcohol use (McPhee et al., 2018). While not entirely consistent, these findings suggest the importance of considering sex differences in examining the cerebral consequences of alcohol use.
1.2. Perceived stress, self-efficacy, and binge drinking
Other investigations identified volumetric correlates of psychological constructs that conduce to heavy alcohol use. For instance, we showed earlier that decreases in GMV of the left thalamus mediated the interaction effects of impulsivity and alcohol expectancy on problem alcohol use (Ide et al., 2017a). Alcohol misuse is also known to be associated with behavioral inhibition traits that implicate emotional processing dysfunction and perceived stress, anxiety and depression (Lannoy et al., 2018). For instance, negative affect along with coping motives support the effects of perceived stress on alcohol use behavior (Anker et al., 2019). Occupational stress contributed to alcohol misuse through social motives for drinking (Temmen and Crockett, 2020). Stress caused by discrepancy in perceived masculine role was associated with binge drinking (Yang et al., 2019). Stress may promote alcohol consumption via glucocorticoid interactions with the reward pathways and, for some individuals, the anxiolytic effects of alcohol influence the motivation to drink (Becker, 2017). As a maladaptive strategy to cope with stress, alcohol use in turn compromises emotional regulation and aggravates emotional distress, perpetuating alcohol use (Lee et al., 2018, Wellman et al., 2014). Importantly, although men are more likely to engage in binge drinking than women (Merlo et al., 2017) (see (Wilsnack et al., 2018) for increasing rates of female binge drinkers), women appear to be more vulnerable to emotional stress and mood disturbance (Kuntsche et al., 2017) and the effects of stress on alcohol consumption (Deguchi et al., 2018). Thus, it is critical to examine sex differences in the relationship between perceived stress and alcohol use and the neural bases of these differences.
Perceived stress vary according to individual variation in self-efficacy across a wide variety of settings both in healthy and clinical populations. Self-efficacy refers to individual’s belief in the competence in executing behaviors to achieve a desired outcome (Bandura, 1990, Haycock et al., 1998) or in the capacity to regulate behavior, thoughts and emotions (Carver and Scheier, 1982). Self-efficacy may mitigate the effects of perceived stress on alcohol use in recovering alcohol dependent individuals (Gu et al., 2020) and on self-care across a variety of clinical conditions, including, for instance, sugar control in diabetics (Indelicato et al., 2017). Thus, it would be of importance to examine how alcohol use, self-efficacy and perceived stress are inter-related.
1.3. Morphometric correlates of perceived stress and self-control
Stress is a health risk, and individuals with higher daily stress demonstrated diminished GMV of the medial prefrontal cortex (mPFC) (Ansell et al., 2012), as observed in patients with depression and posttraumatic stress disorder (Kroes et al., 2011). Higher Perceived Stress Scale scores, an indicator of chronic life stress, predicted lower GMV in the right orbitofrontal cortex and hippocampus (Gianaros et al., 2007). Hippocampal GMV was negatively associated with perceived stress and the severity of depression (Joss et al., 2020, Merz et al., 2018) and anxiety (Gold et al., 2017, Mueller et al., 2013) across neurotypical and clinical populations. Notably, the mPFC is known to play a critical role in self-control (Cook, 2014). In structural imaging studies, for instance, lower mPFC GMV was associated with poor cognitive control in the Stroop task (Wang et al., 2015) and with individually reported lack of self-control (Matsui et al., 2002). Thus, it would be of specific interest to explore how the mPFC may be implicated in the inter-relationship of self-efficacy, perceived stress, and alcohol use severity.
1.4. The present study
We aimed to characterize the clinical characteristics and neural correlates of binge drinking by taking advantage of a large imaging data set of the Human Connectome Project (HCP). We employed group by sex covariance analyses to examine the clinical characteristics and structural brain measures and followed up with mediation and path analyses to reveal the potentially mediating roles of the structural brain markers in the relationship of perceived stress, self-efficacy and alcohol use.
2. Materials and Methods
2.1. Dataset and demographics
For the current study, we have obtained permission from the HCP to use both the Open and Restricted Access data. As in our previous work (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c), we employed the 1200 Subjects Release (S1200) data set, including behavioral and 3 T MR imaging data of 1206 healthy young adult participants (1113 with structural MR scans) collected from 2012 to 2015. Binge drinking was defined as having four or more drinks for women, or five or more drinks for men on a single day (Wechsler et al., 1994). The binge drinking group comprised 180 adults who reported binge drinking at least once a week for the last 12 months (131 males, 72.8%). A total of 312 adults reported no binge drinking in the prior year. However, 56 of the 312 met criteria for life time history of either alcohol abuse or dependence or had binge drinking history in their heaviest 12 months period and were excluded, leaving 256 adults (83 males, 32.4%) in the non-binge drinking group. Thus, the data of a total of 436 adults (214 men, 22–36 with mean ± SD = 27.7 ± 3.5 years; 222 women, 22–36 or 29.8 ± 3.6 years) were obtained in this study, with more men than women in the binge drinking group (x2 = 68.87, p < 0.001, chi-square test). A total of 272 of the 436 subjects were twins or siblings. Thus, family coding was included as an additional covariate in all subsequent analyses. Linear mixed effects model showed a significant group (F(1,432) = 4.39, p = 0.037) and sex (F(1,432) = 17.72, p < 0.001) main as well as group × sex interaction (F(1,432) = 8.05, p = 0.005) effect in age.
All subjects were physically healthy with no severe neurodevelopmental, neuropsychiatric or neurological disorders. Participants provided written informed consent and all aspects of the study, including subject recruitment, experimental procedures were conducted according to a protocol in accordance with the Declaration of Helsinki and approved by the Washington University Institutional Review Board (IRB #201204036; title: “Mapping the Human Connectome: Structure, Function and Heritability”).
2.2. Behavioral and clinical measures
All participants reported the average number of drinks consumed per drinking day (not just binge drinking day) in past 12 months: binge drinking men (mean ± SD = 4.3 ± 1.3), non-binge drinking men (1.2 ± 0.8); binge drinking women (3.3 ± 1.5); non-binge drinking women (1.1 ± 0.8). Linear mixed effects model showed a significant group (F(1,430) = 487.72, p < 0.001) and sex (F(1,430) = 20.73, p < 0.001) main as well as group × sex interaction (F(1,430) = 17.08, p < 0.001) effect. This along with other drinking metrics available from the HCP are summarized in Supplementary Table S1.
All participants were evaluated with the NIH-Toolbox Emotion Measures – 18+ (i.e., > 18 years old) battery – which consists of 4 sub-domains: negative affect, psychological well-being, stress and self-efficacy, and social relationships, and a total of 17 subscales: anger-affect, anger-hostility, anger-physical aggression, fear-affect, fear-somatic arousal, sadness, general life satisfaction, meaning and purpose, positive affect, friendship, loneliness, perceived hostility, perceived rejection, emotional support, instrumental support, perceived stress, self-efficacy.
Perceived stress, characterized by self-reported perceptions about the nature of events and their relationship to the values and coping resources of an individual. There are 10 items in Perceived Stress subscale each scored from 1 to 5 or 5 to 1 (reverse scored), so the score sums to 10–50, with a higher score indicating higher perceived stress. Self-efficacy, characterized by a person’s self-reported belief in his/her capacity to manage functioning and effect control over meaningful events. There are 10 items in Self-Efficacy subscale each scored from 1 to 4, so the score sums from 10 to 40, with a higher score indicating higher self-efficacy. All NIH Toolbox Emotion measures come with T-scores, which are standard scores in which a T-score of 50 represents the mean of the US general population (based on the 2010 Census) and 10 T-score units represents one standard deviation. The Perceived Stress T score ranged from 22.4 to 80.5 and Self-Efficacy T score ranged from 24.1 to 68.4 for the current sample.
For perceived stress and self-efficacy scores, we performed a linear mixed effects model with age and family coding as covariates. We also performed a linear regression each between perceived stress and self-efficacy scores and the average number of daily drinks in the prior 12 months, with age and family coding as covariates.
2.3. Imaging protocol
Magnetic resonance imaging was done using a customized 3 T Siemens Connectome Skyra with a standard 32-channel Siemens receiver head coil and a body transmission coil. T1-weighted high-resolution structural images were acquired using a 3D MPRAGE sequence with 0.7 mm isotropic resolution (FOV = 224 × 224 mm, matrix = 320 × 320, 256 sagittal slices, TR = 2400 ms, TE = 2.14 ms, TI = 1000 ms, FA = 8°). T2-weighted high-resolution structural images were acquired using a 3D T2-SPACE sequence with 0.7 mm isotropic resolution (FOV = 224 × 224 mm, matrix = 320 × 320, 256 sagittal slices, TR = 3200 ms, TE = 565 ms). Images were pre-processed to correct for distortions introduced by gradient non-linearities, remove readout distortions, correct for bias field distortions and align the images to the MNI space template.
2.4. Cortical thickness and gray matter volumes
Cortical thickness was computed by the HCP (Van Essen et al., 2013). Both T1- and T2-weighted images were used to segment the cortical grey and white matter, and a cortical surface reconstruction generated with FreeSurfer. Morphometric parameters are derived from the surface reconstruction, with cortical thickness estimated as the geometric distance between the white and grey matter surfaces. There are a total of 68 regions of interest (ROI). Thus, we evaluated the results of group analyses at a corrected threshold of p < 0.05/68 = 0.00074.
We implemented voxel-based morphometry (VBM) to quantify regional gray matter volumes (GMVs) with the CAT12 toolbox (http://dbm.neuro.uni-jena.de/vbm/), as detailed in the Supplementary Methods and our previous work (Hu et al., 2018, Ide et al., 2020). In group analyses we performed a group by sex flexible factorial with age, family coding and total intracranial volume as covariates and evaluated the group and sex main and interaction effects at voxel p < 0.001, uncorrected in combination with a cluster p < 0.05 FWE-corrected, on the basis of Gaussian random field theory as implemented in the SPM.
2.5. Mediation and path analyses
We performed mediation analyses following published routines (MacKinnon et al., 2007, Wager et al., 2008), as detailed in the Supplementary Methods and our previous work (Hu et al., 2018, Ide et al., 2017b, Le et al., 2020a, Le et al., 2019, Wang et al., 2020, Zhornitsky et al., 2019), to evaluate the relationships between neural markers, self-efficacy and perceived stress (see Results).
With path analysis we evaluated the relationships among neural markers, the severity of recent alcohol use, self-efficacy, and perceived stress in men (see Results). Path analysis involves a set of exogenous variables with variance not accounted for by the model and endogenous variables with variance explained in part by other variables in the model (Le et al., 2020b, Wuensch, 2016). Path analysis is conducted with regression analysis, which predicts the effects of all other variables on the endogenous variables. The β weights from these multiple regressions are the path coefficients. Standardized path coefficients convey assumptions about the directionality of interactions between variables. Model fit is typically assessed with fit indices that include the root mean square estimation of approximation (≤0.08 for an acceptable fit), chi-square (χ2/df, ≤3), comparative fit index (≥0.9), and standardized root mean square residual (≤0.08) (Hu and Bentler, 1995).
3. Results
3.1. Clinical measures
Perceived stress and self-efficacy scores were shown for each of the four groups in Fig. 1A and 1B. Linear mixed effects model with age and family as covariates showed that there were no significant group (F(1,430) = 2.36, p = 0.126) or sex (F(1,430) < 0.01, p = 0.960) main or group by sex interaction (F(1,430) = 0.03, p = 0.860) effect for perceived stress score; and no significant group (F(1,430) = 2.17, p = 0.141) or sex (F(1,430) = 0.02, p = 0.884) main or group by sex interaction (F(1,430) = 0.49, p = 0.483) effect for self-efficacy score. In post-hoc comparisons, binge and non-binge drinking groups did not differ in perceived stress or self-efficacy in men or in women alone (all p’s > 0.090).
Fig. 1.
Perceived stress and self-efficacy scores. (A) Perceived stress and (B) self-efficacy score of men binger (MB), men non-binger (MnB), women binger (WB), and women non-binger (WnB): mean ± SE. Correlation between perceived stress and self-efficacy scores in (C) men and (D) women.
Perceived stress and self-efficacy scores were negatively correlated across all subjects (r = -0.47, p = 1.2 × 10-25) and in men (r = -0.42, p = 2.0 × 10-10; Fig. 1C) and women (r = -0.52, p = 5.0 × 10-17; Fig. 1D) separately.
Linear mixed effects model with age and family as covariates showed a significant group (F(1,430) = 487.72, p < 0.001) and sex (F(1,430) = 20.73, p < 0.001) main as well as group × sex interaction (F(1,430) = 17.08, p < 0.001) effect in the average number of daily drinks consumed in the past 12 months (Fig. 2C). In post-hoc analyses both men and women showed higher daily number of drinks in the binger than non-binger group (men: t = 20.58, p < 0.001; women: t = 8.94, p < 0.001; two-sample t test with age and family as covariates) with a greater effect size in men. However, perceived stress scores and daily number of drinks were not correlated across all subjects (r < 0.01, p = 0.929), in men (r = 0.01, p = 0.843), or in women (r = -0.07, p = 0.315). Self-efficacy scores and daily number of drinks were also not correlated across all subjects (r = 0.06, p = 0.202), in men (r = 0.05, p = 0.437), or in women (r = 0.08, p = 0.231).
Fig. 2.
(A) Left posterior cingulate cortex. (B) The left PCC thickness and (C) average daily number of drinks consumed in the prior year (mean ± SE) of men binger (MB), men non-binger (MnB), women binger (WB), and women non-binger (WnB). Note that these are mean numbers of daily drinks across all days and not just binge drinking days (D) Linear regressions of left PCC thickness vs. average daily drinks in the prior year in men and women. **p ≤ 0.001.
3.2. Cortical thickness and gray matter volumes
For cortical thickness, the results of the linear mixed effects models are shown for all 68 ROIs as available in the HCP data set (Supplementary Table S2). At a corrected threshold of p < 0.05/68 = 0.00074 the thickness of left posterior cingulate cortex (left PCC, Fig. 2A; F(1,430) = 13.2, p < 0.001) showed a significant group main effect but not a sex main or interaction effect. In post-hoc analyses only men showed diminished left PCC thickness in the binger than non-binger group (Fig. 2B; t = -3.75, p < 0.001; two-sample t test with age and family as covariates).
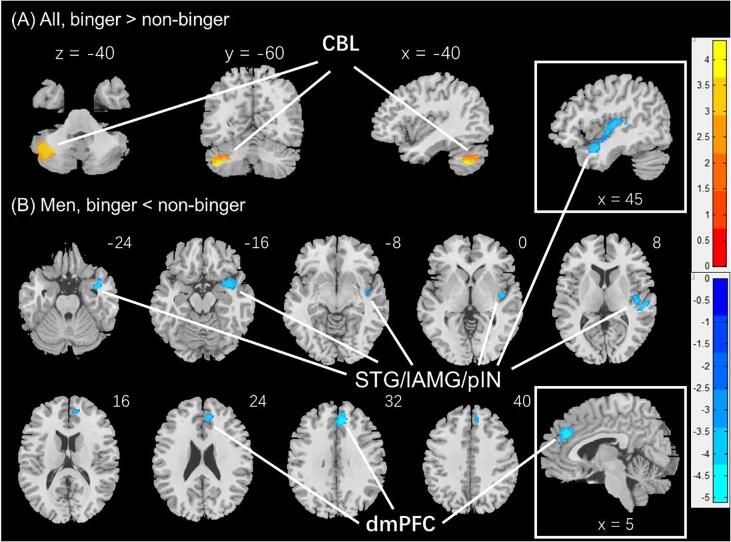
In the analyses of the GMVs, the left cerebellum (-51, −60, −48; Z = 4.49, 3254 mm3) showed higher GMV in binger vs. non-binger. There were no significant group by sex interaction effects. In men alone, the dorsomedial prefrontal cortex (dmPFC), in the area of the anterior cingulate and paracingulate gyri (8, 38, 30; Z = 5.11, 3763 mm3) and a large cluster (42, −12, 3; Z = 4.33, 7840 mm3) comprising the right superior temporal gyrus, probably including the lateral amygdala and posterior insula, showed higher GMV in non-binger vs. binger group (Fig. 3). In post-hoc analyses, the latter cluster also showed a significant group by sex interaction effect (F(1,429) = 4.12, p = 0.043), with men (t = -3.14, p = 0.002) but not women (t = -0.48, p = 0.629) showing the group differences. Women alone did not show differences in GMVs between the two drinking groups. These clusters are summarized in Table 1.
Fig. 3.
Regional gray matter volumes showing differences between binge and non-binge drinkers. (A) All, binger vs. non-binger, (B) Men, binger vs. non-binger. Voxel p < 0.001, uncorrected. All clusters with cluster p < 0.05, corrected for family-wise error, are shown in Table 1. Color bars show voxel t values; warm: binger > non-binger, cool: non-binger > binger. Clusters are overlaid on a T1 structural image in neurological orientation: right = right. CBL: cerebellum; STG/IAMG: superior temporal gyrus/lateral amygdala; pIN: posterior insula; dmPFC; dorsomedial prefrontal cortex.
Table 1.
GMV estimates of the ROIs identified from group analyses and statistics of linear mixed effects model.
| Cluster | MB (n = 131) | MnB (n = 83) | WB (n = 49) | WnB (n = 173) | Main effect |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group |
Sex |
Interaction |
||||||||
| F429 | p | F429 | p | F429 | p | |||||
| All, binger > non-binger | ||||||||||
| CBL | 0.50 ± 0.06 | 0.47 ± 0.06 | 0.46 ± 0.06 | 0.44 ± 0.06 | 9.81 | 0.002 | 0.01 | 0.913 | 0.00 | 0.981 |
| Men, non-binger > binger | ||||||||||
| dmPFC | 0.67 ± 0.08 | 0.68 ± 0.08 | 0.61 ± 0.07 | 0.60 ± 0.07 | 4.33 | 0.038 | 0.10 | 0.751 | 2.12 | 0.146 |
| STG/lAMG/pIN | 0.51 ± 0.05 | 0.53 ± 0.06 | 0.47 ± 0.04 | 0.47 ± 0.04 | 7.34 | 0.007 | 0.15 | 0.698 | 4.12 | 0.043 |
Note: F and p values of linear mixed effects model with age, family and total intracranial volume as covariates. All values are mean ± SD. CBL: cerebellum (MNI: −51–60 −48); dmPFC: dorsomedial prefrontal cortex (8 38 30); STG/lAMG/pIN: superior temporal gyrus/lateral nucleus of the amygdala and posterior insula (42–12 3). MB: men binger; MnB: men non-binger; WB: women binger; WnB: women non-binger.
3.3. Correlation between morphometric and clinical measures
Left PCC thickness was not significantly correlated with perceived stress in entire cohort (r = -0.04, p = 0.416), with age, family and sex as covariates, but significantly correlated with self-efficacy (r = 0.15, p = 0.002) and daily number of drinks (r = -0.24, p < 0.001). In men, with age and family as covariates, left PCC thickness was significantly correlated with perceived stress (r = -0.14, p = 0.044), self-efficacy (r = 0.22, p = 0.001), and daily number of drinks (r = -0.30, p < 0.001); in women, none of the correlations were significant (perceived stress: r = 0.08, p = 0.230; self-efficacy: r = 0.06, p = 0.375; daily number of drinks: r = -0.11, p = 0.111). Slope tests showed that the linear regressions were significantly different in slope between men and women in perceived stress (Z = -2.29; p = 0.022; Fig. 4A) and daily number of drinks (Z = -2.06; p = 0.0394; Fig. 2D), but not in self-efficacy (Z = 1.7; p = 0.0891; Fig. 4B) vs. left PCC thickness.
Fig. 4.
Linear regression between cortical thickness of the left PCC and (A) perceived stress, (B) self-efficacy, and between GMV of dmPFC and (C) perceived stress, (D) self-efficacy in men, (M, blue) and women (W, green).
Two clusters were identified showing lower GMV in binge vs. non-binge drinking men. We derived for all subjects the GMV of these two regions of interest (ROI) for a linear regression each against perceived stress and self-efficacy score, with age, sex, family and TIV as covariates for the entire group. We also performed the same regression for men and women separately with age, family and TIV as covariates. The results showed a negative correlation between dmPFC GMV and the perceived stress (r = -0.23, p = 0.001) and a positive correlation between dmPFC GMV and self-efficacy score (r = 0.20, p = 0.003) in men, but no significant correlations in women. We examined whether men and women differed in these correlations with a slope test (Zar, 1999). The results showed that the two linear regressions were significantly different in slope between men and women (perceived stress: Z = -2.8, p = 0.0051, Fig. 4C; self-efficacy: Z = 2.08, p = 0.0375, Fig. 4D).
The GMV of neither cluster showed a significant correlation with daily number of drinks (both p’s > 0.118).
3.4. Mediation and path analysis
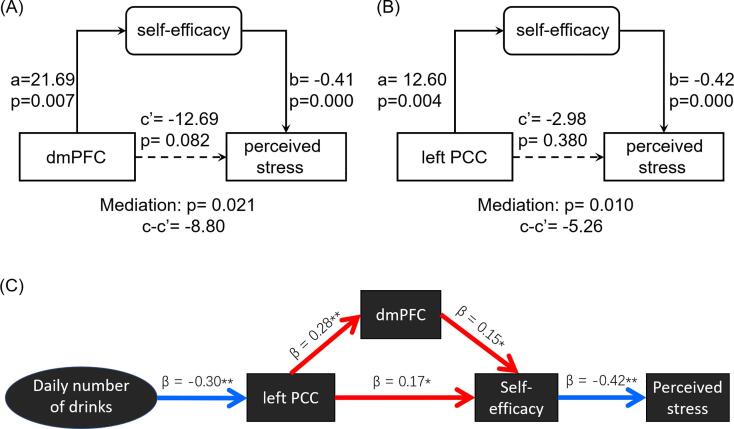
Because the perceived stress and self-efficacy scores were significantly correlated in men, we performed mediation analyses to investigate the inter-relationship between perceived stress, self-efficacy and dmPFC GMV as well as left PCC thickness, with age and family as covariates, in men.
For each set of analysis, we examined all six models and employed a corrected p value of 0.05 to evaluate the mediation effects. In men, the model of dmPFC GMV → self-efficacy → perceived stress showed a significant (path c – c’, p = 0.021) and complete (path c’, p = 0.082) mediation effect (Fig. 5A). The model of left PCC thickness → self-efficacy → perceived stress showed a significant (path c – c’, p = 0.010) and complete (path c’, p = 0.380) mediation effect (Fig. 5B). The results of all other mediation models are shown in Supplementary Figure S1 and S2, respectively.
Fig. 5.
Mediation analyses between perceived stress, self-efficacy and (A) dmPFC GMV and (B) left PCC cortical thickness in men. (C) Path analysis showed the model with a significant fit. Red arrows and blue arrows mean positive and negative relationship, respectively. Age and family as covariates in mediation and path analyses. **p < 0.01, *p < 0.05.
As described earlier, the average daily number of drinks in the prior year was significantly correlated with left PCC thickness (r = -0.30, p < 0.001) in men, in accord with previous findings of diminished PCC thickness and volumes as a result of chronic drinking (Mashhoon et al., 2014, Rando et al., 2011). Thus, we built on the findings of mediation models and conducted path analyses to characterize the relationship among the daily number of drinks, left PCC thickness, dmPFC GMV, self-efficacy and perceived stress. Specifically, we aimed to distinguish the directional influences between left PCC thickness and dmPFC GMV. The results showed a significant model fit with daily drinks → lower PCC thickness → dmPFC GMV → lower self-efficacy → higher perceived stress (Fig. 5C; fit indices: RMSEA = 0.071 [90% CI: 0.042 0.116], χ2/df = 2.401, SRMR = 0.065, and CFI = 0.897) but not with daily drinks → dmPFC GMV → lower PCC thickness → lower self-efficacy → higher perceived stress (fit indices: RMSEA = 0.123 [90% CI: 0.088 0.160], χ2/df = 4.217, SRMR = 0.083, and CFI = 0.649).
4. Discussion
Young adult binge as compared to non-binge drinkers did not demonstrate significant differences in perceived stress or self-efficacy as evaluated by the NIH Emotion Toolbox, whereas perceived stress was negatively correlated with self-efficacy across subjects. Binge relative to non-binge drinkers showed diminished GMV of the dmPFC and thickness of the left PCC in men only. The relationship between dmPFC GMV or left PCC thickness and perceived stress in men was completely mediated by self-efficacy. Further, in path analysis, daily number of drinks in the prior year modulated perceived stress via PCC thickness, dmPFC GMV and self-efficacy in men. Both PCC and dmPFC are implicated in self-control and these structural brain findings may suggest dysfunctional self-control, likely as a result of chronic alcohol consumption, in male drinkers. In contrast, the lack of significant findings suggest the need of multimodal, including functional, imaging markers to investigate the inter-relationship of self-control, perceived stress and problem alcohol use in women. We discuss the main findings in the below.
4.1. Morphometric correlates of binge drinking and potential sex differences
Compared with non-binge drinkers, binge drinkers showed larger GMV in the cerebellum. While not consistent with alcohol’s neurotoxic effects on the brain (Lisdahl et al., 2013), similar findings have been reported in some of the earlier volumetric studies (Anderson et al., 2010, Kühn et al., 2019, Wilson et al., 2015). This along with these previous findings can be considered together with the literature implicating cerebellar dysfunction in alcohol addiction (Pitel et al., 2015). In particular, alcohol is known to alter the development of cerebellar circuitry following the loss of Purkinje cells, and the increases in cerebellar volume may reflect functional compensation, in contrast with findings of volume loss in chronic, dependent drinkers (Sullivan et al., 1995).
In men only, binge drinkers demonstrated smaller GMV in the dmPFC and a cluster encompassing the right-hemispheric superior temporal gyrus, lateral amygdala, and posterior insula. While smaller GMV of the latter regions have been reported in problem drinkers (Jang et al., 2007, Kvamme et al., 2015, Makris et al., 2008), the functional significance remains to be investigated. In men, dmPFC GMV was positively and negatively correlated with self-efficacy and perceived stress, respectively, consistent with previous volumetric studies of heavy drinkers (Cservenka and Brumback, 2017) and impulse control dysfunction in binge drinkers (Banca et al., 2016).
We also observed thinner PCC in binge drinkers, in accord with an earlier report (Mashhoon et al., 2014). Although post-hoc simple comparisons confirmed the difference only in men, the ANOVA did not show a significant sex difference. Binge and non-binge drinking women did not differ in any regional thickness measures at the same statistical threshold. Therefore, in contrast to earlier studies reporting lower cortical GMV and/or thickness in male but the opposite in female binge drinkers (Kvamme et al., 2015, Squeglia et al., 2012), we did not observe morphometric differences in opposite directions between the sexes. The PCC is a hub of the default mode network and, as with the dmPFC, has been implicated in decision-making particularly in situations that require mentalizing and internal representation (Leech and Smallwood, 2019). With its extensive connections with the limbic motor (e.g., midcingulate) and emotional memory (e.g., hippocampus) circuit, the PCC may support emotion-modulated actions (Rolls, 2019). Notably, our studies of the HCP data showed higher PCC activation in men vs. women during identification of facial emotions (Li et al., 2020b) and social interactions (Li et al., 2020a). In a study of alcohol drinkers engaged in a reward go/no-go task, PCC response to avoidance – behavioral inhibition to prevent monetary loss – completely and bidirectionally mediated the relationship between punishment sensitivity and hazardous alcohol use (Le et al., 2019). Thus, the current findings of thinner PCC may suggest higher vulnerability of male binge drinkers to emotion processing dysfunction and affect-laden decision making.
4.2. Perceived stress, self-efficacy, and structural brain markers of drinking
Perceived stress did not differ between drinking groups in this cohort of young adults, consistent with previous findings (Brook et al., 2017, Garcia et al., 2020, Tavolacci et al., 2013). In contrast with earlier reports (Bonar et al., 2011, Martínez-Montilla et al., 2020), we did not observe a significant difference in self-efficacy between binge and non-binge drinkers. On the other hand, perceived stress was negatively correlated with self-efficacy for the entire cohort as well as for all four sub-groups, suggesting self-efficacy as a psychological mediator of perceived stress (Guo et al., 2019, Liu and Aungsuroch, 2019, Makara-Studzińska et al., 2019, Smeds et al., 2020) even in a young, non-clinical population.
In men, dmPFC GMV was negatively and positively correlated with perceived stress and self-efficacy, respectively. Further, self-efficacy mediated the relationship between dmPFC GMV and perceived stress. This finding is broadly consistent with an earlier report of more significant loneliness in men relative to women and of the role of self-efficacy in associating reduced regional white matter density in the dmPFC with loneliness (Nakagawa et al., 2015). As described earlier, self-efficacy refers to individual’s ability in goal-directed behavioral control. Extensive research has implicated dmPFC in cognitive control, including error and conflict monitoring (Barch et al., 2000, Brown and Braver, 2005), reward processing and outcome evaluation (Hadland et al., 2003, Rogers et al., 2004), as well as decision making under risk and uncertainty (Hadland et al., 2003, Kennerley et al., 2006). Thus, the current findings of self-efficacy inter-relating diminished dmPFC GMV to perceived stress in men is consistent with the broad conceptual scheme of dmPFC function.
In path models, we showed that dmPFC GMV mediated the effects of PCC thickness on self-efficacy and perceived stress. When the average number of daily drinks was included in the model, we further demonstrated an effect of the severity of recent alcohol consumption on PCC thickness in its path to influence perceived stress. Although we did not observe a significant correlation between perceived stress and recent alcohol use severity, the finding does not necessarily suggest that perceived stress is inconsequential as a risk factor of alcohol misuse. In fact, a few studies also failed to observe a significant relationship between the level of perceived stress and frequencies of binge drinking (Garcia et al., 2020), or between depression or anxiety levels and hazardous drinking in college students (Nourse, 2017). It is possible that the impact of perceived stress on alcohol use behavior would best reveal in studies with longitudinal assessment of stress and alcohol use.
4.3. Limitations of the study and conclusions
A number of limitations need to be considered for the current study. First, the findings of mediation and path analyses only suggest directional influences. Whether or how lower dmPFC GMV and left PCC thickness aggravates emotional distress via diminished self-control needs be verified experimentally. Second, although alcohol use severity varied significantly across individuals, the HCP data represent largely a non-clinical sample. Thus, whether the current findings extend to addicted individuals needs to be examined. Third, the “negative” findings in women may result from less severe alcohol use in women than in men, as reflected in the average daily number of drinks in the prior year. Studies with multimodal imaging may also be needed in identifying neural markers of binge drinking in women. Investigations of the sex differences in the psychological and neural processes would inform both the pathophysiology and treatment of problem alcohol consumption. Further, the sample comprised more bingers in men and more non-bingers in women and, although we have examined sex differences specifically for the findings revealed in men or women alone, it remains possible that this asymmetry in group representation may have influenced the results. Finally, we conducted mediation and path analyses on cross-sectional data, and the findings need to be interpreted with caution. More broadly, these findings need to be replicated in an independent data set.
To conclude, we showed that morphometric alterations of the dmPFC and PCC as a result of chronic alcohol consumption may alter self-control and aggravate emotional distress. It is likely that, as stress elicits alcohol use, this pathophysiological process perpetuate habitual and heavy drinking.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The current study is supported by NIH grants DA051922, AG072893, AA021449 (C-SRL) and National Key Research and Development Program of China No.2020YFC2007300, No. 2020YFC2007301 (XT). Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102866.
Contributor Information
Xiaoying Tang, Email: xiaoying@bit.edu.cn.
Chiang-Shan R. Li, Email: chiang-shan.li@yale.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anderson C.M., Rabi K., Lukas S.E., Teicher M.H. Cerebellar lingula size and experiential risk factors associated with high levels of alcohol and drug use in young adults. Cerebellum (London, England) 2010;9(2):198–209. doi: 10.1007/s12311-009-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Kummerfeld E., Rix A., Burwell S.J., Kushner M.G. Causal Network Modeling of the Determinants of Drinking Behavior in Comorbid Alcohol Use and Anxiety Disorder. Alcohol. Clin. Exp. Res. 2019;43(1):91–97. doi: 10.1111/acer.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell E.B., Rando K., Tuit K., Guarnaccia J., Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol. Psychiatry. 2012;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., Kang I., Lee B.C., Jeon Y., Cho H.B., Yoon S., Lim S.M., Kim J., Lyoo I.K., Kim J.E., Choi I.-G. Prefrontal Cortical Thickness Deficit in Detoxified Alcohol-dependent Patients. Experimental neurobiology. 2016;25(6):333–341. doi: 10.5607/en.2016.25.6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banca P., Lange I., Worbe Y., Howell N.A., Irvine M., Harrison N.A., Moutoussis M., Voon V. Reflection impulsivity in binge drinking: behavioural and volumetric correlates. Addict. Biol. 2016;21(2):504–515. doi: 10.1111/adb.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Perceived self-efficacy in the exercise of personal agency. Journal of Applied Sport Psychology. 1990;2(2):128–163. [Google Scholar]
- D.M. Barch T.S. Braver F.W. Sabb D.C. Noll Anterior Cingulate and the Monitoring of Response Conflict: Evidence from an fMRI Study of Overt Verb Generation 12 2 2000 2000 298 309. [DOI] [PubMed]
- Becker H.C. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 2017;122:115–126. doi: 10.1016/j.neuropharm.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonar E.E., Rosenberg H., Hoffmann E., Kraus S.W., Kryszak E., Young K.M., Ashrafioun L., Pavlick M., Bannon E.E. Measuring university students' self-efficacy to use drinking self-control strategies. Psychol. Addict. Behav. 2011;25(1):155–161. doi: 10.1037/a0022092. [DOI] [PubMed] [Google Scholar]
- Brook M.J., Christian L.M., Hade E.M., Ruffin M.T. The Effect of Perceived Stress on Epstein-Barr Virus Antibody Titers in Appalachian Ohio Women. NeuroImmunoModulation. 2017;24(2):67–73. doi: 10.1159/000478658. [DOI] [PubMed] [Google Scholar]
- Brown J.W., Braver T.S. Learned Predictions of Error Likelihood in the Anterior Cingulate Cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Carver C.S., Scheier M.F. Control theory: A useful conceptual framework for personality–social, clinical, and health psychology. American Psychological Association. 1982;92(1):111–135. [PubMed] [Google Scholar]
- Cook J.L. Task-relevance dependent gradients in medial prefrontal and temporoparietal cortices suggest solutions to paradoxes concerning self/other control. Neurosci. Biobehav. Rev. 2014;42:298–302. doi: 10.1016/j.neubiorev.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Cservenka A., Brumback T. The Burden of Binge and Heavy Drinking on the Brain: Effects on Adolescent and Young Adult Neural Structure and Function. Front. Psychol. 2017;8:1111. doi: 10.3389/fpsyg.2017.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi Y., Iwasaki S., Kanchika M., Nitta T., Mitake T., Nogi Y., Kadowaki A., Niki A., Inoue K., Chen W. Gender differences in the relationships between perceived individual-level occupational stress and hazardous alcohol consumption among Japanese teachers: A cross-sectional study. PLoS ONE. 2018;13(9):e0204248. doi: 10.1371/journal.pone.0204248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Rouchy E., Galéra C., Tzourio C., Michel G. The relation between ADHD symptoms, perceived stress and binge drinking in college students. Psychiatry Res. 2020;284:112689. doi: 10.1016/j.psychres.2019.112689. [DOI] [PubMed] [Google Scholar]
- Gianaros P.J., Jennings J.R., Sheu L.K., Greer P.J., Kuller L.H., Matthews K.A. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold A.L., Steuber E.R., White L.K., Pacheco J., Sachs J.F., Pagliaccio D., Berman E., Leibenluft E., Pine D.S. Cortical Thickness and Subcortical Gray Matter Volume in Pediatric Anxiety Disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(12):2423–2433. doi: 10.1038/npp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröpper S., Spengler S., Stuke H., Gawron C.K., Parnack J., Gutwinski S., Wiers C.E., Bermpohl F. Behavioral impulsivity mediates the relationship between decreased frontal gray matter volume and harmful alcohol drinking: A voxel-based morphometry study. J. Psychiatr. Res. 2016;83:16–23. doi: 10.1016/j.jpsychires.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Gu M., Lee H.K., Sok S.R. A structural model for quality of life of alcoholics. J. Adv. Nurs. 2020;76(7):1658–1667. doi: 10.1111/jan.14378. [DOI] [PubMed] [Google Scholar]
- Guo J., Yang J., Wiley J., Ou X., Zhou Z., Whittemore R. Perceived stress and self-efficacy are associated with diabetes self-management among adolescents with type 1 diabetes: A moderated mediation analysis. J Adv Nurs. 2019;75(12):3544–3553. doi: 10.1111/jan.14179. [DOI] [PubMed] [Google Scholar]
- Hadland K.A., Rushworth M.F.S., Gaffan D., Passingham R.E. The Anterior Cingulate and Reward-Guided Selection of Actions. J. Neurophysiol. 2003;89(2):1161–1164. doi: 10.1152/jn.00634.2002. [DOI] [PubMed] [Google Scholar]
- L.A. Haycock P. McCarthy C.L. Skay Procrastination in College Students: The Role of Self-Efficacy and Anxiety 76 3 1998 317 324.
- Howell N.A., Worbe Y., Lange I., Tait R., Irvine M., Banca P., Harrison N.A., Bullmore E.T., Hutchison W.D., Voon V., Soriano-Mas C. Increased ventral striatal volume in college-aged binge drinkers. PLoS ONE. 2013;8(9):e74164. doi: 10.1371/journal.pone.0074164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.-T., Bentler P. Sage Publications; Thousand Oaks, CA, US: 1995. Evaluating model fit. [Google Scholar]
- Hu S., Ide J.S., Chao H.H., Castagna B., Fischer K.A., Zhang S., Li C.-S. Structural and functional cerebral bases of diminished inhibitory control during healthy aging. Hum. Brain Mapp. 2018;39(12):5085–5096. doi: 10.1002/hbm.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Li H.-T., Chen Y., Le T.M., Li C.S.P., Zhornitsky S., Li C.-S.-R. Gray matter volumetric correlates of behavioral activation and inhibition system traits in children: An exploratory voxel-based morphometry study of the ABCD project data. NeuroImage. 2020;220:117085. doi: 10.1016/j.neuroimage.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Zhornitsky S., Hu S., Zhang S., Krystal J.H., Li C.-S. Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. NeuroImage. Clinical. 2017;14:750–759. doi: 10.1016/j.nicl.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Zhornitsky S., Hu S., Zhang S., Krystal J.H., Li, C.-s.R., Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. NeuroImage: Clinical. 2017;14:750–759. doi: 10.1016/j.nicl.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indelicato L., Dauriz M., Santi L., Bonora F., Negri C., Cacciatori V., Targher G., Trento M., Bonora E. Psychological distress, self-efficacy and glycemic control in type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases. 2017;27(4):300–306. doi: 10.1016/j.numecd.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Jang D.-P., Namkoong K., Kim J.-J., Park S., Kim I.-Y., Kim S.I., Kim Y.-B., Cho Z.-H., Lee E. The relationship between brain morphometry and neuropsychological performance in alcohol dependence. Neurosci. Lett. 2007;428(1):21–26. doi: 10.1016/j.neulet.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Joss D., Lazar S.W., Teicher M.H. Effects of a mindfulness based behavioral intervention for young adults with childhood maltreatment history on hippocampal morphometry: a pilot MRI study with voxel-based morphometry. Psychiatry Research: Neuroimaging. 2020;301 doi: 10.1016/j.pscychresns.2020.111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley S.W., Walton M.E., Behrens T.E.J., Buckley M.J., Rushworth M.F.S. Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 2006;9(7):940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kroes M.C.W., Rugg M.D., Whalley M.G., Brewin C.R. Structural brain abnormalities common to posttraumatic stress disorder and depression. Journal of psychiatry & neuroscience : JPN. 2011;36:256–265. doi: 10.1503/jpn.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, S., Mascharek, A., Banaschewski, T., Bodke, A., Bromberg, U., Büchel, C., Quinlan, E.B., Desrivieres, S., Flor, H., Grigis, A., Garavan, H., Gowland, P.A., Heinz, A., Ittermann, B., Martinot, J.-L., Nees, F., Papadopoulos Orfanos, D., Paus, T., Poustka, L., Millenet, S., Fröhner, J.H., Smolka, M.N., Walter, H., Whelan, R., Schumann, G., Lindenberger, U., Gallinat, J., Consortium, I., 2019. Predicting development of adolescent drinking behaviour from whole brain structure at 14 years of age. eLife 8, e44056. [DOI] [PMC free article] [PubMed]
- Kuntsche E., Kuntsche S., Thrul J., Gmel G. Binge drinking: Health impact, prevalence, correlates and interventions. Psychology & Health. 2017;32(8):976–1017. doi: 10.1080/08870446.2017.1325889. [DOI] [PubMed] [Google Scholar]
- Kvamme T.L., Schmidt C., Strelchuk D., Chang-Webb Y.C., Baek K., Voon V. Sexually dimorphic brain volume interaction in college-aged binge drinkers. NeuroImage. Clinical. 2016;10:310–317. doi: 10.1016/j.nicl.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy Séverine, Dormal V., Brion Mélanie, Gaudelus B., Billieux J., Maurage P. Affective impairments in binge drinking: Investigation through emotional facial expression decoding. Compr. Psychiatry. 2018;83:59–63. doi: 10.1016/j.comppsych.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Le T.M., Chao H., Levy I., Li C.-S.-R. Age-Related Changes in the Neural Processes of Reward-Directed Action and Inhibition of Action. Front. Psychol. 2020;11:1121. doi: 10.3389/fpsyg.2020.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.M., Zhornitsky S., Wang W., Ide J., Zhang S., Li C.-S. Posterior Cingulate Cortical Response to Active Avoidance Mediates the Relationship between Punishment Sensitivity and Problem Drinking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019;39(32):6354–6364. doi: 10.1523/JNEUROSCI.0508-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.M., Zhornitsky S., Zhang S., Li C.-S.-R. Pain and reward circuits antagonistically modulate alcohol expectancy to regulate drinking. Transl. Psychiatry. 2020;10:220. doi: 10.1038/s41398-020-00909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Bi Lee Sulki Chung HaeKook Lee Jeong Seok Seo The Mutual Relationship Between Men’s Drinking and Depression: A 4-Year Longitudinal Analysis 53 5 2018 2018 597 602. [DOI] [PubMed]
- Leech R., Smallwood J. In: Handbook of Clinical Neurology. Vogt B.A., editor. Elsevier; 2019. Chapter 5 - The posterior cingulate cortex: Insights from structure and function; pp. 73–85. [DOI] [PubMed] [Google Scholar]
- Li G., Chen Y., Wang W., Dhingra I., Zhornitsky S., Tang X., Li C.-S. Sex Differences in Neural Responses to the Perception of Social Interactions. Front. Hum. Neurosci. 2020;14 doi: 10.3389/fnhum.2020.565132. 565132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Zhang S., Le T.M., Tang X., Li C.-S. Neural responses to negative facial emotions: Sex differences in the correlates of individual anger and fear traits. NeuroImage. 2020;221:117171. doi: 10.1016/j.neuroimage.2020.117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G. Li S. Zhang T.M. Le X. Tang C.-S.R. Li Neural Responses to Reward in a Gambling Task: Sex Differences and Individual Variation in Reward-Driven Impulsivity Cerebral cortex communications 1 2020 tgaa025-tgaa025. [DOI] [PMC free article] [PubMed]
- Lisdahl K.M., Thayer R., Squeglia L.M., McQueeny T.M., Tapert S.F. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013;211(1):17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Aungsuroch Y. Work stress, perceived social support, self-efficacy and burnout among Chinese registered nurses. J Nurs Manag. 2019;27(7):1445–1453. doi: 10.1111/jonm.12828. [DOI] [PubMed] [Google Scholar]
- Mackey S., Allgaier N., Chaarani B., Spechler P., Orr C., Bunn J., Allen N.B., Alia-Klein N., Batalla A., Blaine S., Brooks S., Caparelli E., Chye Y.Y., Cousijn J., Dagher A., Desrivieres S., Feldstein-Ewing S., Foxe J.J., Goldstein R.Z., Goudriaan A.E., Heitzeg M.M., Hester R., Hutchison K., Korucuoglu O., Li C.-S., London E., Lorenzetti V., Luijten M., Martin-Santos R., May A., Momenan R., Morales A., Paulus M.P., Pearlson G., Rousseau M.-E., Salmeron B.J., Schluter R., Schmaal L., Schumann G., Sjoerds Z., Stein D.J., Stein E.A., Sinha R., Solowij N., Tapert S., Uhlmann A., Veltman D., van Holst R., Whittle S., Wiers R., Wright M.J., Yücel M., Zhang S., Yurgelun-Todd D., Hibar D.P., Jahanshad N., Evans A., Thompson P.M., Glahn D.C., Conrod P., Garavan H. Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. The American journal of psychiatry. 2019;176(2):119–128. doi: 10.1176/appi.ajp.2018.17040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D.P., Fairchild A.J., Fritz M.S. Mediation analysis. Annu Rev Psychol. 2007;58(1):593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara-Studzińska M., Golonka K., Izydorczyk B. Self-Efficacy as a Moderator between Stress and Professional Burnout in Firefighters. Int. J. Environ. Res. Public Health. 2019;16(2):183. doi: 10.3390/ijerph16020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Oscar-Berman M., Jaffin S.K., Hodge S.M., Kennedy D.N., Caviness V.S., Marinkovic K., Breiter H.C., Gasic G.P., Harris G.J. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Montilla José.M., Mercken L., Lima-Serrano M., de Vries H., Lima-Rodríguez Joaquín.S. Why are Spanish Adolescents Binge Drinkers? Focus Group with Adolescents and Parents. Int. J. Environ. Res. Public Health. 2020;17(10):3551. doi: 10.3390/ijerph17103551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y., Czerkawski C., Crowley D.J., Cohen-Gilbert J.E., Sneider J.T., Silveri M.M. Binge alcohol consumption in emerging adults: anterior cingulate cortical “thinness” is associated with alcohol use patterns. Alcohol. Clin. Exp. Res. 2014;38(7):1955–1964. doi: 10.1111/acer.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Yoneyama E., Sumiyoshi T., Noguchi K., Nohara S., Suzuki M., Kawasaki Y., Seto H., Kurachi M. Lack of self-control as assessed by a personality inventory is related to reduced volume of supplementary motor area. Psychiatry Research: Neuroimaging. 2002;116(1-2):53–61. doi: 10.1016/s0925-4927(02)00070-7. [DOI] [PubMed] [Google Scholar]
- McPhee M.D., Claus E.D., Boileau I., Lee A.C.H., Graff-Guerrero A., Hendershot C.S. Does Family History of Alcohol Use Disorder Relate to Differences in Regional Brain Volumes? A Descriptive Review with New Data. Alcohol. Clin. Exp. Res. 2018;42(12):2369–2384. doi: 10.1111/acer.13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo L.J., Curran J.S., Watson R. Gender differences in substance use and psychiatric distress among medical students: A comprehensive statewide evaluation. Substance Abuse. 2017;38(4):401–406. doi: 10.1080/08897077.2017.1355871. [DOI] [PubMed] [Google Scholar]
- Merz E.C., He X., Noble K.G., Pediatric Imaging N., Genetics S. Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage. Clinical. 2018;20:243–251. doi: 10.1016/j.nicl.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V.L., Owens M.M., Syan S.K., Petker T.D., Sweet L.H., Oshri A., MacKillop J., Amlung M. Associations Between Drinking and Cortical Thickness in Younger Adult Drinkers: Findings From the Human Connectome Project. Alcohol. Clin. Exp. Res. 2019;43(9):1918–1927. doi: 10.1111/acer.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.C., Aouidad A., Gorodetsky E., Goldman D., Pine D.S., Ernst M. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism? Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(2):184–195. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Takeuchi H., Taki Y., Nouchi R., Sekiguchi A., Kotozaki Y., Miyauchi C.M., Iizuka K., Yokoyama R., Shinada T., Yamamoto Y., Hanawa S., Araki T., Hashizume H., Kunitoki K., Sassa Y., Kawashima R. White matter structures associated with loneliness in young adults. Sci. Rep. 2015;5:17001. doi: 10.1038/srep17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse, R., 2017. College Binge Drinking and its Association to Depression and Anxiety. East Asian Archives of Psychiatry. [PubMed]
- Pfefferbaum, A., Rohlfing, T., Pohl, K.M., Lane, B., Chu, W., Kwon, D., Nolan Nichols, B., Brown, S.A., Tapert, S.F., Cummins, K., Thompson, W.K., Brumback, T., Meloy, M.J., Jernigan, T.L., Dale, A., Colrain, I.M., Baker, F.C., Prouty, D., De Bellis, M.D., Voyvodic, J.T., Clark, D.B., Luna, B., Chung, T., Nagel, B.J., Sullivan, E.V., 2016. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cerebral cortex (New York, N.Y. : 1991) 26, 4101-4121. [DOI] [PMC free article] [PubMed]
- Pitel A.L., Segobin S.H., Ritz L., Eustache F., Beaunieux Hélène. Thalamic abnormalities are a cardinal feature of alcohol-related brain dysfunction. Neurosci. Biobehav. Rev. 2015;54:38–45. doi: 10.1016/j.neubiorev.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Powers J., Duffy L., Burns L., Loxton D. Binge drinking and subsequent depressive symptoms in young women in Australia. Drug Alcohol Depend. 2016;161:86–94. doi: 10.1016/j.drugalcdep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Rando K., Hong K.-I., Bhagwagar Z., Li C.-S., Bergquist K., Guarnaccia J., Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. The American journal of psychiatry. 2011;168(2):183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R.D., Ramnani N., Mackay C., Wilson J.L., Jezzard P., Carter C.S., Smith S.M. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. In: Handbook of Clinical Neurology. Vogt B.A., editor. Elsevier; 2019. Chapter 2 - The cingulate cortex and limbic systems for action, emotion, and memory; pp. 23–37. [DOI] [PubMed] [Google Scholar]
- Smeds M.R., Janko M.R., Allen S., Amankwah K., Arnell T., Ansari P., Balters M., Hess D., Ferguson E., Jackson P., Kimbrough M.K., Knight D., Johnson M., Porter M., Shames B.D., Schroll R., Shelton J., Sussman J., Yoo P. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. The American Journal of Surgery. 2020;219(6):907–912. doi: 10.1016/j.amjsurg.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Sousa S.S., Sampaio A., López-Caneda E., Bec C., Gonçalves Ó.F., Crego A. Increased Nucleus Accumbens Volume in College Binge Drinkers - Preliminary Evidence From Manually Segmented MRI Analysis. Front. Psychiatry. 2020;10:1005. doi: 10.3389/fpsyt.2019.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Sorg S.F., Schweinsburg A.D., Wetherill R.R., Pulido C., Tapert S.F. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220(3):529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolle M., Sack P.-M., Thomasius R. Binge drinking in childhood and adolescence: epidemiology, consequences, and interventions. Deutsches Arzteblatt international. 2009;106:323. doi: 10.3238/arztebl.2009.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E.V., Rosenbloom M.J., Deshmukh A., Desmond J.E., Pfefferbaum A. Alcohol and the Cerebellum: Effects on Balance, Motor Coordination, and Cognition. Alcohol health and research world. 1995;19:138–141. [PMC free article] [PubMed] [Google Scholar]
- Syaifullah A.H., Shiino A., Fujiyoshi A., Kadota A., Kondo K., Ito T., Segawa H., Moniruzzaman M., Waki T., Miyagawa N., Tooyama I., Ueshima H., Miura K., Ueshima H., Miura K., Horie M., Nakagawa Y., Yamamoto T., Nakano Y., Ogawa E., Maegawa H., Morino K., Miyazawa I., Watanabe Y., Nozaki K., Tooyama I., Shiino A., Andoh A., Tsuru T., Ogita H., Miyamatsu N., Nakamura Y., Kadota A., Kondo K., Torii S., Kadowaki T., Kadowaki S., Suzuki S., Ito T., Kunimura A., Segawa H., Fujiyoshi A., Higashiyama A., Okamura T., Azuma K., Sawamura T., Igase M., Tabara Y., Sekikawa A., Barinas-Mitchell E.J.M., Edmundowicz D., Ohkubo T., Hozawa A., Murakami Y., Okuda N., Arima H., Satoh A., Kita Y., Hisamatsu T., Yanagita M., Abbott R.D., Ohno S., Takashima N., Miyagawa N., Zaid M., Saito Y. Alcohol drinking and brain morphometry in apparently healthy community-dwelling Japanese men. Alcohol. 2021;90:57–65. doi: 10.1016/j.alcohol.2020.11.006. [DOI] [PubMed] [Google Scholar]
- Tavolacci M.P., Ladner J., Grigioni S., Richard L., Villet H., Dechelotte P. Prevalence and association of perceived stress, substance use and behavioral addictions: a cross-sectional study among university students in France, 2009–2011. BMC public health. 2013;13:724. doi: 10.1186/1471-2458-13-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmen C.D., Crockett L.J. Relations of Stress and Drinking Motives to Young Adult Alcohol Misuse: Variations by Gender. J. Youth Adolesc. 2020;49(4):907–920. doi: 10.1007/s10964-019-01144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R.E., Hagerty S.L., Sabbineni A., Claus E.D., Hutchison K.E., Weiland B.J. Negative and interactive effects of sex, aging, and alcohol abuse on gray matter morphometry. Hum. Brain Mapp. 2016;37(6):2276–2292. doi: 10.1002/hbm.23172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Smith S.M., Barch D.M., Behrens T.E.J., Yacoub E., Ugurbil K. The WU-Minn Human Connectome Project: an overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Jin C., Yuan K., Shakir T.M., Mao C., Niu X., Niu C., Guo L., Zhang M. The alteration of gray matter volume and cognitive control in adolescents with internet gaming disorder. Front. Behav. Neurosci. 2015;9:64. doi: 10.3389/fnbeh.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhornitsky S., Le T.M., Zhang S., Li C.-S. Heart Rate Variability, Cue-Evoked Ventromedial Prefrontal Cortical Response, and Problem Alcohol Use in Adult Drinkers. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2020;5(6):619–628. doi: 10.1016/j.bpsc.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, Davenport, Dowdall, Moeykens, Castillo, 1994. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. Jama the Journal of the American Medical Association. [PubMed]
- Wellman R.J., Contreras Gisèle.A., Dugas E.N., O'Loughlin E.K., O'Loughlin J.L. Determinants of Sustained Binge Drinking in Young Adults. Alcohol. Clin. Exp. Res. 2014;38(5):1409–1415. doi: 10.1111/acer.12365. [DOI] [PubMed] [Google Scholar]
- Wilsnack R.W., Wilsnack S.C., Gmel G., Kantor L.W. Gender Differences in Binge Drinking. Alcohol research : current reviews. 2018;39:57–76. doi: 10.35946/arcr.v39.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S., Malone S.M., Thomas K.M., Iacono W.G. Adolescent drinking and brain morphometry: A co-twin control analysis. Developmental Cognitive Neuroscience. 2015;16:130–138. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo B., Wang K., Tran T. Racial and ethnic differences in associations between psychological distress and the presence of binge drinking: Results from the California health interview survey. Addict. Behav. 2017;65:1–6. doi: 10.1016/j.addbeh.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Wuensch, K.L., 2016. Introduction to path analysis. 1-18.
- Yang X., Lau J.T.F., Wang Z., Lau M.C.M. Prevalence of binge drinking and relationships between masculine role discrepancy and binge drinking via discrepancy stress among Chinese men. Drug Alcohol Depend. 2019;196:57–61. doi: 10.1016/j.drugalcdep.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Zar, J., 1999. Biostatistical analysis.
- Zhornitsky S., Zhang S., Ide J.S., Chao H.H., Wang W., Le T.M., Leeman R.F., Bi J., Krystal J.H., Li C.-shan R. Alcohol Expectancy and Cerebral Responses to Cue-Elicited Craving in Adult Nondependent Drinkers. Biological psychiatry. Cognitive neuroscience and neuroimaging. 2019;4(5):493–504. doi: 10.1016/j.bpsc.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullig K.J., Valois R.F., Huebner E.S., Oeltmann J.E., Drane J.W. Relationship between perceived life satisfaction and adolescents’ substance abuse. J. Adolesc. Health. 2001;29(4):279–288. doi: 10.1016/s1054-139x(01)00269-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.