Abstract
Background/aim
Atherosclerotic heart diseases can occur at an early age in patients with ankylosing spondylitis (AS). Flow-mediated dilation (FMD) and carotid intima-media thickness (cIMT) values are reliable markers for early detection of subclinical atherosclerosis in patients with AS. We aimed to investigate the relationship between visfatin levels and indirect markers of subclinical atherosclerosis and endothelial dysfunction in patients with AS.
Materials and methods
Forty-two patients diagnosed with AS and 42 age, sex, and body mass index (BMI)-matched controls were included in the study. Visfatin levels, FMD, and cIMT were measured using appropriate methods.
Results
Visfatin levels of the patients were significantly higher than controls (p < 0.001). FMD values in patients with AS were significantly lower (p = 0.007) whereas cIMT were significantly higher than the controls (p = 0.003). There was a negative relationship between FMD with visfatin levels (p = 0.004), BASDAI (p = 0.010), and BASFI (p = 0.007). There was a positive relationship between cIMT with visfatin (p = 0.005), BASDAI (p < 0.001), and BASFI (p < 0.001). There was a positive relationship between visfatin with BASDAI (p < 0.001), and BASFI (p < 0.001).
Conclusion
Visfatin levels are increased and associated with impaired FMD and increased cIMT in patients with AS. Increased visfatin levels may be associated with subclinical atherosclerosis in AS.
Keywords: Visfatin, ankylosing spondylitis, flow-mediated dilation, carotid intima-media thickness, subclinical atherosclerosis
1. Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease-causing destruction in the spinal and peripheral joints [1]. Multilevel organ and system involvement including eye, skin, kidney, gastrointestinal, and cardiovascular systems may occur in the course of AS. Cardiovascular system involvement is seen in 2% to 10% of patients with AS and cardiovascular risk is increased compared to the healthy population [2–4]. Cardiac involvement may occur in various forms ranging from asymptomatic atherosclerosis to mortal conduction disorders, ischemic heart disease, aortic valve diseases, aortitis, hypertension, and cardiomyopathy [5]. Findings of atherosclerosis may be detected even at an early stage of the disease, and chronic inflammation is considered as an important contributing factor in the development of atherosclerosis in AS [6].
The carotid intima-media thickness (cIMT) measurement is suggested as a cost-effective, reliable, and noninvasive method for detecting subclinical atherosclerosis in patients with AS [7]. Flow-mediated dilatation (FMD) is another noninvasive method for early detection of endothelial dysfunction which reflects the dilation rate of an artery due to nitric oxide released from endothelial cells [8]. FMD can detect endothelial dysfunction caused by decreased bioavailability of nitric oxide released from the endothelium [9]. Impaired FMD can be detected before apparent atherosclerotic changes may occur and is an early and reliable marker of endothelial damage [10].
Visfatin also called nicotinamide phosphoribosyltransferase (NAMPT) and pre-B cell colony enhancing factor (PBEF), was first described in 2004[11]. Visfatin is an adipokine predominantly released from visceral adipose tissue but also released from all tissues [12].Visfatin is a pro-inflammatory cytokine and also increases the release of other pro-inflammatory cytokines such as interleukin (IL) -1beta, IL-6, and tumor necrosis factor-alpha from monocytes and vascular endothelial cells and results in severe inflammation [13]. Increased visfatin levels are associated with vascular inflammation and carotid plaques [14]. Increased circulatory visfatin levels have been reported in various inflammatory diseases such as rheumatoid arthritis, inflammatory bowel diseases, and psoriasis [12]. Elevated visfatin levels has been reported as a predictor of radiographic progression in patients with AS [15,16]. Visfatin levels were associated with increased cIMT values and impaired FMD [17,18].
In this study, we aimed to investigate the possible relationship between endothelial dysfunction and visfatin levels in patients with AS.
2. Materials and methods
2.1. Patients
Forty-two patients with AS who met the modified New York criteria and 42 age-, sex-, and body mass index (BMI)-matched healthy controls were included in the study. Patients were consequently recruited from Malatya State Hospital rheumatology and physical medicine and rehabilitation outpatient clinics. Individuals who were pregnant or nursing women, or had diagnosis for malign tumors, diabetes mellitus, hypertension, heart disease, hyperlipidemia, acute or chronic infections, acute or chronic renal failure, chronic obstructive pulmonary disease, and obesity (BMI ≥ 30 kg/m2) were excluded.
2.1.1. Sample size
The prevalence of AS is 0.5%–1.4%. The incidence of atherosclerosis in the community is 27.6% [19]. Atherosclerosis is seen 1.4–1.7-fold more in AS patients than in the population [20]. The minimum sample size required to find statistical significance was calculated with the Sample Size Calculator Sample Size Calculator [clincalc.com])., considering 0.05 type I error (alpha), 0.8 power (1-beta), effect size 0.68, and the two-sided alternative hypothesis (H1). The minimum number of patients and controls to be included in the study was calculated as 36 each.
2.1.2. Current smoker
Current smokers were recorded according to the definition of the National Health Interview Survey “Current smoker: An adult who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes.” The smoking duration was calculated as packs-year1.
2.2. Biochemical analysis
Venous blood samples of the patients and controls were collected after 12 h of fasting. Blood samples were taken into dry tubes, divided and separated into small pieces, and stored at –80 °C until analyzed. Complete blood count analysis was performed by flow cytometry device (Mindray BC-6800 Auto Hematology Analyzer, Shenzhen, China). C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN) creatinine values were measured using a spectrophotometry device (Abbot-Architect c8000, Japan). The erythrocyte sedimentation rate (ESR) was evaluated by the Westergren method (Berkhun SDM-100, Turkey).
2.3. Visfatin measurement
Serum blood samples for visfatin were taken from all patients and controls between 08.00–09.00 in the morning after 12 h of fasting. Visfatin measurement of all individuals was made from their serum samples on the same day. Serum visfatin level was measured by enzyme-linked immunosorbent method (ELISA) using the Elisa kit (Elabscience, China). The process was carried out following the manufacturer’s instructions. Absorbance was evaluated at 450 nm by an ELISA reader.
2.4. Ultrasonography
Ultrasonographic (US) measurements were made by the same experienced radiologist using a high-resolution ultrasound device (Logiq S6; General Electric, Milwaukee, WI, USA) and a 12 MHz multi-frequency linear probe. Sonography was performed by the patient in the supine position and the neck turned to the side of examination. US evaluations in patients and controls were performed in the morning (9:00–10:00 AM) after 12 h of fasting. Twelve hours before the study, all subjects were discontinued smoking, alcohol, and caffeine consumption, and exercise are not permitted.
2.5. Measurement of carotid intima-media thickness
Intima-media thickness (IMT) measurements were attained from nonplaque areas of the three different points of the right and left common carotid artery (CCA) and 1 cm distant from the bifurcation. Two bright echogenic lines on the arterial wall were identified as intima and media. A total of three measurements were made for each side of the body, the average of three measurements was calculated as the IMT value.
2.6. Method of evaluating flow-mediated dilation
The participants were placed in the supine position and rested for at least 10 min. Then, the brachial blood pressure was measured and noted. Subsequently, FMD was measured using an echography (Vivid S6, GE, USA) equipped with a linear probe (frequency range, 12 MHz). The basal measurement of the right brachial artery diameter was performed in a linear plane and nearly 2 to 3 cm upper from the antecubital fossa. Afterward, a cuff was placed around the forearm distal to the ultrasonographic evaluation line. The cuff was inflated to supra systolic pressure (50 mm Hg above the previously measured systolic blood pressure) and held in this position for 5 min of ischemia. The diameter of the brachial artery was re-measured 1 min after the cuff was completely deflated. FMD value was obtained by calculating the percentage increase in diameter of the brachial artery.
2.7. Statistical analysis
SPSS program (version 20) was used for the evaluation of all statistical analyzes in the study. Kolmogorov–Smirnov test was used to show the homogeneity of distribution. The t-test or Mann–Whitney U test was used for comparison between groups and Pearson correlation coefficients or Spearman rank test for analyzing relationship between parameters where appropriate. The categorical variables such as age and current smoking were evaluated using the chi-square test. Independent variables affecting cIMT and FMD were determined using linear stepwise regression analysis. Before performing stepwise linear regression analysis, univariate analysis was performed to determine independent variables associated with cIMT and FMD. In univariate analysis, for cIMT, age, male sex, visfatin, Bath ankylosing spondylitis disease activity index (BASDAI), Bath ankylosing spondylitis functional index (BASFI), fasting plasma glucose (FPG), total cholesterol (TC), low-density cholesterol (LDL), triglyceride (TG), CRP, and ESR were determined as independent variables. For FMD, BASDAI, BASFI, disease duration, creatinine, TC, and LDL were determined as independent variables. A p-value of < 0.05 was considered statistically significant.
3. Results
Age, sex, and BMI values of the patients and controls were similar (p > 0.05). Alcohol and tobacco consumptions were similar between groups. The regions where we detect enthesitis in patients are: costochondral joints (n = 4), trochanter major (n = 3), anterior superior iliac spine (n = 3), iliac crest (n = 1), posterior superior iliac spine (n = 1), processus spinosus (n = 6), achilles tendon region (n = 4), and multiple sites (n = 2). The median disease duration of patients with AS was 3.5 years. Sociodemographic characteristics of the patients and healthy controls are shown in Table 1.
Table 1.
Sociodemographic characteristics of the patient and control groups.
| AS(n = 42) | Control(n = 42) | P value | |
|---|---|---|---|
| Age (years) | 39.2 ± 7.3 | 39.4 ± 9.6 | 0.929 |
| Sex (M/F) (n) | 13/29 | 13/29 | 1.000 |
| Disease duration (years) | 3.5 (1.0–45.0) | ||
| Peripheral arthritis n, (%) | 2 (4.8) | ||
| Enthesitis n, (%) | 24 (57.1) | ||
| BASDAI | 3.2 ± 1.3 | ||
| BASFI | 2.8 ± 1.3 | ||
| BMI (kg/m2) | 26.8 ± 3.6 | 25.9 ± 5.0 | 0.361 |
| Current smokers (n) | 13 | 16 | 0.412 |
| Smoking (packet-years) | 18.5 ± 4.3 | 14.6 ± 8.0 | 0.108 |
| Drinking (n) | 1 | 0 | 1.000 |
| NSAID (n) | 21 | ||
| MTX (n) | 14 | ||
| Infliximab (n) | 5 | ||
| Adalimumab (n) | 4 | ||
| Etanercept (n) | 6 | ||
| Certolizumab (n) | 4 | ||
| Salazopyrin (n) | 2 | ||
| Topical steroid | 1 | ||
| Systemic steroid | 0 |
Abbreviations: AS, ankylosing spondylitis; BASDAI, bath ankylosing spondylitis disease activity index; BASFI, bath ankylosing spondylitis functional index; BMI, body mass index; NSAID, nonsteroidal antiinflammatory drug; MTX, methotrexate.
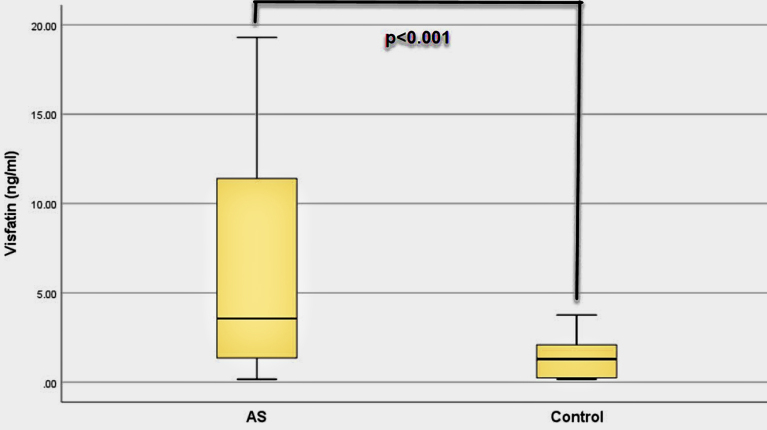
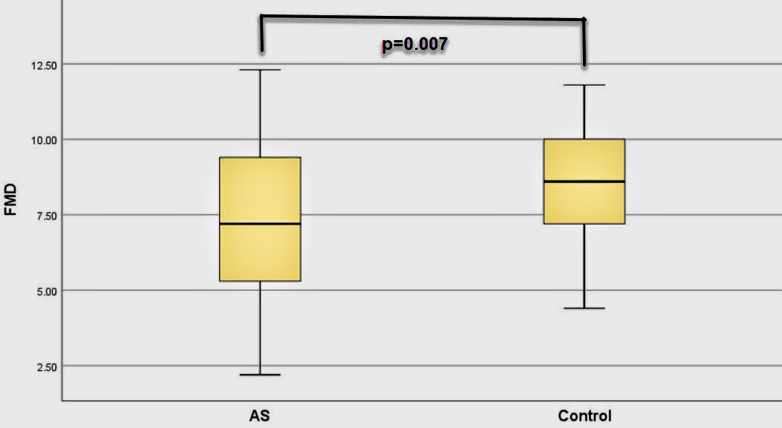
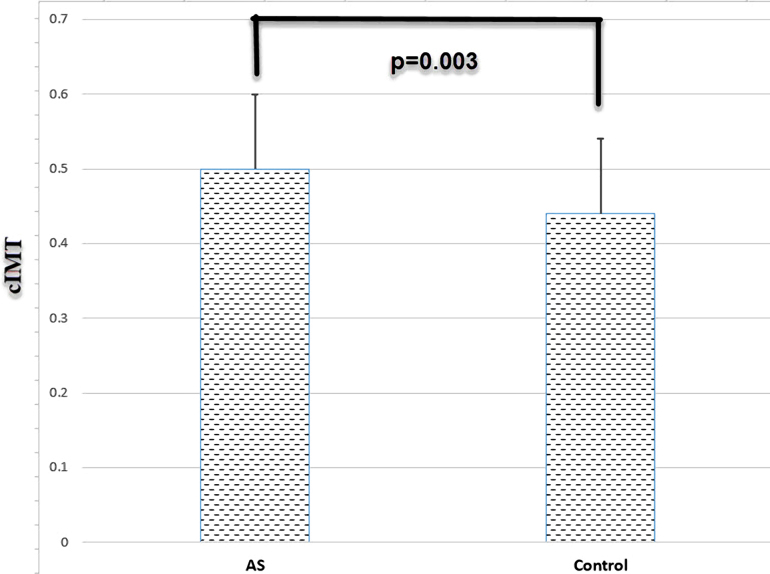
Visfatin levels of the patients were significantly higher than healthy controls (p < 0.001) (Table 2). FMD values of the patients were lower than controls (p = 0.007), whereas their cIMT values were higher than the controls (p = 0.003). Serum uric acid, CRP and ESR values of the patients were higher than the controls (Table 2). High-density lipoprotein (HDL) values of the patients were lower than healthy controls. Visfatin, FMD, and cIMT values of the patients and controls are shown in Figures 1, 2, and 3, respectively. All biochemical results of the patients are shown in Table 2.
Table 2.
Biochemical results of the patient and control group.
| AS (n = 42) | Control (n = 42) | P value | |
|---|---|---|---|
| Visfatin (ng/mL) | 3.5 (0.19–19.3) | 1.3 (0.17–7.0) | <0.001 |
| FMD (%) | 7.2 ± 2.8 | 8.7 ± 1.7 | 0.007 |
| cIMT mm | 0.50 ± 0.1 | 0.44 ± 0.1 | 0.003 |
| Carotid plaque (n) | 0 | 0 | 1.000 |
| FPG (mg/dL) | 99.6 ± 22.3 | 93.4 ± 16.1 | 0.144 |
| BUN (mg/dL) | 25.8 ± 7.5 | 29.9 ± 8.2 | 0.020 |
| Creatinine (mg/dL) | 0.7 ± 0.08 | 0.7 ± 0.18 | 0.106 |
| AST (IU/L) | 22.0 ± 11.9 | 22.8 ± 10.2 | 0.733 |
| ALT (IU/L) | 27.4 ± 18.7 | 24.8 ± 16.3 | 0.502 |
| SUA (mg/dL) | 4.3 ± 0.9 | 3.3 ± 0.6 | <0.001 |
| CRP (mg/dL) | 0.20 (0.10–4.82) | 0.10 (0.10–2.15) | 0.008 |
| ESR (mm/h) | 23.2 ± 17.5 | 14.7 ± 11.3 | 0.007 |
| WBC (×109/L) | 7.5 ± 2.0 | 7.2 ± 1.4 | 0.463 |
| Hb (g/dL) | 13.5 ± 1.9 | 13.3 ± 1.3 | 0.668 |
| TSH (mIU/L) | 1.7 ± 0.9 | 1.8 ± 1.1 | 0.788 |
| TC (mg/dL) | 191.3 ± 39.7 | 199.8 ± 25.2 | 0.250 |
| TG (mg/dL) | 131.9 ± 52.5 | 128.8 ± 53.7 | 0.792 |
| HDL (mg/dL) | 40.2 ± 8.6 | 48.3 ± 9.8 | <0.001 |
| LDL (mg/dL) | 124.7 ± 35.6 | 125.7 ± 19.8 | 0.883 |
Abbreviations: AS, ankylosing spondylitis; FMD, flow-mediated dilation; cIMT, carotid intima-media thickness; FPG, fasting plasma glucose; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; SUA, serum uric acid; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell count; Hb, hemoglobin; TSH, thyroid stimulating hormone; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 1.
Figure 2.
Figure 3.
There was a negative correlation between FMD values with visfatin (p = 0.004), BASDAI (p = 0.010), and BASFI (p = 0.007). There was positive relationship between cIMT with visfatin (p = 0.005), BASDAI (p < 0.001), and BASFI (p < 0.001). There was a negative relationship between cIMT and HDL. There was a positive relationship between visfatin with BASDAI (p < 0.001), and BASFI (p < 0.001). There was a negative relationship between visfatin and HDL. All results of correlation analysis are shown in Table 3.
Table 3.
Correlation analysis results of patients.
| Parameters | FMD | cIMT | Visfatin | |||
|---|---|---|---|---|---|---|
| R value | P value | R value | P value | R value | P value | |
| Visfatin | –0310 | 0.004 | 0.301 | 0.005 | ||
| BASDAI | –0.281 | 0.010 | 0.396 | <0.001 | 0.479 | <0.001 |
| BASFI | –0.294 | 0.007 | 0.427 | <0.001 | 0.441 | <0.001 |
| SUA | –0.280 | 0.010 | 0.257 | 0.018 | ||
| Age | 0.506 | <0.001 | ||||
| FPG | 0.219 | 0.045 | ||||
| CRP | 0.314 | 0.004 | ||||
| ESR | 0.229 | 0.036 | 0.236 | 0.030 | ||
| Disease duration | 0.368 | <0.001 | ||||
| TC | –0.222 | 0.041 | 0.342 | 0.001 | ||
| HDL | –0.218 | 0.036 | –0.229 | 0.036 | ||
| LDL | 0.322 | 0.003 | ||||
| TG | 0.407 | <0.001 | ||||
Stepwise linear regression analysis was performed after finding independent variables related to cIMT and FMD according to univariate analysis. In the stepwise linear regression analysis (r2 = 0.551, F = 24.2, p < 0.001 for cIMT; r2 = 0.251, F: 6.6, p < 0.001 for FMD); there was an independent inverse relationship between FMD with visfatin (beta [β] = 0.223, p = 0.045), and BASFI (β = 0.290, p = 0.011). There was an independent association between cIMT with age (β = 0.431, p < 0.001), BASFI (β = 0.371, p < 0.001), and male sex (β = 0.298, p < 0.001). All results of univariate and stepwise linear regression analysis are shown in Table 4 and Table 5.
Table 4.
Independent variables associated with cIMT and FMF in univariate analysis.
| cIMT | FMD | |||
|---|---|---|---|---|
| Beta | P value | Beta | P value | |
| Age | 0.506 | <0.001 | 0.028 | 0.799 |
| Male sex | 0.255 | 0.019 | 0.140 | 0.203 |
| Visfatin | 0.301 | 0.005 | 0.310 | 0.004 |
| BMI | 0.111 | 0.314 | 0.021 | 0.847 |
| BASDAI | 0.396 | <0.001 | 0.281 | 0.010 |
| BASFI | 0.427 | <0.001 | 0.294 | 0.007 |
| FPG | 0.219 | 0.045 | 0.041 | 0.713 |
| BUN | 0.069 | 0.533 | 0.013 | 0.909 |
| Creatinine | 0.020 | 0.854 | 0.281 | 0.010 |
| TC | 0.342 | 0.001 | 0.222 | 0.042 |
| HDL | 0.212 | 0.053 | 0.107 | 0.302 |
| LDL | 0.322 | 0.003 | 0.280 | 0.010 |
| TG | 0.407 | <0.001 | 0.060 | 0.585 |
| SUA | 0.104 | 0.344 | 0.198 | 0.070 |
| CRP | 0.314 | 0.004 | 0.007 | 0.949 |
| ESR | 0.229 | 0.036 | 0.082 | 0.457 |
| AST | 0.101 | 0.359 | 0.139 | 0.208 |
| ALT | 0.184 | 0.094 | 0.184 | 0.094 |
| WBC | 0.022 | 0.845 | 0.132 | 0.232 |
| Hb | 0.054 | 0.623 | 0.014 | 0.896 |
| TSH | 0.040 | 0.715 | 0.062 | 0.574 |
| Smoking | 0.096 | 0.546 | 0.250 | 0.111 |
| Disease duration | 0.245 | 0.117 | 0.346 | 0.025 |
Abbreviations: cIMT, carotid intima-media thickness; FMD, flow-mediated dilation; BMI, body mass index; BASDAI, bath ankylosing spondylitis disease activity index; BASFI, bath ankylosing spondylitis functional index; FPG, fasting plasma glucose; BUN, blood urea nitrogen; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; SUA, serum uric acid; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, white blood cell count; Hb, hemoglobin; TSH, thyroid stimulating hormone.
Table 5.
Stepwise linear regression analysis.
| Dependent variable | Independent variables | Beta regression coefficient | P value |
|---|---|---|---|
| FMD | Visfatin | –0.223 | 0.045 |
| Creatinine | –0.263 | 0.010 | |
| BASFI | –0.290 | 0.011 | |
| LDL | –0.219 | 0.031 | |
| cIMT | Age | 0.431 | <0.001 |
| BASFI | 0.371 | <0.001 | |
| Male sex | 0.298 | <0.001 | |
| TG | 0.242 | 0.003 |
Abbreviations: FMD, flow-mediated dilation; cIMT, carotid intima-media thickness; BASFI, bath ankylosing spondylitis functional index; LDL, low-density lipoprotein; TG, triglyceride.
4. Discussion
Our results revealed that cIMT, a marker of subclinical atherosclerosis, was higher in patients with AS than matched healthy controls, and FMD, a marker of endothelial dysfunction, is lower in patients than controls, indication poorer endothelial functions. Our results also showed that serum levels of visfatin were higher in patients with AS compared to controls, and higher levels of visfatin may be associated with higher disease activity and poorer physical functions.
The increased risk of atherosclerosis is known in patients with AS. Chronic inflammation which disrupts endothelial functions and steroid and nonsteroid antiinflammatory drugs used in the treatment are factors contributing to the increased cardiovascular risk in patients with AS [3,21,22].
Visfatin is a multiple immunomodulatory protein that stimulates the release of pro-inflammatory cytokines. Visfatin activates leukocytes and causes pro-inflammatory cytokine release, resulting in an increase in inflammation and reactive oxygen species [23]. Increased visfatin levels were shown associated with insulin resistance and increased cardiac events[24]. An independent relationship was found between visfatin levels with coronary artery disease and coronary slow-flow phenomenon[25]. Zheng et al. found a strong relationship between increased visfatin levels (>8.799 ng/mL) and major adverse cardiovascular events (MACEs) in acute myocardial infarction [26]. Stejskal et al. reported that the 20 ng/mL cut-off value of visfatin is an independent marker for AMI with high sensitivity (84%) and specificity (90%)[27]. Miranda–Filloy et al. found visfatin levels higher in AS patients than healthy controls, but they did not find any relationship between visfatin level with lipid parameters and BASDAI[28]. Hulejova et al. found a positive relationship between visfatin and BASDAI in patients with axial spondyloarthritis[28]. In the current study, we found a relationship between serum visfatin levels with both BASDAI and BASFI. We found a negative relationship between visfatin with HDL, which has a potent antiatherosclerotic effect.
Atherosclerosis and cardiac events can be seen at an early age in AS[29]. Impaired FMD is a good marker of subclinical atherosclerosis. Bodnar et al. found that FMD values are significantly lower and cIMT values remarkably higher in patients with AS compared to controls[30]. Wang et al. found that in 120 AS patients, the FMD value was pronouncedly lower than the control group[31]. There is an inverse relationship between the circulating visfatin levels with FMD, an early marker of endothelial dysfunction[32]. Yilmaz et al. showed a relationship between endothelial dysfunction and visfatin in 406 patients with chronic renal failure[33].
The cIMT value has been proven to be a reliable marker for early detection of subclinical atherosclerosis in patients with AS [7,34].A positive correlation was shown between serum visfatin levels and cIMT in diabetic and nondiabetic hemodialysis patients[35]. Zhong et al. reported that the relationship between serum visfatin levels and carotid plaque, and an increase in visfatin levels was a predictive marker for the carotid plaque with 70% sensitivity and 67% specificity[36].
Regarding rheumatic diseases which progress with aberrant inflammation, higher visfatin levels with respect to controls and high disease activity compatible with visfatin levels were shown in patients with rheumatoid arthritis and Behçet’s disease but no relationship between cIMT and glucose intolerance[37]. On the other hand, contradictory results were reported in patients with systemic lupus erythematosus and systemic sclerosis indicating similar levels of visfatin compared with controls[35]. Syrbe et al. reported higher serum visfatin levels in patients with AS[15].
To the best of our knowledge, our study is the first to investigate cIMT and FMD together and possible interactions between circulating visfatin levels and disease parameters in patients with AS.
Possible interactions between visfatin and FMD with uric acid levels and estimated glomerular filtration rate (eGFR) has been described in different disease groups [38,39].In our study, we found a relationship between visfatin and FMD and serum creatinine. Low-density lipoprotein (LDL) is a highly proatherogenic molecule and Matsui et al., revealed a strong relationship between LDL and impaired FMD in statin naive individuals[40]. In our study, we also found a relationship between LDL and FMD.
5. Limitation of the study
Our study has some limitations. The study was conducted with a small number of subjects. Current study is a pilot study and studies with broad participation are needed. Another missing point in our study is the absence of the diseased-control group. The number of patients with high disease activity (BASDAI> 4) was quite low. The relationship between visfatin and cIMT and FMD in AS patients should be investigated in further studies.
6. Conclusion
Circulating visfatin levels are associated with disease activity and functional ability in patients with AS, and along with cIMT and FMD may be associated with increased risk of subclinical atherosclerosis and endothelial dysfunction.
Funding
All authors declare that this study has received no financial support.
Informed consent
The study was approved by the ethics committee of Ankara Numune Education and Research Hospital, Turkey. All participants were informed of the study protocol and signed consents were obtained.
Acknowledgment
The cost of the visfatin Elisa kit and biochemical tests used in this study was provided by the first author, RAB.
Footnotes
https://www.smokingpackyears.com/.
References
- Sieper J Braun J Rudwaleit M Boonen A Zink A Ankylosing spondylitis: an overview. Annals of the Rheumatic Diseases. 2002;61:8–18. doi: 10.1136/ard.61.suppl_3.iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente RL Valente JM De Castro GR Zimmermann AF Fialho SC Subclinical atherosclerosis in ankylosing spondylitis: is there a role for inflammation? The Revista Brasileira de Reumatologia. 2013;53:377–381. [PubMed] [Google Scholar]
- McCarey D Sturrock RD Comparison of cardiovascular risk in ankylosing spondylitis and rheumatoid arthritis. Clinical and Experimental Rheumatology. 2009;27:124–126. [PubMed] [Google Scholar]
- Balciūnaitė A Budrikis A Rumbinaitė E Sabaliauskienė J Patamsytė V Ankylosing spondyloarthritis resulting severe aortic insufficiency and aortitis: exacerbation of ankylosing spondyloarthritis and stenosis of the main left coronary artery after mechanical aortic valve implantation with cardiopulmonary bypass. Case Reports in Rheumatology. 2020;10 doi: 10.1155/2020/9538527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan Y. Cardiac involvement in ankylosing spondylitis. Journal of Clinical Medicine Research. 2016;8:427–430. doi: 10.14740/jocmr2488w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YM Chang WP Wei JC Chou P Wang PY Midlife ankylosing spondylitis increases the risk of cardiovascular diseases in males 5 years later: a national population-based study. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure E Icli A Uslu AU Sakiz D Cure MC Atherogenic index of plasma: a useful marker for subclinical atherosclerosis in ankylosing spondylitis: AIP associate with cIMT in AS. Clinical Rheumatology. 2018;37:1273–1280. doi: 10.1007/s10067-018-4027-0. [DOI] [PubMed] [Google Scholar]
- Korkmaz H Onalan O Evaluation of endothelial dysfunction: flow-mediated dilation. Endothelium. 2008;15:157–163. doi: 10.1080/10623320802228872. [DOI] [PubMed] [Google Scholar]
- Al-Qaisi M Kharbanda RK Mittal TK Donald AE Measurement of endothelial function and its clinical utility for cardiovascular risk. Vascular Health and Risk Management. 2008;4:647–652. doi: 10.2147/vhrm.s2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitakari OT Celermajer DS Flow-mediated dilatation. The British Journal of Clinical Pharmacology. 2000;50:397–404. doi: 10.1046/j.1365-2125.2000.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug C Lodish HF Visfatin: a new adipokine. Science. 2005;307:366–367. doi: 10.1126/science.1106933. [DOI] [PubMed] [Google Scholar]
- Filippatos TD Randeva HS Derdemezis CS Elisaf MS Mikhailidis DP Visfatin/PBEF and atherosclerosis-related diseases. Current Vascular Pharmacology. 2010;8:12–28. doi: 10.2174/157016110790226679. [DOI] [PubMed] [Google Scholar]
- Zheng LY Xu X Wan RH Xia S Lu J Association between serum visfatin levels and atherosclerotic plaque in patients with type 2 diabetes. Diabetology & Metabolic Syndrome. 2019;11:60–60. doi: 10.1186/s13098-019-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X Chen M Association between elevated visfatin and carotid atherosclerosis in patients with chronic kidney disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38:553–559. doi: 10.3969/j.issn.1672-7347.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Syrbe U Callhoff J Conrad K Poddubnyy D Haibel H Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression. Arthritis and Rheumatology. 2015;67:678–685. doi: 10.1002/art.38968. [DOI] [PubMed] [Google Scholar]
- Hulejova H Kropackova T Bubova K Krystufkova O Filkova M Serum visfatin levels in patients with axial spondyloarthritis and their relationship to disease activity and spinal radiographic damage: a cross-sectional study. Rheumatology International. 2019;39:1037–1043. doi: 10.1007/s00296-019-04301-z. [DOI] [PubMed] [Google Scholar]
- Kadoglou NP Sailer N Moumtzouoglou A Kapelouzou A Tsanikidis H Visfatin (nampt) and ghrelin as novel markers of carotid atherosclerosis in patients with type 2 diabetes. Experimental and Clinical Endocrinology and Diabetes. 2010;118:75–80. doi: 10.1055/s-0029-1237360. [DOI] [PubMed] [Google Scholar]
- Takebayashi K Suetsugu M Wakabayashi S Aso Y Inukai T. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus. Metabolism. 2007;56:451–458. doi: 10.1016/j.metabol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Song P Fang Z Wang H Cai Y Rahimi K Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. The Lancet Global Health. 2020;8:e721–e729. doi: 10.1016/S2214-109X(20)30117-0. [DOI] [PubMed] [Google Scholar]
- Eriksson JK Jacobsson L Bengtsson K Askling J Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Annals of the Rheumatic Diseases. 2017;76:364–370. doi: 10.1136/annrheumdis-2016-209315. [DOI] [PubMed] [Google Scholar]
- Papadakis JA Sidiropoulos PI Karvounaris SA Vrentzos GE Spanakis EK High prevalence of metabolic syndrome and cardiovascular risk factors in men with ankylosing spondylitis on anti-TNFalpha treatment: correlation with disease activity. Clinical and Experimental Rheumatology. 2009;27:292–298. [PubMed] [Google Scholar]
- Tsai WC Ou TT Yen JH Wu CC Tung YC. Long-term frequent use of non-steroidal anti-inflammatory drugs might protect patients with ankylosing spondylitis from cardiovascular diseases: a nationwide case-control study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc H Dogru T Kara M Tapan S Ercin CN Association of plasma visfatin with hepatic and systemic inflammation in nonalcoholic fatty liver disease. Annals of Hepatology. 2013;12:548–555. [PubMed] [Google Scholar]
- Romacho T Sánchez-Ferrer CF Peiró C Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators of Inflammation. 2013;10 doi: 10.1155/2013/946427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak HA Aslan S Yalcin AA Akturk IF Yalcin B Relationship between serum visfatin levels and coronary slow-flow phenomenon. Herz. 2015;40:921–928. doi: 10.1007/s00059-015-4313-4. [DOI] [PubMed] [Google Scholar]
- Zheng M Lu N Ren M Chen H Visfatin associated with major adverse cardiovascular events in patients with acute myocardial infarction. BMC Cardiovascular Disorders. 2020;20:271–271. doi: 10.1186/s12872-020-01549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskal D Sigutova R Svestak M Vaclavik J Kusnierova P Measurement of novel adipokine visfatin in young patients with acute myocardial infarction. Clinical testing of a new ELISA. Biomedical papers of the Medical Faculty of the University Palacky. Czechoslovakia 2020;164:138–140. doi: 10.5507/bp.2020.024. [DOI] [PubMed] [Google Scholar]
- Miranda-Filloy JA López-Mejias R Genre F Carnero-López B Ochoa R Leptin and visfatin serum levels in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clinical and Experimental Rheumatology. 2013;31:538–545. [PubMed] [Google Scholar]
- Rueda-Gotor J Genre F Corrales A Blanco R Fuentevilla P Detection of high cardiovascular risk patients with ankylosing spondylitis based on the assessment of abdominal aortic calcium as compared to carotid ultrasound. Arthritis Research and Therapy. 2018;20:195–195. doi: 10.1186/s13075-018-1684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnár N Kerekes G Seres I Paragh G Kappelmayer J Assessment of subclinical vascular disease associated with ankylosing spondylitis. The Journal of Rheumatology. 2011;38:723–729. doi: 10.3899/jrheum.100668. [DOI] [PubMed] [Google Scholar]
- Wang HH Wang QF Low vaspin levels are related to endothelial dysfunction in patients with ankylosing spondylitis. The Brazilian Journal of Medical and Biological Research. 2016;49:1414–1414. doi: 10.1590/1414-431X20165231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa SS Hamdy SM El-Sheikh RG Serum visfatin as a non-traditional biomarker of endothelial dysfunction in chronic kidney disease: an Egyptian study. European Journal of Internal Medicine. 2010;21:530–535. doi: 10.1016/j.ejim.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Yilmaz MI Saglam M Carrero JJ Qureshi AR Caglar K Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrology Dialysis Transplantation. 2008;23:959–965. doi: 10.1093/ndt/gfm727. [DOI] [PubMed] [Google Scholar]
- Kucuk A Uğur Uslu A Icli A Cure E Arslan S The LDL/HDL ratio and atherosclerosis in ankylosing spondylitis. Zeitschrift für Rheumatologie. 2017;76:58–63. doi: 10.1007/s00393-016-0092-4. [DOI] [PubMed] [Google Scholar]
- El-Shishtawy SH Mosbah O Sherif N Metwaly A Hanafy A Association between serum visfatin and carotid atherosclerosis in diabetic and non-diabetic patients on maintenance hemodialysis. Electronic Physician. 2016;8:1966–1972. doi: 10.19082/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M Tan HW Gong HP Wang SF Zhang Y Increased serum visfatin in patients with metabolic syndrome and carotid atherosclerosis. Clinical Endocrinology (Oxford) 2008;69:878–884. doi: 10.1111/j.1365-2265.2008.03248.x. [DOI] [PubMed] [Google Scholar]
- Ozgen M Koca SS Aksoy K Dagli N Ustundag B Visfatin levels and intima-media thicknesses in rheumatic diseases. Clinical Rheumatology. 2011;30:757–763. doi: 10.1007/s10067-010-1649-2. [DOI] [PubMed] [Google Scholar]
- Hsu CY Huang PH Chen TH Chiang CH Leu HB Increased circulating visfatin is associated with progression of kidney disease in non-diabetic hypertensive patients. American Journal of Hypertension. 2016;29:528–536. doi: 10.1093/ajh/hpv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbay M Yilmaz MI Sonmez A Turgut F Saglam M Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. The American Journal of Nephrology. 2011;33:298–304. doi: 10.1159/000324847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S Kajikawa M Hida E Maruhashi T Iwamoto Y Optimal target level of low-density lipoprotein cholesterol for vascular function in statin naïve individuals. Scientific Reports. 2017;7:8422–8422. doi: 10.1038/s41598-017-09043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]