Abstract
Background/aim
Sjögren’s syndrome (SS) is an autoimmune disease and its pathogenesis is still not completely clear. The wingless (Wnt)/β-catenin pathway has recently been shown to play an important role in inflammation. This study aims to determine the serum and saliva levels of Dickkopf (DKK)1 and sclerostin and to evaluate Wnt-1 and Wnt-3a expression in the salivary gland in patients with primary SS.
Materials and methods
This study included 30 patients diagnosed with SS, 30 patients diagnosed with systemic lupus erythematosus (SLE), and 29 healthy controls. Serum and saliva levels of DKK1 and sclerostin were measured and the expressions of Wnt1 and Wnt3a in the salivary gland were measured immunohistochemically.
Results
Serum DKK1 and sclerostin levels were lower in the SS and SLE groups compared to the control group (both p < 0.001). Saliva DKK1 levels were higher in the SS group compared to the control and SLE groups (p = 0.004 and p = 0.009, respectively). Wnt1 and Wnt3a expression were found in salivary gland tissue samples in 71.4% of primary SS patients and relatively frequent than control group.
Conclusions
Serum DKK1 and sclerostin levels in primary SS and SLE were decreased. Moreover, levels of Wnt1 and Wnt3a expression in the salivary gland were also elevated in primary SS. Therefore, it can be concluded that the Wnt/β-catenin pathway activities may be altered in case of glandular inflammation.
Keywords: Sjögren syndrome, wingless, sclerostin, dickkopf -1
1. Introduction
Sjögren’s syndrome (SS) is a systemic autoimmune disease that is prevalent in women [1,2]. Its pathogenesis is not completely clear; however, genetic disposition, viral stimulation, hormonal factors, and natural and acquired immunity have been implicated [36]. The chief symptoms of the disease include dryness in the mouth and eyes, fatigue, and joint pain due to exocrinopathy. These symptoms, particularly fatigue, decrease the patient’s life quality and result in an impairment of cognitive capacity [79]. Currently, there is no completely effective treatment for these symptoms [10]. Thus, determining the pathogenesis of this disease is important. Accordingly, studies are being conducted to identify the pathologic pathways.
The (Wnt) signaling pathway plays an important role in the development of the immune system, at the organogenesis stage, and in the regulation of various biological events such as the proliferation and differentiation of cells [11,12]. The Wnt/Wingless β-catenin signaling pathway is activated by proteins such as low-density lipoprotein receptor-related protein (LRP) 5, LRP6 and frizzled, and inhibited by extracellular proteins such as sclerostin and Dickkopf (DKK) 1 [1216]-. Studies have been conducted on the Wnt pathway in relation to various rheumatic diseases such as rheumatoid arthritis (RA), spondyloarthritis, and systemic lupus erythematosus (SLE); and this pathway was shown to be linked to the pathogenesis [17,18].
The present study aims to evaluate the serum and saliva levels of sclerostin and DKK1, and to immunohistochemically evaluate the levels of Wnt1 and Wnt3a expression in the salivary gland, in patients with primary SS. Moreover, the relationship between these parameters and clinical activity were also determined.
2. Materials and methods
2.1. Study subject
The study was carried out by the departments of rheumatology at FiratUniversity. Ethical committee approval was obtained from FiratUniversity clinical research ethics committee. We conducted our study in accordance with the approved guidelines of ethical principles for medical research involving human subjects. Written informed consent was provided from all participant. This study included 30 primary SS patients, 30 patients diagnosed with SLE (as a patient control group), and 29 healthy controls.
The SS and SLE patient groups were composed of patients who applied to the rheumatology outpatient clinic, met the respective diagnostic criteria [19,20], and agreed to participate in the study. The healthy control group was composed of volunteers who applied to the rheumatology outpatient clinic but did not have rheumatic inflammatory pathology according to the evaluations. Individuals younger than 18 years and older than 65 years; pregnant women; breastfeeding women; and patients with active infections, poorly controlled diabetes, heart failure, and malignant diseases were excluded from the study.
In addition, archived salivary gland tissue samples of 14 patients, diagnosed with SS and included in the study, and of 12 patients who had provided salivary gland tissue specimens for a preliminary diagnosis of SS but were not diagnosed with SS (pathological control group) were included in the study to be evaluated immunohistochemically for Wnt1 and Wnt3 expression levels. Therefore, time to harvest salivary gland tissue samples was different time periods from saliva and serum sample harvesting.
2.2. Clinical Evaluation
The patients were evaluated with regard to the parameters of disease activity used in the follow-up of SS and SLE. Disease activity was determined using ESSDAI (EULAR SS disease activity index) and ESSPRI (EULAR SS patient reported index) in SS patients [21], and using SLEDAI (systemic lupus erythematosus disease activity index) in SLE patients [22].
2.3. Laboratory evaluation
The results of the routine tests (fasting blood sugar, creatinine, ALT, blood count, erythrocyte sedimentation rate [ESR], and C-reactive protein [CRP]) were recorded in the patient and the control groups. Additionally, for the SS group, results indicating ANA, anti-Ro, anti-La; and for the SLE group ANA, anti-dsDNA, anti-Sm, C3 and spot urine protein were recorded from the patient files. In addition to these routine tests, 5blood and 2m-ml - saliva samples were collected from all participants after overnight fasting. Blood samples were centrifuged at 3000 rpm for 5 , and 2 m of serum was separated. The obtained serum and saliva samples were stored at 20 lminutesl-C until the day of the analysis. the end of the study, the stored samples were assessed to determine serum and saliva levels of sclerostin and DKK1 with the ELISA method, using an appropriate commercial kit °In (YH Biosearch, Pudong District, China).
2.4. Histopathological evaluation
Salivary gland tissue samples of 14 SS patients that had been collected for diagnostic purposes, and archived tissue samples of 12 patients who had provided minor salivary gland tissue samples due to a preliminary diagnosis of SS but were not diagnosed with SS (pathology control group) were included in the study. Tissue specimens were paraffin embedded. Formalin-fixed paraffin-embedded tissue sections that were cut 3-μm thick were placed onto positively charged glass slides (Isotherm, Objektträger, Braunschweig, Germany) .Immunohistochemical (IHC) staining was performed using tissue sections to determine Wnt1 and Wnt3a expression. The following antibodies were used for immunohistochemistry: Wnt1 (Mouse Wnt1 primary antibody (10C8), NBP1-51575, Novus biologicals, USA) and Wnt3a (Mouse Wnt3a primary antibody, NBP1-74183, Novus biologicals, USA). IHC staining of tissue sections were used the Ventana BenchMark Ultra Autostainer (Ventana, Tuscon, AZ-85755, USA) and the UltraView Univerversal DAB kit (Ventana, Tuscon, AZ-85755, USA), following the manufacturer’s instructions. Tissue sections were imaged with an Olympus B×51 upright light microscope (Olympus America, Center Valley, , USA) and images captured in digital color camera (DP71, Olympus)
PennsylvaniaIn the evaluations, the criteria for positivity was defined as cytoplasmic staining in the ducts. The findings were graded based on staining intensity as follows; [23]
-: no staining
+: mild staining
++: moderate staining
+++: strong staining2.5. Statistical analysis
The continuous data obtained in the study were presented as mean ± standard deviation, while nonparametric data without normal distribution were expressed as median (minimum-maximum). Statistical analyses were conducted using the IBM-SPSS, 22.0 software (International Business Machines-Statistical Product and Service Solutions (version )IBM Corp., Armonk, NY, USA). The Chi-square test was used for categorical data. Normal distributions were tested with the KolmogorovSmirnov test. The significant difference among groups was determined by the KruskalWallis variance analysis and the MannWhitney U test for dual comparisons in the nonnormal distributed and nonparametric data. One-way ANOVA and the student’s t-test for dual comparisons were performed parametric data with normal distribution. Bonferroni correction was applied since there were three study groups. Analysis of covariance (ANCOVA) was also used to adjust variables for age and . P-values < 0.05 were considered significant.
3. Results
3.1. Baseline characteristics
The demographic and clinical data of the study groups were presented in the Table 1. The median (min-max) age was determined as 51 (3665) -years for the SS group, as 37 (2250) -years for the SLE group, and 29 (2357) -years for the healthy control group. The percentage of females in the healthy control, SLE, and SS groups were determined as 51.7%, 80%, and 100%, respectively. ANCOVA was used to adjust variables for age and , since the differences between the groups in terms of age and gendergender were statistically significant (both p < 0.05). The median (min-max) disease duration was 1.7 (011) years for SS patients and 1.5 (013) years for the SLE group. The groups did not differ the disease durations (p > 0.05).
Table 1.
Demographic and clinical data of the study groups.
| Data× | HC(n = 29) | SLE(n = 30) | SS(n = 30) | P1* | P2* | P3* |
|---|---|---|---|---|---|---|
| Age, years | 29 (23–57) | 37 (22–50) | 51 (36–65) | 0.068 | <0.001 | 0.001 |
| Disease duration, years | - | 1.7 (0–11) | 1.5 (0–13) | - | - | 0.738 |
| Female, % | 51.7 | 80 | 100 | 0.029 | < 0.001 | 0.023 |
| Hemoglobin, g/dL | 14.7 ± 1.2 | 12.4 ± 2.1 | 13.3 ± 1.5 | < 0.001 | 0.004 | 0.104 |
| WBC, 103/µL | 7.2 ± 1.9 | 6.2 ± 2.4 | 5.9 ± 1.8 | 0.140 | 0.069 | 0.907 |
| ESR, mm/h | 5 (1–39) | 18.5 (3–66) | 12 (5–71) | <0.0001 | <0.0001 | 0.361 |
| CRP, mg/dL | 3.4 (3–16.8) | 3.4 (1.9–14.6) | 3.4 (3.2–70) | 0.612 | 0.368 | 0.778 |
| Serum DKK1, ng/mL | 52.9 (24.1–74.5) | 26.5 (2.1–63.2) | 33.9 (20.6–59) | < 0.001 | < 0.001 | 0.046 |
| Saliva DKK1, ng/mL | 30.6 ± 5.9 | 31.1 ± 6.9 | 36.3 ± 6.9 | 0.944 | 0.004 | 0.009 |
| Serum sclerostin, ng/mL | 15.5 (3.4–18.3) | 4.6 (2.1–18.1) | 4.5 (2–18.6) | < 0.001 | < 0.001 | 0.984 |
| Saliva sclerostin, ng/mL | 16.1 ± 3.4 | 15.7 ± 2.5 | 15.6 ± 3.7 | 0.877 | 0.838 | 0.996 |
3.2. Basic laboratory tests
Hemoglobin levels were significantly lower in the SS and SLE groups compared to the healthy control group (p = 0.004 and p < 0.001, respectively). However, there were no statistically significant differences with regard to leukocyte, platelet, and CRP levels. ESR was higher in the SLE and SS groups compared to the control group (p < 0.001 for both).
3.3. Disease activity score of SLE and SS patients
Systemic lupus erythematosus patients showed a median SLEDAI score of 10 (029), -and SS patients showed a median ESSDAI score of 1 (03)-. ANA positivity was 96% in the SLE group and 83.3% in the SS group.
3.4. The sclerostin and DKK1 levels of the patient and healthy control groups
The SS group demonstrated lower serum DKK1 and sclerostin levels compared to the control group (both p < 0.001), even after adjustment for age and by ANCOVA analysis. However, saliva levels of DKK1 and sclerostin were comparable across the SS and healthy control groups (p > 0.05). Serum DKK1 and sclerostin levels were lower in SLE patients compared to healthy controls (p < 0.001 for both). However, the SLE group did not differ from the control group in terms of saliva DKK1 and sclerostin levels. In addition, serum and saliva levels of DKK1 were higher in patients diagnosed with SS compared to the SLE group (p = 0.046 and p = 0.009, respectively). However, the SS and SLE groups were not significantly different with regard to serum and saliva levels of sclerostin.
A positive correlation was determined between serum DKK1 levels and serum sclerostin levels in the healthy control, SLE, and SS groups (respectively; r = 0.829, p < 0.001; r = 0.783, p < 0.001; and r = 0.677, p < 0.001). Serum and saliva levels of sclerostin and DKK1 were not correlated with ESSDAI and SLEDAI in these groups (p > 0.05).
3.5. Wnt1 and Wnt3a expression were increased in the salivary gland of the patients with SS
Wnt1 and Wnt3a positivity were higher in the salivary gland biopsy samples of patients diagnosed with SS compared to the control group (Figures 1a, 1b for Wnt1; Figures 2a, 2b for Wnt3a). However, this difference was not statistically significant (Table 2).
Table 2.
Wnt1 and Wnt3a expressions in the salivary glands of patients with primary SS.
| Control | Sjögren’s Syndrome | P* | |
|---|---|---|---|
| Wnt1 positive patients, % | 46.2 | 71.4 | 0.182 |
| Mild, % | 7.7 | 42.9 | |
| Moderate, % | 15.4 | 14.3 | |
| Strong, % | 23.1 | 14.3 | |
| Wnt3a positive patients, % | 53.8 | 71.4 | 0.345 |
| Mild, % | 30.8 | 7.1 | |
| Moderate, % | 15.4 | 14.3 | |
| Strong, % | 7.7 | 50 |
Figure 1.
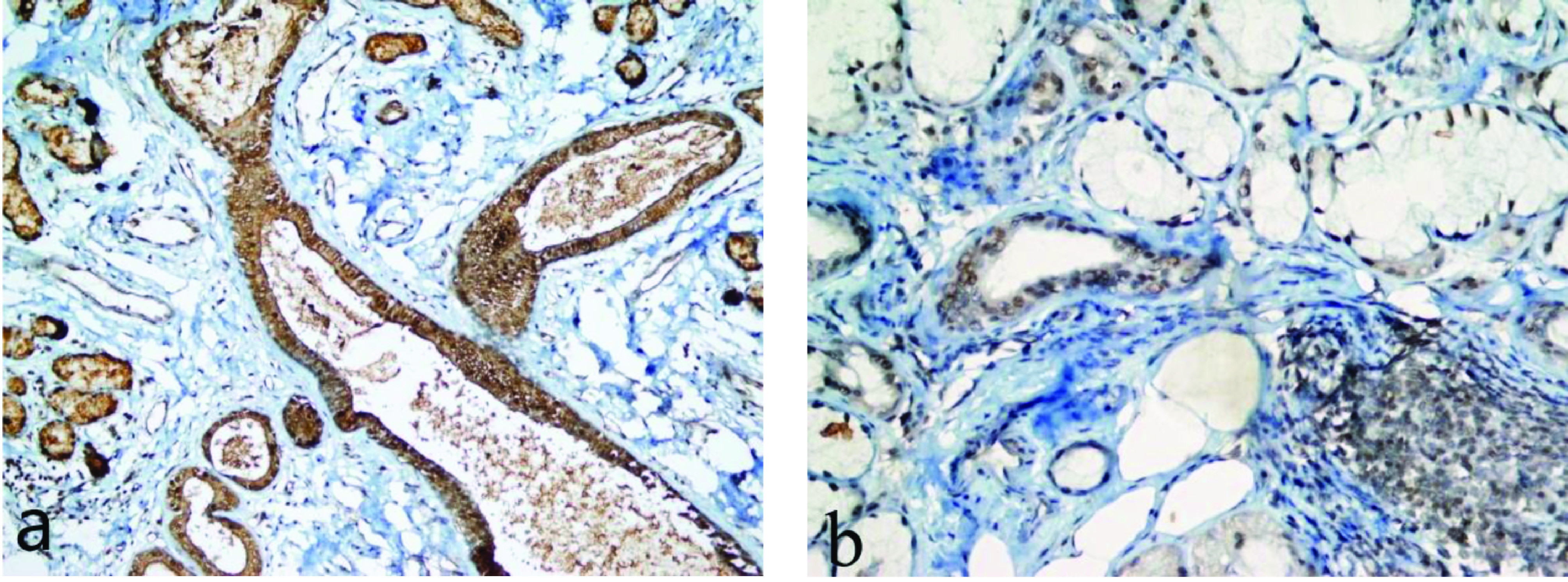
Immunohistochemical Wnt1 expression in the salivary gland. (a) Strong nuclear and cytoplasmic Wnt1 positivity in the ducts and acini in controls (×400), (b) Mild nuclear and cytoplasmic Wnt1 positivity in the ducts and acini in SS patients (×400).
Figure 2.
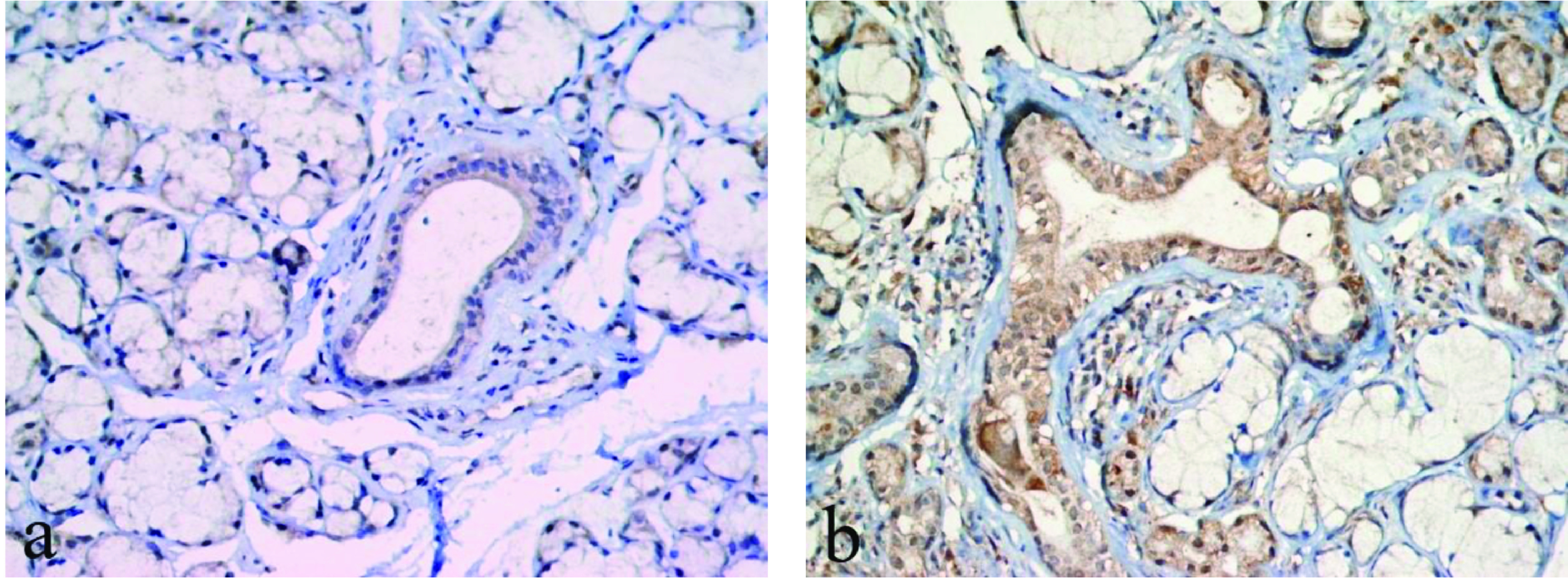
Immunohistochemical Wnt3a expression in the salivary gland. (a) Mild nuclear and cytoplasmic Wnt3a positivity in the ducts and acini in controls (×400), (b) Strong nuclear and cytoplasmic Wnt3a positivity in the ducts and acini in SS patients (×400).
SS patients with Wnt1- and Wnt3a-positive salivary glands and those with Wnt1- and Wnt3a-negative salivary glands did not have significantly different serum and saliva levels of DKK1 and sclerostin (for each p > 0.05). Similarly, Wnt1- and Wnt3a-positive SS patients and Wnt1- and Wnt3a-negative SS patients were not significantly different with regard to ANA, anti-Ro, and anti-La antibody positivity (for each p > 0.05) (Table 3).
Table 3.
Differences between Wnt1- and Wnt3a-positive and Wnt1- and Wnt3a-negative patients with primary SS.
| Wnt1 | Wnt3a | |||||
|---|---|---|---|---|---|---|
| Data× | Negative | Positive | P* | Negative | Positive | P* |
| Anti-Ro titre, IU/L | 131.2 ± 79.9 | 80.4 ± 95 | 0.1 | 98.2 ± 96.6 | 93.6 ± 2 | 0.7 |
| Anti-La titre, IU/L | 6.5 ± 2 | 76.7 ± 107.8 | 0.7 | 112.2 ± 134.3 | 32.6 ± 68.8 | 0.3 |
| Serum DKK1, ng/mL | 28.6 ± 5.8 | 36.9 ± 9 | 0.06 | 31.9 ± 4.3 | 35.6 ± 10.2 | 0.6 |
| Saliva DKK1, ng/mL | 38 ± 10.5 | 35.8 ± 7.2 | 0.8 | 37.9 ± 9.5 | 35.8 ± 7.7 | 0.6 |
| Serum sclerostin, ng/mL | 4.5 ± 2.5 | 5.2 ± 4.8 | 1 | 3.8 ± 1.1 | 5.5 ± 4.9 | 0.6 |
| Saliva sclerostin, ng/mL | 14.7 ± 2 | 14.2 ± 4.9 | 0.6 | 15.5 ± 1.7 | 13.9 ± 4.8 | 0.3 |
4. Discussion
The aim of this study was to determine the activity of Wnt/β-catenin signaling pathway in primary SS. For this purpose, serum and salivary sclerostin and DKK1 levels and Wnt1 and Wnt3a immunohistochemical expressions in salivary gland tissue were evaluated.
SS is a chronic, autoimmune disease that mainly affects the tear and salivary glands. Lymphocyte infiltration and dysfunction of the exocrine glands result in dry mouth and eyes [24]. Hereditary and environmental factors have been implicated in the pathogenesis. One of the most important theories in the pathogenesis is that the SS is a process that starts with epithelitis and then continues with lymphocyte infiltration. Therefore, the disease is considered by some researchers as autoimmune epithelitis [25]. Moreover, recent data show that salivary gland epithelial cells play an active role in initiating inflammatory and autoimmune response [26].
Wnt/β - catenin signaling pathway plays an important role in embryonic development, tissue homeostasis, cellular proliferation and also the regulation of the immune system such as T, B and dendritic cells [27,28]. Genes associated with this pathway have been expressed in a wide variety of cell types and tissues such as adipocytes, osteoblasts, platelets, lymph gland, adrenal gland, thyroid, pituitary and salivary gland [29,30]. Wnt/β-catenin signaling pathway is known to play a role in the pathogenesis of cancer and autoimmune diseases [28,3134]. In this respect, there are a number of studies investigating biomarker in these diseases [35,36]. Wnt/-β-catenin signaling pathway is an important regulatory pathway in bone formation and destruction [37,38].
The blocking DKK1, which is an inhibitor of Wnt/β-catenin signaling pathway, decreased osteoclastic activity and decreased bone erosion in RA patients [39]. It has also been found that DKK1 levels increased in RA patients and correlated with bone erosion [37]. Wehmeyer et al. [40] have found that the sclerostin levels in the synovium of RA are significantly higher than in patients with osteoarthritis. In a subsequent study [41], it has been observed that the level of DKK1 is higher in patients with RA and glucocorticoid treatment decreases serum DKK1 level.
In our study, it is observed that serum DKK1 and sclerostin levels are decreased in patients with primary SS. There are controversial results in terms of DKK1 and sclerostin levels in inflammatory diseases. Contrary to the articles documenting increased serum DKK1 and sclerostin levels in RA [40,41], Zhang et al. [42] have reported that serum DKK1 level in patients with RA is similar in healthy subjects. There are two controversial study reporting increased [42] and decreased [43] serum DKK1 levels in patients with ankylosing spondylitis.
Dovjak et al. [44] have found that DKK1 levels are correlated with age and the presence of osteoporosis. Furthermore, serum DKK1 level is higher in males. Gifre et al. [41] have documented that treatment alters serum DKK1 levels. Therefore, it can be speculated that the differences on demographical data, clinical manifestations and treatment modalities of the mentioned studies are the potential reasons of controversial results of DKK1 in inflammatory diseases.
In our study, it is documented that serum the level of DKK1, an inhibitor of Wnt signaling pathway is decreased, while glandular tissue Wnt1 and Wnt3a expressions are relatively higher, in patients with primary SS. Wnt signaling pathways are an important regulator in salivary gland organogenesis. Wnt/β-catenin signaling plays a role in both mesenchymal and ductal maturation of salivary glands. On the other hand, the Wnt/β-catenin signal and the noncanonical Wnt pathway work together to regulate maturation in the morphogenesis stage [45,46]. However, the Wn/-t β-catenin signal is significantly activated in the channel epithelium during functional regeneration in the adult salivary gland [46]. Fernandez-Torres et al. [47] have found that gene polymorphisms such as LRP5, FRZB, and ADIPOQ associated with Wnt/β-catenin signaling pathway lead to increased risk of primary SS.
The present study has few limitations. The sample size was not calculated before enrolling study participants. Age and were not matched; however, adjustment for age and gender by ANCOVA was performed during statistical analysis. Moreover, salivary gland tissue and serum samples were not harvested same time.
In present study, Wnt1 and Wnt3a expression are found in salivary gland tissue samples in 71.4% of primary SS patients. High salivary DKK1 levels in primary SS group can be explained by glandular inflammation and damage. According to the best of our knowledge, the present study is the first to evaluate the efficacy of Wnt/gender β-catenin pathway in primary SS. In conclusion, in present study, serum levels of DKK1 and sclerostin, Wnt/β-catenin signaling pathway inhibitors, are decreased and Wnt1 and Wnt3a are accumulated in the glandular tissue in primary SS patients. These results indicate that Wnt/β-catenin signaling pathway is affected in primary SS.
Acknowledgment/Conflict of interest/Disclaimers
We thank Batuhan Selvi for helpful in the English language editing the article.
References
- Bowman SJ Ibrahim GH Holmes G Hamburger J Ainsworth JR Estimating the prevalence among Caucasian women of primary Sjögren’s syndrome in two general practices in Birmingham, UK. Scandinavian Journal of Rheumatology. 2004;33:39–43. doi: 10.1080/03009740310004676. [DOI] [PubMed] [Google Scholar]
- Daniels TE Fox PC Salivary and oral components of Sjögren’s syndrome. Rheumatic Disease Clinics of North America. 1992;18:571–589. [PubMed] [Google Scholar]
- Kramer JM Early events in Sjögren’s Syndrome pathogenesis: the importance of innate immunity in disease initiation. Cytokine. 2014;67:92–101. doi: 10.1016/j.cyto.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Cafaro G Croia C Argyropoulou OD Leone MC Orlandi M One year in review 2019: Sjögren’s syndrome. Clinical and Experimental Rheumatology. 2019;37118:3–15. [PubMed] [Google Scholar]
- Sandhya P Kurien BT Danda D Scofield RH Update on pathogenesis of Sjogren’s Syndrome. Current Rheumatology Reviews. 2017;13:5–22. doi: 10.2174/1573397112666160714164149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carrasco M Fuentes-Alexandro S Escárcega RO Salgado G Riebeling C Pathophysiology of Sjögren’s syndrome. Archives of Medical Research. 2006;37:921–932. doi: 10.1016/j.arcmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Miyamoto ST Valim V Fisher BA Health-related quality of life and costs in Sjögren’s syndrome. Rheumatology. 2019;15 doi: 10.1093/rheumatology/key370. [DOI] [PubMed] [Google Scholar]
- Maarse F Jager DH Forouzanfar T Wolff J Brand HS Tooth loss in Sjögren’s syndrome patients compared to age and gender matched controls. Medicina Oral Patologia Oral y Cirugia Bucal. 2018;23:e545–e551. doi: 10.4317/medoral.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY Lee J Choi YS Kim JW Kwok SK Do I sound dry? Comparative voice analysis of primary Sjögren’s syndrome. Clinical and Experimental Rheumatology. 2018;112:130–136. [PubMed] [Google Scholar]
- Brito-Zerón P Retamozo S Kostov B Baldini C Bootsma H Efficacy and safety of topical and systemic medications: a systematic literature review informing the EULAR recommendations for the management of Sjögren’s syndrome. RMD Open. 2019;5 doi: 10.1136/rmdopen-2019-001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ Luis TC Tiemessen MM WNT signalling in the immune system: WNT is spreading its wings. Nature Reviews Immunology. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Clevers H Nusse R. Wnt /β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Komiya Y Habas R Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K Semenov M Kato Y Spokony R Liu C LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Malinauskas T Jones EY Extracellular modulators of Wnt signalling. Current Opinion in Structural Biology. 2014;29:77–84. doi: 10.1016/j.sbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Niehrs C Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Cici D Corrado A Rotondo C Cantatore FP Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. International Journal of Molecular Sciences. 2019;20:5552–5552. doi: 10.3390/ijms20225552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD Huang XF Yan QR Bao CD Aberrant activation of the WNT/β-catenin signaling pathway in lupus nephritis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiboski SC Shiboski CH Criswell L Baer A Challacombe S American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care & Research. 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri M Orbai AM Alarcón GS Gordon C Merrill JT Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis & Rheumatology. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seror R Bootsma H Saraux A Bowman SJ Theander E Defining disease activity states and clinically meaningful improvement in primary Sjögren’s syndrome with EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI) Annals of the Rheumatic Diseases. 2016;75:382–389. doi: 10.1136/annrheumdis-2014-206008. [DOI] [PubMed] [Google Scholar]
- Bombardier C Gladman DD Urowitz MB Caron D Chang CH Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis & Rheumatology. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Denysenko T Annovazzi L Cassoni P Melcarne A Mellai M WNT/β-catenin signaling pathway and downstream modulators in low- and high-grade glioma. Cancer Genomics Proteomics. 2016;13:31–45. [PubMed] [Google Scholar]
- Thorne I Sutcliffe N. Sjögren’s syndrome. British Journal of Hospital Medicine. 2017;78:438–442. doi: 10.12968/hmed.2017.78.8.438. [DOI] [PubMed] [Google Scholar]
- Takeda K Akira S Toll-like receptors. Current Protocols in Immunology. 2015;109:14–14. doi: 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- Manoussakis MN Kapsogeorgou EK The role of intrinsic epithelial activation in the pathogenesis of Sjögren’s syndrome. Journal of Autoimmunity. 2010;35:219–224. doi: 10.1016/j.jaut.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Roberts EW Broz ML Binnewies M Headley MB Nelson AE Critical role for CD103(+)/CD141(+) dendritic cells bearing ccr7 for tumor antigen trafficking and priming of t cell immunity in melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecarpentier Y Schussler O Hébert JL Vallée A Multiple targets of the canonical WNT/β-catenin signaling in cancers. Frontiers in Oncology. 2019;9:1248–1248. doi: 10.3389/fonc.2019.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M Patel MS Levasseur R Lobov I Chang BH -independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. Journal of Cell Biology. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RD Carulli JP Del Mastro RG Dupuis J Osborne M A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. The American Journal of Human Genetics. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L Wang Y Zhang N Zhang Y Lin J Heterozygous deletion of LRP5 gene in mice alters profile of immune cells and modulates differentiation of osteoblasts. BioScience Trends. 2018;12:266–274. doi: 10.5582/bst.2018.01013. [DOI] [PubMed] [Google Scholar]
- Kawazoe M Kaneko K Shikano K Kusunoki N Nanki T Glucocorticoid therapy causes contradictory changes of serum Wnt signaling-related molecules in systemic autoimmune diseases. Clinical Rheumatology. 2018;37:2169–2178. doi: 10.1007/s10067-017-3689-3. [DOI] [PubMed] [Google Scholar]
- Gözel N Duran F Yildirim A Yolbaş S Önalan E Paricalcitol inhibits Wnt/β-catenin signaling pathway and ameliorates dermal fibrosis in bleomycin induced scleroderma model. Archives of Rheumatology. 2017;33:288–294. doi: 10.5606/ArchRheumatol.2018.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olferiev M Jacek E Kirou KA Crow MK Novel molecular signatures in mononuclear cell populations from patients with systemic lupus erythematosus. Clinical Immunology. 2016;172:34–43. doi: 10.1016/j.clim.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Pishvaian MJ Byers SW Biomarkers of WNT signaling. Cancer Biomarkers. 2007;3:263–274. doi: 10.3233/cbm-2007-34-510. [DOI] [PubMed] [Google Scholar]
- Santiago L Daniels G Wang D Deng FM Lee P Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. American Journal of Cancer Research. 2017;7:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- Rossini M Viapiana O Adami S Fracassi E Idolazzi L In patients with rheumatoid arthritis, Dickkopf-1 serum levels are correlated with parathyroid hormone, bone erosions and bone mineral density. Clinical and Experimental Rheumatology. 2015;33:77–83. [PubMed] [Google Scholar]
- Daoussis D Andonopoulos AP The emerging role of Dickkopf-1 in bone biology: is it the main switch controlling bone and joint remodeling? Seminars in Arthritis. Rheumatism. 2011;41:170–177. doi: 10.1016/j.semarthrit.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Diarra D Stolina M Polzer K Zwerina J Ominsky MS Dickkopf-1 is a master regulator of joint remodeling. Nature Medicine. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Wehmeyer C Stratis A Pap T Dankbar B The role of the WNT inhibitor sclerostin in rheumatoid arthritis bone/cartilage biology. Annals of the Rheumatic Diseases. 2010;69:21–22. [Google Scholar]
- Gifre L Ruiz-Gaspà S Monegal A Nomdedeu B Filella X Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone. 2013;57:272–276. doi: 10.1016/j.bone.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Zhang L Ouyang H Xie Z Liang ZH Wu XW Serum DKK-1 level in the development of ankylosing spondylitis and rheumatic arthritis: a meta-analysis. Experimental & Molecular Medicine. 2016;48 doi: 10.1038/emm.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SR Lim MJ Suh CH Park SG Hong YS Dickkopf-1 level is lower in patients with ankylosing spondylitis than in healthy people and is not influenced by anti-tumor necrosis factor therapy. Rheumatology International. 2012;32:2523–2527. doi: 10.1007/s00296-011-1981-0. [DOI] [PubMed] [Google Scholar]
- Dovjak P Dorfer S Föger-Samwald U Kudlacek S Marculescu R Serum levels of sclerostin and dickkopf-1: effects of age, gender and fracture status. Gerontology. 2014;60:493–501. doi: 10.1159/000358303. [DOI] [PubMed] [Google Scholar]
- Patel N Sharpe PT Miletich I. Coordination of epithelial branching and salivary gland lumen formation by Wnt and FGF signals. Developmental Biology. 2011;358:156–167. doi: 10.1016/j.ydbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Häärä O Fujimori S Schmidt-Ullrich R Hartmann C Thesleff I Wnt pathways are required for salivary gland branching morphogenesis. Development. 2011;138:2681–2691. doi: 10.1242/dev.057711. [DOI] [PubMed] [Google Scholar]
- Fernández-Torres J Pérez-Hernández N Hernández-Molina G Martínez-Nava GA Garrido-Rodríguez D Risk of Wnt/β-catenin signalling pathway gene polymorphisms in primary Sjögren’s syndrome. Rheumatology. 2020;59:418–425. doi: 10.1093/rheumatology/kez269. [DOI] [PubMed] [Google Scholar]


