Abstract
Cytotoxic T lymphocytes (CTL) can trigger an apoptotic signal through the Fas receptor or by the exocytosis of granzyme B and perforin. Caspase activation is an important component of both pathways. Granzyme B, a serine proteinase contained in granules, has been shown to proteolytically process and activate members of the caspase family in vitro. In order to gain an understanding of the contributions of caspases 8 and 3 during granule-induced apoptosis in intact cells, we have used target cells that either stably express the rabbitpox virus-encoded caspase inhibitor SPI-2 or are devoid of caspase 3. The overexpression of SPI-2 in target cells significantly inhibited DNA fragmentation, phosphatidylserine externalization, and mitochondrial disruption during Fas-mediated cell death. In contrast, SPI-2 expression in target cells provided no protection against granzyme-mediated apoptosis, mitochondrial collapse, or cytolysis, leading us to conclude that SPI-2-inhibited caspases are not an essential requirement for the granzyme pathway. Caspase 3-deficient MCF-7 cells were found to be resistant to CTL-mediated DNA fragmentation but not to CTL-mediated cytolysis and loss of the mitochondrial inner membrane potential. Furthermore, we demonstrate that granzyme B directly cleaves the proapoptotic molecule Bid, bypassing the need for caspase 8 activation of Bid. These results provide evidence for a two-pronged strategy for mediating target cell destruction and provide evidence of a direct link between granzyme B activity, Bid cleavage, and caspase 3 activation in whole cells.
A critical event during apoptosis is the proteolytic cleavage and activation of a family of cysteine proteases which have been collectively referred to as caspases (8, 52). Caspase activation requires sequence-specific proteolytic processing at internal aspartate residues in order to convert the inactive zymogen to an active protease. Fourteen caspases have now been identified, which can be divided into three subfamilies based upon their distinct substrate specificities (77). Caspases 1, 4, and 5 (group 1) play a role in cytokine maturation and inflammation, whereas group 2 (caspases 2, 3, and 7) and group 3 (caspases 6, 8, 9, and 10) caspases are directly involved in apoptosis. Group 2 and Group 3 can be further divided into initiator and effector caspases. Initiator caspases, such as caspase 8, 9, and 10, are responsible for the activation of downstream effector caspases, such as caspases 3, 6, and 7, which in turn are responsible for the cleavage and inactivation of a multitude of intracellular proteins and the eventual demise of the cell (69).
A wide range of external stimuli induce caspase activation and apoptosis, including the interaction of cytotoxic T lymphocytes (CTL) with target cells. CTL are important for the removal of both virus-infected and malignant cells and can destroy target cells by two distinct caspase-dependent mechanisms (2). The first and best-characterized pathway involves ligation of the Fas death receptor on the surface of target cells (54). Engagement of the Fas receptor culminates in the recruitment of caspase 8 via the adapter molecule FADD (also called MORT1) (3, 50). Recruitment of caspase 8 results in the autocatalytic activation of this caspase (51, 80), which is then capable of activating downstream caspases either by direct proteolytic cleavage or indirectly through the activation of Bid and the release of mitochondrial apoptosis-inducing proteins such as cytochrome c (21, 28, 37, 40, 62, 82). The second pathway of CTL-induced cell death involves the calcium-dependent exocytosis of cytolytic granules from CTL. The granules of CTL are composed of a variety of proteins necessary for inducing target cell death (20, 66). Included among these are perforin, a pore-forming protein that is thought to facilitate the entry of other granule-contained proteins, and a family of the serine proteases known as granzymes.
Granzyme B displays unique substrate specificity for a member of the serine proteinase family, proteolytically cleaving proteins following aspartate residues (4, 53, 55). Significantly, the first substrate identified for granzyme B was found to be a member of the caspase family, caspase 3 (9). Multiple caspase proteins have now been identified, and many serve as substrates for granzyme B in vitro (6, 9, 13, 14, 22, 50, 57, 74, 78), suggesting that granzyme B induces apoptosis by triggering the activation of multiple caspases within intact cells. Additionally, studies have shown that caspases 1, 2, 3, 6, 7, and 8 are proteolytically processed in cells following granule-mediated apoptosis (1, 6, 9, 15, 16, 48, 64). Thus far, however, only caspases 8 and 3 have been shown to be direct substrates for granzyme B in intact cells (1, 48, 81), indicating that the activations of caspases 8 and 3 are potentially critical events during granule-mediated apoptosis.
Caspase 8 is the first caspase activated during Fas-mediated cell death and is essential for activating the proapoptotic molecule Bid, resulting in release of cytochrome c from the mitochondria (21, 28, 40, 82). Caspase 8 therefore represents a crucial step for Fas-induced apoptosis. Although granule-mediated apoptosis is also known to result in the activation of caspases, including caspase 8, information is still lacking regarding the specific role that activation of each caspase plays within whole cells. Since caspase 8 is a direct substrate for granzyme B in whole cells (48) and can activate other members of the caspase family, including caspase 3 (67, 68), it can be argued that the activation of caspase 8 by granzyme B may be an important step during granule-mediated cell death. The contribution of caspase 8 activity during granule-mediated apoptosis, however, has not been fully evaluated.
Many viruses encode proteins that specifically interfere with caspase 8 (49), providing unique macromolecular tools for dissecting caspase cascades induced by proapoptotic stimuli like CTL. One example is the poxvirus-encoded serine proteinase inhibitor designated CrmA in cowpox virus, which is also referred to as SPI-2 in other members of the poxvirus family. CrmA/SPI-2 is a potent inhibitor of both caspase 1 and caspase 8 and has been used extensively for elucidating apoptotic cascades (83). In order to investigate the contribution of caspase 8 during granule-mediated cell death in whole cells, we utilized Jurkat cells transfected with the rabbitpox virus crmA gene, SPI-2. As anticipated, SPI-2 was an excellent inhibitor of apoptosis induced through the Fas receptor. In contrast, SPI-2 expression provided no protection against granule-mediated cell death. Although caspase 8 was proteolytically cleaved during granule-mediated cell death, our data indicate that granule-mediated CTL killing can short-circuit the need for caspase 8 activity. In support of this, we demonstrate for the first time that granzyme B directly cleaves the proapoptotic molecule Bid. Additionally, MCF-7 cells, a breast carcinoma cell line which is naturally devoid of caspase 3, were found to be refractory to granzyme-induced DNA fragmentation but were still susceptible to CTL-mediated lysis, mitochondrial dysfunction, and death. The ability of CTL to activate multiple caspase members in addition to inducing cell death via caspase-independent processes through the activation of Bid indicates that CTL are extremely well equipped to ensure target cell death.
MATERIALS AND METHODS
Cell lines.
Jurkat cells were grown in RPMI 1640 medium (Gibco BRL Life Technologies Inc.) supplemented with 10% fetal calf serum (FCS) (Hyclone), 25 mM HEPES, 100 μM 2-mercaptoethanol, 100 μg of penicillin per ml, and 100 μg of streptomycin per ml (RHFM). Stably transfected Jurkat cells were maintained in RHFM supplemented with 800 μg of G418 (Gibco BRL Life Technologies Inc.) per ml. MCF-7 cells were purchased from the American Type Culture Collection and routinely cultured in RHFM supplemented with 100 μM nonessential amino acids (Gibco BRL Life Technologies Inc.). Human CTL (hCTL) were generated as previously described (1) and maintained in RHFM containing 90 U of interleukin 2 (Chiron) per ml.
Reagents.
The purification of human granzyme B from YT cells was performed as previously described (5, 23). The replication-deficient adenovirus type 5 dl1-70 was supplied by J. Gauldie, McMaster University, Hamilton, Ontario, Canada. Murine anti-human Fas antibody (clone CH-11) was purchased from Upstate Biotechnology Incorporated and routinely used at 250 ng/ml to induce DNA fragmentation. Staurosporine was purchased from Sigma and used at 5 μM. The caspase inhibitors zVAD-fmk and zIETD-fmk were purchased from Kamiya Biomedical. Jurkat cells were pretreated with caspase inhibitors for 60 min prior to the addition of apoptosis-inducing reagents. Polyclonal rabbit anti-caspase 3 and anti-caspase 8 antiserum was provided by D. W. Nicholson, Merck Frosst Centre for Therapeutic Research, Pointe Claire, Quebec, Canada. The murine anti-SPI-2 monoclonal antibody was provided by R. W. Moyer, University of Florida, Gainesville. The polyclonal rabbit anti-Bid antibody was provided by X. Wang, University of Texas Southwestern Medical Center, Dallas.
Generation of stable transfected cell lines.
The rabbitpox virus SPI-2 gene was subcloned into the XhoI site of eucaryotic expression vector BMGneo (31). Jurkat cells (5 × 106) were stably transfected with 10 μg of NotI-linearized DNA by electroporation (250 V, 250 μF). Stably transfected cells were selected with 1 mg of G418 (GIBCO BRL Life Technologies Inc.) per ml and cloned by limiting dilution. Once selected, the resulting cells were routinely grown in RHFM containing 800 μg of G418 (Gibco BRL Life Technologies Inc.) per ml. Two clones (clones 4 and 5) generated from this transfection were chosen for further analysis.
Apoptosis induction.
Jurkat cells were resuspended at 106 cells/ml in RHFM. Granzyme B (1 μg/ml) and adenovirus (10 PFU/cell) were added directly to the cell suspension. Cells were incubated at 37°C for either 2 or 4 h. For Fas killing, 250 ng of anti-Fas antibody (clone CH11) per ml was added directly to the cells, and the cells were incubated at 37°C and then analyzed at the times indicated.
Immunoblotting.
Cellular lysates were collected by directly harvesting 106 cells into 100 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel loading buffer and subjected to SDS-PAGE analysis. Proteins were transferred to nitrocellulose (Micron Separations Inc.) by using a semidry transfer apparatus (Tyler Corp.) for 1 h at 150 mA. Membranes were blocked in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (ICN Biomedicals Inc.) and 5% skim milk for 16 h. Caspases 3 and 8 were detected using polyclonal rabbit anti-caspase 3 or anti-caspase 8 antiserum at a dilution of 1:10,000 or 1:2,000 respectively. Expression of SPI-2 in stably transfected Jurkat cells was verified by immunoblotting using a murine anti-SPI-2 monoclonal antibody at a dilution of 1:20. Bid expression and cleavage were detected using polyclonal rabbit anti-Bid antiserum at a dilution of 1:3,000. All primary antibodies were incubated with the membrane for at least 2 h, after which the blot was washed three times in PBS containing 0.1% Tween 20. The membranes were probed with either a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Bio-Rad Laboratories) at a 1:20,000 dilution or a horseradish peroxidase-conjugated goat anti-murine secondary antibody (Jackson ImmunoResearch Laboratories) at a 1:3,000 dilution. Transferred proteins were visualized with a chemiluminescence detection system (Amersham Inc.) for caspase 3 and 8 detection or with SuperSignal substrate (Pierce) for SPI-2 expression according to the manufacturers' directions.
Flow cytometric analysis of DNA fragmentation.
DNA fragmentation in Jurkat cells was monitored by flow cytometric analysis via the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) method (19). Cells (106) were harvested and washed in PBS containing 1% FCS. The cells were then fixed in 200 μl of 2% paraformaldehyde for 30 min at room temperature with constant agitation. Following fixation, cells were washed three times in PBS containing 1% FCS prior to permeabilization in 100 μl of 0.1% Triton X-100–0.1% sodium citrate for 2 min on ice. The cells were washed again in PBS containing 1% FCS and then incubated for 1 h at 37°C in 30 μl of 30 mM Tris (pH 7.2)–140 mM cacodylate–0.6 nmol of fluorescein-12-dUTP–3 nmol of dATP–1 mM CoCl2–25 U of terminal deoxynucleotidyltransferase (Boehringer Mannheim). After incubation, cells were washed in PBS containing 1% FCS prior to flow cytometric analysis performed on a Becton Dickinson FACScan flow cytometer equipped with an argon-ion laser with 15 mW of excitation at 488 nm. Emission wavelengths were detected through the FL1 channel equipped with a 530-nm filter (20-nm band-pass). Data were acquired on 10,000 cells per sample with light scatter signals at linear gain and fluorescence signals at logarithmic gain.
Detection of phosphatidylserine externalization.
Phosphatidylserine externalization was monitored using the ApoAlert annexin V apoptosis kit (Clontech) according to the protocol provided by the manufacturer. Flow cytometric analysis was performed on a Becton Dickinson FACScan flow cytometer equipped with an argon-ion laser with 15 mW of excitation at 488 nm. Emission wavelengths were detected through the FL1 channel equipped with a 530-nm filter (20-nm band-pass). Annexin V-fluorescein isothiocyanate binding was quantitated using flow cytometric analysis by examining 10,000 cells per sample.
Chromium and [3H]thymidine release assays.
51Cr and [3H]thymidine release assays were performed as previously described (18). Briefly, target cells were preincubated with 51Cr (Dupont NEN) or with [3H]thymidine (Dupont NEN) at 37°C for 1 or 24 h, respectively. Labeled target cells were incubated with hCTL at the indicated effector-to-target ratios. 51Cr release was quantitated after 4 h, and [3H]thymidine release was quantitated after 2 h. 51Cr and [3H]thymidine releases were calculated as follows: percent lysis = 100 × (sample release − spontaneous release)/(total release − spontaneous release).
Detection of mitochondrial transmembrane potential and production of reactive oxygen species.
Changes in mitochondrial transmembrane potential and production of reactive oxygen species were quantitated as previously described (24). Briefly, cells were simultaneously loaded with 40 nM 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] (Molecular Probes) and 2 μM hydroethidine (Molecular Probes) prior to flow cytometric analysis in order to detect changes in mitochondrial transmembrane potential and the production of reactive oxygen species, respectively. As a control, cells were also treated with the membrane uncoupler carbonyl cyanide m-chlorophenylhydrazone (mClCCP) (Sigma) at a final concentration of 5 μM (27).
In vitro cleavage of Bid.
Bid was in vitro transcribed and translated in the presence of [35S]methionine using the coupled transcription-translation TNT kit (Promega). Purified granzyme B was added to in vitro-transcribed and translated Bid at a range of amounts (0, 0.001, 0.0025, 0.01, 0.025, 0.1, 0.25, and 1.0 μg). Following digestion, reaction products were analyzed by SDS-PAGE and visualized by phosphorimager analysis using a Molecular Dynamics Storm 860.
RESULTS
SPI-2 expression protects cells from anti-Fas-mediated apoptosis.
To determine whether caspase 8 activity was a critical component of granule-mediated cell death, we stably transfected Jurkat cells with the rabbitpox virus serpin SPI-2. Expression of SPI-2 in various G418-resistant Jurkat clones was monitored by Western blotting analysis using a monoclonal antibody that was raised against recombinant SPI-2 protein. Figure 1A demonstrates that two Jurkat clones, clones 4 and 5, transfected with BMGneo-SPI-2 both expressed SPI-2 protein (lanes 1 and 2) but that Jurkat cells transfected with empty vector alone did not (lane 3).
FIG. 1.
SPI-2 expression inhibits Fas-mediated DNA fragmentation and caspase 3 activation. (A) Immunoblot analysis of SPI-2 expression in stably transfected Jurkat cells. Jurkat cells transfected with SPI-2 BMGneo [JSPI-2(4) and JSPI-2(5)] (lanes 1 and 2) express SPI-2, whereas cells transfected with the empty vector (Jneo) (lane 3) do not. (B) Jurkat cells were treated with 250 ng of anti-Fas antibody per ml for 8 h, and DNA fragmentation was monitored by TUNEL assay as described in Materials and Methods. Panels: a, untreated Jneo cells; b, Jneo cells treated with anti-Fas; c, Jneo cells treated with anti-Fas in the presence of 100 μM zIETD-fmk; d, untreated JSPI-2 (clone 4); e, JSPI-2 (clone 4) treated with anti-Fas; f, untreated JSPI-2 (clone 5); g, JSPI-2 (clone 5) treated with anti-Fas. Representative data from three experiments are shown. (C) Caspase 3 activation in untreated Jneo cells (lane 1), Jneo cells treated with anti-Fas antibody for 8 h (lane 2), untreated JSPI-2 (clone 4) cells (lane 3), JSPI-2 (clone 4) cells treated with anti-Fas (lane 4), untreated JSPI-2 (clone 5) cells (lane 5), and JSPI-2 (clone 5) cells treated with anti-Fas (lane 6) was monitored by Western blotting.
Previous studies have shown that expression of cowpox virus CrmA and rabbitpox virus and vaccinia virus SPI-2 protects cells from Fas-mediated apoptosis (12, 25, 33, 41, 76). The functionality of SPI-2 in our cloned cell lines was therefore assessed by determining whether the presence of SPI-2 could inhibit DNA fragmentation initiated via triggering the Fas surface receptor. Cells were treated with anti-Fas antibody for 8 h, and DNA fragmentation was assessed using flow cytometry by quantitating the terminal deoxynucleotidyl transferase-mediated addition of fluorescein-labeled dUTP onto the ends of fragmented DNA. Untreated Jurkat cells transfected with the empty vector demonstrated 2% of the cells undergoing DNA fragmentation (Fig. 1B, panel a). Following 8 h of anti-Fas treatment, 59% of the cells transfected with the empty vector underwent DNA fragmentation (Fig. 1B, panel b). This DNA fragmentation could be completely abolished by preincubating the cells for 1 h with the peptide-based caspase inhibitor zIETD-fmk (Fig. 1B, panel c). Jurkat cells transfected with SPI-2 in the absence of anti-Fas antibody demonstrated little DNA fragmentation (Fig. 1B, panels d and f). In contrast to cells transfected with the empty vector and treated with anti-Fas antibody, both SPI-2-expressing Jurkat clones showed significantly less DNA fragmentation (15 and 17%, respectively) (Fig. 1B, panels e and g), indicating that the SPI-2 expressed in these cells was functional and provided protection against Fas-mediated apoptosis. Similar results were obtained using a 3H release assay (data not shown). In agreement with previous studies, the expression of SPI-2 in our Jurkat clones did not protect cells from staurosporine-induced DNA fragmentation (data not shown), further indicating that our cloned cell lines exhibited the normal spectrum of SPI-2 activities (7).
To confirm that SPI-2 was in fact operating upstream of caspase 3 activation, we also monitored Fas-mediated caspase 3 processing, since caspase 3 is thought to be the major target for activated caspase 8 (63, 68). Caspase 3 activation was assessed by Western blotting analysis using an antibody raised against the large subunit of the active caspase. Jurkat cells treated with anti-Fas antibody showed the conversion of full-length 32 kDa procaspase 3 to the mature 19- and 17-kDa forms (Fig. 1C, lane 2). In contrast, both Jurkat clones that express SPI-2 demonstrated only minor amounts of active caspase 3 in response to Fas ligation (Fig. 1C, lanes 4 and 6).
Caspase 8 is processed during CTL-mediated cytotoxicity.
The observation that purified granzyme B can proteolytically cleave and activate members of the caspase family in vitro suggests that caspases may be directly activated by granzyme B in intact cells. Since we and others have previously shown that granzyme B directly cleaves and activates caspase 3, it is possible that the activation of initiator caspases, such as caspase 8, is not a necessary step during granule-mediated cell death (1, 81). Caspase 8, however, has previously been shown to be a substrate for granzyme B in vitro and is processed during granule-mediated cell death in HeLa cells (48, 50).
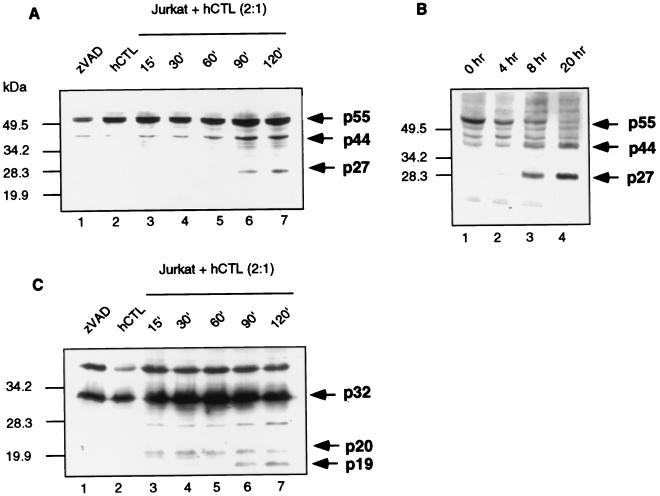
To determine whether caspase 8 was in fact processed in Jurkat cells during granule-mediated cell death, we monitored its proteolytic activation by Western blotting analysis. Jurkat cells were treated with hCTL, which have previously been demonstrated to kill these cells via the calcium-dependent granule-mediated pathway, at an effector-to-target ratio of 2:1 (1). Caspase 8 processing was visualized using an antibody raised against the large 20-kDa subunit of the active caspase. Jurkat cells treated with zVAD-fmk in the absence of hCTL demonstrated full-length 55-kDa caspase 8 (Fig. 2A, lane 1). Unprocessed caspase 8 was also detected in hCTL (Fig. 2A, lane 2). Processing of caspase 8 to a 44-kDa fragment was first detected after 60 min, and increased amounts were seen at 90 and 120 min (Fig. 2A, lanes 5, 6, and 7). Generation of the 44-kDa product occurs as a result of removal of the small 10-kDa subunit from the full-length caspase (47, 48). We also routinely observed the generation of a 27-kDa fragment, which was first observed 90 min after the addition of hCTL (Fig. 2A, lane 6). Since the caspase 8 antibody was raised against the 20-kDa subunit of the active caspase, processing to the 27-kDa fragment probably represents removal of the prodomain from a fragment containing both the 20- and 10-kDa subunits of the active caspase. Curiously, we were unable to observe further processing to the 20-kDa subunit, which the antibody was raised against, possibly due to the rapid turnover of this subunit, as previously suggested (58). Additionally, when Jurkat cells were treated with anti-Fas antibody for 4, 8, or 20 h, we also observed processing of caspase 8 to a 44-kDa fragment and a 27-kDa fragment (Fig. 2B, lanes 2 to 4) but could not observe further processing to the 20-kDa subunit. Taken together, these observations indicate that during granule-mediated cell death, caspase 8 undergoes proteolytic processing.
FIG. 2.
Caspase 8 is processed in target cells following the addition of whole CTL or anti-Fas antibody. (A) Jurkat cells were incubated with whole hCTL at an effector-to-target ratio of 2:1. Cell lysates were generated at the times indicated and immunoblotted for caspase 8 activation. (B) Jurkat cells were treated with 250 ng of anti-Fas antibody per ml for 0, 4, 8, and 20 h, and caspase 8 activation was assessed by Western blotting analysis. (C) Jurkat cells were incubated with whole hCTL at an effector-to-target ratio of 2:1. Cell lysates were generated at the times indicated and immunoblotted for caspase 3 activation.
In order to determine the timing of caspase 8 activation with respect to caspase 3 activation, we monitored caspase 3 cleavage by Western blotting. Untreated Jurkat cells and hCTL demonstrated full-length caspase 3 (Fig. 2C, lanes 1 and 2) which was rapidly converted to the p20 fragment following the addition of hCTL. We were able to detect activation of caspase 3 as early as 15 min after hCTL addition (Fig. 2C, lane 3). At 30 min the initial conversion of p20 to p19 was evident (Fig. 2C, lane 4), and this increased with time (Fig. 2C, lanes 4 to 7).
Caspase 8 activity is not a necessary component of CTL-mediated cytotoxicity.
Since multiple caspases are activated during CTL-mediated killing and since both caspase 8 and caspase 3 have been shown to be activated directly by granzyme B, we investigated the contribution of caspase 8 activity during granule-mediated cell death using cells expressing the poxvirus caspase 8 inhibitor SPI-2. Cells undergoing apoptosis display a number of characteristic biochemical features, such as DNA fragmentation, mitochondrial disruption, and membrane alterations which include the externalization of phosphatidylserine residues on the outer leaflet of the plasma membrane and 51Cr release. These morphological changes can be monitored biochemically using a variety of assays.
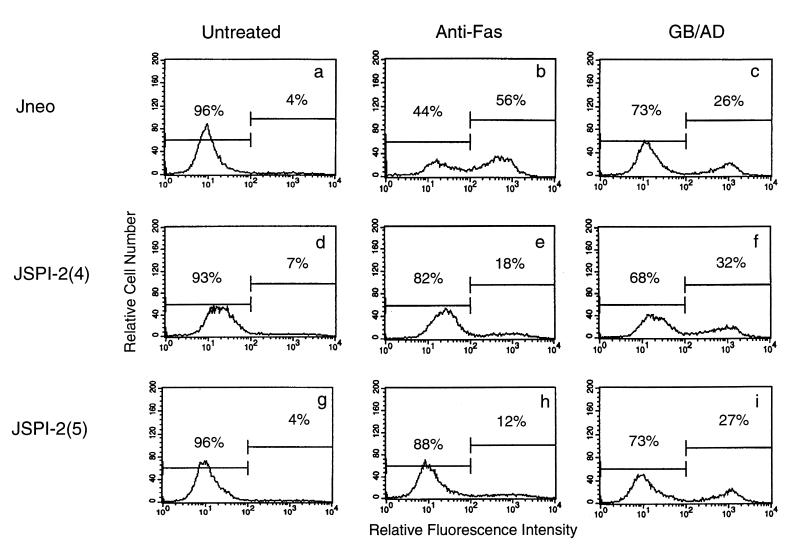
Externalization of phosphatidylserine onto the surface of the plasma membrane is an early indicator of apoptosis and is important for the recognition and clearance of apoptotic cells by phagocytes (35, 44, 61). We used flow cytometric analysis to monitor the externalization of phosphatidylserine by quantitating the amount of fluorescein-labeled annexin V binding. Cell death was induced by treatment either with anti-Fas or with granzyme B and adenovirus, which has previously been shown to replace the need for perforin (16). Jurkat cells transfected with the empty vector or transfected with SPI-2 demonstrated little externalization of phosphatidylserine in the absence of proapoptotic stimuli (Fig. 3a, d, and g). After exposure to anti-Fas treatment for 8 h or exposure to granzyme B and adenovirus for 4 h, 56 and 26%, respectively, of the control cells displayed phosphatidylserine on the outer leaflet of the plasma membrane (Fig. 3b and c). The expression of SPI-2 resulted in an inhibition of phosphatidylserine externalization in response to anti-Fas (Fig. 3e and h) but not after treatment with granzyme B and adenovirus (Fig. 3f and i). This indicates that the presence of SPI-2 does not protect cells from granzyme B-mediated phosphatidylserine exposure and also presumably the subsequent engulfment of apoptotic cells by phagocytes. Pretreatment of Jurkat cells with the pan-specific caspase inhibitor zVAD-fmk completely blocked phosphatidylserine externalization in response to either anti-Fas or granzyme B and adenovirus treatment, demonstrating that caspase activation is a necessary component of granzyme B- and adenovirus-induced phosphatidylserine exposure (reference 24 and data not shown). SPI-2 expression in target cells, however, had no effect on granzyme-mediated phosphatidylserine externalization, indicating that caspases not inhibited by SPI-2 are important for this phenomenon during granzyme-mediated death.
FIG. 3.
SPI-2 expression inhibits phosphatidylserine exposure in response to anti-Fas but not in response to granzyme B. Phosphatidylserine exposure was quantitated by annexin V-fluorescein isothiocyanate binding following treatment with 250 ng of anti-Fas per ml or treatment with granzyme B (1 μg/ml) and adenovirus (10 PFU/cell). (a) Untreated Jneo cells; (b) Jneo cells treated with anti-Fas; (c) Jneo cells treated with granzyme B and adenovirus; (d) untreated JSPI-2 (clone 4); (e) JSPI-2 (clone 4) cells treated with anti-Fas; (f) JSPI-2 (clone 4) cells treated with granzyme B and adenovirus; (g) untreated JSPI-2 (clone 5); (h) JSPI-2 (clone 5) cells treated with anti-Fas; (i) JSPI-2 (clone 5) cells treated with granzyme B and adenovirus. Representative data from three experiments are shown.
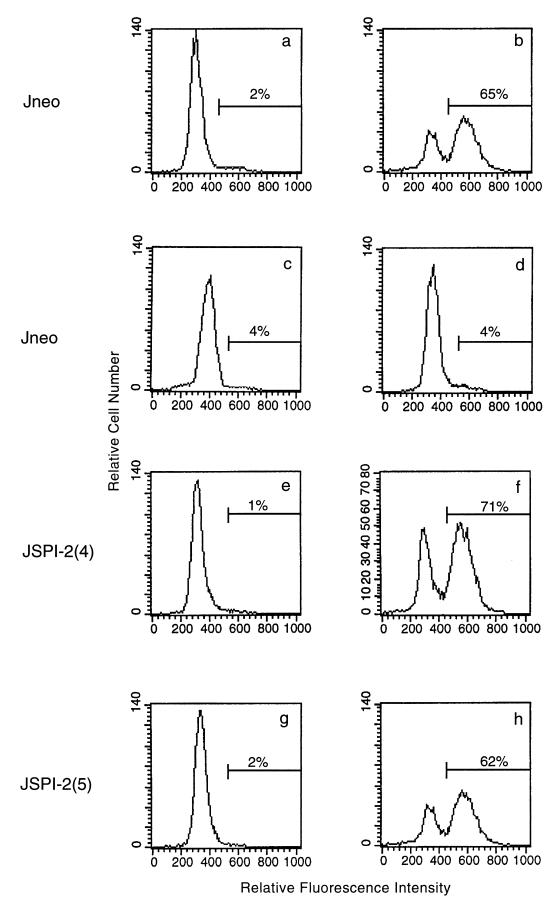
Since the presence of granzyme B plays an essential role in the induction of target cell DNA fragmentation during CTL-mediated death (26), we also examined the ability of SPI-2 expression to inhibit granzyme B-induced DNA fragmentation. Jurkat cells were treated with purified granzyme B and adenovirus, and the percentage of cells undergoing DNA fragmentation was quantitated by flow cytometry. Cells transfected with the empty vector showed little DNA fragmentation in the presence of either granzyme B alone or adenovirus alone (Fig. 4a, c, and d). When purified granzyme B and adenovirus were added together to these same cells, 65% of the cells displayed DNA fragmentation (Fig. 4b). Likewise, Jurkat clones 4 and 5 expressing SPI-2 showed no DNA fragmentation in the absence of proapoptotic stimuli (Fig. 4e and g). When granzyme B and adenovirus were added, 71 and 62% of the cells, respectively, were observed to undergo DNA fragmentation (Fig. 4f and h). Thus, we were unable to detect any significant difference in the amount of granzyme B-mediated DNA fragmentation in Jurkat cells transfected with the empty vector or expressing SPI-2, although SPI-2 expression inhibited Fas-mediated cell death, as shown in Fig. 1B.
FIG. 4.
SPI-2 expression in target cells does not inhibit granzyme B-induced DNA fragmentation. Jurkat cells transfected with the empty vector or expressing SPI-2 were treated with purified granzyme B (1 μg/ml) and adenovirus (10 PFU/cell), and DNA fragmentation was assessed by the TUNEL protocol. (a) Untreated Jneo cells; (b) Jneo cells treated with granzyme B and adenovirus; (c) Jneo cells treated with granzyme B alone; (d) Jneo cells treated with adenovirus alone; (e) untreated JSPI-2 (clone 4) cells; (f) JSPI-2 (clone 4) cells treated with granzyme B and adenovirus; (g) untreated JSPI-2 (clone 5) cells; (h) JSPI-2 (clone 5) cells treated with granzyme B and adenovirus. Representative data from three experiments are shown.
We also assessed the effect of SPI-2 expression on the ability of whole CTL to induce DNA fragmentation and membrane damage as measured by [3H]thymidine and 51Cr release. As shown in Fig. 5A, the expression of SPI-2 provided no protection against whole CTL-mediated membrane damage over a range of effector-to-target ratios. The addition of 10 mM EGTA inhibited chromium release, demonstrating that in these experiments Jurkat cells were killed by the calcium-dependent granule-exocytosis pathway and not by the calcium-independent Fas pathway (Fig. 5A). In addition, no significant difference was observed between the empty-vector-transfected cells and SPI-2 expressing Jurkat cells when [3H]thymidine release was measured over the same range of effector-to-target ratios (Fig. 5B). This further suggested that caspases inhibited by SPI-2, including caspase 8, are not a necessary component of granzyme-mediated DNA fragmentation.
FIG. 5.
SPI-2 expression does not provide protection against whole CTL-mediated DNA fragmentation or membrane damage. Labeled target cells were incubated with whole CTL at a range of effector-to-target ratios. (A) 51Cr release was measured after 4 h. (B) [3H]thymidine release was measured after 2 h. The means and standard deviations from triplicate samples are shown.
Much experimental evidence now indicates that mitochondrial disruption is an important event during apoptosis (reviewed in reference 36). These mitochondrial changes include the production of reactive oxygen species (ROS), loss of the inner membrane transmembrane potential (ΔΨm), and the release of apoptosis-inducing proteins, such as the recently identified apoptosis-inducing factor (71, 73), cytochrome c (38, 39), and caspases (43, 70). During Fas-mediated cell death, activation of caspase 8 is essential for the initiation of these mitochondrial changes and subsequent activation of caspase 3 (72, 79). Although loss of ΔΨm, production of ROS, and release of cytochrome c have also recently been shown to occur during granzyme-mediated cell death, the precise mechanisms by which this occurs are not yet well understood (24, 45). We therefore assessed the ability of SPI-2 to affect mitochondrial disruption during both Fas- and granzyme-mediated apoptosis. Loss of ΔΨm and production of ROS can be monitored simultaneously by flow cytometry. The lipophilic dye DiOC6(3) targets to the negatively charged environment of the mitochondria, and when the ΔΨm dissipates during apoptosis, DiOC6(3) leaks out of the mitochondria, leading to decreased fluorescence. The induction of ROS is measured by treating cells with hydroethidine, which is oxidized to ethidium in the presence of ROS. Using this assay, Jurkat cells transfected with the empty vector or transfected with SPI-2 in the absence of proapoptotic stimuli demonstrated normal mitochondria that retained the DiOC6(3) dye with no production of ROS (Fig. 6a, e, and i). As a control, cells were also treated with a membrane uncoupler, the protonophore mClCCP (27). Treatment of cells with mClCCP resulted in a loss of DiOC6(3) and an increase in ethidium fluorescence as demonstrated by the dramatic redistribution of cells from the lower right quadrant to the upper left quadrant (Fig. 6b, f, and j). Cells transfected with the empty vector and treated with anti-Fas antibody for 8 h also showed a loss of cells from the lower right quadrant compared to untreated cells (Fig. 6a and c). The loss of DiOC6(3) and production of ROS following anti-Fas treatment were significantly inhibited by expression of SPI-2 (Fig. 6g and k). As previously documented, treatment of cells with granzyme B and adenovirus for 2 h also resulted in a redistribution of cells from the lower right quadrant, representing a loss of ΔΨm and production of ROS (Fig. 6d) (24). In contrast to Fas-induced mitochondrial disruption, however, loss of ΔΨm and production of ROS mediated by granzyme B and adenovirus treatment were not inhibited by the expression of SPI-2.
FIG. 6.
SPI-2 expression inhibits ΔΨm and ROS production during Fas-mediated cell death but not during granzyme-mediated cell death. Jurkat cells were treated either with anti-Fas or with granzyme B and adenovirus (GB/AD). Mitochondrial transmembrane potential was determined using 40 nM DiOC6(3), and the production of ROS was assessed with 2 μM hydroethidine (HE). (a) Untreated Jneo cells; (b) Jneo cells treated with the membrane uncoupler mClCCP; (c) Jneo cells exposed to anti-Fas for 8 h; (d) Jneo cells treated with purified granzyme B and adenovirus for 2 h; (e) untreated JSPI-2 (clone 4) cells; (f) JSPI-2 (clone 4) cells treated with the membrane uncoupler mClCCP; (g) JSPI-2 (clone 4) cells exposed to anti-Fas for 8 h; (h) JSPI-2 (clone 4) cells treated with purified granzyme B and adenovirus for 2 h; (i) untreated JSPI-2 (clone 5) cells; (j) JSPI-2 (clone 5) cells treated with the membrane uncoupler mClCCP; (k) JSPI-2 (clone 5) cells exposed to anti-Fas for 8 h; (l) JSPI-2 (clone 5) cells treated with purified granzyme B and adenovirus for 2 h. Representative data from three experiments are shown.
Caspase 3 activation occurs in SPI-2-expressing cells and is an essential component in caspase-dependent, granzyme-mediated DNA fragmentation.
Although caspase 8 is directly processed by granzyme B in intact cells (48), we and others have previously shown that caspase 3 is also directly activated by granzyme B (1, 48, 81), implicating caspase 3 as an important caspase during granzyme-induced apoptosis. To confirm that caspase 3 was normally processed in the SPI-2-expressing cells during granule-induced cell death, we treated Jurkat cells with hCTL at an effector-to-target ratio of 2:1 and monitored caspase 3 activation by Western blotting analysis. SPI-2-expressing Jurkat cells and hCTL alone showed no caspase 3 processing, and only full-length 32-kDa caspase could be detected (Fig. 7, lanes 1 and 2). Following the addition of hCTL to the SPI-2-expressing cells, caspase 3 processing to a 20-kDa form and a 19 kDa form could clearly be detected over a period of 120 min (Fig. 7, lanes 3 to 7), which was similar in control Jurkat cells (Fig. 7, lane 9).
FIG. 7.
Caspase 3 is normally activated in SPI-2 expressing cells. Cells were treated with whole CTL at an effector-to-target ratio of 2:1, and caspase 3 processing in both SPI-2-expressing cells (lanes 1 and 3 to 7) and Jneo cells (lanes 8 and 9) was monitored by Western blotting.
To further examine the requirement for caspase 3 activation during granzyme-induced cell death, we took advantage of the breast carcinoma cell line MCF-7. MCF-7 cells express no caspase 3 due to a deletion within exon three that is critical for correct processing of the mRNA (30). When whole CTL at a range of effector-to-target ratios were added to MCF-7 cells, no DNA fragmentation could be detected (Fig. 8A). In contrast, when Jurkat cells that express caspase 3 were used as targets, increasing amounts of [3H]thymidine release were detected, indicative of cells undergoing DNA fragmentation. In support of these observations, we were also unable to detect any DNA fragmentation in MCF-7 cells following treatment with granzyme B and adenovirus (data not shown). These results indicated that caspase 3 was crucial for granule-induced DNA fragmentation when whole CTL are used as effectors. Caspase 3, however, was found to be completely dispensable for CTL-induced membrane damage, since caspase 3-deficient MCF-7 cells treated with whole CTL demonstrated 51Cr release (Fig. 8B). We and others have found that peptide-based caspase inhibitors are insufficient to inhibit CTL-mediated membrane damage as measured by 51Cr release (references 24, 45, and 60 and unpublished data). These results reiterate that CTL can destroy cells via a caspase 3-independent pathway (24, 45, 60).
FIG. 8.
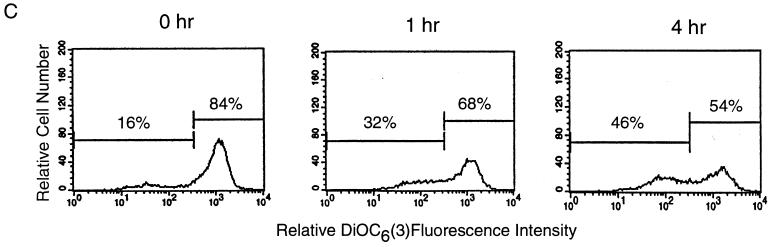
Caspase 3 activation is a necessary component for granule-mediated DNA fragmentation. MCF-7 cells lacking caspase 3 are unable to undergo DNA fragmentation after treatment with whole CTL but still undergo CTL-mediated membrane damage. (A) MCF-7 cells and Jurkat cells were labeled with [3H]thymidine. Labeled cells were incubated with a range of effectors-to-target ratios in the presence of 2 μg of concanavalin A per ml, and [3H]thymidine release was measured after 2 h. Data from triplicate samples are shown. (B) MCF-7 cells were labeled with 51Cr and incubated with whole CTL at a range of effector-to-target ratios in the presence and absence of 5 mM EGTA. 51Cr release was measured after 4 h. The means and standard deviations from triplicate samples are shown. (C) MCF-7 cells were treated with hCTL at an effector-to-target ratio of 2.5:1. Mitochondrial transmembrane potential was determined using 40 nM DiOC6(3). Representative data from three independent experiments are shown.
To further understand the contribution of caspase 3 during CTL-mediated killing, we measured the loss of mitochondrial membrane potential, using DiOC6(3), and phosphatidylserine exposure by quantitating the amount of annexin V binding. MCF-7 cells were incubated with hCTL at an effector-to-target ratio of 2.5:1, and DiOC6(3) loss from the mitochondria was monitored by flow cytometry. MCF-7 cells loaded with DiOC6(3) for the duration of the experiment demonstrated no loss of fluorescence (data not shown). Following the addition of hCTL at 0, 1, and 4 h, MCF-7 cells showed a loss of DiOC6(3), which is indicative of cells undergoing loss of mitochondrial potential (Fig. 8C). This result indicates that caspase 3 is not essential for mitochondrial collapse (Fig. 8C, 1 and 4 h) and further indicates that granzyme B-mediated mitochondrial collapse is a caspase-independent event. In contrast, we were unable to detect any phosphatidylserine exposure in MCF-7 cells treated with hCTL in the same experiments (data not shown), indicating that the presence of caspase 3 is an important prerequisite for this phenomenon in MCF-7 cells.
Granzyme B is responsible for the cleavage and activation of Bid.
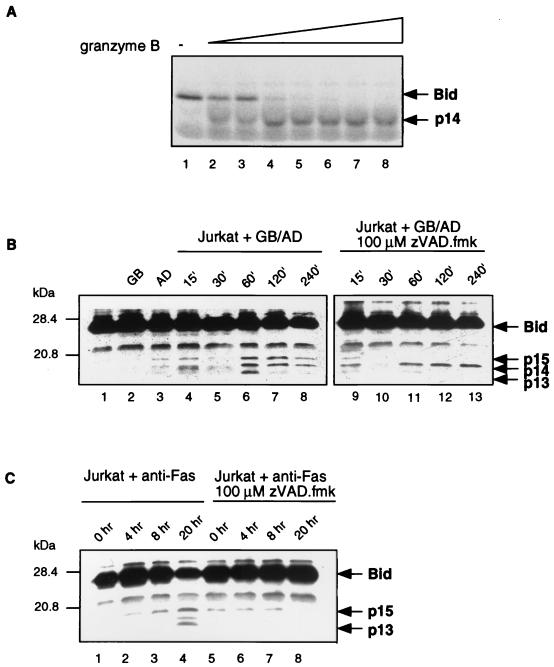
Recently the proapoptotic molecule Bid has been identified as a major player in Fas-mediated apoptosis. Since the direct cleavage of Bid by granzyme B may be responsible for the caspase-independent mitochondrial collapse seen during granule-mediated killing, we examined the ability of granzyme B to activate Bid (21, 28, 40, 82). The involvement of Bid in the granzyme B pathway was examined both in vitro and in vivo. First, to verify that Bid was a substrate for granzyme B, Bid was translated in vitro in the presence of [35S]methionine. The translated product was treated with increasing amounts of purified granzyme B, and proteolytic cleavage was analyzed by SDS-PAGE and autoradiography. Figure 9A demonstrates that translated Bid was clearly cleaved by granzyme B in vitro. Granzyme B cleaves Bid following aspartate 75, resulting in N-terminal and C-terminal fragments of similar size that are indistinguishable by SDS-PAGE (28).
FIG. 9.
Granzyme B is responsible for cleaving Bid. (A) Granzyme B cleaves Bid in vitro. Bid was translated in the presence of [35S]methionine, and purified granzyme B was added in increasing amounts (0, 0.001, 0.0025, 0.01, 0.025, 0.1, 0.25, and 1.0 μg). (B) Bid is cleaved in intact cells by granzyme B. Jurkat cells were untreated (lane 1), treated with granzyme B (GB) (lane 2), treated with adenovirus (AD) (lane 3), or treated with adenovirus and granzyme B simultaneously for 15, 30, 60, 120, and 240 min (lanes 4 to 8, respectively). Jurkat cells were pretreated with 100 μM zVAD-fmk prior to the simultaneous treatment with granzyme B and adenovirus for 15, 30, 60, 120, and 240 min (lanes 9 to 13, respectively). (C) Fas activation results in Bid cleavage. Jurkat cells were untreated (lane 1), treated with anti-Fas antibody for 4, 8, and 20 h (lanes 2 to 4, respectively), or pretreated with 100 μM zVAD-fmk prior to the addition of anti-Fas antibody (lanes 5 to 8).
Having determined that purified granzyme B could in fact proteolytically cleave Bid, we asked if Bid was cleaved in intact cells during granzyme B-mediated killing. To perform these experiments, we assessed the cleavage of Bid using Western blotting analysis. Jurkat cells were treated with granzyme B and adenovirus in the absence and presence of zVAD-fmk over a period of 4 h. Using this approach, full-length Bid was found to be unprocessed in untreated cells or cells treated with granzyme B alone or adenovirus alone (Fig. 9B, lanes 1 to 3). Following the simultaneous addition of granzyme B and adenovirus, processing of Bid into three fragments (p15, p14, and p13) could clearly be detected after 60 min (Fig. 9B, lanes 6 to 8). The activation of Bid by caspase 8 results in the cleavage of Bid at residue 59, causing the appearance of p15 and p13 fragments (28), whereas granzyme B is predicted to cleave Bid at residue 75, resulting in two fragments of equal size corresponding to p14 (28). In the presence of zVAD-fmk, only one cleavage product of approximately 14 kDa (p14) could be detected, indicating that granzyme B was responsible for cleaving Bid in the absence of caspase activity (Fig. 9B, lanes 11 to 13). In contrast, Fas-mediated cleavage of Bid, which results in the generation of a p15 fragment and a p13 fragment, was completely inhibited in the presence of the pan-specific caspase inhibitor zVAD-fmk (Fig. 9C). These data demonstrate for the first time that granzyme B is responsible for the direct activation of Bid during cell death.
DISCUSSION
It is now generally accepted that an important component of CTL killing is via induction of the internal cell suicide pathway resulting in apoptosis. Activation of members of the caspase family is a critical component of apoptosis, and many members of the caspase family can be proteolytically processed by purified granzyme B in vitro (reviewed in reference 10). These observations have led to the suggestion that granzyme B initiates apoptosis by directly activating caspases in intact cells. In order to gain insight into the contributions of specific caspases during granule-mediated apoptosis in intact cells, we have used target cells stably transfected with a poxvirus macromolecular caspase inhibitor, SPI-2, and MCF-7 cells, which are naturally devoid of caspase 3.
Viruses express proteins that specifically interfere with caspase activity (49), thus presenting an opportunity to utilize these virus-encoded caspase inhibitors for the dissection of apoptotic cascades. The cowpox virus-encoded caspase inhibitor CrmA, also referred to as SPI-2 in other poxviruses, has been widely utilized for this purpose. The crmA gene product is related to members of the serine protease inhibitor family and inhibits apoptosis induced by various apoptotic stimuli by directly inhibiting caspases (56; reviewed in references 11 and 46). CrmA is a potent inhibitor of both caspases 1 and 8 (34, 83) and also displays activity against other group 1 caspases as well as caspases 9 and 10 (17). Rabbitpox virus SPI-2 is a CrmA-related serine proteinase inhibitor that displays 93% amino acid identity with cowpox virus-encoded CrmA and inhibits caspase 1 with an efficiency equal to that of CrmA (42). As expected, previous studies have shown that like CrmA, rabbitpox virus SPI-2 is an excellent inhibitor of Fas-mediated apoptosis during virus infection (41). Our studies have extended this observation by further demonstrating that the heterologous expression of SPI-2 in Jurkat cells results in significant protection against anti-Fas-mediated apoptosis. Caspase 8 is the first caspase activated during Fas-mediated cell death and is processed by granzyme B in intact cells (3, 47, 48, 50). Using an antibody that was raised against the large subunit of caspase 8, we provide further evidence that caspase 8 is processed during granule-mediated cell death (Fig. 2A). Although caspase 8 is directly activated by granzyme B (48), our data indicate that the activation of caspase 8 is not a critical component of CTL killing. This conclusion is based upon the fact that expression of the serine protease inhibitor SPI-2 has no detrimental effect on granule-mediated cell death. Our data are supported by previous experimental results with other cell lines showing that the presence of SPI-2 or CrmA in rabbitpox- and cowpox virus-infected cells or the heterologous expression of CrmA does not significantly inhibit granule-induced 51Cr release (41, 76). We have expanded upon these observations by also examining DNA fragmentation, phosphatidylserine externalization, and mitochondrial disruption in addition to 51Cr release mediated by whole CTL as well as by purified granzyme B and adenovirus. SPI-2 expression in cells does not prevent cell death measured by any of these criteria. In an additional study, expression of FLIP in target cells, which interacts with both caspase 8 and FADD, also did not interfere with cell death induced by granzyme B and perforin, granzyme B and adenovirus, or whole CTL (32). These results further indicate that caspase 8 activity is not an essential component of granule-mediated cytotoxicity and in combination with results presented here suggest that CTL can bypass the need for caspase 8 activity by activating other caspases within the cell. For example, although caspase 8 is normally activated, under conditions where caspase 8 is inhibited, like during virus infection, alternate caspase pathways may compensate.
In addition to inhibiting group 1 caspases and caspase 8, CrmA is also a good inhibitor of caspases 9 and 10 (17). Since caspases 8, 9, and 10 function primarily during the initiation stage of apoptosis, extrapolation of our data indicates that granzyme-mediated cell death can in fact bypass the need for activation of initiator caspases. Studies have shown that although CrmA is an effective inhibitor of initiator caspases, it displays little activity against caspases 2, 3, 6, and 7, suggesting that activation of these caspases may be critical during granule-mediated apoptosis (17). In fact studies from our laboratory have demonstrated that granzyme B can directly activate caspase 3 in intact cells (1), further suggesting that granzyme B can bypass the need for activation of initiator caspases in whole cells. Experiments reported here demonstrate no interference with caspase 3 activation in cells expressing SPI-2 after treatment with whole CTL (Fig. 7), and we routinely detect activation of caspase 3, by Western blotting, prior to caspase 8 activation (Fig. 2C). When MCF-7 cells, which are devoid of caspase 3, were treated with whole CTL, no DNA fragmentation could be detected, indicating that caspase 3 was essential for granule-mediated DNA fragmentation (Fig. 9A). Since MCF-7 cells express caspases 2, 5, 7, 8, 9, and 10 (29), the data indicate that none of these caspases can replace the need for granzyme-induced caspase 3-mediated DNA fragmentation in these cells. Recently, caspase 3 activation has been linked to the generation of a caspase-activated deoxyribonuclease activity necessary for DNA fragmentation (59), which is dependent upon caspase 3 expression in MCF-7 cells (75). In addition, in vitro experiments have demonstrated that caspase 3 activity is required for the activation of caspases 2, 6, 8, and 10 (65). The proteolytic activation of caspase 3 in intact cells may therefore represent a critical component of granzyme B-mediated apoptotic cell death.
Although caspase 3-deficient MCF-7 cells were resilient to CTL-mediated DNA fragmentation, our data demonstrate that these cells are not resistant to CTL-mediated killing. When MCF-7 cells were incubated with whole CTL, membrane damage was clearly apparent as monitored by 51Cr release (Fig. 8B). Additionally, MCF-7 cells treated with whole CTL also demonstrated loss of mitochondrial inner membrane potential (Fig. 8C). Experiments reported here demonstrate that mitochondrial collapse can occur in the absence of caspase 3 (Fig. 8C) or in the absence of caspase 8 activity due to the expression of SPI-2 (Fig. 6). Additionally, we have recently demonstrated that mitochondrial collapse and cytochrome c release occur independent of caspase activation during granule-mediated cell death (24). The recent finding that activation of the proapoptotic molecule Bid results in mitochondrial damage and cytochrome c release during Fas-mediated cell death led us to examine the involvement of Bid in the granzyme B pathway (21, 28, 40, 82). We demonstrate that Bid is indeed a substrate for granzyme B in vitro (Fig. 9A) and, more significantly, that Bid is cleaved in intact cells (Fig. 9B). Under conditions where caspase activation is inhibited, Bid is proteolytically cleaved into two fragments of approximately 14 kDa, indicating that during CTL-mediated granule killing, granzyme B directly activates Bid (Fig. 9B). In contrast, in Fas-mediated apoptosis, Bid is cleaved into 15- and 13-kDa fragments, and this cleavage is inhibited in the presence of zVAD-fmk (Fig. 9C). During Fas-mediated death, the cleavage of Bid by caspase 8 results in translocation of Bid to the mitochondria, which is essential for mitochondrial disruption and cytochrome c release (21, 28, 40). We predict that in a similar fashion, granzyme B-activated Bid also translocates to the mitochondria, resulting in the caspase-independent release of cytochrome c during CTL-mediated death. Thus, we demonstrate for the first time that Bid is directly activated by granzyme B in intact cells, leading to caspase-independent mitochondrial collapse. Our results support the view that CTL can destroy target cells via both caspase-dependent and caspase-independent mechanisms (24, 45, 60). In addition, the ability of granzyme B to activate a variety of members of the caspase family in addition to Bid adds another dimension to this inherent flexibility.
In conclusion, we have demonstrated biochemically the absolute requirement for caspase 8 activity during Fas-mediated apoptosis. This is in stark contrast to the results observed with the granzyme pathway. Under conditions where caspase 8 is inhibited by expression of SPI-2, all of the hallmarks of apoptosis occurred unabated. Most importantly, we demonstrate that granzyme B can directly cleave the proapoptotic molecule Bid, resulting in caspase-independent mitochondrial collapse and cell death. Clearly, CTL have evolved to counter virus-encoded and tumor cell immune evasion strategies. In this case, cells expressing a poxvirus protein that can block the initiator caspase 8 and tumor cells devoid of caspase 3 can still be effectively destroyed by CTL. These findings have important implications for the design of immunomodulatory molecules that seek to block these effectors of cell-mediated immunity.
ACKNOWLEDGMENTS
This work was supported by the National Cancer Institute of Canada, the Medical Research Council of Canada, and the Howard Hughes Foundation (grants to R.C.B.). M.B. is the recipient of an Alberta Heritage Foundation for Medical Research postdoctoral fellowship, and J.A.H. is the recipient of a Medical Research Council of Canada studentship. M.J.P. is the recipient of a Medical Research Council of Canada postdoctoral fellowship. R.C.B. is a Medical Scientist of the Alberta Heritage Foundation for Medical Research, a Howard Hughes International Research Scholar, and a Distinguished Scientist of the Medical Research Council of Canada.
We thank D. W. Nicholson for providing anti-caspase 3 and -caspase 8 antisera, X. Wang for providing anti-Bid antisera, J. Gauldie for providing the replication-deficient adenovirus, G. McFadden for helpful discussions, H. Everett for critically reading the manuscript, and T. Sawchuk and I. Shostak for technical assistance.
REFERENCES
- 1.Atkinson E A, Barry M, Darmon A J, Shostak I, Turner P C, Moyer R W, Bleackley R C. Cytotoxic T lymphocyte-assisted suicide. J Biol Chem. 1998;273:21261–21266. doi: 10.1074/jbc.273.33.21261. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson E A, Bleackley R C. Mechanisms of lysis by cytotoxic T cells. Crit Rev Immunol. 1995;15:359–384. doi: 10.1615/critrevimmunol.v15.i3-4.90. [DOI] [PubMed] [Google Scholar]
- 3.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 4.Caputo A, James M N G, Powers J C, Hudig D, Bleackley R C. Conversion of the substrate specificity of mouse proteinase granzyme B. Nat Struct Biol. 1994;1:364–367. doi: 10.1038/nsb0694-364. [DOI] [PubMed] [Google Scholar]
- 5.Caputo A, Parrish J C, James M N G, Powers J C, Bleackley R C. Electrostatic reversal of serine proteinase substrate specificity. Proteins. 1999;35:415–424. [PubMed] [Google Scholar]
- 6.Chinnaiyan A M, Orth K, Hanna W L, Duan H J, Poirier G G, Froelich C J, Dixit V M. Cytotoxic T cell-derived granzyme B activates the apoptotic protease ICE-LAP-3. Curr Biol. 1996;6:897–899. doi: 10.1016/s0960-9822(02)00614-0. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan A M, Orth K, O'Rourke K, Duan H, Poirier G G, Dixit V M. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-XL function upstream of the Ced-3 like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darmon A J, Nicholson D W, Bleackley R C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 10.Darmon A J, Pinkoski M J, Bleackley R C. Granule-mediated cytotoxicity. In: Kumar S, editor. Apoptosis: biology and mechanisms. Berlin, Germany: Springer-Verlag; 1999. pp. 103–125. [DOI] [PubMed] [Google Scholar]
- 11.Dbaibo G S, Hannun Y A. Cytokine response modifier A (crmA): a strategically deployed viral weapon. Clin Immunol Immunopathol. 1998;86:134–140. doi: 10.1006/clin.1997.4476. [DOI] [PubMed] [Google Scholar]
- 12.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan H, Orth K, Chinnaiyan A M, Poirier G G, Froelich C J, He W, Dixit V M. ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem. 1996;271:16720–16724. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes-Alnemri T, Armstrong R C, Krebs J, Srinivasula S M, Wang L, Bullrich F, Fritz L C, Trapani J A, Tomaselli K J, Litwack G, Alnemri E S. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes-Alnemri T, Takahashi A, Armstrong R, Krebs J, Fritz L, Tomaselli K J, Wang L, Yu Z, Croce C M, Earnshaw W C, Litwack G, Alnemri E S. Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res. 1995;55:6045–6052. [PubMed] [Google Scholar]
- 16.Froelich C J, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, Shah G M, Bleackley R C, Dixit V M, Hanna W. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem. 1996;271:29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Calvo M, Peterson E P, Leiting B, Ruel R, Nicholson D W, Thornberry N A. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 18.Garner R, Helgason C D, Atkinson E A, Pinkoski M J, Ostergaard H L, Sorensen O, Fu A, Lapchak P H, Rabinovitch A, McElhaney J E, Berke G, Bleackley R C. Characterization of a granule-independent lytic mechanism used by CTL hybridomas. J Immunol. 1994;153:5413–5421. [PubMed] [Google Scholar]
- 19.Gavriell Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths G M, Argon Y. Structure and biogenesis of lytic granules. In: Griffiths G M, Tschopp J, editors. Pathways for cytolysis. Berlin, Germany: Springer-Verlag; 1995. pp. 39–58. [DOI] [PubMed] [Google Scholar]
- 21.Gross A, Yin X M, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved Bid targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, Sarnecki C, Fleming M A, Lippke J A, Bleackley R C, Su M S S. Processing and activation of CMH-1 by granzyme B. J Biol Chem. 1996;271:10816–10820. doi: 10.1074/jbc.271.18.10816. [DOI] [PubMed] [Google Scholar]
- 23.Hanna W L, Zhang X, Turbov J, Winkler U, Hudig D, Froelich C J. Rapid purification of cationic granule proteases: application to human granzymes. Protein Exp Purif. 1993;4:398–404. doi: 10.1006/prep.1993.1052. [DOI] [PubMed] [Google Scholar]
- 24.Heibein J A, Barry M, Motyka B, Bleackley R C. Granzyme B-induced loss of mitochondrial inner membrane potential (ΔΨm) and cytochrome c release are caspase-independent. J Immunol. 1999;163:4683–4693. [PubMed] [Google Scholar]
- 25.Heinkelein M, Pilz S, Jassoy C. Inhibition of CD95 (Fas/APO-1)-mediated apoptosis by vaccinia virus WR. Clin Exp Immunol. 1996;102:8–14. doi: 10.1046/j.1365-2249.1996.927619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 27.Heytler P G, Pritchard W W. A new class of uncoupling agents—carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962;7:272. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- 28.Honglin L, Zhu H, Xu C, Juan Y. Cleavage of Bid by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 29.Janicke R U, Ng P, Sprengart M L, Porter A G. Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998;273:15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 30.Janicke R U, Sprengart M L, Wati M R, Porter A G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 31.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4, or 5, using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka T, Schroter M, Hahne M, Schneider P, Irmler M, Thome M, Froelich C J, Tschopp J. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol. 1998;161:3936–3942. [PubMed] [Google Scholar]
- 33.Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith G L. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1β-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1β-induced fever. J Gen Virol. 1997;78:677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 34.Komiyama T, Ray C A, Pickup D J, Howard A D, Thornberry N A, Peterson E P, Salvesen G. Inhibition of interleukin-1β converting enzyme by cowpox virus serpin crmA. J Biol Chem. 1994;269:19331–19337. [PubMed] [Google Scholar]
- 35.Koopman G, Reutelingsperger C P M, Kuijten G A M, Keehnen R M J, Pals S T, van Oers M H J. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 36.Kroemer G, Zamzami N, Susin S A. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 37.Kuwana T, Smith J J, Muzio M, Dixit V, Newmeyer D D, Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 38.Li F, Srinivasan A, Wang Y, Armstrong R C, Tomaselli K J, Fritz L C. Cell-specific induction of apoptosis by microinjection of cytochrome c. J Biol Chem. 1997;272:30299–30305. doi: 10.1074/jbc.272.48.30299. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 40.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 41.Macen J L, Garner R S, Musy P Y, Brooks M A, Turner P C, Moyer R W, McFadden G, Bleackley R C. Differential inhibition of the Fas-mediated and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc Natl Acad Sci USA. 1996;93:9108–9113. doi: 10.1073/pnas.93.17.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macen J, Takahashi A, Moon K B, Nathaniel R, Turner P C, Moyer R W. Activation of caspases in pig kidney cells infected with wild-type CrmA/SPI-2 mutants of cowpox and rabbitpox viruses. J Virol. 1998;72:3524–3533. doi: 10.1128/jvi.72.5.3524-3533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancini M, Nicholson D W, Roy S, Thornberry N A, Peterson E P, Casciola-Rosen L A, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin S J, Reutelingsperberg C P, McGahon A J, Rader J, van Schie R C A A, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald G, Shi L, Vande Velde C, Lieberman J, Greenberg A H. Mitochondria-dependent and -independent regulation of granzyme B-induced apoptosis. J Exp Med. 1999;189:131–143. doi: 10.1084/jem.189.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFadden G, Barry M. How poxviruses oppose apoptosis. Semin Virol. 1998;8:429–442. [Google Scholar]
- 47.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medema J P, Toes R E M, Scaffidi C, Zheng T S, Flavell R A, Melief C J M, Pete M E, Offringa R, Krammer P H. Cleavage of FLICE (caspase-8) by granzyme B during cytotoxic T lymphocyte-induced apoptosis. Eur J Immunol. 1997;27:3492–3498. doi: 10.1002/eji.1830271250. [DOI] [PubMed] [Google Scholar]
- 49.Meinl E, Fickenscher H, Thome M, Tschopp J, Fleckenstein B. Anti-apoptotic strategies of lymphotropic viruses. Immunol Today. 1998;19:474–479. doi: 10.1016/s0167-5699(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 50.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex (DISC) Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 51.Muzio M, Stockwell B R, Stennicke H R, Salvesen G S, Dixit V M. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 52.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 53.Odake L M, Kam C M, Narasimhan L, Poe M, Blake J T, Krahenbuhl O, Tschopp J, Powers J C. Human and murine cytotoxic T lymphocyte serine proteases: subsite mapping with peptide thioester substrates and inhibition of enzyme activity and cytolysis by isocoumarins. Biochemistry. 1991;30:2217–2227. doi: 10.1021/bi00222a027. [DOI] [PubMed] [Google Scholar]
- 54.Peter M E, Krammer P H. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 55.Poe M, Blake J T, Boulton D A, Gammon M, Sigal N H, Wu J K, Zweerink H J. Human cytotoxic lymphocyte granzyme B. Its purification from granules and the characterization of substrate and inhibitor specificity. J Biol Chem. 1991;266:98–103. [PubMed] [Google Scholar]
- 56.Quan L T, Caputo A, Bleackley R C, Pickup D J, Salvesen G S. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J Biol Chem. 1995;270:10377–10379. doi: 10.1074/jbc.270.18.10377. [DOI] [PubMed] [Google Scholar]
- 57.Quan L T, Tewari T M, O'Rourke K, Dixit V, Snipas S J, Poirier G G, Ray C, Pickup D J, Salvesen G S. Proteolytic activation of the cell death protease Yama/CPP32 by granzyme B. Proc Natl Acad Sci USA. 1996;93:1972–1976. doi: 10.1073/pnas.93.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasper D M, Vaillancourt J P, Hadano S, Houtzager V M, Seiden I, Keen S L C, Tawa P, Xanthoudakis S, Nasir J, Martindale D, Koop B F, Petersen E P, Thornberry N A, Huang J, MacPherson D P, Black S C, Hornung F, Lenardo M J, Hayden M R, Roy S, Nicholson D W. Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Diff. 1998;5:271–288. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- 59.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 60.Sarin A, Williams M S, Alexander-Miller M A, Berzofsky J A, Zacharchuk C M, Henkart P A. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity. 1997;6:209–215. doi: 10.1016/s1074-7613(00)80427-6. [DOI] [PubMed] [Google Scholar]
- 61.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 62.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schlegel J, Peters I, Orrenius S, Miller D K, Thornberry N A, Yamin T-T, Nicholson D W. CPP32/apopain is a key interleukin-1β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1995;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 64.Shi L F, Chen G, MacDonald G, Bergeron L, Li H L, Miura M, Rotello R J, Miller D K, Li P, Seshadri T, Yuan J Y, Greenberg A H. Activation of an interleukin 1 converting enzyme-dependent apoptosis pathway by granzyme B. Proc Natl Acad Sci USA. 1996;93:11002–11007. doi: 10.1073/pnas.93.20.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slee E A, Harte M T, Kluck R M, Wolf B B, Casiano C A, Newmeyer D D, Wang H-G, Reed J C, Nicholson D W, Alnemri E S, Green D R, Martin S J. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smyth M J, O'Connor M D, Trapani J A. Granzymes: a variety of serine protease specificities encoded by genetically distinct subfamilies. J Leukoc Biol. 1996;60:555–562. doi: 10.1002/jlb.60.5.555. [DOI] [PubMed] [Google Scholar]
- 67.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Molecular ordering of the Fas pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stennicke H R, Jurgensmeier J M, Shin H, Deveraux Q, Wolf B B, Yang X, Zhou Q, Ellerby H M, Ellerby L M, Bradesen D, Green D R, Reed J C, Froelich C J, Salvesen G S. Pro-caspase 3 is a major physiologic target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 69.Stroh C, Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Diff. 1998;5:997–1000. doi: 10.1038/sj.cdd.4400451. [DOI] [PubMed] [Google Scholar]
- 70.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost M C, Alzari P M, Kroemer G. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med. 1999;189:381–393. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett D R, Aebersold R, Siderovski D P, Penninger J M, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 72.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H G, Geley S, Fassy F, Reed J C, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1342. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Talanian R V, Yang X, Turbov J, Seth P, Ghayur T, Casiano C A, Orth K, Froelich C J. Granule-mediated killing: pathways for granzyme B-initiated apoptosis. J Exp Med. 1997;186:1323–1331. doi: 10.1084/jem.186.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang D, Kidd V J. Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J Biol Chem. 1998;273:28549–28552. doi: 10.1074/jbc.273.44.28549. [DOI] [PubMed] [Google Scholar]
- 76.Tewari M, Telford W G, Miller R A, Dixit V M. CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte-mediated apoptosis. J Biol Chem. 1995;270:22705–22708. doi: 10.1074/jbc.270.39.22705. [DOI] [PubMed] [Google Scholar]
- 77.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 78.Van de Craen M, Van den Brande I, Declercq W, Irmler M, Beyaert R, Tschopp J, Fiers W, Vandenabeele P. Cleavage of caspase family members by granzyme B: a comparative study in vitro. Eur J Immunol. 1997;27:1296–1299. doi: 10.1002/eji.1830270535. [DOI] [PubMed] [Google Scholar]
- 79.Van de Craen M, Van Loo G, Declercq W, Schotte P, Van den Brande I, Mandruzzato S, van der Bruggen P, Fiers W, Vandenabeele P. Molecular cloning and identification of murine caspase 8. J Mol Biol. 1998;284:1017–1026. doi: 10.1006/jmbi.1998.2226. [DOI] [PubMed] [Google Scholar]
- 80.Yang X, Chang H Y, Baltimore D. Autoproteolytic activation of procaspases by oligomerization. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 81.Yang X, Stennicke H R, Wang B, Green D R, Janicke R U, Srinivasan A, Seth P, Salvesen G S, Froelich C J. Granzyme B mimics apical caspases. Description of a unified pathway for trans-activation of executioner caspase-3 and -7. J Biol Chem. 1998;273:34278–34283. doi: 10.1074/jbc.273.51.34278. [DOI] [PubMed] [Google Scholar]
- 82.Yin X M, Wang K, Gross A, Zhao Y, Zhao Y, Zinkel S, Klocke B, Roth K A, Korsmeyer S J. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. Target protease specificity of the viral serpin CrmA. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]