Abstract
Background and Aims
We aimed to validate newly proposed noninvasive criteria for diagnosing clinically significant portal hypertension (CSPH) using liver stiffness measurements (LSM) by transient elastography (TE) and platelet count.
Methods
Diagnostic performance of these new criteria for CSPH (LSM ≥ 25 kPa to rule in and Plt ≥ 150 × 109/L + LSM ≤ 15 kPa to rule out CSPH) were retrospectively tested in an independent cohort of consecutive patients who underwent hepatic venous pressure gradient (HVPG) measurements and liver biopsy due to suspicion of compensated advanced chronic liver disease. Suspicion of cACLD was based on LSM ≥ 10 kPa by TE or results of liver imaging, without overt signs of CSPH. Patients with conditions known to affect results of LSM (ALT > 5 × ULN, liver congestion, extrahepatic biliary obstruction, infiltrative liver neoplasms) were excluded.
Results
Seventy six (76) patients were included: 78.9% males, mean age 62 years, 36.8% suffered from alcoholic, 30.3% nonalcoholic fatty liver disease, 14.5% chronic viral hepatitis, 30.3% were obese, 52.6% had HVPG ≥ 10 mmHg, 56.6% had platelet count ≥ 150 × 109/L. LSM ≥ 25 kPa had 88.9% specificity (95% CI 73.9–96.9) to rule in, whereas Plt ≥ 150 + LSM ≤ 15 kPa had 100% sensitivity (95% CI 91.1–100) to rule out CSPH.
Conclusion
By using these simple noninvasive criteria 49/76 (64.5%) patients could be classified correctly for the presence/absence of CSPH, thus obviating the need for HVPG measurements.
Keywords: Chronic liver disease, Liver cirrhosis, Portal hypertension, Elastography
Introduction
Clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) ≥ 10 mmHg, represents a significant milestone in the natural history of chronic liver disease [1]. According to the results from PREDESCI trial, the introduction of non-selective beta blockers (NSBB) to patients with CSPH (as measured by HVPG) reduces the risk of liver decompensation or death [2]. Previously, NSBB were recommended only when large esophageal varices are present on screening endoscopy [3]. Although HVPG is the gold standard method for diagnosing CSPH, it is invasive and limited to specialized centers [4]. Consequently there is much interest in development of noninvasive methods to diagnose CSPH. Recently, new diagnostic criteria have been proposed based on results of a multicenter study conducted in cohorts of patients with compensated advanced chronic liver disease (cACLD) using results of liver stiffness measurement (LSM) by transient elastography (TE) and platelet count (Plt) [5]. These criteria were derived from the international cohort of patients suffering from alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD) and chronic viral hepatitis.
We aimed to evaluate diagnostic performance of these newly proposed noninvasive criteria in our cohort of patients evaluated for the suspicion of cACLD.
Materials and Methods
Patients
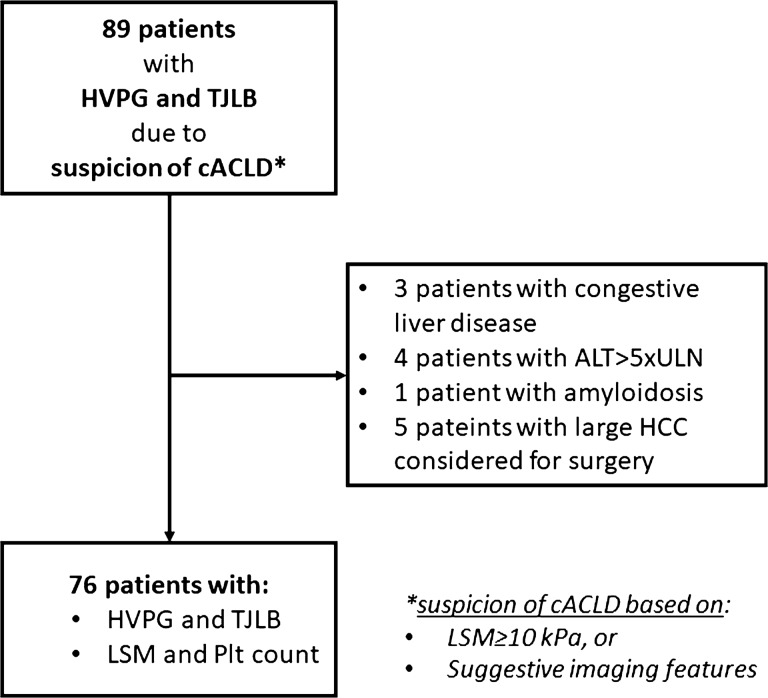
Consecutive patients with available results of HVPG measurements and liver biopsy performed due to suspicion of cACLD over a period of three years were included in this retrospective study approved by the Institutional Ethics Committee. Suspicion of cACLD was based on LSM ≥ 10 kPa by TE or results of liver imaging (ultrasound, computerized tomography or magnetic resonance that revealed coarse liver parenchyma with irregular liver surface or irregular interface of the hepatic veins,), without overt signs of CSPH (presence of portosystemic collaterals or signs of decompensation—presence of ascites, not otherwise detectable by physical examination) [3, 6, 7]. All patients underwent standardized clinical workup in order to define etiology and clinical stage of liver disease. Only patients over 18 years of age, with available results of LSM by TE performed within 1-month period before HVPG measurement, and no previous episodes of liver decompensation (ascites, variceal bleeding, portal encephalopathy, jaundice) were included. Patients with conditions known to affect results of LSM (ALT > 5 × ULN, liver congestion, extrahepatic biliary obstruction, infiltrative liver neoplasms) were excluded (Fig. 1) [8].
Fig. 1.
Flowchart of the study. ALT alanine aminotransferase; cACLD compensated advanced chronic liver disease; HCC hepatocellular carcinoma; HVPG hepatic venous pressure gradient; LSM liver stiffness measurements; Plt platelet count; TJLB transjugular liver biopsy; ULN upper limit of normal
Methods
We evaluated the criteria for diagnosing CSPH (LSM ≥ 25 kPa by TE to rule in and Plt ≥ 150 × 109/L + LSM ≤ 15 kPa to rule out CSPH) as proposed by Pons et al.[5]. LSM and Plt results were tested against the results of HVPG measurements available for each patient, performed as per the method described elsewhere [4]. cACLD was confirmed in patients with bridging fibrosis or cirrhosis on histopathology [3]. Since Pons et al. [5] suggested that the “rule-in” (LSM ≥ 25 kPa) criterion might not perform well in obese (body mass index > 30 kg/m2) patients with nonalcoholic fatty liver disease (NAFLD), we repeated the analysis after exclusion of a subset of such patients.
Institutional Review Board Statement
This study was conducted in accordance with the World Medical Association Declaration of Helsinki, and the study protocol was approved by the Institutional Ethics committee (No 2021/2202-07). Due to the retrospective design of the study, informed consent was waived by the ethics committee.
Data Analysis
We fitted logistic models to probability of HVPG ≥ 10 mmHg among the cohort of patients who met inclusion criteria to determine sensitivity and specificity for LSM alone or combined with platelet counts at the proposed cutoffs [5] by using SAS 9.4 for Windows (SAS Inc., Cary, NC).
Results
The majority of 76 included patients suffered from alcoholic (36.8%) or nonalcoholic fatty (30.3%) liver disease (Table 1), 40 (52.6%) had HVPG ≥ 10 mmHg, 56.6% had platelet counts ≥ 150 × 109/L and 30.3% were obese (body mass index, BMI > 30 kg/m2) (Table 1).
Table 1.
Patient characteristics—overall and by etiology
| All patients | ALD | NAFLD | HBV or HCVa | All other causesb | |
|---|---|---|---|---|---|
| N | 76 | 28 (36.8% of all) | 23 (30.3% of all) | 11 (14.5% of all) | 14 (18.4% of all) |
| Men | 60 (78.9) | 24 (85.7) | 19 (82.6) | 10 (90.9) | 7 (50.0) |
| Age (years) | 62 (53–67; 34–76) | 60 (52–66; 34–74) | 62 (59–67; 37–74) | 58 (54–64; 39–69) | 64 (49–68; 35–76) |
| Hepatic venous pressure gradient (mmHg) | 10.0 (5.0–15.0; 1.5–30) | 13.5 (7.0–16.8; 3.4–30) | 6.0 (4.0–11.0; 1.5–23.0) | 7.0 (4.0–17.0; 3.0–20.0) | 8.0 (4.5–16.6; 2.5–18.6) |
| Hepatic venous pressure gradient ≥ 10 mmHg | 40 (52.6) | 21 (75.0) | 8 (34.8) | 5 (45.5) | 6 (42.9) |
| High-risk varices | 17 (22.4) | 10 (35.7) | 3 (13.0) | 3 (27.3) | 1 (7.1) |
| Platelets (× 109/L) | 161 (103–225; 22–320) | 120 (78–217; 59–320) | 179 (107–246; 76–273) | 162 (141–253; 90–299) | 161 (135–198; 22–257) |
| Platelets ≥ 150 × 109/L | 43 (56.6) | 12 (42.9) | 14 (60.9) | 8 (72.7) | 9 (64.3) |
| Body mass index (kg/m2) | 28.3 ± 5.0 (18.6–49.8) | 29.1 ± 5.9 (22.8–49.8) | 29.9 ± 4.0 (21.0–35.9) | 25.9 ± 4.4 (18.6–33.8) | 25.5 ± 3.1 (21.6–33.7) |
| Body mass index > 30 kg/m2 (obese patients) | 23 (30.3) | 10 (35.7) | 10 (43.5) | 2 (18.2) | 1 (7.1) |
| Liver stiffness measurement (kPa) | 24.1 ± 16.6 (2.8–69.1) | 31.1 ± 15.7 (8.3–69.1) | 19.3 ± 15,6 (2.8–69.1) | 22.0 ± 18.4 (9.9–63.9) | 19.5 ± 15.4 (3.4–63.9) |
| HPE: bridging fibrosis or cirrhosis (cACLD) | 61 (80.3) | 27 (96.4) | 16 (69.6) | 8 (72.7) | 10 (71.4) |
| HPE: cirrhosis | 53 (69.7) | 27 (96.4) | 11 (47.8) | 5 (45.5) | 10 (71.4) |
| Bilirubin (µmol/L) | 16.4 (12–25; 3.1–49) | 20.7 (12.9–37.2; 9–49) | 15.0 (11–21.9; 8.6–44) | 13.7 (11.1–19.1; 3.1–31.4) | 14.5 (11.7–22.8; 5–42.3) |
| Albumin (g/L) | 42 (36–45; 25–51) | 36 (33–44; 25–50) | 43 (41–45; 35–51) | 43.5 (41–45; 39–50) | 39 (32–45; 26–48) |
| International normalized ratio | 1.1 (1.0–1.4; 1.0–2.3) | 1.3 (1.1–1.5; 1.0–1.9) | 1.0 (1.0–1.1; 1.0–2.3) | 1.0 (1.0–1.1; 1.0–1.6) | 1.1 (1.0–1.7; 1.0–1.8) |
| Creatinin (µmol/L) | 71 (62–83; 37–125) | 68 (61–81; 51–121) | 71 (64–92; 52–119) | 70 (56–95; 52–122) | 74 (62–79; 37–125) |
| Aspartate transaminase (U/L) | 44 (32–68; 15–170) | 52 (35–78; 15–170) | 34 (28–42; 19–82) | 48 (39–54; 30–103) | 54 (35–77; 29–158) |
| Alanine transaminase (U/L) | 43 (27–69; 12–153) | 35 (24–66; 12–146) | 45 (28–76; 15–139) | 50 (31–71; 26–115) | 44 (33–75; 15–153) |
| Gamma glutamyl transferase (U/L) | 99 (52–196; 14–1625) | 165 (66–25; 19–1568) | 93 (51–150; 25–1625) | 65 (43–106; 19–509) | 80 (44–195; 14–477) |
| Alkaline phosphatase (U/L) | 100 (79–130; 47–342) | 97 (79–137; 60–197) | 92 (68–124; 51–280) | 105 (66–124; 56–186) | 120 (74–175; 47–342) |
Data are counts (percent), median (quartiles, range) or mean ± SD (range)
ALD alcoholic liver disease, cACLD compensated advanced chronic liver disease, HBV/HCV chronic hepatitis B/C, NAFLD nonalcoholic fatty liver disease, HPE histopathology examination
aAll patients (8 HCV, 3 HBV, no dual infection) were viremic at the time of diagnostic evaluation (i.e., were not treated by antivirals)
bDiagnoses: autoimmune hepatitis (n = 7), cryptogenic cirrhosis (n = 5), Wilson’s disease (n = 1), drug-induced liver injury (n = 1)
LSM ≥ 10 kPa was detected in 65/76 (85.5%) patients, and 59 (90.8%) of them had bridging fibrosis or cirrhosis (cACLD) on histopathology examination. Of the remaining 11 patients with LSM < 10 kPa, 2/11 had bridging fibrosis or cirrhosis. A wide range of LSM values were recorded, overall and across all etiology subgroups (Table 1). The suggested LSM cutoff (≥ 25.0 kPa) for CSPH had moderate sensitivity and specificity and positive (PPV) and negative (NPV) predictive values, yet enabled correct identification of 25/40 patients with CSPH (“CSPH adequately ruled in”) (Table 2). Combination of platelets ≥ 150 × 109/L and LSM of ≤ 15.0 kPa showed 100% sensitivity for the absence of CSPH and 24/36 patients without CSPH could be correctly identified as such (“CSPH adequately ruled out”) with no false negatives (Table 2). BMI altered the performance of LSM and Plt to predict CSPH. For instance, as BMI increased, higher LSM measurements were required for a given platelet count to predict CSPH, whereas for a given LSM a lower platelet count was required to accurately define CSPH. (Fig. 2). When obese NAFLD patients were excluded (n = 10), prevalence of patients with CSPH only slightly changed (35/66, 53.0%). The “rule-in” criterion (sensitivity 67.5% [50.9–81.4], specificity 88.9% [73.9–96.9], PPV 87.1% [70.2–96.4], NPV 71.1% [55.7–83.6]), and the “rule-out” criterion (sensitivity 100% [90.0–100], specificity 64.5% [45.4–80.8], PPV 76.1% [61.2–87.4], NPV 100% [83.2–100]) performed practically identically as in the entire cohort.
Table 2.
Diagnostic performance of liver stiffness measurement (LSM) by transient elastography in respect to clinically significant portal hypertension (CSPH, defined as hepatic venous pressure gradient, HVPG ≥ 10 mmHg)
| Criterion to rule in: LSM ≥ 25 | Criterion to rule out: Plt ≥ 150 and LSM ≤ 15a | |
|---|---|---|
| Sensitivity (%) (95%CI) | 67.5 (50.9–81.4) | 100 (91.1–100)b |
| Specificity (%) (95%CI) | 88.9 (73.9–96.9) | 66.6 (49.0–81.4) |
| Positive predictive value, PPV (%) (95%CI) | 87.1 (70.2–96.4) | 76.9 (63.2–87.5) |
| Negative predictive value, NPV (%) (95%CI) | 71.1 (55.7–83.6) | 100 (85.8–100)b |
| Ruled in as CSPH | 29/76 | – |
| True positives (CSPH, adequately ruled in) | 25/40 | – |
| False positives (ruled in, but no CSPH) | 4/29 | – |
| “Missed” CSPH (CSPH, but not ruled in) | 15/40 | – |
| Ruled out as CSPH | – | 24/76 |
| True negatives (no CSPH, adequately ruled out) | – | 24/36 |
| False negatives (ruled out, but CSPH) | – | 0/24 |
| “Missed” non-CSPH (no CSPH, but not ruled out) | – | 12/36 |
| Patients adequately ruled in or ruled out | 25 + 24 = 49/76 (64.5%) | |
| Patients who could not be ruled in or ruled out | 27/76 (35.5%), 4 misclassified as CSPH | |
Data are shown for cutoff values as suggested by Pons et al. [5] to rule-in CSPH (LSM ≥ 25 kPa) and to rule it out—platelet (Plt) counts ≥ 150 × 109/L and LSM ≤ 15 kPa. Among the 76 included patients, 40 (event prevalence 52.6%) suffered CSPH
aThis diagnostic test has a reverse logic as compared to the test/criterion defined to rule-in CSPH: a “positive test” (high platelet counts combined with low LSM) here serves to detect a lack of CSPH. Therefore, its “target” is a “non-condition.” In this sense, reliable recognition of the target would actually result in high specificity and high PPV. In order to avoid confusion, data are presented in the same direction as for the rule-in test/criterion
bOne-sided 97.5% confidence interval
Fig. 2.
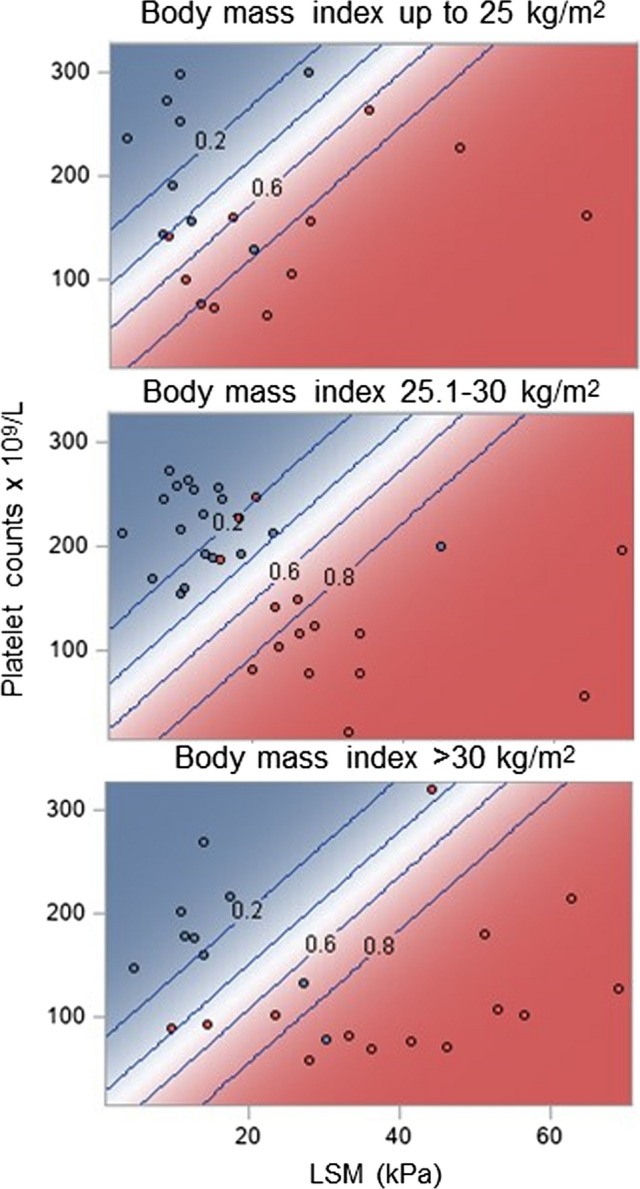
Relationship between liver stiffness measurement (LSM) by transient elastography and platelet counts and probability of clinically significant portal hypertension as defined by hepatic venous pressure gradient (HVPG) ≥ 10 mmHg conditional on the body mass index (BMI) level. Diagonal lines (and numbers) depict levels of probability of HVPG ≥ 10 mmHg. Higher LSM and lower platelet counts were consistently associated with a higher probability of HVPG ≥ 10 mmHg irrespective of BMI. However, with increasing BMI, at any given level of platelet counts, higher LSM values were needed for a certain probability of HVPG ≥ 10 mmHg; conversely, at any given LSM value, lower platelet counts were needed for a certain probability of HVPG ≥ 10 mmHg
Discussion
In this study, we have validated the newly proposed noninvasive criteria for diagnosing CSPH [5] in patients with the suspicion of cACLD. This is the first study to validate these criteria in an independent cohort of patients with available HVPG measurements and liver biopsy as the referent diagnostic methods. According to our results, the new criteria are highly reliable for ruling out CSPH with 100% sensitivity, but have somewhat lower specificity in the present cohort for ruling in CSPH. Considering the entire cohort, 53/76 (69.7%) patients would fit into the suggested criteria, thus obviating the need for HVPG measurement as an invasive and relatively unavailable procedure, with the cost of falsely ruling in CSPH in 4/76 (5.3%) patients, or alternatively expressed—4/29 (13.8%) of the patients with LSM ≥ 25 kPa were false positives.
The combination of platelet count and LSM by TE (at other cutoffs) has been already extensively validated for ruling out the presence of high-risk esophageal varices as suggested by Baveno VI, and this has become even more important during the COVID-19 pandemic where access to endoscopy services may be limited [3]. Recently, the introduction of non-selective beta blockers to patients with CSPH as measured by HVPG has been shown to prevent decompensation and improve survival [2]. Accurate noninvasive criteria to diagnose CSPH would facilitate treatment of patients without the need for HVPG measurement. Due to important prognostic meaning of CSPH in cACLD in general as well as when considering the possibility of liver resection and extrahepatic surgery, these noninvasive criteria should be considered even more important in the era of precision medicine [1, 9, 10]. In fact, they represent a paradigm shift in the management of portal hypertension allowing clinicians to accurately classify patients and either avoid invasive investigations such as HVPG measurements or allow timely prescription of prophylactic medications to at risk patients. This would mean that in patients with LSM by TE ≥ 25 kPa introduction of NSBB might be considered, provided no contraindication exists for these drugs. However, if adopted, this approach would result in unnecessarily treating almost 14% of patients as suggested by the present cohort, and 10% as suggested by Pons et al. across different etiologies, rising to 23% in patients with NALFD [5]—exposing these patients potentially to the adverse effects of NSBB. Therefore, for the time being, the most practical benefit from using Pons’ criteria would be to avoid unnecessary HVPG measurements in patients in whom CSPH has been ruled out by these noninvasive criteria. In our opinion, the decision to introduce NSBB for patients with LSM ≥ 25 kPa should be weighted individually, with probably higher likelihood for the presence of CSPH in patients with higher ranges of LSM.
Interestingly, in keeping with observation by Pons et al. [5] our patients with NAFLD had the lowest prevalence of CSPH and accurate prediction of CSPH by noninvasive methods required either a higher LSM or lower Plt count with increasing body mass index. This phenomenon remains an issue for further investigation but might be due to the physical influence of liver fat on the TE readings, and possibly due to technical characteristics of the Fibroscan XL probe [11, 12]. Nevertheless, when obese NAFLD patients were excluded (n = 10), the performance of tested criteria only marginally changed, probably due to very small influence of this number of patients to the overall cohort.
Of the 4 patients who were misclassified as CSPH (did not have HVPG ≥ 10 mmHg but had TE ≥ 25 kPa) in our study 3 suffered ALD and 1 suffered NAFLD. We assume that high LSM obtained in these 3 patients with ALD resulted from the ongoing alcohol drinking (known for its influence on LSM, i.e., patients who actively drink have higher LSM in comparison with patients with the same fibrosis stage who are not drinking currently) [13, 14]. As for the remaining 1 patient with NAFLD, he probably fits the rule observed in Pons' and our study that higher LSM is needed for accurate prediction of CSPH among patients with NAFLD.
The limitation of this study is relatively small number of tested individuals and the fact 20% of patients did not meet histological criteria for cACLD. The latter specifically refers to patients in whom the suspicion of cACLD was based on “imaging” criteria. Indeed, only 2/11 (18.2%) of them had histologically confirmed cACLD, as opposed to the recently published results that revealed almost equal performance of imaging criteria and LSM for this purpose [7]. In our study, conventional ultrasound was used in 8/11 of the patients to define the morphological features suggestive of cACLD. Accordingly, it appears that conventional ultrasound probably overestimates the stage of liver fibrosis, especially if not using high-frequency probes and with high prevalence of obesity and fatty liver as encountered in the present study, both known for their negative influence on the quality of the ultrasound image. Therefore, we believe that LSM ≥ 10 kPa, as originally suggested by Baveno VI consensus, rather than the imaging features of the liver, is probably more reliable (less equipment-, operator-, and patient-dependent) criterion for considering the presence of cACLD. Additionally, the cohort comprised patients with mixed etiology of chronic liver disease, with a possible influence of NAFLD on the overall results as already explained. It is important to stress that the criteria by Pons, et al. were developed in alcohol, viral, and NAFLD patients, and consequently, they may not work well in other etiologies of liver disease. On the other hand, our patients were thoroughly evaluated, using “gold standard” methods (HVPG and liver biopsy) to serve as the reference background for the investigated TE, and all the methods were performed by well-trained and highly experienced operators. Also, these data represent a real-world experience outside of the strict protocol of a clinical trial and thus reflective of modern hepatology practice.
In conclusion, we have observed a good performance of the newly proposed noninvasive diagnostic criteria for CSPH in an independent cohort of patients with cACLD. By using LSM and Plt count, it was possible to classify correctly almost 65% of patients with respect to the presence or absence of CSPH. These criteria are best used to rule out the presence of CSPH, whereas they tend to somewhat overestimate the presence of CSPH.
Abbreviations
- ALD
Alcoholic liver disease
- BMI
Body mass index
- cACLD
Compensated advanced chronic liver disease
- CSPH
Clinically significant portal hypertension
- HVPG
Hepatic venous pressure gradient
- LSM
Liver stiffness measurements
- NAFLD
Nonalcoholic fatty liver disease
- Plt
Platelet count
- TE
Transient elastography
Author's contribution
Kristian Podrug and Ivica Grgurevic had contributed to conception and design, acquisition and interpreting data, drafting the manuscript, revising it critically, and final approval; Vladimir Trkulja was involved in conception and design, analysis and interpretation of data, drafting the manuscript, revising it critically, and final approval; Marko Zelenika, Tomislav Bokun, Anita Madir, and Tajana Filipec Kanizaj, participated in acquisition and interpreting data, revising it critically, and final approval. James O’Beirne participated in conception and design, interpreting data, revising the manuscript critically, and final approval
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest. Each author has approved the final draft submitted.
Footnotes
An editorial commenting on this article is available at 10.1007/s10620-021-07283-w.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R; Baveno V Faculty. Expanding consensus in portal hypertension. Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. [DOI] [PubMed]
- 4.Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 5.Pons M, Augustin S, Scheiner B et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2020. 10.14309/ajg.0000000000000994. Epub ahead of print. PMID: 33038131. [DOI] [PubMed]
- 6.Berzigotti A, Abraldes JG, Tandon P, et al. Ultrasonographic evaluation of liver surface and transient elastography in clinically doubtful cirrhosis. J Hepatol. 2010;52:846–853. doi: 10.1016/j.jhep.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Souhami A, Sartoris R, Rautou PE, et al. Similar performance of liver stiffness measurement and liver surface nodularity for the detection of portal hypertension in patients with hepatocellular carcinoma. JHEP Rep. 2020;2:100147. doi: 10.1016/j.jhepr.2020.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version) Ultraschall Med. 2017;38:e16–e47. doi: 10.1055/s-0043-103952. [DOI] [PubMed] [Google Scholar]
- 9.Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2015;61:526–536. doi: 10.1002/hep.27431. [DOI] [PubMed] [Google Scholar]
- 10.Reverter E, Cirera I, Albillos A, et al. The prognostic role of hepatic venous pressure gradient in cirrhotic patients undergoing elective extrahepatic surgery. J Hepatol. 2019;71:942–950. doi: 10.1016/j.jhep.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Petta S, Wai-Sun Wong V, Bugianesi E, et al. Impact of obesity and alanine aminotransferase levels on the diagnostic accuracy for advanced liver fibrosis of noninvasive tools in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2019;114:916–928. doi: 10.14309/ajg.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 12.Wong VW, Irles M, Wong GL, et al. Unified interpretation of liver stiffness measurement by M and XL probes in non-alcoholic fatty liver disease. Gut. 2019;68:2057–2064. doi: 10.1136/gutjnl-2018-317334. [DOI] [PubMed] [Google Scholar]
- 13.Mueller S, Millonig G, Sarovska L, et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966–972. doi: 10.3748/wjg.v16.i8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahon P, Kettaneh A, Tengher-Barna I, et al. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol. 2008;49:1062–1068. doi: 10.1016/j.jhep.2008.08.011. [DOI] [PubMed] [Google Scholar]