Abstract
The high incidence of human herpesvirus-6 (HHV-6) reactivation, potentially interfering with engraftment after umbilical cord blood (UCB) hematopoietic cell transplantation (HCT), remains a major challenge. To potentially address this problem, we evaluated the effect of prophylactic foscarnet administered twice daily beginning on day +7 and continuing through engraftment in 25 patients. To determine the impact of foscarnet on HHV-6, engraftment, and other transplantation outcomes, we compared results in 61 identically treated patients with hematologic malignancies. Treatment and control groups underwent reduced-intensity conditioning UCB HCT with a conditioning regimen of fludarabine, cyclophosphamide, and total body irradiation 200 cGy with or without antithymocyte globulin (ATG), using sirolimus plus mycophenolate mofetil immune suppression. The treatment and control groups were similar in terms of age, disease risk, use of ATG, Hematopoietic Cell Transplantation Comorbidity Index, and graft CD34 cell dose; however, foscarnet-treated patients were less likely to receive a double UCB graft and to be treated more recently (2016 to 2018). The cumulative incidence of HHV-6 reactivation by day +100 was 63% for all patients (95% confidence interval [CI], 51% to 75%) and was not significantly different between the 2 groups. HHV-6 reactivation occurred at a median of 34 days in the foscarnet group and 25.5 days in the control group. The incidence of neutrophil engraftment at day 42 was higher in the foscarnet group compared with the control group (96%; [95% CI, 83% to 100%] versus 75% [95% CI, 64% to 85%]; P< .01). The cumulative incidence of platelet engraftment by 6 months was 92% (95% CI, 69% to 100%) for the foscarnet group versus 75% (95% CI, 60% to 90%) for the control group (P= .08), and multivariate analysis identified the use of foscarnet as an independent predictor of better platelet engraftment. No patients died as a result of graft failure in recipients of foscarnet, whereas 5 patients died from graft failure in the control group. Six-month overall survival (OS) and nonrelapse mortality (NRM) were better in the foscarnet group (96% versus 72% [P= .02] and 4% versus 18% [P= .07], respectively). Even though foscarnet prophylaxis did not prevent HHV-6 viremia, we observed a delay in time to HHV-6 reactivation, a trend toward differences in engraftment, NRM, and OS compared with historical controls.
Keywords: Hematopoietic cell, transplantation, Foscarnet, HHV-6, Toxicity, Graft failure
INTRODUCTION
Umbilical cord blood (UCB) is a valuable alternative for allogeneic hematopoietic stem cell transplantation (HCT). However, delayed engraftment, graft failure, and increased risk for opportunistic infections remain major challenges when considering this donor source. Human herpesvirus-6 (HHV-6) reactivation occurs in the majority of UCB recipients [1-3]. Thus, UCB recipients are a particularly vulnerable population for HHV-6 viremia and post-transplantation complications with severe sequelae, such as delayed hematopoietic recovery, graft failure, and limbic encephalitis [4-9].
In an attempt to decrease the incidence of HHV-6 reactivation and disease, various prophylactic and preemptive therapies have been explored [10-14]. To date, these strategies have not been effective in preventing HHV-6-related disease, and the effect of HHV-6 viremia prophylaxis on hematopoietic recovery has not been examined. In our center, we observed a high incidence of HHV-6 reactivation associated with delayed engraftment and graft failure in patients undergoing reduced-intensity conditioning (RIC) UCB HCT using sirolimus (Siro) and mycophenolate mofetil (MMF) immune suppression [15]. We modified the infection prophylaxis by adding foscarnet in the pre-engraftment period to recipients of RIC UCB HCT to suppress HHV-6 viremia and improve engraftment. Here we report the outcomes of patients receiving foscarnet prophylaxis compared with historical controls who received the same conditioning and immune suppression regimens.
METHODS
Study Design and Eligibility
We retrospectively evaluated all consecutive patients with hematologic malignancies receiving a standardized RIC followed by a UCB transplantation and post-transplantation immune suppression with Siro/MMF with or without HHV-6 prophylaxis at the University of Minnesota between September 2012 and December 2018, excluding 2 patients who also received an adoptive transfer of regulatory T cells. In January 2016, foscarnet was added uniformly as antiviral prophylaxis for recipients of UCB transplantation after RIC to potentially limit HHV-6 reactivation. This clinical treatment protocol was approved by the university’s Institutional Review Board (ClinicalTrials.gov identifier NCT02722668).
Treatment Regimen and Supportive Care
The conditioning regimen, immune suppression, and supportive care have been reported previously [15]. In brief, all patients received fludarabine (Flu; 150 mg/m2), cyclophosphamide (Cy; 50 mg/kg), and low-dose total body irradiation (TBI; 200 cGy). Antithymocyte globulin (equine ATG) at a dose of 90 mg/kg on days −6 to −4 was included in the conditioning regimen for patients not exposed to potently immunosuppressive chemotherapy in the previous 3 months. All patients received Siro with a target serum concentration of 3 to 12 mg/mL and MMF 3 g/day starting on day −3 as GVHD prophylaxis. Patients seropositive for cytomegalovirus (CMV) received high-dose acyclovir prophylaxis (800 mg 5 times daily versus 400 mg twice daily) until day +6, followed by maintenance acyclovir from the time of foscarnet discontinuation until day +100. In addition to standard supportive care measures [16,17], foscarnet (45 mg/kg twice daily) was added in January 2016 as antiviral prophylaxis starting at day +7 and continuing until neutrophil engraftment, with a plan for at least 10 days of therapy when clinically feasible at the discretion of the treating physician. All patients were planned to receive 500 mL of normal saline infusion before foscarnet.
HHV-6 Assessment
HHV-6 reactivation was monitored weekly starting on day +7 and continuing until engraftment for patients receiving foscarnet prophylaxis. Testing was done by whole blood quantitative HHV-6 DNA PCR. HHV-6 DNA quantification was performed at weekly intervals after total nucleic extraction. Viral loads were expressed as the number of viral DNA copies per milliliter of blood. An active infection was defined by more than one test with a viral load >2.7 log/mL of blood, the assay sensitivity. After engraftment, HHV-6 PCR was tested as clinically indicated including, but not limited to, persistent fevers, blood counts, rash, and mental status changes. Institutional guidelines suggested treatment on detection of >25,000 copies/μL of HHV-6, even in the absence of clinical symptoms.
Statistical Analysis
The cumulative incidence of neutrophil and platelet engraftment was estimated with nonengraftment death as a competing risk, and the cumulative incidence of nonrelapse mortality (NRM) by 6 months was estimated with relapse as a competing risk [18]. Kaplan-Meier curves were used to estimate the probability of overall survival (OS) with log-rank testing of comparisons [19]. Neutrophil engraftment was defined as absolute neutrophil count ≥5 × 108/L on the first of 3 consecutive measurements. Platelet engraftment was defined as the first of 7 consecutive days with a nontransfused count of ≥20 × 109/L. Gray’s test was used to compare differences in overall curves, and the chi-square test was used to compare point estimates.
Cox regression was used to examine the independent effect of foscarnet use on 6- and 12-month OS [20]. Fine and Gray regression was used to test the independent effect of foscarnet on neutrophil and platelet engraftment and on NRM [21]. Logistic regression was also used to evaluate the independent effect of foscarnet on the odds of neutrophil engraftment without respect to time. Factors considered in regression analyses were use of foscarnet (no versus yes), age (per increase by decade), donor type (single UCB [sUCB] versus double UCB [dUCB]), conditioning (Cy/Flu/TBI versus Cy/Flu/TBI/ATG), Karnofsky Performance Status (KPS) score (<90 versus 90 to 100), Disease Risk Index (DRI; low risk versus intermediate risk versus high risk), CD34 dose (continuous), and recipient CMV serostatus (negative versus positive).
All reported Pvalues were 2-sided. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
Patient, disease, and transplantation characteristics for the 2 groups are summarized in Table 1. The cohort comprised 86 patients with hematologic malignancies, including 25 patients who received foscarnet prophylaxis and 61 historical controls treated similarly but without foscarnet. The median age at transplantation was 62 years (range, 16 to 73 years). More patients received sUCB than dUCB in the foscarnet group compared with the historical control group (60% versus 10%; P < .01). Acute leukemia was the most frequent diagnosis, and most patients had a DRI of intermediate to high risk. ATG was part of the conditioning regimen in 36% of the patients in the foscarnet group compared with 49% of controls (P = .26). Eight percent of the patients in the foscarnet group had a KPS <80%, compared with 2% of controls. The HCT Comorbidity Index score was similar in the 2 groups. CMV-positive serostatus was slightly more frequent in the foscarnet group (72% versus 52%; P = .1). The median infused total nucleated cell dose was 3.4 × 107/kg (range, 2.4 to 8.4 × 107/kg) in the foscarnet group and 4.1 × 107/kg (range, 2.2 to 9.6 × 107/kg) in the control group (P = .02). This difference reflects in part the less frequent use of dUCB grafts in the control group. The median infused total CD34 cell dose was .4 × 106/kg (range, .1 to 1.5 × 106/kg) in the foscarnet group and .5 × 106/kg (range, .02 to 2.4 × 106/kg) in the control group (P= .04).
Table 1.
Patient and Disease Characteristics
| Characteristic | Foscarnet | No Foscarnet |
P Value |
|---|---|---|---|
| Number of patients | 25 | 61 | |
| Male sex, n (%) | 14 (56) | 42 (69) | .26 |
| Age at transplantation, yr, median (range), (IQR) | 62 (45-69), (59-66) | 63 (16-73), (58-67) | .69 |
| Year of transplantation, n (%) | |||
| 2012-2015 | – | 58 (95) | |
| 2016-2018 | 25(100) | 3 (5) | |
| Donor type, HLA match, n (%) | <.01 | ||
| sUCB, 4/6 | 4 (16) | 1 (2) | |
| sUCB, 5-6/6 | 11 (44) | 5 (8) | |
| dUCB | 10 (40) | 55 (90) | |
| Diagnosis, n (%) | .88 | ||
| ALL | 2 (8) | 7 (12) | |
| AML | 12 (38) | 18 (30) | |
| MDS | 2 (8) | 15 (25) | |
| CLL | 0 | 4 (7) | |
| NHL/Hodgkin lymphoma | 5 (20) | 11 (18) | |
| Multiple myeloma | 3 (12) | 1 (2) | |
| Other malignancy | 1 (4) | 5 (8) | |
| DRI, n (%) | .62 | ||
| Low | 1 (4) | 6 (10) | |
| Intermediate | 17 (68) | 39 (64) | |
| High | 7 (28) | 14 (23) | |
| Very high | 0 | 2 (3) | |
| Conditioning, n (%) | .26 | ||
| RIC: Cy/Flu/TBI | 16 (64) | 31 (51) | |
| RIC: Cy/Flu/TBI/ATG | 9 (36) | 30 (49) | |
| GVHD prophylaxis: Siro/MMF, n (%) | 25 (100) | 61 (100) | |
| KPS at admission, n (%) | <.01 | ||
| <80 | 2 (8) | 1 (2) | |
| ≥80 | 23 (92) | 60 (98) | |
| HCT-CI, n (%) | |||
| Low risk (0) | 5 (20) | 15 (25) | |
| Intermediate risk (1-2) | 10 (40) | 29 (48) | |
| High risk (3+) | 10 (40) | 17 (28) | |
| Positive CMV serostatus, n (%) | 18 (72) | 32 (52) | .10 |
| TNC dose, × 107/kg, median (range), (IQR) | 3.4 (2.4-8.4), (3.0-4.1) | 4.1 (2.2-9.6), (3.4-5.1) | .02 |
| CD34 dose, × 106/kg, median (range), (IQR) | .4 (.1-1.5), (.2-.5) | .5 (.02-2.4), (.3-.8) | .04 |
| Grade II-IV acute GVHD, %, median (95% CI) | 28 (11-45) | 36 (24-48) | .42 |
| Duration of foscarnet therapy, d, median (range), (IQR) | 16 (2-23), (10-20) | ||
| Follow-up, yr, median (range) | 3.2 (.8-3.7) | 5.5 (2.3-7.0) |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CLL, chronic lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; CR, complete remission; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index.
HHV-6 and CMV Reactivation
The cumulative incidence of HHV-6 reactivation by day +100 was 63% for all patients (95% CI, 51% to 75%), 72% in the foscarnet group, and 59% in controls (P= .83). The median time to HHV-6 reactivation was 34 days (range, 2 to 100 days; inter-quartile range [IQR], 27 to 37 days) in the foscarnet group and 26 days (range, 13 to 81 days; IQR, 19.5 to 32.5 days) in the control group (P = .83 ). The incidence of HHV-6 reactivation requiring treatment was similar in the 2 groups (41% versus 40%; P = .53). There was 1 case of HHV-6 pneumonitis in the control group and no cases in the foscarnet group. None of the patients had HHV-6-related encephalitis. The median duration of foscarnet prophylaxis administration was 16 days (range, 2 to 23 days; IQR, 10 to 20 days). Of the 25 patients receiving foscarnet, premature discontinuation occurred in 3 patients, for transient renal insufficiency in 2 and foscarnet-related headache in 1. The cumulative incidence of CMV reactivation by day +100 was 37% in the foscarnet group and 18% in the control group (P= .08) and was not significantly different in CMV-seropositive recipients (50% versus 28%; P = .17).
Hematopoietic Recovery
The median time to neutrophil engraftment was 19.5 days (range, 8 to 35 days). The rate of neutrophil engraftment at day +42 was 96% (95% CI, 83% to 100%) in the foscarnet group and 75% (95% CI, 64% to 85%) in the control group (P < .01) (Figure 1). Sixteen patients did not achieve neutrophil engraftment by day +42. These 16 patients included only 1 patient from the foscarnet group, and 15 patients from in the control group. Of these 15 control patients, 11 had preceding HHV-6 reactivation. After day 42, 7 achieved neutrophil engraftment (the patient in the foscarnet group and 6 of 15 patients in the control group), and the remaining 9 control patients had primary graft failure. Of the 9 patients with primary graft failure, 4 had underlying myelodysplastic syndrome, 4 had acute myelogenous leukemia, and 1 had non-Hodgkin lymphoma. The median time to HHV-6 reactivation in these 9 patients was 28 days (range, 19 to 33 days). Recipients of sUCB grafts were more likely than recipients of dUCB grafts to achieve neutrophil engraftment by day +42 (100% versus 75%; P = .04), but neither ATG administration nor CMV serostatus influenced neutrophil engraftment. In multiple regression models, after adjusting for age, donor type (sUCB versus dUCB), and CD34 cell dose, the odds of patients achieving neutrophil engraftment by day 42 was 3.5-fold higher in the foscarnet group (OR, 3.5; 95% CI, .4 to 31.2; P= .27).
Figure 1.
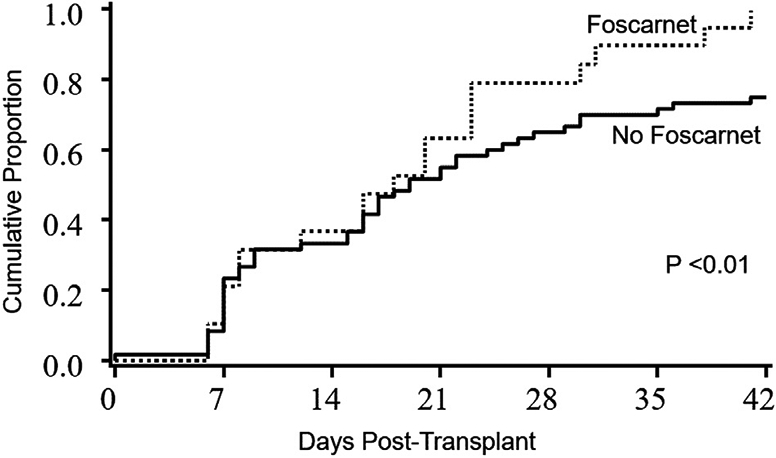
Neutrophil engraftment in the foscarnet and no foscarnet groups. The incidence of neutrophil engraftment at day 42 was 96% (95% CI, 83% to 100%) in the foscarnet group and 75% in the control group (95% CI, 64% to 85%).
The median time to platelet engraftment of ≥20 × 109/μL was 59 days (range, 43 to 105 days). The cumulative incidence of platelet engraftment by 6 months was 92% (95% CI, 69% to 100%) for the foscarnet group and 75% (95% CI, 60 to 90%) for the control group (P = .08) (Figure 2). Seventeen patients did not achieve platelet engraftment by 6 months, including 2 patients in the foscarnet group and 15 controls. Thirteen of these 15 control patients, 13 had HHV-6 reactivation. In univariate analysis, there was no significant difference in the incidence of platelet engraftment with donor units (sUCB versus dUCB). Patients receiving conditioning regimens without ATG had a higher incidence of platelet engraftment (89% versus 69%; P = .02), and negative CMV serostatus was marginally associated with better engraftment (89 versus 74%; P= .08). In multiple regression models, after adjusting for age, donor type (sUCB versus dUCB), CMV serostatus, and CD34 cell dose, the proportion of patients achieving platelet engraftment by 6 months was 1.7-fold higher in the foscarnet group compared with controls (hazard ratio, 1.7; 95% CI, 1.0 to 2.8; P= .04).
Figure 2.
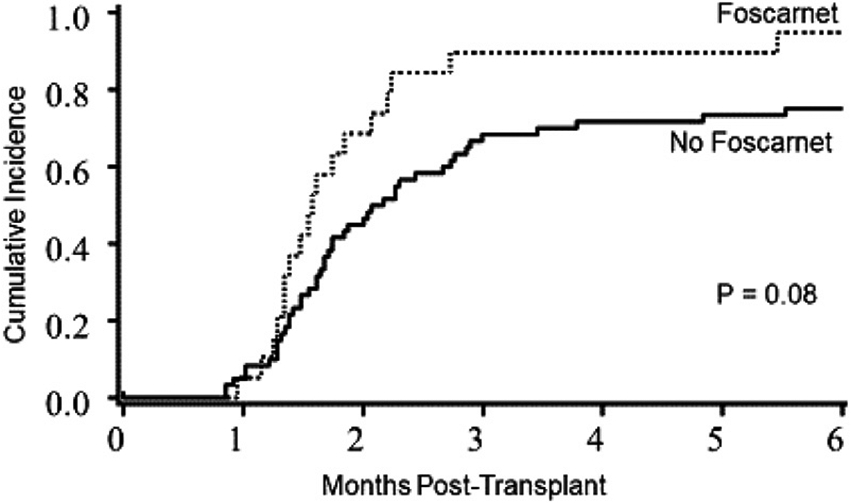
Platelet engraftment in the foscarnet and no foscarnet groups. The cumulative incidence of platelet engraftment by 6 months was 92% (95% CI, 69% to 100%) in the foscarnet group versus 75% (95% CI, 60% to 90%) in the control group.
OS
Six-month OS was 79% (95% CI, 69% to 86%) for all patients and was higher in the foscarnet group compared with the control group (96% versus 72%; P= .02) (Figure 3). In univariate analysis, sUCB (95% versus 74%; P = .04) and lower DRI (100% in low risk, 82% in intermediate risk, and 64% in high/very high risk; P= .02) predicted better 6-month OS. In multiple regression analysis, after adjusting for age, donor type (sUCB versus dUCB), and DRI, only DRI remained significant at 6 months, with high/very high DRI patients having a 3.1-fold higher risk of mortality (hazard ratio, 3.1; 95% CI, 1.2 to 8.0; P= .02). Foscarnet did not independently influence 6-month OS (P> .1).
Figure 3.
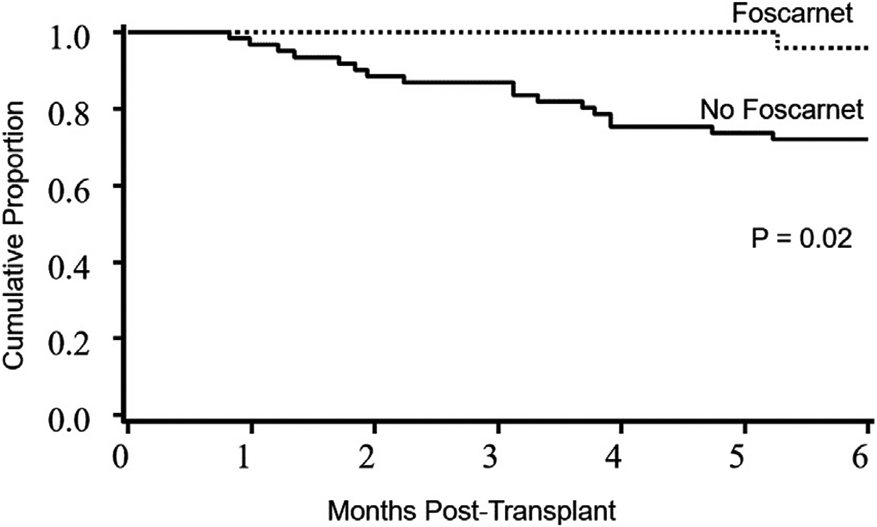
OS in the foscarnet and no foscarnet groups. The 6-month OS was 96% in the foscarnet group (95% CI, 74% to 99%) and 72% in the control group (95% CI, 59% to 82%).
NRM, Causes of Death, and Toxicity
NRM at 6 months was 4% in the foscarnet group and 18% in the control group (P= .07) (Figure 4). NRM was not influenced by donor type, conditioning regimen, KPS, HCT-CI, DRI, or CMV serostatus.
Figure 4.
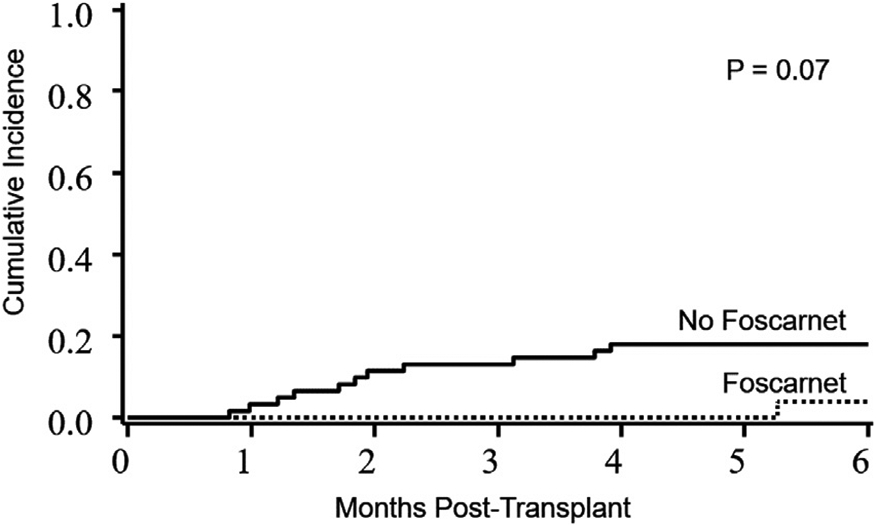
NRM in the foscarnet group versus the no foscarnet group. The 6-month NRM was 4% in the foscarnet group (95% CI, 0 to 12%) and 18% in the control group (95% CI, 8% to 28%).
Among 17 of 61 control patients who died, graft failure was the primary cause of death in 5 patients Figure 5. Four patients died of acute GVHD complications, 6 had disease relapse, 1 had leukoencephalopathy not due to HHV-6 encephalitis, and 1 had multiple organ failure without HHV-6 reactivation. In the foscarnet group, no patients died of graft failure or acute GVHD, and 2 of 25 patients experienced CTCAE grade 2 renal toxicity, which was reversible.
Figure 5.
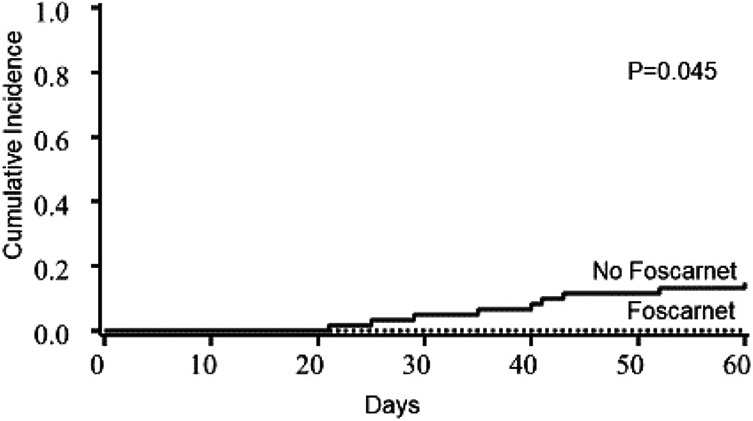
Primary graft failure in the foscarnet and no foscarnet groups. Nine patients had primary graft failure in the control group compared with none in the foscarnet group.
Acute and Chronic GVHD
The incidence of acute GVHD by 100 days after transplantation was similar in the foscarnet and control groups: grade II-IV, 28% versus 36% (P = .41); grade III-IV, 16% versus 20% (P = .64). The incidence of chronic GVHD was similar in the 2 groups at 6 months (4% versus 5%; P = .67) and 1 year (8% versus 7%; P= .97).
DISCUSSION
Our data suggest that foscarnet prophylaxis should be provided after RIC UCB HCT for hematologic malignancies when used with Siro/MMF immunosuppression. These promising pilot results require larger studies confirming improvement in hematopoietic recovery, prevention of graft failure, and effect on NRM and survival among patients receiving foscarnet. Prophylactic foscarnet did not prevent HHV-6 viremia, but we observed no associated graft failure with or without HHV-6 viremia, a decrease compared with our historical controls. Even though foscarnet prophylaxis starting day +7 did not directly prevent HHV-6 viremia, we did observe a delay in the median time to HHV-6 reactivation compared with historical controls.
In our previous study, we observed a higher risk of HHV-6 reactivation and delayed engraftment in patients receiving Siro/MMF immune prophylaxis [15]. Our main goal with the short-course of low-dose foscarnet was to suppress HHV-6 reactivation and improve hematopoietic recovery. Improved graft selection and perhaps the lower HHV-6 burden might have contributed to quicker and more successful engraftment as well.
It is unclear whether Siro/MMF is more immunosuppressive and therefore increases the risk of HHV-6 reactivation compared with GVHD prophylaxis with a calcineurin inhibitor (CNI)/MMF; however, the observed HHV-6 reactivation rate, but not the CMV reactivation rate, was high with this approach. Importantly, although CNI-based GVHD prophylaxis has also been associated with a high incidence of HHV-6 reactivation, concomitant foscarnet potentially would be more nephrotoxic and likely would compromise CNI administration.
Other approaches to decrease the incidence of HHV-6 reactivation and disease have been explored. Early studies suggested that HHV-6-related limbic encephalitis was mostly seen in UCB recipients when HHV-6 DNA copy number exceeded 1 × 104/mL [10]. Preemptive use of foscarnet in the pre-engraftment period was deemed safe in allogeneic HCT recipients but did not prevent HHV-6 encephalitis [11]. Subsequently, prophylactic foscarnet was added to either pre-engraftment regimens from day +7 to day +25 [12] or for 10 days from time of engraftment [13] in recipients of allogeneic HCT from different donor sources. Those strategies did not decrease the incidence of HHV-6 reactivation or encephalitis [12,13]. A multicenter single-arm observational study investigated the effect of pre-engraftment prophylactic foscarnet given at a dose of 90 mg/kg from day +7 until day +27 in the setting of myeloablative and RIC UCB HCT using a CNI as GVHD prophylaxis [14]. They reported a decrease in the incidence of high-level HHV-6 reactivation, defined as a plasma level ≥104 copies/mL at day +60, in patients receiving prophylactic foscarnet compared with historical controls; however, this strategy did not decrease the incidence of HHV-6 encephalitis [14].
Although promising and associated with successful engraftment, our prophylactic strategy did not prevent HHV-6 viremia, and if that is an important goal to limit morbidity, future investigations should focus on risk-adapted prevention measures, perhaps with new and more potent antiviral agents.
ACKNOWLEDGMENTS
This research was supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core of the Masonic Cancer Center, University of Minnesota and the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR002494. This work was supported in part by grants from the National Cancer Institute P01 CA65493 (C.G.B, J.E.W, T.E.D). We would like to acknowledge Michael Franklin, MS, for assistance in editing this manuscript.
Financial disclosure:
This research was supported by National Institutes of Health Grant P30 CA77598 to the Biostatistics and Bioinformatics Core of the Masonic Cancer Center, University of Minnesota and National Cancer Institute Grant P01 CA65493 (to C.G.B, J.E.W, and T.E.D). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Ogata M, Oshima K, Ikebe T, et al. Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:1563–1570 [DOI] [PubMed] [Google Scholar]
- 2.Betts BC, Young JAH, Ustun C, Cao Q, Weisdorf DJ. Human herpesvirus 6 infection after hematopoietic cell transplantation: is routine surveillance necessary? Biol Blood Marrow Transplant. 2011;17:1562–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inui Y, Yakushijin K, Okamura A, et al. Human herpesvirus 6 encephalitis in patients administered mycophenolate mofetil as prophylaxis for graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2019;21:e13024. [DOI] [PubMed] [Google Scholar]
- 4.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:932–940 [DOI] [PubMed] [Google Scholar]
- 5.Radonić A, Oswald O, Thulke S, et al. Infections with human herpesvirus 6 variant B delay platelet engraftment after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2005;131:480–482 [DOI] [PubMed] [Google Scholar]
- 6.Isomura H, Yoshida M, Namba H, et al. Suppressive effects of human herpesvirus-6 on thrombopoietin-inducible megakaryocytic colony formation in vitro. J Gen Virol. 2000;81(Pt 3):663–673 [DOI] [PubMed] [Google Scholar]
- 7.Isomura H, Yamada M, Yoshida M, et al. Suppressive effects of human herpesvirus 6 on in vitro colony formation of hematopoietic progenitor cells. J Med Virol. 1997;52:406–412 [DOI] [PubMed] [Google Scholar]
- 8.Lagadinou ED, Marangos M, Liga M, et al. Human herpesvirus 6-related pure red cell aplasia, secondary graft failure, and clinical severe immune suppression after allogeneic hematopoietic cell transplantation successfully treated with foscarnet. Transpl Infect Dis. 2010;12:437–440 [DOI] [PubMed] [Google Scholar]
- 9.Drobyski WR, Dunne WM, Burd EM, et al. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipients: evidence of a marrow-suppressive role for HHV-6 in vivo. J Infect Dis. 1993;167:735–739 [DOI] [PubMed] [Google Scholar]
- 10.Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193:68–79 [DOI] [PubMed] [Google Scholar]
- 11.Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant. 2011;46:863–869 [DOI] [PubMed] [Google Scholar]
- 12.Ishiyama K, Katagiri T, Ohata K, et al. Safety of pre-engraftment prophylactic foscarnet administration after allogeneic stem cell transplantation. Transpl Infect Dis. 2012;14:33–39 [DOI] [PubMed] [Google Scholar]
- 13.Ogata M, Satou T, Inoue Y, et al. Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transplant. 2013;48:257–264 [DOI] [PubMed] [Google Scholar]
- 14.Ogata M, Takano K, Moriuchi Y, et al. Effects of prophylactic foscarnet on human herpesvirus-6 reactivation and encephalitis in cord blood transplant recipients: a prospective multicenter trial with an historical control group. Biol Blood Marrow Transplant. 2018;24:1264–1273 [DOI] [PubMed] [Google Scholar]
- 15.Bejanyan N, Rogosheske J, DeFor TE, et al. Sirolimus and mycophenolate mofetil as calcineurin inhibitor-free graft-versus-host disease prophylaxis for reduced-intensity conditioning umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2016;22:2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bejanyan N, Rogosheske J, DeFor T, et al. Higher dose of mycophenolate mofetil reduces acute graft-versus-host disease in reduced-intensity conditioning double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2015;21:926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 16. 1997. 19971997:901–910 [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481 [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J R Stat Soc Series B Methodol. 1972;34:187–220 [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509 [Google Scholar]


