Abstract
Background:
Death is an important but often unmeasured endpoint in public health HIV surveillance. We sought to describe HIV among deaths using a novel mortuary-based approach in Nairobi, Kenya.
Methods:
Cadavers aged ≥15 years at death at Kenyatta National Hospital (KNH) and City Mortuaries were screened consecutively from January 29th to March 3rd 2015. Cause of death was abstracted from medical files and death notification forms. Cardiac blood was drawn and tested for HIV infection using the national HIV testing algorithm followed by viral load testing of HIV-positive samples.
Results:
Of 807 eligible cadavers, 610 (75.6%) had an HIV test result available. Cadavers from KNH had significantly higher HIV positivity at 23.2% (95% CI 19.3–27.7) compared to City Mortuary at 12.6% (95% CI 8.8–17.8), p<0.001. HIV prevalence was significantly higher among women than men at both City (33.3% versus 9.2%, p=0.008) and KNH Mortuary (28.8% versus 19.0%, p=0.025). Half (53.3%) of HIV infected cadavers had no diagnosis prior to death and an additional 22.2% were only diagnosed during hospitalization leading to death. Although not statistically significant, 61.9% of males had no prior diagnosis compared to 45.8% of females (p=0.144). Half (52.3%) of 44 cadavers at KNH with HIV diagnosis prior to death were on treatment, and one in five (22.7%) with a prior diagnosis had achieved viral suppression.
Conclusions:
HIV prevalence was high among deaths in Nairobi, especially among women, and prior diagnosis among cadavers was low. Establishing routine mortuary surveillance can contribute to monitoring HIV-associated deaths among cadavers sent to mortuaries.
Keywords: HIV infections, cadaver, ART use, Kenya, public health surveillance
Introduction
Globally, the number of people living with HIV (PLHIV) in 2015 was estimated at 36.7 million persons (1). Between 2010 and 2015, antiretroviral therapy (ART) use for HIV-infected persons increased by 126% in parallel with a 26% reduction in HIV-related deaths, from 1.5 million deaths in 2010 to 1.1 million deaths in 2015 (1,2). Sub-Saharan Africa bears the greatest burden of HIV. In 2015, there were 470,000 HIV-related deaths in East and Southern Africa representing 43% of global deaths due to HIV (1–3).
Kenya is estimated to have the fourth-largest HIV epidemic in the world, with 1.5 million PLHIV (4). The country reported an estimated 30% decline in HIV-related deaths between 2010 and 2015, from 52,314 to 35,822 respectively (5). This is attributed to the rapid increase of number of ART sites and increased ART coverage (6). However, HIV remains a serious public health challenge despite these achievements. Nairobi, the national capital, has the highest burden of HIV in the country with 171,510 residents estimated to be living with HIV and 2,500 HIV-related deaths occurring per year (5). Antiretroviral therapy coverage among PLHIV in Nairobi was estimated at 72% in 2014, nine percentage points below the treatment coverage target of 81% set in UNAIDS 90–90-90 fast-track targets for epidemic control by 2020 (6,7).
Although the impact of ART on mortality in African settings has been widely demonstrated (8–10) monitoring HIV-associated mortality is vital for tracking the success of the national ART program. Although standardized mathematical models based on indirect methods are used to estimate and project the number of people dying due to HIV (11), these models rely on assumptions that are subject to uncertainty, and can limit understanding of the true level of HIV-associated mortality. The national vital statistics system also tracks cause of death, but limited standardization of cause of death coding, lack of availability of HIV status at time of certification of cause of death, and the low estimated coverage of death reporting (<50%) limit the generalizability of these statistics (12,13). Early studies demonstrated the ability of enzyme-based assays to detect HIV in post mortem blood specimens (13–15). A mortuary-based surveillance system could help estimate the proportion of deaths that are HIV-associated, the proportion of deaths that are directly due to HIV, provide the actual age and sex distribution of HIV mortality, and relate HIV mortality to important factors such as viral suppression, ART use and HIV diagnosis, and hence to missed opportunities for epidemic control. We conducted a surveillance study in the two largest mortuaries in Nairobi. A prior publication from this study describes the fraction of deaths attributable to HIV in the Nairobi population by comparing the observed sero-prevalence among deaths with that expected based on demographic models (16). In this manuscript, we map HIV deaths to the steps in the cascade of care including HIV diagnosis, treatment, and achieving viral suppression. We describe the prevalence of HIV among cadavers by age, sex and other factors, and the proportion of HIV-infected cadavers whose underlying cause of death was classified as due to HIV and other causes. We also explore important behavioral and biological covariates such as HIV test history and viral suppression among HIV-associated deaths in order to better define the gaps that, if addressed, may help prevent excess HIV-related mortality.
MATERIALS AND METHODS
This study was conducted in the two largest mortuaries in Nairobi – Kenyatta National Hospital (KNH) Mortuary and City Mortuary covering 51% of all reported deaths in Nairobi county (18). The KNH Mortuary is attached to Kenyatta National Hospital which is a national public referral and teaching hospital, while City Mortuary is managed by the Nairobi County Health Services and receives cadavers from other facilities as well as medico-legal cases brought by the police. All cadavers admitted to both mortuaries were screened consecutively between January 29th and March 3rd 2015. Cadavers aged ≥ 15 years at time of death were eligible for the study. We abstracted cause of death and demographic variables from death notifications and pathology reports. For eligible deaths that occurred at KNH, additional variables regarding history of HIV diagnosis and ART were abstracted from patient files. Cadavers brought in by the police (which require autopsy to establish cause of death) are referred to as medico-legal cases and are generally taken to City Mortuary.
Cardiac blood samples were collected for this study using transthoracic aspiration; although cadavers were not excluded based on time from death to admission or collection, those from which HIV test results were not obtained were excluded from analysis. HIV testing was conducted using the national diagnostic algorithm (17): screening was done using Colloidal Gold assay (KHB Shangai Kehua Bio-Engineering Co, Ltd, Shanghai, China), confirmation of HIV-positive samples was done using First Response HIV 1–2-0 assay (PMC Medical India Pvt Ltd, Mumbai, India), and samples with discrepant results were tested using Unigold HIV assay (Trinity Biotech PLC, Bray, Ireland) as a tie breaker. HIV RNA testing was performed on HIV-positive samples using the Abbott m2000 real time RNA system (Abbott Molecular, Inc., Des Plaines, IL) to determine the HIV RNA concentration. Viral suppression was defined as HIV-1 RNA concentration < 1000 copies/milliliter.
Measures
Sex, age, and cause of death were abstracted from death certificates completed by the attending physician or, in case of autopsy, by the pathologist. Where age was not available from records it was estimated by mortuary technicians. Prior HIV diagnosis and ART use were ascertained from the clinical history documented in patient files for deaths occurring at KNH. HIV positivity was based on HIV serology performed during the study.
Data analysis
Data were analyzed in Stata 14 (StataCorp, Texas, USA). We conducted bivariate analysis to assess the relationship between HIV and select characteristics, and the chi-square test for independence and Fisher’s exact test were used to assess statistical significance. The logit transformation was used to compute confidence interval limits. Multiple logistic regression was used to further explore associations between characteristics and outcome of HIV status. Predictive margins of HIV prevalence as a function of sex and age group were plotted to describe interaction between these variables when predicting HIV status of cadavers. In stratified analyses, the numerator, proportion and confidence intervals were replaced with an asterisk where the denominator was less than 20.
Ethics approvals
This public health surveillance study was approved by the US Centers for Disease Control and Prevention Center for Global Health Associate Director for Science as research that did not involve human subjects (because subjects were deceased). Kenyatta National Hospital/University of Nairobi Ethical Review Board also approved the study. Nairobi County Health Services and the Kenyatta National Hospital Research Office gave administrative approval.
RESULTS
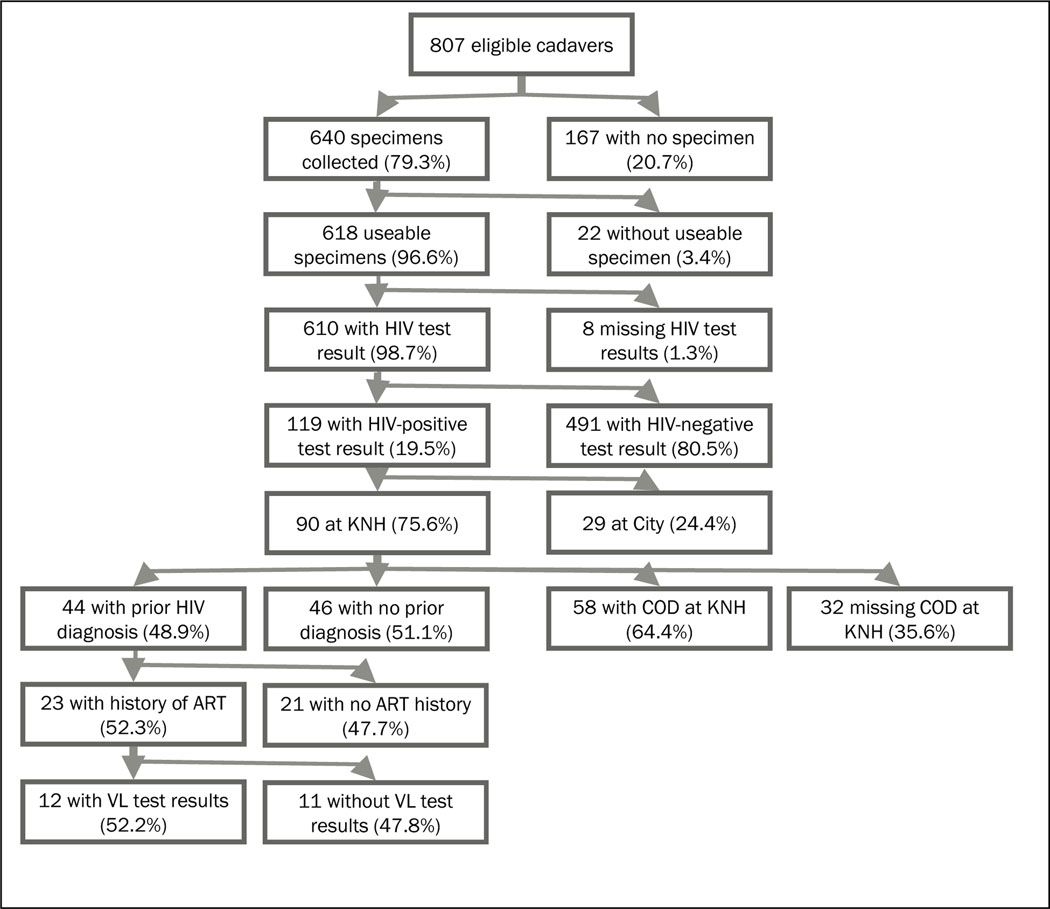
A total of 807 cadavers received at the mortuaries were found to be eligible and enrolled into the study (Figure 1). Specimens were not available for 167 (20.7%) cadavers due to various reasons including logistical/staffing (n=91), extensive burns or putrefaction (n=26), other/legal/discharge or transfer of cadaver (n=31), and failed collection attempt (n=19). Of the 640 (79.3%) with a blood specimen collected, 610 (95.3%) had sufficient quality for HIV testing, and 119 (19.5%) were HIV-positive. Of the 119 HIV-positive specimens, 90 (75.6%) were from cadavers at KNH mortuary for which medical records were abstracted. Of the 90 HIV-positive specimens at KNH, 44 (48.9%) had a prior HIV diagnosis, 23 of whom had a documented history of ART use, while of these, 12 had viral load testing results available in the study.
Figure 1:
Data flow of eligible cadavers aged ≥ 15 years, HIV mortuary survey.
Notes: KNH = Kenyatta National Hospital, ART = antiretroviral treatment, VL = viral load, COD = cause of death
Cadavers were more likely to be males at City (86.0%) versus KNH (67.2%, p<0.001). Cadavers also differed between mortuaries in terms of age: the median age was 33 years at City Mortuary (inter-quartile range [IQR] 28–42) and 44.5 years at KNH (IQR 33–59.5, p<0.001) (Table 1). Given the demographic differences between the deaths enrolled at City and KNH mortuaries, the remaining analyses are stratified by mortuary.
Table 1.
Demographic characteristics of cadavers aged ≥ 15 years by mortuary, HIV mortuary surveillance study, Nairobi, Kenya 2015 (n=610)
| City Mortuary | KNH† Mortuary | Total | Significance* | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Selected characteristics | n | (%) | n | (%) | n | (%) | χ2 (degrees of freedom), p-value |
|
| |||||||
| Sex | |||||||
| Male | 184 | (86.0) | 226 | (57.1) | 410 | (67.2) | χ2 (1) = 52.7, p<0.001 |
| Female | 30 | (14.0) | 170 | (42.9) | 200 | (32.8) | |
| Total | 214 | 396 | 610 | ||||
| Age in years | |||||||
| 15–24 | 36 | (16.8) | 26 | (6.6) | 62 | (10.2) | χ2 (5) = 64.3, p<0.001 |
| 25–34 | 75 | (35.0) | 79 | (19.9) | 154 | (25.2) | |
| 35–44 | 57 | (26.6) | 93 | (23.5) | 150 | (24.6) | |
| 45–54 | 30 | (14.0) | 72 | (18.2) | 102 | (16.7) | |
| 55–74 | 12 | (5.6) | 89 | (22.5) | 101 | (16.6) | |
| 75+ | 4 | (1.9) | 37 | (9.3) | 41 | (6.7) | |
| Total | 214 | 396 | 610 | ||||
| Median (IQR)‡ | 33.0 | (28.0–42.0) | 44.5 | (33.0–59.5) | 40.0 | (33.0–53.0) | |
| Medico-legal cases | |||||||
| Yes | 164 | (76.6) | n/a | n/a | 164 | (76.6) | n/a |
| No | 50 | (23.4) | n/a | n/a | 50 | (23.4) | |
| Total | 214 | n/a | n/a | 214 | |||
Notes:
chi-square test for independence between mortuary and sex or age.
KNH = Kenyatta National Hospital.
IQR = Inter-quartile range.
HIV prevalence differed by sex at both mortuaries: among 30 females at City Mortuary, HIV positivity was 33.2%, while among 184 males it was 9.2% (p=0.008); at KNH, HIV positivity was 28.8% among 170 females, compared with 19.0% among 226 males (p=0.025) (Table 2). Specimen availability was not associated with age, sex or mortuary but specimens were less likely to be available for medico-legal cases at City Mortuary (p=0.004) (Table S1). At City Mortuary, the HIV positivity was significantly higher among cadavers admitted as non-medico-legal cases at 30% (95% CI 18.9–44.1) compared to medico-legal cases at 7.3% (95% CI 4.2–12.5), p=0.001.
Table 2.
HIV positivity rates among cadavers aged ≥ 15 years at KNH and City Mortuaries, Nairobi, Kenya 2015 (n=610)
| City Mortuary | Significance† | KNH Mortuary | Significance† | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Selected characteristics | n | % | (95% CI) | F(df1,df2), p-value | n | % | (95% CI) | F(df1,df2), p-value |
|
| ||||||||
| Sex | ||||||||
| Male | 184 | 9.2 | (5.8–14.4) | F(1,609) = 7.15, p=0.008‡ | 226 | 19.0 | (14.4–24.7) | F(1,609) = 5.06, p=0.025‡ |
| Female | 30 | 33.3 | (18.9–51.8) | 170 | 28.8 | (22.5–36.1) | ||
| Male | ||||||||
| 15–24 | 30 | 6.7 | (1.7–23.2) | F(2,609) = 0.21, p=0.813§ | 17 | 11.8 | (2.9–37.0) | F(2,609) = 0.69, p=0.502§ |
| 25–44 | 119 | 10.1 | (5.8–17.0) | 96 | 21.9 | (14.7–31.3) | ||
| 45+ | 35 | 8.6 | (2.8–23.5) | 113 | 17.7 | (11.7–25.9) | ||
| Total | 184 | 9.2 | (5.8–14.4) | 226 | 19.0 | (14.4–24.7) | ||
| Female | ||||||||
| 15–24 | 6 | 50 | (16.6–83.4) | F(2,609) = 3.19, p=0.042§ | 9 | 33.3 | (11.0–66.9) | F(2,609) = 11.98, p<0.001§ |
| 25–44 | 13 | 46.2 | (22.2–72.0) | 76 | 46.1 | (35.1–57.4) | ||
| 45+ | 11 | 9.1 | (1.2–44.3) | 85 | 12.9 | (7.3–22.0) | ||
| Total | 30 | 33.3 | (18.9–51.8) | 170 | 28.8 | (22.5–36.1) | ||
| Age in years | ||||||||
| 15–24 | 36 | 13.9 | (5.9–29.4) | F(2,609) = 0.50, p=0.604║ | 26 | 19.2 | (8.2–38.8) | F(2,609) = 7.35, p<0.001║ |
| 25–44 | 132 | 13.6 | (8.8–20.6) | 172 | 32.6 | (26.0–39.9) | ||
| 45+ | 46 | 8.7 | (3.3–21.0) | 198 | 15.7 | (11.2–21.4) | ||
| Total | 214 | 12.6 | (8.8–17.8) | 396 | 23.2 | (19.3–27.7) | ||
| Police case | ||||||||
| Yes | 164 | 7.3 | (4.2–12.5) | F(1,213)=10.94, p=0.001¶ | n/a | F(1,213)=10.94, p=0.001¶ | ||
| No | 50 | 30.0 | (18.9–44.1) | n/a | ||||
| Total | 214 | 12.6 | (8.8–17.8) | n/a | ||||
| Total | 214 | 12.6 | (8.8–17.8) | n/a | 396 | 23.2 | (19.3–27.7) | F(1,609)=11.63, p<0.001 π |
Notes:
n/percentages not shown are based on <20 cases.
F-tests for differences in HIV prevalence by group as follows:
by mortuary, sex
by mortuary and sex, age
by mortuary, age;
by medico-legal status (at City mortuary only)
overall between mortuaries.
As medical chart abstraction was performed for deaths at KNH, additional HIV diagnosis and care information was available for HIV-infected deaths at this mortuary (Table 3). About one quarter (24.4%) of HIV-infected deaths had a diagnosis prior to the hospitalization just prior to their death, while an additional 22.2% were diagnosed while hospitalized. The remaining half (53.3%) did not have a diagnosis prior to death. Although 61.9% of males had no prior diagnosis compared to 45.8% of females, the difference was not significant (p=0.144). Two thirds (66.7%) of HIV infected cadavers aged 45 years and above did not did not have a prior HIV diagnosis, while under half (46.7%) of those aged between 15–44 years had no prior HIV diagnosis, again the difference was not significant (p=0.079). In contrast, the majority (84.1%) of the deaths where HIV was assigned as the underlying cause had a prior HIV diagnosis, compared to only 35.7% being diagnosed among those for which the underlying cause assigned was not HIV (p=0.001).
Table 3.
HIV testing history among HIV-infected cadavers at KNH Mortuary, Nairobi, 2015 (N=90)
| Ever Diagnosed with HIV | No prior HIV diagnosis | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Prior to hospitalization | During hospitalization |
Total | Fischer’s exact test p-value† | |||||||
| Selected characteristics | % | (95% CI) | % | (95% CI) | % | (95% CI) | % | (95% CI) | n | |
|
| ||||||||||
| Sex | ||||||||||
| Male | 16.7 | (8.0–31.4) | 21.4 | (11.4–36.6) | 38.1 | (24.6–53.7) | 61.9 | (46.3–75.4) | 42 | |
| Female | 31.3 | (19.6–45.9) | 22.9 | (13.0–37.1) | 54.2 | (39.8–67.8) | 45.8 | (32.2–60.2) | 48 | p=0.144 |
| Total | 24.4 | (16.5–34.6) | 22.2 | (14.7–32.2) | 46.7 | (36.5–57.2) | 53.3 | (42.8–63.5) | 90 | |
| Age | ||||||||||
| 15–44 | 25 | (15.5–37.7) | 28.3 | (18.2–41.2) | 53.3 | (40.5–65.7) | 46.7 | (34.3–59.5) | 60 | |
| 45+ | 23.3 | (11.4–41.9) | 10 | (3.2–27.3) | 33.3 | (48.0–81.3) | 66.7 | (31.0–57.1) | 30 | p=0.079 |
| Total | 24.4 | (16.5–34.6) | 22.2 | (14.7–32.2) | 46.7 | (42.8–63.5) | 53.3 | (48.0–81.3) | 90 | |
| Underlying cause of death* | ||||||||||
| Death due to HIV | 43.2 | (29.1–58.4) | 40.9 | (27.1–56.3) | 84.1 | (69.7–92.4) | 15.9 | (7.6–30.3) | 44 | p=0.001 |
| Death due to any other cause | 21.4 | (6.8–50.4) | 14.3 | (3.4–43.8) | 35.7 | (15.3–63.2) | 64.3 | (36.8–84.7) | 14 | |
| Total | 37.9 | (26.1–51.4) | 34.5 | (23.1–47.9) | 72.4 | (59.2–82.6) | 27.6 | (17.4–40.8) | 58 | |
Notes:
cause of death was only available for 58 HIV-positive cadavers with HIV testing history available.
test for difference between having prior HIV diagnosis or not and selected characteristic.
In a multiple logistic regression model including mortuary, age and sex as independent predictors, the adjusted odds of HIV infection were twice as high at KNH Mortuary compared with City Mortuary (adjusted odds ratio [aOR] 1.80, 95% CI 1.08–3.01), higher for women than men (aOR=6.39, 95% CI 1.47–27.68) among cadavers aged 15–24 years old, and significantly lower for cadavers aged 45+ years (aOR=0.18, 95% CI 1.64–18.67) versus 15–24 years among women (Table S2). There was a significant interaction between sex and age, consistent with higher prevalence among younger females and older males. When modeling finer-grained age groups, the peak risk of HIV infection for females was seen in the 35–44 years age group, while for males it was in the 45–54 years age group (Figure S1). No significant interaction was found between sex and mortuary.
Half (52.3%) of the 44 cadavers with HIV diagnosis prior to death were on treatment, although treatment status was missing for 10 cadavers, so the true prevalence of treatment may have been higher. Among those with a prior HIV diagnosis, 10 had achieved viral suppression (Table 4). Only two of the 23 with evidence of treatment were not virally suppressed at time of death, though an additional 11 cadavers did not have viral load available.
Table 4.
HIV testing and ART history among cadavers diagnosed with HIV prior to death, KNH Mortuary, Nairobi, 2015 (N=44)
| Indicator | n | % | (95% CI) |
|---|---|---|---|
|
| |||
| Total treated for HIV | 23 | 52.3 | (37.6–66.5) |
| Treated and virally suppressed | 10 | 22.7 | (12.6–37.4) |
| Treated and not suppressed | 2 | 4.5 | (1.1–16.6) |
| Treated and viral load unknown | 11 | 25.0 | (14.4–39.9) |
| Not treated | 11 | 25.0 | (14.4–39.9) |
| Treatment unknown | 10 | 22.7 | (12.6–37.4) |
Notes: Includes two cases excluded from previous table where timing of HIV diagnosis was not known
DISCUSSION
In this study we estimated HIV positivity in cadavers at the two largest mortuaries in Nairobi, HIV diagnosis prior to death, subsequent use of ART and viral suppression for hospital-based deaths. Females continue to bear the greatest burden of HIV infection. The percentage of female cadavers that were HIV-infected was greater than that for males at both mortuaries. While greater HIV-associated mortality in women than men was not surprising earlier in the epidemic, given the high ART coverage, especially among women, further research is warranted to confirm and interpret this finding.
We found that one in eight cadavers at City Mortuary and one in four cadavers at KNH Mortuary were HIV infected. The higher HIV positivity among cadavers at KNH compared to City Mortuary may be due to KNH’s status as a referral hospital, the majority of whose patients are hospitalized prior to death and who might have sought care for HIV complications, while conversely City Mortuary primarily receives medico-legal deaths, which are likely to be due to external causes.
The last national survey conducted in 2012 estimated HIV prevalence among adults at 4.9% and that only 57.5% of adults living with HIV in Kenya had been previously diagnosed (18). We found that more than eight in ten hospitalized patients for whom the underlying cause of death assigned was HIV had been diagnosed prior to death, however only about half of these were known to have been diagnosed prior to admission. The remaining 15.9% of deaths due to HIV did not have a documented HIV diagnosis at time of death. These scenarios represent missed opportunities for diagnosis prior to life-threatening illness and death. Among those with prior HIV diagnosis, the approximately half of deaths with no evidence of treatment represent additional missed opportunities to save lives. About one fifth of those with a prior HIV diagnosis were on treatment and virally suppressed at time of death. These deaths are difficult to explain in the absence of a detailed clinical history, including duration on treatment, and diagnosed comorbidities and opportunistic infections.
The study had important limitations. Medical history was only available for hospital-based deaths at KNH and in those cases, was limited to the inpatient records for their hospitalization just prior to death, representing only a third (199/610) of all deaths with HIV test results captured in this study, and 50% (199/396) of those captured at KNH Mortuary. This limited our ability to establish whether a detectable viral load was due to treatment failure, poor adherence, or lack of treatment. Cause of death was also missing for 38.5% (235/610) of sampled mortuary deaths. Our study relied on routinely recorded causes of death, and full autopsies were not performed specifically for this study, so the underlying cause of death may have been ascertained incorrectly. While we included the two largest mortuaries in Nairobi in the study, a demographic analysis reported elsewhere found coverage to be 51% of expected deaths in the city (16), hence our findings are not necessarily representative of all deaths in Nairobi. Although inclusion of City Mortuary may have led to over-representation of external causes among males, excluding City from the analysis did not have a significant impact on the association between sex and death due to HIV. Additionally, the fact that three-quarters of the deaths at City Mortuary were medico-legal cases may also explain why no deaths were assigned HIV as a cause of death at this mortuary.
In spite of these limitations, the implementation of a novel mortuary-based surveillance study in Kenya provided an estimate of the proportion of HIV-related deaths at the two largest mortuaries in Nairobi, as well as important ancillary information on ART use, viral load, and diagnosis of HIV prior to death. Although studies have documented a reduction in HIV associated mortality attributable to ART, the prevalence of HIV among deaths in Nairobi is still higher than in the general population (19–22). In assessing impact of HIV treatment programs, measurement of mortality is rarely undertaken. As national ART programs continue to scale-up in an effort to save lives and achieve epidemic control, it is critical that appropriate surveillance systems are implemented that can monitor reductions in mortality as well as the patterns of specific causes of death among PLHIV, including those that are not being reached by care and treatment programs. Although measuring the level of HIV infection among deaths can help interpret declining trends in HIV prevalence in Nairobi, increasing the representativeness of the catchment of mortuary surveillance and developing methods for generalizing to the population of all deaths in the community is key. Although strengthened national vital statistics and HIV case-based surveillance would provide more robust and comprehensive data, establishment of routine mortality surveillance in sentinel sites could play an important role in monitoring outcomes among people living with HIV in Kenya. This study shows that routine testing of cadavers and linking results to clinical records is a feasible approach that can provide important insights into the levels of HIV-associated mortality in the era of widespread ART availability. The findings also highlight the need to determine why PLHIV continue to experience high mortality even as ART is believed to be widely accessible, so that appropriate actions can be taken.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contributions of the mortuary and laboratory staff for without whom this manuscript would not have been possible. We would also like to appreciate the thoughtful comments of reviewers and referees that improved the manuscript.
Funding
This publication was made possible by support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreements [#PS001814, GH000069].
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Footnotes
Declaration of Interests
All authors declare no competing interests.
REFERENCES
- 1.UNAIDS. Global AIDS Update. 2016. Available from: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf
- 2.UNAIDS_FactSheet_en.pdf. [cited 2016 Oct 11]. Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- 3.UNAIDS. AIDS by the numbers, AIDS is not over, but it can be 2016. Available from: http://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf
- 4.UNAIDS. AIDSinfo Online Database. 2017. [cited 2017 May 5]. Available from: aidsinfoonline.org [Google Scholar]
- 5.National AIDS Control Council (NACC) Kenya HIV Estimates, 2015. Kenya: NACC; 2016 Oct. [Google Scholar]
- 6.UNAIDS. 90–90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. Oct [cited 2016 Aug 19]. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf [Google Scholar]
- 7.UNAIDS. Fast-Track: Ending the epidemic by 2030. Available from: http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf
- 8.Floyd S, Marston M, Baisley K, et al. The effect of antiretroviral therapy provision on all-cause, AIDS and non-AIDS mortality at the population level – a comparative analysis of data from four settings in Southern and East Africa. Tropical Medicine and International Health 2012; 17(8): E84–E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gargano JW, Laserson K, Muttai H, et al. The adult population impact of HIV care and antiretroviral therapy in a resource poor setting, 2003–2008. AIDS 2012, 26:1545–1554. [DOI] [PubMed] [Google Scholar]
- 10.Reniers G, Slaymaker E, Nakiyingi-Miiro J, et al. Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA). AIDS 2014; 28 (Suppl 4):S533–S542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stover J, Andreev K, Slaymaker E, et al. Updates to the Spectrum model to estimate key HIV indicators for adults and children. AIDS. 2014;28(4):S427–S434. doi: 10.1097/QAD.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Civil Registration Services (CRS). Kenya vital statistics report 2014. Nairobi, Kenya: Civil Registration Services, CRS; Statistics Division. Government of Kenya; 2015. [Google Scholar]
- 13.Karhunen PJ, Brummer-Korvenkontio H, Leinikki P, Nyberg M. Stability of human immunodeficiency virus (HIV) antibodies in postmortem samples. J Forensic Sci. 1994. [PubMed] [Google Scholar]
- 14.Klatt EC, Shibata D, Strigle SM. Postmortem enzyme immunoassay for human immunodeficiency virus. Arch Pathol Lab Med. 1989. doi:S0004-27302009000500011 [pii] [PubMed] [Google Scholar]
- 15.Leshchinskaia N, Smol’skaia T. [Postmortem detection of antibodies to human immunodeficiency virus]. Klin Lab Diagn. 1993;May-Jun(3):47–50. [PubMed] [Google Scholar]
- 16.Young PW, Kim AA, Wamicwe J, et al. HIV-associated mortality in the era of antiretroviral therapy scale-up – Nairobi, Kenya, 2015. PLoS One. 2017. doi: 10.1371/journal.pone.0181837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health and Sanitation, Kenya. Circular No. MPHS/ADM/1/12. Nairobi, Kenya: NASCOP; 2013. Feb. [Google Scholar]
- 18.Kim AA, Mukui I, Young PW, et al. Undisclosed HIV infection and art use in the Kenya AIDS indicator survey 2012: relevance to targets for HIV diagnosis and treatment in Kenya. AIDS. 2016:1. doi: 10.1097/QAD.0000000000001227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mberu B, Wamukoya M, Oti S, Kyobutungi C. Trends in causes of adult deaths among the urban poor: Evidence from Nairobi Urban Health and Demographic Surveillance System, 2003–2012. J Urban Heal. 2015. doi: 10.1007/s11524-015-9943-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubaihayo J, Tumwesigye NM, Konde-Lule J, et al. Trends and predictors of mortality among HIV positive patients in the era of highly active antiretroviral therapy in Uganda. Infect Dis Rep. 2015. doi: 10.4081/idr.2015.5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salihu HM. Global reduction in HIV-related maternal mortality: ART as a key strategy. Int J MCH AIDS. 2015;4(2):8. [PMC free article] [PubMed] [Google Scholar]
- 22.Rubaihayo J, Tumwesigye NM, Konde-Lule J, et al. Trends and predictors of mortality among HIV positive patients in the era of highly active antiretroviral therapy in Uganda. Infect Dis Rep. 2015. doi: 10.4081/idr.2015.5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.