Abstract
Purpose:
The primary goal of this study was to investigate whether chronic exposures to ultra-high B0 fields can induce long-term cognitive, behavioral or biological changes in C57BL/6 mice.
Methods:
C57BL/6 mice were chronically exposed to 10.5 T or 16.4 T magnetic fields (3-hour exposures, 2 exposure sessions per week, 4 or 8 weeks of exposure). In vivo single-voxel 1H MRS was used to investigate possible neurochemical changes in the hippocampus. In addition, a battery of behavioral tests, including Morris water maze, balance beam, rotarod and fear conditioning, were used to examine long-term changes induced by B0 exposures.
Results:
Hippocampal neurochemical profile, cognitive and basic motor functions were not impaired by chronic magnetic field exposures. However, the balance beam walking test and the Morris water maze testing revealed B0 induced changes in motor coordination and balance. The tight-circling locomotor behavior during Morris water maze tests was found as the most sensitive factor indexing B0 induced changes. Long-term behavioral changes were observed days or even weeks subsequent to the last B0 exposure at 16.4 T but not at 10.5 T. Fast motion of mice in and out of the 16.4T magnet was not sufficient to induce such changes.
Conclusions:
Observed results suggest that the chronic exposure to magnetic field as high as 16.4 T may result in long-term impairment of the vestibular system in mice. Although observation on mice may not directly translate to humans, nevertheless, they indicate that studies focused on human subject safety at very high magnetic fields are necessary.
Keywords: chronic exposure, ultra-high magnetic fields, neurochemical profiling, battery of behavioral test, vestibular system, tight circling
1. INTRODUCTION
Since the introduction of the first 7 tesla magnet for human imaging approximately two decades ago (1), research at this ultra-high magnetic field has demonstrated unique advantages for numerous applications in MR imaging of the human body, particularly functional brain mapping (1–3) and brain MR spectroscopy (4,5). This progress ultimately led to clearance of 7T MRI scanners for clinical use for the human brain and extremities in the United States and the European Union. Based on the successes achieved at 7 T, and anticipated increases in the signal-to-noise ratio with magnetic fields (4–7) there is growing interest in moving significantly beyond 7 T to magnetic fields of 10.5 T (8–10) and beyond. Such a move, however, raises critically important questions about the safety of subjects exposed to such high static magnetic field (B0).
To date, there is no reported evidence that ultra-high B0 field significantly affects vital signs (11,12) and cognitive function (13). A comprehensive investigation of cognitive, vestibular and physiological functions of subjects exposed to 10.5 T has been recently published (14). Initial results of this study suggested that the exposure of research subjects to 10.5 T does not have major or unexpected effects that would slow or halt further enrollment in imaging studies of the head and torso at 10.5 T (8,10). However, increased B0 strength does enhance adverse sensory effects, such as vertigo, dizziness, nausea, metallic taste, nystagmus and magnetophosphenes (13, 15–18). These adverse subjective sensory perceptions affect not only subjects of MRI examination, but also those working in the fringe field surrounding magnets (19).
The effects of B0 on biological and behavioral functions have been investigated using several animal models (20–31). Although adverse biological effects were not observed in male and female adult rats or their progeny when exposed sub-chronically to the 9.4T field (20), rats and mice exposed to ultra-high B0 (4 – 20 T) showed tight circling in an open field (26,27) or circling swimming in a pool (31) immediately after removal from the magnet. The direction of circling (counter-clockwise or clockwise) depended on the B0 orientation (parallel or anti-parallel) relative to the rostral – caudal body axis (25–27). The amplitude of circular motion was modulated by the angle between B0 and rostral – caudal body axis, with maximal and minimal effects corresponding to parallel and perpendicular orientation, respectively (31). Such a circling locomotion has been observed only within a short period (< 3 min) after removal from the magnet (25). Partially restrained rats that were able to move their head and neck showed an immediate and persistent deviation of the head position (rightward tilt if parallel to B0, leftward tilt if anti-parallel to B0) in a 14.1T magnet (22). The tilt of the head during magnet exposure was opposite to the direction of locomotor circling. Fast motion of rats across a steep B0 gradient (inserting and removing from the 14.1T magnet) suppressed the rearing, but was not sufficient to induce the acute circling after removal from the magnet (21). It has been reported that with the exception of circling locomotor activity, mice unrestrained during a 30-min 14.1 T exposure exhibited larger behavioral effects (rearing, conditioned taste aversion) than the restrained group (32). However, the opposite results were observed for partially restrained rats that were exposed with their heads free (22). Repeated exposures of rats to 14.1 T (three 30-min exposures) attenuated the behavioral responses (23). In addition, altered swimming behavior was also observed in zebrafish inside of strong magnets (29). Last but not least, B0 field induced nystagmus was recently studied in mice inside the 4.7T magnet. The direction of nystagmus depended on head orientation relative to the B0 field. It has been recently reported that a long-term continuous exposure (28 days) of mice to high magnetic fields (2 T – 12 T) did not affect routine blood tests and the function of the liver, kidneys or lipid metabolism (30). In addition, histomorphological changes or pathological damage were not observed in any of several mouse organs analyzed (heart, brain, liver, spleen, kidney, lung, testicle and femur) after continuous 28-day-long exposure to the B0 field (30).
The sensation of vertigo (perception of self-motion) reported by MRI subjects and MR technologists as well as the circling locomotion behavior of rodents removed from the magnet suggest direct involvement of the vestibular system in the B0 field interaction with the human or animal body (33). The first mechanism of the vestibular system interaction with the B0 field was proposed nearly three decades ago (34). Since then other mechanisms of magnetic vestibular stimulation have been proposed: (a) forces owing to differences in diamagnetic susceptibility inside the vestibular system, (b) induced electrical currents (Faraday’s law), and (c) motion-induced magnetohydrodynamic effects in endolymph (35). Each of these three mechanisms requires either temporally or spatially varying magnetic field or head motion. However, it has been discovered that all humans with an intact labyrinth experience a long-term robust and persistent nystagmus (involuntary, rhythmic movement of the eyes typically observed during head rotation) when exposed to homogeneous B0 field without any motion (36,37). The direction and the slow phase eye velocity depend on head orientation relative to B0. The proposed mechanism for this effect involves a Lorentz force resulting from the interaction of a strong B0 field with naturally occurring ionic currents flowing through the inner ear endolymph into vestibular hair cells (33,37). The Lorentz force causes the endolymph in the superior and lateral semicircular canals to exert pressure on the cupula, inducing its constant deflection (27,32). Deflection of the cupula bends the underlying hair cells either towards or away from the kinocilium, thus mechanically opening gated ion channels on the hair cells and initiating afferent vestibular signaling. This inner ear stimulation creates a sensation of rotation, and a constant horizontal/torsional nystagmus observed in humans and rodents even in a static magnetic field without any head motion (38,39). The sensations of nausea and dizziness are likely to be the result of semicircular canal inputs to the brain and the conflict with other inputs (from otolith organs and the visual system) rather than a direct consequence of magnetic fields interacting with the central nervous system (36,39,40). The proposed mechanism of the vestibular system interaction with the B0 field mediated by the Lorentz force can also explain why the circling locomotor behavior of rats correlated with the duration of the B0 exposure and not with motion through the B0 gradient (21).
Except for the nystagmus, which has been observed to persist during the entire testing period of human subjects inside of the magnet (25 min) (37), the self-motion perception (vertigo) was reported only for a short period of time after entering the B0 field or shortly after removal from the field. The tight circling locomotor behavior of rodents (in open field or during swimming in a pool) has been investigated and observed only after immediate removal from the B0 field (25–27,31). Thus, knowledge about the persistence of and recovery from adverse effects subsequent to B0 exposure is sparse. The primary goal of this study was to investigate whether chronic exposures to ultra-high B0 fields could induce long-term cognitive, behavioral or biological changes. This idea and the rationale behind this type of investigations was driven by the human subject safety concerns when the human research at ultra-high B0 fields started to explore magnetic fields beyond 7 T (9.4 T – Tübingen and Minneapolis, 10.5 T Minneapolis). Effects of the chronic exposure to ultra-high B0 fields were investigated at 10.5 T and 16.4 T using C57BL/6 mice. Testing of mice included 1H magnetic resonance spectroscopy (MRS) and a battery of behavioral tests (Morris water maze, rotarod, balance beam, and fear conditioning).
2. METHODS
2.1. Protocols for magnetic field exposure and testing
The data presented in the paper are a result of animal experiments conducted under two separate protocols. Results from Protocol I informed and led to a modified study design for Protocol II (Figure 1). Both protocols were used to study the long-term effects of chronic exposure to ultra-high B0 fields. All experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota.
Figure 1.
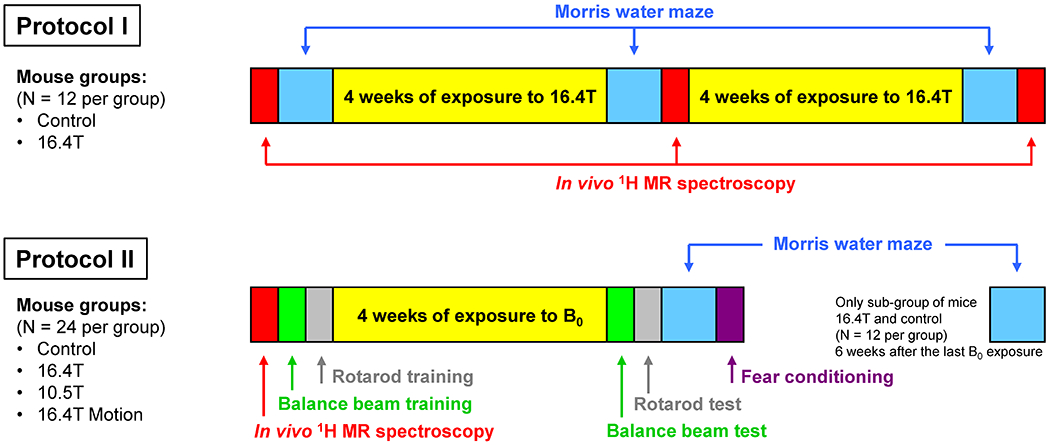
Two protocols for study design used to investigate chronic exposures of C57BL/6 mice to ultra-high magnetic fields that include 1H MRS and a battery of behavioral tests. Protocol I – mice were exposed to 16.4 T for 3 hours per session, two sessions per week, sham exposures were applied for control mice. Protocol II – one group of mice was exposed to 10.5 T, another group of mice were exposed to 16.4 T, each exposure was 3 hours long, and two exposures were applied per week. Control group of mice underwent sham exposures of the same duration. The forth group of mice was exposed to 16.4 T only for a short time (~2 min) that included a vigorous movement of the mouse holder twenty times in and out of the 16.4 T magnet. For a subgroup of mice (16.4T, control, N = 12 each), the MWM test was repeated 6 weeks after the last B0 exposure.
2.1.1. Protocol I
Protocol I used two 4-week long periods with repeated exposures to 16.4 T magnetic field (number of mice N = 12, 3-hour long exposure sessions, two exposure sessions per week). In order to minimize any confounding factors, the control group of mice (N = 12) was exposed to a sham magnetic field using a plastic tube mimicking the magnet bore and exactly the same schedule of exposures as the 16.4T group. Mice were not sedated during the B0 exposure and therefore they could move freely inside the plastic bins of the animal holder, though due to the limited space, their movements would have been somewhat restricted Figure S1). The ability to move during the B0 exposure may have affected the B0 field effects (22,32). Two different methods were used to investigate the long-term effects of the chronic exposure to 16.4 T magnetic field. First, in vivo 1H MRS was utilized to examine the effects of the chronic B0 exposure on brain neurochemistry. Second, the widely used Morris water maze (MWM) test, which is considered as the gold standard in mouse behavioral neuroscience, was chosen as a measure of cognitive ability (41). Both tests were applied before the exposure, after 4-week long exposure to 16.4 T and once again after the second 4-week long exposure to 16.4 T (Figure 1, Protocol I). Mice were 2 and 3 months old when the first and second B0 exposure periods started, respectively.
2.1.2. Protocol II
Protocol II (Figure 1) used only one 4-week long period with repeated exposures of to the B0 field (two exposures per week). This protocol was used with four different groups of mice (N = 12 each). The first three groups followed the same procedures and were exposed to 10.5 T, 16.4 T and a sham magnetic field (Control group) for three hours per session and two sessions per week. The forth mouse group (labeled as 16.4T Motion) was repeatedly exposed to 16.4T (two exposure sessions per week), but instead of three hours per session, exposures lasted for about 2 min. During this 2-min period, the mouse holder was swiftly pushed in and pulled out of the 16.4T magnet 20 times. For protocol II, 1H MRS was used only before the B0 exposure for screening purposes to eliminate mice with high concentration of brain glutamine that randomly appear in the C57BL/6 mouse strain (42). Eliminated mice were replaced from a backup mouse group.
In Protocol II, the MWM test was performed only once after the final exposure to different magnetic fields. In addition, the behavioral testing was expanded to include the balance beam walking, rotarod and fear conditioning tests. Studied mice were first trained for the balance beam and rotarod testing and then they were chronically exposed (4 weeks, 8 exposures total) to the B0 field. The battery of behavioral tests started the next day after the last B0 exposure in the following order: balance beam, rotarod, MWM, and fear conditioning. The whole B0 exposure and testing Protocol II was repeated a second time with another batch of mice to increase the number of mice in each group to N = 24. There was only a small difference in the mouse age between the first and second batch of mice when the B0 field exposure began (1st batch: 2 months old, 2nd batch: 3 months old). For a subgroup of mice (16.4T, control) from the second batch of animals, the MWM test was repeated 40 days after the last B0 exposure.
Out of 96 mice enrolled in this study protocol, only 90 animals accomplished all tests and were included in final statistical analysis. One mouse died due to drowning during MWM testing, one male mouse was injured due to a fight in the cage and four mice were excluded from the study due to poor performances during the balance beam walking training session before the magnetic field exposure.
2.2. Methods described in Supporting Information
All necessary information describing used animals, magnets, magnet holders and testing methods, including in vivo 1H MR spectroscopy, Morris water maze, balance beam walking, rotarod and fear conditioning can be found in Supporting Information. In addition, used statistical analysis is described in this section.
3. RESULTS
3.1. B0 exposure – Protocol I
The primary goal of this study was to investigate possible long-term behavioral or biological effects of a chronic exposure to the ultra-high static magnetic field. Since any long-term effects caused by B0 exposure have not been reported yet, we proposed a protocol that would maximize the B0 field exposure in order to increase the chance to detect any change between exposed and control animals. Therefore, we decided to use the 16.4T magnet, the strongest horizontal bore magnet currently available and to expose mice to this B0 field multiple times for an extensive period of time (Figure 1, Protocol I).
3.1.1. 1H MR spectroscopy
The 1H MR spectra were acquired from the hippocampus. Hippocampus was chosen for its critical role in cognitive functions by regulating learning, memory encoding, memory consolidation, and spatial navigation. In addition, the hippocampus is known to be vulnerable to different adverse effects, such as stress (43), neonatal iron deficiency (44), hyperglycemia (45) or exposure to drugs (46). Twelve female and twelve male mice used in Protocol I were divided equally into two groups (control and exposed to 16.4 T). One animal that had extremely high level of glutamine in the brain ([Gln] = 8.1 μmol/g) due to an inborn liver defect (42), was excluded from the study. That animal was replaced by one from the backup group of identical mice.
Figure 2A shows the spectral quality consistently achieved in this study. The inset shows the default size and location of the VOI in the left hippocampus of C57BL/6 mice. The unsuppressed water signal linewidth was FWHM = 11.5 ± 0.7 Hz (mean ± SD). The consistency of this spectral quality was an essential precondition for a reliable quantification of 16 brain metabolites (Figure 2B,C). The neurochemical profile included the following metabolites: alanine (Ala), ascorbate (Asc), creatine (Cr), phosphocreatine (PCr), γ-aminobutyric acid (GABA), glucose (Glc), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (myo-Ins), lactate (Lac), N-acetyl-aspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), taurine (Tau), glycerophosphocholine (GPC), phosphocholine (PC). In addition, the content of the fast relaxing macromolecules (MM) was also quantified (expressed in a.u.). The 1H MRS data were collected from the mice three times, before any B0 field exposure and subsequently after 4 and 8 weeks of exposure to 16.4 T or to sham magnetic-field (control group). Despite high detection sensitivity and coefficients of variation (Figure 2B and C, error bars indicate SD), no significant differences between 16.4T and control groups were observed (t-test corrected for multiple comparisons using the false discovery rate, q < 0.2) for any metabolite before or after 4 and 8 weeks of a chronic exposure to an ultra-high magnetic field.
Figure 2.
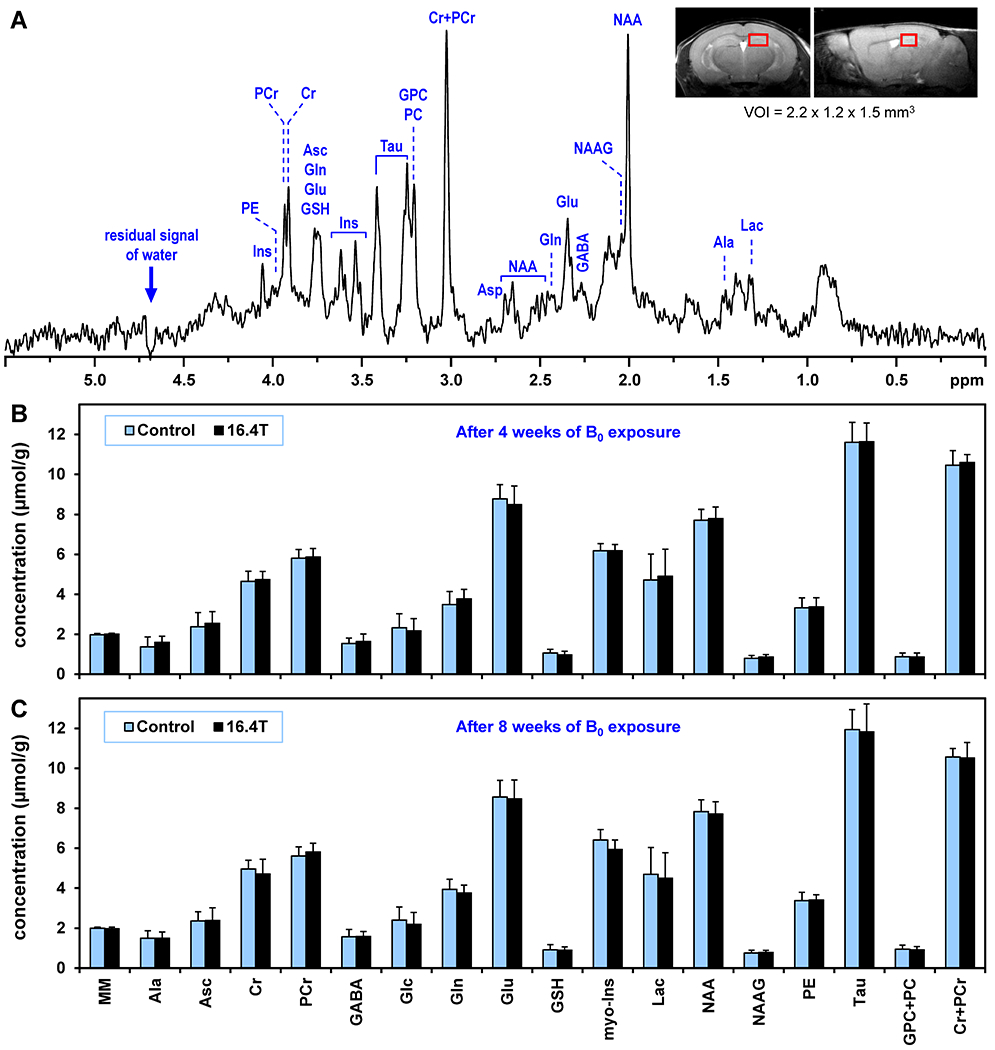
(A) A representative in vivo 1H MR spectrum acquired from the hippocampus of C57BL/6 mouse exposed to 16.4 T. STEAM, TE = 2 ms, TR = 5 s, number of averages = 320, VOI = 3.8 μL. Inset: fast-SE axial and sagittal images show the typical position of VOI centered in dorsal hippocampus. (B, C) Comparison of hippocampal neurochemical profiles of C57BL/6 mice chronically exposed to 16.4 T relative to control mice (N = 12 per group). (B) 4-week long exposure, (C) 8-week long exposure. Error bars indicate SD. Significant differences between groups were not detected for any metabolite (t-test). Used abbreviations: macromolecule (MM), alanine (Ala), ascorbate (Asc), creatine (Cr), phosphocreatine (PCr), γ-aminobutyric acid (GABA), glucose (Glc), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (Ins), lactate (Lac), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), taurine (Tau), glycero-phosphocholine (GPC), phosphocholine (PC).
3.1.2. Morris water maze test
The MWM test was used to access cognitive functions of mice chronically exposed to 16.4 T magnetic field. The test relies on animals’ aversion to swimming and water. During testing in MWM, animals were trained to use visual-spatial cues around the room to navigate an open swimming arena in order to locate a submerged escape platform. Five-day long MWM testing was performed before and then after 4 and 8 weeks of B0 exposures (Figure 1, Protocol I). The escape latency (i.e. time to reach the hidden platform) and the swim distance were assessed from recorded mouse swimming trajectories. The time spent in a target zone, where the hidden platform was located during first four days of testing, was assessed from the “probe” data collected on day 5. The results of three repeated MWM tests were not independent (Figure 3). A typical step-wise decrease in escape latency over consecutive days of testing was observed for both groups of mice (Figure 3A) as they learned to navigate in the MWM. However, the escape latency results measured after 4 weeks of the B0 exposure were very different from the previous MWM test. The escape latency was much shorter on day 1 relative to the previous MWM test and did not change over four testing day (Figure 3B). Both the escape latency and the swim distance for mice exposed to 16.4 T were significantly different relative to the control group (Table 1). A significant difference between 16.4T and control mouse groups was also found in the probe trial (Figure 3H, Table 1). Similar differences were not observed after 8 weeks of the B0 exposure (Figure 3I, Table 1).
Figure 3.
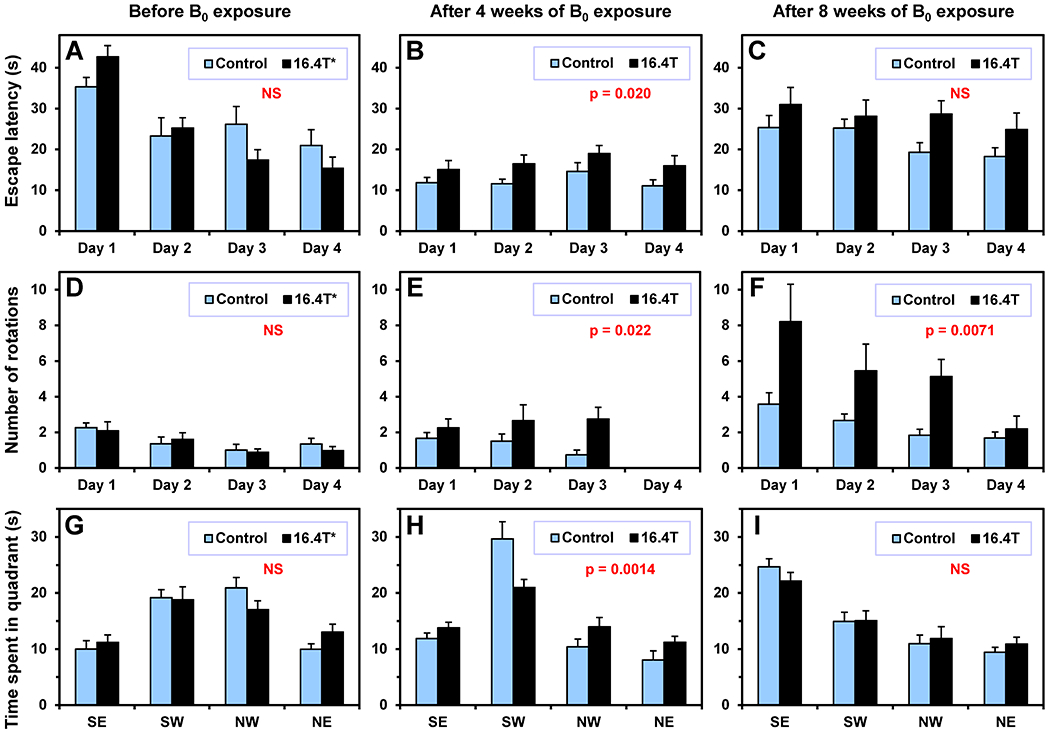
Morris water maze (MWM) test – Protocol I. Comparison of mice chronically exposed to 16.4 T (N = 12) relative to controls (sham exposure, N = 12). Animals were tested in three MWM sessions: (A, D, G) before the B0 exposure, (B, E, H) after 4 weeks of the B0 exposure and (C, F, I) after 8 weeks of the B0 exposure. (A-C). Escape latency, (D-F) angular velocity analysis – number of tight-circling 360° rotations, (G-I) the probe test – time spent in four quadrants. Probe target zones: (G) NW – before exposure, (H) SW – after 4 weeks of exposure, (I) SE – after 8 weeks of exposure. Four trials for each animal were performed every day (Day 1 – 4), only one trial per animal was performed during the probe test. Two-way ANOVA: p-values for B0 exposure factor (A-F), multiple comparison p-values for the target zones (G – NW, H – SW, I – SE). Error bars indicate SEM. (E) Data for Day 4 are missing due to malfunction of the video recording.
Table 1.
Statistical analysis of the Morris water maze tests used in Protocol I. Mice chronically exposed to 16.4 T (N = 12) were compared to mice with sham exposure (control group, N = 12). Exposure duration = 3 hour, two exposures per week. Two-way ANOVA, reported p-values are for the B0 exposure factor, p < 0.05 (blue), non-significant (red). The 2nd factor of ANOVA was the test day (escape latency, swim distance, tight circling) or the MWM quadrant (probe).
| Exposure to magnetic field | Test segment | Test parameter | ANOVA |
|---|---|---|---|
| B0 exposure | |||
| Before B0 exposure | Day 1 - 4 | Escape latency | 0.645 |
| Before B0 exposure | Day 1 - 4 | Swim distance | 0.629 |
| Before B0 exposure | Day 1 - 4 | Tight circling | 0.710 |
| Before B0 exposure | Probe | Time in target zone | 0.309 |
| After 4 weeks of exposure to B0 | Day 1 - 4 | Escape latency | 0.0199 |
| After 4 weeks of exposure to B0 | Day 1 - 4 | Swim distance | 0.0006 |
| After 4 weeks of exposure to B0 | Day 1 - 4 | Tight circling | 0.0220 |
| After 4 weeks of exposure to B0 | Probe | Time in target zone | 0.0014 |
| After 8 weeks of exposure to B0 | Day 1 - 4 | Escape latency | 0.052 |
| After 8 weeks of exposure to B0 | Day 1 - 4 | Swim distance | 0.089 |
| After 8 weeks of exposure to B0 | Day 1 - 4 | Tight circling | 0.0071 |
| After 8 weeks of exposure to B0 | Probe | Time in target zone | 0.941 |
By a visual inspection of mouse swimming trajectories in the MWM pool, we recognized that some mice exposed to 16.4 T showed a tight circling behavior (Figure 4). In order to quantify this behavior, the angular velocity as a function of time was calculated by using the ANY-maze mouse tracking raw data (time, X and Y coordinates). The angular velocity of 90°/s was chosen as a lower threshold to quantify the number of tight circling rotations. This angular velocity corresponds to one 360° rotation per 4 seconds. As expected, a significant difference between two mice groups before any magnetic field exposure was not observed (Figure 3D). However, significant differences were observed after 4 and 8 weeks of exposure to 16.4 T compared to the control group (Figure 3E,F, Table 1). All results are also reported in Supporting Information Tables S1–3.
Figure 4.
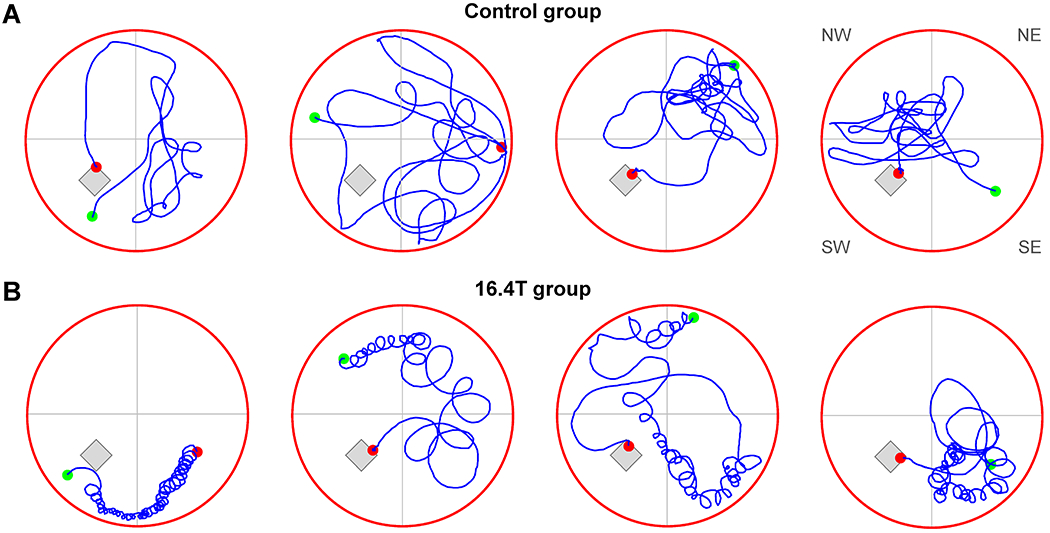
Morris water maze test – Protocol I. Characteristic swimming trajectories of four control mice (A) and four mice exposed to 16.4 T for 8 weeks (B). Maximum duration of the test = 60 s. The hidden platform and virtual division of the pool into quadrants are depicted. Green dots: start points, red dots: end points. The MWM test started in quadrants (from left to right): SW. NW, NE and SE.
3.2. B0 exposure – Protocol II
Long-term behavioral changes observed in Protocol I between mice exposed to 16.4 T and the control group of mice were unexpected. In addition, the MWM results (escape latency, swim distance, probe) were not consistent between tests taken after 4 and 8 weeks of the B0 field exposure (Table 1). Moreover, the tight circling behavior was observed only in a few mice. In order to avoid any possible bias in data interpretation and to confirm long-term behavioral changes induced by a chronic exposure to B0 field observed in Protocol I, we decided to repeat the B0 exposure experiment using a modified protocol (Figure 1, Protocol II). Four mice groups were included in this protocol (control, 16.4T, 10.5T and 16.4T Motion). In addition, the behavioral testing was expanded to include the balance beam walking, rotarod and fear conditioning tests. The whole protocol was repeated twice to increase the statistical power of behavioral tests (N increased from 12 to 24). Finally, 1H MRS data collection after the B0 exposure was excluded from the Protocol II because results acquired in Protocol I did not indicate any measurable change in the hippocampal neurochemical profile.
3.2.1. MRI/MRS mice screening
Out of 100 screened C57BL/6 mice, an extremely high level of Gln (6.9 μmol/g) was detected in one mouse, extensively enlarged ventricles (hydrocephalus) were detected in two mice and finally, a traumatic brain injury was detected in one animal. These mice were eliminated from the study and were replaced by normal healthy mice from the backup group.
3.2.2. Morris water maze test
The B0 exposure was found as a significant factor (two-way ANOVA) for the escape latency and the swim distance (Figure 5A, Table 2). Multiple comparison analysis revealed that only the 16.4T group was significantly different from the control group (Table 2). The analysis of angular velocity and tight circling was found to be the most sensitive test differentiating the 16.4T mouse group from the control (Figure 5B, Table 2). Mice exposed to an extensive motion in and out of 16.4T magnet (16.4T Motion group) did not show the circling swimming behavior observed in the 16.4T group (Table 2). The effect of B0 exposure was not observed in the probe test (Table 2). Not all mice in the 16.4T group were affected by the B0 field in the same way. Some animals showed the tight circling behavior rather consistently, but others showed the circling only sporadically. Figure S2 in Supporting Information shows the box plot representing the distribution of average number of rotations over 4 days of MWM testing for each animal from the 16.4T group. Animals that showed consistent tight circling nearly exclusively spun in the same direction: clockwise or counter-clockwise. Figure 6A shows 8 of 16 swimming trajectories (4 test days, 4 trials per day) of one of tightly circling animals (mouse #16 from Figure S2). As the angular velocity analysis demonstrates (Figure 6B), this mouse was always circling counter-clockwise. This angular velocity analysis was used to quantify the number of tight circles (360° rotations) presented in Figure 5B. It has to be pointed out again that only fast body rotation (angular velocity ≥ 90°/s, i.e. one rotation in ≤ 4 s) were counted for that analysis. Table S4 shows the MWM results in numerical form.
Figure 5.
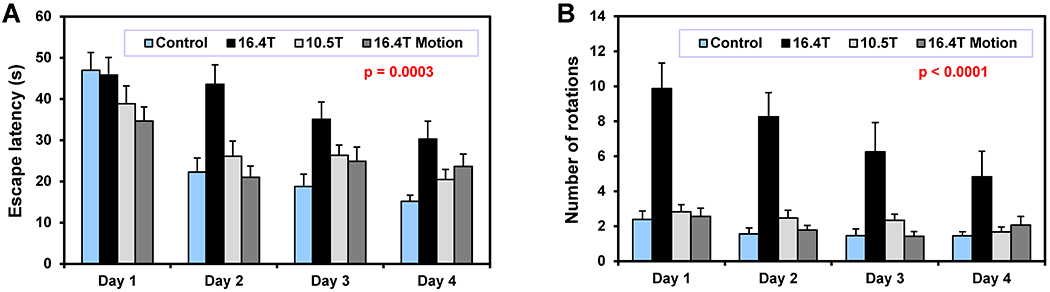
Morris water maze test – Protocol II. Four groups of mice were chronically exposed to magnetic fields (2 exposures per week, 4 weeks): control group – sham exposure (3 hours, N = 23), 16.4T (3 hours, N = 24), 10.5T (3 hours, N = 22), 16.4T Motion (2 min, 20 fast motions in and out of magnet, N = 21). (A) Escape latency; (B) Angular velocity analysis – number of tight-circling 360° rotations. Two-way ANOVA: p-values for B0 exposure factor. Error bars indicate SEM.
Table 2.
Statistical analysis of the battery of behavioral test used in Protocol II. Four mice groups were tested after chronically exposed to magnetic fields (3-hour long exposures, 2 exposures per week, 4 weeks of exposures): 16.4T group (N = 24), 10.5T group (N = 22), control group (sham exposures, N = 23), 16.4T group with motion (N = 21, instead of 3 hours only 2 min exposures with intensive motion in and out of magnet). Morris water maze (MWM), Balance beam (BB), fear conditioning (FC). Only a subgroup of mice was used for an additional MWM test repeated six week later after the last B0 exposure (only control and 16.4T group, N = 12 each). Two-way ANOVA; first factor: B0 exposure; second factor: testing day in MWM (escape latency, swim distance, tight circling), zone in MWM (Probe) and session type in FC; one-way ANOVA (rotarod, BB walking); p < 0.05 (blue), non-significant (red).
| Behavioral test | Test parameter | ANOVA | Multiple comparisons tests# | ||
|---|---|---|---|---|---|
| B0 exposure | 16.4T | 10.5T | 16.4T M | ||
| MWM – Day 1-4 | Escape latency | 0.0003 | 0.0002 | 0.769 | 0.999 |
| MWM – Day 1-4 | Swim distance | 0.0079 | 0.0209 | 0.692 | 0.967 |
| MWM – Day 1-4 | Tight circling | <0.0001 | <0.0001 | 0.066 | 0.691 |
| MWM – Probe | Time in target zone | N/A | 0.825 | 0.999 | 0.320 |
| Rotarod | Latency to fall off | 0.319 | 0.865 | 0.711 | 0.642 |
| BB walking – 15 mm square | Latency to cross | 0.0297 | 0.973 | 0.513 | 0.0489 |
| BB walking – 17 mm round | Latency to cross | 0.0171 | 0.999 | 0.345 | 0.0205 |
| BB walking – 8 mm square | Latency to cross | 0.0057 | 0.835 | 0.110 | 0.064 |
| BB walking – 15 mm square | Number of foot-slips | 0.0119 | 0.0133 | 0.795 | 0.999 |
| BB walking – 17 mm round | Number of foot-slips | 0.183 | 0.323 | 0.999 | 0.854 |
| BB walking – 8 mm square | Number of foot-slips | <0.0001 | 0.0042 | 0.611 | 0.345 |
| FC – Baseline | Freezing | 0.278 | 0.998 | 0.999 | 0.884 |
| FC – Context | Freezing | 0.278 | 0.523 | 0.190 | 0.893 |
| FC - Cue | Freezing | 0.278 | 0.993 | 0.894 | 0.460 |
| MWM Repeat – Day 1-4 | Escape latency | 0.611 | |||
| MWM Repeat – Day 1-4 | Swim distance | 0.0238 | |||
| MWM Repeat – Day 1-4 | Tight circling | 0.0076 | |||
| MWM Repeat – Probe | Time in target zone | N/A | 0.990 | ||
Comparisons relative to the control group.
Figure 6.
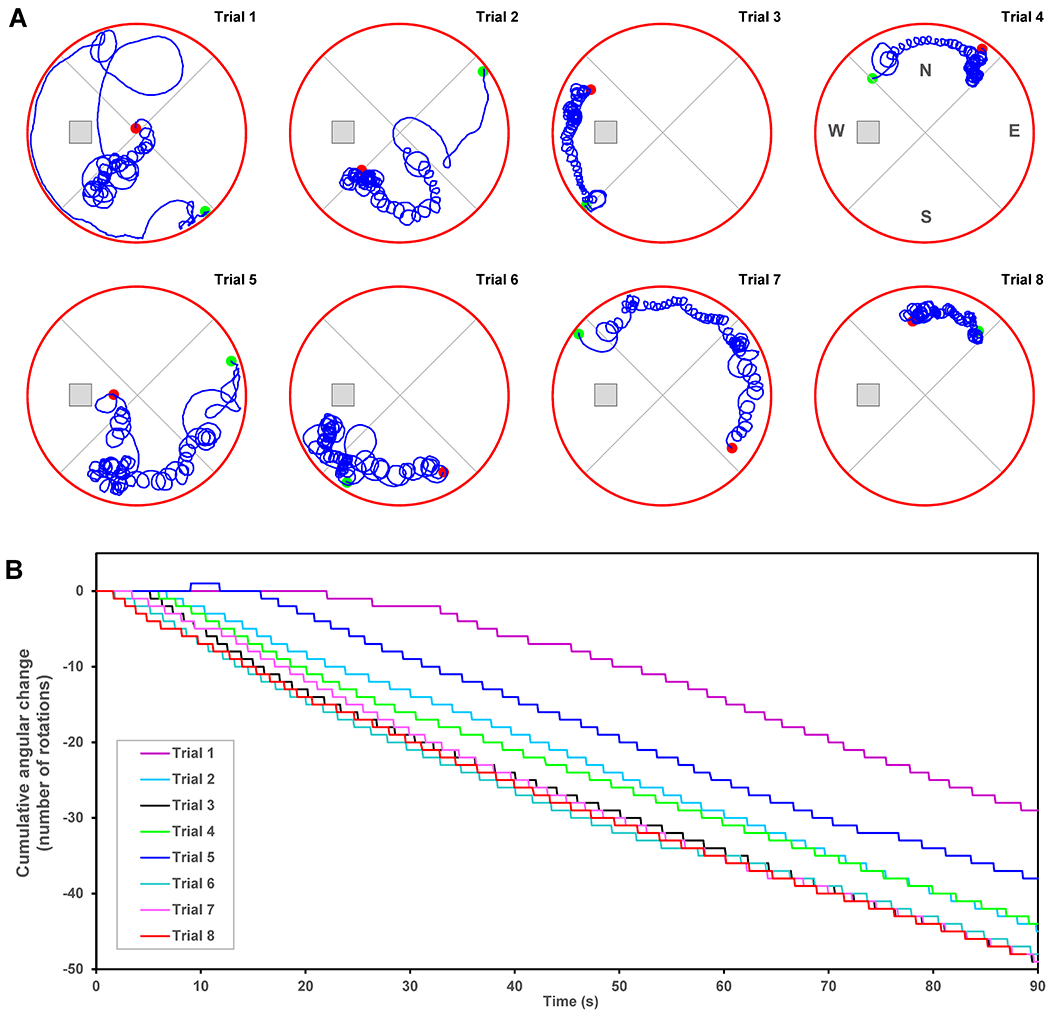
Morris water maze test – Protocol II. (A) Swimming trajectories of a single mouse that showed consistent tight circling (#16 in Supporting Information Figure S2); (B) cumulative angular change analyzed from trajectories shown in panel A. The hidden platform and virtual division of the pool into quadrants are depicted. Green dots: start points, red dots: end points.
A subset of mice exposed to 16.4 T and a corresponding control group (N = 12 per each group, from the second batch of animals included in this study) were tested in MWM once again 6 weeks after the last exposure to 16.4 T. Significant differences between these two groups were found for the total swim distance (Figure 7A, Table 2) and for the number of tight circling rotations (p = 0.008, Figure 7B). Neither the escape latency during training nor the performance during the probe tests was significantly different between 16.4T and the control group (Table 2 and S6).
Figure 7.
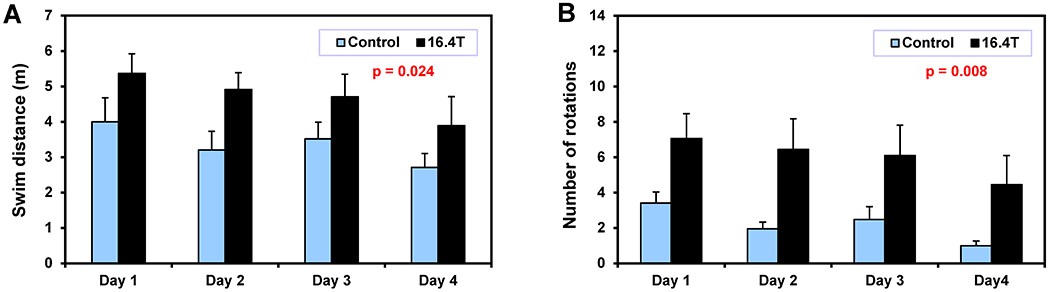
Morris water maze test – Protocol II. Subgroup of mice exposed to 16.4 T (N = 12) and control mice (N = 12) that were repeatedly tested in MWM six weeks later after the last exposure. (A) Swim distance to find a hidden platform; (B) angular velocity analysis – number of tight-circling 360° rotations. Two-way ANOVA: p-values for B0 exposure factor. Error bars indicate SEM.
3.2.3. Balance beam walking test
All mice were trained on the balance beam prior to magnetic field exposures and 4 out of 96 mice were eliminated from the final analysis due to a poor performance during training tests (failure to cross within the time limit). After the B0 field exposure, mice were tested using three different walking rod profiles: 15 mm square, 17 mm round and 8 mm square (2 tests per each rod). The latency to cross and the number of slips were evaluated (Figure 8, Tables 2 and S5). Although the B0 exposure factor (two-way ANOVA) was found significant for the crossing latency on all three types of rods, multiple comparison tests showed modest differences only for two rod types for the 16.4T Motion group relative to controls (Table 2). The number of footslips was significantly increased in the 16.4T group relative to controls on two rod types (Figure 8, Table 2).
Figure 8.
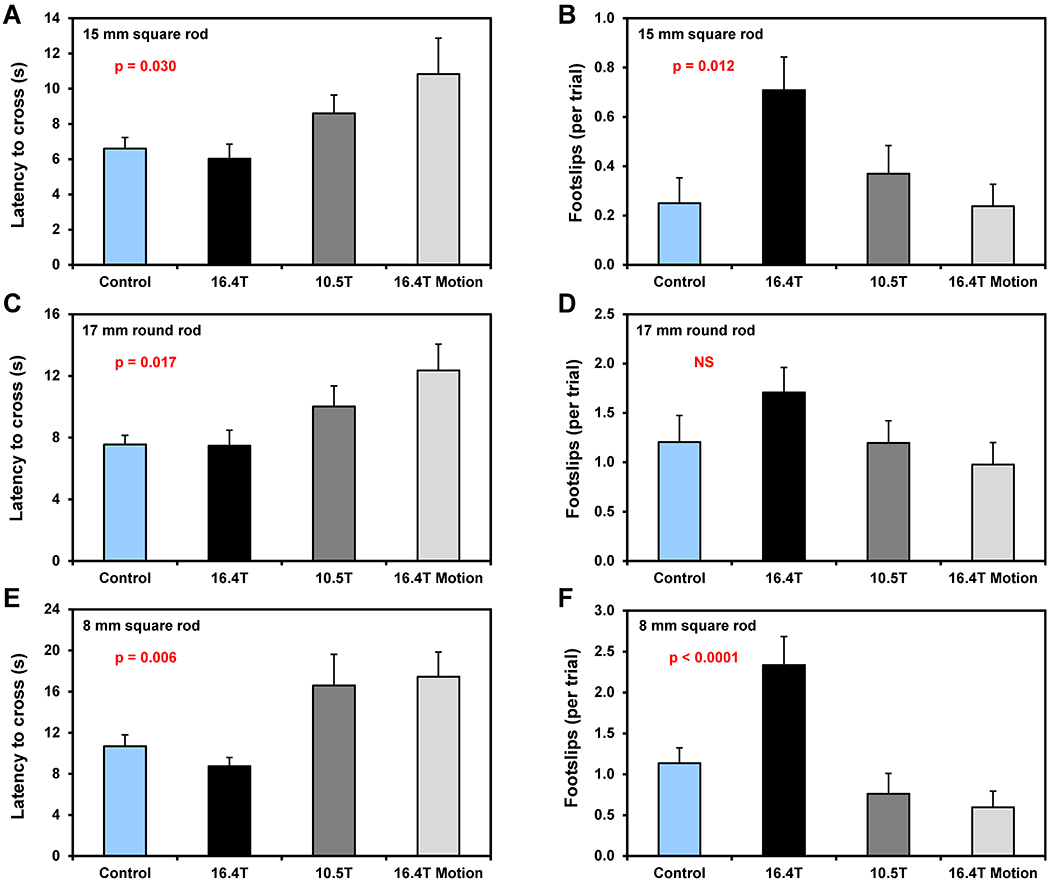
Balance beam walking test. Four groups of mice with different exposure to magnetic field were tested. Latency (duration) to cross the beam (panels A, C, E) and number of foot-slips (panels B, D, F). Walking test were performed using three different rod types: 15 mm square (A, B), 17 mm round (C, D) and 8 mm square (E, F). Two-way ANOVA: p-values for B0 exposure factor. Error bars indicate SEM.
3.2.4. Rotarod test
As expected, significant differences between mice groups were not observed for rotarod tests before the exposure to the magnetic field (three-day training). Rotarod tests performed after chronic 4-week long exposures to 10.5 T and 16.4 T (with and without motion) did not reveal any significant differences between mice groups in the latency to fall from the rotating rod with linearly increasing angular speed (Tables 2 and S5).
3.2.5. Fear conditioning test
Mice were placed into a conditioning chamber and were given pairings of a conditioned stimulus (auditory and visual cues) and an aversive unconditioned stimulus (an electric footshock). No difference between B0 exposed and a control group in freezing behavior was observed in the conditioning session following each cue (auditory noise, light) – shock (mild electrical foot shot) pairing, suggesting that all groups were similarly responsive to the shock. Likewise, there was no significant difference among groups in the cue- (auditory noise, light) or context-driven (test chamber) freezing response (Tables 2 and S5).
4. DISCUSSION
When MR research is moving to magnetic field strengths of 7 T and beyond, safety of subjects and MR technologists must be given the highest priority. Temporal adverse sensational effects resulting from exposures to ultra-high magnetic fields, such as vertigo, dizziness, nausea and metallic taste, have been widely reported (13,15–19). Consequently, an important question arises in our mind as to whether repeated exposures to ultra-high static magnetic fields could ultimately cause some long-term impairment. The safety of subjects undergoing MRI scans at ultra-high fields as well as safety of supporting staff working around these magnets was the rationale for designing and executing this project.
Because the long-term adverse effects of exposure to ultra-high B0 fields have not been reported yet, we proposed a protocol that would maximize the chance to detect even subtle behavioral or biological changes (ultra-high B0 fields, long exposure periods, large number of tested animals and using a battery of behavioral tests). Changes in the hippocampal neurochemical profiles were not detected in mice chronically exposed to 16.4 T for 4 and 8 weeks (Figure 2B,C) despite high metabolite detection sensitivity (average SD = 0.5 ± 0.3 μmol/g). Learning and memory deficits (fear-conditioning test) were not observed between mice exposed to 10.5 T or 16.4 T and control mice (Table 2). The accelerating rotarod test did not reveal a locomotor deficit or an impaired motor coordination in mice chronically exposed to 10.5 T or 16.4T with or without motion (Table 2). However, the balance walking test, which is more sensitive for detecting subtle deficits in motor skills and balance indeed revealed consistently impaired coordination in mice exposed to the highest field strength (Table 2, Figure 8).
The Morris water maze was chosen for Protocol I primarily to investigate whether long-term chronic exposure to 16.4 T can affect the spatial memory and learning of exposed mice. Furthermore, using this type of test offered an opportunity to potentially detect a circling swimming behavior similar to that observed in mice immediately after their exposure to 14.1 T (25,31). Three 5-day-long MWM testing sessions were used in Protocol I, before the field exposure, and after 4 and 8 weeks of exposure to 16.4 T (Figure 1). Significant differences between 16.4T and control group in common MWM indices (escape latency, swim distance, target zone time in probe test) were observed after 4 weeks of exposure, but not after 8 weeks of exposure (Figure 3,Table 1). These counterintuitive results were most likely caused by repeating the whole MWM test three times. The 4-week long period between M\WM tests was probably too long for retaining the platform location from the spatial reference memory. In addition, the platform location was changed between three MWM sessions. However, our data clearly indicate that the previous MWM test affected the spatial navigation working memory of mice during the subsequent MWM test. Indeed, a similar link between MWM test and re-test has been already reported (47). The mice retained the knowledge how to search the pool efficiently to find the platform location, retaining the method to solve the task (47). Moreover, a lowered level of anxiety in subsequent tests probably led to better performances. Therefore, a typical day-by-day decrease in escape latency was observed only in the first MWM test. However, this disadvantage in Protocol I design gave us an opportunity to demonstrate that the tight circling swimming behavior was indeed induced by the exposure to 16.4 T (Figure 3D–F, Table 1) and not by accidently including mice with a vestibular defect in this study. In addition, tight-circling results presented in Figure 3E,F indicates that the exposure to 16.4 T has a cumulative effect (Tables S2–3).
The MWM tests in Protocol II confirmed the initial MWM results from Protocol I that the chronic exposure to 16.4 T induces long-term behavioral changes in C57BL/6 mice. However, similar behavioral changes were neither observed in mice exposed to 10.5 T nor in mice exposed to 16.4 T only for short periods of time (~2 min) during extensive motion in and out of the 16.4T magnet (Figure 5, Table 2). Moreover, the tight circling of mice exposed to 16.4 T observed in Protocol I (Figure 4) was confirmed and validated in Protocol II (Figures 5B, 7B, Table 2). Analysis of the angular velocity and quantification of the tight-circle swimming was found to be the most sensitive parameter to index behavioral changes in mice chronically exposed to ultra-high magnetic field (Table 1 and 2). Not all mice exposed to 16.4 T were affected in a similar way, with some animals consistently showing tight circling (Figure 6), while other animals demonstrating it only sporadically (Figure S2). This is in agreement with previous reports, when the tight-circling behavior immediately after the removal from the magnet was observed just for a fraction of studied rats (23,26) and mice (32). In addition, postural disturbances and instability were also reported only for a fraction of zebrafish examined inside 4.7T and 11.7T magnets (29).
It has been widely reported that the direction of circling depends on the orientation of animal body (head) relative to the magnetic field (25–27,31). When the B0 field was parallel with the rostral/caudal body axis then the animal circled counter-clockwise and when the field was anti-parallel, the circling direction was clockwise. The consequences of the magnetic field exposure were minimal when the rostral/caudal body axis was perpendicular to B0 (31). Therefore, it is not surprising that in this study, where the mice were not restrained inside the holder and could have had any orientation relative to the B0 field, some mice were circling clockwise in the MWM, other circled counter-clockwise while the rest exhibited limited or no circling behavior. Since in previous studies 30-min magnetic field exposures resulted in tight circling, which was observed only immediately after their removal from the magnet (27,31), it is reasonable to suggest that tight circling observed in this study (days or weeks after the last field exposure) required a cumulative dose effect of multiple exposures. Although a quantitative comparison of circling results between Protocols I and II (Figures 3D–F and 5B) is difficult due to multiple factors that affect them (e.g., escape latency, maximum test duration, repeated testing), the circling results after 4 and 8 weeks of B0 exposure (Tables 1 and S2–3) indicate that the B0 field exposure indeed has a cumulative dose effect. Furthermore, as magnetic vestibular stimulation is modulated by head orientation relative to B0, such a cumulative effect would require that certain mice had a preference in magnet orientation across the multiple exposures. Similar preferences in orientation to the magnetic field were observed in zebrafish swimming inside the magnet (29,48). These assumptions may also explain substantial inter-animal variation in the tight circling shown in Supporting Information Figure S2. It is important to emphasize that the direction of circling (clockwise or counter-clockwise) was not random, but mice that consistently exhibited tight circling in multiple MWM trials, were always spinning in the same direction (Figure 6B). While there is a close similarity in the tight-circling locomotor activity of this study and previous reports in rodents (25–27,31), there are two major differences between this and previous experiments. First, in most previous studies animals were exposed to the magnetic field only once for 30 minutes, whereas this study explored substantially longer periods of exposures (3 hours) repeated over 4 or 8 weeks resulting in a total of 24 or 48 hours inside the magnet, respectively (Figure 1). Second, previous studies primarily focused on acute B0 effects observable within the first 2 min after removing animals from the magnet. In contrast, this study reported on long-term B0 effects with the tight-circling behavior being observed days or even weeks later after the last exposure (Figure 3F and 7B).
Behavioral findings suggest that the chronic exposure to ultra-high magnetic field produced task-specific long-term effects on motor function, while assessments of cognition were largely normal. In vivo 1H MRS did not reveal any detectable changes in the neurochemical profile of the hippocampus (Figure 2), which has a critical role in cognition. In addition, the contextual and cued fear conditioning tests did not provide any evidence of an impaired learning and memory of B0 exposed mice (Table 2). Although MWM test is considered as a test of spatial memory and learning, significant differences observed in mice exposed to 16.4 T do not necessarily indicate a deficit in cognitive functions, but most likely were caused by tight-circling, which indicate involvement of the vestibular system (33,39). While the basic motor functions were not affected by B0 (rotarod test), a deficit in fine motor functions and coordination was detected by the balance beam walking test (Figure 8, Table 2). Increased foot-slips were specific for the 16.4T mouse group, while the observed modest increase in the latency to cross was the only behavioral test showing a significant difference for another 16.4T group (16.4T Motion) relative to controls (Table 2).
The tight-circling swimming behavior observed in the MWM test and the increased number of foot-slips detected on the balance beam clearly indicates that the vestibular system was the primary organ of the body that interacted with the magnetic field and was affected by the chronic exposure to B0 (33,39). To the best of our knowledge, this is the first animal model study reporting long-term behavioral changes induced by the magnetic field exposure. Long-term behavioral changes (1 – 4 days) were not previously observed in mice once exposed to 14.1 T for 30 min (23). Repeated long-term exposures to a static 16.4 T magnetic field that resulted in continuous vestibular stimulation were necessary to induce the tight-circling behavior in mice (Figures 4 and 6). In agreement with a previously reported study (21), short exposures combined with fast motion in a spatially inhomogeneous B0 field (16.4T Motion group) were not sufficient to induce such circling behavior (Figure 5B, Table 2). The locomotor circling behavior observed in this study is consistent with a continuous vestibular stimulation induced by a static magnetic field. The mechanism of this stimulation can be explained by a Lorentz force resulting from the interaction of a strong static B0 field with naturally occurring ionic currents flowing through the inner ear endolymph into vestibular hair cells (33,36,37,39). The resulting force within the endolymph is strong enough to displace the horizontal semicircular canal cupula (33). This mechanism does not require motion in the magnetic field, which is consistent with our observation that fast motion of the animal holder in and out of the 16.4T magnet did not result in circling behavior (Figure 5B). Tight-circling was significantly increased in mice exposed to 16.4 T, but the behavior of mice exposed to 10.5 T was not different from the control group with a sham B0 exposure (Table 2). This is in agreement with previously published reports that B0 induced circling was observed only above a specific threshold (≥ 7T in rats) and the intensity of circling was scaled with the B0 field strength (26,27). The locomotor circling previously observed immediately after exposure is a compensatory response to the removal of the persistent vestibular perturbation imposed during magnetic field exposure (31). Such a circling behavior decays within minutes because, once outside of the magnetic field, the brain adapts quickly to the balance and spatial orientation inputs which indicate perturbation has ceased and compensation is no longer needed (49). Therefore, the tight-circling behavior of mice observed days or weeks later after a 4-week-long exposure to 16.4 T (Figure 7B) cannot be explained in the same way. Our data indicate that continuous long-term vestibular stimulation (24 or 48 h) caused a long-term impairment of the vestibular apparatus. It should be emphasized that the Lorentz force mechanism of the B0 interaction with the inner ear implies continuous stimulation over the entire duration of exposure (33,39). This phenomenon was well documented by a long-lasting nystagmus observed in humans (37,50) and mice (28) inside the magnet. Although habituation effects were reported for a limited number of repeated exposures (3 x 30 min in 14.1 T), such a mechanism is most likely insufficient to prevent a long-term vestibular system impairment because the habituation and adaptation processes suppress the perception, but not the stimulation itself (51). These suggestions are in agreement with previously published reports that prolonged or intense stimuli, such as chronic weightlessness (52), rotation (53), changes in atmospheric pressure (54), or even a percussive auditory stimulus (55) can cause long-lasting damage to the semicircular canals or otolith organs (23). The assumption of vestibular apparatus impairment is supported by our observation that the direction of spinning (clockwise or counter-clockwise) remains the same in mice for each animal that showed a consistent tight-circling behavior (Figure 6). The differences in the tight circling between individual mice exposed to 16.4 T (Figure S2) can be explained by differences in preferred orientations inside the magnet, but potentially also by differences in the sensitivity to magnetic vestibular stimulation caused by genetic differences (48) or by minor variations in the anatomy of the inner ear labyrinth. It is important to emphasize again that while mice were not immobilized during B0 exposures, the Lorentz force mechanism of vestibular stimulation does not require any head motion. It is obvious that further experiments are necessary to clarify whether the vestibular impairment caused by chronic B0 field exposure (highlighted in Figure 7B) is ultimately reversible or potentially permanent.
The results highlight important safety concerns regarding human subjects exposed to ultra-high magnetic fields, while adverse sensational effects, such as vertigo, dizziness and nausea are known to be transient. However, the results of this animal model demonstrate that the chronic exposure to ultra-high magnetic field may result in long-term impairment of the vestibular system. Long-term behavioral changes were observed in mice exposed to 16.4 T, but were not observed in mice exposed to 10.5 T (Figure 5, Table 2). These results necessarily raise a series of important questions, including: is it possible that exposure to ultra-high B0 may cause some long-term impairment or even permanent damage to the vestibular system in humans? Is there a safety threshold in magnetic field strength and/or exposure duration for humans? Does the position/orientation of the human head inside the magnet affect the potential long-term effects of chronic exposures to ultra-high fields? It is clear that additional studies are needed to answer these questions while research at higher and higher magnetic fields continues to cautiously move forward. We cannot agree more with the statement of Ward et al., in their recent review article (39) that as the strength of magnetic fields of MRI machines increases for both scientific and clinical use it becomes increasingly important to understand the mechanisms and safety issues related to its use.
5. CONCLUSIONS
Long-term behavioral changes have been observed in mice chronically exposed to 16.4 T, but not to 10.5 T. Angular velocity analysis of tight circling of mice swimming in the Morris water maze was found to be the most sensitive parameter indexing the B0 induced behavioral changes. These findings have serious implications for the safety of subject participation in MRI/MRS research studies at ultra-high magnetic fields.
Supplementary Material
Figure S1. Images of the animal holder. (A,B) Plastic bins of the animal holder, two mice were kept inside each bin during exposures to magnetic field. (C) The animal holder in front of the 16.4 T magnet. The temperature was maintained at 24°C using warm water circulating and the air exchange in mouse containers was maintained by continuous air flow through silicone tubing connected to mouse containers.
Figure S2. Morris water maze test – Protocol II. Angular velocity analysis – number of tight-circling 360° rotations for all mice chronically exposed to 16.4 T. The box plot represents data of 16 MWM trials (4 trial per test day, 4 days of testing). Boxes indicate median with upper and lower quartiles, whiskers indicate maximum and minimum values.
Table S1. Protocol I: Morris water maze tests performed on mice before exposure to magnetic field. Neither control (N = 12) nor 16.4T* group (N = 12) were exposed to any magnetic field. The label 16.4T* means that this group of mice was later exposed to 16.4 T magnetic field. Submerge platform was located in the north-west (NW) quadrant (zone). Table values represent mean ± SD.
Table S2. Protocol I: Morris water maze tests performed on mice after 4-week long period of a chronic exposure to 16.4 T magnetic field. Two mice groups were exposed to B0 fields for a period of 4 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 12), 16.4T group (N = 12). Submerge platform was located in the south-west (SW) quadrant (zone). Table values represent mean ± SD.
Table S3. Protocol I: Morris water maze tests performed on mice after 8-week long period of a chronic exposure to 16.4 T magnetic field. Two mice groups were exposed to B0 fields for a period of 8 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 12), 16.4T group (N = 12). Submerge platform was located in the south-east (SE) quadrant (zone). Table values represent mean ± SD.
Table S4. Protocol II: Morris water maze tests performed on mice chronically exposed to magnetic fields. Four mice groups were exposed to B0 fields for a period of 4 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 23), 16.4T group (N = 24), 10.5T group (N = 22), 16.4T Motion group (N = 21, instead of 3 hours only 2 min exposures with intensive motion in and out of magnet). Submerge platform was located in the west (W) quadrant (zone). Table values represent mean ± SD.
Table S5. Protocol II: Battery of behavioral tests performed on mice chronically exposed to magnetic fields. Four mice groups were exposed to B0 fields for a period of 4 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 23), 16.4T group (N = 24), 10.5T group (N = 22), 16.4T Motion group (N = 21, instead of 3 hours only 2 min exposures with intensive motion in and out of magnet). Balance beam walking (BB), fear conditioning (FC). Table values represent mean ± SD.
Table S6. Protocol II: Morris water maze test performed six weeks later after the last B0 exposure. Subgroup of mice exposed to 16.4 T (N = 12) and control group (sham exposures, N = 12). Submerge platform was located in the west (W) quadrant (zone). Table values represent mean ± SD.
Financial information:
The preparation of this manuscript was supported by NIH grants P41-EB015894, P41-EB027061 and P30-NS076408
REFERENCES
- 1.Ugurbil K Imaging at ultrahigh magnetic fields: History, challenges, and solutions. Neuroimage 2018;168:7–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Martino F, Yacoub E, Kemper V, Moerel M, Uludag K, De Weerd P, Ugurbil K, Goebel R, Formisano E. The impact of ultra-high field MRI on cognitive and computational neuroimaging. Neuroimage 2018;168:366–382. [DOI] [PubMed] [Google Scholar]
- 3.Dumoulin SO, Fracasso A, van der Zwaag W, Siero JCW, Petridou N. Ultra-high field MRI: Advancing systems neuroscience towards mesoscopic human brain function. Neuroimage 2018;168:345–357. [DOI] [PubMed] [Google Scholar]
- 4.Mekle R, Mlynarik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med 2009;61(6):1279–1285. [DOI] [PubMed] [Google Scholar]
- 5.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med 2009;62(4):868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn Reson Med 2016;75(2):801–809. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med 2001;46(1):24–30. [DOI] [PubMed] [Google Scholar]
- 8.He X, Erturk MA, Grant A, Wu X, Lagore RL, DelaBarre L, Eryaman Y, Adriany G, Auerbach EJ, Van de Moortele PF, Ugurbil K, Metzger GJ. First in-vivo human imaging at 10.5T: Imaging the body at 447 MHz. Magn Reson Med 2020;84(1):289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowogrodzki A The world’s strongest MRI machines are pushing human imaging to new limits. Nature 2018;563(7729):24–26. [DOI] [PubMed] [Google Scholar]
- 10.Sadeghi-Tarakameh A, DelaBarre L, Lagore RL, Torrado-Carvajal A, Wu X, Grant A, Adriany G, Metzger GJ, Van de Moortele PF, Ugurbil K, Atalar E, Eryaman Y. In vivo human head MRI at 10.5T: A radiofrequency safety study and preliminary imaging results. Magn Reson Med 2020;84(1):484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson IC, Renteria L, Burd H, Pliskin NH, Thulborn KR. Safety of human MRI at static fields above the FDA 8 T guideline: sodium imaging at 9.4 T does not affect vital signs or cognitive ability. J Magn Reson Imaging 2007;26(5):1222–1227. [DOI] [PubMed] [Google Scholar]
- 12.Kangarlu A, Burgess RE, Zhu H, Nakayama T, Hamlin RL, Abduljalil AM, Robitaille PM. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imaging 1999;17(10):1407–1416. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich A, Szostek A, Meyer P, Nees F, Rauschenberg J, Grobner J, Gilles M, Paslakis G, Deuschle M, Semmler W, Flor H. Cognition and sensation in very high static magnetic fields: a randomized case-crossover study with different field strengths. Radiology 2013;266(1):236–245. [DOI] [PubMed] [Google Scholar]
- 14.Grant A, Metzger GJ, Van de Moortele PF, Adriany G, Olman C, Zhang L, Koopermeiners J, Eryaman Y, Koeritzer M, Adams ME, Henry TR, Ugurbil K. 10.5T MRI static field effects on human cognitive, vestibular, and physiological function. Magn Reson Imaging 2020;73:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatahi M, Demenescu LR, Speck O. Subjective perception of safety in healthy individuals working with 7T MRI scanners: a retrospective multicenter survey. MAGMA 2016;29(3):379–387. [DOI] [PubMed] [Google Scholar]
- 16.Hoff MN, McKinney At, Shellock FG, Rassner U, Gilk T, Watson RE Jr., Greenberg TD, Froelich J, Kanal E. Safety Considerations of 7-T MRI in Clinical Practice. Radiology 2019;292(3):509–518. [DOI] [PubMed] [Google Scholar]
- 17.Patel M, Williamsom RA, Dorevitch S, Buchanan S. Pilot study investigating the effect of the static magnetic field from a 9.4-T MRI on the vestibular system. J Occup Environ Med 2008;50(5):576–583. [DOI] [PubMed] [Google Scholar]
- 18.Uwano I, Metoki T, Sendai F, Yoshida R, Kudo K, Yamashita F, Higuchi S, Ito K, Harada T, Goodwin J, Ogawa A, Sasaki M. Assessment of sensations experienced by subjects during MR imaging examination at 7T. Magn Reson Med Sci 2015;14(1):35–41. [DOI] [PubMed] [Google Scholar]
- 19.Walker M, Fultz A, Davies C, Brockopp D. Symptoms Experienced by MR Technologists Exposed to Static Magnetic Fields. Radiol Technol 2020;91(4):316–323. [PubMed] [Google Scholar]
- 20.High WB, Sikora J, Ugurbil K, Garwood M. Subchronic in vivo effects of a high static magnetic field (9.4 T) in rats. J Magn Reson Imaging 2000;12(1):122–139. [DOI] [PubMed] [Google Scholar]
- 21.Houpt TA, Carella L, Gonzalez D, Janowitz I, Mueller A, Mueller K, Neth B, Smith JC. Behavioral effects on rats of motion within a high static magnetic field. Physiol Behav 2011;102(3-4):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houpt TA, Cassell J, Carella L, Neth B, Smith JC. Head tilt in rats during exposure to a high magnetic field. Physiol Behav 2012;105(2):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houpt TA, Cassell JA, Hood A, DenBleyker M, Janowitz I, Mueller K, Ortega B, Smith JC. Repeated exposure attenuates the behavioral response of rats to static high magnetic fields. Physiol Behav 2010;99(4):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houpt TA, Cassell JA, Riccardi C, DenBleyker MD, Hood A, Smith JC. Rats avoid high magnetic fields: dependence on an intact vestibular system. Physiol Behav 2007;92(4):741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houpt TA, Houpt CE. Circular swimming in mice after exposure to a high magnetic field. Physiol Behav 2010;100(4):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houpt TA, Pittman DW, Barranco JM, Brooks EH, Smith JC. Behavioral effects of high-strength static magnetic fields on rats. J Neurosci 2003;23(4):1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houpt TA, Pittman DW, Riccardi C, Cassell JA, Lockwood DR, Barranco JM, Kwon B, Smith JC. Behavioral effects on rats of high strength magnetic fields generated by a resistive electromagnet. Physiol Behav 2005;86(3):379–389. [DOI] [PubMed] [Google Scholar]
- 28.Ward BK, Lee YH, Roberts DC, Naylor E, Migliaccio AA, Della Santina CC. Mouse Magnetic-field Nystagmus in Strong Static Magnetic Fields Is Dependent on the Presence of Nox3. Otol Neurotol 2018;39(10):e1150–e1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward BK, Tan GX, Roberts DC, Della Santina CC, Zee DS, Carey JP. Strong static magnetic fields elicit swimming behaviors consistent with direct vestibular stimulation in adult zebrafish. PLoS One 2014;9(3):e92109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Luo J, Lv H, Zhang Z, Yang J, Dong D, Fang Y, Hu L, Liu M, Liao Z, Li J, Fang Z, Wei Y, Han W, Shaikh AB, Yin D, Shang P. Safety of exposure to high static magnetic fields (2 T-12 T): a study on mice. Eur Radiol 2019;29(11):6029–6037. [DOI] [PubMed] [Google Scholar]
- 31.Houpt TA, Kwon B, Houpt CE, Neth B, Smith JC. Orientation within a high magnetic field determines swimming direction and laterality of c-Fos induction in mice. Am J Physiol Regul Integr Comp Physiol 2013;305(7):R793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockwood DR, Kwon B, Smith JC, Houpt TA. Behavioral effects of static high magnetic fields on unrestrained and restrained mice. Physiol Behav 2003;78(4-5):635–640. [DOI] [PubMed] [Google Scholar]
- 33.Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Vestibular stimulation by magnetic fields. Ann N Y Acad Sci 2015;1343:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenck JF, Dumoulin CL, Redington RW, Kressel HY, Elliott RT, McDougall IL. Human exposure to 4.0-Tesla magnetic fields in a whole-body scanner. Med Phys 1992;19(4):1089–1098. [DOI] [PubMed] [Google Scholar]
- 35.Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics 2007;28(5):349–361. [DOI] [PubMed] [Google Scholar]
- 36.Antunes A, Glover PM, Li Y, Mian OS, Day BL. Magnetic field effects on the vestibular system: calculation of the pressure on the cupula due to ionic current-induced Lorentz force. Physics in Medicine and Biology 2012;57(14):4477–4487. [DOI] [PubMed] [Google Scholar]
- 37.Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS. MRI Magnetic Field Stimulates Rotational Sensors of the Brain. Current Biology 2011;21(19):1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mian OS, Li Y, Antunes A, Glover PM, Day BL. Effect of head pitch and roll orientations on magnetically induced vertigo. Journal of Physiology-London 2016;594(4):1051–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward BK, Roberts DC, Otero-Millan J, Zee DS. A decade of magnetic vestibular stimulation: from serendipity to physics to the clinic. J Neurophysiol 2019;121(6):2013–2019. [DOI] [PubMed] [Google Scholar]
- 40.Schenck JF. Safety of strong, static magnetic fields. J Magn Reson Imaging 2000;12(1):2–19. [DOI] [PubMed] [Google Scholar]
- 41.Nunez J Morris Water Maze Experiment. J Vis Exp 2008;19:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cudalbu C, McLin VA, Lei H, Duarte JM, Rougemont AL, Oldani G, Terraz S, Toso C, Gruetter R. The C57BL/6J mouse exhibits sporadic congenital portosystemic shunts. PLoS One 2013;8(7):e69782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvina K, Jodeiri Farshbaf M, Mondal AK. Long term effects of stress on hippocampal function: Emphasis on early life stress paradigms and potential involvement of neuropeptide Y. J Neurosci Res 2021;99(1):57–66. [DOI] [PubMed] [Google Scholar]
- 44.Rao R, Tkac I, Unger EL, Ennis K, Hurst A, Schallert T, Connor J, Felt B, Georgieff MK. Iron supplementation dose for perinatal iron deficiency differentially alters the neurochemistry of the frontal cortex and hippocampus in adult rats. Pediatr Res 2013;73(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao R, Nashawaty M, Fatima S, Ennis K, Tkac I. Neonatal hyperglycemia alters the neurochemical profile, dendritic arborization and gene expression in the developing rat hippocampus. NMR Biomed 2018;31(5):e3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traudt CM, Tkac I, Ennis KM, Sutton LM, Mammel DM, Rao R. Postnatal morphine administration alters hippocampal development in rats. J Neurosci Res 2012;90(1):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janus C, D’Amelio S, Amitay O, Chishti MA, Strome R, Fraser P, Carlson GA, Roder JC, St George-Hyslop P, Westaway D. Spatial learning in transgenic mice expressing human presenilin 1 (PS1) transgenes. Neurobiol Aging 2000;21(4):541–549. [DOI] [PubMed] [Google Scholar]
- 48.Takebe A, Furutani T, Wada T, Koinuma M, Kubo Y, Okano K, Okano T. Zebrafish respond to the geomagnetic field by bimodal and group-dependent orientation. Sci Rep 2012;2:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mian OS, Li Y, Antunes A, Glover PM, Day BL. On the Vertigo Due to Static Magnetic Fields. Plos One 2013;8(10):e78748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Magnetic vestibular stimulation in subjects with unilateral labyrinthine disorders. Front Neurol 2014;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell DE, Della Santina CC, Cullen KE. Plasticity within excitatory and inhibitory pathways of the vestibulo-spinal circuitry guides changes in motor performance. Sci Rep 2017;7(1):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Peng Z, Yang M, Zhang X, Wei J, Xu M, Zheng QY. Observation of the morphology and calcium content of vestibular otoconia in rats after simulated weightlessness. Acta Otolaryngol 2005;125(10):1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hozawa J, Kimura N, Kamata S, Ishida T, Usami S, Kamimura T. Influence of long-term repetitive rotatostimulations on lateral semicircular canals. Acta Otolaryngol Suppl 1984;406:245–250. [DOI] [PubMed] [Google Scholar]
- 54.Nakashima T, Itoh M, Watanabe Y, Sato M, Yanagita N. Auditory and vestibular disorders due to barotrauma. Ann Otol Rhinol Laryngol 1988;97(2 Pt 1):146–152. [DOI] [PubMed] [Google Scholar]
- 55.Perez R, Freeman S, Cohen D, Sohmer H. Functional impairment of the vestibular end organ resulting from impulse noise exposure. Laryngoscope 2002;112(6):1110–1114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Images of the animal holder. (A,B) Plastic bins of the animal holder, two mice were kept inside each bin during exposures to magnetic field. (C) The animal holder in front of the 16.4 T magnet. The temperature was maintained at 24°C using warm water circulating and the air exchange in mouse containers was maintained by continuous air flow through silicone tubing connected to mouse containers.
Figure S2. Morris water maze test – Protocol II. Angular velocity analysis – number of tight-circling 360° rotations for all mice chronically exposed to 16.4 T. The box plot represents data of 16 MWM trials (4 trial per test day, 4 days of testing). Boxes indicate median with upper and lower quartiles, whiskers indicate maximum and minimum values.
Table S1. Protocol I: Morris water maze tests performed on mice before exposure to magnetic field. Neither control (N = 12) nor 16.4T* group (N = 12) were exposed to any magnetic field. The label 16.4T* means that this group of mice was later exposed to 16.4 T magnetic field. Submerge platform was located in the north-west (NW) quadrant (zone). Table values represent mean ± SD.
Table S2. Protocol I: Morris water maze tests performed on mice after 4-week long period of a chronic exposure to 16.4 T magnetic field. Two mice groups were exposed to B0 fields for a period of 4 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 12), 16.4T group (N = 12). Submerge platform was located in the south-west (SW) quadrant (zone). Table values represent mean ± SD.
Table S3. Protocol I: Morris water maze tests performed on mice after 8-week long period of a chronic exposure to 16.4 T magnetic field. Two mice groups were exposed to B0 fields for a period of 8 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 12), 16.4T group (N = 12). Submerge platform was located in the south-east (SE) quadrant (zone). Table values represent mean ± SD.
Table S4. Protocol II: Morris water maze tests performed on mice chronically exposed to magnetic fields. Four mice groups were exposed to B0 fields for a period of 4 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 23), 16.4T group (N = 24), 10.5T group (N = 22), 16.4T Motion group (N = 21, instead of 3 hours only 2 min exposures with intensive motion in and out of magnet). Submerge platform was located in the west (W) quadrant (zone). Table values represent mean ± SD.
Table S5. Protocol II: Battery of behavioral tests performed on mice chronically exposed to magnetic fields. Four mice groups were exposed to B0 fields for a period of 4 weeks (3-hour long exposures, 2 exposures per week): Control group (sham exposures, N = 23), 16.4T group (N = 24), 10.5T group (N = 22), 16.4T Motion group (N = 21, instead of 3 hours only 2 min exposures with intensive motion in and out of magnet). Balance beam walking (BB), fear conditioning (FC). Table values represent mean ± SD.
Table S6. Protocol II: Morris water maze test performed six weeks later after the last B0 exposure. Subgroup of mice exposed to 16.4 T (N = 12) and control group (sham exposures, N = 12). Submerge platform was located in the west (W) quadrant (zone). Table values represent mean ± SD.


