Abstract
Background
Implementation and uptake of novel and cost-effective medicines can improve patient health outcomes and healthcare efficiency. However, the uptake of new medicines into practice faces a wide range of obstacles. Earlier reviews provided insights into determinants for new medicine uptake (such as medicine, prescriber, patient, organization, and external environment factors). However, the methodological approaches used had limitations (e.g., single author, narrative review, narrow search, no quality assessment of reviewed evidence). This systematic review aims to identify barriers and facilitators affecting the uptake of new medicines into clinical practice and identify areas for future research.
Method
A systematic search of literature was undertaken within seven databases: Medline, EMBASE, Web of Science, CINAHL, Cochrane Library, SCOPUS, and PsychINFO. Included in the review were qualitative, quantitative, and mixed-methods studies focused on adult participants (18 years and older) requiring or taking new medicine(s) for any condition, in the context of healthcare organizations and which identified factors affecting the uptake of new medicines. The methodological quality was assessed using QATSDD tool. A narrative synthesis of reported factors was conducted using framework analysis and a conceptual framework was utilised to group them.
Results
A total of 66 studies were included. Most studies (n = 62) were quantitative and used secondary data (n = 46) from various databases, e.g., insurance databases. The identified factors had a varied impact on the uptake of the different studied new medicines. Differently from earlier reviews, patient factors (patient education, engagement with treatment, therapy preferences), cost of new medicine, reimbursement and formulary conditions, and guidelines were suggested to influence the uptake. Also, the review highlighted that health economics, wider organizational factors, and underlying behaviours of adopters were not or under explored.
Conclusion
This systematic review has identified a broad range of factors affecting the uptake of new medicines within healthcare organizations, which were grouped into patient, prescriber, medicine, organizational, and external environment factors. This systematic review also identifies additional factors affecting new medicine use not reported in earlier reviews, which included patient influence and education level, cost of new medicines, formulary and reimbursement restrictions, and guidelines.
Registration
PROSPERO database (CRD42018108536).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-021-07196-4.
Keywords: New medicines, Uptake, Implementation, Systematic review, Innovation implementation, Healthcare organizations
Introduction
The uptake of an evidence-based intervention in clinical practice can take on average 17 years before it becomes part of a routine practice [1]. In healthcare, medicines are deemed to be the most common therapeutic intervention requiring significant funds from the system [2]. The slow uptake of cost-effective and novel medicines can delay improvements in patient health outcomes, healthcare efficiency, and even lessen the international competitiveness of the country in the life sciences sector [2–4]. For instance, in the United Kingdom (UK), the relative uptake of nationally recommended new medicines often lags behind other comparative countries’ health systems such as Australia, Canada or France [5].
There is a considerable amount of scientific literature exploring why the implementation of evidence-based interventions succeeds or fails within a complex healthcare environment [6]. Factors affecting implementation outcomes have been grouped into patient, provider, innovation, structural and organizational factors [7]. At the patient level, earlier reviews indicated patients’ socio-demographic and economic characteristics influenced the uptake of new medicines [8–10]. However, patients’ influence through their involvement in decision-making was relatively unexplored [8–10]. At provider level, prescribers’ scientific orientation and prescribing habits were suggested to affect uptake [10]. Furthermore, innovation level factors, such as effectiveness, safety-profile, convenience, and therapeutic novelty of new medicines were considered important aspects. Reviews concluded that cost was of low importance [8–10], but cost could be a factor in current healthcare systems as balancing increasing expenditure on medicines and available funding is becoming harder [2]. At an organizational level, mainly the impact of an organization’s characteristics, e.g., size, ownership, was suggested to have limited impact [8, 10]. Finally, structural level features, such as peer influence, pharmaceutical detailing, scientific literature and meetings, and regulatory pressures were identified as potential factors [8–10].
Although these earlier reviews provided some insight into the determinants of new medicine uptake, the methodological approaches had limitations (e.g., single author, narrative review, narrow search, no quality assessment of reviewed evidence). Also, healthcare systems have changed rapidly over the last ten years with increasing focus on patient-centred care and patient involvement in decision-making [11], use of medicines [2], expenditure on medicines [2], and new policies being developed to improve patient access to new medicines [12]. Studies in earlier reviews might not have captured all factors relevant to current healthcare systems and hence an updated review is warranted. This review, therefore, aims to identify barriers and facilitators affecting the uptake of new medicines into clinical practice, including areas for future research. Also, the review sought to provide more insight on the factors unexplored in earlier reviews such as patient influence and cost of new medicines.
Methods
Protocol and registration
A protocol for this review was registered on PROSPERO (Registration number: CRD42018108536) [13]. The conduct of the systematic review was guided by the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement [14] (see Additional file 1 for the PRISMA checklist).
Eligibility criteria
The inclusion criteria were established using the PICOS framework [15]. Eligible studies focused on adult participants (18 years and older) requiring or taking any new medicine(s) for any condition in the context of healthcare organizations. The World Health Organization definition of health innovation was used to define ‘new medicine’ as new or improved pharmaceutical product which improved people’s health and aimed to “add value in the form of improved efficiency, effectiveness, quality, sustainability, safety and/or affordability” [16]. Studied healthcare organizations were primary or secondary care. Eligible studies identified factors affecting (impeding or facilitating) the uptake of new medicines. The authors of this review considered uptake as the use of a new medicine within a healthcare organization within five years after it had been approved by the regulatory agency of the country where the study was conducted. Studies that only reported prescribing trends and/or patient demographics (age, gender) and clinical comorbidities were excluded. Qualitative, quantitative, or mixed-methods empirical studies published in English were eligible. Grey literature (conference proceedings, theses), review articles, clinical guidelines, and incomplete studies were excluded.
As healthcare systems have changed rapidly over the last ten years in relation to medicine use [2, 11, 12], studies from 2008 and onwards were included in the review to capture studies more relevant to current healthcare systems. Also, the review had a broad search strategy over seven databases, thus the time period limitation allowed to process a manageable number of studies yielded by the search.
Information sources
The search was conducted in seven electronic databases: Medline, EMBASE, Web of Science, CINAHL, Cochrane Library, SCOPUS, and PsychINFO. Hand-searching was conducted using Google Scholar, reference lists, and forward citations of included studies and relevant systematic reviews to identify relevant studies that were inaccurately indexed or unindexed.
Search
The search strategy was developed in collaboration with a subject librarian at the University of Bradford. The search was completed on 4 September 2018 and updated on 23 April 2020. The search terms were developed from four search categories: ‘uptake’, ‘new medicine’, ‘healthcare organization’, and ‘barriers and facilitators’ (see Additional file 2 for Medline search strategy).
Study selection
After the removal of duplicates using the reference management software (EndNote X7®), one reviewer (KM) independently screened titles and abstracts. The second reviewer (JT) screened rejected articles after titles and abstract screening to minimise the removal of potentially relevant studies [17]. Two reviewers (KM, JT) independently reviewed full-texts of potentially relevant studies. The first reviewer (KM) screened the reference lists and forward citations, and the second reviewer (JT) independently reviewed studies deemed to meet the eligibility criteria. Any disagreements were discussed to reach a consensus. If consensus was not reached, the third reviewer (IM) reviewed disagreements.
Data collection process and data items
A standardised proforma was developed by the research team and piloted with five studies before being finalised. One reviewer (KM) independently extracted data for 100% of the studies and the second reviewer (JT) independently checked the data extraction forms for accuracy and completeness. Any disagreements were discussed to reach a consensus. Abstracted data included citation information, study information (aim, design, data source, setting), studied new medicine, participant details, findings relevant to this review, funding source and reported conflict of interest. Lead authors of the studies were contacted via e-mail to provide missing or additional data if required.
Risk of bias in individual studies
Two independent reviewers (KM and JT or IM) assessed risk of bias of included individual studies using the Quality Assessment Tool for Studies with Diverse Designs (QATSDD) [18]. The QATSDD tool consists of 16 criteria and is validated to assess studies with heterogeneous study designs. The following aspects of studies were examined: theoretical framework; aims and objectives; research setting; sample size and representativeness; data collection procedure and rationale; recruitment; appropriateness, reliability and validity of data analysis tools or process; user involvement; strengths and limitations. Reviewers scored each study on a scale of 0 (not at all/not stated) to 3 (complete/explicitly stated) against each criterion. The maximum score was of 42 for quantitative and qualitative studies and 46 for mixed-methods studies. Disagreements were resolved through discussion or by a third reviewer (KM, JT, or IM). The total score for each study was calculated by adding scores for each criterion and expressed as a percentage (0–100%). Studies with scores < 50% were classed as low, 50 to > 70% as moderate, and > 70% as high-quality studies. Although the low methodological quality studies were not excluded, they were given less weight in the synthesis of results and conclusions.
Synthesis of results
The meta-analysis was not feasible due to the heterogeneity of study designs and methods used. Therefore, a narrative synthesis using a ‘best fit’ framework synthesis [19] was conducted to summarise the findings of reviewed studies.
‘Best fit’ framework synthesis is based on framework analysis [20] and is thought to bring a more transparent and pragmatic process than other more interpretive forms of synthesis [19]. It is a two-stage synthesis process. Firstly, an initial framework of preliminary themes was identified against which the data from the reviewed studies would be mapped. The initial framework used in this review was a multi-level framework by Chaudoir et al., which was developed by collecting implementation success factors for health innovations from multiple previous frameworks [7]. The themes in the Chaudoir et al. [7] framework were patient, provider, innovation, structural, and organizational.
The second stage of the ‘best fit’ framework synthesis involved reviewing the studies meeting the inclusion criteria and coding the identified factors affecting the uptake of new medicines against the themes in the multi-level framework by Chaudoir et al. [7]. The reviewing and summarising of the coded data were completed using NVivo11 software. New themes were created for data that could not be coded against the framework through a process of interpretation similar to thematic analysis [19].
Two reviewers (KM, IM) independently coded the material, and any discrepancies were resolved through discussion. Identified factors were finalised in a team discussion (KM, SR, KS, DP).
Results
Study selection
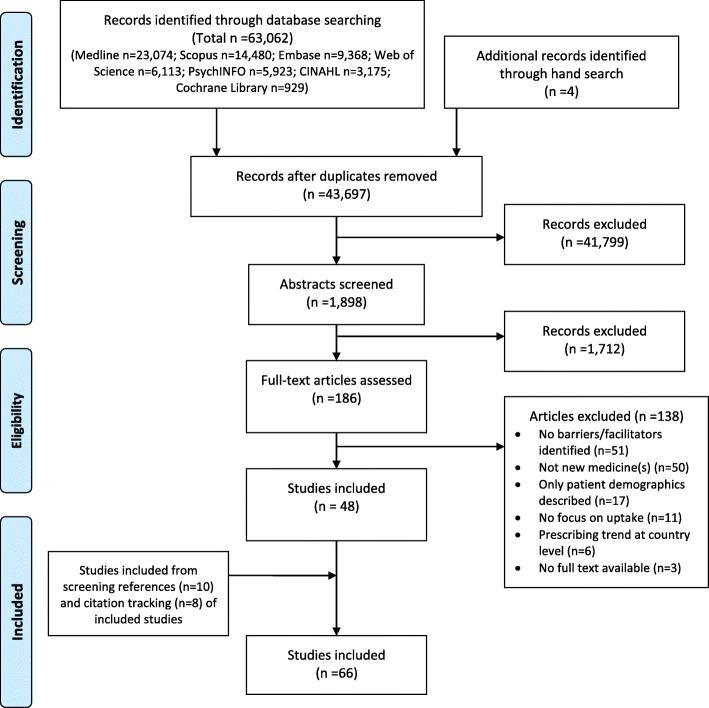
The study selection process is summarised in the PRISMA flowchart (Fig. 1). Of 43,697 unique citations identified in the search strategy, and an additional 22 studies retrieved through alternative methods, 66 studies were eligible for inclusion.
Fig. 1.
PRISMA flow diagram showing the systematic literature search and screening process
Study characteristics
Study characteristic of included studies are presented in Table 1. Most of the studies (n = 62) used quantitative methods [21–49, 51–65, 68–79, 81–86]; three were qualitative [50, 67, 80], and one was mixed-methods [66]. The predominant source of data collection was secondary data (n = 46) from various databases and registries [22–25, 27, 29–34, 36, 40–44, 46, 48, 51–64, 66, 70–72, 74, 78, 79, 81–85, 87]. Other studies (n = 17) used surveys [26, 44, 47, 67, 68, 73], interviews [21, 26, 37, 50, 57, 66, 67, 76, 80], patients’ medical records [28, 45], prescriptions from community pharmacies [75], observations [35], or a focus group [80] to collect primary data. Two studies [38, 49] used both primary and secondary data. Studied new medicines were from twenty different therapeutic classes and five studies described medicines as newly marketed.
Table 1.
Characteristics of included studies looking at factors affecting the uptake of new medicines
| Author(s), publication year, country | Study objective related to this systematic review | Study Design | Data Source | Setting | Medicine(s) | Sample | Key Findings | Funding source and Conflict of interest (COI) |
|---|---|---|---|---|---|---|---|---|
| Abraham et al. (2010), USA [21] | To investigate if participation in clinical trials research network influences adoption of alcohol pharmacotherapies in publicly funded programs | Quantitative | Face-to-face interviews and brief telephone interviews | Primary and secondary care | acamprosate |
244 public programs, 127 Clinical Trail Network (CTN) affiliated program administrators |
Affiliation of programs with CTN; Percentage of master’s level counsellors; Access to a prescribing physician. |
Funding: national funding body COI: not reported |
| AbuDagga et al. (2014), USA [22] | To identify factors associated with dabigatran versus warfarin use | Quantitative | Administrative pharmacy and medical claims database | Primary and secondary care | dabigatran | 20,320 patients | Patient’s clinical and demographic characteristics; Speciality of prescriber; Patient’s health insurance plan type. |
Funding: Daiichi Sankyo COI: one author was employee of Daiichi Sankyo; another received payments from Daiichi Sankyo; four authors-none |
| Anderson et al. (2015), USA [23] | To determine if conflict of interest policies influence psychiatrists’ antipsychotic prescribing and compare prescribing between academic and non-academic psychiatrists | Quantitative | IMS Health databases and physicians’ characteristics database | Primary and secondary care | Nine new and reformulated antipsychotics | 2464 prescribers | Affiliation with academic medical centres with conflict-of-interest policies; Type of prescriber (academic or non-academic). |
Funding: national funding body COI: none |
| Anderson et al. (2018), USA [24] | To explore characteristics of prescribers adopting new cardiovascular medicines | Quantitative | IMS Health databases | Primary and secondary care | dabigatran, aliskiren | 5953 physicians | Speciality of prescriber; Gender of prescriber; Medical school attended by prescriber. |
Funding: national funding body COI: none |
| Baik et al. (2016), USA [25] | To evaluate how patient characteristics are associated with the initiation of anticoagulant for patients newly diagnosed with atrial fibrillation | Quantitative | Pharmacy claims database | Primary and secondary care | dabigatran, rivaroxaban | 17,193 patients | Patient clinical and demographic characteristics; Patient’s health insurance plan type; Out-of-pocket expenses- no effect. |
Funding: national funding body COI: none |
| Boon et al. (2008), Belgium [26] | To examine the impact of reimbursement restrictions on the choice of antiepileptic (AEDs) medicines | Quantitative | Structured face-to-face interviews | Secondary care | 16 AEDs, including old and new | 100 neurologists | Reimbursement condition; Formulary restrictions. |
Funding: GlaxoSmithKline COI: not reported |
| Bourke and Roper (2012), UK [27] | To explore the factors that shape the timing of the first prescription of six new medicines by General Practitioners (GPs) | Quantitative | Prescribing and GP characteristics databases | Primary care | escitalopram, rofecoxib, esomeprazole, desloratadine, nicotine, drospirenone and oestrogen | 625 GP practices | Availability of nurse or clerical support; Participation in national incentive program to reduce prescribing costs; Previous early adoption of new medicines; GP’s prescribing portfolio size; Geographical location of GP practice. |
Funding: not reported COI: not reported |
| Brais et al. (2017), Canada [28] | To identify predictors of oral anticoagulant choice for patients with atrial fibrillation | Quantitative | Electronic medical records | Secondary care | dabigatran, rivaroxaban, apixaban | 439 patients at single teaching hospital | Patient’s demographic and clinical characteristics; Speciality of prescriber. |
Funding: Bayer Inc., and Bristol-Myers Squibb Company-Pfizer alliance COI: not reported |
| Burden et al. (2015), Canada [29] | To examine the impact of formulary changes to the use of zoledronic acid | Quantitative | Pharmacy claim database and prescriber databases | Primary and secondary care |
zolendronic acid, denosumab |
18,226 patients | Formulary status change (removal of prior authorisation); Speciality of prescriber; Gender of prescriber. |
Funding: national funding body COI: none |
| Carracedo-Martínez et al. (2017), Spain [30] | To assess the impact of the removal of prior authorization requirements for two coxibs on their use | Quantitative | Pharmacy claim database | Primary care | celecoxib, etoricoxib | One health district,catchment area of 383,125 people | Formulary prescribing conditions (prior authorisation requirement). |
Funding: none COI: none |
| Chamberlain et al. (2014), UK [31] | To explore the impact of the Cancer Drug Fund (CDF) on access to cancer medicines in England, compared with Wales | Quantitative | IMS Health databases | Secondary care | 15 cancer medicines | Not stated- prescribing volumes milligrams/1000 population used | The CDF was associated with higher prescription volumes in England for most medicines, which NICE had rejected for some or all indications pre-CDF and for medicines, which NICE had not appraised pre-CDF, but subsequently rejected. |
Funding: national funding body COI: none |
| Chitagunta et al. (2009), USA [32] |
To study the role of learning in the diffusion of three Cox-2 Inhibitors before withdrawal of rofecoxib |
Quantitative | Prescription and advertising expenditure databases, published articles | Primary and secondary care | celecoxib, rofecoxib, valdecoxib | 6577 patients and 17,329 prescriptions | Advertising, news and academic articles; Socio-economic status of patient; Patient’s demographic characteristics; Patient’s health insurance plan type; Patient’s satisfaction with existing treatment. |
Funding: not reported COI: not reported |
| Chressanthis et al. (2012), USA [33] | To examine the effect of access limits to pharmaceutical representatives on new medicines prescribing by physicians | Quantitative | IMS Health databases | Primary and secondary care | sitagliptin | 65,131 physicians | Organisation restrictions to pharmaceutical representative access; Speciality and age of prescriber; Size and geographical location of organisation |
Funding: AstraZeneca COI: two authors were employees of AstraZeneca |
| Conti et al. (2012), USA [34] | To examines how evidence of the incremental effectiveness of novel chemotherapy medicines impacts on the adoption by physicians | Quantitative | Chemotherapy order system database | Secondary care | Seven oral chemotherapy medicines | 4,344,711 patients, 122 medical oncology practices in 35 the USA states | Severity of the underlying disease; Clinical trials and media reports concurrent with market launch date; Medicine effectiveness. |
Funding: national funding body COI: not reported |
| DeVore et al. (2018), USA [35] | To identified patient, provider, and practice characteristics associated with sacubitril/valsartan use | Quantitative | Observations | Primary and secondary care | sacubitril/valsartan | 4216 patients, 121 sites across the USA | Patient’s clinical and demographic characteristics; Socio-economic status of patient; Patient’s health insurance plan type; Speciality of prescriber; Size of organisation; Staff composition at the organisation. |
Funding: Novartis COI: five authors in previous receipt of funding from pharmaceutical industry; two acts as consultants to pharmaceutical industry; two were employees of Novartis |
| Donohue et al. (2018), USA [36] | To estimate the effect of peer adoption of three first-in-class medications on physicians’ own adoption of those medications. | Quantitative | IMS Health, insurance, and administrative claims databases | Primary and secondary care | dabigatran, sitigliptin, aliskiren | 11,958 physicians | Peer influence (internal and external). |
Funding: national funding body COI: not reported |
| Ducharme and Abraham (2008), USA [37] | To examine predictors of buprenorphine adoption | Quantitative | Brief telephone interviews and survey database | Primary and secondary care | buprenorphine | Staff members from 49 USA states and a data set of 12,236 substance abuse treatment facilities | Government owned and non-profit facilities; Hospital-based programs and opioid treatment programs; Programs offering detoxification services; Accredited programmes; Programmes serving adult population; Geographical location and size of programme; Government funding; Programs having at least one managed care contract; Coverage of medicine by patient’s health insurance. |
Funding: national funding body COI: none |
| Dybdahl et al. (2011), Denmark [38] | To analyse associations between GPs’ clinical interests and their preference for new medicine | Quantitative | Postal survey and pharmacy prescription database | Primary care | Three COX-2 inhibitors and six angiotensin-II antagonists medicines | 68 GPs | Continuous medical education activities. |
Funding: not reported COI: authors received consultant fees or/and were previously involved in pharmaceutical industry funded research |
| Friedman et al. (2010), USA [39] | To examine the influence of senior managers’ characteristics on the adoption of buprenorphine | Quantitative | Telephone survey | Primary and secondary care | buprenorphine | 547 pairs of administrative directors and clinical supervisors | Gender, age, the length of service and views of programme directors on treatment; Affiliations and accreditation of programme; Breadth of provided medical services and use of other medicines; Staff composition; Gender of patients. |
Funding: national funding body COI: |
| Fuksa et al. (2015), Czech Republic [40] | To evaluate the overall changes in statin utilisation and expenditure with regards to the changing prescribing conditions | Quantitative | Insurance prescription claims database | Primary and secondary care | atorvastatin, rosuvastatin | 774,281 patients | Changes in formulary prescribing conditions. |
Funding: not reported COI: not reported |
| Garjon et al. (2012), Spain [41] | To analyse the diffusion of new medicines during the first months of use and examine the adoption between family physicians and specialists | Quantitative | Prescription database | Primary and secondary care | cefditoren, duloxetine, etoricoxib, ezetimibe, levocetirizine, olmesartan, pregabalin and tiotropium | 1248 physicians | Speciality of prescriber; Therapeutic innovation of medicine; Range of indications for medicine; Prior authorisation requirement. |
Funding: not reported COI: three authors received educational fees from pharmaceutical industry |
| Groves et al. (2010), Canada [42] | To assess relationship between physicians’ characteristics and prescribing of new medicines | Quantitative | Administrative and insurance claims databases | Primary and secondary care | Four COX-2 inhibitors and two non-selective NSAIDs medicines | 925 physicians | Demographic characteristics; Speciality of prescriber; Geographical location of practice; Caseload of prescriber. |
Funding: not reported COI: not declared |
| Haider et al. (2008), Sweden [43] | To examine the association between educational level of patients and the use of newly marketed medicines among elderly patients | Quantitative | Three national registers: prescribed medicines, inpatient, and education | Primary and secondary care | 18 newly marketed medicines with at least 350 users | 626,258 patients | Patient’s educational level and gender; Number of prescribed medicines for patient; Patient’s residential area. |
Funding: not reported COI: none |
| Hickson et al. (2019), USA [44] | To describe trends over time in the initiation of the dipeptidyl peptidase-4 (DPP-4) inhibitors before and after removal of the rosiglitazone black box warning and restricted access program | Quantitative | Administrative claims database | Primary and secondary care | DPP-4 inhibitors | 280,969 patients | Regulatory restrictions to the use of medicines in the same category as new medicines. |
Funding: not reported COI: one author was employee of Truven Health Analytics/IBM Watson Health |
| Hirunrassamee and Ratanawijitrasin (2009), Thailand [45] | To assess access to medicines and other medical technologies under the three government health insurance schemes | Quantitative | Hospital electronic database and paper records | Secondary care | Antiepileptic and antineoplastic lung cancer medicines | 913 patients (antiepileptics), 33 patients (antineoplastics); 3 hospital sites | Patient’s health insurance plan type; Out-of-pocket payments. |
Funding: not reported COI: not reported |
| Hsieh and Liu (2012), Taiwan [46] | To explore issues surrounding utilisation of biologics in Taiwan | Quantitative | National insurance claims database | Secondary care | trastuzumab, rituximab, peginterferon-alfa-2A, etanercept | 590 patients | Size of hospital; Type of hospital ownership; Patient’s clinical characteristics. |
Funding: national funding body, Johnson & Johnson COI: none |
| Huang et al. (2013), USA [47] | To examine factors that influence doctors’ decision in initiating or switching from warfarin to dabigatran | Quantitative | Online survey | Secondary care | dabigatran | 65 physicians | Cost of medicine; Patient’s socioeconomic status; Patient’s clinical characteristics; Speciality of prescriber; Experience of prescriber with the medicine; Perceived benefits of new over ‘old’ therapy. |
Funding: not reported COI: not reported |
| Huskamp et al. (2013), USA [48] | To examined physician adoption of second-generation antipsychotic medications and identified physician-level factors associated with early adoption | Quantitative | IMS Health prescription database | Primary and secondary care |
olanzapine, quetiapine, ziprasidone, and aripiprazole |
30,369 physicians | Age and gender of prescriber; Speciality of prescriber; Size and type of practice; Caseload of prescriber; Medical school location of prescriber. |
Funding: national funding body COI: one author consulted National Railways Labor Conference |
| Iyengar et al. (2011), USA [49] | To assess the impact of social networks on the adoption of a new medicine by physicians | Quantitative | Mailed and online survey, IMS Health databases, and pharmaceutical company sales calls records | Primary and secondary care | A newly launched prescription medicine used to treat a specific type of viral infection (short and long-term) | 185 physicians from three cities | Peers influence- the level of impact is shaped by peer’s usage volume and by the clinicians’ perception of their self-reported opinion leadership. Perceived leaders by colleagues adopted new medicine quicker than self-reported leaders. |
Funding: not reported COI: not reported |
| Karampli et al. (2020), Greece [50] | To explore factors influencing adoption of new antidiabetic medicines for patients with type 2 diabetes mellitus | Qualitative | Semi-structured face-to-face interviews | Primary and secondary care |
DDP-4 inhibitors, GLP-1 agonists, SGLT2 inhibitors, new oral fixed-dose combinations of glycose-lowering medications, new dosage forms |
10 physicians | New medicine’s safety profile, efficacy, degree of relative advantage, formulation, cost, ease of use; Habitual prescribing of physician; Physician’s needs and values of practice; Physician’s experience with established medicines; Patient’s clinical and demographic characteristics; Patient’s preferences and adherence to treatment; Patient’s health insurance plan type; Working place of physician. |
Funding: none COI: none |
| Keating et al. (2018), USA [51] | To examine diffusion of bevacizumab and assess variation in use across oncology practices | Quantitative | Insurance claim database | Secondary care | bevacizumab | 2329 practices | Size and accreditation of organisation; Staff composition at organisation; Patient’s clinical and demographic characteristics; Patient’s socio-economic status. |
Funding: national funding body COI: none |
| Keating et al. (2020), USA [52] | To understand adoption of bevacizumab by oncologists for patients with cancer using network analysis method | Quantitative | Insurance claim database | Secondary care | bevacizumab | 44,012 patients, 3261physicians, 51 hospital referral regions | Patient’s clinical and demographic characteristics; Age of prescriber; Peer influence. |
Funding: national funding body COI: one author received consultant fees from Grail |
| Kennedy et al. (2020), Ireland [53] | To compare the use of direct oral anticoagulants in areas with warfarin clinics compared to those without | Quantitative | Pharmacy claims database shapefiles of warfarin clinics and areas | Primary care | apixaban, dabigatran, edoxaban, rivaroxaban | Presence or absence of hospital-based warfarin clinics- no effect. |
Funding: national funding body COI: none |
|
| Kereszturi et al. (2015), Hungary [54] |
To identify socio-demographic, workplace, practice, prescribing and patient characteristics of the early prescribers of the newly marketed innovative medicines |
Quantitative | DoktorInfo prescription database | Secondary care | vildagliptin with metformin and metformin with sitagliptin combinations | 318 physicians | Portfolio width and prescribing volume of prescriber; Number of patients looked after by prescriber and number of consultations per patient; Prescribing of other branded medicines; Proportion of patients treated with insulin. |
Funding: AXA Research Fund COI: not reported |
| King et al. (2013), USA [55] | To examine the effect of attending a medical school with an active policy on restricting gifts from representatives of pharmaceutical and device industries on subsequent prescribing behaviour | Quantitative | IMS Health database and physicians’ characteristics database | Primary care | lisdexamfetamine, paliperidone, desvenlafaxine | 8602 physicians | Attending a medical school with an active gift restriction policy; Length of exposure to gift restriction policy. |
Funding: national funding body COI: none |
| King and Bearman (2017), USA [56] | To examine how different pharmaceutical detailing regulations and peer influence shaped medicine diffusion processes of newly marketed medicines | Quantitative | IMS Health prescription database | Primary care | lisdexamfetamine, duloxetine | 208,072 physicians for duloxetine, 215,445 physicians for lis-dexamfetamine | Policies limiting or banning gifts from pharmaceutical industry; Peer influence. |
Funding: not reported COI: not reported |
| Knudsen et al. (2009), USA [57] | To examines the adoption of buprenorphine over a 2-year period in community-based treatment programs associated and not with Clinical Trials Network (CTN) | Quantitative | Telephone and face-to-face interviews | Primary care | buprenorphine | 193 community-based treatment programs (CTPs) | Involvement in CTN buprenorphine protocol development; Size of organisation; Access to prescribers; Offering other inpatient services; Type of organisation. |
Funding: national funding body COI: not reported |
| Lin H et al. (2011), USA [58] | To explore the patterns of physician prescribing and medication choice for major depressive disorder between 1993 and 2007 | Quantitative | National survey database | Primary care | Four antidepressant drug classes | 125,605,444 patients | Patient’s health insurance type; Age of patient; Practice geographical location |
Funding: not reported COI: not reported |
| Lin S et al. (2011), Taiwan [59] | To examine how the prescribing decisions made by psychiatrists’ colleagues influence the likelihood of the psychiatrists’ initial prescription | Quantitative | National insurance database | Secondary care | duloxetine | 155 psychiatrists | Speciality of prescriber; Clinical experience of prescriber; Adoption behaviour of colleagues. |
Funding: university funding COI: not reported |
| Liu et al. (2011), Taiwan [60] | To investigate the effect of various economic factors on the diffusion of new medicines | Quantitative | National drug claims database | Primary and secondary care | seven oral anti-glycaemic medicines | 3,384,223 prescriptions | Degree of competition in the pharmaceutical and health service market; Size of the provide; Type of organisation; Disease severity; Geographical location of organisation. |
Funding: national funding body COI: not reported |
| Liu and Gupta (2012), USA [61] | To analyze individual physicians’ adoption of a newly launched prescription medicine | Quantitative | ImpactRx market research database and TNS Media Intelligence data (journal advertising expenditure) | Primary and secondary care |
A newly launched medicine from one of the largest therapeutic classes of prescription medicines in USA, novel mechanism of action |
2129 physicians |
Targeted detailing, journal advertising, meetings and events sponsored by industry, peer influence, and patient requests has positive impact. Specialists and prescribers with larger prescription volumes in the studied therapeutic class and who practice in communities with a larger percentage of patients from a White background adopted the new medicine quicker. |
Funding: not reported COI: not reported |
| Lo-Ciganic et al. (2016), USA [62] | To examine the physician adoption of dabigratran | Quantitative | IMS Health database and physicians’ characteristics database | Primary and secondary care | dabigatran | 3911 prescribers | Speciality of prescriber; Prescribers age; Hospital referral region; Patient’s health insurance plan type. |
Funding: national funding body; university funding COI: none |
| Luo et al. (2017), USA [63] | To assess the prevalence and variation in sacubitril/valsartan prescription among a real-world population with heart failure with reduced ejection fraction | Quantitative | National registry of hospitalised patients | Secondary care | sacubitril/valsartan | 21,078 patients, 241 hospital sites | Geographical location of organisation; Accreditation of organisation-no effect; Patient’s clinical and demographic characteristics; Patient’s health insurance plan type-no effect. |
Funding: Novartis COI: one author was employee and three received consultant fees from Novartis; one received research funding from pharmaceutical companies |
| Luo et al. (2018), USA [64] | To evaluate the early impact of this national treatment guideline update on the use of sacubitril/valsartan | Quantitative | National registry of hospitalised patients and national hospitals survey database | Secondary care | sacubitril/valsartan | 7200 patients | Size, location, accreditation of organisation and available services- no effect; National guideline publication- little/no effect. |
Funding: Novartis COI: one author was employee of Novartis; four received research support from industry |
| Luo et al. (2019), USA [65] | To identify hospital characteristics associated with the use of sacubitril/valsartan | Quantitative | National registry of hospitalised patients; national hospitals survey database, US census region, insurance claim database. | Secondary care | sacubitril/valsartan | 16,674 patients, 210 hospital sites | Size and accreditation of organisation-no effect; Organisation type (profit/non-profit); Geographical location of organisation; Follow-up ambulatory services-no effect. |
Funding: Novartis COI: one author was employee of Novartis; three authors received research support from pharmaceutical companies |
| Manchanda et al. (2008), USA [66] | To explore impact of marketing and interpersonal communication on the adoption of a new medicine in two unrelated markets | Mixed-methods | Pharmacy audit database, pharmaceutical company marketing records, interviews | Primary and secondary care | A new medicine from important medicine category | 466 physicians | Pharmaceutical industry targeted communication; Detailing, detailing stock, and sampling stock by pharmaceutical industry; Peer influence; Direct advertising to patients-no effect. |
Funding: university funding COI: not reported |
| Martin et al. (2017), France [67] | To explore the barriers to the diffusion of newly released oral targeted therapies dedicated to metastatic breast cancer | Qualitative | Semi-structured face-to-face interviews | Secondary care | everolimus | 40 physicians | Amount of new information to be acquired about the medicine; Lack of organisation in patient management; Time required to manage oral cancer treatments; Prescriber’s prescribing habits; No clear position of the new medicine in the therapeutic strategy; Being the only oncologist or multi-organ oncologist in the organisation. |
Funding: Odyssea association COI: none |
| Murphy et al. (2018), Ireland [68] | To explore factors that influence general practitioners prescribing of direct oral anticoagulants | Quantitative | Postal survey | Primary care | apixaban, dabigatran, edoxaban, rivaroxaban | 221 general practitioners | Hospital colleagues’ influence; Local and national guidelines; Conferences and journal articles; Clinical and demographic characteristics of patient; Perceived efficacy of medicine; Monitoring requirements; Size of practice. |
Funding: none COI: none |
| Netherland et al. (2009), USA [69] | To examine factors affecting willingness to adopt buprenorphine by physicians | Quantitative | On-site and online surveys | Primary care | buprenorphine | 172 prescribers, two national programs | Training of clinical staff on new medicine; Access to other services and treatments; Presence of effective referral system for alternative treatment; Adequate time per visit; Patients’ concerns about medicine; Availability of clinical guidelines and medicine; Reimbursement for consultation; Record keeping requirements; Access to an expert prescriber; Gender and ethnicity of prescriber; Experience and speciality of prescriber. |
Funding: national funding body COI: not reported |
| Ohl et al. (2013), USA [70] | To determine rural-urban variation in adoption of raltegravir amongst in national Veterans Affairs healthcare | Quantitative | Health care and residence databases | Primary and secondary care | raltegravir | 1222 patients | Residential area of patient; Patient’s clinical and demographic characteristics; Previous use of antiretroviral medicines. |
Funding: national funding body COI: not reported |
| Ohlsson et al. (2009), Sweden [71] | To investigate determinants of early adoption of rosuvastatin | Quantitative | National drug register | Primary care | rosuvastatin | 73,547 prescriptions from 170 health care practices | Type of ownership; Existence of strong therapeutic traditions; Socioeconomic status of patient. |
Funding: not reported COI: not reported |
| Patel et al. (2015), USA [72] | To characterise the prevalence, patterns, and predictors of direct oral anticoagulants versus warfarin therapy at discharge among atrial fibrillation patients hospitalised with ischemic stroke or transient ischemic attack | Quantitative | National stroke database | Secondary care | dabigatran, rivaroxaban | 61,655 patients from 1542 hospitals | Patient’s clinical characteristics; Ambulatory status of patient; Discharge destination; Patient’s health insurance plan type. |
Funding: national funding body COI: two authors received consultant fees and three research support from pharmaceutical industries |
| Potpara et al. (2017), Balkan countries [73] | To explore the use of direct oral anticoagulants in seven Balkan countries | Quantitative | Online survey | Secondary care | dabigatran, rivaroxaban, apixaban | 2663 patients from 49 centres | Speciality of prescriber; Patient’s clinical characteristics; Atrial fibrillation treatment strategy; Hospital-based centres; Previous use of oral anticoagulants. |
Funding: none COI: six authors received speaker fees and one consultant fees from pharmaceutical industry |
| Rodwin et al. (2020), USA [74] |
To examine patient and hospital-level factors associated with prasugrel and ticagrelor use in acute myocardial infarction |
Quantitative | National hospital registry for patients with myocardial infarction | Secondary care | prasugrel, ticagrelor |
362,354 patients, 801 hospitals |
Patient’s clinical and demographic characteristics; Patient’s health insurance plan type; Number of patients treated in hospital; Geographical location and accreditation of organisation; Speed of adoption of previous innovation. |
Funding: national funding body COI: one author received consultant fees and research support for pharmaceutical industry; one author received salary support from the funding body, funding from insurance companies, and hold equity interest in Medtronic |
| Sato et al. (2012), Japan [75] | To assess the impact of the sitagliptin regulatory safety alert on the prescribing behaviour | Quantitative | Prescription data from 300 pharmacies | Primary and secondary care | sitagliptin | 87,678 patients | Size of hospital; Speciality of prescriber; Safety alert. |
Funding: none COI: two authors received research support from pharmaceutical industry |
| Savage et al. (2012), USA [76] | To examine the extent to which programs’ interorganisational institutional and resource-based linkages predict the likelihood of being an earlier adopter, later adopter, or non-adopter of buprenorphine | Quantitative | Face-to-face interviews and brief telephone interviews | Primary and secondary care | buprenorphine | 345 privately funded substance abuse treatment programs | Membership in national and regional associations; Detailing activities by pharmaceutical companies; Use of National Institute on Drug Abuse website as an information source. |
Funding: national funding body COI: not reported |
| Scholten et al. (2015), Germany [77] | To examine the factors at the organisational level that influence the implementation of systemic thrombolysis in stroke patients. | Quantitative | Hospital structure quality reports registry | Secondary care | alteplase | 286 hospitals | Existence of stroke unit; Hospital size. |
Funding: none COI: none |
| Steinberg et al. (2013), USA [78] | To identify patient and/or provider factors associated with the use of dabigatran in patients with atrial fibrillation | Quantitative | National registry for outpatients with atrial fibrillation | Secondary care | dabigatran |
8794 patients, 176 sites |
Patient’s clinical and demographic characteristics; Patient’s health insurance plan type; Education level of patient; Current antiarrhythmic use; Speciality of prescriber. |
Funding: Janssen Scientific Affairs, national funding body COI: seven author received consultant fees, five research support, two speaker fees from pharmaceutical industry, one author employed by Johnson & Johnson. |
| Tanislav et al. (2018), Germany [79] | To investigate oral anticoagulation in stroke patients documented in a nationwide registry | Quantitative | National hospital quality registry | Secondary care | apixaban, dabigatran, edoxaban, rivaroxaban | 3813 patients | Treatment on stroke unit; Patient’s clinical and demographic characteristics; Previous oral anticoagulant/ antiplatelet use. |
Funding: none COI: none |
| Tobin et al. (2008), Australia [80] |
To identify the factors that influence prescribing of new medicines among general practitioners, endocrinologists and psychiatrists |
Qualitative | Focus groups with semi-structure interview guide | Primary and secondary care |
Medicine that has in the past 1–2 years been in Pharmaceutical Benefit Scheme (PBS) listed, or released to the market, or a new chemical entity |
21 prescribers | Socioeconomic status of patient; Clinical need for medicine; New medicine’s attributes: adverse effects, safety, efficacy; Listing of medicine in PBS; Peer influence; Prescriber’s familiarity with the therapeutic area; Prescriber’s knowledge of the medicine. |
Funding: non-profit organisation COI: not reported |
| Tsai et al. (2010), Taiwan [81] | To examine factors affecting thiazolidinediones penetration into Taiwan’s hospitals | Quantitative | National health insurance database | Secondary care | pioglitazone, rosiglitazone | 580 hospitals | Degree of competition in the pharmaceutical market; Type of hospital; Type of ownership of hospital; Geographical location of hospital; Cost of medicines; Prescribing volume of diabetic medicines by hospital. |
Funding: national funding body COI: not reported |
| Wang et al. (2010), Taiwan [82] | To determine if socioeconomic status impacts adoption of newly reimbursed non-steroidal anti-inflammatory medicines under a universal health insurance program | Quantitative | Eight different electronic databases | Primary and secondary care | rofecoxib, celexocib, nimesultide | 875 patients | Patient’s clinical and demographic characteristics; Patient’s socio-economic status; Patient’s habits of health-care utilisation. |
Funding: not reported COI: not reported |
| Weir et al. (2012), Canada [83] | To explore the impacts of formulary listing changes and regulatory agency warnings on the use of erythropoiesis-stimulating agents in cancer patients | Quantitative | Prescription and physician characteristics databases, province people registry | Secondary care |
Three erythropoiesis-stimulating agents |
171,967 patients | Formulary changes in reducing or removing restrictions for use; Safety warnings from regulatory agencies. |
Funding: national funding body COI: one author received honorarium from Amgen |
| Wen et al. (2011), Taiwan [84] | To characterise how a new medicine class for diabetes mellitus diffused in the health care market | Quantitative | National insurance claim database | Secondary care | rosiglitazone, pioglitazone | 580 hospitals | Accreditation and type of hospital; Type of ownership of hospital; Degree of competition in the pharmaceutical market; Geographical location of hospital; Number of prescribers prescribing these medicines; Prior anti-diabetic prescription capacity. |
Funding: national funding body COI: none |
| Zhang et al. (2019), Australia [85] | To evaluate how physicians’ risk preferences and personality affects their decisions to adopt new prescription medicines | Quantitative |
Database of national panel survey of medical practitioners, insurance claim database |
Primary care | apixaban, dabigatran, rivaroxaban | 576 GPs | Socio-demographic characteristics of prescriber; Prescribing volume; Willingness to take clinical risks; Employment status in the GP practice; Time spent in consultations; Location of GP practice; GP practice affiliations and social practice characteristics-no effect; Patient’s demographic characteristics; Patient’s socio-economic status. |
Funding: national funding body, university COI: none |
| Zhang et al. (2020), China [86] | To obtain information on the use of PD-1/PD-L1 inhibitors by oncologists in China | Quantitative | Online and offline survey | Secondary care | PD-1/PD-L1 checkpoint inhibitors | 588 oncologists | Knowledge and understanding mechanism of action of new medicines; Experience in using new medicines; New medicine’s attributes: cost, efficacy, adverse effects. |
Funding: none COI: none |
Risk of bias within studies
The methodological quality of studies ranged from 45 to 81%, with a mean score of 67% (see Additional file 3, Table S1). Two studies were deemed to be low, 38 medium, and 26 high quality. The most prominent methodological weaknesses were lack of reporting reliability and validity of data measurement tools used in quantitative studies and reliability of analytical process used in qualitative studies. There was no evidence of pilot testing or user involvement across all studies (Table 2).
Table 2.
Summary of the scores for the 16 criteria used to assess the methodological quality shown for all studies, quantitative and qualitative studies*
| QASTDD tool criteria and study design | Range | Mean | Standard deviation | % maximum of possible score achieved |
|---|---|---|---|---|
| 1. Explicit theoretical framework (all studies) | 0–3 | 1.9 | 0.9 | 62% |
| Quantitative studies | 0–3 | 1.9 | 0.9 | 62% |
| Qualitative studies | 1–3 | 1.7 | 1.2 | 56% |
| 2. Statement of aims/objectives in main body of report (all studies) | 2–3 | 2.8 | 0.4 | 93% |
| Quantitative studies | 2–3 | 2.8 | 0.4 | 92% |
| Qualitative studies | 3 | 3 | 0 | 100% |
| 3. Clear description of research setting (all studies) | 2–3 | 2.7 | 0.5 | 89% |
| Quantitative studies | 2–3 | 2.7 | 0.5 | 89% |
| Qualitative studies | 2–3 | 2.7 | 0.5 | 89% |
| 4. Evidence of sample size considered in terms of analysis (all studies) | 0–3 | 1.5 | 0.7 | 50% |
| Quantitative studies | 1–3 | 1.5 | 0.6 | 49% |
| Qualitative studies | 0–3 | 2 | 1.7 | 67% |
| 5. Representative sample of target group of a reasonable size (all studies) | 1–3 | 2.2 | 0.5 | 74% |
| Quantitative studies | 1–3 | 2.2 | 0.5 | 75% |
| Qualitative studies | 2 | 2 | 0 | 67% |
| 6. Description of procedure for data collection (all studies) | 1–3 | 2.4 | 0.7 | 81% |
| Quantitative studies | 1–3 | 2.4 | 0.7 | 81% |
| Qualitative studies | 2–3 | 2.7 | 0.6 | 89% |
| 7. Rationale for choice of data collection tool(s) (all studies) | 0–3 | 1.7 | 0.9 | 58% |
| Quantitative studies | 0–3 | 1.8 | 0.8 | 59% |
| Qualitative studies | 0–2 | 0.7 | 1.2 | 22% |
| 8. Detailed recruitment data (all studies) | 0–3 | 2.0 | 1.0 | 67% |
| Quantitative studies | 0–3 | 2.0 | 1.0 | 68% |
| Qualitative studies | 0–3 | 1.7 | 1.5 | 56% |
| 9. Statistical Assessment of reliability and validity of measurement tool(s) (Quantitative studies only) | 0–3 | 0.9 | 1.1 | 29% |
| 10. Fit between stated research question and method of data collection (Quantitative studies only) | 2–3 | 2.8 | 0.4 | 93% |
| 11. Fit between stated research question and format and content of data collection tool e.g. interview schedule (Qualitative studies only) | 2–3 | 2.3 | 0.5 | 75% |
| 12. Fit between research question and method analysis (all studies) | 2–3 | 2.9 | 0.3 | 97% |
| Quantitative studies | 2–3 | 2.9 | 0.2 | 98% |
| Qualitative studies | 2–3 | 2.3 | 0.6 | 78% |
| 13. Good justification for analytical method selected (all studies) | 0–3 | 2.4 | 0.8 | 81% |
| Quantitative studies | 0–3 | 2.5 | 0.8 | 83% |
| Qualitative studies | 2–3 | 2.7 | 0.6 | 89% |
| 14. Assessment of reliability of analytical process (Qualitative studies only) | 0–2 | 1.0 | 0.8 | 33% |
| 15. Evidence of user involvement in design (all studies) | 0 | 0 | 0 | 0% |
| 16. Strengths and limitations critically discussed (all studies) | 0–3 | 1.9 | 0.7 | 63% |
| Quantitative studies | 0–3 | 1.9 | 0.7 | 63% |
| Qualitative studies | 1–2 | 1.3 | 0.6 | 44% |
*Quantitative studies n = 62, qualitative studies n = 3, mixed-methods study n = 1. As there was only one mixed-methods study, its scores were included in reporting score for all studies and individual scores can be found in Additional file 3, Table S1
Synthesis of results
Factors affecting the uptake of new medicines were grouped into five thematic areas: patient, prescriber, medicine, organizational and external environment factors. Summary of main thematic areas with subthemes are shown in Table 3.
Table 3.
Summary of main thematic areas and developed subthemes of factors affecting the uptake of new medicines
| Thematic area | Subthemes |
|---|---|
| Patient-level factors | Demographic characteristics |
| Socioeconomic status | |
| Health status | |
| Patient engagement with treatment | |
| Prescriber factors | Socio-demographic characteristics |
| Scope of expertise | |
| Knowledge and prescribing habits | |
| Medicine-level factors | Efficacy |
| Safety profile | |
| Cost | |
| Therapeutic innovation | |
| Medicine administrative burden | |
| Organizational-level factors | Ownership status |
| Teaching status | |
| Size | |
| Location | |
| Available services and resources | |
| Staff composition | |
| Care co-ordination and quality | |
| External environment-level factors | Pharmaceutical detailing |
| Reimbursement conditions and formulary status | |
| Peer influence (internal and external) | |
| Guidelines | |
| Other information sources | |
| Organization affiliations |
The thematic area(s) in each included study is reported in Additional file 4, Table S2. External environment, organizational, patient and prescriber factors were reported most frequently (n = 36, n = 34, n = 31 and n = 29 studies respectively) and medicine factors (n = 18) were the least. Summary of factors affecting the uptake of new medicines referred to in the reviewed studies is displayed in Table 4.
Table 4.
Summary of factors affecting the uptake of new medicines referred to in the reviewed studies
| Identified factor | Number of studies referred to the factor | As facilitator | As barrier | No impact | Citations |
|---|---|---|---|---|---|
| Patient factors | |||||
| Age (younger) | 18 | 11 | 4 | 3 | [22, 25, 28, 32, 35, 51, 52, 58, 60, 63, 70–74, 78, 79, 82, 85] |
| Gender (male) | 12 | 4 | 1 | 7 | [22, 28, 29, 58, 60, 71–74, 78, 79, 82] |
| Ethnicity (White) | 10 | 6 | 1 | 3 | [25, 35, 51, 58, 61, 70, 72, 74, 78, 82] |
| Education level (higher) | 5 | 4 | 1 | [32, 35, 43, 78, 82] | |
| Income (higher) | 11 | 11 | 1 | [22, 25, 32, 45, 47, 50, 51, 71, 80, 82, 85] | |
| Insurance (private or more comprehensive) | 9 | 9 | [22, 25, 35, 45, 58, 62, 72, 74, 78] | ||
| Residential area (urban or more affluent) | 3 | 3 | [43, 70, 85] | ||
| Health condition (more severe & comorbidities) | 13 | 5 | 8 | [22, 28, 34, 35, 46, 51, 63, 72–74, 78, 79, 82] | |
| Polypharmacy | 9 | 3 | 4 | [22, 25, 28, 43, 72, 73, 79] | |
| Patient satisfaction, adherence to current therapy & monitoring | 4 | 4 | [32, 47, 50, 80] | ||
| Response to current therapy (poor) | 3 | 3 | [47, 70, 80] | ||
| Patients request & therapy preferences | 5 | 5 | [47, 50, 61, 69, 80] | ||
| Prescriber factors | |||||
| Age (younger) | 7 | 4 | 2 | 3 | [27, 29, 33, 42, 48, 53, 62] |
| Gender (male) | 6 | 4 | 2 | [23, 29, 42, 48, 62, 85] | |
| Graduating from a top-20 medical or foreign school | 3 | 3 | 2 | [23, 48, 62] | |
| Principal or partner GP | 1 | 1 | [85] | ||
| Specialist or secondary care prescriber | 16 | 13 | 4 | 1 | [22, 24, 28, 29, 33, 35, 41, 42, 47, 48, 59, 61, 62, 73, 75, 78] |
| Non-academic prescriber | 1 | 1 | [23] | ||
| Greater prescribing volume or portfolio breadth | 5 | 5 | [27, 48, 54, 61, 85] | ||
| Knowledge of new medicine | 7 | 7 | [47, 50, 67–69, 80, 86] | ||
| Continuing medical education activities | 1 | 1 | [38] | ||
| Early adopter in the past | 1 | 1 | [27] | ||
| Taking clinical risks & spending less time in consultations | 1 | 1 | [85] | ||
| Medicine factors | |||||
| Efficacy | 6 | 6 | [34, 50, 68, 69, 80, 86] | ||
| Safety concerns (adverse & long-term effects) | 6 | 6 | [44, 47, 50, 68, 80, 86] | ||
| Interactions with food/medicines (less) | 3 | 3 | [47, 68, 80] | ||
| High unit cost | 5 | 5 | 3 | [47, 50, 68, 80, 86] | |
| Therapeutic innovation | 5 | 5 | [41, 48, 50, 60, 80] | ||
| Ease of use & administration | 4 | 3 | 1 | 1 | [47, 50, 69, 80] |
| Reduced monitoring & clinic visits | 2 | 2 | [47, 68] | ||
| Organizational factors | |||||
| Ownership status (private) | 10 | 7 | 2 | 1 | [21, 37, 46, 57, 60, 64, 65, 71, 81, 84] |
| Teaching status | 8 | 1 | 7 | [54, 63–65, 72–74, 77] | |
| Size (larger) | 17 | 11 | 3 | 3 | [33, 35, 37, 46, 48, 51, 57, 60, 65, 68, 72, 75–77, 81, 82, 84] |
| Location (more populated) | 10 | 3 | 3 | 5 | [27, 42, 57, 63, 71–73, 77, 84, 85] |
| Availability of supportive services | 11 | 7 | 4 | [27, 35, 37, 39, 53, 57, 65, 69, 76, 77, 79] | |
| Limited consultation time | 2 | 2 | [67, 69] | ||
| Number of specialists, nurses, or healthcare professionals (higher) | 8 | 8 | [21, 35, 39, 48, 51, 57, 59, 67] | ||
| Care co-ordination (fragmented) | 2 | 2 | [67, 69] | ||
| External environment factors | |||||
| Pharmaceutical detailing | 11 | 11 | 1 | [23, 33, 50, 55, 61, 66, 68, 76, 80] | |
| Formulary or reimbursement restrictions | 10 | 10 | [23, 26, 29–31, 37, 40, 50, 80, 83] | ||
| Peer influence (internal & external) | 14 | 14 | [36, 49, 50, 52, 56, 59, 61, 64, 66, 68, 71, 80, 81, 84] | ||
| Recommended by guideline (international, national, or local) | 6 | 5 | 1 | [50, 64, 67–69, 86] | |
| Scientific literature, websites, & conferences | 6 | 6 | 1 | [32, 34, 50, 68, 76, 80] | |
| Organizational affiliations | 6 | 4 | 2 | [21, 39, 50, 51, 57, 76] | |
Patient-level factors
Demographic characteristics (n = 21)
Studies reported mixed results of patients’ age, gender, and ethnicity impact on the uptake of new medicines. Some prescribers tended to prescribe new medicines to younger [22, 28, 35, 51, 52, 58, 63, 71, 72, 74, 78], male [22, 72, 74, 79], female [82], or white ethnicity [61] patients. Others observed use of new medicine in older patients [25, 32, 70, 82, 85], mixed impact of ethnicity [25, 35, 51, 72, 74, 78], or suggested that patients’ age [60, 73, 79], gender [28, 29, 58, 60, 71, 73, 78], ethnicity [58, 70, 82] had no impact on prescribing decisions. Studies were medium to high quality and one study [79] was low quality.
Socioeconomic status (n = 21)
Some prescribers reported that patients’ socioeconomic factors [68], which included education, income, health insurance plan, residential area, influenced their prescribing decisions. Some findings suggested patients with a higher level of education were more likely to receive new medicines [32, 35, 43, 78], regardless of their age, gender, education, type of residential area, number of medicines used, and comorbidity [43]. However, one study [82] observed no impact of patient education level. All studies were high-quality. Prescribers also considered affordability of new medicines by patients [50]. Some studies suggested patients with higher income or ability to pay out-of-pocket expenses were more likely to receive a new medicine [22, 32, 45, 47, 51, 71, 80, 82, 85], but one study observed no difference [25]. Only three studies were high-quality [32, 71, 82]. Furthermore, patients with private health insurance plans covering prescription medicines and medical care services were reported to have a greater access to new medicines [22, 25, 35, 45, 58, 62, 72, 74, 78]; two studies were high-quality [35, 78]. Lastly, some studies indicated that patients living in a capital city [43], urban [70], or more affluent areas [85] were more likely to receive new medicines; two studies were high-quality [43, 70].
Health status (n = 21)
Prescribers highlighted that patients’ clinical characteristics and comorbidities influenced new medicines use [50]. Some prescribers reported prescribing new medicines for patients with more severe disease [34, 35, 46, 63, 82] or polypharmacy [22, 25, 43]; five studies were high-quality [34, 35, 43, 46, 82]. Other low to high quality studies reported new medicines use in patients with fewer comorbidities, or less severe conditions [22, 28, 51, 72–74, 78, 79], and no polypharmacy or concomitant use of medicines increasing the risk of side effects [28, 72, 73, 79]. Medium to high quality studies reported patient’s poor response to the current treatment encouraged [47, 70, 80] and that patient’s satisfaction with the existing treatment discouraged new medicine use [32].
Patient engagement with treatment (n = 5)
Some prescribers stated that patients’ requests for a new medicine [47, 61, 80] and interest in it [67], adherence to current treatment [50, 80] and monitoring [47] were influential in prescribing decisions. Some prescribers were described as aiming for a shared decision-making thus patients’ therapy preference and compatibility with their lifestyle [50] shaped prescribing decisions. Only one study was high-quality [61].
Prescriber factors
Socio-demographic characteristics (n = 11)
Medium to high quality studies suggested younger [33, 48, 53, 62] or older [27, 42], male [23, 42, 48, 85], graduating from a top-20 medical [23, 62] or foreign medical school [48] prescribers were earlier adopters. Other medium to high quality studies reported that age [29, 42, 48], gender [29, 62], prescribers’ length of practice [35, 85], graduating from a top-20 medical school [48, 62] did not influence prescribing decisions. A medium-quality study indicated general practitioners’ (GPs) who were a principal or partner in a practice were more likely to use new medicines than employee GPs [85].
Scope of expertise (n = 23)
Thirteen studies indicated that specialist prescribers adopted new medicines quicker than their other or primary care colleagues [22, 24, 28, 33, 41, 47, 48, 59, 61, 62, 73, 78] but only three were high-quality [24, 61, 78]. Other medium to high quality studies reported the opposite [29, 42, 48, 75] or no impact [35]. A high-quality study observed the clinical interest of primary care prescribers did not influence new medicine prescribing from the same clinical area [38]. Increasing total prescribing volume [48, 54, 85] or greater prescribing portfolio breadth [27, 54] in medium-quality studies, prescribing multiple medicines for the same condition [84] or larger prescription volume in the same therapeutic class [61] in high-quality studies were suggested to increase adoption of new medicines. Also, a high-quality study observed non-academic prescribers were more likely to use new and reformulated antipsychotics [23].
Knowledge and prescribing habits (n = 10)
Medium-quality studies suggested prescribers’ previous experience and knowledge of using new medicines increased their use [47, 67–69, 80, 86], whereas a lack of knowledge and confidence delayed or prevented use [50, 67]. Some prescribers commented that an overwhelming amount of new information for new medicines prescribing discouraged their use [67]. A high-quality study observed that continuing medical education activities supported prescribing of new medicine in one of two studied therapeutic areas [38]. In medium-quality studies, prescribers classed as early adopters in the past [27], or more likely to take clinical risks [85], or spend less time in patient consultations [85] tended to use new medicines quicker.
Medicine-level factors
Efficacy (n = 6)
Some prescribers stated relative effectiveness of a new medicine influenced their prescribing decisions in medium-quality studies [50, 68, 69, 80, 86]. A high-quality study, focusing on novel chemotherapies, suggested that perceived better quality rather than incremental effectiveness influenced new medicine use [34].
Safety profile (n = 9)
Some prescribers reported that concerns over adverse effects [47, 50, 68, 80, 86] and unknown long-term risks [50] discouraged prescribing new medicines. Less interactions with other medicines or food [47, 68, 80], and less reported adverse effects [47] compared to existing treatments, encouraged uptake. All were medium-quality studies. Medium to high quality studies observed that national safety reports, e.g., Food and Drug Administration, highlighting safety concerns contributed to hesitancy of some prescribers to use new medicines [44, 83]. Also, a high-quality study suggested that scientific articles [32] rather than safety alerts influenced prescribing behaviours as safety concerns would be first reported in the scientific literature. Another medium-quality study suggested safety concerns with an existing class of medicines encouraged prescribers to use new medicines from a therapeutically different class [44].
Cost (n = 9)
Some prescribers reported a higher unit cost of a new medicine over existing therapy was a barrier for its use [47, 50, 68, 80, 86]. However, a proportion of prescribers did not consider a medicine’s cost in their prescribing decisions [47, 68, 86]. The unit cost of the new medicine was perceived differently by prescribers and patients. Patients appeared willing to pay more if the new medicine was in their best interest [80]. In contrast, prescribers considered the patient’s ability to pay out of pocket costs [22, 45, 47, 50, 71, 82], which could affect patients’ adherence to therapy and affordability of future prescriber’s visits [50]. Some prescribers also discussed their role in containing spending of social insurance, although others thought cost-savings to public spending was not a prescriber’s job [50]. Only two studies were high-quality [71, 82].
Therapeutic innovation (n = 5)
Two studies suggested new medicines [41] or reformulations [48], perceived as having therapeutic innovation, were adopted quicker than medicines without. Another study indicated the availability of more medicines within the same therapeutic category (i.e., higher competition) had a negative impact on new medicines entering the same category use [60]. Some prescribers reported considering a new medicine’s relative clinical benefits other than safety, efficacy, or cost over existing treatment [50, 80]. For instance, a positive effect on patient’s weight, comorbidities, and cardiovascular protection by new antidiabetic medicines [50]. All studies were medium quality.
Medicine administrative burden (n = 5)
Some prescribers stated the ease of administration [80] or use [50] of the medicine facilitated its uptake. Another study observed that increased complexity of taking a new medicine, e.g., twice a day, was a barrier to a minority of prescribers [47]. The majority of prescribers in the case of oral anticoagulants reported reduced monitoring or clinic visits encouraged their use [47, 68]. Also, concerns about difficulty to initiate new medicines negatively affected the willingness of some prescribers to use them, especially if they were less experienced [69]. All studies were medium quality.
Organizational-level factors
Ownership status (n = 10)
Four high [37, 71, 81, 84] and three medium [57, 60, 65] quality studies suggested private, rather than public organizations, were more likely to use new medicines. Amongst private organizations, for-profit treatment programs were more likely to offer new medicines [37, 57]. In contrast, medium to high quality studies observed public organizations having greater use of new medicines [21, 46] or the ownership status did not influence the uptake [64].
Teaching status (n = 8)
Six medium studies [24, 63–65, 72, 73] and one high [77] quality study observed no difference in the uptake of new medicines between teaching and non-teaching hospitals. One medium-quality study, however, suggested a lower likelihood of new medicine use at a teaching hospital [74].
Size (n = 17)
Six high [35, 37, 46, 81, 82, 84] and five medium [48, 51, 57, 60, 68] quality studies indicated larger hospitals or practices were more likely to use new medicines. Other medium to high quality studies observed it for smaller [33, 75] or medium size [77] organizations. Also, three medium-quality studies suggested organization size did not influence the uptake [65, 72, 76].
Location (n = 16)
In some medium to high quality studies, organizations in urban areas [27, 57, 73], rural locations [42, 84], or in areas with fewer GPs [85] were observed to have a higher use of new medicines. Five medium to high quality studies reported geographical location having no impact on the uptake [27, 63, 71, 72, 77]. Also, nine studies reported regional variation in prescribing of new medicines [46, 60, 63, 65, 72, 74, 81, 85].
Available services and resources (n = 13)
In some cases, organizations providing, or having access to, related supportive services were more likely to adopt new medicines [37, 39, 57, 69, 76, 77, 79]; two were of high [69, 77] and one low [79] quality. For instance, detoxification, mental health services, or substance abuse counselling services for buprenorphine [37, 57, 69, 76] or stroke units for alteplase and direct oral anticoagulants [77, 79] were reported to facilitate the uptake. In other cases, supporting services such as the availability of heart failure clinics [35] or follow-up after hospitalisation [65] for sacubitril/valsartan, availability of hospital-based anticoagulant monitoring clinics for direct oral anticoagulants [53], or presence of dispensing services within general practices [27] had no impact. Also, prescribers reported lack of adequate time per patient visit acted as a barrier [67, 69], especially for less experienced prescribers [69]. Furthermore, some primary care clinicians suggested secondary care colleagues had more learning opportunities available (e.g., participation in clinical trials, education, and learning, access to more patients) supporting new medicine use [50].
Staff composition (n = 9)
Medium-quality studies indicated that lack of specialist prescribers was a barrier to new medicine use [21, 39, 48, 51, 57, 67]. For instance, organizations with more qualified staff [21] and GPs with hospital experience [59] were reported to adopt some of the studied medicines quicker. A high-quality study reported that organizations with higher numbers of nurses, and healthcare professionals with a generalist medical education, were more likely to use new medicines and the number of specialist prescribers had no influence [35]. Another medium-quality study reported the presence of clerical and nursing staff to have limited to no impact on the uptake [27].
Care co-ordination and quality (n = 3)
Some prescribers suggested that lack of organization and fragmentation in the provision of patient care [67], and non-clinical activities of care co-ordination, such as additional record-keeping requirements [69] were barriers to new medicine use. A study looking at heart failure treatment observed a lower uptake of a new medicine within hospitals scoring higher on non-heart failure service quality measures [65]. All studies were medium quality.
External environment-level factors
Pharmaceutical detailing (n = 11)
The pharmaceutical industry was seen to promote awareness of new medicines through pharmaceutical detailing (pharmaceutical marketing aimed at prescribers) and indirectly through conferences, educational events, advertisements in academic and professional journals [50, 61, 80]. Prescribers in medium-quality studies had mixed views on its impact on their prescribing decisions [50, 68, 80] with some reporting pharmaceutical representatives as one of their main information sources about new medicines [50]. Three studies in USA indicated that current and/or past detailing with or without distribution of free samples had a positive impact on new medicine uptake [61, 66, 76]; two studies were high-quality [61, 66]. Also, organizations or areas with restricted access to pharmaceutical detailing or marketing regulation policies in place (e.g., ban of gifts, disclosure policy) had lower and slower uptake of new medicines [23, 33, 66], especially among primary care prescribers [33]; two studies were high-quality [23, 66]. A high quality study suggested gift restrictions having a greater negative impact than disclosure policies [66]. Another high-quality study indicated that prescribers completing training at medical schools with active policies restricting access to the pharmaceutical industry were less likely to use new medicines [55]. A medium-quality study suggested that prescribers with very low access to pharmaceutical detailing were slower in changing their prescribing behaviour when negative information about new medicines was released [33]. Lastly, a high-quality study reported direct-to-consumer advertising aimed at patients had no influence on the uptake [66].
Reimbursement conditions and formulary status (n = 13)
Nine studies suggested that reimbursement conditions for a medicine influenced the use of new medicines [23, 26, 29–31, 40, 50, 80, 83]; two high [29, 83] and one low [30] quality studies. Formulary or reimbursement restrictions [50, 80] or cost-control regulatory measures [23, 50] were suggested to have negative impact on new medicine use. Removing reimbursement restrictions such as a requirement of prior authorisation [29, 30], specialist use only in secondary care [40], only as second-line therapy [26, 83], or providing reimbursement for medicines excluded from a national formulary [31] were suggested to support new medicine use. The inclusion of new medicines in formularies (e.g., public insurance, regional, local, national) was reported to facilitate their use [37, 80] with one study being high-quality [37]. Also, medium to high quality studies suggested financial incentives to reduce prescribing costs had limited to no impact on the uptake of new medicines already included in formularies [27, 34].
Peer influence (internal and external) (n = 14)
Some prescribers indicated that their peers’ adoption of new medicines positively influenced their prescribing behaviour of new medicines in eight high [49, 52, 56, 61, 66, 71, 81, 84] and five medium [36, 50, 59, 68, 80] quality studies. Also, four high-quality studies suggested adoption of new medicines by prescribers after approval was even greater if their peers were early adopters [52, 71, 81, 84]. Four medium-quality studies suggested peers from secondary care or specialist areas influenced primary care prescribers [50, 59, 68, 80]. Some prescribers stated that other colleagues, opinion leaders, and experts influenced the use of new medicines [50, 59, 64, 80]. One high-quality study indicated peer influence being the greatest from month four of the medicine’s launch until month 17 [66]. Another high -quality study observed that peer influence had a greater impact in the states of the USA with policies restricting pharmaceutical marketing [56].
Guidelines (n = 6)
Guidelines (local, national, or international) were indicated to influence prescribing decisions of some prescribers [50, 68, 69, 86], especially of less experienced [50, 68, 69, 86]. Some prescribers reported absence of guidelines prevented [86] or delayed [50] prescribing new medicines till a guideline was released. In one study some prescribers suggested difficulties in determining the position for the new medicine within a clinical pathway was a barrier for the uptake [67]. Contrastingly, one study reported the publication of national guidelines had no impact on the rate of uptake of the studied new medicine [64]. All studies were medium quality.
Other information sources (n = 6)
Some prescribers in medium to high quality studies reported conferences, medical or news articles, scientific societies’ websites, or clinical trial reports discussing new medicines as having impact on prescribing decisions [32, 34, 50, 68, 76, 80]. A high-quality study, looking at cyclooxygenase-2 inhibitors, suggested that medical articles discouraged prescribers to use new medicines, but news articles and media reports encouraged it [32]. Another high-quality study, looking at oral chemotherapy agents, observed that clinical trials and media reports published around the Food and Drug Administration (in USA) approval date had positive impact on uptake [34]. Some prescribers reported scientific literature having greater influence in prescribing decisions than information gathered through social professional networks [50] or news media [34]. Also, prescribers using national research websites were suggested to use new medicines earlier [76].
Organization affiliations (n = 6)
Three studies indicated organizations’ participation in research networks having positive impact on new medicines use [21, 50, 57]. This was attributed to an organization’s experience with treatment protocols and exposure to the process of implementing new treatments. Also, organizational links with professional associations were reported to increase the likelihood of being an early adopter in the case of buprenorphine [76]. However, two studies suggested treatment programs affiliated with medical health centres had the same or slower adoption rates than the independent ones [39, 51]. All studies were medium quality.
Discussion
Summary of evidence
This systematic review has identified a broad range of factors affecting the uptake of new medicines within healthcare organizations. The identified factors were grouped into patient, prescriber, medicine, organizational, and external environment factors, as per the Chaudoir et al. [7] framework, and overlapped with the Consolidated Framework for Implementation Research (CFIR) [87]. They had a varied impact on the uptake of the different studied new medicines.
Our review findings, differently from earlier reviews [8–10], indicated the presence of patient influence on the uptake of new medicines. Patients were reported to influence prescribing decisions through their interest in or request for new medicines, satisfaction with current treatment, and therapy preferences. However, only a small number of studies reported patient influence and further research is required to establish its relative importance in the uptake of new medicines. Also, reviewed studies did not explore the impact of patient involvement in decision-making, availability of patient choice, and patient-clinician relationship, which are suggested to influence implementation of health innovations [7, 87, 88]. Understanding of these factors could offer explanation for why new medicines are used with some patient types but not others, as established in the reviewed studies.
In our review, high-quality studies indicated that patients with a higher education level were more likely to receive new medicines. This was in contrast to Lubloy’s review findings based on one study [10] and not reported in the other two earlier reviews [8, 9]. Patient education level has been associated with health education, literacy, and behaviours [89], potentially translating into the level of patient influence on new medicine use. Also, patient education level is linked with patient income [90]. As in Lubloy’s review [10], patients with higher income (able to pay out-of-pocket costs) and more comprehensive insurance plans were observed to have greater access to new medicines. This was more predominant in countries without universal health coverage, e.g., USA, but was also present in countries with universal health coverage requiring co-payments from patients, e.g., Taiwan. More studies are needed to explore the impact of patient income on new medicines use in countries with a publicly funded national health service, e.g. UK.
Another important finding was the impact of new medicine cost to healthcare organisations and patients on its uptake. Differently to previous reviews [8–10], our review findings indicated that the high cost of a new medicine was a barrier for the uptake, although to a varied extent. Increasing costs and expenditure on medicines and limited available funding to healthcare services is anticipated to influence uptake of high-cost new medicines [90]. Also, none of the reviewed studies considered the overall costs of new medicines compared to the established therapy (e.g., associated monitoring cost) or health economics (e.g., direct health costs), which could offer explanation to observed geographical variation and restrictions of new medicine use in the reviewed studies.
Our review findings indicated that formulary or reimbursement restrictions influence the uptake of new medicines, which was not reported in earlier reviews [8–10]. The purpose of formulary and reimbursement restrictions is to ensure evidence-based and cost-effective prescribing. These could be used as a cost-control measure for high-cost new medicines, limiting their use. Earlier reviews did not report on impact of guidelines [10] or concluded they had no [8] or varied impact [9]. Our review findings suggest that guidelines have an impact on the uptake. Inclusion of a new medicine in local or national guidelines establishes a new medicine’s place in existing clinical pathways and provides assurance to prescribers that they follow the best practice.
Lastly, the review findings reaffirmed that prescribers’ experience and knowledge, peer influence, pharmaceutical detailing, staff composition at organizations, and scientific literature influence uptake of new medicines [8–10]. However, the present review also highlighted that studies reporting factors affecting new medicine use lacked exploration of wider prescriber factors (e.g., motivation, values and goals, or beliefs about new medicines) and organizational factors (e.g., readiness for innovation, culture and climate, implementation process) reported in the implementation literature affecting implementation of health innovations [7, 87, 91]. Deficiency in reporting these factors could be due to the data sources used by the reviewed studies (mostly secondary administrative data from various databases) and a lack of theoretical frameworks used to inform study designs of reviewed studies. Only 20 of the reviewed studies referenced theoretical approaches employed but none of the studies addressed all constructs of the theoretical approach referenced. Future studies employing determinant frameworks or implementation theories [91] for primary data collection are required to address gaps in understanding barriers and facilitators to the implementation of new medicines into clinical practice.
Strengths and limitations
This systematic review had a broad search strategy over seven databases and included studies of all methodological designs, conducted in both primary and secondary care settings. Grey literature and non-English language articles were excluded for pragmatic reasons and only studies published from 2008 and onwards were included, so other relevant studies might have been missed. The synthesis was underpinned by a determinant framework used in implementation science, which allowed the conceptualisation of the findings as provided in the review. Most of the reviewed studies were medium (38 studies) or high (26 studies) quality increasing confidence in the review findings. Finally, included studies covered medicines with varied complexities and expertise required to prescribe them. Therefore, not all influential factors identified in the review are relevant to all healthcare settings and medicines, reducing the generalisability of the review findings.
Conclusions
This systematic review provides a comprehensive exploration of factors affecting the use of new medicines and identified potential gaps in the research literature, through the use of a determinant framework used in implementation science. Factors affecting new medicine use not reported in earlier reviews were identified and included the following: patient influence and education level, cost of new medicines, formulary and reimbursement restrictions, and guidelines. Further research employing determinant frameworks or implementation theories are needed to address identified gaps, especially regarding wider patient, prescriber, and organizational factors, in understanding barriers and facilitators to the uptake of new medicines into clinical practice.
Supplementary Information
Additional File 2. Medline Search Strategy.
Additional File 3. Methodological quality of included studies using the QATSDD tool.
Additional File 4. Summary of thematic areas identified in the included studies.
Acknowledgments
Not applicable.
Abbreviations
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QATSDD
Quality Assessment Tool for Studies with Diverse Designs
- GP
General Practitioner
Authors’ contributions
KM, DP, SR and KS contributed to the conception and design of the study. KM, DP, SR, and KS designed the literature search. KM performed the literature search. KM and JT conducted the screening and study selection process. KM, JT, and IM completed quality appraisal of included studies. KM, IM, DP, SR, and KS were involved in the data interpretation. KM wrote the first draft of the manuscript. DP, SR, KS, JT, and IM provided critical input and aided in the revision of the manuscript. All authors have read and approved the final manuscript.
Funding
This work presents research funded by the Pharmacy Research UK (grant reference: PRUK-2018-GA-1-KM) and Leeds Teaching Hospitals NHS Trust. The views expressed are those of the author and not necessarily those of Pharmacy Research UK or Leeds Teaching Hospitals NHS Trust. The funding source had no role in the design of the study, the data collection, the analysis, the interpretation of data or writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and additional files including articles included in the analysis which are cited in the reference list.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kristina Medlinskiene, Email: k.medlinskiene1@bradford.ac.uk, Email: kristina.medlinskiene@nhs.net.
Justine Tomlinson, Email: J.E.C.Tomlinson@bradford.ac.uk.
Iuri Marques, Email: I.Marques@bradford.ac.uk.
Sue Richardson, Email: s.richardson@hud.ac.uk.
Katherine Stirling, Email: katherine.stirling@nhs.net.
Duncan Petty, Email: D.R.Petty1@bradford.ac.uk.
References
- 1.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J Roy Soc Med. 2011;104(12):510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewbank L, Omojomolo D, Sullivan K, McKenna H. The rising cost of medicines to the NHS: what is the story? The King’s fund. 2018. [Google Scholar]
- 3.World Health Organization. WHO Health Innovation Group. https://www.who.int/life-course/about/who-health-innovation-group/en/. Accessed 28 May 2020.
- 4.Office for Life Sciences and Welcome Trust. Accelerated Access Review: Final Report. 2016. https://wellcome.ac.uk/what-we-do/our-work/accelerated-access-review. Accessed 10 March 2017.
- 5.Office for Life Sciences. Life Sciences Competitiveness Indicators. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/811347/life-sciences-competitiveness-data-2019.pdf. Accessed 29 May 2020.
- 6.Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10(1):53. doi: 10.1186/s13012-015-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudoir SR, Dugan AG, Barr CHI. Measuring factors affecting implementation of health innovations: a systematic review of structural, organizational, provider, patient, and innovation level measures. Implement Sci. 2013;8(1):22–42. doi: 10.1186/1748-5908-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason A. New medicines in primary care: a review of influences on general practitioner prescribing. J Clin Pharm Ther. 2008;3(1):1–10. doi: 10.1111/j.1365-2710.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan D, Mason A. Factors affecting the uptake of new medicines in secondary care- a literature review. J Clin Pharm Ther. 2008;33(4):339–348. doi: 10.1111/j.1365-2710.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 10.Lubloy A. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res. 2014;14(1):469–494. doi: 10.1186/1472-6963-14-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loughlin M, Buetow S, Cournoyea M, Copeland SM, Chin-Yee B, Fulford KWM. Interactions between persons—knowledge, decision making, and the co-production of practice. J Eval Clin Pract. 2019;25(6):911–920. doi: 10.1111/jep.13297. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health. High Quality Care for All: NHS Next Stage Review Final Report. 2008. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/228836/7432.pdf. Accessed 28 May 2020.
- 13.Medlinskiene K, Tomlinson J, Marques I. A systematic review of barriers and enablers to the uptake of new medicines into practice at organization level. PROSPERO 2018 CRD42018108536. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018108536. .
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Loannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Health topics: Innovation. https://www.who.int/topics/innovation/en/. Accessed 28 May 2020.
- 17.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. the PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;354:i4086. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 18.Sirriyeh R, Lawton R, Gardner P, Armitage G. Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract. 2012;18(4):746–752. doi: 10.1111/j.1365-2753.2011.01662.x. [DOI] [PubMed] [Google Scholar]
- 19.Carroll C, Booth A, Leaviss J, Rick J. “Best fit” framework synthesis: refining the method. BMC Med Res Methodol. 2013;13(1):37. doi: 10.1186/1471-2288-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, editors. Analyzing qualitative data. Routledge; 1994. p.173–194, DOI: 10.4324/9780203413081_chapter_9.
- 21.Abraham AJ, Knudsen HK, Rothrauff TC, Roman PM. The adoption of alcohol pharmacotherapies in the clinical trials network: the influence of research network participation. J Subst Abus Treat. 2010;38(3):275–283. doi: 10.1016/j.jsat.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AbuDagga A, Stephenson JJ, Fu AC, Kwong WJ, Tan H, Weintraub WS. Characteristics affecting oral anticoagulant therapy choice among patients with non-valvular atrial fibrillation: a retrospective claims analysis. BMC Health Serv Res. 2014;14(1):310. doi: 10.1186/1472-6963-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson TS, Huskamp HA, Epstein AJ, Barry CL, Men A, Berndt ER, Horvitz-Lennon M, Normand SL, Donohue JM. Antipsychotic prescribing: do conflict of interest policies make a difference? Med Care. 2015;53(4):338–345. doi: 10.1097/MLR.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson TS, Lo-Ciganic WH, Gellad WF, Zhang R, Huskamp HA, Choudhry NK, Chang CCH, Richards-Shubik S, Guclu H, Jones B, Donohue JM. Patterns and predictors of physician adoption of new cardiovascular drugs. Healthcare. 2018;6(1):33–40. doi: 10.1016/j.hjdsi.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baik SH, Hernandez I, Zhang Y. Evaluating the initiation of novel Oral anticoagulants in Medicare beneficiaries. J Manag Care Spec Pharm. 2016;22(3):281–292. doi: 10.18553/jmcp.2016.22.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boon P, Dejonghe P, Legros B, Sadzot B, Rijckevorsel K, Shmedding E. Impact of reimbursement restrictions on the choice of antiepileptic drugs: Belgian study on epilepsy treatment (BESET) Seizure. 2008;17(4):350–357. doi: 10.1016/j.seizure.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Bourke J, Roper S. In with the new: the determinants of prescribing innovation by general practitioners in Ireland. Eur J Health Econ. 2012;3(4):393–407. doi: 10.1007/s10198-011-0311-5. [DOI] [PubMed] [Google Scholar]
- 28.Brais C, Larochelle J, Turgeon MH, Blais L, Farand P, Perreault S, Letemplier G, Beauchesne MF. Predictors of direct oral anticoagulants utilization for thromboembolism prevention in atrial fibrillation. J Pharm Pharm Sci. 2017;20:8–14. doi: 10.18433/J30W4F. [DOI] [PubMed] [Google Scholar]
- 29.Burden AM, Tadrous M, Calzavara A, Cadarette SM. Uptake and characteristics of zoledronic acid and denosumab patients and physicians in Ontario, Canada: impact of drug formulary access. Osteoporos Int. 2015;26(5):1525–1533. doi: 10.1007/s00198-014-3023-8. [DOI] [PubMed] [Google Scholar]
- 30.Carracedo-Martinez E, Pia-Moraneira A, Figueiras A. Trends in celecoxib and etoricoxib prescribing following removal of prior authorization requirement in Spain. J Clin Pharm Ther. 2017;42(2):185–188. doi: 10.1111/jcpt.12490. [DOI] [PubMed] [Google Scholar]
- 31.Chamberlain C, Collin SM, Stephens P, Donovan J, Bahl A, Hollingworth W. Does the cancer drugs fund lead to faster uptake of cost-effective drugs? A time-trend analysis comparing England and Wales. Br J Cancer. 2014;111(9):1693–1702. doi: 10.1038/bjc.2014.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chintagunta PK, Jiang R, Jin GZ. Information, learning, and drug diffusion: the case of Cox-2 inhibitors. Quant Mark Econ. 2009;7(4):399–443. doi: 10.1007/s11129-009-9072-1. [DOI] [Google Scholar]
- 33.Chressanthis GA, Khedkar P, Jain N, Poddar P, Seiders MG. Can access limits on sales representatives to physicians affect clinical prescription decisions? A study of recent events with diabetes and lipid drugs. J Clin Hypertens. 2012;14(7):435–446. doi: 10.1111/j.1751-7176.2012.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conti RM, Bernstein A, Meltzer DO. How do initial signals of quality influence the diffusion of new medical products? The case of new cancer treatments. Adv Health Econ Health Serv Res. 2012;23:123–148. doi: 10.1108/S0731-2199(2012)0000023008. [DOI] [PubMed] [Google Scholar]
- 35.DeVore AD, Hill CL, Thomas L, Sharma PP, Albert NM, Butler J, Patterson JH, Spertus JA, Williams FB, Duffy CI, McCague K, Hernandez AF, Fonarow GC. Patient, provider, and practice characteristics associated with Sacubitril/valsartan use in the United States. Circ Heart Fail. 2018;11(9):e005400. doi: 10.1161/CIRCHEARTFAILURE.118.005400. [DOI] [PubMed] [Google Scholar]
- 36.Donohue JM, Guclu H, Gellad WF, Chang C-CH, Huskamp HA, Choudhry NK, Zhang R, Lo-Ciganic W-H, Junker SP, Anderson T, Richards-Shubik S. Influence of peer networks on physician adoption of new drugs. PLoS One. 2018;13(10):e0204826. doi: 10.1371/journal.pone.0204826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Subst Abuse Treat Prev Policy. 2008;3(1):17–27. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dybdhal T, Sondergaard J, Kragstrup J, Kristiansen IS, Andersen M. Primary care physicians’ adoption of new drugs is not associated with their clinical interests: a pharmacoepidemiologic study. Scand J Prim Health Care. 2011;29(2):117–121. doi: 10.3109/02813432.2011.570024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman PD, Jiang L, Alexander JA. Top manager effects on buprenorphine adoption in outpatient substance abuse treatment programs. J Behav Health Serv Res. 2010;37(3):322–337. doi: 10.1007/s11414-009-9169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuksa L, Vocelka M, Vytrisalova M. The impact of changes in national prescribing conditions for statins on their public expenditure and utilization in the Czech Republic 1997–2013. Health Policy. 205(119):1255–64. [DOI] [PubMed]
- 41.Garjon FJ, Azparren A, Vergara I, Azaola B, Loyassa JR. Adoption of new drugs by physicians: a survival analysis. BMC Health Serv Res. 2012;12(1):56–66. doi: 10.1186/1472-6963-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groves KEM, Schellinck T, Sketris I, MacKinnon NJ. Identifying early prescribers of Cycloxygenase-2 inhibitors (COX-2s) in Nova Scotia, Canada: considerations for targeted academic detailing. Res Social Adm Pharm. 2010;6(3):257–267. doi: 10.1016/j.sapharm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Haider SI, Johnell K, Weitoft G, Thorslund M, Fastbom J. Patient educational level and use of newly marketed drugs: a register-based study of over 600,000 older people. Eur J Clin Pharmacol. 2008;64(12):1215–1222. doi: 10.1007/s00228-008-0549-8. [DOI] [PubMed] [Google Scholar]
- 44.Hickson RP, Cole AL, Dusetzina SB. Implications of removing Rosiglitazone’s black box warning and restricted access program on the uptake of Thiazolidinediones and Dipeptidyl Peptidase-4 inhibitors among patients with type 2 diabetes. J Manag Care Spec Pharm. 2019;25(1):72–79. doi: 10.18553/jmcp.2019.25.1.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirunrassamee S, Ratanawijitrasin S. Does your health care depend on how your insurer pays providers? Variation in utilization and outcomes in Thailand. Int J Health Care Finance Econ. 2009;9(2):153–168. doi: 10.1007/s10754-009-9062-6. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh CR, Liu YM. Availability, healthcare costs, and utilization patterns of biologics in Taiwan. Value Health. 2012;15(Suppl 1):35–42. doi: 10.1016/j.jval.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Huang C, Siu M, Vu L, Wong S, Shin J. Factors influencing doctors’ selection of dabigatran in non-valvular atrial fibrillation. J Eval Clin Pract. 2013;19(5):938–943. doi: 10.1111/j.1365-2753.2012.01886.x. [DOI] [PubMed] [Google Scholar]
- 48.Huskamp HA, O’Malley AJ, Horvitz-Lennon M, Taub AL, Berndt ER, Donohue JM. How quickly do physicians adopt new drugs? The case of second-generation antipsychotics. Psychiatr Serv. 2013;64(4):324–330. doi: 10.1176/appi.ps.201200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iyengar R, Van den Bulte C. Valente TW, Opinion leadership and social contagion in new product diffusion. Market Sci. 2011;30:195–212.
- 50.Karampli E, Souliotis K, Polyzos N, Chatzaki E. Why do physicians prescribe new antidiabetic drugs? A qualitative study in the Greek healthcare setting. Health Policy and Technology. 2020;9(2):166–173. doi: 10.1016/j.hlpt.2020.02.007. [DOI] [Google Scholar]
- 51.Keating NL, Huskamp HA, Schrag D, McWilliams JM, McNeil BJ, Landon BE, Chernew ME, Normand S-LT. Diffusion of Bevacizumab across oncology practices: an observational study. Med Care. 2018;56(1):69–77. doi: 10.1097/MLR.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keating NL, O’Malley AJ, Onnela J-P, Gray SW, Bruce E. Association of physician peer influence with subsequent physician adoption and use of bevacizumab. JAMA Netw Open. 2020;3(1):e1918586. doi: 10.1001/jamanetworkopen.2019.18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy C, Choitir CN, Clarke S, Bennett K, Barry M. Direct oral anticoagulants uptake and an oral anticoagulation paradox. Br J Clin Pharmacol. 2020;86(2):392–397. doi: 10.1111/bcp.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kereszturi JL, Lubloy A, Benedek G. Examination of pharmaceutical innovation diffusion using the cox model. Hungarian Statistical Review. 2015;93:47–66. [Google Scholar]
- 55.King M, Essick C, Bearman P, Cole J, Ross JS. Medical school gift restriction policies and physician prescribing of newly marketed psychotropic medications: difference-in-differences analysis. BMJ. 2013;346(jan30 5):f264. doi: 10.1136/bmj.f264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King M, Bearman PS. Gifts and influence: conflict of interest policies and prescribing of psychotropic medications in the United States. Soc Sci Med. 2017;172:153–162. doi: 10.1016/j.socscimed.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knudsen HK, Abraham AJ, Johnson JA, Roman PM. Buprenorphine adoption in the National Drug Abuse Treatment Clinical Trials Network. J Subst Abus Treat. 2009;37(3):307–312. doi: 10.1016/j.jsat.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin HC, Erickson SR, Balkrishnan R. Physician prescribing patterns of innovative antidepressants in the United States: the case of MDD patients 1993-2007. Int J Psychiatry Med. 2011;42(4):353–368. doi: 10.2190/PM.42.4.b. [DOI] [PubMed] [Google Scholar]
- 59.Lin SJ, Jan KA, Kao JT. Colleague interactions and new drug prescribing behavior: the case of the initial prescription of antidepressants in Taiwanese medical centers. Soc Sci Med. 2011;73(8):1208–1213. doi: 10.1016/j.socscimed.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 60.Liu YM, Yang YHK, Hsieh CR. The determinants of the adoption of pharmaceutical innovation: evidence from Taiwan. Soc Sci Med. 2011;72(6):919–927. doi: 10.1016/j.socscimed.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 61.Liu Q, Gupta S. A micro-level diffusion model for new drug adoption. J Prod Innovat Manag. 2012;29(3):372–384. doi: 10.1111/j.1540-5885.2012.00912.x. [DOI] [Google Scholar]
- 62.Lo-Ciganic WH, Gellad WF, Huskamp HA, Choudhry NK, Chang CCH, Zhang R, Jones BL, Guclu H, Richards-Shubik S, Donohue JM. Who were the early adopters of dabigatran? An application of group-based trajectory models. Med Care. 2016;54(7):725–732. doi: 10.1097/MLR.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo N, Fonarow GC, Lippmann SJ, Mi X, Heidenreich PA, Yancy CW, Greiner M, Hammill BG, Hardy NC, Turner SJ, Laskey WK, Curtis LH, Hernandez AF, Mentz RJ, O’Brien EC. Early adoption of Sacubitril/valsartan for patients with heart failure with reduced ejection fraction: insights from get with the guidelines-heart failure (GWTG-HF) JACC Heart Fail. 2017;5(4):305–309. doi: 10.1016/j.jchf.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 64.Luo N, Ballew NG, O'Brien EC, Greiner MA, Peterson PN, Hammill BG, Hardy NC, Laskey WK, Heidenreich PA, Chang C-L, Hernandez AF, Curtis LH, Mentz RJ, Foranow GC. Early impact of guideline publication on angiotensin-receptor neprilysin inhibitor use among patients hospitalized for heart failure. Am Heart J. 2018;200:134–140. doi: 10.1016/j.ahj.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Luo N, Lippmann SJ, Mentz RJ, Greiner MA, Hammill BG, Hardy NC, Laskey WK, Heidenreich PA, Chang C-L, Hernandez AF, Curtis LH, Peterson PN, Fonarow GC, O’Brien EC. Relationship between hospital characteristics and early adoption of angiotensin-receptor/neprilysin inhibitor among eligible patients hospitalized for heart failure. J Am Heart Assoc. 2019;8(3):e010484. doi: 10.1161/JAHA.118.010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manchanda P, Xie Y, Youn N. The role of targeted communication and contagion in product adoption. INFORMS. 2008;27(6):961–976. doi: 10.1287/mksc.1070.0354. [DOI] [Google Scholar]
- 67.Martin E, Pourtau L, Di Palma M, Delaloge S. New oral targeted therapies for metastatic breast cancer disrupt the traditional patients’ management—a healthcare providers’ view. Eur J Cancer Care. 2017;26(6):e12624–e12633. doi: 10.1111/ecc.12624. [DOI] [PubMed] [Google Scholar]
- 68.Murphy A, Kirby A, Bradley C. Knowledge is power: general practitioners prescribing of new oral anticoagulants in Ireland. BMC Res Notes. 2018;11(1):478–484. doi: 10.1186/s13104-018-3597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Netherland J, Botsko M, Egan JE, Saxon AJ, Cunnigham CO, Finkelstein R, Gourevitch MN, Renner JA, Sohler N, Sullivan LE, Weiss L, Fiellin DA. The BHIVES collaborative. Factors affecting willingness to provide buprenorphine treatment. J Subst Abus Treat. 2009;36(3):244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohl M, Lund B, Belperio PS, Goetz MB, Rimland D, Richardson K, Justice A, Perencevich E, Vaughan-Sarrazin M. Rural residence and adoption of a novel HIV therapy in a national, equal-access healthcare system. AIDS Behav. 2013;17(1):250–259. doi: 10.1007/s10461-011-0107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohlsson H, Chaix B, Merlo J. Therapeutic traditions, patient socioeconomic characteristics and physicians’ early new drug prescribing–a multilevel analysis of rosuvastatin prescription in South Sweden. Eur J Clin Pharmacol. 2009;65(2):141–150. doi: 10.1007/s00228-008-0569-4. [DOI] [PubMed] [Google Scholar]
- 72.Patel PA, Zhao X, Fonarow GC, Lytle BL, Smith EE, Xian Y, Bhatt DL, Peterson ED, Schwamm LH, Hernandez AF. Novel oral anticoagulant use among patients with atrial fibrillation hospitalized with ischemic stroke or transient ischemic attack. Circ-Cardiovas Qual. 2015;8(4):383–392. doi: 10.1161/CIRCOUTCOMES.114.000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potpara TS, Trendafilova E, Dan GA, Goda a, Kusljugic Z, Manola S, music L, Gjini V, Pojskic B, Popescu MI, Georgescu CA, Dimitrova ES, Kamenova D, Ekmeciu U, Mrsic D, Nenezic a, Brusich S, Milanov S, Zeljkovic I. lip GYH, BALKAN-AF investigators. The patterns of non-vitamin K antagonist Oral anticoagulants (NOACs) use in patients with atrial fibrillation in seven Balkan countries: a report from the BALKAN-AF survey. Adv Ther. 2017;34(8):2043–2057. doi: 10.1007/s12325-017-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodwin BA, Lu D, Giaimo A, Annapureddy A, Daggubati R, Curtis J, Sciria CT, Wang TY, Desai NR. Patient and hospital characteristics associated with ticagrelor uptake in acute MI: an analysis of the chest pain-MI registry. Int J Cardiol. 2020;304:14–20. doi: 10.1016/j.ijcard.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 75.Sato D, Sato Y, Masuda S, Kimura H. Impact of the sitagliptin alert on prescription of oral antihyperglycemic drugs in Japan. Int J Clin Pharm. 2012;34(6):917–924. doi: 10.1007/s11096-012-9694-3. [DOI] [PubMed] [Google Scholar]
- 76.Savage SA, Abraham AJ, Knudsen HK, Rothrauff TC, Roman PM. Timing of buprenorphine adoption by privately funded substance abuse treatment programs: the role of institutional and resource-based interorganizational linkages. J Subst Abus Treat. 2012;42(1):6–24. doi: 10.1016/j.jsat.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scholten N, Pfaff H, Lehmann HC, Fink GR, Karbach U. Who does it first? The uptake of medical innovations in the performance of thrombolysis on ischemic stroke patients in Germany: a study based on hospital quality data Implement Sci. 2015;10(1):10. doi: 10.1186/s13012-014-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steinberg BA, Holmes DN, Piccini JP, Ansell J, Chang P, Fonarow GC, Gersh B, Mahaffey KW, Kowey PR, Ezekowitz MD, Singer DE, Thomas L, Peterson ED, Hylek EM. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000535–e000547. doi: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanislav C, Allendorfer J, Pfeilschifter W, Fuest S, Stein M, Meyer M, Reuter I, Kaps M, Misselwitz B. One decade of oral anticoagulation in stroke patients: results from a large country-wide hospital-based registry. Int J Stroke. 2018;13(3):308–312. doi: 10.1177/1747493017733928. [DOI] [PubMed] [Google Scholar]
- 80.Tobin L, Neto AA, Wutzke S, Patterson C. Influences on the prescribing of new drugs. Aust Fam Physician. 2008;37:78–81. [PubMed] [Google Scholar]
- 81.Tsai YW, Wen YW, Huang WF, Kuo KN, Chen PF, Shih HW, Lee YC. Pharmaceutical penetration of new drug and pharmaceutical market structure in Taiwan: hospital-level prescription of thiazolidinediones for diabetes. Eur J Health Econ. 2010;11(3):279–290. doi: 10.1007/s10198-009-0174-1. [DOI] [PubMed] [Google Scholar]
- 82.Wang PJ, Chou YJ, Lee CH, Pu C. Diffusion of new medication across different income groups under a universal health insurance program: an example involving newly enlisted nonsteroidal anti-inflammatory drugs for elderly osteoarthritis patients. Int J Public Health. 2010;55(5):497–506. doi: 10.1007/s00038-010-0132-9. [DOI] [PubMed] [Google Scholar]
- 83.Weir MA, Gomes T, Winquist E, Juurlink DN, Cuerden MS, Mamdani M. Effects of funding policy changes and health warnings on the use of erythropoiesis-stimulating agents. J Oncol Pract. 2012;8(3):179–184. doi: 10.1200/JOP.2011.000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wen YW, Huang WF, Lee YC, Kuo KN, Tsai CR, Tsai YW. Diffusion patterns of new antidiabetic drugs into hospitals in Taiwan: the case of Thiazolidinediones for diabetes. BMC Health Serv Res. 2011;11(1):21–34. doi: 10.1186/1472-6963-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Méndez SJ, Scott A. Factors affecting general practitioners’ decisions to adopt new prescription drugs – cohort analyses using Australian longitudinal physician survey data. BMC Health Serv Res. 2019;19(1):94. doi: 10.1186/s12913-019-3889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang B, Song Y, Fu Y, Zhu B, Wang B, Wang J. Current status of the clinical use of PD-1/PD-L1 inhibitors: a questionnaire survey of oncologists in China. BMC Cancer. 2020;20(1):86. doi: 10.1186/s12885-020-6583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaakkola E, Renko M. Critical innovation characteristics influencing the acceptability of a new pharmaceutical product format. J Mark Manag. 2007;23(3-4):327–346. doi: 10.1362/026725707X196404. [DOI] [Google Scholar]
- 89.Hahn RA, Truman BI. Education improves public health and promotes health equity. Int J Health Serv. 2015;45(4):657–678. doi: 10.1177/0020731415585986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Godman B, Bucsics A, Vella Bonanno P, Oortwijn W, Rothe CC, Ferrario A, Bosselli S, Hill A, Martin AP, Simoens S, Kurdi A, Gad M, Gulbinovič J, Timoney A, Bochenek T, Salem A, Hoxha I, Sauermann R, Massele A, Guerra AA Jr, Petrova G, Mitkova Z, Achniotou G, Laius O, Sermet C, Selke G, Kourafalos V, Yfantopoulos J, Magnusson E, Joppi R, Oluka M, Kwon H-Y, Jakupi A, Kalemeera F, Fadare JO, Melien O, Pomorski M, Wladysiuk M, Marković-Peković V, Mardare I, Meshkov D, Novakovic T, Fürst J, Tomek D, Zara C, Diogene E, Meyer JC, Malmström RE, Wettermark B, Matsebula Z, Campbell S and Haycox A. Barriers for Access to New Medicines: Searching for the Balance Between Rising Costs and Limited Budgets. Front. Public Health 2018;6:328. doi: 10.3389/fpubh.2018.00328. Accessed 22 December 2020. [DOI] [PMC free article] [PubMed]
- 91.Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10(1):53. doi: 10.1186/s13012-015-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 2. Medline Search Strategy.
Additional File 3. Methodological quality of included studies using the QATSDD tool.
Additional File 4. Summary of thematic areas identified in the included studies.
Data Availability Statement
All data generated or analysed during this study are included in this published article and additional files including articles included in the analysis which are cited in the reference list.