Abstract
Tumor-associated angiogenesis is a key target for anti-cancer therapy. The imbalance between pro-angiogenic and anti-angiogenic signals elicited by tumor cells or tumor microenvironment always results in activating “angiogenic switch”. Tumor angiogenesis functions in multi-aspects of tumor biology, including endothelial cell apoptosis, tumor metastasis, and cancer stem cell proliferation. Numerous studies have indicated the important roles of inexpensive and less toxic natural products in targeting tumor angiogenesis-associated cytokines and apoptotic signaling pathways. Our current knowledge of tumor angiogenesis is based mainly on experiments performed on cells and animals, so we summarized the well-established models for angiogenesis both in vitro and in vivo. In this review, we classified and summarized the anti-angiogenic natural agents (Polyphenols, Polysaccharides, Alkaloids, Terpenoids, Saponins) in targeting various tumor types according to their chemical structures at present, and discussed the mechanistic principles of these natural products on regulating angiogenesis-associated cytokines and apoptotic signaling pathways. This review is to help understanding the recent progress of natural product research for drug development on anti-tumor angiogenesis.
Keywords: tumor angiogenesis, natural products, angiogenic factors, endothelial cell apoptosis, anti-angiogenic mechanism, chemical structures
Highlights
The primary mechanism of tumor angiogenesis was depicted.
The current in vivo and in vitro models toward targeting angiogenesis were recapitulated.
Natural agents as therapeutics against tumor angiogenesis were summarized.
Introduction
Angiogenesis refers to the process of new blood vessel formation from pre-existing endothelial cells in established vessels. The main cellular element of newly formed capillaries is endothelial cells (ECs), that are primarily modulated by vascular endothelial growth factor (VEGF) (1). After stimulating by VEGF, ECs can migrate to the lead of growing capillaries, that are referred to as tip cells. Upon the transformation of ECs to tip cells, the following ECs (stalk cells) will proliferate and maintain the structural and functional integrity of nascent vessels (2). Unlike physiological condition, tumor cells exposed to hypoxic condition secrete high levels of pro-angiogenic factors, such as VEGF. VEGF is the key regulator of the proper development of tumor blood vessels. Members of the angiopoietins, platelet-derived growth factor (PDGF-B), transforming growth factor (TGF-β) families and basic fibroblast growth factor (bFGF) are also significant factors in the formation of an immature vascular network in tumors (3). Moreover, recent literature has demonstrated that a variety of pro-angiogenic factors (VEGF, angiopoietin-1, bFGF) have the ability of inhibiting EC apoptosis (4) in tumor angiogenesis.
Natural products isolated from plants have been used as medicine from ancient times. The discovery of natural products for treatment dated back to 2600 BC in Mesopotamia (5). Traditional Chinese medicine (TCM) and Indian Ayurveda system are well documented over thousands of years. Early experiments have verified their properties of anti-inflammatory, anti-allergic and anti-infectious diseases (6, 7). Natural phytochemicals have been applied to the therapy of various diseases, including tumors (8). These phytochemicals can effectively inhibit the initiation, development and progression of neoplasm by regulating cellular proliferation, apoptosis and metastasis (9). Recent studies have indicated the important roles of natural compounds in modulating tumor angiogenesis by promoting the apoptosis of endothelial cell and inhibiting the angiogenesis-associated cytokines (10). In this review, we recapitulate the process of tumor angiogenesis and present some natural products that have the effect of anti-tumor angiogenesis.
A systematic literature search was conducted in PubMed database from 2010 to 2020. The following search terms were used: “natural products (or) natural product (or) polyphenols (or) polysaccharides (or) alkaloids (or) terpenoids (or) saponins” and “tumor angiogenesis (or) anti-angiogenic (or) angiogenic factors (or) vascular endothelial cells”. There were no language restrictions, and the abstracts of the papers identified by the initial search were evaluated by the lead reviewer Peng Song for appropriateness to this review. The inclusion criteria of references was that the abstracts of the references introduced the molecular mechanism investigation of natural products against tumor angiogenesis through in vitro or in vivo experiments.
Physiological Vasculature
The function of an extensive vascular network is to supply nutrients and oxygen and remove metabolic waste. During early embryogenesis, vascular growth is the results of combination of vasculogenesis and angiogenesis (11). Vasculogenesis refers to that new blood vessels are formed from primitive ECs precursors, as opposed to angiogenesis, in which the blood vessels developed from pre-existing ECs in established vessels (12). In adulthood, new blood vessels are rarely formed except in several physiological or pathological processes such as female reproductive cycling and wound healing. The sprouting, migration and proliferation of ECs are regulated by various cytokines, among them VEGF is a pivotal one (13).
Neoplasm Angiogenesis
Angiogenic Switch
At the early stage of neoplasm events, tumors present a balance between cell apoptosis and cell proliferation (14). Tumor cells produce pro-angiogenic factors, such as VEGF, PDGF and anti-angiogenic factors, such as angiostatin and thrombospondin. The secretion of these factors is in balance and tumors maintain at the “dormancy state”. However, once the average volume of a tumor exceeds 1-2 mm3, insufficient supply of oxygen and nutrients to tumor tissues will occur. Under hypoxic and/or acidic conditions, this balance is disturbed, and the tumors switch from a non-vascular to a vascular state, which turns on “angiogenic switch”. At this state, hypoxia inducible factor 1 alpha (HIF1-α) is able to bind to hypoxia-regulators and induce the production of VEGF and other pro-angiogenic factors (15). Oncogene Ras and the mutated tumor suppressor gene TP53 also mediate this pro-angiogenic effect. The synthesis of anti-angiogenic factors are found decreased in these tumors (16). Even if only 1% of tumor cells turns on the “angiogenic switch”, tumor growth occurs. In turn, the growth of neoplasm tissues promotes the initiation of angiogenesis (17).
Activated Factors and ECs Apoptosis in Tumor Angiogenesis
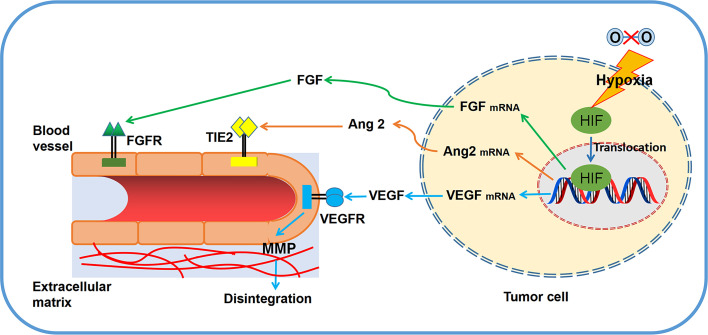
The most well-known receptors for tumor angiogenesis on the membrane of endothelial cells are tyrosine kinase receptors with their co-receptor, neuropilin. These receptors bind to VEGF or bFGF. The transcription of several pro-angiogenic factors can be initiated by the second messenger cascade of these receptors. High levels of VEGF can be found in most tumor types (1, 18). VEGF exerts the key role in the formation of new blood vessels and the proliferation of ECs. There are three tyrosine kinase receptors of VEGF, VEGFR-1 to -3. VEGFR-2 is the most prominent receptor in angiogenesis, which binds to VEGFA with strong tyrosine kinase activity (19). Moreover, latent forms of VEGF ligands are separated in the extracellular matrix, that are regulated by the release and activation of extracellular matrix-degrading proteases (e.g., MMP-9) (20). The up-regulation of other pro-angiogenic signals, such as FGF family, is involved in sustaining tumor angiogenesis (3, 21). Besides the VEGF signaling pathway, other signaling pathways and associated factors are involved in tumor angiogenesis. Angiopoietin 1 (ANGPT1) and ANGPT2 are important ligands from the ANGPT family, that bind to the receptor tyrosine kinase TIE2 on the membrane of ECs. Since ANGPT1 acts as an agonist of TIE2, its function is to promote vessel maturation. The activation of TIE2 mediated by ANGPT2 can induce vascular disruption and increase angiogenesis (22). Another important system that regulates tumor angiogenesis is the Notch-Notch-ligand system. Endothelial-specific ligand Delta-like 4 (DLL4) mediates the formation of the tip and stalk cells following VEGF stimulation (23).
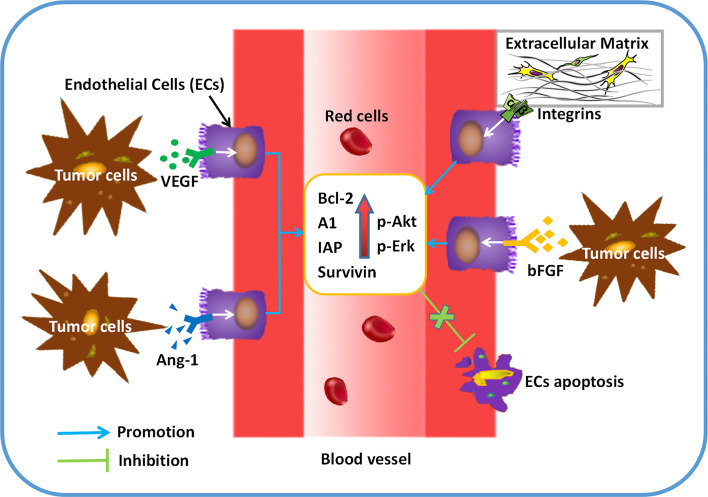
Apparently, neovasculature is strongly governed by ECs apoptosis. Both proangiogenic growth factors and extracellular matrix (ECM) act as the key roles in EC survival and angiogenesis (4). Induction of angiogenic growth factors and associated pathways are capable of inhibiting ECs apoptosis. For instance, ECs sustainment has been demonstrated to be linked with the modulation of cell apoptosis by VEGF (24). By increasing the expression of anti-apoptotic proteins, such as Bcl-2 and survivin, bFGF is able to decrease ECs apoptosis, which guarantees the cells survival (25). Of note, ANGPT1 involves in ECs apoptosis by modulating PI3K/Akt signaling and upregulating the anti-apoptotic protein surviving (26). Regarding the role of cell matrix and cell-cell interactions in angiogenesis, the anti-apoptotic effect of VEGF is dependent on the regulation of αvβ3-integrin and VE-cadherin protein (27, 28). Since the pivotal role of ECs in angiogenesis, many natural products are developed to target EC apoptosis. For example, resveratrol is able to increase Gly-LDL-induced vascular endothelial cell apoptosis (29). Similarly, Physalis alkekengi L. extract can reduce the apoptosis of ECs after exposing to hyperglycemia (30). Overall, angiogenic factors secreted by tumor cells ( Figure 1 ) and EC apoptosis ( Figure 2 ) are of significance in triggering and processing vessel formation in tumor microenvironment.
Figure 1.
Primary principles of angiogenesis in tumor microenvironment. Several significant regulators (VEGF, FGF, MMPs, TIE2) and related pathways are involved in tumor angiogenesis.
Figure 2.
ECs apoptosis can be regulated by pro-angiogenic factors in tumor angiogenesis. After secreting a variety of cytokines (VEGF, bFGF, Angiopoietin-1), tumor cells exerts the role of anti-apoptosis in ECs by binding to its receptors. Up-regulated expression of anti-apoptotic proteins (BCL-2, A1, IAP, etc) and protein kinases (Akt, Erk, etc) can be found in ECs, which results in more survival and less apoptosis in ECs.
Analysis of Vasculature in In Vivo and In Vitro Models
Vasculature has been explored for many decades and the application of in vitro and in vivo assays to evaluate the induction and inhibition of vascularization has exerted a significant role for researchers to study and understand angiogenesis (31). The well-established in vitro models, such as the model of ECs, are easily analyzed and reproducible, yet the cells become senescent in the culture after a few passages (32). Unlike the in vitro models, in vivo models, for example, the model of chick chorioallantoic membrane (CAM), owns the advantages of short experimental period and low cost, which is available for drug screening (33). As an ideal anti-tumor angiogenesis model, zebrafish has been widely used in drug screening due to its small size, transparent embryos, easy large-scale rearing, strong reproductive capacity and short experimental period (34). The aortic ring test is a model of angiogenesis based on organ culture. In this model, the molecular mechanism in regulating angiogenesis can be clarified, but it cannot truly reflect the microvascular environment of tumor growth (35). Another widely used in vivo model is xenograft tumor in mice. Of note, tumor angiogenic environment in animals is different from human, so assays in animals could not replace that in human (31). We have summarized the advantages and disadvantages of the current common angiogenesis models both in vitro and in vivo, which will provide clues for human cancer study ( Table 1 ).
Table 1.
Properties of common experimental models of angiogenesis in vivo and in vitro.
| Assay type | Description | Advantages | Disadvantages | Refs |
|---|---|---|---|---|
| Endothelial cells | The proliferation of EC initializes the formation of blood vessel. Angiogenic-associated factors trigger the activation of EC, thereby degrading local basement membrane, promoting EC migration and invading the proximal ECM, which leads to EC sprout and capillary network formation. Therefore, vitro models of ECs were established to simulate angiogenesis in human body. | Simple, reproducible, and quantitatively analyzed. |
|
(32) |
| ||||
| Tumor cells | Tumor cells are able to secrete a variety of cytokines in tumor growth, which affect tumor angiogenesis. Therefore, we can indirectly evaluate the role of tumor angiogenesis by detecting the expression of related cytokines. | Specifically use for anti-angiogenic drugs development and drug detection of anti-angiogenic effects. | Unequal to human experiments because of the different local environment between tumor cell models and human models. | (36–38) |
| ||||
| Chick chorioallantoic membrane (CAM) | CAMs is one of the most commonly used models to study the anti-angiogenic effect of drugs in vivo, which has been approved by FDA. At 6-8 days after hatching, the development of embryo membrane and vascular network is stable, but the immune system has not been completely established. Therefore, the embryo has no rejection to various foreign materials during this period, which makes it convenient to observe the effects of various drugs on angiogenesis. |
|
The possibility of false positive results due to cellular damage, inflammatory reaction and fibrin degradation products | (33) |
| Methods: The 8-day-old fertile eggs were opened from the end of the air chamber. After the opening, we can observe the growth of blood vessels on CAM and determine the best position of drug intervention, thereby avoiding the damage of chicken embryo caused by mechanical stimulation and eggshell detachment. | ||||
| Rat aortic rings | Aortic ring assay is a model of vascular regeneration based on organ culture. In this assay, the angiogenic vessels developed from a small segment of the aorta. |
|
Hardly reflection of the true microvascular environment of tumor growth | (35) |
| Methods: the rat aorta was removed, cut into 1 mm vascular rings, embedded with fibrin glue or collagen glue, and then cultured in serum-free MCDB131 medium. During the cultural process, the number of new microvessels produced by aortic rings was calculated and analyzed quantitatively. | ||||
| Zebrafish | As an ideal anti-tumor angiogenesis model, zebrafish has been widely used in drug screening: |
|
____ | (34) |
| ||||
| Xenograft mice with tumor cells | The model of mice bearing with tumor cells is used to study the potential antitumor activity of drugs and their effects on tumor size (diameter, area or volume) and survival time of animals. This model can also be used to study the potential anti-angiogenic activity, uptake and distribution of drugs. If a drug has the anti-angiogenic effect, it will prevent or reduce the number of tumor neovascularization. | ____ |
|
(31) |
Natural Anti-Tumor Angiogenic Compounds Derived From Plants
Polyphenols
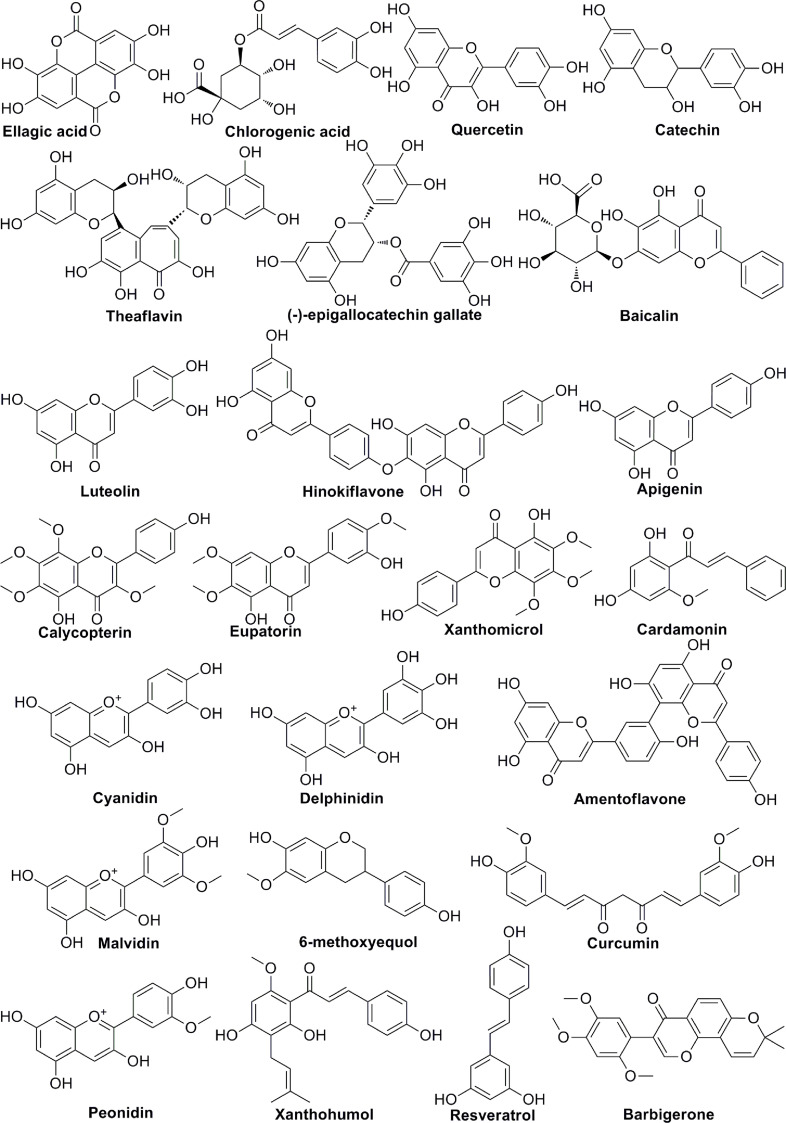
Polyphenols are members of a large family of chemical compounds with several phenolic groups, that can be found in foods and beverages of plant origin, including the spices, dried herbs, tea, red wine, coffee, cocoa products, seeds and nuts, vegetables, and fruits. Polyphenols are usually classified as phenolic acids, flavonoids, stilbenes, lignans, secoiridoids, among others. Polyphenols are of significance in the sustainment of human health. Recent studies have illustrated the pharmacologic functions of polyphenols that include antioxidation, vascular wall protection, improving enterogastric digestion, promoting immune response, and prevention of chronic disorders, such as tumor, atherosclerosis, hypertension, diabetes and so on. In addition to the summary of the chemical structures of polyphenols ( Figure 3 ), the effect of several polyphenols (Ellagic acid, Chlorogenic acid, Quercetin, Catechin, Baicalin, Delphinidin, 6-methoxyequol, et al.) on tumor angiogenesis have also been studied in recent years ( Table 2 ). Ellagic acid can inhibit angiogenesis by inhibiting the expression of VEGFR2 in ECV304 cell line. Moreover, researchers found that ellagic acid was able to regulate angiogenesis by inhibiting VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades in hamster cheek touch carcinogenesis model (39, 40). Chlorogenic acid is one of the hydroxygenic acid derivatives, which derives from Eucommia ulmoides Oliv. Chlorogenic acid suppressed tumor angiogenesis by blocking HIF-1α/Akt signaling pathway in lung cancer A549 cells (41). Quercetin, a flavanol-like compound, is derived from Quercus iberica. Quercetin reduces angiogenesis by inhibiting ERK phosphorylation and VEGFR-2 mRNA expression, and the calcineurin/NFAT signaling pathway (45, 46). Catechin is a flavanol-like compound and derived from black tea, which can affect angiogenesis by inhibiting VEGF (47). Baicalin, originated from Scutellaria baicalensis Georgi, can also inhibit tumor angiogenesis by regulating VEGFR (51). As for Delphinidin, it regulates angiogenesis by affecting the expression of HIF-1α and VEGF in A549 cells (56). 6-Methoxyquol is an isoflavone-like compound derived from soybean and can affect tumor angiogenesis through MAPK signaling pathway (59).
Figure 3.
The chemical structures of polyphenols involved in tumor angiogenesis.
Table 2.
Natural anti-tumor angiogenesis polyphenols and their sources, experimental models and anti-angiogenic mechanisms.
| Category | Name | Source | Experimental model | Anti-angiogenic mechanism | Refs |
|---|---|---|---|---|---|
| Hydroxybenzoic acid derivative | Ellagic acid | blackberry | ECV304 cell line, Hamster cheek pouch carcinogenesis model, HUVEC cells | Inhibits VEGFR2 expression | (39, 40) |
| Inhibits VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades | |||||
| Hydroxycinamic acid derivatives | Chlorogenic acid | Eucommia ulmoides Oliv | A549 cells | Suppresses HIF-1α/Akt pathway | (41) |
| Curcumin | Curcuma aromatica Salisb | A549 and PC-9 cells, Nude mice xenograft tumor model, T24 and UMUC2 cells, HUVEC cells | Blocks c-Met expression and PI3K/Akt/mTOR pathway | (42–44) | |
| Blocks IGF2 and IGF2 mediated PI3K/AKT/mTOR Signaling Pathway | |||||
| Cyclic nucleotide phosphodiesterases inhibition | |||||
| Flavonols | Quercetin | Quercus iberica | MCF-7 cells, BALB/c nude mice xenograft model, HUVEC cells, Transgenic zebrafish embryos TG | Inhibits calcineurin/NFAT pathway, Inhibits ERK phosphorylation and VEGFR-2 expression | (45, 46) |
| Flavanols | Catechin | black tea | HUVEC and HASMC cells | Inhibits VEGF expression | (47) |
| Theaflavin | black tea | OVCAR-3 and A2780/CP70, HUVEC | TF1 reduces VEGF secretion in a HIF1α-independent manner, while the others in a HIF1α-dependent way | (48) | |
| (-)-epigallocatechin gallate | green tea | SW620, HT-29, HCT116, Endothelial cells and xenografts | Suppresses JAK/STAT3/IL-8 pathway and VEGFR2 expression | (49, 50) | |
| Flavones | Baicalin | Scutellaria baicalensis Georgi | A549 cell, Xenograft tumors in nude mice | VEGFR expression inhibition | (51) |
| Apigenin | Apium graveolens L.var.dulce DC. | PC3-M and LNCaP C4-2B cells | Blocks TGF-β1-Smad-VEGF pathway | (52) | |
| Luteolin | Reseda odorata L. | Sprague-Dawley rat | VEGF expression inhibition | (53) | |
| Eupatorin | Isatis tinctoria | HUVEC cells | VEGF expression inhibition | (54) | |
| xanthomicrol | Citrus reticulata Blanco | HUVEC cells and rat aortic rings | VEGF expression inhibition | (55) | |
| calycopterin | Dracocephalum kotschyi | HUVEC cells and rat aortic rings | VEGF expression inhibition | (55) | |
| Anthocyanins | Delphinidin | Pharbitis nil(L.)Choisy | A549 cell | Inhibits HIF-1α and VEGF expression | (56) |
| Cyanidin | purple sweet potato | HUVEC and HRMEC | Blocks ERK pathways | (57) | |
| Peonidin | lycium ruthenicum | HUVEC and HRMEC cells | Blocks ERK pathways | (57) | |
| Malvidin | Malva sinensis Cavan. | SCC131 cells | Suppresses JAK/STAT-3 pathway | (58) | |
| Isoflavone | 6-methoxyequol | soybean | HUVECs A431 cells, Mouse xenograft tumors model | Suppresses MAPK pathway | (59) |
| Pyranoisoflavone | Barbigerone | soybean | B16F10 melanoma cells, Zebrafish and mouse xenograft tumors models | Suppresses MEK 3/6/p38 MAPK signaling pathway | (60) |
| Chalcone | Cardamonin | Alpinia katsumadaiHayata | SKOV3 cells | Inhibis VEGF and HIF-α expression | (61) |
| Xanthohumol | Humulus lupulus L. | BxPC-3 cells | Blocks NF-κB expression | (62) | |
| Biflavonoid | Amentoflavone | Selaginella tamariscina | TSGH8301 cell | Suppresses VEGF expression | (63) |
| Hinokiflavone | Platycladus orientalis (Linn.) Franco | CT26 cell | – | (64) | |
| Stilbens | Resveratrol | Peanut | Rats | MMP-9 expression inhibition | (65) |
Polysaccharides
Polysaccharides are polymeric carbohydrate macromolecules, that are composed of long-chain monosaccharide units connected by glycosidic bonds. As pivotal bioactive macromolecules, polysaccharides are mainly extracted from plants, animals and microorganisms. Polysaccharides are currently applied to various biomedical products and functional foods because of their significant biological functions, such as inhibiting tumor growth, triggering immunity, antioxidation, anti-virus and neuroprotection. Recent studies indicated that polysaccharides extracted from higher plants showed anti-tumor activity by enhancing immunity. In addition to anti-tumor effect through immunomodulation, polysaccharides can directly inhibit tumor angiogenesis. Various polysaccharides, such as dandelion polysaccharide, sulfated polysaccharide, Fucoidan, LEP-2a, and Galactomannan, exert important roles in tumor angiogenesis ( Table 3 ). Dandelion polysaccharide has been proved to inhibit angiogenesis both in vivo and in vitro. The PI3K/Akt signaling pathway is involved in this process, which inhibits the expression of VEGF and HIF-1α (66). Sulfurized polysaccharide is derived from Phellinus ribis. In the Lewis lung carcinoma (LLC) mouse model, sulfurized polysaccharide can inhibit vessel formation by modulating VEGF/VEGFR signaling pathway (67). Fucoidan, extracted from Laminaria japonica, regulates tumor angiogenesis by modulating MAPK and PI3K/Akt signaling pathways (68). Fucoidan is proved to reduce the expression of VEGF and PDGF in two chicken embryo chorioallantoic membrane (CAM) models (69). Moreover, in mice xenografted with DU-145 human prostate cancer cells, fucoidan can decrease tumor growth and angiogenesis by inhibiting JAK/STAT3/VEGF signaling pathway (70). Another important polysaccharide, Lep-2a, can restrain the expression of VEGF, CD105, bFGF, MMP-2 and MMP-9 in hepatocellular carcinoma (72). PSP001 is a galactomannan derived from the fruit rind of Punica granatum L. A recent study found that galactomannan was able to suppress the expression of VEGF, MMP-2 and MMP-9 and up-regulate the expression of TIMP-1 and TIMP-2 in cancer cells (74).
Table 3.
Natural anti-tumor angiogenesis polysaccharides and their sources, experimental models and anti-angiogenic mechanisms.
| Category | Name | Source | Experimental model | Anti-angiogenic mechanism | Refs |
|---|---|---|---|---|---|
| α-type polysaccharide | Dandelion Polysaccharide | the root of dandelion | HUVECs, CAM, Mice xenografted with Hepa1-6 and H22 cancer cells | Inhibits PI3K/AKT/HIF-1α/VEGF pathway | (66) |
| Sulfated polysaccharide | PRP-S16 | Phellinus ribis | LLC (Lewis lung carcinoma) allografts tumor-bearing mice | Inhibits HIF-1α/VEGF/VEGFR-2/AKT pathway | (67) |
| A fucose-rich polysaccharide | Fucoidan | Laminaria japonica, Fucus vesiculosus | TNBC, HUVECs, CAM, Mice xenografted with DU-145 human prostate cancer | Suppresses MAPK and PI3K/AKT signaling pathway | (68–70) |
| Inhibits VEGF and PDGF expression, Inhibits JAK/STAT3/VEGF pathway | |||||
| Homogeneous polysaccharide | HH1-1 | safflower | CAM, BxPC-3 xenograft model and PDX model | Impedes the combination of EGFR and Galectin-3 | (71) |
| Inhibits Galectin-3/GFR/KT/OXO3 signaling pathway | |||||
| Exopolysaccharide | LEP-2a | Lachnum sp. | H22 (hepatocellular carcinoma) allografts tumor-bearing mice | Suppresses VEGF, CD105, bFGF, MMP-2 and MMP-9 expression | (72) |
| Water-soluble polysaccharide | PTP | the roots of Polygala tenuifolia | SKOV3 xenograft, Tumor growth in BALB/c mice | Decreases EGFR, VEGF, and CD34 expression | (73) |
| Galactomannan | PSP001 | the fruit rind of Punica granatum L. | CAM, A375 and A549 cells, B16F10 | suppresses the expression of VEGF, MMP-2 and MMP-9 | (74) |
| Upregulates the expression of TIMP-1 and TIMP-2 | |||||
| Water-soluble polysaccharide | STPC2 | Sargassum thunbergii | HUVECs, A549 cells | Downregulates MMP-2, VEGF and HIF-1α expression | (75) |
| Apigalacturonan-Rich Polysaccharide | ZCMP | The sea grass Zostera caespitosa Miki | HUVECs | – | (76) |
| Pectic polysaccharide | Corn pectic polysaccharide (COPP) | corn (Zea mays L.) | Mice were given B16F10 and injected through the lateral tail vein | Suppresses the expression of VEGF, MMP-2 and MMP-9 | (77) |
| Arabinogalactan | Arabinogalactan | flowers of Panax notoginseng | BxPC-3, Pancreatic cancer cell xenograft tumor in nude mice, HMEC-1 | Inhibits BMP2/Smad/Id1 signaling pathway | (78) |
| Huaier polysaccharide | TP-1 | Huaier fungus | To establish an in vivo pulmonary metastasis model, SMMC-7721 cells were injected into BALB/c nude mice via the tail vein | Inhibits HIF-1α/VEGF pathway | (79) |
| SP-1 | Trametes robiniophila murr (Huaier) | Mice xenografted with SMMC-7721 human hepatocellular carcinoma cells | Suppresses the expression of VEGF, MMP-2, HIF-1α, STAT3 and MMP-9 | (80) |
Alkaloids
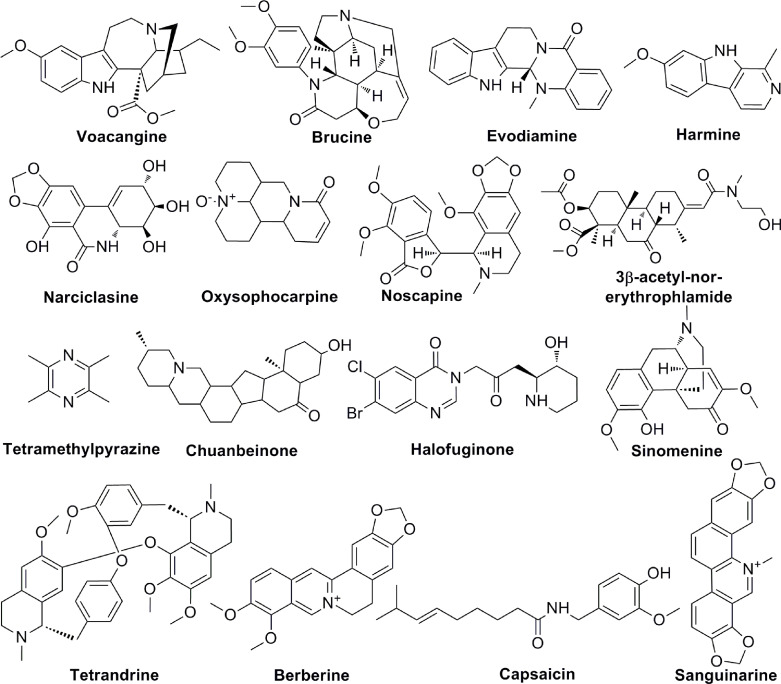
Alkaloids are a group of natural compounds with nitrogen structure and physiological function. Many alkaloids have complex nitrogen heterocyclic structures, and several compounds are organic amines with non-nitrogen heterocycles. Alkaloids have the properties of alkali, that are important active ingredients in Chinese herbal medicine. Although certain vitamins, amino acids and peptides from natural sources are also nitrogenous compounds, they are not included in the category of alkaloids. Alkaloids are ubiquitous in nature, especially in the plant kingdom. Alkaloids have been reported in at least 140 species of plants, especially in Papaveraceae, tetrandridae, Solanaceae, Leguminosae, Apocynaceae, Ranunculaceae, and tillering family, et al. The common alkaloids include pyrrole, pyridine, quinoline, isoquinoline, indole, imidazole, purine, anisodamine, terpenoids, steroids, organic amines, and so on and the chemical structures of these alkaloids are shown in Figure 4 . Current studies proved the biological activities of alkaloids, including anti-tumor, anti-bacterial, anti-inflammatory, antiviral, antiarrhythmic, analgesic, and antispasmodic effects. Voacangine is an indole-like compound, which is rich in Ervatamia Stapf. Treatment with voacangine decreases the VEGFR2 kinase activity in athymic nude mice bearing glioblastoma tumors and HUVECs, suggesting that its anti-angiogenic role is closely associated with VEGFR2 (81). Evodiamine is an indoloquinazoline-like compound, which is derived from Euodia rutaecarpa (Juss.). Evodiamine is able to modulate angiogenesis by inhibiting the expression of VEGF through the c-Mer/Src/STAT3 signaling pathway (82). By inhibiting β-catenin, which reduces VEGF-A expression, Le Shi et al. found the anti-angiogenic role of evodiamine (83). As an isocarbostyril compound, narciclasine regulates HUVECs proliferation by a RhoA-independent activation of the Rho kinase Rock (84). Tetromethylpyrazine is an amide alkaloid, which is extracted from Ligusticum chuanxiong hort. Tetromethylpyrazine combined with paclitaxel inhibited angiogenesis both in vivo and in vitro through blocking ERK1/2 and AKT signaling pathways (85). Previous studies found that harmine, a β-carboline alkaloid, suppressed bladder cancer growth by its anti-angiogenic effect, which was linked to VEGFR2 signaling pathway (86). Oxysophocarpine is a quinolizidine alkaloid and it reduces the levels of HO-1, VEGF, MMP-9 and HIF-1 in OSCC cells (87). 3-acetyl-norerthrophlamide is a cassaditerpene alkaloid, which comes from Erythrophleum fordii. 3-acetyl-norerthrophlamide and exerts the anti-angiogenic function by inhibiting the eNOS activation and NO production (88). Many alkaloids, such as chuanbeinone, halofuginone, tetrandrine, also play significant roles in tumor angiogenesis ( Table 4 ).
Figure 4.
The chemical structures of alkaloids involved in tumor angiogenesis.
Table 4.
Natural anti-tumor angiogenesis alkaloids and their sources, experimental models and anti-angiogenic mechanisms.
| Category | Name | Source | Experimental model | Anti-angiogenic mechanism | Refs |
|---|---|---|---|---|---|
| Indole | Voacangine | Ervatamia Stapf | Athymic nude mice bearing glioblastoma tumors consisting of U87MG glioblastoma, HUVECs | Inhibits VEGFR2 kinase activity and its downstream signaling by binding to the kinase domain of VEGFR2 | (81) |
| Brucine | Strychnos nux-vomica Linn | Human breast cancer cell line MDA-MB-231 | Inhibits VEGF/VE-cadherin/EphA2/MMP-9/MMP-2 pathway | (89) | |
| Indoloquinazoline alkaloid | Evodiamine | Euodia rutaecarpa (Juss.) Benth. (Rutaceae) | Human prostate cancer (PC-3 and DU145), Mouse xenograft model with HCC cell lines (HepG2, SMMC-7721, H22), HUVECs, The number of capillary sprouts from Matrigel embedded rat thoracic aortic rings | Inhibits c-Mer/Src/STAT3 pathway Inhibits VEGF and MMP-9 expression inhibits β-catenin expression | (82, 83) |
| Isocarbostyril alkaloid | Narciclasine | Narcissus and Haemanthus species | HUVECs | By the RhoA-independent activation of the Rho kinase ROCK downregulation of VEGFR2 expression | (84) |
| Amide alkeloid | Tetramethylpyrazine | Ligusticum chuanxiong hort | Ovarian cancer A2780 xenograft mouse models, HUVECs, SKOV3 cell | Inhibits ERK1/2 and Akt pathways | (85) |
| β-carboline alkaloid | Harmine | Pergamum harmala seeds | The xenograft mouse model assay of human bladder cancer used RT4 cell line, HUVECs, Rat aortic ring assay | Inhibits VEGFR2, ERK1/2 and Akt signaling pathways | (86) |
| Quinolizidine alkaloid | Oxysophocarpine | Sophora flavescens Ait. (Kushen), S. alopecuroides L. (Kudouzi or Kugancao), and other leguminous plants of the genus Robinia | HUVECs, BALB/c nude mice were injected with SCC-9 cells | Reduces the levels of HO-1, VEGF, MMP-9 and HIF-1α expression | (87) |
| Opium alkaloid | Noscapine | Papaver somniferum L | HUVECs | Inhibits growth of human endothelial cells, cord formation of human endothelial cells and chemotaxis factors responsible for the angiogenesis | (90) |
| Cassaine diterpene alkaloid | 3β-acetyl-nor erythrophlamide | Erythrophleum fordii | HUVECs, A549 xenograft mouse model | Inhibits the VEGF-mediated eNOS activation and NO production | (88) |
| Isosteroidal Alkaloid | Chuanbeinone | Bulbus of Fritillaria pallidiflora | LLC and S180-bearing mice | Inhibits the expression of VEGF | (91) |
| quinazolinone alkaloid | Halofuginone | Dichroa febrifuga Lour. | NB4 and HUVEC cells, NOD/SCID mice were transplanted with leukemic cells | Reduces VEGF secretion and phosphorylation of SMAD-2 | (92) |
| Blocks TGF-β signaling pathway | |||||
| Bisbenzylisoquinoline alkaloid | Tetrandrine | the roots of the medicinal plant Stephaniae tetrandrae S. Moore (Han-Fang-Ji in Chinese) | Mouse endothelial cells (EOMA cell), Huh7 tumor xenografts | Inhibits EOMA cells proliferation via ROS/Akt pathway | (93) |
| Isoquinoline alkaloids | Berberine | Coptis Rhizome | MHCC-97L cells xenograft mouse model | Inhibits the expression of Id-1, VEGF | (94) |
| Sinomenine | Sinomenium actum Rehd.et wils. | HUVEC, HOS-Luc cells xenograft mouse model | Inhibits the expression of VEGF, MMP-2, MMP-9, RANKL | (95) | |
| Inhibited invasion and metastasis via suppressing the CXCR4-STAT3 pathway | |||||
| Benzophenanthridine alkaloid | Sanguinarine | the root of Sanguinaria canadensis and other poppy-fumaria species | Human microvascular endothelial cells (HMVECs), A549 cells | Inhibits the activation of serum starvation and hypoxia-induced VEGF promoter activity | (96) |
| Amide alkaloids | Capsaicin | Capsicum annuum L. | HUVEC, A549 | Re-activation of p53-SMAR1, inhibits the expression of HIF-1α and Cox-2, down regulate VEGF | (97) |
Terpenoids
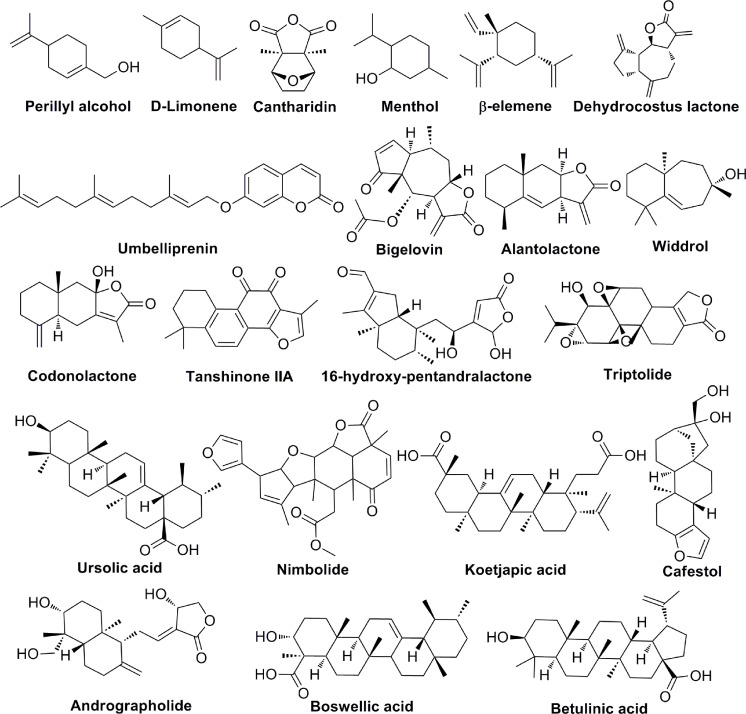
Terpenoids are natural products that derive from mevalonic acid. Terpenoids are composed of multiple isoprene (C5) units with the general formula of (C5H8)n. More than 20,000 terpenoids exist in nature and are widely distributed in the plant kingdom, such as Compositae, Ranunculaceae, Araliaceae, Oleaceae, Magnoliaceae, Lauraceae, Aristolochiaceae, and more. Based on the different molecular structures ( Figure 5 ), terpenoids are divided into five groups: monoterpenes, sesquiterpenes, diterpenoids, triterpenoids and terpenes compound. Most of the bioactive terpenoids have been isolated from medicinal plants. For example, monoterpenes and sesquiterpenes are extracted from essential oils of medicinal plants and triterpenes are primarily available in balsams and resins. Recent studies have found that terpenoids have a variety of biological functions, including the regulation of enzyme system, cell surface signaling transduction, immune function, cell differentiation, tumor proliferation and angiogenesis. Important terpenoids (Perillyl Alcohol, β-elemene, Alantolactone, Tanshinone IIA, Triptolide, Ursolic acid, Koetjapic acid, et al) and their molecular signaling pathways participate in modulating tumor angiogenesis ( Table 5 ). Perillyl Alcohol, hugely available in the essential oils of several plants (Lavendin, Mints, Cherries, etc.), possesses the anti-angiogenic property, the mechanism of which is probably to decrease the release of VEGF in cancer cells and stimulate the expression of Ang2 in endothelial cells (98). β-elemene is a sesquiterpene compound isolated from Curcuma Zedoaria. It inhibits the proliferation and metastasis of melanoma by suppressing VEGF-mediated angiogenesis (102). Alantolactone is also a sesquiterpene compound extracted from Inula Helenium. Alantolactone inhibits angiogenesis by reducing the phosphorylation of VEGFR2 and its downstream protein kinases, including PLCγ1, FAK, Src, and Akt in breast cancer (106). Tanshinone IIA is the key compound of Diterpene, isolated from Salvia Miltiorrhiza Bunge. Tanshinone IIA has anti-angiogenic activity in human EPCs, which is associated with the regulation of VEGF, PLC, Akt and JNK signaling pathways (109). Triptolide is also a Diterpene compound, derived from Tripterygium Wilfordii. Triptolide exerts the anti-angiogenic role in breast cancer by inhibiting ERK1/2-HIF1-α-VEGFA signaling pathway both in vitro and in vivo (113). As an important Triterpene compound, ursolic acid is able to decrease the expression of VEGF and iNOS in ehrlich ascites carcinoma (114). Koetjapic acid is also a significant Triterpene compound. Koetjapic acid is found to reduce the expression of VEGF in various angiogenic models, such as rat aortic ring, CAM and HUVECs (116).
Figure 5.
The chemical structures of terpenoids involved in tumor angiogenesis.
Table 5.
Natural anti-tumor angiogenesis terpenoids and their sources, experimental models and anti-angiogenic mechanisms.
| Category | Name | Source | Experimental model | Anti-angiogenic mechanism | Refs |
|---|---|---|---|---|---|
| Monoterpene | Perillyl alcohol | the essential oils of several plants (lavendin, mints, cherries, etc.) | CAM, HUVECs, BLMVECs, K562 | Inhibits VEGF and Akt pathways | (98) |
| D-limonene rich volatile oil | blood orange (Citrus sinensis (L) Osbeck) | SW480 and HT-29 cells, HUVECs | Reduces the levels of VEGF, MMP-9 expression, inhibits the activation of VEGFR1 | (99) | |
| Cantharidin | Mylabris | Subcutaneous and orthotopic pancreatic xenograft models with PANC-1 cells | Inhibits ERK, JNK, PKC, and NF-κB pathways | (100) | |
| Menthol | Mentha haplocalyx Briq | HepG2 cells | Inhibits the expression of VEGF | (101) | |
| Sesquiterpene | β-elemene | the essential oil of Curcuma zedoaria | Rat aortic ring, Chick embryo chorioallantoic membrane, Melanoma growth and metastasis assay in C57BL/6 mice | Suppresses VEGF pathway | (102) |
| Dehydrocostus lactone | Saussurea lappa and Laurus nobilis | lung cancer cells (A549 and H460 cells), Matrigel plugs were implanted in C57BL/6 mice | Suppresses of HIF-1a, Akt and pAkt, GSK-3β and pGSK-3β, as well as ERK, pERK, mTOR, and p-mTOR | (103) | |
| Inhibits MMP-2 and MMP-9 | |||||
| Umbelliprenin | Different spices of Ferula of umbeliferace | CT26 tumor cells, L929 cells | Inhibits the expression of VEGF, MMP2, MMP-9 and CD31 | (104) | |
| Potentiates immune response by IFN-γ increment and IL-4 decrease | |||||
| Bigelovin | Inula helianthus-aquatica | HCT 116 cells, Orthotopic tumor allografts and experimental metastatic models with colon 26-M01 cells | Interferes IL6/STAT3 and cofilin pathways | (105) | |
| Alantolactone | Inula helenium | HUVECs, human MDA-MB‐231 breast cancer xenograft in mice | Suppresses the phosphorylation of VEGFR2 and its downstream protein kinase including PLCγ1, FAK, Src, and Akt | (106) | |
| Codonolactone | Atractylodes lancea | HUVECs, EA.hy 926 cells | Inhibits MMPs expression and VEGF secretion by down-regulating BMP/Runx2 activation | (107) | |
| Widdrol | Juniperus chinensis | HUVECs, Human colon adenocarcinoma HT29 cells | Suppresses phosphorylation of VEGFR2, AKT, FAK and eNOS | (108) | |
| Diterpene | Tanshinone IIA | The dried root of Salvia miltiorrhiza Bunge | Human EPCs, CAM, Matrigel plug model | Inhibits EPC angiogenesis through the VEGF, PLC, Akt and JNK signaling pathways | (109) |
| 16-hydroxy-pentandralactone | Vitex cofassus | HUVECs | Inhibits VEGF-stimulated HUVEC proliferation | (110) | |
| Cafestol | Unfiltered coffee | HUVECs | Inhibits the phosphorylation of FAK and Akt and decreases nitric oxide production | (111) | |
| Andrographolide | Andrographis paniculate | HUVECs, A549 Cells | Inhibits MMP-9 expression | (112) | |
| Triptolide | Tripterygium wilfordii | HUVECs, Breast cancer cells (Hs578T and MDAMB231) | Inhibits the ERK1/2-HIF1-α-VEGFA signaling pathway | (113) | |
| Triterpene | Ursolic acid | Many plant foods | Ehrlich ascites carcinoma (EAC) cells, Swiss albino mice with EAC cells | Inhibits VEGF and iNOS expression | (114) |
| Nimbolide | Azadirachta indica leaves | HCT-116, HT-29, Caco-2, CRC cells | Inhibits VEGF and MMP-9 expression | (115) | |
| Koetjapic acid | Sandoricum koetjaoe Merr | rat aortic ring, CAM, HUVECs | Inhibits VEGF expression | (116) | |
| Boswellic acid | Boswellia serrata | Ehrlich ascites carcinoma (EAC) cells, Swiss albino mice with EAC cells | Inhibits VEGF and TNF-α expression | (117) | |
| Betulinic acid | The stembark of Betula ssp. and from many other plants | Human endometrial adenocarcinoma (EA) cells | Inhibits prolidase, HIF-1α and VEGF expressions | (118) |
Saponins
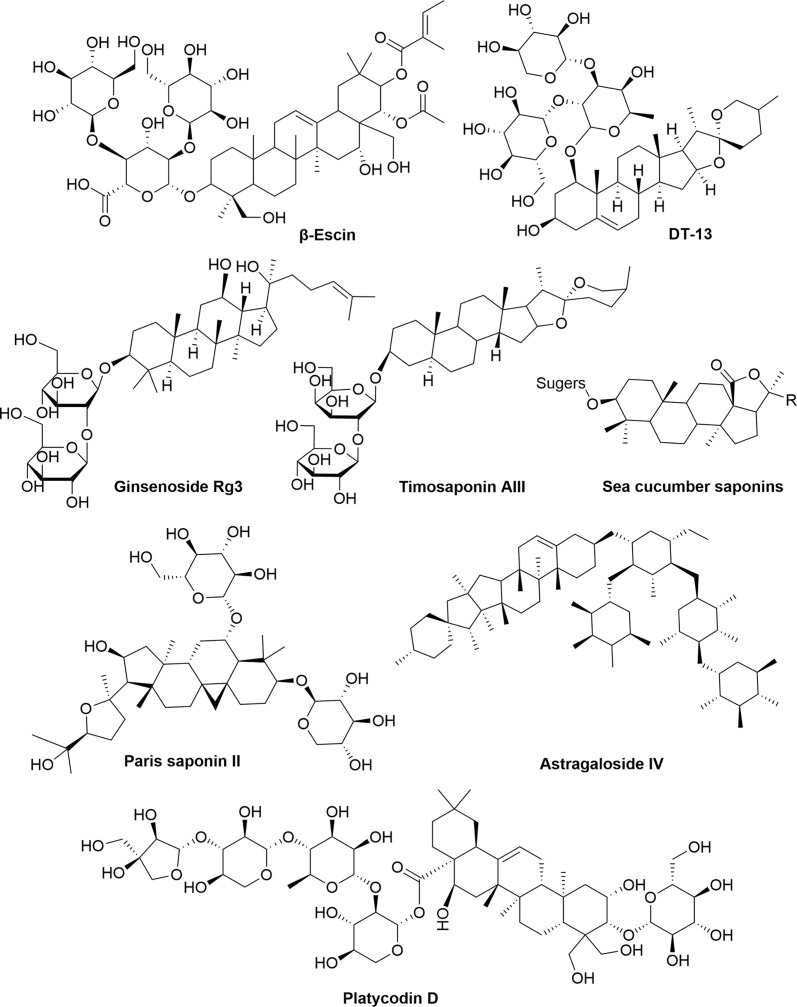
Saponins are composed of sapogenins and sugars. The sapogenins are triterpenes or spirostanes and the make-up sugars are commonly glucose, galactose, rhamnose, arabinose, xylose, glucuronic acid and galacturonic acid. Lipophilic sapogenins and hydrophilic sugar chains make the saponin an excellent surfactant. Saponins are mainly distributed in terrestrial higher plants and marine organisms, such as starfish and sea cucumber. Many Chinese herbal medicines, such as Ginseng, Polygala Tenuifolia, Platycodon grandiflorum, licorice, Anemarrhena and Bupleurum, contain saponins as the main active ingredients. According to the different structures of sapogenins, saponins have the biological functions of anti-tumor, hypoglycemic, cholesterol lowering, liver protection, immune regulation, anti-inflammatory and anti-microbial effects. The chemical structures ( Figure 6 ) and anti-angiogenic property of several saponins (Timosaponin AIII, Ginsenoside Rg3, β-Escin, sea cucumber saponins, et al) has been identified in recent years ( Table 6 ). Timosaponin AIII is a steroidal saponin, derived from Anemarrhena asphodeloides Bge. It exerts anti-angiogenic effect through VEGF/PI3K/Akt/MAPK signaling pathway in the zebrafish embryos model (119). Ginsenoside Rg3 is a triterpene saponin, isolated from Panax ginseng. Ginsenoside Rg3 can decrease the expression of MMP-2, MMP-9 and VEGF in B16 cell tumor mouse model and in vitro B16 cell model (122). β-escin is also a kind of triterpene saponin, extracted from Aesculus hippocastanum seeds. β-escin can inhibit melanoma angiogenesis by increasing the expression of TIMP-1 and TIMP-2 (123). Sea cucumber saponins are crude saponins, that are prevalent in Holothuria leucospilota. The saponins extracted from sea cucumber can inhibit the expression of VEGF-D and TGF-β in MCF7 cells (126).
Figure 6.
The chemical structures of saponins involved in tumor angiogenesis.
Table 6.
Natural anti-tumor angiogenesis saponins and their sources, experimental models and anti-angiogenic mechanisms.
| Category | Name | Source | Experimental model | Anti-angiogenic mechanism | Refs |
|---|---|---|---|---|---|
| Steroidal saponin | Timosaponin AIII | Anemarrhena asphodeloides Bge | Zebrafish embryos, HUVECs | Inhibits VEGF/PI3K/Akt/MAPK signaling cascade | (119) |
| DT-13 | Dwarf lilyturf tuber | HUVECs, CAM | Inhibits the levels of p-VEGFR-2, p-ERK1/2 and p-Akt, Inhibits the expression of VEGF | (120) | |
| Paris saponin II | Rhizoma paridis | HUVECs, SKOV3 cells | Suppresses NF-κB signaling | (121) | |
| Triterpene saponin | Ginsenoside Rg3 | Panax ginseng | B16 melanoma cells, C57BL/6 mice with B16 cells | Inhibits the expression of MMP-2, MMP-9 and VEGF | (122) |
| β-Escin | Aesculus hippocastanum seeds | B16F10 and SK-MEL5 cells | Increases the expression of TIMP-1 and TIMP-2 | (123) | |
| Astragaloside IV | Astragali radix | C57BL/6 mice with LLC cells | Blocks the M2 polarization of macrophages partially through the AMPK signaling pathway | (124) | |
| Platycodin D (PD) | the roots of Platycodon grandiflorum | C57BL/6 mice with H22 cells | Inhibits the expression of VEGF | (125) | |
| Crude saponins | Sea cucumber saponins (SCS) | Holothuria leucospilota (sea cucumber) | MCF7 cells | Inhibits the expression of VEGF-D and TGF-β | (126) |
| Rh.Sp saponins | Rumex hastatus D. Don. | CAM | – | (127) |
Conclusions
This review article shed a light on the role of natural compounds in cancer therapy by modulating angiogenic factors and ECs apoptosis. In addition, the chemical structures of natural compounds involved in tumor angiogenesis were summarized and shown in supplementary material with the format of CS ChemDraw Drawing, which could be conveniently used for the related researchers. Natural products have presented as new stars in the field of anti-tumor neovascularization research for their easy availability and cost-effectiveness (128). A large number of epidemiological studies proved the reduction of cancer incidence upon the high nutritional ingestion of vegetables and fruits (129). Moreover, natural products emerged as SIRT6 modulators can be successfully applied to treat cancer, inflammation, Alzheimer’s Disease, etc (130).
Currently, anti-angiogenic drugs are widely used and recognized in cancer treatments because they enriched the arsenal of chemotherapeutic drugs. The majority of modern targeted drugs fail to achieve the expected therapeutic effects. Consequently, the anti-cancer regimens have shifted to multi-targeted therapies using traditional and integrative natural products. There is a huge number of natural products for anti-angiogenic substances and the study on the complex mechanisms of these compounds is just started. Studies on the role of different structures of natural compounds in inhibiting tumor angiogenesis would assist in anti-cancer drug discovery and development. The anti-angiogenic therapy of human cancer patients is based on pre-clinical models that simulate the pathogenesis of human cancers, and most of them are elucidated in this review. It is certain that future patients will be benefited from the novel discoveries of natural products, thus a lot of research works are warranted.
Perspectives and limitations
As for clinical application of natural products in tumor, issues have emerged during the past few decades. The bioavailability of natural products is the major restriction, since these compounds have the properties of poor aqueous solubility and low absorption rate. Prior to the use of natural products in anti-tumor therapy, the concentration problem needs to be resolved. At present, several carriers (nanoparticles, micelles, lipids, etc) with natural products are being developed to apply to the delivery of these compounds to human body systems. Therefore, as hopeful therapeutic targets towards tumor angiogenesis, more efforts should be made to the development of natural compounds and their modifiers according to their molecular mechanisms and involved signaling pathways in tumors.
Author Contributions
PS, Z-SC, and DD conceived and designed the review. RL and XS created the tables and figures, and wrote the draft manuscript. RL, XS, and YG revised the manuscript and performed the table design. PS, Z-SC, and DD reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81803779); the Gansu Province Science Foundation for Distinguished Young Scholars (20JR10RA348) and the Gansu Province Science Foundation for B Program (1508RJZA018) from Gansu Provincial Sci. & Tech. Department; the Open Project of Research Center of Traditional Chinese Medicine, Gansu Province (zyzx-2020-zx1); the Open Project of Key Laboratory of Prevention and Treatment for Chronic Diseases by TCM in Gansu Province (GSMBKY2015-01); the Gansu Province Health Industry Scientific Research Program Management Project (GWGL2014-53); and the National Natural Science Foundation of Shaanxi Province (2018JQ2051).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks for the hard work of each author.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.772915/full#supplementary-material
References
- 1. Simon T, Gagliano T, Giamas G. Direct Effects of Anti-Angiogenic Therapies on Tumor Cells: VEGF Signaling. Trends Mol Med (2017) 23(3):282–92. doi: 10.1016/j.molmed.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 2. Haralambiev L, Neuffer O, Nitsch A, Kross NC, Bekeschus S, Hinz P, et al. Inhibition of Angiogenesis by Treatment With Cold Atmospheric Plasma as a Promising Therapeutic Approach in Oncology. Int J Mol Sci (2020) 21(19):7098–113. doi: 10.3390/ijms21197098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang H, Yang J, Zhang K, Liu J, Li Y, Su W, et al. Advances of Fibroblast Growth Factor/Receptor Signaling Pathway in Hepatocellular Carcinoma and Its Pharmacotherapeutic Targets. Front Pharmacol (2021) 12:650388. doi: 10.3389/fphar.2021.650388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chavakis E, Dimmeler S. Regulation of Endothelial Cell Survival and Apoptosis During Angiogenesis. Arterioscler Thromb Vasc Biol (2002) 22(6):887–93. doi: 10.1161/01.atv.0000017728.55907.a9 [DOI] [PubMed] [Google Scholar]
- 5. Christensen SB. Natural Products That Changed Society. Biomedicines (2021) 9(5):472–502. doi: 10.3390/biomedicines9050472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng J, Wang C, Jiang Y. Warming Yang Method in Traditional Chinese Medicine for Depression: A Protocol for Systematic Review and Meta-Analysis. Med (Baltimore) (2020) 99(52):e23919. doi: 10.1097/MD.0000000000023919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma B, Dutt V, Kaur N, Mittal A, Dabur R. Tinospora Cordifolia Protects From Skeletal Muscle Atrophy by Alleviating Oxidative Stress and Inflammation Induced by Sciatic Denervation. J Ethnopharmacol (2020) 254:112720. doi: 10.1016/j.jep.2020.112720 [DOI] [PubMed] [Google Scholar]
- 8. Parveen A, Sultana R, Lee SM, Kim TH, Kim SY. Phytochemicals Against Anti-Diabetic Complications: Targeting the Advanced Glycation End Product Signaling Pathway. Arch Pharm Res (2021) 44(4):378–401. doi: 10.1007/s12272-021-01323-9 [DOI] [PubMed] [Google Scholar]
- 9. Gaikwad S, Srivastava SK. Role of Phytochemicals in Perturbation of Redox Homeostasis in Cancer. Antioxid (Basel) (2021) 10(1):83–108. doi: 10.3390/antiox10010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fakhri S, Abbaszadeh F, Jorjani M, Pourgholami MH. The Effects of Anticancer Medicinal Herbs on Vascular Endothelial Growth Factor Based on Pharmacological Aspects: A Review Study. Nutr Cancer (2021) 73(1):1–15. doi: 10.1080/01635581.2019.1673451 [DOI] [PubMed] [Google Scholar]
- 11. Campos-Contreras ADR, Diaz-Munoz M, Vazquez-Cuevas FG. Purinergic Signaling in the Hallmarks of Cancer. Cells (2020) 9(7):1612–35. doi: 10.3390/cells9071612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parmar D, Apte M. Angiopoietin Inhibitors: A Review on Targeting Tumor Angiogenesis. Eur J Pharmacol (2021) 899:174021. doi: 10.1016/j.ejphar.2021.174021 [DOI] [PubMed] [Google Scholar]
- 13. Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, et al. The Role of Microenvironment in Tumor Angiogenesis. J Exp Clin Cancer Res (2020) 39(1):204. doi: 10.1186/s13046-020-01709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho WC, Jour G, Aung PP. Role of Angiogenesis in Melanoma Progression: Update on Key Angiogenic Mechanisms and Other Associated Components. Semin Cancer Biol (2019) 59:175–86. doi: 10.1016/j.semcancer.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 15. Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J Clin Med (2019) 9(1):84–104. doi: 10.3390/jcm9010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wheler JJ, Janku F, Naing A, Li Y, Stephen B, Zinner R, et al. TP53 Alterations Correlate With Response to VEGF/VEGFR Inhibitors: Implications for Targeted Therapeutics. Mol Cancer Ther (2016) 15(10):2475–85. doi: 10.1158/1535-7163.MCT-16-0196 [DOI] [PubMed] [Google Scholar]
- 17. Ribatti D, Vacca A. Role of Endothelial Cells and Fibroblasts in Multiple Myeloma Angiogenic Switch. Cancer Treat Res (2016) 169:51–61. doi: 10.1007/978-3-319-40320-5_5 [DOI] [PubMed] [Google Scholar]
- 18. Yoshida GJ, Azuma A, Miura Y, Orimo A. Activated Fibroblast Program Orchestrates Tumor Initiation and Progression; Molecular Mechanisms and the Associated Therapeutic Strategies. Int J Mol Sci (2019) 20(9):2256–85. doi: 10.3390/ijms20092256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Liu NM, Wang Y, Youn JY, Cai H. Endothelial Cell Calpain as a Critical Modulator of Angiogenesis. Biochim Biophys Acta Mol Basis Dis (2017) 1863(6):1326–35. doi: 10.1016/j.bbadis.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Ieso ML, Yool AJ. Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis. Front Chem (2018) 6:135. doi: 10.3389/fchem.2018.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu G, Chen T, Ding Z, Wang Y, Wei Y, Wei X. Inhibition of FGF-FGFR and VEGF-VEGFR Signalling in Cancer Treatment. Cell Prolif (2021) 54(4):e13009. doi: 10.1111/cpr.13009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monk BJ, Poveda A, Vergote I, Raspagliesi F, Fujiwara K, Bae DS, et al. Anti-Angiopoietin Therapy With Trebananib for Recurrent Ovarian Cancer (TRINOVA-1): A Randomised, Multicentre, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Oncol (2014) 15(8):799–808. doi: 10.1016/S1470-2045(14)70244-X [DOI] [PubMed] [Google Scholar]
- 23. Yang LI, Wang X, Sun J, Liu C, Li G, Zhu J, et al. Neuritin Promotes Angiogenesis Through Inhibition of DLL4/Notch Signaling Pathway. Acta Biochim Biophys Sin (Shanghai) (2021) 53(6):663–72. doi: 10.1093/abbs/gmab039 [DOI] [PubMed] [Google Scholar]
- 24. Gerber HP, Dixit V, Ferrara N. Vascular Endothelial Growth Factor Induces Expression of the Antiapoptotic Proteins Bcl-2 and A1 in Vascular Endothelial Cells. J Biol Chem (1998) 273(21):13313–6. doi: 10.1074/jbc.273.21.13313 [DOI] [PubMed] [Google Scholar]
- 25. O’Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, et al. Control of Apoptosis During Angiogenesis by Survivin Expression in Endothelial Cells. Am J Pathol (2000) 156(2):393–8. doi: 10.1016/s0002-9440(10)64742-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 Is an Apoptosis Survival Factor for Endothelial Cells. FEBS Lett (1999) 448(2-3):249–53. doi: 10.1016/s0014-5793(99)00378-6 [DOI] [PubMed] [Google Scholar]
- 27. Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, et al. Targeted Deficiency or Cytosolic Truncation of the VE-Cadherin Gene in Mice Impairs VEGF-Mediated Endothelial Survival and Angiogenesis. Cell (1999) 98(2):147–57. doi: 10.1016/s0092-8674(00)81010-7 [DOI] [PubMed] [Google Scholar]
- 28. Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of Alphavbeta3 Integrin in the Activation of Vascular Endothelial Growth Factor Receptor-2. EMBO J (1999) 18(4):882–92. doi: 10.1093/emboj/18.4.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sha W, Liu M, Sun D, Qiu J, Xu B, Chen L, et al. Resveratrol Improves Gly-LDL-Induced Vascular Endothelial Cell Apoptosis, Inflammatory Factor Secretion and Oxidative Stress by Regulating miR-142-3p and Regulating SPRED2-Mediated Autophagy. Aging (Albany NY) (2021) 13(5):6878–89. doi: 10.18632/aging.202546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vicas LG, Jurca T, Baldea I, Filip GA, Olteanu D, Clichici SV, et al. Physalis Alkekengi L. Extract Reduces the Oxidative Stress, Inflammation and Apoptosis in Endothelial Vascular Cells Exposed to Hyperglycemia. Molecules (2020) 25(16):3747–65. doi: 10.3390/molecules25163747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song P, Hai Y, Wang X, Zhao L, Chen B, Cui P, et al. Realgar Transforming Solution Suppresses Angiogenesis and Tumor Growth by Inhibiting VEGF Receptor 2 Signaling in Vein Endothelial Cells. Arch Pharm Res (2018) 41(4):467–80. doi: 10.1007/s12272-018-1014-6 [DOI] [PubMed] [Google Scholar]
- 32. Gordon E, Schimmel L, Frye M. The Importance of Mechanical Forces for In Vitro Endothelial Cell Biology. Front Physiol (2020) 11:684. doi: 10.3389/fphys.2020.00684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Victorelli FD, Cardoso VMO, Ferreira NN, Calixto GMF, Fontana CR, Baltazar F, et al. Chick Embryo Chorioallantoic Membrane as a Suitable In Vivo Model to Evaluate Drug Delivery Systems for Cancer Treatment: A Review. Eur J Pharm Biopharm (2020) 153:273–84. doi: 10.1016/j.ejpb.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 34. Brown HK, Schiavone K, Tazzyman S, Heymann D, Chico TJ. Zebrafish Xenograft Models of Cancer and Metastasis for Drug Discovery. Expert Opin Drug Discovery (2017) 12(4):379–89. doi: 10.1080/17460441.2017.1297416 [DOI] [PubMed] [Google Scholar]
- 35. Auerbach R, Akhtar N, Lewis RL, Shinners BL. Angiogenesis Assays: Problems and Pitfalls. Cancer Metastasis Rev (2000) 19(1-2):167–72. doi: 10.1023/a:1026574416001 [DOI] [PubMed] [Google Scholar]
- 36. Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, et al. Glycosylation-Dependent Lectin-Receptor Interactions Preserve Angiogenesis in Anti-VEGF Refractory Tumors. Cell (2014) 156(4):744–58. doi: 10.1016/j.cell.2014.01.043 [DOI] [PubMed] [Google Scholar]
- 37. Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, et al. Interaction Between Bevacizumab and Murine VEGF-A: A Reassessment. Invest Ophthalmol Vis Sci (2008) 49(2):522–7. doi: 10.1167/iovs.07-1175 [DOI] [PubMed] [Google Scholar]
- 38. Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. Cessation of CCL2 Inhibition Accelerates Breast Cancer Metastasis by Promoting Angiogenesis. Nature (2014) 515(7525):130–3. doi: 10.1038/nature13862 [DOI] [PubMed] [Google Scholar]
- 39. Kowshik J, Giri H, Kishore TK, Kesavan R, Vankudavath RN, Reddy GB, et al. Ellagic Acid Inhibits VEGF/VEGFR2, PI3K/Akt and MAPK Signaling Cascades in the Hamster Cheek Pouch Carcinogenesis Model. Anticancer Agents Med Chem (2014) 14(9):1249–60. doi: 10.2174/1871520614666140723114217 [DOI] [PubMed] [Google Scholar]
- 40. Wang N, Wang ZY, Mo SL, Loo TY, Wang DM, Luo HB, et al. Ellagic Acid, a Phenolic Compound, Exerts Anti-Angiogenesis Effects via VEGFR-2 Signaling Pathway in Breast Cancer. Breast Cancer Res Treat (2012) 134(3):943–55. doi: 10.1007/s10549-012-1977-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park JJ, Hwang SJ, Park JH, Lee HJ. Chlorogenic Acid Inhibits Hypoxia-Induced Angiogenesis via Down-Regulation of the HIF-1alpha/AKT Pathway. Cell Oncol (Dordr) (2015) 38(2):111–8. doi: 10.1007/s13402-014-0216-2 [DOI] [PubMed] [Google Scholar]
- 42. Abusnina A, Keravis T, Zhou Q, Justiniano H, Lobstein A, Lugnier C. Tumour Growth Inhibition and Anti-Angiogenic Effects Using Curcumin Correspond to Combined PDE2 and PDE4 Inhibition. Thromb Haemost (2015) 113(2):319–28. doi: 10.1160/TH14-05-0454 [DOI] [PubMed] [Google Scholar]
- 43. Jiao D, Wang J, Lu W, Tang X, Chen J, Mou H, et al. Curcumin Inhibited HGF-Induced EMT and Angiogenesis Through Regulating C-Met Dependent PI3K/Akt/mTOR Signaling Pathways in Lung Cancer. Mol Ther Oncolytics (2016) 3:16018. doi: 10.1038/mto.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tian B, Zhao Y, Liang T, Ye X, Li Z, Yan D, et al. Curcumin Inhibits Urothelial Tumor Development by Suppressing IGF2 and IGF2-Mediated PI3K/AKT/mTOR Signaling Pathway. J Drug Target (2017) 25(7):626–36. doi: 10.1080/1061186X.2017.1306535 [DOI] [PubMed] [Google Scholar]
- 45. Zhao D, Qin C, Fan X, Li Y, Gu B. Inhibitory Effects of Quercetin on Angiogenesis in Larval Zebrafish and Human Umbilical Vein Endothelial Cells. Eur J Pharmacol (2014) 723:360–7. doi: 10.1016/j.ejphar.2013.10.069 [DOI] [PubMed] [Google Scholar]
- 46. Zhao X, Wang Q, Yang S, Chen C, Li X, Liu J, et al. Quercetin Inhibits Angiogenesis by Targeting Calcineurin in the Xenograft Model of Human Breast Cancer. Eur J Pharmacol (2016) 781:60–8. doi: 10.1016/j.ejphar.2016.03.063 [DOI] [PubMed] [Google Scholar]
- 47. Negrao R, Costa R, Duarte D, Gomes TT, Azevedo I, Soares R. Different Effects of Catechin on Angiogenesis and Inflammation Depending on VEGF Levels. J Nutr Biochem (2013) 24(2):435–44. doi: 10.1016/j.jnutbio.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 48. Gao Y, Rankin GO, Tu Y, Chen YC. Inhibitory Effects of the Four Main Theaflavin Derivatives Found in Black Tea on Ovarian Cancer Cells. Anticancer Res (2016) 36(2):643–51. [PMC free article] [PubMed] [Google Scholar]
- 49. Jin G, Yang Y, Liu K, Zhao J, Chen X, Liu H, et al. Combination Curcumin and (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Carcinoma Microenvironment-Induced Angiogenesis by JAK/STAT3/IL-8 Pathway. Oncogenesis (2017) 6(10):e384. doi: 10.1038/oncsis.2017.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu H, Becker CM, Lui WT, Chu CY, Davis TN, Kung AL, et al. Green Tea Epigallocatechin-3-Gallate Inhibits Angiogenesis and Suppresses Vascular Endothelial Growth Factor C/vascular Endothelial Growth Factor Receptor 2 Expression and Signaling in Experimental Endometriosis In Vivo . Fertil Steril (2011) 96(4):1021–8. doi: 10.1016/j.fertnstert.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 51. Yan Y, Yao L, Sun H, Pang S, Kong X, Zhao S, et al. Effects of Wogonoside on Invasion and Migration of Lung Cancer A549 Cells and Angiogenesis in Xenograft Tumors of Nude Mice. J Thorac Dis (2020) 12(4):1552–60. doi: 10.21037/jtd-20-1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mirzoeva S, Franzen CA, Pelling JC. Apigenin Inhibits TGF-Beta-Induced VEGF Expression in Human Prostate Carcinoma Cells via a Smad2/3- and Src-Dependent Mechanism. Mol Carcinog (2014) 53(8):598–609. doi: 10.1002/mc.22005 [DOI] [PubMed] [Google Scholar]
- 53. Cook MT, Mafuvadze B, Besch-Williford C, Ellersieck MR, Goyette S, Hyder SM. Luteolin Suppresses Development of Medroxyprogesterone Acetate-Accelerated 7,12-Dimethylbenz(a)Anthracene-Induced Mammary Tumors in Sprague-Dawley Rats. Oncol Rep (2016) 35(2):825–32. doi: 10.3892/or.2015.4431 [DOI] [PubMed] [Google Scholar]
- 54. Doleckova I, Rarova L, Gruz J, Vondrusova M, Strnad M, Krystof V. Antiproliferative and Antiangiogenic Effects of Flavone Eupatorin, an Active Constituent of Chloroform Extract of Orthosiphon Stamineus Leaves. Fitoterapia (2012) 83(6):1000–7. doi: 10.1016/j.fitote.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 55. Abbaszadeh H, Ebrahimi SA, Akhavan MM. Antiangiogenic Activity of Xanthomicrol and Calycopterin, Two Polymethoxylated Hydroxyflavones in Both In Vitro and Ex Vivo Models. Phytother Res (2014) 28(11):1661–70. doi: 10.1002/ptr.5179 [DOI] [PubMed] [Google Scholar]
- 56. Kim MH, Jeong YJ, Cho HJ, Hoe HS, Park KK, Park YY, et al. Delphinidin Inhibits Angiogenesis Through the Suppression of HIF-1alpha and VEGF Expression in A549 Lung Cancer Cells. Oncol Rep (2017) 37(2):777–84. doi: 10.3892/or.2016.5296 [DOI] [PubMed] [Google Scholar]
- 57. Tanaka J, Nakamura S, Tsuruma K, Shimazawa M, Shimoda H, Hara H. Purple Rice (Oryza Sativa L.) Extract and Its Constituents Inhibit VEGF-Induced Angiogenesis. Phytother Res (2012) 26(2):214–22. doi: 10.1002/ptr.3533 [DOI] [PubMed] [Google Scholar]
- 58. Baba AB, Nivetha R, Chattopadhyay I, Nagini S. Blueberry and Malvidin Inhibit Cell Cycle Progression and Induce Mitochondrial-Mediated Apoptosis by Abrogating the JAK/STAT-3 Signalling Pathway. Food Chem Toxicol (2017) 109(Pt 1):534–43. doi: 10.1016/j.fct.2017.09.054 [DOI] [PubMed] [Google Scholar]
- 59. Bellou S, Karali E, Bagli E, Al-Maharik N, Morbidelli L, Ziche M, et al. The Isoflavone Metabolite 6-Methoxyequol Inhibits Angiogenesis and Suppresses Tumor Growth. Mol Cancer (2012) 11:35. doi: 10.1186/1476-4598-11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang JH, Hu J, Wan L, Chen LJ. Barbigerone Inhibits Tumor Angiogenesis, Growth and Metastasis in Melanoma. Asian Pac J Cancer Prev (2014) 15(1):167–74. doi: 10.7314/apjcp.2014.15.1.167 [DOI] [PubMed] [Google Scholar]
- 61. Xue ZG, Niu PG, Shi DH, Liu Y, Deng J, Chen YY. Cardamonin Inhibits Angiogenesis by mTOR Downregulation in SKOV3 Cells. Planta Med (2016) 82(1-2):70–5. doi: 10.1055/s-0035-1557901 [DOI] [PubMed] [Google Scholar]
- 62. Saito K, Matsuo Y, Imafuji H, Okubo T, Maeda Y, Sato T, et al. Xanthohumol Inhibits Angiogenesis by Suppressing Nuclear factor-kappaB Activation in Pancreatic Cancer. Cancer Sci (2018) 109(1):132–40. doi: 10.1111/cas.13441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chiang CH, Yeh CY, Chung JG, Chiang IT, Hsu FT. Amentoflavone Induces Apoptosis and Reduces Expression of Anti-Apoptotic and Metastasis-Associated Proteins in Bladder Cancer. Anticancer Res (2019) 39(7):3641–9. doi: 10.21873/anticanres.13512 [DOI] [PubMed] [Google Scholar]
- 64. Zhou J, Zhao R, Ye T, Yang S, Li Y, Yang F, et al. Antitumor Activity in Colorectal Cancer Induced by Hinokiflavone. J Gastroenterol Hepatol (2019) 34(9):1571–80. doi: 10.1111/jgh.14581 [DOI] [PubMed] [Google Scholar]
- 65. Pandey AK, Bhattacharya P, Shukla SC, Paul S, Patnaik R. Resveratrol Inhibits Matrix Metalloproteinases to Attenuate Neuronal Damage in Cerebral Ischemia: A Molecular Docking Study Exploring Possible Neuroprotection. Neural Regener Res (2015) 10(4):568–75. doi: 10.4103/1673-5374.155429 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66. Ren F, Wu K, Yang Y, Yang Y, Wang Y, Li J. Dandelion Polysaccharide Exerts Anti-Angiogenesis Effect on Hepatocellular Carcinoma by Regulating VEGF/HIF-1alpha Expression. Front Pharmacol (2020) 11:460. doi: 10.3389/fphar.2020.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ding J, Jia W, Cui Y, Jin J, Zhang Y, Xu L, et al. Anti-Angiogenic Effect of a Chemically Sulfated Polysaccharide From Phellinus Ribis by Inhibiting VEGF/VEGFR Pathway. Int J Biol Macromol (2020) 154:72–81. doi: 10.1016/j.ijbiomac.2020.03.068 [DOI] [PubMed] [Google Scholar]
- 68. Hsu WJ, Lin MH, Kuo TC, Chou CM, Mi FL, Cheng CH, et al. Fucoidan From Laminaria Japonica Exerts Antitumor Effects on Angiogenesis and Micrometastasis in Triple-Negative Breast Cancer Cells. Int J Biol Macromol (2020) 149:600–8. doi: 10.1016/j.ijbiomac.2020.01.256 [DOI] [PubMed] [Google Scholar]
- 69. Oliveira C, Granja S, Neves NM, Reis RL, Baltazar F, Silva TH, et al. Fucoidan From Fucus Vesiculosus Inhibits New Blood Vessel Formation and Breast Tumor Growth In Vivo . Carbohydr Polym (2019) 223:115034. doi: 10.1016/j.carbpol.2019.115034 [DOI] [PubMed] [Google Scholar]
- 70. Rui X, Pan HF, Shao SL, Xu XM. Anti-Tumor and Anti-Angiogenic Effects of Fucoidan on Prostate Cancer: Possible JAK-STAT3 Pathway. BMC Complement Altern Med (2017) 17(1):378. doi: 10.1186/s12906-017-1885-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yao Y, Zhou L, Liao W, Chen H, Du Z, Shao C, et al. HH1-1, a Novel Galectin-3 Inhibitor, Exerts Anti-Pancreatic Cancer Activity by Blocking Galectin-3/EGFR/AKT/FOXO3 Signaling Pathway. Carbohydr Polym (2019) 204:111–23. doi: 10.1016/j.carbpol.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 72. Zong S, Li J, Yang L, Huang Q, Ye Z, Hou G, et al. Synergistic Antitumor Effect of Polysaccharide From Lachnum Sp. In Combination With Cyclophosphamide in Hepatocellular Carcinoma. Carbohydr Polym (2018) 196:33–46. doi: 10.1016/j.carbpol.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 73. Yao H, Cui P, Xu D, Liu Y, Tian Q, Zhang F. A Water-Soluble Polysaccharide From the Roots of Polygala Tenuifolia Suppresses Ovarian Tumor Growth and Angiogenesis In Vivo. Int J Biol Macromol (2018) 107(Pt A):713–8. doi: 10.1016/j.ijbiomac.2017.09.043 [DOI] [PubMed] [Google Scholar]
- 74. Varghese S, Joseph MM, S RA, B SU, Sreelekha TT. The Inhibitory Effect of Anti- Tumor Polysaccharide From Punica Granatum on Metastasis. Int J Biol Macromol (2017) 103:1000–10. doi: 10.1016/j.ijbiomac.2017.05.137 [DOI] [PubMed] [Google Scholar]
- 75. Ou M, Sun X, Liang J, Liu F, Wang L, Wu X, et al. A Polysaccharide From Sargassum Thunbergii Inhibits Angiogenesis via Downregulating MMP-2 Activity and VEGF/HIF-1alpha Signaling. Int J Biol Macromol (2017) 94(Pt A):451–8. doi: 10.1016/j.ijbiomac.2016.10.046 [DOI] [PubMed] [Google Scholar]
- 76. Lv Y, Shan X, Zhao X, Cai C, Zhao X, Lang Y, et al. Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide From the Sea Grass Zostera Caespitosa Miki. Mar Drugs (2015) 13(6):3710–31. doi: 10.3390/md13063710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jayaram S, Kapoor S, Dharmesh SM. Pectic Polysaccharide From Corn (Zea Mays L.) Effectively Inhibited Multi-Step Mediated Cancer Cell Growth and Metastasis. Chem Biol Interact (2015) 235:63–75. doi: 10.1016/j.cbi.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 78. Wang P, Zhang L, Yao J, Shi Y, Li P, Ding K. An Arabinogalactan From Flowers of Panax Notoginseng Inhibits Angiogenesis by BMP2/Smad/Id1 Signaling. Carbohydr Polym (2015) 121:328–35. doi: 10.1016/j.carbpol.2014.11.073 [DOI] [PubMed] [Google Scholar]
- 79. Li C, Wu X, Zhang H, Yang G, Hao M, Sheng S, et al. A Huaier Polysaccharide Restrains Hepatocellular Carcinoma Growth and Metastasis by Suppression Angiogenesis. Int J Biol Macromol (2015) 75:115–20. doi: 10.1016/j.ijbiomac.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 80. Zou Y, Xiong H, Xiong H, Lu T, Zhu F, Luo Z, et al. A Polysaccharide From Mushroom Huaier Retards Human Hepatocellular Carcinoma Growth, Angiogenesis, and Metastasis in Nude Mice. Tumour Biol (2015) 36(4):2929–36. doi: 10.1007/s13277-014-2923-8 [DOI] [PubMed] [Google Scholar]
- 81. Kim Y, Sugihara Y, Kim TY, Cho SM, Kim JY, Lee JY, et al. Identification and Validation of VEGFR2 Kinase as a Target of Voacangine by a Systematic Combination of DARTS and MSI. Biomolecules (2020) 10(4):508–25. doi: 10.3390/biom10040508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hwang ST, Um JY, Chinnathambi A, Alharbi SA, Narula AS, Namjoshi OA, et al. Evodiamine Mitigates Cellular Growth and Promotes Apoptosis by Targeting the C-Met Pathway in Prostate Cancer Cells. Molecules (2020) 25(6):1320–33. doi: 10.3390/molecules25061320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shi L, Yang F, Luo F, Liu Y, Zhang F, Zou M, et al. Evodiamine Exerts Anti-Tumor Effects Against Hepatocellular Carcinoma Through Inhibiting Beta-Catenin-Mediated Angiogenesis. Tumour Biol (2016) 37(9):12791–803. doi: 10.1007/s13277-016-5251-3 [DOI] [PubMed] [Google Scholar]
- 84. Brautigam J, Bischoff I, Schurmann C, Buchmann G, Epah J, Fuchs S, et al. Narciclasine Inhibits Angiogenic Processes by Activation of Rho Kinase and by Downregulation of the VEGF Receptor 2. J Mol Cell Cardiol (2019) 135:97–108. doi: 10.1016/j.yjmcc.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 85. Zou L, Liu X, Li J, Li W, Zhang L, Li J, et al. Tetramethylpyrazine Enhances the Antitumor Effect of Paclitaxel by Inhibiting Angiogenesis and Inducing Apoptosis. Front Pharmacol (2019) 10:707. doi: 10.3389/fphar.2019.00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hai-Rong C, Xiang H, Xiao-Rong Z. Harmine Suppresses Bladder Tumor Growth by Suppressing Vascular Endothelial Growth Factor Receptor 2-Mediated Angiogenesis. Biosci Rep (2019) 39(5):1–10. doi: 10.1042/BSR20190155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu R, Peng J, Wang H, Li L, Wen X, Tan Y, et al. Oxysophocarpine Retards the Growth and Metastasis of Oral Squamous Cell Carcinoma by Targeting the Nrf2/HO-1 Axis. Cell Physiol Biochem (2018) 49(5):1717–33. doi: 10.1159/000493615 [DOI] [PubMed] [Google Scholar]
- 88. Tae N, Hung TM, Kim O, Kim N, Lee S, Na S, et al. A Cassaine Diterpene Alkaloid, 3beta-Acetyl-Nor-Erythrophlamide, Suppresses VEGF-Induced Angiogenesis and Tumor Growth via Inhibiting eNOS Activation. Oncotarget (2017) 8(54):92346–58. doi: 10.18632/oncotarget.21307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mingzhu LP SUO, Mei ZHANG, Yaodong ZHU, Mengran XU, LI W. Brucine Inhibits Vasculogenic Mimicry of Breast Cancer Cell Lines In Vitro and the Related Mechanism. China Oncol (2018) 28(4):241–7. doi: 10.19401/j.cnki.1007-3639.2018.04.001 [DOI] [Google Scholar]
- 90. Meher RK, Naik MR, Bastia B, Naik PK. Comparative Evaluation of Anti-Angiogenic Effects of Noscapine Derivatives. Bioinformation (2018) 14(5):236–40. doi: 10.6026/97320630014236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang D, Li Z, Zhang L, Atanasov AG, Wang S. Characterization of the Isosteroidal Alkaloid Chuanbeinone From Bulbus of Fritillaria Pallidiflora as Novel Antitumor Agent In Vitro and In Vivo . Planta Med (2016) 82(3):195–204. doi: 10.1055/s-0035-1558156 [DOI] [PubMed] [Google Scholar]
- 92. Assis PA, De Figueiredo-Pontes LL, Lima AS, Leao V, Candido LA, Pintao CT, et al. Halofuginone Inhibits Phosphorylation of SMAD-2 Reducing Angiogenesis and Leukemia Burden in an Acute Promyelocytic Leukemia Mouse Model. J Exp Clin Cancer Res (2015) 34:65. doi: 10.1186/s13046-015-0181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xiao W, Jiang Y, Men Q, Yuan L, Huang Z, Liu T, et al. Tetrandrine Induces G1/S Cell Cycle Arrest Through the ROS/Akt Pathway in EOMA Cells and Inhibits Angiogenesis In Vivo . Int J Oncol (2015) 46(1):360–8. doi: 10.3892/ijo.2014.2735 [DOI] [PubMed] [Google Scholar]
- 94. Tsang CM, Cheung KC, Cheung YC, Man K, Lui VW, Tsao SW, et al. Berberine Suppresses Id-1 Expression and Inhibits the Growth and Development of Lung Metastases in Hepatocellular Carcinoma. Biochim Biophys Acta (2015) 1852(3):541–51. doi: 10.1016/j.bbadis.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 95. Xie T, Ren HY, Lin HQ, Mao JP, Zhu T, Wang SD, et al. Sinomenine Prevents Metastasis of Human Osteosarcoma Cells via S Phase Arrest and Suppression of Tumor-Related Neovascularization and Osteolysis Through the CXCR4-STAT3 Pathway. Int J Oncol (2016) 48(5):2098–112. doi: 10.3892/ijo.2016.3416 [DOI] [PubMed] [Google Scholar]
- 96. Xu JY, Meng QH, Chong Y, Jiao Y, Zhao L, Rosen EM, et al. Sanguinarine is a Novel VEGF Inhibitor Involved in the Suppression of Angiogenesis and Cell Migration. Mol Clin Oncol (2013) 1(2):331–6. doi: 10.3892/mco.2012.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chakraborty S, Adhikary A, Mazumdar M, Mukherjee S, Bhattacharjee P, Guha D, et al. Capsaicin-Induced Activation of P53-SMAR1 Auto-Regulatory Loop Down-Regulates VEGF in Non-Small Cell Lung Cancer to Restrain Angiogenesis. PloS One (2014) 9(6):e99743. doi: 10.1371/journal.pone.0099743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Loutrari H, Hatziapostolou M, Skouridou V, Papadimitriou E, Roussos C, Kolisis FN, et al. Perillyl Alcohol Is an Angiogenesis Inhibitor. J Pharmacol Exp Ther (2004) 311(2):568–75. doi: 10.1124/jpet.104.070516 [DOI] [PubMed] [Google Scholar]
- 99. Chidambara Murthy KN, Jayaprakasha GK. Patil BS. D-Limonene Rich Volatile Oil From Blood Oranges Inhibits Angiogenesis, Metastasis and Cell Death in Human Colon Cancer Cells. Life Sci (2012) 91(11-12):429–39. doi: 10.1016/j.lfs.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 100. Xu MD, Liu L, Wu MY, Jiang M, Shou LM, Wang WJ, et al. The Combination of Cantharidin and Antiangiogenic Therapeutics Presents Additive Antitumor Effects Against Pancreatic Cancer. Oncogenesis (2018) 7(11):94. doi: 10.1038/s41389-018-0102-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jin TX-kZX-tWH-cDHC . Effect of Menthol on Proliferation, Migration and Expressions of IL-8, CXCL-12 and VEGF in Hepatoma HepG2 Cells. Chin J Exp Tradit Med Formulae (2019) 25(21):60–5. doi: 10.13422/j.cnki.syfjx.20192124 [DOI] [Google Scholar]
- 102. Chen W, Lu Y, Wu J, Gao M, Wang A, Xu B. Beta-Elemene Inhibits Melanoma Growth and Metastasis via Suppressing Vascular Endothelial Growth Factor-Mediated Angiogenesis. Cancer Chemother Pharmacol (2011) 67(4):799–808. doi: 10.1007/s00280-010-1378-x [DOI] [PubMed] [Google Scholar]
- 103. Sheng W, Mao H, Wang C, Yang N, Zhang Z, Han J. Dehydrocostus Lactone Enhances Chemotherapeutic Potential of Doxorubicin in Lung Cancer by Inducing Cell Death and Limiting Metastasis. Med Sci Monit (2018) 24:7850–61. doi: 10.12659/MSM.911410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Naderi Alizadeh M, Rashidi M, Muhammadnejad A, Moeini Zanjani T, Ziai SA. Antitumor Effects of Umbelliprenin in a Mouse Model of Colorectal Cancer. Iran J Pharm Res (2018) 17(3):976–85. [PMC free article] [PubMed] [Google Scholar]
- 105. Li M, Yue GG, Song LH, Huang MB, Lee JK, Tsui SK, et al. Natural Small Molecule Bigelovin Suppresses Orthotopic Colorectal Tumor Growth and Inhibits Colorectal Cancer Metastasis via IL6/STAT3 Pathway. Biochem Pharmacol (2018) 150:191–201. doi: 10.1016/j.bcp.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 106. Liu YR, Cai QY, Gao YG, Luan X, Guan YY, Lu Q, et al. Alantolactone, a Sesquiterpene Lactone, Inhibits Breast Cancer Growth by Antiangiogenic Activity via Blocking VEGFR2 Signaling. Phytother Res (2018) 32(4):643–50. doi: 10.1002/ptr.6004 [DOI] [PubMed] [Google Scholar]
- 107. Wang S, Cai R, Ma J, Liu T, Ke X, Lu H, et al. The Natural Compound Codonolactone Impairs Tumor Induced Angiogenesis by Downregulating BMP Signaling in Endothelial Cells. Phytomedicine (2015) 22(11):1017–26. doi: 10.1016/j.phymed.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 108. Jin S, Yun HJ, Jeong HY, Oh YN, Park HJ, Yun SG, et al. Widdrol, a Sesquiterpene Isolated From Juniperus Chinensis, Inhibits Angiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 2 Signaling. Oncol Rep (2015) 34(3):1178–84. doi: 10.3892/or.2015.4075 [DOI] [PubMed] [Google Scholar]
- 109. Lee HP, Liu YC, Chen PC, Tai HC, Li TM, Fong YC, et al. Tanshinone IIA Inhibits Angiogenesis in Human Endothelial Progenitor Cells In Vitro and In Vivo . Oncotarget (2017) 8(65):109217–27. doi: 10.18632/oncotarget.22649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rasyid FA, Fukuyoshi S, Ando H, Miyake K, Atsumi T, Fujie T, et al. A Novel Clerodane Diterpene From Vitex Cofassus. Chem Pharm Bull (Tokyo) (2017) 65(1):116–20. doi: 10.1248/cpb.c16-00775 [DOI] [PubMed] [Google Scholar]
- 111. Wang S, Yoon YC, Sung MJ, Hur HJ, Park JH. Antiangiogenic Properties of Cafestol, a Coffee Diterpene, in Human Umbilical Vein Endothelial Cells. Biochem Biophys Res Commun (2012) 421(3):567–71. doi: 10.1016/j.bbrc.2012.04.046 [DOI] [PubMed] [Google Scholar]
- 112. Guo X, Zhao M, Lin Y, Chen W, Wang S, Dai J. [Inhibitory Effect of Andrographolide on Angiogenesis Induced by the Supernatant From Cultured Tumor Cells]. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2018) 43(8):821–5. doi: 10.11817/j.issn.1672-7347.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 113. Liu H, Tang L, Li X, Li H. Triptolide Inhibits Vascular Endothelial Growth Factor-Mediated Angiogenesis in Human Breast Cancer Cells. Exp Ther Med (2018) 16(2):830–6. doi: 10.3892/etm.2018.6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Saraswati S, Agrawal SS, Alhaider AA. Ursolic Acid Inhibits Tumor Angiogenesis and Induces Apoptosis Through Mitochondrial-Dependent Pathway in Ehrlich Ascites Carcinoma Tumor. Chem Biol Interact (2013) 206(2):153–65. doi: 10.1016/j.cbi.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 115. Gupta SC, Prasad S, Sethumadhavan DR, Nair MS, Mo YY, Aggarwal BB. Nimbolide, a Limonoid Triterpene, Inhibits Growth of Human Colorectal Cancer Xenografts by Suppressing the Proinflammatory Microenvironment. Clin Cancer Res (2013) 19(16):4465–76. doi: 10.1158/1078-0432.CCR-13-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nassar ZD, Aisha AF, Ahamed MB, Ismail Z, Abu-Salah KM, Alrokayan SA, et al. Antiangiogenic Properties of Koetjapic Acid, a Natural Triterpene Isolated From Sandoricum Koetjaoe Merr. Cancer Cell Int (2011) 11(1):12. doi: 10.1186/1475-2867-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Agrawal SS, Saraswati S, Mathur R, Pandey M. Antitumor Properties of Boswellic Acid Against Ehrlich Ascites Cells Bearing Mouse. Food Chem Toxicol (2011) 49(9):1924–34. doi: 10.1016/j.fct.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 118. Karna E, Szoka L, Palka JA. Betulinic Acid Inhibits the Expression of Hypoxia-Inducible Factor 1alpha and Vascular Endothelial Growth Factor in Human Endometrial Adenocarcinoma Cells. Mol Cell Biochem (2010) 340(1-2):15–20. doi: 10.1007/s11010-010-0395-8 [DOI] [PubMed] [Google Scholar]
- 119. Zhou ZY, Zhao WR, Xiao Y, Zhou XM, Huang C, Shi WT, et al. Antiangiogenesis Effect of Timosaponin AIII on HUVECs In Vitro and Zebrafish Embryos In Vivo . Acta Pharmacol Sin (2020) 41(2):260–9. doi: 10.1038/s41401-019-0291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhao R, Sun L, Lin S, Bai X, Yu B, Yuan S, et al. The Saponin Monomer of Dwarf Lilyturf Tuber, DT-13, Inhibits Angiogenesis Under Hypoxia and Normoxia via Multi-Targeting Activity. Oncol Rep (2013) 29(4):1379–86. doi: 10.3892/or.2013.2272 [DOI] [PubMed] [Google Scholar]
- 121. Yang M, Zou J, Zhu H, Liu S, Wang H, Bai P, et al. Paris Saponin II Inhibits Human Ovarian Cancer Cell-Induced Angiogenesis by Modulating NF-kappaB Signaling. Oncol Rep (2015) 33(5):2190–8. doi: 10.3892/or.2015.3836 [DOI] [PubMed] [Google Scholar]
- 122. Meng L, Ji R, Dong X, Xu X, Xin Y, Jiang X. Antitumor Activity of Ginsenoside Rg3 in Melanoma Through Downregulation of the ERK and Akt Pathways. Int J Oncol (2019) 54(6):2069–79. doi: 10.3892/ijo.2019.4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kwak H, An H, Alam MB, Choi WS, Lee SY, Lee SH. Inhibition of Migration and Invasion in Melanoma Cells by Beta-Escin via the ERK/NF-kappaB Signaling Pathway. Biol Pharm Bull (2018) 41(10):1606–10. doi: 10.1248/bpb.b18-00251 [DOI] [PubMed] [Google Scholar]
- 124. Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL, et al. Astragaloside IV Inhibits Lung Cancer Progression and Metastasis by Modulating Macrophage Polarization Through AMPK Signaling. J Exp Clin Cancer Res (2018) 37(1):207. doi: 10.1186/s13046-018-0878-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Li W, Tian YH, Liu Y, Wang Z, Tang S, Zhang J, et al. Platycodin D Exerts Anti-Tumor Efficacy in H22 Tumor-Bearing Mice via Improving Immune Function and Inducing Apoptosis. J Toxicol Sci (2016) 41(3):417–28. doi: 10.2131/jts.41.417 [DOI] [PubMed] [Google Scholar]
- 126. Soltani M, Parivar K, Baharara J, Kerachian MA, Asili J. Transcriptional Analysis of VEGF-D and TGFbeta Genes in MCF7 Cells Exposed to Saponin Isolated From Holothuria Leucospilota (Sea Cucumber). Rep Biochem Mol Biol (2015) 4(1):25–31. [PMC free article] [PubMed] [Google Scholar]
- 127. Ahmad S, Ullah F, Ayaz M, Zeb A, Ullah F, Sadiq A. Antitumor and Anti-Angiogenic Potentials of Isolated Crude Saponins and Various Fractions of Rumex Hastatus D. Don. Biol Res (2016) 49:18. doi: 10.1186/s40659-016-0079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dutta S, Mahalanobish S, Saha S, Ghosh S, Sil PC. Natural Products: An Upcoming Therapeutic Approach to Cancer. Food Chem Toxicol (2019) 128:240–55. doi: 10.1016/j.fct.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 129. Zamora-Ros R, Béraud V, Franceschi S, Cayssials V, Tsilidis KK, Boutron-Ruault MC, et al. Consumption of Fruits, Vegetables and Fruit Juices and Differentiated Thyroid Carcinoma Risk in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Study. Int J Cancer (2018) 142(3):449–59. doi: 10.1002/ijc.30880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Akter R, Afrose A, Rahman MR, Chowdhury R, Nirzhor SSR, Khan RI, et al. A Comprehensive Analysis Into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int J Mol Sci (2021) 22(8):4180–202. doi: 10.3390/ijms22084180 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.