Abstract
In plants, negative reproductive interaction among closely related species (i.e., reproductive interference) is known to hamper the coexistence of congeneric species while facilitation can increase species persistence. Since reproductive interference in plants may occur through interspecific pollination, the effective range of reproductive interference may reflects the spatial range of interspecific pollination. Therefore, we hypothesized that the coexistence of congeners on a small spatial scale would be less likely to occur by chance but that such coexistence would be likely to occur on a scale larger than interspecific pollination frequently occur. In the present study, we tested this hypothesis using spatially explicit woody plant survey data. Contrary to our prediction, congeneric tree species often coexisted at the finest spatial scale and significant exclusive distribution was not detected. Our results suggest that cooccurrence of congeneric tree species is not structured by reproductive interference, and they indicate the need for further research to explore the factors that mitigate the effects of reproductive interference.
Keywords: Competition-relatedness hypothesis, Species-to-genus ratio, Reproductive interference, Warm-temperate evergreen forest, Kasugayama forest reserve
Introduction
Understanding how biotic interaction affects species composition and distribution is a major ongoing challenge in community ecology. Among biotic interactions, competition is the most important and well-studied interaction (Goldberg & Barton, 1992) although facilitation plays a major role in supporting biodiversity and shaping community structure (Losapio et al., 2021). A common hypothesis related to the role of competition in community assembly, termed the competition-relatedness hypothesis (CRH; Cahill et al., 2008), states that closely related species compete more intensely than distantly related species, which hypothetically limits the ability of closely related species to coexist (Webb et al., 2002; Slingsby & Verboom, 2006; Prinzing et al., 2008; reviewed by Mayfield & Levine, 2010; HilleRisLambers et al., 2012). The findings of Elton (1946), i.e., that a lower number of species per genus are observed in local areas than in the entire United Kingdom, are considered as evidence for the competitive exclusion of ecologically similar congeners in local habitats. However, the CRH has also been widely discussed (Dayan & Simberloff, 2005), and empirical support for the hypothesis remains inconclusive (Webb et al., 2002; Cavender-Bares et al., 2004; reviewed by Mayfield & Levine, 2010). For plants, Cahill et al. (2008) tested the CRH using experimental data; they revealed that the relationships between phylogenetic relatedness and competitive ability differed between monocots and eudicots.
Although the evidence for the CRH in plants is mixed, recent studies have shown that negative reproductive interaction among closely related species (i.e., reproductive interference) limits the coexistence of closely related species (Weber & Strauss, 2016). Reproductive interference (RI) is defined in the present study as the negative fitness effects of pollen transport among species. In plants, the effect can be generated at various stages and by various mechanisms, e.g., consumption of ovules through seed set failure (Nishida et al., 2014), pollen allelopathy (Murphy, 2000), stigma clogging (Shore & Barrett, 1984) caused by interspecific pollen transfer, and production of unviable hybrids (e.g., Brown & Mitchell, 2001; Takakura et al., 2009; Takakura et al., 2011; Takakura & Fujii, 2010). For this reason, the existence of reproductive interference was reported in both animal pollinated plants and wind pollinated plants (e.g., Takakura et al., 2009; Takakura & Fujii, 2010). Additionally, reproductive interference involves positive frequency-dependence (Kuno, 1992). Under the positive frequency-dependence, the population growth rate of a species will decrease with decreasing relative frequency of conspecifics to heterospecifics (Takakura et al., 2009; Kishi & Nakazawa, 2013). Therefore, it can rapidly lead to the extinction of the affected species with lower population density (Kuno, 1992; Kishi & Nakazawa, 2013 ).
Previous studies of reproductive interference have provided some insight into the pattern and spatial scale of closely related species’ coexistence (Takakura et al., 2011; Whitton, Sears & Maddison, 2017; Nishida et al., 2020). Because shared recent ancestry can yield shared reproductive traits (including similarities in the timing of reproduction, mate recognition, pollination system, and gamete recognition), close relatives (e.g., congeners and sister taxa) are less likely to coexist by chance on a local scale (Whitton, Sears & Maddison, 2017). Additionally, the extent of reproductive interference in plants depends on pollen transfer distance (Takakura et al., 2011). Therefore, it can be hypothesized that the coexistence of congeners on a small spatial scale is less likely to occur by chance, whereas coexistence is more likely to occur on scale larger than pollen flow distance.
In the present study, we aimed to quantitatively assess the distribution patterns of closely related woody plant species in the native forests of Japan to determine the effects of species interactions, especially reproductive interference, on forest community assembly. Using spatially explicit woody plant survey data, we tested the following predictions: (1) congeneric species do not coexist on a fine spatial scale where reproductive interference frequently occurs but coexist on a large spatial scale; and (2) on large scales where reproductive interference does not occur, congeneric species coexist while avoiding each other, resulting in an exclusive or checkerboard distribution (Connor, Collins & Simberloff, 2013).
Materials and method
Study site
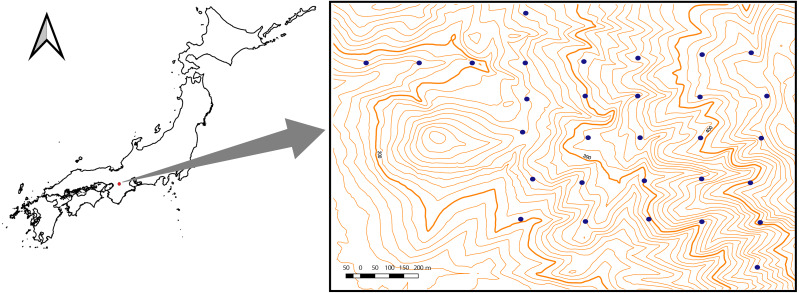
The study area (∼1 km2) was located in the Kasugayama primary forest, Nara prefecture, western Japan (34′41′N135′51′E) (Fig. 1). Because the forest has been preserved as a holy site of the Kasuga Taisha shrine and logging have been prohibited there since 841 AD, the forest is considered not to have been disturbed by humans for a long time (Maesako, Nanami & Kanzaki, 2007). In the area, the mean annual temperature in 2019 was 16.3 °C and the average annual precipitation in 2019 was 1482.5 mm. The highest point of the forest is 498 m. The natural vegetation in the area is evergreen broadleaved forest (Naka, 1982); however, the deer population has recently increased in the forest, causing the spread of alien species such as Sapium sebiferum and Nagia nagi (Maesako, Nanami & Kanzaki, 2007). The field survey for this study was conducted under the permission of the Nara Park Management Office (No. 6-6).
Figure 1. Location of the study site (1 km2) at Kasugayama primary forest, Nara prefecture, western Japan.
The specific locations of the study plots are denoted by dots. Scale bar: 200 m.
Field survey
Field studies were conducted from June to September 2015. In the study area, 30 transect plots (∼0.1 ha in size) at 200 m intervals were established (Fig. 1). Tree species richness was surveyed in each plot; all tree species with heights > 130 cm were recorded. Species were grouped into three life-form categories, namely trees, shrubs, or lianas, following Satake et al. (1981–1982). For categorization of small plants, species with a diameter at breast height (DBH) >10 cm on average were classified as trees while species with a DBH <10 cm were classified as shrubs.
Interspecific competition and null model analysis
In this study, we defined congeners as closely related species based on the definition by Weber & Strauss (2016).
We employed the species-to-genus ratio (S/G ratio) as an indicator of intragenic interactions for the categorized tree, shrub, and liana species. The S/G ratio has long been used to describe community patterns and to infer levels of competitive interactions among species within genera (reviewed by Simberloff, 1970). A low S/G ratio can be interpreted as a product of strong intrageneric competition (Elton, 1946), which could limit congeneric coexistence (Darwin, 1859). First, we calculated an S/G ratio for the whole area and then tested the deviation of this S/G ratio from a 1:1 ratio using a z- test. Second, to test spatial scale dependencies, we calculated S/G ratios at five a priori-defined spatial scales (0.1, 4, 16, 36, and 64 ha). In this study we established 30 plots at 200 m intervals. A priori-defined spatial scales corresponded to the area of each plot extended horizontally.
Distribution exclusiveness among congener species
To evaluate the exclusivity of congeners, we calculated the checkerboard scores (C-scores; Stone & Roberts, 1990) for the genera with multiple species of the same genus distributed within the study area and which occurred in more than three plots (Quercus; wind pollinated, Carpinus; wind pollinated, and Prunus; insect pollinated). Note that there are differing opinions among researchers on whether the genus Prunus should be considered as a single genus or not (Ohba, 1992; Mabberley, 2008). Therefore, the genus Prunus in this study includes the subgenera Cersus and Laurocerasus. In this analysis, we analyzed six Quercus species, two Carpinus species, and two Prunus species.
We set ri and rj as the number of plots in which species i and j, respectively, were present; the checker unit Cij associated with the two species was defined as follows:
| (1) |
where Sij indicates the extent of co-occurrence (i.e., the number of plots shared by the two species).
For N species, there are P = N(N − 1)/2 species pairs; thus, the C-score is calculated as follows:
| (2) |
The C-score becomes larger as the two species occur more commonly across different plots. We simulated null models to compare the observed C-score with stochastic distributions. The null models, which were run 999 times for each species pair, randomly shuffles the number of species (α-diversity) among sampling locations while preserving the species occurrence totals (the plant density). All statistical analyses were performed using R software version 3.6.1 (R Core Team, 2019). EcoSimR package (Gotelli, Hart & Ellison, 2015) was used to compute C-score.
Results
S/G ratio
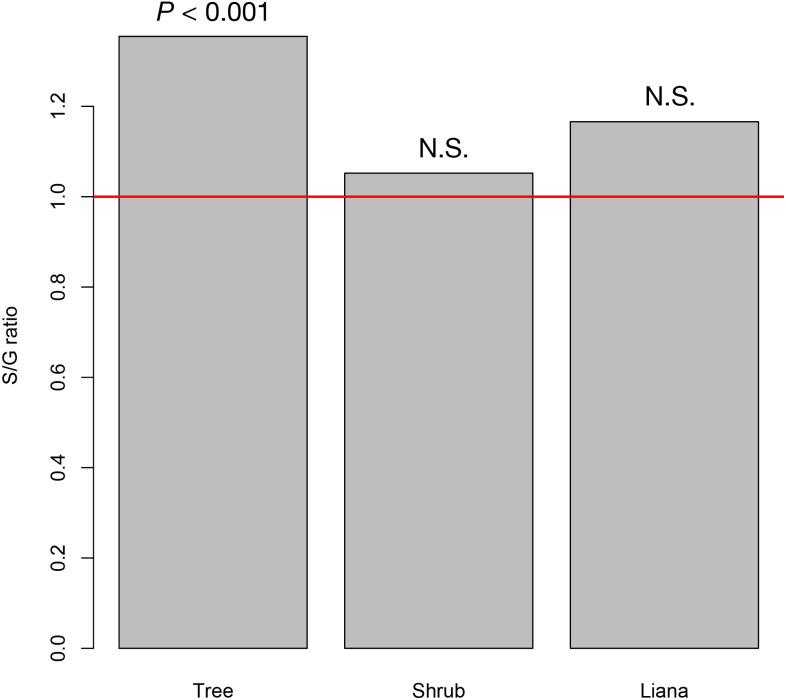
At the study site, we recorded 42 tree species (26 animal pollinated species and 16 wind pollinated species) from 31 genera, 20 shrub species (19 animal pollinated species and 1 wind pollinated species) from 19 genera, and seven liana species from six genera (seven animal pollinated species). The resultant S/G ratios for trees, shrubs, and lianas were 1.350, 1.021, and 1.200, respectively. Only the S/G ratio for tree species significantly deviated from the 1:1 ratio, whereas those for shrub and liana species did not (Fig. 2).
Figure 2. The species–to-genus ratios for the tree, shrub, and liana species categorized in the study site.
This ratio significantly deviated from the 1:1 ratio (red horizontal line) for tree species but not shrub or liana species.
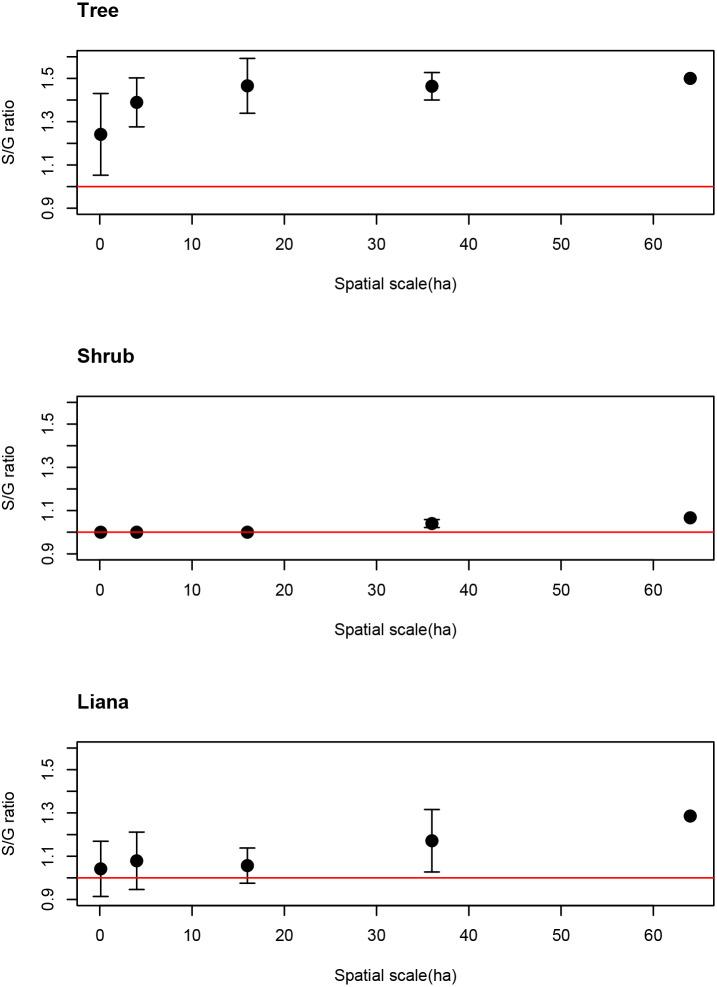
The average S/G for trees increased as spatial scale increased. Even at the smallest spatial scale, however, the average S/G ratio for trees exceeded the 1:1 ratio (Fig. 3), indicating that the coexistence of congeners frequently occurs at the smallest spatial scale for tree species.
Figure 3. Spatial scale dependencies of species-to-genus ratios for the tree, shrub, and liana species categorized in the study site.
Error bars represent standard deviations. Red lines indicate the 1:1 ratio.
C- score
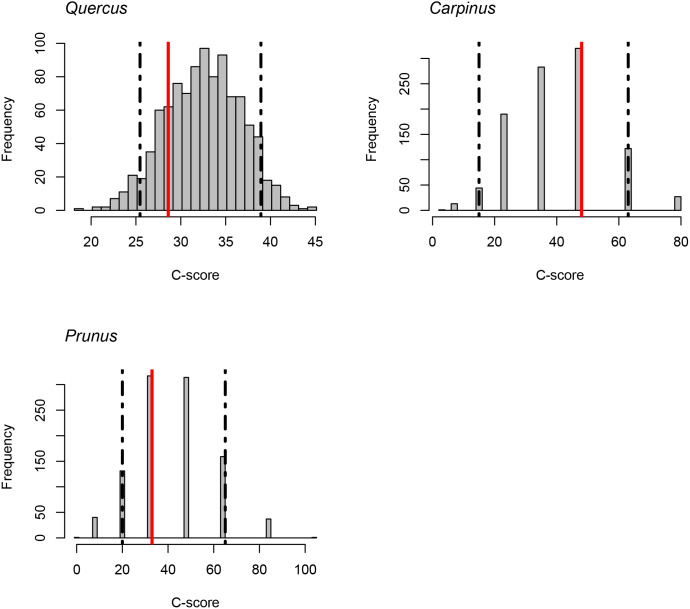
The C-score did not fall outside the 95% confidence intervals of the null model distribution for Quercus, Carpinus, and Prunus. It indicates that statistically significant exclusive distribution of species from the same genus did not occur (Fig. 4).
Figure 4. C-scores (checkerboard score; Stone & Roberts, 1990) for the genera Quercus, Carpinus and Prunus.
Red lines indicate the observed C-score. Histograms indicate the null model distribution. Broken lines indicate the 95% (upper and lower) confidence intervals of the null model distribution.
Discussion
Contrary to out prediction, our results show that, at least in our study area, closely related tree species often coexist even at the finest spatial scale and that statistically significant exclusive distribution of species from the same was not observed. These results suggest that co-occurrence of congeneric tree species is not prevented by reproductive interference. Previous theoretical studies predicted that reproductive interference could more readily prevent coexistence when compared to resource competition (Kuno, 1992; Kishi & Nakazawa, 2013). Additionally, empirical studies have demonstrated that reproductive interference can cause rapid congeneric species exclusion (Takakura et al., 2009; Takakura & Fujii, 2010; Runquist & Stanton, 2012). Therefore, our results did not meet the general expectation of reproductive interference effects on species coexistence, and thereby suggests that competitive exclusion among closely related tree species is somehow mitigated.
In contrast, the S/G ratio for shrubs and lianas did not deviate significantly from the 1:1 ratio, indicating that few congeneric shrub or liana species are distributed in the study area (∼1 km2). Since a low S/G ratio is generally a product of strong intrageneric competition (Elton, 1946), our results might imply the existence of competitive exclusion in shrub and liana species. However, it should be noted that the S/G ratio depends on the number of species present, and it would be expected to decrease in small communities regardless of competition levels (Gotelli & Colwell, 2001). In the study area, the deer population has recently increased, and this has affected the regeneration process and species richness of plant species (Shimoda et al., 1994; Maesako, 2015). As shrubs are likely to be more susceptible to deer grazing, a reduction in the number of shrub species due to deer grazing may have reduced the shrub S/G ratio. Nevertheless, it should be noted that closely related species of Neolitsea aciculate, a dominant shrub in our study area, are distributed about 15 km from the study site (Murata, 1977). This suggests that the low S/G ratio for shrubs and lianas would likely increase if the study area were to be extended by tens of kilometers.
In plants, the spatial extent of reproductive interference corresponds to pollen transfer distance (Takakura et al., 2011); consequently, coexistence of closely related plant species is expected at the spatial extent to which pollen flow does not occur. However, the frequent pollen dispersal range of the dominant tree genera in our study site (i.e., Quercus, Acer, and Machilus) is within a few tens of meters (Nakanishi et al., 2004; Kikuchi et al., 2009; Watanabe et al., 2018). This suggests that the congeneric tree species in our study site coexist on spatial scales at which reproductive interference can occur frequently and that, given the spatial factor, the effects of reproductive interference could not be avoided in this study. A previous theoretical study suggested that recruitment fluctuation could enable coexistence of closely related tree species on a local scale by producing temporal resource partitioning (a mechanism known as the storage effect) (Usinowicz, Wright & Ives, 2012). One of the dominant genera in our study area, Quercus, shows considerable variation in annual seed production (Hirayama et al., 2012), which might contribute to maintaining the coexistence of congener species. Another mechanism that could potentially weaken reproductive interference is reproductive character displacement (Pfennig & Pfennig, 2009). Eaton et al. (2012) showed that disparity in the floral traits of plants could reduce negative reproductive interactions among closely related species. However, there remains a lack of direct evidence to show that reproductive character displacement reduces the effect of reproductive interference. Moreover, experimental evidence of reproductive interference is limited to herbaceous plants.
Previous studies reveal that specialist natural enemies, such as herbivores and pathogens, maintain tree species diversity by the reducing survival rates of conspecific seeds and seedlings located close to reproductive adults or in high conspecific density areas (Janzen–Connell effect; Janzen, 1970; Seiwa et al., 2008; Comita et al., 2014). Additionally, coexistence theory (Chesson, 2000; Barabás, Stump & Stump, 2018) suggests that negative frequency-dependence is essential for stable coexistence of species (Levine & HilleRisLambers, 2009). In contrast, reproductive interference involves positive frequency-dependent selection, it can rapidly lead to species exclusion ( Kishi & Nakazawa, 2013) and eventually hamper species coexistence. Therefore, mitigation of reproductive interference is important to coexist with closely related species (Katsuhara & Ushimaru, 2019). Since positive and negative frequency-dependent processes possibly act simultaneously in nature, it is necessary to compare the relative importance of the two processes in the future.
In future research, investigation on a larger spatial scale with a more complex analysis will be required to determine the relationship between plant life history and the spatial scale of exclusive distribution. Previous studies on herbaceous plants suggest that reproductive interference plays an important role in community assembly (Eaton et al., 2012; Whitton, Sears & Maddison, 2017); however, our results, in concert with prior studies, indicate that reproductive interference is somewhat less effective than expected, especially in long-lived plant species. In this study, we mainly focused on competitive interaction but it has been proposed that shared floral displays might increase reproductive success (facilitation) and that co-flowering plants may, instead of competing, facilitate pollination (Ghazoul, 2006). A study in an alpine plant community reports that facilitative and neutral pollinator-mediated interactions among plants prevailed over competition (Tur et al., 2016). Additionally, recent studies have reported that the interplay between competition and facilitation affects local population persistence (Losapio et al., 2021). Such facilitative interactions might affect the coexistence of closely related species. Further study is therefore necessary to identify the key life history traits that mitigate the effects of reproductive interference.
Supplemental Information
Acknowledgments
We thank two anonymous reviewers for their helpful comments and suggestions. We also thank Tomoya Inada, Minori Hikichi, and Kayo Takasu for fieldwork assistance.
Funding Statement
This article was funded by the Grant-in-Aid for Scientific Research (15K07231 & 21K17915) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Shuntaro Watanabe conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yuri Maesako conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field permission provided by Nagatani Village Hiroshi, Nagatani Park Office (6-6).
Data Availability
The following information was supplied regarding data availability:
The raw measurements and code are available in the Supplemental Files.
References
- Barabás, Stump & Stump (2018).Barabás G, Stump RDM, Stump SM. Chesson’s coexistence theory. Ecological Monographs. 2018;88:277–303. doi: 10.1002/ecm.1302. [DOI] [Google Scholar]
- Brown & Mitchell (2001).Brown BJ, Mitchell RJ. Competition for pollination: effects of pollen of an invasive plant on seed set of a native congener. Oecologia. 2001;129:43–49. doi: 10.1007/s004420100700. [DOI] [PubMed] [Google Scholar]
- Cahill et al. (2008).Cahill JF, Kembel SW, Lamb EG, Keddy PA. Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspectives in Plant Ecology, Evolution and Systematics. 2008;10:41–50. doi: 10.1016/j.ppees.2007.10.001. [DOI] [Google Scholar]
- Cavender-Bares et al. (2004).Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Phylogenetic Overdispersion in Floridian Oak Communities. The American Naturalist. 2004;163:823–843. doi: 10.1086/386375. [DOI] [PubMed] [Google Scholar]
- Chesson (2000).Chesson P. Mechanisms of maintenance of species diversity. Annual Review of Ecology, Evolution, and Systematics. 2000;31:343–366. doi: 10.1146/annurev.ecolsys.31.1.343. [DOI] [Google Scholar]
- Comita et al. (2014).Comita LS, Queenborough SA, Murphy SJ, Eck JL, Xu K, Krishnadas M, Beckman N, Zhu Y. Testing predictions of the Janzen–Connell hypothesis: a meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. Journal of Ecology. 2014;102:845–856. doi: 10.1111/1365-2745.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor, Collins & Simberloff (2013).Connor EF, Collins MD, Simberloff D. The checkered history of checkerboard distributions. Ecology. 2013;94:2403–2414. doi: 10.1890/12-1471.1. [DOI] [PubMed] [Google Scholar]
- Dayan & Simberloff (2005).Dayan T, Simberloff D. Ecological and community-wide character displacement: the next generation. Ecology Letters. 2005;8:875–894. doi: 10.1111/j.1461-0248.2005.00791.x. [DOI] [Google Scholar]
- Darwin (1859).Darwin C. Modern Library, New York: 1859. The Origin of Species. [Google Scholar]
- Mabberley (2008).Mabberley DJ. Mabberley’s Plant-Book: a portable dictionary of plants, their classification and uses. Third edition. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Eaton et al. (2012).Eaton DAR, Fenster CB, Hereford J, Huang S-Q, Ree RH. Floral diversity and community structure in Pedicularis (Orobanchaceae) Ecology. 2012;93:S182–S194. doi: 10.1890/11-0501.1. [DOI] [Google Scholar]
- Elton (1946).Elton C. Competition and the structure of ecological communities. Journal of Animal Ecology. 1946;15:54–68. doi: 10.2307/1625. [DOI] [Google Scholar]
- Ghazoul (2006).Ghazoul J. Floral diversity and the facilitation of pollination. Journal of Ecology. 2006;94:295–304. doi: 10.1111/j.1365-2745.2006.01098.x. [DOI] [Google Scholar]
- Goldberg & Barton (1992).Goldberg DE, Barton AM. Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. The American Naturalist. 1992;139:771–801. doi: 10.1086/285357. [DOI] [Google Scholar]
- Gotelli & Colwell (2001).Gotelli NJ, Colwell RK. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters. 2001;4:379–391. doi: 10.1046/j.1461-0248.2001.00230.x. [DOI] [Google Scholar]
- Gotelli, Hart & Ellison (2015).Gotelli NJ, Hart EM, Ellison AM. EcoSimR: null model analysis for ecological data. R package version 0.1.02015 doi: 10.5281/zenodo.16522. [DOI]
- HilleRisLambers et al. (2012).HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM. Rethinking community assembly through the lens of coexistence theory. Ecology, Evolution, and Systematics. 2012;43:227–248. doi: 10.1146/annurev-ecolsys-110411-160411. [DOI] [Google Scholar]
- Hirayama et al. (2012).Hirayama D, Fujii T, Nanami S, Itoh A, Yamakura T. Two-year cycles of synchronous acorn and leaf production in biennial-fruiting evergreen oaks of subgenus Cyclobalanopsis (Quercus, Fagaceae) Ecological Research. 2012;27:1059–1068. doi: 10.1007/s11284-012-0986-9. [DOI] [Google Scholar]
- Janzen (1970).Janzen DH. Herbivores and number of tree species in tropical forests. The American Naturalist. 1970;104:501–528. doi: 10.1086/282687. [DOI] [Google Scholar]
- Katsuhara & Ushimaru (2019).Katsuhara KR, Ushimaru A. Prior selfing can mitigate the negative effects of mutual reproductive interference between coexisting congeners. Functional Ecology. 2019;33:1504–1513. doi: 10.1111/1365-2435.13344. [DOI] [Google Scholar]
- Kikuchi et al. (2009).Kikuchi S, Shibata M, Tanaka H, Yoshimaru H, Niiyama K. Analysis of the disassortative mating pattern in a heterodichogamous plant, Acer mono Maxim. using microsatellite markers. Plant Ecology. 2009;204:43–54. doi: 10.1007/s11258-008-9564-1. [DOI] [Google Scholar]
- Kishi & Nakazawa (2013).Kishi S, Nakazawa T. Analysis of species coexistence co-mediated by resource competition and reproductive interference. Population Ecology. 2013;55:305–313. doi: 10.1007/s10144-013-0369-2. [DOI] [Google Scholar]
- Kuno (1992).Kuno E. Competitive exclusion through reproductive interference. Researches on Population Ecology. 1992;27:5–284. [Google Scholar]
- Levine & HilleRisLambers (2009).Levine JM, HilleRisLambers J. The importance of niches for the maintenance of species diversity. Nature. 2009;461:254–257. doi: 10.1038/nature08251. [DOI] [PubMed] [Google Scholar]
- Losapio et al. (2021).Losapio G, Schöba C, Staniczenkoc PPA, Carrarad F, Palamara GM, Moraes CMD, Mescher MC, Brooker RW, Butterfieldg BJ, Callaway RM, Cavieres LA, Kikvidze Z, Lortiek CJ, Michaletm R, Pugnaire FI, Bascompte J. Network motifs involving both competition and facilitation predict biodiversity in alpine plant communities. Proceedings of the National Academy of Sciences of the United States of America. 2021;118:1–6. doi: 10.1073/pnas.2005759118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesako (2015).Maesako Y. Kasugayama primary forest and deer. In: Maesako Y, Takatsuki S, editors. The threat of deer and future of forest -efficiency and limitation of deer preventive fence- Bun-ichi; Japan: 2015. in Japanese. [Google Scholar]
- Maesako, Nanami & Kanzaki (2007).Maesako Y, Nanami S, Kanzaki M. Spatial distribution of two invasive alien species, Podocarpus nagi and Sapium sebiferum, spreading in a warm-temperate evergreen forest of the Kasugayama Forest Reserve, Japan. Vegetation Science. 2007;24:103–112. [Google Scholar]
- Mayfield & Levine (2010).Mayfield MM, Levine JM. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecology Letters. 2010;13:1085–1093. doi: 10.1111/j.1461-0248.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- Murata (1977).Murata G. Flora of Kinki. Hyogo Biology. 1977;7(21):149–152. (in Japanese) [Google Scholar]
- Murphy (2000).Murphy SD. Field testing for pollen allelopathy: a review. Journal of Chemical Ecology. 2000;26:2155–2172. doi: 10.1023/A:1005572516948. [DOI] [Google Scholar]
- Naka (1982).Naka K. Community dynamics of evergreen broadleaf forests in southwestern Japan. I. Wind damaged trees and canopy gaps in an evergreen oak forest. Botanical Magazine, Tokyo. 1982;95:385–399. doi: 10.1007/BF02489476. [DOI] [Google Scholar]
- Nakanishi et al. (2004).Nakanishi A, Tomaru N, Yoshimaru H, Kawahara T, Manabe T, Yamamoto S. Patterns of pollen flow and genetic differentiation among pollen pools in quercus salicina in a warm temperate old–growth evergreen broad-leaved forest. Silvae Genetica. 2004;53:258–264. doi: 10.1515/sg-2004-0048. [DOI] [Google Scholar]
- Nishida et al. (2014).Nishida S, Kanaoka MM, Hashimoto K, Takakura K, Nishida T. Pollen–pistil interactions in reproductive interference: comparisons of heterospecific pollen tube growth from alien species between two native Taraxacum species. Functional Ecology. 2014;28:450–457. doi: 10.1111/1365-2435.12165. [DOI] [Google Scholar]
- Nishida et al. (2020).Nishida S, Takakura K-I, Naiki A, Nishida T. Habitat partitioning in native Geranium species through reproductive interference. Annals of Botany. 2020;125:651–661. doi: 10.1093/aob/mcz210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba (1992).Ohba H. Japanese cherry trees under the genus cerasus (Rosaceae) Journal of Japanese Botany. 1992;67:276–281. [Google Scholar]
- Pfennig & Pfennig (2009).Pfennig KS, Pfennig DW. Character displacement: ecological and reproductive responses to a common evolutionary problem. The Quarterly Review of Biology. 2009;3:253–276. doi: 10.1086/605079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzing et al. (2008).Prinzing A, Reiffers R, Braakhekke WG, Hennekens SM, Tackenberg O, Ozinga WA, Schaminée JHJ, Groenendael JMV. Less lineages –more trait variation: phylogenetically clustered plant communities are functionally more diverse. Ecology Letters. 2008;11:809–819. doi: 10.1111/j.1461-0248.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019).R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. [Google Scholar]
- Runquist & Stanton (2013).Runquist RB, Stanton ML. Asymmetric and frequency-dependent pollinator-mediated interactions may influence competitive displacement in two vernal pool plants. Ecology Letters. 2013;16:183–190. doi: 10.1111/ele.12026. [DOI] [PubMed] [Google Scholar]
- Satake et al. (1981–1982).Satake Y, Ohwi J, Kitamura S, Watari S, Tominari T. Wild flowers of Japan. Herbaceous plants (including dwarf subshrubs). I-III. Heibonsha; Tokyo: 1981–1982. (in Japanese) [Google Scholar]
- Seiwa et al. (2008).Seiwa K, Miwa Y, Sahashi N, Kanno H, Tomita M, Ueno N, Yamazaki M. Pathogen attack and spatial patterns of juvenile mortality and growth in a temperate tree, Prunus grayana. Canadian Journal of Forest Research. 2008;38:2445–2454. doi: 10.1139/X08-084. [DOI] [Google Scholar]
- Shimoda et al. (1994).Shimoda K, Kimura K, Kanzaki M, Yoda K. The regeneration of pioneer tree species under browsing pressure of Sika deer in an evergreen oak forest. Ecological Research. 1994;9:85–92. doi: 10.1007/BF02347245. [DOI] [Google Scholar]
- Shore & Barrett (1984).Shore JS, Barrett SCH. The effect of pollination intensity and incompatible pollen on seed set in Turnera ulmifolia (Turneraceae) Canadian Journal of Botany. 1984;62:1298–1303. [Google Scholar]
- Simberloff (1970).Simberloff DS. Taxonomic diversity of island biotas. Evolution. 1970;24:23–47. doi: 10.1111/j.1558-5646.1970.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Slingsby & Verboom (2006).Slingsby JA, Verboom GA. Phylogenetic Relatedness Limits Co-occurrence at Fine Spatial Scales: evidence from the Schoenoid Sedges (Cyperaceae: Schoeneae) of the Cape Floristic Region, South Africa. The American Naturalist. 2006;168:14–27. doi: 10.1086/505158. [DOI] [PubMed] [Google Scholar]
- Stone & Roberts (1990).Stone L, Roberts A. The checkerboard score and species distributions. Oecologia. 1990;85:74–79. doi: 10.1007/BF00317345. [DOI] [PubMed] [Google Scholar]
- Takakura & Fujii (2010).Takakura K-I, Fujii S. Reproductive interference and salinity tolerance differentiate habitat use between two alien cockleburs: Xanthium occidentale and X. italicum (Compositae) Plant Ecology. 2010;206:309–319. doi: 10.1007/s11258-009-9644-x. [DOI] [Google Scholar]
- Takakura et al. (2011).Takakura K-I, Matsumoto T, Nishida T, Nishida S. Effective range of reproductive interference exerted by an alien dandelion, Taraxacum officinale, on a native congener. Journal of Plant Research. 2011;124:269–276. doi: 10.1007/s10265-010-0368-8. [DOI] [PubMed] [Google Scholar]
- Takakura et al. (2009).Takakura K-I, Nishida T, Matsumoto T, Nishida S. Alien dandelion reduces the seed-set of a native congener through frequency-dependent and one-sided effects. Biological Invasions. 2009;11:973–981. doi: 10.1007/s10530-008-9309-z. [DOI] [Google Scholar]
- Tur et al. (2016).Tur C, Sáez A, Traveset A, Aizen MA. Evaluating the effects of pollinator-mediated interactions using pollen transfer networks: evidence of widespread facilitation in south Andean plant communities. Ecology Letters. 2016;19:576–586. doi: 10.1111/ele.12594. [DOI] [PubMed] [Google Scholar]
- Usinowicz, Wright & Ives (2012).Usinowicz J, Wright SJ, Ives AR. Coexistence in tropical forests through asynchronous variation in annual seed production. Ecology. 2012;93:2073–2084. doi: 10.1890/11-1935.1. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. (2018).Watanabe S, Takakura K-I, Kaneko Y, Noma N, Nishida T. Skewed male reproductive success and pollen transfer in a small fragmented population of the heterodichogamous tree Machilus thunbergii. Journal of Plant Research. 2018;131:623–631. doi: 10.1007/s10265-018-1018-9. [DOI] [PubMed] [Google Scholar]
- Webb et al. (2002).Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annual Review of Ecology and Systematics. 2002;33:475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448. [DOI] [Google Scholar]
- Weber & Strauss (2016).Weber MG, Strauss SY. Coexistence in close relatives: beyond competition and reproductive isolation in sister Taxa. Annual Review of Ecology, Evolution, and Systematics. 2016;47:359–381. [Google Scholar]
- Whitton, Sears & Maddison (2017).Whitton J, Sears CJ, Maddison WP. Co-occurrence of related asexual, but not sexual, lineages suggests that reproductive interference limits coexistence. Proceedings of the Royal Society B. 2017;284:20171579. doi: 10.1098/rspb.2017.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements and code are available in the Supplemental Files.