Abstract
Immunohistochemistry (IHC) is a well-established morphology adjunct enabling pathologists to make accurate diagnoses. Metastases to the liver is a common scenario where pathologists may rely heavily on IHC in their interpretation. We conducted this study to audit the patterns of IHC utilization in malignant liver biopsies in 3 practice types (academic, community, and expert) as an initial step toward developing best practice guidelines. A total of 1100 specimens were analyzed and the association between the availability of history of other malignancies and the practice type on IHC utilization was studied. Community pathologists were twice as likely to use IHC and to use more markers per case than academic pathologists or the expert pathologist. When history of another malignancy was available, pathologists were not only 1.5 times more likely to use IHC but they also used more markers per case. IHC was still deemed necessary to reach the diagnosis in 67% of cases with a given history of other malignancy. This study described several variables for consideration in our effort to develop IHC utilization guidelines and its results quantify the variance noted among practice types.
Keywords: IHC utilization, malignant liver, practice patterns, IHC guidelines, IHC algorithms
Immunohistochemistry (IHC) is a well-established adjunct to histomorphology enabling pathologists to make more accurate diagnoses helping their patients and clinical colleagues in their decision-making. Over the past 3 decades, we have been witnessing a steady increase in IHC utilization by pathologists not only because of the introduction of new antibodies but also due to our deeper understanding of its role in tailoring treatment options. In the current era of personalized medicine, we use IHC on a regular basis for genetic and therapeutic determinations, well beyond its traditional use in diagnosis.1,2 Consequently, our paradigm of antibody evaluation and our overall approach to IHC has significantly changed.3 As a complement to this phenomenon, efforts have been made by several research groups and professional organizations to study practice patterns and suggest guidelines for IHC utilization.4 This is to acknowledge that the increased dependency on IHC in our daily pathology practice comes with its own cost both in terms of its technical and professional interpretive aspects. Additional cost, albeit less amenable to quantitation, is the mental effort spent by the pathologists when the IHC results are nonconclusive or contradictory. To date, although some aspects of pathology practice are privileged by having established IHC best practice guidelines,5,6 IHC utilization is left for the most part to the discretion of the individual pathologist and their own interpretation of the literature available in their respective areas. As a result, we see high variability of the same IHC panel utilization even within the same institution.7,8
Biopsy of liver tumors is a relatively common encounter in a tertiary care surgical pathology setting where it is not uncommon for the pathologist to resort to the use of IHC in the workup of the case. Once the histomorphology suggests malignancy, the pathologist would typically move to the next question along the diagnostic algorithm and tries to decide whether this is a primary or a metastatic tumor. If it is the latter, the following step will be to determine the most likely type of tumor and its primary site. Some of the panels of tissue-specific markers have already been described to improve our accuracy in the identification and classification of undifferentiated tumors of uncertain origin irrespective of where they are detected.9 More specific to the liver, various approaches have been documented to help answer the question of whether a tumor is primary or metastatic10 with emphasis on identifying the primary site for the latter type.11–13 The clinical setting of malignant diagnoses in liver specimens offers a practical opportunity to gain insight into the pathologists’ utilization of IHC. We practice in a tertiary care academic setting as part of a citywide health care network, where liver specimens have been, until recently, signed out by general surgical pathologists. The purpose of this study is to audit the patterns of IHC utilization in liver biopsy cases with malignant diagnoses in a health care network that includes an academic center and community hospitals. Evaluating the appropriateness of IHC utilization in this regard would need comparative audits among similar practice settings. This study offers a methodology that can be replicated by other research and performance monitoring teams with the hope of developing best practice guidelines and avoiding superfluous IHC utilization.
MATERIALS AND METHODS
Data Extraction
Sunquest CoPath (Sunquest Information Systems Inc., Tucson, AZ) was implemented as the primary Laboratory Information System in our network in October 2001 (version 2.1). It was used to identify cases for this practice audit analyzing all liver malignancies between 2002 and 2016. Currently, our institution is on version 6.1.1 of CoPath. We searched the database for cases with tissue “part type” entered as “liver” and then data were exported to an Excel spreadsheet (Microsoft Office 2010, Redmond, WA) for further analysis. The spreadsheet was filtered manually by one of the authors (D.E.-R.) to remove all nonmalignant diagnoses. With only cases having malignant diagnoses remaining, the spreadsheet was constructed to maintain the columns representing the fields listed in Table 1; other fields were deleted. It was a standard practice in our system that major clinical findings were consistently abstracted by a trainee or the attending pathologist from the electronic medical record (EMR), Epic (Epic Systems Corporation, Verona, WI). This was entered at the time of sign out in the clinical picture field of the surgical pathology report in CoPath. At the inception of this audit, 30 consecutive cases were pulled from the current series and the information recorded in the clinical picture field of the surgical pathology report was cross-referenced with the physician/operative notes and imaging reports for the same patient in their EMR. This initial review confirmed complete concordance between the 2 data sets and enabled the audit to proceed reliably by confining the review to surgical pathology reports. The EMR was still reviewed on a case-by-case basis as needed. Surgical pathology reports were further reviewed and the information was abstracted from its various fields including the pathologist’s name, clinical picture, diagnosis, diagnosis comment, gross description, and microscopic description.
TABLE 1.
Data Fields Abstracted from CoPath for Further Analysis
| Field | Description |
|---|---|
|
| |
| Case number | Surgical pathology number unique to each case |
| MRN | MRN unique to each patient in the electronic medical records database |
| Collection date | Date of the procedure |
| Accession date | Date the specimen is received and accessioned in the laboratory |
| Sex | Patient’s sex |
| DOB | Date of birth |
| Location | The location where the procedure was performed |
| Ordering physician | The provider who ordered the test |
| Part designator | The letter associated with the specimen when ≥ 1 part/specimen are received per case |
| Part name | The part type name selected as a drop down entry from the system’s dictionary; cannot be altered by staff |
| Part description | The part type description entered by the staff to reflect what has been provided on the surgical requisition |
| Specimen class | Local laboratory discriminator for specimens depending on their source, urgency, and sign out location |
MRN indicates medical record number.
The pathology diagnosis was recorded including whether it was a primary or metastatic tumor, histologic grade of adenocarcinoma, and the antibodies used to reach the final diagnosis. Although in most cases the immunostaining panel was listed in the pathology report, some cases had part or the whole panel reported in an addendum report. These addenda were included in the audit. The number of masses or nodules within the specimen was recorded from the gross description field in cases with wedge biopsy or lobectomy. The number of masses or nodules in the patient’s liver was also collected from the clinical history field of the surgical pathology report as well as from the imaging study report available in EMR when applicable.
History of previous or concurrent malignancy was retrieved from the clinical history field of the surgical pathology report and by checking CoPath for “Other specimens for Patient.” The latter option is a Laboratory Information System function that pulls up reports of prior specimens from the same patient to check whether a prior malignant diagnosis was rendered. This functionality is available to pathologists at the time of sign out of their specimens on a routine basis. History of previous or concurrent malignancy was also retrievable from the EMR system Epic. In addition, this information could also have been obtained by a conversation with a clinician; an activity that was difficult to capture in this audit. The correlation between the final diagnosis and the documented history of previous or concurrent malignancy was categorized into 3 groups as follows.
The pathologist’s diagnosis agreed with the data available.
The pathologist’s diagnosis disagreed with the data available.
The pathologist was unable to give a definitive diagnosis as to whether they agreed or disagreed with the information available. One example of this scenario was when the tumor was morphologically undifferentiated with ambiguous immunophenotype.
Pathologists included in this audit were separated into 2 major “practice types”; an academic group (practiced in the University’s Medical Center) and a community-based group (practiced in 3 different community hospitals within the same network). One additional pathologist practiced in both settings was an experienced hepatic and gastrointestinal pathologist and was included in the analysis as a third practice type.
Statistical Analyses
Descriptive statistics, including frequency, percent, and mean ± SD are presented as appropriate. The association between availability of history of previous or concurrent malignancy and practice type on IHC utilization was studied. The proportions of patients where IHC was used for diagnosis based on pathologist practice group (academic, community, or expert) and the availability of information with regard to the history of malignancy were compared using a generalized estimating equation model to account for the repeated measures within pathologist assuming an exchangeable correlation structure. Odds ratios (ORs) and their associated 95% confidence intervals (CIs) are presented. The mean number of IHC markers tested among those who used IHC were compared by pathologist practice group and availability of history of malignancy using a linear regression mixed model to account for the repeated measures within pathologist assuming an exchangeable correlation structure. Interaction terms for the relationship between pathologist practice group and availability of history of malignancy were considered but not found to be statistically significant so excluded from the final models. Similar models were conducted to explore the relationship between final pathology diagnosis concordance with information available and IHC use and number of IHC markers. Finally, the correlation between number of IHC stains used and tumor grade was determined using Spearman correlation coefficients. Tumor grades were assigned on a 4-point Likert scale (well differentiated = 1, moderately differentiated = 2, poorly differentiated = 3, and undifferentiated = 4). Tumors reported as “high grade” were given the grade score of 3. Data were analyzed using SAS 9.4 (Cary, NC) and P-values <0.05 were considered statistically significant.
RESULTS
Our database included 10,300 liver specimens received in the period of 2002 to 2016, out of which 1100 had a malignant diagnosis. Among patients with malignant diagnoses, 595 (54.1%) were males and 505 (45.9%) were females. Their mean age was 64.7 ± 15.3 years old, with 95% of patients falling between 40 and 86. Over the span of 14 years, 417 cases (37.9%) were diagnosed by 31 academic pathologists, 566 (51.5%) by 20 community-based pathologists and 117 (10.6%) by 1 particular pathologist who is an experienced hepatic and gastrointestinal pathologist. This expert pathologist practiced throughout the entire audited period and overlapped between both the academic and community-based pathology groups.
The 1100 specimens included 983 (89.4%) core needle biopsies, 26 (2.4%) wedge resections, and 25 (2.3%) lobectomies. In the remaining 66 (6.0%) cases, the exact nature of the specimen was not confidently abstractable from the pathology report. Among the 1100 cases, 686 (62.4%) were diagnosed as metastatic and 290 (26.4%) were primary (hepatocellular, gallbladder, or cholangiocarcinoma). There was no conclusive determination of whether the tumor was primary or metastatic in the remaining 124 (11.3%) cases. Details of these diagnoses are provided in Table 2. Among the 686 cases where pathologists concluded the tumor was a metastatic adenocarcinoma, 178 (26.0%) were diagnosed without the use of IHC. Among the 508 (74.1%) cases where IHC was used, pathologists used an average of 6.8 ± 3.8 antibodies (range, 1 to 29).
TABLE 2.
Diagnostic Groups of the 1100 Cases*
| Diagnosis | No. Cases |
|---|---|
|
| |
| Primary tumors | |
| Hepatocellular carcinoma | 218 |
| Biliary adenocarcinoma (cholangiocarcinoma and gallbladder) | 59 |
| Other primary tumors† | 13 |
| Metastatic tumors | |
| From a colorectal primary | 155 |
| From a lung primary | 130 |
| From a pancreatic primary | 115 |
| Lymphoma/leukemia | 73 |
| From another gastrointestinal primary site | 53 |
| From a breast primary | 52 |
| From other primaries‡ | 108 |
| Undetermined tumors | 124 |
When a diagnosis was made in <50 cases, it was lumped under the “other” category.
Other primary tumors included hepatoblastoma and angiosarcoma.
Other metastatic tumors included metastases from renal, adrenal, leiomyosarcoma, thyroid, tonsil, urothelial, vaginal, prostate, larynx, melanoma, endometrial, cervical, ovarian, and unidentified primaries.
Antibodies Used
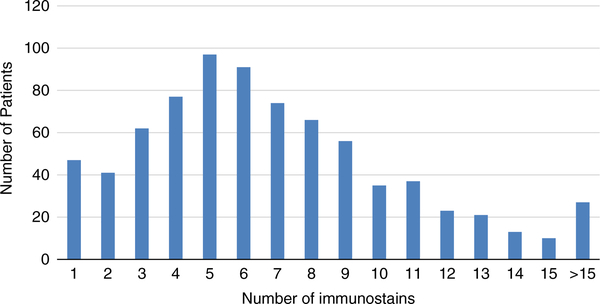
In 323 (29.4%) cases, the final diagnosis was made without the use of any IHC stains either because the pathologist decided none was needed or because core tissue biopsy included scant malignancy that did not enable additional sectioning. However, the workup of the remaining 777 (70.6%) cases utilized IHC with 134 different antibodies being applied. The number of IHC stains used per case in these 777 cases is depicted in Figure 1; the average number of markers used was 6.9 ± 4.1 (range, 1 to 29). Table 3 summarizes the counts of cases where the 5 most frequently utilized antibodies (out of the 134 antibodies) were made. Although CK7 and CK20 are almost always used together when assessing a tumor, the former was ordered by the pathologists slightly more frequently in this series (n = 11 cases).
FIGURE 1.
Number of IHC stains used per case among those where IHC was used (N = 777). IHC indicates immunohistochemistry.
TABLE 3.
Most Frequently Used IHC Markers
| IHC Stain | No. Cases (% Per All 1100 Cases) |
|---|---|
|
| |
| CK7 | 537 (49) |
| CK20 | 526 (48) |
| TTF1 | 401 (36) |
| CDX2 | 322 (29) |
| Hep Par1 | 236 (2l) |
IHC indicates immunohistochemistry.
Tumor Variables
In the 777 cases with IHC, there was a weak positive correlation between tumor grade and the number of markers used by the pathologist for each case. Higher tumor grade was associated with a greater number of markers used by the pathologist with a correlation coefficient of 0.26 (P < 0.0001). The number of liver nodules (in the specimen or in the patient’s liver) was available to the pathologist in 774 cases. In these cases, there was a very weak negative correlation between the number of liver nodules and the number of markers used for each case (correlation coefficient of −0.08, P = 0.02).
Clinical History of Malignancy and IHC Utilization
History of previous or concurrent malignancy was available at the time of specimen submission in 698 (63.5%) cases. In only 230 of these cases (33.0%), the pathologist was able to make the diagnosis, whether their diagnosis agreed or disagreed with the information given, without the use of IHC. This was either because the morphology was convincing or, as was the case in 10 patients, the volume of tumor tissue in the needle core biopsies submitted was too small to allow further sectioning. In these latter cases, the pathologists were unable to confirm or exclude the clinical impression provided. In the remaining 468 cases (67.1%) where the clinical information suggested a metastatic tumor (history of previous or concurrent malignancy), the pathologists still performed IHC. We looked into whether the practice type (academic vs. community vs. expert hepatopathologist) or the availability of clinical information pertaining to previous or concurrent malignancy influenced the number of IHC markers used by the pathologist.
The relationship between the practice type and the availability of history of malignancy and the use of IHC is summarized in Table 4. The interaction was not statistically significant between practice types and history of malignancy available, though for academic pathologists, the availability of history of malignancy was less likely to affect their use of IHC. In general, pathologists were ~1.5 times more likely to use IHC when the history of malignancy was not available compared with when the history of malignancy was available, independent of practice type (OR, 1.55; 95% CI, 1.18, 2.04; P = 0.002). Community-based pathologists were over twice as likely to use IHC than the expert pathologist, independent of the availability of a history of malignancy (OR, 2.23; 95% CI, 1.50, 3.29; P < 0.0001). Academic pathologists and the expert did not appear to differ (OR, 1.20; 95% CI, 0.81, 1.78; P = 0.36).
TABLE 4.
Pathologists’ Practice Type and Availability of Clinical History of Malignancy and Use of IHC
| IHC Not Used [N (%)] | IHC Used [N (%)] | |
|---|---|---|
|
| ||
| Academic Practice Group | ||
| History of malignancy available | 112 (67.1) | 157 (62.8) |
| History of malignancy not available | 55 (32.9) | 93 (37.2) |
| Community Practice Group | ||
| History of malignancy available | 86 (75.4) | 268 (59.3) |
| History of malignancy not available | 28 (24.6) | 184 (40.7) |
| Expert gastrointestinal pathologist | ||
| History of malignancy available | 32 (76.2) | 43 (57.3) |
| History of malignancy not available | 10 (23.8) | 32 (42.7) |
IHC indicates immunohistochemistry.
The relationship between the practice type and availability of history of malignancy and number of IHC markers used among those who used IHC is summarized in Table 5. The interaction was not statistically significant between practice types and history of malignancy available, though for community pathologists, the availability of history of malignancy more greatly affected the number of IHC markers they used. Pathologists used more IHC markers when the history of malignancy was not available compared with when the history of malignancy was available, independent of practice type (P < 0.0001). Community pathologists used more IHC markers than the academic or expert pathologists, independent of the availability of history of malignancy (P = 0.001).
TABLE 5.
Pathologists’ Practice Type and Availability of Clinical History of Malignancy and Number of Markers Used Among Those Who Used IHC
| No. IHC Markers Used |
||
|---|---|---|
| N | Mean (SD) | |
|
| ||
| Academic Practice Group | ||
| History of malignancy available | 157 | 5.7 (3.7) |
| History of malignancy not available | 93 | 6.4 (3.2) |
| Community Practice Group | ||
| History of malignancy available | 268 | 7.0 (3.8) |
| History of malignancy not available | 184 | 8.4 (4.7) |
| Expert gastrointestinal pathologist | ||
| History of malignancy available | 43 | 5.3 (3.9) |
| History of malignancy not available | 32 | 6.3 (3.9) |
IHC indicates immunohistochemistry.
Concordance With the Given Clinical History of Malignancy
The final pathology diagnosis agreed with the information available in 542 of 698 (77.7%) cases, disagreed in 95 (13.6%) cases, whereas in the remaining 61 (8.7%) cases the final diagnosis did neither definitively support nor exclude the information available (Table 6). The disagreement with the information given was more related to the most likely primary tumor site and was less with regard to a metastatic versus a primary tumor. Pathologists were more likely to disagree with available information when IHC was used (P < 0.001). Further, in cases where pathologists rendered a diagnosis different from what the patient’s clinical history had suggested, they used more IHC stains (P < 0.0001).
TABLE 6.
Concordance of Final Pathologist Diagnosis With Given Clinical History of Malignancy and Use of IHC Markers
| IHC Not Used [N (%)] | IHC Used [N (%)] | No. IHC Markers Used |
||
|---|---|---|---|---|
| N | Mean (SD) | |||
|
| ||||
| Agreement | ||||
| Agree | 209 (90.9) | 333 (71.2) | 333 | 5.7 (3.5) |
| Disagree | 11 (4.8) | 84 (18.0) | 84 | 8.3 (3.9) |
| Equivocal | 10 (4.4) | 51 (10.9) | 51 | 8.0 (4.2) |
IHC indicates immunohistochemistry.
IHC Utilization Over Time
We looked into IHC utilization patterns as they evolved over time in cases where the clinical information pertaining to previous or concurrent malignancy was available. Between 2002 and 2006, 158 cases used IHC (54.5%), between 2007 and 2011, 133 cases used IHC (75.6%), and between 2012 and 2016 177 cases used IHC (76.3%); the increase in use of IHC was statistically significant (P = 0.001 and P = 0.002 for comparisons between 2007 and 2011, and 2012 and 2016 with 2002 and 2006, respectively). Similarly, there was an increase in the mean number of IHC markers used over time (2002 to 2006: 6.0 ± 3.5, 2007 to 2001: 7.6 ± 4.2, 2012 to 2016:7.1±4.3; P = 0.0003).
DISCUSSION
IHC is integral to routine contemporary surgical pathology practice, not only to help reach a more accurate diagnosis but also for genetic and therapeutic determinations.1,2 Over the period of this study the battery of IHC stains has expanded greatly, including the advent of some new stains for identification of hepatocellular tumors14 (Hep Par1, arginase, and glypican-3 in addition to the older polyclonal CEA and CD10 and α-fetoprotein) as well as many more tissue-specific stains to assess for potential primary sites of metastatic tumors. For the most part, the use of arginase and Hep Par1 has displaced the use of the older markers.15 Hence, the advent of these new markers has not necessarily increased the number of stains utilized simply to identify hepatocytes and probably have much less impact on the number of stains utilized than the additional tissue-specific stains for metastatic tumors.
The need to rationalize IHC utilization is meant to address its added technical and professional interpretive cost along with the added pathologist’s effort when the IHC results are nonconclusive or contradictory. Guidelines for judicious IHC utilization are only available in a minority of surgical pathology practice scenarios.6 We performed a retrospective review of IHC utilization in a city-wide health care network that included a tertiary care academic center and 3 different community-based pathology practice groups over a 14-year period from 2002 to 2016. The clinical scenario of “malignant liver” offered an opportunity to develop an audit methodology since the liver is one of the most common sites for metastatic cancer and can present diagnostic challenges in terms of morphologic and IHC interpretation.16 Results of this audit are presented as a starting point for more comprehensive studies of IHC utilization appropriateness and developing best practice guidelines avoiding superfluous antibodies utilization in hepatic pathology. A total of 1100 malignant livers were studied that were signed out by 51 general surgical pathologists plus 1 pathologist who specializes in hepatic and gastrointestinal pathology. Community-based pathologists were twice as likely to use IHC and to use more markers per case than academic pathologists or the expert pathologist. The reason for this cannot be determined from the data available, but one could speculate that concern about malpractice and the need to confirm one’s histologic impression with stains may have played a role, especially for less-experienced pathologists. This may be more of a concern for community pathologists, who sometimes have concerns about being “second-guessed” at a later time. On the basis of observations by the 1 pathologist in the study who practices in both settings, availability of clinical data is generally similar between sites (especially in later years with the use of the system-wide EMR system, Epic), although direct access to clinicians is somewhat easier in the smaller community hospital setting.
When the information with regard to a previous or concurrent malignancy was not given, pathologists, in general, were not only ~1.5 times more likely to use IHC but they also used more markers per case. However, it is of note that IHC was still deemed necessary to reach the diagnosis in two-third (67%) of cases with a given history of other concurrent or previous malignancy(ies). In 91% of cases where the diagnosis was finalized without the use of IHC, the pathologist agreed with the clinical impression or history provided. A classic example of this situation is a liver nodule biopsy from a patient who had a history of colorectal cancer and the tumor’s histomorphology is compatible with a metastatic adenocarcinoma from the colorectum. This percentage dropped significantly when pathologists implemented IHC as they agreed with the available history of malignancy in only 71% of cases. This suggests that when the histomorphology did not necessarily agree with the available information or when the case had other complicating features, the pathologists ordered IHC and were more likely to disagree with the available information (18% of cases), mostly with regard to the possible source of the tumor. The fact that the final diagnosis remained equivocal in determining the possible tumor source with the available history in 11% of cases when IHC is used, compared with only 4% of the straightforward cases signed out without IHC suggests that these cases were inherently problematic and that the use of IHC was most likely appropriate.
For this audit of IHC utilization, we chose the scenario of malignant liver specimens because of its inherent interpretive difficulties. Tumors in the liver tend to pose a challenge to pathologists. Hepatocellular carcinoma not only shares overlapping histologic features with benign hepatocellular lesions, but also can closely mimic metastatic tumors such as a neuroendocrine tumor, breast carcinoma, renal cell carcinoma, adrenocortical carcinoma, melanoma, and epithelioid angiomyolipoma.13,17,18 There is myriad of metastatic tumors that can be encountered in the liver, some of which may share similar morphologic features. In such instances, IHC plays an important role in accurate tumor classification. When approaching liver malignancy, the pathologist typically takes into account the gross findings, the status of background liver (with or without advanced chronic liver disease), and the clinical scenario.16 As such, metastases are less commonly encountered in patients with advanced chronic liver disease. If the patient has known primary malignancy, comparing the current sample with the original tumor is very helpful when available, and IHC could possibly be avoided in such cases. Our audit showed that metastatic tumors in 24% to 43% of cases with a known history of primary cancer were signed out without the use of IHC depending on the nature of the practice and expertise of the pathologist.
To date, there are very few studies that suggest a simple systematic approach when dealing with metastatic tumors in the liver.19 Our study showed the need for clear guidelines/recommendations on how to appropriately apply IHC when evaluating liver tumors, incorporating some of the clinical variables discussed in this audit. In most cases, the pathologist is presented with limited material such as a needle biopsy or fine needle aspiration, and therefore, a sequential and/or algorithmic approach is crucial when selecting the appropriate panel. In the most commonly encountered scenario when a gastrointestinal primary site is suspected, a simple CK20/CK7 panel is recommended for initial evaluation as it was shown that CK20+/CK7− tumors have a 78.4% probability of originating from colon, whereas the tumors with CK20+/CK7+ profile have 74.8% probability of originating from the pancreas or biliary tract.7 The CK20− group was found to be too heterogeneous to be accurately classified. A later study showed similar results.20 Another common scenario is when hepatocellular carcinoma is one of the leading differentials. To tackle this issue a recent study suggested performing 2 stains: arginase-1 and CK19. On the basis of the results of these 2 markers, cases fall into 4 categories, and further evaluation could be based on the respective clinicopathologic findings.13 In most cases, a “tailored” approach is needed when selecting an IHC panel for each individual case. It is important to remember that when selecting antibodies for each case, most sensitive and specific markers with proven diagnostic value should be applied to avoid interpretative errors, the unnecessary consumption of the limited diagnostic material provided and the over utilization of laboratory resources.
This practice audit has generated several guiding points that deserve further discussion. When faced with a tumor in the liver that may represent metastatic carcinoma and there is a likely primary site the use of IHC to confirm the primary site is often not warranted. This occurs most likely when the histology is compatible with the known primary site and there are no alternative likely primary sites. Use of IHC under these circumstances is detrimental not only because of the cost involved in performing “confirmatory” stains, but also because these stains may exhaust potentially valuable tissue which might be better used for prognostic or therapeutic molecular studies. In contrast, IHC confirmation with a known potential primary site may be appropriate under several circumstances including (1) cases in which the histologic appearance is not consistent with the stated potential primary site, (2) cases in which there are several potential primary sites and the histology is ambiguous regarding which primary is present in the liver, and (3) cases in which the clinical history makes metastases unlikely (eg, a history of low stage colorectal carcinoma diagnosed 20 y ago). Another important caveat is that clinical histories can often be incomplete or misleading. We recently encountered a case in which the provided history was breast carcinoma but the biopsy demonstrated non-Hodgkin lymphoma. Review of the medical records indicated that while the patient did indeed have breast carcinoma diagnosed 17 years ago, she also had a non-Hodgkin lymphoma diagnosed 5 years ago, which was not mentioned in the provided history on the pathology requisition. Therefore, it is usually prudent to review medical records, when possible, to assure that information submitted with the case is accurate. Oftentimes stains are performed because of fear of future litigation if at some future date the determination of the primary site should be determined to be incorrect, although this should not, in general, be a driving factor. Another common reason for staining is because of requests from clinicians for confirmation, usually with little understanding of the need or lack of same for such confirmation. The latter scenario can be politically problematic and oftentimes stains are performed to make the clinician “happy.” Direct clear communication with clinicians can do much to alleviate this type of request. The conflicting issues we routinely encounter in surgical pathology are (1) time constrains with the increasing tendency to request a diagnosis as soon as possible, and (2) limited tissue consumed by cutting tissue sequentially. Sequential staining only works if adequate ribbon or unstained slides are preserved at the initial recutting of the block to assure that tissue is not exhausted by repeat sectioning of the block. Often there is very little tissue left after the initial 3 levels are cut. The benefit of sequential staining is decreased cost and, to some extent, tissue preservation for molecular studies.
This is a retrospective study and a practice audit of IHC utilization patterns of 3 different practice types within a single system. We found that for malignant liver samples, community-based pathologists were twice as likely to use IHC and to use more markers per case than academic pathologists or the expert pathologist. When history of previous or concurrent malignancy was available, pathologists, in general were not only ~1.5 times more likely to use IHC but they also used more markers per case. Pathologists still deemed IHC necessary to reach the diagnosis in two-thirds of cases with a given history of other malignancy. This study described several variables for consideration in our effort to develop IHC utilization guidelines in the evaluation of malignant liver lesions and its results quantify the variance noted among practice types. Incorporating these results with others from comparable settings would help develop best practice guidelines and avoid superfluous IHC utilization.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Buchanan DD, Tan YY, Walsh MD, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014;32:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao HL, Yeh YC, Lin CH, et al. Diagnostic algorithm for detection of targetable driver mutations in lung adenocarcinomas: comprehensive analyses of 205 cases with immunohistochemistry, real-time PCR and fluorescence in situ hybridization methods. Lung Cancer. 2016;101:40–47. [DOI] [PubMed] [Google Scholar]
- 3.McCourt CM, Boyle D, James J, et al. Immunohistochemistry in the era of personalised medicine. J Clin Pathol. 2013;66:58–61. [DOI] [PubMed] [Google Scholar]
- 4.Colling R, Church DN, Carmichael J, et al. Screening for Lynch syndrome and referral to clinical genetics by selective mismatch repair protein immunohistochemistry testing: an audit and cost analysis. J Clin Pathol. 2015;68:1036–1039. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI, Egevad L, Humphrey PA, et al. Best practices recommendations in the application of immunohistochemistry in the prostate: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38:e6–e19. [DOI] [PubMed] [Google Scholar]
- 6.Taylor CR. The WHO classification of lymphomas: cost-effective immunohistochemistry using a deductive reasoning “decision tree” approach: part II: the decision tree approach: diffuse patterns of proliferation in lymph nodes. Appl Immunohistochem Mol Morphol. 2009;17:470–482. [DOI] [PubMed] [Google Scholar]
- 7.Plourde A, Gross A, Jiang Z, et al. Patterns in immunohistochemical usage in extended core prostate biopsies. Arch Pathol Lab Med. 2013;137:1630–1634. [DOI] [PubMed] [Google Scholar]
- 8.Singh C, Manivel JC, Truskinovsky AM, et al. Variability of pathologists’ utilization of p16 and Ki-67 immunostaining in the diagnosis of cervical biopsies in routine pathology practice and its impact on the frequencies of cervical intraepithelial neoplasia diagnoses and cytohistologic correlations. Arch Pathol Lab Med. 2014;138:76–87. [DOI] [PubMed] [Google Scholar]
- 9.Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasms/tumor of uncertain origin. Arch Pathol Lab Med. 2014;138: 1583–1610. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZE, Lin F. Application of immunohistochemistry in gastrointestinal and liver neoplasms. New markers and evolving practice. Arch Pathol Lab Med. 2015;139:14–23. [DOI] [PubMed] [Google Scholar]
- 11.Tot T Adenocarcinomas metastatic to the liver: the value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer. 1999;85:171–177. [DOI] [PubMed] [Google Scholar]
- 12.Gokden M, Shinde A. Recent immunohistochemical markers in the differential diagnosis of primary and metastatic carcinomas of the liver. Diagn Cytopathol. 2005;33:166–172. [DOI] [PubMed] [Google Scholar]
- 13.Choi W-T, Ramachandran R, Kakar S. Immunohistochemical approach for the diagnosis of a liver mass on small biopsy specimens. Hum Pathol. 2017;63:1–13. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T, Phillips D, Jain D, et al. Comparison of 5 immunohistochemical markers of hepatocellular differentiation for the diagnosis of hepatocellular carcinoma. Arch Pathol Lab Med. 2015;139: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 15.Choi WT, Kakar S. Immunohistochemistry in the diagnosis of hepatocellular carcinoma. Gastroenterol Clin North Am. 2017;46: 311–325. [DOI] [PubMed] [Google Scholar]
- 16.Khadim MT, Jamal S, Ali Z, et al. Diagnostic challenges and role of immunohistochemistry in metastatic liver disease. Asian Pac J Cancer Prev. 2011;12:373–376. [PubMed] [Google Scholar]
- 17.Lau SK, Prakash S, Geller SA, et al. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. 2002;33:1175–1181. [DOI] [PubMed] [Google Scholar]
- 18.Jin M, Zhou X, Yearsley M, et al. Liver metastases of neuroendocrine tumors rarely show overlapping immunoprofile with hepatocellular carcinomas. Endocr Pathol. 2016;27:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S-Y, Kim B-H, Kim J-H, et al. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med. 2007;131:1561–1567. [DOI] [PubMed] [Google Scholar]
- 20.Varadhachary GR, Karanth S, Qiao W, et al. Carcinoma of unknown primary with gastrointestinal profile: immunohistochemistry and survival data for this favorable subset. Int J Clin Oncol. 2014; 19:479–484. [DOI] [PubMed] [Google Scholar]