Abstract
Non-alcoholic fatty liver disease (NAFLD) includes a range of hepatic manifestations, starting with liver steatosis and potentially evolving towards non-alcoholic steatohepatitis (NASH), cirrhosis or even hepatocellular carcinoma. NAFLD is a major health burden, and its incidence is increasing worldwide. Although it is primarily a disease of disturbed metabolism, NAFLD involves several immune cell-mediated inflammatory processes, particularly when reaching the stage of NASH, at which point inflammation becomes integral to the progression of the disease. The hepatic immune cell landscape is diverse at steady state and it further evolves during NASH with direct consequences for disease severity. In this Review, we discuss current concepts related to the role of immune cells in the onset and progression of NASH. A better understanding of the mechanisms by which immune cells contribute to NASH pathogenesis should aid the design of innovative drugs to target NASH, for which current therapeutic options are limited.
Subject terms: Kupffer cells, Metabolic syndrome, Immunology
Non-alcoholic steatohepatitis (NASH) is a serious chronic liver disorder of increasing prevalence worldwide. Metabolic by nature, the disease also mobilizes the immune system. Here, Huby and Gautier discuss current knowledge regarding how diverse immune cell subsets affect NASH onset and progression.
Introduction
The prevalence of metabolic diseases, as exemplified by obesity, has increased at an exponential rate over the past few decades. Importantly, in addition to having its own comorbidities, obesity broadly impacts on the health of a patient and increases their susceptibility to other conditions. For example, patients with obesity are more prone to cancer1 and their susceptibility to infectious diseases is increased, as recently observed for coronavirus disease 2019 (COVID-19)2. Long-recognized comorbidities of obesity include insulin resistance and type 2 diabetes, hypertension, dyslipidaemia, cardiovascular disease and fatty liver disease3. Remarkably, non-alcoholic fatty liver disease (NAFLD) is also a major independent risk factor for the development of cardiovascular diseases, including atherosclerosis4,5. There is growing evidence of a tight association between obesity and NAFLD6, and considerable research attention is now being directed to this association owing to the increasing prevalence of NAFLD and its ability to evolve towards liver dysfunction and failure. The burden of NAFLD is expected to increase and current projections estimate that, by 2030, more than 300 million individuals will develop NAFLD in China, more than 100 million in the United States and 15–20 million in the major European countries7. Current economic costs associated with NAFLD and its comorbidities are extremely high8–10. Thus, strong efforts are underway to develop innovative therapeutics to combat the disease, for which there are currently no approved therapies11.
NAFLD is a heterogeneous condition, the clinical manifestations of which only become problematic at the stage of non-alcoholic steatohepatitis (NASH), when liver injury, inflammation and fibrosis are superimposed on the initial steatosis12. Inflammatory processes are involved in the onset and progression of NASH12 and they also favour its evolution towards more debilitating conditions such as cirrhosis and hepatocellular carcinoma (HCC)12. Here, we first summarize the clinical features of NAFLD and NASH and their pathophysiology. After describing the immune landscape of the resting liver, we focus on the immune cell-mediated and inflammatory aspects of NASH, in particular discussing the role of diverse immune cell populations in NASH and its transition to HCC. However, we do not cover in any detail the inflammatory processes in NASH involving non-immune cells. Finally, we conclude by summarizing the therapies that are currently under development and possible future therapeutic options to combat NASH.
The spectrum of NAFLD
The occurrence of a fatty liver in the absence of significant alcohol consumption was classified as NAFLD 40 years ago13. A consensus group of experts recently proposed that the term ‘metabolic dysfunction-associated fatty liver disease (MAFLD)’ may be used to more accurately describe the key metabolic dimension associated with hepatic steatosis, without excluding other aetiologies of liver disease such as alcohol consumption or viral hepatitis6,14. However, this change in nomenclature is a matter of debate in the field15; thus, we will continue to use NAFLD until the issue is resolved. It should also be noted that the definition of MAFLD does not entirely overlap with that of NAFLD and therefore the terms are not fully interchangeable when discussing individual studies. NAFLD is considered the hepatic manifestation of metabolic syndrome and is highly associated with metabolic comorbidities, including obesity, type 2 diabetes, hyperlipidaemia and hypertension16. Metabolic syndrome is the most common cause of chronic liver disease and has been estimated to be present in about 25% of the adult population worldwide16. NAFLD is a progressive disease whereby steatosis (the excessive accumulation of lipids in hepatocytes) constitutes the first disease stage, which can eventually evolve to the more complex stage of NASH. NASH is characterized by the presence of hepatic steatosis (affecting more than 5% of hepatocytes), hepatocellular damage (with distinctive hepatocyte ballooning), signs of inflammation and varying degrees of fibrosis12. The disease presents with an important heterogeneity in its clinical manifestations and evolution6, such that patients with NASH and advanced fibrosis (10–20% of patients with NAFLD)17,18 may further progress to cirrhosis, HCC and end-stage liver disease, potentially requiring liver transplantation19. Thus, NASH is a key step in the clinical progression of NAFLD.
Histological scoring systems remain the ‘gold standard’ to diagnose NAFLD and to identify patients with NASH who are at greater risk of progression to cirrhosis and HCC. Nevertheless, the disease scoring and staging systems are semiquantitative, subject to sampling error and evaluate only major changes in hepatic tissue remodelling. In this context, emerging techniques that analyse liver biopsies with high-resolution multiphoton microscopy, 3D digital reconstructions and computational simulations are now offering new possibilities for the quantitative characterization of disease progression20. In addition, multiple blood-based biomarkers have been developed that, combined with measurements of liver stiffness, help to guide clinicians in identifying patients with NAFLD who are at risk of progression to NASH21–24. The identification of such patients is a key challenge for disease management but also to improve patient selection and the design of clinical trials for emerging pharmacotherapies. Limiting NASH onset and blocking its transition towards liver failure and HCC are major health challenges worldwide.
Pathophysiology of NASH
NASH is a complex multifactorial disease and its exact aetiology is not completely understood. Overall, multiple triggers are likely to contribute to inducing the hallmarks of NASH, namely steatosis, liver injury and inflammation, which together lead to the onset of fibrosis and sometimes HCC (Fig. 1). Steatosis is, in most cases, an early event in NAFLD but it does not necessarily transition to NASH. Additional stresses are needed to induce NASH onset, such as lipotoxicity, oxidative stress and inflammation, which combine to induce cellular stress pathways leading to hepatocyte death, inflammation and fibrosis development.
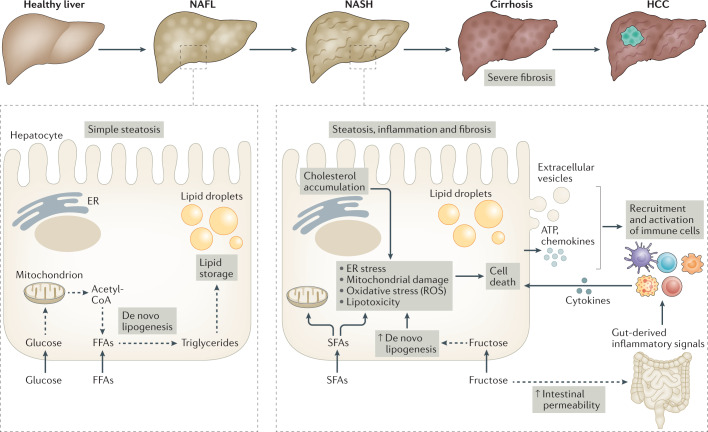
Fig. 1. Contributing factors in NAFLD progression and NASH pathogenesis.
Non-alcoholic fatty liver disease (NAFLD) is a progressive disease that starts with simple steatosis (non-alcoholic fatty liver (NAFL)) and can evolve to a more complex form known as non-alcoholic steatohepatitis (NASH), which is characterized by steatosis, inflammation and fibrosis. NASH can further progress towards cirrhosis and, in some cases, hepatocellular carcinoma (HCC). The NAFL stage is characterized by triglyceride accumulation in hepatocytes through de novo lipogenesis. Lipogenesis is fuelled by the uptake of glucose and free fatty acids (FFAs) and their incorporation into lipid-synthesis pathways. The onset of NASH is associated with multiple cellular stresses in lipid-loaded hepatocytes, including endoplasmic reticulum (ER) stress, mitochondrial damage, oxidative stress (involving the production of reactive oxygen species (ROS)) and/or lipotoxicity. Such cellular stresses can have various causes, including the accumulation of saturated fatty acids (SFAs), increased de novo lipogenesis resulting from increased fructose uptake, or cholesterol accumulation in the ER. In addition to increasing de novo lipogenesis, fructose also increases intestinal permeability, which can lead to the activation of hepatic immune cells by gut-derived inflammatory mediators. This promotes liver inflammation as well as the death of stressed hepatocytes, which also release pro-inflammatory mediators such as ATP, extracellular vesicles and chemokines, further reinforcing the inflammatory process and leading to the development of fibrosis. Exacerbation of this vicious cycle can precipitate the transition towards cirrhosis and eventually the development of HCC.
Liver steatosis, which is usually characterized by increased liver triglyceride storage, can arise from circulating free fatty acids released upon adipose tissue lipolysis but also from hepatic fatty acid synthesis through de novo lipogenesis25. A major substrate for de novo lipogenesis is fructose26, which is sometimes used in combination with fat in dietary mouse models of NASH (Box 1). A recent study showed that fructose intake is sufficient to induce NASH and its evolution towards HCC in the NASH-susceptible MUP-uPA mouse model27. In addition to sustaining de novo lipogenesis, fructose also increases gut permeability, which can lead to hepatic inflammation; together, de novo lipogenesis and increased gut permeability precipitate NASH onset and the NASH–HCC transition27. High-fat diet (HFD) feeding also favours NASH and HCC development in MUP-uPA mice by inducing endoplasmic reticulum stress and liver injury, in part through production of the pro-inflammatory cytokine tumour necrosis factor (TNF)28. Thus, regardless of the substrate (carbohydrate or fat), the induction of hepatic steatosis favours NASH onset in the MUP-uPA mouse model. Importantly, recent studies highlight that diet-induced NASH is accelerated in the presence of dietary cholesterol29,30, favouring the transition to HCC31,32. In HFD-fed MUP-uPA mice, endoplasmic reticulum stress-induced caspase 2 activation leads to the activation of sterol regulatory element-binding proteins (SREBPs), which results in cholesterol and triglyceride synthesis and accumulation in the liver33. Caspase 2 expression is similarly increased in patients with NASH, and genetic or pharmacological inhibition of caspase 2 limits NASH development in HFD-fed MUP-uPA mice33. Overall, the data indicate that hepatic accumulation of triglycerides and cholesterol creates a permissive environment for the development of NASH.
Lipid-mediated stresses can lead to liver injury by increasing hepatocyte susceptibility to apoptosis. Indeed, steatosis induced by long-term feeding of mice with a HFD sensitizes hepatocytes to cytokine-induced cell death34, as may occur during NASH when hepatic leukocytes, such as macrophages, locally secrete inflammatory cytokines35. In humans, hepatocyte apoptosis is greater in patients with NASH than in those with simple steatosis and this correlates with increased levels of fibrosis and inflammation36. However, other forms of cell death, such as necroptosis, might also occur during NASH and contribute to its pathogenesis37. In mice, hepatocyte death alone can trigger NASH development. Indeed, deleting Ikbkg in hepatocytes (HepΔIkbkg mice), which impedes the pro-survival effect of nuclear factor-κB signalling, leads to spontaneous hepatocyte cell death and liver injury in mice fed a standard chow diet38. HepΔIkbkg mice eventually develop steatosis, NASH and HCC38, which recapitulates the natural course of NAFLD. Of note, increased hepatocyte susceptibility to cell death is also a hallmark of the MUP-uPA mouse model. Indeed, increased hepatocyte death is already noticeable in MUP-uPA mice compared with wild-type mice fed a standard chow diet and HFD feeding of MUP-uPA mice further increases hepatocyte death and precipitates NASH onset28. Also in support of a key role for hepatocyte apoptosis in NASH, deletion of AMP-activated protein kinase (AMPK) in hepatocytes leads to their death and accelerates fibrosis in a diet-induced mouse model of NASH39. Mechanistically, AMPK limits caspase 6 activation and thus caspase 6-mediated hepatocyte death during NASH39. Caspase 6 activation is increased in various mouse models of NASH and correlates with increased fibrosis in patients with NASH. In mice, caspase 6 inhibition limits the diet-induced hallmarks of NASH39 and broad-spectrum caspase inhibitors also protect against NASH40,41. Importantly, inflammation has a crucial role in hepatocyte death during NASH as limiting TNF-induced signalling reduces hepatocyte death and liver injury, which translates to reduced liver steatosis and fibrosis42. Thus, hepatocyte death is a cardinal feature of NASH pathogenesis.
In addition to lipid-mediated stress and hepatocyte death, the liver environment during NASH also leads to oxidative stress in patients and in mouse models43,44. Mice deficient for Nfe2l2, which encodes NRF2 (a master regulator of the antioxidant response), are highly susceptible to diet-induced NASH and present with an accelerated transition from simple steatosis to NASH45. In addition, oxidative stress is increased in HepΔIkbkg mice and antioxidant treatment blunts the progression to NASH in this model38. Increased oxidative stress during NASH notably induces the oxidation and inactivation of hepatic protein tyrosine phosphatases, including protein tyrosine phosphatase non-receptor type 2 (PTPN2, also known as TCPTP)46. Ptpn2 deletion in hepatocytes leads to uncontrolled signalling through signal transducer and activator of transcription 1 (STAT1) and STAT3 that accelerates the progression of steatosis to NASH and the transition of NASH to HCC, respectively46. In addition, it is noteworthy that mitochondrial oxidative stress induced upon hepatic accumulation of free cholesterol increases hepatocyte susceptibility to cytokine-induced cell death47. The NASH inflammatory environment, which is mostly controlled by cells of the immune system, has a crucial role here. Indeed, inflammatory mediators, such as TNF, produced by immune cells induce the death of lipid-loaded, stressed hepatocytes that are rendered sensitive to cytokine-mediated cell death48. Finally, oxidized phospholipids, which are non-enzymatic products of oxidant-mediated lipid peroxidation, are also produced during NASH and induce mitochondrial damage as well as further production of reactive oxygen species (ROS). Antibody-mediated neutralization of oxidized phospholipids decreases oxidative stress together with steatosis, inflammation, fibrosis, hepatocyte death and NASH–HCC progression in a diet-induced mouse model49.
Thus, lipid loading of hepatocytes, oxidative stress and inflammation function together during NASH to induce hepatocyte death, which leads to liver injury, further inflammation and tissue fibrosis (Fig. 1). The important role of inflammation in these processes suggests that the immune cell-rich hepatic environment can modulate NASH onset and severity. Here, we focus on how immune cell-mediated inflammation impacts NASH pathogenesis, notwithstanding that non-immune cell types, such as hepatocytes and endothelial cells, can also shape hepatic inflammation through different means. For example, hepatocytes can release pro-inflammatory mediators, such as ATP50 or extracellular vesicles51,52, and endothelial cells also produce inflammatory triggers and chemokines such as TNF, IL-6 and CCL2 (ref.53).
Box 1 Mouse models of fatty liver disease.
Multiple mouse models have been generated to study non-alcoholic fatty liver disease (NAFLD) and the transition of non-alcoholic steatohepatitis (NASH) to hepatocellular carcinoma (HCC). The strong association of NAFLD with overnutrition, obesity and insulin resistance has led to the development of various diet-induced models, using dietary formulas enriched in lipids. Moreover, widely used models of obesity, insulin resistance and atherosclerosis, such as ob/ob mice and Apoe–/– mice, develop features of NAFLD but do not usually transition to NASH and are therefore not discussed in this Review. Mice fed a high-fat diet (HFD) typically develop fatty liver but have limited inflammation and no appreciable fibrosis, which are key components of NASH169. To circumvent this limitation, the HFD has been supplemented with cholesterol, saturated fatty acids such as palmitate, or fructose and/or sucrose to further promote hepatic insulin resistance, inflammation and mild-to-moderate fibrosis49,50,81,151,170. Occasional evidence of HCC development has also been reported with such diets50,81,151. These supplemented diets, and in particular those rich in fat, cholesterol and fructose and/or sucrose, are often referred to as a ‘Western diet’ although its composition has not been standardized and varies across laboratories. A recent systematic analysis of the literature on rodent models of NAFLD suggests that high-fat, high-fructose diets most closely recapitulate the overall characteristic features of human NAFLD171. Along these lines, inbred isogenic C57BL/6J×129S1/SvImJ mice (termed DIAMOND mice) fed a cholesterol-enriched HFD with ad libitum consumption of water with a high fructose and glucose content develop NASH with progressive stages of fibrosis, and a major proportion of these mice develop HCC in the long term (>30 weeks of diet)172. Owing to its mixed genetic background, this model would be more suitable for the preclinical development of therapeutic targets than are knockout or conditional knockout strains, which are more suitable for mechanistic studies. MUP-uPA mice, which express the urokinase plasminogen activator (uPA) under control of the hepatocyte-specific MUP promoter, are another specific model of NASH. When fed a HFD28 or high-fructose diet27, these mice undergo endoplasmic reticulum stress in hepatocytes and develop obesity, insulin resistance, liver fibrosis and liver inflammation. The appearance of liver tumours is also observed in these mice after 8–10 months of diet. Diets deficient in methionine and/or choline, which are indispensable for hepatic mitochondrial β-oxidation of fatty acids and the synthesis of very low-density lipoprotein, respectively, have been widely used in preclinical NASH research151,173. In particular, the methionine-deficient and choline-deficient diet, enriched in fat and sucrose, favours the rapid onset of hepatic macrovesicular steatosis, lobular inflammation and perisinusoidal fibrosis, which recapitulates the ultrastructural changes observed in human NASH174. A disadvantage of this diet resides in the hypercatabolism that it induces, leading to weight loss and the absence of insulin resistance. Thus, a choline-deficient diet supplemented with high levels of fat (60% of calories from fat; known as CD-HFD) has been developed to replace the methionine-deficient and choline-deficient diet. Long-term CD-HFD feeding increases fat mass and impairs glucose homeostasis, as well as induces severe steatosis, ballooned hepatocytes, immune cell infiltration, satellitosis, the formation of Mallory–Denk bodies and glycogenated nuclei, all of which are features of human NASH115. In addition, CD-HFD-fed mice have a 25% incidence rate of liver tumours after 12 months of diet115.
Liver immune landscape at steady state
Besides being an essential metabolic and detoxifying organ, the liver is also an important immunological organ that contains a large number of innate and adaptive immune cells54. The highly vascularized nature of the liver, together with reduced blood flow in its fenestrated capillary-like vessels known as sinusoids, constitutes a unique environment to maximize the exposure of immune cells to blood-borne and gut-derived pathogens55,56. Indeed, approximately one-third of the body’s whole blood volume passes through the liver every minute. Most of that blood flows from the portal vein and drains the gastrointestinal tract, with the remainder (20–25%) originating from the hepatic artery57. Most lymphoid and myeloid cell lineages can be found in the liver, residing mainly in the sinusoids but also in the intravascular and subcapsular compartments54,58 (Fig. 2). Here, we provide an overview of the key hepatic immune cell populations.
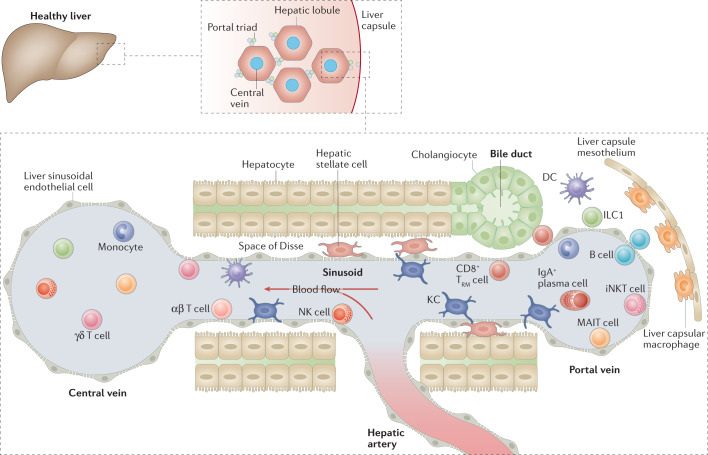
Fig. 2. Immune landscape of the healthy liver.
The liver is composed of lobules that are repeated hexagonal anatomical units. These lobules consist of hepatocytes organized around a central vein that is connected to the hepatic artery and the portal vein via a sinusoidal network. The bile produced by hepatocytes is released into the gastrointestinal tract via bile ducts composed of cholangiocytes. The hepatic artery, portal vein and bile duct together form the portal triads found between hepatic lobules. The blood flow along the portal to central axis (from portal vein and hepatic artery to central vein) creates gradients of oxygen and nutrients, resulting in a spatial division of labour among hepatocytes, a phenomenon known as metabolic zonation. A large spectrum of immune cells is found in the liver. Some circulate or temporarily patrol the hepatic sinusoids or the liver parenchyma, such as natural killer (NK) cells, γδ T cells, CD4+ and CD8+ αβ T cells, monocytes, B cells, invariant NKT (iNKT) cells, mucosal-associated invariant T (MAIT) cells and dendritic cells (DCs). Long-lived resident cells, such as Kupffer cells (KCs), CD8+ tissue-resident memory T (TRM) cells and type 1 innate lymphoid cells (ILC1s) are also found. Liver lobules also have a spatially polarized immune system, known as immune zonation, with KCs, iNKT cells, CD8+ TRM cells and IgA+ plasma cells being particularly enriched in the periportal regions. KCs, which specifically reside in the sinusoids in close contact with liver sinusoidal endothelial cells, are the most abundant immune cell population in the liver. They also establish connections with hepatic stellate cells and hepatocytes in the space of Disse. Another resident macrophage population occupies the liver capsule and these cells are known as liver capsular macrophages.
Innate-like T cell populations, including γδ T cells but mostly natural killer T (NKT) cells, are particularly enriched in mouse livers. Notably, invariant NKT (iNKT) cells, which express a CD1d-restricted semi-invariant αβ T cell receptor and recognize lipid-based antigens, represent up to 50% of intrahepatic T cells compared with much lower levels in other mouse tissues59. iNKT cells expressing the chemokine receptor CXCR6 patrol the hepatic sinusoids in mice (Fig. 2) under the influence of CXCL16 expressed by liver sinusoidal endothelial cells60. It should be noted, however, that iNKT cells are less frequent in human livers (<1% of T cells)61. By contrast, mucosal-associated invariant T (MAIT) cells, which recognize bacteria-derived vitamin B metabolites through MHC-related 1 (MR1), are rare in mice62 but are enriched in the human liver, where they represent 15–45% of the total number of T cells63,64. The liver is also populated with several innate lymphoid cell (ILC) subsets that do not express antigen-specific receptors, including natural killer (NK) cells, ILC1s, ILC2s and ILC3s. In humans, hepatic NK cells predominate over the other ILC subsets65,66. In mice, the main ILC subsets found in the liver are CD49a–CD49b+ NK cells and tissue-resident CD49a+CD49b– ILC1s67–69. As the models used to study ILCs are not specific (for example, models used to deplete NK cells also target ILC1s), the roles of particular subsets in liver physiology and pathology are unclear.
Immune surveillance of the liver also involves conventional CD4+ and CD8+ αβ T cells, with CD4+ T cells present at reduced numbers compared with CD8+ T cells58. A portion of the CD8+ T cells are long-lived resident-memory T cells that are found in the liver parenchyma as well as patrolling the hepatic sinusoids70,71 (Fig. 2), where they confer a first-line response to reinfection70. Naive B cells and antibody-producing plasma cells are also present in the liver72. These plasma cells are mainly found in portal tracts and originate from B cells that are activated in gut-associated lymphoid tissue to produce IgA reactive to commensal bacteria and oral antigens73 (Fig. 2).
With regards to the myeloid compartment, time-of-flight mass cytometry analysis has identified several populations of dendritic cells (DCs), including conventional and plasmacytoid subsets, in mouse livers74. Confocal imaging using CD11c and CX3CR1 markers has suggested that DCs are also distributed under the Glisson capsule, the mesothelium that covers the liver74. However, these CX3CR1+ cells, which have a characteristic dendritic morphology, may in fact correspond to the recently described population of CX3CR1+CD11c+MHC-IIhiF4/80low liver capsular macrophages75 (Fig. 2). Liver capsular macrophages derive from blood monocytes and provide immune protection against peritoneal infections75. However, the vast majority of liver macrophages are represented by Kupffer cells (KCs), which develop during embryogenesis and are maintained in the healthy adult liver by self-renewal independently from blood monocytes76–78. KCs exclusively reside in the liver sinusoids (Fig. 2), where they interact closely with liver sinusoidal endothelial cells, hepatic stellate cells (HSCs) and hepatocytes to acquire their tissue-imprinted signature as well as the signals that enable self-maintenance79. In addition, chemokines produced by liver sinusoidal endothelial cells determine the asymmetric distribution of KCs within the sinusoids, with an enriched periportal localization80.
In summary, the liver is an immune cell-rich environment that, in addition to its role in maintaining homeostasis, undoubtedly has a key role in the pathology of several liver diseases, including NASH.
Immune modulation of NASH pathogenesis
Recent advances in single-cell transcriptomics have allowed for a better appreciation of how the immune cell repertoire is reshaped during NASH in mice81,82 but also in the human cirrhotic liver83. For example, major changes in the myeloid compartment were observed in both mice and humans, with a marked influx of monocytes and monocyte-derived cells81–83. Such changes in the hepatic immune cell landscape are likely to contribute to the uncontrolled inflammatory environment that fuels liver injury and leads to NASH progression. A complex crosstalk between diverse immune cell populations and hepatocytes, HSCs and liver sinusoidal endothelial cells certainly operates during the disease. However, such complexity of interactions is only starting to be uncovered and much of our knowledge so far relates to studies of individual immune cell populations in NASH pathogenesis (Fig. 3), as discussed below. Novel tools, such as single-cell RNA sequencing or single-nucleus RNA sequencing combined with interactome analysis, should allow for a better understanding of the communication axis ongoing during NASH in the near future (Box 2).
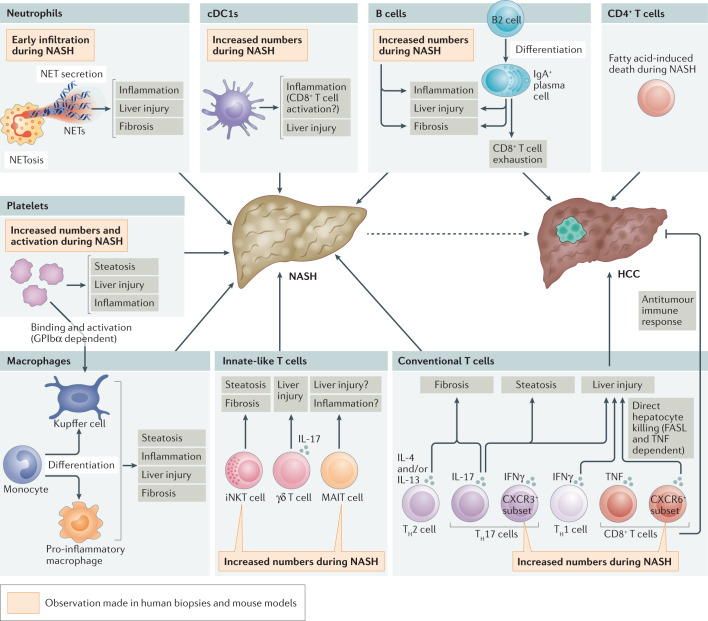
Fig. 3. Immune modulation of NASH pathogenesis.
The hepatic immune cell repertoire is reshaped during non-alcoholic steatohepatitis (NASH) and participates in the uncontrolled inflammatory environment that promotes liver injury (hepatocyte death) and liver fibrosis, further aggravating the disease. These immune cells comprise innate-like T cells, such as invariant natural killer T (iNKT) cells, mucosal-associated invariant T (MAIT) cells and γδ T cells, but also conventional CD8+ T cells and CD4+ T cell subsets, including interferon-γ (IFNγ)-producing T helper 1 (TH1) cells, IL-4-producing and/or IL-13-producing TH2 cells, and IL-17-producing TH17 cells. Neutrophil accumulation is an early event in NASH that promotes inflammation and liver injury, in particular through the secretion of neutrophil extracellular traps (NETs) or their release upon neutrophil cell death (NETosis). Dendritic cells (DCs) and, in particular, type 1 conventional DCs (cDC1s), increase in number and promote liver inflammation (potentially by activating CD8+ T cells) as well as hepatic injury during NASH. Monocytes are also rapidly recruited to the liver, where they can differentiate into pro-inflammatory macrophages or give rise to monocyte-derived Kupffer cells. Platelets are increased in number and activated during NASH; they promote liver steatosis, inflammation and injury, implicating mechanisms that involve direct binding to and activation of Kupffer cells in a glycoprotein GPIbα-dependent manner. B cells and, in particular, IgA+ plasma cells also contribute to NASH by promoting liver inflammation, injury and fibrosis. Furthermore, IgA+ plasma cells can promote the progression to hepatocellular carcinoma (HCC) through their immunosuppressive effects by inducing CD8+ T cell exhaustion. In addition, loss of CD4+ T cells through fatty acid-mediated cytotoxic effects limits their antitumour potential and favours NASH progression to HCC. Finally, CD8+ T cells and, notably, the autoaggressive CXCR6+ subset that is induced by the hepatic microenvironment of steatosis, promote liver damage and the NASH–HCC transition through the secretion of pro-inflammatory cytokines and direct hepatocyte killing in a FASL-dependent and tumour necrosis factor (TNF)-dependent manner. CD8+ T cells can also limit tumour burden through their capacity to mount an antitumour immune response. The contribution of immune cells to the development of NAFLD has been mainly explored in mouse models. Nevertheless, some observations (as indicated in the figure) have been corroborated both in human biopsies and in mouse models of NASH. Our current knowledge is not sufficient to propose an integrated view of NASH pathogenesis involving the crosstalk between immune cells or their temporal involvement and, in many cases, detailed molecular mechanisms are lacking.
Box 2 Crosstalk between immune and parenchymal cells during NASH.
Various immune cell subsets affect different aspects of the pathogenesis of non-alcoholic steatohepatitis (NASH), including liver steatosis, inflammation, injury and fibrosis. Notably, immune cells promote inflammation directly by secreting inflammatory cytokines, such as tumour necrosis factor and IL-1β, but also indirectly by activating neighbouring immune and non-immune cells. In terms of liver injury, which mostly arises from hepatocyte death, immune cells can crosstalk with hepatocytes to promote their cytokine-induced death. Furthermore, the interaction of immune cells with hepatic stellate cells (HSCs)175 can promote fibrogenesis. Overall, it remains poorly defined whether immune cells affect NASH pathogenesis directly by signalling to hepatocytes (liver injury) and HSCs (fibrosis) and/or indirectly through a more complex network of interactions. Indeed, although scattered evidence suggests that there is crosstalk between macrophages and HSCs or liver sinusoidal endothelial cells during NASH176, a clear integrated view is still missing. Methods such as single-cell RNA sequencing or single-nucleus RNA sequencing combined with novel bioinformatic tools that can generate ligand–receptor interaction maps177 would allow for identification of the most relevant pathways of crosstalk. This has recently been attempted in the context of NASH using single-cell RNA sequencing, highlighting HSC-secreted factors that could potentially impact the functions of neighbouring cells82. Of note, single-nucleus RNA sequencing would be particularly interesting to use as it offers the ability to more easily study cells, such as hepatocytes and HSCs, that are difficult to retrieve after classical tissue dissociation methods. This approach would give an integrated view of the intercellular communications operating during NASH and better resolve their complexity. Such an approach has recently been used to study the hepatic cellular communications that enable monocytes to differentiate into Kupffer cells79. In addition, perturbation of a specific immune cell population and analysis of its impact on the ligand–receptor interaction map may help to better understand how a single immune cell population can have broad effects on neighbouring cells. This will aid in uncovering the interactions between several cell types and furthering our understanding of how these interactions coordinate NASH-induced liver injury. This knowledge should eventually define the communication networks and crucial nodes that control the immune-mediated inflammatory processes that promote NASH.
B cells and plasma cells
B cells produce immunoglobulins84, present antigens85 and secrete cytokines upon Toll-like receptor-mediated activation by pathogen-associated molecular patterns86, thereby influencing immune-mediated inflammatory responses in diverse ways. B cells accumulate in the livers of patients with NASH who have high levels of lobular inflammation and fibrosis87, which suggests that they might alter the course of the disease. B cells are pro-inflammatory in mouse models of NASH and this involves both B cell receptor-mediated adaptive immune mechanisms and MYD88-dependent innate immune mechanisms88. In addition, increased gut permeability and microbiota-derived inflammatory mediators probably contribute to the increased activation of intrahepatic B cells during NASH88. Finally, B cell-deficient mice have reduced NASH severity owing to decreased inflammation and fibrosis88.
B cells are heterogeneous and can be subdivided into two major lineages. B2 cells are activated in secondary lymphoid organs and receive help from CD4+ T helper (TH) cells to generate antigen-specific, high-affinity antibodies84. B1 cells secrete ‘natural’ antibodies (germline-encoded immunoglobulins present in the absence of exogenous antigen stimulation) as part of an innate-like immune response89. B2 cell depletion is associated with reduced NASH-associated hepatic fibrosis in mice87, whereas the specific role of B1 cells in NASH has not yet been studied. Further highlighting an important role for B2 cells in NASH pathogenesis, serum levels of B cell-activating factor (BAFF), a cytokine that controls the differentiation and survival of B2 cells but not of B1 cells90, are increased in patients with NASH and further increased in those with fibrosis91. In mice, BAFF neutralization limited liver injury during NASH87.
Upon activation, B2 cells differentiate into antibody-producing B cells or long-lived antibody-producing plasma cells. The liver is particularly rich in IgA-producing plasma cells at the steady state73 and the number of these cells is further increased during NASH in both mice and humans92. In addition, increased serum levels of IgA are associated with advanced hepatic fibrosis in patients with NASH93. HFD-fed MUP-uPA mice that lack IgA develop milder NASH with decreased liver injury and fibrosis92. However, although several lines of evidence implicate B cells and IgA in NASH pathogenesis (Fig. 3), the underlying mechanisms warrant further investigation. In addition, the antigen specificity of the B cells that are generated in patients with NASH and involved in disease progression remains to be elucidated.
Dendritic cells
Upon local activation, DCs can migrate to tissue-draining lymph nodes where they interact with naive T cells94. In addition to their role in inducing an adaptive immune response in this manner, DCs can also participate in local inflammation through their ability to respond to a wide range of pathogen-associated molecular patterns that engage Toll-like receptors and other pattern recognition receptors94. Both subsets of conventional DCs (CD103+ cDC1s and CD11b+ cDC2s) are present in the liver94 and accumulate during NASH in mice95,96 but their impact on NASH pathogenesis remains elusive. In humans, the number of liver cDC1s was increased in patients with NASH and increased cDC1 numbers were associated with increased disease hallmarks of NASH96. NASH induction in Batf3-deficient mice that lack cDC1s led to increased liver triglyceride levels but a similar extent of liver injury97. However, such whole-body deletion of Batf3 could have influenced NASH independently from cDC1 loss. Using a more-specific model of cDC1 depletion, cDC1s were shown to promote liver injury in mice but the mechanisms remain to be clearly defined96. By contrast, the role of cDC2s in NASH has not been addressed so far. Overall, further investigation will be needed to better appreciate the role of cDCs in NASH pathogenesis as well as the underlying mechanisms.
Conventional CD4+ and CD8+ T cells
Conventional CD4+ TH cells adopt a range of polarized cell fates determined by their interactions with specific DC subsets and the cytokine environment94,98. TH1, TH2 and TH17 cell fates are characterized by the expression of interferon-γ (IFNγ), IL-4 and/or IL-13, and IL-17, respectively98. The roles of several of these cytokines and their signalling pathways have been studied in the context of NASH. However, as cell types other than CD4+ T cells also produce these cytokines, it is difficult to specifically assign the phenotype reported to changes in TH cell populations and targeted approaches are required to further our understanding.
Mice deficient in IFNγ, the prototypical TH1 cell cytokine, were markedly protected against liver injury and hepatic fibrosis in a mouse model of NASH99. In these animals, decreased fibrosis is associated with a markedly decreased expression of osteopontin, which is a known inducer of liver fibrogenesis100, but the underlying mechanisms remain largely unexplored. Cellular sources of IFNγ other than TH1 cells, in particular CD8+ T cells, might have contributed to the phenotype. The IFNγ-inducible chemokine CXCL10 is also involved in NASH pathogenesis. CXCL10 induces chemotaxis of CXCR3-expressing cells, including T cells. Circulating levels of CXCL10 are increased in patients with NASH and Cxcl10 deletion or antibody-mediated neutralization of CXCL10 limits steatosis, liver injury and fibrosis in mice101. Cxcr3 deficiency also reduced NASH pathogenesis in mice102. Thus, dampened CXCL10–CXCR3 signalling could partially explain the impact of IFNγ deficiency on NASH.
The role of several TH2 cell-associated cytokines in NASH has also been addressed. Serum levels of IL-13 are increased in patients with NASH and expression levels of its receptor IL-13RA2 are increased in the liver103. IL-13RA2 is expressed by HSCs and cytotoxin-mediated killing of IL-13RA2+ cells impedes liver fibrosis in a rodent model of NASH103. Production of the type 2 cytokines IL-4, IL-5 and IL-13 is induced by IL-33 and, consistent with the known role of type 2 cytokines in extracellular matrix production104, IL-33 treatment promotes hepatic fibrosis in mice105. However, treatment with IL-33 also limits hepatic triglyceride storage and results in a small decrease in liver injury in a mouse model of NASH105. Overall, therefore, the role of TH2 cell-mediated immunity in NASH remains unclear.
TH17 cells can have either homeostatic functions that maintain the intestinal barrier in response to commensals or pathogenic functions that fuel inflammatory diseases when elicited by pathogens106. Hepatic TH17 cells107 and the expression of TH17 cell-associated genes108 are increased in patients with NASH. Similarly, TH17 cells are increased in mouse models of NASH109–111, in particular a subset of pro-inflammatory CXCR3+ TH17 cells that drives NASH pathogenesis112. In mice deficient for unconventional prefoldin RPB5 interactor (Uri1) in hepatocytes (HepΔUri1 mice), NASH is exacerbated owing to DNA damage in hepatocytes and this is associated with the induction of TH17 cells and increased hepatic IL-17A production110. Neutralizing IL-17A using a monoclonal antibody or suppressing the generation of TH17 cells with the RORγt inhibitor digoxin decreases the hallmarks of NASH in HepΔUri1 mice110. Il17a deficiency is equally protective in a different mouse model of NASH111 and recombinant IL-17A treatment increases hepatic DNA damage, steatosis, liver injury and fibrosis in wild-type mice fed a NASH-inducing diet110. Mechanistically, impaired IL-17-induced signalling in myeloid cells protects HepΔUri1 mice from NASH, which suggests the existence of a major pathway of crosstalk between IL-17-producing cells, including TH17 cells, and phagocytes that drives NASH110. Consistent with the pro-fibrotic effects of cytokines produced by TH1, TH2 and TH17 cells reported above, the depletion of all CD4+ T cells in a humanized mouse model of NASH blunted hepatic fibrosis113.
CD8+ T cells mainly produce IFNγ, TNF and cytotoxic molecules such as perforins114. Hepatic CD8+ T cells are increased in number during NASH in both mice and humans92,115, in particular CD8+ T cells expressing CXCR6 (ref.116). Lipid-conditioned CXCR6+ CD8+ T cells induce hepatocyte killing in a perforin-independent, FasL (CD95L)-dependent manner116. Consequently, in a diet-induced mouse model of NASH, CD8+ T cell depletion blunted liver injury115. Perforin 1-deficient mice have exacerbated NASH hallmarks, which was associated with an increased number and activation status of hepatic CD8+ T cells117. Perforin deficiency is known to favour CD8+ T cell activation in viral infection118,119. This effect is cell-extrinsic and involves the persistence of immunostimulatory DCs in the absence of perforin-mediated killing of antigen-loaded DCs120,121. Finally, CXCR6+ CD8+ T cells that accumulate during NASH express the exhaustion marker PD1 and PD1 blockade exacerbated CD8+ T cell activation resulting in accelerated NASH development in mice122. Thus, CD8+ T cells are likely to promote hepatic injury during NASH.
Overall, an integrated mechanistic view of how T cell subsets are activated and cooperate to promote hepatic inflammation during NASH is missing. Most of the current literature refers to cytokines produced by T cell subsets rather than the T cells themselves. In addition, although CD8+ T cell-mediated killing of hepatocytes seems to occur in an antigen-independent manner during NASH116, it remains unknown whether adaptive, antigen-specific T cell responses are also involved. Further investigations will be needed to fill these gaps in our knowledge as well as to elucidate how these pathways could be targeted therapeutically without impairing immune defences.
Innate-like T cells
iNKT cells are enriched in the mouse liver as compared to other organs and are increased in mouse models of NASH as well as in patients with NASH115,123. Their role in NASH pathogenesis has been evaluated using Cd1d-deficient or Traj18-deficient mice124, in which iNKT cells do not develop. iNKT cells promote liver fibrosis124,125 through their ability to increase liver expression of osteopontin124, which is known to stimulate fibrogenesis during NASH in both mice and humans100. A more recent study shows that iNKT cells also favour liver steatosis and, together with CD8+ T cells that induce liver damage, are necessary for NASH development115. iNKT cells are heterogeneous and encompass T-bet+ iNKT1 cells, GATA3+ iNKT2 cells and RORγt+ iNKT17 cells, which selectively produce IFNγ, IL-4 and IL-17, respectively126. As type 2 cytokines, such as IL-4, are known to support collagen production and extracellular matrix deposition104, it would be interesting to assess the specific role of iNKT2 cells in NASH-induced fibrosis.
γδ T cells are another subset of innate-like T cells present in the steady-state liver, where they develop and are maintained in a microbiota-dependent manner127. The number of γδ T cells in the mouse liver increases during NASH and they promote hepatic injury127. Importantly, the development of liver γδ T cells is impaired in Cd1d–/– mice and this may contribute to the dampened NASH phenotype that is observed in these mice124.
One study reported that MAIT cells increase in number during NASH pathogenesis in both mice and humans and that their deficiency promotes liver inflammation and injury128. However, it remains unclear how MAIT cells are protective in the context of diet-induced NASH whereas they are pro-inflammatory and pathogenic during diet-induced obesity129. In addition, the impact of MAIT cells on fibrosis was not addressed in this study but a previous work has described a pro-fibrogenic role of MAIT cells in models of acute liver injury130. Thus, deciphering the role of MAIT cells in NASH, particularly with regards to hepatic fibrosis, warrants further investigation.
Platelets
In addition to their front-line role in coagulation and haemostasis, platelets are also involved in the regulation of inflammatory processes131. For example, the interaction of platelets with blood-borne pathogens facilitates KC-mediated bacterial clearance in the liver132. In addition, circulating platelets interact with monocytes to favour atherosclerotic plaque development by promoting arterial inflammation and further leukocyte recruitment133,134. Platelets are also involved in the development of NASH (Fig. 3). Indeed, anti-platelet therapy was previously shown to dampen NASH pathogenesis in rats135, although the underlying mechanisms remain uncertain. A recent study showed that platelet activation and adhesion (but not aggregation) as well as platelet-derived granules are crucial in the promotion of NASH136. Platelets interact with KCs at both early and late stages of NASH and promote liver steatosis, inflammation and injury in mice136. In addition, platelets promote the accumulation of inflammatory cells in the liver during NASH in a glycoprotein GPIbα-dependent manner. In line with these observations, platelets are a promising therapeutic target in NASH136.
Neutrophils
In contrast to their beneficial roles during infection, neutrophils usually have a detrimental effect on chronic inflammatory disorders through the production of ROS, cytokines, proteases and neutrophil extracellular traps (NETs)137,138. In NASH, hepatic neutrophil infiltration is evident in both mouse models and human biopsies139–142. Neutrophil accumulation is an early event in mouse models of NASH141–143. Neutrophil depletion impairs the early stages of NASH in mice by limiting inflammation and liver injury but such effects are no longer observed as NASH progresses141. Inhibition of the serine protease neutrophil elastase similarly impacts the early phases of NASH141. Neutrophil elastase is released as a constituent of NETs144, which are found very early in the liver during NASH development in mice142 and at increased levels in the circulation of patients with NASH143. Dismantling NETs using deoxyribonuclease I limits hepatic inflammation, liver injury and fibrosis in mice142,143, which indicates the detrimental effect of these structures on NASH progression. Overall, neutrophils seem to have a role in the early pathogenesis of NASH through NET formation (Fig. 3) but their contribution to later stages of the disease remains unclear.
Macrophages
During NASH, inflammatory cues drive the hepatic recruitment of blood monocytes that locally differentiate into monocyte-derived macrophages, thereby increasing the size of the macrophage pool in the liver145. Recent studies have shed light on the diversity of hepatic macrophages in mouse models of NASH35,81,146.
A major finding is that self-maintenance of embryonically derived KCs is impaired in NASH as KCs with low cell surface expression levels of TIMD4 are observed in diseased mouse liver35,81,146. Such TIMD4low KCs are reminiscent of the monocyte-derived KCs that are generated after non-physiological depletion of embryonically derived KCs in mice79,147,148, which suggests that monocyte-derived KCs are generated during NASH. Indeed, fate-mapping experiments document monocyte participation in the mouse KC pool during NASH35,146 and immunostaining studies have shown that these monocyte-derived KCs localize to hepatic sinusoids, similarly to embryonically derived KCs81,146. The generation of monocyte-derived KCs is a response to the increased death of embryonically derived KCs during NASH35 and is aimed at maintaining KC numbers. Transcriptomic analysis reveals the enrichment of a lipotoxicity gene signature in embryonically derived KCs as well as in monocyte-derived KCs during NASH. Such a cellular stress signature probably explains both why embryonically derived KCs die during NASH and their inability to efficiently self-renew. Although the generation of monocyte-derived KCs maintains the KC pool in the liver, their transcriptomic landscape differs from that of embryonically derived KCs35,81,146. In particular, monocyte-derived KCs do not acquire the full gene expression spectrum associated with accessory functions of embryonically derived KCs (such as erythrophagocytosis) and they have a more pronounced inflammatory profile than their embryonically derived counterparts35. Finally, at a functional level, monocyte-derived KCs and embryonically derived KCs differently impact the liver response to NASH. Monocyte-derived KCs limit hepatic triglyceride storage but they promote liver injury to a greater extent than embryonically derived KCs35 (Fig. 4). Thus, KC homeostasis is markedly impaired during NASH and has an impact on liver pathology.
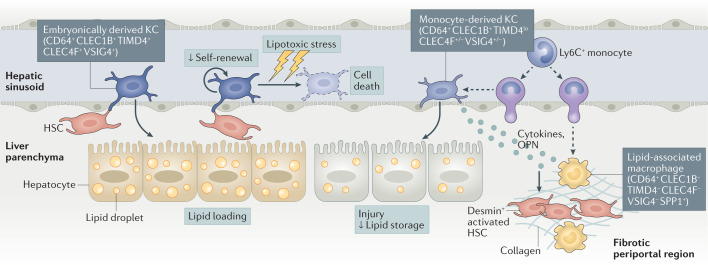
Fig. 4. The diversity of hepatic macrophages during NASH.
During non-alcoholic steatohepatitis (NASH), embryonically derived Kupffer cells (KCs), which are tissue-resident macrophages that inhabit the sinusoids of the healthy liver, are gradually lost. In response to the NASH environment, they lose their capacity to self-renew and show accelerated signs of cell death owing to lipotoxic stress. To maintain the KC pool, circulating Ly6C+ monocytes are recruited to the liver sinusoids, where they differentiate into monocyte-derived KCs. These monocyte-derived KCs express the pan-macrophage marker CD64 and have high levels of expression of CLEC1B (the prototypical marker of embryonically derived KCs) but lower levels of expression of other markers of embryonically derived KCs (such as CLEC4F, VSIG4 and TIMD4). Monocyte-derived KCs are more pro-inflammatory than embryonically derived KCs and they affect the liver response to NASH by limiting liver lipid storage and promoting liver injury. Other macrophages, also derived from circulating monocytes, populate the liver during NASH. They express no typical markers of KCs and are prevalent in fibrotic areas positive for desmin, a marker of activated hepatic stellate cells (HSCs). These monocyte-derived macrophages have been termed lipid-associated macrophages and they resemble the TREM2+CD9+ scar-associated macrophages that have been described in human fibrotic livers. Lipid-associated macrophages and monocyte-derived KCs express high levels of SPP1, the gene encoding osteopontin (OPN), which is a strong inducer of liver fibrogenesis.
In addition to their contribution to the KC pool, monocytes also follow a classical pathway of differentiation during NASH, leading to the generation of monocyte-derived inflammatory macrophages. Of note, the liver environment during NASH has a systemic effect on monocytes as they already show NASH-associated transcriptional alterations in mouse bone marrow149. In the liver, monocyte-derived macrophages express high levels of Spp1, Itgax, Gpnmb, Cd9 and Trem2 (among other markers)81,82,146, which are also found at some levels in monocyte-derived KCs35. In mice, the monocyte-derived macrophages that accumulate in the liver during NASH resemble the lipid-associated macrophages that are present in obese white adipose tissue150, which suggests that metabolic inflammation induces a common gene signature in monocyte-derived macrophages in different tissues and different metabolic contexts. In terms of their function, monocyte-derived macrophages in mouse liver localize to areas of tissue fibrosis in close proximity to desmin+ HSCs81, which suggests that they may participate in hepatic fibrosis (Fig. 4). Similar observations were made in human cirrhotic liver83. Indeed, a TREM2+CD9+ monocyte-derived macrophage population with profibrotic properties accumulates during liver fibrosis in humans83.
Overall, as mentioned above, diverse immune cell populations are involved in NASH pathogenesis and the roles of other immune cell subsets, such as NK cells and ILCs, remain to be clarified. Thus, it is likely that a coordinated response involving crosstalk between immune cells contributes to the hepatic inflammatory environment observed during NASH. However, the contours of such an integrated response typifying NASH pathogenesis have largely been unexplored so far. It is too early to speculate regarding a specific sequence of immune cell activation during NASH, with the exception that neutrophils are known to be recruited early during the disease and have been described to only impact NASH pathogenesis at early stages. Similarly to neutrophils, monocytes are likely to be rapidly recruited to the liver but their contribution to the KC pool might vary in time and magnitude depending on the model. Another important point is that many of the immune cell populations that are known to alter the course of NASH are already present in the steady-state liver and are thus susceptible to being activated locally as soon as NASH triggers are induced. Again, the time and magnitude of such activation might differ between models. Thus, kinetic studies on different mouse models of NASH are needed to better appreciate the dynamic accumulation of immune cells and their activation. Our current knowledge is not sufficient to propose an integrated view of NASH pathogenesis but emerging technologies, such as single-cell RNA sequencing or time-of-flight mass cytometry, are likely to fill this knowledge gap in the near future. Indeed, kinetic, large-scale analysis of the accumulation and activation of immune cell populations over the course of NASH would help to propose a model typifying NASH-driven inflammation.
Immune cells in the NASH–HCC transition
NASH is a major risk factor for developing HCC12, as influenced by the hepatic inflammatory environment involving the immune cell populations described above151. In a mouse model of diet-induced NASH and HCC, CD8+ T cells and NKT cells were crucial for the transition to HCC by promoting both liver steatosis and damage115. In this model, the inability to reach the NASH stage upon depletion of CD8+ T cells and NKT cells is likely to underlie the protection from HCC development. By contrast, CD8+ T cells protect HFD-fed MUP-uPA mice lacking IgA from NASH-induced HCC92. In these mice, CD8+ T cells have a limited effect on promoting NASH and instead resistance to HCC is associated with a decrease in the number of exhausted CD8+ T cells. Consequently, PDL1 blockade reverses T cell exhaustion in MUP-uPA mice fed a HFD, which leads to increased antitumour immunity and reduced tumour incidence92. Thus, CD8+ T cells have a key antitumour role in the HFD-fed MUP-uPA mouse model. In addition, Cd8a-deficient mice develop a higher tumour burden in other models of NASH-induced HCC92. In this study, CD8+ T cells have a limited, albeit significant, effect on NASH severity, which probably explains why their antitumour effects are apparent. In conclusion, the effect of CD8+ T cells in promoting NASH onset might, in other models, mask their well-described antitumour potential152.
CD4+ T cells were shown to impede HCC development in a mouse model of NASH-potentiated HCC153. In this model, fatty acids induce CD4+ T cell death through mitochondrial ROS production, whereas ROS scavenging limits CD4+ T cell loss and reduces tumour burden153. The effects of CD4+ T cells on tumour growth are attributed to their ability to mount a tumour-specific immune response rather than to an effect on NASH progression. However, the opposite effect of CD4+ T cells has been described in another model of NASH-promoted HCC. Indeed, IL-17A-producing TH17 cells drive NASH development and its progression to HCC through IL-17A-induced signalling in myeloid cells110. In this study, the ability of TH17 cells to accelerate NASH progression, rather than their role in the antitumour immune response, is most likely to explain the faster transition towards HCC. Thus, depending on the NASH–HCC model used, CD4+ T cells can modulate NASH-driven HCC through different modes of action. Of note, if NASH progression is affected by an immune cell subset, this will mainly determine the ability of that subset to alter the NASH–HCC transition.
Innate immune cells can also impact NASH-induced HCC. As mentioned above, neutrophils promote the development of NASH through the release of NETs143. Limiting NET production decreases NASH-associated inflammation and reduces NASH-induced HCC143 probably due to limited NASH development. The roles of other myeloid cells, such as KCs, in NASH-induced HCC have not yet been explored, although there is abundant literature on the role of macrophages in HCC development in general.
Overall, immune cells can limit HCC onset either by controlling NASH development or by blocking the NASH–HCC transition. To better understand the mode of action of diverse immune cell types, approaches aimed at modulating specific immune cell populations in a temporal manner need to be developed.
Therapeutic prospects
There are currently no approved therapies for NASH in the clinic12. Approaches targeting the metabolic arm of the disease have been tested. For example, peroxisome proliferator-activated receptor (PPAR) agonists have been previously developed and tested in clinical studies11. Although they are mainly known for their roles in modulating lipid metabolism, PPARγ and PPARδ also have immunomodulatory properties, such as anti-inflammatory actions on macrophages154,155, which may contribute to the potential clinical benefit in NASH. More recently, obeticholic acid (OCA), a ligand for farnesyl X receptor (FXR; a nuclear receptor for bile acids) that is already approved for the treatment of primary biliary cholangitis156, has been repurposed to combat NASH. FXR activation by OCA inhibits bile acid production and promotes their efflux from hepatocytes, thereby limiting their accumulation in the liver157. OCA improves NASH score and decreases fibrosis in an interim analysis of an ongoing phase III study158. However, there are concerns that patients receiving OCA might have dysregulated cholesterol metabolism159, which could increase their risk of cardiovascular disease. Similarly to the PPARs, FXR is also expressed by immune cells and the immunomodulatory effects of OCA cannot be excluded, although firm evidence for such effects is lacking. Finally, the GLP1 receptor agonist semaglutide, which was previously developed to promote weight loss and counteract type 2 diabetes, has shown promising effects on NASH resolution in patients160 and further studies will determine if it could be a potential therapeutic option for NASH.
As discussed above, hepatocyte death is a crucial factor in NASH initiation and perpetuation and caspase inhibitors have been tested in this regard. However, the pan-caspase inhibitor Emricasan, which was shown to limit hepatocyte apoptosis, inflammation and fibrosis in preclinical models40, was unsuccessful in patients with NASH161. This may limit the commercial interest to test drugs targeting apoptosis or, at best, may motivate the use of more specific caspase inhibitors such as those targeting caspase 6, which have shown promising effects in preclinical models39.
Treatments specifically focusing on the immunoinflammatory arm of NASH have also been evaluated, in particular Cenicriviroc (CVC). CVC is a dual CCR2 and CCR5 antagonist that has been shown to limit fibrosis without affecting other aspects of NASH in mice162,163. The chemokine receptors CCR2 and CCR5 are expressed by monocytes as well as T cells and CVC would thus be expected to limit immune cell infiltration of the liver during NASH. Although CVC showed promising results in phase II trials, the phase III study was recently terminated owing to a lack of efficacy11. This is particularly disappointing as CVC was at the frontline of approaches to limit fibrosis in patients with NASH, which is of particular importance as fibrosis can lead to permanent effects on the liver such as cirrhosis164. Thus, use of CVC might be combined with the blocking of other chemokine receptors controlling T cell recruitment to more efficiently limit NASH. Indeed, in addition to CCR2 and CCR5, T cell-specific chemokine receptors, such as CXCR3 or CXCR6, the latter being recently identified to mark a subset of NASH-associated autoaggressive CD8+ T cells116, could be targeted.
At this stage, alternative therapeutic options to limit hepatic fibrosis are not available, which is motivating the design of innovative strategies. Along these lines, a recent study highlights that an immunotherapeutic approach can be used to limit extracellular matrix deposition in a mouse model of NASH. The approach is based on the development of chimeric antigen receptor (CAR) T cells that can target and destroy myofibroblasts165, as previously studied in models of cardiac fibrosis166. In this study, CAR T cells are designed to recognize and destroy urokinase-type plasminogen activator receptor (uPAR)-expressing senescent cells that fuel tissue fibrosis in different organs165. In the NASH liver, CAR T cells target and eliminate uPAR+ HSCs and macrophages, which reduces fibrosis and improves liver function165. Finally, the use of autologous macrophage infusion has been shown to limit liver fibrosis in mice167 and a recent safety profile study has shown the feasibility of such an approach in humans168. This approach might represent an alternative avenue to limit liver fibrosis during NASH.
Conclusions and perspectives
NAFLD is a complex, multifactorial disease with NASH being its first crucial step towards severe liver alterations. Although it develops on a background of altered metabolism, NASH has a strong immunoinflammatory dimension. A vast network of immune cells is mobilized during NASH and we have described here how they can promote the transition from simple steatosis to NASH as well as the evolution of NASH to cirrhosis and HCC. However, our understanding of the inflammatory cues that drive NASH is fragmented and further investigation is required to better appreciate the role of specific immune cell subsets in the disease. In addition, an integrated view of the interactions between different types of immune cells as well as between immune cells and stromal cells is required to appreciate the complexity of NASH. Mouse models of NASH are continuing to improve and are likely to help determine causality between specific immune cell populations and disease progression. This new information will need to be coupled to the better phenotyping of patients using novel methods such as single-cell or single-nucleus RNA sequencing, which would allow for a better appreciation of the immune landscape that characterizes patients with NASH. In addition, comparing the immune landscape in humans with NASH to that observed in different mouse models could be used to determine the superiority of a specific preclinical model over another. Together, these developments should foster a research effort to develop innovative therapeutic strategies to limit the global burden of NASH. Thus, the upcoming years will be the scene of intensive basic, preclinical and clinical research aimed at filling the urgent need for drugs to combat the NASH epidemic.
Acknowledgements
The authors apologize to colleagues whose work could not be cited owing to space limitations. This work was supported by Inserm, Sorbonne Université and grants from the Fondation de France (project number 00056835), the Agence Nationale pour la Recherche (ANR-15-CE14-0015-02, ANR-17-CE14-0009-02, ANR-17-CE14-0023-01 and ANR-20-CE15-0018-02) and the city of Paris (Emergence-s- program) to E.L.G. as well as grants from the Fondation de France (project number 00096295), Alliance Sorbonne Université (Programme Emergence) and the Agence Nationale pour la Recherche (ANR-17-CE14-0044-01) to T.H. The authors thank G. Marcelin for critical reading of the manuscript.
Glossary
- Insulin resistance
A state in which cells are not able to respond fully to insulin or induce signalling pathways downstream of the insulin receptor.
- Steatosis
Describes the abnormal accumulation of lipids in a tissue.
- Metabolic syndrome
A group of five conditions (increased blood pressure, high blood sugar level, excess body fat around the waist, and abnormal cholesterol or triglyceride levels) that can occur together and increase the risk of heart disease, stroke and type 2 diabetes.
- Endoplasmic reticulum stress
A state of cellular stress that occurs when protein folding in the ER is impaired, leading to the accumulation of unfolded and/or misfolded proteins.
- β-oxidation
Catabolic process occurring in the mitochondria by which fatty acids are converted into acetyl-CoA, a major metabolic intermediate.
- Mallory–Denk bodies
Cytoplasmic aggregates of damaged cytoskeletal components found in hepatocytes during NASH or alcoholic liver disease.
- Portal tracts
Areas of the liver, also known as portal triads, that consist of a bile duct, a small branch of the portal vein and a branch of the hepatic artery.
- Gut-associated lymphoid tissue
Intestinal lymphoid structures, including Peyer’s patches and isolated lymphoid follicles, that are particularly rich in antibody-producing plasma cells.
- Hepatic stellate cells
(HSCs). Mesenchymal liver cells found in the area between sinusoids and hepatocytes, known as the space of Disse.
- Neutrophil extracellular traps
(NETs). Structures released by neutrophils that are primarily composed of a scaffold of chromatin fibres and antimicrobial proteins.
- Peroxisome proliferator-activated receptor
(PPAR). Part of a group of ligand-controlled transcription factors of the nuclear receptor family that have major regulatory roles in controlling metabolic functions as well as anti-inflammatory properties.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Immunology thanks X. Revelo and F. Tacke for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer — mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker DJ. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33:479–498. doi: 10.1016/j.cmet.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarma S, Sockalingam S, Dash S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021;23(Suppl. 1):3–16. doi: 10.1111/dom.14290. [DOI] [PubMed] [Google Scholar]
- 4.Pais R, et al. Fatty liver is an independent predictor of early carotid atherosclerosis. J. Hepatol. 2016;65:95–102. doi: 10.1016/j.jhep.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Pais R, Redheuil A, Cluzel P, Ratziu V, Giral P. Relationship among fatty liver, specific and multiple-site atherosclerosis, and 10-year Framingham score. Hepatology. 2019;69:1453–1463. doi: 10.1002/hep.30223. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Sanyal AJ, George J, International Consensus Panel MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 7.Estes C, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 8.O’Hara J, et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020;2:100142. doi: 10.1016/j.jhepr.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schattenberg JM, et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis (nash) in five European countries in 2018: a cost-of-illness analysis. Liver Int. 2021;41:1227–1242. doi: 10.1111/liv.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younossi ZM, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 11.Vuppalanchi R, Noureddin M, Alkhouri N, Sanyal AJ. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021;18:373–392. doi: 10.1038/s41575-020-00408-y. [DOI] [PubMed] [Google Scholar]
- 12.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 14.Eslam M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 15.Younossi ZM, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2021;73:1194–1198. doi: 10.1002/hep.31420. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstedt M, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 19.Pais R, et al. NAFLD and liver transplantation: current burden and expected challenges. J. Hepatol. 2016;65:1245–1257. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segovia-Miranda F, et al. Three-dimensional spatially resolved geometrical and functional models of human liver tissue reveal new aspects of NAFLD progression. Nat. Med. 2019;25:1885–1893. doi: 10.1038/s41591-019-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison SA, et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020;5:970–985. doi: 10.1016/S2468-1253(20)30252-1. [DOI] [PubMed] [Google Scholar]
- 22.Munteanu M, et al. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment. Pharmacol. Ther. 2016;44:877–889. doi: 10.1111/apt.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newsome PN, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020;5:362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava A, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019;71:371–378. doi: 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todoric J, et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2020;2:1034–1045. doi: 10.1038/s42255-020-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa H, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichimura M, et al. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol. Res. 2015;45:458–469. doi: 10.1111/hepr.12358. [DOI] [PubMed] [Google Scholar]
- 30.Savard C, et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang JQ, et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat. Commun. 2018;9:4490. doi: 10.1038/s41467-018-06931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–774. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JY, et al. ER stress drives lipogenesis and steatohepatitis via Caspase-2 activation of S1P. Cell. 2018;175:133–145.e15. doi: 10.1016/j.cell.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imajo K, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16:44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Tran S, et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity. 2020;53:627–640.e5. doi: 10.1016/j.immuni.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Feldstein AE, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/S0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 37.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018;15:738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luedde T, et al. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Zhao P, et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science. 2020;367:652–660. doi: 10.1126/science.aay0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barreyro FJ, et al. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35:953–966. doi: 10.1111/liv.12570. [DOI] [PubMed] [Google Scholar]
- 41.Witek RP, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 42.Wandrer F, et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020;11:212. doi: 10.1038/s41419-020-2411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants. 2021;10:174. doi: 10.3390/antiox10020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 45.Meakin PJ, et al. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol. Cell Biol. 2014;34:3305–3320. doi: 10.1128/MCB.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grohmann M, et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell. 2018;175:1289–1306.e20. doi: 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marí M, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, et al. Neutralization of oxidized phospholipids ameliorates non-alcoholic steatohepatitis. Cell Metab. 2020;31:189–206.e8. doi: 10.1016/j.cmet.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganz M, et al. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J. Transl. Med. 2015;13:193. doi: 10.1186/s12967-015-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J. Lipid Res. 2016;57:233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dasgupta D, et al. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology. 2020;159:1487–1503.e17. doi: 10.1053/j.gastro.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammoutene A, Rautou P-E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019;70:1278–1291. doi: 10.1016/j.jhep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Heymann F, Tacke F. Immunology in the liver — from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 55.Balmer ML, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci. Transl. Med. 2014;6:237ra66. doi: 10.1126/scitranslmed.3008618. [DOI] [PubMed] [Google Scholar]
- 56.McDonald B, et al. Programing of an intravascular immune firewall by the gut microbiota protects against pathogen dissemination during infection. Cell Host Microbe. 2020;28:660–668.e4. doi: 10.1016/j.chom.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J. Gastroenterol. 2010;16:6046–6057. doi: 10.3748/wjg.v16.i48.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ficht X, Iannacone M. Immune surveillance of the liver by T cells. Sci. Immunol. 2020;5:eaba2351. doi: 10.1126/sciimmunol.aba2351. [DOI] [PubMed] [Google Scholar]
- 59.Hammond KJ, et al. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 2001;167:1164–1173. doi: 10.4049/jimmunol.167.3.1164. [DOI] [PubMed] [Google Scholar]
- 60.Geissmann F, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pellicci DG, Koay H-F, Berzins SP. Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat. Rev. Immunol. 2020;20:756–770. doi: 10.1038/s41577-020-0345-y. [DOI] [PubMed] [Google Scholar]
- 62.Rahimpour A, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dusseaux M, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 64.Tang X-Z, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J. Immunol. 2013;190:3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 65.Forkel M, et al. Composition and functionality of the intrahepatic innate lymphoid cell-compartment in human nonfibrotic and fibrotic livers. Eur. J. Immunol. 2017;47:1280–1294. doi: 10.1002/eji.201646890. [DOI] [PubMed] [Google Scholar]
- 66.Vivier E, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 67.Daussy C, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bai L, et al. Liver type 1 innate lymphoid cells develop locally via an interferon-γ-dependent loop. Science. 2021;371:eaba4177. doi: 10.1126/science.aba4177. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez-Ruiz D, et al. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity. 2016;45:889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Steinert EM, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacParland SA, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moro-Sibilot L, et al. Mouse and human liver contain immunoglobulin A-secreting cells originating from peyer’s patches and directed against intestinal antigens. Gastroenterology. 2016;151:311–323. doi: 10.1053/j.gastro.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 74.David BA, et al. Combination of mass cytometry and imaging analysis reveals origin, location, and functional repopulation of liver myeloid cells in mice. Gastroenterology. 2016;151:1176–1191. doi: 10.1053/j.gastro.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 75.Sierro F, et al. A liver capsular network of monocyte-derived macrophages restricts hepatic dissemination of intraperitoneal bacteria by neutrophil recruitment. Immunity. 2017;47:374–388.e6. doi: 10.1016/j.immuni.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 76.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoeffel G, et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Z, et al. Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell. 2019;178:1509–1525.e19. doi: 10.1016/j.cell.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 79.Bonnardel J, et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51:638–654.e9. doi: 10.1016/j.immuni.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gola A, et al. Commensal-driven immune zonation of the liver promotes host defence. Nature. 2021;589:131–136. doi: 10.1038/s41586-020-2977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Remmerie A, et al. Osteopontin expression identifies a subset of recruited macrophages distinct from Kupffer cells in the fatty liver. Immunity. 2020;53:641–657.e14. doi: 10.1016/j.immuni.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong X, et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol. Cell. 2019;75:644–660.e5. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramachandran P, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuseff M-I, Pierobon P, Reversat A, Lennon-Duménil A-M. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat. Rev. Immunol. 2013;13:475–486. doi: 10.1038/nri3469. [DOI] [PubMed] [Google Scholar]
- 86.Fillatreau S. B cells and their cytokine activities implications in human diseases. Clin. Immunol. 2018;186:26–31. doi: 10.1016/j.clim.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bruzzì S, et al. B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD) Free Radic. Biol. Med. 2018;124:249–259. doi: 10.1016/j.freeradbiomed.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 88.Barrow F, et al. Microbiota-driven activation of intrahepatic B cells aggravates nonalcoholic steatohepatitis through innate and adaptive signaling. Hepatology. 2021;74:704–722. doi: 10.1002/hep.31755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 90.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 91.Miyake T, et al. B cell-activating factor is associated with the histological severity of nonalcoholic fatty liver disease. Hepatol. Int. 2013;7:539–547. doi: 10.1007/s12072-012-9345-8. [DOI] [PubMed] [Google Scholar]
- 92.Shalapour S, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551:340–345. doi: 10.1038/nature24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McPherson S, Henderson E, Burt AD, Day CP, Anstee QM. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J. Hepatol. 2014;60:1055–1062. doi: 10.1016/j.jhep.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haas JT, et al. Transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution. Nat. Metab. 2019;1:604–614. doi: 10.1038/s42255-019-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deczkowska A, et al. XCR1+ type 1 conventional dendritic cells drive liver pathology in non-alcoholic steatohepatitis. Nat. Med. 2021;27:1043–1054. doi: 10.1038/s41591-021-01344-3. [DOI] [PubMed] [Google Scholar]
- 97.Heier E-C, et al. Murine CD103+ dendritic cells protect against steatosis progression towards steatohepatitis. J. Hepatol. 2017;66:1241–1250. doi: 10.1016/j.jhep.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 98.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat. Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luo X-Y, et al. IFN-γ deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G891–G899. doi: 10.1152/ajpgi.00193.2013. [DOI] [PubMed] [Google Scholar]
- 100.Syn W-K, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J. Hepatol. 2014;61:1365–1375. doi: 10.1016/j.jhep.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X, et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J. Hepatol. 2016;64:160–170. doi: 10.1016/j.jhep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Shimamura T, et al. Novel role of IL-13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL-13R-directed cytotoxin in a rat model. J. Immunol. 2008;181:4656–4665. doi: 10.4049/jimmunol.181.7.4656. [DOI] [PubMed] [Google Scholar]
- 104.Gieseck RL, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018;18:62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 105.Gao Y, et al. IL-33 treatment attenuated diet-induced hepatic steatosis but aggravated hepatic fibrosis. Oncotarget. 2016;7:33649–33661. doi: 10.18632/oncotarget.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Omenetti S, et al. The intestine harbors functionally distinct homeostatic tissue-resident and inflammatory Th17 cells. Immunity. 2019;51:77–89.e6. doi: 10.1016/j.immuni.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rau M, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/resting regulatory T cell ratio in peripheral blood and in the liver. J. Immunol. 2016;196:97–105. doi: 10.4049/jimmunol.1501175. [DOI] [PubMed] [Google Scholar]
- 108.Tang Y, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin. Exp. Immunol. 2011;166:281–290. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giles DA, et al. Regulation of inflammation by IL-17A and IL-17F modulates non-alcoholic fatty liver disease pathogenesis. PLoS One. 2016;11:e0149783. doi: 10.1371/journal.pone.0149783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gomes AL, et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2016;30:161–175. doi: 10.1016/j.ccell.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 111.Rolla S, et al. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin. Sci. 2016;130:193–203. doi: 10.1042/CS20150405. [DOI] [PubMed] [Google Scholar]
- 112.Moreno-Fernandez ME, et al. PKM2-dependent metabolic skewing of hepatic Th17 cells regulates pathogenesis of non-alcoholic fatty liver disease. Cell Metab. 2021;33:1187–1204.e9. doi: 10.1016/j.cmet.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Her Z, et al. CD4+ T cells mediate the development of liver fibrosis in high fat diet-induced NAFLD in humanized mice. Front. Immunol. 2020;11:580968. doi: 10.3389/fimmu.2020.580968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wolf MJ, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Dudek M, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 117.Wang T, et al. The immunoregulatory effects of CD8 T-cell-derived perforin on diet-induced nonalcoholic steatohepatitis. FASEB J. 2019;33:8490–8503. doi: 10.1096/fj.201802534RR. [DOI] [PubMed] [Google Scholar]
- 118.Matloubian M, et al. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 1999;73:2527–2536. doi: 10.1128/JVI.73.3.2527-2536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood. 2011;118:618–626. doi: 10.1182/blood-2010-12-324533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Terrell CE, Jordan MB. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8+ T cells and dendritic cells. Blood. 2013;121:5184–5191. doi: 10.1182/blood-2013-04-495309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boissonnas A, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 122.Pfister D, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Syn W-K, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Syn W-K, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maricic I, et al. Differential activation of hepatic invariant NKT cell subsets plays a key role in progression of nonalcoholic steatohepatitis. J. Immunol. 2018;201:3017–3035. doi: 10.4049/jimmunol.1800614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li F, et al. The microbiota maintain homeostasis of liver-resident γδT-17 cells in a lipid antigen/CD1d-dependent manner. Nat. Commun. 2017;7:13839. doi: 10.1038/ncomms13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y, et al. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Front. Immunol. 2018;9:1994. doi: 10.3389/fimmu.2018.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Toubal A, et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat. Commun. 2020;11:3755. doi: 10.1038/s41467-020-17307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hegde P, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 2018;9:2146. doi: 10.1038/s41467-018-04450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 132.Wong CHY, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat. Immunol. 2013;14:785–792. doi: 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huo Y, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 134.Koenen RR, et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat. Med. 2009;15:97–103. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 135.Fujita K, et al. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut. 2008;57:1583–1591. doi: 10.1136/gut.2007.144550. [DOI] [PubMed] [Google Scholar]
- 136.Malehmir M, et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019;25:641–655. doi: 10.1038/s41591-019-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 138.Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017;17:248–261. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 139.Gadd VL, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 140.Rensen SS, et al. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am. J. Pathol. 2009;175:1473–1482. doi: 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zang S, et al. Neutrophils play a crucial role in the early stage of nonalcoholic steatohepatitis via neutrophil elastase in mice. Cell Biochem. Biophys. 2015;73:479–487. doi: 10.1007/s12013-015-0682-9. [DOI] [PubMed] [Google Scholar]
- 142.Zhao X, et al. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis. 2020;11:379. doi: 10.1038/s41419-020-2582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van der Windt DJ, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347–1360. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 145.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 146.Seidman JS, et al. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity. 2020;52:1057–1074.e7. doi: 10.1016/j.immuni.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sakai M, et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain Kupffer cell identity. Immunity. 2019;51:655–670.e8. doi: 10.1016/j.immuni.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scott CL, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Krenkel O, et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut. 2020;69:551–563. doi: 10.1136/gutjnl-2019-318382. [DOI] [PubMed] [Google Scholar]
- 150.Jaitin DA, et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178:686–698.e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 152.St Paul M, Ohashi PS. The roles of CD8+ T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30:695–704. doi: 10.1016/j.tcb.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 153.Ma C, et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chawla A. Control of macrophage activation and function by PPARs. Circ. Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lefere S, et al. Differential effects of selective-and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J. Hepatol. 2020;73:757–770. doi: 10.1016/j.jhep.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 156.Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021;18:335–347. doi: 10.1038/s41575-020-00404-2. [DOI] [PubMed] [Google Scholar]
- 157.Kjærgaard K, et al. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J. Hepatol. 2021;74:58–65. doi: 10.1016/j.jhep.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 158.Younossi ZM, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 159.Siddiqui MS, et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J. Hepatol. 2020;72:25–33. doi: 10.1016/j.jhep.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Newsome PN, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 161.Harrison SA, et al. A randomized, placebo-controlled trial of Emricasan in patients with NASH and F1-F3 fibrosis. J. Hepatol. 2020;72:816–827. doi: 10.1016/j.jhep.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 162.Kruger AJ, et al. Prolonged cenicriviroc therapy reduces hepatic fibrosis despite steatohepatitis in a diet-induced mouse model of nonalcoholic steatohepatitis. Hepatol. Commun. 2018;2:529–545. doi: 10.1002/hep4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lefebvre E, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016;11:e0158156. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bataller R, Brenner DA. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Amor C, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aghajanian H, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thomas JA, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 168.Moroni F, et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat. Med. 2019;25:1560–1565. doi: 10.1038/s41591-019-0599-8. [DOI] [PubMed] [Google Scholar]
- 169.Matsumoto M, et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 2013;94:93–103. doi: 10.1111/iep.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang X, et al. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 2020;31:969–986.e7. doi: 10.1016/j.cmet.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Im YR, et al. A systematic review of animal models of NAFLD finds high-fat, high-fructose diets most closely resemble human NAFLD. Hepatology. 2021;74:1884–1901. doi: 10.1002/hep.31897. [DOI] [PubMed] [Google Scholar]
- 172.Asgharpour A, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 2016;65:579–588. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hansen HH, et al. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov. Today. 2017;22:1707–1718. doi: 10.1016/j.drudis.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 174.Machado MV, et al. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015;10:e0127991. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schwabe RF, Tabas I, Pajvani UB. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li H, et al. Crosstalk between liver macrophages and surrounding cells in nonalcoholic steatohepatitis. Front. Immunol. 2020;11:1169. doi: 10.3389/fimmu.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Browaeys R, Saelens W, Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]