Abstract
To assess whether PCR is applicable for monitoring the efficacy of antituberculous treatment, respiratory specimens obtained during treatment and follow-up from sputum smear-positive tuberculosis (TB) patients were examined. First, results of smear, culture, and PCR for Mycobacterium tuberculosis complex (MTB) and an internal inhibition control (MCC) were correlated retrospectively on 1,601 respiratory specimens from patients with no previous cultures of MTB. MTB optical density (OD) values increased to a maximum level of 3.5 to 4.0, with both increasing numbers of acid-fast bacilli and CFU. MTB/MCC OD ratios also increased with both smear and culture grading and correlated significantly better with both than the MTB OD value. Second, changes in MTB OD values and MTB/MCC OD ratios were compared with microscopy and culture for MTB in monthly sputa obtained during treatment and follow-up in 22 smear-positive pulmonary TB patients. Declines in MTB/MCC OD ratios during antituberculous treatment and follow-up were observed. Patients with moderate disease reached the baseline after 6 to 8 months of standard antituberculous treatment regimen, whereas patients with extensive disease were predicted to reach the baseline 1 year or more after the initiation of treatment. Although PCR detects both dead and live bacteria, we believe that PCR can be used to assess the efficacy of antituberculous treatment since increases or slow reductions in MTB/MCC OD ratios would indicate nonoptimal treatment, noncompliance, reduced bioavailability of drugs, or resistant strains of MTB and thereby would identify patients at risk for treatment failure or reactivation.
Follow-up studies of smear-positive pulmonary tuberculosis (TB) patients receiving short-course antituberculous therapy have shown relapse rates of 0 to 6% within 6 to 30 months (reviewed in reference 4) indicating that viable Mycobacterium tuberculosis complex (MTB) may remain after treatment in some patients. Increased relapse rates have been reported in certain subpopulations, i.e., AIDS patients (14, 16), noncompliant patients (15), and patients infected with resistant strains of MTB (13). Methods to identify patients at risk of relapse and methods to monitor response to treatment would be desirable. Clinical evaluation of treatment response can be difficult due to concurrent illness. Since radiographic resolution lags behind clinical improvement (2), microscopy and culture are currently the methods used to monitor the efficacy of treatment and outcome.
Monitoring of treatment by smear examination is a crude method since smear microscopy does not distinguish between dead and live MTB, is not specific for MTB, and is insensitive, with an analytical sensitivity estimated to be 5,000 to 10,000 acid-fast bacilli/ml (6). Sputum culture is superior to smear examination since it is more sensitive and specific. Recently, Epstein and coworkers demonstrated the use of time-to-detection of MTB in serial sputum cultures for monitoring of TB patients (8). However, culturing is time-consuming and, therefore, supplementary rapid and sensitive monitoring methods would be advantageous.
Previous studies (e.g., references 3, 5, and 17) focusing on the performance of various diagnostic PCR assays have demonstrated the persistence of specific nucleic acids from MTB in clinical specimens a long time after the initiation of antituberculous treatment. Few investigations have focused specifically on PCR during treatment and follow-up. In 1993, Yuen et al. showed that an in-house PCR targeting the gene encoding the 38-kDa protein of MTB remained positive 4 weeks after initiation of treatment in 29 of 41 patients (25). In a similar study, Kennedy and coworkers found that PCR targeting IS6110 stayed positive 1 to 2 months longer than culture in smear-positive pulmonary TB patients (11). Also targeting IS6110, Leveé et al. observed that PCR was positive in 25 and 13% of the patients after 2 and 3 months of treatment, respectively, and that 1 of 14 patients stayed positive after 6 months of treatment (12).
In the present study, we used a commercial PCR kit that combines amplification and detection of 16S rDNA from MTB and an internal inhibition control (MCC) to permit evaluation of actual reaction conditions (19). The objectives of this study were to perform PCR on serial sputum samples from smear-positive pulmonary TB patients undergoing antituberculous treatment and to assess whether PCR is applicable for monitoring the treatment of TB patients.
MATERIALS AND METHODS
Correlation of semiquantitative PCR to microscopy and culture grading.
Data on all respiratory specimens received between 20 May 1996 and 11 March 1997 from patients with no documented previous culture-proven TB but with a request for MTB PCR testing and an interpretable culture result were extracted from the laboratory information system at Statens Serum Institut. MTB optical density (OD) values and MTB/MCC OD ratios were compared (rank sum by Spearman) with microscopy and culture results, and P values were approximated by use of Fisher's Z transformation to test significance (18).
Evaluation of PCR on specimens obtained during treatment and follow-up.
For evaluation of PCR results on specimens obtained during treatment and follow-up, 22 consecutive, adult, smear-positive pulmonary TB patients were enrolled between 1 April 1997 and 8 December 1997 at the Clinic of Pulmonary Medicine at Rigshospitalet in Denmark. Patients with human immunodeficiency virus infection were excluded. All but one patient were included at the initiation of treatment, whereas one patient had started treatment 2 months earlier outside Denmark. This patient was still smear and culture positive, and the disease was very extensive. All patients received antituberculous treatment for 6 to 8 months with three to five drugs in the initial phase. The severity of disease was estimated as “moderate” or “extensive” by an experienced chest physician based on the extent of lung involvement at the time of diagnosis as follows: moderate, with cavity present (less than one-third of total lung area affected) or with cavity not present (less than two-thirds of total lung area affected); and extensive, with cavity present (more than one-third of total lung area affected) or with cavity not present (more than two-thirds of total lung area affected). After informed consent was obtained from the patient, three initial sputa and subsequent monthly sputa were obtained as long as the patient was able to produce sputum. The patients were taken out of the study when three consecutive specimens had tested negative by microscopy, culture, and PCR for MTB or if the patient was unable to expectorate.
Ethics.
The study was approved by The Scientific and Ethical Committee of Copenhagen (KF02-103/96). PCR results did not influence treatment, and none of the patients wished to withdraw during the study. Results of smear, culture, and PCR analysis were extracted from the laboratory information system at Statens Serum Institut after approval from The Danish Data Protection Agency (1998-1200-122).
Pretreatment and microbiological analyses.
The specimens were analyzed by microscopy, culture, and genus-specific PCR amplification, followed by detection of MTB- and MCC-specific amplicon in the Department of Mycobacteriology, which is a central diagnostic service for Denmark. After decontamination by either 2% NaOH or 1.5% NaOH plus N-acetyl-l-cysteine (changed during the study period) and centrifugation, the resulting pellets were resuspended in approximately 1 ml of phosphate-buffered saline. One drop of the resuspended pellet was used to make smears that were stained with auramine-rhodamine and examined at ×200 magnification. Smears with 10 or more acid-fast bacteria (AFB) were regarded positive (21) and reported as follows: 1+, 1 to 9 AFB/field; 2+, 1 to 9 AFB/field; 3+, 10 to 90 AFB/field; and 4+, >90 AFB/field.
Next, 150 μl of the resuspended pellet was inoculated onto solid medium (Löwenstein-Jensen slants; Statens Serum Institut), and 500 μl was inoculated into liquid medium (Bactec 12B; Becton Dickinson). Culture media were incubated at 35°C and examined weekly for mycobacterial growth for a maximum of 8 weeks. If present, MTB was identified by DNA-RNA hybridization (AccuProbe; GenProbe, San Diego, Calif.) on the first isolate from each patient and by evaluation of the temperature range and morphology on subsequent specimens. The number of CFU was estimated from the solid medium and reported as follows: 1+, 1 to 10 CFU; 2+, 11 to 99 CFU; and 3+, ≥100 CFU.
PCR analysis.
PCR amplification (Cobas Amplicor; Roche) was performed according to the product insert. In brief, 100 μl of the resuspended pellet was washed, and the resulting pellet was subjected to heating at 60°C for 45 min in the presence of a lysis reagent. After being heated, 100 μl of neutralization reagent was added. In a positive-pressure PCR set-up room, the MCC and the enzyme uracil–N-glycosylase were added to the premade mastermix before aliquots of 50 μl were put into the amplification tubes. Then, 50 μl of lysed specimen was added, and the amplification tubes were transferred to the Cobas Amplicor machine for amplification and detection. After hybridization between amplicon and probes specific for MTB and MCC, the amount of hybridization product was measured spectrophotometrically by determining the OD value. The OD values ranged between 0.000 and 3.999, and a cutoff value of 0.350 was taken to discriminate between positive and negative results as recommended by the manufacturer. Values of 4.000 or more were standardized to 4.000, because those values exceeded the linear range of the photometer. For the three initial specimens, an average was calculated.
Quality assurance.
The Department of Mycobacteriology holds accreditation according to EN45001 for smear examination for AFB, culture of mycobacteria, species identification, and susceptibility testing of MTB. Furthermore, all technicians performing PCR analyses had passed validation panels (Roche Diagnostics, Kaiseraugst, Switzerland) before entering the study. Positive and negative amplification controls were included in all runs, part of the decontaminated specimen was frozen to allow for an eventual retest, and precautions to avoid PCR contamination were taken (separate laboratories, laboratory coats, and pipettes, filter-plugged tips, decontamination of benchtops with sodium hypochlorite, etc.). All PCR controls were within the stipulated limits, and no retesting was performed.
RESULTS
Correlation of semiquantitative PCR to microscopy and culture grading.
To establish the relation between microscopy, culture, and PCR, we analyzed retrospectively 1,601 consecutive respiratory specimens from patients with no documented previous TB verified by culture. Inhibition was detected in 44 specimens (2.7%), leaving 1,557 specimens for further evaluation. A total of 1,509 (96.9%) smear- and MTB culture-negative specimens were investigated first to establish a baseline for PCR MTB OD values and MTB/MCC OD ratios. For MTB OD values, the 0, 5, 50, 95, and 100% percentiles were 0.000, 0.000, 0.004, 0.019, and 0.557, respectively, and two specimens that grew nontuberculous mycobacteria (NTM) had MTB OD values of 0.001 and 0.004, respectively. For MTB/MCC OD ratios, the 0, 5, 50, 95, and 100% percentiles were 0.000, 0.000, 0.001, 0.007, and 0.186, respectively. The specimens that grew NTM had MTB/MCC OD ratios of 0.001 or less.
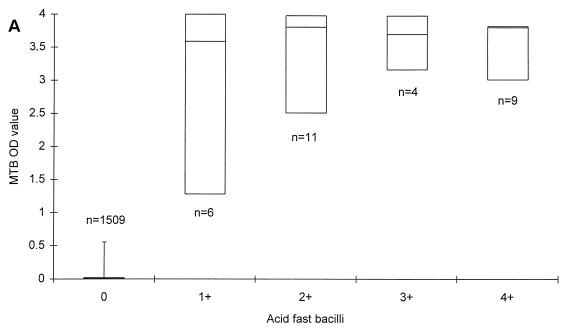
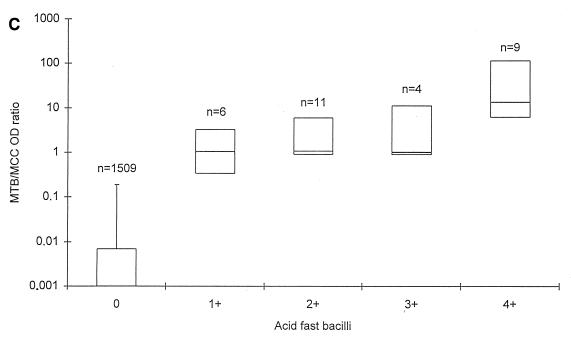
AFB were detected in 32 specimens (2.1%), of which 2 specimens did not grow MTB in culture. Of the remaining 30 specimens, the median MTB OD values were 3.588, 3.807, 3.697, and 3.803 in specimens with 1+, 2+, 3+, and 4+ AFB, respectively (Fig. 1A). The median MTB/MCC OD ratios were 1.041, 1.062, 1.016, and 13.648 in specimens with 1+, 2+, 3+, and 4+ AFB, respectively (Fig. 1C).
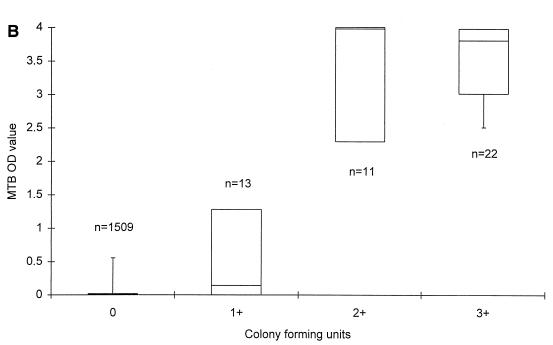
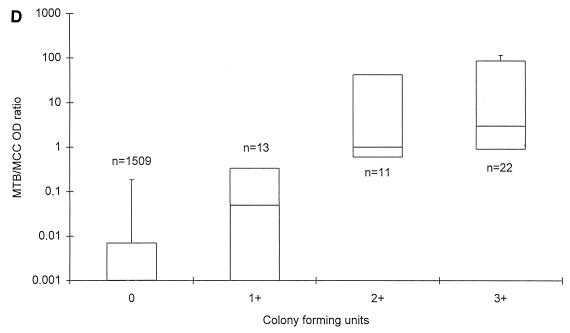
FIG. 1.
MTB OD values (A and B) and MTB/MCC OD ratios (C and D) in relation to microscopy (A and C) and MTB culture grading (B and D) on respiratory specimens from patients with no documentation of previous culture-proven TB. The lines within the boxes represent the median values, and the bars represent the 5th and 95th percentiles.
MTB was recovered by culture from 46 specimens (3.0%). As shown in Fig. 1B, median MTB OD values were 0.140, 3.977, and 3.805 in specimens with CFU 1+, CFU 2+, and CFU 3+, respectively. The median MTB/MCC OD ratios were 0.049, 1.000, and 2.981 in specimens growing CFU 1+, CFU 2+, and CFU 3+, respectively (Fig. 1D).
Statistical analysis on MTB culture-positive specimens demonstrated a better correlation between the MTB/MCC OD ratio to both smear (rs = 0.8452; P = 0.0011) and culture (rs = 0.7993; P = 0.045) than the MTB OD values alone (rs = 0.5206 for smear and rs = 0.6235 for culture) at a 5% significance level. For the 1,557 specimens as a whole, the correlation was also significantly better. Without any retesting of the 1,557 respiratory specimens, a sensitivity of 82.6% (100% in smear-positive and 50% in smear-negative specimens) and a specificity of 99.8% was achieved with this PCR when culture was regarded as the gold standard.
Evaluation of PCR on specimens obtained during treatment and follow-up.
A total of 172 respiratory specimens obtained during antituberculous treatment and follow-up of 22 smear-positive pulmonary TB patients were available for evaluation by microscopy, culture, and PCR. An average of 7.8 specimens (range, 3 to 11) per patient was investigated. From the initial X-ray investigation, the severity of disease was estimated to be moderate in 9 patients and extensive in 13 patients. All but one patient were considered compliant clinically. One patient did not take the antituberculous drugs for the first 6 months and remained smear and culture positive. After 6 months TB medication was taken regularly. PCR results presented from this patient are restricted to the treatment period only.
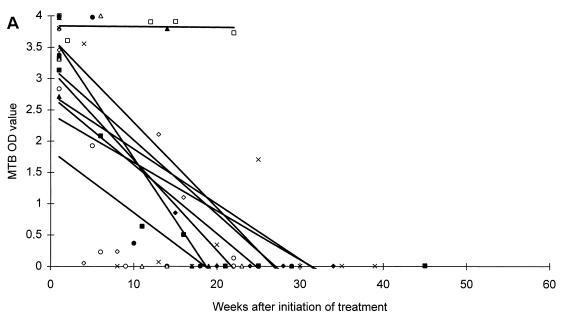
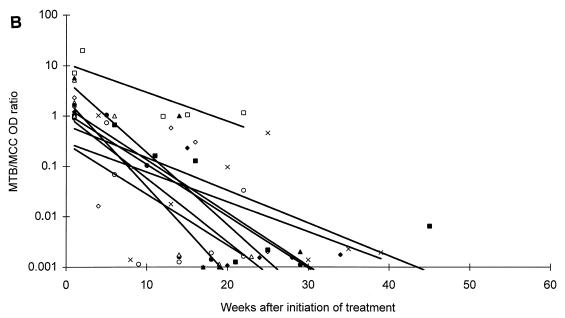
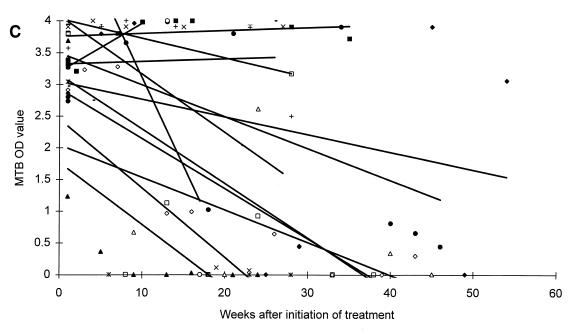
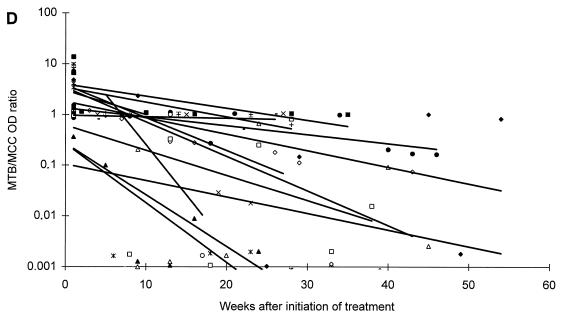
All patients responded microbiologically to treatment as they had either a continuous decrease in CFU or a negative culture result within 2 months of therapy. After 2 and 6 months of antituberculous treatment, one or more specimens were positive by microscopy in 1 of 9 (11.1%) and 0 of 7 (0%) patients, by culture in 1 of 9 (11.1%) and 0 of 7 (0%) patients, and by PCR in 7 of 9 (77.8%) and 0 of 6 (0%) patients with moderate disease, respectively. Similar values for patients with extensive disease were: microscopy positive in 6 of 13 (46.2%) and 1 of 10 (10.0%) patients, culture positive in 0 of 13 (0%) and 0 of 10 (0%) patients, and PCR positive in 11 of 13 (84.6%) and 8 of 10 (80.0%) patients. The changes in MTB OD values during the course of antituberculous treatment are presented in Fig. 2. Regression lines established from the MTB OD values showed a decrease for 19 patients, whereas 3 patients showed an increase during treatment. However, regression lines established from the MTB/MCC OD ratios showed a decrease for all patients (Fig. 2).
FIG. 2.
Changes in MTB OD values (A and C) and MTB/MCC OD ratios (B and D) during antituberculous treatment and follow-up of patients with moderate (A and B) or extensive (C and D) disease. Regression lines for individual patients are inserted.
A difference in the duration time until the patients reached the baseline was also observed. Six of nine (66.7%) patients suffering from moderate disease and three of thirteen (23.1%) patients suffering from extensive disease reached baseline levels at the end of a standard 6 to 8 month of antituberculous treatment regimen, whereas ten of thirteen (76.9%) patients suffering from extensive disease could not be predicted to reach the baseline until 1 year or more after initiation of treatment (Fig. 2).
One patient with moderate disease has suffered a relapse 12 months after termination of the first course of antituberculous chemotherapy. This patient had a PCR-positive specimen with an MTB OD value of 1.704 and an MTB/MCC OD ratio of 0.457 25 weeks after the initiation of antituberculous treatment. Isolates from the first and second episode of TB have shown identical patterns on restriction fragment length polymorphism analysis (23).
DISCUSSION
The aims of the present study were to evaluate PCR results in serial specimens from adult patients with smear-positive pulmonary TB undergoing antituberculous treatment and to assess whether PCR is applicable for monitoring the response to treatment. To pursue this aim, we first established a baseline for MTB OD values and for MTB/MCC OD ratios on smear- and culture-negative respiratory specimens from patients with no record of previous culture proven TB. First, we found that the MTB OD values and the MTB/MCC OD ratios were 0.019 and 0.007 or less in 95% of the specimens, thereby establishing a baseline for those patients, negative by microscopy and culture, and with no previous documentation of TB. Second, we found that the MTB/MCC OD ratio increased with increasing numbers of AFB and CFU, whereas the MTB reached a maximum level in AFB 2+, 3+, and 4+ and CFU 2+ and 3+. MTB/MCC OD ratios correlated better with both microscopy and culture results than the MTB OD values alone, suggesting that this application of PCR is semiquantitative. Thus, the MTB/MCC OD ratio reflects the actual amount of target DNA present in the lysed specimen better than the MTB OD value, probably because annealing of primers to the plasmid carrying the inhibition control site and to potential target DNA takes place in a competitive manner (19). These results are equivalent to results obtained by Afghani et al. by using an in-house competitive PCR targeting IS6110 of MTB (1).
Our results show that half of the patients evaluated 6 months after the initiation of antituberculous treatment had at least one PCR-positive specimen. This finding is in accordance with a report from Hellyer et al. (9), who found 16 of 31 patients to be positive with both qualitative strand displacement amplification and PCR at more than 180 days after initiation of antituberculous treatment. In contrast, this finding conflicts with an earlier report based on IS6110 in-house PCR showing that 1 of 14 patients had PCR-positive specimens after 6 months (12), which may, however, be explained by differences in methodology or patient inclusion criteria. Our study demonstrates also that PCR remains positive much longer in patients suffering from extensive disease than in patients with less-extensive disease. This result supports the findings of Telzak et al., who demonstrated that cavitary disease and large numbers of AFB on smear examination were independent predictors of a longer time to sputum smear and culture conversion (22). In addition, Iinuma et al. reported that the presence of cavitary disease or smear-positive specimens before treatment was significantly associated with a prolonged positivity with a qualitative PCR assay (Amplicor Microwell Plate; Roche) (10).
For all 22 patients, the lines of regression showed a decline in MTB/MCC OD ratios during treatment. Most patients suffering from moderate disease reached baseline levels at the end of a standard 6 to 8 months of antituberculous treatment regimen, whereas most patients suffering from extensive disease were not predicted to reach the baseline until 1 year or more after initiation of treatment. The use of quantitative amplification assays for monitoring treatment of tuberculosis has also been investigated by Desjardin et al. (7) in a study based on the detection of the multicopy element IS6110 in specimens from two patients suffering from cavitary TB. They found that the amount of IS6110 DNA decreased by less than 100-fold during the first 2 months in one patient and by 10- to 100-fold during the first month in another patient during treatment. These results seem similar to the ones obtained during the initial phase of chemotherapy in our study. A continued follow-up of these patients may reveal if results equivalent to ours may be obtained during the late phase of therapy and follow-up.
In 1994, Kennedy et al. described the early detection by IS6110 based PCR in two of four relapse cases (11), and subsequently Scarpellini et al. reported continued PCR-positive results on cerebrospinal fluid from three AIDS patients, who died of disseminated TB (20). Since all patients included in our study responded microbiologically during the course of chemotherapy and were considered compliant, this study suggests that a decline in the MTB/MCC OD ratios should be expected during treatment and that an increase or a slow reduction in the MTB/MCC OD ratio might identify patients at risk of treatment failure due to nonoptimal treatment regimen, reduced bioavailability, noncompliance, or resistance.
Whether MTB/MCC OD ratios above the baseline at the end of treatment is caused by dead or viable bacteria is uncertain from this investigation. As the analytical sensitivity of culture of decontaminated specimens is probably more than 500 bacteria/ml (17, 24), a negative culture result does not exclude the presence of viable M. tuberculosis in clinical specimens. Immunocompetent patients might cope with a few remaining viable bacteria after chemotherapy, while other patients (e.g., AIDS patients or children) could suffer a relapse. A continued follow-up of patients who did not reach the baseline at the end of treatment might reveal whether the MTB/MCC OD ratios can be used to identify patients who would benefit from extended chemotherapy to further reduce the number of bacteria. One patient with moderate disease and a PCR result above the baseline just before the end of therapy did relapse 12 months after the therapy had been completed. At this point, however, the data are too scanty to make general recommendations regarding the duration of either the initial or the continuation phase of antituberculous treatment in the TB population as a whole, and monitoring of standard short-course treatment by culture for MTB is still the established practice.
We regard this as a preliminary study, since we had access to only a few patients and the follow-up to estimate relapse rates was too short.
In conclusion, this study confirms the observation of others that PCR may remain positive for more than 1 year after initiation of antituberculous treatment in patients suffering from severe pulmonary TB. The use of PCR in a semiquantitative manner correlated better with both smear and culture results than the use of a target PCR alone, and the MTB/MCC OD ratio decreased during treatment and follow-up of compliant patients. Although PCR detects both DNA from dead and live bacteria, we believe that PCR can be used to monitor the efficacy of antituberculous treatment because an increase or a slow reduction in the MTB/MCC OD ratio would indicate nonoptimal treatment regimens, reduced bioavailability, noncompliance, or resistant strains of MTB and thereby identify patients at risk of treatment failure or reactivation. Prolonged follow-up of patients with MTB/MCC OD ratios above the baseline after 6 months of antituberculous treatment and further studies, including studies with smear-negative pulmonary and extrapulmonary patients, children, and AIDS patients, are desirable.
ACKNOWLEDGMENTS
First, we acknowledge the patients who participated in this study; without their cooperation the study would not have been possible. We are also grateful to S. Olesen-Larsen from Statens Serum Institut who assisted in the statistical analysis of preliminary data.
Roche Diagnostics paid the fee to have this study evaluated by The Scientific and Ethical Committee of Copenhagen. V. Ø. Thomsen was supported by The Danish Research Academy.
REFERENCES
- 1.Afghani B, Lieberman J M, Duke M B, Stutman H R. Comparison of quantitative polymerase chain reaction, acid-fast bacilli smear, and culture results in patients receiving therapy for pulmonary tuberculosis. Diagn Microbiol Infect Dis. 1998;29:73–79. doi: 10.1016/s0732-8893(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 2.Albert R K, Iseman M D, Sbarbaro J A, Stage A, Pierson D J. Monitoring patients with tuberculosis for failure during and after treatment. Am Rev Respir Dis. 1976;114:1051–1060. doi: 10.1164/arrd.1976.114.6.1051. [DOI] [PubMed] [Google Scholar]
- 3.Bennedsen J, Thomsen V Ø, Pfyffer G E, Funke G, Feldmann K, Beneke A, Jenkins P A, Hegginbothom M, Fahr A, Hengstler M, Cleator G, Klapper P, Wilkins E G L. Utility of PCR in diagnosing pulmonary tuberculosis. J Clin Microbiol. 1996;34:1407–1411. doi: 10.1128/jcm.34.6.1407-1411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan S L. Chemotherapy of tuberculosis. In: Davies P D O, editor. Clinical tuberculosis. London, England: Chapman & Hall Medical; 1994. pp. 141–56. [Google Scholar]
- 5.Clarridge J E, III, Shawar R M, Shinnick T M, Plikaytis B B. Large-scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in a routine mycobacteriology laboratory. J Clin Microbiol. 1993;31:2049–2056. doi: 10.1128/jcm.31.8.2049-2056.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David H L. Bacteriology of mycobacteriosis. Washington, D.C: Government Printing Office; 1976. [Google Scholar]
- 7.Desjardin L E, Chen Y, Perkins M D, Teixeira L, Cave M D, Eisenach K D. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J Clin Microbiol. 1998;36:1964–1968. doi: 10.1128/jcm.36.7.1964-1968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein M D, Schluger N W, Davidow A L, Bonk S, Rom W N, Hanna B. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest. 1998;113:379–386. doi: 10.1378/chest.113.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Hellyer T J, Fletcher T W, Bates J H, Stead W W, Templeton G L, Cave M D, Eisenach K D. Strand displacement amplification and the polymerase chain reaction for monitoring response to treatment in patients with pulmonary tuberculosis. J Infect Dis. 1996;173:934–941. doi: 10.1093/infdis/173.4.934. [DOI] [PubMed] [Google Scholar]
- 10.Iinuma Y, Ichiyama S, Yamori S, Oohama J, Takagi N, Hasegawa Y, Shimokata K, Nakashima N. Diagnostic value of the Amplicor PCR assay for initial diagnosis and assessment of treatment response for pulmonary tuberculosis. Microbiol Immunol. 1998;42:281–287. doi: 10.1111/j.1348-0421.1998.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy N, Gillespie S H, Saruni A O S, Kisyombe G, McNerney R, Ngowi F I, Wilson S. Polymerase chain reaction for assessing treatment response in patients with pulmonary tuberculosis. J Infect Dis. 1994;170:713–716. doi: 10.1093/infdis/170.3.713. [DOI] [PubMed] [Google Scholar]
- 12.Leveé G, Glaziou P, Gicquel B, Chanteau S. Follow-up of tuberculosis patients undergoing standard anti-tuberculosis chemotherapy by using a polymerase chain reaction. Res Microbiol. 1994;145:5–8. doi: 10.1016/0923-2508(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 13.Manalo F, Tan F, Sbarbaro J A, Iseman M D. Community-based short-course treatment of pulmonary tuberculosis in a developing nation. Am Rev Respir Dis. 1990;142:1301–1305. doi: 10.1164/ajrccm/142.6_Pt_1.1301. [DOI] [PubMed] [Google Scholar]
- 14.Nunn P, Porter J, Githui W, Odhiambo J. Treating tuberculosis in HIV-positive Africans. Lancet. 1991;338:1141. [PubMed] [Google Scholar]
- 15.Ormerod L P, Prescott R J. Inter-relation between relapses, drug regimens and compliance with treatment in tuberculosis. Respir Med. 1991;85:239–242. doi: 10.1016/s0954-6111(06)80087-9. [DOI] [PubMed] [Google Scholar]
- 16.Perriëns J H, Colebunders R L, Karahunga C, Willame J-C, Jeugmans J, Kaboto M, Mukadi Y, Pauwels P, Ryder R W, Prignot J, Piot P. Increased mortality and tuberculosis treatment failure rate among human immunodeficiency virus (HIV) seropositive compared with HIV seronegative patients with pulmonary tuberculosis treated with “standard” chemotherapy in Kinshasa, Zaire. Am Rev Respir Dis. 1991;144:750–756. doi: 10.1164/ajrccm/144.4.750. [DOI] [PubMed] [Google Scholar]
- 17.Pfyffer G E, Kissling P, Jahn E M I, Welscher H-M, Salfinger M, Weber R. Diagnostic performance of amplified Mycobacterium tuberculosis direct test with cerebrospinal fluid, other nonrespiratory, and respiratory specimens. J Clin Microbiol. 1996;34:834–841. doi: 10.1128/jcm.34.4.834-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert R, Sokal F, Rohlf J. Significance tests in correlation. In: Sokal R R, editor. Biometry. W. H. New York, N.Y: Freeman and Co.; 1981. p. 583. [Google Scholar]
- 19.Rosenstraus M, Wang Z, Chang S-Y, de Bonville D, Spadoro J P. An internal control for routine diagnostic PCR: design, properties, and effect on clinical performance. J Clin Microbiol. 1998;36:191–197. doi: 10.1128/jcm.36.1.191-197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpellini P, Racca S, Cinque P, Delfanti F, Gianotti N, Terreni M R, Vago L, Lazzarin A. Nested polymerase chain reaction for diagnosis and monitoring treatment response in AIDS patients with tuberculous meningitis. AIDS. 1995;9:895–900. doi: 10.1097/00002030-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Smithwick R W. Laboratory manual for acid-fast microscopy. Atlanta, Ga: Centers for Disease Control (U.S. Department of Health, Education, and Welfare); 1976. [Google Scholar]
- 22.Telzak E E, Fazal B A, Pollard C L, Turett G S, Justman J E, Blum S. Factors influencing time to sputum conversion among patients with smear-positive pulmonary tuberculosis. Clin Infect Dis. 1997;25:666–670. doi: 10.1086/513772. [DOI] [PubMed] [Google Scholar]
- 23.van Embden J D, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yajko D M, Wagner C, Tevere V J, Kocagoz T, Hadley W K, Chambers H F. Quantitative culture of Mycobacterium tuberculosis from clinical sputum specimens and dilution endpoint of its detection by the Amplicor PCR assay. J Clin Microbiol. 1995;33:1944–1947. doi: 10.1128/jcm.33.7.1944-1947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuen K Y, Chan K S, Chan C M, Ho B S W, Dai L K, Chau P Y, Ng M H. Use of PCR in routine diagnosis of treated and untreated pulmonary tuberculosis. J Clin Pathol. 1993;46:318–322. doi: 10.1136/jcp.46.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]