Abstract
Background
Intrauterine insemination (IUI), combined with ovarian stimulation (OS), has been demonstrated to be an effective treatment for infertile couples. Several agents for ovarian stimulation, combined with IUI, have been proposed, but it is still not clear which agents for stimulation are the most effective. This is an update of the review, first published in 2007.
Objectives
To assess the effects of agents for ovarian stimulation for intrauterine insemination in infertile ovulatory women.
Search methods
We searched the Cochrane Gynaecology and Fertility Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL and two trial registers from their inception to November 2020. We performed reference checking and contacted study authors and experts in the field to identify additional studies.
Selection criteria
We included truly randomised controlled trials (RCTs) that compared different agents for ovarian stimulation combined with IUI for infertile ovulatory women concerning couples with unexplained infertility. mild male factor infertility and minimal to mild endometriosis.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane.
Main results
In this updated review, we have included a total of 82 studies, involving 12,614 women. Due to the multitude of comparisons between different agents for ovarian stimulation, we highlight the seven most often reported here.
Gonadotropins versus anti‐oestrogens (13 studies)
For live birth, the results of five studies were pooled and showed a probable improvement in the cumulative live birth rate for gonadotropins compared to anti‐oestrogens (odds ratio (OR) 1.37, 95% confidence interval (CI) 1.05 to 1.79; I2 = 30%; 5 studies, 1924 participants; moderate‐certainty evidence). This suggests that if the chance of live birth following anti‐oestrogens is assumed to be 22.8%, the chance following gonadotropins would be between 23.7% and 34.6%. The pooled effect of seven studies revealed that we are uncertain whether gonadotropins lead to a higher multiple pregnancy rate compared with anti‐oestrogens (OR 1.58, 95% CI 0.60 to 4.17; I2 = 58%; 7 studies, 2139 participants; low‐certainty evidence).
Aromatase inhibitors versus anti‐oestrogens (8 studies)
One study reported live birth rates for this comparison. We are uncertain whether aromatase inhibitors improve live birth rate compared with anti‐oestrogens (OR 0.75, CI 95% 0.51 to 1.11; 1 study, 599 participants; low‐certainty evidence). This suggests that if the chance of live birth following anti‐oestrogens is 23.4%, the chance following aromatase inhibitors would be between 13.5% and 25.3%. The results of pooling four studies revealed that we are uncertain whether aromatase inhibitors compared with anti‐oestrogens lead to a higher multiple pregnancy rate (OR 1.28, CI 95% 0.61 to 2.68; I2 = 0%; 4 studies, 1000 participants; low‐certainty evidence).
Gonadotropins with GnRH (gonadotropin‐releasing hormone) agonist versus gonadotropins alone (4 studies)
No data were available for live birth. The pooled effect of two studies revealed that we are uncertain whether gonadotropins with GnRH agonist lead to a higher multiple pregnancy rate compared to gonadotropins alone (OR 2.53, 95% CI 0.82 to 7.86; I2 = 0; 2 studies, 264 participants; very low‐certainty evidence).
Gonadotropins with GnRH antagonist versus gonadotropins alone (14 studies)
Three studies reported live birth rate per couple, and we are uncertain whether gonadotropins with GnRH antagonist improve live birth rate compared to gonadotropins (OR 1.5, 95% CI 0.52 to 4.39; I2 = 81%; 3 studies, 419 participants; very low‐certainty evidence). This suggests that if the chance of a live birth following gonadotropins alone is 25.7%, the chance following gonadotropins combined with GnRH antagonist would be between 15.2% and 60.3%. We are also uncertain whether gonadotropins combined with GnRH antagonist lead to a higher multiple pregnancy rate compared with gonadotropins alone (OR 1.30, 95% CI 0.74 to 2.28; I2 = 0%; 10 studies, 2095 participants; moderate‐certainty evidence).
Gonadotropins with anti‐oestrogens versus gonadotropins alone (2 studies)
Neither of the studies reported data for live birth rate. We are uncertain whether gonadotropins combined with anti‐oestrogens lead to a higher multiple pregnancy rate compared with gonadotropins alone, based on one study (OR 3.03, 95% CI 0.12 to 75.1; 1 study, 230 participants; low‐certainty evidence).
Aromatase inhibitors versus gonadotropins (6 studies)
Two studies revealed that aromatase inhibitors may decrease live birth rate compared with gonadotropins (OR 0.49, 95% CI 0.34 to 0.71; I2=0%; 2 studies, 651 participants; low‐certainty evidence). This suggests that if the chance of a live birth following gonadotropins alone is 31.9%, the chance of live birth following aromatase inhibitors would be between 13.7% and 25%. We are uncertain whether aromatase inhibitors compared with gonadotropins lead to a higher multiple pregnancy rate (OR 0.69, 95% CI 0.06 to 8.17; I2=77%; 3 studies, 731 participants; very low‐certainty evidence).
Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins (8 studies)
We are uncertain whether aromatase inhibitors combined with gonadotropins improve live birth rate compared with anti‐oestrogens plus gonadotropins (OR 0.99, 95% CI 0.3 8 to 2.54; I2 = 69%; 3 studies, 708 participants; very low‐certainty evidence). This suggests that if the chance of a live birth following anti‐oestrogens plus gonadotropins is 13.8%, the chance following aromatase inhibitors plus gonadotropins would be between 5.7% and 28.9%. We are uncertain of the effect of aromatase inhibitors combined with gonadotropins compared to anti‐oestrogens combined with gonadotropins on multiple pregnancy rate (OR 1.31, 95% CI 0.39 to 4.37; I2 = 0%; 5 studies, 901 participants; low‐certainty evidence).
Authors' conclusions
Based on the available results, gonadotropins probably improve cumulative live birth rate compared with anti‐oestrogens (moderate‐certainty evidence). Gonadotropins may also improve cumulative live birth rate when compared with aromatase inhibitors (low‐certainty evidence). From the available data, there is no convincing evidence that aromatase inhibitors lead to higher live birth rates compared to anti‐oestrogens. None of the agents compared lead to significantly higher multiple pregnancy rates. Based on low‐certainty evidence, there does not seem to be a role for different combined therapies, nor for adding GnRH agonists or GnRH antagonists in IUI programs.
Plain language summary
Which medicines are most effective for women trying to get pregnant by ‘intrauterine insemination’ (where sperm is placed directly in the womb)?
Key messages
‐ ‘Fertility medicines’ are the range of hormones and medicines used to help women get pregnant. This review shows that, in a comparison of two widely‐used fertility medicines – gonadotropins and anti‐oestrogens – gonadotropins probably increase the number of live births.
‐ We have little to no confidence in the evidence comparing the effectiveness of other fertility medicines, both for live birth and multiple pregnancy (expecting more than one baby) rates.
‐ To improve the evidence, future studies of intrauterine insemination (where sperm is placed directly in the womb) should compare fertility medicines with a placebo (dummy drug). More studies comparing anti‐oestrogens with aromatase inhibitors (another widely‐used fertility drug) are also needed.
What is infertility?
Infertility is when a woman is unable to get pregnant after 1 year (or longer) of regular, unprotected sex. Sometimes, doctors distinguish between older and younger women, since natural fertility declines with age. Some providers treat women aged 35 years or older for infertility after 6 months of unprotected sex.
How is infertility treated?
Treatment for infertility depends on what may be causing it. Our review focused on intrauterine insemination combined with various fertility medicines. Fertility medicines work by causing the release of hormones that prompt ovulation – that is, the release of an egg from the ovary. Intrauterine insemination is where sperm is placed directly into the uterus (womb) using a thin, flexible plastic tube inserted through the vagina and cervix.
There are many different fertility medicines. The ones most commonly prescribed include:
‐ gonadotropins (injectable hormones started early in the menstrual cycle to cause multiple eggs to grow to a mature size);
‐ anti‐oestrogens and aromatase inhibitors (oral medicines used to trigger ovulation);
‐ gonadotropin‐releasing hormone (GnRH) agonists and antagonists (medicines used to regulate egg development and ovulation).
What did we want to find out?
We wanted to find out which fertility medicines, combined with intrauterine insemination, are most effective, for women who release an egg during menstruation (ovulatory women).
We were interested in the effects of fertility medicines on:
‐ live births;
‐ multiple pregnancies;
‐ ‘clinical pregnancy’ (defined as evidence of a gestational sac, the fluid‐filled structure around a foetus, with a positive heartbeat);
‐ miscarriages (defined as loss of pregnancy during the first 12 weeks);
‐ ovarian hyperstimulation syndrome (OHSS, a condition where excess hormones can overstimulate the ovaries, leading to various complications); and
‐ ectopic pregnancy, defined as a pregnancy outside the womb.
What did we do?
We searched for studies that compared different fertility medicines for ovulatory women having intrauterine insemination.
We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 82 studies, involving 12,614 women. The women ranged from 18 to 44 years old. Slightly more than two‐thirds (57) of the studies included women or couples with unexplained infertility (the lack of an obvious cause of infertility), male infertility, endometriosis (a painful condition when tissue similar to the lining of the uterus grows outside the uterus), or more than one of these factors.
The studies were conducted in 17 countries around the world, with more than half conducted in India, Iran, Italy, Spain and the USA.
There were more than 20 different comparisons between the various fertility medicines.
Only around one‐fifth (17) of studies reported information about live birth rates.
Main results
Gonadotropins compared to anti‐oestrogens (13 studies): probably increase the chance of live birth. If the chance of a live birth following anti‐oestrogens is assumed to be 22.8%, the chance following gonadotropins would be between 23.7% and 34.6%.
We don’t know if gonadotropins make any difference to multiple pregnancy rate.
Aromatase inhibitors versus anti‐oestrogens (8 studies)
We don’t know if aromatise inhibitors make any difference to:
· live birth rate; or
· multiple pregnancy rate.
Gonadotropins plus GnRH antagonists versus gonadotropins alone (14 studies)
We don’t know if gonadotropins plus GnRH antagonists make any difference to:
· live birth rate; or
· multiple pregnancy rate.
Aromatase inhibitors versus gonadotropins (6 studies): may decrease the chance of a live birth. If the chance of a live birth following gonadotropins is assumed to be 31.9%, the chance following aromatase inhibitors would be between 13.7% and 25%.
We don’t know if aromatase inhibitors make any difference to multiple pregnancy rate.
Aromatase inhibitors plus gonadotropins versus anti‐oestrogens plus gonadotropins (8 studies):
We don’t know if aromatase inhibitors plus gonadotropins make any difference to:
· live birth rate; or
· multiple pregnancy rate.
What are the limitations of the evidence?
Our confidence in the evidence ranged from very low to moderate. More than three‐quarters of the studies had weaknesses in their methods that could affect the reliability of their results, and many of the studies were small.
How up to date is this evidence?
The evidence is up to date to November 2020.
Summary of findings
Summary of findings 1. Gonadotropins versus anti‐oestrogens for intrauterine insemination in women with infertility.
| Gonadotropins versus anti‐oestrogens for intrauterine insemination in women with infertility | ||||||
| Patient or population: intrauterine insemination (IUI) in women with infertility Setting: infertility clinics and university hospitals Intervention: gonadotropins Comparison: anti‐oestrogens | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with anti‐oestrogens | Risk with gonadotropins | |||||
| Live birth rate per couple ‐ all types of infertility | Study population | OR 1.37 (1.05 to 1.79) | 1924 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 228 per 1000 | 289 per 1000 (237 to 346) | |||||
| Multiple pregnancy rate per couple | Study population | OR 1.58 (0.60 to 4.17) | 2139 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 22 per 1000 | 35 per 1000 (14 to 87) | |||||
| Clinical pregnancy rate per couple | Study population | OR 1.34 (1.12 to 1.61) | 2576 (12 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 228 per 1000 | 283 per 1000 (248 to 322) | |||||
| Miscarriage rate per couple | Study population | OR 1.25 (0.88 to 1.77) | 2002 (6 RCTs) | ⊕⊕⊝⊝ Lowc | ||
| 62 per 1000 | 76 per 1000 (55 to 105) | |||||
| OHSS rate per couple | Study population | OR 0.77 (0.19 to 3.14) | 1482 (6 RCTs) | ⊕⊕⊝⊝ Lowd | ||
| 4 per 1000 | 3 per 1000 (1 to 13) | |||||
| Ectopic pregnancy rate per couple | Study population | OR 1.64 (0.67 to 3.98) | 1339 (2 RCTs) | ⊕⊕⊝⊝ Lowd | ||
| 12 per 1000 | 19 per 1000 (8 to 46) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded once for imprecision (total number of events < 300). bWe downgraded once for inconsistency (I2 = 54%). cWe downgraded twice for imprecision (total number of events < 300 and 4/5 were small studies, leading to more uncertainty). dWe downgraded twice for imprecision (total number of events < 300 and wide confidence intervals, leading to more uncertainty).
Summary of findings 2. Aromatase inhibitors versus anti‐oestrogens for intrauterine insemination in women with infertility.
| Aromatase inhibitors versus anti‐oestrogens for intrauterine insemination in women with infertility | ||||||
| Patient or population: intrauterine insemination (IUI) in women with infertility Setting: infertility clinics and university hospitals Intervention: aromatase inhibitors Comparison: anti‐oestrogens | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with anti‐oestrogens | Risk with aromatase inhibitors | |||||
| Live birth rate per couple | Study population | OR 0.75 (0.51 to 1.11) | 599 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 234 per 1000 | 187 per 1000 (135 to 253) | |||||
| Multiple pregnancy rate per couple | Study population | OR 1.28 (0.61 to 2.68) | 1000 (4 RCTs) | ⊕⊕⊝⊝ Lowb | ||
| 24 per 1000 | 30 per 1000 (15 to 61) | |||||
| Clinical pregnancy rate per couple | Study population | OR 1.21 (0.75 to 1.94) | 1160 (8 RCTs) | ⊕⊕⊝⊝ Lowc,d | ||
| 231 per 1000 | 267 per 1000 (184 to 368) | |||||
| Miscarriage rate per couple | Study population | OR 0.91 (0.47 to 1.77) | 967 (3 RCTs) | ⊕⊕⊝⊝ Lowb | ||
| 60 per 1000 | 54 per 1000 (29 to 101) | |||||
| OHSS rate per couple | Study population | ‐ | 813 (2 RCTs) | ⊕⊕⊕⊝ Moderated | No events reported | |
| See comment | See comment | |||||
| Ectopic pregnancy rate per couple | Study population | OR 1.00 (0.29 to 3.50) | 813 (2 RCTs) | ⊕⊕⊝⊝ Lowb | ||
| 12 per 1000 | 12 per 1000 (4 to 42) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded twice for imprecision (total number of events < 300 and only 1 study (lack of external validation)). bWe downgraded twice for imprecision (total number of events < 300 and wide confidence intervals, leading to more uncertainty). cWe downgraded once for inconsistency (I2 = 40%). dWe downgraded once for imprecision (total number of events < 300).
Summary of findings 3. Gonadotropins with GnRH agonists versus gonadotropins alone for intrauterine insemination in women with infertility.
| Gonadotropins with GnRH agonists versus gonadotropins alone for intrauterine insemination in women with infertility | ||||||
| Patient or population: intrauterine insemination (IUI) in women with infertility Setting: university hospitals Intervention: gonadotropins with GnRH agonists Comparison: gonadotropins alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with gonadotropins alone | Risk with gonadotropins with GnRH agonists | |||||
| Multiple pregnancy rate per couple | Study population | OR 2.53 (0.82 to 7.86) | 264 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | ||
| 30 per 1000 | 72 per 1000 (25 to 195) | |||||
| Clinical pregnancy rate per couple | Study population | OR 0.55 (0.32 to 0.95) | 355 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c | ||
| 239 per 1000 | 147 per 1000 (91 to 230) | |||||
| Miscarriage rate per couple | Study population | OR 2.07 (0.18 to 24.15) | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowb,d | ||
| 33 per 1000 | 67 per 1000 (6 to 454) | |||||
| OHSS rate per couple | Study population | OR 1.80 (0.39 to 8.32) | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowb,d | ||
| 100 per 1000 | 167 per 1000 (42 to 480) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded twice for risk of bias (crucial risk of bias for one or multiple criteria, likely to very seriously alter the results). bWe downgraded twice for imprecision (total number of events < 300 and wide confidence intervals, leading to more uncertainty). cWe downgraded once for imprecision (total number of events < 300). dWe downgraded once for risk of bias (plausible risk of bias, likely to seriously alter the results).
Summary of findings 4. Gonadotropins with GnRH antagonist versus gonadotropins alone for intrauterine insemination in women with infertility.
| Gonadotropins with GnRH antagonist versus gonadotropins alone for intrauterine insemination in women with infertility | ||||||
| Patient or population: intrauterine insemination (IUI) in women with infertility Setting: infertility clinics and university hospitals Intervention: gonadotropins with GnRH antagonist Comparison: gonadotropins alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with gonadotropins alone | Risk with gonadotropins with GnRH antagonist | |||||
| Live birth rate per couple | Study population | OR 1.50 (0.52 to 4.39) | 419 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | ||
| 257 per 1000 | 342 per 1000 (152 to 603) | |||||
| Multiple pregnancy rate per couple | Study population | OR 1.30 (0.74 to 2.28) | 2095 (10 RCTs) | ⊕⊕⊕⊝ Moderatec | ||
| 20 per 1000 | 26 per 1000 (15 to 44) | |||||
| Clinical pregnancy rate per couple | Study population | OR 1.35 (1.00 to 1.84) | 2525 (14 RCTs) | ⊕⊕⊝⊝ Lowc,d | ||
| 180 per 1000 | 229 per 1000 (180 to 288) | |||||
| Miscarriage rate per couple | Study population | OR 1.37 (0.84 to 2.22) | 2054 (9 RCTs) | ⊕⊕⊝⊝ Lowb | ||
| 29 per 1000 | 39 per 1000 (25 to 62) | |||||
| OHSS rate per couple | Study population | OR 0.98 (0.12 to 8.32) | 1348 (5 RCTs) | ⊕⊝⊝⊝ Very lowe,f | ||
| 50 per 1000 | 49 per 1000 (6 to 305) | |||||
| Ectopic pregnancy rate per couple | Study population | OR 0.33 (0.01 to 8.23) | 407 (1 RCT) | ⊕⊕⊝⊝ Lowf | ||
| 5 per 1000 | 2 per 1000 (0 to 39) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded twice for inconsistency (I2 = 81%). bWe downgraded twice for imprecision (total number of events < 300 and wide confidence intervals, leading to more uncertainty). cWe downgraded once for imprecision (total number of events < 300). dWe downgraded once for inconsistency (I2 = 47%). eWe downgraded once for inconsistency (I2 = 56%). fWe downgraded twice for imprecision (total number of events < 300 and 1 study with very wide confidence interval, leading to more uncertainty).
Summary of findings 5. Gonadotrophins with anti‐oestrogens versus gonadotropins alone for intrauterine insemination in women with infertility.
| Gonadotropins with anti‐oestrogens versus gonadotropins alone for intrauterine insemination in women with infertility | ||||||
| Patient or population: intrauterine insemination (IUI) in women with infertility Setting: infertility clinics and university hospitals Intervention: gonadotropins with anti‐oestrogens Comparison: gonadotropins alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with gonadotropins alone | Risk with gonadotropins with anti‐oestrogens | |||||
| Multiple pregnancy rate per couple | Study population | OR 3.03 (0.12 to 75.06) | 230 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Clinical pregnancy rate per couple | Study population | OR 1.99 (0.81 to 4.90) | 328 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | ||
| 119 per 1000 | 211 per 1000 (98 to 398) | |||||
| OHSS rate per couple | Study population | OR 0.50 (0.04 to 5.54) | 230 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 17 per 1000 | 9 per 1000 (1 to 89) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded twice for imprecision (total number of events < 300 and wide confidence intervals, leading to more uncertainty). bWe downgraded once for inconsistency (I2 = 50%).
Summary of findings 6. Aromatase inhibitors versus gonadotropins for intrauterine insemination in women with infertility.
| Aromatase inhibitors versus gonadotropins for intrauterine insemination in women with infertility | ||||||
| Patient or population: intrauterine insemination (IUI) in women with infertility Setting: infertility clinics and university hospitals Intervention: aromatase inhibitors Comparison: gonadotropins | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with gonadotropins | Risk with aromatase inhibitors | |||||
| Live birth rate per couple | Study population | OR 0.49 (0.34 to 0.71) | 651 (2 RCTs) | ⊕⊕⊝⊝ Lowa | ||
| 319 per 1000 | 187 per 1000 (137 to 250) | |||||
| Multiple pregnancy rate per couple | Study population | OR 0.69 (0.06 to 8.17) | 731 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,c | ||
| 96 per 1000 | 68 per 1000 (6 to 463) | |||||
| Clinical pregnancy rate per couple | Study population | OR 0.61 (0.46 to 0.82) | 1085 (6 RCTs) | ⊕⊕⊝⊝ Lowd,e | ||
| 274 per 1000 | 187 per 1000 (148 to 237) | |||||
| Miscarriage rate per couple | Study population | OR 0.53 (0.30 to 0.92) | 650 (2 RCTs) | ⊕⊕⊝⊝ Lowc | ||
| 117 per 1000 | 65 per 1000 (38 to 108) | |||||
| OHSS rate per couple | Study population | OR 1.00 (0.14 to 7.20) | 680 (2 RCTs) | ⊕⊕⊝⊝ Lowc | ||
| 3 per 1000 | 3 per 1000 (0 to 21) | |||||
| Ectopic pregnancy per couple | Study population | OR 0.56 (0.21 to 1.48) | 650 (2 RCTs) | ⊕⊕⊝⊝ Lowc | ||
| 34 per 1000 | 19 per 1000 (7 to 49) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded twice for imprecision (total number of events < 300 and 1 small/1 larger study, leading to more uncertainty). bWe downgraded twice for inconsistency (I2 = 77%). cWe downgraded twice for imprecision (total number of events < 300 and wide confidence intervals, leading to more uncertainty). dWe downgraded once for risk of bias (plausible bias likely to seriously alter the results). eWe downgraded once for imprecision (total number of events < 300).
Summary of findings 7. Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins for intrauterine insemination in women with infertility.
| Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins for intrauterine insemination in women with infertility | ||||||
| Patient or population: intrauterine insemination (IUI) in women with infertility Setting: infertility clinics and university hospitals Intervention: aromatase inhibitors with gonadotropins Comparison: anti‐oestrogens with gonadotropins | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with anti‐oestrogens with gonadotropins | Risk with aromatase inhibitors with gonadotropins | |||||
| Live birth rate per couple | Study population | OR 0.99 (0.38 to 2.54) | 708 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| 138 per 1000 | 137 per 1000 (57 to 289) | |||||
| Multiple pregnancy rate per couple | Study population | OR 1.31 (0.39 to 4.37) | 901 (4 RCTs) | ⊕⊕⊝⊝ Lowd | ||
| 10 per 1000 | 13 per 1000 (4 to 42) | |||||
| Clinical pregnancy rate per couple | Study population | OR 0.78 (0.57 to 1.07) | 1244 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,d | ||
| 167 per 1000 | 135 per 1000 (103 to 177) | |||||
| Miscarriage rate per couple | Study population | OR 1.44 (0.75 to 2.77) | 1164 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,d | ||
| 30 per 1000 | 42 per 1000 (22 to 78) | |||||
| OHSS rate per couple | Study population | OR 4.45 (0.75 to 26.43) | 901 (4 RCTs) | ⊕⊕⊝⊝ Lowd | ||
| 2 per 1000 | 11 per 1000 (2 to 62) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded once for risk of bias (plausible bias likely to seriously alter the results). bWe downgraded once for inconsistency (I2 = 69%). cWe downgraded once for imprecision (total number of events < 300). dWe downgraded twice for imprecision (total number of events < 300 and wide confidence intervals, leading to more uncertainty).
Background
Description of the condition
Infertility is estimated to affect as many as 186 million people worldwide. Although male infertility contributes to more than half of all cases of global childlessness, infertility remains a woman's social burden (Inhorn 2015). Infertility is commonly defined as the inability of a couple to conceive after 12 months of regular, unprotected intercourse in women less than 35 years of age, and after six months in women 35 years and older (Practice Committee of the ASRM 2020). The National Health Service (NHS, in the UK) estimates that one in seven couples may have difficulty trying to conceive (NHS 2021). Approximately 15% to 25% of these infertile couples do not show any abnormalities during a routine fertility check‐up (NICE Guidelines 2013). This – the lack of an obvious cause for a couple’s infertility – is defined as ‘unexplained infertility’ (NICE Guidelines 2013). ‘Mild male factor infertility’ is often seen as akin to unexplained infertility. It does not present with a strict definition, but the observation of a single abnormal finding of the semen analysis or a total motile sperm count between 10 and 20 × 106/mL is widely used (Hamilton 2015).
Description of the intervention
Ovarian stimulation (OS) consists of using oral or injectable agents, or both, usually starting from the first days of the menstrual cycle. The cycle is monitored by ultrasound until one or more dominant follicle(s) has developed. The aim of the ovarian stimulation, ideally, is to develop two dominant follicles. When the largest follicle reaches a diameter of greater than 16 mm, ovulation can be induced by human chorionic gonadotropins (hCG), and an intrauterine insemination is performed usually 24 to 48 hours later.
How the intervention might work
Intrauterine insemination (IUI) is often used as first line treatment. The aim of intrauterine insemination with ovarian stimulation (IUI‐OS) is to combine multi‐follicular growth with an accurate timing of insemination with a highly concentrated semen sample. Treatment with IUI‐OS probably results in a higher cumulative live birth rate compared to continuing timed intercourse in couples with a low prediction score of natural conception (Ayeleke 2020). A meta‐analysis by Van Rumste and colleagues showed a significant increase in pregnancy rate when multi‐follicular growth was compared with mono‐follicular growth (odds ratio (OR) 1.6, 99% confidence interval (CI) 1.3 to 2.0) (Van Rumste 2008). However, the chance of multiple gestation also increased. Strict cancellation criteria should be followed to maintain a relatively low incidence of multiple gestation but with the benefit of an increased chance of pregnancy due to multi‐follicular growth. Although in vitro fertilisation (IVF) results in the highest per‐cycle pregnancy rate, it is also the most expensive and invasive treatment (Tjon‐Kon‐Fat 2015). A randomised controlled trial (RCT) conducted by Bensdorp and colleagues showed that live birth rates between three cycles of IVF with single embryo transfer and six cycles of IUI with ovarian stimulation were similar, and therefore IUI‐OS should be advised as the treatment of first choice (Bensdorp 2015).
Various stimulation protocols can be used to obtain multi‐follicular growth. The most commonly used are oral agents, such as anti‐oestrogens (clomiphene citrate (CC)) and aromatase inhibitors (AI), or subcutaneous injections with gonadotropins, such as human menopausal gonadotropin (hMG) or follicle‐stimulating hormone (FSH). Treatment with an anti‐oestrogen, which is a selective E2 receptor‐modulator, is based on an effect on the negative feedback acting as an anti‐oestrogen. The same is true for aromatase inhibitors, such as letrozole, whose effects are due to blocking the formation of oestrogens, resulting in a lack of negative feedback to the pituitary gland. Both gonadotropin‐releasing hormone (GnRH) agonists and GnRH antagonists are known to block the luteinising hormone (LH) trigger, due to desensitisation of the pituitary gland or blocking of the GnRH receptors of the pituitary gland, respectively, preventing premature luteinisation or ovulation, leading to optimal timing of the insemination.
We excluded studies which compared stimulated IUI with IUI in natural cycles, as this is the topic of another Cochrane Review (Ayeleke 2020). The same accounts the number of IUI (Rakic 2021) and timing of the IUI (Cantineau 2014).
Why it is important to do this review
Despite the relatively high prevalence of unexplained and mild male infertility, and the total number of treatment cycles each year worldwide, there is still discussion about the most effective treatment protocol with IUI‐OS. This review was first published in 2007, and many randomised controlled trials have been published since then. This review synthesises and analyses the data emanating from eligible studies to help inform the ongoing discussion about IUI‐OS with the most up‐to‐date evidence.
Objectives
To evaluate agents for ovarian stimulation for intrauterine insemination in infertile ovulatory women.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were considered for inclusion in this review. We included trials with a cross‐over design only if analyses of first‐cycle data were available. We excluded quasi‐RCTs.
Types of participants
Couples who have been trying to conceive for at least one year and for whom OS combined with IUI is a treatment option. This included couples with:
unexplained infertility, defined as no abnormalities found at routine fertility investigations, consisting of cycle analysis, check‐up on fallopian tube patency and semen analysis;
mild male factor infertility, defined as one abnormal semen analysis finding (criteria according to the World Health Organization) or a total motile sperm count between 10 and 20 x 106/mL;
minimal to mild endometriosis, defined as American Society for Reproductive Medicine (ASRM) Grade I or II, diagnosed with laparoscopy.
Types of interventions
IUI combined with different OS drugs or dosages:
gonadotropins versus anti‐oestrogens;
aromatase inhibitors versus anti‐oestrogens;
gonadotropins combined with GnRH agonists versus gonadotropins alone;
gonadotropins combined with GnRH antagonists versus gonadotropins alone;
gonadotropins combined with anti‐oestrogens versus gonadotropins alone;
aromatase inhibitors versus gonadotropins;
aromatase inhibitors combined with gonadotropins versus anti‐oestrogens combined with gonadotropins;
gonadotropins combined with GnRH antagonists versus anti‐oestrogens;
different types of gonadotropins (urinary gonadotropins versus recombinant gonadotropins);
different dosage regimens for anti‐oestrogens or aromatase inhibitors;
different dosage regimens for gonadotropins;
other comparisons.
Types of outcome measures
Primary outcomes
Live birth rate per couple. Live birth was defined as the birth of a child after 24 weeks of gestation showing any sign of life.
Multiple pregnancy per couple, defined as at least two registered heartbeats on ultrasound.
Secondary outcomes
Clinical pregnancy rate per couple. Clinical pregnancy is defined as evidence of a gestational sac with a positive heartbeat.
Miscarriage, defined as a pregnancy loss before 12 weeks of gestation, per couple.
Ovarian hyperstimulation syndrome (OHSS), characterised by cystic enlargement of the ovaries and a fluid shift from the intravascular to the third space due to the increased capillary permeability and ovarian neo‐angiogenesis. This leads to symptoms such as abdominal pain, nausea and vomiting. A serious adverse event is thrombosis.
Ectopic pregnancy, defined as a pregnancy outside the uterine cavity, per couple.
Search methods for identification of studies
We searched for all publications that described RCTs comparing different agents for ovarian stimulation protocols followed by IUI. We imposed no language restrictions or restriction on date of publication. Due to a delay in finalising the review, we performed the electronic searches on several occasions (latest in November 2020).
Electronic searches
We searched the following databases, using the subject headings and keywords shown in the appendices:
The Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of controlled trials, ProCite platform; searched 10 November 2020 (Appendix 1);
CENTRAL via the Cochrane Register of Studies Online (CRSO), Web platform; searched 10 November 2020 (Appendix 2);
MEDLINE, OVID platform; searched from 1946 to 10 November 2020 (Appendix 3);
Embase, OVID platform; searched from 1980 to 10 November 2020 (Appendix 4);
PsycINFO, OVID platform; searched from 1806 to 10 November 2020 (Appendix 5);
Cumulative Index to Nursing and Allied Health Literature (CINAHL), EBSCO platform; searched from 1961 to 24 June 2020 (Appendix 6) (CINAHL search output from June 2020 to 10 November 2020 was included in the CENTRAL 10 November 2020 search output).
Searching other resources
We searched two trial registers, the ClinicalTrials.gov database, a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home), and the World Health Organization International Trials Registry Platform search portal (https://www.who.int/clinical‐trials‐registry‐platform/the‐ictrp‐search‐portal). (Trial registry search output from June 2020 to 10 November 2020 was included in the CENTRAL 10 November 2020 search output.) We handsearched the reference lists of all identified and included studies. Furthermore, we reviewed abstracts of the American Society for Reproductive Medicine (1987 to 2020) and the European Society for Human Reproduction and Embryology (1987 to 2020) meetings. We also contacted experts in the field for any additional trials.
Data collection and analysis
Selection of studies
Two review authors (AGHR, AEPC) independently selected the trials for inclusion according to the aforementioned criteria. Disagreement between review authors was resolved through discussion, involving a third review author when necessary. We produced a PRISMA flow diagram to show the results of the search, including the numbers of included and excluded studies. If trials were published more than once, we included only the most complete and recent data. We contacted authors when necessary. The studies mentioned in the Characteristics of excluded studies table were all excluded on full‐text judgement. We have not reported publications excluded based on their titles and abstracts in this table.
We categorised studies as ‘awaiting classification’ when the trial:
was found in a trial registry and reported to be ongoing;
finalised inclusion without publishing data yet (congress proceeding or journal);
was reported in articles under review for data integrity or due to possible retraction.
Data extraction and management
For all included trials, two review authors (AGHR, AEPC) independently performed data extraction, using a data extraction form to summarise all trial characteristics in a table. We extracted and assessed information about the type of study, type of participants, baseline characteristics, type of interventions and type of outcome measures, as mentioned in the Criteria for considering studies for this review section, as well as the studies' data. If important information was missing from the original publications, we contacted study authors using various means of communication, and sent them a reminder if there was no response. We resolved disagreements through discussion, together with a third review author (BC).
Assessment of risk of bias in included studies
Two review authors (AGHR, AEPC) independently performed an assessment of the risk of bias for each eligible study on each outcome by using the Cochrane risk of bias assessment tool to assess: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias (Higgins 2011). We described all judgements in the risk of bias tables. As blinding was not always possible due to the nature of the intervention, we did score lack of blinding as possible bias, but judged this would not significantly alter the outcomes.
Measures of treatment effect
All outcomes were dichotomous data; therefore, we used the number of events in the control and in the intervention groups to calculate Mantel‐Haenszel odds ratios (ORs), combined with 95% confidence intervals (CIs) for all outcomes. The outcomes live birth and clinical pregnancy were considered a positive consequence of treatment. Therefore, a higher proportion of women with a live birth or a pregnancy was considered a benefit. For adverse outcomes, such as multiple pregnancy, miscarriage and ovarian hyperstimulation syndrome (OHSS) which are negative consequences, higher numbers were considered to be detrimental (increased odds represents relative harm). These considerations need to be taken into account when meta‐analyses are interpreted.
Unit of analysis issues
The outcomes were presented as per woman (or couple) randomised. If there were multiple cycles, the unit of analysis was still per woman randomised. When data per woman were not reported or could not be extracted, we did not include the study in the meta‐analysis. In cross‐over studies, only data before cross‐over were included in the meta‐analysis (when available), as successful treatment prevents a cross‐over, introducing over‐ or underestimation of the effect. For studies where randomisation was performed on a per cycle basis, we extracted first cycle data, or contacted authors when this was not possible from the published data.
Dealing with missing data
We analysed the data on an intention to‐treat basis. If relevant data were missing, we attempted to contact study authors to obtain the data. If we were unsuccessful, we used only the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2 statistic. An I2 measurement greater than 50% was taken to indicate substantial heterogeneity (Deeks 2021).
Assessment of reporting biases
We used Covidence as a tool and database for selecting eligible studies. Duplicates were automatically removed on title by Covidence itself. When a study seemed to be published several times (either in full text or as an abstract), we included the latest version in our data. We investigated publication bias by constructing a funnel graph, plotting sample size versus effect size. We did not construct a funnel plot when there were insufficient studies available (10 or fewer).
Data synthesis
If the studies were sufficiently similar, we combined the data using a fixed‐effect model. Random‐effects model were used in cases of high heterogeneity (>50%). Statistical analysis was performed using Review Manager 5.4.1 (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis for trials comparing two different stimulation protocols in couples with different types of infertility, for the primary outcomes. To make meta‐analysis of subgroups possible, there had to be sufficient studies included (at least two) and the data had to be available for extraction.
Sensitivity analysis
We conducted a sensitivity analysis for the primary outcomes to evaluate whether the results are robust when inclusion in the meta‐analysis was restricted to high‐quality studies (i.e. those having a low risk of selection bias ‐ random sequence generation and allocation concealment). Where data were available, we included sensitivity analysis per dose for the primary outcomes.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables using GRADEpro (GRADEpro GDT), and Cochrane methods (Ryan 2016; Schünemann 2021 ). These tables evaluate the overall quality of the body of evidence for the main review outcomes (live birth, multiple pregnancy, clinical pregnancy, miscarriage, OHSS, ectopic pregnancy) for the main review comparison of gonadotropins versus anti‐oestrogens. We prepared additional summary of findings tables for other important comparisons (aromatase inhibitors versus anti‐oestrogens; gonadotropins with GnRH agonist versus gonadotropins alone; gonadotropins with GnRH antagonist versus gonadotropins alone; different types of gonadotropins; gonadotropins with anti‐oestrogens versus gonadotropins alone; aromatase inhibitors versus gonadotropins; aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins). We assessed the certainty of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. At least two review authors (AGHR, AEPC), working independently, made judgements about evidence certainty (high, moderate, low or very low), and resolved any disagreements through discussion. When both statistical and clinical heterogeneity was present, we downgraded the evidence.
All judgements are justified, documented, and incorporated into the reporting of results for each outcome.
Results
Description of studies
Results of the search
The previous version of this review included 44 trials after full‐text screening (Cantineau 2007). Critical re‐appraisal of these studies resulted in the exclusion of six studies due to the inclusion of a significant percentage of participants with the wrong indication (anovulatory women or the need for use of donor sperm). A total of 82 studies are now included in the current review (Figure 1). We excluded a total of 92 records after full‐text screening (see Characteristics of excluded studies). Five studies are awaiting classification. Of these, we need more information about one published study to determine whether it can be included (Abu Hashim 2012). Of the four remaining studies, identified from trial registries, one is still in the data‐analysing phase (IRCT20090912002445N 2019); one is not recruiting (EUCTR 2006); and we are unsure about the status of two studies due to a lack of response from the authors (IRCT201106256871N 2012; IRCT20180528039878N1 2018) (see Characteristics of studies awaiting classification).
1.
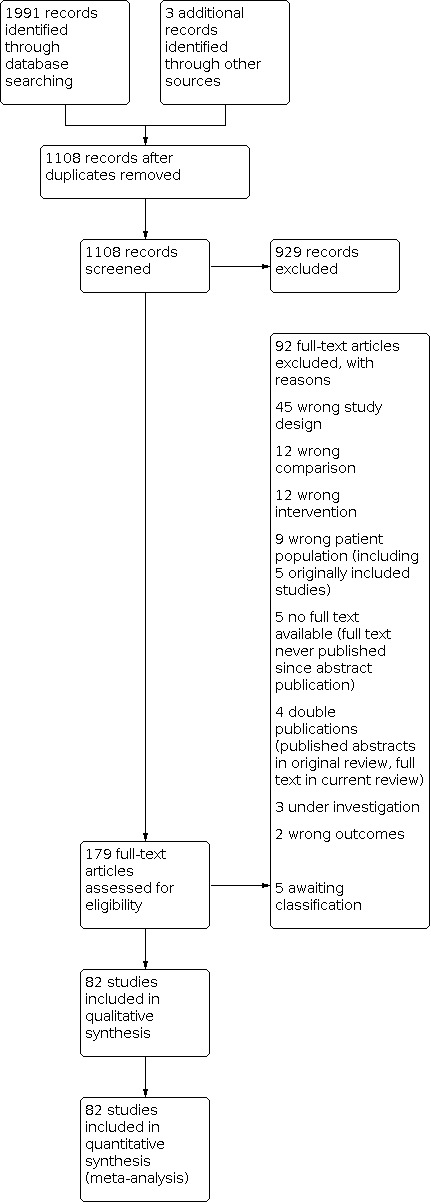
tudy flow diagram.
Included studies
Study design and setting
We included a total of 82 randomised controlled trials (RCTs) in this 2021 updated review. Of these, 77 studies were two‐armed and 5 studies had a three‐arm design (Demirol 2007, Diamond 2015, Hughes 1998, Karthik 2018, Sharma 2011a).
The countries in which the studies were conducted are as follows.
Belgium: Fatemi 2003; Peeraer 2015.
Brazil: Cavagna 2009; Kabouk 2010.
Egypt: Al‐Inany 2010; El Helw 2002; Fouda 2011; Galal 2015; Kamel 1995; Nada 2016.
France: Ecochard 2000.
Greece: Gregoriou 2008.
India: Dhaliwal 2002; Ghosh Dastidar 2009; Jain 2016; Kamath 2013; Karthik 2018; Malhotra 2012; Nayar 2008; Sharma 2011a; Wadhwa 2016.
Iran: Akbari 2012; Davar 2006; Deghani‐Firouzabady 2006; Pourali 2017; Pourmatroud 2013; Rashidi 2013; Sadaghiani 2012; Taravat 2011; Zadehmodares 2012).
Italy: Allegra 2007; Crosignani 2007; Filicori 2001; Filicori 2003; Gerli 1993; Gerli 2004a; Moro 2015; Pattuelli 1996; Ragni 2001; Ragni 2004; Sagnella 2011.
Japan: Sengoku 1994; Sengoku 1999.
Pakistan: Haqnawaz 2013.
Spain: Balasch 1994; Carrera 2002a; Espejo‐Catena 2016; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Labarta 2016; Matorras 2000; Parés 2002.
Sweden: Karlström 1993; Karlström 1998.
Taiwan: Lee 2008; Lin 2008; Wang 2004; Wu 2007.
The Netherlands: Cantineau 2011; Danhof 2018; Dankert 2007; Lambalk 2006.
Turkey: Baysoy 2006; Berker 2011; Dansuk 2015; Demirol 2007; Erdem 2015; Jamal 2005; Ozmen 2005.
USA: Al‐Fozan 2004; Diamond 2015; Dodson 1991; Hughes 1998; Nakajima 1999; Ransom 1996; Sammour 2001; Steward 2011; Williams 2004.
The settings in which patients were recruited were as follows:
Outpatient clinic: Dansuk 2015; Karlström 1993; Karlström 1998.
Infertility clinic: Akbari 2012; Berker 2011; Demirol 2007; Ecochard 2000; El Helw 2002; Filicori 2001, Filicori 2003; Gomez‐Palomares 2008; Ghosh Dastidar 2009; Haqnawaz 2013; Hughes 1998; Jain 2016; Jamal 2005; Pourali 2017; Nayar 2008; Ransom 1996; Rashidi 2013; Sammour 2001; Steward 2011; Zadehmodares 2012.
Department of obstetrics and gynaecology: Al‐Fozan 2004; Al‐Inany 2010; Balasch 1994; Dhaliwal 2002; Erdem 2015; Fouda 2011; Kamel 1995; Karthik 2018; Kim 1996; Kim 2010; Lee 2008; Lin 2008; Malhotra 2012; Moro 2015; Nakajima 1999; Parés 2002; Ragni 2001; Ragni 2004; Sadaghiani 2012; Sagnella 2011; Sengoku 1994; Sengoku 1999; Wadhwa 2016; Wang 2004; Wu 2007.
Division of reproductive endocrinology: Allegra 2007; Baysoy 2006; Cavagna 2009; Kabouk 2010; Kamath 2013; Labarta 2016; Matorras 2000; Ozmen 2005; Pattuelli 1996; Sharma 2011a.
University hospital: Carrera 2002a; Davar 2006; Deghani‐Firouzabady 2006; Dodson 1991; Espejo‐Catena 2016; Al‐Fadhli 2006; Fatemi 2003; Galal 2015; Gregoriou 2008; Kaur 2019; Nada 2016; Peeraer 2015; Pourmatroud 2013.
Multicenter trial: Cantineau 2011; Crosignani 2007; Danhof 2018; Dankert 2007; Diamond 2015; Gerli 1993; Gerli 2004a; Gomez‐Palomares 2005; Lambalk 2006; Taravat 2011; Williams 2004.
Participants
The included studies comprised 12,614 women in total. The age of the women ranged from 18 to 44 years. Fifty‐seven of 82 studies included women or couples with endometriosis, male infertility and/or unexplained infertility (Akbari 2012; Al‐Fadhli 2006; Al‐Fozan 2004; Al‐Inany 2010; Allegra 2007; Balasch 1994; Baysoy 2006; Berker 2011; Cantineau 2011; Carrera 2002a; Crosignani 2007; Danhof 2018; Dankert 2007; Davar 2006; Deghani‐Firouzabady 2006; Demirol 2007; Diamond 2015; El Helw 2002; Erdem 2015; Fatemi 2003; Filicori 2001; Filicori 2003; Fouda 2011; Gerli 1993; Ghosh Dastidar 2009; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Gregoriou 2008; Hughes 1998; Jain 2016; Jamal 2005; Kamel 1995; Karlström 1993; Karthik 2018; Kaur 2019; Labarta 2016; Lambalk 2006; Lee 2008; Lin 2008; Malhotra 2012; Moro 2015; Nada 2016; Nayar 2008; Ozmen 2005; Pattuelli 1996; Peeraer 2015; Ragni 2001; Rashidi 2013; Sagnella 2011; Sammour 2001; Sengoku 1994; Sengoku 1999; Sharma 2011a; Taravat 2011; Wadhwa 2016; Williams 2004; Zadehmodares 2012). Ten studies did not report the type of infertility explicitly (Cavagna 2009; Dansuk 2015; Espejo‐Catena 2016; Galal 2015; Kabouk 2010; Kamath 2013; Kim 2010; Sadaghiani 2012; Wang 2004; Wu 2007). Eleven studies included women with ovulatory dysfunction described as clomiphene citrate (CC) failure or resistance, polycystic ovary syndrome (PCOS), or female factor infertility, as well as women or couples with endometriosis, male infertility and/or unexplained infertility (Dhaliwal 2002; Ecochard 2000; Gerli 2004a; Haqnawaz 2013; Matorras 2000; Parés 2002; Pourali 2017; Pourmatroud 2013; Ragni 2004; Ransom 1996; Steward 2011). Three studies included 'adnexal adhesion', 'tubal disease' and 'cervical factor', as well as women or couples with endometriosis, male fertility and/or unexplained infertility (Dodson 1991; Hughes 1998; Karlström 1998). The remaining study, Kim 2010, included women with endometriosis Grade III‐IV as well as those with mild endometriosis.
Interventions
Thirteen of 82 studies compared gonadotropins with anti‐oestrogens (Balasch 1994; Berker 2011; Danhof 2018; Dankert 2007; Diamond 2015; Ecochard 2000; Erdem 2015; Kamel 1995; Karlström 1993; Karlström 1998; Nakajima 1999; Nayar 2008; Peeraer 2015). One study was not included in the meta‐analysis due to insufficient data on clinical pregnancy rate per couple (Nakajima 1999).
Eight studies compared aromatase inhibitors with anti‐oestrogens (Al‐Fozan 2004; El Helw 2002; Diamond 2015; Fatemi 2003; Fouda 2011; Ozmen 2005; Sammour 2001; Wu 2007).
Four studies compared gonadotropins combined with GnRH agonists with gonadotropins alone (Carrera 2002a; Dodson 1991; Pattuelli 1996; Sengoku 1994).
Fourteen studies compared gonadotropins combined with GnRH antagonists with gonadotropins alone (Allegra 2007; Cantineau 2011; Crosignani 2007; Dansuk 2015; Espejo‐Catena 2016; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Jain 2016; Kamath 2013; Karthik 2018; Lambalk 2006; Ragni 2001; Steward 2011; Williams 2004).
Two studies compared gonadotropins combined with anti‐oestrogens with gonadotropins alone (Al‐Inany 2010; Ransom 1996).
Six studies compared aromatase inhibitors with gonadotropins (Baysoy 2006; Diamond 2015; Galal 2015; Gregoriou 2008; Jamal 2005; Sharma 2011a).
Eight studies compared aromatase inhibitors combined with gonadotropins versus anti‐oestrogens combined with gonadotropins (Akbari 2012; Davar 2006; Haqnawaz 2013; Pourali 2017; Sadaghiani 2012; Taravat 2011; Wang 2004; Zadehmodares 2012).
One study compared gonadotropins combined with GnRH antagonists with anti‐oestrogens (Nada 2016).
Eight studies compared different types of gonadotropins (Demirol 2007; Gerli 2004a; Filicori 2001; Filicori 2003; Labarta 2016; Matorras 2000; Parés 2002; Sagnella 2011).
One study compared different dosage regimens for anti‐oestrogens or aromatase inhibitors (Al‐Fadhli 2006).
Four studies compared different dosage regimens for gonadotropins (Dhaliwal 2002; Hughes 1998; Ragni 2004; Sengoku 1999). One study was not included in the meta‐analysis due to insufficient data per group (Hughes 1998).
Fifteen studies compared interventions that were not defined in the protocol of the study. These were included in the review and are described under 'other comparisons' (Cavagna 2009; Deghani‐Firouzabady 2006; Ghosh Dastidar 2009; Gerli 1993; Kabouk 2010; Kaur 2019; Kim 1996; Kim 2010; Lee 2008; Lin 2008; Malhotra 2012; Moro 2015; Pourmatroud 2013; Rashidi 2013; Wadhwa 2016). Two studies were not included in the meta‐analyses due to insufficient data on clinical pregnancy rate per couple (Kabouk 2010, Kim 2010).
Outcomes
Seventeen of 82 studies reported live birth rate per woman randomised (Akbari 2012; Allegra 2007; Cantineau 2011; Danhof 2018; Dankert 2007; Diamond 2015; Dodson 1991; Erdem 2015; Gomez‐Palomares 2005; Gregoriou 2008; Haqnawaz 2013; Jain 2016; Kim 1996; Peeraer 2015; Ragni 2004; Rashidi 2013; Wang 2004).
Forty‐six studies reported on multiple pregnancies (Al‐Fozan 2004; Al‐Inany 2010; Allegra 2007; Balasch 1994; Baysoy 2006; Berker 2011; Cantineau 2011; Carrera 2002a; Crosignani 2007; Danhof 2018; Dankert 2007; Davar 2006; Deghani‐Firouzabady 2006; Demirol 2007; Dhaliwal 2002; Diamond 2015; Dodson 1991; Erdem 2015; Espejo‐Catena 2016; Filicori 2001; Filicori 2003; Fouda 2011; Gerli 2004a; Gomez‐Palomares 2005 Gomez‐Palomares 2008; Gregoriou 2008; Haqnawaz 2013; Jain 2016; Kaur 2019; Labarta 2016; Lambalk 2006; Matorras 2000; Moro 2015; Nada 2016; Nayar 2008; Parés 2002; Pattuelli 1996; Peeraer 2015; Pourali 2017; Ragni 2001; Rashidi 2013; Sagnella 2011; Sengoku 1999; Steward 2011; Wu 2007; Zadehmodares 2012). One study reported outcomes only per cycle (Nakajima 1999).
Seventy‐eight studies reported clinical pregnancy rate per woman randomised (Akbari 2012; Al‐Fadhli 2006; Al‐Fozan 2004; Al‐Inany 2010; Allegra 2007; Balasch 1994; Baysoy 2006; Berker 2011; Cantineau 2011; Carrera 2002a; Cavagna 2009; Crosignani 2007; Danhof 2018; Dankert 2007; Dansuk 2015; Davar 2006; Deghani‐Firouzabady 2006; Demirol 2007; Dhaliwal 2002; Diamond 2015; Dodson 1991; Ecochard 2000; El Helw 2002; Espejo‐Catena 2016; Erdem 2015; Fatemi 2003; Filicori 2001; Filicori 2003; Fouda 2011; Galal 2015; Gerli 1993; Gerli 2004a; Ghosh Dastidar 2009; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Gregoriou 2008; Haqnawaz 2013; Jain 2016; Jamal 2005; Kamath 2013; Kamel 1995; Karlström 1993; Karlström 1998; Karthik 2018; Kaur 2019; Kim 1996; Labarta 2016; Lambalk 2006; Lee 2008; Lin 2008; Malhotra 2012; Matorras 2000; Moro 2015; Nada 2016; Nayar 2008; Ozmen 2005; Parés 2002; Pattuelli 1996; Peeraer 2015; Pourali 2017; Ragni 2001; Ragni 2004; Ransom 1996; Rashidi 2013; Sadaghiani 2012; Sagnella 2011; Sammour 2001; Sengoku 1994; Sengoku 1999; Sharma 2011a; Steward 2011; Taravat 2011; Wadhwa 2016; Wang 2004; Williams 2004; Wu 2007; Zadehmodares 2012).
Remaining studies (4/82) reported data only per cycle (Kabouk 2010; Nakajima 1999), total pregnancies for the complete study group (Hughes 1998), or no data on pregnancies except that there was no significant difference between groups (Kim 2010).
Forty‐one studies reported on miscarriage rate (Akbari 2012; Al‐Fozan 2004; Balasch 1994; Berker 2011; Cantineau 2011; Carrera 2002a; Cavagna 2009; Crosignani 2007; Danhof 2018; Davar 2006; Demirol 2007; Dhaliwal 2002; Diamond 2015; Dodson 1991; Espejo‐Catena 2016; Erdem 2015; Filicori 2001; Filicori 2003; Fouda 2011; Gerli 2004a; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Gregoriou 2008; Haqnawaz 2013; Jain 2016; Labarta 2016; Lambalk 2006; Matorras 2000 (in Matorras 2002 publication); Moro 2015; Nakajima 1999; Nayar 2008; Parés 2002; Peeraer 2015; Pourali 2017; Rashidi 2013; Sagnella 2011; Sengoku 1999; Steward 2011; Taravat 2011; Wang 2004; Zadehmodares 2012). One study reported miscarriage rate only per pregnancy (Nakajima 1999).
Thirty‐three studies reported on OHSS (Al‐Fadhli 2006; Al‐Inany 2010; Allegra 2007; Balasch 1994, Baysoy 2006; Berker 2011; Cantineau 2011; Carrera 2002a; Crosignani 2007; Dhaliwal 2002; Davar 2006; Deghani‐Firouzabady 2006; Diamond 2015; Dodson 1991; Erdem 2015; Fouda 2011; Gomez‐Palomares 2008; Haqnawaz 2013; Jain 2016; Kaur 2019: Labarta 2016; Moro 2015; Nayar 2008; Parés 2002; Peeraer 2015; Pourali 2017; Pourmatroud 2013; Rashidi 2013; Sagnella 2011; Sengoku 1999; Steward 2011; Wadhwa 2016; Zadehmodares 2012).
Six studies reported on ectopic pregnancy rate (Danhof 2018; Diamond 2015; Dodson 1991; Fouda 2011; Gregoriou 2008; Jain 2016).
Excluded studies
We screened 1108 titles and abstracts, and excluded 92 records after full‐text screening of 179 articles. The main reasons for exclusion of studies were ineligible study design, ineligible participant population or ineligible intervention (see Characteristics of excluded studies).
Risk of bias in included studies
For details of risk of bias judgements for individual studies, see the risk of bias tables following the Characteristics of included studies tables. See also Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged 49 studies to be at low risk of selection bias according to the sequence generation. These studies used computer‐generated randomisation lists. The remaining 33 studies did not describe their method of randomisation explicitly, and we were unsuccessful in contacting study authors. Therefore, these studies were judged as unclear risk or high risk of selection bias.
We judged 18 studies to be at low risk of selection bias as a result of adequate concealment of allocation. These studies used sealed opaque envelopes or a trusted third party was involved during the procedure. The remaining 64 studies did not describe the concealment of allocation explicitly. Therefore, we assessed these as unclear risk of bias.
Blinding
Seventy of 82 studies reported a form of blinding of participants and personnel. Twelve studies reported details on blinding participants and personnel, which we rated as low risk of performance bias. The remaining studies did not provide enough information and we rated them as unclear risk of performance bias.
Eight studies were judged as low risk of bias for detection bias. The remaining studies were rates as unclear risk of detection bias.
Incomplete outcome data
Forty‐nine studies included all or nearly all randomised couples in the analyses. We therefore judged the risk of attrition bias for these studies as low. Thirty‐three of the 82 included studies did not report a flow chart, including information on dropouts and losses to follow‐up, and did not report on whether an intention‐to‐treat analysis was performed. One study did report the eligible women but omitted to report on women randomised per treatment arm (Jain 2016).
Selective reporting
Forty‐nine of the 82 studies mentioned in their results sections the outcomes that were stated in their methods section, resulting in judgement of low risk of bias. The remaining 33 studies reported on less outcomes than expected on the method section rated as unclear risk of reporting bias.
Two funnel plots could be constructed (Comparison 1.3, Figure 4 and Comparison 4.3, Figure 5). Both funnel plots were symmetrical not giving rise to suspected publication bias.
4.

Funnel plot of comparison: 1 Gonadotropins versus anti‐oestrogens, outcome: 1.3 clinical pregnancy rate per couple.
5.

Funnel plot of comparison: 4 Gonadotropins with GnRH antagonist versus gonadotropins alone, outcome: 4.3 clinical pregnancy rate per couple.
Other potential sources of bias
A potential source of heterogeneity is that the significant effect of gonadotropins was only seen in studies where the multiple pregnancy rate was high (14% to 32%).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; and Table 7 for the main comparisons.
1. Gonadotropins compared to anti‐oestrogens
Thirteen studies, involving 2898 women, compared gonadotropins and anti‐oestrogens (Balasch 1994; Berker 2011; Danhof 2018; Dankert 2007; Diamond 2015; Ecochard 2000; Erdem 2015; Kamel 1995; Karlström 1993; Karlström 1998; Nakajima 1999; Nayar 2008; Peeraer 2015).
Primary outcomes
Live birth rate
Five studies, involving 1924 women and comparing gonadotropins with anti‐oestrogens, reported live birth rate (Danhof 2018; Dankert 2007; Diamond 2015; Erdem 2015; Peeraer 2015). One study randomised on cycle level (Peeraer 2015). First‐cycle data could be extracted from the raw data file sent by the authors. Gonadotropins probably improve cumulative live birth rate compared with anti‐oestrogens (OR 1.37, 95% CI 1.05 to 1.79; I2 = 30%; 5 studies, 1924 participants; moderate‐certainty evidence; Analysis 1.1). This suggests that if the chance of live birth following anti‐oestrogens is assumed to be 22.8%, the chance following gonadotropins would be between 23.7% and 34.6%. Sensitivity analysis restricted to studies at low risk of bias for this comparison did not lead to a change in result. Sensitivity analysis restricted to studies with a low‐dose step‐up schedule (Danhof 2018; Dankert 2007; Erdem 2015), starting with 75 international units (IU) gonadotropins per day compared with 100 mg anti‐oestrogens per day, showed that it is uncertain whether gonadotropins improve live birth rate compared with anti‐oestrogens (OR 1.37, 95% CI 0.88 to 2.14; I2 = 54%; 3 studies, 1095 participants; moderate‐certainty evidence). Subgroup analysis according to type of infertility showed no significant difference in live birth rate for male infertility.
1.1. Analysis.

Comparison 1: Gonadotropins versus anti‐oestrogens, Outcome 1: live birth rate per couple
Multiple pregnancy rate
Multiple pregnancy rate was reported in seven studies, involving 2139 women, that compared gonadotropins with anti‐oestrogens (Balasch 1994; Berker 2011; Danhof 2018; Dankert 2007; Diamond 2015; Nayar 2008; Peeraer 2015). We are uncertain whether gonadotropins lead to a higher multiple pregnancy rate compared with anti‐oestrogens (OR 1.58, 95% CI 0.60 to 4.17; I2 = 54%; 7 studies, 2139 participants; low‐certainty evidence; Analysis 1.2). This suggests that if the chance of multiple pregnancy following anti‐oestrogens is 2.2%, the chance following gonadotropins would be between 1.4% and 8.7%. Sensitivity analysis restricted to studies at low risk of bias did not lead to a change in result. Sensitivity analysis restricted to studies with a higher starting dose of gonadotropins (150 IU per day) showed that higher dose schedules probably result in higher multiple pregnancy rates (OR 4.63, 95% CI 2.11 to 10.18; 1 study, 600 participants; low‐certainty evidence).
1.2. Analysis.

Comparison 1: Gonadotropins versus anti‐oestrogens, Outcome 2: multiple pregnancy rate per couple
Secondary outcomes
Clinical pregnancy rate
Twelve studies, involving 2576 women and comparing gonadotropins with anti‐oestrogens, reported a clinical pregnancy rate (Balasch 1994; Berker 2011; Danhof 2018; Dankert 2007; Diamond 2015; Ecochard 2000; Erdem 2015; Kamel 1995; Karlström 1993; Karlström 1998; Nayar 2008; Peeraer 2015). Gonadotropins probably improve clinical pregnancy rate compared with anti‐oestrogens (OR 1.34, 95% CI 1.12 to 1.61; I2 = 0%; 12 studies, 2576 participants; moderate‐certainty evidence; Analysis 1.3). This suggests that if the chance of clinical pregnancy following anti‐oestrogens is 22.8%, the chance following gonadotropins would be between 24.8% and 32.2%.
1.3. Analysis.

Comparison 1: Gonadotropins versus anti‐oestrogens, Outcome 3: clinical pregnancy rate per couple
Miscarriage rate
Six studies comparing gonadotropins and anti‐oestrogens, involving 2002 women, reported miscarriage rate (Balasch 1994; Berker 2011; Danhof 2018; Diamond 2015; Nayar 2008; Peeraer 2015). We are uncertain whether gonadotropins lead to a higher miscarriage rate compared with anti‐oestrogens (OR 1.25, 95% CI 0.88 to 1.77; I2 = 0%; 6 studies, 2002 participants; low‐certainty evidence; Analysis 1.4). This suggests that if the chance of a miscarriage following anti‐oestrogens is 6.2%, the chance following gonadotropins would be between 5.5% and 10.5%.
1.4. Analysis.

Comparison 1: Gonadotropins versus anti‐oestrogens, Outcome 4: miscarriage rate per couple
Ovarian hyperstimulation syndrome (OHSS) rate
OHSS rate was reported in six studies, involving 1482 women, comparing gonadotropins and anti‐oestrogens (Balasch 1994; Berker 2011; Diamond 2015; Erdem 2015; Nayar 2008; Peeraer 2015). We are uncertain whether gonadotropins lead to a higher OHSS rate compared with anti‐oestrogens (OR 0.77, 95% CI 0.19 to 3.14; I2 = 23%; 6 studies, 1482 participants; low‐certainty evidence; Analysis 1.5). This suggests that if the chance of OHSS following anti‐oestrogens is 0.4%, the chance following gonadotropins would be between 0.1% and 1.3%.
1.5. Analysis.

Comparison 1: Gonadotropins versus anti‐oestrogens, Outcome 5: OHSS rate per couple
Ectopic pregnancy rate
Two studies, involving 1339 women and comparing gonadotropins with anti‐oestrogens, reported ectopic pregnancies (Danhof 2018; Diamond 2015). Based on these studies, we are uncertain whether gonadotropins lead to a higher ectopic pregnancy rate compared with anti‐oestrogens (OR 1.64, 95% CI 0.67 to 3.98; I2 = 23%; 2 studies, 1339 participants; low‐certainty evidence; Analysis 1.6). This suggests that if the chance of an ectopic pregnancy following anti‐oestrogens is 1.2%, the chance following gonadotropins would be between 0.8% and 4.6%.
1.6. Analysis.

Comparison 1: Gonadotropins versus anti‐oestrogens, Outcome 6: ectopic pregnancy rate per couple
2. Aromatase inhibitors compared to anti‐oestrogens
Eight studies, involving 1160 women, compared aromatase inhibitors and anti‐oestrogens (Al‐Fozan 2004; Diamond 2015; El Helw 2002; Fatemi 2003; Fouda 2011; Ozmen 2005; Sammour 2001; Wu 2007).
Primary outcomes
Live birth rate
One study comparing aromatase inhibitors and anti‐oestrogens, involving 599 women, reported live birth rate (Diamond 2015). We are uncertain whether aromatase inhibitors lead to a higher live birth rate compared with anti‐oestrogens (OR 0.75, CI 95% 0.51 to 1.11; 1 study, 599 participants; low‐certainty evidence; Analysis 2.1). This suggests that if the chance of live birth following anti‐oestrogens is 23.4%, the chance following aromatase inhibitors would be between 13.5% and 25.3%.
2.1. Analysis.

Comparison 2: Aromatase inhibitors versus anti‐oestrogens, Outcome 1: live birth rate per couple
Multiple pregnancy rate
Four studies, involving 1000 women and comparing aromatase inhibitors and anti‐oestrogens, reported multiple pregnancy rate (Al‐Fozan 2004; Diamond 2015; Fouda 2011; Wu 2007). We are uncertain whether aromatase inhibitors lead to a higher multiple pregnancy rate compared with anti‐oestrogens (OR 1.28, CI 95% 0.61 to 2.68; I2 = 0%; 4 studies, 1000 participants; low‐certainty evidence; Analysis 2.2). This suggests that if the chance of multiple pregnancy following anti‐oestrogens is 2.4%, the chance following aromatase inhibitors would be between 1.5% and 6.1%. Sensitivity analysis restricted to studies of low risk of bias for this comparison did not lead to a change in result.
2.2. Analysis.

Comparison 2: Aromatase inhibitors versus anti‐oestrogens, Outcome 2: multiple pregnancy rate per couple
Secondary outcomes
Clinical pregnancy rate
Eight studies, involving 1160 women and comparing aromatase inhibitors with anti‐oestrogens, reported on clinical pregnancy rate (Al‐Fozan 2004; Diamond 2015; El Helw 2002; Fatemi 2003; Fouda 2011; Ozmen 2005; Sammour 2001; Wu 2007). We are uncertain of the effect of aromatase inhibitors compared with anti‐oestrogens (OR 1.21, 95% CI 0.75 to 1.94; I2 = 40%; 8 studies, 1160 participants; low‐certainty evidence; Analysis 2.3). This suggests that if the chance of a clinical pregnancy following anti‐oestrogens is 23.1%, the chance following aromatase inhibitors would be between 18.4% and 36.8%.
2.3. Analysis.

Comparison 2: Aromatase inhibitors versus anti‐oestrogens, Outcome 3: clinical pregnancy rate per couple
Miscarriage rate
Miscarriage rate was reported in three studies, involving 967 women, comparing aromatase inhibitors and anti‐oestrogens (Al‐Fozan 2004; Diamond 2015; Fouda 2011). We are uncertain of the effect of aromatase inhibitors (OR 0.91, 95% CI 0.47 to 1.77; I2 = 10%; 3 studies, 967 participants; low‐certainty evidence; Analysis 2.4). This suggests that if the chance of a miscarriage following anti‐oestrogens is 6.0%, the chance following aromatase inhibitors would be between 2.9% and 10.1%.
2.4. Analysis.

Comparison 2: Aromatase inhibitors versus anti‐oestrogens, Outcome 4: miscarriage rate per couple
OHSS rate
OHSS rate was mentioned in two studies, involving 813 women, that compared aromatase inhibitors and anti‐oestrogens (Diamond 2015; Fouda 2011). However, there were no events, so no odds ratio could be calculated (Analysis 2.5).
2.5. Analysis.

Comparison 2: Aromatase inhibitors versus anti‐oestrogens, Outcome 5: OHSS rate per couple
Ectopic pregnancy rate
Ectopic pregnancy rate was mentioned in two studies, involving 813 women, that compared aromatase inhibitors and anti‐oestrogens (Diamond 2015; Fouda 2011). We are uncertain of the effect of aromatase inhibitors (OR 1.0, 95% CI 0.29 to 3.50; 2 studies, 813 participants; low‐certainty evidence; Analysis 2.6). This suggests that if the chance of an ectopic pregnancy following anti‐oestrogens is 1.2%, the chance following aromatase inhibitors would be between 0.4% and 4.2%.
2.6. Analysis.

Comparison 2: Aromatase inhibitors versus anti‐oestrogens, Outcome 6: ectopic pregnancy rate per couple
3. Gonadotropins combined with GnRH agonists compared to gonadotropins
Four studies, involving 452 women, compared gonadotropins combined with GnRH agonists to gonadotropins alone (Carrera 2002a; Dodson 1991; Pattuelli 1996; Sengoku 1994).
Primary outcomes
Live birth rate
One study, involving 97 women, comparing gonadotropins combined with GnRH agonists to gonadotropins alone, reported live birth rate (Dodson 1991). However, this study used a cross‐over design: when women did not conceive in the first cycle, they switched to the other treatment arm. The first‐cycle data of 50 women randomised to gonadotropins alone versus 47 women randomised to gonadotropins plus GnRH agonists were not available.
Multiple pregnancy rate
Multiple pregnancy rate was reported by three studies comparing gonadotropins combined with GnRH agonists to gonadotropins alone. Dodson 1991 reported data only per cycle. Two studies, involving 264 women, could be pooled (Carrera 2002a; Pattuelli 1996). We are uncertain whether gonadotropins with GnRH agonists lead to a higher multiple pregnancy rate (OR 2.53, 95% CI 0.82 to 7.86; I2 = 0%; 2 studies, 264 participants; very low‐certainty evidence; Analysis 3.1). This suggests that if the chance of multiple pregnancy following gonadotropins alone is 3%, the chance following gonadotropins combined with GnRH agonists would be between 2.5% and 19.5%. Sensitivity analysis of high‐certainty studies was not possible, since none were available for this comparison.
3.1. Analysis.

Comparison 3: Gonadotropins with GnRH agonist versus gonadotropins alone, Outcome 1: multiple pregnancy rate per couple
Secondary outcomes
Clinical pregnancy rate
Three studies, involving 355 women and comparing gonadotropins combined with GnRH agonists to gonadotropins alone, reported clinical pregnancy rate per couple (Carrera 2002a; Pattuelli 1996; Sengoku 1994). We are uncertain whether gonadotropins combined with GnRH agonists improve the clinical pregnancy rate (OR 0.55, 95% CI 0.32 to 0.95; I2 = 0%; 3 studies, 355 participants; very low‐certainty evidence; Analysis 3.2). This suggests that if the chance of a clinical pregnancy following gonadotropins alone is 23.9%, the chance following gonadotropins combined with GnRH agonists would be between 9.1% and 23%.
3.2. Analysis.

Comparison 3: Gonadotropins with GnRH agonist versus gonadotropins alone, Outcome 2: clinical pregnancy rate per couple
Miscarriage rate
Miscarriage rate per couple was reported by one study, involving 60 women and comparing gonadotropins combined with GnRH agonists to gonadotropins alone (Carrera 2002a). We are uncertain whether gonadotropins combined with GnRH agonists improve miscarriage rate (OR 2.07, 95% CI 0.18 to 24.15; 1 study, 60 participants; very low‐certainty evidence; Analysis 3.3). This suggests that if the chance of miscarriage following gonadotropins alone is 3.3%, the chance following gonadotropins combined with GnRH agonists would be between 0.6% and 45.4%.
3.3. Analysis.

Comparison 3: Gonadotropins with GnRH agonist versus gonadotropins alone, Outcome 3: miscarriage rate per couple
OHSS rate
One study, involving 60 women and comparing gonadotropins combined with GnRH agonists to gonadotropins alone, reported on OHSS per couple (Carrera 2002a). We are uncertain whether gonadotropins with GnRH agonists improve OHSS rate (OR 1.80, 95% CI 0.39 to 8.32; 1 study, 60 participants; very low‐certainty evidence;Analysis 3.4). This suggests that if the chance of OHSS following gonadotropins alone is 10%, the chance following gonadotropins combined with GnRH agonists would be between 4.2% and 48%.
3.4. Analysis.

Comparison 3: Gonadotropins with GnRH agonist versus gonadotropins alone, Outcome 4: OHSS rate per couple
Ectopic pregnancy rate
Only one study, involving 97 women and comparing gonadotropins combined with GnRH agonists and gonadotropins alone, reported on ectopic pregnancy per cycle (Dodson 1991).
4. Gonadotropins combined with GnRH antagonists compared to gonadotropins alone
Fourteen studies, involving 2525 women, compared gonadotropins combined with GnRH antagonists to gonadotropins alone (Allegra 2007; Cantineau 2011; Crosignani 2007; Dansuk 2015; Espejo‐Catena 2016; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Jain 2016; Kamath 2013; Karthik 2018; Lambalk 2006; Ragni 2001; Steward 2011; Williams 2004).
Primary outcomes
Live birth rate
Three studies, involving 419 women, comparing gonadotropins combined with GnRH antagonists to gonadotropins alone, reported on live birth rate (Allegra 2007; Cantineau 2011; Gomez‐Palomares 2005). We are uncertain whether gonadotropins with GnRH antagonist improve live birth rate (OR 1.5, 95% CI 0.52 to 4.39; I2 = 81%; 3 studies, 419 participants; very low‐certainty evidence; Analysis 4.1). This suggests that if the chance of a live birth following gonadotropins alone is 25.7%, the chance following gonadotropins combined with GnRH antagonists would be between 15.2% and 60.3%. Sensitivity analysis restricted to studies at low risk of bias for this comparison did not lead to a change in result.
4.1. Analysis.

Comparison 4: Gonadotropins with GnRH antagonist versus gonadotropins alone, Outcome 1: live birth rate per couple
Multiple pregnancy rate
Multiple pregnancy rate was reported by 10 studies, involving 2095 women, comparing gonadotropins combined with GnRH antagonists to gonadotropins alone (Allegra 2007; Cantineau 2011; Crosignani 2007; Espejo‐Catena 2016; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Jain 2016; Lambalk 2006; Ragni 2001; Steward 2011). There is probably little or no difference between gonadotropins and gonadotropins combined with GnRH antagonists concerning multiple pregnancy rate (OR 1.30, 95% CI 0.74 to 2.28; I2 = 0%; 10 studies, 2095 participants; moderate‐certainty evidence; Analysis 4.2). This suggests that if the chance of multiple pregnancy following gonadotropins alone is 2%, the chance following gonadotropins combined with GnRH antagonists would be between 1.5% and 4.4%. Sensitivity analysis restricted to studies at low risk of bias for this comparison did not lead to a change in result.
4.2. Analysis.

Comparison 4: Gonadotropins with GnRH antagonist versus gonadotropins alone, Outcome 2: multiple pregnancy rate per couple
Secondary outcomes
Clinical pregnancy rate
Fourteen studies, involving 2525 women, comparing gonadotropins combined with GnRH antagonists to gonadotropins alone, reported on clinical pregnancy rate (Allegra 2007; Cantineau 2011; Crosignani 2007; Dansuk 2015; Espejo‐Catena 2016; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Jain 2016; Kamath 2013; Karthik 2018; Lambalk 2006; Ragni 2001; Steward 2011; Williams 2004). We are uncertain whether gonadotropins combined with GnRH antagonists improve the clinical pregnancy rate (OR 1.35, 95% CI 1.00 to 1.84; I2 = 47%; 14 studies, 2525 participants; low‐certainty evidence; Analysis 4.3). This suggests that if the chance of a clinical pregnancy following gonadotropins alone is 18%, the chance following gonadotropins combined with GnRH antagonists would be between 18% and 28.8%.
4.3. Analysis.

Comparison 4: Gonadotropins with GnRH antagonist versus gonadotropins alone, Outcome 3: clinical pregnancy rate per couple
Miscarriage rate
Nine studies, involving 2054 participants, comparing gonadotropins combined with GnRH antagonists to gonadotropins alone reported on miscarriage rate (Allegra 2007; Cantineau 2011, Crosignani 2007; Espejo‐Catena 2016; Gomez‐Palomares 2005; Gomez‐Palomares 2008; Jain 2016; Lambalk 2006; Steward 2011). We are uncertain of the effect of gonadotropins combined with GnRH antagonists (OR 1.37, 95% CI 0.84 to 2.22; I2=0%; 9 studies, 2054 participants; low‐certainty evidence; Analysis 4.4). This suggests that if the chance of miscarriage rate following gonadotropins alone is 2.9%, the chance following gonadotropins combined with GnRH antagonists would be between 2.5% and 6.2%.
4.4. Analysis.

Comparison 4: Gonadotropins with GnRH antagonist versus gonadotropins alone, Outcome 4: miscarriage rate per couple
OHSS rate
Five studies, involving 1348 participants, comparing gonadotropins combined with GnRH antagonists to gonadotropins alone, reported on OHSS rate (Cantineau 2011; Crosignani 2007; Gomez‐Palomares 2005; Jain 2016; Steward 2011). We are uncertain whether gonadotropins with GnRH antagonists improve the OHSS rate (OR 0.98, 95% CI 0.12 to 8.32; I2 = 56%; 5 studies, 1348 participants; very low‐certainty evidence; Analysis 4.5). This suggests that if the chance of OHSS following gonadotropins alone is 5%, the chance following gonadotropins combined with GnRH antagonists would be between 0.6% and 30.5%.
4.5. Analysis.

Comparison 4: Gonadotropins with GnRH antagonist versus gonadotropins alone, Outcome 5: OHSS rate per couple
Ectopic pregnancy rate
Finally, only one study, involving 407 participants, comparing gonadotropins combined with GnRH antagonists to gonadotropins alone, reported on occurrence of ectopic pregnancy (Jain 2016). We are uncertain of the effect of gonadotropins combined with GnRH antagonists (OR 0.33, 95% CI 0.01 to 8.23; 1 study, 407 participants; low‐certainty evidence; Analysis 4.6). This suggests that if the chance of an ectopic pregnancy following gonadotropins alone is 0.5%, the chance following gonadotropins combined with GnRH antagonist would be between 0% and 3.9%.
4.6. Analysis.

Comparison 4: Gonadotropins with GnRH antagonist versus gonadotropins alone, Outcome 6: ectopic pregnancy rate per couple
5. Gonadotropins combined with anti‐oestrogens compared to gonadotropins
Two studies, involving 328 women, compared gonadotropins combined with anti‐oestrogens to gonadotropins alone (Al‐Inany 2010; Ransom 1996).
Primary outcomes
Live birth rate
Neither one of the two studies reported live birth rate.
5.2 Multiple pregnancy rate
One study, involving 230 women, comparing gonadotropins combined with anti‐oestrogens to gonadotropins alone, reported multiple pregnancy rate (Al‐Inany 2010). We are uncertain if there is a difference in multiple pregnancy rate between gonadotropins combined with anti‐oestrogens and gonadotropins alone (OR 3.03, 95% CI 0.12 to 75.06; 1 study, 230 participants; low‐certainty evidence; Analysis 5.1).
5.1. Analysis.

Comparison 5: Gonadotropins with anti‐oestrogens versus gonadotropins alone, Outcome 1: multiple pregnancy rate per couple
Secondary outcomes
Clinical pregnancy rate
Both studies comparing gonadotropins combined with anti‐oestrogens to gonadotropins alone reported on clinical pregnancy rate (Al‐Inany 2010; Ransom 1996). We are uncertain whether gonadotropins combined with anti‐oestrogens improve clinical pregnancy rate (OR 1.99, 95% CI 0.81 to 4.9; I2 = 50%; 2 studies, 328 participants; very low‐certainty evidence; Analysis 5.2). This suggests that if the chance of a clinical pregnancy following gonadotropins alone is 11.9%, the chance following gonadotropins combined with anti‐oestrogens would be between 9.8% and 39.8%.
5.2. Analysis.

Comparison 5: Gonadotropins with anti‐oestrogens versus gonadotropins alone, Outcome 2: clinical pregnancy rate per couple
Miscarriage rate
Not reported.
OHSS rate
Only one study, involving 230 women, comparing gonadotropins combined with anti‐oestrogens to gonadotropins alone, reported OHSS rate (Al‐Inany 2010). We are uncertain of the effect of gonadotropins combined with anti‐oestrogens (OR 0.50, 95% CI 0.04 to 5.54; 1 study, 230 participants; low‐certainty evidence; Analysis 5.3). This suggests that if the chance of OHSS following gonadotropins alone is 1.7%, the chance following gonadotropins combined with anti‐oestrogens would be between 0.1% and 8.9%.
5.3. Analysis.

Comparison 5: Gonadotropins with anti‐oestrogens versus gonadotropins alone, Outcome 3: OHSS rate per couple
Ectopic pregnancy rate
Not reported.
6. Aromatase inhibitors compared to gonadotropins
Six studies reported on comparing aromatase inhibitors to gonadotropins (Baysoy 2006; Diamond 2015; Galal 2015; Gregoriou 2008; Jamal 2005; Sharma 2011a).
Primary outcomes
Live birth rate
Two studies, involving 651 women, comparing aromatase inhibitors and gonadotropins, reported live birth rate (Diamond 2015; Gregoriou 2008). Aromatase‐inhibitors may decrease live birth rate compared with aromatase inhibitors (OR 0.49, 95% CI 0.34 to 0.71; I2 = 0%; 2 studies, 651 participants; low‐certainty evidence; Analysis 6.1). This suggests that if the chance of a live birth following aromatase inhibitors is between 13.7% and 25%, the chance following gonadotropins would be between 31.7%. Sensitivity analysis restricted to the study at low risk of bias did not change the effect (Diamond 2015).
6.1. Analysis.

Comparison 6: Aromatase inhibitors versus gonadotropins, Outcome 1: live birth rate per couple
Multiple pregnancy rate
Three studies, involving 731 women, comparing aromatase inhibitors and gonadotropins, reported on multiple pregnancy rate (Baysoy 2006; Diamond 2015; Gregoriou 2008). We are uncertain of the effect of aromatase inhibitors compared with gonadotropins (OR 0.69, 95% CI 0.06 to 8.17; I2 = 77%; 3 studies, 731 women; very low‐certainty evidence; Analysis 6.2). This suggests that if the chance of a multiple pregnancy following gonadotropins alone is 9.6%, the chance following aromatase inhibitors would be between 0.6% and 46.3%. Sensitivity analysis restricted to the study at low risk of bias revealed a significantly higher prevalence of multiples in the group given gonadotropins (Diamond 2015).
6.2. Analysis.

Comparison 6: Aromatase inhibitors versus gonadotropins, Outcome 2: multiple pregnancy rate per couple
Secondary outcomes
Clinical pregnancy rate
Six studies, involving 1085 women, comparing aromatase inhibitors and gonadotropins, reported clinical pregnancy rate (Baysoy 2006; Diamond 2015; Galal 2015; Gregoriou 2008; Jamal 2005; Sharma 2011a). Aromatase‐inhibitors may decrease clinical pregnancy rate compared with gonadotropins (OR 0.61, 95% CI 0.46 to 0.82; I2 = 0%; 6 studies, 1085 participants; low‐certainty evidence; Analysis 6.3). This suggests that if the chance of a clinical pregnancy following aromatase inhibitors would be between 14.8% and 23.7% the chance following gonadotropins is 27.4%.
6.3. Analysis.

Comparison 6: Aromatase inhibitors versus gonadotropins, Outcome 3: clinical pregnancy rate per couple
Miscarriage rate
Two studies, involving 650 women, comparing aromatase inhibitors and gonadotropins, reported on miscarriage rate (Diamond 2015; Gregoriou 2008). We are uncertain of the effect of aromatase‐inhibitors (OR 0.53, 95% CI 0.30 to 0.92; I2 =0%; 2 studies, 650 participants; low‐certainty evidence; Analysis 6.4). This suggests that if the chance of a miscarriage following aromatase inhibitors would be between 3.8% and 10.8%, the chance following gonadotropins alone is 11.7%.
6.4. Analysis.

Comparison 6: Aromatase inhibitors versus gonadotropins, Outcome 4: miscarriage rate per couple
OHSS rate
Two studies, involving 680 women, comparing aromatase inhibitors and gonadotropins, reported the occurrence of OHSS (Baysoy 2006; Diamond 2015). We are uncertain of the effect of aromatase inhibitors concerning OHSS rate (OR 1.00, 95% CI 0.14 to 7.20; I2 = 0%; 2 studies, 680 participants; low‐certainty evidence; Analysis 6.5). This suggests that if the chance of OHSS following aromatase inhibitors would be between 0% and 2.1%, the chance following gonadotropins alone is 0.3%aromatase inhibitors would be between 0% and 2.1%.
6.5. Analysis.

Comparison 6: Aromatase inhibitors versus gonadotropins, Outcome 5: OHSS rate per couple
Ectopic pregnancy rate
Two studies, involving 650 women, comparing aromatase inhibitors and gonadotropins, reported ectopic pregnancy rate (Diamond 2015; Gregoriou 2008). We are uncertain of the effect of aromatase‐inhibitors (OR 0.56, 95% CI 0.21 to 1.48; I2 = 19%; 2 studies, 650 participants; low‐certainty evidence; Analysis 6.6). This suggests that if the chance of an ectopic pregnancy following aromatase inhibitors would be between 0.7% and 4.9%, the chance following gonadotropins alone is 3.4%.
6.6. Analysis.

Comparison 6: Aromatase inhibitors versus gonadotropins, Outcome 6: ectopic pregnancy per couple
7. Aromatase inhibitors combined with gonadotropins compared to anti‐oestrogens combined with gonadotropins
Eight studies, involving 1244 women, compared aromatase inhibitors combined with gonadotropins to anti‐oestrogens combined with gonadotropins (Akbari 2012; Davar 2006; Haqnawaz 2013; Pourali 2017; Sadaghiani 2012; Taravat 2011; Wang 2004; Zadehmodares 2012).
Primary outcomes
Live birth rate
Three studies, involving 708 women, comparing aromatase inhibitors plus gonadotropins to anti‐oestrogens plus gonadotropins, reported on live birth rate (Akbari 2012; Haqnawaz 2013; Wang 2004). We are uncertain whether aromatase inhibitors plus gonadotropins improve live birth rate (OR 0.99, 95% CI 0.38 to 2.54; I2 = 69%; 3 studies, 708 participants; very low‐certainty evidence; Analysis 7.1). This suggests that if the chance of a live birth following anti‐oestrogens plus gonadotropins is 13.8%, the chance following aromatase inhibitors plus gonadotropins would be between 5.7% and 28.9%. Sensitivity analysis restricted to studies at low risk of bias was not possible since none were available for this comparison.
7.1. Analysis.

Comparison 7: Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins, Outcome 1: live birth rate per couple
Multiple pregnancy rate
Four studies, involving 901 women, comparing aromatase inhibitors plus gonadotropins to anti‐oestrogens plus gonadotropins, reported on multiple pregnancy rate (Davar 2006; Haqnawaz 2013; Pourali 2017; Zadehmodares 2012). We are uncertain whether aromatase inhibitors plus gonadotropins improve multiple pregnancy rate (OR 1.31, 95% CI 0.39 to 4.37; I2 = 0%; 4 studies, 901 participants; very low‐certainty evidence; Analysis 7.2). This suggests that if the chance of a multiple pregnancy following anti‐oestrogens plus gonadotropins is 1.0%, the chance following aromatase inhibitors plus gonadotropins would be between 0.4% and 4.2%. Sensitivity analysis restricted to studies at low risk of bias was not possible since none were available for this comparison.
7.2. Analysis.

Comparison 7: Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins, Outcome 2: multiple pregnancy rate per couple
Secondary outcome
Clinical pregnancy rate
All eight studies, involving 1244 women, comparing aromatase inhibitors plus gonadotropins to anti‐oestrogens plus gonadotropins, reported clinical pregnancy rate (Akbari 2012; Davar 2006; Haqnawaz 2013; Pourali 2017; Sadaghiani 2012; Taravat 2011; Wang 2004; Zadehmodares 2012). We are uncertain whether aromatase inhibitors combined with gonadotropins improve clinical pregnancy rate (OR 0.78, 95% CI 0.57 to 1.07; I2 = 4%; 8 studies, 1244 participants; very low‐certainty evidence; Analysis 7.3). This suggests that if the chance of a clinical pregnancy following anti‐oestrogens with gonadotropins is 16.7%, the chance following aromatase inhibitors plus gonadotropins would be between 10.3% and 17.7%.
7.3. Analysis.

Comparison 7: Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins, Outcome 3: clinical pregnancy rate per couple
Miscarriage rate
For miscarriage rate, seven studies, involving 1164 women, comparing aromatase inhibitors plus gonadotropins to anti‐oestrogens plus gonadotropins reported this outcome (Akbari 2012; Davar 2006; Haqnawaz 2013; Pourali 2017; Taravat 2011; Wang 2004; Zadehmodares 2012). We are uncertain of the effect of aromatase inhibitors plus gonadotropins (OR 1.44, 95% CI 0.75 to 2.77; I2 = 0%; 7 studies, 1164 participants; very low‐certainty evidence; Analysis 7.4). This suggests that if the chance of a miscarriage following anti‐oestrogens plus gonadotropins is 3.0%, the chance following aromatase inhibitors plus gonadotropins would be between 2.2% and 7.8%.
7.4. Analysis.

Comparison 7: Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins, Outcome 4: miscarriage rate per couple
OHSS rate
Four studies, involving 901 women, comparing aromatase inhibitors plus gonadotropins to anti‐oestrogens plus gonadotropins, reported incidence of OHSS (Davar 2006; Haqnawaz 2013; Pourali 2017; Zadehmodares 2012). We are uncertain whether aromatase inhibitors plus gonadotropins improve OHSS rate (OR 4.45; 95% CI 0.75 to 26.4; I2 = 34%; 4 studies, 901 participants; low‐certainty evidence; Analysis 7.5). This suggests that if the chance of OHSS following anti‐oestrogens plus gonadotropins is 0.2%, the chance following aromatase inhibitors plus gonadotropins would be between 0.2% and 6.2%.
7.5. Analysis.

Comparison 7: Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins, Outcome 5: OHSS rate per couple
Ectopic pregnancy rate
Not reported.
Gonadotropins with GnRH agonist compared to anti‐oestrogens
No trials were found comparing gonadotropins combined with GnRH agonists to anti‐oestrogens.
8. Gonadotropins with GnRH antagonists compared to anti‐oestrogens
We found only one study, involving 622 women, that compared gonadotropins with GnRH antagonists to anti‐oestrogens (Nada 2016).
Primary outcomes
Live birth rate
Nada 2016 did not report live births.
Multiple pregnancy rate
Based on the data from one study (Nada 2016), there may be no difference in multiple pregnancy rate between gonadotropins plus GnRH antagonists versus anti‐oestrogens (OR 2.58, 95% CI 0.99 to 6.73; 1 study, 622 participants).
Secondary outcomes
Clinical pregnancy rate
Based on the data from one study involving 622 women (Nada 2016), we are uncertain whether gonadotropins plus GnRH antagonists improve clinical pregnancy rate compared to anti‐oestrogens (OR 2.28, 95% CI 1.51 to 3.46; 1 study, 622 participants).
The other secondary outcomes ‐ miscarriage rate, OHSS rate and ectopic pregnancy rate ‐ were not reported for this comparison.
9. Different types of gonadotropins
Eight studies compared different types of gonadotropins (Demirol 2007; Gerli 2004a; Filicori 2001; Filicori 2003; Labarta 2016; Matorras 2000; Parés 2002; Sagnella 2011). Four of these compared human menopausal gonadotropin (hMG) with recombinant follicle‐stimulating hormone (rFSH) (Filicori 2001; Filicori 2003; Labarta 2016; Sagnella 2011). The other four studies compared rFSH with urinary follicle‐stimulating hormone (uFSH) (Demirol 2007; Gerli 2004a; Matorras 2000; Parés 2002).
Primary outcomes
Live birth rate
None of the eight trials comparing different types of gonadotropins reported live births.
Multiple pregnancy rate
Based on data from the four studies (involving 685 women) comparing hMG to rFSH (Filicori 2001; Filicori 2003; Labarta 2016; Sagnella 2011), there may be no difference in multiple pregnancy rate between hMG and rFSH (OR 0.89, 95% CI 0.35 to 2.31; I2 = 0%; 4 studies, 685 participants). Sensitivity analysis restricted to the studies at low risk of bias did not change the effect. Based on data from the four studies (involving 538 women) comparing rFSH to uFSH (Demirol 2007; Gerli 2004a; Matorras 2000; Parés 2002), there may be no difference in multiple pregnancy rate between rFSH and uFSH (OR 1.02, 95% CI 0.47 to 2.23; I2 = 0%; 4 studies, 538 participants). Sensitivity analysis restricted to the studies at low risk of bias for this comparison did not lead to a change in result.
Secondary outcomes
Clinical pregnancy rate
Based on data from the four studies (involving 685 women) comparing hMG to rFSH (Filicori 2001; Filicori 2003; Labarta 2016; Sagnella 2011), there may be no difference in clinical pregnancy rate between hMG and rFSH (OR 0.95, 95% CI 0.66 to 1.37; I2 = 0%; 4 studies, 685 participants). Sensitivity analysis restricted to studies at low risk of bias was not possible since none were available for this comparison. Based on data from the four studies (involving 538 women) comparing rFSH to uFSH (Demirol 2007; Gerli 2004a; Matorras 2000; Parés 2002), there may be no difference in clinical pregnancy rate between rFSH and uFSH (OR 1.40, 95% CI 0.96 to 2.04; I2 = 0%; 4 studies, 538 participants).
Miscarriage rate
Based on data from the four studies (involving 685 women) comparing hMG to rFSH (Filicori 2001; Filicori 2003; Labarta 2016; Sagnella 2011), there may be no difference in miscarriage rate between hMG and rFSH (OR 1.29, 95% CI 0.50 to 3.3; I2 = 0%; 4 studies, 685 participants). Sensitivity analysis restricted to studies at low risk of bias was not possible since none were available for this comparison. Based on data from the four studies (involving 538 women) comparing rFSH to uFSH (Demirol 2007; Gerli 2004a; Matorras 2000; Parés 2002), there may be no difference in miscarriage rate between rFSH and uFSH (OR 1.37, 95% CI 0.65 to 2.90; I2 = 0%; 4 studies, 538 participants).
OHSS rate
Based on data from two studies (involving 585 women) comparing hMG to rFSH (Labarta 2016; Sagnella 2011), we are uncertain of the effect of hMG on OHSS rate (OR 0.14, 95% CI 0.05 to 0.44; I2 = 0%; 2 studies, 585 participants). Sensitivity analysis restricted to studies at low risk of bias was not possible since none were available for this comparison. Based on data from one study (involving 116 women) comparing rFSH to uFSH (Parés 2002), we are uncertain whether uFSH improves OHSS rate (OR 0.36, 95% CI 0.01 to 9.11; 1 study, 116 participants).
Ectopic pregnancy rate
None of the eight trials for this comparison reported ectopic pregnancy rate.
10. Different dosage regimens for anti‐oestrogens or aromatase inhibitors
Primary outcomes
No trials comparing different dosage regimens for anti‐oestrogens or aromatase inhibitors reported live births or multiple pregnancies.
Secondary outcomes
Clinical pregnancy rate
Based on data from one study (Al‐Fadhli 2006), comparing 5 mg Letrozole with 2,5 mg Letrozole per day we are uncertain whether the 5 mg Letrozole per day improves clinical pregnancy rate (OR 5.71; 95% CI 1.15‐28.32; 1 study, 72 participants).
None of the other secondary outcomes ‐ miscarriage rate, OHSS rate and ectopic pregnancy rate ‐ were reported for this comparison.
11. Different dosage regimens for gonadotropins
Three studies comparing different dosage regimens for gonadotropins could be included in meta‐analysis (Dhaliwal 2002; Ragni 2004; Sengoku 1999).
Primary outcomes
Live birth rate
Based on data from one study (involving 63 women) (Ragni 2004), we are uncertain of the effect of different dosage regimens on live birth rate (OR 13.71, 95% CI 1.62 to 116.34; 1 study, 63 participants).
Multiple pregnancy rate
Based on data from three studies (involving 297 women) (Dhaliwal 2002; Ragni 2004; Sengoku 1999), we are uncertain of the effect of different dosage regimens on multiple pregnancy rate (OR 3.11, 95% CI 0.48 to 20.1; I2 = 0%; 3 studies, 297 participants).
Secondary outcomes
Clinical pregnancy rate
Based on data from three studies (involving 360 women) (Dhaliwal 2002; Ragni 2004; Sengoku 1999), we are uncertain of the effect of different dosage regimens on clinical pregnancy rate (OR 1.81, 95% CI 0.64 to 5.1; I2 = 65%; 3 studies, 360 participants).
Miscarriage rate
Based on data from three studies (involving 360 women) (Dhaliwal 2002; Ragni 2004; Sengoku 1999), we are uncertain of the effect of different dosage regimens on miscarriage rate (OR 0.28, 95% CI 0.08 to 1.05; I2 = 0%; 3 studies, 360 participants).
OHSS rate
Based on data from three studies (involving 360 women) (Dhaliwal 2002; Ragni 2004; Sengoku 1999), we are uncertain of the effect of different dosage regimens on OHSS rate (OR 5.52, 95% CI 1.85 to 16.52; I2 = 0%; 3 studies, 360 participants).
Ectopic pregnancy rate
None of the three studies reported on ectopic pregnancies.
12. Other comparisons
These twelve further comparisons are represented in the included studies:
aromatase inhibitors plus gonadotropins versus aromatase inhibitors alone;
follicle‐stimulating hormone (FSH) plus GnRH antagonist versus human menopausal gonadotropin (hMG) plus GnRH antagonist
different regimens of GnRH agonists or antagonists;
aromatase inhibitors plus tamoxifen and hMG versus aromatase inhibitors plus placebo and hMG;
anti‐oestrogens plus gonadotropins and GnRH antagonist versus anti‐oestrogens with gonadotropins;
anti‐oestrogens plus gonadotropins and human chorionic gonadotropin (hCG) versus anti‐oestrogens with gonadotropins;
anti‐oestrogens plus gonadotropins versus anti‐oestrogens plus hCG;
anti‐oestrogens with hMG versus anti‐oestrogens with rFSH;
GnRH agonist with FSH versus GnRH agonist with hMG;
aromatase inhibitors plus FSH and GnRH antagonist versus aromatase inhibitors plus FSH;
anti‐oestrogens plus gonadotropins and an increasing dose of GnRH antagonist versus anti‐oestrogens plus gonadotropins and a fixed dose of GnRH antagonist; and
rFSH plus recombinant luteinising hormone (rLH) versus hMG.
12.1 Aromatase inhibitors plus gonadotropins versus aromatase inhibitors alone
Multiple pregnancy rate
One study for this comparison reported that there was one multiple pregnancy in the arm with gonadotropins (OR 0.32, 95% CI 0.01 to 8.24; 1 study, 60 participants) (Kaur 2019).
Clinical pregnancy rate
Two studies, involving 128 women, reported on clinical pregnancy rate (Kaur 2019: Malhotra 2012). We are uncertain whether aromatase inhibitors combined with gonadotropins improve clinical pregnancy rate compared to aromatase inhibitors alone (OR 0.31, 95% CI 0.12 to 0.82; I2 = 0%; 2 studies, 128 participants).
OHSS rate
One study, comparing aromatase inhibitors plus gonadotropins to aromatase inhibitors (Kaur 2019), reported that there was one case of OHSS in the arm with gonadotropins.
12.2 FSH plus GnRH antagonist versus hMG plus GnRH antagonist
Clinical pregnancy rate
Based on data from one study (involving 188 women) (Ghosh Dastidar 2009), we are uncertain of the effect of FSH plus GnRH antagonist on clinical pregnancy rate (OR 2.03, 95% CI 0.86 to 4.83; 1 study, 188 participants).
12.3 Different regimens of GnRH agonists or antagonists
Multiple pregnancy rate
One study (Kim 1996), involving 80 women with endometriosis, 39 of whom had minimal to mild endometriosis, compared different regimens of GnRH agonist preceding IUI. We are uncertain of the effect of long and ultralong protocols on multiple pregnancy rates (OR 2.24, 95% CI 0.19 to 26.9).
Clinical pregnancy rate
One study (Kim 1996), involving 80 women with endometriosis, 39 of whom had minimal to mild endometriosis, compared different regimens of GnRH agonist preceding IUI. We are uncertain of the effect of long and ultralong protocols on clinical pregnancy rates (OR 2.02, 95% CI 0.41 to 9.99).
Miscarriage rate
One study (Kim 1996), involving 80 women with endometriosis, 39 of whom had minimal to mild endometriosis, compared different regimens of GnRH agonist preceding IUI. We are uncertain of the effect of long and ultralong protocols on miscarriage rate (OR 1.06, 95% CI 0.06 to 18.2).
12.4 Aromatase inhibitors plus tamoxifen and hMG versus aromatase inhibitors plus placebo and hMG
Clinical pregnancy rate
Based on data from one study, involving 140 women (Pourmatroud 2013), we are uncertain of the effect of aromatase inhibitors plus tamoxifen and hMG on clinical pregnancy rate compared to aromatase inhibitors plus placebo and hMG (OR 2.09, 95% CI 0.50 to 8.73).
Miscarriage rate
Based on data from one study, involving 140 women (Pourmatroud 2013), we are uncertain of the effect of aromatase inhibitors combined with tamoxifen and hMG on miscarriage rate compared to aromatase inhibitors plus placebo and hMG (OR 1.00, 95% CI 0.06 to 16.31).
OHSS rate
Based on data from one study, involving 140 women (Pourmatroud 2013), we are uncertain of the effect of aromatase inhibitors combined with tamoxifen and hMG on OHSS rate compared to aromatase inhibitors plus placebo and hMG (OR 1.52, 95% CI 0.25 to 9.40).
12.5 Anti‐oestrogens plus gonadotropins and GnRH antagonist versus anti‐oestrogens plus gonadotropins
Clinical pregnancy rate
Based on data from one study, involving 76 women (Wadhwa 2016), we are uncertain of the effect of anti‐oestrogens plus gonadotropins and GnRH antagonist on clinical pregnancy rate compared to anti‐oestrogens plus gonadotropins (OR 0.77, 95% CI 0.16 to 3.74).
OHSS rate
Based on data from one study, involving 76 women (Wadhwa 2016), we are uncertain of the effect of anti‐oestrogens plus gonadotropins and GnRH antagonist on OHSS rate compared to anti‐oestrogens plus gonadotropins (OR 0.52, 95% CI 0.04 to 5.42).
12.6 Anti‐oestrogens with gonadotropins and hCG versus anti‐oestrogens with gonadotropins
Multiple pregnancy rate
Based on data from one study, involving 60 women (Deghani‐Firouzabady 2006), we are uncertain of the effect of anti‐oestrogens with gonadotropins and hCG on multiple pregnancy rate per couple compared to anti‐oestrogens with gonadotropins (OR 2.07, 95% CI 0.18 to 24.1).
Clinical pregnancy rate
Based on data from one study, involving 60 women (Deghani‐Firouzabady 2006), we are uncertain of the effect of anti‐oestrogens with gonadotropins and hCG on clinical pregnancy rates per couple compared to anti‐oestrogens with gonadotropins (OR 3.27, 95% CI 0.77 to 13.8).
OHSS rate
Based on data from one study, involving 60 women (Deghani‐Firouzabady 2006), we are uncertain of the effect of anti‐oestrogens with gonadotropins and hCG on OHSS rate compared to anti‐oestrogens with gonadotropins (OR 0.19, 95% CI 0.01 to 4.0).
12.7 Anti‐oestrogens with gonadotropins versus anti‐oestrogens with hCG
Multiple pregnancy rate
One study reported on multiple pregnancy rate and reported that all pregnancies were singletons (Cavagna 2009).
Clinical pregnancy rate
Based on data from one study, involving 38 women (Cavagna 2009), we are uncertain of the effect of anti‐oestrogens with gonadotropins on clinical pregnancy rates per couple compared to anti‐oestrogens with hCG (OR 0.89, 95% CI 0.11 to 7.06).
Miscarriage rate
One study reported on miscarriage rate per couple: no miscarriages were reported (Cavagna 2009).
12.8 Anti‐oestrogens with hMG versus anti‐oestrogens with rFSH
Live birth rate
Based on data from one study, involving 280 women (Rashidi 2013), we are uncertain of the effect of anti‐oestrogens with hMG on live birth rate compared to anti‐oestrogens with rFSH (OR 0.86, 95% CI 0.42 to 1.75).
Multiple pregnancy rate
Based on data from one study, involving 280 women (Rashidi 2013), we are uncertain of the effect of anti‐oestrogens with hMG on multiple pregnancy rate compared to anti‐oestrogens with rFSH (OR 1.04, 95% CI 0.06 to 16.8).
Clinical pregnancy rate
Based on data from one study, involving 280 women (Rashidi 2013), we are uncertain of the effect of anti‐oestrogens with hMG on clinical pregnancy rate compared to anti‐oestrogens with rFSH (OR 0.83, 95% CI 0.43 to 1.62).
Miscarriage rate
Based on data from one study, involving 280 women (Rashidi 2013), we are uncertain of the effect of anti‐oestrogens with hMG on clinical pregnancy rate compared to anti‐oestrogens with rFSH (OR 1.04, 95% CI 0.14 to 7.5).
OHSS rate
Based on data from one study, involving 280 women (Rashidi 2013), we are uncertain of the effect of anti‐oestrogens with hMG on OHSS rate compared to anti‐oestrogens with rFSH (OR 0.86, 95% CI 0.26 to 2.89).
12.9 GnRH agonist with FSH versus GnRH agonist with hMG
Clinical pregnancy rate
One study, involving 32 women (Gerli 1993), comparing FSH with hMG, both combined with a GnRH agonist preceding IUI, reported clinical pregnancy rates. We are uncertain of the effect of a GnRH agonist with FSH (OR 8.0, 95% CI 0.81 to 78.8).
12.10 Aromatase inhibitors plus FSH and GnRH antagonist versus aromatase inhibitors plus FSH
Clinical pregnancy rate
Based on data from one study, involving 61 women (Lee 2008), we are uncertain of the effect of aromatase inhibitors plus FSH and GnRH antagonist on clinical pregnancy rate compared to aromatase inhibitors plus FSH (OR 2.16, 95% CI 0.49 to 9.57).
12.11 Anti‐oestrogens plus gonadotropins and an increasing dose GnRH antagonist versus anti‐oestrogens plus gonadotropins and a fixed dose GnRH antagonist
Multiple pregnancy rate
One study, Lin 2008, comparing anti‐oestrogens plus gonadotropins and an increasing dose GnRH antagonist (from 0.25 mg to 0.5 mg) with anti‐oestrogens plus gonadotropins and a fixed dose GnRH antagonist (0.5 mg) did not reveal a difference in multiple pregnancy rate.
Clinical pregnancy rate
Based on data from one study (Lin 2008), we are uncertain of the effect of anti‐oestrogens combined with gonadotropins and a increasing dose GnRH antagonist (0.5 mg) on clinical pregnancy rate compared to anti‐oestrogens plus gonadotropins and an fixed dose GnRH antagonist (from 0.25 mg to 0.5 mg) (OR 1.18, 95% CI 0.38 to 3.68).
12.12 rFSH plus rLH versus hMG
Multiple pregnancy rate
One study, comparing rFSH plus rLH versus hMG, did not reveal a difference in multiple pregnancy rate (Moro 2015).
Clinical pregnancy rate
Based on data from one study comparing rFSH plus rLH versus hMG (Moro 2015), we are uncertain of the effect of rFSH plus rLH on clinical pregnancy rate (OR 1.39, 95% CI 1.02 to 2.47).
Miscarriage rate
Based on data from one study comparing rFSH plus rLH versus hMG (Moro 2015), we are uncertain of the effect of rFSH plus rLH on miscarriage rate (OR 2.24, 95% CI 0.77 to 6.53).
OHSS rate
Based on data from one study comparing rFSH plus rLH versus hMG (Moro 2015), we are uncertain of the effect of rFSH plus rLH on OHSS rate (OR 7.73, 95% CI 1.51 to 30.1).
None of the above comparisons under comparison 12 included high‐certainty studies, and therefore we did not perform any sensitivity analysis.
Discussion
Summary of main results
This systematic review compared the effectiveness of different agents for ovarian stimulation for IUI with each other in couples who have been trying to conceive for at least one year and for whom ovarian stimulation combined with IUI is a treatment option. Gonadotropins probably improve cumulative pregnancy and live birth rate compared with anti‐oestrogens, without increasing the odds of multiple pregnancy. There was insufficient evidence of differences in terms of live birth, pregnancy rate and multiple pregnancy between aromatase inhibitors and anti‐oestrogens. There was insufficient evidence of differences in terms of live birth, pregnancy rate and multiple pregnancy between gonadotropins with a GnRH agonist and gonadotropins alone. There was insufficient evidence of differences in terms of live birth rate, multiple pregnancy rate and pregnancy rate between gonadotropins combined with GnRH antagonist and gonadotropins alone. There was insufficient evidence of differences in terms of multiple pregnancy rate and pregnancy rate between gonadotropins combined with anti‐oestrogens and gonadotropins alone. Gonadotropins may improve live birth rate and pregnancy rate compared with aromatase inhibitors, with increasing the odds of multiple pregnancy. There was insufficient evidence of differences in terms of live birth rate, pregnancy rate and multiple pregnancy rate between anti‐oestrogens combined with gonadotropins and aromatase inhibitors combined with gonadotropins. The overall certainty of the evidence was very low to moderate due to heterogeneity or imprecision.
Overall completeness and applicability of evidence
In current practice, there is no consensus about which agents for ovarian stimulation protocol are superior. For some comparisons, the total number of couples included in the review was reasonable; therefore, the evidence does appear to be relevant.
Our study population consisted of couples who have been trying to conceive for at least one year and for whom ovarian stimulation combined with IUI is a treatment option. This population included couples with unexplained infertility, mild male factor infertility and minimal to mild endometriosis, making the results widely applicable. Several studies also included women with ovulatory disorders, tubal factor, adnexal adhesions and more severe forms of endometriosis.
We did not emphasise the effect of different medications of the same type (e.g. letrozole and anastrozole or decapeptyl or lucrin).
Costs were not taken into account as an outcome, although this is a relevant outcome.
Quality of the evidence
The strengths of this review include the complete overview of all types of agents used for ovarian stimulation protocols combined with IUI, and the subsequent analyses performed, such as subgroup and sensitivity analysis. However, a major limitation is that a minority of studies reported on the main outcome, live birth. A second limitation is that the quality of the individual studies is often low; only 18/82 included studies scored a low risk on both items concerning selection bias. A third limitation is that the certainty of evidence was low for most comparisons due to the small sample size, which resulted in imprecision and a lack of firm conclusions about the effect of different agents for ovarian stimulation protocols.
Potential biases in the review process
The evidence includes published and unpublished data, and there was no restriction by language. We attempted to keep potential biases to a minimum by having two review authors (AEPC, AGHR), working independently, perform data extraction and assessment of risk of bias, with an independent third person for trials in which review authors were involved.
The studies awaiting classification are trial registrations concerning comparisons 2 (aromatase inhibitors versus anti‐oestrogens), 7 (aromatase inhibitors plus gonadotropins versus anti‐oestrogens plus gonadotropins), and 11 (different dosage regimens for gonadotropins), as well as a new comparison to be made (addition of misoprostol to standard schedules). The results of the trial comparing aromatase inhibitors with anti‐oestrogens is valuable, if adequately powered, to confirm the current evidence based on one high‐certainty randomised trial. The same is true for comparison 7, since the current evidence is of very low certainty.
Agreements and disagreements with other studies or reviews
Two recent Cochrane Reviews both concluded that in couples diagnosed with unexplained infertility with a poor prognosis (defined as a low prediction score for natural conception), treatment with IUI combined with ovarian stimulation probably results in higher live birth rates compared to expectant management (OR 4.48, 95% CI 2.00 to 10.1) (Ayeleke 2020; Wang 2019). The multiple pregnancy rate will increase from 0.6% with expectant management to 5.4% with IUI‐OS (Wang 2019). These reviews do not discuss which agents for ovarian stimulation should be used.
Other reviews on agents for ovarian stimulation combined with IUI were published in recent years.
A review by Gunn 2016 concluded that for women who undergo ovarian stimulation with oral agents, anti‐oestrogens may be more effective than aromatase inhibitors (based on the Diamond 2015 study). Furthermore, they concluded that treatment with gonadotropins seems to be more effective than either oral agent, although it results in a significant risk of multiple gestations that should limit utilisation. All studies in the review by Gunn 2016 were included in the current review and analyses. On the other hand, a review by Eskew 2019 reported no signficant difference between aromatase inhibitors and anti‐oestrogens concerning pregnancy rates. All eight studies included in the review of Eskew 2019 were identified in the current review but only 3 studies were included in the meta‐analyses due to different inclusion criteria such as the combination with IUI.
Since the Diamond 2015 study used 150 IU gonadotropins per day as a starting dose, comparing its efficacy with 5 mg of aromatase inhibitor per day and 100 mg of anti‐oestrogens per day, it is reasonable that a multiple pregnancy rate of 31.8% was detected in the gonadotropins group compared with 9.4% in the anti‐oestrogen and and 13.4% in the aromatase inhibitor group. It is clear that less aggressive stimulation (starting dose 75 IU per day) will lead to a lower rate of multiple pregnancies and no difference between anti‐oestrogens and gonadotropins, as reported in the study by Danhof and colleagues, for example (Danhof 2018). A review by Zolton 2020 reached the same conclusion as Gunn 2016, stating that for every birth gained with the use of gonadotropins, a similar increased risk of multiple gestation occurs. An additional network meta‐analysis did not result in new answers, and stated that gonadotropins ranked highest on live birth rates, but also on the risk of multiple pregnancies (Danhof 2020).
A review by Qin 2020, comparing aromatase inhibitors with anti‐oestrogens, reported a similar pooled effect (OR 1.06, 95% CI 0.93 to 1.20). However, this review included in its analysis two studies that have been retracted. The other two studies included in Qin 2020 were also included in this current review.
A review by Luo 2014 concluded that GnRH antagonists added to gonadotropins result in significantly more pregnancies (OR 1.42, 95% CI 1.13 to 1.78), which is in accordance with the current analysis. Luo 2014 included three studies that we excluded from this meta‐analysis of comparison 4 (Ertunc 2010; Lee 2008; Stadtmauer 2011b). The reasons for exclusion were that two studies included women with polycystic ovary syndrome (PCOS), and the study by Lee 2008 added aromatase inhibitor to both treatment arms and was analysed in comparison 12.10. Another systematic review, by Vitagliano 2018, concluded that GnRH antagonists added to gonadotropins did not significantly increase live birth rate (OR 1.14, 95% CI 0.82 to 1.57), and showed a pooled effect of OR 1.28 (CI 0.97 to 1.69) for the outcome clinical pregnancy rate, including both studies with women with PCOS (Ertunc 2010, Stadtmauer 2011b), as well as a study by Wadhwa 2016. The latter study was described in the current review under comparison 12.5 since it added anti‐oestrogens. Although the GnRH antagonist seems to effectively prevent premature luteinising hormone (LH) rises and luteinisation, the true effect on live birth rates in IUI programs is arguable.
We did not find any relevant, recent systematic reviews for these comparisons: FSH combined with GnRH agonists versus FSH alone; FSH versus FSH combined with anti‐oestrogens; and anti‐oestrogens combined with FSH versus aromatase inhibitors combined with FSH.
Authors' conclusions
Implications for practice.
Based on the available results, gonadotropins probably improve cumulative live birth rate compared with anti‐oestrogens (moderate‐certainty evidence). Additionally, gonadotropins may improve cumulative live birth rate compared with aromatase inhibitors (low‐certainty evidence). From the available data, there is no convincing evidence that aromatase inhibitors lead to higher live birth rates compared to anti‐oestrogens. None of the agents compared lead to significantly higher multiple pregnancy rates. Based on low‐certainty evidence, there does not seem to be a role for different combined therapies, nor for adding gonadotropin‐releasing hormone (GnRH) agonists or GnRH antagonists in intrauterine insemination (IUI) programs.
Implications for research.
In general, it is important to provide data about the efficacy of agents for ovarian stimulation combined with IUI for all ovulatory women suffering from infertility. However, clear definition of the study population is needed to assess the effectiveness of treatment in daily practice. Using placebo treatment in control groups will improve the quality of studies.
In the future, randomised controlled trials are needed that compare aromatase inhibitors with anti‐oestrogens, and gonadotropins combined with IUI in a prospective designed randomised study for mild male factor and unexplained infertility (including power calculation).
What's new
| Date | Event | Description |
|---|---|---|
| 10 November 2020 | New search has been performed | We updated the review. We added 46 new studies. The layout of the review has been changed according to latest Cochrane guidelines. |
| 10 November 2020 | New citation required but conclusions have not changed | The update of the review has strengthened the previous conclusion due to more high quality studies included. New comparisons have been added. Gonadotrophins probably result in higher live birth rates compared to anti‐oestrogens. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 12 November 2010 | Amended | The results of comparison 6.2 and 6.3 have been edited in the text and data/analysis section. |
| 24 January 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank Dr Isaza, Dr Matorras, Dr Karlström and Dr. Gerli, who all responded to our questions related to their publications. For this update, we also thank Dr Danhof and Dr Peeraer, who responded to our requests for additional information. We also thank Dr Maaike Bloemendal and Dr Aart‐Jan Bijkerk for their contributions to the start of the update of this review.We thank Dr Rui Wang, Dr Rik van Eekelen and Dr Noor Danhof for their valuable peer review comments. We thank Faith Armitage for copy‐editing the review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
ProCite platform
Searched 10 November 2020
Keywords CONTAINS "artificial insemination by donor" or "artificial insemination by partner" or "artificial insemination" or "intrauterine insemination" or "intrautero tuboperitoneal insemination" or "IUI" or "Insemination" or "insemination‐artificial by donor" or "insemination‐donor" or "insemination‐intrauterine" or "insemination‐utero tubal" or "conventional insemination" or Title CONTAINS "artificial insemination by donor" or "artificial insemination by partner" or "artificial insemination" or "intrauterine insemination" or "intrautero tuboperitoneal insemination" or "IUI" or "conventional insemination"
AND
Keywords CONTAINS "ovulation" or "ovulatio" or "ovulation induction" or "ovulation rate" or "ovulation stimulation" or "ovulatory IUI" or "superovulation" or "superovulation induction" or "ovarian hyperstimulation" or "ovarian hyperstimulation syndrome" or "ovarian stimulation" or "ovarian stimulation syndrome" or "stimulated cycle" or "Stimulation techniques" or "controlled ovarian "or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "COH" or "COH IUI" or Title CONTAINS "ovulation" or "ovulatio" or "ovulation induction" or "ovulation rate" or "ovulation stimulation" or "ovulatory IUI" or "superovulation" or "superovulation induction" or "ovarian hyperstimulation" or "ovarian hyperstimulation syndrome" or "ovarian stimulation" or "ovarian stimulation syndrome" or "stimulated cycle" or "Stimulation techniques" or "controlled ovarian "or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "COH" or "COH IUI"
(528 records)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 10 November 2020
#1 intra‐uterine insemination* 124
#2 MESH DESCRIPTOR Insemination, Artificial EXPLODE ALL TREES 365
#3 (artificial* inseminat*):TI,AB,KY 250
#4 (intrauter* adj2 inseminat*):TI,AB,KY 998
#5 IUI:TI,AB,KY 894
#6 #1 OR #2 OR #3 OR #4 OR #5 1476
#7 MESH DESCRIPTOR Ovulation Induction EXPLODE ALL TREES 1349
#8 superovulation:TI,AB,KY 210
#9 (ovulat* adj2 induc*):TI,AB,KY 2664
#10 hyperstimulat*:TI,AB,KY 2054
#11 COH:TI,AB,KY 409
#12 (tim* adj2 ovulat*):TI,AB,KY 114
#13 stimulat*:TI,AB,KY 69782
#14 (control* adj2 ovulat*) 127
#15 #7 OR #8 OR #9 OR #10 OR #11 OR #12 or #13 or #14 71959
#16 #6 AND #15 849
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 10 November 2020
1 exp insemination, artificial/ or exp insemination, artificial, heterologous/ or exp insemination, artificial, homologous/ (11769) 2 artificial$ inseminat$.tw. (7485) 3 intrauterine insemination.tw. (2491) 4 intra‐uterine insemination*.tw. (223) 5 IUI.tw. (1808) 6 AIH.tw. (2524) 7 COH‐IUI.tw. (43) 8 or/1‐7 (18730) 9 exp ovulation induction/ or exp superovulation/ (13241) 10 superovulation.tw. (2090) 11 (ovulat$ adj2 induc$).tw. (7885) 12 (ovar$ adj2 stimulat$).tw. (7440) 13 (control* adj2 ovulat*).tw. (781) 14 COH.tw. (1809) 15 COH‐IUI.tw. (43) 16 Hyperstimulat$.tw. (6877) 17 (ovulat$ adj2 stimulat$).tw. (722) 18 (tim$ adj2 ovulat$).tw. (2093) 19 stimulat$.tw. (1148353) 20 or/9‐19 (1165119) 21 8 and 20 (3287) 22 randomized controlled trial.pt. (516561) 23 controlled clinical trial.pt. (93916) 24 randomized.ab. (497951) 25 placebo.tw. (218102) 26 clinical trials as topic.sh. (193591) 27 randomly.ab. (344379) 28 trial.ti. (228192) 29 (crossover or cross‐over or cross over).tw. (86638) 30 or/22‐29 (1355631) 31 exp animals/ not humans.sh. (4753760) 32 30 not 31 (1246822) 33 21 and 32 (484)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 10 November 2020
1 exp artificial insemination/ (17712) 2 artificial$ inseminat$.tw. (7078) 3 intrauterine insemination*.tw. (3889) 4 intra‐uterine insemination*.tw. (423) 5 IUI.tw. (3373) 6 AIH.tw. (5073) 7 COH‐IUI.tw. (85) 8 or/1‐7 (25903) 9 exp ovulation induction/ (14835) 10 superovulation.tw. (2424) 11 (ovulat$ adj2 induc$).tw. (9215) 12 (ovar$ adj2 stimulat$).tw. (11654) 13 (control$ adj2 ovulat$).tw. (785) 14 COH.tw. (2567) 15 COH‐IUI.tw. (85) 16 (Hyperstimulat$ adj2 ovar$).tw. (7738) 17 (ovulat$ adj2 stimulat$).tw. (856) 18 (tim$ adj2 ovulat$).tw. (1970) 19 stimulat$.tw. (1341085) 20 or/9‐19 (1359270) 21 8 and 20 (5344) 22 Clinical Trial/ (983355) 23 Randomized Controlled Trial/ (628181) 24 exp randomization/ (89156) 25 Single Blind Procedure/ (40935) 26 Double Blind Procedure/ (175688) 27 Crossover Procedure/ (65161) 28 Placebo/ (345385) 29 Randomi?ed controlled trial$.tw. (242881) 30 Rct.tw. (39345) 31 random allocation.tw. (2090) 32 randomly allocated.tw. (36719) 33 allocated randomly.tw. (2593) 34 (allocated adj2 random).tw. (833) 35 Single blind$.tw. (25683) 36 Double blind$.tw. (208007) 37 ((treble or triple) adj blind$).tw. (1230) 38 placebo$.tw. (311283) 39 prospective study/ (641093) 40 or/22‐39 (2268067) 41 case study/ (73574) 42 case report.tw. (418983) 43 abstract report/ or letter/ (1129742) 44 or/41‐43 (1611122) 45 40 not 44 (2212924) 46 21 and 45 (1241)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 10 November 2020
1 exp Reproductive Technology/ (1881) 2 artificial$ inseminat$.tw. (275) 3 intrauterine insemination.tw. (33) 4 IUI.tw. (44) 5 AIH.tw. (51) 6 COH‐IUI.tw. (0) 7 exp Infertility/ (2217) 8 or/1‐7 (3825) 9 exp Ovulation/ (406) 10 superovulation.tw. (6) 11 (ovulat$ adj2 induc$).tw. (109) 12 (ovar$ adj2 stimulat$).tw. (69) 13 COH.tw. (135) 14 COH‐IUI.tw. (0) 15 (Hyperstimulat$ adj2 ovar$).tw. (14) 16 (ovulat$ adj2 stimulat$).tw. (16) 17 (tim$ adj2 ovulat$).tw. (123) 18 stimulat$.tw. (138898) 19 or/9‐18 (139536) 20 8 and 19 (114) 21 random.tw. (59858) 22 control.tw. (453410) 23 double‐blind.tw. (23231) 24 clinical trials/ (11806) 25 placebo/ (5779) 26 exp Treatment/ (1068486) 27 or/21‐26 (1472642) 28 20 and 27 (51)
Appendix 6. CINAHL search strategy
EBSCO platform
Searched from 1961 to 24 June 2020 (search output from June 2020 to 10 November 2020 was included in the CENTRAL 10 November 2020 search output)
| # | Query | Results |
| S29 | S16 AND S28 | 164 |
| S28 | S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 | 1,608,947 |
| S27 | TX allocat* random* | 13,390 |
| S26 | (MH "Quantitative Studies") | 30,717 |
| S25 | (MH "Placebos") | 13,747 |
| S24 | TX placebo* | 71,832 |
| S23 | TX random* allocat* | 13,390 |
| S22 | (MH "Random Assignment") | 68,617 |
| S21 | TX randomi* control* trial* | 223,383 |
| S20 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,223,295 |
| S19 | TX clinic* n1 trial* | 296,720 |
| S18 | PT Clinical trial | 110,995 |
| S17 | (MH "Clinical Trials+") | 321,207 |
| S16 | S5 AND S15 | 418 |
| S15 | S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 | 116,792 |
| S14 | TX stimulat* | 114,925 |
| S13 | TX(tim* N2 ovulat*) | 118 |
| S12 | TX (ovulat* N2 stimulat*) | 76 |
| S11 | TX COH | 287 |
| S10 | TX (ovar* N2 hyperstimulat*) | 977 |
| S9 | TX(ovar* N2 stimulat*) | 1,114 |
| S8 | TX (ovulat* N2 induc*) | 2,017 |
| S7 | TX superovulation | 81 |
| S6 | (MM "Ovulation Induction") | 984 |
| S5 | S1 OR S2 OR S3 OR S4 | 1,330 |
| S4 | TX IUI | 412 |
| S3 | TX intrauter* N2 inseminat* | 563 |
| S2 | TX artificial* inseminat* | 924 |
| S1 | (MM "Insemination, Artificial") | 517 |
Data and analyses
Comparison 1. Gonadotropins versus anti‐oestrogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 live birth rate per couple | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 all types of subfertility | 5 | 1924 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [1.05, 1.79] |
| 1.1.2 unexplained subfertility | 4 | 906 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [1.17, 2.12] |
| 1.1.3 male subfertility | 3 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.36, 2.99] |
| 1.2 multiple pregnancy rate per couple | 7 | 2139 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [0.60, 4.17] |
| 1.2.1 all types of subfertility | 7 | 2139 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [0.60, 4.17] |
| 1.3 clinical pregnancy rate per couple | 12 | 2576 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.12, 1.61] |
| 1.4 miscarriage rate per couple | 6 | 2002 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.88, 1.77] |
| 1.5 OHSS rate per couple | 6 | 1482 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.19, 3.14] |
| 1.6 ectopic pregnancy rate per couple | 2 | 1339 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.67, 3.98] |
Comparison 2. Aromatase inhibitors versus anti‐oestrogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 live birth rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 multiple pregnancy rate per couple | 4 | 1000 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.61, 2.68] |
| 2.3 clinical pregnancy rate per couple | 8 | 1160 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.75, 1.94] |
| 2.4 miscarriage rate per couple | 3 | 967 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.47, 1.77] |
| 2.5 OHSS rate per couple | 2 | 813 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.6 ectopic pregnancy rate per couple | 2 | 813 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.29, 3.50] |
Comparison 3. Gonadotropins with GnRH agonist versus gonadotropins alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 multiple pregnancy rate per couple | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.82, 7.86] |
| 3.2 clinical pregnancy rate per couple | 3 | 355 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.95] |
| 3.3 miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 OHSS rate per couple | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.39, 8.32] |
Comparison 4. Gonadotropins with GnRH antagonist versus gonadotropins alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 live birth rate per couple | 3 | 419 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.52, 4.39] |
| 4.2 multiple pregnancy rate per couple | 10 | 2095 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.74, 2.28] |
| 4.3 clinical pregnancy rate per couple | 14 | 2525 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [1.00, 1.84] |
| 4.4 miscarriage rate per couple | 9 | 2054 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.84, 2.22] |
| 4.5 OHSS rate per couple | 5 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.12, 8.32] |
| 4.6 ectopic pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Gonadotropins with anti‐oestrogens versus gonadotropins alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 clinical pregnancy rate per couple | 2 | 328 | Odds Ratio (M‐H, Random, 95% CI) | 1.99 [0.81, 4.90] |
| 5.3 OHSS rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Aromatase inhibitors versus gonadotropins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 live birth rate per couple | 2 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.71] |
| 6.2 multiple pregnancy rate per couple | 3 | 731 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.06, 8.17] |
| 6.3 clinical pregnancy rate per couple | 6 | 1085 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.82] |
| 6.4 miscarriage rate per couple | 2 | 650 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.30, 0.92] |
| 6.5 OHSS rate per couple | 2 | 680 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.20] |
| 6.6 ectopic pregnancy per couple | 2 | 650 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.48] |
Comparison 7. Aromatase inhibitors with gonadotropins versus anti‐oestrogens with gonadotropins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 live birth rate per couple | 3 | 708 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.54] |
| 7.2 multiple pregnancy rate per couple | 4 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.39, 4.37] |
| 7.3 clinical pregnancy rate per couple | 8 | 1244 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.07] |
| 7.4 miscarriage rate per couple | 7 | 1164 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.75, 2.77] |
| 7.5 OHSS rate per couple | 4 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.45 [0.75, 26.43] |
Comparison 8. Gonadotropins with GnRH antagonist versus anti‐oestrogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.2 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
8.1. Analysis.

Comparison 8: Gonadotropins with GnRH antagonist versus anti‐oestrogens, Outcome 1: multiple pregnancy rate per couple
8.2. Analysis.

Comparison 8: Gonadotropins with GnRH antagonist versus anti‐oestrogens, Outcome 2: clinical pregnancy rate per couple
Comparison 9. Different types of gonadotropins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 multiple pregnancy rate per couple | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1.1 A). hMG versus rFSH | 4 | 681 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.31] |
| 9.1.2 B). r‐FSH versus u‐FSH | 4 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.47, 2.23] |
| 9.2 clinical pregnancy rate per couple | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.2.1 A). hMG versus rFSH | 4 | 685 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 9.2.2 B). r‐FSH versus u‐FSH | 4 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.96, 2.04] |
| 9.3 miscarriage rate per couple | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.3.1 A). hMG versus rFSH | 4 | 685 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.32] |
| 9.3.2 B). r‐FSH versus u‐FSH | 4 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.65, 2.90] |
| 9.4 OHSS rate per couple | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.4.1 A). hMG versus rFSH | 2 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.05, 0.44] |
| 9.4.2 B). r‐FSH versus u‐FSH | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.11] |
9.1. Analysis.

Comparison 9: Different types of gonadotropins, Outcome 1: multiple pregnancy rate per couple
9.2. Analysis.

Comparison 9: Different types of gonadotropins, Outcome 2: clinical pregnancy rate per couple
9.3. Analysis.

Comparison 9: Different types of gonadotropins, Outcome 3: miscarriage rate per couple
9.4. Analysis.

Comparison 9: Different types of gonadotropins, Outcome 4: OHSS rate per couple
Comparison 10. Different dosage regimens for anti‐oestrogens or aromatase inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.

Comparison 10: Different dosage regimens for anti‐oestrogens or aromatase inhibitors, Outcome 1: clinical pregnancy rate per couple
Comparison 11. Different dosage regimens for gonadotropins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 live birth rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.2 multiple pregnancy rate per couple | 3 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.48, 20.13] |
| 11.3 clinical pregnancy rate per couple | 3 | 360 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [0.64, 5.10] |
| 11.4 miscarriage rate per couple | 3 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.15, 1.31] |
| 11.5 OHSS rate per couple | 2 | 297 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.52 [1.85, 16.52] |
11.1. Analysis.

Comparison 11: Different dosage regimens for gonadotropins, Outcome 1: live birth rate per couple
11.2. Analysis.

Comparison 11: Different dosage regimens for gonadotropins, Outcome 2: multiple pregnancy rate per couple
11.3. Analysis.

Comparison 11: Different dosage regimens for gonadotropins, Outcome 3: clinical pregnancy rate per couple
11.4. Analysis.

Comparison 11: Different dosage regimens for gonadotropins, Outcome 4: miscarriage rate per couple
11.5. Analysis.

Comparison 11: Different dosage regimens for gonadotropins, Outcome 5: OHSS rate per couple
Comparison 12. Other comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 aromatase inhibitors+gonadotropins versus aromatase inhibitors | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1.1 multiple pregnancy rate per couple | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.24] |
| 12.1.2 clinical pregnancy rate per couple | 2 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.12, 0.82] |
| 12.1.3 OHSS rate per couple | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.24] |
| 12.2 FSH+GnRH antagonist versus hMG+GnRH antagonist | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.2.1 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.3 different regimens of GnRH agonist | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.3.1 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.3.2 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.3.3 miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.4 aromatase inhibitors (AI)+tamoxifen+hMG versus AI+hMG | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.4.1 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.4.2 miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.4.3 OHSS rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.5 anti‐oestrogens with gonadotropins with GnRH antagonist versus anti‐estrogens with gonadotrophins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.5.1 Clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.5.2 OHSS rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.6 anti‐oestrogens with gonadotropins and hCG versus anti‐estrogens with gonadotrophins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.6.1 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.6.2 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.6.3 OHSS rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.7 anti‐oestrogens with gonadotropins versus anti‐estrogens with hCG | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.7.1 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.7.2 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.7.3 miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.8 anti‐oestrogens with hMG versus anti‐oestrogens with rFSH | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.8.1 live birth rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.8.2 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.8.3 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.8.4 miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.8.5 OHSS rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.9 GnRHa+FSH versus GnRHa+hMG | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.9.1 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.10 aromatase inhibitors+FSH+GnRH antagonist versus AI+FSH | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.10.1 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.11 CC+FSH+GnRH antagonist flexible vs CC+FSH+GnRH antagonist fixed | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.11.1 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.11.2 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.12 rFSH+rLH versus hMG | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.12.1 multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.12.2 clinical pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.12.3 miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.12.4 OHSS rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
12.1. Analysis.

Comparison 12: Other comparisons, Outcome 1: aromatase inhibitors+gonadotropins versus aromatase inhibitors
12.2. Analysis.

Comparison 12: Other comparisons, Outcome 2: FSH+GnRH antagonist versus hMG+GnRH antagonist
12.3. Analysis.

Comparison 12: Other comparisons, Outcome 3: different regimens of GnRH agonist
12.4. Analysis.

Comparison 12: Other comparisons, Outcome 4: aromatase inhibitors (AI)+tamoxifen+hMG versus AI+hMG
12.5. Analysis.

Comparison 12: Other comparisons, Outcome 5: anti‐oestrogens with gonadotropins with GnRH antagonist versus anti‐estrogens with gonadotrophins
12.6. Analysis.

Comparison 12: Other comparisons, Outcome 6: anti‐oestrogens with gonadotropins and hCG versus anti‐estrogens with gonadotrophins
12.7. Analysis.

Comparison 12: Other comparisons, Outcome 7: anti‐oestrogens with gonadotropins versus anti‐estrogens with hCG
12.8. Analysis.

Comparison 12: Other comparisons, Outcome 8: anti‐oestrogens with hMG versus anti‐oestrogens with rFSH
12.9. Analysis.

Comparison 12: Other comparisons, Outcome 9: GnRHa+FSH versus GnRHa+hMG
12.10. Analysis.

Comparison 12: Other comparisons, Outcome 10: aromatase inhibitors+FSH+GnRH antagonist versus AI+FSH
12.11. Analysis.

Comparison 12: Other comparisons, Outcome 11: CC+FSH+GnRH antagonist flexible vs CC+FSH+GnRH antagonist fixed
12.12. Analysis.

Comparison 12: Other comparisons, Outcome 12: rFSH+rLH versus hMG
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akbari 2012.
| Study characteristics | ||
| Methods | Randomisation: women were randomly assigned to group A or group B Trial design: parallel Power calculation: no Dropouts: not described Cycle cancellation: not described Blinding: single‐blinded; not described if the participants or outcome assessors were blinded. ITT: not described |
|
| Participants | 160 subfertile women, 160 cycles Age of women: Letrozole: 28.5 ± 4.7 (years); CC: 28.4 ± 4.5 (years) Duration of infertility: Letrozole: 6.3 ± 3.9 (years); CC: 5.05 ± 2.4 (years) Type of infertility: unexplained ( > 1 year of infertility) Previous fertility treatment: not described Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: letrozole 5 mg for 5 days versus clomiphene citrate 100 mg, both followed by hMG from cycle day 8 until day of hCG Trigger for ovulation: Profasi (10,000 IU) i.m. Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | PR per couple Miscarriage rate Live birth per couple Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 2 different ovarian stimulation protocols. However, the exact method of randomisation has not been described. |
| Allocation concealment (selection bias) | Unclear risk | Not described explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The design of the study was single‐blinded; this probably means that the one radiologist who performed the vaginal ultrasound was blinded as to what treatment group the participant was in. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Al‐Fadhli 2006.
| Study characteristics | ||
| Methods | Randomisation: computer generated random table, method of allocation not described. Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 72 women, 72 cycles Age of women: letrozole 2.5 mg: 31.8 ± 0.3 yrs vs letrozole 5 mg: 31.8 ± 0.7 yrs Duration of infertility: letrozole 2.5 mg: 2.7 ± 0.3 vs letrozole 5 mg: 3.0 ± 0.4 Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: letrozole 2.5 mg daily for 5 days; letrozole 5.0 mg daily for 5 days Trigger for ovulation: 10,000 IU hCG Timing of IUI: 24 and 48 hrs after hCG Frequency of IUI: double Semen preparation technique: not stated Number of sperm injected: not stated Type of semen: normal semen analysis, husband semen was likely Catheter used: not stated Cancellation criteria: not stated; one participant developed severe side effects, the treatment was discontinued and she was excluded from the study. |
|
| Outcomes | PR/cycle (started) No multiples or OHSS were encountered Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table. |
| Allocation concealment (selection bias) | Unclear risk | Not described explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding stated, but primary outcome was not likely to be influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Al‐Fozan 2004.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated random table Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 154 women, 238 cycles Age of women: letrozole 30.7 ± 0.5 yrs; CC 31.5 ± 0.5 yrs Duration of infertility: letrozole 2.6 ± 0.2; CC 2.9 ± 0.3 (yrs) Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: letrozole: 44 women; CC: 57 women |
|
| Interventions | Stimulation method/dosage: letrozole 7.5 mg daily for 5 days; CC 100 mg daily for 5 days Trigger for ovulation: 10,000 IU hCG Timing of IUI: 24 and 48 hrs after hCG Frequency of IUI: double Semen preparation technique: not stated Number of sperm injected: not stated Type of semen: normal semen analysis, husband semen was likely Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | OPR/woman PR/cycle Ectopic pregnancy Miscarriage rate per pregnancy Multiple PR/woman Number of ampoules used Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was done using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not described explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding stated, but primary outcome was not likely to be influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Al‐Inany 2010.
| Study characteristics | ||
| Methods | Randomisation: by computer‐generated random number table Trial design: parallel Power calculation: yes Dropouts: no dropouts Cycle cancellation: yes (based on inadequate response or hyper response) Blinding: not described ITT: not described |
|
| Participants | 230 women, 230 cycles Age of women: hpHMG+CC: 27.3 ± 4.7 (yrs); hpHMG: 28.4 ± 4.7 (yrs) Duration of infertility: hpHMG+CC: 3.1±1.9 (yrs); hpHMG: 2.4±1.6 (yrs) Type of infertility: unexplained, mild male factor Previous fertility treatment: not described Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: All subjects received a fixed dose of 75 IU highly purified human menopausal gonadotropin (Merional; IBSA, Lugano, Switzerland), regardless of the response, starting from the third day of the cycle for 5 days. Subsequently, women randomised to the CC+hMG group received 50 mg CC (Clomid; Aventis pharma S.AE, Global Napi Pharmaceuticals, Cairo, Egypt) three times daily starting from the fourth day of hMG and continued until the day of hCG injection. Women randomised to the hMG‐only group maintained hMG injections until the day of hCG injection. Trigger for ovulation: hCG (Choriomon) (10,000 IU) i.m. Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described (husband likely) Catheter used: not described Cancellation criteria: IUI was cancelled if less than two or more than five follicles with a mean diameter of 16 mm were present |
|
| Outcomes | PR/couple Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was performed using sequentially numbered, opaque and sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding stated, but primary outcome was not likely to be influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details on primary outcomes are described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Allegra 2007.
| Study characteristics | ||
| Methods | Randomisation: by code number from a randomisation list in order of enrolment, concealment by third party. Trial design: parallel Power calculation: post hoc power analysis Dropouts: none Cycle cancellation: in 18 cycles no IUI was performed Blinding: not stated ITT: yes, all participants were analysed in the same group |
|
| Participants | 104 women, 320 cycles Age of women: rFSH+GnRH antagonist: 33 ± 4.0 (yrs); rFSH alone: 32.5 ± 3.6 (yrs) Duration of infertility: rFSH+GnRH antagonist: 2.7 ± 1.4 (yrs); rFSH alone: 2.9 ± 1.2 (yrs) Type of infertility: unexplained, mild male factor, endometriosis stage I or II Previous fertility treatment: at least > 6 months ago Primary infertility: rFSH+GnRH antagonist: 80.8% versus rFSH alone: 59.6% |
|
| Interventions | Stimulation method/dosage: Group A: rFSH 75‐150 IU from CD 3, when DF > 14 mm GRnH antagonist, after IUI vaginal progesterone 400 mg/day; Group B: rFSH 75‐150 IU alone Trigger for ovulation: 10,000 IU hCG Timing of IUI: 20 and 34 hrs after hCG Frequency of IUI: double Semen preparation technique: not stated Number of sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: < 2 follicles or > 5 follicles no IUI |
|
| Outcomes | PR/couple Multiple PR OHSS Number of ampoules used Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Code numbers of randomisation list. |
| Allocation concealment (selection bias) | Low risk | Third party. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding stated, but primary outcome was not likely to be influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart of participants and the analysis has been provided; incomplete data are not suspected. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Balasch 1994.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: no Dropouts: not stated Cycle cancellation: not stated Blinding: not stated ITT: not stated |
|
| Participants | 100 women, 192 cycles Age of women: FSH 31.8 ± 3.2 yrs; CC 32.6 ± 2.9 yrs Duration of infertility: FSH: 6.5 ± 2.5 yrs; CC: 6.1 ± 2.3 yrs Type of infertility: unexplained, male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: FSH 75 IU daily from CD 7, CC 50 mg daily for 5 days Trigger for ovulation: hCG (10,000 IU) Timing of IUI: 35‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: swim up into medium No of motile sperm injected: CC: 3.3±1.7 x106 FSH: 3.7±1.9 x106 Type of semen: husband semen Catheter used: IUI catheter Cancellation criteria: not stated |
|
| Outcomes | Ongoing PR/women Miscarriage rate per pregnancy Multiple PR OHSS Number of ampoules used Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised; however, method of randomisation has not been described explicitly. |
| Allocation concealment (selection bias) | Unclear risk | Not described explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding stated, but primary outcome was not likely to be influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Baysoy 2006.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation list, allocation not stated Trial design: not stated Power calculation: sample size was not calculated Dropouts: not stated Cycle cancellation: not stated Blinding: only the specialist who had performed the IUI was blinded to group assignment ITT: no, when an LH surge occurred IUI was performed on the following day but that cycle was not taken into consideration |
|
| Participants | 80 women, 40 in each group, not clear how many cycles Age of women: letrozole 27.2 ± 5.5 yrs; hMG 28.1 ± 4.3 yrs Duration of infertility: letrozole 5.3 ± 2.1 yrs; hMG 5.9 ± 3.2 yrs Type of infertility: unexplained Previous fertility treatment: patients with previous IUI attempts were excluded Primary infertility: 100% |
|
| Interventions | Stimulation method/dosage: 5 mg/d letrozole from days 3 to 7 of the cycle versus 75 IU ( < 30 years) and 150 IU ( > 30 years) of HMG from day 3 onwards for 5 days. After this, the dose and duration of HMG were adjusted during the monitoring. Trigger for ovulation: 10,000 IU hCG Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: husband semen Catheter used: intrauterine catheter Cancellation criteria: not stated |
|
| Outcomes | PR/woman Multiple PR/woman OHSS Premature luteinisation Costs Number of dominant follicles(s) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised according to a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Not described explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The specialist who performed the IUI was blinded to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Berker 2011.
| Study characteristics | ||
| Methods | Randomisation: randomisation was performed by an independent investigator using a computer‐generated number list. Trial design: parallel Power calculation: yes Dropouts: no Cycle cancellation: yes Blinding: not described ITT: not described |
|
| Participants | 189 women, 189 cycles Age of women: CC: 28.0 ± 4.5 (yrs); rFSH: 28.2 ± 4.6 (yrs) Duration of infertility: CC: 44.4 ± 20.3 (months); rFSH: 47.9 ± 19.6 (months) Type of infertility: unexplained infertility and mild male subfertility Previous fertility treatment: yes, at least one cycle of ovulation induction Primary infertility: most participants had primary infertility |
|
| Interventions | Stimulation method/dosage: Women assigned to CC received CC 100 mg/daily orally for five consecutive days starting from cycle days 2–4. Women allocated to rFSH were stratified according to body mass index (BMI). Starting rFSH dosage was 75 IU/day for women with a BMI < 25 kg/m2 and 100 IU/day for women with BMI > 25 kg/m2. rFSH injections were commenced on cycle day 2–4. Trigger for ovulation: (10,000 IU) i.m. Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: Semen samples used for insemination were processed within 1 hour after ejaculation, using a density gradient centrifugation followed by washing with culture medium Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: hCG injection was not given if there were > 3 follicles C17 mm, or > 5 follicles > 14 mm to reduce the risk of multiple pregnancy and/or ovarian hyperstimulation. |
|
| Outcomes | PR/couple PR/cycle Multiple PR OHSS Miscarriage rate Number of dominant follicles ( > 17 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed according to a randomly computer‐generated number list. |
| Allocation concealment (selection bias) | Low risk | An independent secretary prepared and sealed the numbered and opaque envelopes; the envelopes were opened in consecutive order. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding stated, but primary outcome was not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Power calculation, dropout described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Cantineau 2011.
| Study characteristics | ||
| Methods | Randomisation: the couples went to the hospital pharmacist, who distributed the study medication in numbered packages double‐blinded for its content. The numbers were created according to a central computer‐generated list of numbers. Trial design: parallel Power calculation: yes Dropouts: yes Cycle cancellation: yes Blinding: yes, double‐blinded ITT: yes |
|
| Participants | 233 women (113 women in GnRH group, 120 women in placebo group), 572 cycles Age of women: GnRH‐a: 32.6 ± 3.5 (years); Placebo: 32.0 ± 3.7 (years) Duration of infertility: GnRH‐a: 34.8 ± 12.5 (months); Placebo: 36.0 ± 15.1 (months) Type of infertility: unexplained, related to mild male factor or associated with the presence of minimal or mild endometriosis persisting for at least 2 years Previous fertility treatment: not described Primary infertility: both primary and secondary subfertility |
|
| Interventions | Stimulation method/dosage: all participants received 75 IU recombinant FSH s.c. (rFSH, Gonal‐f, Serono Benelux BV) per day from cycle day 2–4 onwards. Monitoring was performed from cycle day 8–9 by transvaginal ultrasound scanning. When a dominant follicle of 14 mm diameter or more was detected, a GnRH antagonist (Cetrorelix, Serono Benelux BV) at a dose of 0.25 mg/day s.c. or a placebo s.c. was added until hCG administration. Trigger for ovulation: (250 mg Ovitrelle IU) im Timing of IUI: 38‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: Either a gradient or swim‐up technique was used for semen specimens, depending on the local protocol of the participating clinic. Number of sperm injected: not described Type of semen: homologue Catheter used: not described Cancellation criteria: cycles were cancelled if > 3 follicles became 15 mm in diameter or larger, > 5 follicles became 11 mm or larger, or no follicle > 10 mm was observed on cycle day 21. |
|
| Outcomes | Live birth rate PR/couple Multiple PR Miscarriage rate Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned central computer‐generated list of numbers. |
| Allocation concealment (selection bias) | Low risk | An independent pharmacist distributed the medication. Packages with the study medication were numbered according to the computer generated list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of the participants and physicians was ensured by treating the women with identically‐appearing injections made by Merck‐Serono B.V. that were administered in the same fashion. Packages were double‐blinded for their content. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were analysed based on coded randomisation numbers without knowledge of whether the participant received GnRH antagonist or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart of the participant flow is provided; data are complete. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Carrera 2002a.
| Study characteristics | ||
| Methods | Randomisation: numeric list Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: rFSH alone (A): 10%; rFSH+GnRH agonist (B): 3% Blinding: not described ITT: not stated |
|
| Participants | 60 women; 60 cycles Age of women: rFSH alone (A): 32.1 ± 2.8 (yrs); rFSH+GnRH agonist (B): 32.5 ± 2.6 (yrs) Duration of infertility: rFSH alone (A) 3.2 ± 1.6 (yrs); rFSH+GnRH agonist (B) 3.4 ± 1.8 (yrs) Type of infertility: unexplained, male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: group A: rFSH 100 IU/d from CD3; group B: GnRH agonist 1 mg/d from CD 21 + rFSH 100 IU/d from CD 3 and 0.5 mg/d GnRHa from CD 3 (Procrin) Trigger for ovulation: hCG (10,000 IU) Timing of IUI: 36‐38 hrs after hCG Frequency of IUI: once Semen preparation technique: Percoll gradient Number of motile sperm injected: rFSH alone (A): 9.6±4.3 x10 6; rFSH+GnRH agonist (B): 8.8±4.9 x 10 6 Type of semen: husband semen Catheter used: Gynetics catheter Cancellation criteria: > 3 foll > 18 mm E2 > 1000 pg/mL |
|
| Outcomes | Clinical pregnancy rate/woman Miscarriage rate/woman Multiple PR/woman Number of ampoules used Number of dominant follicles ( > 17 mm) |
|
| Notes | Number of dominant follicles significantly higher in group B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred by a numeric list; not clear whether the personnel had access to this list and could influence the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding has been described. However, lack of blinding is unlikely to influence the primary outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting on exclusions or loss to follow‐up; in the tables; the number of participants per group is not stated. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected |
Cavagna 2009.
| Study characteristics | ||
| Methods | Randomisation: randomised trial, method of randomisation and allocation not described in abstract Trial design: parallel Power calculation: not described in abstract Dropouts: no cancellations Cycle cancellation: not described in abstract Blinding: not described in abstract ITT: not described in abstract |
|
| Participants | 38 women with an indication for IUI, age < 40 years, BMI > 20 | < 30, FSH < 10 and eumenorrhoeic cycles, 38 cycles Age of women: not described in abstract Duration of infertility: not described in abstract Type of infertility: not described, only indication for IUI treatment Previous fertility treatment: not described in abstract Primary infertility: not described in abstract |
|
| Interventions | Stimulation method/dosage: Group 1 CC 100 mg was supplemented with 75 IU rFSH on day 6, 8 and 10 (n = 20). Group 2 CC 100 mg was supplemented with 200 IU hCG starting on day 8 until day of ovulation. Trigger (n = 18). Trigger for ovulation: hCG ( 5000 IU) im Timing of IUI: not described in abstract Frequency of IUI: not described in abstract Semen preparation technique: not described in abstract Number of sperm injected: not described in abstract Type of semen: not described in abstract Catheter used: not described in abstract Cancellation criteria: not described in abstract |
|
| Outcomes | Ongoing pregnancy ( > 12 wks) PR/couple Multiples PR/woman Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not described explicitly in the abstract. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation has not been described explicitly in the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding has been described. However, this is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | They did report cancellations (none), but did not report dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No judgement possible based on the abstract. |
| Other bias | Low risk | Only data from abstract available. |
Crosignani 2007.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated list. Concealment of allocation with sealed opaque envelopes. Trial design: parallel (one cycle only) Power calculation: yes, 200 couples in each treatment arm necessary to detect a difference of 10% Dropouts: cycle cancellation and thus dropouts Cycle cancellation: yes, 18 rFSH+GnRH antagonist group, 20 rFSH group Blinding: not described ITT: yes |
|
| Participants | 299 women, 299 cycles Age of women: rFSH+GnRH antagonist: 31.3 ± 3.9 (yrs); rFSH alone: 31.2 ± 3.9 (yrs) Duration of infertility: rFSH+GnRH antagonist: 2.8±1.1 (yrs); rFSH alone: 2.8± 1.1 (yrs) Type of infertility: unexplained, male factor, endometriosis stage I or II Previous fertility treatment: not stated Primary infertility: rFSH+GnRH antagonist: 69.6% versus rFSH alone: 73.2% |
|
| Interventions | Stimulation method/dosage: rFSH 50 IU from CD 3, when DF > 13‐14 mm GRnH antagonist; rFSH 50 IU alone from CD 3 Trigger for ovulation: 5000 IU hCG Timing of IUI: 30‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: > 2 follicles >14 mm and/or > 4 follicles > 11 mm and/or serum oestrogen was > 800 pg/mL. Also in the absence of follicular growth |
|
| Outcomes | PR/couple Multiple PR/couple Number of ampoules used Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by means of a computer‐generated list; an independent list was established for each participating centre. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed in sealed opaque envelopes and were opened after inclusion. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding was performed; however this is unlikely to influence the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Writers provided a complete overview of included participants and dropouts; incomplete data are not suspected. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; bias not suspected. |
| Other bias | Low risk | Not suspected. |
Danhof 2018.
| Study characteristics | ||
| Methods | Randomisation: women were randomised using a central password‐protected Internet‐based randomisation program. The randomisation list had been prepared by an independent statistician with a variable block size with randomly selected block sizes that varied between two, four and six. Trial design: parallel Power calculation: yes Dropouts: switched to different treatment Cycle cancellation: yes Blinding: no ITT: yes |
|
| Participants | 738 women with unexplained or mild male subfertility and an unfavourable prognosis according to the Hunault model for natural conception, 2374 cycles Age of women: FSH: 33.1 ± 5.6 (yrs); CC: 33.1 ± 4.6 (yrs) Duration of infertility: FSH: 24.0 (19‐33 (months)); CC: 24.0 (19‐32 (months)) Type of infertility: unexplained, one side tubal pathology and mild male factor Previous fertility treatment: yes, but without hormones Primary infertility: both primary as secondary |
|
| Interventions | Stimulation method/dosage: In the experimental arm, women started with daily subcutaneous injections of 75 IU FSH on Day 3, 4 or 5 of the menstrual cycle and continued these injections until the day of ovulation Triggering: In the standard arm, women started with 100 mg CC on Day 3, 4 or 5 of the menstrual cycle. The tablets were administered orally and stopped after 5 days of daily intake. Trigger for ovulation: hCG (5000 IU or 250 µg) im Timing of IUI: 36‐42 hrs after hCG Frequency of IUI: single Semen preparation technique: the semen was processed according to local protocol. Number of sperm injected: not described Type of semen: mostly homologue Catheter used: not described Cancellation criteria: more than three follicles with a diameter of ≥ 14 mm or five follicles with a diameter of ≥12 mm was seen at transvaginal ultrasound, regardless of the endometrial thickness |
|
| Outcomes | CPR/couple OPR/couple Live birth per couple Multiples PR/couple Number of ampoules used Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a web‐based data system |
| Allocation concealment (selection bias) | Low risk | Couples were randomly allocated after randomisation was performed centrally (third party). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding performed; however lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Prespecified outcomes were reported. Protocol is available, thus not suspected. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Dankert 2007.
| Study characteristics | ||
| Methods | Randomisation: randomisation occurred by computer generated list prepared by another institution, contacted by a central phone number. Trial design: parallel Power calculation: yes Dropouts: yes, described Cycle cancellation: yes, described Blinding: no blinding described ITT: yes |
|
| Participants | 138 women, 406 cycles Age of women: CC: 31.0 (23.5‐37.04) (yrs); FSH: 31.6 (19.7‐38.3) (yrs) Duration of infertility: CC: 33.4 (17‐99) (months); FSH: 34.0 (14‐122) (months) Type of infertility: unexplained or male subfertility Previous fertility treatment: no; previous assisted reproduction attempts were excluded Primary infertility: only primary infertility for at least 24 months. |
|
| Interventions | Stimulation method/dosage: starting dose of CC was 100 mg/day on days 3‐7 of the menstrual cycle, when monofollicular or excessive growth, the dosage was increased/decreased in the next cycle by 50 mg. Starting dose in the rFSH group was 75 IU/day from day 3 of the cycle until follicular maturation was reached, when monofollicular or excessive development occurred the starting dose was increased or decreased by 37.5 IU in the next cycle. When no follicular development was seen on day 11, the dose was increased to 112.5 IU/day Trigger for ovulation: (5000 IU hCG) im Timing of IUI: 38‐40 hrs after hCG Frequency of IUI: single IUI Semen preparation technique: the semen specimen was diluted with 5 mL of human tubal fluid (HTF) medium, containing 10% gepasteuriseerde plasma oplossing (GPO) (pasteurised plasma solution). A gradient technique with Pure Sperm 100 was used. After centrifugation at 600‐900 xg for 20 min, the pellet was resuspended and washed twice with HTF/10GPO. Number of sperm injected: not described Type of semen: husband Catheter used: not described Cancellation criteria: HCG was withheld if more than three follicles with a diameter of > 14mm were seen and unprotected intercourse was strictly advised. |
|
| Outcomes | Live birth PR/couple Multiple PR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated list, prepared at a different institution, contacted by a central phone number. |
| Allocation concealment (selection bias) | Low risk | Central allocation by third party |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Both primary and secondary outcomes have been described in the data section. Furthermore, a full report of intention‐to‐treat analysis and dropouts has been provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Unclear risk | Due to decisions made by the Dutch government during the study period, the study did not reach its necessary number of couples as calculated by the power analysis. It is questionable if there is some kind of bias due to this 'unwanted' event and its influence on the willingness of couples taking part in the study. |
Dansuk 2015.
| Study characteristics | ||
| Methods | Randomisation: participants were randomised using an online research randomiser software program. Trial design: parallel Power calculation: yes Dropouts: yes, 18 women Cycle cancellation: yes Blinding: not described ITT: not described |
|
| Participants | 126 women Age of women: not described, only for the whole group Duration of infertility: group A: 8.39 ± 4.44 (years); Group B: 8.02 ± 4.60 (years) Type of infertility: not described Previous fertility treatment: yes, participants were included if they had a history of two cycles of ovarian stimulation with clomiphene citrate. Participants with previous IUI were excluded. Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: group A received rFSH and GnRH antagonist, most of the patients started with 75 IU, dose was adjusted for the next cycle according to the response, Group B received only rFSH in the same manner. In both groups, the stimulation was started on the third day of the cycle. Trigger for ovulation: Pregnyl (10000 IU) im Timing of IUI: 34‐38 hrs after hCG Frequency of IUI: single Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described. Catheter used: not described Cancellation criteria: premature luteinisation, progesterone level > 1.7 ng/dL during COH, premature LH‐peak, LH level >12.1 on hCG trigger day, probability of multiple gestations due to the presence of more than four follicles > 15mm and poor response to treatment (no follicle > 10mm). |
|
| Outcomes | CPR/couple Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly divided into two groups using online research randomiser software. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No flow chart has been provided; however a seemingly complete overview of the participant selection and exclusion is provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Davar 2006.
| Study characteristics | ||
| Methods | Randomisation: participants were randomised by using a computer‐generated randomisation table Trial design: parallel Power calculation: not described Dropouts: not described Cycle cancellation: not described Blinding: the physician performing the ultrasound was blinded; not clear whether participants and outcome assessors were blinded ITT: not described |
|
| Participants | 115 women, 115 cycles Age of women: LZ+rFSH: 28.7 ± 2.9 (years); CC+rFSH: 25.7 ± 3.8 (years) Duration of infertility: LZ+rFSH: 6.2±2.4 (years); CC+rFSH: 5.45± 2.5 (years) Type of infertility: unexplained and mild male infertility Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: letrozole 5 mg/day from day 3‐7 of the menstrual cycle, combined with rFSH 150 IU/day starting on day 8. Clomiphene citrate 100 mg/day from day 3‐7 of the menstrual cycle, combined with rFSH 150 IU/day starting on day 8. Trigger for ovulation: hCG (10,000 IU) im Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: single IUI Semen preparation technique: swim down procedure Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | CPR/couple Multiples PR/couple Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | One physician performed the ultrasound examination and she was blinded to the group status. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All stated outcomes were reported. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Deghani‐Firouzabady 2006.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation table; concealment not stated. Trial design: parallel Power calculation: no Dropouts: none Cycle cancellation: none Blinding: not stated ITT: yes, all participants were analysed in the same group |
|
| Participants | 60 women, 60 cycles Age of women: CC+150 IU hMG, from DF > 14 mm, hCG 250 IU instead of hMG: 26.4 ± 3.8 (yrs); CC+150 IU hMG: 24 ± 3.4 (yrs) Duration of infertility: CC+150 IU hMG, from DF > 14 mm, hCG 250 IU instead of hMG: 4.1±1.1 (yrs); CC+150 IU hMG: 3.7± 1.2 (yrs) Type of infertility: unexplained, mild male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: Group A: 100 mg CC+150 IU hMG, from DF > 14 mm, hCG 250 IU instead of hMG; Group B: 100 mg CC+ 150 IU hMG. Trigger for ovulation: 10,000 IU hCG Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated No of sperm injected: not stated Type of semen: not stated explicitly but normal semen‐analysis Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple multiple PR/couple OHSS rate Number of dominant follicles ( > 14 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to each group by using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described; however lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the participants who entered the study completed the treatment; a flow chart has been provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Demirol 2007.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation table with sealed envelopes by specialist nurse Trial design: parallel Power calculation: yes, to detect an absolute difference of 15% pregnancy rate, 80 participants in each group would be sufficient Dropouts: none Cycle cancellation: none Blinding: not described ITT: yes |
|
| Participants | 241 women, number of cycles not stated Age of women: rFSH 30.4 ± 2.88 yrs; uFSH 31.5 ± 3.6 yrs; hMG 30.8 ± 3.2 yrs Duration of infertility: rFSH 3.3±1; uFSH 2.9±0.87; hMG 3.2±1 Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: 100% |
|
| Interventions | Stimulation method/dosage: rFSH, uFSH and hMG, BMI < 25 75 IU, BMI> 25 150 IU, from CD 2‐3 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: Puresperm Number of motile sperm injected: not stated, no significant difference in semen characteristics Type of semen: husband semen Catheter used: not stated Cancellation criteria: decreasing E2 levels or more than three follicle of > 16 mm diameter |
|
| Outcomes | PR/couple: rFSH 21/81; uFSH 11/80; hMG 10/80 PR/cycle: not stated Miscarriage rate per pregnancy: rFSH 2/21; uFSH 2/11; hMG 1/10 Multiple PR: rFSH 2/21; uFSH 0/11; hMG 1/10 OHSS rate: rFSH 0%; uFSH 0%; hMG 0% Total dose used: rFSH 825±174; uFSH 1107±178; hMG 1197±212 Number of dominant follicles: rFSH 2.1±1.2; uFSH 1.3±0.8; hMG 1.4±0.9 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with a computer‐generated random number list. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes by third party; unclear whether envelopes were opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart is provided, showing loss to follow‐up/dropouts. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Dhaliwal 2002.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated random table Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described |
|
| Participants | 200 women, 420 cycles Age of women: CC+hMG minimal 28.5 ± 4.2 (yrs); CC+hMG convent 30.1 ± 4.6 (yrs) Duration of infertility: CC+hMG minimal 6.1±2.8; CC+hMG convent 6.9±2.9 (yrs) Type of infertility: unexplained, ovulatory dysfunction with CC failure Previous fertility treatment: CC use Primary infertility: CC+hMG minimal: 74%; CC/hMG conventional: 78% |
|
| Interventions | Stimulation method/dosage: CC+hMG conventional: 100 mg CC daily day 3‐7 + hMG 75‐150 IU daily day 5‐9
CC+hMG minimal: CC 100 mg daily day 3‐7+ hMG 150 IU once day 9 Trigger for ovulation: 5000 IU hCG Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: once Semen preparation technique: swim‐up Number of motile sperm injected: not stated Type of semen: husband semen Catheter used: IUI cannula Cancellation criteria: not stated |
|
| Outcomes | PR/woman Multiple PR Miscarriage rate OHSS Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided into two groups according to random table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation has not been described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not clear. |
Diamond 2015.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation schedule Trial design: parallel Power calculation: yes Dropouts: described Cycle cancellation: not stated explicitly Blinding: AI and CC were coated, sham injection was not feasible |
|
| Participants | 900 women Age of women: CC 32.0±4.6 (yrs); gonadotrophins 32.3±4.1 yrs; AI 32.2±4.3 yrs Duration of infertility: CC 34.2±24.1 months; hMG 34.8±26.2 (months); letrozole 35.2±26.8 (months) Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: CC: 77.7%; hMG: 78.7%; letrozole |
|
| Interventions | Stimulation method/dosage: not stated in study but stated in clinicaltrials.gov: A daily dose of 5 mg of the AI, letrozole, will be administered orally for five days starting on day three of the menstrual cycle. Future cycles can be started at 2.5‐7.5 mg/d. CC will be administered at a dose of 100 mg/d on cycle days 3‐7. Future cycles can be started at 50‐150 mg/d. A daily injection of 150 IU of FSH will be administered subcutaneously starting on day three of the menstrual cycle and continuing until the day of hCG administration. Dosage will be able to be increased or decreased 37.5‐75 IU/d beginning cycle day 7. Future cycles can be started at doses ranging from 75‐225 IU/d. The same type of FSH injections will be used. Trigger for ovulation: 10,000 IU hCG Timing of IUI: 40 hours after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: husband Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Live birth rate Multiple PR Miscarriage rate OHSS Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list with blocked allocation |
| Allocation concealment (selection bias) | Low risk | Third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tablets were coated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiners were neither routinely informed nor explicitly kept unaware of the treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Low risk | Not suspected |
Dodson 1991.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: cross‐over Power calculation: yes Dropouts: not stated Cycle cancellation: hMG: 8 (10%); hMG/leuprolide: 9 (11%) Blinding: not described ITT: not stated |
|
| Participants | 97 women, 159 cycles Age of women of study population: 33.0 ± 4.1 (yrs) Duration of infertility: 4.3 ± 2.7 (yrs) Type of infertility: male factor, endometriosis, adnexal adhesion, unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: hMG: 75 IU daily from CD 7; hMG/leuprolide: 4‐7 days before onset of menstrual period leuprolide 1 mg/day sc. until hCG injection hMG: CD 2‐3 75‐225 IU Trigger for ovulation: 5000 IU hCG Timing of IUI: 40 hrs after hCG Frequency of IUI: once Semen preparation technique: double wash Number of motile sperm injected: not stated Type of semen: not stated explicitly but normal semen‐analysis Catheter used: not stated Cancellation criteria: > 7 follicles > 17 mm E2 > 2000 pg/mL |
|
| Outcomes | Ongoing pregnancy/live births Miscarriage rate for total group Multiple PR for total group Number of ampoules used Number of dominant follicles ( > 16 mm) |
|
| Notes | No first data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to one of the arms; method of randomisation has not been described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was described. |
| Other bias | Low risk | Not suspected. |
Ecochard 2000.
| Study characteristics | ||
| Methods | Randomisation: random number table Trial design: cross‐over Power calculation: yes Dropouts: not stated Cycle cancellation: CC 7 cycles; hMG 2 cycles Blinding: not described ITT: yes |
|
| Participants | 58 women; 56 first cycles; 174 cycles in total Age of women: CC: 30.4 ± 3.5 (yrs); hMG: 31.5 ± 3.7 (yrs). Duration of infertility: CC 4.0 ± 2.0 (yrs); hMG 3.3 ± 2.0 Type of infertility: female factor, male factor, unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: CC: 50‐100 mg daily day 3‐7; hMG: 150 IU/d day 4,6,8,9 Trigger for ovulation: 5000 IU hCG Timing of IUI: 36 hrs after hCG or 24 hrs after LH surge + hCG Frequency of IUI: once Semen preparation technique: Percoll density gradient Number of motile sperm injected: not stated Type of semen: not stated explicitly but normal semen‐analysis Catheter used: not stated Cancellation criteria: > 3 follicles > 14 mm E2 > 1200 pg/mL |
|
| Outcomes | Pregnancy/couple Miscarriage rate Multiple PR OHSS for the total group Number of dominant follicles ( > 16 mm) |
|
| Notes | First cycle data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes, with assignment determined by a random number table; unclear whether envelopes were sealed and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No information about multiple pregnancies. |
| Other bias | Low risk | Not suspected. |
El Helw 2002.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described in abstract ITT: not stated |
|
| Participants | 53 women; cycles not stated Age of women: not stated Duration of infertility: not stated Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: letrozole: 20 mg single dose CD3; CC: 100 mg/d day 3‐7 Trigger for ovulation: 5000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated, but not significantly different between groups Type of semen: not stated explicitly but normal semen‐analysis Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Pregnancy/couple Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information in the abstract to permit judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in abstract for judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in abstract for judgement. |
| Other bias | Low risk | Only data from abstract available. |
Erdem 2015.
| Study characteristics | ||
| Methods | Randomisation: using a software‐generated random allocation sequence (for 2 consecutive cycles within 6 months). Trial design: parallel Power calculation: yes Dropouts: yes (22 for personal reasons) Cycle cancellation: not stated. Blinding: all participants were treated by only two investigators; unclear whether these were blinded for the treatment. ITT: all participants remained in the same group throughout the study |
|
| Participants | 219 women, 341 cycles Age of women: rFSH: 28.8 ± 4.4 (yrs); CC: 29.2 ± 5.8 (yrs) (NS) Duration of infertility: rFSH: 3.4±2.0 (yrs); CC: 3.3± 2.7 (yrs) Type of infertility: unexplained and male infertility Previous fertility treatment: not described Primary infertility: 94% vs 90% was primary infertility |
|
| Interventions | Stimulation method/dosage: CC group received 100 mg/day orally between days 3–7 of the cycle. rFSH group received starting dose of 75 IU/day subcutaneously initiated on day 3 of cycle. Trigger for ovulation: Ovitrelle (250 ucg rhCG) im Timing of IUI: ‐ 36 hrs after hCG Frequency of IUI: single Semen preparation technique: gradient method, with Sperm Grad‐125 Number of sperm injected: not described Type of semen: homologue Catheter used: disposable catheter Cancellation criteria: cycles with more than four dominant follicles and/or serum E2 >1500 pg/mL were cancelled to avoid OHSS and multiple pregnancies. Cycles were cancelled when there was no dominant follicle ( > 14 mm) development in spite of 10 days of maximum dose (150 IU). |
|
| Outcomes | CPR/couple Live birth/couple Multiple PR Miscarriage rate Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred by software‐generated random allocation. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a independent investigator, who was blinded to the participants' identity. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A clear and seemingly complete overview of included and excluded participants has been provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Espejo‐Catena 2016.
| Study characteristics | ||
| Methods | Randomisation: the participants were randomly divided into a treatment group (Group A: 150 participants) and a control group (Group B: 150 participants) using online research randomiser software Trial design: parallel Power calculation: unknown Dropouts: yes (1 for personal reasons, 1 loss to follow‐up) Cycle cancellation: yes (19 due to excess follicle number) Blinding: no blinding described ITT: not described (exclusions were not analysed) |
|
| Participants | 279 women, 279 cycles Age of women: rFSH+GnRH‐ant: 31.26 ± 4.06 (yrs); rFSH only: 32.19 ± 3.95 (yrs) (NS) Duration of infertility: not described Type of infertility: not described Previous fertility treatment: first COS Primary infertility: both primary and secondary |
|
| Interventions | Stimulation method/dosage: Group A maintained the COS treatment and received a subcutaneous daily dose of a GnRH antagonist (0.25 mg, CetrotideR or OrgalutranR) until the day that recombinant hCG was given, while Group B followed controlled ovarian stimulation treatment until the hCG day Trigger for ovulation: Ovitrelle (rhCG 250ug/0.5ml) im Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: swim‐up technique Number of sperm injected: 0.3‐0.5ml Type of semen: homologue (after masturbation) Catheter used: disposable flexible catheter (Gynetics) Cancellation criteria: not described |
|
| Outcomes | CPR/couple Multiple PR Miscarriage rate Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer. |
| Allocation concealment (selection bias) | Unclear risk | By using online randomisation, we assume that allocation could not be influenced. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart has been provided; no suspicion of missing data. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Fatemi 2003.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated random number table Trial design: parallel Power calculation: not performed Dropouts: not stated Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 15 women; cycles not stated Age of women: letrozole: 28.9 (yrs); CC: 28.2 (yrs) Duration of infertility: not stated Type of infertility: unexplained Previous fertility treatment: not stated Primary and secondary subfertility included |
|
| Interventions | Stimulation method/dosage: letrozole: 2.5 mg CD 3‐7; CC: 100 mg/d day 3‐7 Trigger for ovulation: endogenous LH surge Timing of IUI: 24 hrs after LH surge Frequency of IUI: once Semen preparation technique: not stated No of motile sperm injected: not stated Type of semen: not stated explicitly but normal semen analysis Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Pregnancy/couple Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. However, is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full description of the results and primary outcomes. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Small pilot study. |
Filicori 2001.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not performed Dropouts: not stated Cycle cancellation: not stated Blinding: the participants were not blinded to the therapy; the physician performing the pelvic ultrasound was blinded as to which arm the participants belonged. ITT: not stated |
|
| Participants | 50 women; 50 cycles Age of women: FSH: 32±1 (yrs); hMG: 33±1 (yrs) Duration of infertility: not stated Type of infertility: unexplained, mild male factor Previous fertility treatment: ovulation induction in some women Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: LHRH agonist single dose in midluteal‐phase in both groups. r‐FSH 150 IU/d versus hMG: 150 IU/d Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: swim‐up technique No of motile sperm injected: not stated Type of semen: not stated explicitly, but seems husband semen Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Pregnancy/couple Multiple PR OHSS Miscarriages Number of ampoules used Number of dominant follicles ( > 14 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear description of randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No clear description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of physician. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the treatment schedule. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Filicori 2003.
| Study characteristics | ||
| Methods | Randomisation: stated without further description, allocation not stated Trial design: parallel Power calculation: not stated Dropouts: Group A rFSH: 2 participants; Group B hMG: 0 Cycle cancellation: not stated Blinding: the participants were not blinded to their assigned therapy, the physician performing the pelvic ultrasound was blinded as to which treatment protocol the participants belonged. |
|
| Participants | 50 women; 50 cycles Age of women: rFSH: 31.9±0.7 (yrs); hMG: 32.6±0.5 (yrs) Duration of infertility: not stated Type of infertility: unexplained, mild male factor Previous fertility treatment: ovulation induction in some women (9 in rFSH group and 13 in hMG) Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: LHRH agonist single dose in midluteal‐phase in each group. rFSH: 150 IU/d; hMG: 150 IU/d Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: swim‐up technique Number of motile sperm injected: not stated Type of semen: partners' semen Catheter used: not stated Cancellation criteria: when on day 21 no dominant follicles were seen on ultrasound |
|
| Outcomes | Pregnancy/couple Multiple PR Miscarriage rate per pregnancy Number of ampoules used Number of dominant follicles ( > 14 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to two age‐ and weight‐matched groups; however method of randomisation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Not clearly mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded to the treatment; however it is unlikely that this would influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Fouda 2011.
| Study characteristics | ||
| Methods | Randomisation: participants were randomly allocated to extended letrozole group or clomiphene citrate group using a computer‐generated randomisation list and sequentially numbered opaque sealed envelopes, each containing the allocation information written on a card. Envelopes were opened sequentially by a study nurse to allocate participants to the assigned group. Trial design: parallel Power calculation: yes, performed Dropouts: yes, described Cycle cancellation: no Blinding: participants were not blinded to their therapy; the physician who performed the pelvic ultrasound was blinded to the treatment protocol. ITT: not clearly described. |
|
| Participants | 214 women, 421 cycles Age of women: letrozole: 26.68 ± 3.51 (years); CC: 26.13 ± 3.22 (years) Duration of infertility: letrozole: 3.69±1.88 (years); CC: 3.40± 1.62 (years) Type of infertility: unexplained infertility for at least one year Previous fertility treatment: not described Primary infertility: both primary and secondary |
|
| Interventions | Stimulation method/dosage: The extended letrozole group were treated with letrozole (Femara; Novartis pharma AG, Basle, Switzerland) 2.5 mg/day from cycle day 1 to 9. The clomiphene citrate group were treated with clomiphene citrate (Clomid; Aventis pharma S.AE, Global Napi pharmaceuticals, Cairo, Egypt) 100 mg/day from cycle day 3 to 7. All the participants underwent 1 to 3 IUI cycles. Trigger for ovulation: Pregnyl ( 10000 IU) im Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: not described Number of sperm injected: not described Type of semen: probably husband semen Catheter used: soft tip catheter Cancellation criteria: not described |
|
| Outcomes | Cumulative PR/couple Live birth/ongoing PR Multiple PR Miscarriage PR Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated using a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes, each containing the allocation information on a card, were opened sequentially by a study nurse to allocate participants to the assigned group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding was performed of participants; the physician who performed the pelvic ultrasound was blinded to the treatment protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The doctor responsible for ultrasound examination was blinded to the treatment protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full description of participant flow is reported, as well as reporting the negative outcomes (such as miscarriage rate and multiple pregnancies). |
| Selective reporting (reporting bias) | Low risk | All endpoints have been reported. |
| Other bias | Low risk | Not suspected. |
Galal 2015.
| Study characteristics | ||
| Methods | Randomisation: by computer system Trial design: prospective randomised controlled study, parallel Power calculation: sample size calculation by Epi‐Info software Dropouts: not described Cycle cancellation: not described Blinding: not described in abstract ITT: not described |
|
| Participants | 100 women, 100 cycles Age of women: LZ: 26.3 ± 4.4 (yrs); HMG: 25.9 ± 3.8 (yrs) Duration of infertility: not described Type of infertility: not described Previous fertility treatment: not described Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: Group A received a controlled ovarian hyperstimulation done by step‐up protocol of letrozole from day 2 or 3 of menstrual cycle, starting with a dose of 2.5 mg increased daily by 2.5 mg for other 3 days. Group B received controlled ovarian hyperstimulation by HMG ampoules, tailored according to the response. Trigger for ovulation: hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: single Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | CPR/couple | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by computer number system, no details, no full text published. |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstract, insufficient information to make a judgement and no full text. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in abstract; however lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Low risk | Only data from abstract available. |
Gerli 1993.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not performed Dropouts: not stated Cycle cancellation: 3 cycles cancelled Blinding: no ITT: not stated |
|
| Participants | 32 women; 34 cycles Age of women: FSH: 30.9±2.7 (yrs); hMG: 31.4±3.6 (yrs) Duration of infertility: FSH: 2.3±0.6 (yrs); hMG: 2.6±0.8 (yrs) Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: both groups LHRH agonist single dose in midluteal‐phase. r‐FSH 225 IU/d; hMG: 225 IU/d Trigger for ovulation: 5,000 IU hCG Timing of IUI: 12 and 36 hrs after hCG Frequency of IUI: twice Semen preparation technique: swim‐up technique Number of motile sperm injected: not stated Type of semen: not stated explicitly, but seems husband semen Catheter used: not stated Cancellation criteria: participants at risk for OHSS based on ultrasound, hCG was withheld |
|
| Outcomes | Pregnancy/couple OHSS Number of ampoules used Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated explicitly. |
| Allocation concealment (selection bias) | Unclear risk | Not stated explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. However, is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sufficient information about cancelled cycles and inclusions. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected |
Gerli 2004a.
| Study characteristics | ||
| Methods | Randomisation: randomisation table Trial design: parallel Power calculation: not stated Dropouts: 2 participants Cycle cancellation: uFSH: 4; rFSH: 5 Blinding: not described ITT: yes |
|
| Participants | 67 women, 138 cycles Age of women: uFSH: 31.7±3.4 (yrs); rFSH: 31.2±3.2 (yrs) Duration of infertility: uFSH: 2.8± 1.3; rFSH: 2.9±1.5 Type of infertility: ovulatory factor, male factor, unexplained fertility Previous treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: uFSH: 75 IU/d from CD 2; rFSH: 50 IU/d from CD 2 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 32‐40 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated No of motile sperm injected: not stated Type of semen: normal semen analysis thus likely husband semen Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Pregnancy/couple Miscarriage rate Multiple PR Number of ampoules used Number of dominant follicles ( > 17 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not described explicitly |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described; however is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Ghosh Dastidar 2009.
| Study characteristics | ||
| Methods | Randomisation: randomly assigned, method of randomisation not stated Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: 5 (due to poor response or more than 4 follicles) Blinding: not stated ITT: not stated |
|
| Participants | 188 women, 188 cycles Age of women: not stated Duration of infertility: not stated (abstract) Type of infertility: unexplained or mild male factor Previous fertility treatment: not stated Primary infertility: number not stated |
|
| Interventions | Stimulation method/dosage: Group A: participants received 75 IE rFSH daily starting from day 3 of the cycle, when a follicle of 13‐14 mm was observed, Cetrorelix was started and rFSH was replaced by hMG. Women in group B received the same treatment but continued to use rFSH. Trigger for ovulation: 5000 IU hCG Timing of IUI: 35‐38 hrs after hCG Frequency of IUI: single insemination Semen preparation technique: not stated No of sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: poor response or excessive response ( > 4 follicles > 16mm) |
|
| Outcomes | Ongoing PR/couple Live birth rate Miscarriages Ectopic pregnancies |
|
| Notes | Very specific comparison; no comparison of interest yet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation has not been described, only abstract data, no full text. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation has not been described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described if blinding was performed; however lack of blinding is unlikely to influence the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout or loss to follow‐up has not been described. |
| Selective reporting (reporting bias) | Unclear risk | No mention of miscarriage rate or multiple pregnancies (negative outcomes), selective reporting not excluded. |
| Other bias | Low risk | Not suspected. |
Gomez‐Palomares 2005.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated list Trial design: parallel Power calculation: not stated Dropouts: none Cycle cancellation: FSH/GnRH antagonist: 1 cycle; FSH alone: 1 cycle Blinding: not described ITT: not stated |
|
| Participants | 82 women; 82 cycles Age of women: FSH/GnRH antagonist: 33.9±2.6; FSH alone: 32.1±3.3 Duration of infertility: at least 1 year Type of infertility: unexplained, mild male factor Previous fertility treatment: not stated Primary infertility: FSH/GnRH antagonist: 36 women; FSH alone: 39 women |
|
| Interventions | Stimulation method/dosage: FSH/GnRH antagonist: 100 IU/d 5 days GnRH antagonist from DF 16 mm or when E2 > 300 pg/mL 0.25 mg sc; FSH alone: 100 IU/d from CD 3‐4 Trigger for ovulation: 5,000 IU hCG Timing of IUI: 36‐38 hrs after hCG Frequency of IUI: once Semen preparation technique: swim‐up technique Number of motile sperm injected: FSH/GnRH antagonist: 23.4±9.3; FSH alone: 19.9±18.4 Type of semen: normal semen analysis thus husband semen Catheter used: Lee catheter Cancellation criteria: > 4 follicles > 16‐20 mm |
|
| Outcomes | Live birth rate Pregnancy/couple Multiple PR OHSS Miscarriage rate per pregnancy Number of ampoules used Number of dominant follicles ( > 15 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear whether a power analysis was performed nor how many participants were asked to participate. Therefore, no information could be found about missing data or loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; not enough information about missing data or loss to follow‐up. |
| Other bias | Low risk | Not suspected. |
Gomez‐Palomares 2008.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated list; allocation: according to the order of their enrollment Trial design: parallel (only first IUI cycle was included) Power calculation: not described Dropouts: no Cycle cancellation: yes (21 cycles were cancelled) Blinding: not described ITT: not described |
|
| Participants | 367 women, 367 cycles Age of women: GnRH antagonist group: 32.9±2.5 (yrs); rFSH alone: 32.1 ± 3.9 (yrs) Duration of infertility: not stated Type of infertility: mild male factor, unexplained subfertility Previous fertility treatment: no IUI Primary infertility and secondary subfertility included |
|
| Interventions | Stimulation method/dosage: 75‐150 IU rFSH for 5 days, step‐down protocol when leading follicle was 13 mm in diameter; 75‐150 IU rFSH in same fashion + 0.25 mg GnRH antagonist/day added when leading follicle was > 16 mm until the day of hCG. Luteal support was given from IUI 600 mg/d progesterone vaginally Trigger for ovulation: 5,000 IU hCG Timing of IUI: 36‐38 hrs after hCG Frequency of IUI: once Semen preparation technique: swim‐up technique Number of sperm injected: GnRH antagonist: 25±15.1; rFSH alone: 31.5±20.8 Type of semen: husband semen Catheter used: not stated Cancellation criteria: > 4 follicles (16‐20 mm) were present by the day hCG was administered, unexpected ovulation |
|
| Outcomes | PR Multiple PR Miscarriage rate Number of FSH units used Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate is not described, no flow chart of participants available. |
| Selective reporting (reporting bias) | Low risk | Not suspected, outcomes have been described, also negative outcomes such as miscarriage rate. |
| Other bias | Low risk | Not stated explicitly. |
Gregoriou 2008.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated random number, allocation not stated Trial design: parallel Power calculation: expected PR of 7% per letrozole cycle and of 13% per rFSH cycle, sample size required was 400 cycles Dropouts: 1 participant did not completed the study in the remaining 6 months Cycle cancellation: rFSH: 2 cycles; letrozole 7 cycles (due to no follicular development) Blinding: not described ITT: not stated |
|
| Participants | 50 women, 131 cycles. Age of women: rFSH: 31.5 ± 3.7 (yrs); letrozole: 32.1 ± 3.9 (yrs) Duration of infertility: rFSH: 3.9±0.5 (years); letrozole: 3.6 ± 0.4 (years) Type of infertility: unexplained subfertility Previous fertility treatment: failed to conceive after three cycles of ovarian stimulation with CC combined with IUI Primary infertility: rFSH: 0.4±0.1; letrozole: 0.3±01 |
|
| Interventions | Stimulation method/dosage: 150 IU rFSH every 2 days; letrozole 5 mg on days 3‐7 of the cycle, 100 mg vaginal micronised progesterone every day after IUI Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: gradient technique (Percoll) Number of sperm injected: not stated. Type of semen: husband Catheter used: Gynetics flexible IUI cannula Cancellation criteria: no follicular development |
|
| Outcomes | PR/couple Live birth rate Multiple PR Miscarriage rate Ectopic pregnancy rate Number of FSH units used Number of dominant follicles ( > 14 mm) |
|
| Notes | In November 2005, the manufacturing company of the drug issued a statement advising that letrozole should not be used for ovulation induction in premenopausal women; thus, the recruitment was interrupted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. However, is unlikely to influence the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Low risk | All outcomes were stated. |
| Other bias | Unclear risk | Due to manufacturer's statements advising not to use letrozole for ovulation induction, the inclusion of participants stopped prematurely. Therefore the necessary number of participants was not met. |
Haqnawaz 2013.
| Study characteristics | ||
| Methods | Randomisation: participants were assigned to a group by random sampling technique Trial design: parallel Power calculation: not stated Dropouts: none Cycle cancellation: none Blinding: not described ITT: not stated |
|
| Participants | 500 women, 500 cycles Age of women: 24‐44 years Duration of infertility: > 2 years Type of infertility: unexplained, PCOS, early stage endometriosis, borderline male factor infertility Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: group A received letrozole 5 mg five days and gonadotrophins 75 IU once daily for 3‐5 days and group B received clomiphene citrate 50 mg for 5 days and gonadotrophins 75 IU once daily for 3‐5 days. Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: normal semen analysis thus husband semen Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Pregnancy/couple Live birth rate OHSS Number of dominant follicles Multiple PR Miscarriages |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sampling technique. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation by random sampling technique and allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not described. However, lack of blinding is not likely to influence the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up, however no flow chart has been provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Hughes 1998.
| Study characteristics | ||
| Methods | Randomisation: centralised randomisation scheme, allocation not stated explicitly Trial design: parallel Power calculation: yes Dropouts: Group A: 3; Group B: 1 Cycle cancellation: 17% in each group Blinding: not described ITT: not stated |
|
| Participants | 63 women; 59 cycles Age of women: Group A: 32.2±3.4; Group B: 33.0±5.0; Group C: 32.1±4.0 (yrs) Duration of infertility: Group A: 47.2±20; Group B: 51.3±35.1; Group C: 43.9±22.8 (months) Type of infertility: unexplained, endometriosis, tubal disease Previous fertility treatment: in most participants (90%) CC and IUI Primary infertility: 67% |
|
| Interventions | Stimulation method/dosage: Group A: rFSH day 4 150 IU, day 6 and 8: 75 IU/day; group B: rFSH day 4, 6 and 8 150 IU/day; Group C: rFSH: day 4, 6, 8, 10 150 IU/day Trigger for ovulation: 5,000 IU hCG Timing of IUI: 24 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: normal semen analysis thus husband semen Catheter used: not stated Cancellation criteria: no follicle development on day 18. > 2 follicles > 17 mm |
|
| Outcomes | Pregnancy/couple PR/cycle Number of ampoules used Number of dominant follicles ( > 14 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not described. However, lack of blinding is not likely to influence the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected, all outcomes have been described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Jain 2016.
| Study characteristics | ||
| Methods | Randomisation: prospective randomised case‐controlled study Power calculation: yes Dropouts: yes (3) Cycle cancellation: yes (17) Blinding: not described ITT: no |
|
| Participants | 331 women, 427 cycles Age of women: rFSH+GnRH‐a: 30.5 ± 3.69 (yrs); rFSH: 30.0 ± 3.46 (yrs) Duration of infertility: rFSH+GnRH‐a: 3.0, 2.0‐4.5 (yrs); rFSH: 3.0, 2.0‐5.0 (yrs) Type of infertility: unexplained Previous fertility treatment: not described Primary infertility: both |
|
| Interventions | Stimulation method/dosage: Both groups received r‐FSH ranging from 75 IU upward (depending on BMI, age and anticipated ovarian response) starting on day 2/3 of the cycles. In the study group, when a dominant follicle of 15 mm or more was detected, a GnRH antagonist was started at a dose of 0.25 mg until hCG administration. Trigger for ovulation: Ovulation triggering occurred with 5000 IU hCG Frequency of IUI: single IUI Timing of IUI: 44‐48 hrs after hCG Semen preparation technique: double density gradient technique Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | Clinical PR Pregnancy loss rate Ongoing PR Multiple PR Ovarian hyperresponse rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated randomisation was done between study and control groups on the day of initiation of stimulation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected, a detailed flow chart of enrollment was provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Jamal 2005.
| Study characteristics | ||
| Methods | Randomisation: prospective randomised study Trial design: parallel Power calculation: not described in abstract Dropouts: not described in abstract Cycle cancellation: not described in abstract Blinding: not described in abstract ITT: not described in abstract |
|
| Participants | 80 women, 80 cycles Age of women: not described in abstract Duration of infertility: not described in abstract Type of infertility: unexplained Previous fertility treatment: not described in abstract Primary infertility: not described in abstract |
|
| Interventions | Stimulation method/dosage: letrozole 5 mg/day on day 3‐7 of the cycle vs hMG started on day 3 at a dose of 75 IU for women < 30 years old and 150 IU for women > 30 years old and continued until at least one follicle reached 17 mm. Trigger for ovulation: hCG (10,000 IU) im Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: single Semen preparation technique: not described in abstract Number of sperm injected: not described in abstract Type of semen: not described in abstract Catheter used: not described in abstract Cancellation criteria: not described in abstract |
|
| Outcomes | Clinical PR Number of preovulatory follicles |
|
| Notes | 6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective randomised study (however method of randomisation is not described in the abstract) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficent information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | Only data from abstract available. |
Kabouk 2010.
| Study characteristics | ||
| Methods | Randomisation: prospective randomised study Trial design: parallel Power calculation: not described in abstract Dropouts: not described in abstract Cycle cancellation: yes (16) Blinding: not described in abstract ITT: not described in abstract |
|
| Participants | 53 women, 110 cycles Age of women: not described in abstract Duration of infertility: not described in abstract. Type of infertility: not described in abstract Previous fertility treatment: not described in abstract Primary infertility: not described in abstract |
|
| Interventions | Stimulation method/dosage: CC 100 mg on days 3‐7 with supplementation of 75 IU rFSH every other day starting on day 6 of the cycle vs CC 100 mg on days 3‐7 with supplementation of 200 IU hCG starting on day 8 if there was at least 1 follicle > 10 mm. Treatment was continued until the day of ovulation trigger. Trigger for ovulation: hCG (5000 IU) im Timing of IUI: not described in abstract Frequency of IUI: not described in abstract Semen preparation technique: not described in abstract Number of sperm injected: not described in abstract Type of semen: not described in abstract Catheter used: not described in abstract Cancellation criteria: no response and excessive response |
|
| Outcomes | Cancellation rates Number of follicles > 14 mm Endometrial thickness Clinical pregnancy rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation is not described in the abstract; insufficient information to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow chart is provided; however cancellations have been mentioned. Twin pregnancies and miscarriage rate are missing; however these also not stated as outcomes. |
| Selective reporting (reporting bias) | Low risk | Endpoints of the study have been discussed in the results section. |
| Other bias | Low risk | Not suspected. |
Kamath 2013.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated series of random numbers, in blocks of ten Trial design: parallel Power calculation: yes Dropouts: none, only cancellations for medical reasons Cycle cancellation: 11 in antagonist group, 12 in control group Blinding: none described ITT: yes |
|
| Participants | Women 141; 141 cycles Age of women: antagonist: 29.08 ± 3.08 (yrs); control: 28.44 ± 3.5 (yrs) Duration of infertility: antagonist: 7.08 ± 3.07 (yrs); control: 6.35 ± 3.18 (yrs) Type of infertility: not stated Previous fertility treatment: unknown Primary infertility: both primary and secondary subfertility |
|
| Interventions | Stimulation method/dosage: uFSH and when the lead follicle reached 14 mm GnRH antagonist was started until day of hCG trigger versus uFSH without GnRH antagonist Trigger for ovulation: Choragon ( 5000 IU) im if LH < 15 IU/mL, if LH > 15 IU/mL, no trigger was given Timing of IUI: 36 hrs after hCG or 24 hours after LH peak Frequency of IUI: once Semen preparation technique: double density gradient method Number of sperm injected: not stated Type of semen: not stated Catheter used: soft IUI catheter Cancellation criteria: four or more mature follicles (17 mm), poor response (no follicles of greater size than 10 mm observed by day 20 of the cycle) |
|
| Outcomes | PR/couple Live birth Multiple PR Number of ampoules used Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated series of random numbers, in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by placing the group allocation in consecutively numbered opaque sealed envelopes, which were opened after informed consent was obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The principal investigator was blinded for the randomisation sequence; further lack of blinding is unlikely to influence the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected; a detailed flow chart is provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Kamel 1995.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: CC: 4; hMG: 2 Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 60 women; 60 cycles Age of women: not stated Duration of infertility: at least 2 years Type of infertility: unexplained, male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: CC 50 mg/d CD 3‐7; hMG: 75 IU/d CD 3 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36‐42 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: normal semen analysis thus husband semen Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/woman Number of ampoules used Number of dominant follicles ( > 17 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Low risk | Only data from abstract available. |
Karlström 1993.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: CC: 4; hMG: 9 Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 32 women; 32 cycles Age of women: CC 31.7 yrs; hMG 32.0 yrs Duration of infertility: CC: 5.1 yrs; hMG: 4.9 yrs Type of infertility: unexplained, endometriosis Previous fertility treatment: none Primary infertility: not stated for the subgroup IUI |
|
| Interventions | Stimulation method/dosage: CC 100 mg/d CD 3‐7; hMG: 150 IU/d from CD 2‐3 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36‐41 hrs after hCG Frequency of IUI: once Semen preparation technique: method of self‐migration in hyaluronic acid No of motile sperm injected: CC: 10.7 x 106; hMG: 16.6 x 106 Type of semen: husband semen Catheter used: Kremer de la fontaine or TDT catheter Cancellation criteria: not stated |
|
| Outcomes | PR/woman: hMG | |
| Notes | Not only IUI but also DIPI and DIPI with IUI combined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected; extensive reporting of the inclusion and exclusion process, study protocol is approved. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Karlström 1998.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: 32 in total Cycle cancellation: not stated Blinding: not described in abstract ITT: not stated |
|
| Participants | 74 women; 74 cycles Age of women: not stated Duration of infertility: not stated Type of infertility: unexplained, endometriosis, male subfertility, cervical factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: CC 100 mg/d CD 3‐7 versus hMG 150 IU/d from CD 2‐3 Trigger for ovulation: 10,000 IU hCG or LH surge in CC group Timing of IUI: 38 hrs after hCG or day after LH peak Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: husband semen Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/woman | |
| Notes | Extended study from study 1993 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. Only abstract data available |
| Allocation concealment (selection bias) | Unclear risk | Not stated in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Low risk | Only data from abstract available. |
Karthik 2018.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: yes (due to poor ovarian response and mild OHSS) Blinding: not described ITT: not stated |
|
| Participants | 45 women; 83 cycles Age of women: fixed group 31.73±3.01 yrs; flexible group 30.33±2.74 yrs; control 30.60±4.39 (NS) Duration of infertility: fixed 3.93±1.83 yrs; flexible 5.20±3.46 yrs; control 6.27±2.37 yrs (NS) Type of infertility: unexplained (including mild endometriosis) and male subfertility Previous fertility treatment: not stated Primary infertility: primary infertility was seen in 58% (n = 26) and 42% (n = 19) had secondary infertility |
|
| Interventions | Stimulation method/dosage: Each woman received clomiphene citrate 50 mg/d orally on day 2–6 of menstrual cycle followed by human menopausal gonadotrophin (hMG) 75 IU IM, given daily from day 6 to day 8 of cycle and dose increased according to response. In group I (fixed), cetrorelix 0.25 mg SC was given from day 8 of the cycle, and in group II (flexible) it is given on the day of follicle size ≥14 mm, but group III (control) did not receive cetrorelix Trigger for ovulation: 5000 IU hCG Timing of IUI: 36‐38 hrs after hCG Frequency of IUI: once Semen preparation technique: double density gradient technique Number of motile sperm injected: not stated Type of semen: husband semen Catheter used: flexible IUI catheter Cancellation criteria: If four or more mature follicles reach > 16 mm in diameter, the cycle was cancelled, and the participant was advised to avoid intercourse until her next menstrual period. Cycles were also cancelled when poor follicle response was noted until day 18 of the cycle, i.e. no follicle size > 10 mm |
|
| Outcomes | PR/couple Multiple PR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation described. However, method of randomisation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not suspected; however flow chart is missing. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Kaur 2019.
| Study characteristics | ||
| Methods | Randomisation: blocked randomisation design, with block size of four, 30 in each group Trial design: parallel Power calculation: not stated Dropouts: not stated (only exclusions were described) Cycle cancellation: yes Blinding: not described ITT: not stated |
|
| Participants | 60 women; 99 cycles Age of women: letrozole 29.21 (± 3.14 yrs) vs letrozole+hMG 30.8 (± 3.19 yrs) Duration of infertility: letrozole 5.50 (± 3.02 yrs) vs letrozole+hMG 6.33 (±3.14 yrs) Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: both primary and secondary |
|
| Interventions | Stimulation method/dosage: Letrozole: starting from day 2 or 3 of menses with a dose of 2.5 mg, daily increments were made for total of 4 days (total cumulative dose 25 mg). Letrozole+hMG: combination of letrozole and human menopausal gonadotrophin (hMG). Starting from day 2 or 3 of menses, tablet letrozole 2.5 mg twice a day was given for 5 days, intramuscular injection of hMG 150 EH was given every alternate day starting from day 7. Trigger for ovulation: 5,000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: not described number of motile sperm injected: not described Type of semen: not described (assumed homologue) Catheter used: not described Cancellation criteria: hyperstimulation; more than 3 follicles ≥ 16 mm |
|
| Outcomes | PR/couple Multiple PR Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not described; however, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions are described. Dropouts or lost to follow‐up are not described. |
| Selective reporting (reporting bias) | Low risk | Both positive and negative outcomes are reported. |
| Other bias | Low risk | Not suspected. |
Kim 1996.
| Study characteristics | ||
| Methods | Randomisation: blocked randomisation design Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 80 women, 80 cycles Age of women: ultra long: 32.9±2.2 yrs; long: 32.4±2.0 yrs Duration of infertility: ultra long: 3.9±1.3 yrs; long: 3.2±1.0 yrs Type of infertility: endometriosis type I‐IV Previous fertility treatment: in 13 participants previous treatment with GnRHa Primary infertility: ultra long: 59%; long: 61% |
|
| Interventions | Stimulation method/dosage: ultra long protocol: GnRHa 3.75 mg IM 4 weeks before starting daily with GnRHa 0.1 mg combined with FSH/hMG; long protocol: GnRHa 0.1 mg 2 weeks daily followed by FSH/hMG Trigger for ovulation: 10,000 IU hCG Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: once Semen preparation technique: Percoll gradient number of motile sperm injected: not stated Type of semen: normal semen analysis thus husband semen presumed Catheter used: Makler cannula Cancellation criteria: not stated |
|
| Outcomes | Live birth rate/woman PR/woman Multiple PR Miscarriage rate per pregnancy Number of ampoules used Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation design. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Kim 2010.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described in abstract ITT: not stated |
|
| Participants | 80 women Age of women: not stated Duration of infertility: not stated Type of infertility: not stated Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: 80 infertile women were randomised to soft stimulation protocol group (n = 40) or GnRH antagonist MDP group (n = 40) Trigger for ovulation: not stated Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: not stated Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned. However, method of randomisation not stated in available abstract data. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow chart provided. Dropout or loss to follow‐up not described. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes have not been described in the abstract. |
| Other bias | Low risk | Not suspected. |
Labarta 2016.
| Study characteristics | ||
| Methods | Randomisation: prospective randomised controlled trial, computer generated randomisation. allocation: not stated Trial design: parallel Power calculation: yes Dropouts: no Cycle cancellation: hp‐hMG: 3; rFSH+rLH: 4 Blinding: not stated ITT: not stated |
|
| Participants | 67 women Age of women: hp‐hMG: 33.3±2.5 (yrs); rFSH+rLH: 32±2.9 (yrs) Duration of infertility: at least 1 year Type of infertility: unexplained, male factor, mild endometriosis Previous fertility treatment: first or second IUI cycle, excluded when received hormonal treatment in the 3 months prior to the study. Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: 75 IU/d hp‐hMG; 75 IU/d rFSH+ 75 IU/d rLH Trigger for ovulation: 250 µg rhCG (Ovitrelle) Timing of IUI: not stated Frequency of IUI: 2 consecutive days Semen preparation technique: swim‐up procedure Number of sperm injected: 0.5 mL Type of semen: husband semen Catheter used: not stated Cancellation criteria: if 4 or more mature follicles developed |
|
| Outcomes | Ongoing PR/couple Miscarriage rate Multiple PR Number of IU used: hp‐hMG Number of dominant follicles ( > 18 mm) |
|
| Notes | Additional information on allocation concealment was collected after contacting the author in December 2020. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was obtained by computer‐generated block randomisation. |
| Allocation concealment (selection bias) | Low risk | A third party (study coordinator of the clinic), was in charge of ensuring that the participants were treated according to the group that corresponded to them, strictly following the randomisation list. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A detailed flow chart is provided, including cancellations. Protocol is available. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Lambalk 2006.
| Study characteristics | ||
| Methods | Randomisation: blocked randomisation list Trial design: parallel Power calculation: yes Dropouts: GnRH antagonist: 11; FSH alone: 15 Cycle cancellation: GnRH antagonist: 11 cycles; placebo: 15 cycles Blinding: yes ITT: not stated |
|
| Participants | 204 women, 203 cycles Age of women: GnRH antagonist: 32.7±3.3 yrs; FSH alone: 32.5±3.9 yrs Duration of infertility: GnRH antagonist: 3.1±1.7 yrs; FSH alone: 3.4±1.8 yrs Type of infertility: unexplained, male factor Previous fertility treatment: not more than 3 previous IUI attempts Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: GnRH antagonist: rFSH starting dose decided by the investigator + GnRHantagonist when DF >14 mm; FSH alone: rFSH + placebo from DF > 14 mm Trigger for ovulation: 5,000 IU or 10,000 IU hCG Timing of IUI: 34‐42 hr after hCG injection Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not explicitly stated Catheter used: not stated Cancellation criteria: not if more than 3 follicles were ≥ 14 mm |
|
| Outcomes | Ongoing PR/woman: GnRH antagonist Multiple PR: GnRH antagonist Miscarriage rate Number of ampoules used Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised, double blind, placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Lee 2008.
| Study characteristics | ||
| Methods | Randomisation: random number allocation table, method of allocation by sealed envelopes Trial design: parallel Power calculation: yes Dropouts: LL/FSH 0 vs LL/FSH/Cr 1 Cycle cancellation: none blinding: not described ITT: yes |
|
| Participants | 60 women, 30 in each group Age of women: LL/FSH 32.4±0.5yrs vs LL/FSH+GnRHanta 33.0±0.5yrs Duration of infertility: not stated Type of infertility: participants < 38 yrs with unexplained or mild male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: both groups received letrozole on day 3‐7 and received rFSH on stimulation days 4, 6 and 8 and then daily until day of hCG. In addition, participants in group 2 were given a daily injection of cetrorelix when the leading follicle reached 14 mm. Trigger for ovulation: 250 IU hCG Timing of IUI: 36 hr after hCG injection Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not explicitly stated Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Pregancy rate/woman Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number allocation table. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were used; unclear whether envelopes were opaque and numbered sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart of patient inclusions is provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Lin 2008.
| Study characteristics | ||
| Methods | Randomisation: women were randomised by block randomisation into two stimulation protocols. Trial design: parallel Power calculation: yes Dropouts: not described Cycle cancellation: no Blinding: not described in abstract ITT: not stated |
|
| Participants | 74 women; 74 cycles Age of women: group A 31.5 +/‐ 3.3 yrs vs group B 32.5 +/‐ 3.2 yrs Duration of infertility: not stated Type of infertility: male factor or unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: all women were stimulated with CC and HMG. Clomiphene citrate (Clomid; Shionogi, Tokyo, Japan) at 100 mg per day was given from day 3 to day 7 of the cycle. Two ampoules of HMG (Pergonal; Serono, Geneva, Switzerland) were given on days 4, 6 and 8, and then every day from day 9. With protocol A, cetrorelix acetate (Cetrotide; Serono, Bari, Italy) was given at 0.25 mg per day when the leading follicles reached 14 mm. The dose was increased to 0.5 mg per day when the leading follicles were 16 mm. With protocol B, cetrorelix was given at 0.5 mg per day when the leading follicles reached 14 mm, and the dose was maintained until the day of human chorionic gonadotrophin Trigger for ovulation: 5000 IU hCG (Pregnyl) Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: husband semen Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple | |
| Notes | Very specific comparison; standard dosage antagonist vs increasing dosage. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss to follow‐up or dropouts described. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Malhotra 2012.
| Study characteristics | ||
| Methods | Randomisation: stated without further description, allocation not stated Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not stated ITT: not stated |
|
| Participants | 68 women Age of women: not stated Duration of infertility: not stated Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: 2.5 mg/day letrozole from CD 3‐7 versus 2.5 mg letrozole CD 3‐7+ hMG 150 IU alternate day from day 7 until day of ovulation trigger Trigger for ovulation: 5.000 IU hCG Timing of IUI: not stated Frequency of IUI: not stated Semen preparation technique: not stated Number of sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple Multiple PR, miscarriages were comparable in both groups Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. Only abstract data. |
| Allocation concealment (selection bias) | Unclear risk | Not stated in detail. Only abstract data. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Most details on outcomes are missing. |
| Selective reporting (reporting bias) | Unclear risk | Details of the trial and treatment, cycle cancellation are missing. |
| Other bias | Low risk | Only data from abstract available. |
Matorras 2000.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated list Trial design: parallel Power calculation: not stated Dropouts: none Cycle cancellation: rFSH: 24 cycles; uFSH: 27 cycles Blinding: single‐blinded ITT: yes |
|
| Participants | 91 women, 345 cycles Age of women: rFSH: 33.3±3.4 yrs; uFSH: 33.9±3.1 yrs Duration of infertility: rFSH: 4.6±2.0 yrs; uFSH: 5.3±2.5 yrs Type of infertility: unexplained, male factor, ovulatory dysfunction Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: rFSH: 150 IU/d; uFSH: 150 IU/d Trigger for ovulation: 5,000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: Pure sperm Number of motile sperm injected: not stated Type of semen: husband semen Catheter used: not stated Cancellation criteria: > 6 follicles > 15 mm and E2 > 2000 pg/mL |
|
| Outcomes | Pregnancy/couple Multiple PR Miscarriages Number of ampoules used Number of dominant follicles ( > 15 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Third party and sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, ultrasound staff, estradiol analysis and sperm laboratory were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ultrasound staff was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Moro 2015.
| Study characteristics | ||
| Methods | Randomisation: computer generated number list Trial design: parallel Power calculation: yes Dropouts: none Cycle cancellation: rFSH+rLH 13 vs HP‐hMG 5 Blinding: not described ITT: yes |
|
| Participants | 579 women, 579 cycles Age of women: rFSH+rLH 38.4±4.0 yrs; HP‐hMG 37.9±3.6 yrs Duration of infertility: rFSH+rLH 35±15.3 months vs HP‐hMG 36±17.5 months Type of infertility: unexplained and mild male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: rFSH+rLH 150 IU or 150 IU HP‐hMG was started on the 3rd day of the cycle, drug dose was adjusted according to the individual follicular response. Trigger for ovulation: 10,000 IU hCG Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: gradient technique with Percoll Number of motile sperm injected: not stated Type of semen: not clearly stated Catheter used: not stated Cancellation criteria: > 3 follicles > 17 mm or > 4 follicles > 15mm or E2 > 1500 pg/mL |
|
| Outcomes | Clinical PR OPR Interrupted cycles for high risk of OHSS Multiple PR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation list, in order of enrolment. |
| Allocation concealment (selection bias) | Unclear risk | Not stated explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected, a detailed flowchart has been provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Nada 2016.
| Study characteristics | ||
| Methods | Randomisation: randomisation blocks design based Trial design: parallel Power calculation: no Dropouts: 27 in total, 14 and 13 in each group Cycle cancellation: not described apart from dropouts Blinding: not described ITT: yes |
|
| Participants | 622 women, 595 cycles Age of women: CC 30.06 (5.00) yrs; hMG+antagonist 30.4 (5.72) yrs Duration of infertility: CC 5.38 (2.72) yrs vs hMG+antagonist 5.66 (2.21) yrs Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: CC 100 mg CD2‐6 or hMG + antagonist from CD6‐7. Dose of hMG was not stated Trigger for ovulation: 10,000 IU hCG Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: sperm concentration was stated: CC 24.87 (7.80) versus hMG+GnRH antagonist: 23.46 (8.32) millions Type of semen: not clearly stated Catheter used: IUI catheter Cancellation criteria: not stated |
|
| Outcomes | Clinical PR Multiple PR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation blocks design based |
| Allocation concealment (selection bias) | Unclear risk | Not stated explicitly |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected, a detailed flowchart has been provided |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Low risk | Not suspected |
Nakajima 1999.
| Study characteristics | ||
| Methods | Randomisation: open randomised trial Trial design: parallel Power calculation: not stated Dropouts: 2 participants withdrew Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 22 women, 55 cycles Age of women: not stated Duration of infertility: at least 18 months Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: dosages of CC not stated, dosages of rFSH not stated Trigger for ovulation: hCG Timing of IUI: 28‐36 hrs after hCG or after positive ovulation prediction kit Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not described explicitly, since unexplained infertility, assumed husband sperm Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/cycle Multiple PR Miscarriage rate per pregnancy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Open randomised trial, only abstract data. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information in the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | Only data from abstract available. |
Nayar 2008.
| Study characteristics | ||
| Methods | Randomisation: prospective randomised study Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | Age of women: not stated Duration of infertility: not stated Type of infertility: unexplained and mild male factor infertility Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: clomiphene citrate versus low dose recombinant FSH Timing of IUI: not stated Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | CPR | |
| Notes | Authors contacted for additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated explicitly. Abstract data only. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Abstract data only. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | Only data from abstract available. |
Ozmen 2005.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described in abstract ITT: not stated |
|
| Participants | 43 women, 43 cycles Age of women: not stated Duration of infertility: not stated Type of infertility: unexplained, mild‐moderate male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: letrozole: 5 mg/d CD 3‐7; CC: 100 mg/d CD 3‐7 Trigger for ovulation: hCG (dose unknown) Timing of IUI: 33‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: density gradient Number of motile sperm injected: not stated Type of semen: not stated explicitly Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | pregnancy/ couple Number of dominant follicles ( > 17 mm): letrozole |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised study. However, insufficient information in the abstract about exact method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not stated explicitly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
| Other bias | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk' |
Parés 2002.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: no Dropouts: rFSH: 6; uFSH: 4 Cycle cancellation: rFSH: 7/172; uFSH: 6/226 Blinding: not clear ITT: yes |
|
| Participants | 126 women, 398 cycles Age of women: rFSH 33.7± 3.6 (yrs); uFSH 33.2±4.0 (yrs) Duration of infertility: rFSH 4.0±2.1 (yrs); uFSH 4.7±3.8 (yrs) Type of infertility: endometriosis; unexplained; male factor; cervical factor; ovulatory dysfunction Previous fertility treatment: not stated Primary infertility: 80% of each group |
|
| Interventions | Stimulation method/dosage: rFSH 150 IU daily from CD 3; uFSH 150 IU daily from CD 3 Trigger for ovulation: hCG (dose unknown) Timing of IUI: 20 and 40 hrs after hCG Frequency of IUI: twice Semen preparation technique: Percoll gradient Number of motile sperm injected: rFSH: 14.3±13.5; uFSH: 11.3±11.4 x106 Type of semen: normal semen analysis thus husband semen presumed Catheter used: not stated Cancellation criteria: > 4 follicles > 18 mm E2 > 2000 pg/mL or > 6 follicles > 10‐16 mm |
|
| Outcomes | Ongoing PR/couple Miscarriage rate Multiple PR OHSS: rFSH Number of ampoules used Number of dominant follicles ( > 17 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The staff of the lab analysing estradiol and sperm were blinded. Participants and ultrasound staff were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; insufficient information to judge whether 'low risk' or 'high risk'. |
| Other bias | Low risk | Not suspected. |
Pattuelli 1996.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described in abstract ITT: not stated |
|
| Participants | 204 women, 204 cycles Age of women: not stated Duration of infertility: not stated Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: LHRH in midluteal phase, FSH 150 IU CD 1‐5; FSH 150 IU/d CD 2‐6 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 38‐40 hrs after hCG Frequency of IUI: once Semen preparation technique: swim‐up technique Number of motile sperm injected: not stated Type of semen: husband semen Catheter used: not stated Cancellation criteria not stated |
|
| Outcomes | PR/couple Multiple PR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No miscarriages stated. Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Selective reporting (reporting bias) | Unclear risk | Details on study design en patient characteristics are lacking. Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Low risk | Only data from abstract available. |
Peeraer 2015.
| Study characteristics | ||
| Methods | Randomisation: randomised controlled trial, blocked randomisation per 10 envelopes. Trial design:parallel Power calculation: yes Dropouts: yes (25) Cycle cancellation: yes (49) Blinding: not described ITT: yes |
|
| Participants | 330 women, 657 cycles Age of women: CC: 31.6 ± 3.7 (yrs); hMG: 31.9 ± 4.1 (yrs) Duration of infertility: CC: 25.3±19.0 (months); hMG: 28.0± 18.5 (months) Type of infertility: unexplained, mild male infertility Previous fertility treatment: not described Primary infertility: both primary and secondary |
|
| Interventions | Stimulation method/dosage: hMG from day 2 of the menstrual cycle with a starting dose of 37 or 75 IU in a low‐dose step‐up protocol as recommended and based on patient’s age, BMI, basal serum FSH levels and previous medical history. CC group were treated with a starting dose of 50 mg/day from Day 3 until Day 7 of the menstrual cycle. They also received oral ethinyl estradiol (EE2) 50 mg from Day 8 until Day 12 to support endometrial growth, in order to prevent the anti‐estrogenic effect of CC reducing endometrial thickness Trigger for ovulation: hCG (5000 IU) im Timing of IUI: 51‐54 hrs after hCG Frequency of IUI: single Semen preparation technique: the sperm was washed in a 3‐layer discontinuous gradient centrifugation by using ISolatew Number of sperm injected: not stated Type of semen: husband Catheter used: a Frydman Classic Catheter (Laboratoire CCD, Paris, France) equipped with a sterile tuberculin syringe was used for each intrauterine insemination Cancellation criteria: when three or more follicles of 14 mm or larger were present at the time of hCG injection, selective ultrasound‐guided follicular aspiration was offered before IUI or the cycle was cancelled |
|
| Outcomes | PR/ couple Live birth rate Multiple PR Miscarriage rate Number of dominant follicles ( > 18 mm) |
|
| Notes | Dataset from study was obtained from investigator, to calculate the first cycle data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation per 10 envelopes for each recruiting centre, containing 5 in the CC group and 5 in the hMG group. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation for each participant was performed by opening opaque sealed envelopes only after written informed consent of the female partner was obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, is unlikely to influence the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected, description of included participants has been described, as well as adverse events. |
| Selective reporting (reporting bias) | Low risk | Not suspected, 'unwanted' outcomes have also been reported. |
| Other bias | Low risk | Not suspected. |
Pourali 2017.
| Study characteristics | ||
| Methods | Randomisation: randomisation was done by using numbers in closed envelopes Trial design: parallel Power calculation: yes Dropouts: 10 participants were lost to follow‐up Cycle cancellation: 5 cycles were cancelled due to OHSS (CC) and 12 cycles were cancelled due to non‐formation of at least 2 follicles > 16 mm (7 CC, 5 LZ) Blinding: yes ITT: not stated |
|
| Participants | 180 women, 180 cycles Age of women: 28.5±1.7 (CC) vs 28.6 ±1.8 (LZ) (yrs) Duration of infertility: 2.5±1.14 (CC) vs 2.5 ±1.19 (LZ) (yrs) Type of infertility: unexplained (and resistance after three cycles of CC therapy who were candidates for IUI). Previous fertility treatment: yes Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: CC 50 mg twice daily for 5 days, starting on day 3 versus LZ 2.5 mg twice daily for 5 days, starting on day 3. All participants received a daily IM injection with 75 HMG starting on day 6 of the cycle until day of hCG. Trigger for ovulation: 5000 IU hCG Timing of IUI: 36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: if more than 15 follicles were seen in each ovary, it was considered as ovarian hyperstimulation. (Also cancelled when < 2 follicles > 16 mm developed). |
|
| Outcomes | PR/couple OHSS Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done by using numbers in closed envelopes. However, it is unclear if this process used random numbers or consecutive numbers and could be foreseen. |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Gynecologists, radiologists and participants were unaware of study group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Gynecologists, radiologists and participants were unaware of study group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected, flow chart of study design is provided. |
| Selective reporting (reporting bias) | Low risk | Not suspected, also negative outcomes have been reported (twin pregnancies are missing). |
| Other bias | Low risk | Not suspected. |
Pourmatroud 2013.
| Study characteristics | ||
| Methods | Randomisation: double‐blinded prospective study, randomisation by computer randomisation tables. Trial design: parallel Power calculation: no, sample size was chosen on arbitrary basis Dropouts: yes (LZ+T+HMG 5 vs LZ+P+HMG) Cycle cancellation: yes, participants with > 5 follicles of > 16 mm and with 1 follicle > 16mm were excluded Blinding: physicians and participants were blinded about the participants group ITT: no |
|
| Participants | 120 women, 120 cycles Age of women: LZ+Tam 28.8 ± 4.4 (years); LZ+plac: 27.7 ± 4.8 (years) Duration of infertility: LZ+Tam: 4.5 ± 1.8 (years); LZ+plac: 4.6 ± 1.8 (years) Type of infertility: male, unexplained, oligo ovulation, endometriosis, male+ovulation Previous fertility treatment: at least 3 months interval since previous ovulation by induction or IUI cycle Primary infertility: primary or secondary |
|
| Interventions | Stimulation method/dosage: group A: on day 3 LZ 2.5 mg and Tamoxifen 10 mg once a day until day 7, then hMG 75 IU was administered and continued until appearance of one follicle > 18 mm or two follicles > 16 mm versus group B received the same protocol, only Tamoxifen was replaced by placebo. Trigger for ovulation: (10,000 IU) im Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: not stated Number of sperm injected: not stated Type of semen: not stated Catheter used: Wallace catheter Cancellation criteria: participants with > 5 follicles of > 16 mm and with 1 follicle > 16 mm were excluded |
|
| Outcomes | PR/couple Number of ampoules used Number of dominant follicles ( > 18 mm) |
|
| Notes | Very specific comparison; letrozole combined with tamoxifen, both aromatase inhibitors, no meta‐analyses yet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Serial numbers of sealed envelopes, unclear whether envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded prospective trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded prospective trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear description of dropouts. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Unclear risk | No power analysis performed, no information on eligible participants. |
Ragni 2001.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated list Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: FSH + GnRH antagonist: 7 cycles; FSH alone: 9 cycles Blinding: not described ITT: not stated |
|
| Participants | 41 women, 48 cycles Age of women: GnRH antagonist: 33±3.5, FSH alone: 32.9±3 Duration of infertility: more than 2 years Type of infertility: unexplained, male factor Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: Group A: FSH 150 IU/d from CD 3; when DF > 14 0.25 mg GnRH antagonist; Group B: FSH 150 IU from CD 3 Trigger for ovulation: hCG or urinary LH test in group B Timing of IUI: not stated Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: > 6 follicles > 14 mm or < 2 follicles > 14 mm |
|
| Outcomes | Pregnancy/couple Multiple PR Number of ampoules used Number of dominant follicles ( > 14 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No protocol available. |
| Selective reporting (reporting bias) | Unclear risk | Details on participant characteristics are lacking. |
| Other bias | Low risk | Not suspected. |
Ragni 2004.
| Study characteristics | ||
| Methods | Randomisation: blocked randomisation list Trial design: parallel Power calculation: yes Dropouts: Group A: 3 participants withdrew Cycle cancellation: Group A: 2 cycles, Group B: 1 cycle Blinding: not described ITT: not stated |
|
| Participants | 69 women, 69 cycles Age of women: Group A: 33.1±3.0 yrs, Group B: 32.1±6.6 yrs Duration of infertility: Group A: 3.2±1.1 yrs; Group B: 3.0±1.2 yrs Type of infertility: unexplained, male factor, endometriosis, PCOS Previous fertility treatment: no IUI Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: Group A: FSH 50 IU/d; when DF > 14 0.25 mg GnRH antagonist; Group B: FSH 50 IU alternate days/ GnRH antagonist when DF > 14 mm Trigger for ovulation: 5000 IU hCG Timing of IUI: 34 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: normal semen analysis thus husband semen Catheter used: not stated Cancellation criteria: > 2 follicles > 14 mm |
|
| Outcomes | Live births/couple Number of dominant follicles (> 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation list. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes containing treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed description of participant flow. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Ransom 1996.
| Study characteristics | ||
| Methods | Randomisation: random number table Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 98 women, 240 cycles Age of women: hMG: 32.9±4.8 yrs; hMG+CC: 32.3±3.4 yrs Duration of infertility: not stated Type of infertility: unexplained, male factor, endometriosis, ovulatory dysfunction, PCOS, cervical factor Previous fertility treatment: no IUI, max 3 cycles of CC Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: hMG 150 IU/d CD 3; CC 100 mg CD 3‐7 + hMG 150 IU CD 7,9, 11 Trigger for ovulation: 5000 IU hCG Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: standard swim‐up Number of motile sperm injected: hMG: 37.2±25.5; hMG+CC: 42.4±31.7 x106 Type of semen: normal semen analysis thus husband semen Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | Pregnancy/couple Number of dominant follicles: hMG |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Rashidi 2013.
| Study characteristics | ||
| Methods | Randomisation: random allocation (not specified) Trial design: parallel Power calculation: not described Dropouts: not described Cycle cancellation: yes (n=21) Blinding: physicians and participants were blinded to the participants' group ITT: no |
|
| Participants | 280 women, 280 cycles (259 analysed) Age of women: CC+hMG: 29.1 ± 3.1 (yrs); CC+rFSH: 28.7 ± 2.6 (yrs) Duration of infertility: CC+hMG: 3.8 ± 0.9 (yrs); CC+rFSH: 3.9 ± 0.9 (yrs) Type of infertility: unexplained Previous fertility treatment: history of at least 6 cycles of previous induction ovulation, but no IUI, IVF or ICSI attempts Primary infertility: not described explicitly |
|
| Interventions | Stimulation method/dosage: Induction of ovulation in group A was started with CC 100 mg daily during the 3rd–7th days of the menstrual cycle, followed by 75 IU hMG (Menogone, Merck‐Serono, Darmstadt, Germany) given daily on days 7–9. Group B underwent the same protocol, but with hMG replaced by 75 IU rFSH Trigger for ovulation: (10,000 IU) im Timing of IUI: 24‐36 hrs after hCG Frequency of IUI: single Semen preparation technique: swim up method Number of sperm injected: not described Type of semen: not described, probably husband Catheter used: not described Cancellation criteria: In the presence of more than five follicles with a diameter of at least 16 mm, hCG was withheld, the cycle was cancelled, and the couple was advised to have protected intercourse to minimize the risk of multiple pregnancy and ovarian hyperstimulation syndrome |
|
| Outcomes | Clinical PR Live birth rate per couple Miscarriage rate Number of dominant follicles ( > 17 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Couples were randomly allocated to one of two groups. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. Full overview of participant flow is provided, no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Unclear risk | No power analysis, no statement about eligible participants. |
Sadaghiani 2012.
| Study characteristics | ||
| Methods | Randomisation: participants were randomly divided, method of randomisation not described in abstract Trial design: parallel Power calculation: not described in abstract Dropouts: not described Cycle cancellation: not described Blinding: not described in abstract ITT: not described |
|
| Participants | 80 women, 80 cycles Age of women: not described in abstract Duration of infertility: not described in abstract Type of infertility: not described in abstract Previous fertility treatment: not described in abstract Primary infertility: not described in abstract |
|
| Interventions | Stimulation method/dosage: group 1 received clomiphene (100 mg/day) and group 2 received letrozole (5 mg/ day) on the 3‐7th days of menstrual cycle. Both groups received (150 IU/im) HMG on the 7‐9th days of menstrual cycle Trigger for ovulation: hCG (5000 IU) im Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: once Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | PR/couple Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into two groups, method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow chart of participants is provided, loss to follow‐up unclear. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to judge whether low or high risk. |
| Other bias | Low risk | Only data from abstract available. |
Sagnella 2011.
| Study characteristics | ||
| Methods | Randomisation: by computer‐generated random number table, participants received a code number Trial design: non‐inferiority trial, parallel Power calculation: yes Dropouts: 4 (because of lack of response) Cycle cancellation: yes, 25 because of risk of OHSS Blinding: not described ITT: no |
|
| Participants | 523 women, 519 cycles (4 cancellations) Age of women: rFSH: 35.38 ± 3.09 (yrs); HP‐HMG: 35.0 ± 2.98 (yrs) Duration of infertility: rFSH: 36.0 ± 17.95 (months); HP‐HMG: 35.0 ± 18.2 (months) Type of infertility: male factor and unexplained Previous fertility treatment: not described Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: rFSH or HP‐HMG were started on the third day of menstruation. The starting gonadotropin dose used was 75 UI if the woman’s age was < 35 years and 150 UI if the woman’s age was > 35 years. The drug dose was adjusted according to the individual follicular response. Trigger for ovulation: hCG Gonasi (10,000 IU) im Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: single Semen preparation technique: a gradient technique with Percoll (Irvine Scientific, Irvine, CA) was used for semen preparation. Number of sperm injected: 0.35 mL Type of semen: not described, probably husband Catheter used: not described Cancellation criteria: hCG was not administered if > 3 large follicles > 17 mm or > 4 follicles > 16 mm, and/or E2 > 1,500 pg/mL, to minimise the risk of multiple pregnancy and/or OHSS |
|
| Outcomes | CPR/couple Multiple PR Dose total used Number of dominant follicles ( > 17 mm) OHSS rate Miscarriage rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | To guarantee the concealment of allocation, a staff member who was not directly involved in the study was in possession of the randomisation list. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart of inclusions has been provided. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes have been reported. |
| Other bias | Low risk | Not suspected. |
Sammour 2001.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel, prospective randomised trial Power calculation: not stated Dropouts: none Cycle cancellation: none Blinding: double‐blinded ITT: not stated |
|
| Participants | 49 women, cycles not stated Age of women: letrozole: 30.7; CC 32.8 (yrs) Duration of infertility: letrozole: 26; CC: 24 (months) Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: letrozole: 2.5 mg CD 3‐7; CC: 100 mg CD 3‐7 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 24 and 48 hrs after hCG Frequency of IUI: twice Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not stated explicitly Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further details. Abstract data only. |
| Allocation concealment (selection bias) | Unclear risk | Not stated in detail. Abstract data only. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded trial. However, detailed information about blinding cannot be obtained based on the abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Low risk | Only data from abstract available. |
Sengoku 1994.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: cross‐over Power calculation: not stated Dropouts: not stated Cycle cancellation: none Blinding: not described ITT: not stated |
|
| Participants | 91 women, 91 cycles Age of women: Group A: 31.6±3.3; Group B: 32.0±3.7 (yrs) Duration of infertility: Group A: 5.8±3.1; Group B: 5.7±2.9 (yrs) Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: Group A: 32 (71%); Group B: 34 (74%) |
|
| Interventions | Stimulation method/dosage: Group A: hMG 150 IU/day CD 3; Group B: hMG 150 IU/d CD 3 + GnRH agonist 300 IU 3 dd 1 from CD 1 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 24 ‐28 hrs after hCG Frequency of IUI: once Semen preparation technique: washed twice by centrifugation Number of motile sperm injected: hMG: 18.2±8.9; hMG+GnRH agonist: 18.8±9.5 x106 Type of semen: normal semen analysis thus husband semen Catheter used: Tomcat catheter Cancellation criteria: not stated |
|
| Outcomes | Live birth PR/couple Miscarriages Multiple PR not from first cycle only OHSS Number of ampoules used Number of dominant follicles ( > 12 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Sengoku 1999.
| Study characteristics | ||
| Methods | Randomisation: random number table Trial design: parallel Power calculation: yes Dropouts: not stated Cycle cancellation: none Blinding: not described ITT: not stated |
|
| Participants | 97 women, 97 cycles Age of women: high dose uFSH: 31.8±3.5; standard dose uFSH: 32.9±3.3 (yrs) Duration of infertility: high dose uFSH: 4.2± 2.5; standard dose uFSH: 4.6±2.0 (yrs) Type of infertility: unexplained Previous fertility treatment: CC treatment Primary infertility: Group I: 33 (69%); Group II: 35 (71.4%) |
|
| Interventions | Stimulation method/dosage: high dose uFSH: 150 IU/d from CD 3; standard dose uFSH: 75 IU/d from CD 3 Trigger for ovulation: 5000 IU hCG Timing of IUI: 24‐28 hrs after hCG when LH surge was detected IUI was the next morning performed Frequency of IUI: once Semen preparation technique: washed twice Number of motile sperm injected: not stated Type of semen: husband Catheter used: Tomcat catheter Cancellation criteria: not stated |
|
| Outcomes | PR/couple Multiple PR Miscarriages OHSS Number of ampoules used Number of dominant follicles ( > 14 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Random numbers were concealed in sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Sharma 2011a.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated in abstract Dropouts: not stated Cycle cancellation: yes Blinding: not described in abstract ITT: not stated |
|
| Participants | 261 women; 652 cycles Age of women: not stated Duration of infertility: not stated Type of infertility: unexplained Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: Women in Group A received letrozole (5 mg/day from day 3‐7), Group B received letrozole (5 mg/day from day 3‐7) plus u‐FSH 75 IU from day 7 onwards & Group C received continuous u‐FSH 75 IU from day 3 onwards Trigger for ovulation: not described in detail Timing of IUI: not described Frequency of IUI: not described Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the method of randomisation or sequence generation in abstract. |
| Allocation concealment (selection bias) | Unclear risk | No description at all about the method of allocation concealment. However, this may be due to the lack of information in the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding has not been described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the abstract, there is no description of dropouts or incomplete outcomes. |
| Selective reporting (reporting bias) | Low risk | The primary outcomes have been defined in the abstract and all of the mentioned outcomes have been reported. |
| Other bias | Low risk | Not suspected. |
Steward 2011.
| Study characteristics | ||
| Methods | Randomisation: by a computer‐generated random number to one of two protocols after undergoing baseline testing to prove eligibility Trial design: open label RCT, parallel Power calculation: yes Dropouts: not described Cycle cancellation: yes (due to OHSS) Blinding: not described ITT: yes |
|
| Participants | 80 women, 80 cycles Age of women: between 18‐39 yrs Duration of infertility: not described Type of infertility: ovulation dysfunction, mild male factor and unexplained Previous fertility treatment: no, history of prior OI/IUI failure was excluded Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: In protocol A rFSH (Gonal‐f) injections were initiated at a starting dose of 75–150 IU and monitored. All participants received luteal support with P suppositories (200 mg twice daily). Protocol B began in the same fashion. When the leading follicle reached 13 mm in mean diameter or 14 mm in longest diameter, and/or when the serum E2 level reached 400 pg/mL, daily cetrorelix injections were administered (250 mg) until the day of hCG trigger. Trigger for ovulation: Ovidrel (250 µg) im Timing of IUI: 12 and 36 hrs after hCG Frequency of IUI: double Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | CPR/couple Multiple PR Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Not suspected |
| Other bias | Low risk | Not suspected |
Taravat 2011.
| Study characteristics | ||
| Methods | Randomisation: randomisation by random number table. Trial design: parallel Power calculation: yes Dropouts: not described Cycle cancellation: not described Blinding: no blinding described ITT: not described |
|
| Participants | 55 women, 55 cycles Age of women: CC: 28.45 ± 4.56 (yrs); AI: 27.38 ± 3.44 (yrs) Duration of infertility: CC: 4.87 ± 3.13 (yrs); AI: 4.38 ± 1.93 (yrs) Type of infertility: unexplained Previous fertility treatment: not described Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: Group A: Clomiphene Citrate (CC, Razi company, Iran) oral tablet with dose of 100 mg on day 3‐7 of the stimulation cycles plus recombinant FSH (Gonal f 75 IU sc inject. Serono, Frankfort, Germany) on stimulation cycle days of 6‐7‐8. Group B: underwent ovarian stimulation with three day Anastrozole 1 mg oral tablet (Arimedex, Astrazenka, UK limited) on days 3‐5 of stimulation cycles and receiving recombinant FSH (Gonal f 75 IU sc inject. Serono, Frankfort, Germany) on stimulation cycle days 6‐7‐8. Trigger for ovulation: Novarel (hCG 10,000 IU) im Timing of IUI: 34‐36 hrs after hCG Frequency of IUI: two on consecutive days Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | CPR/couple Mean number of dominant follicles ( > 17 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up and dropout were not described, insufficient information available to make a judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement, small group. |
| Other bias | Low risk | Not suspected. |
Wadhwa 2016.
| Study characteristics | ||
| Methods | Randomisation: by randomisation table Trial design: parallel Power calculation: not described Dropouts: no Cycle cancellation: 6 exclusions Blinding: no blinding because of technical problems ITT: not stated |
|
| Participants | 70 women, 70 cycles Age of women: CC+HMG+GnRHanta: 27.88 ± 2.88 (yrs); CC+hMG: 28.22 ± 3.14 (yrs) Duration of infertility: 5–8 years in 41.2% women in group A and 44.4% in group B. Type of infertility: unexplained and mild male factor, one or both tubes patent Previous fertility treatment: not stated Primary infertility: both primary and secondary subfertility |
|
| Interventions | Stimulation method/dosage: all women were treated with clomiphene citrate (CD 3–7) followed by HMG. In group A, a GnRH antagonist was added when one or more follicles of 16 mm diameter or more were visualised in the study group. When at least one follicle reached a size of 18 mm, ovulation was induced by hCG injection Trigger for ovulation: hCG (10,000 IU) im Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: > 4 follicles on day of hCG trigger or serum E2 > 1500 pg/mL |
|
| Outcomes | PR/couple Number of ampoules used |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. However, lack of blinding is unlikely to influence the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart has been provided, excluded participants are described. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes have been reported. |
| Other bias | Low risk | Not suspected. |
Wang 2004.
| Study characteristics | ||
| Methods | Randomisation: stated without further description Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: not stated Blinding: not described ITT: not stated |
|
| Participants | 48 women, 60 cycles Age of women: not stated Duration of infertility: not stated Type of infertility: not stated Previous fertility treatment: super ovulatory cycles with IUI Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: 100 mg CC daily for 5 days; TMX 40 mg daily for 5 days + hMG 150 IU on alternate days from CD 4 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 24‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: not stated Type of semen: not stated Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple Miscarriage rate Multiple PR Number of dominant follicles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated without further description. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in the abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in the abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the abstract to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Low risk | Only data from abstract available. |
Williams 2004.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated random system Trial design: parallel Power calculation: yes Dropouts: not stated Cycle cancellation: Group A: 4 cycles; Group B: 9 cycles Blinding: assessor‐blinded study ITT: not stated |
|
| Participants | 54 women, 118 cycles Age of women: GnRH antagonist: 34.0 (yrs); FSH alone: 33.0 (yrs) Duration of infertility: GnRH antagonist: 23 (months); FSH alone: 17 (months) Type of infertility: unexplained Previous fertility treatment: not IUI or IVF Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: Group A: rFSH 150 IU/d from CD 2‐3 + GnRH antagonist from CD 6; Group B: rFSH 150 IU/d from CD 2‐3 Trigger for ovulation: 10,000 IU hCG Timing of IUI: 34‐40 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of motile sperm injected: FSH+ GnRH antagonist: 34 x 106; FSH alone: 26 x 106 Type of semen: normal semen analysis thus husband semen presumed Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR Multiple PR Number of dominant follicles ( > 16 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised treatment. |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes, unclear if envelopes were sealed and truly random. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not suspected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Not suspected. |
Wu 2007.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation, allocation not stated Trial design: parallel Power calculation: not stated Dropouts: not stated Cycle cancellation: anastrozole: 1 (failure to induce ovulation) CC: 0 Blinding: not described ITT: not stated |
|
| Participants | 33 women, 33 cycles Age of women: anastrozole: 33.2±3.3 (yrs); CC: 32.7±4.2 (yrs) Duration of infertility: anastrozole: 4.0±2.6 (yrs); CC: 3.8±2.6 (yrs) Type of infertility: not explicitly stated Previous fertility treatment: not stated Primary infertility: not stated |
|
| Interventions | Stimulation method/dosage: 1 mg/day CD 3‐7 anastrozole; 100 mg/day CD 3‐7 CC, 2 weeks of luteal support by oral progesterone 100 mg twice per day Trigger for ovulation: 5000 IU hCG Timing of IUI: 24‐32 hrs after hCG Frequency of IUI: once Semen preparation technique: not stated Number of sperm injected: not stated Type of semen: husband Catheter used: not stated Cancellation criteria: not stated |
|
| Outcomes | PR/couple Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
Zadehmodares 2012.
| Study characteristics | ||
| Methods | Randomisation: method of randomisation not described Trial design: parallel Power calculation: not described Dropouts: not described Cycle cancellation: not described Blinding: not described ITT: not stated |
|
| Participants | 106 women, 106 cycles Age of women: CC+FSH: 25.7 ± 4.6 (yrs); Letrozole+FSH: 26.7 ± 3.4 (yrs) Duration of infertility: CC+FSH: 4.6±2.5 (yrs); Letrozole+FSH: 5.3± 2.0 (yrs) Type of infertility: unexplained and mild male factor Previous fertility treatment: none Primary infertility: not described |
|
| Interventions | Stimulation method/dosage: the participants in letrozole group received 5 mg letrozole for 5 days (from cycle day 3 to 7). In the clomiphene group, CC 100 mg was given for 5 days from cycle day 3. In addition, recombinant FSH (Fostimon) 75 IU was administrated the following two days. Trigger for ovulation: hCG (10,000 IU) im Timing of IUI: 36‐40 hrs after hCG Frequency of IUI: single Semen preparation technique: not described Number of sperm injected: not described Type of semen: not described Catheter used: not described Cancellation criteria: not described |
|
| Outcomes | PR/couple Miscarriage rate OHSS Multiples PR Number of dominant follicles ( > 18 mm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. However, lack of blinding is unlikely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow chart has been provided; loss to follow‐up and dropouts not described. |
| Selective reporting (reporting bias) | Low risk | Not suspected. |
| Other bias | Low risk | Not suspected. |
CC: clomiphene citrate; CPR: cumulative pregnancy rate; d: day(s); DF: dominant follicle; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; hCG: human chorionic gonadotropin; hMG: human menopausal gonadotropin; hpHMG: highly purified human menopausal gonadotropin; hrs: hours; HTF: human tubal fluid; i.m./im/IM: intramuscularly; ITT: intention‐to‐treat; IU: international units; IUI: intrauterine insemination; IVF: in vitro fertilisation; LH: luteinising hormone; LZ: letrozole; n/no.: number; OHSS: ovarian hyperstimulation syndrome;OPR: ongoing pregnancy rate; PCOS: polycystic ovary syndrome; plac: placebo; PR: pregnancy rate; rFSH: recombinant follicle‐stimulating hormone; sc/SC: subcutaneously; wks: weeks; yrs: years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abunaila 2020 | Ineligible study design (quasi‐randomised) |
| Akbary‐Asbagh 2007 | Ineligible study design |
| Allegra 1990 | Ineligible study design, ineligible intervention: retrospective study, intracervical insemination |
| Alvarez 1999 | Ineligible study design and ineligible intervention: not randomised. Not only IUI but also timed coitus was advised |
| Arcaini 1996 | Ineligible comparison: superovulation with IUI was compared with superovulation alone |
| Ayaz 2018 | Ineligible study design |
| Azargoon 2013 | Ineligible study design; quasi‐randomised |
| Badawy 2009 | Retracted study |
| Badawy 2010 | Abstract of retracted study |
| Bakas 2011 | Ineligible study design |
| Barros Delgadillo 2010 | Ineligible study design |
| Barroso 2006 | Ineligible study design |
| Bedaiwy 2006 | Ineligible study design |
| Bejarano Velazquez 2016 | Ineligible patient population |
| Bekuretsion 1998 | Published as an abstract in 1998 and no full‐text article published afterwards. No additional information on which women underwent timed intercourse is available. |
| Brami 2004 | Ineligible study design: comment/translation of a review |
| Carrera 2002b | Ineligible patient population |
| Chang 1993 | Ineligible study design |
| Chaudhury 2013 | Ineligible study design |
| Check 1992 | Ineligible study design: quasi‐randomised study (randomised by date of birth) |
| Colombi 1996 | Abstract published in 1996, still no full‐text article was published by 2013. Only the number of cycles were reported, with a large difference between group sizes (192 versus 233 cycles), with no detailed information on randomisation method and concealment of allocation. |
| Crosignani 2005 | Ineligible study design: review article |
| DiMarzo 1992 | Ineligible study design |
| Doyle 1991 | Ineligible comparison: ovarian stimulation with hMG and timed coitus was compared with hMG combined with intrauterine insemination |
| Elnashar 2011 | Abstract of retracted study |
| Eskandar 2007 | Ineligible study design |
| Fernandez 2001 | Abstract published in 2001, still no full‐text article published by 2013. 5% to 6% of the cycles were followed by timed coitus. Not clear whether subfertile couples were included or couples with anovulation only. Additional information was not received. |
| Forghani 2012 | Ineligible intervention |
| Gada 2009 | Ineligible outcomes |
| Gerli 2000 | Included anovulatory women |
| Gerli 2004b | Ineligible patient population |
| Graziano 2013 | Ineligible study design |
| Guzick 2017 | Ineligible intervention |
| Hembram 2017 | Ineligible study design |
| Ibrahim 2012 | Ineligible intervention: not combined with IUI |
| Isa 2014 | Ineligible study design |
| Isaza 2003 | Ineligible study design: quasi‐randomised study; randomised by odds‐even |
| Jacobson 1991 | Ineligible study design: not adequately randomised |
| Jaroudi 1998 | Ineligible comparison: ovarian stimulation combined with IUI was compared with ovarian stimulation combined with timed intercourse |
| Karande 1995 | Randomisation was based on insemination technique and not on ovarian stimulation protocol. Comparison of group CC/IUI: 3/44 versus hMG/IUI: 10/76 seems not correct. |
| Karlström 2000 | 33 couples underwent IUI and 118 couples underwent DIPI (direct intraperitoneal insemination). During weekends no IUI but timed intercourse. No data available for IUI only. |
| Karlström 2002 | Published in 2002 as an abstract with little information on randomisation method. In 2013, still no full text publication available. Unclear which participants underwent IUI or timed intercourse. Additional information was not received. |
| Karmon 2015 | Ineligible study design |
| Khanna 2013 | Ineligible study design |
| Kocak 2010 | Different primary outcomes, randomisation not clear |
| Kotecki 2005 | Published in 2005 as an abstract. In 2020, still no full text publication available. Stated as randomised without further description, with data expressed as pregnancy rate per cycle only. Different group sizes varying from 80 to 178 cycles per group. |
| La Cour Freiesleben 2009 | Ineligible comparison |
| Lorusso 2008 | Ineligible intervention |
| Mahajan 2007 | Ineligible study design |
| Malhotra 2015 | Ineligible intervention |
| Manganiello 1997 | Ineligible study design |
| Martinez Salazar 2009 | Ineligible comparison: does not compare different stimulation protocols with IUI |
| Matorras 2002 | Donor sperm use only |
| Matorras 2006 | Ineligible study design: no randomisation |
| Mitwally 2002 | Ineligible study design |
| Mitwally 2003a | Ineligible study design |
| Mitwally 2003b | Ineligible comparison |
| Mitwally 2004 | Ineligible study design |
| Mitwally 2005 | Ineligible study design |
| Munoz 2011 | Ineligible intervention |
| Nappi 2000 | Ineligible comparison |
| Nava 2004 | Ineligible study design: pseudo‐randomised study |
| Nuojua‐Huttunen 1997 | Ineligible study design: non‐randomised study |
| Papageorgiou 1995 | Ineligible comparison: IUI in natural cycles compared with IUI after mild ovarian stimulation |
| Plowden 2017 | Ineligible intervention |
| Ponzano 2017 | Ineligible study design |
| Prentice 1995 | Ineligible study design, ineligible comparison: ovarian stimulation combined with IUI compared with expectant management; quasi‐randomised by alternating record numbers |
| Reindollar 2010 | Ineligible intervention |
| Ruddock 2004 | Ineligible comparison, ineligible study design |
| Sakhel 2007 | Ineligible intervention |
| Samanta 2008 | Randomisation unclear |
| Scheiber 2003 | Ineligible patient population |
| Sharma 2011b | Ineligible intervention/ineligible comparison |
| Sipe 2006 | Ineligible patient group: anovulatory women, women with PCOS and women with unexplained infertility were included. Data of unexplained infertility could not be extracted from the groups. |
| Steinkampf 1993 | Ineligible intervention: ovarian stimulations compared without IUI |
| Streda 2012 | Ineligible study design/ineligible comparison of interest |
| Taerk 2016 | Ineligible study design/ineligible comparison of interest |
| Taskin 2005 | Ineligible study design: clinical trial, not randomised |
| Tehrani Nejad 2008 | Not truly randomized |
| Topipat 2008 | Ineligible intervention: no IUI used, patients used condoms, only follicle measurements |
| Tummon 1997 | Ineligible comparison: ovarian stimulation combined with IUI compared with no treatment for infertility |
| Unfer 2004 | Ineligible patient population |
| Van Vliet 2014 | Ineligible study design |
| Vasiljevic 2000 | Ineligible study design: non‐randomised study |
| Wang 2008 | Ineligible study design: non‐randomised study, choice of treatment was left to the patient |
| Weiss 2010 | Ineligible comparison |
| Xu 2014 | Ineligible patient population |
| Yun 2013 | Ineligible study design |
CC: clomiphene citrate; hMG: human menopausal gonadotropin; IUI: intrauterine insemination; PCOS: polycystic ovary syndrome
Characteristics of studies awaiting classification [ordered by study ID]
Abu Hashim 2012.
| Methods | Randomisation: computer‐generated blocked randomisation list prepared by independent statistician. Treatment allocation was concealed using sealed opaque envelopes given to a third party who assigned women to a study arm. Trial design: parallel Power calculation: yes, power of 80% Dropouts: yes, aromatase inhibitor: 6, anti‐oestrogens: 5, dropped out after third IUI trial because they moved on to IVF. Cycle cancellation: yes Blinding: outcome assessors were blinded to the treatment groups ITT: yes. |
| Participants | 136 women, 433 cycles Age of women: aromatase inhibitor: 31.3 ± 2.2 (years); anti‐oestrogens: 30.7 ± 2.7 (years) Duration of infertility: aromatase inhibitor: 2.8 ± 0.7 (years); anti‐oestrogens: 2.7 ± 0.8 (years) Type of infertility: unexplained, minimal/mild endometriosis Previous fertility treatment: no Primary infertility: yes |
| Interventions | Stimulation method/dosage: aromatase inhibitor 5 mg daily for 5 days, starting on CD 3, after IUI vaginal progesterone pessaries 400 mg/day; clomid 100 mg daily for 5 days, starting on CD 3 Trigger for ovulation: hCG (10,000 IU) im Timing of IUI: 32‐36 hrs after hCG Frequency of IUI: once Semen preparation technique: 500 µL Ham's F‐10 No of sperm injected: not stated Type of semen: normal semen analysis, thus husband semen Catheter used: Gynetics Cancellation criteria: no cancellations |
| Outcomes | PR/couple: aromatase inhibitor 50.7% vs clomid 46.3% PR/cycle: aromatase inhibitor 15.9% vs clomid 14.5% Live birth: aromatase inhibitor 44.9% vs clomid 40.3% Multiples: aromatase inhibitor 11.4% vs clomid 12.9% Number of ampoules used: not applicable Number of dominant follicle ( > 18 mm): aromatase inhibitor: 1.4 ± 0.2 vs Clomid: 2.8 ± 0.4 |
| Notes | More information is needed to determine whether the study can be included. Editor’s Note: Abu Hashim H, EL Rakhawy M and Abd Elaal I. Randomized comparison of superovulation with letrozole vs clomiphene citrate in an IUI program for women with recently surgically treated minimal to mild endometriosis. Acta Obstetricia et Gynecologica Scandinavica, 2012;91:338–345 |
EUCTR 2006.
| Methods | Prospective randomised multicenter study |
| Participants | Ovulatory women suffering from infertility |
| Interventions | Individual versus standard rFSH doses combined with intrauterine insemination |
| Outcomes | Number of mature follicles |
| Notes |
IRCT20090912002445N 2019.
| Methods | Not stated clearly in the abstract |
| Participants | Individuals with primary and secondary infertility. Age between 18 and 45 years. Candidates for mild ovarian stimulation and intrauterine insemination (IUI) |
| Interventions | Anti‐oestrogens (100 mg daily from day 5 to 9 of menstrual cycle), gonadotropin (75 IU subcutaneously from day 8 to 11 of menstrual cycle), misoprostol (100 mg twice daily from day 5 to 9 of menstrual cycle). Control group: placebo (1 capsule twice daily from day 5 to 9 of menstrual cycle) instead of misoprostol |
| Outcomes | clinical pregnancy rate |
| Notes | keramati@hotmail.com. According to ICRT, trial is completed; no full‐text in PubMed |
IRCT201106256871N 2012.
| Methods | Not stated |
| Participants | Female infertility associated with male factors |
| Interventions | Anti‐oestrogens group (group B) 100 mg, daily, orally, from 3rd to 7th day of menstrual cycle and two gonadotropins in 8th day and one gonadotropin in 9th day of menstrual cycle. Aromatase inhibitor (group A) 10 mg daily, orally, from 3rd to 7th day of menstrual cycle and two gonadotropin in 8th day and one gonadotropin in 9th day of menstrual cycle |
| Outcomes | Endometrial thickness, chemical pregnancy |
| Notes | According to ICTR, study is completed; no full‐text; contacted ahmadishahnaz2005@yahoo.com; no response |
IRCT20180528039878N1 2018.
| Methods | Not stated. Evaluation of the efficiency of anti‐oestrogens + aromatase inhibitor on IUI outcome. |
| Participants | Mild male factor infertility and unexplained infertility. Anovolatory women who have not been pregnant despite the use of ovulation induction techniques and appropriate follicles |
| Interventions | Group A will receive anti‐oestrogens (100 mg, Iran Hormone) on cycle day 3‐7 (for 5 days). Group B will receive aromatase inhibitor (5 mg, SOHA, Iran) on cycle day 3‐7 (for 5 days). Group C will receive anti‐oestrogens (100 mg, Iran Hormone) and aromatase inhibitor (5 mg, SOHA, Iran) on cycle day 3‐7 (for 5 days) |
| Outcomes | Clinical pregnancy |
| Notes | No contact details in trial register. |
hCG: human chorionic gonadotropin; i.m./im/IM: intramuscularly; IU: international units; IUI: intrauterine insemination; IVF: in vitro fertilisation; PR: pregnancy rate; rFSH: recombinant follicle‐stimulating hormone; sc/SC: subcutaneously
Differences between protocol and review
The outcomes of costs of treatment, international units (IU) used (when applicable), and number of dominant follicles were specified in the original protocol. However, we did not include them in the final review.
In the 2021 update, we revised the Methods section according to current Cochrane standards. We changed the title to 'Agents for ovarian stimulation for intrauterine insemination (IUI) in women with infertility'.
The outcome of live birth rate included live birth rates only (thus without ongoing pregnancy rates).
Random‐effects model were used in cases of high heterogeneity (>50%).
We included Sensitivity analysis per dose.
Contributions of authors
AEP Cantineau took the lead in developing the protocol, reading and selecting new studies, and writing the review. AGH Rutten took the lead in reading and selecting new studies, co‐writing the review and grading the evidence. BJ Cohlen commented on drafts of the updated review.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
AEP Cantineau and BJ Cohlen are authors of an included study comparing FSH with a GnRH antagonist with FSH alone. This is an investigators‐initiated trial. Medication used in this trial was supplied by Serono B.V. only. Serono B.V. was unable to interfere with the results of this RCT, and have had no influence on this Cochrane Review. In conclusion, all three review authors have involvement in primary research in the subject area of our review, but no personal financial support has been gained.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akbari 2012 {published data only}
- Akbari S, Roozbahani MA, Roozbahani FA. Comparsion of letrozole versus clomiphene citrate combined with gonadotropins in intrauterine insemination cycles. Iranian Journal of Reproductive Medicine 2012;10(1):29-32. [PMC free article] [PubMed] [Google Scholar]
Al‐Fadhli 2006 {published data only}
- Al-Fadhli R, Sylvestre C, Buckett W, Tulandi T. A randomized trial of superovulation with two different doses of letrozole. Fertility and Sterility 2006;85(1):161-4. [DOI] [PubMed] [Google Scholar]
Al‐Fozan 2004 {published data only}
- Al-Fozan H, Al-Khadouri M, Tan SL, Tulandi T. A randomized trial of letrozole versus clomiphene citrate in women undergoing superovulation. Fertility and Sterility 2004;82(6):1561-3. [DOI] [PubMed] [Google Scholar]
Al‐Inany 2010 {published data only}
- Al-Inany H, Azab H, El-Khayat W, Nada A, El-Khattan E. Suppression in women undergoing intrauterine insemination: a randomized controlled trial. Fertility and Sterility 2010;94(6):2167-71. [DOI] [PubMed] [Google Scholar]
Allegra 2007 {published data only}
- Allegra A, Marino A, Coffaro F, Scaglione P, Sammartano F, Rizza G, et al. GnRH antagonist-induced inhibition of the premature LH surge increases pregnancy rates in IUI-stimulated cycles. A prospective randomized trial. Human Reproduction 2007;22(1):101-8. [DOI] [PubMed] [Google Scholar]
Balasch 1994 {published data only}
- Balasch J, Ballesca JL, Pimentel C, Creus M, Fabregues F, Vanrell JA. Late low-dose pure follicle stimulating hormone for ovarian stimulation in intrauterine insemination cycles. Human Reproduction 1994;9(10):1863-6. [DOI] [PubMed] [Google Scholar]
Baysoy 2006 {published data only}
- Baysoy A, Serdaroglu H, Jamal H, Karatekeli E, Ozornek H, Attar E. Letrozole versus human menopausal gonadotrophin in women undergoing intrauterine insemination. Reproductive BioMedicine Online 2006;13(2):208-2. [DOI] [PubMed] [Google Scholar]
Berker 2011 {published data only}
- Berker B, Kahraman K, Tasken S, Sukur YE, Sonmezer M, Atabekoglu CS. rFSH versus CC for ovarian stimulation in couples with unexplained infertility and male subfertility undergoing IUI: a randomized trial. Archives of Gynecology and Obstetrics 2011;284(6):1561-6. [DOI] [PubMed] [Google Scholar]
Cantineau 2011 {published data only}
- Cantineau AE, Cohlen BJ, Klip H, Heineman MJ, on behalf of the Dutch IUI Study Group Collaborators. The addition of GnRH antagonist in intrauterine insemination cycles with mild ovarian hyperstimulation does not increase live birth rates - a randomized double blinded placebo controlled trial. Human Reproduction 2011;26(5):1104-11. [DOI] [PubMed] [Google Scholar]
Carrera 2002a {published data only}
- Carrera J, Estrada CI. Effect of the addition of GnRH agonist (leuprolide acetate) to FSHr in the protocols of ovarian stimulation for artificial insemination cycles [Effectos de la adición de un análogo de la GnRH (acetato de leuprorelina) a la FSHr en los protocolos de estimulatción ovárica para ciclos de inseminación artificial]. Revista Iberoamericana de Fertilidad y Reproducción Humana 2002;19(2):109-14. [Google Scholar]
Cavagna 2009 {unpublished data only}
- Cavagna M, Scantamburlo V, Ligabo M, Freitas GC, Dzik A. Clomiphene citrate supplemented with either recombinant FSH or low dose hCG for ovarian stimulation for intrauterine insemination: preliminary results. Human Reproduction. European Society of Human Reproduction and Embryology 2009;24 suppl 1:i45. [Google Scholar]
Crosignani 2007 {published data only}
- Crosignani PG, Somigliana E. Effect of GnRh antagonists in FSH mildly stimulated intrauterine insemination cycles: a multicentre randomized trial. Human Reproduction 2007;22:500-5. [DOI] [PubMed] [Google Scholar]
Danhof 2018 {published data only}
- Danhof N, Van Wely M, Koks C, Verhoeve HR, Bruin JP, Verberg M, et al. Ovarian stimulation in IUI cycles in couples with unexplained subfertility: follicle stimulating hormone (FSH) or clomiphene citrate (CC)? Human Reproduction 2017;32 suppl 1:i4-5. [Google Scholar]
- Danhof NA, Van Wely M, Repping S, Koks C, Verhoeve HR, De Bruin JP, et al. Follicle stimulating hormone versus clomiphene citrate in intrauterine insemination for unexplained subfertility: a randomized controlled trial. Human Reproduction 2018;33(10):1866-74. [DOI] [PubMed] [Google Scholar]
Dankert 2007 {published data only}
- Dankert T, Kremer JA, Cohlen BJ, Hamilton CJ, Pasker-de Jong PC, Straatman H, et al. A randomized clinical trial of clomiphene citrate versus low dose recombinant FSH for ovarian hyperstimulation in intrauterine insemination cycles for unexplained and male subfertility. Human Reproduction 2007;22(3):792-7. [DOI] [PubMed] [Google Scholar]
Dansuk 2015 {published data only}
- Dansuk R, Gonenc AI, Sudolmus S, Yucel O, Sevket O, Köroğlu N. Effect of GnRH antagonists on clinical pregnancy rates in ovulation induction protocols with gonadotropins and intrauterine insemination. Singapore Medical Journal 2015;56(6):353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davar 2006 {published data only}
- Davar R, Asgharnia M, Tayebi N, Aflatoonian A. Comparison of the use of letrozole and clomiphene citrate in regularly ovulating women undergoing intrauterine insemination. Middle East Fertility Society Journal 2006;11(2):113-8. [Google Scholar]
Deghani‐Firouzabady 2006 {published data only}
- Dehghani-Firouzabady R, Asgharnia M, Tayebi M, Mehdi Kalantar S. Use of low dose human chorionic gondadotrophin (hCg) for final follicular maturation in ovulatory women treated by intra uterine insemination. Middle East Fertility Society Journal 2006;11(3):210-5. [Google Scholar]
Demirol 2007 {published data only}
- Demirol A, Aksu T, Aydiner F, Gurgan T. Different gonadotropin preparations in IUI cycles: a prospective randomized study. Human Reproduction 2002;17 suppl 1:166-7. [DOI] [PubMed] [Google Scholar]
- Demirol A, Gurgan T. Comparison of different gondadotrophin preparations in intrauterine insemination cycles for the treatment of unexplained infertility: a prospective, randomized study. Human Reproduction 2007;22(1):97-100. [DOI] [PubMed] [Google Scholar]
- Gurgan T, Demirol A. Comparison of COH-IUI results with different gonadotrophin preparations. Human Reproduction 2004;19 suppl 1:i63. [DOI] [PubMed] [Google Scholar]
Dhaliwal 2002 {published data only}
- Dhaliwal LK, Sialy RK, Gopalan S, Majumdar S. Minimal stimulation protocol for use with intrauterine insemination in the treatment of infertility. Journal of Obstetrics and Gynaecology Research 2002;28(6):295-9. [DOI] [PubMed] [Google Scholar]
Diamond 2015 {published data only}
- Diamond MP, Legro RS, Coutfaris C, Alvero R, Robinson RD, Casson P, et al . Letrozole, gonadotrophin, or clomiphene for unexplained infertility. New England Journal of Medicine 2015;373:1230-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodson 1991 {published data only}
- Dodson WC, Walmer DK, Hughes CL Jr, Yancy SE, Haney AF. Adjunctive leuprolide therapy does not improve cycle fecundity in controlled ovarian hyperstimulation and intrauterine insemination of subfertile women. Obstetrics and Gynecology 1991;78(2):187-90. [PubMed] [Google Scholar]
Ecochard 2000 {published data only}
- Ecochard R, Mathieu C, Royere D, Blache G, Rabilloud M, Czyba JC. A randomized prospective study comparing pregnancy rates after clomiphene citrate and human menopausal gonadotropin before intrauterine insemination. Fertility and Sterility 2000;73(1):90-3. [DOI] [PubMed] [Google Scholar]
El Helw 2002 {published data only}
- El Helw B, El Sadek M. Single dose letrozole versus CC for superovulation prior to intrauterine insemination in a prospective RCT. Human Reproduction 2002;17 suppl 1:73. [Google Scholar]
Erdem 2015 {published data only}
- Erdem M, Abay S, Erdem A, Firat Mutlu M, Nas E, Mutlu I, et al. Recombinant FSH increases live birth rates as compared to clomiphene citrate in intrauterine insemination cycles in couples with subfertility: a prospective randomized study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2015;189:33-7. [DOI] [PubMed] [Google Scholar]
Espejo‐Catena 2016 {published data only}
- Espejo-Catena M, Baño I, Puertos J. Effectiveness of gonadotropin-releasing hormone antagonists in the management of multifollicular recruitment in intrauterine insemination cycles. Acta Medica International 2016;3(1):13-8. [Google Scholar]
Fatemi 2003 {published data only}
- Fatemi HM, Kolibianakis E, Tournaye H, Camus M, Van Steirteghem AC, Devroey P. Clomiphene citrate versus letrozole for ovarian stimulation: a pilot study. Reproductive Biomedicine Online 2003;7(5):543-6. [DOI] [PubMed] [Google Scholar]
Filicori 2001 {published data only}
- Filicori M, Cognigni GE, Pocognoli P, Tabarelli C, Ferlini F, Perri T, et al. Luteinizing hormone activity in menotropins optimizes folliculogenesis and treatment in controlled ovarian stimulation. Journal of Clinical Endocrinology and Metabolism 2001;86(1):337-43. [DOI] [PubMed] [Google Scholar]
Filicori 2003 {published data only}
- Filicori M, Cognigni GE, Pocognoli P, Tabarelli C, Ferlini F, Perri T, et al. Comparison of controlled ovarian stimulation with human menopausal gonadotropin or recombinant follicle-stimulating hormone. Fertility and Sterility 2003;80(2):390-7. [DOI] [PubMed] [Google Scholar]
Fouda 2011 {published data only}
- Fouda UM, Sayed AM. Extended letrozole regimen versus clomiphene citrate for superovulation in patients with unexplained infertility undergoing intrauterine insemination: a randomized controlled trial. Reproductive Biology and Endocrinology 2011;21(9):84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galal 2015 {published data only}
- Galal A. Letrozole step-up protocol: the effect of a novel superovulation induction protocol to enhance pregnancy rate in a couple with unexplained infertility undergoing intrauterine insemination. Human Reproduction 2017;32 suppl 1:i7. [Google Scholar]
Gerli 1993 {published data only}
- Gerli S, Villani C. Endocrine changes and follicular development in patients during ovulation induction using Goserelin and different gonadotropin treatments. Clinical & Experimental Obstetrics & Gynecology 1993;20(4):245-50. [PubMed] [Google Scholar]
Gerli 2004a {published data only}
- Gerli S, Casini ML, Unfer V, Costabile L, Bini V, Di Renzo GC. Recombinant versus urinary follicle-stimulating hormone in intrauterine insemination cycles: a prospective, randomised analysis of cost-effectiveness. Fertility and Sterility 2004;83(3):573-8. [DOI] [PubMed] [Google Scholar]
Ghosh Dastidar 2009 {published data only}
- Ghosh Dastidar S, Ghosh Dastidar B. The impact of LH supplementation in controlled ovarian stimulation with recombinant FSH and GnRH antagonist in IUI cycle. Human Reproduction 2009;24 suppl 1:i43. [Google Scholar]
Gomez‐Palomares 2005 {published data only}
- Gomez-Palomares JL, Juliia B, Acevedo-Martin B, Martinez-Burgos M, Hernandez ER, Ricciarelli E. Timing ovulation for intrauterine insemination with a GnRH antagonist. Human Reproduction 2005;20(2):368-72. [DOI] [PubMed] [Google Scholar]
Gomez‐Palomares 2008 {published data only}
- Gomez-Palomares JL, Acevedo-Martin B, Chavez M, Angeles Manzanres M, Ricciarelli E, Hernandez ER. Multifollicular recruitment in combination with gondotrophin-releasing hormone antagonist increased pregnancy rates in intrauterine insemination cycles. Fertility and Sterility 2008;89(3):620-4. [DOI] [PubMed] [Google Scholar]
Gregoriou 2008 {published data only}
- Gregoriou O, Vlahos NF, Konidaris S, Papadias K, Botsis D, Creatsas GK. Randomized controlled trial comparing superovulation with letrozole versus recombinant follicle-stimulating hormone combined with intrauterine insemination for couples with unexplained infertility who had failed clomiphene citrate stimulation and intrauterine insemination. Fertility and Sterility 2008;90(3):678-83. [DOI] [PubMed] [Google Scholar]
Haqnawaz 2013 {published data only}
- Haqnawaz F, Virk S, Qadir T, Imam S, Rizvi J. Comparison of letrozole and clomiphene citrate efficacy along with gonadotrophins in controlled ovarian hyperstimulation for intrauterine insemination cycles. Journal of Reproduction & Infertility 2013;14:138-42. [PMC free article] [PubMed] [Google Scholar]
Hughes 1998 {published data only}
- Hughes EG, Collins JA, Gunby J. A randomized controlled trial of three low-dose gonadotrophin protocols for unexplained infertility. Human Reproduction 1998;13(6):1527-31. [DOI] [PubMed] [Google Scholar]
Jain 2016 {published data only}
- Jain S, Majumdar A. Impact of gonadotropin-releasing hormone antagonist addition on pregnancy rates in gonadotropin-stimulated intrauterine insemination cycles. Journal of Human Reproductive Sciences 2016;9:151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamal 2005 {published and unpublished data}
- Jamal H, Serdaroglu H, Baysoy A, Karatekeli E, Attar E, Ozornek H. Letrozole versus hMG in intrauterine insemination cycles. Human Reproduction 2005;20 suppl 1:i3. [DOI] [PubMed] [Google Scholar]
Kabouk 2010 {unpublished data only}
- Kabouk GB, Donadio NF. A prospective randomized study comparing clomiphene citrate supplemented with recombinant FSH or low-dose HCG in ovarian stimulation for intrauterine insemination. Fertility and Sterility 2010;4 Suppl 1:S160. [Google Scholar]
Kamath 2013 {published data only}
- Kamath MS, Bhave P, Muthukumar RR, Aleyamma MK, George K. Effectiveness of GnRH antagonist in intrauterine insemination cycles. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;166(2):168-72. [DOI] [PubMed] [Google Scholar]
Kamel 1995 {published data only}
- Kamel MA. Effect of induction protocols on pregnancy rate in artificial insemination by husband. Human Reproduction 1995;10 Suppl 1:116. [Google Scholar]
Karlström 1993 {published data only}
- Karlstrom PO, Bergh T, Lundkvist O. A prospective randomized trial of artificial insemination versus intercourse in cycles with human menopausal gonadotropin or clomiphene citrate. Fertility and Sterility 1993;59(3):554-9. [PubMed] [Google Scholar]
Karlström 1998 {published data only}
- Karlström PO, Berkurezion M, Bergh T, Lundkvist O. An extended prospective randomized trial of artificial insemination versus intercourse in cycles stimulated with human menopausal gonadotrophins (hMG) or clomiphene citrate (CC). Fertility and Sterility 1998;70 Suppl 2(3):S420. [PubMed] [Google Scholar]
Karthik 2018 {published data only}
- Karthik S, Kriplani A, Kachhawa G, Khadgawat R, Aggarwal N, Bhatla N. Comparison of two regimens of gonadotropin-releasing hormone antagonists in clomiphene-gonadotropin induced controlled ovulation and intrauterine insemination cycles: randomized controlled study. Journal of Human Reproductive Sciences 2018;11(2):148-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 2019 {published data only}
- Kaur J, Suri V, Gainder S, Arora A. Prospective randomized trial comparing efficacy of letrozole step-up protocol with letrozole plus gonadotropins for controlled ovarian stimulation and intrauterine insemination in patients with unexplained infertility. Archives of Gynecology and Obstetrics 2019;300(6):1767-71. [DOI] [PubMed] [Google Scholar]
Kim 1996 {published data only}
- Kim CH, Cho YK, Mok JE. Simplified ultralong protocol of gonadotrophin-releasing hormone agonist for ovulation induction with intrauterine insemination in patients with endometriosis. Human Reproduction 1996;11(2):398-402. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim Ch, Kang HJ, Kim SR, Jeon GH, Lee HA, Kim SH, et al. Effectiveness of soft stimulation protocol, compared with conventional GnRH antagonist multiple dose protocol in patients undergoing controlled ovarian stimulation with intrauterine insemination. Korean Journal of Reproductive Medicine 2010;37(2):135-42. [Google Scholar]
Labarta 2016 {published data only}
- Labarta E, Bosch E, Simon C, Remohi J, Pellicer A. A prospective, randomized controlled trial comparing highly purified hMG with recombinant FSH + recombinant LH in women undergoing intra-uterine insemination: ovarian response and clinical outcomes. Fertility and Sterility 2007;88:295-6. [Google Scholar]
- Labarta E, Bosch E. Comparative study of highly purified HMG versus recombinant FSH + recombinant LH in ovulation induction for intrauterine insemination: a randomized controlled trial. Medicina Reproductiva y Embriología Clínica 2016;3(1):4-11. [Google Scholar]
Lambalk 2006 {published data only}
- Lambalk CB, Leader A, Olivennes F, Fluker MR, Andersen AN, Ingerslev J, et al. Treatment with the GnRH antagonist ganirelix prevents premature LH rises and luteinization in stimulated intrauterine insemination: results of a double-blind, placebo-controlled, multicentre trial. Human Reproduction 2006;21(3):632-9. [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee TH, Lin YH, Seow KM, Hwang JL, Tzeng CR, Yang YS. Effectiveness of cetrorelix for the prevention of premature luteinizing hormone surge during controlled ovarian stimulation using letrozole and gonadotropins: a randomized trial. Fertility and Sterility 2008;90(1):113-20. [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin YH, Seow KM, Chen HJ, Hsieh BC, Huang LW, Tzeng CR, et al. Effect of cetrorelix dose on premature LH surge during ovarian stimulation. Reproductive Biomedicine Online 2008;16(6):772-7. [DOI] [PubMed] [Google Scholar]
Malhotra 2012 {published data only}
- Malhotra N, Karmakar D, Kumar S. Letrozole alone or letrozole gonadotropin combination as first line for superovulation in women with unexplained infertility undergoing intrauterine insemination (IUI): a prospective randomized trial. Fertility and Sterility 2012;98 Suppl 1(3):S258. [Google Scholar]
Matorras 2000 {published data only}
- Matorras R, Recio V, Corcostegui B, Rodriguez-Escudero FJ. Recombinant human FSH versus highly purified urinary FSH: a randomized study in intrauterine insemination with husbands' spermatozoa. Human Reproduction 2000;15(6):1231-4. [DOI] [PubMed] [Google Scholar]
Moro 2015 {published data only}
- Moro F, Scarinci E, Palla C, Romani F, Familiari A, Tropea A, et al . Highly purified hMG versus recombinant FSH plus recombinant LH in intrauterine insemination cycles in women ≥35 years: a RCT. Human Reproduction 2015;30(1):179-85. [DOI] [PubMed] [Google Scholar]
Nada 2016 {published data only}
- Nada AM, El Setohy KA, Banat MM, Shaheen AF. Antagonist protocol versus clomiphene in unexplained infertility: a randomized controlled study. Taiwanese Journal of Obstetrics & Gynecology 2016;55:326-30. [DOI] [PubMed] [Google Scholar]
Nakajima 1999 {published data only}
- Nakajima AK, Smith LL, Wong B, Scott JZ, Cumming DC, Tataryn IV, et al. A randomized trial of clomiphene citrate plus intrauterine insemination versus recombinant follicle-stimulating hormone plus intrauterine insemination for the treatment of unexplained infertility. Fertility and Sterility 1999;72 Suppl 1:S208. [Google Scholar]
Nayar 2008 {unpublished data only}
- Nayar KD, Uniyal S, Sawhney S. Clomiphene citrate versus low dose recombinant FSH for ovarian hyperstimulation in intrauterine insemination cycles for unexplained and male subfertility. Fertility and Sterility 2008;90 Suppl 1:S476. [Google Scholar]
Ozmen 2005 {published data only}
- Ozmen B, Salih T, Kaan A, Hakan S, Volkan B, Rusen A. Aromatase inhibitor versus clomiphene citrate in IUI cycles. Human Reproduction 2005;20 Suppl 1:i112. [Google Scholar]
Parés 2002 {published data only}
- Parés P, Bordas JR, Sak MJ, Sunol J, Bassas Ll, Viscasillas P, et al. Recombinant FSH versus urinary FSH in ovarian stimulation for intrauterine insemination with husband's sperm. Prospective and randomised study [FSH recombinante versus FSH urinaria en la estimulación ovárica para inseminaciones artificiales conyugales intrauterinas. Estudio prospectivo y randomizado]. Revista Iberoamericana de Fertilidad y repro Humana 2002;19(2):115-21. [Google Scholar]
Pattuelli 1996 {published data only}
- Pattuelli M, Zanasi P, Seracchioli R, Colombi C, Ferlini F, Fabbri R, et al. Supplementation of GnRH agonist to ovarian stimulation and intrauterine insemination does not improve the pregnancy rate. Human Reproduction 1996;11 Suppl 1:128. [Google Scholar]
Peeraer 2015 {published data only}
- Peeraer K, Debrock S, De Loecker P, Tomassetti C, Laenen A, Welkenhuysen M, et al. Low-dose human menopausal gonadotrophin versus clomiphene citrate in subfertile couples treated with intrauterine insemination: a randomized controlled trial. Human Reproduction 2015;30(5):1079-88. [DOI] [PubMed] [Google Scholar]
Pourali 2017 {published data only}
- Pourali L, Ayati S, Tavakolizadeh S, Soleimani H, Sani FT. Clomiphene citrate versus letrozole with gonadotropins in intrauterine insemination cycles: a randomized trial. International Journal of Reproductive Biomedicine 2017;15(1):49-54. [PMC free article] [PubMed] [Google Scholar]
Pourmatroud 2013 {published data only}
- Pourmatroud E, Zargar M, Nikbakht R, Moramazi F. A new look at tamoxifen: co-administation with letrozole in intrauterine insemination cycles. Archives of Gynecology and Obstetrics 2013;287(2):383-7. [DOI] [PubMed] [Google Scholar]
Ragni 2001 {published data only}
- Ragni G, Vegetti W, Baroni E, Colombo M, Arnoldi M, Lombroso G, et al. Comparison of luteal phase profile in gonadotrophin stimulated cycles with or without a gonadotrophin-releasing hormone antagonist. Human Reproduction 2001;16(11):54-8. [DOI] [PubMed] [Google Scholar]
Ragni 2004 {published data only}
- Ragni G, Alagna F, Brigante C, Riccaboni A, Colombo M, Somigliana E, et al. GnRH antagonists and mild ovarian stimulation for intrauterine insemination: a randomized study comparing different gonadotrophin dosages. Human Reproduction 2004;19(1):54-8. [DOI] [PubMed] [Google Scholar]
Ransom 1996 {published data only}
- Ransom MX, Doughman NC, Garcia AJ. Menotropins alone are superior to a clomiphene citrate and menotropin combination for superovulation induction among clomiphene citrate failures. Fertility and Sterility 1996;65(6):1169-74. [DOI] [PubMed] [Google Scholar]
Rashidi 2013 {published data only}
- Rashidi M, Aaleyasin A, Aghahossani M, Loloi S, Kokab A, Najmi Z. Advantages of rFSH over hMG for ovulation stimulation in IUI: a randomised controlled trial in unexplained infertility. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;169(2):244-7. [DOI] [PubMed] [Google Scholar]
Sadaghiani 2012 {published data only}
- Sadaghiani M, Hamdi K, Danaii S, Moosapur P. Comparison of the efficacy of letrozole gonadotropins and clomiphene citrate gonadotropins in ovarian hyperstimulation of infertile women undergoing intrauterine insemination procedure. Iranian Journal of Reproductive Medicine 2012;10:14-5. [Google Scholar]
Sagnella 2011 {published data only}
- Sagnella F, Moro F, Lanzone A, Tropea A, Martinez D, Capalbo A, et al. A prospective randomized noninferiority study comparing recombinant FSH and highly purified menotropin in intrauerine insemination cycles in couples with unexplained infertility and/or mild-moderate male factor. Fertility and Sterility 2011;95(2):689-94. [DOI] [PubMed] [Google Scholar]
Sammour 2001 {published data only}
- Sammour A, Biljan MM, Tan SL, Tulandi T. Prospective randomized controlled trial comparing effect of letrozole (LE) and clomiphene citrate (CC) on follicular development, endometrial thickness and pregnancy rate in patients undergoing superovulation prior to intrauterine insemination (IUI). Fertility and Sterility 2001;76 Suppl 1:S110. [Google Scholar]
Sengoku 1994 {published data only}
- Sengoku K, Tamate K, Takaoka Y, Horikawa M, Goishi K, Komori H, et al. A randomized prospective study of gonadotrophin with or without gonadotrophin-releasing hormone agonist for treatment of unexplained infertility. Human Reproduction 1994;9(6):1043-7. [DOI] [PubMed] [Google Scholar]
Sengoku 1999 {published data only}
- Sengoku K, Tamate K, Takaoka Y, Horikawa M, Goishi K, Komori H, et al. The clinical efficacy of low-dose step-up follicle stimulating hormone administration for treatment of unexplained inferility. Human Reproduction 1999;14(2):349-53. [DOI] [PubMed] [Google Scholar]
Sharma 2011a {published data only}
- Sharma S, Rajani S, Kandula L, Sarkar A, Palchaudhuri P, Chakravarty B. Letrozole gonadotrophins combination is inferior to either letrozole or gonadotrophins alone in women with unexplained infertility after clomiphene failure: a prospective randomised clinical trial. Fertility and Sterility 2011;96 Suppl 1:O-5. [Google Scholar]
Steward 2011 {published data only}
- Steward RG, Gill I, Williams DB, Witz CA, Griffith J, Haddad GF. Cetrorelix lowers premature luteinization rate in gonadotropin ovulation induction-intrauterine insemination cycles: a randomized-controlled clinical trial. Fertility and Sterility 2011;95(1):434-6. [DOI] [PubMed] [Google Scholar]
Taravat 2011 {published data only}
- Taravat F, Hamidreza S, Mansour R, Keshavarzi F, Salma H, Sara D. A comparison of anastrozole and clomiphene citrate for ovulation induction in women with unexplained infertility undergoing intrauterine insemination. Pakistan Journal of Medical Sciences 2011;27(5):985-9. [Google Scholar]
Wadhwa 2016 {published data only}
- Wadhwa L, Khanna R, Gupta T, Gupta S, Arora S, Nandwani S. Evaluation of role of GnRH antagonist in intrauterine insemination (IUI) cycles with mild ovarian hyperstimulation (MOH): a prospective randomised study. Journal of Obstetrics and Gynaecology of India 2016;66:459-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang CW, Horng SG, Chen CK, Wang HS, Huang Hy, Soon YK. Superovulation with tamoxifen citrate and human menopausal gonadotrophin for patients undergoing intrauterine insemination with a thin endometrium. Human Reproduction 2004;19 Suppl 1:i115. [Google Scholar]
Williams 2004 {published data only}
- Williams RS, Hillard JB, De Vane G, Yeko T, Kipersztok S, Rhoton-Vlasak A, et al. A randomized, multicenter study comparing the efficacy of recombinant FSH vs recombinant FSH with Ganirelix during superovulation/IUI therapy. American Journal of Obstetrics and Gynecology 2004;191(2):648-51. [DOI] [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu H, Wang NM, Cheng M, Hsieh J. A randomized comparison of ovulation induction and hormone profile between the aromatase inhibitor anastrozole and clomiphene citrate in women in infertility. Gynaecological Endocrinology 2007;23(2):76-81. [DOI] [PubMed] [Google Scholar]
Zadehmodares 2012 {published data only}
- Zadehmodares S, Niyakan M, Sharafy SA, Yazdi MH, Jahed F. Comparison of treatment outcomes of infertile women by clomiphene citrate and letrozole with gonadotropins underwent intrauterine insemination. Acta Medica Iranica 2012;50(1):18-20. [PubMed] [Google Scholar]
References to studies excluded from this review
Abunaila 2020 {published data only}
- Abunaila R, Al-anbari LA, Abbood MS. The effects of adding gonadotropin-releasing hormone antagonist on cycle characteristics and pregnancy rate in stimulated intrauterine insemination (IUI) cycle. International Journal of Research in Pharmaceutical Sciences 2020;11(3):3053-60. [Google Scholar]
Akbary‐Asbagh 2007 {published data only}
- Akbary-Asbagh F, Heidar Z. Evaulation of letrozole therapeutic effect in infertile women. Acta Medica Iranica 2007;45(3):199-203. [Google Scholar]
Allegra 1990 {published data only}
- Allegra A, Volpes A, Coffaro F. Superovulation with buserelin and gonadotropins dramatically improves the success rate of intrauterine insemination with husband's washed semen [Superovulazione con buserelin e gonadotropine in un programma di aid con seme congelato]. Giornale Italiano di Ostetricia E Ginecologia 1990;12:833-7. [PubMed] [Google Scholar]
- Allegra A, Voples A, Coffaro F, Guida S, Francofonte R. Superovulation with buserelin and gonadotropins dramatically improves the success rate of intrauterine insemination with husbands' washed semen. Acta Europaea Fertilitatis 1990;21(4):191-5. [PubMed] [Google Scholar]
Alvarez 1999 {published data only}
- Alvarez P, Gomez O, Salazar F, Martinez JS, Izquierdo F. Analysis of the follicular stimulation using a low-dose (step-up) protocol with recombinant follicle stimulating hormone (rFSH) and highly purified urinary FSH (FSH-HP) in artificial insemination and directed coitus. Human Reproduction (Oxford, England) 1999;14(Suppl 1):366-7. [Google Scholar]
Arcaini 1996 {published data only}
- Arcaini L, Bianchi S, Baglioni A, Marchini M, Tozzi L, Fedele L. Superovulation and intrauterine insemination vs. superovulation alone in the treatment of unexplained infertility. Journal of Reproductive Medicine 1996;41(8):614-8. [PubMed] [Google Scholar]
Ayaz 2018 {published data only}
- Ayaz R, Asoglu MR, Ayas S. Use of clomiphene citrate alone, urinary follicle-stimulating hormone alone, or both combined sequentially in patients with unexplained subfertility undergoing intrauterine insemination: a randomized trial. Turkish Journal of Obstetrics and Gynecology 2018;15(4):243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azargoon 2013 {published data only}
- Azargoon A, Bahrami M, Alavy Toussy J. Comparison of clomiphene citrate plus HMG with clomiphen citrate plus rFSH in IUI cycles in couples with unexplained or male factor infertility: a prospective randomized study. Iranian Journal of Reproductive Medicine 2013;11(3):243-8. [PMC free article] [PubMed] [Google Scholar]
Badawy 2009 {published data only}
- Badawy A, Elnashar A, Totongy M. Clomiphene citrate or aromatase inhibitors for superovulation in women with unexplained infertility undergoing intrauterine insemination: a prospective randomized trial. Fertility and Sterility 2009;92(4):1355-9. [DOI] [PubMed] [Google Scholar]
Badawy 2010 {published data only}
- Badawy A, Elnashar A, Totongy M. Clomiphene citrate or aromatase inhibitors combined with gonadotropins for superovulation in women undergoing intrauterine insemination: a prospective randomised trial. Journal of Obstetrics and Gynaecology 2010;30(6):617-21. [DOI] [PubMed] [Google Scholar]
Bakas 2011 {published data only}
- Bakas P, Konidaris S, Liapis A, Gregoriou O, Tzanakaki D, Creatsas G. Role of gonadotropin-releasing hormone antagonist in the management of subfertile couples with intrauterine insemination and controlled ovarian stimulation. Fertility and Sterility 2011;95(6):2024-8. [DOI] [PubMed] [Google Scholar]
Barros Delgadillo 2010 {published data only}
- Barros-Delgadillo JC, Trejo-Castaneda H, Ormsby C, Gavino-Gavino F. Differing response to GnRH antagonists in cycles of ovarian hyperstimulation plus intrauterine insemination [Diferencia de respuesta a los antagonistas de GnRH en ciclos de hiperestimulación ovárica más inseminación intrauterina]. Ginecologia y Obstetricia de Mexico 2010;78(1):15-28. [PubMed] [Google Scholar]
Barroso 2006 {published data only}
- Barroso G, Menocal G, Feliz H, Rojas-Ruiz JC, Arslan M, Oehninger S. Comparison of the efficacy of the aromatase inhibitor letrozole and clomiphene citrate as adjuvants to recombinant follicle-stimulating hormone in controlled ovarian hyperstimulation: a prospective, randomized, blinded clinical trial. Fertility and Sterility 2006;86(5):1428-31. [DOI] [PubMed] [Google Scholar]
Bedaiwy 2006 {published data only}
- Bedaiwy MA, Forman R, Mousa NA, Al Inany HG, Casper RF. Cost-effectiveness of aromatase inhibitor co-treatment for controlled ovarian stimulation. Human Reproduction 2006;21(1):2838-44. [DOI] [PubMed] [Google Scholar]
Bejarano Velazquez 2016 {published data only}
- Bejarano Velazquez D, De La Perez LO, Trevino Baez JD, Gonzalez Diaz OA. A randomized trial comparing the efficacy and safety of rFSH+rLH vs. rFSH alone and hMG to induce ovulation in cycles for low complexity techniques. Human Reproduction 2016;31:i304. [Google Scholar]
Bekuretsion 1998 {published data only}
- Bekuretsion M, Lundkvist O, Karlström PO. A randomized study comparing low and high dose of HMG/FSH with and without luteal phase support in intrauterine insemination cycles. Human Reproduction (Oxford, England) 1998;13(Suppl 1):199. [Google Scholar]
Brami 2004 {published data only}
- Brami C. Intrauterine insemination for idiopathic infertility [Place des inséminations intra-utérines dans le traitement des infertilités idiopathiques]. La Revue du Praticien Gynécologie et Obstétrique 2004;80:6. [Google Scholar]
Carrera 2002b {published data only}
- Carrera J, Estrada Ll, Rocas A, Francisco E, Sarquella J. Effects of addition of GnRH agonist (triptoreline acetate) to rFSH over intrauterine insemination cycles in patients with polycystic ovary syndrome [Resultados de la adición de un análogo de la GnRH (acetato de triptorelina) a la FSHr en la estimulación ovárica para ciclos de inseminación intrauterine en pacientes con síndrome de ovarios poliquísticos]. Revista Iberoamericana de Fertilidad y Reproducción Humana 2002;19(6):399-405. [Google Scholar]
Chang 1993 {published data only}
- Chang MY, Huang HY, Lee CL, Lai YM, Chang SY, Soong YK. Treatment of infertility using controlled ovarian hyperstimulation with intrauterine insemination: the experience of 343 cases. Journal of the Formosan Medical Association 1993;92(4):341-8. [PubMed] [Google Scholar]
Chaudhury 2013 {published data only}
- Chaudhury K, Chaudhury S, Khastgir G, Purakaystha S. An effective alternative to only gonadotrophin for controlled ovarian stimulation in unexplained infertility patients undergoing intra-uterine insemination: a clinical trial. Journal of the Indian Medical Association 2013;111(9):589-92, 594. [PubMed] [Google Scholar]
Check 1992 {published data only}
- Check JH, Davies E, Adelson H. A randomized prospective study comparing pregnancy rates following clomiphene citrate and human menopausal gonadotrophin therapy. Human Reproduction 1992;7(6):801-5. [DOI] [PubMed] [Google Scholar]
Colombi 1996 {published data only}
- Colombi C, Pattuelli M, Zanasi P, Seracchioli R, Ferlini F, Magrini O, et al. Small changes in ovarian stimulation protocols do not impair results while largely reducing risks in intrauterine insemination. Human Reproduction (Oxford, England) 1996;(Suppl 1):128. [Google Scholar]
Crosignani 2005 {published data only}
- Crosignani PG, Somigliana E, Colombo M, Riccaboni A, Ragni G, Fahmy I, et al. The current role of intrauterine insemination for the treatment of male factor and unexplained infertility. Middle East Fertility Society Journal 2005;10(1):29-42. [Google Scholar]
DiMarzo 1992 {published data only}
- DiMarzo SJ, Kennedy JF, Young PE, Hebert SA, Rosenberg DC, Villanueva B. Effect of controlled ovarian hyperstimulation on pregnancy rates after intrauterine insemination. American Journal of Obstetrics and Gynecology 1992;166(6):1607-13. [DOI] [PubMed] [Google Scholar]
Doyle 1991 {published data only}
- Doyle MB, DeCherney AH. The value of empiric intrauterine insemination (IUI) with superovulation: a prospective, randomized clinical trial. Fertility and Sterility 1991;56(Abstract book):S34. [Google Scholar]
Elnashar 2011 {published data only}
- Elnashar A, Badawy A, Totongy M. Clomiphene citrate or aromatase inhibitors for superovulation in women with unexplained infertility undergoing intrauterine insemination: a prospective randomized trial. Human Reproduction (Oxford, England) 2011;26:i240. [Google Scholar]
Eskandar 2007 {published data only}
- Eskandar MA. Does the addition of a gondadotrophin-releasing hormone agonist improve the pregnancy rate in intrauterine insemination? A prospective controlled trial. Gynaecological Endocrinology 2007;23(10):551-5. [DOI] [PubMed] [Google Scholar]
Fernandez 2001 {published data only}
- Fernandez-Moris J, Viñuela C, Pizarro V, Guerra J. Recombinant human follicle stimulating hormone (r-hFSH) in patients referred to ovulation induction (OI): low dose step up versus step down protocols. Human Reproduction (Oxford, England) 2001;76(3 Suppl 1):S176. [Google Scholar]
Forghani 2012 {published data only}
- Forghani F. Comparison of urinary and recombinant human chorionic gonadotropin during ovulation induction in intrauterine insemination cycles. International Journal of Fertility and Sterility 2012;6:109. [DOI] [PubMed] [Google Scholar]
Gada 2009 {unpublished data only}
- Gada D, Tomar GS, Khare N, Potnis V, Shah V. A randomized trial comparing duration of follicular phase between letrozole and clomiphene citrate when used for superovulation. In: Abstracts of 25th annual meeting ESHRE. Vol. 24. 2009:87.
Gerli 2000 {published data only}
- Gerli S. Use of ethinyl estradiol to reverse the anti-oestrogenic effects of clomiphene citrate in patients undergoing IUI; a comparative randomized study. Fertility and Sterility 2000;73(1):85-9. [DOI] [PubMed] [Google Scholar]
Gerli 2004b {published data only}
- Gerli S, Casini ML, Unfer V, Costabile L, Mignosa M, Di Renzo GC. Ovulation induction with urinary FSH or recombinant FSH in polycystic ovary syndrome patients: a prospective randomized analysis of cost-effectiveness. Reproductive Biomedicine Online 2004;9(5):494-9. [DOI] [PubMed] [Google Scholar]
Graziano 2013 {published data only}
- Graziano A, Caserta D, Piva I, Lo Monte G, Bordi G, Martini F, et al. The addition of GnRH antagonist in IUI cycles: a pilot study. European Review for Medical & Pharmacological Sciences 2013;17(12):1604-10. [PubMed] [Google Scholar]
Guzick 2017 {published data only}
- Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor-Litvak P, Steinkampf MP, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. New England Journal of Medicine 2017;340(3):177-83. [DOI] [PubMed] [Google Scholar]
Hembram 2017 {published data only}
- Hembram M, Biswas R, Jain A. A study of controlled ovarian stimulation with clomiphene citrate or letrozole in combination with gonadotropins and IUI in unexplained infertility. Journal of Human Reproductive Sciences 2017;10(3):173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2012 {published data only}
- Ibrahim MI, Moustafa RA, Abdel-Azeem AA. Letrozole versus clomiphene citrate for superovulation in Egyptian women with unexplained infertility: a randomised controlled trial. Archives of Gynecology & Obstetrics 2012;286(6):1581-7. [DOI] [PubMed] [Google Scholar]
Isa 2014 {published data only}
- Isa AM, Abu-Rafea B, Al-Asiri S, Al-Motawa J, Almady K, Alwaznah R. Ovarian stimulation medications and patients' responses as prognostic factors in IUI-treated infertile Saudi patients. Iranian Journal of Reproductive Medicine 2014;12(7):493-8. [PMC free article] [PubMed] [Google Scholar]
Isaza 2003 {published data only}
- Isaza V, Requena A, García-Velasco JA, Remohí J, Pellicer A, Simón C. Recombinant vs urinary follicle-stimulating hormone in couples undergoing intrauterine insemination. Journal of Reproductive Medicine 2003;48(2):112-8. [PubMed] [Google Scholar]
Jacobson 1991 {published data only}
- Jacobson A, Galen D, Milani H, Weckstein L, Jacobson J. A novel superovulation regimen: three-day gonadotropin-releasing hormone agonist with overlapping gonadotropins. Fertility and Sterility 1991;56(6):1169-72. [DOI] [PubMed] [Google Scholar]
Jaroudi 1998 {published data only}
- Jaroudi KA, Hollanders H, Sieck U, Zahrani A, Al-Nour A, Atared A. Superovulation and intrauterine insemination for male factor infertility: a controlled randomized study. Middle East Fertility Society Journal 1998;3(3):254-9. [Google Scholar]
Karande 1995 {published data only}
- Karande VC, Rao R, Pratt DE, Balin M, Levrant S, Morris R, et al. A randomized prospective comparison between intrauterine insemination and fallopian sperm perfusion for the treatment of infertility. Fertility and Sterility 1995;64(3):638-40. [DOI] [PubMed] [Google Scholar]
Karlström 2000 {published data only}
- Karlström PO, Bergh T, Lundkvist O. Addition of gonadotrophin-releasing hormone agonist and/or two inseminations with husband's sperm do not improve the pregnancy rate in superovulated cycles. Acta Obstetricia et Gynecologica Scandinavica 2000;79:37-42. [PubMed] [Google Scholar]
Karlström 2002 {published data only}
- Karlström PO, Lundkvist O. A randomized comparison of two low dose protocols of Gonal-F during ovarian stimulation for intrauterine insemination. Human Reproduction (Oxford, England) 2002;17(Suppl):20. [Google Scholar]
Karmon 2015 {published data only}
- Karmon AE, Cardozo ER, Petrozza JC. Clomid compared with follicle-stimulating hormone treatment: similar follicle number means different success rates. Obstetrics & Gynecology 2015;125:60S. [Google Scholar]
Khanna 2013 {published data only}
- Khanna SC, Kumar A, Joy SG, Tanwar R, Sharma S, Prasad S. Is letrozole superior to clomiphene for ovarian stimiulation prior to intrauterine insemination? Archives of Gynecology and Obstetrics 2013;287(3):571-5. [DOI] [PubMed] [Google Scholar]
Kocak 2010 {published data only}
- Kocak M, Dilbaz B. Lyophilised HMG versus rFSH in women with unexplained infertility undergoing a controlled ovarian stimulation with intrauterine insemination: a prospective, randomized study. Gynaecolgical Endocrinology 2010;26:429-34. [DOI] [PubMed] [Google Scholar]
Kotecki 2005 {published data only}
- Kotecki JA, Dzik A, Freitas GC, Soares JB, Gahamondes LG, Cavagna M. Comparison of five different ovarian stimulation protocols for intra-uterine insemination. A prospective randomized trial. Fertility and Sterility 2005;84(Suppl 1):S93. [Google Scholar]
La Cour Freiesleben 2009 {published data only}
- La Cour Freiesleben N, Lossl K, Bogstad J, Bredkjaer HE, Toft B, Rosendahl M, et al. Individual versus standard dose of rFSH in a mild stimulation protocol for intrauterine insemination: a randomized study. Human Reproduction 2009;24(10):2523-30. [DOI] [PubMed] [Google Scholar]
Lorusso 2008 {published data only}
- Lorusso F, Palmisano M, Serratì G, Bassi E, Lamanna G, Vacca M, et al. Intrauterine insemination with recombinant or urinary human chorionic gonadotropin: a prospective randomized trial. Gynecological Endocrinology 2008;24(11):644-8. [DOI] [PubMed] [Google Scholar]
Mahajan 2007 {published data only}
- Mahajan NN, Mahajan KN, Soni RN. Effect of GnRH antagonists in FSH mildly stimulated intrauterine insemination cycles: a multicentre randomized trial. Human Reproduction (Oxford, England) 2007;22(10):2795. [DOI] [PubMed] [Google Scholar]
Malhotra 2015 {published data only}
- Malhotra N, Bansal P, Dadhwal V, Deka D, Sharma A. The role of GnRH analogues in improving outcome at superovulation and intra-uterine insemination (IUI) after surgical correction of mild endometriosis: a randomized controlled trial. International Journal of Gynaecology and Obstetrics 2015;131:E126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manganiello 1997 {published data only}
- Manganiello PD, Stern JE, Stukel TA, Crow H, Brinck-Johnsen T, Weiss JE. A comparison of clomiphene citrate and human menopausal gonadotropin for use in conjunction with intrauterine insemination. Fertility and Sterility 1997;68(3):405-12. [DOI] [PubMed] [Google Scholar]
Martinez Salazar 2009 {published data only}
- Martinez-Salazar J, Cerillo M, Quea G, Pacheco A, Garcia-Velasco JA. GnRH antagonist ganirelix prevents premature luteinization in IUI cycles: rationale for its use. Reproductive BioMedicine Online 2009;19(2):156-61. [DOI] [PubMed] [Google Scholar]
Matorras 2002 {published data only}
- Matorras R, Diaz T, Corcostegui B, Ramón O, Pijoan JI, Rodriguez-Escudero FJ. Ovarian stimulation in intrauterine insemination with donor sperm: a randomized study comparing clomiphene citrate in fixed protocol versus highly purified urinary FSH. Human Reproduction (Oxford, England) 2002;17(8):2107-11. [DOI] [PubMed] [Google Scholar]
Matorras 2006 {published data only}
- Matorras R, Ramon O. Gn-Rh antagonists in intrauterine insemination: the weekend free protocol. Journal of Assisted Reproduction and Genetics 2006;23:51-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitwally 2002 {published data only}
- Mitwally MF, Casper RF. Aromatase inhibition improves ovarian response to follicle-stimulating hormone in poor responders. Fertility and Sterility 2002;77(4):776-80. [DOI] [PubMed] [Google Scholar]
Mitwally 2003a {published data only}
- Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotrophin dose required for controlled ovarian stimulation in women with unexplained infertility. Human Reproduction (Oxford, England) 2003;18(8):1588-97. [DOI] [PubMed] [Google Scholar]
Mitwally 2003b {published data only}
- Mitwally MF, Casper RF. Aromatase inhibitors for the treatment of infertility. Expert Opinion on Investigational Drugs 2003;12(3):353-71. [DOI] [PubMed] [Google Scholar]
Mitwally 2004 {published data only}
- Mitwally MF, Casper RF. Aromatase inhibition reduces the dose of gonadotropin required for controlled ovarian hyperstimulation. Journal of the Society for Gynecologic Investigation 2004;11:406-15. [DOI] [PubMed] [Google Scholar]
Mitwally 2005 {published data only}
- Mitwally MF, Casper RF. Single-dose administration of an aromatase inhibitor for ovarian stimulation. Fertility and Sterility 2005;83(1):229-31. [DOI] [PubMed] [Google Scholar]
Munoz 2011 {published data only}
- Munoz E, Carballo A, Fernandez I, Pabon D, Portela S, Pellicer A. Mild vs conventional ovarian stimulation in intrauterine insemination. A prospective randomized study. Fertility and Sterility 2011;96(3):S256. [Google Scholar]
Nappi 2000 {published data only}
- Nappi L, Carriero C. Efficacy of super ovulatory drugs and intrauterine insemination in the management of infertility. International Journal of Gynaecology and Obstetrics 2000;4:154-6. [Google Scholar]
Nava 2004 {published data only}
- Nava JM, Nava Perez P. Comparison of two ovarian stimulation protocols for intrauterine artificial insemination with FSH-r and with or without a GnRH antagonist [Comparación de dos protocolos de estimulación ovárica para Inseminación artificial intrauterina, con FSH-r y con o sin un antagonista de la GnRH]. Revista Iberoamericana de Fertilidad y Reproducción Humana 2004;21(3):153-8. [Google Scholar]
Nuojua‐Huttunen 1997 {published data only}
- Nuojua-Huttunen S, Tuomivaara L, Juntunen K, Tomás C, Martikainen H. Long gonadotrophin releasing hormone agonist/human menopausal gonadotrophin protocol for ovarian stimulation in intrauterine insemination treatment. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;74:83-7. [DOI] [PubMed] [Google Scholar]
Papageorgiou 1995 {published data only}
- Papageorgiou GA, Papageorgiou A, Zafrakas MA. Intrauterine insemination and mild ovarian hyperstimulation as a treatment for male subfertility. In: Human Reproduction (Oxford, England). Vol. 10. 1995.
Plowden 2017 {published data only}
- Plowden TC, Mumford SL, Wild RA, Cedars M, Steiner AZ, Eisenberg E, et al. Probability of pregnancy with mono versus multiple folliculogenesis among women with unexplained infertility who are undergoing ovulation with gonadotropins, clomiphene or letrozole. Fertility and Sterility 2017;108(3):e233-e234. [Google Scholar]
Ponzano 2017 {published data only}
- Ponzano A, Colangelo EC, Di Biase L, Romani F, Tiboni GM. Clinical experience with an ovarian stimulation protocol for intrauterine insemination adopting a gonadotropin releasing hormone antagonist at low dose. Gynecological Endocrinology 2017;33(3):208-11. [DOI] [PubMed] [Google Scholar]
Prentice 1995 {published data only}
- Prentice A, Sacks GP, Morton NC, Deary AJ, Smith SK. Controlled ovarian stimulation (superovulation) and intrauterine insemination for the treatment of unexplained and minor male factor infertility. In: Human Reproduction. Vol. 10. 1995:112.
Reindollar 2010 {published data only}
- Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, Alper MM, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertility and Sterility 2010;94(3):888-99. [DOI] [PubMed] [Google Scholar]
Ruddock 2004 {published data only}
- Ruddock B. Letrozole for infertility. Canadian Pharmaceutical Journal 2004;137:53-4. [Google Scholar]
Sakhel 2007 {published data only}
- Sakhel K, Khedr M, Schwark S, Ashraf M, Fakih MH, Abuzeid M. Comparison of urinary and recombinant human chorionic gonadotropin during ovulation induction in intrauterine insemination cycles: a prospective randomized clinical trial. Fertility and Sterility 2007;87(6):1357-62. [DOI] [PubMed] [Google Scholar]
Samanta 2008 {unpublished data only}
- Samanta T, Goswami S Sharma S. Comparison of letrozole versus clomiphene citrate in ovulatory women undergoing intrauterine insemination with donor sperm. In: Abstracts of the 24th annual meeting of the ESHRE, Barcelona. Vol. 23. 2008:i121.
Scheiber 2003 {published data only}
- Scheiber M, Behnke EJ, Awadalla SG. Use of GnRH antagonist ganirelix prevents premature LH surge and allows for excellent pregnancy rates in patients with polycystic ovary syndrome undergoing controlled ovarian stimulation with intrauterine insemination. Human Reproduction (Oxford, England) 2003;18(Suppl 1):42. [Google Scholar]
Sharma 2011b {published data only}
- Sharma S, Goswami S, Goswami SK, Ghosh S, Chattopadhyay R, Sarkar A, et al. Efficacy of GnRH agonist Vs hCG as ovulation trigger in IUI cycle: a prospective randomised study. Human Reproduction (Oxford, England) 2011;26:i306. [Google Scholar]
Sipe 2006 {published data only}
- Sipe CS, Davies WA, Maifeld M, Van Voorhis BJ. A prospective randomized trial comparing anastrozole and clomiphene citrate in an ovulation induction protocol using gondadotrophins. Fertility and Sterility 2006;86(6):1676-81. [DOI] [PubMed] [Google Scholar]
Steinkampf 1993 {published data only}
- Steinkampf MP, Banks KS, Liu H. A randomized comparative trial of leuprolide + FSH vs FSH alone in infertile women with polycystic ovarian syndrome (PCO). In: Fertility and Sterility. Vol. Abstract book. 1993:S22.
Streda 2012 {published data only}
- Streda R, Mardesic T, Sobotka V, Koryntova D, Hybnerova L, Jindra M. Comparison of different starting gonadotropin doses (50, 75 and 100 IU daily) for ovulation induction combined with intrauterine insemination. Archives of Gynecology and Obstetrics 2012;286(4):1055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taerk 2016 {published data only}
- Taerk E, Hughes E, Greenberg C, Neal M, Amin S, Faghih M, et al. Choriogonadotropin alpha administration is associated with an increased clinical pregnancy rate among infertile couples undergoing controlled ovarian hyperstimulation with intrauterine insemination. Fertility and Sterility 2016;1:e39-e40. [Google Scholar]
Taskin 2005 {published data only}
- Taskin O, Erman Akar M, Kursun S, Salar Z, Gunduz T, Uner M. Effectiveness of GnRH antagonist use in PCOS patients with repeated premature LH surge in patients undergoing IUI cycles. Fertility and Sterility 2005;84(Suppl 1):S303. [Google Scholar]
Tehrani Nejad 2008 {published data only}
- Tehrani Nejad ES, Abediasi S, Rashidi BH, Azimi Nekoo E, Shariat M, Amirchiaghmaghi E. Comparison of the efficiacy of the aromatase inhibitor letrozole and clomiphen citrate gondadotrophins in controlled ovarian hyperstimulation: a prospective, simply randomized, clinical trial. Journal of Assisted Reproduction and Genetics 2008;25:187-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Topipat 2008 {published data only}
- Topipat C, Choktanasiri W. A comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on superovulation in asian women with normal ovulatory cycles. Gynaecological Endocrinology 2008;24:145-50. [DOI] [PubMed] [Google Scholar]
Tummon 1997 {published data only}
- Tummon IS, Asher LJ, Martin JSB, Tulandi T. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertility and Sterility 1997;68(1):8-12. [DOI] [PubMed] [Google Scholar]
Unfer 2004 {published data only}
- Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. High dose of phytoestrogens can reverse the antiestrogenic effects of clomiphene citrate on the endometrium in patients undergoing intrauterine insemination: a randomized trial. Journal of the Society for Gynecologic Investigation 2004;11(5):323-8. [DOI] [PubMed] [Google Scholar]
Van Vliet 2014 {published data only}
- Van Vliet M, Bensdorp AJ, Custers I, Mochtar MH, Tjon-Kon-Fat RI, Hompes P, et al. Clomiphene citrate and follicle stimulating hormone for controlled ovarian stimulation in intrauterine insemination (INeS trial). Human Reproduction (Oxford, England) 2014;29:i213. [Google Scholar]
Vasiljevic 2000 {published data only}
- Vasiljevic M, Antic N, Prorocic M, Rakic S, Garalejic E, Dragojevic S, et al. Ovulation induction with urinary and recombinant follicle stimulating hormone in infertile women with endometriosis. Gynecological Endocrinology 2000;14(Suppl 2):58. [Google Scholar]
Wang 2008 {published data only}
- Wang C-W, Horng S- G. Ovulation induction with tamoxifen and alternate-day gondadotrophin in patients with thin endometrium. Reproductive Biomedicine Online 2008;17:20-6. [DOI] [PubMed] [Google Scholar]
Weiss 2010 {unpublished data only}
- Weiss A, Geslevich Y. Optimal timing of intra-uterine insemination for controlled ovarian stimulation utilizing gondadotrophins with GnRH antagonists. In: Abstracts of the 26th Annual Meeting of ESHRE, Rome. Vol. 25. June 2010:i45.
Xu 2014 {published data only}
- Xu BF, Wang GY, Fan WM, Chen Q, Zhang AJ. Which is the best protocol of ovarian stimulation prior to artificial insemination by donor. Journal of Reproduction and Contraception 2014;25(1):41-8. [Google Scholar]
Yun 2013 {published data only}
- Yun BH, Chon SJ, Jung YS, Cho S, Choi YS, Lee BS. Comparison of two different regimens of gonadotrophin combined with clomiphene citrate or letrozole for intrauterine insemination. Fertility and Sterility 2013;100(Suppl 3):S458. [Google Scholar]
References to studies awaiting assessment
Abu Hashim 2012 {published data only}
EUCTR 2006 {unpublished data only}
IRCT20090912002445N 2019 {unpublished data only}
- Keramati P, Zarei A. Effects of prostaglandin and clomiphene citrate on pregnancy outcome. Iranian Registry of Clinical Trials (IRCT) 31 July 2019.
IRCT201106256871N 2012 {unpublished data only}
IRCT20180528039878N1 2018 {published data only}
- IRCT20180528039878N1. Evaluation of the efficiency of clomiphene citrate plus letrozole gonadotropins for superovulation in patients undergoing intrauterine insemination: a randomized trial. https://en.irct.ir/trial/32566?revision=53306.
Additional references
Ayeleke 2020
- Ayeleke RO, Asseler JD, Cohlen BJ, Veltman-Verhulst SM. Intra-uterine insemination for unexplained subfertility. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD001838. [DOI: 10.1002/14651858.CD001838.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bensdorp 2015
- Bensdorp AJ, Tjon-Kon-Fat RI, Bossuyt PM, Koks CA, Oosterhuis GJ, Hoek A, et al. Prevention of multiple pregnancies in couples with unexplained or mild male subfertility: randomised controlled trial of in vitro fertilisation with single embryo transfer or in vitro fertilisation in modified natural cycle compared with intrauterine insemination with controlled ovarian hyperstimulation. BMJ 2015;9(350):g7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantineau 2014
- Cantineau AEP, Janssen MJ, Cohlen BJ, Allersma T. Synchronised approach for intrauterine insemination in subfertile couples. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD006942. [DOI: 10.1002/14651858.CD006942.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 8 October 2021. Melbourne, Australia: Veritas Health Innovation, accessed 8 October 2021. Available at covidence.org.
Danhof 2020
- Danhof NA, Wang R, Van Wely M, Van der Veen F, Mol BW, Mochtar MH. IUI for unexplained infertility - a network meta-analysis. Human Reproduction Update 2020;26(1):1-15. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventionsversion 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Ertunc 2010
- Ertunc D, Tok EC, Savas A, Ozturk I, Dilek S. Gonadotropin-releasing hormone antagonist use in controlled ovarian stimulation and intrauterine insemination cycles in women with polycystic ovary syndrome. Fertility and Sterility 2010;93:1179–1184. [DOI] [PubMed] [Google Scholar]
Eskew 2019
- Eskew AM, Bedrick BS, Hardi A, Stoll CRT, Colditz GA, Tuuli MG, et al. Letrozole Compared With Clomiphene Citrate for Unexplained InfertilityA Systematic Review and Meta-analysis. Obstetrics and Gynecology 2019;133(3):437-44. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 9 October 2021. Available at gradepro.org.
Gunn 2016
- Gunn GD, Bates GW. Evidence based approach to unexplained infertility: a systematic review. Fertility and Sterility 2016;105:1566-74. [DOI] [PubMed] [Google Scholar]
Hamilton 2015
- Hamilton AM, Cissen M, Brandes M, Smeenk JM, Bruin JP, Kremer JA, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Human Reproduction (Oxford, England) 2015;30(5):1110-21. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions 6.2 (updated February 2021. Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Inhorn 2015
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update 2015;21(4):411-26. [DOI] [PubMed] [Google Scholar]
Luo 2014
- Luo S, Li S, Jin S, Li Y, Zhang Y. Effectiveness of GnRH antagonist in the management of subfertile couples undergoing controlled ovarian stimulation and intrauterine insemination: a meta-analysis. Plos One 2014;9(10):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS 2021
- National Health Service. Infertility. https://www.nhs.uk/conditions/infertility/ (accessed 15 April 2021).
NICE Guidelines 2013
- National Institute for Clinical Excellence National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: Assessment and Treatment for People with Fertility Problems. London: Royal College of Obstetricians & Gynaecologists 2013. [PubMed]
Practice Committee of the ASRM 2020
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility and Sterility 2020;113(3):533-5. [DOI] [PubMed] [Google Scholar]
Qin 2020
- Qin F, Zhou Y, Huan L, Gui W. Comparison of clomiphene and letrozole for superovulation in patients with unexplained infertility undergoing intrauterine insemination: a systematic review and meta-analysis. Medicine 2020;99(31):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rakic 2021
- Rakic L, Kostova E, Cohlen BJ, Cantineau AEP. Double versus single intrauterine insemination (IUI) in stimulated cycles for subfertile couples. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD003854. [DOI: 10.1002/14651858.CD003854.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence; Version 3.0; December 2016. Cochrane Consumers and Communication Group. Available from cccrg.cochrane.org/author-resources (accessed prior to 26 October 2021). [DOI: 10.26181/5b57d95632a2c] [DOI]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Stadtmauer 2011b
- Stadtmauer LA, Sarhan A, Duran EH, Beydoun H, Bocca S, Pultz B. The impact of a gonadotropin-releasing hormone antagoniston gonadotropin ovulation induction cycles in women with polycystic ovary syndrome: a prospective randomized study. Fertility and Sterility 2011;95(1):216–20. [DOI] [PubMed] [Google Scholar]
Tjon‐Kon‐Fat 2015
- Tjon-Kon-Fat RI, Bensdorp AJ, Bossuyt PM, Koks C, Oosterhuis GJ, Hoek A, et al. Is IVF - served two different ways - more cost-effective than IUI with controlled ovarian hyperstimulation? Human Reproduction 2015;30(10):2331-9. [DOI] [PubMed] [Google Scholar]
Van Rumste 2008
- Van Rumste MM, Custers IM, Van der Veen F, Van Wely M, Evers JL, Mol BW. The influence of the number of follicles on pregnancy rates in intrauterine insemination with ovarian stimulation: a meta-analysis. Human Reproduction Update 2008;14(6):563-70. [DOI] [PubMed] [Google Scholar]
Vitagliano 2018
- Vitagliano A, Saccone G, Noventa M, Borini A, Coccia ME, Nardelli GB, et al. Pituitary block with gonadotrophin-releasinghormone antagonist during intrauterineinsemination cycles: a systematic review andmeta-analysis of randomised controlled trials. BJOG 2019;126:167–75. [DOI] [PubMed] [Google Scholar]
Wang 2019
- Wang R, Danhof NA, Tjon‐Kon‐Fat RI, Eijkemans MJ, Bossuyt PM, Mochtar MH, et al. Interventions for unexplained infertility: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD012692. [DOI: 10.1002/14651858.CD012692.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zolton 2020
- Zolton JR, Lindner PG, Terry N, DeCherney AH, Hill MJ. Gonadotropins versus oral ovarian stimulation agents for unexplained infertility: a systematic review and meta-analysis. Ferility and Sterility 2020;113(2):417-25. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cantineau 2005
- Cantineau AEP, Cohlen BJ, Heineman MJ. Ovarian stimulation protocols (anti‐oestrogens, gonadotrophins with and without GnRH agonists/antagonists) for intrauterine insemination (IUI) in women with subfertility. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD005356. [DOI: 10.1002/14651858.CD005356] [DOI] [PubMed] [Google Scholar]
Cantineau 2007
- Cantineau AEP, Cohlen BJ. Ovarian stimulation protocols (anti‐oestrogens, gonadotrophins with and without GnRH agonists/antagonists) for intrauterine insemination (IUI) in women with subfertility. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD005356. [DOI: 10.1002/14651858.CD005356.pub2] [DOI] [PubMed] [Google Scholar]


