Supplemental Digital Content is available in the text.
Keywords: anticoagulants, atrial appendage, atrial fibrillation, prevention & control, stroke
Background:
Percutaneous closure of the left atrial appendage (LAA) is an alternative to chronic oral anticoagulation to reduce stroke risk in patients with nonvalvular atrial fibrillation. The Amulet IDE trial (Amplatzer Amulet Left Atrial Appendage Occluder IDE Trial) was designed to evaluate the safety and effectiveness of the dual-seal mechanism of the Amulet LAA occluder compared with the Watchman device.
Methods:
Patients with nonvalvular atrial fibrillation at increased risk of stroke were randomly assigned (1:1) to undergo percutaneous implantation of a LAA occluder with the Amulet occluder or Watchman device. The primary end points included safety (composite of procedure-related complications, all-cause death, or major bleeding at 12 months), effectiveness (composite of ischemic stroke or systemic embolism at 18 months), and the rate of LAA occlusion at 45 days. Prespecified secondary end points included a composite of all stroke, systemic embolism, or cardiovascular/unexplained death at 18 months, major bleeding at 18 months, and superiority test of the 3 primary end points.
Results:
A total of 1878 patients were enrolled. The Amulet occluder was noninferior to the Watchman device for the primary safety end point (14.5% versus 14.7%; difference=–0.14 [95% CI, –3.42 to 3.13]; P<0.001 for noninferiority). Major bleeding and all-cause death were similar between groups (10.6% versus 10.0% and 3.9% versus 5.1%, respectively). Procedure-related complications were higher for the Amulet occluder (4.5% versus 2.5%), largely related to more frequent pericardial effusion and device embolization. The Amulet occluder was noninferior to the Watchman device for the primary effectiveness end point (2.8% versus 2.8%; difference=0.00 [95% CI, –1.55 to 1.55]; P<0.001 for noninferiority), and the composite of stroke, systemic embolism, or cardiovascular/unexplained death (5.6% versus 7.7%, difference=–2.12 [95% CI, –4.45 to 0.21]; P<0.001 for noninferiority). The rate of major bleeding was similar between groups (11.6% versus 12.3%; difference=–0.71 [95% CI, –3.72 to 2.31]; P=0.32 for superiority). LAA occlusion was higher for the Amulet occluder than for the Watchman device (98.9% versus 96.8%; difference=2.03 [95% CI, 0.41–3.66]; P<0.001 for noninferiority; P=0.003 for superiority).
Conclusions:
The Amulet occluder was noninferior for safety and effectiveness of stroke prevention for nonvalvular atrial fibrillation compared with the Watchman device and superior for LAA occlusion. Procedure-related complications were higher with the Amulet occluder and decreased with operator experience.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02879448.
Clinical Perspective.
What Is New?
The Amulet IDE trial (Amplatzer Amulet Left Atrial Appendage Occluder IDE Trial) is the first large-scale randomized, multicenter, controlled trial among patients at high risk of stroke or systemic embolism to assess the Amulet occluder compared with the Watchman device.
The dual-seal Amulet occluder was noninferior with respect to safety and effectiveness compared with the single-seal mechanism Watchman device, and superior with respect to left atrial appendage occlusion.
Procedure-related complications were higher for the Amulet occluder, largely related to more frequent pericardial effusion and device embolization. Procedure-related complications decreased with operator experience.
What Are the Clinical Implications?
The Amulet occluder met criteria for the primary safety (composite of procedure-related complications, all-cause death, or major bleeding at 12 months) and primary effectiveness (ischemic stroke or systemic embolism at 18 months) end points versus the Watchman device.
Device-based successful left atrial appendage occlusion was higher with Amulet than with Watchman, but procedural complications were higher when operators had limited experience.
The Amulet occluder offers safety and efficacy similar to the Watchman device with the option to discharge without the use of oral anticoagulants.
Patients with nonvalvular atrial fibrillation are at a 3- to 5-fold increased risk of ischemic stroke1 attributable to the stagnation of blood flow in the left atrial appendage (LAA) that promotes local thrombus formation. Oral anticoagulation (OAC) is effective in preventing thromboembolic events; however, its use is limited by poor adherence and need for long-term treatment, side effects including bleeding, and drug interactions.2 Percutaneous left atrial appendage occlusion (LAAO) can prevent thrombus embolization. In 2015, the Food and Drug Administration approved the single-seal mechanism Watchman device for LAA occlusion that requires 6 weeks of postprocedural anticoagulation. LAAO with a single-seal mechanism may be incomplete because of the complex and variable anatomy of the LAA. A dual-seal device with an outer disc may overcome the limitations of anatomic heterogeneity, provide an improved seal of the LAA ostium, and reduce the risk of leak.
Long-term follow-up of patients in PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) demonstrated that LAAO reduced the risk of hemorrhagic stroke, disabling/fatal stroke, and death but not ischemic stroke compared with warfarin through 5 years.3 Although there are observational studies suggesting comparable implant success, procedural outcomes, and safety events, no randomized multicenter trial comparing different devices with clinical outcome assessment has been performed. The purpose of the Amulet IDE trial was to compare the Amulet occluder with the Watchman device with respect to successful LAA occlusion, and safety and effectiveness for stroke prevention, as well.
Methods
Details about the design of the Amulet IDE trial (Amplatzer Amulet Left Atrial Appendage Occluder IDE Trial) have been published previously.4 In brief, the trial was a multicenter, open label, randomized, controlled trial evaluating the safety and effectiveness of the Amulet occluder. Details about trial organization and a list of participating centers are provided in Table S1 in the Supplemental Material. The sponsor (Abbott) selected investigators qualified by training and experience in percutaneous and transseptal procedures to participate in the trial. Although all implanters met the same minimum criteria for inclusion in the trial and all had previous experience with the Watchman device, physicians outside the United States were more experienced with the Amulet occluder. All physicians had previous experience with the Watchman device. To provide Amulet implant experience at US sites before randomization, up to 3 patients per sponsor-approved implanter could be implanted with the Amulet occluder as part of the roll-in phase. Patients were designated on the basis of physician proctor assessment as a roll-in patient before the procedure. The results in this study represent the main cohort and not the roll-in population. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols require an application that may be submitted to Abbott Structural Heart at iEnvision (www.envisionpharmagroup.com).
The protocol was approved by the institutional review board at each participating center. All patients provided written informed consent. An independent Data and Safety Monitoring Board monitored all safety data and was involved in decisions regarding trial continuation. Adverse events were adjudicated by an independent clinical events committee that was blinded to treatment assignment and device implanted. An independent core laboratory was used to analyze transesophageal echocardiography (TEE) images.
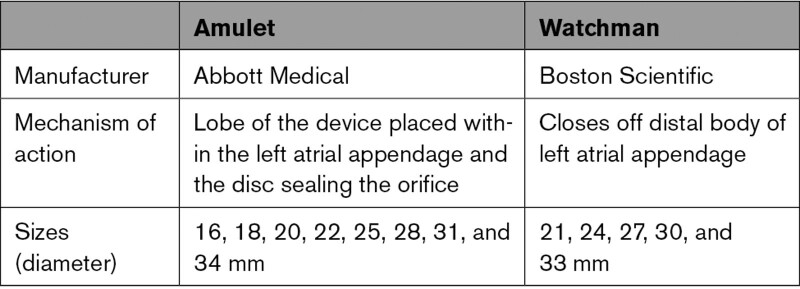
Eligible patients were ≥18 years of age with documented paroxysmal, persistent, or permanent nonvalvular atrial fibrillation and were at increased risk of stroke or systemic embolism defined as CHADS2 score ≥2 or a CHA2DS2-VASc score of ≥3.5, 6 Patients were screened with TEE to ensure suitable LAA anatomy for implanting both devices before enrollment. As required by the directions for use for the Watchman device, patients had to be suitable for anticoagulation therapy for 6 months and have appropriate rationale to seek a nonpharmacological alternative. A complete list of enrollment criteria is provided in Table S2 in the Supplemental Material. Patients were randomly assigned in a 1:1 ratio to undergo percutaneous LAAO with the Amulet occluder (Abbott) or the Watchman device (Boston Scientific) according to a computer-generated randomization scheme (Table 1; Table S3 in the Supplemental Material). Randomization was stratified by investigational site in permuted block sizes of 2, 4, and 6.
Table 1.
Comparison of Devices
Procedures
Implant procedures were guided by TEE and fluoroscopy. Patients implanted with the Amulet occluder were discharged on either aspirin plus clopidogrel or aspirin plus OAC at the discretion of the investigator, whereas patients assigned to and implanted with the Watchman device received aspirin plus warfarin per the device directions for use. When LAAO was confirmed by TEE (residual jet ≤5 mm) at the 45-day visit, cessation of OAC was required for all patients. Patients were then instructed to take aspirin and clopidogrel until the 6-month visit when clopidogrel was discontinued and aspirin continued indefinitely. Details about the trial assessments are shown in Table S4 in the Supplemental Material.
End Points
The primary safety end point was a composite of procedure-related complications, all-cause death, or major bleeding7 through 12 months. The primary effectiveness end point was a composite of ischemic stroke or systemic embolism through 18 months. The primary mechanism of action end point was successful device-based LAA occlusion (residual jet around the device ≤5 mm) assessed by an independent core laboratory on TEE at the 45-day visit. Prespecified secondary end points included (1) a composite of all stroke (ischemic or hemorrhagic), systemic embolism, or cardiovascular/unexplained death at 18 months (noninferiority); (2) major bleeding at 18 months (superiority); (3) superiority test of the primary mechanism of action end point; (4) superiority test of the primary safety end point; and (5) superiority test of the primary effectiveness end point (Tables S5 and S6 in the Supplemental Material).
Statistical Analysis
Assuming a composite event rate of 15% in both groups for the primary safety end point, the sample size required to provide 90% power to reject a noninferiority margin of 5.8% at the 2.5% significance level was 1746 patients. The prespecified primary analysis of the primary safety end point was based on the per-protocol population. In addition, sensitivity analysis was performed in the as-attempted population. Assuming a composite event rate of 4.2% in both groups for the primary effectiveness end point, the sample size required to provide 90% power to reject a noninferiority margin of 3.2% at the 2.5% significance level was 1878 patients. The prespecified primary analysis of the primary effectiveness end point was based on the intention-to-treat population. In addition, sensitivity analysis was performed in the per-protocol population. Both primary safety and effectiveness end point event rates were estimated using the Kaplan-Meier method with the Greenwood formula for the variance of the estimates. Assuming a device-based LAA occlusion rate of 96% for the Amulet occluder and 95% for the Watchman device, the sample size required to provide 90% power to reject a noninferiority margin of 3% at the 2.5% significance level was 1258 patients based on the Farrington Manning test for the difference between 2 binomial proportions. The prespecified primary analysis included patients who received the device as randomly assigned and who had a 45-day occlusion status determined by the core laboratory. Sensitivity analysis including unsuccessful implants was conducted to account for missing data.
Detailed descriptions of analysis populations are provided in Table S7 in the Supplemental Material. Patients who withdrew or were lost to follow-up without experiencing an end point event were censored on the date of withdrawal/loss to follow-up. Sensitivity analysis with multiple imputation was performed to account for missing data.
All 3 primary end points were required to be met to conclude that the trial was successful. If all 3 primary end points of noninferiority were met, the 5 secondary end points would be tested using the Hochberg procedure8 to adjust for multiple comparisons. Other than the preceding analyses, Cox regression model was used to analyze time to first event, and logistic regression was used to analyze binary outcome. A 2-sided P value of <0.05 indicates statistical significance. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute). Data collection for this analysis ended on October 26, 2020.
Role of the Funding Source
The sponsor (Abbott) designed the trial and was responsible for selecting and monitoring sites, and data management and data analysis, as well. The Steering Committee and other coauthors had full access to the data and attest to the integrity of the trial and the accuracy and completeness of the reported data.
Results
Patient Characteristics
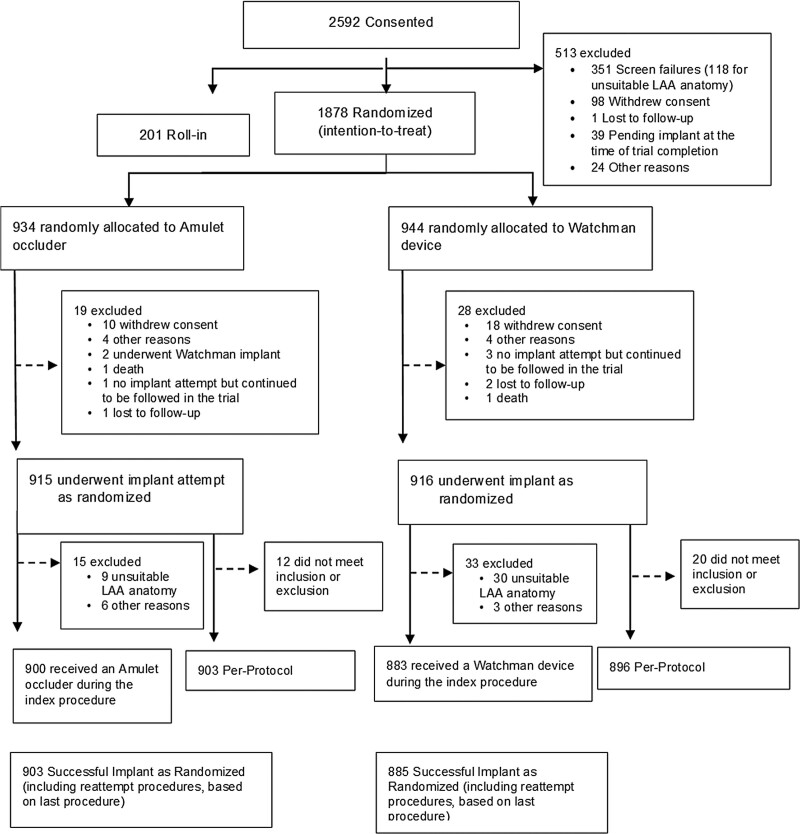
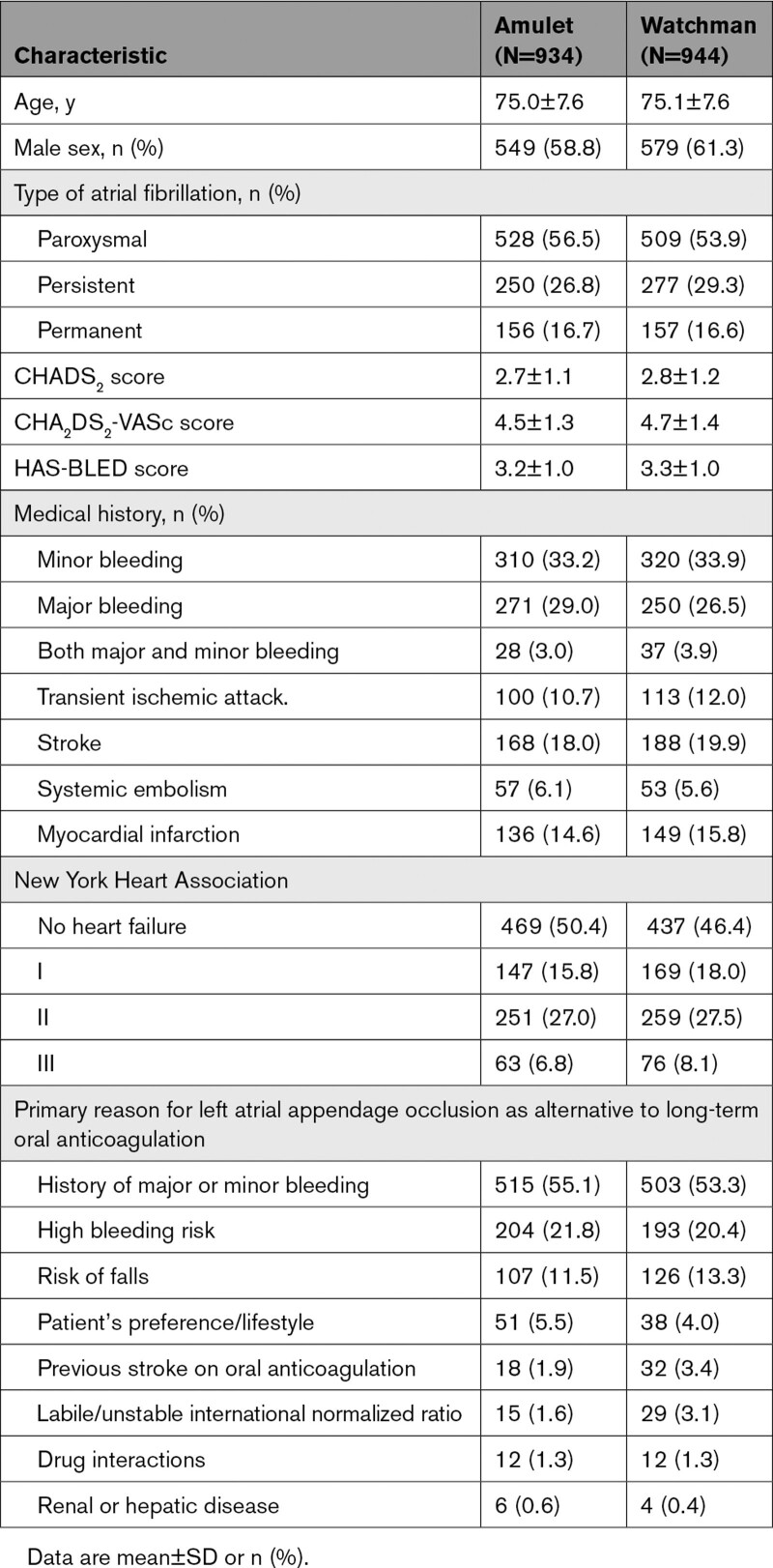
A total of 2592 patients were consented in the Amulet IDE trial (Figure 1). The most common reason for screen failure was LAA anatomy not being appropriate for one or both devices. From September 2016 through March 2019, a total of 1878 patients (1598 at 78 US centers and 280 at 30 centers outside the US) at 108 sites were randomly assigned to receive either an Amulet occluder (934) or Watchman device (944). Baseline characteristics were well-matched between groups (Table 2). The average age was 75 years, and a majority were men. Patients had a high risk for stroke and bleeding as reflected by the average CHA2DS2-VASc (4.5 and 4.7) and HAS-BLED (3.2 and 3.3). History of stroke was present in ≈20% of patients.
Figure 1.
Patient flow chart. Enrollment and disposition of patients in the Amulet IDE trial. LAA indicates left atrial appendage.
Table 2.
Demographics and Baseline Characteristics
Procedural Outcomes
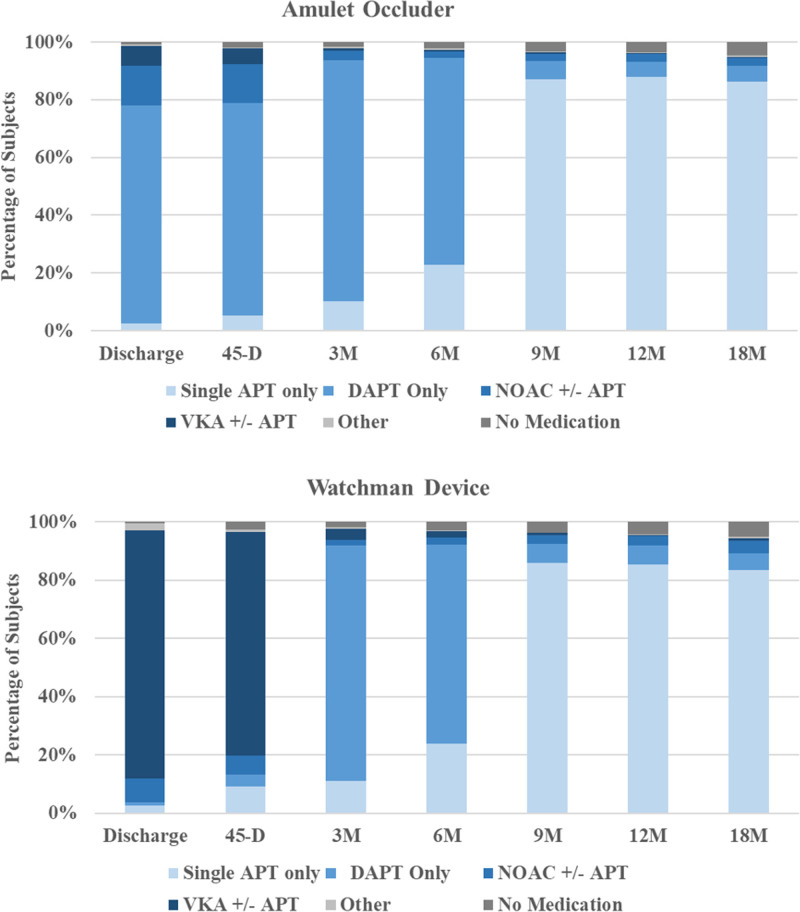
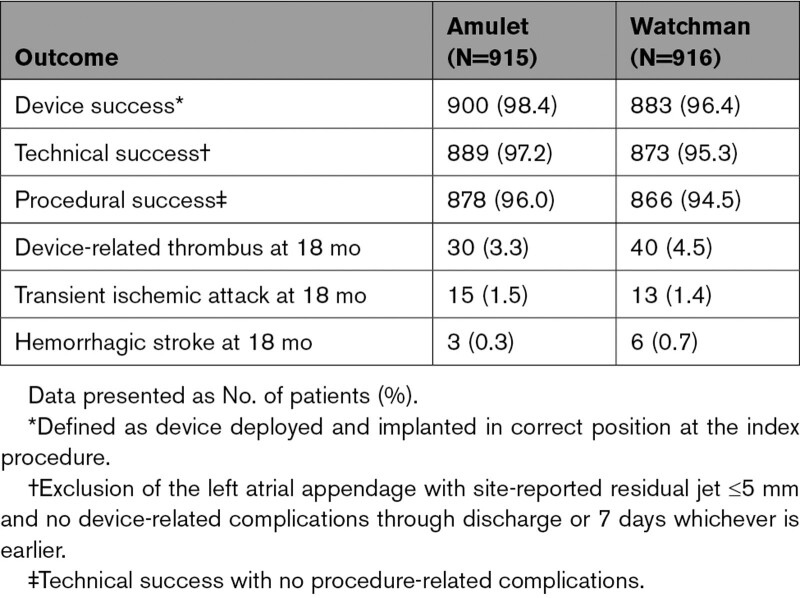
Procedural outcomes are shown in Table 3. Device implant attempt as randomly assigned occurred in 915 patients with Amulet occluders and 916 patients with Watchman devices. Device success9 as randomly assigned was similar between groups (98.4% versus 96.4%). The most common reason for an unsuccessful implant was unsuitable patient anatomy, which was less common in the Amulet group (9 versus 30; Figure 1). Technical and procedural successes were similar between groups (97.2% versus 95.3% and 96.0% versus 94.5%). At hospital discharge, 75.7% of patients with Amulet occluders were on aspirin and clopidogrel and 20.0% were on anticoagulation plus aspirin. Most (82.0%) patients with Watchman devices were discharged on warfarin plus aspirin. At the 9-month follow-up visit and beyond, most patients (≈85%) in both groups were on single-antiplatelet therapy. Details of antithrombotic medication use at each scheduled follow-up visit are provided in Figure 2.
Table 3.
Outcomes
Figure 2.
Antithrombotic medication during follow-up. The distribution of antithrombotic medical regimen on the day of discharge or on the day before each follow-up visit in the 2 groups is presented. APT indicates antiplatelet therapy; DAPT, dual antiplatelet therapy; NOAC, novel oral anticoagulant; and VKA, Vitamin K antagonist.
Primary and Secondary End Points
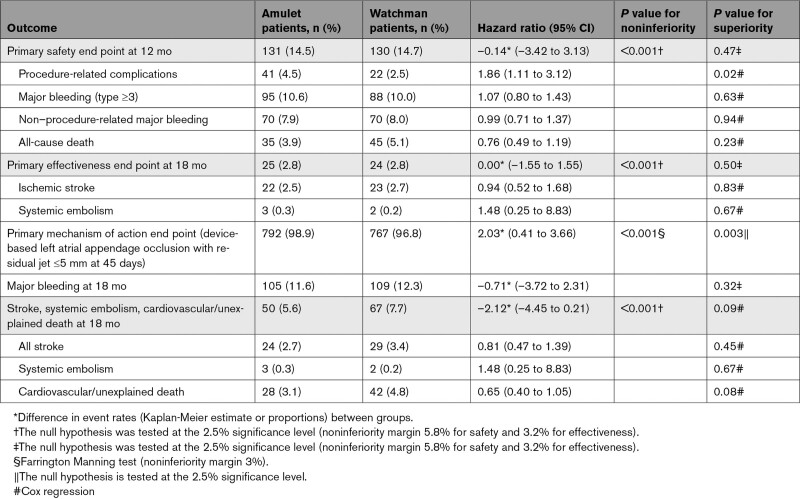
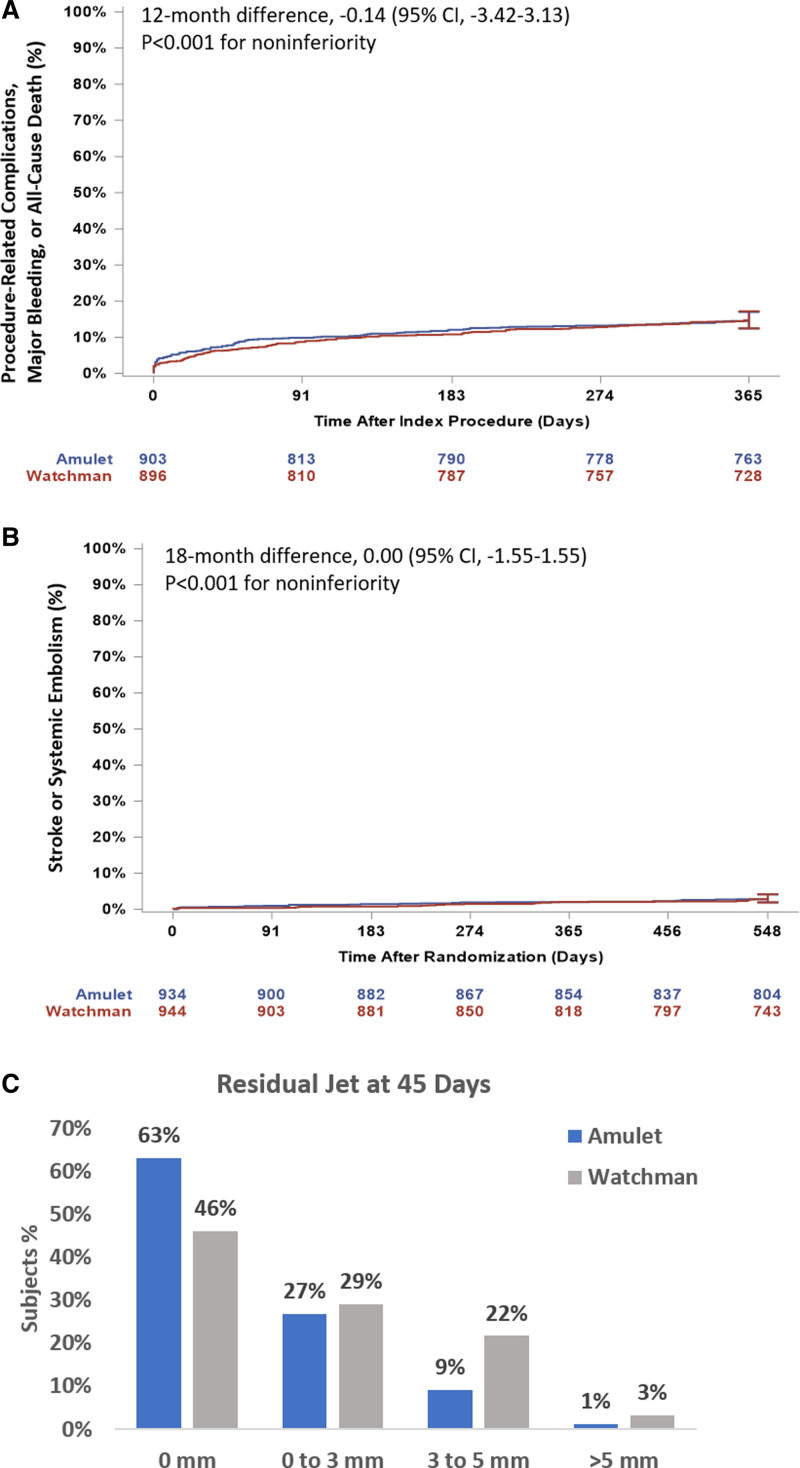
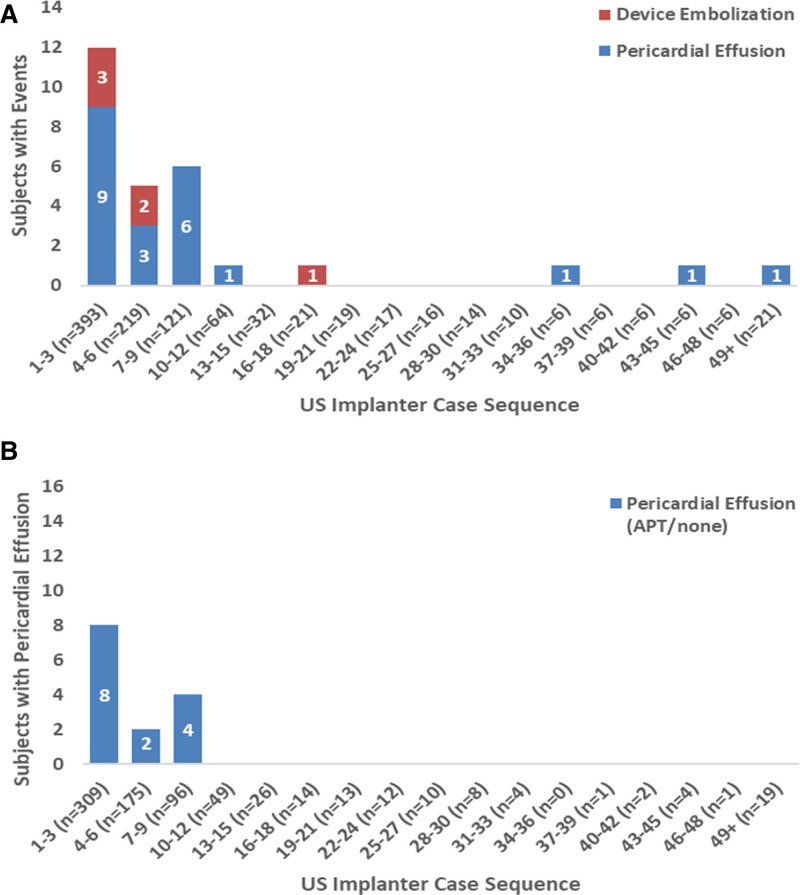
Results of the primary and secondary end points are shown in Table 3 and Table S8 in the Supplemental Material. The rate for the primary safety end point was 14.5% for the Amulet occluder and 14.7% for the Watchman device (Figure 3A; difference, –0.14 [95% CI, –3.42 to 3.13]; P<0.001 for noninferiority, P=0.47 for superiority). Similar results were observed for the as-attempted analysis (Table S8 in the Supplemental Material). Major bleeding and all-cause death were similar between groups (10.6% versus 10.0% and 3.9% versus 5.1%, respectively). Procedure-related complications were higher for the Amulet occluder (Table 3; 4.5% versus 2.5%), largely related to more frequent pericardial effusion and device embolization (Table S4 in the Supplemental Material). Patients discharged on anticoagulation therapy experienced a higher rate of late pericardial effusion than patients discharged on no antithrombotic therapy (Figure S2 in the Supplemental Material). In general, procedure-related complications including pericardial effusion and device embolization occurred early in implanter experience with the Amulet occluder (Figure 4). Although there was a higher rate of pericardial effusion with the Amulet occluder, the event resolved without sequelae in all cases and none required emergency surgery or resulted in death. The rate for the primary effectiveness end point was 2.8% for both groups (Figure 3B; difference, 0.00 [95% CI, –1.55 to 1.55]; P<0.001 for noninferiority, P=0.50 for superiority). The rates of ischemic stroke and systemic embolism were comparable between groups. Similar results were observed for the per-protocol analysis (Table S8 in the Supplemental Material). The ischemic stroke rate was 1.67%/y for the Amulet occluder and 1.94%/y for the Watchman device. Successful device-based LAA occlusion was observed in 98.9% of patients with the Amulet occluder and 96.8% of patients with the Watchman device (Figure 3C; difference, 2.03 [95% CI], 0.41–3.66]; P<0.001 for noninferiority, P=0.003 for superiority). Complete occlusion (ie, no residual jet around the device) was observed in 63.0% of patients with the Amulet occluder and 46.1% of patients with the Watchman device (Figure S1 in the Supplemental Material).
Figure 3.
Primary end point results. A, Kaplan-Meier estimated rates of the primary safety end point in the prespecified analysis population (per protocol). B, Primary effectiveness end point in the prespecified analysis populations (intention-to-treat). C, Distribution of residual jet at 45 days for the images analyzed by the echocardiography core laboratory in the prespecified analysis population (success as randomized).
Figure 4.
US implanters’ Amulet experience and procedure-related complications of pericardial effusion and device embolization. A, Number of patients with Amulet occluder with procedure-related complications of device embolization and pericardial effusion by US implanter case sequence. Cases are grouped in 3s by Amulet case experience across US implanters, including roll-in cases. B, Same type of data for pericardial effusions including only patients discharged on antiplatelet therapy (APT) or no antithrombotic medication (ie, not discharged on oral anticoagulant).
Among the secondary end points, the Amulet occluder was noninferior to the Watchman device for the composite of stroke, systemic embolism, or cardiovascular/unexplained death (5.6% versus 7.7%; difference, –2.12 [95% CI, –4.45 to 0.21]; P<0.001 for noninferiority; Table 3). The rate of major bleeding was similar between groups (11.6% versus 12.3%; P=0.32); therefore, the secondary end point for superiority of major bleeding was not met.
Sensitivity analyses showed that all primary and secondary end point results remained robust to missing data (Table S8 in the Supplemental Material). Prespecified subgroup analyses showed no evidence that the differences in results between groups were different across the different subgroup strata for any of the primary end points (Figures S3–S5 in the Supplemental Material).
Key Descriptive End Points at 18 Months
Device-related thrombosis (DRT) rates were similar between groups (3.3% versus 4.5%; Table 4). Most DRT events were identified during scheduled follow-up visits, and most subjects in both groups were on antiplatelet therapy when the DRT was first identified. In the Watchman group, 2 patients with DRT experienced an ischemic stroke or systemic embolism. In the Amulet group, no patients with DRT experienced an ischemic stroke or systemic embolism. The rates of transient ischemic attack (1.6% versus 1.4%) and hemorrhagic stroke (0.3% versus 0.7%) were similar between groups.
Table 4.
Key Descriptive End Points
Discussion
In the Amulet IDE trial, compared with the single-seal mechanism first-generation Watchman device, the dual-seal mechanism Amulet occluder was noninferior with respect to safety and effectiveness end points, and superior with respect to LAA occlusion. Sensitivity analyses of all primary end points revealed that the results were robust to missing data and similar for different analysis populations.
In patients with nonvalvular atrial fibrillation at risk for stroke, OAC is recommended as first-line therapy.10 Percutaneous LAA occlusion with the Watchman device has been shown to be noninferior to warfarin in patients with nonvalvular atrial fibrillation at moderate stroke risk,3 although direct comparison of LAA closure with the Amulet occluder to non–Vitamin K antagonists remains the subject of ongoing clinical trials. In addition, LAAO may be considered in patients with contraindications for OAC (Class IIB), those who experience major bleeding on OAC, and patients at high bleeding risk, as well. LAAO is associated with device-specific limitations, including the inability to close the appendage because of unsuitable anatomy, incomplete occlusion with leaks, and device-related complications.
The Amulet occluder was noninferior to the Watchman device for the composite safety end point of procedure-related complications, all-cause death, or major bleeding at 12 months. The rate of nonprocedural bleeding was high in both groups, pointing to a high-risk bleeding population. Procedural complications were nearly twice as high with the Amulet occluder and occurred early in an implanter’s experience driven by pericardial effusion and device embolization. In a prospective observational study using the Amulet occluder, rates of device embolization and pericardial effusion requiring surgical or percutaneous intervention were 0.2% and 1.3%, respectively.11 US sites had an existing Watchman program and no previous Amulet experience, which may have contributed to higher complication rates in US sites in the Amulet arm. Moreover, patients with the Amulet occluder who were discharged on OACs experienced a higher rate of late pericardial effusion than those discharged on antiplatelet therapy, suggesting that less intensive antithrombotic therapy may mitigate the risk of late pericardial effusions. Additional study will be required to determine whether rates of device complications diminish with increased operator experience. The Amulet occluder was noninferior with respect to the primary effectiveness end point of stroke and systemic embolism, and the composite end point of stroke, systemic embolism, and cardiovascular/unexplained death, as well, at 18 months. DRT rates were similar between groups despite the reduced rate of postprocedure anticoagulation in the Amulet group.
The Amulet occluder has a dual-seal mechanism and consists of a lobe and a disc connected by a central waist with polyester patches sewn into both the lobe and disc to facilitate effective occlusion. This design may help to overcome the limitations of a single-seal mechanism, including but not limited to short LAA length, proximal lobes near the ostium, and very large ostia. In addition, patients may be treated without the need for OAC postprocedure.
Trial Limitations
The results should be interpreted in view of the following limitations. The trial had many exclusion criteria, and a high number of patients were excluded, which may limit the generalizability of the findings. The echocardiographic core laboratory was not blinded. This trial compared the Amulet occluder with the first-generation Watchman device, not the newer generation of the Watchman FLX.12 At present, it is unknown which antithrombotic regimen provides the best balance between stroke and bleeding risk and the lowest risk for DRT. Moreover, patients continue to experience stroke despite OAC and there may be potential synergy between LAA occlusion and continuation of OAC as demonstrated in the recent LAAOS III trial (Left Atrial Appendage Occlusion Study III).13 Further studies are needed to better understand the higher occurrence of late pericardial effusion in patients with Amulet occluders who are receiving postprocedure OAC. Death as a competing risk for the primary efficacy end point was not accounted for in the primary time-to-event analyses. Last, a direct comparison between LAA occlusion and stroke prevention based on non–Vitamin K antagonists is needed.
Conclusions
Compared with the first-generation Watchman device, LAAO with a dual-seal mechanism using the Amulet occluder demonstrated noninferior safety and effectiveness, with superior LAA occlusion rates but higher device-related complications. The clinical significance of differences in LAA closure will need to be ascertained through longer-term follow-up.
Article Information
Acknowledgments
The authors thank all investigators and institutions participating in the Amulet IDE trial and Dr Zhao and D. Morishetti (Abbott) for their contributions to data analysis. Drs Lakkireddy, Thaler, O’Brien, and Windecker wrote the first draft of the manuscript. All authors had full access to all data in the trial and take responsibility for the integrity of the data and the accuracy of the data analysis. All coauthors provided substantial contributions to the acquisition of data, critically reviewed the manuscript for important intellectual content, provided final approval of the version to be published, and agree to be accountable for all aspects of the work presented.
Sources of Funding
The Amulet IDE trial was funded by Abbott.
Disclosures
Dr Lakkireddy has received research grants from Abbott, atricure, Alta Thera, Medtronic, Biosense Webster, Biotronik, Boston Scientific; speakers honoraria from Abbott, Medtronic, Biotronik and Boston Scientific; and is the principal investigator of the Amulet IDE. Dr Thaler has received consulting fees from Abbott and Occlutech, and research grants from Abbott and the National Institutes of Health (NIH). Dr Ellis has received research grants from Boehringer-Ingelheim, Medtronic, and Boston Scientific and serves as a consultant to Medtronic, Abbott, and Boston Scientific. Dr Swarup has received consulting fees from Biosense Webster, Boston Scientific, and Abbott. Dr Sondergaard has received consultant fees and research grants from Abbott, Boston Scientific, Medtronic, and SMT. Dr Carroll has received consulting fees from Abbott and research grants from Abbott, Medtronic, Edwards Lifesciences, and Philips. Dr Gold has received consultant fees and research grants from Abbott, Boston Scientific, Medtronic, and EBR and consulting fees with CVRx. Dr Hermiller serves as a consultant to Abbott, Edwards, and Medtronic. Dr Diener received honoraria from Abbott, BMS, Boehringer Ingelheim, Daiichi-Sankyo, Novo-Nordisk, Pfizer, Portola and WebMD Global; financial support for research projects was provided by Boehringer Ingelheim; and research grants from the German Research Council, German Ministry of Education and Research, European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. Dr Schmidt has been on the advisory board and proctor for Abbott and Boston Scientific and has received speaker fees from Lifetech. Dr MacDonald, Mansour, and Maini have previously received consultant fees from Abbott. Dr O’Brien is an employee of Abbott. Dr Windecker has received research and educational grants from Abbott, Amgen, Astra Zeneca, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Johnson&Johnson, Medtronic, Medicure, Novartis, Querbet, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi, Terumo, Sinomed, and V-Wave.
Supplemental Materials
Expanded Methods
Tables S1–S8
Figures S1–S5
Supplementary Material
Nonstandard Abbreviations and Acronyms
- DRT
- device-related thrombosis
- LAA
- left atrial appendage
- LAAO
- left atrial appendage occlusion
- OAC
- oral anticoagulation
- TEE
- transesophageal echocardiogram
This work was presented as an abstract at the European Society of Cardiology Congress, August 27–30, 2021.
Supplemental Material is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.057063.
For Sources of Funding and Disclosures, see page 1551.
Contributor Information
David Thaler, Email: dthaler@tuftsmedicalcenter.org.
Christopher R. Ellis, Email: christopher.ellis@vumc.org.
Vijendra Swarup, Email: VSwarup@azheartrhythm.com.
Lars Sondergaard, Email: lars.soendergaard.01@regionh.dk.
John Carroll, Email: JOHN.CARROLL@cuanschutz.edu.
Michael R. Gold, Email: goldmr@musc.edu.
James Hermiller, Email: James.Hermiller@ascension.org.
Hans-Christoph Diener, Email: h.diener@uni-essen.de.
Boris Schmidt, Email: b.schmidt@ccb.de.
Lee MacDonald, Email: leem@southdenver.com.
Moussa Mansour, Email: mmansour@mgh.harvard.edu.
Brijeshwar Maini, Email: brijmaini1@gmail.com.
Laura O’Brien, Email: Laura.OBrien@Abbott.com.
Stephan Windecker, Email: Stephan.Windecker@insel.ch.
References
- 1.Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013; 167:1807–1824. doi: 10.1016/j.ijcard.2012.12.093 [DOI] [PubMed] [Google Scholar]
- 2.Shameem R, Ansell J. Disadvantages of VKA and requirements for novel anticoagulants. Best Pract Res Clin Haematol. 2013; 26:103–14. doi: 10.1016/j.beha.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, Horton RP, Buchbinder M, Neuzil P, Gordon NT, et al. ; PREVAIL and PROTECT AF Investigators. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017; 70:2964–2975. doi: 10.1016/j.jacc.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 4.Lakkireddy D, Windecker S, Thaler D, Søndergaard L, Carroll J, Gold MR, Guo H, Brunner KJ, Hermiller JB, Diener HC, et al. Rationale and design for AMPLATZER Amulet Left Atrial Appendage Occluder IDE randomized controlled trial (Amulet IDE trial). Am Heart J. 2019; 211:45–53. doi: 10.1016/j.ahj.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864–2870. doi: 10.1001/jama.285.22.2864 [DOI] [PubMed] [Google Scholar]
- 6.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011; 123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 8.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance Biometrika. 1988; 75:800–802 [Google Scholar]
- 9.Tzikas A, Holmes DR, Jr, Gafoor S, Ruiz CE, Blomström-Lundqvist C, Diener HC, Cappato R, Kar S, Lee RJ, Byrne RA, et al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace. 2017; 19:4–15. doi: 10.1093/europace/euw141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021; 42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 11.Landmesser U, Tondo C, Camm J, Diener HC, Paul V, Schmidt B, Settergren M, Teiger E, Nielsen-Kudsk JE, Hildick-Smith D. Left atrial appendage occlusion with the AMPLATZER Amulet device: one-year follow-up from the prospective global Amulet observational registry. EuroIntervention. 2018; 14:e590–e597. doi: 10.4244/EIJ-D-18-00344 [DOI] [PubMed] [Google Scholar]
- 12.Kar S, Doshi SK, Sadhu A, Horton R, Osorio J, Ellis C, Stone J, Jr, Shah M, Dukkipati SR, Adler S, Nair DG, Kim J, et al. ; PINNACLE FLX Investigators. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX Trial. Circulation. 2021; 143:1754–1762. doi: 10.1161/CIRCULATIONAHA.120.050117 [DOI] [PubMed] [Google Scholar]
- 13.Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, Reents W, Budera P, Baddour AJ, Fila P, et al. ; LAAOS III Investigators. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021; 384:2081–2091. doi: 10.1056/NEJMoa2101897 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.