Abstract
Delayed-onset nodules (DONs) represent a poorly understood and generally neglected group of complications. It is not a diagnosis. The underlying pathologies and their incidences are largely unknown due to the lack of specificity in clinical signs and the challenges in accessing diagnostic tests, cost implications, or reluctance from patients to undergo them. A lack of presumptive clinical diagnosis, coupled with management ranging from “scatter-gun” polypharmacy to clinical inertia, is believed to result in chronicity and increased morbidity. This paper provides guidance on the identification and understanding of the underlying pathologies and encourages the increased utilization of a medical model of care. The more routine adoption of histopathology, inflammatory markers, and ultrasound will permit a more targeted management and a greater understanding of the incidences and evolution of the pathologies.
Keywords: Delayed onset nodule, delayed hypersensitivity reaction, biofilm, granuloma, ASIA, abscess, dermal filler complications, cross-linked hyaluronic acid, filler, complication, hyaluronidase, hyaluronic acid, non-surgical
Temporary soft-tissue fillers continue to grow in popularity and acceptance, but as their usage increases, so does the occurrence of complications. The occurrence of acute complications has received a great deal of attention and study, resulting in an improved understanding and management of them. Unfortunately, the progress in our understanding of delayed complications has not kept apace. The lack of readily identifiable diagnosis has frequently been met with either a “scatter-gun” polypharmacy or clinician inertia.
The term delayed-onset nodules (DONs) is a descriptive term rather than a diagnosis. It is used to describe nodules or areas of induration which typically occur at least two weeks after filler treatment. We believe that there is no practical merit in separating nodules into “delayed” and “late,” as this variably used distinction only leads to confusion. The prevalence of DONs is largely unknown, but has been reported to be as high as 0.8 percent in one series.1 Potential underlying diagnoses include product redistribution, delayed hypersensitivity reaction, biofilm, granuloma, and systemic manifestations. The variable presentation, potential overlap, or coexistence of numerous underlying pathologies makes for a clinical challenge which has no easy solution. The potential resulting chronicity has been shown to impact quality of life (QoL), often more so than psoriasis and atopic dermatitis.2
This guideline will review the causes, risk factors, diagnostic challenges, and management of DONs. The limited acceptance, validation, and access to further investigations will be considered along with their potential inclusion into the optimized management of our patients.
We aim to assist clinicians who wish to have a better understanding of the topic and offer them an initial management strategy. We also seek to assist and empower clinicians who are keen to collaborate at the highest level to ultimately improve their overall understanding of underlying pathologies, their incidences, and their clinical outcomes.
NORMAL TISSUE RESPONSE TO FILLER
Fillers are usually injected subdermally. As with most foreign materials, this evokes a variable inflammatory response, termed the “foreign body reaction.”3 This cellular response ultimately leads to beneficial neocollagenesis.
Giant cells, or frustrated macrophages, develop when the injected material particles are too large to be phagocytosed by macrophages. These giant cells, and the associated fibroblast activity, have typically disappeared by six months.4,5
Ultimately, the removal of filler is via a combination of phagocytosis and enzymatic breakdown.
POTENTIAL PATHOLOGIES RESPONSIBLE FOR A DON
Previous guidelines have focused on the distinction between inflammatory and noninflammatory nodules and areas of induration. This is overly simplistic and inaccurate, because it fails to allow for the fact that biofilm is, at least initially, a noninflammatory pathology. Figure 1 depicts the potential pathologies and their presentation with respect to inflammation.
FIGURE 1.

Potential pathologies responsible for a delayed-onset nodule and their presentation with respect to inflammation
Redistributed filler. In the first few weeks following filler implantation, nodules can develop as a result of clumping or redistribution. This can be linked to the specific rheological properties of a given filler preparation, the technique (e.g., inappropriate product, volume or depth) or the facial movements that follow (e.g., mechanical clumping). These nodules will typically be singular or small in number, and not associated with any inflammation.
Delayed Hypersensitivity Reactions (DHR). The exact mechanism underlying delayed hypersensitivity to HA is unknown. However, it is thought to be a Type 4 delayed hypersensitivity reaction, which is T-cell mediated, and can progress to a specific granulomatous reaction.4
HA is normally derived from bacterial sources and can be contaminated with bacterial endotoxin, stimulating the immunogenic reaction.6 The product range Restylane® (Galderma Laboratories LP; Fort Worth, Texas) was previously associated with a high incidence of inflammatory reactions.7 Following changes to the raw materials and the manufacturing process, the protein load was reduced six-fold and the incidence of inflammatory reactions fell from 0.15 percent to 0.06 percent.7,8
Reactions typically develop after 24 to 72 hours, but can arise weeks later and can potentially last for months.9 As such, this pathological process might be considered as a DON or an acute complication, depending on the timeline. Typically, these lesions are described as “angry red bumps” and usually occur in all areas treated with the offending filler.10,11 Small seasonal variation to the presentation of this reaction have been reported, and there is speculation that this might be linked to the incidence of viral infections and the winter vaccination schedule.12 Currently there is significant interest in COVID-19 vaccines acting as a potential trigger. It should be stressed that these reactions are nonresponsive to antihistamines.9
Biofilm. A biofilm is a cluster of bacterial cells which is associated with an implant and protected by bacteria-derived extracellular polymeric secretions (EPS).13 This “slime layer” renders the biofilm more tolerant of most antimicrobials and host defences compared to planktonic or free-floating bacterial cells. Biofilms are capable of density-dependent, cell-to-cell communication, known as quorum sensing (QS). Enabling controlled gene-expression, bacteria can coordinate their behavior and virulence, including antibiotic tolerance.14
When an HA filler implant is present, the number of organisms required to cause clinical infection is drastically reduced from 100,000 to 100 per gram of tissue. Infection can follow direct injection of skin flora into the material, or bacteria may be seeded through contiguous direct extension or hematological spread.15 Transient bacteremia, coupled with skin’s physiological immune fluctuations, is believed to impact the likelihood of infection.16 Once the implant is infected, it is impossible to sterilize it or uninfect it in any way.6 Studies suggest that the only way to eradicate a biofilm is to treat with antibiotics at the time of infection.17 Without a great deal of further information, including the incidence of filler-related biofilm, it remains inappropriate to prescribe prophylactic antibiotics.
Infective nodules are more likely to be localized, are unlikely to be symmetrical, and are more likely to be indolent in the periorbital region.18,19 Initial implant infection can occur within hours of injection, but there is usually no inflammatory response at this stage. The development of an inflammatory response might occur after an interval of weeks or months, possibly due to an inciting event, such as a further injection. When biofilm-associated bacteria do arise from their sessile state, they can manifest clinically as acute infection, abscess, granulomatous inflammation, or mimic a Type 4 allergic reaction clinically and microscopically (Figure 2).13
FIGURE 2.
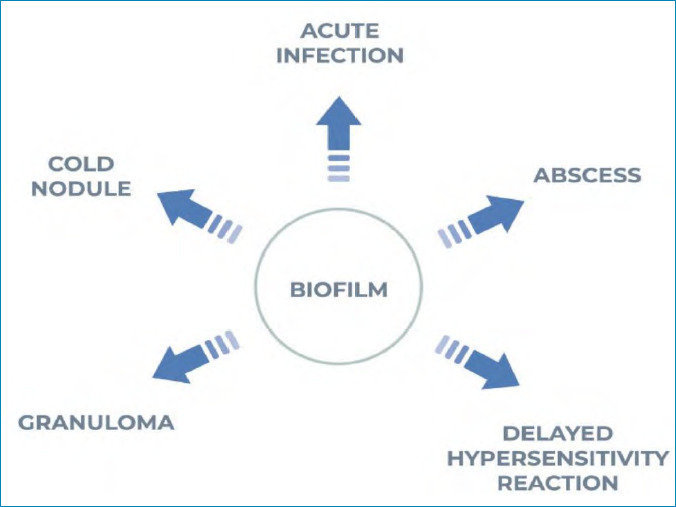
Biofilm presentation
It remains uncertain whether HA filler can sustain bacterial growth, but we recommend consideration of atypical infection as the cause of any new lesion presenting two weeks after filler injection.4,17,20 The judicious use of antibiotics, in concert with a longitudinal diagnostic process, will help avoid delayed or maltreatment of non-infectious, inflammatory, locally-destructive lesions.
Granuloma. The failure of effective phagocytosis leads to a foreign body granuloma, a foreign body surrounded by epithelioid macrophages, and giant cells of different types. A surrounding infiltrate of T lymphocytes secretes cytokines responsible for ongoing macrophage activation. Macrophages orchestrate the recruitment and activation of fibroblasts, while the excessive and prolonged macrophage response ultimately affects destructive fibrosis.4
While a delayed (Type IV) hypersensitivity reaction can lead to granuloma, the majority of granulomas are believed to be of non-allergic origin. This explains why most patients with a history of granuloma can receive further treatment without recurrence.4 Only patients who have had a delayed hypersensitivity-associated granuloma will have a recurrence or positive intradermal skin test.21
HA filler-related granulomas tend to be cystic and have an incidence estimated to be 0.02 to 0.4 percent.5 This systemic response typically presents many months after treatment and affects multiple, if not all, implantation sites.22 Developing slowly or rapidly, there are often periods of eruption and regression. The soft, non-confined nodules have varying degrees of inflammation and can have purplish pigmentation due to congested dermal capillaries.
Some authors call every solitary nodule a granuloma, but the unequivocal diagnosis of foreign body granuloma is based on histologic evidence.13 As patients are often reluctant to permit tissue sampling, it is suggested that the term “non-infectious late-onset inflammatory nodule,” or suspected granuloma, be used in the absence of histology.18
Risk factors for granuloma formation include acute and chronic infections, trauma, and medications.23 The primary biocompatibility of the filler, the volume injected, or the presence of an early strong foreign body reaction do not represent risk factors. However, the superficial placement of HA, particularly in the dermis, does have an associated risk increase.5 Even without treatment, most HA-related granulomas resolve within a year.5
Autoimmune Syndrome Induced by Adjuvants (ASIA)—Systemic manifestations. ASIA is a syndrome which encompasses a number of related, immune-mediated diseases found in susceptible individuals.24 Exposure to an adjuvant, including dermal fillers, results in immune hyperstimulation, the production of autoantibodies, and the development of autoimmune diseases, including sarcoidosis.23
RISK FACTORS FOR DONS
Product. Hyaluronic acid is a complex polysaccharide and a key component of normal, healthy skin. Due to its hygroscopic property, it captures large amounts of water, affording the skin its youthful fullness. Low molecular weight (LMW) HA fragments are generated de novo during inflammation. These fragments alert the immune system to significant tissue damage and induce pro-inflammatory, phenotypic changes in macrophages.25,26
Fillers utilizing LMW-HA might be associated with a higher incidence of DONs, with rates as high as 1 to 4.25 percent being reported.27,28 The normal enzymatic breakdown of HA filler may release LMW fragments which trigger an immune response. As noted previously, the presence of bacterial endotoxin from the raw material is at least partly responsible for triggering the immune response.25,29
Material surface chemistry of a given filler affects the response by the innate immune system, while the macrophage line retains memory of prior exposure.4 Hydrophilic materials like HA have a lower rate of cell adherence, but those that do adhere have a much higher activation level with commensurate production of cytokines.30
Hydrophilic materials are less readily phagocytosed, with cross-linking and higher concentration making them more resistant. While this resistance is associated with greater longevity, it also brings an increased granuloma risk.4
Individual particle size is critical, with those larger than five microns generally requiring macrophage aggregation (foreign body giant cells) to enable phagocytosis. Particles larger than 15 to 20 microns are encapsulated by fibrous tissue and escape phagocytosis.31
Products and their specific rheological properties must be considered in terms of suitable delivery and placement. Superficially placed, high G-prime (G’) products are more likely to be palpable or visible, while products with low cohesivity are more prone to redistribution.32 The use of excessive volumes is likely to increase the risk of nodule formation and will certainly increase the risk of product redistribution. The combination or long-term mixing of products does not appear to increase the risk of adverse reactions (AR). When they do occur, however, they tend to be more severe.33
Technique. “The aesthetic benefit the patient achieves with temporary fillers is 90 percent technique and 10 percent substance.”34
The choice between a blunt cannula and a needle is usually based on clinician preference. Cannula use might be associated with less product redistribution.35,36 However, techniques that excessively dissect the subepidermal plane are associated with an increased risk of local adverse events.37 Smaller needles reduce tissue damage and leave smaller conduits in the skin, lowering the risk of infection.38
Appropriate injection depth is dependent on the rheology of a given filler material. The dermis is the level associated with the greatest sensitivity and tendency to immunologic reactions.5
Extra care should be taken when injecting into the site of previous implants, particularly those utilizing permanent fillers.20 This “stacking” risks potential contamination of the previous filler site and is associated with an increased inflammatory reaction rate.39
We recommend the avoidance of transmucosal injections due to the common presence of organisms such as Streptococcus anginosus, which has a strong association with abscess formation.40
Skin preparation. Skin cleansing has the aim of decreasing the bacterial counts of resident flora. It is not possible to sterilize because approximately 20 percent of skin bacteria are located in deep layers or in other structures where antiseptics cannot penetrate.41 To date, there have been no data with specific, universal guidelines on the appropriate method of preparing the skin.
The following compounds might be considered as agents for skin disinfecting:
Isopropyl alcohol (IPA) is inexpensive and fast-acting, but might cause irritation, has no enduring antimicrobial activity, and is flammable.
Povidone-iodine (PI) is fast-acting but neutralized by blood and sputum.
Chlorhexidine has a highly effective and sustained antimicrobial effect, but carries the potential risks of ocular and ototoxicity.41
Hypochlorous acid (HOCl) is naturally produced during neutrophil activation in the inflammatory phase of wound healing. Non-irritating and non-cytotoxic, it demonstrates high efficacy against a range of bacterial pathogens including those identified in biofilms.43 In-vitro direct comparison with IPA, PI, and chlorhexidine found HOCl to be at least equally efficacious against common skin microorganisms.44
Timing (intercurrent illness). There might be a small seasonal variation in the incidence of nodules, most likely due to the higher incidence of viral infection that can trigger an immune reaction.12 While this is unlikely to affect the delivery of care, treatments should not be carried out in the patients presenting with intercurrent illness or local skin inflammation. It is important for non-dental clinicians to be mindful of periodontal disease, which presents an additional risk for development of a DON.45 It is generally recommended that dental treatment, including cleaning or drilling, is followed by an interval of at least four weeks before injecting dermal fillers. After filler implantation, dental treatment should be delayed by 3 to 4 weeks, if possible.9
Delayed inflammatory reactions might be triggered by vaccinations, including influenza and shingles.46,47 Recent interest has been peaked due to a possible association with the COVID-19 vaccination program. It is believed that there are elevated angiotensin converting enzyme 2 (ACE2) in filler implant sites, assisting in the maintenance of homeostasis. ACE2 is known to be targeted and blocked by the COVID-19 spike protein with a resulting net pro-inflammatory effect.
Early experience with COVID-19 infection and vaccination has prompted consideration of a delay between filler injection and vaccination. There is currently no evidence base for this, but it would seem reasonable to prescribe an interval of two weeks between a vaccination and a filler treatment. It is hoped that our understanding of this situation will evolve quickly, but it is important to be judicious in the utilization of novel agents in this area, particularly that of ACE- inhibitors.48
Patient history. A history of confirmed hypersensitivity to an HA filler is likely to be associated with a significant, and probably more severe, recurrence following repeat exposure to the same product. Presence of autoimmune disease, and even a genetic predisposition, is associated with an increased risk of an immunogenic reaction.49 The use of immunotherapy medications is likely to be associated with an increased risk of inflammatory reactions, while any immunosuppressant medication increases the risk of infection, including biofilm.4,50–53
DIAGNOSIS: THE CLINICAL HISTORY, ASSESSMENT, AND DIFFERENTIAL DIAGNOSIS
Clinical assessment. When a patient presents with a DON-type reaction, it is important to perform a comprehensive history and assessment to establish, where possible, the likely etiology. Table 1 outlines the approach to a clinical assessment.
TABLE 1.
Clinical assessment considerations
| HISTORY | |
| Medical history including allergy history | |
| Surgical and dental history, including implants | |
| Medication history | |
| History of cosmetic injectables including timeline, products used and any history of complications | |
| Current issue including its evolution and associated symptoms | |
| TIMING | Delayed hypersensitivity tends to present after days to weeks |
| Granulomas tend to present after many months | |
| Biofilms can present at any stage, and tend to be single / asymmetric | |
| EXAMINATION | Confirm presence, location and description of lesions including colour |
| Palpate to determine heat, fluctuance, induration or cystic nature | |
| Check for regional lymphadenopathy | |
| FURTHER TESTS TO CONSIDER (SEE DIAGNOSTIC TESTS SECTION) | Blood tests for inflammatory markers |
| Biopsy for histopathology | |
| HFUS—filler identification and initial assessment of nodule54,55 |
HFUS: High-frequency ultrasound scan
The four classic signs, heat, pain, erythema, and growth, should be considered in establishing the degree of inflammation. Mild inflammation presents one or two of the signs, while major inflammation features three or all of these signs. It is important to stress that these positive findings are not highly specific and the presence of major inflammation does not confirm the diagnosis of granuloma, hypersensitivity, or infection.16
MANAGEMENT OF DONS
DONS can be difficult to diagnose, treat, and manage. It is important to consider the treatment plan carefully and avoid unnecessary polypharmacy. We recommend the steps outlined in Figure 3 before starting any treatment.
FIGURE 3.

Management steps for delayed-onset nodules
While it is challenging to establish a working diagnosis without the use of ultrasound and/or histopathology of the area, Table 2 provides guidance based on the clinical presentations of the four main types of DONS etiology. This is extremely important, as it will help to assist in developing the treatment plan.
TABLE 2.
Recommended treatment steps and considerations for delayed-onset nodules
| A. NON-INFLAMMATORY LESION(S) | |
| Redistributed filler or Biofilm | |
|
STEP 1. Consider drainage or aspiration |
|
|
STEP 2. Hyaluronidase is a helpful adjunct and treatment should be to clinical resolution (may require high dose / repeated treatment) |
|
Additional considerations:
|
|
| B. SINGLE / MULTIPLE INFLAMMATORY LESIONS (PATHOLOGY UNCLEAR) | |
| Biofilm, Abscess, Acute infection, Delayed hypersensitivity reaction, Granuloma or Mixed Pathology | |
|
STEP 1. If fluctuant, must be drained (possibly repeatedly), with swab sent for extended culture4,7,9,18,39,56–59 |
|
|
STEP 2. In the presence of moderate to severe inflammation, we strongly recommend consideration of punch biopsy for histopathology and extended culture (this is more likely to yield a true positive culture if done prior to prescribing antibiotics) |
|
|
STEP 3. Broad spectrum antibiotics should be prescribed before commencing other treatments Choices include:
Macrolides have improved accumulation in subcutaneous fat and may block quorum sensing, while azithromycin and clarithromycin specifically possess polymodal immunomodulatory activity16,57 |
|
|
STEP 4. Hyaluronidase may be administered to remove the HA, whether inflammation has settled or not60 |
|
Additional considerations:
|
|
| C. SUSPECTED OR CONFIRMED GRANULOMA(S) | |
|
STEP 1. Biopsy for histopathology and extended culture should be considered to establish a firm diagnosis or diagnoses |
|
|
STEP 2. Inflammation should be first treated with antibiotics as above |
|
|
STEP 3. Subsequent HA removal with hyaluronidase will usually resolve the granuloma(s)—this may necessitate repeated treatments |
|
|
STEP 4. Intralesional fluorinated corticosteroid may be used when the lesions are resistant to repeated hyaluronidase (may need to be repeated at four-week intervals). Steroid should be injected strictly intralesional to minimize the risk of skin atrophy4 |
|
Additional considerations:
|
|
| D. SUSPECTED DELAYED HYPERSENSITIVITY REACTION | |
|
STEP 1. In these cases there may be an obvious immune trigger, causing a reaction in all-filler areas or one filler type. Consider checking inflammatory markers, including ESR and CRP pre-treatment where there is diagnostic uncertainty. |
|
|
STEP 2. *Oral steroids (10-day taper) – 60mg / 50mg / 40mg / 30mg / 20mg / 10mg / 10mg / 5mg / 5mg / 5mg |
|
Additional considerations:
|
|
Note: *Additional consideration should be given to the prescription of oral steroids during a global pandemic
CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; HFUS: High-frequency ultrasound scan
Excision. It would be foolhardy to consider that all chronic HA-related complications can be simply dissolved using hyaluronidase.64 Excision is more likely to be required in the event that a permanent filler has been used, including stacking with a temporary filler.65 Excision remains the last resort for recalcitrant nodules, typically leaving scars unless accessible via a mucosal surface.
Patient counselling. The management of DONs can be protracted and might involve several treatments. It is important to counsel the patient and consider the wider implications of different treatment modalities, including the following:
The path to recovery might be simple or complex.
Additional investigations or treatments might be required.
Complications following use of HA filler are usually relatively straightforward to resolve.
Steroid administration, in the presence of a biofilm, might affect the development of severe panniculitis.66
Consider wider implications of oral steroids in each individual, considering comorbidities and COVID-19 risk.
Other available treatment options. Upon review of the literature, there are many other medications that have been used in conjunction with the main treatment modalities to treat DONs. These medicines are associated with low grades of evidence base supporting their use. Table 3 lists these medications, rationale for use, their evidence base, and limitations.
TABLE 3.
Prescription medicines with limited efficacy and evidence-base
| TREATMENT | INDICATION | RATIONALE | EVIDENCE BASE | LIMITATIONS |
|---|---|---|---|---|
| Allopurinol |
|
|
|
|
| Antihistamine |
|
|
|
|
| 5-fluorouracil |
|
|
|
|
| Hydroxychloroquine |
|
|
|
|
| Imiquimod |
|
|
|
|
| Isotretinoin |
|
|
|
|
| Tacrolimus / Pimecrolimus |
|
|
|
Note: n=number of subjects; significant different if p<0.05
ROLE OF DIAGNOSTIC TESTS
We strongly advocate early consideration of investigations, including checking of inflammatory markers and scanning with high-frequency ultrasound scan (HFUS) in order to:
Have a more targeted treatment strategy
Estimate the underlying prevalence of different pathologies
Better understand the value of simple tests
The merits of blood sampling. Simple blood testing can assist in the diagnostic process. There is merit in checking inflammatory markers, which can help exclude infection. Following treatment of infection with antibiotics and hyaluronidase, an elevated erythrocyte sedimentation rate (ESR) normalizes and the inflammatory signs settle. Patients with a Type IV immunological reaction have a normal ESR.71
Elevated lactate dehydrogenase (LDH) levels may indicate lymphocyte and/or macrophage activation. High angiotensin converting enzyme (ACE) levels may be secondary to macrophagic and granulomatous immune response, in the same way as with other granulomatous immune-mediated disease like sarcoidosis or sarcoidosis-related disorders.68
The role for tissue sampling. It can be argued that the role of histologic assessment in DONs is crucial.72 Some authors suggest that the relevant filler must be identified before any intervention is considered.7 Unfortunately, the majority of patients refuse tissue sampling, rendering the pathway to a solution more arduous.
Sampling typically involves taking a swab or a biopsy. However, a swab can only collect bacteria found on the surface and not those embedded in the wound bed. A biofilm sample typically requires treatment with ultrasound (i.e., sonication) to release bacteria, while the use of antibiotics prior to tissue sampling might render bacteria unculturable. Standard culture is considered inadequate when suspecting biofilm due to the potential for atypical organisms, including mycobacteria.39
To avoid false negative findings, peptide nucleic acid (PNA) fluorescence in-situ hybridization (FISH) might be considered. This analysis provided the first direct visualizations of bacteria and their locations within tissues after filler injections. Polymerase chain reaction (PCR) is also routinely used when bacteria are slow-growing or difficult to culture.72,73 However, PCR does not discriminate between living and dead cells.14
Histologic evaluation can inform the type of filler material used and prove the association between a particular filler agent and the skin reaction.74 It may also offer key information where multiple diagnoses, like DTH, infection, or foreign body granuloma coexist.21
OTHER INVESTIGATIONS AND THEIR MERITS
Ultrasonography represents a non-invasive modality capable of reliable detection and identification of most common types of cosmetic fillers.74,75,76 It can be useful where histopathological assessment fails to elicit specific findings.77
Specialized investigations (complex, unresponsive situations only). Table 4 details other more specialized investigations that might be used in refractory and complex situations, listing benefits and limitations.
TABLE 4.
Specialized investigations for complex or refractory cases
| INVESTIGATION | BENEFITS | LIMITATIONS/ DISADVANTAGES |
|---|---|---|
| MAGNETIC RESONANCE IMAGING |
|
|
| SCINTIGRAPHY |
|
|
| CT / SPECT |
|
|
| INFRARED SPECTROSCOPY |
|
|
CONCLUSION
Little consideration has been given to the impact of long-term complications on quality of life (QoL). Thankfully, the majority of complications will be a cosmetic nuisance, particularly where only HA filler was used.
Düker et al2 used the already validated and accepted, Dermatology Life Quality Index (DLQI) to measure the impact of delayed adverse filler reactions on QoL. Including a wide range of fillers, the impact on QoL caused by adverse filler reactions was high even compared to chronic inflammatory diseases, such as psoriasis and atopic dermatitis. As clinicians, we are charged with marshalling our patients through difficult times. This requires the recognition and support for those most challenged by their complication. We must also keep our eye on the evolution of our practice, including determination of the nature and prevalence of the underlying pathologies. This mandates the need for greater use of a medical model and associated greater use of investigations. If we fail to utilize more testing and invasive techniques, DONs will continue to be the poor relative in comparison with acute complications.
REFERENCES
- Leonhardt JM, Lawrence N, Narins RS. Angioedema acute hypersensitivity reaction to injectable hyaluronic acid. Dermatol Surg. 2005;31(5):577–579. doi: 10.1111/j.1524-4725.2005.31166. [DOI] [PubMed] [Google Scholar]
- Düker D, Erdmann R, Hartmann V et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS) – study. J Eur Acad Dermatol Venereol. 2016;30(6):1013–1020. doi: 10.1111/jdv.13594. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A, Chang DT. Foreign Body reaction to Biomaterials. Semin Immunol. 2008 Apr;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alijotas-Reig J, Fernández-Figueras MT, Puig L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43(2):241–258. doi: 10.1016/j.semarthrit.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Lemperle G, Gauthier-Hazan N, Wolters M et al. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(6):1842–1863. doi: 10.1097/PRS.0b013e31818236d7. [DOI] [PubMed] [Google Scholar]
- Baeva LF, Das SS, Hitchins VM. Bacterial endotoxin detection in hyaluronic acid-based medical devices. J Biomed Mater Res B Appl Biomater. 2017;105(5):1210–1215. doi: 10.1002/jbm.b.33659. [DOI] [PubMed] [Google Scholar]
- Rzany B. Understanding, avoiding, and managing severe filler complications. Plast Reconstr Surgy. 2015;136(5 Suppl):196S–203S. doi: 10.1097/PRS.0000000000001760. [DOI] [PubMed] [Google Scholar]
- Van Dyke S, Hays GP, Caglia AE et al. Severe acute local reactions to hyaluronic acid derived dermal fillers. J Clin Aesthet Dermatol. 2010;3(5):32–35. [PMC free article] [PubMed] [Google Scholar]
- Urdiales-Gálvez F, Delgado NE, Figueiredo V et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510. doi: 10.1007/s00266-017-1063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beleznay K, Carruthers JD, Carruthers A et al. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41(8):929–939. doi: 10.1097/DSS.0000000000000418. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013;(136):1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- Ibrahim O, Overman J, Arndt KA et al. Filler nodules: inflammatory or infectious? A review of biofilms and their implications on clinical practice. Dermatol Surg. 2018;44(1):53–60. doi: 10.1097/DSS.0000000000001202. [DOI] [PubMed] [Google Scholar]
- Cassuto D, Sundaram H. A problem-oriented approach to nodular complications from hyaluronic acid and calcium hydroxylapatite fillers: classification and recommendations for treatment. Plast Reconstr Surg. 2013;132(4 Suppl 2):48S–58S. doi: 10.1097/PRS.0b013e31829e52a7. [DOI] [PubMed] [Google Scholar]
- Graivier MH, Bass LM, Lorenc ZP et al. Differentiating nonpermanent injectable fillers: prevention and treatment of filler complications. Aesthet Surg J. 2018;6;38(suppl 1):S29–S40. doi: 10.1093/asj/sjy032. [DOI] [PubMed] [Google Scholar]
- Hwang CJ. Periorbital injectables: understanding and avoiding complications. J Cutan Aesthet Surg. 2016;9(2):73–79. doi: 10.4103/0974-2077.184049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33(4):561–575. doi: 10.1177/1090820X13484492. [DOI] [PubMed] [Google Scholar]
- Alhede M, Er Ö, Eickhardt S et al. Bacterial biofilm formation and treatment in soft tissue fillers. Pathog Dis. 2014;70(3):339–346. doi: 10.1111/2049-632X.12139. [DOI] [PubMed] [Google Scholar]
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi: 10.2147/CCID.S50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KL, Convery C, Ejikeme I et al. A systematic review of the literature of delayed inflammatory reactions after hyaluronic acid filler injection to estimate the incidence of delayed type hypersensitivity reaction. Aesthet Surg J. 2020;40(5):NP286–NP300. doi: 10.1093/asj/sjz222. [DOI] [PubMed] [Google Scholar]
- Demir E, Perez-Bouza A, Pallua N. Adverse late reactions after cosmetic implantation of hydroxyethylmethacrylate particles suspended in hyaluronic acid: clinics and complication management. Aesthetic Plast Surg. 2013;37(3):576–586. doi: 10.1007/s00266-013-0107-3. [DOI] [PubMed] [Google Scholar]
- López-Pestañaa A, Tuneua A et al. Sarcoid granulomas in facial cosmetic filler material: induction by interferon-α and aibavirin in a patient with Hepatitis C. Actas Dermosifiliogr. 2011;102(9):746–747. doi: 10.1016/j.ad.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Agmon-Levin N. “ASIA”—Autoimmune/autoinflammatory Syndrome Induced by Adjuvants. J Autoimmun. 2011;36(1):4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Filion MC, Phillips NC. Pro-inflammatory activity of contaminating DNA in hyaluronic acid preparations. J Pharm Pharmacol. 2001;53(4):555–561. doi: 10.1211/0022357011775677. [DOI] [PubMed] [Google Scholar]
- Rayahin JE, Buhrman JS, Zhang Y et al. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;13;1(7):481–493. doi: 10.1021/acsbiomaterials.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghpour M, Quatrano NA, Bonati LM et al. Delayed-onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45(8):1085–1094. doi: 10.1097/DSS.0000000000001814. [DOI] [PubMed] [Google Scholar]
- Artzi O, Loizides C, Verner I et al. Resistant and recurrent late reaction to hyaluronic acid-based gel. Dermatol Surg Dermatol Surg. 2016;42(1):31–37. doi: 10.1097/DSS.0000000000000562. [DOI] [PubMed] [Google Scholar]
- Hee CK, Messina D. Role of bacteria on the in vitro immune response to hyaluronic acid fillers. J Am Acad Dermatol. 2018;79(3 Suppl 1):AB248. [Google Scholar]
- Brodbeck WG, Anderson JM. Giant cell formation and function. Curr Opin Hematol. 2009;16(1):53–57. doi: 10.1097/MOH.0b013e32831ac52e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27(5):354–366. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]
- Sánchez O, Rodríguez-Sureda V, Domínguez C et al. Study of biomaterial-induced macrophage activation, cell-mediated immune response and molecular oxidative damage in patients with dermal bioimplants. Immunobiology. 2012;217(1):44–53. doi: 10.1016/j.imbio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Carruthers J. Review of long-lasting dermal fillers. Medical Insight, Inc. 2006:6–23. [Google Scholar]
- Choi MS. Basic rheology of dermal filler. Arch Plast Surg. 2020 Jul;47(4):301–304. doi: 10.5999/aps.2020.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loghem JAJ, Humzah D, Kerscher M. Cannula versus sharp needle for placement of soft tissue fillers: an observational cadaver study. Aesthet Surg J. 2017;38(1):73–88. doi: 10.1093/asj/sjw220. [DOI] [PubMed] [Google Scholar]
- Pavicic T. Calcium hydroxylapatite filler: an overview of safety and tolerability. J Drugs Dermatol. 2013;12(9):996–1002. [PubMed] [Google Scholar]
- Glogau RG, Kane MA. Effect of injection techniques on the rate of local adverse events in patients implanted with nonanimal hyaluronic acid gel dermal fillers. Dermatol Surg. 2008;34(suppl 1):S105–S109. doi: 10.1111/j.1524-4725.2008.34251.x. [DOI] [PubMed] [Google Scholar]
- Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31(1):110–121. doi: 10.1177/1090820X10391083. [DOI] [PubMed] [Google Scholar]
- Wagner RD, Fakhro A, Cox JA et al. Etiology, prevention, and management of infectious complications of dermal fillers. Semin Plast Surg. 2016;30(2):83–86. doi: 10.1055/s-0036-1580734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers JK, Mistry BD. Soft-tissue infection caused by Streptococcus anginosus after intramucosal hyaluronidase injection: a rare complication related to dermal filler injection. Dermatol Surg. 2018;44(Suppl 1):S51–S53. doi: 10.1097/DSS.0000000000001625. [DOI] [PubMed] [Google Scholar]
- Calfee DP, Farr BM. Comparison of four antiseptic preparations for skin in the prevention of contamination of percutaneously drawn blood cultures: a randomized trial. J Clin Microbiol. 2002;40(5):1660–1665. doi: 10.1128/JCM.40.5.1660-1665.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MH, Andriessen A, Bhatia AC et al. Topical stabilized hypochlorous acid: The future gold standard for wound care and scar management in dermatologic and plastic surgery procedures. J Cosmet Dermatol. 2020;19(2):270–277. doi: 10.1111/jocd.13280. [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ, Bhatia N. Status report on topical hypochlorous acid: clinical relevance of specific formulations, potential modes of action, and study outcomes. J Clin Aesthet Dermatol. 2018;11(11):36–39. [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos AG, Rong A, Miller D et al. 0.01% Hypochlorous acid as an alternative skin antiseptic: an in vitro comparison. Dermatol Surg. 2018;44(12):1489–1493. doi: 10.1097/DSS.0000000000001594. [DOI] [PubMed] [Google Scholar]
- Marusza W, Mlynarczyk G, Olszanski R et al. Probable biofilm formation in the cheek as a complication of soft tissue filler resulting from improper endodontic treatment of tooth 16. Int J Nanomedicine. 2012;7:1441–1447. doi: 10.2147/IJN.S27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojani-Lynch T. Late-onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open. 2017;5(12):e1532. doi: 10.1097/GOX.0000000000001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munavalli GG, Guthridge R, Knutsen-Larson S et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2021;9:1–15. doi: 10.1007/s00403-021-02190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munavalli GG, Knutsen-Larson S, Lupo MP et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63–68. doi: 10.1016/j.jdcr.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decates TS, Velthuis PJ, Schelke LW et al. Increased risk of late-onset, immune-mediated, adverse reactions related to dermal fillers in patients bearing HLA-B*08 and DRB1*03 haplotypes. Dermatol Ther. 2021;34(1):e14644. doi: 10.1111/dth.14644. [DOI] [PubMed] [Google Scholar]
- Bisschop C, Bruijn MS, Stenekes MW et al. Foreign body reaction triggered by cytotoxic T lymphocyte-associated protein 4 blockade 25 years after dermal filler injection. Br J Dermatol. 2016;175(6):1351–1353. doi: 10.1111/bjd.14674. [DOI] [PubMed] [Google Scholar]
- Fischer J, Metzler G, Schaller M. Cosmetic permanent fillers for soft tissue augmentation: a new contraindication for interferon therapies. Arch Dermatol. 2007;143(4):507–510. doi: 10.1001/archderm.143.4.507. [DOI] [PubMed] [Google Scholar]
- Descamps V, Landry J, Frances C et al. Facial cosmetic filler injections as possible target for systemic sarcoidosis in patients treated with interferon for chronic hepatitis C: two cases. Dermatology. 2008;217(1):81–84. doi: 10.1159/000128281. [DOI] [PubMed] [Google Scholar]
- Pathmanathan S, Dzienis M. Cetuximab associated dermal filler reaction. BMJ Case Rep. 2019;12(8):e228882. doi: 10.1136/bcr-2018-228882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto Di Santolo M, Massimo C, Tortora G et al. Clinical value of high-resolution (5–17 MHz) echo-color Doppler (ECD) or identifying filling materials and assessment of damage or complications in aesthetic medicine/surgery. Radiol Med. 2019;124(6):568–574. doi: 10.1007/s11547-018-0969-1. [DOI] [PubMed] [Google Scholar]
- Grippaudo FR, Di Girolamo M, Mattei M et al. Diagnosis and management of dermal filler complications in the perioral region. J Cosmet Laser Ther. 2014;16(5):246–252. doi: 10.3109/14764172.2014.946048. [DOI] [PubMed] [Google Scholar]
- Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008;34(Suppl 1):S92–S99. doi: 10.1111/j.1524-4725.2008.34249.x. [DOI] [PubMed] [Google Scholar]
- Rohrich RJ, Monheit G, Nguyen AT et al. Soft-tissue filler complications: the important role of biofilms. Plast Reconstr Surg. 2010;125(4):1250–1256. doi: 10.1097/PRS.0b013e3181cb4620. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg. 2009;35(Suppl 2):1076–1080. doi: 10.1111/j.1524-4725.2009.01346.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Ahn DK, Jeong HS et al. Treatment algorithm of complications after filler injection: based on wound healing process. J Korean Med Sci. 2014;29(Suppl 3):S176–182. doi: 10.3346/jkms.2014.29.S3.S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrich RJ, Bartlett EL, Dayan E. Practical approach and safety of hyaluronic acid fillers. Plast Reconstr Surg Glob Open. 2019;7(6):e2172. doi: 10.1097/GOX.0000000000002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narins RS, Coleman WP, Glogau RG. Recommendations and treatment options for nodules and other filler complications. Dermatol Surg. 2009;35(Suppl 2):1667–1671. doi: 10.1111/j.1524-4725.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- Artzi O, Cohen JL, Dover JS et al. Delayed inflammatory reactions to hyaluronic acid fillers: a literature review and proposed treatment algorithm. Clin Mosmet Investig Dermatol. 2020;13:371–378. doi: 10.2147/CCID.S247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemperle G, Duffy DM. Treatment options for dermal filler complications. Aesthet Surg J. 2006;26(3):356–364. doi: 10.1016/j.asj.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Homsy A, Rüegg EM, Jandus P et al. Immunological reaction after facial hyaluronic acid injection. Case Reports Plast Surg Hand Surg. 2017;4(1):68–72. doi: 10.1080/23320885.2017.1356202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TH, Seo SW, Kim JK et al. Clinical experience with hyaluronic acid-filler complications. J Plast Reconstr Aesthet Surg. 2011;64(7):892–896. doi: 10.1016/j.bjps.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Tolker-Nielsen T, Givskov M et al. Detection of bacteria by fluorescence in situ hybridization in culture-negative soft tissue filler lesions. Dermatol Surg. 2009;35(Suppl 2):1620–1624. doi: 10.1111/j.1524-4725.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- Reisberger EM, Landthaler M, Wiest L et al. Foreign body granulomas caused by polymethylmethacrylate microspheres successful treatment with allopurinol. Arch Dermatol. 2003;139(1):17–20. doi: 10.1001/archderm.139.1.17. [DOI] [PubMed] [Google Scholar]
- Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008;22(2):150–161. doi: 10.1111/j.1468-3083.2007.02354.x. [DOI] [PubMed] [Google Scholar]
- Jansen T, Kossmann E, Plewig G. [Siliconoma. An interdisciplinary problem]. Hautarzt. 1993;44(10):636–643. [PubMed] [Google Scholar]
- Alam M, Gladstone H, Kramer EM et al. ASDS guidelines of care: injectable fillers. American Society for Dermatologic Surgery. Dermatol Surg. 2008;34(Suppl 1):S115–148. doi: 10.1111/j.1524-4725.2008.34253.x. [DOI] [PubMed] [Google Scholar]
- Netsvyetayeva I, Marusza W, Olszanski R et al. Skin bacterial flora as a potential risk factor predisposing to late bacterial infection after cross-linked hyaluronic acid gel augmentation. Infect Drug Resist. 2018;11:213–222. doi: 10.2147/IDR.S154328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khalawany M, Fawzy S, Saied A et al. Dermal filler complications: a clinicopathologic study with a spectrum of histologic reaction patterns. Ann Diagn Pathol. 2015;19(1):10–15. doi: 10.1016/j.anndiagpath.2014.11.004. [DOI] [PubMed] [Google Scholar]
- De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;15;8:205–214. doi: 10.2147/CCID.S80446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarressi A, Nizet C, Lombardi T. Granulomas and nongranulomatous nodules after filler injection: different complications require different treatments. J Plast Reconstr Aesthet Surg. 2020;73(11):2010–2015. doi: 10.1016/j.bjps.2020.08.012. [DOI] [PubMed] [Google Scholar]
- Wortsman X, Wortsman J, Orlandi C et al. Ultrasound detection and identification of cosmetic fillers in the skin. J Eur Acad Dermatol Venereol. 2012;26(3):292–301. doi: 10.1111/j.1468-3083.2011.04047.x. [DOI] [PubMed] [Google Scholar]
- Wortsman X. Identification and complications of cosmetic fillers: sonography first. J Ultrasound Med. 2015;34(7):1163–1172. doi: 10.7863/ultra.34.7.1163. [DOI] [PubMed] [Google Scholar]
- Menis D, Castellanos-Gonzalez M, Llamas-Martin R et al. The utility of skin ultrasound for the diagnosis of complications of tissue filler materials. Actas Dermosifiliogr. 2014;105(8):797–798. doi: 10.1016/j.ad.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Grippaudo FR, Pacilio M, Di Girolamo M et al. Radiolabelled white blood cell scintigraphy in the work-up of dermal filler complications. Eur J Nucl Med Mol Imaging. 2013;40(3):418–425. doi: 10.1007/s00259-012-2305-7. [DOI] [PubMed] [Google Scholar]
- Persichetti P, Palazzolo D, Tenna S et al. Dermal filler complications from unknown biomaterials: identification by attenuated total reflectance spectroscopy. Plast Reconstr Surg. 2013;131(4):597e–603e. doi: 10.1097/PRS.0b013e3182827741. [DOI] [PubMed] [Google Scholar]


