Abstract
ClinicalTrials.gov identifier
NCT04105504.
Background
For Asians, especially women with darker skin tones (Fitzpatrick Skin Types IV and V), clear, bright skin is considered highly desirable, and various topical, oral, or injection-based cosmetic skin-lightening agents with different mechanisms of action are widely available across Asia.
Objective
We sought to investigate the efficacy and safety of an oral glutathione supplement comprising L-glutathione (fermentation), ascorbic acid, alpha-lipoic acid, and zinc (as zinc aspartate) as a skin-lightening agent.
Methods
This randomized, double-blind, controlled clinical trial was carried out at three teaching hospital-based dermatovenereology clinics in Indonesia. Participants were randomized to receive either the glutathione supplement or placebo capsules and were evaluated every four weeks over a 12-week study period. Total reduction in spot ultraviolet, spot polarization, and skin tone were measured and recorded using a Janus Facial Analysis System® (PIE Co., Ltd, Suwon-si, Gyeonggi-do, Korea).
Results
Eighty-three participants, aged between 33 and 50 years, completed the study. Reductions in spot ultraviolet in certain subgroups, spot polarization, and skin tone were greater in the glutathione supplement group than in the placebo group, but the difference was not statistically significant. Both the glutathione supplement and placebo groups experienced only mild side effects in the first four weeks.
Conclusion
The oral glutathione supplement was slightly beneficial for skin lightening in particular subgroups, but the results were not statistically significant. Mild and temporary side effects were reported. Further research is required to more fully evaluate the efficacy of this glutathione supplement as a skin-lightening agent.
Keywords: Glutathione plus, skin-lightening agent, spot ultraviolet, spot polarization, skin tone
For Asians, especially women with darker skin tones (Fitzpatrick Types IV and V), clear, bright skin is considered highly desirable. Various topical, oral, and injection-based cosmetic skin-lightening agents with different mechanisms of action are used widely across Asia, including hydroquinone, azelaic acid, kojic acid, mulberry, alpha-arbutin, beta-arbutin, glutathione, licorice root, papaya, vitamin A (e.g., retinol), vitamin B (i.e., niacinamide), and vitamin C.1,2
One of the widely used systemic agents is glutathione, which is a thiol compound and a regulator of the melanogenic pathway in humans.3 Glutathione is an antioxidant found in the human body, contributing to drug and xenobiotic detoxification.3,4 It is known to be antimelanogenic, thereby associated with melanin production.5,6 It also inhibits tyrosinase activity and melanosome transfer from melanocytes to keratinocytes.4–8 Overall, it is suggested that glutathione might promote pheomelanin synthesis, inhibit intracellular melanogenic enzymes, and exhibit antioxidant and anti-aging effects.3
As demonstrated in in-vitro experiments, glutathione is associated with melanogenesis. Its various mechanisms, including its antioxidant effects, the stimulation of pheomelanin synthesis instead of eumelanin, and interference with intracellular trafficking of melanogenic enzymes, contribute to its antimelanogenic properties.3 Hence, through the histopathological examination of melanocyte activity, location of melanin pigment deposition, and response after therapy, we can determine the effects of glutathione on skin color.6,9 However, Arjinpathana and Asawanonda1 reported many contradictory results regarding the efficacy and safety of glutathione as a skin-lightening agent. Therefore, glutathione is often combined with other compounds, such as ascorbic acid (vitamin C), α-lipoic acid (ALA), and zinc salts, in a cosmetic or skincare product to maximize the result. Similar to glutathione, vitamin C and ALA are also potent antioxidants that have numerous attractive features for use in cosmetic and dermatological products, while zinc salts are widely used in cosmetic formulations and are often included as active or supportive ingredients in a wide range of formulations.10–12
In the present study, we aimed to evaluate the efficacy and safety of an oral glutathione supplement (Lynae® Mazthione; Mazta Farma, Indonesia)—comprising L-glutathione (fermentation), ascorbic acid, ALA, and zinc (as zinc aspartate)—as a skin-lightening agent in healthy Indonesian women, measured using a facial analysis system for assessment every four weeks over a 12-week period.
METHODS
We conducted a multicenter, randomized, double-blind, controlled clinical trial at three dermatovenereology clinics in Indonesia—namely, Presidential-Army Central Hospital Gatot Soebroto in Jakarta, Dr. Wahidin Sudirohusodo Hospital in Makassar, and the Universitas Sumatera Utara Teaching Hospital in Medan. The sample size was calculated using a two-proportion formula.
Participants. Ninety healthy individuals were enrolled in this study. Included participants were aged between 30 and 65 years with Fitzpatrick IV and V Skin Types and were working a maximum of eight hours per day in an office environment. All participants signed an informed consent form. Exclusion criteria included personal or familial history of skin cancer, especially melanoma; use of supplements containing glutathione and/or other antioxidants within one month prior to the study; usage of other skin-lightening agents within one month prior to the study; other dermatoses; pregnancy or breastfeeding; and allergic or other skin reactions to oral therapies.
Procedures. Block randomization was carried out on participants receiving glutathione supplement or placebo capsules by a statistician who was not otherwise involved in this study. Blinding methods were followed for the participants, dermatologist/physicians, and phototechnicians. To ensure blinding, the containers were labeled A and B by a single, blinded pharmacist, who disclosed the codes only at the end of the trial. Glutathione supplement capsules contained 500mg of L-glutathione (fermentation) together with 250mg of ascorbic acid, 50mg of ALA, and 4mg (20mg) of zinc (as zinc aspartate) as additional ingredients, whereas the placebo capsules contained glucose powder in a gelatin coating. Each participant received 30 identically shaped capsules on Weeks 0, 4, 8, and 12. Participants were given topical sunscreen to apply 15 to 30 minutes before sunlight exposure and were asked to keep a drug diary tracking the time of administration and any side effects. All subjects brought their diary to every visit.
Outcome measurements. Participants were evaluated subjectively and objectively on the first, fourth, eighth, and 12th visits. Before evaluation, participants washed their faces using face cleanser, soap, and water.
Objective evaluations were scored according to spot ultraviolet (UV), spot polarization, skin tone, and efficacy on the medial side of the face photographed using a Janus Facial Analysis System® (PIE Co., Ltd, Suwon-si, Gyeonggi-do, Korea), which uses a pigment technology system that assesses the pigment distribution objectively according to three light exposure types—namely, normal, polarizing, and UV light.13 The surface texture of the skin, pores, and wrinkles were assessed by normal light; acne formation and pigmentation were assessed by polarizing light; and the sebum, porphyrins, pigmentation, and keratin levels were assessed by UV light.13
Moreover, we evaluated each parameter in five facial areas, including the forehead, nose, cheeks, temple, and under the eye. Efficacy was calculated using the following formula:
![]()
During a subjective evaluation, participants were involved and a scoring scale was used to indicate minimal, moderate, good, or very good improvement in skin tone. Any side effect of the therapy was reported.
Statistical analysis. All non-normally distributed data were processed using the generalized linear mixed method to compare the efficacies of the glutathione supplement and placebo. The statistical significance was set at p<0.05. Data analyses were performed using the Statistical Package for the Social Sciences for Windows version 28.0 software program (IBM Corporation, Armonk, New York).
Research ethics. The study protocol was reviewed and approved by the ethics committee of the Faculty of Medicine Universitas Indonesia (protocol no. 17-06-0589; granted December 5, 2017). This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments, and all participants provided informed consent to participate in the study.
RESULTS
Demographic data. Seven of the 90 healthy female study participants aged 33 to 50 years were lost to follow-up. Differences in demographics between the glutathione supplement and placebo groups were not significant (Table 1). The participant flowchart for the study is shown in Figure 1.
TABLE 1.
Participant demographics for a 12-week multicentered, randomized, double-blind, controlled clinical trial evaluating the efficacy and safety of glutathione as a skin-lightening agent
| PARTICIPANT DEMOGRAPHICS | GLUTATHIONE (N=45) | PLACEBO (N=45) | |
|---|---|---|---|
| Education† | |||
| Low | 2.3% (1) | 2.3% (1) | |
| Middle | 62.8% (28) | 65.9% (30) | |
| High | 34.9% (16) | 31.8% (14) | |
| Drug history‡ | |||
| Yes | 37.2% (17) | 27.3% (12) | |
| No | 62.8% (28) | 72.7% (33) | |
| Age, year, median (IQR) | 39 (10) | 39 (12) | |
| Duration of exposure (hours/week), median (IQR) | 1 (1) | 1 (1) | |
†low=elementary, middle=junior high/senior high, and high=diploma/bachelor’s degree/higher
‡Drug history included all drugs administered to the participant orally/topically
IQR: interquartile range
FIGURE 1.
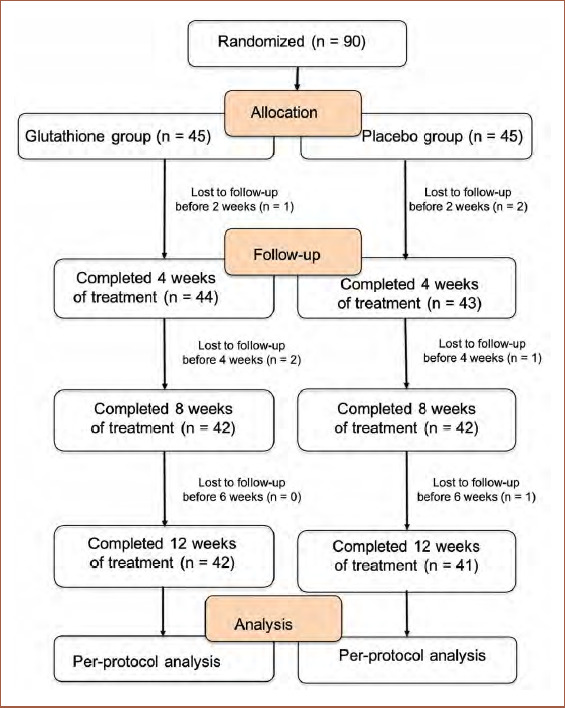
Flowchart showing the participants’ journey over a 12-week multicentered, randomized, double-blind, controlled clinical trial evaluating the efficacy and safety of glutathione as a skin-lightening agent. According to the protocol analysis, only 83 of 90 subjects completed each visit to the end of the study.
Total spot UV, total spot polarization, and total skin tone. Each evaluation on all areas of the face (forehead, cheeks, nose, eye, and temple) showed no significant difference in transformation of total spot UV, total spot polarization, total skin tone, or efficacy between the glutathione supplement and placebo groups. However, there were several interesting findings in total spot UV measurement. If the baseline total spot UV was classified into four groups—namely, 10 or less, 11 to 20, 21 to 30, and greater than 30—the glutathione supplement showed a tendency to halt the increase of total spot UV compared to the placebo (Figures 2 and 3).
FIGURE 2.
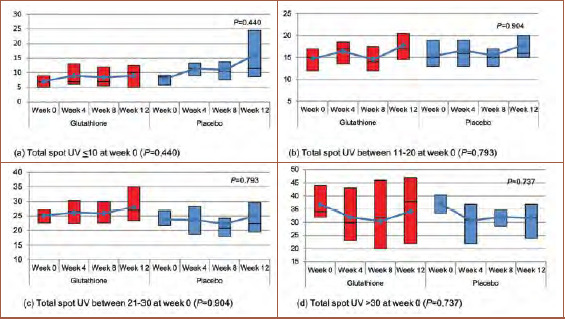
Transformation of total spot UV at Weeks 0, 4, 8, and 12; A) Total spot UV of 10 or less at Week 0 (p=0.440); B) total spot UV of 11 to 20 at Week 0 (p=0.793); C) total spot UV of 21 to 30 at Week 0 (p=0.904), and; D) total spot UV of greater than 30 at Week 0 (p=0.737).
FIGURE 3.
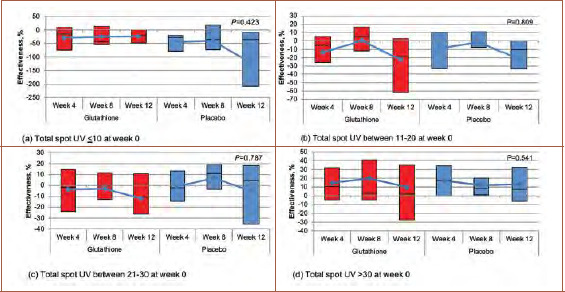
Effectiveness of total spot UV reduction; A) Total spot UV of 10 or less at Week 0 (p=0.440); B) total spot UV of 11 to 20 at Week 0 (p=0.793); C) total spot UV of 21 to 30 at Week 0 (p=0.904), and; D) total spot UV of greater than 30 at Week 0 (p=0.737)
Efficacy of total spot UV, total spot polarization, and total skin tone reduction. Although, overall, there was no significant difference in the efficacy of total spot reduction between the two groups, when dividing participants into four groups according to the efficacy of total spot UV reduction (0%–24.9%, 25%–49.9%, 50%–74.9%, and >75%), there was a higher proportion of participants present in the greater than 75 percent group.
Total spot polarization improved in both groups, and total skin tone improvement was slightly greater in the glutathione supplement group than in the placebo group, although this difference did not reach statistical significance. Total spot UV and total skin tone showed continuous improvement in the placebo group until Week 8, but this trend declined at Week 12. The underlying explanation remains unclear; however, the results did not reach statistical significance (Figure 4).
FIGURE 4.
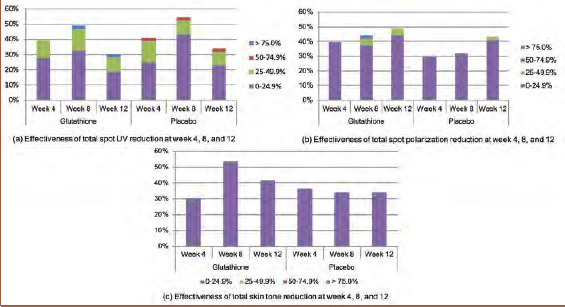
Efficacy of total spot UV, total spot polarization, and total skin tone reduction; A) Effectiveness of total spot UV reduction at Weeks 4, 8, and 12; B) Effectiveness of total spot polarization reduction at Weeks 4, 8, and 12; C) Effectiveness of total skin tone reduction at Weeks 4, 8, and 12
Subjective evaluation by participants. There was no significant difference between the glutathione supplement and placebo groups in terms of subjective efficacy. However, the proportion of participants who experienced an improvement of more than 75 percent was higher in the glutathione supplement group than in the placebo group (Figure 5).
FIGURE 5.
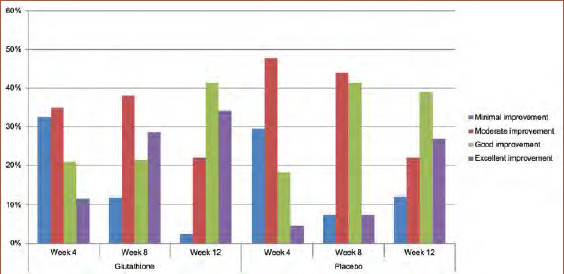
Subjective evaluation of efficacy of glutathione plus in skin tone reduction by participants taking glutathione plus or placebo capsules
Side effects. Mild side effects, such as nausea, skin bumps, and itching, as well as comedonal acne and epigastric pain were experienced by nine percent of the glutathione supplement group and eight percent of the placebo group during the first four weeks. However, these findings were not statistically significant (p=0.546).
DISCUSSION
Although glutathione is quite popular, its efficacy as a skin-lightening agent has yet to be properly evaluated. Our findings raise questions regarding its capacity as a skin-lightening agent.11 Our study results were similar to those of Zubair et al,14 who reported that, similar to the results obtained for the placebo group, after 12 injections of glutathione over six weeks, only six (37.5%) participants experienced skin tone improvement of at least one level. Furthermore, this improvement was not permanent and, after six months of administration, only one (6.2%) participant continued to experience skin tone reduction. In addition to its mediocre efficacy, drug preparation was also costly. Consequently, Zubair et al did not recommend glutathione as a skin-lightening agent.14
In contrast, Weschawalit et al3 reported a statistically significant effect of glutathione as a skin-lightening agent, but this was limited to a group of participants aged older than 40 years who received GSH, a reduced form of glutathione, on the sun-exposed area of the right forearm.3 Arjinpathana et al1 used a Mexameter® (Courage+Khazaka Electronic, Köln, Germany) to measure the melanin index as the main output parameter and found that glutathione was most effective on sun-exposed skin.1 In their systematic review, Dilokthornsakul et al15 likewise reported that glutathione might lighten skin tone in sun-exposed areas; however, its skin-lightening effects remain controversial due to conflicting results from several clinical trials.15 Our study results differed from those of these studies, as glutathione supplement appeared in our investigation to have no significant positive effects on sun-exposed skin.
Theoretically, availability of GSH as an intact molecule declines when consumed orally, which could explain why oral glutathione was less effective.16–20 An open-label, single-arm trial study by Handog et al21 used lozenges to prevent absorption by the gastrointestinal tract and found that the melanin index declined significantly in both sun-exposed and unexposed skin.21 Glutathione is likely pivotal for antioxidant defense in melanoma cells. Campione et al20 found that enzyme glutathione-s-transferase-π (GST-π), present in keratinocytes and melanocytes, protects cells against tumor progression—notably, sun exposure-associated melanoma cells. This study suggests that the enzyme GST-π could be an adjuvant marker for a chronically sun-damaged melanoma pathway.20
Our glutathione capsules contained 500mg of L-glutathione (fermentation), combined with 250mg of ascorbic acid, 50mg of ALA, and 4mg (20mg) of zinc (as zinc aspartate) as additional ingredients. As mentioned before, these other three ingredients were added to support the role of glutathione as a skin-lightening agent to yield a synergistic effect as a depigmenting agent, in absorption, and in capsule formulations.10–12
Zinc aspartate, the zinc salt of aspartic acid, plays a role as a cosmetic biocide, hair-conditioning agent, and skin-conditioning agent. This kind of zinc salt is used in cosmetic formulations.22
Vitamin C, or ascorbic acid, is a water-soluble vitamin found in a variety of foods, particularly fruits and vegetables. It has many functions, including working as an antioxidant to protect cells from oxidative damage. It also maintains the body’s supply of other antioxidants, including glutathione. It is also widely used in dermatology as a treatment modality in depigmentation of hyperpigmented spots on the skin. Vitamin C has been used in conjunction with other treatment modalities.23
Vitamin C helps reprocess glutathione by converting oxidized glutathione back to its active form. A study by Johnston et al24 indicated that vitamin C supplementation (500mg/day) maintains reduced glutathione concentrations in the blood and improves the overall antioxidant protection capacity of the blood. The results of the study indicate that vitamin C supplementation at an amount obtainable by diet significantly raised red blood cell GSH, by 47 percent per day, in healthy adults.24 Another study by Lenton et al25 showed that vitamin C supplements increase glutathione in human lymphocytes. Subjects who took 500 to 1,000mg of vitamin C daily for 13 weeks experienced an 18-percent increase of glutathione in white blood cells.25
Ascorbate is an excellent antioxidant in cells, not only because of its relatively high concentration but also because of the high rate constant of its reaction with free radicals. It even reacts with glutathione radicals in the aqueous phase. The analysis of various markers of oxidative stress suggests that ascorbate acts as an antioxidant in vivo.26
Investigators have also combined ALA with glutathione in previous studies. Tsuji-Naito et al27 demonstrated that DHLHZn (sodium zinc dihydrolipoyl histidinate, compound of Zn2+/dyhydrolipoic acid derivative complex, which was developed for cosmetic/medical use) serves as a potentially effective skin-lightening agent, an effectiveness that is based on the compound’s covalent scavenging of DOPAquinone, resulting in depigmentation.27
Our study revealed that some mild adverse events occurred in the first four weeks, although this occurrence was not statistically significant. Dilokthornsakul et al15 reported that most adverse events were gastrointestinal tract symptoms or skin reactions. Skin reactions, including pruritus, erythema, and red spots, were also observed in the placebo group.15
With the development of analysis technology, such as pattern recognition from a digital image, facial skin measurement systems using a digital image have been developed, and Janus-III has become one of the most widely used facial skin measurement options in the skincare industry in Korea, owing to its convenience and diverse measuring characteristics. A study by Leem et al28 demonstrated that the measured data of facial characteristics using the Janus-III system showed highly correlated patterns with those evaluated by a dermatologist. This device could also be considered for use in specialized research of skin characteristics even with a small sample size.28 After some considerations by the investigators, outcome measurements using the Janus Facial Analysis System® instead of Mexameter® was thought still to be a good option, because of the facility provided in Indonesia.
Limitations. This study was not without limitations. First, effects were measured solely on sun-exposed facial areas. Second, our participant cohort included only Asian women of a certain age group. Third, our study evaluated the efficacy of glutathione supplement capsules only by using the Janus Facial Analysis System®. Finally, this study did not measure any outcomes aside from skin-lightening effects, which meant that other potential cosmetic advantages of glutathione were not examined.
CONCLUSION
We have shown that oral glutathione supplement might have some beneficial effects as a skin-lightening agent, as demonstrated in certain subgroups of our cohort. Reduction of spot UV in one subgroup, skin tone, and spot polarization were greater in the glutathione supplement group, but there were no statistically significant differences in any of our results. Mild and temporary side effects were reported in the first four weeks and resolved by the next evaluation, indicating that the glutathione supplement might be safe to use at the described dosage for this period of time.
Other controlled clinical trials with different measurable outputs are needed to further evaluate the efficacy of glutathione as a skin-lightening agent. Other cosmetic advantages may also be evaluated, including improvements in skin elasticity and wrinkles, to obtain more comprehensive results and information regarding the effects of glutathione.
ACKNOWLEDGMENTS
We acknowledge Indonesian Cosmetic Dermatology Study Group, who supported the study; Sjarif M. Wasitaatmadja, MD, as the initiator of the study; Ahmad Fuady, MD, MSc, as the study statistician; Shafira Ninditya, MD, as the corresponding author’s research assistant who provided editorial assistance in the preparation of this article; and health personnel of the Department of Dermatology and Venereology at each center who participated in the management of the study subjects. We also would like to thank the study participants for their involvement in this study.
REFERENCES
- Arjinpathana N, Asawanonda P. Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat. 2012;23(2):97–102. doi: 10.3109/09546631003801619. [DOI] [PubMed] [Google Scholar]
- Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16(2):101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- Weschawalit S, Thongthip S, Phutrakool P, Asawanonda P. Glutathione and its antiaging and antimelanogenic effects. Clin Cosmet Investig Dermatol. 2017;10:147–153. doi: 10.2147/CCID.S128339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarama CD, Maibach HI. Glutathione as a depigmenting agent: an overview. Int J Cosmet Sci. 2005;27(3):147–153. doi: 10.1111/j.1467-2494.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- Smit N, Vicanova J, Pavel S. The hunt for natural skin whitening agents. Int J Mol Sci. 2009;10(12):5326–5349. doi: 10.3390/ijms10125326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinar VE, Taylor SC, Pandya AG. What’s new in objective assessment and treatment of facial hyperpigmentation? Dermatol Clin. 2014;32(2):123–135. doi: 10.1016/j.det.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263(33):17205–17208. [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Pandya A, Berneburg M, Ortonne JP, Picardo M. Guidelines for clinical trials in melasma. Pigmentation Disorders Academy. Br J Dermatol. 2006;156:21–28. doi: 10.1111/j.1365-2133.2006.07590.x. [DOI] [PubMed] [Google Scholar]
- Telang PS. Vitamin C in dermatology. Indian Dermatol Online J. 2013;4(3):143–146. doi: 10.4103/2229-5178.110593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano C, Calarco P, Ceccarini MR et al. Development and characterization of new topical hydrogels based on alpha lipoic acid-hydrotalcite hybrids. Cosmetics. 2019;6(2):35. [Google Scholar]
- Abendrot M, Kalinowska-Lis U. Zinc-containing compounds for personal care applications. Int J Cosmet Sci. 2018;40(4):319–327. doi: 10.1111/ics.12463. [DOI] [PubMed] [Google Scholar]
- PIE Co., Ltd. Janus Multi Facial Analysis System. https://www.pie.co.kr/eng/product/janus.php Available at: Accessed April 19, 2019.
- Zubair S, Hafeez S, Mujtaba G. Efficacy of intravenous glutathione vs. placebo for skin tone lightening. J Pakistan Assoc Dermatologists. 2017;26:177–181. [Google Scholar]
- Dilokthornsakul W, Dhippayom T, Dilokthornsakul P. The clinical effect of glutathione on skin color and other related skin conditions: a systematic review. J Cosmet Dermatol. 2019;18(3):728–737. doi: 10.1111/jocd.12910. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J, Rupa P, Matsui T et al. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J Agric Food Chem. 2014;62(39):9499–9506. doi: 10.1021/jf503257w. [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB et al. A clinical trial of glutathione supplementation in autism spectrum disorders. Med Sci Monit. 2011;17(12):77–682. doi: 10.12659/MSM.882125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TM, Jones DP. Transepithelial transport of glutathione in vascularly perfused small intestine of rat. Am J Physiol. 1987. 252(5 Pt 1):607 613 [DOI] [PubMed] [Google Scholar]
- Biroccio A, Benassi B, Fiorentino F, Zupi G. Glutathione depletion induced by c-Myc downregulation triggers apoptosis on treatment with alkylating agents. Neoplasia. 2004;6(3):195–206. doi: 10.1593/neo.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione E, Medda E, Paternò EJ et al. Chronically sun-damaged melanomas express low levels of nuclear glutathione-S-transferase-π: an epidemiological and clinicopathological study in Italy. Acta Derm Venereol. 2015;95(1):40–44. doi: 10.2340/00015555-1821. [DOI] [PubMed] [Google Scholar]
- Handog EB, Datuin MS, Singzon IA. An open-lable, single-arm trial of the safety and efficacy of a novel preparation of glutathione as a skin-ligheting agent in Filipino women. Int J Dermatol. 2016;55(2):153–157. doi: 10.1111/ijd.12999. [DOI] [PubMed] [Google Scholar]
- Kim KB, Kim YW, Lim SK et al. Risk assessment of zinc oxide, a cosmetic ingredient used as a UV filter of sunscreens. J Toxicol Environ Health B Crit Rev. 2017;20(3):155–182. doi: 10.1080/10937404.2017.1290516. [DOI] [PubMed] [Google Scholar]
- Sanadi RM, Deshmukh RS. The effect of vitamin C on melanin pigmentation: a systematic review. J Oral Maxillofac Pathol. 2020;24(2):374–382. doi: 10.4103/jomfp.JOMFP_207_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CS, Meyer CG, Srilakshmi JC. Vitamin c elevates red blood cell gluathuone in healthy adults. Am J Clin Nutr. 1993;58(1):103–105. doi: 10.1093/ajcn/58.1.103. [DOI] [PubMed] [Google Scholar]
- Lenton KJ, Sane AT, Therriault H et al. Vitamin C augments lymphocyte glutathione in subjects with ascorbate deficiency. Am J Clin Nutr. 2003;77(1):189–195. doi: 10.1093/ajcn/77.1.189. [DOI] [PubMed] [Google Scholar]
- Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiologic conditions?. FASEB J. 1999;13(9):1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- Tsuji-Naito K, Hatani T, Okada T, Tehara T. Modulating effects of a novel skin-lightening agent, alpha-lipoic acid derivative, on melanine production by the formation of DOPA conjugate products. Bioorg Med Chem. 2007;15(5):1967–1975. doi: 10.1016/j.bmc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Leem S, Kim JS, Kim Y et al. Comparative analysis of skin characteristics evaluation by a dermatologist and the Janus-III measurement system. Skin Res Technol. 2021;27(1):86–92. doi: 10.1111/srt.12915. [DOI] [PubMed] [Google Scholar]


