Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy that is characterized by early metastasis, low resectability, high recurrence, and therapy resistance. The experimental mouse models have played a central role in understanding the pathobiology of PDAC and in the preclinical evaluation of various therapeutic modalities. Different mouse models with targetable pathological hallmarks have been developed and employed to address the unique challenges associated with PDAC progression, metastasis, and stromal heterogeneity. Over the years, mouse models have evolved from simple cell line-based heterotopic and orthotopic xenografts in immunocompromised mice to more complex and realistic genetically engineered mouse models (GEMMs) involving multi-gene manipulations. The GEMMs, mostly driven by KRAS mutation(s), have been widely accepted for therapeutic optimization due to their high penetrance and ability to recapitulate the histological, molecular, and pathological hallmarks of human PDAC, including comparable precursor lesions, extensive metastasis, desmoplasia, perineural invasion, and immunosuppressive tumor microenvironment. Advanced GEMMs modified to express fluorescent proteins have allowed cell lineage tracing to provide novel insights and a new understanding about the origin and contribution of various cell types in PDAC pathobiology. The syngeneic mouse models, GEMMs, and target-specific transgenic mice have been extensively used to evaluate immunotherapies and studying the therapy-induced immune modulation in PDAC yielding meaningful results to guide various clinical trials. The emerging mouse models for experimental parabiosis, hepatic metastasis, cachexia, and image-guided implantation, are increasingly appreciated for their high translational significance. In this article, we describe the contribution of various experimental mouse models to the current understanding of PDAC pathobiology and their utility in evaluating and optimizing therapeutic modalities for this lethal malignancy.
Keywords: Pancreatic cancer, mouse model, targeted therapy, immunotherapy, cachexia, parabiosis, metastasis, vaccine, antibody-targeted therapy
1.0. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most lethal malignancy with a five-year survival of ~10% in the United States. Recent estimated new cases and deaths analysis in the US population suggest PDAC as the fourth leading cause of cancer-related deaths by 2021 [1]. Early detection correlates with better survival in PDAC, still the lack of biomarkers and the asymptomatic nature of disease lead to the diagnosis of PDAC at late stages, leaving the majority of patients’ ineligible for resection [2, 3]. Other challenges associated with poor clinical outcomes in PDAC patients include early metastasis, high recurrence rate, and poor response to the existing therapeutic approaches [4]. The hypovascular, hypoxic, and desmoplastic pancreatic tumor microenvironment (TME) is the major determinant of therapy resistance, which is characterized by disrupted vascular transport [5-7]. The pancreatic TME is primarily comprised of cancer associated fibroblasts (CAFs) that secrete extracellular matrix (ECM) proteins to create a physical barrier that restricts the delivery of systemic therapies to the target tumor cells, leading to poor therapeutic response [8]. Together with CAFs, the tumor associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and other immune-regulatory cells secrete immune modulatory cytokines in the TME, leading to increased tumorigenesis, metastasis, and immunosuppression in pancreatic tumors [5, 9].
Considering the high lethality of PDAC, and challenges associated with poor therapeutic response towards existing treatment modalities, it is important to recapitulate the pathological hallmarks of human PDAC in preclinical models to evaluate therapeutic approaches more accurately for rationalizing the clinical trials. The experimental murine models have served as valuable tools for the development and optimization of targeted therapies for PDAC. These mouse PDAC models can be classified based on the manner of tumor induction, site of tumor implantation/growth, their histopathological characteristics, and clinicopathological relevance. We discuss here the evolution of various mouse models that have been employed for a mechanistic understanding of PDAC pathogenesis, biomarker development, and for evaluation and optimization of chemotherapy, radiation therapy, targeted therapies, and immunotherapy. We also describe the role of animal models in developing novel therapies that have been translated into clinics and highlight the challenges that remain to be addressed to improve their relevance for clinical research.
2.1. Evolution of PDAC experimental mouse models
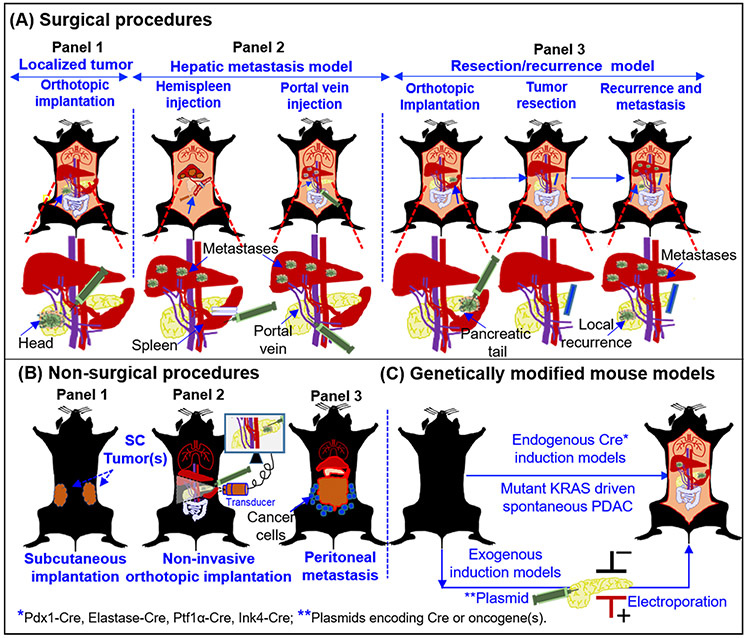
Based on the complexities and experimental requirements, the commonly used mouse models of PDAC can be classified into surgical and non-surgical implantation models, and spontaneous genetically engineered mouse models (GEMMs) (Figure 1). The early experimental mouse models of PDAC involved implanting human PDAC patient-derived cells in athymic nude rats to demonstrate tumor growth [10]. Subsequently, several studies were published to show the development of a reliable preclinical xenograft model of PDAC in different rodent species where human pancreatic tumor derivatives (tumor chunks and cells) were used for orthotopic implantation in the pancreas [11-13]. With the development of various human PDAC cell lines, implantation models remained the mainstay for in vivo studies for studying the gene functions and evaluating therapeutic modalities for several decades till the advent of genetically engineered mouse (GEM) models. The evolution of murine models of PDAC can be divided into pre- and post-GEM periods. Prior to the GEM models, the preclinical models employed for PDAC research were predominantly xenograft mouse models, or carcinogen-induced models in rats, and hamsters [11, 12, 14-16]. Various mouse models currently employed for PDAC research are depicted in Figure 1. Among the mouse models, immunodeficient mice, including the athymic nude mice and severe combined immunodeficiency (SCID) mice, have been extensively used as these models lack xenograft rejection and allow tumor development from the biological material (tissue and cell lines) derived from human PDAC tumors [12, 15, 17, 18]. The orthotopic implantation models where PDAC cells are surgically implanted in the pancreas of the mouse, (Figure 1A; panel 1), are considered superior to the subcutaneous xenograft model (Figure 1B; panel 1), because the tumors grow under the influence of the local organ-specific microenvironment. Since the late 1980s, the orthotopic models of PDAC have been widely used for the optimization of targeted therapies and continue to be used extensively for preclinical evaluation of therapeutic modalities [12, 19]. While implantation and propagation of human PDAC tumor fragments in mice have been practiced since the early 1990s, [20, 21], the use of such models, has increased exponentially in the last decade [22-24]. These models are now called patient-derived xenografts (PDXs) and can be initiated by implantation of tissue fragments or cells from surgical resections or biopsies without the need for in vitro expansion or derivatization. Similarly, the tumor fragments derived from GEMMs or carcinogen-induced murine tumors have been propagated as mouse-derived allografts (MDAs) or mouse derived homograft tumors to evaluate therapeutic modalities in PDAC [19]. In parallel, several models for metastatic PDAC were used to study the underlying mechanisms of metastasis and evaluate therapeutic agents [20, 25, 26]. Peritoneal dissemination model of metastatic PDAC was reported earlier in athymic nude mice and in hamsters with an intent to study the mechanism of peritoneal metastasis and to evaluate its preclinical significance (Figure 1B: panel 3) [16, 27]. Using these models, Yamamura et al. suggested that the peritoneal metastasis could either be fast by direct dissemination or slow via stomata in the diaphragm [16]. Similarly, the peritoneal dissemination model was used to demonstrate that retroviral P53 vector targeted therapy could inhibit primary tumor growth as well as peritoneal metastasis [27].
Figure 1. Major PDAC experimental mouse models employed for preclinical research.
Presentation of a surgical (A), non-surgical (B), and spontaneous models (C), for the experimental therapeutics of PDAC. (A) Panel 1: Orthotopic implantation of tumor source (cells/tumor derivatives) in the pancreas of the mouse. Lower panel depicts the approaches employed for implantation of cells and tumor growth in head of the pancreas. Panel 2: Hepatic metastases models are generated by hemispleen injection and portal vein injection. The lower panels indicate the injection site in the spleen (Left) and in the portal vein (Right). Details of the procedures are mentioned in the section 2.2.4. Panel 3: Resection model for the testing adjuvant and neoadjuvant therapies to evaluate their effect on local recurrence and metastasis. The lower panel depicts implantation of cells and tumor growth in the tail of the pancreas. Such models have allowed the R0 resections. Following resection mice can followed for local recurrence and metastasis .in the presence or absence of therapeutic modalities. (B) Non-surgical procedures: the SC implantation method (panel 1), non-invasive ultrasound guided (USG) injection model (panel 2), and peritoneal dissemination model (panel 3), are primarily the non-surgical methods to evaluate the therapeutics against primary tumor and peritoneal metastasis. (C) Genetically modified mouse models (GEMMs): Both endogenous and exogenous genetic modification can be performed to develop GEMMs of PDAC. Mouse models with mutant KRAS and/or TP53 genes driven by Cre-recombinases under the control of various cell-type specific promoters and described in the in the section 2.2.8. The spontaneous models recapitulate the pathological features of human PDAC with primary tumors and multiple metastases. Alternatively, exogenous induction of oncogenes can be achieved by the introduction of plasmids via electroporation or injectable viral particles with different Cre recombinases to induce the tumor growth in the pancreas.
Mutations in the KRAS gene are the most common early event in the progression of several epithelial malignancies including the cancers of the pancreas, lung, and colon [28, 29]. The development of genetically engineered mouse models harboring oncogenic mutant KRAS that could be conditionally expressed in a tissue-specific manner, ushered the development of gene-driven murine models of spontaneous tumorigenesis (Figure 1 C). The mutant KRAS-driven spontaneous mouse model was first described for non-small cell lung carcinoma [30] and later was modified to generate a model of spontaneous PDAC [31]. Subsequently, Hingorani and coworkers developed murine models harboring TP53R172H mutation along with KRASG12D mutation that developed more aggressive tumors and faithfully recapitulated most of the pathological features of human PDAC [32]. This genetically engineered KRASG12D; TP53R172H; Pdx-1-Cre (KPC) exhibits the early PanIN lesions from the age of 8-10 weeks of age and progresses to PDAC with high incidences of metastasis to liver, lung, diaphragm, and adrenals. The median age of KPC mice was reported to be 24 weeks. KPC tumors closely mimic complex TME of human PDAC, both pathologically and immunologically. Therefore, the KPC model has been widely used for the mechanistic understanding of PDAC progression and the optimization and preclinical evaluation of therapeutic approaches. The introduction of the KPC model revolutionized the progress in the field of biomarker development [33-36], targeted therapies [37-41], and immunotherapies [42-46] of PDAC. The advent of KC and KPC models has not only provided novel insights into the genetic, molecular, and immunological mechanisms driving PDAC, but also broadened the research in the preclinical experimental therapeutics for PDAC and facilitated rapid bench-to-bedside transition. Several KPC tumor-derived cell lines [47, 48], fibroblasts [47], organoids [49-51], and MDAs [52], have emerged as indispensable reagents for understanding pathogenesis and for evaluation of targeted therapies [37, 53-55]. The development of the syngeneic C57BL/6 KPC mouse models and reagents derived from GEMM-derived resources have rejuvenated the field of PDAC immunotherapy. The KPC mouse model has not only served as a valuable tool to understand mechanisms associated with immune activation and suppression, tumor immune microenvironment in PDAC, but also been utilized extensively for testing immunotherapy-based approaches, including therapeutic vaccines [56-58], immune checkpoint inhibition [59, 60], adoptive immune-cell therapy [58, 61], and CAR-T cell therapies [62, 63].
The emerging trend in PDAC clinical trials has been evaluate therapeutic modalities in conjunction with surgical resection either under adjuvant or neoadjuvant settings. While the implantation and spontaneous models of PDAC have been valuable tools for experimental therapeutics, these models are not amenable to surgery due to large size of orthotopic tumors or multifocal nature of spontaneous models. To circumvent these challenges several approaches have been devised to develop models with localized resectable tumors. These include orthotopic implantation of cells in the tail of the pancreas (Figure 1A; panel 3), or injection of plasmids encoding Cre (in the floxed mice) or oncogenes (Figure 1C) (detailed elsewhere in the article). The resultant tumors can be surgically removed, and animals can be followed for local recurrence and metastasis, to evaluate the efficacy of therapeutic agents. Such models are highly relevant for the evaluation of therapeutic modalities in adjuvant and neoadjuvant settings and have been used in several preclinical studies.
2.2. Mouse models specific to different aspects of PDAC pathogenesis
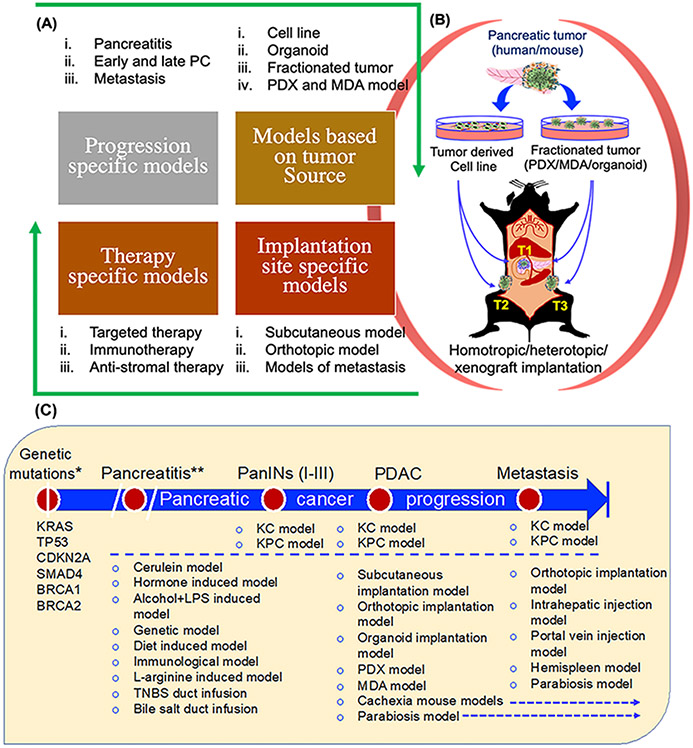
The sequence of genetic and molecular events from early oncogenic mutations to PanINs and to PDAC have been thoroughly investigated to identify actionable targets in PDAC [64, 65]. In this regard, experimental models representing various PDAC progression stages have been used to evaluate targeted therapies (Figure 2). Experimental murine models can be classified based on the stage of progression, mode of tumor initiation, site of tumor development and their suitability of specific therapeutic modalities (Figure 2 A, B). In addition, murine models representing other risk factors for PDAC, such as pancreatitis, diabetes, alcohol, and smoking, have been studied to understand disease pathogenesis and used as experimental models for targeted therapies [66-72] (Figure 2C). Despite well-defined mechanistic cascades involved in PDAC pathogenesis, developing targeted therapies for PDAC remains challenging. Most of the genetic, molecular, and immunological targets reported as key determinants of PDAC pathogenesis failed during preclinical assessment due to multiple challenges associated with disease progression and lack of appropriate evaluation platforms [73]. However, the landscape for preclinical therapeutic evaluation of PDAC has gradually broadened with the emergence of novel experimental models and approaches [74]. Thus far, several murine models have been reported to recapitulate various aspects of PDAC initiation and progression (Figure 2A, 2C), including the experimental mouse models for pancreatitis, models for early and late PDAC, models for metastasis, and those representing the complexities of pancreatic TME.
Figure 2. Classification of murine models of PDAC based on various parameters.
Experimental models are classified based on different parameters including, progression stages, implantation source, implantation site, and therapeutic approaches. (A) Murine models based on progression stages include early and late PDAC and metastasis, including pancreatitis as a major risk factor (Top; left panel); models based on implantation material (Top; right panel), and site of implantation (Bottom; right panel). Lastly, therapy-specific models are described to highlight that based on therapy-specific requirements, models for immunotherapy, anti-stromal therapies, and other targeted therapies can be categorized (Bottom; left panel). (B) Illustration of implantation models based on tumor source and implantation site. Different PDAC sources including cell lines/organoids/PDXs/MDAs derived from tumors of the mouse or human pancreas are implanted surgically into the pancreas or injected subcutaneously. (C) PDAC progression stage-specific experimental mouse models. Experimental models are categorized based on different stages of pancreatic cancer progression. Following oncogenic mutations (Listed below the genetic mutations, which is the first event in PDAC progression), PDAC begins with PanIN lesions and progresses to PDAC with primary tumor and distant metastasis. Pancreatitis is included as a major risk factor for PDAC and based on some reports that suggest the development of PDAC from pancreatitis. In addition to selective models for targeted therapy, spontaneous KC and KPC models are shown in all the stages of PDAC progression. PanIN: pancreatic intraepithelial neoplasia; PDX: Patient-derived xenograft; MDA: Mouse-derived allograft. *Mutant KRAS is reported as driver mutation with others as complementary mutations. **Pancreatitis is not an essential early event for PDAC but is considered as an important risk factor.
2.2.1. Experimental models for inflammation and pancreatitis
Inflammation and pancreatitis are major risk factors in PDAC along with age, race, smoking, obesity, and diabetes [75-77]. Although direct role of pancreatitis, both acute and chronic, is obscure as a single factor, there is ample evidence suggesting that inflammation and pancreatitis could potentially trigger PDAC pathogenesis [78-80]. Both classical and genetically induced models have been used to study inflammation and pancreatitis [81-83]. Cerulein-based experimental models of inflammation and pancreatitis have been extensively employed for mechanistic understanding as well as for therapeutic evaluation. One of the earliest models to study acute pancreatitis was reported in1977 by Lampel and Kern, which involved secretagogue hyperstimulation using cholecystokinin (CCK) in rat [84]. Since then, several studies have used cerulein, an orthologue of CCK, for induction of both acute and chronic pancreatitis (CP) in mice [75, 81]. We have summarized key mouse models that have been used to study pancreatitis in the context of pathophysiology, potentiating PDAC, biomarker discovery, or evaluating the therapeutic modalities (Figure 2C). Previously, it was demonstrated that acute pancreatitis (AP) enhances the aggressiveness of PDAC in K-Ras-dependent manner. In contrast, recent studies have shown that chronic inflammation independent of K-Ras mutations can induce PDAC in the presence of cyclooxygenase-2 or IkB kinase-2 (IKK2) [85]. Further, combining alcohol with cerulein has been reported to induce CP [86]. Earlier, the cerulein-induced models for both AP and CP were used to demonstrate the role of CXCR2 and neutrophil recruitment in pancreatitis. Here, inhibition of CXCR2 in cxcr2−/− GEM or by pharmacological inhibitor significantly delayed pancreatitis [87]. Recently, ruxolitinib, an inhibitor of the JAK-STAT pathway was reported to reduce the severity of CP in the cerulean-induced murine model [88]. Thus, chemically induced and the genetically manipulated mouse models of pancreatitis serve as valuable platforms to evaluate targeted therapies.
2.2.2. Experimental models for pancreatic cancer
Experimental murine models to study therapeutic approaches in PDAC can be classified based on either primary source of tumor, site of implantation, and based on the experimental requirement (Figure 2A). For instance, all experimental studies using human pancreatic tumor as a source have been performed in immunodeficient mice such as athymic and severely combined immunodeficient (SCID) mice. Human PDAC cell lines, organoids, tumoroids, patient-derived xenografts (PDXs) have been used as a source material for the xenograft studies. Due to defined tumor growth kinetics and ease for genetic manipulation, PDAC cell lines have been extensively used for the optimization of therapeutic approaches in xenograft murine models [89, 90]. Depending on the source, the PDAC cell lines differ in genotype, morphology, doubling time, dose-response, invasiveness, tumor growth kinetics, and metastatic nature. Due to easier maintenance under defined culture conditions and their ability to yield consistent and reproducible data, the PDAC cell lines have been a preferred choice for the evaluation of therapeutic approaches for a long time. Moreover, due to their origin from the tumors of human PDAC patients, these cell lines were believed to recapitulate the genomic and molecular diversity of human cancers and their use for targeting cancer cell-intrinsic pathological mechanisms was viewed clinically more relevant [91]. In addition, cell lines are the easiest source for implantation in mice for assessment of therapeutic approaches in vivo. However, multiple in vitro passages and maintenance in a non-physiological environment frequently results in altered phenotypic and functional characteristics of cell lines, which may lead to variability in experimental outcomes [91]. There is a vast literature available for in vivo use of PDAC cell lines in tumorigenesis in mouse models and their use in the preclinical evaluation of various therapies [19, 92]. Of note, these cell lines-based xenograft mouse models are not obsolete yet, rather, are clinically relevant and continued to be used widely in PDAC research. For targets associated with genetic and epigenetic regulation of PDAC, cell line-based xenograft models are still being used for targeted therapies that include siRNA, shRNA, miRNA, and RNAi-based approaches [93-97]. Both genetic and epigenetic determinants of PDAC pathogenesis are conserved in cell lines and cell lines expressing the targets of interest can be selected for in vivo evaluation of targeted therapies in the xenograft mouse model. In contrast, the genetic and epigenetic regulations may differ in cell lines derived from murine and human pancreatic tumors, and therefore, a pre-validation of a target is required to select the right cell line model. Besides, it is important to analyze the cellular localization of a target to develop selective targeted therapy. Recent studies based on PDAC cell line based xenograft models have validated pharmacological inhibitors against targets associated with different oncogenic pathways, including EGFR-STAT3 signaling [98], YAP-TAZ pathway [96], KRAS signaling [99], CDK4/6 [100], SUMO pathway [101], and metabolic pathways [97, 102]. Mechanistically, anti-tumor efficacies of these pharmacological inhibitors have been reported either direct or through chemosensitization [103, 104]. Among other recent developments, using PANC-1 xenograft tumors, it has been demonstrated that iExosomes loaded with siRNA or shRNA targeting KRASG12D suppressed the growth of orthotopic tumors of the pancreas [105]. All these studies strongly suggest that PDAC cell line-based xenograft models remain relevant and will continue to be integral for optimizing targeted therapies for PDAC.
The cell line-based xenograft models have several limitations. First, human pancreatic tumor-derived cells grow in immunodeficient mice and preclude the analysis of immunological parameters of the disease. Second, the PDAC cell lines alter their phenotype as well as tumorigenicity when cultured in vitro for longer passages. Therefore, care must be taken while using the PDAC cell line for xenograft studies. Third, the cell line-based xenograft tumors, particularly the SC implanted tumors, do not recapitulate the stromal complexities of human PDAC. Therefore, preclinical therapeutic assessment is now focusing more on co-implantation models using a combination of cancer-associated fibroblast and cancer cells, organoid models, and tumor fragment-derived xenograft and allograft models [8, 106, 107]. Recent studies have extensively used patient-derived xenografts (PDXs) and mouse derived allografts (MDAs) to evaluate targeted therapies in PDAC [8, 108-110]. Though the PDX and MDA models are of high clinical relevance in PDAC, obtaining fresh tumor tissues to propagate and maintain continuously is challenging. To determine the impact of tumor initiating material on tumor growth, histology, and survival, Tseng et al., compared orthotopic implantation of cell line derived from liver metastasis site of the KPC mouse and the tumor fragments derived from SC homograft implantation of the same cell line [111]. Interestingly, both models showed similar growth and survival, suggesting that the tumorigenic characteristics of the cell are conserved when implanted in the pancreas of the mouse. However, as compared to the cell lines derived from the primary KPC tumors, the liver metastasis derived cell lines and tumor fragments showed significantly higher liver metastases reflecting the preservation of metastatic traits between cell line and homograft implants. . Alternatively, the organoid models have been widely appreciated for drug screening and their therapeutic evaluation in vitro as well as in vivo [49, 50, 110]. More interestingly, organoid based models have been used to understand disease progression and subtyping of PDAC. A recent study reported a xenotransplantation model where the organoid neoplasm from PDAC patient was injected in the pancreatic duct of the mouse [112]. Interestingly, the analysis of global RNA-sequencing data of fast and slow growing organoids suggested that the fast-growing organoids showed “invasive” subtype and the MYC, E2F, mTORC, and KRAS signaling genes were significantly enriched in this set of organoids. In contrast, the slow-growing organoids were enriched in bile and fatty acid metabolism and showed gene signatures associated with “progenitor” and “classical” subtypes. This study suggested that the accurate modeling of molecular subtypes is achievable using organoids and is important for understanding the PDAC progression and for the evaluation of emerging therapies.
2.2.3. Subcutaneous vs. orthotopic implantation
Depending upon the experimental requirement, the subcutaneous (SC) and orthotopic (OT) impanation models have been preferably used to evaluate therapeutic approaches in PDAC [19, 92, 113]. The SC model is a non-surgical method of cancer cell implantation as described in the figure (Figure 1B; panel 1), which is the most used method for the in vivo evaluation of therapeutic modalities because it is convenient to implant tumor cell line and tumor derived fragments without an extensive surgical procedure. In addition, it allows us to assess the tumor growth kinetics longitudinally. The periodic measurements of tumor volumes provide quantitative data of the growth kinetics from the SC tumor models. Most importantly, multiple tumors can be implanted in the same animal to evaluate tumor-specific target(s), e.g., both target positive and negative cells in the same mouse to investigate the effect of targeted therapy. In contrast, the OT tumors grow under the influence of the pancreatic microenvironment and therefore, are considered superior to the SC model in vascular development, stromal organization, and immune infiltration [15, 114]. Previous studies have reported that as compared to SC tumors, OT tumors due to their growth in natural microenvironment are more vascular with high stromal content, which histologically mimics the primary tumors in corresponding human malignancies [75, 114-116]. These vascular and histological differences in SC and OT tumor models cause a variable response to therapeutic agents. For example, the therapeutic response to FOLFIRINOX, a chemotherapeutic regimen for PDAC patients, was found to be variable in the mice bearing SC and OT tumors [114]. Interestingly, the difference in tumor growth, metastatic spread, blood vessel density, and the expression of Ki-67 and Caspase-3 suggested that the therapeutic response to FOLFIRINOX was better in the mice with OT tumors as compared to those with SC tumors. In another study, a comparative analysis of liver metastasis derived KPC cell line and its SC homograft tumor fragments was performed in OT implantation model [111]. The tumor growth, metastasis, histology, immune phenotype, and survival of the mice were not significantly different in the two groups implying that pancreas-specific microenvironment had greater influence on these parameters as compared to the implantation source. Further, the OT model of the pancreas is better for survival analysis as compared to the SC model because the increased tumor burden and associated pathological symptoms such as metastasis and ascites formation cause lethality in tumor-bearing mice. In contrast, the euthanasia in SC model is often dictated by humane endpoints (e. g, tumor volume, ulceration) defined by the local IACUC guidelines. Therefore, the survival data obtained from the SC model may not be truly an outcome of complications associated with tumor burden and metastasis. The OT models are relatively challenging to establish that require expertise in animal surgery to implant tumors. Recently, Erstad et al., have extensively optimized the surgical procedure to implant the PDAC cells in mouse pancreas and provided an excellent description of the techniques with all cautionary steps during the implantation [114]. The site of incision, cell number and volume, site of injection in the pancreas, angle of the needle during injection, and the gauge of the needle, are all important parameters to optimize for OT implantation. For instance, the most common incisions adopted for mouse pancreatic OT implantation are the midline incision, left subcostal incision, and left flank incision. However, the left flank incision with gentle pancreatic dissection provides better exposure of avascular regions of the pancreas to implant the cells [114]. Similarly, the angle of needle while injecting the cells is recommended parallel or at a minimum angle to the pancreas so that the bevel of the needle must not cross the thickness of the pancreas. Although the cell number in a volume of 50-100 μl can vary depending on the aggressiveness of the cell type, the recommendation for KRASG12D; TP53R172H mutant cell lines is in a range of ~104 cells/mouse. Thus, considering these points during OT implantation in the mouse pancreas would help in more appropriate and non-erroneous assessment of therapeutic modalities. Recently, OT mouse model was used for mass screening of > 50 drugs and the study concluded that the combination of HSP70 and MEK inhibitors exerts synergistic effect against PDAC and could be taken for further evaluation [117].
To overcome the leakiness of injected cells during OT implantation, the solidifying gelatinous protein mixture such as Matrigel, which is a brand name of protein mixture secreted by mouse sarcoma cells and resembles the extracellular matrix environment present in different tissues, can be used to implant the cells. Several studies have used Matrigel based implantation method for testing various therapeutic modalities and reported that matrigel provides more consistent and proliferative tumors [118-121]. Alternative to surgical implantation method that is time-intensive and cause aberrant inflammation, a non-invasive method has been developed to implant the cells in the pancreas of the mouse with the help of USG imaging (Figure 1B; panel 2) [122]. Technically, as the transducer comes closer to the abdomen, pancreas can be visualized and the allowing a needle to be guided for the orthotopic implantation of the tumor cells. Like surgical implantation, bolus of cell suspension is the confirmation for accurate cell injection. Intrahepatic injections have also been performed for the orthotopic tumor implantation with the help of USG imaging [123], suggesting that the imaging-based approach could be an alternative to surgical procedures for OT implantation. Besides, there is heterogeneous tumor growth in different mice and requires careful animal randomization before therapy initiation, which could be achieved by bioluminescence imaging or by ultrasound guided (USG) imaging of tumors in appropriate settings. Additionally, if the cancer cells are unlabeled, it is difficult to follow the tumor growth in OT model longitudinally without the use of ultrasound or magnetic resonance imaging tools which are time-consuming and require additional expertise. Despite the challenges in implantation and monitoring, the OT models of the pancreas are clinically more relevant and considered superior to SC model for the evaluation of therapeutic approaches in PDAC.
2.2.4. Mouse models of metastasis and perineural invasion:
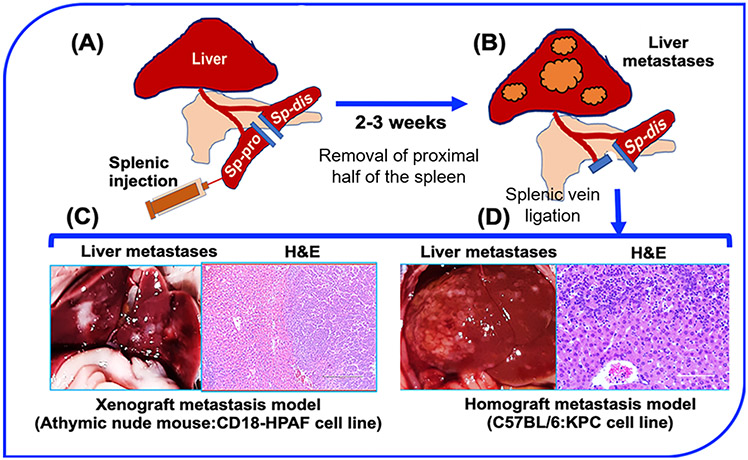
Early metastasis is a hallmark of PDAC and is one of the major causes of cancer-related deaths in PDAC patients [1]. Mechanisms regulating PDAC metastasis are not fully understood, but the genetic and molecular alterations have been reported to drive distant metastasis in PDAC, with the liver as the most favorable metastatic site [124, 125]. Therefore, several attempts have been made to generate experimental models of hepatic metastatic of PDAC. Earlier, studies indicated that orthotopic transplantation of tumor pieces from human pancreatic tumor specimen or after propagation of human PDAC cell lines in SC model resulted in metastatic spread of PDAC to several organs, including the liver [20, 126]. Similarly, the red fluorescent protein (RFP) labeled MIA-PaCa-2 cells were propagated in the SC flank of the mice and transplanted into the pancreas of nude mice [127]. In these transplantation models, metastases to multiple sites including liver, peritoneum, spleen, and lymph nodes, were observed. However, variability in metastatic burden and incidences make it challenging to evaluate targeted therapies specifically against metastases. Recently, OT implantation based model of PDAC metastasis showed that SUIT-2 is a more efficient cell line than Capan-1 for studying distant metastases with higher incidence and reliable dissemination to the liver, lung, and mesenterium [128]. As liver is the most common site for PDAC metastasis, several efforts have been directed to develop liver-specific models of PDAC metastasis. The most common and reliable models for liver metastasis are splenic and portal vein injection models that consistently form hepatic metastases and are considered suitable for evaluating targeted therapies and immunotherapy-based approaches (Figure 1A; panel 2) [129-131]. The highly consistent portal vein injection model has been used for liver metastasis in the multiple cancers including the colon, pancreas, breast, and uvea [132-135]. However, the portal vein injection model is surgically more cumbersome where a surgeon must flip all the abdominal organs to expose the portal vein. Soares et al., have described an alternative highly efficient and reliable model of hepatic metastasis that involves the injection of cancer cells into the hemispleen, as described in the figure (Figure 3) [129]. The hemispleen technique is highly reproducible in generating liver metastases and can be easily performed in xenograft and syngeneic mouse models. Recently, using hemispleen model, radiation-induced immunogenic “abscopal effect” in combination with immunotherapy was demonstrated to inhibit hepatic metastasis [136]. Overall, hemispleen model due to its ability to mimic pathophysiological aspects of hepatic metastasis is ideally suited to study the mechanisms of metastasis and evaluate targeted therapies, including immunotherapies.
Figure 3. Hemispleen injection technique for hepatic metastasis.
(A-B) Illustration of splenic hemispleen injection for hepatic metastasis in mouse mice. (A) Left flank incision is followed by the splitting of spleen into proximal and distal parts after the ligation of the exposing ends with the help of clamps, as described in the main section. Injection of cells in the proximal hemispleen is followed by clamping the splenic blood vessel and removal of proximal hemispleen. (B) Diagram showing the development of liver metastases after 2-3 weeks of hemispleen injection. (C) Representative image showing liver metastases after the hemispleen injection of CD18-HPAF cell line in athymic nude mice with a Hematoxylin and Eosin (H&E) staining of the liver section to show the liver metastases. (D) Representative image showing multiple metastases after hemispleen injection of KPC tumor derived cell line with a representative H&E staining of liver section. Sp-pro: spleen-proximal part; Sp-dis: spleen-distal part.
Perineural invasion (PNI) is a mechanism of cancer cell metastasis through the nerves that is predominantly seen in the tumors lacking vascular and lymphatic invasion [137, 138]. Clinically, the PNI is considered to be a major cause of disease recurrence and pain in PDAC patients [139]. As the pancreatic tumors are hypovascular, lack lymphatic transport system, and located closer to the autonomic plexuses of the abdomen, the disseminating cancer cells tend to follow the perineural route to metastasize. Earlier, the histological analysis of resected pancreatic tumors and regional lymph nodes (N=14 patients) showed that pancreatic head is the primary site of nerve enrichment for the PNI and pattern of PNI is different than lymph node metastasis [140]. Later, Pour et al., demonstrated the evidence of PNI in Syrian hamster model where PNI was observed as most common route of metastasis (~89%) after intrapancreatic implantation of cells, but no vascular invasion [141]. The exact mechanism of PNI is still unknown; however, several oncogenic signals and growth factors such as MMPs, nerve growth factors, adhesion molecules, metabolic alterations, and cytokine signaling, have been reported to contribute in PNI in PDAC [142-151]. In an attempt to explain PNI and PDAC recurrence, Eibl and coworkers used a modified OT implantation model where the xenograft tumors developed after the implantation of MIA PaCa-2 and Capan-2 cells in the pancreas of athymic nude mice were completely resected and followed for disease recurrence [152]. Intriguingly, after 6 weeks of resection, recurrence was observed in the MIA PaCa-2 tumor group and histological evaluation suggested an extensive PNI of retroperitoneal nerve. Similarly, different PDAC cell lines (N=7) with high and low PNI properties were compared at RNA and protein level and used for the analysis of CD74 expression in xenograft mouse model [153]. Interestingly, the invariant chain CD74 was observed overexpressed in PNI high cell line group. In addition, the mouse PNI high cell lines Capan-1 and Capan-2 were found to invade mouse peripheral nerves, suggesting some overlapping human and mouse PNI pathways. Further, the autochthonous KC mouse model has been used to delineate the molecular mechanisms and pathways that regulate PNI, and it was observed that ablation of sensory neuron significantly decreased the PNI [154]. Recently, axon guidance molecule SEMA3D was reported to activate receptor PLXND1 in OT model and knockout of PLXND1 in KPC model significantly decreased perineural invasion, which is highly regulated by dendritic root ganglion cells [155]. Overall, different xenograft and autochthonous mouse models have established the influence of PNI on various pathological aspects of PDAC including its role in local recurrence, metastasis, stromal modulation, and in the alteration of immune TME [138, 155, 156].
2.2.5. Mouse models for pancreatic tumor stroma
Tumor stroma is one of the critical determinants of therapy resistance in PDAC, which has gained greater appreciation with the advent of GEMMs. In fact, GEMMs were used to evaluate several therapeutic modalities targeting stromal components in combination with conventional chemo or radiation therapies [32, 157-159]. In addition to GEM models, recent studies have described several new in vitro and in vivo models to account for the contribution of stroma in evaluating therapeutic regimens [160-162]. Cancer-associated fibroblasts (CAFs) are the predominant stromal cells in pancreatic tumors that contribute to fibro-inflammatory responses within the TME, producing extracellular matrix (ECM) proteins and inflammatory and immunosuppressive cytokines [107, 163]. Therefore, experimental models based on co-implantation of cancer cells and fibroblasts are considered superior to cancer cells implantation alone and recapitulate tumor-stroma in experimental PDAC murine models [164]. Multiple reports suggest CAFs are key determinants of radiation therapy, immunotherapy, and other targeted therapies in PDAC [8, 106, 165]. Earlier, Nguyen and coworkers used the SC co-implantation model to investigate the role of fibroblast-expressed micro-RNAs (miRNAs) on tumor growth and demonstrated that miR 21 promotes tumorigenesis[166]. In contrast, in a recent study, the tumor-restraining role of fibroblasts associated with Meflin protein, a mesenchymal and stemness marker, was demonstrated using the co-implantation model [167]. Interestingly, Meflin knockdown in the fibroblasts rendered the straightening of collagen fibers and led to the tumor aggressiveness in the PDAC transplantation model, whereas the overexpression of Meflin caused the tumor growth suppression. Immunologically, CAFs play a significant role in immunosuppression and contribute to resistance to immunotherapies. Recent studies have used co-implantation models to determine the impact of targeted therapies and immunotherapy-based approaches on TME. For instance, targeting metabolic pathways by small-molecule glutamine analog in the co-implantation model not only reduced metastasis and stemness in cancer cells but also depleted ECM and sensitized tumors to immune checkpoint inhibition therapies [168, 169]. These findings strongly support the significance of including fibroblasts in tumor implantation models for evaluating targeted therapies in PDAC. The co-implantation model is further advanced by the introduction of organoids, PDXs, and MDAs because the tumors growing from these sources constitute high stroma and recapitulate human PDAC more accurately [49, 52, 170].
2.2.6. Mouse models for lineage tracing in PDAC progression
The lineage tracing models are highly relevant for identifying the cell of origin in spontaneous models and understanding the contribution of different cell types in cancer progression, TME constitution, therapy resistance, and immune cell repertoire. Previously, lineage tracing models have been employed to understand the development of pancreas, acinar to ductal trandifferentiation, and contribution of different cell types in the adult pancreas [171-173]. As the pancreatic tumors are highly heterogeneous in cellular composition with extensive phenotypic and functional diversity, lineage tracing approaches have provided a robust tool to understand the unique attributes of each cell lineage. Earlier, to explore the EMT and metastasis of epithelial cells in KPC mice, constructs were used to fluorescently tag the epithelial cells such that when spontaneous tumors develop, these cells could be tracked in vivo [174]. Based on this ‘tag and track’ KPC model, it was observed that yellow fluorescence protein (YFP) labeled tumor epithelial cells of the pancreas undergo EMT at early stage of tumor development as these fluorescently labeled cells were observed in circulation at very early stage, even before the histological confirmation of PDAC development. Similarly, with the help of lineage tracing approaches in KC and KPC models, role of stem cell populations was defined. For example, the stem cell subpopulation characterized by the expression of microtubule marker doublecortin like kinase-1 (DCLK-1) was found to be enriched in preinvasive lesions in mutant KRAS mouse model and play a significant role in pancreatic tumorigenesis [175, 176]. Further, using lineage tracing models of intraductal papillary neoplasm (IPMN) and PanIN lesions, a recent study demonstrated that DCLK-1+ stem cell subpopulation originates from Pdx-1+ progenitors [177]. In addition, the DCLK+ stem cells in IPMNs co-express the tuft cell markers tubulin and COX2, but not the tumor epithelial markers such as MUC5AC, suggesting distinct phenotypic characteristic of DCLK+ stem cells. Likewise, other molecular markers for specific cell lineages and their roles in PDAC progression and drug resistance have been explored using the lineage tracing models [178-181]. Besides, these lineage tracing models have opened avenues to explore the immune cell homing and origin during PDAC progression, which can be valuable tools in designing immunotherapy-based approaches against PDAC.
2.2.7. Mouse models of PDAC-induced cachexia
Cachexia is a complex metabolic and behavioral syndrome that is characterized by anorexia (loss of appetite), muscle wasting, and irreversible weight loss [182]. Up to 85% of PDAC patients exhibit features of oncogenic cachexia and ~30% of patients succumb to cachexia, not due to tumor burden [183-185]. The underlying mechanisms and factors that induce cachexia in PDAC patients remain poorly understood. Activation of proinflammatory cytokines, tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6, and IL-8 in cachectic skeletal muscles lead to the activation of signaling pathways, e.g., JAK2/STAT3 and NF-kB, which cause protein degradation and muscle wasting in PDAC patients [186-188]. Due to the lack of robust animal models and poorly understood mechanisms, cachexia management is highly challenging in PDAC patients. Previously, PDAC cell line xenograft and allograft murine models have been used to study cachexia [183]. Most of these studies analyzed weight loss, muscle wasting, and mRNA analysis of markers associated with cachexia. For instance, in a murine model of metastasis and cachexia, Greco et al. inoculated Panc02 and FC1242 cell lines in C57BL/6 mice and treated these mice with toll-like receptor-7/9 (TLR-7/9) inhibitor or anti-TGF-β antibody to investigate the role of TLR-7/9 or TGF- β mediated pathways [189]. Interestingly, treatment with anti-TGF-β antibody reverted the cachexia symptoms and impeded weight loss and improved survival. Further, several other molecular pathways, including MyD88, TLRs, CD25, and TGF-β, have been shown to regulate cachexia in PDAC [190-192]. In a comparative analysis, the KPC tumor cells were implanted at different sites (subcutaneously, intraperitoneally, and orthotopically) in C57BL/6 mice to study cachexia-related metabolic, phenotypic, and behavioral changes [193]. This study demonstrated that the implantation site is crucial while studying cachexia in mouse models. Both intraperitoneal and orthotopic implantation models developed more severe cachexia as compared to the subcutaneous implantation model. The KPC tumor-bearing mice showed a decrease in food intake and locomotive activity. Furthermore, the muscle catabolism (wasting) was observed in both the skeletal and cardiac muscles. In contrast to subcutaneous tumors, the orthotopic tumors are histologically closer and constitute a mimicking TME as present in human PDAC tumors; therefore, it is likely that factors associated with pancreatic TME play an important role in inducing cachexia. For example, recent studies suggest that tumor-associated macrophages play important role in muscle wasting and cachexia via activating STAT3 signaling [194, 195]. Hence, stroma intact murine PDAC models are more useful in understanding cachexia progression and for evaluating approaches for the management of cachexia.
2.2.8. Genetically engineered mouse models
The genetically engineered mouse models (GEMMs) of PDAC have served as useful tools for the preclinical evaluation of various therapeutic approaches and helped in understanding the disease progression [31, 32]. In fact, the current understanding of the early stages of PDAC progression is based on the observations made in the mutant KRAS mouse models of PDAC [196, 197]. The PDAC GEMMs can be classified based on three important categories: the target gene(s) driving oncogenesis; specificity of promotor used to regulate the Cre-recombinase expression, and the manner of oncogene induction (Figure 1C). In combination with the oncogenic mutant KRAS, several other genes have been targeted to enhance the pathological robustness of these murine models, which include P53, Smad4, lkb1, Tgf-β, and Ink4 [19, 198, 199]. Similarly, the Cre-recombinase has been used under the control of different pancreas specific promoters such as Pdx-1, Elastase, P48, and Ptf-1-α [200-203] that are specific to distinct cell lineages in the pancreas. In another approach to rapidly produce the GEMMs, embryonic stem cells (ESCs) harboring the mutant KRAS, a homing cassette, and other genetic elements, were used at an early developmental stage to produce GEMMs with characteristics to develop tumors [204]. Due to similar histopathological features as human PDAC and rapid tumor progression, Pdx-1-Cre regulated KRASG12D; TP53R172H (KPC) mouse model has been most widely used to understand PDAC biology and for the evaluation of targeted therapies [42, 55, 205-207], gene silencing, and pharmacological inhibition of selective targets [208-212]. There are several advantages of KPC mouse as an experimental model for the optimization of targeted therapies. First, the KPC mouse model recapitulates the pathobiology of human PDAC, including the progression, metastasis, stromal complexities, and immunological consequences of PDAC pathogenesis (Figure 1C), and therefore, it is considered suitable to evaluate the effects of various risk factors and therapeutic agents on PDAC progression. For instance, recent studies have shown that smoking, which is a major risk factor for pancreatitis and PDAC, not only induces the stemness and endothelin-axis upregulation in KC mouse model but also promotes accumulation of myeloid-derived suppressor cells (MDSCs) in TME [71, 213, 214]. Second, PDAC progression is faster in KPC mice with a significant tumor burden by an average age of 20-24 weeks and metastases. Recently, tumors and cell lines derived from KPC mice have been used to demonstrate that selective metabolic reprogramming of stem cell populations promotes metastasis during PDAC progression [215]. Third, KPC mice exhibit high penetrance, with more than 90% of mice developing PDAC and succumbing to metastatic disease. Lastly, the resources generated from the KPC mouse models, including tumor cell lines, age-specific primary and metastatic tissue samples, serum samples, and intact tumor tissues for allograft implantation (MDAs) and tumoroid development, have emerged as valuable tools for PDAC research in immunocompetent animals. As generating ample number of KPC mice is challenging, the KPC cell lines and MDAs are useful alternatives that have been used for evaluating both targeted therapies and immunotherapy. Later, several other K-Ras mutations driven mouse models were introduced with other companion oncogenic and target specific mutations to study their effects in PDAC pathobiology [216, 217]. The KPC mouse model has been further utilized to develop gene specific knockout (KO) models to elucidate their role on PDAC pathobiology. For example, disruption of genes specific to stemness, metabolism, metastasis, glycosylation and immune pathways, have been demonstrated to alter the pathological features of gene specific KPC KO models [207, 210, 218, 219].
Despite several advantages, there is a variability in tumor initiation, progression, and incidences of metastasis in KPC mice, which can impact the experimental readouts during preclinical testing of therapeutic approaches. However, advanced imaging approaches have been employed to analyze tumor volumes, including USG imaging, MRI, and Computed Tomography (CT) in experimental murine models to follow the tumor growth and metastasis [220-222]. In addition, alternative approaches have been used to produce large batches of experimental mice that mimic the tumor progression and clinicopathological features of KPC mouse model. In this regard, tumor-derived homograft implantation and electroporation of oncogenic plasmids have been utilized to generate larger animal cohort and minimize the variability and challenges associated with KPC mice [210, 223, 224]. The electroporation to introduce plasmids encoding oncogenes or Cre recombinase have be used to efficiently produce focal disease in a more reproducible and predictable manner as compared to cumbersome breeding schemes where the yield of mice with the desired genotypes is low.
3.0. Therapeutic approach specific mouse models
Currently, preclinical evaluation of various therapeutic modalities for PDAC relies on sophisticated murine models to facilitate clinical translation. These models can either be immunodeficient to allow the growth of human PDAC cells, or immunocompetent where tumors are induced by carcinogen treatment, genetic manipulation or syngeneic cell implantation. The immune deficient models have been extensively used for evaluating chemotherapeutic agents, targeted therapies and radiation therapy, against tumors derived from human cancer cell lines and patient derived xenografts. In contrast, immunocompetent models are ideally suited for evaluating immunotherapies or to examine the impact other therapeutic modalities on immune system components. Immunocompetent models are being used to evaluate the contribution of immune system in the response or resistance to non-immune therapies.
3.1.0. Murine models for chemotherapy and radiotherapy:
The treatment approach for PDAC patients is based on tumor stage and patient’s response to therapies, which include surgery, chemotherapy, and radiotherapy [225, 226]. The optimization of chemotherapy and radiotherapy regimen in preclinical PDAC models is highly challenging because the small size animals differ in body weight, organ size, and basal metabolic rate (BMR), which are critical parameters for the chemotherapy dose optimization and for the delivery of radiotherapy. Despite that, major chemotherapies including gemcitabine, FOLFIRINOX, nab-paclitaxel, and capecitabine, have been optimized in mouse PDAC models [114, 227-231]. Earlier preclinical evaluations of chemotherapies and radiotherapy were intended to assess their effects on tumor growth, metastasis, and therapeutic resistance [18, 232-236]. However, most of these studies were usually performed in xenograft mouse models that were immune deficient and, it was difficult to make any immunological interpretation. For example, therapeutic agents’ genistein and wortmannin that target DNA repair pathway and angiogenesis respectively, were reported to enhance the gemcitabine sensitivity in OT pancreatic tumors in SCID mice [234, 237]. Similarly, minnelide, a heat shock protein-70 (HSP70) inhibitor, was reported to overcome oxaliplatin resistance in OT PDAC xenografts derived from MiaPaCa cell line w in athymic nude mice [236]. Similarly, preclinical assessment of radiolabeled monoclonal antibodies (mAbs) against the tumor specific targets employed xenograft mouse models [18, 233, 238-240]. SC and xenograft models have also been used to evaluate stereotactic body radiation therapy (SBRT) and chemotherapy-based combination treatment modalities [241-244]. However, the targeted delivery of SBRT to the mouse pancreatic tumors is challenging due to the small animal size and the anatomy of mouse pancreas, which hinders in focused delivery of radiation beam to the pancreatic tumors. However, a recent study showed that the radiation and chemotherapy-induced immunological alterations and their therapeutic effects in combination with immunotherapy-based approaches have been investigated thoroughly in immunocompetent syngeneic mouse models of PDAC [245-249]. Similarly, a combination of SBRT and anti-CD40 has been reported as effective in situ vaccination in PDAC mouse model, suggesting that the SBRT based combination therapies could be more effective in the treatment of PDAC [250]. The growing numbers of experimental mouse models for the preclinical evaluation of different therapeutic approaches are based on their ability to address the unique challenges associated with the pathological hallmarks of the disease and the therapeutic responses to the given treatment (Figure 2A-B). Besides chemo- and radiotherapy, other targeted therapies such as gene silencing approaches, pharmacological inhibitors, small peptides, and monoclonal antibodies, have been preferentially evaluated in SC and OT xenograft models using human PDAC cell lines, organoids, and PDXs.
Efforts have also been directed to generate experimental models to recapitulate clinical settings to optimize the therapeutic interventions in adjuvant and neoadjuvant settings. Several approaches have been employed to develop murine models amenable to surgical resection of primary tumors for evaluating the impact of therapeutic modalities on disease recurrence and survival (Figure 1A; panel 3 & Figure 1C). A recent study demonstrated that there is high local recurrence and metastasis after the early resection of pancreatic tumors that were generated by the electroporation of oncogenic plasmid in the mouse pancreas. However, the treatment with gemcitabine (100 mg/kg) as adjuvant chemotherapy following the surgery significantly reduce the local recurrence and prolonged the survival of the treated mice [223]. Similarly, electroporation of cre-recombinase expressing oncogenic plasmids was performed in the pancreatic tail of the KRASG12D; P53fl/fl B6 mice using the 5 mm diameter tweezer type electrode. A total of four pulses (35-ms/35 V) were given for sufficient plasmid electroporation, as depicted in the figure (Figure 1C). Once the focal tumor growth was observed, resection was performed following the standards commonly used in clinics [251]. For this, mice were laparotomized and surgical area was prepared. Next, the splenic blood vessel (BV) and blood supply to the tumor were stopped by applying the ligation clips. The important stages for this resection model are highlighted in panel 3 of Figure 1A. In this model, early resection coordinated with neoadjuvant and adjuvant therapies of gemcitabine, anti-PD-1 (T cell checkpoint) antagonist, and anti-CD96 (NK cell checkpoint) inhibitor provided long term survival benefit and significantly inhibited the local recurrence [251]. In another study, adjuvant radioimmunotherapy (RIT) was evaluated in a murine model following surgical resection of OT xenograft tumors. Intraperitoneal administration of 22.5 MBq dose of 64Cu-PCTA-retuximab after surgery significantly inhibited the local recurrance, liver metastasis, and peritoneal dissemination, as compared to the resected control group [252]. Recently, adjuvant co-therapy of acetylcholinesterase inhibitors with or without gemcitabine was investigated in orthotopic xenograft resection model [253]. However, it was observed that adjuvant co-therapy was not effective in enhancing the survival in this resection model. Thus, these xenograft and GEM resection models are useful tools for guiding the clinical trials for PDAC patients undergoing surgery with adjuvant or neoadjuvant therapies.
3.2.0. Experimental models for evaluating immunotherapies:
Immunotherapy-based approaches have been extensively evaluated for treating PDAC [254]. Immunotherapies such as immune checkpoint inhibition, cytokine therapy, therapeutic vaccines, and other immune-modulatory therapeutic approaches have been investigated primarily in syngeneic mouse models in C57BL/6 mice. Particularly, the cell lines and homograft’s from KPC mice-derived tumors have served as valuable reagents for immunological studies in PDAC (Figure 2B). Immunotherapy-based approaches can be categorized into two subgroups: antibody-targeted therapies and vaccine-based therapies. While the antibody-targeted therapies may or may not require immunocompetent mice depending upon the target molecules, vaccine-based therapies are reliant on the intact host immune system. The third group of targeted immunotherapies is cell-based immunotherapies which involves adoptive immune cell transfer and chimeric antigen receptor T-cell (CAR T cell). Recently, progress has been made where cell based targeted therapies have been evaluated in the xenograft, allograft, and GEM mouse models of PDAC [255-258].
3.2.1. Antibody-targeted therapies:
Murine models of PDAC have been extensively used for testing the efficacy of various monoclonal antibodies (mAbs) targeting either tumor-associated targets, antigens associated with tumor vasculature, or targets within the tumor stroma. The latter class may include targets on stellate cells, fibroblasts, immune cells, or an acellular stroma component. Two kinds of models are generally used: the implantation models (subcutaneous and orthotopic) and genetically engineered mouse models, where tumors arise spontaneously in an immunocompetent mouse [115, 259, 260]. The SC and OT implantation models have been extensively used for evaluating monoclonal antibodies against PDAC. For example, anti-Her2 mAb Trastuzumab was shown to inhibit the growth of Capan-1 xenograft implanted subcutaneously in nude mice [261]. The antibody did not inhibit the growth of Capan-1 cells in in vitro assays, showing that the predominant mechanism of action of Trastuzumab in PDAC may involve the activation of immune effector mechanisms. Further, Trastuzumab administration exhibited synergy with gemcitabine in reducing the growth of Capan-1 xenografts. Similarly, dual inhibition of HER2 and EGFR exhibited synergy in reducing the growth of PDAC xenografts [262]. Antibodies like CS-1008, a humanized antihuman Death Receptor 5 (DR5) antibody and AMG 655 (a fully human anti-DR5 antibody) also synergized with gemcitabine in reducing the growth of SC and OT PDAC xenograft mouse model (MIA PaCa-2) [263, 264]. A human single chain antibody against DR5 again inhibited the growth of MIA PaCa-2 xenografts and synergized with gemcitabine [265]. Another such anti-MUC1antibody called PankoMab-GEX was also shown to be highly tumor selective and was shown to induce potent ADCC towards its target cells [266]. Various other tumor-associated targets such as mesothelin, IGF-1R have been evaluated in similar xenograft models with varying degrees of success [267-269]. However, many agents that work very well in these models do not translate efficiently to the clinic with human patients. To circumvent these limitations, mAb-based therapies have also been evaluated in GEMMs For example, in KPC mice model anti-CD40 mAb induced potent activation of macrophages in tumor tissue resulting in stromal depletion, improved delivery of chemotherapy, and enhanced tumor cell killing [270].The KPC mouse model has also been extensively used to study the effects of anti-PD-L1 and anti-CTLA-4 immune checkpoint blockade and the poor response obtained in these models correlated well with the results obtained from human trials [271]. These observations further support that the KPC mouse model efficiently recapitulates the attributes of human PDAC in the sense that it is poorly immunogenic, excludes effector T cells, and provides an immunologically “privileged environment” for PDAC cells to thrive [260]. However, in the same study, a combination of anti-PD1 mAb and a CXCR4 inhibitor “AMD3100” induced tumor regressions in the KPC mice model. PD-L1 blockade also exhibited synergy with radiation therapy in both the KPC model and SC transplantation model using Panc02 cells [60]. KPC model was also used to test the efficacy of TME-associated targets such as a LOX-blocking antibody that demonstrated improved efficacy in combination with gemcitabine [189]. Similarly, a combination of an anti-MUC1 mAb called TAB004 with liposomal-MSA-IL2 provided therapeutic benefit in an immunocompetent MUC1 transgenic mice model [272]. This model exhibits the expression of human MUC1 in a spontaneously arising tumor in the pancreas of the mouse, with similar apical luminal MUC1 expression in transformed ducts as seen in human PDAC tumors. KPC mouse model was also used to study the efficacy of targeting stroma as seen by targeting stromal matrix protein CTGF. In this study, it was shown that an antibody against CTGF could synergize with gemcitabine in reducing tumor growth [158].
3.2.2. Vaccine-based therapies:
For immunological studies, the C57BL/6 strain of mice has been a model of choice as it predominantly elicits Th1-type immune response [273, 274], which is critical for anti-tumor immunity. However, the selection of immunogenic vaccine candidates and the development of relevant mouse models remains a challenge. In PDAC, three major types of therapeutic vaccines have reached clinical trials, including whole-cell vaccines, peptide vaccines, and dendritic cell (DC) based vaccines [275]. Preliminary studies are easier for DC-based vaccines because antigen-loaded DCs from the same mouse strain can be used to investigate the efficacy in an allograft mouse model or in a KPC mouse to analyze cytokine release, T cell activation, and anti-tumor effect [58, 276]. However, the anti-tumor immune response needs to be validated in human subjects. Thus far, whole-cell vaccine GVAX and multi-peptide vaccine Algenpantucel-L have advanced to phase II and phase III clinical trials, respectively [277]. Other vaccine candidates that are under investigation include MUC1, mutant K-Ras peptide, GV1001, and KIF20A. Recent studies suggest that other mucins, including MUC4 and MUC16, could be potential vaccine candidates against PDAC [278-280]. Most of the tumor antigens are self-antigens and demonstrate antigenic tolerance that led to their poor immunogenicity. However, recent studies suggested that tumor-specific mutations, splice variants, and post-translational modifications potentiate the immunogenicity of vaccine candidates. Besides, the lack of a reliable preclinical platform rendered slower development of vaccine-based immunotherapies in PDAC. Earlier, studies were performed using transfected murine cell line-based allograft models or using adoptive T-cell transfer methods to demonstrate the therapeutic efficacy of vaccine candidates in PDAC [281]. However, to investigate the therapeutic potential of candidate vaccines, antigen-specific transgenic (Tg) mouse models are required. For example, the development of the MUC1-Tg mouse significantly contributed towards understanding the immunogenic role of MUC1 and in the development of MUC1 specific vaccines [272, 282, 283]. Another approach to investigate the antigen-specific immune response of human PDAC antigens in murine models is by employing the human MHC transgenic mouse model. Human MHC-Tg mice models have demonstrated successful antigen presentation of human T-cell epitopes [284, 285], suggesting that the MHC-Tg mouse model could be used to investigate whether the selected antigen is efficiently presented on human MHC-I molecules to successfully prime T-cells for antitumor immune response. However, the generation of MHC transgenic needs an understanding of patient haplotype and therefore could be challenging to be use for personalized immunotherapy. Overall, vaccine development against PDAC is still an emerging field and it needs more robust and clinically acceptable mouse models for testing the vaccine based therapeutic approaches against PDAC.
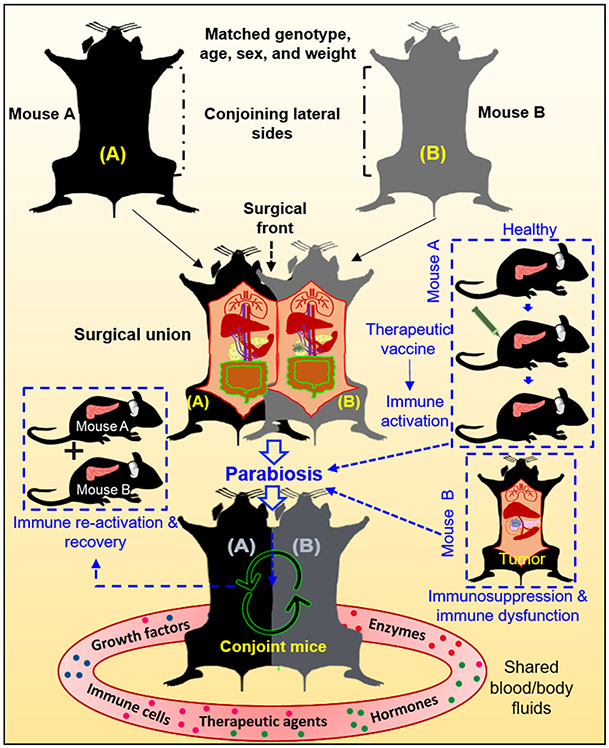
3.2.3. Mouse models of parabiosis
Parabiosis is an experimental approach where two individual animals are joined anatomically to share blood and body fluids (Figure 4). Earlier use of parabiosis in biomedical research was described in the 19th century. In 1908, Sauerbruch and Heyde first coined the word “parabiosis”, and then several other groups showed the utility of parabiosis in experimental animal physiology [286, 287]. Pioneer work from Lambert and co-workers in the rat cancer model showed that the mouse tumor grew when transplanted in the rat in a parabiosis setting. Parabiosis models are of high relevance in studying cancer immunology and immunotherapy. For example, in an earlier attempt, Kross et al. showed that tumor cells when implanted in susceptible mice in immune and susceptible conjoint parabiosis system, exhibit reduced tumor growth in the susceptible mouse [288] suggesting the active exchange of immune components between the conjoined animals. Several recent studies have revived interest in parabiosis murine models for immunological studies [289-292]. For PDAC, a study published in 1964 showed in the rat parabiosis model that when one rat was irradiated while the other was shielded, it showed hormonal imbalance after irradiation but protected the supraradiated mouse from lethal radiation dose. In addition, there was hardly any influence of parabiosis on tumor growth [293]. In another report, to decipher the role of host-derived MMP9 in angiogenesis and pancreatic tumor growth, MMP9 (−/−) and MMP9 (+/+) conjoint mice were used as parabiotic partners [294]. This study suggested that host derived MMP9 is crucial for tumor angiogenesis and growth. Recently, Zhu et al. used the parabiosis model by conjoining CD45.1+KPC mouse and CD45.2+ congenic wild-type mouse to investigate the contribution of circulating monocytes in tumor-associated macrophage population in PDAC [295]. Both the CD45.1+KPC and CD45.2+ wild-type mice were joined surgically at the age of ~14 weeks when KPC mice were pathologically at the PanIN stage and followed for next 6 weeks for chimerism in the partner KPC mouse. Interestingly, 28% of Ly6Chi peripheral chimerism was observed in KPC mice in the first two weeks, which remained stable after 6 weeks of parabiosis. However, only 2.5% of chimerism was observed after 6 weeks of follow-up, suggesting that most TAMs are maintained independently of circulating monocytes. As PDAC is highly heterogeneous in the stroma and immune infiltration, the parabiosis models might help in deciphering the origins, trafficking and kinetics of infiltrating immune cells and fibroblasts.
Figure 4. Depiction of mouse parabiosis model.
Either two male or female mice (A&B) with matching age, sex, weight, and genetic background are housed together for at least two weeks for adaptable cohabitation before the surgical union. For surgical union, the mice are placed together laterally and incisions are made longitudinally (top; dotted lines). The subcutaneous fascia is separated from the skin with the help of forceps, followed by suturing the olecranon with the help of non-absorbable sutures to connect the joints tightly. The surgical front is shown by the dotted back arrow (middle). In two weeks, mice start sharing blood vasculature and blood-borne components, as shown the circular arrows in the figure (bottom). An application of parabiosis models in PDAC research is depicted in boxes with blue dotted lines, where healthy mouse A is immunized with therapeutic vaccine to generate robust immune response and then conjoined to mouse B that has tumor in the pancreas and is can possibly exhibit localized and systemic immunosuppression and immune dysfunction (Right side; Blue dotted panel). Conjoining with immune activated mouse will help in immune reactivation in the tumor bearing mouse and can potentially restore anti-tumor immunity, as shown in the figure (Left side, blue dotted panel).
4. Conclusion and future perspectives
Mouse models have played a crucial role in enhancing the current understanding of PDAC pathobiology and are central for the evaluation of novel therapeutic modalities. Beginning from the simplest cell line-based SC xenograft models to the complex GEMMs that are driven by oncogenic K-Ras mutation, each murine model has unique characteristics that provide flexibility to choose a specific model for the evaluation of targeted therapies against a given pathological hallmark. A catalog of existing PDAC mouse models describing their classification based on various parameters such as genetic background, tumor source and site for implantation, immunocompatibility, and targetable pathological hallmarks of the disease, can be a valuable resource for choosing the appropriate experimental model for evaluating emerging therapeutic approaches. While the cell line-based SC and OT implantation models have remained the mainstay of experimental therapeutics for PDAC, the greater appreciation of the complexities of disease progression, and the role of TME in determining the therapy response, fueled the development of new generation models involving fibroblast-PDAC cell mixture co-implantation, PDXs, MDAs, and organoids. The advanced animal models recapitulate some of the stromal and vascular complexities of PDAC and are thus considered superior to the cell line-based models.
The mutant KRAS driven GEMMs have boosted the progress of immunotherapy and provided a deeper insight into PDAC initiation and progression. In the context of immunotherapy, the renewed interest in the parabiosis models to study immune cell trafficking, tumor-immune cell interactions, and associated immunomodulatory mechanisms, can pay rich dividends, particularly when mouse models for oncogenesis are combined with models engineered for altered immune/stromal cell functions. This can help circumvent challenges associated with simultaneous gene manipulations in multiple cellular compartments. Understanding molecular mechanisms associated with early metastasis and developing therapeutic approaches to prevent and treat PDAC metastasis have been challenging. In this regard, the mouse models of PDAC metastasis, including portal vein injection model, hemispleen injection model, perineural invasion model, and spontaneous models of metastasis, are poised to play a greater role in PDAC experimental therapeutics. To understand PDAC initiation and progression, the molecular cascade of early metastasis, and infiltrating immune cell populations, mouse models for cell lineage tracing have been recently used [296, 297]. These genetically engineered fluorescently labeled cell lineage tracing models will be highly useful in understanding PDAC initiation and for studying the origins and trafficking of metastatic cancer cells and infiltrating immune cells in PDAC. There is growing evidence suggesting that the targeting of pathological hallmarks in PDAC requires specific models. For example, the development of PDAC cachexia models has significantly expanded our understanding of its role in PDAC pathogenies and therapeutic outcome.
Overall, to enrich the pipeline of novel targeted therapies and achieve high success rate in clinical trials, it is imperative to use robust and specific mouse models during preclinical assessment. Although recently evolved mouse models have provided clinically more relevant platforms for targeting various aspects of PDAC pathogenesis, there are still several unaddressed questions related to targeted therapies. For instance, experimental models required for targeted therapies against early PDAC, including precancerous lesions and PanINs, are not well defined. Another challenge in PDAC is to develop experimental models for targeting pre-metastatic phenotypes of PDAC cells, including EMT and stem cell phenotype. Similarly, mouse models for immunotherapy, particularly vaccine-based therapies, need to mimic the human immune system. Introducing humanized mouse models may benefit the development of therapeutic vaccines against PDAC. However, it is essential for a therapeutic vaccine to identify first the PDAC-specific immunogenic target antigen(s) and then develop antigen-specific transgenic mouse models to evaluate its therapeutic effects.
Emerging knowledge from various omics and single-cell sequencing-based studies has revealed extensive heterogeneity and functional dichotomy in tumor-associated fibroblasts, tumor-associated macrophage and immune cells. Some cellular subtypes in the pancreatic TME are tumor-promoting, while others exhibit anti-tumor effects. Further, genomic and transcriptomic, and immunological profiling have identified several subtypes of PDAC [298, 299]. This new knowledge on one hand forces us to rethink and recalibrate therapeutic modalities to combat PDAC, while on the other hand, it makes modeling such complexities in murine models a challenging endeavor. For example, choosing the right class of CAFs for co-implantation studies and predicting their behavior post-implantation will require careful planning and analysis. Similarly, the existing PDAC mouse models, to some extent, can address the cellular heterogeneity; however, designing the mouse models to elucidate the cellular plasticity in the pancreatic TME is still a challenge.
In summary, the new generation PDAC mouse models can address the challenges associated with PDAC progression, metastasis, stromal heterogeneity, and immunosuppression. However, future development of novel therapeutic approaches would need more improvisation in existing mouse models to specifically understand and target the pathological hallmarks of PDAC.
Highlights.
Due to challenges in early detection, and limited efficacy of various treatment modalities, pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies and remains an active area of preclinical research employing animal models for improving diagnosis and therapy.
The experimental mouse models have transformed PDAC research serving as indispensable tools for understanding disease progression and pathophysiology, and ideal platforms for evaluating therapeutic approaches.
Both, the implantation models and genetically engineered mouse models (GEMMs), have been extensively used to optimize the targeted and untargeted therapeutic approaches against PC and facilitated their clinical translation.
The introduction of mutant KRAS-driven syngeneic mouse model and its derivative tumor tissues, tumoroids, and cell lines have kindled the progress in developing the immunotherapy-based approaches for PC therapy.
New generation models including patient derived xenograft (PDX), mouse derived allograft (MDA), organoids, and mouse models for liver metastasis, cachexia, parabiosis, lineage tracing, and perineural invasion, are set to dominate the preclinical research landscape for experimental therapeutics of PDAC.
Acknowledgement
We acknowledge our thanks to scientific manuscript editor for editing the manuscript. We also acknowledge Mansi Gulati, Nidhi Vinay Dwivedi, and Jaganmay Sarkar for their inputs in the manuscript.
Funding
The current work is supported in part by NIH-funded grants: (P01 CA217798, R01 CA195586, U01CA213862, and R01CA247471)
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. The other authors disclosed no potential conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, 70 (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Singhi AD, Koay EJ, Chari ST, Maitra A, Early Detection of Pancreatic Cancer: Opportunities and Challenges, Gastroenterology, 156 (2019) 2024–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Strobel O, Neoptolemos J, Jäger D, Büchler MW, Optimizing the outcomes of pancreatic cancer surgery, Nature Reviews Clinical Oncology, 16 (2019) 11–26. [DOI] [PubMed] [Google Scholar]
- [4].Zhu H, Li T, Du Y, Li M, Pancreatic cancer: challenges and opportunities, BMC Med, 16 (2018) 214–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Karamitopoulou E, Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features, British Journal of Cancer, 121 (2019) 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ligorio M, Sil S, Malagon-Lopez J, Nieman LT, Misale S, Di Pilato M, Ebright RY, Karabacak MN, Kulkarni AS, Liu A, Vincent Jordan N, Franses JW, Philipp J, Kreuzer J, Desai N, Arora KS, Rajurkar M, Horwitz E, Neyaz A, Tai E, Magnus NKC, Vo KD, Yashaswini CN, Marangoni F, Boukhali M, Fatherree JP, Damon LJ, Xega K, Desai R, Choz M, Bersani F, Langenbucher A, Thapar V, Morris R, Wellner UF, Schilling O, Lawrence MS, Liss AS, Rivera MN, Deshpande V, Benes CH, Maheswaran S, Haber DA, Fernandez-Del-Castillo C, Ferrone CR, Haas W, Aryee MJ, Ting DT, Stromal Microenvironment Shapes the Intratumoral Architecture of Pancreatic Cancer, Cell, 178 (2019) 160–175.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nia HT, Munn LL, Jain RK, Mapping Physical Tumor Microenvironment and Drug Delivery, Clinical Cancer Research, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wei L, Ye H, Li G, Lu Y, Zhou Q, Zheng S, Lin Q, Liu Y, Li Z, Chen R, Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer, Cell Death & Disease, 9 (2018) 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Murakami T, Hiroshima Y, Matsuyama R, Homma Y, Hoffman RM, Endo I, Role of the tumor microenvironment in pancreatic cancer, Ann Gastroenterol Surg, 3 (2019) 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Davies G, Duke D, Grant AG, Kelly SA, Hermon-Taylor J, Growth of human digestive-tumour xenografts in athymic nude rats, Br J Cancer, 43 (1981) 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Satake K, Mukai R, Kato Y, Shim K, Umeyama K, Experimental pancreatic carcinoma as a model of human pancreatic carcinoma, Clinical oncology, 10 (1984) 27–34. [PubMed] [Google Scholar]
- [12].Marincola FM, Drucker BJ, Siao DY, Hough KL, Holder WD Jr., The nude mouse as a model for the study of human pancreatic cancer, The Journal of surgical research, 47 (1989) 520–529. [DOI] [PubMed] [Google Scholar]
- [13].Vezeridis MP, Doremus CM, Tibbetts LM, Tzanakakis G, Jackson BT, Invasion and metastasis following orthotopic transplantation of human pancreatic cancer in the nude mouse, Journal of surgical oncology, 40 (1989) 261–265. [DOI] [PubMed] [Google Scholar]
- [14].Rivera JA, Graeme-Cook F, Werner J, Z'Graggen K, Rustgi AK, Rattner DW, Warshaw AL, Fernández-del Castillo C, A rat model of pancreatic ductal adenocarcinoma: targeting chemical carcinogens, Surgery, 122 (1997) 82–90. [DOI] [PubMed] [Google Scholar]
- [15].Schwarz RE, McCarty TM, Peralta EA, Diamond DJ, Ellenhorn JD, An orthotopic in vivo model of human pancreatic cancer, Surgery, 126 (1999) 562–567. [PubMed] [Google Scholar]
- [16].Yamamura S, Onda M, Uchida E, Two types of peritoneal dissemination of pancreatic cancer cells in a hamster model, Nihon Ika Daigaku zasshi, 66 (1999) 253–261. [DOI] [PubMed] [Google Scholar]
- [17].Mohammad RM, Al-Katib A, Pettit GR, Vaitkevicius VK, Joshi U, Adsay V, Majumdar AP, Sarkar FH, An orthotopic model of human pancreatic cancer in severe combined immunodeficient mice: potential application for preclinical studies, Clinical cancer research : an official journal of the American Association for Cancer Research, 4 (1998) 887–894. [PubMed] [Google Scholar]
- [18].Cardillo TM, Ying Z, Gold DV, Therapeutic advantage of (90)yttrium- versus (131)iodine-labeled PAM4 antibody in experimental pancreatic cancer, Clinical cancer research : an official journal of the American Association for Cancer Research, 7 (2001) 3186–3192. [PubMed] [Google Scholar]
- [19].Kong K, Guo M, Liu Y, Zheng J, Progress in Animal Models of Pancreatic Ductal Adenocarcinoma, J Cancer, 11 (2020) 1555–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fu X, Guadagni F, Hoffman RM, A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens, Proceedings of the National Academy of Sciences of the United States of America, 89 (1992) 5645–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM, A novel "patient-like" treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice, Cancer research, 53 (1993) 3070–3072. [PubMed] [Google Scholar]
- [22].Garcia PL, Miller AL, Yoon KJ, Patient-Derived Xenograft Models of Pancreatic Cancer: Overview and Comparison with Other Types of Models, Cancers, 12 (2020) 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rajeshkumar NV, Yabuuchi S, Pai SG, De Oliveira E, Kamphorst JJ, Rabinowitz JD, Tejero H, Al-Shahrour F, Hidalgo M, Maitra A, Dang CV, Treatment of Pancreatic Cancer Patient-Derived Xenograft Panel with Metabolic Inhibitors Reveals Efficacy of Phenformin, Clinical Cancer Research, 23 (2017) 5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Witkiewicz Agnieszka K., Balaji U, Eslinger C, McMillan E, Conway W, Posner B, Mills Gordon B., O’Reilly Eileen M., Knudsen Erik S., Integrated Patient-Derived Models Delineate Individualized Therapeutic Vulnerabilities of Pancreatic Cancer, Cell Reports, 16 (2016) 2017–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].An Z, Wang X, Kubota T, Moossa AR, Hoffman RM, A clinical nude mouse metastatic model for highly malignant human pancreatic cancer, Anticancer research, 16 (1996) 627–631. [PubMed] [Google Scholar]
- [26].Kimura Y, Kobari M, Yusa T, Sunamura M, Kimura M, Shimamura H, Matsuno S, Establishment of an experimental liver metastasis model by intraportal injection of a newly derived human pancreatic cancer cell line (KLM-1), International journal of pancreatology : official journal of the International Association of Pancreatology, 20 (1996) 43–50. [DOI] [PubMed] [Google Scholar]
- [27].Hwang RF, Gordon EM, Anderson WF, Parekh D, Gene therapy for primary and metastatic pancreatic cancer with intraperitoneal retroviral vector bearing the wild-type p53 gene, Surgery, 124 (1998) 143–150; discussion 150–141. [PubMed] [Google Scholar]
- [28].Uprety D, Adjei AA, KRAS: From undruggable to a druggable Cancer Target, Cancer treatment reviews, 89 (2020) 102070. [DOI] [PubMed] [Google Scholar]
- [29].Buscail L, Bournet B, Cordelier P, Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer, Nat Rev Gastroenterol Hepatol, 17 (2020) 153–168. [DOI] [PubMed] [Google Scholar]
- [30].Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T, Somatic activation of the K-ras oncogene causes early onset lung cancer in mice, Nature, 410 (2001) 1111–1116. [DOI] [PubMed] [Google Scholar]
- [31].Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA, Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse, Cancer cell, 4 (2003) 437–450. [DOI] [PubMed] [Google Scholar]
- [32].Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA, Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice, Cancer cell, 7 (2005) 469–483. [DOI] [PubMed] [Google Scholar]
- [33].Ray P, Rialon-Guevara KL, Veras E, Sullenger BA, White RR, Comparing human pancreatic cell secretomes by in vitro aptamer selection identifies cyclophilin B as a candidate pancreatic cancer biomarker, The Journal of Clinical Investigation, 122 (2012) 1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mirus JE, Zhang Y, Li C.l., Lokshin AE, Prentice RL, Hingorani SR, Lampe PD, Cross-Species Antibody Microarray Interrogation Identifies a 3-Protein Panel of Plasma Biomarkers for Early Diagnosis of Pancreas Cancer, Clinical Cancer Research, 21 (2015) 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ligat L, Saint-Laurent N, El-Mrani A, Gigoux V, Al Saati T, Tomasini R, Nigri J, Dejean S, Pont F, Baer R, Guillermet-Guibert J, Cordelier P, Lopez F, Dufresne M, Pancreatic preneoplastic lesions plasma signatures and biomarkers based on proteome profiling of mouse models, British Journal of Cancer, 113 (2015) 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Resovi A, Bani MR, Porcu L, Anastasia A, Minoli L, Allavena P, Cappello P, Novelli F, Scarpa A, Morandi E, Falanga A, Torri V, Taraboletti G, Belotti D, Giavazzi R, Soluble stroma-related biomarkers of pancreatic cancer, EMBO Molecular Medicine, 10 (2018) e8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Song X, Zhu S, Xie Y, Liu J, Sun L, Zeng D, Wang P, Ma X, Kroemer G, Bartlett DL, Billiar TR, Lotze MT, Zeh HJ, Kang R, Tang D, JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice, Gastroenterology, 154 (2018) 1480–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, Jiang H, Fields RC, Pachter JA, Lim KH, DeNardo DG, Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion, Gut, 69 (2020) 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nagathihalli NS, Castellanos JA, Lamichhane P, Messaggio F, Shi C, Dai X, Rai P, Chen X, VanSaun MN, Merchant NB, Inverse Correlation of STAT3 and MEK Signaling Mediates Resistance to RAS Pathway Inhibition in Pancreatic Cancer, Cancer research, 78 (2018) 6235–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Olive KP, Tuveson DA, The Use of Targeted Mouse Models for Preclinical Testing of Novel Cancer Therapeutics, Clinical Cancer Research, 12 (2006) 5277. [DOI] [PubMed] [Google Scholar]
- [41].Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT, Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer, Proceedings of the National Academy of Sciences, 110 (2013) 20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, Zhong X, Zimmers TA, Ostrowski MC, Ludwig T, Bloomston M, Bekaii-Saab T, Lesinski GB, IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer, Gut, 67 (2018) 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, Feo S, Giovarelli M, Novelli F, Vaccination With ENO1 DNA Prolongs Survival of Genetically Engineered Mice With Pancreatic Cancer, Gastroenterology, 144 (2013) 1098–1106. [DOI] [PubMed] [Google Scholar]
- [44].Soares KC, Rucki AA, Kim V, Foley K, Solt S, Wolfgang CL, Jaffee EM, Zheng L, TGF-β blockade depletes T regulatory cells from metastatic pancreatic tumors in a vaccine dependent manner, Oncotarget, 6 (2015) 43005–43015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Keenan BP, Saenger Y, Kafrouni M.l., Leubner A, Lauer P, Maitra A, Rucki AA, Gunderson AJ, Coussens LM, Brockstedt DG, Dubensky TW, Hassan R, Armstrong TD, Jaffee EM, A Listeria Vaccine and Depletion of T-Regulatory Cells Activate Immunity Against Early Stage Pancreatic Intraepithelial Neoplasms and Prolong Survival of Mice, Gastroenterology, 146 (2014) 1784–1794.e1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH, CD40 Agonists Alter Tumor Stroma and Show Efficacy Against Pancreatic Carcinoma in Mice and Humans, Science, 331 (2011) 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dauer P, Zhao X, Gupta VK, Sharma N, Kesh K, Gnamlin P, Dudeja V, Vickers SM, Banerjee S, Saluja A, Inactivation of Cancer-Associated-Fibroblasts Disrupts Oncogenic Signaling in Pancreatic Cancer Cells and Promotes Its Regression, Cancer research, 78 (2018) 1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Torres MP, Rachagani S, Souchek JJ, Mallya K, Johansson SL, Batra SK, Novel pancreatic cancer cell lines derived from genetically engineered mouse models of spontaneous pancreatic adenocarcinoma: applications in diagnosis and therapy, PLoS One, 8 (2013) e80580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tiriac H, Plenker D, Baker LA, Tuveson DA, Organoid models for translational pancreatic cancer research, Current opinion in genetics & development, 54 (2019) 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tiriac H, Belleau P, Engle DD, Plenker D, Deschenes A, Somerville TDD, Froeling FEM, Burkhart RA, Denroche RE, Jang GH, Miyabayashi K, Young CM, Patel H, Ma M, LaComb JF, Palmaira RLD, Javed AA, Huynh JC, Johnson M, Arora K, Robine N, Shah M, Sanghvi R, Goetz AB, Lowder CY, Martello L, Driehuis E, LeComte N, Askan G, Iacobuzio-Donahue CA, Clevers H, Wood LD, Hruban RH, Thompson E, Aguirre AJ, Wolpin BM, Sasson A, Kim J, Wu M, Bucobo JC, Allen P, Sejpal DV, Nealon W, Sullivan JD, Winter JM, Gimotty PA, Grem JL, DiMaio DJ, Buscaglia JM, Grandgenett PM, Brody JR, Hollingsworth MA, O'Kane GM, Notta F, Kim E, Crawford JM, Devoe C, Ocean A, Wolfgang CL, Yu KH, Li E, Vakoc CR, Hubert B, Fischer SE, Wilson JM, Moffitt R, Knox J, Krasnitz A, Gallinger S, Tuveson DA, Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer, Cancer discovery, 8 (2018) 1112–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Öhlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA, Organoid models of human and mouse ductal pancreatic cancer, Cell, 160 (2015) 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li J, Qian W, Qin T, Xiao Y, Cheng L, Cao J, Chen X, Ma Q, Wu Z, Mouse-Derived Allografts: A Complementary Model to the KPC Mice on Researching Pancreatic Cancer In Vivo, Computational and structural biotechnology journal, 17 (2019) 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wallez Y, Dunlop CR, Johnson TI, Koh SB, Fornari C, Yates JWT, Bernaldo de Quiros Fernandez S, Lau A, Richards FM, Jodrell DI, The ATR Inhibitor AZD6738 Synergizes with Gemcitabine In Vitro and In Vivo to Induce Pancreatic Ductal Adenocarcinoma Regression, Molecular cancer therapeutics, 17 (2018) 1670–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Engle DD, Tiriac H, Rivera KD, Pommier A, Whalen S, Oni TE, Alagesan B, Lee EJ, Yao MA, Lucito MS, Spielman B, Da Silva B, Schoepfer C, Wright K, Creighton B, Afinowicz L, Yu KH, Grützmann R, Aust D, Gimotty PA, Pollard KS, Hruban RH, Goggins MG, Pilarsky C, Park Y, Pappin DJ, Hollingsworth MA, Tuveson DA, The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice, Science, 364 (2019) 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG, Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy, Nature medicine, 22 (2016) 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim VM, Blair AB, Lauer P, Foley K, Che X, Soares K, Xia T, Muth ST, Kleponis J, Armstrong TD, Wolfgang CL, Jaffee EM, Brockstedt D, Zheng L, Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies, Journal for ImmunoTherapy of Cancer, 7 (2019) 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yasmin-Karim S, Bruck PT, Moreau M, Kunjachan S, Chen GZ, Kumar R, Grabow S, Dougan SK, Ngwa W, Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer, Front. Immunol, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yang J, Hu S, Shangguan J, Eresen A, Li Y, Pan L, Ma Q, Velichko Y, Wang J, Hu C, Yaghmai V, Zhang Z, Dendritic cell immunotherapy induces anti-tumor effect in a transgenic mouse model of pancreatic ductal adenocarcinoma, Am J Cancer Res, 9 (2019) 2456–2468. [PMC free article] [PubMed] [Google Scholar]
- [59].Ma Y, Li J, Wang H, Chiu Y, Kingsley CV, Fry D, Delaney SN, Wei SC, Zhang J, Maitra A, Yee C, Combination of PD1 Inhibitor and OX40 Agonist Induces Tumor Rejection and Immune Memory in Mouse Models of Pancreatic Cancer, Gastroenterology, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Azad A, Yin Lim S, D'Costa Z, Jones K, Diana A, Sansom OJ, Kruger P, Liu S, McKenna WG, Dushek O, Muschel RJ, Fokas E, PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy, EMBO molecular medicine, 9 (2017) 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sivaram N, McLaughlin PA, Han HV, Petrenko O, Jiang YP, Ballou LM, Pham K, Liu C, van der Velden AW, Lin RZ, Tumor-intrinsic PIK3CA represses tumor immunogenecity in a model of pancreatic cancer, J Clin Invest, 129 (2019) 3264–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Watanabe K, Luo Y, Da T, Guedan S, Ruella M, Scholler J, Keith B, Young RM, Engels B, Sorsa S, Siurala M, Havunen R, Tahtinen S, Hemminki A, June CH, Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses, JCI Insight, 3 (2018) e99573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ma X, Shou P, Smith C, Chen Y, Du H, Sun C, Porterfield Kren N, Michaud D, Ahn S, Vincent B, Savoldo B, Pylayeva-Gupta Y, Zhang S, Dotti G, Xu Y, Interleukin-23 engineering improves CAR T cell function in solid tumors, Nature Biotechnology, 38 (2020) 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Waddell N, Pajic M, Patch A-M, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MCJ, Robertson AJ, Fadlullah MZH, Bruxner TJC, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, lacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempera MA, Biankin AV, Johns AL, Mawson A, Chang DK, Scarlett CJ, Brancato M-AL, Rowe SJ, Simpson SH, Martyn-Smith M, Thomas MT, Chantrill LA, Chin VT, Chou A, Cowley MJ, Humphris JL, Jones MD, Scott Mead R, Nagrial AM, Pajic M, Pettit J, Pinese M, Rooman I, Wu J, Tao J, DiPietro R, Watson C, Steinmann A, Ching Lee H, Wong R, Pinho AV, Giry-Laterriere M, Daly RJ, Musgrove EA, Sutherland RL, Grimmond SM, Waddell N, Kassahn KS, Miller DK, Wilson PJ, Patch A-M, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu C, Nones K, Lynn Fink J, Christ A, Bruxner T, Cloonan N, Newell F, Pearson JV, Bailey P, Quinn M, Nagaraj S, Kazakoff S, Waddell N, Krisnan K, Quek K, Wood D, Fadlullah MZH, Samra JS, Gill AJ, Pavlakis N, Guminski A, Toon C, Asghari R, Merrett ND, Pavey D, Das A, Cosman PH, Ismail K, O’Connnor C, Lam Duncan McLeod VW, Pleass HC, Richardson A, James V, Kench JG, Cooper CL, Joseph D, Sandroussi C, Crawford M, Gallagher J, Texler M, Forest C, Laycock A, Epari KP, Ballal M, Fletcher DR, Mukhedkar S, Spry NA, DeBoer B, Chai M, Zeps N, Beilin M, Feeney K, Nguyen NQ, Ruszkiewicz AR, Worthley C, Tan CP, Debrencini T, Chen J, Brooke-Smith ME, Papangelis V, Tang H, Barbour AP, Clouston AD, Martin P, O’Rourke TJ, Chiang A, Fawcett JW, Slater K, Yeung S, Hatzifotis M, Hodgkinson P, Christophi C, Nikfarjam M, Mountain A, Eshleman JR, Hruban RH, Maitra A, lacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Hodgin M, Scarpa A, Lawlor RT, Beghelli S, Corbo V, Scardoni M, Bassi C, Tempera MA, Biankin AV, Grimmond SM, Chang DK, Musgrove EA, Jones MD, Nourse C, Jamieson NB, Graham JS, Biankin AV, Chang DK, Jamieson NB, Graham JS, Oien K, Hair J, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM, I. Australian Pancreatic Cancer Genome, R. Garvan Institute of Medical, B. Queensland Centre for Medical Genomics / Institute for Molecular, H. Royal North Shore, H. Bankstown, H. Liverpool, H. Westmead, H. Royal Prince Alfred, Fremantle H, H. Sir Charles Gairdner, H. St John of God, H. Royal Adelaide, C. Flinders Medical, H. Greenslopes Private, P. Envoi, H. Princess Alexandria, H. Austin, B. Victorian Cancer, I. Johns Hopkins Medical, A.R.-N.C.f.A.R.o. Cancer, S.F. University of California, G. University of, G. Greater, S. Clyde National Health, Whole genomes redefine the mutational landscape of pancreatic cancer, Nature, 518 (2015) 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chan-Seng-Yue M, Kim JC, Wilson GW, Ng K, Figueroa EF, O’Kane GM, Connor AA, Denroche RE, Grant RC, McLeod J, Wilson JM, Jang GH, Zhang A, Dodd A, Liang S-B, Borgida A, Chadwick D, Kalimuthu S, Lungu I, Bartlett JMS, Krzyzanowski PM, Sandhu V, Tiriac H, Froeling FEM, Karasinska JM, Topham JT, Renouf DJ, Schaeffer DF, Jones SJM, Marra MA, Laskin J, Chetty R, Stein LD, Zogopoulos G, Haibe-Kains B, Campbell PJ, Tuveson DA, Knox JJ, Fischer SE, Gallinger S, Notta F, Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution, Nature Genetics, 52 (2020) 231–240. [DOI] [PubMed] [Google Scholar]
- [66].Chen K, Qian W, Jiang Z, Cheng L, Li J, Sun L, Zhou C, Gao L, Lei M, Yan B, Cao J, Duan W, Ma Q, Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer, Mol Cancer, 16 (2017) 131–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Brereton MF, Rohm M, Shimomura K, Holland C, Tornovsky-Babeay S, Dadon D, Iberl M, Chibalina MV, Lee S, Glaser B, Dor Y, Rorsman P, Clark A, Ashcroft FM, Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells, NatCommun, 7 (2016) 13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cifarelli V, Lashinger LM, Devlin KL, Dunlap SM, Huang J, Kaaks R, Poliak MN, Hursting SD, Metformin and Rapamycin Reduce Pancreatic Cancer Growth in Obese Prediabetic Mice by Distinct MicroRNA-Regulated Mechanisms, Diabetes, 64 (2015) 1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang L, Bai YY, Yang Y, Hu F, Wang Y, Yu Z, Cheng Z, Zhou J, Diabetes mellitus stimulates pancreatic cancer growth and epithelial-mesenchymal transition-mediated metastasis via a p38 MAPK pathway, Oncotarget, 7 (2016) 38539–38550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Edderkaoui M, Xu S, Chheda C, Morvaridi S, Hu RW, Grippo PJ, Mascariñas E, Principe DR, Knudsen B, Xue J, Habtezion A, Uyeminami D, Pinkerton KE, Pandol SJ, HDAC3 mediates smoking-induced pancreatic cancer, Oncotarget, 7 (2016) 7747–7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kumar S, Torres MP, Kaur S, Rachagani S, Joshi S, Johansson SL, Momi N, Baine MJ, Gilling CE, Smith LM, Wyatt TA, Jain M, Joshi SS, Batra SK, Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages, Oncogene, 34 (2015) 2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Xu S, Chheda C, Ouhaddi Y, Benhaddou H, Bourhim M, Grippo PJ, Principe DR, Mascarinas E, DeCant B, Tsukamoto H, Pandol SJ, Edderkaoui M, Characterization of Mouse Models of Early Pancreatic Lesions Induced by Alcohol and Chronic Pancreatitis, Pancreas, 44 (2015) 882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ho WJ, Jaffee EM, Zheng L, The tumour microenvironment in pancreatic cancer — clinical challenges and opportunities, Nature Reviews Clinical Oncology, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bisht S, Feldmann G, Animal models for modeling pancreatic cancer and novel drug discovery, Expert Opinion on Drug Discovery, 14 (2019) 127–142. [DOI] [PubMed] [Google Scholar]
- [75].Saluja AK, Dudeja V, Relevance of animal models of pancreatic cancer and pancreatitis to human disease, Gastroenterology, 144 (2013) 1194–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kalapothaki V, Tzonou A, Hsieh C.-c., Toupadaki N, Karakatsani A, Trichopoulos D, Tobacco, ethanol, coffee, pancreatitis, diabetes mellitus, and cholelithiasis as risk factors for pancreatic carcinoma, Cancer Causes & Control, 4 (1993) 375–382. [DOI] [PubMed] [Google Scholar]
- [77].Maisonneuve P, Lowenfels AB, Risk factors for pancreatic cancer: a summary review of meta-analytical studies, International Journal of Epidemiology, 44 (2014) 186–198. [DOI] [PubMed] [Google Scholar]
- [78].Momi N, Kaur S, Krishn SR, Batra SK, Discovering the route from inflammation to pancreatic cancer, Minerva Gastroenterol Dietol, 58 (2012) 283–297. [PMC free article] [PubMed] [Google Scholar]
- [79].Li J.-s., Liu F, The Relationship of Acute Pancreatitis and Pancreatic Cancer, Pancreas, 48 (2019). [DOI] [PubMed] [Google Scholar]
- [80].Aigul H, Treiber M, Lesina M, Schmid RM, Mechanisms of Disease: chronic inflammation and cancer in the pancreas—a potential role for pancreatic stellate cells?, Nature Clinical Practice Gastroenterology & Hepatology, 4 (2007) 454–462. [DOI] [PubMed] [Google Scholar]
- [81].Klauss S, Schorn S, Teller S, Steenfadt H, Friess H, Ceyhan GO, Demir IE, Genetically induced vs. classical animal models of chronic pancreatitis: a critical comparison, The FASEB Journal, 32 (2018) 5778–5792. [DOI] [PubMed] [Google Scholar]
- [82].Leal AS, Liby KT, Murine Models of Pancreatitis Leading to the Development of Pancreatic Cancer, Curr Protoc Pharmacol, 83 (2018) e48. [DOI] [PubMed] [Google Scholar]
- [83].Lerch MM, Gorelick FS, Models of Acute and Chronic Pancreatitis, Gastroenterology, 144 (2013) 1180–1193. [DOI] [PubMed] [Google Scholar]
- [84].Lampel M, Kern HF, Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue, Virchows Archiv. A, Pathological anatomy and histology, 373 (1977) 97–117. [DOI] [PubMed] [Google Scholar]
- [85].Swidnicka-Siergiejko AK, Gomez-Chou SB, Cruz-Monserrate Z, Deng D, Liu Y, Huang H, Ji B, Azizian N, Daniluk J, Lu W, Wang H, Maitra A, Logsdon CD, Chronic inflammation initiates multiple forms of K-Ras-independent mouse pancreatic cancer in the absence of TP53, Oncogene, 36 (2017) 3149–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ahmadi A, Nikkhoo B, Mokarizadeh A, Rahmani M-R, Fakhari S, Mohammadi M, Jalili A, An optimised mouse model of chronic pancreatitis with a combination of ethanol and cerulein, Cent Eur J Immunol, 41 (2016) 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Steele CW, Karim SA, Foth M, Rishi L, Leach JD, Porter RJ, Nixon C, Jeffry Evans T, Carter CR, Nibbs RJ, Sansom OJ, Morton JP, CXCR2 inhibition suppresses acute and chronic pancreatic inflammation, 237 (2015) 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Komar HM, Serpa G, Kerscher C, Schwoegl E, Mace TA, Jin M, Yang M-C, Chen C-S, Bloomston M, Ostrowski MC, Hart PA, Conwell DL, Lesinski GB, Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo, Scientific Reports, 7 (2017) 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ, Phenotype and genotype of pancreatic cancer cell lines, Pancreas, 39 (2010) 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kim S-C, Shin Y-K, Kim S-W, Seo H-Y, Kwon W, Kim H, Han Y, Lee J-O, Jang J-Y, Ku J-L, Establishment and Characterization of 10 Human Pancreatic Cancer Cell Lines Including a HER2 Overexpressed Cell Line, Pancreas, 48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Knudsen ES, Balaji U, Mannakee B, Vail P, Eslinger C, Moxom C, Mansour J, Witkiewicz AK, Pancreatic cancer cell lines as patient-derived avatars: genetic characterisation and functional utility, Gut, 67 (2018) 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Swayden M, Soubeyran P, lovanna J, Upcoming Revolutionary Paths in Preclinical Modeling of Pancreatic Adenocarcinoma, Frontiers in oncology, 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Moriya C, Taniguchi H, Miyata K, Nishiyama N, Kataoka K, Imai K, Inhibition of PRDM14 expression in pancreatic cancer suppresses cancer stem-like properties and liver metastasis in mice, Carcinogenesis, 38 (2017) 638–648. [DOI] [PubMed] [Google Scholar]
- [94].Duxbury MS, Matros E, Ito H, Zinner MJ, Ashley SW, Whang EE, Systemic siRNA-mediated gene silencing: a new approach to targeted therapy of cancer, Ann Surg, 240 (2004) 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Morimoto M, Horikoshi Y, Nakaso K, Kurashiki T, Kitagawa Y, Hanaki T, Sakamoto T, Honjo S, Umekita Y, Fujiwara Y, Matsura T, Oncogenic role of TYRO3 receptor tyrosine kinase in the progression of pancreatic cancer, Cancer Letters, 470 (2020) 149–160. [DOI] [PubMed] [Google Scholar]
- [96].Santoro R, Zanotto M, Simionato F, Zecchetto C, Merz V, Cavallini C, Piro G, Sabbadini F, Boschi F, Scarpa A, Melisi D, Modulating TAK1 Expression Inhibits YAP and TAZ Oncogenic Functions in Pancreatic Cancer, Molecular cancer therapeutics, 19 (2020) 247. [DOI] [PubMed] [Google Scholar]
- [97].Lee D-E, Yoo JE, Kim J, Kim S, Kim S, Lee H, Cheong H, NEDD4L downregulates autophagy and cell growth by modulating ULK1 and a glutamine transporter, Cell Death & Disease, 11 (2020) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dosch AR, Dai X, Reyzer ML, Mehra S, Srinivasan S, Willobee BA, Kwon D, Kashikar N, Caprioli R, Merchant NB, Nagathihalli NS, Combined Src/EGFR inhibition targets STAT3 signaling and induces stromal remodeling to improve survival in pancreatic cancer, Molecular Cancer Research, (2020) molcanres.0741.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, Shemi A, Golan T, Domb AJ, Yavin E, Giladi H, Rivkin L, Simerzin A, Eliakim R, Khalaileh A, Hubert A, Lahav M, Kopelman Y, Goldin E, Dancour A, Hants Y, Arbel-Alon S, Abramovitch R, Shemi A, Galun E, Mutant KRAS is a druggable target for pancreatic cancer, Proceedings of the National Academy of Sciences, 110 (2013) 20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kumarasamy V, Ruiz A, Nambiar R, Witkiewicz AK, Knudsen ES, Chemotherapy impacts on the cellular response to CDK4/6 inhibition: distinct mechanisms of interaction and efficacy in models of pancreatic cancer, Oncogene, 39 (2020) 1831–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Biederstädt A, Hassan Z, Schneeweis C, Schick M, Schneider L, Muckenhuber A, Hong Y, Siegers G, Nilsson L, Wirth M, Dantes Z, Steiger K, Schunck K, Langston S, Lenhof HP, Coluccio A, Orben F, Slawska J, Scherger A, Saur D, Muller S, Rad R, Weichert W, Nilsson J, Reichert M, Schneider G, Keller U, SUMO pathway inhibition targets an aggressive pancreatic cancer subtype, Gut, (2020) gutjnl-2018-317856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gibson AR, Leary BR, Du J, Sarsour EH, Kalen AL, Wagner BA, Stolwijk JM, Falls-Hubert KC, Alexander MS, Carroll RS, Spitz DR, Buettner GR, Goswami PC, Cullen JJ, Dual Oxidase-Induced Sustained Generation of Hydrogen Peroxide Contributes to Pharmacologic Ascorbate-Induced Cytotoxicity, Cancer research, 80 (2020) 1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lipner MB, Peng XL, Jin C, Xu Y, Gao Y, East MP, Rashid NU, Moffitt RA, Herrera Loeza SG, Morrison AB, Golitz BT, Vaziri C, Graves LM, Johnson GL, Yeh JJ, Irreversible JNK1-JUN inhibition by JNK-IN-8 sensitizes pancreatic cancer to 5-FU/FOLFOX chemotherapy, JCI Insight, 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ricciardiello F, Gang Y, Palorini R, Li Q, Giampá M, Zhao F, You L, La Ferla B, De Vitto H, Guan W, Gu J, Zhang T, Zhao Y, Chiaradonna F, Hexosamine pathway inhibition overcomes pancreatic cancer resistance to gemcitabine through unfolded protein response and EGFR-Akt pathway modulation, Oncogene, (2020). [DOI] [PubMed] [Google Scholar]
- [105].Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R, Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer, Nature, 546 (2017) 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wang FT, Sun W, Zhang JT, Fan YZ, Cancer-associated fibroblast regulation of tumor neo-angiogenesis as a therapeutic target in cancer, Oncology letters, 17 (2019) 3055–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Whittle MC, Hingorani SR, Fibroblasts in Pancreatic Ductal Adenocarcinoma: Biological Mechanisms and Therapeutic Targets, Gastroenterology, 156 (2019) 2085–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pausch TM, Aue E, Wirsik NM, Freire Valls A, Shen Y, Radhakrishnan P, Hackert T, Schneider M, Schmidt T, Metastasis-associated fibroblasts promote angiogenesis in metastasized pancreatic cancer via the CXCL8 and the CCL2 axes, Scientific Reports, 10 (2020) 5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Raimondi G, Mato-Berciano A, Pascual-Sabater S, Rovira-Rigau M, Cuatrecasas M, Fondevila C, Sánchez-Cabús S, Begthel H, Boj SF, Clevers H, Fillat C, Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses, EBioMedicine, 56 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, Stigter ECA, Burgering B, Geurts V, Gracanin A, Bounova G, Morsink FH, Vries R, Boj S, van Es J, Offerhaus GJA, Kranenburg O, Garnett MJ, Wessels L, Cuppen E, Brosens LAA, Clevers H, Pancreatic cancer organoids recapitulate disease and allow personalized drug screening, Proceedings of the National Academy of Sciences, 116 (2019) 26580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tseng WW, Winer D, Kenkel JA, Choi O, Shain AH, Pollack JR, French R, Lowy AM, Engleman EG, Development of an Orthotopic Model of Invasive Pancreatic Cancer in an Immunocompetent Murine Host, Clinical Cancer Research, 16 (2010) 3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Miyabayashi K, Baker LA, Deschênes A, Traub B, Caligiuri G, Plenker D, Alagesan B, Belleau P, Li S, Kendall J, Jang GH, Kawaguchi RK, Somerville TDD, Tiriac H, Hwang C-I, Burkhart RA, Roberts NJ, Wood LD, Hruban RH, Gillis J, Krasnitz A, Vakoc CR, Wigler M, Notta F, Gallinger S, Park Y, Tuveson DA, Intraductal Transplantation Models of Human Pancreatic Ductal Adenocarcinoma Reveal Progressive Transition of Molecular Subtypes, Cancer discovery, 10 (2020) 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Guerin MV, Finisguerra V, Van den Eynde BJ, Bercovici N, Trautmann A, Preclinical murine tumor models: A structural and functional perspective, eLife, 9 (2020) e50740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Erstad DJ, Sojoodi M, Taylor MS, Ghoshal S, Razavi AA, Graham-O'Regan KA, Bardeesy N, Ferrone CR, Lanuti M, Caravan P, Tanabe KK, Fuchs BC, Orthotopic and heterotopic murine models of pancreatic cancer and their different responses to FOLFIRINOX chemotherapy, Disease models & mechanisms, 11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Herreros-Villanueva M, Hijona E, Cosme A, Bujanda L, Mouse models of pancreatic cancer, World J Gastroenterol, 18 (2012) 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhang W, Fan W, Rachagani S, Zhou Z, Lele SM, Batra SK, Garrison JC, Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy, Scientific Reports, 9 (2019) 11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Grbovic-Huezo O, Pitter KL, Lecomte N, Saglimbeni J, Askan G, Holm M, Melchor JP, Chandwani R, Joshi S, Haglund C, lacobuzio-Donahue CA, Chiosis G, Tammela T, Leach SD, Unbiased in vivo preclinical evaluation of anticancer drugs identifies effective therapy for the treatment of pancreatic adenocarcinoma, Proceedings of the National Academy of Sciences, 117 (2020) 30670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Maeda A, Chen Y, Bu J, Mujcic H, Wouters BG, DaCosta RS, In Vivo Imaging Reveals Significant Tumor Vascular Dysfunction and Increased Tumor Hypoxia-Inducible Factor-1α Expression Induced by High Single-Dose Irradiation in a Pancreatic Tumor Model, International Journal of Radiation Oncology*Biology*Physics, 97 (2017) 184–194. [DOI] [PubMed] [Google Scholar]
- [119].Fridman R, Benton G, Aranoutova I, Kleinman HK, Bonfil RD, Increased initiation and growth of tumor cell lines, cancer stem cells and biopsy material in mice using basement membrane matrix protein (Cultrex or Matrigel) co-injection, Nat Protoc, 7 (2012) 1138–1144. [DOI] [PubMed] [Google Scholar]
- [120].Benton G, Kleinman HK, George J, Arnaoutova I, Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells, International Journal of Cancer, 128 (2011) 1751–1757. [DOI] [PubMed] [Google Scholar]
- [121].Burrack AL, Spartz EJ, Raynor JF, Wang I, Olson M, Stromnes IM, Combination PD-1 and PD-L1 Blockade Promotes Durable Neoantigen-Specific T Cell-Mediated Immunity in Pancreatic Ductal Adenocarcinoma, Cell Rep, 28 (2019) 2140–2155.e2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hay CA, Sor R, Flowers AJ, Clendenin C, Byrne KT, Ultrasound-Guided Orthotopic Implantation of Murine Pancreatic Ductal Adenocarcinoma, JoVE, (2019) e60497. [DOI] [PubMed] [Google Scholar]
- [123].McVeigh LE, Wijetunga I, Ingram N, Marston G, Prasad R, Markham AF, Coletta PL, Development of orthotopic tumour models using ultrasound-guided intrahepatic injection, Scientific Reports, 9 (2019) 9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tuveson David A., Neoptolemos John P., Understanding Metastasis in Pancreatic Cancer: A Call for New Clinical Approaches, Cell, 148 (2012) 21–23. [DOI] [PubMed] [Google Scholar]
- [125].Yachida S, lacobuzio-Donahue CA, The Pathology and Genetics of Metastatic Pancreatic Cancer, 133 (2009) 413–422. [DOI] [PubMed] [Google Scholar]
- [126].Büchler P, Reber HA, Lavey RS, Tomlinson J, Buchler MW, Friess H, Hines OJ , Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model, The Journal of surgical research, 120 (2004) 295–303. [DOI] [PubMed] [Google Scholar]
- [127].Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M, An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer, Clinical & experimental metastasis, 21 (2004) 7–12. [DOI] [PubMed] [Google Scholar]
- [128].Higuchi T, Yokobori T, Naito T, Kakinuma C, Hagiwara S, Nishiyama M, Asao T, Investigation into metastatic processes and the therapeutic effects of gemcitabine on human pancreatic cancer using an orthotopic SUIT-2 pancreatic cancer mouse model, Oncology letters, 15 (2018) 3091–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, Jaffee E, Schulick RD, Yoshimura K, Edil B, Zheng L, A preclinical murine model of hepatic metastases, J Vis Exp, (2014) 51677–51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Enomoto T, Oda T, Aoyagi Y, Sugiura S, Nakajima M, Satake M, Noguchi M, Ohkohchi N, Consistent liver metastases in a rat model by portal injection of microencapsulated cancer cells, Cancer research, 66 (2006) 11131–11139. [DOI] [PubMed] [Google Scholar]
- [131].Mierke F, Hempel S, Distler M, Aust DE, Saeger HD, Weitz J, Welsch T, Impact of Portal Vein Involvement from Pancreatic Cancer on Metastatic Pattern After Surgical Resection, Annals of surgical oncology, 23 (2016) 730–736. [DOI] [PubMed] [Google Scholar]
- [132].Goddard ET, Fischer J, Schedin P, A Portal Vein Injection Model to Study Liver Metastasis of Breast Cancer, J Vis Exp, (2016) 54903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Enomoto T, Oda T, Aoyagi Y, Sugiura S, Nakajima M, Satake M, Noguchi M, Ohkohchi N, Consistent Liver Metastases in a Rat Model by Portal Injection of Microencapsulated Cancer Cells, Cancer research, 66 (2006) 11131. [DOI] [PubMed] [Google Scholar]
- [134].Limani P, Borgeaud N, Linecker M, Tschuor C, Kachaylo E, Schlegel A, Jang J-H, Ungethum U, Montani M, Graf R, Humar B, Clavien P-A, Selective portal vein injection for the design of syngeneic models of liver malignancy, American Journal of Physiology- Gastrointestinal and Liver Physiology, 310 (2016) G682–G688. [DOI] [PubMed] [Google Scholar]
- [135].Sugase T, Lam BQ, Danielson M, Terai M, Aplin AE, Gutkind JS, Sato T, Development and optimization of orthotopic liver metastasis xenograft mouse models in uveal melanoma, Journal of Translational Medicine, 18 (2020) 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hegde S, Krisnawan VE, Herzog BH, Zuo C, Breden MA, Knolhoff BL, Hogg GD, Tang JP, Baer JM, Mpoy C, Lee KB, Alexander KA, Rogers BE, Murphy KM, Hawkins WG, Fields RC, DeSelm CJ, Schwarz JK, DeNardo DG, Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer, Cancer cell, 37 (2020) 289–307.e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ, Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche, American journal of cancer research, 9 (2019) 1–21. [PMC free article] [PubMed] [Google Scholar]
- [138].Tan X, Sivakumar S, Bednarsch J, Wiltberger G, Kather JN, Niehues J, de Vos-Geelen J, Valkenburg-van Iersel L, Kintsler S, Roeth A, Hao G, Lang S, Coolsen ME, den Dulk M, Aberle MR, Koolen J, Gaisa NT, Olde Damink SWM, Neumann UP, Heij LR, Nerve fibers in the tumor microenvironment in neurotropic cancer-pancreatic cancer and cholangiocarcinoma, Oncogene, 40 (2021) 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Bapat AA, Hostetter G, Von Hoff DD, Han H, Perineural invasion and associated pain in pancreatic cancer, Nature Reviews Cancer, 11 (2011) 695–707. [DOI] [PubMed] [Google Scholar]
- [140].Matsuda M, Nimura Y, Perineural invasion of pancreas head carcinoma, Nihon Geka Gakkai Zasshi, 84 (1983) 719–728. [PubMed] [Google Scholar]
- [141].Pour PM, Egami H, Takiyama Y, Patterns of growth and metastases of induced pancreatic cancer in relation to the prognosis and its clinical implications, Gastroenterology, 100 (1991) 529–536. [DOI] [PubMed] [Google Scholar]
- [142].Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ, Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells, Clinical & experimental metastasis, 21 (2004) 285–292. [DOI] [PubMed] [Google Scholar]
- [143].Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA, MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion, Cancer research, 67 (2007) 10222–10229. [DOI] [PubMed] [Google Scholar]
- [144].Li J, Ma Q, Hyperglycemia promotes the perineural invasion in pancreatic cancer, Med Hypotheses, 71 (2008) 386–389. [DOI] [PubMed] [Google Scholar]
- [145].Abiatari I, Gillen S, DeOliveira T, Klose T, Bo K, Giese NA, Friess H, Kleeff J, The microtubule-associated protein MAPRE2 is involved in perineural invasion of pancreatic cancer cells, International journal of oncology, 35 (2009) 1111–1116. [DOI] [PubMed] [Google Scholar]
- [146].Ben QW, Wang JC, Liu J, Zhu Y, Yuan F, Yao WY, Yuan YZ, Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma, Annals of surgical oncology, 17 (2010) 2213–2221. [DOI] [PubMed] [Google Scholar]
- [147].Li X, Ma G, Ma Q, Li W, Liu J, Han L, Duan W, Xu Q, Liu H, Wang Z, Sun Q, Wang F, Wu E, Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells, Mol Cancer Res, 11 (2013) 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Huang C, Li Y, Guo Y, Zhang Z, Lian G, Chen Y, Li J, Su Y, Li J, Yang K, Chen S, Su H, Huang K, Zeng L, MMP1/PAR1/SP/NK1R paracrine loop modulates early perineural invasion of pancreatic cancer cells, Theranostics, 8 (2018) 3074–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Fujisawa T, Shimamura T, Goto K, Nakagawa R, Muroyama R, Ino Y, Horiuchi H, Endo I, Maeda S, Harihara Y, Nakajima A, Matsuhashi N, Kato N, Isayama H, Puri A, Suzuki A, Bellayr I, Leland P, Joshi BH, Puri RK, A Novel Role of Interleukin 13 Receptor alpha2 in Perineural Invasion and its Association with Poor Prognosis of Patients with Pancreatic Ductal Adenocarcinoma, Cancers (Basel), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Furuhashi S, Sakaguchi T, Murakami T, Fukushima M, Morita Y, Ikegami K, Kikuchi H, Setou M, Takeuchi H, Tenascin C in the Tumor-Nerve Microenvironment Enhances Perineural Invasion and Correlates With Locoregional Recurrence in Pancreatic Ductal Adenocarcinoma, Pancreas, 49 (2020) 442–454. [DOI] [PubMed] [Google Scholar]
- [151].Zhang JF, Tao LY, Yang MW, Xu DP, Jiang SH, Fu XL, Liu DJ, Huo YM, Liu W, Yang JY, Hua R, Lu P, Sun YW, CD74 promotes perineural invasion of cancer cells and mediates neuroplasticity via the AKT/EGR-1/GDNF axis in pancreatic ductal adenocarcinoma, Cancer Lett, 508 (2021) 47–58. [DOI] [PubMed] [Google Scholar]
- [152].Eibl G, Reber HA, A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer, Pancreas, 31 (2005) 258–262. [DOI] [PubMed] [Google Scholar]
- [153].Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, Aiura K, Shimazu M, Hirohashi S, Nimura Y, Sakamoto M, Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer, Clinical cancer research : an official journal of the American Association for Cancer Research, 12 (2006) 2419–2426. [DOI] [PubMed] [Google Scholar]
- [154].Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, Rhim AD, Davis BM, Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer, Proceedings of the National Academy of Sciences of the United States of America, 113 (2016) 3078–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Jurcak NR, Rucki AA, Muth S, Thompson E, Sharma R, Ding D, Zhu Q, Eshleman JR, Anders RA, Jaffee EM, Fujiwara K, Zheng L, Axon Guidance Molecules Promote Perineural Invasion and Metastasis of Orthotopic Pancreatic Tumors in Mice, Gastroenterology, 157 (2019) 838–850.e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Yang MW, Tao LY, Jiang YS, Yang JY, Huo YM, Liu DJ, Li J, Fu XL, He R, Lin C, Liu W, Zhang JF, Hua R, Li Q, Jiang SH, Hu LP, Tian GA, Zhang XX, Niu N, Lu P, Shi J, Xiao GG, Wang LW, Xue J, Zhang ZG, Sun YW, Perineural Invasion Reprograms the Immune Microenvironment through Cholinergic Signaling in Pancreatic Ductal Adenocarcinoma, Cancer research, 80 (2020) 1991–2003. [DOI] [PubMed] [Google Scholar]
- [157].Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA, Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer, Science, 324 (2009) 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA, CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) 12325–12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popović Z, Huang P, Bawendi MG, Boucher Y, Jain RK, Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels, Nat Commun, 4 (2013) 2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Hwang HJ, Oh MS, Lee DW, Kuh HJ, Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells, J Exp Clin Cancer Res, 38 (2019) 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, Ridinger-Saison M, DelGiorno KE, Antal CE, Liang G, Atkins AR, Erikson G, Sun H, Meisenhelder J, Terenziani E, Woo G, Fang L, Santisakultarm TP, Manor U, Xu R, Becerra CR, Borazanci E, Von Hoff DD, Grandgenett PM, Hollingsworth MA, Leblanc M, Umetsu SE, Collisson EA, Scadeng M, Lowy AM, Donahue TR, Reya T, Downes M, Evans RM, Wahl GM, Pawson T, Tian R, Hunter T, Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring, Nature, 569 (2019) 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Suklabaidya S, Dash P, Das B, Suresh V, Sasmal PK, Senapati S, Experimental models of pancreatic cancer desmoplasia, Lab Invest, 98 (2018) 27–40. [DOI] [PubMed] [Google Scholar]
- [163].Helms E, Onate MK, Sherman MH, Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment, Cancer discovery, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Suklabaidya S, Dash P, Das B, Suresh V, Sasmal PK, Senapati S, Experimental models of pancreatic cancer desmoplasia, Laboratory Investigation, 98 (2018) 27–40. [DOI] [PubMed] [Google Scholar]
- [165].Steer A, Cordes N, Jendrossek V, Klein D, Impact of Cancer-Associated Fibroblast on the Radiation-Response of Solid Xenograft Tumors, Front Mol Biosci, 6 (2019) 70–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Nguyen AH, Li L, Toste PA, Wu N, Donahue TR, MicroRNAs in Pancreatic Fibroblast Induced by Tumor Cells, Journal of the American College of Surgeons, 219 (2014) S130. [Google Scholar]
- [167].Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, Esaki N, Ushida K, Mii S, Shiraki Y, Ando K, Weng L, Ishihara S, Ponik SM, Conklin MW, Haga H, Nagasaka A, Miyata T, Matsuyama M, Kobayashi T, Fujii T, Yamada S, Yamaguchi J, Wang T, Woods SL, Worthley D, Shimamura T, Fujishiro M, Hirooka Y, Enomoto A, Takahashi M, Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis, Cancer research, 79 (2019) 5367–5381. [DOI] [PubMed] [Google Scholar]
- [168].Sharma NS, Gupta VK, Garrido VT, Hadad R, Durden BC, Kesh K, Giri B, Ferrantella A, Dudeja V, Saluja A, Banerjee S, Targeting tumor-intrinsic metabolic node sensitizes pancreatic cancer to anti-PD1 therapy, bioRxiv, (2019) 519462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Sharma NS, Gupta VK, Garrido VT, Hadad R, Durden BC, Kesh K, Giri B, Ferrantella A, Dudeja V, Saluja A, Banerjee S, Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy, The Journal of clinical investigation, 130 (2020) 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Pergolini I, Morales-Oyarvide V, Mino-Kenudson M, Honselmann KC, Rosenbaum MW, Nahar S, Kem M, Ferrone CR, Lillemoe KD, Bardeesy N, Ryan DP, Thayer SP, Warshaw AL, Fernandez-Del Castillo C, Liss AS, Tumor engraftment in patient-derived xenografts of pancreatic ductal adenocarcinoma is associated with adverse clinicopathological features and poor survival, PLoS One, 12 (2017) e0182855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP, In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia, Gastroenterology, 133 (2007) 1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC, Lineage tracing reveals the dynamic contribution of cells to the developing and adult pancreas, Development, 138 (2011) 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gu G, Brown JR, Melton DA, Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis, Mechanisms of Development, 120 (2003) 35–43. [DOI] [PubMed] [Google Scholar]
- [174].Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ, EMT and dissemination precede pancreatic tumor formation, Cell, 148 (2012) 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, Maitra A, Leach SD, DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer, Gastroenterology, 146 (2014) 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y, May R, Cho Y, Asfaha S, Worthley DL, Hayakawa Y, Urbanska AM, Quante M, Reichert M, Broyde J, Subramaniam PS, Remotti H, Su GH, Rustgi AK, Friedman RA, Honig B, Califano A, Houchen CW, Olive KP, Wang TC, Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis, Cell Stem Cell, 18 (2016) 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Qiu W, Remotti HE, Tang SM, Wang E, Dobberteen L, Lee Youssof A, Lee JH, Cheung EC, Su GH, Pancreatic DCLK1(+) cells originate distinctly from PDX1(+) progenitors and contribute to the initiation of intraductal papillary mucinous neoplasm in mice, Cancer Lett, 423 (2018) 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Glenn ST, Jones CA, Sexton S, LeVea CM, Caraker SM, Hajduczok G, Gross KW, Conditional deletion of p53 and Rb in the renin-expressing compartment of the pancreas leads to a highly penetrant metastatic pancreatic neuroendocrine carcinoma, Oncogene, 33 (2014) 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Farrell AS, Joly MM, Allen-Petersen BL, Worth PJ, Lanciault C, Sauer D, Link J, Pelz C, Heiser LM, Morton JP, Muthalagu N, Hoffman MT, Manning SL, Pratt ED, Kendsersky ND, Egbukichi N, Amery TS, Thoma MC, Jenny ZP, Rhim AD, Murphy DJ, Sansom OJ, Crawford HC, Sheppard BC, Sears RC, MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance, Nat Commun, 8 (2017) 1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Kopp JL, Dubois CL, Schaeffer DF, Samani A, Taghizadeh F, Cowan RW, Rhim AD, Stiles BL, Valasek M, Sander M, Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice, Gastroenterology, 154 (2018) 1509–1523.e1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Mameishvili E, Serafimidis I, Iwaszkiewicz S, Lesche M, Reinhardt S, Bölicke N, Buttner M, Stellas D, Papadimitropoulou A, Szabolcs M, Anastassiadis K, Dahl A, Theis F, Efstratiadis A, Gavalas A, Aldh1b1 expression defines progenitor cells in the adult pancreas and is required for Kras-induced pancreatic cancer, Proceedings of the National Academy of Sciences of the United States of America, 116 (2019) 20679–20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Tisdale MJ, Biology of cachexia, J Natl Cancer Inst, 89 (1997) 1763–1773. [DOI] [PubMed] [Google Scholar]
- [183].Henderson SE, Makhijani N, Mace TA, Pancreatic Cancer-Induced Cachexia and Relevant Mouse Models, Pancreas, 47 (2018) 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wigmore SJ, Plester CE, Richardson RA, Fearon KC, Changes in nutritional status associated with unresectable pancreatic cancer, Br J Cancer, 75 (1997) 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Ronga I, Gallucci F, Riccardi F, Uomo G, Anorexia-cachexia syndrome in pancreatic cancer: recent advances and new pharmacological approach, Adv Med Sci, 59 (2014) 1–6. [DOI] [PubMed] [Google Scholar]
- [186].Fearon KC, Glass DJ, Guttridge DC, Cancer cachexia: mediators, signaling, and metabolic pathways, Cell Metab, 16 (2012) 153–166. [DOI] [PubMed] [Google Scholar]
- [187].Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS, Role of NF-kappaB and cytokine in experimental cancer cachexia, World J Gastroenterol, 9 (2003) 1567–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA, JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia, Am J Physiol Endocrinol Metab, 303 (2012) E410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Greco SH, Tomkotter L, Vahle AK, Rokosh R, Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A, Zambirinis C, Mohaimin T, Rendon M, Levie E, Pansari M, Torres-Hernandez A, Daley D, Barilla R, Pachter HL, Tippens D, Malik H, Boutajangout A, Wisniewski T, Miller G, TGF-beta Blockade Reduces Mortality and Metabolic Changes in a Validated Murine Model of Pancreatic Cancer Cachexia, PLoS One, 10 (2015) e0132786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Yakovenko A, Cameron M, Trevino JG, Molecular therapeutic strategies targeting pancreatic cancer induced cachexia, World J Gastrointest Surg, 10 (2018) 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Pu N, Zhao G, Yin H, Li JA, Nuerxiati A, Wang D, Xu X, Kuang T, Jin D, Lou W, Wu W, CD25 and TGF-beta blockade based on predictive integrated immune ratio inhibits tumor growth in pancreatic cancer, J Transl Med, 16 (2018) 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Zhu X, Burfeind KG, Michaelis KA, Braun TP, Olson B, Pelz KR, Morgan TK, Marks DL, MyD88 signalling is critical in the development of pancreatic cancer cachexia, J Cachexia Sarcopenia Muscle, 10 (2019) 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Michaelis KA, Zhu X, Burfeind KG, Krasnow SM, Levasseur PR, Morgan TK, Marks DL, Establishment and characterization of a novel murine model of pancreatic cancer cachexia, J Cachexia Sarcopenia Muscle, 8 (2017) 824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Markov SD, Gonzalez D, Mehla K, Preclinical Models for Studying the Impact of Macrophages on Cancer Cachexia, Current Protocols in Pharmacology, 91 (2020) e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Shukla SK, Markov SD, Attri KS, Vernucci E, King RJ, Dasgupta A, Grandgenett PM, Hollingsworth MA, Singh PK, Yu F, Mehla K, Macrophages potentiate STAT3 signaling in skeletal muscles and regulate pancreatic cancer cachexia, Cancer Lett, 484 (2020) 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Leach SD, Mouse models of pancreatic cancer: the fur is finally flying!, Cancer cell, 5 (2004) 7–11. [DOI] [PubMed] [Google Scholar]
- [197].Shen R, Wang Q, Cheng S, Liu T, Jiang H, Zhu J, Wu Y, Wang L, The biological features of PanIN initiated from oncogenic Kras mutation in genetically engineered mouse models, Cancer Letters, 339 (2013) 135–143. [DOI] [PubMed] [Google Scholar]
- [198].Westphalen CB, Olive KP, Genetically engineered mouse models of pancreatic cancer, Cancer J, 18 (2012) 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Guerra C, Barbacid M, Genetically engineered mouse models of pancreatic adenocarcinoma, Molecular Oncology, 7 (2013) 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Krah NM, De La O J-P, Swift GH, Hoang CQ, Willet SG, Chen Pan F, Cash GM, Bronner MP, Wright CV, MacDonald RJ, Murtaugh LC, The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma, eLife, 4 (2015) e07125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ma L, Saiyin H, LSL-KrasG12D; LSL-Trp53R172H/+; Ink4flox/+; Ptf1/p48-Cre mice are an applicable model for locally invasive and metastatic pancreatic cancer, PLOS ONE, 12 (2017) e0176844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Veite-Schmahl MJ, Joesten WC, Kennedy MA, HMGA1 expression levels are elevated in pancreatic intraepithelial neoplasia cells in the Ptf1a-Cre; LSL-KrasG12D transgenic mouse model of pancreatic cancer, British Journal of Cancer, 117 (2017) 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Feng J, Lin A, Chen X, Wang D, Wong M, Zhang G, Na J, Zhang T, Chen Z, Chen Y-T, Nancy Du Y-C, High levels of truncated RHAMM cooperate with dysfunctional p53 to accelerate the progression of pancreatic cancer, bioRxiv, (2021) 2021.2002.2019.432042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Saborowski M, Saborowski A, Morris J.P.t., Bosbach B, Dow LE, Pelletier J, Klimstra DS, Lowe SW, A modular and flexible ESC-based mouse model of pancreatic cancer, Genes & development, 28 (2014) 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Xie Y, Zhu S, Zhong M, Yang M, Sun X, Liu J, Kroemer G, Lotze M, Zeh HJ 3rd, Kang R, Tang D, Inhibition of Aurora Kinase A Induces Necroptosis in Pancreatic Carcinoma, Gastroenterology, 153 (2017) 1429–1443.e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Humpton TJ, Alagesan B, DeNicola GM, Lu D, Yordanov GN, Leonhardt CS, Yao MA, Alagesan P, Zaatari MN, Park Y, Skepper JN, Macleod KF, Perez-Mancera PA, Murphy MP, Evan GI, Vousden KH, Tuveson DA, Oncogenic KRAS Induces NIX-Mediated Mitophagy to Promote Pancreatic Cancer, Cancer discovery, 9 (2019) 1268–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Hamada S, Taguchi K, Masamune A, Yamamoto M, Shimosegawa T, Nrf2 promotes mutant K-ras/p53-driven pancreatic carcinogenesis, Carcinogenesis, 38 (2017) 661–670. [DOI] [PubMed] [Google Scholar]
- [208].Das M, Shen L, Liu Q, Goodwin TJ, Huang L, Nanoparticle Delivery of RIG-I Agonist Enables Effective and Safe Adjuvant Therapy in Pancreatic Cancer, Molecular therapy : the journal of the American Society of Gene Therapy, 27 (2019) 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Na'ara S, Amit M, Gil Z, L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression, Oncogene, 38 (2019) 596–608. [DOI] [PubMed] [Google Scholar]
- [210].Chugh S, Barkeer S, Rachagani S, Nimmakayala RK, Perumal N, Pothuraju R, Atri P, Mahapatra S, Thapa I, Talmon GA, Smith LM, Yu X, Neelamegham S, Fu J, Xia L, Ponnusamy MP, Batra SK, Disruption of C1galt1 Gene Promotes Development and Metastasis of Pancreatic Adenocarcinomas in Mice, Gastroenterology, 155 (2018) 1608–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, Brunton VG, Pilarsky C, Winkler TH, Brabletz S, Stemmler MP, Brabletz T, The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer, Nature cell biology, 19 (2017) 518–529. [DOI] [PubMed] [Google Scholar]
- [212].Zhou C, Qian W, Ma J, Cheng L, Jiang Z, Yan B, Li J, Duan W, Sun L, Cao J, Wang F, Wu E, Wu Z, Ma Q, Li X, Resveratrol enhances the chemotherapeutic response and reverses the stemness induced by gemcitabine in pancreatic cancer cells via targeting SREBP1, Cell proliferation, 52 (2019) e12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Nimmakayala RK, Seshacharyulu P, Lakshmanan I, Rachagani S, Chugh S, Karmakar S, Rauth S, Vengoji R, Atri P, Talmon GA, Lele SM, Smith LM, Thapa I, Bastola D, Ouellette MM, Batra SK, Ponnusamy MP, Cigarette Smoke Induces Stem Cell Features of Pancreatic Cancer Cells via PAF1, Gastroenterology, 155 (2018) 892–908.e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Gupta S, Prajapati A, Gulati M, Gautam SK, Kumar S, Dalal V, Talmon GA, Rachagani S, Jain M, Irreversible and sustained upregulation of endothelin axis during oncogene-associated pancreatic inflammation and cancer, Neoplasia, 22 (2020) 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Nimmakayala RK, Leon F, Rachagani S, Rauth S, Nallasamy P, Marimuthu S, Shailendra GK, Chhonker YS, Chugh S, Chirravuri R, Gupta R, Mallya K, Prajapati DR, Lele SM, T CC, J LG, Grandgenett PM, Hollingsworth MA, Murry DJ, Batra SK, Ponnusamy MP, Metabolic programming of distinct cancer stem cells promotes metastasis of pancreatic ductal adenocarcinoma, Oncogene, 40 (2021) 215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA, Hingorani SR, Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas, Cancer cell, 11 (2007) 229–243. [DOI] [PubMed] [Google Scholar]
- [217].Yin X, Su J, Zhou X, Guo J, Wei W, Wang Z, K-ras-driven engineered mouse models for pancreatic cancer, Discov Med, 19 (2015) 15–21. [PubMed] [Google Scholar]
- [218].Rossi Sebastiano M, Pozzato C, Saliakoura M, Yang Z, Peng R-W, Galiè M, Oberson K, Simon H-U, Karamitopoulou E, Konstantinidou G, ACSL3–PAI-1 signaling axis mediates tumor-stroma cross-talk promoting pancreatic cancer progression, Science Advances, 6 (2020) eabb9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Mucciolo G, Curcio C, Roux C, Li WY, Capello M, Curto R, Chiarle R, Giordano D, Satolli MA, Lawlor R, Scarpa A, Lukac P, Stakheev D, Provero P, Vannucci L, Mak TW, Novelli F, Cappello P, IL17A critically shapes the transcriptional program of fibroblasts in pancreatic cancer and switches on their protumorigenic functions, Proceedings of the National Academy of Sciences, 118 (2021) e2020395118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Vohra R, Park J, Wang YN, Gravelle K, Whang S, Hwang JH, Lee D, Evaluation of pancreatic tumor development in KPC mice using multi-parametric MRI, Cancer Imaging, 18 (2018) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Kawano T, Murata M, Kang JH, Piao JS, Narahara S, Hyodo F, Hamano N, Guo J, Oguri S, Ohuchida K, Hashizume M, Ultrasensitive MRI detection of spontaneous pancreatic tumors with nanocage-based targeted contrast agent, Biomaterials, 152 (2018) 37–46. [DOI] [PubMed] [Google Scholar]
- [222].Goetze RG, Buchholz SM, Patil S, Petzold G, Ellenrieder V, Hessmann E, Neesse A, Utilizing High Resolution Ultrasound to Monitor Tumor Onset and Growth in Genetically Engineered Pancreatic Cancer Models, J Vis Exp, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gürlevik E, Fleischmann-Mundt B, Brooks J, Demir IE, Steiger K, Ribback S, Yevsa T, Woller N, Kloos A, Ostroumov D, Armbrecht N, Manns MP, Dombrowski F, Saborowski M, Kleine M, Wirth TC, Oettle H, Ceyhan GO, Esposito I, Calvisi DF, Kubicka S, Kuhnel F, Administration of Gemcitabine After Pancreatic Tumor Resection in Mice Induces an Antitumor Immune Response Mediated by Natural Killer Cells, Gastroenterology, 151 (2016) 338–350.e337. [DOI] [PubMed] [Google Scholar]
- [224].Li J, Betzler C, Lohneis P, Popp MC, Qin J, Kalinski T, Wartmann T, Bruns CJ, Zhao Y, Popp FC, The IL-17A/IL-17RA Axis Is Not Related to Overall Survival and Cancer Stem Cell Modulation in Pancreatic Cancer, Int J Mol Sci, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I, From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer, Nat Rev Clin Oncol, 17 (2020) 108–123. [DOI] [PubMed] [Google Scholar]
- [226].Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH, Therapeutic developments in pancreatic cancer: current and future perspectives, Nat Rev Gastroenterol Hepatol, 15 (2018) 333–348. [DOI] [PubMed] [Google Scholar]
- [227].Fanchon LM, Russell J, Pillarsetty N, O'Donoghue I, Gangangari K, Yu KH, Humm JL, Comparing the intra-tumoral distribution of Gemcitabine, 5-Fluorouracil, and Capecitabine in a murine model of pancreatic ductal adenocarcinoma, PLoS One, 15 (2020) e0231745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA, nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer, Cancer discovery, 2 (2012) 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Bhutiani N, Li Y, Zheng Q, Pandit H, Shi X, Chen Y, Yu Y, Pulliam ZR, Tan M, Martin RCG 2nd, Electrochemotherapy with Irreversible Electroporation and FOLFIRINOX Improves Survival in Murine Models of Pancreatic Adenocarcinoma, Annals of surgical oncology, 27 (2020) 4348–4359. [DOI] [PubMed] [Google Scholar]
- [230].Russell J, Pillarsetty N, Kramer RM, Romesser PB, Desai P, Haimovitz-Friedman A, Lowery MA, Humm JL, In Vitro and In Vivo Comparison of Gemcitabine and the Gemcitabine Analog 1-(2'-deoxy-2'-fluoroarabinofuranosyl) Cytosine (FAC) in Human Orthotopic and Genetically Modified Mouse Pancreatic Cancer Models, Mol Imaging Biol, 19 (2017) 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, Ellenrieder V, Jodrell DI, Tuveson DA, SPARC independent drug delivery and antitumour effects of <em>nab</em>-paclitaxel in genetically engineered mice, Gut, 63 (2014) 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Blanquicett C, Saif MW, Buchsbaum DJ, Eloubeidi M, Vickers SM, Chhieng DC, Carpenter MD, Sellers JC, Russo S, Diasio RB, Johnson MR, Antitumor efficacy of capecitabine and celecoxib in irradiated and lead-shielded, contralateral human BxPC-3 pancreatic cancer xenografts: clinical implications of abscopal effects, Clinical cancer research : an official journal of the American Association for Cancer Research, 11 (2005) 8773–8781. [DOI] [PubMed] [Google Scholar]
- [233].Gold DV, Modrak DE, Schutsky K, Cardillo TM, Combined 90Yttrium-DOTA-labeled PAM4 antibody radioimmunotherapy and gemcitabine radiosensitization for the treatment of a human pancreatic cancer xenograft, Int J Cancer, 109 (2004) 618–626. [DOI] [PubMed] [Google Scholar]
- [234].Ng SS, Tsao MS, Nicklee T, Hedley DW, Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice, Clinical cancer research : an official journal of the American Association for Cancer Research, 7 (2001) 3269–3275. [PubMed] [Google Scholar]
- [235].Zhang X, Galardi E, Duquette M, Lawler J, Parangi S, Antiangiogenic treatment with three thrombospondin-1 type 1 repeats versus gemcitabine in an orthotopic human pancreatic cancer model, Clinical cancer research : an official journal of the American Association for Cancer Research, 11 (2005) 5622–5630. [DOI] [PubMed] [Google Scholar]
- [236].Modi S, Kir D, Giri B, Majumder K, Arora N, Dudeja V, Banerjee S, Saluja AK, Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer, Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract, 20 (2016) 13–23; discussion 23-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH, Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer, Cancer research, 65 (2005) 9064–9072. [DOI] [PubMed] [Google Scholar]
- [238].Alisauskus R, Wong GY, Gold DV, Initial studies of monoclonal antibody PAM4 targeting to xenografted orthotopic pancreatic cancer, Cancer research, 55 (1995) 5743s–5748s. [PubMed] [Google Scholar]
- [239].Gold DV, Cardillo T, Vardi Y, Blumenthal R, Radioimmunotherapy of experimental pancreatic cancer with 131I-labeled monoclonal antibody PAM4, Int J Cancer, 71 (1997) 660–667. [DOI] [PubMed] [Google Scholar]
- [240].Yamamoto A, Sawada T, Yamashita Y, Nishihara T, Ho JJ, Kim Y, Chung KH, Radioimmunotherapy of orthotopically transplanted pancreatic cancer with 131I-labeled chimeric monoclonal antibody Nd2, Oncol Rep, 6 (1999) 179–184. [DOI] [PubMed] [Google Scholar]
- [241].Song CW, Griffin RJ, Lee Y-J, Cho H, Seo J, Park I, Kim HK, Kim DH, Kim M-S, Dusenbery KE, Cho LC, Reoxygenation and Repopulation of Tumor Cells after Ablative Hypofractionated Radiotherapy (SBRT and SRS) in Murine Tumors, Radiation Research, 192 (2019) 159–168. [DOI] [PubMed] [Google Scholar]
- [242].Taniguchi CM, Molkentine J, Fujimoto TN, Colbert LE, Deorukhkar A, Herman JM, Effect of stereotactic radiation (SBRT) on survival in a preclinical model of spontaneous pancreatic cancer with induction gemcitabine/nab-paclitaxel, Journal of Clinical Oncology, 36 (2018) 273–273. [Google Scholar]
- [243].Dobiasch S, Kampfer S, Habermehl D, Duma MN, Felix K, Strauss A, Schilling D, Wilkens JJ, Combs SE, MRI-based high-precision irradiation in an orthotopic pancreatic tumor mouse model, Strahlentherapie und Onkologie, 194 (2018) 944–952. [DOI] [PubMed] [Google Scholar]
- [244].Taylor E, Zhou J, Lindsay P, Foltz W, Cheung M, Siddiqui I, Hosni A, Amir AE, Kim J, Hill RP, Jaffray DA, Hedley DW, Quantifying Reoxygenation in Pancreatic Cancer During Stereotactic Body Radiotherapy, Scientific Reports, 10 (2020) 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Gajiwala S, Torgeson A, Garrido-Laguna I, Kinsey C, Lloyd S, Combination immunotherapy and radiation therapy strategies for pancreatic cancer-targeting multiple steps in the cancer immunity cycle, J Gastrointest Oncol, 9 (2018) 1014–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Mills BN, Connolly KA, Ye J, Murphy JD, Uccello TP, Han BJ, Zhao T, Drage MG, Murthy A, Qiu H, Patel A, Figueroa NM, Johnston CJ, Prieto PA, Egilmez NK, Belt BA, Lord EM, Linehan DC, Gerber SA, Stereotactic Body Radiation and Interleukin-12 Combination Therapy Eradicates Pancreatic Tumors by Repolarizing the Immune Microenvironment, Cell Rep, 29 (2019) 406–421.e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Rech AJ, Dada H, Kotzin JJ, Henao-Mejia J, Minn AJ, Twyman-Saint Victor C, Vonderheide RH, Radiotherapy and CD40 Activation Separately Augment Immunity to Checkpoint Blockade in Cancer, Cancer research, 78 (2018) 4282–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, Monkhorst K, Baas P, Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial, JAMA Oncol, 5 (2019) 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Yasmin-Karim S, Bruck PT, Moreau M, Kunjachan S, Chen GZ, Kumar R, Grabow S, Dougan SK, Ngwa W, Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer, Front Immunol, 9 (2018) 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Yasmin-Karim S, Bruck PT, Moreau M, Kunjachan S, Chen GZ, Kumar R, Grabow S, Dougan SK, Ngwa W, Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer, Frontiers in Immunology, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Brooks J, Fleischmann-Mundt B, Woller N, Niemann J, Ribback S, Peters K, Demir IE, Armbrecht N, Ceyhan GO, Manns MP, Wirth TC, Kubicka S, Bernhardt G, Smyth MJ, Calvisi DF, Gürlevik E, Kühnel F, Perioperative, Spatiotemporally Coordinated Activation of T and NK Cells Prevents Recurrence of Pancreatic Cancer, Cancer research, 78 (2018) 475–488. [DOI] [PubMed] [Google Scholar]
- [252].Yoshii Y, Matsumoto H, Yoshimoto M, Oe Y, Zhang MR, Nagatsu K, Sugyo A, Tsuji AB, Higashi T, (64)Cu-Intraperitoneal Radioimmunotherapy: A Novel Approach for Adjuvant Treatment in a Clinically Relevant Preclinical Model of Pancreatic Cancer, J Nucl Med, 60 (2019) 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Pfitzinger PL, Fangmann L, Wang K, Demir E, Gürlevik E, Fleischmann-Mundt B, Brooks J, D'Haese JG, Teller S, Hecker A, Jesinghaus M, Jäger C, Ren L, Istvanffy R, Kuhnel F, Friess H, Ceyhan GO, Demir IE, Indirect cholinergic activation slows down pancreatic cancer growth and tumor-associated inflammation, J Exp Clin Cancer Res, 39 (2020) 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Balachandran VP, Beatty GL, Dougan SK, Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities, Gastroenterology, 156 (2019) 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, Taunk PS, Wu N, Su W, Wu J, Ahsan A, Kurz E, Chen T, Yaboh I, Li F, Gutierrez J, Diskin B, Hundeyin M, Reilly M, Lich JD, Harris PA, Mahajan MK, Thorpe JH, Nassau P, Mosley JE, Leinwand J, Kochen Rossi JA, Mishra A, Aykut B, Glacken M, Ochi A, Verma N, Kim JI, Vasudevaraja V, Adeegbe D, Almonte C, Bagdatlioglu E, Cohen DJ, Wong KK, Bertin J, Miller G, RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer, Cancer cell, 34 (2018) 757–774.e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen H, Cuevas C, Dotson AM, Tan X, Hotes JL, Greenberg PD, Hingorani SR, T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma, Cancer cell, 28 (2015) 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Raj D, Yang MH, Rodgers D, Hampton EN, Begum J, Mustafa A, Lorizio D, Garces I, Propper D, Kench JG, Kocher HM, Young TS, Aicher A, Heeschen C, Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma, Gut, 68 (2019) 1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Kinkead HL, Hopkins A, Lutz E, Wu AA, Yarchoan M, Cruz K, Woolman S, Vithayathil T, Glickman LH, Ndubaku CO, McWhirter SM, Dubensky TW Jr., Armstrong TD, Jaffee EM, Zaidi N, Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer, JCI Insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Gopinathan A, Morton JP, Jodrell DI, Sansom OJ, GEMMs as preclinical models for testing pancreatic cancer therapies, Disease models & mechanisms, 8 (2015) 1185–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Lee JW, Komar CA, Bengsch F, Graham K, Beatty GL, Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+) ;LSL-Trp53(R172H/+) ;Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery, Curr Protoc Pharmacol, 73 (2016) 14.39.11–14.39.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Kimura K, Sawada T, Komatsu M, Inoue M, Muguruma K, Nishihara T, Yamashita Y, Yamada N, Ohira M, Hirakawa K, Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine, Clinical cancer research : an official journal of the American Association for Cancer Research, 12 (2006) 4925–4932. [DOI] [PubMed] [Google Scholar]
- [262].Larbouret C, Gaborit N, Chardes T, Coelho M, Campigna E, Bascoul-Mollevi C, Mach JP, Azria D, Robert B, Pelegrin A, In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors' down-regulation and dimers' disruption, Neoplasia, 14 (2012) 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].DeRosier LC, Huang ZQ, Sellers JC, Buchsbaum DJ, Vickers SM, Treatment with gemcitabine and TRA-8 anti-death receptor-5 mAb reduces pancreatic adenocarcinoma cell viability in vitro and growth in vivo, Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract, 10 (2006) 1291–1300; discussion 1300. [DOI] [PubMed] [Google Scholar]
- [264].Derosier LC, Vickers SM, Zinn KR, Huang Z, Wang W, Grizzle WE, Sellers J, Stockard CR Jr., Zhou T, Oliver PG, Arnoletti P, Lobuglio AF, Buchsbaum DJ, TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth, Molecular cancer therapeutics, 6 (2007) 3198–3207. [DOI] [PubMed] [Google Scholar]
- [265].Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, Compaan D, Lowman H, Hymowitz S, Ashkenazi A, Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5, Cell death and differentiation, 15 (2008) 751–761. [DOI] [PubMed] [Google Scholar]
- [266].Danielczyk A, Stahn R, Faulstich D, Loffler A, Marten A, Karsten U, Goletz S, PankoMab: a potent new generation anti-tumour MUC1 antibody, Cancer immunology, immunotherapy : CII, 55 (2006) 1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, Chao Q, Jacob S, Turchin H, Gibbs L, Phillips MD, Mudali S, Iacobuzio-Donahue C, Jaffee EM, Moreno M, Pastan I, Sass PM, Nicolaides NC, Grasso L, Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin, Cancer immunity, 7 (2007) 20. [PMC free article] [PubMed] [Google Scholar]
- [268].Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL, IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor, Clinical cancer research : an official journal of the American Association for Cancer Research, 13 (2007) 5549s–5555s. [DOI] [PubMed] [Google Scholar]
- [269].Hassan R, Williams-Gould J, Steinberg SM, Liewehr DJ, Yokokawa J, Tsang KY, Surawski RJ, Scott T, Camphausen K, Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts, Clinical cancer research : an official journal of the American Association for Cancer Research, 12 (2006) 4983–4988. [DOI] [PubMed] [Google Scholar]
- [270].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH, CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans, Science, 331 (2011) 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT, Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) 20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Dreau D, Moore LJ, Wu M, Roy LD, Dillion L, Porter T, Puri R, Momin N, Wittrup KD, Mukherjee P, Combining the Specific Anti-MUC1 Antibody TAB004 and Lip-MSA-IL-2 Limits Pancreatic Cancer Progression in Immune Competent Murine Models of Pancreatic Ductal Adenocarcinoma, Frontiers in oncology, 9 (2019) 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Song HK, Hwang DY, Use of C57BL/6N mice on the variety of immunological researches, Lab Anim Res, 33 (2017) 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A, Innate immune response in Th1- and Th2-dominant mouse strains, Shock (Augusta, Ga.), 22 (2004) 460–466. [DOI] [PubMed] [Google Scholar]
- [275].Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, Aithal A, Bhatia R, Wannemuehler MJ, Narasimhan B, Solheim JC, Batra SK, Jain M, Emerging trends in the immunotherapy of pancreatic cancer, Cancer Letters, 417 (2018) 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Shangguan A, Shang N, Figini M, Pan L, Yang J, Ma Q, Hu S, Eresen A, Sun C, Wang B, Velichko Y, Yaghmai V, Zhang Z, Prophylactic dendritic cell vaccination controls pancreatic cancer growth in a mouse model, Cytotherapy, 22 (2020) 6–15. [DOI] [PubMed] [Google Scholar]
- [277].Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E, Ziogas D, Psyrri A, Karamouzis MV, Immunotherapy for pancreatic cancer: A 2020 update, Cancer treatment reviews, 86 (2020) 102016. [DOI] [PubMed] [Google Scholar]
- [278].Gautam SK, Kumar S, Dam V, Ghersi D, Jain M, Batra SK, MUCIN-4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy, Seminars in Immunology, 47 (2020) 101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Banerjee K, Gautam SK, Kshirsagar P, Ross KA, Spagnol G, Sorgen P, Wannemuehler MJ, Narasimhan B, Solheim JC, Kumar S, Batra SK, Jain M, Amphiphilic polyanhydride-based recombinant MUC4β-nanovaccine activates dendritic cells, Genes & cancer, 10 (2019) 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O, Johns AL, Mead RS, Gill AJ, Chang DK, McKay SH, Chantrill LA, Chin VT, Chou A, Humphris JL, Pajic M, Steinmann A, Arshi M, Drury A, Froio D, Morgan A, Timpson P, Hermann D, Vennin C, Warren S, Pinese M, Wu J, Pinho AV, Mead RS, Tucker K, Andrews L, Gill AJ, Samra JS, Arena J, Pavlakis N, High HA, Mittal A, Chang DK, Biankin AV, Bailey P, Martin S, Musgrove EA, Jones MD, Nourse C, Jamieson NB, Chou A, Chantrill LA, Stoita A, Williams D, Spigelman A, Waddell N, Pearson JV, Patch A-M, Nones K, Newell F, Mukhopadhyay P, Addala V, Kazakoff S, Holmes O, Leonard C, Wood S, Xu C, Grimmond SM, Hofmann O, Wilson PJ, Christ A, Bruxner T, Asghari R, Merrett ND, Pavey D, Das A, Goodwin A, Cosman PH, Ismail K, O’Connor C, Cooper CL, Goodwin A, Grimison P, Kench JG, Sandroussi C, Lam VW, McLeod D, Nagrial AM, Kirk J, James V, Texler M, Forest C, Epari KP, Ballal M, Fletcher DR, Mukhedkar S, Zeps N, Beilin M, Feeney K, Nguyen NQ, Ruszkiewicz AR, Worthley C, Chen J, Brooke-Smith ME, Papangelis V, Clouston AD, Martin P, Barbour AP, O’Rourke TJ, Fawcett JW, Slater K, Hatzifotis M, Hodgkinson P, Nikfarjam M, Eshleman JR, Hruban RH, Wolfgang CL, Hodgin M, Scarpa A, Lawlor RT, Beghelli S, Corbo V, Scardoni M, Bassi C, Gönen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, lacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD, I. Australian Pancreatic Cancer Genome, R. Garvan Institute of Medical, H. Prince of Wales, H. Royal North Shore, G. University of, H. St Vincent’s, Q.B.M.R. Institute, C.f.C.R. University of Melbourne, I.f.M.B. University of Queensland, H. Bankstown, H. Liverpool, C.O.B.L. Royal Prince Alfred Hospital, H. Westmead, Fremantle H, H. St John of God, H. Royal Adelaide, C. Flinders Medical, P. Envoi, H. Princess Alexandria, H. Austin, I. Johns Hopkins Medical, A.R.-N.C.f.A.R.o. Cancer, Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer, Nature, 551 (2017) 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Spear S, McNeish IA, Capasso M, Generation of Orthotopic Pancreatic Tumors and Ex vivo Characterization of Tumor-Infiltrating T Cell Cytotoxicity, J Vis Exp, (2019). [DOI] [PubMed] [Google Scholar]
- [282].Morikane K, Tempero R, Sivinski CL, Kitajima S, Gendler SJ, Hollingsworth MA, Influence of organ site and tumor cell type on MUC1-specific tumor immunity, International immunology, 13 (2001) 233–240. [DOI] [PubMed] [Google Scholar]
- [283].Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ, Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model, Cancer research, 58 (1998) 315–321. [PubMed] [Google Scholar]
- [284].Huang M, Zhang W, Guo J, Wei X, Phiwpan K, Zhang J, Zhou X, Improved Transgenic Mouse Model for Studying HLA Class I Antigen Presentation, Scientific Reports, 6 (2016) 33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Zeng Y, Gao T, Zhao G, Jiang Y, Yang Y, Yu H, Kou Z, Lone Y, Sun S, Zhou Y, Generation of human MHC (HLA-A11/DR1) transgenic mice for vaccine evaluation, Hum Vaccin Immunother, 12 (2016) 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Eggel A, Wyss-Coray T, A revival of parabiosis in biomedical research, Swiss Med Wkly, 144 (2014) w13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Conboy MJ, Conboy IM, Rando TA, Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity, Aging Cell, 12 (2013) 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Kross I, Parabiosis and Tumor Growth, Cancer research, 10 (1921) 121–126. [Google Scholar]
- [289].Pradeep S, Kim SW, Wu SY, Nishimura M, Chaluvally-Raghavan P, Miyake T, Pecot CV, Kim SJ, Choi HJ, Bischoff FZ, Mayer JA, Huang L, Nick AM, Hall CS, Rodriguez-Aguayo C, Zand B, Dalton HJ, Arumugam T, Lee HJ, Han HD, Cho MS, Rupaimoole R, Mangala LS, Sehgal V, Oh SC, Liu J, Lee JS, Coleman RL, Ram P, Lopez-Berestein G, Fidler IJ, Sood AK, Hematogenous metastasis of ovarian cancer: rethinking mode of spread, Cancer cell, 26 (2014) 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Feng N, Luo JM, Guo X, The immune influence of a parabiosis model on tumour-bearing mice, Swiss Med Wkly, 148 (2018) w14678. [DOI] [PubMed] [Google Scholar]
- [291].Arina A, Idel C, Hyjek EM, Alegre ML, Wang Y, Bindokas VP, Weichselbaum RR, Schreiber H, Tumor-associated fibroblasts predominantly come from local and not circulating precursors, Proceedings of the National Academy of Sciences of the United States of America, 113 (2016) 7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Franklin RA, Li MO, Determining Leukocyte Origins Using Parabiosis in the PyMT Breast Tumor Model, Bio Protoc, 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Warren S, Carlstein RG, Steinke J, Chute RN, Island-Cell Tumors in Irradiated Rats in Parabiosis, Proc Soc Exp Biol Med, 115 (1964) 910–912. [DOI] [PubMed] [Google Scholar]
- [294].Nakamura T, Kuwai T, Kim JS, Fan D, Kim SJ, Fidler IJ, Stromal metalloproteinase-9 is essential to angiogenesis and progressive growth of orthotopic human pancreatic cancer in parabiont nude mice, Neoplasia, 9 (2007) 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, Yokoyama WM, Hawkins WG, Fields RC, Randolph GJ, DeNardo DG, Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression, Immunity, 47 (2017) 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Lee AYL, Dubois CL, Sarai K, Zarei S, Schaeffer DF, Sander M, Kopp JL, Cell of origin affects tumour development and phenotype in pancreatic ductal adenocarcinoma, Gut, 68 (2019) 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Maddipati R, Stanger BZ, Pancreatic Cancer Metastases Harbor Evidence of Polyclonality, Cancer discovery, 5 (2015) 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Bailey P, Chang DK, Nones K, Johns AL, Patch A-M, Gingras M-C, Miller DK, Christ AN, Bruxner TJC, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, lacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey U-MH, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM, I. Australian Pancreatic Cancer Genome, Genomic analyses identify molecular subtypes of pancreatic cancer, Nature, 531 (2016) 47–52. [DOI] [PubMed] [Google Scholar]
- [299].Collisson EA, Bailey P, Chang DK, Biankin AV, Molecular subtypes of pancreatic cancer, Nat Rev Gastroenterol Hepatol, 16 (2019) 207–220. [DOI] [PubMed] [Google Scholar]