Abstract
Lung transplantation has been well described for patients with coronavirus disease 2019 (COVID-19) in the acute setting, but less so for the resulting pulmonary sequelae. This report describes a case of lung transplantation for post–COVID-19 pulmonary fibrosis. A 52-year-old woman contracted COVID-19 in July 2020 and mounted a partial recovery, but she went on to have declining function over the ensuing 3 months, with development of fibrocystic lung changes. She underwent bilateral lung transplantation and recovered rapidly, was discharged home on postoperative day 14, and has done well in follow-up. This case report demonstrates that lung transplantation is an acceptable therapy for post–COVID-19 pulmonary fibrosis.
Over the past year, the lung failure and transplantation community has met unprecedented challenges in the care of patients affected by coronavirus disease 2019 (COVID-19). The pandemic has led not only to an increase in patients with acute lung injury, but also to a population of survivors with long-term sequelae of COVID-19. Many of the early lung transplants for COVID-19 were performed for patients with acute lung failure during extracorporeal membrane oxygenation support rather than for long-term effects of the disease.1, 2, 3, 4, 5, 6 Here we report a case of lung transplantation for post–COVID-19 pulmonary fibrosis.
An otherwise healthy 52-year-old woman contracted COVID-19 pneumonia in July 2020. At baseline, she worked full time and jogged several miles per day. After infection, her condition progressed to severe acute respiratory distress syndrome (ARDS), and she was treated with remdesivir, tocilizumab, and corticosteroids. She spent 7 days on bilevel positive airway pressure with a fraction of inspired oxygen (Fio 2) of 100% at rest, followed by an additional 5 weeks on continuous positive airway pressure alternating with a humidified high-flow nasal cannula (HFNC). After 6 weeks, she was discharged to a long-term assisted care facility, where she utilized HFNC with an Fio 2 of 70% at rest and bilevel positive airway pressure with an Fio 2 of 50% during short walks and time on a recumbent bicycle. The result of follow-up COVID-19 polymerase chain reaction testing was negative in August 2020, and she additionally became COVID-19 immunoglobulin G antibody positive. Unfortunately, progressive pulmonary fibrosis developed, prompting referral for consideration of advanced lung failure therapies.
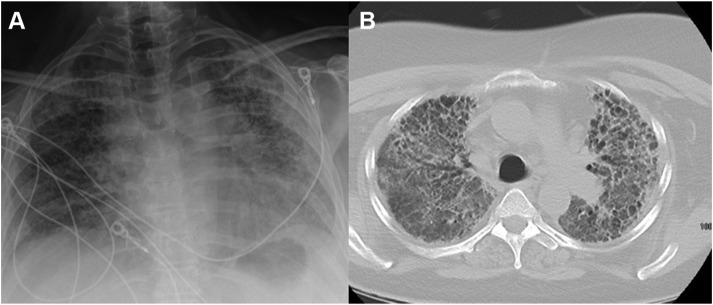
She was transferred to our institution (University of Wisconsin School of Medicine and Public Health, Madison, WI) in October 2020 for transplantation evaluation. Imaging demonstrated fibrotic changes predominantly in the bilateral upper lobes, with some apical cystic change and traction bronchiectasis with mild bilateral hilar mediastinal lymphadenopathy (Figure 1 ). Her exertional hypoxia progressed to the point of precluding safe ambulation despite HFNC at 100% Fio 2 and 55 L/min. In an effort to maintain transplantation candidacy and avoid severe deconditioning, venovenous extracorporeal membrane oxygenation (ECMO) was initiated with a right internal jugular 28-F dual-lumen cannula.
Figure 1.
Pretransplantation representative chest imaging demonstrating fibrotic changes in the bilateral upper lobes greater than in the lower lobes with some apical cystic change and traction bronchiectasis with mild bilateral hilar mediastinal lymphadenopathy.
After less than 24 hours of ECMO, she underwent bilateral sequential lung transplantation with central venoarterial ECMO support though bilateral thoracosternotomy. Intraoperatively, there were no significant pleural adhesions, but robust mediastinal and hilar lymphadenopathy with stigmata of acute inflammation was identified. The procedure was uncomplicated, and ischemic times were 268 minutes for the left lung and 391 minutes for the right lung. Postoperatively, she did not require vasopressor support. Bronchoscopy on postoperative day (POD) 1 demonstrated intact anastomoses, and her P/F ratio was >400. She was extubated early on POD 2, and the remainder of her stay was unremarkable, with quick improvement and discharge to home on POD 14. It has now been more than a year since her lung transplantation, and she has done well, with no evidence of rejection and stable pulmonary function tests: most recent forced expiratory volume in 1 second, 1.81 L (86% of predicted); and forced vital capacity, 2.26 L (89% of predicted).
The right native lung weighed 492 g and the left weighed 377 g. The bilateral lung surfaces were intact with smooth, glistening pleura. The pleura demonstrated innumerable subcentimeter nodules, or cobblestoning, bilaterally. Scattered emphysematous blebs were also present. The cut surfaces of the bilateral lungs showed diffuse consolidation involving all lung lobes. There were focal hemorrhagic changes and focal peripheral bronchiectasis without bronchopneumonia.
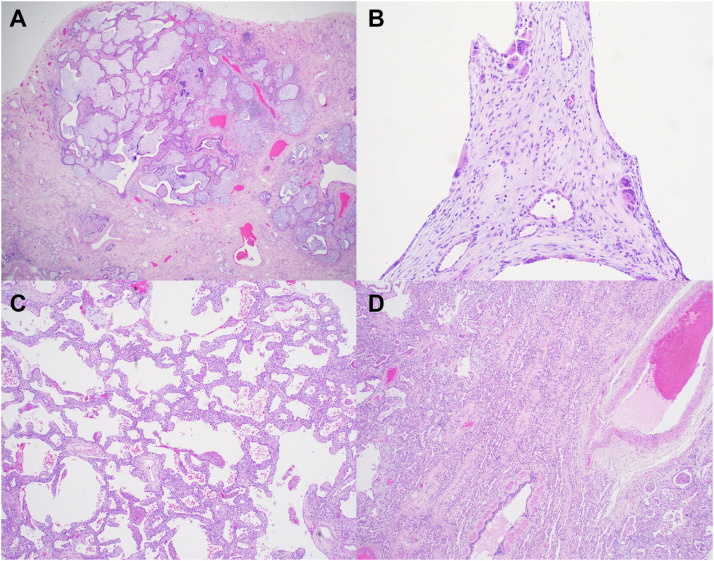
The histologic sections were notable for diffuse fibrosing interstitial pneumonia (Figure 2 ). The subpleural compartment showed more dense fibrosis and microscopic honeycomb change. Also in the peripheral lung, there were dilated cystic spaces with giant cell reaction, consistent with reaction to interstitial air. In the central portion of the lungs, the fibrosis diffusely widened the existing alveolar spaces in a nonspecific interstitial pneumonia–like pattern of fibrosis. In other areas, the fibrosis showed a sieve-like pattern with alveoli arranged in parallel slits. Overall, the pattern of fibrosis was inconsistent with a named interstitial lung disease.
Figure 2.
Representative photomicrographs from pathologic examination of the explanted lungs. (A) Peripheral section of the lung showing the subpleural area with dense fibrosis and microscopic honeycombing (hematoxylin and eosin [H&E]; original magnification ×20). (B) Peripheral cystic spaces with giant cell reaction (H&E; original magnification ×100). (C) Lung parenchyma showing a nonspecific interstitial pneumonia–like pattern of fibrosis (H&E; original magnification ×40). (D) Sieve-like pattern of fibrosis in the central lung (H&E; original magnification ×40).
Comment
At the time of this patient’s operation, fewer than 20 lung transplantations had been performed worldwide for COVID-19.1, 2, 3, 4, 5, 6 Although the natural history of COVID-19 and pulmonary remodeling after infection are poorly understood, severe pulmonary fibrosis itself is a widely accepted indication for lung transplantation. As the pandemic continues into its second year and beyond, the lung failure and transplantation community will shift to treating survivors who have long-term effects. Here we describe a case of lung transplantation performed for pulmonary fibrosis after COVID-19 pneumonia in a patient who had a successful and rapid postoperative recovery.
Initial reports of explanted lungs detailed the pathologic changes that developed in patients after the initial onset of symptoms of COVID-19.1 , 2 , 5 , 6 Some reports described generic interstitial fibrosis, whereas others mentioned that hyaline membranes were present, thus raising the possibility of organizing diffuse alveolar damage instead of true interstitial fibrosis.2 , 5 Bharat and colleagues1 reported that cystic space formation was common and that some of the cystic spaces demonstrated “complete fibrosis.” Similar to these earlier reports, our patient showed cystic space formation with giant cells, but we also identified true microscopic honeycomb change in the absence of hyaline membrane formation.
In contrast to previously published reports of lung transplantations for severe COVID-19–related ARDS, our patient did not undergo lung transplantation for ARDS but instead for irreversible post–COVID-19 fibrosis. She met listing criteria established for interstitial lung disease and was more than 3 months into her disease course. She had become seronegative for COVID-19 and antibody positive, and with no evidence of an acute superimposed disease process on her final native lung disease. Post–COVID-19 pulmonary fibrosis has been well characterized in recent literature on the basis of clinical, radiologic, and pathologic information used to describe the nonidiopathic pulmonary fibrosis associated with COVID-19.7 , 8 Our experience adds to what may become a large series of patients who undergo lung transplantation for fibrosis as a long-term effect of COVID-19, and we urge the transplantation community as a whole to explore how best to care for this important population.
References
- 1.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for pulmonary fibrosis secondary to severe COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J.Y., Qiao K., Liu F., et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J (Engl) 2020;133:1390–1396. doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han W., Zhu M., Chen J., et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann Surg. 2020;272:e33–e34. doi: 10.1097/SLA.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang C., Jaksch P., Hoda M.A., et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8:1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo W.R., Yu H., Gou J.Z., et al. Histopathologic findings in the explant lungs of a patient with COVID-19 treated with bilateral orthotopic lung transplant. Transplantation. 2020;104:e329–e331. doi: 10.1097/TP.0000000000003412. [DOI] [PubMed] [Google Scholar]
- 6.Aesif S.W., Bribriesco A.C., Yadav R., et al. Pulmonary pathology of COVID-19 following 8 weeks to 4 months of severe disease: a report of three cases, including one with bilateral lung transplantation. Am J Clin Pathol. 2020;155:506–514. doi: 10.1093/ajcp/aqaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambardar S.R., Hightower S.L., Hyprikar N.A., et al. Post-COVID-19 pulmonary fibrosis: novel sequelae of the current pandemic. J Clin Med. 2021;10:2452. doi: 10.3390/jcm10112452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweiger T, Hoetzenecker K. Commentary: post-COVID-19 acute respiratory distress syndrome and post-COVID-19 fibrosis—the new kids in town. J Thorac Cardiovasc Surg. 2022;163:869-870. [DOI] [PMC free article] [PubMed]