Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged from Wuhan in China before it spread to the entire globe. It causes coronavirus disease of 2019 (COVID-19) where mostly individuals present mild symptoms, some remain asymptomatic and some show severe lung inflammation and pneumonia in the host through the induction of a marked inflammatory ‘cytokine storm’. New and efficacious vaccines have been developed and put into clinical practice in record time, however, there is a still a need for effective treatments for those who are not vaccinated or remain susceptible to emerging SARS-CoV-2 variant strains. Despite this, effective therapeutic interventions against COVID-19 remain elusive. Here, we have reviewed potential drugs for COVID-19 classified on the basis of their mode of action. The mechanisms of action of each are discussed in detail to highlight the therapeutic targets that may help in reducing the global pandemic. The review was done up to July 2021 and the data was assessed through the official websites of WHO and CDC for collecting the information on the clinical trials. Moreover, the recent research papers were also assessed for the relevant data. The search was mainly based on keywords like Coronavirus, SARS-CoV-2, drugs (specific name of the drugs), COVID-19, clinical efficiency, safety profile, side-effects etc.This review outlines potential areas for future research into COVID-19 treatment strategies.
Keywords: Therapeutic, COVID-19, SARS-CoV-2, Cytokine storm, Anti-viral compounds
1. Introduction
Coronaviruses in the last two decades have caused serious infection and mortality in humans. In December 2019, a third novel strain of the severe acute respiratory syndrome coronavirus, SARS-CoV-2 surfaced from a seafood market in Wuhan, China. The differences in sequences in the spike protein in SARS-CoV-2 results in its enhanced binding to angiotensin-converting enzyme 2 (ACE2) in human lung cells (Asrani et al., 2020). The greater transmission rates of SARS-CoV-2 as compared to previous coronaviruses, resulted in the infection of > 179 million people and 3.8 million deaths across the globe (World Health Organization, 2020). Despite of effective guidelines and safety protocols being released in diagnosis of COVID-19 (Asrani et al., 2021), worst implications of different waves of COVID-19 were witnessed in many parts of the world including India whose health infrastructure was extremely strained with scarcity of oxygen concentrates and other essential treatment aids (Asrani et al., 2021). Many individuals also struggled to get the diagnosis done through RT-PCR within the specified time putting an undue pressure to the government and health sectors in this time of crisis (Asrani et al., 2020). This has led to severe global lockdown causing huge physiological, psychological, social and economic losses to governments and their citizens.
Infection of human airway and lung cells with SARS-CoV-2 results in acute pneumonia-like symptoms and an increased incidence of death among vulnerable older population or those with pre-existing comorbidities such as diabetes, cardiovascular and chronic obstructive pulmonary disease (COPD) (Channappanavar and Perlman, 2017, Johansen and Irving, 2020). Angiotensin-converting enzyme 2 (ACE2) expression is increased in the lower airways of susceptible older and male healthy individuals, while there is reduced expression of ACE2 in patients with asthma (Wark et al., 2021). Administration of renin-angiotensin aldosterone system inhibitors to the COVID-19 infected patients with history of smoking and comorbidity like COPD should not be encouraged as elevated expression of ACE2 is observed in these cases (Haug et al., 2020). Viruses and other invading microorganisms express multiple pathogen associated molecular patterns (PAMPs) that are recognized by pattern recognition receptors (PRRs) such as toll-like receptors (TLRs), which then elicit inflammatory and innate immune responses in the host (Hansbro et al., 2017). Innate immune responses include the release of inflammatory factors, type I interferons (IFNs) and maturation of macrophages and dendritic cells leading to adaptive immune responses (Lu et al., 2011). The SARS-CoV-2 is capable of escaping surveillance by the host innate immune system by either inducing senescence or apoptotic conditions in macrophages or through suppression of interferon type-1 (IFN-1). In the first case, the inflammatory cytokines associated with cellular senescence are produced, which in turn promotes a pro-infectious environment for virus multiplication and subsequent disease manifestations ( Fig. 1) (Fu and Cheng, 2020, Johansen and Irving, 2020). Later, infiltration of immune cells into lung tissue results in the production of more pro-inflammatory cytokines and reactive oxygen species (ROS) (Asrani and Hassan, 2020). Secondly, IFN-1 induction plays a central role as an immune defense of the host in response to a viral infection (Stetson and Medzhitov, 2006). Since, production of IFN-β and induction of host antiviral state depends upon activation of a signaling complex located on mitochondrial outer membrane (Liu et al., 2010), the ORF9b gene of coronavirus involved in targeting the mitochondria inhibits the production of IFN-1 (Shi et al., 2014).
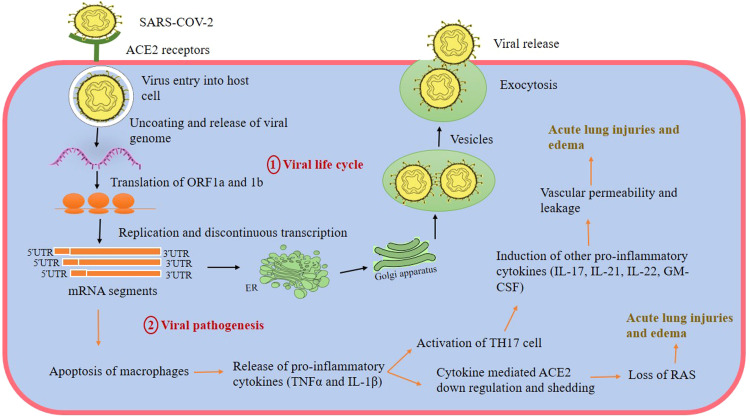
Fig. 1.
Replication cycle and inflammatory response of SARS-CoV-2 in human host cells. The entry of virus occurs through the interaction of spike glycoproteins to ACE2 on the host cell. Once, the virus enters by fusion with the host cell membrane, it starts synthesis of non-structural proteins. These proteins further cause replication and discontinuous transcription of structural genes producing fragments of mRNA. Translation of these fragments leads to the production of four structural proteins which are then assembled to form mature virus particles. Exocytosis is shown by the virus to exit from the infected host through endomembrane system. The cycle repeats when a virus approaches a new host for its replication (Brian and Baric, 2005, Shereen and Khan, 2020). The second part of the figure shows the viral pathogenesis mediated because of production of a cytokine storm. Early virus replication causes apoptosis of macrophages resulting in release of pro-inflammatory cytokines. This causes further activation of TH17 cells and down regulation of ACE2 receptors leading to more production of inflammatory cytokines and acute lung injuries. Abbreviations: ORF- Open Reading Frame, Interleukin 1β (IL-1 β), Tumor necrosis factor (TNF-α), Interleukin 17 (IL-17), Interleukin 21 (IL-21), Interleukin 22 (IL-22), Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), T helper cells (TH 17), angiotensin-converting enzyme-2 (ACE2), Renin-angiotensin system (RAS).
Adaptive immunity is induced through recognition of viral antigens presented by antigen presenting cells (APCs) to T cell (TCRs) and B cell (BCRs) receptors. In case of SARS, spike (S) and nucleocapsid (N) protein of coronavirus possesses immunogenic epitopes which are recognized by APCs for inducing humoral immunity. This leads to direct activation of B cells to produce antibodies, as well as activation of CD4+ T helper 2 (Th2) and follicular helper T cells (Tfh) that further activate B cells. CD4+ Th1 cells are also activated to produce pro-inflammatory cytokines such as IFN-γ (Mortaz et al., 2020), and CD4+ Th17 cells that produce IL-17 among other cytokines. Th17 cell activation in SARS-CoV-2 infection leads to increased vascular permeability and leakage. Finally, activated CD8+ cytotoxic T cells (CTL) are able to kill virus-infected cells directly. The two most important antibodies that are produced by B cells includes IgM and IgG. Among these, IgM can be detected after 3–6 days post-viral infection while, IgG could be detected after 8 days of viral infection (Zhuoyue et al., 2003). IgG antibodies are more likely to provide protective roles for longer duration as SARS-specific IgM antibodies were found to disappear by the end of week 12 (Li et al., 2003). In patients with MERS infection it is suggested that suppression of T cell functionality results in greater production of pro-inflammatory cytokines, free radicals and chemokines in patients; therefore, SARS-CoV-2 is likely to follow a similar destructive pattern affecting lungs and other organs (Niu et al., 2018). Therefore, in later stages of infection, SARS-CoV-2 impacts adaptive immunity by suppressing T cell functions and causing lymphopenia. The exact mechanism behind lymphocyte reduction remains unclear but few hypotheses have been laid in this direction. Some studies supports apoptosis of lymphocytes occurs in patients of severe SARS-CoV-2 infection (Qu et al., 2020) as earlier in case of SARS-CoV-1 acute infection, higher levels of plasma Fas-ligand and cleaved caspase-3-positive CD4 and CD8 lymphocytes were found in patients (Chen et al., 2006). Other studies speculate IL-β induced pyroptosis as primary cause of lymphopenia (Tay et al., 2020). Other than this, suppression of bone marrow during cytokine storm, direct infection of T-cells with SARS-CoV-2 (Wang et al., 2020) or during pneumonia, sequestration of immune cells in the infected lung airways could also be probable cause of reduced number of lymphocytes (Azkur et al., 2020).
Post SARS-CoV-2 invasion, downregulation and shedding of ACE2 receptors which leads to loss of renin-angiotensin system (RAS) function has also been observed. Following recovery, COVID-19 occurs with long-term symptoms of breathing difficulty and lethargy which may involve tissue remodeling and fibrosis although this is yet to be confirmed. Some studies have shown evidence that SARS-CoV-2 promote lung fibrosis by inducing transcriptional signatures in human epithelial cells (Xu et al., 2020).
Despite the enormous efforts of researchers to produce anti-viral drugs against COVID-19, the search for an effective treatment has been elusive. Remdesivir, though is not very effective, still has been approved as the first emergency drug for the treatment of COVID-19 in several countries and its extreme shortages necessitates the identification of further treatment options. Here, we describe in detail the different drugs that are currently in clinical trials or possess the potential to target specific SARS-CoV-2 infection ( Fig. 2). They are classified based on their mode of action and mechanisms. We also describe the previous success of drugs in overcoming other viral and non-viral infections to illustrate their potential for repurposing to combat COVID-19.
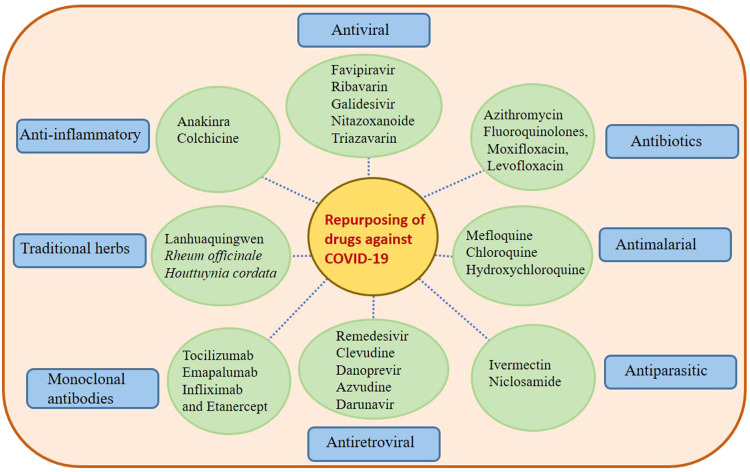
Fig. 2.
An overview and scenario of drugs considered for repurposing in the COVID-19 pandemic. The figure describes different classes of drugs that are previously known to exhibit activities against other viral and non-viral diseases. These are classified on the basis of their mode of action and may have a role in suppressing the viral loads of SARS-CoV-2 as reported by various small scale randomized studies as of now.
2. Pharmacological drugs classes and their viral targets
Different classes of pharmacological drugs were FDA approved for the treatment of earlier episodes of highly pathogenic human CoVs (Kumari et al., 2020). These include the anti-diarrheal agent loperamide hydrochloride (de Wilde et al., 2014); the antimalarial agent chloroquine diphosphate (Dyall et al., 2014); cyclophilin inhibitors such as cyclosporin A (de Wilde et al., 2013); the kinase inhibitor Wortmannin (Kindrachuk et al., 2015) and the neurotransmitter inhibitors chlorphenoxamine (Dyall et al., 2014). In vitro studies with Vero-E6 cell lines infected with mouse-adapted SARS-CoV showed > 50% inhibition against the viruses and < 30% cytotoxicity by neurotransmitter inhibitors such as chlorpromazine hydrochloride and triflupromazine hydrochloride; kinase inhibitors such as nilotinib; the DNA synthesis inhibitor gemcitabine hydrochloride; and the oestrogen inhibitor toremifene citrate (Dyall et al., 2014).
Since the distribution of effective vaccines is ongoing, and there will likely be vaccine-escape mutants and people who do not respond or remain unvaccinated there remains an important need to identify effective drugs for treating COVID-19 patients to reduce their viral burden and/or disease severity (Dhama et al., 2020). The WHO has approved various drugs for experimental trials that might become promising agents in treating SARS-CoV-2 infection ( Fig. 3). Furthermore, different classes of other drugs are currently under clinical investigation to assess their efficacy against COVID-19. These include broad spectrum antivirals, antiretrovirals, antimalarials and antiparasitic drugs, antibiotics, monoclonal antibodies, traditional medicines and immune-modulators and anti-inflammatory drugs ( Table 1).
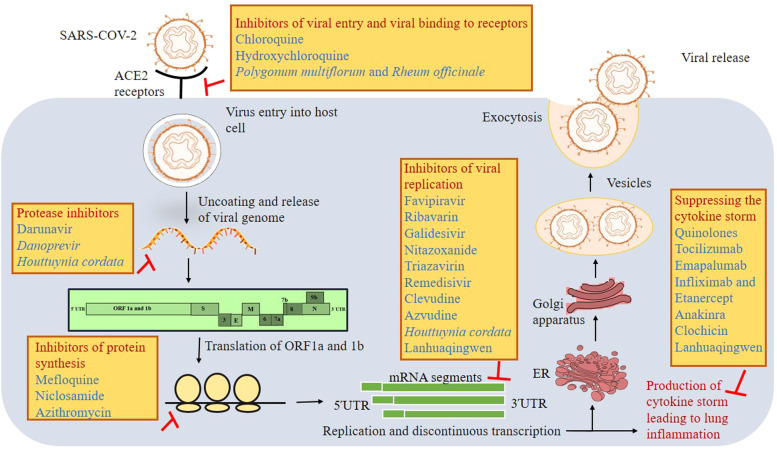
Fig. 3.
Mode of action of various drugs under different stages of viral life cycle. The figure shows different viral targets and their associated drugs within the life cycle of SARS-CoV-2. Virus entry inhibitors, protease inhibitors, protein synthesis inhibitors, viral replication inhibitors and drugs that suppresses the cytokine storm and other inflammatory responses of the host could be targeted for achieving the planetary health. The combination of these drugs may also provide better efficacy to contain and control the COVID-19.
Table 1.
Repurposing of different classes of drugs against COVID-19.
| Classes of drug | Name of drug and its current status | Mechanism of action | Activity against previously known diseases |
|---|---|---|---|
| Antiviral compounds | Favipiravir (further studies are required) |
Targets RNA-dependent RNA polymerase (RdRp) and prevents replication and transcription of virus. It many also induce lethal transition mutagenesis. |
Influenza viruses. |
| Ribavirin (further studies are required) |
It inhibits the enzyme inosine monophosphate dehydrogenase required for the conversion of guanine precursors to the guanosine and causes destabilization of viral mRNA. It is also an analog of guanosine and prevents replication of the virus. | Hepatitis C virus (HCV). | |
| Galidesivir (further studies are required) |
An analog of adenosine nucleoside which blocks the action of RNA polymerase. | Marburg virus, Yellow fever, HCV and Ebola | |
| Nitazoxanide (further studies are required) | Inhibits viral replication by inhibiting the host- regulatory pathways. It also inhibits the inflammatory cytokines. | Influenza viruses, HIV AIIDS and HCV. | |
| Triazavirin (further studies are required) |
Inhibits viral replication by being an analog of purine nucleoside. | Influenza and other respiratory infections. | |
| Antiretroviral compounds | Remdesivir (A FDA approved drug for treatment of COVID-19). |
Inhibits RNA dependent RNA polymerase required for multiplication of viruses in lung epithelial cells. It is also an analog of adenine therefore, prevents viral replication. |
Ebola virus and Nipah virus. |
| Danoprevir (further studies are required) |
Inhibits viral proteases. Possesses structural similarity with chymotrypsin like protease of SARS-CoV-2. | HCV and HIV. | |
| Azvudine (further studies are required) |
Nucleoside inhibitor of reverse transcriptase enzyme required for the replication of various RNA viruses. | HIV, HCV, HBV and EV71. | |
| Darunavir (further studies are required) |
Viral protease inhibitor. | HIV. | |
| Antimalarial compounds | Mefloquine (further studies are required) | Inhibits the protein synthesis. | Plasmodium falciparum causative agent of malaria. |
| Chloroquine (No longer considered to be effective in treating COVID-19) |
Prevents the virus binding to the cellular receptors on the host cell by inhibiting the enzyme quinone reductase 2 required for the synthesis of sialic acid (significant role in ligand reduction). | Malarial parasites. | |
| Hydroxychloroquine (No longer considered to be effective in treating COVID-19) |
Increases the pH of endosomes disrupting the fusion of virus in the host cell. Interferes with glycosylation of ACE2 receptors. |
Malarial parasites, lupus, rheumatoid arthritis etc. | |
| Antiparasitic compounds | Ivermectin (Under clinical trials but is no longer considered to be effective in treating COVID-19 as of now). |
Disturbs the nuclear transport of viral proteins by dissociating IMPα/β1 heterodimer. | Strongyloidiasis (roundworm infection). |
| Niclosamide (further studies are required) |
Inhibits the oxidative phosphorylation and stimulates the activity of adenosine triphosphatase in mitochondria. | Anticestodal (tapeworm infection). | |
| Antibiotics | Azithromycin (No longer considered to be effective in treating COVID-19) |
Macrolide antibiotic that interferes with the synthesis of proteins. | Zika virus, Rhino virus and respiratory bacterial infections. |
| Quinolones (further studies are required) |
Inhibits the production of IL-1 and TNF-α and thus suppresses the lung inflammation response. Exhibits NO regulatory and anti-oxidative effects on lung injuries. |
Pneumonia and influenza viral infection. | |
| Monoclonal antibodies | Tocilizumab (Found to be effective against COVID-19). |
Blocks both membrane-bound and soluble IL-6 receptors and their associated signaling pathways. | Rheumatoid diseases and immunotherapy in cancer patients. |
| Emapalumab (further studies are required) |
Exhibits high affinity towards INF-γ receptors and blocks its associated signaling. | Multiple organ failure caused by hyper-inflammation. | |
| Infliximab and Etanercept (further studies are required) |
Infliximab targets TNF-α and Etanercept is a protein that fuses with the TNF-α receptor causing its inactivation. | Rheumatoid arthritis and other immune disorders. | |
| Sarilumab | Inhibitor of IL-6 receptor in both soluble and membrane bound form. | Rheumatoid arthritis | |
| Immune modulators or anti-inflammatory compounds | Anakinra (further studies are required) |
Blocks the receptors of IL-1β and IL-1α and further the signaling cascade for the cytokine storm production. | Rheumatoid arthritis. |
| Colchicine (No longer considered to be effective in treating COVID-19) |
Prevents the formation and polymerization of microtubules. It also inhibits the production of TNF-α from macrophages and prevents their interaction with the endothelial cells required in initiating the process of inflammation. |
Gout, arthritis and myocardial infraction. | |
| Traditional herbs | Lanhuaqingwen | Inhibits the viral replication and regulates the cytokine storm. | Influenza viruses and SARS-CoV. |
| Polygonum multiflorum and Rheum officinale | Inhibits the spike protein of SARS-CoV-2 and prevents the viral binding to ACE2 receptors. | SARS-CoV. | |
| Houttuynia cordata | Inhibits RdRp and chymotrypsin like protease. It also enhances the effect of CD4+ and CD8+ T cells for inhibiting the replication of virus. |
SARS-CoV. |
2.1. Antiviral drugs
Antiviral drugs specifically aim to target viral entry or replication, or virus-specific enzymes by blocking essential viral factors and cellular processes required for virus survival in the host (Chen-Yu Hsu and Starkey, 2015, Durantel and Zoulim, 2016). Here, we discuss the mode of action of different antiviral drugs that might have activity against SARS-CoV-2 and describe their targets.
Favipiravir was first authorized in Japan for its activity against influenza virus. The drug is activated upon intracellular phosphoribosylation and then inhibits RNA-dependent RNA polymerase (RdRp) (Furuta, 2017). In a randomized clinical trial, favipiravir was administered to 200 patients with COVID-19 in Wuhan, China. A significant reduction in pneumonia and other COVID-19 symptoms was observed (4 days versus 11 days in the control group). Further, the treated group had a shorter time to negative SARS-CoV-2 PCR result, suggesting that there was a reduced viral load in these patients (Scavone et al., 2020). The treated group also had a quicker reduction in elevated body temperature (2.5 days versus 4.5 days with placebo) (Chang Chen et al.). Several mechanisms of action are suggested. One is the likely inclusion of the drug into the nascent viral RNA chain or binding into polymerase domains preventing nucleotide incorporation that is required for RNA replication and transcription (Furuta et al., 2017). Another study showed that favipiravir induces a lethal transition mutation of viral RNA nucleotides from C→T or C→U and G →A which might target the viral replication and survival in the host (Baranovich et al., 2013).
Ribavirin is an another antiviral drug that blocks viral replication (Crotty et al., 2001). It is a structural analog of the nucleotide guanosine which interferes with host polymerases that recognize viral RNA and induce replication (Agostini et al., 2018). This drug also inhibits the enzyme inosine monophosphate dehydrogenase required for the conversion of guanine precursors to guanosine (Markland and McQuaid, 2000, Shu and Nair, 2008). With the absence of guanosine residues on the mRNA cap, viral RNA is destabilized leading to its breakdown (Graci and Cameron, 2006). A randomized control trial with ribavirin, 500 mg twice (BID) or thrice daily (TID) is recommended in the revised Treatment Plan Edition 5 in China (Khalili and Zhu, 2020, Khalili and Zhu, 2020). Ready access and low cost makes Ribavirin the preferred choice over other drugs in this category. However, the use of this drug is controversial, as it induced anemia, depression, insomnia, and irritability in patients infected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) (Martin and Jensen, 2008). Hence, the true potential of ribavirin can only be determined in large randomized clinical trials with COVID-19 patients (Khalili and Zhu, 2020, Khalili and Zhu, 2020).
Galidesivir (BioCryst Pharmaceuticals) functions against a broad spectrum of viral infections including those caused by Marburg, yellow fever, HCV and Ebola viruses (Rele, 2020). It is a structural analog of adenosine nucleoside and, like Ribavirin, blocks the action of viral RNA polymerase (Taylor et al., 2016). The drug has been demonstrated to be potentially active against some 20 different RNA viruses including those from the families of coronaviruses, bunyaviruses, togaviruses, filoviruses, arenaviruses, flaviviruses, and paramyxoviruses (Basha, 2020, Li and De Clercq, 2020). A dose-dependent inhibition of MERS-CoV was found with increasing concentration of this drug in an in vitro study conducted with a Vero-E6 cell line (Li et al., 2020). Similar inhibition rates were observed with SARS-CoV-1 (Ataei and Hosseinjani, 2020). The high efficiency of this drug against previous CoV outbreaks suggests its potential in treating COVID-19 patients (Zhang et al., 2020). Importantly this drug is well tolerated and can be administered either orally or intramuscularly (Ataei and Hosseinjani, 2020). Larger studies are currently underway to assess the potential of Galidesivir as a therapeutic intervention in COVID-19 (Shah et al., 2020).
Nitazoxanide (NTZ), a broad range antiviral drug, is in the thiazolide class of drugs and also has antiparasitic and antibacterial properties (Zumla et al., 2015). It is FDA approved and licensed in the USA, predominantly against gastrointestinal parasites such as Giardia lamblia and Cryptosporidium parvum (Amadi et al., 2002). In vitro studies with NTZ showed suppressive effects on SARS-CoV-2 replication (Mahmoud et al., 2020), which occurs through interference with host-regulated pathways. These in vitro studies have shown promising results against influenza viruses, HIV and HCV (Rossignol and Keeffe, 2008). Mechanistically, this drug has been shown to suppress various inflammatory cytokines such as IL-2, -4, -5, -6, -8, -10 and TNF-α in peripheral blood mononuclear cells (PBMC) isolated from healthy donors and later cultured in the presence and absence of three different doses of tizoxanide (actively circulating metabolite of NTZ) i.e. 0.5, 1.0 and 10 ng/ml (Clerici et al., 2011). It has been demonstrated that the peak plasma concentration and trough concentration i.e. 4.6 and 0.8 mg/ml of this drug could be achieved in humans during phase 2b/3 clinical trials by twice daily dosing of NTZ controlled release tablets (Rossignol, 2016). Since, in vitro studies have demonstrated that the low percent inhibitory concentration (IC50) of 0.1 and 1 µg/ml is required to treat influenza and other respiratory viruses therefore, the levels of NTZ achieved in phase 2b/3 clinical trials are sufficient to be used as antiviral therapy of respiratory concern. (Rossignol, 2016). This extended-release tablet holds potential in treating viral respiratory infections by suppressing viral loads and other major symptoms associated with viral entry and replication, such as in influenza (Rossignol, 2014).
Triazavirin (TZV) is another purine nucleoside that inhibits viral replication (Loginova et al., 2014). Phase 2 clinical trials showed promising results in reducing the duration of symptoms of influenza (respiratory symptoms and fever) and related complications (pneumonia, diabetes, asthma, lung and heart diseases) associated with secondary influenza virus infections (Kiselev et al., 2012). A recent pilot randomized control trial (RCT) conducted with TZV in patients with COVID-19 showed reductions in signs of inflammation in the lungs and other vital organs as measured through radiological findings (such as hydrothorax, consolidations, abnormalities in chest); laboratory findings (including WBC, monocytes, platelets, neutrophil count, creatine kinase, CRP, blood urea nitrogen etc.) and various other serious and adverse effects (acute hepatic injury, urinary tract infection, anemia, hyperproteinemia, hyperalbuminemia etc.) (Wu et al., 2020). A total of 52 patients were recruited, half were administered TZV and the other half placebo. Although no significant difference in time to clinical improvement was recorded, it was noted that the TZV arm had lower frequency of reliance on concomitant medicines for cardiac, renal, respiratory, hepatic and coagulation support (Wu et al., 2020).
2.2. Antiretroviral drugs
Remdesivir, a drug used for the treatment of the Ebola virus and Nipah virus, has also shown some positive effects on the patients with COVID-19 (Wang et al., 2020). It is a nucleoside analog possessing antiviral activity (Szychlinska et al., 2019) which works by inhibiting the RNA-dependent RNA polymerase required for the multiplication of virus in lung epithelial cells (Gordon et al., 2020). As an analog of adenine it incorporates itself into the viral RNA and causes premature termination of transcription (Siegel et al., 2017).
This drug has been licensed for its emergency and provisional use in different countries for the patients that have been hospitalized with COVID-19 or those who are under severe effects of this infection. The countries where this is currently licensed includes United States, Japan, United Kingdom, South Korea, Taiwan, Singapore, Israel, Egypt, India, European Union and Australia (https://www.reuters.com/article/healthcoronavirus-gilead-remdesivir-idUSL1N2HD1UX). A randomized study conducted on 1062 patients from whom 541 had received remdesivir and 521 received placebo showed reduction in the median recovery rate from 15 days in the placebo group (95% confidence interval [CI], 13–18) to 10 days (95% CI, 9–11) in COVID-19 patients receiving remdesivir (Madsen, 2020). Initial assessment of COVID-19 patients determined that this drug reduced viral load significantly if it was administered before the peak of viral replication. Similar observations were noted in animal studies. Administration of remdesivir prior to 24 h of inoculation in MERS-CoV infected rhesus monkeys showed complete inhibition of viral replication and formation of respiratory lesions; while administration 12 h post-inoculation in the same model resulted in reduced viral replication, symptoms, and pulmonary lesions (de Wit et al., 2020). However, it was found not to be effective in SARS-CoV-2 infected mouse models once viral replication peaked (Sheahan et al., 2020).
Danoprevir has been licensed in China for treating HCV-infected patients since 2018. It works by inhibiting the HCV protease that is required for virus survival in the host cell. Ritonavir is a similar drug that inhibits CYP3A4 and can reduce the required concentration of Danoprevir in human plasma. It also acts against HIV proteases. The structural similarities of the chymotrypsin-like protease of SARS-CoV-2 with HCV and HIV provide an incentive to test the ability of Danoprevir to treat COVID-19. A small-scale open label interventional clinical study was performed on 11 COVID-19 patients. Following four to 12 days of treatment with Danoprevir plus the pharmacokinetic enhancer Ritonavir resulted in two consecutive negative tests for SARS-CoV-2 determined by RT-PCR of nasopharyngeal swabs. There was also improvement in lung imaging as assessed by high resolution chest CT scans with overall improvement in clinical respiratory symptoms, and normal body temperature was achieved rapidly in treated patients (Chen et al., 2020).
Azvudine (FNC) possesses broad-spectrum antiviral activity against HIV (Tyack et al., 2015), HCV (Nilsson et al., 2012), HBV (Zhou et al., 2012) and Enterovirus 71 (EV71) (Xu et al., 2020) as demonstrated in various clinical trials. It is a nucleoside inhibitor of the reverse transcriptase required for the replication of various RNA viruses (Wang et al., 2014). A block randomized clinical trial of 172 patients demonstrated the potential of FNC as an inhibitor of HIV-1 and has been approved by National Medical Products Administration (NMPA) for further determination of the effective concentrations and doses of FNC for clinical research on patients with naïve HIV infection (Ren et al., 2020). Its activity and efficacy against SARS-CoV-2 has now been demonstrated in several studies (Ren and Luo, 2020, Zhai and Ding, 2020). A pilot study was performed on 20 patients of mild COVID-19 symptoms who were randomly chosen to receive FNC and other received standard antiviral treatment (as described in Diagnosis and Treatment Program Trial version 5 or 6 guidelines) as a control group in an open label controlled clinical trial. These standard antiviral therapies included antiviral drugs, antibiotic drugs, Chinese medications, oxygen support therapies, adjuvant medications etc. The results showed conversion to a negative PCR result from the start of treatment to 11.3 days in the control group (patients receiving other standard anti-viral treatment) and 4.5 days in the patients belonging to FNC group. Moreover, no adverse effects were recorded during the clinical trial suggesting that larger scale studies are warranted to better analyze FNC’s potential in combating COVID-19 (Ren et al., 2020).
Darunavir (DRV) is another viral protease inhibitor which inhibits the HIV-1 protease and subsequently decreases HIV replication (Rabi et al., 2013). It is taken in combination with Cobicistat and Ritonavir which increase its concentration in the plasma (Gallant et al., 2016). The tolerability and efficacy of this drug have been proven and therefore, it is licensed drug for HIV patients, suggesting its possible application to COVID-19 patients as well (Orkin et al., 2013). Also, its adverse side effect profile is minimal. However, recent studies conducted on patients of COVID-19 did not show encouraging results. In a randomized experiment, group 1 received a single pill of DRV/C (800 mg of Darunavir and 150 mg of cobicistat) for 5 days while no oral antiviral drugs were given to the patients of group 2 (control group). It was observed on the fifth day after the treatment, DRV administration did not reduce the duration of conversion to negative PCR compared to controls (Chen et al., 2020). This study was on a pilot scale in which only patients with mild symptoms were enrolled (Chen et al., 2020). Similarly, another study reported disappointing results after DRV administration in SARS-CoV-2 patients with pre-existing HIV infection (Riva et al., 2020).
2.3. Antimalarial drugs
Mefloquine is a quinolone-methanol compound structurally related to quinine. The drug functions against all known malarial parasite types including most drug-resistant forms of Plasmodium falciparum (White, 1994). It functions by inhibiting protein synthesis through its interaction with the 80S ribosome of P. falciparum (Wong et al., 2017). In-silico studies on docking of Mefloquine against spike protein of SARS-CoV-2 and virus main protease (MPro) using Schrodinger software showed that it may have activity against the virus by interacting and inhibiting the main viral protease. (Sachdeva et al., 2020). Furthermore, in vitro studies with Vero-E6 cell lines show that Mefloquine-artesunate combination therapy has the greatest antiviral effect against SARS-CoV-2 as compared to the other artemisinin-based combination therapy (ACT) drugs which are used for treating malaria. It was observed that 72.1 ± 18.3% inhibition of SARS-CoV-2 was achieved with Mefloquine-artesunate concentrations of 550 mg/250 mg (equivalent blood concentration 8.3 and 5 µM respectively). (Gendrot et al., 2020).
Chloroquine (CQ) is a well-established antimalarial drug which has been used for decades. With respect to SARS-CoV-2 several mechanisms are proposed for the antiviral potential of the drug (Devaux et al., 2020). One is interference with the biosynthesis of sialic acid by directly inhibiting the quinone reductase-2 enzyme (Kwiek et al., 2004). Sialic acid plays a significant role in ligand recognition, and CQ may prevent the virus from binding to the cellular sialic acid receptors on the host cell (Varki, 1997). Another mechanism is based on endosome-mediated viral entry into host cells (Gay et al., 2012). Increase in the pH of the endosome prevents the fusion of the viral membrane to the endosome, and, therefore, interferes with viral entry (Vianney et al., 2010). Similar pH modulations affect the maturation of the viral particles (Randolph et al., 1990). The accumulation of membrane proteins of SARS-CoV-2 inside golgi bodies determines the fate of virion budding that may be affected by CQ treatment (Klumperman et al., 1994). In vitro studies have demonstrated the activity of CQ against SARS-CoV-2 (Gao et al., 2020), however it’s administration is strictly controlled in clinical trials because of potential drug interactions and side effects (Hossen et al., 2020).
Hydroxychloroquine (HCQ) is another quinolone-based anti-malarial drug which works by increasing the pH of endosomes thereby disrupting the fusion of the parasite with the host cell (Savarino et al., 2003). It has also been shown that it interferes with the glycosylation of ACE2 receptors (Vincent et al., 2005). These effects led to the analysis of its potential as a COVID-19 treatment across multiple countries. China followed by France used this drug along with azithromycin and showed positive effects in reducing viral load in COVID-19 patients (Savarino et al., 2003). In an attempt to treat increasing patient numbers in the USA, the FDA approved its use as a prophylactic agent (Eapen et al., 2020). However, it was soon observed that patients did not show improvement in treatment with this drug (Molina et al., 2020). A population-based cohort study through OpenSAFELY platform on the rheumatoid arthritis patients using hydroxychloroquine before SARS-CoV-2 infection assessed the effects of this drug on mortality (Rentsch et al., 2021). They included 30,569 patients who were taking hydroxychloroquine from six months prior to what was the start of the COVID-19 pandemic in England as well as 16,4068 patients with rheumatoid arthritis who were not taking hydroxychloroquine. They found no significant difference in COVID-19 mortality in patients with or without hydroxychloroquine treatment (0.23% mortality rate in hydroxychloroquine versus 0.22% in non-users) (Rentsch et al., 2021). Several other human clinical trials also fail to support the use of hydroxychloroquine for either treatment or prophylaxis of COVID-19 (Ferner and Aronson, 2020, Jorge, 2021).
2.4. Antiparasitic drugs
Ivermectin is an antiparasitic drug that also possesses antiviral properties against some viruses. It mainly interferes with the nuclear transport of viral proteins by dissociating importin (IMPα/β1) heterodimers (Wagstaff et al., 2012). Since nuclear transport is important for the replication of viruses, targeting this process may be a powerful approach against viral infection (Caly et al., 2012). Upon exposing SARS-CoV-2 infected vero-hSLAM cells to ivermectin at a concentration of 5 µM, a 5000 fold reduction in SARS-CoV-2 viral activity was reported 48 h post infection (Caly et al., 2020). However; Schmith and his team (Schmith et al., 2020) has shown that the approved concentration of ivermectin as a single drug is not sufficient to reach a successful clinical trial. The total bound and unbound concentrations of plasma ivermectin is still far to reach the IC50, even when the level of dose is 10x higher than the approved dose. Therefore, this drug is still under the clinical trials where some studies have shown no benefit including the randomized trials. One of the largest trails of ivermectin showing it to be the most benefit drug against COVID-19 has been retracted because of the falsified data and hence, it has been suggested by Dr. Josh Davis of University of Newcastle to “not to recommend ivermectin for routine use outside the setting of clinical trials”.
An FDA-approved anthelminthic drug Niclosamide has also been used against various bacteria, viruses and parasites known to infect humans (Fan et al., 2019). It is among the top drugs listed in WHO’s essential medicines (World Health Organization, 2019). It is recommended for use against tapeworms due to its inhibition of oxidative phosphorylation and stimulation of adenosine triphosphatase in mitochondria (Weinbach and Garbus, 1969). Immunoblot studies with Niclosamide conducted on Vero E6 cells revealed its ability to block viral replication and abolish antigen synthesis at a concentration of 1.56 µM (Wu et al., 2004). Moreover, a concentration as low as 1 μM of the drug was sufficient to suppress the cytopathic effect of SARS-CoV-1 in Vero-E6 cell lines with an EC50 of < 0.1 μM (Wen et al., 2007). Since Niclosamide is an inexpensive and well-tolerated drug it is an attractive candidate for treating COVID-19 in economically poorer countries (Mahmoud et al., 2020).
2.5. Antibiotics
Azithromycin is a macrolide antibiotic that interferes with the synthesis of proteins (Sultana et al., 2020). It is specifically used to treat respiratory bacterial infections and reduce severity of respiratory symptoms and chronic lung inflammation (Gibson and Yang, 2017, Rizk and Kalantar-Zadeh, 2020). It also possesses activity against several respiratory RNA virus infections (Schögler et al., 2015). Several earlier studies support its ability to inhibit Zika virus and rhinovirus replication (Gielen et al., 2010). It also has an in vitro antiviral effect against SARS-CoV-2 (Oldenburg and Doan, 2020) and infected bronchial epithelial cells (Gielen et al., 2010). It is a relatively safe and commonly prescribed drug (Oldenburg and Doan, 2020). Some studies suggest that azithromycin, along with HCQ, is efficacious in treating COVID-19 (Gautret and Lagier, 2020, Juurlink, 2020, Rosenberg and Dufort, 2020); while others claim that azithromycin activity in COVID-19 is restricted to the inhibition of superimposed bacterial infections rather than direct action against SARS-CoV-2 (Oldenburg and Doan, 2020, Sultana and Cutroneo, 2020). An open label randomized clinical trial in 447 adult participants in Brazil showed no benefit of azithromycin on clinical outcomes including clinical status and mortality in COVID-19 (Furtado et al., 2020). This drug is now dropped from the clinical trials because of not being of much clinical importance against COVID-19.
Various other antibacterial drugs have been repurposed as potential therapies for COVID-19 (Karampela and Dalamaga, 2020). The fluoroquinolones including moxifloxacin and levofloxacin are commonly used antibiotics to treat pneumonia (Metlay et al., 2019). Recent in silico studies showed their ability to bind to the protease of SARS-CoV-2 and thus their potential activity against its replication (Marciniec et al., 2020). In vivo studies in mice model of H1N1 influenza A virus infection showed levofloxacin can scavenge oxidative stress markers (neutrophil-derived hydroxyl radicals) and nitric oxide (NO) metabolites in the lungs (Enoki et al., 2015). Fluoroquinolones inhibited the production of IL-1 and TNF-α in both in vitro and in vivo studies (H1N1 influenza A virus infection mouse model) as demonstrated by immunohistochemistry and electron spin trapping experiments. Thus, levofloxacin, a type of fluoroquinolone derivative contributed to attenuating inflammatory responses with reduced symptoms of lung inflammation associated with SARS-CoV-2 replication (Enoki et al., 2015). There are few studies of the safety profile and pharmacokinetics of moxifloxacin needed for treatment of lower respiratory tract infections. Notably, other studies highlight that various chronic conditions (cardiac arrhythmias, long term QT prolongation and tendon rupture) are associated with the long term use of quinolones (Cornett et al., 2017).
2.6. Monoclonal antibodies
Tocilizumab is a monoclonal antibody specifically designed against IL-6 receptors and their signaling pathways (Zhang et al., 2020). Since IL-6 acts as a ligand for the activation of the JAK-STAT pathway leading to T-helper cells 17 (TH-17) cell differentiation, that can lead to a cytokine storm, blocking the ligand could prevent the signaling cascade that mediates SARS-CoV-2 driven lung inflammation (Chen and Zhang, 2020, Chen and Zhang, 2020). Clinical studies show encouraging results in patients with COVID-19 including improved oxygenation capacity, reduced respiratory symptoms and fever (Michot et al., 2020). A retrospective analysis of changes in clinical manifestations, laboratory examinations, and CT scan images of 21 patients with severe COVID-19, showed improved recovery and reduced death following the administration of tocilizumab in comparison to the patients who received routine treatment for a week before tocilizumab administration. This routine treatment did not improve the patient’s deteriorating conditions rather led to hypoxemia, sustained fever and worsening of CT scan results and hence, TCZ was given in these patients later to judge its efficacy. It was observed that following TCZ administration, fever returned to normal on the first day and other symptoms reduced within 5 days. The level of supplemental oxygen intake reduced considerably (75%) among 15 out of 20 patients while one patient did not require O2 therapy. CT scan images showed absorption of lung lesion opacities in 19 patients. The percentage of lymphocytes in peripheral blood returned to normal in 52.6% patients on the fifth day of treatment. On average, patients were discharged ~15 days after tocilizumab treatment (Xu et al., 2020). Chinese authorities have approved its use for treating pneumonia associated with COVID-19 and tocilizumab-based therapy has been included in “Diagnosis and Treatment Program of COVID-19 of the National Health Commission of China” since 3th March 2020 (Fu et al., 2020).
Emapalumab is also a monoclonal antibody (IgG1) against IFN-γ (Al-Salama, 2019) and is recommended for patients with hyper-inflammatory conditions with multiple organ failure (Locatelli et al., 2020). It has been approved in the US for the treatment of hemophagocytotic lymphohistiocytosis (HLH) disorder. An open label, single group, phase 2–3 study on 34 patients suggested that it is safe in adolescent and pediatric patients suffering from HLH (Locatelli et al., 2020). Although few clinical trials have been conducted in analyzing the potential of emapalumab to combat COVID-19, the use of Emapalumab in controlling the hyper-inflammation generated upon SARS-CoV-2 replication and through apoptosis and pyroptosis of macrophages is supported (Scala and Pacelli, 2020).
Infliximab and Etanercept both inhibit TNF-α, a pro-inflammatory cytokine produced by macrophages during SARS-CoV-2 infection (Kristensen et al., 2006). Since TNF-α is one of the prominent initial cytokines released during viral infections, it is possible that its active inhibition could diminish the SARS-CoV-2 mediated lung inflammation in host cells (Asrani and Hassan, 2020).
Sarilumab is one of the other antibodies that serves to inhibit the signal transduction by binding to IL-6 receptors both in soluble and membrane bound form. It belongs to the subclass 1 of immunoglobulin G antibody (IgG1). The approval of FDA for its use as anti-RA drug by inhibiting IL-6 signaling suggests their probable use for the patients of COVID-19 (Khiali et al., 2021). A prospective study on eight COVID-19 patients in Italy was carried out where all the patients first received a standard anti-viral therapy such as hydroxychloroquine 400 mg, darunavir 800 mg, azithromycin 500 mg, enoxaparin 100 U per kg, cobicistat 150 mg (Benucci et al., 2020). After their hospital administration, these patients received three additional doses of intravenous infusions of sarilumab- 400 mg after 24 h, 200 mg after 48 h and another 200 mg after 96 h of hospital admission. Comparisons of the functional parameters of seven patients at the baseline, 96 h and after one week from the first infusion were made. Lymphocyte count, oxygen saturation/fraction of inspired oxygen, IL-6 levels increased in contrary to C-reactive protein, D-dimer, echo score, serum amyloid A and lactate dehydrogenase levels decreased. From the day of hospitalization, seven patients were discharged within the 14 days however; one patient did not show improvement in the oxygen saturation and died on the 13th day. A mild increase in the IL-6 levels occurred because of decreased IL-6 clearance after massive blockage of IL-6 receptors by this antibody.
2.7. Immune-modulators or anti-inflammatory drugs
Anakinra blocks IL-1 receptors and the synthesis of IL-1β and IL-1α, two stimulatory cytokines produced by macrophages to initiate the inflammation cycle (Zeng et al., 2020) that are oftenly associated with severe respiratory diseases like asthma, chronic obstructive pulmonary diseases (COPD) including COVID-19 (Kim et al., 2015; Kim et al., 2017). A small scale open label study of the efficacy of anakinra showed improvement in the oxygenation capacity and reduced the need for life-saving mechanical ventilation in COVID-19 patients in comparison to the patients before treatment (Aouba et al., 2020). It is administered by subcutaneous injection and sometimes induces a reaction at the injection site limiting its use (Ramírez and Cañete 2018). Some studies show serious side-effects with predisposition to infections like pneumonia, gangrene, cellulitis and herpes zoster virus with high dose (> 100 mg) treatment for rheumatoid arthritis. (Salliot et al., 2009). The German National Registry (RABBIT) showed that adverse effects linked with the administration of anakinra occur at a rate of 17.5/100 patient years while serious adverse events occurred at 3.2/100 patient years (Lampropoulos et al., 2015). Since, no serious side effects are associated with its use however; determination of optimal concentration of this drug along with close monitoring of patients is required before this could be repurposed in the treatment of COVID-19. (Aouba et al., 2020).
Colchicine is an anti-inflammatory drug recommended for the treatment of gout and arthritis (Tardif et al., 2019). Its potential is currently being explored for treating COVID-19 (Schlesinger et al., 2020). Low dose colchicine prevents the formation of microtubules required for mitosis and higher doses prevent microtubule polymerization (Bhattacharyya et al., 2008). Since microtubules play a significant role in the maintenance of cell shape, motility, cell signaling, and signal transduction, several important cellular processes are targeted at once (Ben-Chetrit and Levy, 1998). It also possesses anti-inflammatory effects on neutrophils by reducing the expression of adhesion molecules, motility and migration, thereby interfering with their interactions with endothelial cells (Li et al., 1996). It also inhibits the production of TNF-α by macrophages required to initiate inflammatory processes and prevents their interaction with the endothelial cells (Ding et al., 1990). Coronaviruses use tubulins to enter into host cells and microtubules during their replication cycle (Sims et al., 2008). The virions require the assembly of spike proteins mediated by microtubules for the formation of their viral structures (de Haan and Rottier, 2005). Various randomized open-label clinical studies are underway exploring the potential of this drug in COVID-19 (Deftereos et al., 2020) (ClinicalTrials.gov Identifier: NCT04322682). Recently, some studies have denied their efficacy in treating the COVID-19 infected patients and it has been now dropped from the list of potential clinical drug against coronavirus.
2.8. Traditional medicines
Traditional plants have an age old history of being used for the treatment of various diseases (Asrani et al., 2019). Lanhuaqingwen (LH), is a Chinese traditional medicine made up of 13 herbs. The antiviral activity of LH on SARS-CoV-2 was assessed using cytopathic effect inhibition (CPE) and plaque reduction assay in Vero E6 cells lines and further its effect was observed in the form of morphological characteristics under transmission electron microscope. Further, cell viability assay of LH on SARS-CoV-2 infected huh-7 cells showed cytotoxicity at the concentration of 600 µg/ml in comparison to 50 µM remdesivir concentration as a positive control. Similarly, the expression level of pro-inflammatory cytokines were measured in huh-7 cells infected with SARS-CoV-2 using real time quantitative PCR assays (Runfeng et al., 2020). The results exhibited decreased concentration of cytokines such as INF-α, CCL-2/MCP-1, CXCL-10/IP-10 and IL-6 in concentration dependent LH-treated cells in comparison to mock-treated huh-7 cells (Runfeng et al., 2020). LH exhibits broad-spectrum activity against influenza viruses and SARS-CoV-1 by impairing the nuclear export of the viral ribonucleoprotein and thus inhibiting the viral replication. The suppression of virus induced NF-kβ activation was also observed followed by alleviation of virus-induced gene expression of cytokines such as IP-10, IL-6, Il-8, MCP-1, MIP1A, TNF-α, those particularly involved in the COVID-19 cytokine storm, in dose dependent manner (Ding et al., 2017). This medicine has been recommended for use by 20 health commissions across China and the National Administration of Traditional Chinese Medicine. It is also incorporated in the Guidelines for the Diagnosis and Treatment of Novel Coronavirus Pneumonia Trials issued by the National Health Commission of the People’s Republic of China (Runfeng et al., 2020). In previous studies, the IC50 for anti-influenza activity of LH was 200–2000 µg/ml (Ding et al., 2017). LH has antiviral activity against SARS-CoV-2 of 411.2 µg/ml IC50 by CPE assay. Transmission electron microscopy studies of the virus after exposure to 600 µg/ml of LH showed decreased assembly of virus particles at the plasma membrane, plasma vesicles and cytoplasm of cells infected with SARS-CoV-2. The in vitro studies on cytokine profile of huh-7 cells infected with SARS-CoV-2 also showed the change by suppression of cytokine gene expression following intake of LH in a dose-dependent manner (600 µg/ml) (Runfeng et al., 2020).
Various other traditional herbs from different geographical locations are being studied to identify their potential role in treating COVID-19 (Mirzaie et al., 2020). Microorganisms also play an important role in modulating plant bioactive compounds for metabolic and immune fitness (Shinde et al., 2020). Plants such as Pyrrosia lingua, Lindera aggregata, Lycoris radiata, and Artemisia annua showed anti-SARS-CoV-1 activity at 2.4–88.2 μg/ml (Li et al., 2005). The plant extract of Lycoris radiate which when subjected to fractionation, purification and CPE/MTS viability assays led to the extraction of a phytochemical compound called Lycorine. This compound exhibited an anti-SARS-CoV-2 ability by showing half maximal effective concentration (EC50) value of 15.7 ± 1.2 mM; cytotoxic concentration (CC50) value of 1498.0 ± 912.0 mM in cytotoxicity assay and selective index greater than 900. This supports its use as an excellent candidate for anti-viral activity (Lau et al., 2008). Other medicinal compounds like Polygonum multiflorum and Rheum officinale inhibit interactions of the spike protein of SARS-CoV-1 and prevents its binding to ACE2 receptors (Ho et al., 2007). Aqueous extract of the traditional herb Houttuynia cordata inhibits RNA-dependent RNA polymerase (RdRp) and chymotrypsin-like protease from SARS-CoV-1 (Luo et al., 2019). It also enhances the ability of CD4+ and CD8+ T cells to inhibit the replication of viruses (Chiow et al., 2016). These traditional herbs are hypothesized to possess similar antiviral effects against SARS-CoV-2 owning to high similarity with SARS-CoV-1 (Nugraha et al., 2020).
2.9. Probiotics as a preventive measure against COVID-19
General health and well-being is important to prevent or tackle the widespread diseases by naturally boosting the immunity of an individual (Kurian et al., 2021). From past, the concept of probiotics and prebiotics have continued to shape the lives of individuals and are common household items which are taken in different forms for maintaining a good health.
Probiotics consists of live microorganisms which when administered orally benefits the human health by reconstituting the composition of gut microbiota (Gohil et al., 2021). The close association of gastrointestinal and respiratory system has also been established suggesting the alternative way of treating the infections of respiratory origin (Spagnolello et al., 2021). A bidirectional communication between gut and lung referred to as gut-lung axis has been evident which is strongly associated with immune homeostasis (Dang and Marsland, 2019, Olaimat and Aolymat, 2020).
Four clinical trials have been found to have the positive results when probiotics were used as an adjunctive treatment in COVID-19. One of the study has shown a misbalance of certain essential microbial population such as Lactobacillus and Bifidobacterium in the intestine of the COVID-19 patients and therefore, probiotics could potentially aid in restoration of these natural microbial populations (Xu et al., 2020). Another study emphasized that the large dose of probiotics helped in dissolving the symptoms associated with COVID-19 accompanied by reduction in the signs of inflammation (Wu et al., 2020).
There are number of ways through which probiotics work. They remodulate the microbial flora in the gut and prevent the entry of pathogenic microorganisms (Peng et al., 2021). Probiotics bind to the gut epithelium and undergo competitive inhibition with the pathogens who enter via gut route (Walton et al., 2021). They may release substances that modulates the permeability of intestine (Weiland-Bräuer et al., 2020). Some probiotics may result in secretion of mucin which forms a mucus layer as a part of first line defense against invading microorganisms (Din et al., 2020). They are also know to function by strengthening the epithelial barrier and in attenuating the signs of inflammation (Hiippala et al., 2018).
An another approach called bioengineered probiotic has been proposed by Verma et al. (2019) where Lactobacillus paracasei (LP) expressing secretory human ACE2 (in fusion with the non-toxin subunit B of cholera toxin) was used as a live vector for oral delivery of human ACE2. The validation of this probiotic was performed in the mouse model of diabetic retinopathy. The successful implementation of this bioengineered technology has also indicated its possible use for preventing the COVID-19. Apart from interfering with the entry of SARS-CoV-2, these probiotics might boosts an innate immunity or might be useful in controlling the dysbiosis in COVID-19 patient (Senapati et al., 2020).
3. Conclusion and future prospects
The alarming rate of occurrence of COVID-19 in different countries underscores the urgent need for effective treatments. Different classes of drugs have shown promise in treating SARS-CoV-2 infection and COVID-19 in small-scale studies. However, larger well-powered randomized controlled clinical trials are required to ensure their safety and efficacy. Even as vaccines become widely available throughout the world, SARS-CoV-2 is here to stay and cases will continue to present, therefore the quest for effective treatments remains imperative.
Funding
SSS is funded by grants from Clifford Craig Foundation Launceston General Hospital, Australia and Rebecca L. Cooper Medical Research Foundation, Australia. MIH acknowledge Science & Engineering Research Board, Department of Science and Technology, Government of India, for the financial assistance (Project no: EMR/2015/002372). PMH is funded by grants from the National Health and Medical Research Council, Medical Research Future Fund and Rainbow Foundation.
Acknowledgments
Authors sincerely thank to the Department of Science and Technology, Government of India for the FIST support (FIST program No. SR/FST/LSI-541/2012).
Conflicts of interest
S.S. Sohal reports personal fees from Chiesi outside the submitted work. All the other authors do not have any conflict of interest to declare.
References
- Agostini M.L., Andres E.L., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9:2. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salama Z.T. Emapalumab: first global approval. Drugs. 2019;79(1):99–103. doi: 10.1007/s40265-018-1046-8. [DOI] [PubMed] [Google Scholar]
- Amadi B., Mwiya M., et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360(9343):1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- Aouba A., Baldolli A., et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- Asrani P., Afzal Hussain K.N., et al. Guidelines and safety considerations in the laboratory diagnosis of SARS-CoV-2 infection: a prerequisite study for health professionals. Risk Manag. Healthc. Policy. 2021;14:379. doi: 10.2147/RMHP.S284473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrani P., Eapen M.S., et al. Diagnostic approaches in COVID-19: clinical updates. Expert Rev. Respir. Med. 2020:1–16. doi: 10.1080/17476348.2021.1823833. [DOI] [PubMed] [Google Scholar]
- Asrani P., Eapen M.S., et al. Implications of the second wave of COVID-19 in India. Lancet Respir. Med. 2021 doi: 10.1016/S2213-2600(21)00312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrani P., Hasan G.M., et al. Molecular basis of pathogenesis of coronaviruses: a comparative genomics approach to planetary health to prevent zoonotic outbreaks in the 21st century. OMICS: J. Integr. Biol. 2020 doi: 10.1089/omi.2020.0131. [DOI] [PubMed] [Google Scholar]
- Asrani P., Hassan M.I. SARS-CoV-2 mediated lung inflammatory responses in host: targeting the cytokine storm for therapeutic interventions. Mol. Cell. Biochem. 2020:1–13. doi: 10.1007/s11010-020-03935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrani P., Patial V., et al. Production and Management of Beverages. Elsevier; 2019. Production of fermented beverages: shedding light on Indian culture and traditions; pp. 409–437. [Google Scholar]
- Ataei M., Hosseinjani H. Molecular mechanisms of galidesivir as a potential antiviral treatment for COVID-19. J. Pharm. Care. 2020;8(3):150–151. [Google Scholar]
- Azkur A.K., Akdis M., et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranovich T., Wong S.-S., et al. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J. Virol. 2013;87(7):3741–3751. doi: 10.1128/JVI.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha S.H. Corona virus drugs–a brief overview of past, present and future. J. PeerScientist. 2020;2(2) [Google Scholar]
- Ben-Chetrit E., Levy M. Colchicine: 1998 update. Semin. Arthritis Rheum. 1998 doi: 10.1016/s0049-0172(98)80028-0. (Elsevier) [DOI] [PubMed] [Google Scholar]
- Benucci M., Giannasi G., et al. COVID‐19 pneumonia treated with sarilumab: a clinical series of eight patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya B., Panda D., et al. Anti‐mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008;28(1):155–183. doi: 10.1002/med.20097. [DOI] [PubMed] [Google Scholar]
- Brian D., Baric R. Coronavirus Replication and Reverse Genetics. Springer; 2005. Coronavirus genome structure and replication; pp. 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., et al. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Wagstaff K.M., et al. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antivir. Res. 2012;95(3):202–206. doi: 10.1016/j.antiviral.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Chang Chen, M., Jianying Huang, M., et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial.
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017 doi: 10.1007/s00281-017-0629-x. (Springer) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Yu Hsu A., Starkey M.R., et al. Targeting PI3K-p110α suppresses influenza virus infection in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015;191(9):1012–1023. doi: 10.1164/rccm.201501-0188OC. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhang X., et al. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang za zhi=Zhonghua Shaoshang zazhi=Chin. J. Burns. 2020;36 doi: 10.3760/cma.j.cn501120-20200224-00088. E005-E005. [DOI] [PubMed] [Google Scholar]
- Chen, H., Zhang, Z., et al., 2020. First clinical study using HCV protease inhibitor danoprevir to treat naive and experienced COVID-19 patients, medRxiv. [DOI] [PMC free article] [PubMed]
- Chen J., Xia L., et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect. Dis. 2020 doi: 10.1093/ofid/ofaa241. (Oxford University Press US) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.-F., Chang J.-C., et al. Role of vascular cell adhesion molecules and leukocyte apoptosis in the lymphopenia and thrombocytopenia of patients with severe acute respiratory syndrome (SARS) Microbes Infect. 2006;8(1):122–127. doi: 10.1016/j.micinf.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiow K., Phoon M., et al. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016;9(1):1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Trabattoni D., et al. The anti-infective Nitazoxanide shows strong immumodulating effects (155.21) Am. Assoc. Immnol. 2011 [Google Scholar]
- Cornett E., Novitch M.B., et al. Macrolide and fluoroquinolone mediated cardiac arrhythmias: clinical considerations and comprehensive review. Postgrad. Med. 2017;129(7):715–724. doi: 10.1080/00325481.2017.1362938. [DOI] [PubMed] [Google Scholar]
- Crotty S., Cameron C.E., et al. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc. Natl. Acad. Sci. USA. 2001;98(12):6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang A.T., Marsland B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Jochmans D., et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Raj V.S., et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J. Gen. Virol. 2013;94(Pt 8):1749. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., Feldmann F., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftereos S.G., Giannopoulos G., et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw. Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.13136. e2013136-e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.-M., et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Sharun K., et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din A.U., Mazhar M., et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2020 doi: 10.1016/j.biopha.2020.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A.H., Porteu F., et al. Downregulation of tumor necrosis factor receptors on macrophages and endothelial cells by microtubule depolymerizing agents. J. Exp. Med. 1990;171(3):715–727. doi: 10.1084/jem.171.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Zeng L., et al. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantel D., Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J. Hepatol. 2016;64(1):S117–S131. doi: 10.1016/j.jhep.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Dyall J., Coleman C.M., et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58(8):4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen M.S., Lu W., et al. Dysregulation of endocytic machinery and ACE2 in small airways of smokers and COPD patients can augment their susceptibility to SARS-CoV-2 (COVID-19) infections. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020 doi: 10.1152/ajplung.00437.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki Y., Ishima Y., et al. Pleiotropic effects of levofloxacin, fluoroquinolone antibiotics, against influenza virus-induced lung injury. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Xu J., et al. Dual activity of niclosamide to suppress replication of integrated HIV-1 and Mycobacterium tuberculosis (Beijing) Tuberculosis. 2019;116:S28–S33. doi: 10.1016/j.tube.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner, R., Aronson, J., 2020. Hydroxychloroquine for COVID-19: what do the clinical trials tell us, The Centre for Evidence-Based Medicine.
- Fu B., Xu X., et al. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020;18(1):1–5. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Cheng Y., et al. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020:1–6. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado R.H., Berwanger O., et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396(10256):959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Komeno T., et al. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad., Ser. B. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J.E., Daar E.S., et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3(4):e158–e165. doi: 10.1016/S2352-3018(16)00024-2. [DOI] [PubMed] [Google Scholar]
- Gao J., Tian Z., et al. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay B., Bernard E., et al. pH-dependent entry of chikungunya virus into Aedes albopictus cells. Infect., Genet. Evolut. 2012;12(6):1275–1281. doi: 10.1016/j.meegid.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Gendrot M., Duflot I., et al. Antimalarial artemisinin-based combination therapies (ACT) and COVID-19 in Africa: In vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate. Int. J. Infect. Dis. 2020;99:437–440. doi: 10.1016/j.ijid.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P.G., Yang I.A., et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- Gielen V., Johnston S.L., et al. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- Gohil K., Samson R., et al. Probiotics in the prophylaxis of COVID-19: something is better than nothing. 3 Biotech. 2021;11(1):1–10. doi: 10.1007/s13205-020-02554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., et al. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16(1):37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbro P.M., Haw T.J., et al. Toll-like receptors in COPD. Eur. Respir. Soc. 2017 doi: 10.1183/13993003.00739-2017. [DOI] [PubMed] [Google Scholar]
- Haug G., Eapen M.S., et al. Renin-angiotensin-aldosterone system inhibitors in Covid-19. N. Engl. J. Med. 2020;382(24) doi: 10.1056/NEJMc2013707. [DOI] [PubMed] [Google Scholar]
- Hiippala K., Jouhten H., et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10(8):988. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.-Y., Wu S.-L., et al. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen M.S., Barek M.A., et al. A review on current repurposing drugs for the treatment of COVID-19: reality and challenges. SN Compr. Clin. Med. 2020:1–13. doi: 10.1007/s42399-020-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen M., Irving A., et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13(6):877–891. doi: 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge A. Hydroxychloroquine in the prevention of COVID-19 mortality. Lancet Rheumatol. 2021;3(1):e2–e3. doi: 10.1016/S2665-9913(20)30390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink D.N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192(17):E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karampela I., Dalamaga M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili J.S., Zhu H., et al. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID‐19. J. Med. Virol. 2020 doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili J.S., Zhu H., et al. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID‐19. J. Med. Virol. 2020;92(7):740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiali S., Rezagholizadeh A., et al. A comprehensive review on sarilumab in COVID-19. Expert Opin. Biol. Ther. 2021;21(5):615–626. doi: 10.1080/14712598.2021.1847269. [DOI] [PubMed] [Google Scholar]
- Kim R.Y., Pinkerton J.W., et al. Role for NLRP3 inflammasome–mediated, IL-1β–dependent responses in severe, steroid-resistant asthma. Am. J. Respir. Crit. Care Med. 2017;196(3):283–297. doi: 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
- Kim R.Y., Pinkerton J.W., et al. Inflammasomes in COPD and neutrophilic asthma. Thorax. 2015;70(12):1199–1201. doi: 10.1136/thoraxjnl-2014-206736. [DOI] [PubMed] [Google Scholar]
- Kindrachuk J., Ork B., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev O., Deeva E., et al. A new antiviral drug triazavirin: results of phase II clinical trial. Vopr. Virusol. 2012;57(6):9–12. [PubMed] [Google Scholar]
- Klumperman J., Locker J.K., et al. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 1994;68(10):6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen L.E., Saxne T., et al. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res. Ther. 2006;8(6):R174. doi: 10.1186/ar2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P., Singh A., et al. Potential diagnostics and therapeutic approaches in COVID-19. Clin. Chim. Acta; Int. J. Clin. Chem. 2020;510:488–497. doi: 10.1016/j.cca.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian S.J., Unnikrishnan M.K., et al. Probiotics in prevention and treatment of COVID-19: current perspective and future prospects. Arch. Med. Res. 2021 doi: 10.1016/j.arcmed.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiek J.J., Haystead T.A., et al. Kinetic mechanism of quinone oxidoreductase 2 and its inhibition by the antimalarial quinolines. Biochemistry. 2004;43(15):4538–4547. doi: 10.1021/bi035923w. [DOI] [PubMed] [Google Scholar]
- Lampropoulos C.E., Orfanos P., et al. Adverse events and infections in patients with rheumatoid arthritis treated with conventional drugs or biologic agents: a real world study. Clin. Exp. Rheumatol. 2015;33(2):216–224. [PubMed] [Google Scholar]
- Lau K.-M., Lee K.-M., et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118(1):79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Chen X., et al. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 2003;349(5):508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- Li G., De Clercq E. Nature Publishing Group; 2020. Therapeutic Options for the 2019 Novel Coronavirus (2019-nCoV) [DOI] [PubMed] [Google Scholar]
- Li S.-y, Chen C., et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Geng M., et al. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Davis G.S., et al. Inhibition of LPS-induced tumor necrosis factor-α production by colchicine and other microtubule disrupting drugs. Immunobiology. 1996;195(4–5):624–639. doi: 10.1016/s0171-2985(96)80027-1. [DOI] [PubMed] [Google Scholar]
- Liu X.-Y., Wei B., et al. Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res. 2010;20(9):994–1011. doi: 10.1038/cr.2010.103. [DOI] [PubMed] [Google Scholar]
- Locatelli F., Jordan M.B., et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N. Engl. J. Med. 2020;382(19):1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- Loginova S., Borisevich S., et al. Investigation of triazavirin antiviral activity against tick-borne encephalitis pathogen in cell culture. Antibiotiki. 2014;59(1–2):3–5. [PubMed] [Google Scholar]
- Lu X., Pan J. a, et al. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Wang C.-Z., et al. Application of Chinese medicine in acute and critical medical conditions. Am. J. Chin. Med. 2019;47(06):1223–1235. doi: 10.1142/S0192415X19500629. [DOI] [PubMed] [Google Scholar]
- Madsen L.W. Remdesivir for the treatment of Covid-19 – final report. N. Engl. J. Med. 2020;338(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud D.B., Shitu Z., et al. Drug repurposing of nitazoxanide: can it be an effective therapy for COVID-19? J. Genet. Eng. Biotechnol. 2020;18(1):1–10. doi: 10.1186/s43141-020-00055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniec K., Beberok A., et al. Ciprofloxacin and moxifloxacin could interact with SARS-CoV-2 protease: preliminary in silico analysis. Pharmacol. Rep. 2020:1–9. doi: 10.1007/s43440-020-00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., McQuaid T., et al. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 2000;44(4):859–866. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Jensen D.M. Ribavirin in the treatment of chronic hepatitis C. J. Gastroenterol. Hepatol. 2008;23(6):844–855. doi: 10.1111/j.1440-1746.2008.05398.x. [DOI] [PubMed] [Google Scholar]
- Metlay J.P., Waterer G.W., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michot J.-M., Albiges L., et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaie A., Halaji M., et al. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19) Complement. Ther. Clin. Pract. 2020 doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.M., Delaugerre C., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020:30085–30088. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E., Tabarsi P., et al. The immune response and immunopathology of COVID-19. Front. Immunol. 2020:11. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M., Kalayanov G., et al. Discovery of 4′-azido-2′-deoxy-2′-C-methyl cytidine and prodrugs thereof: a potent inhibitor of Hepatitis C virus replication. Bioorgan. Med. Chem. Lett. 2012;22(9):3265–3268. doi: 10.1016/j.bmcl.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Niu P., Zhang S., et al. Ultrapotent human neutralizing antibody repertoires against Middle East respiratory syndrome coronavirus from a recovered patient. J. Infect. Dis. 2018;218(8):1249–1260. doi: 10.1093/infdis/jiy311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugraha R.V., Ridwansyah H., et al. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid.-Based Complement. Altern. Med. 2020:2020. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaimat A.N., Aolymat I., et al. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. npj Sci. Food. 2020;4(1):1–7. doi: 10.1038/s41538-020-00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg C.E., Doan T. Azithromycin for severe COVID-19. Lancet. 2020;396(10256):936–937. doi: 10.1016/S0140-6736(20)31863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin C., DeJesus E., et al. Final 192–week efficacy and safety of once‐daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV‐1–infected treatment‐naive patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59. doi: 10.1111/j.1468-1293.2012.01060.x. [DOI] [PubMed] [Google Scholar]
- Peng J., Zhang M., et al. Probiotics as adjunctive treatment for patients contracted COVID-19: current understanding and future needs. Front. Nutr. 2021;8:304. doi: 10.3389/fnut.2021.669808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu R., Ling Y., et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J. Med. Virol. 2020;92(9):1533–1541. doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabi S.A., Laird G.M., et al. Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J. Clin. Investig. 2013;123(9):3848–3860. doi: 10.1172/JCI67399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J., Cañete J.D. Anakinra for the treatment of rheumatoid arthritis: a safety evaluation. Expert Opin. Drug Saf. 2018;17(7):727–732. doi: 10.1080/14740338.2018.1486819. [DOI] [PubMed] [Google Scholar]
- Randolph V.B., Winkler G., et al. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174(2):450–458. doi: 10.1016/0042-6822(90)90099-d. [DOI] [PubMed] [Google Scholar]
- Rele S. Emerging outbreaks and epidemic threats: The practicality and limitations in the development and manufacturing of treatments for Coronavirus (COVID-19) Polymorphism. 2020;4:45–52. [Google Scholar]
- Ren Z., Luo H., et al. A randomized, open‐label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID‐19, a pilot study. Adv. Sci. 2020;7(19) doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch C.T., DeVito N.J., et al. Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol. 2021;3(1):e19–e27. doi: 10.1016/S2665-9913(20)30378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A., Conti F., et al. Darunavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk J.G., Kalantar-Zadeh K., et al. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020:1–26. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Dufort E.M., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J.-F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antivir. Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health. 2016;9(3):227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol, J.-F. Keeffe, E.B. 2008. Thiazolides: a new class of drugs for the treatment of chronic hepatitis B and C. [DOI] [PubMed]
- Runfeng L., Yunlong H., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva C., Wadhwa A., et al. In silico potential of approved antimalarial drugs for repurposing against COVID-19. OMICS: J. Integrative Biol. 2020;24(10):568–580. doi: 10.1089/omi.2020.0071. [DOI] [PubMed] [Google Scholar]
- Salliot C., Dougados M., et al. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann. Rheum. Dis. 2009;68(1):25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., et al. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala S., Pacelli R. Fighting the host reaction to SARS-COv-2 in critically Ill patients: the possible contribution of off-label drugs. Front. Immunol. 2020;11:1201. doi: 10.3389/fimmu.2020.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone C., Brusco S., et al. Current pharmacological treatments for COVID‐19: What’s next? Br. J. Pharmacol. 2020 doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger N., Firestein B.L., et al. Colchicine in COVID-19: an old drug, new use. Curr. Pharmacol. Rep. 2020;6(4):137–145. doi: 10.1007/s40495-020-00225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmith V.D., Zhou J., et al. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID‐19. Clin. Pharmacol. Ther. 2020;108(4):762–765. doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schögler A., Kopf B.S., et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur. Respir. J. 2015;45(2):428–439. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- Senapati S., Dash J., et al. Bioengineered probiotics to control SARS-CoV-2 infection. Res. Ideas Outcomes. 2020;6 [Google Scholar]
- Shah B., Modi P., et al. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life Sci. 2020 doi: 10.1016/j.lfs.2020.117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020 doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.-S., Qi H.-Y., et al. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193(6):3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde T., Hansbro P.M., et al. Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms. 2020;8(6):921. doi: 10.3390/microorganisms8060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Q., Nair V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med. Res. Rev. 2008;28(2):219–232. doi: 10.1002/med.20104. [DOI] [PubMed] [Google Scholar]
- Siegel D., Hui H.C., et al. ACS Publications; 2017. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo [2, 1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. [DOI] [PubMed] [Google Scholar]
- Sims A.C., Burkett S.E., et al. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res. 2008;133(1):33–44. doi: 10.1016/j.virusres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolello O., Pinacchio C., et al. Targeting microbiome: an alternative strategy for fighting SARS-Cov-2 infection. Chemotherapy. 2021;66(1–2):24–32. doi: 10.1159/000515344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D.B., Medzhitov R. Type I interferons in host defense. Immunity. 2006;25(3):373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Sultana J., Cutroneo P.M., et al. Azithromycin in COVID-19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 2020;43(8):691–698. doi: 10.1007/s40264-020-00976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychlinska M.A., Castrogiovanni P., et al. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019;58(2):565–581. doi: 10.1007/s00394-018-1632-2. [DOI] [PubMed] [Google Scholar]
- Tardif J.-C., Kouz S., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R., Kotian P., et al. BCX4430–a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health. 2016;9(3):220–226. doi: 10.1016/j.jiph.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyack P.L., Calambokidis J., et al. Formal Comment on Schorr GS, Falcone EA, Moretti DJ, Andrews RD (2014) First Long-Term Behavioral Records from Cuvier’s Beaked Whales (Ziphius cavirostris) Reveal Record-Breaking Dives. PLoS ONE 9 (3): e92633. doi: 10.1371/journal. pone. 0092633. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0142287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11(4):248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- Verma A., Xu K., et al. Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol. Ther. – Methods Clin. Dev. 2019;14:161–170. doi: 10.1016/j.omtm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianney, T., NguyetN., et al., 2010. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. [DOI] [PMC free article] [PubMed]
- Vincent M.J., Bergeron E., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2(1):69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff K.M., Sivakumaran H., et al. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012;443(3):851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G.E., Gibson G.R., et al. Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19. Br. J. Nutr. 2021;126(2):219–227. doi: 10.1017/S0007114520003980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.-R., Yang Q.-H., et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xu W., et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020:1. doi: 10.1038/s41423-020-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark P.A., Pathinayake P.S., et al. ACE2 expression is elevated in airway epithelial cells from older and male healthy individuals but reduced in asthma. Respirology. 2021;26(5):442–451. doi: 10.1111/resp.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland-Bräuer N., Pinnow N., et al. The native microbiome is crucial for offspring generation and fitness of Aurelia aurita. MBio. 2020;11(6) doi: 10.1128/mBio.02336-20. e02336-02320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbach E.C., Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969;221(5185):1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- Wen C.-C., Kuo Y.-H., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50(17):4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- White N. Mefloquine. Br. Med. J. 1994 (Publishing Group) [Google Scholar]
- Wong W., Bai X.-C., et al. Mefloquine targets the Plasmodium falciparum 80S ribosome to inhibit protein synthesis. Nat. Microbiol. 2017;2(6):17031. doi: 10.1038/nmicrobiol.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2019. World Health Organization Model List of Essential Medicines: 21st List 2019. [Google Scholar]
- World Health Organization, 2020. WHO coronavirus disease (COVID-19) dashboard.
- Wu C.-J., Jan J.-T., et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004;48(7):2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C., XuQ., et al., 2020. The volatile and heterogenous gut microbiota shifts of COVID-19 patients over the course of a probiotics-assisted therapy. [DOI] [PMC free article] [PubMed]
- Wu X., Yu K., et al. Efficacy and safety of triazavirin therapy for coronavirus disease 2019: a pilot randomized controlled trial. Engineering. 2020 doi: 10.1016/j.eng.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xu X., et al. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir. Res. 2020;21(1):1–12. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Cai H., et al. Management of COVID-19: the Zhejiang experience. J. Zhejiang Univ. (Med. Sci.) 2020;49(2):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Yang J., et al. The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses. J. Virol. 2020;94:9. doi: 10.1128/JVI.00204-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q.-L., Yu Z.-J., et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J. Infect. Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P., Ding Y., et al. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Stephen P., et al. Novel coronavirus polymerase and nucleotidyl-transferase structures: potential to target new outbreaks. J. Phys. Chem. Lett. 2020 doi: 10.1021/acs.jpclett.0c00571. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang Y., et al. Novel nucleoside analogue FNC is effective against both wild-type and lamivudine-resistant HBV clinical isolates. Antivir. Ther. 2012;17(8):1593–1599. doi: 10.3851/IMP2292. [DOI] [PubMed] [Google Scholar]
- Zhuoyue W., Xin Z., et al. IFA in testing specific antibody of SARS coronavirus. South China J. Prev. Med. 2003;29(3):36–37. [Google Scholar]
- Zumla A., Azhar E.I., et al. Elsevier; 2015. Host-directed Therapies for Improving Poor Treatment Outcomes Associated with the Middle East Respiratory Syndrome Coronavirus Infections. [DOI] [PMC free article] [PubMed] [Google Scholar]