Abstract
Individuals with type 2 diabetes (T2DM) are at high risk for nonalcoholic fatty liver disease (NAFLD), and evidence suggests that poor glycemic control is linked to heightened risk of progressive NAFLD. We conducted an observational study based on data from a telehealth trial conducted in 2018-2020. Our objectives were to (1) characterize patterns of NAFLD testing/care in a cohort of individuals with poorly controlled T2DM; and (2) explore how laboratory based measures of NAFLD (eg, liver enzymes, fibrosis-4 [FIB-4]) vary by glycemic control. We included individuals with poorly controlled T2DM (n = 228), defined as hemoglobin A1c (HbA1c) ≥ 8.5% despite clinic-based care. Two groups of interest were (1) T2DM without known NAFLD; and (2) T2DM with known NAFLD. Demographics, medical history, medication use, glycemic control (HbA1c), and NAFLD testing/care patterns were obtained by chart review. Among those without known NAFLD (n = 213), most were male (78.4%) and self-identified as Black race (68.5%). Mean HbA1c was 9.8%. Most had liver enzymes (85.4%) and platelets (84.5%) ordered in the outpatient department over a 2-year period that would allow for FIB-4 calculation, yet only 2 individuals had FIB-4 documented in clinical notes. Approximately one-third had abnormal liver enzymes at least once over a 2-year period, yet only 7% had undergone liver ultrasound and 4.7% had referral to hepatology. Among those with known NAFLD (n = 15), mean HbA1c was 9.5%. Only 4 individuals had undergone transient elastography, half of whom had advanced fibrosis. NAFLD is underrecognized in poorly controlled T2DM, even though this is a high-risk group for NAFLD and its complications.
Keywords: diabetes, NAFLD, glycemic control
Nonalcoholic fatty liver disease (NAFLD) disproportionately impacts individuals with type 2 diabetes (T2DM), with a prevalence of 60% to 70% [1-4]. Importantly, the presence of T2DM is associated with higher odds of having progressive forms of NAFLD [5, 6] such as nonalcoholic steatohepatitis and advanced liver fibrosis, with a prevalence as high as 10% to 15% for advanced fibrosis in T2DM [3, 4]. T2DM also leads to a substantial increase in the risk of NAFLD complications, including a 2-fold increase in hepatocellular carcinoma and liver-related mortality risk [7, 8]. Recent evidence also suggests that beyond the presence of T2DM, glycemic control may be linked to greater severity of liver fibrosis in NAFLD [9]. Therefore, individuals with poor glycemic control may be at particular risk of progressive NAFLD and poor liver outcomes.
Despite an increasing clinical burden of NAFLD, and calls to action across the medical community to prepare for this rising public health threat [10-14], NAFLD remains underrecognized in primary care settings [15]. It is likely that this underrecognition extends to T2DM populations—even in those with poor glycemic control—though data specific to poorly controlled T2DM are lacking. The primary purpose of this study was to examine NAFLD testing and care patterns in a cohort of individuals with poorly controlled T2DM. Since laboratory-based measures (liver enzymes, noninvasive scores, eg, fibrosis-4 [FIB-4] index) constitute initial testing for NAFLD that may prompt additional work-up, we also aimed to explore how these measures vary by glycemic control.
Materials and Methods
Data Source and Sample
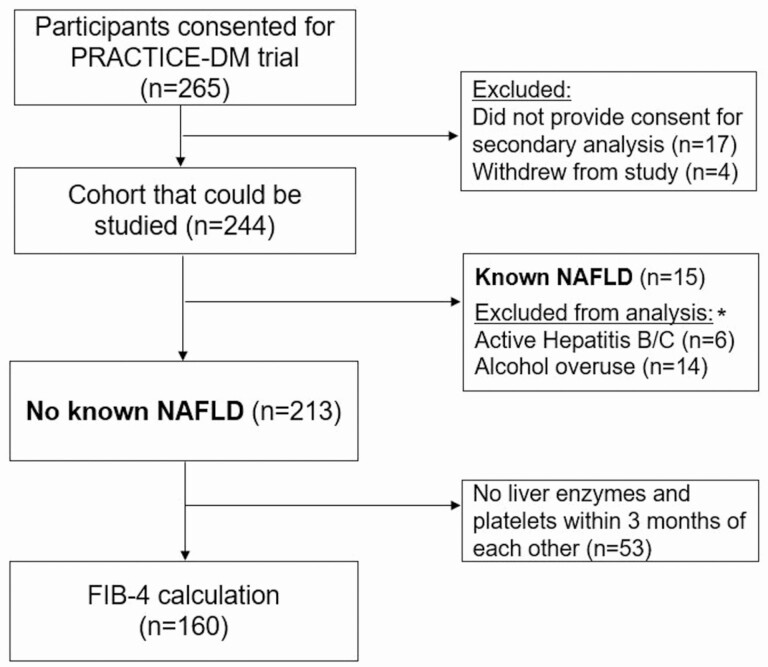
We performed a secondary analysis of patients enrolled in the “Practical Telehealth to Improve Control and Engagement for Patients with Clinic-refractory Diabetes Mellitus” (PRACTICE-DM, NCT03520413) trial [16]. This is a multilevel telehealth intervention aimed at reducing costs and complications in individuals with persistent poorly controlled diabetes (ie, T2DM with HbA1c consistently ≥8.5% despite clinic-based care). Individuals in this study were recruited from 2 clinical sites: the Durham and Richmond Veterans Affairs Medical Centers (VAMC). This study included individuals consented for PRACTICE-DM (Fig. 1). The 2-year study period was unique to each individual and spanned from 1 year pre- and postenrollment (2018-2020 in most cases). This work was reviewed and approved by the Internal Revenue Boards of the Durham (#2130) and Richmond (#2398) Veterans Affairs Health Care Systems.
Figure 1.
Study flowchart. *Due to overlap of conditions, the number of unique patients is 16.
We examined 2 groups of interest: (1) T2DM without known NAFLD (but at risk); and (2) T2DM with known NAFLD. Individuals without known NAFLD were defined by no diagnosis of NAFLD, alcohol overuse, or hepatitis B/C at the beginning of the study period, and these diagnoses were determined by chart review. Alcoholism and viral hepatitis were excluded as NAFLD cannot be diagnosed in the presence of these conditions under current guidelines [17]. Alcohol use was defined by an AUDIT-C score ≥4 in men or ≥3 in women at any time during the study period [18], or by a diagnosis of alcoholism in the problem list or notes. Hepatitis B and C were defined by serologies (ie, positive hepatitis B surface antigen, hepatitis C antibody), and verified by chart review. In the case of cured hepatitis C, individuals were included if cure was ≥5 years preceding the study period. We defined individuals as having undiagnosed NAFLD using 2 different criteria: (1) a previously validated approach requiring 2 elevated alanine aminotransferase (ALT) values (≥40 IU/mL in males, ≥31 IU/mL in females) at least 6 months apart [19], and (2) a similar approach but using ALT cut-offs that correlate with healthy ranges for ALT (2 values ≥30 IU/mL in males, ≥19 IU/mL in females, at least 6 months apart) [20]. Known NAFLD was defined by inclusion of this diagnosis in the problem list, or if it was acknowledged in clinical notes.
Baseline Measures
We used baseline data from the PRACTICE-DM trial [16] to characterize our population in terms of demographics, medical history, and medication use.
Additional Data Collection
In order to capture testing and care patterns for NAFLD in T2DM, we conducted a chart review to collect the following additional data: (1) up to 10 sets of outpatient liver enzymes (aspartate aminotransferase [AST], ALT]) and platelet counts; (2) documentation of NAFLD risk stratification scores (eg, FIB-4, NAFLD fibrosis score) in primary care, endocrinology, or hepatology notes; (3) relevant imaging studies (ultrasound, transient elastography, computed tomography or magnetic resonance imaging scan); (4) hepatology referrals; and (5) liver biopsy data.
FIB-4 Calculation and Categories
Among noninvasive measures of liver fibrosis, the FIB-4 index has the best diagnostic accuracy within the Veterans Health Administration [21], and it has also been recommended by societies such as the American Association for the Study of Liver Disease and the American Gastroenterological Association due to its low cost, high specificity, and reasonable area under the receiver operator characteristic curve (0.76-0.84) for identifying advanced fibrosis [11, 21, 22]. We calculated FIB-4 scores using the following equation: (age × AST)/(platelets × √(ALT) [23]. FIB-4 was calculated when outpatient liver enzyme and platelet data were drawn within 3 months of each other. For individuals in whom multiple FIB-4 scores were calculated, the highest score during the study period was selected—this approach was chosen because even 1 high FIB-4 score should prompt consideration of further testing for NAFLD. For individuals <65 years, FIB-4 scores were categorized based on the following established cut-offs: low (<1.3), indeterminate (1.3–2.67), or high (>2.67) risk of advanced fibrosis [23]. Since FIB-4 has lower specificity for advanced fibrosis in individuals ≥65 years of age, we used a higher threshold of 2.0 for defining low (<2.0) vs indeterminate (2.0-2.67) risk in this group [24]. In T2DM, FIB-4 of >2.67 has 97% specificity for detecting advanced fibrosis [25]. Prior to this study, FIB-4 had been automatically generated in the electronic health record (EHR) at the Richmond VAMC based on same-day liver enzyme and platelet data.
Data Analysis
We conducted a cross-sectional, descriptive examination of individuals without known NAFLD, stratified by clinical site. Laboratory-based measures of NAFLD were then compared across the following glycemic control groups: HbA1c <8.5%, HbA1c 8.5–9.5%, HbA1c >9.5%. We conducted a similar descriptive examination of those with known NAFLD. Descriptive statistics included calculation of proportions (n, %) and mean with SD. No statistical comparisons between groups were made.
Results
We included 228 individuals enrolled in PRACTICE DM. Of these individuals, 213 (93.4%) had no known NAFLD. The mean age of this cohort was 58.1 (SD 8.1), 21.6% (n = 46) were female, and 68.5% (n = 146) were of Black race (Table 1). Most were on metformin (79.2%, n = 168) and insulin (70.9%, n = 151). The majority had liver enzymes (85.4%, n = 182) and platelets (84.5%, n = 180) checked at least once within a 2-year period, though FIB-4 or other risk stratification scores were rarely documented in clinical notes (0.9%, n = 2). As seen in Table 1, liver ultrasound was performed in 7.0% (n = 15) of this cohort, and hepatic steatosis was noted in 80% of these studies. Very few individuals underwent transient elastography (2.3%, n = 5) or were referred to Hepatology (4.7%, n = 10). Rates of NAFLD testing and care were similarly low across both sites, even though the Richmond VAMC had an automatic FIB-4 calculator embedded into the EHR.
Table 1.
Characteristics of T2DM population without known NAFLD, stratified by clinical site
| Whole cohort (n = 212) | Durham VAMC (n = 117) | Richmond VAMC (n = 96) | |
|---|---|---|---|
| Demographic | |||
| Age, mean (SD) | 58.1 (8.1) | 57.8 (7.8) | 58.5 (8.6) |
| Male, n (%) | 167 (78.4) | 93 (79.5) | 74 (77.1) |
| Race, n (%) | |||
| White | 50 (23.5) | 26 (22.2) | 24 (25.0) |
| Black or African American | 146 (68.5) | 80 (68.4) | 66 (68.8) |
| Other | 17 (8.0) | 11 (9.4) | 6 (6.3) |
| Ethnicity, n (%)a | |||
| Hispanic or Latino/a | 9 (4.2) | 3 (2.6) | 6 (6.3) |
| ≤40 miles from nearest VA clinic (%) | 163 (76.5) | 88 (75.2) | 75 (78.1) |
| Medical history | |||
| Hypertension, n (%) | 181 (85.0) | 99 (84.6) | 82 (85.4) |
| Hyperlipidemia, n (%)a | 178 (83.6) | 99 (84.6) | 79 (82.3) |
| BMI (kg/m2), mean (SD) | 35.0 (6.2) | 35.6 (6.4) | 34.4 (5.9) |
| Diabetes duration (years), mean (SD) | 12.5 (8.0) | 12.6 (7.6) | 12.5 (8.6) |
| T2DM medication use and controla | |||
| Metformin, n (%) | 168 (79.2) | 98 (83.8) | 70 (73.6) |
| Sulfonylurea, n (%) | 86 (40.6) | 49 (41.9) | 37 (38.9) |
| Thiazolidinedione, n (%) | 20 (9.4) | 19 (16.2) | 1 (1.1) |
| DPP4 inhibitor, n (%) | 2 (0.9) | 1 (0.8) | 1 (1.1) |
| GLP-1 receptor agonist, n (%) | 34 (16.0) | 10 (8.5) | 24 (25.3) |
| SGLT2 inhibitor, n (%) | 26 (12.2) | 15 (12.8) | 11 (11.6) |
| Insulin, n (%) | 151 (70.9) | 87 (74.4) | 64 (66.7) |
| HbA1c, %, mean (SD)a | 9.8 (1.5) | 9.7 (1.4) | 9.9 (1.7) |
| NAFLD testing/care patterns | |||
| Liver enzymes, n (%) | 182 (85.4) | 99 (84.6) | 83 (86.4) |
| # of checks, mean (SD) | 2.3 (1.8) | 2.4 (2.0) | 2.1 (1.6) |
| Platelets, n (%) | 180 (84.5) | 92 (78.6) | 88 (91.7) |
| # of checks, mean (SD) | 2.9 (2.6) | 3.0 (3.3) | 2.8 (1.7) |
| FIB-4 or other score documented, n(%)b | 2 (0.9) | 0 (0) | 2 (2.1) |
| Liver ultrasound, n (%) | 15 (7.0) | 8 (6.8) | 7 (7.3) |
| Proportion with fatty liver, n (%) | 12 (80.0) | 6 (75.0) | 6 (85.7) |
| CT/MRI with fatty liver, n (%) | 6 (2.8) | 3 (2.6) | 3 (3.1) |
| Transient elastography, n (%)c | 5 (2.3) | 2 (1.7) | 3 (3.1) |
| F0-1 (%) | 2 (40.0) | 0 (0) | 2 (40.0) |
| F2 (%) | 1 (20.0) | 1 (20.0) | 0 (0) |
| F3 (%) | 2 (40.0) | 1 (20.0) | 1 (20.0) |
| F4 (%) | 0 (0) | 0 (0) | 0 (0) |
| Hepatology referral, n (%) | 10 (4.7) | 4 (3.4) | 6 (6.3) |
Abbreviations: BMI, body mass index; DPP4, dipeptidyl peptidase-4; GLP-1, glucagon-like pepetide-1; SGLT2, sodium glucose cotransporter-2; HbA1c, hemoglobin A1c; FIB-4, fibrosis-4; CT, computed tomography; MRI, magnetic resonance imaging.
a 1 Richmond participant was missing HbA1c and data on noninsulin medications; 1 Richmond participant responded “Don’t Know” to Latino/Hispanic origin; 1 Richmond participant responded “Don’t Know” to diagnosis of hyperlipidemia.
b 40 individuals (27 Durham 13 Richmond) were not included in this calculation because they did not have outpatient liver enzymes or platelets checked. We only included outpatient liver enzyme and platelet data in this table.
c Proportions and percentages of the individuals who underwent transient elastography (n = 5).
Our cohort had poor glycemic control by design, with a mean HbA1c of 9.8% (SD 1.5) in those without known NAFLD. Mean AST and ALT values tended to be lower with worse glycemic control (HbA1c >9.5%), which was most prominent for AST (Table 2). Despite this finding, undiagnosed NAFLD by ALT-based criteria [19] was most prevalent in the highest HbA1c (>9.5%) group (20.8% vs 11.9-15.4% in lower HbA1c groups)—overall, 17% (n = 36) of individuals had undiagnosed NAFLD based on these criteria. Notably, most individuals in the highest HbA1c group had FIB-4 scores in the low-risk range (mean FIB-4 score 0.9, SD 0.5).
Table 2.
Laboratory-based measures in cohort without known NAFLD, stratified by glycemic control
| Glycemic control groupsc | |||
|---|---|---|---|
| Mean HbA1c <8.5% (n = 39) | Mean HbA1c 8.5-9.5% (n = 67) | Mean HbA1c >9.5% (n = 106) | |
| Liver enzyme testing | |||
| AST, mean (SD)d | 22.9 (8.0) | 23.0 (18.0) | 19.3 (7.3) |
| ALT, mean (SD)d | 36.4 (12.2) | 36.0 (21.9) | 34.0 (11.9) |
| ALT >40 IU/L, n (%)d | 16 (44.4) | 16 (29.1) | 38 (42.2) |
| Platelet count, mean (SD)e | 236.2 (63.1) | 258.9 (57.4) | 260.3 (61.8) |
| Undiagnosed NAFLDa, n (%) | 6 (15.4) | 8 (11.9) | 22 (20.8) |
| Undiagnosed NAFLDb, n (%) | 15 (38.5) | 19 (28.4) | 43 (40.6) |
| FIB-4 score, mean (SD)f | 1.4 (1.4) | 1.0 (0.4) | 0.9 (0.5) |
| FIB-4 score risk categories | |||
| Low risk, n (%) | 24 (72.7) | 42 (85.7) | 72 (93.5) |
| Indeterminate risk, n (%)c | 7 (21.2) | 7 (14.3) | 5 (6.5) |
| High risk, n (%) | 2 (6.1) | 0 (0) | 0 (0) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; NAFLD, nonalcoholic fatty liver disease; FIB-4, fibrosis-4.
a Based on Kanwal et al. criteria (ALT ≥40 IU/mL in males, ALT ≥31 IU/mL in females at least 6 months apart) [19].
b Based on Prati et al. criteria for healthy ALT ranges (ALT ≥30 IU/mL in males, ALT ≥19 IU/mL in females at least 6 months apart) [20].
c 1 individual did not have a measurable A1c collected thus is excluded from this table.
d 31 individuals had no AST or ALT values measured (3 with HbA1c <8.5%, 12 with HbA1c 8.5-9.5%, and 16 with HbA1c >9.5%).
e 47 individuals had no platelets measured (5 with HbA1c <8.5%, 18 with HbA1c 8.5-9.5% and 24 in with HbA1c >9.5%).
f 53 individuals had no FIB-4 score calculated due to missing values (6 with HbA1c <8.5%, 18 with HbA1c 8.5-9.5%, and 29 with HbA1c >9.5%). We used liver enzymes and platelets collected within 3 months of each other for FIB-4 calculation.
Of the 15 individuals with known NAFLD (Table 3), mean age was 57.9 (SD 9.3), 20% were female (n = 3), and 60% were of Black race (n = 9). All had hypertension and hyperlipidemia, and mean HbA1c was 9.5%. Few individuals were on thiazolidinediones (n = 2) or T2DM medications that promote weight loss (ie, glucagon-like peptide 1 receptor agonist [n = 3], sodium-glucose cotransporter 2 inhibitor [n = 2]), and the majority were followed by endocrinology (n = 13). Mean FIB-4 score was 1.65 (SD 0.94), which is indeterminate risk of advanced fibrosis based on mean age of this cohort. Of the 4 individuals who had transient elastography done, 2 had advanced fibrosis (stage 3-4).
Table 3.
Characteristics of population with T2DM and known NAFLD
| Demographic | Whole cohort (n = 15) |
|---|---|
| Age, mean (SD) | 57.9 (9.3) |
| Male, n (%) | 12 (80.0) |
| Race, n (%) | |
| White | 5 (33.3) |
| Black or African American | 9 (60.0) |
| Mixed race | 1 (6.7) |
| Ethnicity, n (%) | |
| Hispanic or Latino/a | 2 (13.3) |
| ≤40 miles from nearest VA clinic (%) | 9 (60.0) |
| Medical history | |
| Hypertension, n (%) | 15 (100.0) |
| Hyperlipidemia, n (%) | 15 (100.0) |
| Diabetes duration (years) mean (SD) | 15.1 (5.7) |
| BMI (kg/m2), mean (SD) | 37.8 (6.8) |
| T2DM medication use and control | |
| Metformin, n (%) | 13 (86.7) |
| Sulfonylurea, n (%) | 5 (33.3) |
| Thiazolidinedione, n (%) | 2 (13.3) |
| DPP4 inhibitor, n (%) | 0 (0) |
| GLP-1 receptor agonist, n (%) | 3 (20.0) |
| SGLT2 inhibitor, n (%) | 2 (13.3) |
| Insulin, n (%) | 12 (80.0) |
| HbA1c, %, mean (SD) | 9.5 (1.8) |
| NAFLD testing/care patterns | |
| Liver enzymes, n (%) | 15 (100) |
| # of checks, mean (SD) | 3.3 (1.8) |
| Platelets, n (%) | 15 (100) |
| # of checks, mean (SD) | 3.2 (2.1) |
| FIB-4 or other score documented, n (%) | 0 (0) |
| FIB-4 score, mean (SD)a | 1.65 (0.94) |
| Liver ultrasound, n (%) | 6 (40.0) |
| Transient elastography, n (%) | 4 (26.7) |
| F0-1 (%) | 1 (25.0) |
| F2 (%) | 1 (25.0) |
| F3 (%) | 2(50.0) |
| F4 (%) | 0 (0) |
| Following with hepatology, n (%) | 5 (33.3%) |
| Following with endocrinology, n (%)b | 13 (86.7) |
Abbreviations: BMI, body mass index; DPP4, dipeptidyl peptidase-4; GLP-1, glucagon-like pepetide-1; SGLT2, sodium glucose cotransporter-2; HbA1c, hemoglobin A1c; FIB-4=fibrosis-4.
a 1 individual had missing data due to lack of liver enzymes and platelets done within 3 months of each other. Only outpatient laboratory data were included in this table.
b Had to be following endocrinology for T2DM care.
Discussion
Our study reveals that NAFLD is underrecognized in T2DM, even in those with poorly controlled disease where risk for NAFLD-related complications can be high. Greater action is needed by diabetes providers to detect NAFLD in this high-risk population, and to initiate appropriate care to prevent end-stage liver disease.
In our study, approximately one-third of individuals without known NAFLD had abnormal liver enzymes, yet only 7% had a liver ultrasound, which is the first-line imaging test to diagnose NAFLD. Even fewer (<5%) were referred for assessment of liver fibrosis by transient elastography or hepatology referral. To put these findings into context, current guidelines suggest a high index of suspicion for progressive forms of NAFLD in T2DM, and they recommend use of noninvasive risk assessment tools, such as FIB-4 or transient elastography, to assess for risk of advanced fibrosis [17]. At minimum, abnormal liver enzymes and/or fatty liver noted on imaging should be followed by some form of liver fibrosis assessment [26]. Despite the vast majority of our cohort (≥85%) having liver enzyme and platelet data available for calculation of FIB-4 or other noninvasive (fibrosis) risk score, only 2 individuals had documentation of a risk score in the clinical notes. Overall, our data highlight large gaps between recommended and current practices.
While it is agreed that abnormal liver enzymes should prompt further work-up, it is known that liver enzymes are not reliable indicators of fibrosis [27]. Our data demonstrate a decrease in AST levels with worsening glycemic control, as well as a correlated decline in FIB-4 scores. Since higher HbA1c has been associated with more severe fibrosis on liver biopsy [9, 27] (ie, the gold standard for diagnosing fibrosis), our data suggest that the performance of FIB-4 may vary by glycemic control. While evidence so far suggests good accuracy of FIB-4 in well-controlled T2DM populations, it is important to note that its specificity may be high in T2DM (97%), but sensitivity can be as low as 22% [25]. Thus, FIB-4 is likely to miss many cases of advanced fibrosis, and our findings suggest the possibility of even lower sensitivity of FIB-4 in individuals with very poor glycemic control. To better inform its applicability in practice, the performance of FIB-4 across the full glycemic spectrum should be explored in future studies.
The high proportion of individuals deemed low risk by FIB-4 may explain some of the observed clinical inertia in this study; however, lack of recognition of FIB-4 scores in notes, combined with low rates of testing despite a high prevalence of abnormal liver enzymes (~33%), suggests that other factors are at play. Prior studies have identified a number of factors underlying poor recognition of NAFLD in practice, including limited awareness of NAFLD and its complications, confusion surrounding guidelines, and lack of knowledge regarding approaches that may reverse nonalcoholic steatohepatitis and fibrosis [10, 28-34]. Lack of knowledge and awareness may also underlie the low usage of pioglitazone in our study, ie, 13.3% of individuals with known NAFLD. Other groups have noted similar reluctance to use pioglitazone in practice, even in patients with NAFLD where there is clear benefit [28, 35]. This is likely driven by concerns regarding its safety, even though risks of pioglitazone are minimal in individuals without established heart failure [28]. Given NAFLD is a metabolic disease that is concentrated in T2DM, greater efforts are needed to improve awareness and knowledge of NAFLD in T2DM care.
Our data suggest that even abnormal liver enzymes and EHR-integrated FIB-4 calculation may be insufficient to trigger clinical action related to NAFLD. In order to promote high-quality care of NAFLD, novel approaches are needed to effectively educate providers on the importance of detection and initial care of NAFLD (including when to refer to hepatology), and, concurrently, to develop machine learning approaches to proactively detect high-risk cases of NAFLD in the EHR, so they may be directly referred to hepatology or multidisciplinary clinics for appropriate care.
Strengths of this study include its well-characterized group of individuals with poor T2DM control, which is traditionally an understudied population. Our cohort was racially diverse, with 68.5% having self-identified as Black or African American. We also conducted a detailed chart review assessment of alcohol overuse and viral hepatitis to exclude these other common forms of chronic liver disease.
Our study is limited in that we only examined a 2-year period, so we may have missed relevant testing or care that occurred before or after this timeframe. However, even if we did not consider studies that indicated fatty liver prior to the study period, lack of inclusion of NAFLD in the problem list during our study period would still suggest underrecognition of this condition. Another limitation is that we had very few women in this study, as well as individuals of Hispanic or Latino/a ethnicity, and the latter is known to be a high-risk population for NAFLD and its complications [36]. This was also a veteran population, so these results may not generalize to the general population.
Conclusion
In conclusion, NAFLD is underrecognized in poorly controlled T2DM, even though risk of progressive liver disease in this population is high. As such, there is a great need for primary care physicians and endocrinologists alike to incorporate detection and risk stratification of NAFLD into their diabetes practice, and to join in multidisciplinary efforts to tackle this rising public health threat.
Acknowledgments
Financial Support: This study was supported by a grant from VA Health Services Research & Development (IIR 16-213). The authors also acknowledge support from the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) [CIN 13-410] within the Durham VA Health Care System. There were no other funding sources for this study.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- FIB-4
fibrosis-4
- HbA1c
hemoglobin A1c
- NAFLD
nonalcoholic fatty liver disease
- T2DM
type 2 diabetes
- VAMC
Veterans Affairs Medical Centers
Additional Information
Disclosures: Authors have no conflicts of interest to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793-801. [DOI] [PubMed] [Google Scholar]
- 2. Dai W, Ye L, Liu A, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltimore). 2017;96(39):e8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciardullo S, Monti T, Perseghin G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care. 2021;44(2):519-525. [DOI] [PubMed] [Google Scholar]
- 4. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loomba R, Abraham M, Unalp A, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network . Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56(3):943-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelusi S, Petta S, Rosso C, et al. Renin-angiotensin system inhibitors, type 2 diabetes and fibrosis progression: an observational study in patients with nonalcoholic fatty liver disease. PLoS One. 2016;11(9):e0163069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stepanova M, Rafiq N, Makhlouf H, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2013;58(10):3017-3023. [DOI] [PubMed] [Google Scholar]
- 8. Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107(1):46-52. [DOI] [PubMed] [Google Scholar]
- 9. Alexopoulos AS, Crowley MJ, Wang Y, et al. Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology. 2021;74(3):1220-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419-430. [DOI] [PubMed] [Google Scholar]
- 11. Kanwal F, Shubrook JH, Younossi Z, et al. Preparing for the NASH epidemic: a call to action. Gastroenterology. 2021;161(3):1030-1042.e8. [DOI] [PubMed] [Google Scholar]
- 12. Kanwal F, Shubrook JH, Younossi Z, et al. Preparing for the NASH epidemic: a call to action. Gastroenterology. 2021;161(3):1030-1042.e8. [DOI] [PubMed] [Google Scholar]
- 13. Kanwal F, Shubrook JH, Younossi Z, et al. Preparing for the NASH epidemic: a call to action. Gastroenterology. 2021;161(3):1030-1042.e8. [DOI] [PubMed] [Google Scholar]
- 14. Kanwal F, Shubrook JH, Younossi Z, et al. Preparing for the NASH epidemic: a call to action. Metabolism. 2021;122:154822. [DOI] [PubMed] [Google Scholar]
- 15. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10-14. [DOI] [PubMed] [Google Scholar]
- 16. Kobe EA, Edelman D, Tarkington PE, et al. Practical Telehealth to Improve Control and Engagement for Patients with Clinic-refractory Diabetes Mellitus (PRACTICE-DM): Protocol and baseline data for a randomized trial. Contemp Clin Trials. 2020;98:106157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Veterans Affairs. Using AUDIT-C alcohol screening data in VA research: interpretation, strengths, limitations, & sources. Accessed April 13, 2021. https://www.hepatitis.va.gov/alcohol/treatment/audit-c.asp#S1X
- 19. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1-10. [DOI] [PubMed] [Google Scholar]
- 21. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259-2266. [DOI] [PubMed] [Google Scholar]
- 22. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486-1501. [DOI] [PubMed] [Google Scholar]
- 23. Sterling RK, Lissen E, Clumeck N, et al. ; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317-1325. [DOI] [PubMed] [Google Scholar]
- 24. McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112(5):740-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alkayyali T, Qutranji L, Kaya E, Bakir A, Yilmaz Y. Clinical utility of noninvasive scores in assessing advanced hepatic fibrosis in patients with type 2 diabetes mellitus: a study in biopsy-proven non-alcoholic fatty liver disease. Acta Diabetol. 2020;57(5):613-618. [DOI] [PubMed] [Google Scholar]
- 26. American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S40-S52. [DOI] [PubMed] [Google Scholar]
- 27. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100(6):2231-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rinella ME, Lominadze Z, Loomba R, et al. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern Med J. 2013;43(3):247-253. [DOI] [PubMed] [Google Scholar]
- 30. Matthias AT, Fernandopulle ANR, Seneviratne SL. Survey on knowledge of non-alcoholic fatty liver disease (NAFLD) among doctors in Sri Lanka: a multicenter study. BMC Res Notes. 2018;11(1):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med J. 2018;48(2):144-151. [DOI] [PubMed] [Google Scholar]
- 32. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758-765. [PubMed] [Google Scholar]
- 34. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(10):2809-2816. [DOI] [PubMed] [Google Scholar]
- 35. Le P, Chaitoff A, Rothberg MB, McCullough A, Alkhouri N. Trends in pioglitazone use among U.S. adults with type 2 diabetes and suspected nonalcoholic fatty liver disease. Expert Opin Investig Drugs. 2020;29(2):205-208. [DOI] [PubMed] [Google Scholar]
- 36. Samji NS, Snell PD, Singal AK, Satapathy SK. Racial disparities in diagnosis and prognosis of nonalcoholic fatty liver disease. Clin Liver Dis (Hoboken). 2020;16(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.