Abstract
In May 2020, a pregnant woman in her 37th pregnancy week was diagnosed with COVID-19 in St. Petersburg in Russia. All treatments failed and the patient died after 11 days due to acute respiratory distress syndrome. A stillborn child was removed by caesarian section. Pathological investigations showed that the child died due to antenatal asphyxia with aspiration pneumonia. The child was positive for SARS-CoV-2 and immunohistochemical investigations showed viral infection and cellular changes in several organs such as pancreas, brain, spleen, and adrenals. These results emphasize the importance of vaccinating pregnant women against SARS-CoV-2.
Keywords: SARS-CoV-2, COVID-19, Pregnancy, Stillbirth, Viral infiltration, Pancreas
Introduction
For pregnant women infected with SARS-CoV-2, there may be an increased risk for hospitalization, preterm birth, and maternal mortality [1], [2], [3]. Therefore, attention to the importance of a global COVID-19 maternal immunization plan has been drawn [4], as it has been indicated that mRNA COVID-19 vaccines are safe and without significant side effects for pregnant women [5].
Most neonates born to infected women tested negative for SARS-CoV-2, and the majority of those testing positive for the virus presented with mild symptoms [6], [7]. Infections of infants with SARS-CoV-2 have primarily been observed when the mother was infected within one week of delivery [7]. For most cases, it is unclear whether the mother-to-child transmission occurred in utero, intrapartum, or early in the postnatal period. Rare cases of SARS-CoV-2 in utero vertical transmission has been reported [8], [9], but so far, only placental involvement was reported, whereas fetal organ infection has not been documented.
Here, we report a pregnant woman that died due to COVID-19 in the 39th gestation week. Investigation of her stillborn child showed in utero infection with SARS-CoV-2 and that the child also died due to the infection. Immunohistochemical investigations of the child showed viral infiltration and cellular changes in several organs.
Case presentation
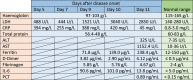
In May 2020, a 34-year-old woman at gestational week 38 was admitted to hospital with respiratory symptoms and slightly increased temperature three days after the first symptoms appeared. Two days later, the respiratory symptoms aggravated and she was transferred to our hospital (S.P. Botkin Infectious Hospital), where she tested positive for SARS-CoV-2 by PCR. CRP, D-dimer, and IL-6 values were highly increased (Table 1) and she received amoxiclav, azithromycin and kaletra. Intravenous immunoglobulin prepared from convalescent plasma from recovered COVID-19 patients or other antibody-based therapies were not considered at this time of the pandemic. At this stage, the uterus had a normal tone and the fetus was alive and moving. Blood values, however, worsened and eleven days after disease onset, the patient died due to severe viral pneumonia with acute respiratory distress syndrome (ARDS). A stillborn boy of 4240 g and 55 cm was removed by caesarian section. Fetal heart rate monitoring was not performed continuously and therefore the exact time of death is unknown. Viral placentitis with chronic insufficiency and acute placental decompensation was observed.
Table 1.
Laboratory data of a pregnant woman infected with SARS-CoV-2. At day 6 after disease onset, she was admitted to hospital. At day 11, she died due to acute respiratory distress syndrome.
 |
During autopsy of the child, lung and trachea swabs were positive for SARS-CoV-2 and pathological investigations showed antenatal asphyxia with aspiration pneumonia. In all organs, endothelial cells with enlarged light nuclei appeared and proliferative overgrowth of bronchial epithelium and macrophages was observed. Proliferative changes in the lungs as observed in adults infected with SARS-CoV-2 were noticed, but without signs of ARDS (Fig. 1A). Nodular hyperplasia in the adrenal cortex was detected and mononuclear infiltration and cellular changes were documented in liver and kidney and more pronounced in the pancreas (Fig. 1B,C) as found in adult COVID-19 patients. Virus antigen was detected in brain, kidney, heart, spleen, adrenal, and pancreas (Fig. 1D).
Fig. 1.
Organs from stillborn child with COVID-19 show viral infiltration. (A) H&E stained lung section showing lymphocytic infiltration (red) and proliferation of epithelial cells. 100x magnification. (B) H&E stained pancreas section showing lymphocytic infiltration (red). Islets are indicated in black. 100x magnification. (C) H&E stained sections demonstrating cellular changes in an endocrine pancreatic islet. 400x magnification. (D) Coronavirus spike proteins in the islets detected by immunohistochemistry (brown). 400x magnification.
Discussion
We describe a case of a pregnant woman with COVID-19 leading to intrauterine infection with SARS-CoV-2 causing the death of both mother and child. Thus, we confirm the possibility of intrauterine transplacental viral challenge of the fetus. In several organs of the stillborn child, we observed viral infiltration and cellular changes.
In adult COVID-19 patients, SARS-CoV-2 were shown to infect and replicate in cells of the endocrine and exocrine pancreas [10], [11]. Moreover, in both the exocrine and endocrine pancreata from COVID-19 patients, infiltration with immune cells and signs of necroptosis have been observed [11]. This implies that beta-cell infection with SARS-CoV-2 might lead to either direct or indirect impairment of the beta-cells functions causing variable degrees of metabolic dysregulation [12]. Several studies report hyperglycemia and ketoacidosis after an infection with SARS-CoV-2 [13], [14], and new-onset diabetes after COVID-19 has been reported in a number of studies [15], [16]. However, it remains a subject of discussion if COVID-19 can indeed directly or indirectly lead to new-onset diabetes or accelerate pre-existing unrecognised diabetes or prediabetes.
Previously, a direct infection of the adrenal gland with SARS-CoV-2 has been detected of COVID-19 patients [17], and as the stillborn child was positive for SARS-CoV-2 in the adrenal, a direct cytopathic effect may possibly be confirmed.
Whether an intrauterine infection with SARS-CoV-2 might lead to lasting consequences for surviving children, as observed in adults, still needs to be investigated. At present, pregnancy and neonatal outcomes are being investigated in different registries of pregnant women with suspected or confirmed SARS-CoV-2 infection in order to guide treatment and prevention [18], [19], [20].
Currently, a fierce discussion if pregnant women should be vaccinated against coronavirus is taking place. Therefore, this case is of particular interest as we show that a stillborn boy had viral infiltration in brain, kidney, pancreas, heart, adrenals and probably other organs. These data indicate the importance of vaccination during pregnancy in order to protect both the mother and the child.
Consent
Unfortunately, the patient and her child died before written and signed consent to publish was obtained. According to the rules of the ethics committee in Russia, written consent from relatives to deceased patients is not necessary. Therefore, only verbal consent was obtained from the husband after the death of the patient and her child.
Ethical approval
Consent from relatives to the patient was obtained according to the rules of the local ethics committee.
CRediT authorship contribution statement
MAV were involved in clinical care of the patient and clinical documentation. TN and YS made autopsies, VZ, TN, YS, and NS performed the histopathological study. CS reviewed the literature and wrote the first draft. SRB edited the initial draft. All authors read and approved the final submitted version.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research foundation) project no. 314061271, TRR 205/1: “The Adrenal: Central Relay in Health and Disease” and project no. 288034826, IRTG 2251: “Immunological and Cellular Strategies in Metabolic Disease”.
References
- 1.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed sars-cov-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lokken E.M., Huebner E.M., Taylor G.G., Hendrickson S., Vanderhoeven J., Kachikis A., et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in washington state. Am J Obstet Gynecol. 2021;225(1) doi: 10.1016/j.ajog.2020.12.1221. 77 e71-77 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lokken E.M., Taylor G.G., Huebner E.M., Vanderhoeven J., Hendrickson S., Coler B., et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am J Obstet Gynecol. 2021;225(1) doi: 10.1016/j.ajog.2021.02.011. 75 e71-75 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardají A., Sevene E., Cutland C., Menéndez C., Omer S.B., Aguado T., et al. The need for a global covid-19 maternal immunisation research plan. Lancet. 2021;397(10293):e17–e18. doi: 10.1016/S0140-6736(21)00146-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., et al. Preliminary findings of mrna covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De, et al. Synthesis and systematic review of reported neonatal sars-cov-2 infections. Nat Commun. 2020;11(1):5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodworth K.R., Olsen E.O., Neelam V., Lewis E.L., Galang R.R., Oduyebo T., et al. Birth and infant outcomes following laboratory-confirmed sars-cov-2 infection in pregnancy - set-net, 16 jurisdictions, march 29-october 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., et al. Analysis of sars-cov-2 vertical transmission during pregnancy. Nat Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., et al. Transplacental transmission of sars-cov-2 infection. Nat Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller J.A., Groß R., Conzelmann C., Krüger J., Merle U., Steinhart J., et al. Sars-cov-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 11.Steenblock C., Richter S., Berger I., Barovic M., Schmid J., Schubert U., et al. Viral infiltration of pancreatic islets in patients with covid-19. Nat Commun. 2021;12(1):3534. doi: 10.1038/s41467-021-23886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornstein S.R., Rubino F., Ludwig B., Rietzsch H., Schwarz P.E.H., Rodionov R.N., et al. Consequences of the covid-19 pandemic for patients with metabolic diseases. Nat Metab. 2021;3(3):289–292. doi: 10.1038/s42255-021-00358-y. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. Covid-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montefusco L., Ben Nasr M., D’Addio F., Loretelli C., Rossi A., Pastore I., et al. Acute and long-term disruption of glycometabolic control after sars-cov-2 infection. Nat Metab. 2021;3(6):774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khunti K., Del Prato S., Mathieu C., Kahn S.E., Gabbay R.A., Buse J.B. Covid-19, hyperglycemia, and new-onset diabetes. Diabetes Care. 2021 doi: 10.2337/dc21-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steenblock C., Schwarz P.E.H., Ludwig B., Linkermann A., Zimmet P., Kulebyakin K., et al. Covid-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Y., Xu B., Guan W., Xu D., Li F., Ren R., et al. The adrenal cortex, an underestimated site of sars-cov-2 infection. Front Endocrinol. 2020;11:11. doi: 10.3389/fendo.2020.593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee J., Mullins E., Townson J., Playle R., Shaw C., Kirby N., et al. Pregnancy and neonatal outcomes in covid-19: Study protocol for a global registry of women with suspected or confirmed sars-cov-2 infection in pregnancy and their neonates, understanding natural history to guide treatment and prevention. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullins E., Hudak M.L., Banerjee J., Getzlaff T., Townson J., Barnette K., et al. Pregnancy and neonatal outcomes of covid-19: coreporting of common outcomes from pan-covid and aap-sonpm registries. Ultrasound Obstet Gynecol. 2021;57(4):573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap M., Debenham L., Kew T., Chatterjee S.R., Allotey J., Stallings E., et al. Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of covid-19 in pregnancy and postpartum: a living systematic review protocol. BMJ Open. 2020;10(12) doi: 10.1136/bmjopen-2020-041868. [DOI] [PMC free article] [PubMed] [Google Scholar]



