Abstract
We obtained and analyzed whole genome shotgun sequences of all 845 species of butterflies recorded from Canada and the United States. Genome-scale phylogenetic trees constructed from the data reveal several non-monophyletic genera and suggest improved classification of species included in these genera. Here, these changes are formalized and 2 subgenera are described: Amblyteria Grishin, subgen. n. (type species Goniloba exoteria Herrich-Schäffer, 1869, parent genus Amblyscirtes Scudder, 1872), and Coa Grishin, subgen. n. (type species Hesperia baracoa Lucas, 1857, parent genus Polites Scudder, 1872). Furthermore, we resurrect 3 genera and 2 subgenera from synonymy, change the rank of 6 currently used genera to subgenus, synonymize 2 genera, transfer 3 (2 resurrected) subgenera and 11 additional species to different genera than those these taxa were assigned to, and raise one name from synonym to species rank. Namely, Hedone Scudder, 1872 and Limochores Scudder, 1872 are valid genera and not synonyms of Polites Scudder, 1872; Pendantus K. Johnson & Kroenlein, 1993 is a valid genus and not a synonym of Electrostrymon Clench, 1961; and Sphaenogona Butler, 1870 and Lucidia Lacordaire, 1833 are valid subgenera of Abaeis Hübner, [1819] (new placement) and not synonyms of Eurema Hübner, [1819]. The following taxa are best treated as subgenera: Mimoides Brown, 1991 of Eurytides Hübner, [1821] (sensu lato); Philotiella Mattoni, [1978] of Euphilotes Mattoni, [1978]; Neominois Scudder, 1875 of Oeneis Hübner, [1819]; Agraulis Boisduval & Le Conte, [1835] of Dione Hübner, [1819]; Copaeodes Speyer, 1877 of Oarisma Scudder, 1872; and Problema Skinner & R. Williams, 1924 of Atrytone Scudder, 1872. Phaeostrymon Clench, 1961 and Saliana Evans, 1955 are junior subjective synonyms of Satyrium Scudder, 1876 and Calpodes Hübner, [1819], respectively. The entire subgenus Erynnides Burns, 1964 is transferred from Erynnis Schrank, 1801 to Gesta Evans, 1953. New genus-species combinations resulting from transfer of species between genera are: Nastra perigenes (Godman, 1900) (not Vidius Evans, 1955); Troyus fantasos (Cramer, 1780), Troyus onaca (Evans, 1955), Troyus aurelius (Plötz, 1882), Troyus marcus (Fabricius, 1787), Troyus diversa (Herrich-Schäffer, 1869), and Troyus drova (Evans, 1955) (not Vettius Godman, 1901); Oligoria percosius (Godman, 1900), Oligoria rindgei (H. Freeman, 1969), Oligoria lucifer (Hübner, [1831]), and Oligoria mustea (H. Freeman, 1979) (not Decinea Evans, 1955). Urbanus alva Evans, 1952 is a valid species and not a synonym of Urbanus belli (Hayward, 1935), new status.
Keywords: taxonomy, classification, genomics, phylogeny, biodiversity
INTRODUCTION
Genome-scale phylogenetic approaches show promise to revolutionize our understanding of butterfly evolution and refine their taxonomy (Allio et al. 2019; Li et al. 2019; Zhang et al. 2019a; Zhang et al. 2019b). Accurate phylogenetic trees constructed from millions of base pairs give better confidence in the results and frequently reveal inconsistencies with the current classification. Most taxonomists agree that a genus-group taxon should be monophyletic, i.e., a group of species that consists of all descendants of their common ancestor. Thus, if a strongly supported by statistics phylogenetic tree reveals that a genus is not monophyletic (i.e., its member species are distributed across several clades in the tree intermixed with species from other genera, or the clade with this genus includes some species that are not currently placed in this genus), some action needs to be taken to restore monophyly of the genus. The genus could either be split into several genera, or species that are currently not included into it, but are in the same clade with it, could be transferred into this genus. An important consideration is to follow the type species of the genus, because it defines the genus. Only the clade with the type species could carry this genus name. In addition to monophyly, attention should be paid to the prominence of the genus and consistency with how other genera in this group of organisms are defined, see Taxonomic Appendix to Li et al. (2019) for discussion. In brief, the genus should be a major phylogenetic group, i.e., it is best if the phylogenetic tree branch leading to the last common ancestor of the genus is longer compared to other nearby branches, and the genetic diversity within different genera should be comparable, so that genera define more or less equivalent groups.
We obtained and analyzed whole genome shotgun sequences of all butterfly species recorded from Canada and the United States (Pelham 2008; Pelham 2019). The manuscript describing this work is available from bioRxiv (Zhang et al. 2019c). Focusing on general evolutionary principles we can learn from butterflies, the manuscript does not go into taxonomic details. However, accurate phylogenetic trees we obtained from the genomic data reveal that some genera are not monophyletic, and some are either too broad or too narrow in terms of genetic divergence. Here, we propose taxonomic rearrangements that are supported by these phylogenetic trees. The following sections are in the standardized format. Taxonomic act is the title of each section. Relevant genera, subgenera and their type species, are listed giving valid names if the type species are synonyms. When the species are listed with their original genus name, author names are given without parenthesis. Currently used genus name for these species is clear from the context. Each section is illustrated by a small segment of a nuclear genomic tree with species that are needed to support the conclusion. The trees showing all US and Canada species are available from the bioRxiv preprint (Zhang et al. 2019c). Previous genus-species combinations (per Pelham 2019, version revised 7 October 2019) are used in the figures. New combinations are given in the text. Species of major focus are shown in red, other species in the genus of interest are shown in blue. Trees on gray background include species not recorded from the US. The section ends with a conclusion and in many cases with a list of species with revised genus-species names combinations. The sections are ordered by family and in their taxonomic order.
Family Papilionidae Latreille, [1802]
Mimoides Brown, 1991 is a subgenus of Eurytides Hübner, [1821]
Previously placed in the genus Mimoides Brown, 1991 (type species Papilio ariarathes, Esper, 1788), Papilio phaon Boisduval, 1836 is sister to Eurytides marcellus (Cramer, 1777) among butterflies of Canada and the US, and is phylogenetically close to Eurytides philolaus (Boisduval, 1836), suggesting that it should be placed in the genus Eurytides Hübner, [1821] (type species Eurytides iphitas Hübner, [1821]) (Fig. 1). Additionally taking into account that Mimoides species are close to each other as evidenced by their morphology (Tyler et al. 1994) and COI barcodes (Ratnasingham & Hebert 2007), we propose to treat Mimoides as a subgenus of Eurytides among its other subgenera: Protesilaus Swainson, [1832] (type species Papilio protesilaus Linnaeus, 1758) and Neographium Möhn, 2002 (type species Papilio philolaus Boisduval, 1836). Curiously, Papilio marcellus Cramer, 1777 is in the same clade with Mimoides, but is in a different clade from Neographium, and therefore should be included in the subgenus Mimoides despite the similarity in wing patterns to Eurytides (Neographium) philolaus. Finally, application of the genus Protographium Munroe (1961) (type species Papilio leosthenes E. Doubleday, 1846) to the New World is unwarranted, because genomic data show that the Australian endemic P. leosthenes is sister to another Old World genus Graphium Scopoli, 1777 (type species Papilio sarpedon Linnaeus, 1758) and is in a different clade from Eurytides (including Mimoides and Neographium) (Fig. 1). Eurytides versus Protographium is yet another case of striking wing pattern convergence in butterflies.
Fig. 1.
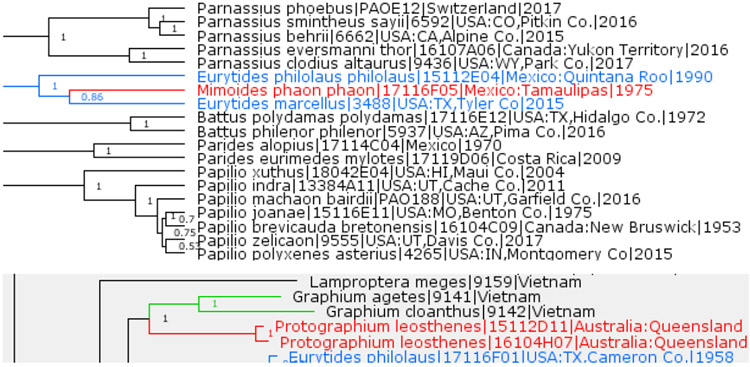
Eurytides, Mimoides and Protographium.
Family Pieridae Swainson, 1820
Sphaenogona Butler, 1870 and Lucidia Lacordaire, 1833 are subgenera of Abaeis Hübner, [1819] and not of Eurema Hübner, [1819], new placement
Previously junior subjective synonyms of Eurema Hübner, [1819] (type species Papilio delia Cramer, [1780], a junior subjective synonym of Pieris daira Godart, 1819), Sphaenogona Butler, 1870 (type species Terias bogotana C. & R. Felder, 1861, which is treated as a subspecies of Terias mexicana Boisduval, 1836) and Lucidia Lacordaire, 1833 (type species Papilio albula Cramer, 1775) are not monophyletic with Eurema, but are instead in the same clade with Abaeis Hübner, [1819] (type species Papilio nicippe Cramer, 1779) (Fig. 2). This genomic tree shows notable genetic divergence among Abaeis, Sphaenogona and Lucidia that is only slightly less than the divergence between Eurema and Pyrisitia Butler, 1870 (type species Papilio proterpia Fabricius, 1775) suggesting that Sphaenogona and Lucidia are not synonyms, but can be treated as subgenera of Abaeis. As a result, we use the following new or revised combination for the US species: Abaeis (Sphaenogona) boisduvaliana (C. Felder & R. Felder, 1865), Abaeis (Sphaenogona) mexicana (Boisduval, 1836), Abaeis (Sphaenogona) salome (C. Felder & R. Felder, 1861), and Abaeis (Lucidia) albula (Cramer, 1775). Consequently, only a single US species remains in Eurema, the type species of the genus: Eurema daira. Our proposed changes keep the number of genera in this group at 3 (Eurema, Abaeis, and Pyrisitia), and simply rearrange species between these genera. This rearrangement agrees with wing pattern characters on the dorsal side, making identification of the genus in the US easier. Both Eurema and Pyrisitia lack darker expanded area near forewing tornus and their males possess darker scaling along the outer margin of hindwing, at least by the veins. Eurema males can be distinguished by a long dark bar near forewing inner margin, which Pyrisitia species lack. Males of Abaeis species either have a dark forewing tornus (forewing mostly orange with a dark cell spot in the nominal subgenus and yellower, without the cell spot in the subgenus Sphaenogona), or lack dark scaling by the hindwing outer margin (USA only) and wings are mostly white with variable extent of dark margins (subgenus Lucidia). Although it is tempting to unite all these medium-sized white-yellow-orange butterflies in a single genus Eurema, their genetic divergence is very large (Fig. 2), and the group is divided into two prominent clades (Eurema + Pyrisitia and Abaeis), one of which splits into two more (Eurema and Pyrisitia). Therefore, we keep the three-genus arrangement of the group. Moreover, Abaeis as defined here is a broad and diverse genus. Comprehensive sequencing of the worldwide fauna of the group is likely to substantiate further splits rather than lumps.
Fig. 2.
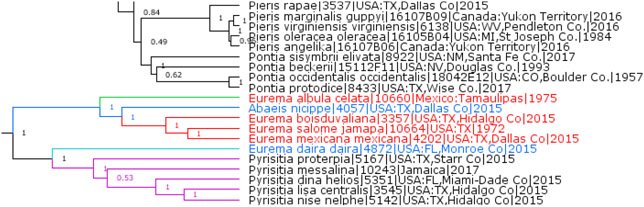
Eurema, Abaeis, Sphaenogona, and Lucidia.
Family Lycaenidae [Leach], [1815]
Philotiella Mattoni, [1978] is a subgenus of Euphilotes Mattoni, [1978]
Philotiella Mattoni, [1978] (type species Lycaena speciosa Hy. Edwards, 1877) is phylogenetically close to Euphilotes Mattoni, [1978] (type species Lycaena enoptes Boisduval, 1852) (COI barcodes differ by only 3.3%) and does not prominently stand out from it in the tree (Fig. 3). Therefore Philotiella is better treated as a subgenus, new status. Euphilotes is a genus distinct from Philotes Scudder, 1876 (type species Lycaena regia Boisduval, 1869, a junior subjective synonym of Lycaena sonorensis C. Felder & R. Felder, 1865), because they are not monophyletic. Philotes is sister to Glaucopsyche Scudder, 1872 (type species Polyommatus lygdamus E. Doubleday, 1841) (Fig. 3). However, these three genera are close to each other and, if one prefers, can be combined under Glaucopsyche. The tight cluster of species comprising Euphilotes, (Fig. 3) contrasts with the two rather distant species of Glaucopsyche and the monotypic Philotes at about the same distance from them. Apparently, not all genera form prominent assemblages of species, and some genera may not be that distinct from each other, like Glaucopsyche and Philotes.
Fig. 3.

Euphilotes, Philotiella, and Philotes.
Phaeostrymon Clench, 1961 is a junior subjective synonym of Satyrium Scudder, 1876
Phaeostrymon Clench, 1961 (type and the only species Thecla alcestis W. H. Edwards, 1871) was previously considered a valid genus, but it originates within Satyrium Scudder, 1876 (type species Lycaena fuliginosa W. H. Edwards, 1861), thus rendering Satyrium paraphyletic (Fig. 4). Because Satyrium is a tight (but diverse) group of close relatives prominently separated from other genera (Fig. 4), Phaeostrymon is a close sister to the clade with the type species of Satyrium, and Phaeostrymon is not prominently distinct from this clade, it is best to treat Phaeostrymon as a junior subjective synonym of Satyrium, new status.
Fig. 4.
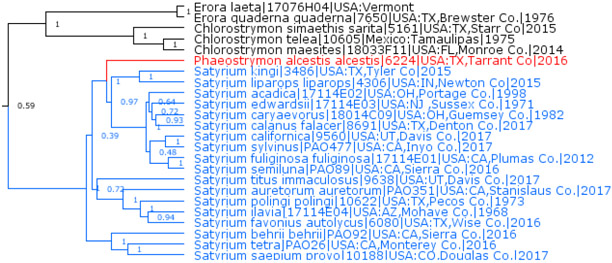
Satyrium and Phaeostrymon.
Pendantus K. Johnson & Kroenlein, 1993 is a valid genus
Previously placed in the genus Electrostrymon Clench, 1961 (type species Papilio endymion Fabricius, 1775), Thecla guzanta Schaus, 1902 is not monophyletic with it and instead is sister to Calycopis Scudder, 1876 (type species Rusticus poeas Hübner, [1811], which is a junior subjective synonym of Hesperia cecrops Fabricius, 1793) (Fig. 5). Not willing to place guzanta in Calycopis due to genetic and morphological divergence of about the same magnitude as that between Electrostrymon and Kisutam K. Johnson, 1993 (Thecla syllis Godman & Salvin, 1887), we resurrect from synonymy the genus Pendantus K. Johnson & Kroenlein, 1993 (type species Thecla plusios Godman & Salvin 1887, currently treated as a junior subjective synonym of Tmolus denarius Butler & H. Druce, 1872) and form new or revised combinations: Pendantus guzanta, Pendantus thurman (Thompson & Robbins, 2016), Pendantus denarius (A. Butler & H. Druce, 1872), and Pendantus perisus (H. Druce, 1907).
Fig. 5.
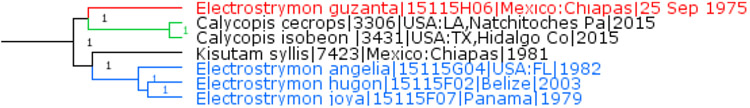
Electrostrymon, Calycopis, and Pendantus.
Family Nymphalidae Rafinesque, 1815
Neominois Scudder, 1875 is a subgenus of Oeneis Hübner, [1819]
In agreement with the previous study (Kleckova et al. 2015), we find that Neominois Scudder, 1875 (type species Satyrus ridingsii W. H. Edwards, 1865) originates within Oeneis Hübner, [1819] (type species Papilio norna Thunberg, 1791, represented by O. polixenes (Fabricius, 1775) in the US) as it is currently defined, and is sister to the subgenus ueneis. COI barcode difference between ridingsii and polixenes is only 6.2%. However, subgenus Protoeneis Gorbunov, 2001 (type species Chionobas nanna Ménétriés, 1858, represented by Oeneis uhleri (Reakirt, 1866) in the US fauna) that is placed in the genus Oeneis, is a sister to the clade consisting of Neominois and subgenus Oeneis (Fig. 6). The entire group of 3 taxa is compact (Fig. 6), prominent, and genetic divergence within it agrees with the expected divergence within a genus. Therefore, we treat Neominois as a subgenus of Oeneis.
Fig. 6.

Oeneis and Neominois.
Agraulis Boisduval & Le Conte, [1835] is a subgenus of Dione Hübner, [1819]
Monotypic genus Agraulis Boisduval & Le Conte, [1835] (type species Papilio vanillae Linnaeus, 1758) is a close sister to Dione Hübner, [1819] (type species Papilio juno Cramer, 1779) (Fig. 7). COI barcode difference between A. vanillae and Dione moneta Hübner, [1825] is 7.9%. Time-calibrated nuclear genomic tree shows that genetic divergence (Fig. 7) between Agraulis and Dione is nearly the same as the divergence among species of Boloria Moore, 1900 (type species Papilio pales [Denis & Schiffermüller], 1775) and smaller than the divergence between Heliconius Kluk, 1780 (type species Papilio charithonia Linnaeus, 1767) and Eueides Hübner, 1816 (type species Nereis dianasa Hübner, [1806]) (Fig. 7). Therefore, we treat Agraulis as a subgenus of Dione.
Fig. 7.

Agraulis and Dione.
Family Hesperiidae Latreille, 1809
Urbanus alva Evans, 1952, new status
Described as a subspecies of Urbanus viterboana (Ehrmann, 1907) by Evans (1952), alva (the holotype, male, from Mexico: Veracruz, Atoyac, examined by NVG) was placed in synonymy with Urbanus belli (Hayward, 1935) (the holotype, female, from Argentina: Salta, photographs examined) by Steinhauser (1981), who was unable to see the belli holotype. Our genomic analysis of U. belli from Argentina indicates that it is not conspecific with belli-like specimens from Mexico or anywhere else in North America due to genetic divergence between them. Evans noted shorter hindwing tails in alva compared to belli, and this character holds true comparing the holotypes of these taxa and additional belli specimens from Argentina we sequenced, including males. For these reasons we resurrect alva from synonymy with belli and treat it as a distinct species Urbanus alva, new status. Consequently, we exclude Urbanus belli (Hayward, 1935) from the North American fauna.
Erynnides Burns, 1964 is a subgenus of Gesta Evans, 1953 and not of Erynnis Schrank, 1801, new placement
Gesta Evans, 1953 (type species Thanaos gesta Herrich-Schäffer, 1863) is a sister to subgenus Erynnides Burns, 1964 (type species Nisoniades propertius Scudder & Burgess, 1870), with the exclusion of subgenus Erynnis Schrank, 1801 (type species Papilio tages Linnaeus, 1758, represented by Erynnis brizo (Boisduval & Le Conte, [1837]) and Erynnis icelus (Scudder & Burgess, 1870) in the US), thus rendering Erynnis paraphyletic (Fig. 8). There are three possible solutions. First (splitting), treat all three (Erynnis, Gesta, and Erynnides) as valid genera. However, genetic divergence between Gesta, and Erynnides is moderate (about 8% in the COI barcode), and no prominent tree branches separate the two taxa. Their genitalia are also quite similar. Therefore, these two taxa are best viewed as subgenera. Second (lumping), treat all three as subgenera of Erynnis, thus eliminating genus-species combinations involving Gesta. However, genetic divergence between Erynnis (sensu stricto) and Gesta + Erynnides is prominent (Fig. 8), comparable to that between other Erynnini Brues & F. Carpenter, 1932 genera. While the lumping solution is more compatible with how these taxa were viewed historically, it is not consistent with how other members of Erynnini are partitioned into genera. Third (middle ground), is a two-genus solution, i.e., to transfer Erynnides from Erynnis to Gesta. Phylogenetic trees show the two prominent clades corresponding to these two genera, and the clade leading to their common ancestor is shorter and thus less prominent (Fig. 8). Genetic divergence between these two genera is the same magnitude as between other sister genera of Erynnini. This divergence is equally profound in nuclear (autosomes and Z chromosome) and mitochondrial genomes. Therefore, we prefer this two-genus solution. As a result, all species formerly placed in the subgenus Erynnides of Erynnis would change their genus name to Gesta. This action results in many name changes, but highlights deep genetic divergence between mostly Old Worth Erynnis and exclusively New World Gesta and thus seems to be more biologically meaningful. Although the switch of names is bothersome in short run, it may be beneficial long term.
Fig. 8.
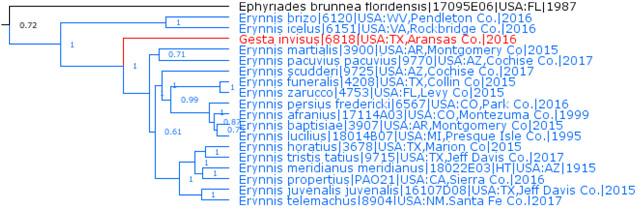
Erynnis and Gesta.
Copaeodes Speyer, 1877 is a subgenus of Oarisma Scudder, 1872
Previously placed in the genus Oarisma Scudder, 1872 (type species Hesperia powesheik Parker, 1870), Thymelicus edwardsii W. Barnes, 1897 is not monophyletic with it, and is a close sister to the two US species from the genus Copaeodes Speyer, 1877, including its type species Heteropterus procris W. H. Edwards, 1871, a junior subjective synonym of Ancy-loxipha [sic!] aurantiaca Hewitson, 1868 (Fig. 9). Investigation of other Oarisma and Copaeodes species reveals that they are close to each other and difficult to partition between the two genera (Fig. 9). Only the close cluster of 3 species O. poweshiek (Parker, 1870), O. garita (Reakirt, 1866), and O. era Dyar, 1927 constitute Oarisma sensu stricto. Others fall in a different and broad clade, which in addition to Copaeodes includes other species previously placed in Oarisma, e.g., O. edwardsii (W. Barnes, 1897) and O. nanus (Herrich-Schäffer, 1865), and we treat this clade as a subgenus Copaeodes within a broader-defined Oarisma.
Fig. 9.
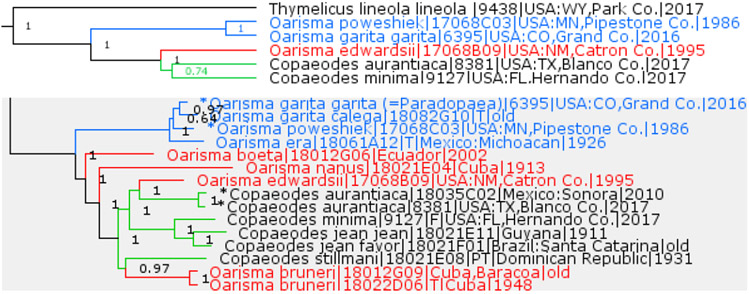
Oarisma and Copaeodes. US species (tree above) and including others (gray tree below, asterisks mark type species).
Saliana Evans, 1955 is a junior subjective synonym of Calpodes Hübner, [1819]
Monotypic genus Calpodes Hübner, [1819] (type species Papilio ethlius Stoll, 1782) is genetically close to Saliana Evans, 1955 (type species Papilio salius Cramer, 1775) (Fig. 10) and their type species differ by only about 6.5% in COI barcodes. Moreover, Calpodes + Saliana clade experienced rapid radiation over a short period of time and, as a result, the tree looks more like a comb than a bi-branching structure (Fig. 10). It is not clear whether Saliana is monophyletic: 64% bootstrap in the nuclear genome tree suggests that it is not, and that Saliana fusta Evans, 1955 is sister to the rest of Saliana + Calpodes. Due to rapid radiation, this genus is difficult to partition into meaningful subgenera, because no clear clusters of species are apparent in the tree (Fig. 10). For all these reasons, we propose that Saliana is a junior subjective synonym of Calpodes, new status. Interestingly, C. ethlius diverged strongly in wing shapes and patterns from all other members of the genus (although their male genitalia are similar) and this new synonymy is unexpected. Therefore, we tested the results using multiple specimens of C. ethlius (7 shown in Fig. 10) and they all cluster together, within former Saliana. The newly expanded Calpodes is a strongly supported (bootstrap 100%) and prominent (long tree branch is leading to it) genus.
Fig. 10.
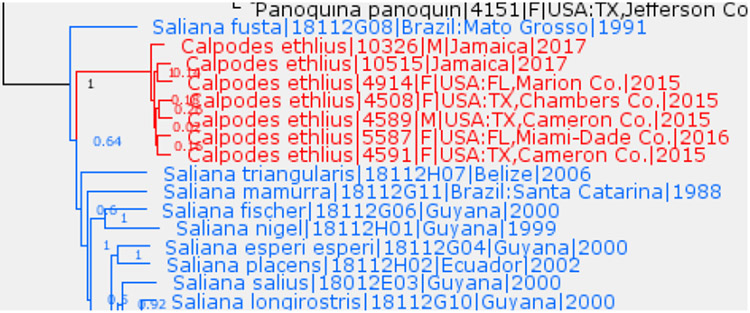
Calpodes and Saliana.
Nastra perigenes (Godman, 1900), new combination
Previously placed in the genus Vidius Evans, 1955 (type species Narga vidius Mabille, 1891), Mastor perigenes Godman, 1900 is not monophyletic with the type species of Vidius, and instead forms a clade with Nastra Evans, 1955 (type species Hesperia lherminier Latreille, [1824]) (Fig 11) and therefore is transferred to this genus to form a new combination Nastra perigenes.
Fig. 11.
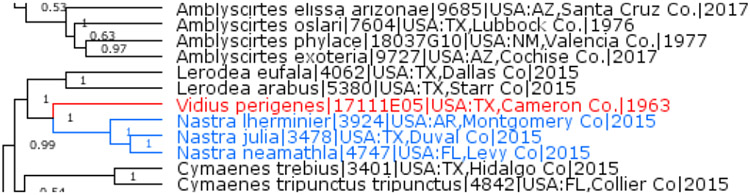
Nastra and no Vidius.
Amblyteria Grishin, new subgenus
http://zoobank.org/C1F98F8D-C366-4065-9317-EA039D15CB22
Type species.
Goniloba exoteria Herrich-Schäffer, 1869.
Definition.
A prominent clade within Amblyscirtes Scudder, 1872 (type species Hesperia vialis W. H. Edwards, 1862) without a name (Fig. 11, 12). Keys to N.2.2, 6, 8, or 22 in Evans (1955). Phenotypically diverse lineage of species that differs from its relatives by the following combination of characters: males either with stigma long, narrow and rather straight, hindwing below with many small white spots in some species, and size small (forewing length mostly < 15mm), or if larger, then forewing pale spot in cell M1-M2 strongly offset towards outer margin; or with brands at the base of vein CuA2, and brands either very conspicuous on bronze-colored wings, or wings black, head orange and fringes not orange but pale. Due to this pronounced phenotypic variation, the subgenus is best defined by DNA characters. A combination of the following base pairs in the standard DNA COI barcode region (658 bp) is diagnostic: T548C, A550T (these two characters are synapomorphic), 343(not G as in Stomyles), T346T(not C as in Stomyles), T553T(not A as in the nominal subgenus), and A637A(not T as in Stomyles).
Fig. 12.
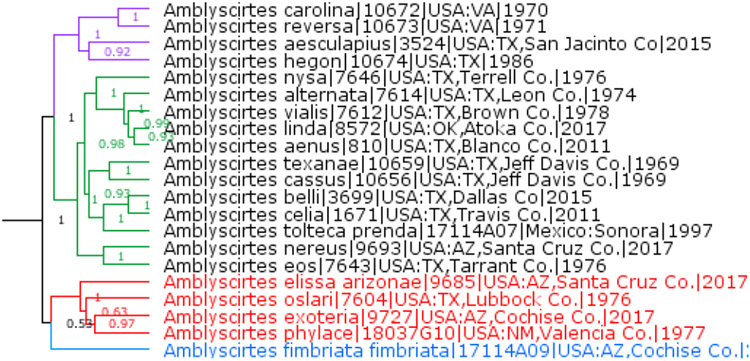
Amblyscirtes.
Etymology.
The name is a feminine noun in the nominative singular, a fusion of the type species name epithets: Amby[scirtes] + [exo]teria.
Species included.
Amblyscirtes elissa Godman, [1900], Pamphila oslari Skinner, 1899, Amblyscirtes brocki Freeman, 1992, Goniloba exoteria Herrich-Schäffer, 1869, and Pamphila phylace Edwards, 1878.
Parent taxon.
Genus Amblyscirtes Scudder, 1872.
Assignment of species to subgenera.
With the description of Amblyteria subgen. n., we consider that the genus Amblyscirtes consists of 4 subgenera (Fig. 12). Subgenus Stomyles Scudder, 1872 (Eastern US clade) consists of A. carolina (Skinner, 1892), A. reversa F. Jones, 1926, A. aesculapius (Fabricius, 1793), and A. hegon (Scudder, 1863). Subgenus Mastor Godman, [1900] (Southern clade) consists of A. fimbriata (Plötz, 1882) (the only USA species), A. anubis (Godman, 1900), A. novimmaculatus A. Warren, 1998, A. raphaeli H. Freeman, 1973, A. patriciae (E. Bell, 1959), and A. folia Godman, 1900. Names of type species are underlined. Other Amblyscirtes species (Mielke 2005; Pelham 2008) not mentioned here belong to the nominal subgenus.
Comment.
A surprising result is that two very similar-looking species A. (Amblyteria) phylace and A. (Mastor) fimbriata are not each other's closest relatives and belong to different subgenera (Fig. 12).
Troyus fantasos (Cramer, 1780), Troyus onaca (Evans, 1955), Troyus aurelius (Plötz, 1882), Troyus marcus (Fabricius, 1787), Troyus diversa (Herrich-Schäffer, 1869), and Troyus drova (Evans, 1955), new combinations
Previously placed in the genus Vettius Godman, 1901 (type species Papilio phyllus Cramer, 1777), Papilio fantasos Cramer, 1780 is not monophyletic with Vettius type species, and is a close relative of the monotypic genus Troyus A. Warren & Turland, 2012 (type and the only included species Troyus turneri A. Warren & Turland, 2012). Their sister genus is Monca Evans, 1955 (type species Cobalus telata Herrich-Schäffer, 1869) (Fig. 13). Therefore, we establish a new combination Troyus fantasos Due to genetic and morphological similarities, we additionally propose the following new combinations: Troyus onaca (Evans, 1955), Troyus aurelius (Plötz, 1882), Troyus marcus (Fabricius, 1787), Troyus diversa (Herrich-Schäffer, 1869), and Troyus drova (Evans, 1955). All these species were in Vettius before.
Fig. 13.

Troyus, Monca, and no Vettius.
Hedone Scudder, 1872 is a valid genus
We find that Polites Scudder, 1872 (type species Hesperia peckius W. Kirby, 1837) is paraphyletic with respect to Wallengrenia Berg, 1897 (type species Hesperia premnas Wallengren, 1860). Genetic divergence within Polites that includes Wallengrenia is too large compared to how most Hesperiidae genera are defined. Therefore, instead of including Wallengrenia into Polites, it makes sense to restore monophyly of these genera by splitting Polites into genera with divergence consistent with that of most other Hesperiidae genera. Previously a junior subjective synonym of Polites Scudder, 1872 (type species Hesperia peckius W. Kirby, 1837), Hedone Scudder, 1872 (type species Hesperia brettus Boisduval & Le Conte, [1837], a junior subjective synonym of Thymelicus vibex Geyer, 1832) forms a clade sister to the rest of Polites + Wallengrenia (Fig. 14). Therefore, Hedone is a valid genus, and Hedone vibex (Geyer, 1832) is a revised combination. Due to morphological similarities, we additionally propose the following new combinations: Hedone bittiae (Lindsey, 1925), Hedone vibicoides (de Jong, 1983), and Hedone dictynna (Godman & Salvin, 1896).
Fig. 14.

Polites, Wallengrenia, Hedone, and Limochores.
Limochores Scudder, 1872 is a valid genus
Sister to the rest of Polites + Wallengrenia excluding Hedone, Limochores Scudder, 1872 (type species Hesperia manataaqua Scudder, 1863, which is a junior subjective synonym of Hesperia origenes Fabricius, 1793) was treated as a junior subjective synonym of Polites (Fig. 14). For the reasons given above for Hedone, we propose the following new or revised combinations: Limochores origenes (Fabricius, 1793), Limochores mystic (W. H. Edwards, 1863), Limochores sonora (Scudder, 1872), Limochores puxillius (Mabille, 1891), and Limochores pupillus (Plötz, 1882). Interestingly, two difficult-to-distinguish species that frequently fly together at the same location, L. origenes and Polites themistocles (Latreille, [1824]), ended up in different genera. Even though their genitalia are similar, they belong to distant from each other clades in the tree (Fig. 14): P. themistocles is closely related to P. peckius, the type genus of Polites, while L. origenes is closer to L. mystic.
Coa Grishin, new subgenus
http://zoobank.org/CD8143FC-839D-408E-A114-3FFEEA7AE349
Type species.
Hesperia baracoa Lucas, 1857.
Definition.
A sister to subgenus Yvretta Hemming, 1935 (type species Pamphila citrus Mabille, 1889, which is treated as a junior subjective synonym Hesperia subreticulata Plötz, 1883), Hesperia baracoa Lucas, 1857 prominently stands out from other Polites (Fig. 14). Therefore, this lineage is given a subgenus status and a name. This new subgenus keys to M.13.4 in Evans (1955). Distinguished from its relatives within Polites by the combination of the following characters: presence of apiculus (longer than 1 segment); diagnostic shape of stigma: rather short, relatively straight and narrower than in other species with defined apiculus; the lack of spot before the end of discal cell on plain gray-brown ventral hindwing without dark spots (but sometimes with a row of pale discal spots) combined with orange area by the forewing costa below, stemming from the wing base and reaching apical spots.
Etymology.
The name is a feminine noun in the nominative singular, the ending of the type species name.
Species included.
Only the type species.
Parent taxon.
Genus Polites Scudder, 1872.
Assignment of species to subgenera.
With the description of Coa subgen. n., we consider that Polites consists of 3 subgenera (Fig. 14). Subgenus Yvretta consists of P. subreticulata (Plötz, 1883), P. carus (W. H. Edwards, 1883), and P. rhesus (W. H. Edwards, 1878). Other Polites species (Mielke 2005; Pelham 2008) not mentioned in this work belong to the nominal subgenus. Interestingly, Wallengrenia Berg, 1897 (type species Hesperia premnas Wallengren, 1860) is not prominently distinct genetically from Polites (green branch in Fig. 14) and may therefore be included in Polites as the 4th subgenus, depending on researcher's taste.
Problema Skinner & R. Williams, 1924 is a subgenus of Atrytone Scudder, 1872
Currently monotypic genus Atrytone Scudder, 1872 (type species Hesperia iowa Scudder, 1868) is sister to Problema Skinner & R. Williams, 1924 (type genus Pamphila byssus W. H. Edwards, 1880), a genus of two species, including also Hesperia bulenta Boisduval & Le Conte, [1837]. All three species are close in their genomic sequences (Fig. 15). Their genetic divergence is more consistent with them being congeners. Therefore, we treat Problema as a subgenus of Atrytone to form new or revised combinations Atrytone byssus and Atrytone bulenta.
Fig. 15.
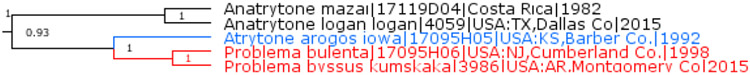
Atrytone and Problema.
Oligoria percosius (Godman, 1900), Oligoria rindgei (H. Freeman, 1969), Oligoria lucifer (Hübner, [1831]), and Oligoria mustea (H. Freeman, 1979), new combinations
Previously placed in the genus Decinea Evans, 1955 (type species Hesperia decinea Hewitson, 1876), Cobalus percosius Godman, 1900 is not monophyletic with Decinea type species, which is in the same clade with Buzyges Godman, 1900. Instead, percosius is a very close sister of the monotypic genus Oligoria Scudder, 1872 (type species Hesperia maculata W. H. Edwards, 1865) (Fig. 16), which is in the same clade with Xeniades Godman, 1900. COI barcode difference between maculata and percosius is only 4%. Therefore, we establish a new combination: Oligoria percosius. Due to morphological and genetic DNA similarities, we additionally propose the following new combinations: Oligoria rindgei (H. Freeman, 1969), Oligoria lucifer (Hübner, [1831]), and Oligoria mustea (H. Freeman, 1979) (Fig. 16). All these species were previously placed in Decinea.
Fig. 16.
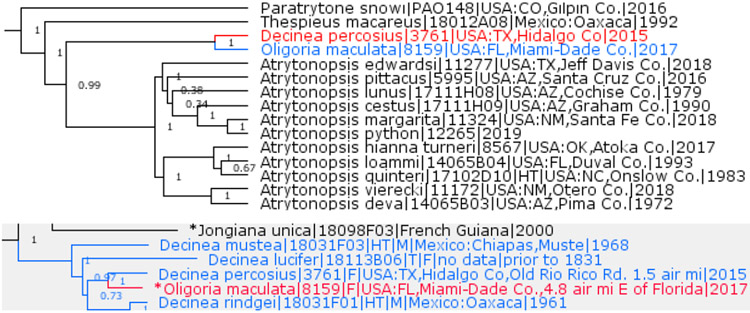
Oligoria and no Decinea. US species (tree above) and including others (gray tree below, asterisks mark type species).
ACKNOWLEDGMENTS
We acknowledge Leina Song and Ping Chen for excellent technical assistance. We are grateful to David Grimaldi and Courtney Richenbacher (AMNH: American Museum of Natural History, New York, NY, USA), Jason Weintraub (ANSP: Academy of Natural Sciences of Drexel University, Philadelphia, PA, USA), Jonathan P. Pelham (BMUW: Burke Museum of Natural History and Culture, Seattle, WA, USA), Vince Lee and the late Norm Penny (CAS: California Academy of Sciences, San Francisco, CA, USA), Boris Kondratieff (CSUC: Colorado State University Collection, Fort Collins, CO, USA), Crystal Maier and Rebekah Baquiran (FMNH: Field Museum of Natural History, Chicago, FL, USA), Weiping Xie (LACM: Los Angeles County Museum of Natural History, Los Angeles, CA, USA), Andrew D. Warren and Debbie Matthews-Lott (MGCL: McGuire Center for Lepidoptera and Biodiversity, Gainesville, FL, USA), Edward G. Riley, Karen Wright, and John Oswald (TAMU: Texas A&M University Insect Collection, College Station, TX, USA), Alex Wild (TMMC: University of Texas Biodiversity Center, Austin, TX, USA), Jeff Smith and Lynn Kimsey (UCDC: Bohart Museum of Entomology, University of California, Davis, CA, USA), Robert K. Robbins, John M. Burns, and Brian Harris (USNM: National Museum of Natural History, Smithsonian Institution, Washington, DC, USA) for granting access to the collections under their care and for stimulating discussions; to Jim P. Brock, Jack S Carter, Bill R. Dempwolf, James McDermott, the late Edward C. Knudson (TLS: Texas Lepidoptera Survey, specimens now at MGCL), Harry Pavulaan, James A. Scott, John A. Shuey, and Mark Walker for specimens and leg samples, to Jonathan P. Pelham for insightful discussions and critical review of the manuscript. Greg Kareofelas (Davis, CA) and Matthew Garhart (Grand Junction, CO) collected selected species and placed them in RNAlater for molecular analysis. Boris Kondratieff, Chuck Harp, and James Scott curated the butterfly collection at the C. P. Gillette Museum of Arthropod Diversity, Colorado State University, which facilitated the accurate sampling of remaining species needed to complete the analysis. Evi Buckner-Opler assisted by providing emotional and logistic support and helped to collect specimens. We are indebted to Texas Parks and Wildlife Department (Natural Resources Program Director David H. Riskind) for the research permit 08-02Rev, to U. S. National Park Service for the research permits: Big Bend (Raymond Skiles) for BIBE-2004-SCI-0011 and Yellowstone (Erik Oberg and Annie Carlson) for YELL-2017-SCI-7076 and to the National Environment & Planning Agency of Jamaica for the permission to collect specimens. We acknowledge the Texas Advanced Computing Center (TACC) at The University of Texas at Austin for providing HPC resources. The study has been supported in part by grants from the National Institutes of Health GM127390 and the Welch Foundation I-1505.
Footnotes
ZooBank registration: http://zoobank.org/57AAF2C1-C1A5-4B90-8FAF-69E26D95B5C8
LITERATURE CITED
- Allio R, Scornavacca C, Benoit N, Clamens AL, Sperling FAH, and Condamine FL. 2019. Whole genome shotgun phylogenomics resolves the pattern and timing of swallowtail butterfly evolution. Systematic Biology: ahead of print May 7. pii: syz030. [DOI] [PubMed] [Google Scholar]
- Evans WH 1952. A catalogue of the American Hesperiidae indicating the classification and nomenclature adopted in the British Museum (Natural History). Part II. Pyrginae. Section I. British Museum (Natural History). London. v + 178 pp., pls. 10–25. [Google Scholar]
- Evans WH 1955. A catalogue of the American Hesperiidae indicating the classification and nomenclature adopted in the British Museum (Natural History). Part IV. Hesperiinae and Megathyminae. British Museum (Natural History); London. v + 499 pp., pls. 54–88. [Google Scholar]
- Kleckova I, Cesanek M, Fric Z, and Pellissier L. 2015. Diversification of the cold-adapted butterfly genus Oeneis related to Holarctic biogeography and climatic niche shifts. Molecular Phylogenetics and Evolution 92: 255–265. [DOI] [PubMed] [Google Scholar]
- Li W, Cong Q, Shen J, Zhang J, Hallwachs W, Janzen DH, and Grishin NV. 2019. Genomes of skipper butterflies reveal extensive convergence of wing patterns. Proceedings of the National Academy of Sciences of the United States of America 116(13): 6232–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke OHH 2005. Catalogue of the American Hesperioidea: Hesperiidae (Lepidoptera). Sociedade Brasileira de Zoologia; Curitiba, Paraná, Brazil. xiii + 1536 pp. [Google Scholar]
- Pelham JP 2008. Catalogue of the Butterflies of the United States and Canada. Journal of Research on the Lepidoptera 40: 1–658. [Google Scholar]
- Pelham JP 2019. Catalogue of the Butterflies of the United States and Canada. Revised 7 Oct 2019. Accessed 8 Oct 2019 (http://www.butterfliesofamerica.com/US-Can-Cat.htm). [Google Scholar]
- Ratnasingham S, and Hebert PD. 2007. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes 7(3): 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser SR 1981. Revision of the proteus group of the genus Urbanus Hubner (Lepidoptera: Hesperiidae). Bulletin of the Allyn Museum 62: 1–41. [Google Scholar]
- Tyler HA, Brown KS, and Wilson KH. 1994. Swallowtail butterflies of the Americas: a study in biological dynamics, ecological diversity, biosystematics, and conservation. Scientific Publishers; Gainesville, FL. 376 pp. [Google Scholar]
- Zhang J, Cong Q, Shen J, Brockmann E, and Grishin NV. 2019a. Genomes reveal drastic and recurrent phenotypic divergence in firetip skipper butterflies (Hesperiidae: Pyrrhopyginae). Proceedings of the Royal Society B: Biological Sciences 286(1903): 20190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cong Q, Shen J, Brockmann E, and Grishin NV. 2019b. Three new subfamilies of skipper butterflies (Lepidoptera, Hesperiidae). Zookeys 861: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cong Q, Shen J, Opler PA, and Grishin NV. 2019c. Genomics of a complete butterfly continent. bioRxiv preprint. [Google Scholar]


