Abstract
Background
Keratoconus (KC) is the most common ectatic corneal disease, characterized by significantly localized thinning of the corneal stroma. Genetic, environmental, hormonal, and metabolic factors contribute to the pathogenesis of KC. Additionally, multiple comorbidities, such as diabetes mellitus, may affect the risk of KC.
Main Text
Patients with diabetes mellitus (DM) have been reported to have lower risk of developing KC by way of increased endogenous collagen crosslinking in response to chronic hyperglycemia. However, this remains a debated topic as other studies have suggested either a positive association or no association between DM and KC. To gain further insight into the underlying genetic components of these two diseases, we reviewed candidate genes associated with KC and central corneal thickness in the literature. We then explored how these genes may be regulated similarly or differentially under hyperglycemic conditions and the role they play in the systemic complications associated with DM.
Conclusions
Our comprehensive review of potential genetic factors underlying KC and DM provides a direction for future studies to further determine the genetic etiology of KC and how it is influenced by systemic diseases such as diabetes.
Keywords: Keratoconus, Genetics, Diabetes, Collagen crosslinking, Oxidative stress
Highlights
-
•
Keratoconus (KC) could be attributed to genetic, environmental, hormonal, metabolic factors.
-
•
Among many comorbidities, diabetes mellitus (DM) could be associated with KC negatively.
-
•
We provided a comprehensive review on the potential connections between KC and DM.
-
•
Underlying genes may be involved in biomechanics, extracellular matrix dynamics, inflammation, and oxidative stress.
1. Introduction
Keratoconus (KC) is a bilateral, progressive ectatic corneal disorder characterized by localized thinning of the corneal stroma and alteration of corneal curvature.1 As the cornea adopts a conical shape, this results in myopia, irregular astigmatism, and eventual visual impairment.1 The histopathology of KC includes stromal thinning, breaks in Bowman's layer, focal fibrosis, thickening of the epithelium, and keratocyte apoptosis in the anterior stroma.2,3 Although KC is known to be multifactorial with genetic, metabolic, hormonal, and environmental influences, the exact etiology remains elusive.4, 5, 6 The risk of developing KC has been associated either positively or negatively with many systemic disorders, including but not limited to, Down syndrome,7 connective tissue diseases,8, 9, 10 autoimmune diseases,11 and diabetes mellitus (DM).9,12, 13, 14, 15 However, the exact etiology of KC and its relationship to systemic diseases, such as DM, remains elusive.
DM may have multiple effects on the cornea, including keratopathy, neuropathy, inflammation, alterations in collagen fibrils, and endothelial cell loss.16 Multiple studies have suggested that DM is inversely associated with the risk of KC, suggesting a protective role against the development and/or severity of KC.13,14,17,18 This is in contrast to other studies that have reported either 1) a positive association in both prevalence and severity between KC and DM or 2) no significant correlation between the two diseases.14,19, 20, 21, 22 This discrepancy may be reflective of the varying sample sizes, inclusion/exclusion criteria, sample ascertainment approaches, and populations analyzed (Table 1).
Table 1.
List of human studies evaluating the relationship between keratoconus and diabetes
(adapted and modified from 26).
| Study | Study size | Design | Population Characteristics | Findings | Association |
|---|---|---|---|---|---|
| 13 | KC patients (n=571) Non-KC controls (n=571) |
Retrospective case-control study |
|
T2DM showed a protective effect against KC development (odds ratio = 0.2195) | Inverse association of KC development with DM |
| 14 | KC patients without DM (n=269) KC patients with DM (n=26) |
Retrospective cross-sectional study |
|
T2DM showed a protective effect against more severe KC (odds ratio = 0.2); No difference in DM prevalence in KC population | Inverse association of DM with KC severity |
| 17 | KC patients (n=1383) non-KC controls (n=1383) | Retrospective case-control study |
|
T2DM showed a protective effect against KC development (odds ratio = 0.350) | Inverse association of KC development with DM |
| 18 | KC patients (n=16,053) non-KC controls (n=16,053) | Retrospective longitudinal cohort study |
|
20% lower odds of KC development with uncomplicated DM; 52% lower odds of KC development with DM-associated organ failure | Inverse association of KC development with DM |
| 19 | KC patients (n=2679) non-KC controls (n=26,7900 | Retrospective longitudinal cohort study |
|
No significant difference in DM prevalence in KC patients. Total DM odds ratio=1.03, T1DM odds ratio=0.87, T2DM odds ratio=1.07 | No significant association between KC development and DM |
| 298 | KC patients (n=575) non-KC controls (n=2875) | Retrospective longitudinal cohort study |
|
No significant difference in DM prevalence in KC patients. Multivariate odds ratio=1.02 | No significant association between KC development and DM |
| 299 | 29 studies incorporating 50,358,341 subjects | Systematic review and meta-analysis |
|
Odds of developing KC were 23% lower in T2DM, but relationship was not significant | No significant association between KC development and DM |
| 22 | KC patients (n=2051) non-KC controls (n=12,306 | Retrospective case-control study |
|
No significant association in KC and DM, with odds ratio 1.60 (0.89–2.89) and p-value 0.149. | No significant association between KC development and DM |
| 20 | KC patients (n=1377) non-KC controls (n=4131) AND T2DM KC patients (n=75) non-DM KC controls (n=225) |
Retrospective case-control and Cross-sectional study |
|
Higher prevalence of T2DM in KC population compared to controls (6.75% and 4.84%, respectively); Higher severity of KC in DM patients (odds ratio = 2.691) | Positive association of KC development with T2DM |
| 21 | KC patients (n=1552) non-KC controls (n=7.760) | Retrospective cohort study |
|
Higher prevalence of T2DM in KC population compared to controls (19.2% and 14.5%, respectively); Positive association of KC with DM (odds ratio = 1.35) | Positive association of KC development with DM |
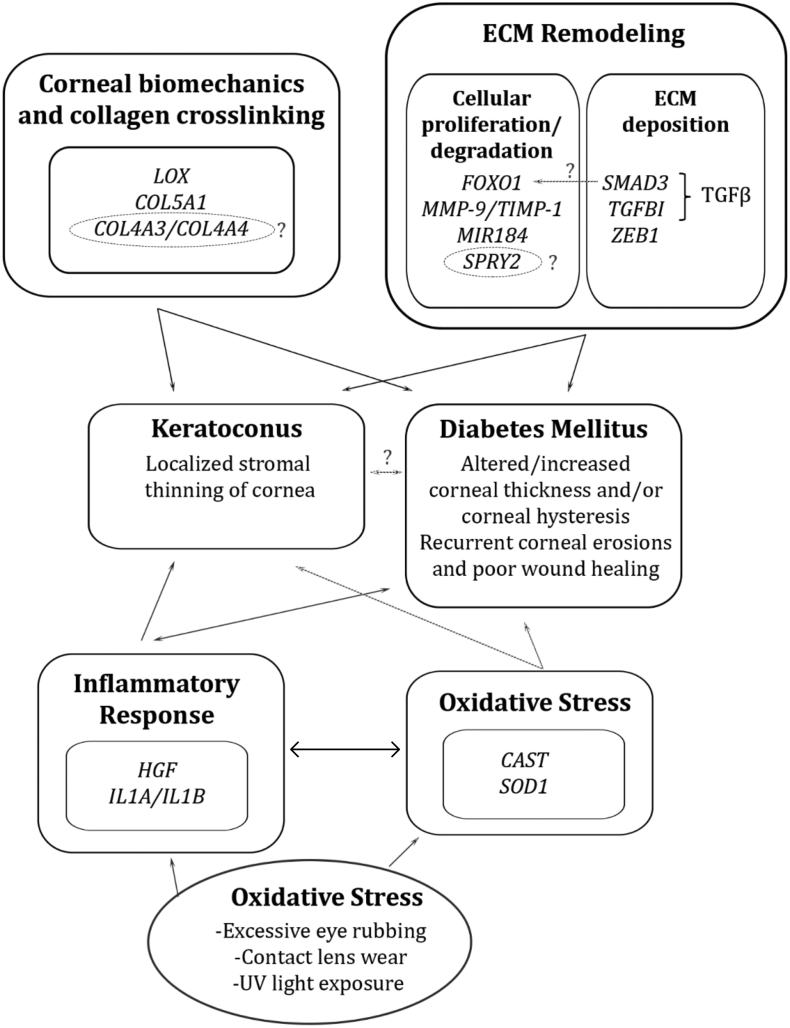
Our goal was to provide a comprehensive review of potential genetic associations between DM and KC. We have outlined various genes implicated in KC risk and summarized their potential roles in DM-induced corneal changes (Table 2). These genes may work through several mechanisms including alterations in corneal biomechanics and collagen crosslinking, alterations in ECM composition and proteolytic activity, as well as increased inflammation and oxidative stress.
Table 2.
List of genes that could mediate the potential correlation between keratoconus and diabetes.
| Gene | Functions | CHR | KC/CCT effects | DM effects | References |
|---|---|---|---|---|---|
|
Cornea biomechanics and collagen crosslinking | |||||
| LOX | Lysyl oxidase, participates in collagen crosslinking | 5q23.2 | Reduced LOX expression in corneal stroma and reduced activity in KC-derived corneal fibroblasts | Increased LOX expression and activity in retinal endothelial cells; unclear effect in DM cornea | 33,45,47,58 |
|
COL5A1 |
Collagen type V, alpha-1 chain |
9q34.2-q34.3 |
COL5A1 haploinsufficiency results in corneal stroma thinning, reduced collagen fibers |
Possible interaction between COL5A1 and HbA1c in DR study; no known direct effect on COL5A1 in cornea |
65,66,72,81 |
|
ECM remodeling | |||||
| FOXO1 | Transcription factor | 13q14.1 | SNP in FOXO1 linked to CCT, FOXO1 expression/activity unknown in cornea | FOXO1 linked to AGE-mediated disruption of autophagic flux and vascular endothelial cell autophagic apoptosis, role in cornea unknown | 66,85,90 |
| SMAD3 | Transcription factor | 15q22.23 | SNP in SMAD3 linked to CCT, Increased pSMAD3 and increased TGFβ signaling in KC cells | SMAD3 linked to ECM remodeling in DN; role in cornea unclear | 49,98,100,106 |
| TGFBI | Transforming growth factor beta induced | 5q31.1 | SNP in TGFBI linked to KC with decreased levels of TGFBIp in KC cornea | Unknown effect in DM cornea, shown to be upregulated in response to high glucose and TGFβ in DM proximal tubules | 124, 125, 126,132 |
| ZEB1 | Zinc finger transcription factor | 10p11.22 | Mutations in ZEB1 associated with KC and PPCD; possible genotype/phenotype correlation | Unknown effect in DM cornea, implicated in epithelial-to-mesenchymal transition under hyperglycemic conditions | 152,154,155,158 |
| MMP-9 | Matrix metalloproteinase- 9 | 20q11.2-q13.1 | Increased MMP-9 activity noted in tear sample with corresponding upregulation in MMP-9 mRNA; SNP identified in MMP-9 | Increased MMP-9 activity in tears from DM patients; SNP identified in MMP-9 associated with T2DM susceptibility | 170,173,176,181,185 |
| TIMP-1 | Tissue inhibitor of metalloproteinases-1 | Xp11.23 | Decreased TIMP-1 levels detected in KC patients, SNP in TIMP-1 associated with increased KC risk | Increased TIMP-1 levels in tears of pediatric T1DM patients, but overall role of TIMP-1 in DM remains inconclusive | 83,178,182,185 |
|
MIR184 |
microRNA |
15q22-q25 |
Mutations in miR-184 implicated in KC pathogenesis, but extent of association with KC alone remains unclear |
miR-184 expression decreased in pancreatic β –cells in response to extracellular glucose; decreased in islet cells of T2DM patients |
187,196,200,201,310,311 |
|
Inflammation and ROS production | |||||
| HGF | Hepatocyte growth factor | 7q21.1 | Increased HGF protein in KC corneal epithelium; increased HGF and c-Met mRNA in corneal wound healing | Increased HGF with decreased HGF receptor c-Met expression in DM cornea | 3,214,217,220 |
| CAST | Calpain/calpastatin, proteolytic degradation | 5q15 | SNP in CAST strongly linked to KC; CAST expression/activity unclear | High glucose induces calpain activity, increasing ROS production and vascular endothelial dysfunction; unknown effect in cornea | 226,229,230 |
| SOD1 | Superoxide dismutase 1 cytoplasmic antioxidant enzyme | 21q22.11 | Deletion mutation in SOD1 in several cohorts; decreased SOD1 expression in KC corneal fibroblast cultures | Associated polymorphisms in SOD1 identified; decreased SOD1 expression in DM cornea with associated increase in RAGE | 238, 239, 240, 241,254,256 |
|
IL1A/IL1B |
Interleukin 1alpha/beta, inflammatory cytokine |
2q13 |
Increased IL-1α expression in KC corneas, SNPs identified in IL1A and IL1B |
Imbalance in IL-1β to IL-1Ra in DM cornea, SNPs identified in IL1A, IL1B, and IL1RN in DM patients |
119,162,203,262,268 |
|
Additional genes of interest | |||||
| SPRY2 | Sprouty 2 | 13q31.1 | SNP in SPRY2 linked to CCT and corneal epithelium proliferation; SPRY2 expression activity in KC cornea unknown | SNP near SPRY2 linked to increase DM susceptibility; unclear effect in SPRY2 cornea | 49,278,280, 281, 282 |
| COL4A3, COL4A4 | Collagen type IV, alpha-3/4 chain, structural portion of corneal membranes | 2q36.3 | Alterations in collagen type IV reported in KC, but unclear if genetic polymorphisms play a role | Alterations in collagen type IV reported in DN and in the cornea under hypoxic conditions, unknown genetic association with DM cornea | 48,283,290,291 |
2. Clinical significance
By gathering information regarding the potential overlapping genetic factors in KC and DM, this review discusses potential therapeutic targets that may slow or halt the progression of KC. In other words, it might be beneficial to manage KC by utilizing the same mechanisms that strengthen the cornea in DM. These mechanisms include but are not limited to shifting the balance in the cornea microenvironment towards increased endogenous collagen crosslinking, decreased extracellular matrix (ECM) remodeling and proteolytic degradation, and decreased inflammation. By regulating local gene expression, this may serve as an alternative form of therapy for those patients in which corneal crosslinking is not an option, such as those with prior history of herpetic keratitis and history of poor epithelial wound healing.23 Furthermore, this may reduce the need for corneal transplantation in patients with more severe forms of KC.
3. Corneal biomechanics and collagen crosslinking in KC and DM
The cornea is naturally a viscoelastic structure, in that it must be elastic enough to expand into an aspheric half-sphere, but stiff enough to maintain its shape and resist the intraocular pressure (IOP).24,25 There is a delicate interplay between the multiple corneal layers and the composition within each layer that contributes to the overall corneal shape. This involves the organization of the collagen structure within each layer, the attachment of proteoglycans and glycosaminoglycans to collagen fibers, the corneal swelling pressure, and the production/degradation of extracellular matrix components.24 There are three major mechanisms of collagen crosslinking important in corneal biology, as previously outlined in McKay et al., 2019.26 Those include an enzymatic reaction: lysyl oxidase-mediated crosslinking as well as two non-enzymatic reactions: 1) advanced glycation end product (AGE)-mediated crosslinking and 2) photooxidative crosslinking mediated by riboflavin as a treatment option for KC. We will discuss the crosslinking mechanisms in more detail as they relate to the genes involved in KC and DM.
In order to quantify differences in corneal shape and structure, two measurements are commonly obtained: the central corneal thickness (CCT) and corneal hysteresis (CH). While CCT is a gross measure of the overall thickness of the cornea, CH reflects the viscous damping ability of the cornea. CH combines elasticity and viscosity and provides further information on the structural integrity of the cornea. Increased edema in the cornea may give the appearance of stromal thickening and increased CCT. This must be taken into account in systemic diseases such as DM, in which glucose induces increased water retention in multiple tissues, including the cornea.27,28
KC is associated with alterations in the corneal biomechanics.1 Previous studies have shown altered expression or abnormal localization of extracellular matrix (ECM) components in KC,29 with decreased levels of proteoglycan core proteins and abnormal collagen synthesis.25 Furthermore, age has been shown to be inversely correlated with the risk of KC30 and KC progression,31 suggesting that age-related crosslinking is protective against KC and other ectasias.32
Conversely, diabetes is known to be an independent cause of endogenous, non-enzymatic crosslinking as high levels of glucose causes glycosylation of corneal fibers and increases AGE-mediated crosslinking in the cornea, causing the cornea to become stiffer.13,32, 33, 34 As a result, increased CCT and alteration in corneal endothelial cells have been observed in patients with diabetes.35, 36, 37, 38, 39 Lee et al., 2006 reported that CCT was significantly correlated with the duration of diabetes after controlling for age.37 Interestingly, Hager et al., 2009 observed a significant increase in CH in patients with diabetes, although they did not observe a significant increase in CCT after correcting for age, IOP, and gender.40 Schler et al., 2012 also reported significantly higher CH and corneal resistance factor (CRF) in patients with poorly-controlled diabetes as compared to healthy subjects and well-controlled diabetes.41 Since CH and CRF are correlated to HbA1c, this suggests that the cornea biomechanics could be altered depending on extent of glucose control.41 These findings suggest that DM may induce structural alterations in the cornea. In terms of corneal biomechanics, in this section we discussed two genes, LOX and COL5A1, and their roles in relationship to the corneal biomechanics observed in KC and DM.
3.1. LOX
Lysyl oxidase (LOX) is a copper amine oxidase that initiates the physiologic crosslinking of collagens and elastin by oxidizing the side chain of peptidyl lysine, thus generating reactive aldehydes on lysine residues that may cross-react with nearby groups.26,42,43 In the eye, the LOX enzyme has been detected in the trabecular meshwork, ciliary body, lens, retina, and cornea.44, 45, 46 In addition to LOX, there are four LOX-like proteins (LOXL1, LOXL2, LOXL3, and LOXL4) that also catalyze the oxidative deamination of lysine residues in collagen and elastin45.
Multiple genome-wide association studies (GWAS) and candidate association studies have identified the association between sequence variants in LOX to both KC risk and CCT, suggesting its potential role in KC pathogenesis.47, 48, 49 Specifically, a particular SNP rs2956549 in LOX has been associated with KC in various ethnicities as well as a meta-analysis.50, 51, 52, 53 Dudakova et al., 2012 reported altered distribution of LOX expression in the corneal stroma of KC patients, with a reduction in total LOX (both LOX and LOXL) protein activity in corneal fibroblasts derived from KC corneas.45 This was consistent with a recent study that reported reduced pre-existing LOX and collagen levels in a patient who developed ectasia after small incision lenticule extraction (SMILE), despite normal preoperative biomechanical evaluation.54 Furthermore, ectopic LOX expression in the human corneal fibroblast induced significantly more collagen gel contraction in vitro, confirming the role that it plays in strengthening the corneal stroma.54
Given LOX's role in collagen crosslinking, reduced LOX activity in KC may lead to impaired cross-linking which results in corneal ectasia.45,54 Although there is a confirmed genetic association of LOXL1 to pseudoexfoliation syndrome and open-angle glaucoma,46,55,56 it is still unclear the role that LOXL proteins may play in the cornea. Dudakova et al., 2016 confirmed that LOXL1-4 enzymes were present in all layers of the cornea in cryosection samples and reported lower LOXL2 expression in KC corneas using IHC and Western blot analyses.57 Further studies will be required to better understand the expression patterns and activity of LOXL proteins in the cornea.
The exact role of LOX in DM-induced corneal changes remains unclear, as one study failed in observing an increase in LOX-mediated crosslinking in the cornea of DM patients.33 However, Chronopoulous et al., 2010 revealed that hyperglycemic conditions increased LOX expression and LOX activity in rat retinal endothelial cells in vitro and in diabetic rat retinas in vivo.58 This is consistent with the finding of increased LOX-dependent crosslinking in skin collagen in patients with diabetes.59 Coral et al., 2013 confirmed increased LOX mRNA expression in ARPE-19 cells exposed to high glucose.60 Further studies will be required to investigate the effects of DM on LOX activity in the cornea. The increase in LOX activity and expression in response to high glucose in retinal studies suggests that high glucose may have a similar effect in corneal stromal and endothelial cells. Given that the cornea is an avascular structure, there may be differential upstream regulation of LOX in response to high glucose concentrations as compared to the retina.
3.2. COL5A1
Since collagen is the most abundant protein in the cornea,26 it is not surprising that various types of collagen have been implicated in association with CCT and KC, including COL1A1 and COL1A2,61 COL8A2,62 and COL5A1.37,63 The genetic association of COL5A1 with CCT, in particular, reached genome-wide significance after combining data from European, Australian, and Singaporean GWAS.62, 63, 64, 65, 66 Collagen type V is a regulatory fibril-forming collagen involved in the formation of heterotypic fibrils with collagen type I.67, 68, 69, 70 Together, both collagen type I and V are the dominant collagen isoforms in the human corneal stroma.71 Although collagen type V only comprises 2–5% of the total collagen in most tissues, it determines 10–20% of the fibrillary collagens in the cornea.67,72 Interestingly, the most common molecular mechanism in classic Ehler-Danlos syndrome (EDS), a generalized connective tissue disorder, is a functional loss of one COL5A1 allele.73,74 Segev et al., 2006 investigated the corneal phenotype in EDS patients with COL5A1 haploinsufficiency and Col5a1+/− mouse models. Both EDS patients and knockout mice exhibited consistent corneal thinning and the Col5a1+/− mice also exhibited a decrease in total collagen content with a 25% reduction in the number of stromal fibrils.72 These findings suggest that alterations in COL5A1 expression serves as a strong genetic predisposition towards the development of corneal thinning and KC.
In DM, collagen is influenced by hyperglycemia primarily through nonenzymatic AGE-mediated crosslinking. This occurs by way of the Maillard reaction, in which a primary amine found on amino acid residues, such as lysine or arginine, is converted to a reactive Schiff base that rearranges to form the Amadori product.26 This serves as an early-stage product that leads to the formation of AGE products. A classic example of the Maillard reaction is the glycation of hemoglobin, which gives rise to the glycated HbA1c, the established biomarker for sustained glucose levels.26,75 Studies have identified increased end products of the Maillard reaction, including pentosidine, within the diabetic cornea.26,33 This increase in AGE-mediated crosslinking in DM has been associated with increased tendon stiffness and higher mechanical strength, which could be inhibited by insulin supplementation.26,76,77 As a result, the increased crosslinked collagen is resistant to enzymatic degradation in the cornea.78 AGE-mediated crosslinked adducts can then bind to the receptor for advanced glycation end products (RAGEs), which are pro-inflammatory receptors expressed in a wide variety of tissues. Although originally described for its ability to bind AGEs, RAGEs have the capability to bind multiple other ligands and play a crucial role in homeostasis and inflammatory processes.79,80
To our knowledge no direct associations between COL5A1 expression and DM have been established. Very recently, Ng et al., 2020 evaluated possible gene-environment interactions between genetic variants identified via GWAS, and the effect of glycemic control (indicated by HbA1c) on the risk of severe diabetic retinopathy (DR).81 Interestingly, the SNP COL5A1 rs59126004 exhibited a protective effect against DR in patients with adequate glycemic control (HbA1c <7%), but not in patients with inadequate glycemic control (HbA1c ≥ 7%),81 suggesting a potential interaction between COL5A1 rs59126004 and glucose levels in the retina.
Priyadarsini et al., 2016 quantified the expression of collagen type I, III, and V in the human corneal stroma of Type 1 and Type 2 DM, and discovered elevated levels of collagen type I and type III, but not collagen type V.27 Given that this was a small study with four to eight donor corneas in each category, it would be worth expanding the study with a larger sample size. Collagen type I and V are the predominant types of a healthy cornea, while collagen type III is primarily associated with fibrosis and wound healing in the setting of injury. This finding suggests that hyperglycemia may induce collagen expression in an isoform-dependent manner.27 We expect more studies of COL5A1 to better understand DM's direct effects on COL5A1 in the cornea.
4. Alterations in extracellular matrix (ECM) remodeling in the cornea
In line with collagen crosslinking, alterations in corneal ECM remodeling have been observed in KC and DM including variations in cellular proliferation, disruption in autophagic flux, and increased ECM fibrosis. This is complemented by the observation of an imbalance between matrix metalloproteinase (MMP) and tissue inhibitors of matrix metalloproteinase (TIMPs) in KC patients.82,83 Here, we take a close look at several KC- and CCT-associated transcription factors including FOXO1, SMAD3, TGFBI, and ZEB1 (Table 2). We then searched the literature to determine how they may be differentially regulated under hyperglycemic conditions in the cornea. We also included ECM remodeling genes MMP-9, TIMP-1, and MIR184.
4.1. FOXO1
Transcription factor Forkhead box protein O1 (FOXO1) is ubiquitously expressed in mammalian cells.84 It mediates multiple different pathways, including regulation of metabolic homeostasis, oxidative stress, cell proliferation, and autophagy. Sequence variants near or within FOXO1 have been associated with CCT,66,85,86 but its effect on FOXO1 expression and activity is not well-described in the KC cornea. FOXO1 is regulated by a variety of environmental factors, including insulin, which normally inhibits FOXO1 activity.87 In settings of insulin resistance and increased glucose, such as T2DM, increased FOXO1 has been shown to induce gluconeogenesis abnormalities, cell apoptosis, uncontrolled autophagy, and inhibition of proliferation.84,88
Although much remains to be understood about FOXO1's role in KC and DM, some studies have analyzed its effects on vascular endothelial cells. Wilhelm et al., 2016 indicated that FOXO1 decreases the metabolic and proliferative activities of vascular endothelial cells by attenuation of glycolysis and mitochondrial respiration.84,89 This study reported that the endothelial-restricted deletion of FOXO1 in mice caused a profound increase in endothelial cell proliferation, such that it induced vascular hyperplasia and vessel enlargement. In contrast, overexpression of FOXO1 resulted in vessel thinning and reduced branching of vasculature.89 A separate study reported that FOXO1 signaling was involved in AGE-induced vascular endothelial cell autophagy through impairment of autophagosome-lysosomal fusion. This impaired autophagic flux then resulted in endothelial cell autophagic apoptosis.90 These findings are intriguing as they suggest a correlation in FOXO1 activity with AGE-induced endothelial damage and decreased endothelial cell proliferation in the vasculature. Thus, it would be interesting to perform similar studies in the microenvironment of the cornea to determine if differential regulation of FOXO1 may contribute to altered endothelial cell proliferation and/or endothelial cell apoptosis in KC and DM corneas.
4.2. SMAD3
There are three functional classes of SMAD proteins, including the receptor-regulated SMAD (R-SMAD), the Co-mediator SMAD (Co-SMAD), and the inhibitory SMAD (I-SMAD) proteins.91 SMAD3 is an R-SMAD that is directly phosphorylated and activated by type I receptor kinases, forming activated SMAD complexes that then activate the transcription of target genes,91 specifically those involved in the TGFβ signaling pathway. TGFβ signaling is tightly regulated by I-SMAD proteins, SMAD6 and SMAD7, which compete for binding of SMAD3 to co-mediators.92 TGFβ signaling is known to play an important role in ECM remodeling and MMP expression.93, 94, 95
Several studies have identified involvement of TGFβ signaling in KC.96, 97, 98, 99 Priyadarsini et al., 2015 observed a significant increase in pSMAD3 expression with TGFβ3 signaling in human KC cells compared to normal human corneal fibroblasts.98 This was paralleled by significant downregulation of SMAD6/7 at baseline and failure of SMAD6/7 upregulation in response to stimulation with TGFβ in the human KC cells.98 Recently, variants near or within SMAD3 were identified to be associated with KC susceptibility in GWAS of CCT,49,100 further reinforcing its potential role in KC pathogenesis. Taken together, it has been suggested that the lack of inhibition by SMAD6/7, along with potential alterations in SMAD3 activity, results in increased TGFβ signaling that may promote the formation of a fibrotic ECM in KC.98
TGFβ/SMAD signaling plays an important role in regulating glucose and energy homeostasis as well.101 The TGFβ/SMAD3 pathway is activated downstream of the AGE/RAGE signaling pathway in the setting of hyperglycemia.102,103 This is suggested to be the main driving force in the development of diabetic nephropathy (DN) due to increased ECM deposition in mesangial cells.104,105 Ono et al., 2018 demonstrated that AGE stimulation resulted in significant activation of Smad1 and Smad3 in mesangial cells in mice, likely as a result of increased TGBβ signaling.106 Additionally, the loss of Smad3 prevented renal dysfunction under diabetic conditions by reduced mesangial matrix accumulation and reduced GBM thickening.106 This reinforces the critical role that TGFβ/SMAD3 plays in ECM remodeling, and shows that this pathway is also affected under hyperglycemic conditions. However, this pathway has not been analyzed in the DM cornea. Interestingly, TGFβ/SMAD3 signaling promoted gluconeogenesis in hepatocyte cells through interaction with FOXO1, another established KC-susceptibility gene.101 It is necessary to determine if the TGFβ/SMAD interaction with FOXO1 is present in the human cornea.
4.3. TGFBI
The transforming growth factor beta-induced (TGFBI) gene has been implicated in the pathogenesis of KC and a heterogeneous group of corneal dystrophies that are characterized by the progressive loss of corneal transparency.107 It encodes transforming growth factor beta-induced protein (TGFBIp), which is also known as keratoepithelin, BIGH3, or βigh3.108 In the cornea, it is expressed primarily in the corneal epithelium, stroma, and retrocorneal fibrous membranes.108, 109, 110, 111, 112 TGFBIp is known to interact with multiple extracellular macromolecules, including the proteoglycan decorin113 and collagen type I, II, IV, VI, and XII.114, 115, 116, 117 It is thought to link cells to the ECM through various integrin binding sites116,118, 119, 120, 121, 122 and thus play important roles in corneal wound healing and maintenance of the ECM.
In a cDNA library constructed from KC corneas, TGFBI was found to be the second most abundant transcript.123 Sequence variants in TGFBI were also identified in Chinese and Polish KC patients.124,125 However, the exact relationship between TGFBI, TGFBIp, and KC is still unclear. Researchers have suggested that mutations in TGFBI could contribute to decreased mechanical stability in the cornea, thus resulting in corneal thinning as seen in KC.126 This is supported by findings of decreased TGFBIp in KC corneas.126 Conversely, elevated levels of TGFBIp have been found in areas of corneal scarring, likely due to TGFB1-mediated upregulation in response to corneal injury.126, 127, 128 Interestingly, two KC patients had stromal amyloid deposits that were associated with TGFBIp in the corneal buttons, but no TFGBI mutation was present.99 It is possible that there was concurrent scarring in these two patients, or that local factors in the KC corneas predispose to development of TGFBIp amyloid deposits, thus disrupting the structural integrity of the cornea.99
TGFBI has also been identified as a potential risk gene for the development of both T1DM and T2DM after detecting the association of several SNPs in human genetic studies.129 Aside from the cornea, TGFBIp is produced by smooth muscle cells, fibroblasts, and proximal tubular epithelial cells130,131 upon TGFβ or high-glucose stimulation.127,132 In studies investigating DM, it was observed that high glucose levels increased TGFBIp expression in renal proximal tubule epithelial cells by activating TGFβ. This coincided with findings of a high glucose-stimulated increase in collagen and fibronectin production in mesangial cells and proximal tubular cells, which is mediated by TGFβ activation.133, 134, 135 Although the pathologic consequences of increased TGFBIp in the proximal tubules remains unclear, it may play an important role in the degree of renal interstitial fibrosis, which is closely correlated with a progressive decline in renal function in DM.132,136,137 To date, no studies have analyzed TGFBI and TGFBIp in the diabetic cornea, but TGFBI has been shown to be upregulated in vitro in the human corneal epithelial cell line in response to TGFβ.128 Given these findings, it is possible that TGFBIp may also be increased in the cornea in response to high glucose, promoting TGFβ-mediated corneal thickening and fibrosis. Additionally, it has been suggested that TGFBIp mutations occur in a genotype-phenotype fashion, in which various mutations account for the different degrees of phenotypic severity seen in KC and corneal dystrophies.138 Thus, the high glucose environment seen in DM could result in a variation on the phenotypic spectrum of TGFBI mutations and/or epigenetic modifications.
4.4. ZEB1
The zinc finger E box-binding homeobox 1 (ZEB1), also known as transcription factor 8 (TCF8)139,140 or δEF1,141 can function as either a transcriptional enhancer or repressor for different genes.142 ZEB1 has been implicated in the regulation of type 1 collagen expression, particularly in osteoblasts,141 and repression of the epithelial phenotype.140,143,144 It has been identified as a potent epithelial to mesenchymal transition (EMT) activator and stimulator of angiogenesis in tumor biology studies,145, 146, 147 as well as a regulator of TGFβ signaling with its counterpart, ZEB2.148
Mutations in ZEB1 have been reported in posterior polymorphous corneal dystrophy (PPCD),139,140,149 Fuch's endothelial corneal dystrophy (FECD),127,150,151 and keratoconus.152 This highlights another example of genetic heterogeneity that results in a variety of phenotypic presentations, like that of TGFBI. A heterozygous frameshift mutation in ZEB1 was found to induce ectopic expression of COL4A3 by corneal epithelial cells in PPCD, implicating COL4A3 as a possible target of ZEB1 regulation.140 In contrast, a missense ZEB1 mutation resulted in markedly reduced COL4A1, COL4A2, and COL4A3 expression in corneal keratocytes.152 Reduced expression of COL4A1 and COL4A3 has also been reported in KC,29,153 but further work is required to determine the role of COL4A1/COL4A3 and how they may be regulated by ZEB1 in KC. Based on current findings, Lechner et al., 2013 suggested that missense mutations in ZEB1 are associated with FECD and KC, while protein truncating mutations result in PPCD.152 This is supported by the finding of unique mutations in a family with both KC and FECD, as well as a patient with triple corneal dystrophy consisting of KC, FECD, and epithelial basement membrane corneal dystrophy.152,154
In efforts to better understand ZEB1's role in wound healing and angiogenesis, it was recently found that persistent hyperglycemia, as seen in DM, potently induced ZEB1 expression in human dermal microvascular endothelial cells (HMEC).155 This corresponded with increased ZEB1 expression in laser capture microdissection endothelial tissue obtained from the wounded edge of diabetic wound patients.155 In Singh et al., 2019, immunoprecipitation-mass spectrometry was performed to gain further mechanistic insights into the differential action of ZEB1 under normoglycemic and hyperglycemic conditions, thus revealing putative proteins that physically associated with ZEB1.155 It was found that hyperglycemia diminished the physical association of ZEB1 with E-cadherin, resulting in a loss of control over E-cadherin repression which is known to cause the microvascular endothelial dysfunction commonly observed in DM.155,156 Additionally, hyperglycemic conditions impaired the binding of several pro-inflammatory proteins, suggesting that alterations in cellular ZEB1 may contribute to the inflammation seen in DM.155
A different study analyzed the role of long noncoding RNA (lncRNA) ZEB1 antisense 1 (ZEB1-AS1) in DM, as it has been shown to increase ZEB1 expression.157,158 By increasing ZEB1 expression, ZEB1-AS1 is thought to play an antifibrotic role in DM through modulation of EMT, which is considered to be the main pathogenic factor of renal fibrosis.158, 159, 160 Wang et al., 2018 confirmed this in ZEB1-AS1 knockdown studies, which increased high glucose-induced ECM accumulation by downregulation of ZEB1 expression, resulting in renal fibrosis.161 This was investigated further by Meng et al., 2020, who reported that ZEB1-AS1 was down-regulated in kidney tissues of DM patients as well as hyperglycemic-induced HK-2 cells.158 Furthermore, ZEB1-AS1 improved the high glucose-induced EMT and fibrogenesis by mediating miR-216a-5p and BM7.158 While the exact mechanisms are beyond the scope of this review, it is clear that there is a very complex mechanism of regulation surrounding ZEB1. It is important to study the role of ZEB1 in the cornea of DM patients as it may have the potential to alter the expression of inflammatory cytokines and disrupt corneal endothelial/epithelial structure under hyperglycemic conditions.
4.5. MMP-9 and TIMP-1
MMPs are a family of 24 zinc-dependent proteases involved in multiple physiological processes including tissue remodeling and the degradation of ECM.162,163 The activity of the MMPs is balanced by four inhibitory proteins, the tissue inhibitors of metalloproteinases (TIMPs1-4).164,165 Multiple MMPs have been implicated in the pathogenesis of KC, including MMP-1, MMP-2, MMP-3, MMP-7, and MMP-13.166,167 MMP-9, also known as gelatinase B, is among the most well-studied in relation to KC.164 Multiple studies of tear composition have shown increased levels of MMP-9 protein in KC, including one patient with asymmetrical KC in which MMP-9 was upregulated only in the tears of the affected eye.168, 169, 170This increase in MMP-9 was confirmed with an accompanying upregulation of MMP-9 mRNA in the corneal epithelium.170 Interestingly, the MMP-9 mRNA in KC patients was significantly higher in cells from the cone apex as compared to the corneal periphery, which may contribute to the focal structural weakness of the cornea.171 An additional study revealed an increase in MMP-9 protein level in the blood of KC patients compared to controls.172
TIMP-1 exhibits a unique binding interaction with MMP-9, as it usually exhibits a high level of coordinated expression with MMP-9, is frequently secreted as a TIMP-1/MMP-9 complex, and binds MMP-9 with high affinity.173 Recent studies revealed a decrease in the levels of TIMP-1 in KC corneas.83,174,175 Taken together, the increased MMP-9 and decreased TIMP-1 activity seen in KC may reflect in an imbalance of proteolytic activity, thus contributing to ECM degradation and corneal thinning. This is reinforced by the analyses of genetic polymorphisms in MMP-9 and TIMP-1 in KC patients that were associated with findings of higher MMP-9 and lower TIMP-1 activity in KC tear samples.176 It is important to note that the TIMP-1 SNP was only associated with increased KC risk in females.177 Given that TIMP-1 is located on the X chromosome,178 this likely accounts for differences in gender susceptibility.
In regards to DM, studies have indicated that elevated glucose levels also disrupt the MMP/TIMP balance in macrophages and endothelial cells, primarily through an amplification in MMP expression and activity.179 Takahashi et al., 2000 reported enhanced MMP activity in human corneal epithelial cells under hyperglycemic conditions as well as increased MMP-9 activity in the cornea of diabetic rat models.180 In support of this finding, two studies reported: 1) increased MMP-9 and TIMP-1 protein levels in the tears of pediatric T1DM patients along with 2) increased MMP-9 activity in tears of T2DM patients.181,182 Additionally, genetic polymorphisms in MMP-9 have been associated with susceptibility to T2DM and diabetic nephropathy.183, 184, 185 However, no genetic association has been identified with TIMP-1 and DM in patients.185 The role of TIMP-1 in DM remains inconclusive. Taken together, differences in the imbalance of MMP-9/TIMP-1 activity may contribute to different degrees of ECM remodeling in the cornea of KC and DM patients.
4.6. MIR184
MicroRNAs (miRNAs) bind to the complementary sequences located in the 3′-UTR region of the target genes, resulting in degradation of the mRNA or suppression of translation.186 MIR184, in particular, encodes for miR-184, which is expressed in the central corneal epithelial cells and the lens epithelium.187, 188, 189 It is the most abundant miRNA in both the cornea and the lens and it is known to competitively inhibit the binding of miR-205 to the mRNA of the inositol polyphosphate phosphatase-like 1 gene (INPPL1, also known as SHIP2).189,190 This neutralizes the inhibitory activity of miR-205 on INPPL-1 and subsequently downregulates the Akt pathway, which has been shown to markedly increase keratinocyte apoptosis and cell death.190 Interestingly, this situation is unique to the corneal epithelium as it is the only known epithelium that exhibits overlapping expression of miR-184 and miR-205.189,190 Additionally, miR-184 has been shown to regulate differentiation of human-induced pluripotent stem cells into corneal epithelial-like cells.191,192
Given its role in the cornea, it is not surprising that mutations in MIR184 may lead to KC. To date, multiple studies have identified a heterozygous mutation in MIR184 in a Northern Ireland family with KC and cataracts,187 a family with EDICT (endothelial dystrophy, iris hypoplasia, congenital cataract, and stromal thinning) syndrome,193 and an European Spanish family with various corneal abnormalities including severe KC.194 However, the lack of mutations in KC patients from Iran,191 Turkey,195 and Saudi Arabia196 suggests a limited role of MIR184 in KC pathogenesis.196
Specific miRNAs have also been associated with T2DM cellular processes, including apoptosis, response to cytokines, and insulin secretion.197 miR-184, in particular, has been identified as an important modulator of compensatory pancreatic β-cell expansion during insulin resistance.198, 199, 200 Generally, the expression of miR-184 is increased in islet cells in periods of fasting, demonstrating an active role in pancreatic β-cells as the glucose levels decrease.201 Likewise, the miR-184 expression levels have been shown to decrease in the presence of increasing extracellular glucose.201 Together, this highlights the potential role of miR-184 in glucose metabolism. Furthermore, its expression is strongly decreased in the pancreatic islet cells of insulin-resistant mouse models and human patients with T2DM.200 miR-184 expression and activity needs to be further studied in the DM cornea. Given its regulation by glucose levels in pancreatic islet cells, it is possible that miR-184 may be similarly downregulated by high glucose levels in the DM cornea, thus inhibiting cellular apoptosis and resulting in corneal thickening.
5. Inflammation and oxidative stress in the cornea
Although KC has traditionally been described as a noninflammatory degenerative condition, there is emerging evidence suggesting that inflammation within the epithelium and stroma are involved in the pathogenesis of KC.3 Multiple studies have observed significant increases in proinflammatory molecules such as IL-6, IL-4, IL-5, IL-8, and IL-12169,202 in KC tears compared to controls. Additionally, KC keratocytes have been reported to express more IL-1α receptors,203 which may trigger keratocyte apoptosis since IL-1α is a proinflammatory cytokine.204
In parallel, several studies have found that oxidative stress is involved in the development and progression of KC.83,205, 206, 207 KC corneas have exhibited abnormalities such as increased levels of inducible nitric oxide synthase, nitrotyrosine, malondialdehyde, and glutathionine S-transferase,208 as well as decreased activities of extracellular superoxide dismutase.205 This is supported by multiple observations of KC corneas and fibroblasts exhibiting increased levels of ROS and relatively greater mitochondrial DNA damage as compared to controls.207,209,210 Interestingly, the combination of oxidative stress and hyperglycemia, particularly in T2DM, accelerates AGE formation.80 Thus, with increased AGE accumulation, this may create the potential for increased inflammation, enhanced production of ROS, and impairment of DNA repair mechanisms80 in the DM cornea as well.
The increase in inflammatory markers and ROS within the KC cornea may be the result of environmental stimuli such as eye-rubbing and external oxidants such as UV light, which drives the pathological thinning of the stroma.211 DM itself is an inflammatory systemic disease, so we wanted to explore its effects on the KC-associated genes. In this section, we focused on HGF, CAST, SOD1, and IL1A/IL1B and their roles in DM and KC pathophysiology (Table 2).
5.1. HGF
Hepatocyte growth factor (HGF) is a pleiotropic growth factor that binds to its receptor, mesenchymal-epithelial transition factor (c-Met/Met) and activates multiple downstream pathways, including MAPK, PI3K-Akt axis, and activators of transcription (JAK/STAT) pathways.212 It is primarily involved in cell proliferation and migration, particularly in corneal epithelial cells, as well as inflammatory-related signaling cascades.3,213,214 Injured corneas have exhibited increased HGF and c-Met mRNA expression during corneal wound healing.214, 215, 216
The genetic association between HGF variants and KC susceptibility was identified with several HGF SNPs in the European and Australian cohort studies.50,217,218 Given that all the currently identified SNPs are located in a noncoding region upstream of HGF, it was suggested that they regulate gene expression by way of RNA splicing, transcription factor binding, and miRNA regulation.3,219 Additionally, there is recent evidence of increased HGF protein in the KC epithelium compared to control corneal epithelium.3 This suggests that poorly regulated and overexpressed HGF may have detrimental effects on the ECM due to inflammation in KC.
In diabetic corneas, both ex vivo and organ culture, HGF expression was noted to be increased with a corresponding decrease in HGF receptor c-Met expression.220 This suggests a disruption in the HGF/c-Met system, such that there is reduced cell migration and poor epithelial healing, which is characteristic of diabetic corneas.221, 222, 223 This hypothesis is supported by the finding that overexpression of c-met in diabetic corneas resulted in restoration of nearly normal epithelial wound healing times.223 In other DM studies, HGF has been shown to play a role in the metabolic flux of glucose, manage β-cell homeostasis, and modulate the inflammatory response.224 Thus, it is possible that reduced receptor c-Met expression in DM corneas could serve a protective role against the inflammatory response mediated by HGF.
5.2. CAST
CAST encodes calpastatin, an inhibitor of calpains. CAST is a calcium-dependent cysteine protease that is involved in a variety of cellular processes, including proliferation, apoptosis, and cell migration.225 The calpain/calpastatin system is present in the corneal epithelium where it is suspected to play a role in epithelial cell turnover and wound healing.226,227 It has also been localized to corneal endothelial cells and fibroblasts.226,228 Genotyping of both Caucasian and Han Chinese patients with KC revealed a consistent association between variants near or within CAST, with KC susceptibility.226,229
In DM, calpain activity has been shown to be increased in vascular endothelial cells in response to excess glucose.127,230, 231, 232, 233 Calpain may also play a role in mitochondrial ROS generation, such that it contributes to diabetic vascular injury by way of vascular inflammation232,234 and glucose-induced apoptosis in endothelial cells.230,235 This is supported by the finding that genetic inhibition of calpain through over-expression of calpastatin reduces vascular ROS production.230 Increased glucose-induced calpain activity has also been shown to initiate vascular endothelial dysfunction by inactivating prostacyclin (PGI2), as overexpression of endogenous calpastatin inhibited this effect.236 A similar mechanism may be observed in DM corneas, as calpain is activated by high-glucose levels. Analyzing the expression and activity levels of CAST in KC and DM corneas will give us further insight into this pathway.
5.3. SOD1
Superoxide dismutase 1 (SOD1) encodes a copper and zinc-dependent cytoplasmic enzyme that is directly involved in the antioxidative processes associated with ROS elimination and reduction of oxidative stress in the cornea.205 It has been shown to localize to the corneal epithelium, endothelium, and keratocytes.205 Given that there have been increased levels of oxidative stress markers in the KC cornea, several studies have suggested that mutations in SOD1 might be involved in development of KC.48,83,237 A specific SOD1 deletion was detected in two non-related American families with an autosomal dominant form of KC as well as in the Greek and Brazilian KC population.238, 239, 240 Mutational analyses revealed that this deletion excluded the SOD1 protein active site, which suggests a loss of enzyme function.239 Although the specific SOD1 deletion has had a low frequency in cohort studies, the fact that it has been identified in various populations suggests that it might serve as a potential genetic component in the development of KC. This is supported by the finding of suppressed levels of SOD1 expression in KC corneal fibroblast cultures as compared to controls.241 However, this is a controversial topic as other studies have refuted SOD1's involvement as there was not enough evidence in mutational analyses.48,127,237,242, 243, 244, 245, 246
Mutations in the SOD1 gene has also long been associated with amyotrophic lateral sclerosis (ALS). It is generally accepted that mutations in SOD1 results in conformational instability of the protein, resulting in the formation of SOD1 aggregates that exert a cytotoxic effect in motor neurons, which then results in the progressive degeneration of motor neurons observed in ALS.247 Interestingly, ALS has recently been shown to share several genetic pathways with DM.248 Additionally, several genetic variations of SOD1 polymorphism have been associated with diabetes and diabetic complications.249, 250, 251, 252, 253, 254 One study implicated a role for AGE/RAGE signaling in DM-mediated vascular calcification through activation of Nox-1 and decreased expression of SOD1, which increased oxidative stress.255 Recently, a separate study investigated ocular surface damage in diabetic mice and found an accumulation of ROS, increased expression of RAGE, and decreased SOD1 expression in the cornea.256 This study also reported that topical treatment with pigment epithelium-derived factor (PEDF) was shown to improve corneal epithelial damage by decreasing RAGE and increasing SOD1 expression.256 Together, this suggests that SOD1 may play an important role in alleviating the oxidative stress seen in corneal pathology. SOD1's role in KC pathogenesis is currently under debate, but it is possible that increased AGE/RAGE activity in the DM cornea results in decreased SOD1 expression.
5.4. IL1A and IL1B
Interleukin (IL)-1 is a proinflammatory cytokine involved in various cellular activities, including cell proliferation, differentiation, and apoptosis.257 The IL-1 family includes two proinflammatory cytokines, IL-1α and IL-1β, and the IL-1 receptor antagonist (IL-1Ra), encoded by IL1A, IL1B, and IL1RN, respectively.257 IL-1 has been shown to upregulate keratocyte expression of collagenases, metalloproteinases, and other enzymes involved in collagen remodeling during corneal wound healing.258,259 Early studies detected increased keratocyte apoptosis in KC corneas and suggested that it might be triggered by increased basal IL-1 release.260,261 This is supported by the finding of increased IL-1α binding sites in KC corneal fibroblasts compared to control corneas.203 However, genetic association analyses for polymorphisms in IL1A and IL1B in KC have remained controversial. Genetic associations with KC have been identified with variants in IL1B in the Han Chinese,262 Korean,263 and Japanese KC population,257 but the involvement of IL1A was only observed in the Han Chinese KC population.262 Furthermore, no genetic associations with variants in or near IL1A or IL1B were observed in the Turkish population.264 To date, it is still unclear how the mRNA expression levels of IL1A and IL1B relates to the expression of cytokines IL-1α and IL-1β in KC pathophysiology.
The IL-1 family of cytokines has also been implicated in DM and DM-related complications by way of inflammation.265, 266, 267, 268 To better understand the role of IL-1 in the diabetic cornea and corneal wound healing, a recent study utilized a genome-wide cDNA array analysis in normal and DM mouse corneas.268 This study by Yan et al., 2016 reported upregulation of IL-1β expression in the healing corneal epithelium of both normal and DM corneas, with no difference in IL-1α expression.268 There was a corresponding increase in IL1RN expression in the normal cornea, but a decrease in the DM cornea, suggesting a disturbed balance of IL-1β to IL-1Ra.268 This disruption in IL-1Ra signaling had multiple adverse effects in corneal wound healing, including suppression of proinflammatory cytokine/chemokine expression and a decrease in the overall early inflammatory response to wounding in DM mouse corneas.268 Interestingly, increased production of IL-1β has also been observed in macrophages in response to prolactin stimulation.269 Given that prolactin-induced protein has recently been suggested to be a novel biomarker for KC,270,271 this is an intriguing association between the balance of IL-1β to IL-1Ra signaling and hormonal influences.
A separate cross-sectional analysis reported increased levels of IL-1Ra in patients with prevalent DM or metabolic syndrome.162 It was suggested that the increased IL-1Ra expression levels predicted the progression of metabolic syndrome to clinically incident diabetes, independently of CRP and other risk factors.162 This study also revealed genetic variants in IL1A, IL1B, and IL1RN that may have gender-specific associations with DM.162 Further studies will be required to better understand how the genetic variants in IL1A and IL1B influence expression of IL-1α and IL-1β and whether there is differential regulation of the IL-1 family in KC and DM.
6. Additional genes of interest
Multiple other genes have been associated with CCT and KC pathophysiology, including but not limited to SPRY2 and COL4A3/COL4A448,49 (Table 2). Here, we discuss how these genes may potentially have overlapping or differing roles in KC and DM pathogenesis.
6.1. SPRY2
The Sprouty family consists of four members, SPRY1-4. All are direct targets and negative feedback regulators of fibroblast growth factor (FGF) signaling,272, 273, 274 playing important roles in the early development of multiple organs.273,275,276 SPRY2, in particular, has been shown to modulate the apoptotic actions induced by pro-inflammatory cytokine, TNF-α.277 Kuchara et al., 2011 revealed an important role for Spry1 and Spry2 in the regulation of lens vesicle separation and corneal epithelial proliferation in mouse models.278 This study generated Spry1;Spry2 double-null mutants and observed increased corneal epithelial proliferation and an inhibition in terminal differentiation.278 A later study revealed that eyelid closure was impaired due to increased proliferation of conjunctival epithelial cells in Spry conditional knock-out mutants.279 This suggests that Spry-1 and Spry-2 normally suppress ectopic growth in the corneal epithelial tissue. A recent GWAS identified SPRY2 as a novel candidate gene significantly associated with CCT inter-individual variation.49 Although no studies have analyzed SPRY2 in KC pathogenesis, it is possible that it may contribute to alterations in the corneal epithelium.
Recently, a variant near SPRY2 was found to be associated with increased susceptibility to T2DM in both the Han Chinese and Japanese population.280,281 SPRY2 KO in human hepatocyte cells resulted in increased glucose intake, suggesting a possible role for SPRY2 in glucose metabolism in hepatocytes.282 This same study also reported the upregulated genes in SPRY2 KO cells to be involved with DNA replication and cell cycle regulation, which is consistent with its established role in inhibition of cellular proliferation.282 Taken together, deletions in SPRY2 may have pathologic effects in both the cornea and metabolic homeostasis.
6.2. COL4A3/COL4A4
COL4A3 and COL4A4 both encode for collagen type IV, the major structural component in epithelial and endothelial basement membranes in the human cornea.283,284 Collagen type IV mutations have been implicated in a variety of clinical manifestations, including Alport syndrome and PPCD.140,283,285 Both genes have been reported to be differentially expressed in KC corneas, suggesting a role for collagen type IV in KC pathogenesis.153,286 However, in genetic analyses of COL4A3 and COL4A4, no pathogenic variants were identified in KC patients, although common polymorphisms are present in the affected and healthy populations.48,86,283,287,288 Thus, the role of collagen type IV mutations in KC pathogenesis is unclear.
Collagen type IV is also a major structural component of the glomerular basement membrane (GBM), and alterations in the collagen composition have been implicated in the pathogenesis of nephropathy in DM.289 GWAS meta-analysis of T1DM revealed a SNP rs55703767 that resulted in thinner GBM in patients with DM but was protective against renal complications.290 Interestingly, Onochie et al., 2020 reported that hypoxia induced a reduction in laminin and collagen type IV in the cornea, which resulted in a delay in wound healing and increased corneal stiffness.291 This was in parallel to the finding of impaired wound healing and a decrease in laminin along the basal lamina in the diabetic cornea.284 Given that diabetes can cause a transition to a hypoxic state, this suggests a potential role for collagen type IV in the induction of increased corneal stiffness that has been reported in DM.291 Further studies will be needed to see if collagen type IV mutations have a potential effect on the DM cornea and to better understand how hypoxia affects collagen type IV.
7. Discussion
Multiple retrospective epidemiological studies have suggested that patients with DM have reduced risk in the development and/or severity of keratoconus (Table 1).13,14,17,18 Seiler et al., 2000 was among the first to report this protective role of DM in a retrospective case-control study of the German population.13 This finding was later confirmed by Naderan et al., 2014 in the Iranian population,17 and Kuo et al., 2006 in the United States population.14 One limitation of the above studies is that the data was extracted from the hospital/clinic population. Woodward et al., 2016 addressed this by conducting a retrospective longitudinal population-based cohort study in the United States.18 With a larger sample size, Woodward et al., 2016 found that there were 20% lower odds of KC development with uncomplicated DM and 52% lower odds of KC development with DM-associated complications.18
A common working hypothesis in support of this observation is that chronic hyperglycemia promotes glycosylation of corneal fibers and induces endogenous collagen crosslinking within the corneal stroma, thus preventing the biomechanical weakening of the cornea.13,34 Although the cornea is an avascular structure, previous reports have indicated that corneas in diabetic patients are still exposed to increased glucose concentration,292, 293, 294 which supports this hypothesis. Another working hypothesis is that DM disrupts corneal endothelial cell function, resulting in stromal edema and increased CCT35,37,295, 296, 297 (Fig. 1).
Fig. 1.
In KC and DM, several overlapping mechanisms may contribute to the discussed pathology, including alterations in corneal biomechanics and collagen crosslinking, alterations in ECM composition and proteolytic activity, as well as increased inflammation and oxidative stress.
This remains a debated topic as other studies have either 1) suggested a positive association between KC and DM or 2) did not identify a significant correlation (Table 1).14,19, 20, 21, 22 For example, Kosker et al., 2014 conducted a retrospective case-control and cross-sectional study in the United States population and reported a higher prevalence of T2DM in the KC population as well as greater severity of KC in DM patients.20 However, this was clinic-based, and the prevalence of DM in clinic populations as compared to the general population may account for differences in these findings. Subsequently, Moon et al., 2020 conducted a retrospective cohort population-based study in the Korean population and they also reported a higher prevalence of T2DM in the KC population with a positive association of KC and DM.21 In contrast, Bak-Nielsen et al., 201919 and Lee et al., 2020298 conducted a retrospective cohort study in the Danish and Korean population, respectively, and found no significant differences in DM prevalence in KC patients. In efforts to consolidate the findings, Hashemi et al., 2020299 conducted a meta-analysis and reported that although the odds of developing KC were 23% lower, this relationship was not significant. A limitation of this meta-analysis is that all studies included were either cross-sectional or case-control studies, which can be more prone to information and selection bias compared to cohort studies.299 Thus, the variations in study designs likely contribute to differences in findings.
The multiple effects of DM on the cornea are complex and have been reviewed elsewhere.16,26,300,301 These DM effects include, but are not limited to, keratopathy, neuropathy, inflammation, alterations in collagen fibrils, endothelial cell loss, and increased glucose in the aqueous humor.16,292 Patients with DM are often predisposed to ocular surface complications such as dry eye, recurrent corneal erosions, and bacterial infection.26,302, 303, 304 However, the mechanism of correlation between DM and KC pathogenesis remains unclear. In efforts to better understand KC pathophysiology, multiple candidate and genome-wide association studies have identified the genetic and metabolic components involved in KC.48,49,100,305 Additionally, a recent review outlined important biological and chemical pathways related to collagen crosslinking in DM and KC.26
In terms of corneal biomechanics, KC is characterized by localized stromal corneal thinning and reduced CCT,1 while DM generally causes the cornea to become stiffer with increased CCT and/or CH.13,34,35,37 This is likely reflected by changes in the collagen composition of the cornea, as outlined by McKay et al., 2019.26 Two genes, LOX and COL5A1, may play important roles in corneal collagen crosslinking. Current evidence suggests that overall LOX activity (including LOX and LOXL proteins) is decreased in KC, leading to impairment of lysyl oxidase-mediated crosslinking and weakening of the cornea.45,54 In contrast, hyperglycemic conditions have been shown to upregulate LOX expression and activity in retinal cells.58,60 If a similar effect may be seen in corneal cells under hyperglycemic conditions, this could explain the reduced risk of KC development in DM patients. Given that COL5A1 reached genome-wide significance in KC patients, and that patients with COL5A1 haploinsufficiency exhibited corneal thinning, we discussed whether COL5A1 shared similar genetic significance in DM corneal pathology. To our knowledge, no studies have analyzed DM's direct effects on COL5A1 in the cornea. A recent study did find differential collagen composition with elevated levels of collagen types I and type III, but not collagen type V, in the human corneal stroma of DM patients. This finding suggests that hyperglycemia may induce collagen modulation in an isoform-dependent manner. Further studies are needed to better understand the effects of hyperglycemia and increased AGE-mediated crosslinking on the various collagen isoforms in DM corneas, and to determine whether sequence variants in or near COL5A1 are associated with KC and DM cornea pathology.
Alterations in corneal biomechanics is also characterized by disruption in the ECM composition of the cornea. Thus, we explored several genes that play a role in ECM remodeling and were identified in KC and CCT-associated GWAS. This includes several transcription factors including FOXO1, SMAD3, TGFBI, and ZEB1. It became readily apparent that the investigation of these genes is lacking in the DM cornea, but many exhibited an association with AGE-mediated signaling and differential expression under hyperglycemic conditions in other tissues. This suggests that a similar effect could be seen in the DM cornea, but further research studies will be needed to confirm this. TGFβ signaling would be worth exploring further in the KC and DM cornea, as it has been associated with alterations in SMAD3, TGFBIp and FOXO1 signaling. For example, increased TGFβ signaling and SMAD3 activity has been suggested to increase ECM deposition in mesangial cells, leading to the development of DN. This, in combination with an imbalance in MMP-9/TIMP-1 activity, may contribute to different degrees of ECM remodeling in the cornea of KC and DM patients. Additionally, MIR184 has a very unique role in the remodeling of the corneal epithelium. Given its downregulation by high glucose levels in pancreatic islet cells, MIR184 may potentially link elevated glucose levels and alterations in corneal epithelial cell apoptosis in the DM cornea.
Interestingly, there was genetic overlap in several inflammatory proteins and regulators of oxidative stress in both KC and DM. Although KC was previously described as a noninflammatory condition, it has been suggested that external environmental factors such as excessive eye rubbing may induce an increase in inflammatory markers, contributing to KC pathogenesis. Likewise, DM has been associated with low-grade inflammation that is thought to contribute to insulin resistance observed in T2DM.306 In this review, we closely examined the role of HGF, CAST, SOD1, and IL1A/IL1B. While HGF's role remains unclear, the imbalance in HGF and HGF receptor c-Met expression in DM corneas is intriguing, which could lend towards a protective role against inflammatory degradation in the ECM of DM. We found that SOD1 likely plays a similar role in reducing oxidative stress, as decreased SOD1 expression has been suggested in both KC and DM. Further studies into the calpain/calpastatin system, as well as the ratio of IL-1α/IL-1β to IL-1Ra, will be needed before an association can be made between DM and KC. It is possible that imbalances in both systems may contribute to the differing clinical pathology observed in the cornea.
Last but not least, multiple other genes have been associated with KC pathophysiology, but their roles in DM are unclear. We highlighted a few remaining KC candidate genes, including SRPY2 and COL4A3/COL4A4. However, much more extensive studies are required before correlations can be made with DM. We suggest exclusively studying the DM cornea with a focus on KC-associated genes and analyzing the expression patterns of associated proteins.
From a clinical perspective, the age of onset for KC often ranges from the teenage years to the 30s and 40s while the age of onset for type 2 DM ranges from teenage years to the 70s.1,271,307, 308, 309 Type 2 DM is becoming increasingly prevalent in children and adolescents worldwide, including the US. The average age of diagnosis for children and adolescents in the US is 14 years.307 The incomplete overlap in age-onset between KC and DM may complicate efforts in interpreting the potential protective effect of DM on KC. This raises an important question to all the reported studies: what is the distribution of DM age-onset for those with KC or without KC? It will be more beneficial to only include DM patients with age-onset less than 30–40 years.
8. Conclusions
Despite the conflicting literature evidence between KC and DM, our comprehensive discussion explained whether and how DM is associated with KC and how DM may function as a protective mechanism (Fig. 1). At this time, there is more evidence to support the protective role of DM in KC patients. If true, we hope to identify potential localized therapeutic targets in KC management that act by strengthening the KC cornea through similar mechanisms that may alter the DM cornea. One such example would be targeting LOX in the KC cornea and increasing its expression such that LOX-mediated collagen-crosslinking is increased, thus resulting in corneal thickening. Additionally, by targeting inflammatory cascades such as the calpain/calpastatin system and the IL-1α/IL-1β to IL-1Ra ratio, we can then potentially halt the inflammatory-mediated corneal thinning seen in KC. To conclude, by analyzing potential genetic associations in KC and DM, this review illustrates the areas where the current literature is lacking, with the hope of providing direction for future studies in elucidating the pathophysiology of KC and DM in the cornea. Future research would have tremendous benefit in identifying potential therapeutic targets in clinical management of KC.
9. Literature search
A comprehensive literature search completed by the end of September 2020 was performed on Pubmed. All selected articles were reviewed thoroughly by the authors to consolidate candidate genes that have been identified in genetic analyses and genome wide studies of keratoconus and central corneal thickness variations. We then explored how those respective genes may be similarly or differentially regulated under hyperglycemic conditions and the role they play in the systemic complications associated with diabetes.
Study Approval
Not applicable.
Author Contributions
Kristin M. Ates: Methodology, Investigation, Writing – Original Draft, Writing - Review & Editing, Amy J. Estes: Writing - Review & Editing, Yutao Liu: Conceptualization, Writing - Review & Editing, Supervision, Funding Acquisition.
Acknowledgements
We acknowledge the support of The Graduate School of Augusta University and the Medical College of Georgia.
Funding
This work was supported by the National Institutes of Health [grant numbers R01EY023242, R21EY028671, and P30EY031631]; and the startup fund from the Medical College of Georgia at Augusta University, Augusta, GA, USA.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work. reported in this paper.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- AGE
advanced glycation end product
- CAST
calpastatin
- CCT
central corneal thickness
- CH
corneal hysteresis
- CRF
corneal resistance factor
- DM
diabetes mellitus
- DN
diabetic nephropathy
- DR
diabetic retinopathy
- ECM
extracellular matrix
- EDS
Ehler-Danlos syndrome
- EMT
epithelial to mesenchymal transition
- FECD
Fuch's endothelial corneal dystrophy
- FOXO1
forkhead box protein O1
- GBM
glomerular basement membrane
- GWAS
genome-wide association studies
- HGF
hepatocyte growth factor
- IHC
immunohistochemistry
- KC
keratoconus
- lncRNA
long noncoding RNA
- LOX
lysyl oxidase
- miRNA
microRNA
- MMP
matrix metalloproteinases
- PPCD
posterior polymorphous corneal dystrophy
- RAGE
receptor for advanced glycation end products
- SOD1
superoxide dismutase 1
- TIMP
tissue inhibitors of metalloproteinases
- TGFBI
transforming growth factor beta-induced
- TGFBIp
transforming growth factor beta-induced protein
- VSX1
visual system homeobox 1
- ZEB1
zinc finger E box-binding homeobox 1
References
- 1.Rabinowitz Y.S. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Sykakis E., Carley F., Irion L., et al. An in depth analysis of histopathological characteristics found in keratoconus. Pathology. 2012;44(3):234–239. doi: 10.1097/PAT.0b013e3283511b42. [DOI] [PubMed] [Google Scholar]
- 3.You J., Wen L., Roufas A., et al. Expression of HGF and c-Met proteins in human keratoconus corneas. J Ophthalmol. 2015 doi: 10.1155/2015/852986. 2015852986, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon-Shaag A., Millodot M., Shneor E., et al. The genetic and environmental factors for keratoconus. BioMed Res Int. 2015 doi: 10.1155/2015/795738. 2015795738, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas S.E.M., Burdon K.P. Genetic and environmental risk factors for keratoconus. Annu Rev Vis Sci. 2020:625–646. doi: 10.1146/annurev-vision-121219-081723. [DOI] [PubMed] [Google Scholar]
- 6.Sugar J., Macsai M.S. What causes keratoconus? Cornea. 2012;31(6) doi: 10.1097/ICO.0b013e31823f8c72. [DOI] [PubMed] [Google Scholar]
- 7.Alio J.L., Vega-Estrada A., Sanz P., et al. Corneal morphologic characteristics in patients with down syndrome. JAMA Ophthalmol. 2018;136(9):971–978. doi: 10.1001/jamaophthalmol.2018.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckh U., Schonherr U., Naumann G.O. Autosomal dominant keratoconus as the chief ocular symptom in Lobstein osteogenesis imperfecta tarda. Klin Monbl Augenheilkd. 1995;206(4):268–272. doi: 10.1055/s-2008-1035438. [DOI] [PubMed] [Google Scholar]
- 9.Khaled M.L., Helwa I., Drewry M., et al. Molecular and histopathological changes associated with keratoconus. BioMed Res Int. 2017 doi: 10.1155/2017/7803029. 20177803029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson I. Keratoconus and the Ehlers-Danlos syndrome: a new aspect of keratoconus. Med J Aust. 1975;1(18):571–573. doi: 10.5694/j.1326-5377.1975.tb111590.x. [DOI] [PubMed] [Google Scholar]
- 11.Nemet A.Y., Vinker S., Bahar I., et al. The association of keratoconus with immune disorders. Cornea. 2010;29(11):1261–1264. doi: 10.1097/ICO.0b013e3181cb410b. [DOI] [PubMed] [Google Scholar]
- 12.Billcliff P.G., Lowe M. Inositol lipid phosphatases in membrane trafficking and human disease. Biochem J. 2014;461(2):159–175. doi: 10.1042/BJ20140361. [DOI] [PubMed] [Google Scholar]
- 13.Seiler T., Huhle S., Spoerl E., et al. Manifest diabetes and keratoconus: a retrospective case-control study. Graefes Arch Clin Exp Ophthalmol. 2000;238(10):822–825. doi: 10.1007/s004179900111. [DOI] [PubMed] [Google Scholar]
- 14.Kuo I.C., Broman A., Pirouzmanesh A., et al. Is there an association between diabetes and keratoconus? Ophthalmology. 2006;113(2):184–190. doi: 10.1016/j.ophtha.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Naderan M., Shoar S., Rezagholizadeh F., et al. Characteristics and associations of keratoconus patients. Contact Lens Anterior Eye. 2015;38(3):199–205. doi: 10.1016/j.clae.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Ljubimov A.V. Diabetic complications in the cornea. Vis Res. 2017:139138–139152. doi: 10.1016/j.visres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naderan M., Naderan M., Rezagholizadeh F., et al. Association between diabetes and keratoconus: a case-control study. Cornea. 2014;33(12):1271–1273. doi: 10.1097/ICO.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 18.Woodward M.A., Blachley T.S., Stein J.D. The association between sociodemographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology. 2016;123(3):457–465. doi: 10.1016/j.ophtha.2015.10.035. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bak-Nielsen S., Ramlau-Hansen C.H., Ivarsen A., et al. A nationwide population-based study of social demographic factors, associated diseases and mortality of keratoconus patients in Denmark from 1977 to 2015. Acta Ophthalmol. 2019;97(5):497–504. doi: 10.1111/aos.13961. [DOI] [PubMed] [Google Scholar]
- 20.Kosker M., Suri K., Hammersmith K.M., et al. Another look at the association between diabetes and keratoconus. Cornea. 2014;33(8):774–779. doi: 10.1097/ICO.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 21.Moon J.Y., Lee J., Park Y.H., et al. Incidence of keratoconus and its association with systemic comorbid conditions: a nationwide cohort study from South Korea. J Ophthalmol. 2020 doi: 10.1155/2020/3493614. 20203493614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claessens J.L.J., Godefrooij D.A., Vink G., et al. Nationwide epidemiological approach to identify associations between keratoconus and immune-mediated diseases. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2021-318804. bjophthalmol-2021-318804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galvis V., Tello A., Ortiz A.I., et al. Patient selection for corneal collagen cross-linking: an updated review. Clin Ophthalmol. 2017:11657–11668. doi: 10.2147/OPTH.S101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kling S., Hafezi F. Corneal biomechanics - a review. Ophthalmic Physiol Opt. 2017;37(3):240–252. doi: 10.1111/opo.12345. [DOI] [PubMed] [Google Scholar]
- 25.Ma J., Wang Y., Wei P., et al. Biomechanics and structure of the cornea: implications and association with corneal disorders. Surv Ophthalmol. 2018;63(6):851–861. doi: 10.1016/j.survophthal.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 26.McKay T.B., Priyadarsini S., Karamichos D. Mechanisms of collagen crosslinking in diabetes and keratoconus. Cells. 2019;8(10) doi: 10.3390/cells8101239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priyadarsini S., McKay T.B., Sarker-Nag A., et al. Complete metabolome and lipidome analysis reveals novel biomarkers in the human diabetic corneal stroma. Exp Eye Res. 2016 doi: 10.1016/j.exer.2016.10.010. 15390-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weston B.C., Bourne W.M., Polse K.A., et al. Corneal hydration control in diabetes mellitus. Invest Ophthalmol Vis Sci. 1995;36(3):586–595. [PubMed] [Google Scholar]
- 29.Kenney M.C., Nesburn A.B., Burgeson R.E., et al. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997;16(3):345–351. [PubMed] [Google Scholar]
- 30.Ertan A., Muftuoglu O. Keratoconus clinical findings according to different age and gender groups. Cornea. 2008;27(10):1109–1113. doi: 10.1097/ICO.0b013e31817f815a. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Yang H., Rabinowitz Y.S. Longitudinal study of keratoconus progression. Exp Eye Res. 2007;85(4):502–507. doi: 10.1016/j.exer.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackburn B.J., Jenkins M.W., Rollins A.M., et al. A review of structural and biomechanical changes in the cornea in aging, disease, and photochemical crosslinking. Front Bioeng Biotechnol. 2019;7(66):66. doi: 10.3389/fbioe.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sady C., Khosrof S., Nagaraj R. Advanced Maillard reaction and crosslinking of corneal collagen in diabetes. Biochem Biophys Res Commun. 1995;214(3):793–797. doi: 10.1006/bbrc.1995.2356. [DOI] [PubMed] [Google Scholar]
- 34.Goldich Y., Barkana Y., Gerber Y., et al. Effect of diabetes mellitus on biomechanical parameters of the cornea. J Cataract Refract Surg. 2009;35(4):715–719. doi: 10.1016/j.jcrs.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Busted N., Olsen T., Schmitz O. Clinical observations on the corneal thickness and the corneal endothelium in diabetes mellitus. Br J Ophthalmol. 1981;65(10):687–690. doi: 10.1136/bjo.65.10.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keoleian G.M., Pach J.M., Hodge D.O., et al. Structural and functional studies of the corneal endothelium in diabetes mellitus. Am J Ophthalmol. 1992;113(1):64–70. doi: 10.1016/s0002-9394(14)75755-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.S., Oum B.S., Choi H.Y., et al. Differences in corneal thickness and corneal endothelium related to duration in diabetes. Eye (Lond) 2006;20(3):315–318. doi: 10.1038/sj.eye.6701868. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Thorin J.C. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38(2):19–36. [PubMed] [Google Scholar]
- 39.Su D.H., Wong T.Y., Wong W.L., et al. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology. 2008;115(6):964–968 e1. doi: 10.1016/j.ophtha.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Hager A., Wegscheider K., Wiegand W. Changes of extracellular matrix of the cornea in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1369–1374. doi: 10.1007/s00417-009-1088-4. [DOI] [PubMed] [Google Scholar]
- 41.Scheler A., Spoerl E., Boehm A.G. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta Ophthalmol. 2012;90(6):e447–e451. doi: 10.1111/j.1755-3768.2012.02437.x. [DOI] [PubMed] [Google Scholar]
- 42.Kagan H.M., Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 43.Lucero H.A., Kagan H.M. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19-20):2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coral K., Angayarkanni N., Madhavan J., et al. Lysyl oxidase activity in the ocular tissues and the role of LOX in proliferative diabetic retinopathy and rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2008;49(11):4746–4752. doi: 10.1167/iovs.07-1550. [DOI] [PubMed] [Google Scholar]
- 45.Dudakova L., Liskova P., Trojek T., et al. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res. 2012:10474–10481. doi: 10.1016/j.exer.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Sethi A., Mao W., Wordinger R.J., et al. Transforming growth factor–β induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Investig Ophthalmol Vis Sci. 2011;52(8):5240–5250. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bykhovskaya Y., Li X., Epifantseva I., et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci. 2012;53(7):4152–4157. doi: 10.1167/iovs.11-9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bykhovskaya Y., Margines B., Rabinowitz Y.S. Genetics in Keratoconus: where are we? Eye Vis (Lond) 2016;316 doi: 10.1186/s40662-016-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choquet H., Melles R.B., Yin J., et al. A multiethnic genome-wide analysis of 44,039 individuals identifies 41 new loci associated with central corneal thickness. Commun Biol. 2020;3(1):301. doi: 10.1038/s42003-020-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dudakova L., Palos M., Jirsova K., et al. Validation of rs2956540:G>C and rs3735520:G>A association with keratoconus in a population of European descent. Eur J Hum Genet. 2015;23(11):1581–1583. doi: 10.1038/ejhg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao X.D., Chen P., Chen Z.L., et al. Evaluating the association between keratoconus and reported genetic loci in a Han Chinese population. Ophthalmic Genet. 2015;36(2):132–136. doi: 10.3109/13816810.2015.1005317. [DOI] [PubMed] [Google Scholar]
- 52.Hasanian-Langroudi F., Saravani R., Validad M.H., et al. Association of lysyl oxidase (LOX) polymorphisms with the risk of keratoconus in an Iranian population. Ophthalmic Genet. 2015;36(4):309–314. doi: 10.3109/13816810.2014.881507. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J., Zhang L., Hong J., et al. Association of common variants in LOX with keratoconus: a meta-analysis. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shetty R., Kumar N.R., Khamar P., et al. Bilaterally asymmetric corneal ectasia following SMILE with asymmetrically reduced stromal molecular markers. J Refract Surg. 2019;35(1):6–14. doi: 10.3928/1081597X-20181128-01. [DOI] [PubMed] [Google Scholar]
- 55.Schlotzer-Schrehardt U. Molecular pathology of pseudoexfoliation syndrome/glaucoma--new insights from LOXL1 gene associations. Exp Eye Res. 2009;88(4):776–785. doi: 10.1016/j.exer.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Thorleifsson G., Magnusson K.P., Sulem P., et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317(5843):1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 57.Dudakova L., Sasaki T., Liskova P., et al. The presence of lysyl oxidase-like enzymes in human control and keratoconic corneas. Histol Histopathol. 2016;31(1):63–71. doi: 10.14670/HH-11-649. [DOI] [PubMed] [Google Scholar]
- 58.Chronopoulos A., Tang A., Beglova E., et al. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes. 2010;59(12):3159–3166. doi: 10.2337/db10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buckingham B., Reiser K.M. Relationship between the content of lysyl oxidase-dependent cross-links in skin collagen, nonenzymatic glycosylation, and long-term complications in type I diabetes mellitus. J Clin Invest. 1990;86(4):1046–1054. doi: 10.1172/JCI114807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coral K., Madhavan J., Pukhraj R., et al. High glucose induced differential expression of lysyl oxidase and its isoform in ARPE-19 cells. Curr Eye Res. 2013;38(1):194–203. doi: 10.3109/02713683.2012.720341. [DOI] [PubMed] [Google Scholar]
- 61.Dimasi D.P., Chen J.Y., Hewitt A.W., et al. Novel quantitative trait loci for central corneal thickness identified by candidate gene analysis of osteogenesis imperfecta genes. Hum Genet. 2010;127(1):33–44. doi: 10.1007/s00439-009-0729-3. [DOI] [PubMed] [Google Scholar]
- 62.Vithana E.N., Aung T., Khor C.C., et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Genet. 2011;20(4):649–658. doi: 10.1093/hmg/ddq511. [DOI] [PubMed] [Google Scholar]
- 63.Vitart V., Bencic G., Hayward C., et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum Mol Genet. 2010;19(21):4304–4311. doi: 10.1093/hmg/ddq349. [DOI] [PubMed] [Google Scholar]
- 64.Hoehn R., Zeller T., Verhoeven V.J., et al. Population-based meta-analysis in Caucasians confirms association with COL5A1 and ZNF469 but not COL8A2 with central corneal thickness. Hum Genet. 2012;131(11):1783–1793. doi: 10.1007/s00439-012-1201-3. [DOI] [PubMed] [Google Scholar]
- 65.Li X., Bykhovskaya Y., Canedo A.L., et al. Genetic association of COL5A1 variants in keratoconus patients suggests a complex connection between corneal thinning and keratoconus. Invest Ophthalmol Vis Sci. 2013;54(4):2696–2704. doi: 10.1167/iovs.13-11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu Y., Vitart V., Burdon K.P., et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013;45(2):155–163. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birk D.E. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32(3):223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 68.Birk D.E., Fitch J.M., Babiarz J.P., et al. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988;106(3):999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun M., Chen S., Adams S.M., et al. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011;124(Pt 23):4096–4105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birk D., Bruckner P. 2011. The Extracellular Matrix: An Overview, Biology of Extracellular Matrix. [Google Scholar]
- 71.Birk D.E., Fitch J.M., Babiarz J.P., et al. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95(Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 72.Segev F., Heon E., Cole W.G., et al. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci. 2006;47(2):565–573. doi: 10.1167/iovs.05-0771. [DOI] [PubMed] [Google Scholar]
- 73.Schwarze U., Atkinson M., Hoffman G.G., et al. Null alleles of the COL5A1 gene of type V collagen are a cause of the classical forms of Ehlers-Danlos syndrome (types I and II) Am J Hum Genet. 2000;66(6):1757–1765. doi: 10.1086/302933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wenstrup R.J., Florer J.B., Willing M.C., et al. COL5A1 haploinsufficiency is a common molecular mechanism underlying the classical form of EDS. Am J Hum Genet. 2000;66(6):1766–1776. doi: 10.1086/302930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rohlfing C.L., Wiedmeyer H.M., Little R.R., et al. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the diabetes control and complications trial. Diabetes Care. 2002;25(2):275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 76.Andreassen T.T., Seyer-Hansen K., Bailey A.J. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys Acta. 1981;677(2):313–317. doi: 10.1016/0304-4165(81)90101-x. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Fessel G., Georgiadis M., et al. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013;32(3-4):169–177. doi: 10.1016/j.matbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Spoerl E., Wollensak G., Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29(1):35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 79.Neeper M., Schmidt A.M., Brett J., et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(21):14998–15004. [PubMed] [Google Scholar]
- 80.Reynaert N.L., Gopal P., Rutten E.P.A., et al. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int J Biochem Cell Biol. 2016;81(Pt B):403–418. doi: 10.1016/j.biocel.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 81.Ng K.K.K., Cheung C.Y.Y., Lee C.H., et al. Possible modifying effect of hemoglobin A1c on genetic susceptibility to severe diabetic retinopathy in patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 2020;61(10):7. doi: 10.1167/iovs.61.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith V.A., Matthews F.J., Majid M.A., et al. Keratoconus: matrix metalloproteinase-2 activation and TIMP modulation. Biochim Biophys Acta. 2006;1762(4):431–439. doi: 10.1016/j.bbadis.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Kenney M.C., Chwa M., Atilano S.R., et al. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005;46(3):823–832. doi: 10.1167/iovs.04-0549. [DOI] [PubMed] [Google Scholar]
- 84.Xing Y.Q., Li A., Yang Y., et al. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018:193124–193131. doi: 10.1016/j.lfs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 85.Gao X., Gauderman W.J., Liu Y., et al. A genome-wide association study of central corneal thickness in Latinos. Invest Ophthalmol Vis Sci. 2013;54(4):2435–2443. doi: 10.1167/iovs.13-11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abu-Amero K.K., Helwa I., Al-Muammar A., et al. Case-control association between CCT-associated variants and keratoconus in a Saudi Arabian population. J Negat Results Biomed. 2015;1410 doi: 10.1186/s12952-015-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J., Waldron R.T., Su H.Y., et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2016;311(4):G675–G687. doi: 10.1152/ajpgi.00251.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kitamura T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9(10):615–623. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- 89.Wilhelm K., Happel K., Eelen G., et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529(7585):216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H., Ge S., He K., et al. FoxO1 inhibits autophagosome-lysosome fusion leading to endothelial autophagic-apoptosis in diabetes. Cardiovasc Res. 2019;115(14):2008–2020. doi: 10.1093/cvr/cvz014. [DOI] [PubMed] [Google Scholar]
- 91.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 92.Nakao A., Afrakhte M., Moren A., et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389(6651):631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 93.Gomes L.R., Terra L.F., Wailemann R.A., et al. TGF-beta1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;1226 doi: 10.1186/1471-2407-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schiller M., Javelaud D., Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35(2):83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 95.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200(4):448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 96.Engler C., Chakravarti S., Doyle J., et al. Transforming growth factor-beta signaling pathway activation in Keratoconus. Am J Ophthalmol. 2011;151(5):752–759. doi: 10.1016/j.ajo.2010.11.008. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maier P., Broszinski A., Heizmann U., et al. Active transforming growth factor-beta2 is increased in the aqueous humor of keratoconus patients. Mol Vis. 2007:131198–131202. [PubMed] [Google Scholar]
- 98.Priyadarsini S., McKay T.B., Sarker-Nag A., et al. Keratoconus in vitro and the key players of the TGF-beta pathway. Mol Vis. 2015:21577–21588. [PMC free article] [PubMed] [Google Scholar]
- 99.Tai T.Y., Damani M.R., Vo R., et al. Keratoconus associated with corneal stromal amyloid deposition containing TGFBIp. Cornea. 2009;28(5):589–593. doi: 10.1097/ICO.0b013e31818c9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hosoda Y., Miyake M., Meguro A., et al. Keratoconus-susceptibility gene identification by corneal thickness genome-wide association study and artificial intelligence IBM Watson. Commun Biol. 2020;3(1):410. doi: 10.1038/s42003-020-01137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yadav H., Devalaraja S., Chung S.T., et al. TGF-beta1/Smad3 pathway targets PP2A-AMPK-FoxO1 signaling to regulate hepatic gluconeogenesis. J Biol Chem. 2017;292(8):3420–3432. doi: 10.1074/jbc.M116.764910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abe H., Tominaga T., Matsubara T., et al. Scleraxis modulates bone morphogenetic protein 4 (BMP4)-Smad1 protein-smooth muscle alpha-actin (SMA) signal transduction in diabetic nephropathy. J Biol Chem. 2012;287(24):20430–20442. doi: 10.1074/jbc.M111.275610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flyvbjerg A., Denner L., Schrijvers B.F., et al. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53(1):166–172. doi: 10.2337/diabetes.53.1.166. [DOI] [PubMed] [Google Scholar]
- 104.Belghith M., Bluestone J.A., Barriot S., et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9(9):1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 105.Huang C., Kim Y., Caramori M.L., et al. Cellular basis of diabetic nephropathy: II. The transforming growth factor-beta system and diabetic nephropathy lesions in type 1 diabetes. Diabetes. 2002;51(12):3577–3581. doi: 10.2337/diabetes.51.12.3577. [DOI] [PubMed] [Google Scholar]
- 106.Ono H., Abe H., Sakurai A., et al. Novel interplay between Smad1 and Smad3 phosphorylation via AGE regulates the progression of diabetic nephropathy. Sci Rep. 2018;8(1):10548. doi: 10.1038/s41598-018-28439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kannabiran C. Genetics of corneal endothelial dystrophies. J Genet. 2009;88(4):487–494. doi: 10.1007/s12041-009-0067-1. [DOI] [PubMed] [Google Scholar]
- 108.Han K.E., Choi S.I., Kim T.I., et al. Pathogenesis and treatments of TGFBI corneal dystrophies. Prog Retin Eye Res. 2016:5067–5088. doi: 10.1016/j.preteyeres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 109.El-Shabrawi Y., Kublin C.L., Cintron C. mRNA levels of alpha1(VI) collagen, alpha1(XII) collagen, and beta ig in rabbit cornea during normal development and healing. Invest Ophthalmol Vis Sci. 1998;39(1):36–44. [PubMed] [Google Scholar]
- 110.Escribano J., Hernando N., Ghosh S., et al. cDNA from human ocular ciliary epithelium homologous to βig-h3 is preferentially expressed as an extracellular protein in the corneal epithelium. 1994;160(3):511–521. doi: 10.1002/jcp.1041600314. [DOI] [PubMed] [Google Scholar]
- 111.Hirano K., Klintworth G.K., Zhan Q., et al. Beta ig-h3 is synthesized by corneal epithelium and perhaps endotheliumin Fuchs' dystrophic corneas. Curr Eye Res. 1996;15(9):965–972. doi: 10.3109/02713689609017642. [DOI] [PubMed] [Google Scholar]
- 112.Leung E.W., Rife L., Smith R.E., et al. Extracellular matrix components in retrocorneal fibrous membrane in comparison to corneal endothelium and Descemet's membrane. Mol Vis. 2000:615–623. [PubMed] [Google Scholar]
- 113.Reinboth B., Thomas J., Hanssen E., et al. Beta ig-h3 interacts directly with biglycan and decorin, promotes collagen VI aggregation, and participates in ternary complexing with these macromolecules. J Biol Chem. 2006;281(12):7816–7824. doi: 10.1074/jbc.M511316200. [DOI] [PubMed] [Google Scholar]
- 114.Hanssen E., Reinboth B., Gibson M.A. Covalent and non-covalent interactions of betaig-h3 with collagen VI. Beta ig-h3 is covalently attached to the amino-terminal region of collagen VI in tissue microfibrils. J Biol Chem. 2003;278(27):24334–24341. doi: 10.1074/jbc.M303455200. [DOI] [PubMed] [Google Scholar]
- 115.Kim J.E., Park R.W., Choi J.Y., et al. Molecular properties of wild-type and mutant betaIG-H3 proteins. Invest Ophthalmol Vis Sci. 2002;43(3):656–661. [PubMed] [Google Scholar]
- 116.Poulsen E.T., Runager K., Nielsen N.S., et al. Proteomic profiling of TGFBI-null mouse corneas reveals only minor changes in matrix composition supportive of TGFBI knockdown as therapy against TGFBI-linked corneal dystrophies. FEBS J. 2018;285(1):101–114. doi: 10.1111/febs.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Runager K., Klintworth G.K., Karring H., et al. The insoluble TGFBIp fraction of the cornea is covalently linked via a disulfide bond to type XII collagen. Biochemistry. 2013;52(16):2821–2827. doi: 10.1021/bi400212m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bae J.S., Lee S.H., Kim J.E., et al. Betaig-h3 supports keratinocyte adhesion, migration, and proliferation through alpha3beta1 integrin. Biochem Biophys Res Commun. 2002;294(5):940–948. doi: 10.1016/S0006-291X(02)00576-4. [DOI] [PubMed] [Google Scholar]
- 119.Kim H.J., Kim I.S. Transforming growth factor-beta-induced gene product, as a novel ligand of integrin alphaMbeta2, promotes monocytes adhesion, migration and chemotaxis. Int J Biochem Cell Biol. 2008;40(5):991–1004. doi: 10.1016/j.biocel.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Kim J.E., Jeong H.W., Nam J.O., et al. Identification of motifs in the fasciclin domains of the transforming growth factor-beta-induced matrix protein betaig-h3 that interact with the alphavbeta5 integrin. J Biol Chem. 2002;277(48):46159–46165. doi: 10.1074/jbc.M207055200. [DOI] [PubMed] [Google Scholar]
- 121.Kim J.E., Kim S.J., Lee B.H., et al. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, betaig-h3. J Biol Chem. 2000;275(40):30907–30915. doi: 10.1074/jbc.M002752200. [DOI] [PubMed] [Google Scholar]
- 122.Ohno S., Noshiro M., Makihira S., et al. RGD-CAP ((beta)ig-h3) enhances the spreading of chondrocytes and fibroblasts via integrin alpha(1)beta(1) Biochim Biophys Acta. 1999;1451(1):196–205. doi: 10.1016/s0167-4889(99)00093-2. [DOI] [PubMed] [Google Scholar]
- 123.Rabinowitz Y.S., Dong L., Wistow G. Gene expression profile studies of human keratoconus cornea for NEIBank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest Ophthalmol Vis Sci. 2005;46(4):1239–1246. doi: 10.1167/iovs.04-1148. [DOI] [PubMed] [Google Scholar]
- 124.Guan T., Liu C., Ma Z., et al. The point mutation and polymorphism in keratoconus candidate gene TGFBI in Chinese population. Gene. 2012;503(1):137–139. doi: 10.1016/j.gene.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 125.Karolak J.A., Polakowski P., Szaflik J., et al. Molecular screening of keratoconus susceptibility sequence variants in VSX1, TGFBI, DOCK9, STK24, and IPO5 genes in Polish patients and novel TGFBI variant identification. Ophthalmic Genet. 2016;37(1):37–43. doi: 10.3109/13816810.2014.926375. [DOI] [PubMed] [Google Scholar]
- 126.Takacs L., Csutak A., Balazs E., et al. Expression of betaig-h3 is lower than normal in keratoconus corneas but increases with scarring. Cornea. 1999;18(5):599–605. [PubMed] [Google Scholar]
- 127.Group T.D.R.S.R. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88(7):583–600. [PubMed] [Google Scholar]
- 128.Wang M., Munier F., Araki-Saski K., et al. TGFBI gene transcript is transforming growth factor-beta1-responsive and cell density-dependent in a human corneal epithelial cell line. Ophthalmic Genet. 2002;23(4):237–245. doi: 10.1076/opge.23.4.237.13884. [DOI] [PubMed] [Google Scholar]
- 129.Han B., Luo H., Raelson J., et al. TGFBI (betaIG-H3) is a diabetes-risk gene based on mouse and human genetic studies. Hum Mol Genet. 2014;23(17):4597–4611. doi: 10.1093/hmg/ddu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Billings P.C., Herrick D.J., Kucich U., et al. Extracellular matrix and nuclear localization of beta ig-h3 in human bladder smooth muscle and fibroblast cells. J Cell Biochem. 2000;79(2):261–273. doi: 10.1002/1097-4644(20001101)79:2<261::aid-jcb90>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 131.Park S.W., Bae J.S., Kim K.S., et al. Beta ig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with alpha3beta1 integrin. Exp Mol Med. 2004;36(3):211–219. doi: 10.1038/emm.2004.29. [DOI] [PubMed] [Google Scholar]
- 132.Lee S.H., Bae J.S., Park S.H., et al. Expression of TGF-beta-induced matrix protein betaig-h3 is up-regulated in the diabetic rat kidney and human proximal tubular epithelial cells treated with high glucose. Kidney Int. 2003;64(3):1012–1021. doi: 10.1046/j.1523-1755.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 133.Phillips A.O., Steadman R., Morrisey K., et al. Exposure of human renal proximal tubular cells to glucose leads to accumulation of type IV collagen and fibronectin by decreased degradation. Kidney Int. 1997;52(4):973–984. doi: 10.1038/ki.1997.419. [DOI] [PubMed] [Google Scholar]
- 134.Ziyadeh F.N., Sharma K. Role of transforming growth factor-beta in diabetic glomerulosclerosis and renal hypertrophy. Kidney Int Suppl. 1995 51S34-6. [PubMed] [Google Scholar]
- 135.Ziyadeh F.N., Sharma K., Ericksen M., et al. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994;93(2):536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lane P.H., Steffes M.W., Fioretto P., et al. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993;43(3):661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- 137.Nath K.A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20(1):1–17. doi: 10.1016/S0272-6386(12)80312-X. [DOI] [PubMed] [Google Scholar]
- 138.Munier F.L., Frueh B.E., Othenin-Girard P., et al. BIGH3 mutation spectrum in corneal dystrophies. Invest Ophthalmol Vis Sci. 2002;43(4):949–954. [PubMed] [Google Scholar]
- 139.Aldave A.J., Yellore V.S., Yu F., et al. Posterior polymorphous corneal dystrophy is associated with TCF8 gene mutations and abdominal hernia. Am J Med Genet A. 2007;143A(21):2549–2556. doi: 10.1002/ajmg.a.31978. [DOI] [PubMed] [Google Scholar]
- 140.Krafchak C.M., Pawar H., Moroi S.E., et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am J Hum Genet. 2005;77(5):694–708. doi: 10.1086/497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Terraz C., Toman D., Delauche M., et al. Delta Ef1 binds to a far upstream sequence of the mouse pro-alpha 1(I) collagen gene and represses its expression in osteoblasts. J Biol Chem. 2001;276(40):37011–37019. doi: 10.1074/jbc.M104185200. [DOI] [PubMed] [Google Scholar]
- 142.Vandewalle C., Van Roy F., Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66(5):773–787. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frisch S.M. E1a induces the expression of epithelial characteristics. J Cell Biol. 1994;127(4):1085–1096. doi: 10.1083/jcb.127.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grooteclaes M.L., Frisch S.M. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene. 2000;19(33):3823–3828. doi: 10.1038/sj.onc.1203721. [DOI] [PubMed] [Google Scholar]
- 145.Chiu L.Y., Hsin I.L., Yang T.Y., et al. The ERK-ZEB1 pathway mediates epithelial-mesenchymal transition in pemetrexed resistant lung cancer cells with suppression by vinca alkaloids. Oncogene. 2017;36(2):242–253. doi: 10.1038/onc.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y., Pathak N., Kramer-Zucker A., et al. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134(6):1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 147.Zhang P., Sun Y., Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14(4):481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Postigo A.A. Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. EMBO J. 2003;22(10):2443–2452. doi: 10.1093/emboj/cdg225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liskova P., Tuft S.J., Gwilliam R., et al. Novel mutations in the ZEB1 gene identified in Czech and British patients with posterior polymorphous corneal dystrophy. Hum Mutat. 2007;28(6):638. doi: 10.1002/humu.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mehta J.S., Vithana E.N., Tan D.T., et al. Analysis of the posterior polymorphous corneal dystrophy 3 gene, TCF8, in late-onset Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2008;49(1):184–188. doi: 10.1167/iovs.07-0847. [DOI] [PubMed] [Google Scholar]
- 151.Riazuddin S.A., Zaghloul N.A., Al-Saif A., et al. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am J Hum Genet. 2010;86(1):45–53. doi: 10.1016/j.ajhg.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lechner J., Dash D.P., Muszynska D., et al. Mutational spectrum of the ZEB1 gene in corneal dystrophies supports a genotype-phenotype correlation. Invest Ophthalmol Vis Sci. 2013;54(5):3215–3223. doi: 10.1167/iovs.13-11781. [DOI] [PubMed] [Google Scholar]
- 153.Stachs O., Bochert A., Gerber T., et al. [The extracellular matrix structure in keratoconus] Ophthalmologe. 2004;101(4):384–389. doi: 10.1007/s00347-003-0902-3. [DOI] [PubMed] [Google Scholar]
- 154.Mazzotta C., Traversi C., Raiskup F., et al. First identification of a triple corneal dystrophy association: keratoconus, epithelial basement membrane corneal dystrophy and fuchs' endothelial corneal dystrophy. Case Rep Ophthalmol. 2014;5(3):281–288. doi: 10.1159/000367937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Singh K., Sinha M., Pal D., et al. Cutaneous epithelial to mesenchymal transition activator ZEB1 regulates wound angiogenesis and closure in a glycemic status-dependent manner. Diabetes. 2019;68(11):2175–2190. doi: 10.2337/db19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pankow J.S., Decker P.A., Berardi C., et al. Circulating cellular adhesion molecules and risk of diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabet Med. 2016;33(7):985–991. doi: 10.1111/dme.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu C., Lin J. Long noncoding RNA ZEB1-AS1 acts as an oncogene in osteosarcoma by epigenetically activating ZEB1. Am J Transl Res. 2016;8(10):4095–4105. [PMC free article] [PubMed] [Google Scholar]
- 158.Meng Q., Zhai X., Yuan Y., et al. lncRNA ZEB1-AS1 inhibits high glucose-induced EMT and fibrogenesis by regulating the miR-216a-5p/BMP7 axis in diabetic nephropathy. Braz J Med Biol Res. 2020;53(4):e9288. doi: 10.1590/1414-431X20209288. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159.Loeffler I., Wolf G. Epithelial-to-Mesenchymal transition in diabetic nephropathy: fact or fiction? Cells. 2015;4(4):631–652. doi: 10.3390/cells4040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun J., Wang Y., Cui W., et al. Role of epigenetic histone modifications in diabetic kidney disease involving renal fibrosis. J Diabetes Res. 2017 doi: 10.1155/2017/7242384. 20177242384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang J., Pan J., Li H., et al. lncRNA ZEB1-AS1 was suppressed by p53 for renal fibrosis in diabetic nephropathy. Mol Ther Nucleic Acids. 2018:12741–12750. doi: 10.1016/j.omtn.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luotola K., Pietila A., Zeller T., et al. Associations between interleukin-1 (IL-1) gene variations or IL-1 receptor antagonist levels and the development of type 2 diabetes. J Intern Med. 2011;269(3):322–332. doi: 10.1111/j.1365-2796.2010.02294.x. [DOI] [PubMed] [Google Scholar]
- 163.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.di Martino E., Ali M., Inglehearn C.F. Matrix metalloproteinases in keratoconus - too much of a good thing? Exp Eye Res. 2019:182137–182143. doi: 10.1016/j.exer.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 165.Parks W.C., Wilson C.L., Lopez-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 166.Balasubramanian S.A., Mohan S., Pye D.C., et al. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90(4):e303–e309. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 167.Smith V.A., Easty D.L. Matrix metalloproteinase 2: involvement in keratoconus. Eur J Ophthalmol. 2000;10(3):215–226. doi: 10.1177/112067210001000305. [DOI] [PubMed] [Google Scholar]
- 168.Lema I., Duran J.A. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112(4):654–659. doi: 10.1016/j.ophtha.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 169.Lema I., Sobrino T., Duran J.A., et al. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. 2009;93(6):820–824. doi: 10.1136/bjo.2008.144253. [DOI] [PubMed] [Google Scholar]
- 170.Shetty R., Ghosh A., Lim R.R., et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci. 2015;56(2):738–750. doi: 10.1167/iovs.14-14831. [DOI] [PubMed] [Google Scholar]
- 171.Pahuja N., Kumar N.R., Shroff R., et al. Differential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest Ophthalmol Vis Sci. 2016;57(13):5372–5382. doi: 10.1167/iovs.16-19677. [DOI] [PubMed] [Google Scholar]
- 172.Sobrino T., Regueiro U., Malfeito M., et al. Higher expression of toll-like receptors 2 and 4 in blood cells of keratoconus patiens. Sci Rep. 2017;7(1):12975. doi: 10.1038/s41598-017-13525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Farina A.R., Mackay A.R. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers (Basel) 2014;6(1):240–296. doi: 10.3390/cancers6010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brown D., Chwa M.M., Opbroek A., et al. Keratoconus corneas: increased gelatinolytic activity appears after modification of inhibitors. Curr Eye Res. 1993;12(6):571–581. doi: 10.3109/02713689309001835. [DOI] [PubMed] [Google Scholar]
- 175.Lee J.E., Oum B.S., Choi H.Y., et al. Evaluation of differentially expressed genes identified in keratoconus. Mol Vis. 2009:152480–152487. [PMC free article] [PubMed] [Google Scholar]
- 176.Abdullah O.A., El Gazzar W.B., Salem T.I., et al. Role of extracellular matrix remodelling gene SNPs in keratoconus. Br J Biomed Sci. 2020;77(1):13–18. doi: 10.1080/09674845.2019.1654346. [DOI] [PubMed] [Google Scholar]
- 177.Saravani R., Yari D., Saravani S., et al. Correlation between the COL4A3, MMP-9, and TIMP-1 polymorphisms and risk of keratoconus. Jpn J Ophthalmol. 2017;61(3):218–222. doi: 10.1007/s10384-017-0503-3. [DOI] [PubMed] [Google Scholar]
- 178.Hardcastle A.J., Thiselton D.L., Nayudu M., et al. Genomic organization of the human TIMP-1 gene. Investigation of a causative role in the pathogenesis of X-linked retinitis pigmentosa 2. Invest Ophthalmol Vis Sci. 1997;38(9):1893–1896. [PubMed] [Google Scholar]
- 179.Death A.K., Fisher E.J., McGrath K.C., et al. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis. 2003;168(2):263–269. doi: 10.1016/s0021-9150(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 180.Takahashi H., Akiba K., Noguchi T., et al. Matrix metalloproteinase activity is enhanced during corneal wound repair in high glucose condition. Curr Eye Res. 2000;21(2):608–615. [PubMed] [Google Scholar]
- 181.Iyengar M.F., Soto L.F., Requena D., et al. Tear biomarkers and corneal sensitivity as an indicator of neuropathy in type 2 diabetes. Diabetes Res Clin Pract. 2020:163108143. doi: 10.1016/j.diabres.2020.108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Symeonidis C., Papakonstantinou E., Galli A., et al. Matrix metalloproteinase (MMP-2, -9) and tissue inhibitor (TIMP-1, -2) activity in tear samples of pediatric type 1 diabetic patients: MMPs in tear samples from type 1 diabetes. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):741–749. doi: 10.1007/s00417-012-2221-3. [DOI] [PubMed] [Google Scholar]
- 183.Ahluwalia T.S., Khullar M., Ahuja M., et al. Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS One. 2009;4(4):e5168. doi: 10.1371/journal.pone.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nazir N., Siddiqui K., Al-Qasim S., et al. Meta-analysis of diabetic nephropathy associated genetic variants in inflammation and angiogenesis involved in different biochemical pathways. BMC Med Genet. 2014;15103 doi: 10.1186/s12881-014-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saravani S., Yari D., Saravani R., et al. Association of COL4A3 (rs55703767), MMP-9 (rs17576)and TIMP-1 (rs6609533) gene polymorphisms with susceptibility to type 2 diabetes. Biomed Rep. 2017;6(3):329–334. doi: 10.3892/br.2017.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 187.Hughes A.E., Bradley D.T., Campbell M., et al. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am J Hum Genet. 2011;89(5):628–633. doi: 10.1016/j.ajhg.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Karali M., Peluso I., Gennarino V.A., et al. miRNeye: a microRNA expression atlas of the mouse eye. BMC Genom. 2010;11715 doi: 10.1186/1471-2164-11-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ryan D.G., Oliveira-Fernandes M., Lavker R.M. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006:121175–121184. [PubMed] [Google Scholar]
- 190.Yu J., Ryan D.G., Getsios S., et al. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A. 2008;105(49):19300–19305. doi: 10.1073/pnas.0803992105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Farzadfard A., Nassiri N., Moghadam T.N., et al. Screening for MIR184 mutations in Iranian patients with keratoconus. J Ophthalmic Vis Res. 2016;11(1):3–7. doi: 10.4103/2008-322X.180715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shalom-Feuerstein R., Serror L., De La Forest Divonne S., et al. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cell. 2012;30(5):898–909. doi: 10.1002/stem.1068. [DOI] [PubMed] [Google Scholar]
- 193.Iliff B.W., Riazuddin S.A., Gottsch J.D. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Invest Ophthalmol Vis Sci. 2012;53(1):348–353. doi: 10.1167/iovs.11-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bykhovskaya Y., Caiado Canedo A.L., Wright K.W., et al. C.57 C > T mutation in MIR 184 is responsible for congenital cataracts and corneal abnormalities in a five-generation family from Galicia, Spain. Ophthalmic Genet. 2015;36(3):244–247. doi: 10.3109/13816810.2013.848908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cagil N., Ugurlu N., Sahan C.Y., et al. Lack of MIR143, MIR145, MIR184, MIR1224, and MIR29b1 mutations in keratoconus pathogenesis. Turk J Med Sci. 2017;47(5):1669–1671. doi: 10.3906/sag-1702-92. [DOI] [PubMed] [Google Scholar]
- 196.Abu-Amero K.K., Helwa I., Al-Muammar A., et al. Screening of the seed region of MIR184 in keratoconus patients from Saudi arabia. BioMed Res Int. 2015 doi: 10.1155/2015/604508. 2015604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Martinez-Sanchez A., Rutter G.A., Latreille M. MiRNAs in beta-cell development, identity, and disease. Front Genet. 2016;7226 doi: 10.3389/fgene.2016.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Martinez-Sanchez A., Nguyen-Tu M.S., Cebola I., et al. MiR-184 expression is regulated by AMPK in pancreatic islets. Faseb J. 2018;32(5):2587–2600. doi: 10.1096/fj.201701100R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nesca V., Guay C., Jacovetti C., et al. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013;56(10):2203–2212. doi: 10.1007/s00125-013-2993-y. [DOI] [PubMed] [Google Scholar]
- 200.Tattikota S.G., Rathjen T., McAnulty S.J., et al. Argonaute2 mediates compensatory expansion of the pancreatic beta cell. Cell Metabol. 2014;19(1):122–134. doi: 10.1016/j.cmet.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tattikota S.G., Rathjen T., Hausser J., et al. miR-184 regulates pancreatic beta-cell function according to glucose metabolism. J Biol Chem. 2015;290(33):20284–20294. doi: 10.1074/jbc.M115.658625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jun A.S., Cope L., Speck C., et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fabre E.J., Bureau J., Pouliquen Y., et al. Binding sites for human interleukin 1 alpha, gamma interferon and tumor necrosis factor on cultured fibroblasts of normal cornea and keratoconus. Curr Eye Res. 1991;10(7):585–592. doi: 10.3109/02713689109013850. [DOI] [PubMed] [Google Scholar]
- 204.Efron N., Hollingsworth J.G. New perspectives on keratoconus as revealed by corneal confocal microscopy. Clin Exp Optom. 2008;91(1):34–55. doi: 10.1111/j.1444-0938.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 205.Behndig A., Karlsson K., Johansson B.O., et al. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Invest Ophthalmol Vis Sci. 2001;42(10):2293–2296. [PubMed] [Google Scholar]
- 206.Buddi R., Lin B., Atilano S.R., et al. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002;50(3):341–351. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- 207.Joseph R., Srivastava O.P., Pfister R.R. Differential epithelial and stromal protein profiles in keratoconus and normal human corneas. Exp Eye Res. 2011;92(4):282–298. doi: 10.1016/j.exer.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 208.Gondhowiardjo T.D., van Haeringen N.J. Corneal aldehyde dehydrogenase, glutathione reductase, and glutathione S-transferase in pathologic corneas. Cornea. 1993;12(4):310–314. doi: 10.1097/00003226-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 209.Atilano S.R., Coskun P., Chwa M., et al. Accumulation of mitochondrial DNA damage in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46(4):1256–1263. doi: 10.1167/iovs.04-1395. [DOI] [PubMed] [Google Scholar]
- 210.Chwa M., Atilano S.R., Reddy V., et al. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Invest Ophthalmol Vis Sci. 2006;47(5):1902–1910. doi: 10.1167/iovs.05-0828. [DOI] [PubMed] [Google Scholar]
- 211.Kenney M.C., Brown D.J., Rajeev B. Everett Kinsey lecture. The elusive causes of keratoconus: a working hypothesis. CLAO J. 2000;26(1):10–13. [PubMed] [Google Scholar]
- 212.Organ S.L., Tsao M.S. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Spix J.K., Chay E.Y., Block E.R., et al. Hepatocyte growth factor induces epithelial cell motility through transactivation of the epidermal growth factor receptor. Exp Cell Res. 2007;313(15):3319–3325. doi: 10.1016/j.yexcr.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wilson S.E., Walker J.W., Chwang E.L., et al. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993;34(8):2544–2561. [PubMed] [Google Scholar]
- 215.Li Q., Weng J., Mohan R.R., et al. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest Ophthalmol Vis Sci. 1996;37(5):727–739. [PubMed] [Google Scholar]
- 216.Wilson S.E., Liang Q., Kim W.J. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest Ophthalmol Vis Sci. 1999;40(10):2185–2190. [PubMed] [Google Scholar]
- 217.Burdon K.P., Macgregor S., Bykhovskaya Y., et al. Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Invest Ophthalmol Vis Sci. 2011;52(11):8514–8519. doi: 10.1167/iovs.11-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sahebjada S., Schache M., Richardson A.J., et al. Association of the hepatocyte growth factor gene with keratoconus in an Australian population. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0084067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huang Y.Y., Neuhauss S.C. The optokinetic response in zebrafish and its applications. Front Biosci. 2008:131899–131916. doi: 10.2741/2810. [DOI] [PubMed] [Google Scholar]
- 220.Saghizadeh M., Kramerov A.A., Tajbakhsh J., et al. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci. 2005;46(10):3604–3615. doi: 10.1167/iovs.04-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen W.L., Lin C.T., Ko P.S., et al. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology. 2009;116(6):1038–1047. doi: 10.1016/j.ophtha.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 222.Kabosova A., Kramerov A.A., Aoki A.M., et al. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003;77(2):211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Saghizadeh M., Kramerov A.A., Yu F.S., et al. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest Ophthalmol Vis Sci. 2010;51(4):1970–1980. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Oliveira A.G., Araujo T.G., Carvalho B.M., et al. The role of hepatocyte growth factor (HGF) in insulin resistance and diabetes. Front Endocrinol (Lausanne) 2018;9503 doi: 10.3389/fendo.2018.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zatz M., Starling A. Calpains and disease. N Engl J Med. 2005;352(23):2413–2423. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- 226.Li X., Bykhovskaya Y., Tang Y.G., et al. An association between the calpastatin (CAST) gene and keratoconus. Cornea. 2013;32(5):696–701. doi: 10.1097/ICO.0b013e3182821c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shearer T.R., Azuma M., David L.L., et al. Calpain and calpastatin in rabbit corneal epithelium. Curr Eye Res. 1990;9(1):39–44. doi: 10.3109/02713689009000053. [DOI] [PubMed] [Google Scholar]
- 228.Persson H., Kawashima S., Karlsson J.O. Immunohistochemical localization of calpains and calpastatin in the rabbit eye. Brain Res. 1993;611(2):272–278. doi: 10.1016/0006-8993(93)90513-m. [DOI] [PubMed] [Google Scholar]
- 229.Zhang J., Wu D., Li Y., et al. Evaluating the association between calpastatin (CAST) gene and keratoconus in the Han Chinese population. Gene. 2018:65310–65313. doi: 10.1016/j.gene.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 230.Chen B., Zhao Q., Ni R., et al. Inhibition of calpain reduces oxidative stress and attenuates endothelial dysfunction in diabetes. Cardiovasc Diabetol. 2014;1388 doi: 10.1186/1475-2840-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ohara T., Sussman K.E., Draznin B. Effect of diabetes on cytosolic free Ca2+ and Na(+)-K(+)-ATPase in rat aorta. Diabetes. 1991;40(11):1560–1563. doi: 10.2337/diab.40.11.1560. [DOI] [PubMed] [Google Scholar]
- 232.Stalker T.J., Gong Y., Scalia R. The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes. 2005;54(4):1132–1140. doi: 10.2337/diabetes.54.4.1132. [DOI] [PubMed] [Google Scholar]
- 233.Wang S., Peng Q., Zhang J., et al. Na+/H+ exchanger is required for hyperglycaemia-induced endothelial dysfunction via calcium-dependent calpain. Cardiovasc Res. 2008;80(2):255–262. doi: 10.1093/cvr/cvn179. [DOI] [PubMed] [Google Scholar]
- 234.Bryk D., Zapolska-Downar D., Malecki M., et al. Trans fatty acids induce a proinflammatory response in endothelial cells through ROS-dependent nuclear factor-kappaB activation. J Physiol Pharmacol. 2011;62(2):229–238. [PubMed] [Google Scholar]
- 235.Cifarelli V., Geng X., Styche A., et al. C-peptide reduces high-glucose-induced apoptosis of endothelial cells and decreases NAD(P)H-oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia. 2011;54(10):2702–2712. doi: 10.1007/s00125-011-2251-0. [DOI] [PubMed] [Google Scholar]
- 236.Randriamboavonjy V., Kyselova A., Elgheznawy A., et al. Calpain 1 cleaves and inactivates prostacyclin synthase in mesenteric arteries from diabetic mice. Basic Res Cardiol. 2017;112(1):10. doi: 10.1007/s00395-016-0596-8. [DOI] [PubMed] [Google Scholar]
- 237.Arnal E., Peris-Martinez C., Menezo J.L., et al. Oxidative stress in keratoconus? Invest Ophthalmol Vis Sci. 2011;52(12):8592–8597. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- 238.Moschos M.M., Kokolakis N., Gazouli M., et al. Polymorphism analysis of VSX1 and SOD1 genes in Greek patients with keratoconus. Ophthalmic Genet. 2015;36(3):213–217. doi: 10.3109/13816810.2013.843712. [DOI] [PubMed] [Google Scholar]
- 239.Udar N., Atilano S.R., Brown D.J., et al. SOD1: a candidate gene for keratoconus. Invest Ophthalmol Vis Sci. 2006;47(8):3345–3351. doi: 10.1167/iovs.05-1500. [DOI] [PubMed] [Google Scholar]
- 240.Gadelha D.N.B., Feitosa A.F.B., da Silva R.G., et al. Screening for novel LOX and SOD1 variants in keratoconus patients from Brazil. J Ophthalmic Vis Res. 2020;15(2):138–148. doi: 10.18502/jovr.v15i2.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Atilano S.R., Lee D.H., Fukuhara P.S., et al. Corneal oxidative damage in keratoconus cells due to decreased oxidant elimination from modified expression levels of SOD enzymes, PRDX6, SCARA3, CPSF3, and FOXM1. J Ophthalmic Vis Res. 2019;14(1):62–70. doi: 10.4103/jovr.jovr_80_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Al-Muammar A.M., Kalantan H., Azad T.A., et al. Analysis of the SOD1 gene in keratoconus patients from Saudi arabia. Ophthalmic Genet. 2015;36(4):373–375. doi: 10.3109/13816810.2014.889173. [DOI] [PubMed] [Google Scholar]
- 243.Gajecka M., Radhakrishna U., Winters D., et al. Localization of a gene for keratoconus to a 5.6-Mb interval on 13q32. Invest Ophthalmol Vis Sci. 2009;50(4):1531–1539. doi: 10.1167/iovs.08-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stabuc-Silih M., Strazisar M., Hawlina M., et al. Absence of pathogenic mutations in VSX1 and SOD1 genes in patients with keratoconus. Cornea. 2010;29(2):172–176. doi: 10.1097/ICO.0b013e3181aebf7a. [DOI] [PubMed] [Google Scholar]
- 245.De Bonis P., Laborante A., Pizzicoli C., et al. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol Vis. 2011:172482–172494. [PMC free article] [PubMed] [Google Scholar]
- 246.Saee-Rad S., Hashemi H., Miraftab M., et al. Mutation analysis of VSX1 and SOD1 in Iranian patients with keratoconus. Mol Vis. 2011:173128–173136. [PMC free article] [PubMed] [Google Scholar]
- 247.van Zundert B., Brown R.H., Jr. Silencing strategies for therapy of SOD1-mediated ALS. Neurosci Lett. 2017:63632–63639. doi: 10.1016/j.neulet.2016.07.059. [DOI] [PubMed] [Google Scholar]
- 248.Logroscino G. Motor neuron disease: are diabetes and amyotrophic lateral sclerosis related? Nat Rev Neurol. 2015;11(9):488–490. doi: 10.1038/nrneurol.2015.145. [DOI] [PubMed] [Google Scholar]
- 249.Flekac M., Skrha J., Hilgertova J., et al. Gene polymorphisms of superoxide dismutases and catalase in diabetes mellitus. BMC Med Genet. 2008;930 doi: 10.1186/1471-2350-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Haldar S.R., Chakrabarty A., Chowdhury S., et al. Oxidative stress-related genes in type 2 diabetes: association analysis and their clinical impact. Biochem Genet. 2015;53(4-6):93–119. doi: 10.1007/s10528-015-9675-z. [DOI] [PubMed] [Google Scholar]
- 251.Mohammedi K., Maimaitiming S., Emery N., et al. Allelic variations in superoxide dismutase-1 (SOD1) gene are associated with increased risk of diabetic nephropathy in type 1 diabetic subjects. Mol Genet Metabol. 2011;104(4):654–660. doi: 10.1016/j.ymgme.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 252.Neves A.L., Mohammedi K., Emery N., et al. Allelic variations in superoxide dismutase-1 (SOD1) gene and renal and cardiovascular morbidity and mortality in type 2 diabetic subjects. Mol Genet Metabol. 2012;106(3):359–365. doi: 10.1016/j.ymgme.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 253.Panduru N.M., Cimponeriu D., Cruce M., et al. Association of +35A/C (intron3/exon3) polymorphism in SOD1-gene with diabetic nephropathy in type 1 diabetes. Rom J Morphol Embryol. 2010;51(1):37–41. [PubMed] [Google Scholar]
- 254.Yin J., Wang X., Li S., et al. Interactions between plasma copper concentrations and SOD1 gene polymorphism for impaired glucose regulation and type 2 diabetes. Redox Biol. 2019:24101172. doi: 10.1016/j.redox.2019.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kay A.M., Simpson C.L., Stewart J.A., Jr. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J Diabetes Res. 2016 doi: 10.1155/2016/6809703. 20166809703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu X., Liu H., Lu X., et al. PEDF attenuates ocular surface damage in diabetic mice model through its antioxidant properties. Curr Eye Res. 2021;46(3):302–308. doi: 10.1080/02713683.2020.1805770. [DOI] [PubMed] [Google Scholar]
- 257.Mikami T., Meguro A., Teshigawara T., et al. Interleukin 1 beta promoter polymorphism is associated with keratoconus in a Japanese population. Mol Vis. 2013:19845–19851. [PMC free article] [PubMed] [Google Scholar]
- 258.Strissel K.J., Rinehart W.B., Fini M.E. Regulation of paracrine cytokine balance controlling collagenase synthesis by corneal cells. Invest Ophthalmol Vis Sci. 1997;38(2):546–552. [PubMed] [Google Scholar]
- 259.West-Mays J.A., Sadow P.M., Tobin T.W., et al. Repair phenotype in corneal fibroblasts is controlled by an interleukin-1 alpha autocrine feedback loop. Investig Ophthalmol Vis Sci. 1997;38(7):1367–1379. [PubMed] [Google Scholar]
- 260.Kim W.J., Rabinowitz Y.S., Meisler D.M., et al. Keratocyte apoptosis associated with keratoconus. Exp Eye Res. 1999;69(5):475–481. doi: 10.1006/exer.1999.0719. [DOI] [PubMed] [Google Scholar]
- 261.Wilson S.E., He Y.G., Weng J., et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62(4):325–327. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 262.Wang Y., Wei W., Zhang C., et al. Association of interleukin-1 gene single nucleotide polymorphisms with keratoconus in Chinese Han population. Curr Eye Res. 2016;41(5):630–635. doi: 10.3109/02713683.2015.1045083. [DOI] [PubMed] [Google Scholar]
- 263.Kim S.H., Mok J.W., Kim H.S., et al. Association of -31T>C and -511 C>T polymorphisms in the interleukin 1 beta (IL1B) promoter in Korean keratoconus patients. Mol Vis. 2008:142109–142116. [PMC free article] [PubMed] [Google Scholar]
- 264.Palamar M., Onay H., Ozdemir T.R., et al. Relationship between IL1beta-511C>T and ILRN VNTR polymorphisms and keratoconus. Cornea. 2014;33(2):145–147. doi: 10.1097/ICO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 265.Banerjee M., Saxena M. Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin Chim Acta. 2012;413(15-16):1163–1170. doi: 10.1016/j.cca.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 266.Helqvist S. Interleukin 1 beta-mediated destruction of pancreatic beta-cells in vitro. A model of beta-cell destruction in insulin-dependent diabetes mellitus? Dan Med Bull. 1994;41(2):151–166. [PubMed] [Google Scholar]
- 267.Mandrup-Poulsen T., Zumsteg U., Reimers J., et al. Involvement of interleukin 1 and interleukin 1 antagonist in pancreatic beta-cell destruction in insulin-dependent diabetes mellitus. Cytokine. 1993;5(3):185–191. doi: 10.1016/1043-4666(93)90003-n. [DOI] [PubMed] [Google Scholar]
- 268.Yan C., Gao N., Sun H., et al. Targeting imbalance between IL-1beta and IL-1 receptor antagonist ameliorates delayed epithelium wound healing in diabetic mouse corneas. Am J Pathol. 2016;186(6):1466–1480. doi: 10.1016/j.ajpath.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tripathi A., Sodhi A. Prolactin-induced production of cytokines in macrophages in vitro involves JAK/STAT and JNK MAPK pathways. Int Immunol. 2008;20(3):327–336. doi: 10.1093/intimm/dxm145. [DOI] [PubMed] [Google Scholar]
- 270.Sharif R., Bak-Nielsen S., Sejersen H., et al. Prolactin-Induced Protein is a novel biomarker for Keratoconus. Exp Eye Res. 2019:17955–17963. doi: 10.1016/j.exer.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sharif R., Bak-Nielsen S., Hjortdal J., et al. Pathogenesis of Keratoconus: the intriguing therapeutic potential of Prolactin-inducible protein. Prog Retin Eye Res. 2018:67150–67167. doi: 10.1016/j.preteyeres.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chambers D., Mason I. Expression of sprouty2 during early development of the chick embryo is coincident with known sites of FGF signalling. Mech Dev. 2000;91(1-2):361–364. doi: 10.1016/s0925-4773(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 273.Faedo A., Borello U., Rubenstein J.L. Repression of Fgf signaling by sprouty1-2 regulates cortical patterning in two distinct regions and times. J Neurosci. 2010;30(11):4015–4023. doi: 10.1523/JNEUROSCI.0307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Minowada G., Jarvis L.A., Chi C.L., et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126(20):4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 275.Basson M.A., Akbulut S., Watson-Johnson J., et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8(2):229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 276.Shim K., Minowada G., Coling D.E., et al. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8(4):553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 277.Ding W., Warburton D. Down-regulation of Sprouty2 via p38 MAPK plays a key role in the induction of cellular apoptosis by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2008;375(3):460–464. doi: 10.1016/j.bbrc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kuracha M.R., Burgess D., Siefker E., et al. Spry1 and Spry2 are necessary for lens vesicle separation and corneal differentiation. Invest Ophthalmol Vis Sci. 2011;52(9):6887–6897. doi: 10.1167/iovs.11-7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kuracha M.R., Siefker E., Licht J.D., et al. Spry1 and Spry2 are necessary for eyelid closure. Dev Biol. 2013;383(2):227–238. doi: 10.1016/j.ydbio.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Imamura M., Iwata M., Maegawa H., et al. Genetic variants at CDC123/CAMK1D and SPRY2 are associated with susceptibility to type 2 diabetes in the Japanese population. Diabetologia. 2011;54(12):3071–3077. doi: 10.1007/s00125-011-2293-3. [DOI] [PubMed] [Google Scholar]
- 281.Shu X.O., Long J., Cai Q., et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 2010;6(9) doi: 10.1371/journal.pgen.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Cook N.L., Pjanic M., Emmerich A.G., et al. CRISPR-Cas9-mediated knockout of SPRY2 in human hepatocytes leads to increased glucose uptake and lipid droplet accumulation. BMC Endocr Disord. 2019;19(1):115. doi: 10.1186/s12902-019-0442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Stabuc-Silih M., Ravnik-Glavac M., Glavac D., et al. Polymorphisms in COL4A3 and COL4A4 genes associated with keratoconus. Mol Vis. 2009:152848–152860. [PMC free article] [PubMed] [Google Scholar]
- 284.Ljubimov A.V., Burgeson R.E., Butkowski R.J., et al. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72(4):461–473. [PubMed] [Google Scholar]
- 285.Lemmink H.H., Schroder C.H., Monnens L.A., et al. The clinical spectrum of type IV collagen mutations. Hum Mutat. 1997;9(6):477–499. doi: 10.1002/(SICI)1098-1004(1997)9:6<477::AID-HUMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 286.Bochert A., Berlau J., Koczan D., et al. Gene expression in keratoconus. Initial results using DNA microarrays. Ophthalmologe. 2003;100(7):545–549. doi: 10.1007/s00347-003-0808-0. [DOI] [PubMed] [Google Scholar]
- 287.Kokolakis N.S., Gazouli M., Chatziralli I.P., et al. Polymorphism analysis of COL4A3 and COL4A4 genes in Greek patients with keratoconus. Ophthalmic Genet. 2014;35(4):226–228. doi: 10.3109/13816810.2014.946055. [DOI] [PubMed] [Google Scholar]
- 288.Mas Tur V., MacGregor C., Jayaswal R., et al. A review of keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol. 2017;62(6):770–783. doi: 10.1016/j.survophthal.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 289.Yagame M., Kim Y., Zhu D., et al. Differential distribution of type IV collagen chains in patients with diabetic nephropathy in non-insulin-dependent diabetes mellitus. Nephron. 1995;70(1):42–48. doi: 10.1159/000188542. [DOI] [PubMed] [Google Scholar]
- 290.Salem R.M., Todd J.N., Sandholm N., et al. Genome-Wide association study of diabetic kidney disease highlights biology involved in glomerular basement membrane collagen. J Am Soc Nephrol. 2019;30(10):2000–2016. doi: 10.1681/ASN.2019030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Onochie O.E., Onyejose A.J., Rich C.B., et al. The role of hypoxia in corneal extracellular matrix deposition and cell motility. Anat Rec (Hoboken) 2020;303(6):1703–1716. doi: 10.1002/ar.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Davies P.D., Duncan G., Pynsent P.B., et al. Aqueous humour glucose concentration in cataract patients and its effect on the lens. Exp Eye Res. 1984;39(5):605–609. doi: 10.1016/0014-4835(84)90060-5. [DOI] [PubMed] [Google Scholar]
- 293.Foulks G.N., Thoft R.A., Perry H.D., et al. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol. 1979;97(6):1076–1078. doi: 10.1001/archopht.1979.01020010530002. [DOI] [PubMed] [Google Scholar]
- 294.Kaji Y., Usui T., Oshika T., et al. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci. 2000;41(2):362–368. [PubMed] [Google Scholar]
- 295.Goldstein A.S., Janson B.J., Skeie J.M., et al. The effects of diabetes mellitus on the corneal endothelium: a review. Surv Ophthalmol. 2020;65(4):438–450. doi: 10.1016/j.survophthal.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 296.Saini J.S., Mittal S. In vivo assessment of corneal endothelial function in diabetes mellitus. Arch Ophthalmol. 1996;114(6):649–653. doi: 10.1001/archopht.1996.01100130641001. [DOI] [PubMed] [Google Scholar]
- 297.El-Agamy A., Alsubaie S. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin Ophthalmol. 2017:11481–11486. doi: 10.2147/OPTH.S126217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lee H.K., Jung E.H., Cho B.J. Epidemiological association between systemic diseases and keratoconus in a Korean population: a 10-year nationwide cohort study. Cornea. 2020;39(3):348–353. doi: 10.1097/ICO.0000000000002206. [DOI] [PubMed] [Google Scholar]
- 299.Hashemi H., Heydarian S., Hooshmand E., et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39(2):263–270. doi: 10.1097/ICO.0000000000002150. [DOI] [PubMed] [Google Scholar]
- 300.Bikbova G., Oshitari T., Tawada A., et al. Corneal changes in diabetes mellitus. Curr Diabetes Rev. 2012;8(4):294–302. doi: 10.2174/157339912800840479. [DOI] [PubMed] [Google Scholar]
- 301.Markoulli M., Flanagan J., Tummanapalli S.S., et al. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf. 2018;16(1):45–57. doi: 10.1016/j.jtos.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 302.Dogru M., Katakami C., Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108(3):586–592. doi: 10.1016/s0161-6420(00)00599-6. [DOI] [PubMed] [Google Scholar]
- 303.Inoue K., Okugawa K., Amano S., et al. Blinking and superficial punctate keratopathy in patients with diabetes mellitus. Eye (Lond). 2005;19(4):418–421. doi: 10.1038/sj.eye.6701497. [DOI] [PubMed] [Google Scholar]
- 304.Miller D.D., Hasan S.A., Simmons N.L., et al. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. 2019:13325–13335. doi: 10.2147/OPTH.S157430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Valgaeren H., Koppen C., Van Camp G. A new perspective on the genetics of keratoconus: why have we not been more successful? Ophthalmic Genet. 2018;39(2):158–174. doi: 10.1080/13816810.2017.1393831. [DOI] [PubMed] [Google Scholar]
- 306.Lontchi-Yimagou E., Sobngwi E., Matsha T.E., et al. Diabetes mellitus and inflammation. Curr Diabetes Rep. 2013;13(3):435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 307.Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4(6):270–281. doi: 10.4239/wjd.v4.i6.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Szczotka L.B., Barr J.T., Zadnik K. A summary of the findings from the collaborative longitudinal evaluation of keratoconus (CLEK) study. CLEK study group. Optometry. 2001;72(9):574–584. [PubMed] [Google Scholar]
- 309.Zadnik K., Barr J.T., Edrington T.B., et al. Baseline findings in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Invest Ophthalmol Vis Sci. 1998;39(13):2537–2546. [PubMed] [Google Scholar]
- 310.Wheeler J., Hauser M.A., Afshari N.A., et al. The genetics of keratoconus: a review. Reprod Syst Sex Disord. 2012;(suppl 6) doi: 10.4172/2161-038x.S6-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lechner J., Bae H.A., Guduric-Fuchs J., et al. Mutational analysis of MIR184 in sporadic keratoconus and myopia. Invest Ophthalmol Vis Sci. 2013;54(8):5266–5272. doi: 10.1167/iovs.13-12035. [DOI] [PubMed] [Google Scholar]