Abstract
The coronavirus disease 2019 (COVID-19) pandemic is a global health threat with particular risk for severe disease and death in older adults and in adults with age-related metabolic and cardiovascular disease. Recent advances in the science of ageing have highlighted how ageing pathways control not only lifespan but also healthspan, the healthy years of life. Here, we discuss the ageing immune system and its ability to respond to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We specifically focus on the intersect of severe COVID-19 and immunosenescence to highlight pathways that may be determinant for the risk of complications and death following infection with SARS-CoV-2. New or adapted therapeutics that target ageing-associated pathways may be important tools to reduce the burden of death and long-term disability caused by this pandemic. Proposed interventions aimed at immunosenescence could enhance immune function not only in the elderly but in susceptible younger individuals as well, ultimately improving complications of severe COVID-19 for all ages.
INTRODUCTION
The immune system changes with age in nearly every aspect, generally resulting in a decline in pathogen immunity with increased age. This diminished capacity of the aged immune system is clinically evident, as ageing is associated with high morbidity and mortality rates for various infections and significant reductions in vaccine efficacy1-6. Thus, as the emergent SARS-CoV-2 coronavirus began to circulate the globe early in 2020, it was reasonable to expect the elderly population may be especially susceptible to poorer outcomes of COVID-19, the disease caused by SARS-CoV-2. Indeed, data from Wuhan, China showed that age was the primary risk factor associated with COVID-19 progression to acute respiratory distress syndrome (ARDS) and end-organ failure7, which has since been corroborated by many others8-10.
COVID-19 has taken a devastating toll on the entire population, but particularly older adults. As of May 24, 2021, the Centers for Disease Control and Prevention (CDC) reported nearly 590,000 total deaths from COVID-19 in the United States alone, with an estimated 80% of those deaths occurring in individuals aged 65 years or older11,12. Compared to a 5-17 year-old reference group, the rate of hospitalization and death due to COVID-19 is approximately 1,300 times higher in individuals between the age of 65-74, and 8,700 times higher in individuals 85 years and older in the United States11. While aged individuals have a higher prevalence of comorbidities that are also independently associated with increased risk to severe COVID-19, including cardiovascular disease, diabetes, chronic obstructive pulmonary disease, chronic kidney disease, cancer, and others10, chronological age is still the single greatest risk factor for COVID-19 mortality13.
Thus, this review will focus on the intersect between ageing and detrimental SARS-CoV-2 host-pathogen interactions during severe COVID-19 and detail the physiological and immunological mechanisms underpinning both circumstances. We will also highlight targets for new or adapted therapeutics that may improve aspects of immunosenescence implicated in these shared pathways. Directed enhancement of the ageing immune system may subsequently reduce the burden of death and long-term disability caused by COVID-19.
AGEING, INFLAMMAGEING, and IMMUNOSENESCENCE: AN OVERVIEW
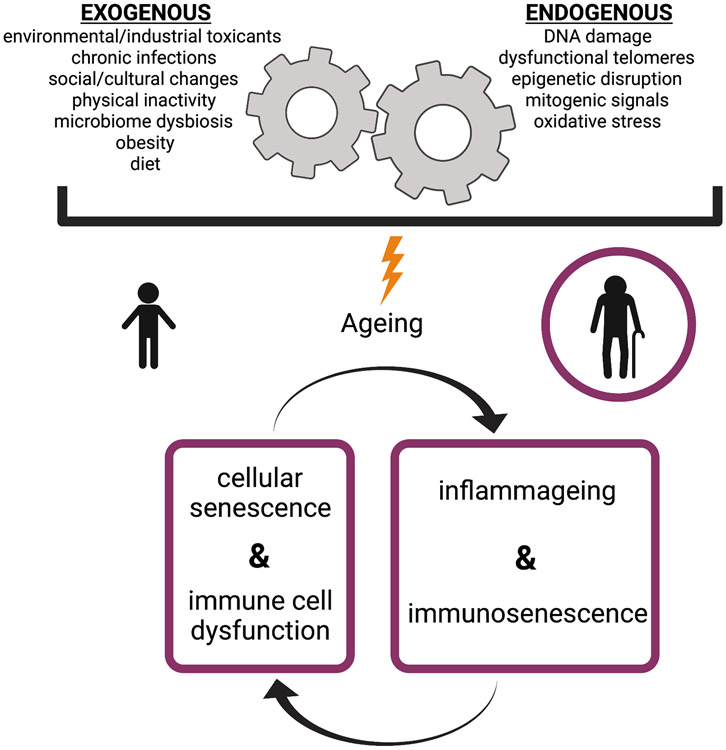
As humans age, the presence of systemic basal inflammatory mediators increases independently of acute immune challenges in a phenomenon known as inflammageing. This persistent, low-grade, chronic inflammation is speculated to drive many chronic diseases associated with ageing, and is also a main contributor to immunosenescence, a term defined as the overall changes to the immune system as we age, including its reduced ability to fight novel infection14. It is generally accepted that inflammageing occurs in response to an accumulation of exogeneous and endogenous physiological stresses over time and is primarily mediated by immune cells and senescent cells (Figure 1)14,15. The relative variability in these many stressors from person to person likely explains why humans have wide variances in their biological inflammatory age across set chronological age time-points15,16. This is important to consider when studying the elderly, as those aged ≥65 years old are not a monolithic group.
Figure 1. Factors that contribute to inflammageing and immunosenescence.
Both exogenous and cell-endogenous factors contribute to chronic inflammation with age, which is primarily mediated by immune cells and senescent cells. Senescent cells persist and accumulate during ageing, and they display an abnormal secretory phenotype, characterized by the production of inflammatory mediators, matrix metalloproteinases (MMPs), fibronectin, and reactive oxygen species. These inflammatory mediators contribute to inflammageing, which, over time, affects immune cell function, promoting immunosenescene. The endogenous and exogenous factors listed here can also directly affect the inflammatory potential of the immune system, which further promotes a feed-forward loop of inflammageing and immunosenescence.
Immune cells produce many of the inflammatory mediators found in ageing tissues, which can also impact their own function. The increased presence of proinflammatory cytokines or danger signals leads to constitutive low-level engagement of immune cell signaling events such as JAK-STAT, MyD88, NF-κB, and inflammasomes, resulting in high basal activation but impaired immune cell responses to further cytokine and pattern recognition receptor (PRR) stimulation17,18. Thus, many immune cell subsets become hyporesponsive to acute challenges as we age17,19-25. This may help explain why mediators of systemic chronic inflammation have been linked to suboptimal vaccine responses in not only the elderly26,27 but in younger individuals as well28. Immunosenescence is further characterized by an impairment in antigen presentation and naive T cell priming, a propensity for myeloid lineage differentiation in the bone marrow, altered type I IFN responses, diminished CD8+ T cell cytotoxic function, decreased phagocytic function for many innate immune cell types, a restricted naive T cell and B cell repertoire, and impaired production of high-avidity antibodies29,30. These immunological changes weaken the immune responses to most viruses, leaving the elderly particularly vulnerable to influenza, SARS-CoV-2, and other lethal coronaviruses, such as SARS-CoV-1 and Middle East Respiratory Syndrome (MERS)-CoV31. The effects of ageing on the human immune system have been extensively reviewed elsewhere30,32-34; thus, we will focus on aspects of the ageing immune system that specifically overlap with characteristics of the immune response during severe COVID-19 outcomes.
COVID-19: AN OVERVIEW IN PATHOGENESIS
SARS-CoV-2 is the causative agent of COVID-19, declared a global pandemic by the World Health Organization on March 11, 202035. SARS-CoV-2 has a single-stranded, positive-sense RNA genome36. Attachment and entry of SARS-CoV-2 to target cells is initiated when the virus engages its cognate receptor, angiotensin-converting enzyme 2 (ACE2) via the receptor-binding domain (RBD) of the viral Spike protein, also called the S protein37. Host transmembrane protease serine 2 (TMPRSS2) promotes S protein priming and facilitates viral entry38. Primary cell types of SARS-CoV-2 entry into the body include nasal epithelium, alveolar type II pneumocytes, superficial conjunctival cells, and many types of enterocytes in the gut39. Virus entry into such cells leads to viral replication, destruction of infected cells, and triggering of an innate immune response. These processes occur early during the SARS-CoV-2 incubation period of approximately 5 days. SARS-CoV-2 is likely initially detected by host cells via toll-like receptor (TLR)-7 and −8, which sense single-stranded RNA, and potentially TLR3, sensing double stranded RNA intermediates40. TLR signaling engages interferon regulatory factors (IRFs) and MyD88/NF-κB signaling pathways, leading to the production of type I interferons (IFNs) and pro-inflammatory cytokines (IL-6, TNFα, and IL-1β)40. Host cell damage resulting from SARS-CoV-2 infection can also lead to release of endogenous damage-associated molecular patterns (DAMPs), such as ATP, DNA, and oxidized phospholipids41. DAMPs serve as danger signals for inflammasomes, multi-protein immune complexes which cleave and activate IL-1β and IL-18, and are also directly sensed via PRRs including TLRs, to further propagate inflammation40,42. Proinflammatory cytokine and chemokine production recruits and activates innate immune cells, including neutrophils, natural killer (NK) cells, dendritic cells (DC)s, and monocytes. Activated DCs and viral antigens eventually migrate to the draining lymph nodes where they engage the adaptive immune system, T and B cells, with potential to clear the virus.
After the viral incubation period, in most cases, a young healthy individual will clear the virus with this coordinated immune response. However, patient symptoms can vary greatly from asymptomatic to severe, the latter being more common in the elderly43,44. These severe COVID-19 patients have higher levels of circulating cytokines and chemokines, contributing to enhanced risk for critical outcomes of COVID-19: pneumonia, cytokine storm, acute respiratory distress syndrome (ARDS)45, sepsis and coagulopathy46,47. ARDS is a severe and often fatal complication of COVID-19. It is clinically defined by the acute onset of respiratory failure, hypoxaemia, bilateral lung infiltrates on chest imaging not fully explained by effusions/collapse/nodules, and lack of cardiogenic related edema48. During COVID-19, ARDS manifests as a diffuse alveolar damage pattern seen on autopsies of COVID-19 patients, and is linked to a combination of lung immune cell infiltration, cytokine storm, and tissue damage from secreted proteases, reactive oxygen species (ROS), and viral killing 49,50. Cytokines that mediate manifestations of ARDS, like IL-6 and TNFα, can also facilitate vascular permeability, systemic shock and multi-organ failure41,51. Many patients with severe COVID-19 also develop bacteria pneumonia, a secondary bacterial infection of the lungs52. These bacterial infections bolster proinflammatory responses that perpetuate the cytokine storm, systemic inflammation, and ARDS phenotypes53.
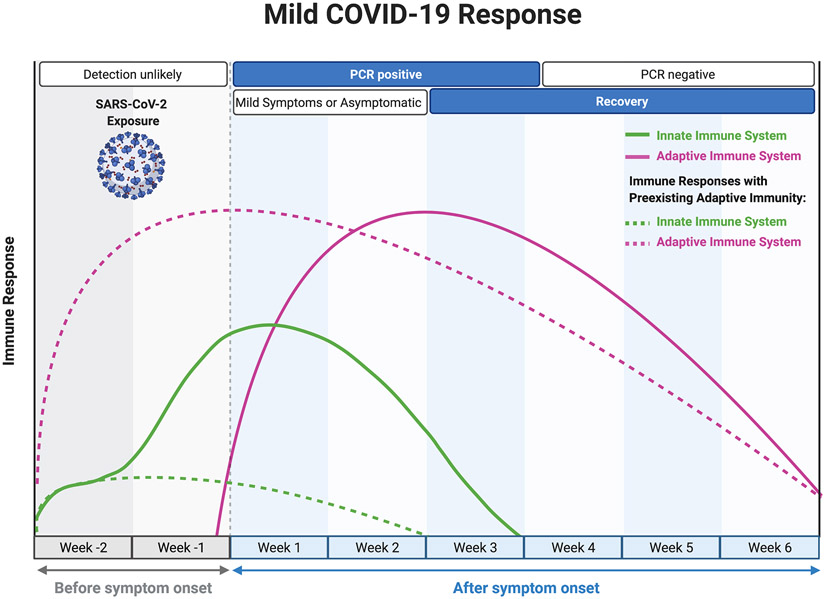
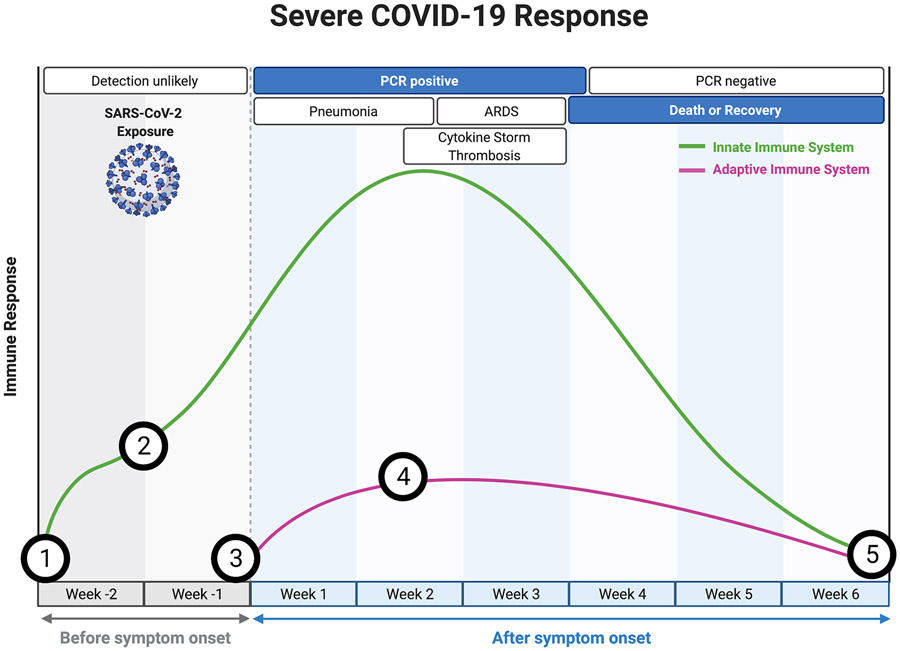
Mouse studies of SARS-CoV-2 show a higher viral burden in older mice in the first few days post-infection, suggesting that aging may also lead to a delay or dysfunction in the initial triggering and priming stages of the immune response54,55 that is propagated in part by suboptimal antigen-specific adaptive immunity56. The onset of ARDS in severe cases typically occurs around day 12 post-symptom onset44,57, when viral shedding appears to be mostly resolved58, followed by death or recovery around 7-12 days later44,57. Therefore, severe cases of COVID-19 are not simply due to an inability to clear the viral infection, but rather due to a sustained, dysregulated, and highly destructive inflammatory response. A basic summary of the clinical progression and associated immune response during mild and severe COVID-19 is depicted in Figure 2.
Figure 2. Immune response patterns and clinical disease courses for mild and severe COVID-19.
For (a) mild COVID-19 and (b) severe COVID-19, graphs show the general disease course timeline and the magnitude of the innate (green) and adaptive (purple) immune responses over time. Dashed lines represent the time course for response in patients with pre-existing adaptive immunity. For (b) severe COVID-19, there are five main points of interest in the immune response that could be influenced by an ageing immune system: 1) heightened basal inflammation (inflammageing) at the point of SARS-CoV-2 inoculation may predispose the elderly to an already pro-inflammatory local environment; 2) innate immune cell dysfunction associated with aging may alter early immune responses; 3) delayed and/or diminished adaptive responses due to poor naive clonal diversity, weak or ineffective pre-existing immunity, and poor T cell priming; 4) altered T effector function and antibody responses due to immunosenescence; and 5) the possibility of a reduced memory response and potential long-lasting effects on the immune system that may further promote immunosenescent phenotypes.
IMMUNE SYSTEM DYSREGULATION ASSOCIATED WITH AGEING AND SEVERE COVID-19: INNATE IMMUNITY
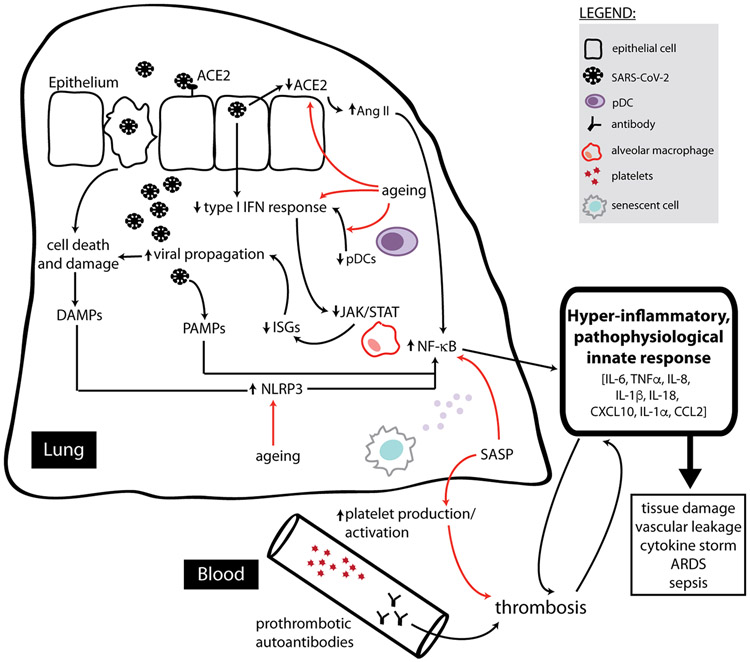
Primary goals of the innate immune system in response to a viral infection are: (1) initiate a local inflammatory response to activate and recruit immune cells, (2) directly eliminate virally infected cells, and (3) prime the adaptive immune response. As we age, the ability to achieve these three goals is either diminished or dysregulated. Here, we discuss components of the innate immune system, the inflammatory processes implicated in both inflammageing and severe COVID-19, and provide a summary in Figure 3.
Figure 3. An ageing innate immune system may predispose a patient to severe COVID-19.
Consequences of the virus are indicated by black arrows, while consequences of ageing are indicated by red arrows. SARS-CoV-2 first enters cells by binding to ACE2, and viral entry then leads to the shedding of ACE2. Ageing may also be associated with reduced expression of ACE2, and the low expression of ACE2 after infection potentially mediates a pro-inflammatory state through the production of angiotensin (Ang) II. Severe COVID-19 has also been correlated with reduced type I IFN responses, a defect which may be compounded by aberrant type I IFN signaling with age. This could be made worse by the notable reduction in total number of pDCs found in severe COVID-19 patients. Aged pDCs also have a reduced ability to produce type I IFNs, and the pro-inflammatory nature of the SASP in older individuals desensitizes JAK/STAT signaling in innate immune cells. All of this would compound the loss of type I IFN signaling during severe COVID-19, ultimately leading to reduced expression of antiviral IFN-stimulated genes (ISGs) and permitting enhanced viral propagation. This viral replication leads to cell death and damage, causing the production of immunostimulatory DAMPs and PAMPs. Signaling in response to these signals generally activates the NF-κB transcription factor, which is also the main immune signaling pathway engaged by SASP mediatorsand the persistent basal stimulation of pattern recognition receptors in older adults. NF-κB signaling promotes production of pro-inflammatory cytokines. DAMPs linked to both aging and viral infection can also trigger activation of inflammasomes such as NLRP3, potentiating cytokines such as IL-1β and IL-18, as well as triggering further cell death through pyroptosis. SASP mediators and the autoimmune-prone environment associated with aging may also predispose aged individuals to a prothrombotic environment, and thrombosis and inflammation continue to promote one another in a feed-forward loop which further contributes to the hyperinflammatory, pathophysiological innate response that causes devastating outcomes observed in severe COVID-19.
Anti-inflammatory effects of ACE2
The primary SARS-CoV-2 cellular receptor, ACE2, can play a direct role in early inflammatory processes through the renin-angiotensin-aldosterone-system (RAAS) signaling pathway, thereby aiding in one of the primary goals of the innate immune system: initiating a local inflammatory response to activate and recruit immune cells. ACE2 converts Angiotensin II (an inflammatory mediator) to angiotensin 1-7 (an anti-inflammatory mediator). Angiotensin II signaling generates a proinflammatory state in vascular cells, leading to enhanced vascular permeability and local inflammation59. In mouse models of ARDS, the anti-inflammatory effect of ACE2 was shown to be protective against severe acute lung injury60. Angiotensin 1-7 has also been shown to decrease the production of pro-inflammatory cytokines, specifically IL-6, TNFα, and IL-8, through inhibition of the p38-MAPK/NF-κB signaling pathway, while also upregulating the expression of the anti-inflammatory cytokine, IL-1061. An increased presence of Angiotensin II during the early response to SARS-CoV-2 has been postulated to play a role in promoting cytokine storm phenotypes associated with severe COVID-19, most likely due to the excessive production of TNFα and local macrophage activation62. Interestingly, there is a reduction of ACE2 expression in the lungs of old rats when compared to their younger counterparts63, and one study has shown a decrease in ACE2 mRNA in multiple tissues of elderly humans when compared to their younger counterparts64. ACE2 is also downregulated in humans with cardiovascular disease and diabetes, which also correlate with severe COVID-1965. Decreased expression of ACE2 could mean less potential for SARS-CoV-2 invasion of host cells, and further ACE2 reduction caused by viral entry may exacerbate a heightened proinflammatory response that helps mediate cytokine storm, acute lung injury, and ARDS. This hypothesis is supported by research showing elevated levels of Angiotensin II in the plasma of patients with severe COVID-1966. Moreover, a study of SARS-CoV-2 infection in old and juvenile rhesus macaques showed lower levels of ACE2 in old versus young macaques after SARS-CoV-2 infection, supporting the hypothesis that ACE2 is dysregulated in the elderly, even across species67.
A propensity for proinflammatory cytokine production
An uncontrolled inflammatory response to SARS-CoV-2 can lead to harmful and even irreversible tissue damage, both locally and systemically. The majority of patients with severe COVID-19 demonstrate substantially elevated serum levels of proinflammatory cytokines and chemokines, including IL-6, IL-1β, IL-2, IL-8, IL-17, G-CSF, GM-CSF, CXCL10, CCL2, CCL3 and TNF49,54,68. In mouse studies of SARS-CoV-2 where aging mice were directly interrogated, similar cytokines, including IL-6, IL-1α, IL-1β, TNFα, and the chemokine, CCL2, were also linked to the aged host response to SARS-CoV-254,55. Many of these cytokines are also elevated basally in the elderly due to inflammageing. For instance, elevated IL-6 has been an especially stable indicator of poor outcomes in patients with COVID-19, and studies have demonstrated that IL-6 is also among the most reliable ageing parameters69. The presence of IL-6 is a clinical hallmark of vascular NF-κB activation70, a major transcription factor that regulates many of the proinflammatory genes of innate immune cells. There is a close link between ageing, NF-κB signaling, and inflammation71. Moreover, an anti-IL-6 receptor antibody could attenuate COVID-19 severity in a cohort of predominantly older patients (median age 67 years), pointing to some cytokines as important drivers of exaggerated disease72. IL-1β and IL-18 are also critical in inflammageing; these cytokines are products of the NLRP3 inflammasome, and contribute to the pathology of ageing-related diseases73. In ageing patients, SARS-CoV-2 can potentiate NLRP3 inflammasome activation and IL-1β and IL-18 levels, pyroptosis, and the release of DAMPs (including ATP), further heightening inflammation and COVID-19 pathogenesis74,75. ROS increase with age due to mitochondrial dysfunction and inflammation, and can also drive NLRP3 inflammasome activation, likely further contributing to COVID-19 pathophysiology in the elderly58,76,77. Interestingly, metformin may lower risk of severe COVID-19 since it reduces ATP levels and mitochondrial ROS which can fuel NLRP3 mediated IL-1β production78. Additionally, treatment with an IL-1R antagonist has led to improvements in mortality in severe COVID-1979, and the NLRP3 inflammasome is considered an emerging target for COVID-1980.
Despite elevated NF-κB signaling observed at baseline during ageing, many immune cells (including B cells, T cells, and DCs) isolated from an inflammageing environment have been shown to be hyporesponsive to acute ex vivo stimuli, although monocytes appear to standout as being hyperresponsive81. Consistent with these observations, mass cytometry and single cell RNA-seq analysis of monocytes from older COVID-19 patients showed they were enriched for IFNγ response, and the expression of TNFα, IL-1β, and CXCL876. Aged monocytes were also enriched for broad pathways linked to TLR signaling, oxidative stress, MAPK, NF-κB, as well as the senescent cell marker p21, leading the authors to speculate the presence of inflammatory aged or senescent cells with a senescence associated secretory phenotype (SASP) as one mechanism for exuberant inflammatory tone in these cells during COVID-1976. Indeed, senescent cells persist and accumulate during ageing82 and they display an abnormal secretory phenotype characterized by the presence of inflammatory cytokines that contributes to inflammageing. This SASP is characterized by the production of cytokines, chemokines, growth factors, matrix metalloproteinases (MMPs), fibronectin, and ROS83. In support of a role of cellular senescence in COVID-19 pathology, a recent manuscript showed that in vitro exposure of senescent mouse and human cells to PAMPs and the viral S-protein leads to significantly increased SASP production and expression of viral entry genes ACE2 and TMPRSS2 compared to non-senescent cells. Using a β-coronavirus mouse hepatitis virus (MHV), which has some relation to SARS-CoV-2, the study demonstrated that targeting senescent cells in genetically modified mice and with senolytics (Fisetin, and Dasatinib plus Quercetin), was able to reduce inflammation and improve survival84.
Neutrophils from elderly individuals exhibit some hyporesponsive attributes: decreased bactericidal activity, decreased respiratory burst, and decreased neutrophil extracellular traps (NET) formation85. However, neutrophils from the elderly also display aberrant migration and enhanced degranulation, suggesting the neutrophils of aged individuals are not able to fight off pathogens efficiently but still produce highly inflammatory and damaging molecules in a potentially non-localized fashion86. Moreover, elevated levels of IL-6, as either part of the host pathogen response or due to inflammageing, engenders prolonged neutrophil survival through reducing apoptosis87, and neutrophilia is in fact an indicator of poor clinical outcomes for COVID-1988.
Thus, aged individuals may have dysregulated innate immune cell function and inflammatory responses to SARS-CoV-2. Consistently, studies have shown that the sustained presence of increased neutrophils and monocytes in the blood are associated with severe COVID-19 disease89,90. As mentioned, these changes occur in part due to age-related high basal TLR activation, changes in oxidative stress pathways, inflammasome activation, increased senescent cells burden and SASP, as well as other pathways such as reduced autophagy and DNA damage with age91. Thus, SARS-CoV-2 infection combined with inflammageing leads to an exaggerated innate immune response and worsened outcomes of COVID-19. However, more studies are needed to tease apart the complex interconnections between inflammageing and susceptibility to severe COVID-19.
The pro-thrombotic nature of senescent cells and severe COVID-19
While the connection between the proinflammatory profile of senescent cells and the exuberant proinflammatory environment of severe COVID-19 is important to note, there is another intriguing connection between the two: thrombosis. Senescent cells have a paracrine pro-coagulation effect92. Induction of cellular senescence via doxorubicin (DOXO) treatment in a senescence reporter mouse, p16-3MR was associated with significantly shorter tail bleed times, greater platelet count, more highly activated platelets, and higher levels of thrombopoietin (Tpo) in the serum92. Elimination of senescent cells in vivo reversed these pro-thrombotic phenotypes. SILAC analysis of human senescent cells and ex vivo experiments also showed human platelets sensitized by senescent cell supernatants were more highly activated92.
Pro-thrombotic coagulopathy, high levels of D-dimer, venous thromboembolism, arterial thromboses, and fibrin-based occlusion of small blood vessels have all been associated with cases of severe COVID-197,44,49,93-99. Mechanistically proinflammatory cytokines lead to the expression of tissue factor (CD142), the initiator of blood coagulation, on platelets, monocytes, macrophages, and endothelial cells. Complement activation, NETs, and lung hypoxia further propagate pro-thrombotic conditions, which work in a feed-forward loop with ongoing inflammation, connecting thrombosis and the innate immune system100. A study also described the production of viral-induced prothrombotic autoantibodies against phospholipids and phospholipid-binding proteins during cases of severe SARS-CoV-2 infection101. Thus, the combination of a heightened inflammatory response to SARS-CoV-2 with the already thrombotic-prone microenvironment of the elderly may further potentiate the coagulopathy associated with worsened outcomes of COVID-19.
Loss of a type I interferon responses
Reduced type I IFN levels have been observed in the serum of patients with life-threatening COVID-19102. In a rhesus macaque model of SARS-CoV-2 infection, old macaques also had reduced type I IFN and Notch signaling pathways in their lungs when compared to juvenile counterparts67. The reduced type I IFN responses in humans is in part linked to a reduction of plasmacytoid DCs (pDCs), strong producers of IFNα, which has also been reported in cases of severe COVID-19103. Ten percent of all patients with life-threating COVID-19, however, also harbor autoantibodies against type I IFNs104. Moreover, severe patients can also carry loss of function variants in multiple genes linked to TLR3 and IRF7 pathways, involved in both the induction and amplification of type I IFNs105. If type I IFNs are present early and properly localized to sites of infection, they can effectively limit viral propagation, as type I IFNs are responsible for optimal activation of macrophages, antigen presentation by DCs, and enhanced anti-viral effector T cell responses106. Likewise, IFN induced transmembrane family (IFITM) proteins may inhibit SARS-CoV-2 entry, as demonstrated for previous coronaviruses107,108.
Studies have revealed the importance of type I IFN responses in regulating monocyte and neutrophils early after SARS-CoV-2 infection. Peripheral blood of mild COVID-19 patients contained more classical monocytes (CD14+CD16−) exhibiting an early and transient type I IFN signature109,110. Conversely, monocytes and neutrophils in severe COVID-19 patients expressed more genes involved with NF-κB signaling and ROS/NOS production throughout the course of disease109. Non-classical (CD14+CD16++) and intermediate (CD14++CD16+) subsets of monocytes are proinflammatory and known to expand during viral infections111. Thus, their expansion in response to SARS-CoV-2 infection is expected112,113, although a dysregulated exuberant response from these cells would also contribute to the highly inflammatory environment observed in severe COVID-19 pathophysiology. Intriguingly, non-classical monocytes have also been shown to produce the type I IFN, IFNα, in response to TLR3114. Thus, the kinetics of the dysregulation of non-classical monocytes in severe outcomes is important to consider, as an early loss of this subset of monocytes has been described by some109,110, and may further contribute to the reduced type I IFN response observed in patients with severe COVID-19.
Ageing leads to a delay in type I IFN responses, linked to changes in viral sensing, which was also seen in SARS-CoV-131. While the mechanisms are not entirely known, ageing compromises both the primary and secondary RIG-I signaling pathways that control expression of many type I IFN genes115. This is associated with reduced production of type I IFNs in those >65, impairing their anti-viral responses, like to respiratory influenza A virus115. During SARS-CoV-1, type I IFN signaling proteins downstream of RIG-I are further reduced due to infection-induced mitochondrial dysfunction linking to viral sensor MAVS116. Given basal mitochondrial dysfunction, as well as a reduction in TRAF adaptor proteins and phosphorylated IRF3 linked to RIG-I signaling in the elderly115,117, they are particularly vulnerable to such changes of RIG-I insufficiency. Interestingly, RIG-I can restrain SARS-CoV-2 replication in human lung cells, though the mechanism does not require its type I IFN signaling ability118. The elderly also display reduced total numbers of pDCs at baseline and their pDCs often show reduced TLR7 expression117, and have diminished capacity to produce type I IFNs119-121. While there is a strong overlap of reduced type I IFN responses in the elderly and in severe COVID-19 patients, additional mechanistic connections between the two requires further investigation.
Decline in antigen presentation and T cell priming
Innate immunity plays a critical role in the initiation of adaptive immune responses by ensuring proper activation of T lymphocytes. To achieve effective T cell priming, innate immune cells must do two things: (1) present antigen via MHC molecules alongside costimulatory receptors via cell-cell interactions between the T cell and the antigen presenting cell (APC), and (2) produce the proper cytokines to skew CD4+ T cell differentiation towards the appropriate effector response specific to the invading pathogen. Defects in the ability of APCs to accomplish either of these tasks can have detrimental effects on the adaptive immune response and alter disease outcome.
APCs, such as DCs and monocytes taken from the blood of patients with acute COVID-19 display impaired antigen presenting abilities. When stimulated ex vivo, DCs taken from the blood of COVID-19 patients had minimal expression of CD80, CD86, CCR7, and HLA-DR103. HLA-DR expression on monocytes from severe COVID-19 patients is also reduced relative to those from mild patients109,110,112,122. Ageing negatively affects antigen presentation as well. Monocyte populations shift in the elderly, and there is an accumulation of non-classical monocytes that significantly downregulate HLA-DR123. Other work suggests reduced levels of MHC class II, CD40, and CD86 on aged DC subsets post activation with TLR agonists; although there are conflicting reports, as previously reviewed124. During SARS-CoV-1 infection, lung DCs from older mice displayed an impaired ability to migrate to the draining lymph node, which negatively affected subsequent T cell priming125. This migration defect was caused by increased levels of prostaglandin D(2) in the elderly mouse lungs, which directly reduced the surface expression of CCR7 on DCs125.
Ageing predisposes an individual to impaired antigen presentation and T cell priming. Thus, a virus that further reduces the immune system’s capacity for these essential tasks could render the elderly especially susceptible to worsen disease outcome due to improper adaptive immune responses.
IMMUNE SYSTEM DYSREGULATION ASSOCIATED WITH AGEING AND SEVERE COVID-19: ADAPTIVE IMMUNITY
The role of adaptive immunity in controlling SARS-CoV-2 infection has been a major scientific focus, as generating robust immunological memory is the primary goal of current vaccines. Adaptive immunity is carried out by three broad populations of lymphocytes: B cells, CD4+ T cells, and CD8+ T cells. B cells are responsible for producing anti-viral antibodies. CD4+ T cells act as ‘helper’ cells, producing cytokines to bolster the antiviral immune response and aid B cells in the production of long-lasting, neutralizing antibodies. CD8+ T cells directly kill virally infected cells while also producing antiviral cytokines. Collectively, these three arms of the adaptive immune response have proven critical in controlling viral infection, including SARS-CoV-2. With age, adaptive immunity wanes, potentially allowing for SARS-CoV-2 to evade or subvert this arm of the immune system. Here, we review the adaptive immunological responses to SARS-CoV-2 and comment on how features of the ageing immune system may contribute to a defective adaptive response during severe COVID-19. A summary is depicted in Figure 4.
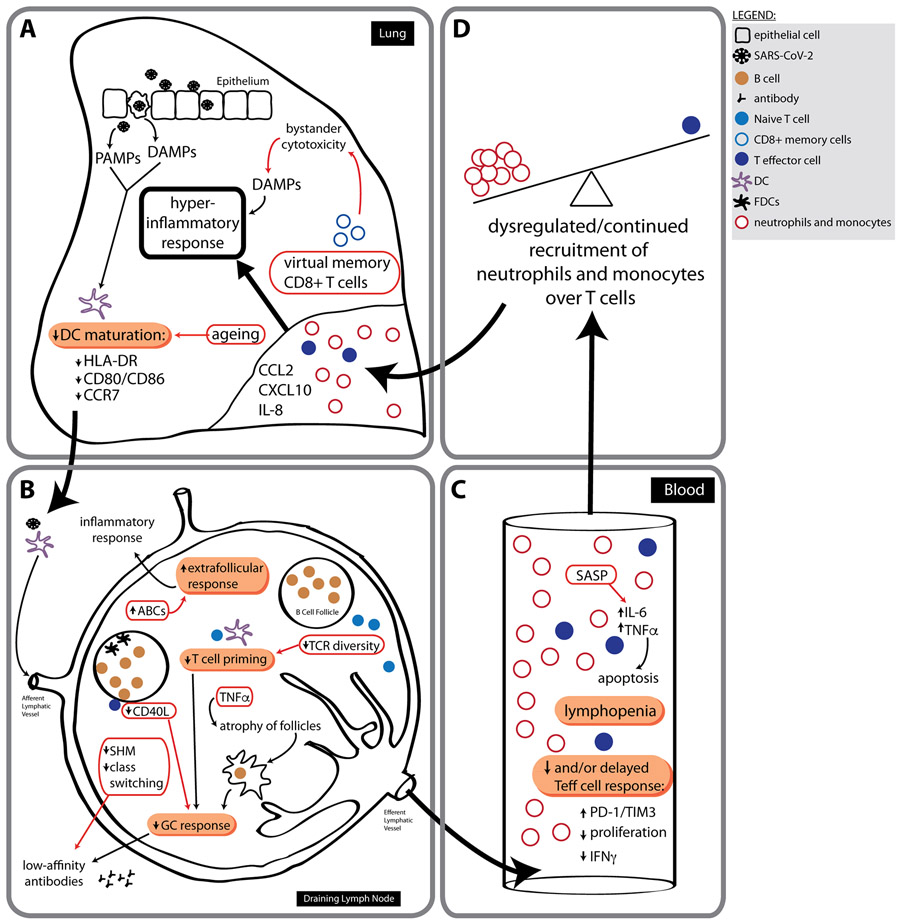
Figure 4. Ageing adaptive immune system responses during severe COVID-19.
a-d, It has been hypothesized that a delayed and/or weak adaptive immune response helps drive the dysregulated and continued recruitment of neutrophils and monocytes. These cells facilitate a non-specific and highly destructive hyperinflammatory response that contributes to the outcomes observed in patients with severe COVID-19. Failures of the adaptive system would begin at sites of infection (a), where DCs are initially activated by PAMPs and DAMPs in response to SARS-CoV-2. Patients with severe COVID-19 have DCs with a reduced maturation profile. (b) Ageing also negatively affects DC maturation, furthering the potential for improper priming of naive T cells in lymph nodes in older adults. There appears to be a loss of GC reactions and atrophy of the secondary lymphoid organs (SLOs) in fatal cases of COVID-19. These factors, combined with decreased CD40L expression on aged CD4+ T cells and reduced AID expression in aged B cells, could culminate in greatly impaired high-avidity, long-lasting antibody production that is dependent upon the success of GC reactions. Reductions in levels of GC reactions are also linked to extrafollicular and age-associated B cell (ABC) responses, which may potentiate ‘lupus-like autoimmune inflammation’. As we age, the naive TCR repertoire also decreases, making DC accessibility to SARS-CoV-2-specific naive T cells potentially even more challenging, delaying priming responses. (c,d) Lymphopenia is also strongly associated with severe COVID-19 outcomes. The aged microenvironment may contribute to this phenotype, as the SASP mediators IL-6 and TNFα can cause enhanced T cell apoptosis. Furthermore, the presence of virtual memory CD8+ T cells at sites of SARS-CoV-2 infection may contribute to a hyperinflammatory response through the action of bystander cytotoxicity, which occurs in a non-antigen-specific manner and can be highly destructive if left unregulated. AID, activation-induced cytidine deaminase; FDC, follicular dendritic cells; SHM, somatic hypermutation; Teff cell, effector T cell. Immune responses to the virus are indicated by black arrows, and consequences of aging are shown by red arrows.
T Lymphocytes
Adaptive responses to SARS-CoV-2 begin around one week after symptoms (Figure 2), with SARS-CoV-2 specific CD4+ and CD8+ T cells being observed as early as four days post-symptom onset126. SARS-CoV-2 infection elicits a Th1 CD4+ T cell response, with IFNγ and IL-2 being the primary cytokines produced by these cells and some TNFα production as well112,126-129. Indeed, many of the chemokines that are elevated in the blood of COVID-19 patients, like CXCL9 and CXCL10, are involved in recruiting and/or differentiating naive T cells into Th1 cells. Both CD4+ and CD8+ T cells have been determined to confer protection against previous coronaviruses130. Current evidence suggests an early, robust, and diverse T cell response directed against SARS-CoV-2 correlates with milder COVID-19 outcomes126,131,132; however, excessive cytokine release from T cells could also contribute to aberrant activation of monocytes during cytokine storm. Since ageing impacts many aspects of the T cell biology, it is important to understand how these changes may impact worsening outcomes to SARS-CoV-2 in the elderly.
Reduced naive lymphocyte clonal diversity
Maintaining a diverse TCR repertoire is critical for the optimal functioning of the immune system, but naive CD4+ and CD8+ TCR diversity diminishes as we age133. There are many factors that may contribute to the restricted TCR repertoire of the elderly, but the exact mechanisms underpinning this phenomenon are still debated. Thymic involution, a process wherein the thymus gradually atrophies as we age, prevents the development of new T cells in the elderly, thus restricting the TCR repertoire. Evidence suggests newly generated naive T cells that do manage to enter the aged T cell repertoire display reduced T effector qualities upon activation and generate less effective memory cells134. Nascent T cell generation, however, may only be a part of the story, especially in regards to CD8+ T cells. In humans, tightly regulated homeostatic proliferation and active maintenance of T cell quiescence are imperative for a stable peripheral naive T cell compartment, and these two factors may play a prominent role in sustaining naive T cell diversity as we age135. Additionally, chronic antigen stimulation can lead to an expanded, oligoclonal T cell memory pool that further exacerbates the diminished presence of polyclonal naive T cells.
Severe COVID-19 has been associated with lower TCR diversity against SARS-CoV-2 epitopes131,132. Specifically, when compared to those with mild COVID-19, patients with severe disease generated a weaker T cell response to the N-terminal portion of the SARS-CoV-2 S protein, a region that includes the critical receptor-binding domain (RBD)128. Low frequencies of naive T cells have also been correlated with severe COVID-19 outcomes126. These findings could suggest a connection between the diminished naive TCR repertoire of the elderly prior to SARS-CoV-2 infection and worsened disease outcome.
Lymphopenia
COVID-19 severity is associated with lymphopenia, and many studies have shown a correlation between poor disease outcome and reduced total numbers of peripheral T cells in the blood136. It has even been suggested that a patient’s blood lymphocyte percentage can be used clinically as an independent prognostic measure to identify moderate, severe, and critically ill disease trajectories to inform early therapeutic decisions137. The lymphopenia observed in severe COVID-19 may prove even more detrimental for aged individuals, as immunosenescence leads to poorer T cell responses that could be further exacerbated by a decline in T cell numbers.
Initially, it was speculated that COVID-19 associated lymphopenia may be a direct result of increased T cell migration to the sites of infection. However, while lymphocytes do accumulate in the lungs of deceased COVID-19 patients, this migration probably does not completely account for the magnitude of the observed lymphopenia. One group analyzing bronchoalveolar lavage fluid (BALF) by single-cell RNA sequencing found that the total number of CD8+ T cells with a tissue resident phenotype were actually greater in patients with moderate disease compared to those with severe disease138. The same study also concluded that lung macrophages present in BALF of severe COVID-19 patients expressed chemokines more likely to recruit inflammatory monocytes and neutrophils, while the lung macrophages of moderate COVID-19 patients expressed higher levels of T cell-recruiting chemokines, supporting the hypothesis that T cell migration to the lungs does not account for the lymphopenia observed in the blood in severe disease138. Other hypotheses to explain the depletion of T cells during SARS-CoV-2 infection include direct infection of T cells with the virus, and/or increased prevalence of activation-induced cell death (AICD) in response to either cognate antigen stimulation or as an effect of the cytokine milieu41. Lymphopenia does correlate with serum levels of IL-6, IL-10, and TNFα139,140, and the IL-6 receptor antagonist, tocilizumab, increased the number of circulating lymphocytes in COVID-19 patients122.
One cohort of 522 COVID-19 patients revealed a strong association between lymphopenia and age in addition to the association between lymphopenia and disease severity, such that patients older than 60 years had the lowest total number of T cells in their blood139. In the case of influenza virus, aged CD8+ T cells showed reduced expansion or proliferative capacity, and this decrease in virus-specific CD8+ T cells negatively affected viral clearance in the elderly134. Studies aimed at further delineating a similar association between lymphopenia, ageing, T cell expansive capacity, and SARS-CoV-2 infection may be of great interest for future investigation.
Decline in T effector cell function and enhanced cellular exhaustion
T cell effector functions tend to decrease with age, while T cell exhaustion increases. Analysis of blood from severe versus mild COVID-19 patients during the acute phase of illness has revealed contradictory reports about whether T cells are functioning properly following SARS-CoV-2 infection. Some studies have found reduced effector functions for CD4+ T cells, i.e. IFNγ/IL-2/TNFα production, in patients with severe disease141, while others have seen no differences142. For CD8+ T cells, there are reports of diminished CD8+ cytotoxicity and cytokine production in severe cases126,132, while others report the opposite141, and still others find no differences142. The differences across many studies may be due in part to the timing of cytokine sampling. Given that ageing reflects a state of impaired adaptive immunological function, we favor a model whereby ageing predisposes an individual to exhausted or compromised effector T cells with reduced cytokine production and/or cytotoxicity, compromising viral clearance during acute stages of the disease. Consistent with this hypothesis, one study identified the presence of IFNγ producing CD8+ T cells during the acute stages of disease as the strongest predictor of mild COVID-19 outcomes126. Moreover, CD4+ and CD8+ T cells also display greater expression of exhaustion markers (PD-1, Tim-3, etc.) in severe COVID-19 cases compared to mild ones139; however, these markers may simply represent activation rather than functional exhaustion, as one paper recently suggested143.
Pre-existing immunity to SARS-CoV-2
SARS-CoV-2 reactive CD4+ T cells are seen in up to 40-60% of unexposed individuals127. One study utilized cell sorting experiments to show that the SARS-CoV-2-reactive T cells identified from SARS-CoV-2 unexposed individuals were primarily from the memory T cell compartment, and they were also reactive to other human coronaviruses144. Therefore, many individuals have existing memory T cells that were generated from endemic human coronavirus infections and can cross-react with SARS-CoV-2 epitopes, known as SARS-CoV-2 cross-reactive memory T cells128. Another study concluded that severe COVID-19 may even be associated with a lack of these pre-existing SARS-CoV-2 cross-reactive memory T cells in the TCR repertoire131.
Given the relationship between COVID-19 severity and age, these findings require further investigation. For instance, it will be important to know if the elderly have been exposed to more human coronavirus infections, and whether this exposure translates into robust memory T cell responses. Indeed, the lack of TCR diversity with age may prevent broad memory T cell development from prior exposures to the ‘common cold’ endemic human coronaviruses. Memory T cells generated by the elderly may also not be maintained as well in the peripheral repertoire, perhaps due to niche competition from the effects of memory inflation145. COVID-19 severity has been linked to the incidence of CMV, a primary pathogen associated with memory inflation, but more work is needed to establish how CMV and other latent viruses of the elderly affect new memory T cell formation, function, and longevity146.
As we age, CD8+ T cells can also lose their naive quiescent state and differentiate in response to IL-15 signaling without the presence of their cognate antigen. These ‘virtual memory’ CD8+ T cells have been most widely studied in mice147, but a human equivalent population has been identified (CD45RA+KIR+NKG2A+Eomes+) and similarly positively correlates with age147. Virtual memory CD8+ T cells are innate-like in that they can become activated by cytokines (IL-15, IL-18, and type I IFNs) and mediate cytotoxic effects without the need for their cognate antigen during viral infections. While this could provide a benefit in the clearance of the virus, it could also prove highly damaging to the host if left unregulated148. Whether or not these cells play a role in COVID-19 outcomes has yet to be studied.
B Lymphocytes
In response to SARS-CoV-2 infection, B cells produce detectable levels of IgM, IgG, and IgA antibodies against SARS-CoV-2 at around one-week post-symptom onset, and by two weeks the majority of patients seroconvert for IgG and IgM. This response occurs concurrently with the detection of circulating SARS-CoV-2 specific T Follicular Helper cells, suggesting a role for T-dependent antibody production126. Neutralizing antibodies against the RBD of SARS-CoV-2 have been discovered in both mice and humans149,150, and passive transfer of these antibodies reduces disease severity and offers protection in mouse models of SARS-CoV-2. Convalescent patient serum samples have also provided promising results in the clinic151. However, the natural role antibodies play during the course of COVID-19 progression remains contested.
Many studies have reported a correlation between higher IgG antibody titers and severe disease, but others have seen either the opposite or no correlation between antibody production and disease severity126. These seemingly contradictory results might possibly be explained by the dual role played by antibodies during viral infections: although neutralizing antibodies are generally beneficial to viral clearance, the early production or prior existence of already circulating, non-neutralizing antibodies can lead to antibody-mediated enhancement of viral entry and induce a severe inflammatory response. This is called antibody-dependent enhancement (ADE) of disease; however, there is currently no evidence supporting a role for ADE during SARS-CoV-2 infection152. Interestingly, the presence of IgG antibodies with an afucosylated IgG-Fc tail during acute COVID-19 infection have also been associated with severe COVID-19 outcomes, perhaps owing to their stronger pro-inflammatory activity via FcγRIIIa153.
The quality of the humoral immune response declines with age, as aged B cells display a diminished potential to undergo somatic hypermutation154, which could prevent the aged from generating robust neutralizing antibody titers to aid the clearance of natural infection and generate effective immunity against re-infections. Consistently, the levels of viral spike-protein-specific IgG during early time-points of acute SARS-CoV-2 infection in rhesus macaques are reduced in older macaques compared to young ones, consistent with age crippling the formation of class-switched antibody titers needed to clear the viral infection155.
Another change in B cells that occurs in animal models and in humans with aging is the accumulation of age-associated B cells (ABCs) with unique properties156. In mice, these cells are promoted by TLR7 responses156, which is relevant being a major PRR for SARS-CoV-2. In humans, these cells are found within the late memory fraction (IgD-CD27-, also called double negative B cells), secrete inflammatory mediators like TNFα, IL-6, IL-8, and have been implicated in autoimmune disease, chronic viral infections, and more recently in COVID-19156-158. During COVID-19, critically ill patients show expansion of such cells, which also expressed CD11c and T-bet, in a skewed extrafollicular B cell response, with signatures of lupus-like autoimmune disease158.
Consistent with a dominant extrafollicular B cell response, studies have consistently reported defective germinal center (GC) responses in the secondary lymphoid organs of patients who have succumbed to SARS-CoV-2 infection129,159. One of these studies also noted diminished GC formation that occurred concurrently with high levels of TNFα in lymph node follicles, and TNFα has previously been demonstrated to inhibit Tfh differentiation and subsequent GC formation160. TNFα production also increases as we age and could contribute to this observed phenotype161-163. Other hallmarks of ageing also affect GC formation. Expression of CD40L, a costimulatory molecule critical for successful T and B cell interactions, decreases on aged CD4+ T cells134. Additionally, the lymphopenia observed in the elderly as well as severe COVID-19 patients could imply a reduction in the availability of CD4+ T cells to engage with B cells. This dilemma would be heightened by the lack of TCR and BCR clonal diversity in aged individuals, as the probability for cognate T:B interactions would be greatly reduced.
Another consequence of ageing is the loss of tolerance and the emergence of autoantibodies. Recent work in SARS-CoV-2 has linked life-threatening COVID-19 to the presence of autoantibodies against type I IFNs, especially in men104. These autoantibodies were found across multiple ages, though there was a higher prevalence in patients over 65. Thus, it remains to be determined if the loss of tolerance with ageing facilitates production of these and other autoantibodies that might worsen clinical outcome in COVID-19. Moreover, the mechanistic basis in the sex bias to produce such autoantibodies specifically in males also warrants further investigation.
BOLSTERING IMMUNITY IN THE ELDERLY TO COMBAT COVID-19
Biomarkers of ageing
While we associate immunosenescence with the chronological process of ageing, this paradigm can be misleading. People of the same chronological age can have a widely variable ‘immunological age’. Important work is being done to identify the biomarkers associated with immunological ageing, in hope of developing prognostic tools for assessing an individual’s risk of developing specific age-related diseases16. Identifying biomarkers indicative of immunosenescent phenotypes would also be of great interest for the development of therapeutics that could preemptively bolster viral immunity not only in the elderly, but also in susceptible younger individuals as well.
Identifying biomarkers of immunosenescence represents an especially exciting area of research, as effects of ageing have been shown to be amenable to interventions. For example, epigenetic changes associated with ageing, including Horvath’s methylation clock164, appear to be responsive to unique pharmacological interventions. One year of treatment with DHEA, metformin, and recombinant human growth hormone therapy in elderly men resulted in a 1.5 year reduction in ‘epigenetic age’ and significantly regenerated the thymus165. Many strategies have already been proposed to ameliorate the declining immune system in the elderly166. Below, we will highlight how current strategies to combat ageing could be linked to improve immune function in the face of SARS-CoV-2.
Interventions for immunosenescence and COVID-19
Rapamycin and rapalogs
Sirolimus (rapamycin) and rapalogs, derivatives and mimetics of rapamycin, have often been studied in the field of ageing. These drugs target critical factors in the rapamycin (TOR) pathway and are commonly used clinically as immunosuppressants. Animal models exploring the effect of rapamycin and rapalogs on longevity have determined their ability to extend lifespan167-169. While they are immunosuppressive at high doses, these compounds exert immunostimulatory effects at lower doses170. Administration of a rapalog, mTOR inhibitor RAD001, showed improved response to the influenza vaccine in the elderly by around 20%171.
The mechanism of immunostimulatory action by rapamycin and rapalogs is not quite clear. One possibility is that inhibition of mTORC1 could be relieving feedback inhibition on other inflammatory and metabolic pathways, thus boosting immunity. One such pathway could be insulin signaling. Insulin is a critical mediator of adaptive immune effector function against respiratory infections172, and mTORC1 activation of S6 kinase blocks insulin signaling173,174. mTOR has also been shown to regulate STAT signaling175, and studies in older humans show that the elevation in baseline phosphorylation of STAT proteins in T cells distinguishes healthy ageing from unhealthy ageing subjects based on cardiovascular ageing phenotypes17. However, rapamycin has also been observed to enhance expression of IL-6176-178 which is associated with poor COVID-19 outcomes, and patients with type 2 diabetes and COVID-19 had significantly increased mortality rates when receiving insulin treatment during the course of their hospitilization179. Thus, before any treatment or adjuvant uses for rapamycin and rapalogs are considered, further investigation into how mTOR and other nutrient sensing pathways alter immunosenescence and COVID-19 outcomes are necessary.
Senolytics
Numerous clinical trials are also underway testing the role of senolytic compounds on ageing. Senolytics, such as Fisetin and Quercetin and Dasatinib (D+Q), clear senescent cells and reduce the proinflammatory and pro-thrombotic effects of SASP. Several small studies have found an improvement in symptoms of inflammation with the administration of senolytics. For example, D+Q resulted in the reduction of SASP-associated proinflammatory cytokines during idiopathic pulmonary fibrosis, an age-related lung disease180, and a recent study showed that Fisetin and D+Q reduced SASP and improved survival in a mouse model of β-coronavirus84. Quercetin may also have beneficial renal-protective and senolytic-independent effects during SARS-CoV-2 infection181. However, more work is needed to determine if senolytics could be a viable option in humans to rejuvenate an aged immune system as either a preventative measure before SARS-CoV-2 infection or as a treatment option during acute infection.
NAD+ precursors
Therapeutics targeting NAD+ metabolism have also been proposed as potential treatments for age-related immune decline, since reduced NAD+ levels are associated with impaired mitochondrial function, immune cell metabolic reprogramming, and cell function182. A recent paper described the downregulation of nuclear-encoded mitochondrial genes related to cellular respiration and Complex I during infection with SARS-CoV-2, suggesting that proper mitochondrial function of immune cells is essential for containing viral propagation183. Immune system activation can further reduce NAD+ levels, such as was recently shown in macrophages treated with PAMPS, including viral TLR ligands, and from ageing mouse tissues which contain CD38-dependent NADase activity184. Studies of the NAD+ precursor, nicotinamide mononucleotide (NMN) administration in mice have been somewhat promising at restoring NAD+ levels and ameliorating mitochondrial function185. A similar NAD+ precursor, nicotinamide riboside (NR), was also able to restore NAD+ levels in mice and humans186.
Diet modulation
There is a mutual interaction between nutrition, immune function, and inflammatory state, as detailed among the hallmarks of immunosenescence187. Notably, a high rate of long-living people and the low incidence of cardiovascular disease in many Mediterranean countries suggests the importance of a diet rich in fruits, vegetables, whole grains, legumes, fish high in omega-3 fatty acids, and extra virgin olive oil188. This particular diet results in a reduction in both oxidative stress and inflammation and regulation of eubiosis of the gut microbiota, which in turn all contribute to an improvement in immune responses189. The use of probiotics in older adults has also been shown to regulate inflammatory conditions: specifically, probiotics can attenuate the production of both IL-1β and IL-6190, which are associated with severe COVID-19. The Mediterranean diet also modulates the level of many biomarkers of inflammation, including IL-6187.
Another possible dietary approach to the reversion of immunosenescence is caloric restriction. NF-κB, mTOR, AMPK, and MAPK are pathways that are involved in both ageing and inflammation and have been shown to be affected by caloric restriction, resulting in downregulation of inflammatory markers, such as IL-6 and IL-1β191,192. Clinical trials utilizing metformin, which activates AMPK, to target ageing have already begun193. Additionally, ketones, which are largely produced during either ketogenic diets or fasting periods, can impact immune function and may have possible therapeutic effects for COVID-19194. If caloric restriction can reverse age-related upregulation of inflammatory genes, future studies should compare COVID-19 responses in elderly patients segmented by caloric intake and fasting regimes. Undernutrition, however, is common in older people, and is also linked to impaired immune responses187. Restricting glucose utilization can also be fatal in mice during viral infection, even though it proves beneficial for fighting bacterial infections195. Therefore, more work is needed to determine whether reduced caloric intake or specific types of nutritional supplementation may boost or hinder immune responses with age in the specific context of SARS-CoV-2 infection.
CONCLUSIONS AND PERSPECTIVES
The serious effects of SARS-CoV-2 infection are likely caused by a pathological hyper-inflammatory response initiating uncontrolled local tissue damage, vascular leakage, systemic cytokine storm, and thrombosis. Research has begun to identify the pathophysiological mechanisms underlying these events in response to SARS-CoV-2, and some recurring observations in cases of severe disease have emerged: 1) early defects in type I IFN production and signaling, 2) sub-optimal T cell responses, and 3) dysregulated monocyte and neutrophil inflammation. While there is much work left in elucidating why some develop severe disease while others are asymptomatic, we can begin to propose why an aged immune system may be especially vulnerable to these severe outcomes.
Basal inflammageing is driven by NF-κB signaling and generally renders immune cells initially hyporesponsive to acute activation, with the possible exception of the monocyte subsets71,81. A lack of early type I IFN responses during severe COVID-19, compounded with the JAK/STAT hyporesponsiveness of aged immune cells17, could facilitate early viral replication and overburden the immune system in early elderly responses to SARS-CoV-2 invasion. Once the aged immune system overcomes initial signaling thresholds and local viral load peaks, massive production of proinflammatory cytokines would be released and tissue destruction and vascular permeability may become tipped too far in favor of propagating continued, hyper-inflammatory, pathological innate responses. This ‘tipping point’ may also be reached through further TLR4 priming due to secondary bacterial superinfections resulting from the initial viral invasion53. Meanwhile, poor adaptive responses to SARS-CoV-2 in the elderly could be partly driven by ineffective T cell priming, a lack of naive T cell diversity, diminished antibody maturation, and/or inefficient preexisting memory; these characteristics of immunosenescence are most likely compounded by viral evasion tactics and improper early innate responses. Without the help of a fully functional antigen-specific immune response, the broadly acting innate immune system will continue to drive highly destructive effects of inflammation in an unregulated manner, leading to the enhanced morbidity of severe COVID-19.
SARS-CoV-2 has highlighted gaps in our knowledge of the aged immune system. Even basic understanding of how specific immune cell subsets behave to insult as we age remains a major topic of research. Most studies involve the interrogation of human immune cells taken from the blood, but analysis of human cells from specific tissues is lacking. While mouse studies allow us to glean information on immunosenescence, differences between mouse and human immune-ageing have already been discovered, such as in mechanisms controlling naive T cell repertoire diversity135. Furthermore, immune cell subsets are not always perfectly conserved across the two species, such as in the case of monocyte subsets196. These examples emphasize the need for caution when extrapolating data from one model to the next. Ageing immunology studies should also better integrate emerging insights from geroscience, especially from inflammageing research, which has recently described pivotal roles for mitochondrial dysfunction, proteostasis, nutrient sensing, and physical changes in the tissue microenvironment in modulating immune pathways197-200.
Recent early successes of the Pfizer and Moderna mRNA COVID-19 vaccines appear promising for the elderly population. In stage III clinical trials, the Pfizer vaccine (BNT162b2) had a 94.7% success rate for those aged 65 years or older (with a 95% CI of 66.7-99.9)201, and the Moderna vaccine (mRNA-1273) was 86.4% efficient in the same age group (with a 95% CI of 61.4-95.2)202. While these preliminary results are exciting, both studies only assessed vaccine efficiency for about 90 days after complete dosing. A more recent study with BNT162b2 showed that people over the age of 80 had lower anti-SARS-CoV-2 antibody titers seventeen days after the second dose when compared to those under 60 years of age, including 31% with no detectable neutralizing antibodies203. Thus, long-term follow-up studies are needed to determine if there is sustained efficacy in older individuals. Still, many people have already been infected with SARS-CoV-2, and long-term effects of this infection have yet to be extensively detailed. Chronic infections, such as CMV, greatly affect ageing phenotypes, and it is possible COVID-19 may leave a lasting imprint on human physiology as well.
The field of geroscience has been given a boost of attention from the COVID-19 pandemic, which will hopefully lead to even more advances and breakthroughs in the near future. Indeed, our first defense against any virus is a properly functioning immune system; thus, we need to further elucidate the mechanisms of immunosenescence and pursue the development of therapeutics that will enhance ‘healthspan’ in a preemptive way.
Acknowledgments
J.M.B is a recipient of a National Institutes of Health (NIH) T32 (AG000266) postdoctoral fellowship award. D.A.W. and D.F. were funded in part through the Impact Circle at the Buck Institute for Research on Aging. Drafts of Figures 1 and 2 were created with BioRender (A.J.C.).
Footnotes
Competing Interests
J.M.B, D.F., A.J.C, E.V. and D.A.W. declare no competing interests related to this manuscript. D.R. is a Chief Science Officer at Hooke by Healthy, Longevity Optimisation, a longevity clinic and research centre.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1.Yoshikawa TT Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis 30, 931–933 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Goodwin K, Viboud C & Simonsen L Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24, 1159–1169 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Melegaro A & Edmunds WJ The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol 19, 353–363 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Wolters B, Junge U, Dziuba S & Roggendorf M Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine 21, 3623–3628 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Kaml M, et al. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine 24, 6808–6811 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Hainz U, et al. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 23, 3232–3235 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Wu C, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 180, 934–943 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santesmasses D, et al. COVID-19 is an emergent disease of aging. Aging Cell 19, e13230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Z, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 81, e16–e25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajgain KT, Badal S, Bajgain BB & Santana MJ Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am J Infect Control 49, 238–246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control COVID Data Tracker. Vol. 2021 (2021). [cited 2021 January 27] Available from: https://covid.cdc.gov/covid-data-tracker. [Google Scholar]
- 12.Gold JAW, et al. Race, Ethnicity, and Age Trends in Persons Who Died from COVID-19 - United States, May-August 2020. MMWR Morb Mortal Wkly Rep 69, 1517–1521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho FK, et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One 15, e0241824 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrucci L & Fabbri E Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15, 505–522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furman D, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 25, 1822–1832 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpert A, et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med 25, 487–495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen-Orr SS, et al. Defective Signaling in the JAK-STAT Pathway Tracks with Chronic Inflammation and Cardiovascular Risk in Aging Humans. Cell Syst 3, 374–384 e374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rea IM, et al. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol 9, 586 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chougnet CA, et al. Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells Is Associated with Mitochondrial Dysfunction. J Immunol 195, 2624–2632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cumberbatch M, Dearman RJ & Kimber I Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin-1beta. Immunology 105, 466–477 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manser AR & Uhrberg M Age-related changes in natural killer cell repertoires: impact on NK cell function and immune surveillance. Cancer Immunol Immunother 65, 417–426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metcalf TU, et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 14, 421–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metcalf TU, et al. Human Monocyte Subsets Are Transcriptionally and Functionally Altered in Aging in Response to Pattern Recognition Receptor Agonists. J Immunol 199, 1405–1417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Duin D, et al. Age-associated defect in human TLR-1/2 function. J Immunol 178, 970–975 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Zacca ER, et al. Aging Impairs the Ability of Conventional Dendritic Cells to Cross-Prime CD8+ T Cells upon Stimulation with a TLR7 Ligand. PLoS One 10, e0140672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourati S, et al. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat Commun 7, 10369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verschoor CP, et al. Serum C-Reactive Protein and Congestive Heart Failure as Significant Predictors of Herpes Zoster Vaccine Response in Elderly Nursing Home Residents. J Infect Dis 216, 191–197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDade TW, Adair L, Feranil AB & Kuzawa C Positive antibody response to vaccination in adolescence predicts lower C-reactive protein concentration in young adulthood in the Philippines. Am J Hum Biol 23, 313–318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derhovanessian E, Solana R, Larbi A & Pawelec G Immunity, ageing and cancer. Immun Ageing 5, 11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiskopf D, Weinberger B & Grubeck-Loebenstein B The aging of the immune system. Transpl Int 22, 1041–1050 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Nikolich-Zugich J, et al. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience 42, 505–514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolich-Zugich J The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 19, 10–19 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Sadighi Akha AA Aging and the immune system: An overview. J Immunol Methods 463, 21–26 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Boraschi D & Italiani P Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol Lett 162, 346–353 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li F Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol 3, 237–261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 181, 894–904 e899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann M, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271–280 e278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sungnak W, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26, 681–687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khanmohammadi S & Rezaei N Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol 93, 2735–2739 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merad M & Martin JC Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20, 355–362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onofrio L, et al. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA 6, FSO605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakraborty C, Sharma AR, Sharma G, Bhattacharya M & Lee SS SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci 24, 4016–4026 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iba T, Levy JH, Raj A & Warkentin TE Advance in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. J Clin Med 8(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons J & Pittet JF The coagulopathy of acute sepsis. Curr Opin Anaesthesiol 28, 227–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papazian L, et al. Diagnostic workup for ARDS patients. Intensive Care Med 42, 674–685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8, 420–422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai N, et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat Commun 11, 6319 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruan Q, Yang K, Wang W, Jiang L & Song J Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46, 846–848 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaillancourt M & Jorth P The Unrecognized Threat of Secondary Bacterial Infections with COVID-19. mBio 11(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang A, et al. Specific sequences of infectious challenge lead to secondary hemophagocytic lymphohistiocytosis-like disease in mice. Proc Natl Acad Sci U S A 116, 2200–2209 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinnon KH 3rd, et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586, 560–566 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leist SR, et al. A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell 183, 1070–1085 e1012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia LF Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front Immunol 11, 1441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torres Acosta MA & Singer BD Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur Respir J 56(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tay MZ, Poh CM, Renia L, MacAry PA & Ng LFP The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20, 363–374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M, Jiang L, Monticone RE & Lakatta EG Proinflammation: the key to arterial aging. Trends Endocrinol Metab 25, 72–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imai Y, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436, 112–116 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu X, et al. Angiotensin-converting enzyme 2-angiotensin (1-7)-Mas axis prevents pancreatic acinar cell inflammatory response via inhibition of the p38 mitogen-activated protein kinase/nuclear factor-kappaB pathway. Int J Mol Med 41, 409–420 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Banu N, Panikar SS, Leal LR & Leal AR Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci 256, 117905 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie X, Chen J, Wang X, Zhang F & Liu Y Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 78, 2166–2171 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 19(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tikellis C & Thomas MC Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int J Pept 2012, 256294 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63, 364–374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosa BA, et al. IFN signaling and neutrophil degranulation transcriptional signatures are induced during SARS-CoV-2 infection. Commun Biol 4, 290 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin C, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71, 762–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson TE Recent results: biomarkers of aging. Exp Gerontol 41, 1243–1246 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Brasier AR The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 86, 211–218 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salminen A, et al. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev 7, 83–105 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Guaraldi G, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2, e474–e484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youm YH, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 18, 519–532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Junqueira C, et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. medRxiv (2021). [Google Scholar]
- 75.Rodrigues TS, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 218(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng Y, et al. A human circulating immune cell landscape in aging and COVID-19. Protein Cell 11, 740–770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mishra SR, et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int J Biochem Cell Biol, 106013 (2021). [DOI] [PubMed] [Google Scholar]
- 78.Xian H, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barkas F, et al. Anakinra in hospitalized non-intubated patients with coronavirus disease 2019: a systematic review and meta-analysis. Rheumatology (Oxford) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah A Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19. Front Immunol 11, 1021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sayed N, et al. An Inflammatory Clock Predicts Multi-morbidity, Immunosenescence and Cardiovascular Aging in Humans. bioRxiv (2019). [Google Scholar]
- 82.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114, 1299–1307 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coppe JP, Desprez PY, Krtolica A & Campisi J The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camell CD, et al. Senolytics reduce coronavirus-related mortality in old mice. Science (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ortmann W & Kolaczkowska E Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res 371, 473–488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sapey E, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 123, 239–248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asensi V, et al. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect Immun 72, 3823–3828 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 81, e6–e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuri-Cervantes L, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 5(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lucas C, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bajaj V, et al. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front Physiol 11, 571416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiley CD, et al. SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells. Cell Rep 28, 3329–3337 e3325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang N, Li D, Wang X & Sun Z Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18, 844–847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guan WJ, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382, 1708–1720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui S, Chen S, Li X, Liu S & Wang F Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 18, 1421–1424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klok FA, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191, 145–147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lodigiani C, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191, 9–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fox SE, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 8, 681–686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Price LC, McCabe C, Garfield B & Wort SJ Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J 56(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zuo Y, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 12(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hadjadj J, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou R, et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 53, 864–877 e865 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bastard P, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Q, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McNab F, Mayer-Barber K, Sher A, Wack A & O'Garra A Type I interferons in infectious disease. Nat Rev Immunol 15, 87–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Channappanavar R, et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 19, 181–193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Channappanavar R, et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest 129, 3625–3639 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silvin A, et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 182, 1401–1418 e1418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schulte-Schrepping J, et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 182, 1419–1440 e1423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wong KL, et al. The three human monocyte subsets: implications for health and disease. Immunol Res 53, 41–57 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Zhou Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. National Science Review 7, 998–1002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang D, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv (2020). [Google Scholar]
- 114.Boyette LB, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One 12, e0176460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molony RD, et al. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci Signal 10(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi CS, et al. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol 193, 3080–3089 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feng E, Balint E, Poznanski SM, Ashkar AA & Loeb M Aging and Interferons: Impacts on Inflammation and Viral Disease Outcomes. Cells 10(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamada T, et al. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat Immunol (2021). [DOI] [PubMed] [Google Scholar]
- 119.Shodell M & Siegal FP Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol 56, 518–521 (2002). [DOI] [PubMed] [Google Scholar]
- 120.Perez-Cabezas B, et al. Reduced numbers of plasmacytoid dendritic cells in aged blood donors. Exp Gerontol 42, 1033–1038 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Jing Y, et al. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol 70, 777–784 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giamarellos-Bourboulis EJ, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 27, 992–1000 e1003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seidler S, Zimmermann HW, Bartneck M, Trautwein C & Tacke F Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol 11, 30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wong C & Goldstein DR Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol 25, 535–541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao J, Zhao J, Legge K & Perlman S Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest 121, 4921–4930 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rydyznski Moderbacher C, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 183, 996–1012 e1019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grifoni A, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181, 1489–1501 e1415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Braun J, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 587, 270–274 (2020). [DOI] [PubMed] [Google Scholar]
- 129.Kaneko N, et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 183, 143–157 e113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao J, Zhao J & Perlman S T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol 84, 9318–9325 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nelde A, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol 22, 74–85 (2021). [DOI] [PubMed] [Google Scholar]
- 132.Peng Y, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 21, 1336–1345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Britanova OV, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol 192, 2689–2698 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Gruver AL, Hudson LL & Sempowski GD Immunosenescence of ageing. J Pathol 211, 144–156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goronzy JJ & Weyand CM Mechanisms underlying T cell ageing. Nat Rev Immunol 19, 573–583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang I & Pranata R Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care 8, 36 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tan L, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 5, 33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liao M, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26, 842–844 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Diao B, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol 11, 827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv (2020). [Google Scholar]
- 141.Zheng HY, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 17, 541–543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mazzoni A, et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest 130, 4694–4703 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rha MS, et al. PD-1-Expressing SARS-CoV-2-Specific CD8(+) T Cells Are Not Exhausted, but Functional in Patients with COVID-19. Immunity 54, 44–52 e43 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mateus J, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370, 89–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Klenerman P & Oxenius A T cell responses to cytomegalovirus. Nat Rev Immunol 16, 367–377 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Shrock E, et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 370(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.White JT, et al. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun 7, 11291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim TS & Shin EC The activation of bystander CD8(+) T cells and their roles in viral infection. Exp Mol Med 51, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alsoussi WB, et al. A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. J Immunol 205, 915–922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zost SJ, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 584, 443–449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Devarasetti PK, Rajasekhar L, Baisya R, Sreejitha KS & Vardhan YK A review of COVID-19 convalescent plasma use in COVID-19 with focus on proof of efficacy. Immunol Res (2021).
- 152.Arvin AM, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 584, 353–363 (2020). [DOI] [PubMed] [Google Scholar]
- 153.Larsen MD, et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science (2020).
- 154.Frasca D, Diaz A, Romero M & Blomberg BB Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol 87, 113–120 (2017). [DOI] [PubMed] [Google Scholar]
- 155.Singh DK, et al. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat Microbiol 6, 73–86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cancro MP Age-Associated B Cells. Annu Rev Immunol 38, 315–340 (2020). [DOI] [PubMed] [Google Scholar]
- 157.Frasca D Senescent B cells in aging and age-related diseases: Their role in the regulation of antibody responses. Exp Gerontol 107, 55–58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Woodruff MC, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol 21, 1506–1516 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duan YQ, et al. Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr Med Sci 40, 618–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Popescu M, Cabrera-Martinez B & Winslow GM TNF-alpha Contributes to Lymphoid Tissue Disorganization and Germinal Center B Cell Suppression during Intracellular Bacterial Infection. J Immunol 203, 2415–2424 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fagiolo U, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol 23, 2375–2378 (1993). [DOI] [PubMed] [Google Scholar]
- 162.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK & Jeune B Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med 115, 278–283 (2003). [DOI] [PubMed] [Google Scholar]
- 163.Penninx BW, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc 52, 1105–1113 (2004). [DOI] [PubMed] [Google Scholar]
- 164.Horvath S & Raj K DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet 19, 371–384 (2018). [DOI] [PubMed] [Google Scholar]
- 165.Fahy GM, et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 18, e13028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aspinall R & Lang PO Interventions to restore appropriate immune function in the elderly. Immun Ageing 15, 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kaeberlein M, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196 (2005). [DOI] [PubMed] [Google Scholar]
- 168.Robida-Stubbs S, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 15, 713–724 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferrer IR, Araki K & Ford ML Paradoxical aspects of rapamycin immunobiology in transplantation. Am J Transplant 11, 654–659 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med 6, 268ra179 (2014). [DOI] [PubMed] [Google Scholar]
- 172.Tsai S, et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab 28, 922–934 e924 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Tremblay F, Jacques H & Marette A Modulation of insulin action by dietary proteins and amino acids: role of the mammalian target of rapamycin nutrient sensing pathway. Curr Opin Clin Nutr Metab Care 8, 457–462 (2005). [DOI] [PubMed] [Google Scholar]
- 174.Um SH, D'Alessio D & Thomas G Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 3, 393–402 (2006). [DOI] [PubMed] [Google Scholar]
- 175.Saleiro D & Platanias LC Intersection of mTOR and STAT signaling in immunity. Trends Immunol 36, 21–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cappoli N, et al. The mTOR kinase inhibitor rapamycin enhances the expression and release of pro-inflammatory cytokine interleukin 6 modulating the activation of human microglial cells. EXCLI J 18, 779–798 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang Y, et al. Rapamycin upregulates glutamate transporter and IL-6 expression in astrocytes in a mouse model of Parkinson's disease. Cell Death Dis 8, e2611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–577 (2008). [DOI] [PubMed] [Google Scholar]
- 179.Yu B, Li C, Sun Y & Wang DW Insulin Treatment Is Associated with Increased Mortality in Patients with COVID-19 and Type 2 Diabetes. Cell Metab 33, 65–77 e62 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Justice JN, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gu YY, et al. Quercetin as a potential treatment for COVID-19-induced acute kidney injury: Based on network pharmacology and molecular docking study. PLoS One 16, e0245209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Covarrubias AJ, Perrone R, Grozio A & Verdin E NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 22, 119–141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miller B, et al. Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples. Sci Rep 11, 3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Covarrubias AJ, et al. Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages. Nat Metab 2, 1265–1283 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kiss T, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 42, 527–546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Trammell SA, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7, 12948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aiello A, et al. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front Immunol 10, 2247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.O'Keefe JH, et al. A Pesco-Mediterranean Diet With Intermittent Fasting: JACC Review Topic of the Week. J Am Coll Cardiol 76, 1484–1493 (2020). [DOI] [PubMed] [Google Scholar]
- 189.Mitsou EK, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr 117, 1645–1655 (2017). [DOI] [PubMed] [Google Scholar]
- 190.Landete JM, et al. Probiotic Bacteria for Healthier Aging: Immunomodulation and Metabolism of Phytoestrogens. Biomed Res Int 2017, 5939818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chung KW, et al. Recent advances in calorie restriction research on aging. Exp Gerontol 48, 1049–1053 (2013). [DOI] [PubMed] [Google Scholar]
- 192.Chung HY, Kim HJ, Kim JW & Yu BP The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci 928, 327–335 (2001). [PubMed] [Google Scholar]
- 193.Justice JN, et al. Development of Clinical Trials to Extend Healthy Lifespan. Cardiovasc Endocrinol Metab 7, 80–83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stubbs BJ, et al. Investigating Ketone Bodies as Immunometabolic Countermeasures against Respiratory Viral Infections. Med (N Y) 1, 43–65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang A, et al. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell 166, 1512–1525 e1512 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ziegler-Heitbrock L Reprint of: Monocyte subsets in man and other species. Cell Immunol 291, 11–15 (2014). [DOI] [PubMed] [Google Scholar]
- 197.Thompson HL, et al. Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates. Aging Cell 18, e12865 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chakraborty M, et al. Mechanical Stiffness Controls Dendritic Cell Metabolism and Function. Cell Rep 34, 108609 (2021). [DOI] [PubMed] [Google Scholar]
- 199.Desdin-Mico G, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bharath LP, et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab 32, 44–55 e46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Polack FP, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Baden LR, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Muller L, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.