Elevated gut serotonin inhibits autophagy via mTOR promoting a disrupted microbiota and enhanced susceptibility to colitis.
Abstract
Autophagy, an essential intracellular recycling process, is linked to the pathogenesis of various diseases including Crohn’s disease (CD). Factors that lead to the development of impaired autophagy during intestinal inflammation remain largely unexplored. Here, we report the impact of the interaction between serotonin [5-hydroxytryptamine;(5-HT)] and autophagy in colitis in mouse and human studies. In mice, increased gut 5-HT inhibited autophagy and led to enhanced colitis susceptibility. Reciprocally, mice with reduced 5-HT exhibited up-regulated autophagy via the mammalian target of rapamycin pathway, which resulted in significantly decreased colitis. Deletion of autophagy gene, Atg7, in an epithelial-specific manner, in concert with reduced 5-HT, promoted the development of a colitogenic microbiota and abolished the protective effects conferred by reduced 5-HT. Notably, in control and patient peripheral blood mononuclear cells, we uncovered that 5-HT treatment inhibited autophagy. Our findings suggest 5-HT as a previously unidentified therapeutic target in intestinal inflammatory disorders such as CD that exhibits dysregulated autophagy.
INTRODUCTION
Crohn’s disease (CD), a major form of inflammatory bowel disease (IBD), is a serious chronic inflammatory condition of the human bowel (1). Genome-wide association studies have identified an increased risk of developing CD in individuals with autophagy gene polymorphisms including autophagy related 16 like 1 (ATG16L1) and immunity-related guanosine triphosphatase family M (IRGM) (2, 3). Autophagy is a vital conserved catabolic process that provides energy during stress and protects the cell by removing damaged organelles and infectious agents (4, 5). Environmental stressors prime autophagy through the activation of 5′ adenosine monophosphate–activated protein kinase (AMPK) and the inhibition of mammalian target of rapamycin complex 1 (mTORC1) (6). Downstream components, Beclin-1, Atg12-5 (autophagy related 12-5) complex, LC3-II (microtubule associated protein 1 light chain 3), and Atg7 (autophagy related 7), participate in the formation of the double membrane-bound vesicle, the autophagosome (6). The adapter protein, p62/sequestosome 1, delivers substrates to the autophagosome for degradation. Subsequently, fusion of the autophagosome with lysosome forms the autolysosome, a site where lysosomal enzymes degrade the aforementioned substrates (6). Autophagy-deficient mice are highly susceptible to experimental colitis and produce more proinflammatory cytokines (7, 8). Studies demonstrate that autophagy-enhancing pharmacological agents such as rapamycin and salicylates (or other mTOR-inhibiting and AMPK-activating drugs) reduce the severity of experimental colitis and refractory CD, suggesting that these substances may promote a protective role of autophagy in gut inflammation (9–12). However, therapies that inhibit mTOR/enhance autophagy such as rapamycin have many side effects including altered insulin sensitivity, nephrotoxicity, hyperlipidemia, thrombocytopenia, and diminished wound healing (13). Therefore, studying the process of autophagy within the context of IBD presents a unique opportunity for the development of previously unidentified therapeutic strategies for intestinal inflammatory conditions, particularly by identifying new targets to overcome defective autophagy.
In the gastrointestinal tract, the microbiota plays a fundamental role in digestion, prevention of pathogen invasion, and the development of the immune system (14). Disruption of the composition and function of microbiota is a risk factor for the development of IBD (15). The gut microbiota is influenced by signals from the host epithelium. Studies show that mice with intestinal epithelial cell (IEC)–specific Atg7 or Atg5 deficiency exhibit altered gut microbiota composition and diversity (8, 16). These microbial changes are similar to the changes seen in CD, signifying the role of impaired autophagy in gut dysbiosis and inflammation (16). Furthermore, activation of the mTORC2 signaling pathway followed by inhibition of mTORC1 by resveratrol decreased the abundance of obesity-associated gut microbiota and reduced intestinal inflammation in diet-induced obese mice (17). Although some of the mechanisms by which dysfunctional autophagy changes the gut microbiota have been studied (4), little is known about the factors that lead to the development of impaired autophagy during intestinal inflammation.
Enterochromaffin (EC) cells, a subset of enteroendocrine cells, produce approximately 90% of serotonin [5-hydroxytryptamine (5-HT)] in the gut (18, 19). In EC cells, the synthesis of 5-HT begins with the conversion of dietary tryptophan to 5-hydroxy-l-tryptophan (5-HTP), a process catalyzed by the rate-limiting enzyme, tryptophan hydroxylase (Tph) (18). There are two isoforms of the Tph enzyme, Tph1, present mainly in EC cells, and Tph2, found in the brain stem and enteric neurons (18, 20). 5-HTP is converted into 5-HT by aromatic l-amino acid decarboxylase. Apical and basolateral release of 5-HT from EC cells occurs in response to mechanical and various chemical stimulants (20). Once released, 5-HT is taken up by surrounding IECs and immune cells by the serotonin reuptake transporter (SERT) (20). In patients with active IBD, increases in EC cell numbers and 5-HT content have been documented (21–23). Recent studies on experimental models of intestinal inflammation illustrate that inhibiting 5-HT production by genetic deletion of Tph1 or by pharmacological manipulation ameliorates inflammation (24–26). We recently demonstrated the impact of 5-HT on microbial composition and subsequent effects on the susceptibility to inflammation in the intestine (27). Furthermore, Fung et al. (28) have also reported that 5-HT has direct effects on gut bacteria and their colonization. However, the signaling pathway by which 5-HT regulates gut microbiota remains to be determined.
Evidence that increased 5-HT mediates impaired autophagy in human hepatocellular carcinoma cells, murine lacrimal gland, and rat dorsal raphe nucleus (29–31) supports potential interactions between 5-HT and autophagy. While dysregulated 5-HT signaling, autophagy, and microbiota are implicated in intestinal inflammation, the potential interactions among these three factors in the pathogenesis of intestinal inflammation remain unclear. In this study, by using in vitro cell culture systems, experimental models of colitis, and biopsy and blood samples from patients with CD, we investigated interactions between mucosal 5-HT signaling, autophagy, and microbiota in the regulation of intestinal inflammation. Our study demonstrates that increased 5-HT in the colon inhibits autophagy, resulting in altered microbiota composition and increased severity of inflammation, and thus identifies 5-HT as a novel regulator of autophagy-microbiota axis in gut inflammation.
RESULTS
Reduced 5-HT in the gut increases autophagy in the colon and decreases the severity of intestinal inflammation
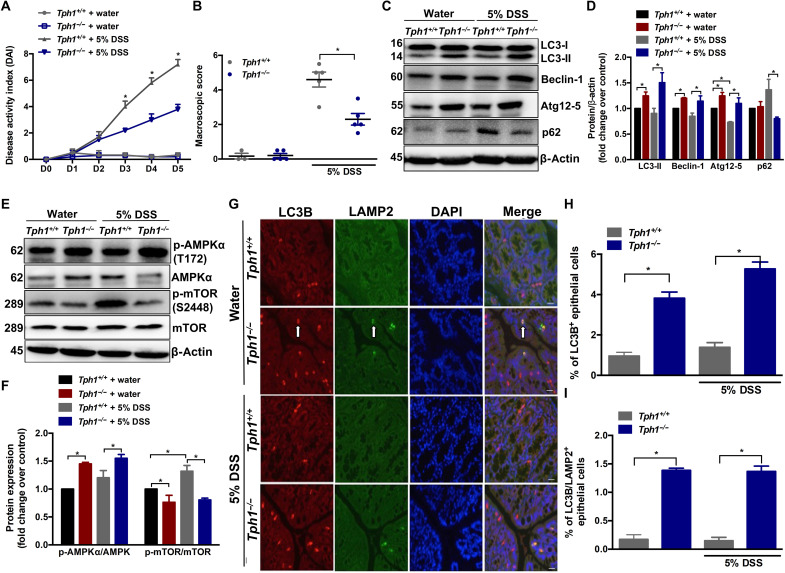
To investigate the linkage between 5-HT and autophagy in the context of intestinal inflammation, we induced acute colitis by administration of either 5% dextran sulfate sodium (DSS) for 5 days or 2.5% DSS for 7 days in drinking water to Tph1–deficient mice (Tph1−/− mice that have significantly reduced 5-HT in gut) and determined the level of autophagy in whole sections of colonic tissues. Similar to our previous findings (24), there was a significant reduction in the severity of colitis in Tph1−/− mice compared to Tph1+/+ mice during both 5 and 2.5% DSS administration (Fig. 1, A and B, and fig. S1, A to E). However, the difference in severity of colitis between Tph1+/+ and Tph1−/− mice was less distinct at 2.5% DSS when compared to the difference in colitis at 5% as indicated by the disease activity index (DAI) (Fig. 1A and fig. S1A). On day 5 after 5% DSS, the Tph1−/− mice had significantly higher levels of p-AMPKα and the autophagy markers, the lipidated form of LC3 (LC3-II), Beclin-1 and Atg12-5 conjugate, and reduced p62 and p-mTOR (S2448), in colon tissues compared to Tph1+/+ mice (Fig. 1, C to F). Control Tph1−/− mice also had increased levels of autophagy proteins compared to control Tph1+/+ group (Fig. 1, C to F). Lysosome-associated membrane protein 2 (LAMP2) is an integral membrane protein of lysosomes and autolysosomes. Since costaining of cells for LC3 and LAMP2 reflects the fusion of autophagosomes and lysosomes, it was used to measure functional autophagy (32). Immunofluorescence analysis revealed significantly increased LC3B+ IECs and LC3B/LAMP2+ double-stained IECs in the Tph1−/− mice regardless of DSS administration (Fig. 1, G to I). On day 7 after 2.5% DSS, the Tph1−/− mice had significantly increased levels of autophagy markers p-AMPKα, LC3-II, and Atg12-5 and decreased p62 and p-mTOR (S2448) in colon tissues compared to Tph1+/+ group (fig. S1, F to I). In separate experiments, Tph1+/+ and Tph1−/− mice were administered 5% DSS and euthanized, and mucosal scrapings were taken. Autophagy markers LC3-II, Beclin-1, and Atg12-5 were significantly increased in the mucosal scraping isolated from Tph1−/− mice compared to Tph1+/+ counterparts (fig. S2, A and B).
Fig. 1. Effects of reduced 5-HT on autophagy in the whole colon of mice with DSS-induced colitis.
DSS (5%) was given to Tph1+/+ and Tph1−/− mice in drinking water for 5 days to induce colitis. Control groups received autoclaved drinking water. Each group contained three to five mice. (A) DAI. *P < 0.05 between colitic Tph1+/+ and Tph1−/− mice on days 3 to 5. (B) Macroscopic damage score on day 5 after DSS and in control mice. (C) Western blot images and (D) quantification of autophagy proteins LC3, Beclin-1, Atg12-5, and p62. (E) Western blot images and (F) quantification of p-AMPKα (T172) and p-mTOR (S2448). Representative Western blot with β-actin is presented as loading controls. The blot shown is representative of three to five random mice in each group. (G) Representative micrograph. Arrows indicate LC3B, LAMP2, and double-stained IECs. Scale bars, 100 μm. (H) Percentage of LC3B+ and (I) percentage of LC3B/LAMP2+ double-stained IECs in colonic sections immunostained for LC3B and LAMP2. Bar graph represents means ± SEM. *P < 0.05 for graphs (B), (D), (F), (H), and (I).
To determine whether blocking 5-HT signaling in the gut can promote autophagy and alleviate the severity of inflammation, we treated colitic C57BL/6 mice orally with the Tph1 inhibitor, LX1031, on days 2 to 5 of 5% DSS administration. Treatment with LX1031 resulted in significantly reduced 5-HT level in the whole colon tissue (fig. S3A) along with decreased day 5 DAI, macroscopic scores, histological damage scores, and levels of the proinflammatory cytokine interleukin (IL)-6 (fig. S3, B to F) compared to the vehicle-treated group. LX1031 promoted autophagy in the colitic mice as indicated by the significant increase in the autophagy proteins LC3-II, Beclin-1, and Atg12-5 and by the reduction of p62 (fig. S3, G and H).
Replenishing 5-HT in Tph1−/− mice impairs autophagy and increases the severity of intestinal inflammation
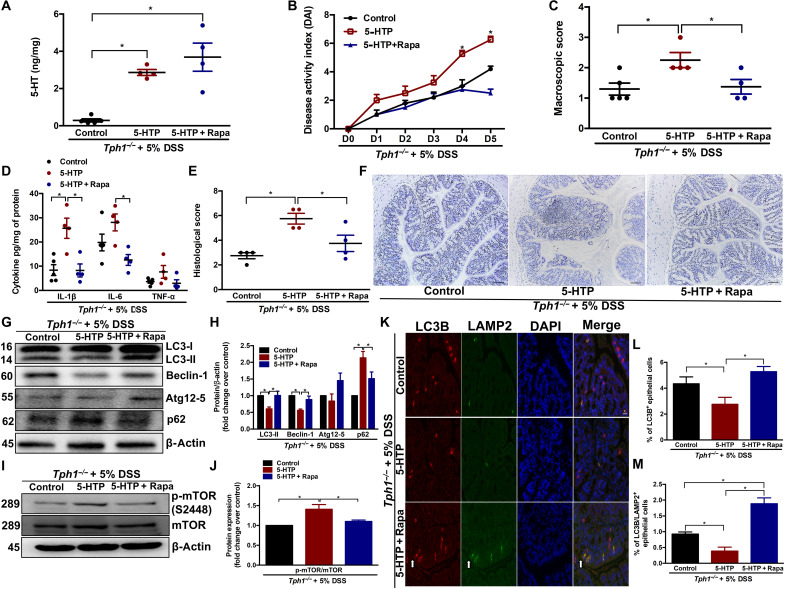
To determine whether the increased autophagy in Tph1−/− mice was due to the lack of 5-HT, we restored 5-HT levels in Tph1−/− mice by subcutaneous injection of 5-HTP, the immediate precursor of 5-HT. As expected, 5-HTP–treated Tph1−/− mice had significantly increased amounts of 5-HT in the colon (Fig. 2A) compared to vehicle-treated Tph1−/− mice. Restoring 5-HT in the Tph1−/− mice increased the severity of DSS colitis (24) as determined by increased day 4 and day 5 DAI, macroscopic scores, histological damage scores, and levels of the proinflammatory cytokine IL-1β (Fig. 2, B to F). The increase in DSS colitis severity in Tph1−/− mice after restoration of 5-HT levels was associated with impaired autophagy in the colon as indicated by a reduction in LC3-II and Beclin-1 and accumulation of p62 and p-mTOR (Fig. 2, G to J). In addition, functional autophagy indicated by LC3B/LAMP2 costaining in the IECs was significantly decreased in Tph1−/− mice after restoration of 5-HT compared to vehicle-treated Tph1−/− mice (Fig. 2, K to M).
Fig. 2. Effect of 5-HTP and rapamycin treatment in Tph1−/− mice with DSS-induced colitis.
DSS (5%) was given to Tph1−/− mice in drinking water for 5 days to induce colitis along with 5-HTP (50 mg/kg twice a day), 5-HTP + rapamycin (1.5 mg/kg once a day), or vehicle treatment. Each group contained four to five mice. (A) Colonic 5-HT levels. (B) DAI. *P < 0.05 between control and 5-HTP as well as between 5-HTP and 5-HTP + Rapa (rapamycin) on days 4 and 5. (C) Macroscopic damage score on day 5 after DSS. Colon tissue was collected to determine (D) IL-1β, IL-6, and TNF-α; (E) histological score; and (F) representative micrographs on day 5 after DSS. Scale bars, 100 μm. (G) Western blot images and (H) quantification of autophagy proteins LC3, Beclin-1, Atg12-5, and p62. (I) Western blot images and (J) quantification of p-mTOR (S2448). Representative Western blot with β-actin as loading controls. Blots are representative of three to four random mice in each group. (K) Representative micrograph. Arrows indicate LC3B, LAMP2, and double-stained IECs. Scale bar, 100 μm. (L) Percentage of LC3B+ and (M) percentage of LC3B/LAMP2+ double-stained IECs in colonic sections. Data are represented as means ± SEM. *P < 0.05 for graphs (A), (C), (D), (E), (H), (J), (L), and (M).
To investigate whether inducing autophagy reverses the enhanced severity of DSS colitis in Tph1−/− mice after 5-HT restoration, we simultaneously treated a separate group of Tph1−/− mice with 5-HTP and rapamycin, an mTOR-dependent autophagy inducer. Mice cotreated with rapamycin and 5-HTP had restored autophagy as indicated by the increase in LC3-II and Beclin-1 and reduction of p62 and p-mTOR (Fig. 2, G to J). In addition, functional autophagy indicated by LC3B/LAMP2 costaining in the IECs was significantly increased in mice cotreated with rapamycin and 5-HTP compared to mice receiving only 5-HTP (Fig. 2, K to M). Compared to mice receiving only 5-HTP, the rapamycin-treated group showed significant reduction in inflammation severity as indicated by reduced day 4 and day 5 DAI, macroscopic scores, histological damage scores, and expression of the proinflammatory cytokines IL-1β and IL-6 (Fig. 2, B to F).
Elevated 5-HT in SERT-deficient mice impairs autophagy and increases the severity of intestinal inflammation
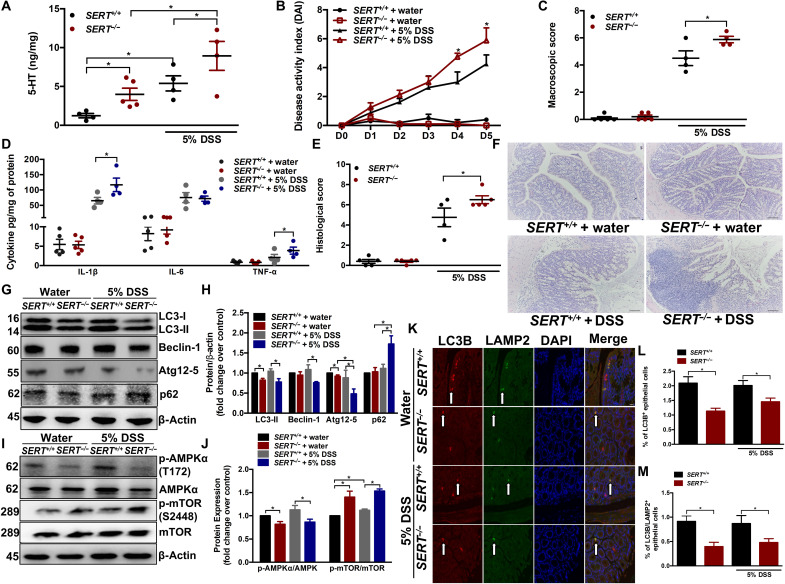
To further substantiate the effect of 5-HT on autophagy in intestinal inflammation, we evaluated autophagy in mice deficient in SERT (SERT−/−) after DSS administration. Deletion of SERT increases the availability of 5-HT in the bowel (33). SERT−/− mice had a significantly increased amount of 5-HT in the colon compared to SERT+/+ mice (Fig. 3A). Intestinal inflammation was exacerbated in SERT−/− mice compared to SERT+/+ mice, as demonstrated by higher day 4 and day 5 DAI, macroscopic scores, histological damage scores, and increased IL-1β and tumor necrosis factor–α (TNF-α) levels (Fig. 3, B to F). Reduction in p-AMPKα, LC3-II, Beclin-1, and Atg12-5 and accumulation of p62 and p-mTOR in SERT−/− mice compared to SERT+/+ counterparts on day 5 after DSS were observed, suggesting an impairment in autophagic flux (Fig. 3, G to J). In the noncolitic group, SERT−/− mice also had decreased amounts of autophagy proteins LC3-II, Atg12-5, and p-AMPKα along with elevated p-mTOR level compared to SERT+/+ mice (Fig. 3, G to J). The percentage of LC3B/LAMP2+ IECs was significantly reduced in both colitic and noncolitic SERT−/− mice compared to SERT+/+, signifying a decrease in autophagic flux (Fig. 3, K to M). These findings further demonstrate an important role of gut 5-HT levels in influencing susceptibility to intestinal inflammation.
Fig. 3. Effect of elevated intestinal 5-HT on DSS-induced colitis and autophagy.
DSS (5%) was given to SERT+/+ and SERT−/− mice in drinking water for 5 days to induce colitis. Control groups received autoclaved drinking water. Each group contained four to five mice. (A) Colonic 5-HT amount. (B) DAI. *P < 0.05 between colitic SERT+/+ and SERT−/− on days 4 and 5. (C) Macroscopic damage score on day 5 after DSS and in control mice. Colon tissue was collected to determine (D) IL-1β, IL-6, and TNF-α; (E) histological score; and (F) representative micrographs on day 5 after DSS. Scale bars, 100 μm. (G) Western blot images and (H) quantification of autophagy proteins LC3, Beclin-1, Atg12-5, and p62. (I) Western blot images and (J) quantification of p-AMPKα (T172) and p-mTOR (S2448). Representative Western blot with β-actin as loading controls. Data are representative of three to four random mice in each group. (K) Representative micrograph. Arrows indicate LC3B, LAMP2, and double-stained IECs. Scale bar, 100 μm. (L) Percentage of LC3B+ and (M) percentage of LC3B/LAMP2+ double-stained IECs in colonic sections. Data are represented as means ± SEM. *P < 0.05 for graphs (A), (C), (D), (E), (H), (J), (L), and (M).
Reduced 5-HT in the gut up-regulates autophagy in IECs
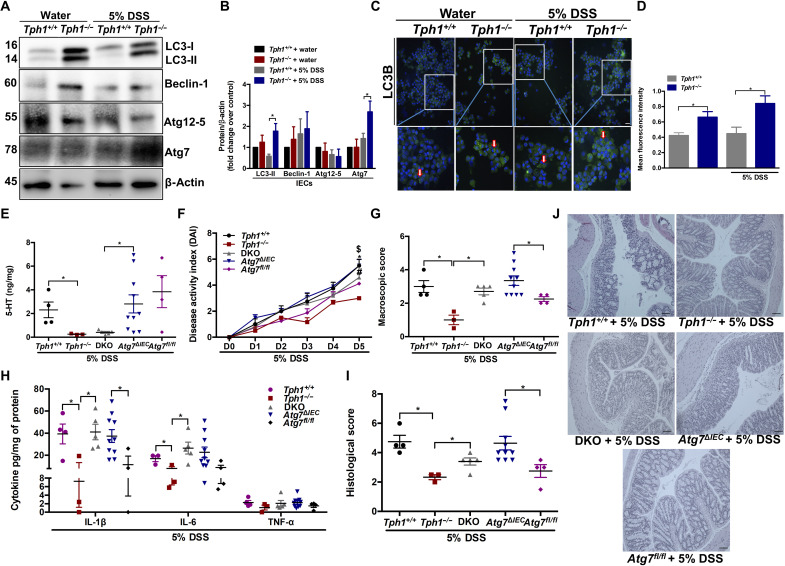
On the basis of the importance of IECs in the pathogenesis of intestinal inflammation (4, 5) and our observations regarding increased autophagy in the mucosal scrapings of Tph1−/− mice, we next investigated whether 5-HT influences autophagy in IECs. Tph1−/− and Tph1+/+ mice were administered DSS and euthanized, and IECs were isolated and confirmed by E-cadherin staining (fig. S4). Western blotting and immunofluorescence analysis revealed significant increase in LC3-II and Atg7 in IECs isolated from Tph1−/− mice on day 5 after DSS (Fig. 4, A to D).
Fig. 4. Impact of 5-HT on autophagy in IECs and the effects of impaired IEC 5-HT–autophagy axis on colitis.
DSS (5%) was given to Tph1+/+ and Tph1−/− mice in drinking water for 5 days to induce colitis. Control groups received autoclaved drinking water. Each group contained three to four mice. IECs were isolated from the colon. (A) Western blot images and (B) quantification of autophagy proteins LC3, Beclin-1, Atg12-5, and Atg7 in the IECs. Representative Western blot with β-actin as loading controls. Data are representative of three to four random mice in each group. (C) Representative micrograph. Arrows indicate LC3B+ puncta in the IECs. Scale bar, 100 μm. (D) Mean fluorescence intensity of LC3B staining in isolated IECs. DSS (5%) was given to Tph1+/+, Tph1−/−, DKO, Atg7ΔIEC, and Atg7fl/fl mice in drinking water for 5 days to induce colitis. Each group contained 3 to 10 mice. (E) Colonic 5-HT amount. (F) DAI. $P < 0.05 between Tph1+/+ and Tph1−/−, *P < 0.05 between Atg7ΔIEC and Atg7fl/fl, and #P < 0.05 between DKO and Tph1−/−. (G) Macroscopic damage score on day 5 after DSS. Colon tissue was collected to determine (H) IL-1β, IL-6, and TNF-α; (I) histological score; and (J) representative micrographs on day 5 after DSS. Scale bars, 100 μm. Data are represented as means ± SEM. *P < 0.05 for graphs (B), (D), (E), (G), (H), and (I).
In addition, 5-HT treatment significantly reduced LC3-II, Beclin-1, and Atg12-5 levels and enhanced expression of p62 and p-mTOR in human colonic epithelial HT-29 cells (fig. S5, A and B). 5-HT also reduced LC3-II and increased p62 expression in mouse primary IECs compared to the control (fig. S5, C and D). Furthermore, inhibition of autophagy by 5-HT treatment of HT-29 cells significantly increased the secretion of proinflammatory cytokine IL-8 compared to the control group (fig. S6C).
5-HT influences autophagy of IECs via 5-HT receptors
5-HT mediates various functions in the gut by acting on 5-HT receptors. Of the 14 subtypes of 5-HT receptors, 5-HT3, 5-HT4, and 5-HT7 receptors are mainly expressed in IECs and HT-29 cell line (34, 35). To determine which 5-HT receptor expressed on the enterocytes is involved in regulation of autophagy, we evaluated the effects of 5-HT3, 5-HT4, and 5-HT7 receptor–specific antagonists (tropisetron, RS39604, and SB269970) on autophagy in HT-29 cells and in mouse primary IECs. In HT-29 cells, 5-HT–induced alteration in the autophagy proteins Atg12-5 and p62 was significantly blocked by the 5-HT3 receptor antagonist tropisetron (fig. S5, A and B). 5-HT4 receptor antagonist, RS39604, reversed the 5-HT–induced changes in Beclin-1, Atg12-5, and p62 in HT-29 cells (fig. S5, A and B). In HT-29 cells, 5-HT–induced reduction in the autophagy proteins LC3-II, Beclin-1, and Atg12-5 and elevation of p-mTOR and p62 were completely reversed by the 5-HT7 receptor antagonist, SB269970 (fig. S5, A and B). In addition, in mouse primary IECs, 5-HT–induced alteration in the autophagy proteins LC3-II and p62 was significantly blocked by the 5-HT3 receptor antagonist tropisetron, 5-HT4 receptor antagonist RS39604, and 5-HT7 receptor antagonist SB269970 (fig. S5, C and D). These findings indicate that although 5-HT impairs autophagy via all three 5-HT receptors expressed on IECs, 5-HT7 receptor might be playing a major role. Together, these findings suggest an important role of 5-HT and its receptors in the regulation of autophagy in IECs.
Disruption of the autophagy gene, Atg7, in IECs abolishes the protective effects of reduced 5-HT in Tph1−/− mice during DSS-induced colitis
Atg7, an E1-like enzyme, is a critical factor for Atg12 and LC3 conjugation and autophagosome formation and is thus essential for autophagy (8). To determine whether reduced severity of inflammation in the intestines of Tph1−/− mice arises from up-regulated autophagy in IECs, double-knockout (DKO) mice deficient in both Tph1 and IEC-specific Atg7 were generated (fig. S7). Deletion of Atg7 from IECs was confirmed by a marked reduction in immunofluorescence staining of LC3B+ IECs isolated from the colon of Atg7ΔIEC mice (fig. S8A). The level of LC3-II was markedly reduced in the DKO mice compared to Tph1−/− and increased in comparison to Atg7ΔIEC mice (fig. S8, B and C). In addition, there was no difference between the 5-HT levels in the colon of the naïve Atg7ΔIEC mice and the Atg7fl/fl mice (fig. S9).
Acute colitis was induced in Tph1+/+, Tph1−/−, DKO, Atg7ΔIEC, and Atg7fl/fl mice by oral administration of DSS. Both the Tph1−/− and DKO mice had reduced 5-HT levels in the colon (Fig. 4E). As demonstrated previously, Atg7ΔIEC mice exhibited exacerbated inflammation compared to the Atg7fl/fl mice (8), reflected by elevation in several parameters of inflammation such as DAI, macroscopic scores, histological damage scores, and proinflammatory cytokine IL-1β (Fig. 4, F to J). The protective effect of reduced 5-HT in the Tph1−/− mice was abrogated when mice lacked the autophagy protein, Atg7, in the IECs, demonstrated by an increase in day 5 DAI, macroscopic scores, histological damage scores, and proinflammatory cytokines IL-1β and IL-6 in DKO mice compared to Tph1−/− mice on day 5 after DSS (Fig. 4, F to J).
Tph1 and IEC-specific Atg7 DKO mice exhibit altered gut microbiota composition
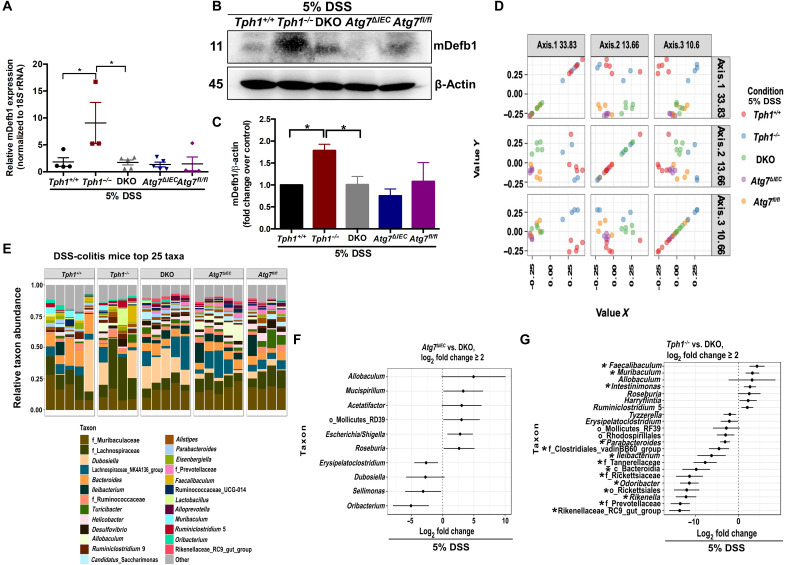
Because the severity of DSS-induced colitis was markedly increased in DKO mice compared to Tph1−/− mice, we hypothesized that the disruption of autophagy in IECs abolished the protective effects in colitis in Tph1−/− mice by modulating gut microbial composition via influencing antimicrobial peptide production from IECs. IECs constitutively produce antimicrobial peptide, β-defensin-1 (mDefb1) (36). We observed that DSS-receiving Tph1−/− mice had increased mDefb1 mRNA expression and protein levels compared with colitic DKO mice, whereas colitic DKO and Atg7ΔIEC mice had similar levels of mDefb1 (Fig. 5, A to C). To examine the gut microbiota, we compared cecal bacterial profiles by 16S ribosomal RNA (rRNA) sequencing of Tph1+/+, Tph1−/−, DKO, Atg7ΔIEC, and Atg7fl/fl mice after DSS administration. For each group, the microbial composition was dominated by Firmicutes, Proteobacteria, and Bacteroidetes (fig. S10A). The five groups of mice separated into distinct clusters shown by the visualization of Bray-Curtis diversity by principal coordinate ordination (PCoA) (Fig. 5D). In all pairings, the differences between the groups were significant with a false discovery rate of <5%. Mouse genotype significantly accounted for 54% of the variation, while 46% of the variation was due to residual factors that were not significant. Taxonomic comparisons revealed greater similarity between the DKO and Atg7ΔIEC mice and more dissimilarity between DKO and Tph1−/− mice (Fig. 5E and fig. S10B). Despite deficiencies in both Tph1 and IEC Atg7, the microbial composition of DKO mice was not an aggregate of Tph1−/− and Atg7ΔIEC mice but showed higher similarity to Atg7ΔIEC mice. The graphs in Fig. 5 (F and G) and table S2 illustrate that 16 bacterial taxa in Tph1−/− mice and no bacterial taxa in Atg7ΔIEC mice were significantly different from those of DKO mice. In Tph1−/− mice, beneficial bacteria such as Faecalibaculum were higher, while colitogenic bacteria such as Parabacteroides and Rikenella were decreased compared to DKO mice (Fig. 5G).
Fig. 5. Effect of impaired IEC 5-HT–autophagy axis on gut microbial composition during DSS colitis.
DSS (5%) was given to Tph1+/+, Tph1−/−, DKO, Atg7ΔIEC, and Atg7fl/fl mice in drinking water for 5 days to induce colitis. Each group contained four to five mice. (A) Quantification of mDefb1 mRNA in the colonic tissue. (B) Western blot images and (C) quantification of mDefb1 protein in the colonic tissue. *P < 0.05 for graphs (A) and (C). 16S rRNA sequencing of cecal content of all groups was performed. (D) PCoA of Bray-Curtis dissimilarity. (E) Taxonomic comparison of gut microbiota in the five experimental groups. (F) With DKO as the baseline, all taxa in the Atg7ΔIEC group for which one log2 fold change is ≥2. (G) With DKO as the baseline, all taxa in the Tph1−/− group for which one log2 fold change is ≥2. *P < 0.01 for graph (G).
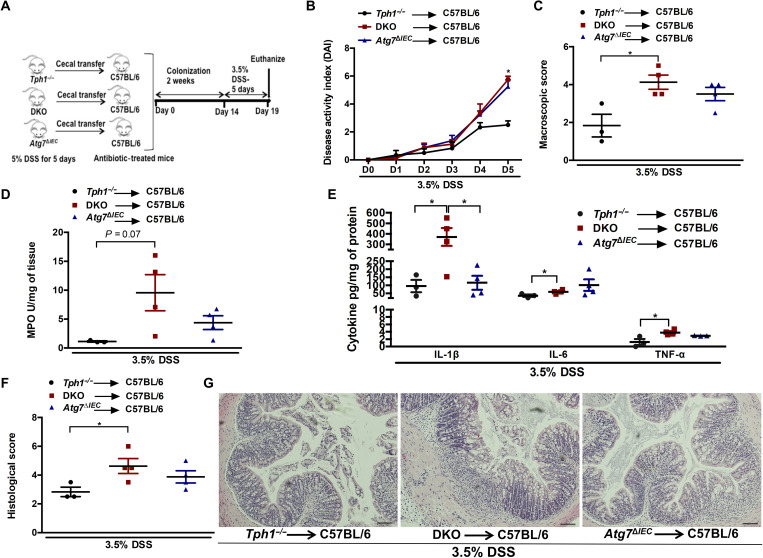
Transfer of colitogenic gut microbiota from DKO mice increases the severity of DSS-induced colitis in antibiotic-treated naïve mice
To further determine whether the altered microbiota in the DKO mice influences the severity of intestinal inflammation, we transferred the cecal content of DSS-receiving Tph1−/− or DKO or Atg7ΔIEC mice to antibiotic-treated C57BL/6 mice, followed by administration of DSS to induce colitis (Fig. 6A). The microbiota profile of the DSS-treated donor and their recipient mice was analyzed by 16S rRNA sequencing of fecal samples to determine how closely the recipients resemble their donors. Bray-Curtis analysis, Jaccard plot, taxonomic composition, and the mean relative abundance demonstrate that among the three pairs of donors and recipients, the Tph1−/− recipients are closest to the Tph1−/− donors (fig. S11, A to D). The clustering of the DKO donors and recipients and Atg7ΔIEC donors and recipients is apparent in axis 1 of the Jaccard plot (fig. S11B). The microbiota of the DKO and Atg7ΔIEC recipients grouped together, whereas the Tph1−/− recipients formed distinct separate clusters (fig. S11, A and B). The recipient mice microbiota had a significant proportion of the donor amplicon sequence variants (ASVs) present, indicated by 75.2, 61.2, and 59.4% of ASVs for Tph1−/−, DKO, and Atg7ΔIEC donors, respectively (fig. S11E).
Fig. 6. Assessment of severity of colitis following adoptive transfer of DSS-treated microbiota to antibiotic-treated C57BL/6 mice.
(A) Schematic of cecal microbiota transfer followed by induction of colitis with 3.5% DSS. Each group had three to four mice. (B) DAI. *P < 0.05 between C57BL/6 mice receiving DKO microbiota and C57BL/6 mice receiving Tph1−/− microbiota. (C) Macroscopic damage score on day 5 after DSS. Colon tissue was collected to determine (D) MPO activity; (E) IL-1β, IL-6, and TNF-α; (F) histological score; and (G) representative micrographs on day 5 after DSS. Scale bars, 100 μm. Data are represented as means ± SEM. *P < 0.05 for graphs (C), (D), (E), and (F).
Higher levels of day 5 DAI, macroscopic and histological damage scores, myeloperoxidase (MPO) activity, and proinflammatory cytokines IL-1β, IL-6, and TNF-α were observed in antibiotic-treated mice colonized with microbiota from DKO mice as compared to those colonized with microbiota from Tph1−/− mice (Fig. 6, B to G). There was no significant difference in colitis severity between the mice receiving microbiota from DKO mice and mice receiving microbiota from Atg7ΔIEC mice except for IL-1β levels. These results suggest that deletion of this autophagy-related gene Atg7 in Tph1−/− mice promotes the development of a colitogenic microbiota in DKO mice that contributes to the differences in severity of inflammation. The comparison of donor and recipient microbiota revealed that the more colitic DKO and Atg7ΔIEC donor and recipient groups had significantly higher levels of bacteria belonging to the genera Dubosiella and Turicibacter compared to the less colitic Tph1−/− donor and recipient (figs. S12 and S13). However, some members of Lachnospiraceae were increased, while others were reduced in the more colitic groups (figs. S12 and S13).
Naïve DKO mice have altered gut microbiota composition that mediates the increased severity of DSS-induced colitis
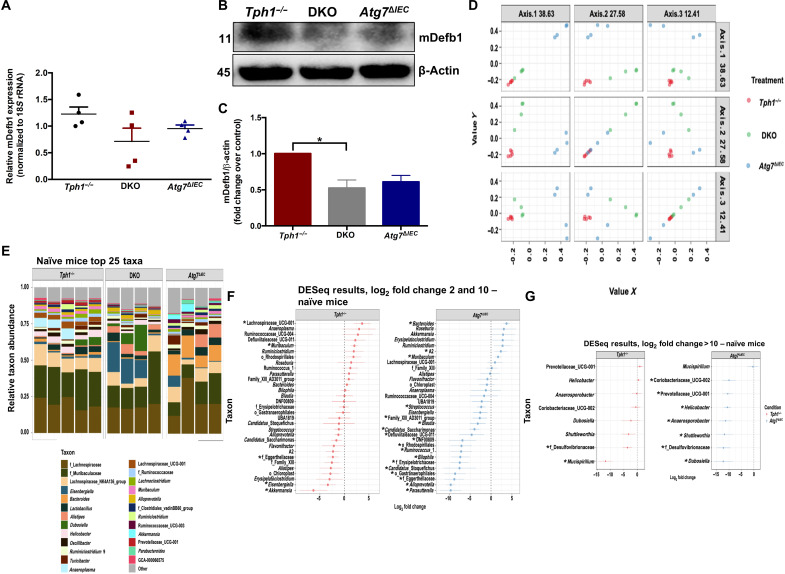
To further investigate whether the microbial changes seen in DKO mice were due to deficiency of the 5-HT–autophagy axis in the IECs, we analyzed the expression of the antimicrobial peptide mDefb1 in the naïve state and the naïve microbiota of Tph1−/−, DKO, and Atg7ΔIEC mice. We observed that naïve Tph1−/− mice had increased mDefb1 mRNA and protein levels compared with naïve DKO mice, whereas naïve DKO and Atg7ΔIEC mice had similar levels of mDefb1 (Fig. 7, A to C). 16S rRNA sequencing revealed that the naïve DKO mice were separated into distinct clusters compared to Tph1−/− and Atg7ΔIEC groups, as shown by the PCoA and taxonomic composition of the gut microbiota (Fig. 7, D and E, and fig. S14A). Genetic differences were found to be a significant driver of variation, with 63% of the variation attributable to genotype. In all pairings, the differences between the groups are significant with a false discovery rate of <5%. Seven bacterial taxa in the Tph1−/− mice and 26 taxa in the Atg7ΔIEC mice were significantly different from those in the DKO mice (Fig. 7, F and G, and table S3). Tph1−/− mice had increased amounts of Lachnospiraceae and reduced levels of Mucispirillum and Desulfovibrionaceae compared to DKO mice (Fig. 7, F and G).
Fig. 7. Effect of impaired IEC 5-HT–autophagy axis on gut microbial composition of naïve mice.
(A) Quantification of mDefb1 mRNA in the colonic tissue. (B) Western blot images and (C) quantification of mDefb1 protein in the colonic tissue. *P < 0.05 for graph (C). 16S rRNA sequencing was performed on the cecal content from naïve Tph1−/−, DKO, and Atg7ΔIEC mice. Each group had four to five mice. (D) PCoA of Bray-Curtis dissimilarity. (E) Taxonomic composition of the gut microbiota. (F) With DKO as the baseline, all taxa in the Tph1−/− and Atg7ΔIEC groups for which one log2 fold change is 2 to 10. (G) With DKO as the baseline, all taxa in the Tph1−/− and Atg7ΔIEC groups for which one log2 fold change is >10. *P < 0.01 for graphs (F) and (G).
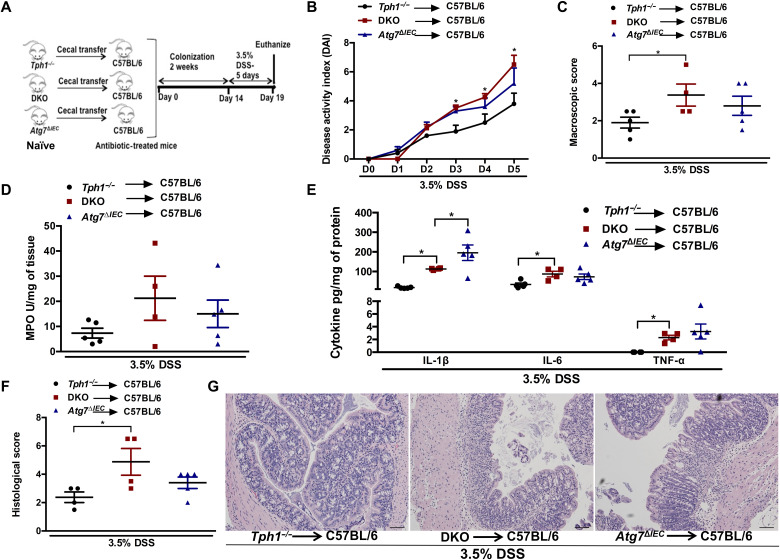
Following transplantation of naïve microbiota, mice receiving microbiota from DKO mice showed elevated parameters of intestinal inflammation such as day 3 to day 5 DAI, macroscopic and histological damage scores, and proinflammatory cytokines IL-1β, IL-6, and TNF-α compared to those receiving Tph1−/− microbiota (Fig. 8, B, C, and E to G). There was no difference in colitis severity between the groups receiving microbiota from DKO and Atg7ΔIEC mice except for IL-1β levels. These findings further suggest that the change in microbiota caused by disruption of the 5-HT–autophagy axis in the IECs mediates the severity of inflammation.
Fig. 8. Assessment of severity of colitis following adoptive transfer of naïve microbiota to antibiotic-treated C57BL/6 mice.
(A) Schematic of cecal microbiota transfer followed by induction of colitis with 3.5% DSS. Each group had four to five mice. (B) DAI. *P < 0.05 between C57BL/6 mice receiving DKO microbiota and C57BL/6 mice receiving Tph1−/− microbiota. (C) Macroscopic damage score on day 5 after DSS. Colon tissue was collected to determine (D) MPO activity; (E) IL-1β, IL-6, and TNF-α; (F) histological score; and (G) representative micrographs on day 5 after DSS. Scale bars, 100 μm. Data are represented as means ± SEM. *P < 0.05 for graphs (C), (D), (E), and (F).
5-HT treatment of human peripheral blood mononuclear cells inhibits autophagy and is reversed by rapamycin
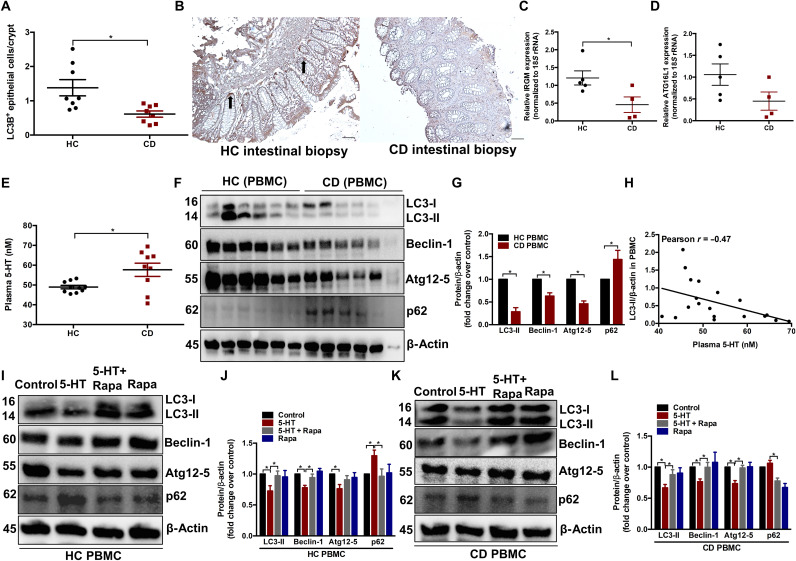
To substantiate our findings in experimental models, we next sought to investigate whether 5-HT plays a role in autophagy in human individuals with or without CD using biopsy and blood samples. In the initial cohort, colonic biopsy samples were obtained from eight healthy controls (HCs) and eight patients with active CD. LC3B+ IECs were significantly lower in the inflamed tissue of patients with CD compared to the noninflamed tissue of the HCs (Fig. 9, A and B). Four to five HCs and active CD colonic biopsy samples were randomly selected from the initial cohort to measure the key CD risk factors IRGM and ATG16L1, which are critical in the autophagy pathway (3). IRGM mRNA expression was significantly reduced in patients with active CD compared to HCs (Fig. 9C).
Fig. 9. Effect of 5-HT on autophagy in HCs and patients with CD.
(A) Number of LC3B-expressing IECs per crypts in colonic sections in cohort 1 HCs (n = 8) and patients with CD (n = 8). (B) Representative micrograph. Arrows indicate LC3B+ IECs. Scale bars, 100 μm. (C) Quantification of IRGM and (D) ATG16L1 mRNA expression in the colonic mucosal biopsies from four to five randomly selected HCs and patients with active CD in cohort 1. (E) Comparison of plasma 5-HT concentration between HCs (n = 10) and patients with CD (n = 10) in cohort 2. (F) Western blot images and (G) quantification of autophagy proteins (LC3, Beclin-1, Atg12-5, and p62) performed on protein extracts obtained from PBMCs of cohort 2. Each lane represents an individual participant, and the bar graph represents means ± SEM. (H) Significant negative correlation between plasma 5-HT and PBMC LC3-II levels. PBMCs from either HCs or CD in cohort 2 were treated in the presence of phytohemagglutinin (PHA; 5 μg/ml) with vehicle, 5-HT (10 μM), or rapamycin (50 nM) or cotreated with 5-HT and rapamycin for 6 hours, and the extracted protein was analyzed for expression of LC3, Beclin-1, Atg12-5, and p62. (I and J) Western blot images and quantification graphs in HCs and (K and L) Western blot images and quantification graphs in CD. Representative Western blot with β-actin as loading controls. Blots shown are representative of 10 HCs and 10 patients with CD from cohort 2. Bar graph represents means ± SEM. *P < 0.05 for graphs (A), (C), (E), (G), (J) and (L).
In another cohort, blood samples were collected from 10 HCs and 10 patients with active CD indicated by symptoms, endoscopy, and high C-reactive protein (CRP) and fecal calprotectin levels (table S1). To determine the effect of 5-HT signaling in the regulation of autophagy in the gut, we used peripheral blood mononuclear cells (PBMCs) isolated from whole blood, since abnormalities in these cells are often reflective of disorders within the gut (37). The higher plasma 5-HT in patients with CD (Fig. 9E) was associated with impaired autophagy in PBMCs, determined by significantly reduced LC3-II, Beclin-1, and Atg12-5 and accumulated p62 compared to HCs (Fig. 9, F and G). In addition, there was a significant negative correlation between levels of plasma 5-HT and autophagic flux in the PBMC denoted by LC3-II/β-actin levels (Fig. 9H). Compared to the control, treatment of PBMCs with 5-HT resulted in the down-regulation of autophagy proteins LC3-II, Beclin-1, and Atg12-5 and accumulation of p62 in both HCs and patients with CD. This was reversed by cotreatment with rapamycin (Fig. 9, I to L). These findings indicate that 5-HT–mediated impairment of autophagy in both patients with CD and HCs is through the activation of the mTOR signaling pathway.
DISCUSSION
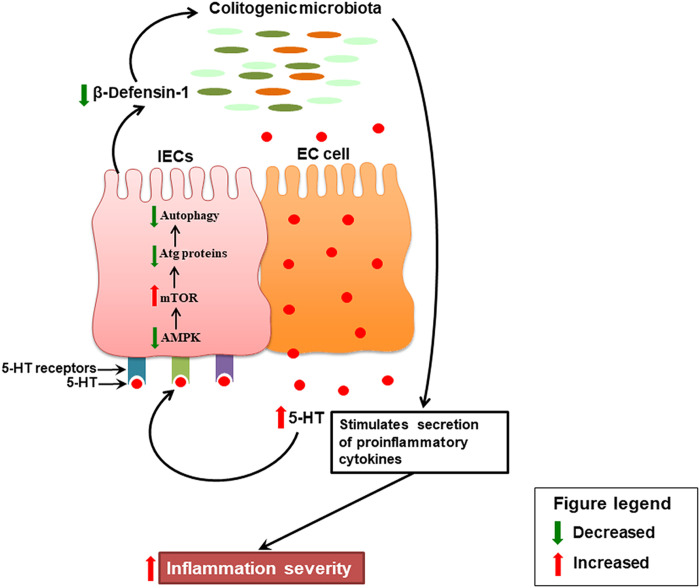
IBD is a chronic inflammatory condition of the human bowel currently affecting several million people worldwide (1). Although the exact etiology of IBD is unknown, studies provide evidence that dysregulated immune responses, genetic factors, gut microbiota, and environmental factors contribute to the pathogenesis of IBD (38). 5-HT is a key enteric signaling molecule, and changes in gut 5-HT signaling are observed in IBD and in experimental colitis (21, 23, 39, 40). Evidence suggests that autophagy, the body’s process of clearing out old and damaged cells, is disrupted in IBD (38). Polymorphisms in autophagy genes result in impaired stress activation of autophagy, establishing a state of chronic inflammation that predisposes individuals to CD (41). Although changes in 5-HT, microbiota, and autophagy are associated with IBD, it remained to be determined whether a tripartite relationship exists among these factors. As illustrated in Fig. 10, in the current study, we find that increased 5-HT signaling impairs AMPKα/mTOR-dependent autophagy in the colon, which results in up-regulation of inflammation severity by increasing the production of proinflammatory cytokines and by selecting for a colitogenic microbiota.
Fig. 10. Schematic representation of the data.
During colitis, increased mucosal 5-HT secreted from the EC cells in the colon binds to 5-HT3, 5-HT4, and 5-HT7 receptors on the IECs and inhibits AMPK. Inhibition of AMPK subsequently activates mTOR. Activation of mTOR inhibits formation of autophagy (Atg) proteins that leads to impairment in autophagy in the IECs. This leads to the reduced production of the antimicrobial peptide, b-defensin-1, by the IECs, which alters the composition of the normal gut microbiota. The colitogenic microbiota stimulates the secretion of proinflammatory cytokines (IL-8, IL-1β, IL-6, and TNF-α) from IECs and immune cells and, ultimately, exacerbates the severity of inflammation.
To investigate the impact of 5-HT on autophagy, we studied Tph1−/− and SERT−/− mice, with different levels of 5-HT in the gut. Tph1−/− mice with low levels of 5-HT (24) had decreased severity of colitis and elevated autophagic flux, while SERT−/− mice, with elevated levels of 5-HT, had reduced autophagy and increased severity of colitis. Apart from the colon, it is also reported that SERT gene knockdown in the dorsal raphe nucleus in rats increased 5-HT level and impaired hippocampal autophagy (31). We did not observe differences in autophagy markers between the wild-type colitic and noncolitic mice. Cabrera and colleagues (7) reported up-regulated autophagy in DSS colitis as a protective mechanism by which cells adapt to different types of stressors, whereas Ortiz-Masia et al. (42) observed reduced autophagy in association with colitis. The disparities between these observations might be due to the differences in DSS concentration and duration in these studies. Our findings suggest that an increase in 5-HT in DSS colitis decelerates autophagy that contributes to disease severity. Blocking 5-HT signaling by the administration of Tph1 inhibitor released this brake, promoted autophagy, and alleviated the severity of colitis. Furthermore, our data indicate that increased 5-HT levels before inflammation inhibit autophagy and make the host more susceptible to colitis.
To further understand the role of 5-HT in autophagy in the context of intestinal inflammation, we restored 5-HT level in Tph1−/− mice and observed up-regulated severity of DSS-induced colitis along with impairment in autophagy. Notably, in Tph1−/− mice, the elevation in the severity of intestinal inflammation attributed to the restoration of 5-HT was attenuated by the mTOR inhibitor, rapamycin. Rapamycin also regulates autophagy-independent cellular processes that can result in immunosuppression (13, 43) and alleviation of colitis. Thus, although other mechanisms cannot be ruled out, our results in Fig. 2 strongly suggest that 5-HT–induced impairment of autophagy in the inflamed colon is reversed by rapamycin-mediated rescue of autophagy. These findings were further confirmed by the loss of the protective action of reduced 5-HT in the Tph1−/− mice when the autophagy protein Atg7 was deficient in the IECs. This suggests that 5-HT impairs mTOR-dependent autophagy in the colon of DSS-treated mice and increases the severity of intestinal inflammation. In addition, we observed that increased 5-HT in the colon reduced the expression of p-AMPKα, a known upstream regulator of mTOR (44). Similar to our findings, Guragain et al. (45) reported that 5-HT treatment of colon epithelial cells induced dephosphorylation of AMPKα in a dose-dependent manner. This suggests that increased 5-HT inhibits phosphorylation of AMPKα, leading to activation of mTOR, since it is known that AMPK regulates mTORC1 signaling (6, 44). Although the role of AMPK in autophagy has been reported before (6), this is the first demonstration that 5-HT is an important regulator of the interaction between AMPK and autophagy.
IECs form the barrier between the gut lumen and mucosa and act as the first line of defense against invading microbes and potential pathogens (46). Functional autophagy in the IECs is required to maintain homeostasis and prevent intestinal inflammation (4, 5). The presence of 5-HT receptors on IECs (18), as well as their proximity to 5-HT–secreting EC cells, suggests that 5-HT plays a critical role in maintaining IEC homeostasis. Our study demonstrates that during DSS-induced colitis, reduced 5-HT in the gut promotes autophagy in the IECs. To further study the 5-HT–autophagy axis in the IECs, we generated DKO mice that were deficient in Tph1 globally and in Atg7 in the IECs. The protective effect of reduced 5-HT in the Tph1−/− mice against DSS-induced colitis was abrogated when the Tph1−/− mice were deficient in autophagy in the IECs. Recently, we demonstrated that blocking 5-HT7 receptor signaling with an antagonist or genetic deletion of this receptor reduced the severity of chemical colitis and increased antimicrobial peptide mDefb1 and mDefb3 productions (27, 47). Another study demonstrated that 5-HT4 receptor stimulation via enema administration had a protective effect in experimental colitis but was not protective when delivered by intraperitoneal injection (48). This protective effect was associated with enhanced proliferation of IECs and increase in gut motility. These findings suggest that 5-HT may incite divergent actions on different cell types in the context of colitis. Our findings suggest that 5-HT–mediated disruption of autophagy in the IECs is arbitrated by 5-HT3, 5-HT4, and 5-HT7 receptor, with 5-HT7 receptor playing a more potent role. It is unclear how the activation of the G protein–coupled receptors 5-HT4 and 5-HT7 and the ligand-gated ion channel 5-HT3 receptors is linked with the stimulation of the mTOR pathway. A possible mechanism for the 5-HT4 and 5-HT7 receptors to activate mTOR is through the elevation of the second messenger cyclic AMP (49) and subsequent inhibition of AMPK. Activation of the 5-HT3 receptor results in the influx of extracellular calcium ions that mobilizes and increases the concentration of intracellular calcium (49). The regulation of mTOR-dependent autophagy by intracellular calcium remains controversial (50). Therefore, further studies are deserved to elucidate the role of 5-HT receptors expressed on IECs in the regulation of autophagy in CD. In addition to receptor sensing, 5-HT can alter gene expression through serotonylation of histone H3 (51). While little is known on the direct evidence of autophagy regulation via serotonylation, recently, it has been demonstrated that 5-HT treatment of human colon cancer cells activates mTORC1 through serotonylation (52). Exploring the role of serotonylation in autophagy in gut inflammation will further enhance our understanding on the interaction between 5-HT signaling and autophagy.
Disruption of the balanced composition of gut microbiota is associated with IBD. Clinical and animal studies suggest that changed gut microbiota structure and function trigger and perpetuate chronic intestinal inflammation (53). Both the process of autophagy and 5-HT signaling are implicated in the regulation of gut microbiota (8, 27, 54). Despite these findings, it is currently unknown whether increased 5-HT influences autophagy in the IECs and results in altered production of the antimicrobial peptide mDefb1 and thus contributes to dysbiosis. In this study, we observed that, in the context of colitis, the protective effects associated with Tph1−/− microbiota are lost when the IECs have impaired autophagy. These findings also coincided with reduced expression of mDefb1 when the IECs were deficient in autophagy. During colitis in Tph1−/− mice, beneficial bacteria such as Faecalibaculum (55) were higher, while bacteria associated with inflammation such as Parabacteroides and Rikenella (15) were decreased compared to DKO mice. Under the naïve condition, Tph1−/− mice had elevated levels of protective bacteria such as Lachnospiraceae and reduced levels of colitogenic bacteria such as Mucispirillum and Desulfovibrionaceae compared to DKO mice (56, 57). Moreover, Dubosiella and Turicibacter that are increased during colitis (58, 59) were significantly higher in the more colitic DKO and Atg7ΔIEC donors and recipients. Furthermore, the transfer of colitic or naïve DKO microbiota to antibiotic-treated mice was associated with increased inflammation severity. Together, these findings identify the 5-HT–autophagy axis in the IECs as a previously unknown key regulator of gut microbiota during intestinal inflammation.
An increase in plasma/serum 5-HT levels is observed in patients with IBD, and the use of selective 5-HT reuptake inhibitors is associated with microscopic colitis (21, 23, 39, 40, 60, 61). In addition, dysfunction in the autophagic process is implicated in the development of intestinal inflammation in patients with IBD (5). Previous studies demonstrated reduced expression of the autophagy proteins in the inflamed intestinal mucosa of patients with CD (42, 62). In this study, we observed impaired autophagy in the colonic biopsy samples and PBMCs along with elevated plasma 5-HT levels in patients with active CD compared with HCs. The inhibitory role of 5-HT in autophagy was further supported by 5-HT–mediated down-regulation of autophagy in PBMCs isolated from both HCs and patients with CD, which was reversed by treatment with the autophagy enhancer, rapamycin. These findings reflect the translational significance of the 5-HT–autophagy axis in CD. However, since autophagy gene polymorphisms have been associated with CD, an important area for future research is investigating the effect of 5-HT on autophagy in CD patients with and without autophagy gene polymorphisms.
This study is the first to report the important role of the 5-HT–autophagy axis in IECs and its contribution to the regulation of microbiota in relation to susceptibility to intestinal inflammation. Our findings suggest that blocking 5-HT signaling may promote autophagy and alleviate the severity of intestinal inflammation. Given recent reports of impaired autophagy in patients with CD (2, 42) and the established changes in 5-HT production in IBD (20–22), this research sheds light on 5-HT as a novel regulator of autophagy in gut inflammation. These findings may ultimately lead to the discovery of novel therapeutic strategies in intestinal inflammatory conditions such as IBD and other health disorders that exhibit dysregulated autophagy.
MATERIALS AND METHODS
Animals
All mice were 8 to 12 weeks old and on a C57BL/6 background. Both male and female mice were used. Breeding pairs of Tph1−/− mice and Tph1+/+ littermates on a C57BL/6 background were obtained from the Université Pierre et Marie Curie, France (63). These mice express normal amounts of 5-HT in the brain and show no observed differences in food intake or body weight as compared with Tph1+/+ mice. SERT+/+ and SERT−/− as well as Atg7fl/fl and Atg7fl/fl villin-Cre (Atg7ΔIEC) mice, generated as described previously (64, 65), were provided by G.R.S. (McMaster University, ON) and D.P. (University of Toronto, ON), respectively. Tph1−/− mice were crossed with Atg7ΔIEC mice to generate Tph1−/-Atg7flox/floxvillin-Cre DKO (fig. S7). C57BL/6 mice were purchased from Charles River Laboratories in Massachusetts, USA. All mice were housed under specific pathogen–free conditions in the central animal facility and were approved by the McMaster University Animal Care Committee and conducted according to Canadian Council guidelines for animal research.
Experimental design
DSS (molecular mass, 40 kDa; catalog no. 02160110, MP Biomedicals Inc.) was added to autoclaved drinking water at 5% (w/v) for 5 days to induce acute colitis. The control mice received autoclaved drinking water. In a separate experiment, Tph1−/− mice were injected subcutaneously with 5-HTP (50 mg/kg) twice daily for 8 days beginning 3 days before induction of 5% DSS colitis, whereas the control Tph1−/− mice received saline as vehicle. Another group of Tph1−/− mice received a similar dosage and duration of 5-HTP along with rapamycin at a dose of 1.5 mg/kg per day for 5 days starting with DSS administration. All interventions were performed during the light cycle.
Adoptive microbiota transfer
C57BL/6 mice received a cocktail of broad-spectrum antibiotics neomycin, ampicillin, vancomycin, and metronidazole (all 0.5 g/liter) in sterile drinking water for 10 days. After 10 days, 200 μl of cecal contents from naïve or DSS-treated Tph1−/− or DKO or Atg7ΔIEC mice was gavaged to three groups of C57BL/6 mice for 3 days. Following 2 weeks of colonization, 3.5% (w/v) DSS was added to the drinking water for 5 days to induce colitis.
Drugs and reagents
5-HT (catalog no. H9523, Sigma-Aldrich), 5-HTP (catalog no. H9772, Sigma-Aldrich), rapamycin (catalog no. 37094, Sigma-Aldrich), and phytohemagglutinin (PHA; catalog no. L1668, Sigma-Aldrich) were prepared according to the manufacturer’s instructions.
Isolation of murine IECs
Murine colon epithelial cells were isolated according to the protocol by Gracz et al. (66). A total of 1 × 106 IECs (100 μl) were plated on slides using cytospin. IECs were stained with rabbit monoclonal anti-LC3A/B antibody [1:200; catalog no. 12741, Cell Signaling Technology (CST)] or with rabbit monoclonal anti–E-cadherin antibody (1:200; catalog no. 3195, CST) according to the CST protocol. The remaining cell pellet was homogenized and used for Western blot analysis.
Isolation of PBMCs from human whole blood and human colonic biopsy collection
The study was approved by the Hamilton Integrated Research Ethics Board (Project# 12-239) (Clinicaltrials.gov #NCT01650311). All biopsy and blood samples were obtained at McMaster University Medical Centre from consenting adults. The exclusion criteria for HCs for both cohorts included history of gastrointestinal complaints, use of drugs such as antidepressants, steroids, nonsteroidal anti-inflammatory drugs, and biologics. Any participant from the HC or CD groups was excluded if he/she was considered unfit by the gastroenterologist. The first cohort included eight HCs and eight patients with CD who donated colonic biopsy samples for immunohistochemistry. In the second cohort, 10 HCs and 10 patients with CD donated 30 ml of whole blood. One milliliter of blood was used to measure plasma 5-HT levels by enzyme-linked immunosorbent assay (ELISA). The remaining 29 ml was used to isolate PBMCs according to the STEMCELL Technologies protocol using Lymphoprep (STEMCELL Technologies). Isolated PBMCs were cultured in RPMI media and 1% penicillin/streptomycin. The PBMCs were treated in the presence of PHA (5 μg/ml) with 5-HT (10 μM), rapamycin (50 nM), or both for 6 hours at 37°C at 5% CO2. The cell lysates were collected for the determination of levels of autophagy proteins by Western blotting.
Evaluation of inflammation and autophagy proteins
Colitis was assessed using DAI, macroscopic score, histological score of hematoxylin and eosin–stained colonic sections, and MPO activity based on established systems (25). DAI is a combined score of weight loss, stool consistency, and fecal bleeding as previously published (25). This scoring system is defined as weight loss: 0, no loss; 1, 1 to 5%; 2, 5 to 10%; 3, 10 to 20%; 4, >20%; stool: 0, normal; 2, loose stool; 4, diarrhea; and bleeding: 0, no blood; 2, Hemoccult positive (Hemoccult II; Beckman Coulter); 4, gross blood (blood around anus). DAI was measured on all 5 days of DSS administration. Levels of colonic tissue proinflammatory cytokines IL-1β, IL-6, and TNF-α were determined by ELISA. Autophagy proteins were analyzed by Western blot and immunofluorescence staining of proteins extracted either from whole colon, mucosa, or IECs.
Western blotting
Murine colonic tissue was homogenized, and total protein was extracted using radioimmunoprecipitation assay buffer containing a protease inhibitor cocktail and phosphatase inhibitor. Total protein concentration of homogenized tissue was determined using the DC Protein Assay Kit (catalog no. 5000111, Bio-Rad). Equal amounts of protein from each group were loaded and separated by 7 to 20% SDS–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane using Trans-Blot Turbo transfer system (Bio-Rad) as per the manufacturer’s instructions. Membranes were blocked with 5% bovine serum albumin in tris-buffered saline–Tween 20 (TBST) for 1 hour at room temperature (r.t). Membranes were incubated overnight at 4°C with primary antibodies against LC3 (1:1000; catalog no. 12741, CST), Beclin-1 (1:1000; catalog no. 3495, CST), Atg12-5 (1:1000; catalog no. 4180, CST), Atg7 (1:1000; catalog no. 8558, CST), p62 (1:1000; catalog no. 5114, CST), p-AMPKα (T172) (1:1000; catalog no. 2535, CST), AMPKα (1:1000; catalog no. 5831, CST), p-mTOR (S2448) (1:1000; catalog no. 5536, CST), mTOR (1:1000; catalog no. 2983, CST), mDefb1 (1:500; catalog no. PA575666, Thermo Fisher Scientific), and β-actin (1:1000; catalog no. 4970, CST). Membranes were washed three times with TBST and incubated with anti-rabbit horseradish peroxidase–linked antibody (1:5000; catalog no. 7074, CST) for 1 hour at r.t. Proteins were treated with Clarity Max Western enhanced chemiluminescence substrate (catalog no. 1705062, Bio-Rad) and exposed to a luminescent image analyzer (ChemiDoc Touch Imaging System) to visualize the proteins. Densitometric analysis was performed on Western blots with ImageJ software (version 1.51), normalized to total β-actin.
Immunohistochemistry/immunofluorescence
Formalin-fixed, paraffin-embedded human mucosal biopsies were stained for LC3B. Colonic tissue sections were deparaffinized with CitriSolv (catalog no. 04355121, Thermo Fisher Scientific) and rehydrated in graded concentrations of ethanol. Sections were subjected to heat-induced epitope retrieval, blocked with 3% bovine serum albumin, and incubated with a polyclonal rabbit anti-LC3B (1:200; catalog no. 48394, Abcam) antibody for 1 hour at r.t. Sections were washed with phosphate-buffered saline/0.5% Tween 20 and incubated with EnVision (horseradish peroxidase–coupled anti-rabbit secondary reagent; DakoCytomation, catalog no. K4003, Dako) for 30 min. Sections were developed using 3,3′-diaminobenzidine solution (SIGMAFAST, catalog no. 079K8208, Sigma-Aldrich) and counterstained with Mayer’s hematoxylin solution (catalog no. MHS1, Sigma-Aldrich). Sections were visualized using a Nikon Eclipse 80i microscope (Nikon Instruments Inc.). The number of LC3B+ cells per 10 glands was counted in four different areas for each section.
For detecting autophagosome and lysosome fusion in the murine colonic tissue sections, double immunofluorescence staining for LC3B and LAMP2 was performed. Colon tissues were fixed in 4% paraformaldehyde and processed for paraffin embedding. Paraffin sections of 4 μm were cut, mounted, dewaxed, and rehydrated. Antigen retrieval was performed on paraffin sections before immunostaining by heating the slides in 0.01 M citrate buffer (pH 6.0) for 30 min. Sections were blocked with 5% normal goat serum (Sigma-Aldrich) and incubated with primary antibodies overnight at 4° C., followed by secondary antibodies for 1 hour at r.t. Primary antibodies were polyclonal rabbit anti-LC3B (1:100; catalog no. 48394, Abcam) and monoclonal rat anti-LAMP2 (1:100; catalog no. B4247, LSBio); secondary antibodies were Alexa Fluor 594–conjugated goat anti-rabbit immunoglobulin G (IgG) (1:100; catalog no. A11037, Invitrogen) for LC3 staining and Alexa 488–conjugated goat anti-rat IgG (1:100; catalog no. A11006, Invitrogen) for LAMP2 staining. After washing, sections were mounted with Fluoroshield mounting medium with DAPI (4′,6-diamidino-2-phenylindole; catalog no. 104139, Abcam) for nuclear counterstaining. Immunostaining was examined, and images were captured using a fluorescence microscope (Nikon Eclipse 80i) with 40× objective lens. All pictures were taken with a Nikon digital camera attached to the microscope using a NIS-Elements Basic Research imaging software. The percentage of LC3B/LAMP2+ epithelial cells was determined by counting the number of LC3/LAMP2 coexpressed epithelial cells and total epithelial cells in three to four different areas for each section.
Enzyme-linked immunosorbent assay
5-HT levels were measured using commercially available ELISA kits (catalog no. IM1749, Beckman Coulter) following its extraction from murine colonic tissue by established protocol (27) or in human plasma. For intestinal cytokine measurement, colonic tissues were homogenized in TBS containing a protease inhibitor mixture (catalog no. P8340, Sigma-Aldrich), centrifuged for 5 min at 3300g, and the resulting supernatants used for measuring cytokine levels (IL-1β, catalog no. SMLB00C; IL-6, catalog no. SM6000B; TNF-α, catalog no. SMTA00B) according to the manufacturer’s instructions (Quantikine Murine, R&D Systems). Total protein levels were quantified in the homogenates using the DC Protein Assay Kit.
Quantitative real-time polymerase chain reaction
Total RNA from human intestinal mucosal biopsy or mouse colon tissue was extracted using TRIzol reagent (catalog no. 15596026, Thermo Fisher Scientific). Total RNA was measured using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), followed by complementary DNA (cDNA) preparation from 1 μg of total RNA using the iScript cDNA Synthesis Kit (catalog no. 1708891, Bio-Rad). Quantitative real-time polymerase chain reaction (qRT-PCR) assay was completed using SsoFast EvaGreen SYBR Green PCR Master Mix (catalog no. 1725201, Bio-Rad) and CFX96 real-time PCR system (Bio-Rad). Commercially available primers for ATG16L1 and IRGM were used (Bio-Rad assay IDs qHsaCED0042534 and qHsaCED0004388). The primer used for mDefb1 has been previously reported (27). The reference gene selected was human or mouse 18S rRNA as described previously (21, 27). The data were analyzed according to the 2−ΔΔCT method and expressed as relative abundances (means ± SEM).
Microbiome profiling and analysis
DNA was extracted from the cecal samples of mice, and the 16S rRNA variable 3 (V3) gene region was amplified as previously described (67). The forward and reverse primer sequences used for the amplification of the V3 gene region have been specified in tables S4 and S5. Cutadapt was used to filter and trim adapter sequences and PCR primers from the raw reads with a minimum quality score of 30 and a minimum read length of 100 base pairs. Sequence variants were then resolved from the trimmed raw reads using DADA2, an accurate sample inference pipeline from 16S amplicon data (68). DNA sequence reads were filtered and trimmed on the basis of the quality of the reads for each Illumina run separately, and error rates were learned and sequence variants were determined by DADA2. Sequence variant tables were merged to combine all information from separate Illumina runs. Bimeras were removed, Ribosomal Database Project classifier was used to assign taxonomy using the SILVA reference database version 1.3.2, and analysis was conducted on DESeq2 R package and using GraphPad Prism version 6.0 for Mac OS X (GraphPad Software) (69, 70). The ASV table was filtered to remove taxa whose mean abundance was 10 or less, samples with a sampling depth of <2500, and those reads not assigned to bacteria or archaea. After filtering, the dataset had 3,473,842 reads in 479 ASVs across 36 samples. The sample read depth ranged from 54,329 to 137,507. To measure beta diversity, we used Bray-Curtis distances based on taxon relative abundance.
Statistical analysis
Three to 10 biological replicates were used for every experiment. Results are represented as means ± SEM. Unpaired Student’s t test (two-tailed) or one-way analysis of variance, followed by Newman-Keuls multiple comparison post hoc test or Pearson correlation coefficient test, was performed to analyze data using GraphPad Prism version 6 (GraphPad Software). P < 0.05 was considered statistically significant. For microbial analysis, statistically significant differences in beta diversity were examined using permutational multivariate analysis of variance. DESeq2 was used to determine significantly different taxa between mice groups, where the difference was considered significant, if P < 0.01 after adjustment for multiple testing via Benjamini-Hochberg multiple testing adjustment procedure (70).
Acknowledgments
We thank N. Pai and S. Shajib for assistance in recruiting patients with CD. We also thank J. J. Kim, X. Wang, J. C. Szamosi, F. M. Nazmul Hassan, and P. M. Far for support in this study.
Funding: This work was supported by grants from the following: Canadian Institutes of Health Research (CIHR) to W.I.K. (PJT 156262); Canadian Association of Gastroenterology (CAG) doctoral fellowship and Farncombe Student Award to S.H.; and Diabetes Canada Investigator Award, a Tier 1 Canada Research Chair, and the J. Bruce Duncan Chair in Metabolic Diseases to G.R.S. G.R.S. is a cofounder of Espervita Therapeutics and received research funding from Espervita Therapeutics, Esperion Therapeutics, Poxel Pharmaceuticals, and Novo Nordisk.
Author contributions: S.H. and W.I.K. conceived the idea and designed the research. S.H. performed all experiments with help from H.W., J.G., S.B., and Y.H.K. S.H., U.C., and J.K.M. recruited study participants. S.H. and I.I.K. analyzed and interpreted the data. S.H. and W.I.K. wrote, edited, and revised the manuscript. M.S., F.C., D.P., J.H.B., and G.R.S. critically appraised the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S14
Tables S1 to S5
References
REFERENCES AND NOTES
- 1.Caruso R., Lo B. C., Nunez G., Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 20, 411–426 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Hampe J., Franke A., Rosenstiel P., Till A., Teuber M., Huse K., Albrecht M., Mayr G., De La Vega F. M., Briggs J., Gunther S., Prescott N. J., Onnie C. M., Hasler R., Sipos B., Folsch U. R., Lengauer T., Platzer M., Mathew C. G., Krawczak M., Schreiber S., A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39, 207–211 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Massey D. C., Parkes M., Genome–wide association scanning highlights two autophagy genes, ATG16L1 and IRGM, as being significantly associated with Crohn’s disease. Autophagy 3, 649–651 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Larabi A., Barnich N., Nguyen H. T. T., New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 16, 38–51 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haq S., Grondin J., Banskota S., Khan W. I., Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 26, 19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z., Klionsky D. J., Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera S., Fernandez A. F., Marino G., Aguirre A., Suarez M. F., Espanol Y., Vega J. A., Laura R., Fueyo A., Fernandez-Garcia M. S., Freije J. M., Kroemer G., Lopez-Otin C., ATG4B/autophagin-1 regulates intestinal homeostasis and protects mice from experimental colitis. Autophagy 9, 1188–1200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuboi K., Nishitani M., Takakura A., Imai Y., Komatsu M., Kawashima H., Autophagy protects against colitis by the maintenance of normal gut microflora and secretion of mucus. J. Biol. Chem. 290, 20511–20526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macias-Ceja D. C., Cosin-Roger J., Ortiz-Masia D., Salvador P., Hernandez C., Esplugues J. V., Calatayud S., Barrachina M. D., Stimulation of autophagy prevents intestinal mucosal inflammation and ameliorates murine colitis. Br. J. Pharmacol. 174, 2501–2511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S., Chen M., Wang Y., Wang Z., Pei Y., Fan R., Liu X., Wang L., Zhou J., Zheng S., Zhang T., Lin Y., Zhang M., Tao R., Zhong J., mTOR inhibition attenuates dextran sulfate sodium-induced colitis by suppressing T cell proliferation and balancing Th1/Th17/Treg profile. PLOS ONE 11, e0154564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massey D. C., Bredin F., Parkes M., Use of sirolimus (rapamycin) to treat refractory Crohn’s disease. Gut 57, 1294–1296 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Banskota S., Wang H., Kwon Y. H., Gautam J., Gurung P., Haq S., Hassan F. M. N., Bowdish D. M., Kim J. A., Carling D., Fullerton M. D., Steinberg G. R., Khan W. I., Salicylates ameliorate intestinal inflammation by activating macrophage AMPK. Inflamm. Bowel Dis. 27, 914–926 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Kim S. G., Blenis J., Rapamycin: One drug, many effects. Cell Metab. 19, 373–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kau A. L., Ahern P. P., Griffin N. W., Goodman A. L., Gordon J. I., Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imhann F., Vich Vila A., Bonder M. J., Fu J., Gevers D., Visschedijk M. C., Spekhorst L. M., Alberts R., Franke L., van Dullemen H. M., Ter Steege R. W. F., Huttenhower C., Dijkstra G., Xavier R. J., Festen E. A. M., Wijmenga C., Zhernakova A., Weersma R. K., Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 67, 108–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L., Liu C., Zhao W., He C., Ding J., Dai R., Xu K., Xiao L., Luo L., Liu S., Li W., Meng H., Impaired autophagy in intestinal epithelial cells alters gut microbiota and host immune responses. Appl. Environ. Microbiol. 84, e00880-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung M. J., Lee J., Shin N. R., Kim M. S., Hyun D. W., Yun J. H., Kim P. S., Whon T. W., Bae J. W., Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci. Rep. 6, 30887 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spohn S. N., Mawe G. M., Non-conventional features of peripheral serotonin signalling - The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 14, 412–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banskota S., Ghia J. E., Khan W. I., Serotonin in the gut: Blessing or a curse. Biochimie 161, 56–64 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Shajib M. S., Baranov A., Khan W. I., Diverse effects of gut-derived serotonin in intestinal inflammation. ACS Chem. Nerosci. 8, 920–931 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Shajib M. S., Chauhan U., Adeeb S., Chetty Y., Armstrong D., Halder S. L. S., Marshall J. K., Khan W. I., Characterization of serotonin signaling components in patients with inflammatory bowel disease. J. Can. Assoc. Gastroenterol. 2, 132–140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidd M., Gustafsson B. I., Drozdov I., Modlin I. M., IL1β- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol. Motil. 21, 439–450 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manocha M., Khan W. I., Serotonin and GI disorders: An update on clinical and experimental studies. Clin. Transl. Gastroenterol. 3, e13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghia J. E., Li N., Wang H., Collins M., Deng Y., El-Sharkawy R. T., Cote F., Mallet J., Khan W. I., Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137, 1649–1660 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Kim J. J., Wang H., Terc J. D., Zambrowicz B., Yang Q. M., Khan W. I., Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G455–G465 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Margolis K. G., Stevanovic K., Li Z., Yang Q. M., Oravecz T., Zambrowicz B., Jhaver K. G., Diacou A., Gershon M. D., Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63, 928–937 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon Y. H., Wang H., Denou E., Ghia J. E., Rossi L., Fontes M. E., Bernier S. P., Shajib M. S., Banskota S., Collins S. M., Surette M. G., Khan W. I., Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell. Mol. Gastroenterol. Hepatol. 7, 709–728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fung T. C., Vuong H. E., Luna C. D. G., Pronovost G. N., Aleksandrova A. A., Riley N. G., Vavilina A., McGinn J., Rendon T., Forrest L. R., Hsiao E. Y., Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 4, 2064–2073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soll C., Jang J. H., Riener M. O., Moritz W., Wild P. J., Graf R., Clavien P. A., Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology 51, 1244–1254 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Imada T., Nakamura S., Hisamura R., Izuta Y., Jin K., Ito M., Kitamura N., Tanaka K. F., Mimura M., Shibuya I., Tsubota K., Serotonin hormonally regulates lacrimal gland secretory function via the serotonin type 3a receptor. Sci. Rep. 7, 6965 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z. M., Zheng C. H., Zhu Z. H., Wu F. T., Ni G. L., Liang Y., SiRNA-mediated serotonin transporter knockdown in the dorsal raphe nucleus rescues single prolonged stress-induced hippocampal autophagy in rats. J. Neurol. Sci. 360, 133–140 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Sharifi M. N., Mowers E. E., Drake L. E., Macleod K. F., Measuring autophagy in stressed cells. Methods Mol. Biol. 1292, 129–150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bischoff S. C., Mailer R., Pabst O., Weier G., Sedlik W., Li Z., Chen J. J., Murphy D. L., Gershon M. D., Role of serotonin in intestinal inflammation: Knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G685–G695 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Regmi S. C., Park S. Y., Ku S. K., Kim J. A., Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic. Biol. Med. 69, 377–389 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Mawe G. M., Hoffman J. M., Serotonin signalling in the gut‑functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallo R. L., Hooper L. V., Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 12, 503–516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuura T., West G. A., Youngman K. R., Klein J. S., Fiocchi C., Immune activation genes in inflammatory bowel disease. Gastroenterology 104, 448–458 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Xavier R. J., Podolsky D. K., Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Coates M. D., Mahoney C. R., Linden D. R., Sampson J. E., Chen J., Blaszyk H., Crowell M. D., Sharkey K. A., Gershon M. D., Mawe G. M., Moses P. L., Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126, 1657–1664 (2004). [DOI] [PubMed] [Google Scholar]
- 40.MacEachern S. J., Keenan C. M., Papakonstantinou E., Sharkey K. A., Patel B. A., Alterations in melatonin and 5-HT signalling in the colonic mucosa of mice with dextran-sodium sulfate-induced colitis. Br. J. Pharmacol. 175, 1535–1547 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murthy A., Li Y., Peng I., Reichelt M., Katakam A. K., Noubade R., Roose-Girma M., DeVoss J., Diehl L., Graham R. R., van Lookeren Campagne M., A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature 506, 456–462 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Ortiz-Masia D., Cosin-Roger J., Calatayud S., Hernandez C., Alos R., Hinojosa J., Apostolova N., Alvarez A., Barrachina M. D., Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: Relevance in IBD. Mucosal Immunol. 7, 929–938 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Sehgal S. N., Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 35, S7–S14 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J., AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guragain D., Gurung P., Chang J. H., Katila N., Chang H. W., Jeong B. S., Choi D. Y., Kim J. A., AMPK is essential for IL-10 expression and for maintaining balance between inflammatory and cytoprotective signaling. Biochim Biophys Acta Gen Subj 1864, 129631 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Benjamin J. L., Sumpter R. Jr., Levine B., Hooper L. V., Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 13, 723–734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J. J., Bridle B. W., Ghia J. E., Wang H., Syed S. N., Manocha M. M., Rengasamy P., Shajib M. S., Wan Y., Hedlund P. B., Khan W. I., Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J. Immunol. 190, 4795–4804 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Spohn S. N., Bianco F., Scott R. B., Keenan C. M., Linton A. A., O’Neill C. H., Bonora E., Dicay M., Lavoie B., Wilcox R. L., MacNaughton W. K., De Giorgio R., Sharkey K. A., Mawe G. M., Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology 151, 933–944.e3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannon J., Hoyer D., Molecular biology of 5-HT receptors. Behav. Brain Res. 195, 198–213 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Hu Y. X., Han X. S., Jing Q., Ca(2+) ion and autophagy. Adv. Exp. Med. Biol. 1206, 151–166 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Farrelly L. A., Thompson R. E., Zhao S., Lepack A. E., Lyu Y., Bhanu N. V., Zhang B., Loh Y. E., Ramakrishnan A., Vadodaria K. C., Heard K. J., Erikson G., Nakadai T., Bastle R. M., Lukasak B. J., Zebroski H. III, Alenina N., Bader M., Berton O., Roeder R. G., Molina H., Gage F. H., Shen L., Garcia B. A., Li H., Muir T. W., Maze I., Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 567, 535–539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye D., Xu H., Xia H., Zhang C., Tang Q., Bi F., Targeting SERT promotes tryptophan metabolism: Mechanisms and implications in colon cancer treatment. J. Exp. Clin. Cancer Res. 40, 173 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campieri M., Gionchetti P., Bacteria as the cause of ulcerative colitis. Gut 48, 132–135 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavoie S., Conway K. L., Lassen K. G., Jijon H. B., Pan H., Chun E., Michaud M., Lang J. K., Gallini Comeau C. A., Dreyfuss J. M., Glickman J. N., Vlamakis H., Ananthakrishnan A., Kostic A., Garrett W. S., Xavier R. J., The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. eLife 8, e39982 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Wang X., Chen Q., Luo L., Ma M., Xiao B., Zeng L., Camellia sinensis and Litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol. Nutr. Food Res. 64, e1900943 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Gao X., Cao Q., Cheng Y., Zhao D., Wang Z., Yang H., Wu Q., You L., Wang Y., Lin Y., Li X., Wang Y., Bian J. S., Sun D., Kong L., Birnbaumer L., Yang Y., Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. U.S.A. 115, E2960–E2969 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rooks M. G., Veiga P., Wardwell-Scott L. H., Tickle T., Segata N., Michaud M., Gallini C. A., Beal C., van Hylckama-Vlieg J. E., Ballal S. A., Morgan X. C., Glickman J. N., Gevers D., Huttenhower C., Garrett W. S., Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 8, 1403–1417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheng K., Zhang G., Sun M., He S., Kong X., Wang J., Zhu F., Zha X., Wang Y., Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 11, 7817–7829 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Shang L., Liu H., Yu H., Chen M., Yang T., Zeng X., Qiao S., Core altered microorganisms in colitis mouse model: A comprehensive time-point and fecal microbiota transplantation analysis. Antibiotics 10, 643 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manzella C. R., Jayawardena D., Pagani W., Li Y., Alrefai W. A., Bauer J., Jung B., Weber C. R., Gill R. K., Serum serotonin differentiates between disease activity states in Crohn’s patients. Inflamm. Bowel Dis. 26, 1607–1618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez-Banares F., Esteve M., Espinos J. C., Rosinach M., Forne M., Salas A., Viver J. M., Drug consumption and the risk of microscopic colitis. Am. J. Gastroenterol. 102, 324–330 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Lees C. W., Barrett J. C., Parkes M., Satsangi J., New IBD genetics: Common pathways with other diseases. Gut 60, 1739–1753 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Cote F., Thevenot E., Fligny C., Fromes Y., Darmon M., Ripoche M. A., Bayard E., Hanoun N., Saurini F., Lechat P., Dandolo L., Hamon M., Mallet J., Vodjdani G., Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl. Acad. Sci. U.S.A. 100, 13525–13530 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bengel D., Murphy D. L., Andrews A. M., Wichems C. H., Feltner D., Heils A., Mossner R., Westphal H., Lesch K. P., Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 53, 649–655 (1998). [DOI] [PubMed] [Google Scholar]
- 65.Sorbara M. T., Foerster E. G., Tsalikis J., Abdel-Nour M., Mangiapane J., Sirluck-Schroeder I., Tattoli I., van Dalen R., Isenman D. E., Rohde J. R., Girardin S. E., Philpott D. J., Complement C3 drives autophagy-dependent restriction of cyto-invasive bacteria. Cell Host Microbe 23, 644–652.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Gracz A. D., Puthoff B. J., Magness S. T., Identification, isolation, and culture of intestinal epithelial stem cells from murine intestine. Methods Mol. Biol. 879, 89–107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whelan F. J., Verschoor C. P., Stearns J. C., Rossi L., Luinstra K., Loeb M., Smieja M., Johnstone J., Surette M. G., Bowdish D. M., The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann. Am. Thorac. Soc. 11, 513–521 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J., Holmes S. P., DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F. O., The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Y., Cai J., Zhang S., Yuan N., Li X., Fang Y., Song L., Shang M., Liu S., Zhao W., Hu S., Wang J., Loss of autophagy leads to failure in megakaryopoiesis, megakaryocyte differentiation, and thrombopoiesis in mice. Exp. Hematol. 43, 488–494 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S14
Tables S1 to S5
References