Abstract
Background
Management of rotator cuff disease may include use of electrotherapy modalities (also known as electrophysical agents), which aim to reduce pain and improve function via an increase in energy (electrical, sound, light, or thermal) into the body. Examples include therapeutic ultrasound, low‐level laser therapy (LLLT), transcutaneous electrical nerve stimulation (TENS), and pulsed electromagnetic field therapy (PEMF). These modalities are usually delivered as components of a physical therapy intervention. This review is one of a series of reviews that form an update of the Cochrane review, 'Physiotherapy interventions for shoulder pain'.
Objectives
To synthesise available evidence regarding the benefits and harms of electrotherapy modalities for the treatment of people with rotator cuff disease.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 3), Ovid MEDLINE (January 1966 to March 2015), Ovid EMBASE (January 1980 to March 2015), CINAHL Plus (EBSCOhost, January 1937 to March 2015), ClinicalTrials.gov and the WHO ICTRP clinical trials registries up to March 2015, unrestricted by language, and reviewed the reference lists of review articles and retrieved trials, to identify potentially relevant trials.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐randomised trials, including adults with rotator cuff disease (e.g. subacromial impingement syndrome, rotator cuff tendinitis, calcific tendinitis), and comparing any electrotherapy modality with placebo, no intervention, a different electrotherapy modality or any other intervention (e.g. glucocorticoid injection). Trials investigating whether electrotherapy modalities were more effective than placebo or no treatment, or were an effective addition to another physical therapy intervention (e.g. manual therapy or exercise) were the main comparisons of interest. Main outcomes of interest were overall pain, function, pain on motion, patient‐reported global assessment of treatment success, quality of life and the number of participants experiencing adverse events.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted the data, performed a risk of bias assessment and assessed the quality of the body of evidence for the main outcomes using the GRADE approach.
Main results
We included 47 trials (2388 participants). Most trials (n = 43) included participants with rotator cuff disease without calcification (four trials included people with calcific tendinitis). Sixteen (34%) trials investigated the effect of an electrotherapy modality delivered in isolation. Only 23% were rated at low risk of allocation bias, and 49% were rated at low risk of both performance and detection bias (for self‐reported outcomes). The trials were heterogeneous in terms of population, intervention and comparator, so none of the data could be combined in a meta‐analysis.
In one trial (61 participants; low quality evidence), pulsed therapeutic ultrasound (three to five times a week for six weeks) was compared with placebo (inactive ultrasound therapy) for calcific tendinitis. At six weeks, the mean reduction in overall pain with placebo was ‐6.3 points on a 52‐point scale, and ‐14.9 points with ultrasound (MD ‐8.60 points, 95% CI ‐13.48 to ‐3.72 points; absolute risk difference 17%, 7% to 26% more). Mean improvement in function with placebo was 3.7 points on a 100‐point scale, and 17.8 points with ultrasound (mean difference (MD) 14.10 points, 95% confidence interval (CI) 5.39 to 22.81 points; absolute risk difference 14%, 5% to 23% more). Ninety‐one per cent (29/32) of participants reported treatment success with ultrasound compared with 52% (15/29) of participants receiving placebo (risk ratio (RR) 1.75, 95% CI 1.21 to 2.53; absolute risk difference 39%, 18% to 60% more). Mean improvement in quality of life with placebo was 0.40 points on a 10‐point scale, and 2.60 points with ultrasound (MD 2.20 points, 95% CI 0.91 points to 3.49 points; absolute risk difference 22%, 9% to 35% more). Between‐group differences were not important at nine months. No participant reported adverse events.
Therapeutic ultrasound produced no clinically important additional benefits when combined with other physical therapy interventions (eight clinically heterogeneous trials, low quality evidence). We are uncertain whether there are differences in patient‐important outcomes between ultrasound and other active interventions (manual therapy, acupuncture, glucocorticoid injection, glucocorticoid injection plus oral tolmetin sodium, or exercise) because the quality of evidence is very low. Two placebo‐controlled trials reported results favouring LLLT up to three weeks (low quality evidence), however combining LLLT with other physical therapy interventions produced few additional benefits (10 clinically heterogeneous trials, low quality evidence). We are uncertain whether transcutaneous electrical nerve stimulation (TENS) is more or less effective than glucocorticoid injection with respect to pain, function, global treatment success and active range of motion because of the very low quality evidence from a single trial. In other single, small trials, no clinically important benefits of pulsed electromagnetic field therapy (PEMF), microcurrent electrical stimulation (MENS), acetic acid iontophoresis and microwave diathermy were observed (low or very low quality evidence).
No adverse events of therapeutic ultrasound, LLLT, TENS or microwave diathermy were reported by any participants. Adverse events were not measured in any trials investigating the effects of PEMF, MENS or acetic acid iontophoresis.
Authors' conclusions
Based on low quality evidence, therapeutic ultrasound may have short‐term benefits over placebo in people with calcific tendinitis, and LLLT may have short‐term benefits over placebo in people with rotator cuff disease. Further high quality placebo‐controlled trials are needed to confirm these results. In contrast, based on low quality evidence, PEMF may not provide clinically relevant benefits over placebo, and therapeutic ultrasound, LLLT and PEMF may not provide additional benefits when combined with other physical therapy interventions. We are uncertain whether TENS is superior to placebo, and whether any electrotherapy modality provides benefits over other active interventions (e.g. glucocorticoid injection) because of the very low quality of the evidence. Practitioners should communicate the uncertainty of these effects and consider other approaches or combinations of treatment. Further trials of electrotherapy modalities for rotator cuff disease should be based upon a strong rationale and consideration of whether or not they would alter the conclusions of this review.
Plain language summary
Electrotherapy modalities for rotator cuff disease
Background
Rotator cuff disease is the most common cause of shoulder pain. People with rotator cuff disease often describe their pain as being worse at night and exacerbated by movement in specific directions, including overhead activity. It is often associated with loss of function and some people describe weakness.
Electrotherapy modalities (also known as electrophysical agents) are types of physical therapy that aim to reduce pain and improve function via an increase in energy (electrical, sound, light, or thermal) into the body. Examples include therapeutic ultrasound, low‐level laser therapy (LLLT), transcutaneous electrical nerve stimulation (TENS), and pulsed electromagnetic field therapy (PEMF). Electrotherapy modalities are delivered by various clinicians, including physiotherapists, chiropractors and osteopaths. In practice, people with rotator cuff disease seldom receive a single electrotherapy modality in isolation from other components of physical therapy treatment (for example manual therapy or exercise, or both).
Study characteristics
This summary of an updated Cochrane review presents what we know from research about the benefits and harms of electrotherapy modalities in people with rotator cuff disease. After searching for all relevant studies published up to March 2015, we included 47 trials (2388 participants). Among the included participants, 67% were women, the average age was 53 years, and the average duration of the condition was eight months. Electrotherapy was delivered for three weeks on average.
Key results
Pulsed therapeutic ultrasound versus placebo (inactive ultrasound) for six weeks in people with calcific tendinitis (based on one trial)
Overall pain (lower scores mean greater pain reduction)
People who had ultrasound had greater pain reduction than people who had placebo. Reduction in pain was 8.60 points more (ranging from 3.72 to 13.48 points more) at six weeks (17% absolute improvement). On a scale of 0 to 52 points, people who had ultrasound rated their reduction in pain score as ‐14.9 points, and people who had placebo rated their reduction in pain score as ‐6.3 points.
Function (higher scores mean more improvement in function)
People who had ultrasound improved more than people who had placebo. Improvement in function was 14.10 points more (ranging from 5.39 to 22.81 points more) at six weeks (14% absolute improvement). On a scale of 0 to 100 points, people who had ultrasound rated their change in function as 17.8 points, and people who had placebo rated their change in function as 3.7 points.
Treatment success
Thirty‐nine more people out of 100 rated their treatment as successful with ultrasound compared with placebo; 39% absolute improvement (ranging from 18% to 60% more improvement). Ninety‐one out of 100 people reported treatment success with ultrasound and 52 out of 100 people reported treatment success with placebo.
Side effects
No participant receiving ultrasound or placebo reported side effects.
Quality of the evidence
Low‐quality evidence suggests that therapeutic ultrasound may improve overall pain, function, global treatment success and quality of life more than placebo at short‐term (six weeks) in people with calcific tendinitis, that LLLT may improve overall pain and function more than placebo at short‐term (up to three weeks), that therapeutic ultrasound and LLLT may produce no clinically important additional benefits in pain and function when combined with other physical therapy interventions alone, and that PEMF may produce no clinically important benefits in pain and function when compared with placebo. Further high quality research is likely to change our confidence in the effect estimates.
We are uncertain whether TENS improves pain and function more than placebo, whether therapeutic ultrasound improves pain and function more than other active interventions (manual therapy, acupuncture, glucocorticoid injection, glucocorticoid injection plus oral tolmetin sodium, or exercise), or whether LLLT improves pain and function more than oral nonsteroidal anti‐inflammatory drugs (NSAID) and glucocorticoid injection, because of the very low quality of the evidence.
Summary of findings
Summary of findings for the main comparison. Therapeutic ultrasound compared to placebo for rotator cuff disease.
| Therapeutic ultrasound compared to placebo for rotator cuff disease | ||||||
| Patient or population: Rotator cuff disease (diagnostic label: calcific tendinitis) Settings: Outpatient clinics and private practices, Austria Intervention: Pulsed therapeutic ultrasound (0.89 MHz frequency, 2.5 W/cm2 intensity for 15 minutes, 3‐5 times a week for 6 weeks) Comparison: Placebo (inactive ultrasound, 3‐5 times a week for 6 weeks) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Therapeutic ultrasound | |||||
|
Overall pain Assessed: Binder's pain scale Scale from: 0‐52 Follow‐up: 6 weeks |
The mean change in overall pain in the control group was ‐6.31 | The mean change in overall pain in the intervention group was 8.6 lower (13.48 lower to 3.72 lower) | ‐ | 61 (1 RCT) | ⊕⊕⊝⊝ LOW2,3 | Lower score denotes greater reduction in pain. Absolute risk difference 17% (7% to 26% more); relative percentage change 42% (18% to 65% more) NNTB 4 (2 to 10) |
|
Function Assessed with Constant‐Murley total score Scale from 0‐100 Follow‐up: 6 weeks |
The mean change in function in the control group was 3.71 | The mean change in function in the intervention group was 14.1 higher (5.39 higher to 22.81 higher) | ‐ | 61 (1 RCT) | ⊕⊕⊝⊝ LOW2,3 | Higher score denotes greater improvement in function. Absolute risk difference 14% (5% to 23% more); relative percentage change 20% (8% to 32% more) NNTB 3 (2 to 7) |
| Pain on motion | See comment | See comment | ‐ | ‐ | ‐ | Not measured |
| Global assessment of treatment success Follow up: 6 weeks | Study population | RR 1.75 (1.21 to 2.53) | 61 (1 RCT) | ⊕⊕⊝⊝ LOW2,3 | Absolute risk difference 39% (18% to 60% more); relative percentage change 75% (21% to 153% more) NNTB 3 (2 to 6) |
|
| 517 per 10004 | 905 per 1000 (626 to 1000) | |||||
|
Quality of life Assessed with Visual analogue scale Scale from: 0‐10 Follow‐up: 6 weeks |
The mean change in quality of life in the control group was 0.41 | The mean change in quality of life in the intervention group was 2.2 higher (0.91 higher to 3.49 higher) | ‐ | 61 (1 RCT) | ⊕⊕⊝⊝ LOW2,3 | Higher score denotes greater improvement in quality of life. Absolute risk difference 22% (9% to 35% more); relative percentage change 33% (14% to 53% more) |
|
Adverse events Follow‐up: 9 months |
Study population | Not estimable | 60 (1 RCT) | ⊕⊕⊝⊝ LOW2,3 | No participant reported any adverse events | |
| 0 per 10004 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Mean score in placebo group in Ebenbichler 1999 used as assumed control group risk.
2Downgraded (‐1) for indirectness. Pulsed ultrasound was delivered to participants with calcific tendinitis, so results may not generalise to people receiving continuous ultrasound, or to other patient subgroups.
3Downgraded (‐1) for imprecision. Sample size was small, with wide 95% CI including effect estimates that are clinically important and unimportant.
4Risk in placebo group in Ebenbichler 1999 used as assumed risk.
Background
Description of the condition
This review is one in a series of reviews aiming to determine the evidence for efficacy of common interventions for shoulder pain. This series of reviews forms the update of an earlier Cochrane review of physical therapy for shoulder disorders (Green 2003). Since our original review, many new clinical trials studying a diverse range of interventions have been performed. To improve usability of the review, we have subdivided the reviews by type of shoulder disorder as people within different diagnostic groupings may respond variably to different interventions. This review focuses on electrotherapy modalities for rotator cuff disease. A separate review of manual therapy and exercise for rotator cuff disease is under review (Page 2016), and reviews of manual therapy and exercise for adhesive capsulitis (frozen shoulder) (Page 2014a) and electrotherapy modalities for adhesive capsulitis (Page 2014b) were published in 2014.
Shoulder pain is common, with a point prevalence ranging from 7% to 26% in the general population (Luime 2004). Although not life‐threatening, it impacts on the performance of tasks essential to daily living, such as dressing, personal hygiene, eating and work, and often results in substantial utilisation of health care resources (Largacha 2006; Mroz 2014; Van der Heijden 1999a; Virta 2012). The most common cause of shoulder pain in primary care is disorders of the rotator cuff (Linsell 2006; Ostor 2005), which comprises the supraspinatus, infraspinatus, subscapularis and teres minor muscles. These muscles facilitate both movement and dynamic stabilisation of the shoulder joint.
Numerous diagnostic labels have been used in the literature to describe disorders of the rotator cuff, for example subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full rotator cuff tear, calcific tendinitis and subacromial bursitis, but the terms are not standardised (Schellingerhout 2008). The term 'rotator cuff disease' was proposed as an umbrella term to classify disorders of the rotator cuff, regardless of the cause of disorder (e.g. degeneration or acute injury) and specific anatomical location (Buchbinder 1996; Whittle 2015). Calcific tendinitis is an uncommon form of rotator cuff disease usually applied to people who present with rapid onset of severe shoulder pain, and who have calcium deposits visible in the rotator cuff tendons on imaging. However, the exact pathophysiologic relevance of calcium deposits in the rotator cuff tendons is unclear and while calcium deposition may be seen in as many as 6.8% of people with shoulder pain, in asymptomatic shoulders the prevalence estimates for calcium deposition range from 2% to 20% (Titchener 2014).
Rotator cuff disease has been found to increase in prevalence with age (Yamamoto 2010) and in those participating in occupational or sporting activities that require repetitive overhead use of the arms (e.g. swimming, tennis) (Edmonds 2014; Walker 2012). People with rotator cuff disease often describe pain in the upper outer arm exacerbated by certain movements (e.g. overhead activity); the pain is often worse at night and when lying on the affected side. Some people also describe weakness and loss of function. However, there are few data regarding the diagnostic accuracy of individual symptoms in rotator cuff disease without tears (Whittle 2015).
In addition to history‐taking and clinical evaluation, the use of physical examination manoeuvres has been recommended for the diagnosis of rotator cuff disease. However there is a wide array of tests and a lack of consensus on the best test or series of tests to use, and varying descriptions of how to execute these tests (Hanchard 2013). Systematic reviews of diagnostic test accuracy studies have found that a positive painful arc test result (pain occurs between 60° and 120° during active abduction of the affected arm) is the most accurate finding for detecting rotator cuff disease, whereas the presence of a positive lag test (external or internal rotation) result was most accurate for diagnosis of a full‐thickness rotator cuff tear (Hanchard 2013; Hermans 2013).
Description of the intervention
Electrotherapy modalities (also known as electrophysical agents) are types of physical therapy that aim to reduce pain and improve function via an increase in energy (electrical, sound, light, or thermal) into the body (Watson 2008a; Watson 2010). There are several electrotherapy modalities used in clinical practice, including therapeutic ultrasound, low‐level laser therapy (LLLT), transcutaneous electrical nerve stimulation (TENS) and pulsed electromagnetic field therapy (PEMF). The delivery of particular electrotherapy modalities in physical therapy practice has varied over time. Between 1990 and 2010, therapeutic ultrasound delivery increased in several countries, LLLT was used at a consistent rate, and TENS administration increased in the UK but declined in Australia (Shah 2012). People seeking treatment for musculoskeletal conditions seldom receive a single electrotherapy modality in isolation. Other physical therapy interventions such as manual therapy and exercise are commonly delivered as co‐interventions (Gebremariam 2014). A brief description of the electrotherapy modalities investigated in this review, and their presumed mechanisms of action, are outlined as follows.
Therapeutic ultrasound delivers energy to deep tissue sites through ultrasonic waves (often at frequencies of 1 or 3 MHz and intensities between 0.1 watts/cm2 and 3 watts/cm2) using a crystal sound head. Treatment can be delivered in two forms, continuous (non‐stop ultrasonic waves) and pulsed (intermittent ultrasonic waves) (Allen 2006; Watson 2008b). The purpose of treatment is to increase tissue temperature and induce non‐thermal physiological changes (such as cell permeability and cell growth), which are believed to promote soft tissue healing and muscle relaxation (O'Brien 2007; Watson 2008b).
Low‐level laser therapy (LLLT) generates a beam of light with a particular wavelength which has the potential to deliver light energy to tissue depths below the dermis (Basford 1989; Bjordal 2010; Peplow 2010). Studies suggest that LLLT contributes to pain relief by reducing pro‐inflammatory cytokines and increasing anti‐inflammatory growth factors and cytokines (Bjordal 2006; Peplow 2010; Sakurai 2000). The effects of LLLT are considered to be dependent on dosage, wavelength, site and duration of treatment, and researchers have suggested that some previous trials of LLLT with inconclusive findings may have delivered dosages that are below that expected to achieve a biological response (Bjordal 2006; Bjordal 2010).
Transcutaneous electrical nerve stimulation (TENS) delivers electrical stimulation via electrodes placed over the intact skin surface near the source of pain to activate underlying nerves (Jones 2009; Sluka 2003). Several types of TENS applications exist; the most common are conventional TENS (high frequency and low intensity, which is sufficient to produce a comfortable tingling sensation) and acupuncture‐like TENS (low frequency and high intensity, which is sufficient to elicit muscle twitching) (Johnson 2008). The development of TENS was based on the Gate Control Theory of Pain (Melzack 1965), which suggests that there is a 'gating' mechanism in the dorsal horn of the spinal cord that regulates the amount of incoming painful stimuli via small diameter afferent nerve fibres, and that stimulation of large diameter afferent nerve fibres using other stimuli (such as TENS) can "close the gate" and reduce the perception of pain (Walsh 2009). Evidence from animal studies suggests that TENS reduces ongoing nociceptive cell activity and inhibits pain facilitatory pathways (DeSantana 2008; Jones 2009).
Pulsed electromagnetic field therapy (PEMF) involves the delivery of pulsing (that is 'on‐off') low‐frequency magnetic fields through the body, which is believed to provide temporary pain relief by influencing tissue generation and cell proliferation (Gordon 2007; Markov 2007).
Continuous short wave diathermy involves delivering a constant stream of short wave (wavelength 3 to 30 m, frequency 10 to 100 MHz) electromagnetic radiation to produce deep heating within tissues (Allen 2006; Shields 2001). The treatment is designed to produce heat at deeper tissue levels than superficial agents (such as a hot pack). The deep tissue heating is believed to induce an increase in metabolic activity, blood flow, collagen extensibility and nerve conduction, which are thought to encourage healing and relieve pain (Allen 2006; Shields 2001).
Interferential current involves crossing two medium frequency currents (most commonly 4000 Hz), which reportedly generates a low‐frequency 'beating' (amplitude‐modulated) effect at between 0 and 150 Hz in the deep tissues (Beatti 2010). These beat frequencies are believed to decrease pain, increase circulation and block nerve conduction.
Two electrotherapy modalities are designed to facilitate delivery of topical medication through the skin (that is transdermal delivery). Phonophoresis is administered using a therapeutic ultrasound device (Machet 2002; Watson 2008b), and iontophoresis is administered using a low‐intensity electrical current (Batheja 2006; Roustit 2014). The therapeutic ultrasound device used in phonophoresis is believed to enhance the absorption of the topically applied medication (Machet 2002). The iontophoretic device is believed to induce electromigration and electro‐osmosis, which are thought to facilitate the movement of positively and negatively charged drugs into the skin (Roustit 2014).
Microcurrent electrical stimulation (MENS) is a novel modality that is claimed to be capable of providing beneficial effects through delivering monophasic or biphasic pulsed microamperage currents with intensities between 1 and 999 uA across the skin (Atya 2012).
In our companion review of electrotherapy modalities for adhesive capsulitis (Page 2014b), we found that LLLT was more effective than placebo in the short‐term, but there was no high quality evidence to support the use of therapeutic ultrasound, TENS, PEMF, continuous short wave diathermy, interferential current, or Iodex iontophoresis for this condition. It is unclear what effect these modalities have on people with rotator cuff disease.
Why it is important to do this review
The previous version of this review (Green 2003) included 10 trials investigating the efficacy of electrotherapy modalities for rotator cuff disease (Berry 1980; Binder 1984; Downing 1986; Ebenbichler 1999; England 1989; Nykänen 1995; Perron 1997; Saunders 1995; Shehab 2000; Vecchio 1993), and concluded that there was little overall evidence to guide treatment. Many new trials have been published since the 2003 review (as summarised in recent systematic reviews, e.g. Alexander 2010; Gebremariam 2014; Kromer 2009; Nyberg 2010). To best inform current practice, an up‐to‐date review which incorporates data from the most recently available trials is needed.
Objectives
To synthesise available evidence regarding the benefits and harms of electrotherapy modalities for the treatment of people with rotator cuff disease.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any design (e.g. parallel, cross‐over, factorial) and controlled clinical trials using a quasi‐randomised method of allocation, such as by alternation or date of birth. We included trials if they reported the methods used to generate the allocation sequence, or if they included a statement such as "random allocation was used". Given that some of these latter, poorly‐reported trials may have used a quasi‐randomised method of allocation, we considered it reasonable to include quasi‐randomised trials that were clearly identified as such. Reports of trials were eligible regardless of the language, date of publication, or publication status.
Types of participants
We included trials that recruited adults (> 16 years of age) with rotator cuff disease as defined by the study authors (e.g. using terminology such as subacromial impingement syndrome, rotator cuff tendinitis or tendinopathy, supraspinatus, infraspinatus or subscapularis tendinitis, calcific tendinitis, subacromial bursitis, or rotator cuff tears), for any duration. We also included trials with participants with non‐specific shoulder pain provided that the inclusion/exclusion criteria were compatible with a diagnosis of rotator cuff disease. If trials included participants with either rotator cuff disease or adhesive capsulitis, we attempted to retrieve the data for rotator cuff disease participants from the trialists. If unsuccessful, we included the trial only if > 75% of participants had rotator cuff disease. We excluded trials that included any participants with a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulders, or pain in the shoulder region as part of a complex myofascial neck/shoulder/arm pain condition.
Types of interventions
We included RCTs comparing any electrotherapy modality to placebo, no treatment, a different electrotherapy modality, or any other intervention. We included RCTs where an electrotherapy modality was used as an adjunct to another treatment only if the comparison provided information on the additional effect of the electrotherapy modality. Electrotherapy modalities included therapeutic ultrasound, laser therapy, transcutaneous electrical nerve stimulation, pulsed electromagnetic field therapy, bipolar interferential current, electromyographic biofeedback, phonophoresis, iontophoresis, and short wave diathermy. Physical therapy interventions such as exercise, mobilisation, massage and manipulation were excluded and are included in a separate Cochrane review.
Types of outcome measures
We did not consider outcomes as part of the eligibility criteria.
Main outcomes
Overall pain (mean or mean change measured by visual analogue scale (VAS), numerical or categorical rating scale).
-
Function. Where trialists reported outcome data for more than one function scale, we extracted data on the scale that was highest on the following pre‐defined list:
Shoulder Pain and Disability Index (SPADI);
Croft Shoulder Disability Questionnaire;
Constant‐Murley Score;
any other shoulder‐specific function scale.
Pain on motion measured by VAS, numerical or categorical rating scale.
Global assessment of treatment success as defined by the trialists (e.g. proportion of participants with significant overall improvement).
Quality of life as measured by generic measures (such as components of the Short Form‐36 (SF‐36)) or disease‐specific tools.
Number of participants experiencing any adverse events.
Other outcomes
Night pain measured by VAS, numerical or categorical rating scale.
Pain with resisted movement measured by VAS, numerical or categorical rating scale.
Range of motion (ROM) (e.g. flexion, abduction, external rotation and internal rotation (measured in degrees or other e.g. hand‐behind‐back distance in centimetres)). Where trialists reported outcome data for both active and passive ROM measures, we extracted the data on active ROM only. We prioritised active ROM because it requires the patient to initiate shoulder movement, and so is a closer proxy to what patients can actually do than passive ROM.
Strength.
Work disability.
Surgery (e.g. surgical decompression, rotator cuff repair).
We extracted efficacy outcome measures (e.g. overall pain, function) at the following time points:
up to three weeks;
longer than three and up to six weeks (this was the main time point);
longer than six weeks and up to six months, and;
longer than six months.
If data were available in a trial at multiple time points within each of the above periods (e.g. at four, five, and six weeks), we only extracted data at the latest possible time point of each period. We extracted adverse events reported at all time points.
We collated the main results of the review into 'Summary of findings' (SoF) tables which provide key information concerning the quality of evidence and the magnitude and precision of the effect of the interventions. We included the main outcomes (see above) in the SoF tables, and presented results at, or nearest, the main time point (six weeks).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2015, Issue 3), Ovid MEDLINE (January 1966 to March 2015), Ovid EMBASE (January 1980 to March 2015), and CINAHL Plus (EBSCOhost, January 1937 to March 2015). The complete search strategies are presented in Appendix 1. Note that the search terms used included clinical terms relevant to adhesive capsulitis and manual therapy and exercise interventions, as the current review and Cochrane reviews of manual therapy and exercise for rotator cuff disease, manual therapy and exercise for adhesive capsulitis, and electrotherapy modalities for adhesive capsulitis were conducted simultaneously.
Searching other resources
We searched for ongoing trials and protocols of published trials in the clinical trials registry that is maintained by the US National Institute of Health (http://clinicaltrials.gov) and the Clinical Trial Registry at the International Clinical Trials Registry Platform of the World Health Organization (http://www.who.int/ictrp/en/). We also reviewed the reference lists of the included trials and any relevant review articles retrieved from the electronic searches, to identify any other potentially relevant trials.
Data collection and analysis
Selection of studies
Two review authors (MJP and BM) independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups.
Possibly relevant ‐ trials that met the inclusion criteria and trials from which it was not possible to determine whether they met the criteria either from their title or abstract.
Excluded ‐ those clearly not meeting the inclusion criteria.
If a title or abstract suggested that the trial was eligible for inclusion, or we could not tell, we obtained a full‐text version of the article and two review authors (MJP and BM) independently assessed it to determine whether it met the inclusion criteria. The review authors resolved discrepancies through discussion or adjudication by a third author (SG or RB).
Data extraction and management
Two review authors (MJP and either MM, BM, SS, JD, or NL) independently extracted data using a standard data extraction form developed for this review. The authors resolved any discrepancies through discussion or adjudication by a third author (SG or RB), until consensus was reached. We pilot tested the data extraction form and modified it accordingly before use. In addition to items for assessing risk of bias and numerical outcome data, we also recorded the following characteristics.
Trial characteristics, including type (e.g. parallel or cross‐over), country, source of funding, and trial registration status (with registration number recorded if available).
Participant characteristics, including age, sex, duration of symptoms, and inclusion/exclusion criteria.
Intervention characteristics, including type of manual therapy or exercise, duration of treatment, use of co‐interventions.
Outcomes reported, including the measurement instrument used and timing of outcome assessment.
One author (MJP) compiled all comparisons and entered outcome data into Review Manager (RevMan) 5.3 (RevMan 2014).
For a particular systematic review outcome there may be a multiplicity of results available in the trial reports (e.g. multiple scales, time points and analyses). To prevent selective inclusion of data based on the results (Page 2013), we used the following pre‐defined decision rules to select data from trials.
Where trialists reported analysis of covariance‐ (ANCOVA) adjusted mean differences along with final values or change from baseline values for the same continuous outcome, we extracted ANCOVA‐adjusted mean differences.
Where trialists reported final values and change from baseline values for the same continuous outcomes, we extracted final values (change from baseline values can be less efficient than final values because measurement of the outcome twice can increase measurement error for outcomes that fluctuate or are difficult to measure precisely (Higgins 2011a)).
Where trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
For cross‐over RCTs, we extracted data from the first period only.
Where trials did not include a measure of overall pain but included one or more other measures of pain, for the purpose of combining data for the primary analysis of overall pain, we combined overall pain with other types of pain in the following hierarchy: unspecified pain; pain with activity; or daytime pain.
Assessment of risk of bias in included studies
Two review authors (MJP and either MM, BM, SS, JD, or NL) independently assessed the risk of bias in included trials using The Cochrane tool for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment (assessed separately for self‐reported and objectively assessed outcomes);
incomplete outcome data;
selective reporting;
other sources of bias (for example, baseline imbalance)
Each item was rated as being at 'Low risk', 'Unclear risk' or 'High risk' of bias. We classified the overall risk of bias as low if all domains were at low risk of bias, as high if at least one domain was at high risk of bias, or as unclear if at least one domain was at unclear risk of bias and no domain was at high risk. We assessed the selective reporting domain for all trials, and documented it in the risk of bias tables, but did not consider it in the overall risk of bias judgement if the only types of selective reporting identified were non‐ or partial reporting of outcomes. Non‐ or partial reporting of outcomes biases the results of meta‐analyses that cannot include the relevant data, not the results of trials, and is therefore considered under the Assessment of reporting biases section (Kirkham 2010). We resolved any discrepancies through discussion or adjudication by a third author (SG or RB).
Measures of treatment effect
We used the Cochrane statistical software, RevMan 5.3 (RevMan 2014), to perform data analysis. We expressed dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous outcomes as mean differences (MDs) with 95% CIs if different trials used the same measurement instrument to measure the same outcome. Alternatively, we analysed continuous outcomes using the standardised mean difference (SMD) when trials measured the same outcome but employed different measurement instruments. To enhance interpretability of dichotomous outcomes, we calculated risk differences and number needed to treat for a beneficial outcome (NNTB) or the number needed to treat for a harmful outcome (NNTH).
Unit of analysis issues
The unit of analysis was the participant for all trials except three (Ebenbichler 1999; Pan 2003; San Segundo 2008), which included participants with bilateral shoulder pain. For these trials, we included the number of shoulders as the denominator in all analyses because the number of participants was not clear. However, only a few participants in both trials had bilateral shoulder pain, so using shoulders as the unit of analysis is likely to have had little impact on the width of the 95% confidence intervals.
Dealing with missing data
When required, we contacted trialists via email (twice, separated by three weeks) to retrieve missing information about trial design, outcome data, or attrition rates such as drop‐outs, losses to follow‐up and post‐randomisation exclusions in the included trials. For continuous outcomes with no standard deviation (SD) reported, we calculated SDs from standard errors (SEs), 95% CIs or P values. If no measures of variation were reported and SDs could not be calculated, we planned to impute SDs from other trials in the same meta‐analysis, using the median of the other SDs available (Ebrahim 2013). Where data were imputed or calculated (e.g. SDs calculated from SEs, 95% CIs or P‐values, or imputed from graphs or from SDs in other trials) we reported this in the tables of Characteristics of included studies.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials. We assessed statistical heterogeneity using the Chi2 statistic and the I2 statistic (Higgins 2002). We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% may not be important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess small study effects, we planned to generate funnel plots for meta‐analyses including at least 10 trials of varying size. If asymmetry in the funnel plot was detected, we planned to review the characteristics of the trials to assess whether the asymmetry was likely due to publication bias or other factors such as methodological or clinical heterogeneity of the trials (Sterne 2011). To assess outcome reporting bias (non‐ or partial reporting of a pre‐specified outcome, which prevents the inclusion of data in a meta‐analysis), we compared the outcomes specified in trial protocols with the outcomes reported in the corresponding trial publications; if trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the trial publications (Dwan 2011; Kirkham 2010).
Data synthesis
For this review update, we identified a large number of trials, which investigated a diverse range of interventions. To define the most clinically important questions to be answered in the review, after data extraction was completed, one review author (MJP) sent the list of all possible trial comparisons to both of the original primary authors of this review (SG and RB). After reviewing the list of possible trial comparisons, both of these review authors discussed and drafted a list of clinically important review questions and categorised each trial comparison under the most appropriate review question. This process was conducted iteratively until all trial comparisons were allocated to a single review question, and was conducted without knowledge of the results of any outcomes. They defined the following review questions.
Are electrotherapy modalities more effective than placebo or no treatment?
Do electrotherapy modalities provide additional benefit when added to other physical therapy interventions (e.g. manual therapy or exercise (or both))?
Are electrotherapy modalities more effective than other active interventions (e.g. glucocorticoid injection, oral NSAID)?
Is one type of electrotherapy modality more effective than another?
As electrotherapy modalities are seldom used in isolation, we considered the first two questions to be the most relevant for clinical practice.
We planned to pool results of trials with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of benefit and harm. Provided trials were homogeneous with respect to other parameters, we planned to pool together trials irrespective of the diagnostic label used in individual trials (e.g. subacromial impingement, rotator cuff tendinitis, supraspinatus tendinitis, impingement) except for calcific tendinitis, which we planned to pool separately. We planned to synthesise effect estimates using a random‐effects meta‐analysis model based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an impact on the results. Where we could not pool data, we presented effect estimates and 95% CIs of each trial in tables and summarised the results in text.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses to investigate the robustness of the treatment effect (of main outcomes) to allocation concealment and participant blinding, by removing the trials that reported inadequate or unclear allocation concealment and lack of participant blinding from the meta‐analysis to see if this changed the overall treatment effect.
Summary of findings tables
We presented the results of the most important comparisons of the review in 'Summary of findings' tables, which summarise the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on outcomes, as recommended by Cochrane (Schünemann 2011a). The 'Summary of findings' tables include an overall grading of the evidence related to each of the main outcomes, using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) approach (Schünemann 2011b).
In the Comments column of the 'Summary of findings' table, we have reported the absolute per cent difference, the relative per cent change from baseline and the NNTB (the NNTB is provided only when the outcome shows a statistically significant difference).
For dichotomous outcomes (global assessment of treatment success, adverse events), we calculated the absolute risk difference using the risk difference statistic in RevMan (RevMan 2014), and expressed the result as a percentage; we calculated the relative per cent change as the risk ratio ‐ 1 and expressed it as a percentage. For continuous outcomes (overall pain, function, pain on motion, quality of life), we calculated the absolute risk difference as the improvement in the intervention group minus the improvement in the control group, expressed in the original units (i.e. mean difference from RevMan divided by units in the original scale), and expressed it as a percentage. The relative per cent change we calculated as the absolute change (or mean difference) divided by the baseline mean of the control group, expressed as a percentage.
In addition to the absolute and relative magnitude of effect provided in the 'Summary of findings' table, for dichotomous outcomes we calculated the NNTB or NNTH from the control group event rate and the risk ratio using the Visual Rx NNT calculator (Cates 2004). For continuous outcomes of function and overall pain, we calculated the NNTB using Wells calculator software, which is available at Cochrane Musculoskeletal editorial office (http://musculoskeletal.cochrane.org). We assumed a minimal clinically important difference (MCID) of 1.5 points on a 10‐point scale (or 15 points on a 100‐point scale) for pain (Hawker 2011), and 10 points on a 100‐point scale for function or disability (for example SPADI, Constant‐Murley, Disabilities of the Arm, Shoulder and Hand (DASH)) for input into the calculator (Angst 2011; Roy 2009; Roy 2010).
Results
Description of studies
Results of the search
The search conducted up to March 2015 resulted in 3488 records across the four databases. Seven additional records were identified from screening reference lists of previously published systematic reviews and included trials. After removal of duplicates, 3166 unique records remained. Of these, 339 were retrieved for full‐text screening based on the title and abstract. We included 47 trials in the review (Abrisham 2011; Aktas 2007; Akyol 2012; Al Dajah 2014; Atya 2012; Bal 2009; Bansal 2011; Baskurt 2006; Berry 1980; Binder 1984; Bingöl 2005; Calis 2011; Celik 2009; Chard 1988; Clews 1987; Dogan 2010; Downing 1986; Ebenbichler 1999; England 1989; Eslamian 2012; Eyigor 2010; Galace de Freitas 2014; Giombini 2006; Grymel‐Kulesza 2007; Johansson 2005; Kelle 2014; Kocyigit 2012; Korkmaz 2010; Kurtai Gursel 2004; Leduc 2003; Montes‐Molina 2012a; Montes‐Molina 2012b; Nykänen 1995; Otadi 2012; Ozgen 2012; Pan 2003; Perron 1997; Polimeni 2003; Rabini 2012; San Segundo 2008; Santamato 2009; Saunders 1995; Shehab 2000; Vecchio 1993; Yavuz 2014; Yeldan 2009; Yildirim 2013). Five additional trials, all of which require translation, are awaiting classification (Dal Conte 1990; Gudmundsen 1987; Güler 2009; Jiménez‐García 2008; Knorre 1990; see table of Characteristics of studies awaiting classification). A flow diagram of the study selection process is presented in Figure 1.
1.
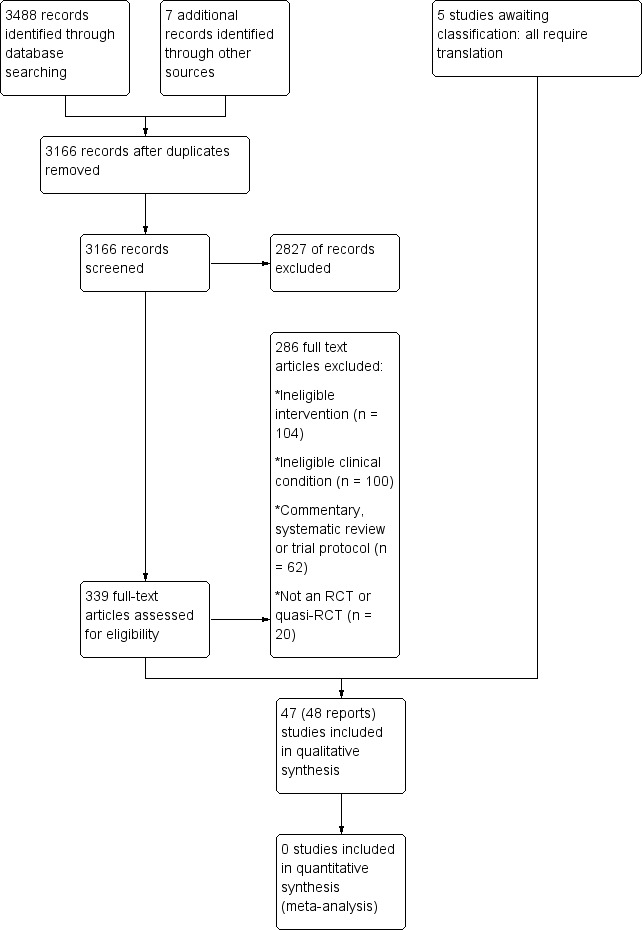
Study flow diagram
Included studies
A full description of all included trials is provided in the Characteristics of included studies tables.
Design
All trials except one were described as RCTs (Kelle 2014 was a quasi‐RCT), and all used a parallel‐group design. Thirty‐nine trials included two intervention arms (Abrisham 2011; Aktas 2007; Akyol 2012; Al Dajah 2014; Atya 2012; Bal 2009; Bansal 2011; Binder 1984; Bingöl 2005; Celik 2009; Chard 1988; Dogan 2010; Downing 1986; Ebenbichler 1999; Eslamian 2012; Eyigor 2010; Galace de Freitas 2014; Grymel‐Kulesza 2007; Johansson 2005; Kocyigit 2012; Korkmaz 2010; Kurtai Gursel 2004; Leduc 2003; Montes‐Molina 2012a; Montes‐Molina 2012b; Nykänen 1995; Otadi 2012; Ozgen 2012; Pan 2003; Perron 1997; Rabini 2012; San Segundo 2008; Santamato 2009; Saunders 1995; Shehab 2000; Vecchio 1993; Yavuz 2014; Yeldan 2009; Yildirim 2013), six included three arms (Baskurt 2006; Calis 2011; Clews 1987; England 1989; Giombini 2006; Kelle 2014), one included four arms (Polimeni 2003) and one included five arms (Berry 1980).
Participants
A total of 2388 participants were included in the 47 trials, and the number of participants per trial ranged from 18 to 200. The median of the mean age of participants was 53 (interquartile range (IQR) 49 to 55) years, and the median of the mean duration of symptoms was 8 (IQR 6 to 13) months. Women comprised 67% of the total sample. Diagnostic labels used by trialists included subacromial impingement syndrome (n = 16: Aktas 2007; Akyol 2012; Al Dajah 2014; Atya 2012; Bal 2009; Baskurt 2006; Calis 2011; Celik 2009; Dogan 2010; Galace de Freitas 2014; Johansson 2005; Kelle 2014; Kocyigit 2012; Yavuz 2014; Yeldan 2009; Yildirim 2013), rotator cuff tendinitis (n = 10: Abrisham 2011; Berry 1980; Binder 1984; Chard 1988; Clews 1987; Eslamian 2012; Eyigor 2010; Otadi 2012; Rabini 2012; Vecchio 1993), supraspinatus tendinitis (n = 10: Bansal 2011; Downing 1986; England 1989; Giombini 2006; Korkmaz 2010; Nykänen 1995; Ozgen 2012; Polimeni 2003; Saunders 1995; Shehab 2000), calcific tendinitis (n = 4: Ebenbichler 1999; Leduc 2003; Pan 2003; Perron 1997), or a mixture of labels (i.e. some participants with impingement, others with tendinitis) (n = 5: Grymel‐Kulesza 2007; Kurtai Gursel 2004; Montes‐Molina 2012a; Montes‐Molina 2012b; San Segundo 2008). However, there were inconsistencies in the diagnostic criteria for (or definitions of) each of the conditions (see Characteristics of included studies tables).
One trial (Bingöl 2005) included participants with non‐specific shoulder pain that was compatible with a diagnosis of rotator cuff disease. One trial (Montes‐Molina 2012a) included participants with rotator cuff disease or adhesive capsulitis, but participants with the latter condition comprised only 5% of the sample. Trials were conducted in Turkey (n = 17), United Kingdom (n = 6), Italy (n = 4), Iran and Spain (n = 3 each), Canada (n = 2), Australia, Austria, Brazil, Egypt, Finland, India, Kuwait, Poland, Saudi Arabia, Sweden, Taiwan, and USA (n = 1 each).
Interventions and Comparisons
A detailed description of the interventions delivered in each trial is presented in the Characteristics of included studies tables, and a summary of the intervention components across trials is presented in Table 2. The trials evaluated physical therapy interventions comprising therapeutic ultrasound (n = 21 trials: Al Dajah 2014; Bansal 2011; Berry 1980; Calis 2011; Celik 2009; Clews 1987; Downing 1986; Ebenbichler 1999; Giombini 2006; Grymel‐Kulesza 2007; Johansson 2005; Kurtai Gursel 2004; Nykänen 1995; Ozgen 2012; Perron 1997; Polimeni 2003; San Segundo 2008; Santamato 2009; Shehab 2000; Yavuz 2014; Yildirim 2013), LLLT (n = 14 trials: Abrisham 2011; Bal 2009; Bingöl 2005; Calis 2011; Dogan 2010; England 1989; Eslamian 2012; Kelle 2014; Montes‐Molina 2012a; Otadi 2012; Saunders 1995; Vecchio 1993; Yavuz 2014; Yeldan 2009), TENS (n = 8 trials: Baskurt 2006; Eyigor 2010; Grymel‐Kulesza 2007; Kocyigit 2012; Korkmaz 2010; Ozgen 2012; Pan 2003; Shehab 2000), PEMF (n = 4 trials; Aktas 2007; Binder 1984; Chard 1988; Galace de Freitas 2014), microwave diathermy (n = 2 trials: Akyol 2012; Rabini 2012), acetic acid iontophoresis (n = 2 trials: Leduc 2003; Perron 1997), high intensity laser therapy (Santamato 2009), light therapy (Montes‐Molina 2012b) and microcurrent electrical stimulation (MENS) (Atya 2012). Sixteen (34%) trials investigated the effect of an electrotherapy modality delivered in isolation (Al Dajah 2014; Atya 2012; Berry 1980; Binder 1984; Chard 1988; Ebenbichler 1999; England 1989; Giombini 2006; Kocyigit 2012; Montes‐Molina 2012a; Montes‐Molina 2012b; Pan 2003; Rabini 2012; Santamato 2009; Saunders 1995; Shehab 2000). The median duration of interventions was three weeks (range 1 to 8) with a median of five treatment sessions delivered per week (range 1 to 10) and a median of 10 treatment sessions provided in total across the treatment period (range 1 to 56). The dosage (e.g. frequency, intensity) of interventions varied, and several trial reports did not include important components such as the duration of each treatment session (Table 2).
1. Characteristics of electrotherapy modalities.
| Therapeutic ultrasound | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Al Dajah 2014 | Frequency: 3 MHz Intensity: 0.5 W/cm2 | 10 minutes | 1 | 1 | 1 |
| Bansal 2011 | Frequency: 1 MHz Intensity: 0.6 W/cm2 | 6‐8 minutes | 10 | 1.5 | 10 |
| Berry 1980 | Frequency: NR Intensity: NR | 10 minutes | 2 | 4 | 8 |
| Calis 2011 | Frequency: 3 MHz Intensity: 1.5 W/cm2 | 5 minutes | 7 | 2 | 15 |
| Celik 2009 | Frequency: 1 MHz Intensity: 1 W/cm2 | 4 minutes | 5 | 3 | 15 |
| Clews 1987 | Frequency: NR Intensity: 0.8 W/cm2 | 15 minutes | 3 | 0.5 | 3 |
| Downing 1986 | Frequency: 1 MHz Intensity: 1.2 W/cm2 | 6 minutes | 3 | 4 | 12 |
| Ebenbichler 1999 | Frequency: 0.89 MHz Intensity: 2.5 W/cm2 | 15 minutes | 3 to 5 | 6 | 24 |
| Giombini 2006 | Frequency: 1 MHz Intensity: 2 W/cm2 | 15 minutes | 3 | 4 | 12 |
| Grymel‐Kulesza 2007 | Frequency: NR Intensity: NR | NR | 5 | 2 | 10 |
| Johansson 2005 | Frequency: 1 MHz Intensity: 1 W/cm2 | 10 minutes | 2 | 5 | 10 |
| Kurtai Gursel 2004 | Frequency: 1 MHz Intensity: 1.5 W/cm2 | 10 minutes | 5 | 3 | 15 |
| Nykanen 1995 | Frequency: 1 MHz Intensity: 1 W/cm2 | 10 minutes | 3 | 3 to 4 | 10 to 12 |
| Ozgen 2012 | Frequency: NR Intensity: 1.5 W/cm2 | 5 minutes | NR | 3 | NR |
| Perron 1997 | Frequency: 1 MHz Intensity: 0.8 W/cm2 | 5 minutes | 3 | 3 | 9 |
| Polimeni 2003 | Frequency: NR Intensity: 1.5 W/cm2 | NR | 7 | 1.5 | 10 |
| San Segundo 2008 | Frequency: 1 MHz Intensity: 2 W/cm2 | 7 minutes | 3 | 3 | 9 |
| Santamato 2009 | Frequency: 1 MHz Intensity: 2 W/cm2 | 10 minutes | 5 | 2 | 10 |
| Shehab 2000 | Frequency: NR Intensity: 0.5 W/cm2 | 10 minutes | 3 to 5 | 3 to 5 | 13 |
| Yavuz 2014 | Frequency: 1 MHz Intensity: 2 W/cm2 | 5 minutes | 5 | 2 | 10 |
| Yildirim 2013 | Frequency: NR Intensity: 1.5 W/cm2 | 4 or 8 minutes | 5 | 3 | 15 |
| Low‐level laser therapy (LLLT) | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Abrisham 2011 | Wavelength: 890 nm Power: 7‐10 W Frequency: 80‐1500 Hz Intensity: 2 to 4 J/cm2 | 6 minutes | 5 | 2 | 10 |
| Bal 2009 | Wavelength: 904 nm Power: 27 W Frequency: 5500 Hz Intensity: 1.6 J/cm2 | 10 minutes | 5 | 2 | 10 |
| Bingol 2005 | Wavelength: 904 nm Power: 50 W Frequency: 2000 Hz Intensity: 2.98 J/cm2 | 5 minutes | 5 | 2 | 10 |
| Calis 2011 | Wavelength: 904 nm Power: 6 mW Frequency: 16 Hz Intensity: 1 J/cm2 | 2 minutes | 7 | 2 | 15 |
| Dogan 2010 | Wavelength: 850 nm Power: 100 mV Frequency: NR Intensity: 3 J/cm2 | 5‐6 minutes | 5 | 3 | 14 |
| England 1989 | Wavelength: 904 nm Power: 10 W Frequency: 4000 Hz Intensity: NR | 5 minutes | 3 | 2 | 6 |
| Eslamian 2012 | Wavelength: 830 nm Power: 100 mW Frequency: NR Intensity: 4 J/cm2 | 5 minutes | 3 | 3 to 4 | 10 |
| Kelle 2014 | Wavelength: 904 nm Power: NR Frequency: 3500 Hz Intensity: 2 J/cm2 | 2.5 minutes | 3 | 3 | 9 |
| Montes‐Molina 2012a | Wavelength: 810 nm Power: 100 mW Frequency: NR Intensity: 1.4 J/cm2 | NR | 3 | 4 | 10 |
| Otadi 2012 | Wavelength: 830 nm Power: 30 mW Frequency: NR Intensity: 1 J/cm2 | NR | 3 | 4 | 10 |
| Saunders 1995 | Wavelength: 820 nm Power: 40 mW Frequency: 5000 Hz Intensity: 30 J/cm2 | 3 minutes | 3 | 3 | 9 |
| Vecchio 1993 | Wavelength: 830 nm Power: 30 mW Frequency: NR Intensity: NR | NR | 2 | 8 | 16 |
| Yavuz 2014 | Wavelength: 850 nm Power: 100 mW Frequency: NR Intensity: 3 J/cm2 | 5 minutes | 5 | 2 | 10 |
| Yeldan 2009 | Wavelength: 904 nm Power: NR Frequency: 2000 Hz Intensity: NR | 8 minutes | 5 | 3 | 15 |
| Transcutaneous electrical nerve stimulation (TENS) | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Baskurt 2006 | Frequency: 100 Hz Pulse duration: 0.1 ms | 20 minutes | 1 | 1 | 1 |
| Eyigor 2010 | Frequency: 100 Hz Pulse duration: 150 µsn | NR | 5 | 3 | 15 |
| Grymel‐Kulesza 2007 | Frequency: 100 Hz Pulse duration: 50 µs | NR | 5 | 2 | 10 |
| Kocyigit 2012 | Frequency: 3 Hz Pulse duration: 250 µs | 30 minutes | 1 | 1 | 1 |
| Korkmaz 2010 | Frequency: 100 Hz Pulse duration: 150 µsn | 20 minutes | 5 | 4 | 20 |
| Ozgen 2012 | Frequency: 60 Hz Pulse duration: 60 µsn | 20 minutes | NR | 3 | NR |
| Pan 2003 | Frequency: 95 Hz Pulse duration: 0.5 ms | 20 minutes | 3 | 4 | 12 |
| Shehab 2000 | Frequency: 50 Hz Pulse duration: NR | 30 minutes | 3 to 5 | 3 to 5 | 13 |
| Pulsed electromagnetic field (PEMF) | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Aktas 2007 | Frequency: 50 Hz Intensity: 30 G | 25 minutes | 5 | 3 | 15 |
| Binder 1984 | Frequency: 73 ± 2 Hz Intensity: NR | 5‐9 hours | 7 | 8 | 56 |
| Chard 1988 | Frequency: 72 ± 3 Hz Intensity: NR | 2 or 8 hours | 7 | 8 | 56 |
| Galace de Freitas 2014 | Frequency: 50 Hz Intensity: 200 G | 30 minutes | 3 | 3 | 9 |
| Microwave diathermy | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Akyol 2012 | Power: 100 W Temperature: NR | 20 minutes | 5 | 3 | 15 |
| Rabini 2012 | Power: 40 W Temperature: 38°C | 30 minutes | 3 | 4 | 12 |
| Acetic acid iontophoresis | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Leduc 2003 | Current: 5 mA | 15‐20 minutes | 1 to 2 | 6 | 10 |
| Perron 1997 | Current: 5 mA | 20 minutes | 3 | 3 | 9 |
| High intensity laser therapy | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Santamato 2009 | Wavelength: 1064 nm Power: 6 W Frequency: NR Intensity: 760 mJ/cm2 | 10 minutes | 5 | 2 | 10 |
| Light therapy | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Montes‐Molina 2012b | Wavelength: 950 nm Power: 310 mW Frequency: NR Intensity: 10.3 J/cm2 | NR | 5 | 2 | 10 |
| Microcurrent electrical stimulation | |||||
| Study ID | Dose | Session duration | No. sessions per week | No. weeks treatment | Total no. sessions |
| Atya 2012 | Intensity: 30‐40 mA Pulse frequency: 10 Hz | 20 minutes | 3 | 6 | 18 |
NR = Not reported
Comparators were also diverse, including placebo (Atya 2012; Berry 1980; Binder 1984; Ebenbichler 1999; England 1989; Galace de Freitas 2014; Kocyigit 2012; Saunders 1995), no intervention (Perron 1997), manual therapy (Al Dajah 2014; Bansal 2011; Clews 1987), exercise (Giombini 2006), glucocorticoid injection (Berry 1980; Eyigor 2010; Kelle 2014; Rabini 2012), acupuncture (Berry 1980; Johansson 2005), oral NSAID (England 1989), extracorporeal shock wave treatment (Pan 2003), sodium hyaluronate injection (Ozgen 2012), hot pack (Baskurt 2006) and cryotherapy (Grymel‐Kulesza 2007).
Twenty‐two trials investigated whether there is benefit in adding an electrotherapy modality to another physical therapy intervention (Abrisham 2011; Aktas 2007; Akyol 2012; Bal 2009; Baskurt 2006; Bingöl 2005; Calis 2011; Celik 2009; Clews 1987; Dogan 2010; Downing 1986; Eslamian 2012; Galace de Freitas 2014; Kelle 2014; Kurtai Gursel 2004; Leduc 2003; Nykänen 1995; Otadi 2012; Polimeni 2003; San Segundo 2008; Vecchio 1993; Yeldan 2009).
Twelve trials compared one type of electrotherapy modality with another (Binder 1984; Calis 2011; Chard 1988; Giombini 2006; Korkmaz 2010; Montes‐Molina 2012a; Montes‐Molina 2012b; Polimeni 2003; Santamato 2009; Shehab 2000; Yavuz 2014; Yildirim 2013).
Outcomes
The outcomes measured in each trial are summarised in Table 3. Of the main outcomes, most trials included a measure of overall pain (n = 40) and function (n = 33), but fewer trials included measures of pain on motion (n = 15), global assessment of treatment success (n = 10), quality of life (n = 5) or adverse events (n = 19). Overall pain was most commonly measured using a zero to 10 or zero to 100 VAS, although several different descriptors for the maximum score on the scale (e.g. "worst imaginable pain", "severe pain", "intolerable pain") were noted. Function was most commonly measured using the Constant‐Murley Score (n = 15) or SPADI (n = 7). Of the other outcomes, most trials included measures of range of motion (n = 26), but fewer included measures of night pain (n = 16), pain with resisted movement (n = 5), strength (n = 10), work disability (n = 1) or surgery (n = 1).
2. Outcome matrix.
| Study ID | Overall pain | Function | Pain on motion | Global assessment | Quality of life | Adverse events |
| Abrisham 2011 | X | X | ||||
| Aktas 2007 | X | X | X | |||
| Akyol 2012 | X | X | X | X | X | |
| Al Dajah 2014 | X | |||||
| Atya 2012 | X | X | ||||
| Bal 2009 | X | X | X | |||
| Bansal 2011 | X | |||||
| Baskurt 2006 | X | |||||
| Berry 1980 | X | X | X | |||
| Binder 1984 | X | X | X | |||
| Bingol 2005 | X | X | ||||
| Calis 2011 | X | X | X | |||
| Celik 2009 | X | X | ||||
| Chard 1988 | X | X | X | |||
| Clews 1987 | X | |||||
| Dogan 2010 | X | X | X | |||
| Downing 1986 | X | X | X | |||
| Ebenbichler 1999 | X | X | X | X | X | X |
| England 1989 | X | X | ||||
| Eslamian 2012 | X | X | ||||
| Eyigor 2010 | X | X | X | X | X | |
| Galace de Freitas 2014 | X | X | ||||
| Giombini 2006 | X | X | X | X | X | |
| Grymel‐Kulesza 2007 | ||||||
| Johansson 2005 | X | X | ||||
| Kelle 2014 | X | X | X | X | X | |
| Kocyigit 2012 | X | |||||
| Korkmaz 2010 | X | X | X | X | X | X |
| Kurtai Gursel 2004 | X | X | X | |||
| Leduc 2003 | X | |||||
| Montes‐Molina 2012a | X | X | X | |||
| Montes‐Molina 2012b | X | X | X | |||
| Nykanen 1995 | X | X | ||||
| Otadi 2012 | X | X | ||||
| Ozgen 2012 | X | X | X | X | X | |
| Pan 2003 | X | X | X | |||
| Perron 1997 | X | |||||
| Polimeni 2003 | X | |||||
| Rabini 2012 | X | X | X | |||
| San Segundo 2008 | X | X | ||||
| Santamato 2009 | X | X | ||||
| Saunders 1995 | X | |||||
| Shehab 2000 | X | |||||
| Vecchio 1993 | X | X | X | X | ||
| Yavuz 2014 | X | X | ||||
| Yeldan 2009 | X | X | X | X | ||
| Yildirim 2013 | X | X | ||||
| FREQUENCY | 40 | 33 | 15 | 10 | 5 | 19 |
Excluded studies
We excluded 286 full‐text articles. Many of these had been retrieved for possible inclusion in one of the other three reviews in this series (i.e. investigated effects of manual therapy and exercise for rotator cuff disease or adhesive capsulitis, or electrotherapy modalities for adhesive capsulitis). The reasons for exclusion were that the intervention was ineligible (n = 104), the clinical condition was ineligible (n = 100), the article was a commentary, systematic review or trial protocol (n = 62), or the study was not an RCT or quasi‐RCT (n = 20). We have listed in the table of Characteristics of excluded studies seven studies which required full‐text screening by a third author (the full list of 286 excluded studies is available on request).
Risk of bias in included studies
A summary of the risk of bias in included trials is presented in Figure 2 and Figure 3.
2.
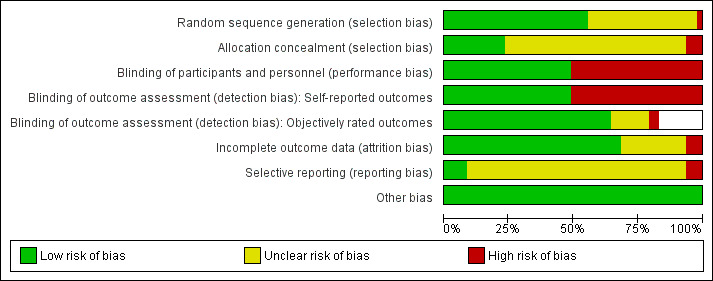
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
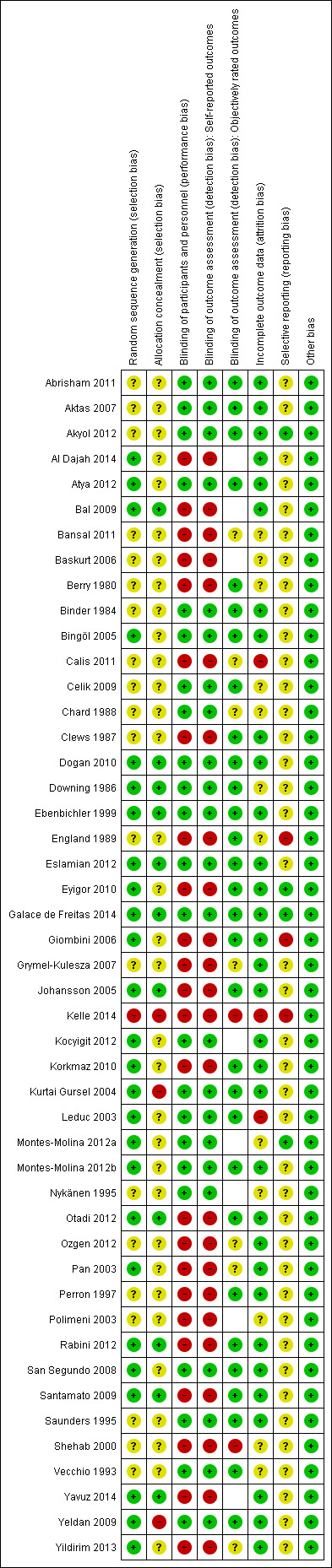
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method used to generate and conceal the allocation sequence was reported in 26 (55%) and 11 (23%) trials, respectively. Only 11 (23%) trials used appropriate methods to both generate and conceal the allocation sequence, and so were rated at low risk of allocation bias. We rated three (6%) trials at high risk of allocation bias because the allocator was aware of the randomisation scheme. In 20 (43%) trials the method of sequence generation was not reported and in 33 (70%) trials the method of allocation concealment was not reported. The risk of allocation bias in these trials was therefore unclear.
Blinding
We rated 23 (49%) trials at low risk of performance bias because participants were successfully blinded. We rated the remaining 24 (51%) trials at high risk of performance bias. Participants in these trial were not blinded, and their beliefs about the intervention they received may have influenced them to deviate from the interventions as planned.
Self‐reported outcomes were measured in all trials. We rated 23 (49%) trials at low risk of detection bias because it was clear that participants were blinded, and the remaining 24 (51%) trials at high risk of detection bias for self‐reported outcomes because participants were not blinded. Of 39 trials with outcome measures that were objectively rated (e.g. range of motion, strength), blinding of outcome assessors was reported in 30 (77%) trials and thus we rated these trials at low risk of detection bias for objective outcomes. In two (5%) trials there was no blinding of assessors of objective outcomes, so the risk of detection bias for objective outcomes was high. In seven (18%) trials it was unclear whether such blinding was done, so the risk of detection bias for objective outcomes was unclear.
Incomplete outcome data
Thirty‐two (68%) trials either had no dropouts, losses to follow‐up or exclusions, or had a small amount of attrition that was deemed unlikely to bias the results. In three (6%) trials there was differential dropout across groups, with reasons that appeared to be related to the treatments received, and thus we rated these trials at high risk of attrition bias. In the remaining 12 (26%) trials the quantity of or reasons for incomplete outcome data were not reported so the risk of attrition bias was unclear.
Selective reporting
We rated four (9%) trials at low risk of selective reporting bias because all outcomes specified in the trial registry entry or trial protocol were fully reported in the trial publication, or all outcomes of importance for rotator cuff disease were reported. We rated three (6%) trials at high risk of selective reporting bias because some of the outcomes that were reported in the trial registry entry or protocol were not reported at all in the results section. We rated the remaining 40 (85%) trials at unclear risk of selective reporting bias for one of two reasons. Firstly, outcome data were completely reported for all outcomes specified in the methods section of the publication, but none of these trials was registered in a trials registry or had an available trial protocol, so it was unclear whether other outcomes were measured but not reported based on the results; or secondly, outcome data were incompletely reported (e.g. reporting means without measures of variation), but it was unclear whether data were incompletely reported based on the nature of the results or because of poor reporting in general (many trials were published before the introduction of reporting guidelines).
Other potential sources of bias
All trials were rated as being free from other potential sources of bias.
Effects of interventions
See: Table 1
Summary data and effect estimates (with 95% CIs) for all trials are presented in the Additional tables section. If an outcome is not referred to within a sub‐section or table, then no data for that outcome was available in the trial(s).
Therapeutic ultrasound
Is therapeutic ultrasound more effective than placebo or no treatment?
In two trials (85 participants), one at high (Berry 1980) and one at low (Ebenbichler 1999) risk of bias overall, therapeutic ultrasound was compared with placebo (i.e. application of an inactive ultrasound device) (Table 4). Ebenbichler 1999 restricted inclusion to patients with calcific tendinitis therefore data were not pooled.
3. Therapeutic ultrasound versus placebo.
|
Study ID: Berry 1980 Participants: Rotator cuff lesions Intervention: Therapeutic ultrasound Control: Placebo ultrasound plus placebo tolmetin sodium | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100, 0 = no pain) at 2 weeks | 33.7 | 34 | 12 | 29.4 | 23.6 | 12 | 4.30 (‐19.12, 27.72) |
| Overall pain (VAS 0‐100, 0 = no pain) at 4 weeks | 41.2 | 36.6 | 12 | 22 | 28.6 | 12 | 19.20 (‐7.08, 45.48) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 2 weeks | 96.3 | 34.2 | 12 | 107.3 | 25.1 | 12 | ‐11.00 (‐35.00, 13.00) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 4 weeks | 95.6 | 37.1 | 12 | 120.8 | 30.1 | 12 | ‐25.20 (‐52.23, 1.83) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (participant does not need a glucocorticoid injection, according to clinician) at 4 weeks | 6 | 12 | 9 | 12 | 0.67 (0.35, 1.28) | ||
|
Study ID: Ebenbichler 1999 Participants: Calcific tendinitis Intervention: Therapeutic ultrasound Control: Placebo ultrasound | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (Binder's pain score 0‐52, 0 = no pain) change from baseline to 6 weeks | ‐14.9 | 9.71 | 32 | ‐6.3 | 9.73 | 29 | ‐8.60 (‐13.48, ‐3.72) |
| Overall pain (Binder's pain score 0‐52, 0 = no pain) change from baseline to 9 months | ‐13.7 | 12.54 | 31 | ‐11.3 | 12.84 | 25 | ‐2.40 (‐9.09, 4.29) |
| Function (Constant‐Murley total score 0‐100, higher = better function) change from baseline to 6 weeks | 17.8 | 16.09 | 32 | 3.7 | 18.40 | 29 | 14.10 (5.39, 22.81) |
| Function (Constant‐Murley total score 0‐100, higher = better function) change from baseline to 9 months | 15.7 | 19.63 | 31 | 12.4 | 18.41 | 25 | 3.30 (‐6.69, 13.29) |
| Quality of life (VAS 0‐10, 0 = excellent quality) change from baseline to 6 weeks | 2.6 | 2.50 | 32 | 0.4 | 2.63 | 29 | 2.20 (0.91, 3.49) |
| Quality of life (VAS 0‐10, 0 = excellent quality) change from baseline to 9 months | 2.4 | 3.27 | 31 | 1.9 | 2.66 | 25 | 0.50 (‐1.05, 2.05) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("clinical improvement", not defined) at 6 weeks | 29 | 32 | 15 | 29 | 1.75 (1.21, 2.53) | ||
| Global assessment of treatment success ("clinical improvement", not defined) at 9 months | 24 | 31 | 14 | 25 | 1.38 (0.93, 2.05) | ||
| Requring surgery during 9 month treatment and follow‐up period | Zero events in both groups | ||||||
| Total adverse events during 9 month treatment and follow‐up period | Zero events in both groups | ||||||
| Work status | "…the number of days lost from work during treatment and follow‐up were moderate...nine patients missed work (four and five, respectively)". | ||||||
Details of the ultrasound were as follows: 0.89 MHz frequency, 2.5 W/cm2 intensity for 15 minutes, three to five times a week for six weeks in Ebenbichler 1999; in Berry 1980, frequency and intensity were not reported, but duration was twice a week for four weeks. The only outcomes measured in both trials were overall pain and global treatment success.
Berry 1980 found no statistically significant differences between ultrasound for four weeks and placebo in overall pain (mean 41.2 versus 22 on a 100‐point scale, MD 19.20, 95% CI ‐7.08 to 45.48, 24 participants), global treatment success (50% (6/12) versus 75% (9/12), RR 0.67, 95% CI 0.35 to 1.28, 24 participants) or shoulder abduction (mean 95.6 versus 120.8 degrees, MD ‐25.20, 95% CI ‐52.23 to 1.83, 24 participants) at four weeks, but the 95% CIs were very wide. The trialists did not report measuring adverse events. We downgraded by one point for high risk of performance bias in this trial (there were additional treatment arms other than ultrasound and placebo, which may have led participants to have different expectations about the treatment they were receiving), and one point for imprecision, and so consider this evidence to be low quality.
Ebenbichler 1999 found clinically important differences favouring therapeutic ultrasound over placebo at six weeks in terms of overall pain (mean change ‐14.9 versus ‐6.3 on a 52‐point scale, MD ‐8.60, 95% CI ‐13.48 to ‐3.72, 61 participants), function (mean change 17.8 versus 3.7 on a 100‐point scale, MD 14.10, 95% CI 5.39 to 22.81, 61 participants), global treatment success (91% (29/32) versus 52% (15/29), RR 1.75, 95% CI 1.21 to 2.53, 61 participants) and quality of life (mean change 2.6 versus 0.4 on a 10‐point scale, MD 2.20, 95% CI 0.91 to 3.49, 61 participants). Between‐group differences were not important at nine months for overall pain (mean change ‐13.7 versus ‐11.3 on a 52‐point scale, MD ‐2.40, 95% CI ‐9.09 to 4.29, 56 participants), function (mean change 15.7 versus 12.4 on a 100‐point scale, MD 3.30, 95% CI ‐6.69 to 13.29, 56 participants), global treatment success (77% (24/31) versus 56% (14/25), RR 1.38, 95% CI 0.93 to 2.05, 56 participants) and quality of life (mean change 2.4 versus 1.9 on a 10‐point scale, MD 0.50, 95% CI ‐1.05 to 2.05, 56 participants). Night pain was measured, but no data were reported. No participant reported adverse events (see Table 1). We downgraded by one point for imprecision and one point for indirectness, as pulsed ultrasound was delivered to participants with calcific tendinitis, so results may not generalise to participants receiving continuous ultrasound, or to other participant subgroups. We therefore consider this evidence to be low quality.
Does therapeutic ultrasound provide additional benefits over other physical therapy interventions (e.g. manual therapy or exercise (or both)) alone?
Eight trials (277 participants) examined whether there is benefit in adding therapeutic ultrasound to another physical therapy intervention (e.g. manual therapy, exercise, TENS, interferential current, ice or multi‐modal physical therapy) (Calis 2011; Celik 2009; Clews 1987; Downing 1986; Kurtai Gursel 2004; Nykänen 1995; Polimeni 2003; San Segundo 2008) (Table 5). The overall risk of bias was high in four trials (Calis 2011; Clews 1987; Kurtai Gursel 2004; Polimeni 2003) and unclear in four trials (Celik 2009; Downing 1986; Nykänen 1995; San Segundo 2008). Due to the variation in comparators, we did not perform any meta‐analyses of the data.
4. Therapeutic ultrasound as add‐on to other physical therapy.
|
Study ID: Calis 2011 Participants: Subacromial impingement syndrome Intervention: Therapeutic ultrasound plus exercise plus hot pack Control: Exercise plus hot pack | |||||||
| Outcome | Intervention | Control | Effect Estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 3 weeks | 2.21 | 2.09 | 21 | 3.96 | 2.71 | 16 | ‐1.75 (‐3.35, ‐0.15) |
| Function (Constant‐Murley total score: 0‐100, higher score = better function) at 3 weeks | 62.85 | 6.85 | 21 | 56.25 | 13.12 | 16 | 6.60 (‐0.46, 13.66) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 3 weeks | 4.24 | 2.26 | 21 | 5.51 | 1.89 | 16 | ‐1.27 (‐2.61, 0.07) |
| Night pain (VAS 0‐10, 0 = no pain) at 3 weeks | 3.74 | 2.18 | 21 | 4.84 | 2.72 | 16 | ‐1.10 (‐2.73, 0.53) |
| Shoulder abduction (degrees, unclear if active or passive) at 3 weeks | 155.95 | 9.21 | 21 | 150.37 | 5.03 | 16 | 5.58 (0.93, 10.23) |
| Shoulder flexion (degrees, unclear if active or passive) at 3 weeks | 177.04 | 3.74 | 21 | 172.18 | 6.93 | 16 | 4.86 (1.11, 8.61) |
| Shoulder internal rotation (degrees, unclear if active or passive) at 3 weeks | 74.85 | 7.29 | 21 | 69.18 | 7.67 | 16 | 5.67 (0.79, 10.55) |
| Shoulder external rotation (degrees, unclear if active or passive) at 3 weeks | 81.66 | 5.82 | 21 | 78.25 | 6.72 | 16 | 3.41 (‐0.72, 7.54) |
|
Study ID: Celik 2009 Participants: Subacromial impingement syndrome Intervention: Therapeutic ultrasound plus TENS plus exercise Control: Placebo ultrasound plus TENS plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 3 weeks | 3 | NR | 20 | 2 | NR | 16 | 1 (95% CI not estimable) |
| Overall pain (VAS 0‐10, 0 = no pain) at 6 weeks | 2 | NR | 20 | 1 | NR | 16 | 1 (95% CI not estimable) |
| Function (Constant‐Murley score 0‐100, higher score = better function) at 3 weeks | 58.3 | 9.07 | 20 | 61.06 | 8.06 | 16 | ‐2.76 (‐8.36, 2.84) |
| Function (Constant‐Murley score 0‐100, higher score = better function) at 6 weeks | 65.65 | 7.65 | 20 | 65.25 | 7.61 | 16 | 0.40 (‐4.61, 5.41) |
| Shoulder forward elevation (degrees) at 3 weeks | 170.2 | 9.87 | 20 | 174.38 | 8.94 | 16 | ‐4.18 (‐10.34, 1.98) |
| Shoulder forward elevation (degrees) at 6 weeks | 175.55 | 6 | 20 | 177.38 | 4.43 | 16 | ‐1.83 (‐5.24, 1.58) |
| Shoulder internal rotation (degrees) at 3 weeks | 75.2 | 14.93 | 20 | 84.19 | 7.57 | 16 | ‐8.99 (‐16.51, ‐1.47) |
| Shoulder internal rotation (degrees) at 6 weeks | 83.15 | 10.9 | 20 | 87.06 | 6.77 | 16 | ‐3.91 (‐9.73, 1.91) |
| Shoulder external rotation (degrees) at 3 weeks | 77.15 | 13.36 | 20 | 79.75 | 14.6 | 16 | ‐2.60 (‐11.84, 6.64) |
| Shoulder external rotation (degrees) at 6 weeks | 84.35 | 9.61 | 20 | 84.63 | 8.36 | 16 | ‐0.28 (‐6.16, 5.60) |
|
Study ID: Clews 1987 Participants: Rotator cuff tendinitis Intervention: Therapeutic ultrasound plus ice Control: Placebo ultrasound plus ice | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Pain after strength test (VAS 0‐10) at 3 days | 3.2 | 1.2 | 6 | 2.7 | 1.9 | 6 | 0.50 (‐1.30, 2.30) |
| Strength (maximal isometric force production, measured in peak force) % change from baseline to 3 days | 11 | 9.5 | 6 | ‐1.5 | 9 | 6 | 12.50 (2.03, 22.97) |
|
Study ID: Downing 1986 Participants: Supraspinatus tendinitis or subacromial bursitis Intervention: Therapeutic ultrasound plus exercise plus NSAID Control: Placebo ultrasound plus exercise plus NSAID | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (4‐point categorical rating scale, 0 = no pain) at 4 weeks | "No significant difference between the sham and true US groups, however, existed in the proportion of patients who improved" | ||||||
| Function (any vs no interference with sleep, dress, work, grooming and sports) at 4 weeks | "Approximately one half of the patients in both groups improved in each category but, again, no significant difference existed between the mean scores of the sham and true US groups" | ||||||
| Global assessment of treatment success at 4 weeks | "Both the patients and the physician recorded that 50% of the patients improved their overall status." | ||||||
| Shoulder flexion (degrees, unclear if active or passive) at 4 weeks | 87 | 6.63 | 11 | 89 | 3 | 9 | ‐2.00 (‐6.38, 2.38) |
| Shoulder abduction (degrees, unclear if active or passive) at 4 weeks | 85 | 13.27 | 11 | 80 | 9 | 9 | 5.00 (‐4.80, 14.80) |
| Shoulder internal rotation (degrees, unclear if active or passive) at 4 weeks | 76 | 23.22 | 11 | 58 | 27 | 9 | 18.00 (‐4.35, 40.35) |
| Shoulder external rotation (degrees, unclear if active or passive) at 4 weeks | 75 | 39.80 | 11 | 72 | 24 | 9 | 3.00 (‐25.27, 31.27) |
|
Study ID: Kurtai Gursel 2004 Participants: Supraspinatus tendinosis, subacromial bursitis, rotator cuff tear or bicipital tendinosis Intervention: Therapeutic ultrasound plus hot pack plus interferential current plus exercise Control: Sham ultrasound plus hot pack plus interferential current plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (0‐3 categorical rating scale, 0 = no pain) at 3 weeks | 1 | 0.1 | 17 | 1.3 | 0.4 | 16 | ‐0.30 (‐0.50, ‐0.10) |
| Function (Dutch SDQ 0‐100, higher = worse function) at 3 weeks | 41.5 | 20.3 | 17 | 38.2 | 15.6 | 16 | 3.30 (‐9.01, 15.61) |
| Pain on motion (0‐3 categorical rating scale, 0 = no pain) at 3 weeks | 1.9 | 0.2 | 17 | 2.1 | 0.2 | 16 | ‐0.20 (‐0.34, ‐0.06) |
| Active shoulder abduction (degrees) at 3 weeks | 150.2 | 20 | 17 | 162.2 | 16.7 | 16 | ‐12.00 (‐24.54, 0.54) |
| Active shoulder flexion (degrees) at 3 weeks | 156.4 | 12.6 | 17 | 160.3 | 12 | 16 | ‐3.90 (‐12.29, 4.49) |
| Active shoulder extension (degrees) at 3 weeks | 51.7 | 9 | 17 | 57.2 | 7.9 | 16 | ‐5.50 (‐11.27, 0.27) |
| Active shoulder external rotation (degrees) at 3 weeks | 81.4 | 15.5 | 17 | 87.8 | 5.4 | 16 | ‐6.40 (‐14.23, 1.43) |
| Active shoulder internal rotation (degrees) at 3 weeks | 71.4 | 18.7 | 17 | 72.2 | 13.4 | 16 | ‐0.80 (‐11.85, 10.25) |
|
Study ID: Nykanen 1995 Participants: Painful arc or supraspinatus tendinopathy/tendinitis Intervention: Therapeutic ultrasound plus massage plus exercises Control: Sham ultrasound plus massage plus exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (Pain Index 1‐5, higher score = worse pain) at 3‐4 weeks | 2.5 | 0.7 | 35 | 2.4 | 0.9 | 37 | 0.10 (‐0.27, 0.47) |
| Overall pain (Pain Index 4‐20, higher score = worse pain) at 4 months | 13 | 5 | 32 | 13 | 4 | 35 | 0.00 (‐2.18, 2.18) |
| Overall pain (Pain Index 4‐20, higher score = worse pain) at 12 months | 13 | 5 | 30 | 13 | 4 | 37 | 0.00 (‐2.21, 2.21) |
| Function (ADL‐score 2‐10, higher score = worse function): at 3‐4 weeks | 4.2 | 1.3 | 35 | 4.4 | 1.4 | 37 | ‐0.20 (‐0.82, 0.42) |
| Function (ADL‐score 3‐14, higher score = worse function): at 4 months | 6.9 | 2.4 | 32 | 7.4 | 2 | 35 | ‐0.50 (‐1.56, 0.56) |
| Function (ADL‐score 3‐14, higher score = worse function): at 12 months | 7 | 2.4 | 30 | 7.3 | 2.3 | 37 | ‐0.30 (‐1.43, 0.83) |
|
Study ID: Polimeni 2003 Participants: Supraspinatus tendinitis or biceps tendinitis Intervention: Therapeutic ultrasound plus mobilisation plus exercises Control: Mobilisation plus exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 10 days | No usable outcome data, though difference between groups not statistically significant | ||||||
| Function (Constant‐Murley total score 0‐100, higher = better function) at 40 days | No usable outcome data, though difference between groups not statistically significant | ||||||
|
Study ID: San Segundo 2008 Participants: Rotator cuff tendinitis or partial rotator cuff tears Intervention: Therapeutic ultrasound plus exercises Control: Placebo ultrasound plus exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐100, 0 = no pain) at 3 weeks | 40.1 | 20.7 | 16 | 44.6 | 20.3 | 15 | ‐4.50 (‐18.94, 9.94) |
| Rest pain (VAS 0‐100, 0 = no pain) at 5 weeks | 35.5 | 21.1 | 16 | 44.9 | 18.9 | 15 | ‐9.40 (‐23.48, 4.68) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 3 weeks | 57.4 | 18.1 | 16 | 50.1 | 15.6 | 15 | 7.30 (‐4.57, 19.17) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 5 weeks | 61.3 | 17.8 | 16 | 51.1 | 16.1 | 15 | 10.20 (‐1.74, 22.14) |
| Night pain (VAS 0‐100, 0 = no pain) at 3 weeks | 20.7 | 21.6 | 16 | 25.2 | 32.5 | 15 | ‐4.50 (‐24.06, 15.06) |
| Night pain (VAS 0‐100, 0 = no pain) at 5 weeks | 15.6 | 20.6 | 16 | 21.6 | 26.3 | 15 | ‐6.00 (‐22.70, 10.70) |
NR = not reported
Apart from one unblinded trial which found less overall pain at three weeks in the 'add‐on' group (Calis 2011), therapeutic ultrasound did not confer additional clinically important benefits compared with other physical therapy interventions alone in the remaining five trials that measured overall pain (Celik 2009; Downing 1986; Kurtai Gursel 2004; Nykänen 1995; San Segundo 2008), seven trials that measured function (Calis 2011; Celik 2009; Downing 1986; Kurtai Gursel 2004; Nykänen 1995; Polimeni 2003; San Segundo 2008) two trials that measured pain on motion (Calis 2011; Kurtai Gursel 2004), one trial that measured global treatment success (Downing 1986), two trials that measured night pain (Calis 2011; San Segundo 2008), four trials that measured range of motion (Calis 2011; Celik 2009; Downing 1986; Kurtai Gursel 2004), or one trial that measured strength (Clews 1987).
None of the trials reported measuring adverse events.
We downgraded the evidence in these eight trials by one point for high or unclear risk of bias overall, and one point for imprecision, and so consider it to be low quality.
Is therapeutic ultrasound more effective than other active interventions (for example, glucocorticoid injection, oral non‐steroidal anti‐inflammatory drug (NSAID))?
Trials compared therapeutic ultrasound with:
manual therapy (3 trials, 82 participants: Al Dajah 2014; Bansal 2011; Clews 1987);
glucocorticoid injection (1 trial, 24 participants: Berry 1980);
glucocorticoid injection plus oral tolmetin sodium (1 trial, 24 participants: Berry 1980);
supervised and home pendular movement and stretching exercises (1 trial, 23 participants: Giombini 2006);
and acupuncture (2 trials, 109 participants: Berry 1980; Johansson 2005)
See Table 6. The overall risk of bias was high in all trials due to the lack of participant blinding.
5. Therapeutic ultrasound versus another active intervention.
|
Study ID: Al Dajah 2014 Participants: Shoulder impingement syndrome Intervention: Therapeutic ultrasound Control: Soft tissue mobilisation and proprioceptive neuromuscular facilitation | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) immediately after 1 treatment session (day 1) | 5.23 | 0.72 | 15 | 3.8 | 0.79 | 15 | 1.43 (0.89, 1.97) |
| External rotation (degrees, unclear if active or passive) immediately after 1 treatment session (day 1) | 40.33 | 5.6 | 15 | 52.4 | 4.9 | 15 | ‐12.07 (‐15.84, ‐8.30) |
|
Study ID: Bansal 2011 Participants: Supraspinatus tendinitis Intervention: Therapeutic ultrasound plus Codman's exercises Control: Deep friction massage technique plus Codman's exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 10 days | 2.1 | NR | 20 | 1.4 | NR | 20 | 0.7 (95% CI not estimable) |
| Active shoulder abduction (degrees) at 10 days | 105.65 | NR | 20 | 107.15 | NR | 20 | ‐1.5 (95% CI not estimable) |
|
Study ID: Berry 1980 Participants: Rotator cuff lesions Intervention: Therapeutic ultrasound Control: Glucocorticoid injection plus tolmetin sodium | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100, 0 = no pain) at 2 weeks | 33.7 | 34 | 12 | 26.2 | 21.3 | 12 | 7.50 (‐15.20, 30.20) |
| Overall pain (VAS 0‐100, 0 = no pain) at 4 weeks | 41.2 | 36.6 | 12 | 29.2 | 24.3 | 12 | 12.00 (‐12.86, 36.86) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 2 weeks | 96.3 | 34.2 | 12 | 95.2 | 22.9 | 12 | 1.10 (‐22.19, 24.39) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 4 weeks | 95.6 | 37.1 | 12 | 93.2 | 25.7 | 12 | 2.40 (‐23.14, 27.94) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (participant does not need a glucocorticoid injection, according to clinician) at 4 weeks | 6 | 12 | 5 | 12 | 1.20 (0.50, 2.88) | ||
|
Study ID: Berry 1980 Participants: Rotator cuff lesions Intervention: Therapeutic ultrasound Control: Glucocorticoid injection plus placebo tolmetin sodium | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100, 0 = no pain) at 2 weeks | 33.7 | 34 | 12 | 20.6 | 20.5 | 12 | 13.10 (‐9.36, 35.56) |
| Overall pain (VAS 0‐100, 0 = no pain) at 4 weeks | 41.2 | 36.6 | 12 | 26.6 | 22.5 | 12 | 14.60 (‐9.71, 38.91) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 2 weeks | 96.3 | 34.2 | 12 | 107.2 | 34.5 | 12 | ‐10.90 (‐38.39, 16.59) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 4 weeks | 95.6 | 37.1 | 12 | 100.6 | 37.7 | 12 | ‐5.00 (‐34.93, 24.93) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (participant does not need a glucocorticoid injection, according to clinician) at 4 weeks | 6 | 12 | 6 | 12 | 1.00 (0.45, 2.23) | ||
|
Study ID: Berry 1980 Participants: Rotator cuff lesions Intervention: Therapeutic ultrasound Control: Acupuncture | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100, 0 = no pain) at 2 weeks | 33.7 | 34 | 12 | 38.6 | 26.7 | 12 | ‐4.90 (‐29.36, 19.56) |
| Overall pain (VAS 0‐100, 0 = no pain) at 4 weeks | 41.2 | 36.6 | 12 | 34.1 | 27.2 | 12 | 7.10 (‐18.70, 32.90) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 2 weeks | 96.3 | 34.2 | 12 | 95.5 | 27.6 | 12 | 0.80 (‐24.07, 25.67) |
| Range of shoulder abduction (degrees, unclear if active or passive) at 4 weeks | 95.6 | 37.1 | 12 | 103.5 | 36.6 | 12 | ‐7.90 (‐37.39, 21.59) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (participant does not need a glucocorticoid injection, according to clinician) at 4 weeks | 6 | 12 | 5 | 12 | 1.20 (0.50, 2.88) | ||
|
Study ID: Clews 1987 Participants: Rotator cuff tendinitis Intervention: Therapeutic ultrasound plus ice Control: Massage plus ice | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Pain after strength test (VAS 0‐10 at strength testing) at 3 days | 3.2 | 1.2 | 6 | 2.8 | 1.2 | 6 | 0.40 (‐0.96, 1.76) |
| Strength (maximal isometric force production, measured in peak force) % change from baseline to 3 days | 11 | 9.5 | 6 | 9.8 | 8.8 | 6 | 1.20 (‐9.16, 11.56) |
|
Study ID: Giombini 2006 Participants: Supraspinatus tendinopathy Intervention: Therapeutic ultrasound Control: Supervised and home exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) at 4 weeks | 5.8 | 0.96 | 12 | 5.3 | 0.65 | 11 | 0.50 (‐0.17, 1.17) |
| Rest pain (VAS 0‐10, 0 = no pain) at 10 weeks | 5.15 | 0.87 | 12 | 4.9 | 0.88 | 11 | 0.25 (‐0.47, 0.97) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 4 weeks | 60 | 3.21 | 12 | 61.2 | 4.28 | 11 | ‐1.20 (‐4.31, 1.91) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 10 weeks | 61.75 | 4.18 | 12 | 63.27 | 5.56 | 11 | ‐1.52 (‐5.57, 2.53) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (ready to return to sport) at 4 weeks | 6 | 12 | 4 | 11 | 1.38 (0.52, 3.61) | ||
| Global assessment of treatment success (ready to return to sport) at 10 weeks | 4 | 12 | 4 | 11 | 0.92 (0.30, 2.81) | ||
| Adverse events | Zero events in both groups | ||||||
|
Study ID: Johansson 2005 Participants: Subacromial impingement syndrome Intervention: Therapeutic ultrasound plus home exercises Control: Acupuncture plus home exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (combined Constant‐Murley, Adolfsson‐Lysholm shoulder score and UCLA‐score, 0‐100, higher score = better function) at 6 weeks | 76 | 11 | 41 | 79 | 9 | 44 | ‐3.00 (‐7.29, 1.29) |
| Function (combined Constant‐Murley, Adolfsson‐Lysholm shoulder score and UCLA‐score, 0‐100, higher score = better function) at 6 months | 83 | 15 | 41 | 83 | 17 | 44 | 0.00 (‐6.81, 6.81) |
| Function (combined Constant‐Murley, Adolfsson‐Lysholm shoulder score and UCLA‐score, 0‐100, higher score = better function) at 12 months | 85 | 14 | 41 | 88 | 13 | 44 | ‐3.00 (‐8.75, 2.75) |
| Total adverse events during 12‐month follow‐up period | Zero events in both groups | ||||||
NR = not reported
There were no clinically important differences in overall pain (i.e. > 1.5 on a 10‐point scale (Hawker 2011)) between therapeutic ultrasound and:
one session of soft tissue mobilisation and proprioceptive neuromuscular facilitation immediately post‐treatment (mean 5.23 versus 3.8 on a 10‐point scale, MD 1.43, 95% CI 0.89 to 1.97, 30 participants, Al Dajah 2014);
deep friction massage daily for 10 days, at 10 days (mean 2.1 versus 1.4 on a 10‐point scale, MD 0.7, 95% CI not estimable, 40 participants, Bansal 2011);
massage daily for three days, at three days (mean 3.2 versus 2.8 on a 10‐point scale, MD 0.40, 95% CI ‐0.96 to 1.76, 12 participants, Clews 1987);
a single glucocorticoid injection, at 4 weeks (mean 41.2 versus 26.6 on a 100‐point scale, MD 14.60, 95% CI ‐9.71 to 38.91, 24 participants, Berry 1980);
a single glucocorticoid injection plus oral tolmetin sodium daily for four weeks, at four weeks (mean 41.2 versus 29.2 on a 100‐point scale, MD 12.00, 95% CI ‐12.86 to 36.86, 24 participants, Berry 1980);
supervised and home pendular movement and stretching exercises weekly for four weeks, at four weeks (mean 5.8 versus 5.3 on a 10‐point scale, MD 0.50, 95% CI ‐0.17 to 1.17, 23 participants, Giombini 2006), and;
acupuncture weekly for four weeks, at four weeks (mean 41.2 versus 34.1 on a 100‐point scale, MD 7.10, 95% CI ‐18.70 to 32.90, 24 participants, Berry 1980).
Function was measured in only two trials, and was similar between groups receiving therapeutic ultrasound and:
supervised and home pendular movement and stretching exercises at four weeks (mean 60 versus 61.2 on a 100‐point scale, MD ‐1.20, 95% CI ‐4.31 to 1.91, 23 participants, Giombini 2006) and 10 weeks (mean 61.75 versus 63.27 on a 100‐point scale, MD ‐1.52, 95% CI ‐5.57 to 2.53, 23 participants, Giombini 2006), and;
acupuncture at six weeks (mean 76 versus 79 on a 100‐point scale, MD ‐3.00, 95% CI ‐7.29 to 1.29, 85 participants, Johansson 2005) and 12 months (mean 85 versus 88 on a 100‐point scale, MD ‐3.00, 95% CI ‐8.75 to 2.75, 85 participants, Johansson 2005).
Adverse events were reported as having been measured in only two of the six trials (Giombini 2006; Johansson 2005) and none were reported by any participant. No important between‐group differences in global treatment success (Berry 1980; Giombini 2006), range of motion (Al Dajah 2014; Bansal 2011; Berry 1980) or strength (Clews 1987) were found.
We downgraded the evidence in these trials by two points for high risk of performance and detection bias, and one point for imprecision, and thus consider it to be very low quality.
Low‐level laser therapy (LLLT)
Is LLLT more effective than placebo or no treatment?
In two trials (44 participants), both at unclear risk of bias overall, LLLT was compared with placebo (i.e. application of an inactive laser) (England 1989; Saunders 1995) (Table 7). The dosage of LLLT differed slightly between the trials; in England 1989, LLLT consisted of 904 nm wavelength, 10 W power, 4000 Hz frequency, intensity not reported, for five minutes, three times a week for two weeks, while in Saunders 1995, LLLT consisted of 820 nm wavelength, 40 mW power, 5000 Hz frequency, 30 J/cm2 intensity, for three minutes, three times a week for three weeks. Different outcomes were measured in each trial so no meta‐analyses were possible.
6. LLLT versus placebo.
|
Study ID: England 1989 Participants: Supraspinatus or bicipital tendinitis Intervention: Low‐level laser therapy (LLLT) Control: Placebo LLLT | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Median difference (95% CI) | |
| Overall pain (VAS 0‐10, higher score = more pain) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 2.5 (2.01, 3) |
| Function (VAS 0‐10, higher score = worse function) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 1.5 (‐0.01, 3.99) |
| Active shoulder abduction (degrees) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 20 (10, 40) |
| Active shoulder flexion (degrees) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 15 (5, 29) |
| Active shoulder extension (degrees) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 6 (0, 20) |
|
Study ID: Saunders 1995 Participants: Supraspinatus tendinitis Intervention: Low‐level laser therapy (LLLT) Control: Placebo LLLT | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Strength (muscle force (N)) at 3 weeks | 172.01 | 40.70 | 12 | 125.55 | 27.44 | 12 | 46.46 (18.69, 74.23) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Overall pain (number of participants with "improved" pain) at 3 weeks | 10 | 12 | 5 | 12 | 2.00 (0.98, 4.09) | ||
NR = not reported
There were favourable effects of LLLT in both trials with respect to overall pain (median difference 2.5 on a 10‐point scale, 95% CI 2.01 to 3.00, 20 participants), function (median difference 1.5 on a 10‐point scale, 95% CI ‐0.01 to 3.99, 20 participants), active shoulder abduction (median difference 20 degrees, 95% CI 10.00 to 40.00, 20 participants), flexion (median difference 15 degrees, 95% CI 5.00 to 29.00, 20 participants) and extension (median difference 6 degrees, 95% CI 0.00 to 20.00, 20 participants) at two weeks, and pain relief (83% (10/12) versus 42% (5/12), RR 2.00, 95% CI 0.98 to 4.09, 24 participants) and strength (MD 46.46, 95% CI 18.69 to 74.23; force (N); 24 participants) at three weeks.
Neither trial reported measuring adverse events.
We considered the evidence from these two trials to be low quality after downgrading by one point for unclear risk of allocation bias, and one point for imprecision.
Does LLLT provide additional benefits over other physical therapy interventions alone?
Ten trials (520 participants) examined whether there is benefit in adding LLLT to another physical therapy intervention (Abrisham 2011; Bal 2009; Bingöl 2005; Calis 2011; Dogan 2010; Eslamian 2012; Kelle 2014; Otadi 2012; Vecchio 1993; Yeldan 2009) (Table 8). The control group received exercise in all trials except for Eslamian 2012, which added LLLT to therapeutic ultrasound plus TENS plus exercise, and Otadi 2012, which added LLLT to therapeutic ultrasound plus exercise. The overall risk of bias was low in two trials (Dogan 2010; Eslamian 2012), unclear in three trials (Abrisham 2011; Bingöl 2005; Vecchio 1993) and high in five trials (Bal 2009; Calis 2011; Kelle 2014; Otadi 2012; Yeldan 2009). The use of different measurement instruments and mixture of final values and change from baseline values across the trials prevented meta‐analysis of data.
7. LLLT as add‐on to other physical therapy.
|
Study ID: Abrisham 2011 Participants: Rotator cuff and bicep tendinitis Intervention: Low‐level laser therapy (LLLT) plus exercise Control: Placebo LLLT plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (10 point scale, 0 = no pain) at 2 weeks | 2.1 | 0.5 | 40 | 3 | 1 | 40 | ‐0.90 (‐1.25, ‐0.55) |
| Active abduction (degrees) at 2 weeks | 102.6 | 6.8 | 40 | 87.9 | 7.9 | 40 | 14.70 (11.47, 17.93) |
| Active flexion (degrees) at 2 weeks | 102.6 | 6.6 | 40 | 88 | 6 | 40 | 14.60 (11.84, 17.36) |
| Active external rotation (degrees) at 3 weeks | 51.3 | 5 | 40 | 49.4 | 4.8 | 40 | 1.90 (‐0.25, 4.05) |
| Total adverse events during 2‐week intervention period | Zero events in both groups | ||||||
|
Study ID: Bal 2009 Participants: Subacromial impingement syndrome Intervention: Low‐level laser therapy (LLLT) plus home exercises Control: Home exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (SPADI total score 0‐100 where higher = worse function) change from baseline to 2 weeks | ‐16.2 | 17.73 | 20 | ‐23.2 | 17.14 | 20 | 7.00 (‐3.81, 17.81) |
| Function (SPADI total score 0‐100 where higher = worse function) change from baseline to 12 weeks | ‐32.7 | 18.58 | 20 | ‐37.2 | 21.28 | 20 | 4.50 (‐7.88, 16.88) |
| Night pain (VAS 0‐100, 0 = no pain) change from baseline 2 weeks | ‐22.7 | 24.36 | 20 | ‐21.7 | ‐19.21 | 20 | ‐1.00 (‐14.60, 12.60) |
| Night pain (VAS 0‐100, 0 = no pain) change from baseline 12 weeks | ‐54.7 | 24.68 | 20 | ‐31.5 | 27.77 | 20 | ‐23.20 (‐39.48, ‐6.92) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("excellent" or "good" result on UCLA) at 2 weeks | 4 | 20 | 3 | 20 | 1.33 (0.34, 5.21) | ||
| Global assessment of treatment success ("excellent" or "good" result on UCLA) at 12 weeks | 17 | 20 | 13 | 20 | 1.31 (0.90, 1.89) | ||
| Total adverse events at 2 weeks | Zero events in both groups | ||||||
| Total adverse events at 12 weeks | Zero events in both groups | ||||||
|
Study ID: Bingol 2005 Participants: Rotator cuff disease Intervention: Low‐level laser therapy (LLLT) plus exercise Control: Placebo LLLT plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | Range | n | Mean | Range | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 2 weeks | 5.65 | (1‐9) | 20 | 5.96 | (0‐9) | 20 | ‐0.31 (95% CI not estimable) |
| Active shoulder abduction (degrees) at 2 weeks | 147.5 | (80‐80) | 20 | 149.5 | (60‐180) | 20 | ‐2 (95% CI not estimable) |
| Active shoulder flexion (degrees) at 2 weeks | 158.5 | (120‐180) | 20 | 160.5 | (120‐180) | 20 | ‐2 (95% CI not estimable) |
| Active shoulder extension (degrees) at 2 weeks | 54 | (30‐60) | 20 | 55.5 | (40‐60) | 20 | ‐1.5 (95% CI not estimable) |
| Active shoulder internal rotation (degrees) at 2 weeks | 63 | (25‐70) | 20 | 61.75 | (30‐70) | 20 | 1.25 (95% CI not estimable) |
| Active external rotation (degrees) at 2 weeks | 69.5 | (30‐90) | 20 | 75 | (30‐90) | 20 | ‐5.5 (95% CI not estimable) |
| Active adduction (degrees) at 2 weeks | 44.75 | (40‐45) | 20 | 43.5 | (25‐45) | 20 | 1.25 (95% CI not estimable) |
| Total adverse events | Zero events in both groups | ||||||
|
Study ID: Calis 2011 Participants: Subacromial impingement syndrome Intervention: Low‐level laser therapy (LLLT) plus exercise plus hot pack Control: Exercise plus hot pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 3 weeks | 2.56 | 2.28 | 15 | 3.96 | 2.71 | 16 | ‐1.40 (‐3.16, 0.36) |
| Function (Constant‐Murley total score: 0‐100, higher score = better function) at 3 weeks | 64.6 | 16.18 | 15 | 56.25 | 13.12 | 16 | 8.35 (‐2.06, 18.76) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 3 weeks | 3.73 | 2.37 | 15 | 5.51 | 1.89 | 16 | ‐1.78 (‐3.30, ‐0.26) |
| Night pain (VAS 0‐10, 0 = no pain) at 3 weeks | 3.68 | 2.85 | 15 | 4.84 | 2.72 | 16 | ‐1.16 (‐3.12, 0.80) |
| Shoulder abduction (degrees, unclear if active or passive) at 3 weeks | 155.8 | 7.35 | 15 | 150.37 | 5.03 | 16 | 5.43 (0.97, 9.89) |
| Shoulder flexion (degrees, unclear if active or passive) at 3 weeks | 174.46 | 6.94 | 15 | 172.18 | 6.93 | 16 | 2.28 (‐2.61, 7.17) |
| Shoulder internal rotation (degrees, unclear if active or passive) at 3 weeks | 70.93 | 6.06 | 15 | 69.18 | 7.67 | 16 | 1.75 (‐3.10, 6.60) |
| Shoulder external rotation (degrees, unclear if active or passive) at 3 weeks | 83.13 | 5.23 | 15 | 78.25 | 6.72 | 16 | 4.88 (0.66, 9.10) |
|
Study ID: Dogan 2010 Participants: Subacromial impingement syndrome Intervention: Low‐level laser therapy (LLLT) plus exercise plus ice Control: Placebo LLLT plus exercise plus ice | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 3 weeks | 3.76 | 1.45 | 30 | 4.63 | 2.1 | 22 | ‐0.87 (‐1.89, 0.15) |
| Function (SPADI total score 0‐100, higher score = worse function) at 3 weeks | 44.33 | 2.8 | 30 | 36.39 | 20.53 | 22 | 7.94 (‐0.70, 16.58) |
| Shoulder flexion (degrees, unclear if active or passive) at 3 weeks | 168 | 22.65 | 30 | 174.31 | 14.98 | 22 | ‐6.31 (‐16.55, 3.93) |
| Shoulder extension (degrees, unclear if active or passive) at 3 weeks | 42.66 | 3.4 | 30 | 42.95 | 3.98 | 22 | ‐0.29 (‐2.35, 1.77) |
| Shoulder abduction (degrees, unclear if active or passive) at 3 weeks | 166.66 | 21.38 | 30 | 172.72 | 16.67 | 22 | ‐6.06 (‐16.41, 4.29) |
| Shoulder adduction (degrees, unclear if active or passive) at 3 weeks | 42 | 4.27 | 30 | 42.04 | 5.26 | 22 | ‐0.04 (‐2.72, 2.64) |
| Shoulder internal rotation (degrees, unclear if active or passive) at 3 weeks | 49.33 | 9.62 | 30 | 49.77 | 4.49 | 22 | ‐0.44 (‐4.36, 3.48) |
| Shoulder external rotation (degrees, unclear if active or passive) at 3 weeks | 44.83 | 5.64 | 30 | 44.09 | 1.97 | 22 | 0.74 (‐1.44, 2.92) |
| Total adverse events during 3‐week treatment period | Zero events in both groups | ||||||
|
Study ID: Eslamian 2012 Participants: Rotator cuff tendinitis Intervention: Low‐level laser therapy (LLLT) plus therapeutic ultrasound, TENS and exercise programme Control: Placebo LLLT plus therapeutic ultrasound, TENS and exercise programme | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 6 weeks | 3.16 | 2.21 | 25 | 5 | 2.67 | 25 | ‐1.84 (‐3.20, ‐0.48) |
| Function (Croft SDQ 0‐22 scale, higher score = greater disability) at 6 weeks | 4.44 | 3.15 | 25 | 8.25 | 5.13 | 25 | ‐3.81 (‐6.17, ‐1.45) |
| Active shoulder abduction (degrees) at 6 weeks | 144.92 | 31.6 | 25 | 132.8 | 31.3 | 25 | 12.12 (‐5.31, 29.55) |
| Active shoulder external rotation (degrees) at 6 weeks | 76.32 | 19.1 | 25 | 78.04 | 19.5 | 25 | ‐1.72 (‐12.42, 8.98) |
|
Study ID: Kelle 2014 Participants: Subacromial impingement syndrome Intervention: Low‐level laser therapy (LLLT) plus home exercises Control: Sham LLLT plus home exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐100) at 3 weeks | 11.1 | 11.6 | 45 | 18.4 | 12.1 | 45 | ‐7.30 (‐12.20, ‐2.40) |
| Rest pain (VAS 0‐100) at 6 months | 11.5 | 13.8 | 45 | 16.3 | 9.5 | 45 | ‐4.80 (‐9.70, 0.10) |
| Function (UCLA 2‐35, higher = better function) at 3 weeks | 25.9 | 4.6 | 45 | 20.2 | 5.5 | 45 | 5.70 (3.61, 7.79) |
| Function (UCLA 2‐35, higher = better function) at 6 months | 26.1 | 5.6 | 45 | 19.9 | 5.5 | 45 | 6.20 (3.91, 8.49) |
| Pain on motion (VAS 0‐100) at 3 weeks | 32.6 | 17.6 | 45 | 43.3 | 17.6 | 45 | ‐10.70 (‐17.97, ‐3.43) |
| Pain on motion (VAS 0‐100) at 6 months | 25.5 | 19.7 | 45 | 40.8 | 18.2 | 45 | ‐15.30 (‐23.14, ‐7.46) |
| Adverse events | Zero events in both groups | ||||||
|
Study ID: Otadi 2012 Participants: Shoulder tendinitis Intervention: Low‐level laser therapy plus therapeutic ultrasound plus exercises Control: Therapeutic ultrasound plus exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant‐Murley score 0‐100, higher = better function) change from baseline to 4 weeks | 19.4 | 19.95 | 21 | 29.95 | 13.05 | 21 | ‐10.55 (‐20.74, ‐0.36) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Overall pain (> 3 point reduction on 0‐10 VAS) at 4 weeks | 15 | 21 | 15 | 21 | 1.00 (0.68, 1.47) | ||
| Overall pain (> 3 point reduction on 0‐10 VAS) at 12 weeks | 8 | 21 | 3 | 21 | 2.67 (0.82, 8.69) | ||
|
Study ID: Vecchio 1993 Participants: Rotator cuff tendinitis Intervention: Low‐level laser therapy (LLLT) plus exercise Control: Placebo LLLT plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) change from baseline to 4 weeks | 2.2 | 2.62 | 19 | 1.4 | 2.40 | 16 | 0.80 (‐0.86, 2.46) |
| Rest pain (VAS 0‐10, 0 = no pain) change from baseline to 8 weeks | 3.9 | 3.05 | 19 | 2.2 | 4.00 | 16 | 1.70 (‐0.69, 4.09) |
| Function (VAS 0‐10, higher = worse function) change from baseline to 4 weeks | 2.9 | 2.62 | 19 | 2 | 3.20 | 16 | 0.90 (‐1.06, 2.86) |
| Function (VAS 0‐10, higher = worse function) change from baseline to 8 weeks | 3.6 | 3.92 | 19 | 2.9 | 4.40 | 16 | 0.70 (‐2.09, 3.49) |
| Pain on motion (VAS 0‐10, 0 = no pain) change from baseline to 4 weeks | 2.7 | 3.49 | 19 | 1.2 | 4.00 | 16 | 1.50 (‐1.01, 4.01) |
| Pain on motion (VAS 0‐10, 0 = no pain) change from baseline to 8 weeks | 3.6 | 3.92 | 19 | 1.8 | 4.80 | 16 | 1.80 (‐1.14, 4.74) |
| Night pain (VAS 0‐10, 0 = no pain) change from baseline to 4 weeks | 3.4 | 3.49 | 19 | 2.1 | 3.60 | 16 | 1.30 (‐1.06, 3.66) |
| Night pain (VAS 0‐10, 0 = no pain) change from baseline to 8 weeks | 4.4 | 3.92 | 19 | 3.2 | 4.80 | 16 | 1.20 (‐1.74, 4.14) |
| Pain on resisted abduction (0‐3 scale, 0 = no pain) change from baseline to 4 weeks | 0.64 | 0.78 | 19 | 0.29 | 1.76 | 16 | 0.35 (‐0.58, 1.28) |
| Pain on resisted abduction (0‐3 scale, 0 = no pain) change from baseline to 8 weeks | 0.71 | 1.05 | 19 | 0.18 | 1.20 | 16 | 0.53 (‐0.22, 1.28) |
| Total range of motion (unclear units, unclear if active or passive) change from baseline to 4 weeks | ‐0.8 | 1.31 | 19 | ‐0.5 | 1.20 | 16 | ‐0.30 (‐1.13, 0.53) |
| Total range of motion (unclear units, unclear if active or passive) change from baseline to 8 weeks | ‐1.5 | 1.31 | 19 | ‐0.8 | 2.00 | 16 | ‐0.70 (‐1.84, 0.44) |
| Total adverse events during 8‐week trial period | Zero events in both groups | ||||||
|
Study ID: Yeldan 2009 Participants: Subacromial impingement syndrome Intervention: Low‐level laser therapy (LLLT) plus exercise plus cold pack Control: Placebo LLLT plus exercise plus cold pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) at 3 weeks | 1.61 | 1.96 | 34 | 1.92 | 1.89 | 26 | ‐0.31 (‐1.29, 0.67) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 3 weeks | 76.67 | 12.73 | 34 | 74.73 | 15.5 | 26 | 1.94 (‐5.40, 9.28) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 3 weeks | 3.7 | 1.69 | 34 | 4.11 | 2.19 | 26 | ‐0.41 (‐1.43, 0.61) |
| Night pain (VAS 0‐10, 0 = no pain) at 3 weeks | 2.29 | 2.06 | 34 | 2.53 | 2.38 | 26 | ‐0.24 (‐1.39, 0.91) |
| Shoulder flexion (degrees, unclear if active or passive) at 3 weeks | 139.52 | 9.89 | 34 | 140.53 | 10.33 | 26 | ‐1.01 (‐6.19, 4.17) |
| Shoulder abduction (degrees, unclear if active or passive) at 3 weeks | 110.67 | 8.79 | 34 | 106.57 | 9.92 | 26 | 4.10 (‐0.72, 8.92) |
| Shoulder external rotation (degrees, unclear if active or passive) at 3 weeks | 63.91 | 5.81 | 34 | 62.5 | 4.66 | 26 | 1.41 (‐1.24, 4.06) |
| Shoulder internal rotation (degrees, unclear if active or passive) at 3 weeks | 71.5 | 4.35 | 34 | 73 | 3.96 | 26 | ‐1.50 (‐3.61, 0.61) |
| Shoulder extension (degrees, unclear if active or passive) at 3 weeks | 41.88 | 6.29 | 34 | 44.3 | 5.04 | 26 | ‐2.42 (‐5.29, 0.45) |
| Strength ‐ shoulder abduction force (kg) at 3 weeks | 18.28 | 2.96 | 34 | 17.88 | 3.43 | 26 | 0.40 (‐1.25, 2.05) |
| Strength ‐ shoulder flexion force (kg) at 3 weeks | 19.54 | 3.21 | 34 | 18.79 | 3.92 | 26 | 0.75 (‐1.10, 2.60) |
| Strength ‐ shoulder external rotation force (kg) at 3 weeks | 20.63 | 3.38 | 34 | 20.74 | 3.21 | 26 | ‐0.11 (‐1.79, 1.57) |
| Strength ‐ shoulder internal rotation force (kg) at 3 weeks | 21.55 | 3.22 | 34 | 20.4 | 4.12 | 26 | 1.15 (‐0.77, 3.07) |
| Strength ‐ shoulder extension force (kg) at 3 weeks | 18.8 | 3.13 | 34 | 17.76 | 2.69 | 26 | 1.04 (‐0.44, 2.52) |
| Total adverse events during 3‐week trial period | Zero events in both groups | ||||||
Of the nine trials that measured overall pain, only one (Eslamian 2012) found that LLLT conferred clinically important benefits when added to therapeutic ultrasound and exercise (at six weeks). Of the eight trials that measured function, only two (Eslamian 2012; Otadi 2012) found that LLLT conferred additional clinically important benefits over other physical therapy interventions alone (at four to six weeks).
Adverse events were reported as having been measured in seven of the 10 trials (Abrisham 2011; Bal 2009; Bingöl 2005; Dogan 2010; Kelle 2014; Vecchio 1993; Yeldan 2009), and none were reported by any participant.
Clinically important differences favouring the 'LLLT add‐on' group were found in two of the four trials measuring pain on motion, and one of the four trials measuring night pain. However these positive results were only found in trials at high overall risk of bias. LLLT did not confer clinically important benefits over the other physical therapy intervention in the one trial that measured global treatment success (Bal 2009), any of the seven trials that measured range of motion (Abrisham 2011; Bingöl 2005; Calis 2011; Dogan 2010; Eslamian 2012; Vecchio 1993; Yeldan 2009), or the single trial that measured strength (Yeldan 2009).
We considered the evidence from these nine trials to be low quality after downgrading by one point for high or unclear risk of bias overall in most trials, and by one point for imprecision in all trials.
Is LLLT more effective than other active interventions?
One trial (20 participants), at high risk of bias overall (England 1989), reported favourable effects of LLLT (three times a week for two weeks) over NSAID (naproxen sodium 550 mg twice daily for two weeks) with respect to overall pain (median difference 2 on a 10‐point scale, 95% CI 1.00 to 3.50, 20 participants), active shoulder abduction (median difference 20 degrees, 95% CI 10.00 to 40.00, 20 participants), flexion (median difference 14.99 degrees, 95% CI 5.00 to 30.00, 20 participants) and extension (median difference 10 degrees, 95% CI 0.00 to 20.00, 20 participants) at two weeks (Table 9). However, function was reported as not significantly different between groups. The evidence was downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and so is considered to be very low quality.
8. LLLT versus another active intervention.
|
Study ID: England 1989 Participants: Supraspinatus or bicipital tendinitis Intervention: Low‐level laser therapy (LLLT) Control: NSAID | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Median difference (95% CI) | |
| Overall pain (VAS 0‐10, higher score = more pain) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 2 (1, 3.5) |
| Function (VAS 0‐10, higher score = worse function) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | "No significant difference" |
| Active shoulder abduction (degrees) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 20 (10, 40) |
| Active shoulder flexion (degrees) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 14.99 (5, 30) |
| Active shoulder extension (degrees) at 2 weeks | NR | NR | < = 10 | NR | NR | < = 10 | 10 (0, 20) |
|
Study ID: Kelle 2014 Participants: Subacromial impingement syndrome Intervention: Low‐level laser therapy (LLLT) plus home exercises Control: Glucocorticoid injection plus home exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐100) at 3 weeks | 11.1 | 11.6 | 45 | 10.0 | 11.3 | 45 | 1.10 (‐3.63, 5.83) |
| Rest pain (VAS 0‐100) at 6 months | 11.5 | 13.8 | 45 | 8.9 | 10.4 | 45 | 2.60 (‐2.45, 7.65) |
| Function (UCLA 2‐35, higher = better function) at 3 weeks | 25.9 | 4.6 | 45 | 27.4 | 4.1 | 45 | ‐1.50 (‐3.30, 0.30) |
| Function (UCLA 2‐35, higher = better function) at 6 months | 26.1 | 5.6 | 45 | 26.8 | 5.4 | 45 | ‐0.70 (‐2.97, 1.57) |
| Pain on motion (VAS 0‐100) at 3 weeks | 32.6 | 17.6 | 45 | 23.6 | 15.6 | 45 | 9.00 (2.13, 15.87) |
| Pain on motion (VAS 0‐100) at 6 months | 25.5 | 19.7 | 45 | 22.1 | 17.9 | 45 | 3.40 (‐4.38, 11.18) |
| Adverse events | Zero events in both groups | ||||||
NR = not reported
One trial (90 participants), at high risk of bias overall (Kelle 2014) reported no clinically important differences between LLLT (three times a week for three weeks) plus home exercises and glucocorticoid injection (administered twice, with second injection delivered 10 days after the first) plus home exercises with respect to rest pain at three weeks (mean 11.1 versus 10.0 on a 100‐point scale, MD 1.10, 95% CI ‐3.63 to 5.83, 90 participants) and six months (mean 11.5 versus 8.9 on a 100‐point scale, MD 2.60, 95% CI ‐2.45 to 7.65, 90 participants), function at three weeks (mean 25.9 versus 27.4 on a 33‐point scale, MD ‐1.50, 95% CI ‐3.30 to 0.30, 90 participants) and six months (mean 26.1 versus 26.8 on a 33‐point scale, MD ‐0.70, 95% CI ‐2.97 to 1.57, 90 participants), or pain on motion at three weeks (mean 32.6 versus 23.6 on a 100‐point scale, MD 9.00, 95% CI 2.13 to 15.87, 90 participants) and six months (mean 25.5 versus 22.1 on a 100‐point scale, MD 3.40, 95% CI ‐4.38 to 11.18, 90 participants).
No participant in the Kelle 2014 trial reported adverse events, while England 1989 did not report measuring adverse events.
We downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and so consider this evidence to be very low quality.
Transcutaneous electrical nerve stimulation (TENS)
Is TENS more effective than placebo or no treatment?
Only one trial (20 participants), at unclear risk of bias overall, compared TENS to placebo (i.e. application of an inactive TENS machine) (Kocyigit 2012). The trial was conducted as part of an investigation of the effect of shoulder pain on regions of the brain believed to play a role in pain perception (as measured using functional magnetic resonance imaging). The only outcome of interest to our review, overall pain, was lower in the TENS group immediately after one treatment session, but 95% CIs could not be estimated (intervention group mean (range) 34.8 (12 to 68); control group mean (range) 64.5 (38 to 95); 100‐point scale; 20 participants). The trialists did not report measuring adverse events. The evidence was downgraded by one point for unclear risk of allocation bias, one point for imprecision and one point for indirectness, and so is considered to be very low quality.
Does TENS provide additional benefits over other physical therapy interventions alone?
One trial (62 participants), at high risk of bias overall (Baskurt 2006), found that one session of TENS plus hot pack resulted in less overall pain than hot pack alone, but the difference was not clinically important (mean 4.67 versus 5.38 on a 10‐point scale, MD ‐0.71, 95% CI ‐1.41 to ‐0.01, 62 participants; Table 10). The trialists did not report measuring adverse events. We downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and so consider this evidence to be very low quality.
9. TENS as add‐on to other physical therapy.
|
Study ID: Baskurt 2006 Participants: Shoulder impingement syndrome Intervention: TENS plus hot pack Control: Hot pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) immediately post 1 treatment session | 4.67 | 1.37 | 31 | 5.38 | 1.45 | 31 | ‐0.71 (‐1.41, ‐0.01) |
Is TENS more effective than other active interventions?
Trials have compared TENS with hot pack (one trial, 61 participants: Baskurt 2006), glucocorticoid injection (one trial, 40 participants: Eyigor 2010) and extracorporeal shockwave treatment (one trial, 62 participants: Pan 2003) (Table 11). Pan 2003 restricted inclusion to patients with calcific tendinitis. The overall risk of bias was high in all trials due to lack of participant blinding.
10. TENS versus another active intervention.
|
Study ID: Baskurt 2006 Participants: Shoulder impingement syndrome Intervention: TENS Control: Hot pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) immediately post 1 treatment session | 5.36 | 1.35 | 30 | 5.38 | 1.45 | 31 | ‐0.02 (‐0.72, 0.68) |
|
Study ID: Eyigor 2010 Participants: Rotator cuff tendinitis Intervention: TENS plus home exercises Control: Glucocorticoid injection plus home exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) at 1 week | 2.3 | 1.2 | 20 | 1.5 | 1 | 20 | 0.80 (0.12, 1.48) |
| Rest pain (VAS 0‐10, 0 = no pain) at 4 weeks | 1.8 | 1.5 | 20 | 0.6 | 0.4 | 20 | 1.20 (0.52, 1.88) |
| Rest pain (VAS 0‐10, 0 = no pain) at 12 weeks | 1 | 0.7 | 20 | 0.2 | 0.4 | 20 | 0.80 (0.45, 1.15) |
| Function (SDQ 0‐100, 0 = no disability) at 1 week | 67.6 | 15.9 | 20 | 37.9 | 22.6 | 20 | 29.70 (17.59, 41.81) |
| Function (SDQ 0‐100, 0 = no disability) at 4 weeks | 42.5 | 14.7 | 20 | 22.1 | 15.9 | 20 | 20.40 (10.91, 29.89) |
| Function (SDQ 0‐100, 0 = no disability) at 12 weeks | 28.5 | 13.5 | 20 | 13.7 | 11.5 | 20 | 14.80 (7.03, 22.57) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 1 week | 4.5 | 1 | 20 | 3.5 | 1.4 | 20 | 1.00 (0.25, 1.75) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 4 weeks | 2.6 | 1.6 | 20 | 1.9 | 1.2 | 20 | 0.70 (‐0.18, 1.58) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 12 weeks | 2.1 | 1.3 | 20 | 1.2 | 0.7 | 20 | 0.90 (0.25, 1.55) |
| Night pain (VAS 0‐10, 0 = no pain) at 1 week | 4.2 | 1.8 | 20 | 2.1 | 2 | 20 | 2.10 (0.92, 3.28) |
| Night pain (VAS 0‐10, 0 = no pain) at 4 weeks | 2.7 | 1.6 | 20 | 1.7 | 1.2 | 20 | 1.00 (0.12, 1.88) |
| Night pain (VAS 0‐10, 0 = no pain) at 12 weeks | 2 | 0.9 | 20 | 1.2 | 0.9 | 20 | 0.80 (0.24, 1.36) |
| Active shoulder flexion (degrees) at 1 week | 144.9 | 17.6 | 20 | 152.5 | 21.6 | 20 | ‐7.60 (‐19.81, 4.61) |
| Active shoulder flexion (degrees) at 4 weeks | 160 | 11.9 | 20 | 162.7 | 14.7 | 20 | ‐2.70 (‐10.99, 5.59) |
| Active shoulder flexion (degrees) at 12 weeks | 165.3 | 8.8 | 20 | 170.5 | 9.1 | 20 | ‐5.20 (‐10.75, 0.35) |
| Active shoulder abduction (degrees) at 1 week | 124.3 | 23.2 | 20 | 143.5 | 22.9 | 20 | ‐19.20 (‐33.49, ‐4.91) |
| Active shoulder abduction (degrees) at 4 weeks | 149.8 | 14.6 | 20 | 163.7 | 16.1 | 20 | ‐13.90 (‐23.43, ‐4.37) |
| Active shoulder abduction (degrees) at 12 weeks | 159.3 | 11.8 | 20 | 170 | 13.3 | 20 | ‐10.70 (‐18.49, ‐2.91) |
| Active shoulder external rotation (degrees) at 1 week | 56.8 | 15.7 | 20 | 59.3 | 20.9 | 20 | ‐2.50 (‐13.96, 8.96) |
| Active shoulder external rotation (degrees) at 4 weeks | 64.5 | 9.9 | 20 | 68.3 | 10.8 | 20 | ‐3.80 (‐10.22, 2.62) |
| Active shoulder external rotation (degrees) at 12 weeks | 70.3 | 8.7 | 20 | 69.9 | 8.9 | 20 | 0.40 (‐5.05, 5.85) |
| Active shoulder internal rotation (degrees) at 1 week | 48.3 | 13.3 | 20 | 59 | 14.8 | 20 | ‐10.70 (‐19.42, ‐1.98) |
| Active shoulder internal rotation (degrees) at 4 weeks | 63 | 11.3 | 20 | 66.7 | 14.2 | 20 | ‐3.70 (‐11.65, 4.25) |
| Active shoulder internal rotation (degrees) at 12 weeks | 68.4 | 11.8 | 20 | 68.6 | 7.9 | 20 | ‐0.20 (‐6.42, 6.02) |
| Quality of life (SF‐36 physical function 0‐100, higher = better) at 12 weeks | 74.4 | 16.9 | 20 | 68.5 | 17.4 | 20 | 5.90 (‐4.73, 16.53) |
| Quality of life (SF‐36 physical role 0‐100, higher = better) at 12 weeks | 63.8 | 15.1 | 20 | 51.2 | 36.7 | 20 | 12.60 (‐4.79, 29.99) |
| Quality of life (SF‐36 bodily pain 0‐100, higher = better) at 12 weeks | 61.3 | 18 | 20 | 68.6 | 16.6 | 20 | ‐7.30 (‐18.03, 3.43) |
| Quality of life (SF‐36 general health 0‐100, higher = better) at 12 weeks | 58.6 | 17.1 | 20 | 50 | 19.2 | 20 | 8.60 (‐2.67, 19.87) |
| Quality of life (SF‐36 vitality 0‐100, higher = better) at 12 weeks | 54.3 | 12.6 | 20 | 51.5 | 12.1 | 20 | 2.80 (‐4.86, 10.46) |
| Quality of life (SF‐36 social functioning 0‐100, higher = better) at 12 weeks | 73.3 | 14 | 20 | 68.1 | 25.8 | 20 | 5.20 (‐7.66, 18.06) |
| Quality of life (SF‐36 emotion role 0‐100, higher = better) at 12 weeks | 53.8 | 18.5 | 20 | 58.2 | 21.2 | 20 | ‐4.40 (‐16.73, 7.93) |
| Quality of life (SF‐36 mental health 0‐100, higher = better) at 12 weeks | 55.1 | 16.3 | 20 | 56.1 | 13.9 | 20 | ‐1.00 (‐10.39, 8.39) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (participant reported "good results" or "very good results") at 1 week | 4 | 20 | 14 | 20 | 0.29 (0.11, 0.72) | ||
| Global assessment of treatment success (participant reported "good results" or "very good results") at 4 weeks | 12 | 20 | 15 | 20 | 0.80 (0.52, 1.24) | ||
| Global assessment of treatment success (participant reported "good results" or "very good results") at 12 weeks | 13 | 20 | 17 | 20 | 0.76 (0.53, 1.11) | ||
| Total adverse events during 12‐week treatment and follow‐up period | Zero events in both groups | ||||||
|
Study ID: Pan 2003 Participants: Calcific tendinitis Intervention: TENS Control: Extracorporeal shockwave therapy | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n* | Mean | SD | n* | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) change from baseline to 4 weeks | ‐1.1 | 1.94 | 29 | ‐3 | 2.41 | 33 | 1.90 (0.82, 2.98) |
| Overall pain (VAS 0‐10, 0 = no pain) change from baseline to 12 weeks | ‐1.74 | 2.2 | 29 | ‐4.08 | 2.59 | 33 | 2.34 (1.15, 3.53) |
| Function (Constant‐Murley score 0‐100, higher = better function) change from baseline to 4 weeks | 9.59 | 9.62 | 29 | 24.21 | 13.68 | 33 | ‐14.62 (‐20.45, ‐8.79) |
| Function (Constant‐Murley score 0‐100, higher = better function) change from baseline to 12 weeks | 11.86 | 13.32 | 29 | 28.31 | 13.1 | 33 | ‐16.45 (‐23.04, ‐9.86) |
| Events* | Total* | Events* | Total* | Risk ratio (95% CI) | |||
| Strength (improvement on Manual Muscle Testing) at 4 weeks | 15 | 29 | 21 | 33 | 0.81 (0.53, 1.26) | ||
| Strength (improvement on Manual Muscle Testing) at 12 weeks | 18 | 29 | 23 | 33 | 0.89 (0.62, 1.28) | ||
| Total adverse events during 12‐week trial period (soreness in the upper arm after treatment) | 0 | 27 | 5 | 32 | 0.11 (0.01, 1.85) | ||
*Unit of analysis is shoulders, not participants
Baskurt 2006 found that both one session of TENS and application of a hot pack had similar effects on overall pain (mean 5.36 versus 5.38 on a 10‐point scale, MD ‐0.02, 95% CI ‐0.72 to 0.68, 61 participants). The trialists did not report measuring adverse events. We downgraded the evidence in this trial by two points for high risk of performance and detection bias, and one point for imprecision, and so consider it to be very low quality.
In Eyigor 2010, clinically important differences favouring a single glucocorticoid injection plus home exercises over TENS plus home exercises (five times a week for three weeks) were found for function at one week (mean 67.6 versus 37.9 on a 100‐point scale, MD 29.70, 95% CI 17.59 to 41.81, 40 participants), four weeks (mean 42.5 versus 22.1 on a 100‐point scale, MD 20.40, 95% CI 10.91 to 29.89, 40 participants), and 12 weeks (mean 28.5 versus 13.7 on a 100‐point scale, MD 14.80, 95% CI 7.03 to 22.57, 40 participants), global treatment success at one week (20% (4/20) versus 70% (14/20), RR 0.29, 95% CI 0.11 to 0.72, 40 participants), and night pain at one week (mean 4.2 versus 2.1 on a 10‐point scale, MD 2.10, 95% CI 0.92 to 3.28, 40 participants). Further, statistically significant differences favouring glucocorticoid injection were found for rest pain, pain on motion, night pain at four and 12 weeks, and active shoulder abduction at one, four and 12 weeks, but none of these differences were considered clinically important. Also, nearly all other measures of active range of motion and all measures of quality of life were not significantly different between groups. No participant reported adverse events. We downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and so consider this evidence to be very low quality.
In Pan 2003, clinically important differences favouring extracorporeal shockwave treatment (two sessions delivered over a four‐week period) over TENS (three times a week for four weeks) were found for overall pain at four weeks (mean change ‐1.1 versus ‐3 on a 10‐point scale, MD 1.90, 95% CI 0.82 to 2.98, 62 participants) and 12 weeks (mean change ‐1.74 versus ‐4.08 on a 10‐point scale, MD 2.34, 95% CI 1.15 to 3.53, 62 participants), and function at four weeks (mean change 9.59 versus 24.21 on a 100‐point scale, MD ‐14.62, 95% CI ‐20.45 to ‐8.79, 62 participants) and 12 weeks (mean change 11.86 versus 28.31 on a 100‐point scale, MD ‐16.45, 95% CI ‐23.04 to ‐9.86, 62 participants). However, improvement in strength was no different between groups (at four weeks 52% (15/29) versus 64% (21/33), RR 0.81, 95% CI 0.53 to 1.26; at 12 weeks 62% (18/29) versus 70% (23/33), RR 0.89, 95% CI 0.62 to 1.28, 62 participants). None of the participants receiving TENS reported adverse events, whereas 16% (5/32) of participants receiving shockwave treatment reported soreness in the upper arm after treatment (RR 0.11, 95% CI 0.01 to 1.85, 59 participants). This evidence was considered to be very low quality due to the high risk of performance and detection bias (downgraded by two points) and imprecision (downgraded by one point).
Pulsed electromagnetic field (PEMF)
Is PEMF more effective than placebo or no treatment?
PEMF was compared to placebo (application of an inactive PEMF machine) in two trials (75 participants), one at low (Galace de Freitas 2014) and one at unclear risk of bias overall (Binder 1984) (Table 12). The dosage and frequency of administration varied substantially between the trials (five to nine hours every day for eight weeks in Binder 1984; 30 minute sessions, three times a week for three weeks in Galace de Freitas 2014).
11. PEMF versus placebo.
|
Study ID: Binder 1984 Participants: Rotator cuff tendinitis Intervention: PEMF for 4 weeks Control: Placebo PEMF for 4 weeks | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) change from baseline to 2 weeks | ‐5 | NR | 15 | ‐1.14 | NR | 14 | ‐3.86 (95% CI not estimable) |
| Overall pain (VAS 0‐10, 0 = no pain) change from baseline to 4 weeks | ‐8.2 | NR | 15 | ‐2.97 | NR | 14 | ‐5.23 (95% CI not estimable) |
| Total active range of motion (degrees) change from baseline to 2 weeks | 59.08 | NR | 15 | 13.23 | NR | 14 | 45.85 (95% CI not estimable) |
| Total active range of motion (degrees) change from baseline to 4 weeks | 75.89 | NR | 15 | 17.15 | NR | 14 | 58.74 (95% CI not estimable) |
|
Study ID: Galace de Freitas 2014 Participants: Shoulder impingement syndrome Intervention: PEMF for 3 weeks Control: Placebo PEMF for 3 weeks | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 where higher score = worse pain) at 3 weeks | 4.8 | 2.4 | 22 | 6 | 2.1 | 24 | ‐1.20 (‐2.51, 0.11) |
| Function (Constant‐Murley total score 0‐100 where higher score = better function) at 3 weeks | 40.7 | 12.6 | 22 | 35.6 | 11.7 | 24 | 5.10 (‐1.95, 12.15) |
| Strength (kg): external rotation at 3 weeks | 26.8 | 12.9 | 22 | 21.6 | 10.3 | 24 | 5.20 (‐1.59, 11.99) |
| Strength (kg): internal rotation at 3 weeks | 38.1 | 17 | 22 | 33.7 | 12 | 24 | 4.40 (‐4.17, 12.97) |
Incomplete reporting prevented calculation of 95% CIs in Binder 1984, although effect estimates favouring PEMF were noted with respect to overall pain and range of motion at two and four weeks. Galace de Freitas 2014 found no clinically important differences between groups in overall pain (mean 4.8 versus 6 on a 10‐point scale, MD ‐1.20, 95% CI ‐2.51 to 0.11, 46 participants), function (mean 40.7 versus 35.6 on a 100‐point scale, MD 5.10, 95% CI ‐1.95 to 12.15, 46 participants), and strength measures at three weeks. Neither trial reported measuring adverse events. We downgraded by one point for unclear risk of allocation bias in one trial (Binder 1984), and one point for imprecision in both trials, and so consider this evidence to be low quality.
Does PEMF provide additional benefits when added to other physical therapy interventions alone?
Two trials (86 participants) examined whether there is benefit in adding PEMF to an exercise programme (Aktas 2007; Galace de Freitas 2014) (Table 13). The overall risk of bias was unclear in one trial (Aktas 2007) and low in the other (Galace de Freitas 2014). Pooling was not possible because of the different timing of outcome assessment (three weeks in Aktas 2007, three months in Galace de Freitas 2014).
12. PEMF as add‐on to other physical therapy.
|
Study ID: Aktas 2007 Participants: Subacromial impingement syndrome Intervention: Pulsed electromagnetic field (PEMF) plus exercise plus cold pack Control: Placebo PEMF plus exercise plus cold pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 10 = intolerable pain) at 3 weeks | 0.9 | 1.55 | 20 | 0.85 | 1.56 | 20 | 0.05 (‐0.91, 1.01) |
| Function (Constant total score 0‐100 where higher = better function) at 3 weeks | 72.65 | 17.99 | 20 | 72 | 12.78 | 20 | 0.65 (‐9.02, 10.32) |
| Pain on motion (VAS 0‐10, 10 = intolerable pain) at 3 weeks | 2.7 | 2.51 | 20 | 2.75 | 2.22 | 20 | ‐0.05 (‐1.52, 1.42) |
| Night pain (VAS 0‐10, 10 = intolerable pain) at 3 weeks | 0.8 | 1.59 | 20 | 2.25 | 3.27 | 20 | ‐1.45 (‐3.04, 0.14) |
| Active range of motion (Constant sub‐score 0‐40, higher = better ROM) at 3 weeks | 35.9 | 6.91 | 20 | 36.7 | 3.13 | 20 | ‐0.80 (‐4.12, 2.52) |
| Strength (Constant sub‐score 0‐25, higher = better strength) at 3 weeks | 12.25 | 7.33 | 20 | 11.5 | 7.17 | 20 | 0.75 (‐3.74, 5.24) |
|
Study ID: Galace de Freitas 2014 Participants: Shoulder impingement syndrome Intervention: PEMF plus exercises for 9 weeks Control: Placebo PEMF plus exercises for 9 weeks | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 where higher score = worse pain) at 3 months | 2.7 | 3 | 22 | 3.4 | 3.1 | 24 | ‐0.70 (‐2.46, 1.06) |
| Function (Constant‐Murley total score 0‐100 where higher score = better function) at 3 months | 52.7 | 11.7 | 22 | 50.4 | 12 | 24 | 2.30 (‐4.55, 9.15) |
| Strength (kg): external rotation at 3 months | 32.7 | 14.5 | 22 | 24.9 | 10.2 | 24 | 7.80 (0.49, 15.11) |
| Strength (kg): internal rotation at 3 months | 43.8 | 4 | 22 | 36.6 | 13.2 | 24 | 7.20 (1.66, 12.74) |
| Strength (kg): elevation at 3 months | 28.5 | 11.4 | 22 | 22.2 | 8.8 | 24 | 6.30 (0.38, 12.22) |
No clinically important difference between groups was found in overall pain at three weeks (mean 0.9 versus 0.85 on a 10‐point scale, MD 0.05, 95% CI ‐0.91 to 1.01, 40 participants) and three months (mean 2.7 versus 3.4 on a 10‐point scale, MD ‐0.70, 95% CI ‐2.46 to 1.06, 46 participants), function at three weeks (mean 72.65 versus 72 on a 100‐point scale, MD 0.65, 95% CI ‐9.02 to 10.32, 40 participants) and three months (mean 52.7 versus 50.4 on a 100‐point scale, MD 2.30, 95% CI ‐4.55 to 9.15, 46 participants), pain on motion (mean 2.7 versus 2.75 on a 10‐point scale, MD ‐0.05, 95% CI ‐1.52 to 1.42, 40 participants), night pain (mean 0.8 versus 2.25 on a 10‐point scale, MD ‐1.45, 95% CI ‐3.04 to 0.14, 40 participants) and active range of motion (mean 35.9 versus 36.7 on a 40‐point scale, MD ‐0.80, 95% CI ‐4.12 to 2.52, 40 participants) at three weeks, and strength measures at three weeks and three months.
Neither trial reported measuring adverse events.
The evidence in these trials was downgraded by one point for unclear risk of allocation bias in one trial, and one point for imprecision, and so is considered to be low quality.
Is PEMF more effective than other active interventions?
We did not find any trials comparing PEMF with another active intervention.
Other electrotherapy modalities: microcurrent electrical stimulation (MENS), microwave diathermy, acetic acid iontophoresis, and multiple modalities
Are other electrotherapy modalities more effective than placebo or no treatment?
One trial (40 participants), at unclear risk of bias overall, compared MENS with placebo (Atya 2012) (Table 14). Participants receiving MENS (three times a week for six weeks) had statistically significantly less overall pain (mean 6 versus 6.8 on a 10‐point scale, MD ‐0.80, 95% CI ‐1.47 to ‐0.13, 40 participants) and better function (mean 60.65 versus 67.6 on a 100‐point scale, MD ‐6.95, 95% CI ‐11.49 to ‐2.41, 40 participants) at six weeks than participants receiving placebo. However we did not consider these differences to be clinically important. The trialists did not report measuring adverse events. We downgraded by one point for unclear risk of allocation bias, and one point for imprecision, and so consider this evidence to be low quality.
13. Microcurrent electrical stimulation (MENS) versus placebo.
|
Study ID: Atya 2012 Participants: Subacromial impingement syndrome Intervention: Microcurrent electrical stimulation (MENS) Control: Placebo MENS | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 6 weeks | 6 | 1.07 | 19 | 6.8 | 1.08 | 21 | ‐0.80 (‐1.47, ‐0.13) |
| Function (Dutch SDQ total score 0‐100 where higher = worse function) at 6 weeks | 60.65 | 7.7 | 19 | 67.6 | 6.88 | 21 | ‐6.95 (‐11.49, ‐2.41) |
One trial (21 participants), at high risk of bias overall, compared multi‐modal electrotherapy (acetic acid iontophoresis plus therapeutic ultrasound) with no treatment in participants with calcific tendinitis (Perron 1997) (Table 15). The trialists found no difference between groups in pain on motion (mean 1.38 versus 1.59 on a five‐point scale, MD ‐0.21, 95% CI ‐0.95 to 0.53, 21 participants) or passive shoulder abduction (mean 113.18 versus 93.75 degrees, MD 19.43, 95% CI ‐8.75 to 47.61, 21 participants). The trialists did not report measuring adverse events. The evidence was downgraded to very low quality (downgraded by two points for high risk of performance and detection bias and by one point for imprecision).
14. Multiple electrotherapy modalities versus no treatment.
|
Study ID: Perron 1997
Participants: Calcific tendinitis Intervention: Acetic acid iontophoresis plus therapeutic ultrasound Control: No treatment | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Pain on motion (passive abduction) (0‐5 scale, 0 = no pain) at 3 weeks | 1.38 | 0.81 | 11 | 1.59 | 0.91 | 10 | ‐0.21 (‐0.95, 0.53) |
| Passive shoulder abduction (degrees) at 3 weeks | 113.18 | 38.94 | 11 | 93.75 | 26.23 | 10 | 19.43 (‐8.75, 47.61) |
Do other electrotherapy modalities provide additional benefits over other physical therapy interventions alone?
Two trials (67 participants) examined whether there is benefit in adding an electrotherapy modality to an exercise programme plus hot pack (Akyol 2012; Leduc 2003). Akyol 2012, which was at unclear risk of bias overall, examined the additional effects of microwave diathermy (Table 16), whereas Leduc 2003, which was also at high risk of bias overall, examined the additional effects of acetic acid iontophoresis in participants with calcific tendinitis (Table 17).
15. Microwave diathermy as add‐on to other physical therapy.
|
Study ID: Akyol 2012 Participants: Subacromial impingement syndrome Intervention: Microwave diathermy plus exercise plus superficial heat Control: Placebo microwave diathermy plus exercise plus superficial heat | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (10‐point scale, 0 = no pain) change from baseline to 3 weeks | ‐2.65 | 1.98 | 20 | ‐2.95 | 2.74 | 20 | 0.30 (‐1.18, 1.78) |
| Overall pain (10‐point scale, 0 = no pain) change from baseline to 7 weeks | ‐2.8 | 2.23 | 20 | ‐2.8 | 3.33 | 20 | 0.00 (‐1.76, 1.76) |
| Function (SPADI total score 0‐100 where higher = worse function) change from baseline to 3 weeks | ‐48.2 | 2.96 | 20 | ‐48.85 | 2.74 | 20 | 0.65 (‐1.12, 2.42) |
| Function (SPADI total score 0‐100 where higher = worse function) change from baseline to 7 weeks | ‐49.75 | 3 | 20 | ‐54.2 | 2.82 | 20 | 4.45 (2.65, 6.25) |
| Pain on motion (10‐point scale, 0 = no pain) change from baseline to 3 weeks | ‐4.05 | 2.35 | 20 | ‐3.45 | 3.2 | 20 | ‐0.60 (‐2.34, 1.14) |
| Pain on motion (10‐point scale, 0 = no pain) change from baseline to 7 weeks | ‐5.1 | 2.65 | 20 | ‐4.1 | 2.77 | 20 | ‐1.00 (‐2.68, 0.68) |
| Night pain (10‐point scale, 0 = no pain) change from baseline to 3 weeks | ‐3.85 | 2.64 | 20 | ‐4.1 | 2.31 | 20 | 0.25 (‐1.29, 1.79) |
| Night pain (10‐point scale, 0 = no pain) change from baseline to 7 weeks | ‐4.1 | 2.9 | 20 | ‐4.5 | 3.2 | 20 | 0.40 (‐1.49, 2.29) |
| Active shoulder abduction (degrees) change from baseline to 3 weeks | 29.5 | 3.23 | 20 | 23.75 | 2.34 | 20 | 5.75 (4.00, 7.50) |
| Active shoulder abduction (degrees) change from baseline to 7 weeks | 33.5 | 3.75 | 20 | 27 | 11.96 | 20 | 6.50 (1.01, 11.99) |
| Active shoulder flexion (degrees) change from baseline to 3 weeks | 26 | 2.32 | 20 | 18.5 | 1.76 | 20 | 7.50 (6.22, 8.78) |
| Active shoulder flexion (degrees) change from baseline to 7 weeks | 28.25 | 2.31 | 20 | 20.5 | 1.82 | 20 | 7.75 (6.46, 9.04) |
| Active shoulder internal rotation (degrees) change from baseline to 3 weeks | 11.5 | 1.31 | 20 | 19.25 | 1.71 | 20 | ‐7.75 (‐8.69, ‐6.81) |
| Active shoulder internal rotation (degrees) change from baseline to 7 weeks | 17.25 | 1.78 | 20 | 22.5 | 1.88 | 20 | ‐5.25 (‐6.38, ‐4.12) |
| Active shoulder external rotation (degrees) change from baseline to 3 weeks | 12.5 | 1.8 | 20 | 21.5 | 1.37 | 20 | ‐9.00 (‐9.99, ‐8.01) |
| Active shoulder external rotation (degrees) change from baseline to 7 weeks | 15.25 | 1.9 | 20 | 22.75 | 1.44 | 20 | ‐7.50 (‐8.54, ‐6.46) |
| Quality of life (SF‐36 physical function 0‐100, higher = better) change from baseline to 3 weeks | 0.08 | 0.89 | 20 | 0.14 | 0.19 | 20 | ‐0.06 (‐0.46, 0.34) |
| Quality of life (SF‐36 physical function 0‐100, higher = better) change from baseline to 7 weeks | 0.11 | 0.1 | 20 | 0.19 | 0.18 | 20 | ‐0.08 (‐0.17, 0.01) |
| Quality of life (SF‐36 social function 0‐100, higher = better) change from baseline to 3 weeks | 0.19 | 0.15 | 20 | 0.12 | 0.12 | 20 | 0.07 (‐0.01, 0.15) |
| Quality of life (SF‐36 social function 0‐100, higher = better) change from baseline to 7 weeks | 0.25 | 0.21 | 20 | 0.17 | 0.18 | 20 | 0.08 (‐0.04, 0.20) |
| Quality of life (SF‐36 physical role limitation 0‐100, higher = better) change from baseline to 3 weeks | 0.31 | 0.47 | 20 | 0.46 | 0.44 | 20 | ‐0.15 (‐0.43, 0.13) |
| Quality of life (SF‐36 physical role limitation 0‐100, higher = better) change from baseline to 7 weeks | 0.43 | 0.57 | 20 | 0.56 | 0.47 | 20 | ‐0.13 (‐0.45, 0.19) |
| Quality of life (SF‐36 emotional role limitation 0‐100, higher = better) change from baseline to 3 weeks | 0.26 | 0.44 | 20 | 0.06 | 0.23 | 20 | 0.20 (‐0.02, 0.42) |
| Quality of life (SF‐36 emotional role limitation 0‐100, higher = better) change from baseline to 7 weeks | 0.35 | 0.45 | 20 | 0.05 | 0.3 | 20 | 0.30 (0.06, 0.54) |
| Quality of life (SF‐36 mental health 0‐100, higher = better) change from baseline to 3 weeks | 0.04 | 0.04 | 20 | 0.03 | 0.05 | 20 | 0.01 (‐0.02, 0.04) |
| Quality of life (SF‐36 mental health 0‐100, higher = better) change from baseline to 7 weeks | 0.04 | 0.06 | 20 | 0.06 | 0.11 | 20 | ‐0.02 (‐0.07, 0.03) |
| Quality of life (SF‐36 energy 0‐100, higher = better) change from baseline to 3 weeks | 0.04 | 0.07 | 20 | 0.02 | 0.07 | 20 | 0.02 (‐0.02, 0.06) |
| Quality of life (SF‐36 energy 0‐100, higher = better) change from baseline to 7 weeks | 0.06 | 0.09 | 20 | 0.04 | 0.09 | 20 | 0.02 (‐0.04, 0.08) |
| Quality of life (SF‐36 pain 0‐100, higher = better) change from baseline to 3 weeks | 0.39 | 0.21 | 20 | 0.38 | 0.17 | 20 | 0.01 (‐0.11, 0.13) |
| Quality of life (SF‐36 pain 0‐100, higher = better) change from baseline to 7 weeks | 0.43 | 0.24 | 20 | 0.46 | 0.26 | 20 | ‐0.03 (‐0.19, 0.13) |
| Quality of life (SF‐36 general health 0‐100, higher = better) change from baseline to 3 weeks | 0.1 | 0.11 | 20 | 0.11 | 0.14 | 20 | ‐0.01 (‐0.09, 0.07) |
| Quality of life (SF‐36 general health 0‐100, higher = better) change from baseline to 7 weeks | 0.13 | 0.15 | 20 | 0.18 | 0.16 | 20 | ‐0.05 (‐0.15, 0.05) |
| Isokinetic strength (60º/s internal rotation) change from baseline to 3 weeks | 2.6 | 5.66 | 20 | 5.15 | 6.37 | 20 | ‐2.55 (‐6.28, 1.18) |
| Isokinetic strength (60º/s internal rotation) change from baseline to 7 weeks | 0.75 | 5.01 | 20 | ‐1.6 | 3.36 | 20 | 2.35 (‐0.29, 4.99) |
| Isokinetic strength (60º/s external rotation) change from baseline to 3 weeks | 0.7 | 4.34 | 20 | 3.5 | 3.7 | 20 | ‐2.80 (‐5.30, ‐0.30) |
| Isokinetic strength (60º/s external rotation) change from baseline to 7 weeks | 1.4 | 5.25 | 20 | 2.45 | 4.32 | 20 | ‐1.05 (‐4.03, 1.93) |
| Isokinetic strength (180º/s internal rotation) change from baseline to 3 weeks | 1.6 | 2.89 | 20 | 2.8 | 4.56 | 20 | ‐1.20 (‐3.57, 1.17) |
| Isokinetic strength (180º/s internal rotation) change from baseline to 7 weeks | 2.4 | 5.09 | 20 | 3.15 | 4.78 | 20 | ‐0.75 (‐3.81, 2.31) |
| Isokinetic strength (180º/s external rotation) change from baseline to 3 weeks | 0.9 | 3.21 | 20 | 1.65 | 3.6 | 20 | ‐0.75 (‐2.86, 1.36) |
| Isokinetic strength (180º/s external rotation) change from baseline to 7 weeks | 0.2 | 3.2 | 20 | 1.25 | 2.46 | 20 | ‐1.05 (‐2.82, 0.72) |
| Total adverse events at 3 weeks | Zero events in both groups | ||||||
| Total adverse events at 7 weeks | Zero events in both groups | ||||||
16. Acetic acid iontophoresis as add‐on to other physical therapy.
|
Study ID: Leduc 2003
Participants: Calficic tendinitis Intervention: Acetic acid iontophoresis plus exercise plus heat pack Control: Sham iontophoresis plus exercise plus heat pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (SPADI total score 0‐100, higher = worse function) at 6 weeks | 23 | 15 | 17 | 40 | 17 | 10 | ‐17.00 (‐29.72, ‐4.28) |
| Active shoulder abduction (degrees) at 6 weeks | 133 | 24 | 17 | 130 | 30 | 10 | 3.00 (‐18.81, 24.81) |
| Active shoulder flexion (degrees) at 6 weeks | 154 | 12 | 17 | 143 | 48 | 10 | 11.00 (‐19.29, 41.29) |
| Active shoulder external rotation (degrees) at 6 weeks | 75 | 11 | 17 | 72 | 16 | 10 | 3.00 (‐8.21, 14.21) |
| Active shoulder internal rotation (degrees) at 6 weeks | 69 | 20 | 17 | 71 | 26 | 10 | ‐2.00 (‐20.71, 16.71) |
Microwave diathermy (five times a week for three weeks) did not provide clinically important benefits over exercise plus hot pack in terms of overall pain at three weeks (mean change ‐2.65 versus ‐2.95 on a 10‐point scale, MD 0.30, 95% CI ‐1.18 to 1.78, 40 participants) and seven weeks (mean change ‐2.8 versus 2.8 on a 10‐point scale, MD 0, 95% CI ‐1.76 to 1.76, 40 participants), function at three weeks (mean change ‐48.2 versus ‐48.85 on a 100‐point scale, MD 0.65, 95% CI ‐1.12 to 2.42, 40 participants) and seven weeks (mean change ‐49.75 versus ‐54.2 on a 100‐point scale, MD 4.45, 95% CI 2.65 to 6.25, 40 participants), pain on motion at three weeks (mean change ‐4.05 versus ‐3.45 on a 10‐point scale, MD ‐0.60, 95% CI ‐2.34 to 1.14, 40 participants) and seven weeks (mean change ‐5.1 versus ‐4.1 on a 10‐point scale, MD ‐1.00, 95% CI ‐2.68 to 0.68, 40 participants), or quality of life, night pain, active range of motion and strength at three weeks and seven weeks. No participant reported adverse events.
Acetic acid iontophoresis (one to two times a week for six weeks) conferred clinically important benefits over exercise plus hot pack with respect to function at six weeks (mean 23 versus 40 on a 100‐point scale, MD ‐17.00, 95% CI ‐29.72 to ‐4.28, 27 participants), but not active range of motion. However, there was a high amount of attrition in this very small trial, which may have biased results in favour of the 'add‐on' group. The trialists did not report measuring adverse events.
We downgraded the evidence from these two trials by two points for high risk of attrition bias in one trial and unclear risk of allocation bias in both trials, and one point for imprecision, and thus consider it to be very low quality.
Are other electrotherapy modalities more effective than other active interventions?
Two trials (54 participants), both at high risk of bias overall, compared therapeutic ultrasound plus TENS plus other physical therapy with either cryotherapy (CO2 vapours at ‐75 degrees Celsius for three minutes) (Grymel‐Kulesza 2007) or sodium hyaluronate injection (one per week for three weeks) plus other physical therapy (Ozgen 2012) (Table 18). In Grymel‐Kulesza 2007, night pain at the end of two weeks' treatment was reported by 73% (11/15) of participants receiving cryotherapy but not by any participant receiving therapeutic ultrasound plus TENS (RR 0.04, 95% CI 0.00 to 0.68, 30 participants). However, there were no important differences between groups in active range of motion and strength. Ozgen 2012 only reported medians and IQRs so MDs and 95% CIs could not be calculated. There were no or very small between‐group differences in median scores for rest pain, function, pain on motion, global treatment success, night pain, and active range of motion at three weeks, three months and four years. Further, no participant reported any adverse events. The evidence from these two trials was downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and so is considered to be very low quality.
17. Multiple electrotherapy modalities versus another active intervention.
|
Study ID: Grymel‐Kulesza 2007 Participants: Chronic rotator cuff injuries Intervention: Therapeutic ultrasound plus TENS plus exercise plus massage Control: Cryotherapy plus exercise plus massage | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Active shoulder abduction (degrees) at 2 weeks | 129 | 37.71 | 15 | 122.3 | 22.03 | 15 | 6.70 (‐15.40, 28.80) |
| Active shoulder extension (degrees) at 2 weeks | 30.67 | 7.04 | 15 | 24.67 | 6.11 | 15 | 6.00 (1.28, 10.72) |
| Active shoulder external rotation (degrees) at 2 weeks | 69.33 | 14.62 | 15 | 55 | 15.12 | 15 | 14.33 (3.69, 24.97) |
| Active shoulder internal rotation (degrees) at 2 weeks | 67.67 | 12.37 | 15 | 58.67 | 13.69 | 15 | 9.00 (‐0.34, 18.34) |
| Strength (Jobes' 5‐point scale) ‐ supraspinatus at 2 weeks | 4.37 | 0.48 | 15 | 3.9 | 0.11 | 15 | 0.47 (0.22, 0.72) |
| Strength (Jobes' 5‐point scale) ‐ subscapularis at 2 weeks | 4.3 | 0.49 | 15 | 4.03 | 0.48 | 15 | 0.27 (‐0.08, 0.62) |
| Strength (Jobes' 5‐point scale) ‐ infraspinatus at 2 weeks | 4.2 | 0.32 | 15 | 4 | 0.38 | 15 | 0.20 (‐0.05, 0.45) |
| Strength (Jobes' 5‐point scale) ‐ biceps at 2 weeks | 4.6 | 0.39 | 15 | 4.37 | 0.44 | 15 | 0.23 (‐0.07, 0.53) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Night pain (number of participants with any night pain) at 2 weeks | 0 | 15 | 11 | 15 | 0.04 (0.00, 0.68) | ||
|
Study ID: Ozgen 2012 Participants: Supraspinatus tendinitis Intervention: Therapeutic ultrasound plus TENS plus hot pack plus home exercises Control: Sodium hyaluronate injection plus home exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Median | IQR | N | Median | IQR | N | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) at 3 weeks | 0 | 0, 0 | 12 | 0 | 0, 2.5 | 12 | 0 (95% CI not estimable) |
| Rest pain (VAS 0‐10, 0 = no pain) at 3 months | 0 | 0, 0 | 12 | 0 | 0, 1.5 | 12 | 0 (95% CI not estimable) |
| Rest pain (VAS 0‐10, 0 = no pain) at 4 years | 0 | 0, 0 | 10 | 0 | 0, 0 | 11 | 0 (95% CI not estimable) |
| Function (ASES 0‐60, higher score = better function) at 3 weeks | 56.5 | 40, 59 | 12 | 56.5 | 52, 60 | 12 | 0 (95% CI not estimable) |
| Function (ASES 0‐60, higher score = better function) at 3 months | 56 | 46, 59 | 12 | 60 | 59.5, 60 | 12 | ‐4.00 (95% CI not estimable) |
| Function (ASES 0‐60, higher score = better function) at 4 years | 60 | 60, 60 | 10 | 60 | 60, 60 | 11 | 0 (95% CI not estimable) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 3 weeks | 0 | 0, 3 | 12 | 0.5 | 0, 4 | 12 | ‐0.5 (95% CI not estimable) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 3 months | 2.5 | 0, 4 | 12 | 0 | 0, 0 | 12 | 2.5 (95% CI not estimable) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 4 years | 0 | 0, 0 | 10 | 0 | 0, 0 | 11 | 0 (95% CI not estimable) |
| Night pain (VAS 0‐10, 0 = no pain) at 3 weeks | 0 | 0, 1 | 12 | 2 | 0, 4.5 | 12 | ‐2 (95% CI not estimable) |
| Night pain (VAS 0‐10, 0 = no pain) at 3 months | 1 | 0, 4 | 12 | 0 | 0, 3 | 12 | 1 (95% CI not estimable) |
| Night pain (VAS 0‐10, 0 = no pain) at 4 years | 0 | 0, 0 | 10 | 0 | 0, 0 | 11 | 0 (95% CI not estimable) |
| Active shoulder abduction (degrees) at 3 weeks | 180 | 170, 180 | 12 | 180 | 135, 180 | 12 | 0 (95% CI not estimable) |
| Active shoulder abduction (degrees) at 3 months | 180 | 162.5, 180 | 12 | 180 | 180, 180 | 12 | 0 (95% CI not estimable) |
| Active shoulder abduction (degrees) at 4 years | 180 | 180, 180 | 10 | 180 | 180, 180 | 11 | 0 (95% CI not estimable) |
| Active shoulder flexion (degrees) at 3 weeks | 175 | 147.5, 180 | 12 | 177.5 | 163.5, 180 | 12 | ‐2.50 (95% CI not estimable) |
| Active shoulder flexion (degrees) at 3 months | 180 | 150, 180 | 12 | 180 | 177.5, 180 | 12 | 0 (95% CI not estimable) |
| Active shoulder flexion (degrees) at 4 years | 180 | 180, 180 | 10 | 180 | 180, 180 | 11 | 0 (95% CI not estimable) |
| Active shoulder extension (degrees) at 3 weeks | 52.5 | 35, 60 | 12 | 60 | 45, 60 | 12 | ‐7.50 (95% CI not estimable) |
| Active shoulder extension (degrees) at 3 months | 60 | 45, 60 | 12 | 60 | 60, 60 | 12 | 0 (95% CI not estimable) |
| Active shoulder extension (degrees) at 4 years | 60 | 60, 60 | 10 | 60 | 60, 60 | 11 | 0 (95% CI not estimable) |
| Active shoulder external rotation (degrees) at 3 weeks | 78.5 | 40, 90 | 12 | 90 | 67.5, 90 | 12 | ‐11.50 (95% CI not estimable) |
| Active shoulder external rotation (degrees) at 3 months | 90 | 70, 90 | 12 | 90 | 90, 90 | 12 | 0 (95% CI not estimable) |
| Active shoulder external rotation (degrees) at 4 years | 90 | 90, 90 | 10 | 90 | 90, 90 | 11 | 0 (95% CI not estimable) |
| Active shoulder internal rotation (degrees) at 3 weeks | 87.5 | 70, 90 | 12 | 90 | 76.5, 90 | 12 | ‐2.50 (95% CI not estimable) |
| Active shoulder internal rotation (degrees) at 3 months | 90 | 75, 90 | 12 | 90 | 90, 90 | 12 | 0 (95% CI not estimable) |
| Active shoulder internal rotation (degrees) at 4 years | 90 | 90, 90 | 10 | 90 | 90, 90 | 11 | 0 (95% CI not estimable) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (excellent improvement) at 3 months | 7 | 12 | 8 | 12 | 0.88 (0.47, 1.63) | ||
| Global assessment of treatment success (excellent improvement) at 4 years | 10 | 10 | 11 | 11 | 1.00 (0.84, 1.19) | ||
| Total adverse events during 4 year trial period | Zero events in both groups | ||||||
Rabini 2012, which included 82 participants and was at high risk of bias overall, compared microwave diathermy (three times a week for four weeks) with glucocorticoid injection (one injection every two weeks for total of three injections) (Table 19). There was no clinically important difference between groups with respect to overall pain at four weeks (mean 35.1 versus 29.6 on a 100‐point scale, MD 5.50, 95% CI ‐2.65 to 13.65, 82 participants), 12 weeks (mean 38.4 versus 28.9 on a 100‐point scale, MD 9.50, 95% CI 1.19 to 17.81, 82 participants) and 24 weeks (mean 37.6 versus 29 on a 100‐point scale, MD 8.60, 95% CI ‐2.07 to 19.27, 82 participants), or function at four weeks (mean 90.1 versus 82.4 on a 100‐point scale, MD 7.70, 95% CI 0.61 to 14.79, 82 participants), 12 weeks (mean 86.6 versus 83.2 on a 100‐point scale, MD 3.40, 95% CI ‐1.55 to 8.35, 82 participants) and 24 weeks (mean 88.1 versus 89.9 on a 100‐point scale, MD ‐1.80, 95% CI ‐9.08 to 5.48, 82 participants). No participant reported any adverse events. We considered this evidence to be very low quality (downgraded by two points for high risk of performance and detection bias and by one point for imprecision).
18. Microwave diathermy versus another active intervention.
|
Study ID: Rabini 2012 Participants: Rotator cuff tendinopathy, with or without partial thickness tendon tears Intervention: Microwave diathermy Control: Glucocorticoid injection | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100, 0 = no pain) at 4 weeks | 35.1 | 24.3 | 40 | 29.6 | 10.3 | 42 | 5.50 (‐2.65, 13.65) |
| Overall pain (VAS 0‐100, 0 = no pain) at 12 weeks | 38.4 | 22.9 | 40 | 28.9 | 14.3 | 42 | 9.50 (1.19, 17.81) |
| Overall pain (VAS 0‐100, 0 = no pain) at 24 weeks | 37.6 | 30 | 40 | 29 | 17.3 | 42 | 8.60 (‐2.07, 19.27) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 4 weeks | 90.1 | 15 | 40 | 82.4 | 17.7 | 42 | 7.70 (0.61, 14.79) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 12 weeks | 86.6 | 12.7 | 40 | 83.2 | 9.9 | 42 | 3.40 (‐1.55, 8.35) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 24 weeks | 88.1 | 20 | 40 | 89.9 | 12.6 | 42 | ‐1.80 (‐9.08, 5.48) |
| Total adverse events during 24‐week trial period | Zero events in both groups | ||||||
Is one type of electrotherapy modality more effective than another?
In 12 trials (674 participants), one type of electrotherapy modality was compared with another.
Therapeutic ultrasound versus LLLT (Calis 2011; Yavuz 2014)
Therapeutic ultrasound versus microwave diathermy (Giombini 2006)
Therapeutic ultrasound versus radar (with mobilisation and exercise in both groups) (Polimeni 2003)
Therapeutic ultrasound versus diadynamic current (with mobilisation and exercise in both groups) (Polimeni 2003)
Therapeutic ultrasound versus high intensity laser therapy (Santamato 2009)
Therapeutic ultrasound versus TENS (with exercise and cold pack in both groups) (Shehab 2000)
Therapeutic ultrasound for four minutes versus therapeutic ultrasound for eight minutes (with superficial heat plus TENS plus exercise in both groups) (Yildirim 2013)
PEMF for six weeks versus PEMF for two weeks (Binder 1984)
PEMF for eight weeks versus PEMF for four weeks (Binder 1984)
PEMF for eight hours per day versus PEMF for two hours per day (Chard 1988)
TENS versus pulsed radiofrequency treatment (with exercise in both groups) (Korkmaz 2010)
Interferential LLLT versus continuous LLLT (Montes‐Molina 2012a)
Interferential light therapy generated by two light probes versus conventional light therapy generated by one light probe (Montes‐Molina 2012b)
The overall risk of bias was unclear in four (Binder 1984; Chard 1988; Montes‐Molina 2012a; Montes‐Molina 2012b), and high in eight trials (Calis 2011; Giombini 2006; Korkmaz 2010; Polimeni 2003; Santamato 2009; Shehab 2000; Yavuz 2014; Yildirim 2013).
In Giombini 2006, the authors observed clinically important differences favouring microwave diathermy over therapeutic ultrasound (both delivered three times a week for four weeks) in terms of rest pain at four weeks (MD 3.40, 95% CI 2.81 to 3.99; 10‐point scale, 26 participants) and 10 weeks (MD 3.95, 95% CI 3.36 to 4.54; 10‐point scale, 26 participants), function at four weeks (MD ‐18.10, 95% CI ‐20.96 to ‐15.24; 100‐point scale, 26 participants) and 10 weeks (MD ‐20.25, 95% CI ‐24.07 to ‐16.43; 100‐point scale, 26 participants), and global treatment success (number of participants returning to sport) at 10 weeks (RR 0.39, 95% CI 0.17 to 0.89; 26 participants). No participant reported adverse events.
Santamato 2009 observed clinically important differences favouring high intensity laser therapy over therapeutic ultrasound (both delivered five times a week for two weeks) in terms of overall pain (MD ‐2.02, 95% CI ‐2.67 to ‐1.37; 10‐point scale, 70 participants), but not function (MD 3.80, 95% CI 0.53 to 7.07; 100‐point scale, 70 participants) at two weeks. The trialists did not report measuring adverse events.
Nine trials found no clinically important or statistically significant differences between groups on any outcome (Binder 1984; Calis 2011; Chard 1988; Korkmaz 2010; Montes‐Molina 2012a; Montes‐Molina 2012b; Polimeni 2003; Shehab 2000; Yavuz 2014). In one trial (Yildirim 2013), statistically significant differences favouring a longer duration of therapeutic ultrasound were found in overall pain, function and active range of motion at five weeks.
Adverse events were reported as having been measured in six of the 12 trials (Binder 1984; Chard 1988; Giombini 2006; Korkmaz 2010; Montes‐Molina 2012a; Montes‐Molina 2012b), and none were reported by any participant (see Table 20).
19. One electrotherapy modality versus another.
|
Study ID: Binder 1984 Participants: Rotator cuff tendinitis Intervention: PEMF for 6 weeks Control: Placebo PEMF for 4 weeks followed by active PEMF for 2 weeks | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) change from baseline to 6 weeks | ‐8.98 | NR | 15 | ‐7.75 | NR | 14 | ‐1.23 (95% CI not estimable) |
| Total active range of motion (degrees) change from baseline to 6 weeks | 101.4 | NR | 15 | 63.45 | NR | 14 | 37.95 (95% CI not estimable) |
|
Study ID: Binder 1984 Participants: Rotator cuff tendinitis Intervention: PEMF for 8 weeks Control: Placebo PEMF for 4 weeks followed by active PEMF for 4 weeks | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) change from baseline to 4 months | ‐11.12 | NR | 15 | ‐10.37 | NR | 14 | ‐0.75 (95% CI not estimable) |
| Total active range of motion (degrees) change from baseline to 4 months | 122.37 | NR | 15 | 115.77 | NR | 14 | 6.6 (95% CI not estimable) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (participants symptomless) at 4 months | 9 | 15 | 10 | 14 | 0.84 (0.49, 1.43) | ||
| Total adverse events during 4 months | "Although many patients found the coils cumbersome, especially at night, no untoward reactions were reported during the controlled study" | ||||||
|
Study ID: Calis 2011 Participants: Subacromial impingement syndrome Intervention: Therapeutic ultrasound plus exercise plus hot pack Control: Low‐level laser therapy plus exercise plus hot pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 3 weeks | 2.21 | 2.09 | 21 | 2.56 | 2.28 | 15 | ‐0.35 (‐1.81, 1.11) |
| Function (Constant‐Murley total score: 0‐100, higher score = better function) at 3 weeks | 62.85 | 6.85 | 21 | 64.6 | 16.18 | 15 | ‐1.75 (‐10.45, 6.95) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 3 weeks | 4.24 | 2.26 | 21 | 3.73 | 2.37 | 15 | 0.51 (‐1.03, 2.05) |
| Night pain (VAS 0‐10, 0 = no pain) at 3 weeks | 3.74 | 2.18 | 21 | 3.68 | 2.85 | 15 | 0.06 (‐1.66, 1.78) |
| Shoulder abduction (degrees, unclear if active or passive) at 3 weeks | 155.95 | 9.21 | 21 | 155.8 | 7.35 | 15 | 0.15 (‐5.27, 5.57) |
| Shoulder flexion (degrees, unclear if active or passive) at 3 weeks | 177.04 | 3.74 | 21 | 174.46 | 6.94 | 15 | 2.58 (‐1.28, 6.44) |
| Shoulder internal rotation (degrees, unclear if active or passive) at 3 weeks | 74.85 | 7.29 | 21 | 70.93 | 6.06 | 15 | 3.92 (‐0.45, 8.29) |
| Shoulder external rotation (degrees, unclear if active or passive) at 3 weeks | 81.66 | 5.82 | 21 | 83.13 | 5.23 | 15 | ‐1.47 (‐5.10, 2.16) |
|
Study ID: Chard 1988 Participants: Rotator cuff tendinitis Intervention: PEMF for 8 hrs per day Control: PEMF for 2 hrs per day | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain | "The improvement in pain score over the 8 weeks of treatment consistently favoured Group 2 (8 hrs per day) for pain at night, on movement, at rest, and total pain score, but this failed to reach significance" | ||||||
| Pain on motion | See above quote | ||||||
| Night pain | See above quote | ||||||
| Pain with resisted movement | No statistically significant difference between groups over the 8‐week treatment period | ||||||
| Total active range of motion | "…when considering the range of active movements…there was no significant difference between the groups at 8 weeks" | ||||||
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (had no further significant problems over the following year) | 14 | 24 | 12 | 19 | 0.92 (0.57, 1.50) | ||
| Total adverse events during 8 week treatment period | Zero events in both groups | ||||||
|
Study ID: Giombini 2006 Participants: Supraspinatus tendinopathy Intervention: Therapeutic ultrasound Control: Microwave diathermy | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) at 4 weeks | 5.8 | 0.96 | 12 | 2.4 | 0.46 | 14 | 3.40 (2.81, 3.99) |
| Rest pain (VAS 0‐10, 0 = no pain) at 10 weeks | 5.15 | 0.87 | 12 | 1.2 | 0.63 | 14 | 3.95 (3.36, 4.54) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 4 weeks | 60 | 3.21 | 12 | 78.1 | 4.23 | 14 | ‐18.10 (‐20.96, ‐15.24) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 10 weeks | 61.75 | 4.18 | 12 | 82 | 5.73 | 14 | ‐20.25 (‐24.07, ‐16.43) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (ready to return to sport) at 4 weeks | 6 | 12 | 11 | 14 | 0.64 (0.34, 1.19) | ||
| Global assessment of treatment success (ready to return to sport) at 10 weeks | 4 | 12 | 12 | 14 | 0.39 (0.17, 0.89) | ||
| Adverse events | Zero events in both groups | ||||||
|
Study ID: Korkmaz 2010 Participants: Supraspinatus tendinopathy or partial tears of the supraspinatus tendon Intervention: TENS plus exercise Control: Pulsed radiofrequency treatment plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) at 1 week | 2.2 | 1.3 | 20 | 2.4 | 1.4 | 20 | ‐0.20 (‐1.04, 0.64) |
| Rest pain (VAS 0‐10, 0 = no pain) at 4 weeks | 1.8 | 1.43 | 20 | 1.3 | 0.9 | 20 | 0.50 (‐0.24, 1.24) |
| Rest pain (VAS 0‐10, 0 = no pain) at 12 weeks | 0.95 | 0.68 | 20 | 0.8 | 0.7 | 20 | 0.15 (‐0.28, 0.58) |
| Function (SPADI total score 0‐130, higher = worse function) at 1 week | 93.9 | 31.3 | 20 | 81.4 | 21 | 20 | 12.50 (‐4.02, 29.02) |
| Function (SPADI total score 0‐130, higher = worse function) at 4 weeks | 54.7 | 26.7 | 20 | 45.9 | 14.5 | 20 | 8.80 (‐4.52, 22.12) |
| Function (SPADI total score 0‐130, higher = worse function) at 12 weeks | 32.4 | 20.5 | 20 | 25.5 | 10.1 | 20 | 6.90 (‐3.12, 16.92) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 1 week | 4.8 | 2 | 20 | 5.2 | 1.8 | 20 | ‐0.40 (‐1.58, 0.78) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 4 weeks | 2.7 | 1.55 | 20 | 2.9 | 1 | 20 | ‐0.20 (‐1.01, 0.61) |
| Pain on motion (VAS 0‐10, 0 = no pain) at 12 weeks | 2.1 | 1.29 | 20 | 2.3 | 0.8 | 20 | ‐0.20 (‐0.87, 0.47) |
| Night pain (VAS 0‐10, 0 = no pain) at 1 week | 4.6 | 1.8 | 20 | 4.4 | 2 | 20 | 0.20 (‐0.98, 1.38) |
| Night pain (VAS 0‐10, 0 = no pain) at 4 weeks | 3 | 1.41 | 20 | 2.7 | 1.2 | 20 | 0.30 (‐0.51, 1.11) |
| Night pain (VAS 0‐10, 0 = no pain) at 12 weeks | 2.1 | 0.96 | 20 | 1.8 | 0.9 | 20 | 0.30 (‐0.28, 0.88) |
| Active shoulder abduction (degrees) at 1 week | 128.3 | 23 | 20 | 138.5 | 26.9 | 20 | ‐10.20 (‐25.71, 5.31) |
| Active shoulder abduction (degrees) at 4 weeks | 152.8 | 15.5 | 20 | 157.7 | 18.1 | 20 | ‐4.90 (‐15.34, 5.54) |
| Active shoulder abduction (degrees) at 12 weeks | 161.3 | 11.8 | 20 | 164 | 13.1 | 20 | ‐2.70 (‐10.43, 5.03) |
| Active shoulder flexion (degrees) at 1 week | 145 | 18.4 | 20 | 151.2 | 23.4 | 20 | ‐6.20 (‐19.25, 6.85) |
| Active shoulder flexion (degrees) at 4 weeks | 161 | 12.9 | 20 | 161.7 | 16.9 | 20 | ‐0.70 (‐10.02, 8.62) |
| Active shoulder flexion (degrees) at 12 weeks | 166.3 | 9 | 20 | 168.5 | 10.1 | 20 | ‐2.20 (‐8.13, 3.73) |
| Active shoulder external rotation (degrees) at 1 week | 55.8 | 14.7 | 20 | 57.2 | 19.9 | 20 | ‐1.40 (‐12.24, 9.44) |
| Active shoulder external rotation (degrees) at 4 weeks | 63.5 | 10 | 20 | 66 | 11.9 | 20 | ‐2.50 (‐9.31, 4.31) |
| Active shoulder external rotation (degrees) at 12 weeks | 69.3 | 7.8 | 20 | 67.7 | 9.7 | 20 | 1.60 (‐3.86, 7.06) |
| Active shoulder internal rotation (degrees) at 1 week | 46.5 | 14.5 | 20 | 58 | 16 | 20 | ‐11.50 (‐20.96, ‐2.04) |
| Active shoulder internal rotation (degrees) at 4 weeks | 61 | 12.3 | 20 | 68 | 11.5 | 20 | ‐7.00 (‐14.38, 0.38) |
| Active shoulder internal rotation (degrees) at 12 weeks | 66.5 | 10.8 | 20 | 70 | 8.7 | 20 | ‐3.50 (‐9.58, 2.58) |
| Quality of life (SF‐36 physical function 0‐100, higher = better) at 12 weeks | 74.25 | 10.03 | 20 | 69.5 | 16.09 | 20 | 4.75 (‐3.56, 13.06) |
| Quality of life (SF‐36 physical role 0‐100, higher = better) at 12 weeks | 60 | 23.5 | 20 | 55.35 | 14.9 | 20 | 4.65 (‐7.54, 16.84) |
| Quality of life (SF‐36 bodily pain 0‐100, higher = better) at 12 weeks | 61.25 | 17.07 | 20 | 67.37 | 14.83 | 20 | ‐6.12 (‐16.03, 3.79) |
| Quality of life (SF‐36 general health 0‐100, higher = better) at 12 weeks | 56.73 | 13.95 | 20 | 55.85 | 18.67 | 20 | 0.88 (‐9.33, 11.09) |
| Quality of life (SF‐36 vitality 0‐100, higher = better) at 12 weeks | 56.25 | 12.65 | 20 | 55.95 | 10.02 | 20 | 0.30 (‐6.77, 7.37) |
| Quality of life (SF‐36 social functioning 0‐100, higher = better) at 12 weeks | 74.37 | 16.95 | 20 | 81.24 | 16.09 | 20 | ‐6.87 (‐17.11, 3.37) |
| Quality of life (SF‐36 emotion role 0‐100, higher = better) at 12 weeks | 54.93 | 19.54 | 20 | 59.15 | 20.48 | 20 | ‐4.22 (‐16.63, 8.19) |
| Quality of life (SF‐36 mental health 0‐100, higher = better) at 12 weeks | 56.2 | 16.02 | 20 | 54.74 | 16.67 | 20 | 1.46 (‐8.67, 11.59) |
| Global assessment of treatment success | "In all weeks, there was no statistically significant difference between the two groups regarding the...physician–patient satisfaction rate (P > 0.05)". | ||||||
| Total adverse events during 12 week follow‐up period | Zero events in both groups | ||||||
|
Study ID: Montes‐Molina 2012a Participants: Rotator cuff tendinitis (53%), bicipital tendinitis (3%), calcific tendinitis (25%), rotator cuff partial tears (16%), impingement syndrome (5%), frozen shoulder (5%), dislocations (10%), bursitis (5%) Intervention: Interferential low‐level laser therapy (LLLT) Control: Continuous LLLT | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) change from baseline to 4 weeks | 0.3 | 1.87 | 86 | 0.4 | 5.95 | 83 | ‐0.10 (‐1.44, 1.24) |
| Function (SPADI total score 0‐100; 0 = no disability) change from baseline to 4 weeks | 6.8 | 26.12 | 86 | 7.3 | 11.45 | 83 | ‐0.50 (‐6.54, 5.54) |
| Night pain (VAS 0‐10, 0 = no pain) change from baseline to 4 weeks | 1.3 | 2.80 | 86 | 1.4 | 2.75 | 83 | ‐0.10 (‐0.94, 0.74) |
| Total adverse events during 4‐week trial period | Zero events in both groups | ||||||
|
Study ID: Montes‐Molina 2012b Participants: Rotator cuff tendinitis, calcific tendinitis or partial rotator cuff tears Intervention: Interferential light therapy generated by two light probes Control: Conventional light therapy generated by one light probe | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10, 0 = no pain) at 2 weeks | 2.1 | 2.5 | 13 | 1.9 | 2.3 | 13 | 0.20 (‐1.65, 2.05) |
| Function (UCLA shoulder scale 1‐35, higher score = better function) at 2 weeks | 22.3 | 6.7 | 13 | 23.9 | 6.8 | 13 | ‐1.60 (‐6.79, 3.59) |
| Night pain (VAS 0‐10, 0 = no pain) at 2 weeks | 4 | 3.8 | 13 | 5.8 | 3.7 | 13 | ‐1.80 (‐4.68, 1.08) |
| Total adverse events during 2 week trial period | Zero events in both groups | ||||||
|
Study ID: Polimeni 2003 Participants: Supraspinatus tendinitis or biceps tendinitis Intervention: Therapeutic ultrasound plus mobilisation plus exercises Control: Radar plus mobilisation plus exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 10 days | No usable outcome data. Difference between groups reported as not statistically significant. | ||||||
| Function (Constant‐Murley total score 0‐100, higher = better function) at 40 days | No usable outcome data. Difference between groups reported as not statistically significant. | ||||||
|
Study ID: Polimeni 2003 Participants: Supraspinatus tendinitis or biceps tendinitis Intervention: Therapeutic ultrasound plus mobilisation plus exercises Control: Diadynamic current plus mobilisation plus exercises | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 10 days | No usable outcome data. Difference between groups reported as not statistically significant. | ||||||
| Function (Constant‐Murley total score 0‐100, higher = better function) at 40 days | No usable outcome data. Difference between groups reported as not statistically significant. | ||||||
|
Study ID: Santamato 2009 Participants: Subacromial impingement syndrome Intervention: High intensity laser therapy Control: Therapeutic ultrasound | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain) at 2 weeks | 2.42 | 1.42 | 35 | 4.44 | 1.37 | 35 | ‐2.02 (‐2.67, ‐1.37) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 2 weeks | 75.91 | 7.02 | 35 | 72.11 | 6.95 | 35 | 3.80 (0.53, 7.07) |
|
Study ID: Shehab 2000 Participants: Supraspinatus tendinitis, subdeltoid bursitis or bicipital tendinitis Intervention: TENS plus exercise plus cold pack Control: Therapeutic ultrasound plus exercise plus cold pack | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Median | 5th to 95th percentile | n | Median | 5th to 95th percentile | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, 0 = no pain): at 3 to 5 weeks | 0 | 0, 0.65 | 26 | 0.5 | 0, 2.75 | 24 | ‐0.5 (95% CI not estimable) |
| Shoulder flexion (degrees, unclear if active or passive) at 3 to 5 weeks | 140 | 120, 160 | 26 | 175 | 115, 180 | 24 | ‐35 (95% CI not estimable) |
| Shoulder abduction (degrees, unclear if active or passive) at 3 to 5 weeks | 130 | 116.7, 156.5 | 26 | 180 | 101.2, 180 | 24 | ‐50 (95% CI not estimable) |
|
Study ID: Yavuz 2014 Participants: Subacromial impingement syndrome Intervention: Low‐level laser therapy plus hot pack plus exercise Control: Therapeutic ultrasound plus hot pack plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overal pain (VAS 0‐100, 0 = no pain) at 1 month | 39 | 14.29 | 16 | 37.43 | 15.07 | 15 | 1.57 (‐8.78, 11.92) |
| Overal pain (VAS 0‐100, 0 = no pain) at 3 months | 37 | 14.37 | 16 | 38.04 | 13.67 | 15 | ‐1.04 (‐10.91, 8.83) |
| Function (SPADI total score 0‐100, higher = worse function) at 1 month | 32.6 | 13.72 | 16 | 34.25 | 14.07 | 15 | ‐1.65 (‐11.44, 8.14) |
| Function (SPADI total score 0‐100, higher = worse function) at 3 months | 29.8 | 13.6 | 16 | 30.57 | 14.47 | 15 | ‐0.77 (‐10.67, 9.13) |
|
Study ID: Yildirim 2013 Participants: Subacromial impingement syndrome Intervention: Therapeutic ultrasound for 4 minutes plus superficial heat plus TENS plus exercise Control: Therapeutic ultrasound for 8 minutes plus superficial heat plus TENS plus exercise | |||||||
| Outcome | Intervention | Control | Effect estimate | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, higher = worse pain) at 5 weeks | 5.2 | 1.26 | 50 | 3.38 | 1.46 | 50 | 1.82 (1.29, 2.35) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 5 weeks | 59.38 | 15.32 | 50 | 66.8 | 19.43 | 50 | ‐7.42 (‐14.28, ‐0.56) |
| Active shoulder abduction (Constant‐Murley sub‐score, higher = better ROM) at 5 weeks | 6.6 | 1.62 | 50 | 7.52 | 1.54 | 50 | ‐0.92 (‐1.54, ‐0.30) |
| Active shoulder flexion (Constant‐Murley sub‐score, higher = better ROM) at 5 weeks | 7.32 | 2 | 50 | 8.22 | 2.37 | 50 | ‐0.90 (‐1.76, ‐0.04) |
| Active shoulder external rotation (Constant‐Murley sub‐score, higher = better ROM) at 5 weeks | 6.2 | 3.39 | 50 | 7.24 | 2.58 | 50 | ‐1.04 (‐2.22, 0.14) |
| Active shoulder internal rotation (Constant‐Murley sub‐score, higher = better ROM) at 5 weeks | 5.72 | 2.27 | 50 | 7.04 | 2.53 | 50 | ‐1.32 (‐2.26, ‐0.38) |
| Strength (Constant‐Murley sub‐score, higher = better ROM) at 5 weeks | 15.5 | 12.26 | 50 | 16.38 | 11.36 | 50 | ‐0.88 (‐5.51, 3.75) |
The results of all of the above trials should be interpreted with caution given that small, single trials evaluated each comparison. We considered the evidence from these 12 trials to be very low quality (downgraded by two points for high risk of performance and detection bias or unclear risk of allocation bias, and by one point for imprecision).
Assessment of reporting bias
Three trials either did not report or partially reported a pre‐specified outcome; however, we were unable to assess the impact of this outcome reporting bias on meta‐analyses since no meta‐analyses were performed. We were unable to generate funnel plots to assess small study effects. Despite this, we considered the risk of publication bias to be low because nearly all of the published studies reported statistically non‐significant results for most outcomes. While some unpublished studies with non‐significant results may exist, their inclusion in the review is unlikely to change our conclusions.
Discussion
Summary of main results
We have considered the results of 47 trials investigating the benefits and harms of various electrotherapy modalities for rotator cuff disease. The trials were heterogeneous in terms of population, intervention and comparator, so data could not be combined in a meta‐analysis. The findings need to be interpreted with caution given they are often based on a single small trial at high risk of bias overall.
Therapeutic ultrasound
Based upon low quality evidence from one small trial of people with rotator cuff disease without calcification, pulsed therapeutic ultrasound was no more effective than placebo with respect to overall pain, global treatment success or shoulder abduction at four weeks (Berry 1980). Based on low quality evidence from another small trial in people with calcific tendinitis, pulsed therapeutic ultrasound was more effective than placebo with respect to overall pain, function, global treatment success and quality of life at six weeks (Ebenbichler 1999). By nine months, groups had similar overall pain and function, likely because participants in both groups experienced natural recovery.
Based upon low quality evidence from eight trials, therapeutic ultrasound produced no clinically important additional benefits when combined with other physical therapy interventions in terms of overall pain, function, pain on motion, night pain, global treatment success, range of motion and strength (Calis 2011; Celik 2009; Clews 1987; Downing 1986; Kurtai Gursel 2004; Nykänen 1995; Polimeni 2003; San Segundo 2008). Further, there were no clinically important differences between therapeutic ultrasound and manual therapy (Al Dajah 2014; Bansal 2011; Clews 1987), glucocorticoid injection (Berry 1980), glucocorticoid injection plus oral tolmetin sodium (Berry 1980), exercise (Giombini 2006) and acupuncture (Berry 1980; Johansson 2005) with respect to overall pain, function, global treatment success, range of motion and strength; however we are uncertain about these results because the evidence is very low quality.
None of the participants in any of the three trials that measured harms reported adverse events (Ebenbichler 1999; Giombini 2006; Johansson 2005).
Low‐level laser therapy (LLLT)
Based on low quality evidence from two placebo‐controlled trials (England 1989; Saunders 1995), there were favourable effects of LLLT with respect to overall pain, function, active range of motion and strength up to three weeks. Based on low quality evidence, LLLT produced few additional benefits when combined with other physical therapy interventions with respect to overall pain, function, pain on motion, global treatment success, night pain, range of motion and strength (Abrisham 2011; Bal 2009; Bingöl 2005; Calis 2011; Dogan 2010; Eslamian 2012; Kelle 2014; Otadi 2012; Vecchio 1993; Yeldan 2009). Also, LLLT had favourable effects over oral NSAID in overall pain and function (England 1989), while an additional trial comparing LLLT to glucocorticoid injection observed no between‐group differences (Kelle 2014); however we are uncertain about these results because the evidence is very low quality.
None of the participants in any of the seven trials that measured harms reported adverse events of LLLT (Abrisham 2011; Bal 2009; Bingöl 2005; Dogan 2010; Kelle 2014; Vecchio 1993; Yeldan 2009).
Transcutaneous electrical nerve stimulation (TENS)
Based on a small single trial, TENS was found to provide greater pain relief immediately after treatment compared with placebo (Kocyigit 2012), but this trial was conducted in a setting not reflective of clinical practice. Further, TENS was found to provide similar pain relief to a hot pack (Baskurt 2006) and no additional pain relief when added to a hot pack (Baskurt 2006). It was also less effective than glucocorticoid injection with respect to function up to 12 weeks, although there were no between‐group differences observed in pain, global treatment success and active range of motion (Eyigor 2010). TENS was also less effective than extracorporeal shockwave treatment in terms of pain and function up to 12 weeks in people with calcific tendinitis (Pan 2003). However, we are uncertain about all of these results because the evidence is very low quality.
None of the participants in either of the two trials that measured harms reported adverse events of TENS (Eyigor 2010; Pan 2003).
Pulsed electromagnetic field (PEMF)
Based on low quality evidence, PEMF provided no clinically important benefits when compared with placebo (Binder 1984; Galace de Freitas 2014), or when added to exercise (Aktas 2007; Galace de Freitas 2014).
None of the trials investigating the effects of PEMF measured adverse events.
Other electrotherapy modalities
Based upon low or very low quality evidence, there were no clinically relevant between‐group differences in outcome in trials comparing MENS versus placebo (Atya 2012), acetic acid iontophoresis plus therapeutic ultrasound versus no treatment (Perron 1997), microwave diathermy versus glucocorticoid injection (Rabini 2012), therapeutic ultrasound plus TENS versus cryotherapy (Grymel‐Kulesza 2007) or therapeutic ultrasound plus TENS versus sodium hyaluronate injection (Ozgen 2012). Further, both microwave diathermy (Akyol 2012) and acetic acid iontophoresis (Leduc 2003) produced no additional benefits over exercise plus hot pack.
None of the participants receiving microwave diathermy reported any adverse events (Akyol 2012; Rabini 2012). None of the trials investigating the effects of MENS or acetic acid iontophoresis measured adverse events.
One type of electrotherapy modality versus another
There was very low quality evidence from 12 single trials comparing one electrotherapy modality to another (Binder 1984; Calis 2011; Chard 1988; Giombini 2006; Korkmaz 2010; Montes‐Molina 2012a; Montes‐Molina 2012b; Polimeni 2003; Santamato 2009; Shehab 2000; Yavuz 2014; Yildirim 2013). Only two found clinically important differences between groups: one trial favouring microwave diathermy over therapeutic ultrasound (Giombini 2006); and another trial favouring high intensity laser therapy over therapeutic ultrasound (Santamato 2009). The results of all of these trials should be interpreted with caution given that small, single trials evaluated each comparison.
Overall completeness and applicability of evidence
Participants in the included trials were mostly representative of populations most affected by rotator cuff disease. Nearly all trials enrolled a community sample of people attending routine physical therapy care. Across the trials, the median age was 53 (IQR 49 to 55) years. Thus, results are applicable to those likely to be seen in practice (Linsell 2006; Yamamoto 2010). Further, trials were conducted in 18 different countries, including a range of high‐ and low‐ to middle‐income countries. However, it is difficult to determine how representative participants in the included trials were with respect to duration of symptoms, as this characteristic was not reported in 17 (36%) trials.
A comprehensive range of treatment comparisons were captured across the trials. The review was dominated by trials investigating whether electrotherapy modalities provided benefit when added to manual therapy or exercise, or whether one electrotherapy modality was more effective than another. Several placebo‐controlled trials were also included (Atya 2012; Berry 1980; Binder 1984; Ebenbichler 1999; England 1989; Galace de Freitas 2014; Kocyigit 2012; Saunders 1995), and electrotherapy modalities were compared to many other active interventions (glucocorticoid injection, sodium hyaluronate injection, oral NSAID, acupuncture, extracorporeal shock wave treatment, hot pack and cryotherapy). Participants underwent treatment for a median of three weeks (interquartile range 2 to 4), so the findings may not generalise to treatment packages delivered over a longer period.
In several trial reports, the components of the electrotherapy modalities were incompletely described. For example, some trialists did not specify the frequency (e.g. Hz or MHz), intensity (e.g. W/cm2), power (e.g. W), duration of session (e.g. five minutes) or frequency of administration (e.g. three times a week for three weeks). This poor reporting is not surprising given that many trials were published prior to the dissemination of reporting guidelines (e.g. CONSORT (Schultz 2010)). Nevertheless, incomplete intervention descriptions hinder trial replication, and limit reliable implementation of the intervention into clinical practice. We recommend that future trialists follow recommendations for reporting of intervention details, as outlined in the template for intervention description and replication (TIDieR) checklist (Hoffman 2014).
Another concerning issue is the variable choice of outcomes measured in the trials. Overall pain and function were measured in most trials (85% and 70%, respectively), but these domains should be measured in all rotator cuff disease trials given that pain and functional limitations are the most common presenting symptoms of the condition (Whittle 2015). Further, adverse events were measured in less than a half the trials (40%). The other main outcomes of the review were measured in even fewer trials: pain on motion (32%), global assessment of treatment success (21%), and quality of life (11%). Outcome measurement has improved since the first version of our review (Green 1998), where function was measured in only 26% of trials (none with a validated disability index), and none of the trials measured quality of life. However, a core domain set and core outcome measurement set for rotator cuff disease trials would likely improve measurement of patient‐important outcomes in future trials, and would facilitate efforts to synthesise the evidence in future (Buchbinder 2003; Page 2015). Together with an international panel, we are currently developing these core sets according to the guidance of the Outcome Measures in Rheumatology (OMERACT) initiative, who have approved a special interest group session on shoulder pain at the OMERACT 2016 meeting, and the Core Outcome Measures in Effectiveness Trials (COMET) initiative.
Quality of the evidence
We used the GRADE approach (Schünemann 2011b) to assess the quality of all included trials. We downgraded all the trials to either low or very low quality based on three factors: firstly, the risk of allocation bias was unclear because trialists did not report whether the allocation sequence was concealed; secondly, the risk of performance and detection bias was high for self‐reported outcomes because participants were not blinded; and thirdly, evidence was based on small, single trials, leading to concerns about imprecision of effect estimates. Trials with unclear allocation concealment have been found to overestimate treatment effects by 7% (ratio of odds ratios 0.93, 95% credible interval 0.87 to 0.99), and unblinded assessment of self‐reported outcomes (such as pain and function) is estimated to exaggerate the treatment benefit by about 22% (ratio of odds ratios 0.78, 95% credible interval 0.65 to 0.92) (Savovic 2012). Given that 77% of trials included in our review had unclear or no allocation concealment, and 51% had unclear or non‐blinded assessment of self‐reported outcomes, further high quality trials may show even smaller effect estimates than those reported in this review.
Potential biases in the review process
We searched CENTRAL, MEDLINE, EMBASE and CINAHL, but not PEDro, a database of randomised trials, systematic reviews and clinical practice guidelines relevant to physiotherapy. An empirical study comparing the indexing of 400 physiotherapy trials in eight bibliographic databases found that almost all were indexed in CENTRAL (95%), PEDro (92%) MEDLINE (89%) and EMBASE (88%). Further, only one of the 400 trials was uniquely indexed in PEDro (Michaleff 2011). Therefore, we think it is very unlikely that we missed relevant trials that would change the conclusions of our review. Two review authors independently assessed the trials for inclusion in this review, extracted data and assessed the risk of bias, and a third review author adjudicated when any discrepancy arose. Review questions of interest were defined with full knowledge of the possible comparisons that could be undertaken, but no knowledge of the results of any comparisons. To prevent selective inclusion of results (Page 2013), we used pre‐defined decision rules to select data from trials when multiple measurement scales, time points and analyses were reported.
A potential limitation was that we excluded one trial (Taverner 2014) which may have included participants with rotator cuff disease, but the eligibility criteria and participant characteristics were not reported in enough detail for us to determine this. Further, we excluded two trials (Ainsworth 2007; Herrera‐Lasso 1993) where approximately two thirds of participants had rotator cuff disease and a third had adhesive capsulitis, but we were unable to obtain data for the rotator cuff disease subgroup. Further, we were unable to translate five trials reported in a language other than English, but will endeavour to include these trials in the next update of this review. In addition, we did not undertake a search for grey literature (e.g. proceedings of specific conferences, theses or unpublished reports). However, since the majority of the evidence we included had "negative" findings, we believe that identification and inclusion of unpublished studies with non‐significant results is unlikely to have changed our conclusions.
Agreements and disagreements with other studies or reviews
Following the earlier Cochrane review of physical therapy for shoulder pain (Green 2003), there have been three systematic reviews of electrotherapy modalities and other physical therapy interventions for rotator cuff disease (Gebremariam 2014; Kromer 2009; Nyberg 2010), and one systematic review of therapeutic ultrasound for shoulder pain (Alexander 2010). All of these reviews have been narrower in scope than ours. Review authors either restricted their participant eligibility criteria according to the diagnostic label used by trialists (e.g. focusing only on subacromial impingement syndrome), or used broad participant eligibility criteria but focused on one electrotherapy modality (i.e. therapeutic ultrasound). Therefore, to our knowledge, ours is the most comprehensive review of electrotherapy modalities for rotator cuff disease. Our conclusions that there may be little or no important benefits of electrotherapy modalities for rotator cuff disease are consistent with the conclusions of all other systematic reviews.
Authors' conclusions
Implications for practice.
Based on low quality evidence, therapeutic ultrasound may have short‐term benefits over placebo in people with calcific tendinitis, and LLLT may have short‐term benefits over placebo in people with rotator cuff disease. In contrast, based on low quality evidence, PEMF may not provide clinically relevant benefits over placebo, and therapeutic ultrasound, LLLT and PEMF may not provide additional benefits when combined with other physical therapy interventions. We are uncertain whether TENS is superior to placebo, and whether any electrotherapy modality provides benefits over other active interventions (e.g. glucocorticoid injection) because of the very low quality of the evidence. Until further evidence confirms or refutes these results, practitioners should communicate the uncertainty of effect and consider other approaches or combinations of treatment.
Implications for research.
High quality placebo‐controlled trials are needed to confirm the favourable effects of therapeutic ultrasound for calcific tendinitis and LLLT for rotator cuff disease observed in previous trials. Further trials of other electrotherapy modalities for rotator cuff disease should be based upon a strong rationale and consideration of whether or not they would alter the conclusions of this review. Novel multi‐modal interventions combining electrotherapy modalities such as ultrasound or LLLT, with manual therapy and exercise, should be compared with a realistic placebo (e.g. use of inactive ultrasound and application of an inert gel) in high quality randomised trials. The interventions should be described in enough detail to inform interpretation of findings and allow replication. Trials should use strategies designed to minimise the potential for bias, including adequate allocation concealment and blinding of participants and outcome assessors. Development of a core set of outcomes for trials of rotator cuff disease and other shoulder disorders would facilitate our ability to synthesise the evidence in future.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2016 | New search has been performed | The original review, 'Physiotherapy interventions for shoulder pain' (Green 2003) was split into four reviews upon updating: 'Manual therapy and exercise for rotator cuff disease' (ongoing), this review, 'Electrotherapy modalities for rotator cuff disease', 'Manual therapy and exercise for adhesive capsulitis (frozen shoulder)' (Page 2014a), and 'Electrotherapy modalities for adhesive capsulitis (frozen shoulder)' (Page 2014b). The review has also been broadened by including all randomised and quasi‐randomised clinical trials regardless of whether outcome assessment was blinded. |
History
Review first published: Issue 6, 2016
| Date | Event | Description |
|---|---|---|
| 1 May 2008 | Amended | Converted to RM5. CMSG ID C067‐R |
| 24 February 2003 | Amended | This review is based on the original review of 'Interventions for shoulder pain'. Please see published notes for further details. |
| 24 February 2003 | New citation required and conclusions have changed | Substantive amendment |
Notes
The original review, 'Physiotherapy interventions for shoulder pain' (Green 2003) was split into four reviews upon updating: 'Manual therapy and exercise for rotator cuff disease' (ongoing), this review, 'Electrotherapy modalities for rotator cuff disease', 'Manual therapy and exercise for adhesive capsulitis (frozen shoulder)' (Page 2014a), and 'Electrotherapy modalities for adhesive capsulitis (frozen shoulder)' (Page 2014b). The review has also been broadened by including all randomised and quasi‐randomised clinical trials regardless of whether outcome assessment was blinded.
Acknowledgements
We are grateful to Steve McDonald from the Australasian Cochrane Centre for his help with the search strategy.
Appendices
Appendix 1. Search strategies
Search strategy for CENTRAL:
MeSH descriptor: [Shoulder Pain] explode all trees
MeSH descriptor: [Shoulder Impingement Syndrome] explode all trees
MeSH descriptor: [Rotator Cuff] explode all trees
MeSH descriptor: [Bursitis] explode all trees
((shoulder* in All Text or rotator* in All Text) and (bursitis in All Text or frozen in All Text or impinge* in All Text or tendonitis in All Text or tendonitis in All Text or tendinopathy in All Text or pain* in All Text))
"rotator cuff" in All Text
"adhesive capsulitis" in All Text
#1 or #2 or #3 or #4 or #5 or #6 or #7
MeSH descriptor: [Rehabilitation] explode all trees
MeSH descriptor: [Physical Therapy Modalities] explode all trees
MeSH descriptor: [Exercise Movement Techniques] explode all trees
MeSH descriptor: [Ultrasonography, Interventional] explode all trees
rehabilitat* in All Text or physiotherapy* in All Text or "physical therap*" in All Text or "manual therap*" in All Text or exercis* in All Text
(ultrasound in All Text or ultrasonograph* in All Text or tns in All Text or tens in All Text or shockwave in All Text or electrotherap* in All Text or mobili* in All Text)
#9 or #10 or #11 or #12 or #13 or #14
#8 and #15
Search strategy for Ovid MEDLINE:
shoulder pain/
shoulder impingement syndrome/
rotator cuff/
exp bursitis/
((shoulder$ or rotator cuff) adj5 (bursitis or frozen or impinge$ or tendinitis or tendonitis or tendinopathy or pain$)).mp.
rotator cuff.mp.
adhesive capsulitis.mp.
or/1‐7
exp rehabilitation/
exp physical therapy techniques/
exp musculoskeletal manipulations/
exp exercise movement techniques/
exp ultrasonography, interventional/
(rehabilitat$ or physiotherap$ or physical therap$ or manual therap$ or exercis$ or ultrasound or ultrasonograph$ or TNS or TENS or shockwave or electrotherap$ or mobili$).mp.
or/9‐14
clinical trial.pt
random$.mp.
((single or double) adj (blind$ or mask$)).mp.
placebo$.mp.
or/16‐19
8 and 15 and 20
Search strategy for Ovid EMBASE:
‘shoulder pain'/exp
‘shoulder impingement syndrome'/exp
‘rotator cuff'/exp
‘bursitis'/exp
((shoulder* OR rotator*) AND (‘bursitis'/de OR frozen OR impinge* OR ‘tendonitis'/de OR ‘tendinitis'/de OR ‘tendinopathy'/de OR pain*))
‘rotator cuff'
‘adhesive capsulitis'
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
‘rehabilitation'/exp
‘physiotherapy'/exp
‘kinesiotherapy'/exp
‘endoscopic echography'/exp
rehabilitat* OR physiotherapy* OR ‘physical therapy' OR ‘manual therapy' OR kinesiotherap* OR exercis*
‘ultrasound'/de OR ultrasonograph* OR ‘transcutaneous nerve stimulation' OR ‘transcutaneous electrical nerve stimulation' OR shockwave OR electrotherap* OR mobili*
#9 OR #10 OR #11 OR #12 OR #13 OR #14
‘randomized controlled trial'/exp
#8 AND #15 AND #16
Search strategy for CINAHL Plus (EBSCOhost):
S1 MH "shoulder pain"
S2 MH "shoulder impingement syndrome"
S3 MH "rotator cuff"
S4 MH bursitis+
S5 TX (shoulder* N5 bursitis) or TX(shoulder* N5 frozen) or TX(shoulder* N5 impinge*) or TX(shoulder* N5 tend?nitis) or TX(shoulder* N5 tendinopathy) or TX(shoulder* N5 pain*)
S6 TX (rotator cuff N5 bursitis) or TX(rotator cuff N5 frozen) or TX(rotator cuff N5 impinge*) or TX(rotator cuff N5 tend?nitis) or TX(rotator cuff N5 tendinopathy) or TX(rotator cuff N5 pain*)
S7 TX rotator cuff
S8 TX adhesive capsulitis
S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8
S10 MH Rehabilitation+
S11 MH physical therapy+
S12 MH Manual Therapy+
S13 MH Therapeutic Exercise+
S14 MH Ultrasonography+
S15 TX rehabilitat* or physiotherapy* or physical therap* or manual therap* or exercise* or ultrasound or ultrasonograph* or TNS or TENS or shockwave or electrotherapy* or mobili*
S16 S10 or S11 or S12 or S13 or S14 or S15
S17 PT clinical trial
S18 TX random*
S19 TX(single blind*) or TX(single mask*)
S20 TX(double blind*) or TX(double mask*)
S21 placebo*
S22 S17 or S18 or S19 or S20 or S21
S23 S9 and S16 and S22
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abrisham 2011.
| Methods |
Study design: Parallel group RCT Setting: Physiotherapy clinic Intervention: Laser treatment (pulsed infrared laser) plus exercise therapy Control: Placebo laser plus exercise therapy Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff and bicep tendinitis Criteria for defining the shoulder condition being treated: Subacromial syndrome (rotator cuff and bicep tendinitis) defined by:
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above):
Baseline characteristics Intervention: Low‐level laser Number randomised: 40 Number included in analyses: 40 Age: 55.2 ± 5.7 years old Sex: F/M 24/16 Duration of symptoms: not reported Control: Placebo laser Number randomised: 40 Number included in analyses: 40 Age: 51.2 ± 6.7 years old Sex: F/M 26/14 Duration of symptoms: not reported |
|
| Interventions |
Intervention: Low‐level laser therapy Description of modality used: infrared laser radiation delivered by a Mustang‐024 device at 890 nm wavelength in pulsed mode. Three points on the shoulder (anterior/coracoid, posterior/glenohumeral joint, lateral/rotator cuff tendon) were irradiated. The biceps tendon was irradiated if applicable Dose: 2 min over 3 areas with an energy density of 2‐4 J/cm2
Frequency of administration: 10 sessions over 2 weeks Control: Placebo laser Description of modality used: infrared laser radiation delivered by a Mustang‐024 device. Three points on the shoulder (anterior/coracoid, posterior/glenohumeral joint, lateral/rotator cuff tendon) were irradiated. The biceps tendon was irradiated if applicable Dose: no lasers were emitted Frequency of administration: 10 sessions in 2 weeks Both groups Description of modality used: in the clinic ‐ pulley and shoulder wheel exercises; at home ‐ pendular shoulder exercises for the first 2 sessions and isometric exercises and active assisted exercises from the third session Dose: not reported Frequency of administration: 10 sessions in 2 weeks |
|
| Outcomes | Outcomes assessed at 2 weeks
|
|
| Notes |
Conflict of interest: the authors reported that they had nothing to declare Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised in two groups by using sealed envelopes method" Comment: The method used to generate the allocation sequence was not clearly reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method used to conceal the allocation sequence was not clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients in the second group were treated with placebo laser therapy. The same device which seemed to be working was used but no laser beams were transferred to the treated area." Comment: Patients were likely blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Shoulder ROM was measured by a blinded physician" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of the 80 participants completed the treatment." Comment: There were no losses to follow‐up, drop‐outs or post‐randomisation exclusions |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes specified in the methods section. However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Aktas 2007.
| Methods |
Study design: Parallel group RCT Setting: Hospital, Turkey Intervention: Pulsed electromagnetic field plus exercise plus cold pack Control: Sham pulsed electromagnetic field plus exercise plus cold pack Source of Funding: This study was supported by the Department of Physical Medicine and Rehabilitation, Cerrahpasa Medical Faculty, Istanbul University. |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated: Diagnosis of shoulder impingement syndrome by:
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: PEMF Number randomised: 20 Number included in analyses: 20 Age: 48 ± 7.9 years old Sex: F/M 15/5 Duration of symptoms: 4.82 ± 3.75 Control: Sham PEMF Number randomised: 20 Number included in analyses: 20 Age: 53.9 ± 11.12 years old Sex: F/M 15/5 Duration of symptoms: 4.80 ± 3.47 |
|
| Interventions |
Intervention: PEMF Description of modality used: Magnetoterapia model MG/3P (Elettromed) Dose: Frequency 50 Hz with a field intensity of 30 G for 25 min per session Method of administration: the switch that allowed the machine to produce waves was set to ‘on' and a U‐shaped applicator 30 x 15 cm in size was used Frequency of administration: 5 sessions per week for 3 weeks Control: Sham PEMF Description of modality used: Magnetoterapia model MG/3P (Elettromed) Dose: none for a 25‐min session Method of administration: the switch that allowed the machine to produce waves was set to ‘off' and a U‐shaped applicator 30 x 15 cm in size was used Frequency: 5 sessions per week for 3 weeks Both Groups Description of modality used
Dose
Method of administration
Frequency
Any additional treatment during trial
|
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes |
Conflict of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two equal groups of 23 patients in a simple systematic manner (x + 1) according to the therapeutic PEMF or sham PEMF application." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A separate individual was provided the randomization list and informed therapist." Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and physicians remained blind to the group allocation throughout the study." Quote: "One group was given PEMF; the other group was given sham PEMF. A magnetic field treatment unit was used with a concealed switch for either the presence or absence of waves when activated by the patient's attendant." Comment: Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quotes: "Patients and physicians remained blind to the group allocation throughout the study." Comment: Assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Forty patients completed the study. Three patients from each group could not continue treatment program. Therefore, six patients dropped out of the study." Comment: The rate and reasons for attrition were equal between groups. Also, analysis was based on all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Akyol 2012.
| Methods |
Study design: Parallel group RCT Setting: University, Turkey Interventions: Microwave diathermy plus superficial heat plus exercise Control: Sham microwave diathermy plus superficial heat plus exercise Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion criteria
Exclusion criteria
Baseline characteristics Total n randomised = 40 participants Total n analysed = 40 participants Intervention: Microwave diathermy Number randomised: 20 Number included in analyses: 20 Mean ± SD (range) age: 55.35 ± 14.50 (21‐78) years Sex: F/M 15/5 Mean ± SD (range) duration of symptoms: 10.5 ± 8.59 (3‐36) months Control: Sham microwave diathermy Number randomised: 20 Number included in analyses: 20 Mean ± SD (range) age: 51.2 ± 6.82 (42‐65) years Sex: F/M 15/5 Mean ± SD (range) duration of symptoms: 14.1 ± 18.38 (3‐84) months |
|
| Interventions |
Intervention: Microwave diathermy Components of intervention: microwave diathermy (Curadar 409 (Enraf–Nonius, The Nederland) equipped with 2,450 MHz microwaves generator with a maximum output power of 100 W, applied for 20 min) Control: Sham microwave diathermy Components of intervention: As above but device was set to the "on" mode, dials were lit but no energy was delivered to the tissue Both groups Superficial heat via hot pack (20 min) plus exercise (15 min shoulder active range of motion (Codman's pendulum, wall‐climbing, and shoulder wheel), 5 min stretching and 10 min strengthening exercise including rotator cuff muscles, rhomboids, levator scapulae, and serratus anterior with an elastic band). Both of the programmes were performed 5 days a week, for 3 weeks, and a total of 15 sessions as an inpatient The use of NSAID, other analgesic drugs, and antidepressant drugs was not permitted during the study period; any pretreatment with these drugs had to be discontinued 7 days before the start of study. The use of other medication for comorbid diseases was permitted during study period |
|
| Outcomes | Outcomes assessed at the end of 3 weeks' treatment and at 1 month follow‐up (i.e. 7 weeks)
|
|
| Notes |
Conflict of interest: none Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Forty patients were randomized (using concealed envelopes) into one of two groups". Comment: There was not enough information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was not enough information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In control group, MD [microwave diathermy] device was set to the "on" mode, dials were lit but no energy was delivered to the tissue." Comment: Participants were blinded to treatment received |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Patients were assessed three times by the same physician (YA), who was blinded with regard to the type of treatment the patients receive" Comment: Blinded assessor measured objective outcomes (e.g. ROM, strength) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No dropouts occurred during the trial, and all subjects in both groups completed the treatment program." |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication. Despite the absence of a protocol, all clinically important outcomes were measured (according to the trial publication) so selective reporting bias is not suspected |
| Other bias | Low risk | Comment: No other sources of bias identified |
Al Dajah 2014.
| Methods |
Study design: Parallel group RCT Setting: Physiotherapy outpatient department, Saudi Arabia Intervention: Therapeutic ultrasound Control: Soft tissue mobilisation and proprioceptive neuromuscular facilitation Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Shoulder impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics
|
|
| Interventions |
Intervention: Therapeutic ultrasound Components of intervention: the arm was abducted to 45° and the forearm was rested on the pillow for support. Ultrasound therapy was given to the subscapularis muscle insertion at the shoulder region Dose: frequency ‐ 3 MHz; intensity ‐ 0.5 W/cm2; duration: 10 min Frequency of administration: once Control: Soft tissue mobilisation (STM) and proprioceptive neuromuscular facilitation (PNF) Components of intervention: the subjects were positioned with the humerus abducted to 45° with elbow flexed to 90°, and the humerus was externally rotated to a midrange position, typically about 20° to 25° of external rotation. The subscapularis was palpated in the axilla to identify areas of myofascial mobility restrictions, taut bands, or trigger points. Identified restrictions were treated with STM utilising a combination of sustained manual pressure, and slow deep strokes to the subscapularis myofascia for 7 min. The STM was followed by contract‐relax PNF for the subscapularis and other glenohumeral medial rotators, beginning in the same position used for the STM. The participants were instructed to perform maximal glenohumeral internal rotation against an opposing, isometric, manual resistance applied by the treating physical therapist for 7 seconds. Afterwards, the participant actively moved the humerus into full available external rotation. This position was maintained for 15 seconds. This 7‐second internal rotation contraction against resistance followed by full active external rotation was repeated 5 times. Subjects were then instructed to actively move through the PNF flexion‐abduction external‐rotation diagonal pattern for 5 repetitions with manual facilitation. Dose: 10 min Frequency of administration: once |
|
| Outcomes | Outcomes assessed immediately after one treatment session (day 1)
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were assigned randomly into two groups by lot method" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no attrition because all participants were treated and assessed in a single session |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Atya 2012.
| Methods |
Design: Parallel group RCT Setting: Outpatient clinic of Faculty of Physical Therapy, Cairo University, Egypt Interventions: Microcurrent electrical stimulation (MENS) Control: Placebo microcurrent electrical stimulation Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Inclusion criteria
Exclusion criteria
Baseline characteristics Total n randomised = 40 participants (40 shoulders) Intervention: MENS Number randomised: 19 Age: 48.8 ± 6 years (range not reported) Sex: F/M 9/10 Mean ± SD (range) duration of symptoms: 5.67 ± 3.13 months (range not reported) Control: Placebo MENS Number randomised: 21 Age: 9.1 ± 3.3 years (range not reported) Sex: F/M 12/9 Mean ± SD (range) duration of symptoms: 6.55 ± 2.21 months (range not reported) |
|
| Interventions |
Intervention: MENS Description of modality used: HARLY physio 3000 unit. MENS is a novel electrotherapeutic modality. It is claimed to be capable of providing beneficial effects through delivering monophasic or biphasic pulsed microamperage currents with intensities between 1 and 999 uA across the skin Components of intervention: participants received a microcurrent stimulation with the following parameters: intensity 30‐40 mA, pulse frequency 10 Hz, pulse width 50 ms, with duration 20 min/session. Current was applied via two skin surface carbon fibre electrodes containing an integral coupling gel Control: Placebo MENS Description of modality used: delivered in the same way as described above with the exception that the electrodes were not connected to the microcurrent device Components of intervention: each participant received 18 treatment sessions at a rate of 3 sessions per week for 6 weeks |
|
| Outcomes | Outcomes assessed at the end of 6 weeks' treatment
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned by means of a computer generated schedule, with random permuted block size of 2" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The staff administering the treatments were not blinded." Quote: "The setting for patients enrolled into the placebo group was identical with the exception that the electrodes were not connected to the microcurrent device. As the administered microcurrent does not induce any sensations nor muscle twitching, patients were not able to distinguish between placebo or verum treatment" Comment: Participants were blinded to treatment, but personnel delivering the intervention were not (though this is unlikely to have affected the outcomes) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The staff administering the treatments were different from the staff administering the outcome measures: the latter were blinded to which treatment group (active or control) each patient was about to receive or had just received" Comment: Blinded assessors measured objective outcomes (proprioception accuracy) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no drop‐outs, losses to follow‐up or exclusions |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data fully reported for all outcomes specified in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Bal 2009.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic, Turkey Intervention: Low‐level laser therapy (LLLT) plus home exercise programme Control: Home exercise programme Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms:
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: LLLT plus exercise Number randomised: 22 Number included in analyses: 20 Age: 51.7 ± 14.1 years old Sex: F/M 15/5 Duration of symptoms: not reported Control: Exercise Number randomised: 22 Number included in analyses: 20 Age: 53.1 ± 8.4 years old Sex: F/M 13/7 Duration of symptoms: not reported |
|
| Interventions |
Intervention: LLLT therapy Description of modality used: LLLT applied over the tuberculum majus and minus, the anterior and posterior faces of the capsule and the subacromial regions. The head of the instrument was held perpendicular to the body surface without pressure. A Ga‐As diode laser instrument (Roland Serie, Elettronica Pagani) was used Dose: 10 min sessions with each body point being treated for 120 seconds. Wavelength 904 nm, 5500 Hz frequency, 27 W maximum power output per pulse was used, with a 13.2 mW average power, 0.8 cm2 spot size, 1.6 J of total energy was delivered per point at each session at a power density of 16.5 mW/cm2. The cumulative energy per point for all sessions was 16 J Frequency of administration: 5 times per week for 2 weeks Control: Home exercise programme No direct comparator was used in the control group. Participants only received the same home exercise programme as intervention group Both groups: Home exercises Description of modality used: comprehensive home exercise programme comprising pendulum circumduction and passive shoulder self‐stretching followed by isometrics in all planes; theraband exercises with three different therabands (low, medium, and high resistances); strengthening exercises for the muscles of scapular stabilisation; and advanced muscle‐ strengthening exercises with dumbbells. Progress was checked at the clinic twice weekly when the new exercises were taught. hot pack use before and cold pack use after each session was encouraged Dose: not reported Frequency of administration: over a period of 12 weeks Any additional treatment during trial: oral paracetamol (1500 mg/d) as needed |
|
| Outcomes | Outcomes assessed at 1 week, 2 weeks and 12 weeks
|
|
| Notes |
Conflict of interest: "No conflicting financial interests exist." Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised into two groups after initial evaluation by selecting a sealed unmarked envelope containing a letter indicating their group assignment." Comment: An adequate method was likely used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: An adequate method was likely used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "All patients were informed about the nature of the study procedure" Comment: Given the nature of the interventions, participants were not blinded, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of each intervention, self‐reported all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients in group 1 and two patients in group 2 were lost to follow‐up" Comment: While reasons for loss to follow‐up were not reported, the numbers were the same across both treatment and control group, so attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Bansal 2011.
| Methods |
Design: Parallel group RCT Setting: University, India Intervention: Therapeutic ultrasound plus Codman's exercises Control: Deep friction massage plus Codman's exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus tendinitis Criteria for defining the shoulder condition being treated Supraspinatus tendinitis defined by:
Inclusion Criteria (not listed above)
Exclusion criteria (not listed above)
Baseline characteristics Intervention: Therapeutic ultrasound and Codman's exercises Number randomised: 20 Mean (SD) age: 30.35 (5.76) years Sex: F/M 111/9 Duration of symptoms: not reported Control: Deep friction massage and Codman's exercises Number randomised: 20 Mean (SD) age: 30.90 (5.33) years Sex: F/M 8/12 Duration of symptoms: not reported |
|
| Interventions |
Intervention: Therapeutic ultrasound Components of intervention: pulsed ultrasound applied to the supraspinatus tendon with the participants positioned with hand behind back Dosage: intensity 0.6 W/cm2, frequency 1 MHz, pulse rate 4:1 for 6‐8 min for 10 sessions over 10 days Frequency of administration: not explicitly reported, assumed daily for 10 days Control ‐ Deep friction massage Components of intervention: deep friction massage to supraspinatus tendon in a transverse direction with the tip of the index finger, reinforced by middle finger. Participants were positioned half‐lying with hand behind back (shoulder adduction and internal rotation) Dosage: 10‐12 min for 10 sessions over 10 days Frequency of administration: not explicitly reported, assumed daily for 10 days Both groups All participants were instructed in Codman's exercises consisting of pendulum or swinging motion of the arm in flexion, extension, horizontal abduction, adduction and circumduction. Dosage: not reported. Frequency of administration: Intensity (arc of motion) was increased as tolerated. Participants were also advised to avoid strenuous work involving the affected upper limb |
|
| Outcomes | Outcomes assessed at 5 days and 10 days
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The individuals were randomly divided into two groups" Comments: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Unclear risk | Comment: No information was reported regarding the assessors of the objective outcome (active range of shoulder abduction) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Only mean scores (no measures of variation) were reported for all outcomes. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Baskurt 2006.
| Methods |
Study design: Parallel group RCT Setting: Orthopaedic physiotherapy unit, Turkey Intervention 1: Transcutaneous electrical nerve stimulation (TENS) Intervention 2: Hot pack Intervention 3: TENS plus hot pack Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Shoulder impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: TENS Number randomised: 30 Number included in analyses: 30 Age (mean and SD, or range): 57.10 ± 4.43 years Number of men and women: F/M 20/10 Duration of symptoms: not reported Intervention 2: Hot pack Number randomised: 31 Number included in analyses: 31 Age (mean and SD, or range): 56.54 ± 9.99 years Number of men and women: F/M 22/9 Duration of symptoms: not reported Intervention 3: TENS plus hot pack Number randomised: 31 Number included in analyses: 31 Age (mean and SD, or range): 57.32 ± 10.61 years Number of men and women: F/M 18/13 Duration of symptoms: not reported |
|
| Interventions |
Intervention 1: TENS Description of modality used: TENS delivered to participant who was comfortably seated in a chair with back support and a pillow in the lap for arm support Dose: 100 Hz 0.1 ms pulse duration, symmetric biphasic wave form of tolerable intensity for 20 min Frequency: 1 session only Any additional treatment during trial: none Intervention 2: Hot pack Description of modality used: hot pack delivered to participant who was comfortably seated in a chair with back support and a pillow in the lap for arm support Dose: 39 degrees Celsius for 20 min Frequency: 1 session only Any additional treatment during trial: none Intervention 3: TENS plus hot pack Description of modality used: the third group was a combination of the methods previously mentioned (i.e. 20 min of TENS and 20 min of heat) Dose: See above Frequency: 1 session only Any additional treatment during trial: none |
|
| Outcomes | Outcome assessed immediately post treatment
|
|
| Notes |
Conflict of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into three groups." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Berry 1980.
| Methods |
Study design: Parallel group RCT Setting: Outpatient hospital. UK Intervention 1: Therapeutic ultrasound Intervention 2: Glucocorticoid injection plus active tolmetin sodium Intervention 3: Glucocorticoid injection plus placebo tolmetin sodium Intervention 4: Acupuncture Control: Placebo ultrasound plus placebo tolmetin sodium Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff lesions Criteria for defining the shoulder condition being treated Pain arising from the shoulder due to a rotator cuff lesion defined as:
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: Therapeutic ultrasound Number randomised: 12 Number included in analyses: 12 Age (SD): 55.1 (12.7) years Sex: F/M 7/5 Duration of symptoms (SD): 16.3 (14.5) weeks Intervention 2: Glucocorticoid injection/tolmetin sodium Number randomised: 12 Number included in analyses: 12 Age (SD): 51.2 (14.6) years Sex: F/M 8/4 Duration of symptoms (SD): 28.3 (15.2) weeks Intervention 3: Steroid injection/placebo tolmetin sodium Number randomised: 12 Number included in analyses: 12 Age (SD): 54.1 (16.7) years Sex: F/M 6/6 Duration of symptoms (SD): 23.6 (27.9) weeks, excluding one participant with a duration of 10 years Intervention 4: Acupuncture Number randomised: 12 Number included in analyses: 12 Age (SD): 52.3 (10.8) years Sex: F/M 4/8 Duration of symptoms (SD): 20.3 (16.9) weeks Control: Placebo ultrasound plus placebo tolmetin sodium Number randomised: 12 Number included in analyses: 12 Age (SD): 56.2 (11.2) years old Sex: F/M 6/6 Duration of symptoms (SD): 27.5 (35) weeks |
|
| Interventions |
Intervention 1: Therapeutic ultrasound Description of modality used: therapeutic ultrasound delivered by a qualified physiotherapist Dose: 10 min (intensity and frequency not reported) Frequency of administration: 8 sessions over 4 weeks Intervention 2: Glucocorticoid injection plus tolmetin sodium Description of modality used: methyl prednisolone and lignocaine injection given by the same person using the anterior approach to the shoulder joint plus tolmetin sodium(1200 mg) Dose: injection ‐ 40 mg methyl prednisolone with 2 mL 2% lignocaine; tolmetin sodium ‐ 2 x 200 mg tablets 3 times a day) Frequency of administration: 1 injection; tolmetin sodium 2 tablets 3 times per day for 4 weeks Intervention 3: Glucocorticoid injection plus placebo tolmetin sodium Description of modality used: methyl prednisolone and lignocaine injection given by the same person using the anterior approach to the shoulder joint plus placebo tolmetin sodium Dose: Injection ‐ 40 mg methyl prednisolone with 2 mL 2% lignocaine; placebo tolmetin sodium ‐ 2 tablets 3 times a day for 4 weeks Frequency of administration: 1 injection; placebo tolmetin sodium 2 tablets 3 times per day Intervention 4: Acupuncture Description of modality used: classical Chinese acupuncture with moxibustion administered by a medically qualified doctor Dose: NA Frequency of administration: once per week for 4 weeks Control: Placebo ultrasound plus placebo tolmetin sodium Description of modality used: placebo ultrasound delivered by a qualified physiotherapist. The participant sat in front of the machine, which was not turned on Dose: none Frequency of administration: 8 sessions over 4 weeks All groups Paracetamol as needed, up to 8 tablets per day |
|
| Outcomes | Outcomes assessed at 2 weeks and 4 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each group contained 12 patients who were allocated treatment according to a random code." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "...the following indices were recorded by a blind, external observer at the start of the study and at 2 and 4 weeks" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Binder 1984.
| Methods |
Study design: Parallel group RCT Setting: Hospital, UK Intervention: Pulsed electromagnetic field therapy (PEMF) for 8 weeks Control: Placebo PEMF for 4 weeks followed by active PEMF for 4 weeks Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention‐ PEMF for 8 weeks Number randomised: 15 Number included in analyses: 15 Age: mean of 54.4 years old Sex: F/M 5/10 Diagnosis:
Duration of symptoms mean (range): 9.2 (3 ‐ 24) months Control‐ Placebo PEMF for 4 weeks followed by active PEMF for 4 weeks Number randomised: 14 Number included in analyses: 14 Age: 53.2 years old Sex: F/M 2/11 Diagnosis:
Duration of symptoms mean (range): 9.5 (3 – 24) months |
|
| Interventions |
Intervention: PEMF for 8 weeks Description of modality used: a single ovoid coil (12.2 ±1.2 x 13.2 ± 0.7 cm2) consisting of 50 turns of copper wire 1.4 mm in diameter was fitted over padding to the outer aspect of the affected shoulder so that the coils protruded from the centre of the pad. Two Velcro straps held it in place Dose: the pulse generators were set at 73 ± 2 Hz and a waveform varying by less than 7%. Participants were instructed to use the coil for 5–9 hours per day with each session lasting at least 1 hour Frequency of administration: 8 weeks Any additional treatment during trial: paracetamol if required Control: Placebo PEMF for 4 weeks followed by active PEMF for 4 weeks Description of modality used: Same as above Dose: same as above, except there was no dose during the first 4 weeks Frequency of administration: 8 weeks Any additional treatment during trial: paracetamol if required |
|
| Outcomes | Outcomes assessed at 2, 4, 6, 8, 12 and 16 weeks
|
|
| Notes |
Conflict of interest: no conflict of interests reported Funding: Arthritis and Rheumatism Council Mean values reported graphically only, so were extracted from the graphs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients fulfilling the criteria were randomly allocated to the treatment group (A), or the control group (B)" Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither patient not medical assessor was aware of the treatment group. At the end of 4 weeks and without breaking the code, both groups were given active coils and therapy was continued for another 4 weeks (phase II). Treatment was then stopped but patients continued to be reviewed for another 8 weeks (phase III), at the end of which the grouping was revealed to patient, medical assessor, and others involved in the study." Comment: participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Neither patient nor medical assessor was aware of the treatment group" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no loss to follow‐up within the study and analysis was based on the number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Data for all continuous outcomes was either partially reported (only means presented on figures). However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the nature of the results |
| Other bias | Low risk | Quote: "4 patients (all group A) refused therapy after 4 weeks since symptoms had resolved. Thus 11 patients had active therapy over 8 weeks and 18 patients over
only 4 weeks. However, the duration of therapy did not affect the outcome." Comment: No other sources of bias identified |
Bingöl 2005.
| Methods |
Study design: Parallel group RCT Setting: Physical therapy clinic, Turkey Intervention: Low‐level laser therapy (LLLT) plus exercises Control: Placebo LLLT plus exercises Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: None Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: LLLT plus exercises Number randomised: 20 Number included in analyses: 20 Age: 63.80 ± 9.77 years old Sex: F/M 12/8 Duration of symptoms: not reported Control: Placebo LLLT plus exercises Number randomised: 20 Number included in analyses: 20 Age: 57.25 ± 10.21 years old Sex: F/M 19/1 Duration of symptoms: not reported |
|
| Interventions |
Intervention: LLLT Description of modality used: laser was applied over the tuberculum majus and minus, bicipital groove, and anterior and posterior faces of the capsule, regardless of the existence of sensitivity, using a GaAs diode laser instrument (Roland Serie Elettronica Pagani) Dose: Wavelength 904 nm. Laser density and spot size 2.98 J/cm2 (at peak power = 50 W, frequency = 2000 Hz, for a duration of 60 s) and 0.8 cm2 respectively for each target point Frequency of administration: 10 sessions over 2 weeks Control: Placebo LLLT Description of modality used: same as above, but while laser was switched on, no laser was applied Dose: none Frequency of administration: 10 sessions over 2 weeks Both groups Description of modality used: supervised exercise programme using Codman, shoulder wheel and finger‐stair components Dose: 15 min Frequency: 10 sessions over 2 weeks Any additional treatment during trial: paracetamol not exceeding 2000 mg/day |
|
| Outcomes | Outcomes assessed at 2 weeks
|
|
| Notes |
Conflict of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the start of the study, another staff physician who was unaware of the examination results of the patients allocated the individuals into two groups of 20 each (either active laser treatment, Group I, or placebo laser [control], Group II) by drawing one card for each patient from a bag where cards numbered from 1 to 40 were placed." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: It is unclear if the cards drawn from the bag had "intervention" or "control" written on them (and thus it is unclear if the physician drawing the cards knew which group the presenting participant would be allocated to) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A physiotherapist instructed and supervised the exercises and performed laser applications in Group I and placebo laser in Group II, where the instrument was switched on and the patients thought they were receiving laser treatment but no laser was applied. Thus, a double‐blind study model was formed." Comment: participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "All evaluations before and after treatment were performed by a third staff physician who was not informed about the group of any patient." Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of the 12 females and eight males in Group I, and 19 females and one male in Group II completed the study" Comment: All participants completed follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Calis 2011.
| Methods |
Study design: Parallel group RCT Setting: Outpatient physical medicine and rehabilitation unit, Turkey Intervention 1: Therapeutic ultrasound plus exercise plus hot pack Intervention 2: Laser plus exercise plus hot pack Control: Exercise plus hot pack Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: Ultrasound (plus hot pack and exercise) Number randomised: 22 Number included in analyses: 21 Age: 50.42 ± 12.41 years Sex: F/M 14/7 Duration of symptoms (range): 3 (1–12) months Intervention 2: Laser (plus hot pack and exercise) Number randomised: 22 Number included in analyses: 15 Age: 46.2 ± 12.14 years Sex: F/M 10/5 Duration of symptoms: 3 (1–24) months Control: hot packplus exercise Number randomised: 22 Number included in analyses: 16 Age: 50.34 ± 13.69 years Sex: F/M 11/5 Duration of symptoms: 3 (1‐24) months |
|
| Interventions |
Intervention 1: Ultrasound Description of modality used: therapeutic ultrasound applied to the shoulder using a Model Sonopuls 463 (Enraf Nonius Co.) with a 20 mm diameter probe, in a continuously circular mode Dose: Intensity of 1.5 W/cm2, frequency of 3 MHz, continuously circular mode for 5 min Frequency of administration: daily for 15 days Intervention 2: Laser Description of modality used: A Ga As laser (Laserpet 100, Petas Co.) was used continuously in a direct contact technique with a 90 degree straight angle to the shoulder Dose: 904 nm wavelength. 6 mW average power, 1 J/cm2 dosage, at 16 Hz frequency for 2 min Frequency: for 15 days All groups: Exercise and hot pack Description of modality used: hot pack applied to the affected shoulder, and an exercise programme (starting with passive ROM exercises and Codman's exercises, later switching to shoulder stretching and strengthening exercises. These were delivered by a physiotherapist) Dose:
Frequency:
Any additional treatment during trial: paracetamol |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes |
Conflict of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients included in the study were offered sealed envelopes containing treatment groups in writing and were allocated accordingly" Comment: There was no information on how the allocation sequence was generated prior to putting into envelopes |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All patients included in the study were offered sealed envelopes containing treatment groups in writing and were allocated accordingly" Comment: There was no information on who disseminated the envelopes and whether they were sequentially numbered, opaque and consecutively disseminated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Unclear risk | Comment: There was no information on whether the assessor of objective outcomes was blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In the beginning of the study groups of twenty two patients are planned. However, one from group one, seven from group two, six from group three are excluded from the study because of incompliance to the study" Comment: There was unequal attrition between groups, and analysis was based on the per‐protocol sample. Excluding participants because of non‐compliance to treatment is likely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Celik 2009.
| Methods |
Study design: Parallel group RCT Setting: Private clinic, Turkey Intervention: Therapeutic ultrasound plus TENS plus exercises Control: Placebo ultrasound plus TENS plus exercises Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: assumed 36 (20 to active and 16 to placebo) Number included in analyses: 36 Age (mean and SD, or range): 51.4 (40‐69) years old Number of men and women: F/M 29/7 Duration of symptoms: not reported |
|
| Interventions |
Intervention: Ultrasound Description of modality used: pulsed ultrasound applied to an area 12 cm2 along the supraspinatus while the affected arm was in a position of adduction, 90 degrees' internal rotation and 30 degrees' hyperextension Dose: Frequency 1 mHz, intensity 1 W/cm2, for 4 min Frequency of administration: 15 sessions over 3 weeks Control: Placebo ultrasound Description of modality used: placebo ultrasound, where the arm was placed in the same position as active treatment Dose: none Frequency of administration: 15 sessions over 3 weeks Both groups Description of modality used: wand exercises, posterior and inferior capsule stretching exercises and exercises to strengthen the rotator cuff, carried out individually with a physiotherapist and at home. TENS and ice were also applied Dose
Frequency
Any additional treatment during trial: NSAIDS |
|
| Outcomes | Outcomes assessed at 3 weeks and 6 weeks
|
|
| Notes |
Conflict of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were divided randomly into two groups according to the type of ultrasound to be used." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The second group received placebo ultrasound with the arm placed in the same position." Comment: Participants were likely blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Before treatments, at the end of the third and sixth weeks, a medical practitioner blind to the treatments used in the study assessed the results". Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Chard 1988.
| Methods |
Study design: Parallel group RCT Setting: Rheumatology research unit, United Kingdom Intervention 1: High dose pulsed electromagnetic field therapy (PEMF) (8 hours/day) Intervention 2: Low dose PEMF therapy (2 hours/day) Source of funding: "E.B.I Medical Systems provided the apparatus and generously provided support" |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Inclusion criteria
Exclusion criteria
Baseline characteristics Total n randomised: 49 participants (49 shoulders) Total n analysed: 43 participants Intervention 1: High dose PEMF Number randomised: 24 Number completed: 24 Mean age: 52.8 years Sex: F/M 8/16 Mean duration of symptoms: 14.2 months Intervention 2: Low dose PEMF Number randomised: 25 Number completed: 19 Mean age 50.1 years Sex: F/M 10/9 Mean duration of symptoms: 14.6 months |
|
| Interventions |
Intervention: High dose coil (8 hours/day) PEMF therapy Components of intervention: participants were instructed to use the treatment coil which consisted of an ovoid concave coil (8.5 ± 0.6 x 11.5 ± 1 cm2) consisting of 120 turns of copper wire (0.8 mm diameter) covered with insulating tape. This was fitted over padding to the outer aspect of the affected shoulder with the coil protruding from the centre of the pad. An elasticated chest strap and Velcro arm strap held it in place. A bi‐osteogen pulse generator was used. This was a portable unit operated by rechargeable nickel cadmium batteries. The signal was set at 72 ± 3 Hz with a pulse duration of 380 ± 10 µs. When not being used the unit was kept on charge Dose: continuously for an 8‐hour period Frequency of administration: daily for 8 weeks Control: Low dose coil (2 hours/day) PEMF therapy Components of intervention: participants were instructed to use the same apparatus as described above Dose: continuously for an 8‐hour period (but the coil switched itself off after 2 hours without indication to the participant) Frequency of administration: daily for 8 weeks |
|
| Outcomes | Outcomes assessed at 2, 4, 6, and 8 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: E.B.I Medical Systems provided the apparatus and support |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to a 2 h treatment (Group I) or an 8 h treatment (Group II)." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Group I received treatment with a 'live unit' and coil that switched itself off after 2 h without indication to the patient, and for group II a standard unit without any automatic switch off was used." Quote: "All patients were instructed to use the apparatus for a continuous 8 h period each day, and neither assessor nor patient was aware of the treatment group" Comment: Participants were blind to treatment whereas personnel were not (though this is unlikely to have affected the outcomes) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Unclear risk | Comment: There was no information on whether the assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Forty nine patients fulfilled the entry criteria and were entered into the study, 25 patients in the 2 h Group I and 24 in the 8 h Group II. Unfortunately, 6 patients in Group I failed to co‐operate in using the equipment for a continuous 8 h treatment. This resulted in interrupted treatment which reset the timing device, and hence they received more than 2 h PEMF per day. Thus, these patients had to be excluded from further analysis, and so the data of the remaining 19 patients in Group I were used." Comment: There was more drop‐out in the control group (all for the same reason), and it is unclear what impact this could have had on the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: For some outcomes, outcome data were reported in Figure (as means with unlabelled error bars), whereas for other outcomes, the trialists only indicated that there was no significant difference between groups. However, this pattern of reporting was not associated with whether results were significant or not (i.e. some non‐significant findings were presented in Figure format). However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Clews 1987.
| Methods |
Study design: Parallel group RCT Setting: Australian Institute of Sport, Australia Intervention 1: Therapeutic ultrasound plus ice Intervention 2: Massage plus ice Control: Placebo ultrasound plus ice Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 18 (6 per group) Age (mean and SD, or range): not reported Sex: not reported Duration of symptoms: not reported |
|
| Interventions |
Intervention 1: Therapeutic ultrasound Components of intervention: pulsed ultrasound Dose: 15 min at an intensity 0.8 W/cm2 Frequency of administration: every day for 3 days Intervention 2: Massage Components of intervention: massage of the long head of biceps, biceps tendon, pectorals, supraspinatus and infraspinatus muscle Dose: 15 min Frequency of administration: every day for 3 days Control 2: Sham ultrasound Components of intervention: sham ultrasound Dose: 15 min Frequency of administration: every day for 3 days All groups Components of intervention: ice packs applied to the affected shoulder, and NSAIDs Dose: Ice for 15 min twice daily and 1 tablet of diclofenac sodium (Voltaren) taken with meals Frequency of administration: every day for 3 days |
|
| Outcomes | Outcomes assessed at 3 days
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the diagnosis had been made and inclusion in the study was confirmed, each subject was randomly assigned to one of these three groups." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "One co‐author did all the testing and was not aware of the subjects' group assignment" Comment: Outcome assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was complete follow‐up of all randomised participants in the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Dogan 2010.
| Methods |
Study design: Parallel group RCT Setting: University, Turkey Intervention: Low‐level laser therapy (LLLT) plus exercise plus ice Control: Placebo LLLT plus exercise plus ice Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: LLLT plus exercise program plus ice Number randomised: 30 Number included in analyses: 30 Age mean (SD): 53.7 ± 12.6 years Sex: F/M 20/10 Duration of symptoms mean (SD): 11.66 ± 18.04 months Control: Placebo LLLT plus exercise program plus ice Number randomised: 22 Number included in analyses: 22 Age mean (SD): 53.45 ± 9.64 years Sex: F/M 13/9 Duration of symptoms mean (SD): 15.27 ± 25.13 months |
|
| Interventions |
Intervention: LLLT Description of modality used: LLLT using a Gallium‐Aluminum‐Arsenide (GaAlAs, infrared laser) diode laser device (Chattanooga group) with a wave‐length of 850 nm, power output of 100 mV, continuous wave and 0.07 cm2 spot area. The laser was applied at a maximum of 5‐6 painful points for 1 min at each point over subacromial region of the shoulder Dose: 3 J/cm2 at each point for 1 min Frequency of administration: once per day, 5 times per week for 14 sessions Control: Placebo LLLT Description of modality used: Placebo laser was applied in the same way as above but the device was turned off during treatment sessions Dose: none Frequency: once per day, 5 times per week for 14 sessions Both groups Description of modality used: cold pack applied by a physiotherapy and exercise programme which included range of motion, stretching and progressive resistance exercises Dose: cold pack (10 min); exercise (10‐15 repetitions) Frequency: once per day, 5 times per week for 14 sessions Any additional treatment during trial: none |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was allocated by numbered envelopes method. Treatment program either LLLT or placebo was written in these closed envelopes and patients selected one of them and randomly assigned into two groups." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo laser was applied in the same way but the device was turned off during treatment sessions. Patients and physiotherapist were asked to use protective eyeglasses during therapy for safety." Quote: "Both of the physicians and patients were blinded. Only the physiotherapist was aware of the procedure." Comment: participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "At the beginning, sociodemographic (age, sex) and clinic (disease duration, localization of shoulder pain) characteristics of the patients were recorded. Pain severity, range of motion and functional status of all patients were evaluated before and after the treatment by different physicians. Both of the physicians and patients were blinded." Comment: Assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients were able to complete the therapy program". |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Downing 1986.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic, USA Intervention: Therapeutic ultrasound plus exercise plus NSAID Control: Sham ultrasound plus exercise plus NSAID Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus tendinitis or subacromial bursitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Therapeutic ultrasound Number randomised: 11 Number included in analyses: 11 Mean age: 54 years Sex: F/M 5/6 Mean duration of symptoms: 6.5 months Control: Sham ultrasound Number randomised: 9 Number included in analyses: 9 Mean age: 52 years Sex: F/M 7/2 Mean duration of symptoms: 6.2 months |
|
| Interventions |
Interventions: Therapeutic ultrasound Description of modality used: the applicator (sound head) had a radiating surface of 10 cm2 and a continuous output was used. Aquasonic gel was the coupling medium applied to the shoulder. Gel was warmed in a beaker of water on a hot plate before each application so that the gel was hot to touch but tolerable. Ultrasound covered a field size of 150 cm2. If the participant appeared to have localised tendinitis, ultrasound was localised to the particular area in addition to the anterior, medial and posterior aspects of the glenohumeral joint. If spasm existed in the trapezius muscle of supraspinatous muscle area, ultrasound was applied to that area for an addition 5 min Dose: frequency of 1 MHz. Intensity used throughout the study was determined by participant's tolerance. The maximal dosage was defined as the intensity at which the participant experienced a dull ache in the joint. An intensity 10% lower than the maximal (submaximal dosage) was used for each treatment. Mean intensity: 1.2 W/cm2. Each treatment lasted 6 min and covered a field size of about 150 cm2 Frequency of administration: 3 treatments per week for 4 weeks (total 12 sessions) Control: Sham ultrasound Description of modality used: ultrasound was administered in the same manner as the true ultrasound except the machine was disconnected to the power outlet Dose: mean intensity: 1.3 W/cm2; no frequency Frequency of administration: 3 treatments per week for 4 weeks (total 12 sessions) Both groups Description of modality used: range of motion exercises (active, active assisted and passive) following each true or sham ultrasound treatment, home exercise, and 5 participants per group were also receiving NSAIDs Frequency of administration: 3 treatments per week for 4 weeks (total 12 sessions) |
|
| Outcomes | Outcomes assessed at 4 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: National Institutes of Health Multipurpose Arthritis Center; from the National Arthritis Foundation; and from the Arthritis Foundation, Connecticut chapter |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly assigned the patients according to a table of random numbers to receive the true or sham US." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "We randomly assigned the patients according to a table of random numbers to receive the true or sham US. After the therapist turned the intensity of US to the submaximal dosage, she covered the controls of the machine so that neither she nor the patient were aware of whether true US was being administered. A third party kept the envelopes containing numbers that assigned the patients to the true or sham group. This person was responsible for leaving the machine connected to the electrical outlet if the patient was to receive true US or disconnecting the machine from the electrical plug if the patient was to receive sham US." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We heated the gel to blind the sham patients, because the coupling medium becomes warm during the administration of true US". Quote: "After the therapist turned the intensity of US to the submaximal dosage, she covered the controls of the machine so that neither she nor the patient were aware of whether true US was being administered." Quote: "As an extra precaution the therapist avoided touching the gel during and after each US application to prevent knowing, by the coolness or warmth, which treatment the patient received." Quote: "Both the patients and the therapist were inaccurate in guessing whether the sham or true US was used. Six patients (2 sham, 4 true) guessed correctly, and 3 (1 sham, 2 true) guessed incorrectly. Eleven (6 sham, 5 true) were uncertain whether they had received the US. The therapist guessed 11 patients (3 sham, 8 true) correctly and 6 (4 sham, 2 true) incorrectly. She was uncertain about 3 patients (2 sham, 1 true)" Comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The therapist and physician evaluated the patients independently of one another recording present and past medical history." Quote: "After the therapist turned the intensity of the US to the submaximal dosage, she covered the controls of the machine so that neither she nor the patient were aware of whether true US was being administered." Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Ebenbichler 1999.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinics and private practices, Austria Intervention: Pulsed therapeutic ultrasound Control: Sham ultrasound Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Calcific tendinitis of the shoulder Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Pulsed ultrasound Number randomised: 35 shoulders Number included in analyses: 32 shoulders Mean age: 49 ± 11 years Sex: not reported Median duration of symptoms: 8 weeks, IQR: 4‐20 weeks Control: Sham treatment Number randomised: 35 shoulders Number included in analyses: 29 shoulders Mean age: 54 ± 10 years Sex: not reported Median duration of symptoms: 8 weeks, IQR: 4‐19weeks |
|
| Interventions |
Intervention: Pulsed ultrasound Description of modality used: ultrasound therapy used with pulsed mode (1:4) over the calcifications. The transducer was 5 cm2 and an aquasonic gel was used as the couplant. To optimise treatment of the affected areas in the supraspinatus and infraspinatus muscles and tendons, the transducer was moved slowly in circles distal to the lateral acromion and the acromial part of the clavicle while the participant flexed his or her upper arm and internally rotated the forearm. Treatment of calcium deposits in the subscapularis muscle was performed with the participant's upper arm in an abducted and externally rotated position. The device was standardised initially, and output was monitored regularly by means of a simple underwater radiation balance. An on–off key introduced into the transducer circuit allowed normal ultrasonic output as well as mock insonation (sham treatment) Dose: frequency: 0.89 MHz; intensity: 2.5 W/cm2; administered for 15 min per session Frequency of administration: 24 x 15 min sessions; first 15 treatments given daily 5 times per week and the remaining were given 3 times a week for 3 weeks Control: Sham ultrasound Description of modality used: ultrasound therapy used in same method as true treatment however ultrasonic generator was not turned on Dose: none Frequency of administration: 24 x 15 min sessions; first 15 treatments given daily 5 times per week and the remaining were given 3 times a week for 3 weeks Any additional treatment during trial: occasional pain relief ‐ analgesic drugs (usually tramadol); NSAIDs were not allowed |
|
| Outcomes | Outcomes assessed at 6 weeks and 9 months
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported Trialists randomised shoulders rather than participants, but did not control for the correlation between outcomes in participants with bilateral shoulder pain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A spreadsheet program (Lotus Symphony, Lotus) was used to generate a list of random numbers. Since patients could have calcific tendinitis in one or both shoulders, randomization was conducted according to shoulders rather than patients. Thus, a patient could receive sham treatment for one shoulder and ultrasound treatment for the other." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "A therapist who was not involved with treatment handed out the treatment assignments, which were in sealed, opaque envelopes." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients and the therapists applying the therapy were blinded to the treatment assignments. The therapist who handed out the treatment assignments also switched the ultrasonic generator to either active or sham mode so that they therapist applying the therapy were blinded. Since the intensity of ultrasound therapy was usually below the threshold of sensitivity, patients were theoretically unable to distinguish between genuine and sham ultrasonography." Comment: Patients and personnel were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Radiography was performed at each follow‐up visit, and the results were assessed independently by two radiologists who were unaware of the patients' treatment assignments". Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 63 consecutive patients (70 shoulders) were enrolled. Nine patients (nine shoulders, 13 percent) did not complete treatment: seven (seven shoulders; three in the ultrasound‐treatment group and four in the sham‐treatment group) dropped out soon after the first session, and two patients (two shoulders) in the sham‐treatment group withdrew because of excessive pain. The characteristics of these patients did not differ significantly from the characteristics of those who completed the study. A total of 54 patients (61 shoulders: 32 in the ultrasound‐treatment group and 29 in the sham‐treatment group) completed the treatment. Of the seven patients who received bilateral treatment, five received ultrasound treatment for one shoulder and sham treatment for the other, one received bilateral ultrasound treatment, and one received bilateral sham treatment. Of these, 50 patients (56 shoulders: 31 in the ultrasound‐treatment group and 25 in the sham‐treatment group) also completed
the nine‐month follow‐up." Comment: The characteristics of the patients who did not complete the study did not differ significantly from the characteristics of those who did complete the study. Reasons for dropping out of the study were reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: No outcome data for rest pain at night and pain on motion at night was reported, despite these outcomes being specified in the methods section. Further, "clinical improvement" was reported as an outcome in the results section but was not specified in the methods section, and it was not clear how improvement was defined. Also, without a trial protocol it is unclear if other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
England 1989.
| Methods |
Study design: Parallel group RCT Setting: Rheumatology outpatient clinic, UK Intervention 1: Laser therapy Intervention 2: NSAID Control: Placebo laser therapy Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus or bicipital tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants if reported Number of participants at enrolment: 30 Number randomised: 30 (10 in each group) Number included in analyses: not reported Age: mean: 48 years (range: 18‐78 years) Sex: 15 males, 15 females Diagnosis: equal number of supraspinatus tendinitis and bicipital tendinitis Duration of symptoms: mean: 12.5 weeks (range: 5‐56 weeks) |
|
| Interventions |
Intervention 1: Laser therapy Description of modality used: active infrared laser therapy ‐ gallium‐arsenic semiconductor diode operating in the infrared region at 904 nm wavelength. Laser was applied to point of maximal tenderness with the shoulder abducted, slightly extended and medially rotated 90 degrees Dose: 4000 Hz frequency with 180 nanosecond pulses, peak power output 10 W for 5 min of 3 mW therapy Frequency of administration: 3 times weekly for 2 weeks Intervention 2: Drug therapy Description of modality used: naproxen sodium 550 mg twice daily for the 2‐week treatment period Control: Dummy laser therapy Description of modality used: same laser used as active laser therapy however laser not turned on. A cardboard screen was used to blind the participant to light emission from the laser Dose: none Frequency of administration: 3 times weekly for 2 weeks |
|
| Outcomes | Outcomes assessed at 2 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to three treatment groups." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A cardboard screen was used to blind the patient to light emission from the laser. Thus the patient and assessor were blind to therapy though the therapists were not for reasons of safety and practicality." Comment: Participants receiving active or placebo laser were blinded, but were not blinded in regards to laser therapy versus NSAID |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The patient and assessor were blind to therapy". Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | High risk | Comment: Outcome data only fully reported for outcomes that were statistically significant. Also, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Eslamian 2012.
| Methods |
Design: Parallel group RCT Setting: Physical Medicine and Rehabilitation Clinic of Tabriz Shohada Hospital, Iran Intervention: Low‐level laser therapy (LLLT) plus routine physiotherapy (therapeutic ultrasound, TENS and exercise programme) Control: Placebo laser plus routine physiotherapy Source of funding: Not reported, but stated that "The authors also certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript." |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Inclusion criteria
Exclusion criteria
Baseline characteristics Total n randomised = 50 participants Total n analysed = 50 participants Intervention: LLLT Number randomised: 25 Mean ± SD (range) age: 50.16 ± 12.10 (25–68) years Sex: F/M 10/15 Mean ± SD (range) duration of symptoms: not reported Control: Placebo LLLT Number randomised: 25 Sex: F/M 16/9 males Mean ± SD (range) age: 50.2 ± 11.72 (25–75) years Mean ± SD (range) duration of symptoms: not reported |
|
| Interventions |
Intervention: LLLT Components of intervention: LLLT was performed by gallium‐aluminum‐arsenide (Ga‐Al‐As) infrared diode laser 476, wavelength 830 nm, average power output of 100 mW, and energy density or intensity of 4 J/cm2. Laser irradiation was delivered in continuous‐wave mode on 1‐cm2 surface area with 20‐s irradiation for each point and total treatment duration of 5 min over the painful regions of shoulder up to 10 painful points Frequency of administration: 3 times a week with 10 sessions in total (i.e. 3‐4 weeks) Control: PlaceboLLLT Components of intervention: wearing eyeglasses and using a probe laser on the shoulder, but in off mode Frequency of administration: 3 times a week with 10 sessions in total (i.e. 3‐4 weeks) Both groups Components of intervention: therapeutic parameters for deep‐heat or ultrasound application consisted of pulse mode, 1‐MHz frequency, pulse intensity: 1.5–2 W/cm2 and duty factor: 25% for 5‐min treatment duration with slowly circular movements of ultrasound probe over painful regions of shoulder. Therapeutic parameters for TENS therapy included high frequency currents of 100 Hz, low current intensity of 10–30 mA, and short pulse width or 50 μs. Treatment duration for both surface heat and TENS was approximately 20 min for each modality. Also, participants were given an exercise program that included range of motion, and stretching and strengthening exercises of shoulder abductors and flexors. Each exercise was performed once a day with 10 repetitions Frequency of administration: 3 times a week with 10 sessions in total (i.e. 3‐4 weeks) |
|
| Outcomes | Outcomes assessed at 6 weeks (3 weeks post treatment cessation)
|
|
| Notes |
Conflicts of interest: "The authors declare that they have no conflicts of interest." Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All of the patients, who had inclusion criteria, were referred to physical medicine and rehabilitation clinic and assigned to two equal groups randomly. After obtaining the written consent, the patients were given closed packets including letters A and B, and in this way they were allocated into an experimental group (A: laser+ physiotherapy) and a control group (B: physiotherapy only)." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: An adequate method was used to generate the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All treatment regimes were administrated by an expert physical therapist. To form a double‐blind study, only the physiotherapists knew the patients in the experimental and control groups. Patients were not aware of being given or not being given the effective laser therapy and the examiner physician was not aware of the group's label. The sham laser was also used to induce a placebo laser effect in the control group of patients. Wearing eyeglasses and using a probe laser on the shoulder, but in off mode, was in fact the method of using the sham laser in our study. Finally, the physiotherapist announced the patient's experimental or control group label." Comment: Participants were blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "To form a double‐blind study, only the physiotherapists knew the patients in the experimental and control groups." Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no drop‐outs, exclusions or losses to follow‐up, and outcome data were reported as based on the total number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Eyigor 2010.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic, Turkey Intervention: Transcutaneous electrical nerve stimulation (TENS) plus home exercises Control: Glucocorticoid injection plus home exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: TENS plus home exercises Number randomised: 20 Number included in analyses: 20 Age: mean: 57.60 ± 9.92 years Sex: female: 14; male: 6 Duration of symptoms: mean: 8.6 ± 4.5 months Control: Glucocorticoid injection plus home exercises Number randomised: 20 Number included in analyses: 20 Age: mean: 60.8 ± 12.5 years old Sex: female: 15; male: 5 Duration of symptoms: mean: 8.9 ± 5.1 months |
|
| Interventions |
Intervention: TENS Description of modality used: TENS on the anterior and posterior aspects of the joint Dose: mean frequency of 100 Hz, 15 mA amplitude, 150 µsn Frequency of administration: 5 times per week for 15 sessions (i.e. 3 weeks) Control: Glucocorticoid injection Description of modality used: all injections were performed by single physician specialised in the field. The injection procedure was standardised. In order to perform the surgical procedure under sterile conditions, the intra‐articular injection procedure was performed in the operating room. Each participant was placed in a supine position, and the skin overlying the operating area was prepared and draped. Fluoroscopy was adjusted to show the shoulder joint in antero‐lateral position. Acromioclavicular joint entry point was marked and local anaesthetic was applied to the skin (0.5 cc prilocaine). A 22 G spinal needle was inserted into the acromioclavicular joint. The injection was placed through the subacromial space and it was observed to penetrate into the glenohumeral joint. Entry into the joint was proved by giving 0.5 cc contrast substance. The prepared mixture was injected as 3.5 cc to glenohumeral joint, 2.5 cc to subacromial space and 1 cc to acromioclavicular joint Dose: the prepared mixture consisted of 0.5 cc triamcinolone (40 mg/ml) (Kenacort‐A), 3.5 cc bupivacaine (5 mg/ml) (Marcaine), 3 cc serum physiologic Frequency of administration: once Both groups: Home exercises Exercises for increasing the range of motion, strengthening exercises and finger ladder exercises were recommended. For each of the exercises, participants were provided with simple, step‐by‐step written instructions with illustrations Any additional treatment during trial: only paracetamol (maximum 4 g daily) allowed |
|
| Outcomes | Outcomes assessed at 1 week, 4 weeks and 12 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised to the two groups by using double randomisation from the random number table." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The assessments were performed by the same physician who was blinded to the treatment protocols." Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no losses to follow‐up. All outcome data were reported as based on all randomised participants |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the methods section. No protocol was available, but all patient‐important outcomes were measured in this trial so it is unlikely that other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Galace de Freitas 2014.
| Methods |
Study design: Parallel group RCT Setting: Outpatient rehabilitation of a public hospital, Brazil Intervention: Pulsed electromagnetic field (PEMF) for 3 weeks followed by exercises for 6 weeks Control: Placebo PEMF for 3 weeks followed by exercises for 6 weeks Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Shoulder impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: PEMF plus exercises Number randomised: 26 Number included in analyses: 26 Age: mean: 50.1 ± 8.2 years old Sex: female: 16; male: 10 Duration of symptoms: mean: 22 ± 17.7 months Control: Placebo PEMF plus exercises Number randomised: 30 Number included in analyses: 30 Age: mean: 50.8 ± 9.6 years Sex: female: 20; male: 10 Duration of symptoms: mean: 21.2 ± 19 months |
|
| Interventions |
Intervention: PEMF Components of intervention: electrodes were positioned on the anterior and posterior part of the shoulder joint with the subject positioned in lateral decubitus. The equipment used was a previously calibrated Magnetherp 330 Dose: device pulsed with a frequency of 50 Hz and an intensity of 20 mT or 200 G for 30 min Frequency of administration: 3 times a week for 3 weeks Control: Placebo PEMF Components of intervention: same equipment used and participants remained in the same position as the active group Dose: device kept on standby mode without any electromagnetic field being applied, for 30 min Frequency of administration: 3 times a week for 3 weeks Both groups: Exercises Components of intervention and Dose: after 3 weeks of active or placebo PEMF, all subjects initiated a therapeutic exercise programme, comprised of range of motion and strengthening exercises (see below) Range of motion exercises
Strengthening exercises
Frequency of administration: twice a week for 6 weeks (after the 3‐week PEMF/placebo PEMF treatment period) |
|
| Outcomes | Outcomes assessed at 3 weeks, 9 weeks and 3 months
|
|
| Notes |
Conflicts of interest: "No commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer a benefit on the authors or on any organization with which the authors are associated." Funding: not reported Trial registered in ClinicalTrials.gov (NCT01452204) Participants did not receive the exercise component until the end of 3 weeks of PEMF or placebo PEMF, so there are two comparisons in this trial:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The assignment of subjects to the 2 groups was performed randomly using opaque, sealed envelopes, each containing the name of 1 of the groups (active PEMF or placebo PEMF). The envelopes were selected by an individual not involved in the study." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignment of subjects to the 2 groups was performed randomly using opaque, sealed envelopes, each containing the name of 1 of the groups (active PEMF or placebo PEMF). The envelopes were selected by an individual not involved in the study." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A single therapist (T.Y.F.) was responsible for setting up the equipment (active or placebo) before treatment in order to maintain the randomized, double‐blind design. This therapist did not remain beside the patient during the session to avoid influencing the results. Two therapists (F.B.M., S.G.R.) were trained in delivering the exercise protocols used for the study and provided all treatment. These 2 therapists and all patients were blinded in relation to active PEMF or placebo PEMF treatment." Comment: Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Quote: "These 2 therapists and all patients were blinded in relation to active PEMF or placebo PEMF treatment." Comment: Participants, who self‐reported some outcomes, were blinded |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Finally, the examiner (D.G.F.) was blind to the group assignment of the patients and did not participate in the interventions." Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "At 3 months, 4 subjects in the active PEMF group and 6 subjects in the placebo PEMF group were lost during follow‐up. Therefore, all per‐protocol data analyses were performed with 22 subjects in the active PEMF group and 24 subjects in the placebo PEMF group." Quote: "After the per‐protocol data analysis, an intention‐to‐treat analysis was performed using the mean value obtained from the remaining subjects of each group." Quote: "The results of the intention‐to‐treat analysis were consistent with the per‐protocol analysis, providing evidence that the missing data had no substantial influence on the overall results." Comment: The number and reasons for attrition were balanced between groups, so attrition is unlikely to have biased the results. |
| Selective reporting (reporting bias) | Low risk | Comment: Trialists only specified strength as an outcome in the ClinicalTrials.gov registry entry (NCT01452204), yet reported data for pain and function in the manuscript. However, both pain and function are important outcomes to measure, so their addition to the trial is unlikely to be a reporting bias issue |
| Other bias | Low risk | Comment: No other sources of bias identified |
Giombini 2006.
| Methods |
Study design: Parallel group RCT Setting: Athletes who attended the Physiotherapy Department of the Sport Science Institute, Italy Intervention 1: Therapeutic ultrasound Intervention 2: Microwave diathermy Intervention 3: Exercise Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus tendinopathy Criteria for defining the shoulder condition being treated: diagnosis of supraspinatus tendinopathy of the dominant shoulder based on following 3 criteria:
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: Therapeutic ultrasound Number randomised: 12 Mean (SD, range) age: 28.6 ± 6.6 years, range 19‐43 years Sex: F/M 4/8 Duration of symptoms: not reported Intervention 2: Microwave diathermy Number randomised: 14 Mean (SD, range) age: 25.3 ± 4.8 years, range 19‐37 years Sex: F/M 2/12 Duration of symptoms: not reported Intervention 3: Exercises Number randomised: 11 Mean (SD, range) age: 26.3 ± 6.2 years, range 20‐38 years Sex: F/M 2/9 Duration of symptoms: not reported |
|
| Interventions |
Intervention 1: Therapeutic ultrasound Components of intervention: continuous ultrasound was administered with the participant in the same position as participants receiving hyperthermia and by slowly moving the transducer in a circular fashion along the area distal to the anterior border of the acromion and the inferior third of a line between the glenoid fossa and the humeral head. A gel complant was used between the ultrasound transducer and the skin of the area undergoing treatment. A Level 730 device was used. It was equipped with an emission probe of 1‐MHz frequency, a sound head with an effective radiating area of 10 cm2 and a maximum output power of 22 W Dose: 1 MHz at an intensity of 2.0 W/cm2; each session lasted 15 min Frequency of administration: 3 times a week for 4 weeks Intervention 2: Microwave diathermy Components of intervention: an ALBA Hyperthermia System was used which was equipped with a 433.92‐MHz microwaves generator with a maximum output power of 100 W; a microstrip antenna applicator, with a curve shape specific for semicylindrical joint volumes of 20 to 30 cm in diameter and with a total radiating area of 240 cm2 and an effective field size; and a pad of silicone 0.5 cm thick, filled with thermostatic deionized water that allows the greatest energy transfer to be achieved while preventing overheating of superficial tissues near the radiant source. A hydraulic thermoregulation and 1 or 2 skin temperature sensors were also used. The thermocouple was placed on the shoulder with the participant lying supine and the arm at 60 degrees of abduction and externally rotated. It was placed over the middle third of the joint line between the glenoid fossa and the humeral head. The thermocouple on the skin was perpendicular to the electromagnetic field Dose: 434 MHz; administered at a power between 50 and 70 W, a pilot temperature on the skin between 38 and 40 degrees centigrade, and a water pad temperature between 35 and 37 degrees centigrade according to the depth of the subcutaneous fat of each participant. Each session lasted 30 min Frequency of administration: 3 times a week for 4 weeks Intervention 3: Exercises Components of intervention: supervised and home exercises, consisting of pendular swinging in the prone position in flexion and extension of the shoulder and passive glenohumeral stretching exercises to tolerance Frequency of administration: supervised exercises once a week for 4 weeks; home exercises 5 min per day, every day for 4 weeks |
|
| Outcomes | Outcomes assessed at 4 weeks and 10 weeks
|
|
| Notes |
Conflicts of interest: the authors stated that they had no conflicts of interest Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised into 3 groups using a computer‐generated list." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes. |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The subjects were assessed by fully trained sports physicians who had never seen the patients and were unaware as to which intervention the patients had been allocated." Comment: Assessor of objective outcome was likely blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no loss to follow‐up and all randomised participants were analysed. |
| Selective reporting (reporting bias) | High risk | Comment: Data for pain on resisted movement was reported in figure only as means with no error bars. No data for night pain, pain on movement, rest pain and painful arc were reported, despite being listed as outcomes in the methods section of the trial report. |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Grymel‐Kulesza 2007.
| Methods |
Study design: Parallel group RCT Setting: Rehabilitation centre, Poland Intervention 1: Therapeutic ultrasound plus TENS plus exercise plus massage Intervention 2: Cryotherapy plus exercise plus massage Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Chronic rotator cuff injuries Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms: 1‐7 months history of shoulder pain Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: Therapeutic ultrasound plus TENS plus exercise plus massage Number randomised: 15 Number included in analyses: 15 Age: mean: 57.6 years; range: 50–65 years Sex: male: 4; female: 11 Duration of symptoms: mean: 4.6 months; range: 1–7 months Intervention 2: Cryotherapy plus exercise plus massage Number randomised: 15 Number included in analyses: 15 Age: mean: 57.5 years; range: 50–65 years Sex: male: 3; female: 12 Duration of symptoms: mean: 4.2 months; range: 2–7 months |
|
| Interventions |
Intervention 1: Therapeutic ultrasound plus TENS Description of modality used: therapeutic ultrasound and TENS covered 4 muscles i.e. the supraspinatus, the infraspinatus, the teres major muscle and the biceps muscle of arm
Dose
Frequency of administration: 10 sessions over 2 weeks Intervention 2: Cryotherapy Description of modality used: painful shoulder joints were cooled with CO2 vapours at ‐75 degrees Celsius for 3 min Frequency of administration: 10 sessions over two weeks Both groups: Exercise and massage Description of modality used: massage and non‐weight bearing exercises as well as self‐assisted exercises according to a uniform programme. Each massage procedure covered the entire shoulder girdle, including the painful joint. Kinesitherapy included non‐weight bearing and self‐assisted exercises. Treatment started with ultrasound plus TENS or cryotherapy followed 15‐20 min later by therapeutic exercises for 20 min followed by massage for 15‐20 min Frequency of administration: every day for 2 weeks |
|
| Outcomes | Outcomes assessed at 2 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: They were randomly assigned to two subgroups (A and B)." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Unclear risk | Comment: There was no information on whether the assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no losses to follow‐up. Data presented were based on the number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Johansson 2005.
| Methods |
Study design: Parallel group RCT Setting: Outpatient urban primary health care centres, Sweden Intervention 1: Therapeutic ultrasound plus home exercises Intervention 2: Acupuncture plus home exercises Source of funding: "This study was supported by funding and facilities provided by the County Council of Ostergotland and Linkopings Universitet, Sweden." |
|
| Participants |
Diagnostic label used by trialist: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms: at least 2 months' duration of the current episode Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: Therapeutic ultrasound plus home exercises Number randomised: 41 Number included in analyses: 30 Age: mean: 49 years; SD: 8 years Sex: female: 27; male: 14 Duration of symptoms: 2‐3 months (n = 11); 4‐6 months (n = 10); 7‐12 months (n = 11); > 12 months (n = 9) Intervention 2: Acupuncture plus home exercises Number randomised: 44 Number included in analyses: 44 Age: mean: 49 years; SD: 7 years Sex: female: 32; male: 12 Duration of symptoms: 2‐3 months (n = 13); 4‐6 months (n = 8); 7‐12 months (n = 10); > 12 months (n = 13) |
|
| Interventions |
Intervention 1: Therapeutic ultrasound Description of modality used: continuous ultrasound with gel coupling administered by 4 physical therapists at the same primary health care centre. The size of the transducer was 4 cm2, and the skin area treated was twice this size, covering an area of about 8–10cm2inferior to the anterior and lateral part of the acromion. The transducer head was moved in small circles covering the area. The participants were seated with the glenohumeral joint extended and medially rotated in order to make the muscle insertion of the supraspinatus muscle appear beneath and anterior to the acromion. This joint position was maintained by placing the arm behind the back of the chair. The equipment used was a Phyaction 190 ultrasound device Dose: frequency = 1 MHz, spatial‐average intensity = 1 W/cm2; 10 min duration Frequency of administration: twice a week for 5 weeks Intervention 2: Acupuncture Description of modality used: standardised needle placement at 4 local points (L1 14 (Binao), L1 15 (Jianyu), LU 1 (Zhongfu), and TE 14 (Jianliao)) and 1 distal point (L1 4 (Hegu)). All physical therapists were trained to locate these points. The type of needle used was a HEGU sterile and single‐packaged one‐time needle no. 8 (30 mm long and 0.30 mm in diameter). The participants lay on a treatment table on their unaffected side. After insertion into the defined points, the needle was rotated a few seconds until "de qui" (described as sensation of heaviness, numbness and radiating paraesthesia) was experienced by the participant. In total 3 stimulations were performed (at insertion, after 15 min and after 30 min). De qi was to be experienced at every stimulation at each acupuncture point, if not the needle was adjusted until this was the case Frequency of administration: 10 treatment sessions in total. 30 min treatment sessions repeated twice a week for 5 weeks Both groups: Home exercises Description of modality used: 2‐step home exercise programme. Part 1: exercises targeted to maintain or restore motion as well as to stimulate circulation in the rotator cuff using many repetitions of low‐intensity exercises, without provoking pain from involved tissues. Part 2: exercises targeted to strengthen the rotator cuff muscles with the upper arm in a neutral position to avoid impingement. In all exercises, the position of a retracted shoulder was emphasised. At the first treatment visit, the participants received instructions from the physical therapist and practiced the exercises in part one of the programme. They were instructed to perform the programme daily for 5 weeks. After the first half of the treatment period, the participants received instruction and practiced the second part of the exercise programme. All rotations were performed with a pillow in the axilla to decrease the activity in the deltoid muscle. Pain during the exercises was not to last more than 10–15 min after the programme. If pain persisted longer than that, the participants were instructed to decrease either the resistance or the force produced. Adherence to the exercise programme was monitored by a home‐exercise adherence log, and the use of additional medications was reported Frequency of administration: daily for 5 weeks. Exercises repeated every other day in the fourth and fifth weeks. |
|
| Outcomes | Outcomes assessed at 6 weeks and 3, 6 and 12 months
|
|
| Notes |
Conflicts of interest: not reported Funding: County Council of Ostergotland and Linkopings Universitet, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed randomization, based on a random list, with the treatment alternative in envelopes was carried out beforehand. The intervention was then introduced and performed by 4 physical therapists at the same primary health care center". Comment: An adequate method was likely used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: An adequate method was likely used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The research physical therapist, who performed the examinations and all assessments, was uninformed of treatment group assignments throughout the study." Comment: Assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients were adherent to the study protocol (no missed or additional interventions) during the 5 weeks of acupuncture or ultrasound. At the 3‐, 6‐, and 12‐month visits, the number of patients who were adherent to the study protocol changed, as shown in Figure 2. In total, 64 patients were adherent to the study protocol throughout the study. The data were analyzed both for the group adhering to the study protocol and with an
"intention‐to‐treat" (ITT) application model for analysis of data for clinical trials. The latter analysis included all patients who were randomly assigned to groups. The principle of last observation carried forward (LOCF) was used in both analyses, using the scores recorded just prior to the missing scores in case of missing posttreatment values. The number of patients where LOCF was used is illustrated in Figure 2." Quote: "The between‐group analysis, including the mean scores from all 4 assessment visits (after 5 weeks of acupuncture or ultrasound and at 3, 6, and 12 months), showed a larger change (P.045, ANCOVA) in the combined score for the acupuncture group, analyzed with those adhering to the study protocol. This effect was seen already at the first assessment visit and was maintained over time. In the ITT analyses, no differences were found across the 4 data collection periods." Comment: Loss to follow‐up was slightly different between groups but an appropriate analysis was used to deal with attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Kelle 2014.
| Methods |
Study design: Parallel group quasi‐randomised trial Setting: Physical Medicine and Rehabilitation outpatient clinic, Turkey Intervention 1: Low‐level laser treatment (LLLT) plus home exercises Intervention 2: Glucocorticoid injection plus home exercises Control: Sham LLLT plus home exercises Source of Funding: Scientific Research Projects Coordination Unit of Cukurova University (grant number TF2006LTP19) |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: LLLT plus home exercises Number randomised: 45 Number included in analyses: 45 Age: 50.7 (range 29‐74) years old Sex: F/M 36/9 Duration of symptoms: 15 (range 2‐120) months Intervention 2: Glucocorticoid injection plus home exercises Number randomised: 45 Number included in analyses: 45 Age: 48.7 (range 18‐77) years old Sex: F/M 35/10 Duration of symptoms: 16.6 (range 1‐120) months Contol: Sham LLLT plus home exercises Number randomised: 45 Number included in analyses: 45 Age: 48 (range 19 to 76) years old Sex: F/M 34/11 Duration of symptoms: 18.7 (range 1‐120) months |
|
| Interventions |
Intervention 1: LLLT Description of modality used: Gallium arsenide laser at a wavelength of 904 nm was administered using the direct contact technique, with a 90‐degree angle on the subacromial space and the most painful area of the affected shoulder accessible to palpation. During LLLT, the laser device was positioned so that the participant could not see it, and both the participant and the therapist wore protective eyewear Dose: 2 J/cm2, 3,500 Hz, for 150 seconds Frequency of administration: 3 times weekly for 3 weeks (total of 9 sessions) Intervention 2: Glucocorticoid injection Description of modality used: betamethasone dipropionate and betamethasone sodium phosphate with lidocaine (3 ml, 1%) were injected into the subacromial region of the affected shoulder. The injection was administered via the lateral approach. The lateral side of the acromion was palpated, and the injection was administered from below the acromion and was directed upward Dose: Betamethasone dipropionate (6.43 mg) and betamethasone sodium phosphate (2.63 mg) Frequency of administration: twice (second injection delivered 10 days after the first) Control: Sham LLLT Description of modality used: Same as LLLT group, except the laser device was not turned on Dose: none Frequency of administration: 3 times weekly for 3 weeks (total of 9 sessions) All groups: Home exercises Description of modality used: a home exercise programme, including shoulder pendulum exercises, posterior capsule stretching, and range of motion and isometric shoulder exercises Dose: 10 repetitions during each session Frequency of administration: twice daily for 3 weeks Any additional treatment: all of the participants were allowed to use up to 1000 mg of paracetamol per day for analgesia when necessary |
|
| Outcomes | Outcomes assessed at 3 weeks, 3 months and 6 months
|
|
| Notes |
Conflict of interest: "The authors declare that there are no conflicts of interest." Funding: Scientific Research Projects Coordination Unit of Cukurova University (grant number TF2006LTP19) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were allocated to three groups according to their order of admission. The first patient was allocated to group I, the second was allocated to group II, and so on." Comment: Alternation (a quasi‐random method of allocation) was used |
| Allocation concealment (selection bias) | High risk | Comment: Alternation (a quasi‐random method of allocation) was used, so the allocation sequence could not be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the patients nor the assessor and therapist were blinded in the study." Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "Neither the patients nor the assessor and therapist were blinded in the study." Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | High risk | Quote: "Neither the patients nor the assessor and therapist were blinded in the study." Comment: Assessor of objective outcomes was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A total of 135 patients with stage I or stage II subacromial impingement syndrome were included in the study. The patients had normal routine laboratory results. Although 114 patients completed the study, the data analysis was performed on an intention‐to‐treat basis, so we included all 135 patients. Seven patients in groups II [sham LLLT] and III [LLLT] did not complete the sessions. Additionally, seven patients in group II [sham LLLT] did not come to their follow‐up visits.". Quote: "In our trial, there was a high dropout rate in the sham laser group, whereas the dropout rate in the low‐level laser treatment group was acceptable in comparison. This outcome might have been due to the slower improvement in the sham laser group, as evidenced by the lack of dropouts in the local corticosteroid injection group." Comment: There were no losses to follow‐up in the glucocorticoid injection group, 7 in the LLLT group, and 14 in the sham LLLT group. Reasons for loss to follow‐up were not recorded, but the amount per group suggests that dropout was related to the intervention. It is unclear what method was used to impute missing data in the intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Comment: Outcome data were fully reported for all outcomes specified in the methods section of the publication except for 4 of the 6 sub‐scales of the Nottingham Health Profile, which were only reported as not significantly different between groups. Also, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Kocyigit 2012.
| Methods |
Study design: Parallel group RCT Setting: University, Turkey Intervention: Transcutaneous electrical nerve stimulation (TENS) Control: Sham TENS Source of funding: Not reported |
|
| Participants |
Diagnostic label used trialist: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: TENS Number randomised: 10 Number included in analyses: 10 Age mean (range): 49.2 (40–55) years Sex: F/M 5/5 Duration of symptoms mean (range): 5.5 (1.5–12) months Control: Sham TENS Number randomised: 10 Number included in analyses: 10 Age mean (range): 44.7 (24 – 64) years Sex: F/M 7/3 Duration of symptoms mean (range): 7.8 (1–24) months |
|
| Interventions |
Intervention: TENS Description of modality used: Low‐frequency TENS. 2 carbon silicone electrodes were placed on the anterior and the posterior aspect of the shoulder. The participants were observed for displacement of electrodes, and continuation of muscle contraction during the TENS treatment Dose: 3 Hz, 250 μs, for 30 min. Intensity of the current was chosen as submaximal value causing visible muscle contractions. In one of the participants, the current intensity was changed because of discontinuation of contractions or irritation of the current Frequency of administration: once Control: Sham TENS Description of modality used: 2 carbon silicone electrodes were placed on the anterior and the posterior aspect of the shoulder. No current was passed through these electrodes Dose: none over a 30 min session Frequency: once |
|
| Outcomes | Outcomes assessed immediately post‐treatment (day 1)
|
|
| Notes |
Conflict of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized to receive either low‐frequency TENS or sham TENS by random number table." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "There is the possibility of unblinding in TENS studies as it delivers electrical current through the skin. There are several attempts to decrease unblinding in the literature: inclusion of patients who were not applied TENS previously, and the use of devices that display an activator light but do not deliver current. In this study, both the strategies were applied to decrease the risk of unblinding. Patients who did not have any kind of electrotherapy earlier were included in the study. The timer of the device was set in the sham TENS group so an indicator light was on during which time the electrodes were connected. All the patients were told that they may or may not feel contractions during application. All the patients were inspected on separate days, so the patients did not see other patients." Comment: Participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported the outcome of interest of our review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no loss to follow‐up in this study |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Korkmaz 2010.
| Methods |
Study design: Parallel group RCT Setting: Outpatient physical therapy and rehabilitation clinic, Turkey Intervention 1: Transcutaneous electrical nerve stimulation (TENS) plus exercise Intervention 2: Pulsed radiofrequency treatment plus exercise Source of funding: "We have no financial relationship for this study". |
|
| Participants |
Diagnostic label used by trialist: Supraspinatus tendinopathy or partial tears of the supraspinatus tendon Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: TENS plus exercise Number randomised: 20 Number included in analyses: 20 Age: mean: 55.80 ± 9.82 years Sex: female: 14; male: 6 Diagnosis: supraspinatus tendinopathy: 10; partial tears of the supraspinatus tendon: 9; acromioclavicular joint osteoarthritis: 1 Duration of symptoms: mean: 8.85 ± 9.05 months Intervention 2: Pulsed radiofrequency plus exercise Number randomised: 20 Number included in analyses: 20 Age: mean: 54.80 years ± 12.09 Sex: female: 14; male: 6 Diagnosis: supraspinatus tendinopathy: 11; partial tears of the supraspinatus tendon: 9; acromioclavicular joint osteoarthritis: 0 Duration of symptoms: mean: 10.45 ± 8.31 months |
|
| Interventions |
Intervention 1: TENS Description of modality used: TENS (Enraf Nonius Sonopuls 492) on the anterior and posterior aspects of the joint Dose: mean frequency of 100 Hz, 15 mA amplitude, 150 μsn; 20 min session Frequency of administration: 5 times per week for 4 weeks (20 sessions) Intervention 2: Pulsed radiofrequency Description of modality used: procedure performed in an operating room with sterile conditions maintained. Each participant was placed in the prone position and the skin within the operation area was prepared and draped. Fluoroscopy was adjusted to show the scapular notch at approximately 15 degrees lateral and 30 degrees of the cephalocaudal angle. The entry point was marked, and local anaesthesia was applied. A radiofrequency needle was introduced through the skin 3 cm along the line of the spine in the upper, outer quadrant, and then guided to the edge of the suprascapular notch with the use of an image intensifier. With 2 Hz motor stimulation (< 0.5 V), a 5 cm long radiofrequency needle with a 0.5 cm active tip was advanced under fluoroscopic guidance. Motor stimulation muscle response was observed, and the correct entry of the needle was confirmed again by a 50 Hz sensorial stimulation (< 0.7 V). Finally, a placement of the needle was verified by both imaging and stimulations. After determining that the needle was in the correct position, pulse radiofrequency was applied to participants Dose: 45 V, 200 msn, 42 degrees; total treatment time: 4 min Frequency of administration: once Both groups: Exercise Description of modality used: supervised exercise programme. All participants in both groups were recommended the following exercises: exercises for increasing the range of motion (active‐passive range of motion, stretching exercise); strengthening exercises; Codman exercises; pulley exercises; and finger ladder exercises. For each of these, participants were provided with simple, step‐by‐step written instructions with illustrations Frequency of administration: exercises were performed 5 days a week for a period of 4 weeks at the rehabilitation unit. Each participant completed the exercise programme on a daily basis and it lasted at least 30 min |
|
| Outcomes | Outcomes assessed at 1, 4 and 12 weeks
|
|
| Notes |
Conflicts of interest: authors state that they have no financial relationship for this research Funding: No specific funding for this trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Forty patients were randomized...by using double randomization from the random number table". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "A physician blinded to the treatment protocols performed the following assessments before and after the procedure." Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication except for global assessment of treatment success, but this did not appear to be related to the lack of statistical significance for this outcome (as many other non‐significant outcomes were fully reported). However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Kurtai Gursel 2004.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic, The Netherlands Intervention: Therapeutic ultrasound plus hot pack plus interferential current plus exercise Control: Sham ultrasound plus hot pack plus interferential current plus exercise Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Supraspinatus tendinosis, subacromial bursitis, rotator cuff tear or bicipital tendinosis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Therapeutic ultrasound plus other physical therapy Number randomised: 20 Number included in analyses: 19 Age: mean: 54.16 ± 8.22 years; range: 38–69 Sex: female: 12; male: 7 Diagnosis: supraspinatus tendinosis: 6; supraspinatus partial rupture: 11; rotator cuff rupture: 1; biceps tendinosis: 8 Duration of symptoms: mean: 8.68 ± 8.84 months; range: 1–36 months Control: Sham ultrasound plus other physical therapy Number randomised: 20 Number included in analyses: 19 Age: mean: 54.00 ± 9.8; range: 35–69 Sex: female: 14; male: 5 Diagnosis: supraspinatus tendinosis: 6; supraspinatus partial rupture: 7; rotator cuff rupture: 3; biceps tendinosis: 7 Duration of symptoms: mean: 8.11 ± 10.81 months; range: 1–42 months |
|
| Interventions |
Intervention: True ultrasound Description of modality used: continuous ultrasound using a Petsan 250 device. The transducer head had an area of 6.2 cm2, an effective radiating area of 5 cm2, and a beam non‐uniformity ratio of 1:6. While sitting on a table, each participant placed an arm with the hand supinated on his or her lap. Using slow circular movements, the treating physical therapist applied the transducer head over the superior and anterior periarticular regions of the participant's glenohumeral joint, covering an area of approximately 15 cm2 Dose: frequency of 1 MHz, intensity of 1.5 W/cm2. The treatment duration was 10 min Frequency of administration: 15 days (5 days each week) Comparator: Sham ultrasound Description of modality used: the ultrasound device was set to "off" mode. The transducer was applied to the same area as the real ultrasound group and Aquasonic transmission gel was used Dose: none Frequency of administration: 15 days (5 days each week for 3 weeks) Both groups: Other physical therapy interventions Description of modality used
Frequency of administration: 15 days (5 days each week) Any additional treatment during trial: paracetamol (500 – 1000 mg maximum daily) if needed |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were randomly assigned by the use of random numbers." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | High risk | Quote: "The selector, who did not perform any assessment, was aware of the randomisation scheme and opened the codes at the statistical evaluation stage." Comment: The allocation sequence was not concealed from the person allocating participants to groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The subjects were not informed about the true nature of the US application. The treating physical therapist was aware of the nature of this intervention and the physical findings of the subjects, but did not change the intervention according to the symptoms during the study". Comment: Participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The assessor and the subjects, however, were not informed about the true nature of US application". Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One subject from the true‐US group and 1 subject from the sham‐US group withdrew from the study because they could not spare time for the physical therapy sessions. Another subject from the true‐US group and 2 other subjects from the sham‐US group withdrew without any explanation". Comment: The amount of attrition was low and relatively equal between groups so was unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results. |
| Other bias | Low risk | Comment: No other sources of bias identified |
Leduc 2003.
| Methods |
Study design: Parallel group RCT Setting: Ambulatory academic hospital in Quebec, Canada Intervention: Acetic acid iontophoresis plus thermotherapy plus exercises Control: Sham iontophoresis plus thermotherapy plus exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Calcifying tendinitis of the shoulder Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Acetic acid iontophoresis Number randomised: 18 Number included in analyses: 17 Age: mean: 51.5 years; range: 39‐71 years Sex: female: 10; male: 7 Duration of symptoms: mean: 27.5 months; range: 3‐144 months Control: Sham iontophoresis Number randomised: 18 Number included in analyses: 10 Age: mean: 47.9 years; range: 31‐63 years Sex: female: 8; male: 5 Duration of symptoms: mean: 33 months; range: 3‐120 months |
|
| Interventions |
Intervention: Acetic acid iontophoresis Description of modality used: an electrotherapy apparatus, Dynaplus 421, was used to administer the treatment. The participant was seated with their arm resting on a table. The active electrode (cathode) was made of easily malleable lead, had a surface of 5 x 7.5 cm and was placed on three compresses saturated with 20 mL of 5% acetic acid applied approximately at the site of calcification of the shoulder. The second electrode (anode), also of malleable lead, had a 4 x 5 cm surface and was fixed to the anterior side of the distal segment of the ipsilateral arm. The acetic acid iontophoresis material was prepared by physiotherapist A who used 2 different techniques. Once the shoulder and arm of all subjects of both groups had been wrapped with identical elastic bandage, the acetic acid iontophoresis treatment was administered by physiotherapist B. After the treatment was completed, the iontophoretic material was removed by physiotherapist A Dose: a galvanic current of 5 mA for 15‐20 min was administered Frequency of administration: 10 sessions: 3 per week for 2 weeks followed by 1 per week for 4 weeks (6 weeks in total) Control: Sham iontophoresis Description of modality used: same as acetic acid iontophoresis group except a plastic film was used to cover the upper surface of the active electrode, and the compresses that were saturated with acetic acid were placed above the active electrode and not between the skin and the electrode, as technically required to ensure iontophoresis Dose: none Frequency of administration: 10 sessions: 3/week for 2 weeks followed by 1/week for 4 weeks (6 weeks in total) Both groups: Thermotherapy and exercises Description of modality used: thermotherapy (no details provided) and range of motion exercises Frequency of administration: 10 sessions: 3/week for 2 weeks followed by 1/week for 4 weeks (6 weeks in total) Any additional treatment during trial: paracetamol if needed |
|
| Outcomes | Outcomes assessed at 6 weeks
|
|
| Notes |
Conflicts of interest: "No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors(s) or upon any organization with which the author(s) is/are associated". Funding: Centre Hospitalier Universitaire de Montreal Foundation, Physiatry Division |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the participants were divided randomly according to a stratified randomisation table..." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Physiotherapist A prepared and installed the material needed for the acetic acid iontophoresis treatment of all participants in both groups; neither the participants nor physiotherapist B were aware of the true nature of the treatments (acetic acid iontophoresis or placebo) administered to participants; physiotherapist B administered the treatments, followed by thermotherapy and ROM exercises. At all times, only the main investigator and physiotherapist A were aware of the actual allocation of patients." Comment: Participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "...neither the participants nor physiotherapist B were aware of the true nature of the treatments (acetic acid iontophoresis or placebo) administered to participants..." Quote: "...The amplitude of active anterior flexion, abduction, and external and internal rotation of the shoulder was assessed by physiotherapist B by using a manual goniometer" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Thirty‐six subjects fitting the inclusion criteria were recruited and randomized in 2 equal groups of 18 participants. Quote: "Nine participants were removed from the study, 5 from the control group for superficial second‐degree burns under the negative electrode; 2 participants were removed after being treated with cortisone injection in the shoulder, and 2 patients failed to show up for the posttreatment radiography. Therefore, a total of 27 subjects remained in the study, 17 in the treatment group and 10 in the control group". Comment: The amount of attrition is unbalanced (higher in the placebo) and authors only reported a per‐protocol analysis, which is likely to have yielded biased results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Montes‐Molina 2012a.
| Methods |
Study design: Parallel group RCT Setting: Physiotherapy Unit and Rehabilitation Department of Ramon y Cajal University Hospital, Spain Intervention: Interferential laser therapy Comparator: Continuous laser therapy Source of funding: Instituto de Salud Carlos III, Fondo de Investigacion Sanitaaria (FIS) |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis, bicipital tendinitis, calcific tendinitis, rotator cuff partial tears, impingement syndrome, frozen shoulder, or bursitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Interferential laser therapy Number randomised: 99 Number included in analyses: 86 Age: mean: 57 years old; range: 52–63 years Sex: male: 26; female: 73 Diagnosis: rotator cuff tendinitis (53%), bicipital tendinitis (3%), calcific tendinitis (25%), rotator cuff partial tears (16%), impingement syndrome (5%), frozen shoulder (5%), dislocations (10%), bursitis (5%) Duration of symptoms: acute (< 90 days): 8; chronic (> 90 days): 91 Control: Continuous laser therapy Number randomised: 99 Number included in analyses: 83 Age: mean: 54 years old; range: 48–62 years Sex: male: 24; female: 75 Diagnosis: rotator cuff tendinitis (50%), bicipital tendinitis (5%), calcific tendinitis (13%), rotator cuff partial tears (17%), impingement syndrome (8%), frozen shoulder (3%), dislocations (10%), bursitis (3%) Duration of symptoms: acute (< 90 days): 6; chronic (> 90 days): 93 |
|
| Interventions |
Intervention: Interferential laser therapy Description of modality used: two independent identical infra‐red GaAIAs diode lasers (Sys Stim 540), Mettler Electronics Corp, Anaheim, CA, USA) with a wavelength 810 +/‐ 10 nm, pulse width of 100 milliseconds and maximum power output of 100 +/‐ 10 mW were used. This type of laser has an elliptical beam spot with an irradiation area of 9.2 mm2 at the aperture and the treatment area is illuminated with three 7400‐nm blue light‐emitting diodes. One applicator was placed perpendicular to the painful arm of the shoulder and the other was placed on the opposite side. Both lasers were switched on with the hand‐held probes pressed against the skin. The area was treated in 5 different points: 1 at the site of maximal pain and the other 4 at adjacent locations immediately above, below, right and left of the central point. Both probes were active and both lasers delivered the same dose at the same time. Participants were seated with the shoulder at rest in adduction and medial rotation Dose: laser was applied using continuous wave mode at a power density of 1.1 W/cm2. The energy dose per point was 7 J in 70 seconds. The energy density was 1.4 J/cm2. Total energy delivered per session was 70 J Frequency of administration: 10 treatment sessions in total, 3 per week (4 weeks) Any additional treatment during trial: some participants performed supervised shoulder exercises. The exercises were the same for all participants – Codman, finger‐stair and shoulder wheels Control: Continuous laser therapy Description of modality used: same as above, except one applicator was placed perpendicular to the painful area of the shoulder and the other applicator was switched off and placed on the opposite side. Both probes were pressed against the skin, as in the interferential group. The same points were treated as the interferential group. Participants were seated with the shoulder at rest in adduction and medial rotation Dose: total energy delivered per session was 35 J. Frequency of administration: 10 treatment sessions in total, 3 per week (4 weeks) Any additional treatment during trial: some participants performed supervised shoulder exercises. The exercises were the same for all participants – Codman, finger‐stair and shoulder wheels |
|
| Outcomes | Outcomes assessed at 4 weeks
|
|
| Notes |
Conflicts of interest: "None declared". Funding: Instituto de Salud Carlos III, Fondo de Investigación Sanitaria (FIS), Project no. PI 07/0046 and FEDER funds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before starting the study, a randomisation list was produced using a random generator. Patients were assigned to one of two groups." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patient blinding was implemented in two ways. First, the laser protective goggles worn by the patients prevented them from noticing if one or both laser applicators were active. Second, laser equipment was placed behind the subjects, preventing them from seeing the probes. The observer was also blinded to the group allocation. Only the physiotherapist who applied the laser therapy knew which treatment was received by each patient." Comment: participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The initial number of 100 patients in each group was reduced to 99 because one patient in each group did not sign the informed consent form. In addition, 16 subjects in the conventional group and 13 subjects in the interferential group dropped out of laser treatment before completion of the 10 sessions. Considering these losses, the number of patients actually studied was 83 in the conventional group and 86 in the interferential group." Comment: The number of losses to follow‐up are relatively similar between groups but no reasons are reported |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the ClinicalTrials.gov registry entry (NCT00694538) |
| Other bias | Low risk | Comment: No other sources of bias identified |
Montes‐Molina 2012b.
| Methods |
Study design: Parallel group RCT Setting: Physical Medicine and Rehabilitation Service (Unit of Physiotherapy) at of Ramon y Cajal University Hospital, Spain Intervention: Interferential light therapy generated by 2 light probes Comparator: Conventional light therapy generated by 1 light probe Source of funding: This work was supported by the Carlos III Health Institute and the Feder Funds, with grant number PI 07/0046 |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis, calcific tendinitis or partial rotator cuff tears Inclusion criteria
Exclusion criteria
Baseline characteristics Total n randomised = 30 participants Total n analysed = 26 participants Intervention: Interferential light therapy Number randomised: 15 randomised; Number completed: 13 Sex: F/M 12/3 Mean ± SD (range) age: 59.2 ± 11.0 years Mean ± SD (range) duration of symptoms: not reported Control: Conventional light therapy Number randomised: 15 randomised Number completed: 13 Sex: F/M 10/5 Mean ± SD (range) age: 9.0 ± 8.9 years Mean ± SD (range) duration of symptoms: not reported |
|
| Interventions | The therapy was applied in all cases with 2 independent and identical devices (Mettler Electronics Sys Stim 540, Anaheim, CA, USA) equipped with a multi‐diode cluster applicator combining 7 light‐emitting diodes at 660 nm and 12 superluminescent diodes at 950 nm wavelength, with a peak power of 500 mW and an average power of 310 mW. Diodes were distributed on each applicator in a circular arrangement covering an area of 4.50 cm2. The output activation was achieved by using a capacitance switch on the handheld applicator Intervention: Interferential light therapy generated by two light probes Components of intervention: two applicators were active and placed on opposite sides of the shoulder joint, covering the pain‐affected zone. In each session, treatment was applied in 2 successive applications. After the first application, the pair of applicators were slightly moved a short distance, and a second application was made. The energy delivered in each application was 84 J, 42 J per applicator with a power density of 67 mW/cm2. The resulting energy density at the skin was 10.3 J/cm2 in all cases. The total energy dose per session (two applications) was 168 J. The accumulated energy delivered during the entire treatment was 1680 J Comparator: Conventional light therapy Components of intervention: for blinding purposes of the study, the procedure was the same as in the interferential group, except that now only 1 of the 2 applicators was active, so the total dose per session (in 2 applications) was 84 J. The accumulated energy delivered in this case during the entire treatment was 840 J Both groups: The treatment technique chosen in both groups was the contact mode, applying the cluster probes and holding them firmly pressed to the skin. Participants were always in a seated position with the shoulder at rest and in medial rotation. The mode selected was pulsed, and the pulse modulation frequency was automatically applied step‐by‐step by the device along 10 interval values, from 10 Hz to 5 kHz, with a cycle duration of 10 seconds, 1 second at each step. The sessions were given over 2 weeks, at a rate of 5 per week |
|
| Outcomes | Outcomes assessed at 2 weeks
|
|
| Notes |
Conflicts of interest: no specific conflicts of interest reported Funding: Carlos III Health Institute (contract number PI 07/0046) and FEDER funds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For the allocation of the 30 remaining participants, block randomization was made by a computer‐generated random number list of elements with two possible random values (1 or 2), prepared by an investigator with no clinical involvement in the trial. The selected patients were consecutively assigned a number on the random list when they first came for treatment. Patients assigned with 1 received interferential light therapy (group 1) and those assigned with 2 received conventional light therapy (group 2)." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: "To preserve the allocation concealment, the random list was handled only by the non‐clinical investigator, who was also responsible for giving the daily sequence of treatments to the physiotherapist." Comment: Insufficient information was reported to determine whether an adequate method of allocation concealment was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: For blinding purposes of the study, the procedure was the same as in the interferential group, except that now only one of the two applicators was active." Quote: "The patient‐blinding procedure consisted in a twofold action. First, the two applicators were applied to all patients, regardless of whether one or both of them were active. Patients wore a pair of goggles that besides giving protection, prevented them from seeing the light spot of the applicator that was switched on. The front panel of the power supply was located behind the patients and outside their visual field." Comment: participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Evaluations were performed by a physiotherapist who was not informed about the technique each patient received." Comment: Assessor of objective outcomes (i.e. objectively measured components of UCLA shoulder scale) was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 30 patients were randomized, assigning 15 to each group. Two subjects per group dropped out over the six‐month period of the study, leaving 13 patients per group to be analysed." Comment: The participants' flow diagram shows that in each group, 1 participant was lost to follow‐up and 1 discontinued treatment. Thus, the number of drop‐outs and reasons for drop‐out were balanced between groups and are unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Nykänen 1995.
| Methods |
Study design: Parallel group RCT Setting: Inpatient rehabilitation centre, Finland Intervention: Therapeutic ultrasound plus massage plus exercises Control: Placebo ultrasound plus massage plus exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Painful arc or supraspinatus tendinopathy/tendinitis Criteria for defining the shoulder condition being treated: Shoulder pain with one of the following:
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Therapeutic Ultrasound Number randomised: 36 Number included in analyses: 35 Age: 66 ± 6 years old Sex: F/M 6/29 Duration of symptoms: not reported Control: Placebo ultrasound Number randomised: 37 Number included in analyses: 37 Age: 67 ± 9 years old Sex: F/M: 5/32 Duration of symptoms: not reported |
|
| Interventions |
Intervention: Therapeutic ultrasound Description of modality used: pulsed ultrasound using a EST301‐machine with Ultra‐Phone ultrasonic coupling medium Dose: Pulsed on‐to‐off ratio 1:4, frequency 1.0 mHz, intensity 1.0 W/cm2, pulse repetition rate 100 mHz, pulse duration 2 ms, radiating area 5 cm2 over a 10‐min treatment period Frequency of administration: 10‐12 treatments over 3‐4 weeks Control: Placebo ultrasound Description of modality used: same as above except the transducer plug was manipulated to leave it off during the sessions Dose: none for 10 min Frequency: 10‐12 treatments over 3‐4 weeks Both groups: Massage and exercises Description of modality used: neck and shoulder massage and group gymnastics attempting to stretch and strengthen the humero‐scapular and cervical musculature. Analgesia and NSAIDs were kept to a minimum but given for pain disturbing sleep |
|
| Outcomes | Outcomes assessed at 3‐4 weeks, 4 months and 12 months
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...the subjects were randomly assigned to groups A or B" Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Before treatment the therapist chose a transducer plug labelled either A or B according to the respective group of patients. A technician, also responsible for the regular checking of the ultrasonic output of the machines, had made the other plug nonfunctioning. Apart from him, no other person knew which plug was manipulated. Manipulation affected only the function of the applicator head, with no difference in machine appearance". Comment: participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Seventy‐two patients (35 in the ultrasound group and 37 in the placebo group) completed the treatment period (one patient suffered a fatal myocardial infarction after one week's treatment). At the 4‐month follow‐up, 67 responded (32 in the ultrasound group and 35 in the placebo group) and at one‐year follow‐up, 68 responded (30 and 37, respectively)". Comment: The experimental group had a larger loss to follow‐up, but reasons for this were not reported. Therefore it is unclear if attrition biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Otadi 2012.
| Methods |
Study design: Parallel group RCT Setting: Physiotherapy ward (by referral from orthopaedic surgeon or rheumatologist), Iran Intervention: Low‐level laser therapy (LLLT) plus therapeutic ultrasound plus exercise Control: Therapeutic ultrasound plus exercise Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Shoulder tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: LLLT, US, exercise and laser Number randomised: 23 Number included in analyses: 21 Age: 49.48 ± 8.5 years old Sex: all female Duration of symptoms: not reported Control: US and exercise Number randomised: 21 Number included in analyses: 21 Age: 48.05 ± 7.9 years old Sex: all female Duration of symptoms: not reported |
|
| Interventions |
Intervention: LLLT Description of modality used: LLLT with Class 3B solid state GA‐AS‐AI infrared laser (Endolaser 476, Enraf Nonius, Holland, type 1476.751) with pencil probe. Laser treatment applied over 1 cm2 areas marked out with a dematographic pencil Dose: Wavelength 830 nm, power 30 mW, 1 J/cm2, beam diameter 4 mm, 1 mm at 10 mm from the probe, angle of divergence 2.5° Frequency of administration: 3 sessions per week for 10 sessions (4 weeks) Control: no placebo LLLT delivered Both Groups: Description of modality used
Dose
Frequency of administration
|
|
| Outcomes | Outcomes assessed at 4 weeks and 12 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported Trial registered in the Iranian Registry of Clinical Trials (IRCT138712101719N1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were randomly assigned into two groups, using unmarked envelopes in clinic to achieve simple randomisation. There were 50 envelopes, 25 of which contained the word 'US and exercise' and 25 of which contained the word 'adding laser'". Comment: An adequate method was likely to used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: An adequate method was likely to used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The staff that assessed the outcomes was differed from the staff that administered the treatments; and they were blinded to the type of treatments" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two diabetic patients reported increase of pain in adding laser group and then withdrew from the study." Comment: The attrition may be related to the laser intervention, but the amount is small so is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Ozgen 2012.
| Methods |
Study design: Parallel group RCT Setting: Pamukkale University School of Medicine, Physical Medicine and Rehabilitation, Turkey Intervention 1: Transcutaneous electrical nerve stimulation (TENS) plus therapeutic ultrasound plus hot pack plus home exercises Intervention 2: Sodium hyaluronate injection plus home exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Supraspinatus tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: TENS plus therapeutic ultrasound plus hot pack plus home exercises Number randomised: 12 Number included in analyses: 11 Mean (SD) age: 52.50 (8.83) years old Sex: F/M 9/3 Duration of symptoms: 9.17 ± 9.90 months Intervention 2: Sodium hyaluronate injection plus home exercises Number randomised: 12 Number included in analyses: 10 Mean (SD) age: 58.67 (9.80) years old Sex: F/M 9/3 Duration of symptoms: 8.75 ± 4.96 months |
|
| Interventions |
Intervention 1: TENS plus therapeutic ultrasound plus hot pack Description of modality used
Dose
Frequency: not reported (assumed 3 weeks in total) Intervention 2: Sodium hyaluronate injection Description of modality used: administered to the shoulder joint by posterior approach. The administration zone cleaned with 10% polyvinylpyrrolidone iodine solution, then a 2 ml (16 mg) of G‐F 20 preparation with a molecular weight of 6 x 106 was administered into the joint cavity using a 21‐gauge injector Dose: 2 ml (16 mg) of G‐F 20 preparation with a molecular weight of 6 x 106 Frequency of administration: 3 times with weekly intervals Both groups: Home exercises Description of modality used: range of motion, stretching and strengthening exercises |
|
| Outcomes | Outcomes assessed at 3 weeks, 3 months and 4 years
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomized into two groups." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Unclear risk | Comment: There was no information on whether the assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "On the other hand, we determined that the effectiveness of treatment in the remaining 11 people in Group I and 10 people in Group II who could be reached was evaluated as 'very good'" Comment: The amount of attrition was small and unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Pan 2003.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinics, Taiwan Intervention 1: Transcutaneous electric nerve stimulation (TENS) Intervention 2: Extracorporeal shock wave therapy (ESWT) Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Chronic calcific tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: TENS Number randomised: 30 shoulders in 28 participants Number included in analyses: 29 shoulders in 27 participants Age: 58.00 ± 1.83 years Sex: F/M 19/9 Duration of symptoms: 23.90 ± 5.32 months Intervention 2: ESWT Number randomised: 33 shoulders in 32 participants Number included in analyses: 33 shoulders in 32 participants Age: 55.21 ± 2.01 years Sex: F/M 20/12 Duration of symptoms: 24.55 ± 6.45 months |
|
| Interventions |
Intervention 1: TENS Description of modality used: Hydrocollator pack and Neurosan50 electrostimulator (TENS) delivered constant square‐wave pulse stimulation current with a 0.5 ms pulse width and a 10 ms interval length to an active electrode secured firmly on the skin at the subacromion painful area Dose: frequency of 95 Hz and intensity increased until local contraction of adjacent muscles. Total session time was around 20 min Frequency: 3 times a week for 4 weeks Intervention 2: ESWT Description of modality used: The OrthospecTM was used to deliver ESWT. The OrthospecTM is a spark gap generator in a mobile unit. The therapeutic zone is ellipsoid in shape, 95 mm in height and 25 mm in diameter. There is about 0.29 mJ/cm2 of energy density at the edge of the therapeutic zone. The contact head was positioned at the marked painful area, which was defined by sonography before each treatment so that the acoustic shock wave could be transmitted effectively Dose: 2 Hz with 2000 shock waves and the energy level ranged from 0.26 mJ/mm2 to 0.32 mJ/mm2, depending on the intensity, which was adjusted to the participant's tolerance Frequency of administration: 2 sessions over 4 weeks |
|
| Outcomes | Outcomes assessed at 2 weeks, 4 weeks and 12 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients were randomly assigned to ESWT or TENS groups by draw" Comment: An adequate method was likely used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Unclear risk | Comment: There was no information on whether the assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "With the exception of 1 patient in the TENS group who dropped out after the first session because of severe pain, all patients completed the scheduled treatments and follow‐up". Comment: The very small amount of attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Perron 1997.
| Methods |
Study design: Parallel group RCT Setting: General community, private practice, Canada Intervention: Acetic acid iontophoresis plus therapeutic ultrasound Control: No treatment Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Calcifying tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Acetic acid iontophoresis plus therapeutic ultrasound Number randomised: 11 Number included in analyses: 11 Age: 43 years (32–57) Sex: F/M 7/4 Diagnosis: Type I lesion: 3, Type II lesion: 8 Duration of symptoms: 45 (0.2–180) months Control: No Treatment Number randomised: 11 Number included in analyses: 10 Age: 40 years (33–50) Sex: F/M 8/2 Diagnosis: Type I lesion n = 2, type II lesion n = 8 Duration of symptoms: 31 (0.5 – 120) months |
|
| Interventions |
Intervention: Acetic acid iontophoresis plus therapeutic ultrasound Description of modality used
Dose
Frequency of administration: 3 treatments per week for 3 weeks Control: No Treatment Both Groups Asked to avoid activities requiring overhead arm movements or repetitive tasks with the involved shoulder |
|
| Outcomes | Outcomes assessed at 1, 2, and 3 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: Ordre des Physiotherapeutes du Quebec Outcome data extracted from Figures using DigitizeIt software |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients in each stratum were then randomly assigned to th experimental (EXP) or control (CTL) groups". Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Four physiotherapists participated in the functional evaluations, but each patient was reevaluated by the same physiotherapist. Evaluators were unaware of the group assignment, and the patients were reminded not to make any statement that would unblind the evaluators" Comment: Assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Although all 22 patients completed the study, results from one patient were rejected because the incidence of X‐ray films taken at each evaluation did not allow a fair comparison of the CD area." Comment: One participant did not complete evaluation, and was removed due to technical problems with their X‐rays. This is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Polimeni 2003.
| Methods |
Study design: Parallel group RCT Setting: Ambulatory academic hospital, Canada Intervention 1: Therapeutic ultrasound plus mobilisation plus exercises Intervention 2: Diadynamic current plus mobilisation plus exercises Intervention 3: Radar plus mobilisation plus exercises Control: Mobilisation plus exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Supraspinatus tendinitis or biceps tendinitis Criteria for defining the shoulder condition being treated:
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 18 into each group Number included in analyses: not reported Age (mean and SD, or range): 56 ± 16 years Number of men and women: F/M 36/14 Duration of symptoms: not reported |
|
| Interventions |
Intervention 1: Therapeutic ultrasound Description of modality used: no details reported Dose: frequency not reported; intensity 1.5 W/cm2 Frequency of administration: 10 days Intervention 2: Diadynamic current Description of modality used: no details reported Dose: long interval of 7 min per session Frequency: 10 days Intervention 3: Radar Description of modality used: no details reported Dose: 60 W/cm2 in increasing 1min steps per day Frequency: 10 days Control: Nothing other than mobilisation plus exercises, which all groups received All Groups: Mobilisation plus exercises Description of modality used: mobilisation of all planes of movement, passive and active assisted exercises Dose: 10 min of passive exercises, 20 min of active assisted exercises Frequency of administration: 10 days |
|
| Outcomes | Outcomes assessed at 5 days, 10 days, and 40 days
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to 4 groups" Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported all outcomes of interest to this review |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Medians with no measures of variation reported for the Constant‐Murley score, however this was not related to the lack of statistical significance for this outcome. Without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Rabini 2012.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic of the Department of Orthopaedics and Traumatology, University Hospital, Rome, Italy Intervention 1: Microwave diathermy Intervention 2: Glucocorticoid injection Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Rotator cuff tendinopathy, with or without partial thickness tendon tears Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: Microwave diathermy Number randomised: 46 Number included in analyses: 40 Age: 59.2 ± 7.1 years Sex: F/M 30/16 Duration of symptoms: 15.5 ± 20.4 months Intervention 2: Glucocorticoid injection Number randomised: 46 Number included in analyses: 42 Age: 56.6 ± 11.6 years Sex: F/M 31/15 Duration of symptoms: 13.1 ± 9.1 months |
|
| Interventions |
Intervention 1: Microwave diathermy Description of modality used: administered using a Smarterapia Sigma Hyperthermia System with a 434 mHz microwave generator and a maximum output power of 100 W. It also utilised a microstrip antenna applicator specific for semicylindrical joint volumes of 20 to 30 cm in diameter. It had a total radiating area of 240 cm2 and an effective field size on a surface of 96 cm2. A 0.5 cm thick silicone pad filled with thermostatic deionised water was applied on the shoulder to allow the greatest energy transfer to be achieved. The pad was placed over the middle third of the joint line (between the glenoid and humeral head) with the participant supine and arm at 60 degrees of abduction and externally rotated. Dose: 40 W power with silicone pad temperature of 38°C. The aim was to achieve 1.5° C difference between cutaneous and deep temperature according to the thickness of the cutaneous fat of each participant. Each session lasted 30 min Frequency: 3 sessions per week for 4 weeks Intervention 2: Glucocorticoid injection Description of modality used: experienced physician injected at the subacromial space of the affected shoulder, using a 21‐gauge needle, aseptic conditions, through a posterolateral access Dose: 1 mL 40 mg methylprednisolone acetate containing 10 mg lidocaine chlorhydrate Frequency of administration: 1 injection every 2 weeks for total of 3 injections |
|
| Outcomes | Outcomes assessed at 4 weeks, 12 weeks and 24 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned…using a random sequence generator (www.random.org)" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization list was kept by an independent researcher not involved in the study. Allocation concealment was performed using closed envelopes, and the assignment code of each patient was revealed to the researcher who performed the treatment only at the beginning of the therapeutic protocol" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Primary and secondary outcome measures were determined at baseline and follow‐up visits by an investigator blind to participants' allocation" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Missing data at follow‐up were managed according to the last‐observation‐carried‐forward (LOCF) method. Data were analysed according to the intention‐to‐treat principle". Quote: "A total of 8 participants (8.7%) were lost to follow‐up, 2 in the corticosteroid group and 6 in the hyperthermia group. The follow‐up was thus completed in 82 patients (89%)" Quote: "Finally, reasons for lack of follow‐up were not recorded. However, only a few participants were lost to follow‐up (8.7%) and dropouts occurred to a similar extent in the 2 treatment groups, which did not substantially affect the results" Comment: The small amount of attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
San Segundo 2008.
| Methods |
Study design: Parallel group RCT Setting: Outpatient rehabilitation service, Spain Intervention: Therapeutic ultrasound plus exercise Control: Placebo ultrasound plus exercise Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Rotator cuff tendinitis or partial rotator cuff tears Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Therapeutic ultrasound plus exercise Number randomised: 17 shoulders Number included in analyses: 16 shoulders Age (mean and SD, or range): 52.6 (10.9) years old Number of men and women: F/M 80%/20% Diagnosis: tendinitis: 88.2%; partial rotator cuff tear: 11.8% Duration of symptoms (SD): 10.2 (11.4) months Control: Placebo ultrasound plus exercise Number randomised: 17 shoulders Number included in analyses: 15 shoulders Age (mean and SD, or range): 56.9 (9.4) years Number of men and women: F/M 87.5%/12.5% Diagnosis: tendinitis: 82.3%; partial rotator cuff tear: 17.7% Duration of symptoms (SD): 12.6 (11.1) months |
|
| Interventions |
Intervention: Ultrasound plus exercises Description of modality used: pulsed ultrasound delivered with standard technique Dose: intensity 2 W/cm2 1:4 at frequency 1 mHz for 7 min Frequency of administration: 3 days a week for 3 weeks Control: Placebo Ultrasound plus exercises Description of modality used: application of non‐functioning ultrasound device Dose: none Frequency of administration: 3 days a week for 3 weeks Any additional treatment during trial: analgesia if required Both groups Description of modality used: active assisted exercises for mild mobility impairment and strengthening exercises for rotator cuff, using an elastic band Dose: not reported Frequency of administration: daily sessions for 3 weeks followed by sessions twice a week for an additional 2 weeks once the ultrasound sessions were finished |
|
| Outcomes | Outcomes assessed at time points: baseline, 3 weeks, 5 weeks, 3 months and 6 months
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported Article is written in Spanish but translated into English using https://translate.google.com.au/ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients included were assigned by a third person, one of the two treatment groups in a sequence generated by a random number table. In patients with bilateral shoulder each shoulder was assigned to a group, randomized consecutively". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Initially, both the physician and the therapist and the patient were blinded to the type of treatment assignment. However, once started the study found that it was possible to blinding therapists who performed the treatment, since the proceeding routine device check told him when the U.S. is not functioning. " Comment: Participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Comment: According to the above quote, the physician (who was the outcome assessor) was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 29 patients completed the study at 5 weeks, but at 3 and 6 months the percentage of patients lost was very high (44.1% overall, 23.5% in group 1 and 20.6 % in group 2), so results could not be analyzed" Comment: There was no attrition at short‐term follow‐up, and authors decided not to analyse data at 3‐ and 6‐month follow‐up due to high attrition at this later time point |
| Selective reporting (reporting bias) | Unclear risk | Comment: Due to the high attrition rate, the authors chose not to publish data for their planned 3‐ and 6‐month follow‐up. However, outcome data were fully reported for all outcomes specified in the methods section of the publication at short‐term follow‐up. Though without a trial protocol it is unclear if other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Santamato 2009.
| Methods |
Study design: Parallel group RCT Setting: Outpatients in a university hospital, Italy Intervention 1: High intensity laser therapy Intervention 2: Therapeutic ultrasound Source of funding: "Work was supported by the Italian Longitundinal Study on Aging (ILSA) ‐ Italian National Research Council" |
|
| Participants |
Diagnostic label used by trialist: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: High intensity laser therapy Number randomised: 35 Number included in analyses: 35 Age (mean and SD, or range): 54.2 years (8.2 SD) Number of men and women: F/M 20/15 Duration of symptoms: 8.7 months (8.8 SD) Intervention 2: Therapeutic ultrasound Number randomised: 35 Number included in analyses: 35 Age (mean and SD, or range): 54.0 years (9.8 SD) Number of men and women: F/M 22/13 Duration of symptoms: 8.1 months (10.8 SD) |
|
| Interventions |
Intervention 1: High intensity laser therapy Description of modality used: high intensity laser therapy with a neodymium yttrium aluminum garnet laser that has a pulsating waveform produced by an HIRO 1.0 device. Administered by a physiatrist using a standard handpiece endowed with fixed spacers, with consistent distance from the skin, verticality of 90 degrees to the treatment zone and a bright spot diameter of 5 mm. Each session involved 3 phases:
Dose: the treatment consisted of a high peak power (1 kW), a wavelength of 1064 nm, a maximum energy for a single impulse of 150 mJ, an average power of 6 W, a fluency of 760 mJ/cm2, and a duration for the single impulse of less than 150 ms. Three steps were predicted in the starting/initial and final phases of the treatment; the fluencies used were 510, 610, and 710 mJ/cm2, respectively. Therefore, the total dose of energy administered was approximately 2050 J over 10 min Frequency of administration: 5 days a week for 2 weeks Intervention 2: Therapeutic ultrasound Description of modality used: continuous ultrasound using a Sonopuls 492 with a 5.8 cm2 transducer head and an effective radiating area of 4.6 cm2. The treating physical therapist, using the technique of slow circular movements, applied the transducer head over the superior and anterior periarticular regions of the participant's glenohumeral joint and on the shoulder trigger points, covering an area of approximately 20 cm2 Dose: frequency of 1 MHz and an intensity of 2 W/cm2 with a duty cycle of 100%. Duration 10 min Frequency of administration: 5 days a week for 2 weeks |
|
| Outcomes | Outomes assessed at 2 weeks
|
|
| Notes |
Conflicts of interest: authors stated that the funding agencies had no role in the design, conduct or reporting of the study Funding: Italian Longitudinal Study on Aging (ILSA) (Italian National Research Council‐CNR‐Targeted Project on Aging grants 9400419PF40 and 95973PF40 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed allocation was performed with random numbers generated from the Web site http://www.random.org/ before the beginning of
the study. The procedure Random Integer Generator allowed us to generate random integers. A priori it generated 100 random integers and, before the beginning of the study, the randomization number was already present." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Individual, sequentially numbered index cards with the random assignments were prepared. The index cards were folded and placed in sealed opaque envelopes. A physician who was unaware of the baseline examination findings opened the envelopes to attribute the interventions according to the group assignments." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "...the physicians who performed the clinical evaluations of the participants were unaware of the group assignments" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 70 participants completed the trial and were included in the analysis." Comment: There was no attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Saunders 1995.
| Methods |
Study design: Parallel group RCT Setting: Physiotherapy department, UK Intervention: Low level laser therapy (LLLT) Control: Placebo laser Source of funding: Not reported |
|
| Participants |
Diagnostic tool used by trialist: Supraspinatus tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number of men and women: F/M 12/12 Intervention: LLLT Number randomised: 12 Number included in analyses: 12 Age mean (SD): 49.8 (8.12) years old Number of men and women: not reported Duration of symptoms: 3.86 (2.4 SD) months Control: Placebo LLLT Number randomised: 12 Number included in analyses: 12 Age mean (SD): 50.7 (8.31 SD) years old Number of men and women: not reported Duration of symptoms: 3.32 (1.9 SD) months |
|
| Interventions |
Intervention: LLLT Description of modality used: a 50 mW, 820 nm (infrared) laser probe was pressed firmly into the tissue, at an angle of 1.57 radian to the tendon. Two areas were irradiated:
Dose: 40 mW, 30 J/cm2 treatment, operated for 90 seconds at a frequency of 5000 Hz for both areas (i.e. 180 seconds in total) Frequency of administration: 9 treatments over 3 weeks (3 treatments per week) Any additional treatment during trial: none reported Control: Placebo LLLT Description of modality used: same as above except laser device switched off Dose: Zero power Frequency of administration: 9 treatments over 3 weeks (3 treatments per week) Both Groups: Advice Description of modality used: a recording of a physiotherapist explaining to the participants how to use their arms, and a transcript of the recording Frequency of administration: the tape was played once, but the transcript was the participant's to keep |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes |
Conflicts of interest: Not reported Funding: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned to two treatment groups" Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The standardized treatments were administered by two physiotherapy helpers who had been given on‐the‐job training and training on laser safety procedures. The helpers used probe A or B depending on the treatment group of the patient. The helpers did not know which of the probes was real and which was the dummy" Quote: "There was no way for the helpers or therapists to distinguish between the probes" Comment: Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The subjects were tested by the same independent 'blind' assessor before and after the course of nine treatments" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No attrition was reported, and outcome data were based on the number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the Methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Shehab 2000.
| Methods |
Study design: Parallel group RCT Setting: Outpatient rehabilitation unit, Kuwait Intervention 1: Trancutaneous electrical nerve stimulation (TENS) plus exercise plus cold pack Intervention 2: Therapeutic ultrasound plus exercise plus cold pack Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Supraspinatus tendinitis, subdeltoid bursitis or bicipital tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 50 (26 in TENS group and 24 in ultrasound group) Number included in analyses: 50 Age mean and SD, or range): 50 ± 5.89 years old Number of men and women: all women Diagnosis: most had supraspinatus tendinitis, subdeltoid bursitis or bicipital tendinitis Duration of symptoms: at least 1 month |
|
| Interventions |
Intervention 1: TENS Description of modality used: TENS through electrodes applied to the anterior and posterior shoulder area Dose: frequency 50 Hz for 30 min Frequency of administration: 3‐5 times per week for 13 sessions (i.e. 3‐5 weeks) Intervention 2: Therapeutic ultrasound Description of modality used: ultrasound around the glenohumeral joint (not reported whether continuous or pulsed) Dose: Intensity 0.5 W/cm2 increasing by 0.1 each session; frequency not reported; duration 10 min Frequency of administration: 3‐5 times per week for 13 sessions (i.e. 3 ‐5 weeks) Both groups: cold packs for 20 min and stretching and range of motion exercises for the shoulder after each treatment |
|
| Outcomes | Outcomes assessed at 3‐5 weeks
|
|
| Notes |
Conflicts of interest: Not reported Funding: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of two groups." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | High risk | Quote: "We realize that not having the outcome measures blinded is a limitation of the study" Comment: Assessor of objective outcomes was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Vecchio 1993.
| Methods |
Study design: Parallel group RCT Setting: Outpatient rheumatology clinics, UK Intervention: Low‐level laser therapy (LLLT) plus exercise Control: Placebo LLLT plus exercise Source of funding: No specific source of funding reported but the authors acknowledge "CM Medico for use of their laser equipment" |
|
| Participants |
Diagnostic label used by trialist: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 35 Number included in analyses: 35 Age mean (range): 54.4 years (17–77) Number of men and women: F/M 25/10 Duration of symptoms: 14.9 months (4–48) LLLT plus exercise Number randomised: 19 Number included in analyses: 19 Age (mean and SD, or range): not reported Number of men and women: F/M 11/8 Duration of symptoms: not reported Placebo LLLT plus exercise Number randomised: 16 Number included in analyses: 16 Age (mean and SD, or range): not reported Number of men and women: F/M 14/2 Duration of symptoms: not reported |
|
| Interventions |
Intervention: LLLT Description of modality used: continuous irradiation laser with a CB Medico Master III hand held single probe laser (Gallium aluminium arsenide diode of class 3B). Each session consisted of three pulses (3 J) to each of a maximum of 5 tender points found on clinical examination. As far as possible, treatment was concentrated in the subacromial or anterior shoulder regions. The laser was held perpendicular to the body and skin contact delivered without pressure Dose: 3 pulses (3 J); wavelength of 830 nm; mean power of 30 mW with a wavelength divergence of ± 1.5 nm and a beam diameter of 3 mm Frequency of administration: twice weekly for 8 weeks Control: Placebo LLLT Description of modality used: same as above except laser device switched off Dose: none Frequency: twice weekly for 8 weeks Both groups: Supervised exercises Description of modality used: exercises including pendular swing and wall climbing exercises. A physiotherapist taught exercises on the first session. Pendular swinging was performed in flexion and extension, abduction and adduction. Participants were also asked to stand facing a wall with both hands placed on the wall and shoulder elevation gradually increasing bilaterally (wall climbing exercises). On their second visit, participants were asked to repeat the exercises as shown previously to determine whether or not the participant had performed them correctly and if not, they were reinstructed Dose: not reported Any additional treatment during trial: paracetamol to a maximum of 2 g per day |
|
| Outcomes | Outcomes assessed at 2 weeks, 4 weeks, 6 weeks and 8 weeks. However, data were analysed at 4 weeks and 8 weeks only
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to treatment..." Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "One physiotherapist set up the appropriate probe (active or placebo) whilst the second 'blinded' physiotherapist administered the treatment." Comment: Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "Patients were assessed by another observer unaware of the treatment code" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Trialists did not report whether there were any dropouts, losses to follow‐up or exclusions, or the number of participants included in each analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Yavuz 2014.
| Methods |
Study design: Parallel group RCT Setting: Outpatient physical medicine and rehabilitation clinic, Turkey Intervention 1: Low‐level laser therapy (LLLT) plus hot pack plus exercises Intervention 2: Therapeutic ultrasound plus hot pack plus exercises Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms: At least 4 weeks Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1: LLLT Number randomised: 16 Number included in analyses: 16 Age: 44.2 ± 8.2 years old Sex: F/M 7/9 Duration of symptoms: 6.7 ± 4.8 months Intervention 2: Therapeutic ultrasound Number randomised: 15 Number included in analyses: 15 Age: 45.3 ± 9.8 years old Sex: F/M 7/8 Duration of symptoms: 6.3 ± 5.2 months |
|
| Interventions |
Intervention 1: LLLT Description of modality used: a gallium‐aluminum‐arsenide (GaAlAs, infrared laser) diode laser device (Chattanooga Group, USA) with a wavelength of 850 nm, a power output of 100 mV, continuous wave, and a 0.07 cm2 spot area laser was used for the laser therapy. The LLLT was applied at a maximum of 5 painful points for 1 min at each point over the subacromial region of the shoulder Dose: 3 J/cm2 to 5 painful points (total 15 J); power output of 100 mV; duration 5 min Frequency of administration: 5 times a week for 2 weeks (10 sessions) Intervention 2: Therapeutic ultrasound Description of modality used: administered to the area over the subacromial region of the shoulder using a technique of slow circular movement, with continuous mode Dose: frequency 1 MHz; intensity 2 W/cm2; duration 5 min Frequency of administration: 5 times a week for 2 weeks (10 sessions) Both groups: Hot pack and exercises Description of modality used: hot pack therapy was applied to all participants in both groups for 10 min. In addition, all participants received an exercise programme. These exercises included range of motion, stretching, and progressive resistive exercises Dose: hot pack for 10 min; each exercise was performed once a day with 10 repetitions Frequency of administration: 5 times a week for 3 weeks (10 sessions) |
|
| Outcomes | Outcomes assessed at 1 and 3 months
|
|
| Notes |
Conflict of interest: "The authors declare that there is no conflict of interest." Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "These participants were randomly assigned into two groups via a numbered‐envelope system: “LLLT” or “US therapy” was written on a piece of paper in each sealed envelope, and each patient selected one envelope". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "These participants were randomly assigned into two groups via a numbered‐envelope system: “LLLT” or “US therapy” was written on a piece of paper in each sealed envelope, and each patient selected one envelope". Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported all outcomes of interest to the review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of the 31 participants completed the trial and were included in the analysis." Comment: All randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Yeldan 2009.
| Methods |
Study design: Parallel group RCT Setting: Outpatients recruited from the Medicine Faculty of Istanbul, University of Istanbul, Turkey Intervention: Low‐level laser therapy (LLLT) plus exercise plus cold pack Control: Placebo LLLT plus exercise plus cold pack Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialist: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated: at least 3 of the folllowing:
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: LLLT plus exercise plus cold pack Number randomised: 34 Number included in analyses: 34 Age: 55.32 ± 8.73 years old Sex: F/M 25/9 Duration of symptoms: 6.5 ± 4.52 months Control: Placebo LLLT plus exercise plus cold pack Number randomised: 33 Number included in analyses: 26 Age: 55.0 ± 8.75 years old Sex: F/M 22/4 Duration of symptoms: 6.42 ± 4.79 months |
|
| Interventions |
Intervention: LLLT Description of modality used: application of GaAs diode laser instrument (Roland Serie Elettronica Pagani), with wavelength 904 nm, frequency range of 5–7000 Hz and maximum peak power of 27, 50 or 27 x 4 W). Laser was applied while sitting on a chair; each participant placed an arm with the hand supinated in his or her lap. The transducer head was placed on the superior and anterior periarticular parts of glenohumeral joint, covering an area of approximately 15 cm2. Three pulses (3 J) were applied to a maximum of 5 tender points found on clinical examination (pain with palpation). As far as possible, treatment was concentrated on the subacromial and anterior shoulder regions. The laser was held perpendicular to the skin without pressure Dose: 90 seconds at each location with a frequency of 2000 Hz. The treatment duration was approximately 8 min Frequency of administration: 5 days per week for 3 weeks Control: Placebo LLLT Description of modality used: same as above except the device was set to "off" mode Dose: none Frequency: 5 days per week for 3 weeks Both groups: Supervised and home exercises and cold pack Description of modality used: progressive exercise programme including range of motion exercises, strengthening and stretching exercises, followed by a cold pack application. Exercises were performed under supervision in the clinic and at home. First week exercises included inferior and posterior capsule stretching, wand exercises (shoulder flexion, abduction, extension, internal and external rotation), active‐assisted range of motion exercises and internal rotator exercise (with a towel). In later weeks, these were performed actively and with Theraband resistance (The Hygenic Corporation). In the second and third weeks, supraspinatus exercise (empty can) was added. The cold pack was applied around the shoulder. To promote compliance with the therapy, participants were asked to write a diary of the exercise programme which was reviewed weekly Dose: between 15 and 30 min of exercise and 15 min of cold pack Frequency: twice daily for 3 weeks |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done using Microsoft Excel 'RAND()' function. Command was =IF(RAND()<=0.5;"laser group";"placebo laser group")." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | High risk | Quote: "The selector (ARO), who did not perform any assessment, was aware of the randomisation scheme." Comment: The allocation sequence was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The subjects were not informed about the true nature of laser application" Quote:"The treating physical therapist (EC) was aware of the nature of this intervention, the physical findings of subjects and the treatment group to which subjects had been allocated." Comment: Participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Low risk | Quote: "The assessor (IY) was blind to which group the subjects had been allocated." Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seven patients in the placebo laser group were unable to complete the therapy; 26 patients were able to complete the study. The reasons for dropping out of the study were surgery (2 subjects), scheduling problems (n=3) or personal circumstances that prevented weekly visits (n=2)." Comment: The dropouts were all in the placebo group, however the reasons for loss to follow‐up were all given. These were unrelated to the study treatments. Therefore, the results are unlikely to be biased due to this attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Yildirim 2013.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic of the Istanbul Physical Therapy and Rehabilitation Education and Research Hospital, Turkey Intervention: Therapeutic ultrasound for 4 min plus superficial heat plus TENS plus exercise Control: Therapeutic ultrasound for 8 min plus superficial heat plus TENS plus exercise Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Any restriction on duration of symptoms
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention: Therapeutic ultrasound for 4 min plus other physical therapy Number randomised: 50 Number included in analyses: 50 Age: mean: 55.4 ± 7.63 years old Sex: female: 34; male: 16 Duration of symptoms: mean: 8.34 ± 4.86 months Control: Therapeutic ultrasound for 8 min plus other physical therapy Number randomised: 50 Number included in analyses: 50 Age: mean: 54.7 ± 8.67 years Sex: female: 27; male: 23 Duration of symptoms: mean: 6.66 ± 4.91 months |
|
| Interventions |
Intervention: Therapeutic ultrasound for 4 min Components of intervention: continuous ultrasound applied using circular motions. A Chattanooga brand ultrasound machine with a transducer head size of 5 cm2 was used Dose: 4 min duration; intensity 1.5 W/cm2; frequency not reported Frequency of administration: 5 times a week for 3 weeks Control: Therapeutic ultrasound for 8 min Components of intervention: continuous ultrasound applied using circular motions. A Chattanooga brand ultrasound machine with a transducer head size of 5 cm2 was used Dose: 8 min duration; intensity 1.5 W/cm2; frequency not reported Frequency of administration: 5 times a week for 3 weeks Both groups: Superficial heat plus TENS plus exercise Components of intervention: TENS, infrared therapy, and exercises. The initial exercise programme consisted of Codman's pendulum exercises, passive range of motion exercises and stretching exercises. Posterior capsular stretching exercises and wall walking exercises were also performed. The exercises were taught to the participants at the beginning of the physical therapy programme. After the participants achieved full or nearly full range of motion, shoulder strengthening exercises were performed. participants were instructed to not to use their affected arm for daily activities, in particular overhead activities, in order to properly rehabilitate their shoulders. After the participants' shoulders were properly strengthened, they were allowed to abduct their shoulder greater than 90 degrees and use their arm for daily activities. The exercises were performed under observation in the outpatient clinic, twice a week, and the participants were instructed to carry out the exercise programme at home twice a day with 20 repetitions per exercise Dose: TENS (30 min, no other details reported); infrared therapy (20 min, no other details reported); exercises (20 repetitions per exercise) Frequency of administration: TENS (unclear); infrared therapy (unclear); exercises (twice a week for 3 weeks in clinic, and twice a day for 3 weeks at home) |
|
| Outcomes | Outcomes assessed at 5 weeks
|
|
| Notes |
Conflicts of interest: "The writers have no conflict of interest to declare." Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 100 patients included in this study were divided into 2 groups each consisting of 50 patients using consecutive sequential randomization". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A prospective, randomized, single‐blind study was performed" Comment: The trialists did not specify who was blinded in this trial (participants, personnel or outcome assessors). It is likely participants were not blinded (and personnel certainly were not blinded). Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objectively rated outcomes | Unclear risk | Quote: "A prospective, randomized, single‐blind study was performed" Comment: The trialists did not specify who was blinded in this trial (participants, personnel or outcome assessors). It is therefore unclear whether assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No losses to follow‐up, withdrawals or post‐randomisation exclusions were reported, and outcome data is analysed based on all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data for all outcomes specified in the methods section of the publication were fully reported, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ainsworth 2007 | Ineligible condition: 29% of participants were classified as "capsular pattern positive", suggesting that they had adhesive capsulitis. We were unable to obtain data for the subgroup of participants with rotator cuff disease |
| Dickens 2005 | Ineligible intervention: multi‐modal physiotherapy, where effect of electrotherapy modality could not be isolated |
| Ginn 2005 | Ineligible intervention: multi‐modal physiotherapy, where effect of electrotherapy modality could not be isolated |
| Hay 2003 | Ineligible intervention: multi‐modal physiotherapy, where effect of electrotherapy modality could not be isolated |
| Herrera‐Lasso 1993 | Ineligible condition: 31% of participants had periarthritis and we were unable to obtain data for the subgroup of participants with rotator cuff disease |
| Taverner 2014 | Ineligible condition: participants were only reported as having "shoulder pain", and it was unclear if participants with adhesive capsulitis, myofascial neck and shoulder pain condition, rheumatoid arthritis or pain due to trauma were excluded |
| Van der Heijden 1999b | Ineligible condition: most participants had pain radiating below the elbow, ˜10% had shoulder pain caused by trauma, and the number of participants with adhesive capsulitis unclear |
Characteristics of studies awaiting assessment [ordered by study ID]
Dal Conte 1990.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Gudmundsen 1987.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Güler 2009.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Jiménez‐García 2008.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Knorre 1990.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
The original review outcomes were pain, range of motion (active and passive), function/disability and quality of life, strength, return to work, participants' perception of overall effect, global preference, physicians' preference and adverse effects. The outcomes reported in this review have been modified from the original review to make them as consistent as possible with other Cochrane reviews on shoulder disorders and other chronic pain conditions. To improve succinctness of the review, we only included one measurement instrument per outcome domain. We assessed study risk of bias using The Cochrane 'Risk of bias' tool in this update of the review (Higgins 2011b). We have included a 'Summary of findings' table.
Contributions of authors
MJP was responsible for writing the review, performing the searches, selecting trials, performing risk of bias assessment, data extraction, analysing the data and interpreting the results of the updated review. SG was responsible for performing the searches, selecting trials and performing the data extraction and quality assessment for the original review, defining the review comparisons and outcomes of interest of the original and updated review, analysing and interpreting the results, and contributing to writing both the original and updated review. BM was responsible for selecting trials, performing risk of bias assessment, data extraction and contributing to writing the manuscript for the updated review. MM, SS, JD, and NL were responsible for performing risk of bias assessment, data extraction and contributing to writing the manuscript for the updated review. RB was responsible for performing the data extraction and quality assessment for the original review, defining the review comparisons and outcomes of interest of both the original and updated review, analysing and interpreting the results, and contributing to writing both the original and updated review.
Sources of support
Internal sources
Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Australia.
Australasian Cochrane Centre, Australia.
External sources
Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088535), Australia.
Declarations of interest
RB is Joint Co‐ordinating Editor of Cochrane Musculoskeletal. To avoid bias, RB was excluded from the editorial and publication process for this review. SG is a practicing physiotherapist in part‐time private physiotherapy practice (self employed), and as such receives remuneration for the delivery of physiotherapy interventions. BM is a practicing physiotherapist in private physiotherapy practice and as such receives remuneration for the delivery of physiotherapy interventions.
New
References
References to studies included in this review
Abrisham 2011 {published data only}
- Abrisham SM, Kermani‐Alghoraishi M, Ghahramani R, Jabbari L, Jomeh H, Zare M. Additive effects of low‐level laser therapy with exercise on subacromial syndrome: a randomised, double‐blind, controlled trial. Clinical Rheumatology 2011;30:1341‐6. [DOI] [PubMed] [Google Scholar]
Aktas 2007 {published data only}
- Aktas I, Akgun K, Cakmak B. Therapeutic effect of pulsed electromagnetic field in conservative treatment of subacromial impingement syndrome. Clinical Rheumatology 2007;26:1234‐9. [DOI] [PubMed] [Google Scholar]
Akyol 2012 {published data only}
- Akyol Y, Ulus Y, Durmus D, Canturk F, Bilgici A, Kuru O, et al. Effectiveness of microwave diathermy on pain, functional capacity, muscle strength, quality of life, and depression in patients with subacromial impingement syndrome: a randomized placebo‐controlled clinical study. Rheumatol International 2012;32(10):3007‐16. [DOI] [PubMed] [Google Scholar]
Al Dajah 2014 {published data only}
- Al Dajah SB. Soft tissue mobilization and PNF improve range of motion and minimize pain level in shoulder impingement. Journal of Physical Therapy Science 2014;26(11):1803‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atya 2012 {published data only}
- Atya AM. Efficacy of microcurrent electrical stimulation on pain, proprioception accuracy and functional disability in subacromial impingement : RCT. Indian Journal of Physiotherapy & Occupational Therapy 2012;6(1):15‐8. [Google Scholar]
Bal 2009 {published data only}
- Bal A, Eksioglu E, Gurcay E, Gulec B, Karaahmet O, Cakci A. Low‐level laser therapy in subacromial impingement syndrome. Photomedicine and Laser Surgery 2009;27:31‐6. [DOI] [PubMed] [Google Scholar]
Bansal 2011 {published data only}
- Bansal K, Padamkumar S. A comparative study between the efficacy of therapeutic ultrasound and soft tissue massage (deep friction massage) in supraspinatus tendinitis. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:80‐4. [Google Scholar]
Baskurt 2006 {published data only}
- Baskurt Z, Baskurt F, Ozcan A, Yilmaz O. The immediate effects of heat and TENS on pressure pain threshold and pain intensity in patients with Stage I shoulder impingement syndrome. Pain Clinic 2006;18:81‐5. [Google Scholar]
Berry 1980 {published data only}
- Berry H, Fernandes L, Bloom B, Clark RJ, Hamilton EB. Clinical study comparing acupuncture, physiotherapy, injection and oral anti‐inflammatory therapy in shoulder‐cuff lesions. Current Medical Research and Opinion 1980;7:121‐6. [DOI] [PubMed] [Google Scholar]
Binder 1984 {published data only}
- Binder A, Parr G, Hazleman B. Pulsed electromagnetic field therapy of persistent rotator cuff tendonitis. Lancet 1984;1:695‐8. [DOI] [PubMed] [Google Scholar]
Bingöl 2005 {published data only}
- Bingöl U, Altan L, Yurtkuran M. Low‐power laser treatment for shoulder pain. Photomedicine and Laser Surgery 2005;23:459‐64. [DOI] [PubMed] [Google Scholar]
Calis 2011 {published data only}
- Calis HT, Berberoglu N, Calis M. Are ultrasound, laser and exercise superior to each other in the treatment of subacromial impingement syndrome? A randomized clinical trial. European Journal of Physical and Rehabilitation Medicine 2011;47:375‐80. [PubMed] [Google Scholar]
Celik 2009 {published data only}
- Celik D, Atalar AC, Sahinkaya S, Demirhan M. The value of intermittent ultrasound treatment in subacromial impingement syndrome. Acta Orthopaedica et Traumatologica Turcica 2009;43:243‐7. [DOI] [PubMed] [Google Scholar]
Chard 1988 {published data only}
- Chard MD, Hazleman BL, Devereaux MD. Controlled trial to investigate dose‐response patterns to portable, pulsed electromagnetic fields in the treatment of rotator cuff tendinitis: a review trial. Journal of Orthopedics & Rheumatology 1988;1:33‐40. [Google Scholar]
Clews 1987 {published data only}
- Clews W, Wajswelner H. Beneficial effects of massage in athletes with rotator cuff tendinitis. Proc Natl Annu Sci Conf Aust Sports Med Fed 1987;2:555‐564. [Google Scholar]
Dogan 2010 {published data only}
- Dogan SK, Ay S, Evcik D. The effectiveness of low laser therapy in subacromial impingement syndrome: a randomized placebo controlled double‐blind prospective study. Clinics 2010;65:1019‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Downing 1986 {published data only}
- Downing DS, Weinstein A. Ultrasound therapy of subacromial bursitis. A double blind trial. Physical Therapy 1986;66:194‐9. [DOI] [PubMed] [Google Scholar]
Ebenbichler 1999 {published data only}
- Ebenbichler GR, Erdogmus CB, Resch KL, Funovics MA, Kainberger F, Barisani G, et al. Ultrasound therapy for calcific tendinitis of the shoulder. New England Journal of Medicine 1999;340:1533‐8. [DOI] [PubMed] [Google Scholar]
England 1989 {published data only}
- England S, Farrell A, Coppock J, Struthers G, Bacon P. Low laser therapy of shoulder tendonitis. Scandanavian Journal of Rheumatology 1989;18:427‐43. [DOI] [PubMed] [Google Scholar]
Eslamian 2012 {published data only}
- Eslamian F, Shakouri SK, Ghojazadeh M, Nobari OE, Eftekharsadat B. Effects of low‐level laser therapy in combination with physiotherapy in the management of rotator cuff tendinitis. Lasers in Medical Science 2012;27(5):951‐8. [DOI] [PubMed] [Google Scholar]
Eyigor 2010 {published data only}
- Eyigor C, Eyigor S, Kivilcim Korkmaz O. Are intra‐articular corticosteroid injections better than conventional TENS in treatment of rotator cuff tendinitis in the short run? A randomized study. European Journal of Physical and Rehabilitation Medicine 2010;46:315‐24. [PubMed] [Google Scholar]
Galace de Freitas 2014 {published data only}
- Galace de Freitas D, Marconded FB, Monteiro RL, Rosa SG, Maria de Moraes Barros Fucs P, Fukuda TY. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: a randomized, double‐blind, placebo‐controlled clinical trial. Archives of Physical Medicine and Rehabilitation 2014;95(2):345‐52. [DOI] [PubMed] [Google Scholar]
Giombini 2006 {published data only}
- Giombini A, Cesare A, Safran MR, Ciatti R, Maffulli N. Short‐term effectiveness of hyperthermia for supraspinatus tendinopathy in athletes: a short‐term randomized controlled study. American Journal of Sports Medicine 2006;34:1247‐53. [DOI] [PubMed] [Google Scholar]
Grymel‐Kulesza 2007 {published data only}
- Grymel‐Kulesza E, Polak A, Kubacki J, Skrzep‐Poloczek B, Krol P. The effect of a multi‐modality therapy including active exercises, classic massage, cryotherapy and a combination of ultrasound and electrical stimulation on rotator cuff injuries. Fizjoterapia Polska 2007;7:107‐23. [Google Scholar]
Johansson 2005 {published data only}
- Johansson KM, Adolfsson E, Foldevi MO. Effects of acupuncture versus ultrasound in patients with impingement syndrome: randomized clinical trial. Physical Therapy 2005;85:490‐501. [PubMed] [Google Scholar]
Kelle 2014 {published data only}
- Kelle B, Kozanoglu E. Low‐level laser and local corticosteroid injection in the treatment of subacromial impingement syndrome: a controlled clinical trial. Clinical Rehabilitation 2014;28(8):762‐71. [DOI] [PubMed] [Google Scholar]
Kocyigit 2012 {published data only}
- Kocyigit F, Akalin E, Gezer NS, Orbay O, Kocyigit A, Ada E. Functional magnetic resonance imaging of the effects of low‐frequency transcutaneous electrical nerve stimulation on central pain modulation: a double‐blind, placebo‐controlled trial. Clinical Journal of Pain 2012;28(7):581‐8. [DOI] [PubMed] [Google Scholar]
Korkmaz 2010 {published data only}
- Korkmaz OK, Capaci K, Eyigor C, Eyigor S. Pulsed radiofrequency versus conventional transcutaneous electrical nerve stimulation in painful shoulder: a prospective, randomized study. Clinical Rehabilitation 2010;24:1000‐8. [DOI] [PubMed] [Google Scholar]
Kurtai Gursel 2004 {published data only}
- Kurtai Gürsel Y, Ulus Y, Bilgiç A, Dinçer G, Heijden G J. Adding ultrasound in the management of soft tissue disorders of the shoulder: a randomized placebo‐controlled trial. Physical Therapy 2004;84:336‐43. [PubMed] [Google Scholar]
Leduc 2003 {published data only}
- Leduc BE, Caya J, Tremblay S, Bureau NJ, Dumont M. Treatment of calcifying tendinitis of the shoulder by acetic acid iontophoresis: a double‐blind randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2003;84:1523‐7. [DOI] [PubMed] [Google Scholar]
Montes‐Molina 2012a {published data only}
- Montes‐Molina R, Prieto‐Baquero A, Martinez‐Rodriguez M E, Romojaro‐Rodriguez A B, Gallego‐Mendez V, Martinez‐Ruiz F. Interferential laser therapy in the treatment of shoulder pain and disability from musculoskeletal pathologies: a randomised comparative study. Physiotherapy 2012;98:143‐50. [DOI] [PubMed] [Google Scholar]
Montes‐Molina 2012b {published data only}
- Montes‐Molina R, Martinez‐Rodriguez ME, Romojaro‐Rodriguez AB, Martinez‐Ruiz F, Prieto‐Baquero A. Interferential light therapy in the treatment of shoulder tendinopathies: a randomized controlled pilot study. Clinical Rehabilitation 2012;26(12):1114‐22. [DOI] [PubMed] [Google Scholar]
Nykänen 1995 {published data only}
- Nykänen M. Pulsed ultrasound treatment of the painful shoulder a randomized, double‐blind, placebo‐controlled study. Scandinavian Journal of Rehabilitation Medicine 1995;27:105‐8. [PubMed] [Google Scholar]
Otadi 2012 {published data only}
- Otadi K, Hadian MR, Olyaei G, Jalaie S. The beneficial effects of adding low level laser to ultrasound and exercise in Iranian women with shoulder tendonitis: a randomized clinical trial. Journal of Back & Musculoskeletal Rehabilitation 2012;25:13‐9. [DOI] [PubMed] [Google Scholar]
Ozgen 2012 {published data only}
- Ozgen M, Firat S, Sarsan A, Topuz O, Ardic F, Baydemir C. Short‐ and long‐term results of clinical effectiveness of sodium hyaluronate injection in supraspinatus tendinitis. Rheumatology International 2012;32:137‐44. [DOI] [PubMed] [Google Scholar]
Pan 2003 {published data only}
- Pan PJ, Chou CL, Chiou HJ, Ma HL, Lee HC, Chan RC. Extracorporeal shock wave therapy for chronic calcific tendinitis of the shoulders: a functional and sonographic study. Archives of Physical Medicine and Rehabilitation 2003;84:988‐93. [DOI] [PubMed] [Google Scholar]
Perron 1997 {published data only}
- Perron M, Malouin F. Acetic acid iantophoresis and ultrasound for the treatment of calcifying tendinitis of the shoulder: A randomized control trial. Archives Physical Medicine Rehabilitation 1997;78:379‐84. [DOI] [PubMed] [Google Scholar]
Polimeni 2003 {published data only}
- Polimeni V, Panuccio A, Furfari P, Crupi D, Barreca G, Forgione C, et al. Preliminary study on the efficacy of various rehabilitation therapies for shoulder pain. Europa Medicophysica 2003;39:59‐63. [Google Scholar]
Rabini 2012 {published data only}
- Rabini A, Piazzini DB, Bertolini C, Deriu L, Saccomanno MF, Santagada DA, et al. Effects of local microwave diathermy on shoulder pain and function in patients with rotator cuff tendinopathy in comparison to subacromial corticosteroid injections: a single‐blind randomized trial. Journal of Orthopaedic & Sports Physical Therapy 2012;42:363‐70. [DOI] [PubMed] [Google Scholar]
San Segundo 2008 {published data only}
- San Segundo RM, Molins J, Valdés M, Fernández TR. Conservative treatment in shoulder pain. Ultrasounds respect sham insonation. A clinical trial [Tratamiento conservador del síndrome subacromial. Ultrasonidos frente a placebo. Un ensayo clínico]. Rehabilitacion 2008;42:61‐6. [Google Scholar]
Santamato 2009 {published data only}
- Santamato A, Solfrizzi V, Panza F, Tondi G, Frisardi V, Leggin BG, et al. Short‐term effects of high‐intensity laser therapy versus ultrasound therapy in the treatment of people with subacromial impingement syndrome: a randomized clinical trial. Physical Therapy 2009;89:643‐52. [DOI] [PubMed] [Google Scholar]
- Santamato A, Solfrizzi V, Panza F, Tondi G, Frisardi V, Leggin BG, et al. Short‐term effects of high‐intensity laser therapy versus ultrasound therapy in the treatment of people with subacromial impingement syndrome: a randomized clinical trial. [Erratum appears in Phys Ther. 2009 Sep;89(9):999]. Physical Therapy 2009;89:643‐52. [DOI] [PubMed] [Google Scholar]
Saunders 1995 {published data only}
- Saunders L. The efficacy of low level laser therapy in supraspinatus tendinitis. Clinical rehabilitation 1995;9:126‐34. [Google Scholar]
Shehab 2000 {published data only}
- Shehab D, Adham N. Comparative effectiveness of ultrasound and transcutaneous electrical stimulation in treatment of periarticular shoulder pain. Physiotherapy Canada 2000;52:208. [Google Scholar]
Vecchio 1993 {published data only}
- Vecchio P, Cave C, King V, Adebajo AO, Smith M, Hazleman BL. A double–blind study of the effectiveness of low level laser treatment of rotator cuff tendinitis. British Journal of Rheumatology 1993;32:740‐2. [DOI] [PubMed] [Google Scholar]
Yavuz 2014 {published data only}
- Yavuz F, Duman I, Taskaynatan MA, Tan AK. Low‐level laser therapy versus ultrasound therapy in the treatment of subacromial impingement syndrome: A randomized clinical trial. Journal of Back & Musculoskeletal Rehabilitation 2014;27(3):315‐20. [DOI] [PubMed] [Google Scholar]
Yeldan 2009 {published data only}
- Yeldan I, Cetin E, Ozdincler AR. The effectiveness of low‐level laser therapy on shoulder function in subacromial impingement syndrome. Disability and Rehabilitation 2009;31:935‐40. [DOI] [PubMed] [Google Scholar]
Yildirim 2013 {published data only}
- Yildirim MA, Ones K, Celik EC. Comparision of ultrasound therapy of various durations in the treatment of subacromial impingement syndrome. Journal of Physical Therapy Science 2013;25(9):1151‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ainsworth 2007 {published data only}
- Ainsworth R, Dziedzic K, Hiller L, Daniels J, Bruton A, Broadfield J. A prospective double blind placebo‐controlled randomized trial of ultrasound in the physiotherapy treatment of shoulder pain. Rheumatology 2007;46:815‐20. [DOI] [PubMed] [Google Scholar]
Dickens 2005 {published data only}
- Dickens VA, Williams JL, Bhamra MS. Role of physiotherapy in the treatment of subacromial impingement syndrome: a prospective study. Physiotherapy 2005;91:159‐64. [Google Scholar]
Ginn 2005 {published data only}
- Ginn KA, Cohen ML. Exercise therapy for shoulder pain aimed at restoring neuromuscular control: a randomized comparative clinical trial. Journal of Rehabilitation Medicine 2005;37:115‐22. [DOI] [PubMed] [Google Scholar]
Hay 2003 {published data only}
- Hay EM, Thomas E, Paterson SM, Dziedzic K, Croft PR. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Annals of the Rheumatic Diseases 2003;62:394‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrera‐Lasso 1993 {published data only}
- Herrera‐Lasso I, Mobarak L, Fernandez‐Dominguez L, Cardiel MH, Alarcon‐Segovia D. Comparative effectiveness of packages of treatment including ultrasound or transcutaneous electrical nerve stimulation in painful shoulder syndrome. Physiotherapy 1993;79:251‐3. [Google Scholar]
Taverner 2014 {published data only}
- Taverner M, Loughnan T. Transcutaneous pulsed radiofrequency treatment for patients with shoulder pain booked for surgery: a double‐blind, randomized controlled trial. Pain Practice 2014;14(2):101‐8. [DOI] [PubMed] [Google Scholar]
Van der Heijden 1999b {published data only}
- Heijden GJ, Leffers P, Wolters PJ, Verheijden JJ, Mameren H, Houben JP, et al. No effect of bipolar interferential electrotherapy and pulsed ultrasound for soft tissue shoulder disorders: a randomised controlled trial. Annals of the Rheumatic Diseases 1999;58:530‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dal Conte 1990 {published data only}
- Dal Conte G, Rivoltini P, Combi F. Pulsed magnetic field therapy for calcific periarthritis of the shoulder joint. Riabilitazione 1990;23:27‐33. [Google Scholar]
Gudmundsen 1987 {published data only}
- Gudmundsen J, Vikne J. Laser treatment for epicondylitis humeri and rotator cuff syndrome. Nord Tidsskr Idrettsmed 1987;2:6‐15. [Google Scholar]
Güler 2009 {published data only}
- Güler H, Turhanoglu AD, Inanoglu K, Inanoglu D, Özer C. Comparison of ketoprofen phonophoresis with ketoprofen and lidocaine‐ prilocaine phonophoresis in patients with subacromial impingement syndrome. Turkish Journal of Rheumatology 2009;24:88‐93. [Google Scholar]
Jiménez‐García 2008 {published data only}
- Jiménez‐García D, López‐Dolado E, López‐Zarzuela M C. Treatment of calcifying tendinitis of the shoulder: iontophoresis with acetic acid or shortwave?. Rehabilitacion 2008;42:239‐45. [Google Scholar]
Knorre 1990 {published data only}
- Knorre von B, Keitel W. Comparative study of therapy: ultrasound, cryotherapy and intra‐articulare cortisonoids to treat alterations of the shoulder joint due to inflammation [Vergleichende therapiestudie: ultraschall, kryotherapie und intraartikulare kortisonoide bei veranderungen des schultergelenkes aus entzundlicher ursache]. Z. Physiother. Jg. 1990;42:221‐225. [Google Scholar]
Additional references
Alexander 2010
- Alexander LD, Gilman DR, Brown DR, Brown JL, Houghton PE. Exposure to low amounts of ultrasound energy does not improve soft tissue shoulder pathology: a systematic review. Physical Therapy 2010;90(1):14‐25. [DOI] [PubMed] [Google Scholar]
Allen 2006
- Allen RJ. Physical agents used in the management of chronic pain by physical therapists. Physical Medicine and Rehabilitation Clinics of North America 2006;17:315‐45. [DOI] [PubMed] [Google Scholar]
Angst 2011
- Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care and Research 2011;63:S174‐88. [DOI] [PubMed] [Google Scholar]
Basford 1989
- Basford JR. Low‐energy laser therapy: controversies and new research findings. Lasers in Surgery and Medicine 1989;9:1‐5. [DOI] [PubMed] [Google Scholar]
Batheja 2006
- Batheja P, Thakur R, Michniak B. Transdermal iontophoresis. Expert Opinion on Drug Delivery 2006;3:127‐38. [DOI] [PubMed] [Google Scholar]
Beatti 2010
- Beatti A, Raynor A, Souvlis T, Chipchase L. The analgesic effect of interferential therapy on clinical and experimentally induced pain. Physical Therapy Reviews 2010;15:243‐52. [Google Scholar]
Bjordal 2006
- Bjordal JM, Johnson MI, Iverson V, Aimbire F, Lopes‐Martins RAB. Photoradiation in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo controlled trials. Photomedicine and Laser Surgery 2006;24:158‐68. [DOI] [PubMed] [Google Scholar]
Bjordal 2010
- Bjordal JM, Lopes‐Martins RAB, Joensen J, Iversen VV. The anti‐inflammatory mechanism of low level laser therapy and its relevance for clinical use in physiotherapy. Physical Therapy Reviews 2010;15:286‐93. [Google Scholar]
Buchbinder 1996
- Buchbinder R, Goel V, Bombardier C, Hogg‐Johnson S. Classification systems of soft tissue disorders of the neck and upper limb: do they satisfy methodological guidelines?. Journal of Clinical Epidemiology 1996;49(2):141‐9. [DOI] [PubMed] [Google Scholar]
Buchbinder 2003
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2004 [Computer program]
- Dr Chris Cates EBM website. Available from http://www.nntonline.net/. Visual Rx NNT Calculator. Dr Chris Cates EBM website. Available from http://www.nntonline.net/, 2004 (accessed 15 November 2015).
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
DeSantana 2008
- DeSantana J, Walsh DM, Vance C, Rakel B, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports 2008;10:492‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebrahim 2013
- Ebrahim S, Akl EA, Mustafa RA, Sun X, Walter SD, Heels‐Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2013;66(9):1014‐21. [DOI] [PubMed] [Google Scholar]
Edmonds 2014
- Edmonds EW, Dengerink DD. Common conditions in the overhead athlete. American Family Physician 2014;89(7):537‐41. [PubMed] [Google Scholar]
Gebremariam 2014
- Gebremariam L, Hay EM, Sande R, Rinkel WD, Koes BW, Huisstede BM. Subacromial impingement syndrome ‐ effectiveness of physiotherapy and manual therapy. British Journal of Sports Medicine 2014;48(16):1202‐8. [DOI] [PubMed] [Google Scholar]
Gordon 2007
- Gordon GA. Designed electromagnetic pulsed therapy: clinical applications. Journal of Cellular Physiology 2007;212:579–82. [DOI] [PubMed] [Google Scholar]
Hanchard 2013
- Hanchard NC, Lenza M, Handoll HH, Takwoingi Y. Physical tests for shoulder impingements and local lesions of bursa, tendon or labrum that may accompany impingement. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD007427.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawker 2011
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care and Research 2011;63:S240‐52. [DOI] [PubMed] [Google Scholar]
Hermans 2013
- Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma‐Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease?: The Rational Clinical Examination systematic review. JAMA 2013;310(8):837‐47. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors),Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Johnson 2008
- Johnson M. Transcutaneous electrical nerve stimulation. In: Watson T editor(s). Electrotherapy: Evidence based practice. 12th Edition. Edinburgh: Churchill Livingstone, 2008. [Google Scholar]
Jones 2009
- Jones I, Johnson MI. Transcutaneous electrical nerve stimulation. Continuing Education in Anaesthesia, Critical Care & Pain 2009;9:130‐5. [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Kromer 2009
- Kromer TO, Tautenhahn UG, Bie RA, Staal JB, Bastiaenen CHG. Effects of physiotherapy in patients with shoulder impingement syndrome: a systematic review of the literature. Journal of Rehabilitation Medicine 2009;41(11):870‐80. [DOI] [PubMed] [Google Scholar]
Largacha 2006
- Largacha M, Parsons IM, Campbell B, Titelman RM, Smith KL, Matsen F. Deficits in shoulder function and general health associated with sixteen common shoulder diagnoses: A study of 2674 patients. Journal of Shoulder and Elbow Surgery 2006;15(1):30‐9. [DOI] [PubMed] [Google Scholar]
Linsell 2006
- Linsell L, Dawson J, Zondervan K, Rose P, Randall T, Fitzpatrick R, et al. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology 2006;45:215‐21. [DOI] [PubMed] [Google Scholar]
Luime 2004
- Luime JJ, Koes BW, Hendriksen IJM, Burdof A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population: a systematic review. Scandinavian Journal of Rheumatology 2004;33:73‐81. [DOI] [PubMed] [Google Scholar]
Machet 2002
- Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. International Journal of Pharmaceuticals 2002;243:1‐15. [DOI] [PubMed] [Google Scholar]
Markov 2007
- Markov MS. Expanding use of pulsed electromagnetic field therapies. Electromagnetic Biology and Medicine 2007;26:257–74. [DOI] [PubMed] [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971‐9. [DOI] [PubMed] [Google Scholar]
Michaleff 2011
- Michaleff ZA, Costa LO, Moseley AM, Maher CG, Elkins MR, Herbert RD, et al. CENTRAL, PEDro, PubMed, and EMBASE are the most comprehensive databases indexing randomized controlled trials of physical therapy interventions. Physical Therapy 2011;91(2):190‐7. [DOI] [PubMed] [Google Scholar]
Mroz 2014
- Mroz TM, Carlini AR, Archer KR, Wegener ST, Hoolachan JI, Stiers W, et al. Frequency and cost of claims by injury type from a state workers' compensation fund from 1998 through 2008. Archives of Physical Medicine and Rehabilitation 2014;95(6):1048‐54. [DOI] [PubMed] [Google Scholar]
Nyberg 2010
- Nyberg A, Jonsson P, Sundelin G. Limited scientific evidence supports the use of conservative treatment interventions for pain and function in patients with subacromial impingement syndrome: randomized control trials. Physical Therapy Reviews 2010;15(6):436‐52. [Google Scholar]
O'Brien 2007
- O’Brien WD. Ultrasound ‐ biophysics mechanisms. Progress in Biophysics and Molecular Biology 2007;93:212‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostor 2005
- Ostör AJ, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology 2005;44(6):800‐5. [DOI] [PubMed] [Google Scholar]
Page 2013
- Page MJ, McKenzie JE, Forbes A. Many scenarios exist for selective inclusion and reporting of results in randomized trials and systematic reviews. Journal of Clinical Epidemiology 2013;66(5):524‐37. [DOI] [PubMed] [Google Scholar]
Page 2014a
- Page MJ, Green S, Kramer S, Johnston RV, McBain B, Chau M, et al. Manual therapy and exercise for adhesive capsulitis (frozen shoulder). Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2014b
- Page MJ, Green S, Kramer S, Johnston RV, McBain B, Buchbinder R. Electrotherapy modalities for adhesive capsulitis (frozen shoulder). Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2015
- Page MJ, McKenzie JE, Green SE, Beaton DE, Jain NB, Lenza M, et al. Core domain and outcome measurement sets for shoulder pain trials are needed: systematic review of physical therapy trials. Journal of Clinical Epidemiology 2015;68(11):1270‐81. [DOI: 10.1016/j.jclinepi.2015.06.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016
- Page MJ, Green SE, McBain B, Surace S, Deitch J, Lyttle N, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database of Systematic Reviews 2016;6:CD012224. [DOI: 10.1002/14651858.CD012224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peplow 2010
- Peplow PV, Chung T‐Y, Baxter D. Application of low‐level laser technologies for pain relief and wound healing. Physical Therapy Reviews 2010;15:253‐85. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roustit 2014
- Roustit M, Blaise S, Cracowski JL. Trials and tribulations of skin iontophoresis in therapeutics. British Journal of Clinical Pharmacology 2014;77:63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2009
- Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis and Rheumatism 2009;61:623‐32. [DOI] [PubMed] [Google Scholar]
Roy 2010
- Roy JS, MacDermid JC, Woodhouse LJ. A systematic review of the psychometric properties of the Constant‐Murley score. Journal of Shoulder and Elbow Surgery 2010;19:157‐64. [DOI] [PubMed] [Google Scholar]
Sakurai 2000
- Sakurai Y, Yamaguchi M, Abiko Y. Inhibitory effect of low‐level laser irradiation on LPS‐stimulated prostaglandin E2 production and cyclooxygenase‐2 in human gingival fibroblasts. European Journal of Oral Sciences 2000;1081:29‐34. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schellingerhout 2008
- Schellingerhout JM, Verhagen AP, Thomas S, Koes BW. Lack of uniformity in diagnostic labeling of shoulder pain: time for a different approach. Manual Therapy 2008;13:478‐83. [DOI] [PubMed] [Google Scholar]
Schultz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152:Epub 24 March. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shah 2012
- Shah SGS, Farrow A. Trends in the availability and usage of electrophysical agents in physiotherapy practices from 1990 to 2010: a review. Physical Therapy Reviews 2012;17:207‐26. [Google Scholar]
Shields 2001
- Shields N, Gormley J, O'Hare. Short‐wave diathermy: a review of existing clinical trials. Physical Therapy Reviews 2001;6:101‐18. [Google Scholar]
Sluka 2003
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. Journal of Pain 2003;4:109‐21. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Titchener 2014
- Titchener AG, White JJ, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case‐control study. Journal of Shoulder and Elbow Surgery 2014;23:1282‐8. [DOI] [PubMed] [Google Scholar]
Van der Heijden 1999a
- Heijden GJ. Shoulder disorders: a state‐of‐the‐art review. Baillieres Best Practice & Research Clinical Rheumatology 1999;13:287‐309. [Google Scholar]
Virta 2012
- Virta L, Joranger P, Brox J, Eriksson R. Costs of shoulder pain and resource use in primary health care: a cost‐of‐illness study in Sweden. BMC Musculoskeletal Disorders 2012;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2012
- Walker H, Gabbe B, Wajswelner H, Blanch P, Bennell K. Shoulder pain in swimmers: a 12‐month prospective cohort study of incidence and risk factors. Physical Therapy in Sport 2012;13(4):243‐9. [DOI] [PubMed] [Google Scholar]
Walsh 2009
- Walsh DM, Howe TE, Johnson MI, Moran F, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006142.pub2] [DOI] [PubMed] [Google Scholar]
Watson 2008a
- Watson T. Electrotherapy: Evidence based practice. 12th Edition. Edinburgh: Churchill Livingstone, 2008. [Google Scholar]
Watson 2008b
- Watson T. Ultrasound in contemporary physiotherapy practice. Ultrasonics 2008;48:321‐9. [DOI] [PubMed] [Google Scholar]
Watson 2010
- Watson T. Key concepts with electrophysical agents. Physical Therapy Reviews 2010;15:351‐9. [Google Scholar]
Whittle 2015
- Whittle S, Buchbinder R. Rotator cuff disease. Annals of Internal Medicine 2015;162(1):ITC1. [DOI: 10.7326/AITC201501060] [DOI] [PubMed] [Google Scholar]
Yamamoto 2010
- Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, et al. Prevalence and risk factors of a rotator cuff tear in the general population. Journal of Shoulder and Elbow Surgery 2010;19(1):116‐20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Green 1998
- Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment and efficacy. BMJ 1998;316:354‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2003
- Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004258] [DOI] [PMC free article] [PubMed] [Google Scholar]


