Abstract
Background
Management of rotator cuff disease often includes manual therapy and exercise, usually delivered together as components of a physical therapy intervention. This review is one of a series of reviews that form an update of the Cochrane review, 'Physiotherapy interventions for shoulder pain'.
Objectives
To synthesise available evidence regarding the benefits and harms of manual therapy and exercise, alone or in combination, for the treatment of people with rotator cuff disease.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 3), Ovid MEDLINE (January 1966 to March 2015), Ovid EMBASE (January 1980 to March 2015), CINAHL Plus (EBSCO, January 1937 to March 2015), ClinicalTrials.gov and the WHO ICTRP clinical trials registries up to March 2015, unrestricted by language, and reviewed the reference lists of review articles and retrieved trials, to identify potentially relevant trials.
Selection criteria
We included randomised and quasi‐randomised trials, including adults with rotator cuff disease, and comparing any manual therapy or exercise intervention with placebo, no intervention, a different type of manual therapy or exercise or any other intervention (e.g. glucocorticoid injection). Interventions included mobilisation, manipulation and supervised or home exercises. Trials investigating the primary or add‐on effect of manual therapy and exercise were the main comparisons of interest. Main outcomes of interest were overall pain, function, pain on motion, patient‐reported global assessment of treatment success, quality of life and the number of participants experiencing adverse events.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted the data, performed a risk of bias assessment and assessed the quality of the body of evidence for the main outcomes using the GRADE approach.
Main results
We included 60 trials (3620 participants), although only 10 addressed the main comparisons of interest. Overall risk of bias was low in three, unclear in 14 and high in 43 trials. We were unable to perform any meta‐analyses because of clinical heterogeneity or incomplete outcome reporting. One trial compared manual therapy and exercise with placebo (inactive ultrasound therapy) in 120 participants with chronic rotator cuff disease (high quality evidence). At 22 weeks, the mean change in overall pain with placebo was 17.3 points on a 100‐point scale, and 24.8 points with manual therapy and exercise (adjusted mean difference (MD) 6.8 points, 95% confidence interval (CI) ‐0.70 to 14.30 points; absolute risk difference 7%, 1% fewer to 14% more). Mean change in function with placebo was 15.6 points on a 100‐point scale, and 22.4 points with manual therapy and exercise (adjusted MD 7.1 points, 95% CI 0.30 to 13.90 points; absolute risk difference 7%, 1% to 14% more). Fifty‐seven per cent (31/54) of participants reported treatment success with manual therapy and exercise compared with 41% (24/58) of participants receiving placebo (risk ratio (RR) 1.39, 95% CI 0.94 to 2.03; absolute risk difference 16% (2% fewer to 34% more). Thirty‐one per cent (17/55) of participants reported adverse events with manual therapy and exercise compared with 8% (5/61) of participants receiving placebo (RR 3.77, 95% CI 1.49 to 9.54; absolute risk difference 23% (9% to 37% more). However adverse events were mild (short‐term pain following treatment).
Five trials (low quality evidence) found no important differences between manual therapy and exercise compared with glucocorticoid injection with respect to overall pain, function, active shoulder abduction and quality of life from four weeks up to 12 months. However, global treatment success was more common up to 11 weeks in people receiving glucocorticoid injection (low quality evidence). One trial (low quality evidence) showed no important differences between manual therapy and exercise and arthroscopic subacromial decompression with respect to overall pain, function, active range of motion and strength at six and 12 months, or global treatment success at four to eight years. One trial (low quality evidence) found that manual therapy and exercise may not be as effective as acupuncture plus dietary counselling and Phlogenzym supplement with respect to overall pain, function, active shoulder abduction and quality life at 12 weeks. We are uncertain whether manual therapy and exercise improves function more than oral non‐steroidal anti‐inflammatory drugs (NSAID), or whether combining manual therapy and exercise with glucocorticoid injection provides additional benefit in function over glucocorticoid injection alone, because of the very low quality evidence in these two trials.
Fifty‐two trials investigated effects of manual therapy alone or exercise alone, and the evidence was mostly very low quality. There was little or no difference in patient‐important outcomes between manual therapy alone and placebo, no treatment, therapeutic ultrasound and kinesiotaping, although manual therapy alone was less effective than glucocorticoid injection. Exercise alone led to less improvement in overall pain, but not function, when compared with surgical repair for rotator cuff tear. There was little or no difference in patient‐important outcomes between exercise alone and placebo, radial extracorporeal shockwave treatment, glucocorticoid injection, arthroscopic subacromial decompression and functional brace. Further, manual therapy or exercise provided few or no additional benefits when combined with other physical therapy interventions, and one type of manual therapy or exercise was rarely more effective than another.
Authors' conclusions
Despite identifying 60 eligible trials, only one trial compared a combination of manual therapy and exercise reflective of common current practice to placebo. We judged it to be of high quality and found no clinically important differences between groups in any outcome. Effects of manual therapy and exercise may be similar to those of glucocorticoid injection and arthroscopic subacromial decompression, but this is based on low quality evidence. Adverse events associated with manual therapy and exercise are relatively more frequent than placebo but mild in nature. Novel combinations of manual therapy and exercise should be compared with a realistic placebo in future trials. Further trials of manual therapy alone or exercise alone for rotator cuff disease should be based upon a strong rationale and consideration of whether or not they would alter the conclusions of this review.
Keywords: Adult, Humans, Male, Middle Aged, Musculoskeletal Manipulations, Rotator Cuff, Exercise Therapy, Exercise Therapy/methods, Muscular Diseases, Muscular Diseases/therapy, Randomized Controlled Trials as Topic, Shoulder Pain, Shoulder Pain/etiology, Shoulder Pain/therapy
Plain language summary
Manual therapy and exercise for rotator cuff disease
Background
Rotator cuff disease is a common cause of shoulder pain. People with rotator cuff disease often describe their pain as being worse at night and exacerbated by movement in specific directions including overhead activity. It is often associated with loss of function and some people describe weakness.
Manual therapy comprises movement of the joints and other structures by a healthcare professional (e.g. physiotherapist). Exercise includes any purposeful movement of a joint, muscle contraction or prescribed activity. The aims of both treatments are to relieve pain, increase strength and joint range, and improve function.
Study characteristics
This summary of an updated Cochrane review presents what we know from research about the benefits and harms of manual therapy and exercise compared with placebo, no intervention or any other intervention in people with rotator cuff disease. After searching for all relevant studies published up to March 2015, we included 60 trials (3620 participants), however only 10 looked at manual therapy and exercise in combination. Among the included participants, 52% were women, average age was 51 years and average duration of the condition was 11 months. The average duration of manual therapy and exercise interventions was six weeks.
Key results: one trial of manual therapy and exercise compared with placebo (inactive ultrasound therapy) for 10 weeks in people with chronic rotator cuff disease
Overall pain (higher scores mean more improvement in pain reduction)
People who had manual therapy and exercise had improvements in pain that were little or no different to people who had placebo. Improvement in pain was 6.8 points more (ranging from 0.7 points less to 14.3 points more) at 22 weeks (7% absolute improvement).
People who had manual therapy and exercise rated their change in pain score as 24.8 points on a scale of 0 to 100 points.
People who had placebo rated their change in pain score as 17.3 points on a scale of 0 to 100 points.
Function (higher scores mean more improvement in function)
People who had manual therapy and exercise improved slightly more than people who had placebo. Improvement in function was 7.1 points more (ranging from 0.3 to 13.9 points more) at 22 weeks (7% absolute improvement).
People who had manual therapy and exercise rated their change in function as 22.4 points on a scale of 0 to 100 points.
People who had placebo rated their change in function as 15.6 points on a scale of 0 to 100 points.
Treatment success
16 more people out of 100 rated their treatment as successful with manual therapy and exercise compared with placebo, 16% absolute improvement (ranging from 2% less to 34% more improvement).
Fifty‐seven out of 100 people reported treatment success with manual therapy and exercise.
Forty‐one out of 100 people reported treatment success with placebo.
Side effects
23 more people out of 100 people had minor side effects such as temporary pain after treatment with manual therapy and exercise compared with placebo.
Thirty‐one out of 100 people reported side effects with manual therapy and exercise.
Eight out of 100 people reported side effects with placebo.
Quality of the evidence
High quality evidence from one trial suggested that manual therapy and exercise improved function only slightly more than placebo at 22 weeks, was little or no different to placebo in terms of other patient‐important outcomes (e.g. overall pain), and was associated with relatively more frequent but mild adverse events.
Low quality evidence suggested that there may be little or no difference in overall pain and function when manual therapy and exercise is compared with glucocorticoid injection, there may be little or no difference in overall pain and function when manual therapy and exercise is compared with arthroscopic subacromial decompression, and people who receive acupuncture plus dietary counselling and Phlogenzym supplement may have less pain and better function than people receiving manual therapy and exercise.
We are uncertain whether firstly, manual therapy and exercise improves function more than oral non‐steroidal anti‐inflammatory drugs (NSAID), and secondly, combining manual therapy and exercise with glucocorticoid injection provides additional improvement in function over glucocorticoid injection alone, because the quality of the evidence was very low.
Summary of findings
Summary of findings for the main comparison. Manual therapy and exercise compared to placebo for rotator cuff disease.
| Manual therapy and exercise compared to placebo for rotator cuff disease | ||||||
| Patient or population: rotator cuff disease Settings: Public hospital physiotherapy units and private physiotherapy practices, Australia Intervention: soft tissue massage, glenohumeral joint mobilisation, thoracic spine mobilisation, cervical spine mobilisation, scapular retraining, postural taping and supervised exercises in 10 sessions over 10 weeks along with home exercises for 22 weeks Comparison: inactive ultrasound therapy and application of an inert gel in 10 sessions over 10 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | manual therapy and exercise | |||||
|
Overall pain Assessed with SPADI pain score Scale from 0‐100 (higher score denotes less pain) Follow‐up: 22 weeks |
The mean improvement in overall pain score in the control group was 17.31 | The mean improvement in overall pain score in the intervention group was 6.8 points higher (‐0.7 lower to 14.3 higher) | ‐ | 120 (1 RCT) | ⊕⊕⊕⊕ HIGH | Absolute risk difference 7% (1% fewer to 14% more); relative percentage change 14% (1% fewer to 30% more) NNTB not applicable |
|
Function Assessed with SPADI total score Scale from 0‐100 (higher score denotes greater function) Follow‐up: 22 weeks |
The mean improvement in function score in the control group was 15.61 | The mean improvement in function score in the intervention group was 7.1 points higher (0.3 higher to 13.9 higher) | ‐ | 120 (1 RCT) | ⊕⊕⊕⊕ HIGH | Absolute risk difference 7% (1% to 14% more); relative percentage change 16% (1% to 32% more) NNTB 6 (3 to 103) |
|
Pain on motion Assessed with VAS Scale from 0‐10 (higher score denotes less pain) Follow‐up: 22 weeks |
The mean improvement in pain on motion score in the control group was 1.61 | The mean improvement in pain on motion score in the intervention group was 0.9 points higher (‐0.03 lower to 1.7 higher) | ‐ | 120 (1 RCT) | ⊕⊕⊕⊕ HIGH | Absolute risk difference 9% (1% to 17% more); relative percentage change 18% (1% fewer to 35% more) NNTB not applicable |
|
Global assessment of treatment success Follow‐up: 22 weeks |
Study population | RR 1.39 (0.94 to 2.03) | 112 (1 RCT) | ⊕⊕⊕⊕ HIGH | Absolute risk difference 16% (2% fewer to 34% more); relative percentage change 39% (6% fewer to 103% more) NNTB not applicable |
|
| 414 per 10002 | 575 per 1000 (393 to 840) | |||||
|
Quality of life Assessed with AQoL Scale from ‐0.4 to 1 (higher score denotes higher quality of life) Follow‐up: 22 weeks |
The mean improvement in quality of life score in the control group was 01 | The mean improvement in quality of life score in the intervention group was 0.07 points higher (0.04 higher to 0.1 higher) | ‐ | 120 (1 RCT) | ⊕⊕⊕⊕ HIGH | Absolute risk difference 5% (3% to 7% more); relative percentage change 10% (5% to 14% more) NNTB not applicable |
|
Adverse events Follow‐up: 11 weeks |
Study population | RR 3.77 (1.49 to 9.54) | 116 (1 RCT) | ⊕⊕⊕⊕ HIGH | Absolute risk difference 23% (9% to 37% more); relative percentage change 277% (49% to 854% more) NNTH 5 (26 to 2) Adverse events were mild, including short‐term pain during or after treatment in the clinic, short‐term pain after home exercises, or mild irritation with taping. |
|
| 82 per 10002 | 309 per 1000 (122 to 782) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
This table summarises data from the Bennell 2010 trial.
1Mean score in the placebo group in Bennell 2010 used as the assumed control group mean.
2Risk in placebo group in Bennell 2010 used as assumed risk
Summary of findings 2. Manual therapy and exercise compared to glucocorticoid injection for rotator cuff disease.
| Manual therapy and exercise compared to glucocorticoid injection for rotator cuff disease | ||||||
| Patient or population: rotator cuff disease Settings: Military hospital–based outpatient clinic, USA; Primary care (general practitioner), UK Intervention: Either joint and soft tissue mobilisation, manual stretches and supervised and home exercises twice a week for three weeks or active and passive mobilisation, home exercises and therapeutic ultrasound once a week for six weeks Comparison: glucocorticoid injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Glucocorticoid injection | manual therapy and exercise | |||||
|
Overall pain Assessed with NRS Scale from 0‐10 (lower score denotes less pain) Follow‐up: 1 month |
The mean overall pain score in the control group was 1.71 | The mean overall pain score in the intervention group was 0.1 points lower (0.92 lower to 0.72 higher) | ‐ | 88 (1 RCT) | ⊕⊕⊝⊝ LOW2 | Absolute risk difference 1% (9% fewer to 7% more); relative percentage change 3% (28% fewer to 22% more) NNTB not applicable |
|
Function Assessed with SPADI total score Scale from 0‐100 (lower score denotes greater function) Follow‐up: 1 month |
The mean function score in the control group was 23.21 | The mean function score in the intervention group was 1 point lower (8.77 lower to 6.77 higher) | ‐ | 88 (1 RCT) | ⊕⊕⊝⊝ LOW2 | Absolute risk difference 1% (9% fewer to 7% more); relative percentage change 2% (19% fewer to 15% more) NNTB not applicable |
| Pain on motion | See Comments column | See Comments column | ‐ | ‐ | ‐ | Outcome not measured |
|
Global assessment of treatment success Follow‐up: 6 weeks |
Study population | RR 0.33 (0.14 to 0.79) | 198 (1 RCT) | ⊕⊕⊝⊝ LOW2,4 | Absolute risk difference 12% (21% to 3% fewer); relative percentage change 67% (86% to 21% fewer) NNTB 9 (7 to 26) |
|
| 184 per 10003 | 61 per 1000 (26 to 145) | |||||
|
Quality of life Assessed with Global Rating of Change Scale Scale from ‐7 to 7 (higher score denotes higher quality of life) Follow‐up: 1 month |
The mean quality of life score in the control group was 31 | The mean quality of life score in the intervention group was no different (1.37 lower to 1.37 higher) | ‐ | 88 (1 RCT) | ⊕⊕⊝⊝ LOW2 | Absolute risk difference 0% (10% fewer to 10% more); relative percentage change 0% (46% fewer to 46% more) NNTB not applicable |
|
Adverse events Follow‐up: 12 months |
Study population | not estimable | 94 (1 RCT) | ⊕⊕⊝⊝ LOW2 | "Other than transient pain from the CSI [injection], there were no other adverse events reported by patients in either group." | |
| 0 per 10005 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
This table summarises data from the Rhon 2014 and Hay 2003 trials.
1Mean score glucocorticoid injection group in Rhon 2014 used as assumed control group mean
2Downgraded (‐2) for risk of bias. Participants could not be blinded (risk of performance bias and detection bias)
3Risk in glucocorticoid injection group in Hay 2003 used as assumed risk
4Downgraded (‐1) for indirectness. Only 75% of participants had rotator cuff disease (25% had adhesive capsulitis)
5Risk in glucocorticoid injection group in Rhon 2014 used as assumed risk
Summary of findings 3. Manual therapy and exercise compared to arthroscopic subacromial decompression for rotator cuff disease.
| Manual therapy and exercise compared to arthroscopic subacromial decompression for rotator cuff disease | ||||||
| Patient or population: rotator cuff disease Settings: Hospital, Ringkjoebing County, Denmark Intervention: 12 weeks of manual therapy (soft tissue treatment) plus supervised exercises (stabilising and strengthening) Comparison: arthroscopic subacromial decompression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Arthroscopic subacromial decompression | Manual therapy and exercise | |||||
|
Overall pain Assessed with Constant‐Murley pain score Scale from 0‐15 (higher score denotes less pain) Follow‐up: 6 months |
The mean improvement in overall pain score in the control group was 3.81 | The mean improvement in overall pain score in the intervention group was 0.1 lower (1.68 lower to 1.48 higher) | ‐ | 84 (1 RCT) | ⊕⊕⊝⊝ LOW2 | Absolute risk difference 1% (11% fewer to 10% more); relative percentage change 2% (40% fewer to 35% more) NNTB not applicable |
|
Function Assessed with Constant‐Murley total score Scale from 0‐100 (higher score denotes greater function) Follow‐up: 6 months |
The mean improvement in function score in the control group was 19.91 | The mean improvement in function score in the intervention group was 1.4 points higher (7.63 lower to 10.43 higher) | ‐ | 84 (1 RCT) | ⊕⊕⊝⊝ LOW2 | Absolute risk difference 1% (7% fewer to 10% more); relative percentage change 4% (23% fewer to 31% more) NNTB not applicable |
| Pain on motion | See Comments column | See Comments column | ‐ | ‐ | ‐ | Outcome not measured |
|
Global assessment of treatment success Follow‐up: 4‐8 years |
Study population | RR 1.14 (0.82 to 1.61) | 79 (1 RCT) | ⊕⊕⊝⊝ LOW2 | Absolute risk difference 9% (13% fewer to 30% more); relative percentage change 14% (18% fewer to 61% more) | |
| 590 per 10003 | 673 per 1000 (484 to 950) | |||||
| Quality of life | See Comments column | See Comments column | ‐ | ‐ | ‐ | Outcome not measured |
| Adverse events | See Comments column | See Comments column | ‐ | ‐ | ‐ | Outcome not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
This table summarises data from the Haahr 2005 trial.
1Mean score in arthroscopic subacromial decompression group in Haahr 2005 used as assumed control group risk
2Downgraded (‐2) for risk of bias. Participants were not blinded (risk of performance and detection bias)
3Risk in arthroscopic subacromial decompression group in Haahr 2005 used as assumed risk
Background
Description of the condition
This review is one in a series of reviews aiming to determine the evidence for efficacy of common interventions for shoulder pain. This series of reviews form the update of an earlier Cochrane review of physical therapy for shoulder disorders (Green 2003). Since our original review, many new clinical trials studying a diverse range of interventions have been performed. To improve usability of the review, we have subdivided the reviews by type of shoulder disorder, as patients within different diagnostic groupings may respond variably to different interventions. This review focuses on manual therapy and exercise interventions alone or in combination for rotator cuff disease. A separate review of electrotherapy modalities for rotator cuff disease is underway. Reviews of manual therapy and exercise for adhesive capsulitis (frozen shoulder) (Page 2014a) and electrotherapy modalities for adhesive capsulitis (Page 2014b) were published in 2014.
Shoulder pain is common, with a point prevalence ranging from 7% to 26% in the general population (Luime 2004). Although not life‐threatening, it impacts on the performance of tasks essential to daily living (such as dressing, personal hygiene, eating and work), and often results in substantial utilisation of healthcare resources (Largacha 2006; Mroz 2014; Van der Heijden 1999; Virta 2012). The most common cause of shoulder pain in primary care is disorders of the rotator cuff (Linsell 2006; Ostör 2005), which comprises the supraspinatus, infraspinatus, subscapularis and teres minor muscles. These muscles facilitate both movement and dynamic stabilisation of the shoulder joint (Whittle 2015).
Numerous diagnostic labels have been used in the literature to describe disorders of the rotator cuff (for example, subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full rotator cuff tear, calcific tendinitis and subacromial bursitis) but the terms are not standardised (Schellingerhout 2008). The term 'rotator cuff disease' was proposed as an umbrella term to classify disorders of the rotator cuff regardless of the cause of disorder (e.g. degeneration or acute injury) and specific anatomical location (Buchbinder 1996; Whittle 2015).
People with rotator cuff disease often describe their shoulder pain as being worse at night and exacerbated by overhead activity, and some describe weakness or loss of function; however, there are few data regarding the diagnostic accuracy of individual symptoms in rotator cuff disease without tears (Whittle 2015). In addition to history‐taking and clinical evaluation, the use of physical examination manoeuvres has been recommended for the diagnosis of rotator cuff disease. A systematic review of diagnostic test accuracy studies found that a positive painful arc test result and a positive external rotation resistance test result were the most accurate findings for detecting rotator cuff disease, whereas the presence of a positive lag test result (external or internal rotation) was most accurate for diagnosis of a full‐thickness rotator cuff tear (Hermans 2013).
Rotator cuff disease has been found to increase in prevalence with age (Yamamoto 2010) and in those participating in occupational or sporting activities (e.g. swimming, tennis) that require repetitive overhead use of the arms (Edmonds 2014; Walker 2012). The condition is often self‐limiting (Reilingh 2008; Whittle 2015), though 14% of patients, particularly the elderly, have been found to continue consulting their GP for shoulder pain beyond two years after initial presentation (Linsell 2006).
Description of the intervention
Manual therapy and exercise, usually delivered together as components of a physical therapy intervention, are commonly used in the management of rotator cuff disease (Whittle 2015). Manual therapy includes any clinician‐applied movement of the joints and other structures, for example mobilisation (of which several types exist, e.g. Kaltenborn 1976; Maitland 1977) or manipulation. Exercise includes any purposeful movement of a joint, muscle contraction or prescribed activity, which may be performed under the supervision of a clinician or unsupervised at home. Commonly prescribed exercises include range of motion (ROM), stretching, stabilising and strengthening (Dewhurst 2010).
Manual therapy and exercise are delivered by various clinicians, including physiotherapists, physical therapists, chiropractors, and osteopaths. The aims of both types of interventions are to improve function, promote healing, increase joint range, strengthen weakened muscles and correct imbalance in the stabilising function of the rotator cuff (Brantingham 2011; Kelly 2010; Kuhn 2009). In practice, people with rotator cuff disease seldom receive a single intervention in isolation (i.e. manual therapy alone or exercise alone) (Dziedzic 1999; Glazier 1998; Kooijman 2013; Roberts 2014). Often, electrotherapy modalities (e.g. therapeutic ultrasound, laser therapy) are also delivered as part of a multimodal physical therapy intervention (Kooijman 2013; Struyf 2012), and manual therapy and exercise may also be used in conjunction with other interventions such as non‐steroidal anti‐inflammatory drugs (NSAIDs) or glucocorticoid injection, or both.
How the intervention might work
Manual therapy and exercise interventions are hypothesised to produce a number of beneficial physiological and biomechanical effects. Manual therapy is employed to reduce pain by stimulating peripheral mechanoreceptors and inhibiting nociceptors, and to increase joint mobility by enhancing exchange between synovial fluid and cartilage matrix (Bialosky 2009). Exercise aims to improve muscle function and range of motion by restoring shoulder mobility, proprioception and stability (Kay 2012).
When delivered together, it is unclear whether the effects of manual therapy with exercise represent the effects of manual therapy, the effects of exercise, or an interaction between the two. It has been suggested that the short‐term analgesic effects of manual therapy may allow people with other musculoskeletal conditions (e.g. neck pain) to perform exercises designed to produce long‐term changes in muscle function and range of motion (Miller 2010; Miller 2014). A similar mechanism of action may occur in people with rotator cuff disease.
Why it is important to do this review
The previous version of this review (Green 2003) included four trials investigating the efficacy of manual therapy or exercise (or both) for rotator cuff disease (Bang 2000; Brox 1993; Conroy 1998; Winters 1997), and concluded that firstly, exercise alone was more effective than placebo and secondly, mobilisation was an effective add‐on to exercise for people with this condition. However, it was unclear whether manual therapy alone, or manual therapy and exercise, were effective. Many new trials have been published since the 2003 review (as summarised in recent systematic reviews, including Brantingham 2011, Braun 2013, Gebremariam 2014, Hanratty 2012, Littlewood 2012 and Van den Dolder 2014). To best inform current practice, an up‐to‐date review which incorporates the most recently available evidence is needed.
Objectives
To synthesise available evidence regarding the benefits and harms of manual therapy and exercise, alone or in combination, for the treatment of people with rotator cuff disease.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any design (e.g. parallel, cross‐over, factorial) and controlled clinical trials using a quasi‐randomised method of allocation, such as by alternation or date of birth. Reports of trials were eligible regardless of the language or date of publication.
Types of participants
We included trials that recruited adults (> 16 years of age) with rotator cuff disease, as defined by the authors (e.g. using terminology such as subacromial impingement syndrome, rotator cuff tendonitis or tendinopathy, supraspinatus, infraspinatus or subscapularis tendonitis, subacromial bursitis, or rotator cuff tears), for any duration.
We also included trials with participants with unspecified shoulder pain provided that the inclusion/exclusion criteria were compatible with a diagnosis of rotator cuff disease. If trials included participants with either rotator cuff disease or adhesive capsulitis, we attempted to retrieve the data for rotator cuff disease participants from the trialists; if unsuccessful, we included the trial only if more than 75% of participants had rotator cuff disease.
We excluded trials that included any participants with a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulders, or pain in the shoulder region as part of a complex myofascial neck/shoulder/arm pain condition.
Types of interventions
We included trials comparing any manual therapy or exercise intervention to placebo, no treatment, a different type of manual therapy or exercise, or another active intervention (e.g. glucocorticoid injection). Trials evaluating the primary or add‐on effects of manual therapy and exercise, manual therapy alone, and exercise alone were eligible.
Eligible manual therapy interventions included mobilisation, manipulation and massage. Eligible exercise interventions included supervised or home exercises, which could be land‐based or water‐based, but had to comprise tailored shoulder exercises rather than just general activity, for example, swimming.
We excluded trials primarily evaluating the effect of electrotherapy modalities such as therapeutic ultrasound, laser therapy, transcutaneous electrical nerve stimulation (TENS), pulsed electromagnetic field therapy, interferential current, phonophoresis, iontophoresis, or short wave diathermy. Electrotherapy modalities for rotator cuff disease have been analysed in a separate Cochrane review.
Types of outcome measures
We did not consider outcomes as part of the eligibility criteria.
Main outcomes
Overall pain (mean or mean change measured by visual analogue scale (VAS), numerical or categorical rating scale).
-
Function. Where trialists reported outcome data for more than one function scale, we extracted data on the scale that was highest on the following a priori defined list:
Shoulder Pain and Disability Index (SPADI) (Roach 1991);
Croft Shoulder Disability Questionnaire (Croft 1994);
Constant‐Murley Score (Constant 1987);
any other shoulder‐specific function scale.
Pain on motion measured by VAS, numerical or categorical rating scale.
Global assessment of treatment success as defined by the trialists (e.g. proportion of participants with significant overall improvement).
Quality of life as measured by generic measures (such as components of the Short Form‐36 (SF‐36)) or disease‐specific tools).
Number of participants experiencing an adverse event in the trial (however defined by the authors).
Other outcomes
Night pain measured by VAS, numerical or categorical rating scale.
Pain with resisted movement measured by VAS, numerical or categorical rating scale.
Range of motion (ROM) (e.g. flexion, abduction, external rotation and internal rotation (measured in degrees or other e.g. hand‐behind‐back distance in centimetres)). Where trialists reported outcome data for both active and passive ROM measures, we extracted the data on active ROM only.
Strength.
Work disability.
Surgery (e.g. surgical decompression, rotator cuff repair).
We extracted efficacy outcome measures (e.g. function or overall pain) at the following time points:
up to three weeks;
longer than three and up to six weeks (this was the main time point);
longer than six weeks and up to six months, and;
longer than six months.
If data were available in a trial at multiple time points within each of the above periods (e.g. at four, five, and six weeks), we only extracted data at the latest possible time point of each period.
We extracted adverse events reported at all time points.
We collated the main results of the review into 'Summary of findings' (SoF) tables which provide key information concerning the quality of evidence and the magnitude and precision of the effect of the interventions. We included the main outcomes (see above) in the SoF tables, with results at, or nearest, the main time point (six weeks) presented.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2015, Issue 3), Ovid MEDLINE (January 1966 to March 2015), Ovid EMBASE (January 1980 to March 2015), and CINAHL Plus (EBSCO, January 1937 to March 2015). The complete search strategies are presented in Appendix 1. Note that the search terms used also included clinical terms relevant to adhesive capsulitis and electrotherapy interventions as the current review and Cochrane reviews of electrotherapy modalities for rotator cuff disease, manual therapy and exercise for adhesive capsulitis, and electrotherapy modalities for adhesive capsulitis, were conducted simultaneously.
Searching other resources
We searched for ongoing trials and protocols of published trials in the clinical trials registry that is maintained by the US National Institute of Health (http://clinicaltrials.gov) and the Clinical Trial Registry at the International Clinical Trials Registry Platform of the World Health Organization (http://www.who.int/ictrp/en/). We also reviewed the reference lists of the included trials and any relevant review articles retrieved from the electronic searches, to identify any other potentially relevant trials.
Data collection and analysis
Selection of studies
Two review authors (MJP and BM) independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups.
Possibly relevant: trials that met the inclusion criteria and trials from which it was not possible to determine whether they met the criteria either from their title or abstract.
Excluded: those clearly not meeting the inclusion criteria.
If a title or abstract suggested that the trial was eligible for inclusion, or we could not tell, we obtained a full‐text version of the article and two review authors (MJP and BM) independently assessed it to determine whether it met the inclusion criteria. The review authors resolved discrepancies through discussion or adjudication by a third author (SG or RB).
Data extraction and management
Pairs of review authors (MJP, BM, SS, JD, NL and MM) independently extracted data using a standard data extraction form developed for this review. The authors resolved any discrepancies through discussion or adjudication by a third author (SG or RB), until consensus was reached. We pilot tested the data extraction form and modified it accordingly before use. In addition to items for assessing risk of bias and numerical outcome data, we also recorded the following characteristics.
Trial characteristics, including type (e.g. parallel or cross‐over), country, source of funding, and trial‐registration status (with registration number recorded if available).
Participant characteristics, including age, sex, duration of symptoms, and inclusion/exclusion criteria.
Intervention characteristics, including type of manual therapy or exercise, duration of treatment, use of co‐interventions.
Outcomes reported, including the measurement instrument used and timing of outcome assessment.
One author (MJP) compiled all comparisons and entered outcome data into Review Manager (RevMan) 5.3 (RevMan 2014).
For a particular systematic review outcome there may be multiple results available in the trial reports (e.g. from multiple scales, time points and analyses). To prevent selective inclusion of data based on the results (Page 2013), we used the following a priori‐defined decision rules to select data from trials.
Where trialists reported analysis of covariance‐ (ANCOVA) adjusted mean differences along with either final values and change from baseline values for the same continuous outcome, we extracted ANCOVA‐adjusted mean differences.
Where trialists reported final values and change from baseline values for the same continuous outcomes, we extracted final values.
Where trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data;
For cross‐over RCTs, we extracted data from the first period only.
Assessment of risk of bias in included studies
Pairs of review authors (MJP, BM, SS, JD, NL and MM) independently assessed the risk of bias in included trials using The Cochrane Collaboration's tool for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment (assessed separately for self‐reported and objectively assessed outcomes).
Incomplete outcome data.
Selective reporting.
Other sources of bias (for example, baseline imbalance).
We rated each item as being at 'low risk', 'unclear risk' or 'high risk' of bias. We classified the overall risk of bias as low if all domains were at low risk of bias, as high if at least one domain was at high risk of bias, or as unclear if at least one domain was at unclear risk of bias and no domain was at high risk. We assessed the selective reporting domain for all trials, and documented it in the risk of bias tables, but did not consider it in the overall risk of bias judgement if the only types of selective reporting identified were non‐ or partial reporting of outcomes. Non‐ or partial reporting of outcomes biases the results of meta‐analyses that cannot include the relevant data, not the results of trials, and is therefore considered under the Assessment of reporting biases section (Kirkham 2010). We resolved any discrepancies through discussion or adjudication by a third author (SG or RB).
Measures of treatment effect
We used the Cochrane Collaboration statistical software, Review Manager 5.3, (RevMan 2014) to perform data analysis. We expressed dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous outcomes as mean differences (MDs) with 95% CIs if different trials used the same measurement instrument to measure the same outcome. Alternatively, we analysed continuous outcomes using the standardised mean difference (SMD) when trials measured the same outcome but employed different measurement instruments. To enhance interpretability of dichotomous outcomes, we calculated risk differences and number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH).
Unit of analysis issues
The unit of analysis was the participant. No trials included participants with bilateral shoulder pain.
Dealing with missing data
When required, we contacted trialists via email (twice, separated by three weeks) to retrieve missing information about trial design, outcome data, or attrition rates such as drop‐outs, losses to follow‐up and post‐randomisation exclusions in the included trials. For continuous outcomes with no standard deviation (SD) reported, we calculated SDs from standard errors (SEs), 95% CIs or P values. If no measures of variation were reported and SDs could not be calculated, we planned to impute SDs from other trials in the same meta‐analysis, using the median of the other SDs available (Ebrahim 2013). We have reported in the tables of Characteristics of included studies where outcome data were imputed.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials. We assessed statistical heterogeneity using the Chi2 statistic and the I2 statistic (Higgins 2002). We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% may not be important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess small study effects, we planned to generate funnel plots for meta‐analyses including at least 10 trials of varying size. If asymmetry in the funnel plot was detected, we planned to review the characteristics of the trials to assess whether the asymmetry was likely due to publication bias or other factors such as methodological or clinical heterogeneity of the trials (Sterne 2011). To assess outcome reporting bias (non‐ or partial reporting of a pre‐specified outcome, which prevents the inclusion of data in a meta‐analysis), we compared the outcomes specified in trial protocols with the outcomes reported in the corresponding trial publications; if trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the trial publications (Dwan 2011; Kirkham 2010).
Data synthesis
For this review update, we identified a large number of trials, which studied a diverse range of interventions. To define the most clinically important questions to be answered in the review, after completing data extraction, one review author (MJP) sent the list of all possible trial comparisons to both of the original primary authors of this review (SG and RB). After reviewing the list of possible trial comparisons, both of these review authors discussed and drafted a list of clinically important review questions and categorised each trial comparison under the most appropriate review question. This process was conducted iteratively until all trial comparisons were allocated to a single review question, and was conducted without knowledge of the results of any outcomes. We defined the following review questions.
Is manual therapy and exercise (with or without electrotherapy) more effective than placebo, no intervention, or another active intervention (e.g. glucocorticoid injection, oral non‐steroidal anti‐inflammatory drug (NSAID), arthroscopic subacromial decompression)?
Is manual therapy and exercise delivered in addition to another active intervention more effective than the other active intervention alone?
Is manual therapy alone more effective than placebo, no intervention, or another active intervention?
Is manual therapy delivered in addition to another active intervention more effective than the other active intervention alone?
Are supervised or home exercises alone more effective than placebo, no intervention, or another active intervention?
Are supervised or home exercises delivered in addition to another active intervention more effective than the other active intervention alone?
Is one type of manual therapy or exercise more effective than another (i.e. one type of manual therapy versus another type of manual therapy, or one type of exercise versus another type of exercise)?
We considered the first two to be the main questions of the review, as a multi‐modal intervention comprising manual therapy and exercise is most reflective of current clinical practice (Klintberg 2015; Kooijman 2013; Roberts 2014; Struyf 2012).
We planned to pool results of trials with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of benefit and harm. We planned to synthesise effect estimates using a random‐effects meta‐analysis model based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an impact on the results. Where we could not pool data, we presented effect estimates and 95% CIs of each trial in tables and summarised the results in the text.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses.
Sensitivity analysis
We planned to perform a sensitivity analysis to investigate the robustness of the treatment effect (of main outcomes) to allocation concealment and participant blinding, by removing the trials that reported inadequate or unclear allocation concealment and lack of participant blinding from the meta‐analysis to see if this changed the overall treatment effect.
Summary of findings tables
We presented the results of the most important comparisons of the review in 'Summary of findings' tables, which summarise the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on outcomes, as recommended by Cochrane (Schünemann 2011a). The 'Summary of findings' tables include an overall grading of the evidence related to each of the main outcomes, using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) approach (Schünemann 2011b).
In the Comments column of the 'Summary of findings' table, we report the absolute per cent difference, the relative per cent change from baseline and the number needed to treat for an additional beneficial outcome (NNTB) (the NNTB is provided only when the outcome shows a statistically significant difference).
For dichotomous outcomes (global assessment of treatment success, adverse events), the absolute risk difference was calculated using the risk difference statistic in RevMan (RevMan 2014), and the result expressed as a percentage; the relative per cent change was calculated as the risk ratio ‐1 and was expressed as a percentage. For continuous outcomes (overall pain, function, pain on motion, quality of life), the absolute risk difference was calculated as the improvement in the intervention group minus the improvement in the control group, expressed in the original units (i.e. mean difference from RevMan divided by units in the original scale), expressed as a percentage. The relative per cent change is calculated as the absolute change (or mean difference) divided by the baseline mean of the control group, expressed as a percentage.
In addition to the absolute and relative magnitude of effect provided in the 'Summary of findings' table, for dichotomous outcomes we calculated the NNTB or the number needed to treat for an additional harmful effect (NNTH) from the control group event rate, and the risk ratio (RR) using the Visual Rx NNT calculator (Cates 2004). For continuous outcomes of function and overall pain, we calculated the NNTB using Wells calculator software, which is available at the Cochrane Musculoskeletal (CMS) editorial office (http://musculoskeletal.cochrane.org). We assumed a minimal clinically important difference (MCID) of 1.5 points on a 10‐point scale (or 15 points on a 100‐point scale) for pain (Hawker 2011), and 10 points on a 100‐point scale for function or disability (for example SPADI, Constant‐Murley, Disabilities of the Arm, Shoulder and Hand (DASH)) (Angst 2011; Roy 2009; Roy 2010) for input into the calculator.
Results
Description of studies
Results of the search
The search conducted up to March 2015 resulted in 3488 records across the four databases. Seven additional records were identified from screening reference lists of previously published systematic reviews and included trials. After removal of duplicates, we screened the titles and abstracts of 3166 unique records. We screened 339 full‐text articles and identified 60 trials (77 reports) which were included in the review (Ainsworth 2009; Al Dajah 2014; Atkinson 2008; Bae 2011; Bang 2000; Bansal 2011; Barbosa 2008; Barra 2011; Barra Lopez 2013; Baskurt 2011; Beaudreuil 2011; Bennell 2010; Bialoszewski 2011; Blume 2014; Brox 1993; Celik 2009; Citaker 2005; Clews 1987; Cloke 2008; Conroy 1998; Cook 2014; Dickens 2005; Djordjevic 2012; Engebretsen 2009; Ginn 2005; Giombini 2006; Haahr 2005; Haik 2014; Hay 2003; Heredia‐Rizo 2013; Holmgren 2012; Janse van Rensburg 2012; Kachingwe 2008; Kardouni 2014; Kassolik 2013; Kaya 2014; Kromer 2013; Littlewood 2014; Lombardi 2008; Ludewig 2003; Maenhout 2013; Martins 2012; Marzetti 2014; McClatchie 2009; Moosmayer 2014; Munday 2007; Osteras 2008; Rhon 2014; Senbursa 2007; Senbursa 2011; Struyf 2013; Subasi 2012; Surenkok 2009; Szczurko 2009; Teys 2008; Van den Dolder 2003; Walther 2004; Wang 2006; Winters 1997; Yiasemides 2011).
Eleven additional trials are awaiting classification. Six require translation (Acosta 2009; Bicer 2005; Just 2009; Leblebici 2007; Werner 2002; Wiener 2005) and five are only available as a conference abstract (Bube 2010; Ellegaard 2013; Ginn 2009; Pribicevic 2006; Wies 2008); see table of Characteristics of studies awaiting classification). Two ongoing trials (Roddy 2014; Van den Dolder 2010) were identified in clinical trials registries (see table of Characteristics of ongoing studies). A flow diagram of the study selection process is presented in Figure 1.
1.
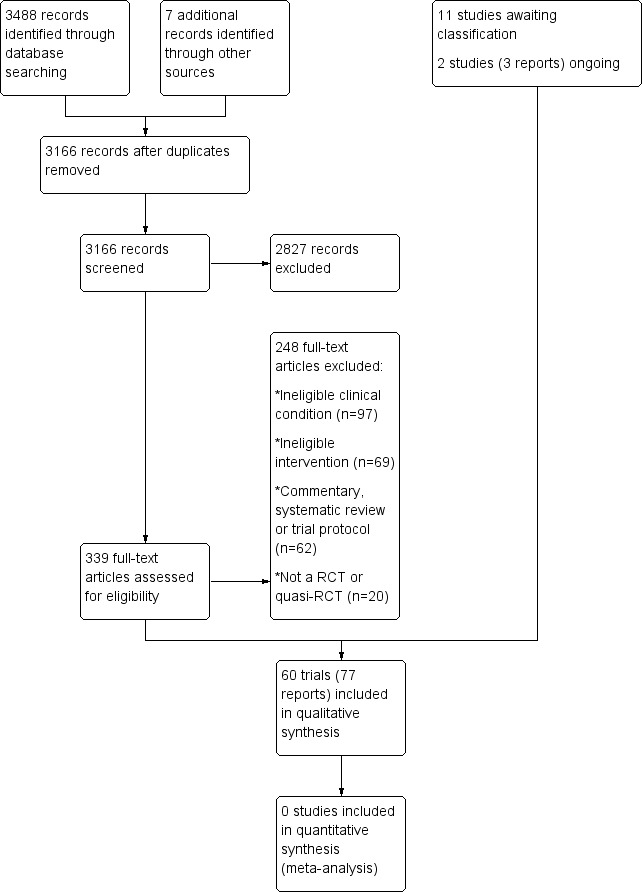
Study flow diagram.
Included studies
A full description of all included trials is provided in the Characteristics of included studies table.
Design
All trials except one were described as RCTs (Kassolik 2013 used a quasi‐random method of allocation). All trials except two used a parallel‐group design (McClatchie 2009 and Teys 2008 used a cross‐over design). Forty‐eight trials included two intervention arms (Ainsworth 2009; Al Dajah 2014; Atkinson 2008; Bae 2011; Bang 2000; Bansal 2011; Barbosa 2008; Barra 2011; Baskurt 2011; Beaudreuil 2011; Bennell 2010; Bialoszewski 2011; Blume 2014; Celik 2009; Citaker 2005; Conroy 1998; Cook 2014; Dickens 2005; Djordjevic 2012; Engebretsen 2009; Haahr 2005; Haik 2014; Hay 2003; Heredia‐Rizo 2013; Holmgren 2012; Janse van Rensburg 2012; Kardouni 2014; Kassolik 2013; Kaya 2014; Kromer 2013; Littlewood 2014; Lombardi 2008; Ludewig 2003; Maenhout 2013; Martins 2012; Marzetti 2014; McClatchie 2009; Moosmayer 2014; Munday 2007; Osteras 2008; Rhon 2014; Senbursa 2007; Struyf 2013; Subasi 2012; Szczurko 2009; Van den Dolder 2003; Wang 2006; Yiasemides 2011), 10 included three arms (Barra Lopez 2013; Brox 1993; Clews 1987; Ginn 2005; Giombini 2006; Senbursa 2011; Surenkok 2009; Teys 2008; Walther 2004; Winters 1997) and two included four arms (Cloke 2008; Kachingwe 2008).
Participants
A total of 3620 participants were included in the 60 trials, and the number of participants per trial ranged from nine to 207. The median of the mean age of participants was 51 (interquartile range (IQR) 46 to 56) years, and the median of the mean duration of symptoms was 11 (IQR 5.5 to 25) months. Fifty‐two per cent of the participants were women.
Diagnostic labels used by trialists included subacromial impingement syndrome (n = 36: Al Dajah 2014; Bae 2011; Bang 2000; Barra 2011; Barra Lopez 2013; Baskurt 2011; Beaudreuil 2011; Blume 2014; Brox 1993; Celik 2009; Citaker 2005; Conroy 1998; Cook 2014; Dickens 2005; Djordjevic 2012; Engebretsen 2009; Haahr 2005; Haik 2014; Heredia‐Rizo 2013; Holmgren 2012; Janse van Rensburg 2012; Kachingwe 2008; Kardouni 2014; Kaya 2014; Kromer 2013; Lombardi 2008; Ludewig 2003; Maenhout 2013; Martins 2012; Munday 2007; Osteras 2008; Rhon 2014; Senbursa 2007; Struyf 2013; Subasi 2012; Walther 2004), rotator cuff tendinitis (n = 4: Atkinson 2008; Clews 1987; Littlewood 2014; Szczurko 2009), supraspinatus tendinitis (n = 3: Bansal 2011; Barbosa 2008; Giombini 2006), painful arc (n = 2: Cloke 2008; McClatchie 2009), rotator cuff tear (n = 2: Ainsworth 2009; Moosmayer 2014), chronic rotator cuff disease (n = 1: Bennell 2010), chronic rotator cuff injury (n = 1:Bialoszewski 2011) or a mixture of labels (i.e. some participants with impingement, others with tendinitis) (n = 3: Senbursa 2011; Surenkok 2009; Van den Dolder 2003). However, there were inconsistencies in the diagnostic criteria for (or definitions of) each of the conditions (see Characteristics of included studies).
Six (10%) trials (Ginn 2005; Kassolik 2013; Teys 2008; Wang 2006; Winters 1997; Yiasemides 2011) included participants with non‐specific shoulder pain that was compatible with a diagnosis of rotator cuff disease. Two trials (Hay 2003; Surenkok 2009) included patients with either rotator cuff disease or adhesive capsulitis, but participants with the latter condition comprised less than 25% of the sample.
Trials were conducted in USA (n = 9), Turkey (n = 8), UK (n = 7), Australia (n = 6), Brazil, Norway (n = 4 each), Spain (n = 3), Belgium, Canada, Italy, Poland, The Netherlands (n = 2 each), Denmark, France, Germany, India, Republic of Korea, Saudi Arabia, Serbia, South Africa and Sweden (n = 1 each).
Interventions and Comparisons
A detailed description of the interventions delivered in each trial is summarised in the Characteristics of included studies and a summary of the intervention components across trials is presented in Table 4. The median duration of the physical therapy interventions was six weeks (range one to 24), with a median of two treatment sessions delivered per week (range one to seven). The types of manual therapy and exercise delivered were very heterogeneous across the trials.
1. Characteristics of manual therapy and exercise interventions.
| Study ID | Manual therapy component(s) | Exercise component(s) | Duration of session (minutes) | Number of sessions per week | Number of weeks treatment |
| Ainsworth 2009 | None | The participant was taught to start with a flexed elbow and to raise the arm to a vertical position. The participant was then taught to control the arm with sways in a 20‐degree arc before elevating and lowering the arm using a weight of approximately 0.75 kg. When the participant could carry out these activities supine, the head of the treatment couch was gradually inclined until they were able to perform the exercises in a sitting position. The participants also carried out stretching exercises to improve ranges of elevation, internal and external rotation, resistance band exercises into internal and external rotation, activities to improve proprioception, posture correction and adaptation of functional activities | Not reported | Not reported | Not reported (assumed to be 6 weeks) |
| Al Dajah 2014 | Soft tissue mobilisation for the subscapularis for 7 minutes and 5 repetitions of the contract‐relax proprioceptive neuromuscular facilitation (PNF) technique for the shoulder internal rotator muscles followed by 5 repetitions of a PNF‐facilitated abduction and external rotation diagonal pattern | None | 10 | 1 | 1 |
| Atkinson 2008 | Manipulation (high velocity, low‐amplitude, gentle‐impulse, shoulder adjustive thrust based on extensive motion palpation) | None | Not reported | 3 | 2 |
| Bae 2011 | None | Motor control and strengthening exercises | 30 | 3 | 4 |
| Bang 2000 | Passive accessory or passive physiological joint mobilisation Maitland grades I‐V; soft tissue massage and muscle stretching | Home exercises: simple cervical and thoracic postural exercises such as chin tucks, and self‐mobilisation such as caudal glides of the glenohumeral joint | 30 | 2 | 3 |
| Bansal 2011 | Deep friction massage to supraspinatus tendon in a transverse direction | Codman's exercises consisting of pendulum or swinging motion of the arm in flexion, extension, horizontal abduction, adduction and circumduction. Intensity (arc of motion) was increased as tolerated | 10 | 7 | 10 days |
| Barbosa 2008 | Front, back, lower longitudinal and lateral relaxations of the glenohumeral joint, anteroposterior movements of the acromioclavicular (squeeze) joint and anteroposterior, inferior‐superior and superior‐ inferior movements of the sternoclavicular joint | Eccentric training exercises: the 'empty the can' movement (the participant performs abduction movements of the shoulder in the scapular plane, with medial rotation) when treating the supraspinatus muscle, or the 'right curl' movement (the participant flexes his elbow, with the arm abducted beside the body) when treating biceps brachii dysfunctions. Movement resistance was offered manually, always by the same researcher and respecting the participant's pain limit | Not reported | 3 | 4 |
| Barra 2011 | Diacutaneous fibrolysis: application of a metallic hook as deeply as possible following the intermuscular septum between the muscles of the cervico‐scapular and shoulder region in a centripetal direction towards the pain location | None | 15 | 1 | 1 |
| Barra Lopez 2013 | Diacutaneous fibrolysis: application of a metallic hook as deeply as possible following the intermuscular septum between the muscles of the cervico‐scapular and shoulder region in a centripetal direction towards the pain location | No details provided | Not reported | 2 | 3 |
| Baskurt 2011 | None | Intervention group: scapular proprioceptive neuromuscular facilitation (PNF) exercises, scapular clock exercise, standing weight shift, double arm balancing, scapular depression, wall push up, wall slide exercises, strengthening and stretching exercises Control group: strengthening and stretching exercises |
Not reported | 3 | 6 |
| Beaudreuil 2011 | Passive mobilisation of the shoulder with a painless ROM | Dynamic humeral centring: learning the lowering of the humeral head during passive abduction of the shoulder, and actively lowering the humeral head by co‐contraction of the pectoralis major and latissimus dorsi during active abduction of the shoulder. Home exercise: no details provided |
Not reported | 3 x wk 1‐3; 2 x wk 4‐6 | 6 |
| Bennell 2010 | Soft tissue massage, glenohumeral joint mobilisation, thoracic spine mobilisation, cervical spine mobilisation, scapular retraining, postural taping | Most exercises required the participant to incorporate their scapular retraining with strengthening of the rotator cuff muscles. Some exercises reinforced and facilitated correct posture. Resistance for specific exercises was provided by hand weights or elastic theraband. Exercises were taught and performed during each treatment session and were otherwise self‐administered at home | 30 to 45 | 2 x wk 1‐2; 1 x wk 3‐6; fortnightly wk 7‐10 | 10 |
| Biasoszewski 2011 | Mobilisation of the glenohumeral joint and soft tissues using Kaltenborn's roll‐glide techniques, Cyriax deep transverse massage, Mulligan's mobilisation with movement and typical techniques of joint mobilisation in the anteroposterior direction | Standard passive and active exercises used to improve the ROM and restore muscle strength. The rotator cuff was initially strengthened in the painless ROM by performing active, passive and self‐assisted exercises. Once the full ROM has been achieved, strengthening exercises were applied, ranging from flexion, abduction and external rotation to internal rotation adduction and extension | Not reported | Not reported | Not reported |
| Blume 2014 | None | Supervised exercises: eccentric or concentric exercises included the seated 'full can', sidelying internal rotation (IR), sidelying external rotation (ER) with towel roll, supine protraction, sidelying horizontal abduction, sidelying abduction, and prone shoulder extension. All exercises were performed using a dumbbell for resistance. Home exercises: stretching and postural correction exercises |
60 | 2 | 8 |
| Brox 1993 | None | Supervised exercises: ROM and strengthening exercises | 60 | 2 | 24 |
| Celik 2009b | None | Intervention group: supervised shoulder flexion below 90 degrees, abduction, T‐bar (wand) exercises containing internal‐external rotation and extension, posterior capsule stretching and internal rotation exercises and rotator cuff strengthening exercises Control group: supervised shoulder flexion exercises above 90 degrees, posterior and inferior capsule stretching exercises, rotator cuff strengthening and internal rotation exercises |
Not reported | 7 | 2 |
| Citaker 2005 | Manual mobilisation (details not provided) | Theraband exercises permitting concentric and eccentric strengthening of the shoulder muscles. The exercises began with the elbow flexed 90 degrees and the shoulder in the neutral position. The exercises were performed through an arc of 45 degrees in each of the 5 planes of motion. In addition, Codman pendulum exercises were utilised as a home programme | Not reported | Not reported | Not reported |
| Clews 1987 | Massage of the long head of biceps, biceps tendon, pectorals, supraspinatus and infraspinatus | None | 15 | 3 | 1 |
| Cloke 2008 | Manual therapy (details not provided) | Exercises (details not provided) | Not reported | 6 sessions over 18 weeks | 18 |
| Conroy 1998 | Mobilisation: depending on the direction of restriction in capsular extensibility, inferior glide, posterior glide, anterior glide or long axis traction could be applied to the participant with oscillatory pressure. Stretch could also be applied in the case of muscle spasm. |
|
15 | 3 | 3 |
| Cook 2014 | Grade III posterior‐anterior mobilisations to the neck | Self‐ and externally‐applied stretching, isotonic strengthening, and restoration of normative movement | Not reported | Varied per participant | Mean of 8 (varied per participant) |
| Dickens 2005 | Acromioclavicular joint, thoracic, cervical spine and glenohumeral joint mobilisation. The physiotherapist assessed the range of accessory movement available in each participant's glenohumeral (anteroposterior, longitudinal caudad), acromioclavicular (anteroposterior, longitudinal caudad), cervical (posterior–anterior) and thoracic spine joints (posterior–anterior and transverse) with passive accessory movements. Any joints that were found to have restricted movement were addressed with mobilisations into the direction of resistance and pain to help restore full pain‐free range of movement | Exercises for the recruitment and strength of scapulothoracic muscles (especially lower trapezius and serratus anterior). The exercise programme was progressed to involve strengthening of infraspinatus, subscapularis and teres minor relative to the supraspinatus and deltoid. The rotator cuff exercises were done with the use of resistance and participants were given Theraband for home use. The exercises started in neutral positions with isometric contractions and were progressed to inner range, through range, outer range and into functional positions. The resistance and speed of these exercises were altered and progressed | Dependent on participant | Not reported | Not reported |
| Djordjevic 2012 | Mobilisation with movement (MWM): during the MWM treatment, the participant was seated, and the therapist was positioned on the opposite side of participant's painful shoulder. The therapist applied the thenar of one hand on the anterior aspect of the participant's humeral head and the other hand on his/her scapula. The hand on the humeral head performed a posterolateral glide, while the other hand stabilised the scapula. During this manoeuvre, the participant was encouraged to perform active shoulder movement to the point of the first onset of pain | Pendulum exercises and pain‐limited, active ROM exercises of shoulder elevation, depression, flexion, abduction, rotations, and strengthening exercises. Strengthening exercises were isometric in nature, working on the external shoulder rotators, internal rotators, biceps, deltoid, and scapular stabilizers (rhomboids, trapezius, serratus anterior, latissimus dorsi, and pectoralis major) | Not reported | 7 | 10 days |
| Engebretsen 2009 | None | Supervised exercises: the initial aim was to unload the stress on the rotator cuff and subacromial structures. During this phase, a mirror for awareness of posture, an elastic rubber band and a sling fixed to the ceiling were used. The participants received immediate feedback and correction (supervision) by the physiotherapist. Once dysfunctional neuromuscular patterns were normalised, endurance exercises were performed with gradually increasing resistance. Participants had an adjusted programme at home, which consisted of correction of alignment during daily living and simple low loaded exercises with a thin elastic cord to provide assistance and resistance to the movement | 45 | 2 | 12 |
| Ginn 2005 | Passive joint mobilisation at the sternoclavicular and acromioclavicular joints | ROM exercises: exercises were upgraded from active assisted to active to resisted active exercises using free weights or elastic resistance | Not reported | 2 | 5 |
| Giombini 2006 | None | Supervised and home exercises, consisting of pendular swinging in the prone position in flexion and extension of the shoulder and passive glenohumeral stretching exercises to tolerance | Not reported | 1 | 4 |
| Haahr 2005 | Soft tissue treatments (details not provided) | Supervised exercises: active training of the periscapular muscles (rhomboid, serratus, trapezoid, levator scapulae, pectoralis minor muscles) and strengthening of the stabilising muscles of the shoulder joint (rotator cuff). This was done within the limits of pain | 60 | 3 x wk 1‐2; 2 x wk 3‐5; 1 x wk 6‐12; 2‐3 x wk 13‐19 | 12 |
| Haik 2014 | Low‐amplitude, high velocity thrust thoracic spine manipulation | None | 3 | 1 | 1 |
| Hay 2003 | Active and passive mobilisation | Home exercise programme | 20 | 1 to 2 | 6 |
| Heredia‐Rizo 2013 | Manual therapy based on soft tissue techniques: micro‐mobilisations of the cervical structures in all movement axes, relaxation manoeuvres performed to fascial restrictions involving the cervical and scapulohumeral region, and a repositioning of the head of the humerus as recommended by Kaltenborn | Supervised exercises: pendular movements using 1 kg of weight in prone, assisted active movements with a pulley, and proprioceptive exercises with a ball in the horizontal plane | 40 | 5 | 3 |
| Holmgren 2012 | None | Supervised exercises: two eccentric exercises for the rotator cuff (supraspinatus, infraspinatus, and teres minor), three concentric/eccentric exercises for the scapula stabilisers (middle and lower trapezius, rhomboideus, and serratus anterior), and a posterior shoulder stretch | 30 | 1 x wk 1‐2; fortnightly wk 3‐12 | 12 |
| Janse van Rensburg 2012 | Thoracic spinal manipulation: a specific high velocity low amplitude "extension with rotation" thrust manipulation was applied to the shoulder | Exercises to stimulate the lower fibres of trapezius and specific rotator cuff‐strengthening | 30 | 1 | 6 |
| Kachingwe 2008 | Intervention group: glenohumeral joint mobilisation was administered based on assessment of glenohumeral joint anterior, posterior and inferior glides and long‐axis distraction passive accessory motions using a 0‐6 accessory motion scale. For situations where there was reactivity within the capsular ROM, grade I‐II mobilisation were applied. For situations where there was no reactivity but capsular hypomobility, grade III‐IV accessory motions were applied. Control group: glenohumeral joint mobilisation with movement (Mulligan technique) involved the therapist applying a sustained posterior accessory glide to the glenohumeral joint while the subject simultaneously actively flexed the shoulder to the pain‐free endpoint and applied a gentle overpressure force using the contralateral arm |
Supervised exercises including posterior capsule stretching, postural correction exercises, and an exercise programme focusing on rotator cuff strengthening and scapular stabilisation. Participants were instructed to perform a home exercise programme mimicking the exercises performed in the clinic | Not reported | 1 | 6 |
| Kardouni 2014 | Thoracic spinal manipulation: a high velocity, low amplitude thrust applied to the lower thoracic spine, mid thoracic spine, and cervicothoracic junction | None | Not reported | 1 | 1 |
| Kassolik 2013 | Classic massage of the shoulder girdle and glenohumeral joint was performed in a side recumbent position. During the massage, typical classic massage techniques (Swedish) were used ‐ stroking with the palms (effleurage), friction with the palms, kneading (petrissage), percussion (tapottement), and vibration | None | Not reported | 5 | 2 |
| Kaya 2014 | Scapular mobilisation (superoinferior gliding, rotations, and distractions to the scapula), neuromuscular facilitation techniques for scapula motions at anterior elevation–posterior depression and posterior elevation–anterior depression planes, glenohumeral joint mobilisation with long axis traction and posterior or inferior glide techniques to improve shoulder internal rotation limitations, and soft tissue massage and joint mobilisation of the neck, thoracic region, and elbow areas | Supervised and home exercises, including strengthening, flexibility (ROM) and Codman's pendulum exercises | 90 | 1 | 6 |
| Kromer 2013 | Painful and angular and/or translatory restricted peripheral joints were treated with manual glide techniques according to the concept of Kaltenborn. Comparable signs of the spine segments were treated with posterior‐anterior glides or coupled movements. Shortened muscles were stretched according to the description of Evjenth & Hamberg. Neural tissue was treated according to Butler | Core exercise programme ‐ dynamic exercises started with 2 sets of 10 repetitions and with low resistance (yellow rubber band); shoulder and neck stretches were held for 10 seconds and repeated twice; isometric scapular training positions were held for 10 seconds and repeated twice | 20 to 30 | 2 x wk 1‐5; 3 x wk 6‐12 | 12 |
| Littlewood 2014 | Manual therapy or massage (no details provided) | Self‐managed loaded exercise: involved exercising the affected shoulder against gravity, a resistive therapeutic band or hand weight over 3 sets of 10 to 15 repetitions completed twice per day. The exercise was prescribed and operationalised within a self‐managed framework which included focus upon knowledge translation, exercise/skill‐acquisition, self‐monitoring, goal‐setting, problem‐solving and pro‐active follow‐up. | Not reported | 1 | 8 |
| Lombardi 2008 | None | Progressive resistance training programme. The exercises were flexion, extension, medial rotation and lateral rotation of the shoulder | Not reported | 2 | 8 |
| Ludewig 2003 | None | Home exercise programme involving: two stretches (pectoralis minor stretch and posterior shoulder stretch), a muscle relaxation exercise for the upper trapezius performed in front of a mirror, and progressive resistance strengthening exercises for two muscle groups (serratus anterior muscle and humeral external rotation) | Not reported | 7 | 10 |
| Maenhout 2012 | None | Eccentric exercise consisted of full can (thumb up) abduction in the scapular plane, which was performed with a dumbbell weight | Not reported | 7 | 12 |
| Martins 2012 | None | Intervention group only: proprioception exercises: exercises with joint position, rhythmic stabilisation and repositioning of the members, unstable base, proprioceptive neuromuscular facilitation, and speed and accuracy Both groups: pendulum exercises of the shoulder, stretching of the cervical spine and shoulder muscles, exercises with a stick (to maintain or improve ROM), exercises to strengthen the muscles of the rotator cuff and scapular stabilisers |
Not reported | 2 | 6 |
| Marzetti 2014 | None | Supervised exercises: neurocognitive exercises (intervention group) or strengthening exercises focused on the rotator cuff and scapular stabilising muscles, stretching exercises, Codman's pendulum exercises and exercises against elastic band resistance (control group) | 60 | 3 | 5 |
| McClatchie 2009 | Lateral cervical glide mobilisations: the lateral aspect of the spinous processes of C5, C6, and C7 was landmarked on the ipsilateral side of the patient's painful shoulder. The examiner's thumb remained on the lateral aspect of the spinous process of C5, with the opposite hand placed on the patient's non‐affected shoulder or head for counterbalance as a lateral movement toward the non‐painful side was applied with the mobilising hand | None | Not reported | 1 | 1 |
| Moosmayer 2014 | None | Supervised exercises only, with particular attention directed towards correction of upper quarter posture and restoration of scapulothoracic and glenohumeral muscular control and stability. Local glenohumeral control was addressed by exercises to centre the humeral head in the glenoid fossa. Isometric exercises and exercises against eccentric and concentric resistance for shoulder rotators were given. When local glenohumeral control was achieved, exercises were given with increasing loads and progressed from neutral to more challenging positions | 40 | 2 x wk 1‐12; > 2 x wk 12‐24 | 24 |
| Munday 2007 | Shoulder girdle adjustments: high‐velocity, low‐amplitude manipulation in the direction of restricted end feel or joint play was performed. Participants sat in a comfortable position with the shoulder girdle exposed. Adjustments to the acromioclavicular joint were most common, although adjustments to the ribs, scapula and glenohumeral joints were made as well. The spine was not adjusted in this trial | None | None | 3 | 3 |
| Osteras 2008 | None | Supervised progressive resistance exercise therapy, comprising global aerobic exercises using a stationary bike, a treadmill, or a step machine, and semiglobal and local exercises using such medical exercise therapy equipment as wall pulley apparatus, lateral pulley apparatus, inclines board, angle bench, multiple purpose bench, shoulder rotator, dumbbells or barbells | 40 | 3 | 12 |
| Rhon 2014 | Combination of joint and soft‐tissue mobilisations; manual stretches; and contract–relax techniques | Supervised exercises: reinforcing exercises directed to the shoulder girdle or thoracic or cervical spine. Home exercises: wand ROM exercises, scapular retraction, scapular protraction, thoracic self‐mobilisation, butterfly stretch | 30 | 2 | 3 |
| Senbursa 2007 | Joint and soft tissue mobilisation: deep friction massage on supraspinatus muscle tendon, radial nerve stretching, scapular mobilisation, glenohumeral joint mobilisation, proprioceptive neuromuscular facilitation techniques including rhythmic stabilisation and hold‐relax | An active ROM, stretching and strengthening exercise programme including rotator cuff muscles, rhomboids, levator scapulae and serratus anterior which was self‐administered using an elastic band at home after being taught by a physiotherapist | Not reported | 3 | 4 |
| Senbursa 2011 | Deep friction massage on the supraspinatus muscle, radial nerve stretching, scapular mobilisation, glenohumeral joint mobilisation, and proprioceptive neuromuscular facilitation techniques | ROM, stretching and strengthening exercises for the rhomboid, levator scapulae, serratus anterior and rotator cuff muscles supervised and at home | Not reported | 3 | 12 |
| Struyf 2013 | Manual mobilisations, stretching and motor control training of the scapula, including: passive manual mobilisation (to improve passive scapular upward rotation and posterior tilting) | Home stretching exercises for the levator scapulae, stretching of the pectoralis minor muscle length by the physiotherapist and scapular motor control training with emphasis on a scapular orientation exercise | 30 | 1 to 3 | 3 to 9 |
| Subasi 2012 | None | Intervention group: supervised land‐based exercises. For the first 10 days, ROM and stretching exercises, and for the following 10 days, strengthening exercises. Control group: supervised water‐based exercises. For the first 10 days, ROM and stretching exercises, and for the following 10 days, strengthening exercises in water by using dumbbells |
Not reported | 7 | 3 |
| Surenkok 2009 | Scapular mobilisation: application of superior and inferior gliding, rotations, and distraction to the scapula of the affected shoulder | None | Not reported | 1 | 1 |
| Szczurko 2009 | Hands‐on shoulder muscle and joint therapy | Standardised exercise programme consisting of passive, active assisted and active ROM muscle strengthening and joint therapy | 30 | 1 | 12 |
| Teys 2008 | Postero‐lateral glide (Mulligans' mobilisation with movement) | None | Not reported | 1 | 1 |
| Van den Dolder 2003 | Soft tissue massage of the shoulder performed as seen fit by the treating therapist. The areas focused on were the lateral border of the scapula in full shoulder flexion; posterior deltoid at end of range horizontal flexion; anterior deltoid at end of range hand‐behind‐back; and pectoralis major in the stretch position. | None | 15 to 20 | 3 | 2 |
| Walther 2004 | None | Intervention: standardised self‐training programme of centring and stretching exercises that affected the shoulder. For most of the exercises, an elastic Thera‐Band was used that was chosen according to the results of the initial force measurements Control: physiotherapy consisting of centring training for the rotator cuff. Stretching was added in case of any limitation of the ROM at the first examination |
10 to 15 | 5 | 12 |
| Wang 2006 | None | Intervention group: customised supervised and home self‐stretching and strengthening exercises for scapular stabilisers, rotator cuff and scapulohumeral muscles Control group: standardised strengthening exercises ‐ shoulder flexors, abductors, extensors, external rotators and internal rotators |
Not reported | 1 | 8 |
| Winters 1997 | Intervention group 1: massage (details not provided), with no mobilisation techniques or manipulative techniques allowed Intervention group 2: mobilisation and manipulation of the cervical spine, upper thoracic spine, upper ribs, acromioclavicular joint and the glenohumeral joint |
Supervised exercises (details not provided) | Not reported | 1 | 6 |
| Yiasemides 2011 | Individually tailored low‐velocity passive joint mobilisations to any of the shoulder region joints and passive mobilisation of the scapula. Either sustained or oscillatory techniques were delivered | Supervised and home exercises including stretching, strengthening and motor retraining | Individually determined | 1 to 2 | 8 |
Manual therapy interventions included:
joint mobilisation (glenohumeral or acromioclavicular joint) (n = 21: Bang 2000; Barbosa 2008; Bennell 2010; Bialoszewski 2011; Conroy 1998; Dickens 2005; Djordjevic 2012; Ginn 2005; Hay 2003; Kachingwe 2008; Kaya 2014; Kromer 2013; McClatchie 2009; Rhon 2014; Senbursa 2007; Senbursa 2011; Surenkok 2009; Struyf 2013; Teys 2008; Winters 1997; Yiasemides 2011);
soft tissue mobilisation or massage of the shoulder (n = 9: Al Dajah 2014; Bennell 2010; Clews 1987; Haahr 2005; Heredia‐Rizo 2013; Kaya 2014; Rhon 2014; Senbursa 2007; Van den Dolder 2003);
spinal or neck mobilisation or manipulation (n = 5: Bennell 2010; Cook 2014; Haik 2014; Kardouni 2014; Kaya 2014);
shoulder manipulation (n = 4: Atkinson 2008; Janse van Rensburg 2012; Munday 2007; Winters 1997);
deep friction massage (n = 4: Bansal 2011; Bialoszewski 2011; Senbursa 2007; Senbursa 2011);
proprioceptive neuromuscular facilitation stretching techniques (n = 4: Al Dajah 2014; Kaya 2014; Senbursa 2007; Senbursa 2011);
diacutaneous fibrolysis (n = 2: Barra 2011; Barra Lopez 2013).
Two trials (Citaker 2005; Cloke 2008), did not report details about the type of manual therapy delivered.
Many different types of exercises were delivered in the trials. These included:
strengthening exercises (n = 23: Ainsworth 2009; Bae 2011; Baskurt 2011; Bennell 2010; Bialoszewski 2011; Brox 1993; Celik 2009; Conroy 1998; Cook 2014; Dickens 2005; Djordjevic 2012; Haahr 2005; Janse van Rensburg 2012; Kachingwe 2008; Kaya 2014; Martins 2012; Marzetti 2014; Rhon 2014; Senbursa 2007; Senbursa 2011; Subasi 2012; Wang 2006; Yiasemides 2011);
stretching exercises (n = 18: Ainsworth 2009; Baskurt 2011; Blume 2014; Celik 2009; Conroy 1998; Cook 2014; Giombini 2006; Holmgren 2012; Kromer 2013; Ludewig 2003; Martins 2012; Marzetti 2014; Senbursa 2007; Senbursa 2011; Subasi 2012; Walther 2004; Wang 2006; Yiasemides 2011);
range of motion exercises (n = 13: Ainsworth 2009; Bialoszewski 2011; Brox 1993; Celik 2009; Conroy 1998; Cook 2014; Djordjevic 2012; Ginn 2005; Kaya 2014; Rhon 2014; Senbursa 2011; Subasi 2012; Szczurko 2009);
progressive resistance training (n = 7: Dickens 2005; Engebretsen 2009; Littlewood 2014; Lombardi 2008; Ludewig 2003; Marzetti 2014; Osteras 2008);
Codman's pendulum exercises (n = 7: Bansal 2011; Citaker 2005; Giombini 2006; Heredia‐Rizo 2013; Kaya 2014; Martins 2012; Marzetti 2014);
eccentric training exercises (n = 6: Barbosa 2008; Blume 2014; Citaker 2005; Holmgren 2012; Maenhout 2013; Moosmayer 2014);
postural exercises (n = 5: Ainsworth 2009; Bang 2000; Bennell 2010; Blume 2014; Kachingwe 2008);
motor control exercises (n = 5: Bae 2011; Marzetti 2014; Moosmayer 2014; Struyf 2013; Yiasemides 2011);
proprioceptive exercises (n = 4: Ainsworth 2009; Baskurt 2011; Heredia‐Rizo 2013; Martins 2012);
dynamic humeral centring (Beaudreuil 2011).
Two trials (Barra Lopez 2013; Cloke 2008) did not report the specific type of exercises delivered. Exercises were performed:
only under supervision in 20 trials (Ainsworth 2009; Bae 2011; Barbosa 2008; Barra Lopez 2013; Baskurt 2011; Bialoszewski 2011; Conroy 1998; Djordjevic 2012; Engebretsen 2009; Heredia‐Rizo 2013; Janse van Rensburg 2012; Lombardi 2008; Martins 2012; Marzetti 2014; Moosmayer 2014; Osteras 2008; Senbursa 2011; Szczurko 2009; Walther 2004; Winters 1997);
only at home in seven trials (Cook 2014; Littlewood 2014; Ludewig 2003; Senbursa 2007; Senbursa 2011; Walther 2004; Yiasemides 2011);
or both under supervision and at home in 21 trials (Bang 2000; Bansal 2011; Beaudreuil 2011; Bennell 2010; Blume 2014; Brox 1993; Celik 2009; Citaker 2005; Dickens 2005; Ginn 2005; Giombini 2006; Haahr 2005; Hay 2003; Holmgren 2012; Kaya 2014; Kromer 2013; Maenhout 2013; Rhon 2014; Struyf 2013; Subasi 2012; Wang 2006).
Trialists investigated the primary or add‐on effects of:
manual therapy and exercise in 10 trials (Bennell 2010; Cloke 2008; Dickens 2005; Ginn 2005; Haahr 2005; Hay 2003; Kachingwe 2008; Rhon 2014; Szczurko 2009; Winters 1997);
manual therapy alone in 29 trials (Al Dajah 2014; Atkinson 2008; Bang 2000; Bansal 2011; Barbosa 2008; Barra 2011; Barra Lopez 2013; Bialoszewski 2011; Citaker 2005; Clews 1987; Conroy 1998; Cook 2014; Haik 2014; Heredia‐Rizo 2013; Janse van Rensburg 2012; Kachingwe 2008; Kardouni 2014; Kassolik 2013; Kaya 2014; Kromer 2013; McClatchie 2009; Munday 2007; Senbursa 2007; Senbursa 2011; Surenkok 2009; Teys 2008; Van den Dolder 2003; Winters 1997; Yiasemides 2011); or
exercise alone in 26 trials (Ainsworth 2009; Bae 2011; Baskurt 2011; Beaudreuil 2011; Blume 2014; Brox 1993; Celik 2009; Djordjevic 2012; Engebretsen 2009; Ginn 2005; Giombini 2006; Holmgren 2012; Kachingwe 2008; Littlewood 2014; Lombardi 2008; Ludewig 2003; Maenhout 2013; Martins 2012; Marzetti 2014; Moosmayer 2014; Osteras 2008; Senbursa 2011; Struyf 2013; Subasi 2012; Walther 2004; Wang 2006).
Comparators were also diverse, including:
placebo (Barra 2011; Bennell 2010; Brox 1993; Haik 2014; Kardouni 2014; McClatchie 2009; Munday 2007; Surenkok 2009; Teys 2008);
no intervention (Dickens 2005; Kachingwe 2008; Lombardi 2008; Ludewig 2003; Surenkok 2009; Teys 2008; Van den Dolder 2003);
glucocorticoid injection (Cloke 2008; Ginn 2005; Hay 2003; Rhon 2014; Winters 1997);
surgery (Brox 1993; Haahr 2005; Moosmayer 2014);
electrotherapy modalities (e.g. therapeutic ultrasound, microwave diathermy) (Al Dajah 2014; Bansal 2011; Giombini 2006);
naturopathic care (Szczurko 2009);
oral NSAID (Cloke 2008);
extracorporeal shock wave treatment (Engebretsen 2009);
kinesiotaping (Kaya 2014);
a functional brace (Walther 2004).
Nineteen trials investigated whether there was benefit in adding manual therapy or exercise to another physical therapy intervention (Ainsworth 2009; Atkinson 2008; Bae 2011; Bang 2000; Barbosa 2008; Barra Lopez 2013; Baskurt 2011; Beaudreuil 2011; Bialoszewski 2011; Clews 1987; Conroy 1998; Cook 2014; Janse van Rensburg 2012; Kachingwe 2008; Kromer 2013; Maenhout 2013; Martins 2012; Senbursa 2011; Yiasemides 2011), and in 18 trials, one type of manual therapy or exercise was compared with another (Blume 2014; Celik 2009; Citaker 2005; Djordjevic 2012; Heredia‐Rizo 2013; Holmgren 2012; Kachingwe 2008; Kassolik 2013; Littlewood 2014; Marzetti 2014; Osteras 2008; Senbursa 2007; Senbursa 2011; Struyf 2013; Subasi 2012; Walther 2004; Wang 2006; Winters 1997).
Outcomes
The outcomes measured in each trial are summarised in Table 5. Of the main outcomes, most trials included a measure of overall pain (n = 48) and function (n = 44), but fewer included measures of pain on motion (n = 16), global assessment of treatment success (n = 17), quality of life (n = 13) or adverse events (n = 17). Overall pain was most commonly measured using a zero to 10 or zero to 100 VAS, though several different descriptors for the maximum score on the scale (e.g. "worst imaginable pain", "severe pain", "intolerable pain") were noted. Function was most commonly measured using the SPADI or the Constant‐Murley Score. Of the other outcomes, most trials included measures of range of motion (n = 38), but fewer included measures of night pain (n = 9), pain with resisted movement (n = 1), strength (n = 19), work disability (n = 7) or surgery (n = 2).
2. Outcome matrix.
| Study ID | Overall pain | Function | Pain on motion | Global assessment | Quality of life | Adverse events |
| Ainsworth 2009 | X | X | ||||
| Al Dajah 2014 | X | |||||
| Atkinson 2008 | X | X | ||||
| Bae 2011 | X | |||||
| Bang 2000 | X | X | ||||
| Bansal 2011 | X | |||||
| Barbosa 2008 | X | X | ||||
| Barra 2011 | X | X | X | |||
| Barra Lopez 2013 | X | X | X | |||
| Baskurt 2011 | X | X | X | |||
| Beaudreuil 2011 | X | X | ||||
| Bennell 2010 | X | X | X | X | X | X |
| Biasoszewski 2011 | X | |||||
| Blume 2014 | X | |||||
| Brox 1993 | X | X | X | X | X | |
| Celik 2009 | X | X | ||||
| Citaker 2005 | X | X | X | |||
| Clews 1987 | X | |||||
| Cloke 2008 | X | X | ||||
| Conroy 1998 | X | X | ||||
| Cook 2014 | X | X | X | X | ||
| Dickens 2005 | X | |||||
| Djordjevic 2012 | ||||||
| Engebretsen 2009 | X | X | X | X | ||
| Ginn 2005 | X | X | X | |||
| Giombini 2006 | X | X | X | X | X | |
| Haahr 2005 | X | X | ||||
| Haik 2014 | X | |||||
| Hay 2003 | X | X | X | X | ||
| Heredia‐Rizo 2013 | X | |||||
| Holmgren 2012 | X | X | X | X | X | |
| Janse van Rensburg 2012 | X | X | ||||
| Kachingwe 2008 | X | X | ||||
| Kardouni 2014 | X | X | X | |||
| Kassolik 2013 | X | |||||
| Kaya 2014 | X | X | X | |||
| Kromer 2013 | X | X | X | |||
| Littlewood 2014 | X | X | ||||
| Lombardi 2008 | X | X | X | X | X | |
| Ludewig 2003 | X | X | ||||
| Maenhout 2012 | X | X | X | |||
| Martins 2012 | X | X | ||||
| Marzetti 2014 | X | X | X | X | ||
| McClatchie 2009 | X | |||||
| Moosmayer 2014 | X | X | X | |||
| Munday 2007 | X | X | ||||
| Osteras 2008 | X | X | ||||
| Rhon 2014 | X | X | X | X | ||
| Senbursa 2007 | X | X | X | |||
| Senbursa 2011 | X | X | X | |||
| Struyf 2013 | X | X | X | |||
| Subasi 2012 | X | X | X | |||
| Surenkok 2009 | X | X | X | |||
| Szczurko 2009 | X | X | X | X | ||
| Teys 2008 | X | |||||
| Van den Dolder 2003 | X | X | ||||
| Walther 2004 | X | X | X | X | ||
| Wang 2006 | X | X | ||||
| Winters 1997 | X | X | ||||
| Yiasemides 2011 | X | X | X | X | ||
| FREQUENCY | 48 | 44 | 16 | 17 | 13 | 17 |
We contacted authors of three trials to retrieve missing data for unreported or partially reported outcomes (Cloke 2008; Dickens 2005; Kachingwe 2008), but received no responses.
Excluded studies
We excluded 248 full‐text articles. Many of these were excluded because they were eligible for inclusion in one of the other three reviews in this series (i.e. focused on electrotherapy modalities for rotator cuff disease or adhesive capsulitis, or manual therapy or exercise for adhesive capsulitis). The reasons for exclusion were that the clinical condition was ineligible (n = 97), the intervention was ineligible (n = 69), the article was a commentary or systematic review (n = 62) or the study was not a RCT or quasi‐RCT (n = 20). We have listed in the table of Characteristics of excluded studies 16 studies which required full‐text screening by a third author (the full list of 248 excluded studies is available on request).
Risk of bias in included studies
A summary of the risk of bias in included trials is presented in Figure 2 and Figure 3.
2.
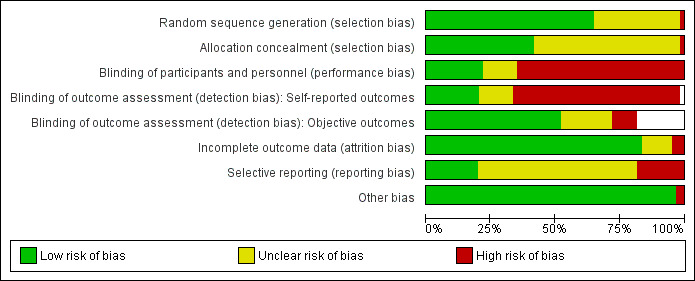
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. White areas mean that either subjective or objective outcomes were not measured in some of the trials, so an assessment of the risk of bias due to lack blinding of such outcomes was not applicable.
3.
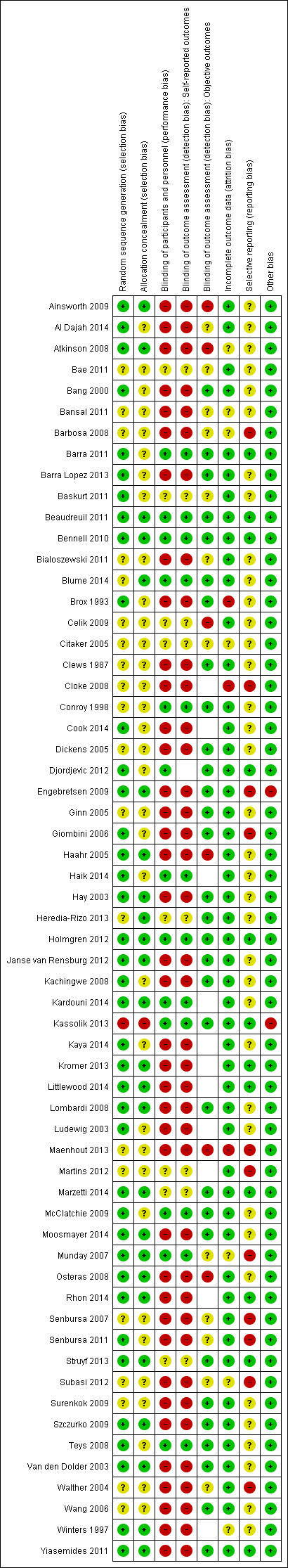
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Empty cells mean that either subjective or objective outcomes were not measured in the trial, so an assessment of the risk of bias due to lack blinding of such outcomes was not applicable.
Allocation
We rated 39 (65%) trials at low risk of allocation bias because the method used to generate the random allocation sequence was adequate. We also rated 25 (42%) trials at low risk of allocation bias because the method used to conceal the allocation sequence was adequate. We rated one trial at high risk of allocation bias because participants were allocated to groups using a quasi‐random sequence. In 20 (33%) trials the method of sequence generation was not reported and in 34 (57%) trials the method of allocation concealment was not reported; the risk of allocation bias in these trials was therefore unclear.
Blinding
We rated 13 (22%) trials at low risk of performance bias because participants were successfully blinded. We rated eight (13%) trials at unclear risk of performance bias because participants received different types of manual therapy or exercise, but it is unclear whether they were provided with any information that would make them perceive the type of manual therapy or exercise they received as superior or inferior to the alternative type of manual therapy or exercise. We rated all 39 (65%) remaining trials at high risk of performance bias because participants were not blinded, which may have led them to deviate from the interventions as planned because of their beliefs about the intervention they received.
Self‐reported outcomes were measured in all but one trial, and of these, we rated 12 (20%) at low risk of detection bias because it was clear that participants were blinded, eight (14%) at unclear risk of detection bias because it was unclear whether participants were blinded, and the 39 remaining trials (66%) at high risk of detection bias for self‐reported outcomes because participants were not blinded. Of 49 trials with outcome measures that were objectively rated (e.g. range of motion, strength), blinding of outcome assessors was reported in 31 (63%) and thus we rated these trials at low risk of detection bias for objective outcomes. In six (12%) trials there was no blinding of assessors of objective outcomes, so the risk of detection bias for objective outcomes was high. In 12 (24%) trials it was unclear whether such blinding was done, so the risk of detection bias for objective outcomes was unclear.
Incomplete outcome data
Fifty (83%) trials either had no dropouts, losses to follow‐up or exclusions, or had a small amount of attrition that was deemed unlikely to bias the results. In three (5%) trials there was differential dropout across groups, with reasons that appeared to be related to the treatments received, and thus we rated these trials at high risk of attrition bias. In the remaining seven (12%) trials the quantity of or reasons for incomplete outcome data were not reported so the risk of attrition bias was unclear.
Selective reporting
We rated 12 (20%) trials at low risk of selective reporting bias because all outcomes specified in the trial registry entry or the trial protocol were fully reported in the trial publication. We rated 11 (18%) trials at high risk of selective reporting bias because data for at least one outcome that was listed in the trial registry entry or the methods section of the publication were not reported in the results section at all. We rated the remaining 37 (62%) trials at unclear risk of selective reporting bias for one of two reasons. Firstly, outcome data were completely reported for all outcomes specified in the methods section of the publication, but none of these trials was registered in a trials registry or had an available trial protocol, so it was unclear whether other outcomes were measured but not reported based on the nature of the results; or secondly, outcome data were incompletely reported (e.g. reporting means without measures of variation), but it was unclear whether data were incompletely reported based on the statistical significance or magnitude of the results.
Other potential sources of bias
In Engebretsen 2009 (supervised exercises versus radial extracorporeal shockwave treatment), there was an imbalance between groups in the number of additional treatments received outside of the trial setting, which was likely to bias the results in favour of the shockwave treatment group. In Kassolik 2013 (Swedish massage versus massage based on the tensegrity principle), there was baseline imbalance in range of motion, which may have biased results in favour of the group receiving massage based on the tensegrity principle. We rated both trials at high risk of other bias, and all other trials (97%) as free from other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
We were unable to perform any meta‐analyses because of clinical heterogeneity or incomplete outcome reporting. Summary data and effect estimates (with 95% CIs) for all trials are presented in the Additional tables section. To enhance readability, we have reported in the following section the summary data, effect estimates and 95% CIs for main outcomes reported in trials addressing the two primary questions of the review (i.e. "Is manual therapy and exercise (with or without electrotherapy) more effective than placebo, no intervention or another active intervention?" and "Is manual therapy and exercise delivered in addition to another active intervention more effective than the other active intervention alone?"). For the remaining questions, the relevant data are presented in the Additional tables. If an outcome is not referred to within a sub‐section or table, then no data for that outcome were available in the trial(s).
Is manual therapy and exercise (with or without electrotherapy) more effective than placebo, no intervention or another active intervention (e.g. glucocorticoid injection, oral non‐steroidal anti‐inflammatory drug (NSAID), arthroscopic subacromial decompression)?
In 10 trials, manual therapy and exercise was compared with either placebo (Bennell 2010), no intervention (Dickens 2005; Kachingwe 2008) or another active intervention (Cloke 2008; Ginn 2005; Haahr 2005; Hay 2003; Rhon 2014; Szczurko 2009; Winters 1997).
Manual therapy and exercise versus placebo
See Table 1. In one trial of 120 participants with chronic rotator cuff disease, judged at low risk of bias overall, a manual therapy and exercise package was compared with placebo (Bennell 2010). The package comprised soft tissue massage, glenohumeral joint mobilisation, thoracic spine mobilisation, cervical spine mobilisation, scapular retraining and postural taping in 10 sessions over 10 weeks along with home exercises primarily focused upon strengthening the rotator cuff muscles for 22 weeks. The placebo consisted of inactive ultrasound therapy and application of an inert gel in 10 sessions over 10 weeks.
No data were available at our primary time point (three to six weeks). At 22 weeks, the mean change in overall pain with placebo was 17.3 points on a 100‐point scale, and 24.8 points with manual therapy and exercise (adjusted mean difference (MD) 6.8 points, 95% confidence interval (CI) ‐0.70 to 14.30 points). Mean change in function with placebo was 15.6 points on a 100‐point scale, and 22.4 points with manual therapy and exercise (adjusted MD 7.1 points, 95% CI 0.30 to 13.90 points). Mean change in pain on motion with placebo was 1.6 points on a 10‐point scale, and 2.6 points with manual therapy and exercise (adjusted MD 0.9 points, 95% CI ‐0.03 to 1.70 points). Fifty‐seven per cent (31/54) of participants reported treatment success with manual therapy and exercise compared with 41% (24/58) of participants receiving placebo (risk ratio (RR) 1.39, 95% CI 0.94 to 2.03). Mean change in quality of life with placebo was 0 points on a 1.4‐point scale, and 0.07 points with manual therapy and exercise (adjusted MD 0.07 points, 95% CI 0.04 to 0.10 points). None of these differences were considered to be clinically important (Table 6). Thirty‐one per cent (17/55) of participants reported adverse events with manual therapy and exercise compared with 8% (5/61) of participants receiving placebo (RR 3.77, 95% CI 1.49 to 9.54). However adverse events were mild and short‐lived (short‐term pain following treatment). We considered the evidence from this trial to be high quality.
3. Manual therapy and exercise versus placebo.
| Study ID: Bennell 2010 Intervention: soft tissue massage, joint mobilisation, scapular retraining and supervised and home exercises Control: sham ultrasound | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (SPADI pain score 0‐100) change from baseline to 22 weeks | 24.8 | 23.7 | 59 | 17.3 | 19.6 | 61 | 6.8 (‐0.7, 14.3)* |
| Function (SPADI total score 0‐100) change from baseline to 22 weeks | 22.4 | 22 | 59 | 15.6 | 17.8 | 61 | 7.1 (0.3, 13.9)* |
| Pain on movement (VAS 0‐10) change from baseline to 22 weeks | 2.6 | 2.9 | 59 | 1.6 | 2.4 | 61 | 0.9 (‐0.03, 1.7)* |
| Quality of life (AQoL ‐0.4 to 1) change from baseline to 22 weeks | 0.07 | 0.2 | 59 | 0 | 0.1 | 61 | 0.07 (0.04, 0.1)* |
| Quality of life (SF‐36 PCS 0‐100) change from baseline to 22 weeks | 10.8 | 25 | 59 | 4.7 | 22.3 | 61 | 6.3 (‐2, 14.5)* |
| Quality of life (SF‐36 MCS 0‐100) change from baseline to 22 weeks | ‐1 | 19.7 | 59 | 1.8 | 15.8 | 61 | 0.6 (‐5.2, 6.4)* |
| Strength: abduction (kg) change from baseline to 22 weeks | 1.1 | 4.4 | 59 | 0.4 | 2.5 | 61 | 1.2 (0.1, 2.3)* |
| Strength: external rotation (kg) change from baseline to 22 weeks | 0.3 | 4.3 | 59 | ‐0.1 | 1.9 | 61 | 0.9 (‐0.1, 1.9)* |
| Strength: internal rotation (kg) change from baseline to 22 weeks | 1.3 | 3.4 | 59 | 0 | 2.7 | 61 | 1.5 (0.4, 2.5)* |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Total adverse events during 11‐week intervention period | 17 | 55 | 5 | 61 | 3.77 (1.49, 9.54) | ||
| Adverse events: short‐term pain during or after the treatment session (during 11‐week intervention period) | 3 | 55 | 5 | 61 | 0.67 (0.17, 2.66) | ||
| Adverse events: increased short‐term pain with the home exercises (during 11‐week intervention period) | 12 | 55 | 0 | 61 | 27.68 (1.68, 456.77) | ||
| Adverse events: mild irritation to the tape used for postural taping (during 11‐week intervention period) | 2 | 55 | 0 | 61 | 5.54 (0.27, 112.84) | ||
| Total adverse events during 11‐week follow‐up period (i.e. from 11‐22 weeks) (note the only adverse event was increased short‐term pain with the home exercises) | 7 | 49 | 0 | 58 | 17.70 (1.04, 302.29) | ||
| Global assessment of treatment success (successful outcome ("much better") compared with those reporting an unsuccessful outcome (either "slightly better", "no change", "slightly worse", or "much worse")) at 22 weeks | 31 | 54 | 24 | 58 | 1.39 (0.94, 2.03) | ||
*ANCOVA adjusted mean differences presented (adjusted for baseline score on outcome)
Manual therapy and exercise versus no treatment
Two trials (89 participants), both at high risk of bias overall, compared manual therapy and exercise with no active intervention other than advice to maintain normal activities (Dickens 2005) or advice regarding posture and overhead activities (Kachingwe 2008). The physical therapy intervention in Dickens 2005 comprised mobilisation of the glenohumeral joint, acromioclavicular joint and thoracic and cervical spine, exercise therapy including attention to muscle imbalance, postural advice, strapping and occasionally electrotherapy, while in Kachingwe 2008, physical therapy comprised either glenohumeral mobilisation and supervised and home exercises or mobilisation with movement and supervised and home exercises.
In Dickens 2005, at six months the mean change in function with no treatment was 0.65 on a 100‐point scale, and 20 points with manual therapy and exercise (MD 19.35, 73 participants) but the 95% CI was not estimable. No other outcomes were reported in this trial. Usable outcome data were not available in Kachingwe 2008, although the authors claimed that there were no statistically significant differences between groups in overall pain, function and active shoulder flexion at six weeks (Table 7). We downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and so consider this evidence to be very low quality.
4. Manual therapy and exercise versus no treatment.
| Study ID: Dickens 2005 Intervention: mobilisation and supervised and home exercises Control: advice to maintain normal activities | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | Range | n | Mean | Range | n | Mean difference (95% CI) | |
| Function (Constant score 0‐100 with higher scores denoting better function) change from baseline to 6 months | 20 | 4 to 45 | 42 | 0.65 | ‐16 to 14 | 31 | 19.35 (95% CI not estimable) |
| Study ID: Kachingwe 2008 Intervention: glenohumeral mobilisation plus supervised and home exercises Control: advice to regarding posture and overhead activities | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 with higher scores denoting worse pain) % change from baseline to 6 weeks | 44.2 | 38.6 | 9 | 14.4 | 119.8 | 7 | Not estimable |
| Function (SPADI total score 0‐130 with higher scores denoting worse function) % change from baseline to 6 weeks | 56.7 | 29.8 | 9 | 34.2 | 58.9 | 7 | Not estimable |
| Active range of flexion % change from baseline to 6 weeks | ‐15.9 | 116.6 | 9 | 42.6 | 15.8 | 7 | Not estimable |
| Study ID: Kachingwe 2008 Intervention: mobilisation with movement plus supervised and home exercises Control: advice to regarding posture and overhead activities | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 with higher scores denoting worse pain) % change from baseline to 6 weeks | 55.2 | 31.9 | 9 | 14.4 | 119.8 | 7 | Not estimable |
| Function (SPADI total score 0‐130 with higher scores denoting worse function) % change from baseline to 6 weeks | 55.5 | 20.1 | 9 | 34.2 | 58.9 | 7 | Not estimable |
| Active range of flexion % change from baseline to 6 weeks | 46.7 | 31.9 | 9 | 42.6 | 15.8 | 7 | Not estimable |
Manual therapy and exercise versus glucocorticoid injection
See Table 2. Five trials (507 participants), all at high risk of bias overall, compared manual therapy and exercise with glucocorticoid injection (Cloke 2008; Ginn 2005; Hay 2003; Rhon 2014; Winters 1997). The physical therapy interventions comprised: six sessions over 18 weeks of manual therapy and exercise (no details provided) (Cloke 2008); passive joint mobilisation and range of motion exercises twice a week for five weeks (Ginn 2005); active and passive mobilisation, home exercises and therapeutic ultrasound once a week for six weeks (Hay 2003); joint and soft tissue mobilisation, manual stretches and supervised and home exercises twice a week for three weeks (Rhon 2014); and massage and exercises once a week for six weeks (Winters 1997). The total number of glucocorticoid injections delivered varied across the trials, from a single injection at baseline only (Ginn 2005; Hay 2003), injection at six, 12 and 18 weeks (Cloke 2008), injection at baseline, one week later and two weeks later if necessary (Winters 1997), and as many as three injections administered one month apart during the one‐year period (Rhon 2014). Due to the heterogeneity in interventions, comparators, diagnoses of shoulder pain, outcome measures, timing of outcome assessment and incomplete reporting of outcome data in some trials, we were unable to synthesise any data in meta‐analyses.
Based on results of single trials, there was no clinically important difference between manual therapy and exercise and glucocorticoid injection with respect to overall pain at four weeks (mean 1.6 versus 1.7 on a 10‐point scale, MD ‐0.10, 95% CI ‐0.92 to 0.72, 88 participants), 11 weeks (mean 11.5 versus 9.2 on a 28‐point scale, MD 2.30, 95% CI 0.50 to 4.10, 82 participants), six months (mean 1.7 versus 2.2 on a 10‐point scale, MD ‐0.50, 95% CI ‐1.32 to 0.32, 84 participants) or 12 months (mean 2.1 versus 2.5 on a 10‐point scale, MD ‐0.40, 95% CI ‐1.23 to 0.43, 94 participants). Further, there was no clinically important difference between groups in function at four weeks (mean 22.2 versus 23.2 on a 100‐point scale, MD ‐1.00, 95% CI ‐8.77 to 6.77, 88 participants), five weeks (mean change 5.3 versus 5.2 on a 27‐point scale, MD 0.10, 95% CI ‐1.62 to 1.82, 84 participants), six weeks (mean change 2.56 versus 3.03 on a 23‐point scale, MD ‐0.47, 95% CI ‐2.11 to 1.17, 197 participants), 18 weeks (mean 27.73 versus 29.81 on a 48‐point scale, MD ‐2.08, 95% CI ‐10.46 to 6.30, 49 participants), six months (mean 21.5 versus 22.2 on a 100‐point scale, MD ‐0.70, 95% CI ‐8.52 to 7.12, 84 participants) or 12 months (mean 21.6 versus 23.1 on a 100‐point scale, MD ‐1.50, 95% CI ‐9.07 to 6.07, 94 participants). Only one trial (Rhon 2014) measured adverse events, and found that apart from transient pain due to the injection, there were no other adverse events reported in either group (Table 8).
5. Manual therapy and exercise versus glucocorticoid injection.
| Study ID: Cloke 2008 Intervention: manual therapy and exercise (no details provided) Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Oxford Shoulder Score (12‐60) with a higher score indicating worse disability) at 18 weeks | 27.73 | 16 | 22 | 29.81 | 13.4 | 27 | ‐2.08 (‐10.46, 6.30) |
| Function (Oxford Shoulder Score (12‐60) with a higher score indicating worse disability) at 12 months | 28.94 | NR | NR | 26.47 | NR | NR | 2.47 (95% CI not estimable) |
| Study ID: Ginn 2005 Intervention: mobilisation, range of motion exercises and electrotherapy modalities Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | 95% CI | n | Mean | 95% CI | n | Mean difference (95% CI) | |
| Pain (VAS 0‐10) change from baseline to 5 weeks | 1 | 0 ‐ 2.5 | 39 | 0.2 | 0 ‐ 1.7 | 45 | 0.80 (‐1.26, 2.86) |
| Function (categorical rating scale, 0‐27 with higher scores denoting worse function) change from baseline to 5 weeks | 5.3 | 4.1 ‐ 6.5 | 39 | 5.2 | 3.9 ‐ 6.5 | 45 | 0.10 (‐1.62, 1.82) |
| Active range of abduction (degrees) change from baseline to 5 weeks (subgroup with decreased ROM and shoulder pain) | 97 | 81 to 113 | ? | 98 | 82 to 114 | ? | ‐1 (95% CI not estimable) |
| Active range of abduction (degrees) change from baseline to 5 weeks (subgroup with full ROM despite shoulder pain) | 30 | 8 to 52 | ? | 28 | 13 to 44 | ? | 2 (95% CI not estimable) |
| Active range of flexion (degrees) change from baseline to 5 weeks (subgroup with decreased ROM and shoulder pain) | 104 | 92 to 116 | ? | 111 | 102 to 120 | ? | ‐7 (95% CI not estimable) |
| Active range of flexion (degrees) change from baseline to 5 weeks (subgroup with full ROM despite shoulder pain) | 1 | 0 to 14 | ? | 0 | 0 to 8 | ? | 1 (95% CI not estimable) |
| Active hand‐behind‐back distance change from baseline to 5 weeks | 7.3 | 4.7 to 10 | 39 | 7.5 | 4.9 to 10.2 | 45 | ‐0.20 (‐3.77, 3.37) |
| Strength (isometric abduction force %) change from baseline to 5 weeks | 60 | 46 to 75 | 39 | 66 | 55 to 76 | 45 | ‐6.00 (‐23.27, 11.27) |
| % range | Total | % range | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (% participants rated as "improved") at 5 weeks | 33 to 85% | 39 | 35 to 78% | 45 | Not estimable | ||
| Study ID: Hay 2003 Intervention: physiotherapy (all participants: advice and instructions on pain relief and active shoulder exercises at home; dependent on participant: ultrasound and active and passive mobilisation) Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Croft SDQ, 0‐23 with higher scores denoting worse disability) change from baseline to 6 weeks | 2.56 | 5.4 | 99 | 3.03 | 6.3 | 98 | ‐0.47 (‐2.11, 1.17) |
| Function (Croft SDQ, 0‐23 with higher scores denoting worse disability) change from baseline to 6 months | 5.97 | 5.4 | 99 | 4.55 | 5.9 | 97 | 1.42 (‐0.16, 3.00) |
| Median | IQR | n | Median | IQR | n | Mean difference (95% CI) | |
| Night pain (VAS 0‐10) at 6 weeks | 2 | 1 to 4 | 99 | 3 | 0 to 6 | 98 | Not estimable |
| Night pain (VAS 0‐10) at 6 months | 1 | 0 to 3 | 99 | 1 | 0 to 4 | 97 | Not estimable |
| Quality of life (EuroQoL, scored from ‐1 to 1) at 6 weeks | 0.76 | 0.66 ‐ 0.8 | 99 | 0.76 | 0.59 ‐ 0.8 | 98 | Not estimable |
| Quality of life (EuroQoL, scored from ‐1 to 1) at 6 months | 0.76 | 0.69 ‐ 0.88 | 99 | 0.76 | 0.66 ‐ 1 | 97 | Not estimable |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (completely recovered) at 6 weeks | 6 | 100 | 18 | 98 | 0.33 (0.14, 0.79) | ||
| Global assessment of treatment success (completely recovered) at 6 months | 23 | 99 | 17 | 97 | 1.33 (0.76, 2.32) | ||
| Active range of abduction (restriction of > 50% compared with non‐involved arm) at 6 weeks | 40 | 99 | 53 | 98 | 0.75 (0.55, 1.01) | ||
| Active range of abduction (restriction of > 50% compared with non‐involved arm) at 6 months | 31 | 99 | 38 | 97 | 0.80 (0.55, 1.17) | ||
| Active range of external rotation (restriction of > 50% compared with non‐involved arm) at 6 weeks | 8 | 99 | 12 | 98 | 0.66 (0.28, 1.54) | ||
| Active range of external rotation (restriction of > 50% compared with non‐involved arm) at 6 months | 7 | 99 | 8 | 97 | 0.86 (0.32, 2.27) | ||
| Study ID: Rhon 2014 Intervention: joint and soft tissue mobilisations, manual stretches, contract‐relax techniques, supervised exercises and home exercises Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (NPRS 0‐10) at 1 month | 1.6 | 1.93 | 42 | 1.7 | 2.02 | 46 | ‐0.10 (‐0.92, 0.72) |
| Overall pain (NPRS 0‐10) at 6 months | 1.7 | 1.85 | 39 | 2.2 | 2.00 | 45 | ‐0.50 (‐1.32, 0.32) |
| Overall pain (NPRS 0‐10) at 12 months | 2.1 | 2.02 | 46 | 2.5 | 2.07 | 48 | ‐0.40 (‐1.23, 0.43) |
| Function (SPADI total score, 0‐100 where higher scores denote worse function) at 1 month | 22.2 | 18.61 | 42 | 23.2 | 18.52 | 46 | ‐1.00 (‐8.77, 6.77) |
| Function (SPADI total score, 0‐100 where higher scores denote worse function) at 6 months | 21.5 | 17.89 | 39 | 22.2 | 18.64 | 45 | ‐0.70 (‐8.52, 7.12) |
| Function (SPADI total score, 0‐100 where higher scores denote worse function) at 12 months | 21.6 | 18.86 | 46 | 23.1 | 18.60 | 48 | ‐1.50 (‐9.07, 6.07) |
| Quality of life (Global Rating of Change scale, ‐7 to +7) at 1 month | 3 | 3.21 | 42 | 3 | 3.37 | 46 | 0.00 (‐1.37, 1.37) |
| Quality of life (Global Rating of Change scale, ‐7 to +7) at 6 months | 3 | 6.17 | 39 | 3 | 3.33 | 45 | 0.00 (‐2.17, 2.17) |
| Quality of life (Global Rating of Change scale, ‐7 to +7) at 12 months | 3 | 3.37 | 46 | 3 | 3.44 | 48 | 0.00 (‐1.38, 1.38) |
| Adverse events | "Other than transient pain from the CSI [injection], there were no other adverse events reported by patients in either group." | ||||||
| Study ID: Winters 1997 Intervention: exercise and massage Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (shoulder pain score 0‐28, higher score denotes worse pain) at 11 weeks | 11.5 | 4.4 | 35 | 9.2 | 3.7 | 47 | 2.30 (0.50, 4.10) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("cured") at 11 weeks* | 18 | 35 | 42 | 47 | 0.58 (0.41, 0.81) | ||
*Outcome data extracted from Figure using DigitizeIt software
Three trials measured global treatment success (Ginn 2005; Hay 2003; Winters 1997). While Ginn 2005 found no difference between groups at five weeks (effect not estimable), Hay 2003 found 6% (6/100) of participants receiving manual therapy and exercise reported global treatment success at six weeks compared with 18% (18/98) of participants receiving glucocorticoid injection (RR 0.33, 95% CI 0.14 to 0.79; 198 participants). A similar effect was found in Winters 1997 at 11 weeks (51% [18/35] manual therapy and exercise versus 89% [42/47] glucocorticoid injection, RR 0.58, 95% CI 0.41 to 0.81; 82 participants). However, the difference between groups was less certain at six months in Hay 2003 (23% [23/99] manual therapy and exercise versus 18% [17/97] glucocorticoid injection, RR 1.33, 95% CI 0.76 to 2.32; 196 participants). Quality of life was measured in two trials (Hay 2003; Rhon 2014). In Hay 2003, scores were no different between groups at six weeks or six months (95% CIs not estimable). In Rhon 2014, quality of life scores were no different between groups at one month (mean 3 versus 3 on a ‐7 to +7 scale, MD 0.00, 95% CI ‐1.37 to 1.37, 88 participants), six months (mean 3 versus 3 on a ‐7 to +7 scale, MD 0.00, 95% CI ‐2.17 to 2.17, 84 participants) or 12 months (mean 3 versus 3 on a ‐7 to +7 scale, MD 0.00, 95% CI ‐1.38 to 1.38, 94 participants). Night pain scores at six weeks and six months in one trial (Hay 2003) were no different between groups (95% CIs not estimable). Active range of motion was reported in two trials (Ginn 2005; Hay 2003). In Ginn 2005, mean differences between groups in active shoulder abduction, flexion and hand‐behind‐back distance were very small and the precision could not be estimated due to incomplete reporting. In Hay 2003, fewer participants in the manual therapy and exercise group had impairment in active shoulder abduction and external rotation but these differences were not statistically significant (Table 8).
In summary, the overall impression from these five trials is that there were no clinically important differences between manual therapy and exercise and glucocorticoid injection with respect to overall pain, function, quality of life, night pain and active range of motion at both short‐ (four to six weeks) and long‐term (six to 12 months). However, global treatment success was more common up to 11 weeks in participants receiving glucocorticoid injection. The lack of difference at long‐term is not surprising given that glucocorticoid injections are short‐acting interventions which only have evidence of benefit over placebo at short‐term follow‐up (Buchbinder 2003). A key limitation of these trials is the lack of participant blinding, which may have biased results in either direction if participants had different pre‐conceived beliefs about the efficacy of physical therapy and glucocorticoid injection. Therefore, we downgraded by two points for high risk of performance and detection bias, and consider this evidence to be low quality.
Manual therapy and exercise versus NSAID
One trial (39 participants) at high risk of bias overall (Cloke 2008), compared six sessions over 18 weeks of manual therapy and exercise (no details provided) with regular NSAID or "simple analgesic intake" (dose and duration not reported). There was no clinically important difference between groups in function at 18 weeks (mean 27.73 versus 30.47 on a 48‐point scale, MD ‐2.74, 95% CI ‐10.21 to 4.73) or 12 months (mean 28.94 versus 30.07 on a 48‐point scale, MD ‐1.13, 95% CI not estimable, unclear number of participants) (Table 9). We downgraded by two points for high risk of performance and attrition bias, and one point for imprecision, and thus consider this evidence to be very low quality.
6. Manual therapy and exercise versus NSAID.
| Study ID: Cloke 2008 Intervention: manual therapy and exercise (no details provided) Control: NSAIDs | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Oxford Shoulder Score (12‐60) with a higher score indicating worse disability) at 18 weeks | 27.73 | 16 | 22 | 30.47 | 7 | 17 | ‐2.74 (‐10.21, 4.73) |
| Function (Oxford Shoulder Score (12‐60) with a higher score indicating worse disability) at 12 months | 28.94 | NR | NR | 30.07 | NR | NR | ‐1.13 (95% CI not estimable) |
Manual therapy and exercise versus arthroscopic subacromial decompression
See Table 3. One trial (84 participants) at high risk of bias overall (Haahr 2005), compared 12 weeks of manual therapy (soft tissue treatment) and supervised exercises (stabilising and strengthening) with arthroscopic subacromial decompression. There was no clinically important difference between groups in overall pain at six months (mean change 3.7 versus 3.8 on a 15‐point scale, MD ‐0.10, 95% CI ‐1.68 to 1.48, 84 participants), 12 months (mean change 3.7 versus 3.6 on a 15‐point scale, MD 0.10, 95% CI ‐1.49 to 1.69, 84 participants) or four to eight years (mean change 3 versus 1.9 on a 10‐point scale, MD 1.10, 95% CI ‐0.14 to 2.34, 79 participants). There was also no clinically important difference between groups in function at six months (mean change 21.3 versus 19.9 on a 100‐point scale, MD 1.40, 95% CI ‐7.63 to 10.43, 84 participants), 12 months (mean change 23 versus 18.8 on a 100‐point scale, MD 4.20, 95% CI ‐5.03 to 13.43, 84 participants) and four to eight years (mean change 11.4 versus 9.1 on a 36‐point scale, MD 2.30, 95% CI ‐2.06 to 6.66, 79 participants). With respect to the remaining outcomes, there was no clinically important difference between groups in global treatment success at four to eight years (68% [27/40] versus 59% [23/39], RR 1.14, 95% CI 0.82 to 1.61; 79 participants), active range of motion at six months (mean change 10.3 versus 9.6 on a 40‐point scale, MD 0.70, 95% CI ‐3.83 to 5.23, 84 participants) and 12 months (mean change 11.6 versus 8.2 on a 40‐point scale, MD 3.40, 95% CI ‐1.34 to 8.14, 84 participants), or strength at six months (mean change 2.7 versus 2.9 on a 25‐point scale, MD ‐0.20, 95% CI ‐2.50 to 2.10, 84 participants) and 12 months (mean change 3.2 versus 3.3 on a 25‐point scale, MD ‐0.10, 95% CI ‐2.68 to 2.48, 84 participants) (Table 10). The lack of patient blinding may have influenced patients in both groups to pursue alternative interventions and influenced their responses to self‐reported outcomes, which may have biased results in either direction. For this reason we downgraded by two points for high risk of performance and detection bias, and consider this evidence to be low quality.
7. Manual therapy and exercise versus arthroscopic subacromial decompression.
| Study ID: Haahr 2005 Intervention: exercises plus heat, cold packs or soft tissue treatment (i.e. not all participants received soft tissue treatment) Control: arthroscopic subacromial decompression | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (Constant‐Murley pain sub‐score, 0‐15 with higher = less pain) change from baseline to 6 months | 3.7 | 3.57 | 43 | 3.8 | 3.80 | 41 | ‐0.10 (‐1.68, 1.48) |
| Overall pain (Constant‐Murley pain sub‐score, 0‐15 with higher = less pain) change from baseline to 12 months | 3.7 | 3.25 | 43 | 3.6 | 4.12 | 41 | 0.10 (‐1.49, 1.69) |
| Overall pain (VAS 0‐9, 0 = no pain) change from baseline to 4‐8 years | 3 | 2.50 | 40 | 1.9 | 3.08 | 39 | 1.10 (‐0.14, 2.34) |
| Function (Constant‐Murley total score, 0‐100 with higher = better function) change from baseline to 6 months | 21.3 | 19.17 | 43 | 19.9 | 22.81 | 41 | 1.40 (‐7.63, 10.43) |
| Function (Constant‐Murley total score, 0‐100 with higher = better function) change from baseline to 12 months | 23 | 19.82 | 43 | 18.8 | 23.13 | 41 | 4.20 (‐5.03, 13.43) |
| Function (total PRIM score 0‐36, higher = worse function) change from baseline to 4‐8 years | 11.4 | 8.44 | 40 | 9.1 | 11.11 | 39 | 2.30 (‐2.06, 6.66) |
| Active ROM (Constant‐Murley ROM sub‐score, 0‐40 with higher scores denoting better ROM) change from baseline to 6 months | 10.3 | 10.40 | 43 | 9.6 | 10.77 | 41 | 0.70 (‐3.83, 5.23) |
| Active ROM (Constant‐Murley ROM sub‐score, 0‐40 with higher scores denoting better ROM) change from baseline to 12 months | 11.6 | 10.72 | 43 | 8.2 | 11.41 | 41 | 3.40 (‐1.34, 8.14) |
| Strength (Constant‐Murley force sub‐score, 0‐25 with higher scores denoting better strength) change from baseline to 6 months | 2.7 | 3.57 | 43 | 2.9 | 6.65 | 41 | ‐0.20 (‐2.50, 2.10) |
| Strength (Constant‐Murley force sub‐score, 0‐25 with higher scores denoting better strength) change from baseline to 12 months | 3.2 | 4.87 | 43 | 3.3 | 6.97 | 41 | ‐0.10 (‐2.68, 2.48) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (recovered or improved) at 4‐8 years | 27 | 40 | 23 | 39 | 1.14 (0.82, 1.61) | ||
| Work disability (self‐reporting as currently working) at 4 to 8 years | 21 | 40 | 20 | 39 | 1.02 (0.67, 1.57) | ||
Manual therapy and exercise versus naturopathic care (dietary counselling, acupuncture, and Phlogenzym supplement)
One trial (85 participants) at high risk of bias overall (Szczurko 2009), compared naturopathic care comprising dietary counselling and acupuncture once per week and daily Phlogenzym supplement (recommended by some naturopaths for pain relief) for 12 weeks with manual therapy and supervised exercises (range of motion and strengthening) once per week and daily placebo tablet for 12 weeks. The authors observed clinically important differences favouring naturopathic care over manual therapy and exercise at 12 weeks with respect to overall pain (mean 2.75 versus 4.05 on a seven‐point scale, MD 1.30, 95% CI 0.56 to 2.04) and function (mean 35.3 versus 56.24 on a 130‐point scale, MD 20.94, 95% CI 6.40 to 35.48). Further, several domains of the SF‐36 quality of life measure (physical functioning, role limitations due to physical health, bodily pain, and general health), and active shoulder abduction, flexion and extension were statistically significantly lower in the manual therapy and exercise group at 12 weeks (see Table 11). However, the lack of participant blinding may have biased results in either direction if participants had different pre‐conceived beliefs about the efficacy of physical therapy and naturopathic care. Therefore, we downgraded by two points for high risk of performance and detection bias, and thus consider this evidence to be low quality.
8. Manual therapy and exercise versus naturopathic care.
| Study ID: Szczurko 2009 Intervention: physical exercise, hands on shoulder muscle and joint therapy and placebo tablets Control: naturopathic care (dietary counselling, acupuncture, and Phlogenzym supplement) | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐7) at 12 weeks | 4.05 | 1.69 | 42 | 2.75 | 1.77 | 43 | 1.30 (0.56, 2.04) |
| Function (SPADI total score 0‐130, with higher scores denoting worse function) at 12 weeks | 56.24 | 36.57 | 42 | 35.3 | 31.57 | 43 | 20.94 (6.40, 35.48) |
| Quality of life (SF‐36 Mental Component Score, 0‐100 with higher scores better) at 12 weeks | 50.05 | 10.4 | 42 | 50.08 | 10.97 | 43 | ‐0.03 (‐4.57, 4.51) |
| Quality of life (SF‐36 physical functioning score, 0‐100 with higher scores better) at 12 weeks | 61.29 | 22.4 | 42 | 74.02 | 25.2 | 43 | ‐12.73 (‐22.86, ‐2.60) |
| Quality of life (SF‐36 role‐physical score, 0‐100 with higher scores better) at 12 weeks | 61.61 | 22.85 | 42 | 72.71 | 24.68 | 43 | ‐11.10 (‐21.21, ‐0.99) |
| Quality of life (SF‐36 bodily pain score, 0‐100 with higher scores better) at 12 weeks | 47.81 | 19.67 | 42 | 59.6 | 19.7 | 43 | ‐11.79 (‐20.16, ‐3.42) |
| Quality of life (SF‐36 general health score, 0‐100 with higher scores better) at 12 weeks | 56.6 | 24.99 | 42 | 70.44 | 19.08 | 43 | ‐13.84 (‐23.31, ‐4.37) |
| Quality of life (SF‐36 vitality score, 0‐100 with higher scores better) at 12 weeks | 56.08 | 18.21 | 42 | 63.72 | 20.55 | 43 | ‐7.64 (‐15.89, 0.61) |
| Quality of life (SF‐36 social functioning score, 0‐100 with higher scores better) at 12 weeks | 74.13 | 23.54 | 42 | 77.44 | 24.72 | 43 | ‐3.31 (‐13.57, 6.95) |
| Quality of life (SF‐36 role‐emotional score, 0‐100 with higher scores better) at 12 weeks | 74.12 | 27.24 | 42 | 80.08 | 23.63 | 43 | ‐5.96 (‐16.81, 4.89) |
| Quality of life (SF‐36 mental health score, 0‐100 with higher scores better) at 12 weeks | 71.81 | 18.83 | 42 | 74.51 | 18.83 | 43 | ‐2.70 (‐10.71, 5.31) |
| Active range of abduction (degrees) at 12 weeks | 105.36 | 45.05 | 42 | 148.63 | 34.73 | 43 | ‐43.27 (‐60.40, ‐26.14) |
| Active range of flexion (degrees) at 12 weeks | 121.08 | 40.53 | 42 | 159.39 | 25.97 | 43 | ‐38.31 (‐52.82, ‐23.80) |
| Active range of extension (degrees) at 12 weeks | 35.44 | 10.26 | 42 | 42.39 | 11.18 | 43 | ‐6.95 (‐11.51, ‐2.39) |
| Active range of adduction (degrees) at 12 weeks | 36.28 | 11.05 | 42 | 35.39 | 7.42 | 43 | 0.89 (‐3.12, 4.90) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Adverse events | 5 | 42 | 2 | 43 | 2.56 (0.53, 12.47) | ||
Is manual therapy and exercise delivered in addition to another active intervention more effective than the other active intervention alone?
Manual therapy and exercise and glucocorticoid injection versus glucocorticoid injection
One trial (47 participants) at high risk of bias overall (Cloke 2008), compared six sessions over 18 weeks of manual therapy and exercise (no details provided) along with glucocorticoid injection (single injection at six, 12 and 18 weeks) with glucocorticoid injection alone. There was no clinically important difference between groups in function at 18 weeks (mean 27.8 versus 29.81 on a 48‐point scale, MD ‐2.01, 95% CI ‐13.09 to 9.07) or at 12 months (mean 23.79 versus 26.47 on a 48‐point scale, MD ‐2.68, 95% CI not estimable) (Table 12). We downgraded by two points for high risk of performance and attrition bias, and one point for imprecision, and thus consider this evidence to be very low quality.
9. Manual therapy and exercise and glucocorticoid injection versus glucocorticoid injection.
| Study ID: Cloke 2008 Intervention: exercise and manual therapy package plus glucocorticoid injection Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Oxford Shoulder Score (12‐60) with a higher score indicating worse disability) at 18 weeks | 27.8 | 22.5 | 20 | 29.81 | 13.4 | 27 | ‐2.01 (‐13.09, 9.07) |
| Function (Oxford Shoulder Score (12‐60) with a higher score indicating worse disability) at 12 months | 23.79 | NR | NR | 26.47 | NR | NR | ‐2.68 (95% CI not estimable) |
Is manual therapy alone more effective than placebo, no intervention or another active intervention?
Twelve trials compared manual therapy alone with either placebo (Barra 2011; Haik 2014; Kardouni 2014; McClatchie 2009; Munday 2007; Surenkok 2009; Teys 2008), no intervention (Surenkok 2009; Teys 2008; Van den Dolder 2003), or another active intervention (Al Dajah 2014; Bansal 2011; Kaya 2014; Winters 1997).
Manual therapy alone versus placebo
The types of manual therapy investigated in the seven trials that compared it to placebo were diacutaneous fibrolysis (Barra 2011), thoracic spinal manipulative therapy (Haik 2014; Kardouni 2014), lateral cervical glide mobilisation (McClatchie 2009), chiropractic shoulder girdle adjustments (Munday 2007), scapular mobilisation (Surenkok 2009), and mobilisation with movement (Teys 2008). The placebo treatment consisted of sham mobilisation or manipulation in six trials (Barra 2011; Haik 2014; Kardouni 2014; McClatchie 2009; Surenkok 2009; Teys 2008) and detuned ultrasound in one trial (Munday 2007). Six trials were considered to be at unclear risk of bias overall due to unclear allocation concealment (Barra 2011; Haik 2014; McClatchie 2009; Surenkok 2009; Teys 2008) or unclear blinding of outcome assessment and attrition (Munday 2007), while one was rated at low risk of bias overall (Kardouni 2014).
Barra 2011 found that participants receiving one session of diacutaneous fibrolysis were more likely to report global treatment success (RR 2.14, 95% CI 1.06 to 4.34; 50 participants) and have greater improvement in active shoulder abduction, flexion and internal rotation immediately post‐treatment (see Table 13). Across the trials there were no clinically important differences between groups in overall pain (Barra 2011, Kardouni 2014; McClatchie 2009, Munday 2007, Surenkok 2009), function (Kardouni 2014; Surenkok 2009), pain on motion (Haik 2014), or quality of life (Kardouni 2014). Three trials measured adverse events (Barra 2011; Munday 2007; Teys 2008); none were reported in Barra 2011 and Teys 2008 while in Munday 2007 there were no reports of serious adverse reactions to shoulder girdle adjustment (such as persistent severe stiffness or pain) although there were five reports of minor, temporary post‐treatment soreness (Table 13). We downgraded by one point for unclear risk of allocation bias in most trials, and one point for imprecision in all trials, and thus consider this evidence to be low quality.
10. Manual therapy alone versus placebo.
| Study ID: Barra 2011 Intervention: diacutaneous fibrolysis Control: placebo diacutaneous fibrolysis | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) change from baseline to immediately post 1 treatment session (i.e. same day) | 9.2 | 15.2 | 25 | 7.5 | 13.7 | 25 | 1.70 (‐6.32, 9.72) |
| Active range of abduction (degrees) change from baseline to immediately post 1 treatment session (i.e. same day) | 7.9 | 9.6 | 25 | 0.6 | 8.7 | 25 | 7.30 (2.22, 12.38) |
| Active range of flexion (degrees) change from baseline to immediately post 1 treatment session (i.e. same day) | 9.5 | 10.9 | 25 | ‐1.9 | 9 | 25 | 11.40 (5.86, 16.94) |
| Active range of extension (degrees) change from baseline to immediately post 1 treatment session (i.e. same day) | 2.1 | 5.7 | 25 | 0.2 | 6.4 | 25 | 1.90 (‐1.46, 5.26) |
| Active range of external rotation (degrees) change from baseline to immediately post 1 treatment session (i.e. same day) | 0.8 | 9.6 | 25 | 0.2 | 5.2 | 25 | 0.60 (‐3.68, 4.88) |
| Active range of internal rotation (hand behind back distance in cm) change from baseline to immediately post 1 treatment session (i.e. same day) | 4.5 | 6.8 | 25 | 1.4 | 3.1 | 25 | 3.10 (0.17, 6.03) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("some or a lot of improvement") immediately post 1 treatment session (i.e. same day) | 15 | 25 | 7 | 25 | 2.14 (1.06, 4.34) | ||
| Total adverse events | Zero events in both groups | ||||||
| Study ID: Haik 2014 Intervention: thoracic spine manipulation Control: sham manipulation | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Pain on motion (VAS 0‐10) immediately post 1 treatment session (i.e. same day) | 2.4 | 2.7 | 25 | 2.2 | 2.3 | 25 | 0.20 (‐1.19, 1.59) |
| Study ID: Kardouni 2014 Intervention: thoracic spinal manipulative therapy Control: sham manipulative therapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10, higher = more pain) at 1‐2 days | 2.4 | 1.6 | 24 | 2 | 1.5 | 21 | 0.40 (‐0.51, 1.31) |
| Function (Penn Shoulder Score 0‐100, higher = better function) at 1‐2 days | 80.6 | 11.1 | 24 | 83 | 9.8 | 21 | ‐2.40 (‐8.51, 3.71) |
| Quality of life (Global Rating of Change, from ‐7 (a great deal worse) to +7 (a great deal better)) at 1‐2 days | 1.3 | 2 | 24 | 2 | 2.2 | 21 | ‐0.70 (‐1.94, 0.54) |
| Study ID: McClatchie 2009 Intervention: lateral cervical glide mobilisation Control: placebo mobilisation | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 1‐4 days | 2.4 | 2.1 | 7 | 3.2 | 2.5 | 14 | ‐0.80 (‐2.83, 1.23) |
| Strength (shoulder abduction strength, kilogram‐force) change from baseline to 1‐4 days | ‐0.01 | 1.1 | 7 | ‐0.4 | 0.9 | 14 | 0.39 (‐0.55, 1.33) |
| Study ID: Munday 2007 Intervention: shoulder girdle adjustments (chiropractic) Control: placebo ultrasound | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) at 3 weeks | 16.73 | 18.19 | 15 | 24 | 11.72 | 15 | ‐7.27 (‐18.22, 3.68) |
| Overall pain (VAS 0‐100) at 7 weeks | 10.73 | 18.72 | 15 | 19.83 | 12.26 | 15 | ‐9.10 (‐20.42, 2.22) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Adverse events | "In this study there were not reports of serious adverse reactions to shoulder girdle adjustment (such as persistent severe stiffness and/or pain) although there were 5 reports of minor, temporary post‐treatment soreness" | ||||||
| Study ID: Surenkok 2009 Intervention: scapular mobilisation Control: sham mobilisation | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) immediately post‐treatment (day 1) | 35.84 | 21.62 | 13 | 20.15 | 24.7 | 13 | 15.69 (‐2.15, 33.53) |
| Function (Constant‐Murley total score 0‐100, with higher scores denoting better function) immediately post‐treatment (day 1) | 54.3 | 5.9 | 13 | 51.84 | 6.51 | 13 | 2.46 (‐2.32, 7.24) |
| Pain on motion (VAS 0‐100) immediately post‐treatment (day 1) | 61.3 | 23.45 | 13 | 42.76 | 32.84 | 13 | 18.54 (‐3.40, 40.48) |
| Active range of abduction (degrees) immediately post‐treatment (day 1) | 149.07 | 38.21 | 13 | 132.3 | 36.17 | 13 | 16.77 (‐11.83, 45.37) |
| Active range of flexion (degrees) immediately post‐treatment (day 1) | 167.3 | 15.89 | 13 | 154.38 | 16.78 | 13 | 12.92 (0.36, 25.48) |
| Study ID: Teys 2008 Intervention: mobilisation with movement Control: sham mobilisation | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Active ROM (elevation, degrees) immediately post‐treatment (day 1) | NR | NR | NR | NR | NR | NR | 9.9 (4.3, 15.6) |
| Adverse events | 0 events in both groups | ||||||
Manual therapy alone versus no treatment
We judged all three trials that compared manual therapy to no treatment to be at high risk of bias overall. Van den Dolder 2003 found that two weeks of soft tissue massage led to less overall pain (MD ‐22.00, 95% CI ‐41.19 to ‐2.81; 100‐point scale, 29 participants), better function (MD 7.20, 95% CI 2.20 to 12.20; 30‐point scale, 29 participants), and more active shoulder abduction, flexion and internal rotation than no treatment. Further, one session of mobilisation with movement increased active shoulder elevation in Teys 2008. In contrast, Surenkok 2009 found that one session of scapular mobilisation led to no important differences in overall pain (MD ‐8.96, 95% CI ‐33.01 to 15.09; 100‐point scale, 26 participants), function (MD 9.07, 95% CI ‐12.09 to 30.23; 100‐point scale, 26 participants), pain on motion (MD ‐2.08, 95% CI ‐19.49 to 15.33; 100‐point scale, 26 participants) and active shoulder abduction or flexion when compared with no treatment (Table 14). We downgraded by two points for high risk of performance and detection bias, one point for imprecision and one point for inconsistency, and thus consider this evidence to be very low quality.
11. Manual therapy alone versus no treatment.
| Study ID: Surenkok 2009 Intervention: scapular mobilisation Control: no treatment | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) immediately post‐treatment (day 1) | 35.84 | 21.62 | 13 | 44.8 | 38.6 | 13 | ‐8.96 (‐33.01, 15.09) |
| Function (Constant‐Murley total score 0‐100, with higher scores denoting better function) immediately post‐treatment (day 1) | 54.3 | 5.9 | 13 | 45.23 | 38.48 | 13 | 9.07 (‐12.09, 30.23) |
| Pain on motion (VAS 0‐100) immediately post‐treatment (day 1) | 61.3 | 23.45 | 13 | 63.38 | 21.81 | 13 | ‐2.08 (‐19.49, 15.33) |
| Active range of abduction (degrees) immediately post‐treatment (day 1) | 149.07 | 38.21 | 13 | 144.07 | 29 | 13 | 5.00 (‐21.08, 31.08) |
| Active range of flexion (degrees) immediately post‐treatment (day 1) | 167.3 | 15.89 | 13 | 160.69 | 21.53 | 13 | 6.61 (‐7.94, 21.16) |
| Study ID: Teys 2008 Intervention: mobilisation with movement Control: no treatment | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Active ROM (elevation, degrees) immediately post‐treatment (day 1) | NR | NR | NR | NR | NR | NR | 11.4 (2.3, 20.5) |
| Adverse events | Zero events in both groups | ||||||
| Study ID: Van den Dolder 2003 Intervention: soft tissue massage Control: wait‐list control | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100 on Short‐Form McGill Pain Questionnaire) at 2 weeks | 31.8 | 26.4 | 15 | 53.8 | 26.3 | 14 | ‐22.00 (‐41.19, ‐2.81) |
| Function (Patient Specific Functional Disability Measure (PSFDM), 0 – 30 with higher score denoting better function) at 2 weeks | 17.6 | 8 | 15 | 10.4 | 5.6 | 14 | 7.20 (2.20, 12.20) |
| Active range of abduction (degrees) at 2 weeks | 135.6 | 24.1 | 15 | 91.2 | 28.6 | 14 | 44.40 (25.08, 63.72) |
| Active range of flexion (degrees) at 2 weeks | 129.5 | 18.5 | 15 | 103.4 | 23.1 | 14 | 26.10 (10.80, 41.40) |
| Active hand behind back distance (cm) at 2 weeks | 19.9 | 10.2 | 15 | 8.1 | 16.2 | 14 | 11.80 (1.87, 21.73) |
Manual therapy alone versus another active intervention
We judged the four trials that compared manual therapy to another active intervention to be at high risk of bias overall. Al Dajah 2014 found soft tissue mobilisation plus proprioceptive neuromuscular facilitation resulted in statistically significantly less overall pain (MD ‐1.43, 95% CI ‐1.97 to ‐0.89; 10‐point scale, 30 participants) and greater external rotation immediately post‐treatment compared with therapeutic ultrasound, though these differences were not clinically important. Bansal 2011 compared deep friction massage with therapeutic ultrasound, and found no clinically important differences between groups in overall pain (MD ‐0.7, 95% CI not estimable; 10‐point scale, 40 participants) and active shoulder abduction at the end of 10 days' treatment. Kaya 2014 found no clinically important differences between manual therapy and kinesiotaping with respect to rest pain (MD ‐0.32, 95% CI ‐1.48 to 0.84; 10‐point scale, 54 participants), function (MD ‐3.10, 95% CI ‐11.40 to 5.20; 100‐point scale, 54 participants), and pain on motion (MD 1.19, 95% CI ‐0.02 to 2.40; 10‐point scale, 54 participants) at 6 weeks, but night pain was higher in the manual therapy group (MD 1.91, 95% CI 0.47 to 3.35; 10‐point scale, 54 participants). Winters 1997 found that participants receiving shoulder manipulation once a week for six weeks had overall pain that was statistically significantly higher (MD 3.40, 95% CI 1.34 to 5.46; 28‐point scale, 79 participants) and were half as likely to have global treatment success (RR 0.49, 95% CI 0.33 to 0.73; 79 participants) at 11 weeks than participants receiving glucocorticoid injection (Table 15). We downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and thus consider this evidence to be very low quality.
12. Manual therapy alone versus another active intervention.
| Study ID: Al Dajah 2014 Intervention: soft tissue mobilisation plus proprioceptive neuromuscular facilitation Control: therapeutic ultrasound | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) immediately after one treatment session (day 1) | 3.8 | 0.79 | 15 | 5.23 | 0.72 | 15 | ‐1.43 (‐1.97, ‐0.89) |
| External rotation (degrees, unclear if active or passive) immediately after one treatment session (day 1) | 52.4 | 4.9 | 15 | 40.33 | 5.6 | 15 | 12.07 (8.30, 15.84) |
| Study ID: Bansal 2011 Intervention: deep friction massage technique plus Codman's exercises Control: ultrasound therapy plus Codman's exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 10 days | 1.4 | NR | 20 | 2.1 | NR | 20 | ‐0.7 (95% CI not estimable) |
| Active range of abduction (degrees) at 10 days | 107.15 | NR | 20 | 105.65 | NR | 20 | 1.5 (95% CI not estimable) |
| Study ID: Kaya 2014 Intervention: manual therapy and exercise plus cold pack Control: kinesiotaping plus exercise plus cold pack | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10) at 6 weeks | 1.5 | 2.28 | 26 | 1.82 | 2.05 | 28 | ‐0.32 (‐1.48, 0.84) |
| Function (DASH 0‐100, higher = worse function) at 6 weeks | 35.61 | 15.66 | 26 | 38.71 | 15.41 | 28 | ‐3.10 (‐11.40, 5.20) |
| Pain on motion (VAS 0‐10) at 6 weeks | 5.11 | 2.68 | 26 | 3.92 | 1.71 | 28 | 1.19 (‐0.02, 2.40) |
| Night pain (VAS 0‐10) at 6 weeks | 3.19 | 3.28 | 26 | 1.28 | 1.88 | 28 | 1.91 (0.47, 3.35) |
| Study ID: Winters 1997 Intervention: manipulation Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (shoulder pain score 0‐28, higher score denotes worse pain) at 11 weeks | 12.6 | 5.1 | 32 | 9.2 | 3.7 | 47 | 3.40 (1.34, 5.46) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("cured") at 11 weeks | 14 | 32 | 42 | 47 | 0.49 (0.33, 0.73) | ||
Is manual therapy delivered in addition to another active intervention more effective than the other active intervention alone?
Thirteen trials examined whether there is benefit in adding manual therapy (either mobilisation, manipulation or massage) to another physical therapy intervention (either an exercise programme, an electrotherapy modality or multi‐modal physical therapy) (Atkinson 2008; Bang 2000; Barbosa 2008; Barra Lopez 2013; Bialoszewski 2011; Clews 1987; Conroy 1998; Cook 2014; Janse van Rensburg 2012; Kachingwe 2008; Kromer 2013; Senbursa 2011; Yiasemides 2011). All except one trial were rated at high risk of bias overall due to lack of participant blinding; Conroy 1998 blinded participants but was at unclear risk of bias overall due to unclear allocation concealment. Due to the heterogeneous diagnoses of shoulder pain and content of interventions, we chose not to synthesise any data in meta‐analyses.
For overall pain, seven out of nine trials reported mean differences favouring the group with manual therapy as an add‐on, but the difference was clinically important in only three of these trials (Bang 2000; Bialoszewski 2011; Conroy 1998). For function, five out of eight trials had mean differences favouring the group with manual therapy as an add‐on; however none of the differences in any trial were clinically important. Only four trials measured adverse events (Atkinson 2008; Cook 2014; Janse van Rensburg 2012; Kromer 2013) and none were reported by any participant. Pain on motion was measured in one trial (Bang 2000), where a clinically important difference favouring the group with manual therapy as an add‐on was noted. There were only slight differences between groups in the number of participants with global treatment success in the three trials which measured this outcome (Barra Lopez 2013; Kromer 2013; Yiasemides 2011). Of seven trials measuring range of motion (Atkinson 2008; Barra Lopez 2013; Bialoszewski 2011; Conroy 1998; Janse van Rensburg 2012; Kachingwe 2008; Yiasemides 2011), only Barra Lopez 2013 and Bialoszewski 2011 found a statistically significant, albeit small, difference between groups on some measures (Table 16). The overall impression from these trials is that adding manual therapy to another physical therapy intervention infrequently conferred clinically important benefits over the other physical therapy intervention alone. We downgraded by two points for high risk of performance and detection bias, and one point for imprecision, and thus consider the evidence from these 13 trials to be very low quality.
13. Manual therapy alone as an add‐on to another physical therapy intervention versus the other physical therapy intervention.
| Study ID: Atkinson 2008 Intervention: manipulation plus mobilisation Control: sham laser plus mobilisation | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (NRS 0‐100) at 2 weeks | 23.2 | 15.5 | 30 | 30.1 | 17.4 | 30 | ‐6.90 (‐15.24, 1.44) |
| Range of abduction (degrees, unclear if active or passive) at 2 weeks | 157.2 | 27.5 | 30 | 155.8 | 26.7 | 30 | 1.40 (‐12.32, 15.12) |
| Range of flexion (degrees, unclear if active or passive) at 2 weeks | 167.9 | 17.4 | 30 | 165.8 | 18.4 | 30 | 2.10 (‐6.96, 11.16) |
| Range of extension (degrees, unclear if active or passive) at 2 weeks | 71.8 | 9.3 | 30 | 70 | 10 | 30 | 1.80 (‐3.09, 6.69) |
| Range of adduction (degrees, unclear if active or passive) at 2 weeks | 66.2 | 12.2 | 30 | 64.5 | 12.6 | 30 | 1.70 (‐4.58, 7.98) |
| Range of external rotation (degrees, unclear if active or passive) at 2 weeks | 75.5 | 16.6 | 30 | 72.9 | 17.9 | 30 | 2.60 (‐6.14, 11.34) |
| Range of internal rotation (degrees, unclear if active or passive) at 2 weeks | 58.3 | 9.1 | 30 | 57.4 | 10.6 | 30 | 0.90 (‐4.10, 5.90) |
| Total adverse events | Zero events in both groups | ||||||
| Study ID: Bang 2000 Intervention: manual physical therapy plus supervised flexibility and strengthening exercises Control: supervised flexibility and strengthening exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (composite of five 0‐100mm VAS pain scores, total score range 0‐500) at 8 weeks | 174.41 | 183.06 | 27 | 360.64 | 272.32 | 22 | ‐186.23 (‐319.33, ‐53.13) |
| Function (Functional Assessment Questionnaire 0‐45, with higher scores denoting better function) at 8 weeks | 38.22 | 4.68 | 27 | 33.26 | 7.84 | 23 | 4.96 (1.30, 8.62) |
| Pain on motion (active abduction, 0‐100mm VAS) at 8 weeks | 16.82 | 21.02 | 27 | 37.54 | 29.01 | 23 | ‐20.72 (‐34.98, ‐6.46) |
| Resisted abduction pain (0‐100mm VAS) at 8 weeks | 22.7 | 26.27 | 27 | 32.64 | 29.45 | 23 | ‐9.94 (‐25.53, 5.65) |
| Resisted external rotation pain (0‐100mm VAS) at 8 weeks | 15.85 | 21.92 | 27 | 30.23 | 29.72 | 23 | ‐14.38 (‐29.07, 0.31) |
| Resisted internal rotation pain (0‐100mm VAS) at 8 weeks | 21.04 | 27.97 | 27 | 33.5 | 27.57 | 23 | ‐12.46 (‐27.90, 2.98) |
| Isometric abduction strength (Newtons) at 8 weeks | 225.3 | 111.86 | 27 | 147.14 | 81.11 | 23 | 78.16 (24.50, 131.82) |
| Isometric external strength (Newtons) at 8 weeks | 159.05 | 77.83 | 27 | 101.88 | 42.06 | 23 | 57.17 (23.15, 91.19) |
| Isometric internal strength (Newtons) at 8 weeks | 191.96 | 82.29 | 27 | 153.62 | 58.63 | 23 | 38.34 (‐0.87, 77.55) |
| Study ID: Barbosa 2008 Intervention: mobilisation plus eccentric muscle training plus therapeutic ultrasound Control: eccentric muscle training plus therapeutic ultrasound | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant‐Murley total score, 0‐100) at end of 4 weeks treatment | 84.43 | 6.97 | 14 | 74.14 | 5.18 | 14 | 10.29 (5.74, 14.84) |
| Study ID: Barra Lopez 2013 Intervention: diacutaneous fibrolysis plus standardised physiotherapy Control: placebo diacutaneous fibrolysis plus standardised physiotherapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) change from baseline to 3 weeks | 22.5 | 19.3 | 40 | 18.9 | 28.9 | 40 | 3.60 (‐7.17, 14.37) |
| Overall pain (VAS 0‐100) change from baseline to 3 months | 28.6 | 24.1 | 40 | 27.1 | 32 | 40 | 1.50 (‐10.91, 13.91) |
| Function (Constant‐Murley score 0‐100) change from baseline to 3 weeks | 9.9 | 8.5 | 40 | 6.2 | 6.3 | 40 | 3.70 (0.42, 6.98) |
| Function (Constant‐Murley score 0‐100) change from baseline to 3 months | 12.4 | 11.7 | 40 | 8.7 | 9.8 | 40 | 3.70 (‐1.03, 8.43) |
| Active range of abduction (degrees) change from baseline to 3 weeks | 12 | 21.2 | 40 | 8.3 | 15.8 | 40 | 3.70 (‐4.49, 11.89) |
| Active range of abduction (degrees) change from baseline to 3 months | 14.3 | 28.1 | 40 | 9.6 | 17 | 40 | 4.70 (‐5.48, 14.88) |
| Active range of flexion (degrees) change from baseline to 3 weeks | 12.3 | 17.5 | 40 | 8.3 | 18.3 | 40 | 4.00 (‐3.85, 11.85) |
| Active range of flexion (degrees) change from baseline to 3 months | 16.5 | 21.5 | 40 | 12.1 | 19.9 | 40 | 4.40 (‐4.68, 13.48) |
| Active range of extension (degrees) change from baseline to 3 weeks | 5.7 | 9 | 40 | 3.3 | 8.3 | 40 | 2.40 (‐1.39, 6.19) |
| Active range of extension (degrees) change from baseline to 3 months | 6.3 | 9.5 | 40 | 4.4 | 8.2 | 40 | 1.90 (‐1.99, 5.79) |
| Active range of external rotation (degrees) change from baseline to 3 weeks | 5.9 | 9.5 | 40 | 4.7 | 12.2 | 40 | 1.20 (‐3.59, 5.99) |
| Active range of external rotation (degrees) change from baseline to 3 months | 6.1 | 11.4 | 40 | 5.1 | 12.4 | 40 | 1.00 (‐4.22, 6.22) |
| Active range of internal rotation (hand behind back distance in cm) change from baseline to 3 weeks | 2.7 | 5.9 | 40 | 2.2 | 5.1 | 40 | 0.50 (‐1.92, 2.92) |
| Active range of internal rotation (hand behind back distance in cm) change from baseline to 3 months | 3.5 | 8.3 | 40 | 2.7 | 7.8 | 40 | 0.80 (‐2.73, 4.33) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success self‐reported as "better" or "much better") at 3 weeks | 33 | 37 | 28 | 37 | 1.18 (0.95, 1.46) | ||
| Study ID: Barra Lopez 2013 Intervention: diacutaneous fibrolysis plus standardised physiotherapy Control: standardised physiotherapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) change from baseline to 3 weeks | 22.5 | 19.3 | 40 | 15.1 | 24.6 | 40 | 7.40 (‐2.29, 17.09) |
| Overall pain (VAS 0‐100) change from baseline to 3 months | 28.6 | 24.1 | 40 | 22.7 | 26.6 | 40 | 5.90 (‐5.22, 17.02) |
| Function (Constant‐Murley score 0‐100) change from baseline to 3 weeks | 9.9 | 8.5 | 40 | 4.2 | 7.8 | 40 | 5.70 (2.12, 9.28) |
| Function (Constant‐Murley score 0‐100) change from baseline to 3 months | 12.4 | 11.7 | 40 | 9.9 | 8.9 | 40 | 2.50 (‐2.06, 7.06) |
| Active range of abduction (degrees) change from baseline to 3 weeks | 12 | 21.2 | 40 | 4.5 | 22.5 | 40 | 7.50 (‐2.08, 17.08) |
| Active range of abduction (degrees) change from baseline to 3 months | 14.3 | 28.1 | 40 | 14.6 | 24.9 | 40 | ‐0.30 (‐11.94, 11.34) |
| Active range of flexion (degrees) change from baseline to 3 weeks | 12.3 | 17.5 | 40 | 2.5 | 19.4 | 40 | 9.80 (1.70, 17.90) |
| Active range of flexion (degrees) change from baseline to 3 months | 16.5 | 21.5 | 40 | 14.8 | 21.1 | 40 | 1.70 (‐7.64, 11.04) |
| Active range of extension (degrees) change from baseline to 3 weeks | 5.7 | 9 | 40 | ‐1.3 | 7.3 | 40 | 7.00 (3.41, 10.59) |
| Active range of extension (degrees) change from baseline to 3 months | 6.3 | 9.5 | 40 | 0.6 | 8.3 | 40 | 5.70 (1.79, 9.61) |
| Active range of external rotation (degrees) change from baseline to 3 weeks | 5.9 | 9.5 | 40 | ‐0.6 | 9.2 | 40 | 6.50 (2.40, 10.60) |
| Active range of external rotation (degrees) change from baseline to 3 months | 6.1 | 11.4 | 40 | 0 | 12 | 40 | 6.10 (0.97, 11.23) |
| Active range of internal rotation (hand behind back distance in cm) change from baseline to 3 weeks | 2.7 | 5.9 | 40 | 0.9 | 4.9 | 40 | 1.80 (‐0.58, 4.18) |
| Active range of internal rotation (hand behind back distance in cm) change from baseline to 3 months | 3.5 | 8.3 | 40 | 2.7 | 6.6 | 40 | 0.80 (‐2.49, 4.09) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success self‐reported as "better" or "much better") at 3 weeks | 33 | 37 | 26 | 38 | 1.30 (1.02, 1.66) | ||
| Study ID: Biasoszewski 2011 Intervention: Manual therapy plus TENS plus ultrasound plus exercise Control: TENS plus ultrasound plus exercise | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) change from baseline to end of 4 treatment sessions (exact timing unclear) | 5.27 | 2.28 | 15 | 3.2 | 1.32 | 15 | 2.07 (0.74, 3.40) |
| Active range of abduction (degrees) at the end of 4 treatment sessions (exact timing unclear) | 147 | 37.93 | 15 | 130 | 26.19 | 15 | 17.00 (‐6.33, 40.33) |
| Active range of flexion (degrees) at the end of 4 treatment sessions (exact timing unclear) | 156.67 | 93.4 | 15 | 143 | 23.74 | 15 | 13.67 (‐35.10, 62.44) |
| Active range of external rotation (degrees) at the end of 4 treatment sessions (exact timing unclear) | 50.67 | 8.63 | 15 | 40.33 | 8.55 | 15 | 10.34 (4.19, 16.49) |
| Active range of internal rotation (degrees) at the end of 4 treatment sessions (exact timing unclear) | 61.67 | 13.18 | 15 | 54.67 | 13.43 | 15 | 7.00 (‐2.52, 16.52) |
| Study ID: Clews 1987 Intervention: massage plus ice Control: therapeutic ultrasound plus ice | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Pain after strength test (VAS 0‐10) at 3 days | 2.8 | 1.2 | 6 | 3.2 | 1.2 | 6 | ‐0.40 (‐1.76, 0.96) |
| Strength (maximal isometric force production, measured in peak force) % change from baseline to 3 days | 9.8 | 8.8 | 6 | 11 | 9.5 | 6 | ‐1.20 (‐11.56, 9.16) |
| Study ID: Clews 1987 Intervention: massage plus ice Control: sham ultrasound plus ice | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Pain after strength test (VAS 0‐10 at strength testing) at 3 days | 2.8 | 1.2 | 6 | 2.7 | 1.9 | 6 | 0.10 (‐1.70, 1.90) |
| Strength (maximal isometric force production, measured in peak force) % change from baseline to 3 days | 9.8 | 8.8 | 6 | ‐1.5 | 9 | 6 | 11.30 (1.23, 21.37) |
| Study ID: Conroy 1998 Intervention: mobilisation plus standardised physiotherapy Control: standardised physiotherapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) at 3 weeks | 12.5 | 14.93 | 7 | 45.86 | 33.26 | 7 | ‐33.36 (‐60.37, ‐6.35) |
| Active range of abduction (degrees) at 3 weeks | 125.71 | 26.21 | 7 | 133.86 | 27.82 | 7 | ‐8.15 (‐36.46, 20.16) |
| Active range of external rotation (degrees) at 3 weeks | 75.71 | 17.51 | 7 | 81.14 | 18.05 | 7 | ‐5.43 (‐24.06, 13.20) |
| Active range of internal rotation (degrees) at 3 weeks | 44.86 | 12.25 | 7 | 49.57 | 16.42 | 7 | ‐4.71 (‐19.89, 10.47) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Function: number of participants who can reach to external occipital protuberance at 3 weeks | 4 | 7 | 5 | 7 | 0.80 (0.36, 1.77) | ||
| Function: number of participants who can reach overhead 135 degrees at 3 weeks | 5 | 7 | 5 | 7 | 1.00 (0.52, 1.94) | ||
| Function: number of participants who can reach to the spinous processes at 3 weeks | 2 | 7 | 2 | 7 | 1.00 (0.19, 5.24) | ||
| Study ID: Cook 2014 Intervention: neck manual therapy plus standardised physiotherapy Control: standardised physiotherapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (numerical rating scale 0‐10) at discharge (mean of 56 (SD 55) days) | 2.3 | 1.8 | 36 | 2.2 | 1.2 | 32 | 0.10 (‐0.62, 0.82) |
| Function (QuickDASH Questionnaire, 1‐5 where higher scores denote worse dysfunction) at discharge (mean of 56 (SD 55) days) | 13.6 | 10.5 | 36 | 13.6 | 6.6 | 32 | 0.00 (‐4.12, 4.12) |
| Total adverse events | Zero events in both groups | ||||||
| Study ID: Janse van Rensburg 2012 Intervention: thoracic spinal manipulation plus mobilisation plus exercises Control: mobilisation plus exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (DASH, 0‐100 with higher scores denoting worse functionality) at 6 weeks | 11.92 | 6.48 | 6 | 20.35 | 12.37 | 2 | ‐8.43 (‐26.34, 9.48) |
| Range of abduction (degrees, unclear if active or passive) at 6 weeks | 142.17 | 12.73 | 6 | 130 | 14.14 | 2 | 12.17 (‐9.92, 34.26) |
| Range of flexion (degrees, unclear if active or passive) at 6 weeks | 142.33 | 7.31 | 6 | 135 | 0 | 2 | 7.33 (95% CI not estimable) |
| Adverse events | Zero events in both groups | ||||||
| Study ID: Kachingwe 2008 Intervention: glenohumeral mobilisation plus exercises Control: exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 with higher scores denoting worse pain) percent change from baseline to 6 weeks | 44.2 | 38.6 | 9 | 20.8 | 112.3 | 8 | Not estimable |
| Function (SPADI total score 0‐130 with higher scores denoting worse function) percent change from baseline to 6 weeks | 56.7 | 29.8 | 9 | 61.6 | 35.9 | 8 | Not estimable |
| Active range of flexion percent change from baseline to 6 weeks | ‐15.9 | 116.6 | 9 | 27.6 | 41.7 | 8 | Not estimable |
| Study ID: Kachingwe 2008 Intervention: mobilisation with movement plus exercises Control: exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 with higher scores denoting worse pain) percent change from baseline to 6 weeks | 55.2 | 31.9 | 9 | 20.8 | 112.3 | 8 | Not estimable |
| Function (SPADI total score 0‐130 with higher scores denoting worse function) percent change from baseline to 6 weeks | 55.5 | 20.1 | 9 | 61.6 | 35.9 | 8 | Not estimable |
| Active range of flexion percent change from baseline to 6 weeks | 46.7 | 31.9 | 9 | 27.6 | 41.7 | 8 | Not estimable |
| Study ID: Kromer 2013 Intervention: manual therapy and exercises Control: exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 5 weeks | 2.9 | 1.6 | 46 | 3.3 | 1.6 | 44 | ‐0.40 (‐1.06, 0.26) |
| Overall pain (VAS 0‐10) at 12 weeks | 2.3 | 1.8 | 46 | 2.3 | 1.8 | 44 | 0.00 (‐0.74, 0.74) |
| Overall pain (SPADI pain sub‐score 0‐100) at 1 year | 17.7 | 21.8 | 44 | 12.4 | 16.9 | 43 | 5.30 (‐2.89, 13.49) |
| Function (SPADI total score, 0‐100 with higher scores denoting worse function) at 5 weeks | 23.5 | 17.5 | 46 | 26.8 | 17.8 | 44 | ‐3.30 (‐10.60, 4.00) |
| Function (SPADI total score, 0‐100 with higher scores denoting worse function) at 12 weeks | 16.1 | 17.2 | 46 | 19.8 | 19.5 | 44 | ‐3.70 (‐11.31, 3.91) |
| Function (SPADI total score, 0‐100 with higher scores denoting worse function) at 1 year | 15.3 | 20.3 | 44 | 10.2 | 15.2 | 43 | 5.10 (‐2.42, 12.62) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("much better" on PGIC) at 5 weeks | 22 | 46 | 20 | 44 | 1.05 (0.68, 1.64) | ||
| Global assessment of treatment success ("much better" on PGIC) at 12 weeks | "No difference between groups" | ||||||
| Global assessment of treatment success ("much better" on PGIC) at 1 year | "No difference between groups" | ||||||
| Work disability (on sick leave) between 0 and 5 weeks | 1 | 40 | 5 | 38 | 0.19 (0.02, 1.55) | ||
| Work disability (on sick leave) at 6 and 12 weeks | 1 | 38 | 3 | 35 | 0.31 (0.03, 2.82) | ||
| Work disability (on sick leave) at 12 weeks and 1 year | 2 | 38 | 3 | 37 | 0.65 (0.11, 3.67) | ||
| Adverse events during 1 year trial period | "One patient had a 12‐point deterioration and another patient a 38‐point deterioration after an accident involving the shoulder." | ||||||
| Study ID: Senbursa 2011 Intervention: joint and soft tissue mobilisation plus supervised exercises Control: supervised exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| % Events | Total | % Events | Total | Risk ratio (95% CI) | |||
| Rest pain (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 83% | Unclear | 64% | Unclear | Not estimable | ||
| Rest pain (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 97% | Unclear | 92% | Unclear | Not estimable | ||
| Night pain (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 47% | Unclear | 36% | Unclear | Not estimable | ||
| Night pain (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 83% | Unclear | 88% | Unclear | Not estimable | ||
| Pain on motion (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 23% | Unclear | 16% | Unclear | Not estimable | ||
| Pain on motion (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 63% | Unclear | 36% | Unclear | Not estimable | ||
| Study ID: Senbursa 2011 Intervention: joint and soft tissue mobilisation plus supervised exercises Control: home exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| % Events | Total | % Events | Total | Risk ratio (95% CI) | |||
| Rest pain (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 83% | Unclear | 82% | Unclear | Not estimable | ||
| Rest pain (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 97% | Unclear | 91% | Unclear | Not estimable | ||
| Night pain (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 47% | Unclear | 45% | Unclear | Not estimable | ||
| Night pain (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 83% | Unclear | 82% | Unclear | Not estimable | ||
| Pain on motion (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 23% | Unclear | 14% | Unclear | Not estimable | ||
| Pain on motion (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 63% | Unclear | 41% | Unclear | Not estimable | ||
| Study ID: Yiasemides 2011 Intervention: passive mobilisation plus exercise and advice Control: exercise and advice | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (SPADI pain sub‐score, 0‐100 with higher scores denoting worse pain) at 1 month | 38 | 22 | 47 | 41 | 21 | 51 | ‐3.00 (‐11.53, 5.53) |
| Overall pain (SPADI pain sub‐score, 0‐100 with higher scores denoting worse pain) at 6 months | 18 | 20 | 47 | 18 | 20 | 51 | 0.00 (‐7.93, 7.93) |
| Function (SPADI disability sub‐score, 0‐100 with higher scores denoting worse function) at 1 month | 32 | 23 | 47 | 30 | 19 | 51 | 2.00 (‐6.39, 10.39) |
| Function (SPADI disability sub‐score, 0‐100 with higher scores denoting worse function) at 6 months | 13 | 18 | 47 | 12 | 16 | 51 | 1.00 (‐5.76, 7.76) |
| Active range of abduction painful arc (degrees) at 1 month | 28 | 24 | 47 | 36 | 25 | 51 | ‐8.00 (‐17.70, 1.70) |
| Active range of abduction painful arc (degrees) at 6 months | 7 | 15 | 47 | 6 | 11 | 51 | 1.00 (‐4.24, 6.24) |
| Active range of flexion painful arc (degrees) at 1 month | 14 | 23 | 47 | 19 | 19 | 51 | ‐5.00 (‐13.39, 3.39) |
| Active range of flexion painful arc (degrees) at 6 months | 3 | 9 | 47 | 3 | 6 | 51 | 0.00 (‐3.05, 3.05) |
| Global assessment of treatment success (6‐point Likert scale, 0 = much worse, 5 = fully recovered) at 1 month | 4.2 | 0.8 | 47 | 3.9 | 0.8 | 51 | 0.30 (‐0.02, 0.62) |
| Global assessment of treatment success (6‐point Likert scale, 0 = much worse, 5 = fully recovered) at 6 months | 4.6 | 1 | 47 | 4.8 | 0.7 | 51 | ‐0.20 (‐0.54, 0.14) |
Are supervised or home exercises alone more effective than placebo, no intervention or another active intervention?
In nine trials, an exercise programme delivered alone was compared with either placebo (Brox 1993), no intervention (Kachingwe 2008; Lombardi 2008; Ludewig 2003) or another active intervention (Brox 1993; Engebretsen 2009; Ginn 2005; Giombini 2006; Moosmayer 2014; Walther 2004).
Exercises alone versus placebo
In Brox 1993, supervised and home exercises were compared with inactive (placebo) laser (each delivered twice a week for six weeks). The trial was judged to be at unclear risk of bias overall due to unclear allocation concealment. Mean differences favouring the exercises group were noted for overall pain (MD 10; 35‐point scale, 80 participants) and function (MD 10; 30‐point scale, 80 participants) at six months, although the data were incompletely reported so 95% CIs were not estimable. The authors stated that there were no statistically significant differences between groups in pain on motion, global treatment success, night pain, range of motion, or number of days on sick leave (Table 17). No participant in either group reported adverse events. We downgraded by one point for unclear risk of allocation bias, and one point for imprecision, and thus consider this evidence to be low quality.
14. Exercises alone versus placebo.
| Study ID: Brox 1993 Intervention: supervised exercises Control: placebo laser | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Median | IQR | n | Median | IQR | n | Mean difference (95% CI) | |
| Overall pain (Neer shoulder score pain score 0‐35 with higher scores denoting less pain) at 6 months | 25 | NR | 50 | 15 | NR | 30 | 10 (95% CI not estimable) |
| Function (Neer shoulder score function score 0‐30 with higher scores denoting better function) at 6 months | 25 | NR | 50 | 15 | NR | 30 | 10 (95% CI not estimable) |
| Night pain (0‐9, 1 = no pain, 9 = worst possible pain) at 6 months | 3 | NR | 50 | 4 | NR | 30 | ‐1 (95% CI not estimable) |
| Pain on motion (0‐9, 1 = no pain, 9 = worst possible pain) at 6 months | 3 | NR | 50 | 6 | NR | 30 | ‐3 (95% CI not estimable) |
| ROM (Neer shoulder score ROM score 0‐25 with higher scores denoting better ROM) at 6 months | 23 | NR | 50 | 19 | NR | 30 | 4 (95% CI not estimable) |
| Work disability: days of sick leave | Reported as not significantly different between groups | ||||||
| Global assessment of treatment success (number of participants with a good or an excellent Neer shoulder score (> 80 points)) | Reported as not significantly different between groups | ||||||
| Total adverse events | Zero events in both groups | ||||||
Exercises alone versus no treatment
Benefits of exercise alone when compared with no treatment were observed in two trials (Lombardi 2008; Ludewig 2003), both at high risk of bias overall. Lombardi 2008 found participants receiving progressive resistance training exercises twice a week for eight weeks had less overall pain (MD ‐1.90, 95% CI ‐3.27 to ‐0.53; 10‐point scale, 60 participants), disability (MD ‐15.50, 95% CI ‐28.94 to ‐2.06; 100‐point scale, 60 participants), and pain on motion (MD ‐1.90, 95% CI ‐3.05 to ‐0.75; 10‐point scale, 60 participants) at two months; these differences were clinically important. Also, statistically significant differences favouring the exercise group were noted for active shoulder internal rotation and measures of strength (Table 18). However, active shoulder abduction, flexion and external rotation were not significantly different between groups, nor were quality‐of‐life scores on the SF‐36. In Ludewig 2003, participants receiving a daily home exercise programme for 10 weeks had better function (MD 6.90, 95% CI 0.59 to 13.21; 83‐point scale, 62 participants) and less work‐related pain (MD ‐1.30, 95% CI ‐2.10 to ‐0.50; 10‐point scale, 62 participants) and work‐related disability (MD ‐1.20, 95% CI ‐2.00 to ‐0.40; 10‐point scale, 62 participants) at 10 weeks; however, these effects were not clinically important. Usable outcome data were not available in Kachingwe 2008, though the authors claimed that there were no statistically significant differences between groups in overall pain, function and active shoulder flexion at six weeks (Table 18). We downgraded by two points for high risk of performance and detection bias, one point for imprecision and one point for indirectness because the trial by Ludewig 2003 was restricted to construction workers. Therefore, this evidence was considered very low quality.
15. Exercises alone versus no treatment, wait‐list control or usual care.
| Study ID: Kachingwe 2008 Intervention: supervised exercises Control: advice regarding posture and overhead activities | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 with higher scores denoting worse pain) % change from baseline to 6 weeks | 20.8 | 112.3 | 8 | 14.4 | 119.8 | 7 | Not estimable |
| Function (SPADI total score 0‐130 with higher scores denoting worse function) % change from baseline to 6 weeks | 61.6 | 35.9 | 8 | 34.2 | 58.9 | 7 | Not estimable |
| Active range of flexion % change from baseline to 6 weeks | 27.6 | 41.7 | 8 | 42.6 | 15.8 | 7 | Not estimable |
| Study ID: Lombardi 2008 Intervention: progressive resistance training exercises Control: wait‐list control | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (DASH laborious function, 0‐100 with higher scores denoting worse functionality) at 2 months | 28.7 | 24.8 | 30 | 44.2 | 28.2 | 30 | ‐15.50 (‐28.94, ‐2.06) |
| Function (DASH activities of daily living, 0‐100 with higher scores denoting worse functionality) at 2 months | 33.2 | 18.7 | 30 | 43.4 | 22.8 | 30 | ‐10.20 (‐20.75, 0.35) |
| Overall pain (VAS 0‐10) at 2 months | 2.4 | 2.1 | 30 | 4.3 | 3.2 | 30 | ‐1.90 (‐3.27, ‐0.53) |
| Pain on motion (VAS 0‐10) at 2 months | 5.2 | 2 | 30 | 7.1 | 2.5 | 30 | ‐1.90 (‐3.05, ‐0.75) |
| Active range of abduction (degrees) at 2 months | 136.9 | 28.5 | 30 | 127.2 | 31.6 | 30 | 9.70 (‐5.53, 24.93) |
| Active range of flexion (degrees) at 2 months | 137.1 | 24.8 | 30 | 130.6 | 27.4 | 30 | 6.50 (‐6.72, 19.72) |
| Active range of internal rotation (degrees) at 2 months | 45.3 | 13.3 | 30 | 35.6 | 15.7 | 30 | 9.70 (2.34, 17.06) |
| Active range of external rotation (degrees) at 2 months | 82.7 | 18 | 30 | 70.5 | 31.7 | 30 | 12.20 (‐0.84, 25.24) |
| Active range of extension (degrees) at 2 months | 54.5 | 8.8 | 30 | 46.9 | 12.2 | 30 | 7.60 (2.22, 12.98) |
| Strength: Peak torque (Nm) at velocity of 60 degrees/second ‐ flexion | 29.2 | 11.46 | 30 | 21.97 | 13.46 | 30 | 7.23 (0.90, 13.56) |
| Strength: Peak torque (Nm) at velocity of 60 degrees/second ‐ extension | 40.03 | 18.42 | 30 | 28.4 | 19.27 | 30 | 11.63 (2.09, 21.17) |
| Strength: Peak torque (Nm) at velocity of 60 degrees/second ‐ abduction | 24.37 | 11.98 | 30 | 15.8 | 11.44 | 30 | 8.57 (2.64, 14.50) |
| Strength: Peak torque (Nm) at velocity of 60 degrees/second ‐ adduction | 33.8 | 18.39 | 30 | 21.73 | 18.42 | 30 | 12.07 (2.76, 21.38) |
| Strength: Peak torque (Nm) at velocity of 60 degrees/second ‐ internal rotation | 22.23 | 9.28 | 30 | 17.13 | 7.99 | 30 | 5.10 (0.72, 9.48) |
| Strength: Peak torque (Nm) at velocity of 60 degrees/second ‐ external rotation | 12 | 5.12 | 30 | 9.53 | 4.15 | 30 | 2.47 (0.11, 4.83) |
| Quality of life (SF‐36 physical functioning score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 64.3 | 19 | 30 | 62.8 | 22.3 | 30 | 1.50 (‐8.98, 11.98) |
| Quality of life (SF‐36 role‐physical score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 36.7 | 41.4 | 30 | 30.8 | 39.8 | 30 | 5.90 (‐14.65, 26.45) |
| Quality of life (SF‐36 bodily pain score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 54.3 | 16 | 30 | 46.7 | 24.1 | 30 | 7.60 (‐2.75, 17.95) |
| Quality of life (SF‐36 general health score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 73.9 | 20.3 | 30 | 68.2 | 25.3 | 30 | 5.70 (‐5.91, 17.31) |
| Quality of life (SF‐36 vitality score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 54.8 | 24.7 | 30 | 49.4 | 26.9 | 30 | 5.40 (‐7.67, 18.47) |
| Quality of life (SF‐36 social functioning score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 76.7 | 27.4 | 30 | 65.4 | 27.2 | 30 | 11.30 (‐2.52, 25.12) |
| Quality of life (SF‐36 role‐emotional score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 62.22 | 40.8 | 30 | 55.5 | 42.3 | 30 | 6.72 (‐14.31, 27.75) |
| Quality of life (SF‐36 mental health score, 0‐100 where a higher score indicates a better quality of life) at 2 months | 62.9 | 22 | 30 | 56.5 | 25.1 | 30 | 6.40 (‐5.54, 18.34) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success at 2 months | "The Likert scale evaluating pain revealed that the experimental group exhibited a greater number of "much better" and "a little better" responses than the control group. This difference was statistically significant between groups (P = 0.001)." | ||||||
| Study ID: Ludewig 2003 Intervention: home exercise programme Control: no treatment | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Shoulder Rating Questionnaire, 17‐100, with a higher score indicating better function) at 8‐12 weeks | 78 | 12.65 | 30 | 71.1 | 12.67 | 32 | 6.90 (0.59, 13.21) |
| Work‐related pain (1‐10 scale with higher scores denoting worse work‐related pain) at 8‐12 weeks | 2.8 | 1.59 | 30 | 4.1 | 1.64 | 32 | ‐1.30 (‐2.10, ‐0.50) |
| Work disability score (1 to 10 scale, with higher scores indicating greater difficulty with work performance) at 8‐12 weeks | 2.5 | 1.59 | 30 | 3.7 | 1.64 | 32 | ‐1.20 (‐2.00, ‐0.40) |
Exercises alone versus another active intervention
Other active interventions which have been compared with an exercise programme alone include tendon repair surgery for rotator cuff tear (Moosmayer 2014), radial extracorporeal shockwave treatment (Engebretsen 2009), microwave diathermy (Giombini 2006), therapeutic ultrasound (Giombini 2006), glucocorticoid injection (Ginn 2005), arthroscopic subacromial decompression (Brox 1993), and a functional brace (Walther 2004).
In Moosmayer 2014, mini‐open or open tendon repair surgery was compared with supervised exercises (twice weekly for 12 weeks, with increasing intervals during the following six to 12 weeks). We judged the trial to be at high risk of bias overall. Overall pain was higher in the exercise group at six months (MD 1.60, 95% CI 0.90 to 2.30; 10‐point scale, 103 participants), 12 months (MD 1.20, 95% CI 0.60 to 1.80; 10‐point scale, 103 participants) and five years (MD 1.00, 95% CI 0.20 to 1.80; 10‐point scale, 103 participants), although the difference was clinically important only at six months. The authors observed no clinically important difference between the supervised exercise group and surgery group in function at six months (MD ‐2.80, 95% CI ‐10.10 to 4.50; 100‐point scale, 103 participants), 12 months (MD ‐8.50, 95% CI ‐15.00 to ‐1.90; 100‐point scale, 103 participants) or five years (MD ‐6.50, 95% CI ‐13.60 to 0.70; 100‐point scale, 103 participants). Active shoulder abduction was lower in the exercise group at 12 months (MD ‐16.80, 95% CI ‐32.40 to ‐1.20; 103 participants) and five years (MD ‐14.70, 95% CI ‐29.40 to ‐0.10; 103 participants), although differences in active shoulder flexion and strength were not clinically important. The lack of participant blinding may have influenced participants in both groups to pursue alternative interventions and influenced their responses to self‐reported outcomes, which may have biased results in either direction. For this reason we downgraded by two points for high risk of performance and detection bias, and consider this evidence to be low quality.
In Engebretsen 2009, radial extracorporeal shockwave treatment (once weekly for four to six weeks) was compared with supervised exercises (twice weekly for up to 12 weeks). We judged the trial to be at high risk of bias overall. There was no clinically important difference between groups in overall pain at six weeks (MD ‐0.3, 95% CI ‐0.9 to 0.4; 9‐point scale, 103 participants), 18 weeks (MD ‐0.2, 95% CI ‐0.7 to 0.3; 9‐point scale, 103 participants) and 1 year (MD ‐0.5, 95% CI ‐1.22 to 0.22; 9‐point scale, 94 participants), or in pain on motion at six weeks (MD ‐0.7, 95% CI ‐1.6 to 0.1; 9‐point scale, 103 participants), 18 weeks (MD ‐0.6, 95% CI ‐1.3 to 0.2; 9‐point scale, 103 participants) and 1 year (MD ‐0.2, 95% CI ‐1.13 to 0.73; 9‐point scale, 94 participants). The authors found that participants receiving supervised exercises had less disability at six weeks (MD ‐10, 95% CI ‐17.6 to ‐2.3; 100‐point scale, 103 participants), 18 weeks (MD ‐8.4, 95% CI ‐16.5 to ‐0.6; 100‐point scale, 103 participants), and one year (MD ‐3.9, 95% CI ‐14.04 to 6.24; 100‐point scale, 94 participants), although none of these differences were considered to be clinically important. The number of participants working at 18 weeks was higher in the supervised exercise group (RR 1.46, 95% CI 1.07 to 1.99; 100 participants), but work disability occurred at a similar frequency in both groups at 1 year (RR 1.1, 95% CI 1.0 to 1.2; 91 participants). One participant in the exercise group and two in the shockwave group had the adverse event of aggravation of pain after treatment (RR 0.50, 95% CI 0.05 to 5.34; 100 participants). A limitation of the trial was that more participants receiving shockwave treatment sought additional care outside of the trial setting, which may have biased results against the exercise group. We downgraded by two points for high risk of performance and detection bias, and consider this evidence to be low quality.
In Giombini 2006, microwave diathermy (three times a week for four weeks) was compared with exercises (once a week supervised and daily at home for four weeks). We judged the trial to be at high risk of bias overall. The authors observed clinically important differences favouring microwave diathermy over exercises in terms of overall pain at four weeks (MD 2.90, 95% CI 2.45 to 3.35; 10‐point scale, 25 participants) and 10 weeks (MD 3.70, 95% CI 3.08 to 4.32; 10‐point scale, 25 participants), function at four weeks (MD ‐16.90, 95% CI ‐20.26 to ‐13.54; 100‐point scale, 25 participants) and 10 weeks (MD ‐18.73, 95% CI ‐23.18 to ‐14.28; 100‐point scale, 25 participants), and global treatment success (number of patients returning to sport) at 10 weeks (RR 0.42, 95% CI 0.19 to 0.95; 25 participants). No participant reported adverse events. We downgraded by two points for high risk of performance and detection bias, one point for imprecision and one point for indirectness because the trial was restricted to professional athletes, and thus consider this evidence to be very low quality.
All differences in outcomes between exercise and the remaining active interventions (therapeutic ultrasound (Giombini 2006), glucocorticoid injection (Ginn 2005), arthroscopic subacromial decompression (Brox 1993), and functional brace (Walther 2004)) were not clinically important or statistically significant (see Table 19). We downgraded the evidence from these four trials by two points for high risk of performance and detection bias, and one point for imprecision, and thus consider it to be very low quality.
16. Exercises alone versus another active intervention.
| Study ID: Ginn 2005 Intervention: exercise therapy Control: glucocorticoid injection | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | 95% CI | n | Mean | 95% CI | n | Mean difference (95% CI) | |
| Pain (VAS 0‐10) change from baseline to 5 weeks | 0.3 | 0 to 2.3 | 48 | 0.2 | 0 ‐ 1.7 | 45 | 0.10 (‐2.33, 2.53) |
| Function (categorical rating scale, 0‐27 with higher scores denoting worse function) change from baseline to 5 weeks | 4.6 | 3.5 to 5.6 | 48 | 5.2 | 3.9 ‐ 6.5 | 45 | ‐0.60 (‐2.26, 1.06) |
| Active range of abduction (degrees) change from baseline to 5 weeks (subgroup with decreased ROM and shoulder pain) | 116 | 100 to 132 | ? | 98 | 82 to 114 | ? | 18 (95% CI not estimable) |
| Active range of abduction (degrees) change from baseline to 5 weeks (subgroup with full ROM despite shoulder pain) | 24 | 10 to 37 | ? | 28 | 13 to 44 | ? | ‐4 (95% CI not estimable) |
| Active range of flexion (degrees) change from baseline to 5 weeks (subgroup with decreased ROM and shoulder pain) | 114 | 104 to 124 | ? | 111 | 102 to 120 | ? | 3 (95% CI not estimable) |
| Active range of flexion (degrees) change from baseline to 5 weeks (subgroup with full ROM despite shoulder pain) | 1 | 0 to 10 | ? | 0 | 0 to 8 | ? | 1 (95% CI not estimable) |
| Active hand‐behind‐back distance change from baseline to 5 weeks | 6.1 | 3.1 to 9.1 | 48 | 7.5 | 4.9 to 10.2 | 45 | ‐1.40 (‐5.26, 2.46) |
| Strength (isometric abduction force %) change from baseline to 5 weeks | 70 | 58 to 82 | 48 | 66 | 55 to 76 | 45 | 4.00 (‐11.85, 19.85) |
| % range | Total | % range | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (% participants rated as "improved") at 5 weeks | 33 to 77% | 48 | 35 to 78% | 45 | Not estimable | ||
| Study ID: Brox 1993 Intervention: supervised exercises Control: arthroscopic subacromial decompression | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Median | IQR | n | Median | IQR | n | Mean difference (95% CI) | |
| Overall pain (Neer shoulder score pain score 0‐35 with higher scores denoting less pain) at 6 months | 25 | NR | 50 | 25 | NR | 45 | 0 (95% CI not estimable) |
| Function (Neer shoulder score function score 0‐30 with higher scores denoting better function) at 6 months | 25 | NR | 50 | 28 | NR | 45 | ‐ 3 (95% CI not estimable) |
| Night pain (0‐9, 1 = no pain, 9 = worst possible pain) at 6 months | 3 | NR | 50 | 2 | NR | 45 | 1 (95% CI not estimable) |
| Pain on motion (0‐9, 1 = no pain, 9 = worst possible pain) at 6 months | 3 | NR | 50 | 3 | NR | 45 | 0 (95% CI not estimable) |
| ROM (Neer shoulder score ROM score 0‐25 with higher scores denoting better ROM) at 6 months | 23 | NR | 50 | 22 | NR | 45 | 1 (95% CI not estimable) |
| Work disability: days of sick leave | Reported as not significantly different between groups | ||||||
| Global assessment of treatment success (number of participants with a good or an excellent Neer shoulder score (> 80 points)) | Reported as not significantly different between groups | ||||||
| Total adverse events | Zero events in both groups | ||||||
| Study ID: Moosmayer 2014 Intervention: supervised exercises Control: rotator cuff tear repair surgery | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 6 months | 2.7 | 2.2 | 51 | 1.1 | 1.3 | 52 | 1.6 (0.9, 2.3)* |
| Overall pain (VAS 0‐10) at 12 months | 1.6 | 1.6 | 51 | 0.5 | 1.2 | 52 | 1.2 (0.6, 1.8)* |
| Overall pain (VAS 0‐10) at 5 years | 1.6 | 1.6 | 51 | 0.6 | 1.4 | 52 | 1 (0.2, 1.8)* |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 6 months | 63.9 | 20.2 | 51 | 65.6 | 16.3 | 52 | ‐2.8 (‐10.1, 4.5)* |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 12 months | 70.3 | 19.1 | 51 | 77.7 | 13.4 | 52 | ‐8.5 (‐15, ‐1.9)* |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 5 years | 74.2 | 20.3 | 51 | 79.8 | 15 | 52 | ‐6.5 (‐13.6, 0.7)* |
| Active range of abduction (degrees) at 6 months | 135.4 | 47.9 | 51 | 135.4 | 41.7 | 52 | ‐2.2 (‐20.3, 15.8)* |
| Active range of abduction (degrees) at 12 months | 143.8 | 43.9 | 51 | 158.4 | 33.7 | 52 | ‐16.8 (‐32.4, ‐1.2)* |
| Active range of abduction (degrees) at 5 years | 155.1 | 41.2 | 51 | 167.3 | 30.6 | 52 | ‐14.7 (‐29.4, ‐0.1)* |
| Active range of flexion (degrees) at 6 months | 146.6 | 46.3 | 51 | 147.3 | 34.5 | 52 | ‐2.1 (‐18.1, 13.9)* |
| Active range of flexion (degrees) at 12 months | 155.6 | 38.4 | 51 | 166.1 | 27.5 | 52 | ‐10.3 (‐23.6, 3.1)* |
| Active range of flexion (degrees) at 5 years | 163.5 | 35.4 | 51 | 170.6 | 27.9 | 52 | ‐8.3 (‐21, 4.4)* |
| Strength (Constant‐Murley strength sub‐score, kg) at 6 months | 10.6 | 5.4 | 51 | 8 | 4.6 | 52 | 2.5 (0.7, 4.2)* |
| Strength (Constant‐Murley strength sub‐score, kg) at 12 months | 11.9 | 5.1 | 51 | 11.1 | 4 | 52 | 0.8 (‐0.9, 2.4)* |
| Strength (Constant‐Murley strength sub‐score, kg) at 5 years | 11.4 | 5.4 | 51 | 12.1 | 4.7 | 52 | ‐0.8 (‐2.7, 1.1)* |
| Study ID: Engebretsen 2009 Intervention: supervised exercises Control: radial extracorporeal shockwave treatment | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (9‐point Likert scale, 1 = no pain, 9 = severe pain) at 6 weeks | 2.6 | 1.9 | 51 | 2.9 | 2.1 | 52 | ‐0.3 (‐0.9, 0.4)* |
| Overall pain (9‐point Likert scale, 1 = no pain, 9 = severe pain) at 18 weeks | 2.5 | 1.9 | 51 | 2.7 | 2 | 52 | ‐0.2 (‐0.7, 0.3)* |
| Overall pain (9‐point Likert scale, 1 = no pain, 9 = severe pain) at 1 year | 2.1 | 1.5 | 48 | 2.6 | 2 | 46 | ‐0.5 (‐1.22, 0.22) |
| Function (SPADI total score 0‐100) at 6 weeks | 25.8 | 21.5 | 51 | 33.5 | 23.3 | 52 | ‐10 (‐17.6, ‐2.3)* |
| Function (SPADI total score 0‐100) at 18 weeks | 24.5 | 25.6 | 51 | 29.2 | 25.9 | 52 | ‐8.4 (‐16.5, ‐0.6)* |
| Function (SPADI total score 0‐100) at 1 year | 24 | 23.4 | 48 | 27.9 | 26.6 | 46 | ‐3.9 (‐14.04, 6.24) |
| Pain on motion (9‐point Likert scale, 1 = no pain, 9 = severe pain) at 6 weeks | 3.9 | 2 | 51 | 4.6 | 2.4 | 52 | ‐0.7 (‐1.6, 0.1)* |
| Pain on motion (9‐point Likert scale, 1 = no pain, 9 = severe pain) at 18 weeks | 3.6 | 2.3 | 51 | 4.1 | 2.5 | 52 | ‐0.6 (‐1.3, 0.2)* |
| Pain on motion (9‐point Likert scale, 1 = no pain, 9 = severe pain) at 1 year | 3.5 | 2.2 | 48 | 3.7 | 2.4 | 46 | ‐0.2 (‐1.13, 0.73) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Work disability (number of participants working) at 18 weeks | 38 | 50 | 26 | 50 | 1.46 (1.07, 1.99) | ||
| Work disability (number of participants working < 50% or unemployed) at 1 year | 38 | 45 | 30 | 46 | 1.1 (1.0, 1.2)* | ||
| Adverse events (aggravation of pain after treatment) within 1 year study period | 1 | 50 | 2 | 50 | 0.50 (0.05, 5.34) | ||
| Study ID: Giombini 2006 Intervention: supervised and home exercises Control: microwave diathermy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 4 weeks | 5.3 | 0.65 | 11 | 2.4 | 0.46 | 14 | 2.90 (2.45, 3.35) |
| Overall pain (VAS 0‐10) at 10 weeks | 4.9 | 0.88 | 11 | 1.2 | 0.63 | 14 | 3.70 (3.08, 4.32) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 4 weeks | 61.2 | 4.28 | 11 | 78.1 | 4.23 | 14 | ‐16.90 (‐20.26, ‐13.54) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 10 weeks | 63.27 | 5.56 | 11 | 82 | 5.73 | 14 | ‐18.73 (‐23.18, ‐14.28) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (ready to return to sport) at 4 weeks | 4 | 11 | 11 | 14 | 0.46 (0.20, 1.06) | ||
| Global assessment of treatment success (ready to return to sport) at 10 weeks | 4 | 11 | 12 | 14 | 0.42 (0.19, 0.95) | ||
| Adverse events | Zero events in both groups | ||||||
| Study ID: Giombini 2006 Intervention: supervised and home exercises Control: therapeutic ultrasound | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 4 weeks | 5.3 | 0.65 | 11 | 5.8 | 0.96 | 12 | ‐0.50 (‐1.17, 0.17) |
| Overall pain (VAS 0‐10) at 10 weeks | 4.9 | 0.88 | 11 | 5.15 | 0.87 | 12 | ‐0.25 (‐0.97, 0.47) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 4 weeks | 61.2 | 4.28 | 11 | 60 | 3.21 | 12 | 1.20 (‐1.91, 4.31) |
| Function (Constant‐Murley total score, 0‐100, higher = better function) at 10 weeks | 63.27 | 5.56 | 11 | 61.75 | 4.18 | 12 | 1.52 (‐2.53, 5.57) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (ready to return to sport) at 4 weeks | 4 | 11 | 6 | 12 | 0.73 (0.28, 1.91) | ||
| Global assessment of treatment success (ready to return to sport) at 10 weeks | 4 | 11 | 4 | 12 | 1.09 (0.36, 3.34) | ||
| Adverse events | Zero events in both groups | ||||||
| Study ID: Walther 2004 Intervention: standardised self‐training of centring and stretching exercises Control: functional brace | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐100) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Function (Constant‐Murley total score) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Pain on motion (VAS 0‐100) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Strength (Constant‐Murley strength sub‐score 0‐25) at 12 weeks | 10.9 | 4.6 | 20 | 14.4 | 5.4 | 20 | ‐3.50 (‐6.61, ‐0.39) |
| Work disability (number of months with inability to work) | 1.2 | NR | 20 | 1.5 | NR | 20 | ‐0.3 (95% CI not estimable) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Number of adverse events | 0 | 20 | 2 | 20 | 0.20 (0.01, 3.92) | ||
| Adverse events | "None of the patients treated with physiotherapy or self‐training dropped out of the therapy regimen. However, one of the patients treated with the brace complained about being bothered by the brace at work, especially while working overhead. Another patient had eczema of the skin develop underneath the pads. Both patients continued to wear the brace during the remainder of the 12‐week therapy period" | ||||||
| Study ID: Walther 2004 Intervention: supervised stretching exercises Control: functional brace | |||||||
| OUTCOME | INTERVENTION | OUTCOME | EFFECT ESTIMATRE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐100) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Function (Constant‐Murley total score) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Pain on motion (VAS 0‐100) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Strength (Constant‐Murley strength sub‐score 0‐25) at 12 weeks | 11.8 | 5.4 | 20 | 14.4 | 5.4 | 20 | ‐2.60 (‐5.95, 0.75) |
| Work disability (number of months with inability to work) | 1.6 | NR | 20 | 1.5 | NR | 20 | 0.1 (95% CI not estimable) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Number of adverse events | 0 | 20 | 2 | 20 | 0.20 (0.01, 3.92) | ||
| Adverse events | "None of the patients treated with physiotherapy or self‐training dropped out of the therapy regimen. However, one of the patients treated with the brace complained about being bothered by the brace at work, especially while working overhead. Another patient had eczema of the skin develop underneath the pads. Both patients continued to wear the brace during the remainder of the 12‐week therapy period" | ||||||
*Adjusted mean differences or risk ratios (adjusted for baseline score)
Are supervised or home exercises delivered in addition to another active intervention more effective than the other active intervention alone?
Six trials investigated the effects of exercise as an add‐on to another intervention (Ainsworth 2009; Bae 2011; Baskurt 2011; Beaudreuil 2011; Maenhout 2013; Martins 2012). The trials investigated the effects of adding a package of strengthening, stretching, and range‐of‐motion exercises to ultrasound, glucocorticoid injection and advice (Ainsworth 2009), adding motor control and strengthening exercises to heat pack, TENS and ultrasound (Bae 2011), adding scapular stabilisation exercises to stretching and strengthening exercises (Baskurt 2011), adding Dynamic Humeral Centering to massage and exercise (Beaudreuil 2011), adding heavy load eccentric training to traditional rotator cuff training (Maenhout 2013), or adding proprioception exercises to stretching and strengthening exercises plus cryotherapy (Martins 2012). The overall risk of bias was low in one study (Beaudreuil 2011), unclear in two trials (Bae 2011; Baskurt 2011), and high in three trials (Ainsworth 2009; Maenhout 2013; Martins 2012).
The addition of exercises resulted in better function in Ainsworth 2009, Bae 2011, Beaudreuil 2011, and Maenhout 2013, but the difference was only clinically important in Bae 2011. No clinically important differences between groups were observed for overall pain (Baskurt 2011; Beaudreuil 2011; Martins 2012), pain on motion (Baskurt 2011), global treatment success (Maenhout 2013), quality of life (Ainsworth 2009; Baskurt 2011; Martins 2012) or strength (Bae 2011; Baskurt 2011; Beaudreuil 2011; Maenhout 2013), although Bae 2011 found that the "exercise add‐on" group had better active range of motion (Table 20). We downgraded the evidence from these six trials by two points for high or unclear risk of performance and detection bias in all but one trial, and one point for imprecision, and thus consider it to be very low quality.
17. Exercises alone as an add‐on to another physical therapy intervention versus the other physical therapy intervention.
| Study ID: Ainsworth 2009 Intervention: supervised exercises, ultrasound, glucocorticoid injection (if needed) and advice Control: ultrasound, glucocorticoid injection (if needed) and advice | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Oxford Shoulder Score 0‐48 with higher scores denoting better function) change from baseline to 6 months | 9.42 | 6.23 | 26 | 4.43 | 7.23 | 30 | 4.99 (1.46, 8.52) |
| Function (Oxford Shoulder Score 0‐48 with higher scores denoting better function) change from baseline to 12 months | 8.96 | 5.3 | 24 | 6.27 | 7.93 | 30 | 2.69 (‐0.85, 6.23) |
| Quality of life (SF‐36 physical functioning score, 0‐100 where higher = better) change from baseline to 6 months | 4.42 | 22.33 | 26 | ‐3 | 13.93 | 30 | 7.42 (‐2.51, 17.35) |
| Quality of life (SF‐36 physical functioning score, 0‐100 where higher = better) change from baseline to 12 months | 5.21 | 13.39 | 24 | ‐3.17 | 17.19 | 30 | 8.38 (0.22, 16.54) |
| Quality of life (SF‐36 role‐physical score, 0‐100 where higher = better) change from baseline to 6 months | 2.89 | 49.16 | 24 | 14.17 | 42.89 | 30 | ‐11.28 (‐36.23, 13.67) |
| Quality of life (SF‐36 role‐physical score, 0‐100 where higher = better) change from baseline to 12 months | ‐5.21 | 44.22 | 26 | 18.33 | 37.1 | 30 | ‐23.54 (‐45.11, ‐1.97) |
| Quality of life (SF‐36 bodily pain score, 0‐100 where higher = better) change from baseline to 6 months | 3.65 | 22.64 | 26 | 4.58 | 28.01 | 30 | ‐0.93 (‐14.20, 12.34) |
| Quality of life (SF‐36 bodily pain score, 0‐100 where higher = better) change from baseline to 12 months | 1.56 | 30.3 | 24 | 4.42 | 29.88 | 30 | ‐2.86 (‐19.02, 13.30) |
| Quality of life (SF‐36 general health score, 0‐100 where higher = better) change from baseline to 6 months | 5 | 15.36 | 26 | 4.33 | 17.75 | 30 | 0.67 (‐8.00, 9.34) |
| Quality of life (SF‐36 general health score, 0‐100 where higher = better) change from baseline to 12 months | ‐4.79 | 15.77 | 24 | ‐1.17 | 16.64 | 30 | ‐3.62 (‐12.30, 5.06) |
| Quality of life (SF‐36 vitality score, 0‐100 where higher = better) change from baseline to 6 months | 2.5 | 15.64 | 26 | 0.67 | 19.38 | 30 | 1.83 (‐7.35, 11.01) |
| Quality of life (SF‐36 vitality score, 0‐100 where higher = better) change from baseline to 12 months | ‐3.54 | 15.91 | 24 | 2.5 | 17.94 | 30 | ‐6.04 (‐15.08, 3.00) |
| Quality of life (SF‐36 social functioning score, 0‐100 where higher = better) change from baseline to 6 months | ‐0.48 | 26.81 | 26 | 0.83 | 28.03 | 30 | ‐1.31 (‐15.69, 13.07) |
| Quality of life (SF‐36 social functioning score, 0‐100 where higher = better) change from baseline to 12 months | ‐7.81 | 34.74 | 24 | 5 | 28.16 | 30 | ‐12.81 (‐29.98, 4.36) |
| Quality of life (SF‐36 role‐emotional score, 0‐100 where higher = better) change from baseline to 6 months | 6.41 | 47.44 | 26 | 0 | 41.98 | 30 | 6.41 (‐17.22, 30.04) |
| Quality of life (SF‐36 role‐emotional score, 0‐100 where higher = better) change from baseline to 12 months | ‐5.56 | 30.56 | 24 | 3.33 | 29.49 | 30 | ‐8.89 (‐25.04, 7.26) |
| Quality of life (SF‐36 emotional wellbeing score, 0‐100 where higher = better) change from baseline to 6 months | 4.15 | 15.11 | 26 | 3.33 | 18.59 | 30 | 0.82 (‐8.01, 9.65) |
| Quality of life (SF‐36 emotional wellbeing score, 0‐100 where higher = better) change from baseline to 12 months | ‐4.17 | 18.89 | 24 | 4.93 | 17.44 | 30 | ‐9.10 (‐18.90, 0.70) |
| Passive external rotation (degrees) change from baseline to 6 months | 8.75 | NR | 26 | ‐3.7 | NR | 30 | 12.45 (95% CI not estimable) |
| Passive external rotation (degrees) change from baseline to 12 months | 7.43 | NR | 24 | 4.4 | NR | 30 | 3.03 (95% CI not estimable) |
| Study ID: Bae 2011 Intervention: motor control exercises and strengthening exercises plus hot packs plus TENS plus ultrasound Control: hot packs plus TENS plus ultrasound | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (SPADI total score 0‐100 with higher scores denoting worse function) at 4 weeks | 20.7 | 4.1 | 17 | 32.1 | 6 | 18 | ‐11.40 (‐14.79, ‐8.01) |
| Active range of abduction (degrees) at 4 weeks | 129.1 | 19.7 | 17 | 106.6 | 10.4 | 18 | 22.50 (11.97, 33.03) |
| Active range of flexion (degrees) at 4 weeks | 155.6 | 8.4 | 17 | 146.2 | 10.9 | 18 | 9.40 (2.97, 15.83) |
| Active range of extension (degrees) at 4 weeks | 40.2 | 4.8 | 17 | 36.2 | 5.8 | 18 | 4.00 (0.48, 7.52) |
| Active range of external rotation (degrees) at 4 weeks | 76.5 | 4.5 | 17 | 70.1 | 6.3 | 18 | 6.40 (2.79, 10.01) |
| Active range of internal rotation (degrees) at 4 weeks | 47 | 8.5 | 17 | 43.7 | 7.7 | 18 | 3.30 (‐2.08, 8.68) |
| Isokinteic strength: peak torque (Nm) of external rotator 60 degrees/sec at 4 weeks | 21.1 | 5.4 | 17 | 14.5 | 4.6 | 18 | 6.60 (3.27, 9.93) |
| Isokinteic strength: peak torque (Nm) of external rotator 180 degrees/sec at 4 weeks | 65.2 | 6.6 | 17 | 68.4 | 7.3 | 18 | ‐3.20 (‐7.81, 1.41) |
| Isokinteic strength: peak torque (Nm) of internal rotator 60 degrees/sec at 4 weeks | 24.7 | 4.5 | 17 | 22.8 | 5.7 | 18 | 1.90 (‐1.49, 5.29) |
| Isokinteic strength: peak torque (Nm) of internal rotator 180 degrees/sec at 4 weeks | 26.1 | 6.3 | 17 | 21.9 | 7.5 | 18 | 4.20 (‐0.38, 8.78) |
| Study ID: Baskurt 2011 Intervention: scapular stabilisation exercises (PNF) plus stretching and strengthening exercises Control: stretching and strengthening exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (rest pain VAS 0‐10) at 6 weeks | 0.85 | 1.08 | 20 | 1.4 | 1.78 | 20 | ‐0.55 (‐1.46, 0.36) |
| Pain on activity (VAS 0‐10) at 6 weeks | 3 | 1.55 | 20 | 3.2 | 2.11 | 20 | ‐0.20 (‐1.35, 0.95) |
| Range of abduction (degrees, unclear if active or passive) at 6 weeks | 179.75 | 11.11 | 20 | 177 | 13.4 | 20 | 2.75 (‐4.88, 10.38) |
| Range of flexion (degrees, unclear if active or passive) at 6 weeks | 179.75 | 1.11 | 20 | 178.5 | 4.61 | 20 | 1.25 (‐0.83, 3.33) |
| Range of internal rotation in 90 degrees abduction (degrees, unclear if active or passive) at 6 weeks | 88.5 | 3.66 | 20 | 87.5 | 5.5 | 20 | 1.00 (‐1.90, 3.90) |
| Range of external rotation in 90 degrees abduction (degrees, unclear if active or passive) at 6 weeks | 87.5 | 4.13 | 20 | 84.5 | 10.9 | 20 | 3.00 (‐2.11, 8.11) |
| Quality of life (WORC 0‐2100, with higher scores denoting worse quality of life) at 6 weeks | 82.61 | 10.33 | 20 | 70.82 | 19.7 | 20 | 11.79 (2.04, 21.54) |
| Strength: lower trapezium (kg) at 6 weeks | 10.96 | 1.2 | 20 | 9.22 | 1.24 | 20 | 1.74 (0.98, 2.50) |
| Strength: middle trapezium (kg) at 6 weeks | 11.15 | 1.41 | 20 | 10.21 | 1.31 | 20 | 0.94 (0.10, 1.78) |
| Strength: upper trapezium (kg) at 6 weeks | 12.19 | 1.28 | 20 | 11.24 | 1.59 | 20 | 0.95 (0.06, 1.84) |
| Strength: serratus anterior (kg) at 6 weeks | 10.19 | 1.61 | 20 | 8.78 | 1.59 | 20 | 1.41 (0.42, 2.40) |
| Strength: supraspinatus (kg) at 6 weeks | 11.64 | 1.25 | 20 | 10.79 | 1.55 | 20 | 0.85 (‐0.02, 1.72) |
| Strength: subscapularis (kg) at 6 weeks | 6.59 | 1.44 | 20 | 6.02 | 1.31 | 20 | 0.57 (‐0.28, 1.42) |
| Strength: infraspinatus (kg) at 6 weeks | 7.05 | 1.3 | 20 | 6.81 | 1.13 | 20 | 0.24 (‐0.51, 0.99) |
| Study ID: Beaudreuil 2011 Intervention: Dynamic Humeral Centering plus massage and home exercise Control: non‐specific mobilisation plus massage and exercise | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (Constant‐Murley pain sub‐score 0‐15, with higher scores denoting less pain) at 3 months | 12.2 | 2.8 | 30 | 9.9 | 2.9 | 32 | 2.30 (0.88, 3.72) |
| Overall pain (Constant‐Murley pain sub‐score 0‐15, with higher scores denoting less pain) at 12 months | 13.1 | 2 | 22 | 10.8 | 3.7 | 26 | 2.30 (0.65, 3.95) |
| Function (Constant‐Murley total score 0‐100) at 3 months | 63.8 | 16.9 | 30 | 54 | 19.8 | 32 | 9.80 (0.65, 18.95) |
| Function (Constant‐Murley total score 0‐100) at 12 months | 68.9 | 17 | 22 | 62 | 21.1 | 26 | 6.90 (‐3.88, 17.68) |
| ROM (Constant‐Murley ROM sub‐score 0‐40, with higher scores denoting better ROM) at 3 months | 26.7 | 9.6 | 30 | 22.2 | 9.7 | 32 | 4.50 (‐0.31, 9.31) |
| ROM (Constant‐Murley ROM sub‐score 0‐40, with higher scores denoting better ROM) at 12 months | 30.3 | 10 | 22 | 27.8 | 11.2 | 26 | 2.50 (‐3.50, 8.50) |
| Strength (Constant‐Murley strength sub‐score 0‐25, with higher scores denoting better strength) at 3 months | 8.6 | 5 | 30 | 8 | 6.8 | 32 | 0.60 (‐2.36, 3.56) |
| Strength (Constant‐Murley strength sub‐score 0‐25, with higher scores denoting better strength) at 12 months | 8 | 6 | 22 | 7.9 | 5.6 | 26 | 0.10 (‐3.20, 3.40) |
| Study ID: Maenhout 2013 Intervention: heavy load eccentric training plus traditional rotator cuff training Control: traditional rotator cuff training | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (SPADI total score 0‐100 with higher scores denoting worse function) at 6 weeks* | 25.4 | 11.9 | 31 | 17.7 | 12 | 30 | 7.70 (1.70, 13.70) |
| Function (SPADI total score 0‐100 with higher scores denoting worse function) at 12 weeks* | 17 | 11.4 | 31 | 14.5 | 11.7 | 30 | 2.50 (‐3.30, 8.30) |
| Isometric strength at 0º abduction (Newton) at 6 weeks* | 150.8 | 27.6 | 31 | 142.7 | 27.5 | 30 | 8.10 (‐5.73, 21.93) |
| Isometric strength at 0º abduction (Newton) at 12 weeks* | 154.3 | 27.6 | 31 | 147.1 | 27.2 | 30 | 7.20 (‐6.55, 20.95) |
| Isometric strength at 45º abduction (Newton) at 6 weeks* | 79.7 | 12 | 31 | 81.7 | 12 | 30 | ‐2.00 (‐8.02, 4.02) |
| Isometric strength at 45º abduction (Newton) at 12 weeks* | 81.6 | 12.2 | 31 | 83.5 | 11.8 | 30 | ‐1.90 (‐7.92, 4.12) |
| Isometric strength at 90º abduction (Newton) at 6 weeks* | 74.8 | 12.3 | 31 | 72.5 | 12.3 | 30 | 2.30 (‐3.87, 8.47) |
| Isometric strength at 90º abduction (Newton) at 12 weeks* | 78 | 12.5 | 31 | 70 | 12.2 | 30 | 8.00 (1.80, 14.20) |
| Isometric strength external rotation (Newton) at 6 weeks* | 94.3 | 12.2 | 31 | 90.5 | 12.5 | 30 | 3.80 (‐2.40, 10.00) |
| Isometric strength external rotation (Newton) at 12 weeks* | 96 | 12.4 | 31 | 92.7 | 12.3 | 30 | 3.30 (‐2.90, 9.50) |
| Isometric strength internal rotation (Newton) at 6 weeks* | 126.5 | 17.6 | 31 | 123.2 | 17.5 | 30 | 3.30 (‐5.51, 12.11) |
| Isometric strength internal rotation (Newton) at 12 weeks* | 129 | 17.9 | 31 | 125 | 17.2 | 30 | 4.00 (‐4.81, 12.81) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("very large improvement or "large improvement") at 6 weeks | 14 | 30 | 10 | 27 | 1.26 (0.68, 2.35) | ||
| Global assessment of treatment success ("very large improvement or "large improvement") at 12 weeks | 14 | 27 | 13 | 20 | 0.80 (0.49, 1.30) | ||
| Study ID: Martins 2012 Intervention: proprioception exercises plus standardised physiotherapy Control: standardised physiotherapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Quality of life (WORC, 0‐2100, with higher score denoting worse QoL) at 6 weeks | 0.01 | NR | 8 | 0.06 | NR | 8 | ‐0.05 (95% CI not estimable) |
| Work disability (Occupational Stress Indicator, 22‐123) at 6 weeks | 90.2 | 20.8 | 8 | 87 | 18.2 | 8 | 3.20 (‐15.95, 22.35) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Overall pain (number participants with mild pain i.e. score < = 3 on VAS 0‐10) at 6 weeks | 6 | 8 | 5 | 8 | 1.20 (0.61, 2.34) | ||
| Overall pain (number participants with moderate pain i.e. score 4 < = 7 on VAS 0‐10) at 6 weeks | 2 | 8 | 3 | 8 | 0.67 (0.15, 2.98) | ||
| Overall pain (number participants with severe pain i.e. score > 7 on VAS 0‐10) at 6 weeks | 0 | 8 | 0 | 8 | Not estimable | ||
*Mean scores in Maenhout 2013 adjusted for baseline score
Is one type of manual therapy or exercise more effective than another?
Eighteen trials compared one type of manual therapy or exercise with another. Trials compared:
eccentric progressive resistance exercises versus concentric progressive resistance exercises (Blume 2014)
exercises below 90 degrees flexion versus exercises above 90 degrees flexion (Celik 2009)
manual mobilisation versus proprioceptive neuromuscular facilitation (Citaker 2005)
mobilisation with movement and taping versus supervised exercises (Djordjevic 2012)
soft tissue techniques versus mobilisation, proprioceptive neuromuscular facilitation and exercise (Heredia‐Rizo 2013)
specific exercise programme targeting the rotator cuff and scapular stabilisers versus non‐specific movement exercises for the neck and shoulder (Holmgren 2012)
glenohumeral mobilisation versus mobilisation with movement (Kachingwe 2008)
classic Swedish massage versus massage based on the tensegrity principle (Kassolik 2013)
self‐managed loaded exercise programme versus multi‐modal physiotherapy (Littlewood 2014)
neurocognitive therapeutic exercise versus traditional therapeutic exercise (Marzetti 2014)
high‐dose exercise programme versus low dose exercise programme (Osteras 2008)
manual therapy programme versus self‐training programme (Senbursa 2007)
supervised exercises versus home exercises (Senbursa 2011)
scapular‐focused treatment versus stretching, muscle friction and eccentric rotator cuff training (Struyf 2013)
water‐based exercise programme versus land‐based exercise programme (Subasi 2012)
self‐training centring and stretching exercises versus supervised stretching exercises (Walther 2004)
customised exercises versus standardised exercises (Wang 2006)
massage and supervised exercises versus manipulation (Winters 1997).
The overall risk of bias was low in one trial (Holmgren 2012), unclear in eight due to unclear allocation concealment, participant blinding or attrition (Blume 2014; Citaker 2005; Djordjevic 2012; Heredia‐Rizo 2013; Kachingwe 2008; Marzetti 2014; Struyf 2013; Winters 1997), and high in nine due to lack of participant blinding or allocation concealment (Celik 2009; Kassolik 2013; Littlewood 2014; Osteras 2008; Senbursa 2007; Senbursa 2011; Subasi 2012; Walther 2004; Wang 2006)
One participant‐blinded trial (Holmgren 2012) investigated the effects of a 12‐week specific exercise programme targeting the rotator cuff and scapular stabilisers (strengthening eccentric exercises for the rotator cuff and concentric/eccentric exercises for the scapula stabilisers) compared with 12 weeks of non‐specific movement exercises for the neck and shoulder (without any external load). All participants received glucocorticoid injection prior to the exercise programme. For all outcomes assessed at three months, statistically significant differences favouring the specific exercise programme were found, most of which were clinically important: overall pain (MD ‐10.00, 95% CI ‐18.18 to ‐1.82; 100‐point scale, 97 participants), function (MD 20.00, 95% CI 11.55 to 28.45; 100‐point scale, 97 participants), pain on motion (MD ‐16.00, 95% CI ‐26.57 to ‐5.43; 100‐point scale, 97 participants), global treatment success (RR 2.87, 95% CI 1.66 to 4.96; 97 participants), night pain (MD ‐12.00, 95% CI ‐21.87 to ‐2.13; 100‐point scale, 97 participants), quality of life (MD 0.13, 95% CI 0.05 to 0.21; scale range from ‐0.59 to 1, 97 participants), and having surgery at some point between three months and one year follow‐up (RR 0.37, 95% CI 0.22 to 0.64; 97 participants). We considered the evidence from this trial to be high quality.
Of the remaining 17 trials, 11 found no clinically important differences between groups on any outcome (Blume 2014; Celik 2009; Citaker 2005; Kachingwe 2008; Littlewood 2014; Marzetti 2014; Senbursa 2007; Senbursa 2011; Walther 2004; Wang 2006; Winters 1997). In the other six trials (Djordjevic 2012; Heredia‐Rizo 2013; Kassolik 2013; Osteras 2008; Struyf 2013; Subasi 2012), some statistically significant differences in outcomes were noted (see Table 21). We downgraded the evidence from these 17 trials by two points for high or unclear risk of performance and detection bias, and one point for imprecision, and thus consider it to be very low quality.
18. One type of manual therapy or exercise versus another.
| Study ID: Blume 2014 Intervention: supervised eccentric progressive resistance exercises plus ice plus home exercises Control: supervised concentric progressive resistance exercises plus ice plus home exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (DASH 0‐100 where higher = worse function) at 5 weeks | 14.6 | 8.7 | 17 | 11.7 | 6.6 | 13 | 2.90 (‐2.57, 8.37) |
| Function (DASH 0‐100 where higher = worse function) at 8 weeks | 11.5 | 11.8 | 17 | 8.4 | 6.7 | 13 | 3.10 (‐3.59, 9.79) |
| Active ROM: scaption (degrees) at 5 weeks | 143.4 | 24 | 17 | 141.9 | 25.7 | 13 | 1.50 (‐16.54, 19.54) |
| Active ROM: scaption (degrees) at 8 weeks | 145.7 | 23.6 | 17 | 143.7 | 31.3 | 13 | 2.00 (‐18.38, 22.38) |
| Strength: abduction torque (lbs) at 5 weeks | 239.3 | 132.8 | 17 | 193.9 | 110.6 | 13 | 45.40 (‐41.78, 132.58) |
| Strength: abduction torque (lbs) at 8 weeks | 281.5 | 165.6 | 17 | 259.4 | 138.4 | 13 | 22.10 (‐86.79, 130.99) |
| Strength: external rotation torque (lbs) at 5 weeks | 173.5 | 121.7 | 17 | 140.2 | 103.1 | 13 | 33.30 (‐47.25, 113.85) |
| Strength: external rotation torque (lbs) at 8 weeks | 203.5 | 121.7 | 17 | 186.1 | 122.5 | 13 | 17.40 (‐70.81, 105.61) |
| Study ID: Celik 2009 Intervention: exercise below 90 degrees plus standardised physiotherapy Control: exercise above 90 degrees standardised physiotherapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 2 weeks | 2.9 | 1.7 | 15 | 4.1 | 1.6 | 15 | ‐1.20 (‐2.38, ‐0.02) |
| Overall pain (VAS 0‐10) at 4 months | 1.1 | 1 | 15 | 1.6 | 1.6 | 15 | ‐0.50 (‐1.45, 0.45) |
| Global assessment of treatment success (patient satisfaction scale, 0‐4, higher score higher satisfaction) at 2 weeks | 2.6 | 0.7 | 15 | 2.2 | 0.6 | 15 | 0.40 (‐0.07, 0.87) |
| Global assessment of treatment success (patient satisfaction scale, 0‐4, higher score higher satisfaction) at 2 weeks | 3.3 | 0.4 | 15 | 3.1 | 0.1 | 15 | 0.20 (‐0.01, 0.41) |
| Study ID: Citaker 2005 Intervention: manual mobilisation plus standardised physiotherapy Control: proprioceptive neuromuscular facilitation plus standardised physiotherapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (total UCLA score, 0‐35, where higher scores denote better function) at 3 weeks | 33.22 | 2.95 | 20 | 29.97 | 4.6 | 20 | 3.25 (0.86, 5.64) |
| Day pain at rest (VAS 0‐10) at 3 weeks | 0.75 | 1.45 | 20 | 0.25 | 0.91 | 20 | 0.50 (‐0.25, 1.25) |
| Night pain at rest (VAS 0‐10) at 3 weeks | 1.75 | 2.55 | 20 | 1.65 | 2.54 | 20 | 0.10 (‐1.48, 1.68) |
| Day pain on motion (VAS 0‐10) at 3 weeks | 0.6 | 1.27 | 20 | 0.6 | 1.19 | 20 | 0.00 (‐0.76, 0.76) |
| Night pain on motion (VAS 0‐10) at 3 weeks | 1.5 | 2.3 | 20 | 1.85 | 2.64 | 20 | ‐0.35 (‐1.88, 1.18) |
| Range of abduction (degrees, unclear if active or passive) at 3 weeks | 170.5 | 21.52 | 20 | 174.75 | 9.8 | 20 | ‐4.25 (‐14.61, 6.11) |
| Range of flexion (degrees, unclear if active or passive) at 3 weeks | 170.4 | 11.74 | 20 | 173 | 10.44 | 20 | ‐2.60 (‐9.49, 4.29) |
| Range of external rotation (degrees, unclear if active or passive) at 3 weeks | 77.5 | 19.23 | 20 | 80.25 | 10.57 | 20 | ‐2.75 (‐12.37, 6.87) |
| Range of internal rotation (degrees, unclear if active or passive) at 3 weeks | 85.5 | 13.11 | 20 | 85.25 | 9.1 | 20 | 0.25 (‐6.74, 7.24) |
| Study ID: Djordjevic 2012 Intervention: mobilisation with movement and kinesiotaping Control: supervised exercise programme | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Active range of abduction (degrees) at 10 days | 170 | 17.89 | 10 | 60.5 | 15.72 | 10 | 109.50 (94.74, 124.26) |
| Active range of flexion (degrees) at 10 days | 166 | 20.59 | 10 | 86 | 21.89 | 10 | 80.00 (61.37, 98.63) |
| Study ID: Heredia‐Rizo 2013 Intervention: soft tissue techniques Control: mobilisation, proprioceptive neuromuscular facilitation and exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (DASH, 0‐100 with higher scores denoting worse functionality) at 3 weeks | 34.69 | 11.21 | 11 | 38.61 | 20.8 | 11 | ‐3.92 (‐17.88, 10.04) |
| Active range of abduction (degrees) at 3 weeks | 142.5 | 25.3 | 11 | 115.55 | 20.68 | 11 | 26.95 (7.64, 46.26) |
| Active range of flexion (degrees) at 3 weeks | 153 | 20.97 | 11 | 140.01 | 16 | 11 | 12.99 (‐2.60, 28.58) |
| Active range of extension (degrees) at 3 weeks | 65.5 | 12.79 | 11 | 51.66 | 11.72 | 11 | 13.84 (3.59, 24.09) |
| Active range of external rotation (degrees) at 3 weeks | 68 | 19.32 | 11 | 52.77 | 21.95 | 11 | 15.23 (‐2.05, 32.51) |
| Active range of internal rotation (degrees) at 3 weeks | 85 | 8.49 | 11 | 73.33 | 16 | 11 | 11.67 (0.97, 22.37) |
| Study ID: Holmgren 2012 Intervention: specific exercise programme Control: non‐specific exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) at 3 months | 10 | 14 | 51 | 20 | 25 | 46 | ‐10.00 (‐18.18, ‐1.82) |
| Function (Constant‐Murley total score, 0‐100 with higher scores denoting better function) at 3 months | 72.5 | 19 | 51 | 52.5 | 23 | 46 | 20.00 (11.55, 28.45) |
| Pain on motion (VAS 0‐100) at 3 months | 25 | 26 | 51 | 41 | 27 | 46 | ‐16.00 (‐26.57, ‐5.43) |
| Night pain (VAS 0‐100) at 3 months | 15 | 22 | 51 | 27 | 27 | 46 | ‐12.00 (‐21.87, ‐2.13) |
| Quality of life (EuroQoL EQ‐5D, ‐0.59 to 1 where lower scores denote worse QoL) at 3 months | 0.82 | 0.14 | 51 | 0.69 | 0.24 | 46 | 0.13 (0.05, 0.21) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("recovered" or "large improvement") at 3 months | 35 | 51 | 11 | 46 | 2.87 (1.66, 4.96) | ||
| Had surgery between 3 months and 1 year | 12 | 51 | 29 | 46 | 0.37 (0.22, 0.64) | ||
| Study ID: Kachingwe 2008 Intervention: glenohumeral mobilisation plus exercises Control: mobilisation with movement plus exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 with higher scores denoting worse pain) % change from baseline to 6 weeks | 44.2 | 38.6 | 9 | 55.2 | 31.9 | 9 | Not estimable |
| Function (SPADI total score 0‐130 with higher scores denoting worse function) % change from baseline to 6 weeks | 56.7 | 29.8 | 9 | 55.5 | 20.1 | 9 | Not estimable |
| Active range of flexion % change from baseline to 6 weeks | ‐15.9 | 116.6 | 9 | 46.7 | 31.9 | 9 | Not estimable |
| Study ID: Kassolik 2013 Intervention: classic Swedish massage Control: massage based on the tensegrity principle | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10 in Short‐Form McGill Pain Questionnaire) at 2 weeks | 3.16 | 1.77 | 15 | 1.66 | 0.48 | 15 | 1.50 (0.57, 2.43) |
| Overall pain (VAS 0‐10 in Short‐Form McGill Pain Questionnaire) at 6 weeks | 3.7 | 1.88 | 15 | 1.06 | 0.45 | 15 | 2.64 (1.66, 3.62) |
| Active range of abduction (degrees) at 2 weeks | 145.8 | 18.6 | 15 | 136.2 | 11.9 | 15 | 9.60 (‐1.57, 20.77) |
| Active range of abduction (degrees) at 6 weeks | 144.4 | 17.6 | 15 | 136.6 | 10.6 | 15 | 7.80 (‐2.60, 18.20) |
| Active range of flexion (degrees) at 2 weeks | 156.2 | 20.9 | 15 | 143.3 | 13.9 | 15 | 12.90 (0.20, 25.60) |
| Active range of flexion (degrees) at 6 weeks | 154.4 | 20.4 | 15 | 144.6 | 15 | 15 | 9.80 (‐3.01, 22.61) |
| Active range of extension (degrees) at 2 weeks | 36.1 | 5.4 | 15 | 29.6 | 8.3 | 15 | 6.50 (1.49, 11.51) |
| Active range of extension (degrees) at 6 weeks | 33.6 | 5.6 | 15 | 29.6 | 7.9 | 15 | 4.00 (‐0.90, 8.90) |
| Active range of external rotation (degrees) at 2 weeks | 14.9 | 5.4 | 15 | 13.8 | 5.2 | 15 | 1.10 (‐2.69, 4.89) |
| Active range of external rotation (degrees) at 6 weeks | 13.8 | 5.4 | 15 | 13.6 | 5.5 | 15 | 0.20 (‐3.70, 4.10) |
| Active range of internal rotation (degrees) at 2 weeks | 25.2 | 5.9 | 15 | 24.4 | 5.9 | 15 | 0.80 (‐3.42, 5.02) |
| Active range of internal rotation (degrees) at 6 weeks | 23.2 | 6.4 | 15 | 24.4 | 6.1 | 15 | ‐1.20 (‐5.67, 3.27) |
| Study ID: Littlewood 2014 Intervention: self‐managed loaded exercise Control: usual physiotherapy (might include advice, stretching, exercise, manual therapy, massage, strapping, acupuncture, electrotherapy, corticosteroid injection at the discretion of the treating physiotherapist) | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (SPADI total score, 0‐100 with higher scores denoting worse function) at 3 months | 20.9 | 19.2 | 12 | 20.7 | 20.3 | 12 | 0.20 (‐15.61, 16.01) |
| Quality of life (SF‐36 physical functioning score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 78.2 | 17.7 | 12 | 73.3 | 29.3 | 12 | 4.90 (‐14.47, 24.27) |
| Quality of life (SF‐36 role‐physical score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 88.5 | 18 | 12 | 79.2 | 20 | 12 | 9.30 (‐5.92, 24.52) |
| Quality of life (SF‐36 bodily pain score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 61.4 | 13.4 | 12 | 71.8 | 18.2 | 12 | ‐10.40 (‐23.19, 2.39) |
| Quality of life (SF‐36 general health score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 74.2 | 20.3 | 12 | 72.9 | 11.6 | 12 | 1.30 (‐11.93, 14.53) |
| Quality of life (SF‐36 vitality score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 69.3 | 12.1 | 12 | 70.8 | 21.5 | 12 | ‐1.50 (‐15.46, 12.46) |
| Quality of life (SF‐36 social functioning score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 45.8 | 11.1 | 12 | 50 | 10.7 | 12 | ‐4.20 (‐12.92, 4.52) |
| Quality of life (SF‐36 role‐emotional score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 95.8 | 10.4 | 12 | 97.2 | 7.4 | 12 | ‐1.40 (‐8.62, 5.82) |
| Quality of life (SF‐36 mental health score, 0‐100 where a higher score indicates a better quality of life) at 3 months | 84.6 | 12.9 | 12 | 82.5 | 13.1 | 12 | 2.10 (‐8.30, 12.50) |
| Study ID: Marzetti 2014 Intervention: neurocognitive therapeutic exercise Control: traditional therapeutic exercise | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10) at 5 weeks | 0.95 | NR | 24 | 2.19 | NR | 24 | ‐1.24 (‐2.93, 0.46) |
| Rest pain (VAS 0‐10) at 24 weeks | 0.45 | NR | 24 | 2.05 | NR | 24 | ‐1.59 (‐3.29, 0.10) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 5 weeks | 75.5 | NR | 24 | 74.57 | NR | 24 | 0.93 (‐11.94, 13.8) |
| Function (Constant‐Murley total score 0‐100, higher = better function) at 24 weeks | 83.27 | NR | 24 | 76.95 | NR | 24 | 6.32 (‐6.55, 19.19) |
| Pain on motion (VAS 0‐10) at 5 weeks | 3.73 | NR | 24 | 4.1 | NR | 24 | ‐0.37 (‐2.35, 1.62) |
| Pain on motion (VAS 0‐10) at 24 weeks | 1.86 | NR | 24 | 3.33 | NR | 24 | ‐1.47 (‐3.46, 0.52) |
| Adverse events | Zero events in both groups | ||||||
| Study ID: Osteras 2008 Intervention: high dose medical exercise therapy Control: low dose medical exercise therapy | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 3 months | 2.1 | 1.6 | 29 | 4.1 | 1.7 | 27 | ‐2.00 (‐2.87, ‐1.13) |
| Overall pain (VAS 0‐10) at 15 months | 1.2 | 1 | 26 | 4.2 | 2.1 | 23 | ‐3.00 (‐3.94, ‐2.06) |
| Function (Shoulder Rating Questionnaire, 17 ‐ 90 with a higher score indicating better function) at 3 months | 69.1 | 13.3 | 29 | 51.5 | 14.2 | 27 | 17.60 (10.38, 24.82) |
| Function (Shoulder Rating Questionnaire, 17 ‐ 90 with a higher score indicating better function) at 15 months | 79.1 | 7.6 | 26 | 54.7 | 18.7 | 23 | 24.40 (16.22, 32.58) |
| Range of abduction (degrees, unclear if active or passive) change from baseline to 3 months | 42 | 42.0632 | 29 | 12 | 35.3905 | 27 | 30.00 (9.69, 50.31) |
| Range of abduction (degrees, unclear if active or passive) change from baseline to 9 months | 49 | 40.4462 | 27 | 14 | 38.7616 | 25 | 35.00 (13.47, 56.53) |
| Range of flexion (degrees, unclear if active or passive) change from baseline to 3 months | 31 | 63.0949 | 29 | 7 | 22.751 | 27 | 24.00 (‐0.51, 48.51) |
| Range of flexion (degrees, unclear if active or passive) change from baseline to 9 months | 34 | 22.751 | 27 | 8 | 16.9582 | 25 | 26.00 (15.14, 36.86) |
| Strength (isometric strength in abduction, Newtons) change from baseline to 3 months | 34 | 26.2895 | 29 | 17 | 20.2231 | 27 | 17.00 (4.76, 29.24) |
| Strength (isometric strength in abduction, Newtons) change from baseline to 9 months | 45 | 42.9741 | 27 | 14 | 46.0294 | 25 | 31.00 (6.74, 55.26) |
| Strength (isometric strength in flexion, Newtons) change from baseline to 3 months | 33 | 34.1764 | 29 | 4 | 12.6395 | 27 | 29.00 (15.68, 42.32) |
| Strength (isometric strength in flexion, Newtons) change from baseline to 9 months | 49 | 60.6694 | 27 | 28 | 62.9876 | 25 | 21.00 (‐12.66, 54.66) |
| Strength (isometric strength in external rotation, Newtons) change from baseline to 3 months | 28 | 52.579 | 29 | 9 | 30.3347 | 27 | 19.00 (‐3.30, 41.30) |
| Strength (isometric strength in external rotation, Newtons) change from baseline to 9 months | 36 | 63.1973 | 27 | 3 | 31.4938 | 25 | 33.00 (6.16, 59.84) |
| Strength (isometric strength in internal rotation, Newtons) change from baseline to 3 months | 15 | 21.0316 | 29 | 13 | 20.2231 | 27 | 2.00 (‐8.81, 12.81) |
| Strength (isometric strength in internal rotation, Newtons) change from baseline to 9 months | 21 | 22.751 | 27 | 9 | 24.226 | 25 | 12.00 (‐0.80, 24.80) |
| Study ID: Senbursa 2007 Intervention: manual therapy program Control: self‐training program | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10) at 3 months | 0.7 | 1.4 | 15 | 0.9 | 0.2 | 15 | ‐0.20 (‐0.92, 0.52) |
| Night pain (VAS 0‐10) at 3 months | 2.2 | 2.4 | 15 | 1.2 | 1.6 | 15 | 1.00 (‐0.46, 2.46) |
| Pain on motion (VAS 0‐10) at 3 months | 3.1 | 2 | 15 | 2.5 | 1.5 | 15 | 0.60 (‐0.67, 1.87) |
| Study ID: Senbursa 2007 Intervention: supervised exercises Control: home exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| % Events | Total | % Events | Total | Risk ratio (95% CI) | |||
| Rest pain (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 64% | Unclear | 82% | Unclear | Not estimable | ||
| Rest pain (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 92% | Unclear | 91% | Unclear | Not estimable | ||
| Night pain (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 36% | Unclear | 45% | Unclear | Not estimable | ||
| Night pain (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 88% | Unclear | 82% | Unclear | Not estimable | ||
| Pain on motion (number of participants with no pain as measured on VAS 0‐10) at 4 weeks | 16% | Unclear | 14% | Unclear | Not estimable | ||
| Pain on motion (number of participants with no pain as measured on VAS 0‐10) at 12 weeks | 36% | Unclear | 41% | Unclear | Not estimable | ||
| Study ID: Struyf 2013 Intervention: scapular‐focused treatment Control: stretching, muscle friction and eccentric rotator cuff training | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐10) at 4‐8 weeks | 1.3 | 1.5 | 10 | 2.3 | 2.6 | 10 | ‐1.00 (‐2.86, 0.86) |
| Function (SDQ‐NL, 0‐100 with higher scores denoting worse function) at 4‐8 weeks | 35 | 14 | 10 | 48.7 | 11.3 | 10 | ‐13.70 (‐24.85, ‐2.55) |
| Pain on motion (VAS 0‐10) at 4‐8 weeks | 3 | 1.9 | 10 | 5.1 | 2 | 10 | ‐2.10 (‐3.81, ‐0.39) |
| Strength (isometric elevation strength, Newtons) at 4‐8 weeks | 55.79 | 18.71 | 10 | 74.11 | 34.28 | 10 | ‐18.32 (‐42.53, 5.89) |
| Study ID: Subasi 2012 Intervention: water‐based exercise programme Control: land‐based exercise programme | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 4 weeks | 3.2 | 1.4 | 29 | 3.7 | 1.4 | 28 | ‐0.50 (‐1.23, 0.23) |
| Overall pain (VAS 0‐10) at 8 weeks | 2.8 | 1.5 | 29 | 4.1 | 1.7 | 28 | ‐1.30 (‐2.13, ‐0.47) |
| Function (SPADI total score 0‐100, with higher scores denoting worse function) at 4 weeks | 16.7 | 12.6 | 29 | 20.1 | 10.5 | 28 | ‐3.40 (‐9.41, 2.61) |
| Function (SPADI total score 0‐100, with higher scores denoting worse function) at 8 weeks | 12 | 9.1 | 29 | 20.9 | 10.2 | 28 | ‐8.90 (‐13.92, ‐3.88) |
| Quality of life (WORC, 0‐2100, where 2100 is worst score) at 4 weeks | 599.7 | 417.5 | 29 | 739.7 | 332.9 | 28 | ‐140.00 (‐335.69, 55.69) |
| Quality of life (WORC, 0‐2100, where 2100 is worst score) at 8 weeks | 475 | 269.9 | 29 | 733.1 | 331.6 | 28 | ‐258.10 (‐415.37, ‐100.83) |
| Study ID: Walther 2004 Intervention: standardised self‐training of centring and stretching exercises Control: supervised stretching exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Rest pain (VAS 0‐100) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Function (Constant‐Murley total score) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Pain on motion (VAS 0‐100) at both 6 and 12 weeks | "No significant difference between groups" | ||||||
| Strength (Constant‐Murley strength sub‐score 0‐25) at 12 weeks | 10.9 | 4.6 | 20 | 11.8 | 5.4 | 20 | ‐0.90 (‐4.01, 2.21) |
| Work disability (number of months with inability to work) | 1.2 | NR | 20 | 1.6 | NR | 20 | ‐0.4 (95% CI not estimable) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Adverse events | 0 | 20 | 0 | 20 | Not estimable (no adverse events) | ||
| Study ID: Wang 2006 Intervention: customised exercises Control: standardised exercises | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) at 4 weeks | 20.1 | 14.5 | 15 | 23.1 | 18 | 15 | ‐3.00 (‐14.70, 8.70) |
| Overall pain (VAS 0‐100) at 8 weeks | 21.6 | 12.5 | 15 | 21.2 | 17.6 | 15 | 0.40 (‐10.52, 11.32) |
| Function (Flexi‐SF Score, 1‐50 with a higher score indicating better function) at 4 weeks | 32.3 | 9.8 | 15 | 32.4 | 6.6 | 15 | ‐0.10 (‐6.08, 5.88) |
| Function (Flexi‐SF Score, 1‐50 with a higher score indicating better function) at 8 weeks | 36.2 | 6.5 | 15 | 33.8 | 7 | 15 | 2.40 (‐2.43, 7.23) |
| Active range of abduction (degrees) at 4 weeks | 147.1 | 36.5 | 15 | 140.4 | 40.6 | 15 | 6.70 (‐20.93, 34.33) |
| Active range of abduction (degrees) at 8 weeks | 149.3 | 32.5 | 15 | 143.4 | 37.4 | 15 | 5.90 (‐19.17, 30.97) |
| Active range of external rotation (degrees) at 4 weeks | 81 | 22.1 | 15 | 71.9 | 27.3 | 15 | 9.10 (‐8.67, 26.87) |
| Active range of external rotation (degrees) at 8 weeks | 81.8 | 18.4 | 15 | 73.1 | 26.9 | 15 | 8.70 (‐7.79, 25.19) |
| Active range of internal rotation (degrees) at 4 weeks | 40.6 | 14.7 | 15 | 45.8 | 14.8 | 15 | ‐5.20 (‐15.76, 5.36) |
| Active range of internal rotation (degrees) at 8 weeks | 44.9 | 15.7 | 15 | 44.5 | 16.9 | 15 | 0.40 (‐11.27, 12.07) |
| Strength (isometric strength of abductors in N‐m) at 4 weeks | 48.3 | 20.9 | 15 | 36.3 | 20 | 15 | 12.00 (‐2.64, 26.64) |
| Strength (isometric strength of abductors in N‐m) at 8 weeks | 53.9 | 21.9 | 15 | 42.2 | 24.4 | 15 | 11.70 (‐4.89, 28.29) |
| Strength (isometric strength of external rotators in N‐m) at 4 weeks | 34.3 | 14 | 15 | 27.7 | 17.4 | 15 | 6.60 (‐4.70, 17.90) |
| Strength (isometric strength of external rotators in N‐m) at 8 weeks | 36.3 | 15.1 | 15 | 29.3 | 15.9 | 15 | 7.00 (‐4.10, 18.10) |
| Strength (isometric strength of internal rotators in N‐m) at 4 weeks | 31.7 | 11.9 | 15 | 28 | 16.6 | 15 | 3.70 (‐6.64, 14.04) |
| Strength (isometric strength of internal rotators in N‐m) at 8 weeks | 37.5 | 15.7 | 15 | 28.4 | 14.6 | 15 | 9.10 (‐1.75, 19.95) |
| Strength (isometric strength of middle trapezius in N‐m) at 4 weeks | 34.8 | 15.5 | 15 | 28.1 | 17.9 | 15 | 6.70 (‐5.28, 18.68) |
| Strength (isometric strength of middle trapezius in N‐m) at 8 weeks | 41 | 19.4 | 15 | 30.8 | 17.9 | 15 | 10.20 (‐3.16, 23.56) |
| Strength (isometric strength of lower trapezius in N‐m) at 4 weeks | 31.8 | 16.3 | 15 | 26 | 16.2 | 15 | 5.80 (‐5.83, 17.43) |
| Strength (isometric strength of lower trapezius in N‐m) at 8 weeks | 37.2 | 20.3 | 15 | 29.4 | 18 | 15 | 7.80 (‐5.93, 21.53) |
| Study ID: Winters 1997 Intervention: exercise and massage Control: manipulation | |||||||
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (shoulder pain score 0‐28, higher score denotes worse pain) at 11 weeks | 11.5 | 4.4 | 35 | 12.6 | 5.1 | 32 | ‐1.10 (‐3.39, 1.19) |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success ("cured") at 11 weeks | 18 | 35 | 14 | 32 | 1.18 (0.71, 1.95) | ||
Subgroup and sensitivity analyses, and assessment of publication bias
Given the inability to conduct meta‐analyses, no subgroup or sensitivity analyses were undertaken. Also, we were unable to generate funnel plots to assess small study effects. Despite the lack of funnel plots, we considered the risk of publication bias to be low because nearly all of the published studies reported statistically non‐significant results for most outcomes. It is possible that some unpublished studies with non‐significant results exist, but their inclusion in the review is unlikely to change our conclusions.
Discussion
Summary of main results
In this systematic review we have considered the results of 60 trials investigating the benefits and harms of manual therapy and exercise for rotator cuff disease. The combination of manual therapy and exercise (the most clinically relevant intervention (Klintberg 2015)) was examined in 10 trials (Bennell 2010; Cloke 2008; Dickens 2005; Ginn 2005; Haahr 2005; Hay 2003; Kachingwe 2008; Rhon 2014; Szczurko 2009; Winters 1997), but the variation in intervention content and comparators meant trials could not be pooled.
High quality evidence from one trial of 120 participants with chronic rotator cuff disease indicated no clinically important differences between manual therapy and exercise and placebo with respect to overall pain, function, pain on motion, global treatment success, quality of life and strength at 22 weeks, although manual therapy and exercise was associated with relatively more frequent but mild adverse events (short‐term pain following treatment) (Bennell 2010). Very low quality evidence from two trials that compared manual therapy and exercise to no treatment was broadly consistent with these results (Dickens 2005; Kachingwe 2008).
Low quality evidence from five trials (Cloke 2008; Ginn 2005; Hay 2003; Rhon 2014; Winters 1997) revealed no clinically important differences between manual therapy and exercise and glucocorticoid injection with respect to overall pain, function, quality of life, night pain and active range of motion up to 12 months. However, global treatment success was more common up to 11 weeks in participants receiving glucocorticoid injection (based on low quality evidence). Low quality evidence from one trial showed no important differences between manual therapy and exercise and arthroscopic subacromial decompression with respect to overall pain, function, active range of motion and strength at six and 12 months, or global treatment success at four to eight years (Haahr 2005). Low quality evidence from one trial found that manual therapy and exercise may not be as effective as acupuncture plus dietary counselling and Phlogenzym supplement with respect to overall pain, function, active shoulder abduction and quality‐of‐life at 12 weeks in postal workers (Szczurko 2009). Very low quality evidence from one trial suggested that firstly, there was no important difference between manual therapy and exercise compared with oral NSAID, and secondly, no added benefit of manual therapy and exercise over glucocorticoid injection alone with respect to function at 18 weeks and 12 months (Cloke 2008).
Overall completeness and applicability of evidence
Participants in the included trials were mostly representative of populations most affected by rotator cuff disease. Nearly all trials enrolled a community sample of people attending routine physical therapy care. Across the trials, the gender ratio was equal, and the median age was 51 (IQR 46 to 56) years. Thus, results are applicable to both male and female older adult populations, which is useful given that the incidence of rotator cuff disease increases with age (Linsell 2006; Yamamoto 2010). Further, trials were conducted in 21 different countries, including a range of high‐ and low‐ to middle‐income countries. However, it is difficult to determine how representative participants in the included trials were with respect to duration of symptoms, as this characteristic was not reported in 31 (52%) trials.
Manual therapy and exercise are most often delivered together in physical therapy practice (Glazier 1998; Kooijman 2013; Roberts 2014; Struyf 2012), yet the effects of this multi‐modal intervention were investigated in only 10 (17%) trials. Our review was dominated by trials investigating whether manual therapy or exercise provided benefit when added to another physical therapy intervention (e.g. manual therapy plus therapeutic ultrasound versus therapeutic ultrasound alone), or whether one type of manual therapy or exercise intervention was more effective than another.
In several trial reports, the components of the exercise programmes were incompletely described. For example, some trialists specified the type of exercise broadly (e.g. "range of movement exercises") without specifying the planes of movement addressed, the frequency of exercises (e.g. once or twice per day) or the setting in which they were undertaken (e.g. clinic or home). Incomplete descriptions such as these hinder replication of the trial, and limit reliable implementation of the exercise programme into clinical practice. A standardised and internationally agreed template for explicit reporting of exercise programmes has recently been developed (Slade 2014; Slade 2016). This guidance will hopefully improve the quality of reporting in future trials.
Another concerning issue is the variable choice of outcomes measured in the trials. Overall pain and function were measured commonly (80% and 73%, respectively), but these domains should be measured in all rotator cuff disease trials given that pain and functional limitations are the most common presenting symptoms of the condition (Whittle 2015). Further, adverse events were measured in less than a third of trials (28%). The proportions of trials measuring the other main outcomes of the review were relatively low: pain on motion (27%), global assessment of treatment success (28%), and quality of life (22%). Outcome measurement has certainly improved since the first version of our review (Green 1998), where function was measured in only 26% of trials (none with a validated disability index), and quality of life was measured in no trials. A core domain set and core outcome measurement set for rotator cuff disease trials would likely improve measurement of patient‐important outcomes in future trials, and would facilitate efforts to synthesise the evidence in future (Buchbinder 2003; Page 2015). We are currently developing these core sets with the support of the Outcome Measures in Rheumatology (OMERACT) initiative, who approved a special interest group session on shoulder pain at the OMERACT 2016 meeting.
Quality of the evidence
Although we presented 'Summary of findings' tables only for trials addressing the primary questions of the review, we used the GRADE approach (Schünemann 2011b) to assess the quality of all the evidence examined. Most of the evidence was downgraded to low quality for a combination of two out of three reasons. Firstly, the risk of performance and detection bias for self‐reported outcomes was high, secondly, evidence was based on small, single trials, leading to concerns about imprecise effect estimates, and thirdly, trialists examined a sample of people whose outcomes may not apply to the general population (e.g. construction workers, professional athletes). Regarding the first of these concerns, we rated very few trials (22% and 20%, respectively) at low risk of performance bias and detection bias for self‐reported outcomes because participants and personnel were not blinded.
Blinding of participants and personnel is difficult to achieve in procedural trials, so performance bias and detection bias are often difficult to minimise. However, this is problematic because it is estimated that trials with unblinded assessment of subjective outcomes (such as function and pain) exaggerate treatment benefits by 22% on average (ratio of odds ratios 0.78, 95% credible interval 0.65 to 0.92) (Savovic 2012). For most comparisons and outcomes in our review, the true effects of the interventions may be different to the effect estimates observed because of this bias. On the other hand, Bennell 2010 found that they achieved a moderate to high degree of participant blinding with the use of inactive ultrasound therapy and application of an inert gel as a placebo control, increasing confidence that their results present an unbiased estimate of the true effect of manual therapy and exercise. This control intervention has also been found to be a realistic placebo for physical therapy in other trials performed by this group (Bennell 2014; Buchbinder 2007).
Potential biases in the review process
We searched CENTRAL, MEDLINE, EMBASE and CINAHL, but not PEDro, a database of randomised trials, systematic reviews and clinical practice guidelines in physiotherapy. A study comparing the indexing of 400 physiotherapy trials in eight bibliographic databases found that almost all were indexed in CENTRAL (95%), PEDro (92%) MEDLINE (89%) and EMBASE (88%), and only one of the 400 trials was uniquely indexed in PEDro (Michaleff 2011). Therefore, it is very unlikely that we missed relevant trials that would change the conclusions of our review. Two review authors independently assessed the trials for inclusion in this review, extracted data and assessed the risk of bias, and a third review author adjudicated when any discrepancy arose. Two of the review authors (SG and RB) are authors of one of the trials included in this review (Bennell 2010). To avoid bias, the paper was sent to an independent review author for eligibility assessment. Neither review author was involved in data extraction or assessment of risk of bias of this trial. Review questions of interest were defined with full knowledge of the possible comparisons that could be undertaken, but no knowledge of the results of any comparisons. To prevent selective inclusion of results (Page 2013), we used pre‐defined decision rules to select data from trials when multiple measurement scales, time points and analyses were reported.
A limitation of the review process was that several trials addressing the main questions of the review did not present data for all measured outcomes, or presented outcome data incompletely, which prevented us from calculating effect estimates and 95% CIs; attempts to obtain unpublished data from trialists were unsuccessful. Another potential limitation was that we excluded four trials (Miller 2004; Mörl 2011; Seok‐Hwa 2013; Tachibana 2012) which may have included participants with rotator cuff disease, but the eligibility criteria and participant characteristics were not reported in enough detail for us to determine this. Further, we excluded one trial (Ginn 1997) that included participants with shoulder pain due to either rotator cuff disease, adhesive capsulitis, osteoarthritis, biceps muscle tear or no specific diagnosis, and we were unable to obtain data on the rotator cuff disease subgroup, which comprised 65% of the sample. However, none of these trials addressed the two main questions of the review, so our overall conclusions remain unchanged. In addition, we did not search for grey literature (e.g. proceedings of specific conferences, theses or unpublished reports). However, we believe that had we identified unpublished studies with non‐significant results, their inclusion in the review would be unlikely to change our conclusions since the majority of the evidence we considered had 'negative' findings.
Agreements and disagreements with other studies or reviews
Following the earlier Cochrane review of physical therapy for shoulder disorders (Green 2003), there have been several systematic reviews of manual therapy (Brudvig 2011; Camarinos 2009; Ho 2009; Pribicevic 2010), exercise (Dewhurst 2010; Hanratty 2012; Kelly 2010; Kuhn 2009; Littlewood 2012; Marinko 2011), and manual therapy and exercise (Brantingham 2011; Braun 2010; Braun 2013; Gebremariam 2014; Kromer 2009; Nyberg 2010; Saltychev 2015; Van den Dolder 2014) for rotator cuff disease. All of these reviews have been narrower in scope than ours. Review authors either restricted their study eligibility criteria according to the diagnostic label used by trialists (e.g. focusing only on subacromial impingement syndrome or rotator cuff tendinopathy), or used broad participant eligibility criteria but focused on only one type of manual therapy (e.g. soft tissue massage). Therefore, to our knowledge ours is the most comprehensive review of manual therapy and exercise interventions for rotator cuff disease.
Our conclusions about the benefits and harms of manual therapy and exercise, manual therapy alone and exercise alone are consistent with nearly all other systematic reviews. In a few cases, other review authors (Dewhurst 2010; Kuhn 2009; Marinko 2011) had more favourable conclusions than ours. However, these discrepancies appear to be driven by less frequent consideration of the overall quality of evidence in these reviews (i.e. while study risk of bias was assessed, other domains of the GRADE approach (imprecision, inconsistency, indirectness and publication bias) were not).
Authors' conclusions
Implications for practice.
Despite identifying 60 trials meeting the inclusion criteria for this review, only one trial compared a combination of manual therapy and exercise reflective of common current practice to placebo. Based upon high quality data from this trial, there was no clinically important benefit of manual therapy and exercise over placebo. Adverse events were relatively more frequent with manual therapy and exercise but mild in nature (short‐term pain following treatment). Effects of manual therapy and exercise may be similar to those of glucocorticoid injection and arthroscopic subacromial decompression, but this is based on low quality evidence. Until further evidence confirms or refutes these results, practitioners should communicate the uncertainty of effect and consider other approaches or combinations of treatment.
Implications for research.
Novel combinations of manual therapy and exercise should be compared with realistic placebo (e.g. use of inactive ultrasound therapy and application of an inert gel) in high quality randomised trials. Further trials of manual therapy alone or exercise alone for rotator cuff disease should be based upon a strong rationale and consideration of whether or not they would alter the conclusions of this review. The interventions should be described in enough detail to inform interpretation of findings and allow replication. Trials should use strategies designed to minimise the potential for bias, including adequate allocation concealment and blinding of participants by delivering a realistic physical therapy placebo. Development of a core set of outcomes for trials of rotator cuff disease and other shoulder disorders would facilitate our ability to synthesise the evidence in future.
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2016 | New search has been performed | The original review, 'Physiotherapy interventions for shoulder pain' (Green 2003) was split into four reviews upon updating: this review, 'Manual therapy and exercise for rotator cuff disease', 'Electrotherapy modalities for rotator cuff disease' (ongoing), 'Manual therapy and exercise for adhesive capsulitis (frozen shoulder)' (Page 2014a), and 'Electrotherapy modalities for adhesive capsulitis (frozen shoulder)' (Page 2014b). The review has also been broadened by including all randomised and quasi‐randomised clinical trials regardless of whether outcome assessment was blinded. |
History
Review first published: Issue 6, 2016
| Date | Event | Description |
|---|---|---|
| 1 May 2008 | Amended | Converted to RM5. CMSG ID C067‐R |
| 24 February 2003 | New citation required and conclusions have changed | Substantive amendment |
| 24 February 2003 | Amended | This review is based on the original review of 'Interventions for shoulder pain'. Please see published notes for further details. |
Notes
The original review, 'Physiotherapy interventions for shoulder pain' (Green 2003) was split into four reviews upon updating: this review, 'Manual therapy and exercise for rotator cuff disease', 'Electrotherapy modalities for rotator cuff disease' (ongoing), 'Manual therapy and exercise for adhesive capsulitis (frozen shoulder)' (Page 2014a), and 'Electrotherapy modalities for adhesive capsulitis (frozen shoulder)' (Page 2014b). The review has also been broadened by including all randomised and quasi‐randomised clinical trials regardless of whether outcome assessment was blinded.
Acknowledgements
We are grateful to Steve McDonald from the Australasian Cochrane Centre for his help with the search strategy. We thank Madeleine Hill for cross‐checking the data extracted against the trial reports. We thank Renea Johnston for assisting with the co‐ordination of data extraction.
Appendices
Appendix 1. Search strategies
Search strategy for CENTRAL:
MeSH descriptor: [Shoulder Pain] explode all trees
MeSH descriptor: [Shoulder Impingement Syndrome] explode all trees
MeSH descriptor: [Rotator Cuff] explode all trees
MeSH descriptor: [Bursitis] explode all trees
((shoulder* in All Text or rotator* in All Text) and (bursitis in All Text or frozen in All Text or impinge* in All Text or tendonitis in All Text or tendonitis in All Text or tendinopathy in All Text or pain* in All Text))
"rotator cuff" in All Text
"adhesive capsulitis" in All Text
#1 or #2 or #3 or #4 or #5 or #6 or #7
MeSH descriptor: [Rehabilitation] explode all trees
MeSH descriptor: [Physical Therapy Modalities] explode all trees
MeSH descriptor: [Exercise Movement Techniques] explode all trees
MeSH descriptor: [Ultrasonography, Interventional] explode all trees
rehabilitat* in All Text or physiotherapy* in All Text or "physical therap*" in All Text or "manual therap*" in All Text or exercis* in All Text
(ultrasound in All Text or ultrasonograph* in All Text or tns in All Text or tens in All Text or shockwave in All Text or electrotherap* in All Text or mobili* in All Text)
#9 or #10 or #11 or #12 or #13 or #14
#8 and #15
Search strategy for MEDLINE (Ovid):
shoulder pain/
shoulder impingement syndrome/
rotator cuff/
exp bursitis/
((shoulder$ or rotator cuff) adj5 (bursitis or frozen or impinge$ or tendinitis or tendonitis or tendinopathy or pain$)).mp.
rotator cuff.mp.
adhesive capsulitis.mp.
or/1‐7
exp rehabilitation/
exp physical therapy techniques/
exp musculoskeletal manipulations/
exp exercise movement techniques/
exp ultrasonography, interventional/
(rehabilitat$ or physiotherap$ or physical therap$ or manual therap$ or exercis$ or ultrasound or ultrasonograph$ or TNS or TENS or shockwave or electrotherap$ or mobili$). mp.
or/9‐14
clinical trial.pt
random$.mp.
((single or double) adj (blind$ or mask$)).mp.
placebo$.mp.
or/16‐19
8 and 15 and 20
Search strategy for EMBASE (Ovid):
‘shoulder pain’/exp
‘shoulder impingement syndrome’/exp
‘rotator cuff’/exp
‘bursitis’/exp
((shoulder* OR rotator*) AND (‘bursitis’/de OR frozen OR impinge* OR ‘tendonitis’/de OR ‘tendinitis’/de OR ‘tendinopathy’/de OR pain*))
‘rotator cuff’
‘adhesive capsulitis’
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
‘rehabilitation’/exp
‘physiotherapy’/exp
‘kinesiotherapy’/exp
‘endoscopic echography’/exp
rehabilitat* OR physiotherapy* OR ‘physical therapy’ OR ‘manual therapy’ OR kinesiotherap* OR exercis*
‘ultrasound’/de OR ultrasonograph* OR ‘transcutaneous nerve stimulation’ OR ‘transcutaneous electrical nerve stimulation’ OR shockwave OR electrotherap* OR mobili*
#9 OR #10 OR #11 OR #12 OR #13 OR #14
‘randomized controlled trial’/exp
#8 AND #15 AND #16
Search strategy for CINAHL Plus (EBSCO):
S1 MH “shoulder pain”
S2 MH “shoulder impingement syndrome”
S3 MH “rotator cuff”
S4 MH bursitis+
S5 TX (shoulder* N5 bursitis) or TX(shoulder* N5 frozen) or TX(shoulder* N5 impinge*) or TX(shoulder* N5 tend?nitis) or TX(shoulder* N5 tendinopathy) or TX(shoulder* N5 pain*)
S6 TX (rotator cuff N5 bursitis) or TX(rotator cuff N5 frozen) or TX(rotator cuff N5 impinge*) or TX(rotator cuff N5 tend?nitis) or TX(rotator cuff N5 tendinopathy) or TX(rotator cuff N5 pain*)
S7 TX rotator cuff
S8 TX adhesive capsulitis
S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8
S10 MH Rehabilitation+
S11 MH physical therapy+
S12 MH Manual Therapy+
S13 MH Therapeutic Exercise+
S14 MH Ultrasonography+
S15 TX rehabilitat* or physiotherapy* or physical therap* or manual therap* or exercise* or ultrasound or ultrasonograph* or TNS or TENS or shockwave or electrotherapy* or mobili*
S16 S10 or S11 or S12 or S13 or S14 or S15
S17 PT clinical trial
S18 TX random*
S19 TX(single blind*) or TX(single mask*)
S20 TX(double blind*) or TX(double mask*)
S21 placebo*
S22 S17 or S18 or S19 or S20 or S21
S23 S9 and S16 and S22
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ainsworth 2009.
| Methods |
Design: Parallel group RCT Setting: One district general hospital and 9 local community hospitals, United Kingdom Intervention: Exercise, therapeutic ultrasound, glucocorticoid injection and advice Control: Therapeutic ultrasound, glucocorticoid injection and advice Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tear Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion criteria (not listed above)
Baseline characteristics Intervention Number randomised: 30; mean (range) age = 78.4 (65‐96) years; male/female = 14/16; duration of symptoms: not reported Control Number randomised: 30; mean (range) age = 78 (68‐88) years; male/female = 15/15; duration of symptoms: not reported |
|
| Interventions |
Intervention: exercise‐based rehabilitation programme Components of intervention: the programme was usually started with the participant lying in a supine position. The participant was taught to start with a flexed elbow and to raise the arm to a vertical position. The participant was then taught to control the arm with sways in a 20 degree arc before elevating and lowering the arm using a weight of approximately 0.75 kg. When the participant could carry out these activities supine, the head of the treatment couch was gradually inclined until they were able to perform the exercises in a sitting position. The participants also carried out stretching exercises to improve ranges of elevation, internal and external rotation, resistance band exercises into internal and external rotation, activities to improve proprioception, posture correction and adaptation of functional activities. Dose: not reported Frequency of administration: 6 treatment sessions (unclear how many sessions per week) Control: therapeutic ultrasound, glucocorticoid injection and advice Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: therapeutic ultrasound, glucocorticoid injection if needed for pain, and advice Dose: not reported Frequency of administration: 6 treatment sessions (unclear how many sessions per week) |
|
| Outcomes | Outcomes assessed at 3, 6 and 12 months
|
|
| Notes | Conflicts of interest: the authors declared that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated a numbered envelope in the sequential order that they were recruited to the trial. Inside each envelope was the allocation which was generated from a random allocation table by an independent statistician". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: See quote above. An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The weakness in this study, as with many physiotherapy trials, was the lack of blinding. Efforts were made to reduce bias in this study but the lack of blinding is acknowledged to be a potential source of bias" Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Comment: Assessors of objective outcomes (ROM) were not blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 60 patients recruited to the trial six were lost to follow up. Two patients withdrew from the trial after recruitment but before treatment had begun. One patient became too ill to proceed with the trial and one patient was widowed and no longer wished to engage with rehabilitation. One patient died of unrelated causes after completing treatment but before the 3 month assessment. Two further patients died, one before the 6 month assessment from kidney failure and the other before the 12 month assessment from bladder cancer. One patient was lost to follow up before the 12 month assessment when he moved out of the area without leaving a forwarding address." Quote: "Although six patients in total were lost to follow up during the course of the trial and all of them belonged to the intervention group, none of them withdrew due to lack of confidence in their treatment. Three patients who had received the intervention treatment died from unrelated factors during the course of the trial. Data for all six patients were included in the analysis of baseline characteristics and up to the point where they dropped out of the trial. Analysis of data was on an intention‐to‐treat basis whereby patients were compared in the groups to which they were originally randomly assigned." Comment: The approach to dealing with missing participant data was likely to have minimised bias in the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were incompletely reported for ROM, but unclear if this was related to the nature of the results. Also, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Al Dajah 2014.
| Methods |
Study design: Parallel group RCT Setting: Physiotherapy outpatient department, Saudi Arabia Intervention: Soft tissue mobilisation and proprioceptive neuromuscular facilitation Control: Therapeutic ultrasound Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Shoulder impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics: not reported |
|
| Interventions |
Intervention: soft tissue mobilisation (STM) and proprioceptive neuromuscular facilitation (PNF) Components of intervention: the subjects were positioned with the humerus abducted to 45 degrees with elbow flexed to 90 degrees, and the humerus was externally rotated to a midrange position, typically about 20 degrees to 25 degrees of check, degrees used elsewhere external rotation. The subscapularis was palpated in the axilla to identify areas of myofascial mobility restrictions, taut bands, or trigger points. Identified restrictions were treated with STM utilising a combination of sustained manual pressure, and slow deep strokes to the subscapularis myofascia for 7 min. The STM was followed by contract‐relax PNF for the subscapularis and other glenohumeral medial rotators, beginning in the same position used for the STM. The participants were instructed to perform maximal glenohumeral internal rotation against an opposing, isometric, manual resistance applied by the treating physical therapist for 7 seconds. Afterwards, the participant actively moved the humerus into full available external rotation. This position was maintained for 15 seconds. This 7‐second internal rotation contraction against resistance followed by full active external rotation was repeated 5 times. Subjects were then instructed to actively move through the PNF flexion‐abduction external‐rotation diagonal pattern for 5 repetitions with manual facilitation Dose: 10 min Frequency of administration: once Control: therapeutic ultrasound Components of intervention: the arm was abducted to 45 degrees and the forearm was rested on the pillow for support. Ultrasound therapy was given to the subscapularis muscle insertion at the shoulder region Dose: frequency ‐ 3 MHz; intensity ‐ 0.5 W/cm2; duration: 10 min Frequency of administration: once |
|
| Outcomes | Outcomes assessed immediately after one treatment session (day 1):
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were assigned randomly into two groups by lot method" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no attrition because all participants were treated and assessed in a single session |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Atkinson 2008.
| Methods |
Study design: Parallel group RCT Setting: University, South Africa Intervention: Manipulation plus mobilisation Control: Placebo laser treatment plus mobilisation Source of Funding: Department of Chiropractic at Durban University of Technology |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinopathy Criteria for defining the shoulder condition being treated Diagnosis of rotator cuff tendinopathy by the researcher and confirmed by a specially trained physician and doctor of chiropractic and 3 of:
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 30; mean (range) age = 41.53 (18‐63) years; male:female = 22:8; duration of symptoms: not reported Control Number randomised: 30; mean (range) age = 42.00 (20‐76) years; male:female = 21:9; duration of symptoms: not reported |
|
| Interventions |
Intervention: manipulation Components of intervention: high velocity, low‐amplitude, gentle‐impulse, shoulder adjustive thrust based on extensive motion palpation (completed at the 1st, 3rd and 6th visits) of the shoulder to detect restriction. The participant was positioned either sitting or lying Dose: not reported Frequency of administration: 6 sessions across a 2‐week period Control: sham laser Components of intervention: motion palpation (equivalent to grades 3 and 4 mobilisation) of the shoulder. Laser unit set to zero. The participant was seated in a comfortable position with the shoulder girdle exposed Dose: 5 min Frequency of administration: 6 sessions across a 2‐week period Both groups Both groups received full‐motion palpation of the shoulder prior to randomisation and before the 1st visit, to assess restriction. This equated to grades 3 and 4 mobilisations of the shoulder |
|
| Outcomes | Outcomes assessed at the 1st, 3rd and 6th visits in the 2‐week period, except for ROM in the Manipulation group, which was measured at each of the 6 visits
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was accomplished by folding 60 sheets of paper, 30 marked Group 1, 30 marked Group 2, and mixing them together thoroughly to assure discontinuity. They were then placed in a box. At each subject randomization time point, the box was held to ensure all folded slips were completely obscured". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was accomplished by folding 60 sheets of paper, 30 marked Group 1, 30 marked Group 2, and mixing them together thoroughly to assure discontinuity. They were then placed in a box. At each subject randomization time point, the box was held to ensure all folded slips were completely obscured". Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants were informed they might be randomized into either group, treatment or placebo". Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "This study would have been improved by a fully powered sample, the addition of a blind assessor." Comment: ROM and algometry were assessed by a non‐blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Five patients dropped out of Group 2 (placebo) and were replaced. Therefore out of a total of 35 [control] patients, 30 patients completed treatment. No patients dropped out of Group 1 and no patients complained or dropped out of the trial because of significant side effects such as persistent severe stiffness and/or pain" Comment: It is not clear when in the process and why these participants dropped out |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Bae 2011.
| Methods |
Study design: Parallel group RCT Setting: University, South Korea Intervention: Motor control exercises and strengthening exercises plus hot packs plus TENS plus ultrasound Control: Hot packs plus TENS plus ultrasound Source of Funding: Supported by Sahmyook University |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated At least one of:
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 17; mean age: 49.9 ± 5.5 years old; sex: F/M 11/6; duration of symptoms: not reported Control Number randomised: 18; mean age: 48.3 ± 4.3 years old; sex: F/M 12/6; duration of symptoms: not reported |
|
| Interventions |
Intervention: motor control and strengthening exercises Components of intervention
Dose: 30 min of exercise
Frequency of administration: 3 times per week for 4 weeks Control: hot packs plus TENS plus ultrasound Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: conservative physical therapy including applied hot packs, TENS and ultrasound. No other detailed provided Dose: 45 min per day (20 min of hot packs), (20 min of TENS), (5 min of therapeutic ultrasound) Frequency of administration: 3 times per week for 4 weeks |
|
| Outcomes | Outcomes assessed at 4 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The thirty‐five participants were randomly assigned to two groups" Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different multimodal interventions, but it is unclear whether they were provided any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain and function, but it is unclear whether they were provided any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of strength and ROM were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No attrition was reported and outcome data were reported as based on the total number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Bang 2000.
| Methods |
Study design: Parallel group RCT Setting: Physical therapy clinic, US Intervention: Manual physical therapy plus supervised flexibility and strengthening exercises Control: Supervised flexibility and strengthening exercises Source of Funding: Kaiser Foundation Research Institute, Northern California |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 28; mean age: 42 ± 10.1 years old; sex: F/M 10/18; duration of symptoms: 5.6 ± 3.7 months Control Number randomised: 24; mean age: 45 ± 8.4 years old; sex: F/M 12/12; duration of symptoms: 4.4 ± 2.8 months |
|
| Interventions |
Intervention: manual physical therapy Components of intervention: techniques applied to movement limitations in the upper quarter that were relevant to the participant's problem. Mostly passive accessory or passive physiological joint mobilisation Maitland grades I‐V were used. Typical initial treatment involved manual therapy techniques to enhance glenohumeral caudal glide in positions of flexion or abduction, and increase physiological flexion or internal rotation, but were adapted and progressed based on evaluation. Typical subsequent treatment involved manual therapy techniques to improve the combined physiological movements of hand behind back or shoulder quadrant, increase upper thoracic extension and side bend, or enhance extension, rotation, or side bend of the cervical spine. Techniques also included soft tissue massage and muscles stretching, and 1‐2 extra home exercises designed to specifically support their mobilisation therapy (e.g. simple cervical and thoracic postural exercises such as chin tucks, and self‐mobilisation such as caudal glides of the glenohumeral joint) Dose: 30 min in total Frequency of administration: twice weekly for 3 weeks Control: supervised flexibility and strengthening exercises Components of intervention: participants only received the treatment common to both groups (see below) Both Groups Components of intervention: standardised flexibility and strengthening exercise programme consisting of 2 passive stretching exercises (1 for the anterior musculature and 1 for the posterior capsule and musculature) and 6 strengthening exercises (flexion, scaption, rowing, horizontal extension‐external rotation, seated press‐up and elbow push‐ups). Four of the 6 strengthening exercises were performed with Theratubing. Each stretch was held for 30 seconds, performed 3 times and with a 10‐second rest between each exercise. Each Theratubing strengthening exercise was performed to a maximum of 10 repetitions for 3 sets with 60 seconds' rest between each exercise. The two other strengthening exercises were performed till fatigue or 25 reps Dose: 30 min in total Frequency of administration: manual therapy group = daily stretching exercises and 3‐times weekly strengthening exercises; control group = twice weekly sessions for 3 weeks in‐clinic and on other days, daily stretching exercises and 3‐times weekly strengthening exercises at home |
|
| Outcomes | Outcomes assessed at 8 weeks (pain and function); isometric strength measured sometime between 4‐8 weeks (not specified)
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On day 1, subjects signed the informed consent and were appointed to either the exercise group or the manual therapy group using the table of random numbers." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The testers were responsible for measurements of all dependent variables and were blinded to group assignment for each subject." Comment: Outcome assessors of objective outcomes were likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two subjects did not complete the study. One subject from the manual therapy group was excluded from the study after the second visit due to injuries sustained in a motor vehicle accident. The other subject, from the exercise group, elected to drop from the study after day 1 citing job related issues. All home exercise program compliance logs were returned and indicated that patients from both groups were fully compliant" Comment: The amount and reasons for attrition are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Bansal 2011.
| Methods |
Design: Parallel group RCT Setting: University, India Intervention: Deep friction massage plus Codman's exercises Control: Therapeutic ultrasound plus Codman's exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus tendinitis Criteria for defining the shoulder condition being treated Supraspinatus tendinitis defined by:
Inclusion Criteria (not listed above)
Exclusion criteria (not listed above)
Baseline characteristics Intervention Number randomised: 20; mean (SD) age = 30.90 (5.33) years; male:female = 12:8; duration of symptoms: not reported Control Number randomised: 20; mean (SD) age = 30.35 (5.76) years; male:female = 9:11; duration of symptoms: not reported |
|
| Interventions |
Intervention: deep friction massage Components of intervention: deep friction massage to supraspinatus tendon in a transverse direction with the tip of the index finger, reinforced by middle finger. Participants were positioned half‐lying with hand behind back (shoulder adduction and internal rotation) Dose: 10‐12 min for 10 sessions over 10 days Frequency of administration: not explicitly reported, assumed daily for 10 days Control: therapeutic ultrasound Components of intervention: ultrasound applied to the supraspinatus tendon with the participants positioned with hand behind back Dose: intensity 0.6 w/cm2, frequency 1 MHz, pulse rate 4:1 for 6‐8 min for 10 sessions over 10 days Frequency of administration: not explicitly reported, assumed daily for 10 days Both groups All participants were instructed in Codman's exercises consisting of pendulum or swinging motion of the arm in flexion, extension, horizontal abduction, adduction and circumduction. Dosage was not reported. Intensity (arc of motion) was increased as tolerated. Participants were also advised to avoid strenuous work involving the affected upper limb |
|
| Outcomes | Outcomes assessed at 5 days and 10 days
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The individuals were randomly divided into two groups" Comments: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported regarding the assessors of the objective outcome, active range of shoulder abduction |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, however it is unclear whether the outcome data reported were based on the total number of randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Only mean scores (no measures of variation) were reported for all outcomes. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Barbosa 2008.
| Methods |
Design: Parallel group RCT Setting: Hospital (orthopaedics clinic), Brazil Intervention: Mobilisation plus eccentric muscle training plus therapeutic ultrasound Control: Eccentric muscle training plus therapeutic ultrasound Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus tendinopathy Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics: Intervention Number randomised: 7; mean age: 43.57 ± 7.49; sex: F/M 4/3; duration of symptoms: not reported Control Number randomised: 7; mean age: 48.71 ± 7.27; sex: F/M 5/2; duration of symptoms: not reported |
|
| Interventions |
Intervention: joint mobilisation Components of intervention: front, back, lower longitudinal and lateral relaxations of the glenohumeral joint, anteroposterior movements of the acromioclavicular (squeeze) joint and anteroposterior, inferior‐superior and superior‐ inferior movements of the sternoclavicular joint. Dose: 1 min of mobilisation for each movement (2‐3 cycles per second), and 1 min of active free abduction movement in the scapular plane, over the arc of movement without pain Frequency of administration: 3 sessions per week for 4 weeks Control: eccentric muscle training plus therapeutic ultrasound Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention
Dose
Frequency of administration: 3 sessions per week for 4 weeks |
|
| Outcomes | Outcomes assessed at 4 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly selected to participate in one of the treatment protocols (A or B)." Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, however it is unclear whether the outcome data reported were based on the total number of randomly assigned participants |
| Selective reporting (reporting bias) | High risk | Comment: No outcome data for pain on motion or ROM were reported (despite being listed as outcomes in the methods section of the review). Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Barra 2011.
| Methods |
Study design: Parallel group RCT Setting: Primary health care centre in the Spanish National Health Service, Spain Intervention: Diacutaneous fibrolysis (type of manual therapy) Control: Placebo diacutaneous fibrolysis Source of Funding: Jordi Gol Institute of Research in Primary Health Care funded translation of the manuscript into English. Funding of the trial not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 25; mean age mean (SD): 56.8 ± 10.3 years old; sex: F/M 16/9; duration of symptoms mean (SD): 14.5 ± 16.2 months Control Number randomised: 25; mean age mean (SD): 60.8 ± 10.1 years old; sex: F/M 13/12; duration of symptoms mean (SD): 17.9 ± 27.6 months |
|
| Interventions |
Intervention: diacutaneous fibrolysis Components of intervention: applied by means of a set of metallic hooks ending in a spatula with bevelled edges that allow a better distribution of the pressure on the skin. The aim is to release adherences between the different musculoskeletal structures, such as muscles, aponeurosis, tendons and others. Three consecutive steps are carried out. In the first one, or manual palpation step, the hand that is not holding the hook, the so‐called palpatory hand, localises the intermuscular septum and remains in permanent control of the implementation of the technique. In the second step, or instrumental palpation step, the hook is placed deeper in the intermuscular septum close to the index finger of the palpatory hand and both together (finger and hook) slide along the intermuscular septum, making short and brief movements perpendicular to the muscular fibres, allowing the detection of areas of movement restriction where the presence of adherences is presumed. The third step, the fibrolysis step, wherever a sensation of movement restriction is felt, a brief supplementary traction is carried out with the hook in order to tear the hypothetical adherent connective fibres. For this study, regardless of pain location, all the participants were treated with the same standardised protocol which involves the musculature of the scapula, the lateral region of the shoulder and arm, and the front part of the shoulder and chest. In the case of bilateral pain, this treatment was applied only to the most painful shoulder Dose: 15 min Frequency of administration: 1 session Control: placebo diacutaneous fibrolysis Components of intervention: the steps of manual and instrumental palpation occur as for the intervention group, but strictly at a superficial level. In the third step, instead of fibrolysis, a pinch of skin is held with the thumb of the palpatory hand and the tip of the spatula, so that the participant feels the hook distinctly but without any action taking place on the deep tissue levels Dose: 15 min Frequency of administration: 1 session |
|
| Outcomes | Outcomes assessed immediately post‐treatment (1 day)
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients who consented to participate in this study were randomly allocated to one of two groups: the intervention group or the placebo group. The randomization was stratified by centre. The DatInf RandList 1.2 software (DatInf GmbH, Tu ¨ bingen, Germany) was used." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A physiotherapist was in charge of recruiting the participants, collecting the demographic data, measuring the initial variables and assigning a correlative number to each participant. Subsequently, the second physiotherapist, who was the only person with access to the random allocation list generated by the randomization software, implemented the technique according to the group assigned to the participant’s number." Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: When asked about their group allocation, 42/50 participants did not know and 5/8 of those who chose a group were correct. Four of these participants were in the intervention group. The physiotherapist applying the intervention was not blinded, although this is unlikely to have affected outcomes given that no adjuvant treatment was provided |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain and treatment success |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "After the participant had received the treatment, the first physiotherapist, still blinded to the technique applied, took the final measurements of the variables" Comment: Assessor of objective outcomes was likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no loss to follow‐up of participants |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data fully reported for all outcomes specified in the ClinicalTrials.gov registry entry |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Barra Lopez 2013.
| Methods |
Study design: Parallel group RCT Setting: Primary healthcare centres, Spain Intervention: Diacutaneous fibrolysis plus standardised physiotherapy (exercise, electrotherapy and cryotherapy) Control 1: Sham diacutaneous fibrolysis plus standardised physiotherapy Control 2: Standardised physiotherapy alone Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 40; mean (SD) age = 56.2 (12) years; male:female = 15:25; mean (SD) duration of symptoms = 17.4 (24.9) months Control 1 Number randomised: 40; mean (SD) age = 60 (10) years; male:female = 13:27; mean (SD) duration of symptoms = 24.2 (59.1) months Control 2 Number randomised: 40; mean (SD) age = 59.1 (11.5); male:female = 17:23; mean (SD) duration of symptoms = 14.7 (21.6) |
|
| Interventions |
Intervention: diacutaneous fibrolysis Components of intervention: application of a metallic hook as deeply as possible following the intermuscular septum between the muscles of the cervico‐scapular (trapezius, rhomboideus major, rhomboideus minor and levator scapulae) and shoulder region (infraspinatus, teres minor, teres major, triceps brachii long head, deltoid, pectoralis major and biceps brachii long head tendon) in a centripetal direction towards the pain location Dose: not reported Frequency: 2 sessions per week for 3 weeks Control 1: sham diacutaneous fibrolysis Components of intervention: application of a metallic hook over the same muscles and in the same direction as the intervention group, but only at a superficial level and without any mechanical action taking place on the deep tissue layers. For the participant to feel the hook distinctly, a pinch of skin was held with the thumb of the palpatory hand and the tip of the hook Dose: not reported Frequency: 2 sessions per week for 3 weeks Control 2: standardised physiotherapy alone Components of intervention: participants only received the treatment common to all groups (see below) All groups Components of intervention: all participants received a protocolised treatment of therapeutic exercises, analgesic electrotherapy and cryotherapy. No other details provided Dose: not reported Frequency: 5 sessions per week for 3 weeks |
|
| Outcomes | Outcomes assessed at post‐treatment (3 weeks) and 3 months
|
|
| Notes |
Conflicts of interest: not reported Trial registered in ClinicalTrials.gov (NCT01424579) Those participants who were suffering from bilateral subacromial impingement syndrome were provided with the protocolised treatment in both shoulders but, for the purpose of the study, only the most symptomatic shoulder was evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence was determined before the study using a computer generated randomisation list. It was stratified for each centre and treating physiotherapist (two in Cornellà, one in Ponteareas). As a result, the treating physiotherapist in Ponteareas has treated 10 patients per group and the two treating physiotherapists in Cornellà have each treated 15 patients per group." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "As Diacutaneous Fibrolysis is a manual technique, the therapist could not be blinded." Quote: "When asked about the technique they thought they had received, 41% of the participants in the intervention group (sham n = 1, do not know n = 14) and 89% of the participants in the placebo group (real n = 21, do not know n = 12) did not correctly determine the right response. Only four people (10%) in the placebo group correctly determined that they had received sham Diacutaneous Fibrolysis treatment. As expected, all the participants in the control group answered “no additional technique” Comment: Participants in the diacutaneous fibrolysis (DF) and sham DF groups were blinded to whether they received DF or sham DF, but knew they did not receive the control condition. All participants in the control group were not blinded. Personnel delivering treatment were not blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations regarding the benefits of the intervention they received, self‐rated pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Assessment was carried out pre‐treatment, post‐treatment, and at a three‐month follow‐up by a different physiotherapist to the treating physiotherapist, and who was blinded to the group assignment." Comment: Assessors of ROM were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analyses followed intention‐to‐treat principles. Missing observations due to lost to follow‐up were completed with the last value observed from each subject" Quote: "Twenty‐four participants were lost to follow‐up. Seven participants (6%) dropped out during the treatment phase due to personal reasons unrelated to the trial, and seventeen participants (14%) did not take the follow‐up evaluation. It was not possible to contact thirteen of the participants for follow‐up and four people were living in another city at that point in time." Comment: The number of participants lost to follow‐up was similar across groups. The reasons for drop out for majority of those lost to follow‐up is unclear (they were unable to be contacted). Where reasons for drop‐out were reported, it is unclear whether these were evenly distributed across groups. Analysis by ITT and missing data are unlikely to affect continuous outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: One outcome (participants' perception of results) is reported in the publication but was not listed on the registered trial information on the U.S. National Institutes of Health ClinicalTrials.gov website. However, it is unclear whether this outcome was introduced based on its results and the results of other outcomes |
| Other bias | Low risk | Comment: No other sources of bias identified |
Baskurt 2011.
| Methods |
Study design: Parallel group RCT Setting: Orthopaedic physiotherapy unit, Turkey Intervention: Scapular stabilisation exercises (PNF) plus stretching and strengthening exercises Control: Stretching and strengthening exercises Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 20; mean (SD) age: 51.50 (8.40) years old; male/female: not reported; mean (SD) duration of symptoms: 8.55 (10.78) months Control Number randomised: 20; mean (SD) age: 51.25 (11.55) years old; male/female: not reported; mean (SD) duration of symptoms: 11.60 (9.52) months |
|
| Interventions |
Intervention: scapular stabilisation exercises Components of intervention: scapular proprioceptive neuromuscular facilitation (PNF) exercises, scapular clock exercise, standing weight shift, double arm balancing, scapular depression, wall push up, wall slide exercises. Sessions were performed under physiotherapist supervision. When participants were able to do 3 sets of 10 repetitions without feeling substantial pain or fatigue, then the strongest elastic band was used Dose: 3 sets of each exercise Frequency of administration: 3 times per week for 6 weeks Control: stretching and strengthening exercises Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: flexibility exercises consisted of anterior, posterior and inferior capsule stretching, forward flexion ROM, abduction ROM and internal rotation stretching (with towel). Strengthening exercises consisted of subscapularis, infraspinatus, supraspinatus, and the anterior and posterior part of deltoid strengthening. Sessions were performed under physiotherapist supervision. When participants were able to do 3 sets of 10 repetitions without feeling substantial pain or fatigue, then the strongest elastic band was used. At the beginning of the study participants were educated on how to best use their injured shoulders (avoiding above the head work etc.) Dose: 3 sets of each exercise Frequency of administration: 3 times per week for 6 weeks |
|
| Outcomes | Outcomes assessed at 6 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients meeting the criteria were separated into 2 groups according to simple random table." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different multimodal interventions, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All randomised participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: Without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Beaudreuil 2011.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic, France Intervention: Dynamic Humeral Centering plus massage and home exercise Control: Non‐specific mobilisation plus massage and exercise Source of Funding: Assistance Publique–Hôpitaux de Paris and the French Society of Rheumatology |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 35; mean age: 57.9 ± 10.7 years old; sex: F/M 21/14; mean duration of symptoms: 35.7 ± 81.6 months Control Number randomised: 35; mean age: 59.4 ± 10.0 years old; sex: F/M 26/8; mean duration of symptoms: 20.9 ± 27.6 months |
|
| Interventions |
Intervention: dynamic humeral centring Components of intervention: the programme consisted of two parts:
Home exercises consisted of 10 co‐contractions of the pectoralis major and latissimus dorsi three times a day, the shoulder actively positioned in abduction and the elbow in the flexed or extended position according to the stage of progression Dose: see above Frequency of administration: dynamic humeral centring ‐ 15 sessions over 6 weeks (3 times a week for the first 3 weeks, and twice a week for the next 3 weeks); home exercises ‐ daily for 6 weeks Control: non‐specific mobilisation Components of intervention: the programme consisted of three stages
Dose: see above Frequency of administration: manual therapy ‐ 15 sessions over six weeks (3 times a week for the first 3 weeks, and twice a week for the next 3 weeks); home exercises ‐ daily for 6 weeks Both groups Components of intervention: each session began with massage of the neck and shoulder region with the participant lying on one side or sitting. Physiotherapists were allowed to adjust the intensity of the treatment according to the participant's capabilities. Participants also performed exercises at home depending on the intervention group Dose: 10 min for massage Frequency of administration: 15 sessions over 6 weeks (3 times a week for the first 3 weeks, and twice a week for the next 3 weeks) |
|
| Outcomes | Outcomes assessed at 3 months and 12 months
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned in permuted blocks of six". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocations were sealed in opaque and consecutively numbered envelopes. Envelopes were opened by an independent investigator who was not involved in the eligibility assessment, outcome assessment or treatment. Allocation was revealed to the physiotherapist before the patients presented for treatment." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were informed that two treatment procedures were being evaluated, with no further information on the superiority of one treatment over the other. Patients were therefore blinded to the study hypothesis" Comment: Participants, but not personnel, were blind to treatment (though the latter is unlikely to have affected outcomes given that no adjuvant treatment was provided) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "An assessor blinded to treatment assessed all outcomes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 34 patients underwent DHC and 35 control treatment. At 3 months, 90% of included patients were available for assessment, and at 12 months 70%." Comment: The rate of dropout was relatively similar between groups, though no reasons for dropout were provided. However, analysis was by intention‐to‐treat, where missing data were imputed using the multiple imputation method |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the ClinicalTrials.gov registry entry (NCT 01022775) |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Bennell 2010.
| Methods |
Study design: Parallel group RCT Setting: Public hospital physiotherapy units and private physiotherapy practices, Australia Intervention: Physiotherapy (soft tissue massage, joint mobilisation, scapular retraining, postural taping, supervised and home exercises) Control: Placebo physiotherapy (sham ultrasound only) Source of Funding: National Health and Medical Research Council |
|
| Participants |
Diagnostic label used by trialists: Chronic rotator cuff disease (encompassing several conditions) Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 59; mean age: 59.3 ± 10.1 years old; sex: F/M 25/34; mean duration of symptoms: 24 months Control Number randomised: 61; mean age: 60.8 ± 12.4 years old; sex: F/M 31/30; mean duration of symptoms: 14 months |
|
| Interventions |
Intervention: physiotherapy Components of intervention: directed at improving dynamic scapular control, strengthening scapular stabiliser and rotator cuff muscles, improving shoulder and thoracic posture, and increasing ROM of thoracic extension. The treatment was administered in a standardised way by a trained physiotherapist. Analgesia as required was permitted, and behaviour modification strategies such as education, goal setting, motivation and positive reinforcement were provided
Dose: total 30 ‐ 45 min each session
Frequency of administration: in‐clinic sessions were performed twice weekly for the first 2 weeks, once weekly for the next 4 weeks and then once fortnightly for the last 4 weeks, totaling 10 sessions over 10 weeks. After the 10‐week programme, participants were instructed to maintain their daily home exercise programme for 12 weeks Control: sham ultrasound Components of intervention: sham ultrasound and light application of a non‐therapeutic gel to the shoulder region Dose: 10 min for the ultrasound and 10 for the gel Frequency of administration: twice weekly for the first 2 weeks, once weekly for the next 4 weeks and then once fortnightly for the last 4 weeks, totaling 10 sessions over 10 weeks. After the 10‐week programme, participants did not receive any intervention and were not instructed to do any home exercises Co‐interventions Use of analgesics and non‐steroidal anti‐inflammatory drugs was similar in the active and placebo groups over both the intervention period (analgesics: 11/55 (20%) active v 14/61 (23%) placebo; non‐steroidal anti‐inflammatories: 12/55 (22%) v 13/61 (21%)) and the follow‐up period (analgesics: 8/49 (16%) v 8/55 (15%); non‐steroidal anti‐inflammatories 6/49 (12%) v 8/55 (15%)) |
|
| Outcomes | Outcomes assessed at 11 weeks and 22 weeks post‐randomisation
|
|
| Notes | Conflicts of interest: authors declared that they have no financial or non‐financial interests that may be relevant to the submitted work | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants had a baseline assessment and were randomised in permuted blocks of six and eight, stratified by treating physiotherapist, to receive either active manual therapy and home exercise treatment or placebo treatment according to a computer generated table of random numbers created by the study biostatistician". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocations were sealed in opaque and consecutively numbered envelopes kept in a central locked location. An independent administrator opened the envelopes in sequence and then revealed the group allocation to the relevant physiotherapist by facsimile just before the participant presented for treatment." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: While the personnel were not blinded to treatment allocation, when asked which treatment they were receiving participants’ answers showed a moderate to high degree of blinding with a blinding index of 0.7 (1 indicates complete blinding, 0 indicates no blinding, and 0.5 would be expected if participants were randomly guessing) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The same blinded assessor (EW) evaluated all participants at baseline, at 11 weeks (at the conclusion of the supervised active or placebo intervention), and at 22 weeks after randomisation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Attrition rates were low and similar across both groups, and data were analysed according to an intention‐to‐treat analysis, where missing data were replaced by the last score carried forward |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the study protocol |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Bialoszewski 2011.
| Methods |
Study design: Parallel group RCT Setting: University, Poland Intervention: Manual therapy plus TENS plus ultrasound plus exercise Control: TENS plus ultrasound plus exercise Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Chronic rotator cuff injury Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 15; mean (range) age: 52.6 (38–61) years old; sex: F/M 5/10; mean duration of symptoms: 4.8 months Control Number randomised: 15; mean (range) age: 50 (38–60) years old; sex: F/M 7/8; mean duration of symptoms: 4.4 months |
|
| Interventions |
Intervention: manual Therapy Components of intervention: mobilisation of the glenohumeral joint and soft tissues using Kaltenborn’s roll‐glide techniques, Cyriax deep friction massage, Mulligan’s mobilisation with movement and typical techniques of joint mobilisation in the anteroposterior direction Dose: not reported Frequency of administration: not reported Control: TENS plus ultrasound plus exercise Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention Standard rehabilitation involving TENS, therapeutic ultrasound and kinesiotherapy
Dose
Frequency of administration: not reported |
|
| Outcomes | Outcomes assessed at 4 time points, but it is not clear when these occurred (in terms of weeks) nor how long the intervention lasted
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to two matched groups" Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information on whether assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Blume 2014.
| Methods |
Study design: Parallel group RCT Setting: Outpatient rehabilitation department, USA Intervention 1: Supervised eccentric progressive resistance exercises plus ice plus home exercises Intervention 2: Supervised concentric progressive resistance exercises plus ice plus home exercises Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1 Number randomised: 17 (17 completed); mean age: 48.8 ± 16.5 years old; sex: F/M 9/8; duration of symptoms: 28.2 ± 23.6 months Intervention 2 Number randomised: 17 (13 completed); mean age: 47.2 ± 14.7 years old; sex: F/M 7/6; duration of symptoms: 23 ± 27.8 months |
|
| Interventions |
Intervention 1: eccentric progressive resistance exercises Components of intervention: supervised eccentric progressive resistance exercises for the rotator cuff and scapular muscles. Exercises included the seated 'full can', sidelying internal rotation (IR), sidelying external rotation (ER) with towel roll, supine protraction, sidelying horizontal abduction, sidelying abduction, and prone shoulder extension. All exercises were performed using a dumbbell for resistance and all were performed in the participant’s pain‐free AROM. The eccentric exercise group performed the lowering portion of the exercises with the therapist repositioning the weight to the starting position to avoid resistance in the lifting portion of each exercise Dose: 3 sets of 12 repetitions of each exercise; duration of session was 1 hour Frequency of administration: twice a week for 8 weeks Intervention 2: concentric progressive resistance exercises Components of intervention: supervised concentric progressive resistance exercises for the rotator cuff and scapular muscles. Same exercises as above, except this group performed the lifting portion of the exercises with the therapist repositioning the weight to the start position to avoid resistance in the lowering portion of the exercise Dose: 3 sets of 12 repetitions of each exercise; duration of session was 1 hour Frequency of administration: twice a week for 8 weeks Both groups ‐ Ice and home exercises Components of intervention: participants received ice treatment on the shoulder for 15 min at the end of each supervised clinic session. A home exercise programme (HEP) of stretching and postural correction exercises (pectoralis minor and posterior shoulder self‐stretching and thoracic spine self‐mobilisation into extension along with pain‐free AROM in flexion and abduction standing in front of a mirror to monitor for excessive scapular elevation) Dose: 3 sets of 12 repetitions of each exercise Frequency of administration: once daily on the days the participant was not exercising in the clinic, for 8 weeks |
|
| Outcomes | Outcomes assessed at 5 weeks and 8 weeks
|
|
| Notes |
Conflicts of interest: not reported Report only available as a PhD thesis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Low risk | Quote: "The participants were randomly assigned to one of the two intervention groups using pre‐prepared, sealed folders selected in numeric order which became their participant identification number" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Participants were likely blinded, as they were unlikely to have noticed the difference between the two exercise programmes |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Outcomes were assessed by an investigator blinded to the treatment intervention group assignment" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Following group allocation, there were 17 participants in each group. One enrolled participant in the concentric group dropped out at three weeks due to an unrelated medical issue. Sixteen participants in the concentric group and seventeen participants in the eccentric group completed the fifth week assessments. Three participants in the concentric group withdrew after five weeks, one due to travelling out of state and two for financial and work conflict reasons. As a result, a total of thirty participants completed the 8‐week study and the final assessments, 13 in the concentric group and 17 in the eccentric group". Comment: The attrition was unrelated to the interventions, and the amount was small, so is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the thesis, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Brox 1993.
| Methods |
Study design: Parallel group RCT Setting: Hospital orthopaedics department and hospital physical medicine and rehabilitation department, Norway Intervention: Supervised exercise Control 1: Arthroscopic subacromial decompression Control 2: Placebo laser treatment Source of Funding: Norwegian Research Council |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 50; mean age: 47 years old; sex: F/M 28/22; duration of symptoms: < 6 months (n = 6); 6 months to 1 year (n = 6); 1–3 years (n = 13); > 3 years (n = 25) Control 1 Number randomised: 45; mean age: 48 years old; sex: F/M 16/32; duration of symptoms: < 6 months (n = 8); 6 months to 1 year (n = 8); 1–3 years (n = 9); > 3 years (n = 20) Control 2 Number randomised: 30; mean age: 48 years old; sex: F/M 15/15; duration of symptoms: < 6 months (n = 5); 6 months to 1 year (n = 5); 1–3 years (n = 5); > 3 years (n = 14) |
|
| Interventions |
Intervention: supervised exercise Components of intervention: supervised exercises to normalise dysfunctional neuromuscular patterns and to "increase the nutrition of the collagen in the rotator cuff". To eliminate gravitational forces and to start the exercises the arm was suspended in a sling fixed to the roof. Relaxed repetitive movements (first rotation, then flexion‐extension, and finally abduction‐adduction) were performed for about an hour in a daily training session. Resistance was gradually added to strengthen the short shoulder rotator and scapular stabilising muscles. Participants also received 3 lessons on the anatomy and function of the shoulder, pain management and ergonometrics Dose: 1 hour Frequency of administration: participants were supervised twice weekly for 6 weeks. On the other days they followed the same exercise programme at home. The training continued for 3 to 6 months, with the supervision gradually being reduced Control 1: arthroscopic subacromial decompression Components of intervention: the aim of the procedure was to make more space for the rotator cuff to reduce the risk of impingement. Standard treatment consisted of bursectomy and resection of the anterior and the lateral part of the acromion and the coracoacromial ligament. Participants received post‐operative rehabilitation on day one and physiotherapy within the first week. Exercises prescribed by the surgeon were performed against low resistance and repeated many times. Unrestricted activities were advised after 4 to 6 weeks Dose: NA Frequency of administration: 1 surgical procedure Control 2: placebo laser Components of intervention: a detuned soft laser delivered by the hospital physiotherapist Dose: not reported Frequency of administration: twice weekly for 6 weeks |
|
| Outcomes | Outcomes assessed at 3 months and 6 months
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatments were allocated by the method of random permuted blocks" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention. The comparison of supervised exercise versus arthroscopic subacromial decompression is of concern, while the comparison of supervised exercise versus placebo is not |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Blind follow up measurements were carried out at three and six months after the first day of treatment. At follow up tests the patients wore a T‐shirt to hide a possible scar from surgery. They were carefully told not to talk about their treatment." Comment: Outcome assessors of objective outcomes were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Unexpectedly, many patients dropped out from surgery. Four of them did not attend follow up examination at six months, which might have biased our results". The comparison of supervised exercise versus arthroscopic subacromial decompression is of concern; the comparison of supervised exercise versus placebo is not |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Celik 2009.
| Methods |
Study design: Parallel group RCT Setting: Marmara University Faculty of Medicine, Physical Medicine and Rehabilitation, Turkey Intervention: Exercise below 90 degrees plus TENS plus pulsed therapeutic ultrasound plus cold pack plus NSAID Control: Exercise above 90 degrees plus TENS plus pulsed therapeutic ultrasound plus cold pack plus NSAID Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 33; mean (range) age: 52 (34–70) years old; sex: F/M 23/7; duration of symptoms: not reported |
|
| Interventions |
Intervention: exercise below 90 degrees Components of intervention: supervised shoulder flexion below 90 degrees, abduction, T‐bar (wand) exercises containing internal‐external rotation and extension, posterior capsule stretching and internal rotation exercises and rotator cuff strengthening exercises Dose: 30 repetitions in the hospital and 30 repetitions twice more at home Frequency of administration: daily for 2 weeks Control: exercise above 90 degrees Components of intervention: supervised exercises over 90 degrees, posterior and inferior capsule stretching exercises, rotator cuff strengthening and internal rotation exercises Dose: 30 repetitions in the hospital and 30 repetitions twice more at home Frequency of administration: daily for 2 weeks Both groups Components of intervention: TENS, pulsed therapeutic ultrasound, ice application and oral tenoxicam Dose:
Frequency of administration: daily for 2 weeks |
|
| Outcomes | Outcomes assessed at 2 weeks and 16 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who agreed to participate were divided into two groups randomly" Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received slightly different types of exercises, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported some outcomes but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Comment: The only objective outcome (physical therapist satisfaction) was measured by an unblinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "First group had 17 patients, while the second group consisted of 16 patients. In the first group, one patient had difficulty in transportation to the hospital, and another patient had indication of surgery while attending exercise program. For these reasons, two patients were left out of the study. In the second group, one patient left the exercise program due to pain. Patients in 2 groups of 15 people (23 females, 7 males; Age 52; range 34‐70) completed the treatment." Comment: The amount of attrition was low and unlikely to have impacted on the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other source of bias was identified |
Citaker 2005.
| Methods |
Study design: Parallel group RCT Setting: University rehabilitation department, Turkey Intervention: Manual mobilisation plus hot pack plus theraband exercises plus home exercises (Codman) Control: Proprioceptive neuromuscular facilitation (PNF) plus hot pack plus theraband exercises plus home exercises (Codman) Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 20; mean (SD) age: 52.80 ± 9.86 years; sex: not reported; duration of symptoms: not reported Control Number randomised: 20; mean (SD) age: 55.50 ± 8.95 years; sex: not reported; duration of symptoms: not reported |
|
| Interventions |
Intervention: manual mobilisation Components of intervention: no details provided Dose: not reported Frequency of administration: 20 sessions (number of sessions per week and total number of sessions not reported) Control: proprioceptive neuromuscular facilitation Components of intervention: no details provided Dose: not reported Frequency of administration: 20 sessions (number of sessions per week and total number of sessions not reported) Both groups Components of intervention: hot pack plus theraband exercises plus home exercises (Codman). The hot packs which were used for the treatment were Nonius™, with dimensions 30–50 cm. They were applied for 20 min. The elastic therabands used for the treatments (Theraband™) were 7.6 cm wide and 152 cm long. They were fixed to an object such as a doorknob. Six colour‐coded bands were available; each provided increasing resistance from 0.5 to 2.7 kg with increments of 0.5 kg. Theraband exercises permit concentric and eccentric strengthening of the shoulder muscles. The exercises begun with the elbow flexed 90 degrees and the shoulder in the neutral position. The exercises were performed through an arc of 45 degrees in each of the 5 planes of motion. In addition, Codman pendulum exercises were utilised as a home programme in both groups Dose: hot pack applied for 20 min Frequency of administration: 20 clinic sessions (number of sessions per week and total number of sessions not reported) followed by 3 weeks of theraband exercises |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were divided into two equal groups randomly." Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received slightly different multi‐modal interventions, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported some outcomes, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of ROM were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No drop‐outs, losses to follow‐up or exclusions were reported, but it is unclear whether outcome data were based on an analysis of all 40 randomised participants, and it is unclear how many participants were randomised to each group |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data fully reported for all outcomes specified in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other source of bias identified |
Clews 1987.
| Methods |
Study design: Parallel group RCT Setting: Australian Institute of Sport, Australia Intervention: Massage plus ice Control 1: Therapeutic ultrasound plus ice Control 2: Placebo ultrasound plus ice Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 18 (6 per group); age (mean and SD, or range): not reported; sex: not reported; duration of symptoms: not reported |
|
| Interventions |
Intervention: massage Components of intervention: massage of several muscles delivered as follows
Dose: 15 min Frequency of administration: every day for 3 days Control 1: therapeutic ultrasound Components of intervention: pulsed ultrasound Dose: 15 min at an intensity 0.8 w/cm2 Frequency of administration: every day for 3 days Control 2: sham ultrasound Components of intervention: sham ultrasound Dose: 15 min Frequency of administration: every day for 3 days All groups Components of intervention: ice packs applied to the affected shoulder, and NSAIDs Dose: ice for 15 min twice daily and 1 tablet of Voltaren taken with meals Frequency of administration: every day for 3 days |
|
| Outcomes | Outcomes assessed at 3 days
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the diagnosis had been made and inclusion in the study was confirmed, each subject was randomly assigned to one of these three groups." Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "One co‐author did all the testing and was not aware of the subjects' group assignment" Comment: Outcome assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was complete follow‐up of all randomised participants in the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Cloke 2008.
| Methods |
Study design: Parallel group RCT Setting: Primary care, United Kingdom Intervention: Exercise and manual therapy package Control 1: Injections of corticosteroid and local anaesthetic Control 2: Combination of exercise and manual therapy package plus injections of corticosteroid and local anaesthetic Control 3: Controlled medical treatment with regular NSAIDs Source of funding: Chartered Society of Physiotherapy Charitable Trust |
|
| Participants |
Diagnostic label used by trialists: Painful arc (synonymous with the diagnosis of subacromial impingement, subacromial bursitis, subdeltoid bursitis, rotator cuff tendinitis, supraspinatus tendonitis, and rotator cuff tendinopathy) Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 112; mean (range) age: 54.5 (23 – 88); sex: F/M 64/48; duration of symptoms: not reported |
|
| Interventions |
Intervention: exercise and manual therapy package (EMTP) Components of intervention: an exercise and manual therapy regime based on a literature review (described in Kibler W. Shoulder rehabilitation: principles and practice. Med Sci Sports Exerc 1998;30:S40‐50) and the opinions of an expert group of physiotherapists. No other details provided Dose: not reported Frequency of administration: 6 sessions over 18 weeks Control 1: glucocorticoid and anaesthetic injection Components of intervention: a course of injections of methylprednisolone and lidocaine The injections were placed 1 cm inferior to the posterior corner of the acromion, directed upward toward the subacromial region Dose: 40 mg of methylprednisolone and 10 ml of 1% lidocaine Frequency of administration: 1 injection every 6 weeks for a maximum of 3 injections Control 2: combination of EMTP and glucocorticoid and anaesthetic injection See details of each above Control 3: NSAIDs or analgesia Components of intervention: regular NSAIDs or simple analgesia if unable to tolerate NSAIDs Dose: not reported Frequency of administration: not reported |
|
| Outcomes | Outcomes assessed at 18 weeks and one year
|
|
| Notes |
Conflicts of interest: not reported Standard deviations for the Oxford Shoulder Score were not reported in numerical format so were estimated from Figures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Those who consented to enter the trial were randomized by closed envelope" Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Those who consented to enter the trial were randomized by closed envelope" Comment: It is not clear whether envelopes were sequentially numbered or opaque or how the sequence was generated, so it is unclear if the allocation sequence was successfully concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "After randomization, the control group had 27 patients, EMTP had 29, the injection group had 28, and both interventions had 29. Ninety patients (80%) completed the trial: control group, 20; EMTP, 22; injections, 26, both interventions, 22. Of the 22 patients who did not complete the trial, 1 was lost to follow‐up, and 21 withdrew during the duration of the trial (control, 7; EMTP, 7; injection, 2; and both, 5), and (18.75% noncompletion rate). Those who withdrew were invited for follow‐up in the standard outpatient clinic. Sixty‐two patients returned the follow‐up questionnaire at 1 year (55% of those randomized). By 1 year, 2 patients in the injection group and 1 each in the control and EMTP groups had gone on to surgery." Quote: "Analysis was on an intention‐to‐treat basis" Comment: Reasons for dropout were not reported and numbers of dropout were unbalanced between groups. The authors state that an intention‐to‐treat analysis was performed, though report outcome data based on the per‐protocol sample |
| Selective reporting (reporting bias) | High risk | Comment: No measures of variation were reported for any outcomes (except for the Oxford Shoulder Score, where SDs and 95% CIs were reported in Figure format). However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. No usable outcome data were reported for global assessment of treatment success or requiring of surgery. Also, without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Conroy 1998.
| Methods |
Study design: Parallel group RCT Setting: Primary care, USA Intervention: Mobilisation plus comprehensive treatment (hot packs, AROM, stretching, strengthening, soft tissue mobilisation and participant education) Control: Comprehensive treatment alone Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 7; age mean (SD): 55.0 ± 10.2 years old; sex: not reported; duration of symptoms: not reported Control Number randomised: 7; age mean (SD): 50.7 ± 16.5 years old; sex: not reported; duration of symptoms: not reported |
|
| Interventions |
Intervention: joint mobilisation Components of intervention: the Foley method (Foley R, Janos S, Johnson R, Petersen C: Active and Passive Movement Testing of the Extremities, Spine, Pelvis, and Temporomandibular Joint, Petersen C (ed), Chicago, IL: Northwestern University Medical School Programs in Physical Therapy, 1994) was used to deliver the mobilisation. Depending on the direction of restriction in capsular extensibility, inferior glide, posterior glide, anterior glide or long axis traction could be applied to the participant with oscillatory pressure. Stretch could also be applied in the case of muscle spasm Dose: each indicated technique was administered 2‐4 times (30 seconds each). As a result, the intervention group received a maximum of 15 min additional treatment compared with the control group Frequency of administration: 3 times per week for 3 weeks Control: comprehensive treatment alone Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: hot packs, active ROM, physiologic stretching and muscle strengthening exercises, soft tissue mobilisation and participant education
Dose
Frequency of administration: 3 times per week for 3 weeks |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to either the experimental (mobilisation) or the control (no mobilisation) group". Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the subject and examiner were blinded to group assignment." Comment: Participants received different multimodal interventions that were unlikely to be distinguishable to participants. Thus, participants were likely blinded to the intervention they received |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Both the subject and examiner were blinded to group assignment." Comment: Assessors of objective outcomes were likely blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One subject dropped from the control group due to lack of understanding instructions". Comment: The amount of dropout was small and unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Cook 2014.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinical/academic centres (USA or South Africa) Intervention: Neck manual therapy plus standard physiotherapy (shoulder manual therapy to the shoulder, self‐ and externally‐applied stretching, isotonic strengthening, and restoration of normative movement) Control: Standard physiotherapy Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 38; mean (SD) age: 51.1 ± 12.9 years old; sex: F/M 15/23; duration of symptoms: 12.9 ± 17.6 weeks Control Number randomised: 36; mean (SD) age: 51.0 ± 15.5 years old; sex: F/M 22/14; duration of symptoms: 10.4 ± 10.6 weeks |
|
| Interventions |
Intervention: neck manual therapy Components of intervention: the manual therapy interventions to the neck consisted of grade III posterior‐anterior mobilisations, performed in prone for 30 repetitions for 3 sets. Since any comparable shoulder symptoms during mobilisation to the cervical spine was an exclusion criterion, and since none of the subjects exhibited active neck symptoms, the posterior‐anterior mobilisation was performed to the stiffest or the participant's most painful segment. When no joint signs were present, the posterior‐anterior was performed to either C5‐C6, or C6‐C7 at the same side of the neck as the shoulder impingement. Where both pain and stiffness were present at multiple levels the clinician was able to identify the targeted level for mobilisation Dose: 30 repetitions for three sets Frequency of administration: participant discharge, treatment length, and frequency of treatment were determined by the physiotherapists, although some participants terminated treatment themselves. Participants had a mean (SD) of 59.7 (70.2) days in care Control: standard physiotherapy Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: Kuhn's (2009) approach, which advocates the use of a modified treatment that is unique to each individual and is based on their hypothesised underlying dysfunctions/causes, was used. The treatment methods included manual therapy, self‐ and externally‐applied stretching, isotonic strengthening, and restoration of normative movement. The clinical and home‐treatment programmes were modified for all subjects in each phase regardless of presentation, and the dosage of the interventions was specific to the examination findings Dose: not reported Frequency of administration: participant discharge, treatment length, and frequency of treatment were determined by the physiotherapists, although some participants terminated treatment themselves. Participants had a mean (SD) of 52 (29.6) days in care |
|
| Outcomes | Outcomes assessed at 2 days and at discharge
|
|
| Notes |
Conflicts of interest: not reported Trial is registered in ClinicalTrials.gov (NCT01744002) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomised by roll of die into the shoulder treatment plus neck mobilisations or the shoulder treatment only groups". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was a randomised, single blinded, controlled trial." Quote: "Physiotherapists were blinded to the collected self‐report outcomes in the study." Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "The study was a randomised, single blinded, controlled trial." Quote: "Physiotherapists were blinded to the collected self‐report outcomes in the study." Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported all outcomes to this review (e.g. pain, function, patient‐acceptable symptom state, self‐report rate of recovery) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seventy‐four (74) subjects were enrolled and of these, six did not return for a required follow‐up visit." Comment: A CONSORT flow chart (Schultz 2010) shows that two participants were lost to follow‐up from the shoulder plus neck treatment group and four were lost to follow‐up from the shoulder treatment group. The reasons for all six losses are "did not return after initial visit". It is unclear what the reasons for not returning were, but regardless of the reasons, the small amount of attrition and relatively equal distribution of attrition is unlikely to bias the outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for three outcomes specified in the ClinicalTrials.gov registry entry (NCT01744002). However self‐reported rate of recovery was listed as an outcome in the methods section (but not in the registry), and no outcome data for it were reported in the Results section. However it is unclear if this outcome was not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Dickens 2005.
| Methods |
Study design: Parallel group RCT Setting: Orthopaedic department in a district general hospital, UK Intervention: Physiotherapy (joint mobilisation, exercise therapy and/or electrotherapy) Control: Maintain normal daily activities Source of funding: Supported in part by the Physiotherapy Research Foundation, Project Reference No. PRF/99/2 |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 45; age: mean (range) 55 (27–68); sex: F/M 19/26; duration of symptoms: not reported Control Number randomised: 40; age: mean (range) 54 (26–73); sex: F/M 18/22; duration of symptoms: not reported |
|
| Interventions |
Intervention: physiotherapy Components of intervention: personalised training programme, both in‐hospital and at home. All participants received some or all of: acromioclavicular joint, thoracic, cervical spine and glenohumeral joint mobilisation, exercise therapy including attention to muscle imbalance, postural advice, strapping and, very occasionally, electrotherapy. The exercises were carried out once or twice per week in hospital until participants felt comfortable performing their therapy programme without supervision. The aim of the physiotherapy was to reduce inflammation and pain, directly using electrotherapy modalities and indirectly by altering the movement patterns (strapping and exercises) to alter the stresses placed on the subacromial structures, to a level where the exercise programme could be undertaken. The need for joint mobilisations was decided upon at the physiotherapy assessment. The physiotherapist assessed the range of accessory movement available in each participant's glenohumeral (anteroposterior, longitudinal caudad), acromioclavicular (anteroposterior, longitudinal caudad), cervical (posterior–anterior) and thoracic spine joints (posterior–anterior and transverse) with passive accessory movements. Any joints that were found to have restricted movement were addressed with mobilisations into the direction of resistance and pain to help restore full pain‐free range of movement. All participants were given exercises for the recruitment and strength of scapulothoracic muscles (especially lower trapezeius and serratus anterior). The exercise programme was progressed to involve strengthening of infraspinatus, subscapularis and teres minor relative to the supraspinatus and deltoid. The rotator cuff exercises were done with the use of resistance and participants were given Theraband for home use. The exercises started in neutral positions with isometric contractions and were progressed to inner range, through range, outer range and into functional positions. The resistance and speed of these exercises were altered and progressed Dose: dependent on participant Frequency of administration: twice a day for exercises. Overall duration of physical therapy programme not reported Control: normal daily activities Components of intervention: advised to maintain their normal activities of daily living whilst waiting for surgery Dose: NA Frequency: NA |
|
| Outcomes | Outcomes assessed at 6 months
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised into two groups using unmarked envelopes in clinic to achieve simple randomisation. There were 100 envelopes, 50 of which contained the word 'control' and 50 of which contained the word 'physiotherapy'." Comment: The random sequence may have been generated by shuffling of envelopes (given that the envelopes were unmarked), but this is not clear |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported. Envelopes were unmarked rather than sequentially numbered, which suggests that they were shuffled, though this is not clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All patients in both groups were re‐examined and Constant scores were performed at 6 months by JLW. JLW was blinded to the group allocation of the patients." Comment: Assessor of objective outcomes was likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three patients in the physiotherapy group were unable to complete the physiotherapy programme for social reasons and were therefore excluded, leaving 42 patients". Quote: "In the control group, nine patients initially randomised not to undergo a physiotherapy programme refused to attend follow‐up for repeat assessment. These patients felt that surgery was inevitable and that further assessment was not indicated. Although we have not been able to analyse the Constant score results on this group of nine patients, they all underwent surgery. These patients were included in the statistical analysis on an intention‐to‐treat basis." Comment: Participants who did not complete follow‐up were analysed using a worst‐case scenario intention‐to‐treat method where possible (i.e., it was assumed they showed no improvement). This is an appropriate imputation method given the reasons for missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: Only mean and range for the Constant score in each group was reported (i.e. no SDs, SEs or 95% CIs). However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Djordjevic 2012.
| Methods |
Study design: Parallel group RCT Setting: Rehabilitation clinic, Serbia Intervention: Mobilisation with movement and kinesiotaping Control: Supervised exercise programme Source of funding: No funding |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 10; mean (SD) age: 51.8 ± 5.3 years; sex: F/M 6/4; mean (SD) duration of symptoms: 4.7 ± 0.6 months Control Number randomised: 10; mean (SD) age: 54.1 ± 6.8 years; sex: F/M 7/3; mean (SD) duration of symptoms: 4.8 ± 0.9 months |
|
| Interventions |
Intervention: mobilisation with movement (MWM) and kinesiotape Components of intervention
Dose: MWM ‐ 10 repetitions, 3 sets daily, 30‐second rest period between sets, in 10 sessions with 24‐hours' rest between sessions. Kinesiotape ‐ applied after initial ROM measure, removed on day 5 and reapplied following ROM measures Frequency of administration: daily for 10 days Control: supervised exercise Components of intervention: pendulum exercises and pain‐limited, active ROM exercises of shoulder elevation, depression, flexion, abduction, rotations, and strengthening exercises. Strengthening exercises were isometric in nature, working on the external shoulder rotators, internal rotators, biceps, deltoid, and scapular stabilisers (rhomboids, trapezius, serratus anterior, latissimus dorsi, and pectoralis major). The participants were instructed to perform all the exercises to the first onset of pain Dose: 10 repetitions in 1 set daily, 30‐second rest periods between sets of different types of exercises; 10 sessions with 24 hours between sessions Frequency of administration: daily for 10 days |
|
| Outcomes | Outcomes assessed at 5 days and 10 days
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly allocated to group 1 or group 2. To ensure balance between the 2 groups, we used a minimization process as a form of restricted randomization. Minimization was run by Minim version 1.5, a minimization program for allocating patients to treatments in clinical trials". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The third author, a physiotherapist and certified MWM and KT practitioner with experience in orthopedic rehabilitation of more than 15 years, was responsible for both groups' treatments. This third author was blind to the group assignment and also to the ROM measured on days 0, 5, and 10. She was also instructed not to discuss with the subject if his/her treatment was any different from the usual program applied to the painful shoulder." Comment: Participants received different multimodal interventions, but were not provided with any information that would lead them to believe one intervention was superior or inferior to the other |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Outcome measures were measured by the second author, who also remained blind to the group assignment." Comment: Only objective outcomes were measured (ROM) and these were measured by a blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All subjects went through each phase of the study (flow diagram showing the progress of subjects at each stage of the clinical trial)" Comment: There were no drop‐outs, exclusions or losses to follow‐up (therefore all randomised participants were analysed) |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes listed in the Australian and New Zealand Clinical Trials Registry entry |
| Other bias | Low risk | Comment: No other sources of bias identified |
Engebretsen 2009.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic of physical medicine and rehabilitation department in Oslo, Norway Intervention: Supervised exercises (1 session weekly for up to 12 weeks) Control: Radial extracorporeal shockwave treatment (1 session weekly for 4–6 weeks) Source of funding: Supported by Health Region East, Norway |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 52; mean (SD) age: 49 (9.3) years; sex: F/M 26/26; duration of symptoms: 3‐6 months: 19 (37); 6‐12 months: 15 (29); 12‐24 months: 8 (15); > 24 months: 10 (19) Control Number randomised: 52; mean (SD) age: 47 (11.7) years; sex: F/M 26/26; duration of symptoms: 3‐6 months: 15 (29); 6‐12 months: 15 (29); 12‐24 months: 6 (12); > 24 months: 16 (31) |
|
| Interventions |
Intervention: supervised exercises Components of intervention: the principle focus was on relearning of normal movement patterns, which could then be transferred to daily activities. The initial aim was to unload the stress on the rotator cuff and subacromial structures. During this phase, a mirror for awareness of posture, an elastic rubber band and a sling fixed to the ceiling were used. The participants received immediate feedback and correction (supervision) by the physiotherapist. Once dysfunctional neuromuscular patterns were normalised, endurance exercises were performed with gradually increasing resistance. Participants had an adjusted programme at home, which consisted of correction of alignment during daily living and simple low loaded exercises with a thin elastic cord to provide assistance and resistance to the movement. Simple advice was given Dose: 45 min Frequency of administration: 2 sessions weekly for up to 12 weeks Control: radial extracorporeal shockwave treatment Components of intervention: 3 to 5 tender points were treated each time. Points were identified through a participant‐oriented biofeedback process (insertion of supraspinatus tendon, dorsolaterally below the acromion, and a maximum of three trigger points in the rotator cuff muscles). Radial extracorporeal shockwave treatment uses low to medium energy shockwaves generated when a projectile is accelerated by compressed air and hits an applicator. These impulses are delivered into the tissue and spread as spherical 'radial' waves (rather than being focused). Participants were informed that the suggested mechanism for pain relief was hyperstimulation analgesia and increased neurovascularisation that improves regeneration of tissue. Participants were advised to avoid activities that elicited pain Dose: frequency: 12‐8 Hz with 2000 pulses per session, with a pressure between 2.5 and 4.0 Bar, depending on what the participant tolerated without anaesthetic Frequency of administration: 1 session weekly for 4‐6 weeks |
|
| Outcomes | Outcomes assessed at 6 weeks, 12 weeks, 18 weeks and 1 year
|
|
| Notes |
Conflicts of interest: the authors stated that they had no conflicts of interest Trial registered in ClinicalTrials.gov (NCT00653081) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician not involved in data collection or analysis randomly allocated patients to treatment groups in blocks of four to six. Randomisation was stratified by sex." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "A person not involved in the treatments opened the sealed envelopes and assigned appointments according to treatment group." Comment: An adequate method was likely used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants and personnel could not be blinded for this trial." Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "A blinded physiotherapist made the baseline and follow‐up measurements. The patients were instructed not to discuss their treatment with the blinded physiotherapist." Comment: Assessor of objective outcomes was likely blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 10 participants per group did not return for all follow‐up measures, and while reasons for loss to follow‐up were not reported, an intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | High risk | Comment: No outcome data were reported for active ROM despite this outcome being listed in the methods section of the trial report. Several outcomes not specified in the ClinicalTrials.gov registry entry were added to the publication (e.g. function, active ROM, work status) |
| Other bias | High risk | Quote: "Thirteen patients in the radial extracorporeal shockwave group and three patients in the supervised exercise group received additional treatment (cortisone injections, chiropractic treatment, physical therapy/supervised exercises) between 12 and 18 weeks (odds ratio 5.5, 95% confidence interval 1.3 to 26.4; P=0.014)." Quote: "In the follow‐up period [up to 1 year], 10 participants in the SE group and 20 participants in the rESWT group had additional treatments (P = 0.024)". Comment: There was an imbalance between groups in the number of additional treatments received outside of the trial setting, which is likely to have biased the results in favour of the radial extracorporeal shockwave group |
Ginn 2005.
| Methods |
Study design: Parallel group RCT Setting: Metropolitan hospital, Australia Intervention: Physical modalities, passive joint mobilisation and ROM exercises Control 1: Glucocorticoid injection Control 2: Exercise therapy Source of funding: Cumberland Research Grants from The University of Sydney, Sydney, Australia |
|
| Participants |
Diagnostic label used by trialists: none specified Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 42; mean (range) age: 57.4 (29‐90) years sex: F/M 16/26; mean (SD) duration of symptoms: 7.4 (10.9) months Control 1 Number randomised: 48; mean (range) age: 55.4 (29‐87) years; sex: F/M 19/29; mean (SD) duration of symptoms: 7.4 (11.2) months Control 2 Number randomised: 48; mean (range) age: 52.6 (22‐83) years; sex: F/M 21/27; mean (SD) duration of symptoms: 7.3 (8.1) months |
|
| Interventions |
Intervention: physical modalities, passive joint mobilisation and ROM exercises Components of intervention: combination of electrophysical modalities (interferential therapy, ultrasound therapy, hot packs and ice packs), passive joint mobilisation at the sternoclavicular and acromioclavicular joints and ROM exercises (functional movements of the arm and could incorporate the use of aids to achieve additional range of movement). The aim of the exercise component was to increase the range of hand placement but excessive scapular movement was discouraged. There was no requirement that the ROM exercises be performed in a pain‐free manner. Exercises were upgraded from active assisted to active to resisted active exercises using free weights or elastic resistance. The specific treatment for each of the subjects was individually determined by the treating physical therapist using data from the initial interview and musculoskeletal assessment and any additional information gathered Frequency of administration: twice weekly attendance for application of passive joint mobilisation and electrophysical modality components, and daily adherence to prescribed exercise programme for 5 weeks Control 1: glucocorticoid injection Components of intervention: single injection of 40 mg methylprednisolone acetate, administered into the sub‐acromial space under local anaesthesia with lignocaine. Participant was encouraged to attempt to use their affected upper limb in a normal manner and to await contact from the investigators at the end of the 5‐week treatment period to arrange a time for reassessment Components of intervention: single injection given Control 2: exercise therapy Components of intervention: the target exercise treatment was directed toward the restoration of normal shoulder muscle function in order to restore dynamic stability and muscle co‐ordination at the shoulder region. This comprised stretches aimed at lengthening shortened shoulder muscles, exercises aimed at strengthening weakened shoulder muscles, including improving co‐ordination between muscles, and motor retraining aimed at restoring scapulohumeral rhythm during the performance of upper limb tasks. All exercises were to be pain‐free and subjects in this treatment group were also advised to avoid/limit pain producing activities. Particular emphasis was placed on restoring the normal muscle force couple co‐ordination and the dynamic stabilising function of shoulder muscles. The specific exercises for each of the subjects was individually determined by the treating physical therapist, using data from the initial interview and musculoskeletal assessment and any additional information gathered by the treating physical therapist. The exercise treatment was administered as a home‐based, daily exercise programme with supervision by the physical therapist once per week, to correct and upgrade the intensity and complexity of the exercises Frequency of administration: daily for 5 weeks |
|
| Outcomes | Outcomes assessed at 5 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated to 1 of the 3 treatment groups by 1 of the researchers, using intervention assignment schedules previously prepared separately for those subgroups." Comments: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comments: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Two senior physical therapists otherwise not associated with the clinical trial, acted as assessors over the length of the study; the first for the initial 22 months and the second for the remaining 24 months. Subjects were specifically requested not to discuss their treatment with the assessor to ensure she/he remained unaware of the treatment group to which the subject had been allocated." Comment: Assessors of objective outcomes were likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Eleven subjects were unavailable for reassessment at the end of the 5‐week treatment period: 6 from the PR subgroup and 5 from the P subgroup. One subject from the injection group died during the treatment period; 1 subject from the exercise group moved interstate; and 9 subjects, 2 from the injection group, 4 from the exercise group and 3 from the MPM group, were unavailable for unknown reasons" Comment: The amount and reasons for attrition are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Giombini 2006.
| Methods |
Study design: Parallel group RCT Setting: Athletes who attended the Physiotherapy Department of the Sport Science Institute, Italy Intervention: Exercises Control 1: Microwave diathermy Control 2: Therapeutic ultrasound Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus tendinopathy Criteria for defining the shoulder condition being treated: Diagnosis of supraspinatus tendinopathy of the dominant shoulder based on following three criteria
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 11; mean (SD, range) age: 26.3 ± 6.2 years, range 20‐38 years; sex: F/M 2/9; duration of symptoms: not reported Control 1 Number randomised: 14; mean (SD, range) age: 25.3 ± 4.8 years, range 19‐37 years; sex: F/M 2/12; duration of symptoms: not reported Control 2 Number randomised: 12; mean (SD, range) age: 28.6 ± 6.6 years, range 19‐43 years; sex: F/M 4/8; duration of symptoms: not reported |
|
| Interventions |
Intervention: exercises Components of intervention: supervised and home exercises, consisting of pendular swinging in the prone position in flexion and extension of the shoulder and passive glenohumeral stretching exercises to tolerance Frequency of administration: supervised exercises once a week for 4 weeks; home exercises 5 min per day, every day for 4 weeks Control 1: microwave diathermy Components of intervention: an ALBA Hyperthermia System was used which was equipped with a 433.92 MHz microwaves generator with a maximum output power of 100 W; a microstrip antenna applicator, with a curve shape specific for semicylindrical joint volumes of 20‐30 cm in diameter and with a total radiating area of 240 cm2 and an effective field size; and a pad of silicone 0.5 cm thick, filled with thermostatic deionized water that allows the greatest energy transfer to be achieved while preventing overheating of superficial tissues near the radiant source. A hydraulic thermoregulation and one or two skin temperature sensors were also used. The thermocouple was placed on the shoulder with the participant lying supine and the arm at 60 degrees of abduction and externally rotated. It was placed over the middle third of the joint line between the glenoid fossa and the humeral head. The thermocouple on the skin was perpendicular to the electromagnetic field Dose: 434 MHz; administered at a power between 50 and 70 W, a pilot temperature on the skin between 38 and 40 degrees centigrade, and a water pad temperature between 35 and 37 degrees centigrade according to the depth of the subcutaneous fat of each participant. Each session lasted 30 min Frequency of administration: 3 times a week for 4 weeks Control 2: therapeutic ultrasound Components of intervention: continuous ultrasound was administered with the participant in the same position as participants receiving hyperthermia and by slowly moving the transducer in a circular fashion along the area distal to the anterior border of the acromion and the inferior third of a line between the glenoid fossa and the humeral head. A gel couplant was used between the ultrasound transducer and the skin of the area undergoing treatment. A Level 730 device was used. It was equipped with an emission probe of 1 MHz frequency, a sound head with an effective radiating area of 10cm2 and a maximum output power of 22 W. Dose: 1 MHz at an intensity of 2.0 w/cm2; each session lasted 15 min Frequency of administration: 3 times a week for 4 weeks |
|
| Outcomes | Outcomes assessed at 4 weeks and 10 weeks
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised into 3 groups using a computer‐generated list." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The subjects were assessed by fully trained sports physicians who had never seen the patients and were unaware as to which intervention the patients had been allocated." Comment: Assessor of objective outcome was likely blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no loss to follow‐up and all randomised participants were analysed |
| Selective reporting (reporting bias) | High risk | Comment: Data for pain on resisted movement were reported in figure only as means with no error bars. No data for night pain, pain on movement, rest pain and painful arc were reported, despite being listed as outcomes in the methods section of the trial report |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Haahr 2005.
| Methods |
Study design: Parallel group RCT Setting: Herning Hospital, Ringkjoebing County, Denmark Intervention: Exercises plus heat, cold packs or soft tissue treatment (i.e. not all participants received soft tissue treatment) Control: Arthroscopic subacromial decompression Source of funding: Medical Research Unit of Ringkjoebing County, Denmark |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above):
Baseline characteristics Intervention Number randomised: 45; mean (SD): 44.5 (1.2) years; sex: F/M 29/14; duration of symptoms: < 6 months: 3; 6‐12 months: 10; > 1 year: 29 Control Number randomised: 45; mean (SD) age: 44.3 (1.3) years; sex: F/M 29/12; duration of symptoms: < 6 months: 4; 6‐12 months: 3; > 1 year: 34 |
|
| Interventions |
Intervention: physiotherapy Components of intervention: the treatments started with application of heat, cold packs, or soft tissue treatments. This was followed by active training of the periscapular muscles (rhomboid, serratus, trapezoid, levator scapulae, pectoralis minor muscles) and strengthening of the stabilising muscles of the shoulder joint (rotator cuff). This was done within the limits of pain Dose: 60 min Frequency of administration: total 19 sessions. First 2 weeks: 3 times weekly. Next three weeks: twice weekly. Last seven weeks: once weekly. After 12 weeks of the trial, participant encouraged to continue the programme 2 to 3 times per week at home Control: arthroscopic subacromial decompression Components of intervention: investigation for stability of the shoulder joint under general anaesthetic followed by an arthroscopic examination of the glenohumeral joint, the rotator cuff and the subacromial bursa. The treatment consisted of bursectomy with partial resection of the antero‐inferior part of the acromion and the coracoacromial ligament. Before discharge, the participant was instructed in performing light movements of the arm within the limits of pain. Stitches were removed by general practitioners after 10 days. At the same time, the participant was instructed by a physiotherapist to carry out increasingly active exercises, including exercises for strengthening the rotator cuff muscles. The team instructing the physiotherapy group was different from the group treating the surgery group. The surgeon then saw the participants after 6‐8 weeks |
|
| Outcomes | Outcomes assessed at 3, 6 and 12 months, and 4‐8 years
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer program was used to generate a random sequence of allocation." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The same specialist (SØ) carried out all the assessments, obtained informed consent for participation, and randomised the patients into one of two intervention groups by opening a sealed envelope containing the result of randomisation, which was unknown to SØ." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "The same specialist (SØ) carried out all the assessments, obtained informed consent for participation, and randomised the patients into one of two intervention groups by opening a sealed envelope containing the result of randomisation, which was unknown to SØ." Quote: "Physiotherapists were not blinded to the treatment given when assessing the Constant score". Comment: Assessor of objective outcomes was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Ninety consecutive patients with subacromial impingement agreed to participate. Forty five cases were randomised to conservative treatment and 45 to surgical treatment. Among those assigned to conservative treatment, one withdrew from participation because of work problems and one failed to fill in the baseline questionnaire, leaving 43 cases in this group. In the surgery group, four cases dropped out before the start of the study (one because of work problems, one with a tumour in the humerus, one because his wife advised against participation, and one for unknown reasons), leaving 41 cases in this group. Within the conservative treatment group, a further six participants were operated on within the 12 months of the study (five because of unsatisfactory improvement during exercises and in one case because a labral lesion was suspected). In the physiotherapy group 42 persons (93%) were followed for 12 months with the main outcome measure (Constant score). In the surgery group 40 persons (89%) had complete follow up data. Quote: "Seventy‐nine (88%) answered the final questionnaire" [at 4‐8 years' follow‐up] Comment: The amount and reasons for drop‐out are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: no other sources of bias were identified |
Haik 2014.
| Methods |
Study design: Parallel group RCT Setting: University, orthopaedic clinics, and community public places, Spain Intervention: Thoracic spine manipulation Control: Sham manipulation Source of Funding: Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior |
|
| Participants |
Diagnostic label used by trialists: Shoulder impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 26 (25 completed); mean age: 33.8 ± 12.2 years old; sex: F/M 11/14; duration of symptoms: 49 ± 96 months Control Number randomised: 26 (25 completed); mean age: 29.7 ± 9.3 years old; sex: F/M 7/18; duration of symptoms: 42.6 ± 66 months |
|
| Interventions |
Intervention: thoracic spine manipulation Components of intervention: low‐amplitude, high velocity thrust thoracic spine manipulation. The participant assumed a seated position and the physiotherapists performed a thrust technique, targeting the midthoracic spine Dose: if no cavitation was detected with the manipulation, the thrust was repeated up to 3 times Frequency of administration: once Control: sham manipulation Components of intervention: the participant assumed the same seated position and the physiotherapist held the participant in the same position as that of the thrust manipulation intervention. The physiotherapist applied the same forces as those of a thrust manipulation, while holding the position for a few seconds, without actually performing a thrust manipulation Dose: as above Frequency of administration: once |
|
| Outcomes | Outcomes assessed immediately post‐intervention (day 1)
|
|
| Notes |
Conflicts of interest: authors stated they had "no affiliations with or financial involvement in any organisation or entity with a direct financial interest in the subject matter or materials discussed in the article" Trialists also assessed scapular kinematics but these were not included in our review outcomes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using computer‐generated lists, one for the impingement group and the other for the asymptomatic group, subjects were randomly assigned to 1 of 4 groups: a TSM impingement group (n = 25), a sham impingement group (n = 25), a TSM asymptomatic group (n = 24) and a sham asymptomatic group (n = 23)". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The subjects were given only general information about the purpose of the study to control expectations and to conduct an effective sham intervention". Comment: Participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported all outcomes of interest to the review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: One participant from each group was excluded post‐randomisation because of a fault in the equipment used to assess scapular kinematics. However, this small amount of attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Hay 2003.
| Methods |
Study design: Parallel group RCT Setting: Primary care – general practitioner, UK Intervention: Physiotherapy (all participants: advice and instructions on pain relief and active shoulder exercises at home; dependent on participant: ultrasound and active and passive mobilisation) Control: Glucocorticoid injection Source of funding: Arthritis Research Council |
|
| Participants |
Diagnostic label used by trialists: None specified Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 103; mean (SD) age: 57.5 ± 13 years; sex: F/M 50/53; mean (range) duration of symptoms: 51 (21‐120) days Control Number randomised: 104; mean (SD) age: 57.6 ± 14 years; sex F/M 60/44; mean (range) duration of symptoms: 58 (28‐128) days |
|
| Interventions |
Intervention: physiotherapy Components of intervention: the most frequently used modality at the assessment visit was a standardised education and advice leaflet for shoulder pain (85%) followed by a home exercise programme (79%) which was reinforced throughout the trial treatment course. The most frequently utilised modalities over the treatment period were ultrasound (42%), active mobilisations (41%) and passive mobilisations (41%) Dose: 20 min Frequency of administration: 8 individual physiotherapy sessions delivered within a 6‐week period Control: glucocorticoid injection Components of intervention: injection of methylprednisolone with lidocaine into the subacromial space, administered by GP according to standard technique: the tip of the acromium and head of the humerus were identified by palpation and the injection point (just behind the mid‐line in the gap between the acromium and head of the humerus) was marked; the skin was cleaned and the needle inserted perpendicular to the skin pointing slightly upwards under the acromium and local steroid with lidocaine injected easily without resistance. Participants were advised to avoid overuse of the shoulder for 48 hours and told that they could make an appointment to return within 4 weeks if their symptoms persisted. If they did return, they were offered a second injection. Dose: 40 mg of methylprednisolone with 4 ml 1% lidocaine (lignocaine) Frequency of administration: 1 injection; if symptoms persisted participant could make appointment within 4 weeks of initial injection and have a second injection |
|
| Outcomes | Outcomes assessed at 6 weeks and 6 months
|
|
| Notes |
Conflicts of interest: not reported 25% of participants had adhesive capsulitis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was according to the study number. Numbers were issued in a predetermined random sequence, in blocks of 10 by general practice, generated by a random number table." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The number corresponded with that on a sealed envelope issued to the patient by the nurse. Participants were instructed not to open the envelope until the nurse had left. The envelope contained information instructing the participant to either make an appointment with one of the trial physiotherapists or to return to their GP for a local steroid injection." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Outcome assessments were performed by the study nurse, who was unaware of the treatment allocation." Comment: Assessor of objective outcomes was likely blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The completion rate of the trial at six months was 95% (196/207) with the following reasons for loss to follow up: five other medical complications, two personal problems, four could not be contacted/refused visit. Intention to treat analysis was used." Comment: The amount and reasons for dropout are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Heredia‐Rizo 2013.
| Methods |
Study design: Parallel group RCT Setting: Private physiotherapy practice, Spain Intervention: Manual therapy based on soft tissue techniques in the cervical and upper thoracic regions plus infrared plus TENS plus ultrasound Control: Mobilisation, proprioceptive neuromuscular facilitation, supervised exercises plus infrared plus TENS plus ultrasound Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 11; mean (SD) age: 54 ± 9.75 years; sex F/M 4/7; duration of symptoms: not reported Control Number randomised: 11; mean (SD) age: 62 ± 10.96 years; sex: F/M 5/6; duration of symptoms: not reported |
|
| Interventions |
Intervention: manual therapy based on soft tissue techniques Components of intervention: manual therapy was started with micro‐mobilisations of the cervical structures in all movement axes. Thus, a selective traction of each vertebra in the longitudinal axis was performed with additional lateral movements in the transverse axis of the restricted zones. Subsequently relaxation manoeuvres were performed to fascial restrictions involving the cervical and scapulohumeral region, especially of the trapezius, the sternocleidomastoid, levator scapulae, subscapularis and pectoral muscles. Finally, adhering to the principles of orthopaedic manual therapy described by Kaltenborn, a repositioning of the head of the humerus was conducted in different stages:
Dose: 40 min Frequency of administration: 5 days a week for 3 weeks Control: mobilisation, proprioceptive neuromuscular facilitation, supervised exercises Components of intervention: this comprised 20 min of:
In addition they completed a daily programme of 20 min of supervised active exercises, such as pendular movements using 1 kg of weight in prone, assisted active movements with a pulley, and proprioceptive exercises with a ball in the horizontal plane Dose: 40 min Frequency of administration: 5 days a week for 3 weeks Both groups Components of intervention: in the first place, infrared was applied on the shoulder for 15 min (Infra 2000, EnrafNonius). Afterward, TENS was used with a frequency of 80 Hz, 150 ms for 30 min (Med 911, Enraf‐Nonius). Lastly, an ultrasound device (Sonopuls 492, Enraf‐ Nonius) with a power of 1.5 W/cm2 and a frequency of 3 MHz in pulsating mode was applied for 5 min. Frequency of administration: 5 days a week for 3 weeks |
|
| Outcomes | Outcomes assessed at 3 weeks
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After signing an informed consent according to the principles gathered in the Helsinki Declaration (2008 version), the patients were randomized to the Conventional (n = 11) or Experimental Group (n = 11) by means of sealed opaque envelopes." Comment: No information regarding how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Low risk | Quote: "After signing an informed consent according to the principles gathered in the Helsinki Declaration (2008 version), the patients were randomized to the Conventional (n = 11) or Experimental Group (n = 11) by means of sealed opaque envelopes." Comment: An adequate method was probably used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A randomized and single blind (the practitioner carrying out the measurements remained unaware of the subject’s group membership) clinical study was conducted." Comment: Despite being described as assessor‐blinded only, participants received slightly different types of manual therapy and exercise, but it is unclear whether they were provided with any information that would make them perceive the type of manual therapy and exercise they received as superior or inferior to the alternative type of manual therapy and exercise |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported function, but it is unclear whether they were provided with any information that would make them perceive the type of manual therapy and exercise they received as superior or inferior to the alternative type of manual therapy and exercise |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "A randomized and single blind (the practitioner carrying out the measurements remained unaware of the subject’s group membership) clinical study was conducted." Comment: Outcome assessor of objective outcomes (ROM) was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No drop‐outs, losses to follow‐up or exclusions were reported, and the number of participants randomised was reported as the number of participants analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Holmgren 2012.
| Methods |
Study design: Parallel group RCT Setting: Department of orthopaedics, University hospital, Sweden Intervention: Specific exercise programme plus subacromial corticosteroid injection, information about shoulder condition, ergonomic advice and advice on correction of posture, and manual therapy when required Control: Non‐specific exercises plus subacromial corticosteroid injection, information about shoulder condition, ergonomic advice and advice on correction of posture, and manual therapy when required Source of funding: This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated Primary subacromial impingement syndrome, diagnosed by an orthopaedic specialist and on waiting list for arthroscopic subacromial decompression. Shoulder condition defined by:
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 50; mean (SD) age: 52 (9) years; sex: F/M 14/37; duration of symptoms: median 24 months, range: 6–120 months Control Number randomised: 52; mean (SD) age: 52 (8) years; sex F/M 22/24; duration of symptoms: median 12 months, range: 6‐156 months |
|
| Interventions |
Intervention: specific exercise programme Components of intervention: the programme consisted of 6 different exercises: 2 eccentric exercises for the rotator cuff (supraspinatus, infraspinatus, and teres minor), 3 concentric/eccentric exercises for the scapula stabilisers (middle and lower trapezius, rhomboideus, and serratus anterior), and a posterior shoulder stretch. The exercises were individually adjusted and progressed with increased external load by using weights and elastic rubber band at the physiotherapist visits once every other week during the whole rehabilitation period. The individual resistance for each participant was determined by using the pain monitoring model. The participant was not allowed to exceed 5 on this 0‐10 scale when they performed the exercises however, they were recommended to feel some pain during loading. After completion of an exercise session, increased pain had to revert to levels before exercise before the next session otherwise the external load was decreased. Great emphasis was placed on teaching good posture (thoracic spine extension and retracted shoulder) and to maintain this position during the exercises Dose: each strengthening exercise performed 15 times in 3 sets, twice daily for 8 weeks; posterior shoulder stretch performed 30‐60 seconds 3 times daily for 8 weeks. From week 8–12 the exercises were repeated once a day. After 12 weeks when the specific exercise programme had finished, participants were encouraged to maintain the daily home exercises for 2 months Frequency of administration: participants saw research physiotherapist once a week for the first 2 weeks and once every other week for the next 10 weeks (a total of 7 visits). The first visit lasted about 60 min, and the subsequent visits lasted about 30 min. In between these supervised sessions, participants performed home exercises once or twice a day for 12 weeks Control: non‐specific exercises Components of intervention: 6 unspecific movement exercises for the neck and shoulder without any external load (shoulder abduction in the frontal plane, shoulder retraction, shoulder elevation, neck retraction, stretch of upper trapezius and pectoralis major). The unspecific exercise programme was thought to have limited effect in people with subacromial impingement syndrome and therefore acted as a control. Participants did the same programme without any progression during the whole rehabilitation period Dose: each movement exercise was repeated 10 times, and each strengthening exercise 3 times twice daily at home and once every other week at the physiotherapist visits Frequency of administration: participants saw research physiotherapist once a week for the first 2 weeks and once every other week for the next 10 weeks (a total of 7 visits). The first visit lasted about 60 min, and the subsequent visits lasted about 30 min. In between these supervised sessions, participants performed home exercises once or twice a day for 12 weeks Both groups Components of intervention: subacromial glucocorticoid injection at the inclusion visit. When necessary, the physiotherapist performed manual treatment by stretching the posterior glenohumeral capsule and pectoralis minor during the visits. Thorough information about their shoulder condition, ergonomic advice and correction of their posture. Exercises introduced 2 weeks after the injection |
|
| Outcomes | Outcomes assessed at 3 months and 12 months*
|
|
| Notes |
Conflicts of interest: The authors stated that they had no conflicts to declare Trial registered in ClinicalTrials.gov (NCT01037673) *We only extracted 12‐month outcome data for the 'required surgery' outcome, as 12‐month data for all other outcomes were sub‐grouped by whether participants underwent surgery post‐exercise intervention or not |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent physiotherapist prepared the random allocation sequence beforehand. Equal numbers of the two treatment alternatives, 55 of each, were prepared and concealed in opaque envelopes. These were then mixed by hand and numbered. At the inclusion visit, the orthopaedic specialist (HB) coded the patients consecutively." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The two treatment alternatives were prepared and concealed in opaque envelopes. Treatment allocation was performed at the first visit to the physiotherapist, within two weeks of the inclusion visit. The research physiotherapist received the envelope with the corresponding code revealing the assigned treatment alternative out of a central locked location just before the participants presented for one of the two treatments: specific exercises (specific exercise group) or unspecific exercises (control exercise group)." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The control exercise programme consisted of six unspecific movement exercises for the neck and shoulder without any external load (shoulder abduction in the frontal plane, shoulder retraction, shoulder elevation, neck retraction, stretch of upper trapezius and pectoralis major. Each movement exercise was repeated 10 times, and each stretching exercise three times twice daily at home and once every other week at the physiotherapist visits. The patients did the same programme without any progression during the whole rehabilitation period. The unspecific exercise programme was thought to have a limited effect in patients with subacromial impingement syndrome and therefore acted as a control". Comment: Participants (but not personnel) were blind to treatment (non‐specific movements were completed by the control group, who were likely unaware that their movements were placebo exercises) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The same orthopaedic specialist evaluated all primary and secondary outcome measures at the inclusion visit before patients started the exercises (baseline) and after three months when patients had completed their exercise programme. The specialist was blinded to the group assignment throughout the study." Comment: Assessor of objective outcomes was likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 152 patients were eligible for inclusion; 102 patients met the inclusion criteria and provided written informed consent to participate. Three weeks after inclusion, five patients were excluded: two patients developed a frozen shoulder, diagnosed by the physiotherapist three weeks after inclusion, and three patients changed their minds about participating in the study and declined participation at the first physiotherapist visit because of lack of time. A total of 97 patients were compliant with the study protocol from baseline to the three month assessment and were included in the statistical analysis". Quote: "Ninety‐five patients were assessed at 1‐year follow‐up. Two patients in each group did not attend the 1‐year follow‐up due to non‐related disease" Comment: The amount and reasons for attrition are unlikely to have affected the results |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data fully reported for all outcomes specified in the clinical trials registry |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Janse van Rensburg 2012.
| Methods |
Study design: Parallel group RCT Setting: National Health Service physiotherapy department, UK Intervention: Thoracic spinal manipulation plus mobilisation plus supervised exercises Control: Mobilisation plus supervised exercises Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 6; mean (range) age: 53 (41‐60) years; sex: F/M 2/4; mean (range) duration of symptoms: 9 (1‐24) months Control Number randomised: 3; mean (range) age: 60 (58‐65) years; sex: F/M 1/2; mean (range) duration of symptoms: 5 (4‐6) months |
|
| Interventions |
Intervention: thoracic spinal manipulation Components of intervention: a specific high velocity low amplitude ‘extension with rotation’ thrust manipulation was applied to the shoulder Dose: 30 min Frequency of administration: once a week for 6 weeks Control: mobilisation plus supervised exercises Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: physiotherapy comprising active or passive glenohumeral mobilisation; transverse friction massage to the rotator cuff tendons; exercises to stimulate the lower fibres of trapezius and specific rotator cuff‐strengthening. Exercises, and ergonomic and lifestyle advice was provided in line with current physiotherapy practice Dose: 30 min Frequency of administration: Once a week for 6 weeks |
|
| Outcomes | Outcomes assessed at 6 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomized into the control or experimental group using unmarked envelopes. As it was anticipated that 20 subjects would be recruited, there were 20 envelopes, 10 of which contained a sticker with the words ‘control – no manipulation’ and 10 which contained a sticker with the words ‘experimental – manipulation’. The investigator had no involvement in the randomization. As participants arranged their treatment sessions at reception, the administration staff asked each patient to choose one of the unmarked envelopes. Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The participants were randomized into the control or experimental group using unmarked envelopes. As it was anticipated that 20 subjects would be recruited, there were 20 envelopes, 10 of which contained a sticker with the words ‘control – no manipulation’ and 10 which contained a sticker with the words ‘experimental – manipulation’. The investigator had no involvement in the randomization. As participants arranged their treatment sessions at reception, the administration staff asked each patient to choose one of the unmarked envelopes. The administration staff opened the envelope and placed the sticker on the inside of the patient’s treatment card so that the treating therapist would know which group the patient was assigned to and the patient would not. If the investigator happened to see the outside of the treatment card, for instance in the filing cabinet, she would not know which group the patient was in as there would be nothing to identify the patient to a particular treatment group" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Pre and post every treatment session, the investigator or second trained data collector (who did not undertake treatments and were therefore blinded to group allocation) measured the shoulder range of movement of each patient." Comment: Assessor of objective outcome was likely blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Thirty‐five patients were assessed by the researcher over a 4‐week period, nine of whom met the inclusion criteria and were recruited into the pilot study. One subject did not wish to continue with physiotherapy and dropped out after the first treatment session. This participant had been randomized to the control group" Comment: The number of drop‐outs was low and unlikely to affect the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Kachingwe 2008.
| Methods |
Study design: Parallel group RCT Setting: University health centre, USA Intervention 1: Glenohumeral mobilisation plus supervised and home exercises Intervention 2: Mobilisation with movement technique plus supervised and home exercises Control 1: Supervised and home exercise only Control 2: Physician advice only Source of funding: Supported by the California State University, Northride Research, Scholarship and Creative Activity Award |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1 Number randomised: 9; mean (SD) age: 43.4 ± 14.7 years; sex: F/M 5/4; mean (SD) duration of symptoms: 19.2 ± 24.6 months Intervention 2 Number randomised: 9; mean (SD) age: 48.9 ± 13.7 years; sex: F/M 4/5; mean (SD) duration of symptoms: mean: 22.6 ± 17.4 months Control 1 Number randomised: 8; mean (SD) age: 47.3± 20.1 years; sex: F/M 4/4; mean (SD) duration of symptoms: mean: 32.5 ± 60.2 months Control 2 Number randomised: 7; mean (SD) age: 45.6 ± 13 years; sex: F/M 3/4; mean (SD) duration of symptoms: mean: 70 ± 92.4 months |
|
| Interventions |
Intervention 1: glenohumeral joint mobilisations (group 1) Components of intervention: joint mobilisation was administered based on assessment of glenohumeral joint anterior, posterior and inferior glides and long‐axis distraction passive accessory motions using a 0‐6 accessory motion scale. For situations where there was reactivity within the capsular ROM, grade I‐II mobilisation were applied. For situations where there was no reactivity but capsular hypomobility, grade III‐IV accessory motions were applied Dose: 3 sets of 30‐second mobilisations (each mobilisation applied for 30 seconds at a rate of approximately 1 mobilisation every 1‐2 seconds, followed by a 30‐second rest). Frequency of administration: once a week for 6 weeks Intervention 2: glenohumeral joint mobilisation with movement (group 2) Components of intervention: Mulligan technique: involved the therapist applying a sustained posterior accessory glide to the glenohumeral joint while the subject simultaneously actively flexed the shoulder to the pain‐free endpoint and applied a gentle overpressure force using the contralateral arm. Total abolition of pain during the technique was mandatory; if the participant started to experience pain during active motion, the therapist would investigate different force planes and/or grades of force until pain‐free motion was sustained; if pain commenced during any repetition of any set, the technique was terminated Frequency of administration: once a week for 6 weeks Control 1: supervised and home exercises (groups 1, 2 and 3) Components of intervention: supervised exercises including posterior capsule stretching, postural correction exercises, and an exercise programme focusing on rotator cuff strengthening and scapular stabilisation. Each session ended with subjects receiving a cold pack for 10‐15 min to decrease potential inflammation and delayed muscle soreness. Participants were instructed to perform a home exercise programme mimicking the exercises performed in the clinic Frequency of administration: supervised exercise ‐ once a week for 6 weeks; home exercises ‐ once per day Control 2: advice (group 4) Components of intervention: participant education on postural awareness and limitation of overhead activities. Advice administered by the referring physician during their initial physical examination. The physician also provided the subject with a standard shoulder impingement home exercise programme without any input from the physical therapist. |
|
| Outcomes | Outcomes assessed at 6 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of four intervention groups according to the block randomisation method. E.g. subject #1 had an equal chance of drawing an envelope assigning them to A, B, C or D. If they drew 'A', then the card was removed so the next participant had an equal chance of drawing an envelope with B, C or D. etc." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: It is unclear if adequate safeguards were put in place to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Each subject was informed of his/her protocol but remained blinded to other group assignments to avoid subject bias" Comment: Despite not knowing what other participants received, expectations about the effectiveness of interventions received may have differed between groups, particularly between those receiving exercise and mobilisation versus those receiving physician advice only |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "One physical therapist with 12 years of clinical experience performed the pre‐ and post‐treatment assessment measurements. This assessor was blinded to group assignment and all intervention protocols." Comment: Assessor of objective outcomes was likely blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts and outcome data reported as based on total number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Kardouni 2014.
| Methods |
Study design: Parallel group RCT Setting: Physical and occupational therapy offices and physician's clinics, USA Intervention: Thoracic spinal manipulative therapy Control: Sham manipulative therapy Source of Funding: Clinical and Translational Science Award No. UL1TR000058 from the National Center for Advancing Translational Sciences and the AD Williams' Fund of the Virginia Commonwealth University |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 24; mean age: 31.1 ± 12.3 years old; sex: F/M 14/10; duration of symptoms: 40.2 ± 65.9 months Control Number randomised: 24 (21 completed); mean age: 31.2 ± 12.1 years old; sex: F/M 9/12; duration of symptoms: 41.2 ± 56.5 months |
|
| Interventions |
Intervention: thoracic spinal manipulative therapy (SMT) Components of intervention: during administration of the thoracic SMT, a high‐velocity, low‐amplitude thrust was applied at the end of available spinal motion after the participant exhaled. For the mid and lower thoracic SMT, the participants were prone, and the thrust was directed in the posterior to anterior direction. For the cervicothoracic junction SMT, participants were seated, and the thrust was an axial (cephalad) distraction Dose: twice at each of the 3 regions, for a total of 6 manoeuvres Frequency of administration: once Control: sham manipulative therapy Components of intervention: the therapist maintained manual contact through the ROM during exhalation, but no manipulative thrust was delivered Dose: twice at each of the 3 regions, for a total of 6 manoeuvres Frequency of administration: once |
|
| Outcomes | Outcomes assessed immediately post‐treatment (pain) and at 1 to 2 days (pain, function, QoL)
|
|
| Notes | Conflicts of interest: "The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in this study." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization list for treatment group assignments of the participants was computer generated with random blocking using nQuery Advisor software (Statistical Solutions, Saugus, MA)". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment assignments were placed into sequentially numbered privacy envelopes to conceal treatment group allocation". Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were blinded to treatment assignment, and were told prior to the start of testing they could receive an active or a placebo treatment. In an effort to help maintain blinding and prevent participants from knowing that they were receiving the active or placebo treatment, both treatment groups were assigned names representative of active treatments. Participants randomized to the thoracic SMT group were told that they were receiving “spinal manipulative therapy” while those randomized to the sham thoracic SMT group were told they would receive a “therapist‐assisted range of motion” treatment." Comment: Participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported all outcomes of interest to the review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Forty‐eight (n = 48) individuals with SIS were randomly assigned to receive thoracic SMT (n = 24) or sham thoracic SMT (n = 24). Three participants were excluded from the final analysis (all in the sham thoracic SMT group) because it was discovered after testing that they had pain in both shoulders, leaving n = 45 for final analysis". Comment: The amount of attrition is small and reasons were unrelated to the intervention, so attrition is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Kassolik 2013.
| Methods |
Study design: Parallel group RCT Setting: General practice, Poland Intervention: Classic (Swedish) massage Control: Massage using techniques based on the tensegrity principle Source of funding: University School of Physical Education in Wroclaw |
|
| Participants |
Diagnostic label used by trialists: None specified Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 35 (18 in one group and 17 in the other); mean (SD) age of women 53.9 ± 16 years; Mean (SD) age of men 43.6 ± 12.3 years; sex: F/M 19/11; duration of symptoms: not reported |
|
| Interventions |
Intervention: classic (Swedish) massage Components of intervention: classic massage of the shoulder girdle and glenohumeral joint was performed in a side recumbent position. During the massage, typical classic massage techniques (Swedish) were used ‐ stroking with the palms (effleurage), friction with the palms, kneading (petrissage), percussion (tapottement), and vibration Dose: each technique was performed 7 to 8 times in particular body parts (frequency 60 to 70 moves per min, as in normal pulse rate); percussion and vibration were performed for 1 min on average Frequency of administration: 5 times a week for 2 weeks Control: massage using techniques of the tensegrity principle Components of intervention: the techniques used for this method were the same as in the methodology of classic massage but were aimed at additional areas. Before the massage, palpation of the selected anatomical structures was carried out. The purpose of the assessment was to determine which tissues had the greatest sensitivity and which showed increased tension. Based on palpation results, the massage of painful tissues was performed Dose: 20 min Frequency of administration: 5 times a week for 2 weeks |
|
| Outcomes | Outcomes assessed at 2 and 6 weeks
|
|
| Notes |
Conflicts of interest: the authors stated that they had no conflicts of interest Trial was registered in ClinicalTrials.gov (NCT01307826) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients assigned with even numbers were included in the classic group and ones with odd numbers to the tensegrity group". Comment: A quasi‐random allocation sequence was used |
| Allocation concealment (selection bias) | High risk | Comment: A quasi‐random (i.e. predictable) allocation sequence was used, therefore the allocation sequence was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were blinded to which group they were in." Comment: Given the nature of the interventions (i.e. one versus another type of massage), it is unlikely that participants perceived the type of massage they received as superior or inferior to the alternative type of massage |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Quote: "Patients were blinded to which group they were in." Comment: Blinded participants self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Range of motion measurements of the glenohumeral joint by the goniometric method were conducted by a physiotherapist...who had no knowledge of which group the patient was assigned." Comment: Outcome assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The CONSORT flow chart (Schultz 2010) shows that three and two participants, respectively, did not receive the allocated intervention for reasons unrelated to the intervention (e.g. unable to attend appointment because of work), which is unlikely to bias the results. No other drop‐outs, losses to follow‐up or exclusions occurred |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the ClinicalTrials.gov registry entry (NCT01307826) |
| Other bias | High risk | Quote: "Before the therapy, the groups differed in 7 of 10 measured motions (lower range in the group with massage based on the tensegrity rule) while after the therapy, the difference appeared in only 1 case. Statistically significant changes in the group with massage based on the tensegrity rule in some of the motions may prove to be the result of worse ROM at the very beginning of the study in this group. Perhaps, in the opinion of the authors, in such a condition of glenohumeral joint, it is easier to achieve such results." Comment: There was baseline imbalance in ROM, which may have favoured the group receiving massage based on the tensegrity principle |
Kaya 2014.
| Methods |
Study design: Parallel group RCT Setting: Outpatient physiotherapy clinic, Turkey Intervention 1: Manual therapy and exercise plus cold pack Intervention 2: Kinesiotaping plus exercise plus cold pack Source of Funding: No funding |
|
| Participants |
Diagnostic label used by trialists: Subacromial impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention 1 Number randomised: 30 (26 completed); mean age: 47.15 ± 9.44 years old; sex: F/M 16/10; duration of symptoms: 6‐28 weeks Intervention 2 Number randomised: 30 (28 completed); mean age: 50.85 ± 5.17 years old; sex: F/M 17/11; duration of symptoms: 6‐26 weeks |
|
| Interventions |
Intervention 1: manual therapy Components of intervention: general mobilisation, including superoinferior gliding, rotations, and distractions to the scapula, were applied 3 to 5 times. Also, neuromuscular facilitation techniques for scapula motions at anterior elevation–posterior depression and posterior elevation–anterior depression planes were performed up to 5 to 6 repetitions. Glenohumeral joint mobilisation with long axis traction and posterior or inferior glide techniques to improve shoulder internal rotation limitations were applied according to the individual requirements of the participants. Soft tissue massage and joint mobilisation of the neck, thoracic region, and elbow areas, according to the involvement, and deep friction massage with specific ischaemic compression technique were applied to supraspinatus muscle Dose: total duration 1.5 hours Frequency of administration: once a week for 6 weeks Intervention 2: Kinesiotaping Components of intervention: application of kinesiotaping to the tissue that was in need of help. According to the Wright test result and muscle strength tests, the affected weak muscle groups including supraspinatus, upper and lower trapezius, deltoideus, teres minor, and levator scapulae were identified. The muscle technique was applied to the specifically affected muscle with no tension on band with a Y shape. Then, a correction technique for the protracted shoulder and a ligament technique for the overall shoulder were applied Dose: 24 hours per day Frequency of administration: standard 2 inch (5 cm) Kinesio Tex tape was applied once per week for 6 weeks. Each taping was removed after 4 to 5 days in situ Both groups ‐ Exercise plus cold pack Components of intervention: supervised and home exercises, including strengthening, flexibility (ROM) and Codman's pendulum exercises. Flexibility exercises were composed of posterior capsule with "cross‐body stretch", upper thoracic extension stretch, and active ROM stretching for glenohumeral joint for flexion and abduction. Strengthening exercises had 3 sets of 10 repetitions, using a 150 cm long precut section of Thera‐Band. The participants began exercising using the no‐latex yellow band at mild tension, and when able to perform 3 sets of 15 repetitions without significant pain or fatigue, they were progressed to the next colour‐resistive band in the sequence: red, green, and blue. Phase 1 emphasised the strengthening of the rotator cuff with avoidance of excessive upper trapezius activity and serratus strengthening. Shoulder elevation exercises were added in phase 2, and in phase 3, the subject was instructed to continue the exercises from phase 2 in addition to the new exercises such as push‐up on wall and push‐up plus with Thera‐Band. Cold pack gel application on the shoulder was recommended to control pain 5 times a day, especially before and after exercises Dose: total duration 1.5 hours Frequency of administration: once a week for 6 weeks (in clinic); daily at home |
|
| Outcomes | Outcomes assessed at 6 weeks
|
|
| Notes | Conflicts of interest: "No funding sources or conflicts of interest were reported for this study." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was designed according to the random case sample in SPSS program (SPSS, Chicago, IL). The SPSS software randomly assigned participants to one of the groups". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported all outcomes of interest to the review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Four participants of 30 from the manual therapy group (MT group) discontinued the study because of personal reasons. Two participants of 30 from the kinesiotaping group (KT group) left the study; 1 of them had severe skin irritation. The other did not like to use the tape throughout the study." Quote: "The subset of per‐protocol analysis is an “as‐treated” analysis in which only participants adherent to the intervention were included from all randomized participants by using baseline‐post‐intervention analysis." Comment: The amount of attrition is small, and while related to the intervention for one participant, is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Kromer 2013.
| Methods |
Study design: Parallel group RCT Setting: Referred from general practitioners or orthopaedic surgeons, Germany Intervention: Individualised manual physiotherapy plus individually adapted exercises Control: Individually adapted exercises alone Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 46; mean (SD) age: 50.1 ± 12.2 years; sex: F/M 22/24; mean (SD) duration of symptoms: 27.4 ± 28.4 weeks Control Number randomised: 44; mean (SD) age: 53.7 ± 9.9 years; sex: F/M 24/20; mean (SD) duration of symptoms: 40.8 ± 53.4 weeks |
|
| Interventions |
Intervention: individualised manual physiotherapy Components of intervention: painful and angular and/or translatory restricted peripheral joints were treated with manual glide techniques according to the concept of Kaltenborn. Comparable signs of the spine segments were treated with posterior‐anterior glides or coupled movements. Shortened muscles were stretched according to the description of Evjenth & Hamberg. Neural tissue was treated according to Butler. Treatment intensity was limited by pain of > 4/10. Subsequent treatment decisions were made with the help of an adapted clinical reassessment process based on the test‐retest principle described by Maitland Dose: initial duration of the glide techniques and the stretches was 20–30 seconds.Further dosage was based on reassessment results. Session duration was 20‐30 min Frequency of administration: 10 treatment sessions within 5 weeks, followed by exercise programme 3 times a week for 7 more weeks Control: individually adapted exercises alone Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: core exercise programme ‐ dynamic exercises started with 2 sets of 10 repetitions and with low resistance (yellow rubber band); shoulder and neck stretches were held for 10 seconds and repeated twice; Isometric scapular training positions were held for 10 seconds and repeated twice. If participants performed the core programme without problem, sets were increased from 2 to 3, repetitions (respectively seconds for the static exercises) were increased from 10 to 20, and in a last step, resistance was increased from the yellow to the red and to the green rubber band. Exercises from an 'additional programme' could be added if the participant could still perform the core programme without problems. Participants were instructed on how to perform each single exercise. They received a booklet with pictures and descriptions of the exercises and the individually defined dosage. Participants had to stop an exercise if they had pain of more than 3 out of 10 on a VNRS during the exercises or longer than approximately 30 seconds after they had stopped an exercise. Participants recorded performance and difficulties with the programme in their log books which enabled the therapist to check the 24‐hour effect of the programme and to make adaptations. If the total load of the programme was too provocative, participants were allowed to split the programme into 2 parts performing them at different times during the day. For some exercises an alternative version could be used (e.g. exercises C6b instead of C6a). If an exercise could not be performed due to pain, it was left out for the next 2 training sessions and was replaced by exercises AP1 and AP2. Contact time for the control group was 15–20 min Frequency of administration: participants performed the exercises twice a day for the first week, then once daily. Minimum exercises frequency during the week was 4, maximum 7. Thereafter both groups continued their exercise programme for 3 times a week for 7 more weeks |
|
| Outcomes | Outcomes assessed at 5 and 12 weeks and 1 year
|
|
| Notes |
Conflicts of interest: not reported Trial is registered in Current Controlled Trials (ISRCTN86900354) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients who fulfilled the eligibility criteria were asked to sign informed consent, they underwent baseline assessment and were subsequently allocated to treatment groups in blocks of 6 using central randomization via the internet." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "To guarantee allocation concealment, therapists received the information about patient allocation immediately before the first treatment by the Department of Epidemiology, Maastricht University". Comment: Central randomisation (i.e. an adequate method) was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the nature of the intervention it was impossible to blind therapist and participants. However, we blinded therapists for the control group to all clinical information about their patients." Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes (e.g. pain, function, global assessment) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Finally, 90 participants were randomly allocated, with 44 patients in the control group (IAEX) and 46 patients in the intervention group (IAEX + IMPT). At 5 weeks all patients were analysed with no loss to follow‐up. At 12 weeks 2 patients in the intervention group discontinued treatment, 1 without giving a reason, the other reported that treatment took too much effort." Quote: "After 1 year data were available for 87 patients; 44 patients in the IMPT group and 43 in the IEP group". Comment: Only three participants dropped out, one for reasons relating to the intervention. This small dropout rate is unlikely to have had a substantial impact on the results |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the clinical trial registry entry (Current Controlled Trials ISRCTN86900354) and published trial protocol |
| Other bias | Low risk | Comment: No other sources of bias identified |
Littlewood 2014.
| Methods |
Study design: Parallel group RCT Setting: Private physiotherapy clinic, UK Intervention: Self‐managed loaded exercise Control: Usual physiotherapy (might include advice, stretching, exercise, manual therapy, massage, strapping, acupuncture, electrotherapy, corticosteroid injection at the discretion of the treating physiotherapist) Source of funding: International Mechanical Diagnosis and Research Foundation (IMDTRF) |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 12; mean (range) age: 62.6 (46‐76) years; sex: F/M 7/5; mean (range) duration of symptoms: 29 (3‐120) months Control Number randomised: 12; mean (range) age: 63.9 (44‐79) years; sex: F/M 5/7; mean (range) duration of symptoms: 49 (3‐168) months |
|
| Interventions |
Intervention: self‐managed loaded exercise Components of intervention: the intervention was prescribed by the physiotherapist but completed by the participant independently. It involved exercising the affected shoulder against gravity, a resistive therapeutic band or hand weight over three sets of 10 to 15 repetitions completed twice per day. Exercise prescription was guided by symptomatic response requiring that pain was produced during exercise, but overall, symptoms were no worse upon cessation of that exercise. The exercise was prescribed and operationalised within a self‐managed framework which included focus upon knowledge translation, exercise/skill acquisition, self‐monitoring, goal setting, problem solving and pro‐active follow‐up Frequency of administration: mean number of treatment sessions was 3.9 (participants received a maximum of four funded sessions) Control: usual physiotherapy Components of intervention: might include a range of interventions including advice, stretching, exercise, manual therapy, massage, strapping, acupuncture, electrotherapy, glucocorticoid injection at the discretion of the treating physiotherapist Frequency of administration: mean number of treatment sessions was 7.6 (participants received a maximum of eight funded sessions) |
|
| Outcomes | Outcomes assessed at 3 months post‐treatment
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated randomisation sequence was produced by SJW in blocks of two and four to ensure an equal number of participants were randomised to each group". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The treating physiotherapists allocated participants to the self‐managed exercise or usual physiotherapy treatment group by selecting the next consecutively numbered sealed opaque envelope, which concealed the group allocation." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Design: A single‐centre pragmatic unblinded Parallel‐group RCT." Quote: "Similar to other RCTs of physiotherapy interventions, this trial was unblinded which introduces a potential source of bias. Although we initially proposed a double‐blind study, i.e. patient and hence outcome assessor, this was regarded as unacceptable by the ethics committee." Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes (e.g. pain, function, quality of life) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "100% retention was attained with all participants completing the SPADI at three months." Comment: There were no dropouts, losses to follow‐up or exclusions |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the published protocol of this trial |
| Other bias | Low risk | Quote: "The groups appeared well balanced at baseline except that the self‐managed exercise group reported higher baseline shoulder pain and disability via the SPADI and the usual physiotherapy treatment group reported a longer mean duration of symptoms (49 versus 29 months). This estimate is influenced by one participant who reported duration of 168 months. When the influence of this outlier was removed the revised estimate of mean duration of symptoms was 37 months for the usual physiotherapy group." Comment: There was some baseline imbalance in SPADI score and duration of symptoms, though it is unlikely to have had an impact on the results |
Lombardi 2008.
| Methods |
Study design: Parallel group RCT Setting: Outpatients attending clinics of the Federal University of São Paulo, Brazil Intervention: Progressive resistance training programme for the musculature of the shoulder Control: Waiting list control Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 30; mean (SD) age: 56.3 ± 11.6 years; sex: F/M 21/9; mean (SD) duration of symptoms: 13.7 ± 9.6 months Control Number randomised: 30; mean (SD) age: 54.8 ± 9.4 years; sex F/M 25/5; mean (SD) duration of symptoms: 13.9 ± 9.3 months |
|
| Interventions |
Intervention: training programme Components of intervention: progressive resistance training programme. The exercises were flexion, extension, medial rotation and lateral rotation of the shoulder. Participants underwent a muscle strength assessment using a repetition maximum (RM) exercise in which participants performed 6 repetitions with the maximum bearable weight thereby determining the 6‐repetition maximum (6 RM). Once the 6 RM load was determined, training was divided into: 2 series of 8 repetitions, the first series with 50% of the 6 RM and the second series with 70% of the 6 RM, respecting the participant's pain threshold. The exercise was interrupted if the participant felt pain and performed another movement. Between the first and second series, there was a resting period of 2 min. The speed of movement was 2 seconds for both the eccentric and concentric phases. The 6 RM load was re‐evaluted every 2 weeks. Multipulley muscle‐building equipment was used for the exercises. To strengthen the flexors of the shoulder, the participant was positioned with his or her back to the equipment and the elbow flexed at 90 degrees; the participant performed the flexion movement of the shoulder from 0–90 degrees. In the extensor strengthening exercise, the participant faced the equipment with the elbow flexed at 45 degrees and the shoulder at 60 degrees of flexion and 30 degrees of extension. In the strengthening of the medial and lateral rotators, the participant was positioned alongside the equipment with the elbow flexed at 90 degrees; for the medial rotation, the participant started at 45 degrees of lateral rotation and moved to 45 degrees of medial rotation; for the lateral rotation, the participant began the movement at 45 degrees of medial rotation and moved to 30 degrees of lateral rotation Frequency of administration: twice a week for a period of 8 weeks Control: no training programme Components of intervention: participants remained on a waiting list and were informed that they would receive physiotherapeutic treatment after 2 months had passed Both groups Components of intervention: 750 mg of acetaminophen every 8 hours when experiencing pain. In cases where the pain surpassed 7 on the visual pain scale, the participant could take 50 mg of diclofenac every 8 hours until the pain reached a 5 on the pain scale. This was done at the participant's discretion |
|
| Outcomes | Outcomes assessed at 2 months
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization list was utilized to randomly allocate patients into experimental and control groups and a concealed randomization with an opaque sealed envelope was performed." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated randomization list was utilized to randomly allocate patients into experimental and control groups and a concealed randomization with an opaque sealed envelope was performed." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported the SPADI |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Evaluations were carried out at the beginning and end of the treatment program by the same blinded examiner for both groups". Comment: Assessor of objective outcomes was likely blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In cases of interruption or abandonment of treatment, the data was analyzed as intent‐to‐treat." Quote: "Sixty patients were randomly assigned to the experimental and control groups, with 30 patients in each group. Four patients from the control group failed to finish the study: 1 who started having difficulties appearing at the rehabilitation center but appeared for the final evaluation, and 3 who failed to return for the final evaluation, stating difficulties appearing at the evaluation locale. Data from the prior evaluation of the patients from the control group were used for the intent‐to‐treat analysis." Comment: The amount and reasons for dropout are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Ludewig 2003.
| Methods |
Study design: Parallel group RCT Setting: Construction workers recruited through local unions Intervention: Home exercise programme of 5 shoulder stretching and strengthening exercises Control: No treatment Source of funding: Center to Protect Worker’s Rights, the Public Health Service, and the University of Iowa, USA (grant # U60/CCU317202) |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 34; mean (SD) age: 48 (1.8) years; sex: men only; duration of symptoms: not reported Control Number randomised: 33; mean (SD) age: mean: 49.2 (1.8) years; sex: men only; duration of symptoms: not reported |
|
| Interventions |
Intervention: home exercise programme Components of intervention: two stretches (pectoralis minor stretch and posterior shoulder stretch), a muscle relaxation exercise for the upper trapezius performed in front of a mirror, and progressive resistance strengthening exercises for two muscle groups (serratus anterior muscle and humeral external rotation). Participants received instructions from a licensed therapist. Each subject received written/pictorial instructions for home reference and a daily exercise log to monitor compliance Dose: stretches (30 seconds each repetition and 5 repetitions daily); muscle relaxation exercise (5 times daily); progressive resistance strengthening exercises (3 sets of 10 repetitions the first week, progress to 3 sets of 15 repetitions the second week and three sets of 20 days the third week, after which, participants were to continue increasing weight resistance and repeat the repetition sequence) Frequency of administration: daily for 10 weeks Control: no intervention |
|
| Outcomes | Outcomes assessed at an average of 10 weeks (between 8‐12 weeks)
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by an investigator blindly selecting one of two slips of paper indicating group assignment." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: It is unclear if adequate safeguards were put in place to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Researchers were not blinded to group assignment, but were to baseline measurements at the time of follow up." Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Ninety two per cent of subjects completed the study. Seven subjects were lost to follow up, four (11.8 %) in the exercise intervention group, and three control subjects (one symptomatic (3%) and two asymptomatic (8%)). One intervention subject withdrew after experiencing a new injury at work that interfered with continuation of the exercises. Another intervention subject was referred by his physician for additional outpatient physical therapy and subsequently withdrew from the study. A third intervention and one symptomatic control subject were not able to return for follow up for personal reasons (death in the family, custody dispute). The remaining three subjects either were no shows or were unable to be reached after multiple attempts at the time of post‐test. Subjects lost to follow up were similar to the full sample with regard to demographic characteristics." Quote: "The initial analysis included all subjects from whom post‐test data were obtained, regardless of their level of compliance with the exercise programme. A secondary complete “intention to treat” analysis was also performed where all subjects initially enrolled were analysed. Missing post‐test data were replaced with imputed values based on the average observed means from the two symptomatic groups." Comment: The amount and reasons for attrition are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other source of bias were identified |
Maenhout 2013.
| Methods |
Study design: Parallel group RCT Setting: Shoulder surgery clinic, Belgium Intervention: Heavy load eccentric training plus traditional rotator cuff training Control: Traditional rotator cuff training Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 31; mean (SD) age: 40.2 (12.9) years; sex: F/M 16/15; duration of symptoms: not reported Control Number randomised: 30; mean (SD) age: 39.4 (13.1) years; sex: F/M 20/10; duration of symptoms: not reported |
|
| Interventions |
Intervention: heavy load eccentric training Components of intervention: eccentric exercise consisted of full can (thumb up) abduction in the scapular plane, which was performed with a dumbbell weight. Participants were asked to perform the eccentric phase at a speed of 5 min/repetition. Starting position of the eccentric phase at full scapular abduction had to be pain free, and, if not, participants were advised to stretch out the arm at a slightly lower degree of scapular abduction Dose: based on pain monitoring model. Whenever the pain was no longer present during the last set of repetitions, dumbbell weight was increased with 0.5 kg Frequency of administration: 3 sets of 15 repetitions performed twice a day, at home for 12 weeks Control: traditional rotator cuff training Participants only received the treatment common to both groups (see below) Both groups Components of intervention: performed two traditional rotator cuff strengthening exercises at home that involved internal and external rotation resisted with an elastic band (Thera‐Band). Participants were instructed to perform the exercises at a speed of 6 min'/repetition (2 min concentric phase, 2 min isometric phase and 2 min eccentric phase). The colour of the band was chosen so that the participant did not experience significantly more pain during the exercise than at rest. Load was increased by changing colour of the elastic band as soon as pain decreased. In addition, physiotherapy treatment sessions aimed at correcting performance of the exercises, increasing load and emphasising the importance of adherence to the home exercises were delivered. Treatments included glenohumeral mobilisation, scapulothoracic mobilisation, scapula setting and posture correction Frequency of administration: exercises ‐ 1 per day for 3 sets of 10 repetitions, performed at home for 12 weeks. Nine physiotherapy treatments delivered over 12 weeks |
|
| Outcomes | Outcomes assessed at 6 and 12 weeks
|
|
| Notes |
Conflicts of interest: not reported Trial was registered in ClinicalTrials.gov (NTC00782522) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Prior to the intervention, baseline outcome measurements were performed. Subsequently, patients were randomly allocated to the traditional rotator cuff strength training (TT) group or the TT combined with heavy load eccentric training (TT + ET) group." Comment: No information on the method used to generate the allocation sequence was reported (in either the registry entry or publication) |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on the method used to conceal the allocation sequence was reported (in either the registry entry or publication) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes (e.g. pain, function, treatment success) |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "All tests were completed at the laboratory of the Department of Rehabilitation Science and Physiotherapy of Ghent University. This investigator could not be blinded to treatment group." Comment: The assessor of objective outcomes was not blinded and may have assessed outcomes for each group differently based on prior expectations of the benefits of each intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Intention to treat principle was respected, and all patients were included in analysis as randomized." Comment: Trialists report that an intention‐to‐treat analysis was performed, but data for participant‐reported treatment success were reported based on per‐protocol analysis (and it is unclear what sample size other outcomes were based on in the analysis). A CONSORT flow chart (Schultz 2010) shows that more participants in the control group dropped out at week 12 due to "No improvement". This attrition is likely to underestimate the difference between groups in participant‐reported treatment success (i.e. bias in favour of the control group). Other outcomes may also be affected if not based on an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Comment: Some outcomes specified in the registry entry (NTC00782522) were not reported in the publication (ROM, force reproduction, subacromial space). Also, patient‐reported treatment success was not pre‐specified in the registry entry |
| Other bias | Low risk | Comment: No other sources of bias identified |
Martins 2012.
| Methods |
Study design: Randomised, prospective and comparative trial Setting: Rehabilitation Centre, Brazil Intervention: Proprioception exercises plus stretching and strengthening exercises plus cryotherapy Control: Stretching and strengthening exercises plus cryotherapy Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 9; age: 30 ≤ 40 years (n = 0); 41 ≤ 50 years (n = 5); > 50 years (n = 3); sex: F/M 7/1; duration of symptoms: not reported Control Number randomised: 9; age: 30 ≤ 40 years (n = 2); 41 ≤ 50 years (n = 2); > 50 years (n = 4); sex: F/M 7/1; duration of symptoms: not reported |
|
| Interventions |
Intervention: proprioception exercises Components of intervention: exercises with joint position, rhythmic stabilisation and repositioning of the members, unstable base, proprioceptive neuromuscular facilitation, and speed and accuracy Dose: resistance during strength exercises was increased every 3 sessions Frequency of administration: 2 sessions per week for 6 weeks Control: stretching and strengthening exercises plus cryotherapy Components of intervention: participants only received the treatment common to both groups (see below) Both groups Components of intervention: Codman's pendulum exercises of the shoulder, stretching of the cervical spine and shoulder muscles, exercises with a stick (to maintain or improve ROM), exercises to strengthen the muscles of the rotator cuff and scapular stabilisers, cryotherapy (ice pack for 20 min, performed at the end of the treatment session), and education regarding joint protection and posture Frequency of administration: 2 sessions per week for 6 weeks |
|
| Outcomes | Outcomes assessed at 6 weeks
|
|
| Notes |
Conflicts of interest: not reported Trial registration ClinicalTrials.gov NCT01465932 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All subjects were then randomized and allocated to Group 1 (control) or Group 2 (experimental) by a person not participating in the application of data collection instruments and/or rehabilitation programs." Quote: "In order to promote greater homogeneity with the clinical status of the patients, subjects were initially subdivided according to the presence or absence of shoulder movement deficits (measurement of shoulder ROM with a goniometer) and the level of pain intensity, as shown by the Visual Numeric Scale (VNS)...The randomization occurred so that the first subject of each subgroup was randomly assigned to one group and the second subject was assigned to the other group, and so on." Comment: The specific method used to generate the allocation sequence is unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All subjects were then randomized and allocated to Group 1 (control) or Group 2 (experimental) by a person not participating in the application of data collection instruments and/or rehabilitation programs." Comment: The specific method used to conceal the allocation sequence was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different multimodal interventions, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported all outcomes, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 9 participants were randomly allocated to each group, and one dropped out of each group, both for the same reason (lack of commitment to rehabilitation programme). This is unlikely to have biased the results |
| Selective reporting (reporting bias) | High risk | Comment: Mean (SD) data for pain were reported for each group by subgroup only (mild, moderate, severe). No measures of variation were reported for the health‐related quality‐of‐life measure. Pain had not been pre‐specified in the clinical trials registry entry |
| Other bias | Low risk | Comment: No other sources of bias identified |
Marzetti 2014.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic, Italy Intervention: Neurocognitive therapeutic exercise Control: Traditional therapeutic exercise Source of Funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Shoulder impingement syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 24; mean age: 61.6 ± 11.2 years old; sex: F/M 12/12; duration of symptoms: not reported Control Number randomised: 24; mean age: 62.6 ± 13.9 years old; sex: F/M 15/9; duration of symptoms: not reported |
|
| Interventions |
Intervention: neurocognitive therapeutic exercise (NCTE) Components of intervention: the neurocognitive protocol contained 10 exercises. The first 3 aimed at restoring shoulder fragmentation and counterbalance; the second set consisted of 4 exercises aimed at centring the humeral head in the glenoid fossa during active movements and introducing counterbalancing mechanism of the scapula during upper limb movements; the last 3 exercises aimed at recovering maximum range of movement of the affected shoulder. Exercises involving specific instruments (e.g. inclined table with a board with 5 concentric circles) were taught to promote the stimulation of higher cortical functions useful to select the most important proprioceptive information necessary to organise the motor behaviour and recover fine motor skills. The execution of the exercises was facilitated by using motor imagery Dose: duration 1 hour Frequency of administration: 3 times a week for 5 weeks Control: traditional therapeutic exercise (TTE) Components of intervention: the traditional therapeutic exercise protocol contained mainly strengthening exercises focused on the rotator cuff and scapular stabilising muscles, stretching exercises, Codman's pendulum exercises and exercises against elastic band resistance Dose: duration 1 hour Frequency of administration: 3 times a week for 5 weeks |
|
| Outcomes | Outcomes assessed at 5, 12 and 24 weeks
|
|
| Notes |
Conflicts of interest: not reported Trial registered in ClinicalTrials.gov (NCT01785745) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to either NCTE (group 1) or TTE (group 2) using a random sequence generator (www.random.org)" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation sequence was performed using closed envelopes, and the assignment code of each patient revealed to the researcher who performed the treatment only at the beginning of the therapeutic protocol". Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Single‐blind randomized, non‐inferiority trial." Quote: "Outcome measures were determined by an assessor blinded to patient allocation." Comment: It can be inferred from the above quotes that the trialists considered participants to be unblinded. However, it is unclear whether participants were provided with any information that would make them perceive the type of exercise they received as superior or inferior to the alternative type of exercise |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported some outcomes, but it is unclear whether they were provided with any information that would make them perceive the type of exercise they received as superior or inferior to the alternative type of exercise |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Outcome measures were determined by an assessor blinded to patient allocation." Quote: "As a check on blindness, the assessor was asked to guess treatment allocation after the final outcome assessments were completed. The analysis of these guesses showed a correctness of approximately 30%, which is considered not better than chance" Comment: Assessor of objective outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Missing data at follow‐up were managed by the Last Observation Carried Forward (LOCF) method. Analyses were performed according to the intention‐to‐treat principle" Quote: "All of the participants completed the treatment protocol. Two participants in the NCTE group and three in the TTE group did not attend the follow‐up visit at T2. Two participants in the TTE group did not attend the follow‐up visit at T3." Quote: "...reasons for lack of follow‐up were not recorded. However, only a few participants were lost at follow‐up (10.4%) and dropouts occurred to a similar extent in the two treatment groups, which did not substantially affect the results" Comment: No reasons for loss to follow‐up were reported, but the amount is small and relatively balanced between groups, so is unlikely to have biased the results. All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data fully reported for all outcomes specified in the ClinicalTrials.gov registry entry |
| Other bias | Low risk | Comment: No other sources of bias identified |
McClatchie 2009.
| Methods |
Study design: Cross‐over RCT Setting: Private orthopaedic practice, Canada Intervention: Lateral cervical glide mobilisations Control: Placebo mobilisation Source of funding: Financial support provided by the Graduate Department of Rehabilitation Science, University of Toronto, Toronto, Canada |
|
| Participants |
Diagnostic label used by trialists: Painful arc Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 21 (7 mobilisation group, 14 placebo group in first period); mean (SD) age: 49.8 (9.8) years old; sex: F/M 14/7; duration of symptoms: not reported |
|
| Interventions |
Intervention: lateral cervical glide mobilisations Components of intervention: participant was seated with the thoracic spine resting against the back of the chair, head in a neutral position, feet resting flat on the floor, and arms relaxed with hands in their lap. The lateral aspect of the spinous processes of C5, C6, and C7 was landmarked on the ipsilateral side of the participant's painful shoulder. The examiner's thumb remained on the lateral aspect of the spinous process of C5, with the opposite hand placed on the participant's non‐affected shoulder or head for counterbalance as a lateral movement toward the non‐painful side was applied with the mobilising hand Dose: 2 min each at C5, C6 and C7, with small amplitude end range movements (Grade IVþ) Frequency of administration: once, within 4 days of the cross‐over treatment Control: placebo mobilisation: Components of intervention: involved the examiner resting their hands in the same positions as the mobilisation technique, but without the application of external force Dose: hands held at C5, C6 and C7 for 2 min each Frequency of administration: once, within 4 days of the cross‐over treatment |
|
| Outcomes | Outcomes assessed between 1 and 4 days
Note that active cervical spine ROM was also measured but was not extracted as we were only interested in shoulder ROM |
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized by a coin toss..." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each subject recognized that the mobilization and placebo interventions were different from each other, however, no subject realized that the placebo intervention was not therapeutic." Comment: Participants were likely blind to the intervention they received at each session |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All outcome measures were assessed both before and after the intervention and conducted by the first investigator, who was blinded to which treatment intervention was received." Comment: Assessors of objective outcomes were likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no loss to follow‐up. and all randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Moosmayer 2014.
| Methods |
Study design: Parallel group RCT Setting: Recruited by general practitioners and referred to orthopaedic surgeon Intervention: Physiotherapy comprising exercises only Control: Surgery Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Full rotator cuff tear Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above):
Baseline characteristics Intervention Number randomised: 51; mean (range) age: 61 (46‐75) years; sex: F/M 15/36; mean (SD) duration of symptoms: 9.8 (9.8) months Control Number randomised: 52; mean (range) age: 59 (44‐75) years; sex: F/M 15/37; mean (SD) duration of symptoms: 12.3 (18.7) months |
|
| Interventions |
Intervention: physiotherapy Components of intervention: supervised exercises only, with particular attention directed towards correction of upper quarter posture and restoration of scapulothoracic and glenohumeral muscular control and stability. Local glenohumeral control was addressed by exercises to centre the humeral head in the glenoid fossa. Isometric exercises and exercises against eccentric and concentric resistance for shoulder rotators were given. When local glenohumeral control was achieved, exercises were given with increasing loads and progressed from neutral to more challenging positions. During all exercises, scapular stability had to be maintained. Additional exercises were given for specific demands in work, sports and leisure activities. Twelve of 51 participants initially randomised to physiotherapy underwent tendon repair surgery during the 5‐year follow‐up, but were analysed in the group to which they were allocated. Dose: 40 min Frequency of administration: 20 sessions given on average twice weekly for 12 weeks and with increasing intervals during the following 6–12 weeks Control: surgery Components of intervention: tendon repair surgery was performed in a standard manner by mini‐open (9 participants) or open (42 participants) tendon repair. All were performed in the deck‐chair position under interscalene block regional anaesthesia and total intravenous anaesthesia without the use of inhalation agents, by one of three experienced orthopaedic surgeons. Following diagnostic arthroscopy and through a deltoid splitting approach, an anteroinferior acromioplasty was performed. With the arm at the side, the rotator cuff was mobilised until the tear was fully exposed. The footprint was prepared to bleeding bone and tendon repair performed with a combination of tendon‐to‐tendon and tendon‐to‐bone techniques by passing sutures through bone tunnels in the greater tuberosity. The deltoid was repaired to the acromion through drill holes. Tenodesis of the long head of biceps tendon was performed in 18 participants in whom arthroscopy had shown inflammation of a partial tear. Mini‐open tendon repair differed from open repair by a shorter incision and arthroscopic acromioplasty. Post‐operatively the arm was mobilised in a sling and passive range‐of‐movement exercises commenced. Active‐assisted movements where initiated after 6 weeks, and supplemented by strengthening exercises 12 weeks after surgery |
|
| Outcomes | Outcomes assessed at 6 and 12 months, 2 years and 5 years
|
|
| Notes | Conflicts of interest: the authors state "Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other nonprofit organisation with which one or more of the authors are associated." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomisation list (block length 20, ratio 1:1) was drawn up by our statistician." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered, sealed envelopes were used to assign treatment according to the participants’ study number, given at baseline assessment. The randomisation sequence was concealed from the study’s collaborators until treatment was assigned." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Only the outcome assessor remained blinded throughout the study." Comment: Assessor of objective outcomes was likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In order to avoid interpretation bias and loss of power it was decided to perform all outcome analyses 'as randomised' by following an intention‐to‐treat principle. As a consequence, results from patients who withdrew (one from the surgery group) and those who changed treatment (nine from the physiotherapy group) were assessed as originally randomised. For the patient who withdrew from the surgery group, the baseline data carried forward gave him a result far below the mean of his group. The nine patients who changed treatment after failed physiotherapy had all performed at least 15 treatment sessions, and it seemed adequate to interpret their final score pre‐operatively as the best estimate for the final result from physiotherapy. Following these patients without further treatment would have been unethical, and elimination of their results from analysis would have led to an overestimation of the effect of physiotherapy." Quote: "Twelve nonoperative patients reported an insufficient treatment effect. This subgroup had a mean increase of the Constant score of 1.8 points (range, 220 to 22 points) and underwent secondary surgery within the first two years (three patients during the first six months, six patients during the following six months, and three patients during the second year)." Quote: "The five‐year follow‐up rate was 98%." Comment: An appropriate method was used to deal with participants who did not remain in their allocated group. Twelve of 51 participants initially randomised to physiotherapy underwent tendon repair surgery, but were analysed in the group to which they were allocated. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Munday 2007.
| Methods |
Study design: Parallel group RCT Setting: General population, South Africa (participants recruited through local advertising, flyers placed in general practitioners' and chiropractors' offices, and around universities, colleges, schools and businesses) Intervention: Shoulder girdle adjustments (chiropractic) Control: Detuned ultrasound (placebo) Source of funding: Masters in Technology: Chiropractic (Durban University of Technology) |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall Sex: F/M 14/16 Intervention Number randomised: 15; mean (range) age: 22 (16‐38) years; sex: not reported; duration of symptoms: not reported Control Number randomised: 15; mean (range) age: 23 (19‐32) years; sex: not reported; duration of symptoms: not reported |
|
| Interventions |
Intervention: shoulder girdle adjustments Components of intervention: high velocity, low‐amplitude manipulation in the direction of restricted end feel or joint play was performed. Participants sat in a comfortable position with the shoulder girdle exposed. Adjustments to the acromioclavicular joint were most common, although adjustments to the ribs, scapula and glenohumeral joints were made as well. The spine was not adjusted in this trial Frequency of administration: 8 sessions over 3 weeks Control: placebo (detuned ultrasound) Components of intervention: participants were asked to sit in a comfortable position with the shoulder exposed while they received detuned ultrasound Dose: no frequency or times. Duration 6 min Frequency of administration: 8 sessions over 3 weeks |
|
| Outcomes | Outcomes assessed at 3 and 7 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was accomplished by utilising 30 folded sheets of paper (15 marked Group A, 15 marked Group B), thoroughly mixed together to assure discontinuity and then placed in a hat. At each subject randomisation time point, the hat was held so that all folded slips were completely obscured." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "At each subject randomised time point, the hat was held so that all folded slips were completely obscured." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were made aware that they might be randomized into either group (treatment or placebo) A or B. At the end of the trial, those in the placebo group were offered up to 8 free treatments". Comment: Patients were likely blinded to treatment (i.e. unaware that the ultrasound machine was not switched on) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Four patients were excluded due to either non‐compliance with treatment protocols or exclusion factors. Additional patients were recruited and randomised to achieve a total of 30 patients (out of N=34) completing the trial. Intention to treat analysis was used." Comment: The flow of participants throughout the trial is unclear |
| Selective reporting (reporting bias) | High risk | Comment: No outcome data were reported for ROM, which was specified as an outcome in the methods section |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Osteras 2008.
| Methods |
Study design: Parallel group RCT Setting: Primary care and orthopaedic surgeon clinics Intervention: High dose medical exercise therapy Control: Low dose medical exercise therapy Source of funding: No funding |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 31; mean (SD) age: 46.1 ± 11.2 years; sex: F/M: 66.9%/33.1%; mean (SD) duration of symptoms: 3.6 ± 5.1 years Control Number randomised: 30; mean (SD) age: 41.8 ± 14.5 years; sex: F/M 74%/26%; mean (SD) duration of symptoms: 3.1 ± 4.3 years |
|
| Interventions |
Intervention: high dose exercise Components of intervention: supervised high dose progressive resistance exercise therapy, comprising global aerobic exercises using a stationary bike, a treadmill, or a step machine, and semiglobal and local exercises using such medical exercise therapy equipment as wall pulley apparatus, lateral pulley apparatus, inclines board, angle bench, multiple purpose bench, shoulder rotator, dumbbells or barbells. The participants' exercise programme was graded in such a way that it was performed pain free, or close to pain free (a maximum of 3 on a 10‐point VAS) Dose: participants performed eight exercises, each of 3 sets of 30 repetitions. Prior to the semiglobal and local exercises participants warmed up for 15–20 min on an ergometer cycle. Half way through the exercise programme (4 exercises each of 3 sets of 30 repetitions) the participants cycled for 10 min. After the last 4 exercises, the participants did another 10 min' stationary ergometer cycling. The intensity during cycle exercises was moderate to high (i.e. a heart rate frequency of 70%–80% of the maximal heart rate) Frequency of administration: 3 treatments a week for 12 weeks Control: low dose exercise Components of intervention: same components as the high dose exercise group, but at a lower dose (see below) Dose: participants started each treatment with 5–10 min on an ergometer cycle and then performed 5 semiglobal and local exercises using medical exercise therapy equipment performing 2 sets of 10 repetitions of each exercise. The intensity during the cycle exercises was moderate to high (i.e. a heart rate frequency of 70%–80% of the maximal heart rate). The crucial difference between the groups were time on the bike (35 min in the high dose group compared to 10 min in the low dose group), number of exercises (8 compared to 5), and number of repetitions (3 times 30 compared to 2 times 10 per exercise) Frequency of administration: 3 treatments a week for 12 weeks |
|
| Outcomes | Outcomes assessed at 3, 9 and 15 months
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization procedure was concealed from the experimenters and treating physiotherapist. The envelopes containing numbers regarding HD versus LD were randomly drawn from a basket and kept in a locked place." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization procedure was concealed from the experimenters and treating physiotherapist. The envelopes containing numbers regarding HD versus LD were randomly drawn from a basket and kept in a locked place." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (high intensity versus low intensity exercise), participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "The outcome measurements were not obtained by a blinded assessor" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "92% of patients completed the study. Five patients were lost to post‐test; two in the HD group (3%) and three in the LD group (5%). In the HD group, one patient moved away from the city and was therefore unable to keep in touch, and the second HD subject withdrew after experiencing a new injury at work that interfered with the continuation of the exercise treatment. In the LD group, one subject was referred by his physician for additional outpatient therapy and therefore withdrew from the study. The two last patients in the LD interventions were not able to return for the post‐test for personal reasons." Comment: The amount and reasons for attrition are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Rhon 2014.
| Methods |
Study design: Parallel group RCT Setting: Military hospital–based outpatient clinic, USA Intervention: Manual physical therapy (joint and soft tissue mobilisations, manual stretches, contract‐relax techniques, supervised exercises) plus home exercises Control: Glucocorticoid injection plus home exercises Source of funding: Cardon Rehabilitation Products through the American Academy of Orthopaedic Manual Physical Therapists |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 52; mean (SD) age: 40 ± 12 years; sex: F/M: 17/29; mean (SD) duration of symptoms: 4.9 ± 4.4 months Control Number randomised: 52; mean (SD) age: 42 ± 12 years; sex: F/M: 14/38; mean (SD) duration of symptoms: 6.5 ± 13.9 months |
|
| Interventions |
Intervention: manual physical therapy Components of intervention: the manual physical therapy intervention consisted of a combination of joint and soft‐tissue mobilisations; manual stretches; contract–relax techniques reinforcing exercises directed to the shoulder girdle or thoracic or cervical spine. Participants did not receive identical treatments, but the manual physical therapy techniques were matched to individual impairments identified on examination. Home exercises (including wand ROM exercises, scapular retraction, scapular protraction, thoracic self‐mobilisation, butterfly stretch) were prescribed to reinforce clinic interventions Dose: 30 min (manual physical therapy); 2‐3 times per day (home exercises) Frequency of administration: twice weekly over a 3‐week period Control: glucocorticoid injection Components of intervention: injected 40 mg of triamcinolone acetonide to the subacromial space of the symptomatic shoulder. Participants also received printed instructions to perform a gentle gravity‐assisted distraction and oscillatory pendulum exercise Dose: the physician spent approximately 30 min with each subject explaining the rationale for the injection, relevant anatomy, performing the procedure and reviewing the pendulum exercises Frequency of administration: as many as three total injections could be administered by the study physician (1 month apart) during the 1‐year period |
|
| Outcomes | Outcomes were assessed at baseline, 1 month, 3 months, 6 months, and 1 year
|
|
| Notes |
Conflicts of interest: the authors stated that they had no conflicts of interest Trial registered in ClinicalTrials.gov (NCT01190891) and trial protocol published in BMJ Open |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization schedule was computer‐generated" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization schedule was computer‐generated, with assignments placed in opaque, sequentially numbered envelopes by an off‐site investigator not involved with patient care or follow‐up. Treatment allocation was revealed after collection of baseline outcomes" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients and treating clinicians were not blind to the intervention." Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "The research assistant who collected outcome assessments at each time point was blind to group assignment." Comment: Despite having a blinded research assistant record patients' responses, unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Most patients (96%) returned for follow‐up visits at 1 year." Quote: "The primary analyses of effectiveness included all available data from patients who received their assigned treatment (that is, the CSI or at least 1 session of MPT). We used a linear mixed‐effects model, which is flexible in accommodating data assumed to be missing at random with data from 5 time points (0, 1, 3, 6, and 12 months) for the SPADI (primary outcome) and NPRS and 4 time points for GRC. The intervention (MPT or CSI) was the fixed effect with random effects for the repeated measures over time within a patient; the primary treatment comparison was the difference between groups from baseline to 1 year. For the sensitivity analysis to explore the effect of missing data, they were imputed for the 3 outcome variables at all follow‐ups (20 imputations using MULTIPLE IMPUTATION‐FULLY CONDITIONAL SPECIFICATION)" Quote: "We performed a sensitivity analysis with imputation for missing data and the results remained unchanged." Comment: Attrition was dealt with using an appropriate method |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes pre‐specified in the trial protocol |
| Other bias | Low risk | Comment: No other sources of bias identified |
Senbursa 2007.
| Methods |
Study design: Parallel group RCT Setting: Outpatient clinic of Physiotherapy Rehabilitation, Hacettepe University, Ankara, Turkey Intervention: Manual therapy programme (12 clinic‐run sessions of joint and soft tissue mobilisation, ice, stretching and strengthening exercise programmes and education 3 times per week) Control: Self‐training programme (active ROM, strengthening and stretching exercise programme 7 times a week) Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 15; mean (SD) age: 48.1 (7.5) years; sex: not reported; duration of symptoms: not reported Control Number randomised: 15; mean (SD) age: 49.5 (7.9) years; sex: not reported; duration of symptoms: not reported |
|
| Interventions |
Intervention: manual therapy Components of intervention: joint and soft tissue mobilisation: deep friction massage on supraspinatus muscle tendon, radial nerve stretching, scapular mobilisation, glenohumeral joint mobilisation, proprioceptive neuromuscular facilitation techniques including rhythmic stabilisation and hold‐relax. An education programme, ice application, stretching and strengthening exercise programme and home training were also performed. The stretching and strengthening exercise programme was supervised by a physiotherapist and a shoulder exercise brochure also provided instructions. Home training was self‐delivered with an elastic Thera‐band Dose: not reported Frequency of administration: 12 sessions in total, completed in sessions 3 times per week for 4 weeks Control: self‐training: Components of intervention: an active ROM, stretching and strengthening exercise programme including rotator cuff muscles, rhomboids, levator scapulae and serratus anterior which was self‐administered at home using an elastic band at home after being taught by a physiotherapist Dose: 10–15 min Frequency of administration: 7 times a week for 4 weeks |
|
| Outcomes | Outcomes assessed at 3 months
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The short‐term clinical effectiveness of manual physical therapy compared with usual care was assessed in a randomized clinical trial" Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (one delivered by physiotherapist, other delivered at home), participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no attrition reported, and outcome data were reported as based on the number of participants randomised |
| Selective reporting (reporting bias) | High risk | Comments: No outcome data for the Neer function tests scores and strength outcomes were reported, despite being listed as outcomes in the methods section of the trial report. Also, without a trial protocol it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Senbursa 2011.
| Methods |
Study design: Parallel group RCT Setting: Hacettepe University, Physiotherapy and Rehabilitation Department, Turkey Intervention: Joint and soft tissue mobilization plus supervised exercise programme Control 1: Supervised exercise programme Control 2: Home‐based exercise programme Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Supraspinatus tendinopathy or Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 30; mean (SD) age: 50.5 ± 10.6 years old; sex: not reported; duration of symptoms: not reported Control 1 Number randomised: 25; mean (SD) age: 48.2 ± 7.9 years old; sex: not reported; duration of symptoms: not reported Control 2 Number randomised: 22; mean (SD) age: 48.0 ± 9.0 years old; sex: not reported; duration of symptoms: not reported |
|
| Interventions |
Intervention: joint and soft tissue mobilisation Components of intervention: deep friction massage on the supraspinatus muscle, radial nerve stretching, scapular mobilisation, glenohumeral joint mobilisation, and proprioceptive neuromuscular facilitation techniques Dose: not reported Frequency of administration: 3 times per week for 12 weeks Control 1: supervised exercises Components of intervention: ROM, stretching and strengthening exercises for the rhomboid, levator scapulae, serratus anterior and rotator cuff muscles supervised and at home Dose: 3 sets of 10 repetitions Frequency of administration: 3 times per week with a physio and self‐administered daily for 12 weeks Control 2: home exercises Components of intervention: ROM, stretching and strengthening exercises for the rhomboid, levator scapulae, serratus anterior and rotator cuff muscles at home only Dose: 3 sets of 10 repetitions Frequency of administration: daily for 12 weeks |
|
| Outcomes | Outcomes assessed at 4 and 12 weeks
|
|
| Notes | Conflicts of interest: the authors declared that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly assigned by the SPSS software to one of three consecutive treatment groups". Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients underwent rehabilitation for 12 weeks" Comment: There was no attrition reported, and outcome data were reported as based on the number of participants randomised |
| Selective reporting (reporting bias) | High risk | Comment: No outcome data were reported for several outcomes (e.g. ROM and strength); the authors only report that there was no statistically significant difference between groups |
| Other bias | Low risk | Comment: No other sources of bias identified |
Struyf 2013.
| Methods |
Study design: Parallel group RCT Setting: Private medical clinics or private physiotherapy practices, Belgium Intervention: Scapular‐focused treatment (stretching and scapular motor control training) Control: Stretching, muscle friction and eccentric rotator cuff training, plus therapeutic ultrasound Source of funding: This study was financially supported by MSD Europe bvba, Nijverheidsstraat 18, Londerzeel, Belgium, and by research grant (G842) supplied by the Department of Health Sciences, Artesis University College Antwerp, Antwerp, Belgium. The material used in this study was provided by MSD Europe bvba |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 10; mean (SD) age: 45.4 ± 15.1 years; sex: F/M 5/5; duration of symptoms: not reported Control Number randomised: 12; mean (SD) age: 46.2 ± 13.5 years; sex: F/M 7/5; duration of symptoms: not reported |
|
| Interventions |
Intervention: scapular‐focused treatment Components of intervention: manual mobilisations, stretching and motor control training of the scapula, including: passive manual mobilisation (to improve passive scapular upward rotation and posterior tilting), home stretching exercises for the levator scapulae and rhomboids muscles, stretching of the pectoralis minor muscle length by the physiotherapist and scapular motor control training with emphasis on a scapular orientation exercise (SOE) Scapular orientation exercises completed with 10 repetitions, once per day Dose: 30‐min session Frequency of administration: 9 sessions in total, delivered between 1 and 3 times per week (depending on practical issues of the participant) Control Components of intervention
Dose: 30 min session Frequency of administration: 9 sessions in total, delivered between 1 and 3 times per week (depending on practical issues of the participant) |
|
| Outcomes | Outcomes assessed at the end of 9 treatment sessions (4‐8 weeks) and 3 months after the final treatment session
|
|
| Notes |
Conflicts of interest: the authors stated that they had no conflicts of interest Trial registered (ISRCTN20736216) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patient took a form (with a letter A (n=23) or B (n=23)) indicating allocation to either groups from a closed envelop. A list with patient numbers and the group allocation that resulted from this randomization procedure was stored in a sealed envelope. Only the therapist had direct access to the randomization list. In this way, patients were randomly allocated to either treatment group A or B." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received slightly different multi‐modal interventions, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported some outcomes, but it is unclear whether they were provided with any information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All assessments were performed by the same examiner blinded for group allocation. The order of the assessments (primary and secondary outcomes) was randomized to avoid order effects." Comment: A blinded assessors measured objective outcomes (e.g. muscle strength) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Two participants dropped out of the control group, one because of cervical pain, the other was unable to be contacted. No participants dropped out of the intervention group. Analysis was by intention‐to‐treat with last observation carried forward. Missing data unlikely to affect the results |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes reported in the clinical trials registry |
| Other bias | Low risk | Comment: No other sources of bias identified |
Subasi 2012.
| Methods |
Study design: Parallel group RCT Setting: Outpatients Intervention: Water‐based exercise programme and surface heat and TENS and deep heat (ultrasound) Control: Land‐based exercise programme and surface heat and TENS and deep heat (ultrasound) Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated: Subacromial impingement syndrome defined by:
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 28 participants (35 shoulders); mean (SD) age: 58.3 ± 8.6 years old; sex: F/M 21/7; mean (SD) duration of symptoms: 8.9 ± 7.5 months Control Number randomised: 29 participants (35 shoulders); mean (SD) age: 56.2 ± 11.3 years old; sex: F/M 15/14; mean (SD) duration of symptoms: 10.0 ± 13.2 months |
|
| Interventions |
Intervention: land‐based exercise Components of intervention: supervised land‐based exercises. For the first 10 days, ROM and stretching exercises, and for the following 10 days, strengthening exercises were performed. After completion of 20 days' therapy, participants continued a home exercise programme twice daily Dose: not reported Frequency of administration: daily for the first 20 days and then twice daily for 3 months Control: water‐based exercise Components of intervention: supervised water‐based exercises. For the first 10 days, ROM and stretching exercises, and for the following 10 days, strengthening exercises in water by using dumbbells were performed. Water‐based exercises took place in a therapy pool maintained at 28‐30°C, which was 8 metres in width, 12 metres in length, and 1.4 metres at its deepest point. After completion of 20 days' therapy, participants continued a home exercise programme twice daily Dose: Not reported Frequency of administration: daily for the first 20 days and then twice daily for 3 months Both Groups Components of intervention
Dose:
Frequency of administration:
|
|
| Outcomes | Outcomes assessed at end of 20 days treatment and at 3 months post‐treatment initiation (˜8 weeks)
|
|
| Notes | Conflicts of interest: the authors stated that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Of the patients, 28 were randomized into the land‐based exercise group (LG) and 29 were randomized into the water‐based exercise group (WG)." Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: The flow of participants through the trial was not described, and it is unclear whether outcome data reported were based on an analysis of all randomised participants |
| Selective reporting (reporting bias) | High risk | Comment: The authors state in the methods section that specific tests (unspecified) and active and passive ROM were measured, but no data were reported for these outcomes. Also, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Surenkok 2009.
| Methods |
Study design: Parallel group RCT Setting: Sports physiotherapy clinic, Turkey Intervention: Scapular mobilisation Control 1: Sham mobilisation Control 2: No treatment Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: tendinitis or tenosynovitis Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 13; mean (SD) age: 55.07 (13.36) years; sex: F/M 10/3; duration of symptoms: not reported Control 1 Number randomised: 13; mean (SD) age: 54.30 (12.70) years; sex: F/M 2/11; duration of symptoms: not reported Control 2 Number randomised: 13; mean (SD) age: 55.53 (17.15) years; sex: F/M 10/3; duration of symptoms: not reported |
|
| Interventions |
Intervention: scapular mobilisation Components of intervention: application of superior and inferior gliding, rotations, and distraction to the scapula of the affected shoulder. The participants laid the affected forearm on their back. The therapist stood before the participant's affected shoulder, placing the index finger of one hand under the medial scapular border, the other hand grasping the superior border of the scapula. The scapula was moved superiorly and inferiorly for superior and inferior glide, and then the scapula was rotated upward and downward for scapular rotation. Second, while the participant was in the same position the physiotherapist put the ulnar fingers under the medial scapular border and distracted the scapula from the thorax Dose: Sets of 10 repetitions with 30 seconds between each set Frequency of administration: once Control 1: sham mobilisation Components of intervention: the sham condition replicated the treatment condition except for the hand positioning. The therapist placed one hand on the medial aspect of the scapula and the other hand on the affected shoulder. A simulated scapulothoracic movement, but with minimal pressure, was actually applied Dose: sets of 10 repetitions with 30 seconds between each set Frequency of administration: once Control 2: no treatment Components of intervention: the participant was seated for the same length of time, but no manual contact between the therapist and the participant took place Dose: not applicable Frequency: once |
|
| Outcomes | Outcomes assessed immediately post‐treatment (i.e. same day of treatment)
|
|
| Notes |
Conflicts of interest: not reported 22 (56%) participants had tendinitis, 10 (26%) had tenosynovitis, and 7 (18%) of participants had adhesive capsulitis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants received 1 of 3 treatment conditions (SM, sham, or control) in a randomized order known only by the treating therapist." Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The same physiotherapist applied all treatment conditions; a second physiotherapist took pre‐outcome and post‐outcome measurements. Both physiotherapists were blind to the group of each subject." Comment: Given the nature of the interventions, participants and physiotherapists could not be blind to treatment for the comparison of "mobilisation versus no treatment", and participants may have had different expectations about the benefits of each intervention. However, participants in the comparison of "mobilisation versus sham mobilisation" were blind to treatment |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: In the comparison between mobilisation and no treatment, unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes. However, in the comparison between mobilisation and sham mobilisation, blinded participants self‐reported outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The same physiotherapist applied all treatment conditions; a second physiotherapist took pre‐outcome and post‐outcome measurements." Comment: Assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no dropouts, losses to follow‐up or exclusions |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Szczurko 2009.
| Methods |
Study design: Parallel group RCT Setting: Postal employees who were members of the Canadian Union of Postal Workers, Canada Intervention: Physical exercise, hands on shoulder muscle and joint therapy and placebo tablets Control: Naturopathic care (dietary counselling, acupuncture, and Phlogenzym supplement) Source of funding: "Supported by The Canadian Union of Postal Workers and the Canada Post Corporation, Joint Benefits Committee. Mucos Pharma, Puhonice, Czech Republic and Heel Canada, Anjou, Quebec, Canada supplied the study drug" |
|
| Participants |
Diagnostic label used by trialists: Rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Inclusion criteria (not listed above)
Exclusion criteria (not listed above)
Baseline characteristics Intervention Number randomised: 42; mean (SD) age = 50.9 (7.86) years; sex: F/M 25/17; duration of symptoms: not reported Control Number randomised: 43; mean (SD) age = 50.7 (8.16) years; sex: F/M 25/18; duration of symptoms: not reported |
|
| Interventions |
Intervention: physical therapy Components of intervention: standardised exercise programme consisting of passive, active assisted and active ROM muscle strengthening and joint therapy, reportedly consistent with standard physiotherapy for shoulder injuries; hands‐on shoulder muscle and joint therapy; placebo tablets consisting of an inert fibre substance, matched to Phlogenzym in appearance, smell and taste Dose: 30‐min physical therapy consultations; 2 placebo tablets, 3 times per day Frequency of administration: once weekly for 12 weeks Control: naturopathic care Components of intervention: individualised dietary counselling with emphasis on reduction of alcohol intake and increase in consumption of fish, berries, fruits, vegetables, nuts and whole grains; standardised acupuncture treatment – needle insertion at LI15, SJ14, SI19, SI10‐13 and BL41‐46 plus up to 4 Ashi points of pain (needles were inserted and briefly stimulated using perpendicular thrusting technique); phlogenzym supplement (90 mg bromelain, 48 mg trypsin, 100 mg rutin) Dose & Frequency of administration: dietary counselling and acupuncture: one 30‐min session per week for 12 weeks; supplement: 2 tablets, 3 times per day for 12 weeks |
|
| Outcomes | Outcomes assessed at 4, 8 and 12 weeks post randomisation (only 12 week data reported)
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...participants were randomized using age‐ and sex‐matched computer randomization..." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment using central randomization was preserved up to the point of treatment" Comment: An adequate method was used to generate the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...participants were blinded to allocation and supplements were delivered using identical‐looking tablets for all supplements and placebo, it was not possible to mask the interventions from the participants or the clinicians delivery care". Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the interventions they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The flexion, extension, abduction, adduction, internal rotation, and external rotation of the affected shoulder were assessed by a coordinator blinded to treatment and using a goniometer/inclinometer" Comment: Assessor of objective outcomes was blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In total, 89 patients were randomized and enrolled in the study. Four of the 89 patients decided to not start the study after reconsideration or became unreachable before the first treatment visit. None of these 4 participants withdrew with the knowledge of what type of treatment they would be receiving. Of the 85 participants who started treatment, 17 (10 control, 7 active) did not complete the 12‐week course of study: 1 participant broke her leg, 6 became unreachable, and 10 could not commit the time or lost interest. Of the 43 participants who started treatment in the NC group, 41 completed week 8 and 36 completed week 12. Of the 42 participants who started treatment in the PE group, 36 completed week 8 and 32 completed week 12." Comment: The amount and reasons for attrition are unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: No data for some measures of ROM (active internal rotation and external rotation) were reported, but it is unclear if non‐reporting was related to the results. Also, without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Teys 2008.
| Methods |
Study design: Cross‐over RCT Setting: General population in south‐east Queensland, Australia Intervention: Postero‐lateral glide (mobilisation with movement) Control 1: Sham postero‐lateral glide Control 2: No treatment Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: None specified Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Overall cohort of participants Number randomised: 24; mean (SD) age: 46.1 (9.86) years old; sex: F/M 13/11; duration of symptoms: not reported |
|
| Interventions |
Intervention: postero‐lateral glide (Mulligans' mobilisation with movement) Components of intervention: participant was seated and the therapist stood beside the participant on the opposite side to the affected shoulder. One hand was placed over the scapula posteriorly while the thenar eminence of the other hand was placed over the anterior aspect of the head of the humerus. A posterior gliding force was applied to the humeral head. The participant was then asked to raise the affected arm in the plane of the scapula to the point of pain onset while the therapist sustained the gliding force to the humeral head, with care to avoid the sensitive coracoid process. The therapist endeavoured to maintain the glide at right angles to the plane of movement throughout the entire range Dose: 3 sets of 10 repetitions with a 30‐second rest between each set Frequency of administration: once Control 1 ‐ Sham Mobilisation Components of intervention: the therapist stood on the opposite side of the participant and placed one hand along the clavicle and sternum and the other on the posterior aspect of the humeral head of the affected shoulder. A simulated anterior glide was performed but with minimal pressure actually applied. The participant was asked to elevate the affected shoulder in the plane of the scapula through half of their available pain‐free range to minimise the likelihood of pain provocation Dose: 3 sets of 10 repetitions with a 30‐second rest between each set Frequency of administration: once Control 2 ‐ No treatment Components of intervention: participant seated for the same length of time as in the other groups, but no manual contact made Dose: NA Frequency of administration: once |
|
| Outcomes | Outcomes assessed immediately post‐treatment (i.e. same day of treatment)
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The treatment allocation sequence was block randomized using the drawing of lots and concealed from the investigator who took the outcome measurements" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participant blinding was facilitated by recruitment of people who had no experience of the manipulative therapy techniques applied to the shoulder and by careful instruction that did not refer to the study’s aims of evaluation of a treatment technique. Subjects were informed that the study was investigating the effects of manual handling on shoulder pain. An exit questionnaire assessed the adequacy of patient blinding. Results of the exit questionnaire showed that three participants (12%) correctly guessed they had only received active treatment and none had correctly guessed that they had received either a sham or control." Comment: Participants were likely blinded to the intervention they received |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pressure pain threshold |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The outcome measures were taken by an investigator skilled in their application and who remained blind to the allotted treatment condition." Comment: Assessor of ROM was blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There was no loss to follow‐up" |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
Van den Dolder 2003.
| Methods |
Study design: Parallel group RCT Setting: Repatriation hospital in Sydney, Australia Intervention: Soft tissue massage Control: No treatment Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement, supraspinatus/rotator cuff tear, supraspinatus tendinitis, or rotator cuff tendinitis Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 15; mean (SD) age: 63.1 (9.9) years old; sex: F/M 4/11; median (IQR) duration of symptoms: 26 (13–26) weeks No treatment Number randomised: 14; mean (SD) age: 65.9 (9.2) years old; sex: F/M 5/9; median (IQR) duration of symptoms: 30 (23–91) weeks |
|
| Interventions |
Intervention: soft tissue massage Components of intervention: soft tissue massage of the shoulder performed as seen fit by the treating therapist. The areas focused on were the lateral border of the scapula, in full shoulder flexion; posterior deltoid, at end of range horizontal flexion; anterior deltoid, at end of range hand‐behind‐back; and pectoralis major, in the stretch position Dose: 15–20 min Frequency of administration: 6 treatments over 2 weeks Control: no treatment Components of intervention: stayed on a waiting list Frequency: 2 weeks |
|
| Outcomes | Outcomes assessed at 2 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by selection of a sealed envelope from a container of identical envelopes, inside which were instructions regarding which group the patient was to be allocated to." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "A third person, who arranged all necessary follow‐up appointments, opened each envelope. This ensured concealment of allocation to both patients and assessor" Comment: An adequate method was used to generate the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding of the patients to allocation was not possible". Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "A third person, who arranged all necessary follow‐up appointments, opened each envelope. This ensured concealment of allocation to both patients and assessor." Comment: Assessor of objective outcomes was likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no attrition and all outcome data were analysed based on the number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
Walther 2004.
| Methods |
Study design: Parallel group RCT Setting: Not reported (Germany) Intervention: Standardised self‐training Control 1: Conventional physiotherapy (stretching exercises) Control 2: Functional brace Source of funding: Not reported |
|
| Participants |
Diagnostic label used by trialists: Impingement Criteria for defining the shoulder condition being treated Subacromial impingement confirmed by:
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 20; mean (range) age: 52.1 (40 – 66) years; sex: F/M 11/9; mean (range) duration of symptoms: 23 (3 – 72) months Control 1 Number randomised: 20; mean (range) age: 51.5 (37 – 66) years; sex: F/M 9/11; mean (range) duration of symptoms: 32 (2 – 120) months Control 2 Number randomised: 20; mean (range) age: 48.6 (25 – 61) years; sex: F/M 6/14; mean (range) duration of symptoms: 27 (5 – 60) months |
|
| Interventions |
Intervention: standardised self training Components of intervention: standardised self‐training programme of centring and stretching exercises that affected the shoulder. Instructions for the exercise programme were printed with PhysioTools software. For most of the exercises, an elastic Thera‐Band was used that was chosen according to the results of the initial force measurements. The self‐training programme was taught to participants under the guidance of a physiotherapist for a maximum of 4 sessions Dose: 10‐15 min Frequency of administration: 5 times per week for 12 weeks Control 1 ‐ Conventional Physiotherapy Components of intervention: physiotherapy consisting of centring training for the rotator cuff. Stretching was added in case of any limitation of the ROM at the first examination Dose: 10 sessions initially and further sessions prescribed by family doctor Frequency of administration: 2–3 times per week for 12 weeks Control 2 ‐ Functional brace Components of intervention: participants were supplied with a functional shoulder brace (Coopercare Lastrap; Coopercare Inc, Toronto, Ontario, Canada). They were instructed on how to use the brace and told to use it as long as possible during the day and, if comfortable, also at night. The Coopercare Lastrap shoulder brace consists of a cotton sleeve and special Thermovibe pads. The presumed effect of the brace is the absorption of vibrations and the accumulation of heat. The brace is fixed with two elastic Velcro straps Dose: as long as possible during the day and at night if possible Frequency: Every day for 12 weeks |
|
| Outcomes | Outcomes assessed at 6 weeks and 12 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After informed consent was obtained, 60 consecutive patients with painful disabling impingement syndrome of the shoulder were randomized into three different conservative treatment groups" Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: There was no information about whether assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "None of the patients treated with physiotherapy or self‐training dropped out of the therapy regimen. However, one of the patients treated with the brace complained about being bothered by the brace at work, especially while working overhead. Another patient had eczema of the skin develop underneath the pads. Both patients continued to wear the brace during the remainder of the 12‐week therapy period." Comment: There was no attrition |
| Selective reporting (reporting bias) | High risk | Comment: Measures of variation were only reported for the few outcomes that had statistically significant results |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Wang 2006.
| Methods |
Study design: Parallel group RCT Setting: Outpatient physical therapy clinic affiliated with an academic intuition, USA Intervention: Customised exercises Control: Standardised exercises Sources of funding: Texas Physical Therapy Foundation |
|
| Participants |
Diagnostic label used by trialists: one of eight scapular or humeral syndromes ‐ scapular downward rotation syndrome, scapular depression syndrome, scapular abduction syndrome, scapular winging syndrome, humeral anterior glide syndrome, humeral superior glide syndrome, humeral medial rotation syndrome, humeral hypomobility syndrome Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 20; mean (SD) age: 39.3 ± 13.2 years; sex: F/M 9/6; mean (SD) duration of symptoms: 49.6 ± 52.4 weeks Control Number randomised: 18; mean (SD) age: 49.9 ± 18.3 years; sex: F/M 6/9; mean (SD) duration of symptoms: 41.6 ± 51.5 weeks |
|
| Interventions |
Intervention: customised exercises Components of intervention: mainly supervised and home self‐stretching and strengthening exercises for scapular stabilisers, rotator cuff and scapulohumeral muscles. There were 6 exercises for each of the potential 8 categories that participants could be classified into based on the examination of the physical therapist. When subjects were able to complete the shoulder exercises without difficulty or without increasing symptoms, they were progressed to the next level of exercises. In general, strengthening exercises were progressed by increasing repetitions and resistance, while stretching exercises increased by hold time. Resistance was provided by Thera‐band or gravity in weaker muscles. When participants had difficulty or symptoms increased with the prescribed exercises, the exercise level was decreased by decreasing the resistance, decreasing the repetitions, or stopping the exercises that aggravated the symptoms. Dose: 5 repetitions of a 5‐second hold for each exercise. Resistance level or hold time was increased with participant ability Frequency of administration: Supervised ‐ once weekly for 8 weeks; Home ‐ twice daily 5 times per week for 8 weeks Control: standardised exercises Components of intervention: same as above, except that 5 standardised strengthening exercises were delivered: shoulder flexors, abductors, extensors, external rotators and internal rotators. Dose: 5 repetitions of a 5‐second hold for each exercise. Resistance level or hold time was increased with participant ability Frequency of administration: supervised ‐ once weekly for 8 weeks; home ‐ twice daily 5 times per week for 8 weeks |
|
| Outcomes | Outcomes assessed at 4 and 8 weeks
|
|
| Notes | Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were then randomly assigned to one of two exercise groups by selecting a number in a pre‐ prepared sealed envelope” Comment: No information on how the allocation sequence was generated prior to inserting numbers in envelopes |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Subjects were then randomly assigned to one of two exercise groups by selecting a number in a pre‐ prepared sealed envelope” Comment: Not clear if envelopes were sequentially numbered or shuffled by participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This is a single‐blinded randomized clinical trial. The tester was blinded from the subject’s treatment group." Comment: Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “Four outcome measurements were obtained by a tester who was blinded to the assigned treatment group” Comment: Assessor of objective outcomes was likely blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 5 and 3 participants dropped out of each respective group, but the reasons were balanced between groups, so attrition is unlikely to have affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Winters 1997.
| Methods |
Study design: Parallel group RCT Setting: General practices, The Netherlands Intervention: Physiotherapy (exercise, massage) Control 1: Manipulation Control 2: Glucocorticoid injection Source of funding: Ministry of Welfare, Health and Culture |
|
| Participants |
Diagnostic label used by trialists: None specified Criteria for defining the shoulder condition being treated Patients consulting their GP with shoulder complaints as follows:
The participants were then divided into three diagnostic subgroups
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Shoulder Girdle subgroup Intervention Number randomised: 29; Mean (SD) age: 46.4 (11.2) years old; Sex: F/M 18/11; Median duration of symptoms: 4 weeks Control 1 Number randomised: 29; Mean (SD) age: 43.9 (12.6) years old; Sex: F/M 15/14; Median duration of symptoms: 3 weeks Synovial subgroup Intervention Number randomised: 35; Mean (SD) age: 53.1 (12.6) years old; Sex: F/M 14/21; Median duration of symptoms: 4 weeks Control 1 Number randomised: 32; Mean (SD) age: 46.7 (12.1) years old; Sex: F/M 17/15; Median duration of symptoms: 9 weeks Control 2 Number randomised: 47; Mean (SD) age: 53.5 (12.5) years old; Sex: F/M 32/15; Median duration of symptoms: 8 weeks |
|
| Interventions |
Intervention: physical therapy Components of intervention: regimes of "classic" physiotherapy, possibly including physical applications, massage and exercise therapies. No mobilisation techniques or manipulative techniques were allowed Dose: not reported Frequency of administration: twice per week (number of weeks in total not reported) Control 1: manipulation Components of intervention: mobilisation and manipulation of the cervical spine, upper thoracic spine, upper ribs, acromioclavicular joint and the glenohumeral joint Dose: not reported Frequency of administration: once a week for a maximum of 6 sessions Control 2: glucocorticoid Injections Components of intervention: injection of triamcinolone acetonide and lignocaine. Two out of 3 synovial structures (glenohumeral joint capsule, subacromial space and acromioclavicular joint) were injected. The intra‐articular injection was given from the posterior side, the subacromial injection from the lateral side and the acromioclavicular injection perpendicularly from the upper side of the joint Dose: 1 ml of 40 mg/ml triamcinolone acetonide in combination with 9 ml of 10 mg/ml lignocaine for 1–3 injections Frequency of administration: after randomisation, 1 week later and another 2 weeks later if needed |
|
| Outcomes | Outcomes assessed at 11 weeks and 2‐3 years (only for treatment success)
|
|
| Notes |
Conflicts of interest: the authors stated that they had no conflicts of interest Only data for the synovial group were included in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The university's Department of Family Practice was in charge of the randomisation to treatment. For each diagnostic category, we had made a series of closed unnumbered envelopes which contained instructions of the treatment to be given. The participating general practitioners had to call a secretary and state the diagnostic category of each patient. The secretary in turn would draw an envelope to assign treatment" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention. The comparison of physiotherapy versus manipulation is of less concern |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported all outcomes. The comparison of physiotherapy versus manipulation is of less concern |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Drop out because of treatment failure was significantly higher in the physio therapy group (45% (13/29) of patients) than in the manipulation group (20% (6/29) patients)." Quote: "Drop out because of treatment failure was much lower in the injection group (17% (7/47)) than in the physio therapy group (51% (18/35)) and manipulation group (59% (19/32))." Comment: All withdrawals were recorded and their reasons published in the article. An intention to treat analysis was performed but it is not clear how missing data were imputed |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: The study appears to be free of other bias |
Yiasemides 2011.
| Methods |
Study design: Parallel group RCT Setting: Metropolitan teaching hospital, Australia Intervention: Passive shoulder mobilisation and exercise and advice Control: Exercise and advice alone Source of Funding: Partially funded by Musculoskeletal Physiotherapy Australia research Grant (2005) |
|
| Participants |
Diagnostic label used by trialists: None specified Criteria for defining the shoulder condition being treated
Inclusion Criteria (not listed above)
Exclusion Criteria (not listed above)
Baseline characteristics Intervention Number randomised: 47; mean (range) age = 62 (35‐85) years; sex: F/M 27/20; mean (SD) duration of symptoms: 9.7 (12) months Control Number randomised: 51; mean (range) age = 58 (27‐81) years; sex: F/M 24/27; mean (SD) duration of symptoms: 22 (38) months |
|
| Interventions |
Intervention: passive shoulder region joint mobilisation Components of intervention: individually tailored low‐velocity passive joint mobilisations to any of the shoulder region joints and passive mobilisation of the scapula. Either sustained or oscillatory techniques were utilised Dose: individually determined Frequency of administration: as above (minimum of 60% of all treatments involved passive shoulder mobilisation) Control: participants only received the treatment common to both groups (see below) Both groups: Components of intervention: both groups received advice and an individually tailored exercise programme. Advice included avoidance of painful shoulder movements and maintenance of normal scapulohumeral rhythm. Exercises included stretching, strengthening and motor retraining Dose: not reported Frequency of administration: daily home exercise performance. Participants attended therapy sessions for exercise technique revision and progression 1‐2 times per week for 4 weeks, with a maximum of 12 treatment sessions over 8 weeks |
|
| Outcomes | Outcomes assessed 1 month, 3 months and 6 months
|
|
| Notes |
Conflicts of interest: not reported Trial registration: ACTRN: 12605000151639 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation of participants was performed using a previously determined treatment assignment schedule with random numbers generated from the data analysis function in Microsoft Excel." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure concealment, the randomization procedure was carried out by a researcher (K.A.G.) not involved in participant recruitment, treatment, or assessment, and the treatment assignment schedule was stored in consecutively numbered, sealed opaque envelopes." Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations regarding the benefits of the interventions |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "Primary outcome measurements of pain, functional impairment, and self‐rated improvement were obtained from participants who were not blinded to treatment group allocation" Comment: Unblinded participants who may have had different expectations regarding the benefits of the interventions received self‐reported some outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Secondary outcome measurements of painful AROM were obtained by a researcher (R.Y.) blinded to group allocation at the same time points." Comment: Outcome assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were conducted using an intention‐to‐treat approach. Missing data (lost to follow‐up) were replaced with values obtained by imputation using regression models within each variable and group at all available time points. For the 2 control group participants who were lost prior to reassessment at 1 month after recruitment and, therefore, did not have a self‐rated change in symptoms score, the average of the group was used for their missing scores." Comment: Amount of drop out and reasons were similar between groups |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the trial registry entry |
| Other bias | Low risk | Comment: No other sources of bias identified |
AROM = active range of motion; PROM = passive range of motion; ROM = range of motion; VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergman 2004 | Ineligible clinical condition: did not exclude people with pain radiating to the neck region or to the lower part of the arm |
| Bron 2011 | Ineligible clinical condition: participants had non‐specific shoulder pain and myofascial trigger points |
| Chen 2009 | Ineligible clinical condition: mixture of shoulder disorders, where some participants had concomitant neck pain, and the inclusion/exclusion criteria was more compatible with a diagnosis of adhesive capsulitis |
| Geraets 2005 | Ineligible clinical condition: did not exclude patients with pain radiating to the neck region or to the lower part of the arm (>50% participants had a concomitant neck problem) |
| Ginn 1997 | Ineligible clinical condition: mixed shoulder disorders, where patients with rotator cuff disorders comprised only 65% of the sample (remaining patients had osteoarthritis, adhesive capsulitis, biceps muscle tear or no diagnosis) |
| Hakguder 2011 | Not a randomised or quasi‐randomised trial |
| Jinhwa 2012 | Not a randomised or quasi‐randomised trial |
| Krischak 2013 | Ineligible intervention: occupational therapy is ineligible, and both groups received exercise |
| Merolla 2013 | Not a randomised or quasi‐randomised trial |
| Miller 2004 | Ineligible clinical condition: inclusion/exclusion criteria not compatible with a diagnosis of rotator cuff disease, and unclear if participants with adhesive capsulitis, a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulders, or pain in the shoulder region as part of a complex myofascial neck/shoulder/arm pain condition were excluded |
| Molsberger 2010 | Ineligible intervention: compared physical therapy plus NSAID to acupuncture or sham acupuncture. Cannot separate the effect of physical therapy from NSAID |
| Muth 2012 | Not a randomised or quasi‐randomised trial |
| Mörl 2011 | Ineligible clinical condition: inclusion/exclusion criteria not compatible with a diagnosis of rotator cuff disease, and unclear if participants with adhesive capsulitis, a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulders, or pain in the shoulder region as part of a complex myofascial neck/shoulder/arm pain condition were excluded |
| Saggini 2010 | Ineligible intervention: examined the effect of a proprioceptive Multi Joint System device, not manual therapy or exercise |
| Seok‐Hwa 2013 | Ineligible clinical condition: inclusion/exclusion criteria not compatible with a diagnosis of rotator cuff disease, and unclear if participants with adhesive capsulitis, a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulders, or pain in the shoulder region as part of a complex myofascial neck/shoulder/arm pain condition were excluded |
| Tachibana 2012 | Ineligible clinical condition: inclusion/exclusion criteria not compatible with a diagnosis of rotator cuff disease, and unclear if participants with adhesive capsulitis, a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulders, or pain in the shoulder region as part of a complex myofascial neck/shoulder/arm pain condition were excluded |
Characteristics of studies awaiting assessment [ordered by study ID]
Acosta 2009.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Bicer 2005.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Bube 2010.
| Methods | Available only as a conference abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ellegaard 2013.
| Methods | Available only as a conference abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ginn 2009.
| Methods | Available only as a conference abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Just 2009.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Leblebici 2007.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pribicevic 2006.
| Methods | Available only as a conference abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Werner 2002.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wiener 2005.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wies 2008.
| Methods | Available only as a conference abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Roddy 2014.
| Trial name or title | The SUPPORT trial (SUbacromial imPingement syndrome and Pain: a randomised controlled trial Of exeRcise and injecTion) |
| Methods | Factorial RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Outcomes assessed at 6 weeks, 6 months and 12 months
|
| Starting date | 01/03/2011 |
| Contact information | Edward Roddy, Keele University, UK. Email: e.roddy@keele.ac.uk |
| Notes | Trial registration number: ISRCTN42399123 |
Van den Dolder 2010.
| Trial name or title | Is soft tissue massage an effective treatment for mechanical shoulder pain? |
| Methods | Parallel group RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Outcomes assessed at 5 weeks and 4 months
|
| Starting date | 22/06/2007 |
| Contact information | Paul van den Dolder, University of Sydney, Sydney, Australia. Email: pvan0651@mail.usyd.edu.au |
| Notes | Trial registration number: ACTRN12607000336482 |
Differences between protocol and review
The original review outcomes were pain, range of motion (active and passive), function/disability and quality of life, strength, return to work, participants' perception of overall effect, global preference, physicians' preference and adverse effects. The outcomes reported in this review have been modified from the original review to make them as consistent as possible with other Cochrane reviews on shoulder disorders and other chronic pain conditions. To improve succinctness of the review, we only included one measurement instrument per outcome domain. We assessed study risk of bias using The Cochrane Collaboration's 'Risk of bias' tool in this update of the review. We have included a 'Summary of findings' table.
Contributions of authors
MJP was responsible for writing the review, performing the searches, selecting trials, performing risk of bias assessment, data extraction, analysing the data and interpreting the results of the updated review. SG was responsible for performing the searches, selecting trials and performing the data extraction and quality assessment for the original review, defining the review comparisons and outcomes of interest of the original and updated review, analysing and interpreting the results, and contributing to writing both the original and updated review. BM was responsible for selecting trials, performing risk of bias assessment, data extraction and contributing to writing the manuscript for the updated review. SS, JD, NL and MM were responsible for performing risk of bias assessment, data extraction and contributing to writing the manuscript for the updated review. RB was responsible for performing the data extraction and quality assessment for the original review, defining the review comparisons and outcomes of interest of both the original and updated review, analysing and interpreting the results, and contributing to writing both the original and updated review.
Sources of support
Internal sources
Australasian Cochrane Centre, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Australia.
External sources
Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088535), Australia.
Declarations of interest
SG and RB are authors of one of the trials included in this review (Bennell 2010). To avoid any bias, the paper was sent to an independent review author to assess whether it met the inclusion criteria for this review. Neither author was involved in the data extraction or risk of bias assessment of this trial. RB is Joint Co‐ordinating Editor of Cochrane Musculoskeletal. To avoid bias, RB was excluded from the editorial and publication process for this review. SG is a practicing physiotherapist in part‐time private physiotherapy practice (self‐employed), and as such receives remuneration for the delivery of physiotherapy interventions. BM is a practicing physiotherapist in private physiotherapy practice and as such receives remuneration for the delivery of physiotherapy interventions.
New
References
References to studies included in this review
Ainsworth 2009 {published data only}
- Ainsworth R, Lewis J, Conboy V. A prospective randomized placebo controlled clinical trial of a rehabilitation programme for patients with a diagnosis of massive rotator cuff tears of the shoulder. Shoulder & Elbow 2009;1:55‐60. [Google Scholar]
Al Dajah 2014 {published data only}
- Al Dajah SB. Soft tissue mobilization and PNF improve range of motion and minimize pain level in shoulder impingement. Journal of Physical Therapy Science 2014;26(11):1803‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkinson 2008 {published data only}
- Atkinson M, Mathews R, Brantingham JW, Globe G, Cassa T, Bonnefin D, et al. A randomized controlled trial to assess the efficacy of shoulder manipulation vs. placebo in the treatment of shoulder pain due to rotator cuff tendinopathy. Journal of the American Chiropractic Association 2008;45:11‐26. [Google Scholar]
Bae 2011 {published data only}
- Bae YH, Lee GC, Shin WS, Kim TH, Lee SM. Effect of motor control and strengthening exercises on pain, function, strength and the range of motion of patients with shoulder impingement syndrome. Journal of Physical Therapy Science 2011;23:687‐92. [Google Scholar]
Bang 2000 {published data only}
- Bang MD, Deyle GD. Comparison of supervised exercise with and without manual physical therapy for patients with shoulder impingement syndrome. Journal of Orthopaedic and Sports Physical Therapy 2000;30:126‐37. [DOI] [PubMed] [Google Scholar]
Bansal 2011 {published data only}
- Bansal K, Padamkumar S. A comparative study between the efficacy of therapeutic ultrasound and soft tissue massage (deep friction massage) in supraspinatus tendinitis. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:80‐4. [Google Scholar]
Barbosa 2008 {published data only}
- Barbosa RI, Goes R, Mazzer N, Fonseca MCR. The influence of joint mobilization on tendinopathy of the biceps brachii and supraspinatus muscles. Brazilian Journal of Physical Therapy 2008;12:298‐303. [Google Scholar]
Barra 2011 {published data only}
- Barra ME, López C, Fernández G, Murillo E, Villar E, Raya L. The immediate effects of diacutaneous fibrolysis on pain and mobility in patients suffering from painful shoulder: a randomized placebo‐controlled pilot study. Clinical Rehabilitation 2011;25:339‐48. [DOI] [PubMed] [Google Scholar]
Barra Lopez 2013 {published data only}
- Barra Lopez ME, Lopez de Celis C, Fernandez JG, Raya de Cardenas L, Lucha Lopez MO, Tricas Moreno JM. Effectiveness of diacutaneous fibrolysis for the treatment of subacromial impingement syndrome: a randomised controlled trial. Manual Therapy 2013;18(5):418‐24. [DOI] [PubMed] [Google Scholar]
Baskurt 2011 {published data only}
- Baskurt Z, Baskurt F, Gelecek N, Özkan MH. The effectiveness of scapular stabilization exercise in the patients with subacromial impingement syndrome. Journal of Back and Musculoskeletal Rehabilitation 2011;24:173‐9. [DOI] [PubMed] [Google Scholar]
Beaudreuil 2011 {published data only}
- Beaudreuil J, Lasbleiz S, Aout M, Vicaut E, Yelnik A, Bardin T, et al. Effect of dynamic humeral centring (DHC) treatment on painful active elevation of the arm in subacromial impingement syndrome. Secondary analysis of data from an RCT. British Journal of Sports Medicine 2015;49(5):343‐6. [DOI] [PubMed] [Google Scholar]
- Beaudreuil J, Lasbleiz S, Richette P, Seguin G, Rastel C, Aout M, et al. Assessment of dynamic humeral centering in shoulder pain with impingement syndrome: a randomised clinical trial. Annals of the Rheumatic Diseases 2011;70:1613‐8. [DOI] [PubMed] [Google Scholar]
- Beaudreuil J, Lasbleiz S, Yelnik A, Bardin T, Orcel P. Effect of dynamic humeral centering on painful active elevation of the arm in subacromial impingement syndrome: a randomized trial. Annals of Physical and Rehabilitation Medicine 2012;55:e158‐e159+e161. [Google Scholar]
Bennell 2010 {published data only}
- Bennell K, Coburn S, Wee E, Green S, Harris A, Forbes A, et al. Efficacy and cost‐effectiveness of a physiotherapy program for chronic rotator cuff pathology: a protocol for a randomised, double‐blind, placebo controlled trial. BMC Musculoskeletal Disorders 2007;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennell K, Wee E, Coburn S, Green S, Harris A, Staples M, et al. Efficacy of standardised manual therapy and home exercise programme for chronic rotator cuff disease: randomised placebo controlled trial. BMJ 2010;340:c2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bialoszewski 2011 {published data only}
- Bialoszewski D, Zaborowski G. Usefulness of manual therapy in the rehabilitation of patients with chronic rotator cuff injuries. Preliminary report. Ortopedia, Traumatologia, Rehabilitacja 2011;1:9‐20. [DOI] [PubMed] [Google Scholar]
Blume 2014 {published data only}
- Blume CL. Comparison of an eccentric exercise intervention to a concentric exercise intervention in adults with subacromial impingement syndrome. PhD thesis. Texas Women's University. http://poar.twu.edu/handle/11274/3673. 2014. [PMC free article] [PubMed]
Brox 1993 {published data only}
- Brox JI, Staff PH, Ljunggren AE, Brevik JI. Arthroscopic surgery compared with supervised exercises in patients with rotator cuff disease (stage II impingement syndrome). BMJ 1993;307:899‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brox JI, Staff PH, Ljunggren AE, Brevik JI. Arthroscopic surgery compared with supervised exercises in patients with rotator cuff disease (stage II impingement syndrome).[Erratum]. BMJ 1993;307:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celik 2009 {published data only}
- Celik D, Akyüz G, Yeldan I. Comparison of the effects of two different exercise programs on pain in subacromial impingement syndrome. Acta Orthopaedica et Traumatologica Turcica 2009;43:504‐9. [DOI] [PubMed] [Google Scholar]
Citaker 2005 {published data only}
- Citaker S, Taskiran H, Akdur H, Arabaci UO, Ekici G. Comparison of the mobilization and proprioceptive neuromuscular facilitation methods in the treatment of shoulder impingement syndrome. The Pain Clinic 2005;27:197‐202. [Google Scholar]
Clews 1987 {published data only}
- Clews W, Wajswelner H. Beneficial effects of massage in athletes with rotator cuff tendinitis. Proc Natl Annu Sci Conf Aust Sports Med Fed 1987;2:555‐64. [Google Scholar]
Cloke 2008 {published data only}
- Cloke DJ, Watson H, Purdy S, Steen IN, Williams JR. A pilot randomized, controlled trial of treatment for painful arc of the shoulder. Journal of Shoulder and Elbow Surgery 2008;17:17s‐21s. [DOI] [PubMed] [Google Scholar]
Conroy 1998 {published data only}
- Conroy DE, Hayes KW. The effect of joint mobilization as a component of comprehensive treatment for primary shoulder impingement syndrome. Journal of Orthopaedic and Sports Physical Therapy 1998;28:3‐14. [DOI] [PubMed] [Google Scholar]
Cook 2014 {published data only}
- Cook C, Learman K, Houghton S, Showalter C, O'Halloran B. The addition of cervical unilateral posterior–anterior mobilisation in the treatment of patients with shoulder impingement syndrome: A randomised clinical trial. Manual Therapy 2014;19(1):18‐24. [DOI] [PubMed] [Google Scholar]
Dickens 2005 {published data only}
- Dickens VA, Williams JL, Bhamra MS. Role of physiotherapy in the treatment of subacromial impingement syndrome: a prospective study. Physiotherapy 2005;91:159‐64. [Google Scholar]
Djordjevic 2012 {published data only}
- Djordjevic OC, Vukicevic D, Katunac L, Jovic S. Mobilization with movement and kinesiotaping compared with a supervised exercise program for painful shoulder: results of a clinical trial. Journal of Manipulative and Physiological Therapeutics 2012;35(6):454‐63. [DOI] [PubMed] [Google Scholar]
Engebretsen 2009 {published data only}
- Engebretsen K, Grotle M, Bautz‐Holter E, Ekeberg OM, Juel NG, Brox JI. Supervised exercises compared with radial extracorporeal shock‐wave therapy for subacromial shoulder pain: 1‐year results of a single‐blind randomized controlled trial. Physical Therapy 2011;91(1):37‐47. [DOI] [PubMed] [Google Scholar]
- Engebretsen K, Grotle M, Bautz‐Holter E, Sandvik L, Juel NG, Ekeberg OM, et al. Radial extracorporeal shockwave treatment compared with supervised exercises in patients with subacromial pain syndrome: single blind randomised study. BMJ 2009;339:b3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginn 2005 {published data only}
- Ginn KA, Cohen ML. Exercise therapy for shoulder pain aimed at restoring neuromuscular control: a randomized comparative clinical trial. Journal of Rehabilitation Medicine 2005;37:115‐22. [DOI] [PubMed] [Google Scholar]
Giombini 2006 {published data only}
- Giombini A, Cesare A, Safran MR, Ciatti R, Maffulli N. Short‐term effectiveness of hyperthermia for supraspinatus tendinopathy in athletes: a short‐term randomized controlled study. American Journal of Sports Medicine 2006;34:1247‐53. [DOI] [PubMed] [Google Scholar]
Haahr 2005 {published data only}
- Haahr JP, Andersen JH. Exercises may be as efficient as subacromial decompression in patients with subacromial stage II impingement: 4‐8‐years' follow‐up in a prospective, randomized study. Scandinavian Journal of Rheumatology 2006;35(3):224‐8. [DOI] [PubMed] [Google Scholar]
- Haahr JP, Østergaard S, Dalsgaard J, Norup K, Frost P, Lausen S, et al. Exercises versus arthroscopic decompression in patients with subacromial impingement: a randomised, controlled study in 90 cases with a one year follow up. Annals of the Rheumatic Diseases 2005;64:760‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haik 2014 {published data only}
- Haik MN, Alburquerque‐SendÍn F, Silva CZ, Siqueira‐Junior AL, Ribeiro IL, Camargo PR. Scapular kinematics pre‐ and post‐thoracic thrust manipulation in individuals with and without shoulder impingement symptoms: a randomized controlled study. Journal of Orthopaedic & Sports Physical Therapy 2014;44(7):475‐87. [DOI] [PubMed] [Google Scholar]
Hay 2003 {published data only}
- Dziedzic K, Thomas E, Paterson S, Croft P, Hay EM. A pragmatic randomised controlled trial (RCT) in primary care for shoulder pain: content of the physiotherapy intervention. Rheumatology 2001;40(Suppl 1):148. [Google Scholar]
- Hay EM, Thomas E, Paterson SM, Dziedzic K, Croft PR. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Annals of the Rheumatic Diseases 2003;62:394‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M, Stokes EA, Thomas E, Dziedzic K, Hay EM. A cost consequences analysis of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Rheumatology 2005;44(11):1447‐51. [DOI] [PubMed] [Google Scholar]
Heredia‐Rizo 2013 {published data only}
- Heredia‐Rizo AM, Lopez‐Hervas A, Herrera‐Monge P, Gutierrez‐Leonard A, Pina‐Pozo F. Shoulder functionality after manual therapy in subjects with shoulder impingement syndrome: A case series. Journal of Bodywork and Movement Therapies 2013;17(2):212‐8. [DOI] [PubMed] [Google Scholar]
Holmgren 2012 {published data only}
- Hallgren HCB, Holmgren T, Oberg B, Johansson K, Adolfsson LE. A specific exercise strategy reduced the need for surgery in subacromial pain patients. British Journal of Sports Medicine 2014;48(19):1431‐6. [DOI] [PubMed] [Google Scholar]
- Holmgren T, Björnsson Hallgren H, Öberg B, Adolfsson L, Johansson K. Effect of specific exercise strategy on need for surgery in patients with subacromial impingement syndrome: randomised controlled study. BMJ 2012;344:e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janse van Rensburg 2012 {published data only}
- Janse van Rensburg K, Atkins E. Does thoracic manipulation increase shoulder range of movement in patients with subacromial impingement syndrome? A pilot study. International Musculoskeletal Medicine 2012;34(3):101‐7. [Google Scholar]
Kachingwe 2008 {published data only}
- Kachingwe AF, Phillips B, Sletten E, Plunkett SW. Comparison of manual therapy techniques with therapeutic exercise in the treatment of shoulder impingement: a randomized controlled pilot clinical trial. Journal of Manual and Manipulative Therapy 2008;16:238‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kardouni 2014 {published data only}
- Kardouni JR, Shaffer SW, Pidcoe PE, Finucane SD, Cheatham SA, Michener LA. Immediate changes in pressure pain sensitivity after thoracic spinal manipulative therapy in patients with subacromial impingement syndrome: A randomized controlled study. Manual Therapy 2014;20(4):540‐6. [DOI: 10.1016/j.math.2014.12.003] [DOI] [PubMed] [Google Scholar]
Kassolik 2013 {published data only}
- Kassolik K, Andrzejewski W, Brzozowski M, Wilk I, Gorecka‐Midura L, Ostrowska B, et al. Comparison of massage based on the tensegrity principle and classic massage in treating chronic shoulder pain. Journal of Manipulative & Physiological Therapeutics 2013;36(7):418‐27. [DOI] [PubMed] [Google Scholar]
Kaya 2014 {published data only}
- Kaya DO, Baltaci G, Toprak U, Atay AO. The clinical and sonographic effects of kinesiotaping and exercise in comparison with manual therapy and exercise for patients with subacromial impingement syndrome: A preliminary trial. Journal of Manipulative and Physiological Therapeutics 2014;37(6):422‐32. [DOI] [PubMed] [Google Scholar]
Kromer 2013 {published data only}
- Kromer TO, Bie RA, Bastiaenen CH. Effectiveness of individualized physiotherapy on pain and functioning compared to a standard exercise protocol in patients presenting with clinical signs of subacromial impingement syndrome. A randomized controlled trial. BMC Musculoskeletal Disorders 2010;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer TO, Bie RA, Bastiaenen CH. Effectiveness of physiotherapy and costs in patients with clinical signs of shoulder impingement syndrome: One‐year follow‐up of a randomized controlled trial. Journal of Rehabilitation Medicine 2014;46(10):1029‐36. [DOI] [PubMed] [Google Scholar]
- Kromer TO, Bie RA, Bastiaenen CH. Physiotherapy in patients with clinical signs of shoulder impingement syndrome: a randomized controlled trial. Journal of Rehabilitation Medicine 2013;45(5):488‐97. [DOI] [PubMed] [Google Scholar]
Littlewood 2014 {published data only}
- Littlewood C, Ashton J, Mawson S, May S, Walters S. A mixed methods study to evaluate the clinical and cost‐effectiveness of a self‐managed exercise programme versus usual physiotherapy for chronic rotator cuff disorders: Protocol for the SELF study. BMC Musculoskeletal Disorders 2012;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood C, Malliaras P, Mawson S, May S, Walters SJ. Self‐managed loaded exercise versus usual physiotherapy treatment for rotator cuff tendinopathy: a pilot randomised controlled trial. Physiotherapy 2014;100(1):54‐60. [DOI] [PubMed] [Google Scholar]
Lombardi 2008 {published data only}
- Lombardi I, Magri AG, Fleury AM, Silva AC, Natour J. Progressive resistance training in patients with shoulder impingement syndrome: a randomized controlled trial. Arthritis and Rheumatism 2008;59:615‐22. [DOI] [PubMed] [Google Scholar]
Ludewig 2003 {published data only}
- Ludewig PM, Borstad JD. Effects of a home exercise programme on shoulder pain and functional status in construction workers. Occupational and Environmental Medicine 2003;60:841‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maenhout 2013 {published data only}
- Maenhout AG, Mahieu NN, Muynck M, Wilde LF, Cools AM. Does adding heavy load eccentric training to rehabilitation of patients with unilateral subacromial impingement result in better outcome? A randomized, clinical trial. Knee Surgery, Sports Traumatology, Arthroscopy 2013;21(5):1158‐67. [DOI] [PubMed] [Google Scholar]
Martins 2012 {published data only}
- Martins LV, Marziale MHP. Assessment of proprioceptive exercises in the treatment of rotator cuff disorders in nursing professionals: a randomized controlled clinical trial. Brazilian Journal of Physical Therapy 2012;16(6):502‐9. [DOI] [PubMed] [Google Scholar]
Marzetti 2014 {published data only}
- Marzetti E, Rabini A, Piccinini G, Piazzini DB, Vulpiani MC, Vetrano M, et al. Neurocognitive therapeutic exercise improves pain and function in patients with shoulder impingement syndrome: a single‐blind randomized controlled clinical trial. European Journal of Physical & Rehabilitation Medicine 2014;50(3):255‐64. [PubMed] [Google Scholar]
McClatchie 2009 {published data only}
- McClatchie L, Laprade J, Martin S, Jaglal SB, Richardson D, Agur A. Mobilizations of the asymptomatic cervical spine can reduce signs of shoulder dysfunction in adults. Manual Therapy 2009;14:369‐374. [DOI] [PubMed] [Google Scholar]
Moosmayer 2014 {published data only}
- Moosmayer S, Lund G, Seljom U, Svege I, Hennig T, Tariq R, et al. Comparison between surgery and physiotherapy in the treatment of small and medium‐sized tears of the rotator cuff: a randomised controlled study of 103 patients with one‐year follow‐up. Journal of Bone & Joint Surgery [British Volume] 2010;92‐B:83‐91. [DOI] [PubMed] [Google Scholar]
- Moosmayer S, Lund G, Seljom US, Haldorsen B, Svege IC, Hennig T, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five‐year follow‐up. Journal of Bone & Joint Surgery [American Volume] 2014;96(18):1504‐14. [DOI] [PubMed] [Google Scholar]
Munday 2007 {published data only}
- Munday SL, Jones A, Brantingham JW, Globe G, Jensen M, Price JL. A randomized, single‐blinded, placebo‐controlled clinical trial to evaluate the efficacy of chiropractic shoulder girdle adjustment in the treatment of shoulder impingement syndrome. Journal of the American Chiropractic Association 2007;44:6‐15. [Google Scholar]
Osteras 2008 {published data only}
- Osteras H, Torstensen TA. The dose‐response effect of medical exercise therapy on impairment in patients with unilateral longstanding subacromial pain. The Open Orthopaedics Journal 2010;4:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteras H, Torstensen TA, Haugerud L, Osteras BS. Dose‐response effects of graded therapeutic exercises in patients with long‐standing subacromial pain. Advances in Physiotherapy 2009;11:199‐209. [Google Scholar]
- Østerås H, Torstensen TA, Arntzen G, Østerås BS. A comparison of work absence periods and the associated costs for two different modes of exercise therapies for patients with longstanding subacromial pain. Journal of Medical Economics 2008;11:371‐81. [DOI] [PubMed] [Google Scholar]
- Østerås H, Torstensen TA, Østerås B. High‐dosage medical exercise therapy in patients with long‐term subacromial shoulder pain: a randomized controlled trial. Physiotherapy Research International 2010;15:232‐42. [DOI] [PubMed] [Google Scholar]
Rhon 2014 {published data only}
- Rhon DI, Boyles RB, Cleland JA. One‐year outcome of subacromial corticosteroid injection compared with manual physical therapy for the management of the unilateral shoulder impingement syndrome: a pragmatic randomized trial. Annals of Internal Medicine 2014;161:161‐9. [DOI] [PubMed] [Google Scholar]
Senbursa 2007 {published data only}
- Senbursa G, Baltaci G, Atay A. Comparison of conservative treatment with and without manual physical therapy for patients with shoulder impingement syndrome: a prospective, randomized clinical trial. Knee Surgery, Sports Traumatology, Arthroscopy 2007;15:915‐21. [DOI] [PubMed] [Google Scholar]
Senbursa 2011 {published data only}
- Senbursa G, Baltaci G, Atay ÖA. The effectiveness of manual therapy in supraspinatus tendinopathy. Acta Orthopaedica et Traumatologica Turcica 2011;45:162‐7. [DOI] [PubMed] [Google Scholar]
Struyf 2013 {published data only}
- Struyf F, Nijs J, Mollekens S, Jeurissen I, Truijen S, Mottram S, et al. Scapular‐focused treatment in patients with shoulder impingement syndrome: A randomized clinical trial. Clinical Rheumatology 2013;32(1):73‐85. [DOI] [PubMed] [Google Scholar]
Subasi 2012 {published data only}
- Subasi V, Toktas H, Seçil DÜ, Turel A, Çakir T, Kavuncu V. Water‐based versus land‐based exercise program for the management of shoulder impingement syndrome. Turkish Journal of Physical Medicine & Rehabilitation 2012;58:79‐84. [Google Scholar]
Surenkok 2009 {published data only}
- Surenkok O, Aytar A, Baltaci G. Acute effects of scapular mobilization in shoulder dysfunction: a double‐blind randomized placebo‐controlled trial. Journal of Sport Rehabilitation 2009;18:493‐501. [DOI] [PubMed] [Google Scholar]
Szczurko 2009 {published data only}
- Szczurko O, Cooley K, Mills EJ, Zhou Q, Perri D, Seely D. Naturopathic treatment of rotator cuff tendinitis among Canadian postal workers: a randomized controlled trial. Arthritis and Rheumatism 2009;61:1037‐45. [DOI] [PubMed] [Google Scholar]
Teys 2008 {published data only}
- Teys P, Bisset L, Vicenzino B. The initial effects of a Mulligan's mobilization with movement technique on range of movement and pressure pain threshold in pain‐limited shoulders. Manual Therapy 2008;18:37‐42. [DOI] [PubMed] [Google Scholar]
Van den Dolder 2003 {published data only}
- Dolder PA, Roberts DL. A trial into the effectiveness of soft tissue massage in the treatment of shoulder pain. Australian Journal of Physiotherapy 2003;49:183‐8. [DOI] [PubMed] [Google Scholar]
Walther 2004 {published data only}
- Walther M, Werner A, Stahlschmidt T, Woelfel R, Gohlke F. The subacromial impingement syndrome of the shoulder treated by conventional physiotherapy, self‐training, and a shoulder brace: results of a prospective, randomized study. Journal of Shoulder and Elbow Surgery 2004;13:417‐23. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang SS, Trudelle‐Jackson EJ. Comparison of customized versus standard exercises in rehabilitation of shoulder disorders. Clinical Rehabilitation 2006;20:675‐85. [DOI] [PubMed] [Google Scholar]
Winters 1997 {published data only}
- Winters JC, Jorritsma W, Groenier KH, Sobel JS, Meyboom‐de Jong B, Arendzen HJ. Treatment of shoulder complaints in general practice: long term results of a randomised, single blind study comparing physiotherapy, manipulation, and corticosteroid injection. BMJ 1999;318:1395‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom‐de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ 1997;340:1320‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yiasemides 2011 {published data only}
- Yiasemides R, Halaki M, Cathers I, Ginn KA. Does passive mobilization of shoulder region joints provide additional benefit over advice and exercise alone for people who have shoulder pain and minimal movement restriction? A randomized controlled trial. Physical Therapy 2011;91:178‐89. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bergman 2004 {published data only}
- Bergman GJ, Winters JC, Groenier KH, Pool JJ, Meyboom‐de Jong B, Postema K, et al. Manipulative therapy in addition to usual medical care for patients with shoulder dysfunction and pain: a randomized, controlled trial. Annals of Internal Medicine 2004;141:432‐9. [DOI] [PubMed] [Google Scholar]
Bron 2011 {published data only}
- Bron C, Gast A, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. Treatment of myofascial trigger points in patients with chronic shoulder pain: a randomized, controlled trial. BMC Medicine 2011;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen JF, Ginn KA, Herbert RD. Passive mobilisation of shoulder region joints plus advice and exercise does not reduce pain and disability more than advice and exercise alone: a randomised trial. Australian Journal of Physiotherapy 2009;55:17‐23. [DOI] [PubMed] [Google Scholar]
Geraets 2005 {published data only}
- Geraets JJ, Goossens ME, Groot IJ, Bruijn CP, Bie RA, Dinant GJ, et al. Effectiveness of a graded exercise therapy program for patients with chronic shoulder complaints. Australian Journal of Physiotherapy 2005;51:87‐94. [DOI] [PubMed] [Google Scholar]
Ginn 1997 {published data only}
- Ginn KA, Herbert RD, Khouw W, Lee R. A randomized, controlled clinical trial of a treatment for shoulder pain. Physical Therapy 1997;77:802‐9. [DOI] [PubMed] [Google Scholar]
Hakguder 2011 {published data only}
- Hakgüder A, Taştekın N, Bırtane M, Uzunca K, Zaterı C, Süt N. Comparison of the short‐term efficacy of physical therapy in subacromial impingement syndrome patients with stage I and II magnetic resonance imaging findings. Turkish Journal of Rheumatology 2011;26(2):127‐34. [Google Scholar]
Jinhwa 2012 {published data only}
- Jinhwa J, Kihun C, Jaeho Y. Effects of scapular stabilizing exercise in patients with partial‐thickness rotator cuff tear. Journal of Physical Therapy Science 2012;24(11):1173‐5. [Google Scholar]
Krischak 2013 {published data only}
- Krischak G, Gebhard F, Reichel H, Friemert B, Schneider F, Fisser C, et al. A prospective randomized controlled trial comparing occupational therapy with home‐based exercises in conservative treatment of rotator cuff tears. Journal of Shoulder and Elbow Surgery 2013;22(9):1173‐9. [DOI] [PubMed] [Google Scholar]
Merolla 2013 {published data only}
- Merolla G, Bianchi P, Porcellini G. Ultrasound‐guided subacromial injections of sodium hyaluronate for the management of rotator cuff tendinopathy: a prospective comparative study with rehabilitation therapy. Musculoskeletal Surgery 2013;97(Suppl 1):49‐56. [DOI] [PubMed] [Google Scholar]
Miller 2004 {published data only}
- Miller JS, Stanley I, Moore K. Videotaped exercise instruction: a randomised controlled trial in musculoskeletal physiotherapy. Physiotherapy Theory & Practice 2004;20:145‐54. [Google Scholar]
Molsberger 2010 {published data only}
- Molsberger AF, Schneider T, Gotthardt H, Drabik A. German randomized acupuncture trial for chronic shoulder pain (GRASP) ‐ a pragmatic, controlled, patient‐blinded, multi‐centre trial in an outpatient care environment. Pain 2010;151:146‐54. [DOI] [PubMed] [Google Scholar]
Mörl 2011 {published data only}
- Mörl F, Matkey A, Bretschneider S, Bernsdorf A, Bradl I. Pain relief due to physiotherapy doesn't change the motor function of the shoulder. Journal of Bodywork and Movement Therapies 2011;15:309‐18. [DOI] [PubMed] [Google Scholar]
Muth 2012 {published data only}
- Muth S, Barbe MF, Lauer R, McClure P. The effects of thoracic spine manipulation in subjects with signs of rotator cuff tendinopathy. Journal of Orthopaedic and Sports Physical Therapy 2012;42(12):1005‐1016. [DOI] [PubMed] [Google Scholar]
Saggini 2010 {published data only}
- Saggini R, Cavezza T, Pancrazio L, Pisciella V, Saladino G, Zuccaro MC, et al. Treatment of lesions of the rotator cuff. Journal of Biological Regulators and Homeostatic Agents 2010;24(4):453‐9. [PubMed] [Google Scholar]
Seok‐Hwa 2013 {published data only}
- Seok‐Hwa C, Byoung‐Hee L, Eun Jung C. The effects of stability exercises on shoulder pain and function of middle‐aged women. Journal of Physical Therapy Science 2013;25(2):155‐8. [Google Scholar]
Tachibana 2012 {published data only}
- Tachibana K, Ueki N, Uchida T, Koga H. Randomized comparison of the therapeutic effect of acupuncture, massage, and Tachibana‐style‐method on stiff shoulders by measuring muscle firmness, VAS, pulse, and blood pressure. Evidence‐based Complementary and Alternative Medicine 2012;2012:989705. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Acosta 2009 {published data only}
- Acosta TB, Rodríguez EQ, Pérez YL, Tápanes SH, Morales IP, Lotti AG. Physical treatment reoutfitter in the painful shoulder. Revista Iberoamericana de Fisioterapia y Kinesiologia 2009;12:12‐9. [Google Scholar]
Bicer 2005 {published data only}
- Bicer A, Ozisik S, Aksik SC, Erdogan C. Comparison of local corticosteroid injection and conventional physical therapy in management of the painful shoulder. Turkiye Klinikleri Tip Bilimleri Dergisi 2005;25(4):506‐12. [Google Scholar]
Bube 2010 {published data only}
- Bube J, Hettasch J, Ruetz M, Schwerla F. Osteopathic treatment of patients with shoulder pain: a randomized controlled trial. International Journal of Osteopathic Medicine 2010;13:118. [Google Scholar]
Ellegaard 2013 {published data only}
- Ellegaard K, Christensen R, Mortensen SR, Bartholby C, Torp‐Pedersen S, Bandholm T, et al. Exercise therapy and ultrasound guided glucocorticoid injection in patients with painful shoulder: a randomised controlled trial. Arthritis and Rheumatism 2013;65:S1162. [Google Scholar]
Ginn 2009 {published data only}
- Ginn K. Do passive mobilizations applied to shoulder region joints improve the treatment outcome in patients with shoulder pain?. New Zealand Journal of Physiotherapy 2009;37:148. [Google Scholar]
Just 2009 {published data only}
- Just H, Stelzer L. Effectiveness of manual therapy in patients with shoulder pain: randomised controlled trial. Manuelle Therapie 2009;13:212‐8. [Google Scholar]
Leblebici 2007 {published data only}
- Leblebici B, Adam M, Yapgu S, Bags S, Akman MN. Comparing the effects of open versus closed kinetic chain scapulohumeral stability exercises in rotator cuff problems. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2007;53(4):134‐7. [Google Scholar]
Pribicevic 2006 {published data only}
- Pribicevic M, Pollard H. A randomised controlled clinical trial of manual therapy treatment for shoulder pain. Journal of Science and Medicine in Sport 2006;9:23. [Google Scholar]
Werner 2002 {published data only}
- Werner A, Walther M, Ilg A, Stahlschmidt T, Gohlke F. Self‐training versus conventional physiotherapy in subacromial impingement syndrome. Zeitschrift für Orthopädie und ihre Grenzgebiete 2002;140:375‐80. [DOI] [PubMed] [Google Scholar]
Wiener 2005 {published data only}
- Weiner M, Mayer F. Effects of physiotherapy on peak torque and pain in patients with tendinitis of the supraspinatus muscle. Deutsche Zeitschrift für Sportmedizin 2005;56:383‐7. [Google Scholar]
Wies 2008 {published data only}
- Wies JT, Humphreys H, Latham M, Enrico P, Viljoen T, Hazleman B, et al. A randomised placebo‐controlled trial of physiotherapy for rotator cuff tendinopathies [abstract]. The Journal of Bone and Joint Surgery 2008;90‐B:213. [Google Scholar]
References to ongoing studies
Roddy 2014 {published data only}
- Roddy E, Ogollah R, Zwierska I, Datta P, Hall A, Hay E, et al. Clinical effectiveness of exercise and corticosteroid injection for subacromial impingement syndrome: a randomised controlled trial. Arthritis and Rheumatology 2014;66:S489‐S90. [Google Scholar]
- Roddy E, Zwierska I, Hay EM, Jowett S, Lewis M, Stevenson K, et al. Subacromial impingement syndrome and pain: Protocol for a randomised controlled trial of exercise and corticosteroid injection (the SUPPORT trial). BMC Musculoskeletal Disorders 2014;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Dolder 2010 {published data only}
- Dolder P, Ferreira P, Refshauge K. Is soft tissue massage an effective treatment for mechanical shoulder pain? A study protocol. Journal of Manual and Manipulative Therapy 2010;18(1):50‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Angst 2011
- Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care and Research 2011;63:S174‐88. [DOI] [PubMed] [Google Scholar]
Bennell 2014
- Bennell KL, Egerton T, Martin J, Abbott JH, Metcalf B, McManus F, et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA 2014;311(19):1987‐97. [DOI] [PubMed] [Google Scholar]
Bialosky 2009
- Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Manual Therapy 2009;14(5):531‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brantingham 2011
- Brantingham JW, Cassa TK, Bonnefin D, Jensen MT, Globe G, Hicks M, et al. Manipulative therapy for shoulder pain and disorders: expansion of a systematic review. Journal of Manipulative and Physiological Therapeutics 2011;34:314‐46. [DOI] [PubMed] [Google Scholar]
Braun 2010
- Braun C, Hanchard NCA. Manual therapy and exercise for impingement related shoulder pain. Physical Therapy Reviews 2010;15(2):62‐83. [Google Scholar]
Braun 2013
- Braun C, Bularczyk M, Heintsch J, Hanchard NCA. Manual therapy and exercises for shoulder impingement revisited. Physical Therapy Reviews 2013;18(4):263‐84. [Google Scholar]
Brudvig 2011
- Brudvig TJ, Kulkarni H, Sah S. The effect of therapeutic exercise and mobilization on patients with shoulder dysfunction: A systematic review with meta‐analysis. Journal of Orthopaedic and Sports Physical Therapy 2011;41:734‐48. [DOI] [PubMed] [Google Scholar]
Buchbinder 1996
- Buchbinder R, Goel V, Bombardier C, Hogg‐Johnson S. Classification systems of soft tissue disorders of the neck and upper limb: do they satisfy methodological guidelines?. Journal of Clinical Epidemiology 1996;49(2):141‐9. [DOI] [PubMed] [Google Scholar]
Buchbinder 2003
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2007
- Buchbinder R, Youd JM, Green S, Stein A, Forbes A, Harris A, et al. Efficacy and cost‐effectiveness of physiotherapy following glenohumeral joint distension for adhesive capsulitis: a randomized trial. Arthritis and Rheumatism 2007;57:1027‐37. [DOI] [PubMed] [Google Scholar]
Camarinos 2009
- Camarinos J, Marinko L. Effectiveness of manual physical therapy for painful shoulder conditions: a systematic review. Journal of Manual & Manipulative Therapy 2009;17:206‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2004 [Computer program]
- Dr Chris Cates EBM website. Available from http://www.nntonline.net/. Visual Rx NNT Calculator. Dr Chris Cates EBM website. Available from http://www.nntonline.net/, 2004 (accessed 15 November 2015).
Constant 1987
- Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clinical Orthopaedics and Related Research 1987;Jan(214):160‐4. [PubMed] [Google Scholar]
Croft 1994
- Croft P, Pope D, Zonca M, O'Neill T, Silman A. Measurement of shoulder related disability: results of a validation study. Annals of the Rheumatic Diseases 1994;53:525‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dewhurst 2010
- Dewhurst A. An exploration of evidence‐based exercises for shoulder impingement syndrome. International Musculoskeletal Medicine 2010;32(3):111‐6. [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dziedzic 1999
- Dziedzic K, Sim J, Hiller L, Ainsworth RL, Stevenson K, Hay EM. A survey to determine standard physiotherapy treatment for a pragmatic randomised trial of the long‐term effectiveness of local steroid injection versus physiotherapy for shoulder pain. Annals of the Rheumatic Diseases 1999;58:S227. [Google Scholar]
Ebrahim 2013
- Ebrahim S, Akl EA, Mustafa RA, Sun X, Walter SD, Heels‐Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2013;66(9):1014‐21. [DOI] [PubMed] [Google Scholar]
Edmonds 2014
- Edmonds EW, Dengerink DD. Common conditions in the overhead athlete. American Family Physician 2014;89(7):537‐41. [PubMed] [Google Scholar]
Gebremariam 2014
- Gebremariam L, Hay EM, Sande R, Rinkel WD, Koes BW, Huisstede BM. Subacromial impingement syndrome ‐ effectiveness of physiotherapy and manual therapy. British Journal of Sports Medicine 2014;48(16):1202‐8. [DOI] [PubMed] [Google Scholar]
Glazier 1998
- Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R, et al. Management of common musculoskeletal problems: a survey of Ontario primary care physicians. CMAJ 1998;158:1037‐40. [PMC free article] [PubMed] [Google Scholar]
Hanratty 2012
- Hanratty CE, McVeigh JG, Kerr DP, Basford JR, Finch MB, Pendleton A, et al. The effectiveness of physiotherapy exercises in subacromial impingement syndrome: a systematic review and meta‐analysis. Seminars in Arthritis and Rheumatism 2012;42(3):297‐316. [DOI] [PubMed] [Google Scholar]
Hawker 2011
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care and Research 2011;63:S240‐52. [DOI] [PubMed] [Google Scholar]
Hermans 2013
- Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma‐Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease?: The Rational Clinical Examination systematic review. JAMA 2013;310(8):837‐47. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 2009
- Ho CY, Sole G, Munn J. The effectiveness of manual therapy in the management of musculoskeletal disorders of the shoulder: a systematic review. Manual Therapy 2009;14:463‐74. [DOI] [PubMed] [Google Scholar]
Kaltenborn 1976
- Kaltenborn FM. Manual therapy for the extremity joints. Oslo, Norway: Olaf Norlis Bokhandel, 1976. [Google Scholar]
Kay 2012
- Kay TM, Gross A, Goldsmith CH, Rutherford S, Voth S, Hoving JL, et al. Exercises for mechanical neck disorders. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD004250.pub4] [DOI] [PubMed] [Google Scholar]
Kelly 2010
- Kelly SM, Wrightson PA, Meads CA. Clinical outcomes of exercise in the management of subacromial impingement syndrome: a systematic review. Clinical Rehabilitation 2010;24(2):99‐109. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Klintberg 2015
- Klintberg IH, Cools AM, Holmgren TM, Holzhausen AC, Johansson K, Maenhout AG, et al. Consensus for physiotherapy for shoulder pain. International Orthopaedics 2015;39(4):715‐20. [DOI] [PubMed] [Google Scholar]
Kooijman 2013
- Kooijman M, Swinkels I, Dijk C, Bakker D, Veenhof C. Patients with shoulder syndromes in general and physiotherapy practice: an observational study. BMC Musculoskeletal Disorders 2013;14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kromer 2009
- Kromer TO, Tautenhahn UG, Bie RA, Staal JB, Bastiaenen CHG. Effects of physiotherapy in patients with shoulder impingement syndrome: a systematic review of the literature. Journal of Rehabilitation Medicine 2009;41(11):870‐80. [DOI] [PubMed] [Google Scholar]
Kuhn 2009
- Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence‐based rehabilitation protocol. Journal of Shoulder and Elbow Surgery 2009;18(1):138‐60. [DOI] [PubMed] [Google Scholar]
Largacha 2006
- Largacha M, Parsons IM, Campbell B, Titelman RM, Smith KL, Matsen F. Deficits in shoulder function and general health associated with sixteen common shoulder diagnoses: a study of 2674 patients. Journal of Shoulder and Elbow Surgery 2006;15(1):30‐9. [DOI] [PubMed] [Google Scholar]
Linsell 2006
- Linsell L, Dawson J, Zondervan K, Rose P, Randall T, Fitzpatrick R, et al. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology 2006;45:215‐21. [DOI] [PubMed] [Google Scholar]
Littlewood 2012
- Littlewood C, Ashton J, Chance‐Larsen K, May S, Sturrock B. Exercise for rotator cuff tendinopathy: a systematic review. Physiotherapy 2012;98(2):101‐9. [DOI] [PubMed] [Google Scholar]
Luime 2004
- Luime JJ, Koes BW, Hendriksen IJM, Burdof A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population: a systematic review. Scand J Rheumatol 2004;33:73‐81. [DOI] [PubMed] [Google Scholar]
Maitland 1977
- Maitland GD. Peripheral manipulation. London, United Kingdom: Butterworths, 1977. [Google Scholar]
Marinko 2011
- Marinko LN, Chacko JM, Dalton D, Chacko CC. The effectiveness of therapeutic exercise for painful shoulder conditions: a meta‐analysis. Journal of Shoulder and Elbow Surgery 2011;20:1351‐9. [DOI] [PubMed] [Google Scholar]
Michaleff 2011
- Michaleff ZA, Costa LO, Moseley AM, Maher CG, Elkins MR, Herbert RD, et al. CENTRAL, PEDro, PubMed, and EMBASE are the most comprehensive databases indexing randomized controlled trials of physical therapy interventions. Physical Therapy 2011;91(2):190‐7. [DOI] [PubMed] [Google Scholar]
Miller 2010
- Miller J, Gross A, D'Sylva J, Burnie SJ, Goldsmith CH, Graham N, et al. Manual therapy and exercise for neck pain: a systematic review. Manual Therapy 2010;15(4):334‐54. [PubMed] [Google Scholar]
Miller 2014
- Miller J, Gross A, Kay TM, Graham N, Burnie SJ, Goldsmith CH, et al. Manual therapy with exercise for neck pain. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011225] [DOI] [Google Scholar]
Mroz 2014
- Mroz TM, Carlini AR, Archer KR, Wegener ST, Hoolachan JI, Stiers W, et al. Frequency and cost of claims by injury type from a state workers' compensation fund from 1998 through 2008. Archives of Physical Medicine and Rehabilitation 2014;95(6):1048‐54. [DOI] [PubMed] [Google Scholar]
Nyberg 2010
- Nyberg A, Jonsson P, Sundelin G. Limited scientific evidence supports the use of conservative treatment interventions for pain and function in patients with subacromial impingement syndrome: randomized control trials. Physical Therapy Reviews 2010;15(6):436‐52. [Google Scholar]
Ostör 2005
- Ostör AJ, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology 2005;44(6):800‐5. [DOI] [PubMed] [Google Scholar]
Page 2013
- Page MJ, McKenzie JE, Forbes A. Many scenarios exist for selective inclusion and reporting of results in randomized trials and systematic reviews. Journal of Clinical Epidemiology 2013;66(5):524‐37. [DOI] [PubMed] [Google Scholar]
Page 2014a
- Page MJ, Green S, Kramer S, Johnston RV, McBain B, Chau M, et al. Manual therapy and exercise for adhesive capsulitis (frozen shoulder). Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2014b
- Page MJ, Green S, Kramer S, Johnston RV, McBain B, Buchbinder R. Electrotherapy modalities for adhesive capsulitis (frozen shoulder). Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2015
- Page MJ, McKenzie JE, Green SE, Beaton DE, Jain NB, Lenza M, et al. Core domain and outcome measurement sets for shoulder pain trials are needed: systematic review of physical therapy trials. Journal of Clinical Epidemiology 2015;68(11):1270‐81. [DOI: 10.1016/j.jclinepi.2015.06.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pribicevic 2010
- Pribicevic M, Pollard H, Bonello R, Luca K. A systematic review of manipulative therapy for the treatment of shoulder pain. Journal of Manipulative and Physiological Therapeutics 2010;33:679‐89. [DOI] [PubMed] [Google Scholar]
Reilingh 2008
- Reilingh ML, Kuijpers T, Tanja‐Harfterkamp AM, Windt D. Course and prognosis of shoulder symptoms in general practice. Rheumatology 2008;47(5):724‐30. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roach 1991
- Roach KE, Budiman‐Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care and Research 1991;4:143‐9. [PubMed] [Google Scholar]
Roberts 2014
- Roberts D, Li F. The presentation and outcomes of shoulder pain in public hospital physiotherapy departments in NSW: an observational study. Physiotherapy Theory and Practice 2014;30(5):299‐305. [DOI] [PubMed] [Google Scholar]
Roy 2009
- Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis and Rheumatism 2009;61:623‐32. [DOI] [PubMed] [Google Scholar]
Roy 2010
- Roy JS, MacDermid JC, Woodhouse LJ. A systematic review of the psychometric properties of the Constant‐Murley score. Journal of Shoulder and Elbow Surgery 2010;19:157‐64. [DOI] [PubMed] [Google Scholar]
Saltychev 2015
- Saltychev M, Äärimaa V, Virolainen P, Laimi K. Conservative treatment or surgery for shoulder impingement: systematic review and meta‐analysis. Disability and Rehabilitation 2015;37(1):1‐8. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schellingerhout 2008
- Schellingerhout JM, Verhagen AP, Thomas S, Koes BW. Lack of uniformity in diagnostic labeling of shoulder pain: time for a different approach. Manual Therapy 2008;13:478‐83. [DOI] [PubMed] [Google Scholar]
Schultz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152:Epub 24 March. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Slade 2014
- Slade SC, Dionne CE, Underwood M, Buchbinder R. Standardised method for reporting exercise programmes: protocol for a modified Delphi study. BMJ Open 2014;4(12):e006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slade 2016
- Slade SC, Dionne CE, Underwood M, Buchbinder R, Beck B, Bennell K, et al. Consensus on Exercise Reporting Template (CERT): A Modified Delphi Study. Physical Therapy 2016. [DOI: 10.2522/ptj.20150668] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Struyf 2012
- Struyf F, Hertogh W, Gulinck J, Nijs J. Evidence‐based treatment methods for the management of shoulder impingement syndrome among Dutch‐speaking physiotherapists: an online, web‐based survey. Journal of Manipulative and Physiological Therapeutics 2012;35(9):720‐6. [DOI] [PubMed] [Google Scholar]
Van den Dolder 2014
- Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non‐specific shoulder pain: a systematic review with meta‐analysis. British Journal of Sports Medicine 2014;48(16):1216‐26. [DOI] [PubMed] [Google Scholar]
Van der Heijden 1999
- Heijden GJ. Shoulder disorders: a state‐of‐the‐art review. Best Practice & Research Clinical Rheumatology 1999;13:287‐309. [DOI] [PubMed] [Google Scholar]
Virta 2012
- Virta L, Joranger P, Brox J, Eriksson R. Costs of shoulder pain and resource use in primary health care: a cost‐of‐illness study in Sweden. BMC Musculoskeletal Disorders 2012;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2012
- Walker H, Gabbe B, Wajswelner H, Blanch P, Bennell K. Shoulder pain in swimmers: a 12‐month prospective cohort study of incidence and risk factors. Physical Therapy in Sport 2012;13(4):243‐9. [DOI] [PubMed] [Google Scholar]
Whittle 2015
- Whittle S, Buchbinder R. Rotator cuff disease. Annals of Internal Medicine 2015;162(1):ITC1. [DOI: 10.7326/AITC201501060] [DOI] [PubMed] [Google Scholar]
Yamamoto 2010
- Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, et al. Prevalence and risk factors of a rotator cuff tear in the general population. Journal of Shoulder and Elbow Surgery 2010;19(1):116‐20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Green 1998
- Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment and efficacy. BMJ 1998;316:354‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2003
- Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004258] [DOI] [PMC free article] [PubMed] [Google Scholar]


