Abstract
Neuropathic pain in spinal cord injury (SCI) is associated with inflammation in both the peripheral and central nervous system (CNS), which may contribute to the initiation and maintenance of persistent pain. An understanding of factors contributing to neuroinflammation may lead to new therapeutic targets for neuropathic pain. Moreover, novel circulating biomarkers of neuropathic pain may facilitate earlier and more effective treatment. MicroRNAs (miRNAs) are short, non-coding single-stranded RNA that have emerged as important biomarkers and molecular mediators in physiological and pathological conditions. Using a genome-wide miRNA screening approach, we studied differential miRNA expression in plasma from 68 healthy, community-dwelling adults with and without SCI enrolled in ongoing clinical studies. We detected 2367 distinct miRNAs. Of these, 383 miRNAs were differentially expressed in acute SCI or chronic SCI versus no SCI and 71 were differentially expressed in chronic neuropathic pain versus no neuropathic pain. We selected homo sapiens (hsa)-miR-19a-3p and hsa-miR-19b-3p for additional analysis based on p-value, fold change, and their known role as regulators of neuropathic pain and neuroinflammation. Both hsa-miR-19a-3p and hsa-miR-19b-3p levels were significantly higher in those with chronic SCI and severe neuropathic pain versus those with chronic SCI and no neuropathic pain. In confirmatory studies, both hsa-miR-19a-3p and hsa-miR-19b-3p have moderate to strong discriminative ability to distinguish between those with and without pain. After adjusting for opioid use, hsa-miR-19b-3p levels were positively associated with pain interference with mood. Because hsa-miR-19 levels have been shown to change in response to exercise, folic acid, and resveratrol, these studies suggest that miRNAs are potential targets of therapeutic interventions.
Keywords: biomarker, microRNA, neuropathic pain, rehabilitation medicine, spinal cord injury
Introduction
According to the International Association for the Study of Pain (IASP), neuropathic pain is caused by a lesion or disease of the somatosensory nervous system generally classified as central or peripheral.1 There are nearly 17,900 new cases of spinal cord injury (SCI) each year with over 296,000 individuals living with SCI in the United States.2 SCI-induced neuropathic pain represents a significant clinical challenge that affects 50–70% of men and women living with SCI. Chronic neuropathic pain is associated with significant morbidity and there are few effective treatment options. Moreover, the underlying mechanisms contributing to chronic neuropathic pain after SCI are poorly understood. Therefore, the elucidation of pathophysiological mechanisms involved in neuropathic pain associated with SCI is essential for the development of novel mechanism-based therapeutic interventions. Identification of a novel circulating biomarker of neuropathic pain would have great clinical utility as this might facilitate earlier identification and treatment of individuals likely to develop chronic neuropathic pain.
Accumulating evidence suggests that a class of small non-coding inhibitory RNAs known as microRNAs (miRNAs) play an important role in regulating pain processing in a wide range of experimental models.3 miRNAs are non-coding single-stranded RNA of 19–24 nucleotides with the ability to modulate a large proportion of the genome post-transcriptionally. They bind to the 3′ untranslated region (UTR), or occasionally to 5′ UTRs, of the multiple messenger RNA (mRNA) targets to which they exhibit imperfect, or sometimes, perfect complementarity. This enables one specific miRNA to inhibit expression of multiple genes.4–6
Specific changes in protein expression in peripheral nociceptive and central neurons are thought to contribute to the development of hyper-excitability leading to persistent chronic neuropathic pain.1 Because SCI-induced neuropathic pain is mediated by, among other factors, neuronal protein expression, the process can potentially be regulated by miRNAs. Many studies have been done in rodent models of neuropathy investigating changes in miRNA expression both centrally and peripherally. Most have demonstrated dysregulation in numerous miRNAs.7–10 It has been reported that a lesion in the periphery (sciatic nerve) can cause downregulation of specific miRNAs centrally in post-synaptic neurons of the nucleus accumbens.3 Further, these changes may be involved in the development of co-morbid conditions, such as anxiety and sleep disorders, that are associated with neuropathic pain.
There is a general consensus in that miRNA alterations that occur both in the central nervous system (CNS) and the peripheral nervous system (PNS) mediate or are associated with neuropathic pain. Evidence suggests that miRNAs can influence microglial activity and neuroinflammation by controlling expression levels of proteins that stimulate or inhibit microglial activation.11,12 Neuroinflammation is thought to contribute to neuropathic pain after SCI. To date, there is limited information on miRNAs as regulators of neuropathic pain after SCI. To begin to address this gap, we conducted untargeted genome-wide miRNA screenings to assess differential miRNA expression in plasma from healthy, community-dwelling adults with and without SCI and with or without severe neuropathic pain enrolled in ongoing clinical studies.
Methods
Subjects
We studied a convenience sample of participants with and without SCI who were enrolled in one of two ongoing clinical trials to improve bone health or in an observational study assessing surgical treatment for severe neuropathic pain. For all participants, data were derived from baseline testing. The first clinical trial (ClinicalTrials.gov Identifier: NCT02533713 and institutional review board [IRB] ID 919227-45) included participants with and without chronic SCI. Inclusion criteria for participants with SCI were as follows: 18 years of age or older, injury duration of 3 years or more, use of a wheelchair as the primary mode of mobility (more than 50% of the time), SCI level C7–T12, height 155–191 cm, weight less than 113 kg, spasticity in both lower extremities less than 3 on the Modified Ashworth Scale (MAS), and sufficient upper body strength to complete sit-to-sit transfers. Exclusion criteria included enrollment in another clinical trial, pregnancy, orthostatic hypotension with symptomatic fall in blood pressure >30 mm Hg when upright, an active grade 2 or greater pressure ulcer, an unhealed limb or pelvic bone fracture, history of other neurological disease (e.g., stroke, peripheral neuropathy, myopathy), active treatment for epilepsy or thyroid disorders, active use of medications potentially affecting bone health (bisphosphonates, androgenic steroids, estrogenic steroids, lithium, glucocorticoid use for more than 3 months). Participants without SCI were 18 years of age or older and were age, gender, and ethnicity matched to the enrolled participants with SCI. Pregnant women without SCI were excluded.
The second clinical trial (ClinicalTrials.gov Identifier: NCT02946424 and IRB ID 962729-29) included participants with acute SCI (within 3 months of injury) who were 18–60 years of age and who used a wheelchair as their primary mode of mobility (more than 50% of the time). Participants were excluded if they were enrolled in another clinical trial or had contraindications to simvastatin including: drug allergy, history of liver disease, active liver disease (elevated transaminases), moderate or heavy alcohol intake, renal dysfunction (glomerular filtration rate [GFR] of <60 mL/min calculated using the Cockcroft-Gault equation), concurrent use of drugs that cause myopathy or increase the risk of myopathy with simvastatin therapy (gemfibrozil, niacin, cyclosporine, danazol, amiodarone, dronedarone, ranolazine, calcium channel blockers, colchicine), strong CYP34A inhibitors (itraconazole, ketoconazole, posaconazole, voriconazole, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, boceprevir, telaprevir, nefazodone, cobicistat-containing products), uncontrolled or poorly controlled diabetes (hemoglobin [Hb] A1c >8.0%), or unstable anti-coagulation treatment as indicated by International Normalized Ratio (INR).
Additional exclusion criteria included metabolic bone disease, untreated thyroid disorder, history of bilateral oophorectomy, active use of medications potentially affecting bone health including bisphosphonates, androgenic steroids, estrogenic steroids, anti-epileptics, lithium, glucocorticoid use for more than 3 months, and those who received inhaled glucocorticoids in the past year, and pregnant or lactating women and women of childbearing potential who were unwilling or unable to use a reliable form of contraception. The observational study (IRB ID 1235452-13) included adults with SCI planning to undergo dorsal root entry zone lesioning surgery to alleviate severe neuropathic pain.
For this genome-wide miRNA expression profiling screen study, we studied all participants with banked baseline serum samples. This included 14 participants with acute SCI, 26 participants with chronic SCI, 5 participants with chronic SCI and severe neuropathic pain, and 23 participants without SCI who were sex-and age-matched to the chronic SCI group. We therefore studied a total of 68 participants who completed baseline testing between May 15, 2017 and November 05, 2019. The HealthOne IRB approved all protocols prior to initiation of the study, and all participants gave their written informed consent to participate.
Variable definition
Information regarding SCI, medical history, health habits, and medication use was obtained by a questionnaire at the time of enrollment. Injury level was considered dichotomously (paraplegia vs. tetraplegia). Age, age at injury, and injury duration were considered as continuous variables. Participants were asked to report a history of medication use including anti-depressants, nonsteroidal anti-inflammatory agents, opioids, gabapentin, and spasmolytics. For all medications, users were defined as those actively taking the drug at the time of testing. Smokers were defined as smoking 20 or more packs of cigarettes or using 336 g (12 oz) of tobacco or more in a lifetime or smoking 1 or more cigarettes a day for at least 1 year. Current smokers reported cigarette use within 1 month of testing. Smoking status was considered dichotomously (current smoker vs. never/past smoker). For active/ever smokers, cigarette exposure was also considered continuously (pack-years). Lifetime alcohol consumption was calculated based on report of average daily, weekly, or monthly quantity and frequency of alcohol consumption. Each glass of wine (4 oz = 10.8 g), beer (12 oz = 13.2 g), and shot of liquor (1.5 oz = 15.1 g) was converted to grams of alcohol.13,14 Neuropathic pain was assessed using the International Spinal Cord Injury Pain Data Set.15,16 Average pain intensity in the last week (0–10 numerical rating scale), pain interference with mood, pain interference with daily activities, and pain interference with sleep were considered as continuous variables. Neuropathic pain was also considered dichotomously (yes vs. no).
Motor score
Motor level and completeness of injury were confirmed by physical exam at study entry by the study physician according to the American Spinal Injury Association Impairment Scale (AIS) as previously described.17,18 Participants were classified as AIS A (sensory and motor complete, no sensory or motor function below the neurological level of injury), AIS B (motor complete, preservation of sensory but no motor function below the neurological level of injury), or AIS C (motor incomplete, sensory and motor function preserved below the neurological level, and more than half of key muscles below the neurological level are not strong enough to overcome gravity).
Biochemical analyses
Plasma samples were drawn into an EDTA tube and immediately delivered to the core blood research laboratory at our facility. The samples were centrifuged for 15 min at 2600 rpm (1459 × g) at 40℃ and stored at −80℃ until batch analysis. All miRNA analyses were performed at LC Sciences (Houston, TX, USA).
miRNA bioinformatics pipeline
Briefly, comprehensive miRNA/small RNA sequencing service included sample Quality Control (QC), library preparation, and sequencing (50 base pair sequencing, on average a minimum of 7–10 million reads per sample) (Fig. 1). Raw reads were subjected to an in-house program, ACGT101-miR (LC Sciences, Houston, TX, USA) to remove adapter dimers, junk, low complexity, common RNA families (rRNA, tRNA, snRNA, snoRNA), and repeats. Subsequently, unique sequences with length of 18 to 26 nucleotides were mapped to specific species precursors in miRBase 21.0 by BLAST search to identify known miRNAs and novel 3p- and 5p-derived miRNAs. Length variation at both 3’ and 5’ ends and one mismatch inside of the sequence were allowed in the alignment. The unique sequences mapping to specific species of mature miRNAs in hairpin arms were identified as known miRNAs. The unique sequences mapping to the other arm of known specific species precursor hairpin opposite to the annotated mature miRNA-containing arm were considered to be novel 5p- or 3p-derived miRNA candidates. The remaining sequences were mapped to other selected species precursors (with the exclusion of specific species) in miRBase 21.0 by BLAST search, and the mapped pre-miRNAs were further BLASTed against the specific species genomes to determine their genomic locations. The unmapped sequences were BLASTed against the specific genomes, and the hairpin RNA structures containing sequences were predicated from the flank 80 nt sequences using RNAfold software.
FIG. 1.
MicroRNA bioinformatics pipeline.
The criteria for secondary structure prediction were: 1) number of nucleotides in one bulge in stem (≤12); 2) number of base pairs in the stem region of the predicted hairpin (≥16); 3) cutoff of free energy (kCal/mol less than or equal to -15); 4) length of hairpin (up and down stems + terminal loop ≥50); 5) length of hairpin loop (≤20); 6) number of nucleotides in one bulge in mature region (≤8); 7) number of biased errors in one bulge in mature region (≤4); 8) number of biased bulges in mature region (≤2); 9) number of errors in mature region (≤7); 10) number of base pairs in the mature region of the predicted hairpin (≥12); and 11) percent of mature in stem (≥80).
Raw data were filtered using a filter module in an in-house program, ACGT101-miR, to delete low-quality reads, 3' adapter sequences, and contaminations. The sequences ≥18 nt of clean data were annotated in the Rfam database to remove non-coding RNA (rRNA, tRNA, snRNA, snoRNA) and degradation fragments of mRNA. The remaining sequences were aligned against an miRNA database, miRbase (Release 22), and perfectly matched sequences were considered conserved Homo sapiens (hsa) miRNAs. After processed by filter module in ACGT101-miR, unique reads were analyzed using ACGT101-miR to detect conserved and novel hsa miRNAs. Normalization of sequence counts in each sample (or data set) was achieved by dividing the counts by a library size parameter of the corresponding sample. The library size parameter is a median value of the ratio between the counts of a specific sample and a pseudo-reference sample. A count number in the pseudo-reference sample is the count geometric mean across all samples.
where Sj is the library size parameter; cij is the count number of sequence i of sample j; m is the total number of samples involved.
Target prediction analysis
Target genes potentially regulated by miRNAs of interest were predicted using the consensus of three publicly available miRNA target databases: TargetScan, MicroCosm, and miRTarBase. The predicted target false-positive rate was reduced significantly by applying a cutoff of −0.4 in the context scores for TargetScan results; each miRNA target was cross-referenced against this gene set. Enrichment and pathway analysis of predicted miRNA gene targets was undertaken using MetaCoreTM process networks, pathway maps, and GO molecular functions/processes. Top pathways were ranked based on z-score and the additional enriched gene count per pathway. Interactions between miRNAs and their target gene networks for each tissue were visualized using CyTargetLinker v3.0.1, an open source software package for Cytoscape v.4.0.43.
Circos v0.67 was used to display interaction between miRNAs and target genes in a circular layout, facilitating the visualization of the position of the miRNAs and target genes in the respective genome; only target genes passing the described cutoff were visualized in the CircosPlot. hsa-miR-19a-3p and hsa-miR-19b-3p from an experimental data set were loaded. yFiles Organic Layout was performed to better visualize physical and genetic interaction networks. Targets with strong experimental evidence for hsa-miR-19a-3p and hsa-miR-19b-3p were retrieved, following which, an extended literature search of the PubMed database was performed for further confirmation of neuro-associated validated targets.
Statistical analysis
We conducted two screenings: one based on presence or absence of SCI (No SCI n = 23, acute SCI n = 14, and chronic SCI n = 26) and one based on severe neuropathic pain in chronic SCI (n = 5, pain score range 8–10) versus no neuropathic pain in chronic SCI (n = 4). For each comparison, normalized deep-sequencing counts were analyzed by selectively using Fisher's exact test, χ2 2X2 test, χ2 nXn test, Student's t test, or analysis of variance (ANOVA) as appropriate. Because False Discovery Rate (FDR) adjusted p-values may be too conservative for screening studies, we determined differential expression based on log twofold concentration change and raw p-value <0.05. This study is adequately powered to detect twofold differences in expression between groups.19 All subsequent analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). To compare subject characteristics as appropriate, t tests or χ2 tests were used. General linear models (PROC GLM) were then applied including all participants with clinical/demographic data and miRNA values (n = 66, Table 1) to confirm the screening findings. We assessed associations between biomarker levels (hsa-miR-19a-3p and hsa-miR-19b-3p), pain, and pain interference scores. Pain interference with mood was selected for multi-variable model building based on univariate findings. Factors with a p-value of <0.10 in the univariate models were included in the multi-variable models of pain interference with mood. Factors with a p-value of <0.05 were considered statistically significant.
Table 1.
Characteristics of Confirmatory Cohort Based on Presence of Neuropathic Pain
| Variable |
No neuropathic pain (n = 38) |
Neuropathic pain (n = 28) |
Total (n = 66) |
P
|
|---|---|---|---|---|
| Demographics | ||||
| Age (years) [mean ± SD] | 36.62 ± 10.78 | 40.11 ± 12.77 | 38.10 ± 11.70 | 0.86 |
| Male, n (%) | 24 (63.1) | 24 (85.7) | 48 (72.7) | 0.04 |
| Injury characteristics | ||||
|---|---|---|---|---|
| AIS classification |
|
|
|
|
| • A, n (%) |
10 (66.7)a |
20 (71.4) |
30 (69.8) |
0.60 |
| • B, n (%) |
4 (26.7)a |
4 (14.3) |
8 (18.6) |
|
| • C, n (%) |
1 (6.6)a |
4 (14.3) |
5 (11.6) |
|
| Tetraplegia, n (%) |
4 (26.7)a |
6 (21.4) |
10 (23.3) |
0.71 |
| Injury duration (years) [mean ± SD] |
6.67 ± 8.10a |
8.81 ± 9.06 |
8.06 ± 8.70 |
<0.0001 |
| Age at injury (years) [mean ± SD] | 28.73 ± 11.41a | 30.39 ± 12.44e | 29.73 ± 11.91 | 0.003 |
| Medication use | ||||
|---|---|---|---|---|
| Active opioid use, n (%) |
4 (10.5) |
8 (34.8)e |
12 (19.7) |
0.04 |
| Active spasmolytic use, n (%) |
6 (15.8) |
10 (43.5)e |
16 (26.2) |
0.01 |
| Active gabapentin use, n (%) |
3 (7.9) |
10 (43.5)e |
13 (21.3) |
0.002 |
| Active antidepressant use, n (%) | 1 (2.6) | 2 (8.7)e | 3 (4.9) | 0.55 |
| Health habits | ||||
|---|---|---|---|---|
| Current alcohol user, n (%) |
32 (86.5)b |
19 (90.5)f |
51 (87.9) |
1.0 |
| Lifetime alcohol exposure kg/years [mean ± SD] |
147.78 ± 205.11c |
116.27 ± 97.21c |
136.04 ± 172.40 |
0.23 |
| Current smoker, n (%) |
1 (2.7)b |
4 (19.1)f |
5 (8.6) |
0.05 |
| Cigarette exposure (pack-years) [mean ± SD] | 9.67 ± 9.88d | 6.21 ± 5.03d | 7.56 ± 7.24 | 0.005 |
Among SCI n = 15; bavailable data for n = 37; camong ever alcohol users; damong ever smokers; eavailable data for n = 23; favailable data for n = 21.
AIS, American Spinal Injury Association Impairment Scale; SCI, spinal cord injury; SD, standard deviation.
Results
Subject characteristics of genome-wide microRNA expression profile screenings
Subject characteristics are presented in Table 2A,B. For the SCI versus No SCI screening (Table 2A), participants with No SCI were 37.1 ± 11.1 years of age (range 24.2 to 57.9 years) and 57% male. None reported neuropathic pain. Participants with acute SCI were 37.0 ± 10.8 years of age (range 21.2 to 54.7 years), 79% male, and 2.9 ± 0.7 months post-injury (range 1.7 to 4.4 months). Fifty-four percent reported neuropathic pain. Participants with chronic SCI were 36.5 ± 11.0 years of age (range 18.6 to 70.6 years), 77% male, and 10.4 ± 6.6 years post-injury (range 3.1 to 23.4 years). Sixty-five percent reported neuropathic pain. All participants with SCI used a wheelchair as their primary mode of mobility. Those with acute SCI were more likely to be tetraplegic than those with chronic SCI. Medication use (opioids, spasmolytics, and gabapentin) was more common in acute SCI than chronic SCI or No SCI. For the neuropathic pain in SCI screening (Table 2B), those with neuropathic pain in SCI were all males, 50.2 ± 16.9 year of age (range 30.2 to 72.0 years) and 16.6 ± 14.0 years post-injury (range 3.5 to 33.3 years). SCI without neuropathic pain were 75% male, 30.1 ± 6.7 years of age (25.2 to 40.0 years), and 8.0 ± 6.1 years post-injury (3.6 to 17.1 years).
Table 2A.
Characteristics of Genome-Wide MicroRNA Expression Profile Screening
| Variable |
No SCI (n = 23) |
Acute SCI (n = 14) |
Chronic SCI (n = 26) |
Total (n = 63) |
P
|
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) [mean ± SD] | 37.08 ± 11.07 | 36.95 ± 10.77 | 36.54 ± 10.97 | 36.83 ± 10.79 | 0.98 |
| Male, n (%) | 13 (56.5) | 11 (78.6) | 20 (76.9) | 44 (69.8) | 0.21 |
| Pain, n(%) | 11 (47.8) | 13 (100.0)a | 21 (80.8) | 45 (72.6) | 0.001 |
| Neuropathic pain, n (%) | 0 (0.0) | 7 (53.8)a | 17 (65.4) | 24 (38.7) | <0.0001 |
| Injury characteristics | |||||
|---|---|---|---|---|---|
| AIS classification |
N/A |
|
|
|
|
| • A, n (%) |
|
10 (71.4) |
17 (65.4) |
27 (67.5) |
0.88 |
| • B, n (%) |
|
3 (21.4) |
5 (19.2) |
8 (20.0) |
|
| • C, n (%) |
|
1 (7.2) |
4 (15.4) |
5 (12.5) |
|
| Tetraplegia, n (%) |
N/A |
8 (57.1) |
2 (7.7) |
10 (25.0) |
0.001 |
| Injury duration (years) [mean ± SD] |
N/A |
0.24 ± 0.06 |
10.35 ± 6.64 |
6.81 ± 7.21 |
<0.0001 |
| Age at injury (years) [mean ± SD] | N/A | 36.21 ± 10.70 | 25.76 ± 10.69 | 29.42 ± 11.70 | 0.005 |
| Biomarkers | |||||
|---|---|---|---|---|---|
| MiR-19a (normalized deep sequencing count) [mean ± SD] |
1243.83 ± 820.64 |
1671.36 ± 815.03 |
989.53 ± 642.25 |
1233.89 ± 782.86 |
0.02 |
| MiR-19b (normalized deep sequencing count) [mean ± SD] | 2573.04 ± 1440.92 | 3961.14 ± 1833.18 | 2100.00 ± 1137.60 | 2686.29 ± 1574.51 | 0.0009 |
| Medication use | |||||
|---|---|---|---|---|---|
| Active opioid use, n(%) |
0 (0.0) |
5 (35.7) |
7 (26.9) |
12 (19.1) |
0.003 |
| Active spasmolytic use, n (%) |
0 (0.0) |
10 (71.4) |
6 (23.1) |
16 (25.4) |
<0.0001 |
| Active gabapentin use, n (%) |
1 (4.4) |
8 (57.1) |
4 (15.4) |
13 (20.6) |
0.0008 |
| Active antidepressant use, n (%) | 0 (0.0) | 1 (7.1) | 2 (7.7) | 3 (4.7) | 0.43 |
| Health habits | |||||
|---|---|---|---|---|---|
| Current alcohol user, n (%) |
23 (100.0) |
9 (90.0)b |
20 (76.9) |
52 (88.1) |
0.03 |
| Lifetime alcohol exposure kg/years [mean ± SD] |
120.54 ± 109.56 |
72.71 ± 81.86c |
180.42 ± 239.39c |
135.30 ± 170.78 |
0.25 |
| Current smoker, n (%) |
0 (0.0) |
2 (20.0)b |
3 (11.5) |
5 (8.5) |
0.10 |
| Cigarette exposure (pack-years) [mean ± SD] | N/A | 12.51 ± 7.65d | 3.27 ± 3.72d | 7.16 ± 7.24 | 0.01 |
Available data for n = 13; bavailable data for n = 10; camong ever alcohol users; damong ever smokers.
SD, standard deviation.
Table 2B.
Characteristics of Genome-Wide MicroRNA Expression Profile Screening Neuropathic Pain Cohort
| Variable |
No neuropathic pain (n = 4) |
Neuropathic pain (n = 5) |
Total (n = 9) |
P
|
|---|---|---|---|---|
| Demographics | ||||
| Age (years) [mean ± SD] | 30.13 ± 6.72 | 50.16 ± 16.94 | 41.26 ± 16.49 | 0.06 |
| Male, n (%) | 3 (75.0) | 5 (100.0) | 8 (88.9) | 0.44 |
| Injury characteristics | ||||
|---|---|---|---|---|
| AIS classification |
|
|
|
|
| • A, n (%) |
3 (75.0) |
5 (100.0) |
8 (88.9) |
0.44 |
| • B, n (%) |
1 (25.0) |
0 (0.0) |
1 (11.1) |
|
| Tetraplegia, n (%) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
N/A |
| Injury duration (years) [mean ± SD] | 8.03 ± 6.13 | 16.62 ± 14.01 | 12.80 ± 11.52 | 0.29 |
| Biomarkers | ||||
|---|---|---|---|---|
| MiR-19a (normalized deep sequencing count) [mean ± SD] |
857.50 ± 390.35 |
3174.80 ± 1079.10 |
2144.89 ± 1459.80 |
0.006 |
| MiR-19b (normalized deep sequencing count) [mean ± SD] | 1791.75 ± 772.43 | 7005.20 ± 1873.36 | 4688.11 ± 3086.83 | 0.001 |
AIS, American Spinal Injury Association Impairment Scale; SD, standard deviation.
Differential expression of hsa-miR-19a-3p and hsa-miR19-b-3p levels
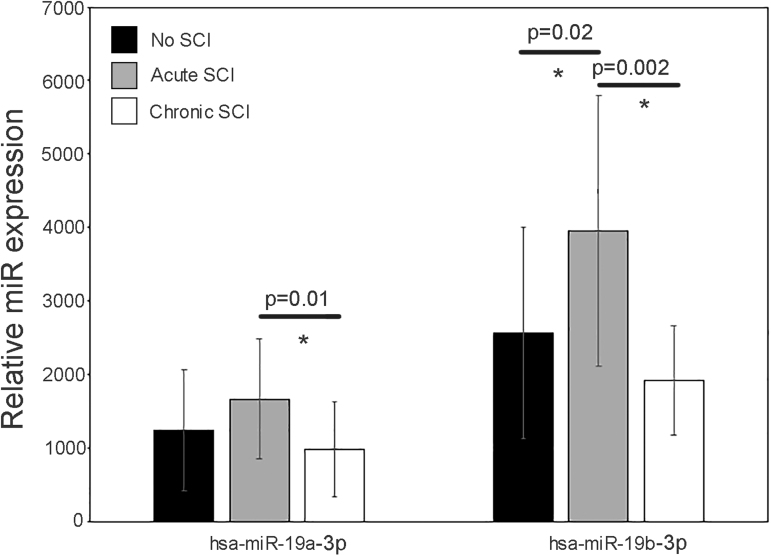
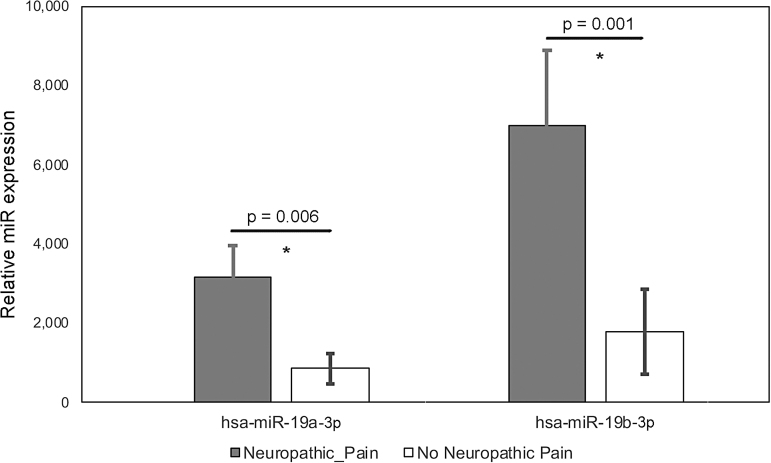
We detected 2367 distinct miRNAs. In acute SCI (146) or chronic SCI (237) versus No SCI, 383 miRNAs were significantly (p < 0.05) up- or downregulated. In acute SCI versus chronic SCI, 208 miRNAs were significantly (p < 0.05) up- or downregulated. In participants with chronic SCI reporting no neuropathic pain versus those reporting neuropathic pain, 71 miRNAs (Fig. 2, Table 2B) were significantly (p < 0.05) up or downregulated. We selected hsa-miR-19a-3p and hsa-miR-19b-3p for further analysis based on log twofold concentration change, p-value, and their reported role in neuropathic pain and neuroinflammation.20 One or both were differentially expressed in acute SCI versus No SCI (Fig. 3, 3961 vs. 2573, p = 0.02 for 19b), acute SCI versus chronic SCI (Fig. 3, 1671> vs. 990, p = 0.01 for 19a and 3961 vs. 2100, p = 0.002 for 19b), and neuropathic pain versus no neuropathic pain (Fig. 4, 3175 vs. 857, p = 0.006 for 19a and 7005 vs. 1792, p = 0.001 for 19b).
FIG. 2.
Volcano plot comparing Log2 (fold change) of miRNA expression values for participants who have a chronic SCI reporting no neuropathic pain (n = 4) versus those who have chronic SCI and report neuropathic pain (n = 5). Significantly upregulated genes shown in red, significantly downregulated genes shown in blue (p < 0.05). Some dots may represent more than one data point. Refer to Table 2B for participants characteristics. 19a: hsa-miR-19a-3p; 19b: hsa-miR-19b-3p. miRNA, microRNA; SCI, spinal cord injury.
FIG. 3.
Expression values of hsa-miR-19a-3p and hsa-miR-19b-3p in participants with no SCI (black bars, mean expression value), participants who have an acute SCI (gray bars, mean expression value), and participants who have a chronic SCI (white bars, mean expression value). Black horizontal lines indicate the p-values for the differences between groups. Error bars are SD. Asterisks (*) indicate significant differences between groups. SCI, spinal cord injury; SD, standard deviation.
FIG. 4.
Expression values of hsa-miR-19a-3p and hsa-miR-19b-3p for participants who have a chronic SCI reporting no neuropathic pain (white bars. mean expression value) versus those who have chronic SCI and report neuropathic pain (gray bars: mean expression value). Black horizontal lines indicate the p-values for the differences between groups. Error bars are SD. Asterisks (*) indicate significant differences between groups. SCI, spinal cord injury; SD, standard deviation.
Validated targets of miR-19a and miR-19b
Targets with strong experimental evidence for hsa-miR-19a-3p and hsa-miR-19b-3p were retrieved from miRTarBase (Fig. 5), following which, we performed an extended literature search of the PubMed database and further confirmed those neuro-associated validated targets. miR-19a and miR-19b representative validated target genes of miR-19a and miR-19b are shown in Table 3A–C. Phosphatase and Tensin Homolog (PTEN),21,22 Rap Guanine Nucleotide Exchange Factor (2Rapgef2),23 voltage-gated potassium channel subunits,20 Tumor Protein p53 (p53),24 and RUNX Family Transcription Factor 3 (RUNX3)25 are common targets shared by both miR-19a and miR-19b. Further, miR-19a has its own validated targets including Suppressor of Cytokine Signaling 1 (SOCS1), Methyl-CpG Binding Protein 2 (MeCP2), Ras Homolog Family Member B (RhoB), Peroxisome Proliferator Activated Receptor Alpha (PPARα), and Leucine Rich Repeats and Immunoglobulin Like Domains 1 (LRIG1).26 miR-19b also has its confirmed targets, FMR1 Autosomal Homolog 1 (FXR1)27 and Signal Transduction and Activator of Transcription 3 (STAT3).28 Among these validated targets, especially, SOCS1 was confirmed as a direct target of miR-19a in neuropathic pain rat models.29 Also, miR-19a and miR-19b regulate their common shared target, multiple functionally related voltage-gated potassium channel in chronic neuropathic pain in rats.20
FIG. 5.

microRNA-target interaction network generated by Cytoscape software demonstrating the density of shared and unique targets for each microRNA. Targets with strong experimental evidence for hsa-miR-19a-3p and hsa-miR-19b-3p were retrieved from miRTarBase. Blue color indicates targets shared by hsa-miR-19a-3p and hsa-miR-19b-3p, and yellow color indicates targets specific for either hsa-miR-19a-3p or hsa-miR-19b-3p.
Table 3A.
Validated Targets of Both MiR-19a and 19b
| Target | Model | Tissue | MicroRNA effect | Relative expression | References |
|---|---|---|---|---|---|
| Phosphatase and Tensin Homolog (PTEN)* |
In vitro Human In vitro Rat |
Malignant glioma cell lines Resected glioma SH-SY5Y cells SCI |
Proliferation Oncogenic Anti-apoptotic Anti-apoptotic |
↑ Gliomagenesis ↓ SCI |
Jia et al. Xu et al. |
| Rap Guanine Nucleotide Exchange Factor (2Rapgef2*) | In vitro | Neural progenitor cell (NPC) | Regulates cell migration | ↑ NPCs ↓ During neuronal development |
Han et al. |
| Voltage-gated potassium channel subunits* | Rat | Primary sensory neurons | Regulate A-type outward potassium currents | ↑ Mechanical allodynia |
Sakai et al. |
| Tumor Protein p53 (p53) |
In vitro Rat |
SH-SY5Y cells Al-malt induced apoptosis in rat brain |
Anti-apoptotic Anti-apoptotic |
↑ Al-induced neural cell apoptosis ↑ Al-induced neural cell apoptosis |
Zhu et al. |
| RUNX Family Transcription Factor 3 (RUNX3) |
In vitro Mouse Human |
U87 and LN229 glioma cell lines Resected glioma Resected glioma |
Inhibition of proliferation and invasion Apoptotic Anti-oncogenic |
↓ Glioma cell lines ↑ Mice implanted with As-miR-19a/b in right forebrain ↓ Gliomagenesis |
Sun et al. |
SCI, spinal cord injury.
Table 3B.
Validated Targets of MiR-19a
| Target | Model | Tissue | MicroRNA effect | Relative expression | References |
|---|---|---|---|---|---|
| Suppressor of Cytokine Signaling 1 (SOCS1) | Rat bilateral chronic constriction injury | Sciatic nerve | Anti-inflammatory | ↑ Neuropathic pain |
Wang et al. |
| Methyl-CpG Binding Protein 2 (MeCP2) |
In vitro Mouse |
Neuro-2a cells Dorsal root ganglia |
N/A | Manners et al. | |
| Ras Homolog Family Member B (RhoB) |
In vitro Human |
LN18, LN229, LN428, SW1783, SW1088, U251, U373, and U87 glioma cell lines Resected glioma |
Promotion of proliferation and invasion Oncogenic |
↑ Following miRNA-19a knockdown ↓ With miRNA-19a N/A (They didn't check RhoB expression at mRNA or protein level in resected glioma. They just checked miR-19a expression.) |
|
| Peroxisome Proliferator Activated Receptor Alpha (PPARα) | Human In vitro Mouse |
Resected glioma U87 and LN229 glioma cells Resected glioma |
Anti-oncogenic Inhibition of glioma cell colony formation, invasion, and glucose consumption Anti-oncogenic |
↓ Gliomagenesis ↑ Following miRNA-19a knockdown ↓ Gliomagenesis |
Shi et al. |
| Leucine Rich Repeats and Immunoglobulin Like Domains 1 (LRIG1) | Human In vitro |
Resected glioma U251, U87, A172, and U118 glioma cell lines |
Oncogenic Inhibition of glioma cell proliferation |
N/A (They didn't check LRIG1 expression at mRNA or protein level in resected glioma. They just checked miR-19a expression.) ↑ Following miRNA-19a knockdown |
Shao et al. |
Table 3C.
Validated Targets of MiR-19b
| Target | Model | Tissue | MicroRNA effect | Relative expression | References |
|---|---|---|---|---|---|
| FMR1 Autosomal Homolog 1 (FXR1 ) | In vitro | SH-SY5Y cells | Apoptotic | ↑ Fragile X syndrome |
Ma et al. |
| Signal Transduction and Activator of Transcription 3 (STAT3) | In vitro | SH-SY5Y cells | Increase in STAT3 phosphorylation |
↓ Alzheimer's disease |
Wu et al. |
Discriminative ability of hsa-miR-19a-3p and hsa-miR-19b-3p to distinguish between pain and no pain
Subject characteristics based on the presence (n = 28) or absence (n = 38) of neuropathic pain are presented in Table 1. The neuropathic pain group was more likely to be male (86% vs. 63%, p = 0.04), to have been injured at an older age (30 vs. 29 years, p = 0.003), to have longer injury duration (9 vs. 7 years, p < 0.0001), and to use medications (opioids, p = 0.04, spasmolytics, p = 0.01 and gabapentin, p = 0.002) than the no pain group. Both hsa-miR-19a-3p (area under the curve [AUC] 0.77) and hsa-miR-19b-3p (AUC 0.78) have moderate to strong discriminative ability to distinguish between those with and without pain. The discriminative ability increased when considering both together in the same model (AUC 0.79, Fig. 6).
FIG. 6.
ROC curve of: hsa-miR-19a-3p (red dotted line), hsa-miR-19b-3p (green dotted line), and hsa-miR-19a-3p and hsa-miR-19b-3p (blue solid line) as a predictor of ISCI pain in participants who have SCI. AUC for hsa-miR-19a-3p is 0.7665 (p = 0.0174), for hsa-miR-19b-3p is 0.7833 (p = 0.0109), and for hsa-miR-19a-3p and hsa-miR-19b-3p together is 0.7899 (p = 0.0389). AUC, area under the curve; ISCI, International Spinal Cord Injury; ROC, receiver operating characteristic; SCI, spinal cord injury.
Univariate factors associated with neuropathic pain interference with mood
In univariate analyses (Table 4), men reported greater pain interference with mood than women, active opioid users reported greater interference than non-users, and active spasmolytic users reported greater interference than non-users. hsa-miR-19a-3p and hsa-miR-19b-3p levels were both positively associated with pain interference with mood. We also found a positive association between hsa-miR-19a-3p and hsa-miR-19b-3p levels and pain interference with activities of daily living and pain interference with sleep (data not shown, p = 0.0003 to 0.03).
Multi-variable factors associated with neuropathic pain interference with mood
In multi-variable models (data not shown) hsa-miR-19a-3p and hsa-miR-19b-3p were no longer associated with pain interference with daily activities or sleep. In separate multi-variable models (Table 5), sex, spasmolytic use, and miR-19a were no longer significantly associated with pain interference with mood. Opioid users had significantly greater pain interference than non-users (3.7 vs. 1.6, p = 0.008). Pain interference with mood score increased by 0.0004 ± 0.0001 units for every 1 unit increase in hsa-miR-19b-3p (p = 0.04). This model explained 18% of the variation in pain interference with mood score (model p = 0.003).
Table 4.
Univariate Predictors of Neuropathic Pain Interference with Mood
| Continuous variable | β ± SE | P |
|---|---|---|
| Age (unit pain interference score/year) | 0.03 ± 0.02 | 0.29 |
| Injury duration (unit pain interference score/year) | 0.07 ± 0.04a | 0.14 |
| Age at injury (unit pain interference score /year) | -0.007 ± 0.03a | 0.84 |
| MiR-19a (unit pain interference score/normalized deep sequencing count) | 0.001 ± 0.0003 | 0.003 |
| MiR-19b (unit pain interference score/normalized deep sequencing count) | 0.0005 ± 0.0001 | 0.0004 |
| Lifetime alcohol use (unit pain interference score/kg/year) | 0.002 ± 0.002b | 0.31 |
| Cigarette exposure (unit pain interference score/pack-year) | -0.05 ± 0.09c | 0.61 |
| Categorical variable | Mean pain interference score ± SE | P |
|---|---|---|
| Sex |
|
0.03 |
| • Male |
2.83 ± 2.82 |
|
| • Female |
1.16 ± 2.25 |
|
| Injury level |
|
0.22 |
| • Paraplegia |
3.57 ± 3.01a |
|
| • Tetraplegia |
2.30 ± 2.11 |
|
| Active opioid use |
|
0.005 |
| • Yes |
3.83 ± 2.85d |
|
| • No |
1.59 ± 2.28 |
|
| Active spasmolytic use |
|
0.03 |
| • Yes |
3.25 ± 2.88d |
|
| • No |
1.60 ± 2.29 |
|
| Active gabapentin use |
|
0.16 |
| • Yes |
2.92 ± 2.62d |
|
| • No |
1.79 ± 2.49 |
|
| Alcohol use |
|
0.96 |
| • Yes |
1.96 ± 2.46e |
|
| • No |
2.00 ± 3.21 |
|
| Smoker status |
|
0.61 |
| • Ever |
2.17 ± 2.69e |
|
| • Never | 1.82 ± 2.45 |
Among SCI n = 43; bamong ever alcohol users; camong ever smokers; davailable data for n = 61; eavailable data for n = 5.8.
SCI, spinal cord injury; SE, standard error of the mean.
Table 5.
Multi-Variable Factors Associated with Neuropathic Pain Interference with Mood
| Model p = 0.003, R2 = 0.18 | ||
|---|---|---|
| Continuous variable | β ± SE | P |
| MiR-19b (normalized deep sequencing count) | 0.0004 ± 0.0001 | 0.04 |
| Categorical variable | Mean pain interference score ± SE | P |
|---|---|---|
| Active opioid use |
|
0.008 |
| • Yes |
3.71 ± 0.67 |
|
| • No | 1.62 ± 0.33 |
SE, standard error of the mean.
Discussion
In this study we performed an untargeted genome-wide miRNA screening to study differential miRNA expression in plasma from 68 healthy, community-dwelling adults with and without SCI. We found 383 miRNAs that were differentially expressed in acute SCI or chronic SCI versus no SCI. In addition, we found 71 miRNAs were differentially expressed in chronic neuropathic pain versus no neuropathic pain. In a subsequent analysis, we selected hsa-miR-19a-3p and hsa-miR-19b-3p based on p-value, fold change, and their known role as regulators of neuroinflammation and neuropathic pain. We found that both hsa-miR-19a-3p and hsa-miR-19b-3p levels are significantly higher in acute SCI compared with chronic SCI and in those with chronic SCI with neuropathic pain versus those without neuropathic pain. hsa-miR-19b-3p levels are also significantly greater in both acute and chronic SCI versus No SCI. Additionally, both hsa-miR-19a-3p and hsa-miR-19b-3p have moderate to strong discriminative ability to distinguish between those with and without pain. We also found that after adjusting for opioid use, hsa-miR-19b-3p is positively associated with pain interference with mood. Our large sample size is a strength of this study as it reduces the risk of small sample size error.19
Neuropathic pain presents at or below the level of injury30,31 and is cited as the most “severe pain” post-SCI.32 Unmitigated pain limits physical activity, negatively impacts rehabilitation, limits work and social activities, and reduces quality of life.33 Unfortunately, neuropathic pain is considered refractory to most available treatments, likely because treatment is offered after pain has already developed. It is possible that substantial and perhaps irreversible neuronal changes have already occurred, limiting the therapeutic potential. Therefore, early preventative strategies to mitigate chronic neuropathic pain after SCI may be more effective. Development of and validation of biomarkers might help to identify those most at risk of chronic sublesional neuropathic pain and therefore who might benefit from preventative strategies, both pharmacological and non-pharmacological.34
A poor understanding of the physiological basis of neuropathic pain continues to hinder the identification of novel, effective interventions. The study of miRNA in neuropathic pain is a relatively new field of research. However, the significance of miRNA alterations in a variety of rodent pain models and in clinical conditions characterized by pain has been clearly established. miRNAs exert post-transcriptional modulation of large sections of the genome by binding to regulatory gene elements and inhibiting the translation of many genes.35,36 Significant alterations in miRNAs expression (together with the resultant changes in protein expression) have been reported in both the affected tissues and in blood from patients suffering from several pain conditions such as complex regional pain syndrome, cystitis-induced chronic pain, and irritable bowel disorder. They are found in every human tissue and biofluid, are resistant to RNAse degradation, and have the ability to cross the blood–brain barrier, making them excellent candidate biomarkers for neurotrauma-related conditions, neurorecovery, and response to various neuroprotective interventions.37
Diverse causes of neuropathic pain are associated with excessive inflammation in both the PNS and CNS, which may contribute to the initiation and maintenance of persistent pain. Chemical mediators, such as cytokines, chemokines, and lipid mediators, released during an inflammatory response have the undesired effect of sensitizing and stimulating nociceptors, their central synaptic targets, or both. Although there is lack of consensus in the literature regarding male and female specific pain pathways, sex dimorphism has been reported in the development and maintenance of neuropathic pain.38 This includes sex differences in immune-mediated pain and the effect of sex hormones on nociception and morphine tolerance.38 In our study men reported higher levels of pain interference with mood in univariate analyses. However, sex was no longer significantly associated with pain interference with mood in multi-variable models. Caution must be taken when interpreting this result as our sample included too few women to make meaningful comparisons based on sex. Understanding the potential impact of sex on these processes better may lead to potentially novel therapeutic targets for neuropathic pain.
There are many validated targets of hsa-miR-19a-3p and hsa-miR-19b-3p, including SOCS1.39 Members of the SOCS family are thought to modulate neuroinflammation.40 In particular, two members, SOCS1 and SOCS3, control inflammatory cytokine signaling in neurons,41–43 Schwann cells,41,44 oligodendrocytes,45,46 astrocytes,47,48 and microglia.49–51 Expression of SOCS1 and SOCS3 by microglia is associated with reduced nitric oxide (NO) production and decreased sensitivity to cytokine-induced signaling, thereby reducing neuroinflammation.52,53 Reduced SOCS1 expression in microglia, on the other hand, promotes pro-inflammatory microglia and aggravation of neuroinflammation.54 Upregulation of these miRNAs is associated with increased inflammation. miR-19a-3p and miR-19b-3p may contribute to neuroinflammation via modulation of SOCS1 expression after SCI, thereby providing a potential mechanistic link between neuroinflammation and neuropathic pain. miR-19a-3p and miR-19b-3p both belong to the miR-17-92 cluster located on human chromosome 13. miRNA clusters result from genome duplication and are classified based on sequence homology. Overexpression of miR-17-92 cluster members is associated with neuropathic pain in a variety of models and clinical contexts, and this is thought to be due to regulation of voltage-gated potassium channels (Table 3).20
Our findings are consistent with prior reports and support a role for the coordinated regulatory effect of miR-17-92 cluster miRNAs on multiple voltage-gated potassium channels in the development and/or maintenance of neuropathic pain. Several studies have demonstrated alterations in miRNA levels due to therapeutic interventions, including exercise and medications. This suggests that miRNAs are therapeutic targets. In fact, miR-19-3p levels have been shown to change in response to exercise, folic acid, and resveratrol.55
SOCS1 was found to play a role in inflammatory reactions by regulating JAK/STAT signaling pathways in innate immune cells and non-immune cells56–59 JAK/STAT blockade in spinal cord microglial cells and astrocytes can attenuate local inflammation and pain hypersensitivity.60,61 Interestingly, SOCS3 can also block JAK/STAT3 activity, preventing the abnormal expression of interleukin (IL)-6, CC chemokine ligand CCL2, and activating transcription factor ATF3 induced in the spinal cord by chronic constriction injury (CCI) of the sciatic nerve and substantially attenuated mechanical hypersensitivity (allodynia) in rats. Qin and colleagues51 reported interferon (IFN)-induced SOCS1 negatively regulates CD40 gene expression in macrophages and microglia. They found IFN-induced expression of the SOCS1 protein, which functioned in a negative regulatory feedback loop to inhibit IFN signaling, and ultimately CD40 expression.51 In addition to SOCS1 and SOCS3, voltage-gated potassium channel subunits as the validated target of both miR-19a-3p and miR-19b-3p provided the opportunity to develop a novel analgesic strategy for therapy for neuropathic pain based on concurrent regulation of multiple functionally related proteins. Overexpression of miR-19a, miR-19b, miR-18, and miR-92a cluster members elicits mechanical allodynia in rats, whereas their blockade alleviates mechanical allodynia in a rat model of neuropathic pain.20 Taken together, these results strongly support a role for miR-19a-3p and miR-19b-3p in the pathogenesis of neuropathic pain.
There are several limitations of the current study to consider. Despite being a comparatively large sample for miRNA expression profile screening studies, the candidate miRNAs identified in this study should be confirmed in a larger, more heterogeneous sample. Moreover, this is a cross-sectional study and therefore we cannot assign causality to any of the identified relationships. Mechanistic studies focused on these miRNAs, SOCS1/SOCS3 pathways, and inflammatory cytokines are needed. Despite these limitations, this expression profile screening study provides important insight into the potential role of two miRNAs and neuropathic pain in SCI.
Abbreviations Used
- 2Rapgef2
Rap Guanine Nucleotide Exchange Factor
- AIS
American Spinal Injury Association Impairment Scale
- ANOVA
analysis of variance
- AUC
area under the curve
- CCI
chronic constriction injury
- CNS
central nervous system
- FDR
False Discovery Rate
- FXR1
FMR1 Autosomal Homolog 1
- GFR
glomerular filtration rate
- Has
Homo sapiens
- Hb
hemoglobin
- IASP
International Association for the Study of Pain
- IFN
interferon
- IL
interleukin
- INR
International Normalized Ratio
- IRB
institutional review board
- ISCI
International Spinal Cord Injury
- LRIG1
Leucine Rich Repeats and Immunoglobulin Like Domains 1
- MAS
Modified Ashworth Scale
- MeCP2
Methyl-CpG Binding Protein 2
- miRNA
microRNA
- mRNA
messenger RNA
- NO
nitric oxide
- P53
Tumor Protein p53
- PNS
peripheral nervous system
- PPARα
Peroxisome Proliferator Activated Receptor Alpha
- PTEN
Phosphatase and tensin homolog
- PTEN
Phosphatase and Tensin Homolog
- QC
Quality Control
- RhoB
Ras Homolog Family Member B
- ROC
receiver operating characteristic
- RUNX3
RUNX Family Transcription Factor 3
- SCI
spinal cord injury
- SD
standard deviation
- SE
standard error of the mean
- SOCS1
Suppressor of Cytokine Signaling 1
- STAT3
Signal Transduction and Activator of Transcription 3
- UTR
untranslated region
Funding Information
This study received support from the Department of Defense (W81XWH-10-1-1043), National Institutes of Health (R01AR064793) and The National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR, 90SI5015-01-00).
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Ye, L, Morse, LR, Falci, SP, Olson, JK, Shrivastava, M, Nguyen, N, Linnman, C, Troy, KL, and Battaglino, RA (2021) hsa-MiR-19a-3p and hsa-MiR-19b-3p are associated with spinal cord injury-induced neuropathic pain: Findings from a genome-wide microRNA expression profiling screen. Neurotrauma Reports 2:1, 424–439, DOI: 10.1089/neur.2021.0011.
References
- 1. Gold, M.S., and Gebhart, G.F. (2010). Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Spinal Cord Injury Statistical Center, University of Alabama at Birmingham. (2021). Spinal Cord Injury Facts and Figures at a Glance. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202021.pdf, last accessed 09/02/2021). [Google Scholar]
- 3. Imai, S., Saeki, M., Yanase, M., Horiuchi, H., Abe, M., Narita, M., Kuzumaki, N., Suzuki, T., and Narita, M. (2011). Change in microRNAs associated with neuronal adaptive responses in the nucleus accumbens under neuropathic pain. J. Neurosci. 31, 15294–15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Behm-Ansmant, I., Rehwinkel, J., and Izaurralde, E. (2006). MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb. Symp. Quant. Biol. 71, 523–530. [DOI] [PubMed] [Google Scholar]
- 5. Fabian, M.R., Sonenberg, N., and Filipowicz, W. (2010). Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379. [DOI] [PubMed] [Google Scholar]
- 6. Fukao, A., Aoyama, T., and Fujiwara, T. (2015). The molecular mechanism of translational control via the communication between the microRNA pathway and RNA-binding proteins. RNA Biol. 12, 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arai, M., Genda, Y., Ishikawa, M., Shunsuke, T., Okabe, T., and Sakamoto, A. (2013). The miRNA and mRNA changes in rat hippocampi after chronic constriction injury. Pain Med. 14, 720–729. [DOI] [PubMed] [Google Scholar]
- 8. Genda, Y., Arai, M., Ishikawa, M., Tanaka, S., Okabe, T., and Sakamoto, A. (2013). microRNA changes in the dorsal horn of the spinal cord of rats with chronic constriction injury: a TaqMan(R) Low Density Array study. Int. J. Mol. Med. 31, 129–137. [DOI] [PubMed] [Google Scholar]
- 9. Bali, K.K., Hackenberg, M., Lubin, A., Kuner, R., and Devor, M. (2014). Sources of individual variability: miRNAs that predispose to neuropathic pain identified using genome-wide sequencing. Mol. Pain 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu, D., Raafat, M., Pak, E., Hammond, S., and Murashov, A.K. (2011). MicroRNA machinery responds to peripheral nerve lesion in an injury-regulated pattern. Neuroscience 190, 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta, N., Jadhav, S., Tan, K.L., Saw, G., Mallilankaraman, K.B., and Dheen, S.T. (2020). miR-142-3p regulates BDNF expression in activated rodent microglia through its target CAMK2A. Front. Cell Neurosci. 14, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashraf, U., Zhu, B., Ye, J., Wan, S., Nie, Y., Chen, Z., Cui, M., Wang, C., Duan, X., Zhang, H., Chen, H., and Cao, S. (2016). MicroRNA-19b-3p modulates Japanese encephalitis virus-mediated inflammation via targeting RNF11. J. Virol. 90, 4780–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawson, D.A. (2003). Methodological issues in measuring alcohol use. Alcohol Res. Health 27, 18–29. [PMC free article] [PubMed] [Google Scholar]
- 14. Garshick, E., Segal, M.R., Worobec, T.G., Salekin, C.M., and Miller, M.J. (1989). Alcohol consumption and chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 140, 373–378. [DOI] [PubMed] [Google Scholar]
- 15. Bryce, T.N., Budh, C.N., Cardenas, D.D., Dijkers, M., Felix, E.R., Finnerup, N.B., Kennedy, P., Lundeberg, T., Richards, J.S., Rintala, D.H., Siddall, P., and Widerstrom-Noga, E. (2007). Pain after spinal cord injury: an evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J. Spinal Cord. Med. 30, 421–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dworkin, R.H., Turk, D.C., Farrar, J.T., Haythornthwaite, J.A., Jensen, M.P., Katz, N.P., Kerns, R.D., Stucki, G., Allen, R.R., Bellamy, N., Carr, D.B., Chandler, J., Cowan, P., Dionne, R., Galer, B.S., Hertz, S., Jadad, A.R., Kramer, L.D., Manning, D.C., Martin, S., McCormick, C.G., McDermott, M.P., McGrath, P., Quessy, S., Rappaport, B.A., Robbins, W., Robinson, J.P., Rothman, M., Royal, M.A., Simon, L., Stauffer, J.W., Stein, W., Tollett, J., Wernicke, J., Witter, J., and Immpact (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113, 9–19. [DOI] [PubMed] [Google Scholar]
- 17. Battaglino, R.A., Sudhakar, S., Lazzari, A.A., Garshick, E., Zafonte, R., and Morse, L.R. (2012). Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone 51, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morse, L.R., Sudhakar, S., Danilack, V., Tun, C., Lazzari, A., Gagnon, D.R., Garshick, E., and Battaglino, R.A. (2012). Association between sclerostin and bone density in chronic spinal cord injury. J. Bone Miner. Res. 27, 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kok, M.G.M., de Ronde, M.W.J., Moerland, P.D., Ruijter, J.M., Creemers, E.E., and Pinto-Sietsma, S.J. (2018). Small sample sizes in high-throughput miRNA screens: a common pitfall for the identification of miRNA biomarkers. Biomol. Detect. Quantif. 15, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakai, A., Saitow, F., Maruyama, M., Miyake, N., Miyake, K., Shimada, T., Okada, T., and Suzuki, H. (2017). MicroRNA cluster miR-17-92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat. Commun. 8, 16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia, Z., Wang, K., Zhang, A., Wang, G., Kang, C., Han, L., and Pu, P. (2013). miR-19a and miR-19b overexpression in gliomas. Pathol. Oncol. Res. 19, 847–853. [DOI] [PubMed] [Google Scholar]
- 22. Xu, X., Cui, L., Zhong, W., and Cai, Y. (2020). Autophagy-associated lncRNAs: promising targets for neurological disease diagnosis and therapy. Neural Plast. 2020, 8881687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han, J., Kim, H.J., Schafer, S.T., Paquola, A., Clemenson, G.D., Toda, T., Oh, J., Pankonin, A.R., Lee, B.S., Johnston, S.T., Sarkar, A., Denli, A.M., and Gage, F.H. (2016). Functional implications of miR-19 in the migration of newborn neurons in the adult brain. Neuron 91, 79–89. [DOI] [PubMed] [Google Scholar]
- 24. Zhu, M., Huang, C., Ma, X., Wu, R., Zhu, W., Li, X., Liang, Z., Deng, F., Zhu, J., Xie, W., Yang, X., Jiang, Y., Wang, S., Wu, J., Geng, S., Xie, C., and Zhong, C. (2016). Modulation of miR-19 in aluminum-induced neural cell apoptosis. J. Alzheimers Dis. 50, 1149–1162. [DOI] [PubMed] [Google Scholar]
- 25. Sun, J., Jia, Z., Li, B., Zhang, A., Wang, G., Pu, P., Chen, Z., Wang, Z., and Yang, W. (2017). MiR-19 regulates the proliferation and invasion of glioma by RUNX3 via beta-catenin/Tcf-4 signaling. Oncotarget 8, 110785–110796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shao, L.M., Yang, J.A., Wang, Y.F., Wu, P., Li, J.Q., and Chen, Q.X. (2014). MicroRNA-19a promotes glioma cell growth by repressing LRIG1. Int. J. Clin. Exp. Med. 7, 5067–5074. [PMC free article] [PubMed] [Google Scholar]
- 27. Ma, Y., Tian, S., He, S., Chen, Q., Wang, Z., Xiao, X., Fu, L., and Lei, X. (2016). The mechanism of action of FXR1P-related miR-19b-3p in SH-SY5Y. Gene 588, 62–68. [DOI] [PubMed] [Google Scholar]
- 28. Wu, Y., Xu, J., Xu, J., Cheng, J., Jiao, D., Zhou, C., Dai, Y., and Chen, Q. (2017). Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer's disease. Tohoku J. Exp. Med. 242, 129–136. [DOI] [PubMed] [Google Scholar]
- 29. Wang, C., Jiang, Q., Wang, M., and Li, D. (2015). MiR-19a targets suppressor of cytokine signaling 1 to modulate the progression of neuropathic pain. Int. J. Clin. Exp. Pathol. 8, 10901–10907. [PMC free article] [PubMed] [Google Scholar]
- 30. Burke, D., Fullen, B.M., Stokes, D., and Lennon, O. (2017). Neuropathic pain prevalence following spinal cord injury: a systematic review and meta-analysis. Eur. J. Pain 21, 29–44. [DOI] [PubMed] [Google Scholar]
- 31. Bryce, T.N., Biering-Sorensen, F., Finnerup, N.B., Cardenas, D.D., Defrin, R., Ivan, E., Lundeberg, T., Norrbrink, C., Richards, J.S., Siddall, P., Stripling, T., Treede, R.D., Waxman, S.G., Widerstrom-Noga, E., Yezierski, R.P., and Dijkers, M. (2012). International Spinal Cord Injury Pain (ISCIP) Classification: Part 2. Initial validation using vignettes. Spinal Cord 50, 404–412. [DOI] [PubMed] [Google Scholar]
- 32. Siddall, P.J., McClelland, J.M., Rutkowski, S.B., and Cousins, M.J. (2003). A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257. [DOI] [PubMed] [Google Scholar]
- 33. Hergenroeder, G.W., Redell, J.B., Choi, H.A., Schmitt, L., Donovan, W., Francisco, G.E., Schmitt, K., Moore, A.N., and Dash, P.K. (2018). Increased levels of circulating glial fibrillary acidic protein and collapsin response mediator protein-2 autoantibodies in the acute stage of spinal cord injury predict the subsequent development of neuropathic pain. J. Neurotrauma 35, 2530–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruener, H., Zeilig, G., Gaidukov, E., Rachamim-Katz, O., Ringler, E., Blumen, N., Engel-Haber, E., and Defrin, R. (2020). Biomarkers for predicting central neuropathic pain occurrence and severity after spinal cord injury: results of a long-term longitudinal study. Pain 161, 545–556. [DOI] [PubMed] [Google Scholar]
- 35. Bartel, D.P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lagos-Quintana, M., Rauhut, R., Lendeckel, W., and Tuschl, T. (2001). Identification of novel genes coding for small expressed RNAs. Science 294, 853–858. [DOI] [PubMed] [Google Scholar]
- 37. Liu, Q., and Paroo, Z. (2010). Biochemical principles of small RNA pathways. Annu. Rev. Biochem. 79, 295–319. [DOI] [PubMed] [Google Scholar]
- 38. Gregus, A.M., Levine, I.S., Eddinger, K.A., Yaksh, T.L., and Buczynski, M.W. (2021). Sex differences in neuroimmune and glial mechanisms of pain. Pain 162, 2186–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang, X., and Chen, Z. (2015). MicroRNA-19a functions as an oncogenic microRNA in non-small cell lung cancer by targeting the suppressor of cytokine signaling 1 and mediating STAT3 activation. Int. J. Mol. Med. 35, 839–846. [DOI] [PubMed] [Google Scholar]
- 40. Baker, B.J., Akhtar, L.N., and Benveniste, E.N. (2009). SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 30, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turnley, A.M., Starr, R., and Bartlett, P.F. (2002). Failure of sensory neurons to express class I MHC is due to differential SOCS1 expression. J. Neuroimmunol. 123, 35–40. [DOI] [PubMed] [Google Scholar]
- 42. Dominguez, E., Mauborgne, A., Mallet, J., Desclaux, M., and Pohl, M. (2010). SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J. Neurosci. 30, 5754–5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mori, H., Hanada, R., Hanada, T., Aki, D., Mashima, R., Nishinakamura, H., Torisu, T., Chien, K.R., Yasukawa, H., and Yoshimura, A. (2004). Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 10, 739–743. [DOI] [PubMed] [Google Scholar]
- 44. Girolami, E.I., Bouhy, D., Haber, M., Johnson, H., and David, S. (2010). Differential expression and potential role of SOCS1 and SOCS3 in Wallerian degeneration in injured peripheral nerve. Exp. Neurol. 223, 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Balabanov, R., Strand, K., Kemper, A., Lee, J.Y., and Popko, B. (2006). Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-gamma. J. Neurosci. 26, 5143–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emery, B., Butzkueven, H., Snell, C., Binder, M., and Kilpatrick, T.J. (2006). Oligodendrocytes exhibit selective expression of suppressor of cytokine signaling genes and signal transducer and activator of transcription 1 independent inhibition of interferon-gamma-induced toxicity in response to leukemia inhibitory factor. Neuroscience 137, 463–472. [DOI] [PubMed] [Google Scholar]
- 47. Qin, H., Niyongere, S.A., Lee, S.J., Baker, B.J., and Benveniste, E.N. (2008). Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J. Immunol. 181, 3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi, W.H., Ji, K.A., Jeon, S.B., Yang, M.S., Kim, H., Min, K.J., Shong, M., Jou, I., and Joe, E.H. (2005). Anti-inflammatory roles of retinoic acid in rat brain astrocytes: suppression of interferon-gamma-induced JAK/STAT phosphorylation. Biochem. Biophys. Res. Commun. 329, 125–131. [DOI] [PubMed] [Google Scholar]
- 49. Qin, H., Roberts, K.L., Niyongere, S.A., Cong, Y., Elson, C.O., and Benveniste, E.N. (2007). Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J. Immunol. 179, 5966–5976. [DOI] [PubMed] [Google Scholar]
- 50. Park, K.W., Lin, C.Y., and Lee, Y.S. (2014). Expression of suppressor of cytokine signaling-3 (SOCS3) and its role in neuronal death after complete spinal cord injury. Exp. Neurol. 261, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qin, H., Wilson, C.A., Lee, S.J., and Benveniste, E.N. (2006). IFN-beta-induced SOCS-1 negatively regulates CD40 gene expression in macrophages and microglia. FASEB J. 20, 985–987. [DOI] [PubMed] [Google Scholar]
- 52. Kim, J.H., Jou, I., and Joe, E.H. (2014). Suppression of miR-155 Expression in IFN-gamma-treated astrocytes and microglia by DJ-1: a possible mechanism for maintaining SOCS1 expression. Exp. Neurobiol. 23, 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park, E.J., Park, S.Y., Joe, E.H., and Jou, I. (2003). 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J. Biol. Chem. 278, 14747–14752. [DOI] [PubMed] [Google Scholar]
- 54. Lofrumento, D.D., Nicolardi, G., Cianciulli, A., De Nuccio, F., La Pesa, V., Carofiglio, V., Dragone, T., Calvello, R., and Panaro, M.A. (2014). Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson's-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 20, 249–260. [DOI] [PubMed] [Google Scholar]
- 55. Li, X., Teng, C., Ma, J., Fu, N., Wang, L., Wen, J., and Wang, T.Y. (2019). miR-19 family: a promising biomarker and therapeutic target in heart, vessels and neurons. Life Sci. 232, 116651. [DOI] [PubMed] [Google Scholar]
- 56. Hashimoto, M., Hiwatashi, K., Ichiyama, K., Morita, R., Sekiya, T., Kimura, A., Sugiyama, Y., Sibata, T., Kuroda, K., Takahashi, R., and Yoshimura, A. (2011). SOCS1 regulates type I/type II NKT cell balance by regulating IFNgamma signaling. Int. Immunol. 23, 165–176. [DOI] [PubMed] [Google Scholar]
- 57. Sachithanandan, N., Graham, K.L., Galic, S., Honeyman, J.E., Fynch, S.L., Hewitt, K.A., Steinberg, G.R., and Kay, T.W. (2011). Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes 60, 2023–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torisu, T., Nakaya, M., Watanabe, S., Hashimoto, M., Yoshida, H., Chinen, T., Yoshida, R., Okamoto, F., Hanada, T., Torisu, K., Takaesu, G., Kobayashi, T., Yasukawa, H., and Yoshimura, A. (2008). Suppressor of cytokine signaling 1 protects mice against concanavalin A-induced hepatitis by inhibiting apoptosis. Hepatology 47, 1644–1654. [DOI] [PubMed] [Google Scholar]
- 59. Yoshimura, A., Suzuki, M., Sakaguchi, R., Hanada, T., and Yasukawa, H. (2012). SOCS, inflammation, and autoimmunity. Front. Immunol. 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Molet, J., Mauborgne, A., Diallo, M., Armand, V., Geny, D., Villanueva, L., Boucher, Y., and Pohl, M. (2016). Microglial Janus kinase/signal transduction and activator of transcription 3 pathway activity directly impacts astrocyte and spinal neuron characteristics. J. Neurochem. 136, 133–147. [DOI] [PubMed] [Google Scholar]
- 61. Tsuda, M., Kohro, Y., Yano, T., Tsujikawa, T., Kitano, J., Tozaki-Saitoh, H., Koyanagi, S., Ohdo, S., Ji, R.R., Salter, M.W., and Inoue, K. (2011). JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 134, 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]