Abstract
Despite reports of parental exposure to stress promoting physiological adaptations in progeny in diverse organisms, there remains considerable debate over the significance and evolutionary conservation of such multigenerational effects. Here, we investigate four independent models of intergenerational adaptations to stress in Caenorhabditis elegans – bacterial infection, eukaryotic infection, osmotic stress, and nutrient stress – across multiple species. We found that all four intergenerational physiological adaptations are conserved in at least one other species, that they are stress -specific, and that they have deleterious tradeoffs in mismatched environments. By profiling the effects of parental bacterial infection and osmotic stress exposure on progeny gene expression across species, we established a core set of 587 genes that exhibited a greater than twofold intergenerational change in expression in response to stress in C. elegans and at least one other species, as well as a set of 37 highly conserved genes that exhibited a greater than twofold intergenerational change in expression in all four species tested. Furthermore, we provide evidence suggesting that presumed adaptive and deleterious intergenerational effects are molecularly related at the gene expression level. Lastly, we found that none of the effects we detected of these stresses on C. elegans F1 progeny gene expression persisted transgenerationally three generations after stress exposure. We conclude that intergenerational responses to stress play a substantial and evolutionarily conserved role in regulating animal physiology and that the vast majority of the effects of parental stress on progeny gene expression are reversible and not maintained transgenerationally.
Research organism: C. elegans
Introduction
Multigenerational effects of a parent’s environment on progeny have been reported to contribute to numerous organismal phenotypes and pathologies in species ranging from plants to mammals (Agrawal et al., 1999; Bozler et al., 2019; Burton et al., 2020; Burton et al., 2017; Dantzer et al., 2013; Dias and Ressler, 2014; Hibshman et al., 2016; Houri-Zeevi et al., 2020; Jordan et al., 2019; Kaletsky et al., 2020; Kishimoto et al., 2017; Klosin et al., 2017; Luna et al., 2012; Ma et al., 2019; Moore et al., 2019; Öst et al., 2014; Palominos et al., 2017; Posner et al., 2019; Veenendaal et al., 2013; Vellichirammal et al., 2017; Webster et al., 2018; Wibowo et al., 2016; Willis et al., 2021). These effects on progeny include many notable observations of intergenerational (lasting 1–2 generations) adaptive changes in phenotypically plastic traits such as the development of wings in pea aphids (Vellichirammal et al., 2017), helmet formation in Daphnia (Agrawal et al., 1999), accelerated growth rate in red squirrels (Dantzer et al., 2013), and physiological adaptations to osmotic stress and pathogen infection in both Arabidopsis (Luna et al., 2012; Wibowo et al., 2016) and Caenorhabditis elegans (Burton et al., 2020; Burton et al., 2017). These intergenerational adaptive changes in development and physiology, which include effects that are sometimes interchangeably described as parental effects, can lead to substantial increases in organismal survival, with up to 50-fold increases in the survival of offspring from stressed parents being reported when compared to the offspring from naive parents (Burton et al., 2020). While many of the most studied intergenerational effects of a parent’s environment on offspring have been identified in plants and invertebrates, intergenerational effects have also been reported in mammals (Dantzer et al., 2013; Dias and Ressler, 2014). Similar to findings in plants and invertebrates, some observations of intergenerational effects in mammals have been found to be physiologically adaptive (Dantzer et al., 2013), but many others, such as observations of fetal programming in humans (de Gusmão Correia et al., 2012; Langley-Evans, 2006; Schulz, 2010) and studies of the Dutch Hunger Winter (Veenendaal et al., 2013), have been reported to be deleterious. Nonetheless, even for these presumed deleterious intergenerational effects, it has been hypothesized that under different conditions the intergenerational effects of fetal programming, such as the effects caused by the Dutch Hunger Winter, might be considered physiologically adaptive (Hales and Barker, 2001; Hales and Barker, 1992).
If intergenerational responses to environmental stresses represent evolutionarily conserved processes, if they are general or stress-specific effects, and whether adaptive and deleterious intergenerational effects are molecularly related remains unknown. Furthermore, multiple different studies have recently reported that some environmental stresses elicit changes in progeny physiology and gene expression that persist for three or more generations, also known as transgenerational effects (Kaletsky et al., 2020; Klosin et al., 2017; Ma et al., 2019; Moore et al., 2019; Posner et al., 2019; Webster et al., 2018). However, if intergenerational effects (lasting 1–2 generations) and transgenerational effects (lasting 3+ generations) represent related or largely separable phenomena remains unclear. Answering these questions is critically important not only in understanding the role that multigenerational effects play in evolution, but also in understanding how such effects might contribute to multiple human pathologies that have been linked to the effects of a parent’s environment on offspring, such as Type 2 diabetes and cardiovascular disease (Langley-Evans, 2006).
Here, we investigated the evolutionary conservation, stress specificity, and potential tradeoffs of four independent models of intergenerational adaptations to stress in C. elegans – bacterial infection, eukaryotic infection, nutrient stress, and osmotic stress. We found that all four models of intergenerational adaptive effects are conserved in at least one other species, but that all exhibited a different pattern of evolutionary conservation. Each intergenerational adaptive effect was stress -specific and multiple intergenerational adaptive effects exhibited deleterious tradeoffs in mismatched environments or environments where multiple stresses were present simultaneously. By profiling the effects of multiple different stresses on offspring gene expression across species we identified a set of 37 genes that exhibited intergenerational changes in gene expression in response to stress in all species tested. In addition, we found that an inversion in the expression of a key gene involved in the intergenerational response to bacterial infection, rhy-1, from increased expression to decreased expression in the offspring of stressed parents, correlates with an inversion of an adaptive intergenerational response to bacterial infection in C. elegans and C. kamaaina to a deleterious intergenerational effect in C. briggsae. Lastly, we report that none of the effects of multiple different stresses on F1 gene expression that we detected here persisted transgenerationally into F3 progeny in C. elegans. Our findings demonstrate that intergenerational adaptive responses to stress are evolutionarily conserved, stress -specific, and are predominantly not maintained transgenerationally. In addition, our findings suggest that the mechanisms that mediate intergenerational adaptive responses in some species might be related to the mechanisms that mediate intergenerational deleterious effects in other species.
Results
Intergenerational adaptations to stress are evolutionarily conserved
To test if any of the intergenerational adaptations to stress that have been reported in C. elegans are evolutionarily conserved in other species we focused on four recently described intergenerational adaptations to abiotic and biotic stresses – osmotic stress (Burton et al., 2017), nutrient stress (Hibshman et al., 2016; Jordan et al., 2019), Pseudomonas vranonvensis infection (bacterial) (Burton et al., 2020), and Nematocida parisii infection (eukaryotic – microsporidia) (Willis et al., 2021). All of these stresses are exclusively intergenerational and did not persist beyond two generations in any experimental setup previously analyzed (Burton et al., 2017; Burton et al., 2020; Willis et al., 2021). We tested if these four intergenerational adaptive responses were conserved in four different species of Caenorhabditis (C. briggsae, C. elegans, C. kamaaina, and C. tropicalis) that shared a last common ancestor approximately 30 million years ago and have diverged to the point of having approximately 0.05 substitutions per site at the nucleotide level (Figure 1A; Cutter, 2008). These species were chosen because they represent multiple independent branches of the Elegans group (Figure 1A) and because we could probe the conservation of underlying mechanisms using established genetics approaches.
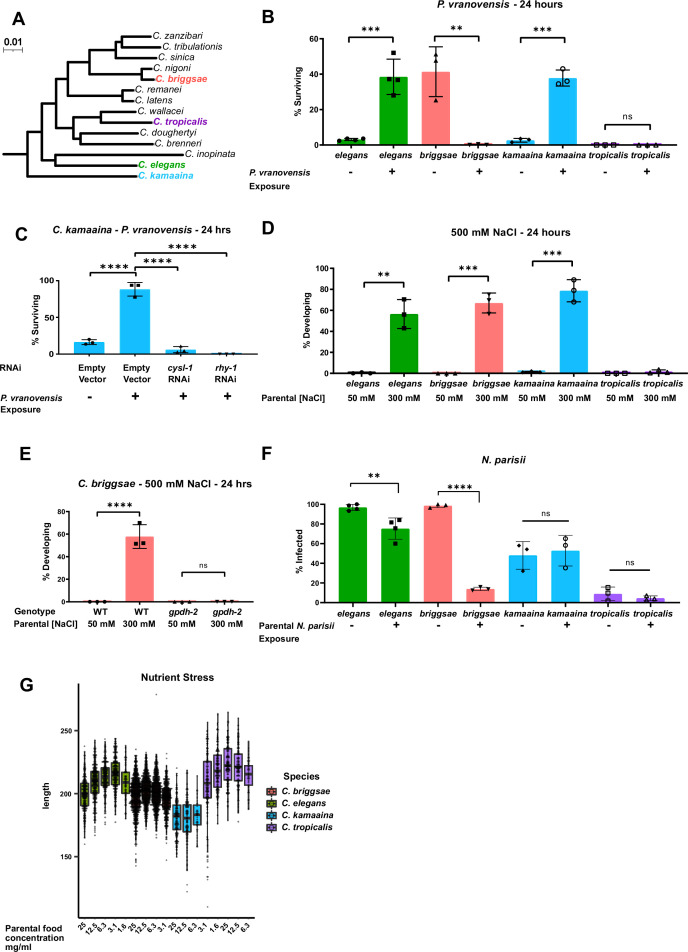
Figure 1. Intergenerational adaptations to multiple stresses are evolutionarily conserved in multiple species of Caenorhabditis.
(A) Phylogenetic tree of the Elegans group of Caenorhabditis species adapted from Stevens et al., 2020. Scale represents substitutions per site. (B) Percent of wild-type C. elegans (N2), C. kamaaina (QG122), C. briggsae (AF16), and C. tropicalis (JU1373) animals surviving after 24 hr on plates seeded with P. vranovensis BIGb0446. Data presented as mean values ± s.d. n = 3–4 experiments of >100 animals. (C) Percent of C. kamaaina wild-type (QG122) animals surviving after 24 hr of exposure to P. vranovensis. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (D) Percent of wild-type animals mobile and developing at 500 mM NaCl after 24 hr. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (E) Percent of wild-type and Cbr-gpdh-2(syb2973) mutant C. briggsae (AF16) mobile and developing after 24 hr at 500 mM NaCl. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (F) Percent of animals exhibiting detectable infection by N. parisii as determined by DY96 staining after 72 hr for C. elegans and C. briggsae, or 96 hr for C. kamaaina and C. tropicalis. Data presented as mean values ± s.e.m. n = 3–4 experiments of 83–202 animals. (G) Boxplots for length of L1 progeny from P0 parents that were subject to the HB101 dose series. Larvae were measured using Wormsizer. Boxplots show median length with four quartiles. n = 3–8 experiments of 50–200 animals. **p < 0.01, ***p < 0.0001, ****p < 0.0001.
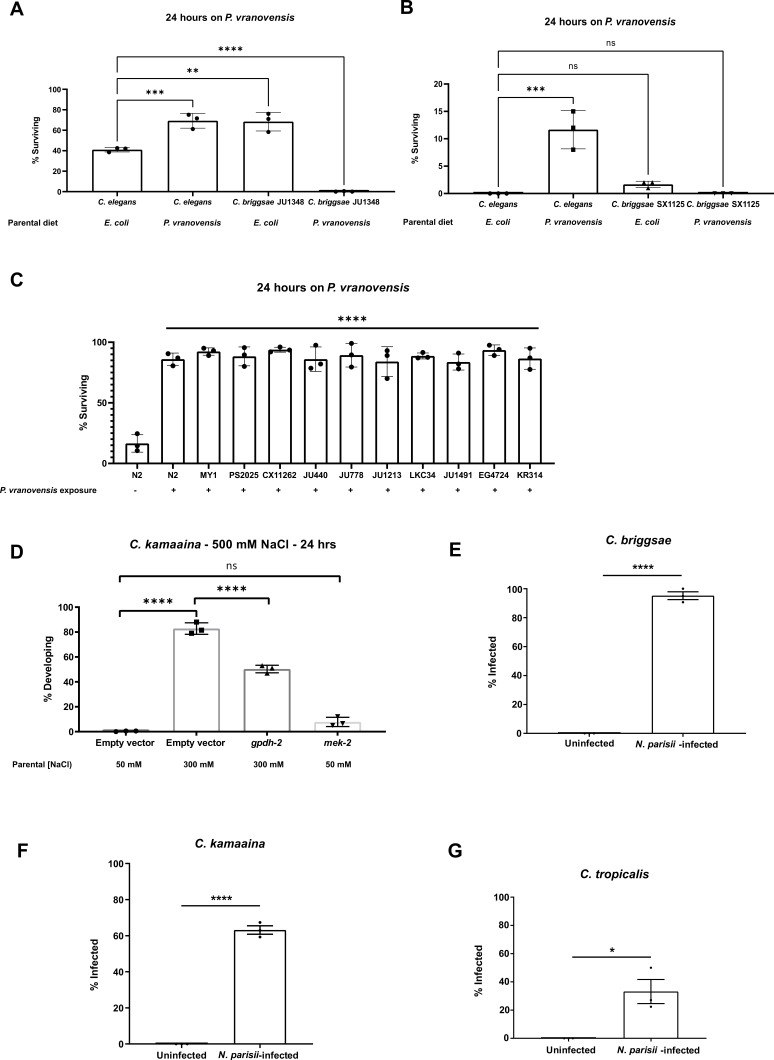
Figure 1—figure supplement 1. Intergenerational responses to environmental stress are conserved in wild isolates of Caenorhabditis species.
We exposed parents of all four species to P. vranovensis and subsequently studied their offspring’s survival rate in response to future P. vranovensis exposure. We found that parental exposure to the bacterial pathogen P. vranovensis protected offspring from future infection in both C. elegans and C. kamaaina (Figure 1B) and that this adaptive intergenerational effect in C. kamaaina required the same stress response genes (cysl-1 and rhy-1) as previously reported for C. elegans (Burton et al., 2020; Figure 1C), indicating that these animals intergenerationally adapt to infection via a similar and potentially conserved mechanism. By contrast, we found that naive C. briggsae animals were more resistant to P. vranovensis than any of the other species tested, but exposure of C. briggsae parents to P. vranovensis caused greater than 99% of offspring to die upon future exposure to P. vranovensis (Figure 1B). We confirmed that parental P. vranovensis exposure resulted in an adaptive intergenerational effect for C. elegans but a deleterious intergenerational effect for C. briggsae by testing multiple additional wild isolates of both species (Figure 1—figure supplement 1A-C). Parental exposure to P. vranovensis had no observable effect on offspring response to infection in C. tropicalis (Figure 1B). We conclude that parental exposure to P. vranovensis causes substantial changes in offspring susceptibility to future P. vranovensis exposure in multiple species, but whether those effects are protective or deleterious for offspring is species-dependent.
Using a similar approach to investigate intergenerational adaptive responses to other stresses, we found that parental exposure to mild osmotic stress protected offspring from future osmotic stress in C. elegans, C. briggsae, and C. kamaaina, but again not in C. tropicalis (Figure 1D). This intergenerational adaptation to osmotic stress in C. briggsae and C. kamaaina required the glycerol-3-phosphate dehydrogenase gpdh-2 (Figure 1E and Figure 1—figure supplement 1D), similar to previous observations for C. elegans (Burton et al., 2017) and indicating that these adaptations are regulated by similar and likely evolutionarily conserved mechanisms.
We then sought to test if intergenerational resistance to infection by the eukaryotic pathogen N. parisii is similarly conserved in Caenorhabditis species. N. parisii is a common natural pathogen of both C. elegans and C. briggsae (Zhang et al., 2016). Here, we show that N. parisii can also infect C. kamaaina and C. tropicalis (Figure 1—figure supplement 1E-G). By investigating the effects of parental N. parisii infection on offspring across species, we found that parental exposure of C. elegans and C. briggsae to N. parisii protected offspring from future infection (Figure 1F). By contrast, parental exposure of C. kamaaina and C. tropicalis to N. parisii had no observable effect on offspring infection rate (Figure 1F).
Lastly, we investigated the intergenerational effects of nutrient stress on offspring. We found that parental nutrient stress by food deprivation resulted in larger offspring in both C. elegans and C. tropicalis, which is predicted to be adaptive (Hibshman et al., 2016), but had minimal effects on offspring size in C. briggsae and C. kamaaina (Figure 1G). Collectively, our findings indicate that all four reported intergenerational adaptive effects in C. elegans are conserved in at least one other species but all four show a different pattern of conservation, which is consistent with each response being regulated by distinct molecular mechanisms (Burton et al., 2020; Burton et al., 2017; Hibshman et al., 2016; Jordan et al., 2019; Willis et al., 2021).
Parental exposure to environmental stresses leads to common and stress-specific gene expression changes in offspring across species
Of the four intergenerational models investigated here, parental exposure of C. elegans to osmotic stress and P. vranovensis infection were previously reported to cause substantial changes in offspring gene expression, including the increased expression of genes that are required for the observed intergenerational adaptations (Burton et al., 2020; Burton et al., 2017). These effects of parental stress exposure on offspring gene expression resemble a subset of the transcriptional stress response observed in parental animals and could potentially prime offspring to respond to the same stress (Burton et al., 2020). Here, we exposed C. elegans, C. briggsae, C. kamaaina, and C. tropicalis to either osmotic stress or P. vranovensis infection and subsequently performed RNA-seq on offspring to test: (1) if the specific heritable changes in gene expression in response to each of these stresses are conserved across species and (2) if any changes in gene expression correlate with the phenotypic differences in intergenerational responses to stress we observed in the different species. This analysis allowed us to compare the effects of parental stress on offspring gene expression of 7587 single-copy orthologs that are conserved across all four species (Supplementary file 1).
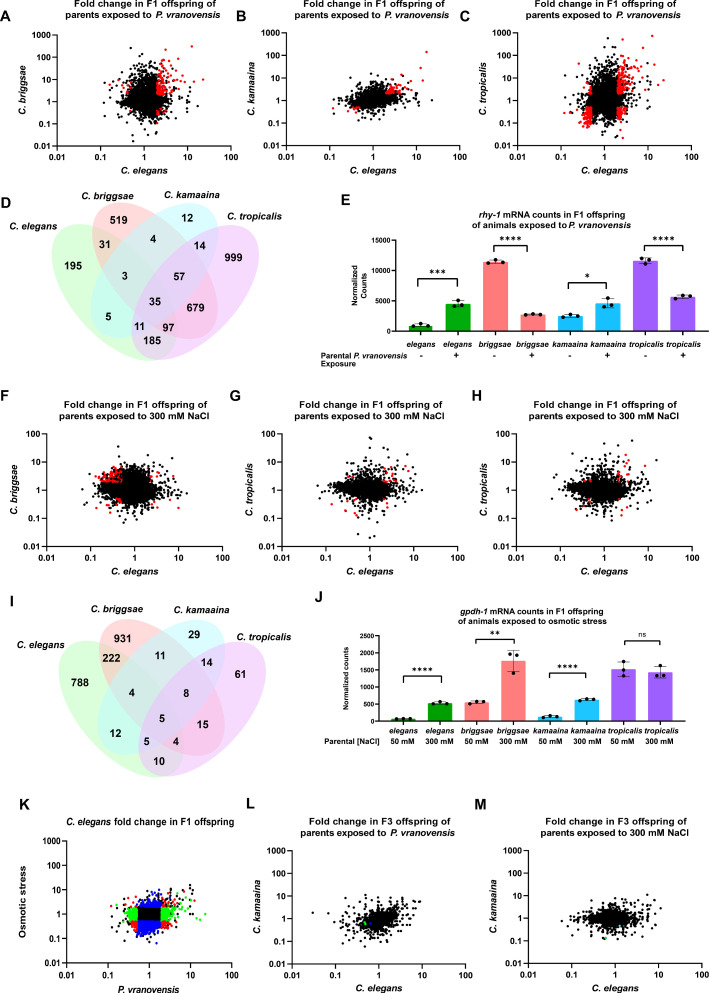
Consistent with previous observations in C. elegans, we found that parental exposure to P. vranovensis resulted in substantial changes in offspring gene expression in all four species we investigated (Figure 2 and Supplementary file 2). Of the 7587 single-copy orthologs shared between the four species, we identified 367 genes that exhibited a greater than twofold change in expression in the offspring of infected animals in C. elegans (padj <0.01) and at least one other species (Figure 2D and Supplementary file 2). Furthermore, we found that 35 genes exhibited a greater than twofold change in expression (padj <0.01) in the offspring of parents exposed to P. vranovensis in all four species (Figure 2D and Table 1). These data indicate that parental exposure to the bacterial pathogen P. vranovensis leads to changes in offspring gene expression at a common set of stress–response genes in diverse species of Caenorhabditis.
Figure 2. Parental exposure to P. vranovensis and osmotic stress have overlapping effects on offspring gene expression across multiple species.
(A) Average fold change of 7587 single-copy ortholog genes in F1 progeny of C. elegans and C. briggsae parents fed P. vranovensis BIGb0446 when compared to parents fed E. coli HB101. Average fold change from three replicates. Red dots represent genes that exhibit >twofold (padj <0.01) changes in expression in both species. (B) Average fold change of 7587 single-copy ortholog genes in F1 progeny of C. elegans and C. kamaaina parents fed P. vranovensis BIGb0446 when compared to parents fed E. coli HB101. Average fold change from three replicates. Red dots represent genes that exhibit >twofold (padj <0.01) changes in expression in both species. (C) Average fold change of 7587 single-copy ortholog genes in F1 progeny of C. elegans and C. tropicalis parents fed P. vranovensis BIGb0446 when compared to parents fed E. coli HB101. Average fold change from three replicates. Red dots represent genes that exhibit > twofold (padj <0.01) changes in expression in both species (D) Venn diagram of the number of genes that exhibit overlapping >2 fold (padj <0.01) changes in expression in F1 progeny of animals exposed to P. vranovensis BIGb0446 in each species. (E) Normalized counts of reads matching orthologs of rhy-1 in the F1 offspring of parents fed either E. coli HB101 or P. vranovensis BIGb0446. Data from Supplementary file 2. n = 3 replicates. (F) Average fold change of 7587 single-copy ortholog genes in F1 progeny of C. elegans and C. briggsae parents grown at 300 mM NaCl when compared to parents grown at 50 mM NaCl. Average fold change from three replicates. Red dots represent genes that exhibit >twofold (padj <0.01) changes in expression in both species. (G) Average fold change of 7587 single-copy ortholog genes in F1 progeny of C. elegans and C. kamaaina parents grown at 300 mM NaCl when compared to parents grown at 50 mM NaCl. Average fold change from three replicates. Red dots represent genes that exhibit >twofold (padj <0.01) changes in expression in both species in both species. (H) Average fold change of 7587 single-copy ortholog genes in F1 progeny of C. elegans and C. tropicalis parents grown at 300 mM NaCl when compared to parents grown at 50 mM NaCl. Average fold change from three replicates. Red dots represent genes exhibit >twofold (padj <0.01) changes in expression in both species. (I) Venn diagram of the number of genes that exhibit overlapping >twofold (padj <0.01) changes in expression in F1 progeny of animals grown at 300 mM NaCl in each species. (J) Normalized counts of reads matching orthologs of gpdh-1 in the F1 progeny of parents grown at either 300 mM NaCl or 50 mM NaCl. Data from Supplementary file 3. n = 3 replicates. (K) Average fold change for 7587 ortholog genes in F1 progeny of C. elegans parents fed P. vranovensis or exposed to 300 mM NaCl when compared to naive parents. Average fold change from three replicates. Red dots – genes that change in expression in response to both stresses. Blue dots – genes that change in expression in response to only osmotic stress. Green dots – genes that change in expression in response to only P. vranovensis. (L) Average fold change of 7512 single-copy ortholog genes in F3 progeny of C. elegans and C. kamaaina fed P. vranovensis BIGb0446 when compared to those fed E. coli HB101. Average fold change from three replicates. Blue dots represent genes that exhibited >twofold (padj <0.01) changes in expression in C. elegans. Green dots represent genes that exhibited >twofold (padj <0.01) changes in expression in C. kamaaina. (M) Average fold change of 7512 single-copy ortholog genes in F1 progeny of C. elegans and C. kamaaina parents grown at 300 mM NaCl when compared to parents grown at 50 mM NaCl. Average fold change from three replicates. Green dots represent genes that exhibited >twofold (padj <0.01) changes in expression in C. kamaaina. *p < 0.05, **p < 0.01, ***p < 0.0001, ****p < 0.0001.
Figure 2—figure supplement 1. Differences in developmental timing are insufficient to explain a majority of the observed differences in gene expression in the offspring of stressed parents.
Table 1. Complete list of genes that exhibited a greater than twofold change in expression in the F1 progeny of parents exposed to P. vranovensis or osmotic stress in all four species tested.
| Genes that change in F1 progeny of all species exposed to P. vranovensis |
Predicted function |
|---|---|
| C18A11.1 | Unknown |
| R13A1.5 | Unknown |
| D1053.3 | Unknown |
| pmp-5 | ATP-binding activity and ATPase-coupled transmembrane transporter activity, ortholog of human ABCD4 |
| C39E9.8 | Unknown |
| nit-1 | Nitrilase ortholog – predicted to enable hydrolase activity |
| lips-10 | Lipase related |
| srr-6 | Serpentine receptor, class R |
| Y51B9A.6 | Predicted to enable transmembrane transporter activity |
| gst-33 | Glutathione S-transferase |
| ptr-8 | Patched domain containing, ortholog of human PTCHD1, PTCHD3, and PTCHD4 |
| ZC443.1 | Predicted to enable D-threo-aldose 1-dehydrogenase activity |
| cri-2 | Conserved regulator of innate immunity, ortholog of human TIMP2 |
| Y42G9A.3 | Unknown |
| ttr-21 | Transthyretin-related, involved in response to Gram-negative bacteria |
| F45E4.5 | Involved in defense against Gram-negative bacteria |
| C42D4.1 | Domain of unknown function DUF148 |
| asp-14 | Predicted to enable aspartic-type endopeptidase activity. Involved in innate immune response |
| cyp-32B1 | Cytochrome P450 family. Ortholog of human CYP4V2 |
| nas-10 | Predicted to enable metalloendopeptidase activity and zinc ion-binding activity |
| W01F3.2 | Unknown |
| nhr-11 | Nuclear hormone receptor |
| F26G1.2 | Unknown |
| F48E3.2 | Predicted to enable transmembrane transporter activity |
| hpo-26 | Unknown, hypersensitive to pore forming toxin |
| R05H10.1 | Unknown |
| C08E8.4 | Involved in innate immune response |
| C11G10.1 | Unknown |
| Y73F4A.2 | Unknown, DOMON domain containing |
| bigr-1 | Predicted to enable hydrolase activity |
| nlp-33 | Neuropeptide like, involved in innate immune response |
| far-3 | Predicted to enable lipid-binding activity |
| Genes that change in F1 progeny of all species exposed to both osmotic stress and P. vranovensis | |
| C30B5.6 | Unknown |
| hphd-1 | Predicted to enable hydroxyacid–oxoacid transhydrogenase activity. Ortholog of human ADHFE1 |
| C42D4.3 | Unknown, DB module and domain of unknown function DB |
| Genes that change in F1 progeny of all species exposed to osmotic stress | |
| ttr-15 | Transthyretin-like family |
| F08F3.4 | Predicted to enable catalytic activity. Involved in innate immune response.Ortholog of human TDH |
We performed the same analysis on the offspring of all four species from parents exposed to osmotic stress. From this analysis, we observed that parental exposure to osmotic stress resulted in 235 genes exhibiting differential expression in both C. elegans and C. briggsae offspring (Figure 2F–J and Supplementary file 3). In addition, we found that these changes in gene expression were largely distinct from the gene expression changes observed in the offspring of parents exposed to P. vranovensis (Figure 2K and Supplementary files 2 and 3), indicating that different parental stresses have distinct effects on offspring gene expression. However, parental exposure to C. kamaaina and C. tropicalis to osmotic stress resulted in approximately fivefold fewer changes in offspring gene expression (Figure 2G–H and Supplementary file 3). In total five genes (C30B5.6, hphd-1, C42D4.3, ttr-15, and F08F3.4) exhibited differential expression in the offspring of parents exposed to osmotic stress in all four species (Figure 2I and Table 1) and three of these five (C30B5.6, hphd-1, and C42D4.3) were also observed to change in the offspring of parents exposed to P. vranovensis (Table 1).
Pairing gene expression and phenotypic data across species significantly enriches for genes required for intergenerational adaptations
To further probe how parental exposure to environmental stresses affects offspring gene expression, we first analyzed the gene ontology of the 37 genes that exhibit changes in expression in the offspring of stressed parents in all four species using g:Profiler (Raudvere et al., 2019). We found that these 37 genes were significantly enriched for extracellular proteins (p < 2.278 × 10–3). However, no additional commonalities were identified and none of these 37 genes have previously been linked to adaptations to P. vranovensis infection or osmotic stress.
We found that different species exhibit different intergenerational responses to both P. vranovensis infection and osmotic stress (Figure 1). We hypothesized that the effects of parental exposure to environmental stresses on offspring gene expression might correlate with how offspring phenotypically respond to stress. Parental exposure of C. elegans and C. kamaaina to P. vranovensis led to increased progeny resistance to future P. vranovensis exposure (Figure 1B). By contrast, parental exposure of C. briggsae to P. vranovensis led to increased offspring susceptibility to P. vranovensis (Figure 1B). We hypothesized that differences in the expression of genes previously reported to be required for adaptation to P. vranovensis, such as the acyltransferase rhy-1, might underlie these differences between species. We therefore investigated whether any genes exhibited specific changes in expression in C. elegans and C. kamaaina that were either absent or inverted in C. briggsae. We found that of the 562 genes that exhibited a greater than twofold change in expression in the offspring of parents exposed to P. vranovensis in C. elegans, only 54 also exhibited a greater than twofold intergenerational change in expression in C. kamaaina (Supplementary file 2). From this refined list of 54 genes, 17 genes either did not exhibit a change in C. briggsae or changed in the opposite direction (Table 2).
Table 2. Complete list of genes that exhibited a consistent and greater than twofold change in expression in the F1 progeny of parents exposed to P. vranovensisor osmotic stress in only species that intergenerationally adapted to stress.
Genes listed for P. vranovensis were identified by comparing genes that change consistently in C. elegans and C. kamaaina, but not C. briggsae. Genes listed for osmotic stress were identified by comparing genes that change consistently in C.elegans, C. briggsae, and C. kamaaina, but not in C. tropicalis. Bold font indicates genes that have already been demonstrated to be involved in C. elegans adaption to these stresses.
| Genes that change consistently in F1 progeny of only species that adapt to P. vranovensis | Predicted function |
|---|---|
| daf-18 | Lipid phosphatase, homologous to human PTEN tumor suppressor |
| gst-38 | Glutathione S-transferase |
| H04M03.3 | Predicted to enable oxidoreductase activity. |
| oops-1 | Oocyte partner of SPE-11 |
| F09G8.10 | Unknown |
| glb-1 | Globin -related |
| F57H12.6 | Unknown |
| elo-6 | Predicted to enable transferase activity, transferring acyl groups, ortholog of human ELOVL3 and ELOVL6 |
| cpr-5 | Predicted to enable cysteine-type peptidase activity |
| xpo-2 | Exportin involved in nuclear export, ortholog of human CSE1L |
| cysl-1 | Cysteine synthase known to be involved in adaptation to P. vranovensis |
| rhy-1 | Regulator of HIF-1 known to be involved in adaptation to P. vranovensis |
| cdc-25.1 | Homolog of human CDC25 phosphatase |
| imb-1 | Importin beta family, ortholog of human KPNB1 |
| VZK882L.2 | Unknown |
| cysl-2 | Cysteine synthase known to be involved in adaptation to P. vranovensis |
| cyk-7 | Involved in intercellular bridge organization |
| Genes that change consistently in F1 progeny of only species that adapt to osmotic stress | |
| T05F1.9 | Unknown |
| grl-21 | Unknown, Ground-like domain containing |
| gpdh-1 | Glycerol-3-phosphate dehydrogenase known to be involved in osmotic stress resposne |
| T22B7.3 | Amidinotransferase, ortholog of human DDAH1 and DDAH2 |
Consistent with our hypothesis that intergenerational gene expression changes across species might correlate with their phenotypic responses, we found that all three genes previously reported to be required for the intergenerational adaptation to P. vranovensis (rhy-1, cysl-1, and cysl-2 – Burton et al., 2020) were among the 17 genes that exhibited differential expression in C. elegans and C. kamaaina but not in C. briggsae. This overlap is significantly above what is expected by chance (P < 1.337e−−08 – hypergeometric probability). We conclude that the effects of parental exposure to P. vranovensis on offspring gene expression correlate with their phenotypic response. Furthermore, we propose that this new list of 17 genes (Table 2) is likely to be enriched in additional conserved genes required for this intergenerational response to pathogen infection. This list includes multiple highly conserved genes including multiple factors involved in nuclear transport (imb-1 and xpo-2), the CDC25 phosphatase ortholog cdc-25.1, and the PTEN tumor suppressor ortholog daf-18.
Notably, of the revised list of 17 genes, we identified a single gene that exhibited a greater than twofold increase in expression in C. elegans and C. kamaaina F1 progeny but had an inverted greater than twofold decrease in expression in C. briggsae F1 progeny. That gene is rhy-1 (Figure 2E), one of the three genes known to be required for animals to intergenerationally adapt to P. vranovensis infection (Burton et al., 2020). This directional change of rhy-1 expression in progeny of animals exposed to P. vranovensis correlates with the observation that parental exposure to P. vranovensis results in enhanced pathogen resistance in offspring in C. elegans and C. kamaaina but has a strong deleterious effect on pathogen resistance in C. briggsae (Figure 1B). Collectively, these findings suggest that molecular mechanisms underlying adaptive and deleterious effects in different species might be related and dependent on the direction of changes in gene expression of specific stress–response genes.
We performed the same analysis pairing our transcriptional data with our phenotypic data for the intergenerational response to osmotic stress. We found that C. elegans, C. briggsae, and C. kamaaina intergenerationally adapted to osmotic stress, but C. tropicalis did not (Figure 1D). We therefore identified genes that were differentially expressed in the F1 offspring of C. elegans, C. briggsae, and C. kamaaina exposed to osmotic stress, but not in C. tropicalis. From this analysis, we identified four genes (T05F1.9, grl-21, gpdh-1, and T22B7.3) that are specifically differentially expressed in the three species that adapt to osmotic stress but not in C. tropicalis (Table 2); this list of genes includes the glycerol-3-phosphate dehydrogenase gpdh-1 which is one of the most upregulated genes in response to osmotic stress and is known to be required for animals to properly respond to osmotic stress (Lamitina et al., 2006). These results suggest that, similar to our observations for P. vranovensis infection, different patterns in the expression of known osmotic stress response genes correlate with different intergenerational phenotypic responses to osmotic stress.
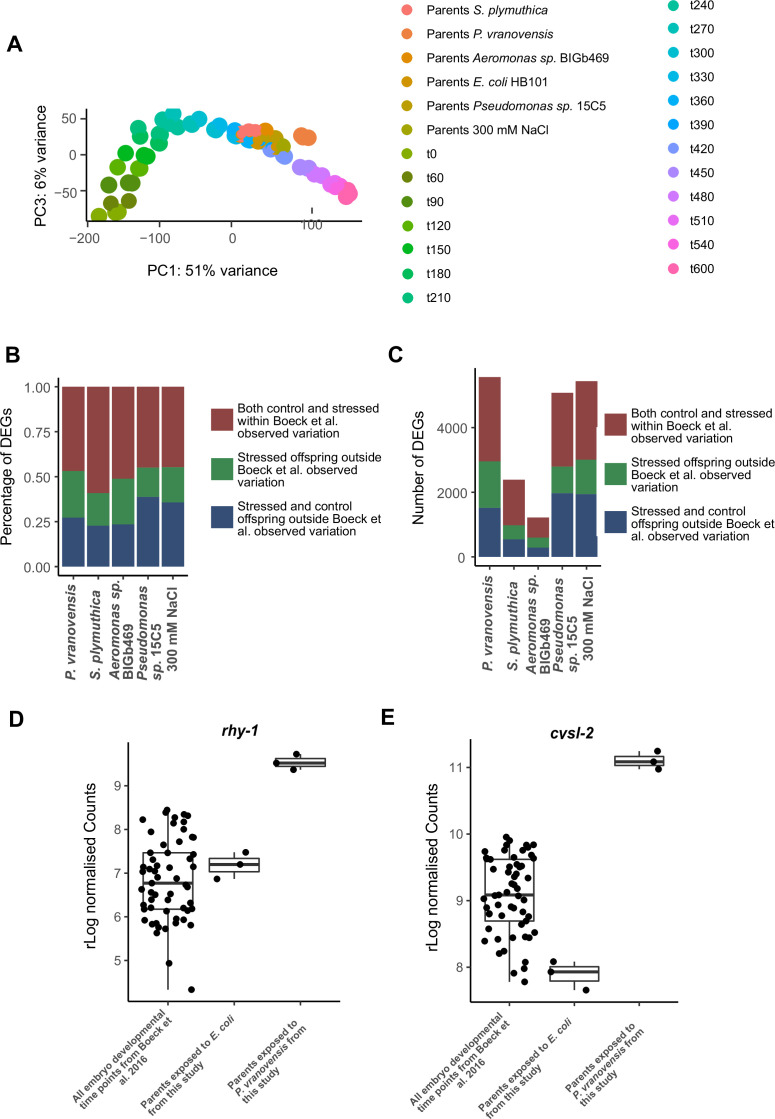
Differences in gene expression in the offspring of stressed parents could be due to programmed changes in expression in response to stress or due to indirect effects caused by changes in developmental timing. To confirm that the embryos from all conditions were collected at the same developmental stage we compared our RNA-seq findings to a time-resolved transcriptome of C. elegans development (Boeck et al., 2016). Consistent with our visual observations that a vast majority of offspring collected were in the comma stage of embryo development, we found that the gene expression profiles of all offspring from both naive and stressed parents overlapped strongly with the 330- to 450-min time points of development (Figure 2—figure supplement 1). In addition, we found that approximately 50% of all genes that were differentially expressed in the offspring of stressed parents when compared to naive parents exhibited a change in gene expression that was more than one standard deviation outside their average expression across all time points of embryo development (Figure 2—figure supplement 1B-C). We similarly found that many of the genes known to be required for intergenerational responses to stress exhibit expression that is outside the range of expression observed at any time point of early development (Figure 2—figure supplement 1D-E). These results suggest that a majority of the expression differences we observed in the offspring of stressed parents were not due to differences in developmental timing.
The effects of parental bacterial infection and osmotic stress on offspring gene expression are not maintained transgenerationally
Determining whether the effects of parental exposure to stress on offspring gene expression are reversible after one generation or if any changes in gene expression persist transgenerationally is a critical and largely unanswered question in the field of multigenerational effects. To test if any of the intergenerational changes in gene expression that we observed persist transgenerationally, we performed RNA-seq of F3 progeny of C. elegans exposed to both P. vranovensis and osmotic stress. We found that none of the 1515 genes that exhibited differential expression in F1 progeny for either P. vranovensis infection or osmotic stress were also differentially expressed in C. elegans F3 progeny (Figure 2L and M and Supplementary file 4). We conclude that, at minimum, the vast majority of intergenerational effects of these stresses on gene expression in C. elegans do not persist transgenerationally.
We hypothesized that transgenerational effects on gene expression could potentially be more robust in other species. We therefore performed the same analysis on F3 gene expression in response to both P. vranovensis infection and osmotic stress in a second species that intergenerationally adapts to both stresses, C. kamaaina. We again found that none of the genes that exhibited differential expression in F1 progeny of parents exposed to P. vranovensis were also differentially expressed in F3 progeny (Figure 2L and Supplementary file 4). We did, however, identify two genes, the C. kamaaina orthologs of C. elegans hphd-1 and C09B8.4, that exhibited differential expression in both the F1 and F3 progeny of parents exposed to osmotic stress (Figure 2M and Supplementary file 4). It is possible that these two genes represent true transgenerational effects on gene expression, but given that these effects were not also observed in C. elegans and that only two genes were identified out of thousands of possible gene comparisons using a false discovery cutoff of 1 %, we cannot rule out that these two genes are false positives. Collectively, our results suggest that neither of these biotic or abiotic stresses that elicit robust intergenerational changes in gene expression cause similar transgenerational changes in gene expression under the same conditions in multiple different species. We note, however, that it remains possible that transgenerational effects of these stresses could persist through other mechanisms, could affect the expression of genes that are not clearly conserved between species, or could exert weaker effects on broad classes of genes that would not be detectable at any specific individual loci as was reported for the transgenerational effects of starvation and loss of COMPASS complex function on gene expression in C. elegans (Greer et al., 2011; Webster et al., 2018). Furthermore, it is possible that transgenerational effects on gene expression in C. elegans are restricted to germ cells (Buckley et al., 2012; Houri-Zeevi et al., 2020; Posner et al., 2019) or to a small number of cells and are not detectable when profiling gene expression in somatic tissue from whole animals.
Intergenerational responses to stress can have deleterious tradeoffs
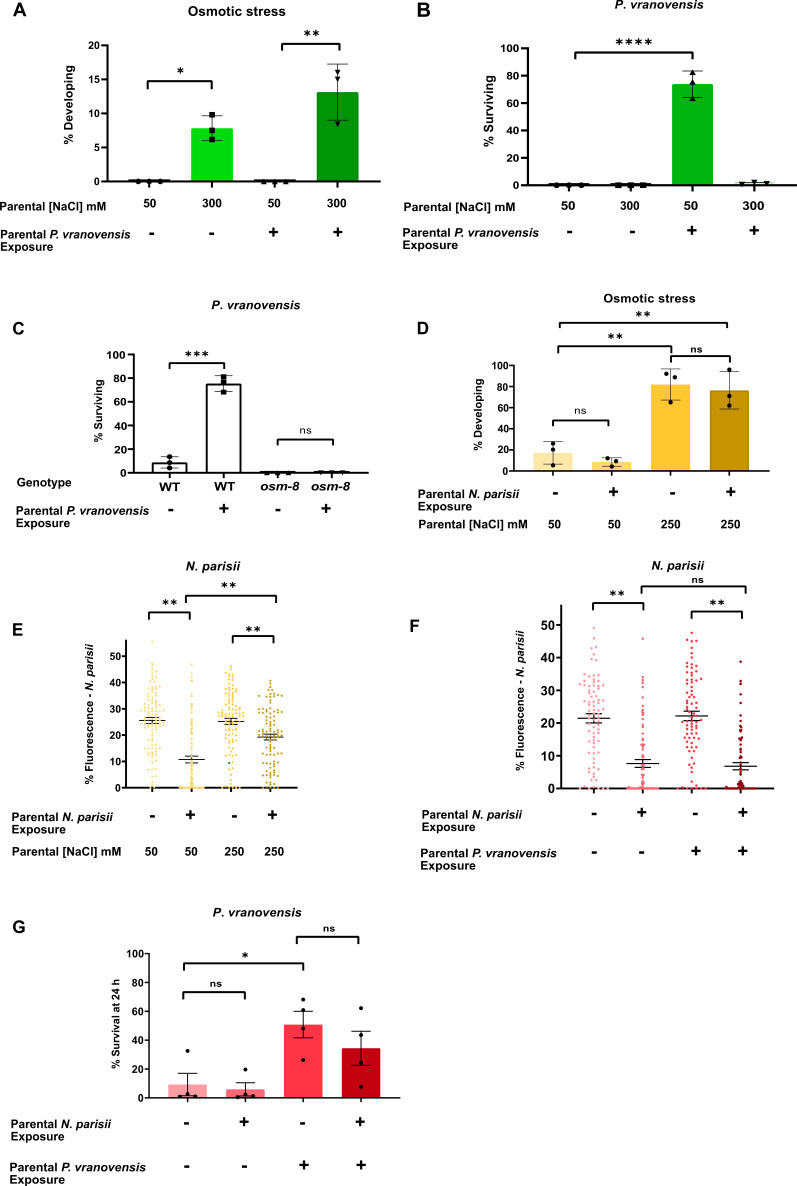
Intergenerational changes in animal physiology that protect offspring from future exposure to stress could be stress-specific or could converge on a broadly stress-resistant state. If intergenerational adaptive effects are stress-specific, then it is expected that parental exposure to a given stress will protect offspring from that same stress but potentially come at the expense of fitness in mismatched environments. If intergenerational adaptations to stress converge on a generally more stress-resistant state, then parental exposure to one stress might protect offspring against many different types of stress. To determine if the intergenerational effects we investigated here represent specific or general responses, we assayed how parental C. elegans exposure to osmotic stress, P. vranovensis infection, and N. parisii infection, either alone or in combination, affected offspring responses to mismatched stresses. We found that parental exposure to P. vranovensis did not affect the ability of animals to intergenerationally adapt to osmotic stress (Figure 3A). By contrast, parental exposure to osmotic stress completely eliminated the ability of animals to intergenerationally adapt to P. vranovensis (Figure 3B). This effect is unlikely to be due to the effects of osmotic stress on P. vranovensis itself, as mutant animals that constitutively activate the osmotic stress response (osm-8) were also completely unable to adapt to P. vranovensis infection (Figure 3C; Rohlfing et al., 2011). We conclude that animals’ intergenerational responses to P. vranovensis and osmotic stress are stress-specific, consistent with our observation that parental exposure to these two stresses resulted in distinct changes in offspring gene expression (Figure 2K).
Figure 3. Intergenerational adaptations to stress are stress-specific and have deleterious tradeoffs.
(A) Percent of wild-type C. elegans mobile and developing at 500 mM NaCl after 24 hr. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (B) Percent of wild-type C. elegans surviving after 24 hr of exposure to P. vranovensis BIGb0446. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (C) Percent of wild-type and osm-8(n1518) C. elegans surviving after 24 hr of exposure to P. vranovensis BIGb0446. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (D) Percent of wild-type C. elegans mobile and developing at 420 mM NaCl after 48 hr. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (E) N. parisii parasite burden of individual C. elegans after 72 hr (as determined by percentage fluorescence from DY96-stained spores after 72 hr). Data presented as mean values ± s.e.m. n = 4 experiments of 25 animals (F) N. parisii parasite burden of individual C. elegans after 72 hr (as determined by percentage fluorescence from DY96-stained spores after 72 hr). Data presented as mean values ± s.e.m. n = 3 experiments of 25 animals. (G) Percent of wild-type C. elegans surviving after 24 hr of exposure to P. vranovensis BIGb0446. Data presented as mean values ± s.e.m. n = 3 experiments of >100 animals. *p < 0.05, **p < 0.01, ***p < 0.0001, ****p < 0.0001.
We performed a similar analysis comparing animals’ intergenerational response to osmotic stress and the eukaryotic pathogen N. parisii. We previously reported that L1 parental infection with N. parisii results in progeny that is more sensitive to osmotic stress (Willis et al., 2021). Here, we found that L4 parental exposure of C. elegans to N. parisii had a small, but not significant effect on offspring response to osmotic stress (Figure 3D). However, similar to our observations for osmotic stress and bacterial infection, we found that parental exposure to both osmotic stress and N. parisii infection simultaneously resulted in offspring that were less protected against future N. parisii infection than when parents are exposed to N. parisii alone (Figure 3E). Collectively, these data further support the conclusion that intergenerational responses to infection and osmotic stress are stress-specific and suggest that intergenerational adaptations to osmotic stress might come at the expense of animals’ ability to properly respond to bacterial or eukaryotic infections when either is paired with osmotic stress.
To compare animals’ intergenerational responses to bacterial infection and eukaryotic infection, we performed a similar comparative analysis. We found that parental exposure to P. vranovnesis had no observable effect on offspring response to N. parisii either alone or when both pathogens were present simultaneously (Figure 1F). Similarly, we found that parental exposure to N. parisii had no observable effect on offspring response to P. vranovensis either alone or when both pathogens were present at the same time (Figure 1G). We conclude that intergenerational adaptations to osmotic stress, P. vranovensis infection and N. parisii infection are largely stress-specific.
Intergenerational responses to Pseudomonas pathogens are distinct from other bacterial pathogens
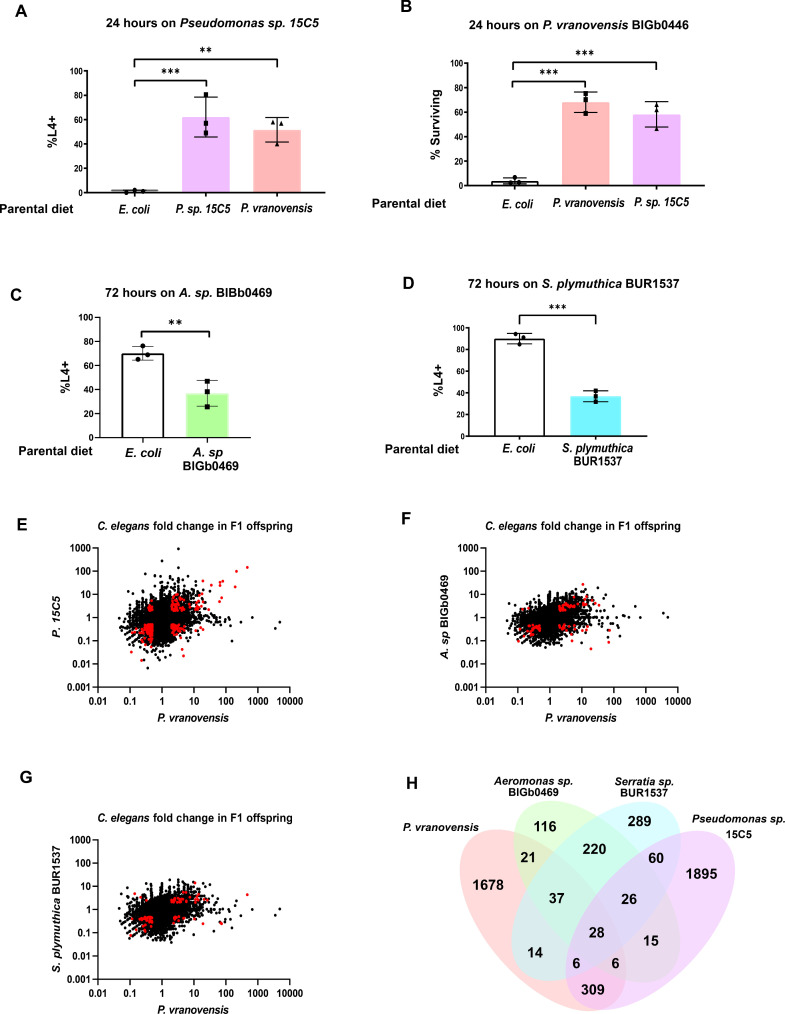
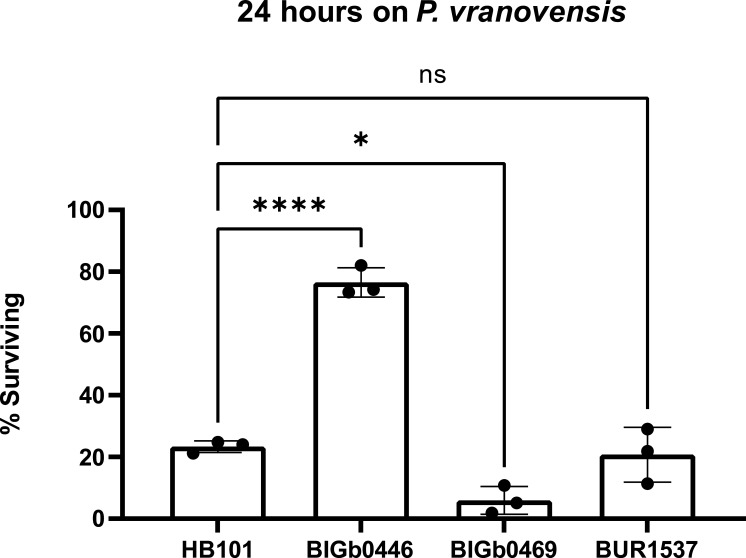
To further probe the specificity of intergenerational responses to stress, we also sought to determine if the substantial changes in pathogen resistance and gene expression observed in C. elegans offspring from parents exposed to the bacterial pathogen P. vranovensis were specific to this pathogen or part of a general response to bacterial pathogens. We previously found that the transcriptional response to P. vranovensis in F1 progeny is distinct from the response to P. aeruginosa (Burton et al., 2020). To further probe the specificity of this intergenerational response, we first screened wild bacterial isolates from France (Samuel et al., 2016) and the United Kingdom (Supplementary file 5) for those that are potential natural pathogens of C. elegans and that also intergenerationally affect C. elegans survival or growth rate. From this analysis, we identified a new Pseudomonas isolate, Pseudomonas sp. 15C5, where parental exposure to Pseudomonas sp. 15C5 enhanced offspring growth rate in response to future exposure to Pseudomonas sp. 15C5 (Figure 4A). This intergenerational effect resembled C. elegans intergenerational adaptation to P. vranovensis and we found that parental exposure to either isolate of Pseudomonas protected offspring from future exposure to the other Pseudomonas isolate (Figure 4A–B). To test if Pseudomonas sp. 15C5 was a new isolate of P. vranovensis or a distinct species of Pseudomonas, we performed both 16S rRNA sequencing and sequenced the gene rpoD of Pseudomonas sp. 15C5. From this analysis, we found that Pseudomonas sp. 15C5 is not an isolate of P. vranovensis and is most similar to Pseudomonas putida – 99.49% identical 16S rRNA and 98.86% identical rpoD by BLAST (Supplementary file 6). These results indicate that parental exposure to multiple different Pseudomonas species can protect offspring from future pathogen exposure. We note, however, that other pathogenic species of Pseudomonas, such as P. aeruginosa, did not cross protect against P. vranovensis (Burton et al., 2020), indicating that not all pathogenic species of Pseudomonas result in the same intergenerational in offspring pathogen resistance.
Figure 4. Many of the intergenerational effects of parental exposure to bacterial pathogens on offspring gene expression are pathogen specific.
(A) Percent of wild-type C. elegans that developed to the L4 larval stage after 48 hr of feeding on Pseudomonas sp. 15C5. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (B) Percent of wild-type C. elegans surviving after 24 hr of exposure to P. vranovensis BIGb0446. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (C) Percent of wild-type C. elegans that developed to the L4 larval stage after 48 hr of feeding on Aeromonas sp. BIGb0469. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (D) Percent of wild-type C. elegans that developed to the L4 larval stage after 48 hr of feeding on Serratia plymuthica BUR1537. Data presented as mean values ± s.d. n = 3 experiments of >100 animals. (E) Average fold change of genes in F1 progeny of C. elegans fed either Pseudomonas sp. 15C5 or P. vranovensis BIGb0446 when compared to parents fed E. coli HB101. Average fold change from three replicates. Red dots represent genes that exhibit statistically significant (padj <0.01) changes in the F1 offspring of parents fed both Pseudomonas sp. 15C5 and P. vranovensis BIGb0446. (F) Average fold change of genes in F1 progeny of C. elegans fed either Aeromonas sp. BIGb0469 or P. vranovensis BIGb0446 when compared to parents fed E. coli HB101. Average fold change from three replicates. Red dots represent genes that exhibit statistically significant (padj <0.01) changes in the F1 offspring of parents fed both Aeromonas sp. BIGb0469 and P. vranovensis BIGb0446. (G) Average fold change of genes in F1 progeny of C. elegans fed either S. plymuthica BUR1537 or P. vranovensis BIGb0446 when compared to parents fed E. coli HB101. Average fold change from three replicates. Red dots represent genes that exhibit statistically significant (padj <0.01) changes in the F1 offspring of parents fed both S. plymuthica BUR1537 and P. vranovensis BIGb0446. (H) Venn diagram of the number of genes that exhibit overlapping statistically significant (padj <0.01) changes in expression in F1 progeny of C. elegans parents fed each different bacterial species. **p < 0.01, ***p < 0.0001.
Figure 4—figure supplement 1. Parental exposure to Aeromonas sp. BIGb0469 and S. plymuthica BUR1537 does not protect offspring from P. vranovensis.
In addition to these intergenerational adaptive effects, we also identified two bacterial isolates that activate pathogen–response pathways, Serretia plymutica BUR1537 and Aeromonas sp. BIGb0469 (Samuel et al., 2016; Hellberg et al., 2015), that resulted in intergenerational deleterious effects (Figure 4C–D). Parental exposure of animals to these potential bacterial pathogens did not intergenerationally protect animals against P. vranovensis (Figure 4—figure supplement 1). We conclude that parental exposure to some species of Pseudomonas can protect offspring from other species of Pseudomonas, but that these effects are likely specific to a subset of Pseudomonas species and not part of a broad response to Gram-negative bacterial pathogens.
To determine how different parental bacterial infections affect offspring gene expression patterns, we profiled gene expression in the offspring of C. elegans parents exposed to each of P. vranovensis BIGb0427, Pseudomonas sp. 15C5, Serretia plymuthica BUR1537, and Aeromonas sp. BIGb0469. We found that only 28 genes exhibit differential expression in the offspring of parents exposed to all four potential pathogens (Figure 4E–H). However, we identified 309 genes that are specifically differentially expressed in the offspring of parents exposed to P. vranovensis and Pseudomonas sp. 15C5 but not in the offspring of parents exposed to S. plymuthica BUR1537 or Aeromonas sp. BIGb0469 (Figure 4H and Supplementary file 7). We conclude that parental exposure to bacterial pathogens that elicit enhanced offspring resistance to P. vranovensis resulted in distinct changes in offspring gene expression that are not observed when parents are exposed to other Gram-negative bacterial pathogens. Collectively, our results suggest that a majority of the intergenerational effects of a parent’s environment on offspring gene expression are both stress and pathogen-specific.
Discussion
Overall, our findings support the conclusion that some types of intergenerational effects are conserved while others diverge, not unlike other important aspects of biology that vary between the species investigated here such as mode of reproduction – C. elegans, C. briggsae, and C. tropicalis are hermaphroditic while C. kamaaina is an exclusively male–female species. Specifically, our findings provide some of the first evidence that the mechanisms underlying intergenerational effects of a parent’s environment on offspring are evolutionarily conserved among different species. While these findings are restricted to the genus Caenorhabditis, to our knowledge they represent the first observation that intergenerational responses to stress are conserved across any evolutionary distance, and they provide a base from which we can compare the numerous different reported observations of multigenerational effects in C. elegans to similar intergenerational responses to stress in more distantly related species. For example, we found that only a subset of the many transcriptional changes that are detectable in the offspring of stressed C. elegans parents are conserved in any other species investigated. In addition, we used our analysis to identify 37 genes that exhibited intergenerational regulation of expression in response to the specific stresses of P. vranovensis infection or osmotic stress in all species studied (Figure 3). We propose that these genes might be particularly tuned for intergenerational regulation and might similarly be involved in intergenerational responses to stress in more distantly related species, including species outside the Caenorhabditis genus.
Notably, we found that the expression of these 37 genes in the offspring of parents exposed to either P. vranovensis infection or osmotic stress were still differentially expressed in C. tropicalis even though parental exposure to these stresses did not appear to affect offspring stress resistance in either assay (Figures 1 and 2). We hypothesize that the molecular consequences of parental stress on offspring, such as changes in the expression of stress–response genes, might be more easily identifiable than the specific physiological consequences of parental stress on offspring. In this case, we might not have detected the unique phenotypic effects of parental exposure to stress on offspring in C. tropicalis using our assay conditions, but such effects might still exist in this species and be related to those observed in other species. Future studies of the phenotypic effects of parental stress on offspring across species will likely shed significant light on how similar molecular mechanisms can mediate different intergenerational responses to stress across evolution.
Consistent with the hypothesis that parental exposure to the same stress might elicit distinct phenotypic effects on offspring in different species via evolutionarily related mechanisms, we found that parental exposure of C. briggsae to P. vranovensis had a strong deleterious effect on offspring pathogen resistance even though parental exposure of C. elegans and C. kamaaina to P. vranovensis resulted in increased offspring resistance to P. vranovensis (Figure 1B). This inversion of an intergenerational effect from a presumed adaptive effect to a presumed deleterious effect correlated with an inversion in the expression of specific pathogen–response genes that were previously reported to be required for animals to intergenerationally adapt to P. vranovensis, such as rhy-1 which exhibits increased expression in C. elegans and C. kamaaina offspring from infected parents but decreased expression in C. briggsae offspring from infected parents (Figure 2E). To our knowledge, these findings are the first to suggest that the molecular mechanisms underlying presumed adaptive and deleterious intergenerational effects in different species are evolutionarily related at the gene expression level. These findings suggest that similar observations of presumed intergenerational deleterious effects in diverse species, such as fetal programming in humans, might also be molecularly related to intergenerational adaptive effects in other species. Alternatively, our findings suggest that presumed intergenerational deleterious effects might in fact represent deleterious tradeoffs that are adaptive in other contexts. We expect that a more complete consideration of the evolution of intergenerational effects and the potential relationship between adaptive and deleterious effects will play an important role in understanding how intergenerational effects contribute to organismal resilience in changing environments, what role such effects play in evolution, and how such effects contribute to multiple human pathologies associated with a parent’s environment (Langley-Evans, 2006).
Lastly, the extent to which intergenerational and transgenerational responses to environmental stress represent related, independent, or even mutually exclusive phenomena represents a major outstanding question in the field of multigenerational effects. Evolutionary modeling of intergenerational and transgenerational effects has suggested that different ecological pressures favor the evolution of either intergenerational or transgenerational responses under different conditions. Specifically, it has been suggested that intergenerational effects are favored when offspring environmental conditions are predictable from the parental environment (Dey et al., 2016; Lind et al., 2020; Proulx et al., 2019; Uller, 2008). Furthermore, it has been speculated that intergenerational adaptations to stress will have costs (Uller, 2008). These costs, such as the costs we observed for animals intergenerational adaptation to osmotic stress (Figure 3), are likely to strongly favor the loss or active erasure of intergenerational effects if the parental environment improves to avoid potential deleterious effects when a stress is no longer present. By contrast, transgenerational effects were found to predominantly be favored when parental environmental cues are unreliable and the maintenance of information across many generations might be worth the potential costs (Uller et al., 2015).
Our findings in this study support either a model in which intergenerational and transgenerational effects represent potentially distinct phenomena or a model in which transgenerational effects only persist or occur under certain conditions with the vast majority of the effects of parental stress on offspring gene expression being lost or actively erased after one generation under other conditions. We strongly suspect that future studies into the mechanisms regulating these intergenerational effects will shed significant light on how intergenerational effects on gene expression are lost and/or erased. In addition, we expect that similar studies of transgenerational effects will potentially elucidate the circumstances under which animals decide if environmental information might be worth maintaining transgenerationally despite any potential tradeoffs and if the growing number of transgenerational effects observed in C. elegans are similarly evolutionarily conserved.
Lastly, future studies of intergenerational effects will be critical in determining the extent to which the mechanisms that mediate intergenerational effects are conserved outside of Caenorhabditis and if similar mechanisms to those uncovered in C. elegans mediate the numerous different adaptive and deleterious intergenerational effects that have been reported in diverse taxa ranging from the intergenerational development of wings in aphids (Vellichirammal et al., 2017) to fetal programming and the role it plays in disease in humans (Langley-Evans, 2006).
Materials and methods
Strains
C. elegans strains were cultured and maintained at 20°C unless noted otherwise. The Bristol strain N2 was the wild-type strain. Wild-isolate strains used in the main figures of this study: N2 (C. elegans), AF16 (C. briggsae), JU1373 (C. tropicalis), and QG122 (C. kamaaina). Wild-isolate strains used in figure supplements of this study: MY1 (C. elegans), PS2025 (C. elegans), CX11262 (C. elegans), JU440 (C. elegans), JU778 (C. elegans), JU1213 (C. elegans), LKC34 (C. elegans), JU1491 (C. elegans), EG4724 (C. elegans), KR314 (C. elegans), SX1125 (C. briggsae), and JU1348 (C. briggsae). Mutant alleles used in this study: osm-8(n1518) and Cbr-gpdh-2(syb2973).
P. vranovensis survival assays
P. vranovensis BIGb0446 or Pseudomonas sp. 15C5 was cultured in LB at 37 °C overnight. 1 ml of overnight culture was seeded onto 50 mm NGM agar plates and dried in a laminar flow hood (bacterial lawns completely covered the plate such that animals could not avoid the pathogen). All plates seeded with BIGb0446 or 15C5 were used the same day they were seeded. Young adult animals were placed onto 50 mm NGM agar plates seeded with 1 ml either E. coli HB101, P. vranovensis BIGb446, or Pseudomonas sp. 15C5 for 24 h at room temperature (22 °C). Embryos from these animals were collected by bleaching and placed onto fresh NGM agar plates seeded with BIGb0446. Percent surviving were counted after 24 hr at room temperature (22 °C) unless otherwise noted.
Osmotic stress and P. vranovensis multiple stress adaptation assays
Young adult animals that were grown on NGM agar plates seeded with E. coli HB101 were collected and transferred to new 50 mM NaCl control plates seeded with E. coli HB101, 300 mM NaCl plates seeded with E. coli HB101, 50 mM NaCl control plates seeded with P. vranovensis BIGb0446, or 300 mM NaCl plates seeded with P. vranovensis BIGb0446. Animals were grown for 24 hr at room temperature (22 °C). Embryos from these animals were collected by bleaching and transferred to new 500 mM NaCl plates seeded with E. coli HB101 or 50 mM NaCl plates seeded with P. vranovensis BIGb0446. Percent of animals developing or surviving was scored after 24 hr at room temperature as previously described in Burton et al., 2017 and Burton et al., 2020.
Preparation of N. parisii spores
Spores were prepared as described previously (Willis et al., 2021). In brief, large populations of C. elegans N2 were infected with microsporidia spores. Infected worms were harvested and mechanically disrupted using 1 mm diameter Zirconia beads (BioSpec). Resulting lysate was filtered through 5 μm filters (Millipore Sigma) to remove nematode debris. Spore preparations were tested for contamination and those free of contaminating bacteria were stored at −80 °C.
N. parisii infection assays and multiple stress adaptation assays
P0 populations of 2500 animals were mixed with 1 ml of 10× saturated E. coli OP50-1 or P. vranovensis and a low dose of N. parisii spores (see Table 3) and plated on a 10 cm plate. This low dose limited the detrimental effects on animal fertility that are observed with higher doses, while ensuring most animals were still infected. F1 populations of 1000 animals were mixed with 400 μl of 10× saturated E. coli OP50-1 and a high dose of N. parisii spores (see Table 3) and plated on a 6 cm plate.
Table 3. Details of N. parisii doses employed.
| N. parisii dose | Plate concentration (spores/cm2) | Millions of spores used | |
|---|---|---|---|
| 6 cm plate | 10 cm plate | ||
| Low | ~32,000 | 2.5 | |
| High | ~88,000 | 2.5 | |
To test for inherited immunity to N. parisii in C. elegans, C. briggsae, C. tropicalis, and C. kamaaina, synchronized animals were infected from the L1 larval stage with a low dose of N. parisii. C. elegans and C. briggsae were grown for 72 hr at 21°C; C. tropicalis and C. kamaaina were grown for 96 hr at 21°C. Ten percent of total P0 animals were fixed in acetone for DY96 staining, as described below. Embryos from the remaining animals were collected by bleaching and synchronized by hatching overnight in M9. Resulting F1 animals were infected from the L1 larval stage with a high dose of N. parisii. C. elegans and C. briggsae were fixed at 72 hr postinfection (hpi) at 21°C; C. tropicalis and C. kamaaina were fixed at 96 hpi at 21°C.
For multiple stress adaptation assays using N. parisii and osmotic stress, animals were grown on NGM agar plates seeded with 10× saturated E. coli OP50-1 until the L4 stage. Next, animals were collected and mixed with 1 ml of either E. coli OP50-1 alone or supplemented with a low dose of N. parisii spores and plated on either 50 mM NaCl or 250 mM NaCl plates. Animals were grown for 24 hr at 21 °C. Embryos from these animals were collected by bleaching. To test adaptation to osmotic stress, 2000 F1 embryos were transferred to 420 mM NaCl plates seeded with E. coli OP50-1. Percentage of animals hatched was scored after 48 hr at 21 °C, as previously described in Burton et al., 2017 and Burton et al., 2020. To test adaptation to N. parisii, the remaining embryos were synchronized by hatching overnight in M9. Resulting F1 animals were either not infected as controls, or infected at the L1 larval stage with a high dose of N. parisii. Animals were fixed after 72 hr at 21 °C for DY96 staining and analysis.
For multiple stress adaptation assays using N. parisii and P. vranovensis, animals were grown on NGM agar plates seeded with E. coli OP50-1 until the L4/young adult stage. Next, animals were collected and mixed with 1 ml of either E. coli OP50-1 alone or E. coli OP50-1 supplemented with a low dose of N. parisii spores, or 1 ml of P. vranovensis BIGb0446 alone or P. vranovensis BIGb0446 supplemented with a low dose of N. parisii spores. Animals were plated on NGM and grown for 24 hr at 21 °C. Embryos from these animals were collected by bleaching. To test adaptation to P. vranovensis, 2000 F1 embryos were transferred to new NGM plates seeded with P. vranovensis BIGb0446. Percentage of animals surviving was scored after 24 hr at 21 °C as previously described in Burton et al., 2017 and Burton et al., 2020. To test adaptation to N. parisii, the remaining embryos were synchronized by hatching overnight in M9. Resulting F1 animals were either not infected as controls, or infected from the earliest larval stage with a high dose of N. parisii. Animals were fixed after 72 hr at 21 °C for DY96 staining and analysis.
Fixation and staining of N. parisii infection
Worms were washed off plates with M9 and fixed in 1 ml acetone for 10 min at room temperature, or overnight at 4 °C. Fixed animals were washed twice in 1 ml PBST (phosphate-buffered saline [PBS] containing 0.1 % Tween-20) before staining. Microsporidia spores were visualized with the chitin-binding dye Direct Yellow (DY96). For DY96 staining alone, animals were resuspended in 500 μl staining solution (PBST, 0.1 % sodium dodecyl sulfate, 20 μg/ml DY96), and rotated at 21 °C for 30 min in the dark. DY96-stained worms were resuspended in 20 μl EverBrite Mounting Medium (Biotium) and mounted on slides for imaging. Note: to pellet worms during fixation and staining protocols, animals were centrifuged for 30 s at 10,000 × g.
Image analysis of N. parisii infection
Worms were imaged with an Axioimager 2 (Zeiss). DY96-stained worms were imaged to determine number of embryos per worm. Worms possessing any quantity of intracellular DY96-stained microsporidia were considered infected. Precise microsporidia burdens were determined using ImageJ/FIJI (Schindelin et al., 2012). For this, each worm was defined as an individual ‘region of interest’ and fluorescence from GFP (DY96-stained microsporidia) subject to ‘threshold’ and ‘measure area percentage’ functions on ImageJ. Images were thresholded to capture the brighter signal from microsporidia spores, while eliminating the dimmer GFP signal from worm embryos. Final values are given as % fluorescence for single animals.
Preparation of OP50 for plating worms
One colony of E. coli strain OP50 was added to 100 ml of LB and grown overnight at room temperature then stored at 4 °C. One or five drops of HB101 were added to 6 or 10 cm plates of NGM, respectively, to use for growing worm strains and recovering them from starvation.
Preparation of HB101 for liquid culture
One colony of E. coli strain HB101 was added to a 5 ml starter culture of LB with streptomycin and grown for 24 hr at 37 °C. The starter cultures were then added to a 1 l culture of TB and grown for another 24 hr at 37 °C. The bacteria was centrifuged for 10 min at 5000 rpm to form a pellet. After being weighed, the bacteria was then resuspended in S-complete to create a 10× (250 mg/ml) stock that was stored at 4 °C. Further dilutions with S-complete were used to create the dilutions for each condition in this experiment.
Dietary restriction/dilution series cultures
For C. elegans, C. briggsae, and C. tropicalis, 10 L4 hermaphrodite worms were picked onto three 10 cm plates seeded with OP50, and for C. kamaaina 10 L4 females and ~20 males were picked onto three 10 cm plates. For all species, adults were removed after 24 hr. C. elegans and C. briggsae were grown for 96 hr before bleaching and C. tropicalis and C. kamaaina were grown for 120 hr before bleaching due to slower growth and longer generation time. After bleaching, worms were aliquoted into 100 ml cultures of S-complete at one worm/100 μl with a concentration of 25 , 12.5 , 6.25 , 3.13 , or 1.6 mg/ml of HB101 and kept in 500 ml flasks in shaking incubators at 20 °C and 180 rpm. Worms were grown in these cultures for 96 hr (C. elegans), 102 hr (C. briggsae), or 120 hr (C. tropicalis and C. kamaaina) before being bleached and prepared for starvation cultures. Due to slow development and inability to properly scale up in liquid culture, 1.6 mg/ml cultures for C. briggsae and 1.6 and 3.13 mg/ml cultures for C. kamaaina were excluded from the rest of this experiment.
Starvation cultures
After bleach, embryos were placed into 5 ml virgin S-basal cultures in 16 mm glass test tubes on a roller drum at 20 °C at one worm/μl. Worms were aliquoted out of this culture using micropipettes for further assays.
Measuring L1 size
Twenty-four hours after bleach (~12 hr after hatch), 1000 L1s were pipetted out of the starvation cultures, spun down in 15 ml plastic conical tubes by centrifuge for 1 min at 3000 rpm then plated onto unseeded 10 cm NGM plates. L1s were imaged with a Zeiss Discovery. V20 stereomicroscope at ×77 and measured using Wormsizer (Moore et al., 2013). Ad libitum concentration was defined as 25 mg/ml and dietary restriction concentration was determined based on what concentration of HB101 produced the largest average L1 size for each strain. For C. elegans, this was 3.13 mg/ml, and eightfold dilution from ad libitum and consistent with previous determinations for dietary restriction in C. elegans (Hibshman et al., 2016). For C. briggsae, peak L1 size varied between 12.5 and 6.25 mg/ml depending on replicate. We chose to use 6.25 mg/ml as the dietary restriction concentration to be consistent with replicates that were already being processed. The peak L1 size and determination of dietary restriction for C. tropicalis were 6.13 mg/ml. C. kamaaina did not show a significant change in L1 size across conditions and was ultimately excluded from the brood size assay due to difficulty interpreting effects of starvation on brood size in a male–female strain.
L1 size statistics
A linear mixed effects model was performed on the L1 size data to see if there was a significant effect of HB101 concentration on L1 size. The lme4 package in R studio was used to perform this linear mixed effects test. The function lmer() was used on data from each species, for example: • lmer(length~ condition + (1 | replicate) + (1 | replicate:condition), data = C_elegans), ‘length’ is the length in microns of each individual worm, ‘condition’ is the fixed effect of the concentration of HB101, ‘1 | replicate’ is the addition of the random effect of replicate to the model, ‘1 | replicate:condition' is the addition of the random effect per combination of replicate and condition, and ‘data’ is the primary spreadsheet restricted by the species of interest.
Gene orthology inference among species
To identify one-to-one orthologs across the four species, we downloaded protein and GFF3 files for C. elegans, C. briggsae, and C. tropicalis genomes from WormBase (Harris et al., 2020) (version WS275) and for the C. kamaaina genome from caenorhabditis.org (version v1). We assessed gene set completeness using BUSCO (Simão et al., 2015) (version 4.0.6; using the parameter -m proteins) using the ‘nematoda_odb10’ lineage dataset. For each species, we selected the longest isoform for each protein-coding gene using the agat_sp_keep_longest_isoform.pl script from AGAT (Jacques Dainat, 2021) (version 0.4.0). Filtered protein files were clustered into orthologous groups (OGs) using OrthoFinder (Emms and Kelly, 2019) (version 2.4.0; using the parameter -og) and one-to-one OGs were selected.
F1 and F3 sample collection for RNA-seq
Young adult animals grown on NGM agar plates seeded with E. coli HB101 were collected and transferred to new plates seeded with either control plates (50 mM NaCl) seeded with E. coli HB101, P. vranovensis BIGb0446, P. vranovensis BIGb0427, S. plymuthica BUR1537, Pseudomonas sp. 15C5, Aeromonas sp. BIGb0469, or plates containing 300 mM NaCl seeded with E. coli HB101. Animals were grown for 24 hr at room temperature (22 °C). Embryos from these animals were collected by bleaching and immediately frozen in 1 ml Trizol.
Analysis of RNA-seq data
RNA libraries were prepared and sequenced by BGI TECH SOLUTIONS using 100PE DNBseq Eukaryotic Transcriptome service. Quality controlled and adapter trimming of RNA reads were performed using fastp-v4.20.0 (Chen et al., 2018) (--qualified_quality_phred 20 --unqualified_percent_limit 40 --length_required 50 --low_complexity_filter --complexity_threshold 30 --detect_adapter_for_pe --correction --trim_poly_g --trim_poly_x \ --trim_front1 2 --trim_tail1 2 --trim_front2 2 --trim_tail2 2) (1). Next, reads were aligned using STAR-2.7.1a (Dobin et al., 2013) (--alignSJoverhangMin 8 --alignSJDBoverhangMin 1 --outFilterMismatchNmax 999 --outFilterMismatchNoverReadLmax 0.04 --alignIntronMin 10 --alignIntronMax 1000000 --alignMatesGapMax 1000000 --outFilterType BySJout --outFilterMultimapNmax 10000 --winAnchorMultimapNmax 50 --outMultimapperOrder Random) (2) against the genome of C. elegans WS275, C. briggsae WS275, C. tropicalis WS275, and the C. kamaaina genome obtained from caenorhabditis.org. Read counts were obtained using subread-2.0.0 (-M -O -p --fraction -F GTF -a -t exon -g gene_id) (Liao et al., 2014) (3) using the annotation for C. elegans PRJNA13758.WS275, C. briggsae PRJNA10731.WS275, C. tropicalis PRJNA53597.WS275, and C. kamaaina Caenorhabditis_kamaaina_QG2077_v1. Counts were imported into R and differential gene expression analysis was performed with DESeq2 (FDR < 0.01) (Love et al., 2014).
For comparisons made between different species, genes were subsetted to include only those 7587 single-copy ortholog groups that were identified between the four species. In addition to the 7203 genes that were identified as single-copy ortholog groups by OrthoFinder, the 7587 contain an additional 385 ortholog groups that were identified as having more than one ortholog in one out four of the species but where all but one of the multiple orthologs had no observable expression in any of the samples collected.
For the comparison between the stress response and gene expression during embryo development, data were downloaded from Boeck et al., 2016 and imported in R with raw counts from this study. The range of embryo expression for each gene was considered as one standard deviation ± the mean of regularized log normalized counts across all embryo time points. DEGs from the stress experiments where the regularized log normalized counts for one or both of the comparison samples (for all replicates) were outside of the embryo range were considered unlikely to be caused by developmental timing.
L4+ developmental rate assays
Young adult animals that were grown on NGM agar plates seeded with E. coli HB101 were collected and transferred to new plates seeded with E. coli HB101, Pseudomonas sp. 15C5, S. plymuthica BUR1537, or Aeromonas sp. BIGb0469. Animals were grown for 24 hr at room temperature (22 °C). Embryos from these animals were collected by bleaching and transferred to new plates seeded with 1 ml of E. coli HB101 Pseudomonas sp. 15C5, S. plymuthica BUR1537, or Aeromonas sp. BIGb0469. Percent of animals that reached the L4 larval stage was scored after either 48 or 72 hr at 22 °C.
Identification of Pseudomonas sp. 15 C5 and S. plymuthica BUR1537
Samples of rotting fruit and vegetation were collected from around Cambridge (UK) in 50 ml vials. For isolation of wild bacteria, the samples were homogenized and resuspended in M9 and plated on LB Agar, Nutrient Agar, or Actinomycete Isolation Agar plates and grown at either 37 °C or 30 °C for 24 hr. Single colonies were isolated from the plates and grown in LB or Nutrient Broth at the same temperature overnight. Stocks were frozen and stored at −80 °C in 20 % glycerol. One thousand five hundred and thirty-seven total isolates were obtained and frozen. C. elegans embryos were placed onto NGM agar plates seeded with each of the 1537 bacterial isolates. Bacterial isolates that caused substantial delays in animal development or lethality were further analyzed for isolates where parental exposure to the isolate for 24 hr modified offspring phenotype when compared to offspring from parents fed the normal laboratory diet of E. coli HB101. Bacterial genus and species were identified by 16 S rRNA profiling and sequencing.
RNAi in C. kamaaina
dsDNA corresponding to the C. kamaaina orthologs of cysl-1, rhy-1, mek-2, and gpdh-2 was synthesized and cloned into the L4440 vector by GENEWIZ (Takeley, UK). Vectors were transformed in E. coli HT115. C. kamaaina embryos were collected by bleaching and placed onto NGM agar plates containing 1 mM IPTG that were seeded with E. coli HT115 transformed with either the L4440 empty vector or each of the new vectors and grown at room temperature (22 °C) for 48 hr. After 48 hr, animals were transferred to new 50 mM NaCl control plates seeded with E. coli HB101, 300 mM NaCl plates seeded with E. coli HB101, or 50 mM NaCl control plates seeded with P. vranovensis BIGb0446. Animals were grown for 24 hr at room temperature (22 °C). Embryos from these animals were collected by bleaching and transferred to new 500 mM NaCl plates seeded with E. coli HB101 or 50 mM NaCl plates seeded with P. vranovensis BIGb0446. Percent of animals developing or surviving was scored after 24 hr at room temperature as previously described in Burton et al., 2017 and Burton et al., 2020.
Statistics and reproducibility
Sample sizes for experiments involving C. elegans were selected based on similar studies from the literature and all animals from each genotype and condition were grouped and analyzed randomly. All replicate numbers listed in figure legends represent biological replicates of independent animals cultured separately, collected separately, and analyzed separately. Unpaired two-tailed Student’s t-test was used for Figures 1B, D, F, 2E, J, 4C and D, and Figure 1—figure supplement 1F-G. Two-way ANOVA was used for Figures 1C, E, 3A–G, and Figure 1—figure supplement 1A-E. One-way ANOVA was used for Figure 4A and B, and Figure 4—figure supplement 1. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Acknowledgements
We would like to thank Buck Samuel and Marie-Anne Felix for bacterial isolates. We also thank Matt Rockman and Luke Noble for prepublication access to the genome of C. kamaaina. We would also like to thank Marie-Anne Felix and the Caenorhabditis Genetic Center, which is funded by the NIH National Center for Research Resources (NCRR), for Caenorhabditis strains. NOB is funded by a Next Generation Fellowship from the Centre for Trophoblast Research. KF and LRB were funded by the National Institutes of Health (GM117408, LRB). AW and AR were funded by the Natural Sciences and Engineering Research Council of Canada (Grant #522691522691) and an Alfred P Sloan Research Fellowship FG2019-12040 (to AWR). This work was also supported by Cancer Research UK (C13474/A18583, C6946/A14492) and the Wellcome Trust (104640/Z/14/Z, 092096/Z/10/Z) grants to EAM.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Nicholas O Burton, Email: nick.burton@vai.org.
Diethard Tautz, Max-Planck Institute for Evolutionary Biology, Germany.
Patricia J Wittkopp, University of Michigan, United States.
Funding Information
This paper was supported by the following grants:
Centre Trophoblast Research Next Generation fellowship to Nicholas O Burton.
National Institutes of Health to Kinsey Fisher, L Ryan Baugh.
National Institutes of Health GM117408 to L Ryan Baugh.
Natural Sciences and Engineering Research Council of Canada Grant #522691522691 to Alexandra Willis, Aaron W Reinke.
Alfred P. Sloan Foundation FG2019-12040 to Aaron Reinke.
Cancer Research UK C13474/A18583 to Eric A Miska.
Cancer Research UK C6946/A14492 to Eric A Miska.
Wellcome Trust 104640/Z/14/Z to Eric A Miska.
Wellcome Trust 092096/Z/10/Z to Eric A Miska.
Additional information
Competing interests
No competing interests declared.
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Data curation, Formal analysis, Methodology, Writing – review and editing.
Data curation, Formal analysis, Investigation, Methodology, Writing – review and editing.
Data curation, Formal analysis, Investigation, Methodology, Writing – review and editing.
Data curation, Formal analysis, Investigation, Methodology, Writing – review and editing.
Formal analysis, Funding acquisition, Methodology, Project administration, Writing – review and editing.
Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Writing – review and editing.
Formal analysis, Funding acquisition, Methodology, Project administration, Writing – review and editing.
Additional files
Data availability
RNA-seq data that support the findings of this study have been deposited at NCBI GEO and are available under the accession code GSE173987.
The following dataset was generated:
Burton N, Price J, Braukmann F, Miska E. 2021. Parental exposure to environmental stress results in evolutionarily conserved intergenerational changes in offspring gene expression. NCBI Gene Expression Omnibus. GSE173987
The following previously published datasets were used:
Boeck M. 2016. The time-resolved transcriptome of C. elegans. NCBI Sequence Read Archive - Supplemental Table 1. PMC5052054
References
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. doi: 10.1038/43425. [DOI] [Google Scholar]
- Boeck ME, Huynh C, Gevirtzman L, Thompson OA, Wang G, Kasper DM, Reinke V, Hillier LW, Waterston RH. The time-resolved transcriptome of C. elegans. Genome Research. 2016;26:1441–1450. doi: 10.1101/gr.202663.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozler J, Kacsoh BZ, Bosco G. Transgeneratonal inheritance of ethanol preference is caused by maternal NPF repression. eLife. 2019;8:e45391. doi: 10.7554/eLife.45391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, Kimble J, Fire A, Kennedy S. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489:447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NO, Furuta T, Webster AK, Kaplan REW, Baugh LR, Arur S, Horvitz HR. sulin-like signalling to the maternal germline controls progeny response to osmotic stress. Nature Cell Biology. 2017;19:252–257. doi: 10.1038/ncb3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NO, Riccio C, Dallaire A, Price J, Jenkins B, Koulman A, Miska EA. Cysteine synthases CYSL-1 and CYSL-2 mediate C. elegans heritable adaptation to P. vranovensis infection. Nature Communications. 2020;11:1741. doi: 10.1038/s41467-020-15555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter AD. Divergence Times in Caenorhabditis and Drosophila Inferred from Direct Estimates of the Neutral Mutation Rate. Molecular Biology and Evolution. 2008;25:778–786. doi: 10.1093/molbev/msn024. [DOI] [PubMed] [Google Scholar]
- Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. Density Triggers Maternal Hormones That Increase Adaptive Offspring Growth in a Wild Mammal. Science. 2013;340:1215–1217. doi: 10.1126/science.1235765. [DOI] [PubMed] [Google Scholar]
- de Gusmão Correia ML, Volpato AM, Águila MB, Mandarim-de-Lacerda CA. Developmental origins of health and disease: experimental and human evidence of fetal programming for metabolic syndrome. Journal of Human Hypertension. 2012;26:405–419. doi: 10.1038/jhh.2011.61. [DOI] [PubMed] [Google Scholar]
- Dey S, Proulx SR, Teotónio H. Adaptation to Temporally Fluctuating Environments by the Evolution of Maternal Effects. PLOS Biology. 2016;14:e1002388. doi: 10.1371/journal.pbio.1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nature Neuroscience. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biology. 2019;20:238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJP. The thrifty phenotype hypothesis: Type 2 diabetes. British Medical Bulletin. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Harris TW, Arnaboldi V, Cain S, Chan J, Chen WJ, Cho J, Davis P, Gao S, Grove CA, Kishore R, Lee RYN, Muller H-M, Nakamura C, Nuin P, Paulini M, Raciti D, Rodgers FH, Russell M, Schindelman G, Auken KV, Wang Q, Williams G, Wright AJ, Yook K, Howe KL, Schedl T, Stein L, Sternberg PW. WormBase: a modern Model Organism Information Resource. Nucleic Acids Research. 2020;48:D762–D767. doi: 10.1093/nar/gkz920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg JE, Matilla MA, Salmond GPC. The broad-spectrum antibiotic, zeamine, kills the nematode worm Caenorhabditis elegans. Frontiers in Microbiology. 2015;6:137. doi: 10.3389/fmicb.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibshman JD, Hung A, Baugh LR. Maternal Diet and Insulin-Like Signaling Control Intergenerational Plasticity of Progeny Size and Starvation Resistance. PLOS Genetics. 2016;12:e1006396. doi: 10.1371/journal.pgen.1006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houri-Zeevi L, Korem Kohanim Y, Antonova O, Rechavi O. Three Rules Explain Transgenerational Small RNA Inheritance in C. elegans. Cell. 2020;182:1186–1197. doi: 10.1016/j.cell.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques Dainat Darío Hereñú, Pascal Pucholt. NBISweden/AGAT: AGAT-v0.6.2 2021
- Jordan JM, Hibshman JD, Webster AK, Kaplan REW, Leinroth A, Guzman R, Maxwell CS, Chitrakar R, Bowman EA, Fry AL, Hubbard EJA, Baugh LR. sulin/IGF Signaling and Vitellogenin Provisioning Mediate Intergenerational Adaptation to Nutrient Stress. Current Biology. 2019;29:2380–2388. doi: 10.1016/j.cub.2019.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R, Moore RS, Vrla GD, Parsons LR, Gitai Z, Murphy CT. C. elegans interprets bacterial non-coding RNAs to learn pathogenic avoidance. Nature. 2020;586:445–451. doi: 10.1038/s41586-020-2699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto S, Uno M, Okabe E, Nono M, Nishida E. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nature Communications. 2017;8:14031. doi: 10.1038/ncomms14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B. Transgenerational transmission of environmental information in C. elegans. Science. 2017;356:320–323. doi: 10.1126/science.aah6412. [DOI] [PubMed] [Google Scholar]
- Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. PNAS. 2006;103:12173–12178. doi: 10.1073/pnas.0602987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC. Developmental programming of health and disease. The Proceedings of the Nutrition Society. 2006;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Lind MI, Zwoinska MK, Andersson J, Carlsson H, Krieg T, Larva T, Maklakov AA. Environmental variation mediates the evolution of anticipatory parental effects. Evolution Letters. 2020;4:371–381. doi: 10.1002/evl3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Bruce TJA, Roberts MR, Flors V, Ton J. Next-generation systemic acquired resistance. Plant Physiology. 2012;158:844–853. doi: 10.1104/pp.111.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Niu R, Huang T, Shao L-W, Peng Y, Ding W, Wang Y, Jia G, He C, Li C-Y, He A, Liu Y. N6-methyldeoxyadenine is a transgenerational epigenetic signal for mitochondrial stress adaptation. Nature Cell Biology. 2019;21:319–327. doi: 10.1038/s41556-018-0238-5. [DOI] [PubMed] [Google Scholar]
- Moore BT, Jordan JM, Baugh LR. WormSizer: high-throughput analysis of nematode size and shape. PLOS ONE. 2013;8:e57142. doi: 10.1371/journal.pone.0057142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RS, Kaletsky R, Murphy CT. Piwi/PRG-1 Argonaute and TGF-β Mediate Transgenerational Learned Pathogenic Avoidance. Cell. 2019;177:1827–1841. doi: 10.1016/j.cell.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öst A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, Ruf M, Rajewsky N, Reuter G, Iovino N, Ribeiro C, Alenius M, Heyne S, Vavouri T, Pospisilik JA. Paternal Diet Defines Offspring Chromatin State and Intergenerational Obesity. Cell. 2014;159:1352–1364. doi: 10.1016/j.cell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Palominos MF, Verdugo L, Gabaldon C, Pollak B, Ortíz-Severín J, Varas MA, Chávez FP, Calixto A. Transgenerational Diapause as an Avoidance Strategy against Bacterial Pathogens in Caenorhabditis elegans. MBio. 2017;8:e01234-17. doi: 10.1128/mBio.01234-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner R, Toker IA, Antonova O, Star E, Anava S, Azmon E, Hendricks M, Bracha S, Gingold H, Rechavi O. Neuronal Small RNAs Control Behavior Transgenerationally. Cell. 2019;177:1814–1826. doi: 10.1016/j.cell.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx SR, Dey S, Guzella T, Teotónio H. How differing modes of non-genetic inheritance affect population viability in fluctuating environments. Ecology Letters. 2019;22:1767–1775. doi: 10.1111/ele.13355. [DOI] [PubMed] [Google Scholar]
- Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update. Nucleic Acids Research. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing AK, Miteva Y, Moronetti L, He L, Lamitina T. The Caenorhabditis elegans mucin-like protein OSM-8 negatively regulates osmosensitive physiology via the transmembrane protein PTR-23. PLOS Genetics. 2011;7:e1001267. doi: 10.1371/journal.pgen.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Rowedder H, Braendle C, Félix MA, Ruvkun G. Caenorhabditis elegans responses to bacteria from its natural habitats. PNAS. 2016;113:E3941–E3949. doi: 10.1073/pnas.1607183113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. PNAS. 2010;107:16757–16758. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Stevens L, Rooke S, Falzon LC, Machuka EM, Momanyi K, Murungi MK, Njoroge SM, Odinga CO, Ogendo A, Ogola J, Fèvre EM, Blaxter M. The Genome of Caenorhabditis bovis. Current Biology. 2020;30:1023–1031. doi: 10.1016/j.cub.2020.01.074. [DOI] [PubMed] [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends in Ecology & Evolution. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Uller T, English S, Pen I. When is incomplete epigenetic resetting in germ cells favoured by natural selection? Proceedings. Biological Sciences. 2015;282:20150682. doi: 10.1098/rspb.2015.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal M, Painter R, de Rooij S, Bossuyt P, van der Post J, Gluckman P, Hanson M, Roseboom T. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG. 2013;120:548–554. doi: 10.1111/1471-0528.12136. [DOI] [PubMed] [Google Scholar]
- Vellichirammal NN, Gupta P, Hall TA, Brisson JA. Ecdysone signaling underlies the pea aphid transgenerational wing polyphenism. PNAS. 2017;114:1419–1423. doi: 10.1073/pnas.1617640114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster AK, Jordan JM, Hibshman JD, Chitrakar R, Baugh LR. Transgenerational Effects of Extended Dauer Diapause on Starvation Survival and Gene Expression Plasticity in Caenorhabditis elegans. Genetics. 2018;210:263–274. doi: 10.1534/genetics.118.301250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibowo A, Becker C, Marconi G, Durr J, Price J, Hagmann J, Papareddy R, Putra H, Kageyama J, Becker J, Weigel D, Gutierrez-Marcos J. Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife. 2016;5:e13546. doi: 10.7554/eLife.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AR, Zhao W, Sukhdeo R, Wadi L, El Jarkass HT, Claycomb JM, Reinke AW. A parental transcriptional response to microsporidia infection induces inherited immunity in offspring. Science Advances. 2021;7:eabf3114. doi: 10.1126/sciadv.abf3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Sachse M, Prevost MC, Luallen RJ, Troemel ER, Félix MA. A Large Collection of Novel Nematode-Infecting Microsporidia and Their Diverse Interactions with Caenorhabditis elegans and Other Related Nematodes. PLOS Pathogens. 2016;12:e1006093. doi: 10.1371/journal.ppat.1006093. [DOI] [PMC free article] [PubMed] [Google Scholar]