Abstract
The class II phosphoinositide 3-kinases (PI3K) PI3K-C2α and PI3K-C2β are two recently identified members of the large PI3K family. Both enzymes are characterized by the presence of a C2 domain at the carboxy terminus and, in vitro, preferentially utilize phosphatidylinositol and phosphatidylinositol 4-monophosphate as lipid substrates. Little is understood about how the catalytic activity of either enzyme is regulated in vivo. In this study, we demonstrate that PI3K-C2α and PI3K-C2β represent two downstream targets of the activated epidermal growth factor (EGF) receptor in human carcinoma-derived A431 cells. Stimulation of quiescent cultures with EGF resulted in the rapid recruitment of both enzymes to a phosphotyrosine signaling complex that contained the EGF receptor and Erb-B2. Ligand addition also induced the appearance of a second, more slowly migrating band of PI3K-C2α and PI3K-C2β immunoreactivity on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Since both PI3K enzymes can utilize Ca2+ as an essential divalent cation in lipid kinase assays and since the catalytic activity of PI3K-C2α is refractory to the inhibitor wortmannin, these properties were used to confirm the recruitment of each PI3K isozyme to the activated EGF receptor complex. To examine this interaction in greater detail, PI3K-C2β was chosen for further investigation. EGF and platelet-derived growth factor also stimulated the association of PI3K-C2β with their respective receptors in other cells, including epithelial cells and fibroblasts. The use of EGF receptor mutants and phosphopeptides derived from the EGF receptor and Erb-B2 demonstrated that the interaction with recombinant PI3K-C2β occurs through E(p)YL/I phosphotyrosine motifs. The N-terminal region of PI3K-C2β was found to selectively interact with the EGF receptor in vitro, suggesting that it mediates the association of this PI3K with the receptor. However, the mechanism of this interaction remains unclear. We conclude that class II PI3K enzymes may contribute to the generation of 3′ phosphoinositides following the activation of polypeptide growth factor receptors in vivo and thus mediate certain aspects of their biological activity.
The binding of polypeptide growth factors to their cell surface receptors triggers the recruitment of numerous molecules to form a localized signaling complex at the plasma membrane. Translocation to the activated receptor from intracellular compartments and conformational and posttranslational modifications all contribute to activate many of the recruited secondary messenger molecules and thus perpetuate the signaling cascade (57). The accumulation of 3′ phosphoinositides has been observed in numerous cell types following their stimulation with polypeptide growth factors, cytokines, and chemotactic agents (19, 25). In quiescent cultures, levels of phosphatidylinositol(3,4)-bisphosphate [PtdIns(3,4)P2] and phosphatidylinositol(3,4,5)-triphosphate [PtdIns(3,4,5)P3] are low but increase rapidly in response to cell stimulation (54). Consequently, the production of these phosphoinositides has been proposed to mediate events such as mitogenesis, cell adhesion and motility, and cellular differentiation and to offer protection against apoptosis (55, 58). In contrast, phosphatidylinositol(3)-phosphate [PtdIns(3)P] appears to be synthesized constitutively, and its levels do not vary greatly following ligand addition. Despite little knowledge about how its production is controlled, PtdIns(3)P is considered to play a pivotal role in the regulation of intracellular membrane trafficking (11). Characterization of the enzymes responsible for the generation of 3′ phosphoinositides has identified several proteins which can be assigned to one of three classes based on structural similarity, substrate specificity, and probable mechanism of activation (15).
The class IA p85-p110 heterodimer was the first phosphoinositide 3-kinase (PI3K) enzyme complex to be purified, and it remains the principle focus of most studies concerned with characterizing a receptor tyrosine kinase-associated PI3K activity. Three mammalian class IA catalytic subunits, termed p110α, p110β, and p110δ, associate with a 50-, 55-, or 85-kDa adapter subunit to form a heterodimeric enzyme. The adapters all contain two tandem Src homology 2 (SH2) domains which facilitate translocation of the catalytic subunit to the plasma membrane upon receptor tyrosine phosphorylation (40, 66). The mechanism by which the activation of lipid kinase activity is achieved remains unclear, although availability of the phospholipid substrate, conformational changes, and tyrosine phosphorylation of the PI3K complex have all been postulated as a regulatory switch (28, 64). A fourth class I enzyme, p110γ, does not associate with either a receptor tyrosine kinase or a p85-like adapter. Instead, it binds a protein termed p101 and is activated by βγ subunits of heterotrimeric GTP-binding proteins (52). Consequently, it is termed a class IB PI3K. All class I enzymes phosphorylate phosphatidylinositol (PtdIns), PtdIns(4)P, and PtdIns(4,5)P2 in vitro but most likely produce PtdIns(3,4,5)P3 in vivo (21, 53). The paradigm class III PI3K is Vps34p, a protein originally identified in yeast (48). Mutational analysis has shown that Vps34p plays a central role in orchestrating vesicular trafficking by its production of PtdIns(3)P (22, 60).
Class II PI3K enzymes are distinguished by a carboxy-terminal C2 (CalB) domain (47). A Drosophila enzyme (31, 35) and three mammalian isoforms have been characterized: PI3K-C2α (mcpk, p170) (14, 35, 59), PI3K-C2β (HsC2-PI3K) (2, 6), and PI3K-C2γ (34, 39). Both PI3K-C2α and PI3K-C2β are ubiquitously expressed, whereas PI3K-C2γ is predominantly found in liver. These enzymes preferentially utilize PtdIns and PtdIns(4)P in vitro, but under certain conditions, they also phosphorylate PtdIns(4,5)P2, albeit poorly (14). Little is known about how these enzymes are activated, but PI3K-C2α lies downstream of the monocyte chemotactic peptide 1 chemokine receptor (56) and the insulin receptor (5). In platelets, PI3K-C2β is activated following stimulation of the integrin αIIbβ3 with fibrinogen (65). Interestingly, of all the mammalian enzymes, PI3K-C2α remains the most refractory to wortmannin, a commonly used inhibitor of PI3K activity (3).
There has been a long-standing observation that antiphosphotyrosine antibodies immunoprecipitate increased PI3K activity from lysates of cells stimulated with epidermal growth factor (EGF) (8, 33, 45). The EGF receptor (EGFR) (ErbB-1) is one member of a family of ErbB kinases which includes ErbB-2 (p185erbB2/neu), ErbB-3 (p180erbB3), and ErbB-4 (p180erbB4) (46). Although EGF can only bind to the EGFR, ligand addition promotes both homo- and heterodimerization of two ErbB receptor subunits. ErbB-3 and ErbB-4 also display selectivity in ligand binding. In contrast, while ErbB-2 displays no direct ligand interaction, it appears to be the preferred binding partner of each of the other ErbB receptor subunits (26). Receptor dimerization activates an intrinsic tyrosine kinase activity which provides a series of phosphotyrosine docking sites for signaling molecules such as Shc, Grb2, Src, and phospholipase C (10). In contrast to results obtained with the platelet-derived growth factor (PDGF) receptor (PDGFR), conflicting data exist regarding the ability of the EGFR to directly associate with a class IA PI3K heterodimer. PI3K activity has been copurified with the EGFR, and EGF stimulation increases receptor-associated PI3K activity (24); however, neither the EGFR nor ErbB-2 possess the Tyr-X-X-Met consensus motif, which is recognized by the SH2 domains on the p85 adapter subunit. In contrast, ErbB-3 contains seven such motifs in a unique region of its carboxy terminus. Thus, heterodimerization of the EGFR with ErbB-3 is postulated to be a mechanism that explains the observed association of PI3K activity (44, 51).
Given the debate that exists in the literature concerning the exact mechanism by which EGF mediates an increase in receptor-associated PI3K activity, we examined whether the class II PI3K enzymes might play a role downstream of the EGFR and the PDGFR.
MATERIALS AND METHODS
Cell cultures.
Stock cultures of mammalian cells were passaged every 3 to 4 days in 90-mm dishes (Nunc) using Dulbecco minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U of penicillin per ml, and 100 μg of streptomycin per ml (Life Technologies). Cultures were incubated in a humidified atmosphere of 10% CO2–90% air at 37°C. For experimental use, cells were switched to DMEM containing either ITS supplement (Sigma) or 0.5% FBS. After 16 to 24 h, cultures were confluent and quiescent. Drosophila Sf9 cells were grown at 27°C in IPL-41 medium containing 10% FBS and supplemented with yeast extract ultrafiltrate, lipid concentrate, and gentamicin (Sigma). Stock cultures were passaged every 3 to 4 days.
Generation of antisera.
To generate antiserum against PI3K-C2α, an N-terminal fragment was expressed as a glutathione S-transferase (GST) fusion protein in the following manner. Nucleotides 4 to 1011 of the PI3K-C2α cDNA sequence (encoding residues 2 to 345) were amplified by PCR using complementary oligonucleotides incorporating SmaI and EcoRI restriction sites at the 5′ and 3′ ends, respectively. Following digestion, this cDNA was ligated into the pGex2T expression vector (Pharmacia) to allow its in-frame expression at the C terminus of GST. This plasmid was used to transform Epicurian coli XL-1 cells, and production of the GST fusion protein was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (30°C for 4 h). Cells were harvested and lysed in phosphate-buffered saline (PBS) containing 1% Triton X-100, 2 mM EDTA, 5 mM benzamidine, 1 mM dithiothreitol (DTT) 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 50 mTIU of aprotinin per ml, 50 μM pepstatin, and 50 μM leupeptin (PBST buffer) at 4°C. The fusion protein was affinity purified using glutathione-Sepharose beads (Pharmacia) (4 h, 4°C) and, after being washed, was eluted by the addition of 10 mM glutathione–150 mM NaCl in 100 mM Tris (pH 8.0). Glutathione was later removed by dialysis against 50 mM Tris (pH 7.4)–150 mM NaCl–1 mM DTT (three times for 4 h each time at 4°C). Aliquots of the expressed fusion protein were frozen and injected (100 μg) into rabbits at monthly intervals. One week after each injection, serum was tested for its ability to immunoprecipitate and Western blot PI3K-C2α protein and PI3K-C2α immunoreactivity from cell lysates. Antiserum to PI3K-C2β was raised in rabbits as previously described (2). Neither antiserum displayed a cross-reaction against the other class II PI3K, the class IA enzymes, or human Vps34p.
Generation of recombinant p85α-p110α heterodimer, PI3K-C2α, and PI3K-C2β.
Sf9 cells (approximately 60% confluent) were infected for 48 to 64 h with plaque-purified baculovirus to Glu-tagged p85α and p110α (28), Glu-tagged PI3K-C2α (14), or Glu-tagged PI3K-C2β (2). Cells were harvested by centrifugation, and the pellet was incubated in Triton lysis buffer at 4°C for 20 min. Lysates were clarified by centrifugation (13,000 × g), and the supernatant was incubated with anti-Glu tag monoclonal antibody (43) coupled to protein G-Sepharose beads (Pharmacia) for 4 h at 4°C. The beads were then washed with Triton lysis buffer, and recombinant protein was eluted by four sequential incubations with 100 mM Tris (pH 7.4)–50 mM DTT–50 μg of Glu tag peptide (MEFMPME) per ml for 10 min at 4°C. Fractions were pooled as required, and the free peptide was removed by gel filtration on a PD-10 column (Pharmacia).
Transient expression in mammalian cells.
HEK293 and Cos 7 cells were grown to 50 to 60% confluence on 150-mm dishes and transfected with a cDNA construct encoding myc-tagged PI3K-C2β in pcDNA3.0 by using calcium phosphate as previously described (2). Cells were harvested 48 h postinfection and examined for protein expression.
Stable expression in mammalian cells.
A431 cells were grown to 40 to 60% confluence and transfected with a myc-tagged PI3K-C2β construct in pcDNA3.0 (2) by using Lipofectamine in accordance with the manufacturer's instructions (Life Technologies). Cultures were passaged 1:10 after 48 h in medium containing 0.75 mg of G418 (Life Technologies) per ml. Colonies of G418-resistant cells were evident after 14 days and were expanded in selection medium. Clones were screened for protein production by immunoprecipitation with anti-myc antibody (9E10; Santa Cruz) followed by Western blotting and lipid kinase assays.
The cDNA construct encoding Glu-tagged PI3K-C2β was subcloned into the pBabe Neo vector and used to transfect BOSC 23 cells in order to generate recombinant retrovirus (41). NIH 3T3 cells were grown to 40 to 60% confluence and infected with the recombinant retrovirus for 48 h at 37°C. The cultures were split 1:10 in DMEM–10% FBS containing 0.75 mg of G418 per ml. Colonies of resistant cells appeared after 1 week and were harvested by trypsinization. Clones were expanded in selection medium and screened for protein expression by immunoprecipitation of cell lysates with anti-Glu tag antibody followed by either Western blotting or PI3K assays.
Immunoprecipitations.
Cultures were washed twice with DMEM and treated in the absence or presence of EGF (100 nM) for various periods of time at 37°C. The cells were lysed at 4°C in 1 ml of 10 mM Tris-HCl (pH 7.6)–5 mM EDTA–50 mM NaCl–30 mM sodium pyrophosphate–50 mM NaF–100 μM Na3VO4–1% Triton X-100–1 mM PMSF (lysis buffer). Lysates were clarified by centrifugation at 13,000 × g, and the supernatants were transferred to a fresh tube. Immunoprecipitations were performed at 4°C over 4 h, and the immune complexes were collected on protein G-Sepharose beads for 1 h at 4°C. Beads were spun and washed three times in lysis buffer prior to further analysis.
Western blotting.
Immunoprecipitates were extracted in sample buffer (200 mM Tris-HCl, 6% sodium dodecyl sulfate [SDS], 2 mM EDTA, 4% 2-mercaptoethanol, 10% glycerol [pH 6.8]) and fractionated by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to polyvinylidene difluoride membranes, which were blocked with 5% nonfat dried milk in PBS (pH 7.2) and then incubated for 3 to 5 h with antibody in PBS containing 3% nonfat milk. Immunoreactive proteins were detected with either anti-mouse or anti-rabbit antibody coupled to horseradish peroxidase (Amersham) and visualized by enhanced chemiluminescence (Amersham).
Assay of PI3K activity.
Lipid kinase assays were performed in a total volume of 50 μl containing 20 mM HEPES (pH 7.4), 100 mM NaCl, 0.1 mM EGTA, 0.1 mM EDTA, and 200 mM phosphoinositide. After preincubation of sonicated lipid with samples for 10 min, reactions were initiated by the addition of divalent cation (6 mM) and 100 μM ATP (0.2 μCi of [γ-32P]ATP). Reaction mixtures were incubated at 30°C for 20 min, and reactions were terminated with acidified chloroform-methanol (1:1 [vol/vol]). The extracted lipid products were fractionated by thin-layer chromatography (TLC). To separate PtdIns(3)P and PtdIns(4)P, a borate solvent system was used (61). Phosphorylated lipids were visualized by either autoradiography or PhosphorImager analysis (Molecular Dynamics). All assays were linear with respect to time and enzyme addition.
Association with EGFR and PDGFR.
The EGFR was immunoprecipitated from cultures of A431 cells using monoclonal antibody R1 (Santa Cruz) and collected on protein G-Sepharose beads. Similarly, ErbB-2, ErbB-3, and ErbB-4 were immunoprecipitated using antibodies Ab2 (Calbiochem), C-17 (Santa Cruz), and Ab-1 (NeoMarkers), respectively. The PDGFR was isolated from NIH 3T3 fibroblasts using antisera against the β chain of the receptor (958; Santa Cruz). In addition, cDNAs encoding the human EGFR mutants Y992F, Y1068F, Y1086F, Y1148F, Y1173F, Y992-1173F, and Y992-1068-1173F in pRK5 (32) were transfected into HEK293 cells, and the recombinant receptor was isolated using anti-EGFR antibody R1 and protein G-Sepharose beads. Immunoprecipitates were washed once in lysis buffer and twice in protein kinase buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 0.02% Triton X-100, 12 mM MgCl2, 2 mM MnCl2, 10% glycerol, 1 mM sodium orthovanadate, 2 mM DTT). Reaction mixtures were incubated for 15 min at 37°C in protein kinase buffer in the absence or presence of 1 mM ATP. Samples were washed twice in lysis buffer and added to lysates of HEK293 cells that had been transfected with either vector alone or vector containing PI3K-C2β. Alternatively, the immobilized receptor was incubated with soluble PI3K-C2β (4 h, 4°C). The beads were washed, proteins were fractionated by SDS-PAGE, and Western blotting or PI3K assays were performed. The phosphopeptides (“p” prefix) corresponding to the EGFR [(p)Y992 (DDVVDADEpYLIP), (p)Y1068 (DTFLPVPEpYINQ), and (p)Y1148 (QISLDNPDpYQQ)] and to ErbB-2 [Y1196 (GGAVENPEpYL)] were purified by high-pressure liquid chromatography after synthesis (Alta Bioscience) and lyophilized. After reconstitution in PBS and neutralization with 0.1 N NaOH, the concentrations were determined by measurement of the optical density at 264 nm. The peptides were added at 25 μM to the EGFR–PI3K-C2β association assay described above.
Expression of recombinant domains of PI3K-C2β and analysis of their binding to the EGFR.
The cDNA fragments encoding amino acid residues 1 to 331 (N-terminal fragment) and 1440 to 1609 (C2 fragment) of PI3K-C2β were subcloned into pGex2T as previously described (2). A putative phosphotyrosine-binding domain (PTB) was predicted by sequence alignment and modelling (data not shown) of amino acid residues 538 to 687. The corresponding cDNA fragment was amplified by PCR and subcloned into pGex2T using BamHI and EcoRI sites. The construct was verified by sequencing. Each domain was expressed in E. coli as a GST fusion protein and purified to homogeneity by glutathione-Sepharose affinity chromatography. The purified, immobilized domains (50 μg/ml) were then assayed for their ability to affinity purify the EGFR from quiescent or EGF-stimulated A431 cell lysates prepared as described above. The NT fragment was also prepared in soluble form by thrombin cleavage and assayed for its ability to interact with purified recombinant EGFR expressed in HEK293 cells. Finally, the recombinant NT domain was also added at 50 μg/ml to the EGFR–PI3K-C2β association assay described above.
RESULTS
Both PI3K-C2α and PI3K-C2β are recruited to phosphotyrosine-containing immune complexes following EGF stimulation.
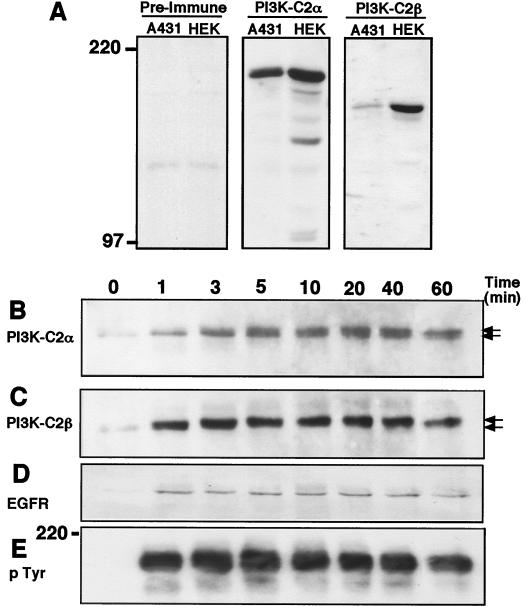
Given their degree of sequence homology, their similar apparent molecular masses, and the ubiquitous distribution of both PI3K isozymes in human tissue and cell lines, we initially determined the ability to resolve PI3K-C2α and PI3K-C2β by SDS-PAGE and to verify the specificity of each antiserum used. Figure 1A shows Western blotting of A431 and HEK293 cell lysates fractionated by SDS-PAGE on a 5% bisacylamide gel and probed with each antiserum. Anti–PI3K-C2α antisera revealed in both A431 and HEK293 cell lysates one predominant band that migrated with an apparent molecular mass of 190 kDa. Antisera raised against PI3K-C2β also revealed a single immunoreactive band of 180 kDa in both lysates. While each lysate contained similar levels of PI3K-C2α, less PI3K-C2β was present in A431 cells than in HEK293 cells. Thus, both PI3K isozymes were clearly distinguished using this system, and the specificity of each antiserum was confirmed by the absence of cross-reacting bands.
FIG. 1.
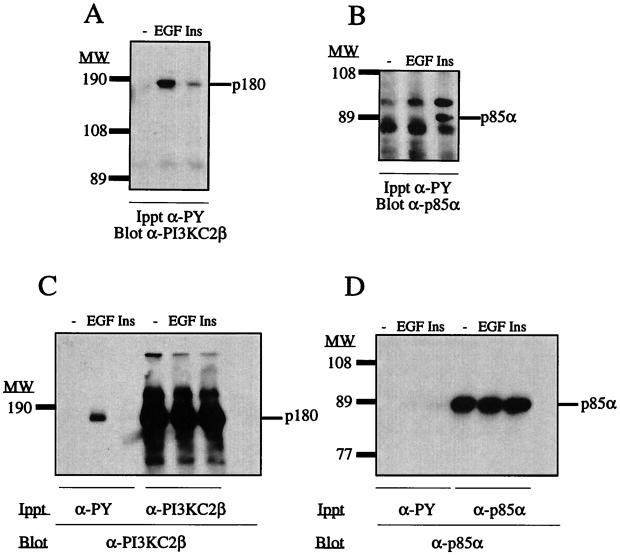
Both PI3K-C2α and PI3K-C2β are immunoprecipitated by antiphosphotyrosine antibody from lysates of EGF-stimulated cultures. (A) Confluent cultures of A431 and HEK293 cells were lysed with Laemmli sample buffer on ice. These lysates were boiled, fractionated by SDS-PAGE, and Western blotted with either anti–PI3K-C2α or anti–PI3K-C2β antiserum. (B to E) Confluent and quiescent cultures of A431 cells were stimulated with EGF (100 nM) for the indicated times. Cells were lysed with buffer containing Triton X-100 at 4°C, and the supernatants were clarified by centrifugation (13,000 × g). Antiphosphotyrosine antibody (PY20) was added (4 h, 4°C), and the resulting immune complexes were collected on protein G-Sepharose beads. Following extraction with sample buffer, proteins were fractionated by SDS-PAGE and Western blotted with either anti–PI3K-C2α antiserum (B), anti–PI3K-C2β antiserum (C), anti-EGFR antibody (R1) (D), or antiphosphotyrosine antibody (PY99) (E). Numbers at left indicate the apparent molecular mass.
It was previously shown that two tyrosine-phosphorylated proteins (210 and 190 kDa) coimmunopreciptiated with the Drosophila class II PI3K Cpk from embryo lysates (35). Since the EGFR initiates its downstream effects via the activation of intrinsic tyrosine kinase activity, we examined if either PI3K-C2α or PI3K-C2β could be coimmunoprecipitated from EGF-stimulated cell lysates with antiphosphotyrosine antibody. Figure 1B and C show that in A431 cells, both PI3K-C2α and PI3K-C2β were recruited to phosphotyrosine-containing complexes in a time-dependent manner following EGF addition. Compared to lysates from quiescent cells (Fig. 1B, lane 0), an accumulation of PI3K-C2α was evident in antiphosphotyrosine antibody immunoprecipitates within 1 min. This accumulation became nearly maximal after 5 min and peaked between 20 and 40 min. Closer inspection revealed that two bands of PI3K-C2α immunoreactivity were immunoprecipitated. The accumulation of a more slowly migrating form increased with time and, at 60 min, this form was clearly the predominant form of PI3K-C2α immunoreactivity. Interestingly, the kinetics with which PI3K-C2β immunoreactivity appeared in antiphosphotyrosine antibody immunoprecipitates differed from those for PI3K-C2α. After EGF stimulation, PI3K-C2β coimmunoprecipitated in these complexes very rapidly (Fig. 1C). Its association was maximal within 1 min and remained at this level for up to 40 min, decreasing only after 60 min of stimulation. EGF also produced a band shift in PI3K-C2β immunoreactivity; however, in contrast to that of PI3K-C2α, all of the PI3K-C2β immunoreactivity appeared to have shifted to the more slowly migrating form by 1 min, making the visualization of a distinct doublet difficult. Western blotting of the antiphosphotyrosine antibody immune complexes with an anti-EGFR antibody (R1) and an antiphosphotyrosine antibody (PY99; Santa Cruz) revealed the immunoprecipitation of the EGFR and the kinetics of EGFR tyrosine phosphorylation. The rate of EGFR activation was in agreement with that reported in previous studies and closely paralleled the recruitment of PI3K-C2β to the phosphotyrosine complexes (Fig. 1D and E). Tyrosine-phosphorylated EGFR was undetectable in quiescent cultures but was evident 1 min after ligand addition and over the next 60 min.
PI3K-C2α and PI3K-C2β associate with EGFR and ErbB-2 following EGF stimulation.
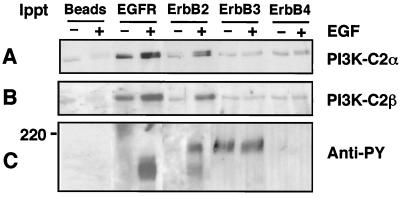
To confirm that PI3K-C2α and PI3K-C2β are present in an EGFR-containing signaling complex, quiescent and confluent cultures of A431 cells were incubated in the absence or presence of EGF (100 nM, 10 min). Lysates were prepared and immunoprecipitated with antibody raised against either EGFR, ErbB-2, ErbB-3, or ErbB-4 (4 h, 4°C). The resultant immune complexes were fractionated by SDS-PAGE and Western blotted with either anti–PI3K-C2α, anti–PI3K-C2β or antiphosphotyrosine antibody. The increased association of both PI3K-C2α (Fig. 2A) and PI3K-C2β (Fig. 2B) was observed in immunoprecipitates of both the EGFR and ErbB-2. Interestingly, both class II PI3K enzymes appeared to be associated with these receptors in quiescent cultures, despite the absence of detectable receptor tyrosine phosphorylation (Fig. 2C). Following EGF stimulation, a band shift in both PI3K enzymes was observed. Western blotting with antiphosphotyrosine antibody revealed the phosphorylated EGFR at 170 to 180 kDa. Immunoprecipitation with anti–ErbB-2 antibody revealed coimmunoprecipitation of the EGFR and a band of approximately 190 kDa from lysates of EGF-stimulated cells. Negligible amounts of PI3K-C2α and PI3K-C2β were coimmunoprecipitated with either anti–ErbB-3 or anti–ErbB-4 antibody. No tyrosine phosphorylation of ErbB-4 was detected, but in ErbB-3 immunoprecipitates, a constitutively tyrosine phosphorylated band at 190 to 200 kDa was observed, in agreement with previous reports stating that ErbB-3 is constitutively tyrosine phosphorylated in situ (27, 44). Our results demonstrate that in A431 cells, both PI3K-C2α and PI3K-C2β preferentially associate with the EGFR and ErbB-2. Both class II PI3Ks were present in a complex with these receptor chains in quiescent cells, but their association increased markedly following EGF stimulation.
FIG. 2.
PI3K-C2α and PI3K-C2β coimmunoprecipitate with EGFR and ErbB-2 following EGF stimulation. Quiescent and confluent cultures of A431 cells were incubated in the absence (−) or presence (+) of 100 nM EGF for 10 min. Clarified lysates were prepared and incubated (4 h, 4°C) with either protein G-Sepharose beads alone (beads) or together with either anti-EGFR (R1), anti–ErbB-2 (Ab2), anti–ErbB-3 (C17), or anti–ErbB-4 (Ab-1) antibody. The resultant immune complexes were washed and, following extraction, associated proteins were fractionated by SDS-PAGE before Western blotting with either anti–PI3K-C2α antiserum (A), anti–PI3K-C2β antiserum (B), or antiphosphotyrosine antibody (PY99) (C).
Both PI3K-C2α and PI3K-C2β utilize Ca2+ as a cofactor for phosphate transfer in lipid kinase assays.
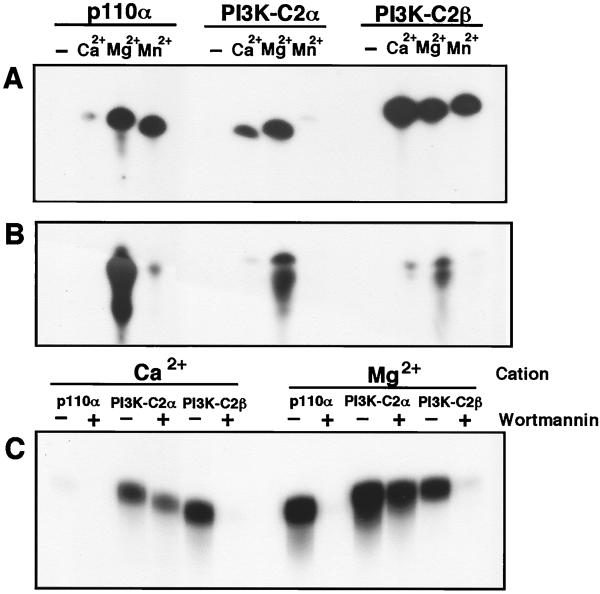
PI3K-C2β was recently shown to utilize Ca2+ as a cofactor for phosphate transfer (2). Consequently, we expressed recombinant Glu-tagged PI3K-C2α in Sf9 cells to examine if it also could phosphorylate inositol-containing phospholipids by using Ca2+ as a source of divalent cation. Assays were performed in the presence of EDTA and EGTA with disodium ATP as the phosphate donor. Under these conditions, recombinant p85α-p110α, PI3K-C2α, and PI3K-C2β were all unable to phosphorylate either PtdIns or PtdIns(4)P in the absence of exogenous cation (Fig. 3A and B). All three enzymes phosphorylated PtdIns and PtdIns(4)P in the presence of Mg2+. In contrast to p110α, both PI3K-C2α and PI3K-C2β phosphorylated PtdIns in the presence of Ca2+, although their ability to generate PtdIns(3,4)P2 was severely attenuated. When Mn2+ was used in these assays, p110α and PI3K-C2β were both able to phosphorylate PtdIns, but PI3K-C2α displayed no kinase activity (14). Under these conditions, only p110α could phosphorylate PtdIns(4)P (Fig. 3B). We previously demonstrated that the catalytic activity of PI3K-C2α was less sensitive to wortmannin than the other mammalian PI3Ks. Given that PI3K-C2α could utilize Ca2+ for phosphate transfer, we examined if these conditions would alter its sensitivity to the inhibitor. Thus, p110α, PI3K-C2α, and PI3K-C2β were incubated with PtdIns in the absence or presence of 50 nM wortmannin for 10 min. After this time, reactions were initiated by the addition of ATP together with either Ca2+ or Mg2+ (Fig. 3C). With either Ca2+ or Mg2+, 50 nM wortmannin completely abolished the lipid kinase activity of PI3K-C2β, while that of PI3K-C2α was only moderately attenuated (15 to 20%). Similarly, in the presence of Mg2+, 50 nM wortmannin abolished the lipid kinase activity of p110α. Again, no lipid kinase activity was observed with p110α in the presence of Ca2+.
FIG. 3.
Cation specificity of p110α, PI3K-C2α, and PI3K-C2β and their sensitivity to inhibition by wortmannin. (A and B) Recombinant p85α-p110α, PI3K-C2α, and PI3K-C2β were assayed for lipid kinase activity using either PtdIns (A) or PtdIns(4)P (B) in the absence (−) or presence of either Ca2+, Mg2+, or Mn2+. Reaction products were extracted, fractionated by TLC, and examined by autoradiography. (C) p85α-p110α, PI3K-C2α, and PI3K-C2β were also examined for their ability to phosphorylate PtdIns in the presence of either Ca2+ or Mg2+ as the divalent cation and in the absence (−) or presence (+) of wortmannin (50 nM).
EGF stimulates the recruitment of class II PI3K lipid kinase activity to the EGFR.
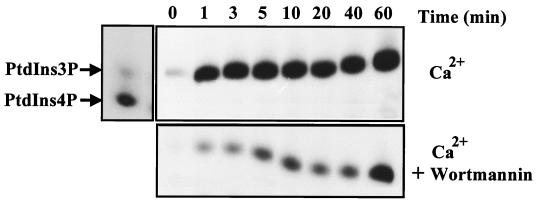
Given that both PI3K-C2α and PI3K-C2β are recruited to the EGFR following ligand addition, we examined if a corresponding increase in PI3K activity could also be observed in receptor immunoprecipitates. Figure 4A shows that in the presence of Ca2+, EGF produced a rapid recruitment of PI3K activity to the EGFR compared to quiescent controls. This association was maximal by 1 min and remained constant over the 60-min period of stimulation. As demonstrated in Fig. 3, in contrast to p110α, both PI3K-C2α and PI3K-C2β could phosphorylate PtdIns under these conditions. To identify the contribution of PI3K-C2α to this response, the assay was repeated in the presence of wortmannin (50 nM). Again, EGFR immunoprecipitates from lysates of quiescent cells displayed minimal PI3K activity. However, upon EGF addition, receptor-associated PI3K activity steadily increased with time (Fig. 4B). This profile of EGFR-associated, Ca2+-dependent PI3K activity correlated well with the results obtained from Western blotting of the class II PI3K enzymes (Fig. 1). Therefore, the rapid association of Ca2+-dependent PI3K activity (1 to 3 min) may be primarily attributed to PI3K-C2β, while at later times (longer than 10 min), the contribution of PI3K-C2α becomes more evident.
FIG. 4.
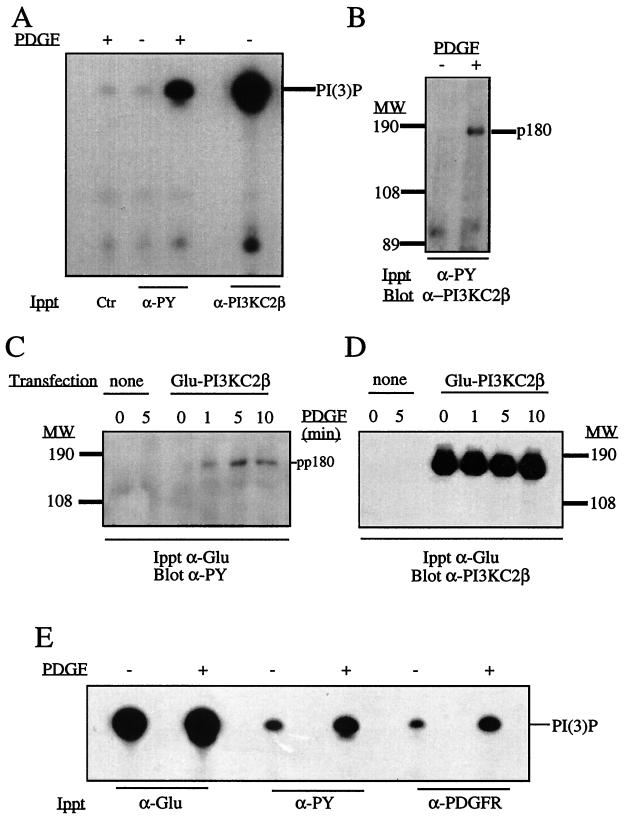
Ca2+-dependent PI3K activity associates with the EGFR following ligand addition. Confluent and quiescent A431 cultures were stimulated with EGF (100 nM) for the times indicated, and lysates were prepared. These were incubated with anti-EGFR antibody (R1) for 4 h at 4°C, and the immune complexes were collected with protein G-Sepharose beads. After washing, these immunoprecipitates were used for lipid kinase assays with PtdIns in the presence of Ca2+ (top panel) or Ca2+ and 50 nM wortmannin (bottom panel). Reactions were terminated, and radiolabeled phospholipids were extracted, separated by TLC, and examined by autoradiography. For reference, a mixture of PtdIns(3)P and PtdIns(4)P was also separated and served as a control (arrows). The slight shift in mobility observed is an artifact of the TLC run.
EGF and PDGF stimulate the recruitment of PI3K-C2β to phosphotyrosine complexes in other cell types.
We also examined if PI3K-C2β was recruited to phosphotyrosine signaling complexes in other cell types and with other polypeptide growth factors. Figure 5 contrasts the regulation of PI3K-C2β with that of the class I p85α adapter subunit in HEK293 and Cos 7 cells. In HEK293 cells, both EGF (100 nM) and insulin (1 μg/ml) recruited PI3K-C2β to phosphotyrosine complexes (Fig. 5A). In contrast, only insulin induced the recruitment of the p85α subunit (Fig. 5B). Similarly, with lysates of EGF-stimulated Cos 7 cells, PI3K-C2β (Fig. 5C) but not the p85α subunit (Fig. 5D) could be detected in phosphotyrosine-containing immunoprecipitates by Western blotting. These data do not demonstrate the absence of class I PI3K in EGFR-containing signaling complexes, only that the levels are below the limit of detection of Western blotting with anti-p85 antibody. Stimulation of NIH 3T3 cells with PDGF (10 nM, 5 min) increased the PI3K activity in antiphosphotyrosine antibody immunoprecipitates when measured in the presence of Ca2+ (Fig. 6A). This finding correlated with the appearance of PI3K-C2β in antiphosphotyrosine antibody immunoprecipitates (Fig. 6B). Since the level of endogenous PI3K-C2β is low in NIH 3T3 cells, these were transfected with a cDNA construct encoding N-terminal Glu-tagged PI3K-C2β by use of a recombinant retrovirus. Clones that expressed recombinant protein were amplified by G418 selection. Following PDGF stimulation of these cells, lysates were prepared and immunoprecipitated with anti-Glu tag antibody. These immune complexes were either analyzed by SDS-PAGE and Western blotted or assayed for PI3K activity in the presence of Ca2+. PDGF stimulation produced a time-dependent increase in a tyrosine-phosphorylated band of 180 kDa (Fig. 6C). The expression of PI3K-C2β was confirmed by Western blotting with anti–PI3K-C2β antisera (Fig. 6D). Increased Ca2+-dependent PI3K activity was also observed in antiphosphotyrosine and anti-PDGFR antibody immunoprecipitates prepared from lysates of PDGF-stimulated cells, compared to the control activity (Fig. 6E). Interestingly, PDGF stimulation did not markedly increase the total pool of PI3K-C2β kinase activity. The PDGFR was also immunoprecipitated from quiescent and PDGF-stimulated NIH 3T3 cells which expressed the Glu-tagged PI3K-C2β enzyme. Western blotting of these immunoprecipitates with anti–PI3K-C2β antisera revealed an association of PI3K-C2β with the activated PDGFR (data not shown).
FIG. 5.
Differential regulation of PI3K-C2β and p85α by EGF and insulin. (A and B) HEK293 cells were stimulated with EGF (100 nM) or insulin (Ins) (1 μg/ml) for 5 min. Lysates were incubated with antiphosphotyrosine antibody (α-PY), and the resulting immunoprecipitates were fractionated by SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes and Western blotted with either anti–PI3K-C2β antiserum (A) or anti-p85α antibody (B). (C and D) Cos 7 cells were also stimulated with either EGF or insulin, lysed, and incubated with either antiphosphotyrosine antibody (α-PY), anti–PI3K-C2β antiserum, or anti-p85α antibody. After SDS-PAGE, the proteins were Western blotted with either anti-PI3K-C2β antiserum (C) or anti-p85α antibody (D). The positions of PI3K-C2β (p180) and p85α are indicated. MW, molecular weight (in thousands).
FIG. 6.
PI3K-C2β is present in antiphosphotyrosine antibody immunoprecipitates of PDGF-stimulated fibroblasts. (A and B) NIH 3T3 cells were stimulated with PDGF (10 nM), and lysates were immunoprecipitated with mouse immunoglobulin antibody (Ctr), antiphosphotyrosine antibody (α-PY), or anti–PI3K-C2β antiserum. The samples were assayed for PI3K in the presence of Ca2+, and the radioactive phosphoinositide products were separated by TLC (A). Antiphosphotyrosine antibody immunoprecipitates from quiescent or PDGF-stimulated cultures were also fractionated by SDS-PAGE and Western blotted with anti–PI3K-C2β antiserum (B). (C and D) Cultures of cells stably expressing epitope-tagged PI3K-C2β were stimulated with PDGF for various times as indicated, and their lysates were immunoprecipitated with anti-Glu tag monoclonal antibody. After SDS-PAGE, immunoprecipitated proteins were Western blotted with antiphosphotyrosine antibody (C), stripped, and reprobed with anti–PI3K-C2β antiserum (D). (E) Lysates from NIH 3T3 cells stimulated with PDGF were also immunoprecipitated with anti-Glu tag antibody, antiphosphotyrosine antibody, or anti-PDGFR antibody. The resultant immune complexes were analyzed for phospholipid kinase activity in the presence of Ca2+. Radiolabeled phosphoinositide products were separated by TLC. MW, molecular weight.
Taken together, our results demonstrate that the stimulation of both epithelial cells and fibroblasts with EGF and PDGF recruits this class II PI3K to phosphotyrosine signaling complexes containing EGFR or PDGFR in vivo.
PI3K-C2β associates with autophosphorylated EGFR and PDGFR in vitro.
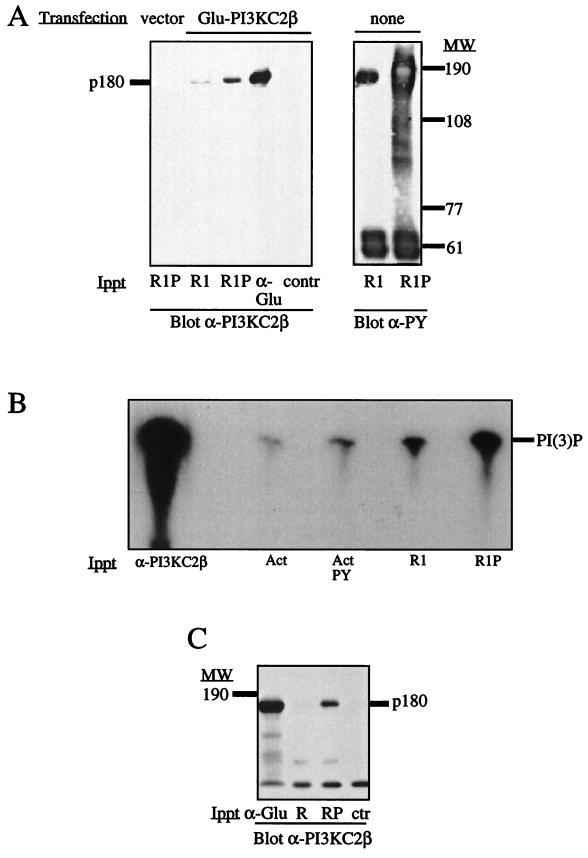
By use of a complementary approach, the EGFR was immunoprecipitated from quiescent A431 cells and autophosphorylated in vitro. The immobilized receptor was then incubated with lysates of HEK293 cells that had been transfected with the cDNA construct encoding Glu-tagged PI3K-C2β. Isolation of the receptor and Western blotting with anti–PI3K-C2β antisera revealed that the phosphorylated EGFR (R1P) coimmunoprecipitated PI3K-C2β more efficiently than the nonphosphorylated receptor (R1) (Fig. 7A). The phosphorylation state of each EGFR preparation was examined by Western blotting with antiphosphotyrosine antibody. To investigate the interaction between PI3K-C2β and the EGFR further, recombinant epitope-tagged PI3K-C2β was affinity purified from lysates of Sf9 cells infected with the corresponding baculovirus. The enzyme was eluted from beads by competition with a peptide corresponding to the epitope tag and incubated with immobilized EGFR that had been previously immunoprecipitated from quiescent A431 cells (R1) and autophosphorylated in vitro (R1P). The receptor association of PI3K-C2β was determined by measuring PI3K activity in the presence of Ca2+ relative to that in the presence of either Actigel beads alone (Act) or Actigel beads coupled to phosphotyrosine (ActPY) (Fig. 7B). A greater association of the PI3K-C2β enzyme was observed with the phosphorylated receptor than with the nonphosphorylated receptor. Under similar conditions, the enzyme interacted poorly with phosphotyrosine-coupled beads. Recombinant PI3K-C2β also associated with the autophosphorylated PDGFR (RP) but not with the nonphosphorylated receptor (R) (Fig. 7C).
FIG. 7.
Interaction of PI3K-C2β with the EGFR and PDGFR in vitro. (A) Immobilized EGFR immunoprecipitated from A431 cells (R1) was autophosphorylated in vitro (R1P) and incubated with a cell lysate from HEK293 cells that had been transfected with either Glu-tagged PI3K-C2β or an empty expression vector. Lysates from transfected cells were also immunoprecipitated with anti-Glu tag antibodies (α-Glu) or mouse immunoglobulins (contr). Each affinity complex was fractionated by SDS-PAGE and Western blotted with anti–PI3K-C2β antiserum. Aliquots of unphosphorylated (R1) or autophosphorylated (R1P) EGFR were also Western blotted in parallel with monoclonal antiphosphotyrosine antibody. (B) Recombinant epitope-tagged PI3K-C2β was purified from infected Sf9 cells and incubated with anti–PI3K-C2β antiserum (α-C2β), Actigel beads (Act), Actigel beads coupled to phosphotyrosine (Act PY), or immobilized purified EGFR (R1) that had been autophosphorylated in vitro (R1P). The samples were assayed for phospholipid kinase activity with PtdIns in the presence of Ca2+. The radiolabeled phospholipid products were analyzed by TLC. (C) Purified epitope-tagged PI3K-C2β was also incubated with either anti-Glu tag antibody–protein G (control) (ctr), immobilized PDGFR immunoprecipitated from resting NIH 3T3 cells (R), or receptor that had been autophosphorylated in vitro (RP). The samples were fractionated by SDS-PAGE and Western blotted with anti–PI3K-C2β antiserum. The position of PI3K-C2β (p180) is shown. MW, molecular weight.
Identification of the PI3K-C2β-binding site on the EGFR.
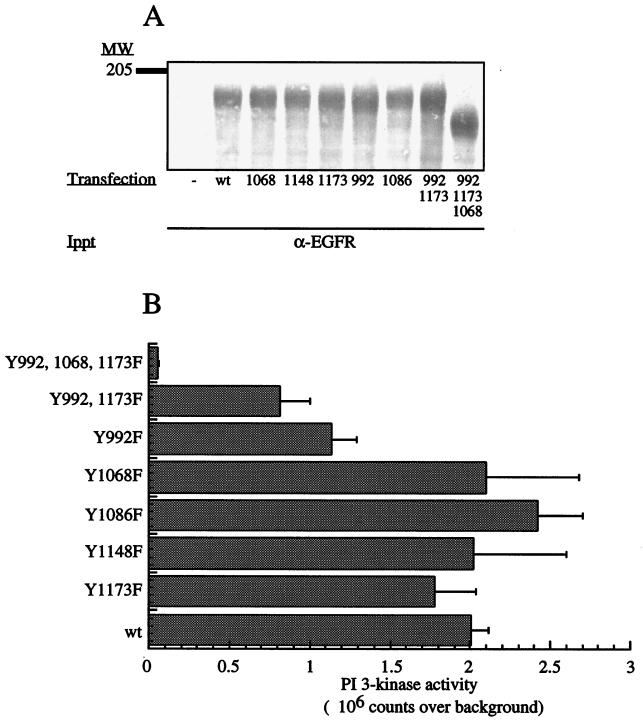
To identify the site(s) on the cytoplasmic domain of the EGFR that mediates its recruitment of PI3K-C2β, a panel of receptor point mutations was expressed in HEK293 cells. The ability of the mutants to interact with this PI3K was examined with lipid kinase assays performed in the presence of Ca2+. The expression of each receptor mutant was similar (Fig. 8A). Mutants Y1068F, Y1086F, Y1148F, and Y1173F all associated with PI3K-C2β in a manner similar to that of the wild-type EGFR (Fig. 8B). The degrees of binding of PI3K-C2β to mutant Y992F and to the double mutant Y992-1173F were reduced by 50 and 65%, respectively. The interaction of PI3K-C2β with the triple mutant Y992-1068-1173F was almost completely abolished.
FIG. 8.
Interactions of EGFR mutants with PI3K-C2β. (A) A mammalian expression vector containing cDNA encoding various human EGFR mutants was transfected into HEK293 cells. Each recombinant EGFR mutant was immunoprecipitated with anti-EGFR antibody and tested for its ability to interact in vitro with recombinant PI3K-C2β isolated from HEK293 cell lysates. Quantification of each mutant receptor present in the immunoprecipitates was done by SDS-PAGE and Coomassie blue staining. The control sample (−) represents EGFR immunoprecipitation from nontransfected cells. (B) The samples were assayed for PI3K activity in the presence of Ca2+. Radiolabeled phospholipid products were analyzed by TLC and quantified by PhosphorImager analysis. Background binding to endogenous EGFR was subtracted from all values, and data are presented as mean ± standard error from six independent experiments. MW, molecular weight; wt, wild type.
Similar results were also obtained when the interaction of PI3K-C2β with the various receptor mutants was examined by Western blotting (data not shown). These findings identify (p)Y992 as an important binding site on the EGFR, while (p)Y1068 and (p)Y1173 also contribute to its interaction with PI3K-C2β. Sequence alignment of these three sites revealed the putative consensus binding site for PI3K-C2β to be E(p)YL/I.
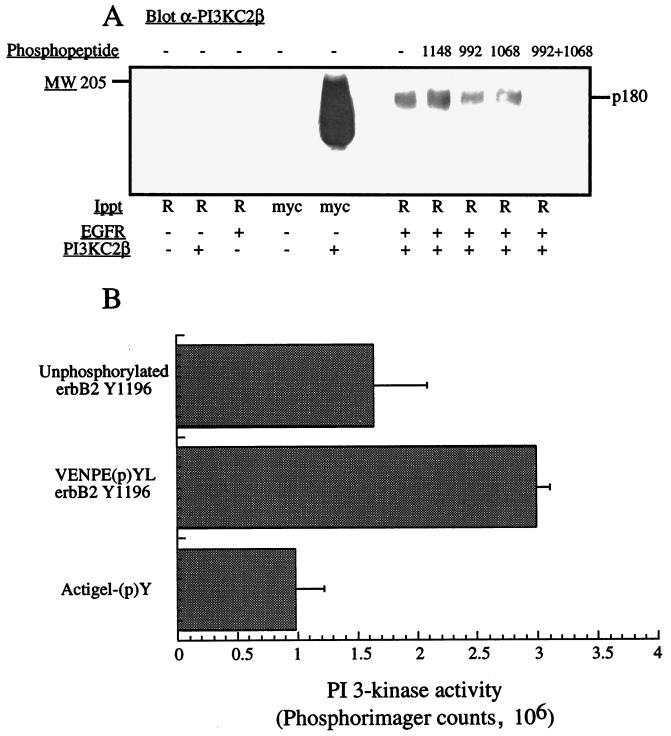
The specificity with which PI3K-C2β interacts with the EGFR was also examined by incubating purified autophosphorylated wild-type EGFR with PI3K-C2β (both transiently expressed in HEK293 cells) in the presence or absence of phosphopeptides corresponding to different sites on the cytoplasmic tail of EGFR. The phosphopeptides corresponding to two binding sites on the EGFR (Y992 and Y1068 at 25 μM) decreased the amount of PI3K-C2β that coimmunoprecipitated with the EGFR, while the phosphopeptide (p)Y1148 had no effect (Fig. 9A). Strikingly, the addition of both (p)Y992 and (p)Y1068 completely abolished the EGFR–PI3K-C2β interaction. The ability of recombinant PI3K-C2β to interact with an immobilized phosphopeptide containing the E(p)YL sequence (ErbB-2 Y1196) was also examined with PI3K assays performed in the presence of Ca2+. The interaction of PI3K-C2β with the phosphorylated peptide was significantly greater than that with either the nonphosphorylated peptide or immobilized phosphotyrosine alone (Fig. 9B).
FIG. 9.
Phosphopeptides containing an E(p)YL sequence bind PI3K-C2β. (A) Lysates from HEK293 cells transfected with either empty vector (−) or wild-type EGFR (+) were immunoprecipitated with anti-EGFR antibodies (R). The isolated EGFR was autophosphorylated in vitro and incubated with lysates of HEK293 cells transfected with either vector (−) or myc-tagged PI3K-C2β in the absence or presence of phosphopeptides corresponding to the EGFR at residues (p)992, (p)Y1068, and (p)Y1148. The receptors were isolated, and the associated proteins were fractionated by SDS-PAGE and Western blotted with anti–PI3K-C2β antiserum. p180 represents PI3K-C2β. (B) Immobilized peptides corresponding to ErbB-2 Y1196, (p)Y1196, or phosphotyrosine were incubated with purified recombinant PI3K-C2β that had been expressed in Sf9 cells. Following incubation, each sample was washed and assayed for PI3K activity in the presence of Ca2+. Radiolabeled phospholipid products were fractionated by TLC, and the spots were quantified by PhosphorImager analysis. Data are presented as mean ± standard error from four independent experiments.
Identification of the N-terminal domain of PI3K-C2β as the site of interaction with the EGFR.
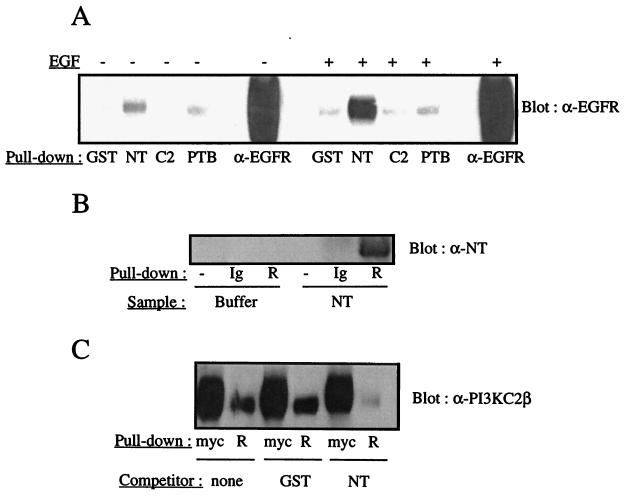
To identify the domain(s) of PI3K-C2β mediating its association with the EGFR, three cDNA fragments, encoding the N-terminal region, the C-terminal C2 domain, and a putative PTB domain identified between residues 538 and 687, were expressed in E. coli and purified. Homogeneous preparations of each GST fusion were tested for their ability to precipitate the EGFR from resting or EGF-stimulated A431 cell lysates. A detectable interaction of the N-terminal region with the EGFR was observed in resting lysates under conditions where GST alone or the GST-C2 domain fusion showed no interaction (Fig. 10A). The GST-PTB domain fusion also displayed a weak but reproducible ability to interact with the EGFR in lysates from quiescent cultures. When lysates from EGF-stimulated cells were examined, the binding of the EGFR to the N-terminal region of PI3K-C2β increased markedly (Fig. 10A), while no significant increase in binding was observed with the other domains.
FIG. 10.
The N-terminal region of PI3K-C2β interacts with the EGFR. (A) Purified recombinant domains corresponding to the PI3K-C2β N terminus (NT), C2 domain (C2), or putative PTB domain were produced in E. coli as GST fusion proteins. The immobilized domains and GST were incubated with lysates from quiescent or EGF-stimulated A431 cells. The fusion proteins were isolated, and the associated proteins were fractionated by SDS-PAGE and Western blotted with anti-EGFR antiserum. (B) Recombinant EGFR was purified from transfected HEK293 cells by immunoprecipitation with immobilized anti-EGFR antibody and autophosphorylated in vitro (R). The receptor was then incubated with either soluble PI3K-C2β N-terminal fragment (NT) or buffer alone. Samples were fractionated by SDS-PAGE and Western blotted with antiserum directed against the PI3K-C2β N terminus. Protein G (−) and immobilized anti-EGFR antibody alone (Ig) served as controls and were assayed in parallel. (C) Purified soluble GST or purified soluble PI3K-C2β N-terminal fragment (NT) was added to the EGFR PI3K-C2β association assay described in the legend to Fig. 9A. The resultant blots were probed with anti–PI3K-C2β antiserum.
The N-terminal domain was then prepared in soluble form and tested for its ability to bind purified recombinant EGFR expressed in HEK293 cells. A detectable interaction of the purified N-terminal fragment with the EGFR was observed by Western blotting (Fig. 10B); together with the results presented in Fig. 10A, these results demonstrate that this region of PI3K-C2β is likely to mediate the association with the EGFR. Finally, the recombinant N-terminal region was added in soluble form to lysates containing recombinant PI3K-C2β. The ability of the full-length enzyme to bind to purified recombinant EGFR was investigated under these conditions. The EGFR–PI3K-C2β association was almost completely abolished in the presence of the N-terminal fragment (Fig. 10C) but not in the presence of purified GST. Together, these observations indicate that an interaction between the N-terminal region and the EGFR is both necessary and sufficient for PI3K-C2β to associate with this receptor. The putative PTB domain of PI3K-C2β appears to play only a modest role in its recruitment to the EGFR.
DISCUSSION
In this study, we demonstrate that PI3K-C2α and PI3K-C2β represent two downstream targets of growth factor receptor signaling cascades. Stimulation of A431 cells with EGF resulted in the rapid recruitment of both class II PI3K enzymes to a phosphotyrosine signaling complex containing the EGFR. Interestingly, the kinetics with which the two molecules were recruited differed markedly (Fig. 1). PI3K-C2β accumulated rapidly with a time course similar to that previously reported for class I PI3K enzyme heterodimers (13). Similar kinetics were observed with HEK293 and Cos 7 cells (Fig. 5) and fibroblasts stimulated with PDGF (Fig. 6). In contrast, while a proportion of PI3K-C2α was present in phosphotyrosine complexes within minutes, this PI3K isozyme accumulated over a much longer period, about 20 to 40 min, following ligand addition. This difference suggests either differential compartmentalization of the class II PI3K isozymes or a difference in their mechanisms of regulation. Recent studies with the EGFR and other ErbB family members have begun to clarify the events that control the endocytic routing of these receptors following ligand addition. Such data demonstrate that the subcellular localization of the activated receptor influences the nature of its downstream signaling events (17, 29).
EGF stimulation of PI3K activity has been described for a large number of primary cells and cell lines. Leydig cells, A431 cells which express EGFR to high levels (approximately 106 receptors per cell), and murine fibroblasts transfected with recombinant EGFR display increased PI3K activity in antiphosphotyrosine antibody immune complexes following EGF addition (50, 51). Furthermore, the human class IA PI3K adapter subunit p85 was cloned by use of a technique that screened for target proteins of receptor tyrosine kinases using the phosphorylated carboxy-terminal tail of the EGFR as bait (49). However, in other cells, including A431 and A549 cells, this association is not seen (51). Since EGFR lacks the YxxM consensus motif recognized by the SH2 domains of the class IA PI3K adapters and ErbB-3 contains seven copies, PI3K signalling was proposed to occur through a heterodimerized EGFR–ErbB-3 complex (44, 51). The validity of this model depends upon the coordinated expression of EGFR and ErbB-3 in addition to the specific heterodimerization of these two receptor chains over other combinations. However, it has been reported that ErbB-2 preferentially heterodimerizes with EGFR, ErbB-3, and ErbB-4 (26). Furthermore, some cells, such as PC12 and A549, do not possess ErbB-3 yet activate PI3K following EGF stimulation. It has been suggested that the adapter protein p120cbl may mediate an interaction between the activated EGFR and the class IA p85-p110 PI3K heterodimer (50). Other groups have reported that the class IA PI3K adapter p85 appears to be poorly recruited to EGFR-containing phosphotyrosine complexes (Fig. 5). Consequently, in certain cell types, the EGF-stimulated increase in 3′ phosphorylated lipids may more accurately represent the activation of class II PI3K enzymes than a class IA p85-p110 heterodimer.
The identification of either EGFR or ErbB-2 in human tumors correlates with a poor prognosis (20). A common alteration of the EGFR in human disease is the deletion of exons 2 to 7. Expression of the truncated receptor, termed EGFRvIII, confers a selective advantage in vivo, resulting in a transformed phenotype. These cells also have been found to have high levels of constitutive PI3K activity (37). This finding illustrates the need to improve the understanding of how EGFR regulates PI3K activity. Since EGF stimulation recruits both PI3K-C2α and PI3K-C2β to the EGFR-containing signaling complex, together with ErbB-2 (Fig. 2), various SH2 domain-containing adapter proteins were examined to exclude any possible interaction with PI3K-C2β in vivo. Under conditions where PI3K-C2β was coimmunoprecipitated with the activated EGFR, there was no coimmunoprecipitation of this enzyme with molecules known to interact with the EGFR, namely p85α (23), c-Src (30), Shc (42), and Grb2 (7) (data not shown).
We demonstrate that the association of PI3K-C2β with EGFR is largely mediated by residue (p)Y992 but that (p)Y1068 and (p)Y1173 are also involved. These phosphotyrosine residues lie within the consensus sequence E(p)YL/I, which is also found on both the α and the β chains of the PDGFR at (p)Y579. Previously, this site on the PDGFR had been shown to bind only members of the Src family tyrosine kinases (36). Such specificity contrasts with the behavior of class I PI3K adapters, whose SH2 domains preferentially bind (p)YxxM motifs. Furthermore, it supports our finding that class IA PI3K adapters do not mediate the recruitment of class II PI3K enzymes to growth factor receptors.
The N-terminal region (residues 1 to 301) of PI3K-C2β is able to associate with the EGFR (Fig. 10). Unfortunately, the mechanism responsible for this interaction is currently unclear, although work is currently in progress to define the nature of this interaction. The fact that this domain associates weakly with the nonphosphorylated receptor may explain our findings that PI3K-C2β is observed in complex with the EGFR in quiescent cells.
It is currently unclear why receptor tyrosine kinases would need to recruit two distinct forms of the PI3K enzyme. While each PI3K enzyme may fulfill a specific biological role, the class I catalytic subunits utilize a broader range of phospholipid substrates in vitro than the class II enzymes. Consequently, stimulation of a class I PI3K enzyme alone could explain the activation of downstream targets, such as p70S6 kinase (9, 62), PDK1 and Akt (1, 18), and noncanonical isoforms of protein kinase C (38). Although the kinetics with which the p85-p110 heterodimer and PI3K-C2α associate with the EGFR suggest temporal specificity, the same is not true for PI3K-C2β. Several ligands have now been demonstrated to mediate the activity of class II PI3K enzymes (5, 56, 65). However, receptor heterogeneity makes it difficult to conclude how this activation is achieved. The results presented here also contrast with those obtained with other ligands, since neither EGF nor PDGF stimulated an increase in the total pool of either PI3K-C2α or PI3K-C2β lipid kinase activity (Fig. 6 and data not shown). This fact may reflect a difference in receptor regulation but perhaps more likely indicates that both growth factors activate only a small proportion of the total pool of enzyme, as previously described for the class IA heterodimers (13). In other cell types, where a larger number of PI3K molecules become activated, higher specific activity of immunoprecipitated PI3K is achieved.
It was previously shown that the lipid kinase activity of PI3K-C2β could be distinguished from those of other PI3K enzymes on the basis of its cation preference (2). We have now expanded this work and demonstrated that in contrast to the class IA PI3K and PI4K enzymes (16), PI3K-C2α and PI3K-C2β both can use Ca2+ as a cofactor for phosphate transfer (Fig. 3). While p110α and PI3K-C2β can phosphorylate PtdIns in the presence of Mn2+, PI3K-C2α cannot. Also, when PtdIns(4)P was used as a substrate, this cation selectivity was no longer observed, and all three enzymes were active only in the presence of Mg2+. How these findings relate to the activity of each enzyme in situ remains uncertain, but this property could be used in future studies to characterize an isolated PI3K enzyme prior to its specific analysis. More importantly, perhaps, the data elegantly show that despite a high degree of sequence homology within the catalytic domains of these molecules, functional differences do exist. Structure-function studies have already begun to characterize the catalytic domains of PI3K enzymes in detail (63) and will doubtless continue until their structures are solved. Ultimately, such differences might be exploited to develop selective antagonists against these enzymes to provide possible therapeutic benefits.
A long-overlooked property of the class IA enzymes is their serine-threonine protein kinase activity. It has been proposed that this activity provides negative feedback regulation, since serine phosphorylation of p85 results in decreased lipid kinase activity of the associated catalytic subunit (12). Potentially, these enzymes could mediate downstream signaling via this protein kinase activity, but aside from IRS-1, no other substrates have been identified. However, in our experiments, neither PI3K-C2α nor PI3K-C2β displayed any protein kinase activity (data not shown), and although it was claimed in an earlier study (34) that PI3K-C2γ can act as a protein kinase, no data were presented in that report. It remains to be seen if the differential regulation of protein kinase activity can provide the required functional specificity. Study of the class IB PI3K p110γ does indicate, however, that its lipid and protein kinase activities may regulate two distinct downstream pathways via Akt and mitogen-activated protein kinase, respectively (4). If the class IA and class II PI3K enzymes do have different downstream targets, the mechanisms of their selective activation will require elucidation.
ACKNOWLEDGMENTS
We thank Christopher Odell for help with automated DNA sequencing, Shun-Cheng Li (Samuel Lunenfield Research Institute, Toronto, Ontario, Canada) for EGFR and ErbB-2 phosphopeptides, Julian Downward and Peter Parker (ICRF, London, United Kingdom) for purified monoclonal anti-Glu and anti-myc tag antibodies, and Parmjit Jat for the pBabeNeo vector and the BOSC 23 cell line.
A.A. was supported by a grant from the Swiss National Science Foundation.
REFERENCES
- 1.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R J, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro A, Volinia S, Zvelebil M J, Stein R, Watton S J, Layton M J, Gout I, Ahmadi K, Downward J, Waterfield M D. Human PI3-kinase C2β—the role of calcium and the C2 domain in enzyme activity. J Biol Chem. 1998;273:33082–33091. doi: 10.1074/jbc.273.49.33082. [DOI] [PubMed] [Google Scholar]
- 3.Arcaro A, Wymann M P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann M. Bifurcation of lipid and protein kinase signals of PI3Kγ to the protein kinases PBK and MAPK. Science. 1998;282:293–296. doi: 10.1126/science.282.5387.293. [DOI] [PubMed] [Google Scholar]
- 5.Brown R A, Domin J, Arcaro A, Waterfield M D, Shepherd P R. Insulin activates the alpha isoform of class II phosphoinositide 3-kinase. J Biol Chem. 1999;274:14529–14532. doi: 10.1074/jbc.274.21.14529. [DOI] [PubMed] [Google Scholar]
- 6.Brown R A, Ho L K, Weber-Hall S J, Shipley J M, Fry M J. Identification and cDNA cloning of a novel mammalian C2 domain-containing phosphoinositide 3-kinase, HsC2-PI3K. Biochem Biophys Res Commun. 1997;233:537–544. doi: 10.1006/bbrc.1997.6495. [DOI] [PubMed] [Google Scholar]
- 7.Bunday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 8.Carter A N, Downes C P. Phosphatidylinositol 3-kinase is activated by nerve growth factor and epidermal growth factor in PC12 cells. J Biol Chem. 1992;267:14563–14567. [PubMed] [Google Scholar]
- 9.Chung J, Grammer T C, Lemon K P, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70 S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 10.Cohen G, Ren B R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 11.De Camilli P, Emr S D, McPherson P S, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 12.Dhand R, Hiles I, Panayotou G, Roche S, Fry M J, Gout I, Totty N F, Truong O, Vicendo P, Yonezawa K, Kasuga M, Courtneidge S A, Waterfield M D. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994;13:522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domin J, Dhand R, Waterfield M D. Binding to the platelet derived growth factor receptor transiently activates the p85α-p110α phosphoinositide 3-kinase complex in vivo. J Biol Chem. 1996;271:21614–21621. doi: 10.1074/jbc.271.35.21614. [DOI] [PubMed] [Google Scholar]
- 14.Domin J, Pages F, Volinia S, Rittenhouse S E, Zvelebil M J, Stein R C, Waterfield M D. Cloning of a human phosphatidylinositol 3-kinase with a C2 domain which displays reduced sensitivity to the inhibitor wortmannin. Biochem J. 1997;326:139–147. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domin J, Waterfield M D. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997;410:91–95. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- 16.Downing G L, Kim S, Nakanishi S, Catt J, Balla T. Characterization of a soluble adrenal phosphatidylinositol 4-kinase reveals wortmannin sensitivity of type III phosphatidylinositol kinases. Biochemistry. 1996;35:3587–3594. doi: 10.1021/bi9517493. [DOI] [PubMed] [Google Scholar]
- 17.Emlet D R, Moscatello D K, Ludlow L B, Wong A J. Subsets of epidermal growth factor receptors during activation and endocytosis. J Biol Chem. 1997;272:4079–4086. doi: 10.1074/jbc.272.7.4079. [DOI] [PubMed] [Google Scholar]
- 18.Franke T F, Yang S-I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 19.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 20.Gullick W J. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull. 1991;47:87–98. doi: 10.1093/oxfordjournals.bmb.a072464. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins P T, Jackson T R, Stephens L R. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992;358:157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- 22.Herman P K, Emr S D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiles I D, Otsu M, Volinia S, Fry M J, Gout I, Dhand R, Panayotou G, Ruiz-Larrea F, Thompson A, Totty N F, Justin Hsuan J, Courtneidge S A, Parker P J, Waterfield M D. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992;70:419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- 24.Hu P, Margolis B, Skolnik E Y, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992;12:981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapeller R, Cantley L C. Phosphatidylinositol 3-kinase. BioEssays. 1994;16:565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- 26.Karunagraran D, Tzahar E, Beerli R R, Chen X, Graus-Porta D, Ratzkin B J, Segar R, Hynes N E, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15:254–264. [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus M H, Fedi P, Starks V, Muraro R, Aaronson S A. Demonstration of ligand dependent signalling by the erbB-3 tyrosine kinase and its constitutive activation in human breast tumor cells. Proc Natl Acad Sci USA. 1993;90:2900–2904. doi: 10.1073/pnas.90.7.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layton M J, Harpur A G, Panayotou G, Bastiaens P I, Waterfield M D. Binding of a diphosphotyrosine-containing peptide that mimics activated platelet-derived growth factor receptor beta induces oligomerization of phosphatidylinositol 3-kinase. J Biol Chem. 1998;273:33379–33385. doi: 10.1074/jbc.273.50.33379. [DOI] [PubMed] [Google Scholar]
- 29.Lenferink A E G, Pinkas-Kramarski R, van de Poll M L M, van Vugt M J H, Klapper L N, Tzahar E, Waterman H, Sela M, van Zoelen E J J, Yarden Y. Differential endocytic routing of homo- and heterodimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luttrell D K, Lee A, Lansing T J, Crosby R M, Jung K D, Willard D, Luther M, Rodriguez M, Berman J, Gilmer T M. Involvement of pp60c-src with two major signalling pathways in human breast cancer. Proc Natl Acad USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDougall L K, Domin J, Waterfield M D. A family of phosphoinositide 3-kinases in Drosophila identifies a new mediator of signal transduction. Curr Biol. 1995;5:1404–1415. doi: 10.1016/s0960-9822(95)00278-8. [DOI] [PubMed] [Google Scholar]
- 32.Margolis B, Li N, Koch A, Mohammadi M, Hurwitz D R, Zilberstein A, Ullrich A, Pawson T, Schlessinger J. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J. 1990;9:4375–4380. doi: 10.1002/j.1460-2075.1990.tb07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller E S, Ascoli M. Anti-phosphotyrosine immunoprecipitation of phosphoinositol 3 ′kinase activity in different cell types after exposure to epidermal growth factor. Biochem Biophys Res Commun. 1990;173:289–295. doi: 10.1016/s0006-291x(05)81055-1. [DOI] [PubMed] [Google Scholar]
- 34.Misawa H, Ohtsubo M, Copeland N G, Gilbert D J, Jenkins N A, Yoshimura A. Cloning and characterisation of a novel class II phosphoinositide 3-kinase containing C2 domain. Biochem Biophys Res Commun. 1998;244:531–539. doi: 10.1006/bbrc.1998.8294. [DOI] [PubMed] [Google Scholar]
- 35.Molz L, Chen Y W, Hirano M, Williams L T. Cpk is a novel class of drosophila PtdIns 3-kinase containing a C2 domain. J Biol Chem. 1996;271:13892–13899. doi: 10.1074/jbc.271.23.13892. [DOI] [PubMed] [Google Scholar]
- 36.Mori S L, Ronnstrand L, Yokote K, Engstrom A, Courtneidge S A, Claesson-Welsh L, Heldin C H. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta receptor: involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993;12:2257–2264. doi: 10.1002/j.1460-2075.1993.tb05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moscatello D K, Holgado-Madruga M, Emlet D R, Montgomery B, Wong A J. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273:200–206. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi H, Brewer K A, Exton J H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 39.Ono F, Nakagawa T, Saito S, Owada Y, Sakagami H, Goto K, Suzuki M, Matsuno S, Kondo H. A novel class II phosphoinositide 3-kinase predominantly expressed in the liver and its enhanced expression during liver regeneration. J Biol Chem. 1998;273:7731–7736. doi: 10.1074/jbc.273.13.7731. [DOI] [PubMed] [Google Scholar]
- 40.Panayotou G, Gish G, End P, Truong O, Gout I, Dhand R, Fry M J, Hiles I, Pawson T, Waterfield M D. Interactions between SH2 domains and tyrosine-phosphorylated platelet-derived growth factor beta-receptor sequences: analysis of kinetic parameters by a novel biosensor-based approach. Mol Cell Biol. 1993;13:3567–3576. doi: 10.1128/mcb.13.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titre helper free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci P G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 43.Porfiri E, Evans T, Chardin P, Hancock J F. Prenylation of Ras proteins is required for efficient hSOS1-promoted guanine nucleotide exchange. J Biol Chem. 1994;269:22672–22677. [PubMed] [Google Scholar]
- 44.Prigent S A, Gullick W J. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raffioni S, Bradshaw R A. Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor and nerve growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci USA. 1992;89:9121–9125. doi: 10.1073/pnas.89.19.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riese D J, II, Stern D F. Specificity within the EGF family/ErbB receptor family signalling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 47.Rizo J, Sudhof T C. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 48.Schu P V, Takegawa K, Fry M J, Stack J H, Waterfield M D, Emr S D. Phosphatidylinositol 3-kinase encoded by the yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–92. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 49.Skolnik E Y, Margolis B, Mohammadi M, Lowenstein E, Fisher R, Drepps A, Ullrich A, Schlessinger J. Cloning of PI3-kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991;65:83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- 50.Soltoff S P, Cantley L C. p120cbl is a cytosolic adapter protein that associates with phosphoinositide 3-kinase in response to epidermal growth factor in PC12 and other cells. J Biol Chem. 1996;271:563–567. doi: 10.1074/jbc.271.1.563. [DOI] [PubMed] [Google Scholar]
- 51.Soltoff S P, Carraway S A, Prigent S A, Gullick W G, Cantley L C. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephens L R, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka A S, Thelen M, Cadwallader K, Tempst P, Hawkins P T. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adapter, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 53.Stephens L R, Hughes K T, Irvine R F. Pathways of phosphatidylinositol (3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 54.Stephens L R, Jackson T R, Hawkins P T. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta. 1993;1179:27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 55.Toker A, Cantley L C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 56.Turner S J, Domin J, Waterfield M D, Ward S G, Westwick J. The CC chemokine monocyte chemotactic peptide-1 activates both the class I p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2α. J Biol Chem. 1998;273:25987–25995. doi: 10.1074/jbc.273.40.25987. [DOI] [PubMed] [Google Scholar]
- 57.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–211. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 58.Vanhaesebroeck B, Leevers S, Panayotou G, Waterfield M D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 59.Virbasius J V, Guilherme A, Czech M P. Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J Biol Chem. 1996;271:13304–13307. doi: 10.1074/jbc.271.23.13304. [DOI] [PubMed] [Google Scholar]
- 60.Volinia S, Dhand R, Vanhaesebroeck B, MacDougall L K, Stein R, Zvelebil M J, Domin J, Panaretou C, Waterfield M D. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J. 1995;14:3339–3348. doi: 10.1002/j.1460-2075.1995.tb07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walsh J P, Caldwell K K, Majerus P W. Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc Natl Acad Sci USA. 1991;88:9184–9187. doi: 10.1073/pnas.88.20.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weng Q P, Andrabi K, Klippel A, Kozlowski M T, Williams L T, Avruch J. Phosphatidylinositol 3-kinase signals activation of p70S6 kinase in situ through site-specific p70 phosphorylation. Proc Natl Acad Sci USA. 1995;92:5744–5748. doi: 10.1073/pnas.92.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wymann M P, Bulgarelli-Leva G, Zvelebil M J, Pirola L, Vanhaesebroeck B, Waterfield M D, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr G A, Backer J M. Regulation of the p85-p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Banfic H, Straforini F, Tosi L, Volinia S, Rittenhouse S. A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. A source of phosphatidylinositol 3-phosphate. J Biol Chem. 1998;273:14081–14084. doi: 10.1074/jbc.273.23.14081. [DOI] [PubMed] [Google Scholar]
- 66.Zhou S Y, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]