Abstract
Background
Patients with primary systemic vasculitis or polymyalgia rheumatica might be at a high risk for poor COVID-19 outcomes due to the treatments used, the potential organ damage cause by primary systemic vasculitis, and the demographic factors associated with these conditions. We therefore aimed to investigate factors associated with COVID-19 outcomes in patients with primary systemic vasculitis or polymyalgia rheumatica.
Methods
In this retrospective cohort study, adult patients (aged ≥18 years) diagnosed with COVID-19 between March 12, 2020, and April 12, 2021, who had a history of primary systemic vasculitis (antineutrophil cytoplasmic antibody [ANCA]-associated vasculitis, giant cell arteritis, Behçet's syndrome, or other vasculitis) or polymyalgia rheumatica, and were reported to the COVID-19 Global Rheumatology Alliance registry were included. To assess COVID-19 outcomes in patients, we used an ordinal COVID-19 severity scale, defined as: (1) no hospitalisation; (2) hospitalisation without supplemental oxygen; (3) hospitalisation with any supplemental oxygen or ventilation; or (4) death. Multivariable ordinal logistic regression analyses were used to estimate odds ratios (ORs), adjusting for age, sex, time period, number of comorbidities, smoking status, obesity, glucocorticoid use, disease activity, region, and medication category. Analyses were also stratified by type of rheumatic disease.
Findings
Of 1202 eligible patients identified in the registry, 733 (61·0%) were women and 469 (39·0%) were men, and their mean age was 63·8 years (SD 17·1). A total of 374 (31·1%) patients had polymyalgia rheumatica, 353 (29·4%) had ANCA-associated vasculitis, 183 (15·2%) had giant cell arteritis, 112 (9·3%) had Behçet's syndrome, and 180 (15·0%) had other vasculitis. Of 1020 (84·9%) patients with outcome data, 512 (50·2%) were not hospitalised, 114 (11·2%) were hospitalised and did not receive supplemental oxygen, 239 (23·4%) were hospitalised and received ventilation or supplemental oxygen, and 155 (15·2%) died. A higher odds of poor COVID-19 outcomes were observed in patients who were older (per each additional decade of life OR 1·44 [95% CI 1·31–1·57]), were male compared with female (1·38 [1·05–1·80]), had more comorbidities (per each additional comorbidity 1·39 [1·23–1·58]), were taking 10 mg/day or more of prednisolone compared with none (2·14 [1·50–3·04]), or had moderate, or high or severe disease activity compared with those who had disease remission or low disease activity (2·12 [1·49–3·02]). Risk factors varied among different disease subtypes.
Interpretation
Among patients with primary systemic vasculitis and polymyalgia rheumatica, severe COVID-19 outcomes were associated with variable and largely unmodifiable risk factors, such as age, sex, and number of comorbidities, as well as treatments, including high-dose glucocorticoids. Our results could be used to inform mitigation strategies for patients with these diseases.
Funding
American College of Rheumatology and the European Alliance of Associations for Rheumatology.
Introduction
Patients with autoimmune conditions could be at an increased risk of hospitalisation or death from COVID-19.1 Previous studies, including analyses from the COVID-19 Global Rheumatology Alliance physician registry, have reported associations between worse COVID-19 outcomes in patients with rheumatic disease and the following risk factors: older age, a high burden of comorbidities, high doses of glucocorticoids, high disease activity, and the use of particular conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs) and biological and targeted synthetic DMARDs.2, 3, 4 However, patients with rheumatic diseases differ greatly in their demographic profiles and in their exposure to immunosuppressive therapies.
Research in context.
Evidence before this study
Data from large registries, including the COVID-19 Global Rheumatology Alliance physician registry, have reported associations between poor COVID-19 outcomes and older age, having comorbidities, receiving a prednisolone-equivalent dose of 10 mg/day or higher, and use of rituximab. However, only small studies or case reports have described outcomes of COVID-19 in patients with primary systemic vasculitis. We searched Pubmed on May 15, 2021, using the search tems “COVID-19”, “vasculitis”, “ANCA vasculitis”, “Giant cell arteritis”, “Polymyalgia Rheumatica”. We searched for primary research including case-series published in any language between Jan 1, 2020, and May 1, 2021. Case reports were excluded. We found five studies describing COVID-19 outcomes in patients with primary systemic vasculitis.
Added value of this study
In this analysis of patients with primary systemic vasculitis or polymyalgia rheumatica, including 155 (15·2%) patients who were reported to have died, older age, male sex, a glucocorticoid dose of 10 mg/day or higher, moderate or severe disease activity, and a high number of comorbidities were associated with poor COVID-19 outcomes. Risk factors for poor outcomes were older age and obesity in patients with giant cell arteritis; older age, moderate, or high or severe disease activity, and rituximab or cyclophosphamide use in patients with ANCA-associated vasculitis; and older age in patients with polymyalgia rheumatica.
Implications of all the available evidence
Different risk factors, including particular treatments and increased disease activity, were associated with poor COVID-19 outcomes in patients with primary systemic vasculitis or polymyalgia rheumatica. The identified risk factors could help to guide physicians in recommending mitigation strategies for their patients.
The primary systemic vasculitides are characterised by vascular inflammation, which can lead to ischaemic events and end-organ damage. Patients with primary systemic vasculitis could be at a high risk for poor outcomes following COVID-19 due to the use of immunosuppressive therapies, such as high doses of glucocorticoids, rituximab, and other DMARDs. Patients with primary systemic vasculitis might also have comorbidities, such as pulmonary or renal disease, which have been associated with poor COVID-19 outcomes in the general population. Finally, in addition to demographic factors, such as older age, there could be an increased susceptibility to the endothelial dysfunction described in COVID-19.5 It is therefore important to understand the factors associated with poor COVID-19 outcomes in patients with primary systemic vasculitis. Similar to these patients, those with polymyalgia rheumatica might also be at high risk for poor COVID-19 outcomes, given that they have similar age demographics and also receive long-term treatment with glucocorticoids.6 The outcomes of COVID-19 in this patient population have not yet been reported.
To our knowledge, no large, well characterised studies done to date have investigated COVID-19 outcomes in patients with specific vasculitis subtypes or polymyalgia rheumatica. The objective of this disease-specific analysis of data from the COVID-19 Global Rheumatology Alliance physician registry was to describe the presentation of COVID-19 among patients with primary systemic vasculitis and polymyalgia rheumatica, and to identify factors associated with poor COVID-19 outcomes.
Methods
Study design and participants
In this retrospective cohort study, we sourced data from the COVID-19 Global Rheumatology Alliance physician registry and the European Alliance of Associations for Rheumatology (EULAR) COVID-19 registry. These registries contain provider-reported cases of COVID-19 among patients with rheumatic diseases.2, 3, 7, 8, 9 Cases are voluntarily entered by rheumatologists or other health-care providers. Data are entered directly into the global or European data entry systems, or transferred from national registries (France, Germany, Italy, Portugal, or Sweden). Patients aged 18 years and older diagnosed with COVID-19 (confirmed or presumptive) between March 12, 2020, and April 12, 2021, who had a history of primary systemic vasculitis or polymyalgia rheumatica were included. Primary systemic vasculitis included antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis, microscopic polyangiitis, or eosinophilic granulomatosis with polyangiitis), giant cell arteritis, Behçet's syndrome, and other vasculitides including Kawasaki disease. A text entry option was available when inputting data to the registry to provide a specific diagnosis or another diagnosis, if not listed. Data quality was assessed by the University of California (San Francisco, CA, USA) and the University of Manchester (Manchester, UK), both of which confirmed that there were no duplicates in the data entries. Given the nature of the data collected, the UK Health Research Authority and the University of California San Francisco institutional review board considered this study exempt from the need to obtain patient consent. Both institutions provided ethics approval for this study.
Procedures
Data from the COVID-19 Global Rheumatology Alliance and EULAR COVID-19 registries were collected for analysis on April 15, 2020, by the GRA data analytic center at the University of California San Francisco. All patients with primary systemic vasculitis or polymyalgia rheumatica were included in the main analysis. Given disease-specific differences in treatments and risk factors for COVID-19 outcomes, subgroup analyses were done for the following specific diagnoses: giant cell arteritis, ANCA-associated vasculitis, polymyalgia rheumatica, Behçet's syndrome, and other vasculitis.
Immunosuppressive therapies for primary systemic vasculitis at the time of COVID-19 infection were included in the analyses and categorised into groups. DMARDs were categorised as conventional synthetic DMARDs (including antimalarials, apremilast, azathioprine or 6-mercaptopurine, colchicine, cyclosporine, cyclophosphamide, leflunomide, methotrexate, mycophenolate mofetil or mycophenolic acid, sulfasalazine, and tacrolimus) and biological and targeted synthetic DMARDs (including abatacept, rituximab, anakinra, canakinumab, tocilizumab, sarilumab, infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol). Rituximab, cyclophosphamide, and glucocorticoids were also analysed separately; glucocorticoids were categorised by the prednisolone-equivalent dose (0 mg/day, 1–5 mg/day, 6–9 mg/day, or ≥10 mg/day).
The primary outcome was COVID-19 outcome, assessed by use of an ordinal COVID-19 severity scale, which was defined as: (1) no hospitalisation (ie, admission to hospital); (2) hospitalisation with no supplemental oxygen; (3) hospitalisation with any supplemental oxygen or mechanical ventilation; and (4) death.
Relevant covariates included age (analysed as a continuous variable and by decade), sex (female or male), race or ethnicity (White, Black, Latin American, or other), time period (on or before June 15, 2020; June 16 to Sept 30, 2020; or Oct 1, 2020, to April 12, 2021),10 comorbidities (hypertension, cardiovascular disease, diabetes, chronic kidney disease, lung disease, interstitial lung disease, or cancer), number of comorbidities (analysed as a continuous variable), body-mass index (BMI; obese [BMI ≥30 kg/m2] or non-obese [BMI <30 kg/mg2]), smoking status (ever or never smoker), disease activity, as per the physician's global assessment (remission, low, moderate, or high or severe), and region (Europe, North America, South America, or other). Other regions included Asia, Eastern Mediterranean, South-East Asia, and Western Pacific region.
Statistical analysis
Categorical variables are reported as numbers and percentages, and continuous variables are reported as means (SD) or medians (IQR). Data were analysed by ordinal logistic regression, and associations were estimated with odds ratios (ORs) and their associated 95% CIs. Only patients with complete outcome data were included in the models. Missing data for other variables were assumed to be missing at random. Multiple imputation was performed for all models to obtain pooled estimates for disease activity, smoking, and glucocorticoid use. An overall model included sex, age, glucocorticoid use as a categorical variable (ie, prednisolone-equivalent dose categories), medication category (no DMARDs, conventional synthetic DMARDs only, biological or targeted synthetic DMARDs only, combined biological or targeted synthetic plus conventional synthetic DMARDs, rituximab only, or cyclophosphamide only), time period, number of comorbidities, smoking status, obesity (ie, a BMI of ≥30 kg/m2), disease activity, and region. Individual ordinal regression models, which included the same covariates but with different medication categories (ie, no DMARDs, methotrexate, leflunomide, IL-6 inhibitor, azathioprine, rituximab, or cyclophosphamide), were also constructed for giant cell arteritis, ANCA-associated vasculitis, and polymyalgia rheumatica. In all models, age was treated as a continuous variable by decade, and a nominal test was used to confirm that the parallel regression assumption was met. An interaction term between prednisolone usage (binary) and disease activity was included as an exploratory analysis in the overall population.3, 4 We also did a sensitivity analysis including independent comorbidities (hypertension, cardiovascular disease, diabetes, chronic kidney diseases, lung disease, or interstitial lung disease) in patients with ANCA-associated vasculitis. Results were considered statistically significant at a two-sided p value of less than 0·05. Analyses were done in R, version 4.0.2.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
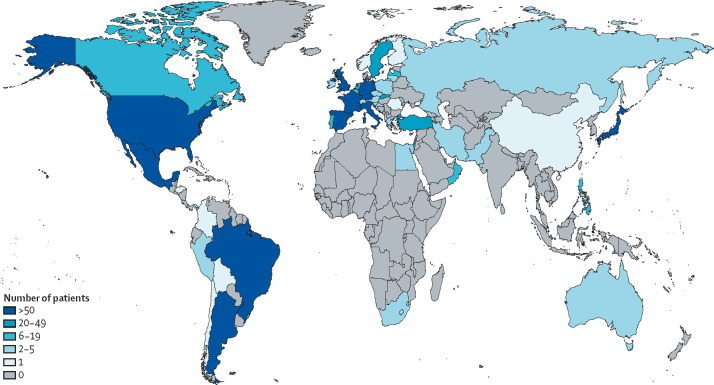
Between March 12, 2020, and April 12, 2021, 1202 cases of COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica were reported to the COVID-19 Global Rheumatology Alliance physician registry and were included in our analysis (figure ). 733 (61·0%) of patients were women and 469 (39·0%) were men, and the mean age of patients was 63·8 (SD 17·1) years. Most patients were from Europe (704 [58·6%] patients) and North America (328 [27·3%]). Polymyalgia rheumatica was the most common diagnosis (374 [31·1%] patients), followed by ANCA-associated vasculitis (353 [29·4%]), giant cell arteritis (183 [15·2%]), other vasculitis (180 [15·0%]), and Behçet's syndrome (112 [9·3%]; table 1 ). The most common comorbidities were hypertension (564 [46·9%]), cardiovascular disease (222 [18·5%]), diabetes (216 [18·0%]), lung disease (212 [17·6%]), and chronic kidney disease (160 [13·3%]). Most patients were in remission (442 [36·8%]) or had low disease activity (370 [30·8%]) at the time of COVID-19 diagnosis. A total of 752 (62·6%) patients were taking glucocorticoids and 631 (52·5%) were taking DMARDs.
Figure.
Global distribution of patients with primary systemic vasculitis and polymyalgia rheumatica who had COVID-19 in the COVID-19 Global Rheumatology Alliance physician registry
Table 1.
Baseline characteristics of patients with primary systemic vasculitis or polymyalgia rheumatica at the time of COVID-19 onset
| All patients (n=1202) | ||
|---|---|---|
| Mean age, years | 63·8 (17·1) | |
| Sex | ||
| Female | 733 (61·0%) | |
| Male | 469 (39·0%) | |
| Race or ethnicity | ||
| White | 724 (60·2%) | |
| Black | 19 (1·6%) | |
| Latin American | 145 (12·1%) | |
| Other | 110 (9·2%) | |
| Missing | 204 (17·0%) | |
| Region | ||
| Europe | 704 (58·6%) | |
| North America | 328 (27·3%) | |
| South America | 90 (7·5%) | |
| Other | 80 (6·7%) | |
| Time period | ||
| June 15, 2020, or before | 502 (41·8%) | |
| June 16, 2020, to Sept 30, 2020 | 164 (13·6%) | |
| Oct 1, 2020, to April 12, 2021 | 536 (44·6%) | |
| Diagnosis | ||
| ANCA-associated vasculitis | 353 (29·4%) | |
| Giant cell arteritis | 183 (15·2%) | |
| Polymyalgia rheumatica | 374 (31·1%) | |
| Behçet's syndrome | 112 (9·3%) | |
| Other vasculitis | 180 (15·0%) | |
| Number of comorbidities | ||
| 0 | 428 (35·6%) | |
| 1 | 388 (32·3%) | |
| ≥2 | 386 (32·1%) | |
| Comorbidities | ||
| Hypertension | 564 (46·9%) | |
| Cardiovascular disease | 222 (18·5%) | |
| Diabetes | 216 (18·0%) | |
| Chronic kidney disease | 160 (13·3%) | |
| Lung disease* | 212 (17·6%) | |
| Interstitial lung disease | 44 (3·7%) | |
| Cancer | 77 (6·4%) | |
| Body-mass index ≥30 mg/kg2 | 240 (20·0%) | |
| Smoking status | ||
| Ever smoker | 265 (22·0%) | |
| Never smoker | 448 (37·3%) | |
| Missing | 489 (40·7%) | |
| Median glucocorticoid dose, mg/day† | 6·0 (5·0–12·0) | |
| Categorical glucocorticoid (prednisolone equivalent) dose, mg/day | ||
| 0 | 369 (30·7%) | |
| 1–5 | 367 (30·5%) | |
| 6–9 | 99 (8·2%) | |
| ≥10 | 286 (23·8%) | |
| Missing | 81 (6·7%) | |
| Disease activity | ||
| Remission | 442 (36·8%) | |
| Low | 370 (30·8%) | |
| Moderate | 112 (9·3%) | |
| High or severe | 54 (4·5%) | |
| Missing | 224 (18·6%) | |
| Medication | ||
| No DMARDs | 571 (47·5%) | |
| Conventional synthetic DMARDs only | 367 (30·5%) | |
| Biological or targeted synthetic DMARDs only | 193 (16·1%) | |
| Combined biological or targeted synthetic plus conventional synthetic DMARDs | 71 (5·9%) | |
| Rituximab only‡ | 128/353 (36·3%) | |
| Cyclophosphamide only | 20 (1·7%) | |
Data are mean (SD), n (%), median (IQR), or n/N (%). ANCA=antineutrophil cytoplasmic antibody. DMARD=disease-modifying antirheumatic drug.
Includes interstitial lung disease, chronic obstructive pulmonary disease, asthma, or other lung diseases.
Excludes non-users of glucocorticoids.
In patients with antineutrophil cytoplasmic antibody-associated vasculitis only.
Among the 1020 patients for whom outcomes were reported, 512 (50·2%) were not hospitalised (table 2 ). The baseline characteristics of these 1020 patients, stratified according to primary systemic vasculitis subtype and polymyalgia rheumatica, are presented in the appendix (pp 1–3). Based on the ordinal COVID-19 severity scale, 114 (11·2%) patients were hospitalised and required no supplemental oxygen, 239 (23·4%) were hospitalised and required ventilation or supplemental oxygen, and 155 (15·2%) died (table 2). In an ordinal regression model that included all disease types, patients had higher odds of worse COVID-19 outcomes if they were older (per each additional decade of life OR 1·44 [95% CI 1·31–1·57]), male compared with female (1·38 [1·05–1·80]), had a greater number of comorbidities (per each additional comorbidity 1·39 [1·23–1·58]), were taking 10 mg/day or more of prednisolone compared with none (2·14 [1·50–3·04]), or had moderate, or high or severe disease activity compared with disease remission or low disease activity (2·12 [1·49–3·02]; table 3 ). Patients were less likely to have worse outcomes if they developed COVID-19 between Oct 1, 2020, and April 12, 2021, compared with on or before June 15, 2020 (0·39 [0·30–0·51]). An exploratory analysis that included an interaction term between prednisone use and disease activity was not significant (p=0·27).
Table 2.
Outcomes according to the ordinal COVID-19 severity scale by type of disease
| All patients (n=1020) | Giant cell arteritis (n=158) | ANCA-associated vasculitis (n=294) | Polymyalgia rheumatica (n=323) | Behçet's syndrome (n=97) | Other vasculitis (n=148) | |
|---|---|---|---|---|---|---|
| Not hospitalised | 512 (50·2%) | 69 (43·7%) | 110 (37·4%) | 187 (57·9%) | 69 (71·1%) | 77 (52·0%) |
| Hospitalisation with no supplemental oxygen | 114 (11·2%) | 19 (12·0%) | 30 (10·2%) | 30 (9·3%) | 15 (15·5%) | 20 (13·5%) |
| Hospitalisation with ventilation or supplemental oxygen | 239 (23·4%) | 38 (24·1%) | 89 (30·3%) | 71 (22·0%) | 11 (11·3%) | 30 (20·3%) |
| Death | 155 (15·2%) | 32 (20·3%) | 65 (22·1%) | 35 (10·8%) | 2 (2·1%) | 21 (14·2%) |
Data are n (%). This analysis excludes 182 patients with missing outcome data. ANCA=antineutrophil cytoplasmic antibody.
Table 3.
Multivariable logistic regression analysis of factors associated with ordinal COVID-19 severity outcomes in patients with primary systemic vasculitis or polymyalgia rheumatica
| Odds ratio (95% CI)* | p value | ||
|---|---|---|---|
| Age, per decade of life | 1·44 (1·31–1·57) | <0·001 | |
| Sex | |||
| Female | 1·00 (ref) | .. | |
| Male | 1·38 (1·05–1·80) | 0·020 | |
| Time period | |||
| June 15, 2020, or before | 1·00 (ref) | .. | |
| June 16, 2020, to Sept 30, 2020 | 0·80 (0·54–1·19) | 0·27 | |
| Oct 1, 2020, to April 12, 2021 | 0·39 (0·30–0·51) | <0·001 | |
| Number of comorbidities | 1·39 (1·23–1·58) | <0·001 | |
| Smoking status | |||
| Never smoker | 1·00 (ref) | .. | |
| Ever smoker | 1·01 (0·70–1·46) | 0·95 | |
| Body-mass index, mg/kg2 | |||
| <30 | 1·00 (ref) | .. | |
| ≥30 | 1·07 (0·78–1·46) | 0·16 | |
| Glucocorticoid (prednisolone equivalent) use, mg/day | |||
| 0 | 1·00 (ref) | .. | |
| 1–5 | 1·14 (0·83–1·57) | 0·41 | |
| 6–9 | 1·22 (0·75–1·97) | 0·43 | |
| ≥10 | 2·14 (1·50–3·04) | <0·001 | |
| Disease activity | |||
| Remission or low | 1·00 (ref) | .. | |
| Moderate, or high or severe | 2·12 (1·49–3·02) | <0·001 | |
This analysis excludes 182 patients with missing outcome data.
Adjusted for age, sex, time period, number of comorbidities, smoking status, obesity, glucocorticoid use, disease activity, region, and medication category.
Among 158 patients with giant cell arteritis, 69 (43·7%) were not hospitalised, 19 (12·0%) were hospitalised and required no supplemental oxygen, 38 (24·1%) were hospitalised and required ventilation or supplemental oxygen, and 32 (20·3%) died. In a multivariable ordinal regression model, factors associated with a higher odds of worse COVID-19 outcomes included older age (per each additional decade of life OR 1·89 [95% CI 1·27–2·83]) and obesity compared with non-obesity (2·98 [1·18–7·55]; table 4 ). Patients diagnosed with COVID-19 between Oct 1, 2020, and April 12, 2021, were less likely to have severe outcomes than those diagnosed on or before June 15, 2020 (0·28 [0·13–0·62]).
Table 4.
Multivariable logistic regression analysis of factors associated with ordinal COVID-19 severity outcomes in patients according to disease type
|
Giant cell arteritis (n=149) |
ANCA-associated vasculitis (n=266) |
Polymyalgia rheumatica (n=291) |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI)* | p value | OR (95% CI)* | p value | OR (95% CI)* | p value | ||
| Age, per decade of life | 1·89 (1·27–2·83) | 0·0019 | 1·60 (1·33–1·91) | <0·001 | 2·75 (2·00–3·80) | <0·001 | |
| Sex | |||||||
| Female | 1·00 (ref) | .. | 1·00 (ref) | .. | 1·00 (ref) | .. | |
| Male | 1·20 (0·56–2·55) | 0·64 | 1·37 (0·83–2·26) | 0·21 | 1·54 (0·89–2·67) | 0·12 | |
| Time period | |||||||
| June 15, 2020, or before | 1·00 (ref) | .. | 1·00 (ref) | .. | 1·00 (ref) | .. | |
| June 16, 2020, to Sept 30, 2020 | 0·72 (0·22–2·34) | 0·59 | 0·82 (0·39–1·71) | 0·59 | 0·59 (0·24–1·44) | 0·25 | |
| Oct 1, 2020, to April 12, 2021 | 0·28 (0·13–0·62) | 0·0015 | 0·47 (0·27–0·81) | 0·0062 | 0·28 (0·16–0·47) | <0·001 | |
| Medication | |||||||
| No DMARD | 1·00 (ref) | .. | 1·00 (ref) | .. | 1·00 (ref) | .. | |
| Methotrexate | 0·97 (0·34–2·71) | 0·95 | 0·79 (0·31–1·99) | 0·61 | 1·61 (0·85–3·07) | 0·15 | |
| Leflunomide | 4·93 (0·34–72·07) | 0·24 | .. | .. | .. | .. | |
| IL-6 inhibitor | 0·52 (0·20–1·33) | 0·17 | .. | .. | .. | .. | |
| Azathioprine | .. | .. | 1·10 (0·54–2·24) | 0·79 | .. | .. | |
| Rituximab | .. | .. | 2·15 (1·15–4·01) | 0·016 | .. | .. | |
| Cyclophosphamide | .. | .. | 4·30 (1·10–16·75) | 0·036 | .. | .. | |
| Number of comorbidities | 1·48 (1·06–2·07) | 0·021 | 1·13 (0·89–1·42) | 0·31 | 1·27 (0·98–1·63) | 0·068 | |
| Smoking status | .. | .. | .. | .. | .. | .. | |
| Never smoker | 1·00 (ref) | .. | 1·00 (ref) | .. | 1·00 (ref) | .. | |
| Ever smoker | 0·93 (0·42–2·06) | 0·86 | 1·12 (0·61–2·05) | 0·71 | 0·80 (0·39–1·62) | 0·52 | |
| Body-mass index, mg/kg2 | |||||||
| <30 | 1·00 (ref) | .. | 1·00 (ref) | .. | 1·00 (ref) | .. | |
| ≥30 | 2·98 (1·18–7·55) | 0·021 | 1·35 (0·73–2·51) | 0·34 | 1·06 (0·55–2·05) | 0·87 | |
| Glucocorticoid (prednisolone equivalent) use, mg/day | |||||||
| 0 | 1·00 (ref) | .. | 1·00 (ref) | .. | 1·00 (ref) | .. | |
| 1–5 | 0·96 (0·39–2·34) | 0·92 | 1·67 (0·92–3·03) | 0·091 | 1·29 (0·60–2·79) | 0·52 | |
| 6–9 | 1·75 (0·44–7·04) | 0·43 | 0·60 (0·21–1·69) | 0·33 | 1·30 (0·50–3·38) | 0·58 | |
| ≥10 | 2·89 (1·16–7·21) | 0·023 | 2·80 (1·36–5·79) | 0·0054 | 1·27 (0·52–3·12) | 0·60 | |
| Disease activity | |||||||
| Remission or low | 1·00 (ref) | .. | 1·00 (ref) | .. | 1·00 (ref) | .. | |
| Moderate, or high or severe | 3·14 (0·71–13·97) | 0·12 | 2·16 (1·01–4·31) | 0·028 | 1·99 (0·81–4·89) | 0·13 | |
This analysis includes only patients with studied factors (ie, medications). ANCA=antineutrophil cytoplasmic antibody. DMARD=disease-modifying antirheumatic drug. IL-6=interleukin 6. OR=odds ratio.
Adjusted for age, sex, time period, medication use category, number of comorbidities, smoking status, obesity, glucocorticoid use, disease activity, and region.
Among 294 patients with ANCA-associated vasculitis, 110 (37·4%) were not hospitalised, 30 (10·2%) were hospitalised and required no supplemental oxygen, 89 (30·3%) were hospitalised and required ventilation or supplemental oxygen, and 65 (22·1%) died. In a multivariable ordinal regression model, factors associated with higher odds of worse COVID-19 outcomes included older age (per each additional decade of age OR 1·60 [95% CI 1·33–1·91]), rituximab use compared with no DMARD use (2·15 [1·15–4·01]), cyclophosphamide use compared with no DMARD use (4·30 [1·10–16·75]), and moderate, or high or severe disease activity compared with low disease activity (2·16 [1·01–4·31]; table 4). Patients diagnosed with COVID-19 between Oct 1, 2020, and April 12, 2021, had a lower odds of severe outcomes than those diagnosed on or before June 15, 2020 (0·47 [0·27–0·81]). In a sensitivity analysis that included individual comorbidities, only chronic kidney disease (2·12 [1·17–3·84]) was associated with a higher odds of worse COVID-19 outcomes compared with not having chronic kidney disease in patients with ANCA-associated vasculitis (appendix pp 4–5).
Among 323 patients with polymyalgia rheumatica, 187 (57·9%) were not hospitalised, 30 (9·3%) were hospitalised and required no supplemental oxygen, 71 (22·0%) were hospitalised and required ventilation or supplemental oxygen, and 35 (10·8%) died. In a multivariable ordinal regression model, factors associated with higher odds of worse COVID-19 severity included older age (per each additional decade of life OR 2·75 [95% CI 2·00–3·80]; table 4). Patients diagnosed with COVID-19 between Oct 1, 2020, and April 12, 2021, had a lower odds of severe outcomes than those diagnosed on or before June 15, 2020 (0·28 [0·16–0·47]).
Among 97 patients with Behçet's syndrome, 69 (71·1%) were not hospitalised, 15 (15·5%) required hospitalisation with no supplemental oxygen, 11 (11·3%) required hospitalisation and ventilation or supplemental oxygen, and two (2·1%) died (table 2). Due to the low number of events among Behçet's syndrome patients, ordinal regression models were not constructed due to insufficient power.
Finally, among 148 patients with other types of vasculitis, 77 (52·0%) did not require hospitalisation, 20 (13·5%) required hospitalisation with no supplemental oxygen, 30 (20·3%) required hospitalisation with ventilation or supplemental oxygen, and 21 (14·2%) died. Text entry diagnoses for this group were only present for nine (6·1%) patients (four had Takayasu's arteritis, one had Cogan's syndrome, one had cryoglobulinaemic vasculitis, one had isolated pulmonary capillaritis, one had deficiency of adenosine deaminase 2 vasculitis, and one had relapsing polychondritis). Given the heterogeneity of diagnoses, ordinal regression models were not constructed.
Discussion
To our knowledge, we report the largest study to date of COVID-19 outcomes in patients with primary systemic vasculitis or polymyalgia rheumatica. Older age, a higher number of comorbidities, higher disease activity, and taking 10 mg/day or more of prednisolone were associated with worse COVID-19 outcomes. In disease-specific analyses, we observed unique factors associated with poor outcomes in individual primary systemic vasculitis categories. Reassuringly, patients with COVID-19 submitted to the registry later in the analysis period (ie, Oct 1, 2020, to April 12, 2021) had a lower rate of poor outcomes than those submitted earlier in the analysis period (ie, on or before June 15, 2020).11
These data extend previous observations from smaller cohort studies to a large and well characterised international cohort of patients with primary systemic vasculitis who had COVID-19. In the pooled cohort, almost half (49·8%) of patients were hospitalised and 15·2% had died. Compared with a recent (2021) study done in the UK and Ireland, which found that 59 (91%) of 65 patients with primary systemic vasculitis were admitted to hospital and 18 (28%) died, our results are reassuring and could reflect an improvement in outcomes over time.11, 12 The cause of this change is not known, but it could plausibly be related to more experience with managing COVID-19 or the use of fewer experimental interventions over time.13 As with the general population, both comorbidities and age were important risk factors for poor outcomes, emphasising the importance of public health measures, risk mitigation, and prioritisation of vaccination in these individuals. Consistent with previous studies, higher doses of glucocorticoids and moderate or high disease activity were associated with worse outcomes; however, no interaction between these two variables was found.3, 4
Among the identified patients with ANCA-associated vasculitis and COVID-19, almost two-thirds were hospitalised and approximately one-fifth died. These results should be interpreted with caution. First, a provider-reported registry is biased toward accumulating patients with severe COVID-19. Second, COVID-19 outcomes have improved over time, and this study includes patients from the early months of the COVID-19 pandemic. Nevertheless, this is the first study to evaluate a large and well characterised population of patients with ANCA-associated vasculitis who had COVID-19. Our results are supported by a smaller published case-series,14 which also reported high rates of poor outcomes in patients with ANCA-associated vasculitis. Our study builds on these previous results by further identifying risk factors associated with poor outcomes. In a sensitivity analysis, patients with chronic kidney disease had worse COVID-19 outcomes than those who did not have chronic kidney disease, which is consistent with other studies done in the general population.1, 13 Glucocorticoid use and having received rituximab or cyclophosphamide were also associated with worse outcomes, which is similar to the results of previous studies in other rheumatic diseases.3 Whether this observations reflects the immunosuppressive effects of these drugs or the selection bias related to the patients who receive them cannot be ascertained from this study.
Similar to patients with ANCA-associated vasculitis, a high proportion of patients with giant cell arteritis reported in this registry had poor COVID-19 outcomes, including death. In addition to the aforementioned limitations of these data, the high mortality rates observed in patients with giant cell arteritis could reflect the importance of age in COVID-19 mortality.15 The high COVID-19 mortality rates could also be associated with an increased risk or severity of cardiometabolic comorbidities, which have been associated with poor outcomes in COVID-19.16, 17, 18 Few cohorts of patients with giant cell arteritis are available to further verify these findings. A study done in France reported eight cases of COVID-19 among 148 patients with large vessel vasculitis, only one of whom died.19 A similar study done in Italy reported four cases of COVID-19 among 151 patients with large vessel vasculitis, none of whom died.20 Given some of the unmodifiable risk factors for outcomes in patients with giant cell arteritis, attention to other factors, such as the prescription of high-dose glucocorticoids, is crucial. Patients with polymyalgia rheumatica in our study had less severe COVID-19 outcomes and lower mortality rates than did patients with giant cell arteritis and ANCA-associated vasculitis. In patients with polymyalgia rheumatica, poor outcomes were associated only with age, which is a known risk factor for the general population. Despite a similar age distribution in this group as in the group of patients with giant cell arteritis, these differences could potentially highlight the role of other important factors, such as the use of higher glucocorticoid doses and obesity.
Overall, few patients with Behçet's syndrome in our cohort had severe COVID-19 outcomes, with only a third of patients requiring hospitalisation and two patients who died. Patients with Behçet's syndrome were younger than those with other disease types. Despite the evident concern of an increased risk of thrombosis associated with both Behçet's syndrome and COVID-19,21 which is associated with poor outcomes, our results showing less severe outcomes in these patients than in those with other disease types are reassuring and consistent with those reported by small case-series.22, 23 Due to a low number of events, patients with Behçet's syndrome who had COVID-19 were not included in the regression analysis. The mortality rate in patients with other types of vasculitis was lower than in those with giant cell arteritis and ANCA-associated vasculitis, but given the smaller sample size, reduced diversity of diagnoses, and absence of information on specific diagnoses, this patient group was not included in regression analyses.
In addition to the limitations already noted, the following factors should also be acknowledged. First, cross-sectional, physician-entered, case-reporting registries might be subject to selection bias toward patients with more severe COVID-19. In particular, the mortality rate should be considered a case fatality rate as opposed to an infection fatality rate, as we have probably overestimated the true mortality risk among patients with primary systemic vasculitis who develop COVID-19. Second, given the nature of the COVID-19 Global Rheumatology Alliance physician registry, participation is dependent on a COVID-19 diagnosis, and particular covariates (eg, age) that were accounted for can lead to a collider bias (by affecting both condition and outcomes).24 Third, the time periods between COVID-19 diagnosis and clinical outcomes were not fully collected, and the attribution of clinical outcomes to COVID-19 was based on the treating physician's opinion. However, the results from this registry are consistent with findings from other data sources, verifying the information collected and the interpretation of our results. Fourth, although we were able to analyse multiple factors associated with COVID-19 outcomes in our models, we cannot exclude other confounders as potential explanations for our findings. We therefore caution against making causal inferences from our data. Finally, the absence of an interaction between prednisolone treatment and disease activity, as well as other medication associations, could have been due to low power rather than an absence of an association.
In conclusion, in this study of patients with primary systemic vasculitis or polymyalgia rheumatica who had COVID-19, we report high rates of severe COVID-19 outcomes, particularly in patients with giant cell arteritis and ANCA-associated vasculitis. Important predictors of poor COVID-19 outcomes include older age, a higher number of comorbidities, moderate, or high or severe disease activity, and the use of specific medications, including high-dose glucocorticoids. Our study identifies risk factors associated with poor COVID-19 outcomes in this patient population and in those with specific disease phenotypes, and stratifies outcomes by specific disease phenotypes. These observations could guide risk mitigation strategies in the treatment of patients with these conditions. Further studies should address the reasons for these concerning outcomes in patients with primary systemic vasculitis who develop COVID-19.
Data sharing
Researchers interested in performing additional analysis from the COVID-19 Global Rheumatology Alliance provider registry are invited to submit proposals through the COVID-19 Global Rheumatology Alliance at https://rheum-covid.org. Data are currently available on reasonable request. For approved projects, after review by the COVID-19 Global Rheumatology Alliance steering committee, summary tables and data analyses will be provided as requested. Raw data is not available to other researchers.
Declaration of interests
SES reports funding from a Vasculitis Clinical Research Consortium (VCRC)–Vasculitis Foundation Fellowship (the VCRC is part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Science [NCATS], and is funded by a collaboration between NCATS and the National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS; U54 AR057319]). RC reports speaker's fees from Janssen, Roche, Sanofi, and Abbvie outside the submitted work. CH received funding under a sponsored research agreement from Vifor Pharmaceuticals, outside the submitted work. SLM has received consulting fees from AbbVie; consulting fees from AstraZeneca; other from Roche-Chugai; consulting fees from Sanofi; and non-financial support from Roche, all outside the submitted work; and is a patron of the patient charity PMRGCAuk. PM is a Medical Research Council-GlaxoSmithKline (MRC-GSK) EMINENT clinical training fellow, who has received project funding from this organisation, outside the submitted work; has received funding from the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre (UCLH BRC); reports grants from MRC-GSK; reports personal fees from Swedish Orphan Biovitrum and Lilly; and reports consultancy fees from Abbvie and Pfizer, all outside the submitted work. LN reports being a trustee of the charity PMR-GRA Scotland. JSA reports grants from the National Institute of Health (NIH) and NIAMS, the Rheumatology Research Foundation, the Brigham Research Institute, the R Bruce and Joan M Mickey Research Scholar Fund, and Amgen; grants and personal fees from Bristol-Myers Squibb; and personal fees from Gilead, Inova, Janssen, Optum, and Pfizer, all outside the submitted work. AD-G is supported by the US Centers for Disease Control and Prevention, the Rheumatology Research Foundation Scientist Development Award, the Robert D and Patricia E Kern Center for the Science of Health Care Delivery, and the Women's Health Career Enhancement Award outside the submitted work. KLH reports receiving speaker's fees from Abbvie; grant income from Bristol-Myers Squibb, UCB Pharma, and Pfizer, all outside the submitted work; and is supported by the NIHR Manchester Biomedical Research Centre outside the submitted work. RG reports non-financial support from Pfizer Australia and Janssen Australia; and personal fees from Pfizer Australia, Cornerstones, Janssen New Zealand, and Novartis, all outside the submitted work. UM-L is supported by grants from the German Ministry of Research and Education and the German Research Foundation outside the submitted work. MAG reports funding from the NIH and the NIAMS. PCR reports personal fees from Abbvie and Gilead; grants and personal fees from Janssen, Novartis, UCB Pharma, and Pfizer; non-financial support from Bristol-Myers Squibb and Pfizer; and personal fees from Lilly and Roche, all outside the submitted work. JY reports no competing interests related to this work; is supported by grants from NIH (K24 AR074534 and P30 AR070155); and reports consulting fees from Eli Lilly, Pfizer, Aurinia, and AstraZeneca, all outside the submitted work. PMM has received consulting or speaker's fees from Abbvie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB Pharma, all outside the submitted work (all <$10 000); and is supported by the NIHR UCLH BRC outside the submitted work. ES is a board member of the Canadian Arthritis Patient Alliance, which is a patient-run, volunteer-based organisation, whose activities are largely supported by independent grants from pharmaceutical companies. JWL reports grants from Pfizer, outside the submitted work. JSH reports no competing interests related to this work; is supported by grants from the Rheumatology Research Foundation; receives salary support from the Childhood Arthritis and Rheumatology Research Alliance; and reports consulting fees for Novartis, Swedish Orphan Biovitrum, and Biogen, all outside the submitted work (<$10 000). PS reports no competing interests related to this work, but reports receiving honorarium for editing social media for the American College of Rheumatology journals (<$10 000). SBh reports receiving non-branded consulting fees from AbbVie, Amgen, Horizon, Novartis, and Pfizer (<$10 000 from each)outside the submitted work. ZSW reports receiving grant support from Bristol-Myers Squibb and Principia-Sanofi; has consulted for Viela Bio and MedPace; and is supported by grants from the National Institutes of Health, all outside the submitted work. AS reports personal fees for lectures from AbbVie, Celltrion, Lilly, Merck Sharp & Dohme, Roche, Bristol-Myers Squibb, and Pfizer outside the submitted work. EFM has received grants from Abbvie, Novartis, Lilly Portugal, Amgen Biofarmacêutica, Grünenthal SA, Merck Sharp & Dohme, Medac, and A Menarini Portugal-Farmacêutica SA; grants and non-financial support from Pfizer; and non-financial support from Grünenthal, all outside the submitted work. LG reports research grants from Amgen, Galapagos, Janssen, Lilly, Pfizer, Sandoz, and Sanofi; consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Biogen, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis, and UCB Pharma, all outside the submitted work. LC declares no competing interests related to this study, but her institute works by contract for laboratories among other institutions, such as Abbvie Spain, Eisai, Gebro Pharma, Merck Sharp & Dohme España SA, Novartis Farmaceutica, Pfizer, Roche Farma, Sanofi Aventis, Astellas Pharma, Actelion Pharmaceuticals España, Grünenthal, and UCB Pharma. NJP reports grants from NIH during the conduct of the study. MU-G reports grants from Pfizer and Janssen, outside the submitted work. SBa reports grants and personal fees from Alexion Pharma, outside the submitted work. RV reports grants from Novartis, Pfizer, and Bristol-Myers Squibb, outside the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the American College of Rheumatology and the European League Against Rheumatism. The views expressed herein are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the UK National Health Service, the NIHR, the UK Department of Health, or any other organisation. Patient research partners (KB and LN) were involved in the design, conduct, reporting and interpretation of the results of this study. Patient partners have participated in the development of this manuscript and are listed as coauthors.
Contributors
SES, RC, MSP, AMS, MAG, KB, CH, DL, SLM, PM, LN, JY, ZSW, and PCR contributed to the study design and the original idea for the manuscript. SES, RC, MSP, AMS, MAG, and JY had full access to and verified the underlying study data, developed the figure and tables, and vouch for the data analyses. AMS and MAG did the statistical analysis and contributed to data quality control, data analysis, and data interpretation. SES, RC, MSP, AMS, MAG, KB, CH, DL, SLM, PM, LN, JY, ZSW, PCL, GG, MIHS, FNM, HAM, SAdSS, NCA, AK, DR, LQ, MSa, SBa, ATJM, PC, RH, UM-L, BFH, RV, RPT, ML, SLER, SA-E, JAS, TY-TH, KMD’S, NJP, LW, EG, MVA, MU-G, LJ, ZI, AS, EFM, KLH, LG, LC, SL-T, LK-F, ES, JSH, PS, SBh, JWL, and PMM contributed to data collection, data analysis, and data interpretation. SES, RC, MSP, JY, and PCR directed the study, data collection, data analysis, and interpretation of the methods, and had final responsibility for the decision to submit the publication. All authors contributed intellectual content during the drafting and revision of the manuscript and approved the final version to be published.
Contributor Information
Global Rheumatology Alliance:
Brahim Dahou, Eva Rath, Yves Piette, Mieke Devinck, Bea Maeyaert, Francinne Machado Ribeiro, Sandra Lucia Euzebio Ribeiro, Marcelo Pinheiro, Rosana Quintana, Gimena Gómez, Karen Roberts, Roberto Miguel Baez, Vanessa Castro Coello, María J. Haye Salinas, Federico Nicolas Maldonado, Alvaro Andres Reyes Torres, Gelsomina Alle, Romina Tanten, Hernán Maldonado Ficco, Romina Nieto, Carla Gobbi, Yohana Tissera, Cecilia Pisoni, Alba Paula, Juan Alejandro Albiero, Maria Marcela Schmid, Micaela Cosatti, Maria Julieta Gamba, Carlevaris Leandro, María Alejandra Cusa, Noelia German, Veronica Bellomio, Lorena Takashima, Mariana Pera, Karina Cogo, Maria Soledad Gálvez Elkin, María Alejandra Medina, Veronica Savio, Ivana Romina Rojas Tessel, Rodolfo Perez Alamino, Marina Laura Werner, Sofía Ornella, Luciana Casalla, Maria de la Vega, María Severina, Mercedes García, Luciana Gonzalez Lucero, Cecilia Romeo, Sebastián Moyano, Tatiana Barbich, Ana Bertoli, Andrea Baños, Sandra Petruzzelli, Carla Matellan, Silvana Conti, Ma. Alicia Lazaro, Gustavo Fabián Rodriguez Gil, Fabian Risueño, Maria Isabel Quaglia, Julia Scafati, Natalia Lili Cuchiaro, Jonathan Eliseo Rebak, Susana Isabel Pineda, María Elena Calvo, Eugenia Picco, Josefina Gallino Yanzi, Pablo Maid, Debora Guaglianone, Julieta Silvana Morbiducci, Sabrina Porta, Natalia Herscovich, José Luis Velasco Zamora, Boris Kisluk, Maria Sol Castaños Menescardi, Rosana Gallo, María Victoria Martire, Carla Maldini, Cecilia Goizueta, Sabrina Solange de la Vega Fernandez, Carolina Aeschlimann, Gisela Subils, Sebastián Ibáñez, Anne-Marie Chassin-Trubert, Lingli Dong, Lui Cajas, Marko Barešic, Branimir Anic, Melanie-Ivana Culo, Tea Ahel Pavelic, Kristina Kovacevic Stranski, Boris Karanovic, Jiri Vencovsky, Marta Píchová, Maria Filkova, Hesham Hamoud, Dimitrios Vassilopoulos, Gabriela Maria Guzman Melgar, Ho So, Márta Király, Mahdi Vojdanian, Alexandra Balbir-Gurman, Fatemah Abutiban, Julija Zepa, Inita Bulina, Loreta Bukauskiene, Beatriz Zaueta, Angel Alejandro Castillo Ortiz, Erick Zamora Tehozol, David Vega, Diana Cervántes Rosete, Eduardo Martín Nares, Tatiana Sofia Rodriguez-Reyna, Marina Rull Gabayet, Deshiré Alpízar-Rodríguez, Fedra Irazoque, Xochitl Jimenez, Lenny Geurts-van Bon, Theo Zijlstra, Monique Hoekstra, Nasra Al-Adhoubi, Babur Salim, Enrique Giraldo, Ariel Salinas, Manuel Ugarte-Gil, Jaroslaw Nowakowski, Samar Al-Emadi, Richard Conway, Rachael Flood, Geraldine McCarthy, Ioana Felea, Ileana Filipescu, Simona Rednic, Laura Groseanu, Maria Magdelena Tamas, Vanda Mlynarikova, Martina Skamlova, Martin Zlnay, Dagmar Miceková, Lubica Capova, Zelmira Macejova, Emoke Štenová, Helena Raffayova, Gabriela Belakova, Eva Strakova, Marieta Sencarová, Sona Žlnayová, Anna Anna Sabová, Daniela Spisakova, Mária Oetterová, Olga Lukacova, Martina Bakosova, Alojzija Hocevar, Natalia de la Torre-Rubio, Juan José Alegre Sancho, Montserrat Corteguera Coro, Juan Carlos Cobeta Garcia, Maria Carmen Torres Martin, Jose Campos, Jose A Gomez Puerta, Gozd Kubra Yardimci, Servet Akar, Ozan Cemal Icacan, Selda Çelik, Viktoriia Vasylets, Su-Ann Yeoh, Claire Vandevelde, Sasha Dunt, Jane Leeder, Elizabeth Macphie, Rosaria Salerno, Christine Graver, Katie Williams, Sheila O'Reilly, Kirsty Devine, Jennifer Tyler, Elizabeth Warner, James Pilcher, Samir Patel, Elena Nikiphorou, Laura Chadwick, Caroline Mulvaney Jones, Beverley Harrison, Lucy Thornton, Diana O'Kane, Lucia Fusi, Audrey Low, Sarah Horton, Shraddha Jatwani, Sara Baig, Hammad Bajwa, Vernon Berglund, Angela Dahle, Walter Dorman, Jody Hargrove, Maren Hilton, Nicholas Lebedoff, Susan Leonard, Jennifer Morgan, Emily Pfeifer, Archibald Skemp, Jeffrey Wilson, Anne Wolff, Eduardo Cepeda, Kristin D'Silva, Tiffany Hsu, Naomi Patel, Jeffrey Sparks, Derrick Todd, Zachary Wallace, Denise Hare, Cassandra Calabrese, Christopher Adams, Arezou Khosroshahi, Adam Kilian, Douglas White, Melanie Winter, Theodore Fields, Caroline Siegel, Nicole Daver, Melissa Harvey, Neil Kramer, Concetta Lamore, Suneya Hogarty, Karen Yeter, Leanna Wise, Faizah Siddique, Byung Ban, Tamar Tanner, Eric Ruderman, William Davis, Robert Quinet, Evangeline Scopelitis, Karen Toribio Toribio, Tameka Webb-Detiege, Jerald Zakem, Khurram Abbass, Gilbert Kepecs, Lilliam Miranda, Michael Guma, Ammar Haikal, Sushama Mody, Daric Mueller, Arundathi Jayatilleke, JoAnn Zell, Alison Bays, Kathryn Dao, Ezzati Fatemeh, Deborah Parks, David Karp, and Guillermo Quiceno
Supplementary Material
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schäfer M, Strangfeld A, Hyrich KL, et al. Response to: ‘correspondence on ‘factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician reported registry” by Mulhearn et al. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220134. published online March 1. [DOI] [PubMed] [Google Scholar]
- 5.Bonaventura A, Vecchié A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315:2442–2458. doi: 10.1001/jama.2016.5444. [DOI] [PubMed] [Google Scholar]
- 7.Gianfrancesco MA, Leykina LA, Izadi Z, et al. Association of race and ethnicity with COVID-19 outcomes in rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician registry. Arthritis Rheumatol. 2021;73:374–380. doi: 10.1002/art.41567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianfrancesco MA, Hyrich KL, Gossec L, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson PC, Yazdany J, Machado PM. Global research collaboration in a pandemic-challenges and opportunities: the COVID-19 Global Rheumatology Alliance. Curr Opin Rheumatol. 2021;33:111–116. doi: 10.1097/BOR.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorge A, D'Silva KM, Cohen A, et al. Temporal trends in severe COVID-19 outcomes in patients with rheumatic disease: a cohort study. Lancet Rheumatol. 2021;3:e131–e137. doi: 10.1016/S2665-9913(20)30422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutherford MA, Scott J, Karabayas M, et al. Risk factors for severe outcomes in patients with systemic vasculitis & COVID-19: a bi-national registry-based cohort study. Arthritis Rheumatol. 2021;73:1713–1719. doi: 10.1002/art.41728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ERA-EDTA Council. ERACODA Working Group Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94. doi: 10.1093/ndt/gfaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kant S, Morris A, Ravi S, et al. The impact of COVID-19 pandemic on patients with ANCA associated vasculitis. J Nephrol. 2021;34:185–190. doi: 10.1007/s40620-020-00881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 16.Buttgereit F, Matteson EL, Dejaco C, Dasgupta B. Prevention of glucocorticoid morbidity in giant cell arteritis. Rheumatol (Oxford) 2018;57(suppl 2):ii11–ii21. doi: 10.1093/rheumatology/kex459. [DOI] [PubMed] [Google Scholar]
- 17.Lai LYH, Harris E, West RM, Mackie SL. Association between glucocorticoid therapy and incidence of diabetes mellitus in polymyalgia rheumatica and giant cell arteritis: a systematic review and meta-analysis. RMD Open. 2018;4 doi: 10.1136/rmdopen-2017-000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, She Z-G, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068. doi: 10.1016/j.cmet.2020.04.021. 77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comarmond C, Leclercq M, Leroux G, et al. Correspondence on ‘impact of COVID-19 pandemic on patients with large-vessels vasculitis in Italy: a monocentric survey’. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-219407. published online Nov 12. [DOI] [PubMed] [Google Scholar]
- 20.Tomelleri A, Sartorelli S, Campochiaro C, Baldissera EM, Dagna L. Impact of COVID-19 pandemic on patients with large-vessel vasculitis in Italy: a monocentric survey. Ann Rheum Dis. 2020;79:1252–1253. doi: 10.1136/annrheumdis-2020-217600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinosa G, Araujo O, Amaro S, et al. COVID-19 and Behçet's disease: clinical case series. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217778. published online July 21. [DOI] [PubMed] [Google Scholar]
- 24.Yurttas B, Poyraz BC, Sut N, et al. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol Int. 2021;41:1105–1114. doi: 10.1007/s00296-021-04841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers interested in performing additional analysis from the COVID-19 Global Rheumatology Alliance provider registry are invited to submit proposals through the COVID-19 Global Rheumatology Alliance at https://rheum-covid.org. Data are currently available on reasonable request. For approved projects, after review by the COVID-19 Global Rheumatology Alliance steering committee, summary tables and data analyses will be provided as requested. Raw data is not available to other researchers.