ABSTRACT
Citrus plants are challenged by a broad diversity of abiotic and biotic stresses, which definitely alter their growth, development, and productivity. In order to survive the various stressful conditions, citrus plants relay on multi-layered adaptive strategies, among which is the accumulation of stress-associated metabolites that play vital and complex roles in citrus defensive responses. These metabolites included amino acids, organic acids, fatty acids, phytohormones, polyamines (PAs), and other secondary metabolites. However, the contribution of PAs pathways in citrus defense responses is poorly understood. In this review article, we will discuss the recent metabolic, genetic, and molecular evidence illustrating the potential roles of PAs in citrus defensive responses against biotic and abiotic stressors. We believe that PAs-based defensive role, against biotic and abiotic stress in citrus, is involving the interaction with other stress-associated metabolites, particularly phytohormones. The knowledge gained so far about PAs-based defensive responses in citrus underpins our need for further genetic manipulation of PAs biosynthetic genes to produce transgenic citrus plants with modulated PAs content that may enhance the tolerance of citrus plants against stressful conditions. In addition, it provides valuable information for the potential use of PAs or their synthetic analogs and their emergence as a promising approach to practical applications in citriculture to enhance stress tolerance in citrus plants.
KEYWORDS: Citrus, polyamines, putrescine, spermidine, spermine, biotic stress, abiotic stress, Xanthomonas citri, Xylella fastidiosa, Candidatus Liberibacter asiaticus
Introduction
In citrus, leaf metabolites play vital and complex roles in the defensive response against biotic and abiotic stress. These metabolites included amino acids,1–6 organic acids,1,3,6 fatty acids,2,6 phytohormones,7,8 polyamines (PAs)4,9–12 and other secondary metabolites. However, the contribution of non-proteinogenic amino acids (NPAAs) and PAs pathways in citrus defensive responses are poorly understood.
Polyamines (PAs) are ubiquitous organic compounds that are found in all living kingdoms from Prokaryote to Archaea and Eukaryotes, including plants.13–16 PAs are low molecular weight, aliphatic, polycationic nitrogen-containing compounds. PAs are containing unsaturated hydrocarbons that are occasionally branched with two or more primary amino groups.13,15,17–20 At physiological pH, PAs are positively-charged,21 therefore, they can bind to negatively-charged macromolecules, such as nucleic acids (DNA and RNA), acidic phospholipids and numerous types of proteins13 to stabilize their structure, particularly under stress conditions. The putrescine, spermidine, and spermine are the major ubiquitously found PAs in higher plants.22–24
In citrus, PAs are synthesized from L-arginine through two different routes (well-reviewed recently by Killiny and Nehela, 2020).25 The first route is where L-arginine is converted by arginaseto ornithine, which is then decarboxylated by ornithine decarboxylase (ODC) to form the diamine putrescine. The second route involving arginine decarboxylation by arginine decarboxylase (ADC) to produce agmatine, which catalyzes toputrescine via some catalytic steps. Once putrescine is produced, it is then converted into the tri-amine spermidine by spermidine synthase (SPDS), with the addition of an aminopropyl moiety donated by decarboxylated S-adenosylmethionine (dcSAM) which produced by the activity of S-adenosyl-methionine decarboxylase (SAMDC; aka AMD1). Subsequently, spermidine is converted into the tetra-amines spermine by the incorporation of another aminopropyl group (also from dcSAM) using spermine synthase (SPMS).
PAs are implicated in several biological and physiological processes for growth and development in both prokaryotes and eukaryotes.22,26 Furthermore, PAs modulate the plant response to various abiotic and biotic stress, including plant-microbe interactions.22 In citrus, these physiological processes include embryogenesis,27,28 root system architecture,29–32 shoot system architecture,33–35 flowering, fruit set, fruit development, and fruit quality,10,36-41 and stomatal closure, gas-exchange, photosynthesis and chlorophyll fluorescence.35 Furthermore, PAs might be involved in protecting the citrus plant from stress-associated damages and enhancing stress tolerance against biotic4,12,42–44 and abiotic stress.9–11,34,43,45–52 However, the molecular mechanisms behind these roles remain ambiguous.
Accordingly, in this review article, we discuss the potential roles of PAs in citrus defensive responses with an emphasis on the recent advances in response to abiotic and biotic stress. We are going to discuss the PAs-based defensive responses of citrus against environmental stressors including extreme temperatures (low and high), salinity, heavy metals, drought stress. As well as, we are going to discuss the recent metabolic, genetic, and molecular evidence illustrating the potential PAs-based defensive responses of citrus plants to biotic stress including virus and viroid infection, fungal and oomycetes infection, and bacterial infection, particularly, the vector-borne phytopathogenic bacteria. We believe that the knowledge gained so far about the roles of PAs in citrus responses underpin our understanding of PAs-based defensive responses and provide valuable information for the potential use of PAs or their synthetic analogs a promising approach to practical applications to enhance stress tolerance in citrus plants.
PAs-Based Defensive Mechanisms Against Abiotic Stress in Citrus
Role of PAs in citrus response(s) to low- and high-temperature
Citrus(family Rutaceae) is one of the most widely grown fruit crops worldwide,53 where it grows within 35°-40° north and south latitude.53,54 Citrus species are considered to be native to the tropical and subtropical geographical areas.53,54 Therefore, citrus trees are not generally chill hardy. Among the common citrus species, C. reticulata was reported to be the hardiest variety to frost. C. reticulata can tolerate the temperature as low as −10°C for a short period, however, realistically temperature above −2°C is required for successful gardening. Although the low, butnon-freezing temperature, may have some pros such as reducing disease severity, controlling insect pests, and/or modify ripening processes, it may also cause chilling injury (CI).55 Low-temperature tolerance in citrus plants is a highly dynamic stress-response phenomenon and has been reported to be associated with complex crosstalk between various biochemical and physiological processes.55 Among the biochemical defensive mechanisms to low-temperature stress, changes in PAs and their biosynthetic genes have given considerable attention as a key player against chilling because of their anti-senescence activity and antioxidant- and free-radical scavenging properties.56–58
Although many previous studies evaluated the changes in the endogenous content of major PAs compounds in citrus and its relative species as affected by low- and/or high-temperature stress (Table 1), very few studies were carried out on leaves tissue,9–11 while most of these studies were done on fruits.59–67 The data presented in Table 1 demonstrate a positivecorrelation between putrescine content and citrus tolerance to low- and/or high-temperature. Generally, putrescine was increased in most cases, except in the studies of Martínez-Romero et al. 1999 and Fu et al. 2016.11,65 For instance, putrescine was decreased when lemon (C. limon cv. Verna) fruits were stored at 2°C for 20 days after treatment with putrescine, calcium, or distilled water.65 Putrescine was decreased also when sweet orange (C. sinensis cv. Jincheng) seedlings were placed at high-temperature stress (45°C) for 3 hours.11
Table 1.
Changes in the endogenous content of major PAs compounds in citrus and its relative species as affected by low- and/or high-temperature stress.
| ‘Cultivar’/Variety/(species) | Tissue | Treatment | Put a | Spd | Spm | Reference |
|---|---|---|---|---|---|---|
| ‘Bearss’ Lemon (Citrus limon) | Flavedo | Fruits were stored at chilling temperatures (1°C) | ▲ | - | - | 59 |
| ‘Marsh’ Grapefruit (C. paradisi) | Flavedo | Fruits were stored at chilling temperatures (4.4°C) | ▲ | - | - | 59 |
| ‘Marsh’ Grapefruit (C. paradisi) | Juice | Fruits were stored at chilling temperatures (4.4°C) | ▲ | - | - | 59 |
| Sour Orange (C. aurantium) | Leaves | Plants were exposed for 3 weeks to cold stress (15.6°C day and 4.4°C night) | ▲ | ▲ | ▼ | 9 |
| ‘Valencia’ Sweet Orange (C. sinensis) | Leaves | Plants were exposed for 3 weeks to cold stress (15.6°C day and 4.4°C night) | ▲ | ▲ | ▲ | 9 |
| Rough Lemon (C. jambhiri) | Leaves | Plants were exposed for 3 weeks to cold stress (15.6°C day and 4.4°C night) | ● | ▲ | ▲ | 9 |
| ‘Washington’ Navel Orange (C. sinensis) | Leaves | Plants subjected to 4 weeks of low-temperature (10°C day and 7°C night) | ▲ | ▲ | ▼ | 10 |
| ‘Marsh’ Grapefruit (C. paradisi) | Flavedo | Fruits were stored at 2°C for 4 weeks ± hot water dips (53°C for 2–3 min) | ▲ | - | - | 60 |
| ‘Eureka’ Lemon (C. limon) | Flavedo | Fruits were stored at 2°C for 4 weeks ± hot water dips (53°C for 2–3 min) | ▲ | - | - | 60 |
| ‘Sweety’ Oroblanco (C. grandis × C. paradisi) | Flavedo | Fruits were stored at 2°C for 4 weeks ± hot water dips (53°C for 2–3 min) | ▲ | - | - | 60 |
| ‘Nagami’ Kumquat (Fortunella margarita) | Flavedo | Fruits were stored at 2°C for 4 weeks ± hot water dips (53°C for 2–3 min) | ▲ | - | - | 60 |
| ‘Tahitian’ Lime (C. latifolia) | Fruits | Fruits stored at 0°C | ↑↓ | - | - | 61 |
| ‘Emperor’ Mandarin (C. reticulata) | Fruits | Fruits stored at 0°C | ↑↓ | - | - | 61 |
| ‘Marsh’ Grapefruit (C. paradisi) | Fruits | Fruits stored at 0°C | ↑↓ | - | - | 61 |
| ‘Valencia’ Sweet Orange (C. sinensis) | Fruits | Fruits stored at 0°C | ↑↓ | - | - | 61 |
| `Fortune’ Mandarin (C. reticulata) | Flavedo | Fruits were stored for 45 days at 2°C | ▲ | ▲ | ● | 62 |
| `Fortune’ Mandarin (C. reticulata) | Flavedo | Fruits conditioned for 1, 2, and 3 days at 37°C and 90-95% RH | ▲ | ▲ | ▲ | 63 |
| `Fortune’ Mandarin (C. reticulata) | Flavedo | Fruits stored at 2°C for 28 days | ▲ | ↑↓ | ↑↓ | 63 |
| `Fortune’ Mandarin (C. reticulata) | Flavedo | Fruits submitted to postharvest hot water dips at 44, 47, 50, 53, 56, and 59°C | ▲ | ▲ | ▲ | 64 |
| `Verna’ Lemon (C. limon) | Fruits | Fruits stored at 2°C for 20 days after treatment with putrescine, Ca, or water | ▼ | ▼ | ↑↓ | 65 |
| `Fortune’ Mandarin (C. reticulata) | Fruits | Heat-conditioning treatment (HCT) at 37°C with air | ▲ | ▲ | ▲ | 66 |
| `Fortune’ Mandarin (C. reticulata) | Fruits | HCT at 37°C, followed by 90 days storage at 2°C | ▲ | ▼ | ▼ | 66 |
| `Fortune’ Mandarin (C. reticulata) | Fruits | HCT at 37°C, then 90 days storage at 2°C, then recovering for 7 days at 20°C | ▲ | ▲ | ▲ | 66 |
| `Verna’ Lemon (C. limon) | Fruits | Fruits dipped in hot water (45°C) for 10 min, then stored at 15°C for 3 weeks | ▲ | ▲ | ● | 67 |
| Sour orange (C. aurantium) | Leaves | Plants were kept at 6 ± 1°C for 6 days. Samples were taken 5 and 24 hpt. | ▲ | ▲ | ▼ | 68 |
| `Jincheng’ Sweet Orange (C. sinensis) | Leaves | Seedlings were placed for 3 hours for high-temperature stress (45°C) | ▼ | ▼ | ▲ | 11 |
a(▲) signifies for increased levels, (▼) signifies for decreased levels, (●) signifies for no change, (↑↓) signifies for a varied response(s) based on the time of exposure,61 ripening stage of the fruit,63 and (˗) signifies for not-reported compounds. Abbreviations: Put: putrescine, Spd: spermidine, and Spm: spermine.
Unlike putrescine, spermidine and spermine are less reported in citrus and its relatives and both have been shown a varied response(s) to low- and high-temperatures based on the time of exposure61 and ripening stage of the fruit.63 For example, spermidine was increased in the leaves of sour orange (C. aurantium), sweet orange (C. sinensis cv. Valencia),andRough Lemon (C. jambhiri) that were exposed for three weeks to low-temperature stress (15.6°C and 4.4°C at daytime and night, respectively)9 and Navel Orange (C. sinensis cv. Washington) plants subjected to four weeks of low-temperature stress (10°C and 7 °C at daytime and night, respectively).10 Likewise, spermidine was increased in fruits (Flavedo) of Fortune’ Mandarin (C. reticulata)62–64,66 and `Verna’ Lemon (C. limon)67 after different low- and high-temperature treatments. By contrast, spermidine was decreased in `Verna’ Lemon (C. limon) fruits when stored at 2°C for 20 days after treatment with putrescine, calcium, or distilled water65 and `Fortune’ Mandarin (C. reticulata) fruits when stored at 2°C for 90 days after heat-conditioning treatment at 37°C.66 Similarly, `Jincheng’ Sweet Orange (C. sinensis) leaves had lower levels ofspermidine when seedlings were placed for three hours at 45°C.11
Spermine, regardless of being the minor PA, showed a varied response(s) based on the temperature, plant species,9,66 time of exposure,61 ripening stage of the fruit.63 For example, spermine was increased in fruits of `Fortune’ mandarin (C. reticulata) when they conditioned heat-conditioning treatment (HCT) at 37°C with air63,66 or submitted to postharvest hot water dips between 44–59°C.64 Spermine accumulated in leaves of `Jincheng’ sweet orange (C. sinensis) seedlings when placed at high-temperature (45°C) for 3 hours11 and in leaves of ‘Valencia’ sweet orange (C. sinensis) and rough lemon (C. jambhiri) when Plants exposure for 3 weeks to cold stress (15.6°C and 4.4°C at daytime and night, respectively).9 On the other hand, spermine content was reduced in leaves of sour orange (C. aurantium) and ‘Washington’ navel orange (C. sinensis) when plants exposed to low-temperature stress (10–15.6°C and 4.4–7 °C at daytime and night, respectively) for 3–4 weeks.9,10 Spermine also decreased in `Fortune’ mandarin (C. reticulata) when fruits conditioned with HCT at 37°C, followed by 90 days of storage at 2°C.66 Interestingly, the endogenous spermine content fluctuated in the fruits of `Fortune’ mandarin (C. reticulata) and `Verna’ lemon (C. limon) when they stored at 2°C for 20–28 days63,65
As we mentioned above, because citrus species are native to tropical and subtropical areas, most of the citrus cultivars are susceptible to cold temperatures. Therefore, many previous studies have been carried out to clone and characterize low-temperature stress-responsive genes from citrus and its relative species (Table 2). Although more than 25 genes were cloned successfully from citrus, very few genes have been reported to be involved in PAs-based defensive mechanisms against low-temperature stress.79,82,88,90 Among the low temperature-responsive genes from citrus, cold-responsive (COR; aka cold-regulated) genes were studied intensively. Most of COR genes are a member of closely related dehydrins (DHNs; late embryogenesis abundant [LEA] protein) from citrus that could potentially contribute to cold tolerance.56,69,70,72,74,75,94
Table 2.
Low-temperature stress-responsive genes that have been cloned from citrus or its relatives.
| Genes | GenBank accession No.a | ‘Cultivar’/Variety/(species) | References |
|---|---|---|---|
| COR19 | L39005 | Trifoliate Orange (Poncirus trifoliata) | 69 |
| CuCOR19 | AB016809 | ‘Satsuma’ Mandarin (Citrus unshiu) | 70 |
| FPAL1 | AJ238753 | ‘Fortune’ Mandarin (C. clementina × C. reticulata) | 71 |
| FPAL2 | AJ238754 | ‘Fortune’ Mandarin (C. clementina × C. reticulata) | 71 |
| COR15 | AY032975 | ‘Marsh Seedless’ Grapefruit (C. paradisi) | 72 |
| CLT (CLTa,CLTb) | AY316308 | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 73 |
| CsDHN | AY297793 | Navel Orange (C. sinensis) | 74 |
| CpDHN | AY160772 | ‘Star Ruby’ Grapefruit (C. paradisi) | 74 |
| CrCOR15 | AY327515.1 | ‘Fortune’ Mandarin (C. clementina × C. reticulata) | 75 |
| CuCOR15 | AB178479 | ‘Satsuma’ Mandarin (C. unshiu) | 56 |
| PtrPII‐C02 | DQ158860 | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 76 |
| PI-B05 | - | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 77 |
| PI-C10 | - | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 77 |
| HOS1 | FJ844367 | Trifoliate Orange (P. trifoliata) | 78 |
| CsSAMDC | FJ496345 | ‘Newhall’ Navel orange (C. sinensis) | 79 |
| CsNAC1 | CX306190 | ‘Cleopatra’ Mandarin (C. reshni) | 80 |
| LOS2 | GQ144341 | Trifoliate Orange (P. trifoliata) | 81 |
| PtADC | HQ008237 | Trifoliate Orange (P. trifoliata) | 82 |
| PtCBF1 | DQ790888.1 | Trifoliate Orange (P. trifoliata) | 83 |
| PtrWRKY2 | JX236033.2 | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 84 |
| PtrbHLH | JX512645 | Trifoliate Orange (P. trifoliata) | 85 |
| PtrWRKY1 | CX065984 | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 86 |
| R2R3MYB | Multiple genes | Sweet orange (C. sinesis | 87 |
| R2R3MYB | Multiple genes | Clementine (C. clementina) | 87 |
| PtrICE1 | KJ812152 | Trifoliate Orange (P. trifoliata) | 88 |
| CsCBF | FJ861084.1 | sweet orange (C. sinensis) | 89 |
| Ptr-miR396b | - | Trifoliate Orange (P. trifoliata) | 90 |
| p35 anti-apoptotic b | - | From Autographa californica moth Nuclear polyhedrosis virus (AcMNPV) expressed in ‘Carrizo’ citrange (C. sinensis × P. trifoliata) | 91,92 |
| CsbHLH18 | - | sweet orange (C. sinensis) | 93 |
a(˗) Signifies for not-reported accession numbers.
For instance, three COR15 genes were cloned and identified including CpCOR15(GenBank accession No. AY032975) from ‘Marsh seedless’ grapefruit (C. paradisi),72 CrCOR15(GenBank Accession No. AY327515.1) from ‘Fortune’ mandarin(C. clementina × C. reticulata),75 and CuCOR15(GenBank accession No. AB178479) from ‘Satsuma’ mandarin (C. unshiu).56 COR15, which is almost completely identical to other reported citrus DHNs proteins, might protect stromal proteins from aggregation under freeze stress conditions via binding metals using a specific sequence containing histidine.56 In addition, Two COR19 were cloned from citrus plants including, PtrCOR19 (GenBank accession No. L39005) from trifoliate orange (P. trifoliata)69 and CuCOR19 (GenBank accession No. AB016809) from ‘Satsuma’ mandarin (C. unshiu).70 Both are DHNs protein and could be orthologues of CsLEA65 with histidine-rich domains.69,70 Additionally, CuCOR15 and CuCOR19 from C. unshiu contain a relatively considerable amount of histidine, arginine, and other reactive amino acid residues on the surface of their folded molecules and exhibited a ROS-scavenging activity and metal ion binding properties.56,70 Moreover, two DHNs were cloned including CsDHN from navel orange (C. sinensis)and CpDHN from ‘star ruby’ grapefruit (C. paradisi) (GenBank accession No. AY297793 and AY160772, respectively).74 Interestingly, CpDHN mRNA levels were up-regulated in the fruit peel tissue when fruits exposed to short hot‐water treatment followed by storage at chilling temperatures.74 These findings suggest that DHNs might play a molecular regulatory role in heat‐induced chilling tolerance in citrus plants.
Unfortunately, the interaction between COR/DHNs genes and PAs in citrus is poorly studied and, to our knowledge, it is not reported yet. However, PAs were reported to be associated with DHN genes to regulate tolerance to water stress in other plant species such as white clover (Trifolium repens). Exogenously applied spermidine (20 μM) to detached leaves of T. repens significantly increased the transcript levels of three DHNs genes including, Dehydrin (Y2SK), DHN1 (Y2 K), and Dhn b (SK2) at different times.95 Nevertheless, further studies are required to explore the relationship between COR/DHNs genes and PAs in citrus plants.
In addition to CORs/DHNs genes, two full-length cDNA clones of L-phenylalanine ammonia-lyase (PAL) were isolated from Fortune mandarin (C. clementina × C. reticulata) and described as FPAL1 and FPAL2 (GenBank accession No. AJ238753 and AJ238754, respectively).71 PAL is generally recognized as a marker of environmental stress in different plant tissues and could be involved in PAs-based defensive response of citrus fruits and other organs to cold temperatures. Interestingly exogenous PAs application significantly enhanced the PAL activity in Scots pines (Pinns sylvestris L.) callus in vitro.96 On the other hand, inhibition of PAL activity in alfalfa (Medicago sativa) cell suspension culture let to a remarkable reduction in endogenous PAs content.97 Taken together, these findings demonstrated the potential role of FPAL1 and FPAL2 in citrus responses to low-temperature stress.
Furthermore, several low temperature-responsive genes were cloned and identified from trifoliate orange (P. trifoliata), an extremely cold hardy citrus rootstock. These genes including citrus low-temperature (PtrCLT; GenBank accession No. AY316308) gene with two transcripts, called CLTa and CLTb,73 PtrPII‐C02 (a really interesting gene- [RING‐] Zn finger gene; GenBank accession No. DQ158860),76 PtrPI-B05 (containing an apetala2/ethylene response factor (AP2/ERF) domain and showed homology with ERF proteins from other plants)and PtrPI-C10 (containing both AP2/ERF and B3 DNA binding domains),77 high expression of osmotically responsive genes 1 (PtrHOS1;GenBank Accession No. FJ844367),78 Low expression of osmotically responsive genes 2 (PtrLOS2; GenBank accession No. GQ144341),81 C-repeat/dehydration-responsive elementbinding factor (PtCBF1; GenBank accession No. DQ790888.1),83 and two WRKY transcription factors described as PtrWRKY1 and PtrWRKY2(GenBank accession No. CX065984 and JX236033.2, respectively)84,86, Interestingly, CBFs bind CRT/DRE motifs that exist in the promoters of numerous COR genes to activate gene transcription98 and might interact with Inducer of CBF Expression 1 (ICE1) gene.88
Likewise, several low temperature-responsive genes were cloned and identified from other citrus species including CsNAC1transcription factor (GenBank accession No. CX306190) from C. reshni,80 multiple R2R3MYBgenes from C. sinesis and C. clementina,87 CsCBF (GenBank accession No. FJ861084.1) from C. sinensis,89 and the basic helix–loop–helix (CsbHLH18) transcription factor from C. sinensis.93 Some of these genes might play a key regulatory role in PAs-based defensive response(s) of citrus plants against chilling, however, more studies are required to clarify this role.
Wang et al. (2010 and 2011) cloned two genes involved in PAs biosynthesis includingCsSAMDC (GenBank Accession No. FJ496345) from navel orange (C. sinensis)79 andPtADC (GenBank accession No. HQ008237) from trifoliate orange(P. trifoliata).82 CsSAMDC is a rate-limiting enzyme for PAs biosynthesis and the temporal alteration in its transcript levels may be part of the PAs-based defensive response(s) of citrus plants since its mRNA levels varied in different citrus genotypes with variable levels of tolerance to salinity or to low-temperature stress.79 In addition, SAMDC (aka AMD1) is a key enzyme that connects both ethylene biosynthesis and PAs biosynthesis pathways. Ethylene plays diverse roles in abiotic stress tolerance,99 however, the molecular mechanisms behind ethylene-SAMDC-PAs defensive role(s) remain poorly understood. Likewise, PtADC is a key enzyme responsible for PAs biosynthesis under stress conditions, which might be involved in the citrus response to low temperature, since its transcript levels were up-regulated by low temperature.82 Moreover, the PtADC overexpressing mutant of A. thaliana (adc1-1) had a higher PAs content, particularly putrescine, and showed enhanced resistance to several abiotic stress types such as dehydration, drought, and cold stress.82 In addition, the adc1-1 transgenic line accumulated less ROS under different stresses but exhibited higher ROS scavenging capacity.82 Interestingly, the ROS scavenging capacity ofthe adc1-1transgenic line was reduced significantly when plants were treated with D-arginine (an ADC inhibitor) prior to stress treatment.82 Taken together, these findings prove that both genes (SAMDC and ADC) are involved in PAs-based defensive responses against low-temperature stress, however, their exact role remains ambiguous and required more studies.
Furthermore, two ICE homologs have been isolated and cloned from trifoliate orange (P. trifoliata) plants including, PtrbHLH (GenBank accession No. JX512645)85 and PtrICE1 (GenBank accession No. KJ812152).88 Both homologs were up-regulated upon cold stress. Overexpression of PtrbHLH or PtrICE1 in tobacco (N. tabacum) or lemon (C. limon) resulted in enhanced tolerance to cold stresses at either chilling (around 4°C) or freezing (0 °C) temperatures85,88 but through two different mechanisms. PtrbHLH is a cold-responsive transcription factor that enhances cold tolerance in citrus plants via activating the POD-mediated and ROS scavenging of hydrogen peroxide (H2O2), 85 while PtrICE1 encodes an MYC-like bHLHtranscription factor that functions as a central regulator of PAs-based defensive response against low-temperature via modulating endogenous PAs levels through interacting with PtrADC.88 Yeast two-hybrid screening revealed that PtrADC was confirmed as a bona fide protein interacting with PtrICE1. Furthermore, overexpression of PtrICE1 in tobacco (N. tabacum) and lemon (C. limon) plants exhibited slightly higher transcript levels of ADC under normal growth condition but significantly induced upon cold stress, consistent with changes in endogenous PAs content, which resulted in enhanced tolerance to cold stresses at either chilling (4°C) or freezing (0°C) temperatures.88
Taken together, these findings suggest that ICE1–CBF–COR signaling cascade plays a critical role in mediating PAs-based defensive responses against low-temperature stress. ICEs functions as a master regulator of this cascade via controlling the expression of CBFs, which sequentially regulate the downstream cold-responsive genes.100,101 For example, in A. thaliana, ICE homologs (AtICE2, and AtICE1) were reported to be involved in cold tolerance via modulation of CBF1 transcript levels.102 Later, and during cold acclimation, CBFs activate COR genes and resulting in the acquisition of low-temperature tolerance.98,103 Furthermore, ICE1 acts as a central regulator of PAs-based defensive response against low-temperature via modulating endogenous PAs content through interacting with PtrADC,88 which adds a new avenue for the ICE1–CBF–COR signaling cascade. However, the complete role of ICE1 in citrus plants is not fully understood, and many other ICE1-based mechanisms remain unexplored. For instance, the ICE1-mediated cold response appeared to be positively regulated by the phytohormone jasmonate (JAs) in Arabidopsis plants via regulation of the ICE-CBF/DREB1 transcriptional cascade,104 though, this role is not studied in citrus yet. Recently, Geng and Liu (2018) showed that the transcription factor CsbHLH18is involved in cold tolerance and ROS homeostasis via the regulation of antioxidant enzymes of C. sinensis.93 Interestingly, CsbHLH18is a potential ICE homolog, thatis localized in the nuclei and has transcriptional activation activity.93 Furthermore, CsbHLH18 directly and specifically binds to the promoter of CsPOD and activates it.85
In addition to all cloned genes, several cold-responsive microRNAs (miRNA) have been computationally identified, however, the functions of most of them are poorly explored. Zhang et al. (2016) investigated the function of miRNA396b, a cold-responsive miRNA, from trifoliate orange (Ptr-miR396b) in citrus response to cold stress and its potential regulatory role in ethylene–PAs homeostasis.90 Overexpression of Ptr-MIR396b, the precursor of Ptr-miR396b, in lemon (C. limon) plants showed higher endogenous free PAs content (putrescine, spermidine, and spermine) and their biosynthetic genes (SAMDC, SPDS, and SPMS), but lower transcript levels of ACO and ethylene content than wildtype plants under cold stress.90 These findings suggest that Ptr-miR396bplay a key role in citrus response to cold stress via regulating ACO and modulating ethylene-PAs homeostasis90
In addition, previous studies provide strong evidence that PAs confers tolerance to low-temperature stressvia modulation of the antioxidative capacity of citrus plants.56,58,88,90,105 Early this century, Sala and Lafuente (2000) showed that catalase (CAT) enzyme activity was associated with greater chilling tolerance in ‘Fortune’ mandarins when the fruits conditioned for 3 days at 37°C with air, then stored at 2°C. Moreover, exogenous treatment with 3-amino-1,2,4-triazole (AT; a chemical inhibitor of CAT activity) reduced the enzymatic activity of CAT in the AT-treated fruits, but not ascorbate peroxidase (APX), glutathione reductase (GR) and superoxide dismutase (SOD). The reduction of CAT activity was associated with greater peel damage in ‘Fortune’ mandarins and in the chilling-tolerant ‘Clementine’ and ‘Clemenules’ cultivars stored at 2°C but not at 12°C.105 Recently, it was reported that the foliar application of PAs enhanced the CAT activity in Bakraii citrus.35 Likewise, exogenous spermine treatment significantly increased the activities of CAT, SOD, and peroxidase enzymes, but reduced both H2O2 and superoxide (O2−) in spermine-pretreated trifoliate orange seedlings when exposed to both drought and high-temperature (45°C).58
Furthermore, the adc1-1 mutant of A. thaliana (a PtADC-overexpressing mutant) exhibited higher PAs content, accompanied by higher ROS scavenging capacity and reduced ROS accumulation in cold-stressed plants, which was reversed when the transgenic lines were treated with the ADC inhibitors.82 Additionally, the overexpression of PtrICE1 that encodes a MYC-like bHLH transcription factor in tobacco (N. tabacum) or lemon (C. limon) showed higher PAs content, up-regulated PtADC transcript levels, and higher activities of antioxidant enzymes (SOD and CAT), but appreciably alleviated the accumulation of ROS (H2O2 and O2–) in the transgenic lines under cold conditions.88 Likewise, overexpression of CsbHLH18 in the same model plants resulted in peroxidase (POD) activity, but significantly less ROS in the transgenic lines after cold treatment.85 On the other hand, knockdown of bHLH18 by RNAi in P. trifoliata conferred lower POD activity, but higher H2O2 accumulation accompanied by enhanced cold susceptibility while exogenous POD supplementation improved cold tolerance of the RNAi line which was inversely correlated with the H2O2 levels.85 Interestingly, CsbHLH18 directly and specifically binds to the promoter of CsPOD and activates it.85 Similarly, overexpression of Ptr-MIR396b, the precursor of Ptr-miR396b, in C. limon plants showed reduced ROS accumulation consistent with higher PAs content and up-regulation of their biosynthetic genes and positively regulates cold tolerance via reducing the expression of ACO and suppressing ethylene biosynthesis.90
A previous model has been proposed for the integration of PAs biosynthesis with ABA-dependent and ABA-independent signaling pathways in the cold acclimation response.98 According to Alcázar’s model, cold stress increases the transcript levels of ADC1, causing significant induction in PAs biosynthesis, particularly putrescine. The high endogenous putrescine content might initiate the ABA-dependent signaling pathway via inducing 9-cis-epoxycarotenoid dioxygenase (NCED3) resulting in ABA accumulation, which, subsequently, activate the abscisic acid-responsive element (ABRE)-COR. Later, the activation of the ABA-dependent signaling pathway resulting in a cold acclimation response after cold treatment.98 Interestingly, exogenous ABA treatment induces ADC2 transcription in an ABA-dependent manner.14 Additionally, cold stress could activate the ABA-independent signaling pathway via inducing the C-repeat (CRT)/dehydration-responsive element (DRE)-binding factors (CBF/DREB1) proteins using ICE1. CBFs bind CRT/DRE motifs in the promoters of COR genes to activate gene transcription and play a key role in cold acclimation.98 The induction of ABA-responsive element (ABRE) binding factor and NCED3 (a major ABA-biosynthetic gene) in spermine-treated trifoliate orange seedlings58 support the idea that PAs are integrated with ABA to establish citrus tolerance against low- and high-temperature. However, the PAs-based defensive mechanisms against low-temperature stress are more complicated than PAs-ABA interaction.
Therefore, we proposed a new hypothetical model for the PAs-based defensive mechanisms against low-temperature (Figure 1). In addition to the integration of PAs biosynthesis with ABA-dependent and ABA-independent signaling pathways suggested byAlcázar et al. 2011,98 we suggest that citrus plants develop a multi-layered defensive system to protect themselves. The defense mechanisms include PAs, phytohormones (such as ethylene, ABA, and JAs), accumulation of low-temperature-responsive genes, alteration of the plant signaling system, induction of some miRNA, and modulation of ROS scavenging capacity by regulating the activities of antioxidant enzymes.
Figure 1.
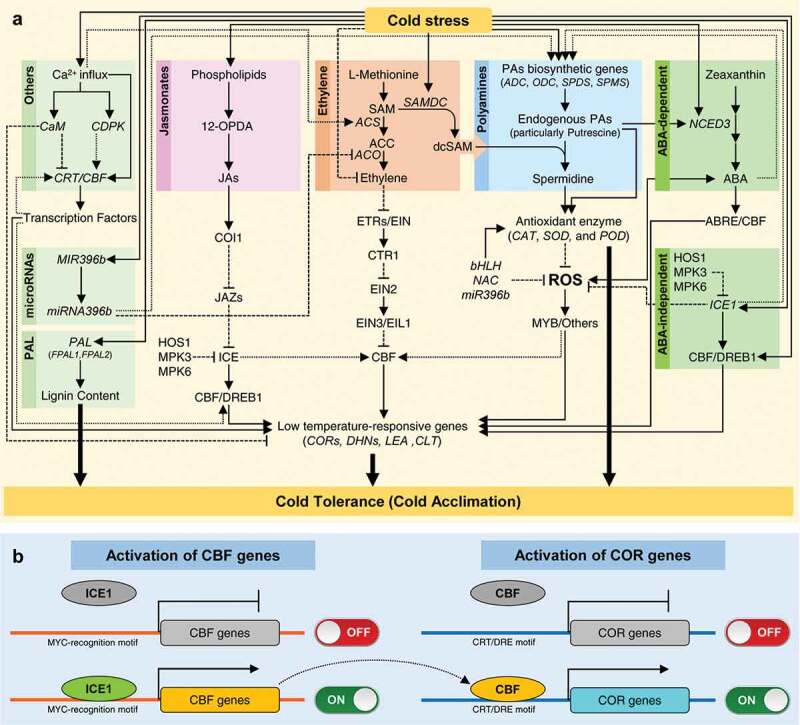
Hypothetical model of the potential polyamines- (PAs-) based responses of citrus plants to cold stress. (a) Integration of PAs with other metabolic pathways in cold acclimation of citrus plants, and (b) Activation of C-repeat/dehydration-responsive element binding factor (CBF) and cold-responsive (COR; aka cold-regulated) genes. Solid lines with arrowhead signify positive reaction, dashed lines with blunt-heads indicate negative interaction, while round-dotted lines with arrows represent hypothetical mechanisms or uncharacterized elements. For more details and abbreviations, see the main text.
According to our model, cold stress induces the PAs’ biosynthetic genes (particularly ADC), causing a significant increase in endogenous PAs content, particularly putrescine. The high endogenous putrescine level directly regulating the antioxidant enzymes,93 leading to enhanced ROS scavenging (Figure 1(a)). Additionally, endogenous PAs levels could be modulated by ICE1 via interacting with ADC.88 Interestingly, ICEs play a key regulatory role in the activation of CBFs (Figure 1(b)) via binding to the MYC-recognition motif in the CBF promoter.100,101,106 Sequentially, CBFs activate the transcription of COR genes through binding to CRT/DRE motifs that exist in the gene promoter(Figure 1(b)).98 Interestingly, the expressions of ICEs, particularly ICE1, are negatively regulated by HOS1-E3 ubiquitin ligase106 or mitogen-activated protein kinase (MKK4/5-MPK3/6) cascade.107 HOS1 ubiquitinates the ICE1, while MPK3 and MPK6 can phosphorylate ICE1, which sequentially promotes the degradation of ICE1.106,107
Furthermore, the high endogenous PAs content might initiate both ABA-dependent and ABA-independent signaling pathway via inducing NCED3 and ICE1, respectively (as described by Alcázar et al., 2011). ABA-dependent signaling pathway enhances the accumulation of ABA and activation of ABRE-CBF, whilethe ABA-independent signaling pathway induces the activation of CBF/DREB1.98 Both pathways resulting in a cold acclimation response via regulating the transcription of COR genes. In addition to ABA pathways, other phytohormones (such as ethylene and JAs) might be involved in PAs-based defensive mechanisms against low-temperature. Briefly, JAs regulate the ICE-CBF/DREB1 transcriptional cascade104 via binding to F-box protein coronatine-insensitive 1 (COI1), while the ethylene pathway supports the PAs biosynthesis via SAMDC (aka AMD1).79 SAMDC is a stress-responsive gene that its transcript levels varied in different citrus genotypes with variable levels of tolerance to salinity or to low-temperature stress79 and highly expressed at the initial stages of either stress treatment in tolerant genotypes.
Moreover, miRNA (such as Ptr-miR396b) might be involved in PAs-based defensive mechanisms against low-temperature. Ptr-miR396b plays a regulatory role in ethylene–PAs homeostasis via regulating the expression of ACO gene.90 Ptr-miR396b negatively regulates the transcript levels of ACO, causing lower ethylene content, but positively regulates the PAs-biosynthetic genes (SAMDC, SPDS, and SPMS), resulting in higher endogenous free PAs content (putrescine, spermidine, and spermine).90
Several previous studies should that role of PAs against low-temperature involve the activation ofCa2+influx108–111 and induction of PAL enzymes.71,96,97 For example, cytosolic Ca2+ levels were increased within seconds of a cold shock in tobacco,108 which was associated with the transcript levels of COR gene.110,111 Plants possess several sensors for the cold-induced Ca2+signature, including calcium-modulated protein (CaM, aka calmodulin) and Ca2+–dependent protein kinase (CDPK).111 Calmodulin is a negative regulator of COR genes,112 while CDPK is a positive regulator of COR genes and cold tolerance in plants.109 Furthermore, PAL could be involved in the citrus response to cold stress71 via modulating lignin biosynthesis113 and/or endogenous PAs contents. The positive correlation between PAL and PAs content was reported in other plant species such as P. sylvestris96 and M. sativa.97
Taken together, citrus plants develop a PAs-based multi-layered defensive system to protect themselves against low-temperature stress. This defensive system requires the orchestration of biochemical, metabolic, transcriptional, and physiological modifications to establish the cold acclimation. Mainly, this defensive system relay on the modulation of ROS homeostasis via the enhancement of antioxidant defense directly and/or the induction of the low temperature-responsive gene expressions. Many genes and transcription factors are implicated in the regulation of ROS homeostasis such as CORs,56,69,70 CsNAC1,80 PtrbHLH,85 PtrICE1,88 CsbHLH18,93 and the miRNA Ptr-miR396b.90 However, the molecular mechanism(s) underlying the PAs-based defensive system against low-temperature is still limited and more investigations are required to gain a better understanding of its regulatory mechanisms.
Role of PAs in citrus response(s) to salinity
More than one-third of irrigated soils worldwide are affected by salt stress (aka salinity).114,115 Salinity is one of the most important environmental factors that threating the growth of many crop plants and reducing their productivity.116,117 Citrus and most of their relative species have been reported previously as salt-sensitive plants.118–122 However, citrus rootstocks are varied in their salinity tolerance,115 and their relative tolerance could be affected by climate conditions, fertilization and nutrition, soil type, irrigation methods, and the genetic background of the rootstock itself.115,122 In citrus, salinity causes various physiological and biochemical alterations due to the concentrations of chloride anion (Cl−) and sodium cation (Na+) in leaves and other plant organs.121 In addition, soil salinization reduces citrus growth, decreases the fruit yield and quality,123 causes nutrient deficiencies or imbalances,115 and impairs photosynthetic activity.124 In addition, salt stress led to a significant reduction in the gas-exchange and chlorophyll fluorescencecharacteristicsin Bakraii (C. reticulata × C. limetta) seedlings including relative chlorophyll contents, chlorophyll fluorescence yields (Fv/Fm), net photosynthetic and respiration rates,51 net photosynthetic rate (PN), stomatal conductance (gs), and intercellular CO2 concentration (Ci).35 Nevertheless, exogenous application of different PAs, regardlessthe concentrations, reversed the negative effects of salinity and enhanced the overall growth of citrus plants.34,35,47,124–126 Moreover, the PAs-treated citrus plants maintained higher gas-exchange and chlorophyll fluorescencecharacteristics when exposed to salt stress.35
Generally, most of the previous studies showed that putrescine and spermidine fluctuated and showed a varied response to salt stress (Table 3).34,45–47,49-52 The varied effects of salinity on the putrescine and spermidine content could be due to the variation in soil type, application method, and the rootstocks. For example, in non-stressful conditions, the salt-tolerant rootstock ‘Cleopatra’ mandarin (C. reshni) had high spermidine and spermine but lower putrescine compared with the salt-sensitive cultivar ‘Troyer’ citrange (P. trifoliata × C. sinensis).49 However, under salinity conditions, putrescine was increased and spermidine and spermine were decreased in salinity-stressed plants.49,50 On the other hand, in most cases, spermine was induced to higher levels after salt stress (Table 3), 34,45–47,51,52 which indicates that the PAs-based defensive mechanisms against salinity stress in citrus mainly depends on spermine, but not other PAs compounds. The PAs induction has been suggested to be a metabolic response to cytoplasm acidification due to anion/cation imbalance caused by salt stress.52 Another point of view for the PAs induction was supported by the ROS-scavenging activities of PAs which can help the stressed plants to overcome the stress-activated physiological damage.52 In addition, the phytohormones ABA, ethylene, and ethylene precursor (ACC; 1-Aminocyclopropane-1-carboxylic acid) were increased after salt stress.46,49,50,127 For instance, the salt-sensitive cultivar ‘Troyer’ citrange (P. trifoliata × C. sinensis) produced ethylene and its precursor (ACC) at a higher rate than the chloride-tolerant rootstock `Cleopatra’ mandarin (C. reshni).50 Taken together, these findings indicate that PAs-based defensive mechanisms against salt stress could be associated with the phytohormones-based defensive system, however, the molecular mechanisms behind this correlation are poorly studied in citrus plants.
Table 3.
Changes in the endogenous content of major PAs, proline (Pro), abscisic acid (ABA), ethylene (ET), and 1-aminocyclopropane-1-carboxylic acid (ACC; ethylene precursor) in citrus and its relative species as affected by salinity/chloride stress.
| ‘Cultivar’/Variety/(species) | Tissue | Treatment | Put a | Spd | Spm | ET | ACC | ABA | Pro | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Cleopatra’ mandarin (Citrus reshni) | leaves | The plants were irrigated with nutrient solutions containing 2, 16, or 48 mM chloride (w/o nitrate) fora 3-month experimental period. | ▲ | ● | ▼ | ▲ | ▲ | ˗ | ˗ | 49,50 |
| ‘Troyer’ citrange (P. trifoliata × C. sinensis) | leaves | The plants were irrigated with nutrient solutions containing 2, 16, or 48 mM chloride (w/o nitrate) for 3-month experimental period. | ▲ | ▼ | ▼ | ▲ | ▲ | ˗ | ˗ | 49,50 |
| ‘Carrizo’ citrange (C. sinensis × P. trifoliata) | leaves | Seedlings were not progressively adapted to saline conditions but treated with salt shock by adding 200 mM NaCl to a modified Hoagland solution. | - | - | - | ▲ | ▲ | ▲ | ▲ | 127 |
| Roots | - | - | - | ˗ | ▲ | ▲ | ˗ | 127 | ||
| ‘Cleopatra’ mandarin (Citrus reshni) | Roots | Seedlings were irrigated with half-strength Hoagland nutrient solution containing 0, 40 or 80 mM NaCl for 12 weeks. | ▼ | ▼ | ▼ | ˗ | ˗ | ˗ | ▲ | 51 |
| leaves | ▼ | ▼ | ▲ | ˗ | ˗ | ˗ | ↑↓ | 51 | ||
| ‘Troyer’ citrange (P. trifoliata × C. sinensis) | Roots Leaves |
Seedlings were irrigated with half-strength Hoagland nutrient solution containing 0, 40 or 80 mM NaCl for 12 weeks. | ▼ | ▼ | ● | ˗ | ˗ | ˗ | ↑↓ | 51 |
| ▼ | ↑↓ | ▲ | ˗ | ˗ | ˗ | ▲ | 51 | |||
| ‘Newhall’ navel orange (C. sinensis) | embryogenic callus | The 21-day old callus was transferred to callus growth medium with 100 mM NaCl and maintained at 25ºC for 7 days. | ▲ | ▲ | ▲ | ˗ | ˗ | ˗ | ˗ | 52 |
| ‘Cleopatra’ mandarin (Citrus reshni) | leaves | Seedlings were grown in Hoagland solution containing 75 mM NaCl | ▲ | ● | ▲ | ˗ | ˗ | ˗ | ▲ | 45 |
| ‘Cleopatra’ mandarin (Citrus reshni) | leaves | The same previous saline solution + 1 mM D-arginine (PAs inhibitor) | ● | ● | ▲ | ▲ | 45 | |||
| ‘Troyer’ citrange (P. trifoliata × C. sinensis) | leaves | Seedlings were grown in Hoagland solution containing 75 mM NaCl | ▲ | ● | ▲ | ˗ | ˗ | ˗ | ▲ | 34 |
| ‘Troyer’ citrange (P. trifoliata × C. sinensis) | leaves | The same previous saline solution + 0.1 mM spermidine | ● | ▲ | ▲ | 34 | ||||
| Ten citrus rootstocks b | leaves | Seedlings were acclimated for 90 days, the four levels of NaCl (0, 30, 60 and 90 mM) were applied along with half-strength Hoagland’s solution for another 90 days. | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | ▲ | 128 |
| Karna khatta sour orange hybrid (C. karna) | leaves | Seedlings were grown in soil with EC around 3.0 dS m−1 vs. garden soil about 0.35 dS m−1 (as control) | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | ▲ | 125 |
| ‘Carrizo’ citrange (C. sinensis × P. trifoliata) | leaves | Seedlings were irrigated with Hoagland solution + 90 mM NaCl for 2 months. | ▼ | ▼ | ● | ˗ | ˗ | ▲ | ▲ | 46 |
| ‘Carrizo’ citrange (C. sinensis × P. trifoliata) | leaves | The same previous saline solution, w/o 5 mM of N-NH4+ (NH4+ treatment) | ▲ | ▲ | ▲ | ˗ | ˗ | ▲ | ▲ | 46 |
| Sour orange (C. aurantium) | leaves | Seedlings were treated with NaCl via soil drench (0.0 vs. 150 mM) | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | ▲ | 126 |
| Sour orange (C. aurantium) | leaves | Seedlings were exposed to 100 mM NaCl + 1 mM exogenous PAs (putrescine, spermidine, or spermine) added to half-strength Hoagland’s nutrient solution | ▼ | ● | ▲ | ˗ | ˗ | ˗ | ▲ | 47 |
| Sour orange (C. aurantium) | Roots | ● | ▼ | ● | ˗ | ˗ | ˗ | ˗ | 47 | |
| nine citrus rootstocks c | leaves | Seedlings were acclimated for 5 months, then treated with four levels of NaCl (0, 25, 50 and 75 mM). NaCl concentration was increased gradually to avoid osmotic shock | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | ▲ | 129 |
| ‘Bakraii’ mandarin (C. reticulata × C. limetta) | leaves | Seedlings were acclimated for 6 months, then treated with 75 mM NaCl at 3-days intervals using 0.5 L of irrigation water. NaCl concentration was increased gradually to avoid osmotic shock. | ˗ | ˗ | ˗ | ˗ | ˗ | ˗ | ▲ | 35 |
a(▲) signifies for increased levels, (▼) signifies for decreased levels, (●) signifies for no change, (↑↓) signifies for varied responses, and (˗) signifies for not-reported compounds.Abbreviations: Put: putrescine, Spd: spermidine, Spm: spermine, ABA: abscisic acid, ET: ethylene, ACC: 1-aminocyclopropane-1-carboxylic acid, and Pro:L-proline.
bThey tested ten citrus rootstocks included: Rough lemon (C. jambhiri), Rubidoux trifoliate (P. trifoliata), Citrus Obvidea, Rangpur Lime (C. limonia), Sanchton Citrumillo (X Citroncirus), Citrumillo-1452 (X Citroncirus), Carrizo Citrange (C. sinensis × P. trifoliata), Yuma Citrange (P. trifoliata × C. sinensis), Gada Dehi, and Bitter Sweet Orange (C. aurantium).128
cThey tested nine citrus rootstocks included: Sour orange (C. aurantium), Bakraii (C. reticulata ×C. limetta), Cleopatra mandarin (C. reshni), Rangpur lime (C. limonia), Rough lemon (C. jambhiri), Macrophylla (C. macrophylla), Swingle citrumelo (P. trifoliata ×C. paradisi), Citrange (P. trifoliata ×C. sinensis) and Trifoliate orange (P. trifoliata).129
Furthermore, the amino acid L-proline usually induced to higher levels in citrus leaves after salt stress (Table 3).34,35,45–47,51,125–129 Proline is a common osmolyte multifunctional amino acid130 and also a major signaling molecule and plant growth regulator that triggers cascade signaling processes.131 In various plant species, proline was reported to be involved in crop tolerance against various abiotic stresses particularly salinity by maintaining ROS homeostasis via modulation of antioxidant enzymes.130–133 Nevertheless, the precise functional mechanisms of proline as an osmolyte in citrus plants are still ambiguous. Proline may function as ROS scavenger, osmotic adjuster, guardian for enzymes and DNA from salt damage(s) and/or signaling/regulatory molecule.134 For example, exogenous application of proline (5 mM) to cultured salt-sensitive Citrus sinensis ‘Valencia late’ cell line positively influenced the symptoms of salinity stress (100 mM NaCl) and increased growth of this cell line.134 Likewise, the addition of proline (particularly at a concentration of 25 mg L−1) to the basal culture media significantly increased the plantlet height and leaf number of C. sinensisplantlets and alleviated the inhibitory effect of salt stress.135 Interestingly, Aziz et al. (1998) reported that stress‐induced changes in PAs contents could regulate the proline accumulation in tomato leaf discs under salt stress.136
Moreover, overexpression of the salt-induced small protein (FcSISP; aka F. crassifoliaStress-Inducible Small Protein) from Meiwa kumquat (Fortunella crassifolia) in trifoliate orange (P. trifoliata) and tobacco (N. nudicaulis)conferred enhanced tolerance to salinity stress in the transgenic plants.137 This higher tolerance to salinity was accompanied by higher levels of proline in tobacco transgenic plants.137 Additionally, transcript levels of two major proline biosynthetic genes including delta 1-pyrroline-5-carboxylate synthetase (P5CS) and pyrroline-5-carboxylate reductase (P5CR) were significantly higher in the N. nudicaulistransgenic plants.137 Interestingly, FcSISP was quickly induced after the salt stress (200 mM NaCl) and rose progressively until reaching its highest peak at 24 hour-post-treatment (hpt), but it mildly induced by other stressors such as dehydration, cold, and even upon the infection with the bacterial phytopathogen causing citrus canker (Xanthomonas citri subsp. citri [Xcc]).137 Together, these findings indicate that FcSISP may acts as a regulator for the proline biosynthesis in citrus upon salinity stress.
In comparison with other fruit trees, citrus species are considered the most salt-sensitive plants118-122 which greatly limit the citrus growth worldwide. Therefore, many previous studies have been carried out to clone and characterize salinity-responsive genes from citrus and its relative species (Table 4). Although about 22 genes were cloned successfully from citrus and two genes were genetically transformed to citrus,142–144 however, very few genes have been reported to be involved in PAs-based defensive mechanisms against salt stress. For example, ‘Carrizo’ citrange (C. sinensis × P. trifoliata) and Rough lemon (C. jambhiri), are the most commonly used citrus rootstocks worldwide, however, both are very sensitive to salinity stress, which restricts their use in salty soils.142,144 Overexpression of the halotolerance (HAL2)gene fromSaccharomyces cerevisiae in ‘Carrizo’ citrange142 and Rough lemon144 improved the tolerance to salinity in transgenic plants.142,144 HAL 2 gene is in the methionine biosynthetic pathway, that encodes for a salt-sensitive biphosphate nucleotidase, which is required for sulfate accumulation in S. cerevisiae.155 HAL 2 gene is involved in salt tolerance mechanism, where it confers tolerance to lithium and sodium ions. Likewise, overexpression of a betaine aldehyde dehydrogenase (AhBADH) gene from Atriplex hortensis in trifoliate orange (P. trifoliata) enhanced the tolerance to salinity in transgenic lines, which may be correlated with the low levels of lipid peroxidation, protection of the photosynthetic machinery, and increase in K+ uptake.143
Table 4.
Salinity-responsive genes that have been cloned/genetically transformed from/to citrus or its relatives.
| Genes | GenBank accession No. a | ‘Cultivar’/Variety/(species) | References |
|---|---|---|---|
| Cit-SAP | - | ‘Shamuti’ Sweet orange (C. sinesis) | 138 |
| CSA (Cit-SAP) | - | ‘Shamuti’ Sweet orange (C. sinesis) | 139 |
| CsLea5 | - | Sweet orange (C. sinensis) | 140 |
| C3 (Oleosin 1) | Z48450.1 | Sweet orange (C. sinensis) | 141 |
| HAL2 b | X72847 | From yeast (Saccharomyces cerevisiae) expressed in ‘Carrizo’ citrange (C. sinensis × P. trifoliata) | 142 |
| CsSAMDC | FJ496345 | ‘Newhall’ Navel orange (C. sinensis) | 79 |
| CsNAC1 | CX306190 | ‘Cleopatra’ Mandarin (C. reshni) | 80 |
| AhBADH b | - | From Atriplex hortensis expressed in P. trifoliata | 143 |
| HAL2 b | X72847 | From yeast (Saccharomyces cerevisiae) expressed in Rough lemon (C. jambhiri) | 144 |
| PIP1 & PIP2 | - | ‘Cleopatra’ mandarin (C. reshni) ‘Carrizo’ citrange (C. sinensis × P. trifoliata) Trifoliate Orange (P. trifoliata) |
145 |
| CsCLCc | GU942490.1 | Trifoliate Orange (P. trifoliata) | 146 |
| FcSISP | KJ819954.1 | Meiwa kumquat (Fortunella crassifolia) | 137 |
| CrNCED1 | DQ028471.1 | ‘Cleopatra’ mandarin (C. reshni) | 147 |
| AtCBF3/DREB1A b | At4g25480 | From Arabidopsis thalianaexpressed in C. macrophylla | 148 |
| CisAL7 | Cs3g01400 | Sweet orange (C. sinensis) | 149 |
| PtrICE1 | KJ812152 | Trifoliate Orange (P. trifoliata) | 88 |
| CsPAO1 | Cs7g02060.1 | Sweet orange (C. sinensis) | 150 |
| CsPAO2 | Cs7g18840.2 | Sweet orange (C. sinensis) | 150 |
| CsPAO3 | Cs6g15870.1 | Sweet orange (C. sinensis) | 150 |
| CsPAO4 | Cs4g141 50.1 | Sweet orange (C. sinensis) | 150,151 |
| CsPAO5 | Cs7g23790.1 | Sweet orange (C. sinensis) | 150 |
| CsPAO6 | Cs7g23670.1 | Sweet orange (C. sinensis) | 150 |
| Four CsWRKYs(Cs30, Cs40, Cs41 and Cs48) | - | Sweet orange (C. sinensis) | 152 |
| Four CcWRKYs(Cc53, Cc54, Cc60, and Cc67) | - | Clementine (C. clementina) | 152 |
| CsCBF | FJ861084.1 | Sweet orange (C. sinensis) | 89 |
| PtrZPT2-1 | KC820894 | Trifoliate orange (P. trifoliata) | 153 |
| p35 anti-apoptotic b | - | From Autographa californica moth Nuclear Polyhedrosis Virus (AcMNPV) expressed in ‘Carrizo’ (C. sinensis × P. trifoliata) | 91,92 |
| PtrCCC | GU942489.1 | Trifoliate orange (P. trifoliata) | 154 |
a(˗) Signifies for not-reported accession numbers.
bThese genes were genetically transformed into citrus species using Agrobacterium tumefaciens-mediated gene transfer.
Moreover, several genes were reported to be involved in citrus response(s) to salt stress (Table 4). These genes including salt-associated protein (Cit-SAP) 138 andCSA (a gene encoding for Cit-SAP)139 from ‘Shamuti’ Sweet orange (C. sinesis) and the plasmamembrane intrinsic proteins (PIP1 and PIP2) from ‘Cleopatra’ mandarin (C. reshni), ‘Carrizo’ citrange (C. sinensis × P. trifoliata) and Trifoliate Orange (P. trifoliata).145 Likewise, three salinity-responsive genes were cloned successfully from Trifoliate Orange (P. trifoliata) including a putative Chloride channel (CLC)-encoding gene (CsCLCc), 146 Cys2/His2 (C2H2) zinc finger proteins (PtrZPT2-1),153 and Cation-chloride cotransporters membrane protein (PtrCCC).154 Furthermore, late-embryogenesis 5 (C-Lea5) gene,140 Oleosin 1 (aka C3),141 Alfin1-like (CisAL7),149 sixpolyamine oxidase (CsPAO)genes(CsPAO1 – CsPAO6), 150,151 and four CsWRKYs(Cs30, Cs40, Cs41 and Cs48)152 were cloned from sweet orange (C. sinensis) and identified as salinity-responsive genes.
Moreover, Gong et al. (2014) isolated a stress-responsive gene from Meiwa kumquat (F. crassifolia)(described as FcSISP).137 Overexpression of FcSISP in tobacco (N. nudicaulis) plants conferred enhanced salt tolerance, accompanied by lower cellular Na+ contents,137 which might be due to the up-regulation of genes associated with Na+ exchange and osmotic adjustment. Interestingly, the transgenic tobacco plants displayed higher transcript levels of genes associated with Na+ exchange including three members of salt overly sensitive (SOS) pathway (SOS1, SOS2, and SOS3)and three other sodium/hydrogen exchanger (NHX) genes (NHX1, NHX2,andNHX3)under normal conditions.137 Although the three hierarchical members of the SOS pathway (SOS1, SOS2, and SOS3) are well-characterized in model plants, particularly A. thaliana,156–159 however, to our knowledge none of these genes was cloned from citrus. Nevertheless, we believe that the SOS pathway plays a key regulatory role in salt tolerance in citrus (Figure 2(a)).
Figure 2.
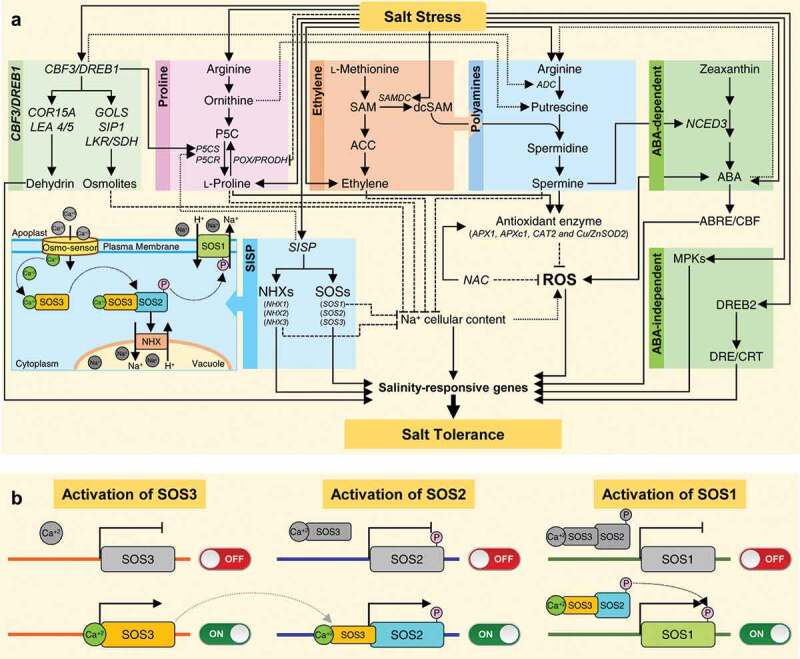
Hypothetical model of the potential polyamines- (PAs-) based responses of citrus plants to salinity stress. (a) Integration of PAs with other metabolic pathways in salt tolerance of citrus plants, (b) Activation of salt overly sensitive (SOS) pathway members SOS3, SOS2, and SOS1, respectively. Solid lines with arrowhead signify positive reaction, while dashed lines with blunt-heads indicate negative interaction. For more details and abbreviations, see the main text.
SOS1 encodes a putative plasma membrane Na+/H+ antiporter,158,159 SOS2 encodes a serine/threonine protein kinase that is required for salt tolerance,157 while SOS3 is calcineurin B-like protein (CBL) related to Ca2+-binding (calcium sensor).156 For the activation of the SOS pathway, SOS3 is activated by sensing the changes in cytosolic Ca2+ and binding to it (Figure 2(b)).156 Then, SOS3 interacts with SOS2 and activates it via forming a SOS2-SOS3 protein complex (Figure 2(c)).157 Later, SOS2 phosphorylates SOS1 (a plasma membrane antiporter) to activate it (Figure 2(d)).158,159 The active SOS1 controls the exchange of Na+ for H+ across the plasma membranes and also controls long-distance transportation of Na+ within the plant, from roots to stem and leaves.158,159
In 2015, Alvarez-Gerding et al. genetically-generated a transgenic rootstock C.macrophylla lines that constitutively express the c-repeat-binding factor (CBF3)/dehydration-responsive element-binding 1A (DREB1A) from A.thaliana.148 CBF3/DREB1A is a well-known salinity tolerance transcription factor. Under salinity stress, the CBF3/DREB1A transgenic lines displayed a higher expression of AtCBF3/DREB1A, as well as the induction of several other genes. For instance, the transgenic lines showed higher expression of ADC2(PAs biosynthetic gene), P5CS (L-proline biosynthetic gene), and compatible osmolites-associated genes such as galactinol synthase (GOLS), lysine ketoglutarate reductase (LKR/SDH), and raffinose synthase (SIP1).148 Interestingly, the overexpression of P5CS from moth bean (Vigna aconitifolia) in the citrus rootstock Swingle citrumelo (C. paradisi cv. Duncan x P. trifoliata) induced the expression of antioxidant enzymes genes including cytosolic ascorbate peroxidases (APX1 and APXc1), catalase 2 (CAT2), and cytosolic superoxide dismutase (Cu/ZnSOD2), and also increased the endogenous proline accumulation under water deficit of drought stress.160 Moreover, the CBF3/DREB1A transgenic lines displayed higher transcripts of dehydrins (COR15A and LEA 4/5) under salt stress.148 Dehydrins were significantly induced in C. clementina in response to water deficit of drought stress.75,161 Collectively, we suggest that CBF3/DREB1 could enhance the salinity tolerance in citrus plants via the induction of several genes involved in multiple metabolic pathways putatively belonging to its activation cascade. PAs, L-proline, compatible osmolytes, and their biosynthetic genes may play a key regulatory role in maintaining the Na+ cellular content or ROS homeostasis (Figure 2(a)), however, more investigations are required to understand the molecular and physiological events associated with these compounds.
Role of PAs in citrus response(s) to heavy metals
The term ‘heavy metal’ refers to any relatively high metal or metalloid and has potential toxicity at low concentrations. Heavy metals are environmentally hazardous and serious toxicants carcinogens to animals and humans.162 Arsenic (As), cadmium (Cd), chromium (Cr), and nickel (Ni) are classified as “category 1 heavy metals; group 1 carcinogens” according to the International Agency for Research on Cancer.163 The phytotoxicity of heavy metals is a significant threat to citriculture worldwide. Previously, the effect of various heavy metals on citrus physiology has been investigated including mercury (Hg).164 cadmium (Cd).165–169 and chromium (Cr).170,171 However, the role of PAs in citrus tolerance to heavy metals is poorly studied. To our knowledge, only one study has been recently published that provides strong evidence about the role of PAs in chromium-tolerance in Kinnow mandarin (C. nobilis × C. deliciosa) grafted on the diploid (2x) and double-diploid (4x) rootstocks of P. trifoliata, C. reshni, and C. limonia.170
Briefly, Shahid et al. (2018a) reported that the free PAs contents, including putrescine, spermidine, spermine, cadaverine, and homospermidine, were significantly higher in chromium-stressed (0.75 mM Cr) plants compared with those grown under normal conditions (0 mM Cr).170 Likewise, plants exposed to Cr-toxicity had higher levels of soluble-conjugated and insoluble-boundPAs (putrescine, spermidine, spermine, cadaverine, and homospermidine) compared with non-stressed plants. All free, soluble-conjugated and insoluble-boundPAs were induced greatly in the plants grafted on double-diploid rootstocks compared with those grafted on diploid rootstocks.170 Surprisingly, the chromium-stress significantly reduced the enzymatic activities of four PAs-biosynthetic enzymes including ADC, ODC, SAMDC, and SPDS, while it induced the enzymatic activities of polyamine oxidase (PAO) and diamine oxidase (DAO) (two major PAs-catabolic enzymes).170
These findings were partially in agreement with the results of Tajti et al. (2018) on wheat (Triticum aestivum) seedling, where putrescine and spermidine were induced by 50 µM cadmium (Cd2+) stress.172 In contrast with these findings, endogenous putrescine and spermidine contents, but not spermine, were decreased by copper- (Cu2+) and Cd2+-induced oxidative damage in sunflower (Helianthus annuus) leaf discs.173 However, pre-treatment with exogenous spermine (1 mM) reverted the negative effects of Cd2+ and Cu2+ on lipid peroxidation almost to control values.173 Similarly, endogenous spermidine and spermine levels were reduced in wheat seedling pre-soaked in H2O and subjected to Cd2+ stress and the pre-soaking in spermidine (2 mM) or spermine (2 mM) mitigated the adverse effects of Cd2+ stress (1 mM) and increased the activities of antioxidant enzymes (SOD and CAT) of wheat seedlings.174 Likewise, the pre-soaking of wheat seeds in aqueous solutions of putrescine (0.5 mM) and spermidine (0.5 mM) modulated the cadmium toxicity and enhancing Cd2+-tolerance in wheat seedlings.172
Citrus response to cadmium particularly, and heavy metals in general, might be associated with the endogenous phytochelatin (PC) but not glutathione (GSH) content.168 For instance, the endogenous levels of three phytochelatins (PC-2, PC-3 and PC-4) were significantly increased in Cd2+-treated roots of two citrus genotypes included Cleopatra mandarin and Carrizo citrange, whereas both GSH and oxidized GSH were significantly reduced in the Cd2+-treated roots.168 Additionally, the role of PAs in plant response(s) to heavy metals could be correlated with increased endogenous PAs content, accumulation of salicylic acid (SA) and proline, alteration of carbon and nitrogen metabolism, and/or homeostasis of ROS via the regulation of antioxidant enzymes.170–176 However, the molecular mechanisms behind the PAs-based response of citrus plants against heavy metals remain poorlyunderstood and required further investigations.
Role of PAs in citrus response(s) to water stress
Due to the long lifespan of citrus species, they are subjected to several biotic and abiotic stresses.177,178 Drought is one of the most intimidating environmental factors for citriculture due to its negative effects on plant growth and development, as well as, productivity.179 Drought-activated physiological damage results from the imbalance between water uptake by the root system and water loss via shoot system, causing a reversible decline in leaf water relations, membrane stability, gas exchange, and photosynthetic activity, leading to ROS accumulation, lipid peroxidation and membrane injury.180,181 A systemic response to abiotic stimuli, including drought, is initiated from plant physiological responses by a signaling cascade termed systemic acquired acclimation (SAA).182 SAA may include changes at the transcriptional, post-transcriptional, proteomic, metabolic changes and/or accumulation,183 as well as, ROS, calcium waves, hydraulic waves, and phytohormones, particularly ABA.182
In citrus, several metabolites are involved in SAA (Table 5). The PAs-based response is an important strategy used by citrus to tolerate and recover from drought events, as well as, to define the other metabolic-based strategies against water stress. A significant accumulation of both putrescine and spermidine were observed in the leaves of drought-stressed plants of ‘Red Tangerine’ (C. reticulata) 57 and ‘Jincheng’ Sweet orange (C. sinensis), 11 but they did not change significantly in water-stressed ‘Owari’ Satsuma orange (C. unshiu).4 Moreover, spermine was slightly increased in leaves of ‘Red Tangerine’,57 however, it was decreased in other studies on ‘Jincheng’ Sweet orange11 and ‘Owari’ Satsuma orange.4 Collectively, these findings indicate that PAs-based response of citrus to water stress is mainly controlled by putrescine and spermidine, but not spermine.
Table 5.
Changes in the endogenous content of major polyamines (PAs), stress-associated amino acids, and stress-associated phytohormones in citrus and its relative species as affected by water stress.
| ‘Cultivar’/Variety/(species) | Tissue | Treatment/experimental design | Pro a | Arg | JA | ABA | Put | Spd | Spm | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Red Tangerine’ (C. reticulata) | Leaves | The role of spermine application (1 mM) in dehydration tolerance after 12 hpt in vitro. | - | - | - | - | ▲ | ▲ | ▲ | 57 |
| Tomato (Solanum lycopersicum ‘Zhongshu No. 4′) | Leaves | Overexpression of PtADC gene from P. trifoliata in transgenic tomato plants | - | - | - | - | ▲ | ▲ | ↑↓ | 82 |
| Tobacco (Nicotiana nudicaulis) overexpressing PtADC | Leaves | Overexpression of PtADC gene from P. trifoliata in transgenic tobacco plants | - | - | - | - | ▲ | ▲ | ● | 82 |
| Citrumelo CPB 4475 (C. paradisi × P. trifoliata) | Roots | Severe drought stress by transplanting plants to dry perlite for different periods | - | - | ▲ | ▲ | - | - | - | 184 |
| ‘Owari’ Satsuma orange (C. unshiu) | Leaves | Dropping the soil moisture content to 20% of the water holding capacity for 5 days | ▲ | ▼ | - | - | ● | ● | ▼ | 4 |
| Tobacco (N. nudicaulis) overexpressing FcWRKY70 | Leaves | Overexpression of FcWRKY70 from ‘Meiwa’ Kumquat (Fortunella crassifolia) | - | - | - | - | ▲ | - | - | 185 |
| Cleopatra mandarin (C. reticulata), | Leaves | Progressive drought was imposed by transplanting plants to perlite, ceasing watering and allowing the substrate to dry for a period of 14 days. | ▲ | - | ▲ | ▲ | - | - | - | 186 |
| Carrizo citrange (C. sinensis × P. trifoliata) | Leaves | Progressive drought was imposed by transplanting plants to perlite, ceasing watering and allowing the substrate to dry for a period of 14 days. | ▲ | - | ▲ | ▲ | - | - | - | 186 |
| ‘Jincheng’ Sweet orange (C. sinensis) | Leaves | The seedlings were dehydrated in vitro at 25°C | - | - | - | - | ▲ | ▲ | ▼ | 11 |
| Rangpur Lime (C. limonia) b | Leaves & Roots | Either ungrafted or grafted subjected to distinct watering regimes (well-watered vs. severe drought) | - | - | - | ▲ | - | - | - | 180 |
| ‘Sunki Maravilha’ mandarin (C. sunki) b | Leaves & Roots | Either ungrafted or grafted subjected to distinct watering regimes (well-watered vs. severe drought) | - | - | - | ▲ | - | - | - | 180 |
| ‘Valencia’ Sweet Orange (C. sinensis) on C. limonia | Leaves & Roots | Responses of Valencia scion grafted on two rootstocks, Rangpur Lime (C. limonia) and ‘Sunki Maravilha’ mandarin (C. sunki), during three successive periods of water deficit | - | - | - | ▲ | - | - | - | 187 |
| ‘Havana’ Tobacco (N. tabacum) overexpressing CsNCED3 | Leaves | Overexpression of CsNCED3 from ‘Rangpur’ lime (C. limonia) | - | - | - | ▲ | - | - | - | 188 |
a(▲) signifies for increased levels, (▼) signifies for decreased levels, (●) signifies for no significant change, (↑↓) signifies for varied responses, and (˗) signifies for not-reported compounds. Abbreviations: Pro: L-proline, Arg:L-Arginine, JA: jasmonic acid, ABA: abscisic acid, Put: putrescine, Spd: spermidine, and Spm: spermine.
bThe two Citrus rootstocks (Rangpur lime and ‘Sunki Maravilha’ mandarin) either ungrafted or grafted with their reciprocal graft combinations or with shoot scions of ‘Valencia’ Sweet Orange (C. sinensis) and Tahiti acid lime (C. latifolia) subjected to distinct watering regimes (well-watered vs. severe drought)
Although several genes have been genetically transformed from/to citrus or its relatives and their transgenic plants were more tolerant to water stress (Table 6), however, very few of them had higher PAs levels. For instance, overexpression of an arginine decarboxylase (PtADC) gene, isolated from Poncirus trifoliata, in tomato (Solanum lycopersicum ‘Zhongshu No. 4′) and tobacco (N. nudicaulis) showed elevated putrescine and spermidine levels in the transgenic lines.82 Likewise, overexpression of FcWRKY70 from ‘Meiwa’ Kumquat (Fortunella crassifolia) in tobacco (N. nudicaulis) plants increased endogenous putrescine content and up-regulated the ADC genes (NtADC1 and NtADC2) in tobacco transgenic plants compared with wild-type plants.185 Interestingly, challenging the transgenic lines with dehydration for 60 min led to significant putrescine accumulation in the tested genotypes.185 Additionally, the transcript levels of both ADC genes (NtADC1 and NtADC2) were induced after dehydration treatment, but they were pronouncedly higher in the transgenic lines than in WT.185
Table 6.
Water stress response-associated genes that have been genetically transformed from/to citrus or its relatives.
| Genes | GenBank accession No. a | Source | ‘Cultivar’/Variety/(species) | References |
|---|---|---|---|---|
| P5CS | - | Moth bean (Vigna aconitifolia) | ‘Carrizo’ citrange (C. sinensis × P. trifoliata) | 178 |
| PtrABF | HM171703.1 | Trifoliate Orange (P. trifoliata) | Tobacco (Nicotiana nudicaulis) | 189 |
| CuLea5 (LeaP600) | DQ424891.1 | Satsuma mandarin (C. unshiu) | Arabidopsis thaliana ecotype ‘Columbia’ | 190 |
| PtrMAPK | - | Trifoliate orange (P. trifoliata) | Tobacco (N. tabacum) | 191 |
| PtrADC | HQ008237 | Trifoliate Orange (P. trifoliata) | Tomato (Solanum lycopersicum ‘Zhongshu No. 4′) | 82 |
| PtrADC | HQ008237 | Trifoliate Orange (P. trifoliata) | Tobacco (Nicotiana nudicaulis) | 82 |
| CpPSY | - | ‘Flame’ Grapefruit (Citrus paradisi) | ‘Havana’ tobacco (N. tabacum) | 192 |
| P5CS | - | Moth bean (Vigna aconitifolia) | ‘Duncan’ Swingle citrumelo (C. paradisi x P. trifoliata) | 160,193 |
| CsGSTUs | - | Sweet orange (C. sinensis) | ‘Wisconsin 38ʹ tobacco (N. tabacum) | 194 |
| FcWRKY70 | - | ‘Meiwa’ Kumquat (Fortunella crassifolia) | Tobacco (N. nudicaulis) | 185 |
| FcWRKY70 | - | ‘Meiwa’ Kumquat (F. crassifolia) | Lemon (C. lemon) | 185 |
| PtrABF | HM171703.1 | Trifoliate Orange (P. trifoliata) | Trifoliate orange (P. trifoliata) | 195 |
| CsTIP2 | - | ‘Rangpur’ Lime (C. limonia) | ‘Havana’ tobacco (N. tabacum) | 196 |
| CsNCED3 b | - | ‘Rangpur’ Lime (C. limonia) | ‘Havana’ tobacco (N. tabacum) | 188 |
a(˗) Signifies for not-reported accession numbers.
bThis gene corresponding to the identical sequence of locus ‘Ciclev10019364 m’ in the citrus reference genome.
Additionally, ectopic expression of ABA-responsive element (ABRE)-binding factor from P. trifoliata (PtrABF) in transgenic tobacco plants enhanced tolerance to both dehydration and drought stresses through scavenging ROS and modulating the gene expression of PAs-biosynthetic genes (NtADC1, NtADC2, and NtSAMDC) and stress-responsive genes (NtLEA5, NtERD10 C, NtCDPK2, and NtDREB).189 Similarly, overexpression of the same gene in trifoliate orange (P. trifoliata) reduced the stomatal density, maintained ROS homeostasis, up-regulated PtrADC, and induced nine stress-associated transcription factors of the NACs, WRKYs, and bZIP families, and seven stress-associated proteins including LEA4-5 and dehydration-induced protein RD22-like.195 Dehydration for 90 min increased the transcript levels of PtrADC in both WT and transgenic lines; however, the induction was greater in the transgenic lines than in wild-type plants.195 In all these studies and under water stress conditions, the transgenic lines exhibited improved dehydration and drought tolerance,82,185,189,195 decreased ROS accumulation,82,189,195 and up-regulated stress-responsive genes.82,185,189,195 In addition, we believe that PAs-based response of citrus to water stress is integrated with other metabolic pathways (Table 5) and water stress response-associated genes/proteins (Table 7). For example, ABA plays a key role in the coordinated regulation of the proline and polyamine biosynthetic pathways. For example, both ABA and osmotic stress, caused by polyethylene glycol altered the proline metabolism differently, however, ABA treatment resulted in higher proline accumulation in wheat seedlings.198 Remarkably, proline metabolism was partly regulated independently and not in an antagonistic manner from PAs biosynthesis.198 Together, these findings support our suggestion that PAs-based response of citrus to water stress is mainly controlled by putrescine and spermidine, but not spermine and regulated by other stress-associated metabolites, particularly ABA and proline.
Table 7.
Water stress response-associated genes that have been cloned from citrus or its relatives.
| Genes | GenBank accession No. a | ‘Cultivar’/Variety/(species) | References |
|---|---|---|---|
| CsLea5 | - | Sweet orange (C. sinensis) | 140 |
| CsNCED1 | DQ028471 | ‘Navelate’ navel orange (C. sinensis) | 197 |
| CsNCED2 | DQ028472 | ‘Navelate’ navel orange (C. sinensis) | 197 |
| PtrRING‐H2 | DQ158860 | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 76 |
| PtrABF | HM171703.1 | Trifoliate Orange (P. trifoliata) | 189 |
| CsSAMDC | FJ496345 | ‘Newhall’ Navel orange (C. sinensis) | 79 |
| CsNAC1 | CX306190 | ‘Cleopatra’ Mandarin (C. reshni) | 80 |
| PtrMAPK | - | Trifoliate orange (P. trifoliata) | 191 |
| PtrADC | HQ008237 | Trifoliate Orange (P. trifoliata) | 82 |
| PtrWRKY2 | JX236033.2 | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 84 |
| PtrWRKY1 | CX065984 | ‘Rubidoux’ Trifoliate Orange (P. trifoliata) | 86 |
| CgWRKY1 | - | ‘Rein King’ Pummelo (C. grandis) | 86 |
| CrNCED1 | DQ028471.1 | ‘Cleopatra’ mandarin (C. reshni) | 147 |
| PtrABF | HM171703.1 | Trifoliate Orange (P. trifoliata) | 195 |
| Four CsWRKYs(Cs30, Cs40, Cs41, and Cs48) | - | Sweet orange (C. sinensis) | 152 |
| Four CcWRKYs (Cc53, Cc54, Cc60, and Cc67) | - | Clementine (C. clementina) | 152 |
| PtrZPT2-1 | KC820894 | Trifoliate orange (P. trifoliata) | 153 |
a(˗) Signifies for not-reported accession numbers.
PAs-Based Defensive Mechanisms Against Biotic Stress in Citrus
It has been suggested that plant metabolites are responsible for securing the energy requirements for plant defensive responses against biotic stress, particularly phytopathogens,199,200 however, this is not the main role. Plant metabolites might contribute directly to plant defense responses, or they could be required to nutritional needs for pathogen growth and multiplication. Previously, we described the chemical composition of citrus phloem sap because it contains all the required nutrients for growth and multiplication of several citrus pathogens such as Candidatus Liberibacter spp., the causal agents of Huanglongbing (aka citrus greening).201 GC-MS analysis revealed that Citrus phloem-sap contains many proteinogenic amino acids (PAAs; such as proline, tryptophan, tyrosine, leucine, isoleucine, and valine), sugars (sucrose, glucose, fructose, and inositol), fatty acids (palmitic, oleic, and stearic acids), and organic acids (succinic, fumaric, malic, maleic, threonic, citric, and quinic acids).201 Proline, the most abundant amino acid, while γ–aminobutyric acid (GABA) was the only NPAA detected in citrus phloem sap.201
Citrus plants are threatening by several biotic stressors such as viral, bacterial, and fungal diseases, in addition to many nematodes, phytoplasmas, spiroplasmas, viroids, and graft-transmissible pathogens. All these pathogens can severely influence many biochemical and metabolic alterations that play a vital and complex role in citrus defensive mechanisms.1–8,12,202,203 The metabolic changes including the alteration of volatile organic compounds,203 amino acids,1–6 organic acids,1,3,6 fatty acids,2,6 phytohormones,7,8 polyamines (PAs)4,12 and other secondary metabolites. However, it is still ambiguous whether these changes are a plant metabolic response(s), or if they are to benefit citrus pathogens or both. In addition, the contribution of PAs and NPAAs pathways in citrus defensive mechanisms against biotic stress are poorly studied and not fully understood.
Role of PAs in citrus response(s) to virus and viroid infection
Citrus species could be infected by many viruses, most of them are asymptomatic until a susceptible scion and/or rootstock is exist.204 Citrus viruses including, but not limited to, Citrus leaf blotch virus (Citrivirus), Citrus leprosis (Cilevirus/Higrevirus), Citrus chlorotic dwarf (Geminiviridae), Citrus psorosis virus (CPsV; Ophiovirus), and Citrus tristeza virus (CTV; Closterovirus).204 In addition, citrus plants could be attacked by a large number of true viroid species; including Citrus Exocortis Viroid (CEVd), Hop Stunt Viroid (HSVd), Citrus Bent Leaf Viroid (CBLVd), Citrus Dwarfing Viroid (CDVd), and Citrus Bark Cracking Viroid (CBCVd).205 Two additional viroids, Citrus Viroid VI (CVd-VI) and Citrus Viroid V (CVd-V) have also been reported.206–208 To our knowledge, the role of PAs in citrus-virus or citrus-viroid pathosystems is not studied yet.
Many attempts have been made to create transgenic resistant plants for CTV and CPsV (Table 8), via introducing the coat protein (CP) genes of CTV209–211,213,214,222,227 or the coat protein (CPsV-54 K) gene and viral 24 k-derived hairpin of CPsV (CPsV-ihp24 K; apolypeptide of unknown function)220,221,225 to virus-sensitive cultivars. However, the role of PAs in citrus response to CTV or CPsV is not reported in these transgenic plants. On the other hand, very few reports about the correlation between CEVd and PAs in tomato (Lycopersicon esculentum) and purple passion (Gynura aurantiaca) plants were found, but not in citrus.228,229 Recently, it has been proved that the nonproteinogenic amino acid, γ-aminobutyric acid (GABA) is associated with tomato response against CEVdafter the treatment with the plant defense inducer, benzothiadiazole (BTH).230
Table 8.
Viral disease tolerance genes that have been cloned/genetically transformed from/to citrus or its related genera.
| Gene | ‘Cultivar’/Variety/(species) | References |
|---|---|---|
| CTV-CP gene | Sour orange (Citrus aurantium) | 209 |
| CTV-CP gene | Mexican lime (C. aurantifolia) | 210 |
| CTV-CP gene | Sour orange (C. aurantium) | 211 |
| CTV-uncp | ‘Rio Red’ grapefruit (C. paradisi) | 212 |
| gna | ‘Rio Red’ grapefruit (C. paradisi) | 212 |
| CTV-p25 CP | Mexican lime (C. aurantifolia) | 213 |
| CTV-CP gene | Grapefruit (C. Paradisi) | 214 |
| CTV-p23 | Mexican lime (C. aurantifolia) | 215,216 |
| CTV-RdRp | ‘Duncan’ grapefruit (C. paradisi) | 217 |
| CTV-392 CTV-p23 hairpin |
Grapefruit (C. paradisi) Grapefruit (C. paradisi) |
218 218 |
| scFv-3DF1 | Mexican lime (C. aurantifolia) | 219 |
| scFv-3CA5 | Mexican lime (C. aurantifolia) | 219 |
| CPsV-CP | Sweet orange (C. sinensis) | 220 |
| CPsV-24 K gene CPsV-48 K gene CPsV-54 Kgene |
Sweet orange (C. sinensis) Sweet orange (C. sinensis) Sweet orange (C. sinensis) |
221 221 221 |
| pCTV-CP pCTV-dsCP pCTV-CS |
Sweet orange (C. sinensis) Sweet orange (C. sinensis) Sweet orange (C. sinensis) |
222 222 222 |
| CTV-p20 hairpin RNA | sour orange (C. aurantium) | 223 |
| CTV-p23 | Mexican lime (C. aurantifolia) | 224 |
| CPsV-ihp24 K | ‘Pineapple’ sweet orange (C. sinensis) | 225 |
| hp2520 | ‘Jincheng’ sweet orange (C. sinensis) | 226 |
| hp252023 | ‘Jincheng’ sweet orange (C. sinensis) | 226 |
| CTV-CP gene | Sour orange (C. aurantium) | 227 |
Abbreviations:
Citruspsorosis virus (CPsV)
Citrus tristeza virus(CTV)
CTV-CP: Citrus tristeza viruscoat protein
CTV-uncp:Citrus tristeza virus untranslatable coat protein gene
Gna: Galanthus nivalis agglutinin gene (a plant-derived insecticidal gene)
CTV-RdRp: Citrus tristeza virus RNA-dependent RNA polymerase gene
scFv: Single-chain variable fragment antibodies
pCTV-CP: Containing the 25 kDa major capsid protein gene (CTV-CP),
pCTV-dsCP: Containing the same CTV-CP gene in an intron-spliced hairpin construct
pCTV-CS: Containing a 559 nt conserved region of the CTV genome
ihp24 K: Citruspsorosis virus (CPsV) viral 24 k-derived hairpin
hp252023: Hairpin RNA targeting p25, p20, and p23
hp2520: Hairpin RNA targeting p25 and p20
CPsV-24 K gene: A 24‐kDa polypeptide of unknown function (24 K) from CPsV
CPsV-48 K gene: A polypeptide of 54.7 kDa of unknown function (54 K) from CPsV
CPsV-54K gene: The coat protein (CP) of 48.6 kDa (48 K protein) from CPsV
CEVd infection stimulated the ethylene production via the activation of 1-Aminocyclopropane-1-carboxylic acid (ACC) synthesis(Figure 3(a)) 231 and enhanced the production of pathogenesis-related (PR) proteins 228 in tomato leaves when systemic symptoms appeared. On the other hand, the CEVd-infected tomato plants displayed a marked decrease in endogenous putrescine content, but no significant changes in either spermidine or spermine content.228 The reduction of free putrescine content caused by CEVd infection was correlated with the reduction in ODC activity, but not ADC activity.229 In addition, treatment with aminoethoxyvinylglycine (AVG; an ethylene biosynthesis inhibitor) or norbornadiene (an ethylene action inhibitor) frustrated the reduction in ODC activity and putrescine content without no significant effect on spermidine or spermine content.228,229 Taken together, these findings suggest that ethylene is negatively regulating the putrescine during the viroid-plant interaction via interfering with the ODC activity (Figure 3(a)).
Figure 3.
Hypothetical model of the potential polyamines- (PAs-) based responses of citrus plants against viroid diseases. (a) Integration of PAs with ethylene in citrus response to the infection with Citrus Exocortis Viroid (CEVd), and (b) The interaction between PAs, ethylene, and salicylic acid during the CEVd infection. Solid lines with arrowhead signify positive reaction, dashed lines with blunt-heads indicate negative interaction, while round-dotted lines with arrows represent hypothetical mechanisms or uncharacterized elements. For more details and abbreviations, see the main text.
Moreover, salicylic acid (SA) is another phytohormone that is involved in the basal resistance of tomato plants to CEVd infection.232 CEVd infection induced the accumulation of SA in the ‘Money Maker’ tomato cultivar, but not in tomato plants expressing the NahG transgene (a mutant prevents the accumulation of endogenous SA).232 In addition, CEVd infection increased the levels of ethylene and putrescine conjugates (caffeoyl-putrescine, and feruloyl-putrescine) in NahG plants earlier and greater than in Money Maker plants.232 These findings suggest that SA also might function as a negative regulator of both ethylene and putrescine conjugates during the viroid-plant interaction (Figure 3(b)).
On the other hand, the viral infection with CTV induced the putrescine accumulation (approximately 6-folds) in the phloem sap of CTV-infected C. macrophylla plants.44 Likewise, PAs were accumulated in hypersensitive, but not susceptible, tobacco plants upon the infection with Tobacco Mosaic Virus (TMV).233 Although the induction of PAs against TMV appears to function independently from SA to activate acidic PR proteins and resistance against virus infection in tobacco plants,234 several transcriptomic and proteomic studies revealed that phytohormones might be implicated in citrus response against CTV.235–239 In citrus, PAs-based defensive mechanisms against CTV might be integrated with phytohormone groups (Figure 4) such as abscisic acid (ABA), 236,238,240 auxins,239,240 brassinosteroids (BRs)and cytokinins (CKs),240 ethylene,235,237–240 gibberellins (GA), 238–240 jasmonates (JA), 237,240 andsalicylic acid (SA).235,238
Figure 4.
Hypothetical model of the potential polyamines- (PAs-) based responses of citrus plants against viral diseases. (a) Integration of PAs with seven phytohormone pathways in citrus response to the infection with Citrus tristeza virus (CTV), and (b) The interaction between PAs, auxins, cytokinins (CKs), and methyl jasmonates (meJA) during the CTV infection. Solid lines with arrowhead signify positive reaction, dashed lines with blunt-heads indicate negative interaction, while round-dotted lines with arrows represent hypothetical mechanisms or uncharacterized elements. For more details and abbreviations, see the main text.
Four instances, four ABA-related genes were up-regulated in CTV-infected sweet orange (C. sinensis cv. ‘Jincheng’) leaves compared with mock-inoculated ones using microarrays.240 These differentially expressed genes (DEGs) including 9-cis-epoxycarotenoid dioxygenase 4 and 5 (NCED4 and NCED5), GRAM (glycine, arginine, alanine and methionine motif) domain-containing protein/ABA-responsive protein-related, and HVA22-like protein F (HVA22 F).240 Likewise, NCED4 wasup-regulated in sweet orange trees affected by the CTV-B6 severe strain, but not the mild strain (CTV-B2).238 Moreover, the ABA-stress ripening-related protein was increased in CTV-infected sweet orange (C. sinensis cv. ‘Westin’) plants.236 Interestingly, NCED3 was up-regulated was induced in spermine-treated trifoliate orange seedlings,58 which supports the idea that PAs-based defensive mechanisms against CTV might be integrated with ABA and its related genes (Figure 4(a)).
Additionally, auxins metabolism and signaling-related genes were differentially regulated upon CTV infection.239,240 Five genes of auxin-responsive family protein were down-regulated in CTV-infected sweet orange leaves compared with mock-inoculated plants.240 Nevertheless,CTV-infection induced the expression of the auxin-responsive GH3 protein (GH3.1)240 and auxin signaling F-box protein 5 (AFB5).239 GH3 gene is involved in the conversion of free active auxins to inactive auxin-amide conjugates via conjugates amino acids to indole-3-acetic acid (IAA).241,242 Together, these findings suggest that CTV infection might decrease the free active auxins level viathe reduction of auxin-responsive family proteins and the induction of GH3(Figure 4(a)). On the other hand, four CKs-related DEGs were down-regulated in CTV-infected sweet orange leaves compared with mock-inoculated plants.240 These DEGs include cytokinin oxidase 5 (CKX5), isopentenyltransferase 3 (IPT3), and two isopentenyltransferases 5 (IPT5).240 The CKs reduction might be a defense mechanism to suppress the development of the symptoms. In Arabidopsis-Rhodococcus fascians interaction, CKs were involved in symptom development via activation of ADC and induction of PAs accumulation.243
Furthermore, four BRs-related DEGs were increased in CTV-infected sweet orange leaves compared with mock-inoculated plants.240 These DEGs include steroid sulfotransferase (ST), FACKEL (FK; encodes a protein with sequence similarity to sterol reductase family), and two squalene epoxidases 3 (SQE3).240 SQE3 is a rate-limiting enzyme in the sterol biosynthesis pathway and might play a key role in citrus defense against CTV. ST gene also might be involved in citrus defense response against CTV, since it found to be induced by SA.244 SA-defense pathway mediated the resistance of sour orange (C. aurantium) rootstock to CTV.245 Additionally, indirect evidence for the alteration of SA in Mexican lime plants infected by the CTV severe strain (CTV-T305),235 since S-adenosyl-methionine salicylic acid carboxyl methyltransferase (SAMT) gene was up-regulated upon CTV-infection. SAMT catalyzes the formation of methylsalicylate (meSA) from SA and induces in systemic acquired resistance (SAR).246 The accumulation of meSA was reported previously from TMV-infected tobacco plants.247 In addition, exogenous application of SA derivative (acetylsalicylic acid; aka aspirin) induced resistance to TMV in tobacco.248 Interestingly, SA and its derivative, meSA, enhanced the accumulation of PAs in other plant species such as maize,249 tobacco,250 and tomato251 via the induction of ADC and/or ODC. Taken together, these findings suggest that PAs-based defensive mechanisms against CTV are mediated by both BRs and SA.
The importance of JA and ethylene pathways as alternative signals in plant defense was magnified252 due to their association with PAs metabolism.253 For example, ACC oxidase (an enzyme that catalyzes ACC to ethylene) was up-regulated in CTV-infected Mexican lime plants.235 Likewise, 29 ethylene-related genes were differentially expressed in CTV-infected sweet orange (C. sinensis cv. ‘Jincheng’) leaves.240 The expression of ethylene-responsive element binding factor 1 (ERF1; an ethylene signal transduction) and two of senescence-related gene 1 (SRG1) were also increased in sweet orange trees infected with a severe strain of CTV (CTV-B6).237
Moreover, nine JA-related genes were differentially expressed in CTV-infected plants.240 JA carboxyl methyltransferase (JMT) was highly up-regulated upon CTV-infection.237,240 Additionally, several JA-biosynthetic genes were expressed at higher levels in CTV-infected plants including two fatty acid desaturase (FAD3and FAD6),hydroperoxide lyase 1 (HPL1), twoallene oxide synthase (AOS), twoallene oxide cyclase 4 (AOC4),and two 12-oxophytodienoate reductase 2 (OPR2).237,240 The induction of JA-biosynthetic genes suggesting that these DEGs might play a key role in citrus defense against CTV through the enhancement of endogenous JA content, which could stimulate the PAs-based defensive mechanisms against CTV. Previous studies showed that the exogenous application of methyl jasmonates (meJA) enhanced the accumulation of conjugated PAs via the up-regulation of gene expression and enzymatic activities of PAs-biosynthetic genes (ADC, ODC, SAMDC).253 However, meJA-induced PAs and their defense-related process are regulated by other phytohormone groups. For example, auxins (such as IAA) synergistically strengthen the meJA-induced PAs, while N6-benzyladenine (a synthetic cytokinin) counteracts it, whereas it appears to function independently of ethylene 253 (Figure 4(b)).
Furthermore, PAs-based defensive mechanisms against CTV might be integrated with the detoxification of ROS (Figure 4(a)). The CTV infection induced the generation of ROS in the infected cells, which subsequently reprogramming both primary and secondary metabolic pathways 240 and influencing the antioxidant enzymes activity.236,254 For instance, the transcripts and enzymatic activity of superoxide dismutase (SOD) and ascorbate peroxidase (APX) were increased in sweet orange (C. sinensis cv. ‘Westin’) plants upon the CTV infection, while the activity of guaiacol peroxidase (GPX) did not change significantly.236 However, The enzymatic activities of SOD and polyphenol oxidase (PPO) were significantly reduced in Mexican lime (C. aurantifolia) plants after ten years of exposure to CTV infection.254 These findings suggest that the antioxidant defense mechanisms might be suppressed by CTV because of continuous and long‐term stress.
In conclusion, we suggest that citrus plants relay on multiple defensive mechanisms against viral infection, particularly CTV. The activation of these defensive mechanisms varies according to virus strain, host genotype, time of exposure to the infection, and environmental conditions. These defensive mechanisms include a combination of PAs-based defensive mechanisms, different phytohormone groups, and antioxidant enzymatic activities. However, we believe that more investigations are required to reconnoiter the molecular mechanisms behind the PAs-phytohormones-ROS crosstalk during the citrus-virus interaction.
Role of PAs in citrus response(s) to fungal and oomycetes infection
Citrus and its relative species are attacked by many fungal and oomycetes phytopathogens that can infect citrus fruits and foliage. The fungal diseases ofcitrus includingAlternaria brown spot (Alternaria alternate; synonyms: A. citri and A. alternate pv. citri), greasy spot (Mycosphaerella citri), melanose (Diaporthe citri; synonyms: Phomopsis citri), scab diseases (Elsinoë fawcettii; synonym: Sphaceloma fawcettii), black spot (Phyllosticta citricarpa; synonyms: Guignardia citricarpa and Phoma citricarpa), post-bloom fruit drop (Colletotrichum acutatum;synonyms: Glomerella acutata and C. abscissum),Pseudocercosperafruit and leaf spot (Pseudocercospora angolensis;synonyms: Phaeoramularia angolensis), blue mold (Penicillium italicum) and green mold (P. digitatum).255,256 In addition, the oomycetous pathogen Phytophthora citrophthora (synonyms: P. citricola and P. parasitica) causes several diseases to citrus plants including foot rot, gummosis, and root rot diseases, and also can infect citrus fruits causing brown rot disease.255,256
Unfortunately, the available studies about the role of PAs in citrus response(s) to the fungal and oomycetes diseases are very limited. As we mentioned above, citrus plants have two routes for the biosynthesis of putrescine, one from arginine via agmatine involving ADC and the other one from ornithine involving ODC. However, fungal phytopathogen and fungi, in general, have only one PAs biosynthesis pathway through.257 The ODC activity plays a key role in the growth of257–259 and pathogenicity257,260 of phytopathogenic fungi (Figure 5(a)). The addition of DL-α-Difluoromethylornithine (DFMO; an inhibitor of ODC) controlled the growth of several phytopathogenic fungi in vitro 257–259 and also prevented the infection by uredospores of the bean rust fungus, Uromyces phaseoli on bean plants Phaseolous vulgaris.257 Interestingly, the transcriptome analysis of the oomycetous pathogen Phytophthora parasitica, the causal agent of the citrus gummosis disease, revealed that only one singleton read was found in the PP/CitEST database that sharing sequence similarity with the ODC gene of Mucor circinelloides f. lusitanicus.260This enzyme might be an important pathogenicity factor,260 and probably could be a target for chemical control because its importance in virulence and growth, however, these hypotheses are not characterized yet and required further investigations.
Figure 5.
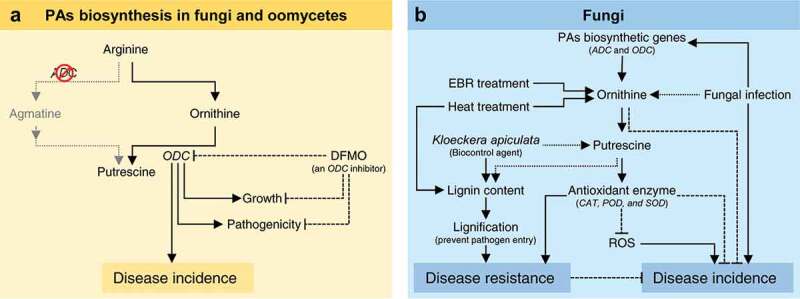
Hypothetical model of the potential polyamines- (PAs-) based responses of citrus plants against fungal diseases. (a) The role of PAs in the pathogenicity of fungi and fungi-like oomycetes and (b) The role of PAs in resistance to fungal diseases of citrus. Solid lines with arrowhead signify positive reaction, dashed lines with blunt-heads indicate negative interaction, while round-dotted lines with arrows represent hypothetical mechanisms or uncharacterized elements. For more details and abbreviations, see the main text.
Furthermore, the exogenous application of 24-epibrassinolide (EBR) on Satsuma mandarin (C. unshiu) fruits significantly enhanced the endogenous ornithine content, which was correlated with a reduced disease incidence of postharvest rots.261 Similarly, theheat treatment significantly increased the ornithine content which was associated with reduced disease incidence, lesion diameter, and improved the disease resistance againstP. italicum on mature ‘Kamei’ Satsuma mandarin (C. unshiu) fruits during postharvest storage.262 In both studies, the accumulation of ornithine was associated with higher enzymatic activities of antioxidant enzymes such as CAT, POD, and SOD.261,262 Collectively, these findings suggest that ornithine might be involved in postharvest disease resistance via regulation of oxidative stress tolerance in citrus plants(Figure 5(b)).
Moreover, transcriptomic profiling of ‘Powell’ orange (C. sinensis) fruit reveal that several genes involved in the PAs pathway were up-regulated after 60-hours of infection with P. digitatum.263 Thesegenes included the major PAs biosynthetic genes (ADC and ODC), putative acetylornithine aminotransferase, arginine biosynthesis protein (argJ1), putative arginine/serine-rich splicing factor, polyamine oxidase 1, and putrescine-binding periplasmic protein-related.263 In the same manner, the endogenous PAs levels, particularly putrescine, were induced in orange fruits after 60-hours of the treatment with the extract of the cell-free culture of the biocontrol yeast Kloeckera apiculata.264 K. apiculata was isolated from the epiphytes of citrus roots265 and showed strong antagonistic activities against both P. digitatum266 and P. italicum.265,267 Together, these findings suggest that the biological control of postharvest fungal pathogens in citrus using K. apiculata is associated with the up-regulation of PAs biosynthesis to enhance citrus responses (Figure 5(b)).
Additionally, at the cell wall level, PAs might help in the prevention of the pathogen entrance by supporting the formation of physical barriers through lignification and suberization.268 For instance, the heat treatment significantly induced the ornithine accumulation in ‘Kamei’ Satsuma mandarin (C. unshiu) pericarp, which was associated with higher lignin content and lower disease incidence of P. italicum.262 Likewise, the lignin content was increased in discs of orange peel after 60-hours of the treatment with K. apiculata or its cell-free extract264 which might be involved in enhancement of fruit resistibility in response to postharvest fungal infection. In conclusion, PAs play a key role in citrus response(s) to fungal infections at multiple levels including regulation of oxidative stress and/or supporting the formation of physical barriers through lignification(Figure 5(b)).
Role of PAs in citrus response(s) to Bacterial infection
Citrus and its relative species are threatened by several bacterial pathogens that cause considerable economic losses and a severe reduction in fruit quality worldwide.269 The citrus bacterial disease including bacterial spot(Xanthomonas alfalfae subsp. citrumelonis), black pit and blast (Pseudomonas syringae), citrus canker (Xanthomonas citri subsp. citri [Xcc]), citrus variegated chlorosis (CVC; Xylella fastidiosa), Huanglongbing (HLB; Candidatus Liberibacter spp.), and stubborn disease (Spiroplasma citri).255,256,269 Among these diseases, citrus canker, CVC, and HLB are the most economically important bacterial disease of citrus, therefore, these three diseases will be our focus in the next paragraphs.
PAs-based defense responses against citrus canker
Citrus canker is a destructive citrus bacterial disease worldwide, that caused by a Gram-negative obligately aerobic, straight rods Gammaproteobacterium belonging to genus Xanthomonas.270,271 Citrus canker is affecting several genera from the Rutaceae family such as Citrus spp., Fortunella spp., and Poncirus spp., including the economically important cultivated hosts such as orange, grapefruit, lime, lemon, pomelo, and citrus rootstock.271 Citrus canker disease is caused by two groups of phylogenetically distinct Xanthomonas strains. The first group (Asiatic group) named Xanthomonas citri (synonyms: X. campestris pv. citri pathotype A and X. axonopodis pv. citri), while the second phylogenetically distinct group (South American group) named X. campestris pv. aurantifolii (synonyms: X. campestris pv. citri and X. axonopodis pv. aurantifolii).270,271 These groups cause identical symptoms using, almost, similar common pathogenicity factors/mechanisms,however, both groups differ in their natural occurrence and host range.269,271
Although enormous work has been focused on creating transgenic citrus plants for canker resistance (Table 9), only one trail was done to target a PAs biosynthetic gene.294 Fu et al. (2011a) showed that the overexpression of apple (Malus sylvestris) spermidine synthase gene (MdSPDS1) in ‘Anliucheng’ sweet orange (C. sinensis) increased the cellular endogenous PAs contents but reduced the susceptibility of transgenic plants to X. axonopodis pv. citri (Xac), the causal agent of citrus canker.294 Upon Xac infection, the transgenic line TG9 had significantly higher spermine levels, PAO activity, antioxidant enzymatic activities (SOD and CAT) and induced H2O2 accumulation.294 The accumulation of H2O2 could be derived from the PAO-mediated oxidation of spermidine or spermine304,305 to trigger the hypersensitive response (HR) or to activate the defense-related genes such as chitinase, cystatin-like protein, pathogenesis-related protein 4A (PR4A) and AOS.294,295 In addition, the genetic modification of PAs biosynthesis pathway may cause transcriptional reprogramming in the transgenic plants, which might be regulated by spermine as it was previously proposed as a signal molecule.234,306 For example, 13 transcription factors (TFs) were upregulated in the canker-resistant transgenic line TG9 that had a higher spermine level.295 The transcriptional up-regulation toward the disease tolerance including AP2 domain-containing transcription factor, three AP2/ERF domain-containing, BT4 (BTB and TAZ domain protein 4) protein, chromatin remodeling complex subunit, FLC-like 1 splice variant 4, guanylyl cyclase, MADS, MYB, NAC, and two unnamed putative TFs295 (Figure 6).
Table 9.
Genetic transformation, transient expression, or over-expression in citrus and its related genera of genes that have been conferred resistance against citrus canker disease.
| Genes | Source | ‘Cultivar’/Variety/(species) | References |
|---|---|---|---|
| Attacin A | Tricloplusia ni | `Hamlin’ Sweet Orange(C. sinensis) | 272 |
|
T. ni T. ni T. ni |
`Valencia’ Sweet Orange(C. sinensis) `Natal’ Sweet Orange(C. sinensis) `Pera’ Sweet Orange(C. sinensis) |
273 273 273 |
|
| Xa21 | Wild rice (Oryza longistaminata) | ‘Early Gold’ sweet orange (C. sinensis) | 274 |
| Wild rice (O. longistaminata) | `Hamlin’ Sweet Orange(C. sinensis) | 275-277 | |
| Wild rice (O. longistaminata) Wild rice (O. longistaminata) Wild rice (O. longistaminata) |
`Valencia’ Sweet Orange(C. sinensis) `Natal’ Sweet Orange(C. sinensis) `Pera’ Sweet Orange(C. sinensis) |
277 277 277 |
|
| Wild rice (O. longistaminata) | ‘Anliucheng’ sweet orange (C. sinensis) | 278 | |
FLS2
|
Tobacco (Nicotiana benthamiana) Tobacco (N. benthamiana) |
`Hamlin’ Sweet Orange(C. sinensis) ‘Carrizo’ citrange (C. sinensis × P. trifoliata) |
279 279 |
| ‘Duncan’ grapefruit (C. paradisi) ‘Sun Chu Sha’ mandarin (C. reticulata) ‘Nagami’ kumquat (Fortunella margarita) |
‘Duncan’ grapefruit (C. paradisi) ‘Duncan’ grapefruit (C. paradisi) ‘Duncan’ grapefruit (C. paradisi) |
280 280 280 |
|
| Bs2 | Pepper (Capsicum annuum) | Lemon (C. limon) | 281 |
| Bs2 | Pepper (C. chacoense) | ‘Pineapple’ sweet orange (C. sinensis) | 282 |
| AtNPR1 | Arabidopsis thaliana | `Hamlin’ Sweet Orange(C. sinensis) | 283,284 |
| Stx 1A | Flesh fly (Sarcophaga peregrina) | Sweet orange (C. sinensis) | 285,286 |
| terf1 | Tomato | ‘Succari’ sweet orange (C. sinensis) | 287,288 |
| hrpN | Erwinia amylovora | `Hamlin’ Sweet Orange(C. sinensis) | 289 |
| CalS1 | C. reshni | Lemon (C. limon) | 290 |
|
pthA-nls pthA-nls |
Xanthomonas axonopodis pv. citri X. axonopodis pv. citri |
‘Succari’ Sweet Orange(C. sinensis) ‘Bingtang’ Sweet Orange(C. sinensis) |
291 291 |
| CtNH1 | Pummelo (C. maxima) | ‘Duncan’ grapefruit (C. paradisi) | 292 |
| CsMAPK1 | Sweet orange(C. sinensis) | Troyer citrange | 293 |
| MdSPDS1 | Apple (Malus sylvestris) | ‘Anliucheng’ sweet orange (C. sinensis) | 294,295 |
| Der | Phyllomedusa sauvagii | ‘Pineapple’ sweet orange (C. sinensis) | 296 |
| CsMAF1 | Sweet Orange(C. sinensis) | `Hamlin’ Sweet Orange(C. sinensis) | 297 |
|
RpfF RpfF |
Xylella fastidiosa X. fastidiosa |
‘Pineapple’ sweet orange (C. sinensis) ‘Carrizo’ citrange (C. sinensis × P. trifoliata) |
298 298 |
| hsr203 J | Tobacco (Nicotiana tabacum cv. Xanthi) | ‘Jincheng’ orange (C. sinensis) | 299 |
| PPP1 | Tobacco (N. tabacum cv. Xanthi) | ‘Jincheng’ orange (C. sinensis) | 299 |
| gst1 | Potato (Solanum tuberosum) | ‘Jincheng’ orange (C. sinensis) | 299 |
| Mthionin | Synthetic modified thionin | ‘Carrizo’ citrange (C. sinensis × P. trifoliata) | 300 |
| CuSTS3-1 | ‘Miyagawawase’ satsuma mandarin (C. unshiu) | `Hamlin’ Sweet Orange(C. sinensis) | 301 |
| CsLOB1 | Sweet Orange (C. sinensis) | ‘Duncan’ grapefruit (C. paradisi) | 302,303 |
Figure 6.
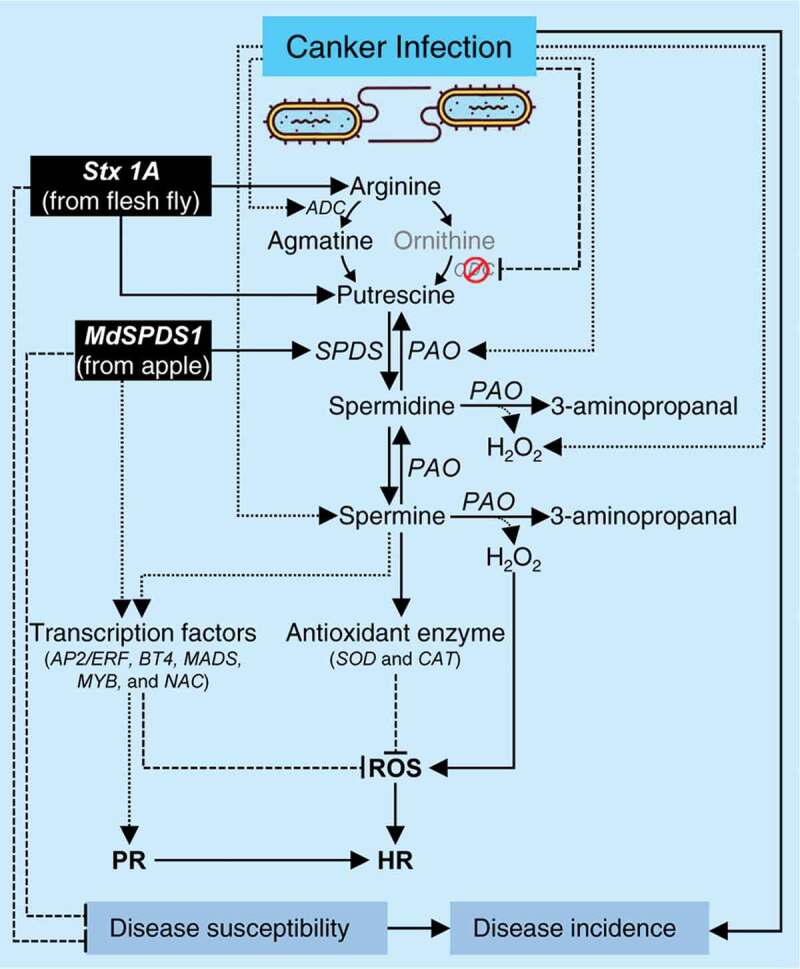
Hypothetical model of the potential polyamines- (PAs-) based response and its integration with different phytohormone pathways in citrus response to citrus canker disease caused by Xanthomonas citri subsp. citri (synonym: X. axonopodis pv. citri). Solid lines with arrowhead signify positive reaction, dashed lines with blunt-heads indicate negative interaction, while round-dotted lines with arrows represent hypothetical mechanisms or uncharacterized elements. For more details and abbreviations, see the main text.
Furthermore, the introduction of antibacterial peptide sarcotoxin IA (Stx 1A) from flesh fly (Sarcophaga peregrina) into ‘Pera’ Sweet orange (C. sinensis) enhanced the canker resistance in the transgenic lines.285 Interestingly, the Stx 1A transgenic lines had higher levels of putrescine and its precursor L-arginine than non-transgenic plants (wild type) under normal growth condition.307 Likewise, the putrescine level has been elevated in non-transgenic ‘Pera’ Sweet orangeleaves upon Xac infection,307 which might play a role in citrus response to Xac. Recently, the exogenous treatment of cancer-infected sweet orange plants with spermidine enhanced the endogenous content of putrescine and spermidine, but not spermine.308 Additionally, the exogenously applied spermidine reduced disease incidence, index, and symptom, whereas it up-regulated the transcript levels of four defense-related genes, including AOS, PR4A,glutathione peroxidase(GPX), and chitinase.308 Collectively, we suggest that PAs might play a key role in citrus defensive responses against citrus canker via regulation of defense-related genes and ROS homeostasis.
Additionally, PAs might be integrated with phytohormones and their metabolism-related genes in citrus response to Xcc. For instance, the transcriptomic analysis of citrus cDNA microarray showed that about 92 phytohormone metabolism-related genes were differentially regulated during the citrus response to Xcc.309 These genes involved in the metabolism ofabscisic acid (ABA),auxins, brassinosteroids (BRs), cytokinins (CKs), ethylene (ET), gibberellins (GAs), jasmonic acid (JA), and salicylic acid (SA), as well as the nitric oxide pathway(NO).309Interestingly, the Xcc-infection induced the accumulation of putrescine and spermidine,but not spermine, at 12- and 24-hours post-inoculation (hpi). The accumulation of putrescine and spermidine was associated with higher levels of JA but not the other phytohormone groups.309 Together,these findings suggest that the JA activation could involve a type of interaction-dependent regulation of free PAs levels, particularly putrescine and spermidine.
Furthermore, the transcriptomic analysis of putative PAs metabolism-related genes in citrus cDNA microarray showed that a putative ornithine decarboxylase (ODC) was down-regulated, while the arginine decarboxylase 1 (ADC) up-regulated after the infection with Xcc.309 These findings suggest that the accumulation of putrescine, and subsequently spermidine, might be due to the activation of PAs biosynthesis via thearginine-agmatine-putrescine route involving ADC, rather than the arginine-ornithine-putrescine route involving ODC. In addition, Xcc-infection induced the transcriptional levels of three polyamine oxidase genes (PAOs).309 PAOs are involved in the back conversion of spermidine and spermine to putrescine, which could partially explain the accumulation of putrescine after Xcc infection. Likewise, the transcriptional levels of a putative copper amine oxidase family protein (CuAO) were induced in the Xcc-infected citrus plants.309 Interestingly, the CuAO was up-regulated in the canker resistant ‘Meiwa’ kumquat (Fortunella crassifolia) and the moderate resistant M-9 cybrid (Cybrid-K type) after Xcc infection.310 On the other hand, under the same conditions, CuAO was down-regulated in the canker susceptible ‘Marsh’ grapefruit (C. paradisi) plants and M-102 cybrid (Cybrid-G type).310 Collectively, these findings suggest that CuAO might play a key role in citrus responses against Xcc infection.
PAs-based defense responses against citrus variegated chlorosis (CVC)
Citrus Variegated Chlorosis (CVC) is a destructive bacterial disease in citrus. CVC is caused by the Gram-negative, xylem-limited, insect-transmitted Gammaproteobacterium Xylella fastidiosa.311,312 Although CVC could be transmitted by grafting, however, it is mainly transmitted by several xylem sap-sucking insects including sharpshooters (Cicadellidae), treehoppers (Membracidae), and spittlebugs (Cercopidae).In Brazil, CVC might be transmitted by 11 species of sharpshooters,313 however, only two of them were found in Florida including the glassy-winged sharpshooter, Homalodisca vitripennis, and the blue-winged sharpshooter, Oncometopia nigricans,314 but the CVC disease is not reported in the USA yet. Since X. fastidiosa colonizes the xylem vessels, it might form a biofilm that blocks the flow of xylem sap, causing a water deficit-like symptom in the upper part of the infected trees.315 X. fastidiosa can infect most of the citrus species and hybrids causing different degrees of symptoms severity. Sweet orange (C. sinensis) varieties are highly susceptible to X. fastidiosa infection, while Rangpur lime, lemon, citron, and pummelo are considered CVC-tolerant varieties and remain symptomless, regardless of the inoculum pressure. Grapefruit, mandarins, mandarin hybrids, lemons, limes, kumquat, and trifoliate orange are moderately susceptible, showing less severe symptoms.
Upon the infection with X. fastidiosa, citrus plants develop a complex multilayered- defensive responses at the physiological, genetic, and metabolic levels.315–317 Due to the difficulties found in the study of X. fastidiosa-citrus pathosystem, very few studies were carried out the elucidate the metabolic changes that happen in citrus plants upon the infection with X. fastidiosa. These metabolic changes included some phytohormones318 and nitrogen metabolism.316 Nitrogen metabolism is a key factor that affects the growth of X. fastidiosa,319 as well as, X. fastidiosa disturbs the nitrogen metabolism of citrus plants.315,316,320 For example, the PAs-associated amino acids, L-proline, and L-arginine were increased atthe initial stages of CVC development in the leaves of ‘Pêra’ sweet orange (C. sinensis) grafted on Rangpur lime (C. limonia).320 Furthermore, the diamine putrescine was detected in higher levels in leaves and petioles of X. fastidiosa-infected plants.316 The accumulation of putrescine might be due to the induction of its precursor arginine which increased in leaves and xylem sap of X. fastidiosa-infected plants.316 Nevertheless, it is not clear yet if the accumulation of both putrescine and arginine is a direct plant response to the X. fastidiosa infection or it is due to the water deficit-like symptom imposed by the xylem blocking since both water deficiency and pathogens attack are known to induce the endogenous PAs levels in plants. However, more investigations are required to clarify the putative PAs-based defensive response against CVC and its integration with other metabolic responses, particularly phytohormones.
Roles of PAs in the Huanglongbing (HLB; citrus greening) pathosystem
Huanglongbing (HLB; aka citrus greening disease) is a destructive bacterial disease of rutaceous plants that can infect most, if not all, citrus cultivars, hybrids, and some citrus relatives. Although Koch’s postulates of HLB have not been fulfilled yet due to the difficulty in culturing the putative bacterium, several pieces of evidence indicate that HLB is associated with a plant fastidious, phloem-limited, phytopathogenic, alphaproteobacterium Candidatus Liberibacter spp..54,321–323 Based on the geographical distribution and the characteristic 16 S rDNA sequence, three bacterial species have been proposed to be associated with HLB.54,324–326 ‘Ca. L. asiaticus’in Arabian Peninsula, Africa, Asia, and the Americas, Ca. L. americanusspreading only in Brazil, andCa. L. africanusinnumerous countries in Africa.54,323–328 Among the three Ca. Liberibacter species, ‘Ca. L. asiaticus’is the dominant species and is causing huge economic losses to citrus production worldwide.54,323The tree-to-tree transmission of Ca. Liberibacter could be by graft inoculation, and they are mainly transmitted by insect vectors called citrus psyllids.329 HLB is transmitted by two insect vectors; the African citrus psyllid, Trioza erytreae Del Guercio (Hemiptera: Triozidae), which transmits Ca. L. africanus323,330 and the Asian citrus psyllid, Diaphorina citriKuwayama(Hemiptera:Liviidae), which transmits both ‘Ca. L. asiaticus’ and Ca. L. americanus.323,331,332 Globally, D. citri, a phloem sap-sucking insect, is one of the most serious pests of citrus, particularly when ‘Ca. L. asiaticus’ is present329 because it harms the new leaves and fruits in addition to its ability to transmit ‘Ca. L. asiaticus’.
Both ‘Ca. L. asiaticus’ and its vector manipulate the host metabolism for their benefit to meet their nutritional needs or to neutralize the host defense responses. In addition, upon ‘Ca. L. asiaticus’-infection and/or D. citri-infestation, citrus plants develop multiple defense mechanisms to protect themselves. Previous studies showed that both ‘Ca. L. asiaticus’-infection and D. citri-infestation can cause many biochemical and metabolic alterations in citrus plants. For instance, both ‘Ca. L. asiaticus’-infection and D. citri-infestation can alter the volatile organic compounds,203,333–336 amino acids,1–6,337,338 organic acids,1,3,6,337 fatty acids,2,6 phytohormones,7,8 polyamines,4,12 carotenoids and leaf pigments202,339 and other secondary metabolites333,337 such as phenolic compounds333,340–342 and flavonoids.339,342–344 However, it remains unclear whether these changes benefit ‘Ca. L. asiaticus’and its vector, or if they are a plant response or both. Although many studies have been carried out to understand the metabolic-based defense responses of citrus plants against ‘Ca. L. asiaticus’ and its vector, only a few studies about the role of PAs pathway in regulating citrus defense responses and HLB symptoms development have been reported.
Based on comparative genomic studies of uncultured strains of ‘Ca. L. asiaticus’, ‘Ca. L. solanacearum’, and their closest cultured species L. crescens BT-1, ‘Ca. L. asiaticus’ was found to lack the biosynthetic pathways and transporters for some amino acids, as well as, PAs biosynthesis pathway.345 For instance, ‘Ca. L. asiaticus’ lack the de novo biosynthetic pathways for six amino acids including L-cysteine, L-histidine, L-phenylalanine, L-proline, L-tryptophan, and L-tyrosine which were reported to be produced by L. crescens.345–347 Likewise, the putrescine biosynthetic pathway was found in L. crescens but it was reported to be partially incomplete in ‘Ca. L. asiaticus’, which lacks the ODC enzyme.345 In other words, ‘Ca. L. asiaticus’ can metabolize the putrescine but it lacks the necessary enzymes for its de novo biosynthesis. Therefore, ‘Ca. L. asiaticus’ might depend on their host plant to get its PAs needs. Recently, we showed that ‘Ca. L. asiaticus’ infection induces the total PAs, ornithine, and putrescine content,12 which may establish a vital host-dependence for its nutrition needs. However,‘Ca. L. asiaticus’ genome does not possess any PAs transporters that could import putrescine or its precursor, ornithine, based on the previous comparative genomic study with L. crescens345 or in-depth prediction of ABC transporters in ‘Ca. L. asiaticus’.348
Herein, we are going to discuss the previous and most recent integrative studies using transcriptomics, proteomics, and metabolomics approaches to understand the citrus response(s) against ‘Ca. L. asiaticus’ and/or its insect vector D. citri. We believe that the metabolic responses of citrus plants may result from host cellular functions for defense reactions, or it may result from the manipulation of metabolic pathways by ‘Ca. L. asiaticus’ and/or D. citri for their benefits to fulfill their nutritional needs, or to induce symptoms development.
Deciphering the Role of Citrus-PAs in HLB Symptoms Development
The most characteristic symptom of HLB is the blotchy mottle, which is an asymmetrical discoloration/chlorosis across the mid-vein of the leaf with patches of yellow and green islands.323,349 Symptoms of HLB are induced by both the pathogen and its vector, due to alteration in many physiological aspects such as phytohormonal levels, carbohydrate status,7 and carotenoids and leaf pigments content.202,339 Additionally, PAs might be involved in HLB symptom development via the production of H2O2. Briefly, diamine oxidase (DAO) and PAO catalyze the oxidation of putrescine and spermidine, respectively, to produce γ-aminobutyric acid (GABA) and resulting in accumulation of H2O2 within the apoplast.304,305 The accumulation of H2O2 in citrus plants after the infection with ‘Ca. L. asiaticus’ has been reported previously.350 In our previous study, GABA was accumulated and both D-amino acid oxidase PA4548 (aka diamine oxidase CsDAO) and CsPAO were expressed at higher levels in ‘Ca. L. asiaticus’ -infected plants,12 which is in agreement with this common feature of pathogen-host interaction in many previous studies.304,305 Thus, we hypothesize that the induction of PAs and their catabolic genes (CsDAO and CsPAO) are correlated with the accumulation of GABA and H2O2 which are working as a response to HLB to create an incompatible interaction. Nevertheless, the alteration in DAO and PAO, and the accumulation of H2O2 have to occur in the appropriate location of the plant to be effective in the creation of incompatible interactions between the host and the pathogen.305 Interestingly, ‘Ca. L. asiaticus’ could survive the toxic effects of accumulated H2O2 using its own peroxidase.350 The detoxification system of citrus plants, however, might not be sufficient to reduce the high H2O2levels, which may eventually become toxic to the leaf tissue and cause the characteristic blotchy mottle symptoms that appear after ‘Ca. L. asiaticus’-infection.350
PAs-based defense responses against HLB
Recently, we showed that ‘Ca. L. asiaticus’-infection induced the PAs biosynthesis, leading to an accumulation of ornithine and putrescine (Figure 7), and their precursor L-arginine, which detected only in ‘Ca. L. asiaticus’-infected plants, but not other treatments.12 Similar putrescine induction was found in the juice from ‘Valencia’ Sweet Orange (C. sinensis) fruits from ‘Ca. L. asiaticus’-infected trees333 and leaves from D. citri-infested trees4 (Table 10). Furthermore, the semi-tolerant varieties such as ‘Volkamer’ lemon (C. volkameriana), Alemow (C. macrophylla),’Mexican’ lime (C. aurantifolia), and ‘Palestine Sweet’ Lime (C. aurantifolia); and the very tolerant variety, Swingle(C. latipes)356 has a greater putrescine content. Moreover, the putrescine conjugate, feruloyl putrescine, was found at high levels in juice from HLB-symptomatic ‘Valencia’ sweet orange (C. sinensis) fruits but did not change significantly in both ‘Hamlin’ or ‘Midsweet’ oranges compared to juice from HLB-asymptomatic and healthy fruit.333 Collectively, these findings indicate that putrescine plays a key role in citrus response/tolerance against ‘Ca. L. asiaticus’. In plants, putrescine is synthesized through two different routes; from arginine using ADC or from ornithine using ODC.360 Interestingly, both CsADC and CsODC were expressed at higher levels in ‘Ca. L. asiaticus’-infected plants indicating that both putrescine biosynthetic pathways are jointly working to enhance the PAs content (Figure 8). Likewise, the arginine pathway metabolites (ornithine, citrulline, and L-proline) were found in much higher abundance in the HLB-susceptible cultivar in response to ‘Ca. L. asiaticus’-infection353
Figure 7.
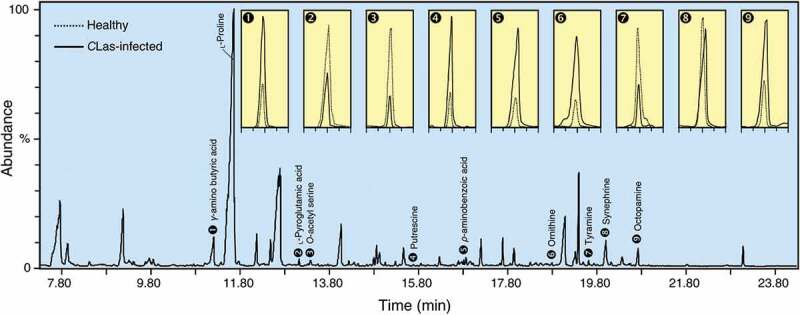
Representative chromatogram of detected non-proteinogenic amino acids (NPAAs) and polyamines (PAs) compoundsof Valencia sweet orange (C. sinensis) leaves after derivatization with MCF using GC-MS running in full-scan mode. Citrus leaf NPAAs and PAs compounds were extracted from frozen tissue using methanol 80% containing 0.1% HCl 6N as described in our previous studies.6,351 Leaf extract was derivatized with methyl chloroformate (MCF) following the protocol of Hijaz and Killiny 2014,201 with slight modifications as described by Killiny and Nehela 2017.6 Derivatized samples were analyzed using a Clarus 680 GC-MS system (Perkin Elmer, Waltham, MA, USA) fitted with a ZB-5 MS GC column (Phenomenex, Torrance, CA, USA) using the GC thermo-program described in our previous study.6 Chromatographic characteristics, MS ion identification, and GC-MS chromatograms analysis were performed according to Killiny and Nehela 2017.6
Table 10.
Changes in the endogenous content of major polyamines (PAs), γ-Aminobutyric acid (GABA), Ornithine (Orn; a PAs precursor), and their associated amino acids in citrus and its relative species as affected by Candidatus Liberibacter asiaticus (the causal agent of Huanglongbing) and/or its vector, Diaphorina citri.
| ‘Cultivar’/Variety/(species) | Tissue | Treatment/experimental design | Pro a | Arg | GABA | Orn | Put | Spd | Spm | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Valencia’ Sweet Orange (Citrus sinensis) b | Fruits | Orange juice from fruits harvested from healthy vs. ‘Ca. L. asiaticus’-infected trees in two seasons | - | - | - | - | ▲ | - | - | 333 |
| ‘Valencia’ Sweet Orange (C. sinensis) | Leaves | Leaves from HLB‐infected, zinc‐deficient, and healthy trees using GC‐MS | ▲ | - | - | - | - | - | - | 1 |
| Four citrus varieties c | Leaves | Two HLB-tolerant citrus varieties vs two HLB-sensitive varieties | ↑↓ | - | - | - | - | - | - | 2 |
| ‘Duncan’ grapefruit (C. paradisi) | Leaves | New flushes of healthy vs HLB-infected trees at 14 and 20 weeks after inoculation | ▲ | - | - | - | - | - | - | 2 |
| ‘Valencia’ Sweet Orange (C. sinensis) | Fruits | Orange juice obtained from healthy, asymptomatic, and symptomaticfruits | ▼ | ▼ | ● | - | - | - | - | 3 |
| ‘Valencia’ and ‘Hamlin’ Sweet Orange (C. sinensis) | Fruits | Orange juice from healthy, HLB-asymptomatic, and -symptomatic oranges | ▼ | - | ▲ | - | ● | - | - | 338 |
| ‘Owari’ Satsuma orange (C. unshiu) | Leaves | Effect of D. citri infestation on leave metabolites (healthy vs D. citri-infested) | ▲ | ▲ | - | - | ▲ | ● | ● | 4 |
| ‘Valencia’ Sweet Orange (C. sinensis) | Leaves & Roots | HLB-asymptomatic vs. -symptomatic tissues from 6-year-old commercial orchard | ● | - | - | - | - | - | - | 352 |
| Six citrus varieties d | Leaves | Comparing five tolerant hybrids with HLB-sensitive cultivar (healthy vs ‘Ca. L. asiaticus’ -infected) | ▲ | ▲ | - | ▲ | - | - | - | 353 |
| Different hosts e | Phloem sap | The phloem sap composition of orange jasmine vs. Curry leaf tree using GC-MS | ▲ | ▲ | ▲ | ▲ | ▲ | - | - | 354 |
| Fourteen citrus varieties f | Phloem sap | 13 citrus varieties with different tolerance degrees to ‘Ca. L. asiaticus’ (MCF derivatization) | ▲ | - | ▲ | - | - | - | - | 5 |
| Alemow (C. macrophylla) | Leaves | The effect of D. citri feeding (0, 5, 15–20, or 25–30 insect/plant) on leaf metabolites. | ● | ▲ | ▲ | - | - | - | - | 355 |
| Fourteen citrus varieties g | Phloem sap | 14 citrus varieties with different tolerance degrees to ‘Ca. L. asiaticus’ (TMS derivatization) | ▲ | ▲ | ▲ | - | ▲ | - | – | 356 |
| ‘Valencia’ Sweet Orange (C. sinensis) | Leaves | Tested the effect of ‘Ca. L. asiaticus’ -infection and/or D. citri-infestation on leaf metabolites | ▲ | - | ▲ | - | - | - | - | 6 |
| Different hosts h | Phloem sap | Four ‘Ca. L. asiaticus’ -permissive host plants vs. three non-permissive Rutaceae plants | ↑↓ | ↑↓ | ↑↓ | - | - | - | - | 357 |
| ‘Ray Ruby’ grapefruit (C. paradisi) i | Leaves | Leaves from HLB‐infected vs. healthy trees using GC‐MS | ▲ | ▲ | - | - | - | - | - | 358 |
| ‘Hamlin’ Sweet Orange (C. sinensis) | Fruits | Orange juice obtained from healthy, asymptomatic, and symptomaticfruits | ▲ | ● | - | - | - | - | - | 359 |
| ‘Valencia’ Sweet Orange (C. sinensis) | Leaves | Tested the effect of ‘Ca. L. asiaticus’ -infection and/or D. citri-infestation on leaf PAs | ▲ | ▲ | ▲ | ▲ | ▲ | - | - | 12 |
a(▲) signifies for increased levels, (▼) signifies for decreased levels, (●) signifies for no significant change, (↑↓) signifies for varied responses, and (˗) signifies for not-reported compounds. Abbreviations: Pro:L-proline, Arg:L-Arginine, GABA:γ-Aminobutyric acid, Orn: Ornithine, Put: putrescine, Spd: spermidine, and Spm: spermine.
bdetermined as feruloyl putrescine333
cThey tested the leave metabolites of two HLB-tolerant citrus varieties (‘Carrizo’ citrange [×Citroncirus webberi] and Trifoliate Orange [P. trifoliata]) and two HLB-sensitive varieties (‘Madam Vinous’ sweet orange [C. sinensis] and ‘Duncan’ grapefruit [C. paradisi]) using GC-MS after derivatization with methoxyamine and N-methyl-N-trifluoroacetamide (MSTFA).2
dThey tested the leave metabolites of five tolerant hybrids in comparison with HLB-sensitive cultivar (healthy vs ‘Ca. L. asiaticus’-infected) using GC-TOF MS after methoximation and trimethylsilylation derivatization. The tested varieties included: Cleopatra mandarin (C. reticulata), Carrizo citrange (C. sinensis × P. trifoliata), US-802 (C. grandis ‘Siamese pummelo’ × P. trifoliata ‘Gotha Road’), US-812 (C. reticulata ‘Sunki’ × P. trifoliata ‘Benecke’), US-897, and US-942 (C. reticulata ‘Sunki’ × P. trifoliata ‘Flying Dragon’).353
eThe author studied the phloem sap composition of Valencia sweet orange (C. sinensis), Curry leaf tree (Bergera koenegii; a specific host for D. citri, but not ‘Ca. L. asiaticus’), and orange jasmine (Murraya paniculate; an alternative host for both D. citri and ‘Ca. L. asiaticus’).354
fThey tested the phloem sap composition of 13 citrus varietieswith different tolerance degrees to ‘Ca. L. asiaticus’ using GC-MS after methyl chloroformate (MCF) derivatization. The tested varieties included: ‘Valencia’ sweet orange (C. sinensis), ‘Duncan’ grapefruit (C. paradise), ‘Ruby Red’ grapefruit (C. paradisi), ‘Madam Vinous’ sweet orange (C. sinensis), Alemow (C. macrophylla), ‘Mexican’ lime (C. aurantifolia), ‘Palestine Sweet’ Lime (C. aurantifolia), ‘Sour’ orange (C. aurantium), ‘Volkamer’ lemon (C. volkameriana), Chinese box orange (Severinia buxifolia), Poncirus trifoliata, Carrizo citrange trifoliate hybrid (‘Washington’ sweet orange [C. sinensis] × P. trifoliata), and Swingle(C. latipes).5
gThey tested the metabolite signature of the phloem sap of 14 citrus varietieswith different tolerance degrees to ‘Ca. L. asiaticus’ using GC-MS after trimethylsilyl (TMS) derivatization. The tested varieties included: ‘Valencia’ sweet orange (C. sinensis), ‘Pineapple’ sweet orange (C. sinensis), ‘Duncan’ grapefruit (C. paradise), ‘Ruby Red’ grapefruit (C. paradisi), ‘Madam Vinous’ sweet orange (C. sinensis), Alemow (C. macrophylla), ‘Mexican’ lime (C. aurantifolia), ‘Palestine Sweet’ Lime (C. aurantifolia), ‘Sour’ orange (C. aurantium), ‘Volkamer’ lemon (C. volkameriana), Chinese box orange (Severinia buxifolia), Poncirus trifoliata, Carrizo citrange trifoliate hybrid (‘Washington’ sweet orange [C. sinensis] × P. trifoliata), and Swingle(C. latipes).356
hThey tested the phloem sap composition of four ‘Ca. L. asiaticus’-permissive host plants vs. three non-permissive Rutaceae plants using a High-Speed Amino Acid Analyzer. The tested ‘Ca. L. asiaticus’-permissive host plants included: ‘Rio Red’ Grapefruit (C.paradisi), ‘Marrs’ Sweet orange (C. sinensis), ‘Valley’ Lemon (C. limon), and ‘Madagascar’ periwinkle (Catharanthus roseus); while the non-permissive plants included: Curry leaf (Murraya koenigii), ‘Lakeview’ Orange jasmine (Murraya exotica), and White sapote (Casimiroa edulis).357
iThey also studied the metabolic changes in leaves from ‘Ca. L. asiaticus’‐infected vs. healthy trees of Hamlin orange (C. sinensis), Murcott mandarin (C. reticulata), but without any significant differences between healthy and ‘Ca. L. asiaticus’‐infected plants for those varieties.358
Figure 8.
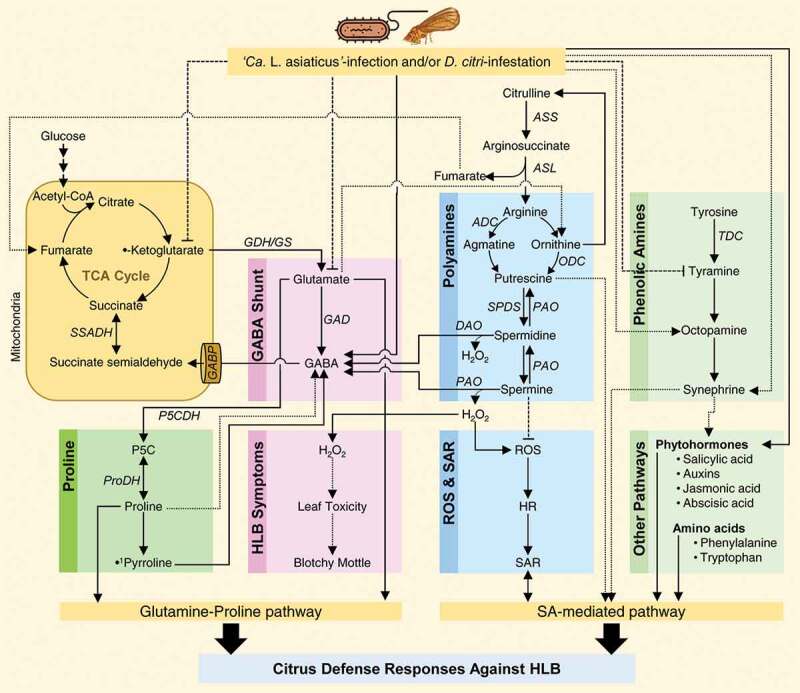
Hypothetical model of the potential polyamines- (PAs-) based response and its integration with other metabolic pathways in citrus response to Huanglongbing (aka citrus greening disease) caused by Candidatus Liberibacter asiaticus. Solid lines with arrowhead signify positive reaction, dashed lines with blunt-heads indicate negative interaction, while round-dotted lines with arrows represent hypothetical mechanisms or uncharacterized elements. For more details and abbreviations, see the main text.
In one of our recent studies, we proposed a hypothetical model for the effect of ‘Ca. L. asiaticus’ infection and/or D. citri infestation on the proteinogenic amino acids, NPAAs, PAs, and tricarboxylic acid- (TCA-) associated compounds of Valencia sweet orange (C. sinensis) leaves.12 In this model, we proposed that citrus plants have an alternative pathway(s) that contribute to the flux toward succinate thereby increasing the availability of fumarate and citrate rather than as an intact TCA cycle (Figure 8). GABA-shunt and PAs biosynthesis pathway are potential candidates for these alternative pathways (from α-ketoglutarate to succinate) and they might be enhanced after under specific abiotic and biotic stresses such as ‘Ca. L. asiaticus’-infection and D. citri-infestation.12 Briefly, in our published model, we proposed that ‘Ca. L. asiaticus’-infection might accelerate the conversion of α-ketoglutarate to glutamate, then to GABA, which results in an accumulation of GABA in the cytosol. Subsequently, GABA is transported from the cytosol to the mitochondria using the GABA permease (GABP), to support the accumulation of succinate directly or indirectly. Interestingly, GABA was detected at high concentration in the HLB-tolerant citrus varieties such as P. trifoliate, Swingle(C. latipes), ‘Volkamer’ lemon (C. volkameriana), Carrizo citrange trifoliate hybrid (‘Washington’ sweet orange [C. sinensis] × P. trifoliata), and Chinese box orange (Severinia buxifolia), 5,356 which support our point of view that GABA plays an important role in citrus response against HLB.
Another pathway that could be involved in the enrichment of the TCA cycle is the PAs biosynthesis pathway.PAs biosynthesis pathway could be involved in the enrichment of the TCA cycle directly through the production of fumarate or indirectly via enhancement of GABA-shunt. The dicarboxylic acid ‘fumarate’ is produced during the conversion of citrulline to argininosuccinate by argininosuccinate synthase (ASS), then to the amino acid arginine using argininosuccinate lyase (ASL; aka argininosuccinase) which splits argininosuccinate to release fumarate and arginine.361 Our findings showed that both CsASS and CsASL were upregulated after ‘Ca. L. asiaticus’-infection, which resulted in the accumulation of arginine (detected only in ‘Ca. L. asiaticus’-infected plants). Together, this might enhance the TCA cycle intermediate fumarate directly (Figure 8). Similar induction of fumarate was observed during the necrotrophic phase of rice blast fungus Magnaporthe grisea362 and the maize anthracnose fungus Colletotrichum graminicola.363
Although several studies have been reported the effect of ‘Ca. L. asiaticus’ infection on citrus metabolites, however, the role of PAs in the HLB pathosystem remains poorly understood because some of these studies reported the effect of ‘Ca. L. asiaticus’ infection on the endogenous content of putrescine only (Table 10) but, to our knowledge, the effect of ‘Ca. L. asiaticus’ infection on both spermidine and spermine was not reported till now. We believe that all PAs, not onlyputrescine, might play a role in citrus defense against ‘Ca. L. asiaticus’ and its vector, via the integration with phytohormones, phenolic amines (such as synephrine), GABA shunt, and stress-associated amino acids, such as proline (Figure 8).
The phenolic monoamine ‘synephrine’ and its precursor, octopamine, are compounds that might be involved with PAs in the defense response against HLB. Synephrine was reported previously in several citrus species and cultivars.364,365 In citrus, synephrine is biosynthesized from tyrosine via a pathway involving tyramine, N-methyltyramine, then to synephrine using tyrosine decarboxylase (TDC), tyramine N-methyltransferase (TNMT), and an unidentified N-methyl-tyramine-β-hydroxylase (NMTβH), respectively.365–367 While it synthesized through tyramine, octopamine, then to synephrine in animals, using TDC, TβH and PNMT, respectively.366 Although octopamine is not an important intermediate in plants as in animals, our results showed that the octopamine level was elevated in the presence of ‘Ca. L. asiaticus’.Furthermore, the transcript level of CsTDC was increasedin all studied treatments. Because only TDC has been isolated and cloned from plants, while other enzymes have not yet been cloned,367 our image about the synephrine-PAs pathway is not fully understood and it might require further studies.
Although many previous studies had investigated the L-proline content after ‘Ca. L. asiaticus’-infection and/or D. citri-infestation (Table 10), however, the integration of L-proline in the PAs-based defense responses of citrus plants against HLB is poorly studied. Higher levels of the amino acids L-proline, in healthy plants were characteristic of the most sensitive variety ‘Madam Vinous’ sweet orange (C. sinensis) when compared to HLB-tolerant varieties such as ‘Carrizo’ orange (Carrizo citrange) and P. trifoliate.2 Likewise, ‘Ca. L. asiaticus’-permissive hosts such as ‘Rio Red’ Grapefruit (C.paradisi), ‘Marrs’ Sweet orange (C. sinensis), ‘Valley’ Lemon (C. limon), and ‘Madagascar’ periwinkle (Catharanthus roseus) had a greater content of L-proline compared to the non-permissive plants such as Curry leaf (Murraya koenigii), ‘Lakeview’ orange jasmine (Murraya exotica), and white sapote (Casimiroa edulis).357 On the other hand, our previous study showed that the phloem sap of orange jasmine (M. paniculate; an alternative host for both D. citri and ‘Ca. L. asiaticus’) had a higher concentration of L-proline compared with the HLB-sensitive variety ‘Valencia’ sweet orange (C. sinensis) and the specific host of D. citri, Curry leaf tree (Bergera koenegii).354 Furthermore, the phloem sap of the semi-tolerant varieties such as’Sour’ orange (C. aurantium) and ‘Volkamer’ lemon (C. volkameriana); tolerant varieties such as Carrizo citrange trifoliate hybrid (‘Washington’ sweet orange [C. sinensis] × P. trifoliata) and Chinese box orange (Severinia buxifolia); and the very tolerant variety Swingle(C. latipes) had a higher L-proline content compared to HLB-sensitive varieties.5,356
In addition, higher levels ofL-proline have been previously reported in symptomatic ‘Ca. L. asiaticus’-infected1,2,6,12,358 and D. citri-infested leaves.4,355 However, L-proline was reported to be reduced in the juice of symptomatic HLB-infected ‘Valencia’ and ‘Hamlin’ Sweet Orange (C. sinensis) fruits.3,338 In disagreement, L-proline was accumulated in the juice obtained from asymptomatic and symptomatic HLB-infected ‘Hamlin’ Sweet Orange (C. sinensis) fruits.359 Nevertheless, in both studies, the PAs precursor amino acid,L-arginine, was not changed or slightly reduced in juice from HLB-symptomatic fruits.3,359 Interestingly, L-arginine was found at higher levels in the phloem sap of the alternative host, orange jasmine (M. paniculate)354 and the semi-tolerant varieties such as ‘Palestine Sweet’ Lime (C. aurantifolia) and ‘Volkamer’ lemon (C. volkameriana).356 Moreover, L-arginine was accumulated in citrus leaves after the infection with ‘Ca. L. asiaticus’12,358 and the infestation with D. citri.4,12,355
In addition, PAs might be integrated with phytohormones to mediate the citrus responses to ‘Ca. L. asiaticus’ and its vector, D. citri(Figure 8). Previously, we showed that multiple phytohormonal signaling mediates citrus responses to the HLB disease.8 Briefly, ‘Ca. L. asiaticus’-infection and D. citri-infestation alert the auxins, SAs, JAs, and ABA profiles of citrus leaves, but do not affect both cytokinins, and gibberellins levels.8 Likewise, the transcriptional analysis of sweet orange trees showed that the solo infection with ‘Ca. L. asiaticus’-B232 disrupted the ABA, auxin, ethylene, JA, and SA, but did not affect the brassinosteroid, cytokinin, or gibberellins pathways.238,239 Interestingly, the accumulation of different phytohormone groups and their related pathways in ‘Ca. L. asiaticus’-infected plants wereassociated with high levels of putrescine and ornithine.12 Furthermore, the exogenous application of GABA increased the level of different phytohormones in ‘Valencia’ sweet orange (C. sinensis).368 Likewise, the exogenous putrescine application (300 mg L−1) increased the IAA, SA, ABA and endogenous putrescine content, while it decreased the gibberellins content of sour orange (C. aurantium) seedlings under salt stress.369 Collectively, these findings support our suggestion that PAs might be integrated with phytohormones to mediate the citrus responses to ‘Ca. L. asiaticus’ and its vector, D. citri, however, more investigations are required for better understanding of PAs-based defense responses against HLB.
Conclusion Remarks and Future Prospects
In this review article, we comprehensively discussed the published literature on the role of PAs in citrus response to abiotic and biotic stress. We explored the PAs-based defensive responses of citrus against abiotic stress such as low- and high-temperature, salinity, heavy metals, drought stress. As well as, we deciphered the potential roles of PAs in citrus response to biotic stressors including virus and viroid infection, fungal and oomycetes infection, and bacterial infection, particularly, the vector-borne phytopathogenic bacteria.
In conclusion, PAs play a key role in protecting the citrus plant from stress-associated damages and enhancing stress tolerance through a multi-layered defense system. The PAs-based defense system involving; 1) the modulation of cellular PA levels, 2) interaction with other metabolic pathways, particularly phytohormones such as salicylic acid, auxins, jasmonic acid, abscisic acid, ethylene, cytokinins, and brassinosteroids, 3) activation of glutamine-Proline pathway and GABA Shunt, 4) activation the cascades of CBF/COR genes and SOS pathway members (SOS3, SOS2, and SOS1), and/or 5) the interaction with other defensive mechanisms such as Ca2+ influx, ROS detoxification, and induction of PAL enzymes and microRNAs.
We believe that the knowledge gained so far about the roles of PAs in citrus responses underpin our understanding PAs-based defensive responses and provide valuable information for the potential use of PAs or their synthetic analogs a promising approach to practical applications to enhance stress tolerance in citrus plants.
Although tremendous efforts have been done to understand the role of PAs in citrus responses to various stressors, however, most of them have been relatively simple and similar. Most previous studies have focused on the alteration of endogenous PAs content under biotic and abiotic conditions, modulation of cellular PAs content via exogenous PAs supplementation, or the induction of endogenous PAs production via genetic manipulation of PAs biosynthetic genes. Nevertheless, very few previous studies have been made to identify, clone, and functionally characterize PAs biosynthetic genes from citrus and its relative species including CsSAMDC79 and PtADC.82 Therefore, further studies are required for cloning, biochemical identification, function characterization, and expression analysis of the genes involved in PAs biosynthesis pathway in citrus, as well as its phytopathogenic microorganisms. For example, the phytopathogenic bacterium ‘Ca. L. asiaticus’ (the causal agent of citrus greening disease) has incomplete PAs biosynthesis pathway, which lacks ODC.345 Thus, ‘Ca. L. asiaticus’ is mainly depending on their host for its nutrition needs of PAs and some other metabolites, which hinders culturing efforts. Therefore, we believe that addition PAs to the ‘Ca. L. asiaticus’ growth medium may improve growth.
Nevertheless, there are still numerous unanswered questions in this exciting area of research, which could be viewed as a promising new area of research and as an inspiring topic for further progress in this field.Firstly, although numerous observations have been addressed the PAs accumulation in response to both biotic and abiotic stresses, however, the molecular mechanisms behind the PAs accumulation are still largely unknown. Thus, further investigations are required for a better understanding ofhow the PAs biosynthetic and catabolic genes are regulated and the regulation network associated with those genes at the transcriptional and post-transcriptional levels. Secondly, the cellular translocation of PAs is poorly studied. Therefore, further studies are required to explore the PAs production sites, the PAs cellular localization, and their transporters within plant cells.Thirdly, although several hypothetical models have been proposed previously to explain the potential mode of action of PAs and their metabolites during the stressful conditions, however, the molecular mechanisms behind their roles in stress tolerance should be clearly deciphered. Most of these previous models focused on the involvement of PAs in the activation of antioxidant enzymes for ROS detoxification. In this review, we discussed the involvement of PAs with different phytohormone groups in citrus response.Nevertheless, further physiological, metabolic, transcriptomic, proteomic and molecular research is required to uncover the exact mode of action of PAs in citrusresponse and to decipher the PAs-phytohormones interactions and the metabolic cross-talk between them during biotic and abiotic stress. Last but not least, our knowledge about the signaling cascades that linking the PAs biosynthetic genes and stress responses in citrus plants is still very limited and very far from being well defined. In other plant species, the transcription factors regulating the ADC genes have been identified previously, while those whose regulate other PAs biosynthetic genes remain unknown. Therefore, deciphering the signaling cascades of PAs in citrus plants under both normal and stressful conditionsis a necessity and could be a new exciting area of research in citrus physiology.
Acknowledgments
We apologize for not being able to cite all related references because of space limitations. The authors would like to acknowledge the members of our laboratory for helpful discussions, detailed comments, and constructive suggestions.
Funding Statement
This work was generously supported by grant No. 2016-70016-24844 for NK from the National Institute of Food and Agriculture (NIFA-USDA).
Author Contributions
N.K., together with Y.N., conceptualized the idea. Y.N. and N.K. contributed to digging into literature and finalizing the figures. Both authors worked together in drafting the manuscript. Finally, N.K. and Y.N. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors declare there is no conflict of interest.
References
- 1.Cevallos-Cevallos JM, García-Torres R, Etxeberria E, Reyes-De-Corcuera JI.. GC-MS analysis of headspace and liquid extracts for metabolomic differentiation of citrus Huanglongbing and zinc deficiency in leaves of “Valencia” sweet orange from commercial groves. Phytochem Anal. 2011;22:1–37. doi: 10.1002/pca.1271. [DOI] [PubMed] [Google Scholar]
- 2.Cevallos-Cevallos JM, Futch DB, Shilts T, Folimonova SY, Reyes-De-Corcuera JI.. GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus Huanglongbing. Plant Physiol Biochem. 2012;53:69–76. doi: 10.1016/j.plaphy.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Slisz AM, Breksa AP, Mishchuk DO, McCollum G, Slupsky CM.. Metabolomic analysis of citrus infection by “Candidatus Liberibacter” reveals insight into pathogenicity. J Proteome Res. 2012;11:4223–4230. doi: 10.1021/pr300350x. [DOI] [PubMed] [Google Scholar]
- 4.Malik NSA, Perez JL, Kunta M, Patt JM, Mangan RL. Changes in free amino acids and polyamine levels in Satsuma leaves in response to Asian citrus psyllid infestation and water stress. Insect Sci. 2014;6:707–716. [DOI] [PubMed] [Google Scholar]
- 5.Killiny N, Hijaz F. Amino acids implicated in plant defense are higher in Candidatus Liberibacter asiaticus-tolerant citrus varieties. Plant Signal Behav. 2016;11:e1171449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Killiny N, Nehela Y. Metabolomic response to huanglongbing: role of carboxylic compounds in citrus sinensis response to ‘Candidatus Liberibacter asiaticus’ and its vector, Diaphorina citri. Mol Plant-Microbe Interact. 2017;30:666–678. doi: 10.1094/MPMI-05-17-0106-R. [DOI] [PubMed] [Google Scholar]
- 7.Rosales R, Burns JK. Phytohormone changes and carbohydrate status in sweet orange fruit from huanglongbing-infected trees. J Plant Growth Regul. 2011;30:312–321. [Google Scholar]
- 8.Nehela Y, Hijaz F, Elzaawely AA, El-Zahaby HM, Killiny N. Citrus phytohormonal response to Candidatus Liberibacter asiaticus and its vector Diaphorina citri. Physiol Mol Plant Pathol. 2018;102:24–35. [Google Scholar]
- 9.Kushad MM, Yelenosky G. Evaluation of polyamine and proline levels during low temperature acclimation of citrus. plant physiol. 1987;84:692–695. doi: 10.1104/pp.84.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali AG, Lovatt CJ. Relationship of polyamines to low-temperature stress-induced flowering of the ‘Washington’ navel orange (Citrus sinensis L. Osbeck). J Hortic Sci. 1995;70:491–498. [Google Scholar]
- 11.Fu XZ, Huang Y, Xing F, Chun CP, Ling LL, Cao L, Peng LZ. Changes in free polyamines and expression of polyamine metabolic genes under drought and high-temperature in Citrus sinensis. Biol Plant. 2016;60:793–798. [Google Scholar]
- 12.Nehela Y, Killiny N. ‘Candidatus Liberibacter asiaticus’ and Its Vector, Diaphorina citri, Augment the Tricarboxylic Acid Cycle of Their Host via the γ-Aminobutyric Acid Shunt and Polyamines Pathway. Mol Plant-Microbe Interact. 2019;32:413–427. doi: 10.1094/MPMI-09-18-0238-R. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SS, Seymour S. A guide to the polyamines. Oxford: Oxford University Press; 1998. [Google Scholar]
- 14.Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 2006;28:1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- 15.Hussain SS, Ali M, Ahmad M, Siddique KHM. Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv. 2011;29:300–311. doi: 10.1016/j.biotechadv.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Michael AJ. Polyamines in eukaryotes, bacteria, and archaea. J Biol Chem. 2016;291:14896–14903. doi: 10.1074/jbc.R116.734780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 18.Tabor CW, Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985;49:81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34:35–45. doi: 10.1007/s00726-007-0501-8. [DOI] [PubMed] [Google Scholar]
- 20.Singh P, Basu S, Kumar G. Polyamines metabolism: A way ahead for abiotic stress tolerance in crop plants. Wani SH, editor. Biochemical, physiological and molecular avenues for combating abiotic stress tolerance in plants. London (United Kingdom):Academic Press; 2018. 39–55. [Google Scholar]
- 21.Wallace HM, A V F, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur-Sawhney R, Tiburcio AF, Altabella T, Galston AW. Polyamines in plants: an overview. J Cell Mol Biol. 2003;2:1–12. [Google Scholar]
- 23.Liu J-H, Wang W, Wu H, Gong X, Moriguchi T. Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci. 2015;6:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michael AJ. Biosynthesis of polyamines and polyamine-containing molecules. Biochem J. 2016;473:2315–2329. doi: 10.1042/BCJ20160185. [DOI] [PubMed] [Google Scholar]
- 25.Killiny N, Nehela Y. Citrus Polyamines: structure, Biosynthesis, and Physiological Functions. Plants. 2020;9:426. doi: 10.3390/plants9030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiburcio AF, Altabella T, Borrell A, Masgrau C. Polyamine metabolism and its regulation. Physiol Plant. 1997;100:664–674. [Google Scholar]
- 27.Liu H-Y, Xiao L-T, Lu X-D, Hu -J-J, Wu S, He C-Z, Deng -X-X. Changes in polyamine levels in Citrus sinensis Osb. cv. Valencia callus during somatic embryogenesis. J Plant Physiol Mol Biol. 2005;31:275–280. [PubMed] [Google Scholar]
- 28.Wu X-B, Wang J, Liu J-H, Deng -X-X. Involvement of polyamine biosynthesis in somatic embryogenesis of Valencia sweet orange (Citrus sinensis) induced by glycerol. J Plant Physiol. 2009;166:52–62. doi: 10.1016/j.jplph.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Mendes A FS, Cidade LC, Otoni WC, Soares-Filho WS, Costa MGC. Role of auxins, polyamines and ethylene in root formation and growth in sweet orange. Biol Plant. 2011;55:375–378. [Google Scholar]
- 30.Wu Q-S, Zou Y-N, Liu M, Cheng K. Effects of exogenous putrescine on mycorrhiza, root system architecture, and physiological traits of glomus mosseae-colonized trifoliate orange seedlings. Not Bot Horti Agrobot Cluj-Napoca. 2012;40:80. [Google Scholar]
- 31.Wu QS, Zou YN, Zhan TT, Liu CY. Polyamines participate in mycorrhizal and root development of citrus (Citrus tangerine) seedlings. Not Bot Horti Agrobot Cluj-Napoca. 2010;38:25–31. [Google Scholar]
- 32.Yao Q, Wang L-R, Xing Q-X, Chen J-Z, Zhu -H-H. Exogenous polyamines influence root morphogenesis and arbuscular mycorrhizal development of Citrus limonia seedlings. Plant Growth Regul. 2010;60:27–33. [Google Scholar]
- 33.Wu Q-S, Zou Y-N. The effect of dual application of arbuscular mycorrhizal fungi and polyamines upon growth and nutrient uptake on trifoliate orange (Poncirus trifoliata) Seedlings. Not Bot Horti Agrobot Cluj-Napoca. 2009;37:95–98. [Google Scholar]
- 34.Anjum MA. Effect of exogenously applied spermidine on growth and physiology of citrus rootstock Troyer citrange under saline conditions. Turkish J Agric For. 2011;35:43–53. [Google Scholar]
- 35.Khoshbakht D, Asghari MR, Haghighi M. Influence of foliar application of polyamines on growth, gas-exchange characteristics, and chlorophyll fluorescence in Bakraii citrus under saline conditions. Photosynthetica. 2018;56:731–742. [Google Scholar]
- 36.Lovatt CJ, Zheng Y, Hake KD. Demonstration of a change in nitrogen metabolism influencing flower initiation in Citrus. Isr J Bot. 1988;37:181–188. [Google Scholar]
- 37.Kushad MM, Orvos AR, Yelenosky G. Relative changes in polyamines during citrus flower development. HortScience. 1990;25:946–948. [Google Scholar]
- 38.Sagee O, Lovatt CJ. Putrescine content parallels ammonia and arginine metabolism in developing flowers of the `Washington’ navel orange. J Am Soc Hortic Sci. 1991;116:280–285. [Google Scholar]
- 39.Ali AG, Zheng Y, Sagee O, Protacio C, Lovatt CJ. Ammonia and/or its metabolites influence flowering, fruit set, and yield of the `washington’ navel orange. HortScience. 1993;28:559. [Google Scholar]
- 40.Arias M, Carbonell J, Agustí M. Endogenous free polyamines and their role in fruit set of low and high parthenocarpic ability citrus cultivars. J Plant Physiol. 2005;162:845–853. doi: 10.1016/j.jplph.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Trénor M, Perez-Amador MA, Carbonell J, Blázquez MA. Expression of polyamine biosynthesis genes during parthenocarpic fruit development in Citrus clementina. Planta. 2010;231:1401–1411. doi: 10.1007/s00425-010-1141-x. [DOI] [PubMed] [Google Scholar]
- 42.Pál M, Janda T. Role of polyamine metabolism in plant pathogen interactions. J Plant Sci Phytopathol. 2017;1:095–100. [Google Scholar]
- 43.Romero FM, Maiale SJ, Rossi FR, Marina M, Ruíz OA, Gárriz A.Polyamine Metabolism Responses to Biotic and Abiotic Stress. Alcázar R, Tiburcio AF. editors. Polyamines: methods and protocols, methods in molecular biology. Vol. 1694. Humana Press;New York (NY). 2018. page 37–49 [DOI] [PubMed] [Google Scholar]
- 44.Killiny N, Hijaz F, Harper SJ, Dawson WO. Effects of Citrus tristeza closterovirus infection on phloem sap and released volatile organic compounds in Citrus macrophylla. Physiol Mol Plant Pathol. 2017;98:25–36. [Google Scholar]
- 45.Anjum MA. Response of Cleopatra mandarin seedlings to a polyamine-biosynthesis inhibitor under salt stress. Acta Physiol Plant. 2010;32:951–959. [Google Scholar]
- 46.Fernández-Crespo E, Camañes G, García-Agustín P. Ammonium enhances resistance to salinity stress in citrus plants. J Plant Physiol. 2012;169:1183–1191. doi: 10.1016/j.jplph.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Tanou G, Ziogas V, Belghazi M, Christou A, Filippou P, Job D, Fotopoulos V, Molassiotis A. Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant, Cell Environ. 2014;37:864–885. [DOI] [PubMed] [Google Scholar]
- 48.Pál M, Szalai G, Speculation: JT. Polyamines are important in abiotic stress signaling. Plant Sci. 2015;237:16–23. doi: 10.1016/j.plantsci.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Bar Y, Apelbaum A, Kafkafi U, Goren R. Polyamines in chloride-stressed citrus plants : alleviation of stress by nitrate supplementation via irrigation water. J Amer Soc Hort Sci. 1996;121:507–513. [Google Scholar]
- 50.Bar Y, Apelbaum A, Kafkafi U, Goren R. Ethylene association with chloride stress in citrus plants. Sci Hortic. 1998;73:99–109. [Google Scholar]
- 51.Anjum MA. Effect of NaCl concentrations in irrigation water on growth and polyamine metabolism in two citrus rootstocks with different levels of salinity tolerance. Acta Physiol Plant. 2007;30:43–52. [Google Scholar]
- 52.Wang J, Liu J-H. Change in free polyamine contents and expression profiles of two polyamine biosynthetic genes in citrus embryogenic callus under abiotic stresses. Biotechnol Biotechnol Equip. 2009;23:1289–1293. [Google Scholar]
- 53.Liu Y, Heying E, Tanumihardjo SA, History G. Distribution, and nutritional importance of citrus fruits. Compr Rev Food Sci Food Saf. 2012;11:530–545. [Google Scholar]
- 54.Gottwald TR. Current epidemiological understanding of citrus Huanglongbing. Annu Rev Phytopathol. 2010;48:119–139. doi: 10.1146/annurev-phyto-073009-114418. [DOI] [PubMed] [Google Scholar]
- 55.Lafuente MT, Zacarías L, Sala JM, Sánchez-Ballesta MT, Gosalbes MJ, Marcos JF, González-Candelas L, Lluch Y, Granell A. Understanding the basis of chilling injury in citrus fruit. Acta Hortic. 2005;682:831–842. [Google Scholar]
- 56.Hara M, Fujinaga M, Kuboi T. Metal binding by citrus dehydrin with histidine-rich domains. J Exp Bot. 2005;56:2695–2703. doi: 10.1093/jxb/eri262. [DOI] [PubMed] [Google Scholar]
- 57.Shi J, Fu X-Z, Peng T, Huang X-S, Fan Q-J, Liu J-H. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010;30:914–922. doi: 10.1093/treephys/tpq030. [DOI] [PubMed] [Google Scholar]
- 58.Fu XZ, Xing F, Wang NQ, Peng LZ, Chun CP, Cao L, Ling LL, Jiang CL. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotechnol Biotechnol Equip. 2014;28:192–198. doi: 10.1080/13102818.2014.909152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald RE, Kushad MM. Accumulation of putrescine during chilling injury of fruits. Plant Physiol. 1986;82:324–326. doi: 10.1104/pp.82.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodov V, Ben-Yehoshua S, Albagli R, Fang DQ. Reducing chilling injury and decay of stored citrus fruit by hot water dips. Postharvest Biol Technol. 1995;5:119–127. [Google Scholar]
- 61.Yuen CMC, Tridjaja NO, Wills RBH, Wild BL. Chilling injury development of ‘Tahitian’ lime, ‘Emperor’ mandarin, ‘Marsh’ grapefruit and ‘Valencia’ orange. J Sci Food Agric. 1995;67:335–339. [Google Scholar]
- 62.Gonzalez-Aguilar G, Zacarias L, Mulas M, Lafuente M. Temperature and duration of water dips influence chilling injury, decay and polyamine content in `Fortune’ mandarins. Postharvest Biol Technol. 1997;12:61–69. [Google Scholar]
- 63.Gonzalez-Aguilar GA, Zacarias L, Lafuente MT. Ripening affects high-temperature-induced polyamines and their changes during cold storage of hybrid fortune mandarins. J Agric Food Chem. 1998;46:3503–3508. [Google Scholar]
- 64.Mulas M, Gonzales-Aguilar G, Lafuente MT, Zacarias L. Polyamine biosynthesis in flavedo of “Fortune” mandarins as influenced by temperature of postharvest hot water dips. Acta Hortic. 1998;377–384. [Google Scholar]
- 65.Martínez-Romero D, Valero D, Serrano M, Marínez-Sánchez F, Riquelme F. Effects of post-harvest putrescine and calcium treatments on reducing mechanical damage and polyamines and abscisic acid levels during lemon storage. J Sci Food Agric. 1999;79:1589–1595. [Google Scholar]
- 66.Gonzalez-Aguilar GA, Zacarias L, Perez-Amador MA, Carbonell J, Lafuente MT. Polyamine content and chilling susceptibility are affected by seasonal changes in temperature and by conditioning temperature in cold-stored “Fortune” mandarin fruit. Physiol Plant. 2000;108:140–146. [Google Scholar]
- 67.Valero D, Martínez-Romero D, Serrano M, Postharvest Gibberellin RF. Heat treatment effects on polyamines, abscisic acid and firmness in lemons. J Food Sci. 2006;63:611–615. [Google Scholar]
- 68.Malik NSA, Perez JL, Patt JE, Zibilske LM, Mangan RL. Increased infestation of Asian citrus psyllids on cold treated sour orange seedlings: its possible relation to biochemical changes in leaves. J Food, Agric Environ. 2012;10:424–429. [Google Scholar]
- 69.Cai Q, Moore GA, Guy CL. An unusual group 2 LEA gene family in citrus responsive to low temperature. Plant Mol Biol. 1995;29:11–23. doi: 10.1007/bf00019115. [DOI] [PubMed] [Google Scholar]
- 70.Hara M, Wakasugi Y, Ikoma Y, Yano M, Ogawa K, Kuboi T. cDNA sequence and expression of a cold-responsive gene in Citrus unshiu. Biosci Biotechnol Biochem. 1999;63:433–437. doi: 10.1271/bbb.63.433. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez-Ballesta MT, Lafuente MT, Zacarias L, Granell A. Involvement of phenylalanine ammonia-lyase in the response of Fortune mandarin fruits to cold temperature. Physiol Plant. 2000;108:382–389. [Google Scholar]
- 72.Porat R, Pavoncello D, Lurie S, McCollum TG. Identification of a grapefruit cDNA belonging to a unique class of citrus dehydrins and characterization of its expression patterns under temperature stress conditions. Physiol Plant. 2002;115:598–603. doi: 10.1034/j.1399-3054.2002.1150414.x. [DOI] [PubMed] [Google Scholar]
- 73.Jia Y, Del Rio HS, Robbins AL, Louzada ES. Cloning and sequence analysis of a low temperature-induced gene from trifoliate orange with unusual pre-mRNA processing. Plant Cell Rep. 2004;23:159–166. doi: 10.1007/s00299-004-0805-z. [DOI] [PubMed] [Google Scholar]
- 74.Porat R, Pasentsis K, Rozentzvieg D, Gerasopoulos D, Falara V, Samach A, Lurie S, Kanellis AK. Isolation of a dehydrin cDNA from orange and grapefruit citrus fruit that is specifically induced by the combination of heat followed by chilling temperatures. Physiol Plant. 2004;120:256–264. doi: 10.1111/j.0031-9317.2004.0242.x. [DOI] [PubMed] [Google Scholar]
- 75.Sanchez-Ballesta MT, Rodrigo MJ, Lafuente MT, Granell A, Zacarias L. Dehydrin from citrus, which confers in vitro dehydration and freezing protection activity, is constitutive and highly expressed in the flavedo of fruit but responsive to cold and water stress in leaves. J Agric Food Chem. 2004;52:1950–1957. doi: 10.1021/jf035216+. [DOI] [PubMed] [Google Scholar]
- 76.Şahin-Çevik M, Moore GA. Isolation and characterization of a novel RING-H2 finger gene induced in response to cold and drought in the interfertile Citrus relative Poncirus trifoliata. Physiol Plant. 2006;126:153–161. [Google Scholar]
- 77.Şahin-Çevik M, Moore GA. Two AP2 domain containing genes isolated from the cold-hardy Citrus relative Poncirus trifoliata are induced in response to cold. Funct Plant Biol. 2006;33:863–875. [DOI] [PubMed] [Google Scholar]
- 78.Liu D-C, He L-G, Wang H-L, Xu M, Sun Z-H. Molecular cloning, characterization and expression analysis of PtrHOS1, a novel gene of cold responses from trifoliate orange [Poncirus trifoliata (L.) Raf.]. Acta Physiol Plant. 2010;32:271–279. [Google Scholar]
- 79.Wang J, Liu J-H, Kurosawa T, Nada K, Ban Y, Moriguchi T. Cloning, biochemical identification, and expression analysis of a gene encoding S-adenosylmethionine decarboxylase in navel orange (Citrus sinensis Osbeck). J Hortic Sci Biotechnol. 2010;85:219–226. [Google Scholar]
- 80.de Oliveira TM, Cidade LC, Gesteira AS, Coelho Filho MA, Soares Filho WS, Costa MGC. Analysis of the NAC transcription factor gene family in citrus reveals a novel member involved in multiple abiotic stress responses. Tree Genet Genomes. 2011;7:1123–1134. [Google Scholar]
- 81.Liu DC, He LG, Wang HL, Xu M, Sun ZH. Expression profiles of PtrLOS2 encoding an enolase required for cold-responsive gene transcription from trifoliate orange. Biol Plant. 2011;55:35–42. [Google Scholar]
- 82.Wang J, Sun -P-P, Chen C-L, Wang Y, Fu X-Z, Liu J-H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J Exp Bot. 2011;62:2899–2914. doi: 10.1093/jxb/erq463. [DOI] [PubMed] [Google Scholar]
- 83.He LG, Wang HL, Liu DC, Zhao YJ, Xu M, Zhu M, Wei GQ, Sun ZH. Isolation and expression of a cold-responsive gene PtCBF in Poncirus trifoliata and isolation of citrus CBF promoters. Biol Plant. 2012;56:484–492. [Google Scholar]
- 84.Şahin-Çevik M. A WRKY transcription factor gene isolated from Poncirus trifoliata shows differential responses to cold and drought stresses. Plant Omics. 2012;5:438–445. [Google Scholar]
- 85.Huang X-S, Wang W, Zhang Q, Liu J-H. A basic helix-loop-helix transcription factor, ptrbhlh, of poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol. 2013;162:1178–1194. doi: 10.1104/pp.112.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Şahin-Çevik M, Moore GA. Identification of a drought- and cold-stress inducible WRKY gene in the cold-hardy Citrus relative Poncirus trifoliata. New Zeal J Crop Hortic Sci. 2013;41:57–68. [Google Scholar]
- 87.Xie R, Li Y, He S, Zheng Y, Yi S, Lv Q, Deng L. Genome-Wide Analysis of Citrus R2R3MYB genes and their spatiotemporal expression under stresses and hormone treatments. PLoS One. 2014;9:e113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang X-S, Zhang Q, Zhu D, Fu X, Wang M, Zhang Q, Moriguchi T, Liu J-H. ICE1 of Poncirus trifoliata functions in cold tolerance by modulating polyamine levels through interacting with arginine decarboxylase. J Exp Bot. 2015;66:3259–3274. doi: 10.1093/jxb/erv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He LG, Jiang YC, Wang HL, Xu M, Sun ZH. Expression and regulation of a cold-responsive gene, CsCBF in Citrus sinensis (L.) Osbeck under low temperature, high salinity and abscisic acid. Acta Hortic. 2016;33–46. [Google Scholar]
- 90.Zhang X, Wang W, Wang M, Zhang H-Y, Liu J-H. The miR396b of Poncirus trifoliata Functions in Cold Tolerance by Regulating ACC Oxidase Gene Expression and Modulating Ethylene–Polyamine Homeostasis. Plant Cell Physiol. 2016;57:1865–1878. doi: 10.1093/pcp/pcw108. [DOI] [PubMed] [Google Scholar]
- 91.Orbović V, Ćalović M, Dutt M, Grosser JW, Barthe G. Production and characterization of transgenic Citrus plants carrying p35 anti-apoptotic gene. Sci Hortic. 2015;197:203–211. [Google Scholar]
- 92.Orbović V, Fields JS, Syvertsen JP. Transgenic citrus plants expressing the p35 anti-apoptotic gene have altered response to abiotic stress. Hortic Environ Biotechnol. 2017;58:303–309. [Google Scholar]
- 93.Geng J, Liu J-H. The transcription factor CsbHLH18 of sweet orange functions in modulation of cold tolerance and homeostasis of reactive oxygen species by regulating the antioxidant gene. J Exp Bot. 2018;69:2677–2692. doi: 10.1093/jxb/ery065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomashow MF. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998;118:1–8. doi: 10.1104/pp.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Z, Zhang Y, Peng D, Wang X, Peng Y, He X, Zhang X, Ma X, Huang L, Yan Y. Polyamine regulates tolerance to water stress in leaves of white clover associated with antioxidant defense and dehydrin genes via involvement in calcium messenger system and hydrogen peroxide signaling. Front Physiol. 2015;6:280. doi: 10.3389/fphys.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laukkanen H, Sarjala T. Effect of exogenous polyamines on Scots pine callus in vitro. J Plant Physiol. 1997;150:167–172. [Google Scholar]
- 97.Cvikrová M, Binarová P, Eder J, Vágner M, Hrubcová M, Zoń J, Macháčková I. Effect of inhibition of phenylalanine ammonia-lyase activity on growth of alfalfa cell suspension culture: alterations in mitotic index, ethylene production, and contents of phenolics, cytokinins, and polyamines. Physiol Plant. 1999;107:329–337. [Google Scholar]
- 98.Alcázar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, Salinas J, Tiburcio AF, Altabella T. Integration of polyamines in the cold acclimation response. Plant Sci. 2011;180:31–38. doi: 10.1016/j.plantsci.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 99.Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20:219–229. doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomashow MF. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol. 2010;154:571–577. doi: 10.1104/pp.110.161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fursova OV, Pogorelko GV, Tarasov VA. Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana. Gene. 2009;429:98–103. doi: 10.1016/j.gene.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 103.Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 104.Hu Y, Jiang L, Wang F, Yu D. Jasmonate regulates the INDUCER OF CBF EXPRESSION–C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 cascade and freezing tolerance in Arabidopsis. Plant Cell. 2013;25:2907–2924. doi: 10.1105/tpc.113.112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sala JM, Lafuente MT. Catalase enzyme activity is related to tolerance of mandarin fruits to chilling. Postharvest Biol Technol. 2000;20:81–89. [Google Scholar]
- 106.Kim YS, Lee M, Lee J-H, Lee H-J, Park C-M. The unified ICE–CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis. Plant Mol Biol. 2015;89:187–201. doi: 10.1007/s11103-015-0365-3. [DOI] [PubMed] [Google Scholar]
- 107.Zhao C, Wang P, Si T, Hsu -C-C, Wang L, Zayed O, Yu Z, Zhu Y, Dong J, Tao WA. et al. MAP Kinase Cascades Regulate the Cold Response by Modulating ICE1 Protein Stability. Dev Cell. 2017;43(618–629.e5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- 109.Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000;23:319–327. doi: 10.1046/j.1365-313x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 110.Lindberg S, Kader MA, Yemelyanov V. Calcium signalling in plant cells under environmental stress. In Ahmad P, Prasad MNV, editors. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. New York (NY): Springer; 2012. p. 325–360. New York;. page.. [Google Scholar]
- 111.Miura K, Furumoto T. Cold signaling and cold response in plants. Int J Mol Sci. 2013;14:5312–5337. doi: 10.3390/ijms14035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Townley HE, Knight MR. Calmodulin as a potential negative regulator of arabidopsis COR gene expression. Plant Physiol. 2002;128:1169–1172. doi: 10.1104/pp.010814. [DOI] [PubMed] [Google Scholar]
- 113.Ji H, Wang Y, Cloix C, Li K, Jenkins GI, Wang S, Shang Z, Shi Y, Yang S, The LX. Arabidopsis RCC1 family protein TCF1 regulates freezing tolerance and cold acclimation through modulating lignin biosynthesis. plos Genet. 2015;11:e1005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sudhir P, Murthy SDS. Effects of salt stress on basic processes of photosynthesis. Photosynthetica. 2004;42:481–486. [Google Scholar]
- 115.Cimen B, Yesiloglu T. Rootstock breeding for abiotic stress tolerance in citrus. In: Shanker A, editor. Abiotic and biotic stress in plants - recent advances and future perspectives. London, UK: IntechOpen; 2016. p. 527–563. [Google Scholar]
- 116.Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- 117.Munns R. Comparative physiology of salt and water stress. Plant, Cell Environ. 2002;25:239–250. [DOI] [PubMed] [Google Scholar]
- 118.García-Agustín P, Primo-Millo E. Selection of a NaCl-tolerant Citrus plant. Plant Cell Rep. 1995;14:314–318. doi: 10.1007/BF00232035. [DOI] [PubMed] [Google Scholar]
- 119.Storey R, Walker R. Citrus and salinity. Sci Hortic. 1998;78:39–81. [Google Scholar]
- 120.Almansa MS, Hernandez JA, Jimenez A, Botella MA, Sevilla F. Effect of salt stress on the superoxide dismutase activity in leaves of citrus limonum in different rootstock-scion combinations. Biol Plant. 2002;45:545–549. [Google Scholar]
- 121.Garcia-Sanchez F, Carvajal M, Cerda A, Martinez V. Response of ‘Star Ruby’ grapefruit on two rootstocks to NaCl salinity. J Hortic Sci Biotechnol. 2003;78:859–865. [Google Scholar]
- 122.Golein B, Rabiei V, Mirabbasi F, Fifaei R, Halaji Sani MF. Effect of salinity stress on physiological and biochemical traits in citrus genotypes. Majallah-i ?ulum-i Baghbani (J Hortic Sci). 2016;29:416–425. [Google Scholar]
- 123.García-Sánchez F, Syvertsen JP. Salinity tolerance of cleopatra mandarin and carrizo citrange citrus rootstock seedlings is affected by CO2 enrichment during growth. J Am Soc Hortic Sci. 2006;131:24–31. [Google Scholar]
- 124.Goswami AK, Dubey AK, Singh AK, Singh SK, Srivastav M, Prakash J, Awasthi OP, Singh K, Goswami S. Effect of polyamines on physio-chemical and biochemical parameters of citrus rootstocks under NaCl stress. Indian J Hortic. 2016;73:496. [Google Scholar]
- 125.Sharma DK, Dubey AK, Srivastav M, Singh AK, Sairam RK, Pandey RN, Dahuja A, Kaur C. Effect of putrescine and paclobutrazol on growth, physiochemical parameters, and nutrient acquisition of salt-sensitive citrus rootstock Karna khatta (Citrus karna Raf.) under NaCl Stress. J Plant Growth Regul. 2011;30:301–311. [Google Scholar]
- 126.Sharma DK, Dubey AK, Srivastav M, Singh AK, Pandey RN, Dahuja A. Effect of paclobutrazol and putrescine on antioxidant enzymes activity and nutrients content in salt tolerant citrus rootstock sour orange under sodium chloride stress. J Plant Nutr. 2013;36:1765–1779. [Google Scholar]
- 127.Gomez-Cadenas A, Tadeo FR, Primo-Millo E, Talon M. Involvement of abscisic acid and ethylene in the responses of citrus seedlings to salt shock. Physiol Plant. 1998;103:475–484. [Google Scholar]
- 128.Balal RM, Ashraf MY, Mumtaz Khan M, Jaskani MJ, Ashfaq M. Influence of salt stress on growth and biochemical parameters of citrus rootstocks. Pak J Bot. 2011;43:2135–2141. [Google Scholar]
- 129.Khoshbakht D, Ramin AA, Baninasab B. Citrus Rootstocks Response to Salinity: physio-biochemical Parameters Changes. Res J Environ Sci. 2014;8:29–38. [Google Scholar]
- 130.Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 131.Yang S-L, Lan S-S GM. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol. 2009;166:1694–1699. doi: 10.1016/j.jplph.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 132.Yildiz M, Terzi̇ H. Effect of NaCl stress on chlorophyll biosynthesis, proline, lipid peroxidation and antioxidative enzymes in leaves of salt-tolerant and salt sensitive barley cultivars. J Agric Sci. 2013;19:79–88. [Google Scholar]
- 133.Mansour MMF, Ali EF. Evaluation of proline functions in saline conditions. Phytochemistry. 2017;140:52–68. doi: 10.1016/j.phytochem.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 134.Lima-Costa ME, Ferreira S, Duarte A, Ferreira AL. Alleviation of salt stress using exogenous proline on a citrus cell line. Acta Hortic. 2010;868:109–112. [Google Scholar]
- 135.Abbas MF, Jasim AM, Al-Taha HA. Effect of exogenous proline on protein pattern changes in Citrus sinensis (L.) Osbeck under in vitro salt stress. Adv Agric Bot. 2012;4:36–41. [Google Scholar]
- 136.Aziz A, Martin-Tanguy J, Larher F. Stress-induced changes in polyamine and tyramine levels can regulate proline accumulation in tomato leaf discs treated with sodium chloride. Physiol Plant. 1998;104:195–202. [Google Scholar]
- 137.Gong X, Zhang J, Liu J-H. A stress responsive gene of Fortunella crassifolia FcSISP functions in salt stress resistance. Plant Physiol Biochem. 2014;83:10–19. doi: 10.1016/j.plaphy.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 138.Ben-Hayyim G, Faltin Z, Gepstein S, Camoin L, Donny Strosberg A, Eshdat Y. Isolation and characterization of salt-associated protein in Citrus. Plant Sci. 1993;88:129–140. [DOI] [PubMed] [Google Scholar]
- 139.Holland D, Eshdat Y, Faltin Z, Ben-Hayyim G. Molecular cloning of CSA, a gene from citrus encoding a protein potentially involved in the defence against oxidative stresses. Acta Hortic. 1993;228–230. [Google Scholar]
- 140.Naot D, Ben-Hayyim G, Eshdat Y, Holland D. Drought, heat and salt stress induce the expression of a citrus homologue of an atypical late-embryogenesis Lea5 gene. Plant Mol Biol. 1995;27:619–622. doi: 10.1007/bf00019327. [DOI] [PubMed] [Google Scholar]
- 141.Naot D, Holland D, Avsian-Kretchmer O, Eshdat Y, Ben-Hayyim G. Induction of a gene encoding an oleosin homologue in cultured citrus cells exposed to salt stress. Gene. 1995;161:171–173. doi: 10.1016/0378-1119(95)00224-t. [DOI] [PubMed] [Google Scholar]
- 142.Cervera M, Ortega C, Navarro A, Navarro L, Peña L. Generation of transgenic citrus plants with the tolerance-to-salinity gene HAL2 from yeast. J Hortic Sci Biotechnol. 2000;75:26–30. [Google Scholar]
- 143.Fu X-Z, Khan EU, Hu -S-S, Fan Q-J, Liu J-H. Overexpression of the betaine aldehyde dehydrogenase gene from Atriplex hortensis enhances salt tolerance in the transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Environ Exp Bot. 2011;74:106–113. [Google Scholar]
- 144.Ali S, Mannan A, El Oirdi M, Waheed A, Mirza B. Agrobacterium-mediated transformation of rough lemon (Citrus jambhiri Lush) with yeast HAL2 gene. BMC Res Notes. 2012;5:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodríguez-Gamir J, Ancillo G, Legaz F, Primo-Millo E, Forner-Giner MA. Influence of salinity on pip gene expression in citrus roots and its relationship with root hydraulic conductance, transpiration and chloride exclusion from leaves. Environ Exp Bot. 2012;78:163–166. [Google Scholar]
- 146.Wei Q, Liu Y, Zhou G, Li Q, Yang C, Peng S. Overexpression of CsCLCc, a chloride channel gene from poncirus trifoliata, enhances salt tolerance in arabidopsis. Plant Mol Biol Report. 2013;31:1548–1557. [Google Scholar]
- 147.Xian L, Sun P, Hu S, Wu J, Liu J-H. Molecular cloning and characterization of CrNCED1, a gene encoding 9-cis-epoxycarotenoid dioxygenase in Citrus reshni, with functions in tolerance to multiple abiotic stresses. Planta. 2014;239:61–77. doi: 10.1007/s00425-013-1963-4. [DOI] [PubMed] [Google Scholar]
- 148.Alvarez-Gerding X, Espinoza C, Inostroza-Blancheteau C, Arce-Johnson P. Molecular and physiological changes in response to salt stress in Citrus macrophylla W plants overexpressing Arabidopsis CBF3/DREB1A. Plant Physiol Biochem. 2015;92:71–80. doi: 10.1016/j.plaphy.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 149.Qu J-W, Liu L-P, Wu J, Yin -Q-Q, Wu -T-T, Guo X-H, Zhang J-L, Yuan F-R, Li N, Deng Z-N. Ectopic expression of CisAF7, an Alfin1-like gene from Citrus sinensis, confers tolerance to several abiotic stresses in Escherichia coli. Plant Omi J. 2015;8:250–256. [Google Scholar]
- 150.Wang W, Liu J-H. Genome-wide identification and expression analysis of the polyamine oxidase gene family in sweet orange (Citrus sinensis). Gene. 2015;555:421–429. doi: 10.1016/j.gene.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 151.Wang W, Liu J-H. CsPAO4 of Citrus sinensis functions in polyamine terminal catabolism and inhibits plant growth under salt stress. Sci Rep. 2016;6:31384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ayadi M, Hanana M, Kharrat N, Merchaoui H, Ben MR, Lauvergeat V, Rebaï A, The MR. WRKY transcription factor family in citrus: valuable and useful candidate genes for citrus breeding. Appl Biochem Biotechnol. 2016;180:516–543. [DOI] [PubMed] [Google Scholar]
- 153.Liu D, Yang L, Luo M, Wu Q, Liu S, Liu Y. Molecular cloning and characterization of PtrZPT2-1, a ZPT2 family gene encoding a Cys2/His2-type zinc finger protein from trifoliate orange (Poncirus trifoliata (L.) Raf.) that enhances plant tolerance to multiple abiotic stresses. Plant Sci. 2017;263:66–78. doi: 10.1016/j.plantsci.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 154.Wei Q, Ma Q, Ning S, Su S, Gu Q. Molecular characterization and functional analysis of a cation-chloride cotransporter gene from trifoliate orange (Poncirus trifoliata L.). Trees. 2018;32:165–173. [Google Scholar]
- 155.Gläser HU, Thomas D, Gaxiola R, Montrichard F, Surdin-Kerjan Y, Serrano R. Salt tolerance and methionine biosynthesis in Saccharomyces cerevisiae involve a putative phosphatase gene. Embo J. 1993;12:3105–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- 157.Liu J, Ishitani M, Halfter U, Kim C-S, Zhu J-K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci. 2000;97:3730–3734. doi: 10.1073/pnas.060034197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shi H, Ishitani M, Kim C, Zhu JK. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci U S A. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi H, Quintero FJ, Pardo JM, Zhu J-K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.de Carvalho K, de Campos MKF, Domingues DS, Pereira LFP, Vieira LGE. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol Biol Rep. 2013;40:3269–3279. doi: 10.1007/s11033-012-2402-5. [DOI] [PubMed] [Google Scholar]
- 161.Gimeno J, Gadea J, Forment J, Pérez-Valle J, Santiago J, Martínez-Godoy MA, Yenush L, Bellés JM, Brumós J, Colmenero-Flores JM. et al. Shared and novel molecular responses of mandarin to drought. Plant Mol Biol. 2009;70:403–420. doi: 10.1007/s11103-009-9481-2. [DOI] [PubMed] [Google Scholar]
- 162.Kim HS, Kim YJ, Seo YR. An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J Cancer Prev. 2015;20:232–240. doi: 10.15430/JCP.2015.20.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163..IARC . IARC monographs on the evaluation of carcinogenic risk of chemicals to human : some metals and metallic cornpounds. Int Agency Res Cancer. 1980;23:438. [PubMed] [Google Scholar]
- 164.Goren R, Siegel SM. Mercury-induced ethylene formation and abscission in citrus and coleus explants. Plant Physiol. 1976;57:628–631. doi: 10.1104/pp.57.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Podazza G, Rosa M, González JA, Hilal M, Prado FE. Cadmium induces changes in sucrose partitioning, invertase activities, and membrane functionality in roots of rangpur lime (Citrus limonia L. Osbeck). Plant Biol. 2006;8:706–714. doi: 10.1055/s-2006-924171. [DOI] [PubMed] [Google Scholar]
- 166.López-Climent MF, Arbona V, Pérez-Clemente RM, Gómez-Cadenas A. Effects of cadmium on gas exchange and phytohormone contents in citrus. Biol Plant. 2011;55:187–190. [Google Scholar]
- 167.Podazza G, Arias M, Prado FE. Cadmium accumulation and strategies to avoid its toxicity in roots of the citrus rootstock Citrumelo. J Hazard Mater. 2012;215–216:83–89. doi: 10.1016/j.jhazmat.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 168.López-Climent MF, Arbona V, Pérez-Clemente RM, Zandalinas SI, Gómez-Cadenas A. Effect of cadmium and calcium treatments on phytochelatin and glutathione levels in citrus plants. Plant Biol. 2014;16:79–87. doi: 10.1111/plb.12006. [DOI] [PubMed] [Google Scholar]
- 169.Podazza G, Arias M, Prado FE. Early interconnectivity between metabolic and defense events against oxidative stress induced by cadmium in roots of four citrus rootstocks. Funct Plant Biol. 2016;43:973. [DOI] [PubMed] [Google Scholar]
- 170.Shahid MA, Balal RM, Khan N, Rossi L, Rathinasabapathi B, Liu G, Khan J, Cámara-Zapata JM, Martínez-Nicolas JJ, Garcia-Sanchez F. Polyamines provide new insights into the biochemical basis of Cr-tolerance in Kinnow mandarin grafted on diploid and double-diploid rootstocks. Environ Exp Bot. 2018;156:248–260. [Google Scholar]
- 171.Shahid MA, Balal RM, Khan N, Zotarelli L, Liu G, Ghazanfar MU, Rathinasabapathi B, Mattson NS, Martínez-Nicolas JJ, Garcia-Sanchez F. Ploidy level of citrus rootstocks affects the carbon and nitrogen metabolism in the leaves of Chromium-stressed Kinnow mandarin plants. Environ Exp Bot. 2018;149:70–80. [Google Scholar]
- 172.Tajti J, Janda T, Majláth I, Szalai G, Pál M. Comparative study on the effects of putrescine and spermidine pre-treatment on cadmium stress in wheat. Ecotoxicol Environ Saf. 2018;148:546–554. [DOI] [PubMed] [Google Scholar]
- 173.Groppa MD, Tomaro ML, Benavides MP. Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci. 2001;161:481–488. [Google Scholar]
- 174.Rady MM, Hemida KA. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol Environ Saf. 2015;119:178–185. doi: 10.1016/j.ecoenv.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 175.Balal RM, Shahid MA, Vincent C, Zotarelli L, Liu G, Mattson NS, Rathinasabapathi B, Martínez-Nicolas JJ, Garcia-Sanchez F. Kinnow mandarin plants grafted on tetraploid rootstocks are more tolerant to Cr-toxicity than those grafted on its diploids one. Environ Exp Bot. 2017;140:8–18. [Google Scholar]
- 176.Hasanuzzaman M, Alhaithloul HAS, Parvin K, Bhuyan MHMB, Tanveer M, Mohsin SM, Nahar K, Soliman MH, Al MJ, Polyamine FM. Action under Metal/Metalloid Stress: regulation of Biosynthesis, Metabolism, and Molecular Interactions. Int J Mol Sci. 2019;20:3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wu Q-S, Srivastava AK, Zou Y-N. AMF-induced tolerance to drought stress in citrus: A review. Sci Hortic. 2013;164:77–87. [Google Scholar]
- 178.Molinari HBC, Marur CJ, Filho JCB, Kobayashi AK, Pileggi M, RPL J, Pereira LFP, Vieira LGE. Osmotic adjustment in transgenic citrus rootstock Carrizo citrange (Citrus sinensis Osb. x Poncirus trifoliata L. Raf.) overproducing proline. Plant Sci. 2004;167:1375–1381. [Google Scholar]
- 179.Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014;202:35–49. doi: 10.1111/nph.12613. [DOI] [PubMed] [Google Scholar]
- 180.DDS S-V, Freschi L, Almeida da H LA, de Moraes DHS, Neves DM, Dos Santos LM, Bertolde FZ, Soares Filho WDS, Coelho Filho MA, Gesteira A da S. Survival strategies of citrus rootstocks subjected to drought. Sci Rep. 2016;6:38775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Snider JL, Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci Rep. 2018;8:4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mittler R, Blumwald E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell. 2015;27:64–70. doi: 10.1105/tpc.114.133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu J-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006;45:523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- 184.De Ollas C, Hernando B, Arbona V, Gómez-Cadenas A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol Plant. 2013;147:296–306. doi: 10.1111/j.1399-3054.2012.01659.x. [DOI] [PubMed] [Google Scholar]
- 185.Gong X, Zhang J, Hu J, Wang W, Wu H, Zhang Q, Liu J-H. FcWRKY70, a WRKY protein of Fortunella crassifolia, functions in drought tolerance and modulates putrescine synthesis by regulating arginine decarboxylase gene. Plant Cell Environ. 2015;38:2248–2262. doi: 10.1111/pce.12539. [DOI] [PubMed] [Google Scholar]
- 186.Vicent Arbona CJ, De Ollas R, Argamasilla MF, López-Climent A, Gómez-Cadenas. Hormone and metabolite traits related to abiotic stress tolerance in citrus. Acta Hortic. 2015;1065:1275–1281. [Google Scholar]
- 187.Neves DM, da H Almeida LA, Santana-Vieira DDS, Freschi L, Ferreira CF, Soares Filho WDS, Costa MGC, Micheli F, Coelho Filho MA, da S Gesteira A. Recurrent water deficit causes epigenetic and hormonal changes in citrus plants. Sci Rep. 2017;7:13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pedrosa AM, Cidade LC, Martins CPS, Macedo AF, Neves DM, Gomes FP, Floh EIS, Costa MGC. Effect of overexpression of citrus 9-cis-epoxycarotenoid dioxygenase 3 (CsNCED3) on the physiological response to drought stress in transgenic tobacco. Genet Mol Res. 2017;16:16019292. doi: 10.4238/gmr16039744. [DOI] [PubMed] [Google Scholar]
- 189.Huang X-S, Liu J-H, Chen X-J. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 2010;10:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim I-J, Lee J, Han J-A, Kim C-S HY. Citrus Lea promoter confers fruit-preferential and stressinducible gene expression in Arabidopsis. Can J Plant Sci. 2011;91:459–466. [Google Scholar]
- 191.Huang X-S, Luo T, Fu X-Z, Fan Q-J, Liu J-H. Cloning and molecular characterization of a mitogen-activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco. J Exp Bot. 2011;62:5191–5206. doi: 10.1093/jxb/err229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cidade LC, de Oliveira TM, Mendes AFS, Macedo AF, Floh EIS, Gesteira AS, Soares-Filho WS, Costa MGC. Ectopic expression of a fruit phytoene synthase from Citrus paradisi Macf. promotes abiotic stress tolerance in transgenic tobacco. Mol Biol Rep. 2012;39(12):10201–10209. doi: 10.1007/s11033-012-1895-2. [DOI] [PubMed] [Google Scholar]
- 193.de Campos MKF, de Carvalho K, de Souza FS, Marur CJ, Pereira LFP, Filho JCB, Vieira LGE. Drought tolerance and antioxidant enzymatic activity in transgenic ‘Swingle’ citrumelo plants over-accumulating proline. Environ Exp Bot. 2011;72:242–250. [Google Scholar]
- 194.Lo Cicero L, Madesis P, Tsaftaris A, Lo Piero AR. Tobacco plants over-expressing the sweet orange tau glutathione transferases (CsGSTUs) acquire tolerance to the diphenyl ether herbicide fluorodifen and to salt and drought stresses. Phytochemistry. 2015;116:69–77. doi: 10.1016/j.phytochem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 195.Zhang Q, Wang M, Hu J, Wang W, Fu X, Liu J-H. PtrABF of Poncirus trifoliata functions in dehydration tolerance by reducing stomatal density and maintaining reactive oxygen species homeostasis. J Exp Bot. 2015;66:5911–5927. doi: 10.1093/jxb/erv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Martins CPS, Neves DM, Cidade LC, Mendes AFS, Silva DC, Almeida -A-AF, Coelho-Filho MA, Gesteira AS, Soares-Filho WS, Costa MGC. Expression of the citrus CsTIP2;1 gene improves tobacco plant growth, antioxidant capacity and physiological adaptation under stress conditions. Planta. 2017;245:951–963. doi: 10.1007/s00425-017-2653-4. [DOI] [PubMed] [Google Scholar]
- 197.Rodrigo M-J, Alquezar B, Zacarías L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J Exp Bot. 2006;57:633–643. doi: 10.1093/jxb/erj048. [DOI] [PubMed] [Google Scholar]
- 198.Pál M, Tajti J, Szalai G, Peeva V, Végh B, Janda T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci Rep. 2018;8:12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bolton MD. Primary metabolism and plant defense–fuel for the fire. Mol Plant Microbe Interact. 2009;22:487–497. doi: 10.1094/MPMI-22-5-0487. [DOI] [PubMed] [Google Scholar]
- 200.Kangasjärvi S, Neukermans J, Li S, Aro E-M, Noctor G. Photosynthesis, photorespiration, and light signalling in defence responses. J Exp Bot. 2012;63:1619–1636. doi: 10.1093/jxb/err402. [DOI] [PubMed] [Google Scholar]
- 201.Hijaz F, Killiny N. Collection and chemical composition of phloem Sap from Citrus sinensis L. Osbeck (Sweet Orange). PLoS One. 2014;9:e101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Killiny N, Nehela Y. One target, two mechanisms: the impact of “Candidatus Liberibacter asiaticus” and its vector, Diaphorina citri, on Citrus Leaf Pigments. Mol Plant-Microbe Interact. 2017;30:543–556. doi: 10.1094/MPMI-02-17-0045-R. [DOI] [PubMed] [Google Scholar]
- 203.Hijaz F, El-Shesheny I, Killiny N. Herbivory by the insect Diaphorina citri induces greater change in citrus plant volatile profile than does infection by the bacterium, Candidatus Liberibacter asiaticus. Plant Signal Behav. 2013;8:e25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee RF. Control of Virus Diseases of Citrus. Adv Virus Res. 2015;91:143–173. doi: 10.1016/bs.aivir.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 205.Murcia N, Hashemian SMB, Serra P, Pina JA, Duran-Vila N. Citrus viroids: symptom expression and performance of washington navel sweet orange trees grafted on carrizo citrange. Plant Dis. 2015;99:125–136. doi: 10.1094/PDIS-05-14-0457-RE. [DOI] [PubMed] [Google Scholar]
- 206.Ito T, Ieki H, Ozaki K, Ito T. Characterization of a new citrus viroid species tentatively termed Citrus viroid OS. Arch Virol. 2001;146:975–982. doi: 10.1007/s007050170129. [DOI] [PubMed] [Google Scholar]
- 207.Serra P, Barbosa CJ, Daròs JA, Flores R, Duran-Vila N. Citrus viroid V: molecular characterization and synergistic interactions with other members of the genus Apscaviroid. Virology. 2008;370:102–112. doi: 10.1016/j.virol.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 208.Serra P, Eiras M, Bani-Hashemian SM, Murcia N, Kitajima EW, Daròs JA, Flores R, Duran-Vila N. Citrus viroid V: occurrence, host range, diagnosis, and identification of new variants. Phytopathology. 2008;98:1199–1204. doi: 10.1094/PHYTO-98-11-1199. [DOI] [PubMed] [Google Scholar]
- 209.Gutiérrez-E MA, Luth D, Moore GA. Factors affecting Agrobacterium-mediated transformation in Citrus and production of sour orange (Citrus aurantium L.) plants expressing the coat protein gene of citrus tristeza virus. Plant Cell Rep. 1997;16:745–753. doi: 10.1007/s002990050313. [DOI] [PubMed] [Google Scholar]
- 210.Domínguez A, Guerri J, Cambra M, Navarro L, Moreno P, Peña L. Efficient production of transgenic citrus plants expressing the coat protein gene of citrus tristeza virus. Plant Cell Rep. 2000;19:427–433. doi: 10.1007/s002990050751. [DOI] [PubMed] [Google Scholar]
- 211.Ghorbel R, Dominguez A, Navarro L, Pena L. High efficiency genetic transformation of sour orange (Citrus aurantium) and production of transgenic trees containing the coat protein gene of citrus tristeza virus. Tree Physiol. 2000;20:1183–1189. doi: 10.1093/treephys/20.17.1183. [DOI] [PubMed] [Google Scholar]
- 212.Yang ZN, Ingelbrecht IL, Louzada E, Skaria M, Mirkov TE. Agrobacterium -mediated transformation of the commercially important grapefruit cultivar Rio Red (Citrus paradisi Macf.). Plant Cell Rep. 2000;19:1203–1211. doi: 10.1007/s002990000257. [DOI] [PubMed] [Google Scholar]
- 213.Domínguez A, de Mendoza AH, Guerri J, Cambra M, Navarro L, Moreno P, Peña L. Pathogen-derived resistance to Citrus tristeza virus (CTV) in transgenic mexican lime (Citrus aurantifolia (Christ.) Swing.) plants expressing its p25 coat protein gene. Mol Breed. 2002;10:1–10. [Google Scholar]
- 214.Febres V, Niblett C, Lee R, Moore G. Characterization of grapefruit plants (Citrus paradisi Macf.) transformed with citrus tristeza closterovirus genes. Plant Cell Rep. 2003;21:421–428. doi: 10.1007/s00299-002-0528-y. [DOI] [PubMed] [Google Scholar]
- 215.Fagoaga C, López C, Moreno P, Navarro L, Flores R, Peña L. Viral-like symptoms induced by the ectopic expression of the p23 gene of citrus tristeza virus are citrus specific and do not correlate with the pathogenicity of the virus strain. Mol Plant-Microbe Interact. 2005;18:435–445. doi: 10.1094/MPMI-18-0435. [DOI] [PubMed] [Google Scholar]
- 216.Fagoaga C, López C, de Mendoza AH, Moreno P, Navarro L, Flores R, Peña L. Post-Transcriptional Gene Silencing of the p23 Silencing Suppressor of Citrus tristeza virus Confers Resistance to the Virus in Transgenic Mexican Lime. Plant Mol Biol. 2006;60:153–165. doi: 10.1007/s11103-005-3129-7. [DOI] [PubMed] [Google Scholar]
- 217.Çevik B, Lee RF, Niblett CL. Genetic transformation of Citrus paradisi with antisense and untranslatable RNA-dependent RNA polymerase genes of Citrus tristeza closterovirus. Turkish J Agric For. 2006;30:173–182. [Google Scholar]
- 218.Ananthakrishnan G, Orbović V, Pasquali G, Ćalović M, Grosser JW. Transfer of citrus tristeza virus (CTV)-derived resistance candidate sequences to four grapefruit cultivars through Agrobacterium-mediated genetic transformation. Vitr Cell Dev Biol - Plant. 2007;43:593–601. [Google Scholar]
- 219.Cervera M, Esteban O, Gil M, Gorris MT, Martínez MC, Peña L, Cambra M. Transgenic expression in citrus of single-chain antibody fragments specific to Citrus tristeza virus confers virus resistance. Transgenic Res. 2010;19:1001–1015. doi: 10.1007/s11248-010-9378-5. [DOI] [PubMed] [Google Scholar]
- 220.Reyes CA, De Francesco A, Peña EJ, Costa N, Plata MI, Sendin L, Castagnaro AP, García ML. Resistance to Citrus psorosis virus in transgenic sweet orange plants is triggered by coat protein–RNA silencing. J Biotechnol. 2011;151:151–158. doi: 10.1016/j.jbiotec.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 221.Reyes CA, Zanek MC, Velázquez K, Costa N, Plata MI, Garcia ML. Generation of sweet orange transgenic lines and evaluation of citrus psorosis virus-derived resistance against Psorosis A and Psorosis B. J Phytopathol. 2011;159:531–537. [Google Scholar]
- 222.Muniz FR, De Souza AJ, Stipp LCL, Schinor E, Freitas W, Harakava R, Stach-Machado DR, Rezende JAM, FAA MF, Mendes BMJ. Genetic transformation of Citrus sinensis with Citrus tristeza virus (CTV) derived sequences and reaction of transgenic lines to CTV infection. Biol Plant. 2012;56:162–166. [Google Scholar]
- 223.Cheng CZ, Yang JW, Bin YH, Bei XJ, Zhang YY, Lu ZM, Zhong GY. Expressing p20 hairpin RNA of Citrus tristeza virus confers Citrus aurantium with tolerance/resistance against stem pitting and seedling yellow CTV strains. J Integr Agric. 2015;14:1767–1777. [Google Scholar]
- 224.Soler N, Fagoaga C, López C, Moreno P, Navarro L, Flores R, Peña L. Symptoms induced by transgenic expression of p23 from Citrus tristeza virus in phloem-associated cells of Mexican lime mimic virus infection without the aberrations accompanying constitutive expression. Mol Plant Pathol. 2015;16:388–399. doi: 10.1111/mpp.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Reyes CA, De Francesco A, Ocolotobiche EE, Costa N, García ML. Uncontrolled Citrus psorosis virus infection in Citrus sinensis transgenic plants expressing a viral 24K-derived hairpin that does not trigger RNA silencing. Physiol Mol Plant Pathol. 2016;94:149–155. [Google Scholar]
- 226.Cheng C, Zhang Y, Yang J, Zhong Y. Expression of hairpin RNA (hpRNA) targeting the three CTV-silencing suppressor genes confers sweet orange with stem-pitting CTV tolerance. J Hortic Sci Biotechnol. 2017;92:465–474. [Google Scholar]
- 227.Ghaderi I, Sohani MM, Mahmoudi A. Efficient genetic transformation of sour orange, Citrus aurantium L. using Agrobacterium tumefaciens containing the coat protein gene of Citrus tristeza virus. Plant Gene. 2018;14:7–11. [Google Scholar]
- 228.Bellés JM, Carbonell J, Conejero V. Polyamines in plants infected by citrus exocortis viroid or treated with silver ions and ethephon. Plant Physiol. 1991;96:1053–1059. doi: 10.1104/pp.96.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bellés JM, Pérez-Amador MA, Carbonell J, Conejero V. Correlation between ornithine decarboxylase and putrescine in tomato plants infected by citrus exocortis viroid or treated with ethephon. Plant Physiol. 1993;102:933–937. doi: 10.1104/pp.102.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.López-Gresa MP, Payá C, Rodrigo I, Bellés JM, Barceló S, Hae Choi Y, Verpoorte R, Lisón P. Effect of Benzothiadiazole on the metabolome of tomato plants infected by citrus exocortis viroid. Viruses. 2019;11:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bellés JM, Granell A, Durán-vila N, Conejero V. ACC synthesis as the activated step responsible for the rise of ethylene production accompanying citrus exocortis viroid infection in tomato plants. J Phytopathol. 1989;125:198–208. [Google Scholar]
- 232.López-Gresa MP, Lisón P, Yenush L, Conejero V, Rodrigo I, Bellés JM. Salicylic acid is involved in the basal resistance of tomato plants to citrus exocortis viroid and tomato spotted wilt virus. PLoS One. 2016;11:e0166938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Marini F, Betti L, Scaramagli S, Biondi S, Torrigiani P. Polyamine metabolism is upregulated in response to tobacco mosaic virus in hypersensitive, but not in susceptible, tobacco. New Phytol. 2001;149:301–309. [DOI] [PubMed] [Google Scholar]
- 234.Yamakawa K, Satoh O. Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol. 1998;118:1213–1222. doi: 10.1104/pp.118.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gandía M, Conesa A, Ancillo G, Gadea J, Forment J, Pallás V, Flores R, Duran-Vila N, Moreno P, Guerri J. Transcriptional response of Citrus aurantifolia to infection by Citrus tristeza virus. Virology. 2007;367:298–306. doi: 10.1016/j.virol.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 236.Dória MS, de Sousa AO, de J Barbosa C, Costa MGC, da S Gesteira A, Souza RM, Freitas ACO, Pirovani CP. Citrus tristeza virus (CTV) Causing Proteomic and Enzymatic Changes in Sweet Orange Variety “Westin. PLoS One. 2015;10:e0130950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fu S, Shao J, Zhou C, Hartung JS. Transcriptome analysis of sweet orange trees infected with “Candidatus Liberibacter asiaticus” and two strains of Citrus Tristeza Virus. BMC Genomics. 2016;17:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fu S, Shao J, Paul C, Zhou C, Hartung JS. Transcriptional analysis of sweet orange trees co-infected with “Candidatus Liberibacter asiaticus” and mild or severe strains of Citrus tristeza virus. BMC Genomics. 2017;18:837. doi: 10.1186/s12864-016-3396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fu S, Shao J, Zhou C, Hartung JS. Co-infection of sweet orange with severe and mild strains of citrus tristeza virus is overwhelmingly dominated by the severe strain on both the transcriptional and biological levels. Front Plant Sci. 2017;8:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cheng C, Zhang Y, Zhong Y, Yang J, Yan S. Gene expression changes in leaves of Citrus sinensis (L.) Osbeck infected by Citrus tristeza virus. J Hortic Sci Biotechnol. 2016;91:466–475. [Google Scholar]
- 241.Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ruiz Rosquete M, Barbez E, Kleine-Vehn J. Cellular Auxin Homeostasis: gatekeeping Is Housekeeping. Mol Plant. 2012;5:772–786. doi: 10.1093/mp/ssr109. [DOI] [PubMed] [Google Scholar]
- 243.Stes E, Biondi S, Holsters M, Bacterial VD. Plant signal integration via D3-type cyclins enhances symptom development in the arabidopsis-rhodococcus fascians interaction. Plant Physiol. 2011;156:712–725. doi: 10.1104/pp.110.171561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rouleau M, Marsolais F, Richard M, Nicolle L, Voigt B, Adam G, Varin L. Inactivation of brassinosteroid biological activity by a salicylate-inducible steroid sulfotransferase from Brassica napus. J Biol Chem. 1999;274:20925–20930. doi: 10.1074/jbc.274.30.20925. [DOI] [PubMed] [Google Scholar]
- 245.Gómez-Muñoz N, Velázquez K, Vives MC, Ruiz-Ruiz S, Pina JA, Flores R, Moreno P, Guerri J. The resistance of sour orange to Citrus tristeza virus is mediated by both the salicylic acid and RNA silencing defence pathways. Mol Plant Pathol. 2017;18:1253–1266. doi: 10.1111/mpp.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ross JR, Nam KH, D’Auria JC, S-Adenosyl-l-Methionine: PE. Salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch Biochem Biophys. 1999;367:9–16. doi: 10.1006/abbi.1999.1255. [DOI] [PubMed] [Google Scholar]
- 247.Seskar M, Shulaev V, Raskin I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1998;116:387–392. [Google Scholar]
- 248.White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- 249.Németh M, Janda T, Horváth E, Páldi E, Szalai G. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci. 2002;162:569–574. [Google Scholar]
- 250.Jang E-K, Min K-H, Kim S-H, Nam S-H, Zhang S, Kim YC, Cho BH, Yang K-Y. Mitogen-activated protein kinase cascade in the signaling for polyamine biosynthesis in tobacco. Plant Cell Physiol. 2009;50:658–664. [DOI] [PubMed] [Google Scholar]
- 251.Zhang X, Shen L, Li F, Meng D, Sheng J. Methyl salicylate-induced arginine catabolism is associated with up-regulation of polyamine and nitric oxide levels and improves chilling tolerance in cherry tomato fruit. J Agric Food Chem. 2011;59:9351–9357. doi: 10.1021/jf201812r. [DOI] [PubMed] [Google Scholar]
- 252.de Jong A, Yakimova E, Kapchina V, Woltering E. A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta. 2002;214:537–545. doi: 10.1007/s004250100654. [DOI] [PubMed] [Google Scholar]
- 253.Biondi S, Scoccianti V, Scaramagli S, Ziosi V, Torrigiani P. Auxin and cytokinin modify methyl jasmonate effects on polyamine metabolism and ethylene biosynthesis in tobacco leaf discs. Plant Sci. 2003;165:95–101. [Google Scholar]
- 254.Hančević K, Radić T, Pasković I, Urlić B. Biochemical and physiological responses to long-term Citrus tristeza virus infection in Mexican lime plants. Plant Pathol. 2018;67:987–994. [Google Scholar]
- 255.Reddy GS, Murti VD. Citrus diseases and their control. New Delhi: Indian Council of Agricultural Research; 1985. [Google Scholar]
- 256.Timmer LW, Dewdney MM, Inserram RN, Duncan LW. Common names of plant diseases: diseases of citrus (Citrus spp.). 2015. Available from: http://www.apsnet.org/publications/commonnames/pages/citrus.aspx
- 257.M V R, Weinstein LH, Galston AW. Prevention of a plant disease by specific inhibition of fungal polyamine biosynthesis. Proc Natl Acad Sci U S A. 1985;82:6874–6878. doi: 10.1073/pnas.82.20.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rajam MV. Polyamine biosynthesis inhibitors - New protectants against fungal plant-diseases. Curr Sci. 1993;65:461–469. [Google Scholar]
- 259.Walters DR. Polyamines and plant disease. Phytochemistry. 2003;64:97–107. doi: 10.1016/s0031-9422(03)00329-7. [DOI] [PubMed] [Google Scholar]
- 260.Rosa DD, Campos MA, Targon MLPN, Souza AA. Phytophthora parasitica transcriptome, a new concept in the understanding of the citrus gummosis. Genet Mol Biol. 2007;30:997–1008. [Google Scholar]
- 261.Zhu F, Yun Z, Ma Q, Gong Q, Zeng Y, Xu J, Cheng Y, Deng X. Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biol Technol. 2015;100:8–15. [Google Scholar]
- 262.Yun Z, Gao H, Liu P, Liu S, Luo T, Jin S, Xu Q, Xu J, Cheng Y, Deng X. Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. BMC Plant Biol. 2013;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tang N, Chen N, Hu N, Deng W, Chen Z, Li Z. Comparative metabolomics and transcriptomic profiling reveal the mechanism of fruit quality deterioration and the resistance of citrus fruit against Penicillium digitatum. Postharvest Biol Technol. 2018;145:61–73. [Google Scholar]
- 264.Liu P, Chen K, Li G, Yang X, Long C. Comparative transcriptional profiling of orange fruit in response to the biocontrol yeast Kloeckera apiculata and its active compounds. BMC Genomics. 2016;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Long C-A, Wu Z, Deng B-X. Biological control of Penicillium italicum of Citrus and Botrytis cinerea of Grape by Strain 34–9 of Kloeckera apiculata. Eur Food Res Technol. 2005;221:197–201. [Google Scholar]
- 265.Long C-A, Wu Z, Deng B-X. Biological control of Penicillium italicum of Citrus and Botrytis cinerea of Grape by Strain 34–9 of Kloeckera apiculata. Eur Food Res Technol. 2005;221:197–201. [Google Scholar]
- 266.Gonzalez-Candelas L, Alamar S, Sanchez-Torres P, Zacarias L, Marcos JF. A transcriptomic approach highlights induction of secondary metabolism in citrus fruit in response to Penicillium digitatum infection. BMC Plant Biol. 2010;10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu P, Luo L, Long C. Characterization of competition for nutrients in the biocontrol of Penicillium italicum by Kloeckera apiculata. Biol Control. 2013;67:157–162. [Google Scholar]
- 268.Prabhavathi V, Polyamine: SS. Role in Fungal Resistance. In: 2ndInternational Conference on Public Health: Issues, challenges, opportunities, prevention, awareness (Public Health: 2016), New Delhi, Delhi, India. 2016. page 107–110. [Google Scholar]
- 269.Mendonça L, Badel J, Zambolim L. Bacterial citrus diseases: major threats and recent progress. J Bacteriol Mycol Open Access. 2017;5:340–350. [Google Scholar]
- 270.Gabriel DW, Kingsley MT, Hunter JE, Gottwald T. Reinstatement of Xanthomonas citri (ex Hasse) and X. phaseoli (ex Smith) to species and reclassification of all X. campestris pv. citri Strains. Int J Syst Bacteriol. 1989;39:14–22. [Google Scholar]
- 271.Brunings AM, Gabriel DW. Xanthomonas citri: breaking the surface. Mol Plant Pathol. 2003;4:141–157. doi: 10.1046/j.1364-3703.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 272.Boscariol RL, Monteiro M, Takahashi EK, Chabregas SM, Vieira MLC, Vieira LGE, Pereira LFP, de AA Mourao Filho F, Cardoso SC, Christiano RSC. et al. Attacin A gene from tricloplusia ni reduces susceptibility to xanthomonas axonopodis pv. citri in transgenic Citrus sinensis `Hamlin’. J Amer Soc Hort Sci. 2006;131:530–536. [Google Scholar]
- 273.Cardoso SC, Barbosa-Mendes JM, Boscariol-Camargo RL, Christiano RSC, Filho AB, Vieira MLC, Mendes BMJ, Mourão Filho de FAA. Transgenic sweet orange (Citrus sinensis L. Osbeck) Expressing the attacin A gene for resistance to Xanthomonas citri subsp. citri. Plant Mol Biol Report. 2010;28:185–192. [Google Scholar]
- 274.Guo WW, Grosser JW. Transfer of a potential canker resistance gene into Citrus protoplasts using GFP as the selectable marker. In: Albrigo LG, Galán Saúco V, editors. Proceedings of the XXVI International Horticultural Congress: Citrus and other subtropical and tropical fruit crops : issues, advances and opportunities. Toronto, Canada: Acta Horticulturae; 2004. page 255–258. [Google Scholar]
- 275.Omar AA, Grosser JW. Protoplast co-transformation and regeneration of transgenic “Hamlin” sweet orange plants containing a cDNA Xa21 Xanthomonas resistance gene and GFP. Acta Hortic. 2007;738:235–243. [Google Scholar]
- 276.Omar AA, Song W-Y, Grosser JW. Introduction of Xa21, a Xanthomonas-resistance gene from rice, into ‘Hamlin’ sweet orange [Citrus sinensis (L.) Osbeck] using protoplast-GFP co-transformation or single plasmid transformation. J Hortic Sci Biotechnol. 2007;82:914–923. [Google Scholar]
- 277.Mendes BMJ, Cardoso SC, Boscariol-Camargo RL, Cruz RB, FAA MF, Bergamin Filho A. Reduction in susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis expressing the rice Xa21 gene. Plant Pathol. 2010;59:68–75. [Google Scholar]
- 278.Li DL, Xiao X, Guo WW. Production of transgenic anliucheng sweet orange (Citrus sinensis osbeck) with xa21 gene for potential canker resistance. J Integr Agric. 2014;13:2370–2377. [Google Scholar]
- 279.Hao G, Pitino M, Duan Y, Stover E. Reduced Susceptibility to Xanthomonas citri in Transgenic Citrus Expressing the FLS2 Receptor From Nicotiana benthamiana. Mol Plant-Microbe Interact. 2016;29:132–142. doi: 10.1094/MPMI-09-15-0211-R. [DOI] [PubMed] [Google Scholar]
- 280.Shi Q, Febres VJ, Jones JB, Moore GA. A survey of FLS2 genes from multiple citrus species identifies candidates for enhancing disease resistance to Xanthomonas citri ssp. citri. Hortic Res. 2016;3:16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sendín LN, Filippone MP, Orce IG, Rigano L, Enrique R, Peña L, Vojnov AA, Marano MR, Castagnaro AP. Transient expression of pepper Bs2 gene in Citrus limon as an approach to evaluate its utility for management of citrus canker disease. Plant Pathol. 2012;61:648–657. [Google Scholar]
- 282.Sendín LN, Orce IG, Gómez RL, Enrique R, Grellet Bournonville CF, Noguera AS, Vojnov AA, Marano MR, Castagnaro AP, Filippone MP. Inducible expression of Bs2 R gene from Capsicum chacoense in sweet orange (Citrus sinensis L. Osbeck) confers enhanced resistance to citrus canker disease. Plant Mol Biol. 2017;93:607–621. doi: 10.1007/s11103-017-0586-8. [DOI] [PubMed] [Google Scholar]
- 283.Zhang X, Francis MI, Dawson WO, Graham JH, Orbović V, Triplett EW, Mou Z. Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. Eur J Plant Pathol. 2010;128:91–100. [Google Scholar]
- 284.Boscariol-Camargo RL, Takita MA, Machado MA. Bacterial resistance in AtNPR1 transgenic sweet orange is mediated by priming and involves EDS1 and PR2. Trop Plant Pathol. 2016;41:341–349. [Google Scholar]
- 285.Bespalhok Filho JC, Kobayashi AK, Pereira LFP, Vieira LGE, Laranja T. Transformação de laranja visando resistência ao cancro cítrico usando genes de peptídeos antibacterianos. Biotecnol Ciência Desenvolv. 2001;28:229–234. [Google Scholar]
- 286.Sala Junior V, Celloto VR, Vieira LGE, Gonçalves JE, RAC G, de Oliveira AJB. Floral nectar chemical composition of floral nectar in conventional and transgenic sweet orange, Citrus sinensis (L.) Osbeck, expressing an antibacterial peptide. Plant Syst Evol. 2008;275:1–7. [Google Scholar]
- 287.Luo SN, Deng ZN, Zhong XH, Yi TY, Huang RF, Zeng BQ. Preliminary results of Succari’s disease resistance enhanced with TERF1 gene. J Cent South Univ For Technol. 2008;5:71–76. [Google Scholar]
- 288.QinL, Luo SL, Deng ZN, Yan JW, Li N, Yuan FR. Enhanced resistance to canker disease and tolerances to biotic and abiotic stresses in terf1 transgenic sweet orange. In: A. Gentile A, La Malfa S, editors. II International Symposium on Citrus Biotechnology. Catania, Italy: Acta Horticulturae; 2011:165–172. [Google Scholar]
- 289.Barbosa-Mendes JM, Filho de FAAM, Filho AB, Harakava R, Beer SV, Mendes BMJ. Genetic transformation of Citrus sinensis cv. Hamlin with hrpN gene from Erwinia amylovora and evaluation of the transgenic lines for resistance to citrus canker. Sci Hortic. 2009;122:109–115. [Google Scholar]
- 290.Enrique R, Siciliano F, Favaro MA, Gerhardt N, Roeschlin R, Rigano L, Sendin L, Castagnaro A, Vojnov A, Marano MR. Novel demonstration of RNAi in citrus reveals importance of citrus callose synthase in defence against Xanthomonas citri subsp. citri. Plant Biotechnol J. 2011;9:394–407. doi: 10.1111/j.1467-7652.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- 291.Yang L, Hu C, Li N, Zhang J, Yan J, Deng Z. Transformation of sweet orange [Citrus sinensis (L.) Osbeck] with pthA-nls for acquiring resistance to citrus canker disease. Plant Mol Biol. 2011;75:11–23. doi: 10.1007/s11103-010-9699-z. [DOI] [PubMed] [Google Scholar]
- 292.Chen X, Barnaby JY, Sreedharan A, Huang X, Orbović V, Grosser JW, Wang N, Dong X, Song W-Y. Over-expression of the citrus gene CtNH1 confers resistance to bacterial canker disease. Physiol Mol Plant Pathol. 2013;84:115–122. [Google Scholar]
- 293.de Oliveira MLP, de Lima Silva CC, Abe VY, Costa MGC, Cernadas RA, Benedetti CE. Increased Resistance Against Citrus Canker Mediated by a Citrus Mitogen-Activated Protein Kinase. Mol Plant-Microbe Interact. 2013;26:1190–1199. doi: 10.1094/MPMI-04-13-0122-R. [DOI] [PubMed] [Google Scholar]
- 294.Fu X-Z, Chen C-W, Wang Y, Liu J-H MT. Ectopic expression of MdSPDS1 in sweet orange (Citrus sinensis Osbeck) reduces canker susceptibility: involvement of H2O2 production and transcriptional alteration. BMC Plant Biol. 2011;11:55. doi: 10.1186/1471-2229-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fu X-Z, Liu J-H. Transcriptional profiling of canker-resistant transgenic sweet orange (Citrus sinensis Osbeck) constitutively overexpressing a spermidine synthase gene. Biomed Res Int. 2013;2013:918136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Furman N, Kobayashi K, Zanek MC, Calcagno J, Garcia ML, Mentaberry A. Transgenic sweet orange plants expressing a dermaseptin coding sequence show reduced symptoms of citrus canker disease. J Biotechnol. 2013;167:412–419. doi: 10.1016/j.jbiotec.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 297.Soprano AS, Abe VY, Smetana JHC, Benedetti CE. Citrus MAF1, a repressor of RNA Polymerase III, binds the Xanthomonas citri canker elicitor PthA4 and suppresses citrus canker development. Plant Physiol. 2013;163:232–242. doi: 10.1104/pp.113.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Caserta R, Picchi SC, Takita MA, Tomaz JP, Pereira WEL, Machado MA, Ionescu M, Lindow S, De Souza AA. Expression of Xylella fastidiosaRpfF in Citrus Disrupts Signaling in Xanthomonas citri subsp. citri and Thereby Its Virulence. Mol Plant-Microbe Interact. 2014;27:1241–1252. doi: 10.1094/MPMI-03-14-0090-R. [DOI] [PubMed] [Google Scholar]
- 299.Zou X, Song E, Peng A, He Y, Xu L, Lei T, Yao L, Chen S. Activation of three pathogen-inducible promoters in transgenic citrus (Citrus sinensis Osbeck) after Xanthomonas axonopodis pv. citri infection and wounding. Plant Cell, Tissue Organ Cult. 2014;117:85–98. [Google Scholar]
- 300.Hao G, Stover E, Gupta G. Overexpression of a modified plant thionin enhances disease resistance to citrus canker and Huanglongbing (HLB). Front Plant Sci. 2016;7:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Shimada T, Endo T, Rodríguez A, Fujii H, Goto S, Matsuura T, Hojo Y, Ikeda Y, Mori IC, Fujikawa T. et al. Ectopic accumulation of linalool confers resistance to Xanthomonas citri subsp. citri in transgenic sweet orange plants. Tree Physiol. 2017;37:654–664. doi: 10.1093/treephys/tpw134. [DOI] [PubMed] [Google Scholar]
- 302.Jia H, Zhang Y, Orbović V, Xu J, White FF, Jones JB, Wang N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol J. 2017;15:817–823. doi: 10.1111/pbi.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Duan S, Jia H, Pang Z, Teper D, White F, Jones J, Zhou C, Wang N. Functional characterization of the citrus canker susceptibility gene CsLOB1. Mol Plant Pathol. 2018;19:1908–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Walters DR. Polyamines in plant–microbe interactions. Physiol Mol Plant Pathol. 2000;57:137–146. [Google Scholar]
- 305.Walters D. Resistance to plant pathogens: possible roles for free polyamines and polyamine catabolism. New Phytol. 2003;159:109–115. [DOI] [PubMed] [Google Scholar]
- 306.Mitsuya Y, Takahashi Y, Berberich T, Miyazaki A, Matsumura H, Takahashi H, Terauchi R, Kusano T. Spermine signaling plays a significant role in the defense response of Arabidopsis thaliana to cucumber mosaic virus. J Plant Physiol. 2009;166:626–643. doi: 10.1016/j.jplph.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 307.Do P Apparecido R, Carlos EF, Lião LM, Vieira LGE, Alcantara GB. NMR-based metabolomics of transgenic and non-transgenic sweet orange reveals different responses in primary metabolism during citrus canker development. Metabolomics. 2017;13:20. [Google Scholar]
- 308.Yang F, Chen C, Fan Q, Shi C, Xie Z, Guo D, Liu J. Influence of temperature and polyamines on occurrence of citrus canker disease and underlying mechanisms. Sci Agric Sin. 2018;51:1899–1907. [Google Scholar]
- 309.Petrocelli S, Pizarro MD, Alet A, De Ollas C, Talón M, Tadeo FR, Gómez-Cadenas A, Arbona V, Orellano EG, Daurelio LD. Phytohormone participation during Citrus sinensis non-host response to Xanthomonas campestris pv. vesicatoria. Plant Gene. 2018;15:28–36. [Google Scholar]
- 310.Murata MM, Omar AA, Mou Z, Chase CD, Grosser JW, Graham JH. novel plastid-nuclear genome combinations enhance resistance to citrus canker in cybrid grapefruit. Front Plant Sci. 2019;9:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lee RF, Beretta MJG, Hartung JH, Hooker ME, Derrick KS. Citrus variegated chlorosis: confirmation of Xylella fastidiosa as the causal agent. Summa Phytopathol. 1993;19:123–125. [Google Scholar]
- 312.Hartung JS, Beretta J, Brlansky RH, Spisso J, Lee RF. Citrus variegated chlorosis bacterium: axenic culture, pathogenicity, and serological relationships with other strains of Xylella fastidiosa. Phytopathology. 2007;84:591. [Google Scholar]
- 313.Almeida RPP, Blua MJ, Lopes JRS, Purcell AH. Vector transmission of Xylella fastidiosa: applying fundamental knowledge to generate disease management strategies. Ann Entomol Soc Am. 2005;98:775–786. [Google Scholar]
- 314.Timmer LW, Lee RF, Allen JC, Tucker DPH. Distribution of sharpshooters in florida citrus groves. Environ Entomol. 1982;11:456–460. [Google Scholar]
- 315.De Souza AA, Takita MA, Do Amaral AM, Coletta-Filho HD, Machado MA. Citrus responses to Xylella fastidiosa Infection, the causal agent of citrus variegated chlorosis. Tree For Sci Biotechnol. 2009;3:73–80. [Google Scholar]
- 316.Purcino RP, Medina CL, de Souza DM, Winck FV, Machado EC, Novello JC, Machado MA, Mazzafera P. Xylella fastidiosa disturbs nitrogen metabolism and causes a stress response in sweet orange Citrus sinensis cv. Pera. J Exp Bot. 2007;58:2733–2744. doi: 10.1093/jxb/erm138. [DOI] [PubMed] [Google Scholar]
- 317.Garcia AL, Torres SCZ, Heredia M, Lopes SA. Citrus responses to Xylella fastidiosa infection. Plant Dis. 2012;96:1245–1249. doi: 10.1094/PDIS-10-11-0868-RE. [DOI] [PubMed] [Google Scholar]
- 318.Gomes MMA, AMMA L, Machado EC, Medina CL. Abscisic acid and indole-3-acetic acid contents in orange trees infected by Xylella fastidiosa and submitted to cycles of water stress. Plant Growth Regul. 2003;39:263–270. [Google Scholar]
- 319.da Silva Neto JF, Koide T, Gomes SL, Marques MV. Global gene expression under nitrogen starvation in Xylella fastidiosa: contribution of the σ54 regulon. BMC Microbiol. 2010;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Medina CL Fotossintese, relações hidricas e alterações bioquimicas em laranjeira “pera” com CVC e submetida a deficiencia hidrica. 2002; 147p. Tese (doutorado) - Universidade Estadual de Campinas, Instituto de Biologia, Campinas (SP). Disponível em. [Google Scholar]
- 321.Jagoueix S, Bove JM, Garnier M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int J Syst Bacteriol. 1994;44:379–386. doi: 10.1099/00207713-44-3-379. [DOI] [PubMed] [Google Scholar]
- 322.Garnier M, Jagoueix-Eveillard S, Cronje PR, Le Roux HF, Bove JM. Genomic characterization of a Liberibacter present in an ornamental rutaceous tree, Calodendrum capense, in the Western Cape province of South Africa. Proposal of “Candidatus Liberibacter africanus subsp. capensis. Int J Syst Evol Microbiol. 2000;50:2119–2125. doi: 10.1099/00207713-50-6-2119. [DOI] [PubMed] [Google Scholar]
- 323.Bové JM. Huanglongbing: a destructive, newly-emerging, century-old diesease of citrus. J Plant Pathol. 2006;88:7–37. [Google Scholar]
- 324.Bové JM, Ayres AJ. Etiology of three recent diseases of citrus in São Paulo State: sudden death, variegated chlorosis and Huanglongbing. IUBMB Life. 2007;59:346–354. doi: 10.1080/15216540701299326. [DOI] [PubMed] [Google Scholar]
- 325.Tatineni S, Sagaram US, Gowda S, Robertson CJ, Dawson WO, Iwanami T, Wang N. In planta distribution of “Candidatus Liberibacter asiaticus” as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathology. 2008;98:592–599. doi: 10.1094/PHYTO-98-5-0592. [DOI] [PubMed] [Google Scholar]
- 326.Wang N, Citrus Huanglongbing: TP. a newly relevant disease presents unprecedented challenges. Phytopathology. 2013;103:652–665. doi: 10.1094/PHYTO-12-12-0331-RVW. [DOI] [PubMed] [Google Scholar]
- 327.da Graça JV. Citrus Greening Disease. Annu Rev Phytopathol. 1991;29:109–136. [Google Scholar]
- 328.Teixeira D Do C, Saillard C, Eveillard S, Danet JL, da Costa PI, Ayres AJ, Bové J. “Candidatus Liberibacter americanus”, associated with citrus Huanglongbing (greening disease) in São Paulo State, Brazil. Int J Syst Evol Microbiol. 2005;55:1857–1862. doi: 10.1099/ijs.0.63677-0. [DOI] [PubMed] [Google Scholar]
- 329.Halbert SE, Manjunath KL, Psyllids AC. (Sternorrhyncha: psyllidae) and Greening Disease of Citrus: a Literature Review and Assessment of Risk in Florida. Florida Entomol. 2004;87:330–353. [Google Scholar]
- 330.APD M, Oberholzer PCJ. Citrus psylla, a vector of the greening disease of Sweet Orange. South African J Agric Sci. 1965;8:297–298. [Google Scholar]
- 331.Capoor SP, Rao DG, Viswanath SM. Diaphorina citri Kuway., a vector of the greening disease of citrus in India. Indian J Agric Sci. 1967;37:572–576. [Google Scholar]
- 332.Martinez AL, Wallace JM. Citrus leaf-mottle-yellows disease in the Philippines and transmission of the causal virus by a Psyllid, Diaphorina citri. Plant Dis Report. 1967;51:692–695. [Google Scholar]
- 333.Baldwin E, Plotto A, Manthey J, Mccollum G, Bai J, Irey M, Cameron R, Luzio G. Effect of Liberibacter infection (Huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: chemical and physical analyses. J Agric Food Chem. 2010;58:1247–1262. doi: 10.1021/jf9031958. [DOI] [PubMed] [Google Scholar]
- 334.Hijaz F, Nehela Y, Killiny N. Possible role of plant volatiles in tolerance against Huanglongbing in citrus. Plant Signal Behav. 2016;11:e1138193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Xu BM, Baker GL, Sarnoski PJ, Goodrich-Schneider RM. A Comparison of the Volatile Components of Cold Pressed Hamlin and Valencia (Citrus sinensis (L.) Osbeck) Orange Oils Affected by Huanglongbing. J Food Qual. 2017;2017:1–20. [Google Scholar]
- 336.Mendoza-Peña E, Cibrián-Tovar J, Velázquez-González J, Tafoya-Rangel F, Azuara-Domínguez A. Volatile compounds of Persian and Mexican lime associated with HLB (Huanglongbing) symptoms. Rev Colomb Entomol. 2018;44:19. [Google Scholar]
- 337.Jones SE, Hijaz F, Davis CL, Folimonova SY, Manthey JA, Reyes-De-Corcuera JI. GC-MS analysis of secondary metabolites in leaves from orange trees infected with HLB: a 9-month course study. In: Proceedings of the 125th Annual Meeting of the Florida State Horticultural Society. Delray Beach, FL, USA: the Florida State Horticultural Society; 2012. p. 75–83. [Google Scholar]
- 338.Chin EL, Mishchuk DO, Breksa AP, Slupsky CM. Metabolite signature of Candidatus Liberibacter asiaticus infection in two citrus varieties. J Agric Food Chem. 2014;62:6585–6591. doi: 10.1021/jf5017434. [DOI] [PubMed] [Google Scholar]
- 339.Balan B, Ibáñez AM, Dandekar AM, Caruso T, Martinelli F. Identifying host molecular features strongly linked with responses to huanglongbing disease in citrus leaves. Front Plant Sci. 2018;9:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Dagulo L, Danyluk MD, Spann TM, Valim MF, Goodrich-Schneider R, Sims C, Rouseff R. Chemical Characterization of Orange Juice from Trees Infected with Citrus Greening (Huanglongbing). J Food Sci. 2010;75:C199–207. doi: 10.1111/j.1750-3841.2009.01495.x. [DOI] [PubMed] [Google Scholar]
- 341.Hijaz FM, Manthey JA, Folimonova SY, Davis CL, Jones SE, Reyes-De-Corcuera JI. An HPLC-MS Characterization of the Changes in Sweet Orange Leaf Metabolite Profile following Infection by the Bacterial Pathogen Candidatus Liberibacter asiaticus. PLoS One. 2013;8:e79485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kiefl J, Kohlenberg B, Hartmann A, Obst K, Paetz S, Krammer G, Trautzsch S. Investigation on key molecules of huanglongbing (HLB)-induced orange juice off-flavor. J Agric Food Chem. 2018;66:2370–2377. doi: 10.1021/acs.jafc.7b00892. [DOI] [PubMed] [Google Scholar]
- 343.Massenti R, Lo Bianco R, Sandhu AK, Gu L, Sims C. Huanglongbing modifies quality components and flavonoid content of ‘Valencia’ oranges. J Sci Food Agric. 2016;96:73–78. doi: 10.1002/jsfa.7061. [DOI] [PubMed] [Google Scholar]
- 344.Dala Paula BM, Raithore S, Manthey JA, Baldwin EA, Bai J, Zhao W, MBA G, Plotto A. Active taste compounds in juice from oranges symptomatic for Huanglongbing (HLB) citrus greening disease. LWT. 2018;91:518–525. [Google Scholar]
- 345.Fagen JR, Leonard MT, McCullough CM, Edirisinghe JN, Henry CS, Davis MJ, Triplett EW. Comparative Genomics of Cultured and Uncultured Strains Suggests Genes Essential for Free-Living Growth of Liberibacter. PLoS One. 2014;9:e84469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Duan Y, Zhou L, Hall DG, Li W, Doddapaneni H, Lin H, Liu L, Vahling CM, Gabriel DW, Williams KP. et al. Complete genome sequence of citrus huanglongbing bacterium, ‘ candidatus liberibacter asiaticus’ obtained through metagenomics. Mol Plant-Microbe Interact. 2009;22:1011–1020. doi: 10.1094/MPMI-22-8-1011. [DOI] [PubMed] [Google Scholar]
- 347.Leonard MT, Fagen JR, Davis-Richardson AG, Davis MJ, Triplett EW. Complete genome sequence of Liberibacter crescens BT-1. Stand Genomic Sci. 2012;7:271–283. doi: 10.4056/sigs.3326772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Li W, Cong Q, Pei J, Kinch L, Grishin N. The ABC transporters in Candidatus Liberibacter asiaticus. Proteins Struct Funct Bioinforma. 2012;80:2614–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Schneider H. Anatomy of greening diseased sweet orange shoots. Phytopathology. 1968;58:1155–1160. [Google Scholar]
- 350.Pitino M, Armstrong CM, Duan Y. Molecular mechanisms behind the accumulation of ATP and H2O2 in citrus plants in response to ‘Candidatus Liberibacter asiaticus’ infection. Hortic Res. 2017;4:17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Nehela Y, Hijaz F, Elzaawely AA, El-Zahaby HM, Killiny N. Phytohormone profiling of the sweet orange (Citrus sinensis (L.) Osbeck) leaves and roots using GC-MS-based method. J Plant Physiol. 2016;199:12–17. doi: 10.1016/j.jplph.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 352.Freitas DDS, Carlos EF, Gil de MCSS, Vieira LGE, Alcantara GB. NMR-Based Metabolomic Analysis of Huanglongbing-Asymptomatic and -Symptomatic Citrus Trees. J Agric Food Chem. 2015;63:7582–7588. doi: 10.1021/acs.jafc.5b03598. [DOI] [PubMed] [Google Scholar]
- 353.Albrecht U, Fiehn O, Bowman KD. Metabolic variations in different citrus rootstock cultivars associated with different responses to Huanglongbing. Plant Physiol Biochem. 2016;107:33–44. doi: 10.1016/j.plaphy.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 354.Killiny N. Metabolomic comparative analysis of the phloem sap of curry leaf tree (Bergera koenegii), orange jasmine (Murraya paniculata), and Valencia sweet orange (Citrus sinensis) supports their differential responses to Huanglongbing. Plant Signal Behav. 2016;11:e1249080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chin E, Godfrey K, Polek M, Slupsky C. 1H NMR analysis of Citrus macrophylla subjected to Asian citrus psyllid (Diaphorina citri Kuwayama) feeding. Arthropod Plant Interact. 2017;11:901–909. [Google Scholar]
- 356.Killiny N. Metabolite signature of the phloem sap of fourteen citrus varieties with different degrees of tolerance to Candidatus Liberibacter asiaticus. Physiol Mol Plant Pathol. 2017;97:20–29. [Google Scholar]
- 357.Sétamou M, Alabi OJ, Simpson CR, Jifon JL. Contrasting amino acid profiles among permissive and non-permissive hosts of Candidatus Liberibacter asiaticus, putative causal agent of Huanglongbing. PLoS One. 2017;12:e0187921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hung W-L WY. A targeted mass spectrometry-based metabolomics approach toward the understanding of host responses to huanglongbing disease. J Agric Food Chem. 2018;66:10651–10661. doi: 10.1021/acs.jafc.8b04033. [DOI] [PubMed] [Google Scholar]
- 359.Hung W-L, Wang Y. Metabolite profiling of candidatus liberibacter infection in hamlin sweet oranges. J Agric Food Chem. 2018;66:3983–3991. doi: 10.1021/acs.jafc.7b05866. [DOI] [PubMed] [Google Scholar]
- 360.Moschou PN, Wu J, Cona A, Tavladoraki P, Angelini R, Roubelakis-Angelakis KA. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J Exp Bot. 2012;63:5003–5015. doi: 10.1093/jxb/ers202. [DOI] [PubMed] [Google Scholar]
- 361.Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol. 2011;2:8–23. [PMC free article] [PubMed] [Google Scholar]
- 362.Parker D, Beckmann M, Zubair H, Enot DP, Caracuel-Rios Z, Overy DP, Snowdon S, Talbot NJ, Draper J. Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J. 2009;59:723–737. doi: 10.1111/j.1365-313X.2009.03912.x. [DOI] [PubMed] [Google Scholar]
- 363.Voll L, Horst RJ, Voitsik AM, Zajic D, Samans B, Pons-Kühnemann J, Doehlemann G, Münch S, Wahl R, Molitor A. et al. Common motifs in the response of cereal primary metabolism to fungal pathogens are not based on similar transcriptional reprogramming. Front Plant Sci. 2011;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Arbo MD, Larentis ER, Linck VM, Aboy AL, Pimentel AL, Henriques AT, Dallegrave E, Garcia SC, Leal MB, Limberger RP. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem Toxicol. 2008;46:2770–2775. [DOI] [PubMed] [Google Scholar]
- 365.Dragull K, Breksa III AP, Cain B. Synephrine Content of Juice from Satsuma Mandarins (Citrus unshiu Marcovitch). J Agric Food Chem. 2008;56:8874–8878. doi: 10.1021/jf801225n. [DOI] [PubMed] [Google Scholar]
- 366.Wheaton TA, Stewart I. Biosynthesis of synephrine in citrus. Phytochemistry. 1969;8:85–92. [Google Scholar]
- 367.Bartley GE, Breksa AP, Ishida BK. PCR amplification and cloning of tyrosine decarboxylase involved in synephrine biosynthesis in Citrus. N Biotechnol. 2010;27:308–316. doi: 10.1016/j.nbt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 368.Hijaz F, Nehela Y, Killiny N. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta. 2018;248:909–918. doi: 10.1007/s00425-018-2947-1. [DOI] [PubMed] [Google Scholar]
- 369.Abdulhussein MAA. Effects of exogenous putrescine application on hormone content of Citrus aurantium L. seedling under salt stress. J Agric Sci Technol A. 2014;4:521–528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Timmer LW, Dewdney MM, Inserram RN, Duncan LW. Common names of plant diseases: diseases of citrus (Citrus spp.). 2015. Available from: http://www.apsnet.org/publications/commonnames/pages/citrus.aspx


