ABSTRACT
We aimed to screen cold-tolerant introgression lines (ILs) of bell pepper and investigate stress responses of these bell peppers under low temperature. Seedlings of cold-resistant wild-type bell pepper CA157, cultivated bell pepper CA52, and their ILs were evaluated for their tolerance to low temperature. Electrical conductivity measurement was performed on ILs and two parents. Then, contents of physiological and biochemical indexes including malondialdehyde (MDA), proline, and soluble sugar content were examined. Moreover, the superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and peroxidase (POD) activities were further investigated. Finally, the chlorophyll fluorescence (PSII) parameters in all pant leaves were examined.
A total of 22 IL plants showed better resistance than their recurrent parent CA52. CL122 was one of the most outstanding plants in ILs that had similar performance with wild bell pepper CA157. Cold resistance analysis based on physiological and biochemical indexes showed that factors such as electrical conductivity, MDA, and PSII were closely related to cold resistance among CA157, CA52, and CL122 under low-temperature stress.
In conclusion, ILs constructed in the current study might be used for cold resistance gene introgression between wild and cultured species. Moreover, CL122 might be a novel bridge material for understanding low-temperature response in bell pepper. Furthermore, electrical conductivity, MDA, and PSII might be used to identify the low-temperature resistance of bell pepper plants in a period of obvious differentiation.
KEYWORDS: Bell pepper, introgression lines, low-temperature stress response, wild species
Introduction
Bell pepper, also known as sweet pepper, is a cultivar group of the species Capsicum annuum,1 and the estimated value of cash receipts for bell peppers in a year is approximately $247.5 million.2 Preferred growing conditions for bell peppers include warm environment, moist soil, and moderate temperate (21–29°C [70°F to 84°F]).3 Currently, low temperature has been regarded as the main limiting factor in pepper cultivation4 as low temperature (under 10°C) affects the growth of bell pepper and its biological yield and even causes plant death. Thus, the screening of introgression lines (ILs) of bell pepper is vital for cold-resistant varieties’ breeding and agricultural production.
In recent years, with the development of technology for quantitative character study of plants, quantitative trait locus (QTL) has been widely used for identifying the main effect of resistance QTL in plants.5 Several QTLs controlling cold resistance at different growth stages of rice have been identified.6 The ILs are useful materials for the mapping of QTLs and evaluation of gene action or interaction in breeding.7 Moreover, multiple backcrosses between IL individuals and receptors can be used to construct QTL-near-isogenic lines (NILs), which will be useful materials for fine mapping and resistance gene cloning.7,8 To our best knowledge, there is no study about the cold-tolerant ILs of bell pepper. It is well known that the wild-type bell pepper is more tolerant to low temperature than cultivated bell pepper.9 The wild-type bell pepper CA157 has a strong resistance to low temperature, which provides an advantageous resource for the exploration of low-temperature resistance-related QTLs/genes in wild sweet pepper. Thus, the construction of QTL-NILs via CA157 and cultivated species is vital for the localization of genes that resist environmental stress.
In this study, in order to evaluate the tolerance of wild-type bell pepper CA157 with cold resistance, cultivated bell pepper CA52, and their ILs to low temperature, the malondialdehyde (MDA), proline, soluble sugar content, superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and peroxidase (POD) activity were investigated. The analysis of low-temperature stress response for current ILs will lay a foundation for further construction of subpopulations and key gene cloning in wild bell pepper.
Materials and methods
Plant material
The seeds of ILs (107 samples) and two parents (wild-type CA157 as donor; processing CA52 as recipient) were obtained from Sweet Peppers Genetics Resource Center (TGRC). The processing CA52 contained more than 85% genome of the wild-type bell pepper. Each IL contains one or more chromosome replacement of bell pepper.
Low-temperature-resistant IL selection
The full-grain seeds of transgenic sweet pepper were selected for sprouting after sowing in a hole plate containing nutritional soil. Some seedlings in the hole plate (20 samples of each group, a total of six group repetition) with the same growth were transplanted to the black nutrition bowl when the seedlings grew to two leaves and one heart stage. Then, all these plants were treated in 4°C low-temperature cold storage (light intensity: 120 μmol m−2 s−1, relative humidity: 70–80%) to reveal the effect of low-temperature treatment on seedling stage at 4°C. Plant phenotypes were observed every 2 h during treatment. Meanwhile, some seedlings in hole plate (20 samples of each group, a total of six group repetition) were cultured under natural light in a glass greenhouse (day temperature: 24–28°C, night temperature: 20–25°C, relative humidity: 70–80%) to reveal the effect of low-temperature treatment on potted seedlings at 4°C. When the seedlings grow to two leaves and one heart stage, the seedlings with the same growth will be transplanted to the black nutrition bowl (vermiculite: peat, perlite: garden soil). After the growth cycle, the plants with the same growth were selected for low-temperature treatment (3 repetitions for each treatment, and 12 strains in each repeat). The selected materials were randomly placed in a white tray. Then, all plants were treated in 4°C low-temperature cold storage (light intensity: 120 μmol m−2 s−1, relative humidity: 70–80%). The phenotype of the plants was observed daily during the process. The grade of blade injury was investigated and evaluated with criteria including grade 0 (the blade is normal), grade 1 (the edge of the leaf is slightly crinkled), grade 2 (the leaf blade atrophy), grade 3 (more than half of the leaves are severely crinkled, the petiole drooping), and grade 4 (the whole plant atrophy, the top drooping).
Electrical conductivity measurement
The electrical conductivity was measured according to Campos et al.,10 with slight modification. A total of 25 ml deionized water was added to the clean cape bottle. Then, totally 20 tablets were punched from the fourth leaves on the plants under normal conditions and low-temperature stress, followed by treatment with rocking bed (60 r/min, 5 h). The conductivity of the soaking solution (S1) was measured by a portable conductance meter (DDB-303A). After treatment of boiling water bath for 30 min, the electrical conductivity of the solution S2 was measured. Relative conductivity (%) = (S1 × 100)/S2.
MDA content examination
MDA content in leaves of bell pepper was examined according to Campos et al.,10 with slight modification. The powder sample (0.2 g) after liquid nitrogen grinding was added in the 10 ml centrifuge tube. After treatment of acetocaustin and 2-thiobarbituric acid, the absorbance values of the supernatant at the wavelength of 450, 532 , and 600 nm were measured, respectively. The MDA concentration (mol/l) was calculated with formula (1):
| (1) |
where A450, A532, and A600 represent the absorption values at wavelengths of 450, 532, and 600 nm, respectively. The MDA content (pmol) in the leaves of bell pepper was further calculated with formula (2):
| (2) |
where V is the sample crystal extract volume (ml), and W represents the quality of the product (g).
Proline and soluble sugar content examination
The standard curve of proline content was constructed according to Bates et al.,11 with slight modification. Then, based on the standard curve, the proline content (%) of bell pepper was calculated with formula (3):
| (3) |
where X represents the proline content in the solution; Vt represents the volume of extraction solution (ml); Vs represents the sample volume (ml); and W is the sample quality (g).
The standard curve of soluble sugar content was determined by anthrone colorimetry according to Fukao et al.,12 with slight modification. Then, the soluble enamel content (%) in bell pepper was calculated with formula (4):
| (4) |
C represents the content (μg) obtained from the standard curve; V represents the total volume of extraction liquid (ml); Vs represents the sample extract volume (ml); N represents the dilution multiple; and W represents the quality of the product (g).
SOD, CAT, APX, and POD activity examination
The SOD activity was examined according to the method of Al-aghabary et al.13 A total of 1.6 ml 50 mmol/l phosphate buffer, 0.3 ml 130 nmol/l Met solution, 0.3 ml 750 mol/l NBT solution, 0.3 ml 100 mol/l EDTA-Na2 solution, 0.3 ml 20 μmol/l riboflavin, and 0.2 ml enzyme extract were added in 10 ml glass test tube each time. Two pairs of tubes were placed to replace the enzyme solution with phosphate buffer. One tube was placed in dark, while the other was placed under light for 20 min. At the end of the reaction, the absorbance of the reaction solution at 560 mn of each tube was measured (tube in black was set as control). The SOD activity (U/g) was calculated with formula (5):
| (5) |
where Ack represents the absorbance of black control, while AE represents the absorbance of sample tube. V represents the sample liquid volume (ml), while Vt represents the sample dosage (ml) at the time of measurement. W represents the sample fresh weight (g).
The CAT activity was examined according to the method of Al-aghabary et al.,13 with slight modification. The CAT activity (U/min×g) was calculated with formula (6):
| (6) |
where V represents the total volume of sample (ml), while Vt represents the sample dosage for examination (ml). T represents the examination time (min), while W represents the sample fresh weight (g).
The APX activity was examined according to the method of Jimenez et al.,14 with slight modification. The activity of APX was calculated with formula (7):
| (7) |
where ΔOD represents the change of absorbance during reaction, while T represents reaction time (min). V represents the volume of extracting solution (ml), while A represents the extinction coefficient (2.8 mm). Vt represents the volume of test solution (ml), while W represents the sample fresh weight (g).
The POD activity was examined using the guaiacol method according to Morohashi,15 with slight modification. POD activity was calculated with formula (8):
| (8) |
where V represents the total volume of the sample solution (ml), while Vt represents the sample consumption (ml). T represents the time of examination (min), while W represents sample fresh weight (g).
Chlorophyll fluorescence parameter examination
The chlorophyll fluorescence for leaves in the plant from the control group and the treatment group (the third leaf from the top) was measured using a portable pulse-modulated fluorescence instrument (FMS-2, Hansatech, UK). The measuring method was according to Wingler et al.16 The blade was fully adapted to 20 min after using dark adaptation clamp. Then, the maximum fluorescence Fm and initial fluorescence Fo were examined under dark. The following equations were used for calculating photosynthetic parameters. The maximum quantum efficiency of photosystem II photochemistry was calculated with Fv/Fm = (Fm − F0)/Fm; quantum efficiency of excitation energy capture by open photosystem II centers was calculated with Fv’/Fm’ = (Fm ‘− F0ʹ)/Fm’; Fm’ represents maximum fluorescence of light‐adapted leaves; Fs represents steady‐state fluorescence; and F0ʹ represents ground fluorescence. Meanwhile, the actual photochemical yield of photosystem II (ΦPSII) was calculated with (Fm’-Fs)/Fm’.
Statistical analysis
All the experimental data in this study were sorted, analyzed, and plotted using SigmaPlot software (version 12, Systat Software Inc.).17 The data were expressed as mean ± SE. The significance of difference was analyzed by one-way analysis of variance. p < 0.05 indicated significant result.
Results
Low-temperature-resistant IL construction
After 3 d of low-temperature (4°C) treatment on CA157 and CA52 plants (6 weeks old), there was a significant difference (p < 0.05) in the occurrence of plant wilt between two groups. In order to identify which chromosomal regions in wild sweet peppers contribute to cold resistance, a total of 93 ILs from CA157 IL population of wild sweet pepper at seedling stage (6 weeks old) were treated at 4°C low temperature. After 3 d of treatment, the ILs showed different degrees of wilting (Figure 1). A total of 22 IL leaves were lighter in atrophy degree than those of the recurrent parent CA52. The low-temperature stress was performed on these 22 ILs, and a plant, named CL122, showed similar performance with wild bell pepper CA157 during the whole treatment, significantly better than other ILs and recurrent female parent CA52. After 2 weeks of low-temperature treatment, the survival rate of CA157 and CL122 was higher than others. Most of the leaves on the top of the plant returned to normal, while most of the other ILs died. Repeated test (three times) showed that the phenotype of CA157 and CL122 was similar under low-temperature stress, and the cold resistance was significantly higher than that of CA52.
Figure 1.

Different chilling injury degree of bell pepper after treated with 4°C for 3 d. (a) chilling injury degree 0 (no chilling injury symptoms); (b) chilling injury degree 1 (the edges of one or two leaves shrunk and curled slightly); (c) chilling injury degree 2 (one or two leaves shrunk and curled); (d) chilling injury degree 3 (more than half of the plant leaves shrunk and curled severely, and the leaf stalk drooped); (e) chilling injury degree 4 (the whole plant leaves shrunk and curled, and stem flexed).
Cold resistance difference among CA157, CA52, and CL122
Plant phenotypes of CA157, CA52, and CL122 in control groups are shown in Figure 2a. After 3 d of low-temperature (4°C) treatment, CA52 plants wilted severely, with the tip of the stem beginning to curve, while the CA157 and CL122 plants showed only a slight curl at the margin of basal leaf blade (Figure 2b). After 10 d of low-temperature treatment, the leaves of CA52 were further dehydrated and withered, and the leaves curled up together (Figure 2c). After 2 weeks of recovery, most of the top leaves of CA157 and CL122 returned to normal. Most of the surviving CA52 plants had completely dried-up leaves, and only lateral lumbar lateral buds or terminal buds had new leaves (Figure 2d). These results indicated that the cold resistance of CA157 and CL122 was significantly higher than that of CA52.
Figure 2.
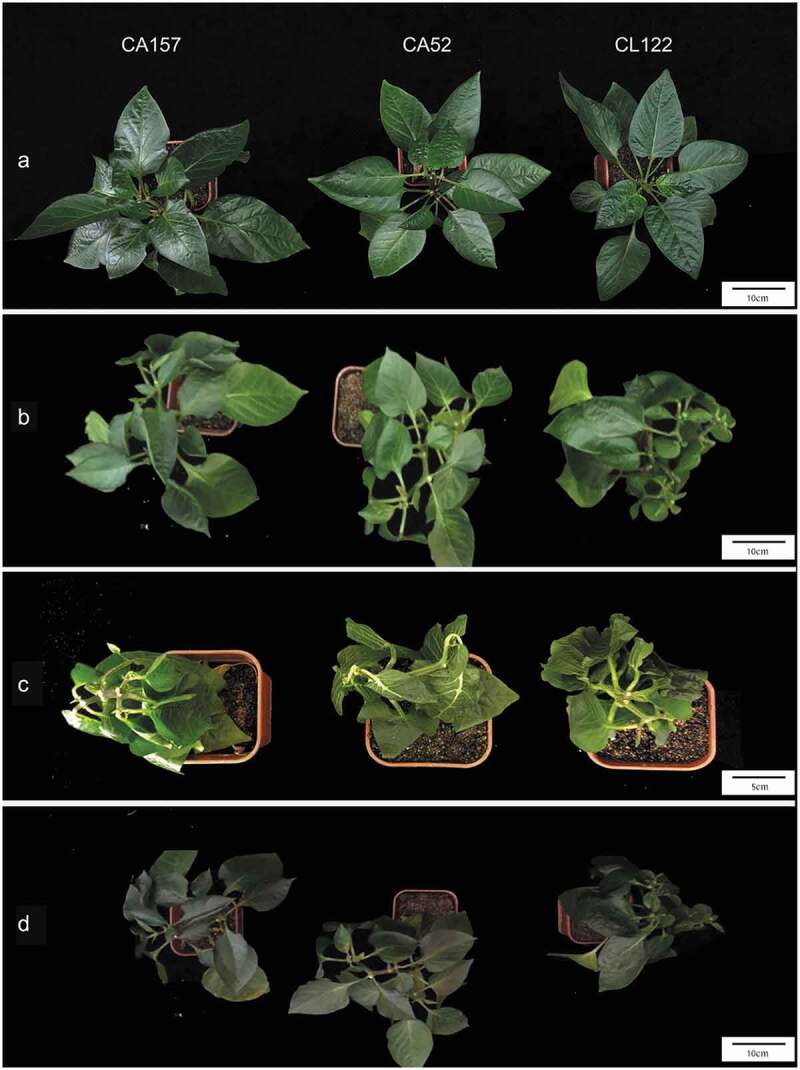
Comparison of phenotypic and physiological responses of seedlings of CA157, CA52, and CL122 under low-temperature stress. Seedlings of CA157, CA52, and CL122 in control (a), treated at 4°C for 3 d (b), treated at 4°C for 10 d (c), and recovered for 2 weeks after 10 d of cold treatment (d).
Effects of low-temperature stress on cell membrane of bell pepper seedlings
Under low-temperature stress, the relative conductivity of CA157, CA52, and CL122 increased with the prolongation of treatment time, but the amplitude was larger in cold-sensitive CA52 (Figure 3a). Under normal conditions and low temperature for 1 d, the difference in the relative conductivity among CA157, CA52, and CL122 was not significant. From the 3rd d, the leaf relative conductivity of CA52 was significantly higher than that of CA157 and CL122 (p < 0.05). The variation trend of MDA was basically consistent with relative conductivity. The MDA content in leaves of CA157 and CL122 was significantly lower than that of CA52 at 5th d and 7th d at low temperature (p < 0.05) (Figure 3b). These results indicated that the changes in cell membrane permeability and plasma membrane peroxidation of cold-resistant plants were significantly lower than those of cold-sensitive plants at the late stage of low-temperature stress.
Figure 3.

Changes in relative conductivity, malondialdehyde, proline, and soluble sugar contents in leaves of CA157, CA52, and CL122 under low-temperature stress. (a) Variation tendency of relative conductivity; (b) variation tendency of malondialdehyde; (c) variation tendency of proline; (d) variation tendency of soluble sugar. Data are presented as mean ± SE; *p < 0.05, **p < 0.01.
Under low-temperature stress, the proline content in CA157 leaves increased continuously, while in the leaves of CA52 and CL122, it showed fluctuations. As shown in Figure 3c, the proline content in CA52 and CL122 leaves increased at first day, decreased at the 5th d, and then increased again. Moreover, after 5 d of low-temperature treatment, the proline content of CA157 was significantly higher than that of CL122 and CA52 (p < 0.05). The soluble sugar content in wild bell pepper CA157 leaves was significantly lower than that of CA52 at the first and third days of low-temperature treatment (p < 0.01), but its content continuously increased and was higher than that of CA52 and CL122 at 7th d. The soluble sugar content in leaves of CA52 and CL122 reached the maximum value on the first day of low-temperature stress, but little change thereafter (Figure 3d).
Effect of low-temperature stress on PSII of bell pepper seedlings
Temperature is a major factor controlling the photosynthetic rates,18 and low-temperature stress mainly accelerates photoinhibition by inhibiting PSII repair. Thus, we detected the changes of Fv/Fm and PSII in three ILs under low temperature. Overall, Fv/Fm and PSII of CA157, CA52, and CL122 decreased gradually with the prolongation of treatment time under low-temperature stress (Figure 4). At the 7th d of low-temperature stress, the Fv/Fm of CA157 and CL122 was significantly higher than that of CA52 (p < 0.05 for CA157; p < 0.01 for CA122). At low-temperature stress for 7 d, the PSII of CA157 and CL122 was significantly higher than that of CA52 (both p < 0.01). These results indicated that the PSII activity of resistant materials was significantly higher than that of cold-sensitive materials under long-term stress.
Figure 4.
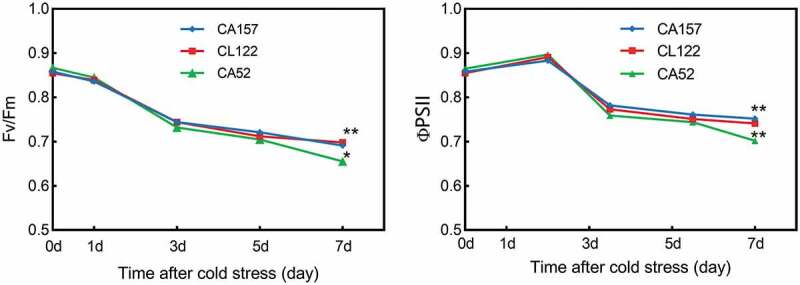
Effect of low-temperature stress on the maximum quantum efficiency of ΦPSII photochemistry (Fv/Fm) and the quantum yield of PSII electron transport (PSII) among CA157, CA52, and CL122. The Y-axis represented the Fv/Fm value; the X-axis represented the 6-week-old seedlings measured at 0, 1, 3, 5, and 7 d of 4°C treatment. Data are presented as mean ± SE. *p < 0.05, **p < 0.01.
Effect of low-temperature stress on antioxidant activity of bell pepper seedlings
Various abiotic stresses result in excessive accumulation of reactive oxygen species (ROS) in plants, and the effective antioxidant enzymes such as CAT, SOD, POD, and APX can eliminate excessive ROS. In this study, the changes of APX, POD, CAT, and SOD activities in CA157, CA52, and CL122 under low-temperature stress were analyzed. The result showed that under low-temperature stress, the activity of APX and POD showed an upward trend (Figure 5a). The activity of APX increased dramatically in CA52. After 3 d of low-temperature stress, the APX activity of CA52 leaves was significantly higher than that of CA157 and CL122 (both, p < 0.05). On the contrary, POD activity in leaves of CA157 and CL122 was significantly higher than that of CA52 at 3 d of low-temperature stress (Figure 5b; p < 0.05). Under low-temperature stress, the activity of CAT and SOD showed a dynamic trend of rising first, then decreasing, and then rising (Figure 5c,d). At 5th to 7th d of low-temperature stress, the CAT activity of CA157 leaves was significantly lower than that of CA52 (p < 0.05). There was no significant difference in CAT activity between CA52 and CL122 during the whole process of low-temperature stress. Moreover, there was no significant difference in SOD activity among CA157, CA52, and CL122 during the whole low-temperature treatment.
Figure 5.

The change in APX, POD, CAT, and SOD activities among CA157, CA52, and CL122. (a) Variation tendency of APX; (b) variation tendency of POD; (c) variation tendency of CAT; (d) variation tendency of SOD; X-axis represented the value of activity; Y-axis represented the time after low-temperature stress. Data are presented as mean ± SE; *p < 0.05.
Discussion
Low temperature is an important inhibitor in the cultivation of bell pepper.19 The establishment of the ILs that constructed by wild bell pepper and cultivated species provides a favorable intermediate resource for low-temperature stress response investigation. In the present study, based on the cold-residence wild-type bell pepper CA157 and cultivated bell pepper CA52, the ILs were established and evaluated for their tolerance to low temperature. The results showed that CL122 was one of the most outstanding plants in ILs that had similar performance with wild bell pepper CA157. Cold resistance analysis based on various physiological and biochemical indexes showed that factors such as electrical conductivity, MDA, and PSII were closely related to cold resistance among CA157, CA52, and CL122 under low-temperature stress. The establishment of the ILs of wild sweet pepper can provide a beneficial intermediate resource for utilizing the resistance resources in wild sweet pepper.
Wild plant species are important carriers of biotic or non-abiotic stress resistance genes because of their long-term growth in harsh natural environments.20 Gene introgression between wild relatives and cultivated species can be used as the bridge materials for the development and utilization of new planting resources.21 A previous study indicates that pepper wild relatives are a very important genetic resource for breeding.22 However, due to linkage barriers, the introduction of target traits always combined with the unfavorable traits.23 Actually, the interspecific ILs constructed by wild and cultured populations are commonly used as a recurrent parent used in pre-breeding and QTL mapping.24 A previous study has shown that ILs of pepper can be used to fine map the location of the traits in the chromosome responsible for fruit production.25 Based on a population-specific genetic linkage map, Eggink et al. indicated that flavor caption from Capsicum baccatum can be realized by ILs in bell pepper.26 In the present study, the ILs that constructed by wild bell pepper and cultivated species were established. These ILs showed better resistance than recurrent parent, further indicating a good value for ILs to realize the gene introgression (such as cold resistance gene) between wild and cultured species. CL122 was one of the most outstanding plants in ILs that had similar performance with wild bell pepper. Thus, we speculated that CL122 might be a novel bridge material for understanding low-temperature response in bell pepper. However, the resistance site or clone of the key gene in CL122 is still unclear. Thus, further hybridization or backcrossing of CL122 with recurrent parent CA52 to construct near-isogenic lines is needed in the further study.
Cold resistance is a complex quantitative trait,27 and hence, it is very difficult to directly clone the key cold resistance genes in plants. It has been reported that plants adapt to stress environment by different mechanisms, including changes in morphological pattern and physiological and biochemical processes. The adaptation associates with the maintenance of osmotic homeostasis by metabolic adjustments, leading to the accumulation of metabolically compatible compounds such as soluble sugar, proline, and MDA. Additionally, it also includes the modification of cell membrane stability and related enzyme activity.28,29 Thus, in the present study, we determined the phenotype, conductivity, contents of soluble sugar, proline and MDA, and antioxidant enzyme activity of CA157, CA52, and CL122 under low-temperature stress. After low-temperature (4°C) treatment, the wilting degree of CL122 was obviously lighter than that of CA52. Relative conductivity is an important index for reflecting cell membrane permeability, which to a certain extent reflects cell damage.30 A previous low-temperature stress on maize root system showed that the electric conductivity increased with the temperature stress increasing.31 Accumulation of the proline and MDA levels can greatly enhance the resistance capability of plants to abiotic stress.28 The present results showed that the leaf relative conductivity of CA52 was significantly higher than that of CA157 and CL122 from the third day. The MDA and proline contents in the leaves of CA52 were significantly higher than those of CA52 after 5 d of low-temperature treatment. These data indicated the excellent cold resistance of CA52.
Various abiotic stresses often result in excessive accumulation of ROS in plants and inflammation damage to various cellular structures.32 In order to maintain the dynamic level of oxidation and reduction in plants, the effective antioxidant enzymes such as CAT, SOD, and APX in plants can eliminate excessive ROS.33 In our study, the APX activity of CA52 leaves was significantly higher than that of CA157 and CL122 after 3 d of low-temperature treatment. It has been reported that in pepper leaves, there is correlation between changes in absorption of light energy by PSII and the variety tolerance under low temperature.34 PSII in pepper seedling leaves decreases under low-temperature stress.35 In the present study, the PSII of CA157 and CL122 was significantly higher than that of CA52 after 7 d of low-temperature stress. Thus, we speculated that electrical conductivity, MDA, and PSII might be used to identify the low-temperature resistance of bell pepper plants in a period of obvious differentiation.
Conclusion
In conclusion, CL122 might be a novel bridge material for understanding low-temperature response in bell pepper. Furthermore, electrical conductivity, MDA, and PSII might be used to identify the low-temperature resistance of bell pepper plants in a period of obvious differentiation.
Funding Statement
This work was supported by the National Natural Science Foundation of China [30671242] and the Natural Science Foundation of Shandong Province Mayor [ZR2017CM009].
Authors’ contributions
Conception and design of the research: SG; acquisition of data: ZS; analysis and interpretation of data: PL; statistical analysis: PL; obtaining funding: LJ; drafting the manuscript: LJ; and revision of the manuscript for important intellectual content: ML. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Moreno Pérez EDC, Mora Aguilar R, Sánchez D, Garcíapérez V.. Phenology and yield of bell pepper (Capsicum annuum L.) hybrids grown hydroponically. Rev Chapingo Serie Hortic. 2011;XVII:1–8. doi: 10.5154/r.rchsh.2011.17.041. [DOI] [Google Scholar]
- 2.Mccoy SR, Hochmuth RC, Laughlin WL, Gazula A, Fenneman DK. Economic analysis of fresh green bell pepper (Capsicum annuum) production under shade structures. Florida State Hortic Soc. 2013 [Google Scholar]
- 3.Sezen SM, Yazar A, Eker S. Effect of drip irrigation regimes on yield and quality of field grown bell pepper. Agric Water Manage. 2006;81:115–131. doi: 10.1016/j.agwat.2005.04.002. [DOI] [Google Scholar]
- 4.Schouten SP. Effects of low temperature, CA conditions and (pre) treatments on quality aspects of Dutch bell peppers. Nucleic Acids Symp. 1995;66:171–173. [Google Scholar]
- 5.Sánchez P, Sánchez IE, Madrid CM, Almela FR, Ballesta MTS, Pardo FBF. A proteomic approach to study the low temperature stress induction in bell pepper fruit. Universidad De Córdoba Servicio De Publicaciones Dialnet; 2011. [Google Scholar]
- 6.Suh JP, Jeung JU, Lee JI, Choi YH, Yea JD, Virk PS, Mackill DJ, Jena KK. Identification and analysis of QTLs controlling cold tolerance at the reproductive stage and validation of effective QTLs in cold-tolerant genotypes of rice (Oryza sativa L.). Theor Appl Genet. 2010;120(5):985–995. doi: 10.1007/s00122-009-1226-8. [DOI] [PubMed] [Google Scholar]
- 7.Tian F, Li DJ, Fu Q, Zhu ZF, Fu YC, Wang XK, Sun CQ. Construction of introgression lines carrying wild rice (Oryza rufipogon Griff.) segments in cultivated rice (Oryza sativa L.) background and characterization of introgressed segments associated with yield-related traits. Theor Appl Genet. 2006;112(3):570–580. doi: 10.1007/s00122-005-0165-2. [DOI] [PubMed] [Google Scholar]
- 8.Monforte AJ, Friedman E, Zamir D, Tanksley SD. Comparison of a set of allelic QTL-NILs for chromosome 4 of tomato: deductions about natural variation and implications for germplasm utilization. Theor Appl Genet. 2001;102:572–590. doi: 10.1007/s001220051684. [DOI] [Google Scholar]
- 9.Miller FP, Vandome AF, Mcbrewster J. Cultivats. Bell pepper. Germany: Alphascript Publishing; 2010. p. 77. [Google Scholar]
- 10.Campos PS, Quartin V, Ramalho JC, Nunes MA. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J Plant Physiol. 2003;160:283–292. doi: 10.1078/0176-1617-00833. [DOI] [PubMed] [Google Scholar]
- 11.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 12.Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-aghabary K, Zhu Z, Shi Q. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr. 2004;27:2101–2115. doi: 10.1081/PLN-200034641. [DOI] [Google Scholar]
- 14.Jimenez A, Hernandez JA, Del Rio LA, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morohashi Y. Peroxidase activity develops in the micropylar endosperm of tomato seeds prior to radicle protrusion. J Exp Bot. 2002;53:1643–1650. doi: 10.1093/jxb/erf012. [DOI] [PubMed] [Google Scholar]
- 16.Wingler A, Mares M, Pourtau N. Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytol. 2004;161:781–789. doi: 10.1111/j.1469-8137.2004.00996.x. [DOI] [PubMed] [Google Scholar]
- 17.Safdari Y, Farajnia S, Asgharzadeh M, Khalili M, Jaliani HZ. Affinity measurement of single chain antibodies: a mathematical method facilitated by statistical software SigmaPlot. Monoclon Antib Immunodiagn Immunother. 2014;33:13–19. doi: 10.1089/mab.2013.0067. [DOI] [PubMed] [Google Scholar]
- 18.Mackey KR, Paytan A, Caldeira K, Grossman AR, Moran D, McIlvin M, Saito MA.. Effect of temperature on photosynthesis and growth in marine Synechococcus spp. Plant Physiol. 2013;163:815–829. doi: 10.1104/pp.113.221937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Zhang G, Feng Z, Li X. Effects of low temperature and weak light on anti-oxidative enzyme activities and plasma membrane permeability of pepper seedlings. Acta Bot Boreali-occidentalia Sin. 2005;25:2478–2483. [Google Scholar]
- 20.Yáñez M, Cáceres S, Orellana S, Bastías A, Verdugo I, Ruizlara S, Casaretto JA.. An abiotic stress-responsive bZIP transcription factor from wild and cultivated tomatoes regulates stress-related genes. Plant Cell Rep. 2009;28:1497–1507. doi: 10.1007/s00299-009-0749-4. [DOI] [PubMed] [Google Scholar]
- 21.Tang Q, Rong T, Song Y, Yang J, Pan G, Li W, Huang Y, Cao M. Introgression of perennial teosinte genome into maize and identification of genomic in situ hybridization and microsatellite markers. Crop Sci. 2005;45:717–721. doi: 10.2135/cropsci2005.0717. [DOI] [Google Scholar]
- 22.Nicolaï M, Cantet M, Lefebvre V, Sage-Palloix AM, Palloix A. Genotyping a large collection of pepper (Capsicum spp.) with SSR loci brings new evidence for the wild origin of cultivated C. annuum and the structuring of genetic diversity by human selection of cultivar types. Genet Res Crop Evol. 2013;60:2375–2390. doi: 10.1007/s10722-013-0006-0. [DOI] [Google Scholar]
- 23.Sobrizal. Steud. introgression lines in rice: identification of genes for reproductive barriers. Advances in Rice Genetics. 2003:128–130. [Google Scholar]
- 24.Arbelaez JD, Moreno LT, Singh N, Tung CW, Maron LG, Ospina Y, Martinez CP, Grenier C, Lorieux M, McCouch S. Development and GBS-genotyping of introgression lines (ILs) using two wild species of rice, O. meridionalis and O. rufipogon, in a common recurrent parent, O. sativa cv. Curinga. Mol Breed. 2015;35:81. doi: 10.1007/s11032-015-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kargbo A, Wang CY. Complex traits mapping using introgression lines in pepper (Capsicum annuum). Afr J Agric Res. 2010;5:725–731. [Google Scholar]
- 26.Eggink PM, Tikunov Y, Maliepaard C, Haanstra JPW, Rooij HD, Vogelaar A, Gutteling EW, Freymark G, Bovy AG, Visser RGF, et al. Capturing flavors from Capsicum baccatum by introgression in sweet pepper. Tagtheoretical Appl Geneticstheoretische Und Angew Gen. 2014;127(2):373–390. doi: 10.1007/s00122-013-2225-3. [DOI] [PubMed] [Google Scholar]
- 27.Vallejo RL, Liu S, Gao G, Fragomeni BO, Hernandez AG, Leeds TD, Parsons JE, Martin KE, Evenhuis JP, Welch TJ, et al. Similar genetic architecture with shared and unique quantitative trait loci for bacterial cold water disease resistance in two rainbow trout breeding populations. Front Genet. 2017;8. doi: 10.3389/fgene.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao H-X, Sun C-X, Shao H-B, Lei X-T. Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. Afr J Biotechnol. 2011;10:2630–2637. doi: 10.5897/AJB10.1272. [DOI] [Google Scholar]
- 29.Liang L, Mei X, Lin F, Xia J, Liu S, Wang J. Effect of low temperature stress on tissue structure and physiological index of cashew young leaves. Ecol Environ Sci. 2009;18:317–320. [Google Scholar]
- 30.Ben-Gal A, Borochov-Neori H, Yermiyahu U, Shani U. Is osmotic potential a more appropriate property than electrical conductivity for evaluating whole-plant response to salinity? Environ Exp Bot. 2009;65:232–237. doi: 10.1016/j.envexpbot.2008.09.006. [DOI] [Google Scholar]
- 31.Luo N, Wei S, Li J. Effects of low-temperature stress on root system characteristics and electric conductivity of maize seedlings. Chin J Ecol. 2014;33:2694–2699. [Google Scholar]
- 32.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Ali S, Jin R, Gill RA, Mwamba TM, Zhang N, Hassan ZU, Islam F, Ali S, Zhou W. Beryllium stress-induced modifications in antioxidant machinery and plant ultrastructure in the seedlings of black and yellow seeded oilseed rape. Biomed Res Int. 2018;2018. 2018,(2018-3-21). doi: 10.1155/2018/1615968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie JM, Ji-Hua YU, Huang GB, Feng Z. Correlations between changes of absorption and transformation of light energy by PSII in pepper leaves and the variety tolerance under low temperature and weak light. Sci Agric Sin. 2011;44:1855–1862. [Google Scholar]
- 35.Yang FF, Zhang GB, Xie JM, Yu JH. Effect of 6-BA pretreatment on chlorophyll fluorescence parameters and lipid peroxidation in pepper seedlings under low temperature and weak light stress. Plant Physiol Commun. 2009;45:575–578. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


