Abstract
Objective: To compare the assessments of 10-year probability by patients and their physicians of cardiovascular complications of hypertension with actual outcomes.
Design: Patients with uncomplicated hypertension treated with at least one antihypertensive drug at inclusion were followed for 10 years through mandatory national health registers.
Setting: 55 primary health care centres, 11 hospital outpatient clinics in Sweden
Patients: 848 patient, 212 physicians.
Main outcome measures: Patients and physicians estimated the probability of hypertension-related complications with treatment (death, heart failure, acute myocardial infarction/AMI, and stroke) for each patient in 848 pairs. Estimates were compared with the clinical outcomes 10 years later using data from the Mortality Register and the National Patient Register.
Results: Patients were significantly better (p < 0.001) than their physicians in estimating the average probability of heart failure compared with actual outcome data (14% vs. 24%, outcome 15%), AMI (16% vs. 26%, outcome 8%), and stroke (15% vs. 25%, outcome 11%). Patients were significantly worse (p < 0.001) at estimating the average probability of death (10% vs. 18%, actual outcome 20%). Neither the patients nor the physicians were able to distinguish reliably between low-risk and high-risk patients after adjustment for age and sex.
Conclusions: Patients were better than their physicians in estimating the average probability of morbidity due to hypertension. Both the patients and their attending physicians had difficulty in estimating the individual patient’s risk of complications. The results support the use of evidence-based tools in consultations for assessing the risk of cardiovascular complications associated with hypertension.
Key points
• Shared decision making relies on a common understanding of risks and benefits. Tools for risk assessment of hypertension have been introduced in the last two decades.
• Without tools for risk assessment, both patients and physicians had difficulties in estimating the individual patient’s risk of cardiovascular morbidity.
• Patients were better than physicians in estimating actual average cardiovascular morbidity due to hypertension during a follow-up of 10 years.
• The results support the use of evidence-based tools in consultations for assessing the risk of cardiovascular complications associated with hypertension.
Keywords: Hypertension; cardiovascular diseases/prevention & control; risk assessment, algorithms; decision making; prospective studies
Introduction
High blood pressure is a leading risk factor for cardiovascular disease (CVD) worldwide with an increased prevalence in low-income countries in South Asia and sub-Saharan Africa [1]. Decision-making by general practitioners in countries with high CVD burden and low life expectancy at age 60 were most likely to treat hypertension in oldest-old based on case-vignettes [2]. Poor adherence to antihypertensive treatment is a significant healthcare challenge [3,4] since low adherence increases the risk of cardiovascular complications [5,6].
Lack of understanding of the relationships between blood pressure, symptoms, and lifestyle may contribute to poor outcomes of antihypertensive treatment [7]. In a recent large study of patients with hypertension treated in primary health care in Sweden, about half did not achieve recommended treatment goals (48% of 88,945 reached BP < 140/90) [8].
Shared decision-making during the consultation is considered important as a means of achieving improved adherence to treatment [9,10]. An essential goal of a consultation is thus to be able to use the best possible risk assessment of the medical condition and to achieve a balance between benefits and risks of different treatment alternatives. Self-reporting by the patient combined with increased patient–health care professional interaction during follow-up consultations can support patients in understanding the blood pressure value in relation to their daily life [11].
Assessing the individual benefit-risk profile of treatment is essential not only when prescribing treatment, but also to ensure that the patient understands the goal of the treatment. This assessment is more straightforward when treating a manifest disease with readily identifiable symptoms that can be influenced by the treatment than when providing treatment to prevent future events such as complications of hypertension. To support shared decision-making in such a situation, the physician needs not only to know the benefits and risks of the alternatives but also needs to develop the ability to discuss this with the patient effectively [12].
One goal of personalised cardiovascular risk assessment is to shift focus from the blood pressure level to the absolute risk level/reduction, thus avoiding undertreatment of elderly and patients with other risk factors [13–15]. Setting a mutual goal for an optimal blood pressure level to reach the desired reduction in cardiovascular risk is a common goal of clinical consultations [5]. However, a recent review found that the majority of participating patients overestimated the benefit of an intervention, and underestimated the possible harm [16].
Decision support systems using personalised cardiovascular risk assessment have been validated against actual outcomes [17,18] and might improve with new machine-learning systems [19]. Reviews have reported effects of clinical decision-support systems on the performance of physicians [20,21], but the evidence on improved clinical outcomes remains sparse [21]. Different guidelines and scoring systems might differ in the assessment of absolute risk [22]. The prognostic models in such decision-support systems ought to be validated for the patient populations where the decision-support systems are to be implemented [23].
This study is a follow-up of an earlier study [24,25] where concordance between patients’ and physicians’ estimates of the risks of hypertension and benefits of treatment during a regular follow-up appointment was studied. The main finding was a high degree of inconsistency, patients assessing risks of untreated hypertension to be higher than their physicians did. The patients also estimated the reduction of risk or benefits of treatment more positively than did their physicians. The objective of this study was to compare the individual 10-year risk assessments of hypertension when treated, both for mortality and morbidity (acute myocardial infarction, stroke, and heart failure), by patients and attending physicians with the actual outcomes 10 years after the consultation studied.
Material and methods
The study is a long-term follow-up of an earlier reported study [24,25] through longitudinal data from mandatory national health data registers. The Swedish national health registers have high coverage, and low loss of data, due to the mandatory use of a unique personal identity number (PIN) recorded every time a person meets a Swedish healthcare professional [26]. The Swedish National Patient Register (NPR) is a mandatory register with more than 99% of all somatic and psychiatric hospital discharges registered with disease classification according to the Swedish version of International Classification of Disease (ICD) system since 1987. The sensitivity of diagnoses for hospital discharges in NPR varies between conditions and over time with high sensitivity for stroke (> 90%) and myocardial infarction (77–92%), but considerably lower for angina pectoris (44%) [26]. The diagnosis of stroke, acute myocardial infarction and heart failure is almost always associated with a hospitalisation period, while minor kidney failure might be diagnosed, and the patient treated in outpatient care. Thus, before data analysis, the decision was made not to include kidney failure as an outcome
Data from the Causes of Death Register (all causes of mortality), the National Patient Register (in- and outpatient diagnoses for ICD10 I00–I99 + N00–N19, for ICD9 390–459 + 580–589), and the Swedish Population Register at Statistics Sweden for 2006 were extracted and linked to the original research database. The resulting file was pseudonymized by the National Board of Health and Welfare.
In the original study [25], patients with a regular follow-up appointment for hypertension who were being treated with at least one antihypertensive drug, and with hypertension as the primary diagnosis were included. The 10-year probabilities of different complications of hypertension were estimated separately by both the patient and his or her attending physician through a visual analogue scale (with anchor points no risk to be affected = 0 and will be affected = 100%, see supplemental material).
The study was performed with care as usual. The patients were recruited from 55 primary healthcare centres and 11 hospital clinics of internal medicine during 1996. At each centre, a nurse listed 10–25 consecutive patients for inclusion in the study, and these were then asked to answer a questionnaire together with a nurse before seeing their physician at a regularly scheduled appointment. The questionnaire included visual analogue scales to assess the probability of different outcomes with and without medication (Supplementary Figure S3).
There were no changes in recruitment, workflow or questionnaire between the pilot and the consecutive full study. For this follow-up study, both the 1013 patients from the original study and the 92 patients in the pilot study were included. The total number of participants was thus 1105 patients.
In all, 138 patients had to be excluded at the 10-year follow-up due to incomplete or illegible PIN, leaving 967 patients accessible for follow-up. The final analysis is based on the 848 patients for whom both the patient and the attending 212 physician had estimated the probability of death within 10 years (on average four patients per physician) (Table 1).
Table 1.
Background data at inclusion for participating patients (n = 848) per healthcare setting.
| Primary healthcare centres | Secondary or tertiary healthcare | |
|---|---|---|
| (n = 674) | (n = 174) | |
| Age 1996, years, mean (range) | 63 (28–87) | 56 (19–83) |
| Male, n (%) | 268 (40%) | 110 (63%) |
| Female, n (%) | 406 (60%) | 64 (37%) |
| Education | ||
| Up to secondary education | 478 (72%) | 86 (50%) |
| High school | 116 (18%) | 41 (24%) |
| University | 67 (10%) | 46 (26%) |
| BMI kg/m2, mean (range) | 27.8 (18.4–51.6) | 27.7 (19.8–49.4) |
| SBP mmHg, mean (range) | 156 (110–235) | 150 (104–210) |
| DBP mmHg, mean (range) | 87 (50–123) | 90 (66–140) |
| Complications from hypertension | 90 (13%) | 59 (34%) |
| Duration of hypertension in years, mean (range) | 12 (0–61) | 12 (0–46) |
| Hypercholesterolemia | 158 (24%) | 45 (26%) |
| Not known | 176 (27%) | 35 (20%) |
| Smoking | 101 (15%) | 17 (10%) |
| Lack of data | 32 (5%) | 15 (9%) |
| Diabetes | 57 (8%) | 13 (8%) |
| Lack of data | 12 (2%) | 2 (1%) |
| Left ventricular hypertrophy | 63 (9%) | 29 (17%) |
| Lack of data | 141 (21%) | 10 (6%) |
| Family history of cardiovascular disease | 471 (70%) | 132 (76%) |
Data documented by attending nurse, except for left ventricular hypertrophy reported by physician.
The patients were stratified in deciles depending on the risk assessment for the four outcomes studied. The stratification was done separately for the assessments by the patients and their physicians. A patient could thus be in separate deciles for the assessments by patients and physicians, respectively. Assessments with the same numerical value were placed in the lower corresponding decile by STATA version 14.1, StataCorp. The average outcomes for patients in each decile (as assessed by patients or physicians) were identified. Z-test was used to compare proportions. Linear regression analysis was used to estimate the association between patient and physician regarding the overall 10-year mortality. In addition, we used logistic regression to compare the actual outcome depending on the estimated 10-year probability in deciles. A p-value of 0.05 was considered significant. Stata MP version 14.1, StataCorp LLC, College Station, TX, USA was used for all statistical analysis.
Results
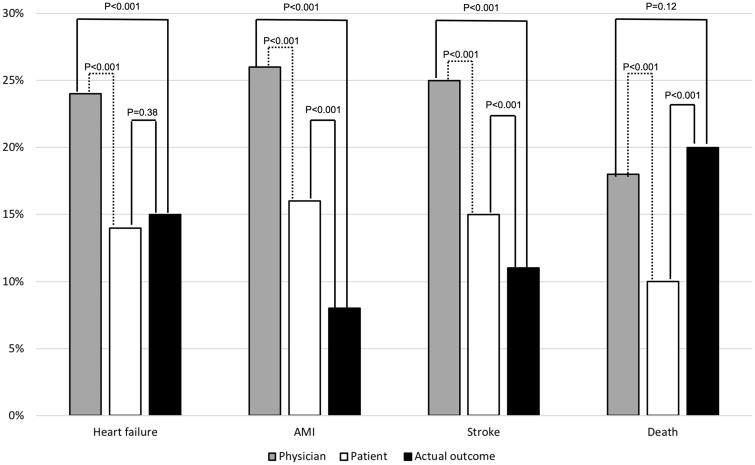
The patients were significantly better (z-test comparing proportions, p < 0.001) than their attending physicians at estimating the average probability of heart failure (patients 14% vs. physicians 24%, actual outcome 15%), acute myocardial infarction (16% vs. 26%, actual outcome 8%), and stroke (15% vs. 25%, actual outcome 11%) (Figure 1, filled lines; Table 2).
Figure 1.
Estimated probability for different cardiovascular complications and death within 10 year by physicians (grey) and patients (white) compared with actual outcomes (black). Dotted line statistical analysis presented in the initial publication [18].
Table 2.
Assessment of 10-year probability, versus actual outcome, of mortality and hypertension-related morbidity for 848 patient–physician pairs (z-test comparing proportions).
| All patients (n = 848) |
Primary healthcare centres (n = 674) |
Secondary/tertiary healthcare (n = 174) |
||||
|---|---|---|---|---|---|---|
| Physician | Patient | Physician | Patient | Physician | Patient | |
| Heart failure (n = 117) | ||||||
| Estimated without treatment | 0.54 (0.51–0.57) | 0.67 (0.63–0.70) | 0.54 (0.51–0.57) | 0.64 (0.60–0.69) | 0.58 (0.50–0.66) | 0.71 (0.66–0.77) |
| Estimated with treatment | 0.24 (0.23–0.26) | 0.14 (0.12–0.15) | 0.24 (0.22–0.26) | 0.13 (0.11–0.14) | 0.25 (0.22–0.31) | 0.14 (0.13–0.19) |
| Outcome | 0.15 | 0.13 | 0.23 | |||
| Outcome vs. estimated with treatment | p < 0.001 | p = 0.38 | p < 0.001 | p = 1.000 | p = 0.32 | p = 0.02 |
| Acute myocardial infarction (n = 66) | ||||||
| Estimated without treatment | 0.54 (0.51–0.57) | 0.75 (0.73–0.78) | 0.55 (0.51–0.58) | 0.72 (0.69–0.76) | 0.51 (0.44–0.59) | 0.78 (0.72–0.81) |
| Estimated with treatment | 0.26 (0.25–0.29) | 0.16 (0.14–0.18) | 0.27 (0.25–0.29) | 0.15 (0.13–0.17) | 0.25 (0.19–0.31) | 0.17 (0.15–0.23) |
| Outcome | 0.08 | 0.08 | 0.10 | |||
| Outcome vs. estimated with treatment | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Stroke (n = 90) | ||||||
| Estimated without treatment | 0.57 (0.54–0.59) | 0.74 (0.71–0.76) | 0.57 (0.53–0.59) | 0.74 (0.71–0.76) | 0.58 (0.54–0.64) | 0.8 (0.77–0.82) |
| Estimated with treatment | 0.25 (0.24–0.27) | 0.15 (0.14–0.17) | 0.25 (0.24–0.27) | 0.15 (0.13–0.17) | 0.27 (0.20–0.30) | 0.16 (0.13–0.21) |
| Outcome | 0.11 | 0.10 | 0.14 | |||
| Outcome vs. estimated with treatment | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.32 |
| Death due to all cases (n = 162) | ||||||
| Estimated without treatment | 0.45 (0.39–0.47) | 0.70 (0.65–0.74) | 0.45 (0.39–0.49) | 0.67 (0.62–0.71) | 0.43 (0.33–0.49) | 0.78 (0.72–0.83) |
| Estimated with treatment | 0.18 (0.16–0.20) | 0.10 (0.09–0.11) | 0.19 (0.16–0.21) | 0.10 (0.09–0.11) | 0.15 (0.12–0.21) | 0.11 (0.08–0.17) |
| Outcome | 0.19 | 0.20 | 0.19 | |||
| Outcome vs. estimated with treatment | p = 0.12 | p < 0.001 | p = 0.49 | p < 0.001 | p = 0.29 | p = 0.005 |
In contrast, the patients were worse (p < 0.001) at estimating the average probability of death due to all causes (patients 10% vs. physicians 18%, actual outcome 20%). In general, patients treated at secondary or tertiary health units tended to be younger, male, more educated and with more complications at the initiation of the study (Table 1). The results were similar for primary healthcare (n = 674) and hospital-based outpatient clinics (n = 174), respectively (Table 2).
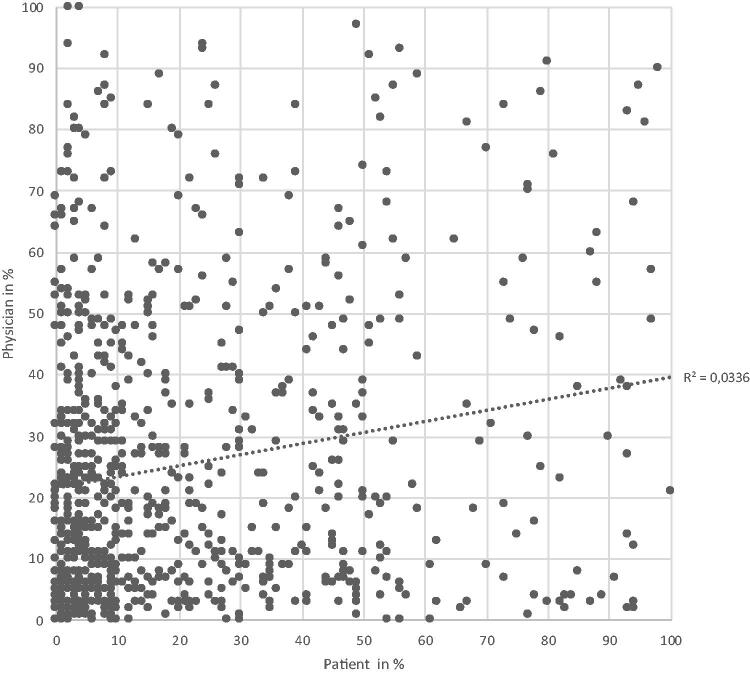
There was no correlation between the estimates by individual patients and the estimates by their attending physicians, either for the 10-year-probability of death, Figure 2, or for heart failure, acute myocardial infarction, or stroke (Supplementary Figure S1(a–c)).
Figure 2.
Estimated mortality within 10 years among 848 patient–physician pairs.
The average of probabilities per decile estimated by patients and physicians respectively are presented in Tables 3 and 4 and Supplementary Figure S2.
Table 3.
Outcome per decile of 10-year probability of hypertension-related morbidity and total mortality as assessed by patients and physicians.
| Deciles according to assessment by the patients |
Deciles according to assessment by the physicians |
||||||
|---|---|---|---|---|---|---|---|
| Decile | Upper limit in assessed probability | Assessed probability – average per decile | Actual average outcome per decile | Upper limit in assessed probability | Assessed probability – average per decile | Actual average outcome per decile | |
| Heart | 1 | 2% | 1.1% | 12.3% | 6% | 3.7% | 6.7% |
| failure | 2 | 4% | 3.6% | 14.3% | 10% | 8.5% | 8.8% |
| 3 | 7% | 5.9% | 16.8% | 15% | 13.2% | 10.0% | |
| 4 | 9% | 8.4% | 10.9% | 19% | 17.4% | 7.4% | |
| 5 | 14% | 12.0% | 10.7% | 24% | 22.1% | 7.2% | |
| 6 | 18% | 16.4% | 13.4% | 31% | 28.2% | 15.5% | |
| 7 | 26% | 22.3% | 13.6% | 40% | 35.3% | 16.7% | |
| 8 | 37% | 32.0% | 13.5% | 51% | 46.6% | 19.1% | |
| 9 | 49% | 44.1% | 13.8% | 64% | 57.8% | 23.2% | |
| 10 | 98% | 66.2% | 20.0% | 100% | 76.2% | 26.0% | |
| AMI | 1 | 2% | 1.1% | 4.0% | 7% | 3.5% | 3.1% |
| 2 | 5% | 4.1% | 10.2% | 11% | 9.3% | 3.9% | |
| 3 | 7% | 6.5% | 5.4% | 17% | 14.6% | 4.4% | |
| 4 | 11% | 9.4% | 9.0% | 22% | 19.9% | 4.1% | |
| 5 | 16% | 13.9% | 10.3% | 27% | 24.8% | 8.5% | |
| 6 | 21% | 18.7% | 7.4% | 32% | 29.7% | 7.7% | |
| 7 | 30% | 26.1% | 10.8% | 41% | 37.0% | 8.5% | |
| 8 | 44% | 37.1% | 3.5% | 51% | 47.0% | 7.7% | |
| 9 | 53% | 48.6% | 2.4% | 63% | 56.7% | 7.6% | |
| 10 | 98% | 71.4% | 15.2% | 96% | 76.1% | 11.5% | |
| Stroke | 1 | 2% | 1.1% | 7.5% | 5% | 2.9% | 5.5% |
| 2 | 4% | 3.4% | 11.0% | 11% | 8.6% | 5.9% | |
| 3 | 7% | 6.1% | 11.4% | 15% | 13.6% | 4.2% | |
| 4 | 10% | 9.0% | 8.8% | 19% | 17.4% | 7.7% | |
| 5 | 15% | 12.9% | 8.0% | 25% | 22.8% | 10.2% | |
| 6 | 21% | 18.3% | 10.2% | 30% | 27.9% | 7.9% | |
| 7 | 30% | 26.3% | 15.5% | 38% | 34.2% | 9.0% | |
| 8 | 42% | 36.1% | 12.4% | 50% | 45.3% | 17.2% | |
| 9 | 54% | 48.3% | 11.9% | 64% | 56.9% | 16.7% | |
| 10 | 98% | 73.1% | 7.8% | 100% | 75.8% | 23.7% | |
| Death | 1 | 1% | 0.5% | 9.8% | 3% | 1.9% | 10.7% |
| 2 | 3% | 2.4% | 16.5% | 6% | 5.1% | 6.4% | |
| 3 | 5% | 4.4% | 18.8% | 9% | 7.9% | 12.5% | |
| 4 | 7% | 6.5% | 21.5% | 13% | 11.2% | 12.7% | |
| 5 | 10% | 8.9% | 15.7% | 18% | 15.8% | 12.4% | |
| 6 | 16% | 13.4% | 13.5% | 25% | 21.8% | 14.6% | |
| 7 | 25% | 20.7% | 17.3% | 33% | 29.3% | 23.5% | |
| 8 | 39% | 31.7% | 25.6% | 47% | 39.5% | 29.4% | |
| 9 | 53% | 47.0% | 28.6% | 62% | 53.1% | 28.4% | |
| 10 | 100% | 74.8% | 25.9% | 100% | 77.9% | 44.9% | |
n = 848 patient–physician pairs. See also Supplementary Figure S2.
Table 4.
Logistic regression of odds ratio (OR) for actual outcome depending on estimated 10-year probability in deciles (increasing estimated probability), by patients and physicians, for death or hypertension-related morbidity in 848 patient–physician pairs.
| Deciles according to assessment by the patients | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for age and sex | Heart failure (n = 832) |
AMI (n = 840) |
Stroke (n = 838) |
Death (n = 848) |
||||||||||||||||||
| Decile | OR | p Value | 95% CI | OR | p Value | 95% CI | OR | p Value | 95% CI | OR | p Value | 95% CI | ||||||||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||||||||
| 2 | 1.06 | 0.90 | 0.44 | – | 2.58 | 2.65 | 0.12 | 0.79 | – | 8.89 | 1.35 | 0.58 | 0.47 | – | 3.88 | 1.22 | 0.66 | 0.50 | – | 3.02 | ||
| 3 | 1.28 | 0.54 | 0.59 | – | 2.78 | 1.10 | 0.90 | 0.23 | – | 5.22 | 1.54 | 0.40 | 0.57 | – | 4.15 | 1.53 | 0.36 | 0.61 | – | 3.85 | ||
| 4 | 0.73 | 0.55 | 0.26 | – | 2.04 | 2.31 | 0.19 | 0.66 | – | 8.06 | 0.96 | 0.95 | 0.30 | – | 3.06 | 1.41 | 0.48 | 0.54 | – | 3.66 | ||
| 5 | 0.78 | 0.56 | 0.33 | – | 1.82 | 2.48 | 0.15 | 0.72 | – | 8.49 | 1.13 | 0.84 | 0.36 | – | 3.57 | 0.83 | 0.71 | 0.32 | – | 2.16 | ||
| 6 | 0.98 | 0.97 | 0.40 | – | 2.45 | 1.70 | 0.43 | 0.46 | – | 6.36 | 1.27 | 0.65 | 0.44 | – | 3.64 | 0.94 | 0.90 | 0.35 | – | 2.54 | ||
| 7 | 1.05 | 0.90 | 0.45 | – | 2.44 | 2.51 | 0.14 | 0.73 | – | 8.61 | 2.00 | 0.17 | 0.75 | – | 5.39 | 1.06 | 0.90 | 0.42 | – | 2.71 | ||
| 8 | 1.09 | 0.85 | 0.45 | – | 2.63 | 0.84 | 0.82 | 0.18 | – | 3.90 | 1.39 | 0.53 | 0.49 | – | 3.92 | 2.25 | 0.07 | 0.93 | – | 5.46 | ||
| 9 | 0.97 | 0.95 | 0.41 | – | 2.30 | 0.47 | 0.39 | 0.08 | – | 2.65 | 1.40 | 0.53 | 0.50 | – | 3.91 | 1.75 | 0.21 | 0.73 | – | 4.20 | ||
| 10 | 1.61 | 0.24 | 0.73 | – | 3.58 | 3.38 | 0.05 | * | 1.02 | – | 11.21 | 0.86 | 0.80 | 0.27 | – | 2.72 | 1.49 | 0.38 | 0.61 | – | 3.61 | |
| Deciles according to assessment by the physicians | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for age and sex | Heart failure (n = 843) |
AMI (n = 842) |
Stroke (n = 842) |
Death (n = 848) |
|||||||||||||||||||
| Decile | OR | p Value | 95% CI | OR | p Value | 95% CI | OR | p Value | 95% CI | OR | p Value | 95% CI | |||||||||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||||||||||
| 2 | 1.23 | 0.71 | 0.41 | – | 3.69 | 0.94 | 0.95 | 0.18 | – | 4.91 | 0.91 | 0.88 | 0.26 | – | 3.12 | 0.43 | 0.12 | 0.15 | – | 1.24 | |||
| 3 | 1.29 | 0.63 | 0.46 | – | 3.66 | 0.89 | 0.89 | 0.19 | – | 4.24 | 0.51 | 0.37 | 0.11 | – | 2.24 | 0.83 | 0.70 | 0.31 | – | 2.20 | |||
| 4 | 0.91 | 0.88 | 0.27 | – | 3.04 | 0.79 | 0.79 | 0.15 | – | 4.17 | 1.00 | 1.00 | 0.29 | – | 3.49 | 0.80 | 0.65 | 0.30 | – | 2.10 | |||
| 5 | 0.82 | 0.73 | 0.26 | – | 2.58 | 1.73 | 0.44 | 0.43 | – | 7.02 | 1.21 | 0.74 | 0.38 | – | 3.89 | 0.49 | 0.15 | 0.19 | – | 1.28 | |||
| 6 | 1.88 | 0.20 | 0.72 | – | 4.94 | 1.51 | 0.58 | 0.35 | – | 6.48 | 0.96 | 0.95 | 0.29 | – | 3.23 | 0.67 | 0.38 | 0.27 | – | 1.64 | |||
| 7 | 2.24 | 0.12 | 0.82 | – | 6.08 | 1.37 | 0.67 | 0.32 | – | 5.94 | 1.07 | 0.92 | 0.32 | – | 3.61 | 1.06 | 0.89 | 0.46 | – | 2.45 | |||
| 8 | 2.33 | 0.09 | 0.89 | – | 6.14 | 2.04 | 0.32 | 0.51 | – | 8.16 | 1.98 | 0.22 | 0.66 | – | 5.97 | 1.21 | 0.66 | 0.52 | – | 2.78 | |||
| 9 | 2.91 | 0.03 | * | 1.12 | – | 7.56 | 2.47 | 0.19 | 0.63 | – | 9.62 | 1.80 | 0.30 | 0.59 | – | 5.52 | 1.11 | 0.81 | 0.48 | – | 2.58 | ||
| 10 | 3.35 | 0.01 | * | 1.30 | – | 8.69 | 2.63 | 0.17 | 0.67 | – | 10.33 | 3.07 | 0.04 | * | 1.04 | – | 9.08 | 1.88 | 0.14 | 0.82 | – | 4.33 | |
See also Supplementary Figure S2.
AMI: acute myocardial infarction; CI: confidence interval.
*p < 0.05.
Logistic regression of odds ratio (OR) for actual outcome depending on estimated 10-year probability in deciles is presented in Table 4. Neither the patients nor the physicians could distinguish patients at high risk from those at low risk of dying after adjusting the odds ratios for age and sex. Patients were not able to distinguish between risk levels adjusted for age and sex for the three conditions, except for the decile with the highest risk of acute myocardial infarction. Physicians managed to identify patients belonging to the two deciles with the highest risk, adjusted for age and sex of developing heart failure, and to the decile with the highest risk of stroke but failed to identify those with a high risk of acute myocardial infarction.
Discussion
Principal findings
Patients treated with antihypertensive drugs estimated on average the 10-year unadjusted probability for three different complications (heart failure, acute myocardial infarction, and stroke) better than their attending physicians did when compared with actual outcomes (Figure 1). A recall bias among physicians could be a partial explanation for this counter-intuitive outcome [27]. Patients with complication will probably be overrepresented overtime at the healthcare centres, thus skewing the apparent probability for complications due to hypertension. On the other hand, physicians were better at estimating the average 10-year survival. A possible explanation for this is that physicians have extensive medical experience of general survival patterns in different age groups while patients might be reluctant to predict their death within the next 10 years.
Neither patients nor their attending physicians were able to perform a correct individualised assessment of the long-term risk of morbidity in the majority of the patients (Tables 3 and 4). After adjustment for age and sex, the physicians, but not patients, managed to partly distinguish individual patients with a high risk of heart failure or stroke from those with low risk. Patients managed to partly distinguish a high risk of acute myocardial infarction from with low risk. The logistic regression contains multiple instances of hypothesis-testing; thus, these findings have to be interpreted with caution. If true, a possible partial explanation might be actual knowledge among physicians about risk factors for heart failure in the individual case, or even a clinical suspicion of early heart failure based on reported symptoms. Also, predicted high risk for complications would constitute an incitement for more aggressive intervention in order to lower the risk. Thus, an early assessment of high individual risk could lead to improved treatment of multiple risk factors leading to a reduced level of actual complications.
The risk assessments by both patients and physicians consist of two components – assessment of the risk associated with untreated hypertension and the assessment of the benefits of treatment. The fact that patients, on average, assessed the actual outcome after 10 years better than the physicians might be a result of different mechanisms. It could be a better assessment by patients of one or both factors. It could also be a result of, for instance, the patients overestimating the risk associated with the treatment, but also overestimating the benefits of treatment even more [16,28].
Strength and weaknesses
A strength of the study is the inclusion of patients from both general practice and hospital-based outpatient clinics. Another strength is that the analysis was predefined at the time of the original study. Also, data on outcomes of morbidity and mortality were collected from national health registers with high coverage and high data quality.
In this study, the estimated individual probability of a hypertension-related complication, given that the patient is treated, is compared with the actual outcome for each patient. Patients with primary hypertension with treatment with at least one drug were included, but we do not know from the data set whether or not the patient continued with the pharmacological treatment during the studied period. However, the comprehensive national healthcare system in Sweden with a low annual maximum individual cost for drugs and consultations has low barriers for healthcare consultations, and thus a low level of loss to follow-up of patients.
The use of a visual analogue scale, VAS, with no intermediate anchoring points for estimating the probability, is another weakness since different groups (patients versus physicians, younger versus older) might interpret the VAS differently. In order to compare the groups, the analyses of the individual pairs of patients and physicians were performed on ranking within each group into deciles, instead of the absolute value of probability. Psychological fallacies might also have influenced the results, such as an otherwise healthy individual finding it hard to imagine or express his/her death or a severe complication, as a possible outcome within 10 years.
Different patients and patients from different cultures may be used to different ways of presenting absolute and relative values. Percentages and visual analogue scales are different (numerical and graphical) ways of presenting fractions. Absolute values expressed as percentages are the preferred presentation in risk prediction tools such as the risk chart of the European Society of Hypertension [29], Score/HeartScore [30] or QRISK3 [31]. Other formats such as odds might be an alternative in a given situation to communicate a probability for a given outcome. The relevant measure for most of the patients is however probably not the average expected risk of a given outcome, with or without treatment, but rather the reduced probability of a negative outcome with treatment [12]. Risk reduction can be expressed either as a relative change calculated as a fraction or percentage, or an absolute reduction in probability measured in percentage points. Both perspectives can also be combined into the number of patients needed to be treated over a specified period to avoid one negative outcome (number needed to treat) or to experience a specified adverse drug reaction (number needed to harm). Visual aids can help overcome the difficulties in presenting the possible effects of different treatment alternatives [32].
A discussion of the risks associated with a disease and the treatment is a normal part of a consultation, especially when initiating treatment. The inclusion criteria for the study included a diagnosis of primary hypertension already treated with at least one drug. If the patient introduced the subject, then patients and physicians could discuss the risk assessments during the consultations based on the patient’s experience with the questionnaire. This could have influenced the risk assessments made by the physicians since these were recorded after the consultation. However, the risk assessments differed sharply in the patient-physician pairs as demonstrated by the scatterplots in Figure 2 and the supplemental material and this would indicate that in most interactions no such discussion took place, or at least was not successful in influencing the physician.
Relation to other studies
The analysis in this study is valid only for Sweden and for the period during which the study was carried out (1995–2006) before physicians had access to digital risk prediction tools. The use of a risk prediction algorithm would not only provide the patient and physician with an estimation of the probabilities of different outcomes but would also over time increase the ability of physicians to assess the risk for an individual patient. Risk assessment tools are also more or less adapted to specific patient populations and show slightly different results [33] and are continuously developed and improved [34]. Also, the epidemiology of hypertension has changed over time [1], as has an increased number of treatment options and the introduction of more ambitious treatment goals. Because of this, the probability of experiencing complications due to hypertension has decreased compared with what was estimated and reported in this study.
It is, however, unclear to what extent such a prediction tool, either as a digital tool or simplified printed risk cards, is used together with the patient in ordinary healthcare today in Sweden. Risk score tools focus on one specific or a combined set of outcomes [18], and even an intermittent use would probably introduce a learning effect. This would then improve the ability of physicians to predict outcomes for different patient groups correctly. To what extent these tools are used in routine clinical care today and how they have influenced how risk is assessed by patients and physicians ought to be studied.
The study focused only on one common risk factor of cardiovascular disease, relatively well-known in the general population. It is thus not possible to generalise the results to other situations with high probabilities of serious adverse outcomes, for instance, in oncology.
Meanings of this study
We conclude that both patients and physicians need evidence-based tools to perform a valid risk assessment of hypertension [16,18,28] in order to support clinical decision making and to support shared decision making. The study was performed before risk prediction algorithms were widely available and used. However, even today, the use of such tools is not routine in day-to-day healthcare. They are designed to present the risk in a specific situation to facilitate the decision to treat the patient, not always to support communication of the risk reduction possible with drug treatment.
This study supports the use of risk assessment tools to improve risk assessment. The findings are relevant for the development of strategies aiming to improve communication between patient and the treating physician about cardiovascular risk.
Ethical approval
The protocol for the original study was approved by the Regional Ethical Review Board in Linköping, Sweden, Study code 95-356. The Swedish Data Protection Authority granted permission in 1995 for the establishment of a register of personal data according to the Data Protection Authority Statute 1993:1, Study code 124.7252-95, to allow for long-term follow-up of personal health data. The protocol for the follow-up study reported here was approved by the Regional Ethical Review Board in Linköping, Sweden, Study code 2014/67-31.
Supplementary Material
Funding Statement
The initial study reported in 1998 [18] was funded by Merck & Co. Inc., the Linköping University, Medical Research Council of Southeast Sweden (FORSS), and the University of Gothenburg (LUA). The prospective cohort study was funded by the NEPI Foundation and the Faculty of Medicine and Health Sciences, Linköping University, Sweden. The NEPI Foundation is a trust initiated by the Swedish Parliament in 1993 to promote the development of pharmaco-epidemiology and is fully financed by the dividend of the capital of the trust. The members of the board are appointed by the Swedish Society of Pharmaceutical Sciences and the Swedish Society of Medicine.
Disclosure statement
All authors have completed the ICMJE uniform disclosure form (available on request from the corresponding author) at www.icmje.org/coi_disclosure.pdf and declare:
no support from any organisation for the submitted work other than stated above;
no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years;
no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.NCD Risk Factor Collaboration - NCD-RisC. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Streit S, Gussekloo J, Burman RA, et al. Burden of cardiovascular disease across 29 countries and GPs’ decision to treat hypertension in oldest-old. Scand J Prim Health Care. 2018;36:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swain S, Hariharan M, Rana S, et al. Doctor-patient communication: Impact on adherence and prognosis among patients with primary hypertension. Psychol Stud. 2015;60:25–32. [Google Scholar]
- 4.Turner JR. Patient and physician adherence in hypertension management. J Clin Hypertens. 2013;15:447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hameed MA, Dasgupta I, Gill P. Poor adherence to antihypertensive drugs. BMJ. 2016;354:i3268. [DOI] [PubMed] [Google Scholar]
- 6.Hameed MA, Tebbit L, Jacques N, et al. Non-adherence to antihypertensive medication is very common among resistant hypertensives: results of a directly observed therapy clinic. J Hum Hypertens. 2016;30:83–89. [DOI] [PubMed] [Google Scholar]
- 7.Marshall IJ, Wolfe CD, McKevitt C. Lay perspectives on hypertension and drug adherence: systematic review of qualitative research. BMJ. 2012;345:e3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ödesjö H, Adamsson Eryd S, Franzén S, et al. Visit patterns at primary care centres and individual blood pressure level - a cross-sectional study. Scand J Prim Health Care. 2019;37:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann TC, Bennett S, Tomsett C, et al. Brief training of student clinicians in shared decision making: a single-blind randomized controlled trial. J Gen Intern Med. 2014;29:844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultberg J, Rudebeck CE. Patient participation in decision-making about cardiovascular preventive drugs - resistance as agency. Scand J Prim Health Care. 2017;35:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengtsson U, Kjellgren K, Hallberg I, et al. Patient contributions during primary care consultations for hypertension after self-reporting via a mobile phone self-management support system. Scand J Prim Health Care. 2018;36:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M, Kjellgren KI, Lind-Åstrand L, et al. Risk communication in consultations about hormone therapy in the menopause: concordance in risk assessment and framing due to the context. Climacteric. 2006;9:347–354. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton-Craig CR, Tonkin AL, Jobling RG. How accurate are hypertension treatment decisions? Absolute risk assessment and prescribing for moderate hypertension - a study of Cambridge general practitioners. Blood Press. 2000;9:323–327. [DOI] [PubMed] [Google Scholar]
- 14.Karmali KN, Lloyd-Jones DM, van der Leeuw J, et al. ; on behalf of the Blood Pressure Lowering Treatment Trialists’ Collaboration . Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data. PLoS Med. 2018;15:e1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho CLB, Breslin M, Doust J, et al. Effectiveness of blood pressure-lowering drug treatment by levels of absolute risk: post hoc analysis of the Australian National Blood Pressure Study. BMJ Open. 2018;8:e017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175:274–286. [DOI] [PubMed] [Google Scholar]
- 17.Collins GS, Altman DG. Predicting the 10 year risk of cardiovascular disease in the United Kingdom: independent and external validation of an updated version of QRISK2. BMJ. 2012;344:e4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karjalainen T, Adiels M, Björck L, et al. An evaluation of the performance of SCORE Sweden 2015 in estimating cardiovascular risk: The Northern Sweden MONICA Study 1999-2014. Eur J Prev Cardiolog. 2017;24:103–110. [DOI] [PubMed] [Google Scholar]
- 19.Weng SF, Reps J, Kai J, et al. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12:e0174944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–1238. [DOI] [PubMed] [Google Scholar]
- 21.Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157:29–43. [DOI] [PubMed] [Google Scholar]
- 22.Szyndler A, Kucharska WA, Dubiela D, et al. ; The Polish Hypertension Registry . SCORE model underestimates cardiovascular risk in hypertensive patients: results of the Polish Hypertension Registry. Blood Press. 2011;20:342–347. [DOI] [PubMed] [Google Scholar]
- 23.Moons KG, Altman DG, Vergouwe Y, et al. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. [DOI] [PubMed] [Google Scholar]
- 24.Kjellgren KI, Ahlner J, Dahlöf B, et al. Perceived symptoms amongst hypertensive patients in routine clinical practice – a population-based study. J Intern Med. 1998;244:325–332. [DOI] [PubMed] [Google Scholar]
- 25.Kjellgren KI, Ahlner J, Dahlöf B, et al. Patients’ and physicians’ assessment of risks associated with hypertension and benefits from treatment. J Cardiovasc Risk. 1998;5:161–166. [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124–1131. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177:407–419. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 30.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 31.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sehestedt T, Jeppesen J, Hansen TW, et al. Risk stratification with the risk chart from the European Society of Hypertension compared with SCORE in the general population. J Hypertens. 2009;27:2351–2357. [DOI] [PubMed] [Google Scholar]
- 34.Sehestedt T, Jeppesen J, Hansen TW, et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31:883–891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.