Abstract
ABSTRACT
Suaeda salsa
L. is a typical euhalophyte and is widely distributed throughout the world. Suaeda plants are important halophyte resources, and the physiological and biochemical characteristics of their various organsand their response to salt stress have been intensively studied. Leaf succulence, intracellular ion localization, increased osmotic regulation and enhanced antioxidant capacities are important responses for Suaeda plants to adapt to salt stress. Among these responses, scavenging of reactive oxygen species (ROS) is an important mechanism for plants to withstand oxidative stress and improve salt tolerance. The generation and scavenging pathways of ROS, as well as the expression of scavenging enzymes change under salt stress. This article reviews the antioxidant system constitute of S. salsa, and the mechanisms by which S. salsaantioxidant capacity is improved for salt tolerance. In addition, the differences between types of antioxidant mechanisms in S. salsaare reviewed, thereby revealing the adaptation mechanisms of Suaeda to different habitats. The review provides important clues for the comprehensive understanding of the salt tolerance mechanisms of halophytes.
KEYWORDS: Suaeda salsa, halophyte, salt-tolerance mechanism, oxidative stress, antioxidant system
Introduction
There exists a set of antioxidant systems responsible for scavenging reactive oxygen species (ROS) in plants.1,2 Under normal circumstances, these systems can scavenge ROS produced during plant growth, to maintain a state of dynamic equilibrium. Under stress conditions, such as salt stress, the amount of ROS synthesized in plants increases,3–6 which results in a relatively inadequate antioxidant scavenging capacity and a disequilibrium that leads to oxidative stress.1,7–10 Plant antioxidant systems can scavenge these ROS to avoid damage by excessive ROS to plants.11,12 The halophytes have strong ROS scavenging ability, and there is complex relationship between stress resistance and antioxidant ability of halophytes.13–15 Suaeda salsa L. (S. salsa) is an annual herbaceous plant of the Suaeda genus, Chenopodiaceae family, which is suitable for saline and alkaline land in the intertidal zone, seaside, lakeside, desert and inland high-salt patches.16 S. salsa can grow in soils with a salt content of 2.5%~3.0% and is a typical salt-tolerant plant,17–20 which can used as a promising model to underst and salt tolerance and to develop saline agriculture.21 In recent years, researchers have conducted many studies on the antioxidant system of S. salsaand revealed the salt-tolerance mechanisms of Suaeda plants from the perspective of oxidative stress. In this paper, the salt tolerance mechanism of S. salsais reviewed mainly in the context of oxidative stress.
The antioxidant systems in S. salsa
Plant antioxidant systems are generally classified into enzymatic defense systems and non-enzymatic defense systems.1,22,23 The former include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), peroxiredoxin reductase (PrxR) and some ascorbic acid (AsA)-glutathione (GSH) cycle enzymes. Among them, POD includes glutathione peroxidase (GPX) and ascorbate peroxidase (APX), and AsA-GSH cycle enzymes include glutathione reductase (GR), dehydroascorbic acid reductase (DHAR) and monodehydroascorbic acid reductase (MDHAR).1,24 Non-enzymatic defense systems include AsA, GSH and some thiol-containing low-molecular-weight compounds.25,26 S. salsascavenges ROS produced by salt stress mainly through the SOD dismutation, CAT pathway, GPX pathway, PrxR pathway and AsA-GSH cycle (Figure 1).
Figure 1.
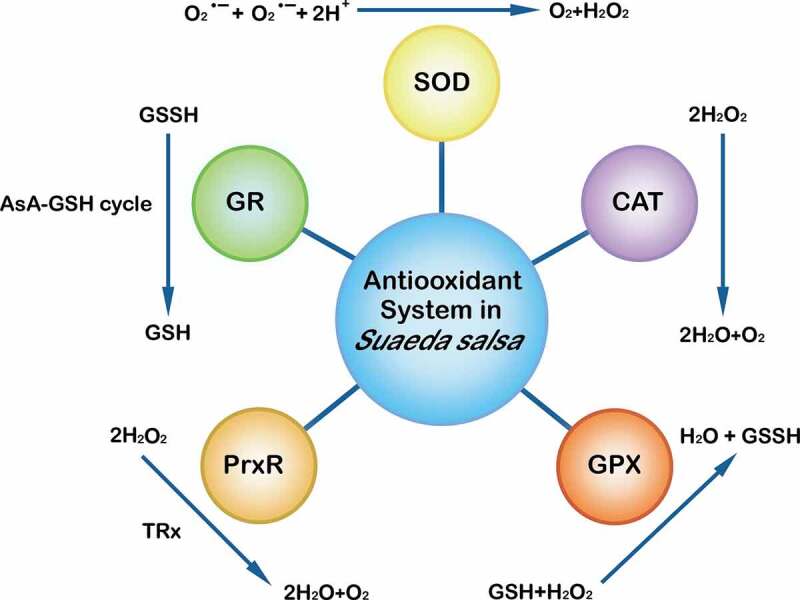
The antioxidant system of Suaeda salsa. ①The superoxide dismutase (SOD) catalyzes the dismutation reaction of two superoxide radicals to form O2 and H2O2, which is then converted to H2O catalyzed by antioxidant enzymes. ②The catalase (CAT) is an enzymatic scavenging agent based on iron porphyrin as the prosthetic group, which can promote the rapid decomposition of H2O2 into H2O and O2. ③The glutathione peroxidase (GPX) catalyzes the binding of GSH to H2O2, generating H2O and GSSH. ④Theperoxidase reductase (PrxR) scavenges H2O2, using thioredoxin (TRx) as a redox carrier to provide electrons. ⑤The glutathione reductase (GR) reduces GSSH to GSH through the AsA-GSH cycle, providing electron donors for the next round of H2O2 scavenging.
The SOD dismutation
SOD is the first defencein the plant antioxidant system and plays an important role in ROS scavenging. SOD catalyzes the dismutation reaction of two superoxide radicals to form O2 and H2O2. H2O2 is then converted to H2O catalyzed by antioxidant enzymes.1 Studies have confirmed that under salt stress, the SOD activity of S. salsa27-29 and S. maritima30 increased gradually as the salt treatment increased. This tendency is mainly due tothe activity of SOD, as an inducing enzyme, is affected by the concentration of the substrate superoxide anion. An increase in the degree of salt stress would increase the production of O2−, thereby inducing the increase in SOD activity.20
The CAT pathway
CAT is an enzymatic scavenging agent based on iron porphyrin as the prosthetic group. This enzyme can promote the rapid decomposition of H2O2 into H2O and O2. CAT is widely found in plant cells and is one of the key enzymes in the biological defense system, providing the organism with an antioxidant defence mechanism. When CAT acts on H2O2 in plants, two H2O2molecules must be bound to the active site of CAT. High concentrations of H2O2 can increase the decomposition rate of CAT, so this pathway can effectively scavenge excessive intracellular H2O2.1 Under salt stress, the CAT activities in S. salsa31and S. maritima30,32 increase, suggesting that this enzymemay play an important role in scavenging H2O2produced by salt stress.
The GPX pathway
GPX is an oxygen radical scavenger. In this pathway, GPX catalyzes the binding of GSH to H2O2, generating H2O and GSSH, and GR then reduces GSSH to GSH, providing electron donors for the next round of H2O2 scavenging.1 Studies have found that salt treatment increasedthe GPX activities in S. salsa31 and S. fruticosa33 leaves. Through a proteomics study, Askari and collaborators found that salt stress could significantly induce the expression of GPX protein in S. aegyptiaca leaves.34
The PrxR pathway
The PrxR pathway is a central link in the intracellular antioxidant defense system in plants. With its reversible disulfide bonds and thiol changes, thioredoxin (TRx) is used as a redox carrier to provide electrons for PrxR to scavenge H2O2.1 The Prx Q gene expression was up-regulated by NaCl in S. salsa, which had a thioredoxin-dependent peroxidase activity, the characteristic of the Prx family.35 The expression of the PrxR protein in S. aegyptiacawas significantly up-regulated by treatment with 150 mmol/L NaCl, indicating that the PrxR protein may play an important role in ROS scavenging under salt stress.34,36
The AsA-GSH cycle
The GR plays a role in the production of GSH through the AsA-GSH cycleinthe mitochondria, chloroplast matrix and cytoplasmof plants, and AsA and GSH in the cycle can inhibit lipid peroxidation and scavenge free-radicals.1An increase of GR activity promotes the production of GSH content, which can directly scavenge ROS.37Treatment of S. salsawith 200 mmol/L NaCl increased the AsA and GSH contents in leaves and decreased the H2O2content, suggesting that the increase of AsA may be important for the decrease in the H2O2content. After treatment of S. salsa with 200 mmol/L NaCl for 7 days, the activities of APX and GR in chloroplast matrix and thylakoids were significantly increased, resulting in a decreased H2O2content and a decreased membrane lipid peroxidation.38
The response of S. salsato salt stress
The effects of different salt concentrations on theS. salsa antioxidant system
Salt stress can lead to increased ROS in plants and thus cause oxidative stress, and the ability to scavenge stress-induced H2O2effectively is crucial to stress resistance.39,40S. salsa can reduce the production of ROS free radicals and the oxidative stress by increasing the activities of antioxidant enzymes.41,42Hence, under salt stress, the activities of various enzymes and the content of non-enzymatic substances involved in the H2O2 scavenging process would reflect the salt-stress resistance ability of S. salsa. Studies have revealed that the effects of salt-stress treatment on the POD activity in S. salsa in different habitats were different.21Upon salt treatment, the POD activity in S. salsa in the intertidal habitat was significantly higher than that in the saline-alkali habitat. With the increase of NaCl concentration, the GR and GSH in S. salsa leaves showed an increase first and then a decrease. Similarly, the GST expression in leavesof S. salsaalso showed a tendency to increase first and then decrease. Some investigators have studied the organ-specific response and the activity of plasma membrane H+-ATPase in callus by studying vacuole H+-ATPase in the NaCl-treated S. salsashoots and roots.43,44 Cheng and collaborators found that salt stress could improve the cold resistance of S. salsa,45 improve seed vigor,46 promote seed maturation47(e.g., salt and nitrate would promote dimorphic seed production and seed germination48) and possibly affect the yield and salt tolerance of S. salsa.49
Effects of different combined treatments of waterlogging and salt onS. salsa antioxidant systems
Using greenhouse control simulation experiments, some papers reported the effects of different groundwater depth and salt concentration on the activities of antioxidant enzymes in multiple Suaeda populations and revealed the mechanism of synergistic regulation of antioxidants in Suaeda caused by waterlogging and salt stress.50–52With waterlogging stress and an increase in salt concentration, the SOD activity increased nearly three fold, and with decreased waterlogging stress, the SOD activity decreased, indicating that waterlogging and salt stress had a positive interaction with SOD and that both salt stress and waterlogging stress could increase the SOD activity. Although the trend of CAT changes under salt stress conditions was similar to that of SOD, the CAT activity decreased during waterlogging stress.31,53,54Under waterlogging and salt stress conditions, MDA content increased with an increase in salt concentration, indicating that when S. salsa is subjected to the dual stress of waterlogging and high salinity, the membrane system of the cells is severely damaged.54–57Based on the changes in water depth at different times, S. salsain coastal saline-alkali soils could adjust the oxidative stress scavenging system to adapt to different environments. The different responses of SOD and CAT to waterlogging stress may be adaptive mechanisms by which S. salsa maintains long-term survival in different water and salt environments.54
The regulation of antioxidant system in S. salsa under some other stresses
Liu and collaborators found that mercury exposure inhibited plant growth of S. salsa and induced significant metabolic responses and increased activities of antioxidant enzymes including SOD and POD.58And after exposure to environmentally relevant lead and zinc for 15 days, the expression levels of CAT genes were significantly upregulated in S. salsa.59Under chilling stress, the SOD and APX activity in S. salsa increased first and then declined, while the production of ROS (O2·− and H2O2) decreased first and then increased.60Co-expression of the S. salsa GST and CAT1genes in transgenic rice resulted in greater increase of CAT and SOD activity following both salt and paraquat stress.61These studies indicated that different stresses could synergistically regulatethe antioxidant system of S. salsa.
The antioxidant abilities of different S. salsaecotypes
S. salsacan be divided into two ecotypes, red-violet and green, depending on the color of the leaves. The former is affected by high salt, low temperature and waterlogging, while the latter is mainly affected by salt stress and drought stress.21,56The two ecotypes of S. salsaalso have different antioxidant pathways. The contents of reduced GSH and AsA and the activities of SOD and APX in the red-violet ecotype of S. salsawere higher than in the green ecotype, while the POD and CAT activities were lower than in the green ecotype. By analysing four antioxidant enzymes, it was found that there were differences in the antioxidant enzyme profiles in the leaves of the two ecotypes of S. salsa. The expression levels of SOD and APX in the red-violet ecotype of S. salsaleaves were higher than those in the green ecotype, while the CAT and POD expression levels in the leaves of the green ecotype of S. salsawere higher than those in the red-violet ecotype.62 The activity and isoenzyme expression of the antioxidant enzymes in the leaves were different between the two ecotypes of S. salsa, suggesting that they relied on different antioxidant enzymes for ROS scavenge.
Researchers compared the antioxidant systems of the two different ecotypes of S. salsain the intertidal habitat and found that the major antioxidant enzymes in the two ecotypes were not identical.63 Under natural conditions, the H2O2content in the leaves of the green ecotype of S. salsawas significantly higher than that in the red-violet ecotype, and the activities of the antioxidant enzymes were not significantly different between these two ecotypes. Wang and collaborators found that the content of betacyanin in S. salsaincreased under salt stress.64The content of betacyanin in the red-violet ecotype of S. salsawas higher than in the green ecotype, and the concentration of exogenous H2O2 was significantly negatively correlated with the content of betacyanin in leaves. These findings indicate that betacyanin may be involved in regulating the ROS levels as a non-enzymatic antioxidant agent in S. salsa, thereby reducing stress-induced oxidative damage.
The activity of extracts in S. salsa
The activity of flavonoid extracts in S. salsa
Flavonoids are a class of plant secondary metabolites with extensive biological activities, including antioxidant, anticancer, anti-inflammatory, anti-allergy, hypoglycemic, hypolipidaemic, immunomodulatory, antibacterial, anti-drug and anti-cardiovascular disease activities.65 Some researchers obtained crude flavonoids from S. salsausing 65% ethanol, which were subjected to polyamide column chromatography to obtain refined flavonoids. The total content of flavonoids was determined by spectrophotometry, and the antioxidant properties were studied using the nitrogen blue tetrazolium (NBT) method, which showed that the flavonoids in S. salsa have an inhibitory effect on the autoxidation of lard.47,66Wang and collaborators obtained ten flavones from the 95% ethanol extract of the leaves and stems of S. salsa, and found that luteolin could clear DPPH and ·OH, with IC50 values 2.89 and 36.7 μg/mL, respectively.67In addition, these flavonoids all have strong inhibitory effects on Escherichia coli and Staphylococcus aureus, with the total flavonoid extracts during the flowering period having the greatest inhibitory effect on the bacteria. Studies have also demonstrated that the total flavonoids in S. salsaextracts could scavenge hydroxyl radicals and oxygen free radicals, and inhibit α-amylase and lipase activities.68
The activity of other extracts in S. salsa
Except for flavonoids, some other compounds from S. salsa have various activities, which can be used as industrial and pharmaceutical materials. Li and collaborators comprehensively analyzed the metabolic response of S. salsa under salinity from the perspective of omics, demonstrating that secondary metabolites, such as quercetin, 2,4-dihydroxybenzoicacid, isorhamnetin and 2-hydroxygenistein, may play an important role as antioxidants and regulatory substances.69Wang and collaborators isolated ten known metabolites from S. salsa using 95% ethanol, and found one of the compounds, (–)-syringaresinol-4-O-β-D-glucopyranoside, showed moderate cytotoxic activity against four carcinoma cell lines, determined by the MTT colorimetric method.70
Perspective
A large number of researches have been conducted on the antioxidant effects of S. salsa, which laid the foundation for revealing the antioxidant mechanism of Suaeda plants and provided important clues for a comprehensive understanding of the salt tolerance mechanisms of euhalophytes. The activity of the enzymes in the antioxidant system is closely related to plant metabolism during stress. However, at present, the production and detection technologies of various ROS forms have not been perfected, and the research on plant scavenging mechanisms needs to be developed. The analysis of ROS generation and properties in halophytes and the action of plant antioxidant systemsis not only important for understanding the physiological metabolism of plants per se, but also has profound significance for improving the stress tolerance of transgenic plants through the bioengineering of antioxidant genes. Therefore, the study of the salt tolerance mechanisms in halophytes, including their antioxidant systems, needs to be strengthened, and in-depth investigations of secondary stress caused by salt stress in Suaeda plants also need to be conducted. Combined with the analysis of stress-induced transcriptional regulation and energy metabolism, the osmotic regulation mechanisms and the antioxidant mechanisms should be clarified and the salt-tolerance mechanisms of Suaeda plants systematically elucidated, laying the foundation for genetic engineering of plant salt-tolerance.
Acknowledgments
This work was supported by grants from the Shandong Provincial Natural Science Foundation of China (ZR2017BC073) and the Project of Shandong Province Higher Educational Science and Technology Program (J16LE07).
Funding Statement
This work was supported by the Department of Education of Shandong Province [J16LE07]; Natural Science Foundation of Shandong Province [ZR2017BC073].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ozgur R, Uzilday B, Sekmen AH, Turkan I.. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct Plant Biol. 2013;40:1–6. doi: 10.1071/Fp12389. [DOI] [PubMed] [Google Scholar]
- 2.Akyol TY, Yilmaz O, Uzilday B, Uzilday RO, Turkan I. Plant response to salinity: an analysis of ROS formation, signaling, and antioxidant defense. Turk J Bot. 2020;44:1–13. doi: 10.3906/bot-1911-15. [DOI] [Google Scholar]
- 3.Shen XY, Wang ZL, Song XF, Xu JJ, Jiang CY, Zhao YX, Ma C, Zhang H. Transcriptomic profiling revealed an important role of cell wall remodeling and ethylene signaling pathway during salt acclimation in Arabidopsis. Plant Mol Biol. 2014;86:303–317. doi: 10.1007/s11103-014-0230-9. [DOI] [PubMed] [Google Scholar]
- 4.Sun W, Li Y, Zhao YX, Zhang H. The TsnsLTP4, a nonspecific lipid transfer protein involved in wax deposition and stress tolerance. Plant Mol Biol Rep. 2015;33:962–974. doi: 10.1007/s11105-014-0798-x. [DOI] [Google Scholar]
- 5.Sun ZB, Qi XY, Wang ZL, Li PH, Wu CX, Zhang H, Zhao Y. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol Biochem. 2013;69:82–89. doi: 10.1016/j.plaphy.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Cao S, Du XH, Li LH, Liu YD, Zhang L, Pan X, Li Y, Li H, Lu H. Overexpression of populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ J Plant Physiol. 2017;64:224–234. doi: 10.1134/s1021443717020029. [DOI] [Google Scholar]
- 7.Yuan F, Leng BY, Wang BS. Progress in studying salt secretion from the salt glands in recretohalophytes: how do plants secrete salt? Front. Plant Sci. 2016:7. doi: 10.3389/fpls.2016.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan F, Lyu MJA, Leng BY, Zhu XG, Wang BS. The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant Mol Biol. 2016;91:241–256. doi: 10.1007/s11103-016-0460-0. [DOI] [PubMed] [Google Scholar]
- 9.Yuan F, Lyu MJA, Leng BY, Zheng GY, Feng ZT, Li PH, Zhu X-G, Wang B-S. Comparative transcriptome analysis of developmental stages of the L imonium bicolor leaf generates insights into salt gland differentiation. Plant Cell And Environment. 2015;38:1637–1657. doi: 10.1111/pce.12514. [DOI] [PubMed] [Google Scholar]
- 10.Sui N, Wang Y, Liu SS, Yang Z, Wang F, Wan SB. Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front Plant Sci. 2018:9. doi: 10.3389/fpls.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu SS, Wang WQ, Li M, Wan SB, Sui N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol Plant. 2017:39. doi: 10.1007/s11738-017-2501-y. [DOI] [Google Scholar]
- 12.Sui N, Tian SS, Wang WQ, Wang MJ, Fan H. Overexpression of glycerol-3-phosphate acyltransferase from suaeda salsa improves salt tolerance in arabidopsis. Front Plant Sci. 2017:8. doi: 10.3389/fpls.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu YG, Liu RR, Sui N, Shi WW, Wang L, Tian CY, Song J. Changes in endogenous hormones and seed-coat phenolics during seed storage of two Suaeda salsa populations. Aust J Bot. 2016;64:325–332. doi: 10.1071/bt16014. [DOI] [Google Scholar]
- 14.Guo YH, Wang D, Jia WJ, Song J, Yang JC, Wang BS. Effects of seed vernalisation and photoperiod on flowering induction in the halophyte Thellungiella halophila. Aust J Bot. 2012;60:743–748. doi: 10.1071/bt12180. [DOI] [Google Scholar]
- 15.Deng YQ, Feng ZT, Yuan F, Guo JR, Suo SS, Wang BS. Identification and functional analysis of the autofluorescent substance in Limonium bicolor salt glands. Plant Physiol Biochem. 2015;97:20–27. doi: 10.1016/j.plaphy.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Ma YC, Yang Y, Liu RR, Li Q, Song J. Adaptation of euhalophyte Suaeda salsa to nitrogen starvation under salinity. Plant Physiol Bioch. 2020;146:287–293. doi: 10.1016/j.plaphy.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Shi WW, Liu RR, Xu YG, Sui N, Zhou JC, Feng G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biology. 2017;32:107–114. doi: 10.1111/1442-1984.12132. [DOI] [Google Scholar]
- 18.Guo JR, Lu CX, Zhao FC, Gao S, Wang BS. Improved reproductive growth of euhalophyte Suaeda salsa under salinity is correlated with altered phytohormone biosynthesis and signal transduction. Funct Plant Biol. 2020;47:170–183. doi: 10.1071/Fp19215. [DOI] [PubMed] [Google Scholar]
- 19.Guo JR, Dong XX, Li Y, Wang BS. NaCl treatment markedly enhanced pollen viability and pollen preservation time of euhalophyte Suaeda salsa via up regulation of pollen development-related genes. J Plant Res. 2020;133:57–71. doi: 10.1007/s10265-019-01148-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QF, Li YY, Pang CH, Lu CM, Wang BS. NaCl enhances thylakoid-bound SOD activity in the leaves of C-3 halophyte Suaeda salsa L. Plant Sci. 2005;168:423–430. doi: 10.1016/j.plantsci.2004.09.002. [DOI] [Google Scholar]
- 21.Song J, Wang BS. Using euhalophytes to understand salt tolerance and to develop saline agriculture: suaeda salsa as a promising model. Ann Bot-London. 2015;115:541–553. doi: 10.1093/aob/mcu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kartashov AV, Radyukina NL, Ivanov YV, Pashkovskii PP, Shevyakova NI, Kuznetsov VV. Role of antioxidant systems in wild plant adaptation to salt stress. Russ J Plant Physl+. 2008;55:463–468. doi: 10.1134/S1021443708040055. [DOI] [Google Scholar]
- 23.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 24.Li K, Pang CH, Ding F, Sui N, Feng ZT, Wang BS. Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S Afr J Bot. 2012;78:235–245. doi: 10.1016/j.sajb.2011.09.006. [DOI] [Google Scholar]
- 25.Meng X, Yang DY, Li XD, Zhao SY, Sui N, Meng QW. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3-MYB factor. Plant Physiol Biochem. 2015;89:24–30. doi: 10.1016/j.plaphy.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Ren XL, Qi GN, Feng HQ, Zhao S, Zhao SS, Wang Y, Wu W-H. Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K + homeostasis in Arabidopsis. Plant J. 2013;74:258–266. doi: 10.1111/tpj.12123. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Luttge U, Ratajczak R. Specific regulation of SOD isoforms by NaCl and osmotic stress in leaves of the C3 halophyte Suaeda salsa L. Journal of Plant Physiology. 2004;161:285–293. doi: 10.1078/0176-1617-01123. [DOI] [PubMed] [Google Scholar]
- 28.Guan B, Yu J, Wang X, Fu Y, Kan X, Lin Q, Han G, Lu Z. Physiological responses of halophyte suaeda salsa to water table and salt stresses in Coastal Wetland of Yellow River Delta. Clean Soil Air Water. 2011;39:1029–1035. doi: 10.1002/clen.201000557. [DOI] [Google Scholar]
- 29.Jithesh MN, Prashanth SR, Sivaprakash KR, Parida AK. Antioxidative response mechanisms in halophytes: their role in stress defence. J Genet. 2006;85:237–254. doi: 10.1007/Bf02935340. [DOI] [PubMed] [Google Scholar]
- 30.Mallik S, Nayak M, Sahu BB, Panigrahi AK, Shaw BP. Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biol Plant. 2011;55:191–195. doi: 10.1007/s10535-011-0029-3. [DOI] [Google Scholar]
- 31.Wu H, Liu X, You L, Zhang L, Zhou D, Feng J, Zhao J, Yu J. Effects of salinity on metabolic profiles, gene expressions, and antioxidant enzymes in halophyte Suaeda salsa. Journal Of Plant Growth Regulation. 2012;31:332–341. doi: 10.1007/s00344-011-9244-6. [DOI] [Google Scholar]
- 32.Sahu BB, Shaw BP. Isolation, identification and expression analysis of salt-induced genes in Suaeda maritima, a natural halophyte, using PCR-based suppression subtractive hybridization. BMC Plant Biology. 2009;9:69. doi: 10.1186/1471-2229-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hameed A, Hussain T, Gulzar S, Aziz I, Gul B, Khan MA. Salt tolerance of a cash crop halophyte Suaeda fruticosa: biochemical responses to salt and exogenous chemical treatments. Acta Physiol Plant. 2012;34:2331–2340. doi: 10.1007/s11738-012-1035-6. [DOI] [Google Scholar]
- 34.Askari H, Edqvist J, Hajheidari M, Kafi M, Salekdeh GH. Effects of salinity levels on proteome of Suaeda aegyptiaca leaves. Proteomics. 2006;6:13. [DOI] [PubMed] [Google Scholar]
- 35.Guo XL, Cao YR, Cao ZY, Zhao YX, Zhang H. Molecular cloning and characterization of a stress-induced peroxiredoxin Q gene in halophyte Suaeda salsa. Plant Sci. 2004;167:969–975. doi: 10.1016/j.plantsci.2004.05.004. [DOI] [Google Scholar]
- 36.Ayarpadikannan S, Chung E, Cho C-W, So H-A, Kim S-O, Jeon J-M, Kwak M-H, Lee S-W, Lee J-H. Exploration for the salt stress tolerance genes from a salt-treated halophyte, Suaeda asparagoides. Plant Cell Reports. 2012;31:35–48. doi: 10.1007/s00299-011-1137-4. [DOI] [PubMed] [Google Scholar]
- 37.Noctor G, Foyer CH. ASCORBATE AND GLUTATHIONE: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 38.Pang CH, Zhang SJ, Gong ZZ, Wang BS. NaCl treatment markedly enhances H2O2-scavenging system in leaves of halophyte Suaeda salsa. Physiol Plantarum. 2005;125:490–499. doi: 10.1111/j.1399-3054.2005.00585.x. [DOI] [Google Scholar]
- 39.Feng ZT, Deng YQ, Zhang SC, Liang X, Yuan F, Hao JL. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Science. 2015;238:286–296. doi: 10.1016/j.plantsci.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Yuan F, Chen M, Yang JC, Song J, Wang BS. The optimal dosage of Co-60 gamma irradiation for obtaining salt gland mutants of exo-recretohalophyte limonium bicolor (bunge) o. Kuntze. Pakistan J Bot. 2015;47:71–76. [Google Scholar]
- 41.Sui N, Li M, Li K, Song J, Wang BS. Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica. 2010;48:623–629. doi: 10.1007/s11099-010-0080-x. [DOI] [Google Scholar]
- 42.Zhao SZ, Sun HZ, Chen M, Wang BS. Light-regulated betacyanin accumulation in euhalophyte Suaeda salsa calli. Plant Cell Tissue Organ Culture. 2010;102:99–107. doi: 10.1007/s11240-010-9710-z. [DOI] [Google Scholar]
- 43.Chen M, Song J, Wang BS. NaCl increases the activity of the plasma membrane H+-ATPase in C-3 halophyte Suaeda salsa callus. Acta Physiol Plant. 2010;32:27–36. doi: 10.1007/s11738-009-0371-7. [DOI] [Google Scholar]
- 44.Yang MF, Song J, Wang BS. Organ-Specific Responses of Vacuolar H+-ATPase in the Shoots and Roots of C-3 Halophyte Suaeda salsa to NaCl. J Int Plant Biol. 2010;52:308–314. doi: 10.1111/j.1744-7909.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- 45.Cheng S, Yang Z, Wang MJ, Song J, Sui N, Fan H. Salinity improves chilling resistance in Suaeda salsa. Acta Physiol Plant. 2014;36:1823–1830. doi: 10.1007/s11738-014-1555-3. [DOI] [Google Scholar]
- 46.Guo JR, Suo SS, Wang BS. Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci Res. 2015;25:335–344. doi: 10.1017/s0960258515000239. [DOI] [Google Scholar]
- 47.Zhou JC, Fu TT, Sui N, Guo JR, Feng G, Fan JL, Song J. The role of salinity in seed maturation of the euhalophyte Suaeda salsa. Plant Biosyst. 2016;150:83–90. doi: 10.1080/11263504.2014.976294. [DOI] [Google Scholar]
- 48.Song J, Zhou JC, Zhao WW, Xu HL, Wang FX, Xu YG, Wang L, Tian C. Effects of salinity and nitrate on production and germination of dimorphic seeds applied both through the mother plant and exogenously during germination in Suaeda salsa. Plant Spec Biol. 2016;31:19–28. doi: 10.1111/1442-1984.12071. [DOI] [Google Scholar]
- 49.Wang FX, Xu YG, Wang S, Shi WW, Liu RR, Feng G, Song J. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol Biochem. 2015;95:41–48. doi: 10.1016/j.plaphy.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Song J, Shi GW, Gao B, Fan H, Wang BS. Waterlogging and salinity effects on two Suaeda salsa populations. Physiol Plant. 2011;141:343–351. doi: 10.1111/j.1399-3054.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 51.Chen TS, Yuan F, Song J, Wang BS. Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Func Plant Biol. 2016;43:244–253. doi: 10.1071/fp15120. [DOI] [PubMed] [Google Scholar]
- 52.Han N, Lan WJ, He X, Shao Q, Wang BS, Zhao XJ. Expression of a Suaeda salsa vacuolar H+/Ca2+ transporter gene in arabidopsis contributes to physiological changes in salinity. Plant Mol Biol Rep. 2012;30:470–477. doi: 10.1007/s11105-011-0353-y. [DOI] [Google Scholar]
- 53.Gomez JM, Jimenez A, Olmos E, Sevilla F. Location and effects of long-term NaCl stress on superoxide dismutase and ascorbate peroxidase isoenzymes of pea (Pisum sativum cv Puget) Chloroplasts. J Exp Bot. 2004;55:119–130. [DOI] [PubMed] [Google Scholar]
- 54.Guan B, Yu JB, Lu ZH, Zhang Y, Wang XH. [Effects of water-salt stresses on seedling growth and activities of antioxidative enzyme of Suaeda salsa in coastal wetlands of the Yellow River Delta]. Huan Jing Ke Xue. 2011;32:2422–2429. [PubMed] [Google Scholar]
- 55.Zhang JX, Wang C, Yang CY, Wang JY, Chen L, Bao XM, Zhao Y-X, Zhang H, Liu J. The role of arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance. Plant J. 2010;62:539–548. doi: 10.1111/j.1365-313X.2010.04173.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang SR, Song J, Wang H, Feng G. Effect of salinity on seed germination, ion content and photosynthesis of cotyledons in halophytes or xerophyte growing in Central Asia. J Plant Ecol. 2010;3:259–267. doi: 10.1093/jpe/rtq005. [DOI] [Google Scholar]
- 57.Zhao KF, Song J, Fan H, Zhou S, Zhao M. Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive maize. Journal Of Integrative Plant Biology. 2010;52:468–475. doi: 10.1111/j.1744-7909.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- 58.Liu XL, Lai YK, Sun HS, Wang YY, Zou N. The interactive effects of mercury and selenium on metabolic profiles, gene expression and antioxidant enzymes in halophyte Suaeda salsa. Environ Toxicol. 2016;31:440–451. doi: 10.1002/tox.22057. [DOI] [PubMed] [Google Scholar]
- 59.Wu HF, Liu XL, Zhao JM, Yu JB. Regulation of metabolites, gene expression, and antioxidant enzymes to environmentally relevant lead and zinc in the halophyte Suaeda salsa. J Plant Growth Regul. 2013;32:353–361. doi: 10.1007/s00344-012-9305-5. [DOI] [Google Scholar]
- 60.Sui N. Photoinhibition of Suaeda salsa to chilling stress is related to energy dissipation and water-water cycle. Photosynthetica. 2015;53:207–212. doi: 10.1007/s11099-015-0080-y. [DOI] [Google Scholar]
- 61.Zhao FY, Zhang H. Salt and paraquat stress tolerance results from co-expression of the Suaeda salsa glutathione S-transferase and catalase in transgenic rice. Plant Cell Tiss Org. 2006;86:349–358. doi: 10.1007/s11240-006-9133-z. [DOI] [Google Scholar]
- 62.Yue XX, Chen M, Duan D, Wang BS. Comparative study on antioxidant system of green and red-violet phenotype suaeda salsa leaves. Journal of Shandong Normal University (Natural Science). 2008;23:121–124. [Google Scholar]
- 63.Li X, Liu Y, Chen M, Song YP, Song J, Wang BS, Feng G. Relationships between ion and chlorophyll accumulation in seeds and adaptation to saline environments in Suaeda salsa populations. Plant Biosyst. 2012;146:142–149. doi: 10.1080/11263504.2012.727880. [DOI] [Google Scholar]
- 64.Wang CQ, Zhao JQ, Chen M, Wang BS. Identification of betacyanin and effects of environmental factors on its accumulation in halophyte Suaeda salsa. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2006;32:195–201. [PubMed] [Google Scholar]
- 65.Juca MM, Cysne FMS, de Almeida JC, Mesquita DD, Barriga JRD, Dias KCF, Barbosa TM, Vasconcelos LC, Leal LKAM, Ribeiro JE, et al. Flavonoids: biological activities and therapeutic potential. Nat Prod Res. 2020;34:692–705. doi: 10.1080/14786419.2018.1493588. [DOI] [PubMed] [Google Scholar]
- 66.Zhou JC, Zhao WW, Yin CH, Song J, Wang BS, Fan JL, Feng G. The role of cotyledons in the establishment of Suaeda physophora seedlings. Plant Biosyst. 2014;148:584–590. doi: 10.1080/11263504.2013.788574. [DOI] [Google Scholar]
- 67.Wang QZ, Zhou DS, Chen Y, Guan FQ, Yin M, Liu F, et al. Flavonoids from Suaeda salsa II. Isolation, Structural Determination, and Antioxidant Activity. Chem Nat Compd+. 2018;54:354–355. doi: 10.1007/s10600-018-2343-x. [DOI] [Google Scholar]
- 68.Shao Q, Han N, Ding TL, Zhou F, Wang BS. SsHKT1;1 is a potassium transporter of the C-3 halophyte Suaeda salsa that is involved in salt tolerance. Funct Plant Biol. 2014;41:790–802. doi: 10.1071/fp13265. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Song J. Analysis of widely targeted metabolites of the euhalophyte Suaeda salsa under saline conditions provides new insights into salt tolerance and nutritional value in halophytic species. Bmc Plant Biol. 2019;19:ARTN 388. doi: 10.1186/s12870-019-2006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang QZ, Zhou DS, Wang M, Zhao YY, Chen Y, Yin M, Feng X. Chemical constituents of Suaeda salsa and their cytotoxic activity. Chem Nat Compd+. 2014;50:531–533. doi: 10.1007/s10600-014-1005-x. [DOI] [Google Scholar]


