ABSTRACT
In plant biology, transient expression analysis plays a vital role to provide a fast method to study the gene of interest. In this study, we report a rapid and efficient method for transient expression in Cannabis sativa seedlings using Agrobacterium tumefaciens-mediated transformation. A. tumefaciens strain EHA105 carrying the pCAMBIA1301 construct with uidA gene was used to transform cannabis seedlings and the GUS assay (a measurement of β-glucuronidase activity) was used to detect the uidA expression. In the current study, we have also established a rapid germination protocol for cannabis seeds. The all three steps seed sterilization, germination and seedlings development were carried out in a 1% H2O2 solution. Transient transformation revealed that both cotyledons and young true leaves are amenable to transformation. Compared with tobacco (Nicotiana benthamiana), cannabis seedlings were less susceptible to transformation with A. tumefaciens. Susceptibility to Agrobacterium transformation also varied with the different cannabis varieties. The method established in this study has the potential to be an important tool for gene function studies and genetic improvement in cannabis.
KEYWORDS: Cannabis sativa, rapid germination, Nicotiana benthamiana, Agrobacterium-mediated transformation, Agrobacterium susceptibility, transient expression, GUS assay
Introduction
Cannabis sativa is an annual dioecious herb that belongs to family Cannabaceae. The male plant is characterized by heterogametic chromosomes (XY) with homogametic chromosomes (XX) conferring the female plant phenotype.1 Historically, cannabis has been widely cultivated as a source of seed oil, fiber and intoxicating resin. First written evidence of using cannabis in medicinal practices is described in the compendium of Chinese medicinal herbs by Emperor Shen Nung, dated 2737 B.C.E.2 In the last decades, the therapeutic potential of cannabinoids has been reported for the treatment of a range of human diseases from complex neurological diseases to cancer.3 Although cannabis is best known for the psychoactive compound D9-tetrahydrocannabinol (THC), it also contains varying levels of non-psychoactive cannabinoids such as cannabidiol (CBD), cannabigerol (CBG), D9-tetrahydrocannabivarin (THCV), and cannabichromene (CBC), that show promising therapeutic properties and in some cases mitigate the psychoactive effects of THC.4
Considering the enormous economic importance, it is worthy to study the functional genomics of cannabis. The transient expression analysis is an important tool for functional genomics study. Agrobacterium-mediated transformation is commonly used to achieve both transient and stable gene expression in plants. Wahby et al.5 reported that C. sativa hypocotyl tissues exhibited high susceptibility to Agrobacterium infection/transformation than other tissues. Recently, Chaohua et al. 20166 established regeneration protocol that uses cotyledons of C. sativa as an explant. In the present study, we employed intact cannabis seedlings for establishment of transient expression protocol. Such protocol can be used for functional genomics study and for the development of stable transformation protocol. In this study, we developed an efficient method for transient expression analysis in C. sativa seedlings using Agrobacterium tumefaciens-mediated transformation and demonstrated that cannabis is less susceptible to Agrobacterium transformation than tobacco. Further, we also displayed that susceptibility to Agrobacterium infection also varied with the different cannabis varieties.
Results and discussion
Transient expression analysis provides a rapid method to study the function of genes. Transient transformation protocols may also be used to develop stable transformation protocols. In this study, we have reported a rapid and efficient method for transient expression in Cannabis sativa seedlings using Agrobacterium tumefaciens-mediated transformation. The Agrobacterium tumefaciens strain EHA105 carrying the pCAMBIA1301 construct was used to transform cannabis seedlings and the GUS assay was used to detect the transgenes.
Hydrogen peroxide (H2O2) has been used as a disinfectant for seeds for decades.7 Nandi et al.8 demonstrated that 1% H2O2 was effective in increasing Chili seed germination percentage, vigor index and inhibition of mycelial growth. In current study, we have established all three steps seed sterilization, germination and seedlings development in a 1% H2O2 solution. The 1% H2O2 solution as a sterilant presents significant advantage over mercuric chloride or bleach that require additional washing of seeds, and separate germination and seedling development steps in Murashige and Skoog (MS) agar medium. The 1% H2O2 treatment resulted in significantly higher and rapid germination than water control at 24 h (Figure 1a) which suggested that H2O2 enhanced the germination frequency and seedling development (Figure 1a-B). This is a very rapid germination method in 1% H2O2 solution as more than 80% germination occurs within 24 h and seedling development to the two cotyledons stage occurred in 72–96 h (Figure 1b,c). After 3–4 days of incubation in 1% H2O2 solution, seedlings emerge from seed coats with two fully opened cotyledons and two immature true leaves (Figure 1b,c); seedlings at this developmental stage were used for transformation. Previous literature reports showed that that different varieties of cannabis showed different germination response and revealed optimal germination within 4–7 days by using various germination methods9 and seedling development in 5–15 days or more.9 In comparison to aforementioned method, the present germination method provides cannabis seedlings in very short period (3–4 days) with least efforts (Figure 1). Similarly, Çavusoglu and Kabar10 demonstrated that exogenous application of H2O2 to seeds of different plant species increases seed germination rates, coleoptile emergence percentages, radicle and coleoptile elongation, and fresh weights of the seedlings.
Figure 1.
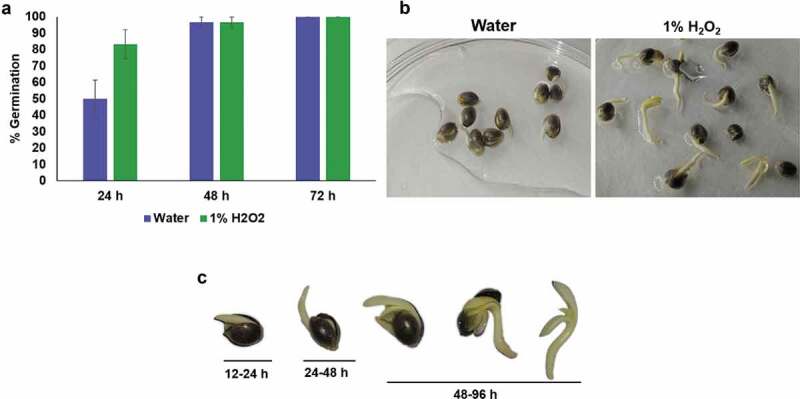
Germination of cannabis seeds in 1% hydrogen peroxide solution and water. (a) Comparison of germination percentage between 1% H2O2 and water. Data were shown as mean ± SE (n = 3). (b) Representative photographs of germinated seeds/seedlings in the 1% H2O2 or water on 4th day. (c) Various stages of germination for cannabis seedlings in 1% H2O2 solution. 12–24 h, cannabis embryo absorbs water until radicle breaks through the seed coat; 24–48 h, further development of radicle; 48–96 h, cotyledons emergence and development of two fully opened cotyledons with two early true leaves.
The overall workflow for the transient transformation of cannabis seedlings is presented in Figure 2. We have used the intact seedlings (two cotyledons stage or two cotyledons with young true leaves stage) for transformation. To enhance the transformation efficiency, we have used the vacuum infiltration followed by 3-days co-cultivation on MS agar media. Vacuum infiltration has been shown to enhance the transformation efficiency of Artemisia annua seedlings.11 To detect the gene transformation in cotyledons and true leaves, the GUS activity assay was employed (Figure 3). GUS analysis revealed that both cotyledons and young true leaves are amenable to transformation (Figure 3). The transformation experiment was repeated four times and in one independent experiment, approximately30 seedlings were evaluated. Previously, Feeney and Punja12,13 successfully demonstrated stable transformation of a hemp cell suspension cultures with A. tumefaciens strain EHA101 carrying the binary vector pNOV3635 with a gene encoding phosphomannose isomerase, although they failed to regenerate fully transgenic cannabis plants. Vacuum infiltration-based Agrobacterium mediated gene delivery system were used in both protocols. However, there are many differences between protocols. The main difference is that Feeney and Punja 201513 used hemp cell suspension culture for transformation, whereas we have used intact cannabis seedlings for transformation. Feeney and Punja 201513 used only one hemp cultivar Anka, while we used three different medical cannabis varieties Nightingale, Holy Grail x CD-1, and Green Crack CBD. Feeney and Punja 201513 used Agrobacterium tumefaciens strain EHA101 carrying binary vector pNOV3635 and we have used EHA105 strain carrying binary vector pCAMBIA 1301. By using our protocol, we have achieved an average transformation frequency with a range of 45–70.6%, while Feeney and Punja 201513 method achieved an average transformation frequency with a range of 15.1–55.3 %. Wahby et al. 20135 reported that hypocotyls tissues were most susceptible to A. rhizogenes infection, while young leaves and cotyledons did not, even when the bacteria were stimulated with acetosyringone. These contradicting results may be due to different Agrobacterium strains or different cannabis varieties used in studies.
Figure 2.
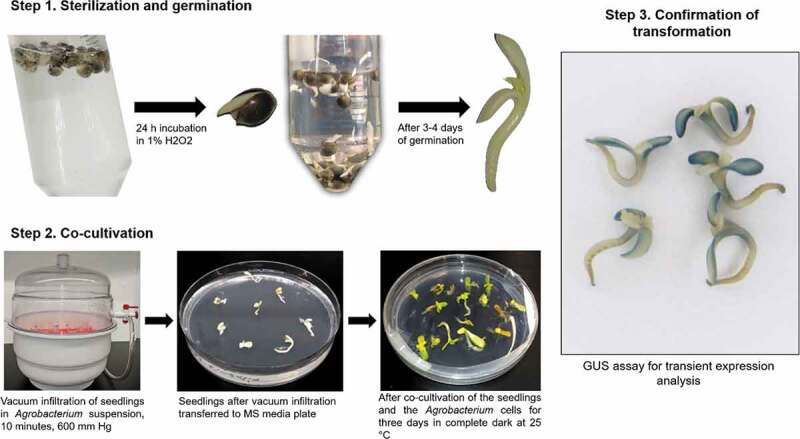
Workflow for Agrobacterium-mediated transient transformation of cannabis seedlings. Step 1. Sterilization and germination, seeds are soaked in 1% H2O2 solution for 24 hours until germination and then transferred into fresh solution. Seeds are then incubated in 1% H2O2 until both cotyledons and epicotyl are visible. Step 2. Co-cultivation, vacuum applied to seedlings submerged in Agrobacterium cell suspension, seedlings are then transferred to MS media plates and incubated for three days in complete dark at 25°C. Step 3. Confirmation of transformation, histochemical GUS assay using transformed seedlings.
Figure 3.
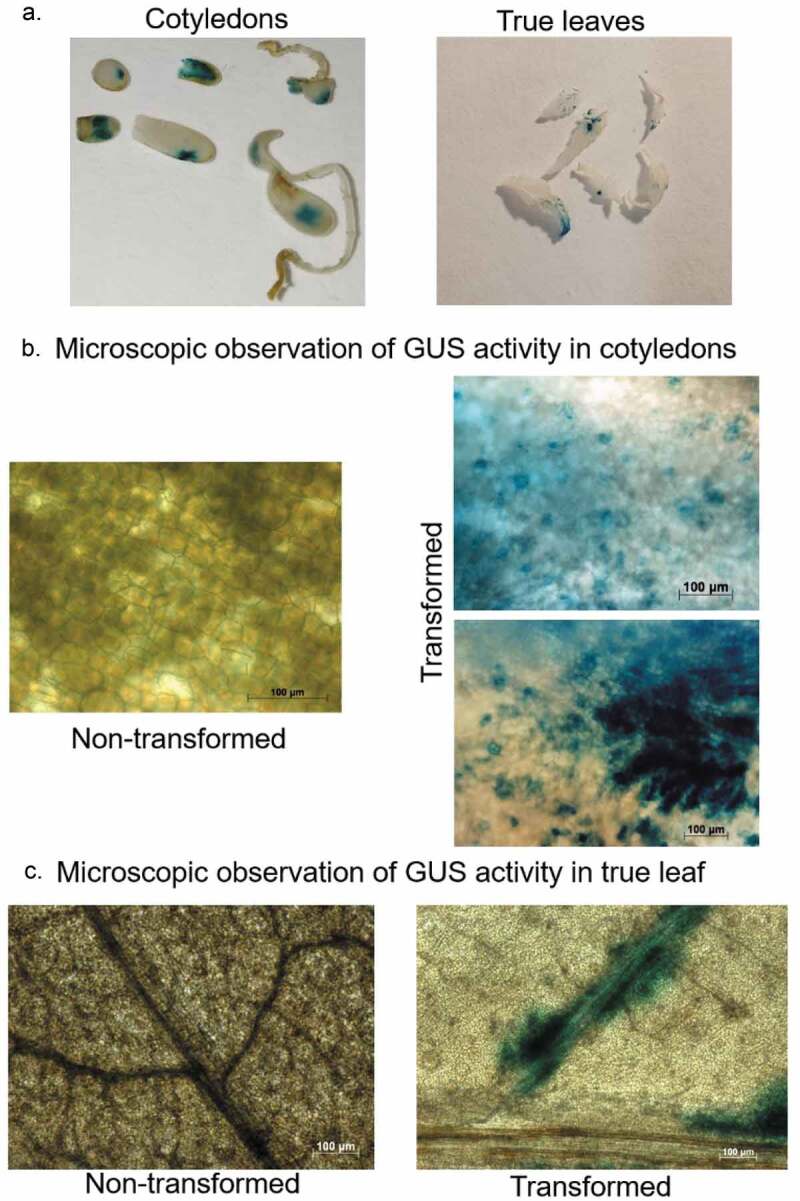
Representative images of GUS activity analysis in cotyledons and leaves tissues of cannabis seedlings to confirm the transient transformation. (a) GUS activity analysis in cotyledons (left panel) and true leaves (right panel). (b) Microscopic observation of GUS activity in cotyledons, non-transformed tissue (left panel) and transformed tissue (right panel). Scale bar 100 µM. (c) Microscopic observation of GUS activity in true leaf, non-transformed tissue (left panel) and transformed tissue (right panel). Scale bar 100 µM.
Comparative qualitative analysis revealed that cannabis seedlings showed less GUS activity than Nicotiana benthamiana (tobacco) suggesting that cannabis is less susceptible to Agrobacterium infection than tobacco (Figure 4). Susceptibility to Agrobacterium infection also varied among the different cannabis varieties. Percentage analysis of transformed seedlings (seedlings which showed at least one visible GUS staining dot in leaves and/or cotyledons) revealed that Nightingale variety showed significantly higher transformed seedlings (70.6%) as compared to Green Crack CBD (45%) and Holy Grail x CD-1 (50%) (p < .05) (Figure 5a). The Nightingale exhibited the GUS staining dots throughout the leaves and cotyledons, however Green Crack CBD and Holy Grail x CD-1 strains showed only fewer GUS staining dots which demonstrates that the Nightingale strain showed higher susceptibility than the Green Crack CBD and Holy Grail x CD-1 (Figure 5b). One possible reason behind this differential susceptibility could be the different secondary metabolite profiles (cannabinoid, terpenoid, alkaloid, and polyphenols) of these cannabis varieties which may impact pathogen defense response. Response to Agrobacterium-infection can be considered as a pathogen response. It is well established that plant host defense response triggered by Agrobacterium infection play crucial role in influencing the susceptibility of plant cells.14-17 Tie et al.18 reported that defense-related genes play a vital role in interplay between Agrobacterium and plant cell. It has been also reported that cultivars of the same species showed differential efficiency to Agrobacterium transformation.18-21 Tie et al. 201218 reported that the transformation efficiency of the indica rice cultivars was lower as compared to japonica cultivars. Further, they demonstrated that the lower T-DNA integrity resulted in lower transformation efficiency in indica rice. The down-regulation of genes involved in DNA repair early after transformation in indica rice may directly lead to the low integration efficiency. Microarray analysis revealed that some genes necessary for the transformation process were down-regulated in the indica cultivar, highlighting the impact of plant defense response on Agrobacterium-mediated transformation.18 Previously, Feeney and Punja22 reported that cannabis is amenable to genetic transformation using Agrobacterium however the plant is recalcitrant to regeneration, impeding the recovery of transgenic cannabis plants.
Figure 4.

Comparative transient expression analysis between cannabis and tobacco using GUS staining.
Figure 5.
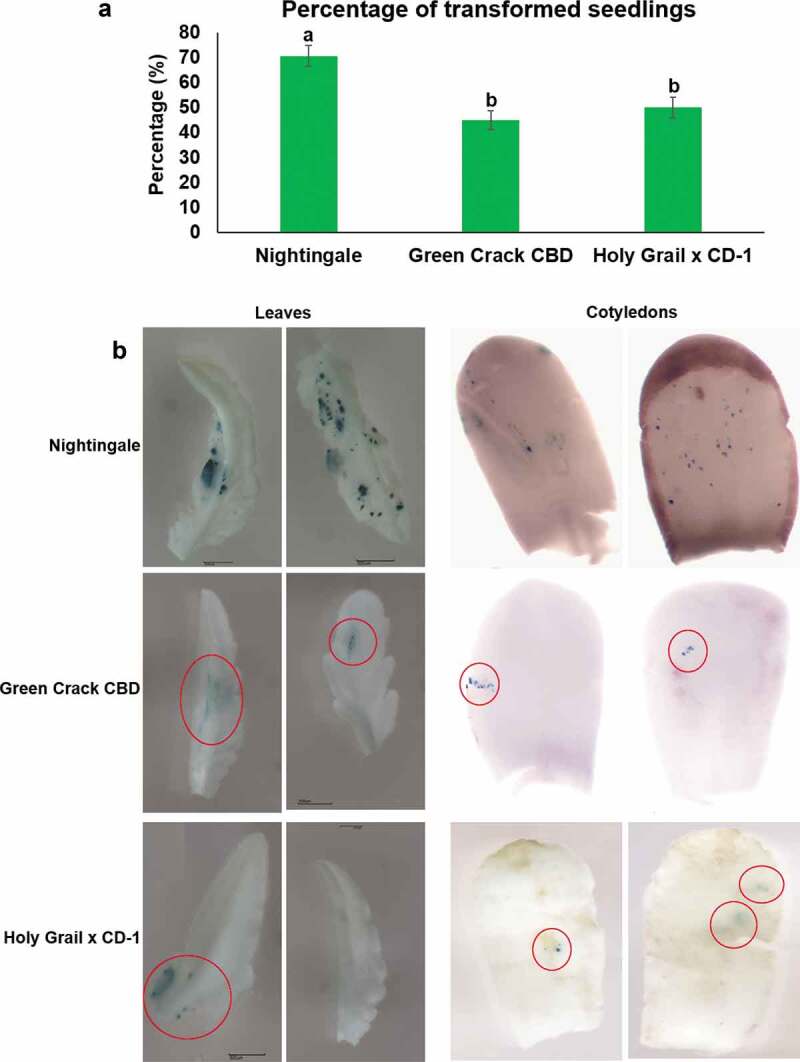
Comparative transient expression analysis among cannabis varieties Nightingale, Green Crack CBD and Holy Grail x CD-1. (a) Percentage of transformed seedlings which showed at least one visible GUS staining dots in leaves and/or cotyledons. Four independent transformation experiments were carried out and in one independent experiment 30 seedlings were used. The data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism version 8.4.2 for Windows. Data are shown as average mean ±SE (n = 4). A p-value less than 0.05 (p ≤ 0.05) were considered statistically significant. Mean values that were significantly different from each other are indicated by different letters. (b) Representative images of comparative GUS staining. The Nightingale exhibited the GUS staining dots throughout the leaves and cotyledons. However Green Crack CBD and Holy Grail x CD-1 varieties showed only fewer GUS staining dots which are red circled.
In conclusion, we developed a rapid and efficient method for transient expression in C. sativa seedlings using Agrobacterium tumefaciens-mediated transformation which has potential to be an important tool for gene-function studies and genetic improvement in C. sativa.
Materials and methods
Materials
Biological materials
Agrobacterium tumefaciens strain (EHA105) carrying binary vector pCAMBIA1301 with uidA gene was used in our study. Agrobacterium strain (EHA105) and the plasmid vector pCAMBIA1301 were a gift from Prof. Barbara Hohn, Friedrich Miescher Institute, Basel, Switzerland.
Cannabis sativa (Candida CD-1, Nightingale, Green Crack CBD, and Holy Grail x CD-1 varieties) and Nicotiana benthamiana seeds were used in this study. All feminized seeds were produced from in-house cannabis varieties. Cuttings from mother plants were subjected to vegetative growth under 18 h light/6 h dark cycle. After 5–6 weeks of vegetative growth selected plants are then masculinized using three times foliar sprays of 3 mM Silver thiosulfate as described by Lubell and Brand 2018.23 To produce feminized seeds, one masculinized plant and 3 female plants are then placed in a separate closed grow tent. Plants are then subjected to 12 h photoperiod for flowering until seed harvesting. For all cannabis varieties, seeds were harvested in our laboratory and were not older than 6 months when employed in the experiments.
Chemicals
Hydrogen Peroxide 30% (Merck®, catalog number: 1072091000)
Agrobacterium liquid growth medium (YEP liquid medium) (see Recipes)
Agrobacterium liquid induction medium (see Recipes)
Histochemical GUS staining solution (see Recipes)
MS solid media (see Recipes)
MgSO4 (Sigma-Aldrich, catalog number: MX0075-1)
Acetosyringone (Sigma-Aldrich, catalog number: D134406)
Murashige & Skoog Basal Medium with Vitamins (PhytoTechnology Laboratories®, catalog number: M519)
Kanamycin sulfate (PhytoTechnology Laboratories®, catalog number: K378)
Rifampicin (Sigma-Aldrich, catalog number: R3501)
Selective antibiotics: Kanamycin, Rifampicin
70% Ethanol
Sucrose (Sigma-Aldrich, catalog number: S0389)
MES (Sigma-Aldrich, catalog number: M3671)
Agar
Yeast extract
NaCl
Peptone
EDTA (pH 8.0) (Sigma-Aldrich, catalog number: E9884)
Sodium phosphate buffer (pH 7.0)
Triton X-100 (Sigma-Aldrich, catalog number: 234729)
Potassium ferricyanide (Sigma-Aldrich, catalog number: 702587)
Potassium ferrocyanide (Sigma-Aldrich, catalog number: P3289)
X-Gluc (Sigma-Aldrich, catalog number: R0852)
Plasticware
Sterile empty 100 × 15 mm Petri plates (VWR International, catalog number: 25384–342)
Sterile disposable 50 ml screw-cap centrifuge tubes (BD, FalconTM, catalog number: 352070)
Plastic pipette tips (20, 200, and 1,000 µl)
Disposable Cuvettes
Sterile filter papers
Equipment
Spectrophotometer
Allegra Benchtop Centrifuge X-12 (Beckman Coulter)
Micro-centrifuge
Laminar flow hood
Eppendorf Research® plus 10, 20, 200, and 1,000 µl
Analytical balance
Top loading electronic balance
pH meter
Vortex mixer
Freezer (- 80°C) (e.g. New Brunswick, model:)
Sterile forceps and scalpel (sterilized by heat treatment using a Bunsen burner)
Sterile inoculating loop
A desiccator attached to a vacuum pump (Brinkman DistiVac)
Growth chamber
Shaker incubator (28°C, 220 rpm)
Incubator 37°C
Fluorescent microscope (Zeiss Observer Z1)
Methods
Rapid germination and seedlings development (performed under sterile conditions)
For germination, seeds were soaked in a 1% hydrogen peroxide solution incubated overnight for 24 hrs at room temperature in the dark. The following day, radicles with hypocotyl are visible (Figure 1).
Transfer germinated seeds into fresh 1% H2O2 solution and further incubate for 3–4 days until cotyledons have fully opened and two early true leaves are visible.
Remove remaining seed coats using sterile scalpel and forceps.
Sterilize seedlings without seed coats by soaking them in 1% hydrogen peroxide for 5 min.
Prior to transformation rinse seedlings in sterile water 3 times to remove remaining hydrogen peroxide.
Preparation of Agrobacterium cells culture (all steps performed under sterile conditions)
Two days before transformation, inoculate 100 ml of YEP (containing 50 µg/mL Kanamycin and 25 µg/mL Rifampicin) with Agrobacterium from glycerol stock and culture at 28°C in an incubator shaker 220 rpm overnight.
Next day centrifuge the Agrobacterium cells culture at 4,000 x g for 15 min at RT.
Remove supernatant and add 3 ml of 10 mM MgSO4, resuspend the Agrobacterium pellet.
Repeat steps 2 and 3.
Centrifuge a third time, remove supernatant.
Resuspend the Agrobacterium pellet in an appropriate volume of induction medium (MS liquid media) so that the final OD600 = 0.6.
Add 100 mM acetosyringone to final concentration 100 µM.
Co-cultivation (all steps performed under sterile conditions)
Place sterilized seedlings in 50 ml Falcon tubes with 30 ml of the Agrobacterium cells suspension (Agrobacterium cells in induction medium supplemented with acetosyringone).
Place the tubes into a sterile vacuum chamber and apply vacuum for 10–20 min.
Transfer seedlings to a sterile filter paper to remove the excess Agrobacterium cell culture.
Transfer the seedlings to 90 mm petri dishes containing MS media (10 seedlings per plate). Spread them evenly on the plate using forceps. Seal the Petri dishes with parafilm.
Co-cultivate the seedlings and the Agrobacterium cells for three days in the dark at 25°C.
After co-cultivation, seedlings can be used directly for GUS staining or can be frozen at −80°C for further analysis e.g. MUG assay, PCR analysis.
Transient expression analysis by GUS assay
After 3-days co-cultivation, rinse seedlings in sterile water.
Place seedlings in 50 ml Falcon tubes with Histochemical GUS staining solution.
Apply vacuum for 10 min.
Incubate overnight at 37°C.
After staining, rinse seedlings in 70% ethanol to remove excessive stain.
Keep seedlings in 70% alcohol for distaining of chlorophyll.
Recipes
YEP liquid medium (1 L)
10 g Yeast extract
10 g Peptone
5 g NaCl
pH 7.0 Autoclave
GM medium (1 L)
4.43 g Murashige & Skoog Basal Medium with Vitamins
10 g Sucrose
500 mg MES
pH 5.7, autoclave
MS sold media (1 L)
4.43 g Murashige & Skoog Basal Medium with Vitamins
8 g Agar
pH 5.7, autoclave
Histochemical GUS stain solution
2 mM Potassium ferrocyanide
2 mM Potassium ferricyanide
100 mM Sodium Phosphate Buffer
500 mg X-Gluc (pre dissolve in dimethyl formamide)
0.1% Triton X-100
1 mM EDTA
Statistical analysis
The data were analyzed by one-way with Tukey’s multiple comparisons test using GraphPad Prism version 8.4.2 for Windows. Data were shown as mean ±SE. A p-value less than 0.05 were considered statistically significant.
Acknowledgments
We thank Natural Sciences and Engineering Research Council of Canada (NSERC) and MITACS for funding our work.
Funding Statement
This work was supported by the Mitacs; Natural Sciences and Engineering Research Council of Canada.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributions
Conceptualization, N.S.Y. and I.K.; Methodology, A.S. and N.S.Y.; Validation, A.S., N.S.Y. and D.G.; Investigation, A.S., N.S.Y. and D.G.; Resources, I.K.; Data Curation, A.S., N.S.Y., D.G. and I.K.; Writing – Original Draft Preparation, N.S.Y; Writing – Review & Editing, A.S., N.S.Y., D.G. and I.K.; Visualization, A.S., N.S.Y. and I.K.; Supervision, N.S.Y. and I.K.; Project Administration, I.K.; Funding Acquisition, I.K.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gaudet D, Yadav NS, Sorokin A, Bilichak A, Kovalchuk I.. Development and optimization of a germination assay and long-term storage for Cannabis sativa pollen. Plants. 2020;9(5):1. doi: 10.3390/plants9050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amar MB. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006;105:(1–2). [DOI] [PubMed] [Google Scholar]
- 3.MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–8. [DOI] [PubMed] [Google Scholar]
- 4.Russo EB, Taming THC. potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahby I, Caba JM, Ligero F. Agrobacterium infection of hemp (Cannabis sativa L.): establishment of hairy root cultures. J Plant Interact. 2013;8:312–320. [Google Scholar]
- 6.Chaohua C, Gonggu Z, Lining Z, Chunsheng G, Qing T, Jianhua C, Xinbo G, Dingxiang P, Jianguang S. A rapid shoot regeneration protocol from the cotyledons of hemp (Cannabis sativa L.). Ind Crops Prod. 2016;83:61–65. [Google Scholar]
- 7.Miché L, Balandreau J. Effects of rice seed surface sterilization with hypochlorite on inoculated Burkholderia vietnamiensis. Appl Environ Microbiol. 2001;67:3046–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandi M, Pervez Z, Alam MS, Islam MS, Mahmud MR. Effect of hydrogen peroxide treatment on health and quality of chilli seed. Int J Plant Pathol. 2017;8:8–13. [Google Scholar]
- 9.Wielgus K, Luwanska A, Lassocinski W, Kaczmarek Z. Estimation of Cannabis sativa L. tissue culture conditions essential for callus induction and plant regeneration. J Nat Fibers. 2008;5:199–207. [Google Scholar]
- 10.Çavusoglu K, Kabar K. Effects of hydrogen peroxide on the germination and early seedling growth of barley under NaCl and high temperature stresses. Eurasia J Biosci. 2010;4:70–79. doi: 10.5053/ejobios.2010.4.0.9. [DOI] [Google Scholar]
- 11.Ma D, Wang H. Transient transformation of Artemisia annua. Bioprotocol. 2015;5(10):e1476. doi: 10.21769/BioProtoc.1476. [DOI] [Google Scholar]
- 12.Feeney M, Punja ZK. Tissue culture and Agrobacterium-mediated transformation of hemp (Cannabis sativa L.). In Vitro Cell Dev Biol Plant. 2013;39:578–585. [Google Scholar]
- 13.Feeney M, Punja ZK. Hemp (Cannabis sativa L.). In: Wang K, editor. Agrobacterium protocols. New York (NY): Springer; 2015. p. 319–329. [Google Scholar]
- 14.Veena, Jiang H, Doerge RW, Gelvin SB. Transfer of T‐DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant J. 2003;35:219–236. [DOI] [PubMed] [Google Scholar]
- 15.Ditt RF, Nester E, Comai L. The plant cell defense and Agrobacterium tumefaciens. FEMS Microbiol Lett. 2005;247:207–213. [DOI] [PubMed] [Google Scholar]
- 16.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. [DOI] [PubMed] [Google Scholar]
- 17.Anand A, Uppalapati SR, Ryu CM, Allen SN, Kang L, Tang Y, Mysore KS. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens.. Plant Physiol. 2008;146:703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tie W, Zhou F, Wang L, Xie W, Chen H, Li X, Lin Y. Reasons for lower transformation efficiency in indica rice using Agrobacterium tumefaciens-mediated transformation: lessons from transformation assays and genome-wide expression profiling. Plant Mol Biol. 2012;78:1–18. [DOI] [PubMed] [Google Scholar]
- 19.Nam J, Matthysse AG, Gelvin SB. Differences in susceptibility of Arabidopsis ecotypes to crown gall disease may result from a deficiency in T-DNA integration. Plant Cell. 1997;9:317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey MA, Boerma HR, Parrott WA. Inheritance of Agrobacterium tumefaciens-induced tumorigenesis of soybean. Crop Sci. 1994;34:514–519. [Google Scholar]
- 21.Lowe BA, Krul WR. Physical, chemical, developmental, and genetic factors that modulate the Agrobacterium-Vitis interaction. Plant Physiol. 1991;96:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feeney M, Punja ZK. The role of Agrobacterium-mediated and other gene-transfer technologies in cannabis research and product development. In: Chandra S, Lata H, ElSohly MA, editor. Cannabis sativa L.-Botany and Biotechnology. Cham, Switzerland: Springer International Publishing; 2017. p. 343–363. [Google Scholar]
- 23.Lubell JD, Brand MH. Foliar sprays of silver thiosulfate produce male flowers on female hemp plants. HortTechnology. 2018;28:743–747. [Google Scholar]


