Abstract
Objective
The present study aimed to perform a systematic review and meta-analysis on the prevalence of one-year hospital readmissions and post-discharge all-cause mortality in recovered COVID-19 patients. Moreover, the country-level prevalence of the outcomes was investigated.
Methods
An extensive search was performed in Medline (PubMed), Embase, Scopus, and Web of Science databases until the end of August 3rd, 2021. A manual search was also performed in Google and Google Scholar search engines. Cohort and cross-sectional studies were included. Two independent reviewers screened the papers, collected data, and assessed the risk of bias and level of evidence. Any disagreement was resolved through discussion.
Results
91 articles were included. 48 studies examined hospital readmissions; nine studies assessed post-discharge all-cause mortality, and 34 studies examined both outcomes. Analyses showed that the prevalence of hospital readmissions during the first 30 days, 90 days, and one-year post-discharge were 8.97% (95% CI: 7.44, 10.50), 9.79% (95% CI: 8.37, 11.24), and 10.34% (95% CI: 8.92, 11.77), respectively. The prevalence of post-discharge all-cause mortality during the 30 days, 90 days and one-year post-discharge was 7.87% (95% CI: 2.78, 12.96), 7.63% (95% CI: 4.73, 10.53) and 7.51% (95% CI, 5.30, 9.72), respectively. 30-day hospital readmissions and post-discharge mortality were 8.97% and 7.87%, respectively. The highest prevalence of hospital readmissions was observed in Germany (15.5%), Greece (15.5%), UK (13.5%), Netherlands (11.7%), China (10.8%), USA (10.0%) and Sweden (9.9%). In addition, the highest prevalence of post-discharge all-cause mortality belonged to Italy (12.7%), the UK (11.8%), and Iran (9.2%). Sensitivity analysis showed that the prevalence of one-year hospital readmissions and post-discharge all-cause mortality in high-quality studies were 10.38% and 4.00%, respectively.
Conclusion
10.34% of recovered COVID-19 patients required hospital readmissions after discharge. Most cases of hospital readmissions and mortality appear to occur within 30 days after discharge. The one-year post-discharge all-cause mortality rate of COVID-19 patients is 7.87%, and the majority of patients' readmission and mortality happens within the first 30 days post-discharge. Therefore, a 30-day follow-up program and patient tracking system for discharged COVID-19 patients seems necessary.
Keywords: Hospital readmission, COVID-19, Re-infection, Mortality
1. Introduction
The coronavirus disease 2019 (COVID-19) has become a global pandemic and the statistics are increasing daily. Numerous mutations in the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have caused an increase in the number of COVID-19 re-infections and related hospital readmissions. There is evidence indicating that these mutations may have reduced the efficacy of current vaccines [1,2]. This re-infection and the decline in immune responses against the SARS-CoV-2 have triggered a re-emergence of the disease in some communities [3,4], bearing in mind that current treatments are not adequately effective in improving the outcome of COVID-19 [[5], [6], [7]].
Re-infection and hospital readmissions are important indicators of controlling the COVID-19 pandemic and healthcare performance quality [8]. Hospital readmissions as a public health concern increase resource utilization and impose an additional burden on the healthcare system [[9], [10], [11]]. At the beginning of the pandemic, studies indicated that recurrence/re-infection of COVID-19 was rare [12,13], but more recent evidence has shown that a significant percentage of patients with COVID-19 develop recurrence of symptoms and require readmission [11,14,15]. The prevalence of hospital readmissions in patients with COVID-19 varies between 1% [16] to 48% [14].
Increasing numbers of recovered COVID-19 patients and their follow-up has shown that that the post-discharge mortality of COVID-19 patients occurs one year after discharge from the index hospitalization. Prevalence of post-discharge mortality of COVID-19 patients was reported between 0% [17] to 37% [18]. However, most studies are single-site researches, and there exists no comprehensive data on post-discharge mortality of recovered COVID-19 patients. There are also significant differences in follow-up time and setting of COVID-19 patients among current studies, making conclusions in this field challenging.
The current systematic review and meta-analysis has investigated the prevalence of hospital readmissions and post-discharge all-cause mortality in follow-up periods of 30 days, 90 days, and one year, to provide comprehensive figures. As a secondary aim, the prevalence of country-level hospital readmissions and post-discharge all-cause mortality has been reported.
2. Method
2.1. Study design
The present study is a systematic review and meta-analysis of observational studies. The protocol of the present study was designed based on Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guideline [19]. The protocol of the current review was not registered and publicly accessed. In all steps, two independent reviewers screened the papers, collected data, and assessed the risk of bias and level of evidence. Any disagreement was resolved through discussion. The agreement rate was 92.3% to 100% for each level of screening and data extraction.
2.2. Eligibility criteria
In the present study, cohort and cross-sectional studies on the prevalence of hospital readmissions and post-discharge all-cause mortality after recovery from COVID-19 were included. Case-control studies were excluded since the nature of sampling in case-control studies overestimates the prevalence of hospital readmissions and post-discharge all-cause mortality. Case reports, duplicate reports, reviews, and pediatric studies were also excluded. Presenting combined data of in-hospital and post-discharge outcomes without any stratification and reporting data on patients discharged from the emergency department without hospitalization in index admission (first admission) were other exclusion criteria. In addition, studies were excluded if they did not assess hospital readmissions or post-discharge all-cause mortality and did not report the total sample size of their COVID-19 patients.
2.3. Search strategy
An extensive search was performed on PubMed, Embase, Scopus, and Web of Science until the end of August 3rd,2021, without time or language limitations. In addition, a manual search was performed on Google and Google Scholar search engines. Since a significant number of COVID-19 papers were accessible as preprints, the manual search was performed cautiously to include relevant preprint articles. The search term is reported in Supplementary material 1.
2.4. Screening and data collection
Records from systematic and manual searches were gathered into EndNote X8.0 software (Clarivate Analytics, Philadelphia, PA, USA), and duplicates were removed. In a two-step process, related articles were selected based on the inclusion and exclusion criteria. In the first step, the titles and abstracts were reviewed and possibly related articles were identified. In the next step, the full texts of the articles were evaluated and related papers were identified and included in the present study. Collected data include study characteristics (name of the first author, year of publication, country), study design (retrospective or prospective), patients' settings, COVID-19 diagnostic criteria, discharge criteria, sample size, age, and gender distribution, underlying diseases, and follow up duration. Underlying disease or infection was defined as the presence of cirrhosis and liver injury, myocardial infarction, cardiovascular disease, autoimmune diseases, cancer, fungal infections, HIV infection, rheumatic and musculoskeletal disease, and acute kidney injury.
2.5. Outcome
The outcomes of interest were hospital readmissions and post-discharge all-cause mortality. Hospital readmissions were defined as the readmission of recovered COVID-19 patients who had previously been hospitalized for COVID-19. Post-discharge all-cause mortality was also considered as all post-discharge deaths in recovered COVID-19 patients.
2.6. Risk of bias assessment
The risk of bias was assessed using National, Heart, Lung, and Blood Institute (NHLBI) tools for cohort and cross-sectional studies [20]. NHLBI risk of bias tools contains 14 signaling questions for the assessment of the quality of included studies (Supplementary Table 1). According to the observational nature of the included studies, participation rate less than 50% (item 3), assessment of exposure prior to outcome assessment (item 6), insufficient timeframe for outcome assessment (item 7), not clear and valid measurement of exposure (item 9) and outcomes (item 11) and more than 20% loss to follow-up (item 13) were defined as fatal errors.
Since most of the studies collected their data from registries, the unblind outcome assessment (item 12) did not have a considerable effect on the quality of the studies. Therefore, unblinded outcome assessment was not considered a fatal error. Sample size calculation was not reported in any of the included studies. Therefore, if the sample size of a study was lower than 100 patients, item 5 was considered as high risk. In addition, item 14 of the NHLBI risk of bias tool was not applicable for this review.
The overall risk of bias was rated as “high” if any concern (high risk; NR or CD) was presented in items 3, 6, 7, 9, 11, and 13 (fatal error). The overall risk of bias was rated as “some concern” if there were no fatal errors and there were a concern (high risk, NR, or CD) in at least two items [21].
2.7. Level of evidence
The level of evidence was determined based on the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework. The GRADE framework classifies the level of evidence for each outcome based on the risk of bias, imprecision, inconsistency, indirectness, and publication bias [22].
2.8. Statistical analyses
Data were recorded as total sample size and number of events (frequency) and were analyzed in STATA 17.0 statistical program (Stata Corp, College Station, TX, USA). Since considerable heterogeneity was expected among the included studies, it was decided to use a random effect model for the meta-analyses. Follow-up time varied between studies, and therefore, analyses were stratified based on follow-up time. The studies were divided into three groups: 30-day follow-up (follow-up between 10 and 30 days after discharge), 90-day follow-up (follow-up between 10 and 90 days), and one-year follow-up (follow-up between 10 days to 365 days).
Heterogeneity between the studies was assessed using I2 statistics and the chi-square test. I2 above 50% was defined as the presence of obvious heterogeneity. In cases of heterogeneity, the possible sources of heterogeneity were investigated using subgroup analysis. Since one-year follow-up after recovery included all 30-day and 90-day follow-up data, subgroup analysis was performed on one-year outcome after discharge. The country type was defined in two categories as developed and developing countries according to the World Bank definition; Developed countries were defined as countries with high-income economies while developing countries were defined as those with low- and middle-income economies [23].
Meta-regression was performed to investigate the relationship between the mean age of patients and the outcomes. For this purpose, the mean age of patients in each study was entered in the analyses as independent variables, and the hospital readmissions and post-discharge all-cause mortality were considered as dependent variables. Sensitivity analyses were performed according to the quality of the included studies and based on the country-level prevalence of hospital readmissions and post-discharge all-cause mortality. For this purpose, only studies on all COVID-19 patients were included and other settings were excluded. Finally, publication bias was investigated using Egger's test and funnel plots.
3. Results
3.1. Article screening process
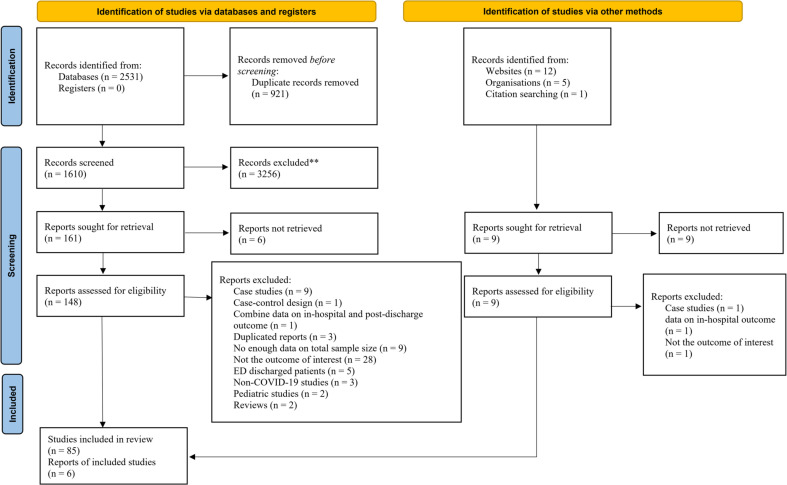
The systematic search yielded 2531 studies, which included 1610 non-duplicated studies. In the manual search of gray literature and preprints, 18 potentially related papers were included. After reviewing the titles and abstracts of the articles, 157 peer-reviewed papers or preprinted manuscripts were reviewed and a total of 91 articles were entered into the present meta-analysis [8,[14], [15], [16], [17], [18],[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108]]. The reasons for excluding articles are shown in Fig. 1 .
Fig. 1.
PRISMA flow diagram for present studies.
3.2. Summary of eligible studies
There were 28 prospective, 61 retrospective, and 2 ambidirectional cohorts. There were 32 studies on the USA population, 18 studies on the Chinese population, 13 studies on the Spanish population, and seven studies on the UK population. Also, three studies were conducted in Iran. These studies included 283,468 patients (51.19% male). The mean age of patients enrolled in the studies ranged from 36.7 to 88.5 years.
The setting of the patients in 76 studies were all COVID-19 patients regardless of the characteristics of included patients. Three studies were performed on the elderly population and two studies were performed on patients with cardiovascular disease. The population of other studies included patients with cirrhosis and liver injury, autoimmune diseases, cancer, fungal infections, human immunodeficiency virus (HIV), rheumatic and musculoskeletal disorders, acute kidney injury, non-severe COVID-19 patients, corticosteroids treated patients, and empiric antibiotics treated patients.
The COVID-19 diagnostic test was Reverse transcription-polymerase chain reaction (RT-PCR) in 63 studies, while 16 studies did not report the diagnostic test. The method of identifying COIVD-19 was mixed in 12 studies. In the mixed-method approach, the diagnostic method was RT-PCR or other diagnostic methods including imaging procedures, serological tests, or clinical symptoms. The discharge criteria were not reported in 69 studies. In 18 studies, discharge criteria included two consecutive negative RT-PCR and clinical improvement. Four studies had discharged patients only based on clinical improvement.
Follow-up time ranged from 10 to 365 days. 48 studies examined hospital readmissions, nine studies examined post-discharge all-cause mortality, and 34 studies examined both outcomes. Table 1 summarizes the characteristics of the studies.
Table 1.
Summary characteristics of included studies.
| Study | Study design | Setting of patients | COVID-19 diagnosis | Discharge criteria | Sample size | Male | Mean age (years) | FU (days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| An, 2020; China [22] | PCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 242 | 116 | 41.3 | 14 | Readmission |
| Atalla, 2021; USA [15] | RCS | All COVID-19 patients | RT-PCR | Clinical improvement | 279 | 191 | 61.3 | 30 | Readmission |
| Ayoubkhani, 2021; UK [24] | RCS | All COVID-19 patients | NR | NR | 47,780 | 26,279 | 65 | 253 | Readmission, post-discharge mortality |
| Bajaj, 2021; USA [14] | PCS | All COVID-19 patients, cirrhosis and liver injury | NR | NR | 122 | 46 | 60.6 | 90 | Readmission, post-discharge mortality |
| Banerjee, 2021; USA [25] | PCS | All COVID-19 patients | RT-PCR | Stable patients with improving clinical trajectory | 621 | 404 | 52.5 | 30 | Readmission, post-discharge mortality |
| Barreto, 2021; Brazil [23] | RCS | All COVID-19 patients | RT-PCR or CT or IgM/IgG | NR | 602 | 247 | 51.8 | 140 | Readmission |
| Bowles, 2021; USA [26] | RCS | All COVID-19 patients | RT-PCR | NR | 1409 | 718 | 67 | 180 | Readmission, post-discharge mortality |
| Cao, 2020; China [27] | RCS | All COVID-19 patients | RT-PCR | NR | 108 | NR | NR | 30 | Readmission |
| Carrillo Garcia, 2021; Spain [28] | PCS | Elderly | RT-PCR or clinical or imaging or laboratory | NR | 165 | 114 | 88.5 | 90 | Readmission, post-discharge mortality |
| Chai, 2021; China [29] | PCS | All COVID-19 patients; cancer | RT-PCR | NR | 588 | 328 | 64.7 | 365 | Post-discharge mortality |
| Chaudhry, 2021; UK [30] | RCS | Corticosteroids treated patients | NR | NR | 196 | 63 | 58.7 | 10 | Readmission |
| Chen J, 2020; China [31] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 1087 | 452 | 60.2 | 52 | Readmission |
| Chen SL, 2020; China [32] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 1282 | 628 | 44 | 28 | Readmission |
| Choi, 2021; USA [33] | RCS | All COVID-19 patients | RT-PCR | NR | 1008 | NR | NR | 30 | Readmission |
| Chopra, 2020; USA [34] | RCS | All COVID-19 patients | NR | NR | 1250 | 648 | 61.3 | 60 | Post-discharge mortality |
| Connolly, 2021; Ireland [35] | PCS | All COVID-19 patients | NR | NR | 502 | 179 | 40 | 12 | Readmission |
| Divanoglou, 2021; Sweden [36] | AmbidiRCSectional | All COVID-19 patients | Laboratory assessment | NR | 433 | 246 | 61.3 | 135 | Post-discharge mortality |
| Donnelly, 2021; USA [37] | RCS | All COVID-19 patients | NR | NR | 1775 | 1688 | 69.8 | 60 | Readmission, post-discharge mortality |
| Frontera, 2021; USA [38] | PCS | All COVID-19 patients | RT-PCR | NR | 380 | 248 | 67.5 | 180 | Readmission, post-discharge mortality |
| Gabriel, 2021; Spain [39] | PCS | All COVID-19 patients | NR | NR | 102 | 48 | 46.2 | 15 | Readmission |
| García Abellán, 2021; Spain [40] | PCS | All COVID-19 patients | RT-PCR | NR | 146 | 88 | 65 | 180 | Readmission, post-discharge mortality |
| Gąsior, 2021; Poland [41] | RCS | MI and cardiovascular | NR | NR | 2988 | 1352 | 69 | 180 | Post-discharge mortality |
| Giannis, 2021; Greece [42] | PCS | All COVID-19 patients | NR | NR | 4906 | 2633 | 61.7 | 92 | Readmission, post-discharge mortality |
| Gordon, 2020; USA [43] | PCS | All COVID-19 patients | RT-PCR or clinical or imaging suspected | NR | 1227 | 674 | 54 | 21 | Readmission |
| Guarin, 2021; USA [44] | RCS | All COVID-19 patients | RT-PCR | NR | 275 | 142 | 64.69 | 180 | Readmission |
| Gudipati, 2020; USA [45] | RCS | All COVID-19 patients | NR | NR | 266 | 125 | 61 | 30 | Readmission |
| Gunster, 2021; Germany [46] | RCS | All COVID-19 patients | RT-PCR | NR | 6518 | 4641 | 68.6 | 180 | Readmission, post-discharge mortality |
| Gutiérrez, 2021; Spain [47] | RCS | All COVID-19 patients, autoimmune Diseases | RT-PCR | NR | 13,940 | 7749 | 67.3 | 30 | Readmission, post-discharge mortality |
| Gwin, 2021; USA [48] | RCS | All COVID-19 patients | RT-PCR | NR | 151 | 88 | 59.6 | 30 | Readmission |
| Hasan, 2021; Bangladesh [17] | PCS | All COVID-19 patients | RT-PCR | NR | 238 | 159 | 61.5 | 30 | Readmission, post-discharge mortality |
| Herc, 2020; USA [49] | RCS | Fungal Infections | NR | NR | 31 | 17 | 66 | 30 | Readmission |
| Hernández-Biette, 2020; Spain [50] | PCS | Non-Severe COVID | RT-PCR | NR | 74 | 35 | 54.6 | 14 | Readmission, post-discharge mortality |
| Holloway, 2021; UK [51] | PCS | All COVID-19 patients | RT-PCR | NR | 141 | NR | NR | 30 | Readmission |
| Huang C, 2021; China [52] | ACS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 1733 | 897 | 56.3 | 199 | Readmission, post-discharge mortality |
| Huang CW, 2021; USA [53] | RCS | All COVID-19 patients | RT-PCR | NR | 2180 | 1238 | 54.7 | 30 | Readmission, post-discharge mortality |
| Islam, 2021; UK [54] | RCS | All COVID-19 patients | RT-PCR | NR | 403 | 211 | 66 | 60 | Readmission, post-discharge mortality |
| Jain, 2020; USA [55] | PCS | All COVID-19 patients | NR | NR | 18 | 10 | 65.3 | 90 | Readmission |
| Jalilian Khave, 2021; Iran [56] | PCS | All COVID-19 patients | RT-PCR | NR | 577 | 449 | 50.1 | 14 | Readmission |
| Jeon, 2020; South Korea [57] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 7590 | 3095 | 47 | 180 | Readmission |
| Kingery, 2021; USA [58] | RCS | All COVID-19 patients | RT-PCR | NR | 1344 | 746 | 60.3 | 30 | Readmission, post-discharge mortality |
| Kirkegaard, 2021; Spain [59] | RCS | All COVID-19 patients | RT-PCR | NR | 629 | 318 | 60.28 | 60 | Readmission, post-discharge mortality |
| Lavery, 2020; USA [60] | RCS | All COVID-19 patients | RT-PCR | NR | 106,543 | 54,080 | 60 | 60 | Readmission |
| Lee, 2020; USA [61] | RCS | HIV patients | RT-PCR | NR | 72 | 44 | 61.3 | 30 | Readmission |
| Leijte, 2020; Netherlands [62] | RCS | All COVID-19 patients | RT-PCR | NR | 596 | 469 | 70 | 90 | Readmission, post-discharge mortality |
| Leon, 2021; Spain [63] | PCS | Rheumatic and musculoskeletal | RT-PCR | NR | 105 | 38 | 66.8 | 210 | Readmission, post-discharge mortality |
| Li, 2020; China [64] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 85 | 55 | 48 | 60 | Readmission |
| Luo, 2020; China [65] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 745 | 424 | 42.4 | 14 | Readmission |
| Maestre-Muñiz, 2021; Spain [66] | RCS | All COVID-19 patients | RT-PCR | NR | 266 | 201 | 71.5 | 365 | Readmission, post-discharge mortality |
| Medler, 2020; USA [67] | RCS | All COVID-19 patients | RT-PCR | NR | 337 | NR | 63.6 | 14 | Readmission |
| Medranda, 2021; USA [68] | RCS | MI and cardiovascular | RT-PCR | NR | 92 | 55 | 63.7 | 30, 90, 180 | Readmission, post-discharge mortality |
| Meije, 2021; Spain [69] | PCS | All COVID-19 patients | RT-PCR | NR | 323 | 171 | 68.8 | 45, 210 | Readmission, post-discharge mortality |
| Menges, 2021; Switzerland [70] | PCS | All COVID-19 patients | RT-PCR | NR | 81 | 43 | 59 | 180 | Readmission |
| Mooney, 2021; UK [71] | RCS | Elderly | RT-PCR | NR | 274 | 161 | 67 | 30 | Readmission |
| Navvas, 2021; UK [72] | RCS | All COVID-19 patients | RT-PCR | NR | 402 | NR | NR | 30 | Post-discharge mortality |
| Nematshahi, 2021; Iran [73] | PCS | All COVID-19 patients | RT-PCR or imaging | NR | 416 | 228 | 58.8 | 180 | Readmission |
| Pan, 2021; China [16] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 1350 | NR | NR | 15 | Readmission |
| Parra, 2020; Spain [74] | RCS | All COVID-19 patients | RT-PCR and imaging | NR | 1368 | 872 | 64.4 | 21 | Readmission |
| Pettit, 2021; USA [75] | RCS | Patients on empiric CABP antibiotics | RT-PCR | NR | 246 | 116 | 60 | 30 | Readmission |
| Pourhoseingholi, 2021; Iran [76] | RCS | All COVID-19 patients | CT scan | NR | 1053 | 773 | 53 | 365 | Readmission, post-discharge mortality |
| Qiao,2020; China [77] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 15 | 8 | 36.7 | 30 | Readmission |
| Quilliot, 2021; France [78] | PCS | All COVID-19 patients | RT-PCR or CT | NR | 296 | 156 | 59.8 | 30 | Readmission, post-discharge mortality |
| Ramos Martínez, 2021; Spain [79] | RCS | All COVID-19 patients | RT-PCR | NR | 7137 | 4022 | 65.4 | 30 | Readmission, post-discharge mortality |
| Reyes Gill, 2021; USA [80] | RCS | All COVID-19 patients | RT-PCR | NR | 150 | 81 | 58.1 | 30 | Readmission, post-discharge mortality |
| Richardson, 2020; USA [81] | PCS | All COVID-19 patients | RT-PCR | NR | 2081 | 1162 | 63.3 | 10 | Readmission |
| Rodriguez, 2021; USA [82] | PCS | All COVID-19 patients | RT-PCR | NR | 3111 | 1250 | 60.9 | 30 | Readmission |
| Roig-Marín, 2021; Spain [83] | RCS | Elderly | RT-PCR | NR | 221 | 152 | 81.6 | 365 | Post-discharge mortality |
| Romero-Duarte, 2021; Spain [84] | RCS | All COVID-19 patients | RT-PCR | NR | 797 | 428 | 63 | 180 | Readmission, post-discharge mortality |
| Saab, 2021; USA [85] | RCS | All COVID-19 patients | RT-PCR | NR | 99 | 64 | 59.8 | 86 | Readmission |
| Shallal, 2020; USA [86] | RCS | All COVID-19 patients | RT-PCR | NR | 585 | 302 | 59.8 | 30 | Readmission |
| Siddiqui, 2021; USA [87] | RCS | All COVID-19 patients; cirrhosis and liver injury | RT-PCR | NR | 11,534 | 972 | 63.8 | 30 | Readmission |
| Somani, 2020; USA [88] | RCS | All COVID-19 patients | RT-PCR | NR | 2864 | 1663 | 65.7 | 14 | Readmission |
| Spence, 2021; UK [89] | RCS | All COVID-19 patients | NR | NR | 106 | 54 | 78.8 | 30, 60, 90 | Readmission, post-discharge mortality |
| Spinicci, 2021; Italy [90] | RCS | All COVID-19 patients | NR | NR | 107 | 59 | 67.3 | 56 | Readmission, post-discharge mortality |
| Stockman, 2021; Germany [91] | RCS | AKI | NR | NR | 37 | NR | 64.5 | 150 | Post-discharge mortality |
| Suárez-Robles, 2020; France [92] | PCS | All COVID-19 patients | RT-PCR | NR | 134 | 62 | 58.53 | 90 | Readmission, post-discharge mortality |
| Tian, 2020; China [93] | PCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 147 | NR | NR | 47 | Readmission |
| Todt, 2021; Brazil [94] | RCS | All COVID-19 patients | RT-PCR | NR | 251 | 150 | 53.6 | 90 | Readmission, post-discharge mortality |
| Uyaroglu, 2021; Turkey [8] | RCS | All COVID-19 patients | RT-PCR or symptoms | Clinical improvement | 154 | 77 | 44.5 | 30 | Readmission |
| van den Borst,2021; Netherlands [95] | PCS | All COVID-19 patients | RT-PCR or symptoms | NR | 98 | 74 | 59 | 90 | Post-discharge mortality |
| Venturelli, 2021; Italy [96] | RCS | All COVID-19 patients | RT-PCR or IgM/IgG or symptoms | Two consecutive negative RT-PCR + clinical improvement | 767 | 512 | 63 | 90 | Post-discharge mortality |
| Verna, 2021; USA [97] | RCS | All COVID-19 patients | Laboratory assessment | NR | 29,659 | 14,965 | 63.5 | 30 | Readmission |
| Wang, 2020; China [98] | PCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 94 | 59 | 49 | 30 | Readmission |
| Weber, 2021; USA [18] | PCS | All COVID-19 patients | NR | NR | 408 | 243 | 62.3 | 180 | Readmission, post-discharge mortality |
| Wu, 2021; China [99] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 132 | 165 | 40.7 | 180 | Readmission, post-discharge mortality |
| Yan, 2020; China [100] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 272 | 154 | 44.3 | 14 | Readmission |
| Yang, 2020; China [101] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 479 | 224 | 42.8 | 90 | Readmission |
| Ye S, 2021; USA [102] | RCS | All COVID-19 patients | RT-PCR | symptoms improvement | 409 | 245 | 57.3 | 14 | Readmission |
| Ye X, 2021; China [103] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 141 | 73 | 45 | 30 | Readmission |
| Yeo, 2021; USA [104] | RCS | All COVID-19 patients | RT-PCR | NR | 1062 | 632 | 56.5 | 30 | Readmission |
| Yuan, 2020; China [105] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 172 | NR | NR | 14 | Readmission |
| Zheng, 2020; China [106] | RCS | All COVID-19 patients | RT-PCR | Two consecutive negative RT-PCR + clinical improvement | 289 | 128 | 48.3 | 14 | Readmission |
COVID-19: Coronavirus disease 2019; CT: Computed tomography scan; FU: Follow up duration; NR: Not reported; PCS: Prospective cohort study; RCS: Retrospective cohort study; RT-PCR: Reverse transcriptase-polymerase chain reaction.
3.3. Hospital readmission rate of recovered COVID-19 patients after hospital discharge
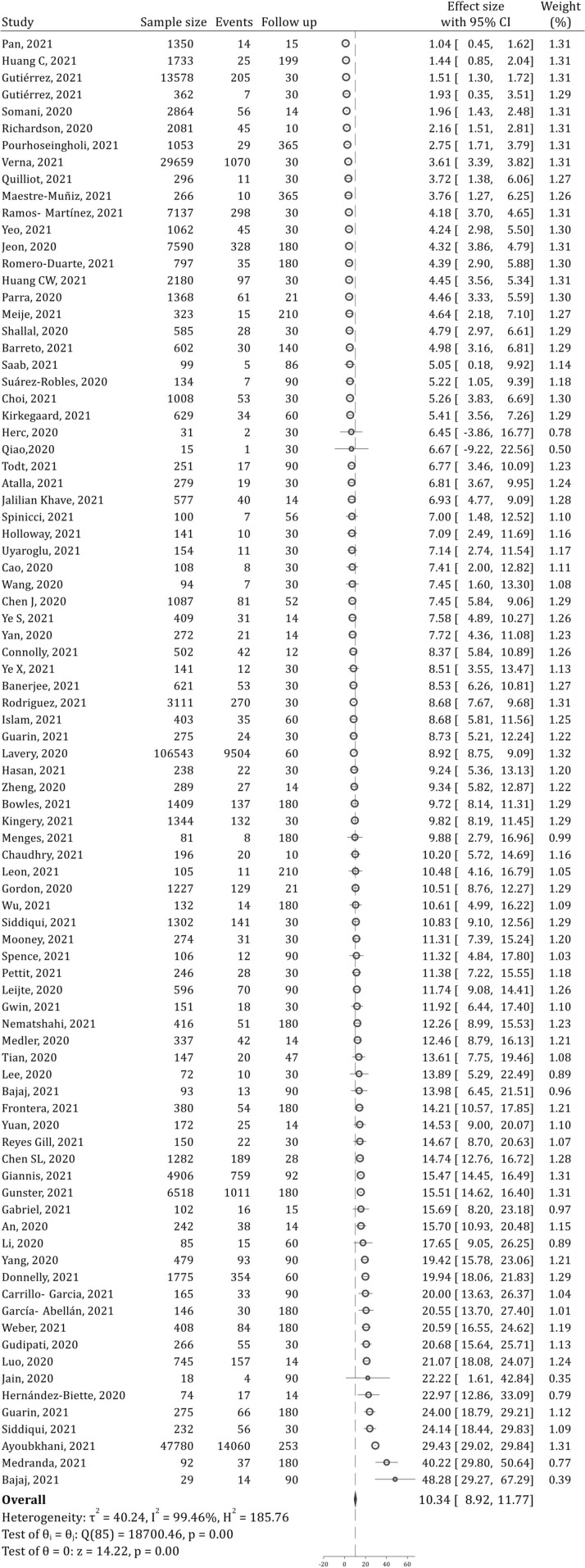
82 studies examined hospital readmissions after patient recovery. These studies contained data from 266,677 patients. Analyses showed that the prevalence of one-year hospital readmissions was 10.34% (95% CI: 8.92, 11.77; 99.46%; I2 = 99.46%) (Fig. 2 ). Hospital readmissions during the first 30 and 90 days after discharge were 8.97% (95% CI: 7.44, 10.50; I2 = 99.04%) and 9.79% (95% CI: 8.37, 11.24; I2 = 99.33%), respectively (Supplementary Figs. 1 and 2). As can be seen, most hospital readmissions occur within the first 30 days.
Fig. 2.
Forest plot for prevalence of hospital readmission during one-year after recovery of COVID-19 patients. CI: Confidence interval.
Subgroup analysis on one-year follow-up showed that differences in country type and patient setting may be potential sources of heterogeneity since stratification of analyses according to these factors led to a decrease in heterogeneity. The hospital readmission rate was 10.68% in developed countries and 6.88% in developing countries. Also, the rate of hospital readmissions in elderly patients with COVID-19 (15.28%) and COVID-19 patients with underlying disease (19.63%) was higher than in other groups (Table 2 ).
Table 2.
Subgroup analysis for determination of source of heterogeneity in assessment of 1-years hospital readmission.
| Variable | Number of analyses⁎ | Prevalence (95% CI) | I2 (p value) |
|---|---|---|---|
| Country type | |||
| Developed | 79 | 10.68 (9.14, 12.22) | 99.53 (<0.0001) |
| Developing | 7 | 6.88 (4.52, 9.24) | 85.48 (<0.0001) |
| Study design | |||
| Prospective | 27 | 11.52 (9.26, 13.79) | 95.42 (<0.0001) |
| Retrospective | 58 | 9.93 (8.15, 11.71) | 99.63 (<0.0001) |
| Ambidirectional | 1 | 1.44 (0.85, 2.04) | NA |
| Setting of patients | |||
| All COVID-19 patients | 77 | 9.71 (8.35, 11.06) | 99.40 (<0.0001) |
| Elderly | 2 | 15.28 (6.80, 23.76) | 80.68 (0.023) |
| Presence of underlying disease or infection⁎⁎ | 7 | 19.63 (7.41, 31.83) | 96.22 (<0.0001) |
| COVID-19 diagnostic criteria | |||
| RT-PCR | 63 | 9.52 (8.04, 11.00) | 99.26 (<0.0001) |
| Mixed criteria⁎⁎⁎ | 9 | 7.22 (4.02, 10.43) | 98.14 (<0.0001) |
| NR | 14 | 16.58 (12.24, 20.92) | 97.89 (<0.0001) |
| Risk of bias score | |||
| Low risk | 36 | 10.38 (8.52, 12.24) | 98.30 (<0.0001) |
| Some concern | 3 | 17.16 (4.67, 38.98) | 96.99 (<0.0001) |
| High risk | 47 | 10.01 (7.99, 12.02) | 99.64 (<0.0001) |
NR: Not reported; CI: Confidence interval.
Since some studies stratified their data according to different subgroups (such as according to underlying disease) the number of analyses is higher than number of studies.
Underlying disease or infection included cirrhosis and liver injury, myocardial infarction, cardiovascular disease, autoimmune diseases, cancer, fungal infections, HIV infection, rheumatic and musculoskeletal and acute kidney injury.
Mixed criteria: RT-PCR or laboratory or clinical or imaging.
3.4. Post-discharge all-cause mortality of COVID-19 patients
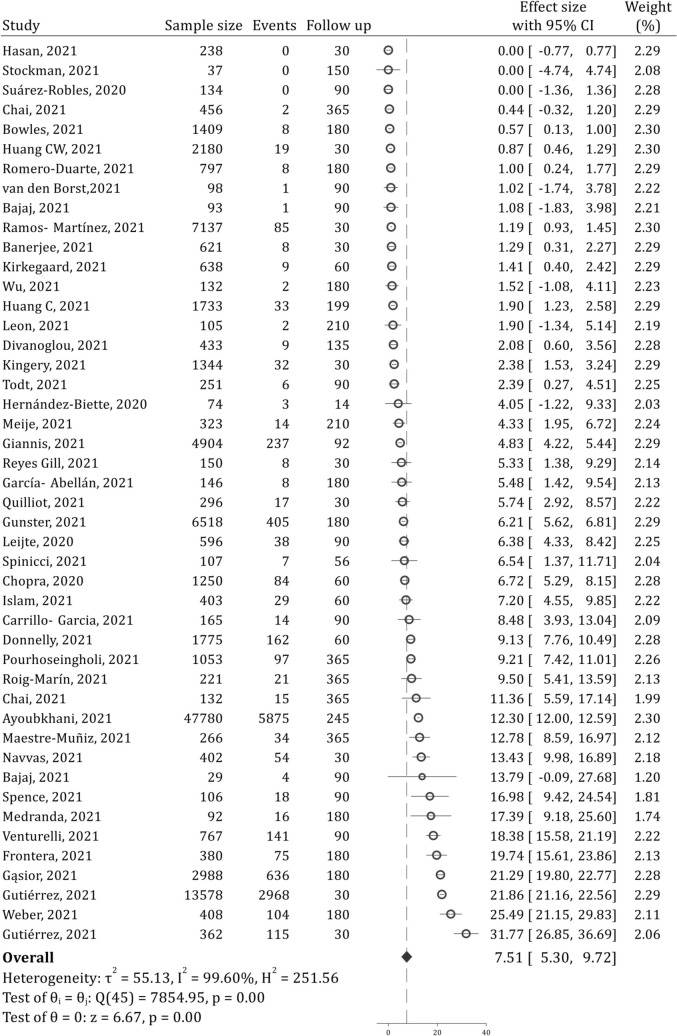
43 studies examined post-discharge all-cause mortality of COVID-19 patients. These studies contained data from 103,107 patients. Analyses showed that the prevalence of all-cause mortality during the one year after discharge was 7.51% (95% CI: 5.30, 9.72; 99.60%; I2 = 99.60%) (Fig. 3 ). All-cause mortality during the first 30 and 90 days were 7.87% (95% CI: 2.78, 12.96; I2 = 99.79%) and 7.63% (95% CI: 4.73, 10.53; I2 = 99.46%), respectively (Supplementary figs. 3 and 4). Most post-discharge deaths occur within the first 30 days.
Fig. 3.
Post-discharge all-cause mortality of COVID-19 patients during one-year after recovery. CI: Confidence interval.
Subgroup analysis showed that diversity in the characteristics of the patients may be a possible source of heterogeneity since stratification of the analyses based on the characteristics of patients led to a decrease in heterogeneity. The post-discharge all-cause mortality rate in COVID-19 patients with underlying disease (12.03%) was almost 100% higher than that of all COVID-19 patients regardless of underlying disease (6.59%) (Table 3 ).
Table 3.
Subgroup analysis for determination of source of heterogeneity in assessment of post-discharge all-cause mortality.
| Variable | Number of analyses⁎ | Prevalence (95% CI) | I2 (p value) |
|---|---|---|---|
| Country type | |||
| Developed | 43 | 7.78 (5.46, 10.11) | 99.63 (<0.0001) |
| Developing | 3 | 3.84 (−1.60, 9.27) | 97.39 (<0.0001) |
| Study design | |||
| Prospective | 17 | 6.01 (2.58, 9.44) | 98.76 (<0.0001) |
| Retrospective | 27 | 8.84 (5.85, 11.84) | 99.73 (<0.0001) |
| Ambidirectional | 2 | 1.93 (1.32, 2.55) | 0.00 (0.833) |
| Setting of patients | |||
| All COVID-19 patients | 37 | 6.59 (4.48, 8.74) | 99.57 (<0.0001) |
| Elderly | 2 | 9.05 (6.00, 12.09) | 0.00 (0.754) |
| Presence of underlying disease or infection⁎⁎ | 7 | 12.03 (3.03, 21.03) | 97.86 (<0.0001) |
| COVID-19 diagnostic criteria | |||
| RT-PCR | 29 | 6.44 (3.68, 9.20) | 99.64 (<0.0001) |
| Mixed criteria⁎⁎⁎ | 6 | 7.45 (2.39, 12.50) | 96.33 (<0.0001) |
| NR | 11 | 10.54 (5.62, 15.46) | 99.43 (<0.0001) |
| Risk of bias score | |||
| Low risk | 19 | 4.00 (2.17, 5.83) | 99.06 (<0.0001) |
| Some concern | 2 | 9.17 (−5.98, 24.32) | 91.54 (<0.0001) |
| High risk | 25 | 10.00 (6.55, 13.46) | 99.36 (<0.0001) |
NR: Not reported; CI: Confidence interval.
Since some studies stratified their data according to different subgroups (such as according to underlying disease) the number of analyses is higher than number of studies.
Underlying disease or infection included cirrhosis and liver injury, myocardial infarction, cardiovascular disease, autoimmune diseases, cancer, fungal infections, HIV patients, rheumatic and musculoskeletal and acute kidney injury.
Mixed criteria: RT-PCR or laboratory or clinical or imaging.
3.5. Meta-regression
Meta-regression was performed to investigate the relationship between the mean age of patients and the outcomes. The findings showed that the mean age of COVID-19 patients at the time of admission was not related to the prevalence of hospital readmissions (meta-regression coefficient = 0.038; p = 0.656). However, the prevalence of post-discharge all-cause mortality increased with age (meta-regression coefficient = 0.360; p = 0.009) (Supplementary Fig. 5).
3.6. Sensitivity analysis
3.6.1. Quality of included studies
The risk of bias was high in 50 studies, some concern in four studies, and low in 37 papers (Supplementary Table 1). The prevalence of one-year hospital readmission in low-risk studies (high-quality studies) was 10.38% (Table 2), while the prevalence of 30-day hospital readmission in low-risk studies was 9.98%.
The prevalence of post-discharge all-cause mortality was 4.00% in low-risk studies (Table 3). Moreover, 30-day post-discharge all-cause mortality in low-risk studies was 3.24%.
3.6.2. Country-level differences of hospital readmissions and post-discharge mortality of COVID-19 patients
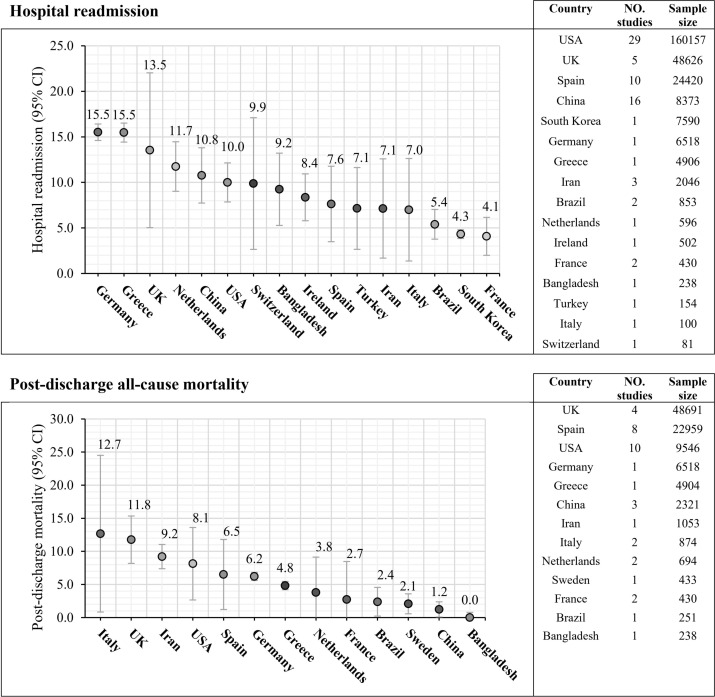
Sensitivity analysis showed that the highest prevalence of one-year hospital readmissions was observed in Germany (15.5%), Greece (15.5%), the UK (13.5%), Netherlands (11.7%), China (10.8%), USA (10.0%), and Sweden (9.9%). While the lowest hospital readmissions rate was seen in Brazil (5.4%), South Korea (4.3%), and France (4.1%).
The highest prevalence of one-year post-discharge all-cause mortality belonged to Italy (12.7%), the UK (11.8%), and Iran (9.2%). The lowest post-discharge all-cause mortality rates were observed in the Netherlands (3.8%), France (2.7%), Brazil (3.4%), Brazil (2.4%), Sweden (2.1%), China (1.2%), and Bangladesh (0.0%) (Fig. 4 and Table 4 ).
Fig. 4.
Country-level hospital readmission and post-discharge mortality of COVID-19 patients.
Table 4.
Country level hospital readmission and post-discharge mortality of COVID-19 patients
| Country | Number of studies | Number of patients | Prevalence | 95% confidence interval |
|---|---|---|---|---|
| Hospital readmission | ||||
| Germany | 1 | 6518 | 15.5 | 14.6, 16.4 |
| Greece | 1 | 4906 | 15.5 | 14.4, 16.5 |
| UK | 5 | 48,626 | 13.5 | 5.2, 21.9 |
| Netherlands | 1 | 596 | 11.7 | 9.1, 14.4 |
| China | 16 | 8373 | 10.8 | 7.8, 13.7 |
| USA | 29 | 160,157 | 10.0 | 7.9, 12.1 |
| Switzerland | 1 | 81 | 9.9 | 2.8, 17.0 |
| Bangladesh | 1 | 238 | 9.2 | 5.4, 13.1 |
| Ireland | 1 | 502 | 8.4 | 5.8, 10.9 |
| Spain | 10 | 24,420 | 7.6 | 3.6, 11.7 |
| Turkey | 1 | 154 | 7.1 | 2.7, 11.5 |
| Iran | 3 | 2046 | 7.1 | 1.8, 12.5 |
| Italy | 1 | 100 | 7.0 | 1.5, 12.5 |
| Brazil | 2 | 853 | 5.4 | 3.8, 7.0 |
| South Korea | 1 | 7590 | 4.3 | 3.9, 4.8 |
| France | 2 | 430 | 4.1 | 2.0, 6.1 |
| Post-discharge all-cause mortality | ||||
| Italy | 2 | 874 | 12.7 | 1.1, 24.3 |
| UK | 4 | 48,691 | 11.8 | 8.3, 15.3 |
| Iran | 1 | 1053 | 9.2 | 7.4, 11.0 |
| USA | 10 | 9546 | 8.1 | 2.8, 13.5 |
| Spain | 8 | 22,959 | 6.5 | 1.3, 11.7 |
| Germany | 1 | 6518 | 6.2 | 5.6, 6.8 |
| Greece | 1 | 4904 | 4.8 | 4.2, 5.4 |
| Netherlands | 2 | 694 | 3.8 | −1.5, 9.0 |
| France | 2 | 430 | 2.7 | −2.9, 8.4 |
| Brazil | 1 | 251 | 2.4 | 0.3, 4.5 |
| Sweden | 1 | 433 | 2.1 | 0.6, 3.6 |
| China | 3 | 2321 | 1.2 | 0.1, 2.4 |
| Bangladesh | 1 | 238 | 0.0 | −0.8, 0.8 |
3.7. Publication bias and level of evidence
There was no evidence of publication bias regarding the hospital readmissions (p = 0.473) and post-discharge all-cause mortality (p = 0.435) assessments (Supplementary Fig. 6).
The overall level of evidence was very low in reporting the hospital readmissions and post-discharge all-cause mortality. According to the GRADE framework, the level of evidence for observational studies starts at low quality. A serious risk of bias and significant inconsistency was observed in the assessment of hospital readmission. Therefore, the quality of evidence was down-rated and reached very low. Also in the post-discharge all-cause mortality study, a high risk of bias and serious inconsistency was observed. Therefore, the certainty of the evidence was rated as very low (Supplementary Table 2).
4. Discussion
The present meta-analysis summarized the available pieces of evidence regarding hospital readmissions and post-discharge all-cause mortality in recovered COVID-19 patients. One-year follow-up showed that the prevalence of hospital readmissions and post-discharge all-cause mortality of recovered COVID-19 patients was 10.34% and 7.87%, respectively. Sensitivity analysis showed that the prevalence of hospital readmissions and post-discharge all-cause mortality in high-quality studies were 10.38% and 4.00%, respectively.
30-day hospital readmissions and post-discharge mortality were 8.97% and 7.87%, respectively. In addition, 30-day hospital readmissions and post-discharge mortality in high-quality studies were 9.98% and 3.24%, respectively. Therefore, most cases of hospital readmissions and mortality appear to occur within the first 30 days after discharge.
One of the interesting points in the present study was the higher hospital readmissions rate in developed countries compared to that of developing countries. The reason for this finding may be attributed to the higher medical benefits (better insurance coverage) provided in developed countries. Health insurance coverage in developing societies is much less widespread than in developed countries. In addition, access to medical services is limited in developing countries. Evidence shows that patients with poor insurance coverage account for a lower rate of readmissions. For example, Jeon et al. showed that the likelihood of readmission for COVID-19 patients with higher medical benefits is up to 5 times more than other patients [59]. Moreover, the data registries and follow-up of patients in developing countries in many instances do not exist, and in some other situations, patients' data loss is another obstacle. Therefore, the hospital readmissions rate may also be underestimated in developing countries.
A similar finding was observed for all-cause mortality. Post-discharge mortality was 7.78% in COVID-19 patients in developed countries and 3.84% in developing countries (Table 3). In addition to inaccurate tracking and recording of deaths in developing countries, the diversity of age distribution among communities should also be considered. The mean age of the population of developing countries is often lower than that of the population of developed countries, so this difference may be another reason for higher post-discharge all-cause mortality in developed countries compared to that of developing countries.
The relationship between age and increased post-discharge all-cause mortality of COVID-19 patients has been studied in some studies, the findings of which are sometimes contradictory. Some of these studies show a significant relationship between age and post-discharge all-cause mortality [39,48,60], while others do not report such a relationship [28,63]. The elderly population is heterogenous and suffers from various underlying diseases such as dementia, Parkinson's, and delirium, all of which affect the outcome of COVID-19 [[109], [110], [111]]. Therefore, differences between the populations included in these studies may be the cause of the contradictory findings. The present meta-analysis showed that age is a possible influencing factor on post-discharge all-cause mortality of COVID-19 patients. However, prospective studies need to be designed to examine the effect of age on mortality, alongside other confounders such as underlying disease.
The present study showed that the prevalence of hospital readmissions and post-discharge mortality is higher in COVID-19 patients with underlying diseases. This finding is somewhat in line with previous studies, showing that underlying diseases are possible risk factors of in-hospital outcomes of COVID-19 patients [[112], [113], [114], [115], [116]]. However, the number of studies examining risk factors for hospital readmissions and post-discharge mortality is small, and sometimes their quality is low due to various reasons. Drewett et al. (sample size = 169) showed that the presence of underlying disease is not associated with hospital readmissions [117]. While Joen et al., in their study of 7590 patients, showed that the risk of hospital readmissions increases to up to five times with increasing Charlson comorbidity index [18]. Also, Ramos-Martínez et al. showed that the presence of underlying respiratory diseases is a risk factor for hospital readmission, but there was no relationship between cardiovascular and kidney diseases and hospital readmissions [81]. Therefore, there seems to be a potential relationship between post-discharge of COVID-19 patients and underlying disease. But the current evidence is contradictory and comes from studies that have a low level of evidence and more research is needed in this field.
There were no eligibility criteria based on comorbidity in the current meta-analysis, and many hospitalized COVID-19 patients have at least one comorbidity [118,119]. Most of the included studies have been performed on a heterogeneous population of COVID-19 patients, from which some patients had a history of an underlying disease and others had no underlying diseases. On the other hand, many of comorbidities are exacerbated by COVID-19, such as cardiovascular diseases and coagulopathies [[112], [113], [114], [115], [116],120]. In general, it seems that even in the presence of comorbidity COVID-19 is likely to be the main cause of readmission, because most of these readmissions occur within the first month after discharge.
Sensitivity analysis showed that hospital readmissions and post-discharge all-cause mortality varied across the countries. Part of this difference is related to socio-economic differences between countries. However, the included sample sizes are small in some countries. The included number of patients in the analysis of hospital readmissions and post-discharge all-cause mortality was less than 1000 patients in 8 (out of 16 countries) and 6 (out of 13 countries) countries, respectively. In other words, in almost half of the countries, the sample size included in the analysis was low, and therefore, care should be taken to interpret the findings regarding countries with low sample sizes.
HIV infection was reported as a risk factor for COVID-19 mortality in previous studies [121] and may cause a severe form of SARS-CoV-2 infection. In our meta-analysis, only one study assessed the post-COVID-19 readmission rate in HIV-infected patients. We performed a sensitivity analysis to assess the effect of this study on the findings. Excluding the HIV population from the analysis did not change the overall readmission rate of COVID-19 patients (10.34% vs 10.31%). Therefore, in the current study HIV infection was not a source of heterogeneity.
Another limitation of the present study was the lack of reporting patients' discharge criteria in the included studies. The standard criterion for discharge of COVID-19 patients is two consecutive negative RT-PCR, in addition to symptoms improvement [122], which was used only in 18 studies. However, in the remaining 73 studies, discharge criteria were not reported or relied only on symptoms improvement. A review study found that of the 10 countries with the highest prevalence of COVID-19, five did not have a discharge criterion, and in the other five countries there was considerable diversity in discharge criteria. This review strongly recommends defining uniform, standard and simple criteria for hospital discharge of COVID-19 patients [123]. During the COVID-19 pandemic and its outbreaks, the lack of hospital beds, medical facilities, and human resources caused patients to be discharged too early, leading to increased hospital readmissions and possible post-discharge deaths. Therefore, it is very important to define and standard discharge criteria in the treatment protocols of COVID-19 patients, to reduce the hospital readmission rates and deaths following the disease. Finally, it should be noted that the included studies were heterogeneous. Nevertheless, the possible sources of heterogeneity were found to be differences in population age, underlying diseases, and country type, the residual source of heterogeneity remained unclear.
5. Conclusion
Although the level of evidence was calculated to be very low, the current meta-analysis showed that 10.34% of recovered COVID-19 required hospital readmissions after discharge. Also, the one-year post-discharge all-cause mortality rate of COVID-19 patients is 7.87%. Most hospital readmissions and post-discharge all-cause mortality appear to occur within 30 days post-discharge. Therefore, in addition to adopting a standard criterion for discharge of COVID-19 patients, a 30-day follow-up program and patient tracking system for discharged COVID-19 patients seems necessary. Further prospective cohort studies are needed to explore the independent risk factors of hospital readmission and post-discharge mortality of COVID-19 patients.
Funding
None.
Author contribution
ZSR participated in designing, data gathering, analysis, and drafting of the paper.
Availability of data
All data used in the present study will be made available to qualified researchers on reasonable request.
Declaration of Competing Interest
There is no conflict of interest.
Acknowledgment
The author would like to thank Dr. Muhammed IM Gubari, for his invaluable contribution in collecting and summarizing the articles.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajem.2021.10.059.
Appendix A. Supplementary data
Supplementary material
References
- 1.Jangra S., Ye C., Rathnasinghe R., Stadlbauer D., Alshammary H., Amoako A.A., et al. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021;2(7):E283–E284. doi: 10.1016/S2666-5247(21)00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hafeez S., Din M., Zia F., Ali M., Shinwari Z.K. Emerging concerns regarding COVID-19; second wave and new variant. J Med Virol. 2021;93(7):4108. doi: 10.1002/jmv.26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherian S., Potdar V., Jadhav S., Yadav P., Gupta N., Das M., et al. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms. 2021;9(7) doi: 10.3390/microorganisms9071542. 1542-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin S.-N., Rui J., Chen Q.-P., Zhao B., Yu S.-S., Li Z.-Y., et al. Effectiveness of potential antiviral treatments in COVID-19 transmission control: a modelling study. Infect Dis Poverty. 2021;10(1):53. doi: 10.1186/s40249-021-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousefifard M., Ali K.M., Aghaei A., Zali A., Neishaboori A.M., Zarghi A., et al. Corticosteroids on the management of coronavirus disease 2019 (COVID-19): a systemic review and meta-analysis. Iran J Public Health. 2020;49(8):1411. doi: 10.18502/ijph.v49i8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousefifard M., Zali A., Ali K.M., Neishaboori A.M., Zarghi A., Hosseini M., et al. Antiviral therapy in management of COVID-19: a systematic review on current evidence. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 8.Uyaroglu O.A., Basaran N.Ç., Özisik L., Dizman G.T., Eroglu I., Sahin T.K., et al. Thirty-day readmission rate of COVID-19 patients discharged from a tertiary care university hospital in Turkey: an observational, single-center study. International J Qual Health Care. 2021;33(1) doi: 10.1093/intqhc/mzaa144. mzaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy M.M. Using readmission rates as a quality indicator in sepsis—addressing the problem or adding to it? JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.12873. e2112873-e. [DOI] [PubMed] [Google Scholar]
- 10.Afriyie D.K., Asare G.A., Amponsah S.K., Godman B. COVID-19 pandemic in resource-poor countries: challenges, experiences and opportunities in Ghana. J Infect Dev Countries. 2020;14(08):838–843. doi: 10.3855/jidc.12909. [DOI] [PubMed] [Google Scholar]
- 11.Kuehn B.M. Hospital readmission is common among COVID-19 survivors. JAMA. 2020;324(24) doi: 10.1001/jama.2020.23910. 2477-. [DOI] [PubMed] [Google Scholar]
- 12.Bonifácio L.P., Pereira A.P.S., Araújo D.C.D.A., Balbão V.D.M.P., Fonseca B.A.L.D., Passos A.D.C., et al. Are SARS-CoV-2 reinfection and Covid-19 recurrence possible? A case report from Brazil. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0619-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gousseff M., Penot P., Gallay L., Batisse D., Benech N., Bouiller K., et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816–846. doi: 10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajaj J.S., Garcia-Tsao G., Wong F., Biggins S.W., Kamath P.S., McGeorge S., et al. Cirrhosis is associated with high mortality and readmissions over 90 days regardless of COVID-19: a multicenter cohort. Liver Transpl. 2021;27(9):1343–1347. doi: 10.1002/lt.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atalla E., Kalligeros M., Giampaolo G., Mylona E.K., Shehadeh F., Mylonakis E. Readmissions among patients with COVID-19. Int J Clin Pract. 2021;75(3) doi: 10.1111/ijcp.13700. [DOI] [PubMed] [Google Scholar]
- 16.Pan L., Wang R., Yu N., Hu C., Yan J., Zhang X., et al. Clinical characteristics of re-hospitalized COVID-19 patients with recurrent positive SARS-CoV-2 RNA: a retrospective study. Eur J Clin Microbiol Infect Dis. 2021;40(6):1245–1252. doi: 10.1007/s10096-020-04151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan M.J., Rabbani R., Anam A.M., Huq S.M.R., Polash M.M.I., Nessa S.S.T., et al. Impact of high dose of baricitinib in severe COVID-19 pneumonia: a prospective cohort study in Bangladesh. BMC Infect Dis. 2021;21(1):427. doi: 10.1186/s12879-021-06119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber B., Siddiqi H., Vieira J., Zhou G., Kim A., Rutherford H., et al. Relationships between myocardial injury, index hospitalization characteristics, and longer-term outcomes in sars-cov-2 infection. J Am Coll Cardiol. 2021;77(18):3025. [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Heart L, and Blood Institute National Heart, Lung, and Blood Institute quality assessment tool for observational cohort and cross-sectional studies USA: National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Available from:
- 21.Yousefifard M., Toloui A., Ahmadzadeh K., Gubari M.I.M., Neishaboori A.M., Amraei F., et al. Risk factors for road traffic injury-related mortality in Iran; a systematic review and meta-analysis. Arch Acad Emerg Med. 2021;2021(1):1–10. doi: 10.22037/aaem.v9i1.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 23.World Bank World Bank Country and Lending Groups United State: The World Bank Group. 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Available from:
- 24.An J.H., Liao X.J., Xiao T.Y., Qian S., Yuan J., Ye H.C., et al. Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Ann Transl Med. 2020;8(17):1084. doi: 10.21037/atm-20-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade Barreto A.P., Duarte L.C., Cerqueira-Silva T., Barreto Filho M.A., Camelier A., Tavares N.M., et al. Post-acute COVID syndrome, the aftermath of mild to severe COVID-19 in Brazilian patients. medRxiv. 2021 doi: 10.1101/2021.06.07.21258520. [Pre-print paper] [DOI] [Google Scholar]
- 26.Ayoubkhani D., Khunti K., Nafilyan V., Maddox T., Humberstone B., Diamond I., et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. The BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee J., Canamar C.P., Voyageur C., Tangpraphaphorn S., Lemus A., Coffey C., Jr., et al. Mortality and readmission rates among patients with COVID-19 after discharge from acute care setting with supplemental oxygen. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowles K.H., McDonald M., Barrón Y., Kennedy E., O’Connor M., Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316–325. doi: 10.7326/M20-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao H., Ruan L., Liu J., Liao W. The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J Med Virol. 2020;92(10):2159–2164. doi: 10.1002/jmv.26017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrillo-Garcia P., Garmendia-Prieto B., Cristofori G., Montoya I.L., Hidalgo J.J., Feijoo M.Q., et al. Health status in survivors older than 70 years after hospitalization with COVID-19: observational follow-up study at 3 months. Eur Geriatric Med. 2021;12:1091–1094. doi: 10.1007/s41999-021-00516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai C., Feng X., Lu M., Li S., Chen K., Wang H., et al. One-year mortality and consequences of COVID-19 in cancer patients: a cohort study. IUBMB Life. 2021;73(10):1244–1256. doi: 10.1002/iub.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhry Z., Shawe-Taylor M., Rampling T., Cutfield T., Bidwell G., Chan X.H.S., et al. Short durations of corticosteroids for hospitalised COVID-19 patients are associated with a high readmission rate. J Infect. 2021;82(6):276–316. doi: 10.1016/j.jinf.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J., Xu X.P., Hu J., Chen Q.D., Xu F.F., Liang H., et al. Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: a retrospective cohort study from Wuhan. China Aging-US. 2020;12(17):16675–16689. doi: 10.18632/aging.103795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S.L., Xu H., Feng H.Y., Sun J.F., Li X., Zhou L., et al. Epidemiological and clinical findings of short-term recurrence of severe acute respiratory syndrome coronavirus 2 ribonucleic acid polymerase chain reaction positivity in 1282 discharged coronavirus disease 2019 cases: a multicenter, retrospective, observational study. Open Forum Infect Dis. 2020;7(10):1–10. doi: 10.1093/ofid/ofaa432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J.J., Contractor J.H., Shaw A.L., Abdelghany Y., Frye J., Renzetti M., et al. COVID-19-related circumstances for hospital readmissions: a case series from 2 New York City hospitals. J Patient Saf. 2021;17(4):264–269. doi: 10.1097/PTS.0000000000000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connolly S.P., Katolo H.W., Cronin C., Creed M., Lambert J.S., Cotter A.G., et al. Home spo2 monitoring of patients with covid-19: the mater cvc project. Top Antiviral Med. 2021;29(1):289–290. [Google Scholar]
- 38.Divanoglou A., Samuelsson K., Sjodahl E.R., Andersson C., Levi R. Rehabilitation needs and mortality associated with the Covid-19 pandemic: a population-based study of all hospitalised and home-healthcare individuals in a Swedish healthcare region. EClinicalMedicine. 2021;36:100920. doi: 10.1016/j.eclinm.2021.100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnelly J.P., Wang X.Q., Iwashyna T.J., Prescott H.C. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2021;325(3):304–306. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426:117486. doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabriel S.G., García M.J.D., Mañas G.P., García N.M., Domínguez M.S., Andrés E.M. Biological markers and follow-up after discharge home of patients with covid-19 pneumonia. Emergencias. 2021;33(3):174–180. [Spanish] [PubMed] [Google Scholar]
- 42.García-Abellán J., Padilla S., Fernández-González M., García J.A., Agulló V., Andreo M., et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021:1–12. doi: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gąsior M., Jaroszewicz J., Wita K., Cieśla D., Hudzik B. High post-discharge mortality in hospitalized COVID-19 patients with cardiovascular comorbidities. Polish Arch Int Med. 2021;131(7–8):749–751. doi: 10.20452/pamw.16026. [DOI] [PubMed] [Google Scholar]
- 44.Giannis D., Allen S.L., Tsang J., Flint S., Pinhasov T., Williams S., et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137(20):2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon W.J., Henderson D., DeSharone A., Fisher H.N., Judge J., Levine D.M., et al. Remote patient monitoring program for hospital discharged COVID-19 patients. Appl Clin Inform. 2020;11(5):792–801. doi: 10.1055/s-0040-1721039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarin G., Lo K.B., Bhargav R., Salacup G., Wattoo A., Coignet J.G., et al. Factors associated with hospital readmissions among patients with COVID-19: a single-center experience. J Med Virol. 2021;93(9):5582–5587. doi: 10.1002/jmv.27104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gudipati S., Ranger J.L., Vahia A.T., Parraga Acosta T.J., Hanna Z.W., Nair S.N., et al. Long-term complications associated with COVID-19 infection. Open Forum Infect Dis. 2020;7(Suppl. 1) S261-S2. [Google Scholar]
- 48.Günster C., Busse R., Spoden M., Rombey T., Schillinger G., Hoffmann W., et al. 6-month mortality and readmissions of hospitalized COVID-19 patients: a nationwide cohort study of 8,679 patients in Germany. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutiérrez M.M.A., Rubio-rivas M., Gómez C.R., Sáez A.M., de Pedro I.P., Homs N., et al. Autoimmune diseases and covid-19 as risk factors for poor outcomes: data on 13,940 hospitalized patients from the spanish nationwide semi-covid-19 registry. J Clin Med. 2021;10(9):1844. doi: 10.3390/jcm10091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gwin M., Saleki M., Lampert H., Meo N., Bann M. Emergency department visits and readmissions after COVID-19 hospitalization: a cross-sectional analysis. Intern Emerg Med. 2021;16(6):1715–1718. doi: 10.1007/s11739-021-02644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herc E., Yared N.F., Kudirka A., Suleyman G. Characteristics and outcomes of COVID-19 patients with fungal infections. Open Forum Infect Dis. 2020;7(Suppl. 1):S253. [Google Scholar]
- 52.Hernández-Biette A., Sanz-Santos J., Boix-Palop L., Navarro Rolón A., Martínez-Palau M., de la Sierra Iserte A. Risk factors for later hospitalization of patients discharged from an emergency department with nonsevere COVID-19 symptoms. Emergencias. 2020;32(6):413–415. [Spanish] [PubMed] [Google Scholar]
- 53.Holloway M., Rickard F., Mitchell E., McCarthy K., Hesford J., Braude P. Readmission to secondary care following COVID-19. Eur Geriatric Med. 2020;11(Suppl. 1):S140. [Google Scholar]
- 54.Huang C.L., Huang L.X., Wang Y.M., Li X., Ren L.L., Gu X.Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang C.W., Desai P.P., Wei K.K., Liu I.A., Lee J.S., Nguyen H.Q. Characteristics of patients discharged and readmitted after COVID-19 hospitalisation within a large integrated health system in the United States. Infect Diseases (London, England) 2021;53(10):800–804. doi: 10.1080/23744235.2021.1924398. [DOI] [PubMed] [Google Scholar]
- 56.Islam N., Lewington S., Kharbanda R.K., Davies J., Varnai K.A., Lacey B. Sixty-day consequences of COVID-19 in patients discharged from hospital: an electronic health records study. Eur J Public Health. 2021;31(2):280–282. doi: 10.1093/eurpub/ckab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain E., Harmon E.Y., Sonagere M.B. Functional outcomes and post-discharge care sought by patients with COVID-19 compared to matched controls after completing inpatient acute rehabilitation. PM R. 2021;13:618–625. doi: 10.1002/pmrj.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jalilian Khave L., Vahidi M., Shirini D., Sanadgol G., Ashrafi F., Arab-Ahmadi M., et al. Clinical and epidemiological characteristics of postdischarge patients with COVID-19 in Tehran, Iran: protocol for a prospective cohort study (tele-COVID-19 study) JMIR Res Protocols. 2021;10(2) doi: 10.2196/23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeon W.H., Seon J.Y., Park S.Y., Oh I.H. Analysis of risk factors on readmission cases of COVID-19 in the Republic of Korea: using nationwide health claims data. Int J Environ Res Public Health. 2020;17(16):5844. doi: 10.3390/ijerph17165844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kingery J.R., Bf Martin P., Baer B.R., Pinheiro L.C., Rajan M., Clermont A., et al. Thirty-day post-discharge outcomes following COVID-19 infection. J Gen Intern Med. 2021;36(8):2378–2385. doi: 10.1007/s11606-021-06924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirkegaard C., Falcó-Roget A., Sánchez-Montalvá A., Valls Á., Clofent D., Campos-Varela I., et al. Incidence and risk factors for early readmission after hospitalization for SARS-CoV-2 infection: results from a retrospective cohort study. Infection. 2021 doi: 10.1007/s15010-021-01662-1. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lavery A.M., Preston L.E., Ko J.Y., Chevinsky J.R., DeSisto C.L., Pennington A.F., et al. Characteristics of hospitalized COVID-19 patients discharged and experiencing same-hospital readmission - United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1695–1699. doi: 10.15585/mmwr.mm6945e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee P.J., Sirichand S., Gutierrez N.R., Corro L.G., Cruz C., Grossberg R., et al. Hiv patients with COVID-19 in the bronx: a retrospective cohort study. Open Forum Infect Dis. 2020;7(Suppl. 1) S335-S6. [Google Scholar]
- 64.Leijte W.T., Wagemaker N.M.M., van Kraaij T.D.A., de Kruif M.D., Mostard G.J.M., Leers M.P.G., et al. Mortality and re-admission after hospitalization with COVID-19. Ned Tijdschr Geneeskd. 2020;164(49):D5423. [PubMed] [Google Scholar]
- 65.Leon L., Perez-Sancristobal I., Garcia A.M., Pedraza L.L., Colomer J.I., Lara S.L., et al. Post discharge persistent symptoms after COVID-19 in rheumatic and musculoskeletal diseases. medRxiv. 2021 doi: 10.1093/rap/rkac008. [Pre-print paper] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C., Luo F., Xie L., Gao Y., Zhang N., Wu B. Chest CT study of fifteen COVID-19 patients with positive RT-PCR retest results after discharge. Quant Imaging Med Surg. 2020;10(6):1318–1324. doi: 10.21037/qims-20-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo L., Liu D., Zhang Z., Li Z., Xie C., Wang Z., et al. Probable causes and risk factors for positive SARS-CoV-2 testing in recovered patients: evidence from Guangzhou. China Front Med. 2021;8:684101. doi: 10.3389/fmed.2021.684101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maestre-Muñiz M.M., Arias Á., Mata-Vázquez E., Martín-Toledano M., López-Larramona G., Ruiz-Chicote A.M., et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10(13):2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medler C.J., Jakupovic L., Weinmann A.J., Kenney R., Davis S.L. Outcomes of empiric antimicrobial therapy in COVID-19 positive patients. Open Forum Infect Dis. 2020;7(Suppl. 1) S264-S5. [Google Scholar]
- 70.Medranda G.A., Fazlalizadeh H., Case B.C., Yerasi C., Zhang C., Rappaport H., et al. Implications of left ventricular function on short-term outcomes in COVID-19 patients with myocardial injury. Cardiovasc Revasc Med. 2021;29:45–49. doi: 10.1016/j.carrev.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meije Y., Duarte-Borges A., Sanz X., Clemente M., Ribera A., Ortega L., et al. Long-term outcomes of patients following hospitalization for coronavirus disease 2019: a prospective observational study. Clin Microbiol Infect. 2021;27(8):1151–1157. doi: 10.1016/j.cmi.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menges D., Ballouz T., Anagnostopoulos A., Aschmann H.E., Domenghino A., Fehr J.S., et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mooney C.J., Hone L., Majid M., Cai J., Mieiro L., Fink D.L. A single Centre study on the thirty-day hospital reattendance and readmission of older patients during the SARS-CoV-2 pandemic. Age Ageing. 2021;50(Suppl. 1):I1. [Google Scholar]
- 74.Navvas J., Varghese R., Selvakannan B., Narayan Y., Newman O., Butt M., et al. COVID-19 post-discharge mortality rate in a London District general hospital. Thorax. 2021;76(Suppl. 1) A186-A7. [Google Scholar]
- 75.Nematshahi M., Neamatshahi M., Rahimi F., Soroosh D. Factors predicting readmission in patients with COVID-19. BMC Res Notes. 2021;14 doi: 10.1186/s13104-021-05782-7. 374-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parra L.M., Cantero M., Morrás I., Vallejo A., Diego I., Jiménez-Tejero E., et al. Hospital readmissions of discharged patients with covid-19. Int J Gen Med. 2020;13:1359–1366. doi: 10.2147/IJGM.S275775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pettit N.N., Nguyen C.T., Lew A.K., Bhagat P.H., Nelson A., Olson G., et al. Reducing the use of empiric antibiotic therapy in COVID-19 on hospital admission. BMC Infect Dis. 2021;21(1):516. doi: 10.1186/s12879-021-06219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pourhoseingholi M.A., Jafari R., Jafari N.J., Rahimi-Bashar F., Nourbakhsh M., Vahedian-Azimi A., et al. Predicting 1-year post-COVID-19 mortality based on chest computed tomography scan. J Med Virol. 2021;93(10):5694–5696. doi: 10.1002/jmv.27146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiao X.M., Xu X.F., Zi H., Liu G.X., Li B.H., Du X., et al. Re-positive cases of nucleic acid tests in discharged patients with COVID-19: a follow-up study. Front Med. 2020;7:349. doi: 10.3389/fmed.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quilliot D., Gérard M., Bonsack O., Malgras A., Vaillant M.F., Di Patrizio P., et al. Impact of severe SARS-CoV-2 infection on nutritional status and subjective functional loss in a prospective cohort of COVID-19 survivors. BMJ Open. 2021;11(7) doi: 10.1136/bmjopen-2021-048948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramos-Martínez A., Parra-Ramírez L.M., Morrás I., Carnevali M., Jiménez-Ibañez L., Rubio-Rivas M., et al. Frequency, risk factors, and outcomes of hospital readmissions of COVID-19 patients. Sci Rep. 2021;11(1):13733. doi: 10.1038/s41598-021-93076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reyes Gil M., Gonzalez-Lugo J.D., Rahman S., Barouqa M., Szymanski J., Ikemura K., et al. Correlation of coagulation parameters with clinical outcomes during the coronavirus-19 surge in New York: observational cohort. Front Physiol. 2021;12:618929. doi: 10.3389/fphys.2021.618929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. Jama. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez V.A., Bhave S., Chen R., Pang C., Hripcsak G., Sengupta S., et al. Development and validation of prediction models for mechanical ventilation, renal replacement therapy, and readmission in COVID-19 patients. JJAMIA. 2021;27(8):1480–1488. doi: 10.1093/jamia/ocab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roig-Marín N., Roig-Rico P. Elderly people with dementia admitted for COVID-19: how different are they? Exp Aging Res. 2021 doi: 10.1080/0361073X.2021.1943794. [Ahead of print] [DOI] [PubMed] [Google Scholar]
- 86.Romero-Duarte A., Rivera-Izquierdo M., de Alba I.G.F., Perez-Contreras M., Fernandez-Martinez N.F., Ruiz-Montero R., et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19:129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saab F.G., Chiang J.N., Brook R., Adamson P.C., Fulcher J.A., Halperin E., et al. Discharge clinical characteristics and post-discharge events in patients with severe COVID-19: a descriptive case series. J Gen Intern Med. 2021;36(4):1017–1022. doi: 10.1007/s11606-020-06494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shallal A., Morrison A.R., Brar I., Suleyman G. Complications and outcomes of obese patients hospitalized with COVID-19. Open Forum Infect Dis. 2020;7(Suppl. 1):S248. [Google Scholar]
- 89.Siddiqui M.A., Suresh S., Simmer S., Abu-Ghanimeh M., Karrick M., Nimri F., et al. Increased morbidity and mortality in COVID-19 patients with liver injury. Dig Dis Sci. 2021 doi: 10.1007/s10620-021-07007-0. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Somani S.S., Richter F., Fuster V., De Freitas J.K., Naik N., Sigel K., et al. Characterization of patients who return to hospital following discharge from hospitalization for COVID-19. J Gen Intern Med. 2020;35(10):2838–2844. doi: 10.1007/s11606-020-06120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spence D., Barclay C., Latham S., Richardson A., Dexter J., Ghosh S. Short-term outcomes in elderly patients with SARS-CoV-2 infections discharged from community hospitals. J Clin Rev Case Rep. 2021;6(9):609–611. [Google Scholar]
- 92.Spinicci M., Vellere I., Graziani L., Tilli M., Borchi B., Mencarini J., et al. Clinical and laboratory follow-up after hospitalization for COVID-19 at an Italian tertiary care center. Open Forum Infect Dis. 2021;8(3) doi: 10.1093/ofid/ofab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stockmann H., Hardenberg J.H.B., Hinze C., Aigner A., Inka G., Stier B., et al. 90-day post-hospital follow-up in survivors after COVID-19-associated acute kidney injury requiring kidney replacement therapy. Kidney Int Rep. 2021;6(4):S24. doi: 10.1016/j.kint.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suárez-Robles M., Iguaran-Bermúdez M.D.R., García-Klepizg J.L., Lorenzo-Villalba N., Méndez-Bailón M. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call check list. Pan Afr Med J. 2020;37:289. doi: 10.11604/pamj.2020.37.289.27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian M., Long Y., Hong Y., Zhang X., Zha Y. The treatment and follow-up of ‘recurrence’ with discharged COVID-19 patients: data from Guizhou. China Environ Microbiol. 2020;22(8):3588–3592. doi: 10.1111/1462-2920.15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Todt B.C., Szlejf C., Duim E., Linhares A.O.M., Kogiso D., Varela G., et al. Clinical outcomes and quality of life of COVID-19 survivors: a follow-up of 3 months post hospital discharge. Respir Med. 2021;184:106453. doi: 10.1016/j.rmed.2021.106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van den Borst B., Peters J.B., Brink M., Schoon Y., Bleeker-Rovers C.P., Schers H., et al. Comprehensive health assessment 3 months after recovery from acute Coronavirus Disease 2019 (COVID-19) Clin Infect Dis. 2020;73(5):e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venturelli S., Benatti S.V., Casati M., Binda F., Zuglian G., Imeri G., et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149 doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verna E.C., Landis C., Brown R.S., Mospan A.R., Crawford J.M., Hildebrand J.S., et al. Factors associated with readmission in the US following hospitalization with COVID-19. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab464. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X., Xu H., Jiang H., Wang L., Lu C., Wei X., et al. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM. 2020;113(9):657–665. doi: 10.1093/qjmed/hcaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Q., Hou X.W., Li H.W., Guo J., Li Y.J., Yang F.F., et al. A follow-up study of respiratory and physical function after discharge in patients with redetectable positive SARS-CoV-2 nucleic acid results following recovery from COVID-19. Int J Infect Dis. 2021;107:5–11. doi: 10.1016/j.ijid.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan N., Wang W., Gao Y.Z., Zhou J.H., Ye J.H., Xu Z.P., et al. Medium term follow-up of 337 patients with coronavirus disease 2019 (COVID-19) in a Fangcang shelter hospital in Wuhan, China. Front Med. 2020;7:373. doi: 10.3389/fmed.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang C., Jiang M., Wang X., Tang X., Fang S., Li H., et al. Viral RNA level, serum antibody responses, and transmission risk in recovered COVID-19 patients with recurrent positive SARS-CoV-2 RNA test results: a population-based observational cohort study. Emerg Microb Infect. 2020;9(1):2368–2378. doi: 10.1080/22221751.2020.1837018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ye S., Hiura G., Fleck E., Garcia A., Geleris J., Lee P., et al. Hospital readmissions after implementation of a discharge care program for patients with COVID-19 illness. J Gen Intern Med. 2021;36:722–729. doi: 10.1007/s11606-020-06340-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ye X., Yuan Y., Huang R., Cheng A., Yu Z., Huang Z., et al. Clinical characteristics of patients with re-admitted of novel coronavirus 2019 (nCOVID-19) in Wenzhou, China. Front Public Health. 2021;9:649178. doi: 10.3389/fpubh.2021.649178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeo I., Baek S., Kim J., Elshakh H., Voronina A., Lou M.S., et al. Assessment of thirty-day readmission rate, timing, causes and predictors after hospitalization with COVID-19. J Intern Med. 2021;290(1):157–165. doi: 10.1111/joim.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan J., Kou S., Liang Y., Zeng J., Pan Y., Liu L. Polymerase chain reaction assays reverted to positive in 25 discharged patients with COVID-19. Clin Infect Dis. 2020;71(16):2230–2232. doi: 10.1093/cid/ciaa398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng J.Z., Zhou R., Chen F.J., Tang G.F., Wu K.Y., Li F.R., et al. Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID-19 patients in Guangzhou, China: a prospective cohort study. PLoS Negl Trop Dis. 2020;14(8) doi: 10.1371/journal.pntd.0008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Putri C., Hariyanto T.I., Hananto J.E., Christian K., Situmeang R.F.V., Kurniawan A. Parkinson’s disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: a systematic review, meta-analysis, and meta-regression. Parkinsonism Relat Disord. 2021;87:155–161. doi: 10.1016/j.parkreldis.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hariyanto T.I., Putri C., Hananto J.E., Arisa J., Fransisca V., Situmeang R., et al. Delirium is a good predictor for poor outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and meta-regression. J Psychiatr Res. 2021;142:361–368. doi: 10.1016/j.jpsychires.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hariyanto T.I., Putri C., Situmeang R.F.V., Kurniawan A. Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID-19) infection. Eur Arch Psychiatry Clin Neurosci. 2021;271(2):393–395. doi: 10.1007/s00406-020-01205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pishgahi M., Toudeshki K.K., Safari S., Yousefifard M. Echocardiographic abnormalities as independent prognostic factors of in-hospital mortality among COVID-19 patients. Arch Acad Emerg Med. 2021;9(1) doi: 10.22037/aaem.v9i1.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rismanbaf A., Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy; a letter to editor. Arch Acad Emerg Med. 2020;8(1) e17-e. [PMC free article] [PubMed] [Google Scholar]
- 114.Safari S., Mehrani M., Yousefifard M. Pulmonary thromboembolism as a potential cause of clinical deterioration in COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 115.Toloui A., Moshrefiaraghi D., Neishaboori A.M., Yousefifard M., Aghajani M.H. Cardiac complications and pertaining mortality rate in COVID-19 patients; a systematic review and meta-analysis. Arch Acad Emerg Med. 2021;9(1) doi: 10.22037/aaem.v9i1.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zali A., Gholamzadeh S., Mohammadi G., Looha M.A., Akrami F., Zarean E., et al. Baseline characteristics and associated factors of mortality in COVID-19 Patients; an analysis of 16000 cases in Tehran, Iran. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 117.Drewett G.P., Chan R.K., Jones N., Wimaleswaran H., Howard M.E., McDonald C.F., et al. Risk factors for readmission following inpatient management of COVID-19 in a low-prevalence setting. Intern Med J. 2021;51(5):821–823. doi: 10.1111/imj.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dorjee K., Kim H., Bonomo E., Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mithal A., Jevalikar G., Sharma R., Singh A., Farooqui K.J., Mahendru S., et al. High prevalence of diabetes and other comorbidities in hospitalized patients with COVID-19 in Delhi, India, and their association with outcomes. Diabetes Metab Syndr Clin Res Rev. 2021;15(1):169–175. doi: 10.1016/j.dsx.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pasquarelli-do-Nascimento G., Braz-de-Melo H.A., Faria S.S., Santos I.D.O., Kobinger G.P., Magalhães K.G. Hypercoagulopathy and adipose tissue exacerbated inflammation may explain higher mortality in COVID-19 patients with obesity. Front Endocrinol. 2020;11(530) doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hariyanto T.I., Rosalind J., Christian K., Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: a systematic review and meta-analysis. South Afr J HIV Med. 2021;22(1):1220. doi: 10.4102/sajhivmed.v22i1.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Europe: 2020. Discharge criteria for confirmed COVID-19 cases –When is it safe to discharge COVID-19 cases from the hospital or end home isolation? 16 Oct 2020. [Google Scholar]
- 123.Sze S., Pan D., Williams C.M.L., Barker J., Minhas J.S., Miller C.J., et al. The need for improved discharge criteria for hospitalised patients with COVID-19—implications for patients in long-term care facilities. Age Ageing. 2020;50(1):16–20. doi: 10.1093/ageing/afaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
All data used in the present study will be made available to qualified researchers on reasonable request.