Abstract
Fecal excretion of hepatitis A virus (HAV) in 18 patients with HAV infection was evaluated by enzyme immunoassay (EIA) to detect viral antigen and by reverse transcription-PCR amplification followed by ethidium bromide staining (PCR-ETBr) or nucleic acid hybridization (PCR-NA) to detect viral genetic material. A gradation of sensitivity was observed in the detection of virus by the three methods. In persons who had detectable virus, serial stool samples were found to be positive by EIA for up to 24 days after the peak elevation of liver enzymes. Viral genetic material could be detected by PCR-ETBr for up to 34 days and by PCR-NA for up to 54 days after the peak elevation of liver enzymes. After intravenous inoculation of tamarins with stool suspensions categorized as highly reactive for HAV (positive by EIA, PCR-ETBr, and PCR-NA), moderately reactive (positive by PCR-ETBr and PCR-NA), or weakly reactive (positive by PCR-NA), only tamarins infected with highly reactive stool suspensions (EIA positive) developed HAV infection. We conclude that positivity of stool specimens for HAV by PCR-ETBr or PCR-NA indicates a lower potential for infectivity, compared to that of EIA-positive stools.
Hepatitis A virus (HAV) is most commonly transmitted by the fecal-oral route. Several aspects of the pathogenesis of HAV infection, including the duration of HAV excretion in stool and the duration of infectivity, are not fully characterized. In addition, the relationship between detection of HAV genetic material in stool and infectivity has not been established. During an outbreak of hepatitis A in an institution for the developmentally disabled in August 1990, we collected serial stool specimens from patients with HAV infection. These stool specimens were evaluated for the presence of HAV and for infectivity in tamarins. The objectives of these studies were (i) to assess the duration of HAV excretion detected by enzyme immunoassay (EIA) and the PCR in adults and (ii) to evaluate the relationship between detection of HAV antigens and genetic material in stools and animal infectivity.
MATERIALS AND METHODS
Patient identification and specimen collection.
In August and September 1990, an outbreak of hepatitis A occurred among residents at an institution for the developmentally disabled in Boulder County, Montana. Overall, 36 of 174 residents at the institution developed HAV infection, defined as positivity for immunoglobulin M antibody to HAV (IgM anti-HAV). The average age of the patients was 39 years (range, 28 to 59 years). In a retrospective cohort study conducted to identify risk factors for the acquisition of hepatitis A, frozen strawberries were implicated as the source of infection. The details of this outbreak investigation have been presented in a previous publication (10). At the outset of the outbreak investigation, 13 residents were identified with symptomatic acute hepatitis A, and immune globulin (IG) was administered to 143 other residents determined to be susceptible by serologic testing. These residents were then monitored by weekly serologic testing for liver enzyme elevations and hepatitis A markers, for up to 8 weeks. During the subsequent 2 weeks, an additional 23 (16%) of these 143 residents became positive for IgM anti-HAV (Abbott Laboratories, North Chicago, Ill.). Of these 23 residents, 7 (30%) had symptomatic HAV infection (jaundice), and 16 (70%) were identified by IgM anti-HAV seroconversion. IgM anti-HAV is found only associated with acute cases of HAV and would therefore not be present in the IG.
Serum specimens were tested at the Hepatitis Branch, Centers for Disease Control and Prevention, for total and IgM anti-HAV by EIA (Abbott Laboratories).
Virus detection.
For patients with elevated liver enzymes, stool specimens were collected and evaluated for evidence of HAV. Stool suspensions were prepared at a 20% (wt/vol) concentration in phosphate-buffered saline (pH 7.4) and clarified by centrifugation at 7,000 × g for 10 min at 4°C. Virus antigen was detected by EIA with rabbit anti-HAV as the capture antibody and hyperimmune chimpanzee anti-HAV serum as the detector antibody (16). The presence of viral nucleic acid was determined by immunocapture of the virus followed by reverse transcription-PCR amplification using primers targeted to the VP1 amino terminus (13). Amplified products were separated by agarose gel electrophoresis followed by detection with ethidium bromide staining (ETBr) or nucleic acid hybridization (NA) (12).
Animal infectivity of virus antigen of RNA-positive stools.
Six anti-HAV-negative tamarins, Saguinus mystax, were divided into three groups of two animals each. The animals were cared for and housed under Association for the Assessment and Accreditation of Laboratory Animal Care International, Inc.-approved animal husbandry conditions at the Animal Resources Branch, Scientific Resources Program, National Center for Infectious Diseases, Centers for Disease Control and Prevention, as outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. Each group was intravenously inoculated with a different stool specimen from patient 1. Intravenous inoculations were used to ensure optimal infectivity. The stool specimens were categorized by the level of detectable viral antigen or RNA. Group 1 was inoculated with 1 ml of filtered human stool suspension that was positive for viral antigen, as determined by EIA, and contained viral nucleic acid as determined by a strong ETBr band. Group 2 was inoculated with 1 ml of stool suspension that did not contain sufficient viral antigen to be detected by EIA but did contain viral nucleic acid, as determined by PCR amplification, and resulted in a weak band by ETBr. The third group of tamarins were inoculated with 1 ml of stool suspension that did not contain sufficient viral antigen for EIA detection but was positive for viral nucleic acid, as determined by PCR amplification followed by NA (PCR-NA). Serum specimens were collected twice a week and tested for liver enzyme levels and total anti-HAV (HAVAB; Abbott laboratories). Stools, collected daily and prepared as 20% suspensions as described above, were evaluated for virus excretion by EIA and PCR-NA.
RESULTS
Virus excretion.
Stool samples were collected from a total of 18 patients, 7 of the original 13 patients identified in the outbreak and 11 of the 23 patients (all of whom had received IG) identified on follow-up.
Of the seven original hepatitis A patients, stools were obtained a median of 8 days (range, 4 days to 42 days) following peak liver enzyme abnormalities. HAV could not be detected in any of these stool specimens by EIA for viral antigen, PCR-ETBr, or PCR-NA.
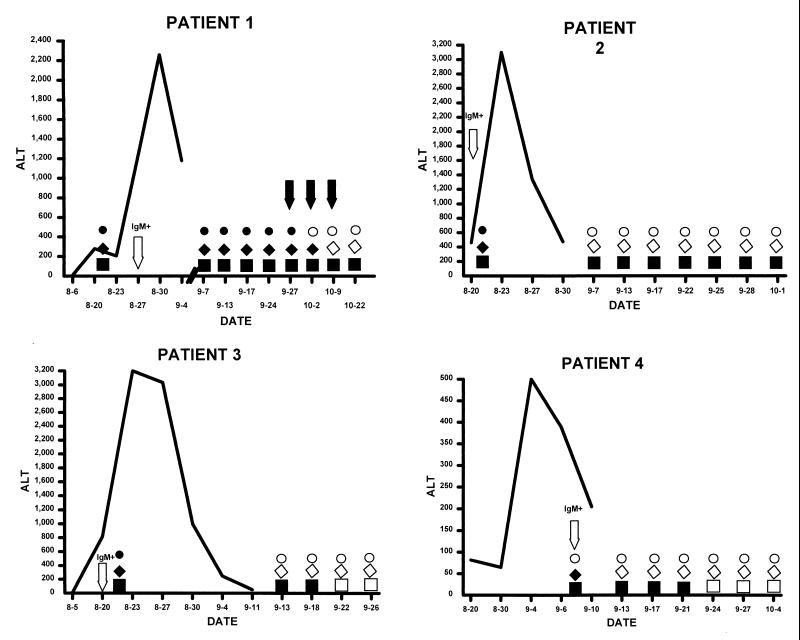
Among the 11 patients for whom stools were obtained on follow-up, HAV could be detected in the stools of 5 patients. One of the patients had detectable HAV by EIA and PCR-ETBr at 13 days following peak liver enzyme abnormalities; however, by 27 days, HAV could no longer be detected. Among the other four patients, HAV was detectable in their stools for periods ranging from 25 to 52 days after peak elevation of alanine amino transferase levels (Fig. 1). In the remaining six patients, HAV could not be detected in stool samples initially collected 4 to 21 days following peak liver enzyme abnormalities.
FIG. 1.
Patterns of disease and virus excretion in four patients who received IG. Elevations of liver enzyme (alanine amino transferase [ALT]) are indicated by the solid line (measured in international units per liter). Solid circles indicate virus excretion as detected by EIA, while solid diamonds indicate virus excretion as detected by PCR-ETBr. Solid squares depict virus detected by PCR-NA. The arrows for patient 1 indicate the specimens that were used for animal infectivity studies (dates, 27 September and 2 and 9 October). Dates on the x axis are given as month-day.
Viral infectivity studies.
During follow-up, stool samples from patient 1 were collected at nine different time points (Fig. 1). The levels of HAV reactivity in the stool samples from this patient were classified into three categories: highly reactive (viral antigen detected with EIA and strongly positive by PCR-ETBr [sample date, 27 September]), moderately reactive (EIA negative but weakly positive by PCR-ETBr [sample date, 2 October]), and weakly reactive (EIA negative and PCR-ETBr negative but PCR-NA positive [sample date, 9 October]).
Six tamarins, two for each category, were administered stools (intravenously) from each of these categories. The two tamarins who were administered the stool suspension classified as highly reactive (EIA positive) developed serologically confirmed hepatitis A (IgM anti-HAV) within 15 to 21 days, developed liver enzyme elevations, and shed virus as detected by PCR-NA. The other tamarins who were inoculated with stool specimens categorized as moderately reactive and weakly reactive did not develop any signs of liver disease, virus replication, or detectable anti-HAV.
DISCUSSION
Early studies using immune electron microscopy conducted shortly after the discovery of HAV in the 1970s suggested that most fecal shedding occurred prior to the onset of disease symptoms and that little or no virus was shed after the peak in liver function abnormalities or the appearance of jaundice (3). Using more sensitive techniques, including radioimmunoassay, others have found that fecal shedding may persist for as long as 3 weeks after the onset of jaundice (1, 2). More recent studies using the highly sensitive PCR have indicated that infected children and adults can excrete HAV for 3 months or longer (7, 17).
The duration of infectivity and the relationship of detection of HAV in stools and infectivity have not been established. Initial studies of infectivity in human volunteers showed that stools collected 3 to 4 weeks after the onset of symptoms did not produce disease (5, 6, 9). In epidemiologic studies, evaluation of the timing when secondary cases occur indicates that the period of infectivity does not last substantially beyond the onset of symptoms (4). In one outbreak investigation, however, HAV shedding in infected neonates was demonstrated by using PCR for up to 5 months following the onset of infection, and a health care worker was determined to have acquired infection from an infected neonate 5 months after the onset of infection (14).
Using sensitive techniques, we have demonstrated that in adults, excretion of HAV antigen and RNA can persist for as long as 54 days following peak liver function abnormalities. At the doses given, infectivity studies revealed that only stool samples containing sufficient HAV to be detected by EIA were infectious when inoculated into tamarins. The stool suspension studied in this investigation that was EIA positive and resulted in tamarin infection was collected approximately 30 days after the peak of liver enzyme elevations.
That PCR-positive, EIA-negative specimens were not infectious when administered to tamarins is somewhat unexpected, since previous studies have shown that inocula titrated in tamarins to contain 106 tamarin infectious doses/ml were positive by PCR-ETBr at 10−1 dilution and PCR-NA at 10−4 dilution (13a). The most likely explanation for the lack of tamarin infectivity despite detection of viral nucleic acid by PCR is a high ratio of physical particles to infectious particles caused by the presence of degraded or defective virus particles (11). A similar phenomenon may explain why PCR-positive samples from factor VIII preparations that were implicated in a hepatitis A outbreak among hemophiliacs failed to cause infection or disease when inoculated into chimpanzees (8). These findings indicate that PCR positivity, while reflecting the presence of viral genetic material and the potential for infectivity, does not necessarily correlate with animal infectivity. Infectivity is dependent upon multiple parameters involving both the virus and the host, including the ratio of physical to infectious particles, the presence of genetically defective virus particles, the inoculum dose given, and the relative sensitivity of the animal model.
In this study, HAV antigen and RNA could not be detected in stools obtained a median of 8 days after the peak of liver enzyme elevation from patients who had not received IG but could be detected at this time point in stools from patients who had received IG during the incubation period. The precise timing of the administration of IG in relation to the time of exposure could not be determined, and it is difficult to determine whether IG may have had an effect on the duration of excretion. While administration of IG late in the incubation period has been shown to increase the likelihood of asymptomatic versus symptomatic infection, a possible effect on duration of excretion has not been observed previously (15). In addition, passively administered IG reflects only a humoral defense against virus replication, and it may be that our inability to detect HAV RNA and antigen in infected persons who had not received IG is a reflection of virus clearance mediated by both humoral and cellular immune responses. However, additional carefully controlled studies are needed to differentiate these possibilities.
ACKNOWLEDGMENT
We thank Janice Lewis for her assistance in these studies.
REFERENCES
- 1.Carl M, Kantor R J, Webster H M, Fields H F, Maynard J E. Excretion of hepatitis A virus in the stools of hospitalized hepatitis patients. J Med Virol. 1982;9:125–129. doi: 10.1002/jmv.1890090207. [DOI] [PubMed] [Google Scholar]
- 2.Coulepis A G, Locarnini S A, Lehmann N I, Gust I D. Detection of hepatitis A virus in the feces of patients with naturally acquired infections. J Infect Dis. 1980;141:151–156. doi: 10.1093/infdis/141.2.151. [DOI] [PubMed] [Google Scholar]
- 3.Dienstag J L, Kapikian A Z, Feinstone S M, Purcell R H, Boggs J D, Conrad M E. Fecal shedding of hepatitis A antigen. Lancet. 1975;i:765–767. doi: 10.1016/s0140-6736(75)92434-4. [DOI] [PubMed] [Google Scholar]
- 4.Hadler S C, Erben J J, Matthews D, Starko K, Francis D P, Maynard J E. Effect of immunoglobulin on hepatitis A in day-care centers. JAMA. 1983;249:48–53. [PubMed] [Google Scholar]
- 5.Havens W P, Ward R, Drill V A, Paul J R. Experimental production of hepatitis by feeding icterogenic materials. Proc Soc Exp Biol Med. 1944;57:206–208. [Google Scholar]
- 6.Havens W P. Period of infectivity of patients with experimentally induced infectious hepatitis. J Exp Med. 1946;83:251–258. [PubMed] [Google Scholar]
- 7.Innis B L, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik C A, Suntayakorn S, et al. Protection against hepatitis A by an inactivated vaccine. JAMA. 1994;27:1328–1333. [PubMed] [Google Scholar]
- 8.Mannucci P M, Gdovin S, Gringeri A, Colombo M, Mele A, Schinaia N, Ciavarella N, et al. Transmission of hepatitis A to patients with hemophilia by factor VIII concentrates treated with organic solvent and detergent to inactivate viruses. Ann Intern Med. 1994;120:1–7. doi: 10.7326/0003-4819-120-1-199401010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Neefe J R, Gellis S S, Stokes J. Homologous serum hepatitis and infectious (epidemic) hepatitis: studies in volunteers bearing an immunological and other characteristics of the etiological agent. Am J Med. 1946;1:3–22. doi: 10.1016/0002-9343(46)90017-4. [DOI] [PubMed] [Google Scholar]
- 10.Niu M T, Polish L B, Robertson B H, Khanna B K, Woodruff B A, Shapiro C N, Miller M A, et al. Multistate outbreak of hepatitis A associated with frozen strawberries. J Infect Dis. 1992;166:518–524. doi: 10.1093/infdis/166.3.518. [DOI] [PubMed] [Google Scholar]
- 11.Nuesch J P, de Chastonay J, Siegl G. Detection of defection genomes in hepatitis A virus particles present in clinical specimens. J Gen Virol. 1989;70:3475–3480. doi: 10.1099/0022-1317-70-12-3475. [DOI] [PubMed] [Google Scholar]
- 12.Robertson B H, Brown V K, Khanna B. Altered hepatitis A VP1 protein resulting from cell culture propagation of virus. Virus Res. 1989;13:207–212. doi: 10.1016/0168-1702(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 13.Robertson B H, Khanna B, Nainan O V, Margolis H S. Epidemiologic patterns of wild-type hepatitis A virus determined by genetic variation. J Infect Dis. 1991;163:286–292. doi: 10.1093/infdis/163.2.286. [DOI] [PubMed] [Google Scholar]
- 13a.Robertson, B. Unpublished data.
- 14.Rosenblum L S, Villarino M E, Nainan O V, Melish M E, Hadler S C, Pinsky P P, Jarvis W R, et al. Hepatitis A outbreak in a neonatal intensive care unit: risk factors for transmission and evidence of prolonged viral excretion among preterm infants. J Infect Dis. 1991;164:476–482. doi: 10.1093/infdis/164.3.476. [DOI] [PubMed] [Google Scholar]
- 15.Stokes J, Jr, Neefe J R. The prevention and attenuation of infectious hepatitis by gamma globulin. JAMA. 1945;127:144–145. [Google Scholar]
- 16.Wheeler C M, Robertson B H, Van Nest G, Dina D, Bradley D W, Fields H A. Structure of hepatitis A virion: peptide mapping of the capsid region. J Virol. 1986;58:307–313. doi: 10.1128/jvi.58.2.307-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yotsuyanagi H, Koike K, Yasuda K, Moriya K, Shintani Y, Fujie H, Kurokawa K, et al. Prolonged fecal excretion of hepatitis A virus in adult patients with hepatitis A as determined by polymerase chain reaction. Hepatology. 1996;24:10–130. doi: 10.1053/jhep.1996.v24.pm0008707246. [DOI] [PubMed] [Google Scholar]